Some sections of this work are incomplete but have been posted as is. They
will be updated and queries resolved as time permits. Apart from files not yet complete, distribution maps, some figures and habitat photographs are the main
items to be added. A question mark may appear in the text appended to items that need to be checked by me. Numerous queries have led me to post incomplete material.
This work has been carried out over a period of 40 years, starting in 1971. I arrived in Iran in January 1976
and, in that year, 7 articles were published
strictly on Iranian fishes (3 on parasites, 1 on pesticides, 1 on fisheries, 1 describing the blind white fish and 1 a summary of the latter; 2 were in Farsi).
A generation later in 2006, over 160 articles on Iranian fishes appeared, along with hundreds of relevant works from neighbouring countries, works on the
aquatic environment in Iran and works on taxonomy and systematics relevant to Iran. The study of fishes is now a very active field within Iran and the Middle
East and much of the newer literature is easily available on-line (see
Bibliography). Accordingly, 2010 is the last year that this work was updated although some systematic and taxonomic studies may still be incorporated.
A wide range of people in Iran, Canada and elsewhere have assisted me in this
work over more than 40 years. Inevitably, I will have forgotten some names, which I
regret. Some people I never met formally, an example being the gentleman nattily
dressed in suit by a stream near Kazerun who jumped fully-clothed into the water to help
me catch fish. Numerous other Iranians have assisted my studies and this website is dedicated to them.
The staff at the Department of Biology, Shiraz (then
Pahlavi) University helped me in numerous ways to collect fishes during a
three-year tenure as an Associate Professor. Dr. Bahman
Kholdebarin was Chairman of the Department for much of my time in Iran and it is
only through his support that I was able to make the collections that enabled
this work to be done. The Research Council of Pahlavi University funded field
trips and is gratefully acknowledged for this support. Collections were made with the help of drivers and assistants and their
efforts over long periods in the field are gratefully acknowledged. They include
H. Assadi, M. H. Jaferi, Sh. Mansoorabadi, A. Shirazi, A. Tofangdar and N.
Yaghar. Various other people assisted too and are mentioned below under the Pahlavi University name.
Studies on Iranian fishes since my residence in Iran have been supported by grants from the Canadian
Museum of Nature, Ottawa (CMN, fish collection acronym CMNFI), by assistance from staff there including Noel
Alfonso, Jadwiga Frank, C. G. Gruchy, Sylvie Laframboise, Alison Murray, Claude Renaud and Michèle
Steigerwald, and by a wide range of students and volunteers. The staff in the
CMN library searched out all the numerous and varied papers on fishes in Iran
and neighbouring countries without which this synthesis would not be possible.
One paper took six years to locate and arrived in the form of a microfilm from
the Soviet Union. I am particularly indebted to Victor Adomaitis who
kindly volunteered for the unrewarding task of scanning hundreds of
images and converting them to thumbnails and usable files.
Mollie MacCormac carried on this task, making a wide variety of images available for the website.
In particular, I should like to acknowledge the support and encouragement of
the late Dr. D. E. McAllister, Curator of Fishes, CMN over many years, in terms
of training and education, both formal and informal, of financial and moral
support, and in practical terms in the ways and means of collecting,
cataloguing, identifying, and studying fishes, and of getting things done.
Various people and their organisations are mentioned below separately for
their particular assistance; these are in alphabetical order.
Dr. Asghar Abdoli collected numerous specimens including exotics and allowed
me to incorporate these discoveries in several papers.
Dr. P. Bănărescu, Institutul de Biologie, Bucureşti has communicated
much information in detailed letters on fishes in the Middle East as well as
loaning and exchanging specimens, for all of which his assistance is acknowledged.
Dr. R. J. Behnke, Colorado State University, Fort Collins is gratefully
acknowledged for his extensive loans of, and access to, collections he and
associates made. These are listed more fully in the Materials and Methods.
Prof. Dr. P. G. Bianco, University of Naples, allowed me free access to
materials, including types, in his possession at the University of Naples and
his hospitality is acknowledged.
Dr. N. Bogutskaya and Dr. A. Naseka, Laboratory of Ichthyology, Zoological Institute, Academy of
Sciences, St. Petersburg are thanked especially for their hospitality, access to
collections, data analyses and interpretations on Iranian fishes.
Dr. C. E. Bond, Department of Fisheries and Wildlife, Oregon State
University, Corvallis allowed extensive loans of fishes from Iran under his care
and these materials are listed in the Material and Methods (see Contents).
Staff at the Fish Section, British Museum (Natural History)
(now the Natural History Museum) have loaned materials and hosted visits on
numerous occasions; their help has been much appreciated for the extensive
collections are a required study to understand the Iranian fauna. They include Dr.
K. Banister, B. Brewster, P. Campbell, O. Crimmen, S. Davidson, Dr. P. H. Greenwood,
A.-M. Hodges, G. Howes, J. Maclaine, Dr. N. Merrett, Dr. D. Siebert, Dr. E. Trewavas, A. Wheeler and Dr. P. J. P. Whitehead.
Dr. T. Hrbek, Washington University School of Medicine, St. Louis is
acknowledged for his complementary studies on tooth-carps using molecular techniques.
Dr. M. Kasparek and Prof. Dr. R. Kinzelbach kindly appointed me to the
Advisory Board of the journal Zoology in the Middle East which has given me an
interesting and valuable overview of studies in that region.
Dr. Yazdan Keivany translated abstracts of his
manuscript reports and first posted my bibliography of Iranian freshwater
fishes on the internet - a stimulus to this work! He is continuing collaborative
efforts aimed at improving knowledge on the ichthyofauna of Iran.
Dr. Bahram Kiabi, Gorgan University
of Agricultural Sciences and Natural Resources is thanked for various items of information on fishes, translations
and gifts of Farsi articles and many interesting fish specimens. His efforts at
facilitating collegiality and his students
have formed the core of modern university researchers on the fishes of Iran.
Dr. F. Krupp, Johannes Gutenburg-Universität Mainz and
Forschungsintitut Senckenberg (NaturMuseum Senckenberg), Frankfurt am Main
contributed a wide variety of information on Middle Eastern fishes, sent me
copies of his theses and in his letters provided many stimulating points of
discussion which helped me clarify my views on the fishes. His published works
are a model for students on fishes in that region. He, with Prof. Dr. Kinzelbach,
kindly invited me to the Symposium on the Fauna and Zoogeography of the Middle East in Mainz, 1985.
Nasser Najafpour, Iranian Fisheries Research and
Training Organization, Jahad-e Sazandegi,Ahvaz was instrumental in arranging
visits to Iran and associated field trips. His enthusiastic cooperation in the
field resulted in many interesting new specimens and his studies on
distributions of fishes in Khuzestan have been very important for this web site.
The team at Ahvaz is acknowledged below individually and in teaching me Farsi
names of fishes. J. Gh. Marammazi was head of that team and his hospitality and
efforts to bring me to Iran are gratefully acknowledged.
Dr. T. T. Nalbant, National Museum of Natural History "Grigore Antipa",
Bucharest, is currently studying loaches I collected in Iran.
Staff at the National Museum of Natural History (Smithsonian Institution), Washington
arranged loans of specimens and allowed access to the collections during several
visits. They include K. A. Bruwelheide, Dr. B. B. Collette, S. Jewett, S.
Karnella and Dr. J. T. Williams.
Staff at the Fischsammlung, Naturhistorisches Museum Vienna have also loaned materials and hosted
visits and their assistance has been essential to studies on Iranian fishes
based on the collections of J. J. Heckel.
They include Dr. H. Ahnelt, Dr. E. Mikschi, Dr. B. Herzig and Dr. R. Hacker.
Dr. J. G. Nielsen and Dr. P. R. Möller, Zoological Museum, University of Copenhagen
facilitated access to collections despite the "orkan".
Dr. P. Bartsch and Mrs. C. Lamour, Museum für Naturkunde, Humboldt-Universität
zu Berlin for access to collections.
M. Rabaniha and F. Owfi, Persian Gulf Fisheries Research Centre, Bushehr and
Iranian Fisheries Research and Training Organization, Jahad-e Sazandegi, Tehran, kindly copied the MMTT catalogue for me
and showed me various specimens of fishes from their work in Bushehr Province and southern Iran.
Dr. Jalal Valiallahi provided stimulating discussions on the limits and the
content of the genus Barbus sensu lato in Iran while working at the CMN as well as a
variety of photographs of these sometimes immense fish. Prof. Dr. H. Wilkens, Zoologisches Institut und Zoologisches Museum der
Universität Hamburg kindly loaned materials and facilitated two visits to the
museum to examine materials.
Various people collected material for me or made gifts of material, sent specimens for identification, identified
material, allowed access to collections under their care, made loans of
material, provided other useful data and general information, and exchanged
ideas. These are listed below in alphabetical order with their affiliations at the time of their
contribution (sometimes only email addresses were known; and apologies if any titles are missing):-
K.
Abbasi, Gilan Fisheries Research Centre, Bandar Anzali, H. A. Abdolhay, Tehran,
I. M. Abd, Nature Iraq, Baghdad, Iraq, A. Abdoli,
Fisheries Research Centre, Sari and Gorgan University of Agricultural Sciences and Natural Resources, S. Abdolmalaki, Gilan Fisheries Research Centre,
Bandar Anzali, S. M. A. Abdullah, Iraq, Dr. T. Abe, University Museum, University of Tokyo, Dr. M. Abedi,
Savadkooh University, H. Abyot, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Ahvaz, T. K. Aday, Iraq, Dr. A. Adhami, Muze-ye Melli-ye Tarikh-e Tabi'i,
Tehran, A. Afzali, Bandar Abbas, Fikret Ahsenböre,
Turkey, Dr. A. Akbary
Pasand, University of Zabol, Zabol, A. Alamdari, Organization of the
Environment, Shiraz, A. A. Al-Attar, Basrah University, A. W. Al-Hakim, University of Nottingham, L. A. J. Al-Hassan,
School of Biological Sciences, University of Auckland,
S. A. S. Al Hatimy, Oman Natural History Museum, Muscat, W. Al-Baharna, Directorate of Fisheries,
Bahrein, Dr. N. M. Ali, Biological Research Centre, University of Baghdad, Dr. T. S. Ali,
University of Basrah, S. Alinejad, Offshore Fisheries Research Centre, Chah
Bahar, Iranian Fisheries Research and Training Organization, Jahad-e Sazandegi,
H. R. Alizadeh, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Tehran, A. R. Al-Jafery, Department of Hydrobiology, Baghdad, Dr. H. Alkahem, King Saud
University, Riyadh, M. A. Al-Mukhtar, Fisheries Research Centre,
Ahvaz, Dr. A. J. Al-Rudainy, University of Baghdad, Iraq, Dr. A. Al-Shamma'a,
Ministry of Science and Technology, Iraq, Nisreen Alwan, Forschungsinstitut
Senckenberg, Germany, Prof. O. A. Amin, Arizona State University, Tempe, Dr. F. Andreone, Museo Regionale di Scienze Naturali, Torino,
Dr. R. Arai, National Science Museum, Tokyo, G. Arbocco,
Museo Civico di Storia Naturale "Giacomo Doria", Genova, Dr. J. D. Archibald,
Yale University, Connecticut, Dr. N. B. Armantrout, Portland, Oregon, Dr. G. Arratia, University of Kansas, Lawrence,
S. Asadollah, Isfahan University of Technology, A. Ashraf, Encyclopædia Iranica,
Columbia University, New York, Dr. J. W. Atz, Department of Herpetology and Ichthyology, American Museum of Natural History, New York,
Prof. S. Balik, Ege University, Izmir, Prof. E. Balletto, Istituto di Zoologia, Genova, G. A. C. Balma, Museo Civico di
Storia Naturale, Carmagnola, Dr. K. Banister, Fish Section, British Museum (Natural History), London, A. J. Bardhun, Shiraz, D. M. Bartley,
Food and Agriculture Organization, Rome, Dr. V. V. Barsukov, Zoological Institute, Academy of Sciences, Leningrad, M. L.
Bauchot, Laboratoire d'Ichtyologie générale et appliquée, Muséum National d'Histoire Naturelle, Paris, R.
Beck, COFAD GmbH, Tutzing, Dr. W. C. Beckman, Opelousas, Louisiana, Dr. A. Ben-Tuvia, Hebrew University of Jerusalem, Dr.
M. Berberian, Uinversity of Cambridge, Dr. P. Berrebi, Université
Montpellier, Dr. A. D. Berrie, Freshwater Biological Association, Wareham, Dr. E. Bertelsen, Zoologisk Museum,
Copenhagen, Prof. Dr. P. G. Bianco, Universita degli Studi di l'Aquila, K. L. Bist, Government
Postgraduate College, Gopeshwar, J. Bohlen, Academy of Sciences, Libechov, Dr. J. E. Böhlke, Academy of Natural Sciences,
Philadelphia, Dr. A. H. Bornbusch, Duke University, Durham, Dr. J. Briggs, King Faisal university, Dammam, Dr. K. E.
Carpenter, Food and Agriculture Organization, Rome, L. A. Cloutier, Department
of the Environment, Tehran, Dr. D. Coffey, Pahlavi University, Shiraz, Dr. M. J.
Collares-Pereira, Museu Bocage, Lisbon, Dr. J. T. Collins, Museum of Natural
History, University of Kansas, Lawrence, Dr. L. J. V. Compagno, J. L. B.
Smith Institute of Ichthyology, Grahamstown, Dr. B. B.
Collette, National Museum of Natural History, Washington, G. H. Copp, Centre for
Environment, Fisheries and Aquaculture Science, Lowestoft, Dr. L. Cornwallis, Oxford, A. S. Creighton,
Division of Fishes, Museum of Zoology, University of Michigan, Ann Arbor, Dr. E. J. Crossman, Department of Ichthyology and
Herpetology, Royal Ontario Museum, Toronto, E. L. Daniel, Encyclopædia Iranica,
Columbia University, New York, F. Darvishi, Mazandaran, S. Deeb, American University of
Lebanon, Beirut, S. Dehqan-Mediseh, Iranian Fisheries Research and Training
Organization, Jahad-e Sazandegi, Ahvaz, Dr. G. B. Delmastro, Museo Civico di Storia
Naturale, Carmagnola, M. Desoutter, Laboratoire d'Ichtyologie générale et
appliquée, Museum National d'Histoire Naturelle, Paris, Dr. M. M. Dick, Museum of Comparative
Zoology, Harvard University, Cambridge, P. Dickinson, National Zoological Garden, Al Ain, Abu Dhabi, W. A. Dill, Davis,
California, J. Dominique, Freshwater and River Ecology Reserach Unit,
Villeurbane, Dr. P. Dugan, Penang, Malayasia, M. Doroudi, Iranian Fisheries
Research and Training Organization, Jahad-e Sazandegi, Bandar-e Lengeh, Dr. J.
D. Durand, ESA CNRS, Villeurbane, Dr. G. Ekingen, Veteriner Fakultesi, Elazig,
O.Elter, Museo ed Istituto di Zoologia Sistematico, Universita di Torino, Dr. B.
Elvira, Ministerio de Agricultura y Pesca, Madrid, G. El Zein, Université
Libanaise, Ksara, Dr. F. Erk'akan, Hacettepe University, Ankara, Dr. W. N.
Eschmeyer, Department of Ichthyology, California Academy of Sciences, San Francisco, Gh. Eskandary,
Fisheries Research Centre, Jahad-e Sazandegi, Ahvaz, Dr. H. R. Esmaeili, Shiraz
University, D. Evans, IUCN, Cambridge, K. Evans, Pahlavi University, Shiraz, K. Fakhro, Directorate of Fisheries,
Bahrein, R. Fatemi, Tehran, Dr. A. M. Fazel, Natural Resources Faculty, Tehran
University, Karaj and Natural History Museum, Department of the Environment,
Tehran, , H. Fazly, Fereydun Kenar, Mazandaran, R. F. Field, Muscat, Dr. E. Firouz, Tehran, Dr. W. Fischer, Food and
Agriculture Organization, Rome, J. Fitzpatrick, Food and Agriculture
Organization, Rome, Dr. R. Fricke, Staatliches Museum für Naturkunde in
Stuttgart, P. A. M. Gaemers, Rijksmuseum van Geologie en Mineralogie, Leiden, M. D. Gallagher, Oman Natural History Museum, Muscat, M. Geerts,
Swalmen, The Netherlands, Prof. Dr. R. Geldiay, Ege University, Izmir, Dr. C.
George, Union College, Schenectady, Dr. H. Ghadirnejad, Iranian Fisheries Research and Training Organization,
Jahad-e Sazandegi, Tehran, A. Ghamoosi, Shahid Beheshti University,
Tehran, S. M. Ghasempouri, Tarbiat Modares University, Noor, Dr. D. I. Gibson,
British Museum (Natural History), London, D. Golani, Zoological Museum, Hebrew University of Jerusalem,
Dr. M. Goren, Tel Aviv University, S. Gorgin, Shiraz, Dr. B. Groombridge, UNEP
World Conservation Monitoring Centre, Cambridge, Dr. S. H. Gruber, University of
Miami, J. M. Gunn, University of Ottawa, R. Haas, California State University, Fresno, M. Hafezieh, Research Centre for
Natural Resources and Animal Husbandry, Jahad-e Sazandegi, Shiraz, Dr. J.
Halpern, Pahlavi University, Shiraz, Dr. K. E. Hartel, Museum of Comparative Zoology, Harvard University, Cambridge, S. S.
Hasan, University of Basrah, Dr. M. R. Hassannia, Jahad-e Sazandegi, Tehran, M. R. Hemami, Isfahan University of
Technology, D. M. Herdson, The Laboratory, Plymouth, E. Holm, Department of
Ichthyology and Herpetology, Royal Ontario Museum, Toronto, Dr. R. A.
Hinrichsen, Shad Foundation, Seattle, A.-M. Hodges, Fish Section, British Museum (Natural
History), London, M. L. Holloway, Fish Section, British Museum (Natural
History), London, L. Honarmond, University of Tehran, Dr. J. Holčík,
Institute of Zoology, Slovak Academy of Sciences, Bratislava, Drs.
F. and Sh. Hosseinie, Shiraz University, Dr. C. Hubbs, University of
Texas, Austin, Dr. J. Huber, Muséum National d'Histoire Naturelle, Paris, J.
Hull, University Museum, Oxford University, Dr. N. A. Hussain, Marine Science
Centre, University of Basrah, Ch. Izadi, Research Centre for
Natural Resources and Animal Husbandry, Jahad-e Sazandegi, Shiraz, Gh.
Izadpanahi, Dr. B. Jalali, ABZIGOSTAR, Tehran, Dr. S. Jahromi, Pahlavi University, Shiraz, Dr. S. Jamili, Iranian Fisheries Research and Training
Organization, Jahad-e Sazandegi, Tehran, Gh. A. Jasimi, Iranian Fisheries
Research and Training Organization, Jahad-e Sazandegi, Ahvaz, Dr. M. N. Javed, Government College,
Lahore, Dr. K. C. Jayaram, Zoological Survey of India, Calcutta, K.
Jazebizadeh, Iranian Fisheries Research and Training Organization, Ahvaz, Dr. J. B. Jensen, Pahlavi University, Shiraz, Dr. R. K. Johnson,
Field Museum of Natural History, Chicago, W. J. Jones, Al Ain, U.A.E., Dr. H. G. Kami, University of Tehran, J. M. Kapetsky,
Food and Agriculture Organization, Rome, Dr. M. H. Karim Koshteh, University of
Guelph, Dr. M. Kasparek, Kasparek Verlag, Heidelberg, Dr. E. J. Keall, Royal Ontario Museum, Toronto, Dr.
A. Keyvanfar, Centre national de Transfusion sanguine-Institut, Paris, Dr. G.
Khalaf, Lebanese University, Mansourieh-el-Metn, Dr. N. R. Khamees, University of
Basrah, S. Khera, Punjab University, Chandigarh, A. Khodady, Shahid Chamran
University, Ahvaz, Prof. Dr. R. Kinzelbach, Zoologisches Institut, Darmstadt,
Dr. W. Klausewitz, Forschungsintitut Senckenberg, Frankfurt, Dr. W. L. Klawe,
Inter-American Tropical Tuna Commission, Scripps Institution of Oceanography, La
Jolla, Dr. M. Kottelat, Zoologsiches Staatsammlung, Munich, Dr. A. Kownacki,
Laboratory of Water Biology, Polish Academy of Sciences, Krakow, Dr. S. O.
Kullander, Swedish Museum of Natural History, Stockholm, Dr. K. Kuronuma, Tokyo
University of Fisheries, Dr. M. Kuru, Hacettepe University, Ankara, P. Lamothe,
Hydro Québec, Montréal, Dr. K. J. Lazara, US Merchant Marine Academy, Kings
Point, New York, A. Lealmonfared, Shahid Beheshti University, Tehran, Dr. R. E. Lee, Pahlavi University, Shiraz,
Dr. K. E. Limburg, State University of New York, Syracuse, Dr. R. Littman,
University of Hawaii, Honolulu, Prof. Dr. H. Loffler, Vienna, R. Lolea, Gorgan University, J. Long, Department of Fisheries
and Wildlife, Oregon State University, Corvallis, O. Lucanus, Montreal, Dr.Mabee, Department of Zoology,
Duke University, Durham, A. A. Mahdi, University of Basrah, A. Mahjoor Azad,
Shahid Beheshti University, Tehran, Dr. P. S. Maitland,
Institute of Terrestrial Ecology, Edinburgh, Dr. H. Malicky, Biologische Station
Lunz, L. Maltz, Tel Aviv University, J. Mansoori, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Ahvaz, J. Gh. Marammazi, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Ahvaz, R. Martino, American Killifish Association,
Dr. M. Masoumian, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Tehran, Dr. A. Matinfar, Iranian Fisheries Research and Training Organization, Jahad-e Sazandegi, Tehran,
Y. Mayahi, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Ahvaz, Dr. R. L. Mayden, Department of Biological Sciences, University of Alabama,
Tuscaloosa, J. J. McAniff, National Underwater Accident Center, University of Rhode Island,
Kingston, M. McDavitt, Alexandria, Virginia, S. Mickleburgh, Fauna and Flora
Preservation Society, London, H.Meeus, Belgische Killifish Vereniging, Wommelgen,
R. Mehrani, Lorestan Research Centre of Natural Resources and Animal Science, Khorramabad,
Dr. A. G. K. Menon, Zoological Survey of India, Calcutta, Dr. S. N. Messieh,
UNDP, Abu Dhabi, Dr. F. T. Mhaisen, University of Baghdad, Dr. A. Miller,
Royal Botanic Garden, Edinburgh, I. D. Miller, United States-Saudi Arabian Joint
Commission, New York, Dr. P. Miller, University of Bristol, Dr. R. R. Miller,
Division of Fishes, Museum of Zoology, University of Michigan, Ann Arbor, Dr. A. A. Mirhosseyni,
National Natural History Museum, Baghdad, Dr. M. R. Mirza, Lahore, A. Mobaraki,
Department of the Environment, Tehran, M. R. Mohaghegh, Tehran, M. Mohammadi, Gorgan Agricultural and
Natural Resources University, Dr. S. Moini, Department of the Environment, Tehran, Dr. B. Mokhayer, University
of Tehran, Dr. K. Molnár, Veterinary Medical Research Institute, Hungarian
Academy of Sciences, Budapest, Dr. F. Moravec, Institute of Parasitology,
Czechoslovak Academy of Sciences, Prague, E. Morin, SOGREAH, Echirolles, Dr. E. O. Murdy,
Bureau of Oceans and International Environmental and Scientific Affairs, Washington,
Dr. G. S. Myers, Scotts Valley, California, M. Naderi, Mazandaran Fishery Research Centre, Sari, S. Naem,
Faculty of Veterinary Medicine, Urmia University, A. Nasrollahzadeh, Gilan,
Prof. Dr. C. M. Naumann, Universität Bielefeld, Dr. S. Nazeeri, Iranian
Fisheries Research and Training Organization, Jahad-e Sazandegi, Khorramabad, R.
B. Nehring, Department of the Environment, Tehran, N. Niameymandi, Persian Gulf Fisheries Research Centre, Bushehr,
Dr. H. Nijssen, Instituut voor Taxonomisch Zoölogie, Zoölogisch Museum, Universiteit van Amsterdam, M.
Nikpaey, Iranian Fisheries Research and Training Organization, Jahad-e Sazandegi,
Ahvaz, N. Nouri, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Tehran, Dr. O. Oliva, Charles University, Prague, Dr. H.-J. Paepke, Museum für
Naturkunde der Humboldt-Universität, Berlin, Dr. A. Paltrinieri, World Health
Organization, Muscat, F. Papahn, Shahid Chamran University, Ahvaz, Dr. L. R.
Parenti, National Museum of Natural History, Washington, J. Parkinson, Edmonton,
A. Parsamanesh, Iranian Fisheries Research and Training Organization, Ahvaz, D. Peck, IUCN, Gland, T. Petr, Food and
Agriculture Organization, Rome, H. Piri Zirkohy, Gilan Fisheries Research
Centre, Bandar Anzali, Dr. E. P. Pister, Desert Fishes Council, Bishop,
California, S. P. Platania, Colorado State University, Fort Collins, T.
Plosch, Ganderkesee, L. Podshadley, Department of Ichthyology, California
Academy of Sciences, San Francisco, Dr. M. Pourgholam, Iranian Fisheries Research and Training Organization,
Jahad-e Sazandegi, Sari, M. Price, Division of Fishes, Museum of Zoology, University of Michigan, Ann Arbor, Dr. G. S.
Proudlove, Department of Environmental Biology, University of Manchester, T. A. Qureshi, Technical
Institute for Agriculture, Amara, M. Rabbaniha, Persian Gulf Fisheries Research
Centre, Bushehr, Dr. H. Rahimian, University of Tehran, Dr. M. Ramin, Iranian
Fisheries Research and Training Organization, Jahad-e Sazandegi, Tehran, F. M.
Razi, Nature and Wildlife Museum, Tehran, Dr. W. J. Rainboth, University of
California, Los Angeles, R. W. Redding, Museum of Zoology, University of
Michigan, Ann Arbor, D. Rees, BBC, London, Dr. K. Relyea, Kuwait Institute for Scientific Research,
H. Rezai, Tehran, Dr. S. Rezvani Gilkolaei, Iranian Fisheries Research and Training
Organization, Jahad-e Sazandegi, Tehran, S. Richards, Murray, Utah, Dr. T. R. Roberts, Kasetsart
University, Bangkok, A. Roohi, Sabzevar Teaching and Training University,
Sabzevar, Khorasan, Dr. I. Rostami, Shahid Chamran University, Ahvaz, B. Saadallah, Iraq
Natural History Museum, Baghdad, M. A. G. Saadati, Department of the
Environment, Mashhad, H. Saadoni, Iranian Fisheries Research and
Training Organization, Jahad-e Sazandegi, Ahvaz, H. R. A. Sabet, Iranian Fisheries Research and
Training Organization, Tehran, A. R. Saeed, University of Kerman, E.
Saderigh-Nejad Massouleh, Iranian Fisheries Research and Training Organization,
Jahad-e Sazandegi, Khorramabad, H. Safikhani, Iranian Fisheries Research and
Training Organization, Jahad-e Sazandegi, Ahvaz, Dr. A. Salnikov, Institute of
Zoology, Academy of Sciences, Ashkhabad, Dr. A. Samaie,
Muse-ye Melli-ye Tarikh-e Tabi'i, Tehran, B. Sanford, Montrose, Colorado and
Port Ludlow, Washington, Dr. A. Sanyal, Zoological Survey of India, Calcutta,
Dr. M. Sarieyyüpoglu, Firat Üniversitesi, Elazig, Dr. A. Savari, Faculty of
Oceanography, Shahid Chamran University, Ahvaz, M. Sayfali, Shahid Beheshti
University, Tehran, D. A. Scott, Dursley, Gloucestershire, Dr. D. E. Sergeant,
Arctic Biological Station, Ste-Anne de Bellevue, Quebec, Gh. Shakhiba, Iranian Fisheries Research and
Training Organization, Ahvaz, A. J. Shams, Directorate of Fisheries,
Bahrein, Dr. I. Sharifpour, Iranian Fisheries Research and
Training Organization, Ahvaz, J. W. Sherman, Academy of Natural
Sciences, Philadelphia, Dr. A. Shiralipour, Pahlavi University, Shiraz, Dr. I. Q. Siddiqui, King Faisal University, Al Hasa, Dr.
P. Skelton, Fish Section, British Museum (Natural History), London, Dr. G. R. Smith, Museum of
Zoology, University of Michigan, Ann Arbor, Dr. W. F. Smith-Vaniz, Academy of
Sciences, Philadelphia, M. Soleymani, Green Front of Iran, Tehran, N. Statman,
Dr. A. N. Svetovidov, Zoological Institute, Academy of Sciences, Leningrad, Dr. C. C. Swift, Natural
History Museum of Los Angeles County, Dr. F. Terofal,
Zoologische Sammlung des Bayreischen Staates, Munich, M. V. Tofighi, Iranian
Fisheries Research and Training Organization, Jahad-e Sazandegi, Tehran, A.
Torfi, Iranian Fisheries Research and Training Organization, Jahad-e Sazandegi,
Ahvaz, Dr. W. Torke, Institut fur Urgeschichte, Tübingen, Dr. E. Tortonese, Museo Civico di Storia Naturale,
Genova, Dr. R. A. Travers, Fish Section, British Museum (Natural History), London, R. G. Tuck,
Muze-ye Melli-ye Tarikh-e Tabi'i, Tehran, Dr. H. Türkmen, Istanbul
Üniversitesi, Dr. E. Unlu, University of Dicle, Diyarbakir, Dr. I. Unsal,
Istanbul Üniversitesi, T. Valinasab, Fisheries Research and Training Organization, Jahad-e
Sazandegi, Tehran, Dr. J. Valiallahi, Tarbiat-e Modarres, Noor, >W. van Neer, Royal Museum of Central Africa,
Tervuren, Prof. Dr. R. Victor, Sultan Qaboos University, Muscat, Prof. Dr. W. Villwock, Zoologisches
Institut und Zoologisches Museum, Hamburg, Dr. V. D. Vladykov, University of
Ottawa, A. Vosughi, Iranian Fisheries Research and Training Organization, Jahad-e
Sazandegi, Tehran, B. Waaland, Pahlavi University, Shiraz, P. Walczak,
Department of the Environment, Tehran, Dr. B. A. Whitton,
University of Durham, Dr. R. Winterbottom, Department of Ichthyology and
Herpetology, Royal Ontario Museum, Toronto, Dr. G.
H. Wossughi, University of Tehran, Dr. T. C. Young, Royal Ontario Museum,
Toronto, M. Zapater, Zaragoza, A. R. Zeanaie, Payam-e Noor University, Bandar Abbas.
Individual Iranians, too numerous to mention here, kindly enunciated
carefully and repeatedly Farsi fish names for my cloth ear.
However it would be remiss not to mention staff at the Iranian
Fisheries Research and Training Organization, Ahvaz including N.
Najafpour, Gh. Marammazi, Gh. Eskandari, and M. A. Al-Mukhtar, as well as
E. Firouz, Tehran, B. Kiabi and A. Abdoli, Gorgan Agricultural and
Natural Resources University, and Y. Keivany, University of Alberta, Edmonton.
And finally I must thank my wife Sylvie and son Nicholas for supporting me in
my obsession with fishes from Iran and Nick for constructing the index page for
this website and linking it to the internet.
This work is meant to provide a guide to the freshwater
fishes of Iran. There are no modern keys to this fauna, some available books are
incomplete or cursory treatments or outdated, and the detailed and diverse scientific
literature is widely scattered in time, languages and journals. Iran lies
at a region of major zoogeographical interchange and has a diverse and
interesting ichthyofauna about which comparatively little is known. An
accurate identification is a pre-requisite for further scientific studies
and this website aims to serve that purpose and to be an introductory guide
to the fishes. The guide is aimed at a mixed audience, including scientists
familiar with ichthyology to whom some introductory sections of this work
will be superfluous, and those whose knowledge of fishes is embryonic or
who may have limited access to literature sources.
This work has been carried out over a period of 40 years from my first
studies on Iranian fishes in 1971 at the University of Ottawa on collections
made by V. D. Vladykov along the Caspian coast, continuing during a three-year
residence in Iran from January 1976. In that year, 7 articles were published
strictly on Iranian fishes (3 on parasites, 1 on pesticides, 1 on fisheries, 1
describing the blind white fish and 1 a summary of the latter; 2 were in Farsi). In 2006, 160 articles on Iranian fishes appeared,
along with many relevant works from neighbouring countries, works on the
aquatic environment in Iran and works on taxonomy and systematics relevant to
Iran. The study of fishes is now a very active field within Iran and the Middle
East. Accordingly, 2010 is the last year that this work is updated although
some systematic and taxonomic studies may still be incorporated.
Literature on
fishes of Iran can be found in Zoological Record (Pisces) and at
the Scientific Information Database (or SID at
http://www.sid.ir/En/Index.asp)which has lists of publications in Iranian journals and abstracts, both in English, as well as in Farsi.
The biological information may be cursory. Many species
are poorly known and their biology has not been studied, especially within
Iran. Some information is available for species shared with Turkey and
Iraq and I have tried to incorporate this literature as being less well
known or accessible. Many Caspian Sea basin species are shared with Europe
and the former U.S.S.R., are comparatively well-known and have an extensive
literature, often summarised in books, bibliographies and synopses. It
is not known in many cases if their biology in Iran is similar. Iranian
populations are often referred to distinct subspecies and occur at the
southern limit of the species range. Only a brief, summary account of their
biology is therefore given from synoptic literature sources. Biological
information generally is a brief summary of literature and readers should
consult the original papers for more details.
Some anecdotal biological information is added from my
field collections where spawning individuals were noted or gut contents
examined superficially. Most fish spawn in the spring. Feeding habits can
often be deduced from morphology. Fish with an arched and ventral mouth,
horny jaw edge, elongate gut and black peritoneum are feeders on detritus
and aufwuchs scraped from rocks. Most fish with a simple, s-shaped gut
feed on invertebrates such as crustaceans and aquatic insect larvae. A
few fish with molar pharyngeal teeth have a diet of molluscs whose shells
are crushed by the heavy teeth. Some fish are piscivorous and have an appropriate
jaw shape and streamlined appearance suitable for catching and holding
their fish prey. Fish with elongate and numerous fine gill rakers filter
phytoplankton or zooplankton from the water column. Very few fish feed
on macrophytes (large plants).
Checklists summarise the diversity of the ichthyofauna. Glossaries
explain both ichthyological terms for those new to the science and
Farsi and geographical terms for those unfamiliar with that
language. A Bibliography comprises books and papers referred to in the text and other relevant
works, which form a good general basis for the
serious student of Iranian freshwater fishes.
The descriptions in this work are founded on original
observations of material and a consideration of the literature. The sources
of this material are various museums which house a scattering of Iranian
species including in particular the Natural History Museum, London (formerly
the British Museum (Natural History)), the Naturhistorisches Museum Wien,
and the Zoological Institute, St. Petersburg which are depositories for
older type material, but the bulk of the research has been based on four
collections. The first of these was made by V. D. Vladykov during 1961
and 1962 when he was an Inland Fisheries Biologist under the Expanded Programme
of Technical Assistance of the Food and Agriculture Organization, UN. This
material was deposited in the National Museum of Natural Sciences, Ottawa
(now the Canadian Museum of Nature) and consists mainly of specimens from
the Caspian Sea basin. The second collection was made by employees of the
Department of the Environment, Tehran, and N. B. Armantrout and R. J. Behnke.
Half this collection was placed in the National Museum of Natural History,
Tehran (Muze-ye Melli-ye Tarikh-e Tabi'i) and half was retained by R. J.
Behnke and formed the basis of Saadati's (1977) thesis at Colorado State
University, Fort Collins. This collection covered the whole of Iran except
the Caspian and Sistan basins. Through the courtesy of Dr. Behnke I have
been able to examine this material in Fort Collins and make extended loans
for study in Ottawa. The Muze-ye Melli-ye Tarikh-e Tabi'i collection is
small (examined in 1995; catalogue 2000) and not as diverse as the Fort Collins material. Oregon State
University contains a collection of fishes made by W. Kinunen, S. Bullock,
R. RaLonde and P. Walczak, who were members of the Peace Corps in Iran
(some of this collection was deposited at the Smithsonian Institution,
Washington, which helped to fund the collection and transport of specimens).
Dr. Carl Bond kindly loaned me much of this material for long periods.
This collection was from all parts of Iran. The last collection, comprising
the bulk of the material, was made by me from 1976 to 1979 while I was
teaching at Pahlavi (now Shiraz) University in Shiraz. This collection
is housed in the Canadian Museum of Nature, Ottawa (formerly NMC, now CMNFI), and
covers all of Iran except the extreme northeast and northwest. Field trips
were funded by the Research Council of Pahlavi University. Subsequently
various Iranian colleagues have sent me specimens and these too are incorporated
in the present work. Principal among these were materials collected by
Asghar Abdoli (then based in Golestan) and Nasser Najafpour and associates of the Iranian Fisheries
Research Organisation (IFRO), Ahvaz. These collections together effectively
cover all the major drainages of Iran and provide the best foundation yet
assembled for a study on this ichthyofauna.
All material stored at the Canadian Museum of Nature,
Ottawa was examined in 45% isopropyl alcohol. Preservative was later changed
to 70% ethanol. The Canadian Museum of Nature also stores extensive field
records including slides, numerous data sheets on most species (counts
and measurements including x-ray plates), an extensive literature base
including translations from foreign languages, and comparative specimens
and literature from other countries in Southwest Asia.
Specimens collected by me were caught by any means that
presented themselves. Gear used included seines of various lengths and
mesh sizes (much repaired and patched!), gill-nets of various stretch meshes
(sometimes used as seines), cast-nets of several diameters (thrown skilfully
by others and poorly by me), by hand, and by purchase from small boys and
anglers using a variety of techniques (of angling on their part and of
persuasion on mine to extract catches from their possession). The object
was to sample any water body for all the kinds of habitat found there within
the limitations of a hasty schedule and the available equipment. Most habitats
were visited for less than one hour, but in the small springs and streams,
which comprise the bulk of Iranian fresh waters outside the large rivers
and lakes of Khuzestan and Sistan and the deep waters of the Caspian Sea,
this was more than adequate to catch a good and varied sample of most species.
This was borne out by repeated visits of longer duration to certain localities
near Shiraz. Pools and flowing sections were seined, gill-netted or cast-netted.
Riffle areas were also attacked in this fashion or seines were used to
block off sections of riffle and upstream rocks disturbed by kicking to
scare secretive species like loaches into the fixed net. In small streams
a dip-net was placed downstream of individual rocks which were kicked over
and the net scooped along the stream bed. Cast-nets proved particularly
useful in rocky streams which had little open water. Draped over the rocks
and only partly in the water, they nevertheless caught large and fast specimens
which were unobtainable by seining. The available fishing gear was less
effective on large rivers and on the Caspian Sea. Here boats, long gill-nets
and trawl gear would have been most useful. The collections are poor in
inhabitants of the main current of large rivers and in the deep water species
of the Caspian Sea. Larger specimens in major water bodies undoubtedly
evaded my nets with ease; some samples of larger individuals were available
from other collections and by purchase from commercial fisheries.
Several criteria were used to select specimens for counts
and measurements. Where few specimens were available, all were counted
and measured. Where several hundred specimens were available selection
was by size (usually larger fish; sometimes much smaller fish as well for
comparison with adult values), by sex to ensure an adequate representation
of males and females, and by locality where geographical variation was
examined. Badly damaged or grossly deformed specimens were excluded but
there was no (conscious) selection for "ideal" specimens.
Wherever a putative species was collected from more than
one drainage basin and material diversity permitted, a comparison was made
between the drainage basins. This work is continuing and details of methods
and materials are to be seen in published results. Students of Iranian
fishes should note that the application of sufficient statistical "weight"
will reveal differences between drainage basin samples and this is especially
true of a desert and semi-desert country like Iran. Springs and streams
may have been colonised by only a few founders. A small population sampled
in the lower reaches of a stream may not have had any contact with conspecifics
higher up in the stream for many generations. Conversely, several seasons
of heavy rain may have afforded recent opportunities for contact and gene
exchange. A one-time sample from a stream may therefore give a quite inaccurate
picture of the character suite of that population. Whether any of the differences
detected have systematic significance requires careful consideration. For
example, Balletto and Spano (1977) described 9 subspecies of Garra
tibanica in the southwest of the Arabian Peninsula using Principal
Components Analysis. This has been termed "statistical overkill" by Alkahem
and Behnke (1983). Also Krupp (1983) has observed that samples of Garra
rufa from the same locality collected in different years or seasons
varied in several characters. Description of subspecies based on limited
material requires a great deal of care therefore.
There are various methods of measuring and counting anatomical
features of fishes. The ones I have used are outlined below. They are based
on Hubbs and Lagler (1958) and Trautman (1981). Some particular characters are
outlined in papers by me in the Bibliography.
The method of counting fin rays differs from that in use
in North America since unbranched and branched rays are counted separately.
A "III,8" count in the European literature would be "9" in the system advocated
by Hubbs and Lagler (1958), i.e. the soft ray count is increased by one
to convert from the "European" to the "American" system. The bulk of the
work on fishes of southwest Asia follows the European system and I have
adopted this methodology to facilitate comparisons, although eschewing Roman numerals.
In this book, scale counts, number of gill rakers and
of vertebrae are usually expressed as ranges based on literature sources
since frequency counts are rarely given. A separate section gives counts
on Iranian fish examined by me followed by a frequency in parentheses (..).
Fin ray counts often show strong modes, but citing the mode alone would
be misleading. Pharyngeal tooth formula is often a modal value from the
literature; loss of or incomplete development of major or minor row teeth
is not uncommon, so counts may vary quite markedly.
Scale counts and paired fin ray counts were made on the
left side of each fish. In some instances, such as a badly deformed fin
or where scales on the left were mostly missing, counts were made on the
right. These instances were rare and restricted to species with low sample sizes.
Not all meristic characters had equal sample sizes; some
material from other museums was not available for x-rays, large series
of pharyngeal tooth counts was not often available because removal of arches
damages specimens, some specimens were damaged in certain characters, time
did not always permit all characters to be counted, some species are well-known
and additional data from Iran is clearly a subset of widely gathered data,
some species were examined in detail to address systematic problems, and so on.
All vertebrae were counted including the hypural plate
as one vertebra. In Cypriniformes and Siluriformes, the four Weberian vertebrae
were included in the count. Almost all counts were made from radiographs.
All rakers on the first gill arch were counted. A lower
limb count in the literature includes any raker at the angle of the upper
and lower limbs. Gill raker counts presented something of a problem when
comparing specimens of disparate sizes. The smaller fish often had very
small rakers at each end of the arch. These were easily missed or torn
off when cleaning a debris-encrusted arch. Removal of arches for a more
careful examination may also damage or destroy the finer rakers which are
intimately associated with the tissues adjacent to the arches. Alizarin
preparations can be of assistance, but the finer rakers may have no bony
content and thereby be omitted. Counts of juvenile fish may therefore give
lower values than counts for larger fish, whether this be due to an increase
in gill raker number with age or because rakers are more easy to count
in larger fish. This kind of variation is only critical where this character
is being used in species identification or in analyses meant to define
and relate species.
The teeth of the modified fifth gill arch in Cyprinidae
were counted in each row and given as a formula from left to right. A count
of 2,5-4,2 consists of two teeth in both the outer left and outer right
rows, five teeth in the inner left row and four teeth in the inner right
row. Pharyngeal teeth rows in Iranian cyprinids varied from one to three
on each side. In certain cases, it was evident from the presence of a socket
that a tooth had been lost. The count then included that tooth.
Fin ray counts were divided into two types. One count
is of spines or hardened soft rays or any unbranched, unpaired unsegmented
rays and this is usually given in Roman numerals in the literature. In
deference to some Iranian unfamiliarity with Roman numerals, the spine
count is given in Arabic numerals in this text. Spine count included rudimentary
rays which, at the anterior dorsal and anal fins, may be obscured by flesh
or scales requiring some probing or dissection. Radiographs were often
useful to confirm counts made under a microscope. The second count is of
soft rays and is also indicated by Arabic numerals. These rays are usually
branched, flexible, segmented and laterally paired. The last two unbranched
rays often arise from a single internal base and were then counted as one.
This is generally the case in Cyprinidae. The branched ray count is the
most diagnostic and variable in such fishes. Some families contain species
with more than one dorsal fin. The first dorsal fin may be composed of
spines and the second dorsal fin of spines and soft rays. In such species
the count is given separately for each fin.
The branched caudal fin rays only were counted. Dorsal
and ventral to these central rays are a series of unbranched rays which
become progressively smaller and may be obscured by flesh and scales where
the caudal fin attaches to the caudal peduncle. Counts in other works often
comprise the branched rays plus one dorsal and one ventral unbranched ray.
Caudal fin ray counts are remarkably uniform within families. In Cyprinidae
the count is almost always 17, except for occasional variants. Garra
persica was unique in having a strong modal count of 16 branched caudal
fin rays.
Paired fin ray counts can be separated into unbranched
and branched rays. A small splint in some species at the origin of the
paired fins was excluded from the count. There is usually one unbranched
ray which is not included in counts cited here. The branched ray counts
were the most important and are the ones given here. However, in the pectoral
fin the innermost rays were often difficult to discern and may increase
with age.
The first scale counted was that scale contacting the
pectoral girdle. The count continued along the flank following the pored
scales and including small, additional scales lying between the large,
regular scales as well as any unpored scales. The small, additional scales
were relatively rare occurrences and any obviously abnormal fish - those
with healed injuries for example - were not counted. The count terminated
with the scale lying over the end of the hypural plate as determined by
flexing the caudal fin. Some works recommend inclusion of a scale overlying
the flexure only if most of its exposed field is closer to the body than
to the caudal fin. Since the flexure of the caudal fin produces a relatively
broad groove, this is difficult to judge in smaller fish. Therefore, the
most posterior scale whose exposed surface touched the groove was the last
scale counted. I have also continued the count onto the caudal fin in some
species for a total count as this sometimes proved useful in comparison
with counts in older literature.
This count commenced with the scale at the origin of the
first dorsal fin and continued down and back to, but not including, the
lateral line scale. Any scale partially or wholly straddling the dorsal
fin origin was counted as one scale. The count followed the natural scale
row and included any small or irregular scales in the row.
This count commenced with the scale at the origin of the
anal fin, followed the natural scale row up and forward to, but not including,
the lateral line scale and included any small or irregular scales. In this,
and the previous count, it sometimes proved necessary to shift the counting
row because of the scale arrangement. This was always a backward shift.
In some instances there were several scales at the anal fin origin which
overlapped each other very closely. All these were counted and account
for the large degree of variation in counts between individuals of some species.
This count was made as in the above count.
All rows of scales between the origin of the dorsal fin
and the head were counted just below the mid-line of the back on the upper
flank. The final "row" at the occiput may consist of a single scale. This
method was used because scales on the mid-line may be small and irregular,
obscured by heavy pigment, or absent.
This was the lowest count of the scale rows around the
caudal peduncle, usually at its narrowest point. Both lateral line scales
were included. Scale rows were counted even when the scale arrangement
was such that occasional alternate rows touched. This count may be quite
consistent between individuals of a species, but it may also vary markedly.
The variation depended on the presence of large scales dorsally and ventrally
on the caudal peduncle connecting the flank scale rows. When such large
scales were present bridging over the top and bottom of the caudal peduncle,
the total count could be, e.g. 12, but in some individuals two or more
smaller scales occupied their positions so that the scale count jumped to 16.
All measurements were to the nearest 0.1 mm using dial calipers.
Measurements were taken on the left side unless a left fin,
for example, was badly deformed or broken. Badly deformed specimens were
not measured. Distortions due to preservation, such as a gaping mouth or
expanded gill covers, were gently adjusted to as natural a position as
possible. The following list explains how the various measurements were
taken. All measurements were taken in a straight line and not over the
curve of the head or body.
From the anteriormost part of the head to the tip of either
lobe of the caudal fin when that fin is normally splayed.
From the anteriormost part of the snout (even when the
lower jaw projects) to the end of the hypural plate (the end of the plate
is found by flexing the caudal fin; in small fish it may be seen by shining
a strong light through the caudal region). Standard length can be an inaccurate
measurement. The end of the hypural plate is obscured by scales, flesh
and caudal rays. Its position is determined by flexing the caudal fin;
this flexure is taken to be the end of the hypural plate. Small fish have
thin, delicate bones and the flexure may be at the anterior base of the
hypural plate, at the origin of the caudal fin rays which articulate with
and overlap the end of the hypural plate, or even between the last whole
vertebra and the hypural plate. Large fish have a broad flexure which can
give a variety of measurements by independent observers. Fortunately, in
this study most fish were comparatively small and strong illumination helped
to discern the end of the hypural plate. For larger fish I can only plead
an attempt at consistency.
From the anteriormost part of the snout to the bony margin
of the opercle (excluding the opercular membrane).
Maximum straight line depth excluding fins or fleshy and
scaly structures at fin bases
Maximum distance from one side of the body to the other.
From the occiput vertically to the breast or lower head
surface.
The distance between the opercles when in their normal,
closed position. The opercles are gently pressed into a closed position
if greatly dilated.
From the anteriormost part of the snout or upper lip at
the mid-line to the bony front margin of the orbit.
Greatest diameter between the bony rims of the orbit.
This distance is not always horizontal.
Greatest distance between the posterior bony orbit margin
and the bony opercular margin.
Least bony width between the orbits over the top of the
head in a straight line.
From the base of the anteriormost dorsal fin ray to the
tip of the snout or upper lip.
From the base of the anteriormost pelvic fin ray to the
anteriormost point on the head (snout or upper lip).
From the base of the anteriormost anal fin ray to the
anteriormost point on the head (snout or upper lip).
The oblique distance from the insertion of the anal fin
to the mid-point of the end of the hypural plate.
The least depth of this structure from the mid-line of
the ventral surface.
From the structural base of the ray to its tip.
From the anteriormost ray base (the origin of the fin)
to the point where the fin membrane contacts the body behind the last ray
(the insertion of the fin).
From the extreme base of the uppermost, outermost or anteriormost
ray to the tip of the fin.
Used principally in Cyprinidae and Cobitidae, this and
the following measurement are from the extreme base of the anteriormost,
uppermost or outermost ray of the appropriate fin to the anterior base
of the next fin.
As above.
From the base of the spine to its tip. In pungent spines,
as in catfishes, this excludes soft rays or membranes distal to the sharp
tip, but in more flexible spines, which may taper gradually as in Cyprinidae,
this measurement includes the soft tip.
Written records extend back to the third millennium B.C.
in Mesopotamia, the plain shared between Iran and Iraq. The Uruk IV symbol
for fish dates to 3100 B.C. or 5050 B.P. Later cuneiform writing on clay
tablets refer to fishes and attempts have been made to identify the species,
with variable results (Scheil, 1918; Diemel, 1926; Civil, 1961: Landsberger, 1962; Salonen,
1970; Sahrhage and Lundbeck, 1992). About 324 Sumerian and Babylonian fish names
have been identified referring to about 90 species (some of which are marine). Fish played a prominent
part in every day life, both as food and as religious
symbols (van Buren, 1948; Salonen, 1970; de Moor, 1998).
Fishing regulations had set penalties and fishing rights were leased. Guilds of
fishermen existed and transport to cities with marketing was organised. Fish
were sun-dried, salted, pickled, fermented and possibly smoked. Fishermen
had to deliver part of their catch to the temples or as duties. Surplus
fish were sold to the public. Consumption of fish was prohibited on certain days
(Sahrhage and Lundbeck, 1992). See also
Freshwater Fishes of Iraq website here.
The Babylonian Epic of Creation mentions nets and splitting fish for drying. Amulets and cylinder seals
depicting fish are common. A hymn which praises Ishtar of Uruk gives the
result of her favour as "whole channels are filled with fish, the channels
swarm with fish and with dates". Fish were offered as sacrifices to gods
and as part of funeral rites, as symbols of life and its renewal, and of
fertility (Wright, 1990). The amount of fish required was clearly stipulated
and whether it should be fresh, roasted or dried. The commoner species
were requested by the basketful but rarer species were requested by numbers
so a practical knowledge of diversity existed in the distant past. So numerous
were sacrificial offerings that at Uruk I the floor of a room or court
was covered with a thick layer of fish scales and fatty waste that gave
it a deep golden-yellow tinge. Some areas had layers of compacted fish,
4-5 cm thick, comprising skeletons, skin and scales, indicative that these
were not kitchen wastes but were sacrifices (van Buren, 1948). An Assyrian
king would have 10,000 fish served at a banquet, although these were cheaper
food items and the Sumerians favoured large, plant-eating carps from muddy pond bottoms (de Moor, 1998).
Archaeological remains containing fish bones at Abu Salabikh, Iraq, dated to 3000 B.C. (and summarised for south
Mesopotamia), have been identified to include Barbus (= Luciobarbus) esocinus,
Barbus (= Tor) grypus, B. (= Luciobarbus) kersin, B.
(= Carasobarbus) luteus, Barbus (= Mesopotamichthys) sharpeyi,
B. (= Luciobarbus) xanthopterus, Aspius vorax, Acanthobrama (presumably A. marmid), Cyprinion sp.,
Alburnus sp., Silurus triostegus, Mystus pelusius, Mastacembelus mastacembelus, Liza abu,
Acanthopagrus sp., and Tenualosa ilisha.
Radcliffe (1926), Salonen (1970) and Sahrhage and Lundbeck (1992) review fishing in Assyrian
and Sumerian-Akkadian times using nets,
spears, traps, weirs and copper hooks and line. Contracts concerned with fish ponds date
from the reign of Darius II, in 422 B.C., and with fishing in 419 B.C.
He also discusses Ea, the god of water dating back to Sumerian times,
for which a fish-god or man-fish was a symbol, still to be seen on ancient
monuments in Iran (see also Green (1986)). The Middle Elamite rock relief at
Tall-i Bakun near Persepolis in Fars depicts a river filled with fish but these
are highly stylised and not identifiable to species.
Fish do appear on bowls and other objects or in the round from archaeological
collections and some are illustrated below courtesy of F. Biglari and the National Museum of Iran:-
A'lam (1999b) briefly reviews fish in pre-Islamic Persian
lore but most, if not all, the fishes referred to are unidentifiable today. Illustrations of fishes
often occur in art work but are generally unidentifiable to species. One example
is a 14.5 cm, 12th century bowl from Iran in the Victoria and
Albert Museum, London. The bowl has shoals of fish in a rotating design painted
in black slip on a frit ware bowl under a turquoise clear glaze (www.iranian.com/Arts/July97/Design/Page6.html,
downloaded 10 June 1997). Governmental revenue from the Caspian fisheries have been recorded as early as
820-873 under the Taherids. Alam (no date) summarises the history of fisheries in Iran.
The Arabic work Aja'ibu-l-Makhluqat or "Wonders of Creation"
by Zakariya b. Muhammad b. Mahmud al-Kammuni al-Qazwini published in 1263
A.D. and later translated into Persian and enlarged in 1275, records sharks
entering rivers at the head of the Persian Gulf to Basrah on the Tigris
and comments on their ferocity and their teeth like points of spears, swords
or saws. Other Arabic and Persian works contain few recognisable species
of freshwater fishes although the tenth century Kitab al-Tabikh
from Baghdad contains some fish names such as bunni (= probably
Mesopotamichthys
sharpeyi) and shabbût (= probably Tor grypus)(Perry,
1998). Probably the best example of an early "scientific" Islamic work
on zoology is the fourteenth century "Nuzhatu-l-Qulub" or "Hearts Delight"
by Hamdullah Al-Mustaufi Al-Qazwini (translated into English by Stephenson
(1928)). Only the "tarikh" is identifiable as a freshwater fish - Alburnus
tarichi from Lake Van in modern Turkey.
Generally paintings of fish on historic items are insufficiently
detailed to allow identification to species (see Stchoukine (1936) for
some examples). However an interesting painting of a fish is found on a
Persian miniature of the fourteenth century stored in the Metropolitan
Museum of Art, New York (Dimand, 1934). The painting shows Jonah leaving the mouth of a fish.
A colour figure of this painting is found in Gould and Atz (1996),
although the image is reversed and a corrected colour version is in Coad et
al. (2000). The painting is from Rashid ad-Din's Jami` al-Tawarikh
or "Universal or World History" which contains accounts of various historical
and mythical events, including the history of China and Mongolia, the Bible
and incidents in the lives of Mohammad and Buddha. As Dimand (1934) points
out, this book was highly favoured by Persian painters of the fourteenth
century and several copies exist, the earliest being 707 A.H. (= 1307 A.D.).
The painting, dating to about 1400 A.D., shows Jonah being cast up by a
fish. The text on Jonah's arms however reads "The disk of the sun entered
into darkness" on the left arm and "Jonah entered the mouth of the fish"
on the right arm. The former, which was taken from the Gulistan
(= Flower Garden) of Sa`di written in 1258, being a more poetic rendering
of the latter. The angel, however, appears to be offering the naked Prophet
a garment, and this, as well as the proximity of terrestrial vegetation,
suggests he is leaving the mouth of the fish.
The fish undoubtedly was copied by the Persian artist
from Chinese paintings (Rice, 1976; Blair, 1995). It most closely approximates
some kind of carp but its mouth has been enlarged to accommodate the squatting
figure, and the opercular opening approaches the eye too closely to make
it a recognisable rendition of any particular species. There also are two
dorsal fins (not found in any member of the carp family), and the pectoral
fins are located too far from the head. Nevertheless, the fish does exhibit
a number of well-observed features such as symmetrical, overlapping scales
on the body with smaller ones on the caudal peduncle, paired and median
fins with fin rays, and the absence of head scales and teeth.
In modern Iran, the fish is still a symbol of prosperity,
blessings, abundance and happiness at Now Ruz, the Persian New Year
on 21 March, when a live fish from a store (usually a goldfish) or local
stream is kept in a bowl. In Persian mythology the earth is balanced on
the horn a gigantic cow and as the new year starts the cow throws the earth
from one horn to the other. The movement of the fish in the bowl when this
happens shows that the new year has begun (Noorbaksh, 1995). Anahita, the
ancient god of water, watched over people in their dealings with water
and fish (Sajaadyeh, 1995).
A general survey of natural history studies in the Muslim
world is given by Mirza (1983), an Islamic approach to the environmental
crisis by Zaidi (1981), and Islamic principles for conservation by Ba Kader
et al. (1983).
Travelers from Europe often wrote up accounts of their
visits to Persia and some commented on the fishes although such comments
were mostly of a general nature and species were rarely identified. An
exception is the trout near Tehran and some of the older comments on these
populations are given in the species description. A summary and translation
into English of the earlier accounts may be found in Pinkerton (1758-1826).
Adam Olearius noted that the king leased fishing in the rivers entering the
Caspian. The lessees blocked the river from September to April near the mouth to
catch migrating fishes. Outside this area anyone was free to fish. Sir John
Chardin, in a series of English and French editions from 1686
to the early nineteenth century of his Description of Persia and Other
Eastern Nations, briefly mentioned fishes (see quote at the beginning
of this work, taken from Sykes (1927)) as did Fraser (1825; 1834), both
authors observing the lack of diversity in a water-poor country but commenting
on the presence of fishes in qanats. Continuing in full the abbreviated
quote from Fraser (1825) at the beginning of this work:-
Cornelius Bruyn (1652-1719) (or Corneille LeBrun, de Bruin)
depicts several fishes from his journey through Russia and Persia, mostly
from the Persian Gulf, but including one called "sjir-majie" (= shir mahi
or milk fish) which Heckel (1843b) identifies as Capoeta trutta
and states that it is from Esfahan. Capoeta trutta is not found
near the city of Esfahan. This illustration appears in volume 1, page 185,
plate 69 of the Amsterdam edition in French published in 1718. However
a reading of the text and examination of the illustration (slides kindly
provided by Martine Desoutter of the Muséum national d'Histoire
naturelle, Paris) show that the fish cannot be identified so clearly. No
scales are shown and the colour pattern is unusual and unlike any Iranian
freshwater fish. The colour pattern is vaguely reminiscent of Barbus
lacerta, although much exaggerated. The illustration is possibly based
on a Barbus or a Capoeta species. The author was in Esfahan
on 23 November 1703 when describing the fish but the specimen is mentioned
in the same paragraph as a "Lezard de mer....prend dans le Golfe Persique"
and I take this to mean that the fish too may come from a locality on or
near the Persian Gulf rather than the neighbourhood of Esfahan as Heckel (1843b) has it.
Floor (2003) devotes some considerable space to fisheries in Qajar Iran, not
repeated here. The most important were the Caspian caviar fishery but also dried
mullets were exported. Mullet were caught on mats stretched across a stream, the
shadow of the mat causing the mullet to jump to avoid it and thus becoming
stranded on the mat surface. The Russians controlled much of the Caspian fishery
although there were also Persian concessionaires.
Scientific works relevant to Iran begin with the Systema
Naturae, 10th edition, by Carolus Linnaeus (1701-1778) published in 1758
and in which scientific naming in zoology has its beginning. Linnaeus adopted
many of the names from the system developed by Petrus Artedi (1705-1735)
who, on a visit to Amsterdam to examine a collection of fishes from the
East and West Indies, drowned in one of the canals. Genera subsequently
found in Iran include Acipenser, Perca, Cobitis, Silurus, Salmo, Esox,
Atherina, Mugil, Cyprinus, and Syngnathus and various species
were described in these and other genera. After this date a variety of
papers were published by authors in many countries describing fishes scientifically
and some of these fishes were eventually found to occur in Iran, as with
the Linnaean genera and species. Examples include Marc Elieser Bloch (1723-1799),
a physician who began to devote himself to ichthyology at the age of 56,
and Johann Gottlob Schneider (1750-1822) who collaborated with Bloch and
published their "Systema Ichthyologiae" in 1801 after Bloch's death. This
work contains all known species at that time (Bloch also wrote "Naturgeschichte
der ausländischen Fische, 1785-1795) and in these works appear such Iranian
species as diverse as the Indian stinging catfish, Heteropneustes fossilis, and the snakehead,
Channa gachua (see Karrer et al., 1994); Johannes Müller
(1801-1858) and Friedrich Gustav Jacob Henle (1807-1885) who published
their "Systematische Beschreibung der Plagiostomen" in 1838-1841, the classical
work on sharks and their relatives; Antoine Risso (1777-1845), an apothecary,
who published in 1810 his "Ichthyologie de Nice" in which are described
two mullet species (Liza aurata and L. saliens) and an atherinid
(Atherina boyeri - see A. caspia) and in a later work (1826) the pipefish (Syngnathus
abaster - see S. caspius) which are now recorded from Iran; and lastly Franz Steindachner
(1834-1919), director of the "Kaiserlich-Königliches Naturhistorisches
Hof-Museum (or Imperial-Royal Natural History Court-Museum - now the Naturhistorisches
Museum at Vienna), who wrote so copiously on fishes from all over the world
that any systematist eventually must consult his works, e.g. for the description
of Schizopygopsis stoliczkae (1866) and Nemacheilus
(= Oxynoemacheilus) angorae
(1897)(see Kähsbauer, 1959; Adler, 1989; Herzig-Straschil, 1997).
A number of fish species are named by others for Ferdinand Stoliczka (1838-1874),
who collected extensively in the Himalayas and was appointed naturalist
to the Second Mission to Yarkand, but who died on the way to Leh through
hardships encountered on this journey (see Day, 1876; 1878).
Fish descriptions from the Middle East begin with the
work of Fredrik Hasselquist (1722-1752) in his "Iter Palaestinum eller
Resa til Heliga Landet Förrättad ifrån År 1749 till
1752" or "Voyage to the Holy Land Undertaken from the Year 1749 to 1752"
which was published by Linnaeus in 1757 after Hasselquist "Succumbed to
the fatigues and cares of the Journey" (Günther, 1869). Although this
work appeared before Linnaeus' 10th Edition and is thus rejected as far
as scientific nomenclature goes, it still contains recognisable and scientific
descriptions of fishes.
Alexander Russell, physician to the British Factory at
Aleppo from 1742?-1753, gave an account of four undescribed fishes from
modern Syria in 1756 (see Russell (1794) for greater detail and illustrations)
of which Mystus pelusius and Mastacembelus mastacembelus
were later found in Iran. The descriptions in this work are attributed
to Daniel Carl Solander (1736-1782) and to Sir Joseph Banks (1743-1820)
and Solander respectively (Wheeler, 1958). Since then a number of works
have appeared on Middle East fishes and although many were restricted to
Syria, the Jordan River basin or drainages of Anatolian Turkey they often
contain descriptions of species also found in Iran (see Bibliography).
Peter Simon Pallas (1741-1811) and Johann Anton von Güldenstädt
(1745-1781) described species from the Caspian Sea basin but outside Iranian
waters (Pallas, 1771, 1776, 1787, 1814; Güldenstaedt, 1772, 1773,
1778). von Güldenstädt was a naturalist on the expedition led
by Pallas charged with exploring the Russian Empire of Catherine II. Pallas
travelled to the Urals and eastwards while Güldenstädt went south
to the Caucasus, only returning to St. Petersburg seven years later (Mearns
and Mearns, 1988). Güldenstädt died in St. Petersburg at only
36 years of age from fever, his resistance weakened by diseases caught
in the Caucasus. Pallas based some of his descriptions on the work of Samuel
Gottlieb Gmelin (1743, 1744 or 1745-1774), an explorer and Professor of
Botany at St. Petersburg employed by the Russian government who visited Gilan and Mazandaran in 1770-1772, living at Anzali for some months. Gmelin
died a captive of a Caucasian chieftain, the Khan of Khaïtakes. A
translated account in English of his travels in northern Iran is given by Floor
(2007). It includes descriptions of fishes and fishing methods such as cast
nets and gill nets.
Other important eighteenth and early nineteenth century
authors describing and collecting fishes eventually found in northern Iran
include A. Lovetzky and Johann Friedrich Brandt (1802-1879), Director of
the Zoological Museum at St. Petersburg, who worked on sturgeons and described
respectively Acipenser nudiventris and Acipenser gueldenstaedtii, and Karl Eduard von Eichwald (Eduard Ivanovich Eikhval'd)
(1795-1876) who travelled to the Caucasus and Caspian Sea including Iran
(1825-1826) and collected fishes although he was prevented from landing
at Anzali by the Persian Governor. Eichwald's "Fauna Caspio-Caucasica"
(1841) was of particular importance as it carried descriptions of new species
and records of a variety of other fishes. Édouard Ménétries
(= Menestrier) (1802-1861) was Curator of the Zoological Collection at
St. Petersburg and collected fishes in the Caucasus during 1829-1830 and
reached the Talish Mountains (Kuhha-ye Tavalesh). He listed a number of
species found in the Caspian Sea and its tributaries in his Catalogue (1832).
Alexander von Nordmann (1803-1866) described the fishes of the Black Sea
in 1840 including gobies (Gobiidae) since found in the Caspian Sea and
the herring Clupeonella cultriventris (= caspia) and the minnow Rutilus frisii.
Several authors worked on marine fishes in the Indian
Ocean and Red Sea, describing species eventually found to penetrate or
live in fresh waters of southern Iran. First among these was Petrus Forsskål
(1732-1763), a Swedish member of a Danish expedition to the Red Sea in
1762 (Nielsen, 1993). Forsskål and four of his companions died and
it was left to the sole survivor, Carsten Niebuhr (1783-1815), to publish
Forsskål's fish descriptions posthumously in 1775. Some of Forsskål's
specimens survive as dried skins in the Zoological Museum of Copenhagen.
Forsskål was the describer of the milkfish, Chanos chanos.
Wilhelm Peter Eduard Simon Rüppell (1794-1884) of the Senckenberg
Museum, Frankfurt collected fishes in the Red Sea in 1822 and published
"Fische des rothen Meeres" in his "Atlas zu der Reise im nördlichen
Afrika" (1828-1830) followed by further field work in 1831 resulting in
a second "Fische des rothen Meeres" in Neue Wirbelthiere zu der Fauna von
Abyssinien gehörig (1835-1838). Rüppell described the tooth-carp
Lebias dispar (= Aphanius dispar) now found throughout southern
Iran. Later works are summarised by Dor (1984) and Dor and Goren (1994)
for the Red Sea. The Persian Gulf fishes have received attention although
there has been no comprehensive review of the fauna and its literature.
Some principal works on this marine fauna include Blegvad and Loppenthin
(1944), White and Barwani (1971), Randall et al. (1978), Relyea
(1981), Sivasubramanian and Ibrahim (1982), Fischer and Bianchi (1984),
Al-Baharna (1986), Kuronuma and Abe (1986) Asadi and Dehqani Posterudi
(1996), and A'lam (1999a).
However, the most important early work on the Middle East
and specifically on Iran is that of Johann Jakob Heckel (1790-1857), Inspector
at the Imperial Royal Court Collection of Natural History in Vienna. He
described the collections sent by Theodor Kotschy (1813-1866) to Vienna
from "Syria" which includes such places as the Quwayq (= Coic, Kueik or
Kuweiq) and Orontes rivers near Aleppo and Antioch, Damascus, the Jordan
River, Mosul on the Tigris River and Kurdistan (Herzig-Straschil, 1997).
In addition, collections were made in Iran from around Shiraz including
the streams of the Maharlu basin in the Shiraz valley, the Kor River basin
north of Shiraz, the Mand River (= Qarah Aqaj) which drains to the Persian
Gulf and Lake Perishan (= Famur) near Kazerun. (Note that measurements
used by Heckel are the "Wiener Zoll" = 26.34 mm comprising 12 "Linien"
(= 2.195 mm) as opposed to the English inch (= 25.40 mm) from information
courtesy of Dr. Barbara Herzig, Naturhistorisches Museum Wien). Heckel's
descriptions appeared in Joseph Russegger's "Reisen in Europa, Asien und
Afrika" in 1843 (volume 1, part 2) for the "Süsswasser-Fische Syriens"
continued in 1846-1849 as a "Naturhistorischer Anhang" followed by "Die
Fische Persiens gesammelt von Theodor Kotschy" (both in volume 2, part
3). The Syrian collections contained a number of species later found in
Iran. In total 70 species were described or mentioned from "Syria" and
many of the specimens are still to be found in excellent condition in the
Naturhistorisches Museum, Wien. Note that these collections contained numerous
specimens (and still do) while the catalogue in Vienna lists relatively
few, presumably those which Heckel intended to be the type series. Heckel's
publications often do not give accurate counts of the specimens on which
the species is founded. It is not always evident which specimens are types
and the whole series from a type locality is regarded as syntypes.
The dating of Heckel's works is not clear for the "Naturhistorischer
Anhang" and the "Die Fische Persiens..." parts which have 1846-1849 on
the cover. According to the International Code of Zoological Nomenclature
the final date is the correct one if it cannot be demonstrated that parts
of the work have their own dates. The copies of Heckel's works I have seen
(mostly xeroxes) do not seem to have individually dated parts or sections
and so I have used 1849 for the date whereas many earlier authors have
used 1846. This does not have any significant taxonomic complications as
there are no other works with potential synonyms in this date range.
The nominal Iranian species numbered 22 and these too
may be found in Vienna. Of 89 species described from Syria and Iran (two
were deemed to be found in both countries and a third is listed merely
as the trout), 72 were described as new species by Heckel, although all
are not now recognised as valid. Heckel's new species from Iran may be
summarised as follows:-
1. Barbus barbulus
(= Luciobarbus barbulus)
2. Systomus albus var. alpina (=
Carasobarbus
luteus)
3. Scaphiodon amir (= Capoeta damascina)
4. Scaphiodon niger (= Capoeta damascina)
5. Scaphiodon macrolepis (= Capoeta aculeata)
6. Scaphiodon saadii (= Capoeta damascina)
7. Cyprinion tenuiradius
8. Discognathus crenulatus (= Garra rufa)
9. Alburnus iblis (= Alburnus mossulensis)
10. Alburnus schejtan (= Alburnus mossulensis)
11. Alburnus caudimacula (= Alburnus mossulensis)
12. Alburnus megacephalus (= Alburnus mossulensis)
13. Cobitis persa (= Oxynoemacheilus persus)
14. Acanthopsis linea (= Cobitis linea)
15. Lebias sophiae (= Aphanius sophiae)
16. Lebias punctata (= Aphanius sophiae)
17. Lebias crystallodon (= Aphanius sophiae)
In all, only 4 new species were discovered according to
the modern interpretation of these taxa. In addition the following 21 species
(under their modern names) described from Syria and Iraq by Heckel
have since been found in Iran: Acanthobrama marmid, Aspius vorax, Barbus lacerta,
Carasobarbus luteus, Luciobarbus
esocinus, Luciobarbus kersin, Luciobarbus pectoralis, Luciobarbus xanthopterus, Capoeta trutta, Alburnus mossulensis, Chondrostoma regium,
Cyprinion kais, C. macrostomum, Garra rufa, G. variabilis, Squalius lepidus,
Tor grypus, Ovynoemacheilus frenatus, Silurus triostegus, Aphanius mento
and Liza abu. Heckel therefore described 25 of the species now known
from Iran, the highest proportion of the fauna by a single scientist.
Some of this material was sent on exchange or as gifts
to other museums although it is not always clear in their records whether
the material comprises types, e.g. the Muséum national d'Histoire
naturelle, Paris contains specimens marked from Vienna or Heckel of Alburnus
sellal from Persepolis (sic, possibly a Heckel species re-identified
as sellal)(1638), Chondrostoma regium from Mosul (1635),
Cyprinion kais from Mosul (1641), Cyprinion tenuiradius from
Perse (1640), Garra rufa obtusa from the Tigris (1633), Garra
rufa rufa from the Orontes (1634), and Squalius lepidus from
Mosul (1636).
The Museum für Naturkunde, Universität Humboldt, Berlin (ZMB) has some Heckel
types listed as such, plus additional material marked as from the Wiener Museum
with type localities such as Aleppo and Mosul but without dates. Some of these
may also be part of Heckel's material but are not indicated as types in the
catalogue. The Senckenberg Museum, Frankfurt also holds some Heckel material. All this additional material has not been investigated in detail by me
as to type status, although some has been examined in these museums as indicated
in the species descriptions.
At the time Heckel's descriptions came out a series of
22 volumes was being published in Paris covering all the fishes then known.
This work by Baron Georges Léopold Chrétien Frédéric
Dagobert Cuvier (1769-1832) and Achille Valenciennes (1794-1865) appeared
from 1828 to 1849 and was a seminal work in ichthyology, the "Histoire
naturelle des poissons" (see Bauchot et al. (1990) for more details).
It contained new species and summaries of descriptions by other authors
for a total of over 4500 fishes. New species from Iran were collected by
Pierre Martin Rémi Aucher-Éloy (1793-1838), a French botanist
and printer, who traveled extensively in Iran from 1835-1838, eventually
dying at Julfa in Esfahan from "an excess of zeal for natural sciences"
(Jaubert, 1843; Cuvier and Valenciennes, 1828-1849 (1844:298); Bauchot
et al., 1990). In 1835 he traveled from Baghdad to Hamadan, Esfahan,
Tehran and Tabriz and in 1837-1838 he visited Shiraz, Bushehr, Bandar Abbas
and the Bakhtiari mountains. The fishes he collected were Leuciscus
maxillaris (= Alburnus mossulensis), Leuciscus albuloides
(= ? Alburnus chalcoides) and Chondrostoma aculeatum
(= Capoeta aculeata) but collection data were poor, stating only "rivers of Persia".
A similar work was undertaken by Albert Carl Ludwig Gotthilf
Günther (1830-1914) whose "Catalogue of the Fishes of the British
Museum" in 8 volumes appeared from 1859 to 1870 and contained new descriptions
and reviews of earlier works with over 6840 species described and over
1680 doubtful species mentioned. New species from Iran or later found there
were Barbus (= Luciobarbus) subquincunciatus and Hemigarra elegans. Günther also founded the Zoological Record,
an annual index of the zoological literature.
Several other works appeared between these major, synoptic
works of Heckel, Cuvier and Valenciennes and Günther and the next
major work on Iranian fishes by Berg (1949) and these are outlined below.
Graf Eugen Keyserling joined a scientific expedition in
1858-1859 sent by the Russian Imperial Government to explore Khorasan under
the direction of the acting privy councilor N. Chanikoff. The difficulty
of baggage transport limited the quantity of alcohol Keyserling could carry
and early fish collections spoiled. However he did draw cyprinid fishes
from nature and gave good descriptions of 9 new species and reported 2
others from what is now northwest and western Afghanistan south of Esfahan,
Yazd and Khabis near Kerman. Only one of his new species is now regarded as a distinct species, namely
Squalius latus.
Filippo de Filippi (1814-1867) an Italian zoologist, Professor
at Turin and Director of the Museum (1848-1865), accompanied an Italian
embassy to Persia in 1862 visiting Tabriz, Qazvin, Tehran, Rasht and the
Caspian Sea. His companion the Marquis Giacomo Doria collected fishes as
far south as Shiraz. Seventeen species were described from the Caspian
basin and inland waters of Iran although locality data were poor in some
instances (Coad, 1985). Seven species were described as new of which 2
are still regarded as full species (Acanthalburnus microlepis and
Cobitis aurata).
Albert Günther, referred to above, also described
collections and new species from the borders of Iran presented to the Natural
History Museum (formerly the British Museum (Natural History)), London.
The earliest of these was the collection made by William Henry Colvill
at Baghdad which Günther referred to 9 extant species in 1874, including
a freshwater shark, and 2 new species, Barbus (= Mesopotamichthys) sharpeyi and Macrones
colvillii (= Mystus pelusius). Barbus faoensis (=
Mesopotamichthys
sharpeyi) was described from Fao (= Faw) in another paper in 1896.
The Afghan Delimitation Commission was dispatched by the British government
to mark the western borders of Afghanistan. J. E. T. Aitchison was appointed
Naturalist and made collections, mostly on the Afghan side of the border,
from Sistan to the Hari Rud which were described in 1889 by Günther.
Seven species were discovered, 3 new, of which only Paraschistura kessleri
is still recognised as valid. Robert Theodore Günther (1869-1940)
was the first curator of the Lewis Evans Collection (1924) which later
became the Oxford Museum for the History of Science in 1935. In the summer
of 1898 he made collections of a variety of animals and fossils in the
Lake Orumiyeh (= Urmia) basin, including fishes, through the assistance
of the Persian authorities and the Archbishop of Canterbury's Mission to
the Assyrian Christians. These were described by Albert Günther in
1899 and comprised 6 species already described elsewhere and 4 new species
which are still regarded as valid names, with the exception of Leuciscus
gaderanus (= Petroleuciscus ulanus also described in this work).
The papers of R. T. Günther, containing some notes on fishes, were examined
in the New Bodleian Library, University of Oxford in 2007.
Karl Fedorovich Kessler (1815-1881) was a Russian zoologist
who helped organise the St. Petersburg Society of Naturalists in 1868 and
later became its President for 11 years. Kessler worked on fishes of the
Volga River and in 1877 published his important monograph on the "Fishes
of the Aral-Caspian-Pontic Ichthyological Region". Kessler described in
this and earlier works a number of species now found in Iran including
the still valid species Caspiomyzon wagneri, Clupeonella grimmi, Alburnus
filippii, Luciobarbus brachycephalus, Capoeta buhsei (from "Persia", apparently
near Tehran (Berg, 1949)), Chondrostoma oxyrhynchum, Oxynoemacheilus brandti,
Paracobitis longicauda, and Pungitius platygaster, plus a number of other
species since synonymised and other valid species reported from the Caspian
Sea basin but not yet recorded from Iran.
Francis Day (1829-1889), Inspector-General of Fisheries
in India and Burma, was the leading nineteenth century ichthyologist of
the Indian subcontinent, attaining this position from his initial career
as a medical officer with the Madras establishment of the East India Company
when fishes were but a hobby. His numerous studies have some items of relevance
to Iran and his 1875-1878 monograph "The Fishes of India" with its 1888
Supplement and the two-volume "Fishes" in the Fauna of British India series
contain useful data and descriptions of over 1400 species.
Henri Emile Sauvage (1844-?) described in 1882 and 1884
the fishes collected by Ernest Chantre of the Lyon Museum on a scientific
expedition to Syria, upper Mesopotamia, Kurdistan and the Caucasus including
several new species from the borders of Iran, namely Silurus chantrei
(= S. triostegus ?) from the Kura River of the Caspian Sea basin
(but Berg (1948-1949) suggests that this species was collected in Syria
or the Tigris basin but without any explanation), Barbus microphthalmus
from the Kura River (= Luciobarbus mursa) and Labeobarbus euphrati
from the Euphrates River (= Luciobarbus esocinus).
Oscar von Grimm described two species of herrings (Clupeidae)
from the Volga River at Astrakhan (Alosa kessleri and A. saposchnikowii),
now known also from Iran.
Aleksandr Mikhailovich Nikol'skii (1858-1942) described
in three papers the fishes collected by N. A. Zarudnyi (see below) in Iran.
Nikol'skii was primarily a herpetologist, head of the herpetological department
of the Zoological Museum of the Academy of Sciences in St. Petersburg,
and later professor at Kharkov University in the Ukraine (Adler, 1989).
These included the first record of Channa orientalis from Iran and
the new species Capoeta fusca, Capoeta nudiventris (= C. fusca),
Capoeta gibbosa (= C. capoeta), Aspiostoma zarudnyi (= Schizothorax
zarudnyi), Barbus bampurensis (= C. watsoni), Cyprinion kirmanense
(= C. watsoni), Nemacheilus (= Paraschistura) bampurensis, Nemacheilus
(= Paraschistura) sargadensis, Discognathus
rossicus
(= Garra rossica) ?
Serghyei Nikolaevich Kamenskii of Kharkov University described
in 1899-1901 "Die Cypriniden der Kaukasusländer" in two volumes which
described a number of new species notably in the genus Barbus since synonymised.
Nikolai Andreevich Borodin (1866-1937) was Chief Specialist
in Fish Culture in the Department of Agriculture and Professor in the Petrograd
Agricultural College and later an exile in the U.S.A., becoming Curator
of Fishes in the Harvard Museum of Comparative Zoology. He wrote a number
of articles on the sturgeons and herrings of the Caspian Sea and discovered
such new species as Acipenser persicus, Alosa braschnikowii,
Clupeonella engrauliformis check others?. In 1908 he co-authored
with E. K. Suvorov "Caspian herrings and their commercial exploitation",
the results of the Caspian Expedition of 1904. Suvorov described Alosa curensis.
Erich Zugmayer (1879-?) collected fishes along the Mekran coast of what is now Pakistani Baluchistan describing, in 1912,
6 freshwater species including 5 new ones from internal and Sea of Oman
basins close to or shared with those of Iran, namely at Panjgur in the
Mashkel (= Mashkid) River drainage and the Dasht River drainage. A later
work (1913) added additional records for Baluchistan. The specimens were
deposited in the Zoological Museum, Munich (Zoologische Staatssammlung, München)
but all fishes were destroyed in World War II on 25 April 1944 (Fritz
Terofal, pers. comm., 1981; Neumann, 2006). Single type specimens were deposited
in the Naturhistorisches Museum Wien (NMW) and the Zoological Survey of India,
Calcutta (ZSI) of Labeo macmahoni (NMW 81256), Scaphiodon daukesi
(NMW 19784, ZSI F8028, ZSI F8032), and Nemacheilus (= Paraschistura) baluchiorum (NMW
19851). None of the species has been collected in Iran.
William Thomas Blanford (1832-1905)(Anonymous, 1905) accompanied
the Persian Boundary Commission in 1872, publishing a two-volume account
in 1876. The Commission mapped the boundary between Persia and Baluchistan.
Major (later Sir) Oliver St. John, with a collector from the Indian Museum,
Calcutta, also made collections from 1869-1871. Fish collections were minor
and not included in Blanford's books. Part of the collections was described
by J. T. Jenkins in 1910 from material deposited in Calcutta. Blanford
and St. John marched from Gwadar through Jalk, Bampur and Kerman to Shiraz,
with Blanford carrying on alone through Esfahan to Tehran. One new species
is from what is now Pakistani Baluchistan, close to the Iranian border
in the Nihing-Dasht drainage (Scaphiodon baluchiorum = Cyprinion watsoni)
while the remaining material, comprising 3 new species of tooth-carps,
is from the neighbourhood of Shiraz. Further discussion about the tangled
nomenclatural history of these little fishes can be found in the relevant Species
Accounts.
(Thomas) Nelson Annandale (1876-1924) was founder and
then Director of the Zoological Survey of India (Anonymous, 1925; Kemp
et al., 1925; Adler, 1989). He and a co-author reviewed the fishes
of Sistan (1920) collected by Colonel Sir A. Henry McMahon and other officers
of the Seistan Arbitration Commission of 1901-1904 and by officers of the
Zoological Survey of India in the winter of 1918. Nine species were described,
one of which, (Nemacheilus macmahoni), formed the basis for a new
genus, Adiposia, since synonymised with Nemacheilus and now
Paracobitis. The
McMahon collection had been examined by Charles Tate Regan (1878-?),
later to be Director of the British Museum (Natural History), London (now
the Natural History Museum) who found 2 new species out of 5 collected
in his 1906 work (Scaphiodon macmahoni (= Cyprinion watsoni)
and Nemacheilus rhadinaeus (= Paracobitis rhadinaea)), by Banawari Lal Chaudhuri of the Indian
Museum, Calcutta in 1909 who reported a new loach (Nemacheilus macmahoni
(= Paracobitis rhadinaea)) and by Annandale in 1919 who described 2 new
species of Discognathus, D. adiscus (= Crossocheilus latius)
and D. phryne (= Garra rossica).
Annandale's co-author on the "Fish of Seistan" was Sunder
Lal Hora (1896-1955) who was to become the leading ichthyologist of India
on a par with Hamilton and Day, and Director of the Zoological Survey of India.
A. Ya. Nedoshivin and B. S. Iljin produced two lengthy
papers in Russian in 1927 and 1929 on fishery capture data for Iranian
waters, forming an important historical record.
Alfons Gabriel and his wife collected fishes in the neighbourhood
of Bandar-e Abbas including the Genu hot spring and the Baschakird Mountains. This material was
described in 1929 by Maximilian Holly of the Naturhistorisches Staatsmuseum in Vienna and contained Cyprinodon
(= Aphanius) ginaonis and Barbus baschakirdi (= Cyprinion
watsoni) from fresh waters.
Viktor Pietschmann (1881-1956), originally Steindachner's
assistant and later (1919-1946) in charge of the fish collection at the
Naturhistorisches Museum Wien, described Mugil pseudotelestes (=
Liza abu) and Glyptothorax steindachneri (identification
uncertain) from the Tigris River basin in Iraq based on materials collected
on the Mesopotamian Expedition in 1910 (Kähsbauer, 1957).
Lev Semenovich Berg (1876-1950) was a leading Soviet physical
geographer and biologist. From 1930 until his death, he was head of the
"Special Laboratory of Ichthyology" of the Zoological Institute of the
Academy of Sciences of the U.S.S.R. in Leningrad and an Academician (Oliva,
1977). His contributions to the ichthyology of the former U.S.S.R. and
to that of Iran appeared in a number of shorter articles and in lengthy
monographs from the late nineteenth century onwards. The shorter works
are listed in the Bibliography and include descriptions of such new species
as Alosa sphaerocephala, Barilius mesopotamicus, Alburnus
atropatenae, Garra persica, Nemacheilus cristatus (=
Metaschistura cristata),
Glyptothorax kurdistanicus, Anatirostrum profundorum,
Knipowitschia caucasica and Knipowitschia iljini. His summary work "Freshwater Fishes of the U.S.S.R.
and adjacent countries" was published in 1948-1949 and in English translation
in 1962-1965 and has much of relevance to northern Iran, although the taxonomy
is now dated. His 1940 work on the "Zoogeography of freshwater fish of
the Near East" placed that fauna in context and included Iran but it was
his 1949 work "Freshwater Fishes of Iran and adjacent countries" which
has been the major modern work on Iranian fishes south of the Caspian Sea
basin and the Lake Orumiyeh basin. This was based on collections deposited
in the U.S.S.R. Academy of Sciences Zoological Institute in Leningrad (acronym
ZIL, now St. Petersburg, Russia with the acronym ZISP). The collections
had been made by two Russian biologists. The first of these was Nikolai
Alekseevich Zarudnyi (1859-1919), a zoologist and ornithologist who made
four journeys to Iran for which he was awarded medals and the Przheval'skii
Prize by the Russian Geographical Society. His first journey in 1896 was
to Kuchan, Sistan and Mashhad, his second in 1898 was to eastern Khorasan
and Beluchistan, the third (1900-1901) was to Khorasan, Sistan and Beluchistan
including the Bampur region and the Makran, and the last journey (1903-1904)
was to Gorgan, western Khorasan, western Kuhistan, southern Irak-Ajemi
and Khuzestan. Zarudnyi's material had previously been examined and described
by Nikol'skii (see above). The second biologist was P. V. Nestorov who
worked with the Turko-Persian Demarcation Commission in 1914 and collected
fishes in the Tigris basin along the present Iran-Iraq frontier.
The Zoological Museum of the Lomonosov Moscow State University
(MSU) contains collections from the Caucasus and Transcaucasia including
the Kura River basin and Azerbaijan but none apparently from Iran (Verigina, 1991).
Several books have appeared in recent years in Farsi on Iranian freshwater
fishes and include "Freshwater Fishes" by Vossughi and Mostajeer (1994),
"Identification of some freshwater fishes of Khuzestan Province" by Najafpour
(1997), "Atlas of Iranian Fishes. Gilan Inland Waters" by Abbasi, Valipour, Talebi Haghighi, Sarpanah and Nezami
(1999), "Freshwater Fishes of Iran" by Mohammadian (1999), "The Inland Water
Fishes of Iran" by Adoli (2000), "A Guide to the Fauna of Iran" by Firouz (2000;
in English as "The Complete Fauna of Iran", 2005), "Iranian sturgeons in the
Caspian Sea (Systematic, biology, artificial propagation, biomass evaluation and
conservation, fishing and production of caviar" by Keyvan (2003),
"Freshwater fishes of Khuzestan Province (Part II)" by Najafpour (2003), "Fish
Species Atlas of South Caspian Sea Basin (Iranian Waters)' by Naderi and Abdoli
(2004), "A Biological Review of Caspian Sturgeons" by Sarafraz and Akbarian
(2005), "Applied Ichthyology" by Hedayatifard and Ramezani (2007),
"Biodiversity of Fishes of the Southern Basin of the Caspian Sea" by Abdoli and
Naderi (2009), and, in
English, "Fishes of Tehran Province and adjacent areas" by Coad (2008).
A report on water laws and institutions in Iran was authored
by Dezfouli (1996) and gives some background on legislation affecting fish
habitats through regulation of water abstraction and pollution prevention.
Several general works on zoogeography of fishes have encompassed
Iran as part of their study. These include Berg (1933b; 1940), Banarescu
(1960; 1977; 1992b) and Por and Dimentman (1989). Most of Iran is part
of the West Asian area, which includes southern Anatolia, the Levant, and
the Arabian Peninsula, or an Iranian Province which excludes the Caspian
Sea, Lake Orumiyeh and Persian Gulf and Sea of Oman drainages. Berg (1940)
lists the following districts within the Iranian Province: the Tehran District
(= Namak Lake basin here), the Turkmen District (= includes the Tedzhen
or Hari River basin here), the Sistan District (= Sistan basin here), and
a Fars District (= the rest, or the basins Dasht-e Kavir, Esfahan, Kerman-Na'in, Sirjan, Lake Maharlu, Kor River,
Hamun-e Jaz Murian, Hamun-e Mashkid, Dasht-e Lut, and Bejestan here). The Caspian Sea drainage is regarded as a separate area. The fauna
is a mixture of elements from the European (western Palaearctic), the Mediterranean,
southern Asia, High Asia and Africa and should be regarded as a transitional
region (various views briefly summarised in Mirza (1994b; 1995)). Zoogeography
is dealt with here in the individual Species Accounts with some mention
in the drainage basin accounts.
A brief history of Afghanistan ichthyology is given in
Coad (1981d) and Petr (1999), of Pakistan in Mirza (1978) and Bilqees et
al. (1995). Literature, and therefore history, on Turkey is summarised
in Coad and Kuru (1986) and Fricke et al. (2007), and on Iraq and the Tigris-Euphrates basin in Coad
and Al-Hassan (1989). Much of the earlier Russian literature on the Caspian
Sea and adjacent waters is given in Romanov (1955).
Freshwater fisheries are increasing in Iran and with this
exploitation there is a commensurate need for an understanding of the
whole ichthyofauna. Coad and Abdoli (1996) and Coad (1998; 1999)
review the biodiversity of Iranian freshwater fishes. Reviews of
fisheries, including aquaculture, can be found in the magazine Abzeeyan, e.g. Anonymous
(1992c) and Madbaygi (1992), at the Food and Agriculture Organization of the
United Nations website (www.fao.org), at www.agri-jahad.org,
the Iranian ministry concerned with fisheries, at the Caspian Environment Programme
(CEP), Baku, Azerbaijan at www.caspianenvironment.org
and in various articles such as Matinfar and Nikouyan (1995), Nash (1997a,
1997b), Mehrabi (2002), Sadeghi and Agheli (2002), Saeedi (2002) and Alam (no date).
Additional information is found under each of the Species Accounts, in particular for
sturgeons (Acipenseridae), the most valuable fishery.
Fisheries data from various sources (and sometimes the same source) are not always
compatible or comparable. The data should be treated as indicative of trends and
relative fishing pressure between species. Some years may have been inadequately
reported, data is incomplete, sources for figures are disparate, poaching levels have varied, and low numbers may
not reflect actual catches.
Early accounts of fisheries along the
Caspian shore of Iran are given by Nedoshivin and Iljin (1927; 1929), Vladykov
(1964) and Keddie (1971).
The freshwater fish catch increased from 6954
tonnes/year in 1974-1976 to 24,613 tonnes/year in 1984-1986, a 254%
increase and five times the world average (Gleick, 1993). Inland fisheries
finfish production was 30,924 tonnes in 1986 and in 1992 Iran
had an inland capture fishery of 40,000 t, as did Turkmenistan;
Kazakhstan had 80,000 t, Uzbekistan 27,439 t, Azerbaijan 36,371 t,
Iraq 4400 t, and Armenia 4500 t (Food and Agriculture Organization,
Rome, Inland Water Resources and Aquaculture Service, Fishery
Resources Division, 1995a). The Caspian Sea fisheries grew from 25,987 t to
98,000 t in the decade 1990-2000 (www.agri-jahad.org,
downloaded 3 November 2003). Saheli (1999) gives figures that show total
aquatic production was dominated by Persian Gulf and Sea of Oman fisheries
in 1995 at 63%, the Caspian Sea occupied 15% and inland waters 15%, the
remainder being from international waters. Petr and Marmulla (2002) give an average catch of
30,000 t for 1995-1999 in inland waters. Kilka was the most important
factor for increased catches in the Caspian and aquaculture in inland fisheries. The catch in 1998 was 75,000 t for
inland waters (IRNA, 15 June 1999) - catch records vary between sources
but give a general idea of the importance of freshwater fisheries. The value of all fish
production in Iran rose to 1046 billion rials in 1996 from 171 billion
rials in 1989 (Tehran Times, 27 July 1998). Freshwater landings
increased from 22,177 t in 1985 to 115,000 t in 1994 (Food and
Agriculture Organization, Fisheries Department, 1996). Cold and warm
water fish production was 67,000 t in 2001 with per capita annual
consumption at 5.2 kg. Production was expected to rise to 220,000 t in
2000-2005 (IRNA, 11 November 2001). Per capita yields for
inland capture fisheries in kilogrammes after Food and Agriculture
Organization, Rome, Inland Water Resources and Aquaculture Service,
Fishery Resources Division (1995a) was as follows and shows marked
increases over these years:-
These values compare with neighbouring countries as follows for the
same period:- Iraq (range 0.182-0.672), Turkey (0.666-0.903),
Afghanistan (0.079-0.102) and Pakistan (0.773-0.874). Per capita supply of
cultured fish was 1.3 kg in 2003 while capture fisheries yielded 5.1 kg (Food
and Agriculture Organization, Fisheries Department, 2006). This same publication
gives fish consumption in kilogrammes per capita as follows:-
Catches in the Caspian Sea for 1991 and 1992 were 3036 t and
2692 t of sturgeons respectively, 13,817 and 21,527 t of kilka
(herrings of the genus Clupeonella, family Clupeidae), and
18,571 and 16,873 t of bony fishes. The herring catch reached 51,000 t
in 1994 from none 10 years previously (Food and Agriculture
Organization, Fisheries Department, 1996). The FAO also records that
the silver carp catch went from none in 1989 to 24,720 t in 1994. In
inland waters the catches of warm water fish were 19,947 t and 21,462
t, of cold water fish 579 t and 775 t (both presumably from fish
farming) and from "natural resources" 24,905 t and 20,183 t.
These catches (totals 80,855 t and 83,512 t) are less than the totals
for the marine catches in the Persian Gulf and Sea of Oman at 277,000
t and 271,000 t but are still significant (Abzeeyan, Tehran, 5(9):III, 1995).
In 1996, the total Caspian Sea catch was 58,000 t while the
southern, marine fisheries reached 265,000 t. The gross value of
all catches (1995) including marine fish and shrimps was U.S.$45
million while fish imports were at $65 million. Caviar made up nearly
60% of exports in 1994 and nearly half of imports are fish meal. The
industry had 111,800 primary employees in 1995, including about 8000
fish farmers. Most fish (70%) is eaten fresh, 15% is frozen and
canned, with some smoked or salted and the remainder is made into fish
meal (Food and Agriculture Organization, Fishery Country Profile,
1997, at www.fao.org/waicent/faoinfo/fishery/fcp/irane.htm). In 1998,
the annual fish catch was listed as 65,000 t with the aim of
raising the catch to 110,000 t by the end of the 1995-1999 economic
development plan. It was estimated that 150,000 t could be obtained
from 500,000 ha of ponds and dam reservoirs (IRNA, 23 October 1998).
TACIS (2002) demonstrates the growth in catches in the Caspian Sea basin of
Iran as follows. The kilka catch was 2000 tonnes per year in 1932-1959, 63,300
t/y in 1996-1998, mullets 390 t/y growing to 4560 t/y, and total catch 7440 t/y
to 81,360 t/y. Nezami et al. (2000) gives the following figures for fish
harvested from Caspian coastal provinces in Iran:-
Golestan:-
This province demonstrates a great variation in mullet catch between years.
Unauthorised fishing in Gorgan Bay in the southeastern Caspian was estimated
at 167,681 kg in 2000-2001 (Kamran, 2006). Mullets (Liza aurata and L.
saliens) comprised 35.7% of the catch.
The biomass of fishes in the Iranian Caspian is estimated at 556,530 t,
12.7% of the total for the sea, with a fish density of 50.6 tonnes/nautical mile
(the lowest values of any Caspian state)(Ivanov and Katunin, 2001). The Caspian
Environment Programme (1998) gives the following tables for bony fish production
in the Iranian Caspian Sea (tonnes) in recent years:-
Abdolmalaki and Psuty (2007) give figures over a wide range of years for Iranian coastal catches in the southern Caspian Sea as follows:-
The Statistical Center of Iran (www.iranworld.com/Indicators/isc-t023.asp,
downloaded 4 April 2005) gives kilka catches for 1997 as 60,400 t, for 1998
as 85,000 t and for 19919.79 as 95,000 t.
The bony fish catches in the Iranian
Caspian Sea waters for 1999-2000 were given by D. Ghaninejad (5th
International Symposium on Sturgeon, Iranian Fisheries Research Organizatio,
9-13 May 2005, Ramsar). Beach seine cooperatives took 11,170 t and the total
catch, allowing for poaching, was estimated at 16,860 t. The total kutum (Rutilus
frisii) catch was 1400 t and this species had an estimated biomass in
Iranian waters of about 22,000 t. The catch of Liza aurata was estimated
at 3559 t with about 22% undersized and the biomass estimated at 11,100 t.
Cyprinus carpio biomass was very low and was estimated at 4200 t. The
Rutilus rutilus (presumably includes R. caspicus) catch was estimated at 1340 t for 2000-2001, mostly poached
with gill nets, and Sander lucioperca at 18 t for the same period, mostly
undersized and immature. The total catch of Abramis brama was estimated to
be 17 t, again undersized and immature.
Catches in the Caspian Sea showed no differences between 7 regions based on
catch-per-unit-effort (cpue) (Mirzajani et al., 2005). Catches varied
from 88 to 459 kg/cpue for 1991-92 and 31-418 kg/cpue for 1994-95. In 2000-01,
the Anzali region had the highest values, significantly different from the
Astara-Hashtpar and east of Gilan province regions.
Beach seines are known as pareh in Farsi. Beach seine cooperatives increased
from 68 in 1989 to 151 in 2004 while the numbers of fishers doubled from 6000 to
12,000. About 85-100 people are members of each beach seine cooperative. The
beach seines are 1000 m long, with a cod-end 10-15 m wide and 100 m long and
with a mesh size legally fixed at 30 mm (smaller meshes are used too). They are
hauled in by tractors. Although there are minimum sizes for fish retention, e.g.
34 cm fork length for Sander lucioperca, fisheries do retain smaller ones
for home consumption or even marketing (Abdolmalaki and Psuty, 2007). Some
further details on Sander lucioperca catches are given in the appropriate
Species Accounts.
Caviar and sturgeon catches from the Statistical Center of Iran (www.iranworld.com/Indicators/isc-t023.asp,
downloaded 4 April 2005) were as follows (note that the Iranian years run
from March to March, so the western years are an approximation here and in the
above table) :-
The whole fisheries industry, including the Persian Gulf marine fin
fisheries and shellfish, received an investment of 500 billion rials
by government and 800 billion rials by the private sector, apparently
for the period 1989-1993. Nine billion rials were allocated to
aquaculture by the government in 1993, planned to rise to 23 billion
rials in 1994, and to 210 billion rials in the next five-year economic
development plan. In 1995, 200 billion rials were allocated to
preparation and provision of infrastructure activities for fish
farming (http://netiran.com/news/IranNews/html/9503131INEC.html). A
national project to expand fish farming within a six-year period would
raise annual production by 50,000 t, create 30,000 jobs, earn $50
million a year and increase consumption of fish to 10 kg per person (IRNA,
22 January 2000). Consumption of fish in Iran is estimated at 5 kg per
capita, having risen from 1 kg in the decade prior to 1999 and is
expected to rise to 6.5 kg in the next five-year economic plan (by the
year 2000) and to 10 kg by 2004 (later revised to 8.5 kg by 2005 (IRNA,
25 September 2000)). Per capita consumption of fish increased due to
increased production but also a government policy of lower prices than
for meat and poultry (IRNA, 6 March 1999; 31 May 1999). In
1993, 350,000 t of seafood products were produced comprising 30%
of the country's protein requirements and a sevenfold increase over
catches before the Islamic Revolution in 1979 (Abzeeyan,
Tehran, 4(9):VI, 1993). The annual fisheries output was expected to
reach 1 million tons by the year 2004 from a 1999 level of 400,000
tons (IRNA, 6 March 1999). Fish exports were expected to earn
Iran $400 million and create 150,000 jobs by 2004. The 1999-2000
government budget allocated 300 billion rials to fisheries (IRNA,
6 March 1999). In 1998, Rana and Bartley (1998) report the average per
capita fish consumption in Iran to be 4.5 kg, low compared to the
world average of 13.5 kg. The Government's plan is to increase
consumption to 6.5 kg by the year 2020 which would require an increase
in fishery production from 382,000 t in 1995 to 670,000 t; these
amounts conflicting with news reports.
Adeli and Shaabanpour (2007) looked at consumption of aquatic products in
Tehran in 2001 and 2005. Per capita consumption rose from 2.8 to 3.46 kg, 16.6%
of people preferred more packaged food, and farmed aquatics were consumed more
than other products, live rainbow trout being preferred the most. Salehi and
Mokhtari (2008) investigated attitudes in fish consumption among Iranian
nutrition experts. The experts listed various factors such as fish market
expansion, advertisements and promotions, health factors, and quality and trust
in the seller as having effects on the increase of fish consumption in Iran.
The Caspian Sea at this time produced 60,000 t and other inland
waters 59,000 t. These waters would have production increased to
420,000 t by 2020. Aquaculture has a high priority in this plan and
expanded at 8.2% per year during 1990-1996, the value in 1996 being
U.S.$306.6 million for a production of 30,000 t. However aquaculture
production for 1988 was only exceeded in 1995 (www.fao.org/fi/publ/circular/c886.1/wasia3.asp).
Over 975 million fingerlings were released into the Caspian Sea and
inland waters from hatcheries or given to fish farmers to be cultured
in ponds during the first five-year plan, 1989-1993. During the next
five-year economic plan, the catch was expected to increase to 2.6
million t from 1.309 million t and 1.9 billion fingerlings
would be released (Abzeeyan, Tehran, 4(9):V, 1993). The
"Iranian Fisheries Research and Training Organization" was
expected to have a budget of 35 billion rials by the end of 1993,
indicative of the importance attached to developing fisheries in Iran
(Abzeeyan, Tehran, 4(5):IV, VII, 1993).
Prior to the Islamic Revolution in 1979, the Iranian fisheries were
divided into two companies, known as Shilat in Farsi, a northern one centred on the
Caspian Sea and a southern one centred on the Persian
Gulf. The combined companies, known as the Iranian Fisheries
Organization or Shilat, were under the Jihad-e Sazandegi Ministry,
starting in 1987. Jihad-e Sazandegi translates as "Construction
Crusade" and is indicative of the attempt to develop the
fisheries to serve the growing population of Iran. The Organisation is now known
as Jihad-e Agriculture as of the year 2000. The Iranian
Fisheries Research and Training Organization officially commenced its
activities in 1990 and is now known as the Iranian Fisheries Research
Organization . It has departments of Research, Training,
Scientific Information and Administration and Research Centres at
Bandar Anzali and Sari in the north of Iran and at Bushehr, Bandar
Abbas, Ahvaz, Bandar Lengeh and Chahbahar in the south. A general
account of the fisheries and their organization in Iran is given at
http://netiran.com/press/economy-domestic/html/000000XXDE0090.html which was
available on the net on 14 April 1997 and a more recent version was at
www.netiran.com/php/artp.php?id=1609, downloaded 19 July 2004.
Aquaculture is now of major significance. Demand for fishery products is
expected to outstrip that available from fisheries (Salehi, 2003). Iran is a major producer of Chinese
carps (Billard and Berni, 2004). For the year 1986-1987
aquaculture production was the largest in Southwest Asia and in 1992
at 42,420 t, it represented 50% of the production for West Asia and by
value it was 62% (Food and Agriculture Organization, Rome, Inland
Water Resources and Aquaculture Service, Fishery Resources Division,
1995b). Yearly cultured fish production climbed from 4753 t in
1985, to 15,000 t in 1986, 18,000 t in 1987, 33,684 t in 1988, 39,913
t in 1989, and to 45,134 t in 1990. In 1995, Iran had 32% of the main
aquaculture production in West Asia (among Turkey, Israel, Iraq and
Syria) although it had been 47% in 1984. The decline was due to a
slower growth rate. The 1995 production was 29,000 t (Shehadeh,
1997). However other sources differ with a freshwater aquaculture
production of 13,615 t for 1995 according to the Food and
Agriculture Organization, Rome, Fisheries Department and Network of
Aquaculture Centres in Asia-Pacific Bangkok (1997). This source
summarises action plans and national objectives for aquaculture. The year
2005-2006 had 96,000 tons of warm and 32,000 tons of cold water production (Iran
Daily, 10 May 2006).
The Food and Agriculture Organization, Rome, Inland Water Resources and
Aquaculture Service, Fishery Resources Division (1995b) also gives
different figures for a range of years:-
The Caspian Environment Programme (1998) gives annual production (in
thousands) of the main cultured fish species in government and private
hatcheries as follows:-
Hosseinzadeh (2003) gives the following figures in tonnes for total fisheries
production in Iran (note that southern waters are marine captures):-
Hosseinzadeh (2003) also gives warmwater fish (major carps, see below)
production by province. Average production (tonnes/ha) increased as follows:
1989 (1 t/ha), 1990 (1.5), 1991 (1.5), 1992 (2.8), 1993 (3.0), 1994 (3.1), 1995
(3.3), 1996 (3.5), 1997 (3.4), 1998 (3.5) and 1999 (3.6). Coldwater fish
production (primarily rainbow trout, Oncorhynchus mykiss) was as follows in tonnes:-
The website www.iranseafoodexpo.ir/portion.asp, downloaded 9 February 2006, gives the following production of freshwater fishes,
presumably in tonnes, with some obvious rounding of figures and conflicts with
figures above:-
Carp culture is the most important fisheries subsector according to Salehi
(1999, 2004a). Chinese major carps are reared in hatcheries and, at about 8 days
of age, they are transferred to nursery ponds. At about 10 g in weight they are
transplanted into water bodies or grown out to market size (1 kg) in farm ponds
(Saheli, 1999). Salehi's 1999 thesis gives an economic, marketing and consumer
study of carp culture in Iran in the 1990s, concentrating on Cyprinus carpio.
He maps fish culture facilities and hatcheries, gives production of carps by
species and by provinces, and also gives an overview of Caspian fisheries apart
from carps. However carp culture is more generally used in the sense of the Chinese major carps (Cyprinus carpio,
Hypophthalmichthys molitrix, Ctenopharyngodon idella and
Hypophthalmichthys nobilis, often reared in polyculture. C. idella
commands the highest price followed by H. molitrix with C. carpio
the cheapest. Polyculture stocking in natural and artificial water bodies is
usually 28-32% Cyprinus carpio, 40-50% Hypophthalmichthys molitrix,
5-10% H. nobilis and the rest Ctenopharyngodon idella. Average
yields varied from 43 kg/ha in 1993, to 40 kg/ha in 1994 to 49 kg/ha in 1995.
Higher yields are cited by Salehi (2004a) at 1540 kg/ha in 2001 but this may be
for growth in summer months and special condition. Total carp production was
54,000 t in 2001 (but see below after FAO, also from Salehi). Salehi's data
differ from those of Hosseinzadeh (2003) above. The following figures are in
tonnes:-
Production by major fish-culturing provinces from Salehi (2004a) for carps is
as follows:-
New aquaculture developments are reported regularly, e.g. see Abzeeyan,
Tehran, 7(4):IV-VI, 1996; Aavakh-Kismi, 1996). The share of
aquaculture compared with total fisheries production more than doubled
between 1980 and 1987, from 5.5% to 12% due to high private sector
investment while the monetary value climbed from 10.9% to 22.2%.
Aquaculture is concentrated in Gilan, Mazandaran, Khuzestan and
Markazi or Tehran provinces where 96% of the total number of existing
establishments are found and 87% of total production (Ahmadi, 1993).
Various other areas of the country are taking on fish culture plans,
e.g. Anonymous (1991b; www.irna.com/newshtm/eng/08151227.htm, IRNA,
29 July 2000) - Lorestan Province; Anonymous (1992b) - Chahar Mahall
va Bakhtiari Province; Anonymous (1996) - Kermanshahan Province;
Islamic Republic News Agency (19 October 1997) - Ilam Province). In
1992 there were over 8047 ha of ponds and 503,500 ha of natural and
semi-natural reservoirs. Consumption of aquaculture products was 800 g
and over 10,400 people were employed in private sector aquaculture (Emadi,
1993a). The number of warm-water fish farms in 1996 was 3736 with an
area of 7989 ha and the number of cold-water fish farms was 79 with an
area of 164,984 ha (Iranian Fisheries Research and Training
Organization Newsletter, 17:4-5, 1997). Lorestan Province produced
772 t of farmed fish in 1997 with 1000 t predicted for 1998 and a
long-term goal of 21,000 t worth 156 billion rials and 10,000 jobs. In
1997, 50 fish farms were under construction along with 125 pools for
fish culture purposes and 10 billion rials were invested (Tehran
Times, 22 September 1998). Yazd Province produced 36 t of
trout from ponds, 16 t of this from saline water, in 1997. In Dehshir
and Marvast, 250 t were to be cultured with 200 t in salt water. For
1999, 500 t were forecast for this province (Tehran Times, 17
September 1998). The Azadegan Fish Farm south of Ahvaz was scheduled
to produce 70,000 t of cold and warm water fishes annually from
342 pools of 15 or 40 ha, employing 4250 people directly and 13,000
indirectly, and with a gross revenue of 305 billion rials annually (IRNA,
11 November 1998). In the Iranian year ending 20 March 2002, warmwater fish
culture produced 3843 t and coldwater culture 12,169 t (www.irna.com, downloaded
6 November 2002). Confusingly, the warmwater fish production in the year
ending 20 March 2003 was expected to be 30,000 t according to IRNA (17
December 2002), and compare tables above.
The following table from www.agri-jahad.org, downloaded 15 November 2002 shows production of fry of various species in
thousands:-
Integrated rice-carp farming and trout farming during the post-harvest period
is also being developed. In 1999 rice-field farming yielded 126 t of fish, as
well as fertilising the fields and controlling the rice stem borer (Petr and Marmulla, 2002).
Salehi and Momen Nia (2006) analysed the benefits of fish and rice integrated
culture in Iran and found it would increase farmer's profits and reduce the need
for fertilisers and pesticides.
Drought conditions have severely affected fish farming in parts of Iran, e.g.
the warm-water farming in Golestan and Mazandaran provinces which lost $6.5
million in 2006 because of low rainfall and the subsequent drought. Output
shrank by 5000 tons in Mazandaran and 1000 tons in Golestan and projected growth
of 15-20% was not attained. This report, from www.agriculturenews.net, downloaded
2 February 2007, noted that Mazandaran alone accounts for 30% of Iran's farm fish production.
Various studies have been carried out on aquaculture facilities or fish farms
in Iran, aimed at improving the yield and combating problems. For example,
Ebrahimzadeh Mousavi and Khosravi (2001) found the toxigenic fungi
Aspergillus flavus, Alternaria spp., Penicillium spp. and
Fusarium spp. at a fish farm for common, grass and silver carp in northern
Iran. Shahsavani et al. (2001) found carp pox in common, grass, silver and
bighead carp in a fish farm in Mashhad; Fathiazad et al. (2002) found clove oil to be a suitable substitute
anaesthetic for MS-222 (which has side effects and 21-day withdrawal period) in
juvenile Cyprinus carpio, Hypophthalmichthys molitrix and
Ctenopharygodon idella; Abtahi et al. (2002) found the LC50
of clove essence was no different from MS-222 for cultivated Acipenser
persicus, Oncorhynchus mykiss and Cyprinus carpio;
Rabani and Nourouzi (2002) studied the quality of the water output from the Neka
Power Station in the eastern Caspian basin for its possible use in warmwater
carp culture, finding it suitable except for dissolved oxygen levels; Yakhchali and Mahmudihesar (2002) surveyed abundance of Ichthyophthirius
multifilis (a protozoan causing white spot disease) in coldwater fish farms
in West Azarbaijan and Seyed Moratzaei et al. (2002) studied this
parasite's in vitro culture; Ebrahimzadeh et al. (2003) examined polyculture of female grass carp x
male bighead carp with silver, bighead and common carp (final weight gain was
not different between hybrids and grass carp, for example); Ghomi Marzdashti and
Azari Takami (2004) studied effects of polyculture of silver, common, grass and
bighead carp (only bighead showed increased growth, for example); Safari (2006) sampled bacteria on 51 farms and examined their use in improving
chemical conditions; Esteki (2006) determined the best conditions for manuring fish farms;
Rahmani and Ehsani (2006) studied ion exchange and air stripping methods for
removing ammonium, which can kill fish in culture systems; Ghorbani Vaghei and
Ahmadi (2007) studied the diversity and abundance of of macrozoobenthos at three
fish farms for Chinese carps in Gilan; etc..
Parasites of fishes are common in aquaculture and wild-caught fishes; the
species are detailed in each of the Species Accounts. Clostridium botulinum is
present in coastal areas of northern Iran and is a potential food hazard if
preservation is inadequate. Contamination rate was 10% in Sander lucioperca
and 6.66% in Salmo trutta (= caspius if native) (Tavakoli
and Razavilar, 2003; Tavakoli and Tabatabei, 2005), 2.2% of smoked carp, 1.1% of
fresh carp, 1.1% of smoked kutum and 1.1% of osetr caviar (R. S. E. Khandaghi in
5th International Symposium on Sturgeon, Iranian Fisheries Research
Organization, 9-13 May 2005, Ramsar).
Shariati and Nikfetrat (2005) survey the attitudes of fishermen to stock enhancement and conservation
efforts in Gilan Province and found a significant positive attitude. Overfishing
and illegal fishing were commonly cited as major problems. Emami and Hosseini
(2004) also assessed the participation of fishery cooperatives from Sari in
preserving fish resources.
Marketing fish in Iran was discussed at www.shilat.com
(downloaded 28 February 2007) and in Salehi (2006) including such items as product quality,
availability, variety, safety, price control, shelf-life, size control,
consumption behaviour, prices, among others. Adeli et al. (2010) found
households in Tehran bought farmed fish 11 times per year, with trout having the
highest demand, and reviewed factors preferred by consumers such as live fish
and price decrease in competition with wild fish.
Quliyev (2006) details fish farming in the neighbouring country of Azerbaijan
with relevance to Iranian Caspian Sea basin species.
Iran is the second largest country in Southwest Asia (after
Saudi Arabia with less than 20 freshwater fish species), has an area of
1,648,000 sq km and ranks fourteenth in the world in size, nearly as large
as the British Isles, France, Italy and Spain combined (Firouz et al.,
1970). It lies between latitudes 25°N and 40°N and longitudes 44°E
and 63°E. Its northern border is shared with
the former U.S.S.R. (Armenia (35 km long) and Azerbaijan (611 km) in the
west opposite Iranian Azarbayjan, and Turkmenistan (992 km) in the east
opposite Mazandaran, Golestan and Khorasan) and includes the southern part
of the Caspian "Sea", by far the world's largest lake (436,284 sq km) and
one of the deepest (1025 m). The Iranian coastline extends for 740 km.
The eastern border is shared with Afghanistan (936 km) and Pakistan (909
km). The southern border fronts on the Sea of Oman and the Persian Gulf,
a coastline of 2440 km. The western border is with Iraq (1458 km) in the
south and Turkey (499 km) in the north. Much of Iran lies at an average
altitude of about 1000 m, a feature found only in a few countries world-wide.
Only Khuzestan, the Caspian Sea coast and the Persian Gulf coast form lowlands.
These lowlands are quite narrow, often less than 20 km wide. Mountains
are the most prominent feature of the Iranian landscape. The two major
chains are the Alborz or Elburz, which rim the Caspian Sea basin in the
north, and the Zagros which form a chain down the western side of the country.
Inland of these chains lies the Iranian plateau, which is flanked on the
east and south by lesser chains of mountains. The country has been likened
to a bowl or saucer. This central plateau has extremely high summer temperatures
and often very cold winters. The deserts of this plateau are barren and
among the driest in the world. Rain falls only in winter. The terminal
basins for streams and springs may be dry for years. There are extensive
salt crusts, known as kavirs, over black, slimy mud and large areas are
composed of hard, gravel plains known as dashts, prominently the Dasht-e
Kavir and the Dasht-e Lut. Water is scarce in these regions, often restricted
to small streams and springs. Larger rivers have their source in distant
mountains. Between the Tigris and the Indus, only the Hirmand River on
the Afghanistan border is large enough to be a river on a world scale -
various "rivers" in the intervening area are really small streams easily
fordable on foot for much of the year.
Fisher (1968) gives a general, physical geography and
Breckle (1983) gives a general account of the features and life (excepting
fishes) of deserts and semi-deserts in Iran. Barthold (1984) gives an historical
geography of Iran and Yarshater (continuing) has many articles on geographical
features. Geological literature is summarised in Dürkoop et al.
(1979) and Davoudzadeh (1997).
It is pertinent here to interject a note on geographical
names. Transliteration of Farsi place names into English is possible by
more than one system. This results in variant spellings for geographical
features in articles and on maps of Iran. For convenience, I have followed
the official standard names approved by the U.S. Board on Geographic Names.
The Board publishes a gazetteer for Iran with a designation of the geographical
place (e.g. lake, populated place, stream, spring, etc.) and its latitude
and longitude. The latest gazetteer is available from the Defense Mapping
Agency, Combat Support Center, Washington, D.C. 20315-0010. Some literature
localities could not be identified from maps or gazetteers. They are placed
in quotes (".....").
I have not included the diacritical marks used in the
Board's system. They would be of little help to those unfamiliar with Farsi
and perhaps unnecessary to those who are. Needless to say, there are variant
diacritical marking systems and in any case pronunciation varies throughout Iran.
The situation is further complicated by transliterations
into other European languages and readers should be aware of this when
reading non-English papers on Iran or Iranian fishes, e.g. the English
Shiraz is Chiraz in French, and Genu, the type locality of Aphanius
ginaonis, has such variants as Ginau, Genow, Gueno, Geno, and finally
Ginao from the German transliteration, hence the trivial name. As if this
were not enough, the vagaries of political fortune are writ large upon
the face of Iran (which used to be Persia). Bandar-e Pahlavi has reverted
to its older name of Anzali (often spelt Enzeli on older maps), Reza'iyeh
to Orumiyeh (= Urmia in older English literature), and Shahreza to Qumisheh
after the fall of the Pahlavi Dynasty in 1979. Other variants are Bandar-e
Khomeyni (formerly Bandar-e Shahpur), Bakhtaran (formerly Kermanshah),
and Khuninshahr or City of Blood (formerly Khorramshahr or City of Joy,
and again Khorramshahr). I have retained names current for the years 1976-1979
recorded in the Board's gazetteer (1984). One exception is the province
of Hormozgan (or Hormozdgan) which I have preferred for its brevity over
the older name on some maps of Saheli-ye Jazayer va Banader-e Khalij-e
Fars va Darya-ye Oman! The province of Mazandaran is now split into two
with the eastern part termed Golestan, and Khorasan and Markazi have also been
split up. Iranian governments have a distressing
tendency to change the names and borders of provinces.
The provinces used here are as follows, being what existed when the data was
compiled:-
Markazi (= Central or Tehran; sometimes split into Tehran and a southeast part called Markazi)
Hormozgan (or Hormozdgan or Saheli-ye Jazayer va Banader-e Khalij-e Fars va Darya-ye Oman)
Another complication is the tendency for long rivers to
have several names along their course, sometimes taken from the nearest
population centre, and for locally used names to be different from map
or gazetteer names. Names also vary with language and through time. One
of the major rivers of Fars Province appears on maps as the Mand River,
but near Shiraz it is called by its Turkic name Qarah Aqaj (also transliterated
Qara Aghach, Qareh Aghaj, Qara Agach, Qareh Aqaj, Qareh Aqach, Kara Agach,
and Kara Agaj). The Kor River, also in Fars, is known in older papers as
the Araxes River which is not the same as that forming the border between
Iran and the former U.S.S.R. (which anyway is often spelt Aras or Araks!).
The early geological history of Iran and neighbouring
areas has necessarily affected the distribution of fishes, facilitating
dispersal or hindering it, isolating or joining species. Some historical
features are discussed under the appropriate drainage basin descriptions
below or under the relevant genus or species but others are more widespread
and are briefly outlined here. Sources include in particular Wolfart (1987)
but also Harrison (1968), Takin (1972), Falcon (1974), Stöcklin (1968,
1974a, 1974b), Krinsley (1970), Stoneley (1974), Kashfi (1976), Shearman
(1976), Booth (1977), Jackson and Wood (1980), Berberian and King (1981a,
1981b), Haynes (1981), Rögl and Steininger (1984), Šengör (1984), Oosterbroek and Arntzen (1992),
Rögl (1998; Rögl, 1999), and Adams et al. (1999). There have been no cladistic analyses of
taxa on which history can be determined. Zoogeographical analyses are based
on present day distribution and suppositions on relationships.
During the Cretaceous and through the Early Oligocene
the Tethys Sea, several thousand kilometres wide, extended from the Mediterranean
Sea to the Indian Ocean, separating the Afro-Arabian and Eurasian continents.
Afro-Arabia was part of Gondwanaland. The usual assumption is that Iran
belongs to Eurasia, perhaps with Central Iran a microcontinent or island
or as a northern continuation of Arabia, and with East Iran a microcontinent
or peninsula of Eurasia. Förster (1976), however, maintains that Central
Iran, and probably North Iran, were part of Gondwana. The Tethys covered
much of what is now Iran and was a barrier to the movement of freshwater
fishes. The ocean regressed during the Late Oligocene except for a Euphrates-Persian
Gulf furrow and the Zagros and Makran troughs. Continental sediments were
deposited in endorheic basins of Iran. The Tethys closed in the Middle
to Late Miocene as evidenced by mammal migrations between Asia and Africa.
The establishment of continental conditions over Iran has been continuous
since the Late Miocene except for an inundation in the Late Pliocene in
the Zagros trough and the Makran coastal region. There may also have been
an early Miocene connection between Arabia and Iran/Iraq allowing movements
of freshwater fishes (Adams et al., 1999). Iran is therefore composed
of parts of Gondwana, which was the continent south of the Tethys, welded
to the northern continent and parts of the Eurasian plate (such as the
central and eastern Iranian microcontinent). The northeastward movement
of the Arabian Plate caused the closure of the Tethys and led to the folding
which in the Miocene/Pliocene orogenies formed the Zagros Mountains, a
prominent feature of western Iran important in zoogeographic studies of
fishes (see Kashfi (1976) for an opposing view). The Zagros orogeny is
related to the opening of the Red Sea which formed a barrier to fish dispersal.
The Alborz Mountains are a northern part of the Alpine-Himalayan orogen
of which the Zagros are a southern part and started to rise in the upper-lower
Pliocene (Krinsley, 1970; Stöcklin, 1974). A continuous land-bridge
between Eurasia and Africa has been in existence since the upper Miocene,
facilitating freshwater fish dispersal. Hora (1937) and Menon (1957) refer
to wet, marshy, tropical conditions and headwater captures along the whole
southern face of the Himalayas and westwards during the Pliocene and early
Pleistocene facilitating the spread of fishes from the east to Iran. Hora
(1937) and Briggs (1987) consider that cyprinids entered Africa from southeast
Asia 18-16 MYA, in the early Miocene, while other groups moved through
Iran and the Arabian Peninsula beginning in the early Eocene. Kosswig (1951;
1952; 1955a; 1955b) notes the similarity at the generic level between Indian
and African fishes, e.g. the cyprinids Barilius, Garra and
Labeo, indicating that these fishes arrived in Africa from India after the desiccation
of the Syrian-Iranian Sea in the Pliocene. The primary route, according
to Kosswig and to Por (1987), was a northern one around the barrier of
the Persian Gulf and Sea of Oman via northern Arabia, Syria and the Levant.
Cooling conditions in these areas during the Pliocene and especially the
Pleistocene glaciations, and arid climates at times, were unsuitable for
tropical forms. These movements left a selection of fishes in what is now
Iran including the cyprinid Garra, the sisorid catfish
Glyptothorax and the spiny eel Mastacembelus.
The Pleistocene fore-deep of the Himalayas may have had
connections with the Tigris-Euphrates basin which extending down the Persian
Gulf as a river valley. The Tigris-Euphrates basin formed during the Pliocene
and was colonised by primary freshwater fishes no earlier than the late
Pliocene (Krupp, 1983). Movements of fishes into Iran from the west and
north were also affected by the presence of the Tethys Sea and a brief
account is given under the genus Barbus sensu lato which has been studied in this regard.
The present picture of the Arabian peninsula is of an
arid desert unsupportive of fish life. The presence of fishes in Arabia
and the Levant, and even Africa, with apparent relationships to fishes
from Iran and the east indicate that fishes must once have traversed this
area. Movements of fishes are thought to have been in a northern arc around
the Fertile Crescent or its earlier version. However this modern picture
is perhaps illusory as there is evidence of a more hospitable environment
in the Arabian Peninsula at various times in the past. Wadis were active
during "pluvial" periods of the Pleistocene as evidenced by deposition
of fluvial material (Al-Asfour, 1978). One of these wadis drained much
of central Arabia to the Kuwait area. The "Kuwait River" once ran from
the Hijaz Mountains in western Saudi Arabia northeastwards for about 850
km to drain into the Persian Gulf via a vast delta occupying much of modern
Kuwait. The river was 8 km wide and over 15 m deep along most of its length
(Hamblin, 1987; Anonymous, 1993b). This river last ran between 11,000 and
6,000 years ago and could have provided a highway for fish dispersal. Earlier
rivers of this nature dating to the Late Miocene (Forey and Young, 1999;
Hill and Whybrow, 1999; Friend, 1999), the Pliocene (Gerson, 1982), and
others like it in other parts of the peninsula, as well as shallow lakes
(e.g. Lake Mundafan in the Rub' al Khali at 36,000-17,000 B.P. and again
at 9000-6000 B.P.) would have facilitated transfer of species across the
Arabian Peninsula, today an impassable desert for fishes, e.g. at the height
of the Würm glaciation 40,000 years ago (Chapman, 1971; McClure, 1976;
Al-Sayari and Zötl, 1978; Brice, 1978; Jado and Zötl, 1984; Wagstaff,
1985). A freshwater connection between Iran and Arabia was almost continuous
from 70,000 to 20,000 years B.P. (Krupp, 1983). However no fish remains
have been found in the late Pleistocene lakes although freshwater molluscs
are frequent, Hippopotamus remains are reported and Neolithic fish
hooks have been found in Al Hasa in eastern Saudi Arabia. Incomplete Miocene
freshwater fish fossils are reported from the Jizan basin in the Tihama
north of the Saudi Arabian-Yemen border (Brown, 1970). One was identified
as a Barbus and the other as a Tilapia. Both these identifications
are of such a general nature (see account on the genus Barbus and related
genera for
example) as to throw little light on past history or relationships with
modern taxa. The Lower Miocene fauna of Al-Sarrar at 15-17 MYA, northwest
of Dhahran in eastern Saudi Arabia, contains pharyngeal teeth thought to
be Barbus sensu lato, and more interestingly several thought to be Labeo
(Thomas et al., 1982). This latter genus is not now found in the
Middle East but occurs in the Indian subcontinent and Africa. The Late
Miocene Baynunah Fauna of Abu Dhabi in the United Arab Emirates contains
Clarias, Bagrus shuwaiensis and Barbus sensu lato in a river connected with an
ancestral Tigris-Euphrates system (Forey and Young, 1999). These fossils
tend to confirm the hypothesis that fishes of Asian origin reached Africa
through the Middle East and could have taken what may be termed a southern
route across the Arabian Peninsula. However Forey and Young (1999) point
out that the modern Arabian fauna may not have a history stretching back
to the Miocene but is due more to a re-invasion after a loss of an earlier
fauna. The modern Iranian fauna, in part, may be a remnant of movements
at various times yet to be resolved in the absence of species-level phylogenies.
In general, the climate of Iran can be classified as arid
to semi-arid, with more than 80% of the country characterised by less than
250 mm annual rainfall. Mountain ranges block off the interior of Iran
and give extremely continental conditions except for the narrow littoral
zones on the Caspian shore and the Persian Gulf. Summers are hot and dry
with little change from day to day. Three main climatic types are found:
warm, temperate and rainy with a dry summer in the Caspian coastal area,
dry, hot desert in the central plateau, and dry, hot steppe in the rest
of the country. Humidity is generally low because of the altitude, much
of Iran being over 1000 m average height. Coastal regions along the Persian
Gulf have a high humidity, especially in summer. Wind patterns are deflected
by the Zagros and Alborz ranges in the west and north. Summer winds are
mainly north and northwest over much of northern and central Iran and are
hot, dry, and strong for long periods. The Sistan "Wind of 120 Days" from
the northwest blows from the end of May to September continuously and is
very hot, dry and sand-laden. The "shamal" blows from the northwest over
Khuzestan and coastal regions of the Persian Gulf from February to October,
most intensely in summer. These summer winds undoubtedly contribute to
the desiccation and, in some cases, filling-in of water courses. In the
south the winds are west and southwest.
Temperature varies greatly over Iran with latitude and
altitude, as well as with the seasons. Winter lows are found in January
and summer highs in July in general, with the Zagros and Alborz mountains
and the Caspian shore having maximum temperatures in August as a result
of the influence of altitude and the sea. The mean monthly temperatures
for January at 15 selected stations across Iran (Ganji, 1968) had a range
of -1°C to 20°C, average about 8°C. For July these figures are 25 to 37°C,
average 30°C. The annual range is 14C° at Jask on the Sea
of Oman and 30.5C° at Mianeh in East Azarbayjan.
Outside the coastal areas of the Caspian and Gulf, the annual range is
considerable, and daily ranges also are large. Nights can be very cold
in the northeast, less so on the plateau. Some areas, like the Khuzestan
plains, have maximum temperatures over 50°C (53°C at Gatvand near Dezful; possibly over
55°C in the interior, hotter than anywhere else on earth) in summer while in the northwest in winter the temperature
can fall below -30°C (to a low of -36°C at Bijar in Kordestan). Five temperature provinces have been delineated
for Iran: the Caspian zone along the littoral which has a low annual temperature
range; the Persian Gulf zone which has a low annual range but high values;
the Zagros zone with a much higher range than the first two zones and a
very low January mean; the Alborz zone which is similar to the Zagros but
has higher temperatures and a greater range; and the interior zone with
the greatest annual range coupled with relatively high values.
Precipitation falls in winter as snow on the mountains
of the north and west. The highest mountains remain snow-covered year round.
The plateau also receives snow but it does not last long and there is no
snow along the Persian Gulf coast. Rain falls mainly in November to May
with a mean annual of 416 mm, although the Caspian littoral is much higher
and the interior plateau much less. Rain is uncommon from May to October
over most of Iran. Maximum rain is found on the outward slopes of the Alborz
and Zagros ranges where the mean annual rainfall is more than 1200 mm,
1950 mm at Anzali. The plateau has less than 120 mm annually, Sistan less
than 70 mm, and Mirjaveh on the Pakistani border only 48 mm annually. The
Caspian littoral has rain in every month at some localities. The plateau
receives most of its rain in spring, the Caspian in autumn, and the Gulf
coast in winter. The result of this pattern of rainfall is heavy runoff
in spring with silt-laden floods and erosion a feature.
Many streams marked on maps are actually dry for much of the year. Even
a major, interior basin river like the Zayandeh which flows through Esfahan
does not reach its terminal basin for much of the year.
Droughts occur and can be devastating for fish habitats.
The drought years 1999-2001 were the worst in 30-40 years and resulted
in a United Nations Technical Mission (see ReliefWeb, 22 August 2000, UN
Office for the Coordination of Humanitarian Affairs (OCHA) at
www.reliefweb.int; Foghi, 2004).
Various effects were noted including the drying of 2500 qanats in Yazd,
in southern Fars groundwater became saline, the Latian, Lar and Karaj dams near
Tehran had water reserves of 51 million cu m, down from 173 million cu m for the
same period in the previous year and were within about 2 months of drying up, several lakes and wetlands
of international importance dried out (Bakhtegan-Neyriz and surrounding
wetlands, Hamun-e Saberi, south end of Hamun-e Puzak and Gav Khuni), rivers
dried completely (Hirmand River and its terminal lake), the Dez and Karkheh
rivers in Khuzestan were depleted by 70% in 2001, water rationing was
implemented in Tehran and 30 other cities, and lower water
levels in rivers that retained flow had reduced oxygen affecting fish (IRNA, various
news reports, 2001). In East Azarbayjan, 190 ha of 220 ha used for fish breeding
were useless through drought (IRNA, 29 August 2001). Marshes south of
Lake Orumiyeh near Mahabad encompassing 30,000 ha dried up (IRNA, 25
August 2001). Water reserves behind dams in Khorasan were depleted by 65% in
2001, the precipitation rate having declined by 40% in the period November
2000-August 2001 (IRNA, 3 September 2001).
Abbaspour and Sabetraftar (2005) reviewed Iranian drought cycles and found
arid conditions were experienced for 13 of the previous 23 years. Drought
affected fishes in the drying of wetlands where hundreds of thousands of fish
died, in Sistan 8-12,000 tons of fish were lost as the lakes dried up, in Fars
fish losses were reported from the Kor River, in East Azarbayjan 174 ha of fish
culture farms were damaged, and rivers draining to the Persian Gulf lost fishes
including migratory species.
The nature of the drainages of Iran is directly related
to climate. The Alborz Mountains in the north block movement of moisture
to the south while the Zagros Mountains in the west block moisture from
that direction. The southeast monsoon is almost completely dry before it
reaches eastern Iran. In consequence the best watered parts of Iran lie
on its northern and western fringes and the interior becomes drier from
west to east and north to south. Interior rivers exist in large part because
of mountain ranges which store water as snow, in the case of the Hirmand
River and the Sistan lakes, far removed from Iran.
There has been many studies on past climates in Iran and
neighbouring countries, attempting to link climate with past environmental
conditions in the Late Pleistocene-Holocene. The Early to Middle Pleistocene,
however, is practically unknown for the Middle East and is not dealt with
here (Butzer, 1978). Past environments have significance for fish habitats,
distributions and zoogeography. The brief summary below is based on Butzer
(1957, 1958a, 1958b, 1961, 1975, 1978), Bobek (1959), Whyte (1961), Hutchinson
and Cowgill (1963), van Zeist and Wright (1963), van Zeist (1967), Wright
et al. (1967), Krinsley (1970), Diester-Haass (1973), Turnbull and
Reed (1974), Nützel (1976), van Zeist and Bottema (1977, 1982), Wright
(1977; 1983), Ganji (1978), Neumann and Sigrist (1978), van Zeist and Woldring
(1978), Woosley and Hole (1978), Farrand (1979), Storch (1980), Coad (1980c),
Kay and Johnson (1981), Lamb (1982), Neumann (1993), Qin and Yu (1998);
Griffiths et al. (2001); Stevens et al. (2001); Snyder et al.
(2001); this being by no means an exhaustive listing of the studies in this field
nor is the below a critical assessment of conflicting views. Evidence for
these past environments is taken from a number of studies in different
fields. The Pleistocene ice has been gradually withdrawing from its last
maximum at 20,000 B.P. and the remains of ice fields and glacial moraines
can be used to determine former conditions such as the snowline. The advance
and retreat of deserts and the use and abandonment of settlements are indicative
of changes. Such erosional physical features as dry riverbeds and other
riverine structures, alluvial fans, sand dunes, and aeolian deposits all
give clues to environmental change. The extent and level of lakes and playas
have been widely studied as indicators of climatic fluctuations. Pollen
and other organisms associated with lake sediments can be used to trace
changing conditions and finally historical records can be analyzed.
Glacial deposits in the outward slopes of the Zagros and
Alborz mountains indicate that the snowline was 600-800 m lower than today,
perhaps as much as 1800 m in some areas, and as much as 1500 m at Shir
Kuh near Yazd and Kuh-e Jupar near Kerman in south-central Iran. Lowered
snowlines cannot be explained by temperature alone but were probably due
to much greater precipitation. Winter would have been longer and colder
in the Pleistocene, more snow would accumulate and summers may have been
cloudier. The runoff period would have been longer and river habitats could
have been less prone to desiccation in late summer.
The climate in the Zagros Mountains of the late Quaternary
in Iran has been examined by means of sediment analyses from lakes Zaribar
and Mirabad and for nearby Turkey at Lake Van. Pollen, chemistry, sediments,
diatoms, cladocerans, ostracods and palaeobotany all confirm geological studies.
The last glacial maximum (the Würm) at about 20,000 B.P. led to local glaciation, a depression
in the snow line and absence of trees. The climate was cool and relatively
dry, with less precipitation than today. The cooler temperatures meant
less evaporation, more runoff and filling of intermontane lakes. The Caspian
Sea and Lake Orumiyeh were much larger than today, being 78 m and 55 m
higher. As the glaciers receded, the land environment or life zones moved
up the mountains. The significance of this for fishes is unknown; there
were few trees and the environment may have resembled modern denuded conditions.
There may have been a higher flow than later when trees developed to hold
runoff and before man chopped them down. However bushes could have retained
water and reduced silt load in rivers. By 12-14,000 B.P the evidence from
Zaribar and Mirabad indicates a warming climate but without increased precipitation.
Indeed rainfall may have been less than today, reducing river flows and
perhaps habitats for fishes. This arid period was succeeded by a more humid
period. An increase in precipitation at Lake Van did not take place until
6500 B.P., about 4000 years later than in western Iran. Climate changed
not only through time but also geographically, just as today. Regional
variations mask general statements about earlier climate for Iran and the
outline given here is perhaps best seen as indicative that change occurred.
The humid period was followed by a period of less rainfall, and then in
the late Holocene by an increase in rainfall. The last 3000 years have
been humid with perhaps two, short, arid episodes. Southern Iran may have
been cool and comparatively moist when the highlands were moderately cold
and relatively dry. Climate probably changed markedly over short periods.
Short cold phases are recorded for Europe in the last several thousand
years, e.g. from about 1400 to 1230 B.C., associated with rises in lake
levels. Similar events may occurred in Iran. Barley harvest dates in Babylonia
derived from clay tablets indicate they were 10-20 days earlier in the
period 1800-1650 B.C. and 10-20 days later in 600-400 B.C. It is concluded
that the former period was warmer and the latter cooler than today.
Pluvial conditions as recognised for more northerly areas
of Europe probably did not occur in Iran during the Pleistocene although
summers may have been less dry because of greater cloudiness and lower
temperatures and evaporation. Lake levels were probably higher 18,000-20,000
years ago (Roberts and Wright, 1993). Krinsley (1970), in his study of
playas in Iran, concluded that the climate was semi-arid rather than pluvial
in the period of maximum cold during the Pleistocene. Lakes, which occupied
endorheic basins and could have facilitated local fish movements, dried
up as the climate warmed with the retreat of ice sheets and glaciers and
evaporation exceeded precipitation. These shallow lakes were found along
the inner mountain front or within basins which received greater discharges.
As distance from the mountains increased, there were only intermittent
lakes and finally playas. An immense lake filling much of central Iran,
as proposed by earlier authors, seems unlikely. Generally conditions over
Iran appear to have varied as much, if not more, in the Pleistocene as
they did in recent centuries through the agency of man. Conditions 9000
years ago were probably drier than today (Roberts and Wright, 1993). The
fishes may have been selected for an ability to survive highly variable
conditions in terms of stream flow, temperature, silt load, local fluctuations
in lake levels and salt content, etc.
The greenhouse effect is apparent in Iran, a rise in temperature
caused by various man-made and released gases. Nasrallah and Balling (1993)
show a temperature increase of 0.09-0.23C°/decade, mean 0.18C°/decade, from 1950-1990.
The major rivers of Iran drain the two mountain chains which retain
enough snow or collect enough rainfall to ensure a constant and
appreciable flow. Afshin (1994) summarises the rivers of Iran. All rivers in Iran are fordable on foot when not in
spring flood with the exception of the Aras and Safid rivers of the
Caspian basin, the Hirmand river of Sistan and the large rivers of
Khuzestan. Most rivers marked on maps are in reality small streams,
with very shallow and clear water. There is little vegetation on the
banks, and fishes, if present, can be seen with ease. A significant
proportion of fish habitat is occupied by small streams, springs and
qanats. Large freshwater lakes or marshes are absent except in Sistan, the
Caspian basin and the plains of Khuzestan. Most large lakes on maps
are salty and do not support a fish fauna. A number of dams have been
built and more are planned (see Bagley (1976), Coad (1980c) and "Aquastat"
from the Food and Agriculture Organization, Rome (http://www.fao.org/ag/agl/aglw/aquastat/iran.htm))
and these form important lacustrine habitats. In 1994, 27 storage dams
were in operation with a capacity of 39.2 km3 and a further
24 were under construction with a capacity of 11.5 km3 (see
also below for more on dams). In 2002 Iran was building 68 dams and the
construction of a further 120 dams were being considered as 33% of the country's
water resources were wasted (IRNA, 2 January 2002). Manouchehri and
Mahmoodian (2002) briefly review environmental impacts of dams in Iran.
The streams may have their origin in a mountain, a spring or a
qanat, but they hold in common a clarity of water, a bare pebble bed,
small dimensions (one to a few metres wide and a few centimetres deep)
and often a short course. They may join another stream but are often
lost in marshes, tapped for irrigation and lost in fields or become
absorbed by the friable and porous ground. Many streams are
intermittent, with flow near their mountain source, dry sections and
perhaps a flow near their mid-course, with subsequent absorption into
the ground. Heavy aquatic vegetation is not common and most plant
material is a thin encrusting layer on the bottom. Banks are often bare of
riparian vegetation and streams are fully exposed to insolation.
Summer temperatures are often high as a result (30°C
and more) yet at higher altitudes streams can be icy cold even in
summer and the typical blue-grey of snow-fed water. Spring floods can
be disastrous, scouring out the stream beds and dumping heavy silt loads
(Melville, 1984). Spring fed streams of shorter course are not affected because
they have a small catchment area and may well provide a refuge for
fishes. The clean water of springs attracts human settlement and these
waters are often blocked off to form ponds or cisterns with water led
off through artificial channels subject to drying as requirements
change. Streams and rivers may also be impounded, forming small ponds
or lakes. Bridges often have small pools beneath them and this may be
the deepest (at ca. 1 m) and most shaded section of a stream.
Marsh areas may be associated with springs. Reeds and other
vegetation develop downstream of the source and may be quite
extensive, occupying several square kilometres. Some areas of marsh
are ponded and provide habitat for larger species as well as shelter
for young. Extensive marshes, lakes and lagoons are developed in
Sistan, the Caspian basin and Khuzestan, all fed by major rivers (50+
m wide and 3+ m deep) draining vast areas of land. These areas vary
widely with season and flood dramatically in spring, inundating vast
tracts of land. The rivers and associated marsh-lake complexes provide
the major freshwater food fishing areas in Iran. The Sistan marshes
have been described in Annandale (1921) and Annandale and Hora (1920),
the Caspian shore by Schüz (1959) and the lowlands of southern Iraq
by Rzóska (1980) and by Thesiger (1985) and Young (1989).
Conservation of aquatic habitats in Iran has been part of a general
programme for biotic conservation summarised in Firouz (1974; 1976),
Firouz and Harrington (1976), Ashtiani-Zarandi (1990) and Kahrom (2000). The Ramsar
Convention on Wetlands of International Importance was named after the
city of Ramsar in northern Iran where the first conference was held in
January 1971. Iran has more Ramsar listed sites than any other country
in Southwest Asia (Scott, 1993). In 1977 there were 11 Park-e Melli
(National Parks), 4 Asar-e Tabii Melli (National Nature Monuments), 24
Manatgheh-Hefazat Shodeh (Protected Regions or Areas) and 31 Panahgah-e
hayat-e Vahsh (Wildlife Refuges) offering varying degrees of
protection to the fish fauna (Firouz et al., 1970; Yachkaschi,
1976; Köpp and Yachkaschi, 1978; Majnunian, 1985). The 1993 United
Nations List of National Parks and Protected Areas at "www.wcmc.org.uk/data/database/un_combo.html
lists" 7 National Parks, 2 National Nature Monuments, 41 Protected
Areas and 18 Wildlife Refuges and the National Report of the
Islamic Republic of Iran for the Convention on Biological Diversity
(Department of the Environment, Tehran) lists 11 National Parks, 47
Protected Areas, 25 Wildlife Refuges, 5 National Nature Monuments, 9
MAB (Man and Biosphere) Sites and 20 Ramsar Sites.
Seven Ramsar sites are priorities for urgent action with the
causes, namely:- Alagol, Ulmagol and Ajigol lakes (impact of
agricultural development), the Anzali Mordab (Talab) complex (falling
water levels and increased eutrophication leading to the rapid spread
of the reed Phragmites australis, south end of Hamun-e Puzak
(water inflow could be reduced because of dam construction in
Afghanistan), Hamun-e Saberi and Hamun-e Hirmand (dam construction in
Afghanistan), Neyriz lakes and Kamjan Marshes (drought and
agricultural activities), Shadegan Marshes and mudflats of Khor al
Amaya and Khor Musa (chemical pollution from the Iran-Iraq war), and
Shurgol, Yadegarlu and Dorgeh Sangi lakes (war and drought effects) (www.ramsar.org/ram_rpt_37e.htm,
downloaded 28 July 2000).
Iran has several unusual habitats for fishes and these are described below.
Only the true hot spring at Genu (27º26'N, 56º20'E)
is known to contain fish including Aphanius ginaonis, Cyprinion
watsoni and Garra persica (Coad, 1980b). A hot spring on
the slopes of Kuh-e Bazman (the mountain is at 28°04'N, 60°01'E) is rumoured to contain
tooth-carps (Cyprinodontidae).
The Ab-e Garm (literally hot water) at Genu emerges at 41°C
and was partially enclosed by brickwork associated with a hammam or
bath-house. The altitude of the spring is about 400 m. Its stream is
10-15 m wide near the source and the bed is composed of stones and
pebbles covered by lime-green algal mats and strings. Only Aphanius
ginaonis was found at the hot spring, not in the main flow but
along the stream margins and in many minor subsidiary springs which
emerge a few metres from the main spring. These minor springs had a
mud bottom, were as shallow as 1 cm and had soap and food debris
pollution in 1977. Side springs and stream margin near the source were
37-40ºC. The other species (along with A.
ginaonis) were found below a cascade and have no access to the
hotter parts of the spring and stream. A. dispar is recorded
from the spring by Werner (1929) but this has not been confirmed by my
collections. The water is clear and colourless, but there is a strong
smell of sulphur. Flow is 30 l/sec. The chemistry of this spring as
given by Joneidi et al. (1971?) was : pH = 6.2, conductivity
14,000 us, dry residue at 180ºC = 9933 mg/l, H2S = 34 (? p.p.b.), r (reacting value) Ca = 22.4, r
Mg = 9.9, r Na + K = 6.1, total cations 162.1 (sic), r Cl =
147, r SO4 = 15.4, r HCO3 = 4.6, total anions =
166 (sic), SiO2 = 10 mg/l, NH4 = 0.7 (no
units given), NO3 = 22 (no units given). There were traces
of CO2 and no measurable Fe, NO2, or CO3.
The hot spring lies in the Genu Protected Area (Biosphere Reserve)
which is described by Zehzad et al. (1997).
Iran is replete with caves but thus far only one has been found to
contain a fish fauna. This cave lies about 12 km north of the railway
station Tang-e Haft in Lorestan at 33°05'N, 48°36'E. Two species are found here, Iranocypris
typhlops (Cyprinidae) and Paracobitis smithi (Nemacheilidae)
(Bruun and Kaiser, 1944; Movaghar, 1973; Greenwood, 1976; Smith, 1978; 1979;
Coad, 1996c; Proudlove, 2001; Romero and Paulson, 2001). The cave lies in the Dez River drainage of the Tigris River
basin and its connection to nearby surface water is intermittent. The
cave is the surface outlet of a subterranean limestone system and the
captures may represent strays from underground. B. Sandford (pers.
comm., 1979) stated that there is some evidence of recent collapse in
the cave system and thus the habitat may be endangered but it is
difficult to assess the extent and nature of underground fissures in the rock.
Qanats are an unusual yet important habitat for fishes in Iran. An
account of their fishes with an extensive bibliography is given in
Coad (1996h); additional literature on this unique environment not
referenced there includes Kuros (1943), Aisenstein (1947), Feylessoufi
(1959), Nesbitt and Bawa (1960; 1961), de Menasce (1966), Jentsch
(1970), Nadji (1970; 1972a; 1972b), Braun (1974), Goblot (1979), Hartl
(1979), Sajjadi (1982), Goldsmith and Hildyard (1984), Behnia (1988), McLachlan
(1988), Beaumont et al. (1989), Harwit (1990), Razavi (1991), Coad (1994b),
Koocheki (1996), Liaqati (1997), Salim Manshadi et al. (1997), Afkhami
(1998), English (1998), Aminpouri (2002),
www.netiran.com/Htdocs/Clippings/DEconomy/200629XXDE05.html, downloaded 8 August 2002), Foltz (2002),
Floor (2003), Wessels and Hoogeveen (2003), and qanats at www.waterhistory.org,
and at www.bibliothecapersica.com/articlenavigation/index.html, under abyari
(irrigation), downloaded 24 December 2004.
The word qanat has various suggested origins including a derivation
from the Akkadian for "reed" according to Goblot (1979) in
contrast to others listed in Coad (1996h).
Over 20% of the irrigated area of Iran is fed by qanats (Redding
and Midlen, 1991) and numbers as high as 60,000 have been estimated.
They are essentially horizontal wells which tap groundwater and
provide a continual, low gradient flow of fresh water. Qanats are an
advantageous habitat for fishes in several ways. The water temperature
is not subject to the extremes found in natural waters, shade within
the qanat provides protection against predators on adults, young and
eggs and against insolation, the gradient and water flow are gentle,
and a certain amount of food is provided by kitchen scraps since
dishes, cooking containers and implements are washed in the jube or channel
and food is cleaned and trimmed there. A school of fish will quickly
gather at a washing site and maintain station in clouds of detergent
in order to pick up scraps of food. Attempts to imitate washing
movements will attract fish momentarily but they soon dart off when no
food is forthcoming. The garden environment with trees and other
vegetation provides shade, energy input from leaf fall and garbage
items, and facilitates development of an invertebrate fauna as a food
source. Aufwuchs on rock surfaces provide a food source along with the
associated invertebrate fauna. The Zoroastrian community, once
widespread in Iran, has a ceremony known as com-e mahi or
"meal for the fishes" in which bread and dried fruit are
thrown into running water as a libation (Boyce, 1977). Feeding of
scraps to fish is also seen in Moslem communities and boys regularly
attempt to attract and catch fish using any available food material
and primitive fishing gear (personal observations; Edwards, 1971).
Qanats are now rapidly being replaced by pump-wells which are
faster and easier to excavate but do not provide fish habitat.
Pump-wells often dry up qanats and natural springs by lowering the
water table (Razavi, 1991; Anonymous, 2001b; Aminpouri, 2002). Also schemes to restrict water
flow from qanats for conservation reasons will presumably affect the available
habitat for fishes (Salim Manshadi et al., 1997).
The qanat fishes comprised 25 species in Coad's study (1996h), 40%
of the fauna on the plateau of Iran. The number of species per qanat
ranges from 1 to 6 although 88% of qanats have only 1-2 species. Areas
with little surface water and low in diversity have 94% of the species
occurring in qanats while better-watered areas with more diversity
have only 29% of species in qanats. The qanat fauna is dominated by
the Cyprinidae, which comprises 76% of the ichthyofauna. The qanat
fauna is a subset of the basin in which the qanat occurs, comprising
small species, broadcast spawners, lacking in specialised food
requirements (usually scrapers of aufwuchs or feeding on
invertebrates), non-migratory, and widely tolerant of environmental conditions.
The fishes in qanats are caught by local people for food but given
the restricted size of this habitat and of most fishes found in them,
this is not a significant dietary item. In the seventeenth century
qanat fishes "were not esteemed as they never saw the light and
were used only for medicinal purposes to cause vomiting" (Ferrier,
1996, quoting Jean Chardin). In the 1950s villagers in Iran believed that qanat
fish lived forever and needed no food, only their own eggs (www.iras.ucalgary.ca/~volk/sylvia/qanat.htm,
downloaded 24 June 2002).
Colonisation is both natural, since loaches are unlikely to be seen
and caught by local people, and deliberate, since larger cyprinids are
found in qanats remote from any surface water. These fish are hardy,
already living in high temperature environments, and are easily
transported for Now Ruz celebrations. At the Zard-Abieh qanat in
Shahrud, a local man remembered putting fish into the qanat 60 years
ago from one now dry (H. Rahimian, pers. comm., 2000).
Salt lakes are common in Iran and are mostly too saline to support
a fish fauna. They are discussed in a world context by Williams
(1996). Fishes do exist in tributary streams (which may be saline in
varying degrees). Rivers and springs around salt lakes are therefore
isolated from one another and might be expected to give rise to unique
populations of fishes. However all these salt lakes are shallow and
liable to desiccate such that tributary streams and springs can
connect and allow faunal interchanges once the lake level falls.
Many streams in Iran are highly mineralized or even salt to taste
yet these support fishes which are usually regarded, at least at the
family level, as salt intolerant. Salinity tolerance studies have not
been carried out on Iranian fishes. The Caspian Sea is at one-third
sea water (12-13‰) yet typical "fresh" water species can
be found there, e.g. Cyprinus carpio.
A number of springs in Iran are said to be "sacred" and
their fish then attain a degree of importance on account of their
inaccessibility to ichthyologists. Howz or tanks at Qumisheh (32°01'N, 51°52'E) were supposed to hold sacred
fish, decorated with gold rings, according to John Fryer in 1698 and
John Chardin in 1711, but G. N. Curzon in 1892 mentioned that the gold
rings were gone and by 1978 so were the fish. A sacred tank or
artificial reservoir at Soh contained fish deemed to be holy. Visitors
were expected to purchase bread to feed these fishes (Anderson, 1880).
The most important "sacred" fish are those of Sa'di's
Tomb in Shiraz (29°37'N, 52°35'E) which were described by Heckel (1849b) as new species Scaphiodon
saadii (= Capoeta damascina) and Discognathus crenulatus
(= Garra rufa). The water is a stream (?qanat) under the tomb and part is
expanded into a hawz-e mahi or fish pond. Fish have been present here since at least the
early nineteenth century as they are mentioned briefly by Waring
(1807). Official permission was gained to collect fishes in Sa'di's
tomb for study but sampling was actively discouraged by local people.
Sa'di was supposed to punish any killing of these fishes with death
but the traveller Chardin was able to catch some to eat by monetary
means. Some of these fish too were reputedly decorated with gold rings
(Ouseley, 1819-1823); regrettably my captures were not.
A mordab is a fresh or brackish water lagoon area found along the
Caspian Sea coast (literally "dead water", the Russian
equivalent is liman). The Anzali Mordab at 37°26'N, 49°25'E is the best known (Firouz, 1968b)
and was formerly called the Pahlavi Mordab. The more modern term is
"talab" (= pool or marsh, which lacks the association with
death) but the older literature refers to mordab and the term is still
in common use. The Anzali Mordab is about 30 km long and 4-8 km wide
with clear water of only 1.5 m average depth. Much of the area is
covered by Phragmites reeds and other plants and only about 15%
is open water. Variations in Caspian Sea level and water abstraction
from feeder streams will affect the mordab level and size. In the
1930s the mordab was 4 to 8 m deep (Vladykov, 1964) and the fall in
level has severely affected the spawning migrations of fishes and the
habitat for developing young. The mordab is the principal breeding
ground for Rutilus frisii kutum and is also important for
several other species. Further details are given below under the
description of the Caspian Sea basin.
Wetlands were originally studied and protected as feeding and
overwintering grounds for important waterfowl but they do protect fish
populations which might otherwise be threatened. Access and hunting is
forbidden or restricted and often fishing too. Anonymous (1971), Carp
(1972) and Dugan (1993) list and describe various wetlands in Iran of
international importance principally:-
See also Scott (1995) where latitude-longitudes are often slightly different.
Lake Kopibalbalch, Hassanlu Marsh, Yadergarlu Marsh and surrounding
marshes (37°00'N, 45°30'E)
Other wetlands are mentioned in the appropriate drainage basin account.
Peritore (1999) gives a general overview of ecological conditions and
attitudes to the environment, Foltz (2001) reviews environmental initiatives, Afasiabi (2003) reviews the
environmental movement in Iran and Valeolahy (2000) reviews the factors
affecting the abundance of fishes and suggests measures for conservation. Jawad (2003) gives an account of the
impact of environmental change on Iraqi fishes which has implications for fishes
in neighbouring waters of Iran.
The drainage basins of Iran are shown in the Figure. The delimitation
of these basins is somewhat arbitrary. Iran is a mountainous country
and much of it is desert. There are thousands of small springs and
streams with no present or recent connection to other water bodies.
Practical considerations require a large scale and I have divided the
country into 19 major basins based on field work, maps, fish
distributions, history of research, works on hydrography and areas
deemed important for an understanding of zoogeography.
There are two main types of basin, exorheic where the rivers and lakes drain to the sea and endorheic,
where rivers drain to an internal basin such as a lake, or are lost in
the desert, and have no connection with the sea. The exorheic basins
all fringe the southern part of Iran. The bulk of the basins, in
number (15) and area (about 78.1% of Iran), are endorheic. These
plateau basins lie at an average altitude of 800 m, alternating with
mountains ridges at an average of 2000 m. The salt lakes and flats of
these basins are fed primarily by groundwater rather than rain (Issar,
1967) and water is lost by evaporation. Wolfart (1987) makes the
valuable point that Quaternary environments in the closed or endorheic
basins of arid Southwest Asia often have marine and brackish fossils.
These are not evidence of marine invasions but of the increasing
salinity derived from the mineral content of rainwater. As the water
evaporates it leaves behind the minerals and over ten thousand years
or less a saline environment develops.
www.bibliothecapersica.com/articlenavigation/index.html, under drainage,
downloaded 24 December 2004 gives four main drainages for Iran as follows:-
Van der Leeden (1975) summarises water resources of Iran with
discharges of principal rivers at various recording stations, lists of
major dams and reservoirs, and resources and demand.
www.bibliothecapersica.com/articlenavigation/index.html, under ab (= water),
downloaded 24 December 2004 also lists major dams and gives a general overview
of hydrology and has descriptions of various rivers under their names. McLachlan (1988)
also considers water resources in Iran. Some of the earlier dam
projects are described by Justin and Taleghani (1955). Later dam
projects can be located by a search at "Netiran.com". Prior to
the Islamic Revolution 13 dams had been built in Iran but the
five-year development plan (1990-1995) designed 110 dams of which 22
were under construction in 1993. 60 dams have been constructed after
the 1979 revolution (IRNA, 31 August 1998).
"Aquastat" from the Food and Agriculture Organization, Rome (www.fao.org/ag/agl/aglw/aquastat/iran.htm) gives an
overview of Iranian water resources and water abstraction and is
updated at intervals. The total domestic, industrial and agricultural
water abstraction was estimated at 70 km3 in 1993, 51% of
the renewable water resources. Annual abstraction from aquifers (57 km3)
is more than the estimated safe yield of 46 km3. An
additional 39 km3 is used annually, 20 km3 for
electricity production, 11 km3 for flood control and 2 km3
for control and thence environmental protection of downstream parts of
rivers, the remainder being surplus. The increasing demands will have
serious effects on the water supply and hence the fish fauna.
Nikravesh (1997) estimates, based on water consumption and population
growth, that Iran will be added to the U.N. list of countries facing
water shortages in the year 2025.
Kuros (1943) gives accounts of historical water resources and the
problems of water supplies in Iran. Lambton (1953) gives an account of
the allocation of water resources in Iran for irrigation. This latter
work is important for an understanding of restrictions on fish
habitats, e.g. in qanats, reservoirs, rivers and springs. Beaumont
(1981) reviews management of water resources in the Middle East and
places the Iranian resources in a wider context. Anonymous (1961c) and
Beaumont (1974) outline water resource development in Iran, the
construction of dams, abstraction for irrigation by traditional and
modern means, and the demands of industry and domestic consumers of
water. All these affect the habitat of fishes, often in deleterious
ways. Noori (1966) describes the hydrology of surface water in Iran.
Pirnia (1951), Anonymous (1961c) and Beaumont (1973b) give accounts of
the river regimes in Iran with discharges and runoffs at various
recording stations. Peak discharges occur in March to May because of
snowmelt. Very low flows occur in summer because of the lack of
precipitation, and because of abstraction for irrigation, and flow is
mostly from groundwater sources. Most rivers are really streams for
much of the year as minimum flows for principal rivers are 0.16-451 cu
m/sec, average about 36 cu m/sec. The Caspian rivers are the only ones
which lack a distinctive annual rhythm and show flows closely related
to precipitation throughout the year. The areas with the largest
runoff values are in the northern and central Zagros Mountains and in
the Alborz Mountains while lowest runoff values per unit area are
found around the deserts in central Iran. In the Zagros and Alborz,
annual runoff values can attain more than 300,000 cu m per sq km.
Löffler (1956; 1961) studied the limnology of several of the major
basins within Iran. The Ramsar Convention on Wetlands has a report on the
Islamic Republic of Iran (No. 37, at www.ramsar.org/ram_rpt_37e.htm, downloaded 4 May 2001).
Peritore (1999) gives a general overview of ecological
conditions and attitudes to the environment in Iran. Zohary (1963) gives a general account of the
vegetation of Iran. A general description of Iran, its structure and
drainage can be found in Harrison et al. (1945), Neumann
(1953), Fisher (1968) and Krinsley (1970). Water policy development is
summarised in Aminipouri (2002). A description of natural areas in
Iran, including a list of National Parks and Protected Rivers, can be found in
Zehzad et al. (2002). The Protected rivers are the Jajrud and Karaj in
the Namak Lake basin, and the Chalus, Sardab, Lar and Haraz rivers of the
Caspian Sea basin.
This basin comprises rivers which drain the southern Zagros
Mountains to the head of the Persian Gulf, but which are not now
tributaries of the Tigris River nor are they the salt streams of
Hormuz. None of these rivers has a significant fishery. At its
northern edge, the Zohreh River flows across the Khuzestan plains and
is close to Tigris River tributaries. Other major rivers are the
Helleh, which debouches into the Gulf north of Bushehr (28°59'N,
50°50'E) and the Mand or Qarah Aqaj (= the classical Sitakos), which, with its tributaries, drains much of
Fars Province to the Gulf south of Bushehr. Near Shiraz it is known as
the Qarah Aqaj or Kavar River. The Band-e Bahman, a weir or small dam on
this river near Kavar, is probably pre-Islamic.
The Mand River is 480 km long and occupies a basin of about 60,000
sq km. Its flow is reduced by a low snow cover (although there can be
torrential spring flow), water seepage, evaporation and abstraction
for irrigation purposes. Discharge has been estimated to range from
10-2025 cu m (Merchant and Ronaghy, 1976). It is also polluted near
Kavar (29°11'N, 52°44'E) by sewage and agriculture residues and does dry up to a series of
isolated pools there. A fish kill, numbering in the many thousands,
occurred in the Mand near Shiraz in 1977 and was attributed to
chemicals used in spraying against malarial mosquitos. The people
hired to spray village houses either dumped quantities of the chemical
into the river to reduce their work load or washed out containers in
the river (Coad, 1980c). Temperature range is at least 20C°
between winter and summer. The delta of the Mand is a Protected Area
of 46,700 ha. There are thin oxbow lakes and associated marshes
The Mand has a number of tributaries, at least two of which are
called Shur (= salt) River. Conductivity near Firuzabad on the Shur
River is 695-715 µM/cm but rises to 20,000 µM/cm below salt domes
further downriver. The more southerly headwaters are close to those of
the Shur River of the Hormuz basin between Darab (28°45'N,
54°34'E) and Fasa (28°56'N, 53°42'E). The headwaters of the Mand lie
north-west of Shiraz near Kuh-e Tabask at 2318 m (29°52'N,
51°49'E) and there are a series of springs in this area called Chehel Chashmeh (= Forty Springs) which
feed the Mand. Nearby is the Dasht-e Arzhan (29°39'N, 51°58'E), a small enclosed basin with a
flooded plain encompassing about 24 sq km at maximum. It is fed by
small springs and streams. The water is fresh since swallow holes in
the southeast corner of the plain drain water away with a salt
flushing effect. Shiraz was once "chiefly supplied with fish from
this lake" (Ouseley, 1819-1823) but it does not now support such
a copious ichthyofauna. A report from Reuters (8 June 2000)
cites a fish kill numbering in the hundreds of thousands from the
"Arjang lagoon, in a suburb of the southern city of Shiraz",
presumably this lake, after it dried up (www.iran-sabz.org/news/fish2.htm).
The Haft Barm-e Kudian lie about 20 km north of Dasht-e Arzhan at 29°49'N,
52°02'E at 2200 m. The seven lakes lie in
rolling country and the largest is about a 1 sq km. Some may dry up in
certain years but fish were found suggesting that there is a perennial
water supply (Cornwallis, 1968a). Scott (1995) says the southern 5
lakes generally dry out completely in summer. In winter the lakes
freeze over. They are about 2-3 m deep and some are slightly saline.
These lakes have been stocked with Esox lucius, Hypophthalmichthys
molitrix, Ctenopharyngodon idella and Gambusia holbrooki.
Surber (1969) gives some spot data on pH, total alkalinity,
calcium-magnesium hardness, chlorides and free CO2 in the
Mand basin. Near Firuzabad, the concentration of total dissolved
solids is 333 mg/l while near Jahrom it reaches 6937 mg/l, indicating
how there can be great variations in habitat within the same river
basin over short distances, depending on local geology.
The Zohreh River and its tributary the Shul, are over 400 km long
and have their headwaters near Kuh-e Barm Firuz at 3673 m (30°25'N,
51°58'E) whose northern flank spawns the
Khersan River, a Karun tributary in the Tigris basin. Its basin is
estimated to be 15,500 sq km. The Kowsar Dam at Gachsaran is 337.5 m
high, its crest is 126 m and the reservoir capacity is 450 million cu
m (http://netiran.com/news/IRNA/html/941126IRGG10.html). Gorjipoor et al.
(2007) carried out a limnological investigation of the Zohreh River.
The Helleh River receives the Dalaki (205 km) and Shapur (231 km)
rivers which drain the lower Zagros ranges west of Shiraz. Its basin
is estimated to be 20,300 sq km (Shiati (1989) gives 10,000 sq km) and
includes Lake Famur. Shiati (1989) gave an account of salinity in the
rivers of this basin. Saline springs and salt domes increase the
salinity about 10 times as the rivers flow down from the mountains.
Total dissolved solids in the upper reaches of this basin are 366
mg/l, rising to 4219 mg/l in the lower reaches. Geological sources of
sulphur also add to the chemical make up of these waters. There are no
important sources of industrial pollution along these rivers but
humans, domestic animals and agriculture are the main pollution
sources. The levels of pollution are in the acceptable range (Gh.
Izadpanahi, pers. comm., 1995). Aquaculture in the area (Helleh and Mand river
basins) has not had obvious effects on coastal water quality (Omidi, 2006). The delta of the Helleh River
is a complex of brackish and fresh marshes and lagoons with a maximum
depth of 3.5 m. It is the largest freshwater marsh system on the
Persian Gulf coast in southern Iran. It is designated as a Protected
Area (42,600 ha). This area developed in the early 1970s when the main
river channel was diverted onto the coastal plain.
A cave at Bishapur above the Shapur River is reputed to house a
deep lake full of fish but this has not been investigated and may only
be a local legend (Mounsey, 1872).
Endorheic Lake Famur, Perishan or Parishan (29°31'N,
51°48'E) is a particular feature of the
Gulf basin which encompasses 42 sq km at about 820 m near Kazerun, is
fed by about 80 fresh and brackish springs with a discharge of about
800 litres/second and supports a fish fauna near the springs. In years
of heavy rainfall the fresh areas expand only to contract in dry years.
An account of the lake is given in Farsi by Maafi (1996a; 1996b;
1996c). The lake is eutrophic and low
concentrations of oxygen periodically cause fish mortalities. The reed
beds are set on fire to increase the available agricultural land and
this results in a sediment input with the consequent decrease in water
depth, fingerling habitat destruction, and fish mortality through
sediments clogging gills. Overfishing is also a problem. Wastewater
and sewage enter the lake untreated and this enhances algal growth and
eutrophication. Fishery ponds are established west of Lake Parishan
resulting in exotic escapes. During periods of low rainfall, Parishan
becomes a shallow saline lake and presumably fish habitat is limited
to the immediate vicinity of freshwater springs.
Lake Parishan and the nearby Dasht-e Arjan (29°37'N, 51°59'E) are a Ramsar Site (World
Conservation Monitoring Centre, 1990). They lie within the Arjan
National Park and International Reserve which encompasses 65,750 ha as
established in 1973. However the Park has been downgraded to a
Protected Area of 52,800 ha with the Ramsar Site being the wetlands of
Lake Parishan at 4200 ha and Dasht-e Arjan at 2400 ha (Khan et al.,
1992). Dasht-e Arjan at 1950 m is a shallow, eutrophic freshwater lake
fed by runoff, precipitation and the Salmon springs. The lake area in
winter may be 1950 ha but shrinks in summer to a few hundred hectares. It dried
completely in 2001. There is an outflow through swallow-holes in the south-east,
traditionally linked to Lake Parishan. The lake margin and the
spring-fed marshes have Phragmites communis, Typha and Juncus
along with aquatic vegetation. Dasht-e Arjan is cooler than the
environs of Lake Parishan because of its higher altitude - 15-35°C
in summer and -10-15°C in winter as opposed to 22-40°C and 5-15°C.
As well as the rivers described above, springs and qanats are
important in the Gulf basin. The Dalaki mineral springs have a temperature range
of 30-38°C and a discharge of 200l/s. They are at 130 m above sea level and
their hydrology, geology and chemistry is reviewed in Kompani-Zare and Moore
(2001). The fishes in this area have not been investigated.
The Shabankareh Dam is a diversion dam in
the lower Helleh River basin and several other dams have been planned
for this basin. Small canals or diversions are also present in this
basin (Borowicka, 1958).
Berg (1940) places this basin, the Hormuz basin and the Makran
basin as part of the Sind Province of the Indian Subregion of the
Sino-Indian Region. Its eastwards extent is the lower and middle Indus
River. The Iranian portion is called the Southern Iranian District.
Small southern Iranian rivers belonged to a single river basin in the
Pliocene, facilitating dispersal according to Berg.
Hormuz
The Hormuz (or Hormozgan) basin comprises a number of intermittent streams and
rivers which drain to the Straits of Hormuz. None of the rivers has a
significant fishery. The basin has a catchment of 55,800 sq km.
Rainfall is low and sporadic at this southern end of the Zagros
Mountains and streams are not always perennial. Qanats are an
important feature and there is a hot spring (41°C) at Genu (27°26'N, 56°20'E)
just north of Bandar-e Abbas. This area of Iran is rich in salt domes
rising to over 1200 m above the surrounding land surface and
consequently surface water is often contaminated and stream banks are
rimed with salt (Lehner, 1944; Shearman, 1976; Kent, 1979). Some of
the islands off this coast are salt plugs, e.g. Hormuz Island.
Temperatures in winter are high in the lower streams, 15-33°C,
and must be much higher in summer. These warm and saline streams are
home to the endemic cichlid, Iranocichla hormuzensis, and so
are distinguished from the fresh waters to the north, east and west. This
species has been collected in the Minab River where my collections in the 1970s
did not find it. The Minab River was therefore included in the Makran basin but
may well form the easternmost part of this basin. However the possibility of an
introduction of this species to the Minab cannot be ruled out.

Salt domes and salt glaciers, southern Iran, NASA and Wikimedia Commons.
The principal river is the Kul with its tributary the Shur (= salt)
River. The upper reaches of the Shur lie west of Darab (28°45'N,
54°34'E) and mountains here exceed 3000 m. The headwaters of the Shur approach those of the eastern
tributaries of the Mand River basin. The lower valleys parallel the
coast and drain eastwards. The Rasul River is a tributary of the Kul,
while the Mehran River drains directly into the sea. The Mehran delta
lies in the Hara Protected Area (Biosphere Reserve) described by
Zehzad et al. (1998). The offshore islands such as Qeshm, are
poor in fresh water, but have not been explored. A number of streams
cross the plain east of Bandar-e Abbas (27°11'N, 56°17'E) draining the Kuh-e Furgun at
3279 m and associated ranges. Although many streams are salty, a
freshwater oasis is found at Sar Khun (27°23'N, 56°26'E).
Several islands in the Persian Gulf are included as part of this
basin. The largest island is Qeshm but it lacks rivers although there are some
small dams to collect rainwater runoff (A. R. Zeanaie, pers. comm., 1999).
Species observed are Aphanius dispar, a mudskipper and the introduced Gambusia
holbrooki. Water temperatures reach 32°C.
This basin comprises the drainages of the eastern highlands north of Birjand (32°53'N, 59°13'E)
flanked by the Dasht-e Kavir basin to the west, the Dasht-e Lut and Sistan basins to
the south, the Tedzhen to the north and the Afghan border to the east.
The Tedzhen basin is separated by three ranges, from west to east, the
Kuh-e Sorkh (35°30'N, 58°36'E) at 3017 m, the Kuh-e Bizak (35°11'N, 60°20'E)
and the Kuh-e Khvaf at 2517 m east of Khvaf (34°33'N,
60°08'E). These receive snow in winter
from moist Caspian Sea air. The highlands are relatively low compared
with other parts of Iran and nowhere exceed 3000 m except for the Kuh-e
Sorkh. The lowest points are in the sumps on the Afghan border at
about 610 m. There are a number of minor sumps and the drainage
patterns have been described as indeterminate. The total area is about
82,000 sq km. Tectonism commonly causes drainage disruptions (Krinsley,
1970).
The distinction of the western parts of the basin from the Dasht-e
Kavir
basin is somewhat arbitrary since the Kavir-e Namak near Bejestan (34°31'N,
58°10'E) lies at a similar level to the
Kavir-e Bozorg and is separated by only a low rise in the land. This
kavir receives intermittent streams from the east and north. The
Bejestan basin does receive tributaries from Afghanistan but these are
minor and do not begin to approach the input received by the Sistan
and Tedzhen basins from the east. Streams drain mostly to the east, to
three small terminal basins straddling the border; from north to south
these are the Namakzar-e Khvaf, the Daqq-e Patargan and the Daqq-e
Tondi.
The Dasht-e Lut basin to the south is separated by the drainage divide of
the Birjand-Qa'in highlands, which trend north-west to south-east. Kuh-e
Kalat is at 2605 m (34°18'N, 58°22'E)
in the north-west and altitudes of 2779 m are reached in the south-east.
This whole basin has seasonal streams and a few springs with qanats
a prominent feature. Water temperatures in qanats is 22-25°C
year round and their is little fluctuation in water flow and chemical
composition. Springs in contrast are influenced by the local geology
and have a variable chemical composition, as well as being influenced
by climate and pollution (Ruttner-Kolisko, 1964; 1966).
The Caspian Sea (Darya-ye Khazar, Darya-ye Mazandaran) basin is
here taken to include both the rivers draining to that sea and the sea
itself within Iranian territorial waters. This basin, in its land
part, is elongate, extending from the Turkish border almost to the
Afghan border and only acquires some width where the Safid River and
its tributaries penetrate the Alborz Mountains in the west. According
to Pirnia (1951) the Caspian basin in Iran (excluding the sea)
encompasses 182,100 sq km while according to Zakeri (1997) this figure
is 256,000 sq km, 15.5% of the whole country. Zakeri (1997) records
864 small and large rivers, including the Safid River with a catchment
of 67,000 sq km. Much of the information on the Caspian Sea itself is
restricted to waters of the former U.S.S.R. and there is relatively
little on Iranian territorial waters. Rozengurt and Hedgpeth (1989),
Kosarev and Yablonskaya (1994), Mandych (1995), Golubev (1996)
and Ivanov (2000) summarise much of the recent Soviet literature, a general review is given by Mamaev (2002)
and Bogutskaya et al. (2008) review early investigations of the sea and
its fish biodiversity with special emphasis on the 1904 expedition led by N. M.
Knipovich.
An ongoing and developing source of
information on this sea, the surrounding land, its history, its management, biodiversity
strategy and action plan, and a wide sweep of environmental problems is the Caspian Environment Programme (CEP), Baku, Azerbaijan
at www.caspianenvironment.org. This site has numerous documents and reports on-line, some with authors, e.g.
Katunin (2000), Ivanov and Katunin (2001), ERM-Lahmeyer International GmbH, DHI Water & Environment and GOPA
Consultants (2001a), others appearing under CEP or TACIS (Technical Assistance to the Commonwealth of
Independent States, European Union), e.g. TACIS and UNDP (2000), TACIS (2002), CEP (1998, 2000b, 2002). These reports include information on
the fishes and fisheries but are best referred to for the interactions between
people and the environment. Kiabi et al. (1999) describe the wetlands and
rivers of Golestan Province at the southeast corner of the Caspian Sea. Razavi
(1999) gives an introduction to the ecology of the sea in Farsi. Nezami et al.
(2000) and CEP (2001) give recent general descriptions of the Iranian Caspian coastal zone,
the important rivers, wetlands, water quality, climate, pollutants, and fisheries.
www.bibliothecapersica.com/articlenavigation/index.html, under Caspian Sea,
downloaded 24 December 2004 also gives an overview of this basin. Nadim et al.
(2006) review the management of coastal areas in the Caspian Sea.
Nasrollahzadeh (2010) reviews the ecological challenges facing thus enclosed sea
and Allahyari (2010) the social sustainability of fishery cooperatives in Gilan..
The Caspian Sea is the largest "lake" or inland water
body in the world at 436,284 sq km, a surface area encompassing 18% of
the total area of all lakes in the world, about the same area as Great
Britain (other surface area figures are 378,400 sq km, 384,400 sq
km and 390,000 sq km - data of this nature varies quite markedly between apparently
authoritative sources). The volume is 78,100 cu km, 44% of the total
volume of inland lakes of the world. Its north-south extent is 1204 km
and width is 204 to 566 km. The shoreline, including islands, extends
for 7000 km, 1000 km of which is Iranian. The catchment area is 3.6 million sq km. Dumont (1998)
presents arguments for this water body being a true lake and not a sea.
North, Middle and South Caspian basins are recognised, divided by
shoals. Iranian waters fall within the South Caspian Basin which
occupies 148,700 sq km and is separated from the Middle Caspian by the
Apsheron Bank. The South Caspian holds over 65% of the sea's water and
is the deepest basin, to -1000 m in depressions, average - 325 m. The northern basin holds only 1% of the water.
The sea receives 291 cu km from river run-off and 87 cu km from
precipitation but loses 374 cu km from evaporation and 11 cu km to
overflow into the Kara Bogaz Gol (Gerasimov, 1978b). The Volga River
accounts for 76.3% (82% according to Dumont (1995)) of the inflow of
rivers, the Kura River 4.9%, the Ural River 3.7%, the Terek River 3.2%
and the remaining rivers including all those of the Iranian shore
11.9%. Iranian rivers account for only 5% of the Caspian inflow, Iran
has 7% of the catchment area, 14% of the coast, contributes 3% of the
settling solids, and 2% of the fishery (Badakhshan and Shayegan in
Glantz and Zonn, 1997). The Volga has its headwaters near Moscow and
is 3688 km long with a catchment area of 1,360,000 sq km and a mean
annual flow at Volgograd of 8380 cu m/sec. The Volga is of prime
importance in the Caspian Sea basin to migratory fishes as a spawning
site and the biology of these species has been studied extensively.
Often these studies provide the basis for much of the knowledge of
Iranian fishes to the south.
Zenkevi(t)ch (1957; 1963) and Barimani (1977) have reviewed the
geography, hydrology and biology of the Caspian Sea, Moiseev (1971)
summarises the living resources of the whole sea, Karpinsky (1992)
aspects of the benthic ecosystem, and Knipovich (1921), Iljin (1927a),
and Nevraev (1929) give accounts of Iranian coastal waters and
regional fisheries in the early twentieth century. Zahmatkesh (1993)
describes the gammarids and bottom sediments, Fallahi (1993) the
plankton and Soleimani (1994) the benthic fauna in Iranian waters.
Mamaev (2002) is a recent general overview.
Water balance for this sea depends on a delicate balance of inflow,
evaporation, precipitation, climate, and abstraction for human needs.
Water 10 m deep or shallower has a bottom of sand and gravel while at
greater depths of 50-100 m clay and softer sediments increase. There
is more sand in these greater depths off Gilan compared with off Mazandaran.
Maximum depth is 1025 m, mean depth is 184 m, and depth below sea
level is -28 m (-27.66 m averaged over the past 2,500 years according
to Dumont (1998)). There are natural water level fluctuations - the
figure cited is from 1983; in 1978 it was -29.02 m, the lowest
recorded since observations began (Voropaev and Velikanov, 1985). Petr
(1987) has pointed out that a decline below -28.5 m would result in a
change in salinity distribution and in water currents mixing riverine
and sea water. A decline in productivity would follow. A fall of only
1 m would cause a 60% reduction in fish food supply and, since this
fall poses barriers to migration to better feeding grounds, a further
20% loss in food supply. Recently however, since 1978, the sea has
begun to rise, by 2.1 m from 1978 to 1993 to -26.95 m, with a possible
rise of 3 m in the next 25 years. Vaziri and Borghei (1995) give an average rise
of 1.2 cm a month for the period 1986-1993. The sea rose 26 cm in 1994. However,
over the past 2500 years the sea level has not exceeded -25 m and is
not anticipated to do so in the near future; the level is cyclical (Rychagov, 1997;
Gorji-Bandpy and Hooman, 2004). The reason for the rise is probably a climatic shift (Mandych, 1995; Shayegan and
Badakshan, 1996; Kobori and Glantz, 1998) but a
sheen of oil from pollution may be helping in the reduced evaporation
of 7-10% observed over two decades. Tectonic shifts of the sea floor
may also be a contributing factor. Predictions of water level changes
have proved unreliable so schemes to ameliorate rises or falls are
unwarranted and could be catastrophic (Abuzyarov, 1999). Georgievskiy (2001)
however, predicts a lowering of the sea level to -27.6-28.9 m by the year 2030
from -27.0 m in 2000. Klige and Myagkov (1992)
examined the water balance of the Caspian Sea and predicted a rise in
sea level to 1995-1997 and then future declines of the order of
several metres in the next century.
The rise in water level is engulfing buildings including industrial
sites which will pollute the waters of the Caspian further. Iranian
towns and cities damaged include Babolsar, Tonekabon, Ramsar,
Ashuradeh, Bandar-e Torkoman, Anzali, Astara and Kolachai (Zonn in
Glantz and Zonn (1997)). Fish caught near Nowshahr in 1999 were
contaminated with oil pollutants (Tehran Times, 1 November
1999). The complex of chemical, petrochemical and metallurgical plants
at Sumgait near Baku in Azerbaijan produces 335,000 tonnes of mostly
toxic waste including dioxins. Hundreds of waste lakes of oil near
Baku are being slowly engulfed by the rising Caspian. Nasrolazadeh Saravi (2001)
and Khatoonabadai and
Dehcheshmeh (2006) describe oil pollution in Iranian coastal waters although it
is much less than near Baku, particularly in Mazandaran and Golestan. Heavy metals
enter down the major rivers from mining and industry and the effects
from the Kura River may have rendered the coast of Azerbaijan almost
untenable for life (Bickham, 1996; Pohlman and Naismith, 1996; Rowe,
1996). Radioactive waste, both liquid and solid, is found in low lying
depressions around nuclear power plants and is liable to enter the
Caspian (Rodionov, 1994; Dumont, 1995).
In contrast to the recent rise in sea level, a series of reports have appeared in
past scientific and popular
literature on the falling level of the Caspian Sea and diversionary
schemes to combat this (e.g. Kovda, 1961; Lamb, 1977; Hollis, 1978; Gribbin, 1979;
Micklin, 1979; 1986; Golden, 1982; Rich, 1982; 1983;
Voropaev and Kosarev, 1982; Voropaev and Velikanov, 1985; Pearce,
1984; Ryan, 1986; Perera, 1989; Rozengurt and Hedgpeth, 1989; among
others). The Caspian dropped 2.3 m between 1930 and 1962 and area has
decreased by 10% or 40,000 sq km. Recent historical levels appear to
be between -25 and -26 m, average -25.8 m. Changes in level of the
Caspian due to natural or other causes in historical and
pre-historical times have been reviewed above. Fall in the sea level
increases salinity, destroys habitat and blocks spawning migrations,
although some effects are less in the southern, Iranian Caspian
because of the larger water mass. The Volga accounts for 76% (some
reports say more than 80%) of the river input to the Caspian Sea. The
Volga is now extensively dammed, as are other rivers in this basin,
and its waters used for industry and agriculture. There are 8 large
dams on the Volga, the largest being the Kuibyshevskaya with a
reservoir area of 6450 sq km and a total volume of 58 cu km. Dams in
the Caspian basin provide almost one third of the hydropower of the
former U.S.S.R. (Rozengurt and Hedgpeth, 1989). Flow into the Caspian
has been cut by at least 25% and in spring, the time of spawning
migrations, by as much as 37% for the Volga-Kama systems. Berka (1990)
reviewed the effects of water level changes on the northern Caspian
fisheries. The North Caspian was designated as an "ecological
disaster area" in 1992 because of water pollution input from the
Volga. The delta is eutrophic with cyanobacterial blooms being common,
affecting fish survival (Saiko in Glantz and Zonn, 1997).
The decline in sea level has been reversed in recent years and a
rise of nearly 2 m was reported and, in Turkmenistan, a shoreline
advance of 2-3 km in places (Rich, 1991; Anonymous, 1992a; Golub,
1992; Ottawa Citizen, 9 July 1994; Priroda, 5:3-25,
1994). This will have positive effects for some fisheries and wetland
conservation but negative effects on recent, low-lying construction
including oil refineries and wells in Azerbaijan and a nuclear waste
dump in Turkmenistan which would cause massive pollution from oil and
radioactive compounds (Pearce, 1995). Environmental hazards to the
fisheries caused by sea level rise include eutrophication from
farmland covered by the sea, pesticides and herbicides from inundated
farmland, salt water penetration into wetlands, input of solid
municipal and industrial wastes and vegetation, destruction of fish
habitat, and input of soil altering the ecosystem (Shayegan and
Badakhshan in Glantz and Zonn, 1997).
It has been suggested that the rise in sea level is due, in part,
to seepage from the Aral Sea basin and that this could be halted by
setting off underground explosions. This smacks of the large-scale
alteration to the environment favoured by Soviet planners to combat
the fall in sea level - both are grandiose and have unknown
consequences for the environment. Climate change is probably a major
factor abetted by the closing off of the Kara Bogaz Gol (responsible
for an estimated 40-45 cm rise alone) and diversion of Siberian rivers
into the Ural River in the northeastern Caspian (Khan et al., 1992).
Much of the former southern U.S.S.R. is water poor and a solution
to this and the falling Caspian level has been advocated. This would
involve diversion of north flowing Siberian rivers at a cost $40
billion. The potential for environmental damage on a local and even
global scale caused this scheme to be shelved in 1986. The project
involved excavations using nuclear explosives, drowning of forests and
construction of canals thousands of kilometres long. Reduced flow into
the Arctic Ocean could affect ice cover which influences atmospheric
pressure and circulation patterns over the whole northern hemisphere. This
Soviet plan has recently been revived (Pearce, 2004).
There is an abundance of historical and other evidence for
variations in Caspian Sea level and its connections with other water
bodies in both recent times and over several million years
(Huntington, 1907; Ehlers, 1971; Lamb, 1977; Gerasimov, 1978b; Hsü,
1978; Coad, 1980c; Rögl and Steininger, 1984; Wossugh-Zamani (1991c);
Oosterbroek and Arntzen, 1992; Sal'nikov, 1995; Mamedov, 1997; Rychagov, 1997;
Caspian Environmental Programme, 2000; Grigorovich et al., 2003; Kotlík
et al., 2008).
Brooks (1949) maintains that the Oxus (= Amu Darya)
flowed into the Caspian in the 14th century instead of the Aral Sea.
Shnitnikov (1969) and Gerasimov (1978a) report flow along the Uzboi
channel north of the Iranian border into the Caspian from the Aral Sea
basin at several periods from the third millennium B.C. to the 16th
century. Sal'nikov (1998) illustrates connections between the Amu
Darya and the Caspian Sea from the Pleistocene to the 20th century.
The connection between the Caspian and Amu Darya and Aral Sea was interrupted about 20,000 years ago when the
Amu Darya turned north, was reconnected about 10,000 years ago, and
essentially interrupted about 4000 years ago. These regular contacts
have resulted in an Aral Sea ichthyofauna with "weakly pronounced
endemics", although the Amu Darya ichthyofauna has a number of
clearly defined endemics which are not yet found in the Caspian Sea
basin (but see below under Tedzhen River basin). Dunin-Barkovsky (1977) records level fluctuations of up to 50 m during
the Holocene due to variations in the general moistening of Eurasia
and intermittent warming and cooling variously associated with changes
in precipitation and evaporation. Ice melt from the Fennoscandian ice
cap, as late as 4000 B.C., added large volumes of water to the Caspian
and an overflow to the Black Sea was then possible. Berg (1948-1949)
maintains that Atherina presbyter (=caspia) and Syngnathus
caspius
entered the Caspian at about this time. Some fishes, such as Salmo
trutta (as then recognised), are probably immigrants from Arctic regions and certain
cyprinoids and percids are freshwater immigrants. Bianco (1990; 1995b)
points out that, at every glacial- interglacial ice melting phase, a
network of connected rivers and lakes allowed primary freshwater
fishes to disperse in the northern Palaearctic. Other fishes are
relicts of earlier transgressions. Such species as herrings (Clupeidae),
gobies (Gobiidae) and possibly sturgeons are believed to have evolved
from the marine fauna of the Tethys Sea which ran from the modern
Atlantic to the Indian Ocean before the Sarmatian basin formed. The
uplift of eastern Anatolia and the Alborz in the Early Miocene between
20 and 17 million years ago (MYBP) closed a seaway from the
Indo-Pacific which had extended into the Eastern Paratethys (=
Black-Caspian-Aral sea in modern terms). The connection reopened in
the Middle Miocene 16.8-16 MYBP) but by the Late Miocene a Sarmatian
basin was cut off from the open seas and developed a unique marine
fauna (Ekman, 1953). This was mostly lost as salinity decreased from
freshwater input and a new fauna developed. A series of connections
and breaks with the Black Sea, Mediterranean Sea and the Atlantic
Ocean in various combinations with brackish and freshwater episodes
gave varying opportunities for faunal interchanges and evolution. The
Caspian fauna differs from the Mediterranean one because its only
communication was via the Black Sea which acted as a
"filter". When the Black and Caspian seas were well
connected, the link to the Mediterranean was broken, and when the
Black and Mediterranean seas were connected, the Caspian connection
was not well developed. Mamedov (1997) and Rychagov (1997) review late
Pleistocene and Holocene changes in Caspian Sea level, Chepalyga
(1984) and Gerasimov (1978b) review water level changes and
connections with the Black Sea over the last 80,000 years, Kosarev
and Yablonskaya (1994) and Mandych (1995) for the last 500,000 years and
Grigorovich et al. (2003) for the last 12.5 million years.
Bianco (1990) gives an overview of the palaeohistory of the Paratethys
Basin. Fluctuations in water level are correlated with climate changes Kotlík
et al. (2008) using multiple gene phylogeography found the Black and
Caspian seas supported separate populations of Rutilus frisii during the
last glaciation, although this separation was not complete and gene exchange
occurred, with the majority of migrations in the Pleistocene.
The total Caspian Sea drainage area is said to be 3,700,000 sq km,
about 25% of the continental land mass of the U.S.A. (Rozengurt and
Hedgpeth, 1989). The basin includes about one fifth of the crops and
one third of total industrial output of the former U.S.S.R. (Rozengurt
and Hedgpeth, 1989). Its northernmost waters are north of St.
Petersburg (= Leningrad) in Russia while its southernmost waters rise
on the flanks of the Zagros Mountains in Iran. This ranges from the
subarctic to the subtropical region and is very diverse in climate and
geology. Natural runoff in the South Caspian Basin ranges from 8 to 18
cu km while in the North Caspian it is 207-375 cu km. However the
North Caspian is very shallow (mean 4-5 m, maximum 20-25 m) compared
to the south Caspian (mean 325-334 m, maximum 980-1025 m). This is
also reflected in the volume, 400-700 cu km compared to 49,000-77,500
cu km. Salinity is about 12-13‰, increasing in isolated bays and
decreasing near river mouths. Summer temperatures in the south reach
27°C and in winter 9°C but the northern parts ice over. The Gorgan River area reached 30.9°C (Laloei,
2006). Surface water temperatures for the South Caspian are reported as 7.0-10.3°C
in winter, 7.9-14.0°C in spring, 25.0-29.0°C in summer and 12.0-19.0°C
in autumn (Rozengurt and Hedgpeth, 1989). These authors also report
salinity ranges of 12.5-13.0, 12.3-13.2, 12.6-13.6 and 12.3-13.5‰
for the same seasons, oxygen levels of 7.0-7.8, 7.0-8.2, 5.0-6.0 and
6.0-8.0 ml/l, and pH values of 8.48, 8.44, 8.44 and 8.50. Vertical
mixing occurs down to 50-150 m in the South Caspian (Mellat-Parast,
1992). There is little oxygen below 200-300 m and no fish life
although changes to the hydrological regime of the Volga have
increased aeration and oxygen content of deeper layers in the south
Caspian, down to 600-800 m. The Caspian has no tides but sustained
winds can cause seiches, local and temporary rises in sea level. There
is a current along the Iranian shore from west to east. The shelf
along the Iranian coast is narrow (6-10 km) and steep (Kosarev and
Yablonskaya, 1994). Beaches are usually sand with shell gravel on the
bottom further out. The extreme western coast has some shingle beaches
and west of Alamdeh in the central part is some rocky shore but there
are no major cliffs or headlands. The shore has coastal dunes, spits
and bars with lagoons inland, either brackish or fresh, grading into
the higher and dryer foothills.
Much of the coast was once forested, but it has been actively cleared and marshes reclaimed as rice
paddy. Rice paddies are now being investigated for fish
cultivation. About 300-500 kg of carp "seed" and a 10% increase in
paddy production per hectare was recorded during the rice cultivation season.
Extending this into the fall gave a production of 750-1000 kg of fish and duck
and in winter 5.5-8.0 t of rainbow trout (Tehran Times, 1 October 2000).
Gilan is attempting a production of 2 kg of trout per sq m of paddy field, with
the aim of harvesting 46,000 t of fish (IRNA, 14 November 2001).
Mazandaran has the highest farm fish production in Iran at 28,000 tonnes
(2006-2007) and is expected to reach 50,000 t by 2010 (www.mehrnews.ir,
downloaded 8 February 2007). The area of forests in northern Iran has been reduced from 3.4
million hectares in 1962 to 1.8 million hectares in 1977 and about 1
million hectares or less in 1995. In Gilan, 975,000 cu m of wood from
the forests are burnt annually by cattle breeders for heating or
cooking purposes or for production of dairy products. Additionally
450,000 cu m of wood are used for industrial purposes. Reforestation
cannot keep up with the losses and forests have been reduced by half
over the past 50 years (Barzegar, The Agricultural and Cattle Breeding
Publication, No. 761, 22 December 1997, from www.netiran.com/Htdocs/Clippings/Deconomy/971222XXDE01.html).
As a result floods now occur with destruction of fish habitat after
30-40 hours of rain where previously no flooding occurred after even 4
days of rain (Hamshahri, Tehran, 628, 20 February 1995).
Abstraction of water for irrigation (60% of water use) has severely
reduced water levels and runoff rates necessary for reproduction of
fishes. Estuarine habitats have been degraded inhibiting the survival
of eggs, larvae and juveniles of anadromous and semi-anadromous
fishes (the latter are species which spawn in the lower stretches and deltas of
rivers where salinity is optimal at 8 g/l for many commercial species, e.g.
Sander lucioperca, Cyprinus carpio, Rutilus caspicus). Over 90% of coastal streams along the Caspian shore are dry in
July in Iran because of irrigation demands. As a result larvae of
spring spawners are flushed into fields where they die, migration and
late summer spawning of Aspius aspius and Luciobarbus
brachycephalus are obstructed, and Salmo caspius and
Rutilus frisii kutum populations are depleted because they
cannot spawn in the shallow, warm, weed-choked water. Nursery and
reproductive areas for Abramis brama, A. sapa, Blicca
bjoerkna, Aspius aspius, and Sander lucioperca among
others are confined because of their low tolerance to salinities above
7-8‰. Without an adequate runoff, the sea encroaches on the estuary. Nasri-Chaari (1994) cites physical obstacles, sand removal from river
banks, overfishing and water pollution for declines in fish migration in recent years.
An earlier, general work including fishes of the Iranian Caspian Sea and
coast is Berg (1948-1049). More recent works are the atlas of the fish species
in the Iranian Caspian Sea in English and Farsi by Jolodar and Abdoli (2004) and
that on the biodiversity of the southern basin by Abdoli and Naderi (2009).
About 25% of
the Iranian total fish catch is from the Caspian coastal area (CEP)
and figures for the Iranian Caspian Sea in tonnes are:-
The fish harvest from the southern Caspian coast of Iran for the 7
month period October 1999-April 2000 dropped by 11% over the same
period from the year before, from 8630 t to 7710 t (IRNA, 10
May 2000). The decline was attributed to a rise in fish prices which
encouraged illegal fishing and to habitat loss. The value for the whole
Caspian fisheries is given as $6 billion by Nezami et al. (2000).
A proposal for a Caspian Fisheries Commission is given by TACIS (1999; 2000b)
and ERM-Lahmeyer International GmbH et al. (2001b). It would aim to conserve and utilise
the living aquatic resources, including the management of fish stocks such as
kilka, herrings and mullets, as well as the famous sturgeons. These species all
have transboundary stocks requiring cooperative management between countries. Articles
aim to protect traditional fishing for sturgeon along the Iranian coast,
establish state monopolies for the export of caviar, set up cooperative research programmes to conserve sturgeon species, establish annual total allowable
catches and fishing regulations, and so on.
About 50,000 tonnes of kilkas are caught each year by the
Industrial Fishing Company and fishing cooperatives using deep conical
nets and air lifting with artificial lights as attractants. About
20,000 t of other species are caught by licensed cooperatives
using beach seines and gill nets although a report in IRNA (27
March 2000) cites more than 16,000 t including whitefish (Rutilus),
Mugilidae, Cyprinidae, "anchovy" (sic), bream (Abramis)
and zander (Sander). An account of site selection for beach
seining is given by Zanoosi (1993). Beach seining has been restricted to the
period from sunrise to 8 p.m., and to 10 p.m. in Miankaleh (www.iranfisheries.net,
downloaded 14 November 2006). The 1994-1995 finfish catch
(excluding sturgeon and kilka) using gill nets, coastal purse seines
and beach seines, was 17,000 t, perhaps over 22,000 t with the
illegal catch included. About 87% of this catch is Rutilus frisii
kutum, Liza auratus and Liza saliens (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 37, 1996). Gill nets showed a 39% decline
compared to the previous year and beach seines were 16% less. Rutilus
frisii kutum comprised 53%, mullet 39% and others 8% of the total
catch (Abzeeyan, Tehran, 6(5, 6):IV, 1995). The harvest from
the southern Caspian Sea coast dropped 11% in the year 2000 from the
same seven month period in the preceding year, to 7710 t, as a
consequence of poaching, neglect of river maintenance, and substandard
capture methods (IRNA, 10 May 2000). The catch in Golestan Province
rose from 470 t in 2000 to 3278 t in 2005, attributed to artificial
propagation, restrictions on beach seining, training about closed seasons and
beach seine standards, increased fishing effort, and a favourable climate (www.iranfisheries.net,
downloaded 14 November 2006).
There are 5 regional fishing centres namely Bandar Anzali with 14
fishing stations, Keyashahr with 12 stations, Babolsar with 13,
Ashuradeh with 9 and Nowshahr with 9 (Iranian
Fisheries Research and Training Organization Newsletter, 7:7, 1995).
The Caspian Environment Programme (2001c) gives 15 stations for Bandar Anzali, 9
for Keyashahr, Babolsar, Ashuradeh and Nowshahr for sturgeon fisheries. Fixed
gill nets are used with a standardised mesh. The Ashuradeh Peninsula, where
more than half of Iran's caviar is processed, was threatened by the
rising Caspian Sea in a 1991 radio report. A 1995 agreement between
Iran, Azerbaijan, Turkmenistan, Kazakhstan and Russia gives each
nation an exclusive fishing zone of 20 nautical miles from shore (Iranian
Fisheries Research and Training Organization Newsletter, 7:7, 1995).
Inland freshwaters of Gilan are divided into three categories by
Bakhshizod-Mahmoodi (1996): natural and impounded ponds, the Safid
River reservoir, and wetlands. The ponds are used primarily for
cyprinid and acipenserid culture, the reservoir is fished by seining,
by spreading wheat grains in littoral areas to attract fish and by
using the shemshad or shaghoul net (a giant dip-net),
and the wetlands are fished by seining, by the salik or mashak
(cast-nets), by the la'kesh (drifting gill net using one and
two boats), by fixed gill nets, by the shemshad and by angling (for ordak mahi).
Pollution is an important factor in the ecology of the sea, from
offshore oil drilling, ship discharges of oil wastes and contaminated
water as well as garbage and even discharges from ship collisions,
radiation from underground, non-military explosions and nuclear waste
dumped in inflowing rivers (radiation levels are 100 times above
normal (Time, 1 November 1993)), manure and pesticides from
farming on the surrounding land mass, city waste water, sewage and
garbage, industrial wastes including mercury and other heavy metals,
discharges from water desalinating plants, extraction of minerals such
as sodium sulphate, mirabelite and espomite, and untreated sewage (see
Sardar (1979), Nuhi and Khorasani (1981), Coad (1980c), Khalili (1994), Raiss-Tousi (1999), Namazi (2000),
Abaee (2001), Charamlambous (2001), Laloei (2006), Zeynali (2009) and Saeidi
et al. (2010) for Iranian problems
and acceptable levels of some elements; Anonymous (1988c),
Edwards (1994), Specter (1994) and Kasymov and Rogers (1996) for former Soviet
waters; Stone (2000b) is a recent, short general overview).
Data collected in 1991 showed the Caspian Sea received effluents comprised of
3000 tonnes of oil products, 28,000 t of sulphites, 315,000 t of chlorides,
200,000 t of tar and 25,000 t of phenols (Namazi, 2000). In Daghestani
rivers, the same author records heavy metals, pesticides, phenol, arsenic, boron
and selenium, among others, at 60-100 times the maximum permissible for
fisheries. The oil industry is considered to be the main source of ecological problems in
the Caspian Sea (Karpyuk, M. and Shavandin, V. 1996. Astrakhaners on
the Caspian Sea. International Affairs, 42(1) from http://home.eastview.com/ia/42_01_15.htm).
Prospecting uses blasting operations which have caused sturgeon deaths
on more than one occasion. A single offshore well during its life
releases into the water 30-120 tonnes of oil, 200-1000 t of sand,
clay and other waste and 150-400 t of drilling mud paraffin
fractions, baryta, lime, detergents, emulsifiers and lubricants. The
ecology is affected 5-12 km from each well. The oil industry in the
Caspian has reserves estimated at $4 trillion and a new oil rush will
further contaminate the sea.
Charamlambous (2001) concludes that municipal wastewater from 11 million
people is the primary pollutant in Iranian coastal waters. Industrial discharge
accounts for 31%. of organic loading, the rest being municipal discharge. The
most industrialised area is around Rasht with waste going into the Anzali Mordab.
The Zarjub River in Rasht is the most polluted river in Gilan, and possibly in
Iran (Ghodrati et al., 2007). TACIS (2000c) reports that in Gilan, 32 of 36 major cities discharge wastewater
untreated into a river and 89 of 90 industries discharge treated wastewater to a
river. Ayati (2003) also reviews pollution in the mordab.
Mirkou (2001) details agro-chemical usage along the Caspian shore
comprising various fertilisers and pesticides. Naderi Jeloudar et al.
(2007), Varedi et al. (2007) and Amirkolaie (2008) describes the
environmental impact on the Haraz River of aquaculture waste water discharge
from rainbow trout farms - pollution levels in this instance were generally too low to
have a significant impact of the river system although phosphorus loading was
increased and levels varied with activity rates of the farms.
Chlorinated pesticides have been used in anti-malarial campaigns
throughout Iran and to eliminate pests on cotton, rice and other
products in Mazandaran. Herbicides and pesticides are widely used in
rice paddies. DDE, DDT, DDD, Lindane, Dieldrin, Eindrin and Kelthane
have been identified in such rivers as the Babol and Chalus (Annual
Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 11-13, 1997). Ebadi and Shokrzadeh (2006) examined
Rutilus frisii, Vimba vimba (= V. persa), Clupeonella delicatula and
Liza aurata for lindane at Chalus, Babolsar, Khazarabad and Miankaleh but
levels detected were less than the FAO/WHO recommended permissible intake and
were no cause for public concern. Similar studies on DDT and DDE and on
chlorobenzilate from the same sites and fish and levels were also less than the
permissible intake (Shokrzadeh and Ebadi, 2005; 2006). Shokrazadeh et al.
(2009) also found that levels of Lindane in dorsal muscle of safid mahi, kefal,
kuli and kilka species were less than FAO/WHO recommended intake. The Chalus River also contains various heavy metals,
such as lead, zinc, copper, iron, cadmium and chromium from mining
activities (Annual Report, 1995-1996, Iranian Fisheries Research
and Training Organization, Tehran, p. 18, 1997). Zeynali et al.
(2009) demonstrated the presence of copper and zinc in muscle tissues of Liza
aurata, Rutilus frisii kutum and Cyprinus carpio from Chalus,
Anzali, Rudsar and Fereydoon Kenar in the Caspian Sea basin although levels were
acceptable for human consumption. Hashemy-Tonkabony and Asadi
Langaroodi (1976) have shown the presence of DDE, DDT, TDE, Dieldrin,
Lindane, Aldrin and Heptachlor in a wide variety of Caspian fishes in
Iran. However, Ebadi and Shokrzadeh (2006) examined Rutilus frisii, Alburnus,
Clupeonella and Liza species in Mazandaran for the organochlorine
pesticide lindane and found levels in muscle tissues to be less than FAO and WHO
recommended permissible intake and so were not a public concern.
Rutilus frisii, Cyprinus carpio, Liza species and Acipenser
stellatus were tested for DDT, aldrin and heptachlor with only the latter
slightly elevated above standard levels at Hashtpar (Iran Daily, 11 January 2006). Phytoplankton diversity in the western Caspian Sea fell from 74
to 40 species, biomass from 8.7 to 2.1 g/ sq m and biomass of benthic
organisms in coastal areas fell from 1724 g/ sq m in 1961 to 21 g/sq m
in 1969 (Clark, 1986). These declines were noted particularly in the
nursery grounds for sturgeon, Abramis brama, Esox lucius
and Cyprinus carpio among other fish species. In the 1980s,
catches of Abramis brama, Cyprinus carpio, Rutilus
rutilus (presumably R. caspicus) and Sander lucioperca fell by as much as 80% and Salmo
trutta (= caspius) and "shad" had almost disappeared. It was estimated
that for 1985, 10,200 tonnes of oil products and 104,200 t of
sewage were dumped in the sea. One-fourth (or 40 billion cubic metres)
of all the wastewater in Russia enters the Caspian Sea and
petrochemical factories alone release 67,000 t of waste annually
(Anonymous, 1988c; Platt, 1995; Hamshahri, Tehran, 3 (639), 7
March 1995). Salinity increased as more water was taken for irrigation
- two-thirds of the Terek and Kura flows did not reach the sea
(Markham, 1989). In Iran, sewage is discharged into the Caspian Sea
from coastal towns, and via rivers, from towns inland. Industrial
solid wastes enter the sea through the larger rivers such as the Safid,
Gohar and Siah as well as the Anzali Mordab complex. The use of
agricultural chemicals such as fertilisers and pesticides has led to
pollution, e.g. in Gilan Province 88,851 t of fertilisers were used in
the year 1992-1993, an 18.7% increase over the previous year. A survey
of 30 towns in Gilan shows that 80% of rubbish dumps are located by
rivers, marshes or the coast (Hamshahri, Tehran, 3 (639), 7
March 1995). An estimated 200,000 fish were killed in the Kacha River,
a branch of the Siyarud in Rasht, poisoned from a dump in the Saravan
region which receives 390 t of rubbish daily. Heavy rains had
washed poison into the river (Tehran Times, 7 October 1998).
As many as 1000 trout (presumably mahi azad, Salmo caspius) died in the
Kileh River in Mazandaran from release of wastes from a dairy manufacturer; sand
extraction was also blamed for affecting fish populations (Iran Daily, 21 July
2005).
The biology of the Volga River and its effects on the Caspian
ecology has been reviewed by Rozengurt and Hedgpeth (1989) and Pavlov
and Vilenkin (1989). This river is of critical importance for marine
fisheries. Fish production is less in the central and southern parts
of the sea as nutrient supply comes from upwelling and circulation
rather than a riverine input. However the Volga has effects even here,
changing the Caspian Sea from its regime in the 1950s. Abstraction of
water for irrigation, industry and household use caused salinity
increases of about 0.2-0.3‰, increased aeration of deep layers and
in their oxygen content down to 600-800 m by as much as 2-3 ml/l due
to convection and thermal winter mixing, an increase in the euphotic
zone to 50 m and depths open to total photosynthesis to 100 m, a
decrease in organic matter and its vertical gradient, and an increase
in wind-driven circulation and its effects on temperature and salinity
layers. In the period 1956-1972, the Caspian Sea was transformed from
a fishery based on valuable species (listed above) to one dependent on
kilka which now occupies 80% of the catch (or 107 times the catch in
1930). Even including the kilka, catches in the 1970s were 245 x 103
tonnes or only 37% of the 1913 catch. The catch of Caspian herrings (a
complex of species in the family Clupeidae) ceased to exist
commercially by the 1970s and in fact was banned. In 1967-1972 it was
0.6-2.1 x 103 compared to 56-62 x 103 in
1945-1953 or 82-307 x 103 in 1900-1917 (Rozengurt and
Hedgpeth, 1989). Moghim et al. (1994) report that, in the
southern areas of the Caspian Sea, nearly 90% of the catch is composed
of Rutilus frisii, Liza saliens and Liza aurata
(with biomasses of 24,000, 7000 and 2400 t respectively and
maximum sustainable yields of 7000, 2900 and 960 t respectively).
The Volga is a major pollutant of the Caspian Sea, carrying sewage,
agricultural waste, PCBs, petrochemical wastes, tannery waste, etc.
from a population base of 60 million people (Golub, 1992). In 1989, 40
million t of polluted wastewater entered the Caspian via the Volga
River, more than a quarter of all the wastewater of Russia (http://www.oneworld.org/patp/pap_overview.html).
A report in 1995 gives the volume of pollutants and industrial wastes
entering the Caspian Sea each year as 11 billion cu m. Russia accounts
for 50%, Azerbaijan 16% and Iran 11% (http://netiran.com/news/IRNA/html/950731IRGG17.html).
The Volga-Don canal in the former U.S.S.R. connected the Caspian
Sea with the Black Sea in 1952 and formed an invasion route for
various benthic organisms while others came in attached to boats
transported by rail or were deliberately introduced (Kasymov, 1982).
The molluscs Abra ovata and Mytilaster lineatus, two
invaders, accounted for over 90% of the total benthic biomass.
Invaders provided 95.1-99.3% of the total benthic biomass in the
western part of the south Caspian Sea in 1976. East of the mouth of
the Safid River, the Azov-Black Sea molluscs Abra ovata and Cerastoderma
lamarcki accounted for 80% of total benthic biomass. In Gorgan
Bay, 99.9% of the benthos fauna is comprised of invaders. The Volga is
also connected to the Baltic and White seas via the White Sea-Baltic
Canal opened in 1933 (Pavlov and Vilenkin, 1989).
The earliest report for the Caspian
appears to be in 1995 by the Iranian Fisheries Research Organization (Bilio and
Niermann, 2004; www.caspianenvironment.org/mnemiopsis/mnem_attach13.htm). The Islamic Republic News
Agency (IRNA) on 12 May 1998 reported that a number of
jellyfish had been observed in the Caspian Sea recently, presumably
brought in the ballast of oil tankers, and its occurrence is documented by Esmaili
Sari et al. (1999) and in numerous other studies by this author and
co-authors. Various studies on the biology of the comb jelly and its impacts have been carried
out in the Iranian Caspian Sea including, e.g. Movahedinia et al. (2002),
Esmaeili et al. (2003), Yussefian (2002)
and Moghim and Rouhi (2009).
The kilka fisheries are now threatened by the comb jelly which spread through the entire sea by
the year 2000. J. Muir (http://news.bbc.co.uk/hi/english/world/middle_east/newsid_1453000/1453117.stm,
downloaded 30 August 2001), Kideys (2002b) and Kideys and Moghim (2003) report a 50% drop in kilka numbers with catches down
from 3-6 t per night to half a tonne for one boat. A 50% decrease in kilka
catches meant a minimum U.S.$15 million loss to the fishermen (Kideys and Moghim,
2003). Iran's kilka fishery fell from 85,000 t in 1999 to 15,000 t in 2004
and losses exceed $125 million (Stone, 2005a). Ghafar Zadeh and Honar Bakhsh
(2008) summarise the economic consequences for Iran. This comb jelly can double in
size in one day, reaches maturity in 2 weeks and then produces 8,000 young every
day. Maximum abundance reached 5122 individuals per square metre in October 2001
and biomass 1024.5 g/sq metre in august-October 2002 (Roohi et al., 2003;
Bagheri, 2004; 2006). The fisheries may recover somewhat after the comb jelly
population collapses (Tidwell, 2001b). The website www.caspianenvironment.org/mnemiopsis/index. htm, downloaded 9 April 2003
and Dumont (2002) have extensive information on this problem and Stone (2002b) and IFRO Newsletter
(29:4, 2001) confirm a severe depression in kilka and herring stocks. Beroe ovata, a comb jelly that
preys on Mnemiopsis, is being cultured in Iran (Kideys, 2002b; Kideys
et al., 2004; Rezvani Gilkolaei et al., 2005; Mirzajani, 2006; Mirzajani et al., 2007) and does
not appear to feed on other organisms in the Iranian Caspian (Iranian
Fisheries Research Organization Newsletter, 38:3, 2004). Reproduction and
growth are slower, and mortality higher, than in the Black Sea, due either to
the lower salinity in the Caspian Sea water or damage to individuals during
transportation for the experiments. If this
comb jelly fails to control Mnemiopsis, the introduction of the exotic
American species, the butterfish (Peprilus triacanthus), known to feed on
ctenophores has been advocated but this fish could also feed on other fishes (Harbison, 2002; Bilio
and Niermann, 2004). The complex politics of the nations surrounding the Caspian
have prevented the introduction of Beroe (Stone, 2005a).
The Kara Bogaz Gol ("Black Throat Bay"), an eastern arm of the Caspian Sea in
Turkmenistan, is 160 km long by 140 km broad (18,389 sq km) but only
2-3 m deep. It acts as a salt precipitator. This water body was
blocked off by a dam to conserve the water lost in it by evaporation
in 1980. The Caspian Sea has a net annual water deficit of 15 cu km
with 5 cu km being lost through the Kara Bogaz Gol alone (Rich, 1982;
1983). However this resulted in salts being spread by the winds,
ruining fish spawning grounds and fish farms in the Caspian basin, and
ultimately would lead to the salinisation of the Caspian Sea. A dike
has now been constructed to allow some flow into the Kara Bogaz Gol
and allow the flushing effect to operate. The refilling process over 3
years prevented a 35 cm rise in the Caspian Sea level (Dumont, 1995).
Use of this water body to reduce level rises in the Caspian Sea and prevent
flooding has been proposed (Wardlaw, 2001). Fish which enter the salty Kara Bogaz Gol lose their swimming
capacity, become blind and thrashing about often come to lie on the
shore. Birds eat them but those that are missed become salted and
dried and may be preserved for a year or so. The Turkmenistan
government re-established natural flow into the Kara Bogaz Gol in 1992
because of the Caspian Sea level rise (Zonn in Glantz and Zonn (1997)).
The Caspian coastal plain in Iran runs for almost 650 km from
Astara (38°26'N, 48°52'E) in the west to Bandar-e Torkeman (= Bandar-e Shah) (36°56'N,
54°06'E) in the east. This plain has a
width of about 25-32 km, but is as narrow as 2 km in places, although
it opens out in the east. The Alborz Mountains are almost 1000 km
long, on average less than 100 km wide but very high. Damavand reaches
5766 m - an estimate - at 35°56'N, 52° 08'E
and is the highest of any mountain to the west of it in Europe and
Asia. It has a continuous snow cover. There are persistent snow fields
and Alam Kuh at 4849 m has small icefields. The north or Caspian slope
is very steep and streams tend to be short and torrential, fed by snow
melt and year-round rain. However there are some longer rivers and the
principal ones are detailed below. There are about 128 small to large
rivers along the Caspian shore. Nümann (1966) gives some limited
biological, chemical and physical data on these streams based on spot
recordings. Surber (1969) gives values of total alkalinity and
calcium-magnesium hardness for a number of streams and reservoirs
along the Caspian shore. Most were moderately to relatively hard and
therefore productive for aquatic organisms such as insect larvae on
which fish feed. The Caspian Environmental Programme (2001b) gives an overview
of habitats and biodiversity along this Iranian shore. Environmentally managed
areas are listed along with factors affecting their status under the headings of
development, drainage, land use alteration, pollution, destruction of
vegetation, over-grazing, mining, hunting and fishing, exotics, dams, and roads.
Of 123 fish species only 10 or just over 8% are protected with one protected
species on the verge of extinction.
Most rivers along the Caspian shore have less than 30% of their
discharge in the two wettest months and 40% in the six driest months
so discharge is well distributed through the year. In contrast, the
Gorgan River at the eastern end of the Caspian basin has 70% of its
discharge in the two wettest months, figures comparable with drier
areas such as Azarbayjan at 50-60% and the Zayandeh and Kor rivers at
40-60%. Annual discharges can vary markedly, e.g. the Lar River had
545 mm on its basin area in 1949-1950 and 1560 mm in 1950-1951 (Ghahraman, 1958).
The Aras (= Araxes or Araks) is a tributary of the Kura River of
Azerbaijan. The Kura rises in Turkey and is 1510 km long. The Aras
forms the border between Iran and the former U.S.S.R. (now Azerbaijan
and Armenia) for 430 km and has its source near Erzurum (39°55'N,
41°17'E) in Anatolia and the headwaters
of the Euphrates River. Its total length is 1072 km. The Aras can be
wide and meandering with braided channels and backwaters. Depth range
of the Aras is 0.5-4.0 m, average 2.5 m (Zakeri, 1997). The Araxes or
Aras Dam was a joint Iranian-Soviet project on this river. Iranian
authorities stocked the dam with 1.8 million fingerlings (species not
specified) weighing over 10 g each in 1997 to enhance fish farming (Islamic
Republic News Agency, 29 December 1997). Akh Gol occupies 600 ha
at 820 m in the Aras River valley in northeastern Iran (Scott, 1995).
It comprises a small brackish lake with associated marshes and springs
and rains to the Aras 5 km away. The area is being converted to
agriculture and the lake is being drained. Principal tributaries of
the Aras in Iran are the Qareh Su (= black water, draining easily eroded,
volcanic soil) draining from the Kuhha-ye Sabalan
at 4810 m (38°15'N, 47°49'E) near Ardabil (38°15'N, 48°18'E)
and the Qotur River draining past Kuh-e Zaki at 3079 m on the Turkish
border through Khvoy (38°33'N, 44°58'E) to the Azerbaijan border near Jolfa (38°57'N,
45°38'E). The Aras and the Safid are two
of the three largest rivers in Iran (with the Karun River of Khuzestan).
The Kura-Araks basin encompasses 225,000 sq km of which 28,000 sq km
or 12.4% is found in Iran (Gleick, 1993). Azerbaijan discharges 303
million cu m of waste into the Caspian Sea annually according to Golub
(1992), presumably through the Kura and other major rivers.
Derzhavin (1929a) gave an interesting account of the formation of a
new channel of the Aras north of the Iranian border in 1896 which led
to the freshening of the Kyzylagach Bay. This favoured migrations of
fishes into the Kura River. However, irrigation schemes on the Mugan
steppe severely reduced catches as well as causing salinisation of
soil. Water abstraction prevented entry of adequate numbers of
sturgeons onto the Kura spawning grounds. This type of water usage is
paralleled along the Caspian shore in Iran with deleterious effects on
a variety of sedentary and migratory fish species.
The Safid (= Sefid or White from its sediment load, up to 60 g/l)
River is the only one to completely pierce the Alborz Mountains and
has a considerable basin (54,100 sq km) on the plateau. Various sources give
differing accounts of its length, up to 800 km. The Safid has
the greatest mean discharge of Iranian Caspian rivers, over three
times that of the Heraz, the next most important. In flood the Safid
discharge is twice that of the Karun, but its minimum is less than a
tenth, because the Karun drains a greater area with higher elevations
and a more extensive snow pack. The Safid discharge is 4000 cu m per
second at maximum, falling to only 15 cu m per second. An average
discharge is 182.17 cu m per second. There used to be two freshets
before the dam was constructed at Manjil, one fed by spring snow melt
in March-May and one by rainfall in the autumn. The rise in water
levels and increased sediment load attracted sturgeons, in particular Acipenser
persicus. The catch of this species and A. gueldenstaedtii
in the Safid River area reached 733,127 kg in 1927/1928 representing
46,500 fish and a caviar yield of 120,958 kg (Vladykov, 1964).
The width of the Safid River varies from 100 to 250 m and depth
from 2 to 8 m. The average instant yield is 128.79 m/sec, range
76.5-288.5 m/sec. The average annual yield is 3,998.4 million cu m (Zakeri, 1997).
The Safid is formed from the Qezel Owzan from the west and the Shah
River from the east which meet on the plateau and flow through a
narrow gorge. This gorge is dammed by what was named the Shahbanou
Farah Dam at Manjil (now the Safid or Manjil Dam) (dam height 106 m,
length 425 m; reservoir 1860 million cu m, surface area 56 sq km
maximum, 14 sq km minimum, maximum depth 80 m, minimum 30 m, summer temperature 24°C, winter 7°C,
pH 7.8, 31 g/l turbid materials, Cl- 229 mg/l, SO4
178 mg/l). Strong water level fluctuations prevent the development of
a belt of vegetation and the heavy sedimentation inhibit a bottom
fauna. Khodjeini and Mohamed (1975) detailed the rate of sediment
accumulation in this dam, 757 cu m/sq km/year, evidence of severe
erosion of a devegetated drainage basin. The reservoir was half filled
with sediment after only 20 years despite an expected life span of 100
years. The reservoir is apparently drained at intervals to remove some
of the accumulated sediment. This would severely affect littoral
spawning and feeding habitats for fishes. Nümann (1966, 1969) gives
details on the limnology of this reservoir. The dam decreased
turbidity in the river, raised water temperatures at the river bed in
summer and caused marked diurnal temperature changes. This prevented
ascent of Salmo caspius to the upper reaches and the dam itself
prevented ascent of Rutilus caspicus. Nümann (1966) recommended
introducing Sander lucioperca, Acanthobrama terraesanctae
(a Levantine species) and cichlids to the reservoir.
Lower dams on the Safid, such as the Tarik (10 m high) and the
Sangar (3 m high), divert water for irrigation purposes on the Gilan
plain, the former through a 16.7 km long tunnel. Construction of the Alamut Dam
in the upper reaches of the Safid River basin would affect such species as
Luciobarbus mursa, prized for sport fishing, which would need full habitat
protection to survive (Aghili et al., 2008). Salmo trutta would not need
protection as its habitat is confined to a stretch of river above the dam.
The Safid breaks up into distributaries near its mouth and its flow
is carried off into a complex of canals and irrigation ditches. The
Safid has changed its delta several times, (Vladykov, 1964). In 1911
it shifted 2-3 km east from the fishing post of 12 Bahman to Hasan
Kiadeh. An account in Farsi on the Safid River is given by Wossugh-Zamani (1991b).
The headwaters of the Qezel Owzan lie in Kordestan, near the Iraqi
border, and so drain part of the northern Zagros Mountains as well as
areas near Lake Orumiyeh such as the Kuh-e Sahand (37°44'N,
46°27'E), mountains near Hamadan (34°48'N, 48°30'E) and the southern slopes of the
Alborz Mountains. The Qezel Owzan is about 550 km long. The Taham Dam
project 12 km northwest of Zanjan lies in the Qezel Owzan basin on the
Taham Chay. This dam is to be 120 m high with a crest length of 450 m
and a capacity of 82.7 million cu m. The fish fauna behind the earth
dam at "Maljiq", 50 km southwest of Hashtrud in the upper
Qezel Owzan basin, suffered severely in the drought of the year 2000.
Twenty-five tonnes of fish died after the reservoir dried up (www.irna.com/newshtm/eng/09151847.htm,
IRNA, 30 July 2000).
The Shah River is much shorter (ca. 175 km) than the Qezel Owzan
and drains the southern Alborz as far east as Takht-e Soleyman at 4819
m (36°22'N, 50°58'E).
The 500 ha Bandar Kiashahr Lagoon (= Bandar-e Farahnaz) Ramsar Site
(World Conservation Monitoring Centre, 1990) at 37°25'N,
49°19'E east of the mouth of the Safid
Rud was a freshwater coastal lagoon and swamp fed by two streams from
the Safid Rud to the west and draining to the Caspian Sea via a
channel to the north. The recent rise in Caspian Sea level has
converted this area into a bay of the sea as it was in the 1950s
before the fall in sea level (Khan et al., 1992). The lagoon
bed is sand and mud and the water was oligotrophic except near the
marshes to the west. There were reedbeds of Phragmites communis,
Typha and Juncus, now restricted to the extreme west
end. There were several factors affecting this habitat including a
fishery with a fish-processing warehouse, grazing, reed cutting,
irrigation abstraction and recreational activities. It was an
important spawning and nursery ground for fishes (effects of recent
changes unknown) and is still an important centre for commercial fishing.
The Heraz (or Haraz) River drains the Alborz east of Tehran and has a number
of longitudinal tributaries in the mountains. These depend on snow
melt and are cold even in summer. Fishes are reported to be present in
these high streams, but were not easily caught. The Heraz debouches
onto a plain and splits up into distributaries. It is polluted from rainbow
trout farms (Kazemzadeh Khajuie et al., 2002) and heavy metals (lead and
cadmium) are present in fish (Riahi Bakhtiyari, 2001; 2002). Banagar et al.
(2008, 2009) record the fish biodiversity as 20 species in 9 families, dominated by
cyprinids at 67.2% and with 70% of species resident, the rest anadromous.
Exotics are Oncorhynchus mykiss, Carassius auratus, Liza
saliens, Gasterosteus aculeatus and Gambusia holbrooki.
The Tajan or Tadjan
River was studied by Ro(o)shan Tabari (1995; 1996) who reported on its
hydrology and biology. Its mouth lies at 36°49'N, 53°05'E. The maximum flow is in April,
decreasing from May onward. In April 1989 flow was 45 cu m/sec falling
rapidly to 0.11 cu m/sec in June. Over 70% of the fishes are
anadromous with sturgeons being the most important species (Acipenser
persicus, A. gueldenstaedtii and Huso huso). Salmo caspius is the most important species in the upper reaches. Other
species found in this river are Cyprinus carpio, Alburnus sp.
(presumably Alburnus hohenackeri), Capoeta capoeta,
Luciobarbus
capito, Vimba vimba (= V. persa), Alburnus chalcoides, Rutilus frisii, Rutilus
rutilus, Liza sp., Gobiidae, and Esox lucius. Rural,
agricultural and industrial pollutants are found in the Tajan and
affect the fishes along with dams and other physical obstacles, sand
removal and overfishing. The Shahid Rajaee Reservoir Dam, inaugurated
in 1997, is found on this river 41 km south of Sari (http://netiran.com/news/IRNA/html/951016IRGG15.html
and http://netiran.com/news/IRNA/current.html#HLNO4). The Independent
(London) reported on 13 July 1994 that tens of thousands of fish died
in this river after poachers poured poison into it about 9 miles (14.4
km) above the estuary. Dead fish covered the river bed for 6 miles (9.6 km).
The south-eastern corner of the Caspian Sea receives two major
rivers, the Gorgan and the Atrak or Atrek (ancient Sarnois). Their courses are roughly
east-west and parallel each other with the Atrak forming part of the
border with Turkmenistan. The Atrak is 495 km long (with 145 km of
this in Turkmenistan; Nezami et al. (2000) state 715 km for the Atrak) and the Gorgan 240 km. The Gorgan drains 10,200
sq km and has an average discharge of 9.39 cu m per second (cf. Safid
River with 182.17 cu m per second; the Chalus River, directly north of
Tehran, has a discharge of 12.65 cu m per second). The Voshmgir or
Sangarsavar Dam at 37°12'N, 54°45'E on the Gorgan stores 60 million cu m of water.
The water level fluctuates markedly, banks are steep and there is little
emergent vegetation. The Golestan Dam (same as preceding?) is 20 km north of Gonbad-e Qabus on
the Gorgan River and has a capacity of 86 million cu m. Keivany et
al. (1990; http://gause.biology.ualberta.ca/Keivany/bsc/html -
1996) report an irregular pH range for the Gorgan River from 6.3 to
7.9 with an average of 7.1. Temperature range was 8 to 33°C.
Conductivity varied greatly from 667 to 10,000 µM/cm, with an average
of 875 µM/cm. Chlorides, especially sodium chloride, were the most
abundant soluble salts. Total dissolved solids varied from 21 mg/l to
4300 mg/l in an inverse relationship with water volume. Water volume
at the dam inlet varied from 2 to 75 m3/second and almost
52% of the sediments entered the dam during a high flood. Water
quality was assessed as polluted. The major fish species were Cyprinus
carpio, Barbus barbus (sic - possibly Luciobarbus capito),
Alburnus spp., Cobitis taenia, Gambusia affinis,
and Carassius carassius (sic - presumably C. auratus).
A fish kill noted by Coad (1980c) in 1978 was attributed by local
informants to careless insecticide spraying on fields neighbouring the
Gorgan. Newspaper and radio reports variously stated that 200 barrels
of a highly toxic chemical spilled into the river when a truck
overturned and that the chemical, identified as Turbidan from the
Trintext chemical plant, was dumped by a technician commissioned to
get rid of the waste product (Kayhan International, 7 May 1978).
The Atrak headwaters are close to those of the Tedzhen basin. The
Atrak basin comprises about 40,000 sq km. The Atrak is only about
10-15 m wide and about 0.5 m deep over much of its lower course. It
only reaches the Caspian Sea during floods. A tributary of the Atrak
from Turkmenistan is the saline Sambar River, about 203 km long. Petr
(1987) reports that efforts were being made to divert this river so as
to increase the water quality in the Atrak. The fresh section of the
Atrak has a conductivity of 2362 µS and the saline section 23,500
µS. The Caspian Sea off the Atrak River is an important fishery
economic zone. Gasan-kuli or Hasan Kuli is a town in Turkmenistan near
the Iranian border referred to in fishery reports from this area. The
catch of Rutilus caspicus, Cyprinus carpio and Sander
marinum was nearly 1.44 x 104 tonnes with only 1.9%
being accounted for by Clupeonella cultriventris (= caspia). However by
1972 the catch of the commercially important species had declined to
1.5% and the less desirable Clupeonella had increased to 5.73 x
104 t or 98.3% of the catch. The causes were reduction
in the Atrak runoff through irrigation withdrawals, pollution from
agriculture, overfishing in the sea and the drop in sea level. Flows of the
Atrak did not reach the sea in 1984, 1986, 1990 and 1991and spawning of species
using the lower reaches did not occur (Caspian Environmental Programme, 2000).
There are 5 lakes along the Atrak, fed by the river, which have
been recently dyked to improve water retention. Their fauna is
dominated by native cyprinids. The lowest lake is saline and they
range in size from 400 to 2500 ha.
The lakes Alagol or Ala-Gol at 37°21-22'N, 54°35'E,
Ulmogol, Alma-Gol or Ulmagol 37°24-25'N, 54°38-39'E
and Ajigol or Adji-Gol at 37°24-25'N, 54°40'E
comprise a Ramsar Site (World Conservation Monitoring Centre, 1990;
Scott, 1995) near the frontier with Turkmenistan just east of the
Caspian Sea. Alagol occupies 1400 ha (Scott (1995) states 900 ha) and
both Ulmogol and Ajigol 200 ha (Scott (1995) states 280 ha and 360 ha
respectively). The Alagol Lake is slightly saline with a mud and sand
bottom. It is fed by springs, seepage and precipitation and may dry
out completely in summer. It overflows westwards when full. Vegetation
is sparse with Juncus, Carex and grasses mainly in the
northeast and small patches of Phragmites communis. It is
oligotrophic and vegetation poor. The other two lakes have seasonal
fresh water fed by precipitation and have a mud and clay bottom. They
are eutrophic and water levels vary greatly so that they may dry up
completely. Ulmogol has little vegetation such as Juncus, the
duckweed Lemna, Phragmites communis, Alhagi and
algae while Ajigol has extensive Phragmites reedbeds at its
eastern end and abundant submerged vegetation. Fishing occurs in the
lakes and the habitats are affected by cattle grazing and reed
cutting. Water is abstracted for irrigation and for a fish hatchery. In Alma-Gol
and Ala-Gol, 90.91% and 82.18% of the total frequency of fishes was comprised of
exotic species. Hemiculter leucisculus was the most frequent in Alma-Gol
(58%) and Adji-Gol (16.82%) and Carassius auratus in Ala-Gol (77.6%).
Other exotics were Gambusia holbrooki, Pseudorasbora parva
and Cyprinus carpio (Patimar and Kiabi, 2005; Patimar, 2007). Patimar (2008) details the
environment of these lakes and lists six native species (Alburnus alburnus (=
hohenackeri),
Barbus (=
Luciobarbus) capito, Capoeta capoeta, Cyprinus carpio, Rutilus
rutilus and Atherina boyeri
(= caspia)) and 4 introduced species (Carassius
auratus, Hemiculter leucisculus, Pseudorasbora parva and
Gambusia holbrooki), variously distributed among the lakes.
The Qareh Su (= Gharesoo) is another river entering the Gorgan Mordab. In its upper
reaches it has a rocky bed and a fauna of Paracobitis malapterura,
Capoeta capoeta and Alburnoides cf. bipunctatus, resembling the grayling
zone of Europe. The central part of the river dries up (the barbel zone) while
the lower river (bream zone) is brackish from gulf input, has high temperatures
and pollution. This lower zone has Carassius auratus, Alburnus
alburnus (= hohenackeri), Cyprinus carpio, Pseudorasbora parva, Gambusia
holbrooki and Gasterosteus aculeatus with Atherina boyeri
(= caspia),
Neogobius kessleri (= Ponticola gorlap), Neogobius melanostomus, Neogobius pallasi,
Knipowitschia caucasica, and Liza saliens feeding in the estuary,
and Acipenser stellatus, Alburnus chalcoides, Cyprinus
carpio, Rutilius rutilus (= R. caspicus) and Vimba vimba (= V. persa) migrating into the
river for reproduction.
Incheh Borun Lake at 37°13'N, 54°30'E is a small and isolated freshwater body of 50 ha about 40 km north of
Gorgan. Lake Bibishervan at 37°09'N, 54°52'E and Lake Eymar at 37°08'N, 54°52'E
are two more small isolated freshwater lakes occupying 300 ha and 250
ha respectively. All three lakes lie on a cultivated plain. The fish
faunas of these lakes are unknown.
The Golestan National Park lies between Bojnurd and Gonbad-e Qabus
and is divided by the Tehran-Mashhad highway. The Iran Nature and
Wildlife Magazine (volume 3, 1999; downloaded from its English
website) states that fish in the Doogh River include rainbow trout and
Umbra krameri (sic), both exotics. The latter species is
an error of translation from Farsi to English of common names (B. Kiabi, pers. comm., 23 February 2000).
A description of the park is given by Kiabi et al. (1994) and of the
Madar-Su Stream in the park, which has been studied ichthyologically, by
Mikaeili et al. (2005).
The Anzali (= Enzeli or Pahlavi) Mordab (37°26'N, 49°25'E) is a freshwater to brackish
lagoon (Firouz, 1968b) separated from the Caspian Sea by a sandy
barrier about 1 km wide. The more modern term is "talab" (= pool or
marsh, which lacks the association with death) but the older literature refers
to mordab and the term is still in common use. It is surrounded by ab-bandans such as the
Selke Ab-bandan of 360 ha at 37°24'N, 49°29'E
which is protected as a Wildlife Refuge. Ab-bandans are a feature of
the Caspian coastal plain, being a shallow and artificial freshwater
impoundment managed in winter for duck hunting and in summer as an
irrigation reservoir. Safaian and Shokri (2003) describe ab-bandans in
Mazandaran based on 423 of these features and Khorasani and Rokni (2001)
examined two Mazandaran ab-bandans in particular. The Anzali Mordab complex of 15,000 ha is a Ramsar Site and this includes the whole mordab, the Siah-Kesheem
marshes, Selke Ab-bandan and several other ab-bandans. The main mordab
comprising open water is 26 km long and 2.0-3.5 km wide encompassing
about 11,000 ha. Reed beds extend the eastern limit by a further 7 km.
The Siah-Kesheem (or Siah-Keshim) Protected Region has a lagoonal
surface area of 4500 ha (Khara, 1994; 6700 ha in Scott, 1995) and is
about 12 km long by 4.5 km wide. It lies to the southwest of the main
mordab, of which is was probably once part, and is fed by the Esfand
River. Note that Khan et al. (1992) state that the Anzali
Mordab is unprotected except for the Siah-Kesheem Protected Region and
the Selke Ab-bandan of 360 ha. A description of the Siah-Keshim
Protected Area is given by Riazi (1996) and of the wetland generally
by Monawari (1990). Pollution in the Sia-Keshim Wetland is reviewed by
Ganjidoust et al. (2009). Important fishes are listed as Sander
lucioperca, Cyprinus carpio, Silurus glanis and Esox
lucius (Iran Nature and Wildlife Magazine, 5, www.neda.net/inwm/no.5/english/pre_sites/pre_sites01.html,
downloaded 8 March 2000).
The main mordab is drained by the Sowsar Roga, Pir Bazar Roga,
"Raste-Khaleh" (? Rasteh Kenar) Roga, Nahang Roga and
Pahlavi or "Koulivar" (? Kolver) Roga over a distance of
about 4 km to the Caspian Sea. Warm, dense and saline sea water is
able to penetrate up these effluent rivers for as much as 10 km, which generally have low
flow because of water abstraction and seasonally low precipitation,
because of the rise in sea level since 1977. Fresh water flows across
the surface of the saline water mixing at depths of 0.5-2.0 m. Salt
water contamination is always a danger as more water is abstracted in
this heavily populated and farmed area (Kimball, 1973; Kimball and Shayegan, 1973;
Sharifi, 2006). Abdolmaleki (1994) gives some data on the benthic
macrofauna of this lagoon. Hosseinpour (1995) surveys the zoobenthic
resources of the Siahdarvishan and Pasikhan, two principal rivers
which enter the lagoon. Other entering rivers are the "Bohambar, Chakoor and Esfand".
Forest clearance around the mordab, rice production and other
agriculture, dams and weirs on inflowing rivers, river bed erosion
through decline in Caspian Sea level, influx of pesticides such as Diazinon (Talebi,
1998), Paraquat, Glyphosite, and chemical fertilisers, domestic and
agricultural sewage, excessive aquatic plant growth and natural decay
of vegetation (Nezami and Khodaparast, 1996; Filizadeh and Khodaparast, 2005),
phytoplankton blooms, some toxic (Nejatkhah et al., 2003) anionic
surfactants (Dadaye Ghandi et al., 2005), siltation from
deforestation of feeder streams, introduction of exotic species of
fish and plants such as Azolla (Iran Daily, 2 November 2006), grazing for livestock, reed
cutting for mats, fences and building materials, and a high urban
population growth of 4.6% per year, all affect the habitat and the
marsh is highly eutrophic (Mirzajani et al., 2010). These factors also contribute to the fall
in commercial fishing success. In the 1930s the catch was dominated by
the valuable Rutilus frisii kutum but in the 1990s the catch
was 50-75 times lower and the mordab now has a low value to fisheries.
The situation is compounded by the absence of effective fishery
management. The introduced Carassius auratus dominates catches.
The mordab was a principal breeding ground for Rutilus frisii kutum,
Abramis brama and Cyprinus carpio, and to a lesser
extent Sander lucioperca, and was an important habitat for Esox
lucius. Fish kills occur, more than 100,000 dying in August 1997
due to a lack of oxygen after "torrential rain and the growth of
aquatic herbs had created an unsuitable environment" (a Reuters
report) and more fish died in 2005 (Iran Daily, 21 August 2005).
Ghahraman and Atar (2003) concluded that the wetland is dying.
The bottom of the shallow west basin was completely covered by
perennial submerged vegetation in the early 1970s (Chara, Nitella,
Ceratophyllum, Myriophyllum, Hydrilla, and Vallisneria).
Water chestnut (Trapa natans) was the predominant floating
plant and covered the central basin in 1966. The Caspian lotus, Nelumbium
caspium is found all across the lagoon and is a significant part
of the standing stock. Phragmites, Sparganium and Typha
are emergent plants which engulfed open water. Reeds were formerly cut
extensively for building purposes but are now replaced by sheet metal
and cement blocks. Falling Caspian Sea water level and eutrophication
from domestic sewage and fertilizers aided plant growth. The fern, Azolla
filiculoides, was introduced as an additive to cattle feed and
rice cultivation from the Philippines in 1986. It soon entered the
mordab from the rice fields and mats up to 20 cm thick covered much of
the open water in 1991 (Holčík
and Oláh, 1992; Filizadeh, 2002). Dense growths of macrophytes have contributed to
declines in commercial fish catches as spawning grounds have
decreased, eutrophication is enhanced, and light penetration is
decreased and so oxygen declines. There are about 200 sq km of marshes
and 30 sq km of shallow open water fed by rivers from the Alborz
Mountains. The area of open water in 1989 was only 22.5% of that in
the late 1930s (Holčík
and Oláh, 1992). However the rise in Caspian Sea level since 1978 has
led to a salt water intrusion during the summer months when the
Caspian level is at its highest and freshwater input from rivers is at
its lowest. Deeper and more saline water may well inhibit plant growth
in the future (Khan et al., 1992).
The marsh is only a few metres higher than the Caspian Sea and had
a maximum depth of 2.5 m in the early 1970s. Caspian Sea level
fluctuations have serious effects on the level of the mordab and hence
its utility as a habitat for fishes. The optimum level for the fish
industry in general in the Caspian basin is given as -27±1 m (Mandych,
1995). The rise in Caspian Sea level since 1977 is gradually returning
the mordab to its supposed, natural brackish state and may improve the
fisheries situation which had declined over the last 50 years.
Emergent and submergent aquatic macrophytes were decreasing and such
fish as Atherina boyeri
(= caspia), Alosa caspia, Liza aurata, Syngnathus caspius and Clupeonella cultriventris
(= caspia) were increasing in
numbers since 1989. However the fishery will require extensive
engineering and management innovations to recover.
Hydrorybproject (1965), Kimball (1973), Kimball and Shayegan
(1973), Kimball and Kimball (1974), Hagh-Panah (1992), Holčík
and Oláh (1992) and Caspian Environmental Programme (2001c) give details of the limnology of the marsh. Water
temperatures vary seasonally from 0° to
28.8°C (average about 16ºC) and dissolved oxygen from 0 to
17.5 mg/l for example. Phytoplankton blooms have killed fish in the mordab, e.g. on 5 June 1997 when dissolved oxygen in the western part
was at 0-0.2 mg/l and hydrogen sulphide was at 2.0-2.5 mg/l (Iranian
Fisheries Research and Training Organization Newsletter, 17:7,
1997).. Conversely, low phytoplankton populations have probably
resulted in lowered fish catches. High water temperatures and
chlorophyll inactivation through high light levels reduce the numbers
of phytoplankton and hence zooplankton, on which fish feed, also
decline. Higgins (1973) found that DDT levels in sturgeon, sturgeon
caviar, Cyprinus carpio and Rutilus frisii taken near
Anzali were not hazardous to humans in flesh (0.2-1.8 p.p.m.) or in
caviar (0.05 to 2.5 p.p.m.), both less than the limit for edible
fishes set by the U.S. Food and Drug Administration at 5 p.p.m., but
that the level in the caviar was a serious threat to sturgeon
reproduction. DDT was more concentrated in the eggs because of their
fats and oils in which DDT is more soluble. Certain heavy metals, lead
and silver, were potentially harmful to the fishes also. Pourang (1995, 1996), Amini Ranjbar (1998), CEP (2001a)
and Sartaj et al. (2005) describe heavy metal concentrations (lead,
chromium, copper, cadmium, zinc, manganese and nickel) in fish, surficial sediments and various macroinvertebrates of
the Anzali wetland. Levels in Carassius auratus and Esox lucius
were below recommended levels for human consumption. Carassius
auratus, Cyprinus carpio, Esox lucius and Hypophthalmichthys
molitrix in the Anzali Mordab have zinc (5.39-27.98, mean 17.28
p.p.m.), cadmium (0-0.08, mean 0.0251 p.p.m.), cobalt (0-1.67, mean
0.6935 p.p.m.), lead (0.11-2.95, mean 1.04 p.p.m.) and mercury
(0.113-0.63, mean 0.3 p.p.m.) in their muscle tissues (Annual
Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 46-47, 1997). Nadim (1977) found the highest
mercury levels in Caspian Sea fish were 0.51 and 0.36 mg/kg in Rutilus frisii
and Esox lucius respectively with the lowest in Liza aurata at
0.07 mg/kg. As the acceptable limit was 0.5 mg/kg, mercury contamination in fish
was not considered a problem. The lowest zinc
concentration was in H. molitrix, the highest lead
concentration was in C. carpio and the highest cobalt
concentration in C. auratus but concentrations were less than
those set by WHO as significant. Södergren et al. (1978)
reported on pollution with organochlorines in Esox lucius from
the mordab and found this predatory fish to have accumulated the DDT
metabolite p,p'-DDE, suggesting that this occurred
over considerable time and was not a recent event. DDT did not appear
to be incorporated in the pelagic food chain, although it has been
used for agriculture and vector control problems. Most DDT probably
attaches to clay and soil particles and settles out on the mordab
bottom. These authors also recorded DDT from sturgeon species and
their eggs in Iranian waters. Pollution continues to be a problem in
this heavily populated, industrial and farming region. Heavy rains in
October 1995 swept industrial wastes including heavy metals such as
lead and zinc, agricultural waste and domestic sewage into the mordab.
A fish kill resulted as evidenced by the mordab being covered with
floating dead fish. The kill was attributed to the heavy metals and to
oxygen depletion (http://netiran.com:80/news/IRNA/html/941029IRGG01.html).
Mercury concentrations in fish and fishermen's hair were studied from the
Caspian shore by Zolfaghari et al. (2008). The mean hair mercury
concentration was below the WHO threshold level and there was a weak correlation
between number of fish meals per month and mercury levels. Levels in Vimba
vimba (= V. persa), Rutilus rutilus (possibly R. caspicus), R. frisii, Liza spp.,
Carassius auratus and Esox lucius exceeded US EPA guidelines.
Amini Rad (2001) assesses the socio-economic importance of fisheries in
Bandar Anzali. Fishes are very popular food items there with an average
consumption of 11.3 kg, 70% more than in the rest of Iran. White fish (safid
mahi, Rutilus frisii) was 1.5 times more expensive than mullets (Mugilidae),
2.6 more than other species and almost 28 times kilka.
Gorgan (= Asterabad or Astrabad) Bay (36°40'N, 53°50'E) is 56 km long by 16 km long and
is brackish (8.7-10.0‰) because of input from rivers although Bayrami et al.
(20030 give 16 p.p.t. The bay encompasses about 400 sq km. A
general description is given by Zanusi (1995) who considers it to be
the second richest resource for caviar in the Caspian Sea after the
Volga River. The Caspian Environmental Programme (2001c) gives an average
surface water temperature of 19.1ºC, oxygen from
2.4 to 11.1 mg/l, pH 8.0-8.5 and total dissolved solids 11.23 mg/l in February
to 15,052 mg/l in March. The bay's ecology has been changed by the recent rise in sea
level which resulted in storm surges over the sand bar between it and
the Caspian Sea. The construction of the Voshmgir Dam on the Gorgan
River in 1970 also had an effect, reducing the amount of fresh water
to the river mouth which provided spawning areas for Cyprinus
carpio and Rutilus rutilus (presumably R. caspicus). Over 40% of the total sturgeon fishing in the Caspian
Sea is centred on Bandar-e Torkeman. There is also a black market in
sturgeon products. Authorised fishing resources shrunk by 33% from
1993-1994 to 1994-1995 through unauthorised fishing, lack of controls
and decrease in controlled sturgeon reproduction. The authorised catch
in 1994 for the region from the Neka River to the Turkmenistan border
was 1500 tonnes and the unauthorised catch was probably of similar
size. The caviar production was 57,000 kg.
The area of the Miankaleh Peninsula, Gorgan Bay and the nearby
freshwater Lapoo-Zaghmarz Ab-bandans is designated as a Ramsar Site
(World Conservation Monitoring Centre, 1990). The Miankaleh Wildlife
Refuge encompasses 81,180 ha and is part of the Miankaleh Protected
Region (97,200 ha). Jones (www.ramsar.org/lib_dir_2_3.htm downloaded
14 April 2000) gives 68,800 ha for the Wildlife Refuge. The Miankaleh
wetland may encompass 40,000 ha, not the larger figures as originally
designated (Khan et al., 1992). The bay has a sand and mud
bottom and is oligotrophic. There are extensive marshes along the
southern and eastern shores which flood in fall and winter. These
marshes are eutrophic from agricultural runoff and stream and
irrigation channel inputs. The bay vegetation comprises principally
glasswort (Salicornia), sedges (Carex) and rushes (Juncus)
with some small reedbeds of Phragmites communis. The ab-bandans
have extensive reedbeds of Phragmites communis with stands of
reedmace (Typha) and abundant submerged vegetation. Several
factors will affect the ichthyofauna including irrigation requirements
limiting freshwater flow into the bay and ab-bandans, a fish
processing plant at Ashuradeh with associated wastes, a new road along
the peninsula which facilitates access and potentially increased
pollution and poaching, reed cutting, heavy livestock grazing,
agricultural wastes, aquaculture ponds using exotics, fishing by local people and a proposed nuclear
power plant. The whole area is an important nursery and breeding
ground for fishes. The ab-bandans are not protected although they are
within the Ramsar Site. The two shallow ab-bandans occupy 950 ha at 36°50'N, 53°17'E northwest of Behshahr. They are
fed by irrigation ditches and drain east into Gorgan Bay.
The Gomishan Marshes at 37°15'N, 53°55'E
extends along the eastern shore of the Caspian Sea from Gomishan north
and northwest to the Turkmenistan border. There are about 4850 ha of
brackish lagoons and marshes, their brackish nature occasioned by the
rise in Caspian Sea level. There is agriculture, livestock grazing and
waterfowl hunting. The fish fauna is mostly unknown but the area is probably and
important breeding ground for the commercially important mullet Liza aurata
(www.ramsar.org/ram_rpt_37e.htm, downloaded 4 May 2001), for Rutilus rutilus
(presumably includes or is R. caspicus) and for Sander
lucioperca, and the latter two are open to hydrocarbon pollution (Ghasempouri
and Esmaili Sari, 2002).
The Astara lagoon at the western end of the Caspian coast of Iran is separated from the Caspian Sea by a sand bar,
and is flooded across this bar during winter storms. The lagoon
encompasses about 950 ha and is fed by a river during August to March,
reducing its salinity to about 7 p.p.m. There is a rich growth of
aquatic plants and the area has potential for fishing and aquaculture
(Petr, 1987). Lavandavil Marsh at 38°20'N, 48°50'E is found about 10 km south of
Astara and lies within a Protected Area of 949 ha. It is a small
swampy woodland and freshwater marsh with extensive stands of Juncus.
Abbasabad Dam at 38°23'N, 48°50'E south of Astara is a 45 ha water storage reservoir. Nur or Neur Gol at
38°00'N, 48°33'E in the northwest Alborz Mountains is a 200 ha freshwater lake at 2300
m about 50 km south of Astara. It lies within the Lisar Protected Area
which includes the whole watershed of the Lisar River. The lake drains
north to an Aras River tributary but freezes over for about 6 months
each year. The submergent vegetation is rich. Rainbow trout (Oncorhynchus
mykiss - see account of this species) were introduced to the lake
in the early 1970s in an attempt to start a sport fishery.
There is also a number of permanent and seasonal lakes along the Sabalan
Mountain range which lies partly in this basin and partly in the Lake Orumiyeh
basin and these are known to have fishes (www.netiran.com,
downloaded 17 June 2004).
The "Lapu" Lake, about 20 km northeast of Sari in Mazandaran, is an example of a smaller water body along the Caspian
shore, covering about 100 ha with a maximum depth of about 2.5 m,
perhaps 3.5 m in winter (Petr, 1987). There is a rich assortment of
aquatic plants. In 1985, 90,000 fingerlings of common carp or kopur (Cyprinus
carpio), grass carp (Ctenopharyngodon idella) and silver
carp (Hypophthalmichthys molitrix) were stocked and 120,000
fingerlings were added in 1986. A good harvest was reported in 1986.
There is a wide variety of reservoirs on the Caspian shore, varying in
size from about 10 to 400 ha. Some completely dry out in summer when
water demands are high but others are stocked with common carp, silver
carp and, to a lesser degree, grass carp. There are also populations
of native fishes such as kopur Cyprinus carpio and ordak mahi (Esox
lucius) but not in commercial quantities.
The "Amirkelayeh" Lake or Lagoon is located between the
cities of Lahijan, Langarud and Kiashahr at 37°17'N,
50°12'E. It is an example of a larger, freshwater lagoon as it
encompasses 1230 ha, being 4.5 km long and up to 1.7 km wide. The lake
is in the Amirkelayeh Wildlife Refuge and is a Ramsar Site (World
Conservation Monitoring Centre, 1990). Average depth is only 1.6 m
although some areas reach 4 m (Scott (1995) states 3-4 m on average
but up to 6 m). The lake is fed by springs and precipitation and is
eutrophic. It lies above the 1980s rise in water level of the Caspian
Sea (Khan et al., 1992). It may flood into marshes or the
Caspian Sea via a small stream into a channel of the Safid River but
is above the recent (1990s) rise in Caspian Sea level. Vegetation is Phragmites
communis and Typha with abundant submerged and floating
plants such as Nelumbium, Lemna, Potamogeton, Hydrilla,
Myriophyllum and Ceratophyllum. The fishes comprise Esox
lucius, Sander lucioperca, Carassius sp. (listed as
Crucian carp, probably C. auratus), Blicca bjoerkna, Syngnathus
caspius, Pungitius platygaster, Silurus glanis, Rutilus
rutilus, Cyprinus carpio, and Tinca tinca. Ctenopharyngodon
idella has been introduced (Nejatsanatee, 1994).
The Fereidookenar or Fereydun Kenar Marshes at 36°35'N, 52°31'E lie 13 km southwest of Babolsar
and occupy 1000 ha. These marshes are artificial, being a damgah or
shallow impoundment for duck hunting and water storage. They are one
of the best protected wetlands along the Caspian shore as the local
duck hunters aggressively restrict access (Khan et al., 1992).
There are fringing reed beds of Phragmites australis and Typha
with abundant floating and submerged vegetation.
"Seyed Mohalli, Zarin Kola (both at 36°44'N,
53°00'E) and Larim Sara (36°45'N, 53°03'E)" are ab-bandans and associated
marshy areas found north of Sari and east of the Tajan
River mouth. The first two occupy 600 ha and the last one 1000 ha.
Aquatic vegetation is rich, both submerged and floating, and there are
extensive stands of Typha and Phragmites. Construction
of a large dam on the Tajan will result in an associated network of
irrigation canals which may cause ab-bandans to be neglected. The ab-bandans, although artificial, have more of the character of a
natural marsh than irrigation channels. Much of this area of the
coastal plain has been converted to agriculture which destroys natural
wetlands so ab-bandans take on a disproportionate importance as a
refuge for wildlife including fishes.
Various dams have been built or are under construction in this
basin including the Gourchye Embankment Dam 15 km southeast of Ardebil
with a capacity of 20 million cu m, the Yamchi Dam 20 km southwest of
Ardebil and the Gaybeglou Dam 40 km south of Meshgin Shahr in East
Azarbayjan Province, the Maku Dam with a 150 million cu m capacity in
West Azarbayjan and the Agh Chay or Ziaeddin Dam near Khvoy (http://netiran.com/news/IRNA/html/950914IRGG06.html;
http://netiran.com/news/IRNA/html/950914IRGG10.html;
http://netiran.com/news/IranNews/html/96102201INEC.html).
The Neka Power Plant in the eastern Caspian basin entrains a large
amount of debris and algae that prevent effective physical systems of fish protection from entrainment. An electrical
fish protection system is used instead. Inflatable rubber dams are now being
constructed in the lower reaches of rivers, e.g. the Babol, to block the rise in
Caspian Sea level such that agricultural water intakes will not be contaminated
with saline water. The effects of these dams on fish migrations and biology is
unknown (www.satujo.com/english/barrage/dams4.htm, downloaded 20 December 2002).
Qanats and springs are not a feature of this basin as in so many
other parts of Iran, except for the drier areas drained by the Qezel
Owzan and other streams of the plateau and in the drier valleys of the
east away from the rainfall of the Alborz-backed Caspian lowlands. One
particular artificial habitat for fishes in the lowlands are the
ab-bandans, shallow freshwater marshes maintained as habitat and
overwintering areas for waterfowl and for conserving water for rice
fields (Beaumont and Neville, 1968). Some ab-bandans around the Anzali
Mordab were set aside as refuges for waterfowl and incidentally would
protect some fish species threatened by the draining of marshes.
Construction of irrigation dams will also lead to abandonment of
ab-bandans. Ab-bandans and damgah (ponds made specifically for duck
trapping) have declined in number but still encompass 10,000 ha (Scott, 1995).
Extensive stocking of commercially important species in the
sturgeon (Acipenseridae) and carp (Cyprindiae) families takes place annually in
the Caspian waters of Iran. These are detailed under the Species Accounts. Varedi and Fazli (2005) examined the rivers Shirud, Tonekabon, Larim, Tajan and
Goharbara of Mazandaran for the physico-chemical properties of estuarine water
in 2000-2001. Only the Shirud and Tonekabon met U.S. Environmental Protection
Agency standards for release of fingerlings, the other rivers failing because of
water abstraction and improper land use development.
A wide variety of parasites have
been recorded from fishes in this basin and these are mostly dealt with in the
Species Accounts. Pazooki et al. (2008), for example, recorded 7
monogenean species from 11 fish species in the Aras, Zangbar and Ghotor rivers
of northwest Iran, namely Dactylogyrus extensus, D. chramuli,
D. lenkorani, D. kendalanicus, Silurodiscoides siluri,
Diplozoon megan and Gyrodactylus varicorhini.
Zoogeographically, Berg (1940) considers this part of Iran to
belong to the Kura-Iranian sector of the Caspian District of the Ponto-Caspian-Aral
Province. This fauna is very similar to that of the Kura River
although certain genera are absent, even in the Safid - a major river,
such as Chondrostoma, Gobio and Leucalburnus.
This basin occupies an immense area of north-central Iran, over
200,000 sq km in the rain shadow of the Alborz Mountains. Mahdavi and
Anderson (1983) detailed the qanat water supply of the margins of this
basin. Intermittent streams drain to several kavirs which are grouped
together under this basin for convenience. The principal kavirs are
the Damghan Kavir in the north, the Sabzevar Kavir in the north-east
and the Kavir-e Bozorg (or Great Kavir) occupying much of the basin,
being about 450 km in east-west extent and 250 km in north-south
extent. The Kavir-e Bozorg receives waters exiting from other kavirs.
The principal streams entering this basin drain the Alborz Mountains
and their eastern extensions in Khorasan. The Alborz peaks exceed 4000
m and even to the east the Kuh-e Binalud (36°30'N,
58°55'E) attains 3416 m near Neyshabur (36°12'N, 58°50'E)
while the lowest points are at an altitude of 650 m. The Damghan Kavir
receives two major streams from the Alborz, the Damghan River and the
Hasanabad River, and other streams dry up in early summer. The
Sabzevar Kavir has numerous small and temporary streams which feed it
as well as two major streams, the Mureh River, 320 km long, and its
tributary, the Kalshur River, 240 km long. The Kalshur drains the Kuh-e
Binalud and flows west to meet the south flowing Mureh. These rivers
drain areas rich in salt domes and samples taken show water to be
saline and some streams are fishless. Qanats support fishes in this
area although the fish only emerge at night in some cases.
Ruttner-Kolisko (1964; 1966) and Ruttner and Ruttner-Kolisko (1972;
1973) studied the chemistry and limnology of natural springs and
qanats in a mountain area separating this basin from the Bejestan
basin. Several factors were found to affect the limnology. Climatic
factors were temperature, precipitation and evaporation, edaphic
factors were geology, salt content of soil and intensity of waterflow,
and pollution by man and animals was a factor. There was a range in
salinity from low (<15 mval/l) to high (>120 mval/l). Qanat
discharges in this area were 20-50 l/sec. Springs were small and many
were dammed to form small pools for livestock.
These large central basins of Iran were once thought to be
desiccating lake basins. However more recent studies have shown that
although there may have been shallow lakes, e.g. saline Lake Damghan,
and rivers carried more flow and were perhaps more closely linked than
today, there was no extensive and continuous freshwater lake over the
whole of central Iran that could have facilitated fish dispersal.
While the hills received increased rainfall, the central deserts
remained arid during Pleistocene "pluvials" and cold phases
(Bobek, 1959; Scharlau, 1968; Krinsley, 1970).
The Dasht-e Lut basin of south-central Iran is ringed by mountains yet has
the lowest point on the plateau at 205 m in the Namakzar-e Shahdad.
The central portions of this basin are some of the most barren and
inhospitable in Iran or indeed the world. Conrad and Conrad (1970) and
Gabriel (1938) give descriptions of this desert basin. Intermittent
streams drain the mountain ranges around Kerman east to the namakzar
or namaksar (= salt waste), north from mountains near Bam (29°06'N,
58°21'E) such as the Kuh-e Jebal Barez (28°30'N, 58°20'E)
and Kuh-e Bazman (28°04'N, 60°01'E)
which delimit the northern edge of the Hamun-e Jaz Murian basin, west from the
slopes of the active volcano Kuh-e Taftan (28°36'N,
61°06'E) and south from the mountain ranges near Birjand (32°53'N, 58°13'E).
High points include the Kuh-e Hazaran west of Bam and south of Kerman
at 4402 m. Such heights retain snow and have more abundant
precipitation which feed streams at least in the mountains. However
many minor and some apparently major streams marked on maps are
completely dry. Much of the water is absorbed into the ground and
tapped by qanats. The Shah River at Birjand is dry through most of the
year (Fisher, 1968). Tabas (33°36'N, 56°54'E)
at the northern end of this basin has numerous qanats (Krinsley, 1970)
but I have not seen samples from this area.
The Shahdad River is presumably in
this basin based on maps and supplies water to Kerman and some nearby villages.
One sample station was polluted by wastes from a rainbow trout farm (Rezaei
Tavabi et al., 2009). The Tahrud is an important stream which drains the Hazaran to a
small sump in the south of the Dasht-e Lut basin and has a continuous flow
which becomes subsurface well east of Bam (compare maps). Its maximum
map extent approaches 250 km. In the mountains, the Tahrud is 1-8 m
wide and up to 50 cm deep. Water temperature was a warm, 15°C on a cool December day.
The Dasht-e Lut includes the largest sand dune field in Iran (ca. 10,000 sq
km) which has developed through aeolian erosion. Sand dunes block
roads and may well fill in or divert streams.
Qanats in this basin can have water temperatures much higher than
the few surface streams. One qanat near Bam had a temperature of 25°C
in a snowstorm, yet stream temperatures below 10°C
are not uncommon.
The principal feature of this basin is the Zayandeh River which
rises in the Zagros Mountains east of Zard Kuh at 4548 m (32°22'N,
50°04'E) and flows east for about 300 km to its terminal basin, the Batlaq-e Gavkhuni at 32°20'N,
52°47'E, a salt marsh with a salinity of 315‰ (Löffler, 1961) and an average depth of about 1 m
(www.netiran.com/php/artp.php?id=1615, downloaded 19 July 2004).
The salt marsh can dry up in summer. Wetlands
associated with the terminal basin are a Ramsar Site of 43,000 ha (or 37,000 ha;
sources vary as does the size of the marsh seasonally and annually).
Associated marshes at the river delta and along its banks are fresh to
brackish. These marshes are fed by flooding and by irrigation canals
but dry up in late spring or early summer. Flooded areas often freeze
over in winter. There is little natural marsh vegetation and flooding
occurs over degraded steppe and cultivated land. Water is diverted for
irrigation and for domestic and industrial uses. It receives
pollution from Esfahan and other urban sources. Esfahan is a major
oasis city on the Zayandeh at 32°40'N, 51°38'E
with a population over 1 million, famous for its bridges (pol in Farsi) among
other sites.
The Zayandeh basin encompasses about
30,480 sq km and is connected to the upper Karun River basin (which
drains to the Persian Gulf) by the Kuhrang Tunnel constructed in 1953
although first proposed in the early sixteenth century (Fitt, 1953;
Afifi, 1966; IRNA, 5 February 2002). Two additional tunnels are under
construction (Stoltz, 2002). A hydroelectric dam at Godar-e Langar (also known as
Karun-4) would also supply piped water to Esfahan 300 km away if it is
completed (Whitley and Gallagher, 1995). Dams have deleterious effects
on a riverine fish fauna and are often stocked with exotic species.
The upper Karun has not been well explored for endemic taxa. Mean
annual flow of the Zayandeh is estimated at 1.2-1.45 billion cu m,
used mostly for agriculture but an increase in population and industry
has necessitated dam construction (Shah Abbas Kabir or Sadd-e Zayandeh
Rud, capacity 1450 million cu m) and diversion schemes. The dam is an oligo-
mesotrophic water body based on phytoplankton studies (Shams and Afsharzadeh,
2009). There is also
the Hana Dam on the Hana River at Semirom with a height of 35 m and a
capacity of 45 million cu m (http://netiran.com/news/IRNA/html931003IRGG04.html)
and the Izadkhast dam to the southwest of the Batlaq-e Gavkhuni (www.irna.com/newshtm/eng/12003142.htm,
IRNA, 2 July 2000). As well as man-made diversions, the upper
Zayandeh basin has captured headwaters from systems tributary to the
Persian Gulf. The Shah Abbas dam has reduced the natural flood flows downstream
and little water now enters the salt desert.
Plans have been made to transfer Zayandeh River water from the
Band-e Cham-e Asseman to Yazd's Shahneh Reservoir by pipeline over a
distance of 375 km (Hamshahri, Tehran, 629:5, 22 February
1995). 78 million cu m of water will be transferred annually and this
will decrease the habitat for fishes in the Zayandeh River basin.
Spring flow is at least 1700 cu m per second, but this drops to 28
cu m per second in autumn (Oberlander, 1968b). Discharge peaks in
April with low values in September-October and decreases dramatically
downstream after abstraction, evaporation and infiltration (Beaumont,
1981). The Zayandeh can be forded on foot at Esfahan in summer and
Buckingham (1829) reported it to be dry. It dried again in 2000, 2001 and 2003 under drought
conditions, partly through water abstraction upstream for irrigation and partly
through aqueducts to other desert cities (Rafsanjan and Yazd) not in the Esfahan basin (Anonymous,
2001b; Foltz, 2002; newspaper reports). The river is polluted by city
sewage, local wastes dumped directly into the river, and industrial
wastes (Moghadam, 1976; Al-Hashimi, 1987; Tehran Times, 15
September 1997). 172,000 cu m of industrial pollutants enter the river
daily. Pollutants include phosphorus, nitrogen, lead, nickel, zinc,
organic substances, iron, manganese, oil products, mineral and organic
dyes and the sewage from villages with a population of 900,000 people.
Nadim (1977) found the highest mercury levels in fish were 0.19 mg/kg. As the
acceptable limit was 0.5 mg/kg, mercury contamination in fish was not considered
a problem. The flow is 1.45 billion cu m annually of which 1.1 billion cu m is
used for agriculture, 150 million cu m for industry and the remainder is used as drinking water.
The basin has a high demand for water supplies and has been under stress in this
regard for the last 50 years. It will be unable to meet water demands in less
than 15 years (Salemi and Heydari, 2006).
Ouseley (1819-1823) noted numerous small "bleak" and
caught several carp-like fish up to 12-14 inches long (ca. 30-36 cm)
in the deeper waters around the bridges over the Zayandeh at Esfahan.
The Batlaq-e Gavkhuni and marshes on the lower Zayandeh are a
Ramsar Site, the lake occupying 12,000 ha, permanent marsh 1000 ha and
temporary marsh 30,000 ha (World Conservation Monitoring Centre,
1990) or 47,000 ha (Mehrabi, 2004). It lies at 1470 m and has an average depth
of 1 m. The Batlaq (= salt lake or marsh, gavkhuni = cowshed because cattle are
put out to pasture in the marshes) is fishless but the marshes
have a freshwater character depending on the input from the Zayandeh
River. The substrate is silt and mud. Much of the marsh has been
converted for agriculture. Flooded areas may freeze over in winter. The salt
lake is said not to dry out completely (Mehrabi, 2004) although flows were down
to 10-100l/s in the dry years 2000-2002 and the lake was dried out (Esteky,
2006).
As with all plateau basins, this one also has springs and qanats
which contain fishes. Surber (1969) gives some data on total
alkalinity and calcium-magnesium hardness in this basin and
characterises it as moderately hard.
The Hamun (= marshy lake, in this instance) is dry for most of the
year, but fills with fresh water in winter (Harrison, 1941). Its
extent is presumably variable, depending on rainfall. It lies at an
altitude of about 300 m, with a still-subsiding depression within the
Jaz Murian plain, and is ringed by mountains.
The two major rivers flowing into the Hamun are the Halil (or
Haliri) River, known as the Kharan or Zar Dasht River in its upper
reaches, which flows from the neighbourhood of Kuh-e Laleh Zar at 4374
m lying to the northwest, and the Bampur River which flows towards the
Hamun from the east but follows a southerly course in its upper
reaches (Tipper, 1921). The source of the Bampur River lies between
1000 and 1500 m. The Halil is a longer river (ca. 390 km) than the Bampur (ca. 315 km) with a stronger and more continuous flow.
However, this river was nearly dry downstream of the Jiroft Dam and there was
only minimum flow upstream in 2008 during a drought (Atabak Mahjoor Azad, pers.
comm., 6 October 2008). There is
a 130 m high dam on the Halil, the Jiroft Dam, 40 km upriver of Jiroft. A flood
water storage dam at Bazman is 37 m high with a capacity of 3.3 million cu m
(www.irna.com, downloaded 26 January 2003). Discharge is only 1-3 m3/second in summer. Floods occur
(including an historical one which destroyed Jiroft in 1000 A.D., and
one in 1993) and river discharge can reach 800 m3/second in
15 hours with an 18 m rise in reservoir level in 40 hours and massive
sediment transport with turbidity reaching 280 gr/liter (sic) (www.stucky.ch/publication/JIRFLOOD.htm
downloaded 19 July 1999). The Bampur River in late November and early
December was flowing in its upper reaches near Karevandar and around
Iranshahr and Bampur but was dry between these two areas. Judging from
its width and depth below Bampur it probably did not reach the Hamun
by surface flow. Most rain at Iranshahr falls in January and February
(15 and 52 mm respectively) with none in the remaining months except
for rare summer monsoonal rains (Ganji, 1960). Irrigation and canal
schemes in the Bampur basin suffer from erosion and siltation problems
as elsewhere in Iran (Borowicka, 1958).
The Hamun-e Jaz Murian basin is ringed by much smaller streams draining the
surrounding mountains. These are all very small, e.g. the Ughin River
was as narrow as 30 cm and maximum depth in pools was about 50 cm when
sampled on 4 December 1977.
The Hamun-e Mashkid (= Mashkel) lies within Pakistan with its
western edge on the border with Iran. In this instance hamun means a
salt waste. The mountain ranges in this area of Iran are parallel with
the Iran-Pakistan border and run in a northwest-southeast direction.
The Mashkid River rises to the east of the mountains ringing the
Hamun-e Jaz Murian basin and flows east into Pakistan where it
receives a right bank tributary, the Rakhshan River, before turning
north to flow into the Hamun-e Mashkid. Its total length is ca. 430
km. Two tributaries of the Mashkid within Iran are the Rutak River and
the Simish (= Sunish River) which drain the lowlands between Kuh-e
Birag (27°35'N, 61°20'E) and the Badamo Range (27°38'N, 62°08'E)
from the northwest to enter the Mashkid River southeast of Saravan (27°22'N,
62°20'E). The upper Mashkid River is a
small mountain stream, probably with a perennial flow. The lower
reaches of this river, and of the Simish, comprise a series of muddy
pools of varying size. Some of these pools were isolated and fishless
in early December 1977, while larger ones, perhaps 1 km long,
contained some emaciated specimens. In this area fish are found more
abundantly in perennially flowing qanat streams.
The Tahlab River and its tributaries drain the eastern slopes of
the mountains south of Zahedan. The Tahlab flows in a southeasterly
direction into the Hamun over a ca. 160 km course. It was dry between
Zahedan and Mirjaveh (29°01'N, 61°28'E)
in early December 1977. The Ladiz River is a short (ca. 80 km) right
bank tributary of the Tahlab flowing from Kuh-e Taftan. In its lower
reach it was a small stream flowing in the bottom of a deep and wide
canyon. The stream banks were white with salt deposits.
This basin occupies 26,440 sq km north and east of Shiraz at a
lowest altitude of ca. 1525 m. Its lowest part is occupied by a
chloride lake, the third largest lake in Iran, composed of two parts,
a northern basin known as Narges or Tashk and a southern basin known
as Neyriz = (Niriz) or Bakhtegan. The two basins are not always
connected and the southern basin is saltier because major freshwater
input is from the north. Löffler (1956; 1957; 1959; 1968; 1981) gives
details of this lake. The lake area varies between 1210 and 2400 sq
km, with a maximum depth of 1.1-1.7 m and a mean depth of 0.5 m.
Salinity is 13.7-101.6 gl-1 and temperatures range from 15°C
to 45°C in the shallows. The lake is reported to have dried out completely in 1871, 1933 and 1966
(Cornwallis, 1968a) and in 2000 (www.irna.com/newshtm/eng/05142727.htm,
IRNA, 26 July 2000). Löffler (1993) considers that this lake
may dry out permanently in the near future if abstraction of water
from the Kor River for irrigation continues to grow. The drought in 2003 reduced
Lake Bakhtegan to a series of puddles. Fluctuations in
lake levels affect the freshwater faunas of springs, including fishes,
which drain into the lake: high levels swamp the springs with water
too saline for fishes to survive. Low levels, however, allow streams to connect
and exchange faunas on the lake bed so they are not as isolated as they might appear.
Bobek (1963) suggests that there may have been an outflow from this
basin to the Gulf at the south-east corner of the lake which was cut
off at the end of the Pleistocene by alluvial fans. However Krinsley
(1970) maintains that any outlet was closed by the late Pliocene.
Major rivers are the Kor (= the classical Araxes) and its tributary
the Pulvar (or Sivan) (= the classical Medus) which rise in the Zagros
Mountains to the north and north-west and drain to the north-west
corner of Lake Tashk. These mountains are high enough (Kuh-e Dinar at
4432 m and 30°50'N, 51°35'E) to have a snow cover and thus there is a continuous flow throughout
the year. However in summer water does not reach the lake because of
the demands of irrigation. Drainage and irrigation canals run through
the basin on the plains at the north end of the lake. Several springs
feed marshes, notably the Lapu'i marshes, a wetland of 150 sq km to
the north-west of the Kor-Pulvar junction, the Zarqan marshes of 4 sq
km, an extension of the Lapu'i marsh (both now severely damaged by
construction of a drainage canal as part of the Dorudzan or Sadd-e Daryush-e
Kabir (dam) at 30°15'N, 52°20'E, a project on the Kor River), the "Gomun", "Gumoon",
"Gumoo" or "Sangare" marshes of 2 sq km at the
north-west corner of Lake Tashk and the Sahlabad marshes of 5 sq km on
the south-east coast of Lake Bakhtegan (Cornwallis, 1968a; 1968b). The
Band-e Amir or Kamjan Marshes at 29°40'N, 53°05'E are formed at the delta of the
Kor River and encompassed about 100 sq km but the Daryush-e Kabir Dam
severely restricts the water flow to these marshes. A dam on the Bolaghi Gorge
is proposed which would affect the flow of the Pulvar but is being opposed on
archaeological grounds (www.netiran.com, downloaded 4 October 2004).
The Neyriz Lakes and Kamjan Marshes are a Ramsar Site (World
Conservation Monitoring Centre, 1990; Khan et al., 1992)
although the Kamjan Marsh area may be deleted because of drought and
other factors such as rice, wheat and cotton growing and livestock
grazing. The "Cheghakhur" and "Gandoman" marshes
in Chahar Mahall and Bakhtiari Province will be substituted for the
Kamjan Marshes as a listed Ramsar Site (Khan et al., 1992). The
"Gumoon" marshes have been partially drained for irrigation
and for conversion into aquaculture ponds (Khan et al., 1992).
The Ghadamghah spring-stream system at 30°15'N, 52°25'E and 1660 m altitude has been
described by Esmaeili et al. (2007) and is a regional hotspot for
biodiversity. The fishes present are Petroleucsicus persidis (Cyrpinidae),
Cobitis linea (Cobitidae), Seminemacheilus tongiorgii,
Oxynoemacheilus farsicus (Nemacheilidae), Aphanius sophiae (Cyprinodontidae) -
all Iranian endemics, and Alburnus mossulensis, Capoeta aculeata
and Capoeta damascina.
The Daryush-e Kabir Dam on the Kor River contains 990 million cu m
of water, is 24 km long and about 9.5 km wide. Its conductivity is 363
µS compared to Lake Bakhtegan at 105,900 µS and consequently it can
support a fish fauna. Band-e Amir on the Kor River is a diversion dam
over 1000 years old and also provides a small reservoir habitat for
fishes (Houtum-Schindler, 1891). At least three other dam sites have
been proposed in this basin (Tang "Boraghi" (= Tang-e Boraq),
"Tang Bulak" and "Ghaderabad" (= Qaderabad)).
Surber (1969) gives some spot data on pH, total alkalinity,
calcium-magnesium hardness, chlorides and free CO2 in this
area. Water is relatively hard. Concentrations of total dissolved
solids vary between 202 mg/l and 436 mg/l in the rivers compared to a
range of 333-6937 mg/l in the Gulf basin.
Kaftar Lake at 30°34'N, 52°47'E is at ca. 2300 m in the Zagros Mountains northeast of Shiraz. It
occupies 4700 ha (500 ha in Khan et al. (1992)) and is a
shallow, semi-permanent freshwater lake which can dry out completely
in summer and is frozen over in winter. The annual mean water temperature is
14.4°C, the mean maximum 23.5°C and the mean minimum about 2°C (B. Jalali,
pers. comm., 1999); and Nowrouzi and Valavi (2011) give various physicochemical
parameters. Lake water has been proposed
for irrigation usage in the past and a recently proposed earthen dam
would reduce the lake area by half (Scott, 1995). It has a mixed ichthyofauna of
native species and exotics. The fishes recolonise from springs and the main
river entering the lake and are also stocked.
The Kor River basin also contains qanats. Some of these flank the
Pulvar River, for example, and serve to bring water to fields above
the incised river bed.
Pollution in this basin has been recorded by Merchant and Ronaghy
(1976) where industry discharges waste untreated into surface and
ground waters. Waste from a sugar mill killed 1 million fish in 1994
and a further 500,000 fish died in 1996 from industrial waste (http://www.iran-e-azad.org/english/noi/noi-83.html
or News on Iran, 83, 15 November 1996). A fish kill was
reported from the Pulvar River in 1978, polluted by wastes from a food
factory (Coad, 1980c). Peritore (1999) and Moussavi and Saber (1999) record the Kor River receiving organic
wastes from animal processing plants, ammonium and mercury from petrochemical
complexes and such heavy metals as cadmium, chromium and arsenic from
electronics manufacturers. Ebrahimi et al. (2008) and Taherianfard et
al. (2008) report lead and mercury levels in
Cyprinus carpio and Capoeta spp. to be less than the maximum
allowable by the European Union but still of concern. Ebrahimi and Taherianfard
(2010a, b), however, found that levels of arsenic, cadmium, lead and mercury for these
species were higher than permissible for human consumption.
Channels started in 1981 to provide more agricultural land drain
through the Kamjan Marshes to Lake Tashk and the Kharameh Marshes to
Lake Bakhtegan. Much of the marsh habitat has been destroyed. The
"Gumoon" Marsh has been drained for agriculture and fish ponds.
Miller (1985) reports on deforestation in this part of Iran during
the fourth to second millennium B.C. Even marsh areas were probably
treed before demands for charcoal and construction materials
increased. The fish faunas must have adapted to increased insolation
and any species sensitive to higher marsh and stream temperatures
would become less common.
The Maharlu basin is the valley of Shiraz (29°36'N, 52°32'E) and encompasses about 4100 sq
km. Lake Maharlu is at an altitude of about 1460 m, has an estimated
average area of 220 sq km, a maximum depth variously cited as 0.5 and
3 m, a salinity of 124‰ or 304.95 gl-1 and is fishless.
The lake dried out completely in 1967 (Cornwallis, 1968a). The lake is
fed by minor streams and springs around its margin. The Khoshk River flowing
through Shiraz is dry for much of the year or composed mostly of
polluted wastes from businesses, domestic sources, industry and agriculture
(Kafilzadeh et al., 2007). The basin also has a number of qanats. Stream
temperatures vary between 8°C in January to 32°C in June while qanats can be warm,
e.g. at Sarvestan (29°16'N, 53°13'E) in December a qanat was 25°C. Surber
(1969) gives some spot data on pH, total alkalinity, calcium-magnesium
hardness, chlorides and free CO2 in this area.
The basin is separated by only a small rise from the Mand River of
the Gulf basin, but is treated separately here because fish
collections have been focused on this valley as Shiraz is the major
city of southern Iran.
Major fresh to brackish springs and their associated marshes (Ab-e
Paravan (2.5 sq km), Barm-e Shur (1.5 sq km) and Soltanabad (7 sq km))
are concentrated at the northern end of the lake (Cornwallis, 1968a).
Larger springs have pools which are about 2 m deep and reed beds of Phragmites
and Typha, some of which are cut. Livestock grazing occurs.
Amphibious tanks were tested in Barm-e Shur, stirring up anoxic bottom
mud and leading to a fish kill.
Numerous small springs around the lake are isolated from one
another by the intervening hypersaline water. Lake levels fluctuate
markedly and allow streams to meet on the exposed salt flat when the
water level is low. At high levels, salty water invades the lower
springs and eliminates their fishes, which only recolonise when the
lake level falls again and connection is made with a stream from a
spring which was above the last rise in lake level. One spring had a
salinity of 34‰ at the source when the lake had risen to
"invade" the spring. Aphanius persicus were
concentrated close to the source but would attempt to evade capture by
swimming into the salt lake where salinity was 180‰. Their
excursions into water of this salinity was brief and fish paled
visibly while darting in and out. Another spring was replete with
tooth-carps at 144‰. Temperature on 8 June 1976 at one spring was 27°C
at the surface and 32°C on the bottom, at about 1 m depth.
Lake Orumiyeh (= Reza'iyeh, Urmia, Urmi, Urumiyeh or Darya-e Shahi)
lies in north-west Iran and is the only Iranian lake large enough to
appear on general maps of the world. This lake is a Ramsar Site and
includes Orumiyeh National Park. Brackish marshes in the northeast,
northwest and southern shores probably support some fishes but the
lake itself is too salty.
Orumiyeh lies at about 1275-1300 m (accounts vary), is about 128-149 km long and 40-60
km wide. This thalassohaline lake has a surface area of 4750-6100 sq km, a volume of
29.4 cu km, a mean depth of 4.9-6.0 m, a maximum depth of 16 m, and a
temperature range of -1.3-27.5°C. Lake level can rise as much as 2 m in one season, as it did in the winter
of 1968-1969. It is a sodium chloride-sulphate system with a salinity up to 340.0 gl-1
(but mostly 217-235gl-1) and consequently is fishless (Abich, 1856;
von Seidlitz, 1858; Rodler, 1887; De Mecquenem, 1908; Plattner, 1955;
Vladykov, 1964; Kelts and Shahrabi, 1986; Ghaheri et al., 1999; www.neda.net/inwm/no.6/english/geology/geology01.html,
downloaded 10 July 2000; Van Stappen et al., 2001; Eimanifar and Mohebbi,
2007; Karbassi et al., 2010). Initially the lake was probably fresh
(Admiralty Naval Staff, 1918). A causeway has divided the lake into
two parts since 1989; a gap allows a limited exchange between the two parts. Its drainage basin approaches 57,000 sq
km (or 51,786 sq km, authors differ) and the lake is the terminal basin for a number of streams and rivers. Annual inflow is
6900 x 106 m3 (Ghaheri et al., 1999). During
spring runoff a freshwater plume covers large areas over the saline
lake near river mouths. Prominent perennial streams include the
Zarrineh River (230 km long) entering from the south and draining part of the
northern Zagros with a range in discharge of 10-510 cu m per second
with the Tata'u or Simineh River (145 km) as a major tributary, the saline
Aji Chay or Talkheh (= bitter) River from the east draining the flanks of Kuhha-ye Sabalan at
4810 m (38°15'N, 47°49'E) and Kuh-e Sahand at 3710 m (37°44'N, 46°27'E),
and the smaller streams from the west such as the Zowla (=
"Zola") Chay (84 km), Nazlu Chay (85 km), Shahr (= "Shaher")
Chay (70 km), Baranduz Chay (70 km) and Gadar (= "Qader") Chay (100 km) (Günther, 1899).
Both the Zarrineh and the Talkheh exceed 200 km in length. The Talkheh
River has a hardness of 820 mg/l according to Surber (1969), who also
gives values of total alkalinity and calcium-magnesium hardness for a
number of streams and lakes around Tabriz. The Talkheh floods extensively in the
spring and forms large marshes. Most streams were
relatively hard like the Talkheh although some were soft such as the
Basmenj Chay draining Kuh-e Sahand at 70 mg/l.
Lake Kobi (= Ghopi) is a Ramsar Site lying at 36°57'N,
45°52'E and 1240 m altitude in this basin. It is south of Lake Orumiyeh and northeast of Mahabad. It
comprises the fresh to brackish lake and associated but discontinuous
marshes of about 1200 ha. The endorheic lake is shallow with a maximum
depth of 1.5 m and a mud bottom. It is fed by precipitation and
springs, and when full floods marshes to the north. It freezes over in
winter. The lake is eutrophic and has reedbeds of Phragmites
communis and abundant submerged vegetation. Livestock grazing and
wildfowl hunting occur.
The Shur Gol and the "Yadegarlu" (= Yadergarlu) and
"Dorgeh Sangi" endorheic lakes are at 37°00', 45°26-35'E south of Lake Orumiyeh and
northwest of Mahabad at 1290 m are also a Ramsar Site comprising 2500
ha of lakes and associated marshes. They are fed by precipitation,
springs and small streams. Shur Gol at 2000 ha is surrounded by the
Hassanlu Marshes. Its water is brackish to saline. The eutrophic
marshes flood in fall and winter and have abundant submerged
vegetation. "Yadegarlu" is a shallow freshwater lake of 350
ha with abundant submerged vegetation and a surrounding of eutrophic
sedge marshes. It may dry out in summer. It apparently suffered in the
Iran-Iraq war (Jones, www.ramsar.org/lib_dir_2_3.htm, downloaded 4
April 2000) and may be deleted as a Ramsar Site. "Dorgeh Sangi"
is 150 ha in extent and is a shallow freshwater and eutrophic lake.
All three lakes may freeze over in winter. Reed cutting, grazing and
waterfowl hunting occurs in this complex and some drainage of wetlands
for agriculture may occur (Khan et al., 1992).
"Gerde Gheet" (Gordeh Git) and "Mamiyand"
(= Meimand?) at 37°02'N, 45°40'E are freshwater marshes south of Lake Orumiyeh and north of Mahabad
occupy 500 ha at 1300 m. The marshes are covered by Phragmites.
Waterfowl hunting occurs here and some livestock grazing.
The "Ghara Gheshlaq" freshwater marshes at 37°10'N,
45°50'E occupy 400 ha at 1290 m south of Lake Orumiyeh and north of Mahabad. The water is about 1 m deep,
eutrophic and freezes over in winter. Large parts of these marshes
were drained by the "Mahabad Multipurpose Drainage and Irrigation
Project" in the 1970s despite environmental concerns. Cornwallis
(1976) notes both the draining of these marshes and the cessation of
freshwater discharge from the Mahabad River. He also points out the
likelihood of chemical contamination from agriculture, choking by
vegetation and the probable use of herbicides. He recommends
introduction of Ctenopharyngodon idella and Hypophthalmichthys
molitrix. The marshes have been proposed as a Ramsar Site.
Lagoons in the Mahabad area dried in the year 2000 (www.irna.com/newshtm/eng/05142727.htm,
IRNA, 26 July 2000).
Gori Gol or Lake Gory at 37°5'N, 46°42'E
is a fresh to brackish lake near Tabriz occupying 120 ha at 1950 m. Depth is 2-3
m on average. It is a Ramsar Site (World Conservation Monitoring Centre, 1990; Scott,
1995). It is fed by precipitation, springs and small streams, with
overflow through a small stream. The lake freezes over in winter. The
submerged vegetation is abundant and there are extensive reedbeds of Phragmites
communis, Juncus, Carex and Scirpus. It is
under pressure from the population of the major city of Tabriz through
sport fishing and wildfowl hunting as well as reed cutting and cattle grazing.
Qanats are found in this basin where surface water is saline. About
225 million cu m of water are produced annually by qanats and wells on
the northern and eastern coast of the lake (Alamouti, 1966). Dams are
found on the Zarrineh River and on the river which flows through
Mahabad paralleling the Zarrineh. The Mahabad Dam has a fish catch of
130 tons (sic) annually and 300,000 fingerlings (species
unspecified) were stocked to save the fish reserves from possible
extinction (IRNA, 7 January 1999). The Mahabad reservoir has a
leech fauna (Codonobdella trunata, Parcanthobdella livanowi,
Baicalobdella torquata, Piscicola geometra) which may
affect local fish farms and fish populations elsewhere if fish are
transplanted (Abdi, 1999: www.mondialvet99.com, downloaded 31 May
2000). The Nowruzlu Dam on the Zarrineh is at 36°55'N, 46°10'E, occupying 1000 ha at 1260 m. It
is water storage reservoir with heavy input from surrounding farming
activities. The Alavian Dam near Maragheh is 80 m high, 935 m long and
has a reservoir of 145 million cu m (http://netiran.com/news/IRNA/html/951214IRGG11.html).
The Nahand Reservoir Dam northeast of Tabriz was inaugurated in 1995
with a capacity of 30 million cu m and a second dam, the Shahid Madani
also near Tabriz, was under construction. Other dams include those at
Ahar, Tabriz, Hashtrud, Hasanlu, Mianeh (= Onligh) and Heris which
were scheduled to be completed in the period 1995-2000 (http://netiran.com/news/TehranTimes/95121601TTEC.html
and www.irna.com/newshtm/eng/12003142.htm, IRNA, 2 July 2000). ? check
basins for dams
The Hassanlou Reservoir Dam at Naqadeh was to open in 1998 with a
height of 10 m, a crest of 5160 m (sic) and a capacity of 107
million cu m (http://netiran.com/news/IRNA/html/950915IRGG06.html). A total of 6 reservoir dams and 10 dams for re-directing water flow will
decrease water input to the lake by 1.04 billion cu m by 2014. The volume
of surface water has fallen from 42 to 22 billion cu m since 1995. The
lake salt has increased to more than 260g/l, up from about 185 g/l. The lake may
well dry up by 2014 (IRNA, 10 September 2001).
Löffler (1993) details the eutrophication threat to this lake
since a traffic embankment was built across the lake 35 km north of
Orumiyeh in 1990. Untreated sewage from Orumiyeh will pollute the
southern part of the lake.
Pollution occurs in various localities on a sporadic basis such as
the Godar River in Naqadeh where a fish kill numbering in the
thousands was reported (Tehran Times, 18 July 1999).
Haji Hassani et al. (2004) found that levels of Ni, Pb and Cu in the
Talkheh Rud were higher than acceptable limits for fish culture while Cr and Fe
were lower. The river receives waste water from agricultural and industrial activities.
Water reservoirs behind the Mahabad, Miandoab and Shahid Kazemi dams were
stocked with 3.6 million fish fry (species not specified) from the Pol-e Dasht
Complex in 2000. This aquaculture site has the capacity to produce 4
million fry. West Azarbayjan produces over 600 tons of fish annually (Tehran
Times, 2 January 2001).
Lake Orumiyeh is the largest natural habitat for brine shrimp in the world and, since 2000, is has been harvested, processed and
used to feed sturgeon in hatcheries (www.worldfishingcompanies.com/html/us/world.report.html?id=1, downloaded 23 October 2001).
The lake was formed during the late Pliocene-Pleistocene and lies
at 1275-1300 m and may well have had a Pleistocene connection to the
Caspian Sea basin although this is in dispute (Scharlu, 1968;
Schweizer, 1975). Pleistocene shorelines from 30 to 115 m above the
present level have been confirmed, and the lake covered twice its
present area, but this would not permit an external discharge. Berg
(1940) reports benches at levels of about 1800 m, 1650-1550 m and
1500-1360 m, which may represent shorelines, and a level of about 1570
m would have had an outlet to the Aras River basin through the Kara-tepe
Pass in the northwest and across the plain near Khvoy. Saadati (1977)
suggests two connections with the Caspian Sea, an early one in the
Pliocene to early Pleistocene resulting in endemic species and a later
one in the late Pleistocene resulting in species which are the same as
the Caspian or only subspecifically distinct. Stream capture may have allowed the entry of some species in recent
times as evidenced by a Salmo cf. trutta/caspius population.
Fish farming is extensive in West Azarbayjan. In the Iranian year ending 20
March 2002, 840 tonnes of coldwater fish were produced and 3000 t of warmwater
fish (Tehran Times, 24 November 2002).
Günther (1899) details a method of catching fish used in the
rivers of this basin. Flour and the pounded berries of Cocculus
indicus are mixed with butter to form a stiff paste. Small pellets
of the paste are thrown into slow flowing water and after 10-15
minutes, if the fish are feeding, they will begin to swim at the
surface in small circles or lie helpless in the shallows and are then
easily scooped up. Some fish can recover from the poison. There is no
effect on humans if poisoned fish are eaten.
Berg (1940) considers that this basin falls within his assignment
of the Iranian shore of the Caspian Sea. Species in common
include Leuciscus (= Squalius) cephalus, Barbus lacerta, Gobio
(= Romanogobio) persus, Capoeta capoeta, Alburnoides bipunctatus
(sic),
and Silurus glanis, and Acanthalburnus urmianus is
related to A. microlepis. Groombridge (1992) notes that the
ichthyofauna of this region is badly in need of re-examination. Naseka (2010)
recognises Urmia (Orumiyeh) Lake as a District within a West Asian Transitional
Region related zoogeographically to the East Transcaucasian District (southern
Caspian Sea area from the Kura River to the Atrek River). Both these Districts
are linked to Iranian endorheic basins, including those listed as ecoregions in
Abell et al. (2008), namely Namak, Kavir, Lut, Esfahan and Sistan, plus
Kavir, Kor, Sirjan, Maharlu Kerman-Na'in and Jaz Murian basins in this work.
This basin is flanked by the Alborz Mountains to the north and the
Zagros Mountains to the west. On the east is the vast expanse of the
Dasht-e Kavir basin and on the south such ranges as the Kuh-e Karkas at 3899 m
(33°27'N, 51°48'E). The basin encloses about 87,600 sq km.
A small sump near Arak (34°05'N, 49°41'E)
is included as part of this basin as it is not separated by any major
landform. A second salt lake is the Howz-e Soltan by the Tehran-Qom
road and this lies in the same depression as the much larger Namak
Lake south of Tehran. The lowest part of this basin is at 765 m and is
covered by water in spring but this generally evaporates by the middle
of summer.
The proximity of the capital, Tehran, to the rivers of this basin
and its rapid growth in population and industry has led to many water
diversionary schemes (Anonymous, 2003). A proposed dam northwest of Tehran would be the largest man-made lake
in the country and the Middle East (sic) (Nouri et al., 2005).
Much of this basin lies in Markazi or Central Province which has 42
dams of varying sizes. The Abbasabad Embankment Dam in Khomein, for
example, is 36 m high, has a crest of 260 m and has a reservoir of
25,000 ha (IRNA, 3 February 1999).
The principal river in the west draining the Alborz south towards the Namak Lake is the Karaj River. Average
temperatures of the Karaj River at the dam site before construction
ranged from 2.5°C in January to 16.4°C
in August (Nümann, 1966). Rieben (1954) and Hariri (1966) give
details of surface and ground water in this river basin. The Amir
Kabir Dam on the Karaj contains 205 million cu m of water and feeds
through pipelines to Tehran. The reservoir has an area of 4 sq km at
high water, 1.1 sq km at low water. Vladykov (1964) and Nümann (1966,
1969) give some details on the limnology of this reservoir,
particularly temperature regimes. The Karaj has a discharge of 124 cu
m per second in spring but this falls to 4.2 cu m per second in
autumn. 55.6% of the annual discharge occurs during spring. There is
no vegetation because of the steep rock sides and water fluctuations.
Nümann (1966) recommended stocking the Karaj reservoir with Coregonus
sp., Sander lucioperca, Acanthobrama terraesanctae (a
Levantine species) and cichlids from Israel as environmental
conditions and plankton levels were suitable.
Nadim (1977) found the highest mercury levels in fish from the Karaj were 0.05
mg/kg. As the acceptable limit was 0.5 mg/kg, mercury contamination in fish was
not considered a problem.
The Abhar River and its tributaries drain the land west of Tehran
and south of Qazvin (36°16'N, 50°00'E).
Its headwaters approach those of the Zanjan River, a Caspian Sea
tributary. The course of the Abhar is about 350 km from its headwaters
to the terminal sump. The lower part of this river is known as the
Shur and is salty. Sewage and untreated factory wastes, as much as
40,000 cu m, flowed into the streams around the city of Qazvin
although waste-water and sewage treatment plants are offsetting this
problem (http://netiran.com/news/IRNA/html/941220IRGG05.html).
Other rivers draining the Alborz are much shorter. The Jajrud (Jaj,
Jaji or Jaje River) to the east of Tehran is dammed at Latian (95
million cu m) for the Tehran water supply also. The Jajrud discharge
is 60.5 cu m per second in spring and 1.5 cu m per second in autumn.
Nümann (1966) reports fish kills in the thousands for Capoeta
buhsei on turbid spring floods of this river. Khorasani (2001) give an
environmental survey of this river. Mirzaei et al. (2010)
give details of Eurasian Otters feeding on Alburnoides bipunctatus (sic,
= namaki),
Squalius cephalus and Capoeta spp. in the Jajrud. The Band Ali Khan River flows
from the Khasrang Mountain (as does the the Jajrud which it receives) and its
branches on the Varamin Plain are used for irrigation. Much of this river is
polluted from wastes in the Jajrud and Tehran's sewage floodway (Rohani, 2004;
Kashefi Alasl and Zaeimdar, 2009). The Lar River, a
Caspian Sea tributary, was scheduled for diversion via a massive
tunnel into the Jajrud (Marwick and Germond, 1975a; 1975b). This would
affect flow in the Heraz River of the Caspian Sea basin and plans to
offset this involved weirs and canal construction no doubt with the
usual deleterious effects on fishes. These major projects are a far
cry from the days in the twentieth century when Tehran depended solely on qanats for its water supply (Rieben, 1954).
The Namak Lake receives the Qareh Su (Gharechay), which flows north of Qom, and
the Qom River from the Zagros Mountains. Discharge of both these
rivers is about 312 cu m per second in flood falling to about 4 cu m
per second in October (Oberlander, 1968b). The Qareh Su exceeds 400 km
in length. The Qom River has captured headwater streams of Persian
Gulf drainage. The Golpayegan River near Golpayegan has a storage
reservoir, the Sadd-e Shah Esma`il. Borowicka (1958) gives some early
figures on siltation and irrigation requirements. The Haroon Canal had
diverted water for irrigation from the Golpayegan River for over 1000
years, and during the summer and fall all river water entered this
canal. The Ghadir or Qadir Dam near Saveh has a volume of 290 million
cu m of water. The 15th Khordad Dam is located 80 km south of Qom on
the Qom-Delijan road (http://netiran.com/news/IranNews/html/95030718INPL.html).
The Khandab Diversionary Dam is near Arak (http://netiran.com/news/IRNA/html/951217IRGG09.html).
Egglishaw (1980) gives some details on the water quality and
environment of rivers and streams of this basin. Imandel et al.
(1978) recorded ground water pollution by detergents in Tehran, where
there was no method of sewage disposal other than discharge to wells
and seepage pits. Södergren et al. (1978) reported on
pollution with organochlorines in the Karaj and Latian reservoirs. Capoeta
buhsei, Oncorhynchus mykiss, Alburnoides bipunctatus (=
namaki)
Coregonus sp. had accumulated the DDT metabolite p,p'-DDE,
particularly in the Latian Reservoir. Direct removal of plants for
fuel and laying bare the roots of such thorny plants as "giavan"
for extracting gum tragacanth leading to plant loss has caused soil
loss by erosion, gullying and affected recharge of groundwater. Poor
farming practices on steep slopes has also led to the loss of topsoil
such that runoff is too fast for infiltration of rain and snow (Rieben,
1954). These factors causing silting of reservoirs, added silt input
to rivers and reduced groundwater recharge with consequent reduction
in spring and qanat flows, all detrimental to fish habitats. Some
areas of southern Tehran receive 300 kg/ha/yr of sulphate ions as acid
rain which lowers river pH and has effects on the fish fauna (Salahi Kojoure, 1997). An effluent leak from a power station in the Vian area of Hamedan sent
40-50,000 litres of furnace oil into 1 km of river in the Qareh Su basin (Iran Network 1,
Persian TV, 1730 GMT, 2 January 2000). Monavari and Mardani (2007) record the
effects of sewage from fish culture ponds in this basin on water quality in the
Jajrud, most factors being within acceptable limits except coliform bacteria.
Qanats are still a major feature of this basin. Alamouti (1966)
records 260 qanats producing 99 million cu m per year on the Varamin
Plain (35°20'N, 51°39'E),
220 qanats producing 161 million cu m per year on the Karaj Plain and
600 qanats producing 200 million cu m per year on the Qazvin Plain.
However numerous pump wells have led to the drying of qanats and a
complex irrigation system has reduced groundwater recharge (Beaumont,
1974). Alibekov (1994) gives a Russian account of qanats in Central
Asia and also refers to those around Tehran in the Namak basin.
The Qazvin area has more than 20 aquarium fish farms producing over 2 million
fish (www.tehrantimes.com, downloaded 28 July 2004). Waters in this area drain
also to the Caspian Sea and there may be potential for escapes of exotics.
Berg (1940) refers this basin to his Tehran District of the Iranian
Province. He notes that some drainages are close to those of the
Caspian Sea basin and that the fauna may be of quite recent origin,
rather than the Pliocene advocated by Derzhavin (1934) for Salmo
trutta or presumably now caspius).
Saadati (1977) considered that the fish fauna of this basin was not
derived from movements through a large freshwater lake connecting all
the tributaries. Some species came from the Caspian Sea basin and
others from the Esfahan and Tigris River basins. The basin may also
have served as a "filter-bridge" allowing such species as Capoeta
aculeata, Capoeta capoeta and the progenitor of Capoeta
fusca to reach the Dasht-e Kavir basin.
The Sirjan basin extends south-east of the Esfahan basin and
parallels the Kerman-Na'in basin. It is named for the town of Sirjan at 29°28'N,
55°42'E which lies at the edge of the
largest salt flat in the basin. It is somewhat higher than the Esfahan
basin which is at 1300 m, being 1448-1710 m. It is distinguished from
the Esfahan basin by its lack of a significant river. There are four
major sumps in this basin, strung out along its length at regular
intervals, and the northern two are connected as are the southern two.
The sumps are fed by intermittent streams. Qanats and minor springs
are found in this basin which has not been extensively explored. The
sump in the north near Abarqu (31°08'N, 53°17'E) receives streams from the west (Kuh-e
Bul at 3661 m and 30°48'N, 52°45'E) and from the east (Khar Kuh at 3512 m and 31°39'N,
53°46'E, and Shir Kuh at 4074 m and 31°37'N, 54°04'E). The southern basins near Sirjan
receive their streams from lower elevations.
The Sistan (= Seistan) basin straddles the Iran-Afghanistan border
and is a north-west to south-east oval in shape. It comprises a number
of minor streams and qanats flowing from the west and the Birjand
highlands, but these are rapidly absorbed or run for only a few days
each year. Its most obvious feature is the vast hamun or swamp
comprising open freshwater lakes, reed beds or neizar, and the
rivers that feed the lakes. This is a major oasis of fresh water surrounded by
hundreds of kilometres of arid plains. Huntington (1905a; 1905b), Annandale
(1919a), Ahmadi and Wossughi (1988), Noorbakhsh (1993), Mansoori
(1994), Ibrahimzadeh (1995), Scott (1995), Weier (2002), CIRSPE (2006a)
and van Beek et al. (2008) give descriptions of this
basin. Note that Weier's (2002) statement (repeated in various newspaper
reports and in UNEP (2003)) that there is nearly 140 species of
fish in Sistan is an error by an order of magnitude! The native ichthyofauna comprises a mixture of endemic species,
species related to or conspecific with high-altitude species from
Central Asia and species from Baluchestan in the wider sense. There is
little relationship to species from Iran to the west. Variations in
water level and crowded conditions lead to disease and parasite
outbreaks in the fishes (Mansoori, 1994).
The principal river is the Helmand (or Hirmand) which flows from
the Paghman Mountains just west of Kabul to end in Sistan after a
journey of 1400 km. Along with the Hari or Tedzhen, this is the only major river
entering Iran. Snow and rain in the Hindu Kush mountains ultimately
reaches Sistan at 427 m from heights of 5300 m. The Helmand is the
most important river between the Tigris and the Indus and drains an
area of 386,000 sq km of which 78,000 sq km or 20.2% lies in Iran (Gleick, 1993).
The Helmand produces 1700-2000 cu m per second in flood and 56 cu m
per second in the dry season. The average annual flow is 78 cu m per second. The river varies between 200 and 900 m
in width and between 2 and 5 m in depth. The annual water income to
Iran is about 6 billion cu m but this varies markedly and was 14,740
million cu m in 1970-1971 and 1976-1977 and 600 cu m in 1985-1986 (Mansoori,
1994). UNEP (2003) gives the following flows in million cu m:-
As it enters the Sistan depression, the Helmand splits into
several branches which feed the swamps, the two main ones being the
Sistan feeding the Hamun-e Helmand (also Hirmand or Hamun Lake) in
Iran and the Parian feeding the Hamun-e Puzak (or Parian) lying mostly
in Afghanistan. The northern part of the Hamun-e Helmand is called
Hamun-e Sabari, or Lake Sistan, which lies half in Afghanistan and
half in Iran, and the southern part is called Hamun-e Hirmand. Hamun-e
Sabari receives water from the Farah River and overflow from Hamun-e
Puzak. The Hamun-e Hirmand receives water from the southern or Sistan
branch of the Helmand River and overflow from Hamun-e Sabari. Other
rivers flowing from Afghanistan are the Harut, Khospas and Khash but
their flow is minor and intermittent compared to the Helmand (Gabriel,
1938). The whole lake area of Sistan is often called the Hamun Lake.
The plentiful natural flow of the Helmand is reduced by irrigation
dams in Afghanistan; the Arqhandab and Kajaki dams extract about half
of the 12 billion cu m which enter the Afghan plain (Michel, 1973;
Mansoori, 1994; Mojtahedzadeh, 2001). A third dam is under construction in Afghanistan
without environmental considerations being taken into account (World
Conservation Monitoring Centre, 1990). The proposed Kamal Khan Dam on
the Helmand in Afghanistan and the "Sistan Drainage and
Irrigation Completion and Rehabilitation Project" in Iran would
lower water level in the lake complex. There are also plans to divert
water from the Sistan area to the city of Zahedan in the south. The
Char-Neimeh (or Chahnimeh) Lake is a depression used as a water
reservoir and is filled from the Parian branch of the Helmand. It has
a surface area of 4,700 ha and is used for irrigation and fish culture
but does reduce flow into the hamuns. However floods in spring 1991
destroyed the Kajaki Dam and associated irrigation controls and the
lakes were more extensive than they had been in over a decade. Rainfall in
Afghanistan increased flow of the Helmand in 2003 and some flooding was expected
in Sistan (www.irna.com, downloaded 23 April 2003).
The Helmand was dry at the Iran-Afghanistan border in 2004 (Gall, 2004). Sadeq
(1999) lists several factors which are threatening the Hamun Lake
namely, fluctuation in incoming water, sedimentation, exotic species,
urbanization and increased population pressure on the hamun resources.
The south end of Hamun-e Puzak and the contiguous Hamun-e Sabari
(or Lake Hamun) are Ramsar Sites (World Conservation Monitoring Centre,
1990). The Lake Hamun Ramsar Site is on the threatened list of
National Parks (Anonymous, 1988b).
Puzak is very shallow, with maximum depth of less than 4 m, and is
the first of the Sistan lakes to flood and may never dry out
completely unlike the other lakes (Khan et al., 1992; Scott,
1995). This lake has extensive reed beds of Phragmites australis
with associated submerged Ceratophyllum demersum and relatively
little open water. Reeds are cut as forage for cattle, burnt to
improve grazing for livestock, used for boats, for wind-breaks and for
cooking and heating. Local people engage in fishing.
The Helmand is very turbid and deposits 8 g of silt for each litre
of water (Fisher, 1968). The sediment load in 1975-1976 was 15,149,000
t and in 1985-1986 280,000 t (Mansoori, 1994). Drinking water looks
like milk! (personal observations, 1977). Rain accounts for little input to the lake, the annual mean
precipitation over 12 years being only 51 mm, most rain falling within
10-15 days (Mansoori, 1994). UNEP (2003) reports evidence of pesticide pollution
in the Helmand and the swamps, e.g. dieldrin.
The lake bottom in Iran is clay and silt and the waters are
markedly alkaline. Water at the edges of the reed swamp were 31°C
in early May, warmer than the inflowing rivers and the irrigation
ditches which were only 22°C at this
time. Annandale (1919a) and Mansoori (1994) give a brief chemistry of
Sistan water. There are marked variations in conductivity,
temperature, pH, oxygen, alkalinity and hardness between sites.
Conductivity ranges from 1280 to 64,000 mmhos (sic), pH from
7.5 to 9.15, oxygen from 0.64 to 11 mg/l, alkalinity from 3.6 to 165
mval and hardness (CaCO3) 180 to 3500 mg/l in Mansoori's
water samples from the Hamun Lake.
Evaporation lowers the water level each year and is caused by
extreme heat and the famous Bad-e Sad-o Bist Ruz (Wind of 120 Days)
which approaches 200 km per hour. This wind causes serious erosion and
marching sand dunes often block streams causing them to change
channel. Evaporation has been measured at 4 m per year because of
temperatures over 40°C in July (Mansoori,
1994). Refilling occurs in February-June and in flood years various
hamuns are joined together into one vast lake. 75% of flooding occurs
in March-May. There are about 3900 sq km of seasonal lake and marsh at
a maximum, dropping to 1930 sq km in July-January. The maximum flood
zone is about 200 km long and 20 km wide, but the lakes have dried up
completely, or almost so, at least 5 times in the past 100 years, e.g.
in 1907, 1962 for 5 years, 1970-1971, 1984 for 4 years, 1988-1989, and
1998-2002, with major fish kills resulting (Tate, 1910; Harrington, 1976; Costantini
and Tosi, 1978; Anonymous, 1992a; Khan et al., 1992; MacFarquahar, 2001;
Foltz, 2002; Weier, 2002; www.netiran.com, downloaded 18
June 2002). There was a big flood in March 1989, spring 1990 and an exceptional flood in
February/March 1991 (Khan et al., 1992). The lakes filled in 2005 (E.
Penning, pers. comm., 28 July 2005). Mansoori (1994)
mentions historical floods, e.g. in 1247 A.D., and droughts, e.g. in 835 A.D.
UNEP (2003) gives satellite photographs showing variations in water extent. The
fish fauna can recolonise newly-flooded marsh areas from the Helmand but
population numbers in the hamun vary greatly between years.
The centre of the hamun is only about 2-3 m deep on average with a
maximum depth of 5 m at highest water level (www.bibliothecapersica.com/articlenavigation/index.html,
under hamun, downloaded 24 December 2004, gives 11 m). Overflow spills into the
salt flat Gowd-e Zereh of Afghanistan through the Shelah River. This
flushing effect probably prevents this endorheic basin from becoming
saline. The Shelah was reduced to isolated and fishless pools in May 1977. The
Gowd-e Zereh is at 467 m at its lowest point.
Extensive canals and ditches form a network over Iranian Sistan and
serve to irrigate and drain fields. These waters contain fish, but may
dry up. The Hirmand is dammed to feed the major canals. The open lake
areas are fringed by reed beds comprised of Typha, Phragmites
and Scirpus which are concentrated at the ends of the detrital
cones of the river deltas (Costantini and Tosi, 1978). Mansoori (1994)
and Ibrahimzadeh (1995) report an absence of Phragmites in area
which was two-thirds covered in previous studies, drought being
advanced as the causative agent along with cattle grazing (Khan et
al., 1992). Usually the reeds recover after drought but in 1991
this did not happen (probably the effects of introduced Ctenopharyngodon
idella on the young shoots since fenced areas excluding fish show
successful reed growth). Two million fish were introduced in early
January 1992 near Kuh-e Khvajeh. Scott (1995) also suggests that local
people may have dug up tubers to use as fuel. A major fish and bird
kill occurred in November 1994 but the cause was never ascertained (Scott, 1995).
Agricultural land around the Sistan lakes is being abandoned
because of increasing soil salinity. Wind-blown salt is becoming a
problem in summer and the area might suffer the same fate as the Aral
Sea (Scott, 1995). A new road running between the Sabari and Helmand
lakes in the Ramsar Site may impede water flow despite bridges having
been constructed. A canal between Puzak and Sabari will also have
major hydrological impacts.
Curiously, both the open lake and the reed beds are poor in fish
but channels among the reeds and areas at the edge of reed beds are
productive. The effluents of the Helmand are particularly productive
and provide a refuge for fish if the lakes dry out. Annandale in
Annandale and Hora (1921) gives an interesting account of the
fisheries of the Sistan lakes in the early years of the 20th century.
Only one species, Schizothorax zarudnyi, was pursued (q.v.)
using reed boats or skiffs called tutin which were still in
evidence in the 1970s. The introduction of exotic species resulted in
an increased fish catch in the 1980s and 1990s and the number of active
fishermen was 1090 (Abzeeyan, Tehran 5(5):III, 1994, M. H. Karim Koshteh,
in litt., 2003). However, Ibrahimzadeh (1995) reports that there is no fish catch in the lake.
Local people took more fish as the population increased (4% per
annum, with added impact from Afghani refugees), as transport facilities
improved and as animal husbandry decreased through degradation of reed beds (M.
H. Karim Koshteh, in litt., 2003). The Islamic Republic
News Agency (IRNA, 22 March 2000) reports a catch of 7000
tonnes from the Hamun Lake; the following figures are from M. H. Karim Koshteh (in
litt., 2003):-
Sistan has fish farming in various water bodies. In 2005, 1.3 million juveniles
of grass carp, common carp, bighead and silver carp produced by the Zahak
hatchery were stocked in farms (www.iranfisheries.net, downloaded 17 January
2005; CIRSPE, 2006b). Goldfish and silver carp are exotics found in the hamuns (E. Penning,
pers. comm., 28 July 2005). CIRSPE (2006a) also lists Rutilus frisii, Abramis
brama and Sander lucioperca, all Caspian Sea basin species, as being
present in Sistan but this may be an error. Gilkolaei (2007) discusses breeding
of Schizothorax zarudnyi, culture of Ctenopharyngodon idella,
Hypophthalmichthys molitrix and Oncorhynchus mykiss and ornamental
fish breeding in this basin.
Berg (1940) places this basin in his Sistan District of the Iranian
Province. It excludes the upper reaches of the Hirmand River. The
schizothoracine fauna is particularly characteristic and had its
origins either by descent from higher altitudes during the Pleistocene
glaciations (favoured by Berg) or are autochthonous as the forms at
high altitudes in the Pamirs and Himalayas rose with mountain
building.
The Tedzhen River is the more familiar, international name and is used here. In Iran this major river is known as the Harirud or Hari
River. The Tedzhen rises in the Selseleh-ye Kuh-e Baba of Afghanistan
and flows west for about 490 km before turning north as the
Iran-Afghanistan border for 160 km. Along with the Hirmand and Aras,
this is the only major river entering Iran. At Sarakhs (36°32'N,
61°11'E) it enters Turkmenistan and is
known there as the Tedzhen, and is eventually lost in the Karakum
desert. The river is usually dry even at Sarakhs (Barthold, 1984).
Most of the water in the Tedzhen remains in Afghanistan where it is
used for irrigation of the Herat valley. Spring floods (March-April)
can increase flow ten-fold for short periods of time. The Jam River is
a southern tributary from Iran, draining the mountains around Torbat-e
Jam (35°14'N, 60°36'E) and the Kashaf River is a northern Iranian tributary draining past
Mashhad from the northern slopes of the Kuh-e Binalud (3416 m at 36°30'N,
58°55'E) and the southern slopes of the
Kuh-e Hazar Masjed (3146 m at 36°52'N, 59°26'E).
The Kashaf is about 310 km long. Its discharge is comparable to, if not
as great as, central Zagros streams and is larger than the plethora of
minor streams draining the Alborz (Oberlander, 1968b). The upper
reaches of the Kashaf approach those of the Atrak River, a Caspian Sea
tributary, and are separated by only a small upfold. This area is very
unstable with frequent earthquakes. The catchment area for the Tedzhen
basin approaches 45,000 sq km (Pirnia, 1951).
Bazangan Lake between Mashhad and Serakhs (36°17'N, 60°29'E) is the largest
natural lake in northeast Iran with an area of 690,000 sq m and a maximum
depth of 6-11 m. It is hyposaline oligotrophic with low phyto- and zooplankton
communities, and with a corresponding low diversity of fishes (Ghassemzadeh, 2004).
A number of minor streams drain northward from the Koppeh Dagh (=
Kopet Dagh or Kopetdag) in the west, a range which straddles the
border of Iran and Turkmenistan in this north-eastern part of Iran,
and from the Hazar Masjed and intervening ranges in the east. These
have not been collected. The Iranian tributaries of the Tedzhen have
not been well collected either, but there is data on the fish fauna
from both Afghanistan and the former U.S.S.R. (now Turkmenistan). Coad
(1981d) lists fishes from Afghanistan, and Aliev et al. (1987; 1988), Starostin (1992) and Salnikov (1994) fishes from Turkmenistan.
Aliev et al. (1987) list rare and endangered species in Turkmenistan.
There is evidently a strong possibility of exotic species from
Turkmenistan entering Iranian waters via the Tedzhen drainage. Fishes,
including exotics, are farmed along the basin of the Karakum Canal, a
1372 km long diversion from the Amu Darya. Some of these exotics can
be expected to enter the Tedzhen River basin via its delta and
eventually the Caspian Sea basin via the Atrek River through runoff
and collector canals (Sal'nikov, 1995; 1998). Potential exotics for
Iran from the Karakum Canal include Pseudoscaphirhyncus kaufmanni
(Acipenseridae), Alburnoides taeniatus, Aristichthys nobilis
(= Hypophthalmichthys nobilis), Aspiolucius esocinus, Aspius
aspius iblioides, Barbus (= Luciobarbus) capito conocephalus, Capoetobrama
kuschakewitschi, Carassius auratus gibelio, Chalcalburnus (= Alburnus)
chalcoides aralensis, Ctenopharyngodon idella, Hemiculter
eigenmanni (= leucisculus), Hypophthalmichthys molitrix,
Mylopharyngodon piceus, Parabramis pekinensis, Pseudogobio
rivularis, Pseudorasbora parva, Rhodeus ocellatus, Rutilus
rutilus aralensis (all Cyprinidae), Cobitis aurata aralensis, Misgurnus
anguillicaudatus (Cobitidae), Barbatula oxiana (Nemacheilidae),
Gambusia holbrooki (Poeciliidae), Oryzias latipes (Oryziatidae),
Channa argus warpachowskii (Channidae), Micropercops cinctus
(Odontobutidae), and Rhinogobius brunneus or Rhinogobius similis (Gobiidae). A Rhinogobius species is
now found in Iran (Coad and Abdoli, 2000a; Abdoli et al., 2000). Other species not native to
the Tedzhen basin but found elsewhere in Iran are also reported such
as Acipenser nudiventris (Acipenseridae), Pelecus cultratus
(Cyprinidae), and Sander lucioperca (Percidae). Cyprinus
carpio stocks are a mix of native and Chinese imports. Silurus
glanis (Siluridae) has also been introduced along with carp from
the Amu Darya although it is also native. Sal'nikov (1995; 1998) also
lists other species which may penetrate the canal eventually. These
exotics have a great potential to cause devastation in the native
fauna through competition and through genetic swamping of related taxa.
The fauna of the Tedzhen basin is found in rivers and streams as
well as springs and qanats. Dams include the Barzou, 40 km north of
Shirvan, which is 85 m high with a crest of 325 m and the Shirnin
Darreh north of Bojnurd which produces 60 million cu m of water for
irrigation (Iran News, 17 September 1997). A dam is scheduled
for completion in 2005 at the Iran-Turkmenistan border. It will have a
capacity of 1,250 million cu m of water (IRNA, 3 September 1999).
Berg (1940) places this basin as a part of his Turkmen District of
the Iranian Province (other parts include the Murgab River of
Afghanistan and Turkmenistan and northslope streams of the Kopet Dagh
in Iran and Turkmenistan). He considers that the Hari River once
belonged to the Amu Darya basin of Central Asia.
The Kerman-Na'in basin extends from Ardestan (33°22'N, 52°23'E) in the north-west to Kerman (30°17'N,
57°05'E) in the south-east. It is an elongate series of small basins combined here for convenience and
named for two major towns at the ends of the basin. Its length exceeds 600 km and its maximum width is 175
km. An almost continuous range of mountains, paralleling the Zagros,
flanks this basin on the west, while the eastern edge is lower and
abuts the Dasht-e Kavir and Dasht-e Lut basins, particularly in the north-east. The
Kerman-Na'in basin lies at a similar altitude to the other interior basins, ca. 1000 m.
In the south-east, streams drain the mountains ringing Kerman, such
as the Kuh-e Hazaran at 4420 m (29°30'N, 57°18'E), the Kuhpayeh at 3142 m (30°35'N,
57°15'E), and the Kuh-e Masahim at 3600 m (30°21'N, 55°20'E),
to a sump just west of Bafq (31°35'N, 55°24'E).
These streams bear names such as Namak and Shur and may well be
inhospitable to fishes. Several streams between Kerman and Yazd marked
prominently on maps were dry in January. Irrigation requirements may
have reduced their flow and most of the fishes from this area are to
be found in qanats. Qanats have temperatures in this region of 17-21°C
in January and have been studied in one village by Smith (1953; 1979).
Around Yazd streams drain the Shir Kuh at 4074 m (31°37'N,
54°04'E) and the Khar Kuh at 3512 m (31°39'N, 53°46'E) but there is no major terminal
sump. Some of the streams enter the Bafq sump while others drain north to a sump near Na'in (32°52'N, 53°05'E)
which also receives intermittent streams from around Na'in.
Intermittent streams from the Kuh-e Karkas at 3899 m (33°27'N, 51°48'E) drain to a sump near Ardestan
but, as in the southern parts of this basin, are not a prominent
feature of the landscape and fishes are mostly to be caught in qanats.
The underground water resources of Yazd Province have been examined
in a newspaper article (Hamshahri, Tehran, 629:5, 22 February
1995) and, although the province is not the same area as the drainage
basin outlined here, it is indicative of the underground water
resources of this part of Iran. These resources comprise 1751
subterranean water canals (probably this means qanats), 2084 semi-deep
wells and 897 deep wells with an annual discharge of 1100 million cu m
of underground water. The authorised capacity is 893 million cu m and
the excess removal has resulted in an annual drop in the water table
of 70 cm. In addition, chemical and biological pollution of
groundwater is a continuing problem and these factors too will affect fish survival.
Much of the fish fauna of the Kerman-Na'in basin appears to be restricted
to qanats, although there may be a fauna in high mountain streams not
readily accessible by road.
The common names of fishes vary with language between countries and within a
country with local usage. This problem is overcome to the scientists' satisfaction by the scientific name, consisting
of two words, the genus name and the specific or trivial name. A genus, e.g.
Luciobarbus, may contain
many species but each species is a unique combination of Luciobarbus and a
specific or trivial name. This scientific name is used the world over whatever the local common name may be. It is always written in Latin
script and the genus and trivial names are derived from and spelt according to
rules of grammar in Latin and Classical Greek. Both these languages are "dead" so the rules and spelling are
fixed and not subject to change with time as modern languages are.
It is generally felt that the advantages of this system outweigh the unfamiliarity of Latin and Greek words and grammar for most people.
As an example of the scientific name, we can consider the first species dealt with
in this work, the Caspian lamprey or Volga lamprey Caspiomyzon wagneri
(Kessler, 1870). The scientific name is underlined or set in italics or bold face to denote its scientific status. Caspiomyzon is the genus name and wagneri
the trivial or specific name. This species was first described by Kessler in a publication dated 1870. Kessler placed this new species in the genus
Petromyzon but L. S. Berg later published reasons for placing it in a
distinct genus, Caspiomyzon. The parentheses around the author (or first describer of the new species) and the
date of description indicate that its generic allocation has been changed.
Sometimes the author of a work (paper or book) is not the describer of the
new species, e.g. the multi-volume work by G. Cuvier and A. Valenciennes (1828-1849, Histoire naturelle des poissons,
22 volumes - see above) continued to appear after Cuvier died. For many years, the species
author appeared in taxonomic works as "Valenciennes in Cuvier and Valenciennes"
to indicate that Valenciennes described the species but the description appeared
in a volume of the book on whose title page both Cuvier and Valenciennes
appeared as authors. Bailey (1951) determined who authored which species in this
case. The trend now is to cite simply a single name, Valenciennes in this
example, and this is seen in "FishBase" and "Catalog of Fishes".
Another
example of a confusing author name involves Francis Buchanan (see above for
details of fishes described by him). His name also appears as Hamilton or
Hamilton-Buchanan or Buchanan-Hamilton - the name Hamilton was assumed on
succeeding to property in Scotland from his mother, formerly a Miss Hamilton.
The scientific name is also used to show relationships between species and it can
therefore be changed if views on the relationships of the
species are changed according to the "International Code of Zoological
Nomenclature". The Fourth Edition of the Code came into effect on 1 January
2000. Errors also arise in giving species scientific names and these must be corrected by name changes
according to the Code. The Code is complicated and detailed explanations based on fishes may be found in Eschmeyer (1998; this
Catalog of Fishes is now online). Some of the more common reasons for name changes are given below.
A single species may be described twice, either by the same person or by two
people. At the time of these descriptions it was genuinely believed that there were two species but
subsequent studies showed that they were the same. This error often arises with confusion between juveniles and
adults and between males and females which may be quite different in
appearance. Older collections from remote areas often comprised only a few specimens and could be in rather poor
condition by the time they came into the hands of an ichthyologist and were
described scientifically. An example of confusion of males and females of the same species is found in the genus Aphanius. In 1910, J. T. Jenkins described three
species of Aphanius (under the genus Cyprinodon as it was
recognised then) from near Shiraz. This material had been collected in 1872 by W. T. Blanford and was comprised of 10
specimens, mostly in good condition. The three species were Cyprinodon blanfordi, C. persicus and C.
pluristriatus. The first of these was thought by Berg (1949) to be a female Aphanius sophiae and the
latter two to be males differing in characters not now considered to be
specifically important. I have a differing opinion! It is also possible,
where two people are concerned, that the author who published his description later
was ignorant of the first author's work. The first name published has priority and the second name is called a
synonym and is no longer used. There may be several synonyms for a species. These are listed in the species descriptions. There is also the problem
of misidentification of specimens. When these specimens are available for study
identification can be confirmed (or amended) but often specimens are discarded
or lost. These errors too may be listed in a synonymy. Krupp (1984a) gives a
synonymy for Aphanius cypris which amply illustrates how a scientific
name may be mis-applied (there are 89 uses of names which all refer to one
species in Krupp's opinion). A. cypris is now thought to be correctly named A. mento.
Occasionally the same name is given to two distinct species because the later author was not
aware that the name had already been used. The name of the species described first is called a senior homonym and
is retained while the later species name, the junior homonym, must by replaced.
The genus name of a species can be changed because an ichthyologist, who has
studied the species and its relatives in detail, considers that it is more
closely related to another species or group of species with a distinct genus
name. A similar case was discussed above with Caspiomyzon wagneri where a new genus was erected for this
species. In both cases, parentheses are placed around the author's name and the date of description to indicate that
the genus name used has been changed. The species placed in a different genus will retain its trivial or
species name unless this trivial name is already in use in the different
genus. Homonymy has then occurred and the species which has priority retains its trivial name and a replacement name
must be given to the more recently described species. It is not unusual for scientists to disagree about the
interpretation of the same data and a species may have a long and complex
career being switched from genus to genus as publications advocate one view or another of its relationships.
There is a higher classification which groups together related genera into Families,
Families into Orders and Orders into Classes. The vast majority of Iranian freshwater fishes belong to the Class
Actinopterygii, the ray-finned bony fishes, with only the Caspian lamprey in
the Class Cephalaspidomorphi. Some sharks penetrate freshwater and these belong to a third Class, Chondrichthyes
or cartilaginous fishes.
A knowledge of fish anatomy is essential in identifying
specimens. The head of a fish carries a number of structures. The eyes
are without eyelids although sharks have a protective membrane, the nictitating
membrane, which acts as an eyelid. Eye size varies with age within a species
but can also be a distinguishing characteristic between species. There
are nostrils, for detecting odours, on the snout, that part of the head
before the eyes. Nostrils are blind sacs and do not connect with the mouth
cavity. Their position and shape may be useful characters. Barbels are
slender, fleshy structures on the snout or chin used for touch and taste.
Their presence, number, position and length are important characters. Sharks
and sturgeons have a small opening near the eye called the spiracle, not
found in the bony fishes. Teeth may be found variously on the tongue, roof
and floor of the mouth and even in the throat. The pharyngeal teeth of
Cyprinidae are often useful characters in identification and may be dissected
out from the posterior part of the gill cavity under the operculum using
dissecting equipment. This requires some practice to avoid damaging the
specimen too extensively. Some teeth are sharp and pointed for piercing
and holding prey, while others are rounded and heavy for crushing food
items covered by a protective shell. The side of the head behind the eye
is the gill cover in bony fishes, composed mainly of one bone, the opercle,
which protects the gills. The gill cover opens posteriorly; bony fishes
have one opening on each side of the head, but lampreys have seven rounded
openings and sharks five to seven vertical slits. The cheek is the area
between the gill cover and the eye. Spines and scales may be found at various
places on the gill cover and cheek. A membrane is found below the gill
cover, supported by thin slivers of bone, the branchiostegals, and connected
with the gill cover on the other side of the head. Under the gill cover
lie the gills which serve in gaseous exchange. Gill rakers on the front
of each gill arch serve to prevent food from damaging the gills and direct
food into the gut. Rakers may be short and widely spaced where food items
are large and easily deflected, or long and close together where food items
are minute like plankton.
The head leads directly to the body; there is no neck.
The body is made up mostly of a trunk. The caudal peduncle or tail stem
starts behind the anal fin and ends at the tail fin. The number and presence
of different types of fins on the body varies with the species of fish
and is often a useful character for identification. The back may carry
1-3 dorsal fins and an adipose fin between the last dorsal fin and the
tail fin. The tail (or caudal) fin is at the end of the body and may be
forked, square cut, rounded, pointed, lanceolate or lunate. Its skeletal
structure may be almost symmetrical or upturned at the end. This upturn
is obvious in sharks and sturgeons which also have a large upper lobe to
the tail fin and a smaller lower lobe. The anal fin, or fins, lies on the
underside of the body surface behind the vent which is the exit for the
intestine, kidney ducts and gonads. The pectoral fins are found behind
the gill cover on each side of the body and a pair of pelvic fins are behind
(abdominal), below (thoracic), or in front (jugular) of the pectorals on
the lower body surface. An axillary pelvic scale above the pelvic fin streamlines
the fin when it is pressed against the body. The pectoral fin may also
have an axillary scale. All the fins except the fleshy adipose fin are
supported by rays. Soft rays are flexible and jointed while spines are
rigid, pointed and unjointed. The number of soft rays and spines in the
various fins is very useful for identification.
Most fishes have a body covering of scales which may extend
onto the head and certain fins. Notable exceptions are the catfish families
Bagridae, Siluridae, Sisoridae and Heteropneustidae, which are completely
naked. Rounded, smooth scales are called cycloid and are found in less
advanced bony fishes. Large cycloid scales may easily detach, as in herrings
(Clupeidae) but small cycloid scales can be embedded and hard to see as
in the eel (Anguillidae). Ctenoid scales bear small teeth on the posterior
margin and feel rough to the touch. Such scales are found in the more advanced
bony fishes such as Percidae. Sturgeons have heavy bony plates called scutes.
Sharks have placoid scales which can be so rough as to scrape the skin
off a human. The teeth of sharks are modified placoid scales. Scales grow
with the fish, laying down rings of material as do trees. In areas with
a change of seasons, the growth rings are widely spaced during the summer
growing season and cramped together in winter when growth is slow. Fish
age can be determined from these rings. The energy expended in spawning
is reflected in the scales which may resorb the edge producing a spawning
check. A fish which lives and grows slowly in fresh water and then migrates
to the rich feeding grounds of the sea will have this history reflected
in the spacing of the growth rings. Scales can be "read" to reveal much
about the life history of an individual fish. The scales also bear radii,
or radiating lines, and their distribution can be useful in identification
along with other scale characters such as shape and focus (growth origin)
position. The scales are covered by an almost undetectable layer of skin.
The skin contains mucus cells which give the fish a slippery feel and colour
cells which give the fish its colour. Some fish are characteristically
more slimy than others, e.g. the eel. Most fish have a distinctive colour
pattern but this can change with age, maturity, behaviour, background,
between sexes, and after death.
Fishes have a sensory lateral line system which runs along
the flank and a similar system on the head. The extent and development
of these systems varies with the species of fish. The lateral line is a
tube in the skin with openings to the outside through pores in the scales.
A lateral line pore count is often used in separating fish species.
The internal structure of a fish may be summarised as
follows. The gills and teeth have already been mentioned. After these structures,
the mouth cavity narrows to an oesophagus which passes to a straight, U-
or J-shaped stomach. Pyloric caeca, which produce enzymes, may be attached
at the junction of the stomach and intestine in some fishes and counts
of these caeca are used in identification of some species. The intestine
ends at the vent. The length of the intestine varies with the diet. Fishes
which feed on plant material have long guts while those that feed on animals
have a short, often s-shaped intestine. Fish have a liver, a reddish organ
at the front of the body cavity. The liver may be very large in sharks
and form a significant part of the body weight. There may be a small, green
gall-bladder associated with the liver. The swimbladder (gasbladder) is
a gas-filled sac with thin walls lying near the top of the body cavity
where it functions as a buoyancy organ and can be used to transmit sounds
to the brain or even produce sounds by means of special drumming muscles.
The swimbladder shape has been used to characterize species. Some fishes
have a poorly developed swimbladder or none at all, since they live on
the bottom of stream beds and must avoid being swept away. Just below the
backbone above the swimbladder are two long, dark-coloured kidneys and
below these are the ovaries, which may be filled with eggs, or the testes
which produce the sperm. A small urinary bladder
lies at the end of the body cavity. The body cavity is lined with a membrane
which may vary in colour from silvery-white to jet black. The main body
muscles are in the form of W-shaped, interlocking blocks and this arrangement
helps produce the sinuous body movements by which fish swim.
Lampreys (Petromyzontidae) differ in structure from bony
fishes. They lack true jaws and have a round, suctorial mouth armed with
teeth. There is a single nostril on top of the head rather than a pair
on each side. There are no scales or paired fins (pectorals and pelvics).
There are seven rounded gill openings in a row behind each eye. The larval
lamprey is called an ammocoete and lives buried in fine sediments, filter
feeding minute particles from the water. In this stage it lacks teeth and
the eye is poorly developed.
Sharks also have a somewhat different structure from bony
fishes. Some species produce living young rather than eggs, while in others
the embryo is laid in a horny egg-case known as a mermaid's purse when
it washes up on a beach. Male sharks have claspers derived from the pelvic
fins, which serve to ensure that sperm are delivered to the female. The
length of time food stays in the gut of sharks, and also sturgeons, is
increased by a spiral valve. The food follows the spiral around rather
than going straight through the gut and so there is more time for digestion
and absorption. There is no swimbladder in sharks, which have to swim constantly
to stay above the bottom. Sharks produce teeth in multiple rows, and as
older teeth at the front of the jaw fall out, new ones move forward to
replace them.
The skeleton includes the skull comprising the cranium, which contains the brain, the jaws, gill
arches, operculum and other associated bones. The cranium also contains
small objects known as otoliths in the inner ear. These aid in sensing
change of direction and in balance. Otoliths can be characteristic of species.
There is a vertebral column with ribs anteriorly enclosing and protecting
the body cavity and its contents. The number of vertebrae is a useful character
and can be counted easily, without damage to the fish, by taking x-rays.
A tail skeleton supports the tail fin and the pectoral and pelvic girdles
support their respective fins. There are fin supports too for the dorsal
and anal fins. Lampreys, sharks and sturgeons have a skeleton composed
of cartilage, a substance not as strong as bone, but when impregnated with
salts (like shark teeth) are remarkably effective.
Most characters used for fish identification are external
for convenience. The most used internal characters are gill raker counts,
pharyngeal teeth counts, gut shape and body cavity lining colour, pyloric
caeca counts and vertebral counts.
The general structure and biology of fishes is covered
in various general works. Coad (1993; 1995b) gives a list of general ichthyology
texts and the Dictionary of Ichthyology
describes various anatomical terms.
Collecting methods and literature are summarised by Coad
(1993; 1995b). Luck plays a part even in scientific collecting as discovery
of new species in areas previously sampled demonstrates (e.g. P. G. Bianco
did not collect Petroleuciscus persidis in Fars, while I found several
populations of that previously unknown species; conversely he found several
populations of Cobitis linea, previously known only from badly damaged
type specimens, while I found none!). Repeated visits to areas already
sampled may prove rewarding.
It is essential that a collector obtain the necessary
licences for scientific purposes. The Department of the Environment, Tehran,
issues licences for set periods and areas. There are about 70 National
Parks, National Nature Monuments, Wildlife Refuges and Protected Areas
or Regions where special licences to collect in these biotic reserves are
only issued if there is no threat to endangered species. The Chalus River,
Sardab River, Karaj River, Jajrud, Lar-Haraz River and all marshes, wetlands,
waterways, deltas and bays along the Caspian Sea coast, and all rivers
of Gilan and Mazandaran provinces that enter those waters are protected
rivers and wetlands. Penalties for unlawful fishing range up to a year
in prison and fines of 50,000 rials. Caspian salmon, cave fishes and trout
are protected specifically and by additional fines (Anonymous, 1977-1978).
Captured fishes which cannot be identified or seem unusual
enough to warrant further attention should be preserved. Labeled, preserved
specimens deposited in a museum are a permanent record of species identity
and distribution. Some taxa present problems of identification even for
experts so that misidentifications are often a nuisance if there is no
material to examine.
There is a developing aquarium industry in Iran that imports fishes from
Singapore and Malaysia. There is a potential for exotics to become
established as there are no controls or statistics are not kept (Tehran Times,
28 July 2001). Various exotics are now established through the aquaculture
industry (Coad and Abdoli, 1993b). Samples from ecological or experimental studies as
well as systematic and distributional works may be preserved and sent to
a museum where their identity can be confirmed and where they are available
to workers in the future. The National Museum of Natural History, Tehran
(Muze-ye Melli-ye Tarikh-e Tabi'i or MMTT) has a small collection of Iranian fishes
but it is not extensive enough for systematic studies. Major museums in
a number of countries welcome exotic material to enhance the variety of
their collections. Acronyms for museum collections can be found in Sabaj Pérez
(2010).
Specimens should be preserved whole, without removal of
the guts or gills so that no key characters are lost. Specimens may be
frozen, or even salted, but the best method and the one used by scientists
is to drop fish into 1 part full-strength formalin to kill the fish quickly
and then immediately add 9 parts of water to form a 10% preserving solution.
Large specimens (larger than about 15 cm) should have a small slit made
in the right side of the belly to allow formalin to penetrate the tissues.
Ichthyologists cut the right side of the fish and leave the left side undamaged
for illustration and scale counting. Hypodermic syringes are used to inject
the abdominal cavity and muscle blocks of very large fish with formalin,
otherwise the preservative will not penetrate all the tissues before decay
sets in. This is especially important in a hot climate like that of Iran.
Syringes should have a capacity of up to 100 ml and be capable of taking
needles of various sizes. Particular care should be taken when injecting
formalin into tissues; the needle should be withdrawn gradually while injecting
the formalin solution to avoid a sudden spurt of liquid under pressure from the injection site.
Wherever possible some specimens should be preserved in 95+% ethanol or other
appropriate solutions for potential molecular studies. Modern DNA techniques may be the only way to
resolve some systematic problems as morphology has proved inadequate.
Formalin should be handled with care as it is a noxious
chemical which irritates the eyes and nose and is painful in skin cuts.
It may be carcinogenic and repeated exposure can trigger allergic reactions
in the skin. Gloves and safety glasses are useful when diluting full-strength
formalin. It should only be handled in well-ventilated rooms or in the
open air. In the field, care should be exercised in packing specimens for
transport so that leakages do not occur. Long-term preservation in formalin
is not advisable as the solution becomes acidic and rots the fish. It also
wrinkles and hardens the specimens.
Most museums store their specimens in alcohol for the
long term. The formalin-fixed specimens are washed briefly in water and
then transferred to 45% iso-propyl alcohol or 70% ethanol. These chemicals
are pleasanter to work with. Some care should be taken such that specimens
are not twisted and bent inside the preserving container. It is difficult
to make counts and measurements necessary for identification on badly deformed
specimens. Each specimen or group of specimens should have at least an
equal volume of preservative as water in the fish tissues tends to dilute
the preserving fluid. Specimens may be stepped through 30%, 50% and 70%
alcohol solutions to reduce wrinkling and ensure a fuller penetration of
alcohol into tissues and a final storage solution of at least 70% ethanol.
Ethanol may be difficult to obtain in Islamic countries and undrinkable
iso-propyl alcohol can be substituted.
The best containers for long-term storage are made of
glass with tightly-sealing polypropylene lids. Plastic containers deteriorate
with time and tend to crack. Metal containers and metal lids eventually
rust. In the field, large plastic buckets with tightly-sealed lids are
less likely to break than glass containers and are not as heavy. Very large
fish may require sone sort of drum, such as a clean oil drum but it should
be noted that formalin corrodes metal and the drums should be lined with
plastic or lacquered. Fluid levels in the collection should be checked
regularly and alcohol concentrations maintained at the recommended values
or the specimens will deteriorate. Collections should be kept in the dark
to reduce fading of pigments and at a constant, cool temperature.
Fish which have been preserved for a week in formalin,
more for larger fishes, or transferred to alcohol can be sent to a museum
for identification. Glass containers full of formalin or alcohol should
not be mailed because of the danger of breakage. The fish should be wrapped
in cheesecloth or some other absorbent packaging, with its label, the cheesecloth
dampened with preservative, and tightly sealed in several, leak-proof plastic
bags before being placed in a padded box for mailing. Spiny fish should
be especially well wrapped to avoid puncturing the plastic bags. A tightly-sealed
package retains the preservative which keeps the fish in good condition.
The box may be labelled "Scientific specimens, no commercial value".
The label is as important as the fish itself. An interesting
specimen is of little or no scientific value if there is no locality data.
Labels should be written at the time of capture. Faulty memory and good
intentions to label specimens later make a poor combination and often result
in collections with no data, or worse with incorrect data. The label should
bear the place of capture, such as a stream, lake, spring, qanat, etc.,
including a reference to the nearest town (local names may not be on maps
or in gazetteers and some village names are very common, e.g. Hoseynabad,
of which there are over 170 in Iran!), latitude and longitude, province,
date, name of collector, notes on the habitat and live colour of the specimens,
and any other items likely to be useful. Colour photographs of fresh fish
are most useful, especially if the fins are pinned erect. Pencil or India
ink should be used on stout, waterproof paper which will not disintegrate
in liquid. The label must be dropped in the jar with the fish. Labels on
the outside of jars always fall off and lids with labels always
get put on the wrong jar!
In fact the amount of information which should be usefully
recorded cannot be put on a small label. Instead extensive field sheets
are used and related to the specimen or sample by a field number. The Canadian
Museum of Nature, Ottawa (formerly National Museum of Natural Sciences) has field sheets with over 70 categories which
can, potentially, be filled in and some categories have as many as 30 alternatives,
e.g. Category 17, Environment includes fresh spring, cave, canal, stream/river,
river-lake junction, flooded area, fresh pool, pond, lake, marsh (treeless),
swamp (with trees), reservoir, ditch, etc. (see below). As an insurance against loss
of field sheets or confusion of numbers, the jar label should carry minimal
locality data as well as the field number.
Glossaries
1. Geographical Glossary
The following glossary of geographical and other terms is mostly in Farsi (which includes words
taken from Turkic, Kurdish and Arabic) with a few Russian words, all
of which appear on maps, in the literature and in the text of the
"Freshwater Fishes of Iran" web site. They may be of help to those
unfamiliar with these languages and avoid tautologies such as Safidrud River.
There are various diacritical marking systems for
Farsi but these do not always transfer accurately across platforms,
appearing as strange symbols or gaps in text. I
have eschewed the use of these as being irrelevant for native speakers
of Farsi and too confusing for those unfamiliar with this language.
ab = water, intermittent stream, stream, spring, lake, well
ab-bandan = shallow, freshwater reservoir on the Caspian plain used for duck hunting in
winter and water storage for irrigation in the dry summer
abad = a suffix indicating an inhabited place
ab-e garm = hot spring
ab ambar = cistern
ab anbar = cistern
abshur rud = salt river, common name of salty rivers
anbar = tank
ateshkade = fire-temple (archaeological feature)
av = stream
`ayn = spring
bagh = garden
bahr = sea
Bahr-e Khazar = Caspian Sea
baksh= municipality
band = dam, reservoir, lake, mountain range (old dams for water storage - see sadd for modern dams)
bandar = port, harbour, anchorage, bay
bankari = constructing temporary weirs for water diversion
bar andaz = halting place
barm = marsh, lake, pond
batlaq = marsh, swamp
berkeh = tank, pool, cistern
biaban = desert (also the name of the coastal plain south of the Minab River to
the cape of Ra's al-Kuh in Hormozgan Province)
birkat = pool, well, marsh, lake
borj = fort, tower
botlaq = marsh, swamp
caviar = sturgeon eggs as food
çay = stream
centner = 100 kg (used in Russian texts as a measure of commercial catches; sometimes given as
50 kg elsewhere but internal evidence in Russian papers indicates 100 kg is correct)
chah = well, spring, cistern
chai = stream
cham = stream, gorge
chashmeh = spring, well
chay = stream
cheng = hill, mountain, promontory
cheshmeh = spring, well
dag = mountain
dagh = mountain
dahaneh = section of a stream, gorge, pass, defile, water gap
damagheh = cape, promontory
damgah = an artificial, freshwater wetland, maintained primarily as a duck-hunting area but
also used for irrigation during the dry summer months
daqq = salt flat, salt depression, salt waste, marsh, intermittent salt lake
darband = gorge or pass
darreh = stream, valley, gorge, ravine
darya = sea, stream, intermittent stream, channel
daryacheh = lake, marshy lake, stream
Darya-ye Mazandaran = Caspian Sea
dasht = plain, desert, steppe, depression, upland, open country, field; usually dry desert
with a firm base of pebbles or silts
deh = village
dehkadeh = village
dez = fortress
echbel = eggs of fishes other than sturgeons as food
emamzadeh = tomb, shrine
eskeleh = jetty
estakhr = pool
gadik, gaduk = pass
galal = stream
gardan, gardaneh = pass
godar = pass
garmsir = hot country, winter quarters in the lowlands
ghadamgah = a religious site; usually no fishing allowed.
gharb(i) = west(ern), as in the province Azarbayjan-e Gharbi)
godar = pass
göl = lake, marsh, swamp
gölü = lake, marsh, swamp
gowd = depression
hammam = bath
hamun = marshy lake, salt waste
hawr = marsh, lake
hesar = fort, castle
hor = marsh
howr = marsh
howz = tank, cistern, pond, pool, lake, reservoir, spring
il'men = a shallow, flood-plain lake heavily overgrown with reeds and rushes (Russian)
ishan = hill
istgah = railway station
jabal = hill, mountain
jangal = forest
jar = stream
jazirat = island
jazireh = island
jebal = hill, mountain
jehil = lake
jolgeh = plain
jonub(i) = south(ern)
ju = stream, irrigation channel
jub, jube = irrigation channel, watercourse, gutter, ditch
juy = stream, watercourse
kal = stream, intermittent stream
kalleh = peak
kamar = hill, mountain, ridge
kani = well
karavansara = caravansary
karez = underground irrigation channel
kaur = stream
kavir = salt waste, salt desert, marsh; usually a salt crust over silt deposits which can be fatal mires of slimy mud (= playa)
khalij = bay, gulf
Khalij-e Fars = Persian Gulf
kharabeh = ruins
khirr = stream
khowr = inlet, stream, channel, bay, bight, tidal creek, estuary
khwar = stream
kizil ala = brown trout (see also qezelala)
kotal = mountain pass
kowr = stream
kuh = mountain, range, hill, peak, ridge, spur
kuhha = mountains, range, hills
liman = a brackish bay of the sea, usually at a river mouth, sometimes cut off from the sea but
still brackish; also an estuary (Russian, mordab in Farsi)
lut = desert
mahur = hill
mandah = stream
markazi = central, as in Markazi Province
masjed = mosque
mordab = lagoon, backwater, creek, swamp, stagnant water (literally dead water, now replaced by talab)
nahr = river, stream, canal, docking basin
naizar = reed swamp (Sistan)
namak = salt; usually a salt lake with open water or a salt crust but without much mud
namaksar = salt waste
naveh = stream
nawah = stream
nehri = stream
neizar = reed swamp in Sistan
ostan = province, governorate-general
ozero = lake
pal = hill, mountain
paskuh = mountain range
pereval = pass
poshteh = hill, mountain
qabr = tomb
qabrestan = cemetery
qal'at = fort
qal'eh = fortress
qanat = underground irrigation channel; an adit shaft
qasr = fort
qolleh = hill, mountain, peak
ramlat = sandy area
ra's = cape, point, promontory
reka = river
reshteh = mountain, mountain range, hill, spur
reshteh kuh = mountain range
rig = sand area, dunes
riz ab = stream
roga = outflow (in the Enzeli or Anzali Mordab)
rud = river, stream, intermittent stream
rudbar = valley drained by a river with flowing water; place watered by many streams
rudkhaneh = river, river bed, watercourse, intermittent stream
rusta = village, inhabited place
sabkhat = salt marsh, lake
sadd = dam, reservoir (used for modern dams)
saddi qanat = a qanat drawing water from a dam
sahel = coast, beach, shore
sar = cape
sarab = spring (in western Iran), literally "beginning water"
saray = caravansaray
sardsir = cold country, summer quarters in the highlands
sarhadd = frontier
sazhen = a marine sazhen equals 1.83 m (Russian)
selseleh = mountain range, mountains
shahr = town, city
shahrdari = municipality
shahrestan = district
shahzadeh = shrine
shamal(i) = north(ern)
sharq(i) = east(ern), as in the province Azarbayjan-e Sharqi
shatt = large river, bank of a river, stream
saydgah = fishing station, as along the Caspian coast
shebh-e jazireh = peninsula
shekasteh = hill, mountain
shil = a wooden barrier erected across a river for catching fish; hence shilat (in Gilaki, the Persian dialect of Gilan)
shilat = fisheries company; Sherkat Shilat = Northern Fisheries Company concerned with the Caspian
Sea; Shilat Jonub = Southern Fisheries Company concerned with the Persian Gulf
shur = salt, brackish, stream
shurab = salt water
shurehzar = salt stream, salt marsh
su = water, stream
suyu = stream
talab = more modern version of mordab.
tall = hill, mountain, spur
tang = pass
tangeh = valley
tappeh = hill, mountain, mound
tell = hill
tepe = hill, mound
vareh = a small dam
vilayet = province
ziarat = shrine
2. Ichthyological Glossary
Technical terms used in ichthyology and in this work are in the Dictionary
of Ichthyology and can also be accessed through the Main Page of www.briancoad.com.
Quotes
A weak fisherman caught a strong fish in his net and not being able to
retain it the fish overcame him and pulled the net from his hand.
A boy went to bring water from the torrent.
The torrent came and took the boy away.
The net brought every time a fish.
This time the fish went and carried off the net.
The other fishermen were sorry and blamed him for not being
able to retain such a fish which had fallen into his net. He replied:
'O brothers, what can be done? My day was not lucky but the fish had
yet one remaining'. Moral: A fisherman cannot catch a fish in the
Tigris without a day of luck and a fish cannot die on dry ground
without the decree of fate. ---- Story 24 from the Gulistan of Sheikh Muslih-uddin Sa'di Shirazi, 1258.
The Caspian sea is marueilous full of fish, but no kind of monstrous fish, as farre as I could vnderstand, yet hath it sundry sortes of fishes which are not
in these parts of the world. ---- Principal Navigations, Voyages, Traffiques and Discoveries of the English Nation, Richard Hakluyt, 1599.
Fresh-water Fish is not so plentiful, because there are but few Rivers in Persia, and they take abundance of Water out
of them, so that very little Fish can breed there..... There are three sorts of Fresh-water Fish in that large Empire; that of Lakes, that of
Rivers, and that of Kerises, or Subterraneous Canals. ---- Sir John Chardin, 1724.
No sea, perhaps, in the world, produces so great a quantity of fish. ---- Said of the Caspian Sea by J. M. Kinneir, 1813.
Thus a man told me that the Caspian Sea, (on the shore of which we conversed) was a Maaden-i-mahi or mine of fish. ---- Sir William Ouseley, 1819.
I may remark as a curious fact in zoology, that many of the cannauts, both here and at Shahrood, swarmed with fish, some of which were of considerable size. ----
James B. Fraser, 1825.
Of fish, in a country which possesses so few rivers, we are not to look for either abundance or variety; nor do the inhabitants make any great use of what they
have." ---- James B. Fraser, 1834.
"If you trip over a pebble on the ground, you can be sure that an Englishman put it there" (Persian saying) - as an Englishman I hope there are not
too many pebbles in this work, and
those that are were inadvertent.
Keys
Introduction
The freshwater fishes of Iran can be identified using these keys,
aided by the Species Accounts.
The keys should not be used for countries bordering Iran
which share many species but also have others not found in Iran.
All keys benefit from use and feed-back - please let me know if you encounter problems.
There are two sets of keys in this work.
There is a general key to families (although all families are recognisable at a
glance in Iran, with a little experience) and a series of keys to genera and
species. Genera keys and species keys may be separate if there are many genera
and species, or combined in a single key if there are few.
Identification keys are based on couplets, a choice between two alternatives, e.g.
1a. Mouth a large crescent; gill membranes joined
to form a free fold over the isthmus = Huso huso
1b. Mouth small and transverse; gill membranes attached to
isthmus---> 2
2a. Lower lip continuous, not split in middle;
more than 48 lateral scutes, usually 55 or more; barbels fimbriate =
Acipenser nudiventris
2b. Lower lip interrupted in the middle; less than 51 lateral scutes, usually less than 45 in Iran; barbels not fimbriate---> 3
If the fish has a large and crescentic mouth, then it is
the fil mahi, Huso huso; if not, then the user is directed to the
next couplet (2), and so on.
Ideally each couplet has a series of characters which reinforce each other
and allow for any loss or damage to characters. Additionally, some characters
are "key" but difficult to interpret without experience or are internal and
require dissection which is not always possible. In some cases, only one character is available since it must
encompass all included species below that point in the key. Since some species
are difficult to identify, additional characters are given in brackets [.....].
These additional characters are not unique to the species but, in combination,
help to identify the species. Definitions of characters are given in the
Dictionary of Ichthyology.
If used properly, a key is more accurate and less time
consuming than flicking through pages of text. The disadvantage of keys is that
the alternative state in each couplet is not at hand if you only have one fish
to examine, and a simple error can lead you widely astray. Some recognised
species have overlapping counts for obvious meristic characters, although means
and modes are significantly different, and differ in other, subtler ways not
readily summarised in a key. Ideally a student of fishes should collect a series
of individuals of different sizes and sexes from each locality, wherever
conservation demands and practicality permits. A series of about 30-40 specimens
allows for character variations dependent on sex and size, and on abnormalities,
and also allows for comparative measurements and counts to be made. And more
careful examination may reveal more than one species in the sample.
Distribution is often an important aid in assigning samples
to a species. Readers should be aware however that fish farming in Iran has led
to the translocation, either deliberately or by accident, of species into basins
where they are not native.
Distribution can appear as a key character when the species is found in basins
exclusive of related species. In brackets [.....], distribution is not exclusive
but can be an additional character as outlined above.
The most important characters for identification are the
general body shape, the number, position and size of the fins, the position and
size of the mouth, whether teeth are obvious or not, the number of scales along
the flank and the number of rays in various fins, among others. Although colour
is often a useful guide, it can also be misleading. Fish vary their colour to
match their background or for spawning rituals. In general, it is best to use
several characters to identify a fish rather than relying on a single one which
can easily be misleading.
Large fishes can be examined for these characters using the
naked eye, but various pieces of equipment are necessary for identification of
smaller species or juveniles. Hand lenses are of some use in magnifying small
characters but by far the best instrument is a binocular microscope which can
magnify up to 50 times. Pharyngeal teeth, fin rays and scales can be counted
with ease using a microscope. Attachments can be used to take photographs or
project images of structures for drawing. Measurements can be taken under a
microscope on small specimens to ensure accuracy, and a microscope leaves both
hands free to handle the specimen and dissecting tools or
calipers. Ichthyologists develop their own techniques for manipulating light
sources and specimens for making structures readily visible. I prefer to have
two light sources. One of these illuminates the surface of the fish for scale
counts and observation of structures. The other bounces light off a white enamel
tray into the microscope and is particularly useful for counting fin rays as the
light travels through the fin enabling clear distinction of rays.
Two types of forceps are very useful. A large pair (25-35 cm
long) enables specimens to be taken out of a jar and sorted without immersing
one's fingers. Preservative solutions will irritate the skin and contact should
be minimised; some ichthyologists wash the specimen in water before handling,
but this may compromise subsequent effectiveness of preservatives. Fine plastic
gloves can be worn, but some people develop allergies to latex. A very fine pair
of forceps with needle-like points is used to spread folded fins to see the rays
and to probe and examine other structures.
Scissors are necessary for slitting the belly and these will
vary in size depending on the size of the fish. Fine scissors can be useful in
dissection. Very large fish may require a sharp knife or scalpel for dissection
or slitting the abdomen. The slit is usually made on the right side of the fish
as the left side (head to left) is used for drawings and photographs.
A needle mounted on a wooden or metal handle can be used for
cleaning gill arches of debris, clearing flesh from pharyngeal arches or lifting
the edges of scales to help in counting them. Most commercial dissecting needles
are too blunt and a fine needle can be taped on the end.
Measurements are best made with calipers for accuracy. Dial
or electronic calipers are available which measure to an accuracy of 0.1 mm,
and are available in several lengths. Very large calipers are usually vernier
calipers, but an accuracy of 0.1 mm for large specimens is not required, or
even attainable.
Examination of minute scales, debris encrusted gill arches or
the lateral and cephalic line canals is facilitated by using compressed air
delivered through a glass tube of 1 mm diameter. The air can come from a
compressor or aquarium air pump, or even from a hand-squeezed bulb.
Key to Families
A little experience will soon make this key to families
redundant as all Iranian freshwater fish families can be recognised at a glance. Separate keys
are given for families with two or more species (Genera and Species below). Only species which I have seen in Iran, examined museum material of, or
have reliable literature records for, are included. The survival of breeding
populations of some exotic fishes is uncertain; nonetheless these species are included here.
Drainage basins are given for families with a limited
distribution; others are widespread, occurring in all or most basins. "Marine" is used here for drainages
entering the Persian Gulf and Sea of Oman including the basins of the Tigris
River, Gulf, Hormuz and Makran. The families under this heading are marine but
have species that regularly enter fresh water in Iran. The terms Tigris River, Gulf, Hormuz and Makran are restricted
here for freshwater residents.
Key characters, e.g. fin ray counts, are restricted to the Iranian species and family members from elsewhere may not key out here.
The families Adrianichthyidae, Mullidae, Percichthyidae and Scophthalmidae have no confirmed records from Iran and are
not included in this key (see figures in Species Accounts for distinctive appearance).
* = exotic species; includes species used in aquaculture which may be widely
distributed. Note that some species have both native and exotic populations,
e.g. Cyprinus carpio.
** = native and translocated. This latter category is liable to change over time as native species are
inadvertently or deliberately moved around Iran.
Only families which key out to a single species, or
whose included species are all exotics, are marked * or ** here - more speciose
families may have both native and exotic components.
Images are of the species
mentioned at that point in the key. Where the image is labelled "e.g." then this
is a representative species or character for that key couplet.
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------delete
these because of wraparoud or just have short one
--------------------------------------------------50
----------------------------------------------------------------------------------------------------100
check books in library for layouts with
figures - note wraparound problem
1a. Seven
lateral gill openings on each side; mouth a sucking disc; no paired (pectoral or pelvic) fins =
Petromyzontidae (Caspiomyzon wagneri - Caspian Sea basin)
1b. Less than seven gill openings (1 or 5) on each side; mouth normal; at least pectoral fins present, usually pelvic fins also ---> 2
2a. Five lateral gill slits on each side; scales placoid (small and prickle-like) =
Carcharhinidae (Carcharhinus leucas - Marine (Tigris River basin))
2b. One gill opening on each side; scales, when present, cycloid, ctenoid or bony scutes --->
3
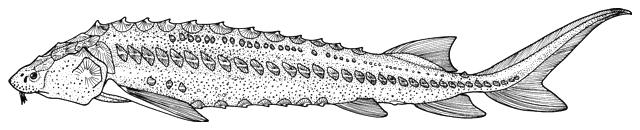
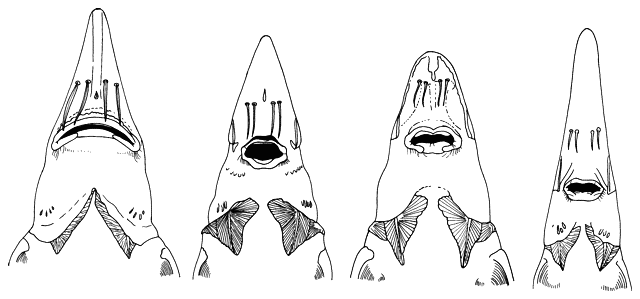
3a. Body
covered with five rows of bony scutes; mouth inferior, behind long snout, with
four barbels in front of mouth = Acipenseridae (Caspian Sea basin)
3b. Body without scutes; barbels, if present, not immediately in
front of mouth on a long snout ---> 4
4a. Chin with
a single barbel at mid-point [no fin spines, 58 or more anal and second dorsal
fin rays] = Lotidae (Lota lota - Caspian Sea basin)
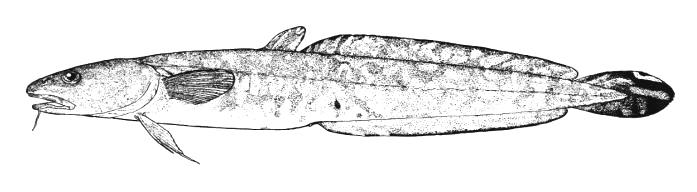
4b. Chin without a barbel; ---> 5
----------------------------------------------------------------------------------
5a. Pelvic fins united to form a disc or funnel = Gobiidae (Caspian Sea,
Tedzhen River and Marine basins) ?pelvic disc pic
5b. Pelvic fins present or absent but not formed into a disc --->
6
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
6a. Pelvic fins absent; body very elongate ("eel-like") ---> 7
6b. Pelvic fins present; body not very elongate ---> 9
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
7a.
First dorsal fin comprising 30-35 short,
sharp spines; flexible snout tip = Mastacembelidae (Mastacembelus
mastacembelus - Gulf, Kor River and Tigris River basins)

7b. Spines lacking in dorsal fin; snout not flexible ---> 8
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
8a. Body extremely thin, bounded by bony rings; dorsal fin short; snout tube-like
with minute mouth
= Syngnathidae (Syngnathus abaster - Caspian Sea basin)

8b. Body robust, covered with minute scales; dorsal fin long; snout not tube-like
and
mouth large = *Anguillidae (Anguilla
anguilla) - Caspian Sea basin)

---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
9a. First dorsal fin with either 3 or 8-12 isolated spines = Gasterosteidae
(Caspian Sea basin; introduced)
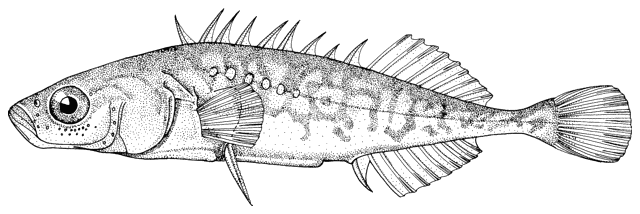 e.g.
Pungitius platygaster
e.g.
Pungitius platygaster
9b. First dorsal fin not composed of isolated spines, spines when
present connected by a membrane --->
10
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
10a. Nostrils each with a single pore; lateral line in two parts, the posterior one lower =
Cichlidae (Iranocichla hormuzensis - Hormuz basin)
10b. Nostrils each with two pores; lateral line a continuous line or absent --->
11
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
11a. Eyes on same side of body; body compressed with left side lying on bottom =
*Pleuronectidae (Platichthys flesus - Caspian Sea basin)
11b. Eyes on opposite sides of body ---> 12
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
12a. Blunt
grinding teeth in jaws = Sparidae (Acanthopgarus latus - Marine
basins)
12b.Teeth absent or, if present, sharp ---> 13
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
13a. Jaws duck-like with strong teeth; dorsal and anal fins far back on body near
tail =
**Esocidae Esox lucius - Caspian Sea basin; translocated)
13b. Jaws and fins not as above --->
14
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
14a. Barbels absent ---> 15
14b. Barbels present ---> 18
Barbels in Paracobitis smithi (dorsal view) and in Barbus lacerta
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
15a. First and second dorsal fins widely separate; scales cycloid --->
16 ?scales
15b. First and second dorsal fins continuous or close
together; scales ctenoid ---> 17
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
16a. First dorsal fin spines 5 or more
(usually 8 or more) and flexible; anal fin spines weak, 1-2 = Atherinidae (Atherina boyeri - Caspian Sea
basin)
16b. First dorsal fin spines 4 and very strong; anal
fin spines strong, 2-4 (usually 3) = Mugilidae (Caspian Sea and Marine
basins)
 e.g.
Liza abu
e.g.
Liza abu
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
17a. Anal fin spines three or more; first dorsal fin spines rarely 11, usually 10 =
*Centrarchidae (Namak Lake basin)
 e.g.
Lepomis macrochirus
e.g.
Lepomis macrochirus
17b. Anal fin spines one or two; first dorsal fin
spines 13 or more = Percidae (Caspian Sea basin)
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
18a. Three or more pairs of barbels present; no scales or scales minute --->
19
18b. Barbels two pairs, one pair, or absent; scales
present and well developed ---> 24
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
19a. Four pairs of barbels present; nasal barbels present = *Heteropneustidae (Heteropneustes
fossilis - Tigris River basin)
19b. Three pairs of barbels present; no nasal barbels ? check this--->
20
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
20a. A thoracic adhesive apparatus ("sucker")
present on the belly between the pectoral fins formed from longitudinal skin
folds = Sisoridae (Gulf and Tigris River basins)
 Sucker in
Glyptothorax silviae
Sucker in
Glyptothorax silviae
20b. No sucker ---> 21
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
21a. Barbels longer than head; no scales; strong
pectoral fin spine ---> 22
21b. Barbels shorter than head; scales minute or absent; no pectoral fin spine
---> 23
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
22a. Dorsal fin spineless, small and short (3-4 rays) and spineless;
anal fin elongate (> 69 rays) = Siluridae (Caspian Sea, Lake Orumiyeh
and Tigris
River basins)
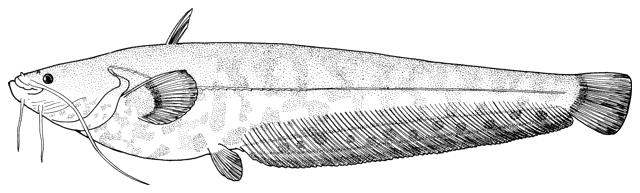
22b. Dorsal fin with a strong spine, well-developed
(7-8 rays); anal fin shorter (6-10 rays) = Bagridae (Mystus pelusius
- Gulf, Hormuz and Tigris River basins)
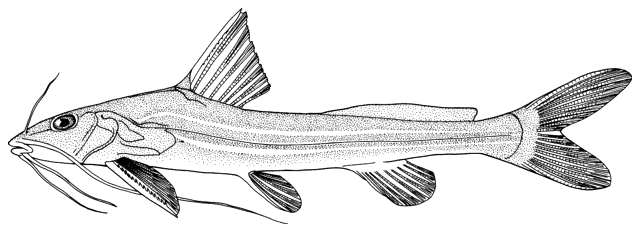
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
23a. Spine below eye folding into a groove; head compressed not rounded =
Cobitidae
(widespread)
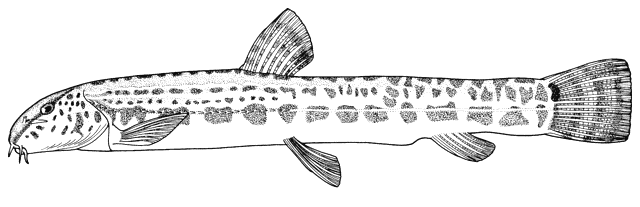 e.g. Cobitis
taenia
e.g. Cobitis
taenia
 Cobitis taenia suborbital spine (enlarged)
?just top pic
Cobitis taenia suborbital spine (enlarged)
?just top pic
23b. No spine below eye; head rounded = Nemacheilidae
(widespread)
 e.g.
Oxynoemacheilus kermanshahensis
e.g.
Oxynoemacheilus kermanshahensis
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
24a. Discrete, short adipose fin present = Salmonidae
(Caspian Sea, Lake Orumiyeh and Namak Lake basins; widely introduced)
 e.g.
Salmo caspius
e.g.
Salmo caspius
24b. No adipose fin ---> 25
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
25a. Dorsal and anal fins long, dorsal with more than 30 rays; head snake-like --->
Channidae (Channa gachua - Hamun-e Jaz Murian basin)
25b. Dorsal and anal fins short, less than 20 rays; head normal ---> 26
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
26a.
No teeth in jaws; lateral line usually obvious ---> 27
26b. Teeth in jaws; no lateral line pores ---> 28
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
27a. Adipose eyelid present; branchiostegal rays 4; alar scales on caudal fin (enlarged
scales) = Chanidae (Chanos chanos - Hormuz basin)
27b. Adipose eyelid absent; branchiostegal rays 3;
alar scales absent = Cyprinidae
(widespread)
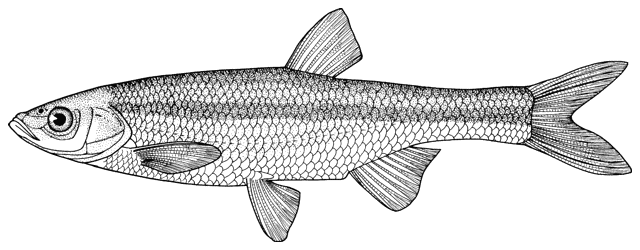 e.g.
Alburnus filippii
e.g.
Alburnus filippii
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
28a. Head naked dorsally; pelvic fins under dorsal fin = Clupeidae (Caspian Sea
and
Marine basins)
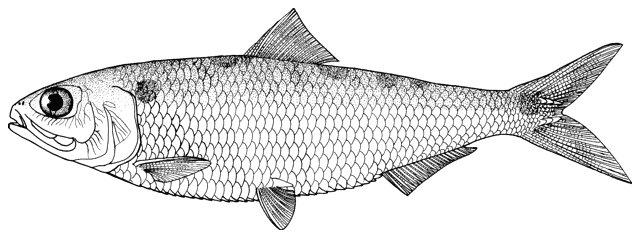 e.g.
Alosa braschnikowii
e.g.
Alosa braschnikowii
28b. Head covered with scales dorsally; pelvic fin bases not under dorsal fin
---> 29
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
29a. Teeth conical; anal fin in males enlarged as a copulatory organ; females without
sheath around anterior anal fin rays; body slender = *Poeciliidae
(widespread)
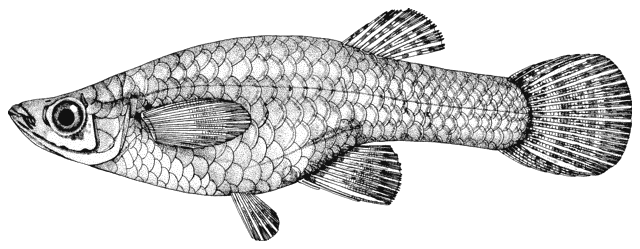 e.g. Gambusia holbrooki
female
e.g. Gambusia holbrooki
female
 Gambusia
holbrooki male anal fin
Gambusia
holbrooki male anal fin
29b. Teeth trifid; anal fin in males normal; females
with a sheath around anterior anal fin rays; body robust = Cyprinodontidae
(widespread)
 e.g.
Aphanius vladykovi
e.g.
Aphanius vladykovi
Keys to Genera and Species
The following keys identify species in the more speciose families and genera.
Some keys identify both genera and species, others have separate keys for each genus. Some species are similar and have overlapping
characters; distribution is then an additional guide to identity. It
should be noted that some species are known to have been introduced to basins
outside their natural range, and the possibility exists that other species may
be translocated.
Keys are arranged alphabetically by family and
by genera within families.
* = exotic species and includes species used in aquaculture which may be widely
distributed. Note that some species have both native and exotic populations,
e.g. Cyprinus carpio, or are native and translocated and marked
as **. This latter category is liable to change over time as native species are
inadvertently translocated.
--|----|----|--
Key to the Genera and Species of Acipenseridae
Sturgeons are restricted to the Caspian Sea basin and, although aquaculture
in internal desert areas has been tried, are unlikely to found as established
translocations.

Ventral view of heads of Huso huso,
Acipenser nudiventris, A. gueldenstaedtii and A. stellatus.
Note that A. persicus
is very similar to A. gueldenstaedtii.
1a. Mouth a
large crescent; gill membranes joined to form a free fold over the isthmus =
Huso huso
1b. Mouth small and transverse; gill membranes attached to
isthmus --->
2
2a. Lower lip continuous, not split in middle; more than 48
lateral scutes, usually 55 or more; barbels fimbriate = Acipenser nudiventris
2b. Lower lip interrupted in the middle; less than 51 lateral scutes (usually
less than 45 in Iranian waters); barbels not fimbriate ---> 3
3a. Snout
long and narrow (more than 61% of head length); barbels closer to mouth than tip
of snout = Acipenser stellatus
3b. Snout
shorter and broader (less than 60% of head length); barbels nearer to tip of
snout than mouth ---> 4
4a. Back
golden-brown, belly yellowish-white; average body depth 12-14% of total length =
Acipenser gueldenstaedtii
4b. Back greyish-blue, belly white; average body depth 16.8% of total length =
Acipenser persicus
--|----|----|--
Key to the Genera and Species
of *Centrarchidae
These two exotic species may not have reproducing populations
in Iran.
1a. Lateral line scales large, 35-50; body compressed
in cross section and deep, maximum body depth 1.7-3.0 (usually 2.5 or less)
times in body length from snout tip to end of scales [Namak Lake basin] = *Lepomis macrochirus

1b. Lateral line scales small, 58-81; body rounded in cross section and
elongate, maximum body depth 2.5-5.0 (usually 3.0 or more) times in body length
from snout tip to end of scales [Namak Lake and Tigris River basins] = *Micropterus salmoides
--|----|----|--
Key to the Genera and Species of Clupeidae
Caspian Sea species have numerous nominal subspecies and keys
to these may be found in Berg (1948-1949) and Svetovidov (1952).
1a. Upper jaw without a median notch, rounded when viewed from
in front; last two anal fin rays enlarged; lower jaw articulation with skull
below or anterior to posterior eye margin; Caspian Sea species ---> 2
1b. Upper jaw with a median notch; last two anal fin rays not
enlarged; lower jaw articulation with skull behind posterior eye margin --->
4
2a. Pectoral fins pointed at tips; head short and wide (interorbital
width 16% or more of head length) ---> 3
2b. Pectoral fins rounded at tips; head large and narrow (interorbital
width 15.5% or less of head length) = Clupeonella grimmi
3a. Body and belly compressed (body depth about 21-27% of
standard length); keeled belly scales evident = Clupeonella caspia
3b. Body cylindrical and belly rounded (body depth 16-19% of
standard length); keeled belly scales weakly developed = Clupeonella
engrauliformis
4a. Branched pelvic fin rays 8; upper gill rakers overlap
lower gill rakers at angle of first arch; Caspian Sea species ---> 5
4b. Branched pelvic fin rays 7; upper gill rakers not overlapping
lower gill rakers at angle of first arch; Gulf and Tigris River basins = Tenualosa ilisha
5a. Body deep and compressed; head large and deep,
wedge-shaped in anterior view; caudal peduncle short; pectoral fins long --->
6
5b. Body not deep and not compressed; head not large and deep,
not wedge-shaped in anterior view; caudal peduncle not short; pectoral fins
short ---> 8
6a. Gill rakers on first arch 60 or more, thin and long, much
longer than gill filaments; teeth weakly developed = Alosa caspia
6b. Gill rakers on first arch 45 or less, shorter, equal to
or somewhat longer than gill filaments; teeth well developed ---> 7
7a. Upper and lower profiles of head straight; lower jaw
protruding and its upper edge straight = Alosa saposchnikowii
7b. Upper and lower profiles of head rounded; jaws equal in
length and lower jaw has a crescentic upper edge = Alosa sphaerocephala
8a. Gill rakers 47 or less, thick and coarse = Alosa
braschnikowii

8b. Gill rakers 59 or more, may be thin and long but can be
coarse and short = Alosa kessleri
--|----|----|--
Key to the Genera of Cobitidae
1a. Caudal fin with 14 branched rays; a row of large and distinct dark spots
laterally, speckles above this row tending to form a row too; sides of body not
distended in front of dorsal fin in males = Cobitis
1b. Caudal fin with 12, rarely 13, branched rays; speckles above the lateral
line not forming horizontal rows; sides of body distended in front of dorsal fin
in males = Sabanejewia
Key to the Species of
Cobitis
? check spines
1a.
Dark brown lateral spots reduced or absent;
Hormuz and Kor River basins = Cobitis linea
1b. Large
dark and obvious spots along the mid-flank numbering 10-20, usually 16-18; Caspian Sea and Tigris River basins = Cobitis
taenia

Key to the Species of Sabanejewia
1a. Row of dark brown spots laterally [branches of suborbital spine differing in length; Caspian Sea and Tedzhen
River basins] = Sabanejewia aurata
1b. No row of large dark spots laterally [Caspian Sea basin] ---> 2
2a. A continuous dark streak
mid-laterally; branches of suborbital spine differing in length; two dark spots
at base of caudal fin = Sabanejewia caspia
2b. Numerous small speckles along flank; suborbital spine strong with branches
of similar length; no dark spot at caudal fin base =
Sabanejewia caucasica
?pic
--|----|----|--
Key to the
Genera and Species of Cyprinidae
?check all genera are in this part of key
?tabulate main charctesr like spine, barbels, sucker?
The cyprinid family is the most speciose in Iranian fresh
waters. Members of the family are more easily identified first to genus and then
to species. Keys are then shorter and less liable to error in use. Additional
characters can be listed under each genus or species which are not unique nor
readily incorporated into keys but which in combination help to identify the
genus or species. These additional characters are given in brackets. Monotypic
genera key out to species in the generic key.
?Add petroleuciscus add Romanogobio, Luciobarbus, Carasobarbus, Kosswigobarbus,
Mesoptamichthys, Tor, *Mylopharyngodon
1a. Branchiostegal membranes not attached to isthmus; gill
rakers fused together; eyes low on side of head, below midline; suprabranchial
organ present = *Hypophthalmichthys spp.
1b. Branchiostegal membranes attached to isthmus; gill rakers not
fused; eyes at or above midline of head; suprabranchial organ absent ---> 2
2a. Serrated stiffened ray (spine-like) in the dorsal and anal
fins; dorsal fin elongate (? rays or more, usually ?); anal fin origin below
dorsal fin ---> 3 see iraq book
2b. No serrated stiffened ray (spine-like) in the anal fin;
dorsal fin short to moderately elongate (?-? rays, usually ?); anal fin origin
behind dorsal fin end ---> 4
3a. Barbels absent; pharyngeal teeth in one row = *Carassius
pic
3b. Barbels present (two pairs); pharyngeal teeth in three rows; ? and exotic =
**Cyprinus carpio
4a. Eyes absent; body pink through lack of pigment; no scales
= Iranocypris typhlops
4b. Eyes present; body pigmented; scales present, sometimes
restricted to anal area ---> 5
mouth structure *Pseudorasbora parva
peculiar short dorsal fin ray
*Pimephales promelas
other species to pull out here
Aspidoparia morar
Barilius mesopotamicus
*Ctenopharyngodon idella
*Hemiculter leucisculus
Leucaspius delineatus
*Pseudorasbora parva
**Rhodeus sericeus
Scardinius erythrophthalmus
5a. Anus and anal fin base sheathed by markedly enlarged
scales ---> 6
5b. Anus and anal fin base not sheathed by markedly enlarged
scales ---> 8
6a. Branched anal fin rays 5; scales mostly absent; pharyngeal teeth in two rows
= Schizopygopsis stoliczkai
6b. Branched anal fin rays 6; scales present; pharyngeal
teeth in three rows =
7a. Barbels absent or vestigial; anal fin branched rays 6; pharyngeal tooth
formula 2,3,4-4,3,2 =
Schizocypris altidorsalis
7b. Barbels present and well-developed; anal fin branched rays 5É see
above;
pharyngeal tooth formula 2,3,5-5,3,2
=
Schizothorax spp.
8a. An adhesive disc prominent on the underside of the head = Garra
Underside of head of Garra persica
8b. No adhesive disc --->
9a. Scaleless keel extending from the
throat to the anal fin; lateral line decurved and wavy = Pelecus cultratus
9b. Not as above ---> 10
10a.
Barbels present ---> x
10b.
Barbels absent ---> 11
11. naked ventral keel Abramis Alburnoides
Alburnus Blicca Alburnus Vimba (also put
alburnoides, alburnus in another couplet)
1. Barbels absent + spine in D D ray count [scales large, 29-35 in lateral line; body
compressed; Tigris River basin; dorsal fin spine smooth, without denticulations]
= Barbus sharpeyi
+ Mesoptamichthys? no barbels
x. Spine in dorsal fin ---> h
No spine in dorsal fin ---> c
h. Spine smooth; mouth not sector-shaped ---> j
Spine with teeth; mouth sector-shaped (u-shaped in young)
---> i
j. Mouth with central tubercles Kosswigobarbus
Mouth without k
k Branched anal fin rays 6, dorsal fin branched rays 10 or more; lateral line
scales ? = Cyprinion
Branched anal fin rays 5; dorsal fin branched rays 9 or less;
lateral line scales ? = Capoeta
j = Carasobarbus luteus
= Tor grypus
c. Scales small, more than ?100 in lateral line = Tinca tinca
Scales larger, less than ? in lateral line ---> q
q. Anal fin branched rays 13 or more = Barilius mesopotamicus
Anal fin branched rays less than 13 ---> t
t. Dorsal fin branched rays 7; pharyngeal teeth in two rows = Gobio gobio
Dorsal fin branched rays 8; pharyngeal teeth in one row
---> s
s. Lateral line complete = Crossocheilus latius
Lateral line incomplete = Hemigrammocapoeta
elegans
Spine no barbel Acanthobrama marmid
chck for more see above and Esmaeili list
Body and caudal peduncle compressed
(caudal peduncle depth at anal fin insertion greater than caudal peduncle
width); well-defined spots on the dorsal and caudal fins; Tedzhen River = Gobio gobio
Body only slightly compressed and the caudal peduncle
cylindrical (caudal peduncle depth at anal fin insertion less than or about
equal to caudal peduncle width); faint spots on the dorsal and caudal fins; Lake Orumiyeh and Caspian Sea = Gobio persus
Key
to the Species of Abramis
Both species are found only in the Caspian Sea basin.
1a. Branched anal fin rays 22-30 = Abramis brama
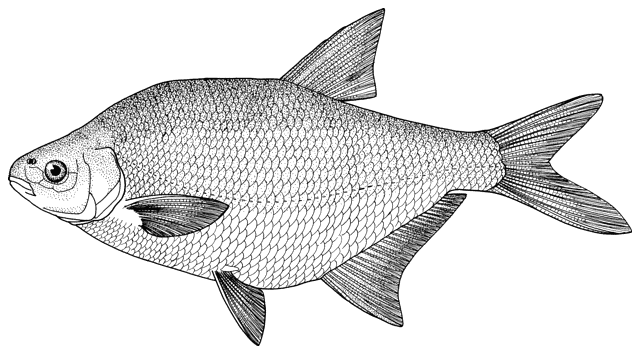
1b. Branched anal fin rays 31-44, mostly 34 or more =
Abramis sapa
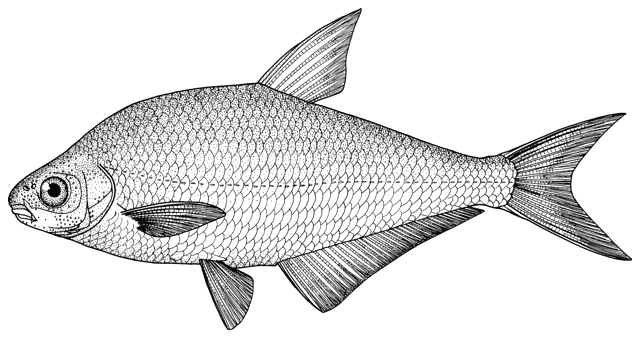
Key to the Species of
Acanthalburnus
1a. Anal fin branched rays 13-19; lateral line scales 60-85;
Caspian Sea basin = Acanthalburnus microlepis
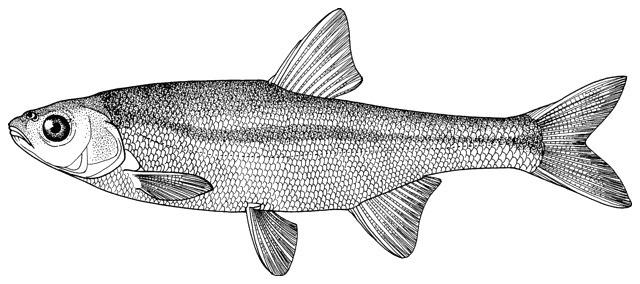
1b. Anal fin branched rays 10-13; lateral line scales 50-68;
Lake Orumiyeh basin = Acanthalburnus urmianus

Key to the Species of
Alburnoides
Populations in the
Esfahan and Tedzhen River basins are not yet identified to species.
1a.
Snout pointed or slightly rounded; mouth terminal
or upturned,
tip of mouth cleft on level from slightly above middle of eye to upper margin of
pupil; lower jaw slightly to moderately projecting relative to upper jaw;
junction of lower jaw and quadrate on about vertical through anterior eye
margin; Kor River basin = Alburnoides qanati
2b. Snout slightly to markedly rounded; mouth terminal
to subterminal,
tip of mouth cleft on level from middle of eye to below lower margin of eye;
upper jaw slightly to moderately projecting relative to lower jaw; junction of
lower jaw and quadrate on about vertical through about middle of eye ---> 2
2a.
Branched anal fin rays 8-11, commonly 9-10; branched dorsal fin rays 7, rarely 8 --->
3
2b. Branched anal fin rays 10-15, commonly 11-13; branched dorsal fin rays 8,
rarely 7 ---> 4
3a. Ventral keel
completely scaled; total vertebrae 40-41; abdominal
vertebrae 20-22, commonly 21; Lake Orumiyeh basin =
Alburnoides petrubanarescui
3b.
Ventral
keel scaleless from one-third to whole keel length; total
vertebrae 38-40, commonly 39; abdominal vertebrae 19-20; Tigris River basin =
Alburnoides nicolausi
4a.
Ventral keel smoothed, scaled along one-third to whole length; Tigris River
basin = Alburnoides idignensis
4b.
Ventral
keel well-pronounced, almost or completely scaleless ---> 5
6a.
Lateral line in alive and preserved fish delineated by dark pigment dots above
and below; 13-15 predorsal vertebrae; mouth terminal, tip of mouth cleft on or
slightly below middle of eye; Caspian Sea basin = A. eichwaldii
6b.
Lateral line
in
alive and preserved fish
somewhat darker than
surrounding flank but no strong dark dots outline to canal;
11-13 predorsal vertebrae;
mouth
almost subterminal, tip of mouth cleft on or below lower margin of eye; Namak
Lake basin =
Alburnoides namaki
Key to the Species of
Alburnus
1a. Dorsal fin branched rays modally 7; strong mid-flank stripe [anal fin
branched rays 9-13, usually 10-12; total gill rakers 12-17; lateral line scales
46-64, usually 50-60; Caspian Sea basin] = Alburnus filippii

1b. Dorsal fin branched rays modally 8; no strong stripe in Caspian Sea
species ---> 2
2a. Total gill rakers 15-31, usually 19 or more [Caspian Sea basin] ---> 3
2a. Total gill rakers 10-18, usually 16 or less ---> 4
3a. Lateral line scales 54-74, usually 55 or more [anal fin branched rays 12-19;
peritoneum light brown; Caspian Sea basin] = Alburnus chalcoides
3b. Lateral line scales 36-53, usually 48 or less [anal fin branched rays 10-21;
peritoneum light silvery; Caspian Sea basin and translocated] = **Alburnus hohenackeri
4a. Lake Orumiyeh basin [anal fin branched rays 9-12, usually 10-11; total gill
rakers 11-16; lateral line scales 46-63, usually 46-58] = Alburnus
atropatenae
4b Tigris River and basins of southern Iran ---> 5
5a. Anal fin branched rays 9-10; upper Tigris River basin near ?; peritoneum silvery
[total gill rakers 12-14; lateral line scales 67-83] = Alburnus zagrosensis
pic?
5b. Anal fin branched rays 10-18, usually 11 or more; elsewhere in southern
Iran; peritoneum brown to black ---> 6
6a. Lateral line scales 43-58; anal fin branched rays 13-18, usually 14-16 [total gill rakers 10-13; Tigris River basin] = Alburnus caeruleus
6b. Lateral line scales 58-89, usually 60 or more; anal fin branched rays 10-14,
usually 11-12 [total gill rakers 11-18; Esfahan, Gulf, Hormuz, Kor River, Lake
Maharlu and Tigris River basins] = Alburnus mossulensis
Key to the Species of Aspius
The distinction of these two species has not been examined
recently and characters overlap, sample sizes for gill rakers and scales
in particular being very small. However, they are found in separate basins.
1a. Lateral line scales 62-105; anal fin branched rays 11-15,
usually 12; total gill rakers 8-11; total vertebrae 50-51; Caspian Sea basin =
Aspius aspius
1b. Lateral line scales 91-110; anal fin branched rays 10-13,
usually 11?; total gill rakers 11-14; total vertebrae 51-53; Tigris River basin =
Aspius vorax
Key to the Species of Capoeta
1a. Dorsal fin branched rays modally 7 [lateral line scales 42-62; total gill rakers 11-20; Bejestan,
Dasht-e Kavir, Dasht-e Lut, Sistan
and Tedzhen River basins] = Capoeta fusca

1b. Dorsal fin branched drays modally 8 or 9 ---> 2
2a. Dorsal fin spine strongly developed, longer than head [lateral line scales 68-90; total gill rakers 23-33;
Gulf and Tigris River basins] = Capoeta trutta
2b. Dorsal fin spine well-developed to weak, not longer than head
---> 3
3a. Total gill rakers 9-17 [lateral line scales 72-99; dorsal
fin spine weak and poorly serrated; Namak Lake basin] = Capoeta buhsei
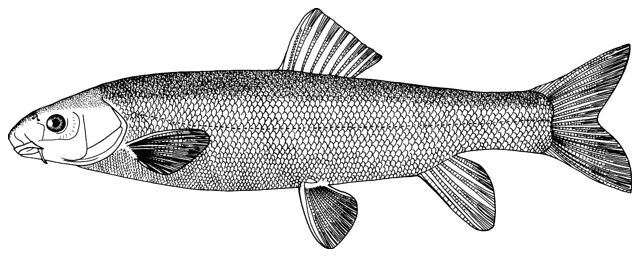
3b. Total gill rakers 16 or more, usually 18 or more ---> 4
4a. Lateral line scales 36-52, mostly 39-48; [Dasht-e Kavir, Esfahan, Kerman-Na'in,
Kor River, Namak Lake and Tigris River basins [total gill rakers 16-25] = Capoeta aculeata
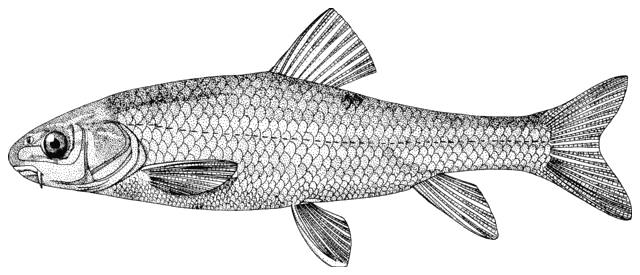
4b. Lateral line scales 46-99; mostly 50 or more ---> 5
5a. Dorsal fin branched rays modally 9; often large black blotches on flank
[lateral line scales 60-99; widespread] =
Capoeta damascina
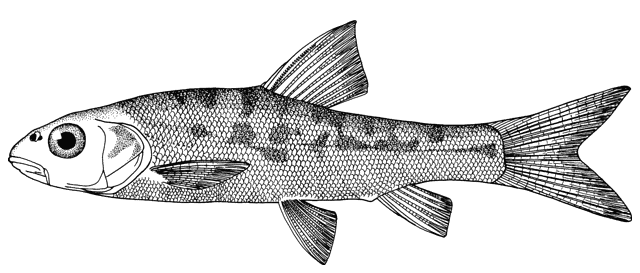
5b. Dorsal fin branched rays modally 8, sometimes 9; without black blotches
---> 6
6a. Irregular brown to black speckles on head and flank [lateral line scales 58-82;
Gulf and Tigris River]
= Capoeta barroisi
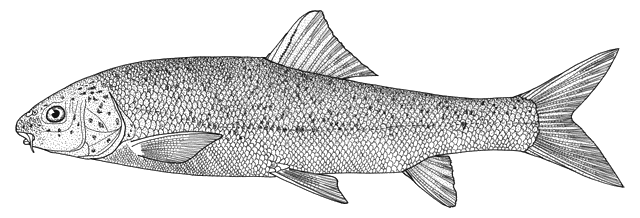
6b. Speckles absent; ? [lateral line scales 46-70; widespread; Tedzhen River fish
often with 4 barbels] = Capoeta capoeta
Key to the Species of
*Carassius
Goldfish have been widely introduced in Iran; presence and distribution of other
species is uncertain. ?gibelio
1a. Lateral line scales 25-34, mostly 31 or less; gill rakers
35-54, size dependent and mostly 39 or more in adults; anal fin branched rays
modally 5; young never with dark spot on caudal peduncle = *Carassius auratus
1b. Lateral line scales 32-36; gill rakers 23-35,
mostly 31 or less; anal fin branched rays modally 6; young usually with dark
spot on caudal peduncle = *Carassius carassius
Key to the Species of
Chondrostoma
1a. Caspian Sea basin [lateral line scales 50-68] = Chondrostoma cyri
1b. Outside Caspian Sea basin ---> 2
2a. Kor River basin [lateral line scales 49-57; dorsal fin branched rays usually
8] = Chondrostoma orientale
?pic
2b. Tigris River basin [lateral line scales 50-69; dorsal fin branched rays
usually 8 or 9] = Chondrostoma regium
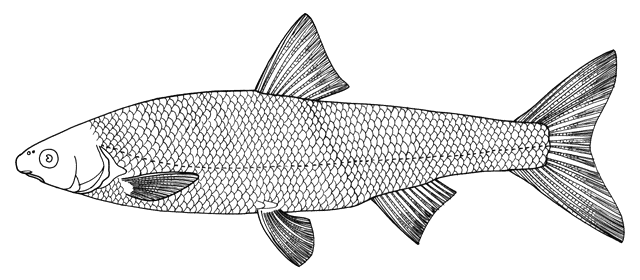
Key to the Species of Cyprinion
?
tabulate characters for comparison
see berg?
1a. Mouth small with large lateral lobes; cartilage may form a tooth-like
structure [dorsal fin branched rays 12-16; total
gill rakers 10-15; Gulf and Tigris River basins] =
Cyprinion kais
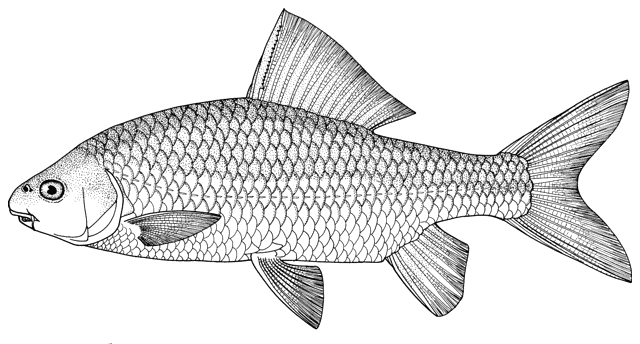
1b. Mouth without large lateral lobes; cartilage arched and not tooth-like --->
2
Mouth in
Cyprinion macrostomum
2a. Mouth oblique and long in lateral view [dorsal fin branched rays 10-13; total gill rakers 11-12; Hamun-e Jaz Murian, Hormuz and
Makran basins] =
Cyprinion milesi
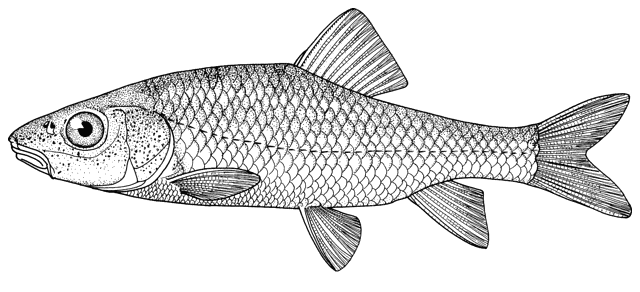
2b. Mouth arched in young, transverse in adults ---> 3
3a. Dorsal fin branched rays 9-12, usually 10-11, means 10.0-10.5; southeastern
and eastern Iran - Dasht-e Lut, Hamun-e Jaz Murian, Hamun-e Mashkid, Hormuz, Makran and Sistan basins =
Cyprinion watsoni
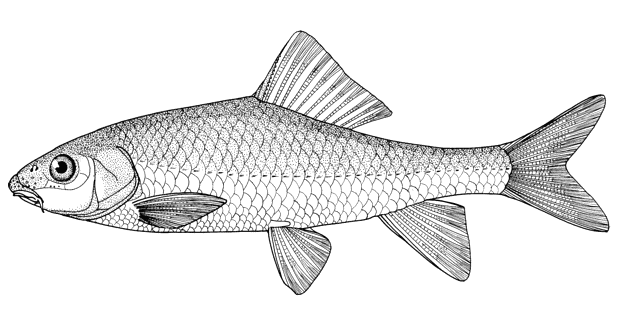
3b. Dorsal fin branched rays 11-17, usually 12-15, means 13.1-13.9; southwestern
Iran - Gulf, Lake Maharlu and Tigris River basins ---> 4
4a. Dorsal fin spine teeth well-developed, even near spine tip [Gulf and Tigris River basins] = Cyprinion macrostomum
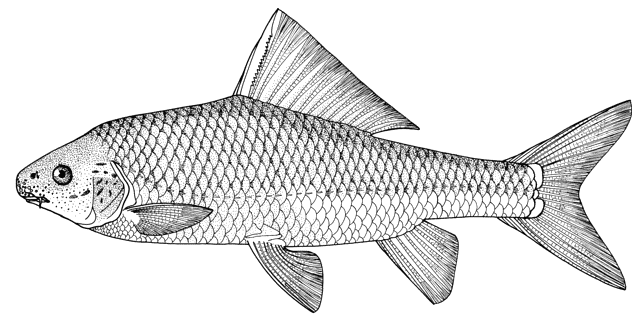
4b. Dorsal fin spine teeth graded in size as near tip and finer [Gulf and Lake Maharlu basins]= Cyprinion tenuiradius
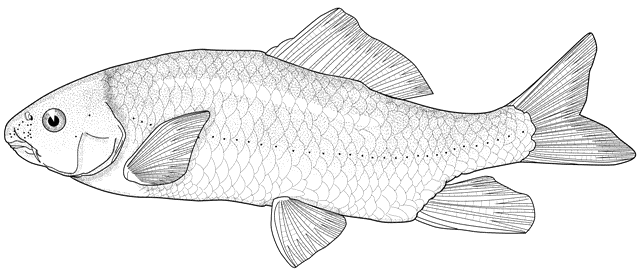
Key to the Species of Garra
?
1a. Caudal fin branched rays modally 16; ? (85.6% for 132 fish, range 15-17)
[Hamun-e Jaz Murian, Hormuz and Makran basins] = Garra persica
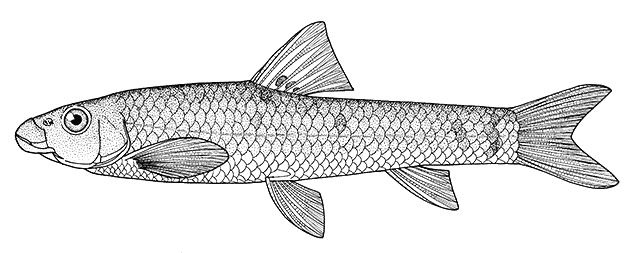

1b. Caudal fin branched rays modally 17, 16 only rarely --->
2
2a. Dorsal fin branched rays modally 8 (87.1% for 534 fish,
range 6-8); ? sucker structure [Gulf, Hormuz, Kor River, Lake Maharlu and
Tigris River basins] = Garra rufa
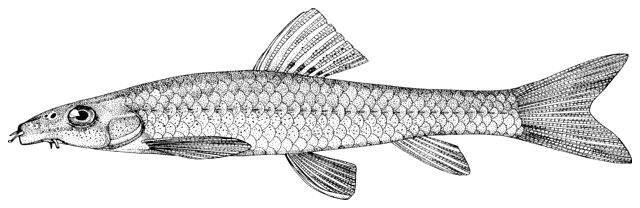
rufa sucker pic? from Berg?
2b. Dorsal fin branched rays modally 7 (91.5% for 59 fish,
range 6-8) ---> 3
3a. ?; eastern Iran (Bejestan, Hamun-e Jaz Murian, Hamun-e Mashkid, Dasht-e
Lut, Makran, Sistan and Tedzhen River basins) = Garra rossica
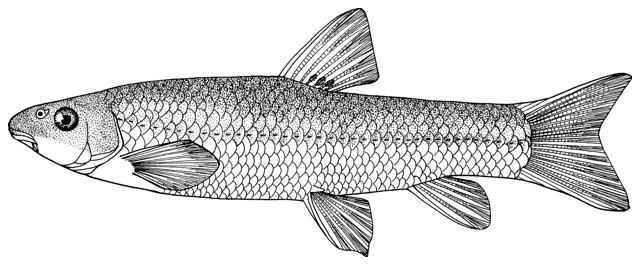
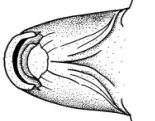
3b. ?; western Iran (Tigris River basin) = Garra variabilis
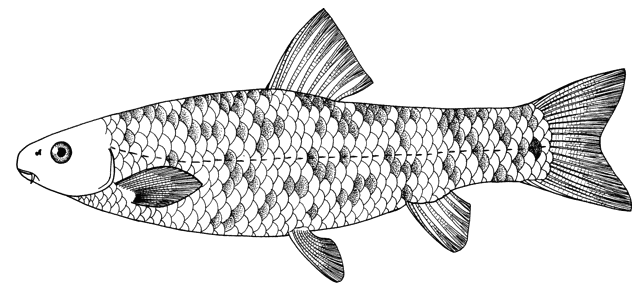
Key to the Species of *Hypophthalmichthys
These two species are widely farmed.
1a. Abdomen with a compressed keel extending from the breast
(pelvic fins) to the vent; pectoral fins short, not extending past the origin of
the pelvic fins; gill rakers a continuous band uniting both sides, roots fused
into a spongy mass = *Hypophthalmichthys molitrix
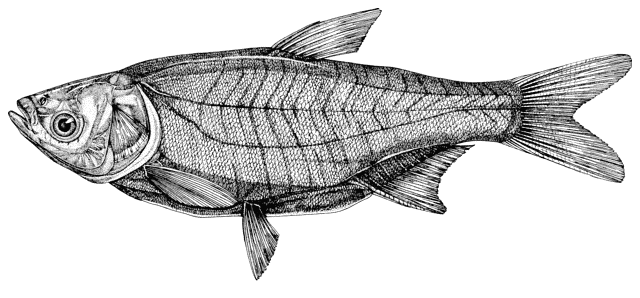
1b. Abdomen with a compressed keel extending from the throat
to vent; pectoral fins long, extending past the origin of the pelvic fins; gill rakers free,
no spongy mass = *Hypophthalmichthys nobilis

Key to the Species of Kosswigobarbus
1a. Lateral line scales 29-41; total vertebrae 39-40; Tigris River basin =
Kosswigobarbus kosswigi
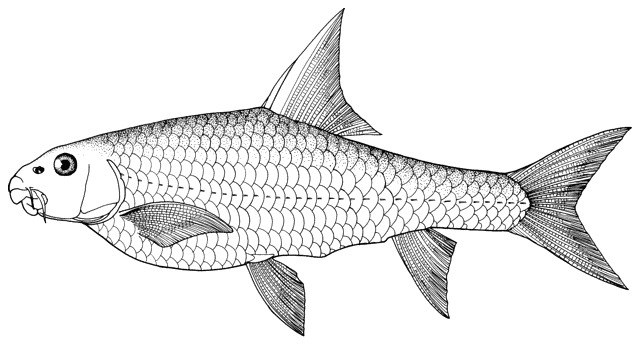
1b. Lateral line scales 24-27; total vertebrae 37-38; A'la River in Khuzestan and the Fahlian
River in Fars = Kosswigobarbus sublimus
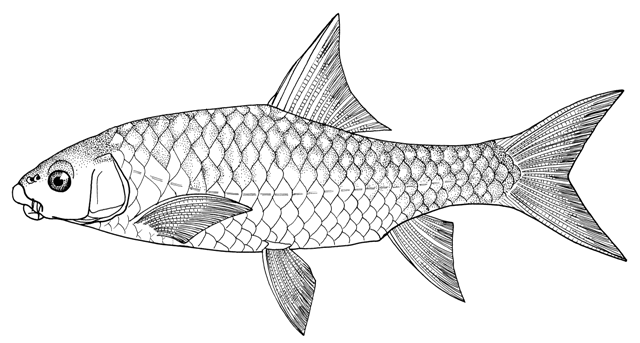
Key to the Species of Luciobarbus
?
1a. Body covered with large dark spots arranged almost in a quincunx (pattern of
five) [Tigris River basin] = Luciobarbus subquincunciatus
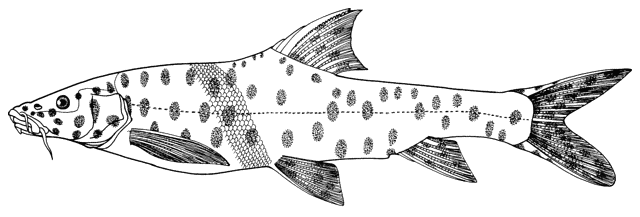 ph
teeth? her and elsewhere
ph
teeth? her and elsewhere
1b. Body without large spots ---> 2
2a. Head elongate, tapering and depressed anteriorly, pike-like, with postorbital distance in standard
length 7.2 or less; adults very large, reputedly over 2 m long [Tigris River and Gulf basins] = Luciobarbus
esocinus
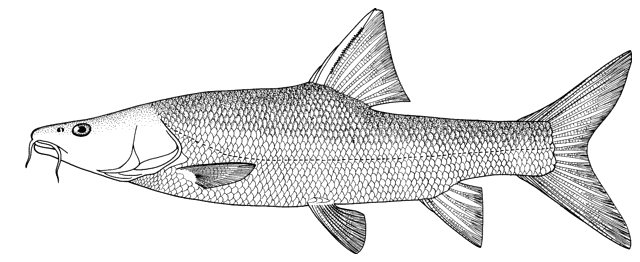
2b. Head not as above; not very large, to ? m ---> 3
3a.Northern and northwestern distribution in the Caspian Sea, Lake Orumiyeh and
Namak Lake basins ---> 4
3b.Southern and western distribution in the Gulf, Kor River and Tigris River basins --->
6
4a. Dorsal fin branched rays modally 7 [predorsal length shorter than postdorsal
length; lateral line scales 62-90, usually
65-77; total gill rakers 16-25; Caspian Sea basin] = Luciobarbus
brachycephalus
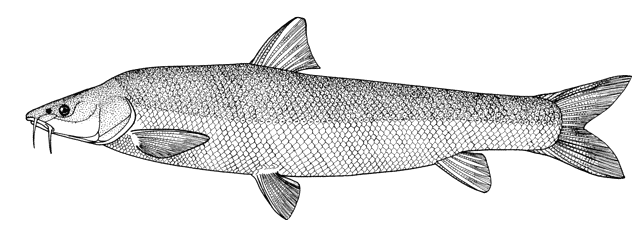
4b. Dorsal fin branched rays modally 8 ---> 5
5a. Lateral line scales 51-72; without three lobes to lower lip; upper dark
flank clearly delineated from lighter lower flank [predorsal length equal to longer
than postdorsal length; total gill rakers 12-19;
Caspian Sea basin] = Luciobarbus capito
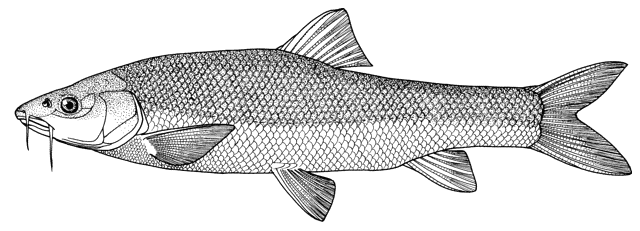
5b. Lateral line scales 74-103, often 85 or more; lower lip usually with three
lobes; body shades from dark to light gradually down flank [?predorsal length; total gill rakers 9-18;
Caspian Sea, Lake Orumiyeh and Namak Lake basins] = Luciobarbus mursa
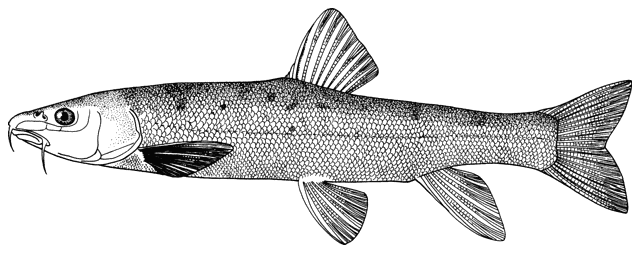
6a. Total gill rakers 7-13 [lateral line scales 57-68; Tigris River basin] = Luciobarbus
xanthopterus
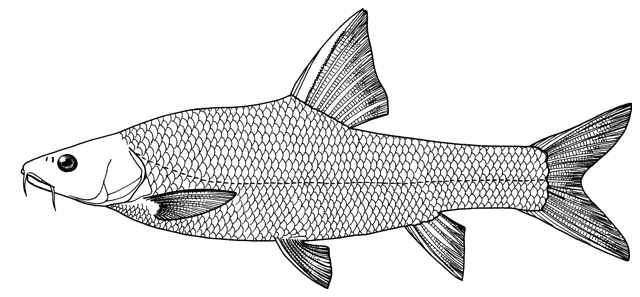
6b. Total gill rakers 14 or more ---> 7
7a. Lips markedly fleshy; fourth major row pharyngeal tooth large and molariform
[scales?; Gulf, Kor River and Tigris River basins] = Luciobarbus barbulus
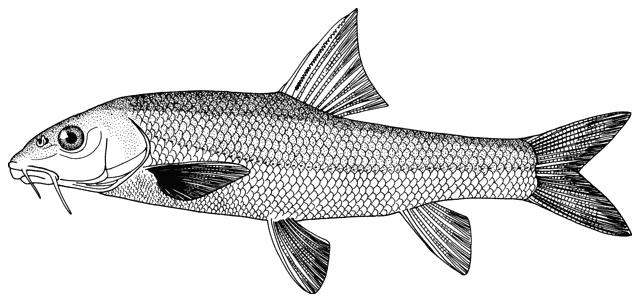
7b. Lips not markedly fleshy; ?Fourth major row pharyngeal tooth similar in size
to third, not molariform?check [scales?] ---> 8
8a. Dorsal fin spine strong, arising from an elevated base; dorsal fin origin at
or ahead of pelvic fins origin [Gulf, Kor River and Tigris River basins] = Luciobarbus
pectoralis
?better pic with stronger spine
8b. Dorsal fin spine present but not markedly strong; dorsal fin origin behind
pelvic fins origin [Gulf and Tigris River basins] = Luciobarbus kersin
? pic needed with weaker spine and D further back - see Iraq book for key
Key to the species of Petroleuciscus
1a. Dorsal fin
branched rays 6-7,
modally 7; anal fin branched rays 7-9,
modally 8; total vertebrae 34-37;
pharyngeal teeth 1.5-4.1;
Gulf, Hormuz and Kor River basins = Petroleuciscus persidis
1b. Dorsal
fin branched rays 7-9,
modally 8 or 9; anal fin branched rays 7-12,
modally 9-11;
total vertebrae 37-42;
pharyngeal teeth usually 2.5-4.2
---> 2
2a. Anal fin
branched rays
9-12,
modally 10 or 11; lateral line scales 45-56;
total vertebrae 41-42;
Esfahan basin =
Petroleuciscus esfahani

2b.
Anal fin branched rays 7-10,
modally 9; lateral line scales 36-45;
total vertebrae 37-38;
Lake Orumiyeh
basin =
Petroleuciscus ulanus
Key to the Species of Romanogobio
Key by A. Naseka,
Zoological Institute, St. Petersburg:-
1a. Number of lateral line scales 41 to 45 with modes of 42
and 43; total vertebrae 38 to 42 with modes of 40 and 41;
connection between the supraorbital and infraorbital head canals usually present; Caspian Sea
basin =
Romanogobio macropterus
1b. Number of lateral line scales 40 to 42 with modes of 40 or
41; total vertebrae 37 to 40 with modes of 38 and 39; connection between the supraorbital and infraorbital head canals usually absent; Lake Orumiyeh
basin = Romanogobio persus
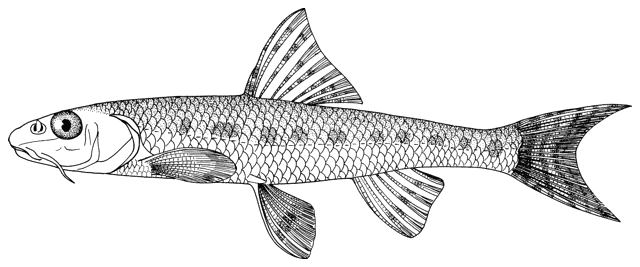
Key to the Species of Rutilus
These species occur only in the Caspian Sea basin.
1a. Lateral line scales 47-64, mostly 55-58; swimbladder
elongate and conical or pointed posteriorly ---> 2
1b. Lateral line scales 39-48, mostly 42-47; swimbladder
rounded posteriorly ---> 3
2a. = Rutilus frisii
2b. = Rutilus kutum
3a. = Rutilus caspicus
3b. = Rutilus rutilus
Key to the Species of
Schizothorax ?
1a. Total gill rakers 24-41[lips thin; Sistan basin] = Schizothorax zarudnyi
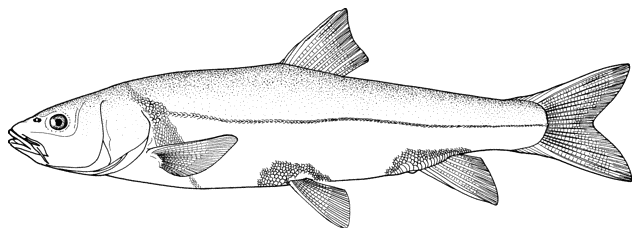
1b. Total gill rakers 18 or less ---> 2
2a.
lips?; Sistan basin = Schizothorax intermedius
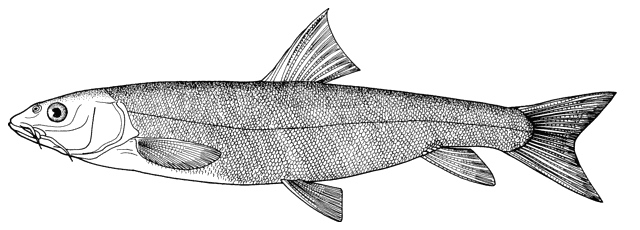
2b. lips thick; Dasht-e Kavir and Tedzhen River basins =
Schizothorax pelzami
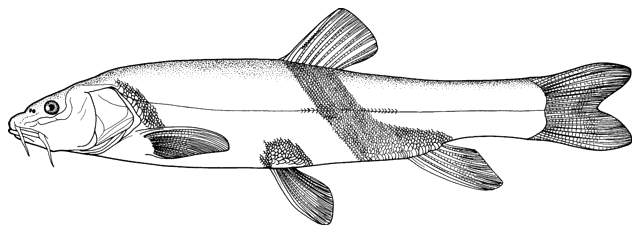
Key to the Species of Squalius
1a. Flank scales outlined by pigment; anal fin rounded distally [Caspian Sea,
Lake Orumiyeh, Namak Lake and Tigris River basins] = Squalius cephalus
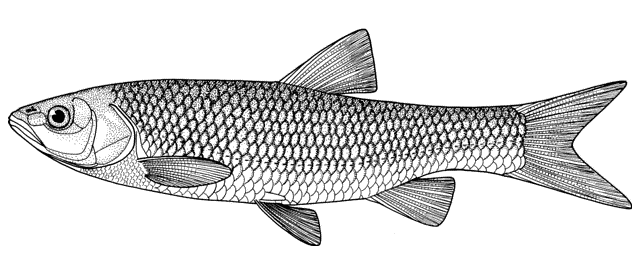
1b. Flank scales not outlined by pigment; anal fin truncate or emarginate
distally ---> 2
2a. Lower jaw not projecting; Tedzhen River basin = Squalius latus
check on fish about lower jaw?
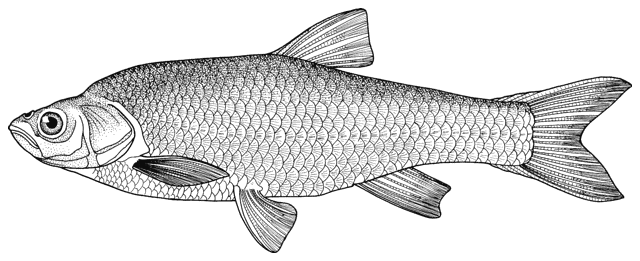
2b. Lower jaw projecting; Tigris River basin = Squalius lepidus
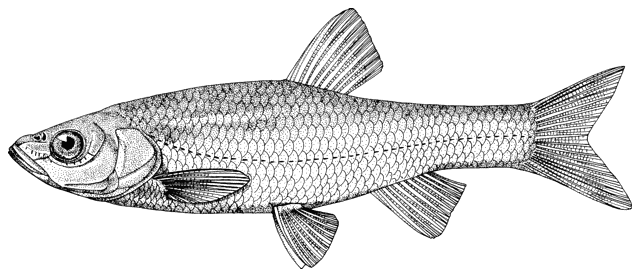
--|----|----|--
Key to the Species of Cyprinodontidae
Almost any sample will contain both males and females,
clearly distinguished by colour and pigment patterns.
?isfahanensis and check over key again
1a. Lateral line scales 36-47 [females finely speckled, no lozenge-shaped
spot at caudal fin base; Tigris River basin] = Aphanius vladykovi
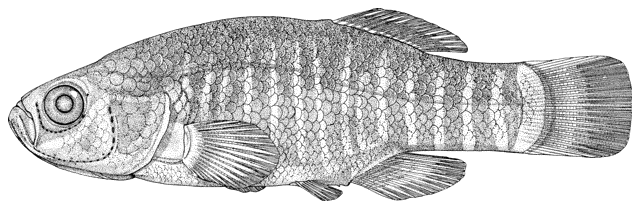 male
male
 female
female
1b. Lateral line scales 24-35, mostly 31 or less ---> 2
2a. Total dorsal fin rays 4-7; total anal fin rays 6-10; gut variably
coiled [Hormuz basin] = Aphanius ginaonis
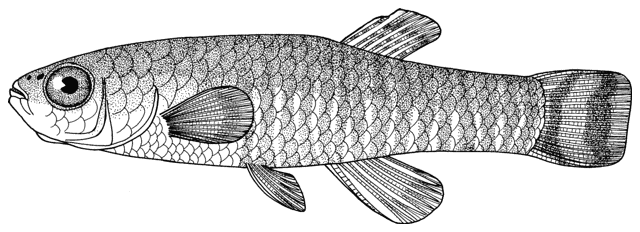 male
?gut pics
male
?gut pics
2b. Total dorsal fin rays 7-13, usually 9 or more; total anal fin rays 8-13,
usually 10 or more; gut regularly coiled ---> 3
3a. Males lemon-yellow with two broad bars on caudal fin;
females ? [Gulf, Hamun-e
Jaz Murian, Hormuz, Makran and Tigris River basins] = Aphanius dispar
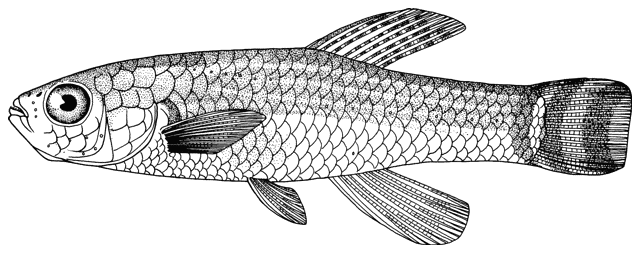
3b. Males not lemon-yellow; ?? ---> 4
4a. Males with blue spots on flank [Tigris River basin] = Aphanius mento
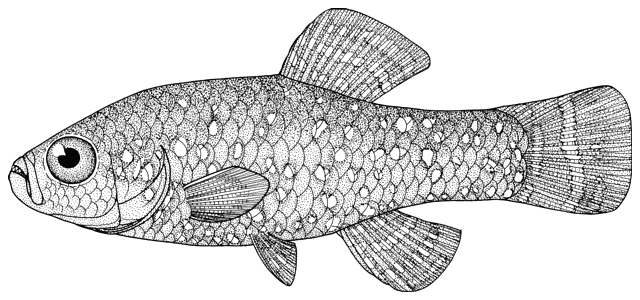 male
male
4b. Males without blue spots on flank ---> 5
5a. Females with obvious flank bars [males barred]; Lake Maharlu basin
= Aphanius persicus
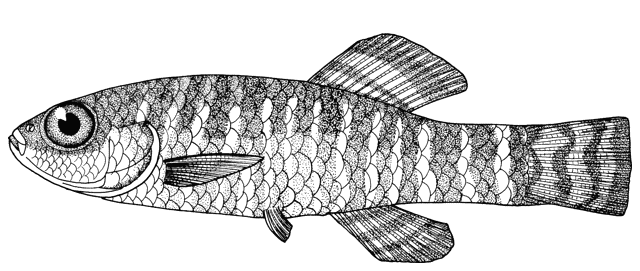 male
male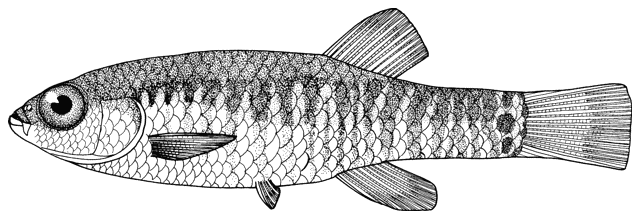 female
female
5b. Females with flank spots ---> 6
6a. ?Females without lozenge-shaped spot at caudal fin base; flank spots large;
Tigris River basin =
Aphanius mesopotamicus
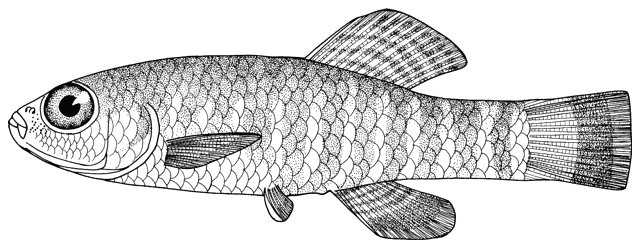 male
male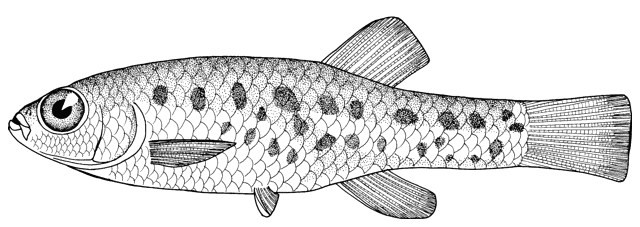 female
female
6b. Females with lozenge-shaped spot at caudal fin base; flank spots small; Kor River basin = Aphanius sophiae
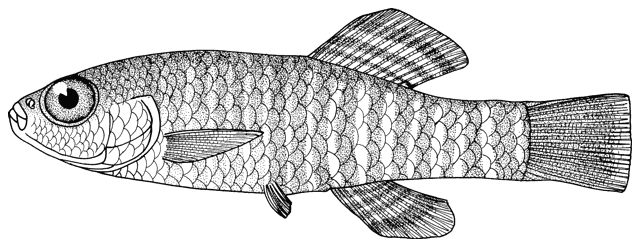 male
male female
female
--|----|----|--
Key to the Genera and Species of Gasterosteidae
1a. Long dorsal fin spines, numbering 3; long pelvic fin
spines, dorsal spines exceeding eye diameter in length; scutes
(vertical bony plates on flank) large [Caspian Sea, Dasht-e Kavir and Tedzhen
River basins] = *Gasterosteus aculeatus
1b. Short dorsal fin spines, numbering 7-11, alternatively
sloping left and right; dorsal spines shorter than eye diameter; scutes small
[Caspian Sea basin] = Pungitius platygaster

--|----|----|--
Key to Persian Gulf and Sea of Oman
Drainage Species of the Gobiidae
1a. Lateral series scales large, 28-36; eyes not protruding = Glossogobius giuris
1b. Lateral series
scales minute, over 90; eyes protruding above dorsal head profile ---> 2
2a. 4-5 first dorsal fin spines; anal fin base and second dorsal fin base 34% or more of
standard length; 2 canine teeth internal to the lower jaw symphysis =
Boleophthalmus dussumieri
2b.10-14 first dorsal fin spines; anal fin base and second
dorsal fin base 27% or less of standard length; no canine teeth internal to the
lower jaw symphysis = Periophthalmus waltoni
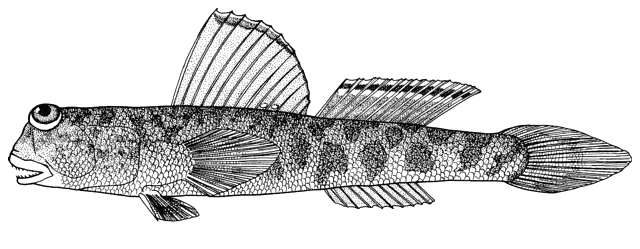
--|----|----|--
Key to Caspian Sea Genera
and Species of the Gobiidae
? check against checklist for all species included eliminate species not
definitely records
Some genera are
monotypic or have only a single species in the Caspian Sea basin and so the keys
terminates there. Speciose genera have separate keys below. The Iranian shore of
the Caspian Sea remains poorly explored in its deeper waters
and keys in Miller (2003), Mitrofanov (2003) and Boldyrev and Bogutskaya (2007)
should be consulted for specimens which do not key out here (see also Species
Accounts for further listings and discussion).
Note that a Rhinogobius species is
recorded from the Tedzhen (= Hari) River basin in Iran as an exotic (see
Species Accounts for
description). This is the only goby outside the Caspian Sea basin and coastal
waters of the Persian Gulf and Sea of Oman in Iran.
The following key is modified after Miller in Miller (2003):- ÉRECHECK!!
1a. Suborbital papillae with longitudinal row a immediately below eye
and having at least one short side row; cheek with several short transverse
rows, none reaching lower eye margin; snout with longitudinal rows s1
and s2 or, if transverse interorbital and snout rows, a
perianal organ is present =
Knipowitschia
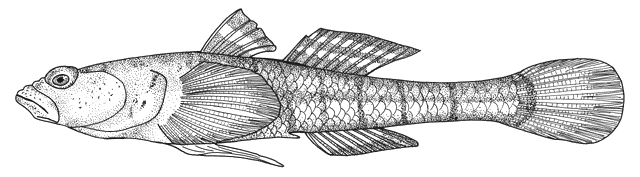 e.g.
Knipowitschia iljini
e.g.
Knipowitschia iljini
1b. Suborbital papillae in transverse rows; no row a;
snout with transverse rows s1 and s2; no
perianal organ ---> 2
2a. Row 5i not below level of row 6i; 6i at or opposite
end of row d; scales normal; canals present or absent ---> 3
2b. Row 5i below level of row 6i; 6i
separated from posterior end of row d by row 5i; scales
non-imbricate or bony tubercles and granules or naked; no canals ---> 7
3a. Anterior nostril an elongate tube hanging over lip = Proterorhinus nasalis
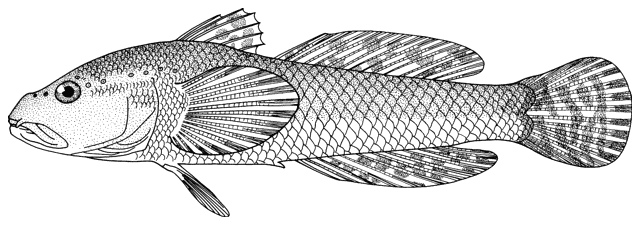
3b. Anterior nostril elongate but not overhanging lip ---> 4
4a. Three rows below row b = Mesogobius nonultimus
?pic
4b. Two rows below row b ---> 5
5a. Five rows before row b = Chasar bathybius
?pic
5b. Four rows before row b =
6
6a. = Neogobius
6b. = Ponticola
7a. No chin barbel or cheek flap; snout a duck-bill shape
= Anatirostrum profundorum
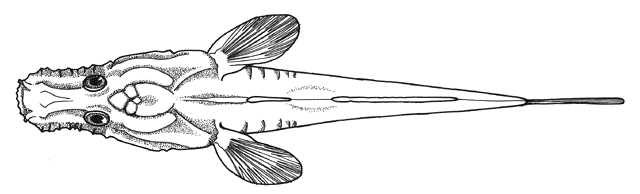
7b. Chin barbel and cheek flap present; snout not a duck-bill shape = Benthophilus
The following key does not use papillae and head canal characters;
see figures above. It is
modified from Mitrofanov (2003) as translated courtesy of Dmitri Ponomarenko:-
RECHECK
1a. Body naked ---> 2
1b. Body covered with regular ctenoid scales ---> 3
2a. Snout narrow and long = Anatirostrum profundorum
2b. Snout regular, not elongated ---> 3
3a. Anterior nostril elongated into a tube that hangs over lip
---> Proterorhinus nasalis
3b. Anterior nostril not as above---> 4
4a. Second dorsal fin
short, with less than 12 branched rays; small fishes less than 50 mm --->
5
4b. Second dorsal long, with more than 12 branched rays ---> 6
5a. Scales on sides of body, with head, throat, belly and back to
second dorsal fin scaleless; eyes lateral; body darkly pigmented without
stripes; tail symmetrical without a dark spot at tail base ---> Knipowitschia
caucasica
5b. Body fairly fully covered with scales; eyes pointed
upwards; body with dark stripes; body glassy and translucent; tail symmetrical
without a basal spot; deepwater species ---> Knipowitschia iljini
6a.
Sinciput not covered with scales = Mesogobius nonultimus
6b. Sinciput and occiput covered with scales ---> 7
7a. =
Neogobius
7b. =
Ponticola
Key to the Species Benthophilus
The following key is modified after Pinchuk and Miller in Miller (2004).
RECHECK See Boldyrev and Bogut too
1a. One or two dermal barbels
behind jaw angle; first dorsal fin with 1-2 spines; tubercles large and high,
not all spinous; tubercles in dorsal row 13-15, in ventral row 10-13 = Benthophilus baeri
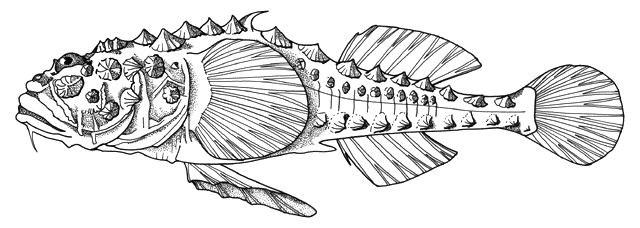
1b. A dermal fold or lobe behind jaw angle (if absent, tubercles
vertically elongated); first dorsal fin spines 3-4 (rarely 2); tubercles in
dorsal row 18 or more, in ventral row14 or more ---> 2
2a. Tubercles vertically
elongated, curved and crest-like, rear edges spinulose; temporal and occipital
region without large tubercles; head narrow, interorbit with median groove
between elevated ridges; dermal filaments present or absent; dermal fold behind
jaw angle when present narrow, with an acute protuberance; back without brown
bands ---> 3
2b. Tubercles conical and tipped by spines ---> 4
3a.
Temporal and occipital region of head with granules; tubercles in dorsal row
usually 30-33 = Benthophilus ctenolepidus
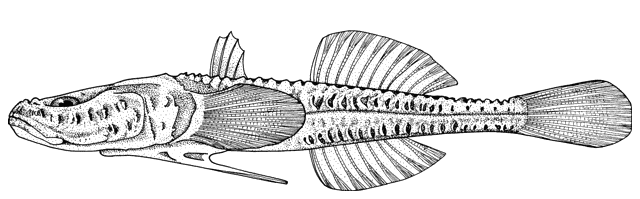
3b. Temporal region of head naked = Benthophilus pinchuki
?pic
4a. Tubercles distinct, relatively large; granules on temporal and occipital
area small and sparse, or if slightly larger then not forming real tubercles;
bands present = B.
leobergius
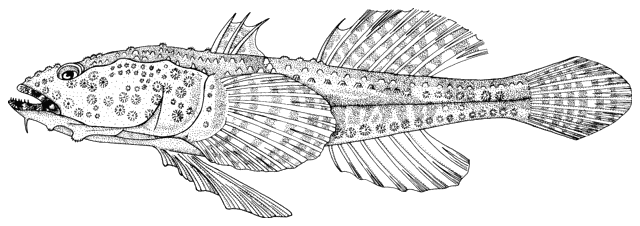
4b. Tubercle rows distinct but tubercles relatively small; upper head and
body densely covered with very small granules; no dark brown bands = B. macrocephalus
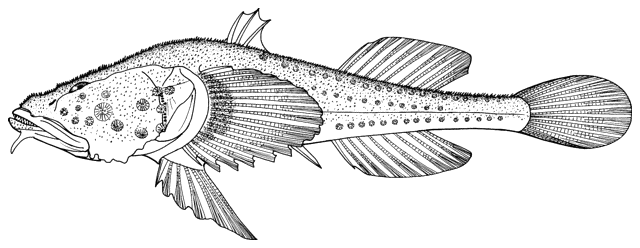
Key to Species
of Knipowitschia
The following key is modified after Miller in Miller (2004).
1a. Males with 0-4 flank bars; anterior oculoscapular canals united at posterior
interorbit, with a single median pore κ, and canals
extending anteriorly to pores λ; preopercular canal present = K. caucasica
1b. Males with 6-10 flank bars; anterior oculoscapular canals more or less separate in midline of
posterior interorbit, with pore κ double, and
canals extending anteriorly through interorbit of variable extent, typically absent; preopercular
canal present or absent = K. iljini

Key to
the Species of Neogobius
The following key is modified after Miller and Vasil'eva
in Miller (2003).
1a. Posterior nostril markedly distant from
edge of orbit; pelvic fin anterior membrane with angular lateral lobes; lobes about
one-sixth to almost one-half width of anterior edge of membrane = N.
caspius
1b. Posterior nostril near edge of orbit; pelvic fin anterior membrane with rounded and shallow lateral
lobes; lobes not more than one-sixth width of anterior edge of membrane, or
lacking entirely ---> 2
2a. At least anterior nape scales
cycloid; first dorsal fin with large dark spot at rear; lateral series scales
usually 49-55 = Neogobius melanostomus
2b. Nape scales ctenoid; first dorsal fin without large dark spot; lateral
series scales usually 55-70 = Neogobius pallasi
Key to the Species of Ponticola
The following key is modified after Miller and Vasil'eva
in Miller (2003). recheck?
1a. Pelvic fin anterior membrane with rounded and shallow lateral
lobes; lobes not more than one-sixth width of anterior edge of membrane, or
lacking entirely = Ponticola syrman
pic?
1b. Pelvic fin anterior membrane with angular lateral lobes; lobes about
one-sixth to almost one-half width of anterior edge of membrane ---> 2
2a. Lateral series scales usually 49-54; lateral lobes of pelvic fin anterior
membrane small, not more than one-fifth width of rear edge; upper lip width
0.4-0.67
lateral preorbital width (lip to orbit); nape scales cycloid;
pelvic fin almost reaches the anal fin (0.9 distance) or extends beyond the anal
fin
origin = Ponticola goebelii
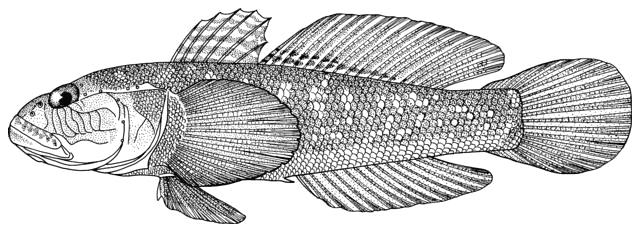
2b. Lateral series scales usually 54-76; lateral lobes of
pelvic fin anterior membrane large, at least one-fifth width of rear edge; upper
lip width at least 0.6
lateral preorbital width (lip to orbit), if less than 0.75,
then nape scales ctenoid; pelvic fin less than nine-tenths distance to anal fin
---> 3
3a. Upper lip not markedly swollen, width about 0.6-0.67
lateral preorbit; interorbital distance 0.8-0.9 eye diameter; caudal peduncle
depth 0.67-0.75 length =
Ponticola gorlap
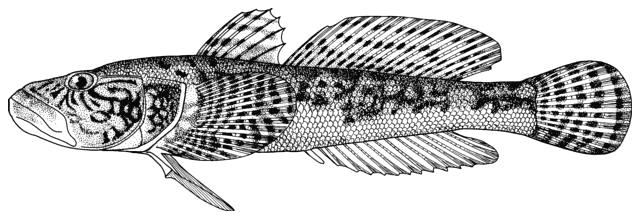
3b. Upper lip moderately swollen, width about 0.75 to more
than length lateral preorbit; interorbital distance 0.4-0.8 eye diameter; caudal
peduncle depth 0.75
to more than length = Ponticola cyrius
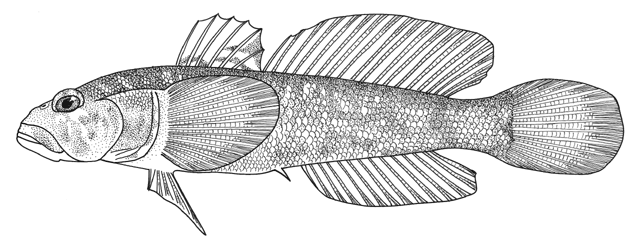
--|----|----|--
Key to the Genera and Species of Mugilidae
?other species see Iraq book subviridis and vaigiensis; premaxilla pics
1a. Posterior end of maxilla not curved below tip of premaxilla, but straight; jaw end on line of gape; adipose eyelid
well-developed, enclosing eye over much of anterior and posterior fields of
iris, so pupil is covered by an oval slit; pyloric caeca 2; Marine, introduced
to Caspian Sea = **Mugil
cephalus
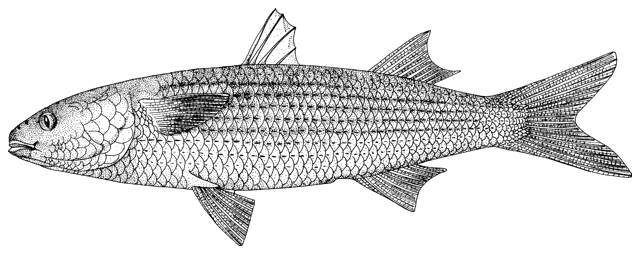
1b. Posterior end of maxilla curved below tip of premaxilla,
visible behind corner of closed mouth; jaw end on below line of gape; adipose
eyelid weakly-developed, not reaching pupil of eye; pyloric caeca 6-9 ---> 2
2a. Branched second dorsal fin rays 7; anal branched rays 8;
pectoral fin long, reaching dorsal fin level; Gulf, Hormuz and Tigris River basins,
translocated to Lake Maharlu basin = **Liza abu
check caeca?

2b. Branched second dorsal fin rays usually 9; anal fin
branched rays 9; pectoral fin short, not reaching dorsal fin level; Caspian Sea
basin
---> 3
3a. Pyloric caeca equal in length; scales of head and back
with one groove; oral edge of preorbital moderately concave ---> *Liza aurata
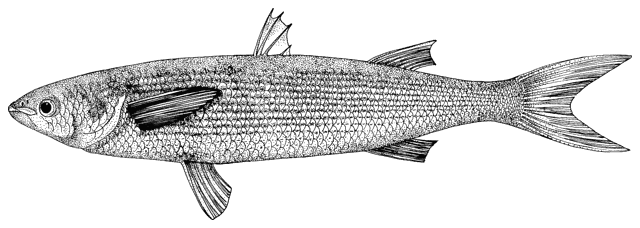
3b. Pyloric caeca in two
groups, 3-5 short and 3-4 long; scales of head and back with 2-7 or more
grooves; oral edge of preorbital bone deeply notched = *Liza saliens
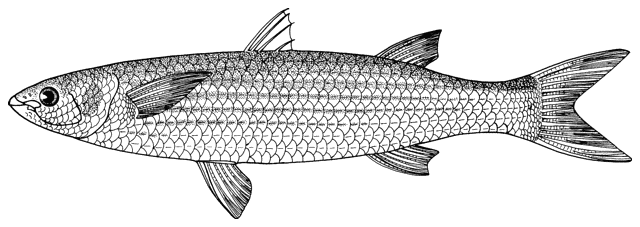
--|----|----|--
Key to the Genera and Species of Nemacheilidae
The following key is modified after Prokofiev (2009).
1a. Anus closer to base of pelvic fins than to anal fin origin; pelvic fin
origin obviously in advance of dorsal fin origin level [Tigris River and
northern Gulf basins] = Turcinoemacheilus kosswigi
1b. Anus closer to anal fin origin than base of pelvic fins, usually at anal
fin origin; pelvic fin origin at or slightly behind dorsal fin origin ---> 2
2a. No c---> 3
2b. Fleshy pelvic fin axillary lobe present ---> 5
3a. Males with fine, brush-like patches of breeding tubercles on pectoral fin
rays (and on sides of head in some species); 3 radial bones in pectoral fin (if
4 then two external ones are flattened, more or less dilated and at least partly
lie over each other) (only visible by dissection or x-ray) = Triplophysa
3b. No such tubercles in males; 4 elongate, cylindrical pectoral fin radial
bones ---> 4
4a. Lateral line short and not extending
end of pectoral fin level; scales absent; manubrium absent or weakly-developed
(in the swimbladder, visible by dissection) [Hormuz and Kor River basins,
northwestern Iran] = Seminemacheilus
tongiorgii
4b.
Lateral line usually complete (if not, then
extends back beyond dorsal fin origin); scales present; manubrium well-developed = Oxynoemacheilus
5a. Cheeks in adult males swollen; no black spot at base of anterior dorsal fin
rays; no bars on body = Paracobitis
5b. Cheeks in adult male snot swollen; strong dark-black spot at base of
anterior dorsal fin rays; usually bars on body ---> 6
6a. Bars restricted to posterior half of body; well-developed adipose crest
supported by 22-25 procurrent caudal fin rays (visible by dissection or x-ray)
[pelvic fin axillary lobe short; no sexual dimorphism; Tedzhen River basin] =
Metaschistura cristata
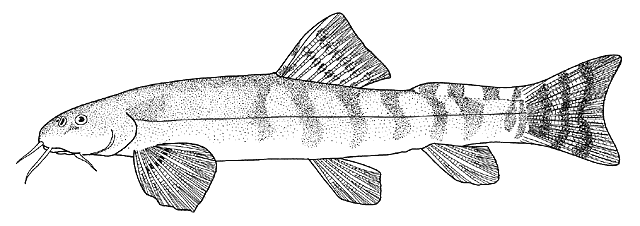
6b. Colour pattern on body not as above; adipose crest weak to absent (if
present supported by less than 15 procurrent caudal fin rays) --->
Paraschistura
Ilamnemacheilus longipinnis ADD to key
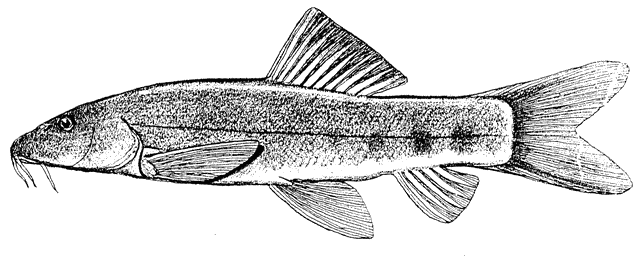
Key to the Species of Oxynoemacheilus
Oxynoemacheilus angorae
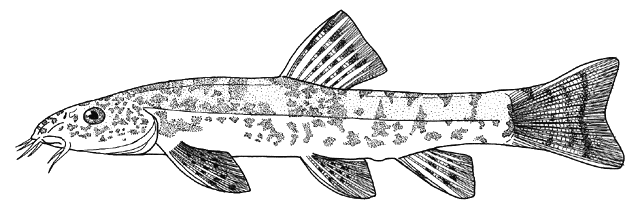
Oxynoemacheilus bergianus
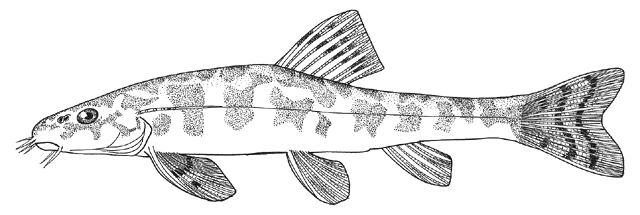
Oxynoemacheilus
brandtii
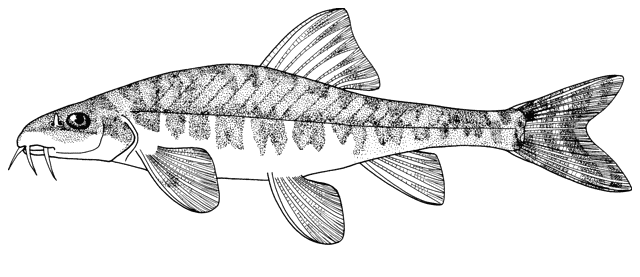
Oxynoemacheilus farsicus
?pic
Oxynoemacheilus
frenatus
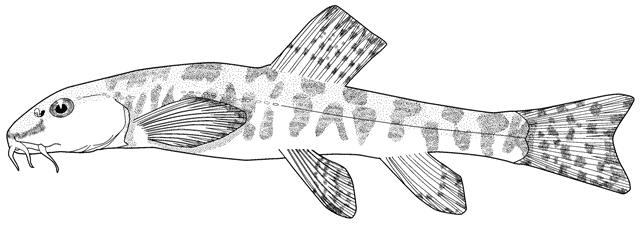
Oxynoemacheilus kermanshahensis

Oxynoemacheilus
persus
1a.
Key to the Species of Paracobitis
Paracobitis iranica
?pic
Paracobitis
longicauda
Paracobitis malapterura
Paracobitis
rhadinaea
?pic
Paracobitis smithi

Dorsal and ventral head views
Paracobitis
vignai
?pic
1a.
Key to the Species of Paraschistura
Paraschistura bampurensis
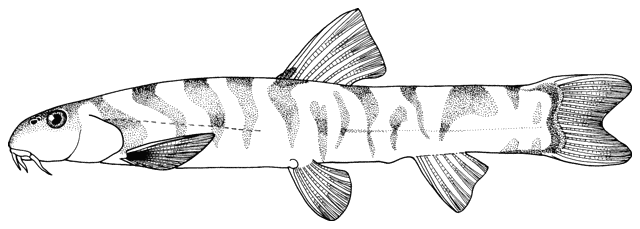
Paraschistura
kessleri
?pic
Paraschistura nielseni
?pic
Paraschistura
sargadensis
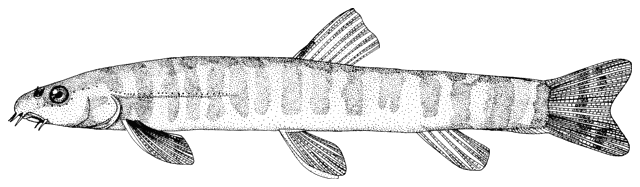
.
--|----|----|--
Key to the
Genera and Species of Percidae
All naturally distributed in the Caspian Sea basin.
1a. Canine teeth absent; prominent bars on flank; anal fin
branched rays usually 8-9, rarely 10; lateral line scales 77 or less = Perca
fluviatilis

1b. Canine teeth present; no bars on flank; anal fin branched
rays rarely 10, usually 11 or more; lateral line scales 78 or more ---> 2
2a. More than 18 branched rays in the dorsal fin; interorbital
width equal to or less than eye diameter [translocated] =
**Sander lucioperca
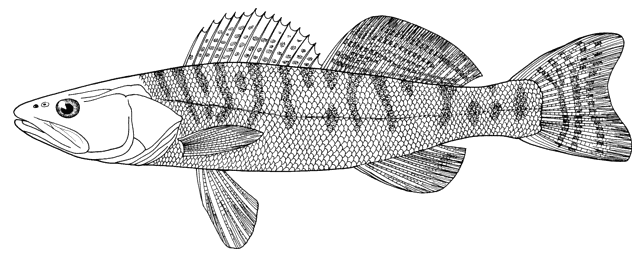
2b. Less than 19 branched
rays in the dorsal fin; interorbital width greater than eye diameter =
Sander
marinus
--|----|----|--
Key to the Genera and Species of *Poeciliidae
1a. Dorsal fin rays 5-9, usually 7; caudal fin not modified in males; widespread
= *Gambusia holbrooki

1b. Dorsal fin rays 11 or more; lower lobe of caudal fin greatly elongated in a
sword-shape in adult males; Gulf and Namak Lake basins = *Xiphophorus hellerii
?pic
--|----|----|--
Key to the Genera and Species of Salmonidae
NEEDS to be checked
?
1a. Teeth in lower jaw absent or weak and brush-like; scales
large, 13 or less from dorsal fin origin to lateral line, 100 or less in lateral
line CHECK; caudal fin clearly forked ---> 2
1b. Teeth in lower jaw strong and conical; lower jaw long,
extending back to or past mid-eye; scales small, 19 or more from dorsal fin
origin to lateral line, 115 or more in the lateral line CHECK; caudal fin
truncate ---> 3
2a. Mouth small? define; snout projects beyond lower jaw; ?, lower jaw not
projecting markedly beyond upper jaw CHECK; teeth in roof of mouth few or
absent; head length usually 4 times or more in standard length; body not
pike-like; Namak Lake basin = *Coregonus lavaretus
2b. Mouth large?; snout not projecting; lower jaw obviously
projecting beyond upper jaw; teeth in roof of mouth in broad bands; head length
usually less than 4 times in standard length; body pike-like ?fusiform?'^;[Caspian
Sea basin =
Stenodus leucichthys
3a. Major anal fin rays 12 or more; anterior edge of preoperculum meeting orbital bones; pyloric caeca 140-249 ---> 4
3b. Major anal fin rays 13 or less; gap between anterior edge
of preoperculum and orbital bones; pyloric caeca 23-66 ---> 5
4a. [Caspian Sea basin] =
*Oncorhynchus keta
4b. [widespread]= *Oncorhynchus mykiss
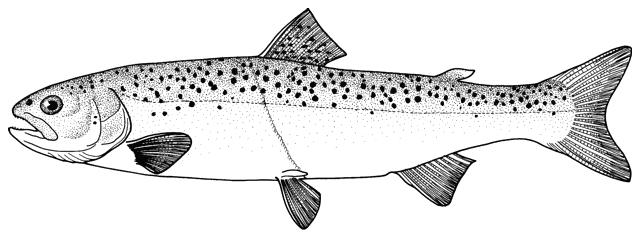
5a. Body with dark spots on light background; vomer with
teeth on head and shaft; lower fins without white leading edge ---> 6
5b. Body with light spots on dark background; vomer with
teeth on head only; lower fins with white leading edge [Namak Lake basin] = *Salvelinus
fontinalis
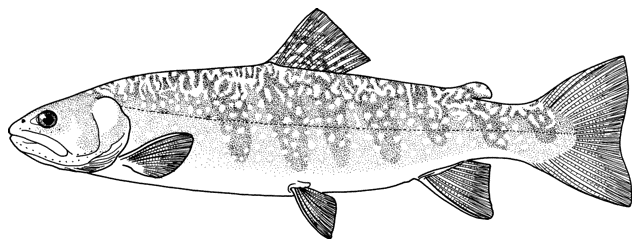
6a. ? [Caspian Sea, Lake Orumiyeh and Namak Lake basins and translocated]=
Salmo caspius

6b. ? [widespread] = *Salmo trutta
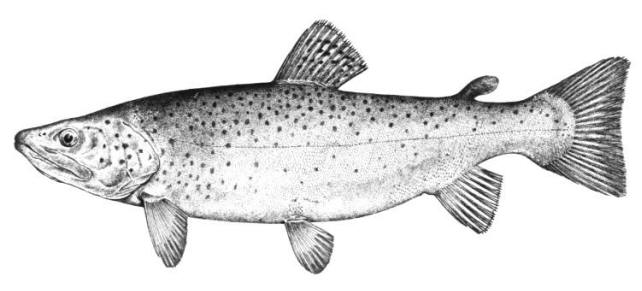
--|----|----|--
Key to the Species of Siluridae
1a. Teeth short and weak (not snaggly); upper and lower jaws meeting at an antero-dorsal position;
finely serrate or smooth pectoral spine posteriorly; colour dark; Caspian Sea,
Lake Orumiyeh and Tedzhen River basins =
Silurus glanis

1b. Teeth robust and long
(snaggly, catching on flesh); the upper and lower jaws meet at a dorsal and
superior position; distinctly and coarsely
serrate pectoral fin spine posteriorly; colour light; Tigris River basin =
Silurus triostegus
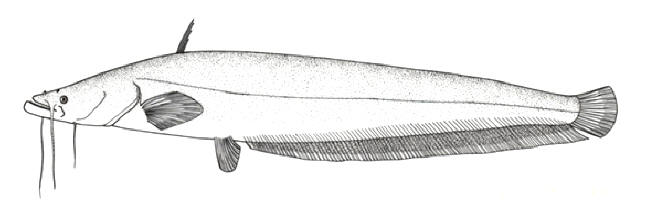
--|----|----|--
Key to the Species of Sisoridae
1a. Head and body dorso-laterally with striated or elongate tubercles;
thoracic adhesive apparatus is wider than long; caudal peduncle short (5.9-6.0
in standard length) [Tigris River basin] =
Glyptothorax kurdistanicus
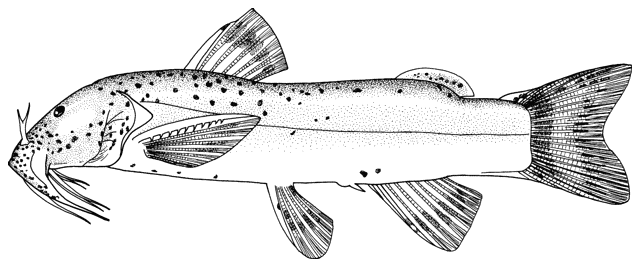
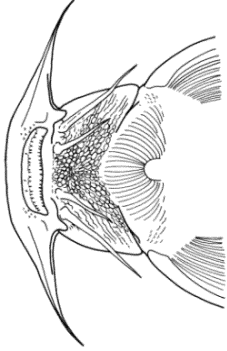
1b.Head and body dorso-laterally without striated or elongate
tubercles; thoracic adhesive apparatus
longer than wide; caudal peduncle long (4.7-5.2 in standard length).
[Gulf and Tigris River basins] = Glyptothorax silviae
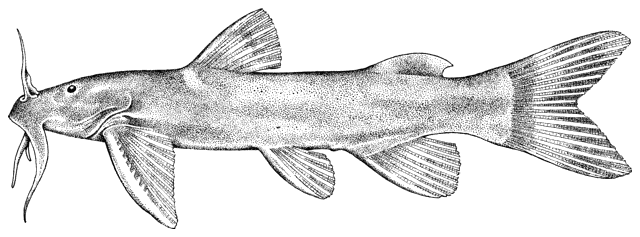

Species AccountsThe species dealt with here in detail
have all been recorded
from Iran and confirmed by specimens. Mention is also made of other species
which occur on the borders of Iran or in drainage basins shared with Iran.
These have no valid Iranian record but may eventually be found in that
country. The listing here is selective from other papers by me on neighbouring
countries as a number of species are unlikely to enter Iranian waters because
their distributions are too remote, e.g. Cobitis elazigensis from
the Tigris-Euphrates basin at Elazig in Turkey or too restricted, e.g.
Typhlogarra widdowsoni from a cave in Iraq (see Coad, 1991b). Coad
(1995a) gives a more complete listing of species found in waters neighbouring Iran.
The most recent checklist on this fauna is by Esmaeili et al. (2010).
The definition of freshwater here includes the southern Caspian Sea
which is at one-third seawater and has both nominally marine and
freshwater species in its fauna.
The choice of introduced species to include in the Species
Accounts is somewhat arbitrary. Soviet authorities introduced a number
of species into the Caspian Sea and its tributaries and some of these became
well established, spreading to Iranian waters, e.g. Liza aurata
and Liza saliens, now commercially important. Other species did
not become established but the potential for spread was there and so they
are mentioned briefly in the Species Accounts. In northeastern Iran, the Tedzhen River flows into Turkmenistan and a number of exotic species are
known from this former Soviet republic (see Aliev et al., 1988;
Shakirova and Sukhanova, 1994; Sal'nikov, 1995). I have listed here only
ones reported from the Tedzhen River basin and its reservoirs. The Tedzhen
(Hari Rud in Iran) connects with the Karakum Canal which harbours a number
of exotics as well as species from the Amu Darya. These may be able to
colonise Iranian waters should they reach the Tedzhen River but are not
included here in the absence of definite records.
A paper in Farsi by Farid-Pak (1957) records the grayling,
Thymallus thymallus (Linnaeus, 1758), the lacustrine smelt Osmerus
eperlamus eperlamus (sic) m. sprinchus (sic)
(= Osmerus eperlanus eperlanus morpha spirinchus Pallas,
1814) and the sculpin Cottus gobio koshewnikowi Grazianov, 1907
from the Caspian coast of Iran but the first two species are distributed
in waters remote from Iran and the last has not been recorded south of
the Caucasus (Abdurakhmanov, 1962; Abbasov, 1980). They are assumed here
to be misreadings of the literature and are not included in the species list.
Some marine species penetrate the fresh waters of
southern Iran from the Persian Gulf and Sea of Oman. These species are
included in a Marine List under Checklist in the
Introduction.
They are not included in keys but more detailed descriptions of these
fishes can be found in the literature listed in the Bibliography
such as Blegvad and Løppenthin (1944), Randall et al. (1978),
Kuronoma and Abe (1986) and Assadi and Dehqani Posterudi (1997). Certain marine species do, however, spend
a significant part of their life cycle in brackish to fresh water and are given full
accounts as freshwater fishes, e.g. Carcharhinus leucas and Tenualosa ilisha.
Choice of other marine species to be given a full treatment is
dependent on frequency of capture, residence time and distance from the sea.
Coad (1991b; 2010) and the website Freshwater Fishes of Iraq
give a list of marine species known from the Tigris-Euphrates
basin but these are mostly records from the Shatt al Arab and Hawr al Hammar
in Iraq which are under tidal influence. Hussain et al. (1989) give
an account of seasonal fluctuations in species composition in the Shatt
al Arab, Iraq. Little or nothing is known of the biology of these species
in fresh and brackish waters. They are listed here to give an idea of the
diversity of species which could be found in Khuzestan and in rivers along
the Persian Gulf coast but are not covered in detail unless verified for
Iran. Al-Daham and Yousif (1990) list additional species in an Iraqi estuary
but do not distinguish the marine species which entered purely fresh water.
Taxonomy and systematics are active disciplines
and scientific names of families, genera and species recognised from Iran can
change. Older literature will be under the former name and searches for
information in such fields as ecology should take this into account. These are
described under the appropriate taxon but some significant changes, relevant to
the Iranian species only, can be simply summarised as:-
Family changes:-
Cobitidae becomes Cobitidae and Nemacheilidae (the
latter formerly Balitoridae).
Gadidae
becomes Lotidae.
Generic changes:-
Caspialosa becomes Alosa.
Barbus becomes Barbus,
Carasobarbus, Kosswigobarbus, Luciobarbus, Mesopotamichthys,
and Tor.
Chalcalburnus
becomes Alburnus.
Gobio
becomes Gobio and Romanogobio.
Leuciscus becomes Petroleuciscus and
Squalius.
Cobitis becomes
Cobitis and Sabanejewia.
Nemacheilus becomes Ilamnemacheilus, Metaschistura,
Oxynoemacheilus, Paracobitis, Paraschistura,
Seminemacheilus, and Triplophysa.
Lebias becomes Aphanius.
Neogobius becomes Babka, Chasar,
Neogobius, and Ponticola.
The Species Accounts are arranged by family after Nelson (2006).
A higher classification can be found in the
Checklist in the Introduction.
Each Species Account is comprised of the following parts:
a) Illustration
The species is illustrated by a line drawing which is accurate in respect of
body shape, number, position and shape of fins, scales and other structures.
This drawing is usually a composite one, based on both a variety of published
illustrations and on specimens.
Further illustrations are from various sources as indicated, are of varying quality and format,
and may include colour and black and white photographs.
Diagrams may also be found in the Keys to illustrate
characters not apparent in the main drawings, such as mouth structure.
b) Map
Distributions are summarized in the form of a map. Often two maps are given,
one for the whole of Iran and one zooming in on distribution if restricted to a
particular part of the country. The
maps are from a world map layer provided by Demis bv (www.demis.nl), accessed
through http://linuxgurrl.agr.ca/mapdata/itis/itisrosa.php.
Maps must be examined in conjunction with the text Distribution (see below). Map
points are are a reflection of adequately documented museum collections and
literature. As such they reflect catchability, ease of identification, rarity,
size (large species not as easily preserved in museums as small ones but perhaps
better documented, even if only in general), field work, available nets and
other equipment, contiguity to research stations and universities, road
accessibility, commercial interest, research interests, and so on. However,
while bearing all these variables in mind and reading the Distribution
summary critically, it is possible to gain a picture of fish distributions and
objective rarity of species.
Other
sources of distributional data are field notes (principally mine and those
of V. D. Vladykov) and sight and field records transmitted to me verbally
by sources judged to be authoritative.
Note that many of these localities were ascertained in pre-GPS days from maps of
varying quality and literature requiring some careful interpretation. Maps
available in the field did not always match maps examined later and once I was
lost for a whole day. Zooming in reduces accuracy proportionately.
Each symbol may represent more than one record
because of the scale of the map or because of repeated visits to the same
locality. Localities have not been sampled on a regular basis so population
trends cannot be given. The general distribution in Iran and elsewhere
is also given textually as outlined below.
The best records are those based on collections in a museum
as these can be re-examined should any questions arise about identity and
field data notes can be re-assessed for accuracy. However, the data associated
with many museum collections are too vague or too contradictory to be included
on maps with a locality symbol.
Criteria for inclusion of literature mapping records are as follows:-
1. Accurate identification (e.g. on geographical grounds;
uniqueness of species so it could not possibly be anything else; lack of
systematic/taxonomic confusion; distinctive characters cited in the text,
drawn or photographed; assessed competence of author in identification),
2. Accurate latitude-longitude data. Latitude-longitude
may be given by the author or derived by me from the literature based on
maps and gazetteers, unique locality names, and my field experience close
in time to when the material was recorded (road/river crossings have changed
in some areas with new construction after the Islamic Revolution). One
exception in accurate latitude-longitude data is that of migratory fish
- if reported from a named river then the river mouth can be recorded since
the fish pass this point on their migration (but few works mention the
extent of upriver migration so no upper limit can be deduced; when an upper
limit is given this is spot mapped; then the species is theoretically present
in a continuous distribution from mouth to upper limit
along the river but this distribution is not filled in and this presence along the
river must be assumed from the known migratory habit).
Criteria for exclusion of literature mapping records are
as follows:-
1. Generalised localities are not accepted, e.g. Safid
River is not accepted since the actual locality along this river is unknown
(except migratory fish - see above); landing ports, fish markets and fish
farms are not included as localities unless the fish capture site or release
site is known,
2. Localities with non-unique names, e.g. Hosseynabad,
a common name for many villages; Shur River, a common name for any brackish
stream, unless these have accurate qualifying data,
3. Descriptions with internal inconsistencies which cannot be resolved to one locality,
4. Named sites which cannot be found in a gazetteer; this
is often a problem with Farsi names transliterated into various European
languages with widely differing orthography,
5. Literature records which conflict with original field
notes, jar labels or catalogues unless the literature explains why it differs.
Under Sources is a partial list of material examined, most with
latitude-longitude. Some material was identified and is used in mapping
distributions but lengths were not taken and that material is not listed.
Sometimes fish were spirited away to be eaten, fell back in the river, leaped
over nets, were kept by another researcher, were seen on market stalls and the
source was given verbally, and so on. Collections in Sources may be
annotated as "no other locality data" indicating that the collection data could
not be interpreted to a latitude/longitude or was internally contradictory.
c) Scientific and Common Names
The use of scientific names is described in the
Introduction.
Scientific names are dynamic and can change as knowledge of the fishes
increases. The ones used here are the latest available.
Common names in Farsi are given with the English translation
in parentheses. Obviously some Farsi names are
merely a translation from the English common name. Note however that some
Iranian names are originally Arabic or Turkic in origin and I have not
always been able to track their meaning. Some species have no common name
and none has been advocated. Others have a common name which is applied
to all members of the same genus (e.g. nemacheilid species are called
mar mahi (= snake fish)) but this has not been repeated under each Species
Account. The common name in Russian, Arabic, Azarbaijanian, English and
from Pakistan is also given to facilitate communication and understanding;
these names are in brackets.
There are often many common "book" names for Caspian Sea
fishes. This is a result of the Russian designation of subspecies and other
categories such as natio. The names are often based on geographical locations.
These names are included here, although many of the taxa are not now recognised,
as an aid to study of the literature. The names are probably not used locally.
Azerbaijani names appear to follow mostly the Russian designations for
these subspecies and again may not be truly local names.
The names cited as by J. J. Heckel in Arabic are also
of dubious value. They are quite old, often from areas remote from Iran,
and may not be in use today. A number of common names whose origin is Arabic
are in use in Khuzestan however, although transmogrified into Farsi.
d) Systematics
An extensive synonymy or historical treatment of the mis-application
of scientific names is not given. Some earlier names can be found in synoptic
works such as Berg (1948-1949; 1949), Coad (1981d; 1985), Krupp (1985)
and others. In certain cases, systematic or nomenclatorial problems remain
unresolved and these are briefly discussed.
Type locality is given for species originally described
from Iran or immediately adjacent waters. This type locality is given as
cited in the original text description in quotes ("....") wherever possible.
Some type localities are not given in quotes, e.g. middle Caspian Sea,
to denote they are a general indication of where the fish was first described
- this is usually applied for older literature not at hand or for fishes
not described from Iran but nearby waters. The original text, jar labels
or catalogues may be compared and interpreted where these are unclear,
contradictory or spellings of place names have changed markedly. Most agree
well between these three sources and are easily located with due allowance
for variant spellings, handwriting skills and transcription errors. Disposition,
number and condition of types may vary with time however. Eschmeyer's on-line
"Catalog of Fishes" has disposition of types but these records are
only as good as the most recent revision of the taxon concerned. Latitude
and longitude are calculated for type localities in Iran wherever possible.
Note that transliteration from Russian names often gives
variant spellings for authors of species names. Actual dates of publication
may vary one or more years subsequent to the date on the journal or article,
i.e. publication may be delayed. This may not be evident from an examination
of the article but may be known to the author or others familiar with the
situation. This has not always been clearly set down in print and accounts
for varying publication dates in different sources.
The disposition and condition of type material is given
where known along with catalogue numbers. Museum acronyms are from Leviton
et al. (1985) but these may change, notably ZIL (Zoological Institute,
Leningrad, U.S.S.R.) became ZISP (Zoological Institute, St. Petersburg,
Russia) and the British Museum (Natural History), London became the Natural
History Museum but retained BM(NH) as its acronym. Note that knowledge
of type material in museums changes as the specimens are examined over
time. Not all new information is published as it is the result of in-house
curatorial work and may only be available in catalogues and jar labels.
The information cited here is the most recent available to me.
Subspecies and lower, non-taxonomic categories have received
names. Such taxa (and non-taxa) have a narrower range of meristic characters and
certain distinguishing other characters compared with the species. Ranges and
descriptions apply to the species as a whole, since many subspecies appear to be
ill-founded where they have been studied in more detail, and indeed some species are not distinct but members
of a wide-ranging and variable species. Certain subspecies may be valid,
or their status is undetermined by recent study, and characters for these
are given separately, either here or in Key characters or Morphology.
e) Key characters
The characters detailed here will separate the species
from any Iranian freshwater fish. These characters (and the keys) should
not be used to identify species from countries bordering Iran as they are
specific to Iran.
f) Morphology
Under this heading are described a number of features
which add to the key characters in describing the fish. Morphometric characters
are not often used since the shape of body parts can be seen in the drawing
and such characters vary greatly with sex and size in contrast to meristic
characters. The accurate explication of morphometric characters depends
on comparative statistics and is beyond the scope of this work. The assessment
of variation between adults and juveniles or between geographical localities
is limited by material and its presentation here by space.
The chief characters summarised here are meristic or countable
characters. These include counts of scale, fin rays, vertebrae, gill rakers,
and teeth. They are summarised as ranges based on literature sources (including
my own data where this expands ranges). In certain cases literature data
is extensive and swamps the few specimens available from Iranian waters.
The literature ranges give an indication of how variable a species may
be in a given character; data on a few Iranian specimens would give a misleading
picture of potential variation which future students of Iranian fishes
may find. Counts from Iranian specimens made by me are given with frequency
in parentheses, e.g. dorsal fin branched rays 7(3), 8(34), 9(5) indicates
that 3 fish had 7 branched dorsal fin rays, 34 fish had 8 branched rays
and 5 fish had 9 branched rays.
g) Sexual dimorphism
Males and females often differ markedly in appearance,
whether in colour, body proportions or in structural features and these
are detailed here to obviate misidentifications.
h) Colour
The colour patterns of fresh and preserved specimens including
males and females, young and adult, and spawning and non-spawning individuals
are given where known. Colour can be a key character in determining the
species but is also variable and should be treated with care in identifications.
Some fish change colour to match their background or pale in response to
a threat. Fish from muddy waters in Iran are often washed out and greyish
in colour. Immersion in ice water enhances the colour patterns and some
of this is retained in preservative.
i) Size
The maximum reported size is recorded as total length
or standard length (if not specified then the source did not indicate which
length was measured) and weight where known. These measures are not restricted
to Iranian specimens since sample sizes are small for some species and
would give a false picture of maximum size.
j) Distribution
This section summarises distribution for the whole range
of the species both within Iran and the rest of the world. Within Iran
the general distribution is given. The detailed mapped distributions are
based on collections or literature with adequate data (see above under Map). Some literature
and museum records are given simply as, e.g. "Safid River", which cannot
be mapped accurately but can be cited in this section. Some literature records
are included here but not every locality based on my field collections as these
are summarised on maps. Not every river
mentioned in the literature is listed here, as common species are assumed to be
widely distributed within a basin; generally only those major rivers or general
localities that are in basins without a mapped distribution are cited.
k) Zoogeography
The relationships of the species, its origins and movements
in the past are given here, where this has been determined.
l) Habitat
The type of habitat favoured by the species is outlined
and includes such factors as altitude, substrate, temperature, salinity,
oxygen, flow regime, pH, vegetation, turbidity, pollution resistance, etc.
There are few detailed studies of habitat requirements for many species:
some can be deduced from morphology. Field data can give a partial picture
but are often limited to one time measurements of seasonal and daily variables
such as temperature which are necessarily of restricted value.
Colour illustrations of habitats are included where available.
m) Age and growth
This section, and the following two sections, either have
no information or masses of information. The Caspian Sea basin species
are often widely known and have books and numerous papers written about
them. There is also a vast "Soviet" literature on some of these species
but I did not have the time nor the resources to digest it all. Here only brief
summaries can be given and it is not always clear whether the Iranian populations,
often at the southern edge of the species range, or recognised as a distinct
subspecies, have the same general ecology as European or more northerly
"Soviet" populations.
Most species outside the Caspian basin are poorly known
ecologically. I have attempted to summarize what is known based on literature
in particular from Iraq and Turkey where ecological studies of varying
quality have been published on some of the species. Morphology can be used
to gain a general picture and knowledge of related species helps.
Generally growth in fishes is fastest in the youngest
age groups, slowing with age and with investment in reproduction. Maximum
age varies considerably, some small species living only a few years while
others are much larger and are reputed to live longer than people. Conventionally,
age may be represented by a number then the + sign, e.g. 0+ = a fish in
its first year of life, less than one year old; 6+ = a fish between 6 and 7 years old.
n) Food
Diet is reported from literature studies and from brief
examination of gut contents by me. Diet varies seasonally, daily, with
age, between sexes, and with changes in environmental conditions but most
fish concentrate on one or a few major groups. These are scrapers, invertebrates
and fishes, and rarely aquatic macrophytes.
o) Reproduction
The spawning season, migrations, egg numbers and diameters, and reproductive
behaviours are recorded here. Some migratory behaviour and ages at spawning may
be recorded in the the Habitats and Age and growth sections.
p) Parasites and predators
This section contains information on the parasites and
predators of the species described. I have recorded only parasites known
from Iranian populations. There is a more extensive literature on Iraqi
populations (see Mhaisen, 1980; Coad and Al-Hassan, 1989) and on European
or Caspian Sea populations (see Romanov, 1955) for species found in Iran.
For eastern waters consult Moravec and Amin (1978) on Afghanistan and Mirza
(1978) on Pakistan. In the absence of definite records for Iran and in
the interests of saving space, I have not cited this extensive literature.
There are a number of piscivorous birds in Iran (see Scott
et al. (1975), Behrouzirad (2007) and general field guides) and these take fishes but
there seems to be little direct observation on the fish species preferred.
q) Economic importance
Note that fishery information may be given on an annual
basis but the year reads 1965-1966 or 1965/66; Iranian years start in March
and run across 2 western calendar years.
r) Conservation
This section details conservation measures undertaken or needed for the species. A
general survey of conservation status of native Iranian freshwater fishes is
given by Coad (2000a).
s) Further work
This section gives some suggestions for knowledge gaps that should be filled.
t) Sources
This section refers to papers or synoptic
works on the species in addition to those cited in the text. It
should be noted that a number of synoptic works refer to several species
in Iran, e.g. Berg's "Freshwater Fishes of the U.S.S.R. and adjacent countries",
and these are not listed repetitively under each Species Account although
they are to be found in the Bibliography. Web sites or URLs are cited as
documentation of statements but it should be noted that these may become broken
links and they are not continually verified as active.
Descriptions are based on Iranian specimens wherever possible but additional
material from neighbouring countries has also been examined. Meristic counts,
for example, are given as frequency distributions for Iranian material while general
ranges for these characters are based on Iranian material, on literature and on
counts of other specimens listed here briefly. Descriptions are also based on
material seen in bazaars or captured in the field but not retained, and on
photographs, drawings, field notes of other collectors, and verbal descriptions
of other scientists.
Details on collections are on file at the Canadian Museum of Nature, Ottawa and in other
institutions as recognised by their acronyms. Locality data is given in short form
and the reader is referred to the website of the relevant museum for further information.
Locality names are taken from U.S. Board on Geographic Names publications and
these may vary from names on labels in museums. The Board names contain both
conventional and local Farsi, Arabic and Turkish names of localities. I have
interpreted names as best I can and have, for example, retained English names
for major water bodies and towns where a strict usage would be bewildering, e.g.
Harirud = Tedzhen River, Sefidrud = Safid River, Al Mawsil = Mosul, Darya-ye
Mazandaran = Caspian Sea, and so on. Sometimes a collection is annotated as "no other locality data", indicating that
no further details are known or localities cited could not be found on maps or
in a gazetteer (and thus there is no latitude-longitude). Collections listed as uncatalogued are mostly held in the Canadian Museum of Nature and may eventually
receive a catalogue number. The collections listed are those examined for morphology.
Map records include these collections, other collections checked for identity and
locality only, and literature sources, all kept in a database held at the Canadian
Museum of Nature: these would be too lengthy to list here.
Petromyzontidae
Back to Contents
Lampreys in the family Petromyzontidae are found in cooler waters of the northern hemisphere,
with a few related species in other families in the southern hemisphere.
Their origins lie at least 300 million years in the past.
There are about 37 lamprey species with only 1 recorded from Iran.
Lampreys are jawless fishes, lacking bone in the skeleton and
having 7 pairs of pore-like gill openings. The eel-like body has no
pectoral or pelvic fins. There are 1 or 2 dorsal fins and a caudal
fin. An anal fin-like fold develops in spawning females. The mouth is
a suctorial disc armed with rows of horny teeth. There are also teeth
on the tongue. The median nostril, or nasohypophyseal opening, is not
connected to the mouth. There is a light-sensitive pineal organ or
"third eye" behind the nostril. The skin is covered in mucus
which is poisonous to fishes and humans. Lampreys are edible if the
mucus is cleaned off.
Their tooth arrangement is used in classification and
identification along with the number of myomeres (muscle blocks along
the body). Both tooth counts and the number of cusps are used, in
particular those on the supraoral lamina (bar above the
"mouth", the oesophageal opening), the infraoral lamina (bar
below the "mouth") and the row of teeth on both sides of the
"mouth". There are various series of smaller teeth and of
course teeth on the tongue. Larval lampreys lack teeth and are
particularly difficult to identify and their determination often
requires specialist knowledge. Characters for the larvae include
counts of myomeres and pigmentation patterns.
Lampreys have an unusual life cycle. Adults die after spawning and
the eggs develop into a larva, known as an ammocoete, which lacks
teeth, has an oral hood, eyes covered by skin, a light-sensitive area
near the tail, and is a filter-feeder while buried in mud and silt.
Fleshy tentacles in the oral hood are used to extract minute organisms
from the water, such as algae (desmids and diatoms) and protozoans.
After several years (up to 19 but usually 7 or less), the ammocoete
transforms into an adult with enlarged eyes, teeth, a different colour
and pronounced dorsal fins. The body shrinks during this metamorphosis
and adults are only larger than ammocoetes if they feed. The adult may
be a parasite on other fishes and marine mammals, or non-feeding.
Individuals of a species may or may not be parasitic and different
species may be parasitic or non-parasitic. The non-parasitic species
are believed to have evolved from a parasitic species so there tends
to be closely related parasitic/non-parasitic species pairs.
Parasitic adults feed mostly on other fishes, attaching to their
bodies by suction and using their toothed tongue to rasp through the
skin and scales to take blood and tissue fragments. Prey is detected
by sight but some lampreys attach to hosts during the night. Perhaps
this reduces their own predation risks and enables them to approach
their quiescent hosts more easily. Lampreys tend to select larger fish
as these survive longer and ensure a good food supply. The flow of
blood is aided by an anti-coagulant in lamprey saliva called
lamphedrin which also serves to break down muscle tissue. The attack
may weaken or even kill the host. Weakened fishes are more prone to
diseases and the wound provides an easy path of entry for them. The
fish (and marine mammal) species parasitised are varied and reflect
availability in the habitat.
Marine lampreys enter fresh water to spawn and freshwater species
may move into or up streams. The scientific name of the family means
"stone sucker" and the adult mouth is used to hold or suck
onto stones as well as on prey. This suction enables the lamprey to
maintain position in fast-flowing streams when spawning and even to
climb over rapids and small waterfalls. Usually spawning occurs in
shallow water with a moderate current, a bottom of gravel and nearby
sand and silt for the ammocoetes to live in. Either or both sexes
build a nest by moving gravel around with their sucking mouths and by
thrashing their bodies. A shallow depression is formed, about 0.5-1.0
metre long. Spawning often occurs in groups and several males may
attach to a female with the sucking disc. The process takes several
days as only a few white to yellow eggs are laid at a time. The eggs
are adhesive.
Adult lampreys are usually caught when attached to a host or when
spawning. Electro-shocking will force ammocoetes out of their u-shaped
burrows to the surface and immobilize adults. They sometimes attach to
boats and occasionally to human swimmers when their skin is cool but are
easily removed, perhaps because nobody has left a lamprey on their
skin long enough to see if the tongue starts rasping flesh!
Genus Caspiomyzon
Berg, 1906
This genus is characterised by having 2 dorsal fins, an oral disc
narrower than the body, teeth are generally low and blunt, the
supraoral lamina is small, oval and sometimes has 2 tubercles and
rarely 2 teeth, the infraoral lamina has 4-6, usually 5, teeth which
may be bicuspid at their tips, there are about 8 small teeth of equal
size in the transverse lingual lamina, the exolaterals, anterials and
posterials are strong and close together, anterior and endolateral
circumorals 9-11, usually 11, and 3 long, papillose velar tentacles are present.
The first illustration below shows a notch at the end of the second dorsal fin
which is an error.
There is a single species in the genus found only in the Caspian
Sea basin. Agnathomyzon Gratzianow, 1906 and its subgenus Haploglossa
Gratzianow, 1906 are synonyms of Caspiomyzon (Eschmeyer et al., 1996).
Caspiomyzon wagneri
(Kessler, 1870)
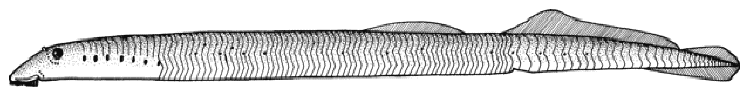
Adult

Adult

Adult

Adult
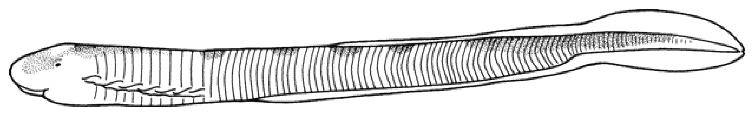
Ammocoete
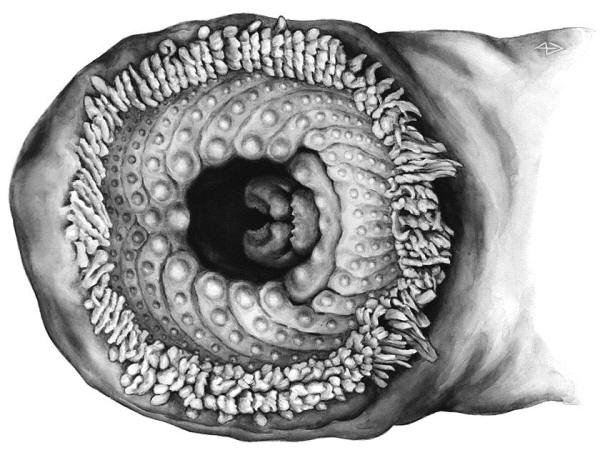
Disc
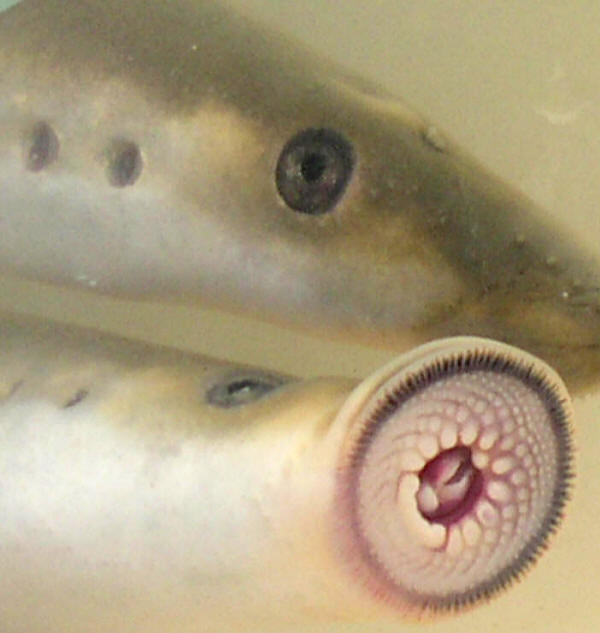
Common names
مارماهي (= mar mahi, meaning snake fish),
مارماهي دهان گرد
(= marmahi-ye dehangerd, meaning round mouth snake fish), mahi dehangerd, mahi dehangerd daryacheh-ye
khazar or dahangerd-e-Daryaye Khazar (= Caspian Sea round mouth fish).
[ilanbaligi or xazar ilanbaligi, djilan-balux or morma in Azerbaijan; kaspiiskaya minoga or Caspian lamprey in Russian; Volga lamprey].
Systematics
The type locality of Petromyzon Wagneri is from the mouth of
the Tvertsa to Astrakhan; Oka and Kama rivers and the 3 syntypes
(29.0-33.0 cm) are in the Zoological Institute, St. Petersburg (ZISP
31) (Holčík, 1986). The Zoological Museum of Moscow University (ZMMU) has one syntype from
the Kura River near Evlakh (P-1393) and one from the Moskva River (P-555) with
P-569 from the Volga River near Kazan being lost (Pavlinov and Borissenko,
2001). The Naturhistorisches Museum Wien in 1997 had one specimen
listed as "? syntype, ? paratype" (sic) under NMW
61053. Agnathomyzon (Haploglossa) caspicus Gratzianow, 1907 is a synonym.
Key characters
This is the only lamprey species in Iran, easily recognised by the
absence of pectoral and pelvic fins, a round, suctorial mouth
containing blunt teeth, and 7 branchial openings.
Morphology
Characters of the species are the same as the genus. Trunk myomeres
number 53-68 in ammocoetes; and 68(2) or 69(1) in adults from Iran. Ginzburg
(1936a) describes ammocoetes from Iran. Renaud et al. (2009) give details
of the feedding apparatus. Nazari et al. (2009) found significant
differences for morphometric, but not meristic, characters, between fish from
the Shirud and Talar River, although a principal components analysis showed
relatively high overlap.
Sexual dimorphism
Females reach larger sizes than males and have a smaller urogenital
papilla. During the spawning migration, the lamprey undergoes certain
morphological changes some of which have been linked to sex of the
fish. The teeth become blunt, fin size increases, the dorsal fins
become almost united at the base in males, and there is a change in
colour. The urogenital papilla length in males increases from a mean
of 1.1 mm to 4.9 mm and in females from a mean of 0.6 to 1.7 mm.
Colour
Adults are dark grey with a silvery-white belly. Spawning adults
become black on the back and flanks with a grey belly covered with
dark oval spots, or are an overall golden colour (Hassan Nazari, pers. comm., 28
July2011, see photo above). Ammocoetes are a pale grey to yellowish with a white belly.
Size
Attains 57.5 cm total length and 205.5 g as the adult and 13.0 cm total
length as the ammocoete. After metamorphosis of the ammocoete there is a shrinkage
in length, the difference between prespawning and spawning adults
being on average 22.3% in Iranian samples (Renaud, 1982). There is
also a small variety which measures 19-31 cm and can attain sexual
maturity at 19.1 cm (forma praecox).
Distribution
Found only in the Caspian Sea and rivers draining to it, in
particular the Volga where it had its largest distribution but is now
known only as far as the Volgograd Reservoir; also in the Ural, Terek,
and Kura rivers. It is recorded in Iran from the upper reaches of the Aras River,
and from the Astara to the Gorgan River along the whole Caspian coast. Specific
localities include the Aras River, Anzali Mordab and the Nahang Roga, Pir Bazar Roga,
Pasikhan River and Siah Darvishan River in the Anzali region, to Kisom on the
Safid River, Cheshmkelya east of the Safid River, Tajan River, Sardab River, Haraz River,
Babol River, Tonekabon River, Pol-e Rud, Gorgan River, and in most
large streams (Derzhavin, 1934; Holčík and Oláh, 1992; Hosseinpour, 1995;
Abbasi et al., 1999; Kiabi et al.,
1999; Abdoli, 2000; Abdoli and Naderi, 2009). Migrations into the Babol, Gorgan and Sardab rivers are
reported by Ghasempouri (1993), the Sardab and Chalus rivers by the Annual
Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran
(1996), and the Shirud (Nazari and Abdoli, 2010), for example.
Zoogeography
Known only from the Caspian Sea, its relationships remain uncertain and
research is ongoing (Claude B. Renaud, pers. comm., 18 May 2007).
Habitat
The habitat of this species in the southern Caspian Sea proper is unknown
although some specimens have been caught in the Caspian at 600-700 m (Jolodar
and Abdoli, 2004). Larvae burrow 1-2 cm into the river bottom and favour areas where
current is moderate at river bends. They can also be found in the
centre of rivers or in backwaters. Fine-grained sand with some ooze
and detritus is preferred at all stages of larval growth but larger
larvae can also be found in a silt-sand bottom with much plant debris
and macrophytes. The ammocoetes select and change habitat according to
sediment size as they grow. They prefer depths greater than 3 m as
protection against drying out, are mostly shallower than 11 m but as
deep as 22 m (Ginzburg, 1970), yet in different rivers or at different
times will be concentrated in water of markedly different depths, e.g.
30-85 cm versus 6-8 m.
Spawning migrations up the Volga River used to exceed 1500 km but
construction of dams now prevents this. The lamprey migrates in
schools with the smaller fish arriving in estuaries first. Larger
lampreys migrate more quickly and travel further. The speed varies
from 1.9 to 15.9 km/day. The migration is triggered by decreasing
water temperature and increasing water level. The strongest migration
is reported at 6-11°C. Movement upriver only occurs at night, near the surface when dark and
on the bottom when the moon is out. During the day, the lampreys hide
among stones. Body fat in the Volga delta was 34% but by the time the
fish reached the spawning grounds upriver it had declined to 1-2%. In
the Kura River of Azerbaijan, the lamprey migrates at the same time as
the Caspian salmon (mahi azad, Salmo caspius) and often attaches to the opercular region of this
species. The peak of this run is in December and January. The
migration in the Volga takes place from the middle of September to the
end of December. Migrating lampreys prefer a current velocity of
0.4-0.6 m/sec and stay close to banks and the bottom. Prespawning
adults overwinter among stones or in the substrate of rivers.
During winter-spring several individuals may be found coiled in a ball under
stones (Askerov et al., 2001). They hardly respond to external stimuli
such as noise or being handled. Transformed lampreys migrate to the Caspian Sea.
Before breeding, males change colour, increase slightly in size, develop their
fins, and become much more active (Askerov et al., 2001).
Nazari and
Abdoli (2010) note a short fall migration in late September to October with the
main migration being in spring (see below). Movement was mostly at night and
involved swimming and resting attached to the concrete of a bridge used as the
observation post.
Age and growth
The growth rates of metamorphosing lampreys and adults are almost
unknown. Length and weight decrease but coefficient of condition
increases in spawning as opposed to pre-spawning adults. The shrinkage
in mean total length is 18-26%. Females are heavier than males up to
about 43 cm but past this point males weigh more. There are 3 age
groups of larvae in the Volga (Ginzburg, 1970), with average lengths
of 3.1 cm, 6.2 cm and 10.1 cm and 2-4 age groups in the Kura. In their
fourth year of life they metamorphose to adults after a downstream
migration into the Caspian Sea. Adult life span is at least 1 year and
5 months. Maturity is attained in May and the beginning of June in the
Volga, and from May to the end of July in the Kura River. Mature
lampreys are mostly 35-41 cm in the Volga and 41-46 in the Kura River.
The female lamprey dies after spawning but the male may live longer
until sperm production ceases.
Nazari et al. (2010) investigated growth parameters in fish from the
Shirud and Talar River. Most fish were were in the 367-369 mm length group,
length-weight relationship was positive, high and significant, growth was
negatively allometric, the coefficient of condition was higher in females, sex
ratio was nearly equal, and growth parameters were similar in the two rivers.
Food
Abakumov (1959) maintains that this lamprey attacks Caspian salmon
(Salmo caspius) based on nineteenth century observations
by Kessler (1870a) and Kavraiskii (1896-1897). Lelek (1987) also
considers it to be parasitic. The lampreys may only have been using
Caspian salmon for transport. Certainly the teeth in this lamprey are
blunt, unlike those in lamprey species known to parasitise fishes. In
contrast, Holčík (1986) states that it is non-parasitic and Ghasempouri (1993) agrees.
Renaud (1982) supposes that adults feed on amphipods since juvenile
acanthocephalans (Corynosoma sp.) are found in prespawners.
This worm has amphipods as the intermediate host. However, Holčík (1986) thinks that
the acanthocephalans are swallowed while the adult
lampreys are feeding on the internal organs of dead fish they
scavenge. Certainly larvae of Corynosoma strumosum (perhaps
correctly C. caspicum: B. Kiabi, in litt., 1994) are
found only in the body cavity of fishes. Renaud et al. (2009) list it as
a carrion feeder but note the well-developed buccal glands which may compensate
for the blunt teeth and it may well feed on fishes. The feeding habits of the
adult of this species remain to be confirmed by direct observation. Gut contents include
aquatic vegetation in Iran and in the Volga delta. Migratory,
transforming and spawning lampreys do not feed. The gut diameter
decreases from 2.7 mm in prespawners to 1.4 mm in spawners in Iran (Renaud,
1982). Ammocoetes feed on detritus and diatoms.
Reproduction
Ginzburg (1969; 1970) examined the reproduction of this species
below the Volgograd Dam on the Volga River and similar conditions may
obtain in Iran. The dam has probably increased fecundity by reducing
the length of the spawning migration so that the fish have more energy
reserves for egg production. A spawning migration exists from December
to May with a peak concentration in the second 10 days of February
although the catches declined in April at least in part because of the
opening of the spillway of the dam. Before the dam was built the
migration from the Caspian Sea passed through the delta from
mid-October to mid-December, with a peak in December. The fish
migrated when water temperatures reached 10-11°C
and moved through channels where the current was strongest. Spawning
begins at 15-16°C, usually in early June but sometimes at the end of March through to the
beginning of July, and temperatures during spawning are usually 15-23°C.
Each female produces up to 60,000 turquoise or blue-green eggs and
spawns once in her lifetime. Eggs are ovate and diameter reaches 1.5
mm. The eggs are laid on coarse to fine-grained, turquoise sand at a
water depth of 3.5-19.0 m, sometimes shallower. The egg colour is
cryptic against the sand substrate. Many eggs are carried downstream
by the current. A redd is excavated in sand or gravel by the male or
by the female (authors differ on this point) and the lamprey attaches
to stones by their suctorial disc. The male attaches to the female's
head with his disc and wraps his body around hers. The tails of both
fish quiver and eggs and sperm are released at the same time. Females
release all their eggs but males may spawn again with other females.
Ammocoetes hatch after 8-10 days at 17-23°C.
Metamorphosis of ammocoetes occurs at 8.0-11.0 cm in October in Iran.
Nazari and Abdoli (2010) examined migration and reproduction in lampreys from
the Shirud in the southern Caspian Sea from 16 March to 2 May at 11.0-21.25°C.
The most intensive migration was at night (peaking at 2100 and declining to 0300
hours) at 16°C (34.4% of the run). About 75% of the run had passed by the
time water temperature reached 16-17°C. Migration stopped when temperature
reached 21°C. Numbers observed each night varied from 1 to 60, average 17, with
peak migrations at 26 March to 10 April and 15 April to 25 April. Sex ratio was
1.07:1 in favour of males but not significantly different. Absolute fecundity
was31,758-51,198 eggs (mean 41,924 eggs) relative fecundity was 80.3-148.1
eggs/mm length (mean 107.2 eggs/mm length) and 260.8-677.4 eggs/g (mean 397.6
eggs/g). Egg diameter was 0.78-1.15 mm (mean 0.92 mm). The gonadosomatic index
of females was 5.83-31.44 (mean 11.22), the peak being in mid-April. Downstream
migrating lampreys were spent but no dead ones were noted so some may survive to
spawn a second time. Two
ammocoetes, 20 and 22 mm long, were found near the mouth of the Shirud River on
18 April 2006 (river bank in a substrate of the sand-mud, water depth <30 cm).
They probably belong to the autumn migratory group (Hassan Nazari, pers.
comm., 28 July2011).
Parasites and predators
See above under Food. Nazari et al. (2010) also record
Corynosoma in their fish. Caspian lampreys are eaten by Silurus
glanis, Lota lota, Sander lucioperca, and Huso huso.
Economic importance
This species was consumed and used for oil extraction in the former
U.S.S.R. (Thomas, 1961; Ginzburg, 1969). Their fat content is so high that they
were once dried and used as candles (Kottelat and Freyhof, 2007) and the high
fat level makes them tasty (Askerov et al., 2001). The catch in the
Volga-Caspian region was 3,420,000 kg or 33.4 million fish in 1913 but
fishing by state organizations ceased after the Volgograd reservoir
was constructed. The mean annual catch in Azerbaijan for 1930-1963
ranged from 10 to 269 tonnes. Local fisheries continue but are of
little significance. It is not commercially important in Iran for
religious reasons but catches of several hundred kilograms can be made
in an hour in such rivers as the Gorgan, Babol and Sardab (Ghasempouri, 1993).
This lamprey is ingested medicinally for treatment of haemorrhoids and besmi
(sic, ?) by Turkmen of the southeastern Caspian (Hassan Nazari, pers.
comm, 29 July 2011).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, in textbooks and
because it is reputedly ichthyosarcotoxic. Intoxication results from
eating the flesh, skin or surface mucus of raw or cooked Caspian
lamprey, the location of the toxin being uncertain. A biogenic amine
is believed to be responsible. Mucus may cause skin irritations.
Poisoning can be avoided by soaking the lamprey in brine as cooking
alone is insufficient. Symptoms develop in a few hours and include
nausea, vomiting, dysenteric diarrhoea, urge to urinate or defecate
without ability to do so, abdominal pain and weakness. Recovery takes
several days and treatment is symptomatic (Coad, 1979b). However
lampreys lack scales and are not eaten in Iran.
Conservation
The Caspian lamprey has been proposed for inclusion in the
"Red Book of the U.S.S.R." which forms the basis for
measures to protect species (Pavlov et al., 1985) and is listed
as "vulnerable" in Europe by Lelek (1987) and Maitland
(1991). It is vulnerable because it migrates into rivers which are
polluted and dammed and because of its restricted and declining
distribution. These conditions apply particularly in Iran, although
there is some evidence for spawning based on captures in the 1990s (Holčík and Oláh, 1992).
Kiabi et al. (1999) consider this species to be near
threatened in the south Caspian Sea basin according to IUCN criteria.
Criteria include medium numbers, habitat destruction, widespread range
(75% of water bodies), absent in other water bodies in Iran, and
absent outside the Caspian Sea basin. Mostafavi (2007) lists it as near
threatened in the Talar River, Mazandaran.
Further work
The question of adult diet remains unresolved and the general
biology of this species in Iran needs to be elucidated.
Sources
The main source of information on this species is the summary by Holčík (1986)
which should be consulted for further details on morphology and biology.
Iranian material: CMNFI 1970-0511, 7 ammocoetes, ? 30-82 mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0514, 33
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0515, 18
ammocoetes, ? 25-98 mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0534, 30
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0535, 14
ammocoetes, ? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0545, 1
adult? see photo?, ?mm total length, Gilan, Safid River (37º01'N, 49º38'E); CMNFI 1970-0546, 2 adults,
352.0-355.0 mm total length, Gilan, Safid River (no other locality data); CMNFI 1970-0547,6
adults and 2 ammocoetes, ? photos? mm total length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0585, 3
adults, 406.0-455.0 mm total length, Gilan, Nahang Roga River (37º28'N, 49º28'E); CMNFI 1971-0327A, 1
adult (part of trunk), Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI
1979-0787, 11 adults, ?mm total length, Gilan, Nahang Roga River (37º28'N, 49º28'E); CMNFI 1980-0118,8
adults, ? mm total length, Gilan, Gelroudkhan River, tributary of the Anzali Mordab (no other
locality data); CMNFI 1980-0119, 10 adults, ? mm total length, Gilan, Gelroudkhan River, tributary of the Anzali Mordab (no other
locality data); CMNFI 1980-0139, 44 ammocoetes, ? mm total length, Gilan, Golshan River estuary (37º26'N, 49º40'E).
Carcharhinidae
Back to
Contens
This family, the requiem or ground sharks, contains more than 50 species of large sharks found
world-wide in tropical to warm-temperate waters. There is only 1
species in Iranian fresh waters. A second species is reported from an Iranian river under special
circumstances and is not regarded as a resident (see
Marine
List). They are distinguished from other sharks by a complex of characters including having an anal fin;
5 gill slits; 2 dorsal fins; no fin spines; nictitating eyelids; and a
scroll intestinal valve. The first dorsal fin base is in front of the pelvic bases; there is a
wavy dorsal tail fin margin; well-developed, knife-like teeth with
cutting edges; usually no spiracles; and precaudal pits.
This is one of the largest and most economically important shark families. Most members are
voracious predators as their common names suggest and they are
frequently dangerous to man. Some of these species enter rivers and
remain there for long periods causing human fatalities. These sharks
are usually viviparous. Food includes a variety of fishes, sharks,
rays, squids, crustaceans, marine reptiles, birds and mammals, and carrion and garbage.
Shark flesh can be eaten and is religiously permissible in Iran.
Genus Carcharhinus
Blainville, 1816
There are about 31 species of gray sharks found world-wide but only one regularly enters fresh water
in Iran. A detailed definition of the genus is given by Compagno (1988).
Carcharhinus leucas
(Müller and Henle, 1839)
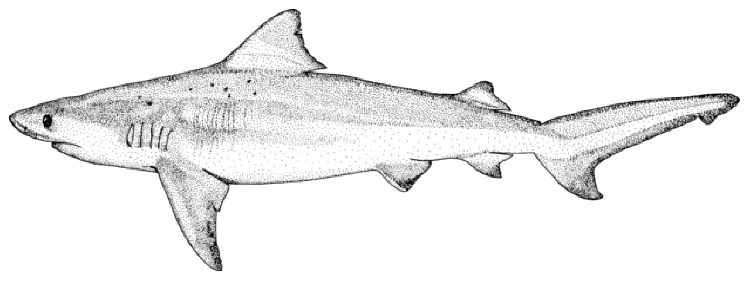
Upper and lower tooth

BM(NH)1924.10.1:1,
Tigris River at Al Karradah near Baghdad

BM(NH)
1874.4.28:9, Tigris River near Baghdad
Common names
kooseh, kuseh, kooseh-kuli, sag mahi (= dog fish).
[kosetch or kossage, jarjur in Arabic; bull shark].
Systematics
Carcharhinus leucas was originally described from the Antilles.
A number of shark species have been reported as entering rivers of the Tigris-Euphrates basin
including Iranian tributaries (Günther, 1874; Day, 1875-1878; Sykes,
1902; Kennedy, 1937; Hunt, 1951; Khalaf, 1961; Mahdi, 1962; Zorzi,
1995; and others). The species appeared under such names as Carcharhinus
gangeticus (Müller and Henle, 1839), Eulamia (= Carcharhinus)
lamia (Blainville, 1820), and Carcharhinus menisorrah (Valenciennes
in Müller and Henle, 1839). A recent revision of carcharhinid sharks
by Garrick (1982) cites only Carcharhinus leucas from fresh
waters of the Tigris-Euphrates basin and Compagno (1984) concurs. Coad
and Papahn (1988) also list specimens which confirm this species to be present.
Key characters
This is the only shark species commonly encountered in Iranian fresh waters and can be
recognised by the 5 gill slits, upper caudal fin lobe larger than
lower, and the arched mouth armed with teeth on the underside of the
head. Distinction from other sharks is given in Compagno (1984).
Morphology
The snout is very short, rounded and ends bluntly. Its length is less than the distance between
the nostrils and much less than the mouth width. There are 12-14,
usually 13, teeth on each side of a median tooth in the upper jaw and
12-13, usually 12, teeth on each side of a median tooth in the lower
jaw. Teeth are heavy, broad, almost triangular, erect near the jaw
symphysis but becoming slightly oblique and more concave or notched
nearer the mouth corners. The teeth are strongly serrated, more so
near the base, and upper teeth more so than lower teeth.
The first dorsal fin lies over or just behind the level of the axil of the pectoral fin. The
apex of the first dorsal fin is pointed to somewhat rounded. The
second dorsal fin is high, has a short posterior lobe and lies just
over the level of the anal fin origin. Pectoral fins are broad and
their tips are narrow and pointed. There is no interdorsal ridge (the
back is smooth between the dorsal fins). The upper precaudal pit is
well-developed while the lower pit is weak.
Sexual dimorphism
Males bear claspers. The pelvic fins are partially modified into grooved, rod-like structures
which are held together to form a tube and are used in mating. They
are not used to clasp the female but as an intromittent organ. Females are larger than males.
Colour
Fin tips are dusky to black, particularly in young. There is no other obvious colour pattern
although the back is darker than the belly, being bluish, grey or
brown. Fins are similar in colour to the neighbouring body.
Size
Attains 3.24 m (Garrick, 1982), 3.40 m (Carpenter et al. 1997), rarely
to 4.0 m and an estimated weight of over 600 kg (McCord and Lamberth, 2009). Fish in Iranian fresh
waters have been estimated as up to 2 m in length but naturally
circumstances were not always favourable for an objective and detached size judgment.
Distribution
Sharks have long been known to enter fresh waters in the Tigris-Euphrates
basin. Zorzi (1995) records a book by Pausanias,
"Guide to Greece", written in the late second century which
refers to sharks in "the Euphrates...., which fatten monsters as
man-eating as any in existence".
One of the earliest distributional records is found in the Arabic work "Wonders of
Creation" by Zakariya al-Qazwini published in 1263 A.D. and later
translated into Persian. The sharks were found at Basrah on the Tigris
River in what is now Iraq and were cited as formidable because of
their voracity and teeth like the points of spears. Shark attacks
still occur at Basrah (Coad and Al-Hassan, 1989).
Subsequently reported in the Tigris River above Baghdad about 850 km from the sea (Günther, 1874;
Kennedy, 1937) before dams were built. Sykes (1902) saw sharks in the
Ab-e Gargar (Karun River in Iran) at Shushtar 420 km from the sea,
Wilson (1942) reporting on events in 1908 records sharks from between
Shushtar and Ahvaz and near Shushtar, Blegvad and Løppenthin (1944)
report then from Khorramshahr, and Hunt (1951) reported them from the
Karun River, Khowr-e Bahmanshir and Shatt al Arab (Arvand River). Coad and Papahn
(1988) report sharks at Ahvaz on the Karun River about 275 km from the
sea as well as further up river at Shushtar and down river in the Khowr-e Bahmanshir.
Zoogeography
This shark is found world-wide in warm temperate to tropical seas and is reported from
fresh waters in Africa, Asia, Australia and the Americas.
Habitat
This is a shark of coastal waters such as harbours, bays and estuaries but unusually it will
penetrate far up rivers, as far as 4000 km up the Amazon River. It is
said to be a sluggish bottom dweller except when attacking prey and in
the sea may be found down to at least 150 m although usually at less
than 30 m. They are said to invade the Khowr-e Bahmanshir and Karun
River of Iran from July to September when freshwater flow is at a
minimum and tidal penetration of salt water is at its highest. However
they do travel well beyond tidal influence in Iran. Local people along
the Bahmanshir River near Tangeh Se in Khuzestan maintain that it is
dangerous to swim there because of these sharks. They are occasionally
trapped in nets set for Tenualosa ilisha and may be caught on
hooks. They are not as common as in the past (N. Najafpour, pers. comm., November 2000).
Age and growth
Maturity in males is attained at 1.60-2.25 m and in females at 1.80-2.30 m. Mature fish are about 6
years old and life span is up to about 14 years.
Food
Food is a wide variety of fishes including tunas, small sharks, and rays, as well as crabs,
shrimps, molluscs, cephalopods, sea urchins, turtles, sea birds and
mammals. Diet in fresh water has not been investigated in Southwest
Asia although Blegvad and Løppenthin (1944) reported that sharks
station themselves under the date palms at Khorramshahr to eat the falling dates!
Reproduction
Birth size is about 56-81 cm
and takes place in estuaries and river mouths. Females may contain up
to 13 embryos and the gestation period is 10-11 months. This species
is known to breed in fresh waters, such as Lake Nicaragua in Central
America, but there have been no reports of reproduction in the Tigris-Euphrates basin.
Parasites and predators
None are reported for Iran.
Economic importance
This shark has a considerable impact on people using water directly in Khuzestan. A number of severe
injuries and fatalities have been reported in fresh waters through
shark attacks. The first comprehensive report in modern times was by
Hunt (1951) although accounts date back to the thirteenth century (Coad
and Papahn, 1988). The latter summarize recorded attacks and add new
ones for a total of 34 in the period 1941-1985, of which about half
were fatal. Additionally Wilson (1942) reports a woman taken by a
shark while drawing water between Shushtar and Ahvaz and a 9 foot (=
2.8 m) near Shushtar which killed two boys and a girl. These Iranian records are a significant proportion of
freshwater attacks worldwide, about 28%. A number of soldiers were
apparently victims during the Iran-Iraq war but no records have come
to light. Men, women and children are attacked as well as horses and
sheep, only the massive water buffalo is said to be safe. Many minor
attacks and narrow misses are probably not reported. Attacks are said
to have declined in recent years since shark oil is no longer used to
caulk boats but this is probably a local legend. People were attacked
while swimming, paddling, bathing, washing vehicles or fishing. There
was no apparent triggering factors for the attacks as victims were
dressed in various colours and types of clothing, engaged in various
activities and environmental conditions where known varied between attack sites.
Freshwater shark attacks have even appeared in a novel "Harem" by Mossanen (2002).
In other parts of the world, this species has been used for its flesh and fins, as leather, for its
liver oil and for fishmeal. Sharks can be eaten by Muslims if
"reliable experts confirmed that shark fell into the category of
cartilaginous and scaly fish" (http://netiran.com:80/news/IRNA/html/950216IRGG13.html)
which appears to be so (netiran.com/news/IranNews/html/95021814INPL.html).
Conservation
This shark appears to still be common in Iranian fresh waters judging from the attacks reported
over the past 50 years or more and no conservation measures are needed
(or likely to be acceptable to the local population).
Further work
The biology of this species in fresh water is unknown for Iran and Iraq and should be thoroughly
investigated as a real hazard to those using rivers of Mesopotamia.
Are the sharks permanent residents or seasonal visitors? Detailed
records of attacks should be kept and analyzed in an attempt to
determine any triggering actions. It may prove possible to make
recommendations for use of water resources so as to avoid shark attacks in future.
Sources
Garrick (1982) and Compagno (1984, 1988) for general anatomy and biology.
Comparative material: BM(NH) 1874.4.28:9, 1, ca. 76.8 cm total length, Iraq, Tigris River near Baghdad (ca.
33º21'N, ca. 44º25'E); BM(NH) 1924.10.1:1 1,
(head only, recorded length 4 ft 1 inch = 1.25 m),
Tigris River at Al Karradah near Baghdad (33º17'N, 44º23'E).
Acipenseridae
Back to
Contents
The family is found in Europe, northern Asia and North America with
4 genera and 25 species. The Caspian Sea basin contains 2 genera and 6
native species, with both genera and 5 species recorded from Iran. The
Caspian population of sturgeons is the largest in the world (Levin,
1997) and Iran is the world's second largest producer of this resource
after the former U.S.S.R. (Josupeit, 1994).
These very large fishes are characterised by 5 longitudinal rows of
well-developed, bony plates along the body. There is a dorsal row, a
lateral row on each side and a ventro-lateral row on each side. In
young fish these plates are sharp and obvious but they become smoother
with age and may disappear completely. The unpaired fins have fulcra,
or flat bony plates, distinct from the scutes, in front of them. Small
plates, grains and denticles cover the remainder of the body and the
head is covered by large bony plates. Sturgeons have an elongate
snout, an inferior protrusible mouth without teeth in adults, fleshy
lips and 4 barbels in a row in front of the mouth (see Keys). The
vertebral column turns upward at the end into the upper lobe of the
tail (known as a heterocercal tail). The first pectoral ray is a
strong spine. There are few gill rakers under a single large gill
cover. The skeleton is cartilaginous, there is a spiral intestinal
valve, 1 branchiostegal ray, fin rays number more than the underlying
basal bones which support them, no gular bones on the lower head
surface and a large swimbladder. The karyotype may be complex with a
very large number of chromosomes, including the very small
microchromosomes, and tetraploidy, e.g. Huso huso, Acipenser
nudiventris and A. stellatus have 2n about 120 while A.
gueldenstaedtii has 2n about 240 and is a tetraploid. Karyotypes
of 120 chromosome species are very similar indicating a slow
evolution, correlated with a slow rate of DNA and protein evolution.
Hybridization is common, even between genera, and hybrids are fertile
and used in aquaculture in Russia (Birstein, 1993). Artyukhin (1995)
gives a phylogenetic tree of Acipenser and Huso. Krieger et al.
(2008) reviewed the molecular phylogeny of the order Acipenseriformes and found
Huso not to be monophyletic, among other unusual placements. They
conclude that some revision of classification may be needed. Rastorguev et al.
(2008) examined mtDNA for Ponto-Caspian sturgeons, although sample sizes were
small, and determined various relationships; Huso was basal with Atlantic
species and all species in the gueldenstaedtii complex were closely
related.
A
general overview of sturgeon systematics and biology is given by
Williot et al. (1991) and Billard (2002). Artyukhin (2006) and Peng et al. (2007)
summarise the relationships aned biogeography of major clades for the order (Acipenseriformes)
which dates back 200 MYA to at least the early Jurassic. A bibliography of sturgeons can be found
at www.geocities.com/CapeCanaveral/Hall/1345/sturgbibl.html.
Sturgeons are subject to overexploitation, a problem addressed by
Lukyanenko (1992), Vadrot (1990), Bemis and Findeis (1994), Faber
(1994), Moghim (1994), Anonymous (1995), Asadollahi (1995), Ivanov et
al. (1995; 1995, 1999), Vlasenko (1995), Waldman (1995), Birstein
(1996), Emadi (1996a; 1996b), DeSalle and Birstein (1996), Hosseinie (1996),
Khodorevskaya et al. (1997), Matthews (1998), Khodorevskaya and Krasikov (1999),
G. Strieker (in CNN.com, downloaded 9 March 2002), Speer et al. (2000),
Raymakers (2002), Oliver (2003), Harrison (2005), Pourkazemi (2006b), Karayev
(2006), Raymakers (2006), and numerous newspaper and
magazine articles. The problems for sturgeon survival in the Caspian
Sea and other waters have been the subject of numerous popular and
scientific articles which cannot all be cited here. A summary of the
problems and management recommendations are found in De Meulenaer and
Raymakers (1996) and The Sturgeon Quarterly published in New
York gives recent information. Caspian populations are Endangered
(high risk of extinction in the near future - Acipenser
gueldenstaedtii, A. nudiventris, Huso huso) or
Vulnerable (high risk of extinction in the medium term future - A.
stellatus, A. persicus) (De Meulenaer and Raymakers, 1996).
In 1997, the Secretariat of the Convention on International Trade in
Endangered Species of Wild Fauna and Flora (CITES) recommended a
proposal to list all sturgeons as a species requiring protection
because of overfishing and pollution. This would result in the close
regulation of the caviar trade and perhaps a trade ban on beluga caviar. Sales of caviar in airport duty-free
shops could end as passengers in a hurry would not be able to obtain
the necessary CITES export permits or certificates from national
authorities. After 1 April 1998 shipped caviar requires export permits
or re-export certificates (Traffic North America, 1(3):14, 1998). In
the year 2000, western countries through CITES (Convention on
International Trade in Endangered Species) gave Iran, Russia, Kazakhstan,
Azerbaijan and Turkmenistan until
31 December to impose quotas on their exports in an effort to save the
sturgeon stocks. Failure to comply would result in a ban on caviar
sales in the west in the year 2002 (IRNA, 25 June 2001). Australia had already banned caviar while the U.K.
banned the import of caviar over 250 g without a permit (IRNA,
26 July 2000; The Times, 1 August 2000). Fishing for sturgeon was halted
after the spring 2001 season in all Caspian states except Iran which has a
well-managed fishery. Fishing quotas will be established after a survey in the
summer of 2001 so as to avoid a complete ban on exports (Ottawa Citizen,
19 June 2001, 22 June 2001).
By 2004, as Profitt (2004) points out, the agreement had not been fully
implemented. Pourkazemi (2006) considers most sturgeon species in the Caspian
Sea will be extinct in the near future.
Stone (2002), Stone and Mervis (2002) and Pearce (2003) give details of a
dispute between scientists and CITES which arose when fishing for beluga was
allowed in 2002. CITES endorsed Russian figures that showed beluga numbers
increased from 7.6 million in 1998, to 9.3 million in 2001 and to 11.6 million
in 2002. Scientific critics felt that there may well be less than half a million
beluga, the differences being based on estimates on how many fish escape
experimental trawling in relation to fish actually caught. The United States
banned beluga caviar imports on 30 September 2005 and Russia advocated a
moratorium on fishing of the major species (Pala, 2005). In April 2006 a global
suspension of trade in caviar and sturgeon products by CITES from the Caspian
Sea was extended indefinitely, with only one species allowed, the Persian
sturgeon from Iran, Iran being the only country that submitted harvest data for
assessment of a sustainable fishery (New York Times (www.nytimes.com),
12 April 2006, downloaded 13 April 2006). The export quota for Iran was set at 100,000 pounds of caviar.
Bemis and Findeis (1994) recommend gourmets restrict their purchases of caviar to that from
fish farms in order to preserve wild stocks of sturgeons.
There was a two-thirds to three-quarters decline in sturgeon
numbers in the Caspian from 1990 to 1995, a result of overfishing and
poaching. References cited above, The Sturgeon Quarterly (5(1/2):15, 1997) and various
newspaper and popular articles reports (e.g. Boston Globe, 8 June 1997 at www.nd.edu/~astrouni/zhiwriter/97/97060808.htm
and New York Times, 23 December 1995 at www.nd.edu/~astrouni/zhiwriter/spool/95122301.htm;
Tidwell (2001a)) give details about poaching in former U.S.S.R. waters of the Caspian
Sea. In 1996, caviar should have sold for £470/kg in Germany but was
available for £100/kg illegally (Nuttall, 1996). Caviar imports to
the U.S.A. increased by 100% from 1991 to 1996 (DeSalle and Birstein,
1996). The international market demand for caviar was 450 t in 1995
but the legal production from the Caspian Sea was only 228 t; the
deficit being made up in part by poaching (Birstein, 1996). Russia officially
exported $25 million worth of caviar in 1999 but smuggling of poached caviar was
valued at $250 million (Speer et al., 2000). As a result, natural
reproduction in the Volga River, the principal spawning ground in the
Caspian Sea has been completely destroyed (Birstein, 1996). Bickham
(1996) states that it is highly likely that the native sturgeon stocks
of the Kura River are extinct or nearly so and Khodorevskaya et al.
(1997) simply record that sturgeons no longer use the Kura and Terek
rivers. Water pollution was given as the cause for a fall in catch in Iran from
34 tons in 2000 to 9.2 tons in 2004 (Iran Daily, 27 August 2005). Legally traded
caviar fell by almost 70% between 1998 and 2003 but illegal sales probably
offset this decline (www.canada.com, downloaded 16 December 2005). The export of Iranian sturgeon was expected to
drop 20-25% in the year ending in March 2006 (Iran Daily, 25 December 2005).
However caviar exports in the 2005-2006 year were given as 18 tons in a later
report, still a drastic fall (Iran Daily, 1 May 2006). The caviar export quota
for Iran in 2006 stood at 44.3 tons (Iran Daily, 11 September 2006).
Azerbaijan increased the allowable catch from 4 tonnes to 30 tonnes
after independence and generally illegal catches made up 90% of all
sturgeon caught (Anonymous, 1996a). The yearly allowable catch for
Iranian sturgeon in 1996 was 1500 tonnes but the total catch for the
Caspian Sea probably exceeds 40,000 tonnes when all countries are
taken into account (Emadi, 1996b). Reduction in stocks was noted in
assessments carried out in Iranian waters from 1988 onward and the it
was decided to reduce the annual catch in 1996 (Iranian Fisheries
Research and Training Organization Newsletter, 14:3, 1996). Iran was
auhorised to take 90 tonnes of caviar for export in 2000 but the government
reduced this to 70 t as a conservation measure (Speer et al., 2000). A
restocking programme in Iranian waters cost about U.S.$33 million and
a buyout of 4000 fixed gillnetters cost U.S.$10 million (Bartley and
Rana, 1998b). Gill nets were trapping young sturgeon, Salmo caspius,
Barbus sensu lato spp., Rutilus spp., and Abramis brama.
Sturgeon fingerling production was 9,124,000 in 1995 and 22 million in
1996-1997 according to the above authors, 25 million according to IRNA
(2 February 1999), and 12 million according to Abdolhay and Tahori (1999). However pollution causes losses of 40-50 million
fingerlings from a production of 108 million, figures at variance with
the preceding (Tehran Times, 5 September 1999). The Iranian Fisheries
Company produced 88.1% A. persicus in 1996, 5.4% A. gueldenstaedtii,
2.7% Huso huso, 2.5% A.stellatus and 1.3% A. nudiventris (Abdolhay
and Tahori, 1999). Keyvanfar and Khanipour (1999) advocate use of trammel nets
to catch broodstock for aquaculture as fish are less stressed. TACIS (2002) and
Raymakers (2002)
give the following table for sturgeon fingerling releases in Iran (in
millions):-
| Species/Year |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
| A. persicus |
4.06 |
5.92 |
2.93 |
3.57 |
4.66 |
8.05 |
11.02 |
18.75 |
22.59 |
17.30 |
| A. gueldenstaedtii |
- |
0.04 |
- |
- |
0.30 |
0.52 |
0.67 |
0.92 |
0.42 |
0.96 |
| A. stellatus |
0.36 |
0.47 |
0.07 |
0.30 |
0.46 |
0.27 |
0.22 |
0.29 |
0.18 |
0.13 |
| H. huso |
0.14 |
0.17 |
0.45 |
0.30 |
0.49 |
0.29 |
0.34 |
1.44 |
0.69 |
0.41 |
| Total |
4.56 |
6.60 |
3.45 |
4.17 |
5.91 |
9.13 |
12.35 |
21.63 |
24.56 |
19.10 |
Abdolhay and Tahori (2006) give descriptions of hatcheries in Iran and the
process of fingerling production, including transportation and incubation
techniques, pond and tank culture, release strategies, and strategic development
plans. Trial production of larvae first occurred in 1922, reaching about 2
million in 1928 but hatchery production first began in 1971. Sturgeon fingerling
production was low between 1981 and 1986 as the focus shifted to Chinese carps
and Rutilus frisii. Brood stock are captured in rivers by beach seines or
selected from fishery stations in February-March. The fish are checked by
sampling eggs and examining germinal vesicle development. Only suitable
fish are injected with ovulation-inducing hormones in March-May over 3-5 days.
The fish are killed and the collected eggs are fertilised with diluted sperm
(1:200 with hatchery water) to avoid polyspermy as eggs have many micropyles.
Eggs are incubated in jars or troughs for 5-10 days and newly emerged larvae are
held in circular tanks. Fry are raised in fertilised ponds for 40-60 days until
they reach 3-5 g. Fingerlings are released in river deltas in June-July. Release
strategies are spot planting of all fish at once, scatter planting at several
sites in the same region and trickle planting over a period of time. Fish are
captured as adults 10-20 years later at a return rate of 1-3%.
Fingerling production in 1000s was:-
|
Year/Species |
H. huso |
A.
nudiventris |
A.
gueldenstaedtii |
A.
persicus |
A.
stellatus |
Total |
| 1993 |
301 |
no data |
no data |
3570 |
300 |
4171 |
| 1994 |
491 |
no data |
300 |
4662 |
456 |
5910 |
| 1995 |
286 |
no data |
522 |
8049 |
268 |
9125 |
| 1996 |
344 |
102 |
673 |
11,018 |
316 |
12,455 |
| 1997 |
1437 |
230 |
919 |
18,751 |
288 |
21,627 |
| 1998 |
687 |
678 |
418 |
22,586 |
181 |
24,552 |
| 1999 |
406 |
304 |
722 |
17,300 |
132 |
18,864 |
| 2000 |
1901 |
114 |
1327 |
13,711 |
226 |
17,279 |
| 2001 |
641 |
1782 |
447 |
16,278 |
820 |
19,970 |
| 2002 |
2404 |
1819 |
1816 |
12,301 |
1300 |
19,642 |
| 2003 |
42 |
1414 |
0 |
18,388 |
196 |
20,041 |
| 2004 |
1464 |
1311 |
617 |
17,412 |
314 |
21,121 |
| Total |
11,175 |
7757 |
7805 |
191,682 |
9774 |
258,567 |
Iranian sturgeons and their caviar increased in importance in the
1990s as the Russian caviar trade was taken over by a black market
system with poor attention to quality. However caviar production in
Iran fell in the 1990s through poaching and oil pollution in other
parts of the Caspian Sea. Production was 130 tonnes per year, down
from 160 tonnes up to 1989 (IRNA, 31 August 1998; Tehran Times,
13 December 1998). Caviar comprises 50% of the seafood exports from
Iran (IRNA, 21 October 1998) and formed 1.2% of Iran's total
exports for the first four months of the Iranian year in 1998 (in 1994 it was
62% (Salehi, 1999)). On the
23 October 1998, the Islamic Republic News Agency (IRNA)
reported that Iran had stopped exporting caviar to protect the
resource, this despite the number of sturgeons in the sea having risen
from 6 to 22 million over the past couple of years. The same article
reports 22 million sturgeon fingerlings stocked in the Caspian Sea by
Iran. The export of 111 tons of caviar in 1998-1999 was worth $29.5
million; catches had been reduced to save the species from extinction
(Tehran Times, 1999). The export amount was over 80 tonnes
since the beginning of the Iranian year (21 March 1999), a 30% drop in
production over the previous year (IRNA, 26 January 2000). The
1999 total export was 90 tonnes of caviar worth 70 million
deutschmarks, a monetary increase of 42% (IRNA, 4 May 2000). The 2000
export of caviar was 70 tonnes (or 71.5 t, or 80-90 t, reports vary) worth 100
million deutschmarks (or $34.4 million) with 80% going to Europe, 10% to Japan
and the rest to various other countries; in addition 100-200 tonnes of sturgeon
meat worth $2-3 million is exported annually (IRNA, 14 July 2001, 7
August 2001, 30 September 2001). The sturgeon catch was 75 t in 2002 with 50 t
being exported for U.S$30 million (IRNA, 11 June 2003). Golestan Province
produced 43% of Iranian caviar, a 17.5% increase presumably in 2000 over the
1999 catch. There are 295 fishermen using 91 fishing boats (IFRO Newsletter, 29:4, 2001).
Sturgeon stocks were evaluated in Iranian waters in 2000 (M. Moghime and F.
Parafkandeh Haghighi, 5th International Symposium on Sturgeon, Iranian Fisheries Research
Organization, 9-13 May 2005, Ramsar; Haghighi, 2006; Moghime, 2006). The catch was 855 t yielding 92.5 t of
caviar, with Acipenser persicus comprising 472 t, A. stellatus 201
t, H. huso 105 t, A. gueldenstaedtii 48 t and A. nudiventris
31.8 t. The catch-per-unit-effort was A. gueldenstaedtii (0.285 kg),
A. persicus (2.296 kg), A. nudiventris (0.089 kg), and A.
stellatus (2.941 kg). Mature females comprised A. gueldenstaedtii
(80.0%), A. persicus (71.8%), A. nudiventris (51.3%), A.
stellatus (74.7%) and H. huso (67.4%). The gonadosomatic value in
terms of body weight was A. gueldenstaedtii (9%), A. persicus
(11%), A. nudiventris (8%), A. stellatus (14%) and H. huso
(2.8%). The catch in Gilan and Golestan provinces was A. stellatus (11%)
and H. huso (35%) of the total catch. In Gilan, the catch was made up of
A. gueldenstaedtii (44.3%), A. persicus (16.4%) and A.
nudiventris (3.8%) and in Golestan these values were 16%, 72% and 0.9%
respectively. The average age in Gilan and Golestan respectively was A.
gueldenstaedtii (15.5 and 18.5 years), A. persicus (20 and 19.2
years), A. nudiventris (15 and 19.3 years), A. stellatus (13.8 and
13.2 years and H. huso (15.8 and 18 years).
Tavakoli et al. (2007)and Kor et al. (2008) surveyed stocks in
the southern (2004-2005) and northern (2006) Caspian Sea respectively. In
the southern Caspian Sea catch of 288 fish total, catch per unit effort was 2
fish in summer and 1.38 fish in winter. The most abundant species was
Acipenser persicus with 1.67 fish per trawl in summer (142 fish) and 0.88 in
winter (75 fish). A. stellatus was 0.22 and 0.48 fish. No Huso huso
were caught in winter and only 4 fish in summer. A. nudiventris comprised
only 4 fish too and and A. gueldenstaedtii 3 fish, both in total. Kor
et al. (2007) examined the population structure of sturgeons in the coastal
waters of Mazandaran, less than 10 m deep, for 2003-2005. The number of fish
captured in 2003-2004 was 301 with catch per unit effort (CPUE) being A.
persicus 4.07, A. stellatus 0.58, A. nudiventris 0.22, and
A. gueldenstaedtii 0.15, and in 2004-2005 the catch was 412 fish with CPUE
A. persicus 6.15, A. stellatus 0.23, A. nudiventris 0.12,
A. gueldenstaedtii 0.35, and Huso huso 0.02.
The world's leading importer of caviar, Caviar House, with an
annual turnover of $100 million took 85% of its caviar from Iran
(Lindberg, 1994; Pala, 1994). The value of the caviar fishery in Iran
was estimated at U.S.$45 million (Bartley and Rana, 1998a; 1998b) and
is the main fish product exported with an international cultural and
culinary significance. The caviar industry in Iran is a state monopoly
under strict control and has not suffered from poaching to the same
extent as happened in the former U.S.S.R. after the collapse of
central authorities. There has been some smuggling reported via Bandar
Abbas to Ras al Khaimah across the Gulf and re-labelling of
Azerbaijani caviar as Iranian to fill Iranian contracts with the U.A.E.
An illegal trade in "bazaar" caviar reached a peak of 70
tonnes in 1983, about 50% of the legal exports (Taylor, 1997). This
caviar was processed poorly in primitive tins with sealant rings made
from old tyres; consequently the price for this product was low. The
Iranian government actively sought to suppress this trade and after 10
years of effort reduced smuggling to 2-4 t annually, a level similar
to that prior to 1979. In 2003 however, 3.8 t of smuggled sturgeon fish and
caviar were reported as confiscated for the previous year (ending 20 March) in
Mazandaran (IRNA, 21 April 2003). Evidence of Iranian control of the industry is
seen in the 1994 setting of a minimum catch size limit of 1 m on all
sturgeon species and limiting fishing sites along the Caspian coast to
90 (Josupeit, 1994; De Meulenaer and Raymakers, 1996). Additionally
Iran now stocks more sturgeons from farms than it catches (The
Times, London, 8 July 1998). However the BBC News (6 May 1998)
reports declines in catches of sturgeon over the past 5-10 years.
The illegal market in caviar has been estimated at £500 million with some caviar fetching up to
£20,000 a kilogramme (The Times, 28 December 2006). In Britain, caviar tins must indicate
their exact source and without this label will be seized by Customs. The label
will carry a species code, source of the caviar, country code, year of harvest,
processing plant registration number and lot identification number, all in an
attempt to regulate and eliminate sales of smuggled caviar. Much of the smuggled
caviar is sold under the counter or to those who have pre-ordered it, or by
shops that then state they were unaware of its illegal status.
Although caviar is the main market item for sturgeons, Iran is
investigating the use of fillets and smoked and salted A. stellatus
in vacuum packs for export (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 45-46, 1997).
Smoked, marinated and canned sturgeon, smoked sturgeon in vegetable oil and
frozen fillets are now available (2001) from several Iranian companies.
Javanmard and Taghavi (2002) investigated the microbiological and chemical
characteristics of these products and only one had a total coliform count more
than the European Community standard. Gelatin has also been produced from sturgeon
fish skin on an experimental basis
(Iranian Fisheries Research Organiztion Newsletter, 30-31:6, 2002;
Koochakian Sabour et al., 2001).
All sturgeon species in the Caspian Sea basin are listed as
"endangered" or "vulnerable" and are maintained in
part by hatchery stocks (http://www.sturgeons.com/htdocs/status.html).
Survival and growth of sturgeon fry in the Caspian Sea is reviewed in
Farsi by Aslaanparviz (1992).
The countries of the Caspian littoral are attempting to conserve
their sturgeon stocks. Even the Swiss company Caviar House has established a
hatchery in Iran to increase stocks (Anonymous, 2001a). An agreement for the "Preservation and
Exploitation of Live Resources in the Caspian Sea" was made
between Iran, Russia, Azerbaijan, Turkmenistan and Kazakhstan in 1996.
Luk'yanernko et al. (1999) point out the need for the agreement to
recognise that sturgeons are sustained by an ecosytem involving the whole
Caspian Sea and the inflowing rivers, that there must be an absolute ban on
uncontrolled fishing for sturgeon in the sea and that national quotas must
reflect the real contribution of a a particular state to overall sturgeon
stocks. Without adequate measures, these authors predict extermination within
5-7 years. Export of caviar is made a monopoly of the governments concerned in an
effort to minimize smuggling of low quality caviar. Jenkins (2001) gives reasons
why an international trade ban would not necessarily help conserve the sturgeons
- most poached caviar is sold within Russia, for example. Sturgeon catches
are restricted to rivers and their estuaries and open-sea trawling is
banned. The five countries are investing $150 million in a fish farm programme to save the sturgeon from extinction: Russia will have 10
new farms and renovate 8 farms on the Volga River, and both Kazakhstan
and Iran have a new farm (Abzeeyan, Tehran, 7(4):II-III, 1996; The
Sturgeon Quarterly, New York, 4(4):1, 1996; newspaper reports). Russian
strategies for conservation of sturgeon are reviewed in Artyukhin et al.
(1999) and the status of the Russian sturgeons is given in Vaisman and Raymakers
(2001). Some sturgeon species are now on Appendix 2 of the U.N. Convention on
International Trade in Endangered Species of Flora and Fauna (CITES)
in an effort to control the import and export of meat and caviar
(Pearce, 1997). The U.S. Fish and Wildlife Service, in an attempt
to combat overfishing of sturgeons, now requires valid CITES permits
for imported caviar (Anonymous, 1998a). DNA tests will be used to
confirm the species of sturgeon listed on the shipment and to
eliminate illegal mixtures with inferior quality roe. Even cats are now used to
detect smuggled sturgeon in Russia. A cat named Rusik is able to detect sturgeon
hidden in trucks better than sniffer dogs (National Post, 9 July 2003, p. A12).
The number of adult fish in the Caspian Sea had declined from 142
million in 1978 to 43.5 million fish in 1994. Ivanov et al. (1999) and
Khodorevskaya and Krasikov (1999) review the status of stocks in the Caspian.
Marked declines are evident and only the Iranian catches are reasonably stable
from 1977 to 1994. All species studied in
Iranian waters had a very low percentage of fish older than 20 years,
are evidently in need of protection (Iranian Fisheries Research and
Training Organization Newsletter, 16:4-5, 1997). An initiative to
make the sale of caviar from threatened sturgeon species illegal is
being proposed by the Species Survival Commission and the IUCN (The
Sturgeon Quarterly, New York, 4(4):1, 1996; Morris, 1997). Part of
this initiative would involve genetic testing of the caviar as a means
of identifying the species of sturgeon. Paddlefish eggs from Montana,
U.S.A. costing less than $5 an ounce have been repackaged as beluga
caviar in Russia and eastern Europe and sold in the U.S.A. for $50 an
ounce. The U.S. Fish and Wildlife Service was to begin monitoring the
caviar trade on 1 April 1998 using DNA tests (U.S.A. Today, 18
November 1997, internet edition). Birstein et al. (1998)
describe a molecular technique for identifying the species source of
commercial caviar (see also Brainard (1998)). They found 23% of
species designations by caviar suppliers to be incorrect, indicating
possible illegal harvesting and poaching. The Iranian Fisheries
Research and Training Organization Newsletter (20:4, 1998) also
reports on nuclear DNA amplification and a marker which distinguishes
species. Additional research is being carried out on egg
identification using ultrastructural characteristics (L. Debus and M.
Winkler, 1998, www.uni-rostock.de).
Sturgeons have been fished since the Neolithic, perhaps 6000 or
more years ago (Tsepkin, 1986) but only in recent years have the
stocks declined significantly. Historical records show it was possible
to catch 500 Huso huso weighing 600-1000 kg in about 2 hours in
the Volga delta at the end of the eighteenth century (Birstein, 1993).
All the sturgeon species were bigger on average and lived longer than
now based on archaeological excavations (Tsepkin and Sokolov, 1971).
The sturgeon catch in the Caspian Sea declined from 27,400 tonnes in
1977 to 8,900 tonnes in 1990 (Vlasenko, 1995). The catch in 1993 was
only 4,200 tonnes because of poaching and pollution of the Volga River
(The Sturgeon Quarterly, 3(1):12, 1995). An estimated 90% of
the Caspian sturgeons are killed before they mature (Platt, 1995).
Catches in Russian waters of the Caspian Sea declined from 7106 tonnes
in 1992 to 3426 tonnes in 1993 to 2960 tonnes in 1994 but 90% of the
real catch is unreported (16,700 tonnes were reported in 1983 for
comparison). The number of adult sturgeons in the Caspian Sea is
estimated to have declined from 142 million fish in 1978 to 43.5
million fish in 1994 (De Meulenaer and Raymakers, 1996). The Caspian Sea
Sturgeon Ranching Programme of the former Soviet Union helped to sustain
fisheries but declines still occurred (Secor et al., 2000).
Catches in Iran, however, increased over a five year period,
perhaps because of heavier fishing pressure. Sternin and Doré (1993)
cite figures for 1986-1990 of 1690 tonnes, 1759 t, 1851 t, 2051 t and
2021 t, while U.S.S.R. catches over the same period were 21,817 t,
20,991 t, 19,027 t, 16,880 t and 15,056 t. A conflicting study noted a
decline from 122,000 sturgeons caught in 1986 to 68,000 in 1993 (Abzeeyan,
Tehran, 6(5, 6):IV-V, 1995). De Meulenaer and Raymakers (1996)
summarise Iranian catches as 700 to 2500 t in the twentieth century,
peaking towards the end of the 1960s, falling to 1000-1500 t in the
1970s and increasing from 1450 t in 1982 to a 1991 high of 3036 t but
falling off rapidly to 1700 t in 1994. Josupeit (1994) gives catches
in Iran in tonnes from 1982 to 1992 as 1450, 1288, 1557, 1650, 1690,
1759, 1851, 2051, 2645, 3036 and 2692 t. The commercial sturgeon catch
in the Safid River delta fell from 6700 tons in 1961 to less than half
a ton in 1993 (http://www.oneworld.org/patp/pap_overview.html).
Spawning may no longer take place in the Safid River (De Meulenaer and
Raymakers, 1996). Zanusi (1995) maintains that over 40% of the total
sturgeon fishing in the Caspian Sea is centred on Bandar-e Torkeman in
Mazandaran, presumably including the acknowledged black market in
sturgeon products. Lewis (1980) gives some information about the
Iranian black market in caviar shortly after the Islamic Revolution
before controls were re-established. A 400 g tin was selling in Paris
black market for $40 compared to $310-315 for the best Russian beluga. Caviar
production in the three Caspian coast provinces of Iran for the 1990s were as
follows in kg after Nezami et al. (2000):-
| Year/Province |
Gilan |
Mazandaran |
Golestan |
| 1991 |
75,974 |
78,713 |
128,446 |
| 1992 |
81,520 |
80,758 |
99,336 |
| 1993 |
51,480 |
58,543 |
83,026 |
| 1994 |
40,368 |
52,162 |
87,576 |
| 1995 |
37,241 |
43,831 |
70,154 |
| 1996 |
41,743 |
41,432 |
79,063 |
| 1997 |
28,641 |
42,329 |
58,304 |
The problem of overexploitation of sturgeons is compounded by their
long life span and their use of rivers as spawning grounds such that
they are easily caught on this migration from the sea. The migration
and spawning is timed differently between species and populations
within species. Some sturgeons migrate long distances up rivers while
others have a shorter migration. Eggs are deposited on stony or gravel
bottoms and hatch after a short incubation. As an example, a study of
sturgeon migrations in the Gorgan and Tajan rivers of Iran showed a
movement of 2 out of 28 fish caught at one station reached the second
station in the Gorgan and no tagged fish reached higher stations in
the Tajan - the rest were caught by fishermen (Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization,
Tehran, p. 53, 1997).
Ramin (1998) studied migration in the Safid River over 35 days in April-May,
from the mouth to 30 km upriver for A. gueldenstaedtii, A. persicus
and A. stellatus. Shallow water caused by sand-clay deposits and illegal
fishing did not prevent successful migration. It was recommended that the Manjil
Dam be used to regulate water flow and a total ban on fishing, especially at the
mouth, during the March-May spawning season be implemented.
In 1998 the comb jelly, Mnemiopsis leidyi, reached the
Caspian Sea via ship ballast and newspapers speculated that the
sturgeon populations would be affected, although how was not
specified.
The young migrate downstream to feed and grow in the sea. Old
reports have sturgeons overwintering in deeper parts of rivers, in a
kind of torpor and with a viscous substance coating the body (Baird,
1873). The barbels are highly sensitive and, as soon as they detect
food, the tubular mouth protrudes to suck in the prey. Food is benthic
organisms although some are predators on larger fishes. Young sturgeon in Iran
feed predominately on polychaetes while crustaceans are a minor food item,
probably caused by lower oxygen conditions favouring the former (Haddadi
Moghaddam and Negaresten, 2003). Pourgholam
(1994) reports the coelenterate Polypodium hydriforme from
sturgeons caught on the Babol Sar and Bandar-e Torkeman fishing
grounds in Mazandaran where up to 25.6% of fishes are infected,
particularly Huso huso and Acipenser gueldenstaedtii.
This parasite destroys the eggs of sturgeons, affecting reproductive
success and the caviar industry (see also Raikova (2002)). Incubated eggs of sturgeons are
susceptible to various species of fungi, with up to 70-90% of eggs
being lost (Czeczuga et al., 1995). Czeczuga et al.
(1995) report 43 species of fungi on eggs of sturgeons from Russian
and Iranian Caspian Sea samples immersed in water from a Polish river,
lake and pond. Huso huso and Acipenser gueldenstaedtii
persicus (sic) eggs carried the fewest species of fungi,
about half the load of other sturgeon species. Ghoroghi (1996) reports
metacercariae of Diplostomum spathaceum in the lens of 22% of
fingerlings on the Shahid Beheshti Fish Farm causing weight loss and
mortality. External parasites on sturgeons include Pseudotracheliastes
stellatus, Nitzschia sturionis, Diclybothrium armatum,
Cystoopsis acipenseris and Diplostomum spathecum with the highest
prevalence in Huso huso at 60% and the lowest in Acipenser persicus
at 13.9% (A. Hajumoradloo in 5th International Symposium on Sturgeon, Iranian
Fisheries Research Organization, 9-13 May 2005, Ramsar). Ghaemi et al.
(2006) found strains of mycobacteria in Iranian sturgeons and Mycobacterium
marinum can cause fish tank granuloma, a disease in humans although none was found in fishermen.
Many sturgeons in former Soviet waters of the Caspian Sea have
developed fatal diseases associated with chemicals such as phenols,
waste fluids and air from gas production facilities associated with
the petrochemical industry. Both the sturgeon and their caviar are now
inedible. Iranian sturgeons are believed to be less affected but since
sturgeons migrate they are susceptible to extra-territorial pollution (Golub, 1992).
Sturgeons are some of the most important commercial species in the
world, with 90% of the total catch coming from the former U.S.S.R. and
only 6% from Iran (but see later under Acipenser gueldenstaedtii
where Iranian production of caviar increased in the 1990s). Over 90%
of all sturgeons are caught in the Caspian Sea. The proportion of
catch is heavily weighted towards the former U.S.S.R. (compared with
Iran in parentheses) with figures from 1971 to 1988 as given by
Sternin and Doré (1993) being 19,100 tonnes (2400 t) for 1971, 20,400
t (2200 t) for 1972, 24,958 t (1801 t) for 1978, 26,322 t (1578 t) for
1979, 26,697 t (1429 t) for 1980, 26,452 t (1496 t) for 1981, 25,704 t
(1450 t) for 1982, 25,570 t (1500 t) for 1983 and 18,470 t (1700 t)
for 1988. The Volga River and its delta provided 75% of the commercial
sturgeon harvest in the Caspian Sea with Acipenser gueldenstaedtii
making up 60-70% of this amount, A. stellatus about 30% and Huso
huso 5-6% (Khodorevskaya et al., 1997). Williot and
Bourguignon (1991) summarise sturgeon catches in Iran from FAO data
for 1965 to 1987 as ranging from a low of 1429 t to a high of 3000 t. Abdolhay
and Tahori (2006) summarise catches as follows:-
| Year |
Total catch
(tonnes) |
A.
stellatus (%) |
Osetra*
(%) |
H. huso
(%) |
| 1972 |
1500 |
34.0 |
36.3 |
29.7 |
| 1991 |
3036 |
49.5 |
41.0 |
9.5 |
| 1994 |
1700 |
49.5 |
41.0 |
9.5 |
| 1997 |
1300 |
35.8 |
54.3 |
9.9 |
| 2000 |
1000 |
35.8 |
61.0 |
3.5 |
| 2001 |
870 |
28.2 |
69.3 |
2.5 |
| 2004 |
600 |
10.7 |
74.6 |
14.7 |
* presumably includes A. persicus and A. gueldenstaedtii
Only 5% of Iranian caviar is consumed in that country, the rest
being exported. Domestic prices are very high at about U.S.$340 per kilogramme
and the caviar is rationed to 100 g per person. Contraband caviar can be bought
at about half this price around Bandar Anzali and at least 30 t are smuggled out
of the country each year (The Daily Star, 7 December 2004, www.dailystar.com, downloaded 17
December 2004). In 1996, 95 t out of an estimated 120 t catch was
exported although formerly as little as 38.7% was exported as in 1978.
Iran is the chief exporter to the European Union, the weight varying
from 95 to 125 t from 1988 to 1994 (De Meulenaer and Raymakers, 1996).
Caviar exports by year for Iran are given by these authors as:-
|
Year |
1988 |
1989 |
1990 |
1991 |
1992 |
|
tonnes |
225 |
249 |
226 |
225 |
169 |
|
U.S.$1000 |
42,155 |
47,865 |
46,005 |
53,800 |
42,004 |
|
U.S.$/kg |
187 |
192 |
204 |
239 |
249 |
The export volume of caviar for 1997-1998 was 105 tonnes worth 62
million German marks (= U.S.$34 million) (IRNA, 16 March 1998).
Prices outside Iran have fluctuated widely because of large amounts
of illegal and often poor quality caviar flooding the world market.
Caviar exports are declining in the 1990s reflecting, it is believed,
the loss of sturgeon stocks in the Caspian Sea (De Meulenaer and Raymakers, 1996).
Caviar is the main product but the flesh is also eaten (a religious
ruling was made in the 1980s to the effect that Iranian ichthyologists
had determined sturgeons to be fish with scales - see below). Early
reports of poisoning from sturgeon eggs have been attributed to poor
preservation and consequent bacterial contamination (Halstead,
1967-1970). The milt of Acipenser sturio contains a toxic
substance known as "sturin" and although this species does
not occur in Iran a similar toxin may occur in Iranian Acipenser (Coad, 1979b).
The swimbladders of sturgeons have been converted to isinglass, a
transparent gelatin used in a variety of products including as a wine
and beer clarifier, in jams and jellies and in glass and pottery. Gmelin (2007)
mentions that in 1770-1774 people along the Langerud were catching large numbers
of sturgeons for their isinglass only, the caviar and flesh not been used. Sabour (2006)
found the swimbladder in Iranian sturgeons to weigh 250-285 g in H. huso,
35-92 g in A. stellatus and 85-160 g in Acipenser spp and could be
was processed to isinglass at 15-20%. Koochekian et al. (2006) found a
higher percentage production of isinglass from A. persicus/A.
gueldenstaedtii than in Huso huso or A. stellatus. Recently Iranian
scientists have investigated production of leather from sturgeon skin
(Iranian Fisheries Research and Training Organization Newsletter,
4:2, 1994; Davarzani, 1995; Iranian Fisheries Research Organization
Newsletter, 22:2, 2000; Iran Daily, 17 January 2006). An estimated 1 million square feet of
leather could be produced and used in handicrafts, book binding,
waterproof products and ornaments.
Various methods to enhance the sturgeon fisheries have been investigated in
Iran. Some are given under the Species Accounts and others are summarised here.
Experiments with pen culture in Gorgan Bay have been carried out to
increase production and with cross-breeding Huso huso and Acipenser
stellatus to create new commercial and resistant stocks (Iranian
Fisheries Research and Training Organization Annual Report,
1992-93). A Farsi review of sturgeon culture is given by Rasoli
(1992). Even surgical procedures under anaesthesia have been tried to
remove eggs through a 15-20 cm incision as part of attempts to
increase caviar production (Mokhayer, 1993; The Times, London,
8 July 1998). Ultrasonagraphy has been used successfully to
distinguish males, females and immature fish without damaging them (Vajhi,
Moghim, Veshkini and Masoudifard (1999) www.mondialvert99.com,
downloaded 31 May 2000; Moghime, 2006). The accuracy was 97.2% for A.
stellatus and 100% for A. gueldenstaedtii, A. nudiventris and
H. huso. Vacuum pumps have also been used to breed
female Acipenser nudiventris and male A. stellatus. The
fish are anaesthetized with xylazine hydrochloride and then eggs and
sperm are pumped out, the advantage being that females can be returned
alive to the sea (Iranian Fisheries Research and Training
Organization Newsletter, 13:5, 1996). Bahmani et al. (2001)
compared haematological parameters in Acipenser persicus and Huso huso
and how these changed with age. Haemtaological indices give insight into
the physiological condition and aid in the selection of broodfish.
Cryopreservation of sperm has been carried out as stripping fish late in the
season is difficult. Sperm in liquid nitrogen with an extender is viable for 1.5
to 2 years (M. Moghim and H. N. Moghadam in 5th International Symposium on
Sturgeon, Iranian Fisheries Research Organization, 9-13 May 2005, Ramsar;
Moghadam, 2006). The colour of gill nets used in the capture of sturgeons has been investigated with
blue nets having a yield of 42.6%, white 29.8% and green 27.5% (5th
International Symposium on Sturgeon, Iranian Fisheries Research Organization,
9-13 May 2005, Ramsar). Studies on the fingerling production of hatcheries
include the nature of the phytoplankton community and the benthic biomass,
parasitic infections (e.g. Diplostomum sp. on the eyes and Trichodina
sp. on the gills were noted at an incidence of 25% and 35.85, productivity
(6,509,185 fingerlings produced from 31 March and 28 July 2000 in two hatcheries
with some transfer of Huso huso fingerlings from another hatchery),
survival rates (56.7% and 25.2% for A. persicus and H. huso
respectively), and growth rate and condition factor (generally low). Kami et
al. (2005) studied the biology of pond turtles (Emys orbicularis)
which live in culture ponds along with sturgeon. One dietary item was
Acipenser persicus. The use of probiotics, microbial cells in the diet, used
to improve health and thus enhance quality of farmed fish is of potential use in
sturgeons as reviewed by Askarian et al. (2006). Bahmani (2006) used both
histology and haematology on Acipenser persicus, A. gueldenstaedtii
and Huso huso to determine physiological condition of fish in ponds
and rearing tanks, comparing the results with natural conditions (similar) and
finding that fibreglass tanks were more suitable than rearing ponds. Banadani (2006) examined
the environmental conditions in the Gorgan River, a major site for release of
sturgeon fingerlings. Mohseni (2006) studied the effect of stocking density of
eggs and larvae in incubators on their survival, growth and appearance of
deformities. Increased density reduced survival and growth and increased
deformities. Parandavar (2006) compared production of sturgeon from broodfish
maintained on farms to those produced from fish taken from the wild. Salehi
(2006) analysed the economics of sturgeon fingerling production and found labour
costs were 55%, food and fertiliser 14%, maintenance 7% and fertilised eggs 5%.
A single fingerling cost 992 rials to produce in Iran, varying between 447
and 1224 rials among hatcheries. Yousefian (2006) gives details of the
production of fingerlings at the Shaid Rajii Fish Farm in 2002. This farm
produced 2,898,086 or 93.27% of the fingerlings released into the Tajan, Larim,
Goharbaran and Sardab rivers. These fingerlings had an average weight of 3.58 g
and condition factors were 0.4 for Acipenser persicus, 0.37 for A.
gueldenstaedtii and 0.31 for A. stellatus, in total and average grade
for the condition factor. Fazlei (no date) summarised the number and quality of
fingerlings released into Mazandaran and Golestan provinces. The most important
rivers for release were the Gorgan (8,659,377 fingerlings, average weight 2.55
g), Tajan (1,453,410, 4.12 g), Larim (1,211,875, 3.4 g) and Goharbaran (743,561,
3.09 g). A. persicus comprised 87.7% of the fingerlings, A.
gueldenstaedtii 6.6%, H. huso 3.3% and A. nudiventris 2.4%.
The International Sturgeon Research Institute has developed a food formula based
on Iranian sturgeon species. Previously food for aquaculture came from Europe
and the domestic version was demonstrated to be superior
(Iranian Fisheries Research
Organization Newsletter, 58 & 59:2, 2009). The hybrid
sturgeon known as bester (female beluga x male sterlet) has been investigated
for expanding sturgeon culture in Iran. Growth was significantly better than in
beluga
(Iranian Fisheries Research
Organization Newsletter, 58 & 59:4, 2009). Jafarian et al. (2009)
studied the use of probiotic bacilli to encapsulate Artemia urmiana
nauplii and the yeast Saccharomyces cerevisiae used to encapsulate
Daphnia magna, Artemia and Daphnia being used as live food for
sturgeon larvae. Both treatments increased growth parameters and feeding
efficiency in A. persicus, A. nudiventris and H. huso.
Lake Orumiyeh has been used as a source for Artemia urmiana or brine shrimp to be used as a
live food in sturgeon aquaculture (Azari Takami, 1987; 1993). Brine shrimp were
found to be a better food than white worms or Daphnia, being cheaper and
easier to prepare, easier to store as cysts, sturgeon fry showed better growth,
pathogens were less, mortality was lower and yield higher. Since 1972 almost 50%
of fry diet has been brine shrimp. The large mouths of sturgeon fry enable them
to take brine shrimp nauplii and even adults a few days after yolk-sac
absorption. Fry are grown to 100-120 mg within 7-10 days and then released into the sea.
Anonymous (1961b) reported on the caviar industry in Iran which at
that time was about 5-6% of the world supply. The Food and Agriculture
Organization of the United Nations in their Yearbook of Fishery
Statistics reported catches of sturgeons from 1980 to 1985 as 1429,
1496, 1450, 1288, 1557 and 1650 tonnes respectively. Soviet catches of
sturgeons in all waters, not just the Caspian Sea, ranged from 22,772
to 26,697 tonnes for the same period. Petr (1987) summarised FAO
statistics for Iran and gave mean landings of sturgeons as 2300 tonnes
(1964-1970), 1800 t (1971-1975), 1500 t (1976-1980), and 1774 t
(1980-1985) but some of this data is very approximate being repeats of
a 1500 t value as an estimate (see also above for more figures). A
pamphlet from the Ministry of Jahad-e Sazandegi (= Construction
Crusade or Rural Development), which is charged with fisheries in
Iran, gave catches for "caviar fish" of 3036 tons
(presumably tonnes) in 1991 and 2692 tons in 1992. The catch in 1995
was 995 tonnes yielding 134 tonnes of caviar with 74% of the catch
from Mazandaran province (http://netiran.com:80/news/TehranTimes/html/95122503TTEC.html).
Other news reports give the 1995 catch as 142 tonnes of caviar, in
1996 112 t and an estimated 140 t in 1997. The 200 t of caviar
produced in 1992 was worth $100 million through export while the 1997
catch was worth only $60 million despite a 50% increase in price. The
Tehran Times (30 May 1998) reported that caviar production was reduced
from 220 tonnes to 40 tonnes during the previous 6 years to preserve
stocks. The allowable catch in 2003 was set at 676.4 t for Iran, a decrease from
685 t in 2002, with caviar exports set at 78.8 t. Figures for other Caspian
states were Azerbaijan 130 t (9.1 t of caviar), Russia 429 t (30.3 t),
Kazakhstan 216 t (23.18 t), and Turkmenistan 56.25 t (5.85 t)(IRNA, 28 December 2002).
Caviar from Iran commanded a higher price than that from the former
U.S.S.R. in the 1990s (Christie, 1995). Catches in the 1952-1957
period yielded an annual average yield of 120 tons (sic,
possibly tonnes here and below) of caviar (Kayhan International, 1
December 1962) which agrees closely with the figure given by Job
(1961a) of 90-115 tons (sic) annually. Catches from 1965/66 to
1968/69 in Iran rendered 208 to 219 tonnes of caviar annually from
1996 to 2290 tonnes of the three main species fished (A.
gueldenstaedtii (presumably including A. persicus), A.
stellatus and Huso huso)(Andersskog, 1970). The catch in
1961-1962 was 170 tons (or 178 tons, V. D. Vladykov, in litt.,
1966; differing data is not unusual as effectiveness of information
gathering varies) and this was the first season when exports to the
U.S.A. exceeded that to the former U.S.S.R., 56 to 46 tons, with 58
tons going to Europe and about 10 tons consumed locally. The caviar
yield in 1956-1957 was low, at 134 tons (or 137 tons, V. D. Vladykov, in
litt., 1966) and averaged 120 tons from 1952-1957 (Kayhan
International, 1 December 1962), a decline over levels prior to
dissolution of the Iran-Soviet company. White (1988) reported a caviar
export of 150 tonnes from Iran with a value of U.S.$20 million out of
a 250 tonnes annual production. Caviar yield in airtight containers
was 233 tonnes (1981), 204 t (1982), 222 t (1983), 247 t (1984), 304 t
(1985), 283 t (1986), 296 t (1987), 281 t (1988), 286 t (1989), and
290 t (1990) (Sternin and Doré, 1993). Production of caviar in the
1990's dropped steadily from 160 tonnes to 120 tonnes as the Caspian
became more polluted (Tehran Times, 5 August 1999) and the
catch for the year ending in March 2000 was expected to be less than
100 tonnes (Reuters News Service, downloaded 1 September 1999).
Pollutants from Russia, Azerbaijan and Kazakhstan include oil spillage
from old equipment at offshore sites and 12 million cubic metres of
sewage from the Volga. The sewage includes toxic PCBs, phenol, heavy
metals, dioxins and DDT as well as household, agricultural and
industrial wastes. A 10-year ban on sturgeon fishing would have to be
placed into effect to allow stocked sturgeon to mature and breed. Research
on qara burun (A. persicus) and uzun burun (A. stellatus) in Iran
has shown heavy metal (cadmium, copper, zinc, lead and mercury) density in
caviar and flesh to be 10 times less than the global safety standard (IRNA,
15 January 2002; IFRO Newsletter,
28:2, 2001). Pourang et al. (2005) examined all five sturgeon species in
Iranian waters and found all toxic trace elements (Cd, Cu, Pb and Zn) to be
markedly below international guidelines for human consumption. Kajiwara et al. (2003) demonstrated contamination by
organochlorines in Iranian sturgeons. DDT and its metabolites predominated at
180-18,000 ng/g on lipid weight followed by PCBs at 110-1900 ng/g. Generally Huso
huso was the most contaminated species and contaminant concentrations were
higher in Azerbaijan and Kazakhstan than Iran, the latter having fewer oil
wells. Gelodar (2006) evaluated four caviar processing plants for their
hygienic standards using the Hazard Analysis and Critical
Control Point (HACCP), an internationally recognized food safety system. Those plants following
the European Community code had decreased their contamination levels.
70% of Iranian caviar is produced in Mazandaran, 130 tonnes in 1994
(Abzeeyan, Tehran, 6(5, 6):III, 1995) although this conflicts
with a report from IRNA for 2 May 1998 where Mazandaran has 35%
of the total Iranian output at 44 tonnes for 1997-1998. 95% of the
Mazandaran caviar is exported along with 60 of 260 tonnes of sturgeon
meat (IRNA, 2 May 1998). The Bandar-e Torkman fisheries
organization in Golestan Province (eastern Caspian Sea) planned to
process 360 tonnes of sturgeon and 48 tonnes of caviar in 1999-2000 (IRNA,
14 December 1999). Newspaper reports in 1995 gave a value of U.S.$40
million for caviar exports from Iran; another report gave U.S.$50
million for 250 t (Food and Agriculture Organization, Fisheries
Department, 1996). This is less than the value of half a day's oil
sales but the caviar fishery is a national symbol (Christie, 1995). Mazandaran
produced 17 tons of caviar in 10 months in 2003-2004 as well as 140 tons of meat
(www.iranmania.com, downloaded 4 October 2004). In
the Iranian fiscal year ending 20 March 1998 Iran exported 105 tonnes
of caviar worth about U.S.$11 million (Anonymous, 1998b). The 2003 allowed share
for Iran was 78.8 t from a catch for the whole Caspian Sea of 148 t (IRNA, 22 September 2003).
The quota for all Caspian caviar in 2004 was 125 tons (www.nytimes.com,
downloaded 12 October 2004).
The sturgeons were little used after eggs were extracted for caviar
although they were sometimes served in small restaurants along the
Caspian coast (personal observations; remarkably tough and tasteless
too!). Sturgeons were "haram" in Iran, forbidden for
religious reasons as scaleless fish although this has been reversed
(Caddy, 1984; Anonymous, 1989; saffron, 2002). Most flesh was exported to Russia (RaLonde
and Walczak, 1970b) although some is dried and pickled for local
consumption (De Meulenaer and Raymakers, 1996) or eaten freshly
grilled (personal observations). Fraser (1834) noted thousands of
sturgeon carcasses lying on the Safid River banks, discarded after
removal of eggs for caviar and swimbladders for isinglass. Export
prices in 1995 ranged from $5.00 per kg of fresh sevryuga fillet to
$14.50 per kg for smoked beluga (fil mahi) fillet (Abzeeyan,
Tehran, 5(9):V, 1995). Shilat now markets sturgeon head-on or
headless, gutted, frozen or in any form required by customers. The
average processed weight is about 20 kg for Huso huso, 8 kg for
Acipenser gueldenstaedtii (probably includes persicus)
and 6 kg for Acipenser stellatus. The meat is served roasted or
smoked (Shilat advertisement in Seafood International, December 1995).
Research has been carried out in Iran on products derived from left-over parts
of sturgeons, the intestines for fish sauce, and skin for gelatin (Sabour et al., 2006).
Capture methods, in the early twentieth century, involved large iron-barbed
hooks attached to ropes stretched across the river mouth to foul-hook the
sturgeon or, further upstream, poles 6-8 feet long armed with an iron hook used
to gaff the sturgeon (Fortescue, 1920). Sturgeons may be caught more recently by large shore seines but mostly they are
taken by gill nets set 1-3 km out to sea although De Meulenaer and
Raymakers (1996) refer to 300 m fixed nets in rivers with passage
space at the sides and bottom as the only authorised method in Iran.
Trawling in the sea is not allowed in Iranian waters. Sturgeons are
taken from March to June and from September to November in each year
(Christie, 1995). The autumn season is best for A. gueldenstaedtii
(and presumably A. persicus) while the spring season is best
for Huso huso and A. stellatus (De Meulenaer and Raymakers, 1996). Autumn is the main season when the sturgeons migrate
to the southern Caspian Sea (De Meulenaer and Raymakers, 1996). The
draft "Agreement on the Conservation and Utilization of the
Biological Resources of the Caspian Sea" in 1995 prohibited
sturgeon fishing in the open Caspian Sea except for traditional
methods by Iran near its coast within quota limits (Vinogradov in Glantz and Zonn, 1997).
Gill nets used to capture bony fishes, mainly cyprinids, are
responsible for an increase in malformations observed in sturgeons in
recent years (Mehdizadeh, 1993). Fins are broken or cut, rostrums
(snouts) deformed and net fragments embedded in flesh. Gill nets were
prohibited in the Caspian Sea off Iran, except for sturgeons, but
during the Iran-Iraq War economic necessity brought back gill netting
for bony fishes and cooperatives were established (Habibnejaad, 1993).
Gill netter cooperatives were changed to kilka or beach seiner
cooperatives and by the end of 1993 no gill nets were allowed in the
Caspian Sea. However it took 12 years to overcome objections to
banning gill nets by fishermen and in parliament. Problems with excess
mortality through inappropriate fishing methods are not new. In the
period 1925-1930 the total length of long-lines used in the Caspian
Sea was 7-8,000 km while sturgeon nets exceeded 10,000 km. Many fish
died in unattended nets or tore lose from long-lines, later to die
from hook injuries (Sternin and Doré, 1993). The prohibition of the use of gill
nets with a less than 12 cm mesh in 1994 by Iran has conserved stocks along the
southern coast of the Caspian. Additionally licenses were restricted and fishing
co-operatives closed down in order to control the take (Raymakers, 2002).
Iranian fisheries have taken place mainly in the sea and so a lot of immature
fish are caught whereas the former Soviet fisheries took place in rivers where
only adults were taken (and ideally could be controlled more easily). However
state control in Iran has meant, as noted above, better control over the
fisheries and more effective conservation, although poaching does occur.
An attempt has been made to raise sevryuga sturgeon in the central Iranian desert
100 km southeast of Yazd (www.iranmania.com, downloaded 13 March 2003 and other
news reports) in a 5000 sq m artificial pond, perhaps more an indication of the
desperate straits of the sturgeon populations than anything else.
Pourkazemi et al. (2000) examined the phylogenetic relationships of
the 5 sturgeon species in Iran using mtDNA. Huso huso and Acipenser
nudiventris showed a close evolutionary relationship as did A.
gueldenstaedtii and A. persicus. The latter two species apparently
diverged about 1 MYA. Birstein and DeSalle (1998) using molecular techniques
found that the Ponto-Caspian species of sturgeons dispersed through the Black,
Azov, Mediterranean and Aral seas during the Pleistocene 1.5 MYA and later, the A.
stellatus-A. persicus lineage originated 6.0-5.5 MYA in the Upper
Miocene-Lower Pliocene, the A. gueldenstaedtii lineage and the Ponto-Caspian
sturgeons originated 15 MYA in the Middle Miocene, Acipenser originated
and diverged 95-65 MYA in the Upper Cretaceous, and Acipenseridae diverged from Polyodontidae, a related family, 200-135 MYA in the Jurassic.
An important, recent literature source on Caspian
sturgeons is Holčík (1989) as well as specific works on Iranian sturgeon biology and
fisheries by Rostami (1961b), Vladykov (1964) and Mobayen (1968). The fisheries
information in these last three works, relating to techniques and
stations, is somewhat dated and not detailed here (Raymakers (2002) gives a map
showing Iranian fisheries stations). A general account
of sturgeons is given in Birstein et al. (1997) and in Hochleithner and
Gessner (1999). Billard (2000) is a recent review of reproduction and
associated methodologies used on fish farms. CITES (2001) gives an
identification guide in English, French and Spanish, with numerous pictures and
diagrams. Pavlov et al. (2001) review
the types of spawning migrations carried out by sturgeons. There are numerous
other popular reports and scientific papers on Caspian Sea sturgeons, not all of
which can be cited or analysed here. A Bibliography of Sturgeons is given by Y.
Keivany and V. J. Birstein at www.geocities.com/keivany/sturgbibl.html. Various
manuscript reports on the biology and rearing of the economically important
sturgeons have appeared in Farsi, e.g. Abdolhay (1997), Abdolhay and Baradaran
Tahori (1998), Baradaran and Abtahee (1998), Fadaee (1997), Kohneshahri and
Azari (1974), Moghim et al. (1996), Nasrichari (1993), Pourkazemi (1996),
Shafizadeh and Vahabi (1996), etc. Regular symposia on sturgeons are held and
extensive presentations and publications result, e.g. The 5th International
Symposium on Sturgeons, papers from it being published in Journal of Applied
Ichthyology 22(s1)(2006). These works have not all been summarised here because
of expense in obtaining copies and because many papers refer to details of
aquaculture physiology and biochemistry.
A general Farsi name for these sturgeons is سگ ماهي (sag mahi = dog fish).
Caviar
خاویار
Further information on the catches of sturgeons and
production of caviar in Iran can be found in the Species Accounts below.
Iran is the second largest producer of caviar, after Russia, with 20% of the
world market valued in excess of $50 million (Khajehpour-Khouei, 2000). Azari
Takami et al. (1997b) outline the historical development of the caviar
trade and Hosseini Seyed et al. (2008) ranks export goal markets for this
commercially important product.Sturgeon roe or eggs are known as caviar and form an expensive
delicacy (Bolourchi, 1997). The word caviar may come from Farsi "kaya-dar",
"khay-dar" or "khay-var" meaning "having eggs",
from خاگآور or khāgāvar for roe-generator,
or from "chav-jar" meaning "a cake of strength or power" or "bread of
lovers" in allusion to its reputed aphrodisiac qualities; havyar
in Turkish means "fish eggs" but the origin of this word seems in some
dispute among etymologists and it may be Greek (Georgacas, 1978; Sternin and Doré, 1993; Bolourchi, 1997).
In addition to sturgeon roe, eggs of other species are eaten in Gilan and
Mazandaran, where the meal is known as ashpal. The species include Rutilus
frisii, Abramis brama, Salmo caspius and less commonly
Cyprinus carpio and Barbus sensu lato spp. The roe can be eaten cured as a
condiment or when fresh, grilled, steamed or mixed with eggs and fried to form
ashpal kuku, a custard-like dish.
The history of the caviar industry in Iran is a complex subject,
variously reported in the popular media and in legend. The Russians
are said to have obtained writs from Moslem leaders in the Caucasus in
the early nineteenth century to the effect that Moslems could not eat
these fishes, leaving the valuable caviar fisheries for Russian
fishermen to monopolise (Kayhan International, 1 December 1962). The
caviar industry was first granted by the Iranian government to Stepan
Martinovitch Lianozoff (or Lionosoff, Lianozov) an Armenian subject of Czarist
Russia in 1873 (or 1876 or 1879, accounts vary), regularly renewed and later transferred to his only
son George. In 1896 the lease was renewed at an annual cost of 450,000 gold
francs (Fortescue, 1920). In one version of events, Martin (the grandson of Stepan)
disappeared in 1923, kidnapped while meeting two ravishing Armenian
sisters, leaving only a letter ceding his rights in the caviar fishery
to the Soviets (Tehran Mossavar Magazine, 18 April 1952; Time, 9
February 1953; L'Illustré, Lausanne, 20 January 1955; Tehran Radio, 6
May 1959). Another version simply has Martin selling his rights to the Soviet
Government (Mirfendereski, 2000) or refusing to pay during the vicissitudes of
the Russian Revolution. In 1919 another Russian subject, Grigor Petrovic
Vanitsof rented the southern Caspian fisheries for 20 years but could not
fulfill his obligations. A joint Irano-Soviet company, "Mahi Iran", formed
under Soviet pressure on the Iranian government, was given a monopoly
of the foreign sale of caviar in 1927 (in 1923 the fisheries of Astara,
Anzali and Hasan Kiadeh had been occupied by Soviet troops and
declared part of the Soviet fisheries). The Irano-Soviet company was
run almost entirely by Soviet technicians and the caviar was marketed
as of Russian origin (Kayhan International, 27 June 1959; Saffron, 2002). One part of the
Persia/U.S.S.R. agreement banned chemical and explosive uses for capturing fish
(Mirfendereski, 2000). The fishery was nationalised in 1953 and administered by the Iranian Fishery
Company (Sherkat Shilat). Most of the catch was sold to the former
U.S.S.R. (Anonymous, 1961b) and Soviet scientists organised caviar
production until the Iranian Revolution in 1979-1980 (Taylor, 1997).
Greenspan (1989) details more recent skullduggery.
Keyvanfar (1988) described the preparation of Iranian caviar from
the various species and the following is taken from that account.
Emadi (1994) and De Meulenaer and Raymakers (1996) also give accounts
of this process and the kinds of caviar obtained. Sturgeons are alive
or very fresh when brought to the processing plant. They are usually killed by a
blow to the head. Sex is determined with an awl-shaped instrument inserted into
the cloaca, pulling out some ova. The female is
split open along the belly and the eggs and the enveloping adipose and
connective tissues removed. The eggs are generally about 10% of the
body weight. However, an average beluga of 68 kg can yield 18 kg of
caviar in Iran (ca. 26%) (V. D. Vladykov, in litt., 1966), and
a 40 kg beluga from Iran yielded 8 kg of caviar (20%) (L'Illustré,
Lausanne, 20 January 1955). The largest amount obtained from a beluga
was 360 kg of caviar (V. D. Vladykov, in litt., 1966). The
other species give an average of 6 kg of caviar in Iran. The eggs are
separated from the tissues by breaking the ovaries into pieces by hand
and delicately pressing the eggs through a 10 x 10 mm screen. This
takes only a few minutes. The eggs are then washed in fresh, cold
(8-12°C) water for 30-40 seconds to remove fragments of ovarian tissue. The eggs are separated from the
washing water by collecting them on a very fine mesh screen, the
process taking 3-4 minutes. This type of washing is not done with A.
stellatus because of the fragility of the egg membrane in this species.
The type and quality of the caviar is determined next and they
depend on the colour, diameter and membrane strength of the egg. Large
eggs with a strong membrane and a clear, grey, dark brown or gold
colour are the best and are packed in metal containers. Small eggs
with fragile membranes and sombre colouring are second quality and
used for pressed caviar or bulk caviar. Pasteurised caviar is made
from eggs with weak membranes since the heat solidifies the membranes.
Salt is added at a rate of 4-6% to the weight of the eggs, varying
with the season and being higher in the warm summer months. The salt
is 99.2% sodium chloride and only 1-10 kg of eggs can be salted at one
time so that salting is uniform. Boric acid and borax are added in a
ratio of 2:3, comprising 20% of the total salt added, to aid in
conserving the caviar. Caviar for export to the U.S.A. is exempt from
this addition of boric acid and borax and only salt is used, 100 g for
each 1 kg of caviar. The salt is mixed delicately with the eggs by
hand for 50-250 seconds. A good salting process is essential for the
preparation of caviar and is evidenced by the eggs having small white
lines on their surface, the membrane becomes stronger and more
resistant, the egg proteins become denser and coagulate, the eggs lose
their adhesiveness, liquid stops coming from the eggs, and the density
of the brine coming from the process increases. When these factors are
detected the salting process is stopped. A salting process which is
too long removes too much protein from the eggs and causes the eggs to
clump together. A process which is too short removes too little water
from the eggs and these eggs lose water gradually over several days in
their container and become soft and semi-liquid. The eggs are then
separated from the brine on a very fine mesh screen.
The U.S. Customs Service produces a description of caviar for the
trade community (www.customs.ustreas.gov/imp-exp1/comply/caviar.htm
downloaded on 20 July 1999). Caviar is graded on grain size, colour,
flavour and firmness. Gold coloured caviar is the rarest and most
desirable followed by light grey. Large grains are preferred over smaller ones.
There is a demand for caviar without borax and boric acid and such
chemicals as methyl parahydroxy benzoate and propyl parahydroxy
benzoate have been examined in Iran as alternatives (Iranian
Fisheries Research and Training Organization Newsletter, 7:3, 1995).
Fresh caviar is not salted and requires careful refrigeration; its
shelf life is short, a maximum of six weeks. Lightly salted caviar is
called "malossol" from the Russian for "little
salt", usually a 2.4% content in Iranian caviar which is very
good quality compared to some caviar which contains up to 11% salt.
The higher the salt content the longer the shelf life. Chilled
malossol kept at -2 to 4°C will be edible
for up to three months. Pressed caviar is prepared in a similar fashion except the salt
content is higher, at 7% in the finished product. It will keep for a
long time at 4-8°C. Borax gives a longer shelf life too and is less
dangerous to human health than the amount of salt needed to give the
caviar an acceptable shelf life. Most caviar consumed world-wide is
pasteurised as some countries do not accept caviar with borax and
higher salt levels are not acceptable to consumers. Pasteurised caviar
has a shelf life of 12-15 months (De Meulenaer and Raymakers, 1996). Caviar
should not be frozen or pasteurised as this affects the taste.
Good quality caviar must be refrigerated. U.S. packaged caviar also
contained tragacanth gum according to labels on jars from the 1960s. Bankehsaz
(2009) found that the quality and grade of exported caviar can be maintained if
storage time is less than 6 months in a -3°C cold room. Razavilar et al.
(2001) found Iranian caviar to have a good microbial condition during processing
and storage in Mazandaran.
The caviar is placed in boxes of 0.5 to 2 kg, each box being filled
to within 1-2 cm of the lid. Sternin and Doré (1993) give tin sizes
of 0.6 and 1.8 kg with a limited amount of 100, 200 and 300 g tins -
most caviar is repacked at its destination in 30 g, 50 g, 125 g, 250
g, 500 g and 1 kg tins and jars). The lid is pressed on centrally to
exclude as much air as possible and the excess brine is allowed to
drain away by stacking the boxes vertically for 1-15 minutes. One
further press is carried out manually, the outside of the box is
cleaned, and boxes are stacked in piles of five for 20-24 hours in the
cold season (October-March) and 12 hours in the warm season. During
this period, the pile of boxes is turned over several times to remove
the last traces of excess brine. After one last press on the centre of
each box to ensure the lid adheres to the eggs and no air remains, the
box is sealed hermetically with a ring of rubber. Well-prepared caviar
has lost 4-6% of its initial weight, has a salt content of 3-5% and
the eggs are separate and non-adhesive. Caviar in this form will keep
for a long time at 0-2°C. Caviar is re-packed in fully airtight tins,
slowing down the maturation process for three months after which the caviar
deteriorates.
A microbiological analysis of Iranian caviar imported to Turkey has been
carried out by Altug and Bayrak (2003) who did not find any pathogenic and toxin
producing Salmonella spp. and Clostridium perfringens. Coliforms,
bacteria and yeasts showed some high counts, perhaps contamination during
production stages.
First quality caviar consists of healthy, non-fragile eggs from one
species with a large or medium size. The caviar is dry, of uniform
colour - between clear-grey and dark-grey - without odour or abnormal
taste. The box is filled within a centimetre of the edge. Second
quality caviar has eggs which may be fragile, are of large, medium or
small size, their colour varies from clear-grey to black, and they may
be damp. Yellowish or brown caviar from A. gueldenstaedtii is
acceptable for these two qualities of caviar. Egg size is determined
by the cubic centimetres occupied by 100 eggs, e.g. for A.
gueldenstaedtii large eggs occupy >1.9 cc, medium 1.4-1.9 cc
and small <1.4 cc and for A. stellatus large eggs occupy
>1.3 cc, medium 0.9-1.3 cc and small <0.9 cc. Egg sizes are not
determined for Huso huso and A. nudiventris. Eggs of the
former are much larger than A. gueldenstaedtii eggs while those
of the latter are nearly the same size as A. stellatus eggs.
.jpg)
Russian and Iranian caviar tins, beluga, osetra and sevruga (Wikimedia Commons).
Sturgeon species cannot be readily identified from the size or
colour of the eggs making up caviar. Diet, pigmentation of the adult,
and age of the fish all appear to influence egg colour. Huso huso
eggs are often light to dark grey, Acipenser gueldenstaedtii
eggs are blackish to brown or almost golden and A. stellatus
eggs are black according to traders. White caviar where the egg has a
red spot on it is from albino fish. Light grey beluga and light yellow
oscietra caviar are now very rare, in the past being found in only a
small proportion of the species population which itself is now in
decline (De Meulenaer and Raymakers, 1996). Le Comptoir du Caviar,
which markets caviar (www.gourmet-tradition.com/en/comptoir_du_caviar.html,
downloaded on 19 March 1999), describes Iranian sevruga as grey to
black with fine grains and an iodised taste, Iranian oscietre as grey-black
with bronze shades, middle-sized grains, very iodised with a little
taste of walnut, and Iranian beluga as very rare, dark or light grey,
large grains and a fine and gusty savour.
The single biggest market for caviar is first-class airline
passengers. Supermarkets, hotels, restaurants and specialised
retailers also market caviar. France consumed the largest amount in
the 1990s, about 60-80 t, while Germany consumed 40-50 t. The Shilat
packages its product carefully to ensure consumers know the caviar is
genuinely Iranian. The large tins in which the caviar is packed keep
their contents edible for 12-18 months at -2 to -3°C
(the oil content and added salt prevent freezing). These tins are
sealed in a piece of net which in turn is sealed on both sides with
consecutive numbers, placed in a sealed linen bag and then in a wooden
box. Each tin is also marked with the loading station number (where
the fish are brought after capture to have their caviar removed) and
also the number of the individual fish scratched on the side. Tins are
shipped by air in "cooltainers" which have their own
refrigeration unit. These large tins are vacuum-packed into smaller
ones in packing centres in Europe (Christie, 1995). The main market in
2000 was Japan to which 30% of Iranian production was exported. Permanent markets in
Europe are Switzerland, Germany, France, Luxembourg and Spain (I.F.R.O.
Newsletter, 26:3, 2001) and the European
Union is often the biggest importer of Iranian caviar. Iran was the top exporter of caviar in
the year 2000 at 71.5 t valued at $34.4 million (IFRO Newsletter,
28:2, 2001). This is a value increase of 17% although the amount was less than in 1999 at
84.9 t. In 2002, 87% of caviar came from Iran
(www.caviar.ru/english/digest.htm, downloaded 12 December 2002) although IRNA (8 December 2002) gives a
figure of of almost 50%. The caviar export quota was 50,505 kg for Iran in 2006 (iran-daily.com, downloaded 28 July 2006)
or 44.3 t (Iran Daily, 11 September 2006).
Iranian caviar sold in major airports like Heathrow in London comes
in several kinds. Caviar House markets imperial, which has large gold
grains and was previously reserved for the Shah's family (from Acipenser
persicus); beluga, light to dark grey and large grained; royal
black consisting of large deep-black grains from a 20-40 year old
osetr; "oscietre", which is dark grey-brown to a golden
yellowish; classic grey, a pale grey with large grains; and sevryuga,
which is dark grey and fine grained. Prices vary with quality and time
as shown below (personal observations):-
|
|
Imperial |
Beluga |
Royal Black |
Oscietre |
Classic Grey |
Sevryuga |
|
December 1993 |
|
|
|
|
|
|
|
50 g |
£82 |
£76 |
£48 |
£36 |
£38 |
£23 |
|
1000 g |
£1411 |
£1318 |
£819 |
£621 |
£656 |
£399 |
|
September 1995 |
|
|
|
|
|
|
|
50 g |
£94 |
£101 |
£54 |
£48 |
£40 |
£36 |
|
1000 g |
£1640 |
£1759 |
£939 |
£836 |
£697 |
£630 |
|
September 1997 |
|
|
|
|
|
|
|
50 g |
£89 |
£88 |
£53 |
£47 |
£39 |
£34 |
|
1000 g |
£1536 |
£1540 |
£912 |
£812 |
£680 |
£590 |
|
November 1999 |
|
|
|
|
|
|
|
50 g |
£140 |
£160 |
£75 |
£60 |
£65 |
£53 |
|
1000 g |
£2420 |
£2770 |
£1312 |
£1060 |
£1138 |
£920 |
|
November 2000 |
|
|
|
|
|
|
|
50 g |
£184 |
£208 |
£114 |
£95 |
£99 |
£79 |
|
1000 g |
£3541 |
£3987 |
£2177 |
£1818 |
£1894 |
£1527 |
| April
2002 |
|
|
|
|
|
|
| 50 g |
£184 |
£208 |
£114 |
£95 |
£99 |
£79 |
| 1000 g |
not given |
not given |
not given |
not given |
not given |
not
given |
The types of caviar listed changed in 2003 as follows:-
|
|
Imperial
XO
|
Beluga |
Beluga XXL |
Royal Black |
Royal Black XL |
Oscietre Gold |
Classic Grey |
|
March 2003 |
|
|
|
|
|
|
|
|
50 g |
£289 |
£160 |
£309 |
£11 6 |
£197 |
£119 |
£125
|
The types of caviar listed changed in 2004 as follows:-
|
|
Imperial
XO
|
Beluga |
Beluga XXL |
Royal Black |
Royal Black XL |
Classic Grey |
Sevruga |
Oscietre |
Imperial |
|
September 2004 |
|
|
|
|
|
|
|
|
|
| 50 g |
£145 |
£160 |
£195 |
£88 |
£114 |
£66 |
£56 |
£75 |
£98 |
| 100 g |
£275 |
£318 |
£385 |
£175 |
£225 |
£129 |
£110 |
£149 |
£195 |
| 200 g |
£540 |
£619 |
£750 |
£340 |
£435 |
£247 |
£221 |
£297 |
£385 |
The types and weights of caviar listed changed in 2006 as follows for
"Prestige Selection":-
|
|
Beluga |
Royal Black |
Classic Grey |
Sevruga |
Oscietre |
Oscietre Gold* |
|
March 2006 |
|
|
|
|
|
|
| 50 g |
£155 |
£125 |
£105 |
£95 |
£120 |
£130 |
| 125 g |
£380 |
£305 |
£255 |
£230 |
£295 |
£320 |
| 250 g |
£750 |
£595 |
£495 |
£450 |
£580 |
£630 |
* = golden-coloured eggs from a mature oscietre.
The types and weights of caviar listed changed in 2006 as follows for "Prunier":-
|
|
Traditional |
Saint James |
Great American |
Paris |
Heritage |
|
March 2006 |
|
|
|
|
|
| 50 g |
£65 |
£85 |
£95 |
£120 |
£155 |
| 125 g |
£155 |
£205 |
£230 |
£295 |
£380 |
| 250 g |
£305 |
£405 |
£455 |
£585 |
£755 |
| 500 g |
£605 |
£805 |
£905 |
£1165 |
£1505 |
| March 2007 (500 g not listed) |
|
|
|
|
|
| 50 g |
£75 |
£110 |
(not listed) |
£120 |
£170 |
| 125 g |
£185 |
£270 |
(not listed) |
£295 |
£415 |
| 250 g |
£370 |
£535 |
(not listed) |
£585 |
£830 |
The types and weights of caviar listed changed in 2006 as follows for
"Private Reserve":-
|
|
Royal Black XL |
Imperial XO |
Beluga XXL |
|
March 2006 |
|
|
|
| 50 g |
£135 |
£145 |
£195 |
| 125 g |
£330 |
£335 |
£480 |
| 250 g |
£650 |
£695 |
£950 |
Prices for these brands were not listed in March 2007, and were only
available on request, indicative of both scarcity and constantly changing
prices. Names of the different types of caviar keep changing so it is
difficult to track price increases. In November 2008, beluga caviar from
Caviar House was selling for
£2170 for 250 g and other types had also continued to increase in price,
often dramatically. Curiously, in July 2010 under "Caspian Sea caviar"
beluga was selling at
£1340 per 250 g. In 2011, all available caviar was Russian or farmed.
Taylor (1997) gives prices in Deutschmarks (DM) per kilogramme net
weight (no duty paid) for Iranian caviar over 12 years including the
approximate "bazaar" or illegal price for smuggled caviar
(note also that A. gueldenstaedti probably includes A.
persicus):-
|
Year |
Huso huso |
A. gueldenstaedti |
A. stellatus |
"Bazaar" |
|
1983 |
540 |
408 |
341 |
200 |
|
1984 |
600 |
424 |
400 |
180 |
|
1985 |
675 |
465 |
404 |
180 |
|
1986 |
650 |
460 |
345 |
200 |
|
1987 |
650 |
414 |
325 |
180 |
|
1988 |
1630 |
445 |
310 |
180 |
|
1989 |
2600 |
510 |
345 |
220 |
|
1990 |
1596 |
432 |
304 |
220 |
|
1991 |
1600 |
450 |
337 |
180 |
|
1992 |
1600 |
470 |
345 |
160 |
|
1993 |
950 |
435 |
345 |
160 |
|
1994 |
950 |
500 |
355 |
80 |
Taylor (1997) also compares demand from western markets with supply
from Iran; for Huso huso demand is 0.2 tonnes while supply is
2.0 t, for A. gueldenstaedti type I.A 2.0 t and 0.5 t, for A.
gueldenstaedti type I.B 60.0 t and 40.0 t, for A.
gueldenstaedti type II 15.0 t and 10.0 t, for A. stellatus
I 100.0 t and 30.0 t, and for A. stellatus type II 100.0 t and 25.0 t.
Friedland (1986) gives a variety of recipes for caviar dishes.
Rehbein (1985) and Keyvanfar et al. (1987) studied soluble caviar proteins of
sturgeon species including A. gueldenstaedtii, A. stellatus, A. nudiventris and Huso
huso. They were able to distinguish the species on this basis and
thus provide a means of detecting fraudulent caviar. Rezvani Gilkolaei (2002)
used DNA PCR amplification and RAPD markers to identify caviar, in particular
that of the endangered Acipenser nudiventris whose caviar has been
substituted for more expensive caviar of A. gueldenstaedtii and A.
persicus. Rehbein et al.
(2008) tested and reviewed different methods for identifying caviar by species,
including DNA, differential scanning colorimetry and determination of stable
isotopes. Gessner et al. (2008) were able to distinguish between farmed
and wild sturgeons based on fatty acid composition and recommend use of specific
fatty acids as additives in the formulated diets of farmed fish. However, Ludwig
(2008) reviewed methods of identifying caviar and other sturgeon products and
detailed difficulties. No single method met the criteria he established
(species-level identification, population identification, wild versus
aquaculture, age of caviar). Cost was also a factor. Keyvanfar (1984)
was unable to find genetic polymorphism in erythrocytes of the four
species listed above using serological techniques.
Genus Acipenser
Linnaeus, 1758
This genus is characterised by a small, transverse mouth (large and
crescentic in Huso), by the gill membranes being joined to the
isthmus and not to each other (joined to each other and free of the
isthmus in Huso), by a rounded or elongate snout, and cylindrical barbels.
Bani et al. (2008) give details of brain morphology in Acipenser
stellatus and A. persicus that suggest sturgeons have evolved
different sensory strategies to cope with life in the deep sea.
There are 16 species in the genus and 4 are reported from Iran.
Ventral view of heads of Huso huso,
Acipenser nudiventris, A. gueldenstaedtii and A. stellatus
(A. persicus is similar to A. gueldenstaedtii)
Acipenser baerii
Brandt, 1869
Introduced to the Caspian Sea basin by Soviet authorities (Karpevich
and Lukonina, 1971; 1972; McNeil, 1979) but no records from Iran.
Acipenser gueldenstaedtii
Brandt and Ratzeburg, 1833
Common names
چالباش (= chalbash or short head),
تاس ماهي (= tas mahi or bald fish; this term includes A.
gueldenstaedtii, A. persicus and A. nudiventris for large eggs, in
fisheries statistics), تاس ماهي روس (= tasmahi-ye Rus
or tasmahi-e-russ), تاس ماهي ايراني
(= tas mahi Irani), osiotra, osyetra, سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), kaviari rusi.
[russkii osetr or Russian sturgeon in Russian; nere or rus neresi in Azerbaijanian; bekra or bekre balyk in Turkmenian].
Systematics
This species was originally described in part from the Volga, Ural
and Terek rivers of the Caspian Sea. Sometimes spelt güldenstädti,
but accents on letters are not used in Latin scientific names.
Birstein et al. (1997) and Reshetnikov et al. (1997)
spell the name gueldenstaedtii, regarding the double "i"
ending correct as opposed to the emended single "i" which
appears in much recent literature.
Acipenser gueldenstaedti persicus natio kurensis Belyaeff, 1932 was described as the
Kura River subspecies but see below under Acipenser persicus.
Comparison of serum proteins have shown antigenic characteristics
distinguishing Volga and Kura River stocks in the Caspian Sea, matched by morphometric characters.
The fishes identified as A. gueldenstaedtii in Iran may well
be almost entirely A. persicus, although this remains to be
determined. Consequently data on morphology and biology are confused in many accounts.
The distinction of A. persicus is questioned by authors (see below).
Some specimens have strong spines on the scutes and have been
described as morpha aculeatus Lovetzky, 1834 although this has no nomenclatural status.
Birstein and Ruban (2004) and Birstein et al. (2005) state that this species has at least three,
morphologically indistinguishable, genetic forms in the Caspian Sea. These are the pure form, one similar to A. baerii
of Siberia, and one to A. naccarii of the Adriatic, with competing
hypotheses to explain this. The most likely hypothesis is that the Caspian forms
are closely related to the ancestral forms of the three species, evolving first
as subdivisions of the original Caspian Sea population and then moving to
different geographical areas when the Caspian was connected to them.
Pourkazemi et al. (1999; Rezvani Gilkolaei, 2000) found two distinctive genotypes and therefore
populations of A. gueldenstaedtii in Iranian waters using
molecular techniques. This species and A. persicus showed great degrees of similarity in a phylogenetic
analysis (Iranian Fisheries Research and Training Organization
Newsletter, 14:4-5, 1996; Pourkazemi et al., 2000). The common origin of the two species
was about 1 million years ago (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 61-62, 1997; Pourkazemi et al., 2000).
Key characters
This sturgeon has a short snout (less than 60% of head length) with
a rounded tip in contrast to the long snout (>60%) and pointed tip
in A. stellatus. Huso huso has an unusual,
crescent-shaped mouth and continuous gill membranes forming a fold on
the isthmus and A. nudiventris has a continuous lower lip and
usually more than 50 lateral scutes. Closely resembling A. persicus,
it is distinguished from that species by the short and blunt snout,
yellowish-white belly and golden-brown back. A drawing in Vlasenko,
Pavlov and Vasil'ev in Holčík (1989) of the two species has a snout length in head length of 4.3 as
opposed to 3.2 for Acipenser persicus but figures of snout
length in total length overlap for the two species. The interorbital
distance is much less in the Persian sturgeon (29.2-30.5% of head
length) than in A. gueldenstaedtii (Artyukhin and Zarkua, 1986)
but in small specimens examined by me from Iran, some had gueldenstaedtii
interorbital distance and persicus snout length. There is a
colour plate and line drawings of the heads of the two species from
the Black Sea in Birstein et al. (1997:8, 220).
Morphology
The lower lip is interrupted at its centre. Barbels are not
fringed, lie nearer the snout tip than the mouth, and do not extend
back to the mouth. Sheibani (2003b) described the posterior alimentary canal in
this species.
Dorsal fin rays 26-51, anal fin rays 18-35. Gill rakers 15-36.
Dorsal scutes 5-19, lateral scutes 21-50 and ventral scutes 6-14.
There are rows of smaller star-shaped scutes between the dorsal and
ventral rows in some fish, rounded in this species and more triangular in A.
persicus. The chromosome number is 2n=250 ± 8 or 2n=247 ± 7 (Klinkhardt
et al., 1995).
Sexual dimorphism
Females are larger than males of the same age.
Colour
The back is usually golden-brown but may be olive-grey to dark
green, the flanks grey-brown, and the belly yellowish-white or rarely
a lemon yellow. Young are blue dorsally and white ventrally.
Size
Attains 160 kg and 2.36 m, perhaps as much as 4 m although not confirmed. In Iran,
fish identified as this species (see Systematics) averaged
16-20 kg and 1.4-1.6 m in the 1950s (Farid-Pak, no date). Tsepkin and
Sokolov (1971) state that Safid River fish reach 2.42 m. De Meulenaer
and Raymakers (1996) give 200 kg and an average length of 2 m.
Distribution
This species is found in the Caspian Sea, particularly in the Volga
River basin, as far as Moscow in the past. Very small numbers are
caught in the Kura and Astara rivers. Also found in the Black Sea
basin. Khodorevskaya et al. (2001) review abundance and distribution in former
Soviet waters of the Caspian Sea. It is less common than Acipenser persicus
in Iranian waters.
In Iran, it is recorded from the Astara River in the west to the Gorgan
River in the east (but see Systematics). Reported recently from
such rivers as the Atrak, Gorgan, Gharasu, Tajan, Babol, Haraz, and
Safid, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea (Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli and Naderi, 2009). V. D. Vladykov's field notes in the early
1960s reported it from Kopurchal, Khadjenafas, Tazeabad, 12 Bahman, Nevissi,
Izadeh and Hasan Kiadeh. Access to many rivers must now be
restricted by reduced water flow, construction, weirs, dams, irrigation canals and pollution.
Zoogeography
Presumably a relict of the isolation of the Caspian and Black seas
from the Mediterranean-Atlantic.
Habitat
There is no marked seasonal variation in depth distribution in the
south Caspian Sea in contrast to the middle Caspian. This species is
found over sand or sandy-silt bottoms in a temperature range of
2.3-24.8°C and a salinity range of 6.28-14.34‰ in the sea. It approaches closer
to the coast in winter (February) than other sturgeons because it
favours colder temperatures. It is found in numbers down to 50 m with
only the occasional specimen being caught below this depth (Legeza,
1972; 1973). Brackish water is favoured because of food
concentrations. High oxygen concentrations are needed, 6-7 mg/l for
adults, although larvae only require a minimum of 1.56 mg/l at 20°C.
Reproduction in the Kura ceases when temperatures reach 26°C.
Eggs are sensitive to oil concentrations of 0.5-1.0 mg/l. Levin (1997)
reports concentrations on the western shelf of the Caspian Sea during
winter, as far south as Azerbaijan, at depths of 5-24 m.
Age and growth
Veshchev and Novikova (1986) and others have recently studied the
spawning run of this species in the Volga River and found fish from 7
to 39 years old with 87.9% 13 to 27 years old. The spawning population
comprises 32 age groups therefore. Males dominate at 63.6%. Males vary
in length from 101 to 185 cm and weigh 3 to 38 kg and females from 116
to 200 cm and 9 to 46 kg. Most males begin to reproduce at 11-13 years
while females begin at 12-16 years. Growth can be rapid,
young-of-the-year reaching as much as 35 cm by autumn. Life span
exceeded 48 years in the past. Khodorevskaya et al. (1993)
cited in Levin (1997) gives Volga River spawning ages of 8 to 35 years
with females 6-8 years older than males. Females have an average
weight of 26-29 kg and a length of 136-163 cm; males are 12.0-14.5 kg
and 130-134 cm. Females mature at 10 years, 2-3 years later than males.
Minimum spawning intervals are 2-3 years for males and 3-4 years for females.
Von Bertalanffy growth parameters in Iranian females are L∞
= 201 cm and K = 0.073 or 192 cm and 0.082 and for males 189 cm and
0.092 depending on the methodology used. Total mortality (Z) was
0.33-0.67 for females and 0.46-0.82 for males, natural mortality (M)
was 0.05 for females and 0.06 for males, fishing mortality (F) was
0.62 for females and 0.39 for males, and optimum fishing mortality was
(F) 0.21 for females and 0.37 for males (Iranian Fisheries Research
and Training Organization Newsletter, 16:4-5, 1997).
Food
This species is primarily a mollusc eater (Polyaninova et al., 1999) but also takes
crustaceans such as chironomids and gammarids and small fishes such as
gobies (Gobiidae) and Clupeonella caspia. The introduced
species of mollusc, Abra ovata, polychaete, Nereis
diversicolor, and crab, Rhithropanopeus harrisii are now
important diet items at 49.3%, 12.3% and 9.2% respectively in the
Caspian Sea. The importance of oligochaetes like Nereis
and Enchytraeus albidus in the diet of sturgeon species is
recognised in Iran and studies on their ecology have been carried
(IFRO Newsletter, 28:3, 2001).
In rivers, fingerlings feed on various benthic organisms. Hajimoradloo et al.
(2002) examined the diet of juvenile fish taken in beach seines from the
Miankaleh peninsula in Golestan and compared it with the diet of A.
persicus. The latter favoured cumaceans while A. gueldenstaedtii
favoured gammarids. Both species had more empty stomachs in autumn and less in
winter. A. gueldenstaedtii had more empty stomachs in all seasons than
A. persicus. The food niche width was less in A. gueldenstaedtii and
food overlap was highest in winter and lowest in spring.
Reproduction
Spawning migrations in sturgeons are triggered by temperature,
daylength and flood discharge. This has been discussed more fully by
Barannikova (1972) along with the effects of dams on this complexly
timed, hormonal process. In northern rivers the water temperatures are
8-18°C (Artyukhin and Zarkua, 1986). The adult loses 25-30% of its weight
after spawning and females are only ready to spawn again after 4-6
years and males after 2-4 years.
The spawning run in the Kura River is complex and four
"races" have been recognised (Gerbilskii, 1955; Berg, 1959).
These are early and late vernal, spawning in their year of entry, and
summer-arriving and autumn-arriving hiemal which overwinter to spawn
the following spring. The chief spawning period in the Kura River is
from the end of May to the beginning of July (Zakharyan, 1972). (Note
that this may in fact apply to A. persicus).
In the Volga River, A. gueldenstaedtii has a run beginning at the
end of March or beginning of April at 1-4°C,
peaking in July. Migration speed in the Volga is 18.1-22.6 km/day.
Eggs are laid on gravel or stone beds at 4-25 m depths and a current
velocity of 1-1.5 m/sec. in the Volga River. Some eggs are laid in
shallower, flooded areas. Egg incubation is optimal at 9-15°C.
The downstream migration of spawned out fish in the Volga begins in
the second half of May and peaks in June and July. Levin (1997)
summarises the migration of Volga River fish as follows. The small
population of the early spring race enters the Volga delta in
April-May and migrates upriver for 600-700 km before spawning in
May-June at 12-15°C. The late spring race migrates to spawning sites in May-June, spawning
in July-August at 19-22°C. In June-July the winter race enters the delta but only migrates
upriver in the next summer. In August-October the late winter race
enters the river. These winter races overwinter in deep parts of the
river and spawn in April-May at 9-13°C.
Volga River sturgeons had a fecundity of 332,900 eggs in one study
(Veshchev and Novikova, 1986), elsewhere reported up to 1,165,000 eggs
for the Volga. The Safid River sturgeon fecundity is said to be less
(this may be A. persicus). Eggs are brownish-grey and ovate, up
to 3.3 x 3.8 mm in dimensions. A 150 kg fish yielded 5 kg of caviar (IFRO
Newsletter, 29:4, 2001).
The sexual cycle lasts 2-3 years on the Iranian coast and is described by
light microscopy in Hedayatifard et al. (2009).
The caviar of this species comprises 4-5 kg on average, making up
16% of the body weight in Iran. In Mazandaran fish enter rivers in
autumn, overwinter and spawn in spring (Iranian Fisheries Research
and Training Organization Newsletter, 9:6, 1995).
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) describe Corynosoma caspicum as a
new species from this and other sturgeon species in Iran. Mokhayer and
Anwar (1973) report the following parasites from Iranian sturgeons in
general. These are the protozoan Trichodina reticulata, the
coelenterate Polypodium hydriforme, the trematodes Skrjabinopsolus
acipenserinus and S. skrjabini, the cestodes Amphilina
foliacea, Bothrimonus fallax and Eubothrium
acipenserinum, the adult nematodes Ascarophis ovotrichuria,
Cyclozone acipenserina and Cucullanus sphaerocephala,
the larval nematodes Contracaecum squalii, Anisakis
schupakowi and Eustrongylides excisus, the acanthocephalans
Leptorhynchoides plagicephalus, Pomphorhynchus laevis
and Corynosoma caspicum, the annelid Piscicola geometra
and the crustacean Pseudotracheliastes stellatus. Polypodium
hydriforme destroys the eggs of sturgeons, up to 80% of the
gonads, rendering reproduction insufficient to maintain the species. Amphilina
foliacea causes parasitic castration in sturgeons. Many of the
parasites provoke anaemia or block the intestine when numbers are
high. Pomphorhynchus laevis is capable of piercing the
intestine. Eustrongylides excisus produces stomach abscesses.
Ectoparasites take blood but also facilitate attack by bacteria and
fungi. On fish farms, Trichodina reticulata can cause high
mortalities while having no apparent effect under natural conditions.
Parasite numbers are controlled on fish farms by immersing the
sturgeons in salty water to remove ectoparasites, by feeding food
items known not to be carriers of parasites and avoiding such natural
foods and intermediate parasite hosts as amphipods. Mokhayer (1976b)
reports gas bubble disease in Iranian sturgeons without specifying the
species of sturgeon as well as the monogenetic trematodes Diclobothrium
armatum and Nitzschia sturionis, the digenetic trematodes Skrjabinopsolus
acipenseris and S. skrjabini, the cestodarian Amphilina
foliacea, the cestodes Bothrimonus fallax and Eubothrium
acipenserinum, the nematode larvae Anisakis schupakowi, Contracaecum
squalii and Eustrongylides excisus, and the nematode adults
Ascarophis ovothricuria, Cucullanus sphaerocephala and Cyclozone
acipenserina, the acanthocephalans Corynosoma caspicum, Leptorhynchoides
plagicephalus and Pomphorhynchus laevis, and the crustacean
Pseudotracheliastes stellatus.
Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in
juveniles at a frequency of 6.42%. Sattari et al. (2002) record
Cucullanus sphaerocephalus, Eustrongylides excisus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus,
Anisakis sp. and Corynosoma strumosum, the fauna being similar to
other sturgeons because of their piscivorous feeding. Hajimoradloo and Ghorbani Nasrabadi (2003)
found the prevalence of metazoan parasites in juveniles of this fish in the
southeast Caspian Sea to be 8 species with Anisakis larvae the highest at
13.3%. Pazooki and Masoumian (2004) report on blood parasites form fish caught
at Anzali, recording Cryptobia acipenseris and Haemogregarina
acipenseris. These parasites caused no pathological effects in the wild fish
but can lead to severe infections and cause anaemia on fish farms. Sattari and
Mokhayer (2005a; 2005b) recorded the occurrence of parasites in this species
from the Iranian southwestern and central coast of the Caspian Sea. The species
found were the nematodes Cucullanus sphaerocephalus, Eustrongyloides
excisus and Anisakis sp., the acanthocephalans Leptorhynchoides
plagicephalus and Corynosoma strumosum, and the digenean trematode
Skrjabinopsolus semiarmatus. General conclusions were that the diversity of
parasites was less in Iranian waters than in the northern Caspian Sea, perhaps a
reflection of the more varied habitat, its productivity and the carbonate ions
differing between the two regions. The diversity of parasite seems to have
declined over time also, perhaps as a result of unfavourable environmental
conditions, particularly in the freshwater ecosystem which limits the waters
available for spawning and parasite acquisition.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
A wide range of fish species are predators on the eggs of this
sturgeon and the young are taken by Silurus glanis, Alosa spp.,
Huso huso, and gobiids.
Economic importance
Chalbash have been fished in the Caspian Sea for at least 6000
years based on excavations at a Neolithic site on the eastern Caspian
coast (Tsepkin, 1986).
This particular species is fished primarily in the months of
September and in April-May in Iran. Caviar yield was 4-7 kg per female
in the 1950s (Farid-Pak, no date). Yields from 1963 to 1967 of meat
(and caviar) were 794.2 tonnes (69.3 tonnes), 918.2 (66.7), 849.0
(71.8), 974.6 (72.8), and 977.1 (75.9) respectively (RaLonde and
Walczak, 1970b). A commercial house maintains (1995) that this species
comprises 27% of the total catch. These data presumably include or are
almost entirely A. persicus in Iran. Spring-caught chalbash produce 2-3
kg of eggs per fish while those caught in the fall have egg weights of
3-4 kg. The former are more suitable for pressed caviar than the
higher priced grain caviar made from the larger eggs of fish caught in
fall (Vladykov, 1964). Figures for tas mahi (this species plus A.
persicus, and also A. nudiventris when eggs are large)
average yearly catches in Iran were given by Vladykov (1964) for the
period 1927/28-1931/31 to 1957/58-1961/62. Body weight varied from
264,105 kg (36.9% of total sturgeon catch) to 842,050 kg (78.9%) while
caviar weight varied from 33,098 kg (69.3%) to 159,931 kg (85.1%)
although the lowest percentage share of caviar for any of the
five-year periods in tas mahi was 28.6%. The category of tas mahi
provided the majority of eggs for caviar up to 1946/1947 (50-89%) but
this fell to 29-31% for the period after 1949/1950 in Vladykov's data.
Earlier data from Nevraev (1929) listed as A. gueldenstaedtii
and A. nudiventris combined for the Astara region of Iran gives
catches of 2002 to 9176 individuals for the period 1901-1902 to
1913-1914, for the Safid Rud region 26,721 to 54,257 individuals for
the period 1899-1900 to 1913-1914, for the Mazandaran region 4065 to
8818 individuals for 1906-1907 to 1913-1914, and for the Astrabad (=
Gorgan) region 2988 to 6044 individuals for 1902-1903 to 1913-1914. The capture
fishery for tas mahi (A. gueldenstaedtii, A. persicus and A.
nudiventris) was 89%, 4.2% and 6.2% respectively in 1973 but by 1993 had
changed to 27%, 69% and 4% due to fingerling production of A. persicus (Abdolhay
and Tahori, 1999). The stock of this species in Iranian waters in 2001 was 9.4
million (0.64 million) specimens comprising 12,900 tonnes (2074 t) with a
commercial stock of 220 t (223 t) (Ivanov and Kanunin, 2001; figures in
parentheses from text which does not agree with table).
Catches of this species in the southern Caspian Sea have declined from 837 t
and 602 kg/boat/day in 1971-1972 to 57 t and 0.34 kg/boat/day in 1999. Young
fish decreased in the decade prior to this study while older fish dominate at
present (Moghim, 2004a). A sharp decrease in sea ranching of fingerlings and a
consequent decrease in young fish abundance, will cause a a considerable decline
in future catches.
Dry-smoked flesh (balyk) is especially favoured in Russia
where this species occupies the first place in catches. Catches in the
period 1898-1913 in the northern Caspian reached 10,000 tonnes a year
only to decline through overfishing. The ban on sea fishing in 1941,
restricting catches to rivers where they could be more closely
controlled, led to a rebound of stocks and by 1977 a record catch of
11,980 tonnes was made.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food.
Conservation
Sturgeons generally are conserved by fish farming and release of
young and fry, attempting to augment natural populations. Stroganov
(1968) reviews Russian fish farming methods. Derzhavin (1923) reported
release of 7,620,000 fingerlings in the Safid River of Iran in 1923. Release
of unfed sturgeon fry was discontinued in Iran in 1965 as
unproductive. A hatchery produced annually 5.5 million sturgeon
juveniles at 3-5 g each (McNeil, 1979), comprising the species A.
stellatus and A. gueldenstaedtii (the latter presumably
includes A. persicus). The Sad-e-Sangar (Dr. Beheshti or Martyr
Beheshti) Fish Farm or Hatchery 27 km from Rasht in Gilan produced
14-15 million sturgeon larvae in 1987 and up to 3 million 2-3 g
sturgeon are produced annually (Petr, 1987). Fingerling production
from four hatcheries in Iran reached a record high of 12 million fish
in 1995-1996 and with a new hatchery in the Gorgan region is expected
to reach 20 million fingerlings (Abzeeyan, Tehran, 7(6):V,
1996). IRNA reported on 31 August 1998 that 24 million fry had
been raised since March of that year, a 15.3% increase over the
previous year and 20 million fry are now released each year. The
Shahid Rajaee Fish Aquaculture Center at Sari, Mazandaran produces 5.5
million sturgeon fingerlings annually, released in 13 Caspian Sea
rivers (IFRO Newsletter, 28:3, 2001). The only
species not produced is Huso huso and the most popular is Acipenser
persicus for its better quality caviar. The young are fed on
daphnia and later oligochaetes (white worms). Fingerlings may be grown
to 10-15 cm length before being released in the Safid River about 20
km from the sea to imprint on the river. In 1987 2.28 million
fingerlings were released and in 1993 6.5 million from the Beheshti
Hatchery. In 1993 a closed system fish culture plant was opened at
this hatchery to produce at least 5 million sturgeon fingerlings
annually (Abzeeyan, Tehran, 4(9):IV, 1993; see also Anonymous
(1993c)). A later report mentions culture of Huso huso in
addition to the sturgeon species mentioned above for the Dr. Beheshti
Sturgeon Hatchery, and production of fingerlings exceeded 60 million
in 1991, the best year from 1973 to 1993 (Abzeeyan, Tehran, 5(3
& 4):IX-X, 1994).
About half a million fingerlings were produced
in autumn 1995 in Mazandaran province (Iranian Fisheries Research
and Training Organization Newsletter, 9:6, 1995). In 1996, it was
expected that 15 million sturgeon fingerlings would be produced from
hatcheries, the main species being Acipenser persicus, Acipenser
stellatus and Huso huso. Fingerlings would be 3-5 g in
weight when released in the Safid River (Abzeeyan, Tehran,
7(2):IV, 1996). The Shaheed Beheshti Fish Propagation and Rearing
Complex of Shilat (Iranian Fisheries Company) produced 9 million
sturgeon fingerlings in 1997, each 3-5 g, for restocking (Bartley and
Rana, 1998b). Eggs are incubated for 5-7 days. Fingerlings are fed on
live Artemia, Daphnia and oligochaetes in 2.5 sq m
circular tanks from day 15 (60-80 mg) and then in earthen ponds for 50
days to the 2-3 g size. The fingerlings remain in the release river
for 10-15 days before entering the Caspian Sea.
The International Sturgeon Research Institute, which opened in 1994 near Rasht, released
22 million fry in 1996-1997 (Bartley and Rana, 1998b). The Institute
carries out varies research programmes, e.g. on the histology of the
gonads of reared sturgeons which have been found to be the same as
sturgeon in nature (Bahmani and Kazemi, 1998).
Abdolhay and Tahori (2006) give fingerling production for this species as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
81 |
74 |
65 |
0 |
31 |
| Injected broodstock |
29 |
24 |
19 |
0 |
10 |
| Spawning rate * (%) |
89.6 |
79 |
66 |
0 |
80 |
| Fertilisation rate (%) |
70 |
55.5 |
49 |
0 |
71 |
| Survival rate in incubators
(%) |
53 |
53.9 |
48 |
0 |
75.1 |
| Survival rate in tanks (%) |
80 |
70.1 |
68 |
0 |
79 |
| Stocking density in ponds
(fish/ha) |
88,333 |
74,580 |
63,752 |
0 |
65,000 |
| Survival rate in ponds (%) |
65 |
79 |
71 |
0 |
65.1 |
| Fingerling production (x
1000) |
1327 |
447 |
1816 |
0 |
617 |
* Rate of response to hormone injection
An experimental approach to conservation of stocks has been the raising of
sturgeon artificially to a size where they produce caviar. The Shahid Beheshti
sturgeon aquaculture centre raised a member of this species to 121 cm, 11.5 kg
and 8 years of age when it yielded 1.4 kg of caviar (Iranian Fisheries Research
Organization Newsletter, 37:2, 2003; Iranian Fisheries Research
Organization Newsletter, 40 & 41:4, 2004).
Shevchenko et al. (1999) summarise rearing technology for A. gueldenstaedtii in Iran. Fingerlings
are raised on artificial feeds in 1-4 cu m plastic tanks for up to 180
days. A mean mass of 120 g is attained, with a maximum of 300 g. Growth rate of
different age groups varied from 1.59 to 0.56% and daily weight gain was from
4.23 to 1.42%. The mean daily increment was affected by stocking density, daily
rations, oxygen content, feed quality and maintenance of feeding routine.
Falahatkar and Amini (2003) give further details on propagation from broodstocks
including maturity duration, oocyte diameter and weight, motility and density of
spermatozoids, time taken to reach 4 and 16 cell divisions, incubation duration,
fertilisation percentage achieved at each stage, mortality rate during
incubation, number of larvae obtained from each broodstock, number of larvae per
gramme, weight of each larva, and morphometric parameters and age for each broodstock.
Akrami et al. (2005) found cladocerans, copepods and chironomid larvae were secondary prey
items of fingerlings in one earthen pond with ostracods occasional prey, while
in another pond all these were secondary prey. Condition factor and growth
decreased as weight and length of fingerlings increased. Growth was was
negatively allometric (b<3).
De Meulenaer and Raymakers (1996) give figures for Iranian hatchery
production from 1983 to 1992 as 1.03 to 6.61 million fingerlings (mean
2.9 million) although mature adults are becoming increasingly
difficult to catch for stripping of eggs and sperm. These Iranian
hatcheries are much smaller than Russian ones which produced about 25
times this number on average annually from the Volga River hatcheries alone.
There is an extensive Russian literature on how to raise sturgeons,
e.g. Mil'shtein (1957; 1972), Marti (1972), Barannikova (1987) and
Dettlaff et al. (1993). A recent (1984-1986) estimate of this
species in the Caspian Sea is 47.7 million fish with 24-28% produced
by artificial means.
All sturgeons are particularly threatened on the spawning migration
when they concentrate in rivers (Rochard et al., 1990).
Sturgeons in the Aras River on the former Soviet-Iranian border, for
example, are threatened by dams and water diversion schemes (Zakharyan,
1972). However this is not an annual migration so the populations are
not subject to loss every year. The common problems encountered by all
Caspian sturgeons are dams and weirs which block reproductive
migrations of adults upriver and also of young and adults returning to
the sea, water abstraction for irrigation which reduces flow or even
dries up a river, degradation of the river bed by extraction of gravel
for construction or the change in silt deposits by the filtering
effects of dams, increased water clarity enables predators to be more
effective changes in the oxygen and temperature regimes caused by
water abstraction, retention of water behind dams or untimely release
from dams, pollution, attraction of adults into irrigation channels by
their strong water flow and changes in the invertebrate fauna on which
the young feed in rivers (Vladykov, 1964; Anonymous, 1970c; Whitney,
1979; Rochard et al., 1990). Variations in Caspian Sea levels
also had effects (Khodorevskaya et al. 1997). For Huso huso
these include lowered accessibility to feeding sites and variations in
food abundance which lead to decreases in relative weight gain and to
a halving in the number of females. The growth and survival of
juvenile Acipenser gueldenstaedtii in the Volga River delta
during their first winter is affected by lower water levels.
Stocks in their sea life were fairly safe until trawling was
introduced. There are restrictions on trawling in the sea to reduce
loss of young sturgeons (Ricker, 1970) and trawling is banned in the
territorial waters of Azerbaijan (Markarova and Alekperov, 1989). It
has been suggested that the Caspian Sea level should be maintained at
-28.5 m or above to retain water productivity on which sturgeons
ultimately rely. A 1 m decline in level can reduce fish food supply by
60% and hinders migration to feeding grounds, another 20% loss (Petr, 1987).
The institution of closed seasons for fishing and restrictions on
techniques used to limit juvenile catches have been implemented in the
former U.S.S.R. The fine for illegal possession of a Huso huso
was about £280 in 1977. Fish lifts have been built on the Volga River
about 5000 km upstream from the Caspian Sea to transport sturgeon
around the Volgograd Dam. The system transports about 10-20% of
migrating Huso huso, Acipenser gueldenstaedtii and A.
stellatus but is relatively inefficient (Rochard et al.,
1990). The poor situation is compounded by the lack of suitable
spawning conditions above the dam and by adults having to migrate
downstream through the dam's turbines. The turbines have wide blades
and rotate slowly so most adults cannot make it through although the
young are short enough to survive the transit. Khodorevskaya et al.
(1997) summarise the decline in catches of this species after the
regulation of the Volga River flow by the Volgograd Dam, built in
1958-1960, which cut off as much as 80% of the spawning grounds.
In Iran baiting hooks with oilcloth or fish was banned in 1952 as
this method took large numbers of immature sturgeon (Vladykov, 1964).
Some problems however may be intractable such as local consumption of
immature fish rather than release or registration in catches. This
lack of registration prevents adequate assessment of the catch and
effective management suffers (Vladykov, 1964). Iran has recently taken
a number of steps to protect the caviar resources including a
reduction in the annual catch from 3000 tons (sic, probably
tonnes) to 1500 tons, restricting export to the government rather than
private companies, combating the illegal caviar trade, and the setting
of export quotas and price controls for Caspian Sea countries (Abzeeyan,
Tehran, 4(5):VI; 4(7):VI, 1993). Gill netting was prohibited in 1995 (Abzeeyan,
Tehran, 6(5, 6):IV-V, 1995). The break-up of the Soviet Union led to
smuggling and overfishing in the newly independent countries around
the Caspian but Iran was able to stabilize world prices by reducing
its caviar exports by 30%. Until 1992 Russian caviar dominated the
world market but more recently Iran became the main supplier with
income for 1989-1994 twice that of 1979 and 1989 (Abzeeyan,
Tehran 5(1 & 2):VII, 1994; Ferguson, 1994). Nevertheless, some
authorities believe overfishing by the five Caspian nations,
particularly in the sea where immature fish are taken along with
adults, will result in the extinction of the sturgeon species there (Los
Angeles Times, Part A, page 1, 28 August 1993). An account of the
caviar black market in Dagestan is given by Chenciner (1998).
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 2% for this species among sturgeons captured.
Lelek (1987) and Maitland (1991) report this species as
"vulnerable" in Europe because it grows and matures slowly,
it is exploited, affected by pollution and killed by river
engineering. Critically endangered in Turkey (Fricke et al., 2007). This species showed the greatest decline among Iranian
sturgeon species through overfishing of younger age groups and habitat
alterations (RaLonde and Walczak, 1970b). Kiabi et al. (1999)
consider this species to be vulnerable in the south Caspian Sea basin
according to IUCN criteria. Criteria include commercial fishing,
medium numbers, habitat destruction, medium range (25-75% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin. IUCN ranks all stocks as endangered (Vecsei, 2001).
Further work
The main concern with all sturgeon species is maintaining a viable
commercial stock. Poaching has caused a decline in the available
number of fish which can be used for breeding and moreover more than
30% of breeders do not respond to hormone stimulation (Kokoza et al.,
1995). There were 6 times more nets in Azerbaijan waters and 4 times
more in the Volga River delta in 1993 than in the 1980s. The legal
catch will probably have to be completely prohibited (Ivanov et al.,
1995). Efron (1993), for example, describes the "caviar
crisis" in the Caspian Sea but problems have long been evident
(Anonymous, 1961a). In 1996, 1 t of caviar was seized from smugglers
in Gilan and one smuggler was fined 20 billion rials (IRNA, 28
July 1997, www.netiran.com). Maintenance of the stock may only be
possible by hatchery production as river regeneration is no longer
feasible because of dams. Mortality in Iran for hatchery reared eggs
of 2 months age was 30-35%, for larvae 20-40%, and for fingerlings
30-40%, a satisfactory level but this could always be improved on (Petr,
1987). Yearly production of sturgeon fingerlings in government
hatcheries in Iran was 1.03 millions in 1983, 1.11 in 1984, 1.13 in
1985, 2.28 in 1986, 3.10 in 1987, 3.16 in 1988, 3.15 in 1989, 4.34 in
1990, 6.60 in 1991, and 3.20 in 1992 (Emadi, 1993a). The 1996 hatchery
production of sturgeon was 12.5 million in 1996 (Bartley and Rana,
1998a). A hatchery facility in Gilan covers 136 ha, produces up to 7
million sturgeon fingerlings a year with plans for up to 20 millions,
and is said to be the largest and most modern sturgeon hatchery in the world.
The Israelis farm osetra and caviar from this species was on sale at
Philadelphia airport at US$75/oz on 19 April 2006.
A detailed comparative study of the morphology of this species and Acipenser
persicus in Iran would enable the young and adults to be clearly
distinguished as well as stocks within each species as a management tool.
Sources
See under the family account.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Acipenser nudiventris
Lovetzky, 1828
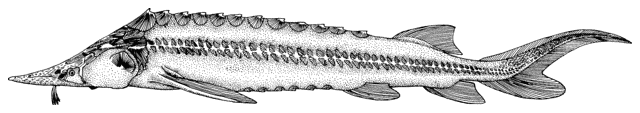
Common names
شيپ (= ship, šep or sheap),
تاس ماهي (= tas mahi, included under this name with A.
gueldenstaedtii and A. persicus when eggs are large for
fisheries statistics), tass mahi shekam brahne, سگ ماهي
(sag mahi), ماهي خاويار (= mahi-ye kaviar,
meaning caviar fish), keshdi, shenavar.
[kalamo, kelemo or kulamo, xazar kalamosu, gaya baligi, girt, ag-gyal or
bich-nyarya in Azerbaijanian; sip or bekre balyk in Turkmenian; spiny sturgeon, thorn sturgeon, fringebarbel sturgeon,
barbel sturgeon,
bastard sturgeon].
Systematics
Acipenser nudiventris was originally described from the Aral Sea.
Acipenser schypa Eichwald, 1831 is a synonym. It is credited
to Linnaeus by Eichwald but not described by Linnaeus; if this name is
available then it is preoccupied by Acipenser schypa
Gueldenstaedt, 1772 (Eschmeyer et al., 1996). Note that Holčík (1989) gives the
spelling as shypa. Acipenser shipa
Lovetsky, 1834 and Acipenser schypa Kessler, 1856 are synonyms.
Acipenser schip Eichwald, 1841 is presumably a misspelling. Acipenser
shyp Forster, 1767 may have priority but this has not been
investigated.
Acipenser nudiventris derjavini Borzenko, 1950 was described
as the Caspian Sea subspecies, as the type locality for the nominate
subspecies is the Aral Sea, but derjavini is no longer recognised (Holčík, 1989).
A hybrid with Acipenser stellatus is reported from the Safid
River (Nedoshivin and Iljin, 1927) and it also hybridises with Huso
huso (Berg, 1948-1949).
V. D. Vladykov points out (in litt., 1973) that ship (in Russian) is probably a Turko-Tartar word referring to a hybrid
since this species has a snout intermediate in length between that of Acipenser
gueldenstaedtii, which is short, and that of Acipenser
stellatus, which is long. The Russian word means prickle or thorn and has given rise to the common names for this
fish in English of "spiny" or "thorn" sturgeon. Acipenser
nudiventris, as its name indicates, has weakly developed or worn
ventral scutes so the names spiny or thorn sturgeon are inappropriate.
Vladykov recommends "sheap" as the common name to avoid
confusion with the word "ship" in Russian (or for that matter in English).
Nucleotide diversity is much lower than other sturgeons in
the Caspian Sea, possibly due to a smaller population size. Haplotypes of
sturgeons from the Ural River in the north Caspian and Iranian waters were
significantly different (Qasemi et al., 2006).
Microsatellite studies indicate that there is more than one population in the
south Caspian Sea and these populations are different from the Ural River one in
the north Caspian Sea (Safari et al., 2007; 2008; 2008).
Key characters
This species has a continuous and thick lower lip, usually more
than 50 scutes laterally, fimbriate barbels, and a transverse mouth.
Morphology
The body is deepest at the first dorsal scute. The rostrum is rounded and
conical in shape in adults, more spatulate in young. Adults are covered with
minute scutes giving a sandpaper texture although visually appearing
smooth. Dorsal fin rays 39-57 and anal fin rays 23-37. Dorsal scutes 11-17,
lateral scutes 49-74 and ventral scutes 10-17. There are no large plates on
the body between the scutes. Scutes lack a hook and even juveniles have this
usual feature barely developed. Ventral scutes are lost or absorbed in large
adults (hence the scientific name). Gill rakers 24-45. Chromosome number is
2n=118 ± 2 (Klinkhardt et al., 1995) or 2n=118 ±
3 (Nowruzfashkhami et al., 2000).
Nourouz Fashkhami et al. (2009) gives details of a method to produce the
most metaphase plates.
Sexual dimorphism
Females are larger than males. Abdurakhmanov (1962) reports a
greater average number of gill rakers in females, a longer postorbital
distance in females, and longer caudal peduncle, pectoral fin, pelvic
fin, snout, eye and snout tip to barbel distance in males.
Colour
The back is olive-green, grey-green or grey-blue, fading to a yellowish-white belly. Fins are
grey.Juveniles mayhave the same colouration as adults or be almost black
dorsally and laterally with a white belly.
Size
Attains 2.21 m and 127 kg. Safid River fish reached 43 kg, weighed
when frozen, with the average being 20.1 kg, in 1914-1915 (Nedoshivin and Iljin, 1927).
Distribution
Found in the Black, Caspian and Aral seas and their drainages but extinct in
the latter. In the Caspian Sea it is most common in the south, being rare in the
Volga River for example. A long residency in fresh water probably
accounts for their scarcity since mortality from winter and predators
is high. Migrations in the Kura River extended 650 km and in the Aras
River 300 km until the Mingechaur Dam was built. Enters the Aras, Astara, Safid, Tajen and Babol rivers in Iran (Derzhavin, 1934; Armantrout, 1980;
CITES website, downloaded 5 April 2004).
Also reported from Hasan Kiadeh by Derzhavin (1934) and by V. D.
Vladykov based on field work notes made in 1962. Rostami (1961) also records
this species from several localities on the Safid River. More recent works
only report it from the Safid River, the southeast Caspian Sea,
southwest Caspian Sea and south-central Caspian Sea (Kiabi et al., 1999;
Abdoli and Naderi, 2009)
and from the Safid River (Abbasi et al., 1999). Vecsei et al.
(2002) consider it as rarely observed in Iran,
Zoogeography
Presumably a relict of the past isolation of the Aral-Caspian-Black
seas from the Mediterranean-Atlantic. This species is reported from
the Karakum Canal and Kopetdag Reservoir in Turkmenistan by Shakirova
and Sukhanova (1994) and Sal'nikov (1995) and may eventually reach the
Tedzhen (= Hari) River basin of Iran.
Habitat
A rare species in trawl catches but known from feeding grounds
along the eastern coast of the south Caspian Sea (Legeza, 1973). Only
100 fish enter the Kura and the Ural stock, an undammed river, is in the low
thousands (Vecsei et al., 2002). This species was never as abundant as
other sturgeons because young spent 2-8 years in fresh water where predators
abound and food is more limited (Vecsei et al., 2002) As an adult,
it favours the areas near river mouths with muddy bottoms. Markarova et al. (1991)
state that its main abundance is south of the mouth of the Kura River
and that it ascends the Safid Rud to spawn, although in smaller
numbers than the Kura River. This species is uncommon in Iranian
waters, only 2.5% in numbers and 4% in weight of the Safid River catch
in 1914-1915 (Nedoshivin and Iljin, 1927; RaLonde, 1970b), and catches
in Azerbaijan are not more than 5% of all sturgeons (Markarova and
Alekperov, 1989). It is usually found over mud near shore at 30-60 m.
Age and growth
Maturity is attained 6-13 years in males and begins at 12-22 years
in females and most are mature at 14 years. Females grow faster than
males. Caspian fish grow faster and larger than those in the Aral Sea.
The oldest fish in the Kura River was 35 years (Markarova et al.,
1991), and maximum age is 36 years. Growth is rapid with one-year-olds
in the Caspian being 23-29 cm long and weighing 40-60 g.
Food
Markarova et al. (1991) found sheap in the south Caspian Sea
to eat fishes such as Atherina, Neogobius (presumably including
related genera), Benthophilus
and Clupeonella, polychaete worms (Nereis), and various
crustaceans. Molluscs play a small part in their diet but eggs of
other sturgeons and the crab Rhithropanopeus harrisii are very
important. The crab, an accidental introduction to the Caspian Sea at
the end of the 1950s, comprises 70% by weight of the food taken. Young
sheap in the Kura River feed on insect larvae such as caddisflies,
dragonflies, mayflies and stoneflies. Hashemyan et al. (2005) found diet
in A. persicus, A. stellatus and A. nudiventris in coastal
waters of Mazandaran and Golestan at depths less than 20 m to consist of
annelids (50.8%), amphipods (41.5%), small fish 4.8%), decapods (2%) and
bivalves (0.9%). Fish shorter than 40 cm fed mostly on shrimps, polychaetes and
gammarids, 41-80 cm fish fed on shrimps, gammarids, polychaetes, bivalves and
smaller fish, while fish greater than 80 cm fed mostly on shrimps and smaller
fish.
Reproduction
A spawning migration to rivers occurs year-round but peaks in
March-April and in October-December in the Kura River of Azerbaijan (Markarova
et al., 1991). The spring run begins at 6.2-13.0°C
while the fall run is at 12.0-17.9°C.
Males predominate over females by 3-6 times. Spawning occurs in
April-May at water temperatures of 10-25°C
and normal development occurs between 11.0 and 17.1°C.
Eggs are laid on pebbly substrates at current speeds of 1-2 m/sec.
Fecundity in sea-caught fish was up to 959,100 eggs (Markarova et
al., 1991). Elsewhere egg numbers may reach 1,290,000 with
diameters up to 3 mm. Fry soon migrate to the sea. Spawning by
individuals is not an annual event but occurs at intervals of 2-3
years for females and 1-2 years for males, allowing for recovery and fattening. Some spent fish may remain
in the Kura River for up to 8 years.
Halajian et al. (2007) used biopsies to determine sex and sexual maturity
stages in 5 and 6 year old fish. Males matured sooner than females. Shalouei and
Imanpour (2009) found that spermatozoa were immotile in ovarian fluid because of
the high concentration of potassium and osmotic pressure.
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Mokhayer and Anwar (1973) report on parasites of sturgeons including
this species (see under Acipenser gueldenstaedtii). Mokhayer
(1976b) reports gas bubble disease in Iranian sturgeons without
specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Sattari et al. (2002) record Cucullanus sphaerocephalus,
Eustrongylides excisus, Skrjabinopsolus semiarmatus,
Leptorhynchoides plagicephalus and Eubothrium acipenserinum, the
fauna being similar to other sturgeons because of their piscivorous feeding.
Sattari and Mokhayer (2005a; 2005b) recorded the occurrence of parasites in this
species from the Iranian southwestern and central coast of the Caspian Sea. The
species found were the nematodes Cucullanus sphaerocephalus and
Eustrongyloides excisus, the cestode Eubothrium acipenserinum, the
acanthocephalan Leptorhynchoides plagicephalus, and the digenean
trematode Skrjabinopsolus semiarmatus. General conclusions were that the
diversity of parasites was less in Iranian waters than in the northern Caspian
Sea, perhaps a reflection of the more varied habitat, its productivity and the
carbonate ions differing between the two regions. The diversity of parasite
seems to have declined over time also, perhaps as a result of unfavourable
environmental conditions, particularly in the freshwater ecosystem which limits
the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Trichodina sp.
Economic importance
The relative scarcity of this species accounts for it being not
more than 1% of the Caspian Sea catch of sturgeons. The highest catch
in the Kura River seems to have been 6000 fish in the 1930s. The Iranian catch
after the CITES website (downloaded 5 April 2004) was:-
| Year |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
| Tonnes |
1.9 |
22.4 |
19.0 |
17.5 |
17.3 |
15.7 |
16.6 |
13.5 |
19.4 |
21.0 |
3.5 (spring only) |
Moghim (2004b) records the total Iranian catch as 2% of the total sturgeon
composition and it is declining. In 1972 the catch per unit effort was 67 tonnes
and 0.5 kg/boat/day but by 2002 it was 15 t and0.09 kg/boat/day.
Conservation
Reduction in flow of the Kura River, the main spawning ground, is
four times less than before regulation (5.5 km3/year
compared to 20-24 km3/year). Sheap find it difficult to
enter the river. Artificial propagation will be the only way to
maintain the population. Between 2.9 and 6.2 million young sturgeon
were released annually in the Caspian Sea from 1966 to 1971. This
situation is mirrored in Iranian rivers such as the Safid Rud. This
species is also particularly sensitive to oil pollution when young.
There are reports that all but the Ural River population are on the
verge of extinction (The Sturgeon Quarterly, 2(2):1, 1994; Vecsei, 2001). It
is already extinct in the Aral Sea (DeSalle and Birstein, 1996). This
species is protected in Iran since populations along the southern
Caspian shore have been greatly reduced and there are not enough mature fish for
fish farming (Bartley and Rana, 1998b; Vecsei et al., 2002).
However the CITES website (downloaded 5 April 2004, but citing September 2000 data) reports
that Iranian hatcheries still obtain some breeders from rivers. CITES also notes
that the number of fishing stations for this species in Iran has been decreased
by half, use of gillnets for Rutilus spp. prohibited as they take
sturgeons too, egg removal by caesarian section instituted, release of fry from
a breeding stock of 3000 fish, and lower export quotas instituted.
Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
15 |
38 |
16 |
50 |
25 |
| Injected broodstock |
14 |
21 |
29 |
32 |
19 |
| Spawning rate * (%) |
86 |
95.2 |
78 |
74 |
75 |
| Fertilisation rate (%) |
80 |
71.5 |
73 |
70 |
72 |
| Survival rate in incubators
(%) |
54 |
61.5 |
51 |
49 |
74 |
| Survival rate in tanks (%) |
70 |
74.7 |
76 |
61 |
66 |
| Stocking density in ponds
(fish/ha) |
92,100 |
77,005 |
56,194 |
87,986 |
61,667 |
| Survival rate in ponds (%) |
71 |
60 |
85 |
34 |
20 |
| Fingerling production (x
1000) |
1143 |
1782 |
1819 |
1414 |
1311 |
* Rate of response to hormone injection
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 1% for this species among sturgeons captured.
This species is sensitive to pesticides such as diazinon. The LC50
(96 h) was 4.6 mg/l and lowered erythrocyte and lymphocyte counts were recorded
with a significant increase in neutrophil counts (Khoshbavar Rostami and Soltani,
2005). Parand Avar et al. (2008) studied the effects of photoperiod
during feeding by juveniles on Daphnia. Uptake was higher in dark
conditions. Shalouei et al. (2009) studied extenders of spermatozoa
motility and Shalouei et al. (2008) the correlation between seminal
plasma indices and spermatozoa motility..
Maitland (1991) lists this species as "endangered" in
Europe because of the declining population, slowness in growth and
maturity, exploitation, and pollution and dams on the spawning
migration. Birstein (1993) and the CITES website (downloaded
5 April 2004) also consider it be endangered. Critically endangered in Turkey
(Fricke et al., 2007). Robins et
al. (1991) list this species as important to North Americans.
Importance is based on its use in aquaculture and as food.
Kiabi et al. (1999) and Moghim (2004b) consider this species to be critically
endangered in the south Caspian Sea basin according to IUCN criteria while the
IUCN gives endangered (Vecsei et al. (2002).
Criteria include commercial fishing, few in numbers, habitat
destruction, limited range (less than 25% of water bodies), absent in
other water bodies in Iran, and present outside the Caspian Sea basin.
See also under A. gueldenstaedtii.
Further work
See under A. gueldenstaedtii.
Sources
See under the family account.
Iranian material: None.
Comparative material: BM(NH) 1879.11.14:56, 1,
255.0 mm total length, U.S.S.R., Tschinas (no other locality data); BM(NH)
1879.11.14:57, 1, 217.0 mm total length, U.S.S.R., Tschinas (no other locality
data); BM(NH) 1897.1.25:9, 1, 411.7 mm total length, Romania, Orsova, lower
Danube (no other locality data).
Acipenser persicus
Borodin, 1897
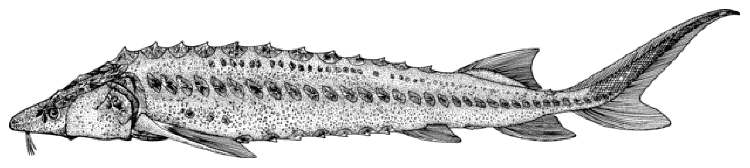
Common name
قره برون (= qara burun, kara burun, kareh burun or ghareburun, meaning black nose),
تاس ماهي
(= tas mahi, this term includes A. gueldenstaedtii), دراكول (= darakul),
تيريج (= tirij),
تاس ماهي ايراني
(= tasmahi-ye Iran), تاس ماهي ايراني
(= tasmahi Irani or tasmahi-e-Iran), سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), cetra.
[nara, nyarya or njara, neresi, Kur narasi for natio kurensis, or bekra in
Azerbaijan; perseya, gunorta perseya, bekre balygy in Turkmenian; kurinskii or persidskii osetr, i.e. Kura or Persian
sturgeon in Russian].
Systematics
The type locality of this species is the Ural and Kura rivers.
Regarded as a not distinct from or a subspecies of Acipenser gueldenstaedtii by
some authors (see Borodin, 1926; Berg, 1948-1949; Whitehead et al.,
1984-1986; Keyvanfar et al., 1987; Keyvanfar, 1988; Ruban et al.,
2008) but Luk'yanenko et al. (1974), Artyukhin and Zarkua (1986), Vlasenko, Pavlov and Vasil'ev in
Holčík (1989), Keyvanfar and Nasrichari (1999), Pourkazemi et al. (2000), Subbotkin and
Subbotkina (2001), Ghorbani and Hajimoradloo (2002), and Gharei et al. (2005) restore it to a full
species on meristic, morphological, ecological, caviar proteins, serum proteins, mtDNA, genomic DNA and
immunological grounds. And again, Birstein et al. (2005) consider it not
to be distinct from Acipenser gueldenstaedtii on the basis of molecular
analyses. Ruban et al. (2008) used meristic, morphometric and
molecular data to come to the same conclusion. Acipenser gueldenstaedtii has a complex intraspecies structure
according to Birstein et al. (2005) and, depending on the rivers and
populations sampled for any given studies, conflicting results can arise. For
the moment, the taxon A. persicus is retained here as distinct until
further resolution of the problem is attained, although given the decimation of
populations this may not be possible.
Moghim et al. (2009) report at least 18 groups that segregate
spatially and temporally for spawning in the Caspian Sea basin.
The type subspecies is found in the Caspian Sea
and Acipenser persicus colchicus Marti, 1940 in the eastern
Black Sea. A natio kurensis Belyaeff, 1932 is reported from the
Kur River of Azerbaijan within Acipenser gueldenstaedtii persicus.
Electrophoretic studies of blood proteins coupled with morphological
data indicate that Gorgan and Safid River populations are two
geographical races (Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 42, 1996).
Hybrids with Acipenser gueldenstaedtii are reported from the
Volga and the Caspian Sea and have been produced artificially (Vasil'eva et al., 2001).
Two syntypes of Acipenser persicus are possibly in the
Zoological Institute, St. Petersburg (ZISP, formerly ZIL) (Eschmeyer et al., 1996).
Key characters
This species has long been confused with A. gueldenstaedtii,
but can be distinguished by a more elongate, massive and downward
curved snout, a white belly and a grey-blue back. A drawing in Vlasenko, Pavlov and Vasil'ev in Holčík
(1989) of the two species has a snout length in head length of 3.2 as
opposed to 4.3 for Acipenser gueldenstaedtii but figures of
snout length in total length overlap for the two species. The
interorbital distance is much less in the Persian sturgeon (29.2-30.5%
of head length) than in A. gueldenstaedtii (Artyukhin and Zarkua, 1986)
but in small specimens examined by me from Iran, some
had gueldenstaedtii interorbital distance and persicus snout length.
Morphology
The Persian sturgeon is slender with an elongate and cylindrical
body, a long head, and a narrow, medium length (5.6% of total length),
massive and usually depressed snout. The snout width near the mouth is
37% of head length. The pectoral fins are relatively small and have
only a weak bony ray. There are usually 1-4 rows of smaller,
longitudinally arranged, bony plaques between the scutes of the dorsal
and lateral rows and sometimes between the lateral and abdominal rows.
The barbels are located relatively closer to the snout tip than those
of A. gueldenstaedtii.
Sheibani and Adib Moradi (2000) described the histology of the pylorus and
pyloric caecum in this species and Sheibani and Pahlavan (2003) the
developmental histology of the liver and pancreas from fry to fingerling.
Dorsal fin rays 27-51, anal fin rays 16-35 according to Holčík (1989)
and 30-49 and 18-32 according to Berg (1948-1949) for Kura
River fish. Gill rakers 15-36. Dorsal scutes 7-19, lateral scutes
23-50 and ventral scutes 7-13 according to Holčík
(1989) while Berg (1948-1949) gives 5-13, 21-42, and 7-14 respectively
for scutes from Kura River fish. Safid River fish have a higher number
of lateral scutes than fish from the Kura River.
The chromosome number is 2n>200 (Nowruz Fashkhami, 1996), later amended to
2n=258 ± 4 (Nowruzfashkhami et al., 2000).
Sexual dimorphism
Females are heavier and longer than males of the same age.
Colour
The back is greyish-blue to dark blue, the flanks with a steel-blue
sheen, the head is lighter than the back, and scutes are lighter in colour
than the background, usually pale yellow in adults but copper-gold in
young. The belly is off-white, sometimes slightly yellowish.
Size
Reaches 2.42 m, possibly 2.50 m, and 76 kg, possibly 80 kg. A specimen caught by
the Bandar-e Torkeman fishery weighed 63 kg, as opposed to the usual
weight of 18-20 kg (Abzeeyan, Tehran, 5(3 & 4):V-VI, 1994). Males
migrating into the Volga River typically weigh 20-30 kg and females 30-35 kg (Vecsei
and Artyukhin, 2001).
Distribution
Found in the Caspian Sea, migrating to the north but mainly in the
south in the Kura River of Azerbaijan and rivers of Iran where it is more common
than A. gueldenstaedtii (Ivanov and Katunin, 2001). Also in the
eastern Black Sea as a distinct subspecies.
In Iran, it is found from the Astara River in the west to the Gorgan
River in the east (Armantrout, 1980), but apparently not the Atrak River on the
border with Turkmenistan (Berg, 1936). Distribution includes the Safid River (to
Kisom and "Musachayu"), Shalman,
Golchan, Langerud, "Djef", "Youssefabad",
"Tchontchenan", Dehkah, "Polrud", Sorkhrud, Feridounkenar,
Talar, Tajan, Neka, "Palarud", Babol, "Mirerud", and "Ferikhabad"
(Kozhin, 1957; Rostami, 1961; Armantrout, 1980). Also
reported from Kargan, Kopurchal, Golshan, Larim, Nirroud, Tazeabad, 12 Bahman, Nevissi, Iz
Deh, and Hasan Kiadeh by V. D. Vladykov based on field
work notes made in 1962. Reported more recently as occurring in the Gorgan,
Babol and Aras rivers by Holčík (1989), in the Gorgan, Gharasu, Tajan, Babol, Haraz, and Safid rivers,
Gorgan Bay, the southeast Caspian Sea,
southwest Caspian Sea and south-central Caspian Sea by Kiabi et al.
(1999) and Abdoli and Naderi (2009) and in the Safid River and Anzali Talab by Abbasi et al. (1999).
It used to ascend the Aras River but numbers in Iranian reaches were always
small. Some literature
records of A. gueldenstaedtii may be this species.
Zoogeography
Presumably a relict of the past isolation of the Black-Caspian seas
from the Mediterranean-Atlantic.
Habitat
This species predominately inhabits the southern part of the
Caspian Sea but does not form dense concentrations. Catches do not
exceed 10-20 fish in 30 minutes of trawling. In winter to spring it is
concentrated in the eastern coastal region and moves north in summer.
In spring, maturing fish are concentrated in the southwest (Legeza,
1973). There is no seasonal variation in depth distribution in the
south Caspian Sea in contrast to the middle Caspian. It is found on
silty bottoms in the south Caspian Sea, sometimes with a sand
admixture, at a temperature range of 4.1-28.0°C
and a salinity range in the sea of 8.59-14.2‰. This species is more
stenohaline than the others, preferring waters with higher salinity as
in typical marine Caspian water and is also more sensitive to lowered
oxygen levels (Legeza, 1972). Kazemi et al. (2003) found that osmoregulatory
ability and development of chloride cells increased during growth, enabling the
fish to transition between fresh and more saline waters. Khodabandeh et al.
(2007) found fry transferred from fresh water to 7.5 and 10‰ sea water
experienced 100% mortality after one hour acclimation; cortisol treatment
increased the ability of fry to withstand these salinities. Ivanov and Katunin (2001) in a trawl survey along
the Iranian coast found 14.2 fish/trawl in the west and 6.7 fish/trawl in the
east with undersized and juvenile fish in the west at 57 fish/trawl. The higher
western catches were attributed to the presence of more rivers, in particular
the Safid River. The general abundance of this species was 8.775 million fish.
This species prefers fast rivers for spawning and migrate long
distances. In the Volga River they migrate at an average speed of 22.6
km/day. They may remain in fresh water after spawning for a year or
more although most return to the sea. These freshwater fish overwinter
in deeper holes and feed intensively on fishes, crustaceans and
molluscs. Larvae move downstream immediately after hatching. Cultured fingerings
can be released safely and optimally into the rivers and estuaries of Iran at an
age of 33-35 days after yolk-sac absorption at a weight of 1.8-2.4 g and 6.2-7.5
cm length (I.F.R.O. Newsletter, 30-31:5, 2002).
Bahmani et al. (2001) have shown that broodfish caught by seines in
the Safid River were less stressed than fish caught by gillnets in estuaries.
Age and growth
Maximum age for accidental catches in the Caspian Sea off
Azerbaijan is 32 years but most (82%) are 14-23 years old. Maturity is
attained between 8 and 13 years in the Kura River (Markarova and
Alekperov, 1989). Most fish entering the Kura River to spawn are 7-34
years old and the main spawning population is 11-24 years. Mean
lengths for Safid River fish are 161 cm for females and 141 cm for
males. Females have a faster growth rate than males. Growth rate is
faster than for A. gueldenstaedtii and in the Volga size and
weight is considerably higher. The numbers of this species and A.
gueldenstaedtii in the southern Caspian are about equal. Maximum
life span is 48 years.
Studies in 2007, however, when 50 stations were sampled in waters less than 10 m
deep, found this species to comprise 82.7% of the absolute frequency and 59% of
the biomass of the total sturgeon catch. A. gueldenstaedtii was last with
5.5% and 2.3% respectively (Iranian
Fisheries Research Organization Newsletter, 51:2, 2007).
Von Bertalanffy growth parameters in Iranian females are L
∞ = 225 cm and K = 0.066 or 207 cm and 0.079 and for males 197 cm and
0.084 or 186 cm and 0.105 depending on the methodology used. Total
mortality (Z) was 0.24-0.57 for females and 0.40-1.1 for males,
natural mortality (M) was 0.04 for females and 0.06 for males, fishing
mortality (F) was 0.47 for females and 0.34 for males, and optimum
fishing mortality was (F) 0.16 for females and 0.34 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
A sample of 31 males and 49 females from the Turkman Sturgeon Fishery Station in
2001 showed sexual maturity at more than 19 years for females and more than 17
years for males (Alavi et al., 2005). Fish taken at 9 fishing stations
along the Iranian coast numbering 4689 individuals had a mean length of 139.1 cm
for males and 153.4 cm for females, weight s19.95 kg and 29.09 kg respectively
and an age of 14.15 years and 16.59 years respectively. The sex ratio was 1:2.2
in favour of females and the majority of females (89.6%) were at level IV
maturity. An increase in sexual maturity of females occurred in autumn while
males were most mature from June to September (Falahatkar, 2006). Samples taken from the whole Caspian shore of Iran from 2002 to 2004 numbering
11,480 fish had a length range of 90-240 cm and growth parameters L∞
= 230 cm and K = 0.058 year-1 (www.shilat.com, downloaded 28
February 2007).
Food
Diet is composed of molluscs, crustaceans including the introduced
crab (Rhithropanopeus harrisii), worms, chironomids and fish
such as gobies (Gobiidae) and small herrings (Clupeonella spp.).
Fish are a large part of the food of young sturgeon at sea. Azari
Takami et al. (1980) found adults to prefer fish, mostly
gobies, followed by crustaceans and two clam species Abra ovata
and Cerastoderma umbonatum in Iran. The zebra mussel is also
eaten as evidenced by a mass of these small clams from the stomach of
a 1.6 m, 35 kg female from Nevissi caught on 29 September 1962.
Reportedly the food diversity of this species is much less than for Huso
huso and Acipenser gueldenstaedtii.
Commercial sized fish feed particularly in the northern Caspian Sea (Ivanov and Katunin, 2001).
Hashemyan et al. (2005) found diet in A. persicus, A. stellatus
and A. nudiventris in coastal waters of Mazandaran and Golestan at depths
less than 20 m to consist of annelids (50.8%), amphipods (41.5%), small fish
4.8%), decapods (2%) and bivalves (0.9%). Fish shorter than 40 cm fed mostly on
shrimps, polychaetes and gammarids, 41-80 cm fish fed on shrimps, gammarids,
polychaetes, bivalves and smaller fish, while fish greater than 80 cm fed mostly
on shrimps and smaller fish. Immature A. persicus, less than two years
old, from fishing stations off Gilan fed on the benthic invertebrates, namely
the polychaetes Hypania sp., Hypaniola sp. and Nereis sp.,
the cumaceans Pterocuma sp. and Stenocuma sp., the clam Abra
ovata, and the crustaceans Paramysis sp. and Gammarus sp.
Adults fed mostly on fish (gobies, smelts and herrings). Haddadi Moghadam et
al. (2009) studied diet in fish collected in summer and winter in the south
Caspian Sea from 2004 to 2006. Food items were fishes (Neogobius sp.,
Atherina caspia, Clupeonella cultriventris (= caspia) and
invertebrates (polychaete worms such as Ampharetidae and Nereis diversicolor;
crustaceans such as Gammarus and Paramysis; and the bivalve
mollusc Abra ovata). The diet varied with season and size group and was
similar to A. stellatus.
The account under A. gueldenstaedtii above gives
some comparative details of diet.
Reproduction
This species was long confused with the chalbash, A.
gueldenstaedtii, and was thought to be a late spring or early
summer spawning population of that species. The spawning run follows that of A.
gueldensatedti. Spawning runs are
dominated by the spring form and winter fish are very rare (Artyukhin
and Zarkua, 1986). Fecundity off Azerbaijan is up to 558,900 eggs (Markarova
and Alekperov, 1989) but may reach 840,000 eggs. In the Safid River it
attains 375,000 eggs. The eggs are brownish-grey and measure up to 3.8 mm in diameter.
The unusually large specimen caught by the Bandar-e Torkeman
fishery gave 22 kg of caviar, almost 35% of the body weight (Abzeeyan,
Tehran, 5(3 & 4):V-VI, 1994).
Spawning takes place at 15-25°C, mainly at 17-23°C,
at higher temperatures than A. gueldenstaedtii (8-18°C).
Spawning sites are gravel, pebble, clay or shell beds, depths are 2-20
m and current speeds 1.0-1.7 m/sec. Catches of what were probably this
species in the estuary of the Safid River for the period
1928/29-1936/37 showed strong peaks in April and May with a minor peak
in September and October (Vladykov, 1964). The Safid is the main
spawning river in Iran (Aslaanparveez, 1993). Spawning takes place in
southern Caspian rivers from April to June and again in August to
September. There is a 2 month interruption in spawning in the Safid
River during summer when water temperatures are 26-30°C.
There is a period of at least 2-4 years before this species can spawn
again. Incubation takes 3-5 days. Shafizadeh and Parivar (1999) state that most
embryos hatch 82-87 hours after fertilisation, most of the yolk is absorbed 6
days after hatching and swimup fry appear from day 7 to 8 at 19-21°C. The timing
of passage of fingerlings into the sea after a hatchery release into the Tajan
River was found to be 12-72 hours after release with a peak migration at 0-3
a.m. Smaller fingerlings stayed longer in the river before leaving (Ramezani,
2003).
Egg size is positively correlated with larval length and weight, yolk sac
volume, hatching time and duration of hatching time, but there was no
correlation with mortality during yolk sac absorption or with mortality during
the first feeding stage (Nazari et al., 2009). Imanpoor et al.
(2009) found the average hydrated egg diameter was 3.64 mm, yolk diameter was
3.26 mm, surface-to-volume ratio was 1.65 and yolk sphere-to-perivitelline space
ratio was 0.75, the latter two being very high. The metabolic rate was low and
spawning can occur in low-temperature waters.
Asadi et al. (2006) have examined serum biochemical parameters that
can be used assessing maturity and managing endangered species.
Parasites and predators
Mokhayer (1976b) reports gas bubble disease in Iranian sturgeons
without specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Most of the data for parasites and diseases summarised under A.
gueldenstaedtii above for Iran may well refer to this species. Soltani et
al. (2000) examined parasites of this species in three locations in Gilan
and found Cucullanus sphaerocephalus and Skrjabinopsolus semiarmatus
had the highest prevalence and intensity. Eustrongylides excisus, Anisakis
sp. and Amphilina foliacea were recorded for the first time from this
sturgeon and diet was strongly correlated with diversity of parasites. Soltani and
Kolbassi (2001) describe the use of different antigens for fingerlings against Aeromonas
hydrophila septicaemia.
Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in
juveniles at a frequency of 5.83%.
Hajimoradloo and Ghorbani Nasrabadi (2003) found the prevalence of metazoan
parasites in juveniles of this fish in the southeast Caspian Sea to be 10
species with Anisakis larvae the highest at 19.7%.
Pazooki and Masoumian (2004) report on blood parasites form fish caught at
Anzali, recording Cryptobia acipenseris and Haemogregarina acipenseris.
These parasites caused no pathological effects in the wild fish but can lead to
severe infections and cause anaemia on fish farms. Gorogi (2006a) recorded the
nematode Cucullanus sphaerocephalus, the the digenean Skrjabinopsolus
semiarmatus and the acanthocephalan Leptorhynchoides plagicephalus
from Iranian waters. Sattari and Mokhayer (2005a; 2005b) recorded the occurrence
of parasites in this species from the Iranian southwestern and central coast of
the Caspian Sea. The species found were the nematodes Cucullanus
sphaerocephalus, Eustrongyloides excisus and Anisakis sp., the
cestode Amphilina foliacea, the acanthocephalan Leptorhynchoides
plagicephalus, the digenean trematode Skrjabinopsolus semiarmatus,
the monogenean trematodes Diclybothrium armatum and Nitzschia
storionis and the crustacean Pseudotracheliastes stellatus. General
conclusions were that the diversity of parasites was less in Iranian waters than
in the northern Caspian Sea, perhaps a reflection of the more varied habitat,
its productivity and the carbonate ions differing between the two regions. The
diversity of parasite seems to have declined over time also, perhaps as a result
of unfavourable environmental conditions, particularly in the freshwater
ecosystem which limits the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Diplostomum
spathaceum, Trichodina sp. and Gyrodactylus sp.
Ebrahimi and Malek (2007) found the helminths Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus and
Eustrongylides excisus.
Haghparast et al. (2007) found Cucullanus sphaerocephalus and
Skrjabinopsolus semiarmatus to have the highest incidence (80 and 55%) in
digestive tracts of broodstocks. Masoumzadeh et al. (2007) examined
broodstocks and found Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Eubothrium acipenserinum, Corynosoma
strumosum, Leptorhynchoides plagicephalus and Amphilina foliacea.
Rajabpour et al. (2008) recorded helminth parasites from fish at three
coastal stations in the southeast Caspian Sea, namely the nematode Cucullanus
sphaerocephalus and the digenean Skrjabinopsolus semiarmatus.
Jalilpour et al. (2009) identified a wide range of fungi on eggs and
larvae of fish from the Shahid Beheshti Sturgeon Rearing Centre.
Bazari Moghaddam et al. (2010) examined larvae and fingerlings in the
Shahid Beheshti Hatchery and observed development of parasitism from the ciliate
Trichodina reticulata and the digenean trematode Diplostomum
spathaceum after release into earthen ponds and the river respectively.
Economic importance
See also under A. gueldenstaedtii where much of the data on
this species is subsumed. The average weight of eggs in this species
in Iran is 4-6 kg per fish and these eggs are ideal for first grade
caviar (Vladykov, 1964). This species has the largest abundance (61.9%), biomass
(50%) and catch-per-unit-effort among all Acipenseridae in Iran in both
2003 and 2004 from sampling 85 stations at 2-100 m depths (followed by A.
stellatus (Iranian Fisheries Research Organization Newsletter, 38:1, 2004)).
Catches of A. persicus declined in the Safid River after
construction of a dam at Manjil which released water for rice farming
and held back sediment, both important triggers for attracting
spawning sturgeon. In 1962, flow was reduced to 7-10 cu m/sec
resulting in water temperatures up to 29°C,
destroying insects and crustaceans on which young sturgeon fed and
making the river narrow and shallow (Vladykov, 1964). Many fish were
attracted into the stronger flow of irrigation canals where they
eventually died. Catches of this and other species also declined
because of the introduction of the more efficient synthetic fibre gill
nets in 1957 (Vladykov, 1964). In Iran this sturgeon is caught both in
the sea and in rivers.
Catches in the Safid River in 1930/31-1934/35 peaked at 13,867 fish
in April with 10,693 fish in May and 3433 fish in March and an annual
total of 32,700 fish (Berg, 1948-1949). Holmes (1845) and Eastwick
(1864) reported on fishing for sturgeon in the Safid River. The
principal method in the first half of the nineteenth century was to
stretch 100 foot (30.5 m) lines across the very shallow, rapid and
murky river with 1 yard (0.9 m) lengths of line attached at intervals
of about 2 feet (0.6 m). These lengths of line were armed with large
hooks which snagged the migrating sturgeon. Sturgeon up to 5 feet (1.5
m) were caught from February to April. At the beginning of February
about 100 fish were taken each day, rising to 600-800 at the end of
the month, 800-2000 in March and to 3500-3800 per day in April. After
May sturgeons had little or no roe. About 125,000 fish were taken
annually and sold for their flesh, caviar and isinglass.
Keyvanfar and Nasrichari (1999) state that from an average 2000 t annual
catch over 10 years (1980-1990) 25% of meat and 24% of caviar were from this
species while 17% of meat and 14% of caviar were from A. gueldensatedtii.
This species produces 51% of Iran's caviar production (I.F.R.O. Newsletter,
30-31:5, 2002). Catches in the Kura River from 1974-1978 varied from 90 to 220 tonnes.
Extensive studies have been carried out on this species, either on hatchery specimens
to improve their survival or using hatchery specimens as experimental organisms. These
studies include rearing using earthworms (Kazerooni Monfared, 1995); ideal stocking densities in tanks (Derakhshandeh Ghazi
Mahale, 1997); stress during transport and confinement of brood stock as
evaluated using blood samples (Bahmani et
al., 2000; Bahmani and Oryan, 2004); on growth performance
using Daphnia magna and Artemia nauplii as food for fry (50% Artemia and 50% Daphnia given at 70% larval body weight
was the best), and on osmoregulation during restocking (Jabbarzadeh Shiadeh et al.,
2000); procedures against infectious diseases using antigens
from Aeromonas hydrophila which causes septicaemia (Kalbassi et al.,
2000); on effective stocking density of eggs and larvae in incubators and
rearing tanks (Mohseni et al., 2000); haematological variables in juveniles and adults at different water
temperatures (Pourgholam and Saeidi, 2000); on optimum feeding rate for
fingerlings (Yousefpour Pirbazari et al., 2000); on blood parameters for
fingerlings in a Gilan fish farm (Shahsavani et al., 2001); a
histological study of the intestines (Sheibani and Pousti, 2001); sperm has been cryo-preserved to conserve the gene pool (Vecsei
and Artyukhin, 2001); on clove oil having no significant difference with MS222,
an anaesthetic used in fish farms (Abtahi
et al., 2002; 2003); food and feeding of fingerlings after release and
their travel time to the estuary (Kamali and Imanpoor, 2002); the relation
between biochemical composition of eggs and their fertilisation rate (Mohammad
Nazari et al., 2002); changes in the levels of sex steroids as oocytes
developed (Nazari et al., 2002); nutrition in fish ponds where cladocerans and chironomids were staples and copepods and their nauplii were
secondary items (Aslan Parviz and Aghaei Moghadam, 2003; Aghaei Moghadam and Aslan Parviz,
2006); the enhancement effect of ozone and physical treatment on the hatching
rate of eggs (Ghomi et al., 2003); purification and partial
characterisation of serum immunoglobulins (Kalbassi et al., 2003);
physiological studies on the liver oxidase system (Karimzadeh et al.,
2003); toxicity of the insecticide diazinon to fingerlings (Pazhand et al.,
2003); dietary levels of fat and protein effecting growth and chemical
composition of fingerlings (Ebrahimi et al., 2004; Mohseni et al.,
2007); on sperm motility (Hadi
Alavi et al., 2004); the identification of fatty acids in
the flesh and the effects of long-term freezing on them (Hedayatifard and Moini,
2004); effect of temperature on fertilisation percentage achieved by broodstock (16.1-18.0ºC
was optimal)(Hosseini Najd Gerami and Hajimoradlu, 2004); the effect of the timing of first feeding with live food on growth and
survival of larvae (Kordjazi et al., 2004); determination
of the 96h LC50 of Saturn, a herbicide, and Malathion, an
insecticide, at 0.007 and 10 mg/l respectively (Nezami et al., 2004);
histology of the gut from hatching to 56 days (Pahlavan Yali et al.,
2004); levels of zinc and copper in muscle tissue and caviar (Sadeghird et al.,
2004); reproductive conditions of broodstock and when they should no longer be
used (Hosseini Najdegrami et al., 2005); the optimal weight and length
for release of fingerlings into rivers and estuaries (1.8-2.4 g, 6.2-7.5 cm,
33-35 days after yolk sac absorption)(Kazemi et al., 2005); the timing of initial feeding
in relation to behaviour (negative phototaxis and assumption of a benthic life
at 5-6 days post-hatching) and expulsion of the melanin plug (larvae can feed
with it present so expulsion cannot be used to determine active
feeding)(Kordjazi et al., 2005); sperm density and fertilisation
rate (Nazari et al., 2005); the toxic effects
on fingerlings of various pollutants such as the oil products phenol and
1-naftol, the herbicide butachlor, and polyaromatic hydrocarbons from oil wells
in the Caspian Sea (Nezami et al., 2005; Padjand et al., 2005;
Soltani et al., 2006); a macroscopic and microscopic study of the spleen
and and associated lymphatic tissue (Sheibani, 2005); evaluation
of hydrogen peroxide against malachite green (possibly toxic and teratogenic)
for fungal disinfection of eggs showed the former to be superior (Vahabzadeh
et al., 2005); antifungal studies on eggs comparing the
utility of formalin, malachite green and potassium permanganate in fish farms,
the latter being safest for controlling Saprolegnia (Abtahi et al., 2005); sperm studies evaluating ionic composition and osmolality
of seminal plasma, sperm density and motility in regard to sperm
cryopreservation (Alavi et al., 2006); inulin-like growth factor-I
inducing oocyte maturation (Bahrami Kanagar et al., 2006); the micro-cesarean method of extracting
eggs from brood stock was better than conventional methods (Feyzbakhsh et al.,
2006);
the use of rotifers (Brachionus plicatilis) in conjunction with
Artemia nauplii as food for larvae (Haddai Moghadam, 2006); use of oxolinic
acid bioencapsulated in Artemia urmiana as a means to increase resistance
to Aeromonas hydrophila infection in larvae (Hajimoradlou and Agh, 2006); studies on blood serum osmotic and ionic regulation in wild adults and
reared juveniles, important in understanding the best use of water with
different salinities in commercial rearing of this species (Kazemi et al.,
2006); induction of ovulation using glycerin as a solvent
for hypophysis powder proved better than physiologic serum (Noroozi et al.,
2006); feeding
formulated diets to larvae and juveniles in hatchery rearing (Pourali Fashtomi
and Mohseni, 2006); establishing blood serum parameters as tools in disease prognosis
and control (Shahsavani et al., 2006a, 2006b); the maximum allowable concentration of Safid
River sediments as determined in aquaria was 1536.74 mg/l (Yosefi Garakoei et al., 2006);
Abedian Kennari
et al. (2007) on use of Daphnia magna enriched with cod liver oil as
a source of highly unsaturated fatty acid on growth, survival, stress resistance
and fatty acid composition of larvae; the effect of stripping frequency on ionic
content and osmolality in seminal plasma composition (Alavi et al.,
2007); details of sperm morphology in comparison to that of fil mahi (Baradaran
Noveyri et al., 2007); comparison of the efficiency of the Yushchenko and
Azarakhash incubators, the latter being better in terms of fertilisation
percentage, mortality rate, active feeding and survival (Farabi et al.,
2007); ability of Artemia urmiana to act as a carrier of oxolinic acid, a
drug used to combat infection in fish larvae (Ghorbani et al., 2007); use
of probiotic bacillus bioencapsulated with Artemia urmiana nauplii to
increase growth of larvae (Jafarian et al., 2007); fatty
acid composition in fresh and frozen tissues, concluding cold storage should not
exceed 12 months (Moeini and Hedayatifard, 2007);
variations in meat quality using dry and mix salting (salt and 1% madder)
(Seyfzadeh et al., 2007); propagation efficiency of broodstock from two
farms in Mazandaran and Golestan were shown to be different (Yousefian and
Farabi, 2007);
Askarian et al. (2008) examined the gastrointestinal tract for lactic
acid bacteria and found the population levels to be significantly lower than in
Huso huso;on amino acids in food pellets increasing consumption (Jafari Shamushaki et al.,
2008); fertilising ability of cryopreserved spermatozoa (Alipour et al.,
2009); on serum biochemical parameters (Asadi et al., 2009); on the median lethal concentration of suspended sediment from the Safid
River, this pecies showing higher tolerance than A. stellatus (Garakouei
et al., 2009);
isolation of Lactobacillus species, which ferment carbohydrates, from the
intestine (Ghanbari et al., 2009); changes in fatty acid composition after freezing and long-term cold
storage (Hedayatifard and Keyvan, 2009); use of Artemia urmiana enriched
with the essential fatty acid docosahexaenoic acid and its effects on growth,
survival and composition of larvae (Hafezieh et al., 2009); the important
influence of temperature on hatching time, start of exogenous feeding, growth
performance and survival of larvae (Jalali et al., 2009);
immunolocalisation of gill chloride cells used in ionic and osmotic regulation
(Khoushnoud et al., 2009); varied effects of egg size on length, weight
growth and survival of prelarval and early feeding stage (Nazari et al.,
2009); recommended use of methyl paraben as a safe preservative in caviar
infected with the bacterium Clostridium botulinum (Salmani et al.,
2009); regulation of water temperature during the embryonic period, temperatures
of 15-18ºC being the upper limit of thermal
optima (Soleymani and Karimabadi 2009); fish effects of
cooking methods on the physico-chemical and nutritional and digestibility
properties of fillets (Alipour et al., 2010); identification of 13 fungal
species in cultivated and natural populations (Firouzbakhsh et al., 2010); positive
effects of Artemia urmiana enriched with highly unsaturated fatty acids
on growth, survival and fatty acids composition of larvae (Hafezieh et al.,
2010); the effects of sex steroids on hormonal control of reproduction (Hajirezaee
et al., 2010); anaesthetic effects of clove essence (400 p.p.m. and 24ºC
was best treatment and for recovery) (Imanpoor et al., 2010); the impact of plasma sex steroids on gonad development
(Nazari, 2010); inhibitory effects on lipid oxidation (or rancidity) of ascorbic
and citric acids compared with vacuum packaging in frozen fillets; Ghanbari and
Jami (2011b) on Lactobacillus species from the guts; etc.
Conservation
See also under A. gueldenstaedtii. Catches in the sea off
Iran are made with large seines and gill nets and many juveniles and
fish below legal size are taken. Netting of sturgeon along the coast of Iran has
been banned and hatchery production in Iran is directed to this species to
maintain stocks. Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 54% for this species among sturgeons captured. Moghiem
(2003) found that catch-per-unit-effort fluctuated from 2.249 to 2.971 kg over
the previous decade, mean length, weight and age declined, the age structure
changed with younger fish increasing in numbers, and catches showed an increase.
Alavi et al. (2005) found overfishing of females in their sample from the
Turkman Sturgeon Fishery Station.
Abdolhay et al. (2006) report on 1062 adults caught in 1998 of which 581
fish were injected with hypophysis extract and produced 22.5 million fingerlings
while in 2002, 802 were caught and 538 produced 12.3 million fingerlings.
Hormonal studies are used to select fertile broodstock to
ensure effective aquaculture (Mojabi et al., 1999; Safi et al.,
1999) and other studies relevant to hatchery success, and thus conservation, are
listed above. Nezami
et al. (2000) maintain that sea-ranching has restored this species in Iran.
Moghim et al. (2001) have used ultrasonography to determine sex and
maturity of this species as there are no obvious external sex characteristics.
Sex and maturity determination were accurate at 100% and 98.6% respectively,
confirmed by necropsy, and thus would prevent the loss of male and immature
female fish if the technique were used in the caviar fisheries.
This species is now found in the northern Caspian Sea, the fish being from
Iranian stocking programmes (Kottelat and Freyhof, 2007).
Amini (2005) and Abdolhay and Tahori (2006) summarise hatchery production for this species:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured
|
661 |
591 |
620 |
2056 |
742 |
| Injected broodstock |
437 |
492 |
528 |
1288 |
436 |
| Spawning rate* (%) |
81 |
86.5 |
410 (sic) |
80 |
85 |
| Fertilisation rate (%) |
72 |
76.1 |
83 |
71 |
75 |
| Survival rate in incubators
(%) |
56 |
52.6 |
75 |
50 |
64 |
| Survival rate in tanks (%) |
76 |
76.4 |
53 |
67 |
74 |
| Stocking density in ponds
(fish/ha) |
84,076 |
89,131 |
76,000 |
97,941 |
95,661 |
| Survival rate in ponds (%) |
56 |
47.4 |
56 |
52 |
56 |
| Fingerling production |
13,711,199 |
16,278,595 |
12,301,214 |
18,388,962^ |
17,412,529 |
* Rate of response to hormone injection; ^ 18,288 in Abdolhay and Tahori
(2006)
Studies on heavy metal contamination (Zn, Cu, Cd,, Pb and Hg) of both flesh
and caviar showed levels were below the maxima allowed for consumption, based on
international standards (Sadeghi Rad et al., 2005; Amini Ranjbar et al.
(2003), Amini Ranjbar and Shariat, 2006; Sadeghi Rad et al., 2009).
Lelek (1987) lists this species as endangered.
Extinct in Turkey (Fricke et al., 2007). Kiabi et al.
(1999) consider this species to be vulnerable in the south Caspian Sea
basin according to IUCN criteria. Vecsei and Artyukhin (2001) list it as
endangered with the IUCN. Criteria include commercial fishing,
abundant in numbers, habitat destruction, widespread range (75% of
water bodies), absent in other water bodies in Iran, and present
outside the Caspian Sea basin. Mostafavi (2007) lists it as vulnerable in the
Talar River, Mazandaran. Kottelat and Freyhof (2007) state that there is
likely no natural reproduction in Iranian waters, fish being from artificial
stocking programmes.
Further work
Fresh samples of sturgeon from Iranian rivers should be examined
systematically and with care to determine if they are indeed this
species and not A. gueldenstaedtii. A detailed comparative
study of the morphology of this species and Acipenser
gueldenstaedtii in Iran would enable the young and adults to be
clearly distinguished as well as stocks within each species as a management tool.
Sources
Holcik (1993) and Shariati (1994) give accounts of this species in Farsi. See also under family above.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Acipenser ruthenus
Linnaeus, 1758
Found in the Caspian Sea basin but no records from Iran proper.
Single specimens have been recorded as entering the Kura River of
Azerbaijan and fishermen reported one fish from off Soviet Astara in
1929 (Berg, 1948-1949) on the border with Iran. The import of 30,000
fingerlings and 20 male parent stock of this species to Iran for
artificial reproduction was envisaged in an agreement with the Russian
Research Centre of Commercial Sturgeon Reproduction in 1995 (Iranian
Fisheries Research and Training Organization Newsletter, 9:3,
1995). Tatina et al. (2010) studied effects of dietary vitamins C and E
on haematological and biochemical parameters in this fish in the breeding centre
in Rasht. Acipenser primigenius Chalikov, 1944 is a hybrid of this
species and Acipenser gueldenstaedtii (Eschmeyer et al., 1996).
The Farsi name is استرلياد (esterliad).
Listed as Endangered in the Volga River (Peterson et al., 2009).
Acipenser stellatus
Pallas, 1771

Common names
ازون برون or اوزون
بورون (uzun burun or ozoonboroon = long nose),
دراكول (= derakul or darakul); tirij
(after Wossugh-Zamani (1991a), meaning shaped like an arrow; see also A. persicus);
سوروگا (= sevruga or sevroga), سگ ماهي (sag mahi),
ماهي خاويار (= mahi-ye kaviar, meaning caviar fish), puze draz.
[uzunburun, Kur uzunburun for natio cyrensis, ag-balyk, all in
Azerbaijanian; tirana in Turkmenian; sevryuga, sevruga or stellate sturgeon (this term also
includes A. nudiventris with small eggs for fisheries
statistics), yuzhnokaspiiskaya sevryuga or South Caspian stellate
sturgeon, both in Russian; star or starred sturgeon].
Systematics
Originally described from the Volga River near Simbirsk.
Acipenser seuruga Güldenstädt, 1772 from the Caspian Sea, Acipenser
hellops Pallas, 1814 from the Black and Caspian seas, Acipenser
Helops Pallas, 1814 from the Araks River, and Acipenser
Ratzeburgii Brandt in Brandt and Ratzeburg, 1833 from the Caspian Sea at the
mouth of the Emba River, are synonyms.
Acipenser stellatus stellatus natio cyrensis Berg, 1932
is described from the southern Caspian Sea and tributary rivers but
has no taxonomic status as an infrasubspecific rank. Morphologically,
this Kura River form is similar to north Caspian members of the
species, differing principally in postorbital distance. Growth and
fecundity are lower in the Kura form and spawning time is different.
M. Poorhazemi (Pourkazemi) finds that A. stellatus is highly polymorphic
with more than one population using molecular techniques (Iranian
Fisheries Research and Training Organization Newsletter, 14:4-5, 1996).
Norouzi et al. (2009) used microsatellite markers to determine that there
is more than one population in the south Caspian Sea which has importance in
terms of stock management, restocking and conservation. Shabani et al. (2003; 2006) found no significant differences between
Volga River and Gorgan, Tajan and Safid River fish of Iran when examining mtDNA.
Norouzi et al. (2008) and Norouzi and Pourkazemi (2009) examined the
population and genetic structure of this species in Iranian waters using
microsatellite markers and found evidence for at least three populations,
particularly a separate one in the Safid River, and probably more than one in
each river such as the Safid and Gorgan rivers.
A hybrid with Acipenser nudiventris is reported from the
Safid River (Nedoshivin and Iljin, 1927). Artificial hybrids with Huso
huso have been produced in Mazandaran for aquaculture projects (Annual
Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran, p. 6, 1996).
Key characters
This sturgeon has a long snout (59-65% of head length) with a
pointed tip in contrast to the short snout and rounded tip in A.
gueldenstaedtii and A. persicus. The continuous lower lip
in A. nudiventris and the large crescentic mouth in Huso
huso distinguish these species.
Morphology
The lower lip is interrupted at its centre, barbels are not
fringed, are short, and do not reach the mouth but are closer to the
mouth than the snout tip.
Dorsal fin rays 38-54 and anal fin rays 20-40; or 40-54 and 22-35
respectively in the Kura for natio cyrensis (Berg, 1948-1949).
Dorsal scutes 9-16, lateral scutes 26-43 and ventral scutes 9-14.
There are smaller scutes between the main rows. Gill rakers 24-29,
usually 25-26 in natio cyrensis. Chromosome number 2n=115 ± 1
(Annual Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 43, 1996), 2n=118 ± 2 or 113 ± 1 (Iranian
Fisheries Research and Training Organization Newsletter, 8:5,
1995) or 2n=118 ± 1 (Nowruz Fashkhami, 1996), 2n=114 (Nowruz
Fashkhami and Khosroshahi, 1999); 2n=146±6 (Chicca et al., 2002).
Sheibani (2003a) described the anterior digestive canal of this species.
Keyvanfar (1986) found a transferrin polymorphism in the serum
proteins of this species but not the other Iranian species of sturgeon and
Keyvanfar (1988) found several variants corresponding to
transferrin in the other species.
Sexual dimorphism
Females are larger than males of the same age; in the Ural River
1.3-1.6 times larger. Head depth and preanal distance differ between
sexes in Kura River fish but only when gonads are ripening.
Abdurakhmanov (1962) reports a longer anal fin, snout, and snout tip
to barbel distance in males, and a longer predorsal length, preanal
length, postorbital length and a greater caudal peduncle depth in females.
Colour
The back is dark grey, ash grey or cinnamon brown, almost black in
some fish, and fades to a white belly. Flanks are yellowish-white. In
small fish, the scutes are lighter than the adjacent body and so are
distinctive. Sea fish are darker than river fish. An eyeless specimen,
1.11 m long, caught in Mazandaran was dark black (Abzeeyan,
Tehran 4(7):V). The eyes were completely absent and their position on
the head was covered with smooth bone.
Size
Attains about 2.21 m and more than 80 kg. Sternin and Doré (1993)
cite a specimen of 2.9 m. Iranian captures averaged 1.3-1.4 m and 9-10
kg in the 1950s (Farid-Pak, no date). One of the largest specimens
ever caught was 2.18 m long and was taken off the Astara River on the
border of Azerbaijan and Iran in 1932. Much larger fish are known from
archaeological sites of the 10th-13th centuries on the Terek River, up
to 2.7 m (Tsepkin and Sokolov, 1971).
Distribution
Found in the Adriatic, Aegean, Black and Caspian seas and their
drainages but the largest populations are in the Caspian. Generally
found from the Astara River in the west to the Gorgan River in the
east in Iran (Berg, 1948-1949; Kozhin, 1957; Armantrout, 1980) but not the Atrak River on the eastern
Caspian border of Iran with Turkmenistan (Berg, 1936). Found in the
Safid River at Kisom and the Mirerud (Derzhavin, 1934; Kozhin, 1957). It used
to ascend the Aras River but numbers in Iranian reaches were always small (Berg,
1948-1949). The Kura River catch was up to 90% of the sturgeons taken. Rostami
(1961) records this species from several localities on the Safid River and from
the Golchan, "Djef", Youssefabad, Tchontchenan, Dehkah, Sorkh, Talar,
Tajan, and Neka rivers. Also
reported from Kargan and Hasan Kiadeh by V. D. Vladykov based on field
work notes made in 1962. Reported more recently from the Gorgan,
Gharasu, Tajan, Babol, Haraz, and Safid rivers, Gorgan Bay, the
southeast Caspian Sea, southwest Caspian Sea and south-central Caspian
Sea by Kiabi et al. (1999) and Abdoli and Naderi (2009) and from the Safid River and Anzali Talab by Abbasi et al. (1999).
Zoogeography
Presumably a relict of the past isolation of waters now
encompassing the Black-Caspian seas.
Habitat
This sturgeon is found in large concentrations in the eastern
coastal region of the south Caspian Sea in August-September with up to
25-30 fish taken in a single trawl, having moved south from northern
waters. Ivanov and Katunin (2001) note the densest concentration in the
per-estuary zone of the Gorgan River, with catches reaching 26 fish/trawl
while along the central part of the Iranian coast catches did not exceed 4
fish/trawl. At the end of winter and particularly in early spring, uzun
burun move onto the Iranian shore. Migrations between the Kura River
lower reaches, the Safid River and elsewhere are reported. They
usually does not descend below 100-130 m except along the southern
shore of the Caspian Sea (Legeza, 1973) where they may descend to 300
m. Uzun burun are common only down to 50 m. There is no seasonal
variation in depth distribution in the south Caspian Sea in contrast
to the middle Caspian. They are often found in surface waters during
the day, and retire to the bottom during the night. Uzun burun are
found on silt and sand-silt bottoms but will also feed on sand and
shell grounds. Temperature range is 4-24°C, in winter 7.5-10.5°C
and 11.0-24.0°C in summer and fall, with an absolute range of 2.4-29.5°C.
Water temperatures below 6°C are unsuitable for feeding however. Salinity range in the sea is
0.1-14.6‰ and this is the most euryhaline sturgeon in the Caspian Sea. This species is the best swimmer among sturgeons in the
Caspian Sea in terms of power to body weight and in the Volga River
migration speed averages 110 km/day (although progress is only 17.6
km/day because of the current).
The effects of diazinon on haematological parameters was examined by
Khoshbavar Rostami et al. (2005) who also found the LC50 was 4.98 mg/l over 96 hours.
Age and growth
Maximum age for accidental catches in the Caspian Sea off
Azerbaijan is 21 years but most are 8-13 years old. Males mature at
11-13 years, the youngest at 7 years, and females at 14-17 years, the
youngest at 8 years in the Kura River. Populations in the Kura River
and Iranian rivers take the longest time to mature, have a slower
growth rate and lower fecundity. Vecsei et al. (2007) give a maturity
range of 5-17 years. Like other sturgeons, this species
does not reproduce every year and in the Caspian and there is a 3-4
year gap between reproductive periods in any individual. Females live
longer than males. Maximum life span is about 41 years. Levin (1997)
summarises the Volga spawning population as being age 6-28 years
(11-16 years on average) with females 150-152 cm and 11-12 kg and
males 128-130 cm and 6-7 kg. Spawning temperature is 16-22°C. The stock on the
Iranian coast was estimated at 3.2 million fish weighing 18,500 tonnes with 6.7%
of fish mature (Ivanov and Katunin, 2001).
Studies in 2007 along the whole Iranian coast when 50 stations were sampled
in waters less than 10 m deep, found this species to comprise 11.8% of the
absolute frequency and 38.7% of the biomass of the total sturgeon catch, second
after A. persicus (Iranian
Fisheries Research Organization Newsletter, 51:2, 2007).
Von Bertalanffy growth parameters in Iranian females are L
∞ = 213 cm and K = 0.062 or 188 cm and 0.104 and for males 190 cm and
0.083 or 171 cm and 0.113 depending on the methodology used. Total
mortality (Z) was 0.52-1.1 for females and 0.62-1.1 for males, natural
mortality (M) was 0.07 for females and 0.08 for males, fishing
mortality (F) was 1.03 for females and 0.54 for males, and optimum
fishing mortality was (F) 0.42 for females and 0.30 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
Samples taken from the whole Caspian shore of Iran from 2002 to 2004 had growth
parameters ∞ = 219 cm and K = 0.06 year-1
(www.shilat.com, downloaded 28 February 2007).
Yelghi et al. (2007) found maximum age frequencies for fish from the
southeastern Caspian Sea were were 9-13 years for male and 12-13 years for
females. Brood fishes more than 15 years old formed little of the total catch.
The oldest and largest individuals were 17 years and 156 cm for males and 27 years
and 178 cm for females.
Growth was negative allometric.
Food
Young specimens feed on crustaceans, older fish on chironomid
larvae and the oldest specimens on fish (Rostami, 1961b). Azari Takami
et al. (1980) found adults to consume gobies (Gobiidae) and
kilka (Clupeonella) with the clams Abra ovata and Cerastoderma
umbonatum as secondary items in Iran. In the Caspian Sea off
Azerbaijan, Zarbalieva (1987) found that the polychaete worm Nereis
diversicolor (82.7% by weight) dominated in the diet of sturgeons
20-80 cm long, being replaced by the mollusc Abra ovata (88.6%)
at 90-120 cm and by Clupeonella spp. (65.1%) and Abra ovata
(31.5%) at 125-140. Sturgeons 50-80 cm long also took the crab Rhithropanopeus
harrisii (21.2%). Other foods include Rutilus rutilus (and presumably
R. caspicus) Cobitis
taenia, mysids, cumaceans, and amphipods. Gobies are generally of
lesser importance than clupeids. Hashemyan et al. (2005) found diet in
A. persicus, A. stellatus and A. nudiventris in coastal waters
of Mazandaran and Golestan at depths less than 20 m to consist of annelids
(50.8%), amphipods (41.5%), small fish 4.8%), decapods (2%) and bivalves (0.9%).
Fish shorter than 40 cm fed mostly on shrimps, polychaetes and gammarids, 41-80
cm fish fed on shrimps, gammarids, polychaetes, bivalves and smaller fish, while
fish greater than 80 cm fed mostly on shrimps and smaller fish. Haddadi Moghadam
et al. (2009) studied diet in fish collected in summer and winter in the
south Caspian Sea from 2004 to 2006. Food items were fishes (Neogobius
sp., Atherina caspia, Clupeonella cultriventris (= caspia) and
invertebrates (polychaete worms such as Ampharetidae and Nereis diversicolor;
crustaceans such as Gammarus and Paramysis; and the bivalve
mollusc Abra ovata). The diet varied with season and size group and was
similar to A. persicus.
In rivers, juveniles feed on gammarids, chironomid larvae, mysids
and worms. Spawning fish eat little or no food and, having used up
much of their fat reserves, return to their feeding grounds in the sea
immediately after spawning. This downstream migration varies from 70 to 80 km/day.
Reproduction
The peak migration in Iran is in April. There is also a peak run in
fall (September-October) in the Kura River, and probably in Iran too
(see below), but it is much less important than the spring run (Berg,
1959). Migrations in the Kura and Safid rivers can be found year round
outside these peaks. The spring run in the Kura begins at about 10°C
and peaks at 18°C, the runs decline in warmer summer temperatures and the fall run begins
as water cools. Water level is also an important factor influencing
runs and spawning. Water level fluctuations exceeding 0.2-0.5 m causes
spawning to stop as fish migrate to deeper water. Summer and fall run
fish do not spawn until the following year. Males arrive on the
spawning ground before females and stay up to 6 weeks; females stay
only 10-12 days. The Volga run begins in March-April with a peak in
May but continues to October-November (Levin, 1997).
Up to 950,000 adhesive eggs are laid although in rivers of the
southern Caspian absolute fecundity is lower, 35,400-362,900 eggs in
the Kura River for example. Fertility is higher in the Volga compared
to the Safid River (Iranian Fisheries Research and Training
Organization Newsletter, 17:6, 1997). The spawning period in the
Kura River is April-September at 15-29°C.
Fish may leap out of the water during spawning and scrape their bodies
on the bottom, leaving scratches and bruises. Eggs are deposited over
gravel, pebbles, or stones mixed with shell fragments and coarse sand
in the river bed or on flooded banks at a current velocity of 0.7-1.8
m/sec. A gravel bottom and a current speed of 1.2-1.5 m/sec are ideal.
Eggs are round to ovate, brownish-grey and up to 3.2 mm in diameter.
The adult loses 25-30% of its weight after spawning and females are
only ready to spawn again after 5-6 years and males after 3-4 years. Spawning
occurs at 15-26°C. Incubation takes 44-80 hours at 20-28°C.
Young fish descend to the sea at 3-4 months of age but in some
populations this occurs immediately after hatching, taking only 12-15 days.
Moghim et al. (2000) have used ultrasonography to determine sex and
maturity stage of this sturgeon. Sex determination had a 97.2% accuracy and took
30 seconds or less per fish. This non-invasive technique reduces stress and
enables immature females caught at sea to be released.
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) record the acanthocephalan Leptorhynchoides
plagicephalus and describe a new species, Corynosoma caspicum,
from this sturgeon in Iran. The coelenterate Polypodium hydriforme
is recorded from the eggs of this sturgeon in the Safid Rud. Mokhayer
and Anwar (1973) report on sturgeon parasites in general (see under Acipenser
gueldenstaedtii). Mokhayer (1976b) also reports gas bubble disease
in Iranian sturgeons without specifying the species of sturgeon as
well as the monogenetic trematodes Diclobothrium armatum and Nitzschia
sturionis. Larvae of the nematode Anisakis is reported from
this species in Iran (Eslami and Mokhayer, 1977). Mokhayer (1989)
reports metacercariae of the eye fluke, Diplostomum spathaceum
from this species in Iran, which can cause complete blindness and
death in commercially important species.
Sattari et al. (2001) found the following parasites in fish from the
southwest Caspian Sea: Skrjabinopsolus semiarmatus, Leptorhynchoides
plagicephalus, Cucullanus sphaerocephalus, Eubothrium
acipenserinum, Bothriomonus fallax, Eustrongylides excisus,
Aniskais sp., Amphilina foliacea and Corynosoma strumosum. Hajimoradloo (2002) records the nematode Cystoopsis acipenseris in adult fish.
Pazooki and Masoumian (2004) report on blood parasites form fish caught at
Anzali, recording Cryptobia acipenseris and Haemogregarina acipenseris.
These parasites caused no pathological effects in the wild fish but can lead to
severe infections and cause anaemia on fish farms. Sattari and Mokhayer (2005a;
2005b) recorded the occurrence of parasites in this species from the Iranian
southwestern and central coast of the Caspian Sea. The species found were the
nematodes Cucullanus sphaerocephalus, Eustrongyloides excisus and
Anisakis sp., the cestodes Eubothrium acipenserinum, Amphilina
foliacea and Bothrimonus fallax, the acanthocephalans
Leptorhynchoides plagicephalus and Corynosoma strumosum, the digenean
trematode Skrjabinopsolus semiarmatus. General conclusions were that the
diversity of parasites was less in Iranian waters than in the northern Caspian
Sea, perhaps a reflection of the more varied habitat, its productivity and the
carbonate ions differing between the two regions. The diversity of parasite
seems to have declined over time also, perhaps as a result of unfavourable
environmental conditions, particularly in the freshwater ecosystem which limits
the waters available for spawning and parasite acquisition.
Shenavar Masouleh
et al. (2006) found hatchery fingerlings to harbour Diplostomum
spathaceum, Trichodina sp. and Gyrodactylus sp.
Ebrahimi and Malek (2007) found the helminths Cucullanus sphaerocephalus,
Skrjabinopsolus semiarmatus, Leptorhynchoides plagicephalus and
Eustrongylides excisus.
Rajabpour et al. (2008) recorded helminth parasites from fish at three
coastal stations in the southeast Caspian Sea, namely the nematode Cucullanus
sphaerocephalus, the digenean Skrjabinopsolus semiarmatus, the
acanthocephalan Leptorhynchoides plagicephalus and the cestode
Amphilina foliacea.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
Predators are most evident on the young and include Silurus
glanis and various gobies (Gobiidae) while eggs are taken by Blicca
bjoerkna, Pelecus cultratus, Gobio sp., and gobies.
Economic importance
Uzun burun are known from a Neolithic site on the eastern Caspian
shore in the former Soviet Union from about 6000 years ago (Tsepkin, 1986).
This sturgeon provided the majority of the caviar produced in Iran
according to reports from the 1960s and beginning of the 1970s (Vladykov,
1964; RaLonde, 1970b), 70% of the total catch according to commercial
suppliers in 1995. It is reputed to have the tastiest flesh and also
the best caviar (Ricker, 1970) but others maintain beluga caviar is
the best. Farid-Pak (no date) gives an average yield of 1.5-2.0 kg for
each female in the 1950s in Iran. Catch records for the Safid River in
1930-1935 showed that 31.7% of fish were caught in May, 18.1% in April
and 9.6% in June, with a small peak in October of 7.9%. Nevraev (1929)
records catches of this species varying from 22,278 to 43,593
individuals in the Astara region of Iran for the period 1901-1902 to
1913-1914, for the Safid Rud region 5536 to 12,670 individuals for the
period 1899-1900 to 1913-1914, for the Mazandaran region 846 to 1490
individuals for 1906-1907 to 1913-1914, and for the Astrabad (= Gorgan)
region 2613 to 5160 individuals for 1902-1903 to 1913-1914. Vladykov
(1964) records average yearly catches in Iran of this species
(including some A. nudiventris with small eggs) from
1927/28-1931/32 to 1957/58-1961/62 with ranges of 59,291-301,218 kg
body weight (9.7-23.8% of the total sturgeon catch; 33.8% in another
five-year period when weight was lower than the maximum shown here)
and 8246-77,780 kg caviar (10.0-48.2%; total range 9.5-54.5%). RaLonde
and Walczak (1970b) summarise yields for the years 1963 to 1967 in
Iran of meat and caviar as 385.2 tonnes (100.4 tonnes), 450.8 (99.3),
436.6 (98.9), 564.4 (113.0), and 584.7 (106.5) respectively. Hassan
Nia (1995) analysed the stocks of this species for a 61-year period
(1927-1987) and calculated projected yields for the period 1988-1992.
Actual yields proved to be the same as projected yields. The catch in
the northern Caspian Sea reached 13,200 tonnes in the latter half of the 1970s.
This species has not been used as extensively as others for studies on
physiology, biochemistry and aquaculture. Some works include Taleban et al. (1998) who studied consumption of this fatty fish and found
a reduction in mean serum triglycerides and very low lipoprotein cholesterol, and
an increase in high density lipoprotein cholesterol; Pourgholam and Saeidi
(2000) investigated haematological variables in juveniles and adults at
different water temperatures; Hedayatifard et al. (2003) studied
variation in fatty acids composition in cold storage and found the best holding
time was three months; Pazhand et al. (2003) on the toxicity of
the insecticide diazinon to fingerlings; Sadeghird et al. (2004) examined
levels of zinc and copper in muscle tissue and caviar; Padjand et al. (2005) examined the toxic
effects on fingerlings of the herbicide butachlor; Hedayatifard and Moeini (2007)
determined the levels of fatty acids in fresh and
frozen samples and their effects on shelf life; Hedayatifard and Yousefian
(2007) looked at shelf life and changes of lipid and fatty acid composition in
frozen storage; Mokaremi Rostami et al. (2007) on the effects on
juveniles of creosote on mortality rate and blood biochemistry with significant
differences from controls; Alipour et al. (2009) on
fertilising ability of cryopreserved spermatozoa; Bahmani et al. (2009)
on seasonal fluctuations of sex steroids in farmed 7-year-old fish; Asadi et
al. (2009) on serum biochemical parameters; Hedayatifard
and Aroujalian (2010) on packaging and shelf life; etc.
The use of 2000 p.p.m. potassium sorbate in processing caviar from
this species gives a better quality product than caviar without
preservatives (Salmani, 1995).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria and as food.
Conservation
See also under A. gueldenstaedtii. Lelek (1987) lists this
species as vulnerable and Birstein (1993) as intermediate in status.
It is now rare in the Safid and Gorgan rivers of Iran because of dam
construction, which inhibits the spawning migration, and irrigation
control structures near river mouths. The ban on sea fishing in 1962
by Soviet authorities led to an increased abundance of this species.
Artificial spawning sites with gravel 3-10 cm in diameter have proved
useful in the former U.S.S.R. and stocking is well established with up
to 23 million young being released in the Volga area annually in the
mid-1970s. However Veshchev (1995) reports that the population of this
species in the Volga could be lost, and this doubtless mirrors the
situation in other Caspian Sea states including Iran. About 30% of all
individuals caught in the Caspian in the late 1980s were hatchery
stock (De Meulenaer and Raymakers, 1996).
Abdolhay et al. (2006) report on 193 adults caught in 1998 which produced
623,000 million fingerlings while in 2002, 290 breeders were caught and 67
produced 1.3 million fingerlings.
Mohseni et al., (2000) have studied
effective stocking density of eggs and larvae in incubators and rearing tanks in
order to maximise production and avoid various morphological deformities.
Moghim et al.
(no date) note that juveniles of this species are caught in the beach seine
fishery for other species in Mazandaran. During 2001-2002, 23,760 seine hauls
had a by-catch of 37% for this species among sturgeons.
Khodorevskaya et al. (1997) summarises the decline of this
species in the Volga and Ural rivers. The problems are the same for
all sturgeons, namely flow alterations affecting the volume of water
on the spawning grounds, reduction in numbers reaching the these
grounds through poaching, and increased pollution affecting
reproductive efficiency.
Studies on heavy metal contamination (Zn, Cu, Cd,, Pb and Hg) of both flesh and
caviar in Iran, however, showed levels were below the maxima allowed for
consumption, based on international standards (Sadeghi Rad et al., 2005;
Abtahi et al. 2007).
The median lethal concentration of suspended sediment from the Safid River has
been studied by Garakouei et al. (2009); who found this species showed a
lower tolerance than A. persicus.
Kiabi et al. (1999) consider this species to be vulnerable
in the south Caspian Sea basin according to IUCN criteria. Criteria
include commercial fishing, abundant in numbers, habitat destruction,
widespread range (75% of water bodies), absent in other water bodies
in Iran, and present outside the Caspian Sea basin. Nezami et al.
(2000) maintain that despite artificial spawning and fingerling production,
restoration of this species in Iran was not very successful.
Mostafavi (2007) lists it as vulnerable in the Talar River, Mazandaran. Critically endangered in Turkey (Fricke et al., 2007). Under IUCN and Appendix II of CITES, this species is now endangered (Vecsei
et al., 2007).
Artificial breeding has been carried out with this species in Iran using
hormones (I.F.R.O. Newsletter, 30-31:4, 2002). In contrast to other sturgeons,
this species does not respond well to pituitary injections used to stimulate
artificial reproduction. Pourkazemi (2006) examined haematological parameters
and found wide fluctuations, with female spawners in particular differing in
sexual maturity and physiological state. Although fish do respond to pituitary
injections, the oocytes do not follow a normal course to maturity, remaining in
the ovary. Oocytes at stage IV had overripe or degenerated oocytes. When
overdosed with pituitary extract, ovulation occurred but oocytes were not mature
and could not be fertilised. Degeneration of the egg membrane was found in 82%
of spawners caught in the wild, presumably due to pollution. Baradaran Tahouri
(1994) examined the effects of pond fertilisation on growth. Haddadi Moghaddam
et al. (2001) studied the growth rate of this sturgeon in fertilised
earthen ponds with added Daphnia. Shahsavani et al. (2001)
determined blood parameters for fingerlings in a Gilan fish farm. Bahmani et al.
(2006) recommended alleviating stress during capture, handling, transport and
confinement, selecting breeders with suitable morphology and correct stage of
sexual maturity, and using the hormone GnRH with domperidone as a substitute for
pituitary extract. Luteinizing hormone releasing hormone
analogue (LHRHa) was also found to be effective at 20.0-31.2
μg/kg body weight (Behmanesh, 2002).
Kazemi et al. (2003) give a detailed histological study of the oocytes of this species.
Caviar and fingerlings have been produced from farmed breeders (Iranian
Fisheries Research Organization Newsletter, 49:3, 2006).).
Sexual maturity was stimulated by injection of GnRH and anti-dopamine, eggs were
extracted surgically, of which more than 80% hatched successfully using sperm
taken by using tubes, and caviar and flesh harvested from one fish was
comparable to natural samples.Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
101 |
58 |
43 |
70 |
52 |
| Injected broodstock |
43 |
38 |
67 |
42 |
12 |
| Spawning rate * (%) |
60.4 |
50 |
49 |
63 |
50 |
| Fertilisation rate (%) |
55 |
58.6 |
51 |
51 |
83 |
| Survival rate in incubators
(%) |
23 |
49.6 |
46 |
44 |
77 |
| Survival rate in tanks (%) |
54 |
93.5 |
60 |
72 |
77 |
| Stocking density in ponds
(fish/ha) |
92,500 |
44,812 |
68,000 |
90,000 |
92,000 |
| Survival rate in ponds (%) |
12.2 |
67.7 |
38 |
61 |
86 |
| Fingerling production (x
1000) |
226 |
820 |
13,009 |
196 |
314 |
* Rate of response to hormone injection
Moghim et al. (2002) used
ultrasonography to determine sex and maturity. This is important in management
of endangered species when external sexual dimorphism is not apparent. Accuracy
was 97.2% and was around 30 seconds or less per fish.
Further work
See under A. gueldenstaedtii.
Sources
See under the family account. Wossugh-Zamani (1991a) gives an account of
this species in Farsi. Derzhavin (1922) and Borzenko (1942) are older
works giving details of the biology of this species.
Iranian material: Hatchery adults examined at Bandar-e Anzali.
Comparative material: BM(NH) 1873.4.21:21-23, 2, 99.6-236.6 mm total length,
Russia, Black Sea (no other locality data); BM(NH) 1929.8.7:4-5 and BM(NH)
1930.3.21:2, 3, 246.4-308.2 mm total length, Ukraine, Sebastopol, Black Sea (no
other locality data).
Genus Huso
Brandt and Ratzeberg, 1833
This genus is characterised by a large and crescentic mouth (small
and transverse in Acipenser) and by the gill membranes being
joined to each other and free of the isthmus (joined to the isthmus in
Acipenser). The snout is short and blunt although Caspian Sea stocks have
a longer snout than Black Sea ones. The barbels are
flattened laterally and gill rakers are rod-like. There are only 2
species in the genus, one in the Caspian, Black and Adriatic seas and
one in the Amur River of eastern Asia.
Birstein and DeSalle (1998) using cytochrome b and 12S and 16S rRNA genes found that Huso may not be
distinct from Acipenser.
Vasil'eva et al. (2009) using cytogenetic and morphological characters
also advocate reverting to the original genus Acipenser for Huso
species.
Huso huso
(Linnaeus, 1758)

Common names
فيل ماهي (= fil mahi, filmahi or
philmahi meaning elephant fish), beluga, beloga, سگ ماهي (sag mahi,
meaning dogfish), ماهي خاويار (= mahi-ye
kaviar, meaning caviar fish), mahi kaviar-e bozorg (= big caviar fish).
[bolka, Kur bolkasi for natio kurensis, ag-kulag-nyarya,
gyuz'gi-burun in Azerbaijanian; doku (akvalyk) in Turkmenian; beluga in Russian; great, giant or European sturgeon].
Systematics
Acipenser huso was originally described from the Danube and rivers of Russia.
Huso huso caspicus Babushkin, 1942 was described as the
subspecies of the Caspian Sea basin (with natio kurensis
Babushkin, 1942 from the Kura River (also spelt incorrectly cyrensis
and curensis)) but Berg (1948-1949) considered Caspian-Volga
populations to be typical and this subspecies description as
unnecessary. No types of Huso huso caspicus are known
(Eschmeyer et al., 1996).
Huso ichthyocolla Bonaparte, 1846 is a synonym (Eschmeyer et
al., 1996) and a nomen nudum (Holčík, 1989).
Acipenser brandtii Günther, 1870 from the "Black
and Caspian Seas, with their rivers" is a hybrid of Huso huso
and Acipenser nudiventris based on Acipenser schypa (in
part) of Brandt and Ratzeberg (Berg, 1948-1949; Eschmeyer et al., 1996).
M. Pourkazemi in PADECO (2002) considers there are two sub-populations in Iran
and Ghadirnejad et al. (2008) using microsatellite loci concluded that
there were possibly two populations in the southern Caspian Sea.
Hybrids of Huso with Acipenser have been bred by the
Aquaculture Department of the Iranian Fisheries Research and Training
Organization (Iranian Fisheries Research and Training Organization
Newsletter, 3:3, 1994; Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 6, 1996; Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization, Tehran,
p. 41, 1997) and natural hybrids with A. gueldenstaedtii, A. nudiventris and A. stellatus are
reported from the Caspian Sea (Berg, 1948-1949).
Key characters
This species is identified by its very large, crescent-shaped mouth
(small and transverse in other sturgeons) and the gill membranes being
joined as a fold across the isthmus.
Morphology
The greatest body depth is slightly anterior to the middle of the body and
large fish appear humpbacked. The lower lip is interrupted at its centre. Barbels are flat
posteriorly, reach almost to the mouth and have foliate appendages.
Experiments on ablading barbels (clipping one, two and four barbels) in 1+ age fish
showed no growth differences with an unclipped control (Abasali Zadeh, 2003).
The dorsal scutes are covered with skin in sexually mature fish,
lateral scutes are smooth and ventro-lateral scutes hidden beneath the skin.
Dorsal fin rays 48-81 and anal fin rays 22-41. Dorsal scutes 9-17,
lateral scutes 28-60 and ventral scutes 7-14. Scutes in adults may be
reabsorbed. The skin is covered in small denticles. Gill rakers 16-36.
The chromosome number is 2n=118 ± 3 or 115 ± 1 (Annual Report,
1994-1995, Iranian Fisheries Research and Training Organization,
Tehran, p. 43, 1996; Iranian Fisheries Research and Training
Organization Newsletter, 8:5, 1995), 2n=116 ± 1 (Nowruz
Fashkhami, 1996), 2n=118 ± 2 or 2n=116 ± 4 (Klinkhardt et al., 1995),
2n=117 (Nowruz Fashkhami and Khosroshahi, 1999). Sex chromosomes are absent or
weakly differentiated in the genome and DNA markers cannot be used to sex
fish; minor surgery has to be used (Keyvan Shokoo et al., 2004;
Keyvanshokooh et al., 2007).
Sexual dimorphism
None found in morphometric and meristic characters although females
are said to be longer and heavier than males of the same age.
Colour
The back is ash-grey, blue-grey to greenish or dark brown, sometimes black, fading to a
white or cream belly. The contrast between the dark back and lighter rest of the
body is marked. Young often have a metallic sheen which fades with age. The snout is yellowish.
Size
Attained weights of 1228 kg yielding 246 kg of caviar or 7.7
million eggs (Berg, 1948-1949), even 1600 kg (Farid-Pak, no date), and
there are newspaper and other reports of fish 1200 kg and 6 m (Ottawa
Citizen 14 May 1986) or even 3200 kg and 9 m but such large fish are
not seen today and the largest sizes are probably exaggerations.
Modern catches are mostly much smaller than these exceptionally large
fish. A recent record with the specimen preserved in the Astrakhan
Museum in Russia is given in Sternin and Doré (1993) for a fish from
the Volga River in 1989 weighing about 980 kg, 4.3 m long and yielding
about 110 kg of caviar (Iran News, 14 July 1998, gives 988 kg, 120 kg
of caviar and an age of 60 years, presumably the same fish). A
photograph of a 1908 capture at Astrakhan in Stein and Bain (1981)
shows a fish weighing about 400 lbs (181.4 kg) containing 200 lbs (90.7 kg) of caviar worth
more than $69,000 in 1981. Tsepkin and Sokolov (1971) give some examples of large fish from former Soviet
waters. Birstein et al. (1997) consider this species to be the largest freshwater fish.
The mean weight of Caspian Sea fish decreased from 110 kg in the
early 1970s to 57 kg in 1991 (De Meulenaer and Raymakers, 1996).
Up to 2.83 m and 450 kg generally in Iran (Azari Takami et al., 1980)
but see below for news reports. Belugas up to 960 kg tried to enter
the Atrak River in 1836 (Vladykov, 1964). The longest fil mahi caught
in Iranian waters is apparently one taken on 23 February 1989 by
Turkmen fishermen at Shilat-e Nahee 4 in Mazandaran (see Abzeeyan,
Tehran, July 1991, page 3). It had a fork length of 4.5 m, a total
weight of 725 kg and a caviar weight of 98.2 kg. This individual was
worth U.S.$140,000 (Abzeeyan, Tehran, November 1992, page 57).
The heaviest fish from Iran is one reported by Hossein Aimani at 3000 lbs
(1360.8 kg) from near Babol in 1973
(www.amarillonet.com/stories/120599/bus_LQ7659.shtml, downloaded 7 March 2000).
Mobayen (1968) gives the largest Iranian specimen as 4.2 m and 850 kg.
Anonymous (1991a) and Sternin and Doré (1993) cite a
fish of 1742 lb (= about 791 kg), 7.5 feet long (= about 2.3 m) and
yielding 220 lb (= about 100 kg) of caviar from Iran in 1989, the
largest caught for 20 years; this may be the same fish as the previous
one as confusion in weights and lengths are common in reports of large
fishes. Other large specimens were taken at Mahmudabad, Mazandaran on
28 October 1992, measuring 3.2 m, weighing 430 kg and with 61.2 kg of
caviar (Abzeeyan, Tehran, November 1992, page 13), at Bandar-e
Torkeman (= Bandar-e Shah) weighing 320 and 410 kg giving 110 kg of
caviar for the two fish (Abzeeyan, Tehran 4(1):IIX, 1993), at
Bandar-e Torkeman, Mazandaran on 27 March 1993, measuring 4.0 m,
weighing 550 kg and with 81 kg of caviar (Abzeeyan, Tehran,
4(2):47, 1993), and in Mazandaran one measuring 3.0 m fork length and
3.4 m total length, weighing 960 kg and yielding 62.5 kg of caviar (Iranian
Fisheries Research and Training Organization Newsletter, 5:8,
1994). Newspaper reports in 1996 listed a fish of 500 kg with 54 kg of
caviar worth $107,000 and a fish caught in October 1997 at Babol Sar
weighed 300 kg, measured 3 m in length and had 45.1 kg of caviar. In
1998, one fish 3.4 m long yielded 43 kg of caviar (Reuters), a fish
caught off Bandar-e Torkeman on 2 February measured 3.75 m, weighed
405 kg and yielded 50 kg of caviar (IRNA (Islamic Republic News
Agency), 3 February 1998), one caught off Bandar Anzali on 25 October
weighed 360 kg, was 3 m long and yielded 24 kg of caviar and
"meat" worth 3.6 million rials (IRNA, 26 October
1998), one caught off Nour, Mazandaran on 15 November measured 3.5 m,
weighed 450 kg, yielded 53 kg of caviar and was 30 years old (IRNA,
16 November 1998), and one caught off Kianshahr, Gilan weighed 290 kg,
was 3.5 m long and yielded 50.6 kg of caviar worth 100 million rials (IRNA,
24 November 1998). In 1999 newspaper reports included one caught off
Bandar Anzali weighing 155 kg, carrying 31 kg of caviar worth $12,400
(IRNA, 31 October 1999), one caught off Talesh weighing 120 kg
with 23.5 kg of caviar worth 150-200 million rials (IRNA, 5
December 1999), and one caught off Bandar-e Torkman weighing over 405
kg with over 52 kg of caviar worth 500 million rials (IRNA, 14
December 1999). One fish caught near Bandar Anzali in weighed 370 kg
and yielded 51 kg of caviar (IRNA, 28 October 2002).
Distribution
Found in the Adriatic, Black and Caspian seas and their drainages.
Derzhavin (1934) reported it from the Babol, Sorkh and Gorgan rivers
but it was rare in the Safid River, although reported up to Kisom and
quite abundant in the sea off its mouth. Nedoshivin and Iljin (1927) record this
species from 10 river mouths while A. stellatus and A. gueldenstaedtii
are reported from 18; the 10 river mouths are Yusufabad, Musachai, Hasan Kiadeh,
Dastak, Safid, Kasumabad, Chalkarud, Sardabrud, Chalus and Kheirud. Kozhin (1957), Rostami (1961) and Armantrout
(1980) stated that it enters the Astara, Safid, Babol and Gorgan
rivers and the Anzali and Gorgan mordabs. It comprised only 0.5% in
numbers and 2.5% in weight of the Safid River catch in 1914-1915
(Nedoshivin and Iljin, 1927). Large numbers were caught in the sea off
Gasan-kuli in Turkmenistan near the Iranian border (Berg, 1948-1949).
Also reported from Hasan Kiadeh by V. D. Vladykov based on field work
notes made in 1962. More recently reported from the Gorgan and
Safid rivers, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea by Kiabi et al. (1999) and Abdoli and Naderi
(2009), from
the Safid River by Abbasi et al. (1999) and from the Safid, Gorgan and
Tedjen rivers. This species was not caught in a survey along the Iranian coast
in 2001 (Ivanov and Katunin, 2001). In 2004 there were plans to introduce this species to isolated,
natural waters bodies in Fars Province (H. R. Esmaeili, in litt., 2004).
Zoogeography
Presumably a relict of past isolation of the Black-Caspian seas from the world ocean.
Habitat
This sturgeon is found in large concentrations in the eastern
coastal region of the south Caspian Sea in all seasons. It is rare in
trawl catches, possibly because it has a more pelagic life than other
sturgeons. Fil mahi descend to greater depths than other sturgeons,
100-140 m in the Caspian and to 180 m in the Black Sea. There is no
seasonal variation in depth distribution in the south Caspian Sea in
contrast to the middle Caspian (Legeza, 1972; 1973). Only the young
are found in shallow, warm areas. On the spawning migration, this
sturgeon usually follows the deepest part of the river.
Most of this sturgeon's life is spent in the sea and it ascends
rivers only to spawn. The new-born sturgeon returns to the sea. Farabi et al.
(2007) examined salinity tolerance and physiology of juvenile fish in Iran. Only
the youngest fish showed mortality on direct transfer from fresh to estuarine
and Caspian sea water. Adults are typically found on silty or muddy bottoms in the sea but may be
found on shelly and coarse sand at a temperature range of 5.6-29.3°C
and depths of 5-140 m. In the southeastern Caspian it remains below 30
m in winter, entering shallower water at depths of 10-20 m in spring
as the temperature ameliorates, dispersing throughout the southeastern
Caspian in summer and migrating into Iranian waters in autumn (Legeza,
1972; Filippov, 1976). Depth distribution depends in large part on the
available food supplies.
Oxygen requirements are high, averaging about 14 mg/l, but they can
survive at 2-3 mg/l. Salinities up to 22‰ are tolerated. Feeding
occurs over a temperature range of 0.5-30°C and the spawning migration at a range of 6-21°C.
The highest densities in the southern Caspian Sea occur at 22-29ºC,
feeding in winter at 10-12ºC (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
Males become sexually mature at age 9-16 years and females at
12-22 years, varying with the spawning river. This is a very late maturation age among fishes
world-wide. Spawning intervals are 4-7 years for males and 5-7 years for females
(Vecsei et al., 2002; see below for other ranges but certainly intervals
are long for a fish species). Spring-spawning females (see below) first spawn at 201-209
cm, 50-60 kg and 17 years. Winter-spawning females first spawn at
181-190 cm, 30-39 kg and 16 years. Most spring females are 230-300 cm
long, weigh 80-160 kg and are 23-28 years of age. Most winter females
are 201-300 cm, 50-160 kg and 17-26 years (Raspopov and Dubinin,
1990). Spawning populations have a complex age structure, the Volga
River in 1936 had 50 age groups for example but only 28 in 1964. There
has been a trend for spawners to be younger. Average catches in former
Soviet waters of the Caspian Sea now weigh only 77 pounds (34.9 kg) each, a
decline caused by overfishing (Los Angeles Times, Part A, page
1, 28 August 1993). A life span of 150 years was reputed for this species but the greatest known
age for a Caspian fish is 75 years (Berg, 1948-1949). Most Caspian fish are now less than 20 years old and made
up of individuals from re-stocking programmes (De Meulenaer and
Raymakers, 1996). Raspopov (1993a; 1993b) gives the life cycle of
Volga River fish as 56 years, although this is not the maximum age. Kura River
sturgeon grow more slowly and mature later than sturgeon from the
Volga River. Growth in this species is rapid with 1-year-old fish in
the Caspian being 51 cm long and weighing 571 g. Growth is slower in
the Caspian than the Black Sea because of the decrease in numbers of Alosa
spp., the prime food item. Growth is also slower in the south Caspian than the
north (Caspian Sea Biodiversity Database, www.caspianenvironment.org). Hedayatai
et al. (2009) were able to correlate weight and length with immature male
gonadal stage, but not for females, in work directed to reducing maturation
time. Moghim et al. (2008) studied sex ratio along the Iranian coast for
the years 1990-2003 and found females dominated at 60-80% of landed fish.
Immature females decreased from 71 to 47% of the catch.
Levin (1997) summarises the spawning population of the Volga River
over the last 10 years as follows although he notes this population is
almost extinct. Rarely spawners enter between August and October and
breed after a winter hibernation. Other fish enter from December to
May with a peak from February to March. Peak spawning is in May with a
downstream migration to the Caspian Sea from June to September.
Females, comprising 20-24% of the spawning population, average 236-261
cm and 106-160 kg and are 17-21 years old with fish larger than 400 cm
being very rare. Males are 199-204 cm and 48-55 kg and are 11-18 years
old. Spawning occurs at 9-11°C.
Farid-Pak (no date) gives approximate weights for Iranian beluga of
75-100 kg and 2.0-2.5 m, and a yield of 17-20 kg of caviar per female.
2608 beluga from Astara in Azerbaijan averaged 168 cm for males and
192 cm for females.
Von Bertalanffy growth parameters in Iranian females are L∞
= 320 cm and K = 0.065 for juveniles, 450 cm and 0.029 for the middle
stanza and 533 cm and 0.023 for older fish and for males 270 cm and
0.086 or 302 cm and 0.072, depending on the methodology used. Total
mortality (Z) was 0.21-0.67 for females and 0.22-0.75 for males,
natural mortality (M) was 0.03 for females and 0.05 for males, fishing
mortality (F) was 0.45 for females and 0.33 for males, and optimum
fishing mortality was (F) 0.07 for females and 0.16 for males (Iranian
Fisheries Research and Training Organization Newsletter, 16:4-5, 1997).
Taghavi Motlagh (2001) gives more complete data (on which the previous summary
was based) on growth, mortality and yield-per-recruit on this species from 1995
to 1999 in the Iranian Caspian Sea. He concluded that fishing mortality should
be stopped. Maximum age in his sample was 46 years.
Food
In contrast to other sturgeons, this species is a pelagic predator as adults.
Even sea birds and seals may be eaten. However, the introduced polychaete worm Nereis is now a mainstay of
the diet of this species in the north Caspian Sea. Other foods are
molluscs, formerly a main food, and small fish such as Rutilus
rutilus (and presumably R. caspicus)and gobies (Gobiidae). Fish are the main diet item when
large, invertebrates when young. This species needs to find thick concentrations
of small or large fishes in order to feed actively; in the north Caspian these
are kilka and fish on migration at fishways and in the midde Caspian spawning
atherinids and commercial herrings (Polyanina et al., 1999). The fish found by Azari Takami et
al. (1980) in Iran were gobies, Cyprinus carpio, Liza,
and Rutilus. Gobies are a favourite food item but bivalves and
crustaceans are taken if fish are absent. Filippov (1976) notes that
large specimens eat sturgeons such as sevryuga, kopur (Cyprinus
carpio), mullets (Mugilidae), birds such as coots, and baby seals
and because of its pelagic life takes the clupeids Alosa
braschnikowii and Clupeonella caspia and also the
shrimp Leander adspersus. Crabs are also eaten. The principal
food as percent by weight in the southeastern Caspian was Neogobius
fluviatilis (= pallasi) (up to 78.1%), gobies accounted for up to 81.2% and
fish 81.6-100%. Crustaceans accounted for up to 7.8% and molluscs only
up to 0.2%. The cyprinid, Chalcalburnus (= Alburnus) chalcoides, is also
eaten (Mageramov and Zarbalieva, 1989).
Reproduction
Roux (1961a) maintained that this species did not reproduce in
Iranian rivers but Rudin (1966) said that they inhabited the Safid and
Gorgan rivers. The main spawning river was the Volga as 90% of the
Caspian stock reproduced there, travelling as far up as the Moskva
River. Males arrive at spawning sites before females. Despite their
size, these sturgeons may leap out of the water on the spawning run
and possibly during spawning. Adhesive eggs are deposited on sandy
substrates, with rocky and gravelly bottoms near the bank, in the
strong current of mid-river (1.5-2.0 m/sec.). Water temperatures are 9-17°C
and eggs develop in 9-10 days (Novikova, 1994; Vecsei et al., 2002). Spawning usually takes
place at a depth of 4-15 m, sometimes as deep as 40 m. Weight loss
after spawning may reach 50% and females are only ready to spawn again
after 5-6 years and males after 3-4 years (4-8 and 4-7 years in Speer et al.,
2000). The migration in the Volga River occurs year-round with peaks in spring (<30% of the stock)
and autumn. The spring race reach the spawning beds in the same year,
reproduce and return to the sea. The winter race, migrating in summer
and fall, overwinter in the river and reproduce the following spring.
The spring run is in March and April and the winter run in September
and October. The chief spawning period in the Kura River is from the
end of May to the beginning of June (Zakharyan, 1972) and fish were
found as far up as Tbilisi (= Tiflis).
Fecundity reaches, exceptionally, 7,729,700 eggs but does not
increase with age for fish of equal length and weight (Raspopov, 1987;
Raspopov and Dubinin, 1990). Mean fecundity for the Volga stock was
531,600 eggs. Normal deposition of eggs is 500/sq m in the Volga but
densities fell below 5/sq m in the 1980s, as low as 0.2/sq m and with
an average of 1.5/sq m (Novikova, 1994). Kura River sturgeon are less
fecund than Volga sturgeon. Egg diameter reaches 4.3 mm. Eggs are a
dark silver and oval. Larvae hatch in 10-14 days, the yolk sac is absorbed in
10-14 days and feeding larvae move downstream at up to 60 km/day (Vecsei et al., 2002).
Parasites and predators
Niak et al. (1970) report infestations of the ciliate Trichodina
sp. in sturgeons (species unspecified) in breeding ponds in Iran.
Golvan and Mokhayer (1973) describe a new species of acanthocephalan, Corynosoma
caspicum, and also Leptorhynchoides plagicephalus from this
sturgeon in Iran. Mokhayer and Anwar (1973) report on sturgeon
parasites in general (see under Acipenser gueldenstaedtii).
Mokhayer (1976b) reports gas bubble disease in Iranian sturgeons
without specifying the species of sturgeon as well as the monogenetic
trematodes Diclobothrium armatum and Nitzschia sturionis.
Pourgholam (1994) reports the coelenterate Polypodium hydriforme
from this species caught on the Babol Sar and Bandar-e Torkeman
fishing grounds in Mazandaran. Larvae of the nematode Anisakis
simplex and the acanthocephalan Corynosoma strumosum are
also reported from this species (Annual Bulletin 1993-94, Iranian
Fisheries Research and Training Organization, Tehran, p. 48-49,
1995). Sattari et al. (2002) record Cucullanus sphaerocephalus,
Eustrongylides excisus, Skrjabinopsolus semiarmatus, Anisakis
sp., Eubothrium acipenserinum and Corynosoma strumosum, the fauna
being similar to other sturgeons because of their piscivorous feeding. Gorogi (2006b) recorded the nematodes Cucullanus sphaerocephalus and
Anisakis schupakovi, the cestode Eubothrium acipsenserinum and the
acanthocephalans Leptorhynchoides plagicephalus and Corynosoma
strumosum from Iranian waters. Sattari and Mokhayer (2005a; 2005b) recorded
the occurrence of parasites in this species from the Iranian southwestern and
central coast of the Caspian Sea. The species found were the nematodes
Cucullanus sphaerocephalus, Eustrongyloides excisus and Anisakis
sp., the cestode Eubothrium acipenserinum, the acanthocephalan
Corynosoma strumosum, the digenean trematode Skrjabinopsolus semiarmatus.
General conclusions were that the diversity of parasites was less in Iranian
waters than in the northern Caspian Sea, perhaps a reflection of the more varied
habitat, its productivity and the carbonate ions differing between the two
regions. The diversity of parasite seems to have declined over time also,
perhaps as a result of unfavourable environmental conditions, particularly in
the freshwater ecosystem which limits the waters available for spawning and parasite acquisition.
Shenavar Masouleh et al. (2006) found hatchery fingerlings to harbour
Diplostomum spathaceum and Trichodina sp.
Barzegar and Jalali (2009), in their summary of crustacean parasites of Iranian
fishes, recorded Pseudotracheliastes stellatus from this sturgeon.
The fil mahi is so large that its predators are only effective on
young fish. They include Sander lucioperca and Silurus
glanis and, needless to say at all sizes, mankind.
Economic importance
This species provides the best caviar according to Borodin (1930).
The large eggs fetch a higher price on the American market. Up to 80% (3000 kg
in 2002) of the legal beluga caviar export is consumed in the U.S.A. (Hamilton,
2002). A 1227 kg specimen caught in Russian waters in 1924 gave 245 kg of caviar worth
£189,350. In the 1990s, a 225 kg fil mahi could yield 22 kg of caviar
worth $120,000 (Trickey, 1995). Catches in the Volga region in the
1970s were in the range 740-2650 tonnes and in the 1980s 460-900
t comprising 4.4-12.2% and 3.7-4.4% respectively by weight of the
total catch of all sturgeons there. The highest catch in the Caspian
Sea was in 1902-1907 (Birstein, 1993). Khodorevskaya et al.
(1997) and Khodorevskaya (1999) summarise the decline in catches and
make the startling observation that 96.3% of all fil mahi in the Volga
River are hatchery reared.
Fil mahi were fished intensively off the Iranian coast in the
southeastern Caspian and in 1950 amounted to 38.6% of the total
sturgeon catch. During the five-year period 1957/1958 to 1961/1962 fil
mahi catches in the Gorgan Division of the Iranian fishery varied
between 86-90% of total Iranian catches. The Atrak River estuary area
was particularly important for this species. Catches of the oldest age
groups has declined and the proportion of young and immature fish has
increased. Iranian rivers suitable for this sturgeon were the Safid
and the Gorgan but both are now regulated so Iranian stocks are
probably maintained by fish reproducing in the rivers of the former
U.S.S.R. (Filippov, 1976). Fil mahi cannot be managed by Iranian
authorities therefore. However the "Gharasoo" Research
Station in Mazandaran is researching the culture and release of fil
mahi up to 1 kg (Madbaygi, 1993b) and farming through pen culture in
Gorgan Bay (Iranian Fisheries Research and Training Organization
Newsletter, 11:6, 1996). Two million "roes" (presumably
young fish) were released into the Caspian Sea from Mazandaran prior
to 1 June 1995 with a further 2 million to be released later in the
year (http://netiran.com/news/IRNA/html/950701IRGG08.html). In 1997,
852 fishermen were fishing for fil mahi on the northern Iranian coast
(Anonymous, 1997c).
Farid-Pak (no date) gives the months of September-October and
March-April as the most important for the fisheries of this species.
Nevraev (1929) gives catch ranges of 109-3100 fil mahi individuals for
the Astara region of Iran over the period from 1901-1902 to 1913-1914,
for the Safid Rud region 104 to 730 individuals for the period
1899-1900 to 1913-1914, for the Mazandaran region 31 to 491
individuals for 1906-1907 to 1913-1914, and for the Astrabad (=
Gorgan) region 688 to 1764 individuals for 1902-1903 to 1913-1914.
Vladykov (1964) records average yearly catches in Iran of this species
from 1927/28-1931/32 to 1957/58-1961/62 with ranges of 57,820-418,059
kg body weight (5.4-33.0% of the total sturgeon catch) and 2038-32,873
kg caviar (2.6-20.4%). There was an upward trend in caviar production
from this species in the 1950s (Vladykov, 1964). RaLonde and Walczak
(1970b) summarise yields for the years 1963 to 1967 in Iran of meat
and caviar as 572.3 tonnes (40.1 tonnes), 583.5 (47.3), 575.8 (39.1),
458.1 (29.5), and 507.2 (30.0) respectively. A commercial house
maintains (1995) that caviar from this species comprises only 3% of
the total catch. Taghavi Motlagh (2001) noted a decline in the share of Iranian
caviar production from 18% in 1971 to 4% in 2000.
This species has been studied in ponds as breeders are used to produce fingerlings which are then
available as experimental fish for chemical and growth studies. Ghorbani et
al. (2003) studied the influence of heavy metals on the level of alfa-amylase
activity in the digestive tract and found decrease in enzyme activity was not
significant. Karimzadeh et
al. (2005) studied cytochrome P4501A1, a major isoenzyme in the
monooxygenase system which can be induced by polycyclic aromatic hydrocarbon
pollutants. Khoshbavar Rostami et al. (2006) studied the effects of
polyaromatic hydrocarbons from Caspian Sea oil wells on 8.5 g fingerlings and
found these chemicals to seriously affect the fish blood and enzyme systems.
Khoshbavar Rostami et al. (2004; 2006) studied the
organophosphate diazinon and its deleterious effects on haematological
parameters in this sturgeon. Sharifpour et al. (2004) studied the effects
of the insecticide endosulfan, sturgeon weighing 3-5 g showing irregular
swimming, whirling, convulsions, with other conditions, and eventually death.
Endosulfan is highly toxic to beluga fingerlings. Sudagar et al. (20050
examined the addition of betaine and methionine (an important nutrient and an
enzyme) to the diet of juvenile beluga. The fish showed improved weight gain,
weight gain percentage, specific growth rate, protein efficiency ratio, net
protein utilisation, condition factor, survival, and price index at enrichment
levels of 0.5% betaine and 1% methionine. Ghorbani et al. (2004) examined
the influence of a series of microelements (zinc, nickel, cobalt, manganese,
iron and copper) on the level of proteolytic enzymes and alkaline phosphatase
activity (used for enzyme inmmunoassays) in the digestive tract of juvenile
beluga. Most treatments showed the level of enzyme activity was less than the
control. Shahsavani (2002) determined blood parameters of fingerlings from fish
farms and found the fish to be healthy. Blood parameters are used to indicate
physiological condition and sublethal stress due to endogenous and exogenous
changes, hence the need to determine normal values. Askarian et al.
(2006) looked at serum osmoregulatory parameters under different light regimes,
one form of physical stressor in aquaculture of this endangered species. No
differences in serum cortisol levels were found between treatments although
elevations of serum cortisol, glucose and triglyceride occurred.in a continuous
dark regime. Gafarian et al. (2007) used probiotic bacillus in the
feeding of larval sturgeon and found that it positively affected feeding
efficiency and levels of carcass nutrient composition. Khoshbavar-Rostami et al. (2007) examined the immune response to
Aeromonas hydrophila bacterin.
Soulati and Falahatkar (2007) looked at stress response in sub-yearlings exposed
to air. Shamloufar et al. (2007) examined the sub-lethal effects of
diazinon on haematological indices in juveniles. Akrami et al. (2008)
studied the effect of prebiotic inulin levels and found it did not increase
growth performance of juveniles. Askarian et al. (2008) examined the gastrointestinal tract for
lactic acid bacteria and found the population levels to be significantly higher
than in Acipenser persicus. Hedayati et al. (2008) studied blood
indices of fish cultured in brackish water. Hosseini et al. (2008) examined the
organochlorine content of four sturgeon species and found fil mahi had four
times more than the next highest species (A. nudiventris); generally
pollutants had been reduced compared to previous studies but some specimens
exceeded guideline levels for food. Soltani et al. (2008) found that
100-200 mg/kg of vitamin C was optimum for rearing this sturgeon. Baghfalaki
et al. (2009) carried out studies on seminal plasma indices in order to
improve short and long-term storage of semen. Darvish
Bastami et al. (2009) found that addition of Daphnia and Artemia extracts
had positive outcomes on growth in juveniles. Akbari et al. (2009)
studied the use of sperm extenders and found that they prolonged spermatozoa
viability in short-term storage and prolonged sperm motility. Ghanbari et al.
(2009) isolated Lactobacillus species, which ferment carbohydrates, from
the intestine. Jalali et al.
(2009) found that Artemia urmiana nauplii on enriched with HUFA and
vitamin C and fed to larval sturgeon improves some growth and stress tolerance. Seifzadeh et al. (2009) examined microbial quality of packaged fillets of
this sturgeon.
Askarian and Kousha (2008) examined food ration on the acute stress response,
those receiving a high ration performing better. Alizadeh et al.
(2009) studied effects of different diets on energy levels and gonad development
for fish reared in inland brackish water, this environment proving suitable. Askarian and Kousha
(2009) studied photoperiod in rearing year-old fil mahi evaluated by growth (no
effect) and serum parameters (various individual responses to stress. Falahatkar
et al. (2009) examined dosages of vitamin C that enhanced immune
responses to disease. Sepahdari et al. (2009) found various skin lesions
in fish fed a diet containing aflatoxin B1 (a naturally occurring
fungal toxin). Sepahdari et al. (2010) found deleterious changes in liver
tissues
n fish fed a diet containing aflatoxin B1. Ghanbari and Jami
(2011b) reported Lactobacillus species from the guts of this species.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria,
as food and in textbooks.
Conservation
See also under A. gueldenstaedtii. Critically endangered in Turkey
(Fricke et al., 2007). Despite loss of 99% of
the Volga River spawning beds to dam construction, natural
reproduction increased over a recent five-year period, but continues
to be dependent on the variable flow-regime (Raspopov and Dubinin,
1990). Novikova (1994) estimated the capacity of the Volga spawning
grounds to be 9-11,000 fish. A major problem in the 1990s was
poaching. Trickey (1995), referring to Russian stocks, expected a
legal harvest of 4400 tonnes with poachers taking twice that amount.
This legal and illegal catch is still less than catches of 20 years
ago, primarily because of pollution. Birstein (1996) records the catch
of the Volga delta hatcheries in 1995 to be only 35 fish, insufficient
for artificial reproduction. Natural spawners are taken by poachers.
The level of poaching in the Ural River is also high, and this was the
only river where some natural reproduction was going on. The fil mahi
has effectively stopped reproducing in the Caspian Sea.
Moghim et al. (no date) note that juveniles of this species are caught
in the beach seine fishery for other species in Mazandaran. During 2001-2002,
23,760 seine hauls had a by-catch of 6% for this species among sturgeons
captured.
Khodorevskaya and Novikova (1995) point out that cooperation among
all the Caspian Sea states is needed to maintain this species along
with an annual release of at least 20 million young from hatcheries.
Fingerlings released per year from 1998 to 2002 range from 6.9 to 12.6 million
for all Caspian states (CITES website). Spawning migrations are now seen only in the Volga and Ural rivers,
the Kura, Terek and Sulak rivers no longer supporting stocks. The
Volga migration was 25,500 fish weighing 2600 t in the early
1970s but has fallen to 11,700 fish weighing 750 t. The
commercial catch fell from 2000 t to 500 t. In the Volga
River 96.3% of the spawning population consists of hatchery fish
although the Ural River maintains a naturally reproducing stock.
Since stocks are maintained mostly by artificial rearing, this
sturgeon has been proposed for inclusion in the "Red Book of the
U.S.S.R." which forms the basis for measures to protect species
(Pavlov et al., 1985; Mina, 1992). Stocks have been increased
through rearing and natural reproduction in the Ural River, the number
rising from 9.6 million in 1976 to 15.3 million in 1983, so the status
of this species was then regarded as acceptable. However Lelek (1987)
and Birstein (1993) list this species as vulnerable to endangered.
Kiabi et al. (1999) consider this species to be endangered in
the south Caspian Sea basin according to IUCN criteria as does IUCN and CITES (Vecsei
et al., 2002). The U. S. Fish and Wildlife Service lists it as threatened
under the U.S. Endangered Species Act as of 21 October 2004 (http://news/fws.gov/newsreleases,
(dated 20 April 2004) and downloaded 22 April 2004) and the Wildlife Service
has been petitioned to make it endangered (Speer et al., 2000).
Endangered status would stop importation of flesh and caviar to the United
States. Suspension of trade in this species from the Black Sea basin by
the U.S. Fish and Wildlife Service was instituted in 2005 (Federal Register,
2005) and imports from Iran are banned for political reasons along with other
sturgeons. Criteria for the various status assessments
include commercial overfishing (fishermen cannot even catch the set quotas),
failure of regulatory oversight, few in numbers, habitat destruction, dams
preventing spawning migrations, medium range (25-75% of water bodies), absent in other water bodies in Iran, poaching,
pollution, diseases due to pollution, and presence outside the Caspian Sea basin. The World Wildlife
Federation (WWF) listed this species as number 4 on the top 10 most
endangered species in the world (www.extravalue.com/sturgeon.shtml,
downloaded 13 March 2000). The species status may be changed to Appendix I on
the CITES listing, when international trade in its caviar would be banned (Vecsei
et al., 2002). The export quota for this sturgeon in the Caspian Sea 2004 was reduced to 4425
kg although an illegal harvest was still substantial (www.tehrantimes.com, downloaded 14 October 2004).
Illegal fishing from 1990 onward and cessation of hatchery releases
will lead to loss of the stock unless an agreement between Caspian
states can be reached to protect this species.
The invasion of the ctenophore Mnemiopsis has led to declines in the
kilka (Clupeonella spp.) stocks, a prime food of fil mahi (Kideys, 2002).
Caviar from Russian caught fil mahi bought in New York stores has
been examined for pollutant content (Boyle, 1994). Three stores
carried caviar with 3.17-3.27 parts per million of DDT plus its
metabolites DDD and DDE, 410-640 parts per billion of the PCB Arclor
1254, and 2.1-2.8 parts per million selenium. These values are below
the U.S. Food and Drug Administration's action levels of 5 parts per
million for DDT, 2 parts per million of PCB and 10-50 parts per
billion of selenium in drinking water. Nevertheless they are cause for concern.
Various studies have been carried out on the aquaculture of this valuable
sturgeon in Iran. Mohseni et al., (2000) have studied effective stocking density of eggs and
larvae in incubators and rearing tanks in order to maximise production and avoid
various morphological deformities.
Abdolhay and Tahori (2006) give fingerling production as:-
| Process/Year |
2000 |
2001 |
2002 |
2003 |
2004 |
| Female broodstock captured |
32 |
29 |
29 |
48 |
16 |
| Injected broodstock |
19 |
14 |
21 |
30 |
9 |
| Spawning rate * (%) |
74 |
71.4 |
62 |
65 |
77 |
| Fertilisation rate (%) |
55 |
65.5 |
65 |
54 |
65 |
| Survival rate in incubators
(%) |
62 |
73.4 |
62 |
32 |
72 |
| Survival rate in tanks (%) |
80 |
62 |
56 |
100 |
79 |
| Stocking density in ponds
(fish/ha) |
82,100 |
51,639 |
51,333 |
52,359 |
65,448 |
| Survival rate in ponds (%) |
73 |
51.3 |
67 |
43 |
59 |
| Fingerling production (x
1000) |
1900 |
640 |
24,037 |
42 |
146 |
* Rate of response to hormone injection
Mohseni et al. (2006) studied the best stocking density for rearing juveniles less than one
year old weighing 92.09 g on average and one-year-old fish weighing 918.14 g on
average. Stocking densities were 1.6, 2.8 and 4.0 kg/m2 for the
juveniles and 1.5, 2.5, 3.5 and 4.5 kg/m2 for the older fish.
Increased density had a negative impact on growth, body weight, specific growth
rate and food conversion ratio in both experiments. Higher concentrations
of fishes even had malformed caudal fins and body injuries from increased
contact. Recommended stocking densities were 1.5-2.0 kg/m2 for fish
up to 90 g and 2.5-3.0 kg/m2 for fish over 900 g.
Cage culture of fingerlings has been carried out in Gorgan Bay
starting in 1992. Cages were 3200 sq m with a depth of 2.5 m and
contained 11,500 fingerlings. Over 16-17 months average weight
increased from 20 g to 1365.5 g, to a maximum of 2200 g. Mean fork
length was 58.6 cm. Food in the first phase was a concentrate of
ground carp and kilka but in later phases natural foods such as
benthos and fry were used. The preliminary results indicate economic
feasibility for cage culture (Iranian Fisheries Research and
Training Organization Newsletter, 7:4-5, 1995; Annual Bulletin
1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 46-47, 1995).
Kamali and Farabi (2005) showed that juveniles weighing
20 g or more adapted better to concentrated feed in fibreglass tanks. Mohseni
et al. (2004) studying growth rate, food conversion ratio and survival in
fingerlings held in fibreglass tanks found these factors to be dependent on
higher feeding frequencies (3, 5 and 8 times per day). Akrami et al.
(2005) found Cladocera were the primary prey of fingerlings in earthen ponds
with chironomid larvae and ostracods secondary prey, and the copepod Cyclops
an occasional prey. Condition factor and growth decreased as weight and length
of fingerlings increased. Growth was was positively allometric (b>3). Mohseni et al.
(2005) found growth of fil mahi was better in fibreglass tanks but later in the
rearing process the trend reversed and earthen tanks showed a better condition.
Mohseni et al. (2006) examined the effects of feeding rates (1, 2, 3 and
4% of biomass) on various factors for fish weighing an average 867.9 g and fed
for 100 days in fibreglass tanks. Increase in feeding ratio directly increased
daily food consumption and negatively affected the feeding efficiency, food
conversion ratio, specific growth rate and price index. When fish were given 2%
of the body weight, one unit of meat was produced from 1.92 units of food. A
second trial with feeding rates 0.75, 1.5, 2.5 and 3% took place with fish
weighing 2096.1 g and fed for 125 days. Feeding with 0.75% produced one unit of
meat per 1.82 units of food consumed. Fatemeh and Armin (2005) studied the
effect of photoperiod on growth in one-year-old fil mahi. Extended day length
had a positive effect on growth rate, specific growth rate, weight and length,
and condition factor. The organophosphate diazinon was studied experimentally by
Khoshbavar Rostami et al. (2006) as to its effects on haematological and
biochemical factors of the blood serum of this fish. Falahatkar et al.
(2006) experimented with various levels of vitamin C as a diet supplement and
recommended 200 mg kg-1 during the first weeks of growth and development.
Mohsen et al. (2008) found that diets supplemented with L-carnitine
improved growth rate, feed utilisation and stimulated protein-sparing effect. L-carnitine
is a vitamin-like compound found naturally in fishes and is involved
transporting long-chain fatty acids in metabolism. Ahmadifar et al.
(2009) found that dietary Ergosan had some positive effects on growth and
haematological parameters (Ergosan comprises algines and
polysaccharides known to strengthen the full range of natural defence systems in
fish).
Nezami et al. (2000) maintain that despite artificial spawning and fingerling
production, restoration of this species in Iran was not very successful.
Abdolhay et al. (2006) report on 17 adults caught in 1998 of which 10
fish were injected with hypophysis extract and produced 1.08 million fingerlings
while in 2002, 29 were caught and 21 produced 2.4 million fingerlings. Azari Takami (1999) cites production of 300-350 kg/ha in 40
days with 106,000 fingerlings produced per 15 females in 40 days with a release
weight of 10-15 g. Spawning fish were captured in the sea as they no longer
migrated into Iranian rivers and propagation results were not as good as in
previous years (420-587 kg/ha in 25 days, 690,000 per 2 females, release weight
5-8 g). About 1 million fingerlings were released into the Caspian Sea.
Iranian releases of fingerlings were 687,400 (1988), 406,100 (1999), 1,900,919
(2000), 700,000 (2001), 2,403,794 (2002) with ca. 4 million proposed for 2003
(CITES website). The annual release of fingerlings weighing 3-5 g into the Caspian from Iran is
1-2 million fish and some of these are tagged for future studies (Iranian
Fisheries Research Organization Newsletter, 39:1, 2004). In 2001, 8 females and 12-14 males were caught in Gilan, about
half of which could be used as broodstock at the Shahid Beheshti hatchery (Raymakers, 2002).
Fingerlings have been raised in fibreglass ponds in brackish and fresh waters
in Iran (Iranian Fisheries Research Organization Newsletter, 35:3, 2003;
H. Pouralifashtomi in the 5th International Symposium on Sturgeon, Iranian Fisheries Research
Organization, 9-13 May 2005, Ramsar; Pouralifashtomi, 2006).
Growth was better in brackish water when fed diets containing 45% protein and
12.8% fat. Studies of cultured male fil mahi show that
they attain maturity at 8-10 years, earlier than fish in natural habitats,
indicative of their potential for caviar production under culture conditions (Iranian
Fisheries Research Organization Newsletter, 39:3, 2004).
Cultivation of this species in earthen ponds in the central
Iranian desert at Bafqh near Yazd has been carried out. After three months at 24ºC
and a salinity of 12.5‰ the fish reached 250 g with a survival rate of
60%, after six months at 16ºC and 11.0‰ the fish weighed 1100 g with a survival rate of 96%. Growth was better during
the cold season (Iranian Fisheries Research Organization Newsletter, 34:3; 36:4, 2003).
Further work
See under A. gueldenstaedtii.
Sources
See under family above. Babushkin (1964) gives a general review of the biology and catch of this species.
Iranian material: None.
Comparative material: CMNFI 1986-0147, 1, ca. 305 mm total length, Romania, Black Sea at Sulina (45°09'N, 29°41'E).
Pseudoscaphirhynchus
Nikolskii, 1900
Pseudoscaphirhynchus kaufmanni
(Kessler, 1877)
This species is reported from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan by Shakirova and Sukhanova (1994) and
Sal'nikov (1995). It may eventually be found in the Tedzhen River and
Caspian Sea basins of Iran. No Iranian record.
Anguillidae
Back to Contents
Freshwater eels are found world-wide in temperate to tropical
waters except for the south Atlantic Ocean and the whole eastern
Pacific Ocean. There are 15 species with 1 found in Iran.
The family is characterised by the elongate body; numerous
vertebrae; small elliptical scales which are difficult to see
casually; a small and elliptical gill opening just in front of the
pectoral fin base; very long dorsal and anal fins confluent with a
reduced caudal fin; a terminal mouth with the lower jaw projecting a
little; small teeth in several rows on the jaws and palate; the dorsal
fin origin well behind the pectoral fin level but in front of the anus
level; no pelvic fins; and by a suite of osteological characters. The
term eel-like is based on the body shape of freshwater eels and
includes the muscular slipperiness associated with this fish and its
mucus-producing skin.
The life cycle of Atlantic eels was unknown until Johannes Schmidt
published his 1922 study based on years of collecting. Where the
adults went on their seaward migration and where the elvers ascending
rivers came from were a mystery. These eels are catadromous, living in
fresh water but migrating to the sea to spawn and die. In the North
Atlantic Ocean spawning occurs in the Sargasso Sea. The young eels or
leptocephali (= thin head larvae) are distinctive, being transparent
and leaf-like. A newspaper can be read through the body of a
leptocephalus. In this form they drift to the shores of America and
Europe, transform into elvers with the more familiar eel-shape and
move into rivers and lakes to feed and grow. Some scientists believe
that the European eel is not a distinct species but merely American
eels (Anguilla rostrata (Le Sueur, 1817)) which develop in
cooler areas of the Sargasso Sea and are carried by different ocean
currents to the shores of Europe. Differences between the American and
European eels overlap and include such characters as vertebral number
which is known to vary with development temperature. Recent studies
using mitochondrial DNA (mt DNA) showed no genetic divergence among
samples of American eels along 4000 km of North American coastline
reflecting a single spawning population. However European eels had a
distinct mtDNA genotype and the conclusion to be drawn is that
American and European eels have separate spawning sites such that
larval dispersal ends up on different continents. The mt DNA
differences are marked but do not prove species distinction as this
level of distinction is known to occur among fishes which are a single
species (though some authorities would argue that these
"single" species are themselves complexes of two or more
species). However Icelandic eels seem to be hybrids between the two
putative species. All other evidence (vertebral and other counts, body
proportions, biology, electrophoresis) suggests that the American and
European eels are the same species but have different spawning sites.
The biology of eels is based almost entirely on the freshwater
phase of their life. Adults in fresh water develop large eyes, the gut
degenerates and coloration changes in preparation for the migration to
the Sargasso Sea. Adults were only caught in the deep ocean, at nearly
2000 m near the Bahamas, in 1977. The Sargasso spawning ground is
deduced from collections of larvae across the Atlantic Ocean - the
smallest and youngest larvae are found around the Sargasso Sea. The
spawning grounds are at about 400 m, at a 17°C
temperature and in saltier water than usual sea conditions according
to some authors but since spawning adults have never been caught this remains dubious.
The theory advanced by D. W. Tucker in 1959 maintained that European
eels lack the energy resources in their migratory, spawning phase to
reach the Sargasso Sea 7000 km from Europe. They are presumed to be
following an instinct to head out to sea, dating from an earlier
geological age when the Atlantic Ocean was narrower before the
separation caused by Continental Drift. All European eels die at sea
and Europe is restocked by larvae drifting there spawned from American
parents. The American populations are closer to the Sargasso and can
make the journey easily. Differences between American and European
eels are merely the consequence of different environmental regimes in
different parts of the Sargasso. This theory has not found general
acceptance but, if true, means that all European eels can be harvested
for food without depleting stocks. Eels are valued as food,
particularly in Europe and Japan,
Hochleithner (2010) gives a review of eel biology and aquaculture.
Genus Anguilla
Schrank, 1798
Characters of the family also serve for the genus.
Anguilla anguilla
(Linnaeus, 1758)
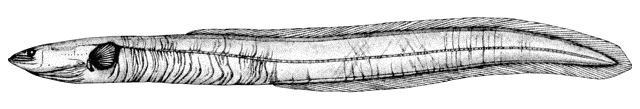
Common names
marmahi-ye ma'muli (= common snake fish), مارماهي
مهاجر (= marmahi mohajer, meaning migrating
snakefish), marmahi-ye haghighi.
[rechnoi ugor' or river eel in Russian; European eel].
Systematics
No major synonyms. Muraena anguilla was originally described from Europe.
Key characters
The eel shape is characteristic along with the long and spineless
dorsal and anal fins and the absence of pelvic fins. The Caspian
lamprey, Caspiomyzon wagneri, has a similar shape but lacks
pelvic fins, has seven gill openings in a row behind the eye, and has
a round suctorial mouth.
Morphology
The scales are small, elliptical in shape and embedded in the skin.
The lateral line is distinct. Some fish in any population may have a
broad or a narrow head. Fish approaching sexual maturity develop very
large eyes, the olfactory organs atrophy, the lateral line becomes
more conspicuous, a tougher and thicker skin develops, and the colour
changes as detailed below.
Dorsal fin rays 243-275, anal fin rays 175-249 and pectoral fin
rays 15-21. Vertebrae 110-119, usually 114-116. The chromosomes are
2n=38 (Klinkhardt et al., 1995).
The leptocephalus and elver stages are not found in Iranian waters
and are not described here (see below under Reproduction).
Sexual dimorphism
At the silver eel stage males are 29-40 cm and females 38-130 cm long.
Colour
Colour is variable but the back is usually grey-brown, olive-brown,
brownish-green, yellowish or black and the belly is whitish to
yellowish. The dorsal fin is dark, other fins are yellowish. The iris
is yellow. This yellow or green eel stage changes to the silver or
bronze eel at maturity. The mature fish is darker on the back, has
silvery or bronze to coppery flanks and belly and a black pectoral fin.
Size
Attains 2.0 m, but rarely, and 12.7 kg, possibly 14.0 kg. Iranian specimens up to 1.0 m long have been caught near Bandar Anzali (Firouz, 2005).
Distribution
Occasionally caught in Iranian waters (P. Walczak, pers. comm., 1978; Holcík
and Razavi, 1992). Holčík and Oláh (1992) report single specimens from the Anzali Mordab (= Talab) and
its exit streams and near Bandar-e Anzali. Also reported generally from the southeast
Caspian Sea, southwest Caspian Sea and south-central Caspian Sea (Kiabi
et al., 1999). Reported from the Safid River and Anzali Talab by Abbasi
et al. (1999). Berra (2001) does not show the Iranian distribution because the fish are introduced.
Elsewhere it is common in Europe including the Mediterranean Sea,
and east to the Black Sea although few young eels migrate naturally as far as this.
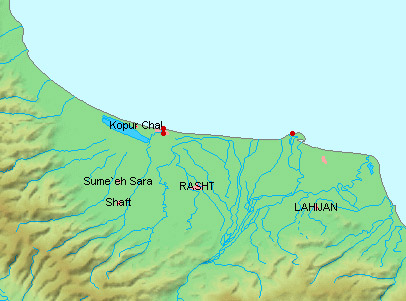
Zoogeography
An exotic species in Iranian waters, arriving there through the
influence of mankind. This species established itself in the Caspian Sea after the
opening of the Volga-Baltic waterway and the introduction of larvae
from France and England and was recorded from fresh waters in
Azerbaijan by Abdurakhmanov and Kuliyev (1968).
Habitat
Eels are caught by fishermen between Bandar-e Anzali and the mouth
of the Safid River in beach seines, in the Anzali Mordab and are
probably present in rivers along the Caspian coast. The catch appears
to be increasing (Holčík and Razavi, 1992). About 10-40 specimens are caught annually weighing
up to 3 kg (Holčík and Oláh, 1992). In Europe freshwater populations show considerable
migratory movements in summer and this helps explain their widening
distribution in the Caspian Sea basin. However, Abbasi (2005) states that the
population has decreased.
Eels will live in almost any kind of water over a wide range of
temperatures; warmer waters being preferred as long as oxygen is not
low. Elvers flourish in sandy areas where grain size is 0.25 mm or in
gravel areas where size is 2 mm or larger, the former for burrowing,
the latter for insinuating between. Adults also prefer a substrate
that can be burrowed into during the day, emerging at night. The
burrows are usually at a 45° angle and
the eel sticks its head out at this angle too. Eels show some
migratory habits within fresh water, moving between summer and winter
areas, over a distance of a few metres to tens of kilometres.
In Europe, the silver eels begin to migrate to the sea in late
summer and autumn on their journey to the Sargasso Sea where they
arrive the following spring. They travel at about 2 km/hour,
particularly at night when the moon is at or a few days after the last
quarter and light levels are low. Iranian fish cannot migrate, being constrained by distance and lack of ready
access to the open ocean.
Age and growth
Eels generally begin to mature only at sizes above 30 cm long.
Females grow much larger than males and usually begin to mature at 54
cm or longer. Maturity is actually attained after leaving European
waters en route to the Sargasso Sea. Eel larvae do not all
metamorphose at the same age (this can vary from 1 to 6 years) with
subsequent effects on age at the same length. In addition, growth
varies widely with the habitat and available food supply. Fish of the
same length often have very different weights. Life span is up to a
reputed 88 years based on a captive specimen.
Food
Eels are principally nocturnal but feed both at night and during
the day. Food includes almost any edible item and includes fish spawn,
small fishes, and larger dead fish which have a mouthful of flesh torn
off by a rapid rotation along the long axis of the eel body. Food
includes insect larvae and algae but fishes, worms, crustaceans and
molluscs are the most important items in order. In the southern
Caspian they have been reported to eat gobies (Gobiidae) and Rutilus
sp. in November, suggesting that feeding continues late in the year in
contrast to other waters where they dig into sand or silt and
hibernate (Abdurakhmanov and Kuliyev, 1968). Eels will lie buried in
mud or gravel with just the head projecting, seizing by a sudden
strike any food item passing by. Eels will feed on commercially
important species such as salmonids and crayfishes. They are reliably
reported to even leave the water and enter fields, presumably to feed
on slugs and worms.
Reproduction
This has not been observed in the wild but under artificial
conditions eels are promiscuous and fertilisation is external. The eel
is believed to spawn in the Sargasso Sea at 100-200 m depths off the
coast of America after a long migration from Europe. Spawning takes
place at the beginning of March. Mature females contain 3 million eggs
per 1 kg body weight. The ovary is a rosy-pink because of numerous
blood vessels. The pelagic eggs are 1.2 mm in diameter. The eggs
develop into a distinctive leptocephalus larva which has a leaf-like
shape quite unlike the adult eel. During its leptocephalus phase, the
eel drifts on ocean currents and actively swims from the American side
of the Atlantic, arriving in Europe in its third summer. It is now
fully grown and 7.5 cm long. The larva gradually transforms into the
elver at depths of 1000 m off the coast of Europe. The elver is a
eel-shaped and transparent and reduces in length and weight during the
autumn when it does not feed. The elvers begin to migrate into rivers
and lakes in Europe in winter. They are regarded as young eels once
they begin to feed and are fully pigmented.
Parasites and predators
There is a heavy toll on elvers which are taken on the migration
into rivers and lakes by a wide variety of fish and birds. Adults are
eaten by large fishes including larger eels and by birds such as
herons and cormorants. A large variety of parasites have been reported from eels.
Economic importance
Not used in Iran for food, probably because its minute scales make
it appear scaleless, and in any case the annual catch is only about
40-60 specimens (Holčík and Razavi, 1992). It is of considerable economic importance in Europe
where annual catches have reached 22,000 tonnes. The 1981 catch in
Turkey, for example, was 374 tonnes. This species is also farmed quite
extensively. The flesh has a high fat content and the eel is often smoked for sale.
The blood of this fish is poisonous but the poison is destroyed by
cooking. Fresh eel blood should never be ingested; a dog injected with
eel serum died within one minute. Symptoms include diarrhoea, bloody
stools, nausea, vomiting, frothing at the mouth, skin eruptions,
cyanosis, apathy, irregular pulse, weakness, numbness, paralysis,
respiratory distress, and death. Severe inflammations will result if
the blood touches the eye or tongue.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria,
as food, for sport and in textbooks.
Conservation
The peculiar migratory behaviour of this species prevents spawning
in Iranian waters and all stocks must be replenished through migration
from European waters or by artificial introductions. As an exotic
species, no conservation measures are required.
Critically endangered in Turkey and throughout its range (Fricke et al.,
2007).
Further work
The spread of this species in Iran should be tracked and its
numbers assessed. Eels can attack commercial fishes trapped in nets
and this should be monitored as a potential loss to fisheries. This
species has potential commercial importance for fish farming although
numbers are too low in Iran to provide adequate supplies to stock a
fish farm.
Sources
There is little information on this species in Iran because of its
scarcity and general biology is taken from Bertin (1956), Tesch
(1973), Sinha and Jones (1975), Deelder (1984), and Hoestlandt (1991).
Clupeidae
Herrings, shads, sardines, pilchards and menhadens are
moderate-sized fishes, usually less than 25 cm long, found in warmer
marine waters with some species anadromous or permanent freshwater
residents. There are about 57 genera and 188 species world-wide (Nelson, 2006), with 8 species in
the Caspian Sea and 1 commonly found in Persian Gulf drainages. Some other species are
known to enter rivers in southern Iran (see Marine List in Checklists in Introduction).
The diversity of this family in the Caspian Sea is seen in the number of subspecies which
have been described, rather than in genera. At the species level there are several endemics.
Curiously, the species and subspecies in the Caspian are generally
of larger size than their relatives in the Black Sea basin. These
observations are attributed to the variable environment in the Caspian
Sea over time, with repeated changes in salinity and temperature which
the fish could not avoid. Black, Mediterranean and Atlantic species
lived under more stable conditions and could, in any case, retreat
from lowered temperatures for example. In addition, the Caspian Sea
clupeids lacked the competitors which entered the Black Sea from the
Mediterranean and Atlantic and some (Clupeonella spp., Alosa
caspia) could occupy the pelagic, planktivore niche taken up by
other species in the Black Sea. There are no other pelagic fish but
these herrings in the stable salinity areas of the Caspian Sea.
These fishes usually have modified scales on the belly forming
abdominal scutes with a saw-like edge. Most species have 2, long,
rod-like postcleithra. The lateral line is usually absent or on only a
few scales. Silvery cycloid scales are easily detached and are found
only on the body. The mouth is usually terminal with jaws about equal
in length. Teeth are small or absent but gill rakers are long and
numerous for sieving plankton. Fins lack spines and there are no
barbels. There is no adipose fin. The pectoral and pelvic fins have a
large axillary scale. The caudal fin is deeply forked. The eye is
partly covered by an adipose eyelid. The flesh is particularly oily
and is highly nutritional.
Members of this family often form immense schools in surface waters
of the ocean and the Caspian Sea where they feed on plankton.
Schooling is an anti-predator device making it difficult for a
predator to pick out an individual from a tight mass of fish. There is
also a "sentry effect" where awareness is increased by the
presence of many fish. The school is maintained by a balance between
visual attraction and lateral line stimulus repulsion. Herring can
feed on the smaller plankton, less than 300-400 µm, at night by
filter-feeding but during the day can also use particulate feeding. In
the latter, they select larger plankton using the area temporalis, a
specialised ventro-posterior region of the retina which improves
vision as herring approach food items from slightly below.
Herring are easily caught and are extremely valuable to commercial
fisheries. They are the most important fishes economically, both as
food for man and also for many other commercial fish species. Wars
have been fought over fisheries for herrings. In one year, members of
the herring family made up 37.3% of all fish caught in the world. Some
are used for fish meal, as fertiliser and as an oil source. The
1994-1995 catch of clupeids in the Iranian Caspian was 98.3 tonnes by
beach seine and 671.5 t by gill nets, a decrease of 200 t in
total over the previous year's catch (Iranian Fisheries Research
and Training Organization Newsletter, 10:4-5, 1995)(but see later
under Clupeonella where catch is much higher). The Caspian Sea shads
account for about 35% of total inland production in Iran which was
117,300 t in 1995 (Bartley and Rana, 1998). These fish are used
in a high value form as frozen whole consumer packs, as fish meal for
poultry and in aquaculture, and in canning (Food and Agriculture
Organization, Fisheries Department, 1996).
The catch of "sprats" (Clupeidae) in Azerbaijani waters
is near extinction through poor fishery management according to Golub (1992).
Major sources for the biology and systematics of Caspian clupeids
remains Svetovidov (1952), now inevitably dated but not yet updated,
Whitehead (1985) and Hoestlandt (1991). There has been no recent,
careful systematic and taxonomic study of these species in the Caspian Sea basin
and extensive new material was not available for examination here.
Genus Alosa
Linck, 1790
The Caspian species of Alosa were formerly placed in the
genus Caspialosa Berg, 1915. Svetovidov (1952) synonymised the
genus Caspialosa Berg, 1915 with Alosa. There are 5
species in Iranian waters and the Caspian Sea as a whole but numerous
subspecies have been described. Alosa species are also found in
the Black Sea, Mediterranean Sea and Atlantic Ocean.
Often distinguished by gill raker counts which in any case overlap,
the various subspecies are difficult to identify. Morphometric
characters are of little help and Zamakhaev (1944) points out that
some named taxa are merely different age groups. This problem is
commented on further in the Species Accounts.
Caspialosa suworowi (Berg, 1913) (also spelt suvorovi
in the literature) has been used for hybrids of various Caspian
herrings and is not a valid species (Berg, 1948-1949). The holotype is
in the Zoological Institute, St. Petersburg under ZISP 15927 (Svetovidov,
1952; Eschmeyer et al., 1996).
Alosa species are distinguished from sympatric Clupeonella
species by larger size (up to 75 cm total length compared to 20 cm), a
large mouth, a black spot on the flank behind the operculum and
sometimes a row of such spots, an elongate scale or ala at the upper
and lower base of the caudal fin, a notch at the mid-line of the upper
jaw and by the last two anal fin rays not being elongated.
Caspian Sea species have a laterally compressed belly with 29-36
spiny scutes running from the throat to the anal fin; the dorsal fin
origin is closer to the snout tip than the caudal fin base; the dorsal
fin lies in a groove formed by enlarged scales; scales are easily
detached; the pelvic fin origin lies below or slightly posterior to
the dorsal fin origin; teeth are usually present on the jaws, roof of
the mouth (on the palatine bone and always on the vomer bone), and on the tongue;
the opercular bone is distinctly striated; eggs are demersal,
semi-pelagic, and lack an oil globule; gill rakers highly variable in
shape and number (18-180); dorsal fin branched rays 11-16, anal fin
branched rays 10-21, scales in lateral series 49-60, and vertebrae 43-55.
Afraei Bandpyi et al. (2004) examined Alosa species from
Mazandaran and Golestan provinces and found the following distinguishing
characters:-
| Species |
Gill rakers |
Ratio of eye diameter to total length (%) |
| A. braschnikowii |
20-40, mean 30.9 |
2.9-5.8, mean 4.7 |
| A. caspia |
110-125, mean 118.3 |
5.7-7.5, mean 6.2 |
| A. pontica (=
kessleri) |
60-73, mean 66.8 |
4.3-6.5, mean 5.5 |
| A. saposchnikowii |
20-48, mean 32.8 |
6.0-9.3, mean 7.3 |
The general Farsi name for these fishes is shag mahi or zalun (both
in Gilaki).
These herrings migrate from the north Caspian Sea to overwinter in the
central and southern parts, returning north in the spring.
Alosa braschnikowii
(Borodin, 1904)
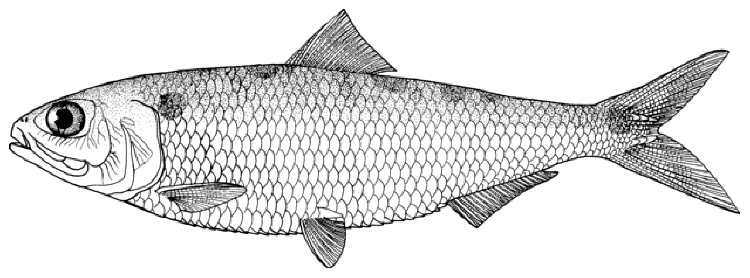
Common names
shagmahi, shagmahi-ye Khazari.
[dolkii siyanayn, Agraxan siyanayi, Sara siyanayi, irikoz siyanak,
hasangulu siyanayi, agbas siyanak, all in Azerbaijan; Caspian marine
shad, Kurinskaya sel'd or Kura herring, poloschataya sel'd or striped
herring, Agrakhanskaya sel'd or Agrakhan herring, bol'sheglazaya sel'd
or bigeye herring, dolginskaya sel'd or dolginka herring, belogolovaya
sel'd or whitehead herring, Astrabadskaya sel'd or Astrabad herring,
sel'd-gonets or driver, zheltospinka or yellow-back, Gasankulinskaya
sel'd or Gasan-Kuli herring, kiselevichevskaya sel'd or Kiselevitch
herring, Krasnovodskaya sel'd or Krasnovodsk herring, vostochnaya
sel'd or eastern herring, obzhorka or glutton, Sarinskaya sel'd or
Sara herring, maiskaya sel'd or May herring, Brashnikovskaya sel'd or Brashhnikov's shad, all in Russian].
Systematics
Originally described as Clupea caspio-pontica var. Braschnikowii.
Reshetnikov et al. (1997) revert to the original double "i"
ending to the specific name. A lectotype from Fort Shevchenko (Aleksandrovsk) is in
the Zoological Institute, St. Petersburg (ZISP 13051) and
paralectotypes were designated by Svetovidov (1952)(ZISP 13051). Clupea
caspio-pontica is an unneeded new name according to Eschmeyer et al. (1996).
Alosa braschnikowii is regarded as a subspecies of Alosa
caspia by some authors. Clupeonella leucocephala Berg, 1913
from Sumgait and Gyurgenchai, Azerbaijan is a synonym (as Caspialosa
brashnikovi leucocephalia (sic) it is listed as a synonym
of C. b. grimmi in Mikhailovskaya (1941)), as is Caspialosa
caspia nigra Kisselevitsh, 1923 from the Caspian Sea opposite
Dzambai (the material also included specimens of Alosa
saposchnikowii) (Whitehead, 1985; Eschmeyer et al., 1996).
Alosa braschnikowii has 9 subspecies in the Caspian Sea
(including Alosa curensis (q.v.) the Kura or striped herring),
namely agrachanica (Mikhailovskaya, 1941) (author also spelt
Mikhaylovsky or Mikhailovsky; dated 1940 in Eschmeyer et al.
(1996) here and below but 1941 on the paper itself and in Svetovidov
(1952) and Berg (1948-1949); species also spelt agrakhanika in
Berg (1948-1949); Caspialosa brashnikovi morpha elata is
a synonym according to Mikhailovskaya (1941)), the Agrakhan herring; autumnalis
(Berg, 1915), the bigeye herring; braschnikowii (Borodin, 1904)
(also spelt brashnikovi in Svetovidov (1952) and Berg
(1948-1949)), the dolginka herring; grimmi (Borodin, 1904), the
whitehead or Astrabad herring, driver or yellow-back; kisselevitshi
(Bulgakov, 1926) (spelt kisselevitschi on the plate in Bulgakov
(1926), kisselevitschi in Mikhailovskaya (1941), kisselevitshi
in Svetovidov (1952) and Whitehead (1985) and kisselewitschi in
Berg (1948-1949)), the Gasan-Kuli or Kiselevitch herring; nirchi
(Morosov, 1928)(author also spelt Morosow in Mikhailovskaya (1941) and
Morozov in Eschmeyer et al. (1996)) (with Caspialosa
brashnikovi kenderlensis Budamshin, 1938 from Kendyrli Bay as a
synonym in Svetovidov (1952) and Berg (1948-1949)), the Krasnovodsk
herring; orientalis (Mikhailovskaya, 1941), the eastern herring
or glutton; and sarensis (Mikhailovskaya, 1941), the Sara or
May herring. Caspialosa brashnikovi derzhavini Tarasevich, 1946
described from the Caspian Sea near the Apsheron Peninsula, Azerbaijan
may be another subspecies. Caspialosa kiselevitschi morpha elata
Morozov, 1928 from the Caspian Sea, Krasnovodsk Bay, Turkmenistan is
an infrasubspecific taxon and its availability and validity as a taxon
have not been examined (Eschmeyer et al., 1996).
This high number of subspecies is an indication of the populational
variation of this shad and not all subspecies may be valid. A modern
revision is required to assess this problem. In light of this
uncertainty and the lack of adequate sample sizes to determine which
of the subspecies occurs in Iranian waters or which taxa are valid,
reference is made here mostly to the species level. Additionally, it
should be noted that hybrids between the various subspecies, and
between this species and other species, do occur to complicate matters even further.
The neotype of Caspialosa brashnikovi agrachanica
was designated by Svetovidov (1952) as the specimen described by Berg
as Caspialosa brashnikovi m. elata taken in front of the
Sulak River mouth, Agrakhan Bay and housed in the Zoological
Institute. St. Petersburg under ZISP 7334.
The neotype of Caspialosa braschnikowi autumnalis was designated by Svetovidov (1952) as a
specimen 26.9 cm long from the eastern shore of the Caspian Sea at
Gasan-Kuli (just north of the Iranian border in Turkmenistan) caught
on 8 April 1948 and housed under ZISP 31749.
The lectotype of Clupea curensis from the Kura River estuary
is under ZISP 13984 with many paralectotypes as established by
Svetovidov (1952) (Eschmeyer et al., 1996).
The neotype of Caspialosa kisselevitshi is also
from Gasan-Kuli caught on 30 June-1 July 1926 and was housed in the
Faculty of Zoology, Central-Asian State University (Sredne-Aziatskogo
Gosudarstvennogo Universiteta), Tashkent.
The neotype of Clupea caspio-pontica var. grimmi
was designated by Svetovidov (1952) as a specimen 34.0 cm long found
at Ashur-ade (= Ashuradeh) near Astrabad Bay (= Gorgan Bay or Khalij-e
Gorgan) on 23 April 1903 is under ZISP 13045.
The neotype of Caspialosa nirchi as designated by
Svetovidov (1952) is from the southern part of the Caspian Sea
opposite North Cheleken Spit and is under ZISP 31780.
The neotype of Caspialosa brashnikovi
orientalis as designated by Svetovidov (1952) is from the southern
part of the Caspian Sea opposite Kara-Ashly and is under ZISP 32187.
The neotype of Caspialosa brashnikovi sarensis from Sara Island is under ZISP 32184 as
established by Svetovidov (1952).
Key characters
Characterised by a relatively elongate and rounded body likened to
a "herring" shape, not as deep as in some related species
which are likened to a "shad" shape. Total gill rakers 18-49
and short (about equal to gill filaments in length, sometimes
shorter). Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-5 unbranched and 12-15, mostly 14, branched rays,
anal fin with 2-4, usually 3, unbranched rays and 10-20, mostly 18,
branched rays. Scales in lateral series 51-54. Teeth are
well-developed on the jaws, tongue and roof of the mouth.
The accompanying table summarises characters of the subspecies and
is taken from Svetovidov (1952) and Mikhailovskaya (1941) but
identification to subspecies should be done with the keys from these works. Some of the
characters used in the keys are not in the table as they do show
individual variation and are difficult to summarise. An example is the
nature of the gill raker (thin, thick, blunt, pointed, bent, straight,
curved, branched, broken off, forked, swollen at the tip, etc.);
another is the degree of protrusion of the lower jaw.
The subspecies grimmi is quite specialised in association
with its benthic mode of life, feeding mostly on gobies (Gobiidae). It
has a unique character in the well-developed callus on the tip of the
lower jaw which adults acquire from rubbing the jaw on the sea bed
while feeding, gill rakers are low in number as fine food is not
taken, and the tips are broken off, broadened, and split owing to
abrasion, and the rakers on the lower arch are reduced in number so
the first raker is far from the tongue base. The subspecies nirchi
is similar. In contrast, the subspecies kisselevitshi has a
high gill raker count, rakers are pointed and not split at the tips,
and the first raker is close to the tongue base. This species lives in
surface waters feeding on Clupeonella, Atherina,
shrimps, gammarids, and gobies (Gobiidae).
|
Character /
Subspecies |
Gill rakers (mostly) |
Pectoral fins as % body length |
Vertebrae |
Head length as % of body length |
|
agrachanica |
20-46
(28-33) |
13.1-15.6 |
47-54 |
22.6-25.2 |
|
autumnalis |
21-37
(28-30) |
16.4-19.9 |
45-53 |
26.0-29.2 |
|
braschnikowii |
24-47
(30-33) |
14.3-16.7 |
48-55 |
23.5-26.6 |
|
curensis |
26-54 |
17.3-18.8 |
47-52 |
25.7-26.5 |
|
grimmi |
18-28
(20-22) |
12.9-15.2 |
45-52 |
22.9-26.4 |
|
kisselevitshi |
29-49
(36-40) |
13.9-16.8 |
43-53 |
24.2-26.9 |
|
nirchi |
20-31
(23-26) |
10.9-14.7 |
48-52 |
23.4-26.3 |
|
orientalis |
20-35
(27-32) |
13.5-18.0 |
45-53 |
25.0-27.8 |
|
sarensis |
20-33
(24-27) |
14.1-16.2 |
45-53 |
23.8-26.6 |
Sexual dimorphism
None reported.
Colour
The back and top of the head are dark with a green or blue tint and
may be grey-green. Some subspecies are paler in colour with a grey or
grass-green back and pale flanks, nirchi has a whitish
blue-green head, light grey back with a slight greenish tint, and
lower jaw and pectoral fins light, while grimmi is also quite
pale with a grey-blue back and top of the head and whitish anterior
head and pectoral fins. There is a dark spot behind the operculum but
no series of spots along the flank in most subspecies, except in rare
cases when there may be up to 7, occasionally 12-13. The subspecies grimmi
regularly has a row of diffused, grey spots almost merging into a
stripe, and nirchi occasionally. Pectoral fins are dark on some
subspecies (braschnikowii, sarensis, kisselevitshi),
pale or whitish on the others, although there is confusion in the
literature over this, perhaps indicative of individual variation (cf. sarensis
in Mikhailovskaya (1941)). The back and upper part of the head may become a deep
black at spawning. The flanks and belly are silvery.
Size
Attains 50 cm standard length but average lengths are about 27-34 cm.
Distribution
All the Caspian subspecies are found widely distributed in the sea
but chiefly in the south in winter, moving north to spawn in spring.
The subspecies sarensis is reported from the Lenkoran coast and
from southwest of Gasan-Kuli (in Turkmenistan just north of the
Iranian border), the subspecies orientalis from Gorgan or
Astrabad Bay, autumnalis from coastal waters at Gasan-Kuli, kisselevitshi
from Astara and Gasan-Kuli, and grimmi from Astara and Gorgan Bay.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
In winter this species moves into deeper water towards the Iranian
coast. In March it approaches coastal waters (Vetchanin, 1984)
including brackish waters but does not enter fresh water. It never enters rivers
in the south of the Caspian Sea (Jolodar and Abdoli, 2004). Salinities
up to 47.6‰ are survived by this species. Spawning and feeding
grounds are in the north Caspian for some populations but others live
permanently in the south Caspian Sea and are of smaller size. The
subspecies kisselevitshi, for example, lives off Gasan-Kuli in
winter at depths below 25 m, not migrating or feeding. In March they
move north to feed and then return south to spawn but lives almost
entirely as a pelagic species in the southern Caspian Sea. Knipovich
(1921) reports this species from depths of 80-98 m in Iranian waters. The
density of this species increased from east to west in a 1999-2001 study in
Iranian waters (Afraei, 2006). Abdoli and Naderi (2009) list it as from the
southwest, southeast and south-central Caspian Sea in Iranian waters.
Age and growth
Maturity is attained at age 2-5 and life span is up to 10 years,
although this varies with the subspecies. Most south Caspian forms
apparently mature at age 2 according to Svetovidov (1952). Growth
rates also vary between subspecies, orientalis being one of the
slowest growing herrings in the Caspian Sea and reaching 10 years of
age. The catch near Astara of sarensis, for example, is mainly
4-5 year olds but this too varies with the subspecies and also with
the year-class strength. Vetchanin (1992) reported on grimmi
catches from the southeastern Caspian where the average length was
27.8-28.6 cm and the average weight 294-313 g. There is a tendency for
length and weight to fall in catches as the summer progressed, from
April to July. Length and weight are less in southern, compared to
northern, waters.
Afraei (2000) found this species to be the largest Alosa in Iranian
waters on average at 395 mm and 760.3 g. Males predominate at 55.8% in Mazandaran
and 69.4% in Golestan catches. Six age classes were present (1+ to
6+) with the 2+ class being the most common at 28.9% and 6+ the rarest at 8.9%.
Food
Diet in the southeastern Caspian Sea in winter comprises 85% Clupeonella
engrauliformis with some gobies (Neogobius) and shrimps (Vetchanin,
1984). From March to November the diet is dominated by Clupeonella
caspia, Atherina boyeri (= caspia) and shrimps. Juvenile Liza
saliens, Syngnathus caspius, molluscs, crabs and higher
aquatic plants are also recorded along with foreign objects such as
rice husks, pieces of wood, foil, polyethylene, etc. This species is a
cannibal. The more southerly populations examined favour Atherina
boyeri (= caspia) and Neogobius species and some of these populations
favour benthic invertebrates. The subspecies grimmi is the most
benthic one and takes primarily gobies with some molluscs as well as Clupeonella.
Feeding intensity rises sharply after spawning. While some herrings,
like Alosa pontica (= kessleri), feed poorly on their migration, this
species feeds intensively on its spring migration.
The feeding regime altered after the invasion of the ctenophore,
Mnemiopsis leidyi. A shift was observed from 85% Clupeonella
engrauliformis to 65% Atherina boyeri (= caspia). Other fishes were also eaten
including Clupeonella grimmi, C. caspia, Cyprinus carpio,
Liza saliens, as well as Palaemon spp. (Iranian Fisheries
Research Organization Newsletter, 49:2, 2006).
Reproduction
Vetchanin (1984) reports spawning of this species in the
southeastern Caspian Sea north of Iranian waters to begin in early
May, continuing to July as it is intermittent. The subspecies sarensis
spawns along the Lenkoran coast from mid-April to the end of June. The
subspecies orientalis spawns in Gorgan Bay from the end of
March to the beginning of April, spawning schools forming at 17-18°C
or higher. The subspecies autumnalis spawns at the same time
off Gasan-Kuli near the Iranian border with Turkmenistan. The
subspecies grimmi spawns in May-June in Gorgan Bay. The
subspecies kisselevitshi has the latest spawning date, June to
July and even in August off Gasan-Kuli when temperatures exceed 25°C.
Spawning takes place in shallow water (1.8-5.8 m) in the sea over sand
or silt bottoms at 15-18°C (some subspecies and populations at 20-22°C,
others beginning as low as 11°C), and a salinity of 8-13‰. Fecundity is up to 178,400 eggs, average
66,000 per fish. There is no feeding while spawning. Early maturers,
like the south Caspian populations, can reproduce up to 7 times in their life.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
The catch for all species of "Caspialosa" in Iran
varied between 5337 kg and 419,518 kg for the years 1956/1957 to
1961/1962 (Vladykov, 1964). In the Anzali region the catch for the
years 1933/1934 to 1961/1962 varied from 1553 kg to 539,710 kg (Vladykov, 1964).
The catch has been as high as 126,900 centners or 12,690 t in the
sea as a whole for the type subspecies alone (1 centner = 100 kg (Svetovidov,
1952)), taken chiefly in spring. Other subspecies were not fished for
as extensively although kisselevitshi was the most numerous of
the south Caspian forms of Alosa braschnikowii, forming 70% of
the drift net catch.
Conservation
Reputedly depleted in Iranian waters. Kiabi et al. (1999)
consider this species to be data deficient in the south Caspian Sea
basin according to IUCN criteria. Criteria include medium numbers,
medium range (25-75% of water bodies), absent in other water bodies in
Iran, and present outside the Caspian Sea basin. Extinct in Turkey (Fricke et
al., 2007).
Further work
The biology of this species in Iranian waters and the stocks or taxa found
there need to be elucidated.
Sources
Iranian material: CMNFI 1970-0581, 5, 226.0-245.0 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1979-0431, 1, 297.2 mm standard length, Mazandaran, bazaar at Now Shahr (no other locality data);
CMNFI 1980-0126, 1, 245.8 mm standard length Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0150, 1, 222.4 mm standard length, Gilan, Safid River estuary (37º24'N, 49º58'E).
Comparative material: BM(NH) 1938.8.2:1, 1, 245.9 mm standard length,
Kazakhstan, Caspian Sea, Kaidak Bay (no other locality data); BM(NH) 1938.8.2:2, 1, ca. 337.5 mm standard length,
Kazakhstan, Caspian Sea, Kaidak Bay (no other locality data); BM(NH)
1939.2.21:17-18, 2, 285.0-305.2 mm standard length, Caspian Sea (no other
locality data); BM(NH) 1939.2.21:19-20, 2, 222.9-273.4 mm standard length,
Caspian Sea (no other locality data).
Alosa caspia
(Eichwald, 1838)
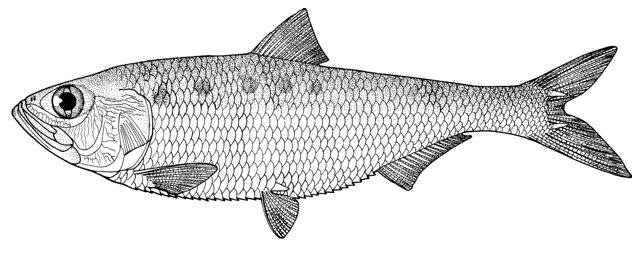
Common names
shagmahi-ye shekambozorg (= big belly herring fish), shagmahi-ye chekameh dar, shagmahi-ye darya-ye
khazar (= Caspian Sea herring fish), شاه ماهي (= shah mahi, meaning king fish),
zalun (in Gilaki), puzanok.
[xazar sisgarini, sara sisgarini in Azerbaijan; Kaspiiskii puzanok
or Caspian shad, severokaspiiskii puzanok or North Caspian shad,
srednekaspiiskii puzanok or Central Caspian shad, il'mennyi puzanok or
il'men shad, Enzeliiskii puzanok or Enzeli (= Anzali) shad, Sarinskii
puzanok or Sara shad, Bakinskii puzanok or Baku shad, Astrabadskii
puzanok or Astrabad shad, all in Russian].
Systematics
Clupea caspia was originally described in Latin from "Hab.
in Caspio mari, meridiem versus" (Caspian Sea, towards the south).
A. caspia has 3 subspecies in the Caspian Sea basin, namely caspia
(Eichwald, 1838) (= North Caspian, Central Caspian, Caspian or il'men
shad); knipowitschi (Iljin, 1927) with natio knipowitschi
(Iljin, 1927) (= Enzeli or Anzali shad) and natio saraica (Svetovidov,
1943) (= Sara or Baku shad); and persica (Iljin, 1927) (=
Astrabad shad). The differences between natio knipowitschi and
natio saraica are small (e.g. gill rakers 122-166 versus
140-150, both averaging 145; vertebrae 43-49 versus 45-51, both with
mostly 47 or 48; growth differences are known, the former grows faster
in the first 2 years of life but the latter reaches a greater size)
and they probably have no taxonomic significance being simply separate
breeding populations. The differences between Alosa caspia caspia
natio caspia (the North or Central Caspian shad) and natio aestuarina
Berg, 1932 (the il'men shad) were found to be based on geography and
growth rate and these names have no taxonomic standing (Svetovidov,
1952). These natio are infrasubspecific ranks and have no validity as names.
Alosa rossica Kessler, 1870 described from the Volga River
is a nomen nudum and is this species. Other taxa now considered
as synonyms of Alosa caspia are Caspialosa caspia salina
Svetovidov, 1936 from Mertvyi Kultuk and Kaidak bays in the northeast
Caspian Sea and Caspialosa caspia kaidakensis Kazancheev, 1936
(spelt kajdakensis in Svetovidov (1952)) from Kaidak, the
latter being in any case a synonym of the former subspecies. Clupeonella
caspia m. elongata Berg, 1913 is also a synonym. Alosa
caspica Yakovlev, 1871 is presumably a misspelling.
Knipovich (1921) records a species, Caspialosa enzeliensis
Iljin, from the southern Caspian Sea which he places as a subspecies
of caspia. I have been unable to locate the original
description of this taxon, which presumably is found in the Anzali
Mordab of Iran. It is probably an unused manuscript name for what
Iljin later described as knipowitschi.
As of 15 July 2007, this scientific name is a Googleblat for this page.
The lectotype of Caspialosa knipowitschi is a specimen 21.2
cm long from Anzali in Iran caught on 15 April 1915 and housed in the
Zoological Institute, St. Petersburg (ZISP 31892). The lectotype of Caspialosa
caspia var. persica is a specimen 147.5 mm long from the
Caspian Sea Bay of Asterabad (= Gorgan Bay or Khalij-e Gorgan) north
of Ashur-ade (= Ashuradeh) at 36°53'N, 53°55'E
caught on 25 April 1904 on the Caspian Expedition of 1904 and housed
in the Zoological Institute, St. Petersburg (ZISP 16413). The
lectotype of Caspialosa caspia knipowitschi n. saraica
is from near Sara Island and is under ZISP 32183. The lectotype of Caspialosa
caspia salina is from Mertvyi Kultuk Bay, 10 km west of Cape
Kizil-kair and is under ZISP 25813. These taxa were designated by
Svetovidov (1952) as none were before or material was not preserved.
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. Total gill
rakers 50-180, variously reported as thin or thick, long (obviously
longer than the gill filaments), and forming a convex outline on the
lower arch. Teeth are poorly developed in both jaws.
Morphology
Dorsal fin with 3-4 unbranched and 12-15 branched rays, anal fin
with 3-4, usually 3, unbranched and 15-20 branched rays. Scales in
lateral series 49-54.
The characters distinguishing subspecies all overlap widely and are
given below after Svetovidov (1952) and Hoestlandt (1991):-
|
Characters /
Subspecies |
Head length as % of body length |
Pectoral length as % of body length |
Vertebrae |
Gill rakers |
|
caspia |
25.5-28.1 |
15.5-18.1 |
45-52
(49-51) |
68-150
(100-140) |
|
knipowitschi |
18.3-24.1* |
16.0-19.1 |
43-51
(47-48) |
120-180
(130-160) |
|
persica |
25.6-27.1 |
16.5-17.7 |
45-51
(47-49) |
50-120
(60-90) |
* The numbers cited in Svetovidov (1952: 256 in the
English version) and Hoestlandt (1991: 128) in the keys to subspecies
do not agree with the numbers on p. 148 and p. 265 respectively in the
species descriptions. The text numbers are used here.
Sexual dimorphism
Females are longer and weigh more than males of the same age.
Colour
The back is blue-green to dark and the flanks
silvery. There is a black spot on the flank behind the upper operculum
margin and sometimes up to 7 spots extending along the upper flank to
a level of the rear of the dorsal fin.
Size
Reaches 28 cm standard length for caspia, to
29.6 cm for knipowitschi, and to 33.8 cm for persica.
Distribution
Found in the Caspian and Black seas. The subspecies caspia
is found mostly in the western half of the Caspian Sea basin but is
the most widely distributed subspecies, found throughout almost the
whole sea. The subspecies knipowitschi is found in the south
near Anzali, Astara and the Baku Archipelago, near the northern shore
of the Apsheron Peninsula in autumn with a few reaching the Gorgan Mordab
in fall and winter; natio saraica is found north of Astara and
spawns near Sara Island, natio knipowitschi spawns in the
Anzali Mordab. The subspecies persica is found in the
southeast, near Gorgan or Astrabad Bay. Holčík and Oláh (1992) report persica from the western basin of the
Anzali Mordab (= Talab) and this species is reported from the Safid River and Anzali Talab as
subspecies persica and from the Anzali Talab as knipowitschi
(Abbasi et al., 1999).
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea, the Anzali Talab and Gorgan Bay in Iranian waters for
both knipowitschi and persica.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
The type subspecies prefers open waters. Caspian shad winter at depths of 30-40 m or more and prefer temperatures not
less than 8-11°C. They rise to surface waters in spring, moving north along the western
shore of the Caspian Sea in waters of about 9-11°C according to Kushnarenko (1986) while Heckman in Hoestlandt (1991) states that
this shad begins to migrate at the end of March at 5-6°C water temperature with a peak at 9-14°C
in mid to late-April, ending in early May. Males migrate in large numbers at the beginning and end of the migration, females in the
middle (Pushbarnek, 1987) while Heckman in Hoestlandt (1991) states that two waves of migration occur, one usually in late April at
7.6-10.2°C comprised of over 80% males and the second in the first half of May at 10.8-14.0°C
comprised of over 70% females. The young, which hatch in the spring, leave the summer feeding grounds before the adults and migrate south
before October-November. Adults follow as temperatures fall. Some populations do not migrate north and spend their whole life in the
southern Caspian Sea. This subspecies will enter fresh waters to spawn in addition to spawning in the open Caspian Sea.
The subspecies knipowitschia prefers water warmer than that of all other Caspian Sea clupeids except for Alosa
caspia persica. Its sea movements are not well known but spawning fish favour waters with freshwater input and some fish enter rivers so
it is classified as semi-anadromous. This subspecies was common in the Anzali Mordab but is now replaced by persica (Holčík and Oláh, 1992).
It is also reported westwards to Astara and eastwards to Gorgan Bay. The winter habitat of persica is unknown. It
is semi-anadromous and remains in the southern Caspian Sea near the shore. From spring to fall this subspecies moves northward along the
eastern Caspian shore towards Krasnovodsk Bay and westwards to the Anzali Mordab.
Age and growth
Pushbarnek (1987) found shad of the type subspecies
up to 7 years of age on the western coast of the middle Caspian Sea.
In the spawning population, the predominant sizes and weighs for males
were 16-21 cm and 60-130 g and for females 18-23 cm and 70-140 g.
Males and females usually mature at 2-3 years although most spawn for
the first time at 3 years. Females grow faster than males. Shad may
spawn up to four times as the period of sexual maturation may continue
for 2-5 years. The age composition of the spawning population is
dependent on year-class strength. First spawners constitute 75.9% of
3-year-olds, 41.7% of 4-year-olds and 23.5% of 5-year-olds. The
Caspian shad is a slow-growing species compared to A. braschnikowii
and A. saposchnikowii, its mean length being 21.2 cm compared
to 32.2 cm and 25.6 cm for the two other species respectively (Shubina, 1981).
Dmitriev (1947) briefly examined the Anzali, Iran
population and found 6 age groups but life span is noted by Heckman in
Hoestlandt (1991) to be up to 9 years. Maturity is attained as early
as 2 years although most fish appear to mature later as most spawners
are 4-5 years old.
The subspecies persica is the slowest growing
of the shad species in the Caspian Sea, sexually mature fish being 13-21 cm
long. Some fish become mature at 2 years of age. Life span is up to 8 years.
The populations of both knipowitschi and persica
are small compared to caspia.
Abbasi and Sabkara (2004c) studied 180 fish from the southeast Caspian Sea coast
of Iran and found fork length to be 103-232 mm, mean 158.8 mm, weight 16-130 g,
mean 52.2 g and age 2-5 years, mean 2.64 years. Afraei (2000) found this species
to be the smallest Alosa in Iranian waters on average at 110 mm and 109 g.
Food
The most intensive feeding period occurs after
reproduction, beginning in June and the highest condition factor is
found at the end of this summer feeding period. Little food is eaten
in winter. Temperature (affecting metabolic rate) and zooplankton
biomass (decreases engender competition with Clupeonella
engrauliformis and other planktivores) are important factors
governing catches of this species (Shubina, 1981). Food is chiefly
copepods, more than 70%, with mysids at 20%, but some phytoplankton
and small fishes are taken. Food in rivers after spawning is mostly
cladocerans and other crustaceans. The above refers to the type
subspecies; food of the other two subspecies is assumed to be similar. The
southeast Caspian Sea fish studied by Abbasi and Sabkara (2004c) fed on
phytoplankton (Rhizosolenia and Sprirogyra) at 4.5%, zooplankton
(Foraminifera, Copepoda, Cirripedia, Bivalvia larvae) at 95.0%, and bony fish
larvae and eggs at 0.5%. The presence of the ctenophore, Mnemiopsis leidyi,
a food competitor reduced the index of fullness and fish growth was reduced.
Abdollapour Bereya et al. (2007) studied diet in fish from beach seines
and gill nets in Gilan. 98.0% of the stomach contents were zooplankton (ostracods,
rhizopods, cladocerans, rotatarians, copepods, cirripedes, mysids, bivalve
larvae and bony fish larvae and eggs), 1.8% was phytoplankton (notably
Rhizosolenia and Spirogyra), and 0.2% was benhthic items (foraminiferans,
sponges, cumaceans, amphipods, insect lavae and palaemonids). Acartia spp.
(copepods) at 83.1% and Balanus (cypris larvae of the cirripede) at 12.9%
were the most abundant. The zooplankton have declined drastically from predation
by Mnemiopsis leidyi, the invasive ctenophore, and the fish have shown a
great reduction in the index of fullness and in growth recently.
Reproduction
Most spawning of the type subspecies occurs in the
north Caspian Sea near the outflow of the major rivers, particularly
the Volga, and the fish overwinter in the south Caspian, migrating
between the two areas (Shubina, 1981). This subspecies spawns
successively, 3 times within a week. Some fish enter fresh water to
spawn. Spawning takes place at the favoured water temperature of
13.8-24.1°C, with mass spawning at 18-22°C,
beginning as early as late April or as late as mid-May and continuing
to mid- or late June. Most eggs are released in the upper 3 m of the
water column. Fecundity reaches 41,000 eggs. The eggs are 1.11-1.38 mm
when ripe but unfertilised and 1.92-2.91 mm in diameter when
fertilised and are semi-pelagic to demersal.
The subspecies knipowitschi spawns in the
Anzali Mordab (and probably the "Chemkhala" River to the
east of the Safid River) in May and June after a spring migration from
the sea, leaving in the fall. Spawning of the subspecies persica takes
place in Gorgan Bay and Holčík and Oláh (1992) suspect from catches of mature and spent fish that it
also occurs in the Anzali Mordab.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator
on this species (Krylov, 1984) and it forms a substantial part of the
diet of Silurus glanis in the Anzali Mordab (Holčík and Oláh, 1992).
Naem et al. (2002) found the monogenean trematode Mazocraes alosae
on the gills of this species in the western branch of the Safid River.
Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Economic importance
The type subspecies was the most important
subspecies in the herring family in the Caspian Sea. It is caught off
the coasts of Dagestan and Azerbaijan for research purposes and
comprises 85% of the clupeid catch (Pushbarnek, 1987), 80-90% of the
Caspian commercial catch (Kushnarenko, 1986). During the 1970s it was
only 2% of the total Caspian fishery production. These herrings
dominated the commercial catch in the Caspian Sea until the 1960s when
commercial fishing was banned except on the western coast of the
central Caspian. Many young of other commercial species were being
killed in the herring fishery, entangled in the gill nets used. Soviet
catches have weighed as much as 75,000 t. This fish is fattier than
other Caspian Clupeidae, except for Alosa kessleri, up
to 18.1% of the body weight. The fat content decreases on the spring migration.
The catch of the subspecies knipowitschi is
of minor economic importance and had been little exploited when
Svetovidov (1952) summarised biology, as the age of captured fish
indicated. About 420 tons (sic, possibly tonnes) were caught in
the Anzali Mordab in 1933 and 1934, but this may be an error in the
report by Vladykov (1964) according to Holčík and Oláh (1992) although Berg (1948-1949) reports 4200 centners for
the same period. The fishing season in the mordab began in mid-April
and ended in mid-June when spent fish appeared. There appears to be no
fishery data on the subspecies persica in the sea. Holčík and Oláh (1992)
report catches of persica, which replaced knipowitschi,
in the Anzali Mordab from the end of April to the beginning of June
but in 1990 this comprised only 5 kg. It is regarded as of inferior quality in Iran.
The Caspian shad is the dominant fish catch in the
Iranian Caspian, comprising 51,000 t in 1994 rising from nothing
a decade earlier (Food and Agriculture Organization, Fisheries Department, 1996).
Robins et al. (1991) list this species as
important to North Americans. Importance is based on its use as food.
Conservation
The stocks of this species in the Anzali Mordab are
likely to increase as the lagoon becomes more saline (Holčík and Oláh, 1992).
Kiabi et al. (1999) consider this species to
be of least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include abundant in numbers, widespread range (75%
of water bodies), absent in other water bodies in Iran, and absent
outside the Caspian Sea basin. Extinct in Turkey (Fricke et al., 2007).
Further work
The biology of this species in Iranian waters and the stocks or taxa found there need to be elucidated.
Sources
See under family heading.
Iranian material: CMNFI 1970-0524, 11, 58.7-88.9 mm standard length, Gilan, Caspian Sea at Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0532, 1, 113.0 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 1, 85.9 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0586, 1, 77.5 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak (36º50'N, 53º56'E);
CMNFI 1970-0587, 2, 107.4-108.6 mm standard length, Mazandaran, Babol Sar (36º43'N, 52º39'E);
CMNFI 1971-0343, 1, 95.5 mm standard length, Gilan, Langarud at Chamkhaleh (37º13'N, 50º16'E);
CMNFI 1979-0430, 1, 118.0 mm standard length, Mazandaran, river east of Now Shahr (36º39'N, 51º31'E);
CMNFI 1979-0431, 7, 120.8-155.1 mm standard length, Mazandaran, Now Shahr bazaar (no other locality data);
CMNFI 1979-0686, 2, 119.7-126.9 mm standard length, Gilan, Safid River (37º24'N, 49º58'E);
CMNFI 1980-0146, 2, 106.9-171.8 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak (36º50'N, 53º56'E).
Comparative material: BM(NH) 1938.8.2:3, 1, 203.8 mm standard length, Caspian
Sea (no other locality data); BM(NH) 1939.2.21:22-23, 2, 175.6-179.2 mm standard
length, Caspian Sea (no other locality data); BM(NH) 1954.6.24:5-7, 3, 164.1-189.1 mm standard
length, Caspian Sea (no other locality data).
Alosa curensis
(Suvorov, 1907)
This species is poorly known and not recorded from Iran but from
Kyzylagach Bay of Azerbaijan. It may, in any case, be a subspecies or
synonym of Alosa braschnikowii (see Svetovidov (1952) and the Alosa
braschnikowii account herein).
Alosa kessleri
(Grimm, 1887)
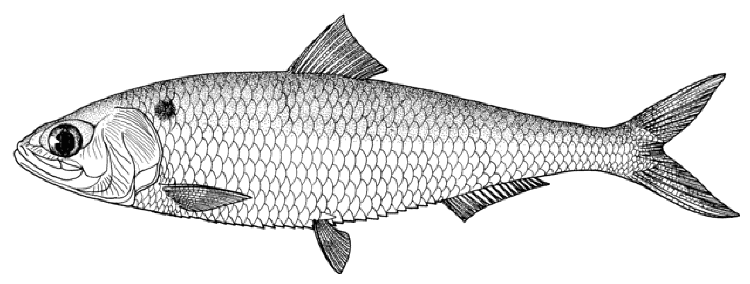
Common names
shagmahi-ye poshtsiah, shagmahi darya-ye siah, shagmahi-ye moohajer or
shagmahi-e-mohajer, zalun (in Gilaki), puzanok.
[Volga siyanayi, garabel siyanak in Azerbaijanian; arkasy gara takgas in
Turkmenian; blackback, Caspian
anadromous shad; chernospinka or black-spined herring, chernonosik or
blacknose, beshenka, zalom, poluzalom, zheleznitsa, veselka,
Volzhskaya mnogotychinkovaya sel'd or Volga many-rakered herring,
Volzhskaya sel'd or Volga herring, Astrakhanskaya sel'd or Astrakhan
herring, all in Russian; Pontic shad, Black Sea herring].
Systematics
Clupea kessleri was originally described from the Volga River delta,
Astrakhan. Clupea pontica was originally described in Latin from "Hab.
in Ponte Euxino prope Odessam" (= Black Sea near Odessa).
Alosa kessleri was formerly considered as a subspecies of A.
pontica. Alosa pontica then had two subspecies in the Caspian Sea, namely kessleri
(Grimm, 1887) (chernospinka or black-spined herring, chernonosik or
blacknose, beshenka, zalom, poluzalom, zheleznitsa, veselka, blackback),
and volgensis (Berg, 1913) (Volzhskaya mnogotychinkovaya sel'd
or Volga many-rakered herring, Volzhskaya sel'd or Volga herring,
Astrakhanskaya sel'd or Astrakhan herring, zheleznitsa, beshenka,
veselka). Kottelat and Freyhof (2007), Abdoli and Naderi (2009) and Naseka
and Bogutskaya (2009) consider Alosa kessleri and A. volgensis to
be valid species.
A lectotype of kessleri, 40.1 cm long, was designated from
the Volga Delta by L. S. Berg under ZISP 15925 (in the Zoological
Institute, St. Petersburg). A lectotype of volgensis, 34.8 cm
long, is under ZISP 15926 and is from the Volga River at Chernyi Yar (Svetovidov, 1952).
A paralectotype of kessleri is under ZIN 15922.
Caspialosa volgensis bergi Tanasiichuk, 1938 described from
the Volga Delta is a synonym of Alosa kessleri (Heckman
in Hoestlandt, 1991). Eschmeyer et al. (1996) give author and
date for Alosa volgensis bergi as Tanassiychuk, 1940, the
variation probably being due to transliteration of a Russian name and
to year of actual publication rather than year on the journal.
Caspialosa kessleri infraspecies volgensis imitans
Berg, 1948 from the Caspian Sea (see Berg (1948-1949) for further
details) is not available because of its infrasubspecific rank (Eschmeyer
et al., 1996).
Clupea caspio-pontica Borodin, 1904 is an unneeded new name
for these fishes from the Black and Caspian seas (Eschmeyer et al., 1996).
Key characters
Characterised by a relatively elongate and rounded body likened to
a "herring" shape, not as deep and compressed as in some
related species which are likened to a "shad" shape. Total
gill rakers 57-158 in the Caspian Sea, 57-95 in kessleri,
87-158 in volgensis. Rakers are usually longer than the gill
filaments in volgensis, shorter in adult kessleri. Teeth
are well developed in both jaws in kessleri and can be felt
with a finger, poorly developed in volgensis such that they
sometimes cannot be felt.
Morphology
Dorsal fin with 3-5 unbranched and 12-16 branched rays, anal fin
with 2-4, usually 3, unbranched and 15-21 branched rays. Vertebrae
47-50 in kessleri (also a report of 50-54, both in Svetovidov
(1952)), 48-54 in volgensis. Pyloric caeca 21-62. Scales in
lateral series 53-56. Gill rakers in adults are thick and often broken
off at the tip or near the base in kessleri, unbroken in volgensis.
The tips of the gill rakers may be swollen and they are arranged in a
straight line. Young fish have long and thin gill rakers with strong
lateral spines. Spines are lost with age. Chromosome number is 2n=48 (Klinkhardt
et al., 1995).
Sexual dimorphism
None reported.
Colour
The overall coloration is dark with a black back which has a violet
tinge in spring in kessleri, light olive green in volgensis.
There is dark, sometimes vague, spot on the flank behind the operculum
and sometimes a series of spots in kessleri, but these are
absent in volgensis. The pectoral fin is black on top. Spawning kessleri
become grey or grey-green on the back and flanks with bronze spots on
the operculum and flanks. A greenish-yellow circle forms around the
eye after spawning.
Size
Reaches 52 cm total length and 2.0 kg for kessleri, 40 cm for volgensis.
Distribution
Found in the Black and Caspian seas and throughout the latter, entering northern rivers to spawn.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
Both subspecies are found in the open sea but kessleri
ascends rivers much higher than volgensis which spawns in the
delta region. Both subspecies overwinter in the southern Caspian Sea
off the Iranian coast and then migrate north to enter the Volga and
other northern rivers to spawn. The subspecies volgensis is
absent from the southern Caspian in summer. The subspecies kessleri
shows a greater affinity than volgensis for cold water.
The subspecies kessleri begins to migrate northward in March
and April mostly along the western shore of the Caspian Sea, beginning
to arrive in northern waters when temperatures are still below 5°C,
most arriving when temperatures are 6-8°C compared to 10-13°C
for volgensis. A mass migration into the lower Volga takes
place in late April or early May for both subspecies when water
temperature reaches 9°C and the peak run begins at 12-15°C,
ending at 22°C. The run of volgensis is about 10 days later than that of kessleri
and spawning takes place earlier as they do not travel as far upriver.
Speed is up to 70 km/day for kessleri and depends on
temperature. This fish used to run 2000 km up the Volga River. Sexually immature fish remain in the south and do not
migrate. Knipovich (1921) reports kessleri as deep as 235-300 m in Iranian waters.
Temperatures up to 25ºC are tolerated.
Age and growth
Males are sexually mature at 3 years and females at 4 years, other
reports give 4-5 years for both sexes in kessleri. Many fish
die after spawning but some survive to spawn two or three times. Four
and five-year- olds dominate on kessleri spawning runs with
some older fish also present. Females predominate in older fish making
the spawning run. Life span is between 7 and 8 years.
Growth of the volgensis subspecies is slower than in kessleri,
which apparently grows faster than any other Caspian clupeid. Life
span in volgensis is 7-8 years with females living longer than
males. Most spawners are 3-4 years old although in some years 5 year
old fish are abundant. Males may mature at 2 years, females later.
Most fish spawn again the next year after their first time but some
may miss a year. An individual may spawn up to four times during its life.
Yılmaz and Polat (2002) compared scales,
vertebrae, otoliths, opercles and subopercles as ageing structures and
determined vertebrae to be the most accurate and reliable for a Turkish Black
Sea population at Samsun. Six age classes were found.
Food
Cladocerans are the main food item of young kessleri which
have a feeding peak at 1800-2200 hours and another at about 0800
hours. Adults in the sea take fishes such as Clupeonella and Atherina
with some crustaceans and insect larvae. Clupeonella caspia
makes up 92% of the diet of kessleri in the northern Caspian in
May, with Sander lucioperca at 6.6% and gammarids at 1.0%.
There is said to be little feeding on the spawning run although some
fish sampled contained cladocerans, copepods, insects, bryozoans and fish fry.
The food of volgensis is similar to the other subspecies,
taking copepods when young and larger items with growth. The main
items are copepods, mysids, cumaceans, amphipods and small fishes.
This subspecies feeds on the spawning migration.
Reproduction
Spawning in kessleri occurs in rivers from mid-May to
mid-August, either the delta or lower reaches when entering in a ripe
condition, or as much as 500 km upriver when entering in an unripe
condition. Larger fish have spawning grounds further upriver than
smaller fish and predominate earlier in the run. The spawning grounds
in the Volga River cover a considerable stretch. Spawning usually
occurs at 18-20°C
between 0300 and 0600 hours or from 1600 hours to sunset. Spawning
occurs in the main channel, over shallow sand banks, or in backwaters.
Batches of eggs are laid at intervals of several days. Eggs are
pelagic as in other Caspian Alosa and develop as they drift
downriver near the bottom. At 22.7°C
incubation takes about 40 hours. The young fish descend in late summer
and early fall. Fecundity in kessleri reaches 344,000 eggs and
egg diameter 1.51 mm. Shed eggs are up to 4.1m in diameter. Some fish may return to spawn in total three times.
Spawning of the first batch of eggs in volgensis may occur
in the sea with the subsequent 2 batches at 7-10 day intervals in the
delta and river. This takes place from mid-May to the beginning of
August. Up to 281,000 eggs are shed. Peak spawning occurs at 15-19°C
and ends at 25-27°C. Most spawning takes place in the evening between the 1600 and 2200
hours. The young appear in the pre-estuarine area of the Volga River
in July and towards October begin to migrate south.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984), larval shad are fed on by other fishes and by various
invertebrates, and adults by various fishes and birds.
Economic importance
The subspecies kessleri and volgensis were caught on
the spawning run with as much as 5750 t being taken annually pre-World
War II. It is the biggest shad in the Caspian Sea. The subspecies kessleri was the most important and
valuable herring in the Caspian Sea. Early spring catches were mostly kessleri
but as the run of volgensis built up it formed an increasingly
significant part of the catch, forming as much as 92% of the total.
The catch of volgensis has declined from this period until the
1970s when the fish taken were mostly kessleri. The catch of Alosa
pontica (= kessleri) on the North Caspian fishing grounds in 1965-1972 has
declined to 2-4% of the 1938-1943 catch. The subspecies volgensis
was one of the most important Caspian herrings, 23-29% of the total
catch from 1936-1939, as high as 69,100 t in 1939.
The subspecies kessleri is said to be the tastiest Caspian
clupeid because of its high fat content, averaging 18.9% of weight
along the coast of Azerbaijan, while in volgensis it was 9.6%.
Post-spawners of kessleri may have a fat content as low as 0.5%.
Catches are processed as canned, salted and pickled fish. Beach seines are used
to catch this fish. Akhondzadeh Basteh et al. (2006) found the bacterial
pathogen Vibrio haemolyticus in fresh and smoked Alosa kessleri.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food.
Conservation
Stocks in Iranian waters are said to be depleted. The subspecies volgensis
was in Category I on the "Red List" of the Russian Republic
(Pavlov et al., 1985). Kiabi et al. (1999) consider this
species (as A. kessleri) to be data deficient in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, numbers unknown, range unknown absent in other
water bodies in Iran, absent outside the Caspian Sea basin.
Further work
Stocks in Iranian waters need to be assessed and protected if required.
Sources
See under family account.
Iranian material:
None available.
Comparative material: BM(NH) 1879.11.14:22-23, 2, 255.9-259.1 mm standard
length, Caspian Sea (no other locality data); BM(NH) 1939.2.21:21, 1, 388.6 mm
standard length, Caspian Sea (no other locality data).
Alosa saposchnikowii
(Grimm, 1887)
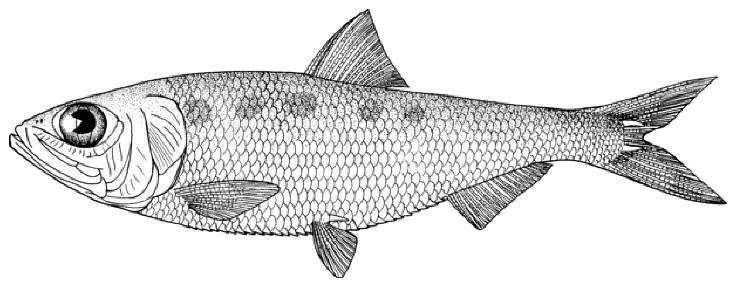
Common names
shagmahi-ye cheshmdorosht, shagmahi, kilka (incorrectly), herring.
[irikoz sisgarin in Azerbaijan; bol'sheglazyi puzanok or bigeye
shad, Sapozhnikovskii puzanok or Saposhnikovi shad, all in Russian].
Systematics
The lectotype of Clupea saposchnikowii from the Volga Delta
is in the Zoological Institute, St. Petersburg under ZISP 15921 (Berg,
1948-1949; Eschmeyer et al., 1996). The name is often spelt saposchnikovi,
in error, or with a single terminal "i"; Reshetnikov et
al. (1997) revert to the original spelling of the specific name.
Caspialosa caspia nigra Kisselevitsh, 1923, in part, from
the Caspian Sea opposite Dzambai is a synonym with a lectotype in the
Zoological Institute, St. Petersburg (ZISP 15938) (Kisselevitsh is
also transliterated Kiselevich and Kisselevitz). The material also
included specimens of Alosa braschnikowii (Whitehead, 1985;
Eschmeyer et al., 1996).
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. The upper
and lower head profiles are straight. The upper edge of the lower jaw
is straight. Total gill rakers 24-41, short (obviously shorter than
the gill filaments), and thick. Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-5, usually 4, unbranched rays and 12-15, mostly
13, branched rays, anal fin with 2-4, usually 3, unbranched rays and
15-21, mostly 18, branched rays. Lateral series scales 52-55.
Vertebrae 47-53. Pyloric caeca 36-59.
Sexual dimorphism
None reported.
Colour
Fish from the southern Caspian Sea are more intensively coloured
than those from the north. The back is violet with green sheen, the
flank has 4 dark stripes which merge with the dark on the back. There
is a spot posterior to the operculum, which may be absent, and there
is no series of spots.
Size
Reaches 36 cm total length and 650 g.
Distribution
Found mainly in the north Caspian Sea and the coast of Dagestan but entering Iranian waters.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
This species spends its whole life in the Caspian Sea and never
enters rivers. It favours colder water and is one of the first clupeid
species to migrate north in spring, principally along the western
coast. Large fish migrate first. Fish first approach the shore of
Azerbaijan in mid-March with a mass approach from late March to
mid-April. It is less frequently encountered in the southern part of
the Caspian Sea, overwintering in the central Caspian and only moving
south if winters are cold. A Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) has it at 400-600 m in the southern Caspian in cold winters but later
states it keeps at 15-32 m. Winter temperatures at which this species
is found are 6-7°C. Depths are 25-32 m in winter, more shallow in summer but below 9 m. Knipovich (1921)
reports this species in a depth range of 52-77 m in
Iranian waters. It tolerates a range of 3-25°C and spawns at salinities of
0.7-11.0‰, although preferring 4.0-7.5‰. The Caspian Sea Biodiversity
Database (from www.caspianenvironment.org) estimates a population of 1.1125 billion fish.
Age and growth
Life span is about 9 years and female lengths and weights exceed
those of males throughout life. On average, males weigh less than half
the weight of females since females carry a heavy egg load. Growth is
most intensive in the first two years of life and slows thereafter
(Chang, 1972). Males mature at age 2 and females at age 3.
Food
A rapacious fish which takes young herrings and kilka, Atherina and
even Benthophilus (Lönnberg, 1900b) as well as large
crustaceans such as mysids and gammarids. It is a cannibal. This shad overwinters and
feeds in the south Caspian Sea (Chang, 1972).
Reproduction
The spring spawning migration (end of April to end of May) enters the north Caspian Sea and
fish are mostly 15-25 cm in body length. Males mature at a younger age
than females as evidenced by fish 3-4 years old predominating among
females and fish 2-4 years old among males in the north Caspian catch.
Spawning takes place in May (peaking in the first 10 days) and most fish are returning for the second
time. Spawning temperatures are lower than in Alosa caspia,
being only 13-14°C although the peak is at 19-20°C.
Spawning occurs in il'mens, the sea where there is a freshwater
discharge such as near the Volga River mouth, and in the northeastern
sea. Females may spawn up to 6 times and males up to 5 times (Chang,
1972). Spawning takes place in shallow water at 1-6 m depths.
Fecundity is up to 318,852 eggs. The young migrate southwards.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
An important commercial species in the central and northern
Caspian, taken on their way to, and on, the spawning grounds. The
fishery in Azerbaijan during 1937 caught fish on average 17 cm long
and 62 g in weight, most fish being 2-3 years old. The Caspian catch
in the period 1936-1939 reached a peak of 8,800 t annually.
Fish are caught with beach seines, stationary nets and drift nets.
Conservation
Stocks in Iranian waters are reputed to be depleted. Kiabi et al.
(1999) consider this species to be data deficient in the south Caspian
Sea basin according to IUCN criteria. Criteria include numbers
unknown, range unknown, absent in other water bodies in Iran, absent
outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters and the stocks or taxa found
there need to be elucidated.
Sources
See under family heading.
Iranian material: CMNFI 1970-0531, 15, 49.9-108.7 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1970-0532, 1, 137.4 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 2, 78.8-80.2 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0581, 1, 102.1 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1979-0788, 3, 96.0-114.9 mm standard length, Mazandaran at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0136, 3, 107.3-127.6 mm standard length, Mazandaran, Fereydun Kenar River (36º41'N, 52º29'E);
CMNFI 1980-0157, 2, 96.6-101.1 mm standard length, Mazandaran, Gorgan River estuary (36º59'N, 53º59'30"E);
CMNFI 1980-0908, 1, 77.9 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E).
Comparative material: BM(NH) 1954.6.24:8-10, 3, 150.5-177.0 mm standard
length, Caspian Sea (no other locality data).
Alosa sphaerocephala
(Berg, 1913)
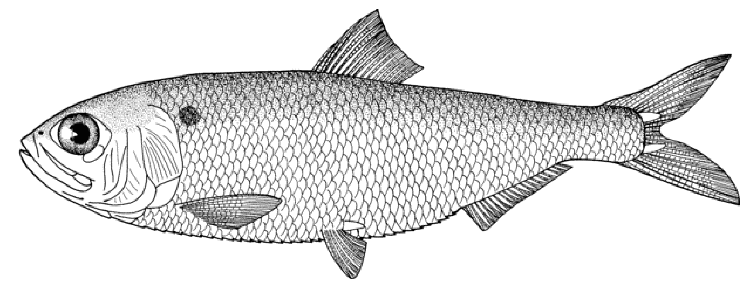
Common names
shagmahi-ye Agrakhan.
[kruglogolovyi puzanok or roundheaded shad, Agrakhanskii puzanok or Agrakhan shad, both in Russian].
Systematics
The holotype of Clupeonella sphaerocephala from Agrakhan
Bay, at Tyulenii Island, Turali in the northern part of the Caspian
Sea is in the Zoological Institute, St. Petersburg under ZISP 15928
with more than 30 paratypes (Eschmeyer et al., 1996).
Key characters
Characterised by a relatively deep and compressed body likened to a
"shad" shape, not as elongate and rounded as in some related
species which are likened to a "herring" shape. The upper
and lower head profiles are obviously rounded. The upper edge of the
lower jaw is crescent-shaped. Total gill rakers 25-45, long (equal to
or longer than the gill filaments), and thin. Teeth are well developed in both jaws.
Morphology
Dorsal fin with 3-4, usually 4, unbranched rays and 13-15 branched
rays, anal fin with 3-4, usually 3, unbranched rays and 17-20 branched rays. Vertebrae 47-51.
Sexual dimorphism
None reported.
Colour
The back is dark with an olive tint, the tip of the snout is
occasionally black and the pectoral fins are dark. There is a black
spot behind the operculum and occasionally a row of such spots.
Size
Reaches 25 cm.
Distribution
Found in the Caspian Sea including Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
This species does not enter fresh waters. It is most common along
the eastern shore of the northern part of the sea in spring where
spawning occurs and along the northern shore of the northern part of
the sea in summer. Knipovich (1921) reports this species from Iranian
waters in a depth range of 52-77 m.
Age and growth
Unknown.
Food
Unknown, although presumably similar to other shads.
Reproduction
Spawning takes place in the northeastern Caspian from mid-May to
the end of June peaking at 18-20°C,
most frequently in a salinity of 8-11‰ and in depths around 3-8 m.
The young move south in late autumn, as late as November, the last
clupeids to leave this area. Fecundity is about 20,000 eggs.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
This species is caught only in small numbers.
Conservation
The status of this species is unknown.
Further work
This species is poorly known biologically and studies in Iranian
waters should be carried out on its life history.
Sources
Iranian material: None available.
Comparative material: BM(NH) 1954.6.24:11-13, 3,
145.6-162.1 mm standard length, Caspian Sea (no other locality data).
Alosa
volgensis
(Berg, 1913)
Recorded from Iranian waters by Kottelat and Freyhof (2007) but
presence needs confirmation by specimens.
Genus Clupeonella
Kessler, 1877
This genus is found in the Black and Caspian seas basins with 5 species, 3
of which are in the Caspian Sea and in Iranian waters.
Clupeonella species are distinguished from sympatric Alosa
species by smaller size, a small and toothless mouth, adipose eyelids
are small or rudimentary, no spots on the flank, no elongate scales
(ala) at the base of the caudal fin, no vomerine teeth, the lack of a
notch at the mid-line of the upper jaw, and by the last two anal fin
rays being elongated.
Species in this genus live entirely in the sea, or in fresh water, or
migrate between the two. Eggs are pelagic and have a large oil globule.
The general Farsi name for these fishes is كيلكا (= kilka or kelka, i.e.
"sprat", although sprat is erroneous according to Berg
(1948-1949) who uses tyulka for these fishes).
The three Clupeonella species have been fished in modern Iran since
December 1971 but the commercial catch did not exceed 15,000 tonnes. Earlier
catches date back only to 1939 with an annual catch of about 100 t in
1943-1949 exported in a marinated form to the Soviet Union (Alam, no date).
Curiously, the abundance of kilka has long been known as Kinneir
(1813) records "and herrings are in such abundance, that after a
storm, the shores of Ghilan and Mazanderaun are nearly
covered with them". Caddy (1984) refers to the kilka fisheries of
the Iranian Caspian by the scientific name Sprattus sprattus
but this is an error.
Caddy (1984) indicated that there were problems
in marketing and utilizing these fishes in Iran even though up to
50,000 t could be caught annually (200,000 t elsewhere in
the same article). Their best use was probably as food for predators
such as Sander lucioperca, Esox lucius and Salmo
caspius. A study by Razavi Sayad (1993b) suggested a ceiling of
100,000 t was possible. The Caspian Sea resources of kilka is
estimated at 800,000 t from which 340,000 t can be exploited
(Abzeeyan, Tehran, 6(8):IV, 1995).
The catch reached 51,000 t
in 1994 from none 10 years previously (Food and Agriculture
Organization, Fisheries Department, 1996) and was 36,000 t in
1997-1998 (IRNA, 31 March 1998) and 85,000 t in 1998-1999 (Fazli and
Roohi, 2002). The catch for the first 6
months of the Iranian year was 17,000 t, taken by 70 trawlers and
a 10% increase over the previous year (IRNA, 20 October 1998).
Fishermen in Gilan caught 50,000 t annually in the late 1990s (Tehran
Times, 5 September 1999). A reported catch of 56,000 t was
made in 1999-2000, a 13% increase over the previous year (IRNA,
27 March 2000). A later estimate expects the kilka catch to reach
66,000 t by the year 2000 (Abzeeyan, Tehran, 5(9):IV, 1995).
Fazli (2006a) records that kilka fishing ships discharge their catches at three
ports, Babolsar and Amirabad in Mazandaran and Anzali in Gilan. The catch
decreased from 28,000 t to 19,600 t in Mazandaran and from 57,000 to
42,600 t in Gilan from 1999 to 2000. The catch per unit effort also
decreased from 3900 kg to 2500 kg over the two years. Anchovy kilka dominated
the catch but the frequency fell from 85-90% to 76% of the catch and common
kilka sharply increased. Common kilka had been caught in spring and summer but
in 2000 they were taken in all months. The average length of anchovy kilka declined
from 96.3 mm in 1997 to 87.3 mm in 2000 and this was also reflected in the
age structure, 5+ and 6+ fish being rare. The presence of the ctenophore,
Mnemiopsis leidyi, was thought to be damaging stocks (Fazli and Roohi, 2002). Darvishi et al.
(200$) studied dietary overlap between the ctenophore and the anchovy kilka (see
below). Fazli (no date) studied kilka catches off Mazandaran in 1996-2000.
Fishing occurred at night and lasted 7.78-8.22 hours. The maximum catch at 42.8%
was taken in October, November and December with a minimum catch in June. The
least annual catch per vessel occurred in 1999-2000 (499,401 kg).
A study utilizing an echo-sounder and a pelagic trawler concludes
that the maximum biomasses for the three Clupeonella species in
the southern Caspian Sea are in winter (422,300 t) and autumn (326,900
t) while in summer and spring values are lower at 275,100 t and
260,800 t respectively. The population consists of 66.1% anchovy kilka (C.
engrauliformis), 18.9% bigeye kilka (C. grimmi) and 15% common kilka
(C. caspia)
(Iranian Fisheries Research and Training Organization Newsletter,
14:6, 1996). Note that later, the Iranian Fisheries Research and
Training Organization Newsletter (17:3, 1997) gives kilka biomass
in the southern Caspian Sea as winter 22,300 t, autumn 26,900 t,
summer 75,100 and spring 60,800 t, presumably lacking the initial
digit, and the percentages of kilka species in the biomass are also
wrong. This is corrected in a subsequent newsletter (Iranian
Fisheries Research and Training Organization Newsletter, Tehran
(18:43, 1997) but the corrected percentage biomasses are given as 66%
for C. engrauliformis, 19% for C. caspia (as C.
delicatula) and 15% for C. grimmi. It is unclear whether grimmi
or caspia is the second most important kilka species. Pourgholam
et al. (1996) give a stock assessment for kilkas in 1995-1995 using the
hydro-acoustic method.
C. engrauliformis dominates the catch in Iran at 91.8%,
followed by C. grimmi at 6.84% and by C. caspia
at only 1.35%. The 2+ and 3+ year classes account for 69.95% of C.
engrauliformis, 81.06% of C. grimmi and 80.88% of C.
caspia catches. Catch rates of kilka on the top ranking 17
fishing grounds of 56 studied range from 800 to 1200 kg per unit
effort per hour while traditional grounds have rates of 400-800 kg per
unit effort per hour. The kilka are caught by attraction to lights and netting
or pumping the catch into specially constructed ships. The kilka fishing
fleet of Iran expanded in the 1980s and 1990s. There were 30 active
vessels in Mazandaran in 1994, each with a capacity up to 30 tons (sic,
probably tonnes here and elsewhere for modern catches) (Abzeeyan, Tehran, 4(10):IV,
1994). The Mazandaran Kilka Cooperative Companies Union had 75 boats in 2000 (Tehran
Times, 31 December 2000). Gilan planned to construct 12 fish meal factories each with an
annual capacity of 1000 t and 10 kilka canneries also with 1000 ton
capacities (Abzeeyan, Tehran, 4(4):III, 1993). Catches off
Gilan alone from April 1994 to January 1995 increased 59% compared to
the same period in 1993-1994, exceeding 20,000 t (Abzeeyan,
Tehran, 6(1):II, 1995). The catch off Mazandaran from March 1994 to
March 1995 was 15,400 t, an increase of 10% over the previous
year. About 1000 t were processed for human consumption and the
rest for fishmeal production (Abzeeyan, Tehran, 6(2):V, 1995).
The total kilka catch for Iran has increased to 45,000 t
annually and efforts were being made to increase it to 110,000 t (Abzeeyan,
Tehran 4(5):IV, 1993). The catch in 1995 was 32,000 t with 64.7%
from Mazandaran and 35.3% from Gilan, with the maximum catch occurring
in April (Abzeeyan, Tehran, 7(6):II, 1996). Catches declined from 95,000
t in 1999 to 15,497 t in 2003 (Sayyad Bourani et al., 2008). Annual Soviet catches reached
37,000 t in 1956 but this declined to 300-1500 t by the end of the 1970s or
0.2-0.8% of all kinds of tyulka or kilka in the Caspian Sea. Turkmenistan
harvested 7660 and 8500 t in 1995 and 1996 although previously
almost 45,000 t valued at $22.5 million had been taken before
equipment deteriorated (http://bisnis.doc.gov/bisnis/isa/9805fish.htm,
downloaded 14 March 2000). Stocks remain large even though kilka are heavily fished.
Kilka are smoked, salted, canned in sauce and oil and marinated according to a traditional recipe and
seasoned with fruits, herbs and vegetables (Keivany and Nasrollahzadeh, 1990;
www.netiran.com/business.html, downloaded 31 October 2003). Moini and Koochekain (2003) give details of fish
sauce production from kilkas using traditional, microbial and enzymatic methods,
along with taste tests. Vacuum packaging of fresh, smoked and salted kilka has been investigated in
Iran (Annual Report, 1995-1996, Iranian Fisheries Research and
Training Organization, Tehran, p. 45-46, 1997) and studies on
processing kilkas as fish balls have also been carried out (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 40, 1996). Koochekian Sabour and Moini (2009)
describe investigations on using Iranian kilkas to produce a fermented fish
sauce for marketing in Southeast Asian countries. One company markets kilka in a clear
package which gives the product a bright and colourful appearance. Kilka have even been made into
crackers (Iranian Fisheries Research and Training Organization
Newsletter, Tehran, 18:6, 1997; Shojaei, 1998). Kilka have also been made
into oil as a by-product of the fish meal industry (Iranian Fisheries Research and
Training Organization Newsletter, 27:3, 2001). Omega-3 fatty acids have been
extracted from kilka oil under laboratory conditions (Salmani Joloudar et al.,
2009). M. Shivazad , H. John Mohammady, A. A. Yousef Hakimi and
H. Fazaely (http://iman.ut.ac.ir/news/agr.htm, downloaded 12 December 2004) discuss the use of Clupeonella engauliformis
as fish meal in animal nutrition and analyse the protein quality and Faeed et
al. (2006) studied spoilage in kilka meal from bacteria and fungi.
The Iranian Fisheries Research and Training Organization Newsletter (20:4,
1998) and Rezaei et al. (2003) report on methods of transporting kilka in cold water and
crushed ice to processing factories which were better than traditional methods.
Salmani et al. (2001) recommend chilled sea water for preservation for human consumption.
Motamedzadegan et al. (2009) found that partial hydrolysis of fish
myofibrillar proteins using papain improves its functionality. Motalebi et al. (2010) investigated the use of whey protein coating on quality
and shelf life of kilkas; it can enhance quality and increase frozen shelf life
in fish stored for up to 4 months.
The kilka fisheries are threatened by the comb
jelly, Mnemiopsis leidyi, which arrived in the Caspian in 1995 in the
ballast water of ships and spread through the entire sea by the year 2000, feeding
voraciously on zooplankton. It is now known as the "Caspian monster"
despite its small size of 5 cm (Muir, 2001). It doubles in size in one
day, reaches maturity in two weeks and then produces 8000 young each day (Muir,
2001). The fisheries collapsed by 50% in a few months, catches by one fisherman
falling from being 3-6 t a night to half a tonne. Ghadirneja (2003) reports
that C. engrauliformis originally dominated the kilka catch at
85-90% but has dropped to 55% through the impact of the comb jelly which has up
to 2285 individuals per cubic metre in the southwest Caspian Sea. Fazli
et al. (2009) describe a multi-species approach for stock management,
allowing for the decline of C. engrauliformis and increase in C.
caspia in Iranian waters through competition with the ctenophore. The fisheries may recover somewhat after
the comb jelly population collapses (Tidwell, 2001b) or if a predator, Beroe ovata,
is introduced and can survive in the less saline waters of the Caspian Sea
(Muir, 2001). Studies indicate it can survive the brackish Caspian Sea water,
feed on the comb jelly and not feed on other plankton (Iranian Fisheries Research Organization
Newsletter, 36:35, 2003). The following catch records for the total kilka catch in
Mazandaran in tonnes is courtesy of F. Darvishi (pers. comm., 2003) and shows
the drastic decline caused by the ctenophore, as well as monthly variations in
catches:-
| Months/Years |
1998 (1377) |
1999 (1378) |
2000 (1379) |
2001 (1380) |
2002 (1381) |
Mean |
| March-April (Farvardin) |
2848 |
2703 |
4644 |
1217 |
876 |
2458 |
| April-May (Ordibehesht) |
1116 |
607 |
972 |
1422 |
195 |
862 |
| May-June (Khordad) |
370 |
763 |
1819 |
125 |
158 |
647 |
| June-July
(Tir) |
1392 |
919 |
194 |
425 |
444 |
675 |
| July-August
(Mordad) |
2152 |
2306 |
433 |
614 |
249 |
1151 |
| August-September
(Shahrivar) |
3117 |
2010 |
581 |
528 |
336 |
1314 |
| September-October
(Mehr) |
3103 |
6184 |
1785 |
432 |
575 |
2416 |
| October-November
(Aban) |
4120 |
3468 |
2305 |
3051 |
1196 |
2828 |
| November-December
(Azar) |
3835 |
3410 |
2655 |
993 |
- |
2723*
(2179) |
| December-January
(Dey) |
2754 |
1735 |
620 |
1082 |
- |
1548*
(1238) |
| January-February
(Bahman) |
3968 |
1262 |
2146 |
1586 |
- |
2241*
(1792) |
| February-March
(Esfand) |
2815 |
1667 |
1192 |
1903 |
- |
1894*
(1515) |
| Total |
31,590 |
28,034 |
19,648 |
13,378 |
4029 |
|
* = averaged over 4 and (5) years.
The species composition of kilkas changed after the introduction of the comb jelly comparing the year 2000
and before with the year 2002 - the common kilka changed from about 1-5% to
about 30%, the bigeye from about 10-15% to 0/2% and the anchovy kilka from about
85-90% to about 70% (Iranian Fisheries Research Organization Newsletter, 36:2, 2003). The catch per unit effort
(catch per vessel per fishing night) fell from 4 t to 1 t.
In 2004, more than 200 fishing boats had been forced to stop operations. The kilka stock has been reduced from 400,000
t to 80,000 t over the past 4 years and the catch fell by 34,000 t
(www.iranmania.com, downloaded 4 October 2004). See also the section on the Caspian Sea basin in the Introduction.
Mamedov (2006) gives details of the biology and decline of kilkas in Azerbaijan
waters.
The Caspian seal was once a major predator on kilkas but the
number of seals has declined on the Kazakhstan and Iranian coasts from 300,000
to 5000 in recent years through DDT pollution, viral infections and food shortages (Hashemi, 2001).
An account on the biology and identification of Caspian kilka in Farsi is given by Emadi (1991)
and Fazli (1990), Fazli and Besharat (1998) and Poorgholam et al.
(1996) give accounts of biology and catches in Iran in Farsi.
Clupeonella caspia
Svetovidov, 1941
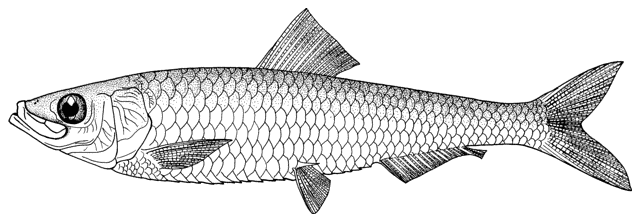
Common names
rizeh keraye (= tiny ?), rizeh kuli, kilka-ye ma'muli or kilka-e-maamooli (= common shad).
[xazar kilkasi in Azerbaijanian; adaty kulke balyk in Turkmenian; Kaspiiskaya tyul'ka or kil'ka (i.e.
Caspian tyulka or kilka), tyulka, obyknovennaya tyul'ka (i.e. common tyulka), all in Russian; common kilka, common Caspian kilka, sardelle,
Caspian sprat, Black Sea sprat].
Systematics
Formerly identified as Clupea cultriventris, originally described from the
northern shore of the Black Sea. Clupea delicatula Nordmann, 1840, described from Odessa
market on the Black Sea, is a synonym of C. cultriventris and a lectotype is in the
Zoological Museum. St. Petersburg under ZISP 2254 with paralectotypes
also under ZISP 2254, as designated by Svetovidov (1952). Clupeonella
delicatula caspia Svetovidov, 1941 was considered to be a synonym and was described
as from the
"Caspian Sea, where it is met with almost everywhere, from very
saline parts (Kaydak Bay) to quite fresh. Enters the mouths of the
Volga and the Ural rivers, ascending sometimes very far
upstream". The holotype is from the Volga Delta and is under ZISP
15883 (Svetovidov, 1952). Kottelat and Freyhof (2007) consider this subspecies
to be a a distinct species found in the Caspian Sea with cultriventris
restricted to the Black Sea. Reshetnikov et al. (1997) consider
recognition of this subspecies as questionable.
The Caspian Sea taxon, Clupeonella caspia,
has a lectotype, 152
mm long, designated by Svetovidov (1952) in the Zoological
Institute, St. Petersburg (ZISP 15883).
Clupea cultriventris is spelled cultiventris
in some parts of Eschmeyer et al. (1996), apparently in error.
Three syntypes of Clupea cultriventris may be in the Muséum
National d'Histoire Naturelle, Paris under MNHN 3681 (Svetovidov,
1952; Eschmeyer et al., 1996).
Clupea cultriventris var. tscharchalensis Borodin,
1896 from Lake Charkhal in the Ural River basin is variously listed as
a variety, morpha or a distinct species
(see Svetovidov (1952) and Kottelat and Freyhof (2007)).
mtDNA studies of fish from Mazandaran and from Gilan showed statistically
significant differences in haplotype frequencies, indicating genetically
different populations (Laloei et al., 2006).
Key characters
This species has a moderately deep body (21-27% of standard
length), a short and wide head (interorbital width 17.5% or more of
head length), a sharply keeled belly, and pointed pectoral fin tips.
The Caspian subspecies is distinguished from the type subspecies of
the Black Sea by having shorter pectoral (15.5-19.0% of standard length) and pelvic fins
(8.5-12.5% of standard length), although ranges overlap, a shallower
body, and a shallower and shorter head. It also grows faster and is
more fatty than the Black Sea subspecies.
Morphology
The dorsal fin has 3-4 unbranched rays, usually 3, followed by
11-14 branched rays and the anal fin has 1-3 unbranched rays, usually
3, and 14-19 branched rays. Scales in lateral series 42-55. There are
24-30 belly scutes and 41-62 (rarely to 64), usually 51 or more, gill
rakers. Vertebrae 40-44 (rarely to 45) compared to 44-47 in the
anchovy kilka and 46-48 in the bigeye kilka, probably as a result of
higher water temperatures during development compared to other kilka
species (Prikhod'ko, 1979b).
Sexual dimorphism
Sexual dimorphism is only evident during egg development when the
belly of females is swollen.
Colour
The back is blue-green or light-green, the flanks silvery and the
belly silvery-white or golden-yellow. Fins are hyaline except the dorsal fin which has
a central dark but faint stripe and the caudal fin which is darkish at
the base. The iris is black.
Size
Reaches 14.5 cm standard length and 19 g.
Distribution
Found in the Black and Caspian seas, tributary rivers and some
adjacent lakes. In Iran, it is reported from sea and also the confluence of the Pasikhan and Pir
Bazar rivers of the Anzali Mordab, the Anzali Mordab and its outlets
by Holčík and Oláh (1992) and from the Safid River and Anzali Talab (= Mordab) by Abbasi
et al. (1999).
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is part of a marine fauna encompassing the Black and Caspian
seas, surviving in the reduced salinity of the latter.
Habitat
The habitat of this species in the Caspian Sea is the coastal zone
of the sea at depths less than 100 m, more usually less than 50-70 m, over a wide range of
temperatures (2.6-27.6°C for adults, higher for larvae, and possibly lower temperatures since
they are found under ice and probably over 28°C
according to some reports), and in fresh and hypersaline waters (to 36‰).
The young can develop in water at 16‰. Southern populations live in
a more saline habitat than northern and central Caspian populations
which are mostly in fresh water. This tyulka may not migrate far but
does move between summer-winter feeding and spring-early summer
spawning grounds. Large schools are found 0.5-2.0 km from shore at
depths of 20-25 m on the eastern coast of the Caspian Sea, descending
deeper if water temperatures rise and coming up to about 8 m in autumn
as temperatures fall. In winter this species is found at about 30-40 m
deep where the temperature range is 7-10°C,
warmer than surface waters. Larvae and young remain in shallow coastal
areas. Knipovich (1921) reports a fish from a depth range of 235-300 m
in Iranian waters but populations at these depths are small (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996). The Caspian Sea Biodiversity Database (from www.caspianenvironment.org)
states that the largest concentrations are found at 3-7‰ with most
intensive spawning at 2-4‰.
It is the most widely distributed kilka and with the other kilka
species the most abundant fish in the Caspian Sea (Prikhod'ko, 1979b).
Large schools can be found by day but these disperse at night. It
overwinters in the southern Caspian Sea and some individuals move
north to spawn and feed in April. The Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) estimates the population to number 224 billion fish, with 96 billion fish
in the south Caspian. The south and north Caspian Sea stocks are about equal in
number after a decline in copepod biomass in the north. The relative frequency
of this species compared to other kilkas increased after the invasion of
Mnemiopsis leidyi, by more than 10% (Fazli, 2006b; Fazli et al., 2006).
Age and growth
Osipov and Kiyashko (2008) found that using otoliths gave more reliable
estimates than using scales for ageing. The Caspian subspecies grows faster than the Black Sea
subspecies. Together with the sturgeons, this species comprises 82.1%
of the fish biomass in the Caspian Sea. Condition in this species is better in winter because of the
summer-autumn feeding period after spring spawning compared to C.
engrauliformis in the Big Kizil-Agach (= Bol'shoy Kyzylagach or
Imeni Kirova) Bay of Azerbaijan (Badalov, 1972). Local populations
have differing growth regimes depending on the productivity of these
areas (Prikhod'ko, 1979b) and there are great variations on a yearly
basis too. Southern populations grow faster than northern ones in
their first year. Females grow somewhat faster than males (9.0 g
versus 7.3 g average weight along the Dagestan coast for example), and
life span is about 6 years. This species is mature there at 1 year and
average life span is about 3 years.
Females dominate the population in
Iran and sexual maturity is attained usually at age 2 and 2-4 year
olds dominate catches but life span is up to 8 years (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996; Abtahi et al., 2002). Fazli (2006b) found age classes 0+ to 5+ in
Iranian waters with 0+ to 3+ making up 95% of the fish in 1997-1999. In 2000,
age classes 0+ and 1+ were reduced in numbers and 2+ to 4+ fish comprised
93.8%. Abtahi et al. (2004) examined fish from the conical net and
light catch at Babolsar and found average fork lengths were 69.82 mm, 83.56 mm,
88.38 mm and 88.43 mm while weights were 2.2 g, 4.18 g, 4.77 g and 5.06 g for
fishes at maturity stages I, II, II and IV. Fazli et al. (2007) studied
this species from 1995 to 2004 in Iranian waters, sampled at landing sites at Amirabad and Babolsar in Mazandaran and Anzali in Gilan. Growth parameters were
L∞ = 132 mm, K = 0.259/yr. t0 = -1.285/yr. The
instantaneous coefficient of natural mortality was 0.506/yr, the instantaneous
coefficient of total mortality (Z) was 1.62/yr and the instantaneous coefficient
of fishing mortality varied over 10 years from 0.125/yr to 1.487/yr. Annual
survival rate (S) was 0.200/yr. Age at first capture was 2.8 years. The von
Bertalanffy growth equation was Lt = 132 (1-e-0.259(t +1.285)).
Ages ranged from 1 to 7 years with age groups 2, 3 and 4 dominating at different
periods. Mean fork lengths were 59.3, 77.5, 87.4, 97.2, 104.5, 111.9 and 116.8
mm. Females dominated in each month except April, averaging 0.47:1, possibly due
to differing attraction to lights used in the fishery. Biomass increased from
16,000 mt in 1995 to more than 41,000 mt in 2002, declining to less than 28,000
mt in 2004. The increase was simultaneous with a sharp decline in anchovy kilka,
changes in zooplankton composition and abundance, and especially an increase in
zooplankton species favoured by this kilka. Currently this kilka is overfished.
Karimzadeh et al. (2010) examined fish from the Babolsar region off
Mazandaran and calculated growth parameters as L∞ = 143.5 mm, K = 0.30/yr-1
and t0 = -1.02/yr, instantaneous coefficient of natural
mortality was 0.671/yr-1 and the current exploitation rate was
estimated as 0.55 and this species is now overfished.
Food
Plankton is the main food and copepods predominate but diet also
includes Cladocera, Balanus larvae and clam larvae. The
dominant food item is the copepod Eurytemora grimmi,
particularly in winter when plankton biomass is lowered in the
Bol'shoy Kyzylagach Bay of Azerbaijan. The food of the common kilka is
more varied than the other kilka species simply because of its habitat
in shallow coastal areas (Badalov, 1972; Prikhod'ko, 1979b). Older
fish take larger and faster crustaceans and consume less food in
proportion to body size as they grow. The most intensive feeding is in
summer and autumn, decreasing in winter and during reproduction. Food
is taken during the day. Roushan Tabari et al. (2009) examined fish from
a fishing vessel of Mazandaran and found highest feeding activity in April with
280±153 prey items per fish weighing 2.9±1.6 mg. Balanus nauplii and
cypris larvae comprised 93% and Acartia 7% at this time with increasing
spring temperatures and reproduction, but the copepod Acartia biomass
dominated from October to February.
Reproduction
Spawning occurs in January-February in the southern Caspian, later
in the north, mainly in depths less than 10 m and where salinity is
low to average for the Caspian Sea (Badalov, 1972; Prikhod'ko, 1979b).
The largest southern Caspian population spawns near the mouths of the
Volga and Ural rivers (Kozlovsky in Hoestlandt, 1991). Spawning is
most intensive at 11°C, but occurs at 10-20°C.
Spawning is intermittent and lasts from mid-April to July. Peak spawning in
Iranian waters of Mazandaran Province is April-May with an average fecundity of
28,240 eggs (Abtahi et al., 2002). Fazli (2006b) recorded mass
spawning in Iranian waters in April, continuing on until August. Eggs are
released in water 0.5-9.0 m deep at a salinity range of 0.02-15‰,
perhaps as high as 29.15‰. Fecundity reaches 60,000 eggs and egg
diameter 1 mm, 0.48-1.46 mm for fertilised eggs. Relative fertility is
4-13 times greater than in Alosa species. Holčík and Oláh (1992)
consider that it may spawn in rivers entering the Anzali Mordab. The studies of Fazli et al. (2006; 2007) showed that reproduction started in March, peaked in
May and finished at the end of August. Half the females were mature at 84.3 mm
fork length.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean parasites Pseudopentagramma symmetrica and Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum, metacercariae of a Bucephalus
species, and larvae of a Contracaecum and an Anisakis
species (Iranian Fisheries Research and Training Organization
Newsletter, 11:4-5, 1996; Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 28, 1997; Shamsi
and Dalimi, 1996; Shamsi et al., 1998).
Varshoie et al. (2010) record the helminths Pseudopentagramma
symmetrica, Bunocotyle cingulata and Mazocreas alosae in this
species from Iranian waters.
Clupeonella species are an important food fish for sturgeons
(59.4% by weight of Acipenser stellatus diet in the Middle
Caspian), Sander, herrings (Clupeidae) and the Caspian seal (Badalov,
1972; Krylov, 1984) as well as Salmo caspius and Stenodus
leucichthys (Kosarev and Yablonskaya, 1994).
Economic importance
It is caught by attraction to underwater electrical lights (Prikhod'ko,
1979b). The other subspecies is also of major importance in the Sea
of Azov. The Caspian subspecies is caught in school seines in spring
and purse seines in summer. In Iranian waters this species formed only
a small proportion (1.35%) of the total kilka catch in a study by
Razavi Sayad (1993b) and Fazli (2006b) gives values of 1.34%, 2.5% and 5.5% for
the years 1990-91, 1997-98 and 1998-99 respectively. However, as the anchovy kilka catch declined, this species increased from 13.7% of the total catch in
1999 to 48.9% in 2003 (Sayyad Bourani et al., 2008).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and as bait.
Conservation
Stocks on the Iranian coast are said to have been depleted but its
ecological specialisation on zooplankton means there is comparatively
little competition with other fishes. It is probably not in any
immediate danger. Kiabi et al. (1999) consider this species to
be of least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include commercial fishing, abundant in numbers,
widespread range (75% of water bodies), absent in other water bodies
in Iran, and present outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Counts are based in part on Svetovidov (1945a). See also under family heading.
Iranian material: CMNFI 1970-0531, 14, 78.0-88.6 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1980-0146, 7, 79.9-96.2 mm standard length, Mazandaran, Gorgan Bay at Ashuradeh-ye Kuchak (36º50'N, 53º56'E);
CMNFI 1993-0146, 3, 80.2-98.2 mm standard length, Mazandaran, Gorgan Bay (no other locality data);
CMNFI 1993-0167, 1, 96.6 mm standard length, Mazandaran, Caspian Sea, 10 km offshore (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 3, 84.9-88.0 mm standard length, Mazandaran, Caspian Sea, 10 km offshore (ca. 36º49'N, ca. 52º39'E).
Clupeonella engrauliformis
(Borodin, 1904)
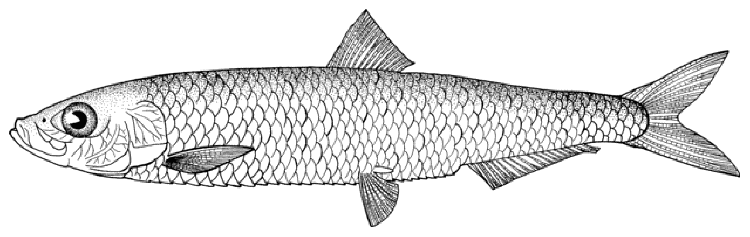
Common names
rizeh keraye (= tiny ?), kilka-ye anchovy or kilka-e-anchovi.
[ancousabanzar kilka in Azerbaijanian; ancous sekilli kulke balyk in
Turkmenian; anchousovidnaya tyul'ka or
anchovy-like tyulka, sardelle or sardel'ka, "sardinka" but
incorrectly, all in Russian; anchovy kilka, anchovy sprat].
Systematics
No major synonyms. Originally described from Buinak, central part
of the Caspian Sea. The lectotype is in the Zoological Institute, St.
Petersburg (ZISP 13860) with paralectotypes as established by
Svetovidov (1952) (Eschmeyer et al., 1996). Eschmeyer et al.
(1996) give the date as 1906 but Reshetnikov et al. (1997) give 1904.
Key characters
This species has a slender body (16-19% of standard length), a
short and wide head (interorbital width 16-18.5% of head length), a
rounded belly, and pointed pectoral fin tips.
Morphology
Dorsal fin with 3 unbranched and 12-14 branched rays, anal fin with
3 unbranched and 15-19 branched rays. Scales in lateral series 45-49.
Vertebrae 44-47, rarely to 48 compared to 41-44 in the common kilka (C.
caspia). Gill rakers number 56-67. Belly scutes 23-31.
Sexual dimorphism
None reported.
Colour
The back and head are dark blue with violet, green or olive tints. These colours become brighter or turn black in dead fish.
The fins are hyaline except the caudal fin which has a black base and the dorsal
fin which has a central dark stripe.
Size
Attains 15.5 cm standard length.
Distribution
Found in the central and southern Caspian Sea, and in Iranian waters the southeast Caspian Sea, southwest Caspian Sea and
the south-central Caspian Sea (Kiabi et al., 1999) as well as the Anzali Mordab,
Babol Sar Beach and Gorgan
Bay (Armantrout, 1980). Abdoli and Naderi (2009) list it as from the southwest,
southeast and south-central Caspian Sea, the Anzali Talab and Gorgan Bay in
Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
The anchovy kilka, along with other kilkas, is the most abundant
fish in the Caspian Sea forming large concentrations in the central
and southern Caspian wherever water depth exceeds 30 m. The anchovy kilka is
estimated to be the most numerous kilka at about 77% (Ivanov and Katunin, 2001;
Daskalov and Mamedov, 2007). It is generally found in the upper water layers but may descend
to 120 m. Nearshore areas, inlets and water of a salinity below 8‰ are
avoided. They can tolerate a salinity range of 8-14‰ but the main part of the
population is found at 10-12‰ (Fazli et al., 2007). Overwintering takes place in the southern Caspian and the
southern part of the central Caspian Sea at 8.5-9.0°C and up to 13.5°C.
Schools extend their range into the central and northern Caspian in
spring to feed (Prikhod'ko, 1979b). This species has a hibernation
period in the south Caspian Sea, a spring migration of part of the
population to the central Caspian, a feeding period in the central and
south Caspian and an autumn prespawning migration to the south Caspian
(Sedov and Rychagova, 1983).
In Iran larvae are found mostly in surface layers at 5-20 m while
adults are found in deeper zones. males dominate in winter while
females dominate in other seasons. The maximum juvenile density (fish
<75 mm), comprising 36% of the population, is seen in the summer (Iranian
Fisheries Research and Training Organization Newsletter, 20:7,
1998). Jolodar and Abdoli (2004) state it is most abundant at 100-150 m.
Age and growth
Abundance of young anchovy kilka, and hence future year-class
strengths, depends on water temperature in autumn (October-November).
Falling water temperatures, in the eastern Caspian for example, are
caused by upwelling which brings nutrients to surface waters and
promotes growth of plankton on which the kilka larvae feed (Prikhod'ko,
1979a). Females are somewhat larger than males in the spawning areas.
Sexual maturity is attained usually at age 2 and 2-4 year olds
dominate catches but life span is up to 8 years (Iranian Fisheries
Research and Training Organization Newsletter, 14:6, 1996). This
species shows the fastest rate of growth in the genus. Of the 8 age
classes, 0+, 1+, 2+ and 3+ form 99.91% of the whole population (Iranian
Fisheries Research and Training Organization Newsletter, 20:7,
1998). The same study showed that 18.6% of the population matures in
the first year of life while 81% matures in the second. The mean age
in coastal areas is 2.9 years, slightly higher than that in deep zones
below 200 m where 0+ fish are more abundant.
The Caspian Sea Biodiversity Database (from www.caspianenvironment.org)
gives a population of up to 293 billion fish in the Caspian Sea.
Fazli et al. (2007) and Sayyad Bourani et al. (2008) studied these kilkas from catches with conical
liftnets carrying underwater lights in the fisheries of Gilan and Mazandaran in
the 1995-2004 period. Fish were aged using the sagittal otoliths. Length and
weight ranges were 40-140 mm and 0.4-18.4 g with averages of 94.0 mm and 5.7 g
(89.2-100.4 mm from 1999 to 2003 in Sayyad Bourani et al., 2008).
The age range was 1-7 years. The dominant age group varied from age 2 to age 4,
making up 40.6% to 57.7% of the catch (Fazli et al., 2007) or 5+ years
with 4+-5+ making up 84.6% for 1999-2003 (Sayyad Bourani et al., 2008). Growth was high for the first year of
life and then gradually decreased. The von Bertalanffy growth equation was Lt
= 148(1-e-0.238(t+1.340)) (Fazli et al., 2007, and following
data). The sex ratio varied with season and was
significantly different from equal at male:female = 0.78:1 for adults. Females
were more abundant from January to June and males predominated from September to
November. Condition factors differed significantly between years, increasing
from 1995 to 1996, being lowest in 1998 and then increasing to 2004, and between
months, being lowest in January and February and then increasing in March. 50%
of fish were mature at 84.5 mm fork length. Annual survival rate was estimated
at 0.32, the instantaneous coefficient of total mortality (Z) was 1.14/year,
natural mortality was 0.473/year. Age at first capture was estimated as 2.92
years. The total biomass declined from 186,000 t in 1996 to less than 12,000 t
in 2004 and the exploitation rate for 1995-2004 varied between 0.340 and 0.815.
Sayyad Bourani et al. (2008) give a K value of 0.598/year and a L∞
of 110.13 mm. Natural, fishing and total mortality coefficients were 0.69, 0.31
and 1 per year respectively and the sex ratio was female:male = 68.2-31.8. These
latter results for the 1999-2003 period show how value scan change when subsets
of data are used. Fatemi et al. (2009) examined fish taken from
commercial vessels in 2007 using lift nets and lights. Age structure ranged from
2 to 7 years and was dominated by the third year class (38.6%). Back-calculation
methods were validated using otoliths to determine lengths. Karimzadeh et al. (2010) examined fish from the
Babolsar region off Mazandaran and calculated growth parameters as L∞ = 151.9 mm, K = 0.28/yr-1
and t0 = -1.12/yr, instantaneous coefficient of natural mortality was
0.633/yr-1 and the current exploitation rate was estimated as 0.41.
Food
Plankton is the main food and copepods predominate but diet also
includes Cladocera, Balanus larvae and clam larvae. The
dominant food item is the copepod Eurytemora grimmi,
particularly in winter when plankton biomass is lowered (Badalov,
1972). It can make up over 70% of its food. This copepod is more
characteristic of the diet of this kilka compared to the other two
species and the daily vertical migrations and seasonal movements of
the copepod are mirrored by the kilka. The most abundant fish species
in the Caspian depends on the most abundant member of the crustacean
zooplankton (Prikhod'ko, 1979b). This species feeds in winter, unlike Clupeonella
caspia. Bankehsaz (1996) surveys the fluctuation in fat
content of this species through the year. Intensive feeding begins in
spring as a preparation for spawning (Sedov and Rychagova, 1983).
Spawning males show a positive response to light and so feed during
the spawning season, while females do not. F. Darvishi (pers. comm., 2003) has
demonstrated that the this species has a similar feeding niche as the exotic
ctenophore Mnemiopsis leidyi and Esmaili Sari et al. (2002)
determined that there is a similar diet in Iranian waters suggesting that a
decline in stocks of the fish is the result of competition.
Darvishi et al. (2004) studied catches of the anchovy kilka and the
ctenophore in the southern Caspian Sea from August 2001 to October 2002. Dietary
overlap was >89 in Babolsar samples and >84 in Nowshahr samples using the
Schoener Index (presumably 0.89 and 0.84 where 0 is no dietary overlap and 1 is
an identical diet). The ctenophore was also feeding on fish eggs but the effect
of this was less than competition for food.
Reproduction
Spawning ends in late autumn and winter food requirements are
higher than in spring-spawning C. caspia (Badalov,
1972). Areas for spawning in this species are extensive. Spawning is
most intensive in July when temperatures are 13-24°C
and salinity 8-13‰ although the Caspian Sea Biodiversity Database
(from www.caspianenvironment.org) gives peak spawning (70%) as in October-November. Fazli (2006a) gives
spawning in Iran as spring and autumn but mass spawning takes place in in
autumn. Spawning takes place in the central and southern
Caspian along both eastern and western shores both in coastal regions
and the open sea from late April to November. Mass spawning takes
place at depths of 50-200 m and as a result eggs and larvae are
carried over a wide area by the Caspian gyral current at these depths
(Prikhod'ko, 1979b). Young hatch mainly in autumn and reach 4.5-8.0 cm
at an age of 8-10 months (Prikhod'ko, 1979a). Eggs are up to 1.82 mm
in diameter and fecundity reaches 39,900 eggs.
In Iran, 80% of the population spawn in autumn and the remainder in
spring. Accordingly the fishery should be closed in October and
November (Iranian Fisheries Research and Training Organization
Newsletter, 19:5, 1998). The subsequent Iranian Fisheries
Research and Training Organization Newsletter (20:7, 1998) states
that 89% of the population spawns in autumn with September, at 68.3%, the major month.
Fazli et al. (2007) found reproduction to start in June, peaking in
October and then declining.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean trematode parasites Pseudopentagramma symmetrica and Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum and
larvae of the nematode Contracaecum sp. (Iranian Fisheries
Research and Training Organization Newsletter, 11:4-5, 1996;
Shamsi et al., 1996; Annual Report, 1995-1996, Iranian Fisheries
Research and Training Organization, Tehran, p. 28, 1997; Shamsi
and Dalimi, 1996; Shamsi et al., 1998). Clupeonella species are an
important food fish for sturgeons (59.4% by weight of sevryuga diet in
the Middle Caspian), Sander (Percidae) and herrings and the
Caspian seal (Badalov, 1972; Krylov, 1984) as well as other fishes. Varshoie
et al. (2010) record the helminths Pseudopentagramma symmetrica,
Bunocotyle cingulata and Mazocreas alosae in this species from
Iranian waters.
Economic importance
This species forms 80-90% of the catches of kilkas in former Soviet
waters (Sedov and Rychagova, 1983) and, as noted above, 91.8% of
catches in an Iranian study (Razavi Sayad, 1993b; Rezaei et al., 2003). High catches are
related to the larger spawning and foraging range of this species
compared to other kilkas and to its habitat in the Caspian gyre, an
area of increased biological productivity (Prikhod'ko, 1979b). It is
caught in former Soviet waters by attraction to underwater electrical
lights attached to the middle of the mouth of a fine-mesh conical net
or the sides of a fish pump (Ben-Yami, 1976). Fishing is suspended at full moons
as the fish are dispersed (Saheli, 1999). Both large and small
individuals are taken by these non-selective methods (Prikhod'ko,
1981). Incidental catches include Mugilidae (common), and Alosa
spp., Atherinidae and the cyprinid Pelecus cultratus (all
occasional) (Ben-Yami, 1976).
It is regarded as a valuable and cheap food resource in Iran where
it is canned, made into sausages and surimi, and processed as fish meal (Shamsi et
al., 1996; Moeini, 2002; Shabanpour et al., 2002,,2006). The catch per unit effort for funnel nets and midwater
trawls is 2321 and 1014 respectively (Iranian Fisheries Research
and Training Organization Newsletter, 20:7, 1998).
Various studies on its preparation and storage as food have been carried out,
e.g. Rezaei et al. (2002; 2003; Moeini et al., 2009).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and as bait.
Conservation
Prikhod'ko (1981) recommends fishing in deeper waters where larger
fish are concentrated to avoid an excessive take of young fish which
favour the upper water layers. Stocks in the southern Caspian Sea are
said to be depleted. Kiabi et al. (1999) consider this species
to be of least concern in the south Caspian Sea basin according to
IUCN criteria. Criteria include commercial fishing, abundant in
numbers, widespread range (75% of water bodies), absent in other water
bodies in Iran, absent outside the Caspian Sea basin. Daskalov and Mamedov
(2007) studied commercial catch data in the Caspian Sea generally and found a
period of high catches from 1991 to 2000 with high spawning-stock biomass and
relatively good recruitment. Catches peaked at 271,400 t, fishing mortality
reached 1.8y-1 in 1999 and overfishing occurred. From 2001 to 2004,
the stock collapsed, recruitment failed in 2001 and catches fell to 54,300 t in
2005. This was attributed to the spread of the ctenophore Mnemiopsis leidyi,
with contributions from overfishing. Fazli et al. (2007) also concur that
both overfishing and the invasive ctenophore caused the collapse of stocks. The
catch in Iran declined from 71% of the total kilka catch in 1999 to 52.5% in
2003 (Sayyad Bourani et al., 2008).
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Iranian material: CMNFI 1993-0167, 1, 99.5 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 4, 89.3-107.6 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E).
Clupeonella grimmi
Kessler, 1877
Common names
kilka-ye cheshmdorosht (= bigeye kilka).
[irikoz kilka in Azerbaijan; sardelle or sardel'ka, bol'sheglazaya
tyul'ka or bigeye tyulka, bol'sheglazaya kil'ka or bigeye kilka, all
in Russian; southern Caspian sprat].
Systematics
Clupeonella Grimmi was originally described from the central
part of the Caspian Sea. The lectotype is in the Zoological Institute,
St. Petersburg under ZISP 10934 as designated by Svetovidov (1952).
Harengula macrophthalma Knipovich, 1921 is a synonym. Four
syntypes are in the Natural History Museum, London under BM(NH)
1897.7.5:41-44 (when examined were numbered 42-44, 3 fish, 29.9-33.5 mm standard
length in poor condition, September 2007), with many others apparently in the Zoological
Institute, St. Petersburg (Eschmeyer et al., 1996).
Key characters
This species has a moderately slender body (17-22% of standard
length), a long and narrow head (interorbital width 13-15% of head
length), a sharply keeled belly, and rounded pectoral fin tips.
Morphology
Dorsal fin unbranched rays 3-4, usually 3, branched rays 13-15, and
anal fin unbranched rays 3, branched rays 14-21. There are 44-49,
usually 46-48, vertebrae, more than in the other two kilka species and
probably a consequence of the low water temperature larvae develop in.
Belly with 26-32 scutes. Gill rakers 42-51.
The bigeye kilka is adapted to life in deeper water having, as its
name indicates, big eyes with more rod cells and a weaker retina but
also more transparent body tissues than other kilkas.
Sexual dimorphism
None reported except size.
Colour
The back and top of the head are dark.
Size
Reaches 14.5 cm standard length.
Distribution
Found in the Caspian Sea and concentrated in the south including Iranian waters.
Abdoli and Naderi (2009) list it as from the southwest, southeast and
south-central Caspian Sea in Iranian waters.
Zoogeography
This species is endemic to the Caspian Sea.
Habitat
The bigeye kilka is found further away from the coast than the
anchovy kilka at depths over 50-70 m, down to 450 m, with large
schools down to 130 m. It does not enter fresh water or low salinity
areas, staying well away from the shore. There is a daily vertical
migration, avoiding sunlight, and following food items. Larvae live in
water temperatures of 5°C.
Overwintering occurs in the southern Caspian at temperatures of 9-11°C,
a migration to the central Caspian takes place in spring, with a
return south in autumn (Prikhod'ko, 1979b).
Age and growth
Sexual maturity is attained usually at age 2, and 2-4 year olds
dominate catches, but life span is up to 8 years (Iranian Fisheries
Research and Training Organization Newsletter, 14:6, 1996). The
female is larger than the male at the same age. Growth is slower than
in C. engrauliformis. Males dominate the population (Iranian
Fisheries Research and Training Organization Newsletter, 14:6,
1996; Fazli et al., 2005) but this study may have sampled spawning fish (see below).
Fazli et al. (2005) examined fish from the main landing ports (Babolsar,
Amirabad and Anzali) found the mean fork length of fish increased from 95.87 mm
in 1997 to 105.0 mm in 2000 but then decreased to 102.3 mm afterwards. Over this
time period, fork length range became wider with specimens in the upper length
classes representing most of the catch. Six age classes were present, 1+ to 6+
years. During 1998-1999, age classes 1+ to 3+ comprised more than 90% of the
catch. In 2000, there was a decrease in age classes 1+ and 2+ and an increase in
3+ to 5+ classes. In 2001, age classes 3+ and 4+ decreased and classes 5+ and 6+
increased. The relative frequency of the bigeye kilka has decreased in recent
years as a result of the introduction of the ctenophore, Mnemiopsis leidyi,
a food competitor and predator on kilka eggs and young. Khorashadizadeh et al.
(2006) found fish in the Babolsar area of the Iranian coast to have 5 age
classes, dominated by the 4+ class. Fazli et al. (2009) examined changes
in the population biology of this kilka over the period 1995 to 2001, attributed
to the inavsive ctenophore. The overall sex ratio was 1.65:1 in favour of males,
length-weight regressions were W = 0.00922L2.851 for females and W=
0.008021L2.907 for males, indicating a negative growth for both
sexes, growth parameters were L∞ = 142 mm, K = 0.28 year-1,
and t0 = -1.39 years, the instantaneous coefficient of natural
mortality was 0.460 year-1, and the instantaneous coefficient of
fishing mortality varied between 0.469 and 0.980 year-1. Biomass
increased from 36,900 mt in 1995 to more than 53,500 mt in 1998 but declined to
less than 5900 mt in 2001. This was attributed to overfishing and the appearance
of the ctenophore, a competitor for zooplankton food.
Karimzadeh et al. (2010) examined
fish from the Babolsar region off Mazandaran and calculated growth parameters as
L∞ = 148.6 mm, K = 0.46/yr-1 and t0 =
-0.18/yr, instantaneous coefficient of natural mortality was 0.881/yr-1
and the current exploitation rate was estimated as 0.26.
Food
Migratory mysids often predominate in the planktonic diet of this
species. Fish fry are also eaten. Its foods are less diverse than that
of other kilkas because the variety is less in the deeper waters this
fish inhabits during the day. The three kilkas share the available
habitat and its foods, the common kilka in shallow, coastal waters,
the anchovy kilka in the upper layers of the open sea and the bigeye
kilka in deeper water of the open sea (Badalov, 1972; Prikhod'ko, 1979b).
Reproduction
Spawning is extended, from January through to September but is most
intense in spring and autumn (Prikhod'ko, 1979b). Males predominate in
the spawning areas, remaining there while females leave immediately
after spawning. Males are mainly at 10-20 m and females at 20-25 m
during the spawning season. Water temperatures at 6-13°C
and salinity 12.6-13.0‰. Fecundity is 28,300 eggs. In Iranian waters, mature
fish ready to spawn are always present in catches in winter and early spring
(Fazli et al., 2005).
Khorashadizadeh et al. (2006) found fish in the Babolsar area of the
Iranian coast to have peak spawning in early January.
Parasites and predators
Samples of this species from Babol Sar and Bandar Anzali contain
the digenean parasites Pseudopentagramma symmetrica, Bunocotyle
cingulata, the acanthocephalan Corynosoma strumosum, Eustrongylides excisus,
and larvae of a Contracaecum and an Anisakis species (Iranian
Fisheries Research and Training Organization Newsletter, 11:4-5,
1996; Annual Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 28, 1997; Shamsi and Dalimi, 1996; Shamsi et al.,
1998; Shamsi et al., 1998).
Varshoie et al. (2010) record the helminths Pseudopentagramma
symmetrica, Bunocotyle cingulata and Mazocreas alosae in this
species from Iranian waters.
Clupeonella species are an important food fish for sturgeons
(59.4% by weight of sevryuga (Acipenser stellatus) diet in the Middle Caspian), Sander
(Percidae) and herrings and the Caspian seal. Predators consume 590
million kg of the three kilka species which themselves are the main
consumers of zooplankton. Kilkas are a very important element in the
life of the Caspian Sea (Badalov, 1972; Prikhod'ko, 1979b; Krylov,
1984). This species is taken to a lesser extent than other Clupeonella
species because it is relatively sparse.
Economic importance
The bigeye kilka catch amounts to about 70 million kg a year in
former Soviet waters of the Caspian by means of electric light. All
three kilka species are caught by using underwater electric lights and
fish pumps (Nikonorov, 1964) but in the case of the bigeye the effect
is avoidance used to drive it to the bottom where it can be caught.
Other kilkas are attracted to the light but the bigeye is a vertical
migrator, avoiding sunlight (Prikhod'ko, 1979b). Light-assisted
catches of kilkas damages young shad (Alosa) stocks which are
an incidental catch (Zakharyan and Teruni, 1979). Catches in Iranian
waters are only 6.84% of the total kilka take (Razavi Sayad, 1993b). The relative
frequency of the bigeye kilka in Iranian catches was ranked second after anchovy
kilka in 1990-1991 at 6.84%, increasing to 12.6% and 21.7% in 1997 and
1998 and then decreasing.
Omega-3 fatty acids from fish oil of this species has been tested as a
dietary supplement and was found to relieve symptoms of dysmenorrhoea (Moghadamnia
et al., 2010).
Conservation
Stocks in Iranian waters are said to be depleted. Kiabi et al.
(1999) consider this species to be of least concern in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, abundant in numbers, widespread range (75% of
water bodies), absent in other water bodies in Iran, and absent
outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters needs to be elucidated.
Sources
Iranian material: CMNFI 1993-0167, 1, 93.0 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E);
CMNFI 1993-0168, 2, 91.8-94.0 mm standard length, Mazandaran, Caspian Sea (ca. 36º49'N, ca. 52º39'E).
Genus Tenualosa
Fowler, 1934
This genus comprises 5 species found from the Indian Ocean to
Indonesia and China. A single species enters rivers of southern Iran.
The genus is defined by a series of characters listed below under Key
characters. These fishes form part of local, artisanal fisheries
throughout their range.
Tenualosa ilisha
(Hamilton, 1822)
Common names
صبور (= sobur, soboor, sobour, sabur, zobur, zabur,
zamur or zomur, all variants of the same word), bari, barak;
mahi-ye khor kuchiku (= small bone fish, at Abadan from www.abadan.com/abadanhistory.html, 15 March 1998).
[zoboor, soboor, sbour in Arabic; hilsa, Indian shad or river shad;
palo, palla or pulla and tikki-palwar in Pakistan].
Systematics
Clupanodon ilisha was originally described from the Ganges
estuaries in India. Formerly placed in the genus Hilsa Regan,
1917. Al-Hassan (1982), citing a personal communication from a Mr. Al-Abaychi
in 1973, suggests that Shatt al Arab fish are distinct from those in
Pakistan on morphometric and meristic grounds but no data have been
published. Milton and Chenery (2001) used
genetic and otolith chemistry data that provided strong evidence for a distinct
stock in Kuwait, compared with stocks from India to Sumatra. Al-Hassan (1999) mentions that people in Basrah can
distinguish two kinds of sobur, based on taste. One is the tastier and
pricier Shatt al-Arab form and the other is the less desirable
estuarine/sea form. This has not been confirmed by systematic studies. Jorfi
et al. (2008; 2009) found differences between populations in Iran and Iraq using
molecular techniques.
Key characters
This species is distinguished from other Indian Ocean clupeids by
the upper jaw with a median notch, the anal fin ray count being less
than 30 rays, a terminal mouth (lower jaw not prominent nor flared at
the corners), scales in lateral series are not perforated posteriorly,
last dorsal fin ray not filamentous, weakly developed lines (the
fronto-parietal striae) on top of the head (usually covered by skin
and not visible), gill rakers on inner arches straight not curled, a
long head 28-32% of standard length, and 30-33 ventral scutes forming a keel
along the belly, 15-18 being prepelvic and 11-15
postpelvic (Al-Nasiri and Al-Mukhtar, 1988a, 1988b; Marammazi et al., 1995).
Morphology
Dorsal fin with 4-5 unbranched rays followed by 14-16 branched
rays, anal fin with 2-3 unbranched rays followed by 16-20 branched
rays, pectoral fin branched rays 12-15 and pelvic fin branched rays 7.
Lateral series scales 44-51. Gill rakers are fine and numerous, up to
about 275 on the lower arch.
Iranian fish examined by Marammazi et al. (1995) from the
Bahmanshir River in Khuzestan have 30-32 total scutes along the belly,
16-18 prepelvic scutes, 13-15 postpelvic scutes, 19-21 dorsal fin
rays, 19-24 anal fin rays, 13-15 pectoral fin rays, 8 pelvic fin rays
and 44-51 scales.
Sexual dimorphism
None reported.
Colour
The back is grey-blue, bluish to green and the sides are silvery
with golden, purplish or pink highlights. The dorsal fin is grey, the
caudal fin grey-blue with a silvery tinge and darkened margin, and the
anal fin is light blue with some silvery tinges. Paired fins are
hyaline. The area behind the gill cover in young fish and many adults
have a dark blotch followed by a series of spots or blotches running
along the upper flank, for a total of 6-7. The blotches may take the
form of bars. The eye is yellow to red. Young have a bronze back,
silvery flanks and a caudal fin margined in black.
Size
Attains 60.6 cm total length and 2.49 kg for females and 43 cm and
0.68 kg for males. A sample of 233 moribund fish from the Ashar Canal,
a branch of the Shatt al Arab, Iraq examined by Al-Nasiri and Al-Mukhtar
(1988a; 1988b) had a total length range of 70-152 mm. Hussain, Jabir
and Yousif (1994) record fish migrating to the Shatt
al Arab for breeding at 21-38 cm for males and 33-43 cm for females.
Mature females in the Shatt al Arab weighed about 0.5-1.1 kg (Jabir
and Faris, 1989). Fishes from Kuwait attained 57 cm (Al-Baz and Grove, 1995).
Fishes from the Arvand, Bahmanshir, Karun and Dez
rivers of Iran were 120-500 mm long (Marammazi et al., 1998; Ghafleh
Marammazi et al., 2004).
Distribution
Reportedly found from the Red Sea and Persian Gulf through the
Indian subcontinent to the Malayan Archipelago in some general works,
or more narrowly from the Persian Gulf to Myanmar. It enters the Shatt al
Arab and Tigris River, once as far north as Baghdad (Kanazawa, 1955),
but the northernmost distribution today in Iraq is the Hawr al Hammar.
Before the construction of dams on the Euphrates the migration was up
to "Yaou" and "Meshkhau" and up to Qal`at Salih
(31°31'N, 47°16'E) in the Tigris of Iraq (van den Eelaart, 1954).
The lower reaches of the Tigris and Euphrates rivers were connected
by a channel to the Khor Al-Zubair in Iraq during 1983. As a
consequence the Khor became oligohaline (at less than 10‰) rather
than hypersaline (at more than 40‰), becoming an estuary with heavy
reed growth. The catch of sobour in the Khor by 1997 exceeded that in
the Shatt al Arab and may involve diversion of stocks from the
original habitat of the Shatt (Hussain, 1997).
In Iran, it is recorded as far north as
the Gargar Shoteit on the Dez River (Marammazi, 1994). Hussain,
Jabir and Yousif (in litt., 1995) record this species from the Shatt al
Arab in Iraq and the Bahmanshir, Jarrahi, Zohreh and Hilleh
rivers in Iran. Marammazi (1994) and Marammazi et al. (1998) report this
species from the Arvand, Bahmanshir, Karun and Dez rivers. Ghafleh Marammazi
et al. (2004) record it from the Zohreh, Bahmanshir, Arvand and Karun rivers in Iran.
It may be found in the Hormuz basin but this has not been verified with
specimens.
In the sea, they are found from Bushehr around to
Kuwait in coastal waters (Blegvad and Løppenthin, 1944; Hussain,
Jabir and Yousif, in litt., 1995).
Zoogeography
Al-Hassan (1982) mentions a study comparing a population of this
species from Basrah, Iraq with one from Pakistan and finding
significant meristic and morphometric differences, perhaps indicative
of distinct stocks.
Habitat
Sobour enter the Shatt al Arab in February and March during high
tides and feed there until the fall according to a study by Al-Nasiri
and Al-Mukhtar (1988a; 1988b) working on fish taken from the Ashar
Canal, Basrah, Iraq. van den Eelaart (1954) reports that most fish
enter the Shatt al Arab in April during the last and first phase of
the moon and anecdotal reports indicate the end of March to be the
peak period of entry. They ascended into the Hawr all Hammar and
from there into the Euphrates as well as into the Tigris (van den Eelaart,
1954). Significant numbers were recording as entering the recovering Hawr al Hammar in 2005-2006
(Hussain et al., 2006). Small specimens (50-100 mm) were observed in the east Hawr al
Hammar in June 2005 and July 2006 (www.iraqmarshes.org, downloaded 29 August
2005; N. A. Hussain, in litt., 2006). In mid-April sbour were found below
the Yaou and Moshkhab regulators which formed the limit of their migration on
the Euphrates in the early 1950s. The limit in the Tigris was beyond Amara. The
main spawning grounds in the Euphrates were probably somewhere between Shinafiya
and Samawa and in the Tigris between Amara and Qalat Saleh.
The last ones leave the Shatt in July and fry
are found in the rivers of Iraq at the end of the June. Hussain, Jabir
and Yousif (1994) record sobour ascending the Shatt
al Arab during March with a continuing migration upstream through
April to July for spawning and a return migration to the sea during
August to October. Al-Hassan (1993) notes that local people believe that sobour
ascend the Shatt al Arab during spring to marshes north of Basrah for spawning,
suggesting that they are the fluvial anadromous type. Al-Hassan (1999) considers they migrate to the sea
in September-November, when they are landed in Kuwait, and they then
migrate to the Iranian coast during December-January. Males and
females move upriver in separate groups according to Iraqi fishermen (Al-Hassan, 1999).
Jorfi
et al. (2008) suggest, based on molecular studies, that a population in the
Persian Gulf chooses the Karun River for spawning and migrates via the
Bahmanshir River, while others migrate up the Tigris and Euphrates rivers in
Iraq via both the Bahmanshir and the Arvand rivers.
Blegvad and Løppenthin (1944) mention this species on sale at
Khorramshahr on 28-29 April. The spawning migration in Iran occurs in
spring (I. Sharifpour, in litt., 1991). It is only found in the
Zohreh River in spring and summer (Marammazi, 1994).
They may be found in deep water, over 18 m, or in shallows, on
their spawning migration. Large concentrations of sobour occur below
dams blocking their migration. Young occur in side branches of the
Shatt al Arab near food, shelter and the spawning grounds (Hussain,
Jabir and Yousif, in litt., 1995).
This species occurs in river estuaries and coastal waters and
appears to be restricted to the northern end of the Persian Gulf
because this is the only part with large spawning rivers (Hussain,
Jabir and Yousif, in litt., 1995). These authors also suggest
that an anadromous stock from the Shatt al Arab migrates to warmer
waters off Bushehr during January, February and March. At the same
time there is a winter decline of Kuwaiti stocks. There may also be a
marine stock inhabiting coastal waters of Kuwait since larvae have
been found in Kuwait Bay during June and November and catches are made
in the Bay year round.
Biogenic and anthropogenic sources were noted for the hydrocarbons in this
species from the Shatt al Arab; n-alkanes attained 31.11 µg/g and hydrocarbons
10.91 µg/g, the highest for the fish species studied (Al-Saad et al.,
1997). The fat content of this shad is a factor in these high levels (Al-Saad,
1990).
Hussain (1997) notes that the changing conditions in the Khawr az Zubayr,
which became oligohaline from hypersaline after it was connected to the
Tigris-Euphrates basin by the Shatt al Basrah Canal. In 1994 fishermen began
catching sbour in the Khawr az Zubayr and by 1997 the numbers caught exceeded the
catch in the Shatt al Arab.
Migrations in the Indus River of Pakistan (Islam and Talbot, 1968)
may last over 7 months and the migration up the Ganges River extends
over 1287 km. Fish may move as much as 70.8 km in one day and may jump
out of the water on the migration.
Age and growth
Al-Nasiri and Al-Mukhtar (1988a; 1988b) give a length-weight relationship of
W = 3.9 x 10-6 L3.16 or log W = 3.16 log L-5.4 for fish
aged at 0+ from the Ashar Canal at Basrah. The mean condition factor was 0.87.
Fishes in the Shatt al Arab are in age groups 5 to 6 for the period May to
August (Hussain et al., 1991). In contrast, a later study on the Shatt al
Arab fish showed there are 5 age groups and the second and third age groups
dominate in catches (Hussain, Jabir and Yousif, 1994). In this latter study,
Shatt al Arab fish mature at 25 cm for males and 33 cm for females, similar to
an Iranian study (see below). The length-weight relationship was log W = -4.7074
+ 3.0479 log L for females and log W = -4.5802 + 3.0193 log L. Condition factor
gradually increased with length groups in males, peaking at 32-33 cm followed by
a sharp decline while females had a nearly stable condition factor from 34 to 43
cm. Mohammed et al. (2001) gave a von Bertalanffy growth equation as L∞
= 60.47 cm and a condition factor of 0.32, slower growth than in Indian and
Bangladesh populations and probably maturing later.
Amodeo (1956) gives lengths of 25 to 35 cm for fish caught in the Shatt al
Arab on their spawning migration. Young grow rapidly, 4.3 cm in
October-November. Most fish on the migration in the Indus River were in age
groups 3 and 4. Life span is up to an estimated 7 years with maturity as early
as 1 year. Jawad et al. (2004) found haematocrit level to increase with
body length up to 40 cm after which it decreased, males showed higher levels
than females, and levels were higher pre-spawning than during spawning and
increased slightly post-spawning, a general correlation with fish activity.
Al-Baz and Grove (1995) studied fish taken from Kuwait fish markets. Females
dominated the catch, male:female ratio being 1:2.4, perhaps because the sexes
moved in different schools. The smallest mature female was 34.4 cm and 50% of
the females are mature at 41.5 cm. They estimated natural mortality (M) based on
von Bertalanffy growth parameters (L∞ and K) and mean annual water
temperature as log M = -0.0066 -0.279 log L∞ + 0.6543 log K + 0.4634
log T. The length-weight relationship was W = 0.011 L2.983 for males
and W = 0.007 L3.104 for females. Growth in the sexes follows
different patterns. Five age groups were detected using otoliths and fish were
fully recruited to the fishery at 3 years of age. von Bertalanffy growth
parameters were L∞ = 52.70 cm and condition factor (K) = 0.28 per
year while using Allen's method they were L∞ =52.50 cm and condition
factor (K) = 0.36 per year Growth curves were given. Annual total mortality was
estimated to be 1.2 using the K value of 0.36. A fishing mortality was
calculated to be 0.8 per year.
In the Bahmanshir River, Iran most fish are 4-5 years old. The
minimum total length and age at maturity are 26.2 cm, 200 g and 2
years for males and 32.18 cm, 450 g and 3 years for females. Von
Bertalanffy growth parameters in Iranian females are L∞
= 57.78 cm and K = 0.282 and in males 46.37 cm and 0.252 (Marammazi,
1995; Iranian Fisheries Research and Training Organization
Newsletter, 12:5, 1996; Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 53-54, 1997).
Hashemi et al. (2009) studied fish landed at Hendigan and Abadan and
recorded L∞ as 42.81 cm, K was 0.9, M
was 1.37, F was 2.41, Z was 3.78 and E was 0.64. Y'/R was 0.048 and B'/R was
0.19, exploitation rate (U) was 0.61, annual stock at the beginning of the year
(P) was 7615 t, annual standing stock (b) was 1927 t and MSY was 3642 t. The
stock was overfished.
Hashemi et al. (2010) studied 9317 fish from landings at Abadan and
Hendijan. Size range was 20-39 cm. The von Bertalanffy growth parameters were L∞
= 43.32 cm, K = 0.78 yr-1, Φ' was 3.16 and t0 was -0.18.
Mortality rates were M = 1.29 and Z = 4.53, and fishing mortality (F) was 3.24
yr-1. The exploitation rate (E) was 0.72 and the stock was overfished.
Values of the sizes where the probability of capture was 50% (L50)
and 100% (L100) were 22.3 and 28.5 cm TL respectively. Fish
were recruited to the fishery at a mean size of L100 =
22.3 cm. The relative yield per recruit (Y'/R) was 0.062, relative biomass per
recruit (B'/R) was 0.12 and exploitation rate (U) was 0.76. The values for
annual catch, total annual stock, standing stock and maximum sustainable yield
were 4645 t, 6635.71 t, 1433.64 t and 3274.19 t respectively. The fishing
pressure must be reduced from 3.24 yr-1 to about 0.97 yr-1for
this population to be adequately managed.
Roomiani and Jamili (2011) examined fish
landed in Iran from a northern Persian Gulf fishery. Growth was isometric.
Maximum total length was 43 cm and weight 949 g. von Bertalanffy growth
parameters were L∞ = 42.74 cm total length, K = 0.77 and
t0 = -0.21 years-1. Total mortality (Z) was 2.55 years-1,
natural mortality was 0.75 years-1, fishing mortality was 1.8 years-1,
and exploitation rate (E) was 0.7 years-1, and paarmeters indicate
overfishing. Maximum sustainable yield was calculated to be 2653 t.
Food
The Ashar Canal study found them to feed on phytoplankton such as
dinoflagellates and diatoms and on zooplankton, mainly copepods, as
well as their own young. The sieve-like gill rakers are used to strain
out planktonic organisms without selection. Presence of some sand
grains indicates that feeding can occur on the river bed. Feeding
intensity may decrease or cease on the spawning migration and is very
high after spawning. The Bahmanshir fish feed principally on copepods
and diatoms. Shatt al Arab juveniles feed mostly on filamentous algae
and diatoms with some organic matter, fish eggs and zooplankton while
adults have empty stomachs on the spawning migration (Hussain, Jabir
and Yousif, in litt., 1995). In the Indus River, the newly hatched
larvae and juveniles graze for five to six months in fresh waters before they
migrate to the sea (http://www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm
, downloaded 18 February 2003).
Reproduction
The spawning migration depends on the flood regime of the rivers. Turbid
water and fast current are probably stimulants to egg deposition. The sbour
depends on river-edge vegetation for egg deposition. Spawning grounds in Iraq
are probably located near the beginning of the side branches of the northern
sector of the Shatt al Arab, 120 km from the sea (Hussain, Jabir and Yousif,
1994). This species is gonochoristic (Blaber et al.,
1997). Males may ascend the river before females but females become
dominant in Indian populations. Males dominate in March in the Shatt
al Arab and the sex ratio reaches equilibrium in the spawning months
of May-July (elsewhere in the same communication spawning is given as
June to August) (Hussain, Jabir and Yousif, 1994; Jawad et al. (2004).
Spawning may occur more than once in a season in India. This has not
been demonstrated for Iran but could occur. The gonadosomatic index
for fishes in the Iraqi Shatt al Arab indicates peaks in March-May and
July-August, suggesting two spawnings (Hussain et al., 1991)
although a later report (Hussain, Jabir and Yousif,
1994) gives spawning as June to July and July to August as evidenced by two
modes of juveniles found in September. Sex ratio is equal during this period.
All females entering the Shatt al Arab were mature with smallest female being 33.0 cm long. Males less
than 25.0 cm were immature, the population reaching 100% maturity at 31-32 cm
(Hussain, Jabir and Yousif, 1994). The Kuwait fish studied by Al-Baz and Grove
(1995) indicated spawning between May and July with a peak in June.
Fecundity in the Indus River population was estimated to be up to
2,917,000 eggs per female, egg diameters reached 0.89 mm, and the hatching takes
place in about 23 to 26 hours (http://www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm,
downloaded 18 February 2003). Estimates for the Hooghly River of India reach 13,230,500 eggs per female (Al-Hassan, 1993). Fecundity
in the Shatt al Arab ranges between 444,960 and 1,616,560 eggs for
fish 33.0-41.5 cm total length although 2 fish 37.3 and 2 fish 39.0 cm
total length had a range in egg numbers of 109,000-233,840, showing
that great variations in fecundity occur between individuals; possibly
some fish had partially spawned before capture (Jabir and Faris, 1989). This
latter study gave a relationship between absolute fecundity and total length as
F = 1.3699 L3.6681 and log F =
0.1367 + 3.6681 log L and between fecundity and weight F = 302.8214 W1.2087
and log F = 2.4812 + 1.2087 log W. Fecundity increased significantly with body
weight, ovary weight and total length. Relative fecundity (ova/gramme body
weight) varied from 737 to 1721, mean 1216.
Hatching can occur within one day at an average temperature of 23°C.
Eggs, larvae and young are found on the spawning grounds but with
growth the young move into estuarine and foreshore areas during winter
months. Hussain, Jabir and Yousif (1994) record the
appearance of juveniles from the northern Shatt al Arab from June to
November. Adults return to their original habitat in the sea after
spawning. There is some evidence for freshwater resident populations
in India which migrate upriver to spawn but do not descend to the sea.
The Bahmanshir fish are thought to spawn from April to July. Only
adults enter the Bahmanshir (Iranian Fisheries Research and
Training Organization Newsletter, 12:5, 1996). Absolute fecundity
of fish from the Arvand, Bahmanshir, Karun and Dez rivers ranges from
374,892 to 1,954,144 eggs for total lengths of 380 to 500 mm
respectively and is related to age. Ova with diameters 0.64-0.795 mm were
released spontaneously in a study of this fish in Khuzestan province, in several
batches along its migration route (Ghafleh Marammazi et al., 2004).
Spawning begins on entry to the Bahmanshir and Arvand rivers in Khuzestan in
April, continuing to September and the end of their migration at the cities of
Shushtar and Dezful higher upriver. Males enter these rivers first in March,
followed by females in April (Ghafleh Marammazi et al., 2004).
Parasites and predators
None reported from Iran other than nematode larvae by Ebrahimzadeh and Nabawi (1975) for fish from the Karun River.
Economic importance
The Ashar Canal study cites 996,308 kg reaching the Ashar fish
market from October 1975 to June 1977 (see also Sharma, 1980). The
catch landed at Fao on the Shatt al Arab estuary of Iraq was 6576
t in 1990-1991 (L. A. J. Al-Hassan, in litt., 1995;
however this seems much too high although the estimate is from the
Food and Agriculture Organization). This species forms the most
important commercial fishery in the Basrah region of southern Iraq,
average catches being 491.086, 319.661 and 267.988 t in 1977, 1978
and 1979 respectively (sic, Jabir and Faris, 1989). There is a drift-net
and stake-net ("hadra") fishery in the sea by Kuwait
in Kuwait Bay and around Falaikah Island (Al-Baz and Grove, 1995).
The fishing season on the Tigris-Euphrates is March to August with a peak in
April, or late April to early June (Jabir and Faris, 1989) or to November (Ali
et al., 1998). van den Eelaart (1954) gave the fishing season for this species as
March-August (peaking in April) in rivers, and March-May (peaking in April) in
Hawr al Hammar, Iraq. Fish are caught at the mouth of the Shatt al Arab as they
enter the river with stationary gill nets, drifting gill nets, in "mailan" and
"odda" traps from March to August. The catch averaged 150-180 kg per ten odda
and in March 1953 the total catch at the mouth of the Shatt al Arab was about
25,000 kg (Amodeo, 1956). Large fish are only caught in the summer (Al-Hassan,
1999).
The catch at Abadan from February to November in 1943 was about 401.42
t and from January to June about 336.67 t (Pillay and Rosa, 1963).
This species is seen on markets at Ahvaz, Khuzestan in November but these are
sea-caught fish. Marjan Iran Company was selling 600-800 g fish for
U.S.$1.40/kg, 800-1000 g fish for U.S.$1.60/kg, 1000-1200 g fish for
U.S.$1.70/kg, and 1200 g and larger fish for U.S.$1.80/kg in August 2003
(http://groups.yahoo.com/groups/hilsa/message/25). The catch in Khuzestan
province in 2000 was 2688 t (Ghafleh Marammazi et al., 2004) and in
2006 was 4989.83 t (about 15% of Khuzestan's total commercial fish landing) (Roomiani
and Jamili, 2011). The catch in Khuzestan Province in 2008 was 4645 t (Hashemi
et al., 2010).
These fish are caught with traps, weirs, gill nets and other
devices in rivers on the spawning migration. They are excellent eating
until spawning occurs after which they lose their flavour. However
this species has been implicated in clupeotoxic poisoning. Hindi et al. (1996a) give the chemical composition of flesh of this
species as 66.41% moisture, 12.12% fat, 18.72% protein and 1.98% ash, indicating
a valuable food fish characterised as fatty. Hindi et al. (1996b) give
chemical indices for assessing fish freshness according to the month of capture
and marketing (pH 6.06, total volatile nitrogen bases 15.32 mgN/100g fish,
thiobarbituric acid 1.35 mg, and free fatty acids 1.33%). Salari and Sadough
(2009) compared heavy metal (Cd, Pb, Cu, Co, Ni) content in muscle, liver and
gill tissues of fish from the Karun River and found levels less than those
considered dangerous in Iran.
In Pakistan, the Indus River fishermen number between 8,000 and 9,000. Jafri
(1994) reviews the Indus fishery which had yields up to 2694 mt. It is the most important Indo-Pacific shad species.
The failure of the Indus River fishery in 2003 through drought resulted in
Iranian fish being flown to Pakistan for marketing there at rupees150-400 per
piece (www.jang-group.com/thenews/feb2003-daily/18-02-2003/business/b2.htm, downloaded 18 February 2003).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, in aquaculture and in textbooks.
Conservation
Hussain, Jabir and Yousif (in litt., 1995) report a decline
in catches over the previous two decades in the Shatt al Arab. Al-Nasiri and Al-Mukhtar (1988a;
1988b) mention that fish enter the polluted Ashar Canal, a side tributary of the
Shatt al Arab, during high tide when waters are diluted. A low tide in October
resulted in severe oxygen depletion and fish suffocated. Das et al.
(1977) found samples from the Ashar fish market in Basrah to be contaminated
with hydrocarbons, emitting a kerosene smell and being unfit for human
consumption. Al-Saad (1990) found petroleum hydrocarbon residues to be high in
Khawr az Zubayr fish at 40.6 μg/g as this species is one that accumulates fat.
Evidently, overfishing and pollution are major factors in the conservation of
this species, to which must be added variations in freshwater flow and quality
from the marshes and Tigris-Euphrates through human processes.
Further work
The migratory habits and ecological requirements of this food fish
need to be examined in more detail for Iranian waters.
Sources
Some aspects of the biology of this species were based on Pillay
and Rosa (1963) and Al-Hassan (1993) writing mostly on Indian and Pakistani populations. Specimens
on markets in Ahvaz, Khuzestan examined.
Iranian material: CMNFI 1991-0153, 1, 243.3 mm standard length, Khuzestan, Zohreh River (no other locality data).
Comparative material: BM(NH) 1875.1.14:11-13, 3, 118.8-135.8 mm standard length, Iraq, Tigris River (no other locality data);
BM(NH) 1920.3.3:178-182, 6, 103.3-132.4 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1989.1.13:1-3, 3, 53.9-59.9 mm standard length, Iraq, Khawr az Zubayr (no other locality data);
BM(NH) 1989.1.13:4-5, 2, 66.6-69.8 mm standard length, Iraq, Khawr az Zubayr (no other locality data).
Chanidae
Back to Contents
This family contains only one species. It is characterised by a compressed and oblong body; small, toothless and terminal mouth; non-protractile upper jaw; lateral
pouches on the posterior part of the branchial chamber forming an epibranchial organ; presence of intermuscular bones; first 3 vertebrae specialised in structure; presence
of an alarm substance; swimbladder present; gill membranes united and not attached to the isthmus; caudal fin deeply forked; the dorsal and pelvic fins opposite and small to
moderate; cycloid scales; and a distinct lateral line.
Genus Chanos
Lacepède, 1803
Characters for the only species in the genus and family are summarised under the family.
Chanos chanos
(Forsskål, 1775)
Common names
khameh mahi (= literally cream fish but probably meant as milkfish).
[sheem in Arabic; milkfish, salmon herring, giant herring].
Systematics
No major synonyms. Mugil chanos was originally described from Jidda on the Red Sea.
Key characters
The milkfish resembles members of the family Clupeidae but is distinguished by a low number of branchiostegal rays (4 as opposed to 6-7), the presence of a
lateral line, and the absence of scutes along the belly.
Morphology
The mouth is small and lacks teeth. There is a notch on the upper jaw in the mid-line into which a lower jaw protuberance fits. The large eyes have an adipose eyelid.
The intestine is very long with many folds. The lower part of the oesophagus has a "gizzard', an area with longitudinal folds.
Lateral line scales 70-92, with 3-11 on the tail fin this latter count varying widely between authors. Total dorsal fin rays 13-17 including usually 2-6 unbranched rays,
branched dorsal fin rays 9-14, usually 11-12; anal fin unbranched rays 2-3,
branched rays 6-10, usually 7-8; pectoral rays 14-18, usually 15-16 and pelvic
branched rays 10-12. Gill rakers and pyloric caeca very numerous. Gut long and complexly coiled.
Vertebrae 42-46. Chromosome number 2n=32, low compared to other primitive teleosts (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- lateral line scales 70(1), 71(2), 72(1), 74(2), 75(1), 76(2) or 79(2); unbranched dorsal fin rays 4(11);
branched dorsal fin rays 11(7) or 12(4); unbranched anal fin rays 3(11); and branched anal fin rays 7(10) or 8(1); pelvic fin branched rays 9(1), 10(4) or 11(6);
vertebrae 42(2) or 43(9).
Sexual dimorphism
None reported.
Colour
The overall colour is silvery with bluish or olive tints dorsally. The flank may have golden tints. The top of the head is yellowish-olive, the sides and ventral surface
bright silvery to whitish. The snout is a light brown. The dorsal and caudal fins are colourless to grey, yellowish or brown with dark margins. The anal and pelvic fins are white,
the anal with a dark margin. The peritoneum is black. The iris is silvery.
Size
Up to 1.85 m and 18.6 kg, although Bagarinao (1994) cites 1.5 m and 14 kg as more reasonable.
Distribution
Found from the African coast, Red Sea and Persian Gulf to the southern Pacific coast of the U.S.A. and to Peru, and north to Japan and south to Australia. Reported to
penetrate 100 km up the Shatt al Arab (McKinnon and Vine, 1992).
In Iran, this species is reported from the Baghu River, Hormozgan near Bandar-e Abbas in 1976, found dead in brackish water about 14 km by river from
the sea (Coad, 1981b). Also reported from the lower Mand River in Bushehr Province by M. Rabbaniha (pers. comm., 1995). Abdoli (2000) illustrates it as entering several rivers around
the Straits of Hormuz including the Minab and Kul rivers. Salehi (1999) records this species from estuaries in Hormozgan and Makran including the "Khoor-Chel",
Shur, "Takhtenze", Tiab, Gask, Heylaru, "Gorginee" and Gabrik rivers.
Zoogeography
The milkfish is unusual in being one of the few Indo-West Pacific fish species found also in the eastern Pacific, although the mechanism of dispersal is uncertain.
Larvae are probably incapable of crossing the 6500 km wide East Pacific Barrier but adults could do so as they can cruise at 2 km/h. However adults have not been caught on the high seas.
Habitat
Usually found in littoral waters of the ocean, rarely entering rivers, but it has been acclimatised to freshwater ponds. It becomes sluggish at temperatures below 15°C and dies at about
12°C but can survive temperatures of 41°C. It seems to prefer waters warmer than about 20°C. The Iranian specimens (Coad, 1981b) were caught at 16°C in a shallow, muddy,
backwater and probably died through exposure to colder temperatures in the main river. Persian Gulf temperatures can fall below 15°C in winter.
It is resistant to salinity changes and can survive in fresh and hypersaline water (0-158 p.p.t.) as well as waters low in oxygen. The dorsal and upper caudal fin lobes
may cut the surface of the water and milkfish are often mistaken for sharks. Milkfish are solitary or found in small schools.
Age and growth
Life span is at least 12 years based on pond specimens but large adults in the sea undoubtedly live much longer. Growth in the sea is poorly known and most data refer to pond-reared
populations. Fish at sea are 20-43 cm long after 1 year, a growth rate considerably less than in ponds. Maturity is reached at 3-5 years in the wild but as long as 8-10 years in ponds.
Food
Young and adult milkfish will feed on surface scum, taking it in with a smacking noise. Benthic and epiphytic organisms are also taken in ponds with the body inclined at an angle of about 30°.
Cyanobacteria, benthic diatoms, foraminiferans, filamentous green algae,
detritus, clams, snails, worms, some crustaceans, and fish eggs and larvae are
taken in from the sea floor. Other reports have this species feeding on plankton
but this may be detritus fallen to the sea floor from surface layers
Reproduction
There are two spawning seasons in India, March to June and September to November but individuals may only spawn once a year. Spawning occurs in clear water of sheltered, sandy bays within
about 6 km of shore, away from river mouths, i.e. saline water, and is probably triggered by rising temperatures in spring (25-30°C) and high tides. These conditions give water deep enough to
avoid eggs being eaten by corals and other benthic organisms yet close enough to shore for larvae to reach their preferred inshore habitats. Eggs are fertilised while floating in
surface waters. Fecundity is reported to reach 7.3 million eggs but this is not based on large fish and fecundity could be considerably more. Egg diameters are up to 1.25 mm when fertilised.
Ribbon-like larvae migrate to coastal areas, metamorphose and may enter creeks and estuaries. About 4 weeks later the young leave coastal waters and spread out in waters where there are adequate
supplies of benthic and planktonic food. Some may remain in estuarine areas for 4 years before returning to the sea. In February-March, and again in October in some populations, the adult
migration to inshore waters for spawning takes place. Adults leave coastal waters after spawning.
Parasites and predators
Young are eaten by a wide variety of predators in nearshore waters as indicated by the high egg production and adult survival rate.
Economic importance
This is the most important tropical marine fish used in aquaculture with a history dating back 500 years. Milkfish are raised in brackish or freshwater ponds throughout Asia, based on larvae captured in shore waters. 1.35 billion larvae were caught in the Philippines in 1974. The Philippines, Indonesia and Taiwan produce about 330,000 tonnes of
milkfish per year. Their wide tolerance of environmental variables and herbivorous diet (rice bran and pelleted foods in captivity) combined with fast growth make them a success in aquaculture.
The fish are marketed at 200-300 g. Adults form part of fisheries aimed at other species.
McKinnon and Vine (1992) report that this species is sold in the fish market at Basrah, Iraq. In the Persian Gulf they can be caught by set nets, gill nets, traps
and hook-and-line (Carpenter et al., 1997).
Milkfish have been cultured in concrete ponds at Tiyab, Hormozgan from March to October. Fry were caught in local estuaries such as the Shur River 30 km east of Bandar Abbas. They were fed
commercial carp food pellets and after 7 months weighed 450 g, or on poor protein food 130 g after 11 months with no growth in the cold season (Annual
Report, 1995-1996, Iranian Fisheries Research and Training Organization, Tehran, p. 40, 1997; Forughi-e-Fard and Gharibnia, 1998; Fourooghi-e-Fard, 2000).
They have also been cultured at Tiyab with Indian white shrimp (Penaeus
indicus) and mean weight of shrimps was found to be higher than in
monoculture (Foroughifard, 2001).
Milkfish have been reported as being ciguatoxic (intermittently poisonous through feeding on toxic food) (Bagnis et al., 1970).
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use as food and as bait, in aquaculture and in textbooks.
Conservation
None required as this species is probably an accidental visitor to Iranian fresh waters.
Further work
The culture of this species in southern Iran could be developed further.
Sources
Biology was based on Schuster (1960) and Bagarinao (1994).
Iranian material: CMNFI 1979-0142, 11, 70.5-98.5 mm standard length, Hormozgan, Baghu River (27º17'N, 56º28'E) (Coad, 1981b).
Cyprinidae
This family contains by far the most species in the Iranian freshwater ichthyofauna
and is divided into two files Abramis to Cyprinus (here), and
Garra to Vimba (see
both in
Contents).
The carp or minnow family is one of the most
widespread and speciose families of fishes in the world, certainly the
most speciose in fresh water and possibly the largest family of
vertebrates (the Gobiidae may be the first). The family is found in
North America, Eurasia and Africa. Other common names in English for
species include barbels, breams, roaches, snow trouts, bitterlings,
shiners, daces, chubs, barbs, "sharks", among many others.
There are about 220 genera and over 2420 species (Nelson, 2006), about 8.5% of the world's fishes. In Iran,
the family is represented by about ?32 native genera (interpretations of genera differ
between authors) and
at least ?73 species (with more to be described) found in all the
major drainage basins.
The minnow or carp family is comprised of small to
very large fishes (1 cm and up to 3 m, with some of the largest members in Iran) characterised by throat or
pharyngeal teeth in 1-3 rows, with a maximum of 8 teeth in a row,
tooth counts and form are often characteristic of the genus or
species, no jaw teeth, body form various from fusiform to compressed,
lips are usually thin and not sucker-like (but can show hypertrophy), the upper jaw is bordered
by the premaxillae bones and usually protrusible, barbels are absent
or present in 1-3 pairs (not more than 2 pairs in Iranian species),
body covered in cycloid scales, in some species easily lost, while the
head is scaleless, no adipose fin, the anterior 4 vertebrae are
modified for conduction of sound from the air bladder to the ear and
are known as the Weberian apparatus, pelvic fins are abdominal in
position, no pyloric caeca, air bladder usually present and
well-developed, connected to the gut by a duct, and not enclosed in a
bony capsule, no true stomach, branchiostegal rays always 3 in number,
no true spines in the fins although in some the last unbranched
dorsal fin ray (at the front of the fin) may be thickened and
spine-like and in Cyprinus and Carassius the last
unbranched anal ray is also thickened. The primitive chromosome number
is 2n=50 but polyploidy is common and seen in Cyprinus, Carassius
and in the schizothoracines. Collares-Pereira (1994) argues that the
polyploid condition (e.g. 2n=100) is primitive or plesiomorphic.
There are 2-4 unbranched rays (including
rudimentary ones) in the dorsal and anal fins followed by the more
numerous branched rays (the last two branched rays are counted as
one). The first pectoral and the first pelvic fin ray are unbranched
and not included in counts. Pharyngeal teeth lie on a modified, fifth
gill arch which can be seen or probed behind the shoulder girdle, just
inside the gill opening. The arch has to be removed with dissecting
equipment to count the teeth. Tooth counts are presented as a formula
such as 2,5-4,1 which indicates 2 teeth in the outer left row and 4 on
the inner right row. Teeth may be lost from major or minor rows so
variant formulae are given after the principal one. A horny pad on the
underside of the basioccipital bone of the skull is used to masticate
the food against. Tooth form varies with the food - molar-shaped teeth
are used to crush molluscs, flat but grooved surfaces for grinding
plant food and sharp edged teeth for slicing various invertebrate foods.
Two subfamilies, the Alburninae and Leuciscinae,
are paraphyletic but together seem to form a monophyletic group with a
radiation about 20 million years ago, based on allozyme, cytochrome b,
16S rDNA and mitochondrial control region data from
European cyprinids (Hänfling and Brandl, 2000; Gilles et al., 2001). These two subfamilies
contain many Iranian genera. Zardoya and Doadrio (1999) analysed the cytochrome b
nucleotide sequence of a variety of cyprinids, mostly European, and found
support for two subfamilies Cyprininae (including barbins) and Leuciscinae
(including cultrins, tincins, gobionins, phoxinins and alburnins + leuciscins).
The origin of cyprinids is estimated at 38.9MYA and the separation of Cyprininae
and Leuciscinae at 27.7MYA. They also found the phylogenetic utility of barbel
possession to be limited as they were acquired independently in the two
subfamilies. The number of rows of pharyngeal teeth were a more reliable
phylogenetic marker, at least at the generic level.
Perea et al.
(2010) using mitochondrial and nuclear DNA give details of major cladogenetic
events in the leuciscin lineages in the circum-Mediterranean, involving genera
and species found in Iran.
Chen and Mayden (2009)
investigated the major clades of cyprinids using a multiple nuclear gene
approach and tentatively recommended elevation of certain subfamilies to family.
This is not in general use at this writing and the Cyprinidae is retained as a
single family here.
Durand et al. (2002) using cytochrome b DNA of Cyprinidae conclude that
the the Middle East is an important interchange area for this freshwater
ichthyofauna rather than a centre of speciation. The Middle East leuciscine
cyprinids have Europe as an important Palearctic influence consistent with the
Lago Mare dispersion while the the cyprinine cyprinids show three highly
divergent lineages, namely one shared with the Euro-Mediterranean area (Barbus/Luciobarbus),
a relict of the Lago Mare dispersion, one shared with Africa (Carasobarbus/Varicorhinus
subgenus) and one with Asia (Garra). The Lago Mare
dispersion occurred during a salinity crisis in the Mediterranean Sea 5.5 MY ago
in the Late Miocene when freshwater fish were able to disperse through
oligohaline or fresh water in the Paratethys Sea to reach the Middle East (Bianco,
1990). Some data of Durand et al. (2002) conflict with this
scenario - the Carasobarbus clade that includes Barbus (= Tor) grypus
shows a separation divergence later than the salinity crisis in the Pliocene
when no migration route was available. But note that some authors place Barbus
grypus in the Indian genus Tor and that evidently more work needs to
be done on its relationships and on those of other species that have no evident
Euro-Mediterranean relatives, but whose origins may well lie in the Oriental Region.
Other Middle Eastern cyprinid genera are regarded by
Durand et al. (2002) as relicts of older colonization waves and show an
eastern influence consistent with an Asian origin of the family
Cyprinidae. Cyprinion has no sister species in the
Euro-Mediterranean area and has been isolated in the Middle East since before
the salinity crisis, 7.8-8.8 MY ago. Cyprinion may have entered the
Middle East during the colonization event that isolated the genera Barbus
sensu lato
and Schizothorax in the European and Asian basins respectively. The
divergence of these species is similar in time to the radiation of the
Leuciscinae supposedly centred in Siberia based on fossil records. Siberia
was probably an important dispersion centre for both Leucicinae and Cyprininae
at that time. Otero (2001) describes a ?Barbus sp. (sic) from the Lower Miocene
of Saudi Arabia showing an early date for the entry of cyprinids to the Afro-Arabian Plate.
Some species may enter brackish water but the
family is primarily a freshwater one. Carps have extremely sensitive
hearing via the Weberian apparatus and this is thought to account for
their success. Carps produce an "alarm substance" when
injured. This chemical stimulates other carps to flee and hide,
another useful adaptation. Carps are remarkable for changes they
undergo during the spawning season. Some fish, which are usually
silvery, develop bright reds and yellows. Nuptial, pearl or breeding
tubercles develop on the head, scales and fin rays often in distinct
patterns, and there are swellings of the head or fin
rays in some species. These changes are most apparent in males. Tubercles and swollen
rays are used to clasp females during the spawning act. Generally
males have longer pectoral fins than females. Tubercles are also used
to fight other males and defend and clean nests. Colour attracts
females for mating. Nest building males are larger than females, the
reverse of the situation in most fishes where egg-bearing females are
the largest. Not all species build nests and some simply broadcast
eggs over weed, gravel or sand. Fractional spawning is common in
carps. This is a prolonged spawning season which ensures no single
batch of eggs is lost to unfavourable, temporary environmental changes
such as floods. Carps are mostly omnivores, feeding on small
crustaceans, insects and some minute plants but some specialise in
eating large plants, or other fishes. Diet is reflected in pharyngeal
tooth shape as mentioned above. Gut length is important too. A long
intestine indicates a reliance on plant material which takes longer to
digest. A simple, s-shaped gut is found in insectivorous fish. A black
peritoneum is thought to protect gut bacteria from damaging light. The
bacteria aid in breaking down the strong cell walls of plants. Size
and shape of the mouth are also indicative of diet. Carps are found in
many diverse habitats from swift, cold streams to warm bogs. These are
schooling fishes, especially when young.
Carps play an important role in fresh waters as
food for other fishes and some species are commercially important as
bait fish, as sport fish or as food in Asian countries. Raising
minnows as bait and as forage fish for sport fish is a big business in
the U.S.A. They are an important element in the commercial aquarium
trade and certain species are used in experimental studies by
scientists. Cyprinids were also important in the past, sacred fish
ponds being reported from Mesopotamia in 3000 B.C., and in Iran today
cyprinids associated with mosques and shrines are "sacred".
A general review of Eurasian cyprinids is given by Bănărescu and Coad (1991).
Carp family members are particularly important in
Iran in aquaculture. The "Chinese carps" (Cyprinus carpio
or common carp, also native to Iran, Ctenopharyngodon idella or
grass carp, Hypophthalmichthys molitrix or silver carp, and to
a lesser extent Hypophthalmichthys nobilis or bighead carp) are
the main species used in warmwater culture in almost all the provinces
of Iran. Common, grass and silver carps are processed into fish
fingers in Iran (Iranian Fisheries Research Organization Newsletter, 25:1, 2000). Danesh-e-Khoshashi (1998) describes facilities and methods used for
spawning Chinese carps in Gilan Province. The production of Chinese
carp fingerlings has been relinquished to the private sector in Iran.
The silver carp catch increased from none in 1989 to 24,720 t in 1994
(Food and Agriculture Organization, Fisheries Department, 1996).
Chinese carp production peaked in 2006 at more than 77000 t according to Salehi
(2009) who also reviews carp farming costs. Chinese carp fingerling production was 22.7 million in 1996 (Bartley
and Rana, 1998a). Stakei (1999) studied nutrients, BOD and COD in
manured polyculture ponds with Chinese carps. A review of world
cyprinid culture, with special reference to the Chinese carps, is
given by Billard (1995).
Rana and Bartley (1998a) give details of carp
aquaculture in Iran. They note that silver carp production increased
11% per year between 1991 and 1996 and bighead carp 7%. Most carp
production occurs in the provinces of Gilan, Mazandaran and Khuzestan
and is a private sector enterprise. Carp broodstock is selected based
on head size, colour and gill structure (surface and shape). Adults
are replaced after 3-4 years. Circular concrete tanks are used for
spawning and have egg collecting and incubation devices which reduce
handling to the minimum. The young carp are grown to market size in
ponds or complex fish farms. In 1994, there were 2583 registered farms
with a water surface area of about 8000 ha. Organic and inorganic
fertilizers are used along with supplementary foods. Fertilizers
include urea (135-1500 kg/ha/yr), ammonium phosphate (80-575 kg/ha/yr)
and manure (3-10 tonnes/ha/yr). Supplementary diets include a variety
of grains (100-6000 kg/ha/yr) or, for intensive monoculture of common
carp, high protein pellets (30-40%). Fingerlings are stocked in
March-April at a density of 2000-6000 per hectare and sold between
November and February. Production is 1.6-5.5 tonnes/ha. Cultivated carps are
susceptible to fungal infections as detailed by Ebrahimzadeh et al.
(2000) for the Safid River Fish Farm Centre where 31 species of fungi were isolated
and Firouzbakhsh et al. (2005) where 39 fungal species were identified
from gill lesions in common, silver and grass carp on five fish farms in
Mazandaran.
Rice fields in Iran are now being considered for
fish culture. Experimental production of 300-500 kg per hectare of
"carp seed" (presumably young fish) an 750-1000 kg of fish
and ducks in the autumn after the paddy is harvested (Iranian
Fisheries Research Organization Newsletter, 22:2, 2000). In the early 1970s
intensive carp culture yielded only half the profits of rice culture (Carl Bond
archives, Oregon State University, Corvallis).
Experiments in the Caspian region for artificial
propagation of Aspius aspius and Barbus (= Luciobarbus) brachycephalus
to enhance stocks and for farming Rutilus frisii and Abramis
brama using mono- and polyculture along with Chinese carps have
been carried out (Iranian Fisheries Research and Training
Organization Annual Report, 1992-93; Annual Bulletin 1993-94,
Iranian Fisheries Research and Training Organization, Tehran, p.
77-78, 1995). There are about 3000 fish farms producing over 98% of
the cultured fish in the country. Yearly production of all cultured
fish has increased from 4753 tonnes in 1985 to 45,134 t in 1990.
Production of carps in government hatcheries has risen as follows:
2.19 million fingerlings in 1983, 5.04 million in 1984, 12.84 million
in 1985, 20.83 million in 1986, 19.05 million in 1987, 50.00 million
in 1988, 50.80 million in 1989, 97.70 million in 1990, 58.00 million
in 1991, and 50.00 million in 1992. In addition private sector
production probably equals these figures (Emadi, 1993a). Polyculture
of common, bighead and silver carp has been tried in Iran (Kamaly,
1991). Fish were stocked in four 200 sq m ponds at three densities in
polyculture (2700, 3750 and 4750 by species) and at one density in
monoculture (9500) fish per hectare. Bighead and silver carp attained
a mean weight of 526 and 498 g in polyculture and common carp averaged
343, 190 and 100 g in the same culture but only 13.6 g in monoculture.
The growth rate in summer averaged 94.4, 93.7 and 76.1% for silver,
bighead and common carp in polyculture and 71.9% for common carp in
monoculture. Pen culture in the Caspian Sea has been investigated for Cyprinus
carpio and the various Chinese carps (Iranian Fisheries
Research and Training Organization Annual Report, Tehran, 1992-93).
Semi-artificial breeding of grass, silver and bighead carps has been
carried out in Iran (Iranian Fisheries Research and Training
Organization Newsletter, 6:3-4, 1994; Annual Report, 1994-1995,
Iranian Fisheries Research and Training Organization, Tehran, p. 39,
1996). Hormone injections were used to induce breeding of fish
held in a round trough for spawning with a rectangular egg collection
trough and a round egg hatching trough. Spawning occurred within
6.5-12.5 hours of injection. The percentage of hatched larvae in this
semi-artificial method was higher than a control artificial method
where eggs are kept in incubators. The increase was 6% for grass carp,
33.72% for silver carp and 16.7% for bighead. Active larvae increased
from 180,000 to 450-500,000 for grass carp, from 157,000 to
400-450,000 for silver carp and from 680,000 to 970,000 for bighead
carp. Additionally female breeder mortality was 3.37% less for grass
carp and 45.19% less for silver carp.
Many carp species can be caught on hook and line by various angling
techniques but outside the larger rivers of Khuzestan and the Caspian
shore this hobby is not much pursued. Even small species and specimens
can give some sport on light tackle such as worm baited hooks
including Luciobarbus barbulus, Carasobarbus luteus, Alburnus mossulensis,
Cyprinion macrostomum and Garra rufa among others.
Fingerlings of Labeo rohita, an Indian carp, were imported to Gilan in
Iran in 2004 to enrich the diversity of cultured fish and increase protein
production. There is always the potential for escapes and establishment of this
exotic.
Genus Abramis
Cuvier, 1816
The bream genus comprises 4 species found in Europe, Asia Minor and the Caspian and Aral Sea basins. There are 2 species in Iran
but see also Blicca and Vimba.
The genus is characterised by a strongly compressed
and deep body, a scaleless keel between the vent and pelvic fins, a
scaleless groove on the back in front of the dorsal fin but not behind
the fin, pharyngeal teeth in 1 row, compressed and with a groove on
the grinding surface, dorsal fin short and spineless, anal fin long to
very long, and lateral line decurved.
Durand et al.
(2002) studying cytochrome b data concluded that this genus is not
monophyletic since A. ballerus and A. sapa are placed basal to a
group of species including A. brama, Blicca bjoerkna, Vimba
species, Acanthalburnus microlepis and Acanthobrama.
.Abramis brama
(Linnaeus, 1758)

Common names
سيم (sim or seam
= silver), ماهي سيم (= mahi-ye sim, meaning silver fish).
[capag, chakag, chapakh or chipakh, all in Azerbaijan; gundogar tarany (topi) in Turkmenian; vostochnyi leshch or
Oriental bream in Russian; common, bronze, eastern or carp bream].
Systematics
Cyprinus Brama was originally described from Sweden.
Abramis brama orientalis Berg, 1949 is reported for the
Caspian and Aral Sea basins but Koshara and Izyumov (1991) restricted
this subspecies to the Aral Sea with the type subspecies in the
Caspian Sea basin. They did not examine any Iranian material. Kozhara
and Mironovskiy (1988) using numbers of pores in the seismosensory
canals for samples taken over a wide range of this species identified
8 population groups but did not recognise subspecies. Some earlier
works also indicate that no subspecies exist (see Reshetnikov et al., 1997).
Caspian material reportedly has more gill rakers, fewer vertebrae
and fewer scales than the type subspecies from the Baltic Sea basin
(Berg, 1948-1949) but further study over the whole range of the
species is needed to clarify the situation, analyzing for clines. The
Iranian populations are referred to the type subspecies for the
moment. The type locality of this subspecies is the Aral Sea at Muinak
and Lake Yaskhan in the Uzboi.
Khara et al. (2007; 2007) compared fish from the Anzali wetland and the Caspian
Sea, and the Caspian Sea and Aras Dam, both meristically and morphometrically. Significant differences were noted
in particular for morphometric characters in the former comparison and
morphometrically and meristically in the second. These differences were attributed to differing
habitats and environmental conditions. Ghasemi et al. (2007) used 5
microsatellite loci in comparing Iranian and Azeri bream and found Iranian
stocks have reduced genetic variability attributed to inbreeding and genetic
drift. Khara et al. (2009) compared fish
from the Anzali Wetland the southern coast of the Caspian Sea in Iran and the
southwest coast in Azerbaijan using mtDNA. The greatest genetic diversity was
found in Azerbaijan which was significantly different from the Iranian samples,
which were not themselves significantly different.
Abramis brama bergi Grib and Vernidub, 1935 (preoccupied by Abramis
sapa bergi Belyaeff, 1930 according to Eschmeyer et al.
(1996)) was originally described from the Aral Sea at Muinak and is
also found in the Uzboi Valley of Turkmenistan, north of the Iranian
border (Berg, 1948-1949). It was replaced by Abramis brama orientalis.
A syntype of Cyprinus brama is in the Natural History
Museum, London as a skin under BM(NH) 1853.11.12:147 (Eschmeyer et al., 1996).
Artificial hybrids with Rutilus frisii kutum and Rutilus
rutilus (may involve R. caspicus) have been bred in Iran (Annual Report, 1994-1995,
Iranian Fisheries Research and Training Organization, Tehran, p. 39-40, 1996).
Key characters
The scaleless keel on the belly, deep body, high number of branched rays in
the anal fin (22-30), modally 9 branched dorsal fin rays, and uniserial
pharyngeal teeth are key characters.
Morphology
The mouth is small but highly protrusible. There is a strong dorsal ridge
anterior to the dorsal fin. Dorsal fin with 3 unbranched and 8-10,
usually 9, branched rays, anal fin with 3 unbranched and 22-30
branched rays. Lateral line scales 48-60. The lateral line is
moderately decurved. Scales are regularly arranged, sheathing the anal
fin base. Scales have numerous fine circuli but only relatively few
posterior and even fewer anterior radii. In a fish about 6 cm long
there are as few as 8 total radii. The focus is almost central and the
anterior scale margin is wavy. There is a pelvic axillary scale. The
ventral keel between the pelvic fin bases and the anal fin is
well-developed. Gill rakers number 18-30 and are short, reaching the
raker below when appressed. They are strongly tuberculate on the inner
surface. Vertebrae 38-47, usually 42-44 in the Caspian populations
(lower counts in literature may not include 4 Weberian vertebrae). The
chromosome number is 2n=50-52 (Klinkhardt et al., 1995).
The chromosome number based on fish from the Iranian coast of the Caspian Sea is 2n = 50 with the number of arms NF = 82 and the karyotype being 8 pairs of metacentric, 8 pairs of submetacentric and 9 pairs of
acrocentric chromosomes (Nahavandi et al., 2001).
Pharyngeal tooth formula modally 5-5, with variants of 6-5
(2.2-4.8%), 5-4 (2.2-4.4%) and 4-5 (8.6%) for collections from the
Caspian and Aral seas basins in former Soviet waters (Vasil'yeva and
Ustarbekov, 1991). Other variants are summarised in Tadajewska (1998).
Teeth bear a small hook at the tip in the main row and have long,
narrow and flat crowns. In young fish, the hook is more pronounced and
the crown has a few tubercles or a series of serrations. The gut is s-shaped with a small anterior loop.
Khar et al. (2007) compared this species from the Caspian Sea and the
Anzali wetland and found significant morphometric, but not meristic,
differences, attributing this to habitat conditions.
Meristic values for Iranian specimens are:- dorsal fin branched rays 9(12) or
10(1); anal fin branched rays 24(3), 25(3), 26(1), 27(4) or 28(2);
pectoral fin branched rays 16(7) or 17(6); pelvic fin branched rays
8(13); lateral line scales 49(2), 50(2), 51(3), 52(3), 54(1) or 55(2);
total gill rakers 23(3), 24(2), 25(6), 26(1), or 27(1); total
vertebrae 44(12) or 45(1); and pharyngeal teeth 5-5(12).
Sexual dimorphism
Males bear tubercles on the head, body and fins. Scale tubercles
appear singly, in pairs or occasionally as 3 per scale. There is some
evidence of differences in gill raker counts between the sexes but
sometimes the males have higher mean counts and sometimes the females.
Abdurakhmanov (1962) reports eye diameter, greatest body depth and
predorsal distance to be greater in females and dorsal fin base
length, pectoral and pelvic fin lengths and interorbital width to be
greater in males from Azerbaijan.
Colour
In Dagestan, the resident form is darker in colour than the semi-anadromous
form (Shikhshabekov, 1969). Overall colour is silvery. The iris is
silvery with a little grey pigment on the upper part. The dorsal and
caudal fins are pale grey, almost transparent, to a greyish-blue, the
pectoral fins may be grey or colourless, and pelvic fins are
colourless. All fins except the pectorals have black tips. Large fish
are a dark olive-green on the back and bronze on the flanks and old
fish may have all fins black. The peritoneum is silvery to light brown
in preserved fish.
Size
Attains 90.0 cm total length and 11.55 kg, possibly 100.0 cm and 16.4 kg.
Distribution
Found from the British Isles across Europe north of the Pyrenees
and Alps eastwards to the Black, Caspian and Aral sea basins although
not in western Transcaucasia. In Iran it is found from the Astara to the Atrak
rivers in the whole Caspian Sea basin (Kozhin, 1957) including the Anzali Mordab, its outlets and tributaries
and the Siah-Keshim Protected Region (Holčík and Oláh, 1992; Riazi, 1996; Kiabi et al., 1999),
the Safid River (Abbasi et al., 1999), Gorgan Bay (Derzhavin, 1934), and freshened areas of the Caspian Sea.
It s also found in the Aras Dam (Khara et al. 2007).
This species is also recorded from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov,
1995) and may eventually reach Iranian waters in the Tedzhen (= Hari)
River basin where it has been reported by Aliev et al. (1988).
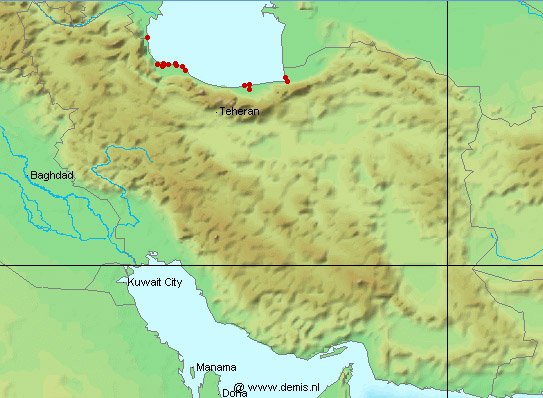
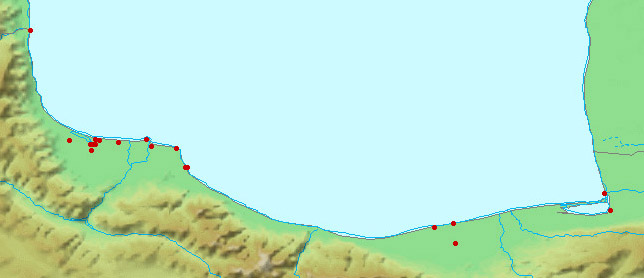
Zoogeography
This species is part of a northern European and northern
Southwest Asian fauna whose zoogeographical history has not been researched. It
origins may lie in a Danubian or Sarmatian fauna.
Habitat
The bream prefers still water and is low in numbers even in rivers
with weak current. Abundant littoral vegetation and a very muddy
bottom are favoured in lakes for reproduction and feeding
respectively. It retreats to deeper water in winter, forming schools
numbering in the many thousands, packed densely together (Muus and
Dahlstrøm, 1999). It is more numerous in the Anzali Mordab along the Caspian coast of
Iran (Jolodar and Abdoli, 2004).
This species can tolerate high temperatures of 33-34°C
in southern areas like Iran for a time but above 28°C growth rate
decreases. Adults can live in a salinity of 12.9‰,
perhaps 14‰, and eggs may be fertilised at a salinity of 10.2‰.
However preferred levels are 2-4‰. Salinity and water level changes have significant effects on abundance
in this species. Population densities vary markedly in both fresh and brackish water populations.
Bream living in the Caspian Sea basin are semi-migratory. They feed
in the brackish sea but spawn and winter in the lower reaches and
deltas of large rivers. A spring migration up rivers begins with ice
melt or warmer temperatures in the sea and after spawning the fish
return to disperse and feed in the sea. In the fall the fish migrate
into the deeper parts of river deltas. In Russian parts of the Caspian
they are found at depths not exceeding 4-5 m but Knipovich (1921)
reports them at 14.6-16.5 m, possibly deeper, in the Iranian Caspian Sea.
There were spring and winter migrants in the southwestern Caspian
including the Anzali Mordab (A. M. Shukolyukov in Berg, 1948-1949).
The spring bream had a longer snout, deeper head, lower body, lower
dorsal and anal fins, and more scales. The spring bream entered the
Mordab for spawning only while the winter bream overwintered in bottom
pools. Changing conditions in the mordab environment in the late 1980s
and the 1990s may have altered this migration. Riazi (1996) reports
that this species migrates into the Siah-Keshim Protected Region of the Anzali Mordab.
Age and growth
The resident form in Dagestan is slightly inferior in length (2-3
cm), weight and age to the semi-anadromous form (Shikhshabekov, 1969).
In Dagestan, the resident form becomes sexually mature at 3 years for
females and 2 years for males at lengths of 23-26 cm and weights of
200-240 g while the semi-anadromous form matures at 4 years and a
length of at least 25-28 cm and a weight of 250-300 g. In Uzbekistan
females mature at lengths ranging from 10.5 to 27 cm in different
reservoirs, usually at age 3 (Kamilov, 1994). Maturity is attained at
a younger age in southern waters generally in this species and this
probably applies in Iran. The maturity range is 2-10 years with males
often maturing a year earlier than females. Females predominate in the
older age groups.
Maximum age exceeds 32 years although in southern waters the
maximum age does not exceed 15 years. Semi-migratory bream of the
Caspian Sea have a fast growth rate and a short life cycle, reaching
37.5 cm standard length by age 8.
Most fish examined by Razivi et al. (1972) from commercial
catches in Iran were 3-6 years old, 25.6-39.8 cm long and weighed
249-950 g. Over a three year period there was a decline in average age.
Young and immature fish formed most of the catch in 1998-1999 when one-year-old
fish comprised 20.3% and two-year-old fish 37.3%. The average length, weight and
age for 1998-1999 were 22.5 cm, 212.2 g and 2.4 years. The rate of recruitment
was 4.6% in 1991 and 2.7% in 1992 (Saiad Borani, 2001). Abdolmalaki (2005b)
studied Caspian Sea fish from Iran and found mean fork length, weight and age to
be 21.7 cm, 191 g and 2.72 years, respectively. The length-weight relationship
was W = 0.2312L2.9 and von Bertalanffy growth parameters were Lt
= 45[1-exp-0.125(t 2.768)], and the instantaneous rate of total (Z),
natural (M) and fishing mortality (F) were 0.92 year-1, 0.28 year-1 and 0.64
year-1, respectively. The exploitation rate (E) was 0.7. Biomass was calculated
as 46.362 t and the maximum sustainable yield was 14.99 t.
Food
Young fish feed on zooplankton. Adults use a strong sucking power
and a tube-like snout to feed on invertebrates and detritus in mud.
This sucking action leaves evident "bream pits" in soft mud,
depressions about 10 cm across. In the northern Caspian Sea food items
include Cumacea, Corophiidae, the clams Adacna (69% by weight)
and Monodacna, Tendipedidae (= Chironomidae), Polychaeta,
Gammaridae, Mysidae, and Oligochaeta. When overcrowded or in turbid
conditions, plankton may be eaten in addition to the normal foods (Muus
and Dahlstrøm, 1999). Large specimens may feed on small fishes. A specimen from the Langarud, Gilan, 158.6 mm
standard length, contained chironomids.
Reproduction
Bream enter the Kura River from December to February with a peak in
January (Berg, 1959) and travel some distance upriver. These fish have
an average length of 31.1 cm and an average weight of 633 g. Length
and weight in Azerbaijan vary from 25.4 to 31.9 cm and 306 to 681 g.
Bream enter the Anzali Mordab, the main spawning area in the southern
Caspian, in the first half of March until the beginning of May. Males
precede females on the spawning ground by about 3 days and males
outnumber females by about 3 to 1. Spawning begins in the first half
of April in shallow water and lasts until mid-May. Fecundity in
Dagestan reservoirs reaches 191,000 eggs (Shikhshabekov, 1969), in
Uzbekistan reservoirs 772,000 eggs (Kamilov, 1994) and a maximum
elsewhere of 941,000 yellowish eggs is reported. Bream spawn
repeatedly with different partners and although most bream spawn only
once a year, multiple spawnings are known. Spawning occurs in masses
over a period of 2-3 days triggered by temperatures of 12-13°C
or above. The commonest spawning temperature for the species overall is 16-18°C.
Spawning is most intensive at night in some populations while others
show late morning and late afternoon peaks. There is much splashing of
the water by their tails and the noise can be heard some distance away
although the fish are easily scared into deeper water by any noise
like human voices. Males probably defend territories, attracting
females and scaring other males away. There can be up to 2.3 million
eggs per sq m however, suggesting that many fish may spawn in the same
area. Eggs are deposited in quiet water, most commonly at depths of
20-80 cm, and they adhere to aquatic plants or flooded land plants.
Eggs are up to 1.9 mm in diameter.
Parasites and predators
Jalali and Molnár (1990a) record the monogenean Dactylogyrus
zandti from this species in the Safid River. Sattari and Faramarzi
(1997) record Caryophyllaeus fimbriceps from 28% of bream in
the Anzali lagoon. Naem et al. (2002) found the following parasites on
the gills of this species from the western branch of the Safid River, namely the
monogenean trematodes Dactylogyrus zandti and D. wonderi. Masoumian et al. (2005) report the protozoan
parasites Ichthyophthirius multifilis and Trichodina perforatafrom
this species in the Aras Dam in West Azarbayjan.
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. elegans from fish in the Safid River.
Sattari et al. (2004, 2005) survey this species in the Anzali wetland, recording
Raphidascaris acus larvae.
Pazooki et al. (2007) record various parasites from localities in West Azarbayjan Province, namely Ligula
intestinalis, Digrama sp., Argulus foliaceus and Caryophyllaeus laticeps. Sattari et al. (2007)
record the cestode Caryophyllaeus fimbriceps, the digenean Diplostomum
spathaceum and the monogeneans Dactylogyrus extensus and
Gyrodactylus sp. in this species in the Anzali wetland of the Caspian shore
and also mention that the monogenean Diplozoon sp. is also known from
this species in the Iranian Caspian Sea.
Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984). Various predatory fishes take bream including Huso huso, Perca
fluviatilis, Sander lucioperca, Aspius aspius and Silurus glanis
but this is comparatively rare especially when bream exceed 20 cm in
length. Birds such as grebes, herons, divers and cormorants are also predators.
Economic importance
This species is an important food fish being both tasty and of
large size. In addition it can live out of water for some time and
thus remain fresh while being transported to market.
Nevraev (1929) gives catches for various fishing regions in Iran in
the early twentieth century. For the Anzali region from 1901-1902 to
1913-1914 the catch was 2283 to 419,117 individuals, for the Safid
River region from 1908-1909 to 1917-1918 the catch was 17,195 to
474,200 individuals (rising steadily but falling in 1917-1918) with no
fish reported in the years 1899-1900 to 1907-1908 and in 1918-1919,
and in Astrabad (= Gorgan) region from 1900-1901 to 1912-1913 the
catch was 20,600 to 1,381,500 individuals with no clear trend, the
catches varying markedly from year to year. The commercial catch in
Iran from 1956/1957 to 1961/1962 varied from 0 to 158 kg (Vladykov,
1964), from 1965/66 to 1968/69 varied from 0 to 29 tonnes (Andersskog,
1970) and from 1963 to 1967 from 0.5 to 16.0 tonnes (with no reported
catch in the first 3 years)(RaLonde and Walczak, 1970b). The catch in
the Bandar-e Anzali region from 1933/34 to 1961/62 varied between only
2 kg and over 1394 t with some years reporting no catches. Holčík
and Oláh (1992) report a catch of 34 kg in the Anzali Mordab for 1990
and for the period 1932-1964 catches ranged from none to 1133.5 tonnes
annually. The total catch of the Northern Shilot (Fisheries Company)
from 1965/66 to 1968/69 varied between 13 and 74 t (RaLonde and
Walczak, 1972). There are obviously wide variations in annual catches
and/or in reporting statistics. The general trend is one of decline in
catches with large fish being caught and the average stock size being
lowered, resulting in a decreased spawning success. This species has a
deep body and immature fish are easily caught. The catch in the Anzali
Mordab was important until the end of the 1940s but had virtually
disappeared by the 1980s (Petr, 1987). Abdolmalaki (2005b) gives a total catch
of 17 t for the 2000-2001 fishing season, only 0.1% of the commercial catch in
Iranian coastal waters of the Caspian Sea. In contrast, the total catch for Iranian waters was
estimated at 26.3 tons of which 15.4 tons was from beach seines; most fish were
immature and undersized (Abdolmalaki, 2006a).
In former Soviet waters of the Caspian Sea, the age composition in
commercial catches was 2-10 years, with the great majority being 3-5
years old. Trawls, seines, pound nets and gill nets are used in the
northern Caspian Sea to catch the bream with 60-70% being taken in
spring. Spawning and breeding farms were established in the former
Soviet Union to rear young fish. Catches in the Volga-Caspian and Ural
regions has been as high as 344,900 centners, prior to 1930, and in
the Aral Sea in 1931 the catch was 115,200 centners.
Mono- and poly-culture of this species has been carried out in Iran
(Annual Bulletin 1993-94, Iranian Fisheries Research and Training
Organization, Tehran, p. 77-78, 1995). Polyculture comprised 70% Abramis
brama, 20% silver carp (Hypophthalmichthys molitrix) and
10% grass carp (Ctenopharyngodon idella) and gave a greater
yield than monoculture. From an average initial weight of 30 g, fish
attained averages of 188 or 211 g in monoculture (average 200 g) and
221 or 278 g (average 250 g) in polyculture with maximum weights of
300 or 580 g at the end of two one-year periods. Water temperatures were 9-33°C
(Annual Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 38-39, 1996; Danesh-e-Khoshashi, 1997).
A state supported stocking programme has released about 70-80
million fingerlings into the Anzali Mordab, all descended from a
single pair mating 8 years ago (Rana and Bartley, 1998a; 1998b). These
fish are intolerant of low oxygen and so perform poorly under pond
conditions. Stocks may be imported from Azerbaijan in the future (Rana
and Bartley, 1998b). The release of 70.46 million fry in 1992-1993 to 1998-1999
period has not been successful in restoring the stocks in Iran. Stock depletion
was attributed to improper fishing methods, pollution, destruction of spawning
grounds, presence of predatory Esox lucius and Silurus glanis in
fry stocking areas, and lack of necessary arrangements in regard to artificial
spawning (Saiad Borani, 2001).
The roe or eggs of this species have been implicated in poisoning
(Halstead, 1967-1970; Coad, 1979b) and should be avoided (see under
the genus Schizothorax for more information on egg poisoning).
Fish should be carefully cleaned in the spawning season to remove the
eggs and ensure against contamination of flesh. Severe cases of egg
poisoning in other species have resulted in death.
This species has been used in Iran for
experimental studies, e.g. on the toxicity and LC50 of phenol and
1-naphthol (Shariati et al., 2004).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and in aquaculture.
Conservation
The subspecies has been proposed for inclusion in the "Red
Book of the U.S.S.R." which forms the basis for measures to
protect species (Pavlov et al., 1985).
RaLonde and Walczak (1970b) reported that 90% of the bream caught
in Iran in 1970 were immature and the stock was in danger of
extinction. About 19-20% of commercial catches in the Volga region are
from hatchery raised stock (Petr, 1987) and it was thought that
stocking could help this species in Iran. During the 1980s and 1990s
there were practically no catch figures for this species in Iran.
Artificial propagation began in 1986 on an experimental basis and 6
million fish were released (Ghenaat Parast, 1993). In 1992-1993 (an
Iranian calendar year), 2.4 million fingerlings were released into the
Anzali Mordab and nearby rivers, a 100% increase over the previous
year (Abzeeyan, Tehran, 4(2):VI, 1993). Total production in
government hatcheries for 1990 was 0.66 million fingerlings, in 1991
2.28 million and in 1992 5.3 million fingerlings (Emadi, 1993a).
Fingerling production was 11.217 million in 1995 and 8.5 million in
1996 (Bartley and Rana, 1998a; 1998b). In 1999-2000, 20 million
juveniles were released (Iranian Fisheries Research Organization
Newsletter Newsletter, 23:4, 2000). From
October to March 2000, 14 million juveniles raised in the Shahid
Ansari aquaculture and breeding centre in Gilan were released into the
Caspian Sea and neighbouring water bodies (Iranian Fisheries Research
Organization
Newsletter, 26:2, 2001). Illegal fishing and non-standard nets threaten the stocks (Annual
Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 19-20, 1997). Billard and Cosson (2002) give an
annual production of 15 million alevins.
Ramin (1997) details studies on the artificial breeding of this
species in Iran, based on 38 brooders, with the goal of saving it from
extinction. Gonadotropic hormone extracted from the pituitary of the
common carp was used to induce brooders. One or two doses at 5-6 mg/kg
body weight gave optimum stripping of eggs at 18°C.
Fertilisation rate was 75-95% and hatching rate was 75-85%. Incubation
took nearly 4 days at 18-21°C.
The grey, pink or yellow eggs numbered 9142-60,050 per spawner with a
swelled diameter of 1.0-1.2 mm. The yolk sac was absorbed after 72
hours and newly hatched larvae were 2.9-3.7 mm long.
Khara et al. (2009) (see above) carried out their molecular study in
order to determine sources for broodstock to increase genetic diversity after
losses from overfishing, pollution and loss of spawning regions.
Kiabi et al. (1999) consider this species to be vulnerable
in the south Caspian Sea basin according to IUCN criteria. Criteria
include commercial fishing, sport fishing, few in number, habitat
destruction, limited range (less than 25% of water bodies), not
present in other water bodies in Iran, and present outside the Caspian
sea basin. Nezami et al. (2000) consider this
species to be endangered because of overfishing, habitat destruction and
spawning groundn degradation.
Further work
Stocks should be carefully monitored on a continuing basis and
efforts made to resurrect this commercial species.
Sources
The chief literature summary for earlier works is Backiel and
Zawisza (1968) although little apparently refers to the Caspian basin
populations and even less to those of the Iranian shore. Nevertheless
this work gives a general overview of biology and general comments
above are based on it.
Iranian material: CMNFI 1970-0542, 4, 75.4-173.7 mm standard length, Gilan, Old Safid River estuary
(37°23'N, 50°11'E); CMNFI 1970-0543A,
1, 70.0 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37°24'N, 49°58'E);
CMNFI 1971-0343,
1, 158.6 mm standard length, Gilan, Langarud at Chamkhaleh (37°13'N, 50°16'E);
CMNFI 1980-0127,
3, 166.1-170.1 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37°24'N, 49°58'E);
CMNFI 1980-0142,
1, 160.6 mm standard length, Gilan, Nahang Roga River (no other locality data);
CMNFI 1980-0906, 3, 105.6-176.0 mm standard length, Gilan, Caspian Sea basin (no
other locality data).
Abramis sapa
(Pallas, 1814)
Common names
سابا, سبا (saba, from the species name),
سيم (sim = silver), ماهي سيم
كند پوزه (= mahi sim kondpuzeh,
meaning bluntsnout silver fish).
[pori or poru, both in Azerbaijan; tarashka, taran' and rybets,
erroneously in Azerbaijan; yuzhnokaspiiskaya beloglazka or South
Caspian white-eye bream in Russian; white-eye bream, southern
white-eye bream, Danube bream].
Systematics
Cyprinus Sapa was originally described from the Sura, Samara
and Kinel' rivers in the Volga River basin. No types known.
May be placed in the genus Ballerus Heckel, 1843 (see Hensel
(1978), Shcherbukha (1973), Howes (1981), Bogutskaya (1986) and Bogutskaya and
Naseka (2004) for various opinions). The nominate subspecies was described from the
Volga River and tributaries.
The subspecies reported from the southern Caspian Sea basin is Abramis
sapa bergi Belyaev, 1929, described from the Kura River in
Azerbaijan. Eschmeyer et al. (1996) date this subspecies to
1930 although the article is dated 1929. Recognition of subspecies is
disputable (Reshetnikov et al., 1997).
Key characters
The scaleless keel on the belly, deep body, very high number of branched rays
in the anal fin (31-44), modally 8 branched dorsal fin rays, and uniserial
pharyngeal teeth are key characters.
Morphology
Dorsal fin with 2-3, usually 3, unbranched and 7-9, usually 8,
branched rays, anal fin with 3 unbranched and 31-44, mostly 34-38
branched rays, pectoral fin branched rays about 15 and pelvic fin branched rays
about 8. Lateral line scales 42-55, mostly 51-52, regularly
arranged over the body. Scales bear numerous very fine circuli, an
almost central focus, numerous to few posterior radii (quite variable
between scales of similar size) and few to none anterior radii. The
anterior scale margin is wavy. A pelvic axillary scale is present.
There is an evident, scaleless keel on the belly between the pelvic fin bases and
the anal fin. Gill rakers 18-25, short, reaching the raker below or
almost the second raker when appressed. Vertebrae 45-48. Pharyngeal teeth
5-5, with elongate, narrow and flattened, concave or rounded crowns below a hooked tip.
The gut is s-shaped with a small anterior loop. The
chromosome number is 2n=50 (Klinkhardt et al., 1995).
Belyaev (1929) for Kura River fish gives lateral line scale counts
as 48(3), 49(6), 50(24), 51(50), 52(54), 53(16) or 54(7) and anal fin
branched rays as 32(1), 33(5), 34(22), 35(32), 36(38), 37(47), 38(25),
39(9), 40(5), 41(2) or 42(1). This subspecies is distinguished from
the type form in the Black Sea (Don River) by fewer lateral line
scales and anal fin branched rays, a longer snout, smaller eyes, less
deep body, lower dorsal fin, shorter anal fin, and longer postorbital length.
Sexual dimorphism
Unknown.
Colour
The Caspian subspecies has a dark back with a bluish tint, flanks
and belly are silvery, fins are a greyish-white and sometimes have a
black margin, and the iris is silvery. The peritoneum is dark brown in
preserved fish.
Size
Attains 41 cm and 0.8 kg.
Distribution
Found in the basins of the Black, Caspian and Aral seas. Reported
from the gut of a Silurus glanis in the Anzali Mordab (Derzhavin,
1934) but not found in recent years (Holčík and Oláh, 1992). Other reports are from the
lower Safid River at Hasan Kiadeh (Belyaev, 1929;
Derzhavin, 1934) and in the Aras River at Karadonly (Berg, 1948-1949).
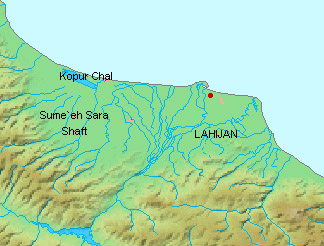
Zoogeography
This species is part of a northern European and northern Southwest Asian
fauna whose zoogeographical history has not been researched.
Habitat
This species feeds in brackish water but spawns and overwinters in
the lower reaches of rivers. It is commonest along the western shore
of the middle and southern Caspian Sea.
Age and growth
Females are 28-29 cm long on average, maximum 39 cm, while males
are about 24 cm, maximum 30 cm (Belyaev, 1929). Males and females
mature at 2-3 years and life span is 5 years in Azerbaijan (Abdurakhmanov, 1962).
Food
Food items include small molluscs, crustaceans and insect larvae as
well as some plant fragments and detritus. Young feed on zooplankton.
Reproduction
A migration into rivers, particularly the Kura, occurs in winter
when temperatures fluctuate from 5 to 10°C (Belyaev, 1929). The run begins in November and peaks in January. The
Kura migration was once over 700 km from the mouth. Spawning occurs in
rivers with gravel bottoms or dense vegetation from April to May.
Fecundity reaches about 150,000 eggs with diameters up to 1.8 mm. Eggs adhere to
stones or plants.
Parasites and predators
Eaten by Silurus glanis (Derzhavin, 1934).
Economic importance
Up to 1-2 million fish were caught in the Kura at spawning (Belyaev,
1929). The annual average catch in Azerbaijan in 1931-1935 was
1,860,000 fish weighing 6200 centners.
Conservation
The subspecies A. sapa bergi has been proposed for inclusion
in the "Red Book of the U.S.S.R." which forms the basis for
measures to protect species (Pavlov et al., 1985). It has
always been very rare in Iran and its absence from the Anzali Mordab
may be due to loss of spawning grounds (Holčík and Oláh, 1992). Lelek
(1987) considers this species to be rare to vulnerable in Europe.
Further work
The status of this species in Iran should be assessed by field
surveys. It is apparently quite rare and was not caught during two
collecting trips along the Caspian shore in the 1970s. It is recorded
only from two localities in Iran in 1929 and 1934.
Sources
Iranian material:- None available, based on literature reports.
Comparative material:- CMNFI 1986-0458, 2, 209.0-211.7 mm
standard length, Germany, Danube River (48º58'N,
12º18'E); BC 59-301, 2, 136.3-154.2 mm standard
length, Ukraine, Tisa, Danube drainage (no other locality data).
Genus Acanthalburnus
Berg, 1916
This genus contains only 2 species, both found in Iran. Berg
(1948-1949) characterises it as similar to Alburnoides but with
the last unbranched dorsal fin ray thickened into a spine which is
strong basally but becomes thinner and flexible on about the last
third of the ray length. Pharyngeal teeth are in 2 rows as opposed to
1 row in Abramis. Durand et al. (2002) include this genus in the Abramis clade based on
cytochrome b data while
Perea et al.
(2010) using mitochondrial and nuclear DNA propose synonymy with Acanthobrama.
Acanthalburnus microlepis
(De Filippi, 1863)
Common names
مرواريد ماهي لب نازك
(= morvaridmahi-e labnazok), kuli.
[garagas or taxta balig, both in Azerbaijan; chernobrovka and napota in Russian; blackbrow bleak].
Systematics
Abramis microlepis was described from the "Kur, presso
Tiflis" (= Kura River near Tbilisi, Georgia) and the holotype is
in the Istituto e Museo di Zoologia della R. Università di Torino
under MZUT N.673 (Tortonese, 1940; Eschmeyer et al., 1996).
Alburnus punctulatus Kessler, 1877, described from the Kura
River at Tiflis (= Tbilisi) and Borzhom, Georgia, is a synonym. A
syntype of Alburnus punctulatus from the St. Petersburg Museum,
84.6 mm standard length, from "R. Kura, Tiflis" is in the
Natural History Museum, London (BM(NH) 1897.7.5:34).
Alburnus Brandtii is apparently a manuscript name for this
species first reported without a formal description in Brandt (1880)
and listed as "Alburnus Brandtii n. sp. 1 ex. Tschaldyr"
and attributed to K. Kessler in the account of the travels of
Professor A. F. Brandt in Transcaucasia (see Kavraiskii, 1897).
Bogutskaya (1997b) lists it as a nomen nudum.
Alburnus microlepis of Kamensky (1901), which is Acanthalburnus
microlepis, should not be confused with Alburnus microlepis
Heckel, 1843, a distinct species described from Aleppo (= Haleb, Syria).
Key characters
This species is distinguished from A. urmianus by having
more lateral line scales, more anal fin branched rays, fewer gill
rakers and gill raker morphology according to Saadati (1977). Gill
raker counts are the same but scale and anal fin ray counts are
generally higher with some overlap. Gill raker morphology does not
appear to differ in the fish examined by me. Distribution is the
easiest separating factor. Both species are distinguished from other cyprinids
in Iran by the dorsal fin spine, 2 rows of pharyngeal teeth, and fin ray and
scale counts.
Morphology
Dorsal fin with 3 unbranched and 7-9, usually 8, branched rays,
anal fin with 2-4, usually 3, unbranched and 12-19, usually 15-17,
branched rays. Pectoral fin branched rays 12-17 and pelvic fin rays
7-9. Lateral line scales 60-87. There is a large pelvic axillary
scale. Scales at the base of the anal fin are somewhat enlarged and
may be vertically elongate, forming a sheath. The scale focus is
sub-central anterior with fine but not numerous circuli and very few
posterior radii (less than 10 main radii in the largest fish seen).
Gill rakers 6-12 and sickle-shaped (Saadati, 1977) but this count
presumably includes only lower arch rakers. Total gill rakers 10-14,
short and only reaching the adjacent raker when appressed. The rounded
raker has a triangular flap on its internal surface with the tip of
the rounded raker projecting. The raker tip may be squarish or even
forked in larger fish. The inner edge of the flap is finely
tuberculate. Vertebrae 40-45. Pharyngeal teeth 2,5-5,2 with variants
2,5-5,1, 1,5-5,2, 1,5-5,1, 3,5-5,2, 2,5-4,2, 2,5-4,1, 2,4-5,1, 2,4-4,1
1,5-4,1, 1,5-4,0, 1,4-5,2, 1,4-5,1 and 2,6-5,2. The teeth are hooked
at the tip with an elongate flat area below and the largest tooth may
be strongly serrated. The posteriormost major row tooth may be almost
vertically above the fourth tooth rather than posterior to it. The
last unbranched dorsal fin ray is thickened in its lower two-thirds
but the last third is thin and flexible. There is an obvious scaleless
keel from the pelvic fins to the vent on the belly mid-line. The mouth
is oblique and subterminal in adults and most young, oblique and
terminal in some young. The gut is relatively short with anterior and
posterior loops.
Both males and females, as well as young, may have fine tubercles
distributed over the head and especially well-developed ventrally and
even on the lips. Belly and lower flank scales have fine tubercles
concentrated at the base of the exposed scale, some lining the scale
margin. Fine tubercles line the dorsal and ventral surfaces of the
pectoral and pelvic fins concentrated on rays but also on membranes,
in a single file or variably dispersed.
Meristic values for Iranian specimens are:- dorsal fin branched rays 7(1) or
8(52); anal fin branched rays 14(4), 15(23), 16(24) or 17(2); pectoral
fin branched rays 14(3), 15(36), 16(11) or 17(3); pelvic fin branched
rays 8(53); lateral line scales 60(1), 62(2), 63(4), 64(6), 65(11),
66(6), 67(7), 68(4), 69(3), 70(5), 72(1) or 73(2); total gill rakers
10(1), 11(4), 12(18), 13(22) or 14(8); total vertebrae 43(2), 44(10) or 45(13); pharyngeal
teeth modally 2,5-5,2(33) with variants 2,5-5,1(6), 1,5-5,2(3) or 1,5-5,1(2).
Sexual dimorphism
Unknown.
Colour
The back and upper head are olive-green to green and the upper flank has a golden sheen. Flanks below are silvery and the abdomen
is silvery-white. There is a dark and wide stripe (about orbit
diameter) on the flank, not always evident in fresh fish. Above the
dark stripe is a narrow golden stripe, about one-third orbit diameter.
Dorsal and caudal fins have black tips while paired fins can have a
reddish or orange base. The peritoneum is brown with dark blotches or speckles.
Size
Reaches 25 cm.
Distribution
Found in the Kura River of Azerbaijan as far down as Mingechaur but
not the lower reaches. In Iran it is found in the Caspian Sea basin including
the Aras River shared with Azerbaijan and
Iran, as far down as Karadonly, and in the Qarasu a
tributary of the Aras. Reported from the Safid River basin (Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004; Abdoli
and Naderi, 2009) and in the Anzali Talab drainage.
Records from the middle Agi Chai or Talkheh River near Tabriz and
the Zarrineh River of the Lake Orumiyeh basin are presumably of A. urmianus (Abdoli, 2000).
Zoogeography
The genus and its two species are restricted to the Caspian Sea basin and the
adjacent Lake Orumiyeh basin and are presumably derived from a common ancestor
related to the Alburnoides-Alburnus lineage.
Habitat
This species inhabits both rivers and lakes.
Age and growth
Females mature at 2 years (Abdurakhmanov, 1962). Spawning probably
occurs in the spring judging from fish caught on 31 January which had
developing eggs. Türkmen et al. (2001) found fish to 7 years of age in
the upper Aras River in Turkey, with three-year-old fish dominant, and also gave
length-weight and length-age relationships. Females attained a greater age and
size than males.
Food
Food includes aquatic insects, crustaceans and snails, and detritus.
Reproduction
Fecundity is up to 19,060 eggs and egg diameter to 1.87 mm. In
Armenia maturity is reached at the end of the second year or beginning
of the third year at 80-120 mm and spawning takes place in late April
to early May and may continue to late August (Pipoyan and Arakelyan,
1999). In the Turkish Aras, maturity for both sexes began at age 2 years, with
all fish mature at 4 years, and spawning started in early May and continued to
the end of July. Fecundity reached a mean value of 9705 eggs and egg size
reached 1.65 mm (Türkmen et al., 2001).
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be conservation
dependent in the south Caspian Sea basin according to IUCN criteria.
Criteria include sport fishing, few in numbers, habitat destruction,
limited range (less than 25% of water bodies), absent in other water
bodies in Iran, and absent outside the Caspian sea basin.
Further work
The biology of this species has not been investigated and its
population biology is also unknown.
Sources
Type material: See above, syntype of Alburnus punctulatus (BM(NH)
1897.7.5:34).
Iranian material:
CMNFI 1970-0522, 2, 55.1-71.3 mm standard length, Gilan, Safid River at Astaneh
Bridge (37º16'30"N, 49º56'E); CMNFI 1970-0536, 4, 70.9-109.3 mm standard length,
Gilan, Siah River estuary (36º53'N, 49º32'E); CMNFI 1970-0538, 1, 70.7 mm
standard length, Gilan, Qezel Owzan River (ca. 36º44'N, ca. 49º24'E); CMNFI
1970-0583, 11, 39.0-79.9 mm standard length, Gilan, Nahang Roga River (37º28'N,
49º28'E); CMNFI 1979-0454, 8, 37.7-64.7 mm standard length, Zanjan, Qezel Owzan
River at Gilavan (36º47'N, 49º08'E); CMNFI 1979-0455, 7, 50.2-123.3 mm standard
length, Markazi, Manjil Dam (36º45'N, 49º17'E); CMNFI 1979-0695, 15, 71.6-112.7
mm standard length, Gilan, Safid River at Manjil Bridge (36º46'N, 49º24'E);
CMNFI 1980-0116, 1, 75.5 mm standard length, Gilan, Safid River at Astaneh
Bridge (37º16'30"N, 49º56'E); CMNFI 2007-0087, 4, mm standard length, Azarbayjan-e
Khavari, Qareh Su (38º22'N, 48º19'E).
Comparative material: CMNFI 1980-0807, 2, 138.2-143.8 mm standard length,
Turkey, Ölçek Suyu (no other locality data); CMNFI 1986-0007, 1, 132.2 mm standard length,
Turkey, Kars River (ca. 41º00'N, ca. 43º00'E).
Acanthalburnus urmianus
(Günther, 1899)
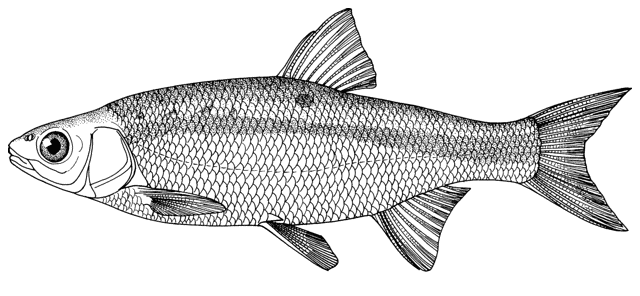
Common names
None.
Systematics
Originally described in the genus Abramis Cuvier, 1816, Berg
(1948-1949) placed this species in the genus Alburnoides
Jeitteles, 1861 but Saadati (1977) places the species in this genus.
The type material in the Natural History Museum, London comprises 2
specimens, 54.9-58.6 mm standard length, from the Urmi River (BM(NH)
1899.9.30:116-117), 1 specimen, 111.7 mm standard length, from the
Ocksa River (BM(NH) 1899.9.30:118) (these three fish being labelled
paralectotypes, with 118 being the lectotype, by P. M. Bănărescu in
1980), and 8 specimens, 50.5-111.7 mm standard length, from the Ocksa
River (BM(NH) 1899.9.30:119-126), these being syntypes. Günther
(1899) refers to the type series as "Five specimens from the
Gader Chai and two small ones from the Urmi River; the largest is only
144 millim. long" so there is some confusion over this material.
Key characters
This species is distinguished from A. microlepis by having
fewer lateral line scales, fewer anal fin branched rays, more gill
rakers and gill raker morphology according to Saadati (1977). Gill
raker morphology does not appear to differ in the fish examined by me.
Gill raker counts are the same but scale and anal fin ray counts are
generally lower with some overlap. Distribution is the easiest
separating factor.
Morphology
Dorsal fin with 3 unbranched and 7-9, usually 8, branched rays,
anal fin with 3 unbranched and 10-13 branched rays. Pectoral fin branched rays
14-16 and pelvic fin branched rays7-8. Lateral line
scales 50-68. Scales bear only a few posterior radii and have a subcentral anterior focus. A pelvic axillary scale is present. Gill
rakers 10-14, short not quite or just reaching the adjacent raker when
appressed; rounded with a projected tip and distinct from its congener
according to Saadati (1977) but closely resembling the structure seen
in A. microlepis according to my observations (see above under A.
microlepis). Pharyngeal teeth usually 2,5-5,2 or 2,5-4,2 with
variants 2,4-5,2, 1,5-4,2 or 2,4-4,2. Posterior teeth are hooked at
the tip, anterior teeth being rounded, and have no, slight, moderate
or even strong serrations. There is a narrow and slightly concave
surface below the tip. Some fish have the anterior margin of the
concave surface higher than the posterior margin, but this is variable
and in some teeth the condition is the reverse. The ventral keel
extends from the anus to the base of the pelvic fins and is fleshy
from half way to the whole length. The intestine is an elongate
s-shaped with a small anterior loop. Total vertebrae 41-43.
Meristic values for Iranian specimens are:- dorsal fin branched rays 7(1), 8(20) or 9(1); anal fin branched
rays 10(1), 11(4), 12(11), or 13(6); pectoral fin branched rays 14(4),
15(17) or 16(1); pelvic fin branched rays 7(3) or 8(19); lateral line
scales 50(1), 52(2), 53(2), 55(1), 56(1), 57(2), 59(2), 60(3), 61(3),
62(2), 63(1), 64(1) or 68(1); total gill rakers 10(1), 11(2), 12(6),
13(8), or 14(5); pharyngeal teeth 2,5-5,2(1), 2,5-4,2(1), 2,4-5,2(1)
or 2,4-4,2(1); total vertebrae 41(5), 42(7) or 43(2).
Sexual dimorphism
Male fish bear tubercles but fully tuberculate fish have not been
examined. One male, 94.7 mm standard length, had a single row of
tubercles on anterior pectoral fin rays.
Colour
Overall colour is silvery with a greenish-olive back and flanks
with numerous minute brown pigment spots which are crowded above the
lateral line to form an inconspicuous darker stripe along the whole
side. The dorsal, caudal and pectoral fins have a light to evident
speckling of melanophores on the rays and membranes but are almost
immaculate in preserved specimens. Larger fish have pigment proximally
on the anterior anal fin rays. The peritoneum is silvery but densely
speckled with melanophores.
Size
Reaches 15.6 cm standard length, almost 20 cm in total length.
Distribution
This species is endemic to the Lake Orumiyeh basin, apparently in southern
and western tributaries (Günther, 1899) although records of A. microlepis
from the middle Agi Chai or Talkheh River near Tabriz are presumably of A. urmianus (Abdoli, 2000).
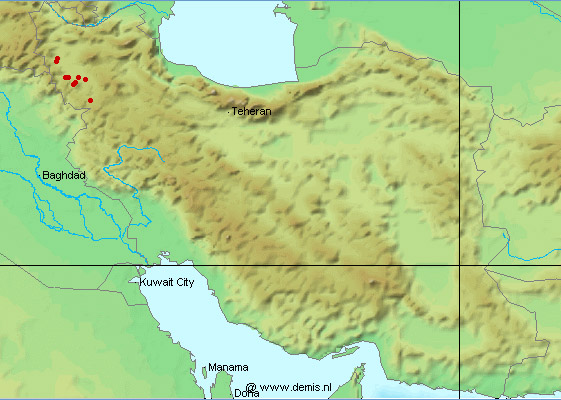
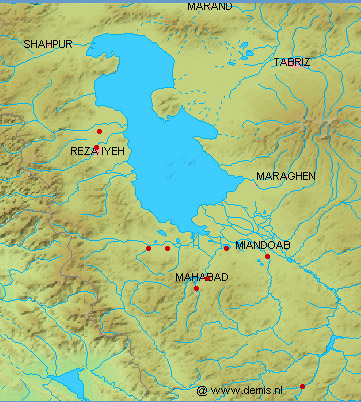
Zoogeography
The closest relative and only congener, Acanthalburnus
microlepis, is found in the Caspian Sea basin. Connections between the Lake
Orumiyeh basin and the Caspian Sea basin have been suggested by Saadati (1977),
an early one in the Pliocene to early Pleistocene resulting in endemic species
and a later one in the late Pleistocene resulting in species which are the same
as the Caspian or only subspecifically distinct. This species presumably dates
from the earlier connection (but see the Lake Orumiyeh drainage basin account
for more details).
Habitat
Details of habitat requirements are unknown but is has been
collected in both river and lakes.
Age and growth
Fish are mature at 14.4 cm. This species is relatively fast-growing,
short-lived species with males attaining 6+ years and females 7+ years in the
Kazemi Dam on the Zarrineh River (Abdoli et al., 2008). The von
Bertalanffy growth curve was estimated as K = 0.427 in males and 0.506 in
females, indicating that females grow faster. The sex ratio was 598♂:912♀ and
there were no significant differences between males and females in the linear
length-weight relationships.
Food
Diet is generally unknown and guts examined were empty except for a
few plant and crustacean remains.
Reproduction
Reproductive data is unknown although this species probably spawns
in the spring as do most members of this family.
Parasites and predators
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. and
Ergasilus sp. on this species.
Economic importance
None.
Conservation
This species is known only from the type series and a few other
specimens in museum collections. Its status is unknown.
Further work
Field work should be carried out to determine the habitat
requirements, ecology and numbers of this uniquely Iranian fish.
Sources
Type material: See above (BM(NH)
1899.9.30:116-117, BM(NH) 1899.9.30:118, BM(NH) 1899.9.30:119-126).
Iranian material: CMNFI 1979-0093, 2,
127.5-130.5 mm standard length, Azarbayjan-e Bakhtari, Lake Qowpi (36º57'N,
45º52'E); CMNFI 2007-0098, 1, 156.3
mm standard length, Azarbayjan-e Bakhtari, river south of Mahabad (ca. 36º42'N,
ca. 45º41'E); CMNFI 2007-0101, 1, 129.3 mm standard length, Azarbayjan-e
Bakhtari, Tata'u River south
of Miandow Ab (ca. 36º54'N, ca. 46º07'E); CMNFI 2007-0105, 1, 90.8 mm standard length, Kordestan, Zarineh River basin south of Saqqez
(ca. 36º06'N, ca. 46º20'E); USNM 205904, 1, 84.7 mm standard
length, Azarbayjan-e Bakhtari, Nazlu-chay near Rezaiyeh (37º40'N, 45º05'E); USNM 205934, 2, 94.5-141.9 mm standard
length, Azarbayjan-e Bakhtari, Lake Qowpi (36º57'N, 45º52'E); uncatalogued, 4, 105.1-134.9 mm standard length, Azarbayjan-e Bakhtari,
Zarineh River (no other locality data);
Genus Acanthobrama
Heckel, 1843
Howes (1981) placed Acanthobrama Heckel, 1843 in the genus Rutilus
Rafinesque, 1820 on osteological grounds but most other authors retain
Acanthobrama as a distinct genus (Coad, 1984a; Krupp, 1985c; Eschmeyer, 1990; Bănărescu,
1992b) based on the scale, keel and anal fin characters listed below. Durand et
al. (2002) include this genus in the Abramis clade based on
cytochrome b data. The genus Trachibrama Heckel, 1843 is a lapsus (Krupp
and Schneider, 1989).
This genus is characterised by a compressed, deep body of small to
moderate size, no barbels, relatively small scales with reduced
numbers of radii, a fleshy keel between the base of the pelvic fins
and the vent, the last unbranched dorsal fin ray is thickened,
spine-like and smooth, and the anal fin is long (9-22 branched rays).
Pharyngeal teeth are usually in a single row on each arch. Gut short.
There are 8 species endemic to Southwest Asia with 1 found in
southwestern Iran (Goren et al., 1973; Coad et al., 1983; Krupp, 1985c).
Acanthobrama marmid
Heckel, 1843
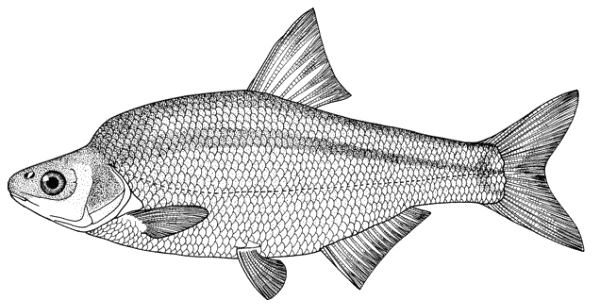
Common names
كلاش پا (= kalashpa),
شبه ساردين (shebeh sardin = pseudo-sardine or resembling sardine),
شبه نازي (= shebeh nazy), mahi sim nama (= bream-like fish).
[semnan arrez; samnan areed; arath (Rahemo et al., 2006); marmid, marmid handscherli (= marmid armed with a dagger), marmid
abbiad (= white marmid), marmid asphar (= yellow marmid) or marmid mablue (= swallowing or devouring marmid) at Aleppo, arrhada (= dove,
lion!) at Mosul (all these latter Arabic names after Heckel (1843b; 1846-1849a), the conflicting names for arrhada included, and are probably antiquated;
Tigris bream].
Systematics
Acanthobrama Arrhada Heckel, 1843, Acanthobrama cupida
Heckel, 1843, Acanthobrama marmid morpha elata Berg,
1949 and Acanthobrama marmid orontis Berg, 1949 are synonyms.
The type locality for Acanthobrama Marmid is "Gewässern
bei Aleppo", for Acanthobrama arrhada "in Mossul",
and for Acanthobrama cupida "in Aleppo" according to
Heckel (1843b) and "Flusse Kueik bei Aleppo" in Heckel
(1846-1849a). The type locality of Acanthobrama marmid morpha elata
is Lake Balikli, 12 km from Erzurum, 8 km from the Karasu River, upper
Euphrates, in Turkey. The type locality of Acanthobrama marmid orontis
is the upper Euphrates region according to Eschmeyer et al.
(1996) (but this is an error, see below).
Details on the syntypes of this species and its synonyms arrhada
and cupida in the Naturhistorisches Museum Wien are given by
Krupp (1985c). Eight syntypes of marmid measuring 41-144 mm
standard length are listed from Mosul (in contrast to Heckel's papers
where the type locality is Aleppo), the number of fish agreeing with
the catalogue in Vienna. These 8 fish are under NMW 55334. Eschmeyer et
al. (1996) do not list these fish as types and the card index in
Vienna in 1997 concurs. A further 15 fish are listed by Krupp from the
Quwayq near Aleppo: 1 fish, 102 mm standard length (NMW 55342 - not in
the 1997 card index; but the following NMW fish are listed), 2,
113-139 mm standard length (NMW 55345), 2, 86-121 mm standard length (NMW
55346), 2, 98-126 mm standard length (NMW 55347), 2, 113-132 mm
standard length (NMW 55348), 2, 114-138 mm standard length (NMW
79068), and 4 fish in the Senckenberg Museum Frankfurt, 82-112 mm
standard length (SMF 543, formerly NMW). Eschmeyer et al.
(1996) list NMW 55345-48 (8), NMW 79068 (2), SMF 543 (4) and in the
Rijksmuseum van Natuurlijke Historie, Leiden RMNH 2537 (4) and RMNH
2539 (2) (both formerly NMW) as the type series.
Two syntypes of A. arrhada from Mosul, 85-92 mm standard
length, are in the Senckenberg Museum Frankfurt (SMF 411, formerly NMW)
(F. Krupp, pers. comm., 1985; 85.7-89.0 mm standard length) while 2 others are in the
Naturhistorisches Museum Wien, ca. 150 mm standard length (NMW 55335)
and 141 mm standard length (NMW 55336) (Krupp, 1985c). However, the
Vienna catalogue lists 6 specimens of A. arrhada and in
addition to the above material there is also NMW 55334 (8 fish) tagged
as syntypes so there is some confusion in what constitutes the type
series. Two possible syntypes are in the Rijksmuseum van Natuurlijke
Historie, Leiden (RMNH 2538) (Eschmeyer et al., 1996).
Krupp (1985c) records syntypes of A. cupida, 151 mm standard
length, (NMW 55340) and 152 mm standard length (NMW 55341). The Vienna
catalogue lists 4 A. cupida which agrees with Heckel's
description although I observed only NMW 55340 (1 fish), NMW 55341 (1)
and also NMW 55342 (1). Eschmeyer et al. (1996) list NMW
55340-43 (1, 1, 1) as syntypes but the numbers indicate 4 fish. The
card index in Vienna in 1997 also lists 55505 (5 fish), one of which
is designated as the lectotype.
The 2 syntypes of Acanthobrama marmid orontis are in
the Zoological Institute, St. Petersburg under ZISP 6720 from "L.
Antioch, 1884, Lortet" according to Berg (1949). This subspecies
is distinguished only by larger scales from the typical form but the 2
syntypes examined by me had lost their scales and were difficult to
count; one seemed to have a count around 64, not as low as 54-55
recorded by Berg (1949). Krupp (1985c) examined type material and new
specimens from the Orontes and found them not to differ from A.
marmid from the Quwayq and Tigris-Euphrates basins. He accordingly
synonymises Acanthobrama marmid orontis with the type subspecies.
Karaman (1972) considered Acanthobrama arrhada to be a subspecies of
A. marmid rather than a synonym based on an unusually strongly ossified
spiny dorsal fin ray in the former. Since A. marmid was described from
Aleppo (= Halab, Syria) and A. arrhada from Mosul, the synonomy of these two taxa may
warrant re-examination.
The fish reported from the Tigris River basin of Iran by Nümann (1966) as Xenocypris macrolepidotus
was this species (Zoologisches Institut und Zoologisches Museum,
Hamburg catalogue number ZMH H2700 examined by me). Saadati (1977)
thought it a new species of Acanthobrama but I disagree.
A hybrid with Chalcalburnus (= Alburnus) mossulensis was reported from
the Hawr al Hammar in southern Iraq by Krupp et al. (1992).
Key characters
The characters of the genus distinguish this species from all other Iranian cyprinids.
Morphology
Mouth nearly horizontal to oblique, equal or lower jaw slightly
behind the upper. The belly has a fleshy keel where the ventral scales
do not meet along the mid-line between the pelvic base and the anus.
The last unbranched dorsal fin ray is a thickened, stiff and smooth
spine, the rigid part varying from 15 to 26% of standard length. The
spine may be strong for much of its length and then abruptly become
thin and flexible or it may taper gradually to a flexible tip. Some
small fish lack an enlarged dorsal fin spine.
Lateral line scales 53-72, scales above the lateral 10-14, scales
between the pelvic fin and lateral line 4-7. There is a pelvic
axillary scale. Radii are restricted to the posterior field on scales
and are few in number. The focus is subcentral anterior to almost central.
Dorsal fin with 3 unbranched rays and 7-9 branched rays. Anal fin
unbranched rays 3, branched rays 13-22. Pectoral fin branched rays
12-18, pelvic fin branched rays 7-9. Total vertebrae 38-43 (38(3), 39(3), 40(7),
41(5), 42(7) or 43(1) combining Iranian and Iraqi material).. Gill rakers
short with a basal swelling, 2-4 on the upper arch, 0-1
at the flexure and 9-12 on the lower arch. Total rakers 12-17. The
rakers reach the one below or to its further base end when appressed.
Pharyngeal teeth usually 5-5, with the anterior tooth compressed and
bluntly pointed, the remainder bevelled with a cutting edge and a
hooked tip. The two anterior teeth are more rounded than the others
although the second one may have a slight hook and is bevelled. Tigris
River basin fish may have 1-2 teeth in a second row. The gut is an
elongate s-shape with a large anterior loop in larger fish. The diploid
chromosome number is 2n=50, with the karyotype consisting of 8
metacentric, 13 submetacentric and 4 pairs of subtelocentric to acrocentric
chromosomes. The karyotype is nearly identical to other Eurasian leuciscine
cyprinids (Gaffaroğlu et al., 2006)..
Different body
forms occur in slow-flowing and fast-flowing waters.
In the former habitat fish have a deep body, often humped behind the
head, while in the latter the body is more streamlined (Karaman,
1972). It seems that A. marmid is founded on the humped form
and A. arrhada and A. cupida on the streamlined one.
Meristic values for Iranian specimens are:- dorsal fin branched rays 7(1)
or 8(8); anal fin branched rays 13(2), 14(2), 15(4) or 17(1); pectoral
fin branched rays 13(2), 14(3), 15(3) or 18(1); pelvic fin branched
rays 7(1), 8(7) or 9(1); lateral line scales 54(1), 55(2), 56(2),
58(1), 59(1) or 63(1); total gill rakers 12(1), 14(6) or 17(1); pharyngeal teeth
5-4(1) or 5-5(7); and total vertebrae 38(3), 39(1), 41(2), 42(2) or 43(1).
Sexual dimorphism
Fine tubercles are found over the top, sides and bottom of the head
in males. Tubercles line the first, unbranched pectoral fin ray
irregularly with up to 2 branching rows. Very fine tubercles are found
on the adjacent membrane and on the lower pectoral fin surface.
Tubercles line the pelvic fin rays in branching rows. The lower caudal
fin rays are lined with tubercles. Anterior upper flank scales, all
belly scales and lower caudal peduncle scales have their margin lined
with tubercles, the peduncle with some tubercles on the mid-scale and
the belly with a concentration on the scale base.
Colour
The overall colour is silvery to whitish with the head and back
reddish-brown. The flanks can be greyish to blackish from numerous
melanophores. There may be a well-developed mid-flank stripe or it may
be poorly developed or evident only posteriorly. The pelvic fins are bright
red, the pectoral and anal fins less red and the dorsal and caudal
fins reddish proximally and black distally. Fin colours may be more
orange or yellow than red. All fin rays and membranes have
melanophores and these can be quite concentrated such that some fish
have dark fins. Young fish in preservative have numerous, distinctive,
small to minute, rounded, square or oblong patches of pigment in 1-3,
irregular, mid-flank rows. Peritoneum black, silvery with a dorsal
concentration of melanophores or with widely scattered melanophores so
it appears silvery.
Size
Reaches 20.8 cm (Berg, 1949)
Distribution
This species is found in the Tigris-Euphrates basin of Turkey, Syria, Iraq and Iran, the Quwayq
(= Kueik) and Orontes rivers, and possibly the Amik Lake and the Bardan
suyu (= stream) near Tarsus (Ladiges, 1960; - Krupp (1985c) suggests
these latter should be checked). In Iran it is found in the Tigris River
basin including the upper reaches of the Karkheh, the Qara Su, and
in marshes such as the Hawr Al Azim.
Zoogeography
The majority of species are found in the Levant which once had connections to
the Tigris-Euphrates basin (Krupp, 1985c).
Habitat
Hussain et al. (1997) report this species to be dominant in
the small fish assemblages in the Shatt Al-Arab near Basrah, Iraq at
70.8% of 14,084 fish caught. It favours side branches off the Shatt al Arab, presumably to avoid
predators which are found in deeper water. Younis et al. (2001b) noted
that this species dominated in the polluted and disturbed environment of a
dockyard on the Shatt al Arab. This was one of the most abundant species in the
recovering marshes of southern Iraq in 2005-2006 (Hussain et al., 2006)
and is also known from large rivers and dams. Also recorded from the Hawr al Azim marsh in Iran.
Age and growth
Al-Nasiri and Salman (1977) studied this species in the Little Zab River, Iraq.
Their largest specimen was 13.7 cm. They described length-weight relationships
and condition factors but some important length groups were missing from their
samples. Condition factor showed a gradual decrease with increasing length and
the means for actual and calculated weights were 1.141 and 1.118 respectively.
Relative condition factor was 1.0009. Younis et al. (2001) examined three
populations of this species in the Shatt al Arab, Iraq and found the 0+ age group to
be represented by fish 2.1-11.0 cm long and 1+ age group by fish 8.3-14.1cm. The
length-weight relationship was W = -3.821 L2.32. Four age groups
with a length range of 4-19 cm were found in the Qarmat Ali River of southern
Iraq, with maturity in the first year (Saud, 1997).
Ünlü et al., (1994) examined a population of this species in the
Tigris River, Turkey and gave figures for growth in length and in weight.
Females grew faster and are larger in size than males at the same age,
particularly for age groups III and IV. Condition factor for males was 1.554 and
for females 1.550. They found 5 age groups with age group III dominant for both
sexes. Overall sex ratio was 1.83 females:1 male. Sexual maturity was attained
by 75% of females and 85% of males in the second year of life and all fish in
age group III were mature.
Food
Heckel (1843b) suggests that they are ravenous feeders based on the
name "swallowing marmid". Gut contents are crustaceans,
insects, and plant and gastropod shell fragments in Iranian specimens. Younis et al. (2001a; 2001b) found Shatt al Arab,
Iraq fish to be detritivores, having organic detritus as the dominant gut content,
followed by phytoplankton (blue-green algae and diatoms), small crustaceans (ostracods,
cyclopoids, cladocerans), and aquatic plants, with dominance varying by month.
Gut contents were crustaceans, insects, and plant and gastropod shell fragments
in fish from Iran examined by me. In a study of the recovering Hammar Marsh,
Iraq, diet was 70.77% insects and 9.81% algae with diatoms, plants, crustaceans and snails
at less than 10% each, in the Hawr al Hawizah 66.4% insects and 14.1% algae, with
amounts of diatoms and various crustaceans being less than 10% each, and in the
Al Kaba'ish (= Chabaish) Marsh 62.7% insects and 17.7% algae with diatoms, plants and various
crustaceans at less than 10% each (Hussain et al., 2006).
Reproduction
Younis et al. (2001) found most females to be ripe in March and July
samples, and some were spent. Well-developed testes are noted in fish caught on 16 May in Turkey
and 7 July near Ravansar, Kermanshahan indicating either a prolonged
breeding season or local variations.
Ünlü et al., (1994) report spawning in May to late June
for their Tigris River, Turkey population. They cite data for a Keban
Dam population (on the Euphrates River in Turkey) where the spawning
season is extended and runs from April to August. Egg diameter exceeds
1.2 mm and egg numbers reach 8125, and elsewhere may reach 11,000 eggs. In the Qarmat Ali River in southern Iraq,
fecundity reached 1759-9293 eggs.
Parasites and predators
None reported from Iran.
Economic importance
None in Iran. In the early 1990s in Iraq, this species was used for human consumption and for fish meal (Younis et al., 2001).
Conservation
This species is rarely reported from
Iranian waters and its status needs to be assessed through further field work.
Endangered in Turkey (Fricke et al., 2007).
Further work
Additional field work is required to
secure more materials and assess conservation status and biology.
Sources
Type material: See discussion above.
Syntypes of Acanthobrama marmid (NMW 55345, NMW
55346, NMW 55347, NMW 55348, NMW
79068, SMF 543); syntypes of Acanthobrama marmid orontis (ZISP
6720), syntypes of A. arrhada (SMF 411, NMW 55335, NMW 55336, NMW 55334);
syntypes of A. cupida (NMW 55340, NMW 55341, NMW 55342, NMW 55505).
Iranian material: CMNFI 1979-0287, 2, 89.9-92.1 mm standard length, Kermanshahan, spring near
Ravansar (ca. 34º42'N, ca. 46º40'E); CMNFI 1979-0360, 1, 40.6 mm standard
length, Khuzestan, Karkeheh River canal (31º40'N, 48º35'E); CMNFI 1979-0377, 2,
28.5-34.6 mm standard length, Khuzestan, Karkheh River (ca. 32º57'N, ca.
47º50'E); CMNFI 1979-0384, 1, 23.1 mm standard length, Khuzestan, Ab-e Shur
drainage (32º00'N, 49º07'E); CMNFI 1991-0154, 1, 113.6 mm standard length,
Khuzestan, Hawr-al-Azim (ca. 31º45'N, ca. 47º55'E); CMNFI 1993-0128, 1, 113.6 mm
standard length, Kermanshahan, Sarab-e Sabz `Ali Khan (34º25'N, 46º32'E); CMNFI
2007-0114, Kermanshahan, Qareh Su basin (ca. 34º28'N, ca. 46º54'E); ZMH H2700,
1, 145.0 mm standard length, Kermanshahan, Gharasu-Gamasiab-Seymarreh (Qareh Su, Gav Masiab
and Simareh rivers, no other locality data); uncatalogued, 1, 101.7 mm standard
length, Kermanshahan, sarabs near Kermanshah (no other locality data).
Comparative material: BM(NH) 1931.12.21:22-25, 4, 65.7-84.6 mm standard
length, Iraq, Mosul (ca. 36º20'N, ca. 43º08'E); BM(NH) 1974.2.22:1084-1091, 7, 105.1-118.3 mm standard
length, Iraq, Najab Bazar (no other locality data); BM(NH) 1974.2.22:1094, 109.3 mm standard length,
Iraq, Great Zab River at Aski Kalak (36º16'N, 43º39'E); BM(NH) 1971.4.2:7, 96.5 mm standard length,
Iraq, River Tigris near Mosul (ca. 36º20'N, ca. 43º08'E); BM(NH) 1974.2.22:1078-1083, 6,
105.2-122.8 mm standard length, Iraq, Najab Bazar (no other locality data); BM(NH) 1974.2.22:1092,
109.5 mm standard length, Iraq, Najab Bazar (no other locality data); CMNFI 1987-0017, 3, 83.8-108.3
mm standard length, Iraq, Hawr al Hammar (no other locality data); BM(NH) 1920.3.3:147-156, 15, 29.5-102.0 mm standard
length, Syria, Ouadi Khneizer (no other locality data); BM(NH) 1968.12.13:108-112, 1 (of
5), 112.6 mm standard length, Syria, Ouadi Khneizer, Khabour (no other locality
data) (collections amalgamated as BM(NH 1968,12.13:105-341 seem to include the
preceding and following collectiosn, 224 (7 as alizarin specimens), 24.1-69.7 mm
standard length); BM(NH) 1968.12.13:113-118, 6, 56.5-117.4 mm standard length,
Syria, River Euphrates at Houreira (no other locality data); ZSM 26136, 5, 55.3-80.3 mm standard length, Syria, Assad
Reservoir, Euphrates basin (no other locality data); CMNFI 1980-0810, 2,
114.8-118.3 mm standard length, Turkey, Göksu in Tigris River basin (no other locality data);
CMNFI 1980-1036, 1, 101.5 mm standard length, Turkey, Keban Dam on Murat Nehri near Elâzığ (no other locality data).
Genus Alburnoides
Jeitteles, 1861
This genus is found in Europe, Asia Minor and Central Asia with ?11
species, with 6 reported in Iran.
The riffle minnows are similar in appearance to the genus Alburnus
but have smooth rather than serrated pharyngeal teeth. Arguably this
distinction is insufficient to warrant a separate genus but it is
retained here as this has not been investigated in depth and the genus
has widespread usage. Certainly it is not uncommon to find individuals
of Alburnus hohenackeri lacking serrations on their pharyngeal teeth.
Pharyngeal teeth in Alburnoides are in 2 rows with strongly
hooked tips but unserrated, scales of medium size, no groove before
the dorsal fin, a keel behind the pelvic fins is usually scaleless but
may be wholly scaled, short dorsal and moderate to long anal fin, last
dorsal fin unbranched ray thickened, decurved lateral line often with
a characteristic spotting pattern above and below each pore, and gill
rakers short and few.
Alburnoides bipunctatus (Bloch, 1782) was the name applied to
most populations across Europe and the Middle East from France north of the Alps
eastwards to the Black, Caspian and Aral Sea basins but ongoing research is
revealing a greater diversity (Bogutskaya and Coad, 2009; Coad and Bogutskaya,
2009).
A record of A. bipunctatus from a qanat at Hormak (29°58'N, 60°51'E) in the Sistan basin by Saadati (1977) is probably an
error of labelling or sorting. It is not mentioned in the collector's (R. J.
Behnke) original field notes nor in a typed version. Also this species was not collected there by me.
Records of parasites for fish identified as "A. bipunctatus" in Iran are
as follows:
Jalali and Molnár (1990a) record the monogeneans Dactylogyrus
alatus and D. chalcalburni from this species in the
Zayandeh Rud. Gussev et al. (1993b) also reports the latter
species and locality. The monogenean Diplozoon paradoxum is
recorded from this species in the Tajan River, Mazandaran (Iranian
Fisheries Research and Training Organization Newsletter, 6:7,
1994). Shamsi et al. (1997) report Clinostomum complanatum,
a parasite causing laryngo-pharyngitis in humans, from this species.
Masoumian and Pazooki (1998) surveyed myxosporeans in this species in Gilan and Mazandaran provinces,
finding Myxobolus ellipsoides. Masoumian
et al. (2005) report the protozoan parasites Ichthyophthirius
multifilis, Trichodina perforata and Chilodonella, sp. from
this species in water bodies in West Azarbayjan.
Mortazavi Tabrizi et al. (2005) record Ligula intestinalis in
this species from the Sattarkhan Dam in East Azerbaijan. Pazooki et al. (2005) record Trichodina
perforata from this species in waterbodies of Zanjan Province. Pazooki et al.
(2006) record the monogeneans Dactylogyrus vistulae, Gyrodactylus
sp. and Paradiplozoon sp. from this fish in Zanjan Province.
Mehdipoor et al. (2004) record the monogeneans Dactylogyrus alatus,
D. chalcalburni and D. pulcher in the Zayandeh River.
To be assigned: CMNFI 1970-0522, 22, 40.4-80.3 mm standard length, Gilan, Safid River at Astaneh Bridge (37º16'30"N, 49º56'E);
CMNFI 1970-0536, 3, 71.9-89.6 mm standard length, Gilan, Siah River estuary (36º53'N, 49º32'E);
CMNFI 1970-0546, 3, 57.1-69.4 mm standard length, Gilan, Safid River canal (no other locality data);
CMNFI 1970-0551, 1, 108.4 mm standard length, Gilan, Ghaleh River near Fowman (37º13'N, 49º19'E);
CMNFI 1970-0583, 16, 40.7-87.3 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1971-0327A, 6, 59.3-81.0 mm standard length, Gilan Shafa River (37º35'N, 49º09'E);
CMNFI 1979-0239, 2, 57.1-79.3 mm standard length, Markazi, Nam River near Firuzkuh (35º43'N, 52º40'E);
CMNFI 1979-0439A, 4, 53.4-72.2 mm standard length, Gilan, Shafa River (37º35'30"N, 49º05'30"E);
CMNFI 1979-0440, 11, 53.7-88.6 mm standard length, Gilan, Lomir River (37º37'N, 49º02'30"E);
CMNFI 1979-0441, 4, 52.4-55.7 mm standard length, Gilan, river 14 km south of Hashtpar (37º42'N, 48º58'E);
CMNFI 1979-0445, 1, 70.6 mm standard length, Gilan, stream 10 km south of Astara (38º21'N, 48º51'E);
CMNFI 1979-0453, 2, 45.8-65.1 mm standard length, Zanjan, Zanjan River (37º06'N, 47º56'E);
CMNFI 1979-0454, 6, 39.6-56.0 mm standard length, Zanjan, Qezel Owzan River at Gilavan (36º47'N, 49º08'E);
CMNFI 1979-0483, 2, 93.0-98.6 mm standard length, Mazandaran, Chashmeh River (37º23'30"N, 55º51'30"E);
CMNFI 1979-0493, 11, 51.1-82.8 mm standard length, Mazandaran, Tajan River drainage (36º19'N, 53º23'E);
CMNFI 1979-0695, 74, 34.1-71.1 mm standard length, Gilan, Safid River at Manjil Bridge (36º46'N, 489º24'E);
CMNFI 1980-0116, 19, 41.1-70.3 mm standard length, Gilan, Safid River at Astaneh Bridge (37º16'30"N, 49º56'E).
check all species correctly transposed from Bogutskaya and Coad?
add
holotype meristics to paratypes frequency
As A. bip from Atrak, Gorgan Gharasu,
Tajan, babol, Jaraz, sardab, Aras, Tonekabon, Pol-e Rud, Safis and Anzali Talab
(Abdoli and Naderi, 2009).
Alburnoides eichwaldii
De Filippii, 1863
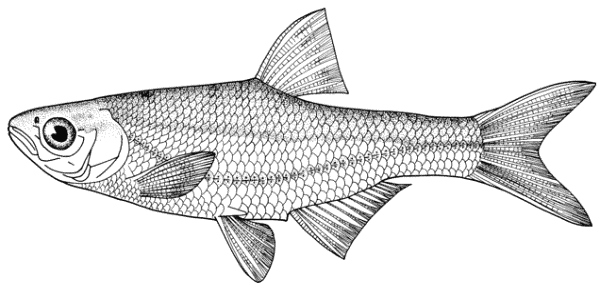
Common names
خياطه (= khayataeh) or ماهي خياطه
(= mahi khayateh, tailor or tailoress fish, possibly from lateral
line pattern like stitches), لپك (= lapak in Mazandaran), پرك (= parak
in Gilaki), sima,
kuli.
[gijovcu in Azerbaijan; vostochnaya bystryanka or oriental
bystranka, zakavkazskaya bystryanka or Transcaucasian bystranka,
Armyanskaya bystryanka or Armenian bystryanka for A. b. armeniensis,
all in Russian; spirlin, riffle minnow or riffle bleak in general].
Systematics
Cyprinus bipunctatus was originally described from the Weser River in Germany.
Alburnus Eichwaldii De Filippi, 1863, described from the
"Kur presso Tiflis" (= Kura River near Tbilisi, Georgia), was
regarded as a Caspian Sea basin subspecies of Alburnoides
bipunctatus but Bănărescu (1991) briefly stated that it cannot be distinguished from Alburnoides
bipunctatus fasciatus (Nordmann, 1840) of the Black Sea basin. Holčík and Jedlička
(1994) considered that the observed variation is clinal and subspecies
are not warranted. Reshetnikov et al. (1997) also consider
subspecies as disputable. There is another nominal subspecies in the
Aras River drainage of Armenia, Alburnoides bipunctatus armeniensis
Dadikyan, 1972, from the rivers Arpa, Vorotan, Vedi, Marmarik, Kasakh and their tributaries,
now regarded as a synonym of eichwaldii (Bogutskaya and Coad, 2009).
Bogutskaya and Coad (2009) resurrect A. eichwaldii, and it is present
in at least in the western part of the Caspian Sea basin, west of the Safid
River.
A syntype of Cyprinus bipunctatus described from the Weser
River, Germany is in the Museum für Naturkunde, Universität
Humboldt, Berlin (ZMB 3357) (Eschmeyer et al., 1996).
Two syntypes of Alburnus eichwaldi from "Tiflis"
are in the Naturhistorisches Museum Wien under NMW 55516 and 4
syntypes are in the Istituto e Museo di Zoologia della R. Università
di Torino under MZUT N.677 (Tortonese, 1940; Eschmeyer et al., 1996).
Syntypes of Alburnoides bipunctatus armeniensis are in the
Zoological Institute, St. Petersburg under ZISP 37502.
Key characters
See B and C 2009 here and below
The pigmentation along the lateral line is distinctive. Total gill raker counts (5-12) are much less than in Alburnus
hohenackeri
(16-29, usually 20 or more) which has similar general scale and fin rays counts.
A. eichwaldii differs from the related A. gmelini by having
fewer branched anal-fin rays (11-14, modally 12-13, vs. 13-16, modally 14-15;
means, 12.2 and 14.3, respectively) and a larger number of total vertebrae (mean
41.3 vs. 40.6, statistically different).
Morphology
see tables for counts?
The original diagnosis of A. eichwaldii gave the following characters: the body is deep, its length
exceeds the depth in four times; eye large;
dorsal-fin rays branched rays 8;
branched anal-fin rays 12; scales
in the lateral series 50, 11 scales above and
7 scales below lateral line. The original description may be added
to by the following combination of characters:
the caudal fin lobes are moderately rounded,
the fin is not deeply forked; the ventral keel
is commonly scaleless but may be variably
scaled (up to completely scaled); the head is
commonly deep and the snout is slightly to
markedly rounded; the upper jaw is slightly
protruding over the lower jaw; the tip of
the mouth cleft is slightly below the level of
the middle of the eye or at about the lower
margin of pupil; the number of dorsal-fin
rays is 8, rarely 7 or 9; the number of
branched anal-fin rays is (10)11-14 with
the modal range of 12-13; pharyngeal
teeth are commonly 2.5-4.2 and other variants
with four teeth in the longer row of the
right ceratobranchial, also, less frequently,
2.5-5.2 or 2.5-5.1; the number of total lateral
line scales 44-56 (Dadikyan, 1972,
1973, gives 39-56, averaging 48.7, in A. bipunctatus
armeniensis); gill rakers 6-10; the
number of total vertebrae is (38, 39)40-43
with a mode of 41; predorsal vertebrae are
(12)13-15 with a mode of 14; the number
of abdominal vertebrae is (18)19-22 with a
mode of 21, and that of caudal vertebrae is
19-22 with a mode of 21; the caudal region
is commonly one vertebra shorter than,
equal to the abdominal region or one vertebra
longer than the abdominal region, and
the difference between the abdominal and
caudal numbers varies from +3 to -1 with a
mode of 0; and the most common vertebral
formulae are 21+21, 21+20 and 20+21.
Dorsal fin with 2-3 unbranched and 6-10, usually 8, branched rays,
anal fin with 2-3 unbranched and 10-18 branched rays, usually 12-13
(but see below for Iran). Lateral line scales 41-58. Gill rakers 5-12, usually
7-10. Vertebrae 37-44. Pharyngeal teeth 2,5-4,2, rarely
2,5-5,2, 2,4-5,2, or 1,5-4,2, with variants being 1,5-4,1, 2,5-4,3, 2,3-4,2,
2,4-4,2, 1,5-4,0, and 1,2,5-4,3. The chromosome number is 2n=50 (Klinkhardt
et al., 1995).
Meristics in Iranian specimens from the Caspian Sea: dorsal fin
branched rays 7(6), 8(121) or 9(3); anal fin branched rays 11(1),
12(26), 13(61), 14(32), 15(9) or 16(1); pectoral fin branched rays
12(3), 13(23), 14(71), 15(24) or 16(9); pelvic fin branched rays 6(3),
7(116) or 8(11); lateral line scales 43(4), 44(5), 45(25), 46(29),
47(23), 48(13), 49(7), 50(10), 51(6) 52(5), 54(1) or 55(2); total gill
rakers 6(7), 7(35), 8(57), 9(30) or 10(1); pharyngeal teeth 2,5-4,2(14),
2,5-5,2(1) or 2,4-5,2(2); and total vertebrae 37(1), 38(1), 39(4), 40(49), 41(32) or 42(2).
The chromosome number is 2n=50 and Nazari et al. (2009) give further
details.
Sexual dimorphism
Abdurakhmanov (1962) reports pelvic fin length greater in males and
snout length greater in females for this species in Azerbaijan.
Colour
?There is a characteristic pigmentation along the lateral line with
a small spot above, and another below, the lateral line opening on
each scale. This only appears in preserved material as live fish are
an overall silvery colour. It can be absent, mostly in lake forms. The
flank has a blue-grey stripe wider than the eye diameter. Above the
lateral line there may be a series of 5-9 black lines formed of
triangular blotches and 3-5 similar lines below the lateral line. The
back and head are dark olive, almost black, dark green or dark brown.
The flank above the lateral line may have purple iridescent tints. The
flanks can be a golden yellow. The belly and lower head are
pearly-white. The dorsal and caudal fins have some grey pigment or may
be dark grey. The bases of the pectoral, pelvic and anal fins have
orange to red pigmentation which is not well developed in young. The
extent and intensity of this pigment is variable between fins,
although in some fish it is equally developed in all these fins.
Size
?
Distribution
Found in river drainages of the southwestern Caspian coast from the Samur
(according to Berg, 1948-1949) down to rivers of the Lenkoran’. The Aras River
basin also harbours this species.
Zoogeography
?This species shows considerable variation over its range from
Europe to southern Iran. Dadikyan (1973) demonstrated variability in
this species in a mountainous region of Armenia within the Aras River
basin. Up to 10 characters could be used to distinguish populations
within the same river but taken at different altitudes. Populations at
similar altitudes but in different rivers (and habitat types, e.g.
rushing rocky streams compared to a bog) also varied but the
characters were not necessarily the same as those distinguishing
altitudinal variants within one river. Local conditions, such as
temperature and flow regime, may govern the characters at any one
site. Gene flow may play a part as fish are carried downstream by
heavy rainfall. Populations living within the same river are
presumably more closely related than populations in different river
systems but may show more differences than populations at similar
altitudes but which have had no gene flow for long periods. These
factors complicate designation of subspecies in this species and
accurate analysis requires large series of specimens.
Habitat
?This species inhabits small streams and is less frequent in the
main flow of large rivers. In Iran, it is one of two most abundant
species in Caspian rivers along with Capoeta capoeta (Iranian
Fisheries Research and Training Organization Newsletter, 19:4,
1998). It prefers well-oxygenated water, low in pollution, with hard
stream beds. In laboratory experiments with European specimens, Bless
(1996) found that reproduction requires a stream velocity of 0.4 ms-1
and a gravel substrate with a diameter of 2-15 cm which allows interstitial flow.
Age and growth
In Azerbaijan, maturity is attained at 1-2 years and life span is 3 years (Abdurakhmanov, 1962).
Food
Food is taken from the bottom or from the water surface, the former
being mostly insect larvae and the latter terrestrial organisms which
fall on the water. Abdoli (2000) lists Simuliidae, Plecoptera, Ephemeroptera,
Chironimidae and Trichoptera. Diatoms are also found in gut contents (Abdurakhmanov, 1962).
Reproduction
?Spawning takes place in spring (April-June) at 13-15.6°C
and adhesive eggs are laid on sand or gravel in fast-flowing water.
Fecundity reaches 6496 eggs and egg diameter 2.16 mm (Abdurakhmanov,
1962). Bless (1996) reports multiple spawning over a period of 15
weeks in laboratory conditions.
Parasites and predators
Barzegar et
al. (2008) record the digenean eye parasite Diplostomum spathaceum
from this fish (as A. bipunctatus).
Economic importance
Unknown. A. bipunctatus is listed as important to North
Americans (Robins et al., 1991). Importance is based on its use as bait and in textbooks. It
is also a known feeder on the larvae of the malaria-carrying mosquito.
Conservation
Lelek (1987) considers A. bipunctatus to be vulnerable to
endangered in Europe through pollution and eutrophication. It is listed as Vulnerable in Turkey
(Fricke et al., 2007). Kiabi et
al. (1999), examining Iranian material, consider A bipunctatus to be of least concern in the
south Caspian Sea basin according to IUCN criteria. Criteria include
abundant in numbers, habitat destruction, widespread range (75% of
water bodies), present in other water bodies in Iran, and present
outside the Caspian Sea basin. These assessments may apply to the current taxon.
Further work
Other populations in Iran related to this taxon are under study (2009).
Sources
Iranian material: CMNFI 2007-0090, ?
Comparative material: See Bogutskaya and Coad (2009).
Alburnoides idignensis
Bogutskaya and Coad, 2009
?
Description of holotype. A ventral keel
between the pelvics and the anal fin is scaleless
along about 1/2 of its length. There is a
pelvic axillary scale and scales extend over
the proximal bases of the anal fin forming
a sheath. The upper body profile is convex,
similar to the lower profile. The caudal
fin lobes are rounded, the fin is shallowly
forked. The snout is markedly rounded,
stout. The mouth is small, between terminal
and subterminal; the tip of the mouth
cleft is on a level of the lower margin of the
pupil.
Dorsal fin rays are 3 unbranched and 8½
branched, anal fin rays are 3 unbranched
and 12½ branched, branched pectoral fin
rays are 14, pelvic fin branched rays are 7.
The anal fin origin is somewhat in front of a
vertical from the posterior end of the dorsal
fin base. Total lateral line scales number 45
and those to posterior margin of hypurals
44, scales around caudal peduncle 15, scales
above lateral line to dorsal fin origin are 9,
scales below lateral line to anal fin origin
are 5, scales below lateral line to pelvic fin
origin are 4, and midline predorsal scales
are 19. Pharyngeal teeth 2.5-4.2. Gill rakers
number 7, they are short and stubby, the
longest touching the adjacent one when appressed.
Total vertebrae are 38, comprising
19 abdominal and 19 caudal vertebrae. Predorsal
vertebrae number 11.
Description of paratypes.
The body is moderately compressed,
relatively thick. The caudal fin lobes are
rounded, the fin is shallowly forked. The
ventral keel between the pelvics and anal
fin is variably scaled: completely scaleless
(4), scaled along about ¼-1/3 of its length
(11), scaled along ½ of its length (7), scaled
along about 2/3 of its length (3) or completely
scaled (4). The anal fin origin is in
front of a vertical from the posterior end
of the dorsal fin base. The snout is moderately
stout, rounded. The mouth is almost
horizontal, its position is between terminal
and subterminal; the tip of the mouth cleft
is between a level of the lower margin of the
pupil and a lower margin of the eye. The
junction of the lower jaw and the quadrate
is on about a vertical through the anterior
margin of the pupil.
Dorsal fin unbranched rays 3, branched
dorsal-fin rays 6½ (1), 7½ (2) and 8½ (10)
(7.7, 0.63). Anal fin unbranched rays 3,
branched anal-fin rays 10½ (1), 11½ (8)
12½ (4) (11.2, 0.60). The dorsal fin outer
margin is truncate to markedly convex
and the anal fin outer margin is clearly
concave. Pectoral fin branched rays 12(2),
13(5), 14(4), 15(2) (13.5, 0.97), pelvic fin
branched rays 6(1), 7(12) (6.9, 0.28).
Pharyngeal tooth counts are 2.5-4.2
(20), 2.4-4.2 (5), 2.5-4.1 (2), 2.5-4.3 (2),
1.5-4.2 (1). The lateral line is complete with
none, 1 or 2 unpored scales at the posterior
end of the lateral series; total lateral line
scales 41(3), 42(2), 43(1), 44(4), 45(1),
46(2) (43.3, 1.80); lateral line scales to the
margin of hypurals 39(1), 40(3), 41(2),
42(1), 43(3), 44(3) (41.9, 1.77). Scales
around caudal peduncle 12(1), 13(-), 14(3),
15(5), 16(-), 17(4) (15.2, 1.52); scales between
dorsal fin origin and lateral line 8(1),
9(11), 10(1) (9.0, 0.41); scales between anal
fin origin and lateral line 4(5), 5(7), 6(1)
(4.7, 0.63); scales between pelvic fin origin
and lateral line 4(10), 5(3) (4.2, 0.44), and
predorsal scales 17(1), 18(2), 19(6), 20(1),
21(2), 22(1) (19.3, 1.38). Total gill rakers in
the outer row on first left arch number 6(2),
7(3), 8(7), 9(1) (7.5, 0.88). Total vertebrae
38(1), 39(11), 40(1) (39.0, 0.41).
Other characters as in holotype.
Paratypes bear pigmentation above and
below the lateral line pores, forming a pale
line margined with dark although this is
obscured by background pigment on the
caudal peduncle. A mid-flank stripe is diffuse
posteriorly and fades anteriorly. A thin
dark stripe at the junction of the hypaxial
and epaxial muscles masses is evident
but also fades anteriorly. The pigment on
scales above and below the lateral line can
be strongly or weakly expressed, forming
stripes, but can be absent. The back is dark
and obscures a predorsal and postdorsal
stripe. A series of strong melanophores is
present on the inner margin of the pectoral
fin unbranched ray. Most fins lack much
pigment, the dorsal fin pigment lining the
rays being the strongest apart from that
noted on the pectoral fin.
Summarized data for the paratypes and
additional material of A. idignensis material
(excluding holotype).
Dorsal fin unbranched rays 3, branched
dorsal-fin rays 6½ (1), 7½ (10), 8½ (50),
9½ (1); among 46 radiographed specimens
6½ (1), 7½ (10), 8½ (35) (7.7, 0.49) (Table
1). Anal fin unbranched rays 3, branched
anal-fin rays 9½ (1), 10½ (2), 11½ (29),
12½ (23), 13½ (6), 14½ (1); among 46
radiographed specimens 9½ (1), 10½ (2),
11½ (23), 12½ (16) (11.3, 0.67). The dorsal
fin outer margin is truncate to markedly
convex and the anal fin outer margin is
slightly concave. Pectoral fin branched rays
12(2), 13(20), 14(23), 15(15), 16(2), pelvic
fin branched rays 6(3), 7(58), 8(1).
Total lateral line scales 41(4), 42(8),
43(2), 44(10), 45(14), 46(10), 47(7), 48(3),
49(2), 50(1), 51(1); lateral line scales to
the margin of hypurals 39(2), 40(7), 41(5),
42(4), 43(13), 44(9), 45(10), 46(7), 47(2),
48(2), 49(1). Scales around caudal peduncle
12(1), 13(-), 14(11), 15(21), 16(14),
17(12), 18(3); scales between dorsal fin
origin and lateral line 8(3), 9(33), 10(21),
11(5); scales between anal fin origin and
lateral line 4(20), 5(32), 6(8), 7(2); scales
between pelvic fin origin and lateral line
3(2), 4(16), 5(29), 6(15), and predorsal
scales 17(2), 18(6), 19(19), 20(12), 21(12),
22(7), 23(3), 24(1). Total gill rakers in the
outer row on first left arch number 6(7),
7(14), 8(32), 9(8), 10(1).
Vertebral counts given below were calculated
in 46 specimens. Total vertebrae
number (37)38-40 with a mode of 39 (39.0,
0.65) (Tables 2 and 4). Predorsal vertebrae
number 11-13(14) (12.2, 0.4) (Tables 2 and
5). Abdominal vertebrae number (18)19-20
(19.5, 0.55) (Tables 3 and 5). Caudal vertebrae
number (18)19-20 (19.5, 0.55) (Tables
3 and 6). The vertebral formulae are 20+19
(16), 19+20 (14), 20+20 (8), 19+19 (6),
19+18 (1), and 18+20 (1). Thus, the mean
difference between abdominal and caudal
counts varies between +3 and -2 averaging
0 (0.0, 0.88) (Tables 3 and 6).
see tables for counts
Common names
شبه زوري (shebeh zury = resembling zury) in Khuzestan
for Alburnoides spp..
Systematics
The holotype (CMNFI 2007-0118) is a male, 106.8 mm
TL, 89.2 mm SL from Kermanshahan, Bid Sorkh
River between Sahneh and Kangavar, Gav Masiab
River drainage, ca. 34°23´N, 47°52´E; 1976 and paratypes (CMNFI 2007-0118A)
number 13, 33.5-90.0 mm SL, same data as holotype. The species is named
for the
Tigris River which was called Idigna in
Sumerian (Akkadian: Idiklat; biblical: Hiddekel;
Arabic: Dijlah; Turkish: Dicle).
Key characters
This species is distinguished by
a combination of characters which includes
an unbranched pectoral fin ray strongly
lined with melanophores on its inner margin;
an eye of an average size, the orbit diameter
larger than the snout length and markedly
smaller than the interorbital width; caudal
fin lobes rounded and fin shallowly forked;
a variably scaled ventral keel though most
commonly scaled along about 1/3-2/3 of its
length; a deep head with a markedly rounded,
stout snout; a small mouth which is between
terminal and subterminal; a tip of the mouth
cleft on a level from the lower margin of the
pupil; commonly 8 branched dorsal-fin
rays; 10-12(13-14) branched anal-fin rays;
41-49(50-51) total lateral line scales (39-49
scales to posterior margin of hypurals); commonly
2.5-4.2 or 2.4-4.2 pharyngeal teeth;
(37)38-40, with a mode of 39, total vertebrae;
11-13(14) predorsal vertebrae, (18)19-20 abdominal vertebrae; (18)19-20 caudal
vertebrae; a caudal vertebral region most
commonly one vertebra shorter or one vertebra
longer than the abdominal region; the
most common vertebral formulae are 20+19
and 19+20, and the difference between the
abdominal and caudal counts averaging 0.
Morphology
Sexual dimorphism
The following characters were significantly
different between sexes (p<0.05).
Greater in females: head width, postorbital
distance, pelvic fin origin to anal fin origin
distance. Greater in males: head length,
pectoral fin length in pectoral fin origin
to pelvic fin origin distance, and pelvic fin
length in pelvic fin origin to anal fin origin
distance.
Colour
The lateral
line is delineated by some darker pigment
above and below but not as strongly as in the A. petrubanarescui holotype and obscured
by background pigmentation on the caudal
peduncle. Some pigment on the flank scales
above and below the lateral line give the impression
of stripes but is not strongly developed.A mid-flank stripe is not developed.
A thin dark stripe separates the epaxial and
hypaxial muscle masses. The back is dark
and obscures a predorsal and postdorsal
stripe. The fins are mostly immaculate, with
some melanophores lining the rays of the
dorsal and pectoral fins in particular. The
unbranched pectoral fin ray is strongly lined
with melanophores on its inner margin. The peritoneum is silvery with fine
melanophores and some spots.
Size
?
Distribution
This species is known from
some upper reaches of tributaries of Karkheh
[Qareh Su] River in the Zagros Mountains.
The Karkheh drains into the Tigris just
below its confluence with the Euphrates.
Zoogeography
?
Habitat
This species was captured in the Sarab Dowrah
River at an altitude of 1370 m, in clear water at 19°C, with pH 6.8, the shore
was bushy,
some plants were present in the water, and the river had a stony bed. Other
species recorded together with this species were Barbus lacerta, a “Nemacheilus” sp.,
Alburnus
mossulensis, Capoeta aculeata, Cyprinion macrostomum and Garra rufa.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
See under A. eichwaldii.
Further Work
This recently
described species needs its conservation status and biology investigated.
Sources
Comparative material: CMNFI 1979-0278, 5,
43.3-52.8 mm SL, Lorestan, Sarab Dowrah
River in Kashkan River drainage, 30 km from
Khorramabad (33°34´N, 48°01´E); CMNFI 2007-0075, 36, 38.1-72.1 mm SL, Hamadan, Qareh Su River system, Malayer River at bridge
5 km from Malayer (ca. 34°17´N, 48°47´E); CMNFI 2007-0115, 8, 43.3-62.7 mm SL, Kermanshahan, stream in Karkheh
River system north of Kermanshah (ca. 34°34´N, 46°47´E).
Alburnoides
namaki
Bogutskaya and Coad, 2009
see tables for counts
Common names
None.
Systematics
The holotype, CMNFI 1979-0461, is a female, 91.2 mm SL from Hamadan, qanat at
Taveh, 35°07´N, 49°02´E.
Paratypes are under CMNFI 1979-0461A, 188, 27.2-96.9 mm SL, same data as
the holotype.
The species is named for the
Namak Lake. Namak means salt in Farsi.
Key characters
Morphology
Sexual dimorphism
The following characters were significantly
different between sexes (p<0.05). Head depth, body
depth, head width, orbit diameter, and predorsal
length were greater in females while pectoral fin
length, pelvic fin length, longest dorsal fin
ray length, pectoral fin length in pectoral
fin origin to pelvic fin origin distance and
pelvic fin length in pelvic fin origin to anal
fin origin distance were greater in males.
One male bore tubercles lining scale margins
and sparsely on the top and sides of the
head. Tubercles are strongest on scales of
the caudal peduncle. The anal-fin rays bear
tubercles which follow the branching of the
distal rays. Tubercles are present on the dorsal,
pectoral and pelvic fin rays but are less
developed than those on the anal fin.
Colour
harmonise within?
The lateral line is somewhat
darker than the surrounding flank but there
are no strong spots or dark outline to canal.
Some pigment on flank scales above and below
the lateral line give a faint impression
of stripes. A mid-flank stripe is only weakly
apparent. A predorsal and postdorsal stripe
is present on the back. The fins are mostly
immaculate, with some melanophores lining
the rays of the dorsal and pectoral fins. The
flanks were a golden-yellow, belly white,
back dark green, base of paired and anal fins
orange, other fins hyaline in life.
Some paratypes bear strong pigmentation
above and below the lateral line pores,
forming an evident pale line margined with
dark. A broad mid-flank stripe can be well developed
or weakly expressed and, on the
caudal peduncle, obscures the lateral line
pigment pattern. However, the lateral line pattern can be weak and this can be seen
over the anal fin where the flank stripe does
not extend down to the decurved lateral
line. The pigment on scales above and below
the lateral line (and below the mid-flank
stripe) can be strongly or weakly expressed,
and in the former case it appears as a series
of thin, discontinuous stripes. Some fish
have a series of strong melanophores on the
inner margin of the pectoral fin unbranched
ray. Dorsal fin membranes may be dusky
and lack pigment lining the rays.
The peritoneum is silvery with a few melanophores.
Size
Distribution
Zoogeography
Habitat
Habitat data is based only on the collection data. Altitude was 1640 m, water
temperature
15.5 °C, pH 6.0, conductivity 1.2 mS, qanat
stream width 1.5 m, maximum depth 75 cm,
vegetation in water encrusting, shore grassy,
gravel and mud bottom, medium current, and
water clear in parts, others cloudy and polluted, The species was collected with
Capoeta buhsei.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
See under A. eichwaldii.
Further Work
This recently
described species needs its conservation status and biology investigated.
Sources
Type material: See above.
Comparative material:
CMNFI 2007-0121, 3,
28.0-74.8 mm SL, Hamadan, stream in
Qareh Chay basin north of Razan, ca. 35°25´N, 49°02´E; CMNFI 2007-0074, 4, 33.1-41.8 mm
SL, Markazi, Qareh Chay, 32 km west
of Arak, 34°03´N, 49°21´E; ZMH 4183
(7, ).?
Alburnoides namaki
Bogutskaya and Coad, 2009
Diagnosis. The species is distinguished by
a combination of characters which includes
the lack of strong spots or dark outline to the
lateral line canal; a small eye, the orbit width
about equal to the snout length but markedly
smaller than the interidth; caudal
fin lobes rounded and fin shallowly forked; a
sharp scaleless ventral keel behind the pelvic
fins along the abdomen to the anus; a deep
head with a stout snout which is markedly
rounded; a tip of the mouth cleft on the level
below the lower margin of the eye; commonly
8½ branched dorsal-fin rays; 10-13½,
commonly 11-12½, branched anal-fin rays;
(43)44-50(52) total lateral line scales (42-51
scales to posterior margin of hypurals); 2.5-
4.2 pharyngeal teeth (or other variants with
four teeth on the right ceratobranchial);
commonly 39-41 total vertebrae; 11-13(14),
commonly12-13, predorsal vertebrae; 19-
20(21) abdominal vertebrae; 19-21 caudal
vertebrae; a caudal vertebral region most
commonly equal to the abdominal region;
and the most common vertebral formulae are
20+20, 20+19 and 19+20.
Description of holotype. A ventral keel
between the pelvics and the anal fin is completely
scaleless. There is a pelvic axillary
scale and scales extend over the proximal
bases of the anal fin forming a sheath. Dorsal
fin rays are 3 unbranched and 8½ branched,
anal fin rays are 3 unbranched and 12½
branched, branched pectoral fin rays are 13,
pelvic fin branched rays are 6. The anal fin
origin is on a vertical from the posterior end
of the dorsal fin base. Total lateral line scales
number 50 and those to posterior margin of
hypurals 51, scales around caudal peduncle
16, scales above lateral line to dorsal fin origin
are 12, scales below lateral line to anal
fin origin are 6, scales below lateral line to
pelvic fin origin are 7, and midline predorsal
scales are 25. Pharyngeal teeth 2.5-4.3. Gill
rakers number 7, they are short and stubby,
the longest touching the adjacent one when
appressed. Total vertebrae are 40, comprising
20 abdominal and 20 caudal vertebrae.
Predorsal vertebrae number 13.
The upper body profile is convex, similar
to the lower profile. The snout is markedly
rounded, stout. The mouth is small, almost
subterminal; the tip of the mouth cleft is
on a level from the lower margin of the eye.
Description of paratypes. The body is
compressed. The ventral keel between the
pelvics and anal fin is completely scaleless,
very sharp and prominent in all specimens.
The anal fin origin is below the posterior
end of the dorsal fin base. The snout is short
and markedly rounded in smaller and larger
individuals. The mouth is almost subterminal,
with the tip of the mouth cleft on a level
of the lower margin of the eye or below. The
junction of the lower jaw and the quadrate
is on about a vertical through the middle of
the eye.
Dorsal fin unbranched rays 3, branched
dorsal-fin rays 7½ (2), 8½ (48), 9½ (8)
(8.1, 0.41). Anal fin unbranched rays 3,
branched anal-fin rays 10½ (5), 11½ (14),
12½ (29), 13½ (9), 14½ (1) (11.8, 0.88)
(see also Tables 1 and 4 for data based on a
set of another 48 specimens which were radiographed).
The dorsal fin outer margin is
truncate to markedly convex and the anal
fin outer margin is slightly concave. Pectoral
fin branched rays 12(6), 13(33), 14(17),
15(2) (13.3, 0.69), pelvic fin branched rays
6(7), 7(51) (6.9, 0.33).
Pharyngeal tooth counts are 2.5-4.2
(20), 2.4-4.2 (5), 2.5-4.1 (2), 2.5-4.3 (2),
1.5-4.2 (1). The lateral line is complete
with none or 1 unpored scales at the posterior
end of the lateral series; total lateral
line scales 43(1), 44(3), 45(3), 46(11), 47(12), 48(16), 49(8), 50(3), 51(-),
52(1)
(47.3, 1.68); lateral line scales to the margin
of hypurals 42(1), 43(4), 44(5), 45(12),
46(15), 47(10), 48(9), 49(1), 50(-), 51(1)
(46.1, 1.70). Scales around caudal peduncle
14(2), 15(9) 16(13), 17(19), 18(14), 19(1)
(16.6, 1.17); scales between dorsal fin origin
and lateral line 9(4), 10(29), 11(24), 12(-),
13(1) (10.4, 0.70); scales between anal fin
origin and lateral line 4(10), 5(37), 6(10),
7(1) (5.0, 0.65); scales between pelvic fin
origin and lateral line 4(3), 5(31), 6(22),
7(2) (5.4, 0.65), and predorsal scales 18(1),
19(4), 20(13), 21(16), 22(13), 23(4), 24(3),
25(4) (21.3, 1.58). Total gill rakers in the
outer row on first left arch number 5(1),
6(14), 7(26), 8(14), 9(3) (7.0, 0.90).
Vertebral counts were calculated for 48
specimens (including holotype). Total vertebrae
number 39-40(41) (39.7, 0.59) (Tables
2 and 4). Predorsal vertebrae number
(11)12-13(14) (12.2, 0.54) (Tables 2 and
5). Abdominal vertebrae number 19-21with
a mode of 20 (19.8, 0.52) (Tables 3 and
5). Caudal vertebrae number 19-21 (19.9,
0.58) (Tables 3 and 6). The vertebral formulae
are 20+20 (in 21 specimens), 19+20
(10), 20+19 (8), 19+21 (3), 20+21 (3), and
21+19 (2). Thus, the mean difference between
abdominal and caudal counts varies
between +3 and -2 with a mode of 0 (-0.1,
0.92) (Tables 3 and 6).
Many
scales are regenerated in various fish from
this collection, perhaps indicating a traumatic
life.
Comparative remarks. Alburnoides namaki
sp. n. differs from all the congeners primarily
by having a combination of a sharp
scaleles keel, a short markedly rounded
snout, an almost subterminal mouth and a
low number of predorsal vertebrae (modally
12). In tree diagrams based on combined
data (Figs 3-6) A. namaki is clustered
together with A. varentsovi sp. n. from the
northern slope of Kopetdag. Alburnoides
namaki which shares with A. varentsovi sp.
n. (and A. idignensis sp. n.) the lowest number
of predorsal vertebrae (modally 12) is
distinguished by a shallowly forked caudal
fin with rounded lobes (vs. clearly forked,
with pointed lobes), a small, almost subterminal
mouth with the tip of the mouth cleft
on a level from the lower margin of the eye
or below (vs. oblique and terminal, the tip
of the mouth cleft on a level from the middle
of the eye or slightly above), a sharp and
commonly completely scaleless ventral keel
(vs. commonly partly scaled).
Common names
None.
Systematics
Key characters
Morphology
Sexual dimorphism
Colour
Size
Distribution
Zoogeography
Habitat
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
Further Work
Alburnoides
nicolausi
Bogutskaya and Coad, 2009
?see tables for counts, check
agaisnt etxt
Diagnosis. The species is distinguished
by a combination of characters which includes
an eye of an average size, the orbit
diameter larger than the snout length and
smaller than the interorbital width; caudal
fin lobes rounded and fin shallowly forked;
a variably scaled ventral keel though most
commonly scaled only along about 1/3 of
its length or scaleless; a deep head with a
moderately stout snout which is slightly
pointed; a tip of the mouth cleft on the level
about the lower margin of the pupil, commonly
7½ branched dorsal-fin rays; 8-11½
branched anal-fin rays; (43)43-47(48-50)
total lateral line scales (42-48 scales to posterior
margin of hypurals); commonly 2.5-
4.2 or 2.4-4.2 pharyngeal teeth; commonly
39-40 total vertebrae; 12-13 predorsal vertebrae;
19-20(21) abdominal vertebrae;
18-20 caudal vertebrae; a caudal vertebral
region most commonly one vertebra shorter
than the abdominal region; and the most
common vertebral formulae are 20+19,
19+20 and 20+20.
Dorsal fin rays are 3 unbranched and 7½
branched, anal fin rays are 3 unbranched
and 10½ branched, branched pectoral fin
rays are 13, pelvic fin branched rays are 7.
The anal fin origin is slightly behind a vertical
from the posterior end of the dorsal fin
base. Total lateral line scales number 47 and
those to posterior margin of hypurals 45,
scales around caudal peduncle 17, scales
above lateral line to dorsal fin origin are 10,
scales below lateral line to anal fin origin
are 5, scales below lateral line to pelvic fin
origin are 5, and midline predorsal scales
are 19. Pharyngeal teeth 2.5-4.2. Gill rakers
number 8, they are short and stubby, the
longest touching the adjacent one when appressed.
Total vertebrae are 38, comprising
20 abdominal and 18 caudal vertebrae. Predorsal
vertebrae number 12.
Description of paratypes.
Dorsal fin unbranched rays 3, branched
dorsal-fin rays 7½ (52) and 8½ (7) (7.1,
0.33). Anal fin unbranched rays 3, branched
anal-fin rays 8½ (2), 9½ (13), 10½ (32),
11½ (12) (10.0, 0.68) (see also Tables 1 and
4 for 42 radiographed specimens). The dorsal
fin outer margin is commonly truncate,
slightly convex or slightly concave, and the
anal fin outer margin is truncate or only
slightly concave. Pectoral fin branched rays
11(1), 12(30), 13(25), 14(3) (12.5, 0.63),
pelvic fin branched rays 6(6), 7(53) (6.9,
0.30).
Pharyngeal tooth counts are 2.5-4.2
(24), 2.4-4.2 (5), 2.4-5.2 (1). The lateral
line is complete with none, 1 or 2 unpored
scales at the posterior end of the lateral series;
total lateral line scales 42(2), 43(11),
44(15), 45(6), 46(13), 47(7), 48(2), 49(2),
50(2) (45.0, 1.82); lateral line scales to the
margin of hypurals 41(3), 42(14), 43(11),
44(11), 45(8), 46(9), 47(-), 48(2), 49(1)
(43.9, 1.83). Scales around caudal peduncle
13(1), 14(9), 15(30) 16(14), 17(5) (15.2,
0.87); scales between dorsal fin origin and
lateral line 8(2), 9(30), 10(25), 11(2) (9.5,
0.62); scales between anal fin origin and lateral
line 3(1), 4(27), 5(28), 6(3) (4.6, 0.62);
scales between pelvic fin origin and lateral
line 4(21), 5(37), 6(1) (4.7, 0.51), and predorsal
scales 18(4), 19(13), 20(18), 21(10),
22(8), 23(4), 24(2) (20.4, 1.49). Total gill
rakers in the outer row on first left arch
number 5(1), 6(1), 7(33), 8(22), 9(2) (7.4,
0.67).
Vertebral counts given below were calculated
in 42 specimens. Total vertebrae
number 38-40 with a mode of 39 (38.9,
0.58) (Tables 2 and 4). Predorsal vertebrae
number 12-13 (12.6, 0.50) (Tables 2 and 5).
Abdominal vertebrae number 19-21with
a mode of 20 (19.8, 0.53) (Tables 3 and 5).
Caudal vertebrae number 18-20 (19.1, 0.68)
(Tables 3 and 6). The vertebral formulae are
20+19 (in 18 specimens), 19+20 (9), 20+18
(6), 20+20 (4), 19+19 (3), 21+18 (1), and
21+19 (1). Thus, the mean difference between
abdominal and caudal counts varies
between +3 and -1 with a mode of 1 (0.6,
1.08) (Tables 3 and 6).
Common names
شبه زوري (shebeh zury = resembling zury) in Khuzestan.
Systematics
The holotype (CMNFI 1979-0281) is a female, 75.0 mm SL, Lorestan, stream
in Simareh River drainage, 5 km south of Nurabad (34°03´30´´N, 47°58´30´´E) and
paratypes (CMNFI 1979-0281A) comprise 164 specimens, 21.3-65.0 mm SL, same data as holotype.
The species is named after a
Latin male name Nicolaus, a derivative of
the Greek Nikolaos (victory of the people),
a compound name composed of the elements
nikē (victory) and laos (the people); a Russian
name Nikolay and an English name
Nicholas, the names of, respectively, Nina
Bogutskaya’s elder son and Brian Coad’s
son, are also derivatives from Nicolaus.
Key characters
This species differs from all the congeners
primarily by having a combination of commonly
7 branched dorsal-fin rays, 8-11
branched anal-fin rays, and 38-40, modally 39, total vertebrae.
Morphology
The body is
moderately compressed, relatively thick.
The upper body profile is convex similar to the lower profile. The snout
is only slightly rounded, almost pointed.
The mouth is oblique, slightly below than
terminal; the tip of the mouth cleft is slightly
below a level of the lower margin of the
pupil.
The junction of the lower jaw and the quadrate
is on about a vertical through the middle
of the eye. The caudal
fin lobes are rounded, the fin is shallowly
forked. A ventral keel between the pelvics and the anal fin is not sharp and is variably scaled: completely scaleless
(9), scaled along about ¼-1/3 of its length
(9), scaled along half of its length (6), scaled
along about two-thirds of its length (4) or completely scaled (2). There
is a pelvic axillary scale and scales extend
over the proximal bases of the anal fin forming a sheath. The anal fin origin is
somewhat behind a vertical from the posterior end of the dorsal fin base.
Sexual dimorphism
The following characters were larger in females: pectoral fin origin to
pelvic fin origin distance, pelvic fin origin to
anal fin origin distance, prepelvic fin length,
and mouth width while the following were larger in males: caudal peduncle
length, pectoral fin length, pelvic fin
length, longest dorsal fin ray length, longest
anal fin ray length, pectoral fin length in
pectoral fin origin to pelvic fin origin distance,
and pelvic fin length in pelvic fin origin
to anal fin origin distance.
Colour
?harmonise
The lateral
line is delineated by some darker pigment
above and below but not as strongly as in
A. petrubanarescui holotype and obscured
by background pigmentation on the caudal
peduncle. Some pigment on the flank scales
above and below the lateral line is weak or
irregular and an impression of stripes is not
very evident. The mid-flank stripe is weak
and diffuse, fading anteriorly under the
dorsal fin. The back is dark but predorsal
and postdorsal stripes are evident. The fins
are mostly immaculate, with some melanophores
lining rays of the dorsal and pectoral
fins in particular. The unbranched pectoral
fin ray is lined with melanophores on its inner
margin, but not as strongly as in some
other samples.
Paratypes can bear strong pigmentation
above and below the lateral line pores,
forming an evident pale line margined with
dark, or this pattern may be quite faint. The
mid-flank stripe is weak or diffuse and fades
anteriorly. A thin line of pigment can be
evident separating the hypaxial and epaxial
muscle masses, fading anteriorly. The pigment
on scales above and below the lateral
line (and below the mid-flank stripe) can be
obvious and form a series of thin, discontinuous
stripes, or it can be absent. Some fish
have a series of strong melanophores on the
inner margin of the pectoral fin unbranched
ray.
The peritoneum is silvery with fine melanophores and some spots.
Size
Distribution
The species is known only
from its type locality, a stream in the Simareh
River drainage at Nurabad. The Simareh (Seymareh) flows into the Karkheh (Qareh Su) River which enters the Hawr al
Hawizeh (Hawr al Azim) on the Iran-Iraq border (Tigris River drainage).
Zoogeography
In tree diagrams (Bogutksaya and Coad, 2009) based
on combined data, this species it is clustered together with another Tigris
River basin species, A. idignensis. ??
Habitat
Habitat data is based on collection data. Fish were collected at
2000 m altitude, 19°C water temperature,
clear water, pH 6.8, forested shore, stony
river bed, moderate amounts of aquatic
plants, and no other species taken.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
See under A. eichwaldii.
Further Work
This recently
described species needs its conservation status and biology investigated.
Sources
Alburnoides
petrubanarescui
Bogutskaya and Coad, 2009
?see tables for counts, check
against etxt
Holotype. CMNFI 1970-0558, female, 109.1 mm TL, 88.8 mm SL; Iran, Azarbaijan-e
Bakhtari, Qasemlou Chay, Orumiyeh [Urmia] Lake basin, ca. 37°21´N, 45°09´E; 27
June 1962; coll. V.D. Vladykov. Paratypes. CMNFI 1970-0558A, 51, 28.7-87.3 mm
SL, counts and measurements on 29 fish 33.6-87.3 mm SL; same data as holotype.
Diagnosis. The species is distinguished by a combination of
characters which includes a small eye; the orbit width about equal to the snout
length but markedly smaller than the interorbital width; caudal fin lobes
rounded and the fin shallowly forked; a scaled ventral keel behind the pelvic
fins along the abdomen to the anus, a deep head with a stout snout which is
markedly rounded; a tip of the mouth cleft on the level below the lower margin
of the eye; commonly 7½ (less frequently 8½) branched dorsal-fin rays; 8-10½,
commonly 9½, branched anal-fin rays; 44-51 total lateral line scales (42-49
scales to posterior margin of hypurals); 2.5- 4.2 pharyngeal teeth (or other
variants with four teeth on the right ceratobranchial); commonly 40-41 total
vertebrae; 13-14 predorsal vertebrae; 20-22, commonly 21, abdominal vertebrae;
19-20 caudal vertebrae; a caudal vertebral region most commonly
shorter than the abdominal region; and the most common vertebral formulae are
21+19 and 21+20.
Description of holotype. The caudal fin lobes are rounded and the fin is
shallowly forked. A ventral keel between the pelvics and the anal fin is smooth
and completely scaled. There is a pelvic axillary scale and scales extend over
the proximal bases of the anal fin forming a sheath. The upper body profile is
convex, similar to the lower profile. The snout is markedly rounded, stout. The
mouth is small, subterminal; the tip of the mouth cleft is on a level below the
lower margin of the eye. The body depth enters SL 3.3 times, HL enters 4.3,
predorsal length 1.8, caudal peduncle depth 7.7, caudal peduncle length 4.1,
length of longest dorsal fin ray 5.2, and length of longest anal fin ray to
scale sheath 6.8. Orbit diameter enters HL 3.5 times, snout length enters 3.6,
and interorbital width 2.6. Pectoral fin length enters pectoral fin origin to
pelvic fin origin distance 1.3 times, and pelvic fin length
enters pelvic fin origin to anal fin origin distance 1.2 times. Dorsal fin rays
are 3 unbranched and 7½ branched, anal fin rays are 3 unbranched and 9½
branched, branched pectoral fin rays are 13, pelvic fin branched rays are 7. The
anal fin origin is on a vertical from the posterior end of the dorsal fin base.
Total lateral line scales number 46 and those to posterior margin of hypurals
45, scales around caudal peduncle 15, scales above lateral line to dorsal fin
origin are 9, scales below lateral line to anal fin origin are 5, scales below
lateral line to pelvic fin origin are 6, and midline predorsal scales are 21.
Pharyngeal teeth 2.5-4.2. Gill rakers number 7, they are short
and stubby, the longest touching the adjacent one when appressed. Total
vertebrae are 41, comprising 21 abdominal and 20 caudal vertebrae. Predorsal
vertebrae number 13.
The peritoneum is silvery with fine melanophores and some large spots. The
lateral line is clearly delineated by darker pigment above and below. Some
pigment on flank scales above and below the lateral line give the impression of
stripes. A mid-flank stripe is evident. The back is dark and obscures a
predorsal and postdorsal stripe. The fins are mostly immaculate, with some
melanophores lining the rays of the dorsal and pectoral
fins. The unbranched pectoral fin ray is strongly lined with melanophores on its
inner margin.
Description of paratypes.
The body is compressed but relatively thick. The ventral keel between the
pelvics and anal fin is not sharp and is completely covered by scales in all
specimens. The anal fin origin is below the posterior end of the dorsal fin
base. The snout is short and markedly rounded in smaller and larger individuals.
The mouth is subterminal, with the tip of the mouth cleft on a level below the
lower margin of the eye. The junction of the lower jaw and the quadrate is on
about a vertical through the anterior eye margin. The following characters
were significantly different between sexes (p<0.05). Greater in females:
postorbital length, predorsal length, pectoral fin origin to pelvic fin origin
distance, pelvic fin origin to anal fin origin distance. Greater in males: HL,
pectoral fin length in pectoral fin origin to pelvic fin origin distance, and
pelvic fin length in pelvic fin origin to anal fin origin distance. Dorsal fin
unbranched rays 3, branched dorsal-fin rays 7½ (19) or 8½ (10) (7.3, 0.48). Anal
fin unbranched rays 3, branched anal-fin rays 8-10½ (9.3, 0.64, including
holotype) (Tables 1 and 4). The dorsal fin outer margin is truncate to markedly
convex and the anal fin outer margin is slightly concave. Pectoral fin branched
rays 13(16), 14(12), 15(1) (13.5, 0.57), pelvic fin branched rays
6(5), 7(24) (6.8, 0.38) Pharyngeal tooth counts are 2.5-4.2 (18), 2.4-4.2 (4),
2.5-4.1 (5), 1.4-4.1 (1), 1.5-4.0 (1). The lateral line is complete with none or
1 unpored scales at the posterior end of the lateral series; total lateral
line scales 43(1), 44(3), 45(2), 46(8), 47(5), 48(6), 49(3), 50(1) (46.7, 1.73);
lateral line scales to the margin of hypurals 42(1), 43(3), 44(4), 45(5), 46(6),
47(6), 48(3), 49(1) (45.6, 1.76). Scales around caudal peduncle 14(2), 15(7)
16(10), 17(9), 18(-), 19(1) (16.0, 1.09); scales between dorsal fin origin and
lateral line 9(7), 10(18), 11(4) (9.9, 0.62); scales between anal fin origin and
lateral line 4(5), 5(23), 6(1) (4.9, 0.44); scales between pelvic fin origin and
lateral line 3(1), 4(8), 5(20) (4.7, 0.55); predorsal scales 20(1), 21(10),
22(14), 23(3), 24(1) (21.8, 0.83). Total gill rakers in the outer row on first
left arch number 6(3), 7(18), 8(8) (7.2, 0.60). Vertebral counts given below
include holotype. Total vertebrae number (39)40-41(42) (40.5, 0.63) (Tables 2
and 4). Predorsal vertebrae number 13-14 with a mode of 13 (13.4, 0.50) (Tables
2 and 5). Abdominal vertebrae number 20-22 with a mode of 21 (21.0, 0.41)
(Tables 3, 5). Caudal vertebrae number 19-20(21) (19.5, 0.57) (Tables 3 and 6).
The vertebral formulae are 21+19 (in 12 specimens), 21+20 (10), 22+19 (3), 20+19
(1), and 21+21 (1). Thus, the caudal vertebral region is shorter than the
abdominal region, rarely equal to it (in 3 specimens), the mean difference
between abdominal and caudal counts being +1.4 (std 0.77) (Tables 3 and 6).
Other characters as in holotype.
Most paratypes bear strong pigmentation above and below the lateral line pores,
forming an evident pale line margined with dark. The broad mid-flank stripe is
well-developed. The pigment on scales above and below the lateral line (and
below the midflank stripe) form a series of thin, discontinuous stripes. Some
fish have a series of strong melanophores on the inner margin of the pectoral
fin unbranched ray. The lateral line over the pectoral and pelvic fins can be
wavy rather than a smooth decurved line.
Comparative remarks. Alburnoides petrubanarescui sp. n.
differs from all the congeners primarily by having a combination of a scaled
keel, the lowest number of branched anal-fin rays (modal value 9½ vs. 10½ and
more), and the highest value of the difference between the abdominal and
vertebral counts. A completely scaled keel is a character shared by A.
petrubanarescui sp. n., A. oblongus distributed in the lower reaches of the Syr
Darya and Alburnoides sp. from Pulvar (Kor River drainage). However, A.
petrubanarescui sp. n. is distinguished from the two other species of this group
by having fewer branched dorsal-fin rays (commonly 7½ vs. 8½) and fewer branched
anal-fin rays (8-10½ vs. 10-12½). Besides, A. petrubanarescui sp. n. is clearly
different from A. oblongus by having larger scales (43-50 total lateral line
scales vs. 50-56), 2.5-4.2 and 2.4-4.2 pharyngeal teeth (vs.
2.5-5.2 or 1.5-5.1), fewer gill rakers (6-9 vs. 10-13), a truncate or rounded
margin of the dorsal fin (vs. concave). A. petrubanarescui sp. n. differs from
Alburnoides sp. from Pulvar, besides some other characters, by fewer dorsal-fin
branched rays (commonly 7½ vs. 8½), fewer anal-fin branched rays (8-10½,
commonly 9½, vs. 10-12½, commonly 11½) and 21+19 or 21+20 vertebrae (vs. 20+20
or 20+21) the difference between abdominal and caudal counts averaging +1.4 (vs.
-0.3).
Etymology. The species is named after the late Petru Bǎnǎrescu, a great
freshwater ichthyologist who contributed significantly to our knowledge of
fishes of Eurasia.
Distribution. This species is described from a river in the
Orumiyeh [Urmia] lake basin and we suppose that it may be an endemic species to
the Orumiyeh lake basin.
Habitat data for the type locality (June 1962): water 18 °C,
fast current in stream, pebbles and sand bottom, shore grassy, much aquatic
plant life, caught with dipnet, other species included Alburnus atropatenae,
Barbus
lacerta, “Nemacheilus” sp.
Common names
None.
Systematics
Key characters
Morphology
Sexual dimorphism
Colour
Size
Distribution
Zoogeography
Habitat
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
See under A. eichwaldii.
Further Work
This recently
described species needs its conservation status and biology investigated.
Sources
Alburnoides
qanati
Coad and Bogutskaya, 2009

Common names
None.
Systematics
The female holotype is in the Canadian Museum of Nature, Ottawa, under CMNFI
1977-0509, 81.5 mm TL, 65.0 mm SL, Fars, at source and along stream of a qanat at Naqsh-e Rostam, Pulvar River system (29°59’30”N, 52°54’00’’E).
Paratypes are under CMNFI 1977-0510, 168 (not 178 as in type description) specimens, 24.9-72.5 mm SL, same
data as holotype. The species was named after the famous qanat system which taps
groundwater to support human survival in desert regions and, incidentally, a
habitat for fishes.
Key characters
The species is distinguished by a combination of characters which includes a
large eye, the orbit width exceeding both the snout length and the interorbital
width, a scaled ventral keel behind the pelvic fins along the abdomen to the
anus, commonly 43-47 lateral line scales to posterior margin of hypurals,
2.5-4.2 pharyngeal teeth, commonly 8 branched dorsal fin rays, 10-12 branched
anal fin rays, 40-41 total vertebrae, an d the caudal vertebral region equal or
longer then the abdominal region (vertebral formulae 20+20 or 20+21).
Morphology
The body is markedly compressed. The upper body
profile is convex or, in larger specimens, slightly to markedly straightened
while the lower profile is considerably convex. The ventral keel between the pelvics and anal fin is not sharp and is completely covered by scales in all
specimens but four possessing a short scaleless portion of keel (about half of keel
length) just in front of the anus. The dorsal fin outer margin is
truncate to slightly rounded and the anal fin outer margin is truncate to
slightly concave. The anal fin origin is behind the posterior
end of the dorsal fin base. A pelvic axillary scale is present and the anal fin base
is proximally overlain by flank scales. The snout is short and slightly pointed. The mouth
is terminal to upturned, with the tip of the mouth cleft on a level from
slightly above the middle of the eye to the upper margin of the pupil. The
mouth cleft is always turned upward, never horizontal, the lower jaw slightly to
moderately projecting relative to the upper jaw, and the junction of the lower
jaw and the quadrate is on about a vertical through the anterior eye margin. The
lateral line is decurved and
only the last few scales are elevated and on the mid-caudal peduncle.
Dorsal fin unbranched rays commonly 3, 4 in 3 specimens only, dorsal fin branched
rays 7(3) or 8(28), anal fin unbranched
rays 3, anal fin branched rays 10(3), 11(22), 12(6),
branched pectoral fin rays 13(4), 14(20) or 15(7), pelvic fin branched rays
7(30). The lateral line is complete with none, 1 or 2 unpored scales
at the posterior end of the lateral series. Lateral line scales to posterior
margin of hypurals 41(1), 42(1), 43(5), 44(6), 45(3), 46(8), 47(5) 48(1) or
49(1), scales above lateral line to dorsal fin origin 9(10), 10(18)
or 11(3), scales below lateral line to pelvic fin origin 3(4),
4(20) or 5(7), and scales below lateral line to anal fin origin
4(17), 5(13) or 6(1). Total scale radii 8(1), 9(1), 10(4), 11(8),
12(20), 13(17), 14(16) 15(12), 16(7), 17(3) or 18(1) (13.2, 1.91). Scale radii
are restricted
to the posterior field encroaching laterally, circuli are eccentric and the
focus is anteriorly located. Total gill rakers in the outer row on first left
arch 6(4), 7(4), 8(21) or 9(1); gill rakers are very short
and widely spaced, not touching the adjacent raker when appressed. Total
vertebrae including 4 Weberian vertebrae and last complex centrum
40(14) or 41(17), abdominal vertebrae (including intermediate ones; precaudal vertebrae auctorum) 20 (29) or 21(12),
predorsal vertebrae (anterior to first dorsal pterygiophore) 13(24) or
14(6), and caudal vertebrae 20(18) or 21(13). The vertebral formula is 20+20(16), 20+21(12) or 21+20(2). Thus, the caudal vertebral region most commonly (in 93% of examined specimens) is
equal to or slightly longer then the abdominal region, the mean difference
between abdominal and caudal counts being -0.3. Pharyngeal tooth counts are
2.5-4.2 in 10 fish examined with one additional fish
being a variant with 2.4-4.0. Teeth are hooked at the tip and not serrated below
it. The gut shape is a simple “S” with an occasional specimen showing a slight
flexure to the left of the anterior loop. The general
topography of cephalic sensory canals and numbers of pores is typical of most
Alburnoides, as described by Bogutskaya (1988). The supraorbital canal is
not lengthened in its posterior section and has 7-11, commonly 8-10 pores, with 2-4 (3 in 90%) and 5-7 (6 in 73%) canal openings
on the nasal and frontal bones, respectively. The infraorbital canal has 10-15
pores (13 in 38%, 12 in 30%) with 4 (93%) or 5 canal openings on the first infraorbital. The preopercular-mandibular canal is complete, with 11-17,
modally 13-16, pores (14 in 38%) with (3)4-6 (5 in 77%) and 7-10 (8 in
62%) canal openings on the dentary and preoperculum, respectively. The
supratemporal canal is complete, with (4)5-7 (7 in 54%) pores.
Sexual dimorphism
Head length is longer in males than in females. Pectoral fin length and
pelvic fin length are also longer in males.
Colour
Pigmentation of the holotype in 5% formalin consisted of a dark lateral line
dividing the hypaxial and epaxial muscle masses and a weakly developed stripe of
black pigment on mid-flank prominent posteriorly on the caudal peduncle but
fading over the pectoral fin and often interrupted anteriorly. The lateral line
pores were lined by pigment dorsally and ventrally. A mid-dorsal line was
apparent before the dorsal fin, weakly developed behind the fin. The fins were
mostly hyaline with some black pigment lining the fin rays of the dorsal and
caudal fins, the dorsal rays of the pectoral fins and the anterior rays of the
anal fin.
Overall colouration is silvery with the bases of the pectoral,
pelvic and anal fins pink in life. An orange line parallels the anal fin base
and the lateral line, lying midway between the two. The ventral surface of the
head between the dentaries may be yellow-orange and similarly coloured spots may
be found on either side of the dorsal mid-line extending along the whole body.
Faint yellow spots occur in rows along the flanks also. Pigmentation in
preserved fish is as described for the holotype although the lateral stripe is
weakly-developed in some specimens, the mid-flank band of spots of black pigment
may be variably developed, and the lateral line may be clearly or only faintly
edged by pigment. The peritoneum is rarely dark brown but usually is
white-grey to light brown with black spots.
Size
Attains 72.5 mm standard length.
Distribution
Known originally from the Pulvar River drainage of the Kor River basin
in southern Iran but also recorded from Harat in the Sirjan basin at 30º01.196'N,
54º20.33'E (material from H. R Esmaeili,
2011).
Zoogeography
This is the southernmost Alburnoides species and may have entered the
Kor River basin by headwater capture from the Tigris-Euphrates River basin.
Habitat
The qanat stream in the Pulvar River basin at 15.00 hours on 6 October 1976 had clear and colourless
water, a temperature of 21°C, pH 6.8, conductivity 0.475 mS, the current was
slow to medium, stream width was about 2 m and maximum depth was up to 1 m, the
shore was grassy, plant life in the stream consisted of encrusting and
submergent types, and the stream bed was gravel and mud. The Harat locality was
at an altitude of 1585 m, pH 8.17, dissolved oxygen 7.25 mg/l, conductivity 816
mS and temperature 22.9-23.3°C.

Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
The numbers and wider distribution of this species should be researched as it
is known from only two localities.
Further Work
See under
Conservation. Biology is unknown.
Sources
Type material: See above.
Other material: CMNFI 1979-0060, 4, 21.0-35.4 mm SL, Fars, spring and
irrigation channel, 7 km north of Sa’adatabad (30°06’N, 53°12’E).
Alburnoides taeniatus
(Kessler, 1874)
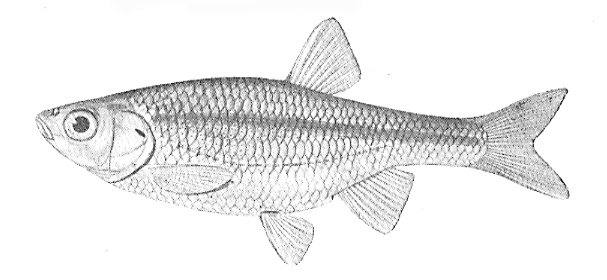
Reported from the Tedzhen River basin (Aliev et al., 1988),
Karakum Canal, Kopetdag Reservoir and Uzboi lakes (Shakirova and
Sukhanova, 1994; Sal'nikov, 1995) in Turkmenistan on the northeastern
border of Iran. It may eventually reach the Caspian Sea basin and the
Tedzhen (= Hari) River basin of Iran. No Iranian record.
Genus Alburnus
Rafinesque, 1820
The bleaks and shemayas are found in Europe and the northern parts of Southwest
Asia with about 38 species (depending on definitions of the taxa). There are 5 species in Iran. Records of Alburnus
orontis Sauvage, 1882 from Iran by Armantrout (1969), Banarescu
(1977) and Wossughi (1978) are in error (Krupp, 1985c). Chalcalburnus
Berg, 1933 is now regarded as a synonym of Alburnus Rafinesque, 1820. There
have been numerous variant views of this synonymy. Bogutskaya (1990)
considers Chalcalburnus to be distinct but later, Bogutskaya
(1997b; Bogutskaya et al., 2000; Bogutskaya and Naseka, 2004), synonymises it with Alburnus.
Reshetnikov et al. (1997) retain Chalcalburnus as a
distinct genus as does Eschmeyer in "Catalog of Fishes" (downloaded, 10 August
2007). Banister (1980) points out that the distinction of the
genus from Alburnus is based on the relative lengths of the
ventral keel and the relative thickness of the last unbranched dorsal
fin ray, characters which he views with suspicion in the absence of
other corroborating evidence.
This genus is characterised by an elongate, compressed, moderately
deep body of small to moderate size, a terminal mouth, no barbels,
scales of moderate size, pharyngeal teeth in 2 rows (2,5-5,2 or
2,5-4,2) with hooked tips and usually serrations (often absent), short
dorsal fin without a thickened ray, a long anal fin, long and
relatively numerous gill rakers, a fleshy keel between the base of the
pelvic fins and the vent (the naked part usually not reaching as far forward as
the pelvic fin bases in species formerly placed in Chalcalburnus), and a light to brown
or black peritoneum. Some authors consider the genus Alburnoides
to be synonyms of Alburnus (e.g. Saadati (1977)) while others
disagree (e.g. Bogutskaya (1990)). These genera are treated separately
here to accord with common usage in Southwest Asia, a conservative
measure when there are conflicting opinions.
Jalali et al. (2002) and Jalali and Barzegar (2006) record several parasites from an undescribed Chalcalburnus
species in Lake Zarivar, namely Ichthyophirius multifilis,
two species of Argulus, a Trichodina species, Dactylogyrus
alatus, Diplostomum spathaceum, Myxobolus molnari and Ligula intestinalis.
Masoumian et al. (2007) record the myxosporean parasite Myxobolus
saidovi from Alburnus maculatus (sic) in the Zayandeh River
and Mehdipoor et al. (2004) record the monogenean Dactylogyrus alatus
from Alburnus maculatus (sic), also in the Zayandeh River..
Alburnus doriae de Filippi, 1865 has a type locality of "dintorni di
Schiraz" but fish resembling this species have not been caught there in late
twentieth and early twenty-first century collections. Krupp (1985c) refers 5
specimens from the type series of Alburnus doriae to his Alburnus sellal and 2
specimens to Squalius lepidus. The lectotype (MZUT N.720 or MZUT P1110) of Alburnus doriae
is stored in the Istituto e Museo di Zoologia della R. Università di
Torino (122.0 mm standard length as measured by me) and 5 paralectotypes (MSNG C.E. 9102) of this nominal species
are in the Museo Civico di Storia Naturale di Genova (Tortonese, 1934;
1940; 1961), only one of which is A. doriae (109.1 mm standard length as
measured by me). Eschmeyer's "Catalog of Fishes" (downloaded 10 August 2007) has
6 specimens in MSNG C.E. 9102, 5 not this species and gives a locality as probably south of Shiraz.
It seems probable that the fish were collected north of Shiraz, presumably in a
Tigris River basin stream based on the other species included in the jar
(although Alburnus sellal is more likely to be A. mossulensis,
q.v.). These materials may, however, have been mixed and the type
locality of this nominal species is obscure.
The species of Alburnus in the Zagros Mountains north of
Shiraz and west of Esfahan are currently under investigation and final species
identities cannot be given at present. Note that materials identified by Coad
(1982d: Alburnus maculatus; 1985: A. doriae) as Leuciscus
lepidus were in error. An illustration of A. doriae is given above
based on the type material.
Small fishes and members of the genus Alburnus are called kuli in
Farsi. In Gilan, kuli are eaten with their heads on and are said to full of
phosphorus, conferring open-mindedness, intelligence and sophistication on the Gilanis.
Alburnus atropatenae
Berg, 1925
Common names
None.
Systematics
The type series is the material called Alburnus filippii by
Günther (1899) from "Sujbulak and Superghan near the mouth of
the Nazlu Chai" as noted in Berg (1925). This material is in the
Natural History Museum, London under BM(NH) 1899.9.30:127, syntype, 1
specimen, 89.7 mm Sl, Azarbayjan-e Bakhtari, Superghan near the mouth
of the Nazlu Chai (Sopurghan on the Nazlu Chay is at 37°45'N,
45°12'E); BM(NH) 1899.9.30:128-30, syntypes, 3, 70.7-96.3 mm Sl, Azarbayjan-e
Bakhtari, Tatawa Chai near Sujbulak (the Tata'u Chay or Simineh River
is not close to Saujbulagh or Mahabad at 36°45'N, 45°43'E
so the exact locality of this collection is unclear).
These syntypes bear an external label, apparently in A. Günther's
handwriting, listing these fish under the name "brevianalis"
which is crossed out and filippii substituted. It appears that
Günther originally intended to describe them as distinct and
subsequently changed his mind.
Berg's (1925) material was not found in a search of the collections
of the Zoological Institute, St. Petersburg (ZISP) in November 1993.
Eschmeyer et al. (1996) give the following data: Syntypes: (46)
ZIL (ZIL being the old acronym for ZISP) but this material is
presumably comparative specimens mentioned by Berg (1925).
Coad and Holčík (1999) demonstrated variation between three populations isolated by
the salt Lake Orumiyeh but considered this variation as insufficiently
different to warrant taxonomic distinction. Nonetheless, the analysis
demonstrated that the three populations have diverged in a measurable
manner, presumably through geographical isolation, although ecological
factors may have played a part as one sample was from a lacustrine
rather than a riverine environment.
Key characters
This species is distinguished from its relatives in the former genus
Chalcalburnus (having a short, naked ventral keel) by a combination of characters:-
|
Species |
Total gill rakers |
Branched anal fin rays |
Pored scales in lateral line |
Peritoneum colour |
|
atropatenae |
11-16 |
9-12 |
46-63 |
black |
|
chalcoides |
18-31 |
12-19 |
54-74 |
light brown |
|
mossulensis |
11-18 |
10-14 |
58-89 |
brown to black |
|
tarichi (Lake Van,
Turkey) |
26-29 |
9-11 |
65-82 |
light brown |
Morphology
Dorsal fin rays branched 7-9, modally 8, after 3 unbranched
rays, anal fin branched rays 9-12 after 3 unbranched rays, pectoral fin branched rays 13-16 and pelvic fin branched rays 7-8. Lateral line
scales 46-63. There is a pelvic axillary scale. The scale focus is slightly anterior or central and there are
relatively few anterior and posterior radii about equal in number. The
exposed fleshy keel in front of the anus is about 1-4 scales lengths,
usually 2, long. Gill rakers lanceolate but short, less than half eye
width, reaching between the first and second adjacent rakers or
touching the second when appressed, total numbering 11-16. Pharyngeal teeth are hooked at the tip
and usually bear a few, large serrations on the larger major row teeth
or more rarely have no serrations, apparently size independent. The posteriormost major row tooth may be dorsal rather than posterior to
the tooth ahead of it. Tooth counts are usually 2,5-4,2. The gut is an
elongate s-shape, sometimes with an anterior loop to the left. Total vertebrae
41-43.
Meristic values for Iranian material: dorsal fin branched rays 7(2), 8(102)
or 9(1); anal fin branched rays 9(5), 10(49), 11(45) or 12(6); pectoral fin
branched rays 13(7), 14(44), 15(41) or 16(13); pelvic fin branched rays 7(17) or
8(88); lateral line scales 46(4), 47(5), 48(12), 49(15), 50(13), 51(15), 52(14),
53(5), 54(5), 55(5), 56(2), 58(6) or 63(1); total gill rakers 11(12), 12(30),
13(35), 14(16), 15(7) or 16(2); pharyngeal tooth counts 2,5-4,2(54), 2,4-4,2(2),
2,4-5,2(1), 2,5-5,2(1), 1,5-4,2(1) or 2,5-3,2(1); and total vertebrae 41(4),
42(12) or 43(3).
Sexual dimorphism
Male specimens have small scattered tubercles on the top of the
head with fewer tubercles on the side of the head. Tubercles are
variably distributed on the head depending on the specimen, or even be
different on each side of a single fish. A distinct row may parallel
the upper lip, another row may follow the upper eye margin, a patch
may be present between the nostril and the upper lip, and there may be
tubercles between the mouth and the eye. Very small tubercles line the
scale margins on the back, flank and belly and belly scales have a
fine row of tubercles on the scale base. Tubercles line the rays of
the pectoral, dorsal, pelvic and anal fins and weakly on the caudal
fin, the rows branching with the fin rays.
Colour
The back is a dark olive brown to grey, with a narrow stripe. The
flank has a dark stripe, as wide as the pupil of the eye, extending
onto the head as far as the eye and back to the middle of the caudal
fin. The stripe is black to dark green. The flank above the stripe is
often lighter in contrast to the darker back and accentuates the
distinctiveness of the stripe. The flank below this stripe, the belly
and the lower head are silvery, and the stripe is clearly set off from
the lower flank. The front of the lower jaw is dark and some of this
pigment extends into the floor of the mouth. The iris is silvery on
the lower half and dark above. The dorsal fin is faintly pigmented
grey along its rays, the caudal fin is grey and the other fins are
colourless. Melanophores are present on the dorsal and caudal fin rays
and the anterior rays of the pectoral, pelvic and anal fin rays. The
nostrils may be dark. The peritoneum is black.
Size
Reaches 21.8 cm.
Distribution
This species is endemic to the Lake Orumiyeh basin and is recorded from the
Kazim-chai, Ozband River, Talkheh, Zarrineh and Tatavi rivers (Günther, 1899;
Berg, 1925; Abdoli, 2000).
Zoogeography
Lake Orumiyeh was formed during the late Pliocene-Pleistocene, lies
at 1275-1295 m, and may well have had a Pleistocene connection to the
Caspian Sea basin although this is in dispute (Scharlu, 1968;
Schweizer, 1975). Pleistocene shorelines from 30 to 115 m above the
present level have been confirmed, and the lake covered twice its
present area, but this would not permit an external discharge. Berg
(1940) reports benches at levels of about 1800 m, 1650-1550 m and
1500-1360 m, which may represent shorelines, and a level of about 1570
m would have had an outlet to the Aras River basin through the Kara-tepe
Pass in the northwest and across the plain near the city of Khvoy.
Saadati (1977) suggests two connections with the Caspian Sea, an early
one in the Pliocene to early Pleistocene resulting in endemic species
and a later one in the late Pleistocene resulting in species which are
the same as the Caspian or only subspecifically distinct. A.
atropatenae may have its origin in the earlier transgression.
Habitat
Unknown.
Age and growth
Unknown.
Food
Gut contents are insects, crustaceans and worms. Filamentous algae
are also present, possibly as accidental inclusions.
Reproduction
Fish captured 25-27 June carried mature eggs.
Parasites and predators
None reported from Iran.
Economic importance
Unknown.
Conservation
Biology is poorly known and numbers and habitat requirements would have to be
examined for a conservation assessment.
Further work
The biology of this species requires a detailed study.
Sources
Type material. See above, Alburnus atropatenae (BM(NH) 1899.9.30:127, 1899.9.30:128-30).
Iranian material: CMNFI 1970-0557, 26, 17.9-31.6 mm standard length, Azarbayjan-e Bakhtari, Shaher Chay (ca. 37º27'N, ca. 44º55'E);
CMNFI 1970-0558, 8, 25.0-88.7 mm standard length, Azarbayjan- e Bakhtari, Qasemlu Chay (ca. 37º21'N, ca. 45º09'E);
CMNFI 1970-0559, 48, 31.4-85.2 mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay (37º25'N, 45º10'E);
CMNFI 1979-0785, 11, 72.6-123.8 mm standard length, Azarbayjan-e Bakhtari, Shaher Chay (ca. 37º27'N, ca. 44º55'E);
CMNFI 1979-0786, 26, 65.0-92.2 mm standard length, Azarbayjan-e Khavari, Guru Lake (37º55'N, 46º24'E);
CMNFI 2007-0096, 1, 54.7 mm standard length, Azarbayjan-e Bakhtari, Qasemlu River in Baranduz Chay basin (ca. 37º25'N, ca. 45º10'E);
CMNFI 2007-0097, 2, 42.0-54.9 mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay basin (ca. 37º16'N, ca. 45º08'E);
CMNFI 2007-0103, 6, 43.3-73.3 mm standard length, Kordestan, Zarrineh River basin (ca. 36º18'N, ca. 46º16'E);
CMNFI 2007-0105, 6, 67.3-112.1 mm standard length, Kordestan, Zarrineh River basin (ca. 36º06'N, ca. 46º20'E);
OSU 8122, 2, 73.1-83.5 mm standard length, Azarbayjan-e Bakhtari, Shaher Chay (ca. 37º27'N, ca. 44º55'E);
USNM 205904, 2, 73.0-82.6 mm standard length, Azarbayjan-e Bakhtari, Nazlu Chay (37º40'N, 45º05'E);
uncatalogued, 1, 81.6 mm standard length, Azarbayjan-e Bakhtari, Haladj River near Mahabad (ca. 36º45'N, ca. 45º43'E) (Coad and Holčík, 1999).
?check against Iraq book
Alburnus caeruleus
Heckel, 1843
Common names
None.
Systematics
The
type locality is Aleppo (= Halab), Syria and material is held inthe Naturhistorisches
Museum Wien. Syntypes are listed in Eschmeyer et al.
(1996) as NMW 16688 (4, 65.7-86.6 mm standard length as measured by me), NMW 55511-13 (2,
64.5-75.4 mm standard length, 2, 61.2-71.1 mm standard length, 2, 74.1-77.4 mm
standard length), 57161 (3, 59.6-71.1 mm standard length) and additionally
?RMNH 2656 [ex NMW] (4); SMF 100 [ex NMW] (4, 61.3-75.7 mm standard length. See also below.
Key characters
Distinguished from its relatives by ? fewer scales along the lateral line (45-58
compared to 60-89) and a deeper body (2.9-3.5 in standard length compared to
4.0-5.1).
Morphology
Dorsal fin with 3 unbranched and 8-9 branched rays, usually 8, anal fin with
3 unbranched and 13-18 branched rays, mostly 14-16, pectoral fin rays 12-15 and pelvic fin
rays 7-8. Lateral line moderately to strongly decurved, scales 43-58. Scales
lack radii on the anterior field. The naked ventral keel is obvious. Pharyngeal
teeth hooked at tip and deeply notched or serrated below. Modally 2,5-4,2, with
variants 2,5-5,2, and 2,5-4,1.Total gill rakers 10-13, just reaching past
adjacent raker when appressed. Total vertebrae 39 (Bogutskaya et al.,
2000). The body is relatively deep with a slight nuchal hump, 2.9-3.5 times in
standard length. The gut is s-shaped.
Sexual dimorphism
Males have tubercles on the lower jaw, the sides and dorsal surface of the
head and on flank scales. Tubercles are evident on the pectoral fin and appear
as traces on the pelvic fins.
Colour
Back blackish, flanks silvery. Horizontal stripe along flank sky-blue, more
diffuse in larger fish but very evident in smaller ones.
Flanks, even lower flanks, and head heavily speckled. The lateral line may bear
pigment spots above and below each pore but the stitched effect is not as marked
as in some Alburnoides species. Fins generally yellowish,
dorsal, anal and pelvic fins apically black to sky blue. The membranes of the
dorsal and anal fins are heavily pigmented while the rays are clearer. This
pigmentation is more evident anteriorly on small fish but in both large and
small fish fins appear dark, especially when the fins are collapsed. On the anal
fin, some fish have dark pigment on all membranes, others, even large fish, have
less pigmentation distally on the posterior membranes. In larger fish, the
pectoral and pelvic fins have dark membranes, the pigmentation fading on the
smaller rays. The pectoral and pelvic fins can be orange. In
some specimens the edge of the caudal fin is quite dark. The peritoneum is brown
to black.
Size
Attains 86.9 mm standard length.
Distribution
Found in the Tigris-Euphrates and Quwayq River systems. The Orontes (= Asi) River is
not a locality (Krupp, 1985c). In Iran, it is recorded by Keyvan Abbasi (Iranian
Fisheries Research Organization Newsletter, 57:2, 2009) from the Gamasiab and Doab
rivers (34º22'16"N, 47º54'51"E at 1412 m altitude and 34º27'11"N, 47º39'34"E at
1322 m) and, given the fishing effort, were quite rare (0.02% of fishing sites,
8 individuals). It may be more widely distributed than museum and
literature records suggest.
Zoogeography
The relationships of this species zoogeographically have not been studied.
Habitat
Khalifa (1989) reported this species as widely distributed in rivers and
ponds, and it is also found in streams, dams and reservoirs in Iraq. Epler et al. (2001) found it to be the third most dominant species
of fish in the Iraqi lakes Habbaniyah, Tharthar and Razzazah, comprising 8.7% of all fish
collected.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Large eggs were visible in fish from Syria caught on 19 May, suggesting
spring spawning.
Parasites and predators
Unknown in Iran.
Economic importance
None.
Conservation
This species is poorly known and documented in Iran so its conservation
status is unknown.
Further work
The biology, distribution and conservation status of this species needs
investigation in Iran.
Sources
Type material:- Syntypes NMW 16688, NMW
55511, NMW 55512, NMW 55513, NMW 57161, SMF 100.
?see Excel file where some fish re-identified
Comparative material:- BM(NH) 1931.12.21:21, 86.9 mm standard length, Mosul,
Mesopotamia but this has 17 rakers. ; BM(NH) 1974.2.22:83, 1, 67.9 mm standard
length, Iraq, Sirwan, Tigris River near Faish Khabour (no other locality data); ZMB 3364 (possibly syntypes as marked from Vienna
Museum), 4, 55.6-65.8 mm standard length, Syria, Aleppo (= Halab); SMF 28638,
14, 69.1-100.7 mm standard length, Syria, Euphrates River, Deir ez zor (35º31'N,
39º54'E); SMF 28678, 3, 59.0-98.8 mm standard length, Syria, Euphrates River
upstream Deir ez zor (35º31'N, 39º57'E); SMF 28698, 4, 84.4-105.6 mm standard
length, Syria, Euphrates River, downstream Baath Lake (35º55.723'N,
39º00.572'E); SMF 28712, 3, 51.8-59.3 mm standard length, Syria, Euphrates
River Raqqa to Halebye-Zalebye (35º36.083'N, 39º00.572'E to 53º50.029'N,
39º20.797'E); BM(NH)
1968.12.13:124-135, 40.1-50.7 mm standard length, Syria, Tigris River at Ain Diwar (?); BM(NH)
1968.12.13:147-154, 4, 38.5-71.1 mm standard length, Syria, Quwayq River at
Behourte (?).
Alburnus chalcoides
(Güldenstaedt, 1772)
Common names
شاه كولي (shah kuli or shah
kooli in Gilaki; kuli is widely used for any small fish and may derive from kul which can mean any pond or sheet of water) or
شاه ماهي (= shah mahi menaing royal fish or king fish in the sense of the best or most important fish); mahi shah kuli;
كاس كولي (= kas-e kuli, meaning
cup or bowl fish?); aslak in Mazandaran, siah kole (= presumably siah kuli, black fish), safid kuli (= white fish).
[samayi, schamay or schumai, Lankaran samayisi for A. chalcoides longissimus, Kur samayisi for
A. chalcoides, all in Azerbaijan; Iranskaya shemaya or Iranian shemaya, Lenkoranskaya shemaya or Lenkoran shemaya,
shemaya or shamaya in Russian; Caspian shemaya; bleak, Danube bleak].
Systematics
Cyprinus chalcoides was originally described from the Terek,
Sulak and Cyrus (= Kura) rivers, Russia.
Cyprinus clupeoides Pallas, 1776 from the Caspian Sea, Terek
and Kura rivers (also spelt clupoides in error), possibly Leuciscus
albuloides Valenciennes, 1844 from
"rivières de Perse", Alburnus longissimus
Warpakhovskii, 1892 from the Geoktapinka River, Lenkoran District,
Azerbaijan and Alburnus latissimus Kamenskii, 1901 from the
mouth of the Kura River, Azerbaijan are synonyms. Since Alburnus
latissimus occurs with Alburnus chalcoides in the Kura
River, its status is necessarily equivocal.
Chalcalburnus chalcoides iranicus Svetovidov, 1945 was
described as the subspecies of the Iranian shore of the Caspian Sea
basin and Alburnus chalcoides longissimus Warpakhovskii,
1892 as the subspecies of the Lenkoran in Azerbaijan neighbouring
Iran. Coad (1996b) examined the types of iranicus and longissimus
and found them not to be distinguishable. The latter name has priority
but both these nominal subspecies, and latissimus, are most
probably not distinct from the type subspecies. They were founded on
small samples from relatively homogenous spawning populations.
Variation may be clinal or related to local temperature and other
environmental variables. A very large series of specimens would be
necessary to define this.
The Caspian Sea species may be Alburnus chalcoides
chalcoides with a distinct subspecies, Alburnus chalcoides
mento (Heckel, 1836), in the Black Sea basin although up to 13
subspecies are named from Anatolia and the basins of the Black,
Caspian and Aral seas.
The type material of Chalcalburnus chalcoides iranicus is in the
Zoological Institute, St. Petersburg (ZISP 31231, holotype (see
below), and 3 paratypes 142.0-199.9 mm standard length), the type
locality being "a small stream near the hospital near Shahi,
Talar River basin" on labels in the Zoological Institute, St.
Petersburg and "a small river in the vicinity of town Shakhi
(basin of the river Talar, running into the Caspian Sea west of the
Gorgan Bay" (Svetovidov, 1945b). Shahi or Qa'emshahr is at 36°28'N,
52°53'E. Svetovidov (1945b) lists the holotype as a female of total length
263.5 mm and body length 226 mm but the holotype in ZISP is 216.7 mm
standard length (Coad, 1996b).
The type material of Alburnus longissimus is in the
Zoological Institute, St. Petersburg (ZISP 8653, 2 syntypes,
164.8-185.9 mm standard length, from "Fl. Geoktapinka" (Lenkoran).
The locality is probably near Prishib at 39°08'N,
48°36'E (Coad, 1996b). ZISP 8654 (6 fish, 121.2-164.4 mm standard length) from
the type locality are listed as types in Berg (1911-1914) but not in
the ZISP catalogue. Also an A. longissimus syntype from St.
Petersburg is in the Natural History Museum, London from "R.
Geotapinka" (BM(NH) 1891.10.7:28).
Bagherian and Rahmani (2007; 2009) examined two populations, from the Haraz River
and the Shirud, morphometrically. The males and the females between the two
populations were different, but this was attributed to environmental factors.
Truss analysis separated the two populations. Rahmani et al. (2007) were able almost to separate the two populations using meristic characters.
Rahmani et al. (2006) were able to separate populations from the Gazafrud
and Haraz rivers using morphometric characters but not meristic ones. Rahmani
et al. (2009) used the 18S rRNA gene and found populations from the Haraz,
Shirud and Gazafrud rivers were homogenous.
A hybrid of Alburnus chalcoides and Vimba vimba persa was reported from the Safid River (Petrov, 1926) and a hybrid between
Leuciscus (= Squalius) cephalus and Alburnus chalcoides is reported from
Turkey (Ünver and Erk'akan, 2005; Ünver et al., 2008).
Key characters
The short, naked ventral keel, usually 8 branched dorsal fin rays,
distribution, and the characters in the table under A. atropatenae can be
used to identify this species.
Morphology
Lateral line scales 54-74. The dorsal and ventral scale margins are
parallel or rounded and the anterior margin is wavy or has a
pronounced central protuberance. The posterior scale margin can be
rounded and more or less smooth or rounded and finely crenulate.
Crenulation may be related to size or sexual maturity but is not
always evident even in spawning males. Circuli are numerous and fine,
radii are few and present on the anterior and posterior fields (a few
fish had some scales with no anterior radii), and the focus is
slightly subcentral anterior. There is a well-developed pelvic
axillary scale. The ventral keel is only naked near the vent and
rarely may be scaled along its entire length although Kottelat and Freyhof
(2007) have an exposed keel of 8-12 scale lengths, up to 80% of the anus to
pelvic fin base distance. Dorsal fin with 2-3,
usually 3, unbranched and 7-9, usually 8, branched rays, anal fin with
3 unbranched and 12-19 branched rays, pectoral fin branched rays
13-16, and pelvic fin branched rays 7-9. Ginzburg (1936b) gives counts
of 13(7), 14(34), 15(52), and 16(7) for anal fin rays from Iranian
material, modally different from my counts below (possibly the last
two rays were counted separately but variation between samples is also
possible). Gill rakers 18-31, serrated medially and elongate, reaching
the second or third adjacent raker when appressed. Total vertebrae
43-45. Pharyngeal teeth 2,5-5,2, more rarely 2,5-5,1, 2,5-5,3,
2,5-5,4, or 3,5-5,3. Teeth are elongate, slender, curved inward,
strongly hooked at the tip and strongly serrated with serrations on
the anterior margin of the long, narrow and concave grinding surface.
The most posterior main row tooth may lie medial to the second tooth.
The swimbladder is pointed posteriorly (rounded in Alburnus
hohenackeri and A. filippii). The gut is an elongate s-shape.
Total vertebrae 41-45.
Meristics in Iranian specimens: dorsal fin branched rays 7(3),
8(55) or 9(2); anal fin branched rays 12(1), 13(4), 14(33), 15(19) or
16(3); pectoral fin branched rays 13(4), 14(9), 15(34) or 16(13);
pelvic fin branched rays 7(2), 8(57) or 9(1); lateral line scales
54(1), 55(2), 56(2), 57(5), 58(8), 59(5), 60(14), 61(7), 62(5), 63(6),
64(2), 65(1), 66(1) or 67(1); total gill rakers 18(1), 19(5), 20(12),
21(15), 22(14), 23(9), 24(3) or 25(1); pharyngeal teeth 2,5-5,2(30),
2,5-4,2(1), 2,4-5,2(1) or 2,5-5,3(1); and total vertebrae 42(2), 43(9), 44(32)
or 45(7).
Sexual dimorphism
Abdurakhmanov (1962) reports the eye diameter and anal fin base to
be larger in males on average for fish from the Kura River basin in
Azerbaijan. Iranian males taken in July have small tubercles scattered
on top of the head and fine tubercles lining the anterior flank scales. Females
are larger than males (Bagherian and Rahmani, 2007)
Colour
The overall colour is metallic silvery and the back is a
contrasting olive-green. The iris is bright silver. There is no dark
band along the sides. The dorsal and caudal fins are greyish and the
other fins colourless to whitish. The peritoneum is light brown but
with numerous melanophores in contrast to the dark peritoneum in A. mossulensis.
Size
Reaches 45.0 cm and 1.5 kg. Shemaya on the Kura River of Azerbaijan
are larger than those in the south Caspian, up to 36 cm as opposed to 29 cm.
Distribution
Found from central Europe to the basins of the Black, western and
southern Caspian and Aral seas. It is recorded from the entire southern coast of the Caspian
Sea and its rivers, including the Atrak, Gorgan, Gharasu, Tajan, Babol, Haraz,
Sardab, Aras, Tonekabon, Pol-e Rud and Safid rivers, the Anzali Talab, Gorgan
Bay, southeast, southwest and south-central Caspian Sea (Derzhavin, 1934; Kozhin, 1957;
Svetovidov, 1945b; Holčík and Oláh, 1992; Shamsi et al.,
1997; Abbasi et al., 1999); Kiabi et al., 1999; Abdoli, 2000;
Bagherian and Rahmani, 2007; 2009; Patimar et al., 2010; Abdoli and
Naderi, 2009).
Alburnus chalcoides aralensis Berg, 1926 is reported
from the Karakum Canal in Turkmenistan (Shakirova and Sukhanova, 1994;
Sal'nikov, 1995) and may eventually be found in the Tedzhen River and
Caspian Sea basins of Iran.
Zoogeography
A widespread species with numerous nominal
subspecies which have not all been fully investigated. It presumably originated
as part of a Danubian or Sarmatian fauna and the subspecies have become isolated
in parts of this former basin.
Habitat
Young are rheophilous (Abdurakhmanov, 1975). A migration to piedmont and
montane zones used to occur before dams and weirs obstructed movements. Some
populations are landlocked while others are semi-anadromous. Knipovich (1921)
reports this species from depths of 23.8-25.6 m in the Iranian Caspian
Sea. Kottelat and Freyhof (2007) record a tolerance of 14‰ salinity. Riazi (1996) reports that this species is native (resident) to
the Siah-Keshim Protected Region of the Anzali Mordab. Shape differences found
by Bagherian and Rahmani (2007) in two Iranian rivers were attributed to
the Haraz River having a muddy estuary, a shallow slope to the bottom, high
turbidity and low water flow in contrast to the Shirud which was sandy with high
water flow and high clarity. The latter population developed a more slender body
due to increased resistance to water flow.
Age and growth
Life span is 5 years with a theoretical limit of 6.5 years in
Azerbaijan (Abdurakhmanov, 1975) and at least 5 years in Iran (Holčík and Oláh, 1992)
and Turkey (Tarkan et al., 2005).
Sexual maturity is attained at 3 years of age in
Azerbaijan and growth is most rapid at an age of 2 years, decreasing
thereafter because of high natural mortality (Abdurakhmanov, 1975).
The fishes on the spring spawning run in the Anzali Mordab are
10.5-29.0 cm standard length, average 14.0 cm, and 2-5 years old with
most (63%) fish in age group 3. Males are mature at 2-4 years and
females at 3-5 years. Growth is high during the first 3 years of life
and then declines (Holčík and Oláh, 1992). Karimpour et al. (1993) found the Anzali
Mordab population to be smaller than the Kura River population but the
mordab fish showed greater growth after maturation. The spawning
migration into the mordab begins in March and peaks in May and at the
beginning of June. Length range was 10.0-24.0 cm, average 16.2 cm with
a mean weight of 64.7 g. Age composition was 2-5 years with
3-year-olds comprising 62.5% of the fish. Females formed 57% of the migrating fish.
Rahmani (2008) investigated this species in the Haraz and Shirud rivers and
found maximum length and weight in a 5- (sic) year old female at 251 mm
and 96 g, the most abundant age groups were 2+ and 3+ years for males and
females respectively, males in the Shirud population were heavier and longer on
average in younger ages while differences in females were not significant, and
females of the Shirud population has isometric growth while Haraz fish had
positive allometry. The von Bertalanffy growth parameters were Lt =
[405.9 (1-e-0.1(t+1.54))] for males in Haraz and Lt =
[442.6 (1-e-0.1(t+1.43))] for females in Haraz, and Lt
= [359.5 (1-e-0.145(t+1.002))] for males in Shirud
and Lt =[405.9 (1-e-0.1(t+1.54))] for females in Shirud.
Females had a higher L∞ while K values
for males were relatively higher in the two rivers.
Rahmani et al. (2009) found growth was better in the Shirud compared with
other populations because this river had desirable biological parameters for
immigration.
Patimar et al. (2010) compared fish from the Siah and Gorgan rivers and
found a five-year life cycle, with negative allometric growth for Siah males and
positive allometric growth for Siah females and for both sexes in the Gorgan,
and sex ratios were unbalanced in favour of females in both rivers. The von Bertalanffy growth parameters were Lt =
[370.08 (1-e-0.15(t+0.70))] for males in Siah and Lt =
[432.52 (1-e-0.11(t+1.21))] for females in Siah, and Lt
= [371.79 (1-e-0.14(t+0.96))] for males in Gorgan
and Lt = [436.10 (1-e-0.11(t+1.34))] for females in Gorgan.
Food
Holčík and Oláh (1992) report a feeding migration in July to September in
the western basin of the Anzali Mordab. Gut contents include diatoms
and algae, dragonfly larvae, and copepods (Abdurakhmanov, 1962).
Iranian fish had plant fragments, sand grains, crustaceans, insect
remains and chironomid larvae in gut contents.
Reproduction
This species is an intermittent spawner with three batches of eggs,
only two of which are laid at an interval of 18-19 days. Fecundity
reaches 54,700 eggs in Azerbaijan but this is less than that of
diadromous populations. Egg diameter is up to 1.9 mm. Spawning takes
place in the second half of July to the end of August at water
temperatures of 18-25°C in the Mingechaur Reservoir in Azerbaijan. Eggs are laid on rocky
bottoms in 15-20 cm of water after a migration into streams or on
rocky grounds of reservoirs (Abdurakhmanov, 1962; 1975; Elanidze,
1983). There is a spawning migration into the Kura River from October
to April, peaking in December-January, with spawning taking place in
spring in the upper reaches (Berg, 1959). In Lake Tuş, Turkey spawning occurred in May-June,
egg numbers reached 20,971 and average egg diameter 1.05 mm (Balık et al., 1996).
Svetovidov (1945b) considers that Iranian populations (his iranicus
subspecies) spawn nearly throughout the year since fish having ripe
sex products were caught in both July and February and young were
found along the Iranian coast throughout the year. Spawning takes
place in the sea, in areas such as Gorgan Bay, and in the lower
reaches of rivers. Khaval (1998) reports a spawning migration into the
Safid River despite construction, sand removal and pollution. Holčík and Oláh (1992)
report a migration into the Anzali Mordab for
spawning in late February to early April (but see above; possibly a
confusion between the migration at an earlier date than the spawning
act). Karimpour et al. (1993) give an absolute fecundity of
6630 eggs in the Anzali Mordab population while mean relative
fecundity is 140 eggs/g of body weight. Iranian fish have 1.5 mm eggs
as early as 13 March (fish standard length 213.2 mm) and 1.7 mm eggs
on 4 June (fish length 154.6 mm) while eggs are only 1.3 mm on 15 July
(fish length 142.8 mm). Larger fish may mature and spawn earlier than younger fish.
Rahmani et al. (2009) found a peak gonadosomatic index for males in May
and for females in early June in the Shirud. Average fecundity was about 3900
eggs with diameter reaching 1.17 mm. Patimar et al. (2010) in their study of Siah and Gorgan River fish found
spawning between April and July in the Siah and March and June in the Gorgan,
peaking in May in both rivers. Absolute fecundity was up to 38,340 eggs, mean
8426 eggs in the Siah and up to 17,263 eggs, mean 4215 eggs in the Gorgan.
Relative fecundity was up to 599 eggs/g, average 212 eggs/g of body weight in
the Siah and up to 696 eggs/g, average 112 eggs/g in the Gorgan. Mean egg
diameters were 1.40 mm in the Siah and 1.27 mm in the Gorgan. These differences
in life history (see also Age and growth above) were attributed to differing
habitat characteristics.
Parasites and predators
Molnár and Jalali (1992) report the monogeneans Dactylogyrus
minor, D. alatus and D. vistulae from this species
in the Ghasemlu River, an inland watershed, with the latter species
also in the Safid Rud. They also describe a new species of monogenean,
Dactylogyrus holciki, from this species in the Beshar River of
the Persian Gulf drainage, possibly confusing this Caspian Sea basin
cyprinid with A. mossulensis. Molnár and Jalali (1992) also
record the monogenean Dactylogyrus chalcalburni from the Safid
and Zayandeh rivers, although this Caspian Sea basin cyprinid does not
occur in the latter locality, possibly again confusing the same
species as noted above. Shamsi et al. (1997) report Clinostomum
complanatum, a parasite causing laryngo-pharyngitis in humans,
from this species. Masoumian and Pazooki (1998) surveyed myxosporeans in this
species in Gilan and Mazandaran provinces, finding Myxobolus pseudodispar.
The helminths Pentagramma symmetrica and Mazocea
alaosa are recorded from the guts of Chalcaburnus tarichi (sic,
presumably A. chalcoides) from the Anzali wetland (Ataee and
Eslami, 1999; www.mondialvet99.com, downloaded 31 May 2000).
Naem et al. (2002) found the following parasites on the gills of this
species from the western branch of the Safid River, namely the monogenean
trematodes Dactylogyrus chalcalburni and Gyrodactylus sp..
Sattari et al. (2004, 2005) surveyed this species in the Anzali wetlands, recording
Anisakis sp. Maleki and Malek (2007) examined fish from the Shirud in the
Caspian Sea basin and recorded the digeneans Posthodiplostomum cuticola,
Diplostomum spathaceum, Clinostomum complanatum and
Allocreadium sp.Sattari et al. (2007) record the nematode Anisakis sp., the
digenean Diplostomum spathaceum and the monogenean Dactylogyrus
extensus in this species in the Anzali wetland of the Caspian shore. Miar
et al. (2008) examined fish in Valasht Lake and the Chalus River, Mazandaran
and found the metazoan Argulus foliaceus.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Argulus foliaceus
on this species.
Economic importance
The shemaya was a valuable edible fish on the Kura River of
Azerbaijan with catches as high as 500 centners (1 centner = 100 kg)
prior to construction of the Kura dam. The catch for Azerbaijan in
1933 was 1950 centners or 2,029,000 fish. Catches in the Mingechaur
Reservoir formed by the dam were 133 centners in 1972 (Abdurakhmanov,
1975). Reputedly delicious eating (Lönnberg, 1900b). They are fished
for on the spawning run when fatty. In Iran they are caught by cast
nets in the inlets and outlets of the Anzali Mordab in spring on the
spawning run and by gill nets in the western basin on the feeding
migration. Holčík and Oláh (1992) report a catch of 956 kg in the Anzali Mordab in 1990
but catches in recent years may have been confused with the exotic Hemiculter
leucisculus (Holčík and Olah, 1990).
Conservation
Holčík and Oláh (1992) report a decline in the numbers of this species owing
to damming of rivers where it used to spawn. Kiabi et al.
(1999) consider this species to be near threatened in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, sport fishing, abundant in numbers, habitat
destruction, widespread range (75% of water bodies), absent in other
water bodies in Iran, and present outside the Caspian Sea basin. Mostafavi
(2007) lists it as near threatened in the Talar River, Mazandaran. Endangered in
Turkey (Fricke et al., 2007).
This species has been artificially bred without hormones on the Shirrud with a
fertilisation rate of 90-98%. Hatching took 6 days and the hatching rate was 57%
(I.F.R.O. Newsletter, 36:4, 2003). On the Tajan River, induction of ovulation
has been carried out using LRH-Aa with metoclopramide and carp pituitary extract
(Yousefian et al., 2008). Fertilisation rate was 83%, hatching rate 90%
and survival of larvae 81%. Shirvani and Jamili (2009) found excessive levels of
cadmium and lead in this fish from regions of Anzali where oil ship traffic was
highest. Daei et al. (2009) reported on the effects of cadmium and lead
on the iron solute in blood.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food.
Lelek (1987) classifies this species as vulnerable to endangered in Europe.
Further work
The various subspecies should be examined using molecular techniques and
numbers of this species in Iranian rivers monitored for conservation management.
Sources
The types of C. chalcoides iranicus are included in the meristic data for Iranian specimens.
Type material: See above, Chalcalburnus chalcoides iranicus (ZISP 31231), Alburnus longissimus (ZISP 8653, ?8645, BM(NH) 1891.10.7:28).
Iranian material: CMNFI 1970-0531, 4, 64.5-74.9 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1970-0553, 2, 101.9-163.1 mm standard length, Gilan, Sowsar Roga River (37º27'N, 49º30'E);
CMNFI 1971-0327A, 2, 54.5-116.9 mm standard length, Gilan, Shafa River (37º35'N, 49º09'E);
CMNFI 1979-0081, 7, 77.8-106.5 mm standard length, Mazandaran, Caspian Sea 3 km west of Chalus (36º41'N, 51º24'E);
CMNFI 1979-0434, 4, 47.3-154.6 mm standard length, Mazandaran, Shir River (36º51'N, 50º49'E);
CMNFI 1979-0435, 1, 170.5 mm standard length, Gilan, stream 10 km west of Ramsar (36º57'N, 50º37'E);
CMNFI 1979-0437, 2, 164.5-175.6 mm standard length, Gilan, Safid River 2 km west of Astaneh (37º16'30"N, 49º56'E);
CMNFI 1979-0438, 12, 114.9-158.9 mm standard length, Gilan, Gholab Ghir River (37º27'N, 49º37'E);
CMNFI 1979-0439, 2, 156.6-173.2 mm standard length, Gilan, Anzali Mordab (ca. 37º27'N, ca. 49º25'E);
CMNFI 1979-0441, 1, 109.8 mm standard length, Gilan, river 14 km south of Hashtpar (37º42'N, 48º58'E);
CMNFI 1979-0443, 1, 159.6 mm standard length, Gilan, river 34 km north of Hashtpar (38º06'N, 48º53'E);
CMNFI 1979-0445, 1, 114.9 mm standard length, Gilan, stream 10 km south of Astara (38º21'N, 48º51'E);
CMNFI 1979-0455, 1, 88.5 mm standard length, Zanjan, Qezel Owzan River at Gilavan (36º47'N, 49º08'E);
CMNFI 1979-0474, 1, 141.0 mm standard length, Mazandaran, Tajan River (36º34'N, 53º05'E);
CMNFI 1979-0686, 23, 25.5-111.0 mm standard length, Gilan, Safid River (37º24'N, 49º598'E);
CMNFI 1979-0788, 48, 35.2-74.7 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0120, 17, 56.4-69.5 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1980-0123, 2, 97.0-106.4 mm standard length, Gilan, Safid River around Dakha (no other locality data);
CMNFI 1980-0126, 3, 182.1-213.2 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0132, 7, 18.7-142.8 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1980-0142, 2, 135.0-187.2 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1980-0908, 3, 45.4-155.2 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E).
Alburnus filippii
Kessler, 1877
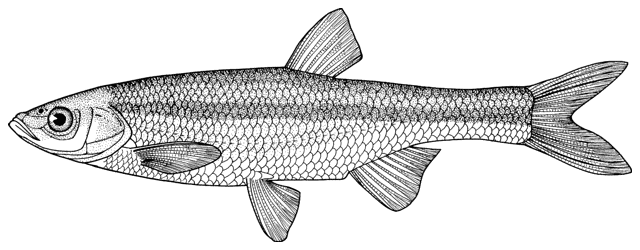
Common names
كولي كورا (= kuli-ye Kura),
ماهي مرواريد
or مرواريد ماهي
(= mahi morvarid or morvarid mahi, meaning pearl fish).
[Kur kumuscasi in Azerbaijan; Kurinskaya ukleika or Kura bleak,
ukleika filippi or Filippi's bleak, both in Russian].
Systematics
The lectotype of Alburnus Filippii as designated by N.
Bogutskaya is in the Zoological Institute, St. Petersburg (ZISP 2926)
and is from "Fl. Kura pr. Tiflis", Acad. Brandt, 1867, 75.3
mm standard length. Paralectotypes are ZISP 2925, 13 fish, same data
as lectotype, 43.0-84.4 mm standard length, ZISP 2914, 2 fish,
"Fl. Kura pr. Borshoma", Acad. Brandt, 1867, 83.6-87.6 mm
standard length, and ZISP 50412, 16 fish, "Reka Kura Tiflis",
Acad. Brandt, 1867, 60.6-88.6 mm standard length. A syntype, 57.3 mm
standard length, is in the Natural History Museum, London from Tiflis
(BM(NH) 1897.7.5:33, formerly in ZISP).
Alburnus filipii var. Kessler in Brandt, 1880 from the
Tchaldyr Lake is also this species.
Knipovich (1921) reports a Caspian basin species Alburnus
philippii Kessler which is presumably a misspelling of filippii.
The specific name is sometimes spelt filippi, which is incorrect.
Abdurakhmanov (1962) compares a sample from the Kura River basin
with one from the Kendalanchaya in the Aras River basin of Azerbaijan
and finds 15 characters are significantly different on average. Fish
from the Kura have a longer head, greater dorsal and anal fin heights,
and longer pectoral, pelvic and upper and lower caudal fin lobes while
fish from the Aras have more scales in the lateral line, a deeper
head, body and caudal peduncle, and a longer anal fin base,
pectoral-pelvic fin distance and snout, and a greater interorbital
width. No taxonomic status is assigned these two populations.
A hybrid with Alburnus charusini hohenackeri (= Alburnus
hohenackeri) was reported by Petrov (1926) from the Safid River and
the Kumbashinka in Lenkoran.
Key characters
This species is distinguished from its relative (Alburnus
chalcoides, also with a long, naked ventral keel) by having modally 7 branched
dorsal fin rays and generally lower anal fin ray counts although these do
overlap (10-21, usually 12-16 in Iran for alburnus; 9-13, usually 10-12,
for filippii). See also table under A. atropatenae.
Morphology
Dorsal fin with 3 unbranched and 6-8, usually 7, branched rays,
anal fin with 2-3, usually 3, unbranched and 9-13 branched rays,
usually 10-12. Pectoral fin branched rays 12-16, pelvic fin branched
rays 6-8, usually 7. Lateral line scales 46-64. Scales have a wavy
anterior margin, an overall vertical oval shape, sometimes tapering to
a rounded posterior point and sometimes more rounded, few anterior and
posterior radii, and a subcentral anterior focus. There is a pelvic
axillary scale. The naked ventral keel usually extends more than half
way from the anal papilla to the pelvic fin insertion but is often
completely scaled, notably in fish from the Safid River basin. The
scaled keel runs from the papilla to the pelvic fin base. Gill rakers
12-21, reaching the second or third adjacent raker when appressed.
Pharyngeal teeth 2,5-5,2 (but see below for Iranian specimens) with
variants 2,5-5,1, 1,5-5,2, 1,5-5,1, 2,5-4,2, 2,4-5,2, 2,5-4,1,
2,4-4,2, 1,5-4,1, 1,4-5,1, and 1,5-4,2. Teeth are stongly hooked and
strongly serrated. Serrations are on the anterior margin of each
tooth. The degree of hook and serration development varies
individually and does not seem to be size related. Some fish have
little development of either character. The area below the hook is an
elongate, flat to concave surface. Vertebrae number 38-43. The
swimbladder has a rounded end in contrast to the pointed end in Alburnus
chalcoides. The gut is an elongate s-shape with a small anterior loop.
The chromosome number is 2n=50 and Nazari et al. (2009) give further
details.
Meristic variation in Iranian specimens: dorsal fin branched rays
6(1), 7(44) or 8(5); anal fin branched rays 9(1), 10(19), 11(24),
12(5) or 13(1); pectoral fin branched rays 12(3), 13(19), 14(20),
15(7) or 16(1); pelvic fin branched rays 6(3), 7(42) or 8(5); lateral
line scales 46(1), 49(1), 50(5), 51(5), 52(4), 53(12), 54(5), 55(2),
56(5), 57(6), 58(1), 60(2) or 63(1); total gill rakers 12(4), 13(8),
14(19), 15(10), 16(6) or 17(3); pharyngeal teeth 2,5-4,2(10),
2,4-5,2(2), 2,4-4,2(2), 2,5-5,2(1), 1,5-4,2(2), 1,5-5,2(1), 1,5-4,1(1)
and 1,4-5,1(1); and total vertebrae 38(2), 39(8), 40(18), 41(9) or 42(1).
Sexual dimorphism
Males and females have moderate-sized tubercles widely scattered on
the top of the head, on the snout and lining the lower edge of the
jaw. Much smaller tubercles are scattered among the ones on top of the head.
Colour
The back is brown, flanks silvery and the belly white. A
characteristic dark streak, as wide as the eye, runs along mid-flank.
Fins are hyaline. The peritoneum is brown or light with large
scattered melanophores.
Size
Reaches 17.0 cm standard length.
Distribution
Found only in the Caspian Sea basin from the Kura River of Azerbaijan to the Safid
River of Iran including headwaters in Turkey, Armenia and Iran at altitudes
over 3000 m. It is distributed from the upper to the lower reaches of
the Aras (Qareh Su) and Safid (Qezel Owzan) rivers in Iran and in the Anzali
Talab (Holčík and Oláh,1992; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
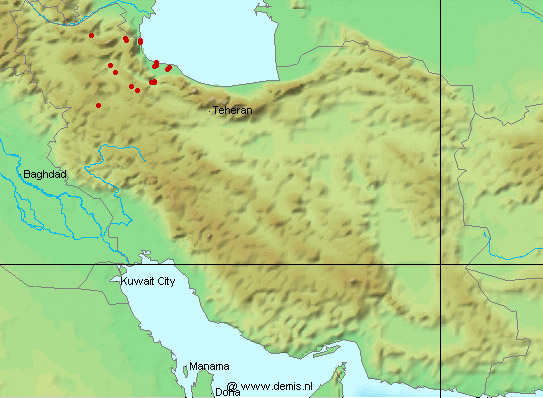
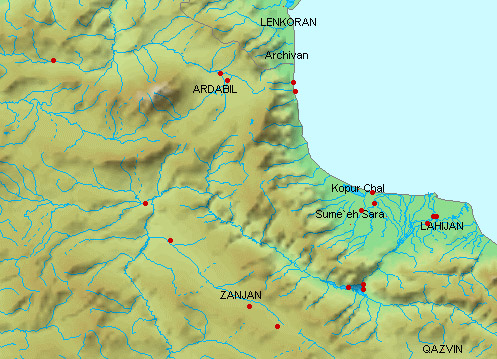
Zoogeography
The relationships of this species with other Alburnus needs to be
examined. It presumably originated as part of a Sarmatian fauna, isolated in the Caspian Sea.
Habitat
Primarily a freshwater species, this minnow may be found in the
brackish outlets of the Anzali Mordab (Holčík and Oláh, 1992). Jolodar and
Abdoli (2004) note that it is found more in upstream waters than A. alburnus.
Age and growth
Life span is about 5 years with maturity at 1 year for males and 2 years for females.
Food
Gut contents include plant remains, mayflies and algae (Abdurakhmanov,
1962). Iranian specimens contain insect remains, a few crustaceans and
sand grains. One sample from the Qareh Su north of Ardebil had been
feeding on water beetles (Hydrophilidae) but also spiders and scarab
beetles (Euoniticellus sp.) indicating food is also taken from the surface.
Reproduction
Eggs number up to 14,210 and diameters up to 1.51 mm. May is the
principal spawning month in Azerbaijan (Abdurakhmanov, 1962). Male
fish caught on 6 June in Iran had tubercles scars on top of the head
while female fish from another locality (Zanjan River) taken on 8 June
had mature eggs measuring 1.2-1.3 mm. Spawning probably occurs in May
and June in Iran, depending on local conditions.
Parasites and predators
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. elegans in the Beheshtabad River. Mortazavi
Tabrizi et al. (2005) record Ligula intestinalis and
Bothriocephalus acheilognathi in this species from the Sattarkhan Dam in
East Azerbaijan. Undoubtedly food for various predatory fishes. Pazooki et al.
(2005) record Trichodina perforata from this species in waterbodies
of Zanjan Province. Pazooki et al.
(2006) record the monogeneans Dactylogyrus vistulae and Gyrodactylus
sp. from this fish in Zanjan Province.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be of least
concern in the south Caspian Sea basin according to IUCN criteria.
Criteria include medium numbers, habitat destruction, medium range
(25-75% of water bodies), absent in other water bodies in Iran, and
absent outside the Caspian sea basin. Vulnerable in Turkey (Fricke et al.,
2007).
Further work
The biology of this species needs investigation.
Sources
Type material: See above, Alburnus Filippii (ZISP 2926, 2925, 2914, 50412, BM(NH) 1897.7.5:33).
Iranian material: CMNFI 1970-0538, 8, 34.9-61.8 mm standard length, Gilan, Qezel Owzan River (ca. 36º44'N, 49º24'E);
CMNFI 1970-0552, 1, 50.1 mm standard length, Gilan, Sowsar Roga River (37º27'N, 49º30'E);
CMNFI 1979-0448, 1, 70.9 mm standard length, Azarbayjan-e Khavari, Ahar Chay 8 km from Ardabil (38º18'30"N, 48º22'E);
CMNFI 1979-0452, 2, 52.4-54.9 mm standard length, Azarbayjan-e Khavari, Qezel Owxan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0453, 9, 43.7-73.3 mm standard length, Zanjan, Zanjan River (37º06'N, 47º56'E);
CMNFI 1979-0455, 17, 42.8-62.5 mm standard length, Markazi, Manjil Dam (36º45'N, 49º17'E);
CMNFI 1979-0695, 3, 61.3-63.5 mm standard length, Gilan, Safid River (36º46'N, 49º24'E);
CMNFI 2007-0081, 1, 51.0 mm standard length, Zanjan, Zanjan River near Soltaniyeh (ca. 36º27'N, ca. 48º45'E);
CMNFI 2007-0082, 11, 41.2-59.6 mm standard length, Zanjan, Zanjan River basin near Zanjan (ca. 36º36'N, ca. 48º32'E);
CMNFI 2007-0087, 6, 55.7-83.1 mm standard length, Azarbayjan-e Khavari, Qareh Su north of Ardebil (38º22'N, 48º19'E);
CMNFI 2007-0107, 3, 41.1-42.3 mm standard length, Kordestan, Qezel Owzan River basin near Bijar (ca. 35º54'N, ca. 47º20'E).
Alburnus
hohenackeri
Kessler, 1870
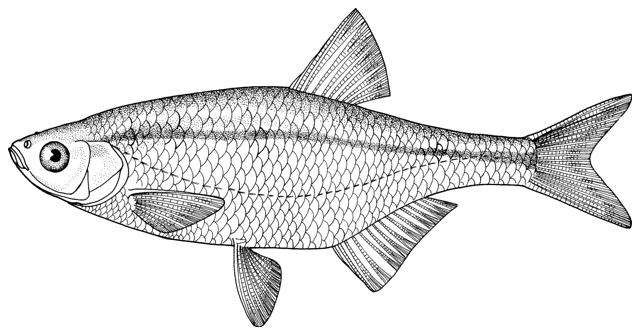

Common names
ماهي مرواريد
or مرواريد ماهي (= mahi morvarid or morvarid mahi,
meaning pearl fish), kuli (= general term for small fish),
كولي ايراني (= kuli-ye Irani).
[simali gafgaz kumuscasi for A. c. charusini or zagafgaziya
kumuscasi for A. c. hohenackeri, both in Azerbaijan; ukleika or
bleak, zakavkazskaya ukleika or Transcaucasian bleak, persidskaya
ukleika or Persian bleak, sefidrudskaya ukleika or Safid River bleak, all in Russian;
Caucasian bleak (as A. hohenackeri)].
Systematics
Alburnus Hohenackeri was originally decsribed from Karabakh, Azerbaijan, on the Kura River.
The taxon in Iran was formerly included within the wide-ranging species
Alburnus alburnus (Linnaesus, 1758). Cyprinus Alburnus was originally described from Europe.
Synonyms are Alburnus charusini
Herzenstein in Zograff and Kavraiskii, 1889 described from the
Kamysh-Samarskie lakes between the Volga and Ural rivers in
Kazakhstan, Alburnus alasanicus Kamenskii, 1901 from the Alasan,
Alazan' or Alazani River, a left bank Kura River tributary in Georgia,
Alburnus lucidus var. macropterus Kamenskii, 1901
described from the Alazan' River, Alburnus alburnus charusini
natio elata Petrov, 1926 from the Prorva River (lower reaches
of the Terek River), the Sulak River and the Divichi Liman, western
Caspian Sea, Alburnus striatus Petrov, 1926 from "Kizil-Agachskogo
Zaliva" (Kizil-Agach Bay, Turkmenistan) and "Astrabadskogo
Zaliva" (= Astrabad or Gorgan Bay, Iran), and Alburnus
alburnus natio dagestanicus Petrov, 1930 (sic) but
later in the same paper given, and probably originally meant, as A.
a. charusini n. dagestanicus) described from the "Kaukasusküste
des Kaspische Meeres".
Alburnus pseudospirlinus Petrov, 1926 from "Novaya
Rechkaya (nizov'ya Sefid-Rud)" (= new stream, lower Safid
River) is a hybrid of this species and Alburnoides bipunctatus
(sic)
(Berg, 1948-1949). A hybrid with Alburnus filippi was described
from the Kumbashinka River in the Lenkoran and from the Safid River (Petrov, 1926).
The holotype of Alburnus hohenackeri is in the Zoological
Institute, St. Petersburg (ZISP 2839). The holotype of Alburnus charusini is in the Zoological
Museum of Moscow State University under MMSU P.1314. Four fish as
listed as questionable syntypes under MMSU P.1812 by Svetovidova
(1978) although according to Eschmeyer et al. (1996) the
original says P.1314 with a unique holotype only.
This species was recognised as Alburnus charusini in Iran
but characters overlap with Alburnus alburnus, a highly
variable species (Gäsowska, 1974). In any case hohenackeri has priority over charusini.
Literature sources conflict on the correct name. Petrov (1926; 1930)
refers to Alburnus alburnus hohenackeri Kessler, 1877 for fish
in northern Iran with natio persicus Petrov, 1926 in the Safid
River, natio dagestanicus Petrov, 1930 in the Dagestan area of
Azerbaijan and natio kumbashensis Petrov,
1926 from the Kumbashinka River and Lake Ol'khovskoye in the Lenkoran
area of Azerbaijan. Natio are not recognised by the Zoological Code of
Nomenclature (Ride et al., 1985). Liška and Pivnička (1985)
refer southern and southeastern populations of this species to Alburnus
alburnus albidus Costa, 1838, and this would include the Iranian
populations. These fish are separated from the type subspecies by
having 39-47 lateral line scales, most frequently 42-44 (44-54, most
frequently 47-50 in A. a. alburnus), branched anal fin rays
10-17, most frequently 13-15 (14-21, most frequently 16-19), and head
length as % of body length 22-27 most frequently 23-25 (19-25, most
frequently 21-23). N. Bogutskaya (pers. comm., 1995) and Reshetnikov et
al. (1997) refer Iranian fish to Alburnus alburnus hohenackeri
as there is a definite character break at the Terek River separating
northern populations from southern ones. Petrov (1930) came to a
similar conclusion on the name of the Iranian populations in his study
as noted above. Aburakhmanov (1962) too refers the taxon hohenackeri
to fish found in the Kura and Aras rivers and in rivers of the
Lenkoran coast (and presumably the Iranian coast) while his charusini
are north of the Apsheron Peninsula. Bogutskaya and Naseka (2004) and Kottelat
and Freyhof (2007) recognise
A. hohenackeri as a distinct species.
Key characters
This species can be confused with Alburnoides eichwaldii
which has similar scale, fin ray and pharyngeal counts. A key
distinction is the total gill raker count of 16-29 (usually 20 or
more) in this species as opposed to 5-12, usually 7-10 in Alburnoides.
Alburnus rakers are more than twice as long as those in Alburnoides
and, being more numerous, are crowded on the arch without the large
gaps between individual rakers which characterises Alburnoides.
Modal dorsal fin branched ray count of 8 separates it from A. filippii.
?separation from other Alburnus in Caspian
Morphology
Dorsal fin branched rays 7-9, usually 8, after 2-4 unbranched rays,
anal fin branched rays 10-21 after 3-4 unbranched rays (note that anal fin count
will be a narrower range if A. hohenackeri is recognised as distinct from
a widespread A. alburnus (see Iranian counts below). Pectoral fin branched rays 11-16
and pelvic fin branched rays 6-9. Lateral line
scales 36-55. Scales bear both anterior and posterior radii with a few
curved radii in the lateral fields. The focus is subcentral anterior
and circuli are numerous and fine. The naked ventral keel is often
wholly or partially covered by scales. Gill rakers 15-29, elongate
reaching the third, or rarely second, below when appressed. Vertebrae
36-46. Pharyngeal teeth 2,5-5,2 with variants 2,5-5,1, 2,5-5,3,
1,5-5,2, 1,5-5,1, 2,5-4,2, 2,4-5,2, 2,4-5,1, 2,4-4,2, 1,5-4,2,
2,5-4,1, 1,5-4,1, 1,4-4,1. The elongate and narrow teeth bear a
strongly hooked tip and have evident serrations in most specimens
although some lack them entirely. The gut is an elongate s-shape with
a small anterior loop. The posterior end of the swimbladder is rounded
(pointed in Alburnus chalcoides).
The chromosome number is 2n=50-52, generally 50 (Klinkhardt et al., 1995).
The natio persicus from the Safid River has dorsal fin
branched rays 7-9, anal fin branched rays 12-16 and lateral line
scales 40-45. Fish from the Kura-Aras basin and Lenkoran (hohenackeri)
have anal fin branched rays 10-15, lateral line scales 38-48,
pharyngeal teeth 2,5-5,2, total gill rakers 16-25 and total vertebrae
37-42 (courtesy of N. Bogutskaya, Zoological Institute, St. Petersburg).
Meristics for Iranian fish including Petrov's (1930) counts of
dorsal and anal branched rays and lateral line scales for Safid River
fish are:- branched dorsal fin rays 7(7), 8(76) or 9(8); branched anal
fin rays 12(6), 13(37), 14(28), 15(16) or 16(2); branched pectoral fin
rays 12(2), 13(18), 14(17) or 15(3); branched pelvic fin rays 7(11) or
8(29); lateral line scales 39(2), 40(8), 41(10), 42(28), 43(13),
44(9), 45(7), 46(1), 47(1), 48(1), or 50(1); total gill rakers 19(1),
20(2), 21(18), 22(7), 23(5), 24(4) or 25(3); pharyngeal teeth
2,5-5,2(13), 2,5-4,2(11), 2,5-4,1(1), 2,4-5,2(2) or 2,4-4,2(1); and
total vertebrae 37(2), 38(24), 39(20), 40(7) or 41(1).
Sexual dimorphism
Tubercles line the edge of each scale and in single file line the
rays of all fins. Fine tubercles cover the whole head.
Colour
The overall colour is bright silvery with the posterior scale
margins grey on the upper flank. The back is dark blue to olive or
bluish-green and is sharply distinct from the lighter flanks. The
mid-line of the back has a narrow dark line. The lateral line and the
area above it have some pigmentation, concentrated along the lateral
line itself, but there is no dark stripe or it is only faintly
developed and is bluish or greyish. Above this stripe is an iridescent
golden-green stripe only visible at a certain angle. The bluish or
greyish stripe is more evident in preserved material. The belly and
lower head surface are pearly-white. The iris is silvery with a yellow
ring along the outer eye rim but very little around the pupil. The
upper part of the iris may have some dark pigment. The dorsal and
caudal fins have dark rays and transparent membranes but may be a
dirty yellow. Membranes may have some pigment, particularly on the
dorsal fin. The upper anterior edge of the pectoral fin has a little
dark pigment while the rest of the fin is colourless to grey or
orange. Some fish have a yellow base to the pectoral fin. The pelvic
and anal fins are usually colourless, although the anal rays may have
some grey or there may be some yellow, orange or red on the fin
generally. The caudal fin tip is dark grey.
In preserved fish, most flank pigment is above the lateral line.
Lateral line scales have pigment both above and below the pore so the
pore stands out. This is not as distinctive as in some Alburnoides
spp.. A mid-dorsal stripe is more evident in smaller fish
and is obscured by the generally darker back and upper flank
pigmentation in larger fish. The peritoneum is a light silvery with
scattered melanophores. A flank stripe may be developed although not
as strongly as in Alburnus filippii; the stripe is more a
darker area along the muscle mass divide between a lighter upper flank
and lower flank.
Size
Reaches 20 cm.
Distribution
Found from England through Europe and east to the Caspian Sea
basin or narrowly the western and southern Caspian Sea basin as A.
hohenackeri. It is reported from the Aras River (including the upper reaches of its
tributary, the Qara Su) to the Atrak River along the Caspian coast of Iran
including the Anzali Talab and Gorgan Bay, and the Gorgan, Gharasu, Tajan, Babol,
Haraz, Sardab, Tonekabon; Pol-e Rud and Safid rivers (Derzhavin,
1934; Holčík and Oláh, 1992; Kiabi et al., 1999; Abbasi et al.,
1999; Abdoli and Naderi, 2009). Also widely introduced across western, central
and eastern Iran, including in the Ab-e Sirvan
in the upper Diyala River, Lake Zarivar, in the Zayandeh River of the Esfahan basin, in the Kalshur, Jajarm and Qareh Su of northeastern Dasht-e Kavir basin, and in the Hamun Kushk, and
Kahak and Sistan dams of the Sistan basin, and possibly in Minab (= Esteghlal)
Dam (A. Abdoli, pers. comm., 1995; J. Holčík,
in litt., 1996;.Abdoli, 2000; Ghorbani Chafi, 2000; A. Afzali, pers. comm., 2002;
Esmaeili et al., 2010).
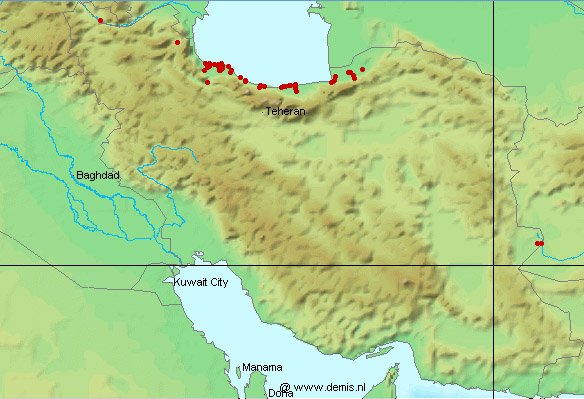
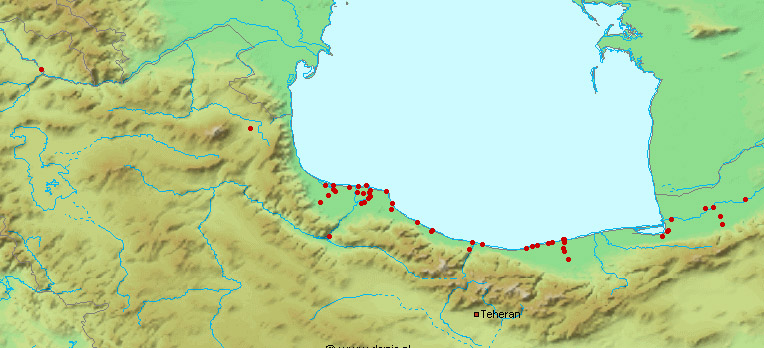
Zoogeography
This is a widespread species showing great morphological variability over its
range, sometimes recognised as taxa. Zoogeographical relationships of these taxa and of
the species to other Alburnus have still to be worked out.
Habitat
This species is found in open waters of lakes along the shore or in
slow rivers, avoiding turbid conditions and heavy vegetation. There
was a mass mortality, presumed to be of this species, on the Babol Sar
beach on 24 June 1963 (USNM 270909). It is found more abundantly at river
estuaries along the Iranian Caspian shore than Alburnus filippii (Jolodar
and Abdoli, 2004).
Age and growth
Maturity is attained at 3 years and life span is up to 9 years. In
more northern waters, most spawning males are 3+ and 4+ years while
females are 5+ and 6+ years. Iranian populations probably have a
similar structure but the age groups would be lower. Mature males
averaged 9.7 cm and females 10.5 cm in one study in Russia (Berg, 1948-1949).
Food
Food is planktonic crustaceans, benthic crustaceans such as
amphipods, flying insects which land on the water surface, aquatic
insects such as backswimmers (Notonectidae), algae, diatoms, and fish
eggs and fry. It is an important prey item for other fishes.
Reproduction
Spawning in Europe takes place from April to July in shallow water
over a hard bottom. June is the main spawning month in Azerbaijan
judging by egg diameters and condition factors (Abdurakhmanov, 1962).
Older fish spawn first. Water temperature is usually at 15-16°C
or more. Spawning takes place in 3-6 stages at intervals of 9-11 days.
The eggs adhere to stones, branches or vegetation. Fecundity is up to
10,000 eggs and egg diameter to 1.4 mm. Incubation lasts about 1 week.
Iranian specimens had 1.1 mm diameter eggs in a sample caught on 11
June and mature males were collected on 10 July. Specimens collected
in September showed egg resorption while those taken in December had
small, developing eggs and those taken in April with better developed
eggs. The specimens were small and spawning probably occurs in July
for these fish and possibly June for larger ones.
Parasites and predators
Molnár and Jalali (1992) record the monogeneans Dactylogyrus
parvus, D. alatus and D. chalcalburni from Alburnus
charusini on the Safid Rud.
Gussev et al. (1993b) report the monogenean, Dactylogyrus
chalcalburni, from this species in the Zayandeh Rud but this fish
does not occur there. The parasite may have been found in Alburnus
mossulensis. Shamsi et al. (1997) report Clinostomum
complanatum, a parasite causing laryngo-pharyngitis in humans,
from this species.
Barzegar et al. (2008) record eye parasites from this fish including the
digeneans Diplostomum
spathaceum and Tylodelphys clavata.
Some European populations of Sander lucioperca feed almost
exclusively on this species. Spent adults are known to eat their own eggs.
Economic importance
The scales contain silvery crystals of guanine which are extracted
and used to make essence d'orient (or pearl essence) for
artificial pearls. About 5000 fish are required for 100 g of essence.
Schools in the lower Don River of the Black Sea number up to 10
million fish weighing 30 tonnes. This abundant species is of indirect
commercial importance as food for more valued fishes but it has also
been used as food for humans.
Conservation
Kiabi et al. (1999) consider this species to be of least
concern in the south Caspian Sea basin according to IUCN criteria.
Criteria include abundant in numbers, habitat destruction, widespread
range (75% of water bodies), and present in other water bodies in Iran.
Endangered in Turkey (Fricke et al., 2007).
Further work
The biology of this species needs investigation, especially in relation to
habitats and other fish species where it has been introduced by accident.
Sources
Iranian material: CMNFI 1970-0510, 8, 44.5-72.1 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1970-0580, 27, 33.9-56.1 mm standard length, Mazandaran, river near Iz Deh (36º36'N, 52º07'E);
CMNFI 1970-0589, 21, 22.5-67.9 mm standard length, Gilan, Safid River (37º12'N, 49º54'E);
CMNFI 1971-0343, 1, 63.5 mm standard length, Gilan, Langarud at Chamkhaleh (37º13'N, 50º16'E);
CMNFI 1979-0265, 30, 61.6-90.4 mm standard length, Gilan, head of Anzali Mordab at Abkenar (37º28'N, 49º20'E);
CMNFI 1979-0432, 22, 34.4-54.3 mm standard length, Mazandaran, Sardab River branch (36º41'N, 51º22'E);
CMNFI 1979-0435, 1, 51.9 mm standard length, Gilan, stream 10 km west of Ramsar (36º57'N, 50º37'E);
CMNFI 1979-0480, 6, 14.4-64.3 mm standard length, Mazandaran, Gorgan Rver at Gonbad-e Kavus (37º15'30"N, 55º09'E);
CMNFI 1980-0122, 41, 29.8-59.0 mm standard length, Mazandaran, Nerissi River (36º38'N, 52º16'E); CMNFI 1980-0147, 5, 44.3-61.5 mm standard length, Gilan, Lashtenesha River (37º21'N, 49º52'E).
Alburnus mossulensis
Heckel, 1843
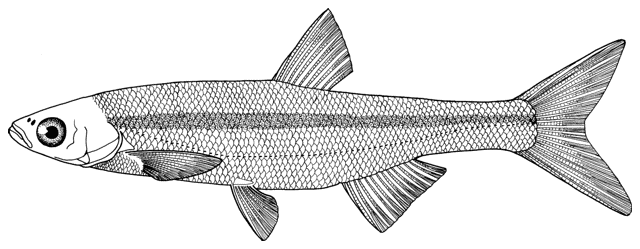
Common names
شاه كولي (shah kuli = king fish), shah kuli-ye jonubi (= southern king fish),
شاه ماهي (= shah mahi, meaning king fish),
shah kuli mosulenzis.
[simnan , semnan or samnan, semnan tuyel; sink, or zurri at Mosul (zurri also used for Chondrostoma regium according to Heckel (1846-1849a),
but is also used for Aphanius spp., Gambusia and any small fishes
or large fishes when young; all in Arabic; Mosul bleak].
Systematics
Leuciscus maxillaris Valenciennes,
1844 from "rivières de Perse", probably Alburnus capito
Heckel, 1843 from "Gebirgsflüssen Kurdistans" (mountain
streams of Kurdistan in Heckel (1843b) or "Gebirgsbache in
Kurdistan" in Heckel (1846-1849a)), Alburnus Iblis Heckel,
1849 described from the "Gegend um Persepolis oder den Gewässern
des Araxes" (= probably the Pulvar (= Sivan) River near
Persepolis and the Kor River, both in Fars), Alburnus Schejtan
Heckel, 1849 described from the "Araxes bei Persepolis", Alburnus
caudimacula Heckel, 1849 described from the "Flusse Kara-Agatsch
und bei dem Dorfe Geré (= Qarah Aqaj or Mand River, Fars; possibly
near Kereft, 29°01'N, 52°52'E), and Alburnus megacephalus Heckel, 1849 described from the
"Araxes" are synonyms (e.g. according to Berg (1949)). The
type locality of Alburnus mossulensis is the "Tigris bei
Mossul" according to Heckel (1843b).
Saadati (1977) considers Alburnus caudimacula to be a
distinct species found in the Mand River of Fars based on head length
being longer (but the ranges overlap) and a shorter scaleless keel
(which is individually variable in these fishes according to my observations).
A subspecies, Alburnus mossulensis delineatus Battalgil,
1942, is reported from Diyarbakir on the Tigris River in Turkey.
A hybrid with Acanthobrama marmid was reported from the Hawr
al Hammar in southern Iraq by Krupp et al. (1992) who also note
that A. mossulensis is probably a synonym of Alburnus
sellal Heckel, 1843, a species originally described the Quwayq
River at Aleppo. However, they retained mossulensis as a
distinct species because of colour differences and the difficulty of
obtaining fresh material of sellal in its polluted habitat at
Aleppo in Syria (see Vesiland (1993) for habitat photograph). Heckel
(1846-1849a) differentiates mossulensis from sellal by
the former being more slender and elongate, the pelvic, dorsal and
anal fins are more anterior so the caudal peduncle is more elongate,
the eyes are larger and lower on the head, and there is a lead-coloured
stripe separating the upper third of the body from the lower part.
Berg (1949) considers that A. mossulensis may be nothing more
than a subspecies of A. sellal.
A Principal Components Analysis on the types of mossulensis and sellal
using 32 morphometric and meristic characters showed some separation between the
two taxa and a Discriminant Function Analysis separated most, but not all,
specimens. The evidence is not conclusive for separation or synonymy and the
taxa are left as distinct in this work.
If mossulensis is a synonym of sellal, then the
nominal taxa Alburnus
hebes Heckel, 1843, Alburnus microlepis Heckel, 1843 and Alburnus
pallidus Heckel, 1843, all from the Kueik (= Quwayq) River at Aleppo
(Heckel, 1843b), would have to be added to the synonymy of sellal
as indicated by Berg (1949), Krupp (1985c) and Eschmeyer's "Catalog of Fishes"
(downloaded 1 September 2007). The 3 syntypes of Alburnus
hebes seen by me in the Naturhistorisches Museum Wien were 58.8-156.5
mm standard length (NMW 17558-17560) (but Eschmeyer et al.
(1996) list NMW 55523 for these syntypes, and the card index had this
number in 1997; possibly they were renumbered). One of these fish is
designated as the lectotype. The holotype of Alburnus microlepis
(NMW 55655) measures 119 mm standard length (Krupp, 1985c). The holotype of
Alburnus pallidus (NMW 55720) measured 76.6 mm standard length.
Krupp (1985c) gives details on the syntypes of Alburnus sellal
held at the Naturhistorisches Museum Wien. Six syntypes of A.
sellal, 124-140 mm standard length, are under NMW 55665 (2 fish, 137.2-141.3
mm standard length, my measurements)
and NMW 55666 (4, 126.9-142.7 mm standard length), and 3, 110-152 mm standard length, are under NMW
55664 (1, 110.5 mm standard length) and 55667 (2, one of which is designated as the lectotype,
140.7-155.4 mm standard length).
Eschmeyer et al. (1996) list NMW 55664-67 as having 1, 2, 4,
and 2 fish in each number in the series and also 2 syntypes (RMNH
2666) in the Rijksmuseum van Natuurlijke Historie, Leiden from NMW.
The catalogue in Vienna lists 8 specimens of A. sellal.
The syntypes of A. mossulensis are under NMW 55656 (2 fish,
111.2-118.4 mm standard length, my measurements), NMW 55717 (2, 83.0-89.4 mm
standard length), and NMW 55718 (2, 101.9-131.5 mm standard length). Two syntypes of Alburnus
mossulensis are in the Senckenberg Museum Frankfurt (SMF 402,
formerly NMW) (F. Krupp, pers. comm., 1985; 80.1-102.7 mm standard length). Eschmeyer et al.
(1996) also list NMW 77723 (2, 90.4-135.4 mm standard length) and 1 possible syntype in the
Rijksmuseum van Natuurlijke Historie, Leiden (RMNH 2644). The
catalogue in Vienna lists 6 specimens of A. mossulensis, with
one specimen from NMW 77723 as the lectotype.
Seven syntypes of Alburnus iblis are in the
Naturhistorisches Museum Wien under NMW 55524 and measure 91-165 mm
standard length (Kähsbauer, 1964; 92.9-172.3 mm standard length by my
measurements). One of these fish is designated as the lectotype. The
catalogue in Vienna lists 8 specimens in one column and 38 in the
adjacent column.
Two syntypes of Alburnus megacephalus are under NMW 55627
and measure 160-162 mm standard length (Kähsbauer, 1964; 162.9-166.1
mm standard length by my measurements); 2 specimens are listed in the
Vienna catalogue. One of these fish is the lectotype.
Fifteen syntypes of Alburnus caudimacula are under NMW 55506
and measure 38.5-118.4 mm standard length; the catalogue in Vienna
lists 8 specimens in one column and what appears to be 26 specimens in
the adjacent column although this may be 20 fish with 6 set aside for A.
schejtan. The Rijksmuseum van Natuurlijke Historie, Leiden has 4
syntypes under RMNH 2654, formerly in NMW (Eschmeyer et al., 1996).
Five syntypes of Alburnus capito measure 48.7-101.9 mm
standard length (NMW 55505) although the catalogue in Vienna only lists 4 fish.
Four syntypes of Alburnus schejtan measure 71.7-112.6 mm
standard length (NMW 22281) and one of these is designated as the
lectotype, 2 syntypes measure 104.5-112.3 mm standard length (NMW
55663), 2 syntypes measure 91.8-100.0 mm standard length (NMW 55719),
and 2 syntypes measure 81.6-94.4 mm standard length (NMW 55721).
Two syntypes of Leuciscus maxillaris, 165-166 mm total
length, are stored in the Muséum national d'Histoire naturelle, Paris
(as 13954 according to Fang (1942) or as A.3954 according to Bertin
and Estève (1948), M. L. Bauchot, in litt., 1982, and my
observations). Fang (1942) regards maxillaris as a distinct
species in Alburnus.
My measurements were 136.7-136.9 mm standard length.
Krupp (1985c) refers 5 specimens from the type series of Alburnus
doriae to his Alburnus sellal and 2 specimens to Leuciscus (=
Squalius) lepidus.
Bianco and Banarescu (1982) felt that their samples showed clinal
variation from northwest to southeast, with numbers of anal fin
branched rays, lateral line scales and gill rakers gradually
decreasing. Their fish from the upper Tigris River basin in Turkey not
far from Mosul (the type locality) and from the Pulvar River (Kor
River basin of Fars) form one subspecies while those from the Mand and
Kul River basin draining to the Persian Gulf in Fars are a distinct
subspecies. Available names for the former subspecies include capito,
iblis, schejtan and megacephalus, the latter
requires a new name according to Bianco and Banarescu (1982).
The Tigris-Kor sample could be A. mossulensis mossulensis and
the Mand-Kul sample A. mossulensis caudimacula (see above).
However, Bianco and Banarescu (1982) are correct to point out that
variation in this species has not been fully examined, local
environmental conditions such as temperature can affect scale counts
and the problem of the relationship of A. sellal remains to be
resolved. They found in 7 specimens of sellal that scale counts
at 71-77 (in contrast to 66-70 in Berg (1949)) overlapped with mossulensis
counts. Berg's (1949) and my counts are very wide for A.
mossulensis, suggesting that local environment may govern meristic
characters as widely demonstrated for fishes. Subspecies recognition
requires much further work as Bianco and Banarescu (1982) acknowledge
by not proposing a new name for the Mand-Kul fish.
Key characters
The short, naked ventral keel, usually 8 branched dorsal fin rays,
distribution, and the characters in the table under A. atropatenae can be
used to identify this species.
Morphology
Dorsal fin with 3 unbranched and 7-9 branched rays, anal fin with 3
unbranched and 10-14 branched rays. Pectoral fin branched rays 13-18,
pelvic fin branched rays 7-9. Lateral line scales 58-89. Gill rakers
11-18. Pharyngeal teeth 2,5-4,2, with hooked tips and serrated edges
to the crowns. Variants include 2,5-5,2, 3,5-5,3 and 2,5-5,3.
Populations vary sympatrically in total vertebral counts: 40-43 and
42-45; and in abdominal counts 20-22 and 22-24 (Bogutskaya et al.,
2000). The karyotype of fish from the Kızılırmak
River in Turkey was 2n=48 (Gül et al., 2000) but this species does not
occur in this area.
Meristics for Iranian specimens:-
branched dorsal fin rays 7(16), 8(303) or 9(13); branched anal fin rays 10(20),
11(200), 12(104) or 13(8); pectoral fin branched rays 13(2), 14(30), 15(110),
16(134), 17(50) or 18(6), branched pelvic fin rays 7(30), 8(288) or 9(18);
lateral line scales 58(1), 59(-), 60(2), 61(1), 62(5), 63(3), 64(9), 65(8),
66(9), 67(12), 68(11), 69(8), 70(18), 71(17), 72(26), 73(21), 74(28), 75(20),
76(19), 77(26), 78(28), 79(21), 80(15), 81(10), 82(5), 83(1), 84(2), 85(2),
86(2), 87(1), 88(1), or 89(1); and total gill rakers 11(7), 12(54), 13(111), 14(101),
15(46), 16(11), 17(1) or 18(1).
Sexual dimorphism
Unknown but males do develop tubercles in the breeding season.
Colour
Overall colour is silvery. The back is a bluish- or reddish-brown,
bluish-black or blackish. A dark, lead-coloured stripe runs along and
above the mid-flank and has a width about the same as the eye
diameter. The stripe may only be evident posteriorly. Scales above the
lateral line have fine melanophores at their base. Lateral line scales can have
pigment spots above and below the tube near the base of each scale, but this is
not as marked as insome Alburnoides spp. The dorsal, anal
and caudal fins are margined with black, the latter the darkest. There
may be a black spot at the caudal fin base and the first pectoral fin
ray may be black dorsally. The pectoral, pelvic and anal fins are
yellowish at their base. Pelvic and anal fins may be reddish. The
peritoneum is brown but may be thickly speckled with black-brown spots
and thus appear almost black.
Size
Reaches about 22 cm (Ergene, 1993).
Distribution
Found in the Tigris-Euphrates basin and adjacent basins. In Iran it is
recorded from the Tigris River, Gulf, Lake Maharlu, Kor River and upper reaches
of the Hormuz basins (M. Hafezieh, pers. comm.; Berg, 1949; Bianco
and Banarescu, 1982; Abdoli, 2000) and questionably from the Esfahan basin (Abdoli,
2000). Records also include the Shapur and Dalaki rivers in the Gulf basin
(Gh. Izadpanahi, pers.
comm., 1995) and the upper Mand including Qara Agaj reach and Shur tributary, Shur tributary
to Dasht-e Palang; upper Zohreh, Marun and Jarrahi, upper Karun and Khersan, Dez,
whole middle to upper Karkheh basin (Simarreh, Qarasu, Gav Masiab)(Abdoli, 2000).
Zoogeography
Its former position in the genus Chalcalburnus indicates a
relationship with fishes occurring in the Black-Caspian seas basin.
Habitat
This species is found in streams, rivers, lakes, reservoirs and marshes.
Al-Habbib (1981) has demonstrated experimentally for specimens taken from the
Aloka River, north of Mosul, Iraq that this species can survive temperatures in the
range of about 1.25-36.2°C when acclimated (fish were identified incorrectly as
Chalcalburnus chalcoides). Epler et al. (2001) found it to be the
second most dominant species of fish (identified as A. sheitan) in lakes
Habbaniyah, Tharthar and Razzazah in Iraq, comprising 10% of all fish collected. This was
one of the most abundant species in the recovering marshes of southern Iraq in
2005-2006 (Hussain et al., 2006).
Age and growth
Jawad (2004) used eye lens diameter for ageing the young (up to age 3) of
this species from the marshes north of Basrah. Ergene (1993) studied the growth
of this species in the Karasu of Turkey and found 4 age groups, and mentions 5
age groups for another Turkish study. Mean fork length is 118.2 mm, 131.0 mm,
145.2 mm and 163.3 mm respectively. Condition factors for these age groups were
0.87, 0.85, 0.84 and 0.86. Türkmen and Akyurt (2000) also working on this
species in the Karasu River found age groups 1 to 6 with age group 3 the most
abundant. The mean condition factor for males and females was 1.023 and 1.047
respectively. Age-length, age-weight (von Bertalanffy equations) and
length-weight relationships were also calculated as lt = 20.41[1-e-0.2485
(t+1.47)], lt = 21.59[1-e-0.1978 (t+2.13)], W = 80.77 (1-e-0.2485
(t+1.47)2.828, W = 103.63 (1-e-0.1978 (t+2.13)3.082,
LogW = -1.796 + 2.828 LogFL (r = 0.943) and LogW = -2.097 + 3.082 LogFL (r = 0.946) respectively.
Length and age at first maturity were 1.26 years and 9.24 cm for males and 1.81
years and 9.65 cm for females in the Karasu River, Turkey; age group 7 was the
oldest recorded (Yıldırım et al., 2007).
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 76 Iranian fish measuring
3.15-8.14 cm standard length. The a-value was 0.0197 and the b-value
2.903 (a b-value < 3 indicating a fish that becomes less rotund as length
increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Younis et al. (2001b) found Shatt al Arab, Iraq fish feeding on
phytoplankton (algae and diatoms) at 44%, followed by organic detritus at 36.7%
(33% in a table), and arthropods at 3.1%, It had a dietary overlap of 89% with
Barbus (= Carasobarbus) luteus in May, the highest in the study. In a study of the
recovering Hammar Marsh, Iraq diet was 67.95% insects and 14.34% algae with diatoms,
plants, crustaceans and fish at less than 10% each, in the Hawr al Hawizah 66.2%
insects and 19.2% algae, with amounts of diatoms and crustaceans being less than
10% each, and in the Al Kaba'ish (= Chabaish) Marsh 73.7% insects and 13.1% algae with diatoms,
plants and crustaceans at less than 10% each (Hussain et al., 2006).
Reproduction
Berg (1949) reports a female 15.5 cm long with mature eggs. Qarmat Ali River, Iraq
fish had a fecundity of 1926-11,779 eggs (Saud, 1997). Yıldırım
et al. (2007) examined this species in the Karasu River of Turkey and
found a male:female sex ratio of 1:1.08, not significantly different from 1:1, a
fecundity range of 3012 to 11,427 eggs, significant correlations between
fecundity and fork length, total weight, age and gonad weight, and a spawning
season from June to August when water temperature attained 15ºC.
Parasites and predators
Molnár and Jalali (1992) describe a new species of monogenean, Dactylogyrus
holciki, from this species in the Beshar River of the Persian Gulf
drainage. Gussev et al. (1993b) report the monogenean, Dactylogyrus
chalcalburni, from Alburnus alburnus in the Zayandeh Rud
but this fish does not occur there. The parasite may have been found
in Alburnus mossulensis. González-Solís et al.
(1997) report Rhabdochona denudata, Contracaecum sp.
larvae and Proleptinae larvae (Nematoda) from this species in the
drainage of Lake Maharlu and Contracaecum sp. larvae in the
drainage of the Kor River, both in Fars. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sp. from the Beshar River of the Tigris
basin in a Chalcalburnus sp., presumably this species.
Barzegar and Jalali (2006) report
parasites in this species from Kaftar Lake as Lernaea cyprinacea and
Diplostomum spathaceum.
Barzegar et al.
(2008) also record the digenean eye parasite Diplostomum spathaceum from
this fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea cyprinacea
on this species.
Economic importance
This species has been used in the preparation of fish meal in Iraq.
Conservation
An abundant species where studied, it appears to be under no threat in Iran.
Endangered in Turkey (Fricke et al., 2007).
Further work
Its taxonomic status in relation to its Levant relative remains unresolved
and the relation between lowland and mountain populations in Iran needs careful
analysis. Its biology in Iran has yet to be studied in detail.
Sources
Type material: See above, Alburnus capito (NMW 55505), Alburnus caudimacula (NMW 55506), Alburnus
hebes ((NMW 17558-17560 or NMW 55523), Alburnus iblis (NMW 55524), Alburnus megacephalus
(NMW 55627), Alburnus microlepis (NMW 55655), Alburnus mossulensis (NMW 55656, 55717, 55718,
77723, SMF 402), Alburnus schejtan (NMW 22281, NMW 55663, 55719, 55721), Alburnus sellal
(NMW 55664, 55665, 55666, 55667), Leuciscus maxillaris (MNHN A.3954).
Iranian material: CMNFI 1977-0510A, 44, 35.7-154.6 mm standard length, Fars, Pulvar River tributary (29º59'30"N, 52º54'E);
CMNFI 1979-0025, 87, 19.1-138.2 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0027, 24, 59.8-105.0 mm standard length, Fars, Chehel Cheshmeh (ca. 29º43'N, ca. 52º04'E);
CMNFI 1979-0028, 55, 19.1-122.6 mm standard length, Fars, Kor River drainage (no other locality data);
CMNFI 1979-0036, 22, 82.3-115.1 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E); CMNFI 1979-0047, 7, 41.4-78.2 mm standard length, Fars, Ab-e Paravan spring (ca. 29º34'N, ca. 52º42'E);
CMNFI 1979-0054, 17, 39.8-95.6 mm standard length, Fars, Shur River tributary (28-29º58-03'N, 52º34-35'E);
CMNFI 1979-0061, 51, 32.9-131.1 mm standard length, Fars, Pulvar River tributary (30º04'N, 53º01'E);
CMNFI 1979-0067, 55, 11.1-107.9 mm standard length, Fars, qanat at Zarqan (ca. 29º46'N, ca. 52º43'E);
CMNFI 1979-0070, 44, 35.0-98.5 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0071, 12, 65.3-104.3 mm standard length, Fars, qanat 23 km from Pol-e Khan (ca. 30º00'N, ca. 52º38'E);
CMNFI 1979-0073, 26, 50.0-93.3 mm standard length, Fars, Mand River (ca. 29º42'30"N, ca. 52º01'30'E);
CMNFI 1979-0074, 39, 23.8-94.0 mm standard length, Fars. Mand River (29º41'N, 52º06'E);
CMNFI 1979-0117, 16, 33.4-130.0 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0128, 9, 43.2-102.5 mm standard length, Fars, Shur River (28º51'N, 52º31'E);
CMNFI 1979-0154B, 1, 46.9 mm standard length, Fars, stream at Koorsiah village (28º45'30"N, 54º24'E);
CMNFI 1979-0155, 2, 56.2-64.7 mm standard length, Fars, spring at Gavanoo village (28º47'N, 54º22'E);
CMNFI 1979-0156, 11, 49.0-74.4 mm standard length, Fars, qanat at Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0157, 53, 31.8-86.6 mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E);
CMNFI 1979-0158, 13, 73.5-108.9 mm standard length, Fars, qanat between Now Bandegan and Qaziabad (28º54'N, 53º53'30"E);
CMNFI 1979-0160, 22, 32.4-106.0 mm standard length, Fars, spring at Arteshkhadeh Pomp (29º09'N, 53º37'E);
CMNFI 1979-0272, 11, 40.5-130.0 mm standard length, Lorestan, river at Nokhor (ca. 33º40-47'N, ca. 48º28-45'E);
CMNFI 1979-0278, 4, 75.5-88.2 mm standard length, Lorestan, Kashkan River drainage (33º34'N, 48º01'E);
CMNFI 1979-0279, 3, 68.7-91.4 mm standard length, Lorestan, Khorramabad River (33º37'N, 48º18'E);
CMNFI 1979-0282, 19, 40.2-131.3 mm standard length, Lorestan, river at Nurabad (34º05'N, 47º58'E);
CMNFI 1979-0284, 30, 73.1-98.3 mm standard length, Kermanshahan, Qareh Su drainage (34º16'N, 46º48'30"E);
CMNFI 1979-0285, 4, 124.7-136.8 mm standard length, Kermanshahan, Qareh Su drainage (34º26'N, 46º37'E);
CMNFI 1979-0289, 2, 125.3-142.1 mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0290, 4, 146.9-171.4 mm standard length, Kermnanshahan, Diyala River drainage (34º31'N, 45º35'E);
CMNFI 1979-0348, 4, 68.0-78.8 mm standard length, Fars, 2 km from Pol-e Berengie (ca. 29º28'N, ca. 52º32'E);
CMNFI 1979-0352, 2, 88.5-93.9 mm standard length, Khuzestan, Jarrahi River drainage (30º33'30"N, 48º48'E);
CMNFI 1979-0499, 3, 104.8-133.6 mm standard length, Fars, irrigation ditch on road to Dariush Dam (30º04'30"N, 52º36'E);
CMNFI 1979-0500, 2, 112.2-116.5 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E).
Comparative material: BM(NH) 1981.4.13:9-11, 3, 64.3-72.8 mm standard length,
Aloka River near Mosul (no other locality data); CMNFI 1980-815, 2, 88.9-107.2 mm; CMNFI 1980-1036, 2,
11.6-145.8 mm standard length, Turkey, ?check this sample for gill raker count ;
Alburnus zagrosensis
Coad, 2009

Common names
None.
Systematics
The holotype
(CMNFI 1979-0248) is a male 81.6 mm SL from Chahar
Mahall va Bakhtiari, stream, 3 km east of Boldaji, upper Karun River basin
(31°55’N, 51°05’E); paratypes are CMNFI 1979-0248A, 128 ♂ 48.4-89.5 mm SL same
locality as holotype and CMNFI 1979-0246, 49, 52.5-92.6 mm SL, Chahar Mahall va
Bakhtiari, stream, 8 km west of Boldaji, upper Karun River basin (31°57’30”N,
50°59’E). The species is named for the Zagros Mountains of western Iran where it
was collected at altitudes over 2300 m.
Key characters
This
species is distinguished from other Iranian and Tigris-Euphrates basin
Alburnus by such characters as high lateral line scale count (67-83; 64 or
lower in alburnus, atropatenae, caeruleus, doriae, filippii), low anal
fin branched ray count (9-10; usually 12 or higher in alburnus, caeruleus,
chalcoides), and low total gill raker count (12-14; 16 or higher in
alburnus, chalcoides). Other
characters include a ventral keel almost absent to
almost complete, a high frequency of 7 branched dorsal fin rays (35.3%), a high
frequency of 7 branched pelvic fin rays (62.7%), a total vertebral count mode at
42, absence of a prominent mid-flank stripe, and small size (females mature at
88.5 mm SL).
Morphology
The
body is elongate and a vertical oval in cross-section, somewhat compressed but
not deep. The upper and lower profiles are a gentle arch. The snout is short and
pointed. The mouth is slightly superior, almost terminal, oblique, and extends
back to a level with the mid-nostril. The mouth tip is on a level with the upper
half of the eye. The anal fin origin is posterior to the dorsal fin insertion.
The dorsal fin margin is rounded and the anal fin margin is straight. The
lateral line is decurved and is on the midline of the body only on the posterior
half of the caudal peduncle. There is a pelvic axillary scale. The naked ventral
keel is flanked for six scale rows anterior to the anal fin.
Dorsal fin with 3 unbranched rays and
7(18) or 8(33) branched rays, anal fin with 3 unbranched rays and 9(29) or
10(22) branched rays, pectoral fin with 14(5), 15(25), 16(15) or 17(6) branched
rays, pelvic fin with 7(32) or 8(19) branched rays, lateral line scales to
hypural fold 67(1), 69(2), 70(3), 71(2), 72(6), 73(6), 74(2), 75(4), 76(6),
77(7), 78(3), 79(3), 80(2), 81(3), or 83(1), scales around caudal peduncle
20(1), 21(10), 22(18), 23(11), 24(10) or 25(1), predorsal scales 32(1), 33(5),
34(1), 35(8), 36(8), 37(5), 38(9), 39(3), 40(6), 41(4) or 42(1), scales between
lateral line and dorsal fin origin 12(1), 13(2), 14(22), 15(17), 16(8), or
18(1), scales between lateral line and anal fin origin 5(6), 6(37) or 7(8),
scales between lateral line and pelvic fin origin 4(6), 6(25), 6(15) or 7(5),
total gill rakers 12(20), 13(19) or 14(12),
short and
usually reach past the anterior base of the adjacent raker when appressed,
abdominal vertebrae 21(14), 22(34) or 23(3), caudal vertebrae 19(17), 20(32) or
21(2), and total vertebrae 40(4), 41(19), 42(27) or 43(1).
From 0 to 5 scales at the end of the
lateral line are not pored. The belly is rounded and has a naked keel in front
of the anal fin, varying in extent. The naked keel may be flanked by one to 11
scales, or from almost no keel to a keel extending from the anal fin almost to
the pelvic fins base. Scales from below the dorsal fin have fine but not
numerous circuli, a subcentral anterior focus and few radii on the anterior and
posterior fields with no radii on the lateral fields. Pharyngeal teeth number
2,5-4,2 (6), 2,5-4,1(2) or 1,5-4,2(2). Teeth are hooked at the tip and have
serrated edges to the narrow grinding surface below the tip. The gut is an
elongate S-shape with a small anterior loop to the left.
Sexual dimorphism
Small tubercles are present on the mature
male, on the sides and top of the head, on the dentaries, as fine ones lining
the scale margins, most evident on caudal peduncle scales, and on the upper
pectoral fin rays. Significant differences between males and females were found
in interorbital width (wider in males), caudal peduncle depth (deeper in males),
and dorsal, anal, pectoral and pelvic fin lengths (longer in males).
Colour
Fins are mostly immaculate with only
slight traces of melanophores. The body is darker dorsally and the upper flank
is much darker than the lower flank with an abrupt transition in pigmentation
between the two halves along the midline. The anterior flank has a less defined
transition with some thin bars extending downward for a short distance. The
mid-flank dark pigmentation forms a weak stripe, well-defined by its ventral
edge above the anal fin level but the dorsal edge is less well-defined and
merges with the upper flank pigmentation. The end of the caudal peduncle has a
broad, fan-shaped pigmentation. A broad stripe is present predorsally and
postdorsally. The predorsal stripe may be separated into two thin stripes, one
on each side of the mid-line with a very thin stripe between them. After 32
years in preservative, this pigment pattern is less evident and the division
between the epaxial and hypaxial muscle masses is marked by an apparent thin
line of dark pigment as the most prominent feature. The peritoneum is silvery
with numerous scattered melanophores grading to fish with continuous
melanophores and dark brown overall.
Size
Attains 92.6 mm standard length.
Distribution
Known only from the type locality in the upper Karun River basin.
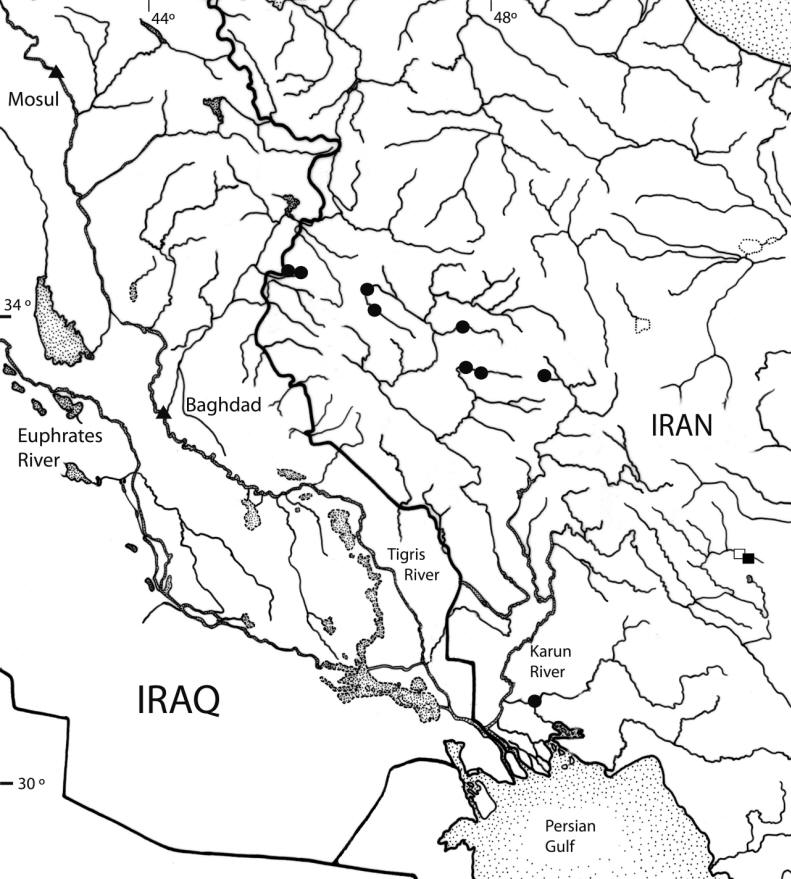
Empty square = holotype, black square = paratypes, of A. zagrosensis.
Black circles = A. mossulensis.
Zoogeography
This species is related to A. mossulensis which is found in same basin
(Tigris-Euphrates) and may have evolved from an isolated population in the upper
Karun River.
Habitat
The type locality was at an altitude of
2360 m, water temperature was 22°C at 1545 hours, pH was 6.2 and conductivity
0.45 mS. The stream width was 3-4 m, depth 1 m maximum, the bottom was muddy and
the water cloudy. Current was slow, the shore grassy and there was a moderate
amount of aquatic vegetation, mostly
Myriophyllum.
Other species caught were the cyprinid
Chondrostoma regium
and the tooth-carp Aphanius
vladykovi.
The
second locality was at an altitude of 2380 m, water temperature was 25°C at 1450
hours, pH was 6.2 and conductivity 0.4 mS. The stream width was 1-2 m, depth 1 m
maximum, the bottom was stones and mud and the water muddy. Current was fast,
the shore grassy and there was some aquatic vegetation. Some of this material
was caught while leaping up a raceway. Other species caught were the cyprinids
Chondrostoma regium and a Capoeta sp.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Although known from only two localities in the upper Karun River basin of Iran,
this species may not be threatened other than by water abstraction and pollution.
Further work
Distribution, conservation and biology of this species needs investigation.
Sources
See Coad (2009).
Type material: See above.
Genus Aspidoparia
Heckel, 1847
This Oriental genus has only 2 species, one of which enters
southeastern Iran. Mirza (2000) proposes that the members of the genus
Aspidoparia be placed in a new subfamily, Aspidoparinae. Cabdio
Hamilton, 1822 is presumably a senior synonym of Aspidoparia but has not
been extensively used in literature on these species so Aspidoparia is
retained here.
It is characterised by an elongate and almost cylindrical body with
a rounded abdomen, the head has a broad ring of suborbital bones, the
mouth is small and inferior, the roof of the mouth has a papillose
nodule, the lower jaw has a sharp, crescentic bony edge, no barbels,
pharyngeal teeth in 2-3 rows, dorsal fin short, anal fins short to
moderate, scales moderate in size, lateral line decurved and running
on the lower half of the caudal peduncle, and the gut is long and
coiled.
Aspidoparia morar
(Hamilton, 1822)
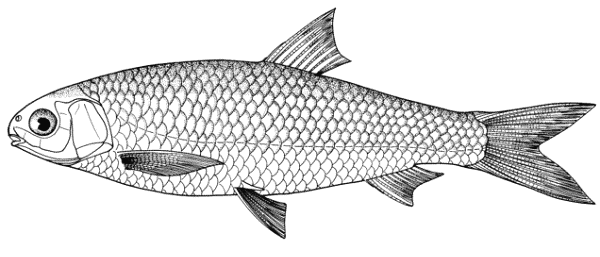
Common names
None.
[waspi or common chilwa in Pakistan].
Systematics
No relevant synonyms. This species was originally described from
the Yamuna and Tista rivers, India. No types are known (Eschmeyer et al., 1996).
Key characters
The suborbital ring of bones is large and distinctive, being almost
as deep as the eye, and this feature is unique in southeastern Iranian cyprinids.
Morphology
The snout is short and rounded and overlaps the upper lip. The
mouth is small, ventral and transverse. The lower jaw is straight with
a slightly horny cutting edge and no lip. The dorsal fin origin is
over or slightly behind the pelvic fin origin. The dorsal fin margin
is straight to very slightly emarginate and the anal fin is emarginate.
Dorsal fin unbranched rays 2-3 (the first unbranched ray is very
small, usually 3 rays are present but not discernible) and branched
rays 6-8, anal fin unbranched rays 2 and branched rays 8-10, pectoral
branched rays 9-16, usually 12 or more, and pelvic fin branched rays 7-8. Lateral line
scales 36-45. Scales have few anterior and more numerous but not many
posterior radii. There is a pelvic axillary scale and several elongate
and overlapping scales in the pectoral axil. Gill rakers are very
short, not touching the adjacent one when appressed, difficult to
count at the fleshy ends of each arch, and numbering about 17-25.
Pharyngeal teeth 2,4,5-5,4,2 in the literature but the main row count
of 4 teeth observed here differs. The main row teeth have large, oval
to oblong flattened crowns. The gut is a very elongate s-shape with a
small anterior loop. Total vertebrae 36-37. The chromosome number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin branched
rays 6(1) or 7(18); anal fin branched rays 8(4) or 9(15); pectoral fin
branched rays 9(1), 12(5), 13(11) or 14(2); pelvic fin branched rays
7(19); lateral line scales 36(1), 37(4), 38(1), 39(5), 40(4), 41(2),
42(1) or 45(1); scales above the lateral line 7(10) or 8(9); scales
below the lateral line to the anal fin 3(1), 4(12) or 5(6); scales
between the lateral line and the pelvic fin 4(13), 5(5) or 6(1);
predorsal scales 17(1), 18(3), 19(2), 20(2), 21(3), 22(3), 23(2) or
24(2); caudal peduncle scales 15(1), 16(5), 17(6), 18(5), 19(1); total
gill rakers 17(1), 18(2), 19(2), 21(2), 22(4), 23(2) or 25(1);
pharyngeal tooth count 2,4,4-4,4,2(5), 2,4,4-4,4,1(1), or
2,3,4-4,4,2(1), and total vertebrae 36(3) or 37(9).
Sexual dimorphism
Unknown.
Colour
Back light brown to brown-green with the flanks very silvery to
silvery-yellow and the belly lighter. There is a golden stripe along
the flank. Fins are a distinct dark yellow. The caudal fin may be yellow to orange
and paired fins a very light orange-yellow. Preserved fish have
immaculate fins except for the caudal fin which has some melanophores
lining the rays, a broad stripe along the midline of the back, and
fine melanophores on the back and upper flank. Some fish have small,
dark dots on the back and upper flank. The peritoneum is black.
Size
Attains 20 cm (Malhotra and Munshi, 1985).
Distribution
This species is reported from the Makran and Mashkid River basins in Pakistan (Mirza, 1992) and
eastwards to Thailand. The Iranian distribution encompasses the
Mashkel (=
Hamun-e Mashkid) and Makran basins, the latter westwards to the Straits of
Hormuz (Kiabi and Abdoli, 2000).
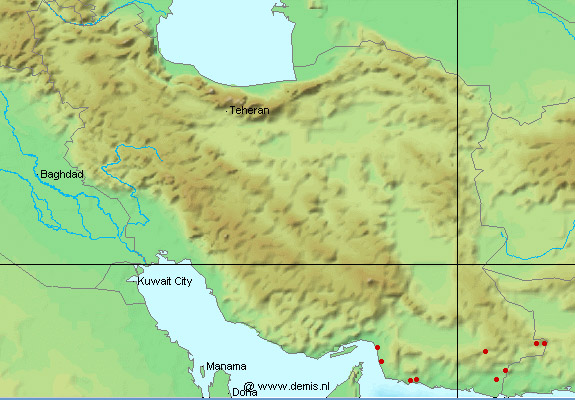
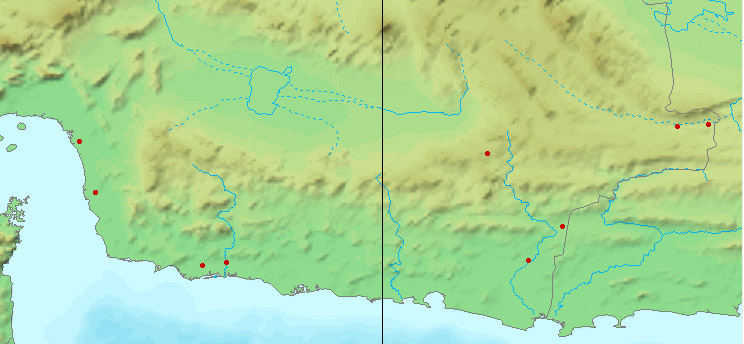
Zoogeography
The species and genus reaches its westernmost limit of distribution
in southeastern Iran. Barriers to further dispersal are unknown but it
may be limited by temperature, habitat availability and poor recent
connections between streams in the Makran and the southern deserts of Iran.
Habitat
This species favours streams with slow current.
Age and growth
A female, 9.8 cm total length, from Iran had mature eggs (Berg, 1949).
Food
This minnow is a carni-omnivore and a voracious feeder (Bhattacharjee
and Dasgupta, 1988). Iranian specimens contained no discernible food
items in their guts.
Reproduction
Spawning occurs from February to April in India (Malhotra and
Munshi, 1985), Iranian specimens caught in December were not mature
suggesting a later spawning season.
Parasites and predators
Jalali et al. (2000) describe two new species of monogeneans,
Dactylogyrus yousefpouri and D. mobedii, from this
species in the Bahu Kalat River of Baluchestan.
Economic importance
Not of any economic importance in Iran but it is eaten in India.
Conservation
Although known from only a few localities in southeastern Iran,
this species may not be threatened other than by water abstraction and pollution.
Further work
The biology of this species, which is at its westernmost range
limit in Iran, is unknown. There are some minor differences in
characters with literature reports, particularly in pharyngeal tooth
count, but sample sizes do not permit an adequate comparison for this
wide-ranging species.
Sources
Iranian material: CMNFI 1979-0316, 1, 22.1 mm standard length, Baluchestan,
stream in Sarbaz River drainage (26º48'N, 61º02'E);CMNFI 1979-0322, 7, 42.3-86.3
mm standard length, Baluchestan, Dashtiari River (ca. 25º45'N, ca. 61º26'E);
CMNFI 1979-0333, 7, 17.7-69.5 mm standard length, Baluchestan, Mashkid River
(ca. 27º05'N, ca. 63º12'E); CMNFI 1979-0334, 10, 22.8-62.0 mm standard length,
Baluchestan, Mashkid River (27º04'N, 62º54'E): OSU 8123, 5, 45.7-50.6 mm
standard length, Baluchestan, Srabaz River (no other locality data).
Comparative material: BC55-61, 2, 67.0-68.2 mm standard length,
India, Barakar River near Tillya Dam (no other locality data).
Genus Aspiolucius
Berg, 1907
Aspiolocius esocinus
(Kessler, 1874)
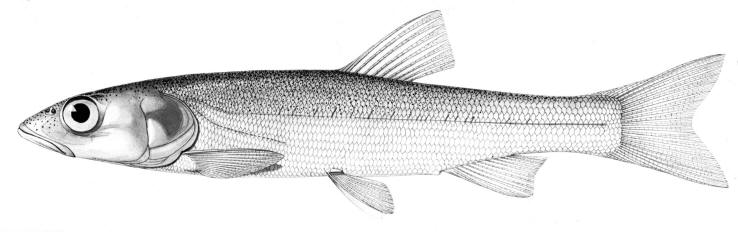
Recorded from the Karakum Canal in Turkmenistan (Sal'nikov, 1995)
and may eventually be found in the Tedzhen (= Hari) River basin of
Iran. No Iranian record.
Genus Aspius
Agassiz, 1832
The asps comprise 2 species found in Europe and Southwest Asia.
Both species are found in Iran.
This genus is characterised by an elongate, rounded and large body,
small scales, a large mouth with the lower jaw projecting, lower jaw
with a tubercle fitting into a notch in the upper jaw, no barbels,
pharyngeal teeth in 2 rows, pointed and hooked, gill rakers short and
wide apart, short dorsal fin without a thickened ray, a long anal fin,
a scaled keel behind the pelvic fins, and gill slits very wide such
that the branchiostegal membranes attach under the posterior end of the eye.
Perea et al.
(2010) using mitochondrial and nuclear DNA propose the synonymy of this genus
with Leuciscus
Cuvier, 1816.
Aspius aspius
(Linnaeus, 1758)
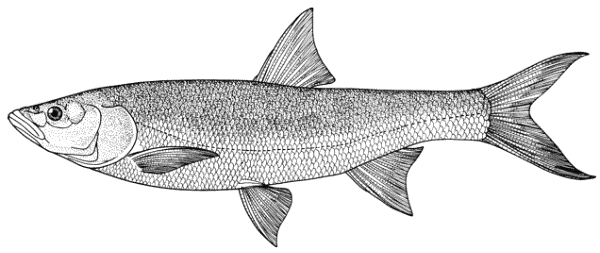
Common names
ماش ماهي
(= mash mahi, not apparently meaning pea fish as the Farsi could indicate), khasham.
[hasam or khasham in Azerbaijan; krasnogubyi zherekh or redlip asp
in Russian; Caspian asp, South Caspian asp].
Systematics
Cyprinus Aspius was described originally from lakes of Sweden.
Cyprinus Rapax Leske, 1774 described from Leipzig, Germany, Cyprinus
taeniatus Eichwald, 1831 described from the Kura River at
Mingechaur, Aspius erytrostomus Kessler, 1877 (sic,
sometimes spelt erythrostomus or erithrostomus)
described in part from the Caspian Sea and Kura River, Azerbaijan and
from the Aral Sea and lower part of the Amu Darya, Uzbekistan, and Aspius
transcaucasicus Warpakhovskii, 1895 from the Lenkoran River and
Lake Bussadagny, Azerbaijan, are synonyms. Aspius aspius taeniatus
(Eichwald, 1831) is the subspecies found in the Caspian Sea.
The types of Cyprinus aspius described from Swedish lakes
are unknown (Eschmeyer et al., 1996).
Eschmeyer et al. (1996) give Aspius transcaucasicus
Varpakhovskii, 1896, although Berg (1948-1949) gives 1895; possibly
the volume year is 1895 but the work did not appear until 1896.
Varpakhovskii is a variant spelling in transliteration from the
Russian. Syntypes of this synonym are in the Zoological Institute, St.
Petersburg under ZISP 10488 (2) and ZISP 10497-48 (sic, in
Eschmeyer et al. (1996) but should read 10497-98 with 5 and 2
specimens respectively (Kottelat, 1997)).
Key characters
The subspecies of the southern Caspian Sea is distinguished from
the type subspecies of Europe and the northern Caspian Sea since the
latter has lower lateral line scale counts of 64-76 as opposed to
62-105, lips never bright red, anal fin branched rays usually 13
instead of 12 (but see Iranian fish below), and height of dorsal fin
usually longer than distance from snout tip to posterior edge of
preopercle. Characters of the genus and distribution serve to separate it from
other cyprinids in Iran.
Morphology
Dorsal fin branched rays 7-10, usually 8, after 2-3, usually 3,
unbranched rays, and anal fin branched rays 11-16, usually 12 (but see below), after
3-4, usually 3, unbranched rays, pectoral fin branched rays 14-17 and pelvic fin
rays 7-9. Lateral line scales 62-105. The
scales have a central focus, fine circuli and few posterior and
anterior radii. There is a pelvic axillary scale. There is a scaled
keel behind the pelvic fins. The lower jaw tip projects and fits into
a notch in the upper jaw. Gill membranes are narrowly attached to the
isthmus, almost under the posterior eye margin. Gill rakers 8-11, very
short and club-shaped, almost reaching or not reaching half way to the
raker below when appressed. Pharyngeal teeth usually 3,5-5,3,
sometimes 2,5-5,3 or with 6 teeth in the main row, teeth elongate,
compressed and obviously hooked. Gut an elongate s-shape. Vertebrae
49-51. The chromosome number is 2n=50-52 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin branched rays 8(6); anal fin branched
rays 13(6); pectoral fin branched rays 18(3); pelvic fin branched rays
8(3); lateral line scales 68(1), 72(2), 73(1), 74(1) or 75(2); total
gill rakers 8(1) or 9(2); pharyngeal teeth 3,5-5,3(3); and total vertebrae 50(2) or 51(1).
Sexual dimorphism
Unknown.
Colour
The overall colour is silvery with the back a blackish-olive or
greenish-grey. The iris is silvery with a narrow golden circle around
the pupil and a little grey pigment on the upper half. Lips are
silvery with a little grey over the upper one. Both lips and iris are
often bright red. The dorsal and caudal fins are grey and the other
fins are transparent without pigment. Fins may be tinged reddish.
Peritoneum silvery to brown.
Size
Reportedly attains 1.2 m and 20.0 kg, possibly over 30.0 kg. The largest of 12,000 fish
from the lower Kura River was 77 cm total length, males averaged 61 cm
and females 64 cm. The average weight of 105,500 fish caught in
1927-1929 was 2.72 kg, females 2.93 kg (based on 1500 fish), males
2.34 kg and the heaviest fish was 5.5 kg (Berg, 1948-1949).
Distribution
Found from the Rhine and north of the Alps in Europe to the
drainages of the Black, Caspian and Aral seas including their southern shores.
This species has been reported from Astara to Gorgan Bay in rivers and
marshes and in the Caspian Sea of Iran (Nedoshivin and Iljin, 1929; Derzhavin,
1934; Berg, 1948-1949; Abbasi et al., 1999; Kiabi et al.,
1999; Abdoli, 2000; Abdoli and Naderi, 2009). Formerly reported from the Anzali Mordab but no longer present
(Holčík and Oláh, 1992) although reported from the Siah-Keshim Protected Region of
the Anzali Mordab by (1996). Found also in the Aras River Dam (Jolodar and Abdoli, 2004).
Also recorded from the Uzboi lakes, Karakum Canal and Kopetdag
Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov,
1995) and may eventually appear in the Tedzhen (= Hari) River basin in Iran.
Zoogeography
The closest relative of this species lies to the south and indicates a
connection between Euro-Mediterranean and/or Black-Caspian-Aral seas basins.
Habitat
In the waters of Dagestan, asp begin to migrate upriver in October,
peaking at the end of November and the beginning of December. They
overwinter in deep holes, emerging in early spring as rivers flood and
move to the spawning grounds. These grounds include river channels,
open lake areas with substantial flow and only rarely places weakly
overgrown with very coarse submerged vegetation such as reeds and
rushes. After spawning the asps return to the Caspian Sea (Shikhshabekov,
1979). Knipovich (1921) reports this species from depths of 14.6-16.5
m, and possibly deeper, in the Iranian Caspian Sea. Riazi (1996)
reports that this species is native (resident) to the Siah-Keshim
Protected Region of the Anzali Mordab.
Age and growth
Life span in the Volga delta is 7-8 years with the bulk of the
population mature at 6 years (Ali, 1974). In the waters of Dagestan
life span is 8 years with maturity at 4 years. Mature males and
females are 41-58 cm long and weigh 840-2800 g (Shikhshabekov, 1979).
Growth is more rapid in the Kura River of Azerbaijan than in other
rivers in the former Soviet Union. Fish taken from commercial catches
in Iran are mostly 3-6 years old, 38.1-56.7 cm long and weigh 631-2241
g (Razivi et al., 1972) or 3-6 years and 33-63 cm total length (Holčík
and Oláh, 1992). Growth is rapid in the latter report, fish reaching
1 kg during the fourth year of life. Maximum life span may be 15 years.
Food
This species is a solitary predator on other fishes such as gobies
(Gobiidae) and silversides (Atherinidae), frogs and even ducklings. An
Iranian specimen had the remains of a large crustacean in its gut.
Young feed on plankton initially but start to take the fry of fishes
at 2-3 months. There is little feeding on the spawning migration.
It may catch other fishes by plunging into shoals at the surface
and may leap out of the water as a result. Abdoli (2000) reports Scardinius
erythrophthalmus, Atherina boyeri and Blicca bjoerkna as food items
in Iran. Surface insects are also eaten.
Reproduction
The spawning season in Gilan is mid-February to late March at 10-13°C
with an incubation period of 9-10 days (Hoseinie, 1995).
Spawning is non-intermittent and the period is short (10-15 days)
in Dagestan (Shikhshabekov, 1979). Fecundity reaches 483,500 eggs in
the south Caspian Sea and maximum egg diameter in the Volga delta is
1.7 mm (Ali, 1974). In Hoseinie's (1995) study of artificial
propagation of this species in Iran, large or swollen eggs number
117-277 per gram, and egg diameters 2.0-2.2 mm. Absolute fecundity
reaches 264,248 eggs. Abdurakhmanov (1962) gives a maximum fecundity
of 342,000 eggs and a maximum egg diameter of 2.4 mm for Azerbaijan
populations. Females with ripe eggs are found between mid-April and
mid-May at water temperatures of 4-12.2°C, optimally 9-11°C.
Up to 20% of Volga asp females do not spawn annually. Eggs develop
while between or adhering to stones on the river bed. Young migrate
downriver from June to August at age 3-4 months and 5-10 cm length.
Parasites and predators
Molnár and Jalali (1992) record the monogenean Dactylogyrus
tuba from this species in the Safid Rud. Masoumian et al. (2005)
report the protozoan parasite Chilodonella, sp. from this species in
the Aras Dam in West Azarbayjan. Masoumian et al. (2002) investigated
parasites from this fish in the Aras and Mahabad dams in northwest Iran and
found the protozoan Myxobolus dispar. Sattari (2004) records the presence
of the nematode, Eustrongylides excisus, in the body cavity. This
parasite can damage muscles in commercial species and render them unsuitable for
sale.
Sattari et al. (2002, 2004, 2005) and Sattari (2004)
records the presence of the nematode, Eustrongylides excisus. This
parasite can damage muscles in commercial species and render them unsuitable for
sale. Pazooki et al. (2007) recorded various parasites from localities in West
Azarbayjan Province, including Argulus foliaceus from this species.
The Caspian seal, Pusa caspica, is a predator (Krylov, 1984).
Economic importance
This fish is taken in Iran as food but comprises only a small
portion of the catch. Nevraev (1929) reports catches of 267 to 2429
fish for the period 1914-1915 to 1917-1918 in the Anzali region. Holčík and
Oláh (1992) record the catch in the Anzali region for 1969-1970
and 1970-1971 as 45.2 t and 36.1 t respectively, these being 84% and
69% of the total Iranian catch. In 1921-1930 the annual catch in the
lower Kura River averaged 249,000 fish and in 1936 for Azerbaijan the
catch weighed 8100 centners and numbered 300,000 fish.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and in sport. The
flesh is white and tasty but rather tough.
Conservation
Recruitment in this species is low in Iran because water is taken
from the summer spawning streams for irrigation purposes. Spawning
success is therefore limited. Larvae of spring spawners are lost when
they enter irrigation channels and become stranded in fields (Razivi et
al., 1972). Holčík and Oláh (1992) consider the decline in this species to be due to
indiscriminate catching of sexually immature fish and, in the Anzali
Mordab at least, environmental changes. The Pol-e Astaneh Fish Farm
has studied propagation of this species (Keivany and Nasrollahzadeh,
1990) and Hoseinie (1995) demonstrates that artificial propagation is
possible. It has also been raised to marketable size
in ponds through artificial feeding with ground kilka and a rice
product (Annual Bulletin 1993-94, Iranian Fisheries Research and
Training Organization, Tehran, p. 81-82, 1995). The Shahid Beheshti hatchery
on the Safid River breeds this species (Raymakers, 2002).The asp is bred in
the Varvarinsk Hatchery and releases up to 1.5 million yearlings are
made into the Kura River, with plans for 8-10 million releases (Kosarev
and Yablonskaya, 1994).
Lelek (1987) classifies this species as vulnerable to endangered in
Europe. Vulnerable in Turkey (Fricke et al., 2007). Kiabi et al. (1999) consider this species to be data
deficient in the south Caspian Sea basin according to IUCN criteria.
Criteria include commercial fishing, habitat destruction, limited
range (less than 25% of water bodies), absent in other water bodies in
Iran, and absent outside the Caspian Sea basin.
Further work
The distribution and abundance of this species in Iranian waters needs
investigation as it is sensitive to environmental changes.
Sources
Iranian material: CMNFI 1970-0526, 2, 236.8-246.1 mm standard length, Gilan,
Safid River below Astaneh (37º19'N, 49º57'30"E); CMNFI 1980-0494, 1, 319.6 mm
standard length, ? Gilan, Caspian Sea basin (no other locality data); ZISP 3917,
1, 402.0 mm standard length, Gilan, Anzali (no other locality data).
Aspius vorax
Heckel, 1843
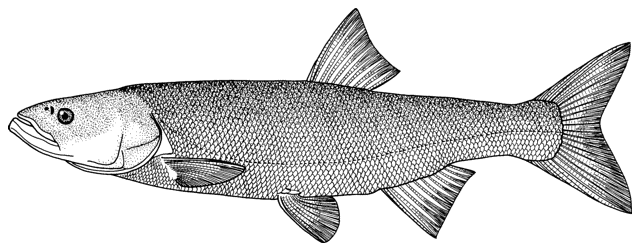
Common names
shelej, shalaj, sholge, sholgeh.
[shillik, shillig, shiliq, shelej, shalaj; bu aliawi, abu elawi; called
"snake" by American soldiers in Iraq because of the name asp being familiar as
the snake that killed Cleopatra; kaschschasch (= voracious) from Heckel (1843b); all in Arabic;
Tigris asp].
Systematics
The type locality for this species is the "Tigris bei Mossul"
according to Heckel (1843b). Krupp (1985c) reports, and I have examined,
a syntype held in the Naturhistorisches Museum Wien under NMW 76776, 261.4 mm standard length.
The catalogue in Vienna in 1997 also lists
NMW 76785 as a type and this specimen is also 261.4 mm standard length. Eschmeyer et al.
(1996) lists a dried skin as a syntype under NMW 16527. The catalogue in Vienna lists 4 fish in
spirits and 2 fish stuffed.
Banister (1980) suggests that this species may be close to Aspius
aspius, perhaps a clinal variant, since the Caspian Sea basin
subspecies, A. a. taeniatus (67-90) has scale counts
intermediate between European populations of A. aspius (65-74)
and A. vorax (93-105) (Banister's figures). However this may be
more apparent than real as there is considerable overlap and frequency
distributions are not given. There was insufficient material on hand
from Iran to investigate this character in more detail.
Key characters
Characters of the genus coupled with distribution serve to identify this species.
Morphology
The head is long and tapers anteriorly. The mouth is oblique and
elongate reaching to the anterior half of the eye. The lower jaw
projects and has a symphysis knob fitting into an upper jaw notch.
There is a hump as the back rises abruptly after the head. The gill
opening is large and extends forward to the posterior eye margin
level. Fins are more falcate than in the line illustration when partially collapsed.
Dorsal fin with 2-3 unbranched and 7-9, usually 8, branched rays. Al-Nasiri
et al. (1975) give a range of 8-11 (probably 7-10 using my system of
counting) dorsal fin rays with a strong mode at 9 (i.e. 8) for 271 fish taken
from the Basrah fish market from January to June. Anal fin with 2-3 unbranched and 9-13 branched rays.
Al-Nasiri et al. (1975) give a range of 10-13 (9-12, 10 modally but high
frequencies at 11 too. Pectoral fin branched rays 16-18 (14-18, modally 16, in Al-Nasiri et
al. (1975)), pelvic fin branched rays 8-9, usually 8. Lateral line scales
82-110, lateral line low on the flank anteriorly, rising to the
midline of the caudal peduncle. There is a pelvic axillary scale.
Scales have a few radii on the posterior field only, a central focus
and numerous, fine, concentric circuli. Pharyngeal teeth 3,5-5,3 with
variants 2,5-5,3 and 2,5-5,2, long, compressed and hooked at the tip.
Gill rakers 9-14, reaching base of adjacent raker when appressed but
widely spaced and not developed anteriorly. Some rakers do reach the
adjacent one when appressed in some fish. Al-Nasiri et al. (1975)
give a range of 11-13 gill rakers with a strong mode at 12. Total vertebrae
51-53 (Al-Nasiri et al. (1975) give 37 as a count which cannot be
reconciled with my counts). The gut is an elongate s-shape.
Meristic values for Iranian specimens are:- dorsal fin branched rays
8(4); anal fin branched rays 10(1) or 11(3); pectoral fin branched
rays 16(1) or 17(3); pelvic fin branched rays 8(4); lateral line
scales 96(1), 98(1) or 100(1); total gill rakers 11(1), 12(2) or 13(1); pharyngeal
teeth 3,5-5,3(3); and total vertebrae 51(3) or 53(1).
Sexual dimorphism
Unknown.
Colour
The back is greenish to blackish but overall colour is silvery-grey
or silvery-white. Fins are said to be all pale yellow in live fish but are dark in some
preserved specimens. A photograph of one freshly caught specimen showed reddish
pectoral, pelvic and anal fins, with the dorsal fin greenish, similar to the
back and flanks. Another freshly caught specimen was overall silvery, with a
brownish-green back, fins overall grey with some yellowish tinges The peritoneum is black to brown.
Size
Reaches over 55.0 cm total length and 6.0 kg in Iraq (van den Eelaart,
1954; Herzog, 1967; Shafi and Jasim, 1982; Bartel et al., 1986) and 1.5 m and 60 kg in the
Euphrates (Gruvel, 1931; if identification is correct). The Suq al-Shouykh
Marsh in April 2005 contained specimens larger than 65.0 cm (www.iraqmarshes.org, downloaded 29 August 2005)
and fish in Baghdad palace ponds were estimated to reach 36-40 inches (91-1.02 m) and 15-20 pounds (6.8-9.1 kg)
(http://members.cox.net/flybox/FishingUpdate.htm, downloaded 9 January 2006).
Distribution
This species is found in the Tigris-Euphrates and the Orontes River basins in
the Middle East. In Iran it is recorded from the lower reaches of rivers in the Tigris River
basin including the Bahmanshir River and also such marshes as the Hawr al Azim (Marammazi, 1995).
Zoogeography
This is one of several species that has a sister taxon in the
Euro-Mediterranean and/or Black-Caspian-Aral seas basin, indicating north-south
connections in the past.
Habitat
van den Eelaart (1954) studied this species in Iraq and found that it lives
in rivers, lakes and marshes in both open and vegetated areas and remains in
shallow water even in summer. It also occurs in smaller water bodies such as
ponds. From spring to fall it is found mainly in marshes
and lakes. The barrages at Hindiyah and Kut blocked the upstream migration of
this species (Mahdi, 1962). Lakes at Camp Slayer in Baghdad contain this species
and, in the shallows, the larger fish chase smaller fish and smaller species
leaving v-shaped wakes with the tail fin exposed. Smaller fish leap out of the
water to escape the shillik (http://members.cox.net/flybox/FishingUpdate.htm,
downloaded 9 January 2006).
Age and growth
Shafi and Jasim (1982) made observations on the biology of this cyprinid in
Habbaniyah Reservoir, Iraq. They report 8 age groups with most rapid growth in summer
months when water temperatures are above 25°C. Growth in weight is about 160.1 g
per year to the fourth year of life and about 331 g per year afterwards.
Condition factor was 0.74-1.18 with a mean of 1.0, stable values probably
related to piscivory. The length-weight relationship was W = 0.0123 x
TL3.0601. The von Bertalanffy equation for growth was lt = 91.0[1-e-0.122
(t-0.25)]. Ali et al. (1986) found the condition factor to range from
0.05 to 1.09 (mean 0.73) and also gave the chemical composition and calorific
value. This species had a higher fat content than Barbus (=
Carasobarbus) luteus with
which it was studied. Al-Dabical and Al-Daham (1995) studied growth in the first
year of life in fish from the Shatt al Basrah Canal, Iraq and gave the length-weight
relationship as loge W = -12.458 + 3.077 loge L and the
growth equation as Lt = 104.118 (1-e -0.0121 (t - 87.871)).
Epler et al. (2001) found the oldest age groups to be 5+, 6+ and 7+ in
Iraqi lakes Razzazah, Habbaniyah and Tharthar respectively. The mean condition factor
was 0.88, 0.76 and 0.87 in lakes Habbaniyah, Tharthar and Razzazah respectively.
The von Bertalanffy parameters were for Lake Tharthar L∞ (cm) =
145.5, K = 0.0803, t0 = -0.3269, W∞ (g) = 32099 and n =
3.2249. These indicate rather uniform growth rates, as L∞ is
relatively high and K very low. Results were considered more reliable than an
earlier study by Jasim (1980) which used inappropriate methods. Annual survival
in Lake Tharthar for fish 2.6-5.5 years was 62.0% (Szczerbowski et al.,
2001). Productivity was low based on chemical and limnological studies, limiting fish production.
Food
This minnow is piscivorous, feeding almost entirely on fish when adult
according to Iraqi studies (Shafi
and Jasim, 1982), although aufwuchs may also be found in gut contents. It is
mainly a mid-water and benthic feeder with limited predation on surface water
organisms (Hussein and Al-Kanaani, 1991). Hussein et al. (1991) examined
diet in the Garma Marshes, Iraq and found aquatic insects and crustaceans to be
important in young shillig in both summer and winter, with molluscs and fish
less important. Even in large shillig, fish were outranked by aquatic insects
and in winter by crustaceans as well. Molluscs were a minor food. Shillig
rejected certain molluscs while taking others, attributed to variations in shell
thickness and a attachment strength to substrates. Liza abu is an
important food fish (Al-Shamma'a and Jasim, 1993). Hussein and Al-Kanaani (1989;
1991; 1993) examined the diet of this species in the Al-Hammar Marsh and found a
gradually reduced feeding intensity towards the winter months, a highest
fullness index in May and lowest in January, and a diet governed by food
accessibility and availability. Crustaceans, fish and aquatic insects are the
main food items in descending order of importance, with fish most important when
using a percentage ranking index in large shillig and even in small shillik by
volume. Benthic molluscs were the third most important food for young shillik
after crustaceans and fish. In a study of the recovering Hammar Marsh, Iraq, diet was
80.0% fish and 20.0% insects, in the Hawr al Hawizah 47.4% fish and 29.4% insects
with shrimps, other crustaceans, algae, diatoms, plants and snails at less than
10% each, and in the Al Kaba'ish (= Chabaish) Marsh 73.0% fish and 16.8% insects with shrimps,
other crustaceans, algae and plants at less than 10% each (Hussain et al.,
2006). Fish are the main diet item of large shillik and
there is a gradual shift from small- to large-sized prey as the shillik grows (Salman
et al., 1994). Frogs, molluscs and aquatic plants and algae were also
found in stomach contents, with frogs being important to large shillik in terms
of prey volume. Plants may be accidental inclusions taken when seizing prey in
weed beds. The fish eaten in descending order of importance were Liza abu,
Gambusia affinis (sic, probably G. holbrooki), Garra
rufa and Cyprinus carpio. The main crustacean eaten was
Metapenaeus affinis along with decapods and amphipods. The gill rakers are
widely spaced, indicative of a piscivorous diet (Salman et al., 1993) and
the gut is a short s-shape, about equal to fish standard length, also indicative
of a piscivorous diet (Salman et al., 1994). Hussain and Ali (2006)
examined feeding relationships among fishes in the Al-Hammar Marsh and found
this species to be a carnivore, 41.9% of the diet being crustaceans, 10.0%
insects and 34.1% fishes. Epler et al. (2001) studied the diet of this
species in Lake Tharthar, Iraq and found year old shillik to be eating oligochaetes,
tendipedids and plants material with only fish in 2-7 year old shillik. Dietary
coincidence with bizz was high in Lake Tharthar, 96.1%.
Reproduction
Shafi and Jasim (1982) record possible spawning in January at 10°C in Iraq with a
fecundity up to 74,509 eggs, a mean of 1157 eggs/g body weight and egg diameter
of about 1.1 mm. van den Eelaart (1954) found this species in deep parts of
Iraqi rivers in December-January, entering marshes and lakes in February to spawn at
the end of February and the beginning of March. Spawning takes place on gravel
beds, the same as those used by Barbus (= Luciobarbus) xanthopterus, but also on plants.
Epler et al. (2001) studied reproduction in Iraqi lakes Tharthar and Habbaniyah
and found males to achieve maturity in the third year of life at 44.2 cm and
females in the fourth at47.2 cm. Spawning occurred in February at 13-14ºC.
Fecundity was 92,000 eggs/kg body mass.
Parasites and predators
Jalali and Molnár (1990a) record the monogeneans Dactylogyrus
pulcher and D. mokhayeri from this species in the Dez River. Moghainemi and Abbasi
(1992) record a wide range of parasites from this species in the Hawr
al-Azim in Khuzestan. Mortazaei et al. (2000) record an
infection rate of 6.6% with the worm Neoechinorhynchus tylosuri
in Khuzestan marshes. Farahnak (2000) and Farahnak et al. (2002) record
Contracaecum sp. and Anisakis sp. from this fish in Khuzestan Province.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish. It is eaten by Silurus triostegus.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Argulus sp.,
Ergasilus sp., Ergasilus sieboldi, Lernaea sp., Lamproglena sp. and
Lamproglena compacta on this species.
Economic importance
Sharma (1980) reports that shillik were an important fish species at the
Basrah, Iraq fish market, accounting for 68,948 kg from October 1975 to June 1977,
although this is an order of magnitude less than for the three most important
species. Its potential for fish farming may be limited by its small gill area
which makes it unfit to maintain gas exchange in oxygen-poor water (Salman et
al., 1991). Kassim et al. (1998) found locally-raised Scenedesmus
acutus algal cultures at 0.5*106 cell/ml with baker's yeast at
0.05 g/L to be the best formula for raising the rotifer Brachionus
calcyflorus as live food for shillik larvae. Growth rate was, however,
higher on an artificial diet of boiled eggs and soybean meal (52%) compared to
48%, in contrast to common carp (q.v.).
van den Eelaart (1954) gave the fishing season in Iraq for this species as
December-February (peaking in January) and February and June-November (peaking
in February and July-August).
Foreign soldiers in Iraq during 2005 regularly caught this species on angling
gear using spoons and streamer flies, e.g. www.carpecapio.com, downloaded 26 August 2005.
Conservation
Few specimens have been caught in Iran and deposited in
museums. This may reflect rarity or inadequate collection methods. It was
commonly caught by American soldiers in Iraq in 2004 as evidenced by emailed
photographs sent to me for identification and is an important food fish in Iraq.
Detailed surveys using appropriate equipment are needed to assess its
distribution and status in Iran.
Vulnerable in Turkey (Fricke et al., 2007).
Further work
Its distribution and status in Iran need so be studied as does its distinction from Aspius aspius.
Sources
Scale counts were taken also from Banister (1980).
Type material: See above (NMW 76776 and NMW 76785).
Iranian material: ZMH 2516, 259.9 mm standard length, Kermanshahan, Karasu-Gamasiab-Seymarreh (no
further locality data); uncatalogued, 3, 105.6-282.5 mm standard length,
Khuzestan, Hawr al Azim and Dez River, (no further locality data).
Comparative material: NMW 91020, 1, 170.6 mm standard length, Iraq, Shatt-al-Arab, Basrah
(30°30'N, 47°47'E); BM(NH) 1920.3.3:127-146, 28, 69.8-284.7 mm standard length, Iraq, Basrah (30°30'N, 47°47'E); BM(NH) 1920.10.8:1, 1, 182.3 mm
standard length, Iraq, Tigris River (no other locality data); BM(NH)
1931.12.21:11, 1, 250.2 mm standard length, Iraq, Mosul (36°20'N, 43°08'E); BM(NH) 1972.3.16:1, 1,
112.1 mm standard length, Iraq, Dokan Lake (no other locality data); BM(NH) 1973.5.21:189-190, 2,
166.2-192.0 mm standard length, Iraq, Shatt-al-Arab (no other locality data); FMNH 51242, 1, 322.6 mm standard. length, Iraq, Halfaya
east of Amara (31°49'N, 47°26'E); uncatalogued, 1, 200.8 mm standard length, Iraq, Hawr al Hammar
(no other locality data). BM(NH) 1968.12.13:182, 1, 251.7 mm standard length, Syria, Cheria
River, tributary to the Orontes River (no other locality data); NMW 90366, 1, 309.0 mm standard length,
Turkey, Cermik on the Euphrates River (39°09'N, 39°27'E); NMW 90807, 1, 214.8 mm standard length, Turkey, Devegeçidi Çayi,
Tigris River basin (no other locality data);
Genus Barbus
Cuvier and Cloquet, 1816
The barbels, genus Barbus sensu lato, are found in Europe, Southwest Asia and Africa and comprise about 800 species with 15
formerly recognised in Iran. Only a single species is now assigned to this
genus.
This genus included a wide variety of species and was something of a catchall, serving to cover groups of species which have not been satisfactorily defined as distinct genera to general acceptance.
Some authors recognise genera not recognised by others or regard these genera as subgenera - this necessarily affects the species count above. Characters in Southwest Asian species include a rounded
or compressed body of moderate to very large size, large to very small scales (lateral line scale count range is at least 26-103), no scale sheath around the anal fin, scales have moderate to high
numbers of radii and numerous fine circuli, the presence of barbels in most species, usually 2 pairs, often 1 pair and sometimes none (and individually variable within species), lips variably developed
from thin to thick and fleshy, the lower lip sometimes with a well-developed median lobe (and lip development individually variable within species), the last unbranched ray in the short dorsal fin
(usually 7-8 branched rays but sometimes more) is thickened and spine-like and may bear teeth or be smooth, a short anal fin, usually with 5 branched rays (but some have 6), pharyngeal teeth in 3 rows
with hooked or spoon-shaped tips but sometimes heavy and massive or molariform, gut short, peritoneum white to brown or black, and colour usually brown without distinctive markings in the form of
stripes, bands or spots (Luciobarbus subquincunciatus is an exception).
Bănărescu and Bogutskaya in Bănărescu and Bogutskaya (2003) restrict Barbus to tetraploid species with scales having divergent striae. These species have 7-8, occasionally
9, branched dorsal fin rays, 5 branched anal fin rays, papillose lips and two pairs of barbels. This then excludes species placed in Carasobarbus, Kosswigobarbus,
Mesopotamichthys
and Tor (see below). Two groups of species can be distinguished in this restricted Barbus according to
Bănărescu and Bogutskaya in Bănărescu and Bogutskaya (2003), namely those with 5 pharyngeal teeth in the main row and a papillose lower lip separated from the chin by a groove and
those with 4 pharyngeal teeth in the main row and a lower lip without papillae and continuous with the chin, this latter group being formerly recognised as the genus Luciobarbus Heckel, 1843. The European/Caucasian member(s) of Barbus
sensu.stricto in Iran is lacerta and of Luciobarbus (treated as a subgenus in Bănărescu and Bogutskaya in
Bănărescu and Bogutskaya (2003)) are brachycephalus and capito.
Berrebi and Tsigenopoulos in Bănărescu and Bogutskaya (2003) and
Tsigenopoulos et al. (2003) review Barbus using molecular markers. They include Barbus cyri (a subspecies of B. lacerta
according to some authors) and B. lacerta in the subgenus Barbus, their Northern Mediterranean Group, and B. brachycephalus, capito, esocinus,
longiceps, mursa, mystaceus, pectoralis, rajanourum, subquincunciatus, xanthopterus and probably barbulus, kersin, sheich and
scincus in the subgenus Luciobarbus, their Southern Group. Levin (2004) studied phenetic relationships of 7 Caucasian taxa and concurred with the division into Barbus and
Luciobarbus. See under the species Kosswigobarbus kosswigi for a discussion about the genus/subgenus Kosswigobarbus.
The genus Barbus sensu lato Cuvier and Cloquet, 1816 has been split
into a number of genera which are now finding general acceptance. Names used in Southwest Asia include Tor Gray, 1834 sensu Karaman, 1971, Labeobarbus Rüppell, 1836,
Systomus McClelland, 1838, Luciobarbus Heckel, 1843, Barynotus Günther, 1868 (preoccupied), Aspiobarbus Berg, 1932, Bertinius Fang, 1943 (and Bertinus
Banister, 1980, a misspelling), Bertinichthys Whitley, 1953 (an unneeded replacement of Bertinius), Mesopotamichthys Karaman, 1971, Carasobarbus Karaman, 1971 and
Kosswigobarbus Karaman, 1971. Labeobarbus is generally considered to be a synonym of Tor, species of which are found mostly in the Oriental Realm, with only
Tor grypus in Iran being a member of the genus Tor (Karaman, 1971; Ekmekçi and Banarescu, 1998). Bertinius is regarded as a synonym of Luciobarbus in Bănărescu
and Bogutskaya in Bănărescu and Bogutskaya (2003). A summary table of generic and/or subgeneric names is given below:-
|
Species |
Original genus |
Proposed genus or subgenus |
|
barbulus |
Barbus |
Luciobarbus |
|
brachycephalus |
Barbus |
Luciobarbus |
|
capito |
Cyprinus |
Luciobarbus |
|
esocinus |
Luciobarbus |
Luciobarbus |
|
grypus |
Barbus |
Tor |
|
kersin |
Barbus |
Luciobarbus |
|
kosswigi |
Cyclocheilichthys |
Kosswigobarbus |
|
lacerta |
Barbus |
Barbus |
|
luteus |
Systomus |
Carasobarbus |
|
mursa |
Cyprinus |
Luciobarbus |
|
pectoralis |
Barbus |
Luciobarbus |
|
sharpeyi |
Barbus |
Mesopotamichthys |
|
sublimus |
Barbus |
Kosswigobarbus |
|
subquincunciatus |
Barbus |
Luciobarbus |
|
xanthopterus |
Luciobarbus |
Luciobarbus |
There are also conflicting views on the validity and synonymy of several nominal
"Barbus" species. An extensive comparison of these views is not given here (see, for example, Myers (1960), Karaman
(1971), Almaça (1983, 1984a, 1984b, 1986, 1990, 1991, 1992, 1994), Krupp (1985c), Howes (1987), Doadrio (1990), Eschmeyer (1990), Berrebi (1995), Berrebi et al. (1996), Tsigenopoulos and Berrebi
(2000)). Karaman's studies have not found general acceptance. Author's views conflict, even when examining the same material. Problems include:- the low number of specimens examined (Almaça (1984a; 1986)
for example, examined 11 nominal taxa relevant to Iran in detail but averaged only about 6 specimens per taxon, often from a single locality or outside Iranian waters); a wide range in size of
individuals of species being compared making age related changes difficult to assess (denticles in the dorsal fin are often lost with age, barbels are shorter, body shape changes, etc); the possibility of
sexual dimorphism; possible variation between populations; ecomorphs being recognised as genera (e.g. Luciobarbus was recognised by having 4, as opposed to 5, teeth in the outer pharyngeal tooth
row; Bertinius is founded on this condition and development of molar teeth for crushing molluscs - but this may have risen independently in response to an ecological opportunity (see Krupp
(1985c)); paedomorphosis and independent origins from a generalised form in different sites (Mina et al., 2001), and the lack of a wide range of new material. An adequate resolution of the
systematics of the Barbus sensu lato species in the Tigris-Euphrates basin in particular would require extensive collections of new material from type localities and from the whole basin
and comparison of this material with the extant types. Not all types are extant and some that do exist are in poor condition. If this were not complication enough,
"Barbus" species are prone to
hybridisation with other "Barbus" species and even other genera, further confusing the resolution of the issue. Almaça (1990) cites a hybridization rate of 5.5-6.0% in
"Barbus" of the Iberian
Peninsula, higher under changed ecological conditions such as the building of dams.
The status of Bertinius longiceps persicus Karaman, 1971 described from the "Karun b. Ahvaz, Persien" (= Karun River at Ahvaz, Khuzestan) on a single specimen is uncertain (lateral
line 56-58, gill rakers 22, subterminal mouth, very short barbels, head somewhat higher and suddenly narrowing compared to the type subspecies of the Jordan and Orontes basins, acuminate snout, dorsal
fin margin concave). It is not "Barbus" longiceps (F. Krupp, in litt., 1986). The holotype is in the Zoologischen Instituts und Zoologischen Museums der Universität Hamburg (ZMH H2509).
The roe or eggs of species in these genera have been implicated in poisoning (Halstead, 1967-1970) and should be avoided (see under the genus Schizothorax for more information on egg poisoning).
Fish should be carefully cleaned in the spawning season to remove the eggs and ensure against contamination of flesh. Severe cases of egg poisoning in other species have resulted in death. Sykes (1927)
however, in his account of the travels of Sir John Chardin in Persia (first published in 1686) quotes "Barbel.... the Spawn of them especially is dangerous, being a certain and a violent
Vomit, by Reason that the Sun never shines on that Fish, and that it breeds in raw Waters; or because they take it with the Nux Vomica or the Vomiting Nut".
Najafpour and Coad (2002) report a case of roe poisoning from eggs of
Carasobarbus luteus.
Barbels are found in running water of streams and rivers although some may inhabit ponds, springs and lakes.
Most show migrations for spawning. A species called soleymani, possibly a "Barbus" species, was considered to be on the verge of extinction in the Gav Masiab River of the Tigris River basin,
through pollution, overfishing, dam building, aquaculture, and introduction of exotics (IranMania.com, 29 December 2006).
"Barbus" species in Khuzestan are thought to be the intermediate hosts of
Heterophyidae flukes found in humans and carnivores (Massoud et al., 1981).
Kazeraani (1994) gives a short account of Iranian "Barbus" species in Farsi. The common names in Farsi for these fishes generally are سس ماهي (= sos, ses or
sas mahi, meaning unknown) and زرده پر (= zardehpar), zardek or zardak and ourange or ourenge (in reference to yellow or orange colorations, probably of the fins).
The origin and movements of "palaearctic" or Euro-Mediterranean "Barbus" species in Southwest Asia have been examined by Banarescu (1976; 1977) and Almaça (1984b; 1988; 1990) and
these works should be consulted for further details. These works are not cladistic analyses but groupings of species based on morphological similarities and may be subject to criticism on this account.
The origin of the genus "Barbus" according to these authors lies in East Asia and reached the Euro-Mediterranean region by a Siberian route.
"Barbus" became extinct in northern East Asia,
Siberia and northern Europe when the climate cooled during either the Pliocene or the Quaternary. Europe was colonised during the Oligocene and it is from Europe through Anatolia that Southwest Asia
received many of its "palaearctic" "Barbus". This route of entry probably did not occur before the Pliocene because the Syrian-Iranian Sea, the last connection between the Tethys Sea and
the Indian Ocean, blocked passage of primary freshwater fishes into what is now Iran and adjacent regions although a connection between a Balkan-Aegean-Anatolian landmass and Iran was possible during the
early Miocene (20-17 MYA). A marine transgression 16.8-11.8 MYA flooding the eastern Paratethys and the rise of mountain barriers led to independent evolution of
"Barbus" in the
Balkan-Aegean-Anatolian landmass and in the Iranian Plateau. During the late Miocene the eastern marine connection of Paratethys closed (11.8-10.5 MYA) allowing an exchange of
"Barbus" between
Iran and Anatolia, continuous from that time. The Paratethys became an intracontinental sea, the Sarmatian Sea, with a basin encompassing the present Black, Caspian and Aral seas and neighbouring
low-lying areas (Bianco, 1990). The Sarmatian Sea freshened as large rivers entered it during the late Miocene and Pliocene, facilitating dispersal of freshwater fishes. A second route of entry for
"Barbus" to northern Iran was via southwestern Siberia and the Aral Sea basin during the early to middle Oligocene. Bănărescu and Bogutskaya in Bănărescu and Bogutskaya (2003)
agree on an east Asian origin for "Barbus", dispersing across Siberia and western Asia. The group split into two branches, one forming
Barbus sensu stricto and using a dispersal
route north of the Ponto-Caspian basin and reaching western Europe and another (Luciobarbus) dispersing across the present-day Mediterranean Sea (see above in discussion of Berrebi and
Tsigenopoulos in Bănărescu and Bogutskaya (2003) and Tsigenopoulos
et al. (2003) for listing of nominal taxa relevant to Iran in these branches or groups).
A recent overview of "Barbus" systematics restricts the genus to Europe, Southwest Asia and Northwest Africa (Berrebi et al., 1996). Barbus sensu stricto is recognised as a lineage
which shares morphological characters, has an ancestral tetraploid origin of 2n=100, and has similar karyotypes, biochemical markers and parasites. Genetic studies indicate four groups of species, namely
West European and Ponto-Caspian, Iberian, Northwest African and Levantine. Iberian barbels are found in Spain and Portugal and along within the Northwest African barbels share no species with Iran. The
West European and Ponto-Caspian barbels include B. brachycephalus, B. capito and B. mursa, and the Levantine barbels include B. barbulus, B. cyri, B. esocinus,
B. lacerta, B. pectoralis, B. rajanorum, B. scincus, B. subquincunciatus and B. xanthopterus. The authors make no comments on the validity of these nominal
species and only B. brachycephalus has been examined in detail for karyotypes and/or nuclear markers. This work is continuing and the authors advocate various methods. They note that accurate
descriptions of many taxa are lacking and that morphology is still the fastest and most cost-efficient way to identify species. Accurate identification is the foundation for all other studies.
Machordom and Doadrio (2001), using ATPase 6 and 8 and cytochrome b, found differentiation in
"Barbus" capito and "B". brachycephalus in the Plio-Pleistocene. A clade of the
subgenus Luciobarbus was found for species from the Caucasus (as above), Greece and North Africa compared to the Iberian Peninsula, isolation having occurred after the Messinian salinity crisis
5.5 MY ago when the Iberian Peninsula broke away from Africa.
Berrebi et al. (1996) recommend that Barbus-like species which cannot be allocated to a clearly defined genus should be placed in a genus called `Barbus', surrounded by single
quotation marks, until the systematic position is elucidated.
In the text of Freshwater Fishes of Iran double
quotation marks (") are used for accounts that referred to Barbus in the
old sense, including all or part of the the species listed here (see table
above).Barbus lacerta
Heckel, 1843



Above photographs courtesy of Atabak Mahjoor Azad
Common names
blizem, bellizem, سس ماهي (= sos or sas mahi), زرده پر (= zardehpar), orenge,
sos mahi Kura.
[Kur sirbiti in Azerbaijan; murtsa, murza, muruza, muruz in Transcaucasia generally; mursa in Armenia; shabout moraqqat in Arabic in Iraq; karrid or karad achmar (red frill or shag, probably from the
colour and the long barbels) and karrid asrak (= blue shaggy one) according to Heckel (1843b) in Arabic in Aleppo; Kurinskii usach or Kura barbel in Russian].
Systematics
Howes (1987) places this species in Barbus sensu stricto. Karaman (1971) assigns many taxa as subspecies of Barbus plebejus Bonaparte, 1832 (dated correctly 1839 in Eschmeyer et
al. (1996), see Bianco (1995a) for details), found throughout Europe and Southwest Asia. Bianco (1995a) considers that Barbus plebejus is restricted to Adriatic drainages of Italy and Croatia.
Valiallahi (2006) considers B. plebejus to be present in Iran and distinct from B. lacerta based mainly on body shape, the relative head length, the body depth and the fourth dorsal fin ray.
Barbus plebejus kosswigi Karaman, 1971 is described as new from the "Oberer Teil des Tigris-Systems" and "Hamam suyu, Beytusebab-Hakkari" (upper Tigris River basin in Turkey).
Almaça (1991) considers it an ecophenotype of his Barbus plebejus scincus since two subspecies of the same species cannot live in the same river basin. Barbus plebejus kosswigi is a
secondary homonym of Cyclocheilichthys (= Kosswigobarbus) kosswigi according to Kottelat (1997).
Barbus plebejus ciscaucasicus Kessler, 1877 is from the western drainages of the Caspian Sea south to Dagestan but only Barbus plebejus lacerta Heckel, 1843 is found in Iran. It is
recognised here as a full species since its relationships to European and other taxa cannot be determined on material available for this study. Bianco and Banarescu (1982) place specimens from the Aras
River near Maku, which are probably this species, in Barbus cyclolepis cyri De Filippi, 1865.
Almaça (1981; 1983; 1984a; 1984b, 1986) gives lacerta specific status, distinguishing it from Barbus plebejus by the strong denticulations on the last dorsal fin unbranched ray, lower
denticle density, number of scales in transverse rows, shorter head and pectoral fin, longer snout, lower body, the decrease in height of the branched dorsal fin rays is gradual and the profile of the
fin is straight, unusual in Barbus with a strongly denticulated dorsal spine. Almaça recognises two subspecies from Iranian drainages:- lacerta from the Tigris-Euphrates basin (and Aleppo)
and cyri from the southern Caspian Sea basin. Berg (1948-1949) also refers Caspian Sea basin specimens to Barbus lacerta cyri but in Berg (1949) has cyri from the Tigris River basin
too. Saadati (1977) suggests that Lake Orumiyeh basin Barbus lacerta are a distinct subspecies based on higher scale counts there (72-89) than in the Caspian Sea basin. However, B. lacerta
as recognised has a wide range in scale counts (see below) and counting methods can differ to include or not supernumerary scales in the lateral line and small scales at the caudal fin base. Fishes
resembling B. lacerta from the Namak Lake basin have higher scale counts than Caspian Sea specimens although sample size is too small for a definitive study. Berg (1948-1949) notes that his
B. lacerta cyri is subject to extremely wide variations in such characters as body depth, fin and barbel lengths, dorsal spine denticle numbers (even absent in some very large fish) and lateral
line scale counts, among others. A large series of specimens would be needed to resolve these problems, allowing for size and sexual variation, new character discoveries and consistent methodologies.
Molecular studies might be helpful.
Barbus Lacerta was described from the "Flüssen Kueik bei Aleppo" (Heckel, 1843b).
The following species are synonyms. Barbus Scincus Heckel, 1843 described from "Aleppo" and later from the "Flusse Kueik bei Aleppo" in Heckel (1846-1849a), Barbus
cyri De Filippi, 1865 described from the "Kur presso Tiflis" (= Kura River near Tbilisi, Georgia) (including Barbus cyri var. tiflissica Kamenskii, 1899 described from the
"Kura bei Tiflis" and Barbus cyri var. chaldanica Kamenskii, 1899 described from the "Andshigan-tschai unweit Chaldan"), Barbus caucasicus Kessler, 1877 from the
Kura and Araks rivers and tributaries, Azerbaijan, Barbus toporovanicus Kamenskii, 1899 described from the "Toporavan See" (= Lake Paravani or Taparavani at 41°26'N, 43°48'E, in the
upper Kura River basin of Georgia), bortschalinicus Kamenskii, 1899 described from the "schwarze Flüsschen (Das schwarze Flüsschen fällt in die Bortschala, rechter Zufluss des Chram,
Nebenfluss der Kura)(tschernaja rjetschka)", Georgia, Barbus sursunicus Kamenskii, 1899 described from "Sursuna in dem Flüsscheu (sic) Kara-tschai, Nebenfluss der Kura, oder
ihrem Zuflusse, erbeutet in einer Höhe von ca 3200', zwischen den Seen Tschaldyr-göll und Tuman-göll, dass kleinere aus dem Flüsschen Abastuman-tschai" (Azerbaijan; later in the same article this
species is spelt zurzunicus), Barbus armenicus Kamenskii, 1899 described from the "See Tschaldyr-göll, 6522' und den Kars-tschai" (Sildir Gölü and the Kars-chai, Turkey), and
Barbus angustatus Kamenskii, 1899 described from the "Kura, bei Borshom". Barbus toporovanicus first appeared in Kamenskii (1887) as a variety of Capoeta fundulus (see
Capoeta capoeta). Type localities from Kamenskii (1899) are, obviously, taken from the German text; there is also an accompanying and preceding Russian text with localities in Latin and Russian
which are very similar, although in some cases abbreviated.
Heckel (1843), the original describer, recognised Barbus scincus as close to his Barbus lacerta but with a shorter head, sharply decurved forehead, small mouth, and small eyes, all
characters not easily quantified without detailed analysis. Berg (1949) placed it in the synonymy of lacerta. Berg's view is followed here; others are described by Almaça (1983; 1984a, 1986) who
favours placing scincus as a subspecies of Barbus plebejus as noted above.
The problem with the conclusions above remains, as pointed out earlier, the lack of new material.
Four syntypes of Barbus lacerta are in the Naturhistorisches Museum Wien (NMW 54227), 1 syntype is in the Senckenberg Museum Frankfurt (SMF 3471, formerly NMW), and 1 syntype is in the Museum
für Naturkunde, Universität Humboldt, Berlin (ZMB 3236, formerly NMW, 110.3 mm standard length, examined February 2006; F. Krupp, pers. comm., 1985; Eschmeyer et al., 1996; Bogutskaya in
Bănărescu and Bogutskaya, 2003). The Vienna card catalogue in 1997 lists one of NMW 54227 as the lectotype. The Vienna catalogue lists 6 specimens. Bogutskaya in Bănărescu and
Bogutskaya (2003) designates 54227-1, 181.6 mm standard length, as the lectotype.
Syntypes of Barbus scincus from "Aleppo", the type locality in Heckel (1843b), are reported in the Naturhistorisches Museum Wien by Almaça (1986) and were also examined by me
(NMW 22272, 2 specimens, 97.6-146.7 mm standard length, in poor condition and NMW 54526, 1 specimen, 158.8 mm standard length, designated as a lectotype by F. Krupp, 31 October 1984). Eschmeyer et
al. (1996) also list NMW 54525 as a syntype and this fish measured 124.2 mm standard length and had been dried at some point before it was examined by me. The Vienna catalogue lists 4
specimens and the card catalogue in 1997 lists these 4 fish with NMW 54526 as "? lectotype" (sic).
Tortonese (1940) and Eschmeyer et al. (1996) list the holotype of Barbus cyri as in the Istituto e Museo di Zoologia della R. Università di Torino (MZUT N.690).
The lectotype of Barbus armenicus, as established by Berg (1948-1949:Fig. 451), is in the Zoological Institute, St. Petersburg under ZISP 5198 with 3 paralectotypes (Eschmeyer et al.,
1996).
The lectotype of Barbus sursunicus is in the Zoological Institute, St. Petersburg under ZISP 14740 as established in Berg (1948-1949:fig. 451).
Abdurakhmanov (1962) compares fish from the Aras and Kura river basins and the Lenkoranchai. Lenkoran fish have fewer scales, longer head length and depth, greater maximum body depth, greater anal fin
height, longer pelvic and ventral fins, a longer lower caudal fin lobe, a shorter caudal peduncle length, a smaller eye, and a shorter interorbital width than Kura and Aras fish; Lenkoran fish have a
longer predorsal distance, greater caudal peduncle depth, and greater dorsal fin height than Kura fish though Aras fish are the same; Lenkoran fish have the dorsal fin base and postorbital distance less
than in Aras, but not Kura, fish. No taxonomic distinction is made for these variations.
Key characters
The spotting on the body is characteristic.
Morphology
The mouth is moderate in size, with moderate to thick tuberculate lips. The median lobe of the lower lip is not developed, being small to absent; however the lip does have a central area which is
thicker and distinct from the lips laterally in small fish. Bogutskaya in Bănărescu and Bogutskaya (2003) gives illustrations of lower lip development and variations in head
shape. Males were thought to have a straight head profile while in females the profile falls steeply in front of the nostrils but Bogutskaya in Bănărescu and Bogutskaya (2003) found some males
with a hump on the snout. Morphology is quite variable. Barbels are thick, the anterior one not extending past the nostril level and the posterior one reaching or exceeding the preopercle level.
Dorsal fin with 3-5, usually 4-5, unbranched rays followed by 7-9, usually 8, branched rays, anal fin with 3 unbranched rays followed by 4-6, usually 5, branched rays. Pectoral fin branched rays 13-19
and pelvic fin branched rays 7-8. Lateral line scales 49-87. Scales are a horizontal oval to rectangular in shape with the anterior margin bearing a central protuberance, and sometimes a wavy form.
Radii are numerous on all scale fields around a subcentral anterior focus with few to moderate numbers of circuli (as scales are small). Scales may be irregularly arranged on the flank because of their
small size giving different counts depending on whether smaller scales are included in the lateral line count. There is a pelvic axillary scale. Gill rakers 5-13, short and just reaching the one adjacent
when appressed. Rakers may not develop on the anterior arch giving a wide range in counts. Vertebrae 39-45. Pharyngeal teeth 2,3,5-5,3,2 with variants 2,3,5-5,3,1, 1,3,5-5,3,2, 1,3,5-5,3,1, 2,3,4-5,3,2,
2,3,5-4,4,2, 2,4,5-4,4,4 and even 1,2,3,5-5,3,2,1. The fourth inner row tooth is usually the largest, slightly larger, or slightly smaller in some, than the third. The fifth inner row tooth is blunt and
other teeth are hooked or pointed. Teeth may be slightly serrated and there is a short concave surface below the hook. The last unbranched ray of the dorsal fin is moderately to strongly developed,
varying between individuals and populations, with denticle density high (up to 65) along three-fifths to two-thirds of its length. Denticle extent appears to be quite variable. Denticles are
proportionately larger in small fish. The tip of the last unbranched ray is thin and flexible. Denticles may be absent in large fish. The gut is elongate with about 2 anterior and 1 posterior loops.
Meristics in Iranian fish: dorsal fin branched rays 8(36); anal fin branched rays 5(36); pectoral fin branched rays 14(5), 15(7), 16(16) or 17(8); pelvic fin branched rays 7(8) or 8(28); lateral line
scales 53(1), 55(1), 56(3), 59(3), 60(3), 63(3), 64(3), 65(2), 66(1), 67(1), 69(3), 70(1), 72(1), 74(2), 76(2), 79(1), 82(3), 85(1) or 87(1); total gill rakers 6(1), 7(9), 8(10), 9(7), 10(6), 11(1) or
13(1); pharyngeal teeth 2,3,5-5,3,2(18), 1,3,5-5,3,1(1), 2,3,4-5,3,2(1) or 2,3,4-4,3,2(1); and total vertebrae ?.
Sexual dimorphism
Females have shorter barbels than males (Berg, 1948-1949) and females have longer anal and ventral fins (Bogutskaya in Bănărescu and Bogutskaya, 2003). Tubercle development in males caught
on 25-26 June consists of minute tubercles thickly developed on the head top, sides and ventrally, lining the margin of anterior belly scales but also 1-2 tubercles in mid-scale, on anterior flank scales
numbering 1-4 becoming 1 tubercle on more posterior scales although most mid-flank scales lack tubercles. Lower flank and lower caudal peduncle scales bear a tubercle. Back scales have a unique
tuberculation consisting of a line rather than a rounded tubercle. The line lies centrally on the scale and extends from the margin part way along the exposed scale. Behind the dorsal fin the back scales
have the central line and one on each side radiating back and up and back and down. Tubercles on the dorsal, caudal and anal fins are small and follow the fin branching. they are weak to absent on the
pectoral and pelvic fins but are found on the first unbranched pectoral ray in two rows. Males are a dark gold dorsally and all fins slightly reddish with a gold iridescence when spawning (Bogutskaya in
Bănărescu and Bogutskaya, 2003). Spawning females have reddish ventral and anal fins.
Colour
The overall colour is yellowish to olive-grey (possibly bluish according to Heckel (1846-1849a)) with numerous, regular dark-brown to black spots on the back, upper flank and dorsal and caudal fins or
irregular mottling. The spots may form a stripe in young fish. In general appearance, fish may be quite light or almost blackish as pigmentation level varies individually. The back is olive-brown to light
or reddish-brown and the flanks silvery to yellowish. The belly and lower head surface are white. The iris is dark to silvery with a narrow silver-golden ring. Barbels are white. The dorsal fin bears dark
spots and extended lines of dark pigment on the rays and membranes. These are not clearly arranged as bars. The margin of the caudal fin is dark in some fish and there may be a band on mid-fin. The caudal
fin is often speckled with dark spots which do not form clear bars. The pectoral fin has dark spots and there are odd dark spots on the pelvic and anal fins. The peritoneum is a light brown with dense
but spaced melanophores.
Size
Reaches 37.5 cm and 460 g, possibly to 550 g.
Distribution
This species is found in the Tigris-Euphrates, Quwaiq and Caspian Sea basins as well as some internal basins of Iran. In Iran, it is recorded from the Caspian Sea basin in the Aras River and its
tributary the Qareh Su, from the Astara to the Atrak rivers including the Anzali Mordab, the upper Safid River drainage in the Qezel Owzan and Shahrud,
in Tajan, Babol, Haraz, Sardab, Aras, Tonekabon, Pol-e Rud and Safid rivers, in the Lake Orumiyeh basin in the middle to upper
Talkheh River, Nazlu Chai, Tatavi and Zarrineh rivers, the Tigris River basin, and the Esfahan basin (Dopolan River)(Günther, 1899; Laptev, 1934; Berg, 1949; Holčík and Oláh, 1992;
Shamsi et al., 1997; Abbasi et al., 1999; Kiabi et al., 1999; Ghorbani Chafi, 2000; Abdoli, 2000).
Zoogeography
Almaça (1991) considers that this species arose from the first wave of colonisers to enter West Asia from South Europe but is more recent in origin than such Barbus
(= Luciobarbus) species as esocinus
and xanthopterus originating from southwestern Siberia.
Habitat
This species is found in fresh waters and is not migratory. It avoids muddy bottoms and prefers sandy or stony substrates (Solak, 1977; Bogutskaya in Bănărescu and Bogutskaya,
2003). These habitats are rich in benthos, cool, with rapid currents and well-oxygenated; however it may congregate in slow waters where temperatures reach 26°C.
Age and growth
Solak (1989a) examined a population of this species in the Aras River in Turkey and found up to 5 age groups. Abdurakhmanov (1962) records 5 years as life span in Azerbaijan. Çalişkan et
al. (1999) also found 5 age groups in Çıldır Lake, Turkey (for Barbus plebejus, probably this species). Fish in age group 2 dominated and the largest fish attained 320 mm and 550 g.
Maturity is attained at 2 years for males and 3 years for females (Bogutskaya in Bănărescu and Bogutskaya, 2003).
Food
Plant remains, crustaceans such as amphipods and insect remains such as chironomids and dragonfly larvae have been found in gut contents. Abdoli (2000) lists Plecoptera, Ephemeroptera and Chironomidae.
Algae is also consumed (Bogutskaya in Bănărescu and Bogutskaya, 2003).
Reproduction
Eggs number up to 19,680 and a diameter of 2.3 mm (Abdurakhmanov, 1962; Bogutskaya in Bănărescu and Bogutskaya, 2003). Spawning may occur 2-3 times in a season judging by oocyte
sizes in mature ovaries and occurs from the end of April to August, varying with locality, once temperatures reach 14°C, ceasing if the temperature exceeds 20°C (Bogutskaya in
Bănărescu and Bogutskaya, 2003). Small Iranian specimens (130.7-157.7 mm standard length) have eggs of 1.0 mm diameter and 1.1 mm on 9 July and 11 May respectively.
Larger eggs were noted in a fish caught on 9 July (1.7 mm). The spawning season is probably spring for large fish.
Parasites and predators
Molnár and Jalali (1992) record the monogeneans Dactylogyrus carpathicus and D. linstowi from Barbus plebejus, presumably this species, in the Safid Rud. Shamsi et
al. (1997) report Clinostomum complanatum, a parasite causing laryngo-pharyngitis in humans, from Barbus barbus plebejus, presumably this species. Masoumian et al.
(2003) record Myxobolus valdogeli while Pazooki et al. (2003) and Pazooki (2006) record Rhabdochona hellichi, Bothriocephalus gowkongensis, Pseudocapillaria
tomentosa, Allocreadium isoporum and Paradiplozoon homoion, all reports from fishes captured in the Tajan and Zarem rivers of Mazandaran. Pazooki et al. (2005) record
Trichodina perforata from this species in waterbodies of Zanjan Province. Pazooki et al. (2006) record the monogeneans Dactylogyrus goktschaicus and Gyrodactylus sp. from this
fish in Zanjan Province. Barzegar et al. (2008) record the digenean eye
parasite Diplostomum spathaceum from this fish. Barzegar and
Jalali (2009) reviewed crustacean parasites in Iran and found Ergasilus
sp. and Lernaea sp. on this species.
Economic importance
Not commercially important although it does provide sport in mountain areas of the former U.S.S.R.
Conservation
Kiabi et al. (1999) consider this species to be near threatened in the south Caspian Sea basin according to IUCN criteria. Criteria include sport fishing, medium in numbers, habitat
destruction, widespread range (75% of water bodies), present in other water bodies in Iran, and present outside the Caspian Sea basin. Mostafavi (2007) lists it as near
threatened in the Talar River, Mazandaran. Endangered in Turkey (Fricke et al., 2007).
Further work
The various populations of this species require more detailed study, especially with molecular methods, to determine their taxonomy.
Sources
Type material: ?
Iranian material: CMNFI 1970-0559, 9, 39.7-114.3 mm standard length, Azarbayjan- eBakhtari, Baranduz Chay (ca. 37º25'N, ca. 45º10'E);
CMNFI 1979-0271, 2, ? mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0289, 1, 131.6 mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0449, 2, 85.7-92.2 mm standard length, Azarbayjan-e Khavari, river 18 km from Khalkhal (ca. 37º42'N, ca. 48º27'E);
CMNFI 1979-0452, ?, ? mm standard length, Azarbayjan-e Khavari, Qezel Owzan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0468, 7, 30.9-96.1 mm standard length, Mazandaran, Haraz River (36º14'N, 52º22'E);
CMNFI 1979-0493, 3, ? mm standard length, Mazandaran, stream in Tajan River drainage (36º19'N, 53º23'E);
CMNFI 1979-0557, ?, ? mm standard length, ();
CMNFI 1979-0558, ?, ? mm standard length, ();
CMNFI 1979-0559, ?, ? mm standard length, ();
CMNFI 1979-0785, 2, 115.7-134.8 mm standard length, Aazrabayan-e Bakhtari, Shaher Chay (37º27'N, 34º55'E);
CMNFI 1979-0786, 1, 84.1 mm standard length, Azarbayjan-e Khavari, Guru Lake (37º55'N, 46º42'E);
CMNFI 1993-0125, 1, 83.1 mm standard length, Kermanshahan, Sarab-e Nilufar (34º24'N, 46º52'E);
CMNFI 1993-0126, 2, 157.7 mm standard length, Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E);
CMNFI 1993-0128, 1, 130.7 mm standard length, Kermanshahan, Sarab-e Sabz 'Ali Khan (34º25'N, 46º32'E);
CMNFI 1993-0136, 1, ?105.5 or 108.2 mm standard length, Mazandaran, Sardabrud (36º39'42"N, 51º22'36"E);
CMNFI 2007-0086, 1, 164.4 mm standard length, Azarbayjan-e Khavari, Qareh Su basin near Nir (ca. 38º02'N, ca. 48º00'E);
CMNFI 2007-0087, 2, ? mm standard length, Azarbayjan-e Khavari, Qareh Su north of Ardebil (38º22'N, 48º19'E);
CMNFI 2007-0088, 2, ? mm standard length, Azarabyjan-e Khavari, Qareh Su east of Lari (38º30'N, 48º03'E);
CMNFI 2007-0093, 1, ? mm standard length, Azarbayjan-e Bakhtari, Qotur River south of Khvoy (38º30'N, 44º58'E);
CMNFI 2007-0095, 4, 25.9-73.3 mm standard length, Azarbaijan-e Bakhtari, Shahr Chay southwest of Orumiyeh (ca. 37º27'N, ca. 44º56'E);
CMNFI 2007-0096, 1, ? mm standard length, Azarbayjan-e Bakhtari, Qasemul River in Baranduz Chay basin (ca. 37º25'N, ca. 45º10'E);
CMNFI 2007-0097, 1, ? mm standard length, Azarbayjan-e Bakhtari, Barunduz Chay basin south of Orumiyeh (ca. 37º16'N, ca. 45º08'E);
CMNFI 2007-0098, 2, 193.1-227.4 mm standard length, Azarbayjan-e Bakhtari, river south of Mahabad (ca. 36º42'N, ca. 45º41'E);
CMNFI 2007-0099, 2, 28.9-132.1 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay west of Mahabad (ca. 36º35'N, ca. 45º25'E);
CMNFI 2007-0100, 1, ? mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0103, 3, 43.6-63.7 mm standard length, Kordestan, Zarineh River basin north of Saqqez (ca. 36º18'N, ca. 46º16'E);
CMNFI 2007-0104, 2, 54.6-71.2 mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º12'N, ca. 46º18'E);
CMNFI 2007-0105, 2, ? mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º06'N, ca. 46º20'E);
CMNFI 2007-0106, 1, 99.1 mm standard length, Kordestan, Qezel Owzan River basin near Divandarreh (ca. 35º52'N, ca. 47º05'E);
CMNFI 2007-0107, 1, 64.6 mm standard length, Kordestan, Qezel Owzan River basin near Bijar (ca. 35º54'N, ca. 47º20'E);
CMNFI 2007-0117, 1, ?66.4 mm standard length, Kermanshahan, Gav Masiab basin near Sahneh (ca. 34º24'N, ca. 47º40'E);
CMNFI 2007-0117, 1, ?67.2 mm standard length, Kermanshahan, Gav Masiab near Sahneh (ca. 34º24'N, ca. 47º40'E);
CMNFI 2007-0118, 1, ? mm standard length, Kermanshahan, Bid Sorkh River between Sangeh and Kangavar (ca. 34º23'N, ca. 47º52'E);
USNM 205931 2, 93.0-115.4 mm standard length, Azarbaijan-e Bakhtari, Baranduz River south of Orumiyeh (37º25'N, 45º05'E);
ZMH 2634, 1, 130.5 mm standard length, ?, Haraz River.
Comparative material: BM(NH) 1974.2.22:1236, 1, 113.8 mm standard length,
Iraq, Karrid Achmar (no other locality data); BM(NH) 1974.2.22:1327-1328, 2, 121.0-129.9 mm standard length, Iraq (no other
locality data); BM(NH) 1974.2.22:1349-1350, 2, 63.1-83.0 mm standard length,
Iraq, Qizillja River, Lesser Zab and Serokani near Diana, Rowanduz, Greater Zab (mixed
sample); BM(NH) 1974.2.22:1351, 1, 146.8 mm standard length, Iraq, Karrid Asrak (no
other locality data).
Genus Barilius
Hamilton, 1822
The members of this genus are found from Pakistan to Thailand with
one species in the Tigris-Euphrates and adjacent basins. Their
systematics is still poorly understood and there may be about 25 species.
This genus is characterised by a compressed but slender and small
body, having small to moderate sized scales, a decurved lateral line,
running for example on the lower part of the caudal peduncle, lateral
line complete, incomplete or absent, a short dorsal fin and a long
anal fin, no fin spines, a moderate and terminal mouth, barbels absent
or in 1 or 2 pairs, short gill rakers, pharyngeal teeth in 3 rows,
and usually with dark bands or spots on the flank.
These fishes are found mostly in mountain streams although some are lowland species.
Barilius mesopotamicus
Berg, 1932
Common names
None.
[sboura iraqia in Arabic, Mesopotamian minnow].
Systematics
The holotype, 44 mm total length and 35.4 mm standard length, is in
the Zoological Institute, St. Petersburg (ZISP 23955) and is
decoloured. The collection date is given by Berg (1949) as 16.IV.1914,
as 3.IV.1914 in the ZISP catalogue and 5.IV.1914 in the jar. The first
two dates are probably correct, one old style and one new style. The
type locality is "Stromgebiete des Tigris, in (Siaret)
Seid-Hassan, an der persisch-türkischen Grenze, unter 33°20'n.
Br., 46°20'ö. L. Seid-Hassan liegt am Flusse Gawi, welcher sich mit dem Kundschian (Gundschian)-tschai
vereinigt; der letztere mündet in den Tigris". Seyyed Hasan (33°06'N,
46°11'E) lies on a tributary of the Kanjan Cham River near the Iranian town of
Mehran on the Iran-Iraq border. The tributary is presumably the Gawi River.
Howes (1980) stated that this species has apomorph characters
shared with species assigned to Leucaspius Heckel and Kner,
1858 but this seems unlikely on general morphological grounds (Coad,
1982b) and Bianco and Banarescu (1982) and Liao et al. (2011) concur, the
latter also incorporating molecular evidence. It resembles other Barilius
in having barbels (none in Leucaspius), a lateral line low on
the body (short and mid-body), broad suborbital bones, and flank bars
(none) while Leucaspius is unique in having in females a fold
of skin in the shape of two, large, rounded papillae around the
genital opening. Bianco and Banarescu (1982) state that this species
may be generically distinct from South Asian Barilius but do
not diagnose a new genus. Bănărescu and Coad (1991) and Bănărescu
(1992b) state that its position and biogeographical affinities are
uncertain. Berg (1949) considers it closer to Indian species of the
genus Barilius than to African ones.
Key characters
The only member of its genus in Iran, this species is easily identified by
the pigment pattern, low lateral line, broad suborbital bones and the barbels.
Morphology
The lower jaw bears a small symphysial knob. The mouth is slightly
subterminal, oblique and elongate with the mouth corner under the
anterior half of the eye. A well-developed barbel has its origin just
anterior to the level of the nostril above the upper lip and lies in a
groove between the upper lip and the beginning of the suborbital bone
series. This barbel can be absent or minute in some fish (females from
Habbaniyah, Iraq (Coad and Krupp, 1983)). In addition to these maxillary barbels, a second
pair of barbels have their origin slightly above the posterior edge of
the mouth in 8 out of 259 fish examined. They are usually rudimentary
but may reach 10.7% of head length. Barbels are difficult to see in
smaller fish without magnification. The suborbital bone series is large.
Dorsal fin unbranched rays 2-3, usually 3, branched rays 7-9; anal
fin unbranched rays 2-3, usually 3, branched rays 10-14, branched
pectoral fin rays 11-15 and branched pelvic fin rays 6-8. Lateral line
scales 42-58. Lateral line incomplete or complete, rarely terminating
at the pectoral fin level. Lateral line decurved and parallel to the
ventral body profile from the pelvic fin origin to the caudal
peduncle, being 2-3 scales above this profile. On the caudal peduncle
the lateral line is below the mid-line while scales on the caudal fin
posterior to the hypural plate are perforated in the mid-line.
Pectoral and pelvic axillary scales present. Scales are regularly
arranged over the whole body but are not strongly imbricate,
particularly on the belly and back anterior to the dorsal fin.
Anterior flank scales are oval with subcentral anterior focus and a
moderate number of circuli. Radii are found principally on the
posterior and lateral fields. Anterior field radii are usually absent
although 1-2 radii may occasionally be found. Scale radii based on 5
anterior flank scales from 5 fish (40.7-50.7 mm standard length)
number 5-11 primary radii, 0-13 secondary radii and 5-23 total radii.
Total gill rakers 7-14. Gill rakers are short and rounded, reaching to
or part way to the raker below when appressed. Total vertebrae 38-41.
Pharyngeal teeth usually 4,5-5,4, often 4,5-5,3 (25% of 20 fish
examined), or more rarely in three rows 1,3,5-5,3,1 or 1,4,5-5,4,1.
Teeth are hooked at the tip, slender and have a concave grinding
surface below the tip. The gut is a simple s-shape.
Meristic values for Iranian specimens are:- branched dorsal fin
rays 7(2), 8(32) or 9(2); branched anal fin rays 10(2), 11(19), 12(13)
13(1) or 14(1); branched pectoral rays 11(1), 12(8), 13(23), 14(2), or
15(1); pelvic fin rays 6(1), 7(33) or 8(2); scales in lateral series
42(1), 43(3), 44(3), 45(2), 46(6), 47(6), 48(1), 49(3), 50(4), 51(4),
52(2) or 54(1); total gill rakers 7(4), 8(6), 9(6), 10(7), 11(6),
12(1), 13(1) or 14(1); pharyngeal teeth 4,5-5,4(8), 4,5-5,3(3) or
1,3,5-5,3,1(1); and total vertebrae 39(8), 40(20) or 41(5).
Sexual dimorphism
Unknown.
Colour
Overall colour is a brilliant silver with a golden-yellow glimmer,
with the back darker and having a thin median stripe. Scales are
highly deciduous and leave a silvery smear on the hand. The flanks
have 6-11 roundish dark, grey-green spots, not clearly apparent in
live fish. In preserved fish the spots are brown. A median dorsal
stripe is variably developed. Fins are lightly pigmented, most
melanophores being on the rays rather than the membranes. The anal and
paired fins are almost entirely hyaline. The caudal fin may show one or
two irregular bars running parallel to the posterior margin. The
peritoneum is light to silvery but bears scattered melanophores which
give a greyish tinge in preserved fish. Some fish from Iraq (Habbaniyah stream)
lacked, or had weakly expressed, flank spots.
Size
Reaches 50.7 mm standard length.
Distribution
This species is found in the Tigris-Euphrates basin, including its Iranian
part and the adjacent Gulf basin (Berg, 1932; 1949; Bianco and Banarescu, 1982). Abdoli (2000)
also records this species from the Jarrahi, the lower Karun, the lower Dez, the
Zohreh, the lower half of the Helleh, and the middle and lower Mand rivers.
Zoogeography
This species is found in the Tigris-Euphrates basin of Turkey,
Syria, Iraq and Iran. It does not appear to be common in Turkey, at
least in the upper reaches of this basin there, nor in upper reaches
of Iranian rivers. The distribution in the Dalaki River of Iran is
outside the modern Tigris-Euphrates basin. It is presumably a relict
of the late Pleistocene when the Tigris-Euphrates flowed down a
drained Gulf receiving tributaries now isolated by the
post-Pleistocene rise in sea level (Coad and Krupp, 1983).
Habitat
Found in both running and still water, from small streams only
1 m wide and irrigation ditches to major rivers more than 200 m
across. Current is slow to fast but generally an obvious flow is
apparent. However one specimen was collected in a fish pond near Ahvaz
(ZSM 25701). The collection localities in Iran are all at low
altitudes and no fish were taken in Zagros Mountain streams and
rivers. Collections were made over mud and pebble substrates in
shallow streams or at river margins. The species may also occur at the
surface in mid-river but no collections confirm this supposition.
Capture temperatures were 12-24°C and conductivity 0.45-10.5 mS. Salinity in drying pools of 20 cm depth
in Syria where this species was caught in March had Cl-1 =
390 mg/l and a salinity of 1.5‰ (Coad and Krupp, 1983).
Age and growth
Unknown.
Food
Gut contents include winged insects (Coleoptera, Heteroptera,
Thysanoptera and Diptera) and spiders, suggestive of surface feeding (Coad
and Krupp, 1983). Abdoli (2000) also reports Hymenoptera, Brachycera and Culicidae.
Reproduction
Most fish were collected in January when eggs were small but developing suggestive of spring spawning.
Al-Rudainy (2008) gives an absolute fecundity of about 200 eggs for Iraq.
aParasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This fish is found in suitable habitats of large rivers and in small ditches and does not appear to be in need of conservation.
Vulnerable in Turkey (Fricke et al., 2007).
Further work
Molecular or detailed osteological analyses might reveal its relationships to taxa from the Oriental region.
Sources
Type material: See above (ZISP 23955).
Iranian material: CMNFI, 1979-0120, 3, 19.3-50.7 mm standard length, Bushehr, Dalaki River near Konar Takhteh (29º28'N, 51º21'E);
CMNFI 1979-0357, 1, 27.6 mm standard length, Khuzestan, Karkheh River drainage (31º34'N, 48º12'E);
CMNFI 1979-0363, 11, 21.4-30.2 mm standard length, Khuzestan, Karkheh River (31º52'N, 48º20'E);
CMNFI 1979-0365, 7, 20.0-34.4 mm standard length, Khuzestan, Doveyrich River drainage (32º25'N, 47º36'30"E);
CMNFI 1979-0367, 1, 34.2 mm standard length, Khuzestan, Meymeh River (32º44'30"N, 47º09'30"E);
CMNFI 1979-0368, 29, 21.6-41.9 mm standard length, Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
CMNFI 1979-0372, 2, 30.7-33.1 mm standard length, Khuzestan, Dez River near Chogha Zanbil (ca. 32º02'N, ca. 48º30'E);
CMNFI 1979-0377, 3, 28.0-39.4 mm standard length, Khuzestan, Karkheh River (ca. 32º57'N, ca. 47º50'E);
CMNFI 1979-0378, 7, 31.9-42.4 mm standard length, Khuzestan, stream tributary to Karkheh River (ca.32º48'N, ca. 48º04'E);
CMNFI 1979-0380, 10, 25.3-41.0 mm standard length, Khuzestan, stream tributary to Dez River (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0381, 7, 24.3-31.2 mm standard length, Khuzestan, stream west of Shushtar (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0382, 4, 25.9-30.8 mm standard length, Khuzestan, Karun River at Shushtar (32º03'N, 48º51'E);
CMNFI 1979-0383, 8, 28.6-34.8 mm standard length, Khuzestan, Ab-e Shur drainage (31º59'30"N, 49º06'E);
CMNFI 1979-0384, 3, 26.8-40.8 mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0392, 3, 35.0-39.3 mm standard length, Khuzestan, Zard River (ca. 31º32'N, ca. 49º48'E);
CMNFI 1979-0396, 35, 25.1-48.8 mm standard length, Khuzestan, Kheyrabad River (30º32'N, 50º23'30"E);
ZSM 25701, 1, 36.5 mm standard length, Khuzestan, fishpond near Ahvaz (no other locality data);
ISSB uncatalogued, 1, 48.7 mm standard length, Bushehr, Helleh River (ca. 29º20'N, ca. 51º15'E) (Coad and Krupp, 1983).
Comparative material:- BM(NH) 1974.2.22:1256-1267, 11, 33.7-46.2 mm standard length, Iraq, stream between Lake Habanniyah and Euphrates River (ca. 33º22'N, 43º34'E);
BM(NH) 1968.12.13:217-220, 4, 18.5-47.4 mm standard length, Syria, Euphrates River at Mayadine (35º01'N, 40º27'E);
BM(NH) 1968.12.13:221-236, 16, 30.8-42.4 mm standard length, Syria, Tigris River at Ain Diwar (37º17'N, 42º11'E);
SMF 16442, 5, 28.2-35.9 mm standard length, Syria, Nahr Balikh at Jisr Shanine (36º03'N, 39º06'E);
SMF 16443, 63, 17.0-34.9 mm standard length, Syria, Nahr Balikh at Jisr Shanine (36º03'N, 39º06'E);
ISSB uncatalogued, 4, 32.8-34.4 mm standard length, Turkey, Batman Suyu (ca. 37º55'N, ca. 40º15'E) (Coad and Krupp, 1983).
Genus Blicca
Heckel, 1843
Shutov (1969) places this genus and species in the genus Abramis
Cuvier, 1817 on the basis of literature data as does analyses by
Shcherbukha (1973) and Howes (1981). Hensel (1978) and Tadajewska
(1998) also place this genus in Abramis on the basis of the
lateral line system structure, pharyngeal teeth, scale and dermal bone
morphology along with data on ecology, behaviour, ontogenesis,
osteology and parasitofauna. Hänfling and Brandl (2000) consider Blicca
a junior synonym to Abramis based on allozyme data. In
contrast, Bogutskaya (1986) using skull morphology reaffirms its generic status.
The white bream genus contains a single species found from Europe
to the Caspian Sea basin including Iran.
The genus is characterised by a deep and strongly compressed body;
scales absent on the back behind the dorsal fin thus forming a narrow
groove; a scaleless keel between the vent and the pelvic fins;
pharyngeal teeth in 2 rows; a small, oblique and subterminal mouth;
moderate number of gill rakers; scales of moderate size; a short and
spineless dorsal fin and a long anal fin; and a light peritoneum.
Blicca bjoerkna
(Linnaeus, 1758)

Common names
simparak or seamparak (= silver scales, possible meaning since parak is a small
feather), سيم نما (sim nama or mahi sim nama, meaning silvery-like fish or sim-like fish in
reference to Abramis brama).
[yastigarin in Azerbaijan; Zakavkazskaya gustera or Transcaucasian
white bream, Armyanskaya gustera for A. b. derjavini, all in
Russian; silver bream, white bream, flat bream].
Systematics
Cyprinus Björkna was originally described from Lake Mälar, Sweden.
Cyprinus Blicca Bloch, 1782 described from lakes in Germany,
Cyprinus gibbosus Pallas, 1814 described from the Sura and
Volga rivers and Blicca argyroleuca Heckel, 1843 are synonyms.
It appears that the latter taxon is first described in Heckel's work
on fishes of Syria, but in the section devoted to classification based
on the pharyngeal teeth of cyprinids; the taxon is later described
from Europe in Heckel and Kner (1858) and is not a Southwest Asian
species. Syntypes of Blicca argyroleuca are in the
Naturhistorisches Museum Wien under NMW 16901 (2 fish), NMW 54918 (6),
NMW 54919 (4) and NMW 54920 (1) (Eschmeyer et al., 1996). The
spelling bjorkna is incorrect (Eschmeyer et al., 1996).
The Caspian Sea basin subspecies is Blicca bjoerkna
transcaucasica Berg, 1916, described from the lower reaches of the
Kura River, Araks, Lenkoran District, Transcaucasia. It is
distinguished by "somewhat" fewer rays in the anal fin
(17-21) and "a tendency to have" fewer lateral line scales
(40-45) than in the type form which mostly has 21-22 anal fin rays and
45-48 lateral line scales (Berg, 1948-1949). Abdurakhmanov (1962)
expands these ranges to 17-22 and 40-48 respectively but gives low
means (± standard error) for 100 fish from Azerbaijan of 19.88±0.13
and 43.56±0.05 respectively. This may be a valid subspecies but the
possibility of clinal variation has not been examined.
Blicca bjoerkna derjavini Dadikyan, 1970 is described from
the "Sevdzhur River, (tributary of Araks River, in Armenian SSR)
and the canal and lake system connected with it". It is
distinguished from transcaucasica by lower mean number of
branched dorsal fin rays and branched anal rays, a higher mean lateral
line scale count, and various morphometric characters.
Key characters
The scaleless ventral keel, postdorsal groove, long anal fin, lateral line
scale count and small and oblique mouth are characteristic.
Morphology
Dorsal fin with 3 unbranched and 7-10 branched rays, usually 8,
anal fin with 3 unbranched and 16-24 branched rays. Pectoral fin
branched rays 14-16, pelvic fin branched rays 7-9. Lateral line scales 40-55.
Scales have numerous fine circuli, an almost central focus, a wavy
anterior margin and a crenulate posterior margin, and few primary
anterior and posterior radii, as few as 2 in each field (there may be
numerous secondary radii which do not reach the focus). There is a
pelvic axillary scale. Gill rakers 12-21, touching the adjacent raker
when appressed. Vertebrae 37-43. Pharyngeal teeth 2,5-5,2 with
variants 2,5-5,1, 1,5-5,2, 1,5-5,1, 2,5-4,2, 2,5-4,1, 1,5-4,1,
3,5-5,2, and 3,5-5,3 (among others, see below and Tadajewska (1998)),
weakly hooked (strongly hooked in young), compressed, concave below
the tip and smooth (anterior tooth margin serrated in young). In young
fish, the first major row tooth may be medial to the second tooth
rather than in line. Tadajewska (1998) gives details of tooth
development. The intestine is s-shaped with a small anterior loop. The
chromosome number is 2n=50 (Klinkhardt et al., 1995; Pourkazemi et al.,
2010).
Meristic values for Iranian specimens are:- dorsal fin branched rays 8(49) or 9(1);
anal fin branched rays 17(3), 18(15), 19(21), 20(9) or 21(2); pectoral
fin branched rays 14(15), 15(24) or 16(7); pelvic fin branched rays
7(1), 8(47) or 9(2); lateral line scales 41(2), 42(10), 43(9), 44(13),
45(10), 46(5) or 47(1); total gill rakers 13(2), 14(25), 15(18), 16(4)
or 18(1); pharyngeal teeth 2,5-5,2(3), 2,5-5,1(4), 1,5-5,2(4),
2,5-4,1(1), 2,5-5,0(1), 0,5-5,2(1), 1,5-5,1(1), 1,5-5,0(2), 1,5-4,1(2)
or 2,4-4,1(1); and total vertebrae 38(8), 39(33) or 40(12).
Sexual dimorphism
Breeding males have fine tubercles on the top of the head,
operculum and lining the exposed scale margins on the flank. There are
occasionally tubercles in mid-scale. Small tubercles are found on the
pectoral fin rays, 1-3 rows on the unbranched ray, 1-2 on the first
branched ray and usually 1 on the other rays, branching to follow the
branching rays. Other fins bear fine tubercles following the fin rays.
Larger tubercles are found in clumps on the scales overlapping the
anal fin base. Tubercles are absent from the belly. Fine unculi are
present on the snout, under the eye and between the tubercles on the
head generally as well as on the underside of the pectoral fin.
Colour
The back is a bluish-green and the rest of the body silvery. The
pectoral and pelvic fins are orange-red with grey tips. The peritoneum
is silvery with scattered melanophores.
Size
Reaches 54.5 cm and 2.25 kg.
Distribution
Found from England through Europe north of the Alps and Pyrenees to
the Caspian Sea basin. Apparently it does not penetrate to the higher
reaches of even major rivers like the Kura and Aras. In Iran it is found from
the Aras River (including its middle reaches in Iran) to the Atrak River in the
Caspian Sea basin including the Gorgan, Tajan, Babol, Haraz, Sardab, Tonekabon,
and Safid rivers, the Anzali Talab (Derzhavin, 1934; Holčík
and Oláh, 1992; Nejatsanatee, 1994; Abbasi et al., 1999; Kiabi et al.,
1999; Abdoli, 2000; Abdoli and Naderi, 2009).
Zoogeography
This species is part of a northern European and northern Southwest Asian
fauna whose zoogeographical history has not been thoroughly researched.
The relationships with similar genera are reviewed under the genus.
Habitat
This species is found in the shallows of warm lakes with heavy
vegetation and in the slower reaches of rivers including river estuaries in Iran
(Jolodar and Abdoli, 2004). It overwinters in
deeper water. There was a mass mortality of this species on the Babol
Sar beach on 24 June 1963 (USNM 271217).
Age and growth
Growth is slow with maturity attained at 3-5 years and 10-12 cm.
Some males may mature at 2 years. Females are much larger than males
of the same age. Life span is up to 16 years. Stunted populations
comprising large numbers of individuals develop where predators are absent.
Food
Food items include insect larvae such as chironomids, worms and
molluscs, and some vegetation. This is a euryphagous species. Young
fish feed principally on copepods and cladocerans. Even adults will
feed on plankton and it is less of a bottom feeder than Abramis brama.
Reproduction
Spawning in the Volga delta takes place about the beginning of May
at around 11°C water temperature but may run from the end of April to the middle of
July in the Volga generally. Spawning in the Aras flood plain occurs
in the middle of April. Generally spawning occurs later than in Abramis
brama and Rutilus rutilus but may overlap and infertile
hybrids result. Shallow weedy areas are preferred. Each female is
pursued by several males. Fecundity reaches 109,000 eggs and egg
diameter 1.44 mm. Eggs adhere to plants or stones on the bottom. There
can be 3 spawnings at intervals of 10-11 days when water temperatures
are at least 16-17°C. Batch spawning shows much individual variation as well as varying
between localities and by year at the same locality.
Parasites and predators
Khara et al. (2006a) record the eye
fluke Diplostomum spathaceum for this fish in the Amirkalayeh Wetland in
Gilan. Khara et al. (2008) found the eye parasite Diplostomum
spathaceum in this fish from Boojagh Kiashar Wetland in Gilan.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish. Tajbakhsh et al. (2010) report the nemtode Philometra rischta
from fish in the Anzali wetland.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984) as are a variety of other fishes such as perch
(Perca fluviatilis) and pike-perch (Sander sp.).
Economic importance
Holčík and Oláh (1992) report a catch of 144 kg in the Anzali Mordab in 1990.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and in textbooks.
Conservation
Lelek (1987) classifies this species as intermediate in Europe
(liable to be transferred to vulnerable or rare categories if their
habitat deteriorates further). Kiabi et al. (1999) consider this
species to be of least concern in the south Caspian Sea basin
according to IUCN criteria. Criteria include sport fishing, abundant
in numbers, habitat destruction, widespread range (75% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin.
Further work
The biology of this species needs study in Iran.
Sources
CMNFI 1970-0510, 1, 56.0 mm standard length, Gilan, Golshan River (37º26'N,
49º40'E); CMNFI 1970-0522, 4, 40.0-62.6 mm standard length, Gilan, Safid River
at Astaneh Bridge (36º16'30"N, 49º56'E); CMNFI 1970-0532, 6, 30.0-63.2 mm
standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0553, 4, 62.1-80.1 mm standard length, Gilan, Sowsar Roga River
(37º27'N, 49º30'E); CMNFI 1970-0579, 2, 52.6-56.9 mm standard length, Gilan, Old
Safid River estuary (37º23'N, 50º11'E); CMNFI 1970-0580, 31, 31.8-86.3 mm
standard length, Mazandaran, river near Iz Deh (36º36'N, 52º07'E); CMNFI
1970-0582, 1, 70.9 mm standard length, Mazandaran, Aliabad Reservoir (36º56'N,
54º50'E); CMNFI 1970-0585, 39, 32.4-52.5 mm standard length, Gilan, Nahang Roga
River (37º28'N, 49º28'E); CMNFI 1970-0587, 36, 34.6-55.4 mm standard length,
Mazandaran, Babol Sar (36º43'N, 52º39'E); CMNFI 1979-0470, 2, 44.5-51.2 mm
standard length, Mazandaran, stream west of Alamdeh (36º35'N, 51º43'E); CMNFI
1979-0472, 30, 38.7-69.6 mm standard length, Mazandaran, stream west of
Mahmudabad (36º37'N, 52º12'E); CMNFI 1979-0685, 3, 63.1-67.1 mm standard length,
Gilan, Safid River (37º24'N, 49º58'E): CMNFI 1980-0117, 1, 80.0 mm standard
length, Gilan, Golshan River (37º26'N, 49º40'E); CMNFI 1980-0122, 15, 38.7-45.3
mm standard length, Mazandaran, Nerissi River (36º38'N, 52º16'E); CMNFI
1980-0149, 6, 60.1-63.7 mm standard length, Gilan, Chabak River (37º21'N, 49º50'E).
Genus Capoeta
Valenciennes, 1842
The genus Capoeta has a wide distribution in Southwest Asia and contains about 20 species of which 7 occur in Iran. Its
affinities are uncertain and may lie with the European Barbus/Aulopyge group or with Cyprinion and its southern and east Asian
relatives (Karaman, 1971; Howes, 1982; Krupp, 1985c; Bănărescu, 1992b).
Varicorhinus Rüppell, 1836 (as used for Southwest Asian cyprinids) is a synonym of Capoeta Valenciennes, 1842 (see Karaman (1969) for further details: Capoeta is distinguished from Varicorhinus of Africa since it has a
denticulate last unbranched dorsal fin ray (as opposed to smooth), very small to medium-sized scales (large), lachrymal bone narrow and
covering only a small part of the upper side of the rostrum (large and covering most of the rostrum), suborbital bones narrow and long (short
and wide), posterior maxillary process not extending back to a level with the centre of the jugal (extends back to a level of the centre of
the suborbitals), lower jaw long (short). Scaphiodon Heckel, 1843 has been used for Capoeta and Cyprinion species in
Southwest Asia. The nomenclatural status of this genus is reviewed by Bănărescu in Bănărescu (1999).
This genus Capoeta is characterised by a compressed to rounded and moderately elongate body, small to moderately large scales
(lateral line counts 37-99), scales at the anal fin base and anus not usually enlarged (sometimes variably enlarged as is the case with
certain cyprinids), an inferior, transverse mouth, the lower jaw with a sharp, horny sheath, barbels absent or in 1 or 2 pairs, dorsal fin
short (usually 7-9 branched rays) with the last unbranched ray thickened and bearing serrations (serrations sometimes reduced to
absent), anal fin short (usually 5 branched rays), gill rakers short, moderate to numerous, pharyngeal teeth in 3 rows with spoon-shaped and
truncate tips, a very long and coiled gut (ca. 7-10 times body length), mostly of uniform colour, and a black peritoneum.
The general name for the members of this genus in northern Iran is سياه ماهي (= siah mahi, meaning black fish)
while in the south they are called twiny or touyeni and even gel cheragh (= mud-eater, mud-grazer). The name Capoeta is
derived from the Armenian and Georgian name for female Capoeta capoeta packed with eggs, namely "Kapwaeti". Other general names for
members of this genus shol khar, ghel khar or choul khar, all variant spoken intonations meaning mud eater.
The origin of Capoeta in Southwest Asia follows the same route as the genus Barbus (q.v.).
CMNFI 1977-0510A, 4, mm standard length, Fars, qanat at Naqsh-e Rostam (29º59'30"N, 52º54'E);
CMNFI 1979-0026, , mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0027, , mm standard length, Fars, Chehel Chashmeh (ca. 29º43'N, ca. 52º02'E);
CMNFI 1979-0036, 2, 83.9-118.3 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0043, , mm standard length, Fars, qanat behind Sarvestan (29º16'N, 53º14'E);
CMNFI 1979-0044, , mm standard length, Fars, qanat at Mian Jangal (29º09'N, 53º27'E);
CMNFI 1979-0053, 6, 47.3-79.5 mm standard length, Fars, Shur River tributary (ca. 28-29º58-03'N, ca. 52º34-35'E);
CMNFI 1979-0054, 16, 35.8-127.9 mm standard length, Fars, Shur River tributary (ca. 28-29º58-03'N, ca. 52º34-35'E);
CMNFI 1979-0057, , mm standard length, Fars, Shapur River 4 km from Shapur (29º49'N, 51º34'E);
CMNFI 1979-0058, 6, 75.6-115.3 mm standard length, Fars, jube over Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0059, 2, 45.0-50.4 mm standard length, ID, more than one species? Fars, Pulver River 8km south of Sivand (30º01'30"N, 52º57'E);
CMNFI 1979-0061, , mm standard length, ID, more than one species? Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0063, 2, 201.0-206.7 mm standard length, Fars, qanat under Sa'adi's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 1979-0067, , mm standard length, Fars, qanat at Zarqan (ca. 29º46'N, ca. 52º43'E);
CMNFI 1979-0068, , mm standard length, Fars, qanat 12 km from Shiraz on Esfahan road (ca. 29º43'N, ca. 52º34'30"E);
CMNFI 1979-0073, 5, 28.9-86.6 mm standard length, Fars, Mand River beyond Chehel Chashhmeh (ca. 29º42'30"N, ca. 52º01'30"E);
CMNFI 1979-0074, , mm standard length, Fars, Mand River backwater (29º41'N, 52º06'E);
CMNFI 1979-0075, , mm standard length, Fars, Mand River at Pol-e Kavar (29º11'N, 52º41'E);
CMNFI 1979-0079, 2, 120.7-149.9 mm standard length, Fars, Mand River 5 km above Band-e Bahman (ca. 29º12'N, ca. 52º38'E);
CMNFI 1979-0079, 1, 159.7 mm standard length, Fars, Mand River 5 km above Band-e Bahman (ca. 29º12'N, ca. 52º38'E);
CMNFI 1979-0090, , mm standard length, Esfahan, Gav Khuni (ca. 32º21'N, ca. 52º49'E);
CMNFI 1979-0093, 1, 73.9 mm standard length, (); BWC 76-77 check CMNFI #
CMNFI 1979-0109, 1, 91.1 mm standard length, Fars, Mand River ar Shahr-e Khafr (28º56'N, 53º14'E);
CMNFI 1979-0109, 1, 103.4 mm standard length, Fars, Mand River ar Shahr-e Khafr (28º56'N, 53º14'E);
CMNFI 1979-0111, 10, 8.7-54.6 mm standard length, Fars, stream 21-22 km from Shiraz (29º37'30"N, 52º21'E);
CMNFI 1979-0113, , mm standard length, Fars, qanat under Sa'adi's Tomb (29º37'N, 52º35'E);
CMNFI 1979-0114, , mm standard length, Fars, Mand River at road bridge (29º41'N, 52º06'E);
CMNFI 1979-0115, 4, 154.4-172.6 mm standard length, qanat under Sa'adi's Tomb (29º37'N, 52º35'E);
CMNFI 1979-0125, 1, 137.8 mm standard length, Bushehr, Dalaki River near Dalaki (ca. 29º28'N, ca. 51º21'E);
CMNFI 1979-0128, 16, 34.6-108.6 mm standard length, Fars, Shur River (28º51'N, 52º31'E);
CMNFI 1979-0128, 18, 17.2-135.3 mm standard length, Fars, Shur River (28º51'N, 52º31'E);
CMNFI 1979-0129, , mm standard length, Fars, spring 2 km north of Farrashband (28º54'N, 52º04'E);
CMNFI 1979-0130, 5, 44.4-93.3 mm standard length, Fars, Shur River 4 km west of Firuzabad (28º51'N, 52º32'E);
CMNFI 1979-0131, 58, 25.5-140.0 mm standard length, Fars, Mand River tributary (28º38'N, 52º49'E);
CMNFI 1979-0132, 23, 51.1-74.4 mm standard length, Fars, Mand River tributary (28º35'N, 52º58'E);
CMNFI 1979-0154B, 6, mm standard length, Fars, upper Shur River drainage near Darab (28º45'30"N, 52º24'E);
CMNFI 1979-0155, 7, 36.2-80.5 mm standard length, Fars, spring at Gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0156, 3, 54.6-122.9 mm standard length, Fars, qanat at Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0157, , mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E);
CMNFI 1979-0158, , mm standard length, Fars, qanat over Qasook River (28º54'N, 53º53'30"E);
CMNFI 1979-0159, 87, 23.1-167.3 mm standard length, Fars, qanat at Qaziabad (ca. 28º54'N, ca. 53º43'E);
CMNFI 1979-0160, 4, 66.3-138.4 mm standard length, Fars, Arteshkadeh Pomp spring (29º09'N, 53º37'E);
CMNFI 1979-0161, 29, 33.2-88.3 mm standard length, Fars, qanat on Neyriz to Shiraz road (29º10'30"N, 53º41'E);
CMNFI 1979-0162, 9, ?-88.3 mm standard length, Fars, qanat behind Sarvestan (29º16'30"N, 53º14'E);
CMNFI 1979-0163, 1, 73.8 mm standard length, ?Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0164, 1, 49.4 mm standard length, ?Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0165, 7, 30.0-96.6 mm standard length, Kerman, qanat at Ahmadabad (30º32'N, 55º38'E);
CMNFI 1979-0166, 67, 37.1-123.1 mm standard length, Kerman, qanat at Hassanabad-e Nuq (30º43'N, 55º50'E);
CMNFI 1979-0168, , mm standard length, Kerman, qanat at Shahabad (29º07'N, 58º16'E);
CMNFI 1979-0169, , mm standard length, Kerman, qanat 10 km from Mahan (30º08'30"N, 57º17'E);
CMNFI 1979-0170, , mm standard length, Kerman, qanat at Baghin (30º12'N, 56º48'E);
CMNFI 1979-0171, , mm standard length, Kerman, qanat at Bardesir (29º56'N, 56º34'E);
CMNFI 1979-0187, , mm standard length, Hormozgan, stream and pools at Sar Khun (27º23'30"N, 56º26'E);
CMNFI 1979-0191, , mm standard length, Fars, stream 10 km east of Furg (ca. 28º16'N, ca. 55º18'E);
CMNFI 1979-0192, , mm standard length, Fars, qanat 2 km east of Rostaq (28º26'30"N, 55º04'E);
CMNFI 1979-0195, , mm standard length, Fars, jube on road to Fasa (ca. 28º54'N, ca. 53º53'30"E);
CMNFI 1979-0198, , mm standard length, Fars, stream at Tadovan (28º47'N, 53º24'30"E);
CMNFI 1979-0199, 6, 70.8-102.1 mm standard length, Fars, qanat 18 km from Jahrom (ca. 28º23-25'N, ca. 53º31-40'E);
CMNFI 1979-0202, , mm standard length, Fars, Mand River (29º01'N, 53º00'E);
CMNFI 1979-0203, , mm standard length, Fars, qanat at Dudej (29º33'N, 52º59'E);
CMNFI 1979-0204, , mm standard length, Fars, qanat on road to Kharameh (29º33'N, 52º59'E);
CMNFI 1979-0205, 12, 45.9-200.5 mm standard length, Fars, jube at Runiz-e Pa'in (29º12'N, 53º42'E);
CMNFI 1979-0206, , mm standard length, Fars, qanat on road to Kharameh (29º12'N, 53º40'E);
CMNFI 1979-0207, 12, 24.2-83.7 mm standard length, Fars, jube 22 km from Neyriz (29º16'N, 54º28'E);
CMNFI 1979-0208, 6, 39.9-130.4 mm standard length, Fars, qanat 47 km from Neyriz (ca. 29º11'N, ca. 54º40'E);
CMNFI 1979-0209, 60, 43.6-138.9 mm standard length, Kerman, qanat at Kuch Kuluh (29º25'N, 56º03'E);
CMNFI 1979-0211, 63, 33.2-94.3 mm standard length, Kerman, river on road to Baft (29º19'N, 56º12'E);
CMNFI 1979-0212, 73, 26.0-99.1 mm standard length, Kerman, qanat on road to Baft (29º14'N, 56º17'E);
CMNFI 1979-0213, 5, 51.4-60.2 mm standard length, Kerman, stream in Kharan River drainage (29º15'N, 56º25'E);
CMNFI 1979-0214, , mm standard length, Kerman, qanat pool on road to Baft (ca. 29º15'N, ca. 56º28'E);
CMNFI 1979-0215, 15, 39.7-125.9 mm standard length, Kerman, Kharan River drainage (29º14'N, 56º37'E);
CMNFI 1979-0216, 11?, 51.1-65.8 mm standard length, Kerman, qanat 9 km from Baft (ca. 29º13'N, ca. 56º42'E);
CMNFI 1979-0217, 15, 39.7-125.9 mm standard length, Kerman, Kharan River drainage (ca. 28º59'30"N, ca. 56º51'30"E);
CMNFI 1979-0221, , mm standard length, Kerman, Halil River drainage (28º51'N, 57º52'E);
CMNFI 1979-0241, , mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0243, , mm standard length, Esfahan, Zayandeh River at Falavarjan (32º33'N, 51º31'E);
CMNFI 1979-0246, , mm standard length, Shahrestan-e Bakhtiari va Chahar Mahall, upper Karun River drainage (31º57'30"N, 50º59'E);
CMNFI 1979-0251, , mm standard length, Esfahan, stream 1 km east of Daran (32º59'N, 50º26'E);
CMNFI 1979-0251, , mm standard length, Esfahan, stream 1 km east of Daran (32º59'N, 50º26'E);
CMNFI 1979-0255, , mm standard length, Markazi, Bar River drainage 2 km west of Shahabiyeh (33º51'30"N, 50º23'E);
CMNFI 1979-0269, 1, 125.0 mm standard length, Lorestan, Dez or Karkheh River drainage (no other locality data);
CMNFI 1979-0271, , mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0272, , mm standard length, Lorestan, river at Nokhor (33º40-47'N, 48º28-45'E);
CMNFI 1979-0273, 7, 66.7-137.6 mm standard length, Lorestan, Kashkan River drainage (33º26'N, 48º19'E);
CMNFI 1979-0274, 3, 28.9-141.8 mm standard length, Lorestan, Kashkan River drainage (33º27'N, 48º11'E);
CMNFI 1979-0276, , mm standard length, Lorestan, Kashkan River drainage (ca. 33º19'N, ca. 47º53'30"E);
CMNFI 1979-0277, 2, 116.2-133.4 mm standard length, Lorestan, Kashkan River drainage (33º30'N, 47º59'30"E);
CMNFI 1979-0278, 3, 93.5-114.7 mm standard length, Lorestan, Kashkan River drainage (33º34'N, 48º01'E);
CMNFI 1979-0279, 1, 126.0 mm standard length, Lorestan, Khorramabad River 16 km from Nurabad (33º37'N, 48º18'E);
CMNFI 1979-0279, 5, 115.6-155.8 mm standard length, Lorestan, Khorramabad River 16 km from Nurabad (33º37'N, 48º18'E);
CMNFI 1979-0280, 3, 104.7-107.7 mm standard length, Lorestan, Kashkan River drainage (33º43-47'N, 48º12-15'E);
CMNFI 1979-0282, 6, 110.3-130.3 mm standard length, Lorestan, Seymareh River drainage at Nurabad (34º05'N, 47º58'E);
CMNFI 1979-0283, 2, 113.7-125.0 mm standard length, Kermanshahan, Qareh Su drainage (34º21'N, 47º07'E);
CMNFI 1979-0285, 3, 125.5-148.0 mm standard length, Kermanshahan, Qareh Su drainage (34º26'N, 46º37'E);
CMNFI 1979-0286, , mm standard length, Kermanshahan, Ravansar River at Ravansar (34º43'N, 46º40'E);
CMNFI 1979-0287, 2, 128.2-136.1 mm standard length, Kermanshahan, Chashmeh Javari 2 km from Ravansar (ca. 34º42'N, ca. 46º40'E);
CMNFI 1979-0288, 62, 37.6-153.7 mm standard length, Ilam and Poshtkuh, Gangir River at Sarab Ewan (33º50'N, 46º18'E);
CMNFI 1979-0289, , mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0290, , mm standard length, Kermanshahan, Diyala River drainage at Qasr-e Shirin (34º31'N, 45º35'E);
CMNFI 1979-0291, , mm standard length, Kermanshahan, Diyala River drainage (34º24'N, 45º37'E);
CMNFI 1979-0306, , mm standard length, Kerman, qanat on road to Baft (29º13'N, 54º33'E);
CMNFI 1979-0307, 5, 50.9-73.4 mm standard length, Kerman, river at Sartal 6 km from Baft (ca. 29º17'N, ca. 56º38'E);
CMNFI 1979-0308, 67, 20.5-246.9 mm standard length, Kerman, river 44 km from Baft (29º02'N, 56º50'E);
CMNFI 1979-0309, , mm standard length, Kerman, Fahraj River at Azizabad (28º57'N, 58º42'E);
CMNFI 1979-0315, 2, 53.5-65.5 mm standard length, Baluchestan, Bampur River 2 km from Karevandar (27º51'N, 60º46'E);
CMNFI 1979-0315, 34?, 53.7-85.1 mm standard length, (); note two collections?
CMNFI 1979-0337, , mm standard length, Baluchestan, stream near Kanowak (ca. 28º40'N, ca. 60º48'E);
CMNFI 1979-0341, 14, 27.2-75.9 mm standard length, Kerman, Tahrud west of Bam (29º23'N, 57º52'E);
CMNFI 1979-0343, , mm standard length, Fars, lake near Deh Bid (ca. 30º32'N, ca. 52º49'E);
CMNFI 1979-0411, 7, 42.2-76.5 mm standard length, Hormozgan, Minab River past Rudan (27º24'N, 57º12'E);
CMNFI 1979-0419, 1, 62.2 mm standard length, Fars, stream 7 km from Rostaq (28º29'N, 55º01'E);
CMNFI 1979-0420, 6, 57.1-150.6 mm standard length, Fars, Rudbar River at Bahregan (30º11'N, 52º03'E);
CMNFI 1979-0422, , mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh, stream in Yasuj valley (30º36'N, 51º34'E);
CMNFI 1979-0424, , mm standard length, Fars, stream on Yasuj to Nurabad road (30º18'N, 51º30'30"E);
CMNFI 1979-0425, , mm standard length, Fars, Haft Barm-e Kudian (29º49'N, 52º02'E);
CMNFI 1979-0426, , mm standard length, Esfahan, qanat at Abbasabad-Natanz (33º36'N, 51º49'E);
CMNFI 1979-0458, 2, 90.7-108.4 mm standard length, Markazi, Khar River 6 km north of Ab-Garm (35º47'N, 49º20'E);
CMNFI 1979-0460, 3, 54.4-65.0 mm standard length, Hamadan, stream 16 km south of Asadabad (34º39'N, 48º05'E);
CMNFI 1979-0462, , mm standard length, Markazi, Mazdaqan River (35º06'30"N, 49º40'30"E);
CMNFI 1979-0466, , mm standard length, Esfahan, qanat at Meymeh (33º27'N, 51º10'E);
CMNFI 1979-0484, , mm standard length, Khorasan, stream 22 km west from Bojnurd (37º28'N, 56º44'E);
CMNFI 1979-0497, 3, 49.8-113.0 mm standard length, Fars, Mand River at Band-e Bahman (29º11'N, 52º40'E);
CMNFI 1979-0497, 7, 102.2-132.0 mm standard length, Fars, Mand River at Band-e Bahman (29º11'N, 52º40'E);
CMNFI 1979-0499, , mm standard length, Fars, ditch 32 km from Kor River bridge (30º04'30"N, 52º36'E);
CMNFI 1979-0501, 6, 34.1-110.9 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1979-0502, , mm standard length, Fars, Haft Barm-e Kudian (29º49'N, 52º02'E);
CMNFI 1993-0126, , mm standard length, Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E);
CMNFI 2007-0030, , mm standard length, Baluchestan, stream near Eskelabad (28º35'N, 60º48'E);
CMNFI 2007-0031, , mm standard length, Baluchestan, headwaters of Bampur River (27º51'N, 60º46'E);
CMNFI 2007-0037, , mm standard length, Kerman, Hosseinabad and Gamatabad qanats at Bam (29º06'N, 58º21'E);
CMNFI 2007-0038, , mm standard length, Kerman, Mehtiabad qanat (29º06'N, 58º21'E);
CMNFI 2007-0039, , mm standard length, Kerman, Tahrud River (ca. 29º23'N, ca. 57º53'E);
CMNFI 2007-0040, , mm standard length, Kerman, Qahariz qanat at Jupar (30º04'N, 57º08'E);
CMNFI 2007-0041, , mm standard length, Kerman, qanat at Baghin (30º12'N, 56º48'E);
CMNFI 2007-0042, , mm standard length, Kerman, qanat at Negar (29º52'N, 56º50'E);
CMNFI 2007-0043, , mm standard length, Kerman, qanat at Emamzadeh Sultan (ca. 29º40'N, ca. 56º45'E);
CMNFI 2007-0044, , mm standard length, Kerman, Qal'eh-ye Askar stream (ca. 29º28'N, ca. 56º38'E);
CMNFI 2007-0045, , mm standard length, Kerman, Kharan River drainage at Baft (29º14'N, 56º38'E);
CMNFI 2007-0047, , mm standard length, Kerman, qanat at Hoshun (29º14'N, 56º19'E);
CMNFI 2007-0048, , mm standard length, Kerman, qanat at Hasanabad (ca. 28º50'N, ca. 55º50'E);
CMNFI 2007-0049, , mm standard length, Hormozgan, upper Kol River basin at Hajjiabad (ca. 28º19'N, ca. 55º55'E);
CMNFI 2007-0063, , mm standard length, Fars Mand River tributary outside Jahrom (28º36'N, 53º37'E);
CMNFI 2007-0065, , mm standard length, Fars, Barm-e Dalak (ca. 29º35'N, ca. 52º38'E);
CMNFI 2007-0066, , mm standard length, Fars, qanat under Sa'adi's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 2007-0067, , mm standard length, Fars, Sivan River (ca. 30º02'N, ca. 52º57'E);
CMNFI 2007-0068, 5, 59.0-89.6 mm standard length, Fars, qanat 4 km south of Abarqu (ca. 31º07'N, ca. 53º14'E);
CMNFI 2007-0069, , mm standard length, Yazd, qanat at Zarej (ca. 31º58'N, ca. 54º17'E);
CMNFI 2007-0070, , mm standard length, Yazd, qanat at Ardakan, (32º19'N, 53º59'E);
CMNFI 2007-0073, , mm standard length, Esfahan, Zayandeh River at Tanderan (32º47'N, 51º02'E);
CMNFI 2007-0075, , mm standard length, Hamadan, Malayer River south of Malayer (ca. 34º17'N, ca. 48º47'E);
CMNFI 2007-0076, , mm standard length, Markazi, Malekabad qanat (34º05'N, 49º53'E);
CMNFI 2007-0083, , mm standard length, Azarbayjan-e Khavari, Qaranqu River basin west of Sar Eskand Khan (ca. 37º25'N, ca. 46º55'E);
CMNFI 2007-0084, , mm standard length, Azarbayjan-e Khavari, Talkheh River basin west of Sarab (ca. 37º56'N, ca. 47º19'E);
CMNFI 2007-0091, , mm standard length, Azarbayjan-e Khavari, Zilber Chay basin west of Marand (38º30'N, 45º23'E);
CMNFI 2007-0100, , mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0108, , mm standard length, Kordestan, Qeshlaq River basin north of Sanandaj (ca. 35º33'N, ca. 47º08'E);
CMNFI 2007-0109, , mm standard length, Kordestan, Qeshlaq River basin south of Sanandaj (ca. 35º16'N, ca. 47º01'E);
CMNFI 2007-0110, , mm standard length, Kordestan, Yuzidar River basin (ca. 35º05'N, ca. 46º56'E);
CMNFI 2007-0115, , mm standard length, Kermanshahan, Qareh Su basin north of Kermanshah (ca. 34º34'N, ca. 46º47'E);
CMNFI 2007-0116, , mm standard length, Kermanshahan, Gav Masiab River basin west of Sahneh (ca. 34º28'N, ca. 47º36'E);
CMNFI 2007-0117, , mm standard length, Kermnashahan, Gav Masiab River basin near Sahneh (ca. 34º24'N, ca. 47º40'E);
CMNFI 2007-0117, , mm standard length, Kermnashahan, Gav Masiab River basin near Sahneh (ca. 34º24'N, ca. 47º40'E);
CMNFI 2007-0122, , mm standard length, Markazi, Khar River basin south of Takestan (ca. 35º56'N, ca. 49º30'E);
USNM 200308, 2, 37.5-47.3 mm standard length, Lorestan, Ab-e Khorramabad (33º30'N, 48º13'E); ? damascina
USNM 205933, 5, 97.5-142.4 mm standard length, Baluchestan, Karavandar Creek (no other locality data); ? damscina
USNM acc. 303854, 14, 29.1-44.6 mm standard length, Fars, Lake Arzhan (29º36'N, 51º59'E). ? damscina
USNM acc. 303854, 4, 55.7-99.0 mm standard length, Fars, pool east of Sangkar (29º48'N, 53º29'E); ?damascina
Capoeta aculeata
(Valenciennes, 1844)
Flank scale
Left pharyngeal arch
Common names
shum (= unlucky or inauspicious, possible meaning), سياه ماهي (= siah mahi),
زرده پر (= zardehpar), siah mahi aculeata.
Systematics
Chondrostoma aculeatum was originally described from "eaux douces de la Perse".
Scaphiodon macrolepis Heckel, 1849 described from the "Confluenten des Araxes bei Persepolis" (probably the Pulvar (= Sivan) River,
Fars near Persepolis) and Varicorhinus bergi Derzhavin, 1929 described in Latin from "Keredsh flumen propea Teherane, Persia
septentrionalis" (Karaj River near Tehran, northern Iran) are synonyms.
Six syntypes (MNHN 2357) of Chondrostoma aculeatum in poor condition are stored in the Muséum national d'Histoire naturelle,
Paris (Bertin and Estève, 1948; Coad and Krupp, 1994). They measure 86-179 mm standard length (Coad and Krupp, 1994) or 105-210 mm total
length (Bertin and Estève, 1948). The largest specimen is designated as the lectotype.
Two syntypes of Scaphiodon macrolepis are in the Naturhistorisches Museum Wien under NMW 55896 and measure 100-175 mm
standard length (Kähsbauer, 1964). Two other fish are marked as syntypes under NMW 51653 and are from Persepolis collected by Th.
Kotschy. The catalogue in Vienna lists 4 fish and the 1997 card index agrees these 4 fish are the syntypes.
Types of Varicorhinus bergi are unknown (Eschmeyer et al. (1996)).
Berg (1949) considers both aculeata and macrolepis as distinct species although very close, the latter distinguished from
the former by a deeper body and a shorter head. Karaman (1969) and Bianco and Banarescu (1982) place both aculeata and macrolepis
in Capoeta capoeta; Karaman does suggest that macrolepis could belong in aculeata. Saadati (1977) considers aculeatus
not more than subspecifically distinct from macrolepis, not realising the former has priority.
Key characters
This species differs from all others in the genus Capoeta in the lower number of lateral line (93% of 314 fish had range of 39-48)
and caudal peduncle scales (90% of 303 fish had a range of 16-20). Capoeta capoeta, a related species, usually has 54 or more lateral line
scales and 20 or more caudal peduncle scales.
Morphology
Dorsal fin with 3-5, modally 4, unbranched and 7-9, modally 8, branched rays. The last dorsal fin unbranched ray is thickened and
serrated, the denticles being long and narrowly spaced but not strongly developed. Distally this spiny ray is flexible. Smaller fish
have proportionately larger and more extensive denticles than larger fish. The extent of denticles from the base distally varies between
about two-thirds and three-quarters. Anal fin with 3 unbranched and 5-6, modally 5, branched rays, pectoral fin with 14-21 branched rays,
and pelvic fin with 7-10 branched rays.
Lateral line scales 36-52. Caudal peduncle scales 13-23. Scale shape is squarish with shallowly rounded to straight dorsal and
ventral margins, sharp corners anteriorly, and a large to moderate central protuberance on the anterior margin. Radii are most numerous
on the posterior field but even there are few, relatively few laterally and few anteriorly. Circuli are very fine but break into
coarser "bubbles" on the posterior field. The focus is subcentral anterior. The pelvic fin axillary scale varies greatly in size.
The mouth is slightly arched or even straight in ventral view. The horny edge to the lower jaw is usually well-developed but may be lost
in preserved specimens. Gill rakers number 16-25 and are short, reaching past the first or second raker when appressed. Rakers are
thick and usually hooked at their tips. Pharyngeal teeth are modally 2,3,4-4,3,2 (in 10 fish). Major row teeth are spatulate with a wide
crown in large fish. Total vertebrae number 39-44. The gut is extremely elongate with numerous anterior and posterior coils.
Meristic characters in Iranian fish are: dorsal fin branched rays 7(50), 8(255) or 9(4); anal fin branched rays 5(177) or
6(1); pectoral fin branched rays 14(2), 15(3), 16(12), 17(52), 18(123), 19(79), 20(27) or 21(6); pelvic fin branched rays 7(23),
8(183), 9(102) or 10(6); lateral line scales 36(1), 37(5), 38(8), 39(15), 40(25), 41(48), 42(56), 43(38), 44(40), 45(23), 46(22),
47(15), 48(10), 49(1), 50(4), 51(2) or 52(1); scales around the caudal peduncle 13(1), 15(5), 16(48), 17(52), 18(73), 19(64), 20(37), 21(12),
22(5) or 23(6); total gill rakers 16(3), 17(13), 18(40), 19(49), 20(62), 21(43), 22(43), 23(27), 24(16) or 25(6); and total vertebrae
39(1), 40(26), 41(90), 42(103) or 44(16).
Sexual dimorphism
Males have moderately large tubercles on the anal fin rays following the ray branching (2-4 tubercles on last 4 branched anal rays), small tubercles on the lowest caudal fin
ray, very fine tubercles on top of the head, larger tubercles on the side of the head, largest on the snout below the eye and nostril as
far as the mouth, connecting across the snout, and numbering 1-5 moderately large tubercles on flank scales variously arranged on each scale and best developed on the posterior part of the body.
Colour
The back is almost entirely black to green-brown or olive-green, the upper flank is brownish, the belly and lower flank are yellow up
to the lateral line, only the belly centre being white. The flanks are generally silvery in live fish. Some fish have small black spots on
the sides and fins. Preserved fish have pigment on the posterior, exposed margin and so are outlined on the flank. The sides of the head
are golden-brown. Flank spots may be in 5 longitudinal rows above, and 2 rows below, the lateral line. Some populations have fish with spots
and mottles on the body and fins but these are probably occasioned by a parasitic infestation. Fins are often reddish-brown to pink although
pelvic and anal fins may be yellowish-green and the dorsal and caudal fins very light to hyaline. Preserved fish have pigment on the rays
and membranes of fins without any distinctive pattern. The dorsal and caudal fins are darker than the lower fins. The iris is golden to
orange. The peritoneum is black.
Size
Reaches 23.4 cm standard length.
Distribution
This species is found in the Tigris River, Namak Lake,
Dasht-e Kavir, Kerman-Na'in, Esfahan, Kor River basins (Rainboth, 1981; Bianco and Banarescu, 1982; Ghorbani Chafi,
2000). Abdoli (2000) maps this species from theKerman-Na'in basin generally; the upper Kal Shur, Jajarm and Jovein rivers in the
Dasht-e Kavir basin; the middle and upper Shur and Abhar,
Qareh Chai and Qom rivers in the Namak Lake basin; the Zayandeh and Shur rivers in the Esfahan basin; the Jarrahi and Marun, upper Karun and Khersan, Dez, Karkheh, Simarreh
and Kashkan rivers in the Tigris River basin.
Zoogeography
Saadati (1977) suggests that this species moved eastward to basins on the plateau during more pluvial periods from the Tigris River basin.
See also above under genus.
Habitat
Unknown in detail.
Age and growth
Unknown.
Food
Gut contents include filamentous algae, plant fragments and diatoms with large amounts of sand. This species has been seen turning belly up to feed (field notes for specimens from Jajarm,
Khorasan).
Reproduction
Reproduction has not been studied in this species. Specimens from the Khorramabad River contained eggs 1.5 mm in diameter on 6 July and some seemed to be reabsorbing eggs. Spawning presumably
takes place in late spring and summer.
Parasites and predators
Barzegar et al. (2004) examined this species for parasites in fish from the Beheshtabad river in Chahar Mahall va Bakhtiari Province and
found Dactylogyrus lenkorani, Gyrodactylus sp. and Myxobolus sp.
Masoumian et al. (2007) record the myxosporean parasite Myxobolus
cristatus from this species in the Zayandeh River. Mehdipoor et al.
(2004) record the monogeneans Dactylogyrus chramuli, D. lenkorani
and D. gracilis in the Zayandeh River. Barzegar and Jalali (2006)
report parasites in this species from Kaftar Lake as Lernaea cyprinacea
and Trichodina sp.
Barzegar et al.
(2008) record the digenean eye parasites Diplostomum spathaceum and
Tylodelphys clavata from this fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea cyprinacea
on this species.
Economic importance
None.
Conservation
This species is widely distributed in Iran and does not appear to be in need of conservation but its biology and habitat requirements are unknown.
Kamali-Far et al.(2009) have used carp pituitary extract in an attempt to
induce spawning in this species. Hatchery production could then be used to
supplement natural stocks. However, the attempt was unsuccessful. Note that the
identity of the species used in this study needs verification judging from the
photograph in the paper.
Further work
The biology of this species needs study as does its habitat requirements and conservation needs.
Sources
Type material: See above, Chondrostoma aculeatum (MNHN 2357).
Iranian material: CMNFI 1979-0025, 2, 65.3-68.1 mm standard length, Fars, Kor River near Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0059, 155, 22.9-67.4 mm standard length, Fars, Pulvar River 8 km south of Sivand (30º01'30"N, 52º57'E);
CMNFI 1979-0061, 6, 28.6-64.9 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0069, 1, 28.7 mm standard length, Fars, qanat at Naqsh-e Rostam (29º59'30"N, 52º54'E);
CMNFI 1979-0070, 16, 25.9-60.0 mm standard length, Fars, Pulvar River near Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0090, 2, 153.6-160.5 mm standard length, Esfahan, Gav Khuni (ca. 32º21'N, ca. 52º49'E);
CMNFI 1979-0116, 49, 24.3-52.1 mm standard length, Fars, Kor River near Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0117, 14, 34.4-44.1 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0252, 3, ?, mm standard length, Markazi, jube at Baqerabad (34º55'N, 50º50'E);
CMNFI 1979-0253, 5, 40.4-103.7 mm standard length, Markazi, stream in Qareh Chay drainage (34º52'N, 50º49'E);
CMNFI 1979-0270, 1, 121.8 mm standard length, Lorestan, Kashkan River draiangae outside Khorramabad (33º26'N, 48º19'E);
CMNFI 1979-0271, 1, 52.1 mm standard length, Lorestan, stream in Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0273, 28, 51.4-104.5 mm standard length, Lorestan, stream in Kashkan River drainage near Khorramabad (33º26'N, 48º19'E);
CMNFI 1979-0274, 6, 20.6-59.2 mm standard length, Lorestan, stream in Kashkan River drainage (33º27'N, 48º11'E);
CMNFI 1979-0275, 1, 50.9 mm standard length, Lorestan, Kashkan River 2 km from Ma'mulan (33º25'N, 47º58'E);
CMNFI 1979-0279, 18, 41.1-129.9 mm standard length, Lorestan, Khorramabad River (33º37'N, 48º18'E);
CMNFI 1979-0282, 7, 99.2-130.8 mm standard length, Lorestan, river at Nurabad (34º05'N, 47º58'E);
CMNFI 1979-0283, 2, 125.2-186.3 mm standard length, Kermanshahan, Qareh Su near Kermanshah (34º21'N, 47º07'E);
CMNFI 1979-0343, 1, 146.6 mm standard length, Fars, lake near Deh Bid (ca. 30º32'N, ca. 52º49'E); check ID?
CMNFI 1979-0365, 1, 25.0 mm standard length, Khuzestan, stream in Doveyrich River drainage (32º25'N, 47º36'30"E);
CMNFI 1979-0396, 9, 32.5-58.7 mm standard length, Khuzestan, Kheyrabad River 20 km from Behbehan (30º32'N, 50º23'30"E);
CMNFI 1979-0427, 2, 100.5-112.2 mm standard length, Markazi, Cheshmeh Fin at Fin (33º57'N, 51º24'E); checkID?
CMNFI 1979-0428, 17, 25.9-104.5 mm standard length, Markazi, stream 3 km south of Sen Sen (34º13'N, 51º16'E); checkID?
CMNFI 1979-0458, 9, 48.5-117.8 mm standard length, Markazi, Khar River 6 km north of Ab-garm (35º47'N, 49º20'E);
CMNFI 1979-0460, 1, 77.6 mm standard length, Hamadan, stream 16 km south of Asadabad (34º38'N, 48º03'E); checkID?
CMNFI 1979-0463, 8, 97.9-135.3 mm standard length, Markazi, Qareh Chay (34º53'N, 50º24'E); checkID?
CMNFI 1979-0464, 1, 74.2 mm standard length, Markazi, qanat at Kheyrabad (34º08'N, 50º00'E);
CMNFI 1979-0465, 18, 35.7-58.3 mm standard length, Markazi, Qom River (34º18'30"N, 50º32'E);
CMNFI 1979-0500, 2, 92.4-98.6 mm standard length, Fars, Pulvar River near Naqsh-e Rostam (29º59'N, 52º54'E); checkID?
CMNFI 1980-0156, 27, ? mm standard length, Markazi, Karaj River (35º47'N, 50º58'E);
CMNFI 1993-0154, 1, mm standard length, Markazi, Sharra River near Far (34º03'N, 49º20'E); checkID?
CMNFI 1993-0156, 1, mm standard length, Markazi, Sharra River (34º03'N, 49º21'E); checkID?
CMNFI 2007-0006, 9, 59.9-127.2 mm standard length, Khorasan, spring in Qareh Su basin south of Garmeh (ca. 36º58'N, ca. 56º15'E);
CMNFI 2007-0007, 8, 59.4-79.3 mm standard length, Khorasan, stream supplemented by qanats, Kal-e Tangeh (ca. 36º59'N, ca. 56º29'E);
CMNFI 2007-0008, 2, 72.1-84.3 mm standard length, Khorasan, qanat at Jajarm (36º57'N, 56º23'E);
CMNFI 2007-0009, 18, 35.9-108.1 mm standard length, Khorasan, qanat at Amirabad (ca. 36º31'N, ca. 56º45'E);
CMNFI 2007-0010, 11, 80.8-123.1 mm standard length, Khorasan, qanat at Haresabad (36º07'N, 57º37'E);
CMNFI 2007-0011, 12, 34.1-85.4 mm standard length, Khorasan, Kalshur River south of Neyshabur (36º05'N, 58º43'E);
CMNFI 2007-0071, 10, 70.4-156.9 mm standard length, Esfahan, qanat at Mohammadiyeh, Na'in (32º51'N, 53º06'E);
CMNFI 2007-0074, 29, 50.6-100.7 mm standard length, Markazi, Qareh Chai west of Arak (34º03'N, 49º21'E);
CMNFI 2007-0075, 16, 29.3-152.5 mm standard length, Hamadan, Hamadan, Malayer River 5 km from Malayer (ca. 34º17'N, ca. 48º47'E);
CMNFI 2007-0076, 5, 56.1-97.4 mm standard length, Markazi, Malekabad qanat east of Arak (34º05'N, 49º53'E);
CMNFI 2007-0078, 8, 37.6-102.8 mm standard length, Markazi, Qom River (ca. 34º18'N, ca. 50º32'E);
CMNFI 2007-0117, ?, mm standard length, Kermanshahan, Gav Masiab River basin near Sahneh (ca. 34º24'N, ca. 47º40'E);
CMNFI 2007-0119, ?, mm standard length, Kermanshahan, Gav Masiab River basin near Kangavar (ca. 34º31'N, ca. 48º03'E);
CMNFI 2007-0120, 15, 29.0-165.5 mm standard length, Hamadan, Ab Chay near Hamadan (ca. 34º49'N, ca. 48º29'E);
CMNFI 2007-0122, 12, 35.0-77.6 mm standard length, Markazi, Khar River basin south of Takestan (ca. 35º56'N, ca. 49º30'E);
BM(NH) 1934.10.29:2, 1, 84.0 mm standard length, Markazi, Tehran (no other locality data);
BM(NH) 1958.11.7:1-6, 6, 25.6-89.9, Khorasan, Jajarm (36º57'N, 56º23'E);
BM(NH) 1975.1.17:255-258, 4, 103.0-161.0 mm standard length, Esfahan, Esfahan (no
other locality data);
MNHN 1960-611, 2, 127.0-144.0 mm standard length, Markazi, Jajrud east of Tehran (ca. 35º45'N, ca. 51º42'E)
USNM 205932, 3, 78.5-159.4 mm standard length, Markazi, stream southwest of Tehran (35º34'N, 51º03'E);
ZMH 5905, 2, 57.0-70.0 mm standard length, ?, Jafar Abad qanat (?);
ZSM 25703, 1, 76.3 mm standard length, ?, Khorramabad River (no other locality data).
Capoeta barroisi
Lortet, 1894
Common names
siah mahi-ye Dasht-e Arzhani (Arzhan Plain black fish).
[tela barroisi in Arabic; spotted barb, Tigris barb].
Systematics
Subspecies are Capoeta barroisi persica Karaman, 1969 described from "See Zariwar, Mariwan, 120 km westlich v. Sannadaj"
(Lake Zaribar near Marivan, Kordestan in the Tigris River basin) and Capoeta barroisi mandica Bianco and Banarescu, 1982 from the "Mand
River near Dasht-e-Arzhan" of Fars Province. Krupp (1985c) considers both these to be synonyms of the nominal subspecies, C. b. barroisi.
The subspecies persica is distinguished from the type subspecies by having a more horseshoe-shaped mouth, 8 branched dorsal
fin rays, 18 gill rakers, blackish pectoral, pelvic and anal fins, few but very large black spots on the body, a shorter anal fin and a
longer pectoral fin, and a deep body, based on a single specimen. Krupp (1985c) considers the characters of mouth form and colour to
fall within the range of the nominal subspecies (and by implication the other characters too).
Özuluğ and Freyhof (2008) found it difficult to reach a conclusion on the
taxonomic status of this subspecies on the basis of a single specimen which
could be abnormal.
C. barroisi mandica differs from the
type subspecies (C. barroisi barroisi) and C. b. persica in number of scales (61-68 in mandica (58-68 in types examined by me), 69-82 in barroisi,
78-79 in persica), number of gill rakers (21-24 in mandica (22-27 in types examined by me and apparently number is related to size of fish),
27-31 in barroisi, 18 in persica), from barroisi in having usually 8 branched dorsal fin rays (barroisi has 9
but persica also has 8), and from persica by a straight mouth (also straight or transverse in barroisi, arched in persica).
Krupp (1985c) considers the scale counts to be within the lower range of the nominal subspecies, gill raker counts and mouth position do not
differ from the nominal subspecies, and the dorsal fin ray count of 8 is seen in the subspecies mandica. Krupp observes that meristic
and morphometric characters are extremely variable in widely distributed Capoeta species.
Özuluğ and Freyhof (2008) examined 5 juvenile specimens from the Mand River and
consider the subspecies mandica to be a valid species. Widespread taxa
like Capoeta species are prime candidates for molecular analyses
which might help resolve conflicting views on - a single widespread, variable
species versus several distinct species.
Berg (1949) considers this species to be close to C. damascina, differing by having a stronger spine in the dorsal fin, hardly an
invariant character. Saadati (1977) considers that C. barroisi of Karaman is in fact C. damascina.
Turan (2008) using mt 16S rDNA concluded on this evidence that C. barroisi
was a subspecies of C. damascina although other genetic markers should be
used for a more reliable assessment.
Syntypes of Capoeta barroisi are in the Musée Guimet d'Histoire Naturelle, Lyon (MGHN 3492, 316 mm standard length, from
the Orontes near Antakya in Turkey collected by E. Chantre and MGHN 3493, 278 mm standard length, from Buhairat Hims in Syria collected by Th. Barrois) (Krupp, 1985c).
The holotype of C. barroisi persica is in the Zoologischen Instituts und Zoologischen Museums der Universität Hamburg (ZMH
H4119, 185.2 mm standard length, Daryacheh-ye Zaribar, 35°32'N, 46°08'E, IV. 1968, W. Nümann (Bianco and Banarescu, 1982; Krupp, 1985c; examined and measured by me).
The holotype of C. b. mandica, 106.9 mm standard length, is in the Istituto di Zoologia dell'Universitá di L'Aquila, Italy (IZA 7890), with 95 paratypes from the same locality
in IZA 7891 (now numbering 84 fish measuring 34.2-84.9 mm standard length) and 5 paratypes in the Institutul de Stiinte Biologice, Bucuresti, Romania (ISBB 3123), these 100 specimens
having a standard length of 34-86 mm. Six paratypes of mandica are in the Canadian Museum of Nature, Ottawa under CMNFI 1982-0366 (from IZA 7891).?lengths
Key characters
The dorsal fin branched ray count of usually 9 rays is characteristic for the type subspecies but not nominal Iranian ones. Gill raker counts, a head length greater than the dorsal fin spine
length and the irregular brownish markings on a silvery-white body are also distinctive.
Morphology
Dorsal fin with 3-4 unbranched and 8-10 branched rays, usually 9 in the type subspecies but 8 in the subspecies mandica, anal fin with 3 unbranched and 5 branched rays.
Pectoral fin branched rays 15-16, pelvic fin rays 7-8. Lateral line scales 61-82, 58-68 in the subspecies mandica. Scales on the belly in front of the pelvic fins are
small and may not be imbricate. Pharyngeal teeth 2,3,4-4,3,2. Gill rakers 18-31, reaching the second adjacent raker when appressed. The last dorsal fin unbranched ray is very strong, but
narrows distally, and bears large denticles or serrations on three-quarters of its length. The snout in the holotype of the mandica subspecies has a depression in front of the nostrils.
ZMH6086 D8, A5 P15, V7, ll 58, gr 24, flanks speckled dorsally, mouth a gentle arch almost straight, large denticles in damaged D spine.
Sexual dimorphism
Tubercles in males are found from eye to eye around the snout with fine tubercles sparse on the top of the head. Most flank scales have a single,
centrally-placed tubercle as do scales on the caudal peduncle. There is a single row of tubercles on the last three anal fin rays. There are some weak tubercles on the side of the head.
Colour
There are numerous, small, distinctive brown to black spots on the head, flank and dorsal and anal fins. The back and upper flank are silvery-white with slate to violet overtones and the
belly is yellowish. The holotype of the subspecies mandica has light specking and mottling on the upper flank and back. All fins have some speckling on the rays and membranes
but no clear rows of spots. Smaller fish (paratypes) have darker and bigger speckles than the holotype which extend lower on the flank.
Size
Reaches 31.6 cm standard length.
Distribution
The subspecies C. barroisi barroisi is found in the Ceyhan, Orontes, Quwayq and Tigris-Euphrates basins, C. b. persica only in Lake Marivan or Zaribar of the Tigris River basin
of Iran, and C. b. mandica in the Mand River of the Gulf basin. Krupp (1985c) includes Iranian Gulf drainages for the type subspecies. Abdoli (2000) has Jarrahi and lower Karun in the
Tigris River basin and the middle and lower Hilleh, lower Mand, and lower Dasht-e Palang rivers in the Gulf basin.
Zoogeography
Taxa in Iran are remote from the type locality of this species and may be indicative of the links between the Levantine fauna and the former tributaries
of the Tigris-Euphrates basin in southern Iran. See also above under genus.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Nothing is known of biology and a conservation assessment cannot be made. Endangered in Turkey (Fricke et al., 2007).
Further work
The biology of this species needs study as a prerequisite for a conservation assessment. The relationships of nominal Iranian taxa to material from the type
locality need further study using molecular techniques.
Sources
Type material: Capoeta barroisi mandica (IZA 7890, 7891, CMNFI 1982-0366 (from IZA 7891)) and C. barroisi persica (ZMH H4119).
Iranian material: ZMH 6086, 1, 73.6 mm standard length, Fars, Shur Fluβ, zufluβ von Mand-Fluβ (= C. b. mandica) P. Bianco ?check this against his paper.
Comparative material: BMNH 1974.2.22:1853-1856, 3, 48.9-60.2 mm standard length, Iraq, Kaliasan near Sulaymaniyah
Capoeta buhsei
Kessler, 1877
Common names
shamshiri (= sword-like), mahi sibili (= moustached fish, from Karaj Lake).
Systematics
Varicorhinus nikolskii Derzhavin, 1929 described in Latin from the "Keredsh flumen" (= Karaj River near Tehran) is a synonym. Saadati (1977) places Capoeta buhsei
in Capoeta damascina.
The 2 syntypes of Capoeta Buhsei, 200.7-211.4 mm standard length, are in the Zoological Institute, St. Petersburg (ZISP 2330) and were collected "iz Persii" (= from Persia)
by Dr. Buhse in 1849. The 11 syntypes of Varicorhinus nikolskii have not been located (Eschmeyer et al., 1996).
Key characters
This species is distinguished by its low total gill raker count of 9-17, mean 12.4, modes 12 and 13, lower arch rakers 7-10 (cf. C. damascina which has 17-25 total rakers, mean
based on ? specimens), the absence of a keel in front of the dorsal fin, the mouth structure, and by a very weak, unserrated or barely serrated dorsal fin spine in large fish (cf.
C. damascina).
Morphology
Dorsal fin unbranched rays 3-4, branched rays 7-9, anal fin unbranched rays 3, branched rays 5., pectoral fin branched rays 14-19, and pelvic fin branched rays 7-9. Lateral line scales 72-99.
Scales are found on the back and on the belly. A pelvic axillary scale is present. Scales have parallel dorsal and ventral margins, a rounded posterior margin and an anterior
margin with a rounded central protuberance. Radii are found on all fields including a few long and curved ones on the lateral fields. The focus is subcentral anterior and circuli are numerous and fine.
Gill rakers 9-19 in literature (but see below), including some counts probably for the lower arm only, and reach the second raker below when appressed but only the next raker in small fish. Pharyngeal
teeth in the main row are spatulate, the crowns flat, narrow and curved. Tooth counts are 2,3,4 or 5-5 or 4,3,2. The fifth tooth in either row is small and variably present. This may be size related
although the fish examined here were all relatively small and showed no clear trend. The gut is elongate with several long coils.
The mouth is large and a shallow horseshoe-shape with the horny lower jaw layer weakly developed but the lower lip corners in particular fleshy and well-developed. The last unbranched dorsal fin
ray is weak with the distal half thin and flexible. Denticles are found on the basal third to two-thirds or more of the ray, their extent and size variable but usually small, weak and less extensive in
large fish, while larger and more extensive in the smallest fish. For fish 48.9-174.0 mm standard length extent of dorsal fin spine serrations in spine length is 0.3-0.8, mean 0.6. The lower lip is
apparent and finely ridged. The upper lip and snout are covered with unculi which occur also over the head but more widely spaced out. The upper lip unculi are densely concentrated and are broader than
other head ones. Unculi are also on the lower head surface and belly scales back to the pelvic fins, and on the anal, pectoral and pelvic fin rays and membranes.
Meristics are as follows: dorsal fin branched rays 8(35) or 9(3); anal fin branched rays 5(38); pectoral fin branched rays 14(1), 17(20), 18(11) or 19(6); pelvic fin branched rays 7(1), 8(5), 9(31);
lateral line scales 72(2), 73(1), 75(3), 76(4), 77(2), 78(1), 79(7), 80(4), 81(5), 82(2), 83(2), 84(2), 86(2) or 91(1); total gill rakers 9(2), 10(2), 11(4), 12(12), 13(11), 14(4), 15(2) or 17(1);
pharyngeal teeth 2,3,4,-4,3,2(7), 2,3,4-5,3,2(6), 2,3,5-4,3,2(5), 2,3,5-5,3,2(1), 1,3,4-4,3,2 (1) or 2,3,4-4,3,1(1); and total vertebrae 43(1) and 44(6) (USNM 20593 and the syntypes).
Sexual dimorphism
One male specimen measuring 94.6 mm standard length bears large tubercles on anal fin rays, fine tubercles scattered on the head, on the back and upper flanks one tubercle per scale at the scale
centre but not on every scale, all along the lateral line at one tubercle per scale, and below the lateral line only in the area above the anal fin.
Colour
Overall colour is brownish in preservative without spots or any distinctive markings. The back is dark. The peritoneum is dark brown to black in preserved fish.
Size
Reaches 25.7 cm.
Distribution
This species is endemic to the Namak Lake basin of Iran (Derzhavin, 1929; Wossughi, 1978; Abdoli, 2000). Abdoli (2000) questionably maps it from the Esfahan basin. A report from Lake Zaribar,
Kordestan (Abzeeyan, 5(5):III, 1994) is presumably a mis-identification and records from springs of Kul River basin near Darab in the Hormuz basin (Bianco and Banarescu (1982) and the
Hamun-e Jaz Murian basin (Vossoughi, 1998) are also questionable.
Zoogeography
An endemic of an interior Iranian basin, its zoogeographical relationships to other Capoeta have not been resolved. See also above under genus.
Habitat
Unknown.
Age and growth
Unknown.
Food
Gut contents include aquatic insect larvae and masses of filamentous algae, suggesting that aufwuchs is an important diet item.
Reproduction
Generally unknown but fish caught on 5 June measuring 121.3-132.6 mm standard length have small eggs, perhaps because this size of fish is not mature. A 174.0 mm standard length caught in January
has larger eggs than those from the June fish. A male fish caught on 5 May and measuring 146.6 mm standard length has mature testes.
Parasites and predators
Williams et al. (1980) report the helminths Khawia armeniaca (a cestode) and Acanthocephalorhynchoides cholodkowskyi (an acanthocephalan) from this species in the
Zayandeh River at Esfahan.
Economic importance
Unknown.
Conservation
The conservation status of this species has not been determined by field studies and assessments can only be done from museum collections.
Further work
The distribution and numbers of this species in the Namak Lake basin should be examined by field studies to determine the population status. This basin is mostly in Markazi (= Central) Province
which contains Tehran and a very large human population with great demands on limited water resources. It is probably not under any immediate threat but is an Iranian endemic.
Sources
Type material: See above, Capoeta buhsei (ZISP 2330).
Iranian material: CMNFI 1970-0588, 19, 42.4-128.9 mm standard length, Markazi, Karaj Lake (35º57'N, 51º06'E);
CMNFI 1979-0094, 2, 143.1-174.0 mm standard length, Markazi, Karaj Lake (35º57'N, 51º06'E);
CMNFI 1979-0266, 2, 52.4-54.3 mm standard length, Esfahan, spring at Nowqan (ca. 33º10'N, ca. 50º05'E);
CMNFI 1979-0458, 1, 94.2 mm standard length, Markazi, Khar River (35º47'N, 49º20'E);
CMNFI 1979-0459, 2 ?check fish, only 1 in catalogue, 27.0-31.6 mm standard length, Hamadan, stream 2 km south of Razan (35º22'N, 49º02'E);
CMNFI 1979-0461, 1, 54.1 mm standard length, Hamadan, qanat at Taveh (35º07'N, 49º02'E);
CMNFI 1979-0495, 1, 42.5 mm standard length, Markzai, Nam River west of Firuzkuh (35º43'N, 52º40'E);
CMNFI 1980-0154, 71, 12.0-34.9 mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1980-0156, 27, 32.4-54.3 mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1993-0151, 1, 146.4 mm standard length, Markazi, Sharra River near Far (34º03'N, 49º19'E);
CMNFI 1993-0152, 2, 121.3-132.6 mm standard length, Markazi, Sharra River near Khosbijan (34º07'N, 49º23'E);
CMNFI 1993-0153, 2, 104.3-138.9 mm standard length, Markazi, Sharra River near Emarat (33º52'N, 49º36'E);
CMNFI 1993-0154, 1, 124.0 mm standard length, Markazi, Sharra River near Far (34º03'N, 49º20'E);
CMNFI 2007-0074, 3, ? mm standard length, Markazi, Qareh Chay (34º03'N, 49º21'E);
CMNFI 2007-0078, 5, ? mm standard length, Markazi, Qom River (ca. 34º18'N, ca. 50º32'E): check ID?
CMNFI 2007-0079, 14, ? mm standard length, Zanjan, Abhar River basin (ca. 36º16'N, ca. 49º08'E);
CMNFI 2007-0120, , mm standard length, Hamadan, Ab Chay (ca. 34º49'N, ca. 48º29'E);
CMNFI 2007-0121, 3, 82.5-141.5 mm standard length, Hamadan, Qareh Su basin north of Razan (ca. 35º25'N, ca. 49º02'E);
CMNFI 2007-0122, , mm standard length, Markazi, Khar River basin south of Takestan (ca. 35º56'N, ca. 49º30'E);
USNM 20593, ?, ? mm standard length, ();
ZMH 2632, 1, 148.2 mm standard length, Dojodje ();
ZMH 2633, ?, ? mm standard length, above Latian ().
Capoeta capoeta
(Güldenstaedt, 1773)
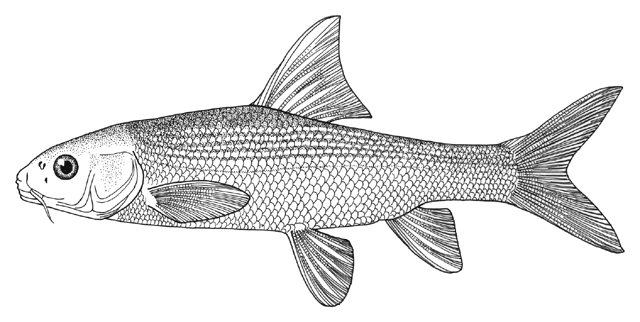

Common names
tilkhos, سياه ماهي (= siah mahi, meaning black fish), sang lisak (= rock snail?); soru (= slippery) in the Dalaki and Shapur river basins.
[gara balig or Lankaran xramulyasi for C. c. gracilis, Kur xramulyasi for C. capoeta, both in Azerbaijan; khramulya, capoeta, kapuit, kaput (all apparently derived from local names in
Georgia and Armenia, namely khramuli and kapweti); Lenkoranskaya khramulya or Lenkoran khramulya, Kurinskaya khramulya or Kura khramulya, Zakaspiiskaya khramulya or Transcaspian khramulya (also
marinka is used locally for the Transcaspian khramulya subspecies but this is an error), Araksinskaya khramulya or Araks khramulya, all in Russian; Transcaucasian barb; khramulia; kersin handscherli
at Aleppo, in Arabic].
Systematics
Cyprinus capoeta was originally described from Tbilisi, Georgia.
Cyprinus fundulus Güldenstaedt, 1787 from the Caspian Sea, Cyrus River (and Capoeta fundulus Valenciennes, 1842), Scaphiodon asmussii Keyserling,
1861 from "Warme Quelle bei Sultan Karaul, 8 Meilen nordöstlich von Herat" (now in Afghanistan, formerly in Persia), Scaphiodon gracilis Keyserling, 1861 from "Wasserleitung
bei Gaes, einige Meilen von Isphahan", Scaphiodon heratensis Keyserling, 1861 described from the "Heri-Rud, ein Fluss bei Herat" (now in Afghanistan, formerly in Persia),
Capoeta Hohenackeri Kessler, 1877 from Caucasia (probably lower Kura and Araks rivers, Azerbaijan), Capoeta (Scaphiodon) Steindachneri Kessler, 1872 and Capoeta Steindachneri
var. platylepida Kessler, 1872 both from the Zeravshan River, Uzbekistan, and probably Capoeta gibbosa Nikol'skii, 1897 described in Latin as from "Bochsani in Persia orientale"
are synonyms. Capöeta Guldenstädtii De Filippi in Tortonese, 1940 from "F. Arasse, Erzerum (Anatolia)" is Capoeta capoeta but it is a manuscript name and is not available
(Tortonese, 1940; Eschmeyer et al., 1966); 2 syntypes are in the Istituto e Museo di Zoologia della R. Università di Torino (MZUT N.729).
Eschmeyer et al. (1996) have the date of Cyprinus capoeta as 1772; the type locality is Tiflis, Caspian Sea: they also have Cyprinus fundulus authored by Pallas, 1814 although
Berg (1948-1949) has Güldenstädt as the author.
A hybrid of Capoeta capoeta heratensis and Schizothorax pelzami is reported from the northern Kopetdag in Turkmenistan (Starostin, 1936).
Capoeta hohenackeri Kessler, 1877 described from tributaries of the Kura and Aras rivers has a high lateral line scale count in the original description (78) and might be a mislabelled
Capoeta tinca (Heckel, 1843) from Black Sea drainages of Georgia and Turkey rather than the Caspian Sea basin.
Capoeta capoeta gracilis is the subspecies of much of Iran and Capoeta capoeta heratensis (figure above) is the subspecies from the Tedzhen River basin (Berg, 1949). The former
usually has one pair of barbels, the latter two pairs (but see below). Bianco and Banarescu (1982) limit C. c. gracilis to basins between the Safid River and the Atrak while C. c. capoeta
is found in the Kura-Aras basin. Holčík and Jedlička (1994) consider that the two subspecies gracilis and heratensis do not exist but that the taxon C. capoeta exhibits
clinal variation.
Bănărescu in Bănărescu (1999) limits C. capoeta gracilis to the Lake Orumiyeh basin and the Safid River in Iran (and the lower Kura River of Azerbaijan) while his C.
capoeta aff. gracilis (an unnamed subspecies related to C. capoeta gracilis) is found along the rest of the Iranian Caspian shore. However his material was limited (and did not
include any from Esfahan, the type locality of gracilis) and the analysis is based on lateral line scale counts only. Bănărescu in Bănărescu (1999) also states that
C. capoeta sevangi de Filippi, 1865 is the subspecies of the Araxes River basin, presumably including Iran, distinguished from the type subspecies, C. capoeta capoeta of
the Kura River basin, by having the dorsal fin margin straight or slightly convex as opposed to slightly to moderately notched. This character does not seem to be significant for such wide ranging and
variable populations, which he admits in one case at least (Kura River at Mingechaur), show differences between samples from the same locality at different times.
Abdurakhmanov (1962) compares fish from the Kura River basin (presumably C. c. capoeta) with fish from the Lenkoranchai and Bilyashchai in Azerbaijan (C. c. gracilis) and finds that the
latter have fewer dorsal fin rays on average, greater head length and depth, smaller eye, longer snout and postorbital distance, greater body depth and caudal peduncle depth, a shorter postdorsal
distance, a shorter dorsal fin base, lesser dorsal fin height, a longer anal fin base, a greater pectoral-pelvic distance and a shorter pelvic-anal fin distance.
Dadikyan (1986) refers to Varicorhinus capoeta araxensis subsp. nov. from the Aras River basin in Armenia.
Günther (1899) points out that the considerable morphological variation shown by these fishes has resulted in numerous specific names and that it is difficult to assess them without a large
comparative series and better information on localities. Berg (1948-1949) also indicates that the various subspecies are very close to each other and that their distributions are not clearly isolated.
C. c. heratensis shows major variations in body form, sometimes called morpha elata with a deep body and morpha elongata with a shallow and elongate body. These are not
taxonomically significant but simply ecomorphs and all intermediates between the two extremes can be found. The deep-bodied form probably formed part of the fishes described as asmussii
(Berg, 1948-1949). Reshetnikov and Shakirova (1993) list Capoeta heratensis as a full species.
Samaee et al. (2006) showed differences in morphometry between fish
from six rivers along the Iranian Caspian shore with an overall assignment of
individuals to a group of 88.6%. The morphometric data were mirrored by
molecular data. Differences in morphometry were attributed to environmental and
habitat conditions (temperature, turbidity, food availability and water depth
and flow) but molecular data indicated a genetic basis, presumably through lack
of gene flow between the river populations. Samaee et al. (2009) examined
morphological variation with this species in the Shirud of the south Caspian Sea
basin. There were no significant differences in meristic characters but
morphometric characters varied and could be used to distinguish 5 groups.
AnvariFar et al. (2011) compared fish from above and below the Shahid
Rajaei Dam (built in 1995) and found the two populations to be morphologically
different.
Records of Capoeta capoeta from the Tigris River basin at least are probably Capoeta damascina with low scale counts (F. Krupp, in litt., 1986).
Wossughi (1978) described, in a dissertation, a subspecies from the Namak Lake basin (from "Tschmeh Jafar Abad bei Araq") but this work may not be published in the sense of the
International Code of Zoological Nomenclature (Ride et al., 1985). In any case, the holotype is Capoeta aculeata and the other material comprises 21 Leuciscus
(= Squalius) cephalus orientalis and 4 Capoeta aculeata (F. Krupp, in litt., 1984). The type material, all female, is stored in the Zoologischen Instituts und Zoologischen
Museums der Universität Hamburg (holotype, 132 mm standard length under ZMH 5946, and 2 paratypes, 115-121 mm standard length, under ZMH 5947).
Bianco and Banarescu (1982) described C. capoeta intermedia from the Mand River in Fars but this is referred to C. damascina here (q.v.).
The types of Capoeta gibbosa are in the Zoological Institute, St. Petersburg (ZISP 11104) but have dried at some point. Their locality is given by Nikol'skii (1897) in Latin as "Bochsani
in Persia orientali. 4.VII.96 (2)". This may be Bozjani at 35°48'N, 59°36'E. Berg (1949) considers that this nominal species is close to C. capoeta gracilis but is distinguished by body
proportions (longer caudal peduncle and a longer head) but it is founded on only 2 specimens, hardly an adequate sample.
Types of Scaphiodon asmussii, S. gracilis and S. heratensis were not kept ? phrasing
Key characters
Berg (1948-1949) and Abdurakhmanov (1962) separate C. c. capoeta from C. capoeta gracilis, both of which may occur in Iran, by the following key:-
1(2). Dorsal fin emarginate above; lateral line scales usually 55-59; dorsal fin spine strong with numerous denticles; back behind occiput and particularly in front of the dorsal fin strongly
compressed.....C. c. capoeta
2(1). Dorsal fin truncated in adults; lateral line scales usually 47-58; dorsal fin weak; back behind occiput not or only weakly compressed; radii on scales with minute recesses
.....C. c. gracilis
Morphology
Dorsal fin unbranched rays 3-5, branched rays 7-10, usually 8-9; anal fin unbranched rays 2-4, branched rays 5; pectoral fin branched rays 15-20; and pelvic branched rays 7-9. Lateral line scales
46-70. In the subspecies gracilis the scales are said to be somewhat larger than in the type form, 47-58, mostly 48-50, and in heratensis the range is given as 50-60 by Berg (1948-1949).
Scales are regularly arranged over the body. There is a pelvic axillary scale. Scales have a wavy anterior edge, few anterior and posterior radii and an almost central focus. Gill rakers 16-30, lower
counts may refer to lower arm rakers only and total counts in the range 25-30 are probably more typical. Vertebrae 42-47. Pharyngeal teeth 2,3,4-4,3,2 or 2,3,5-4,3,2 with a hooked tip, spatulate below on
posterior teeth while anterior teeth are conical. The last unbranched dorsal fin ray is strong with denticles along one half to two-thirds of its length (less strong in gracilis than in the type
form). The number of barbels is variable - in fish from Uzbekistan 2 barbels (126 fish or 58.6%), 3 barbels (15 or 7.0%) or 4 barbels (74 or 34.4%)(Amanov, 1970). The subspecies heratensis
is characterised by having 4 barbels but this is probably variable in Iran as in Uzbekistan. Six specimens from the Hari River basin of Iran all had 4 barbels.
Levin et al. (2005) found gracilis and heratensis (and steindachneri) to be oligovertebrate with 41-45 vertebrae, modes 42 to 44, compared to the multivertebrate type
subspecies capoeta and sevangi with 45-48 vertebrae, mode 46. Morphometry and longevity also differ between these two groups and it was assumed they belong to different phyletic lines.
The Lake Sevan, Armenia subspecies (sevangi) has 2n=150 and is closer to
"Barbus" than to African Varicorhinus, a genus in which Southwest Asian Capoeta were once
placed (Krysanov, 1999). C. capoeta from the Safid River, the Shahrud in Rudbar and the Madarso River in Golestan National Park also have 2n=150, NF=230-234 (Pourali et al., 2000;
Pourali Darestani et al., 2006). C. c. umbla from the Tigris River of Turkey had 2n=150, possibly hexaploid, with 43 meta-submetacentric chromosomes, 32 pairs of
subtelo-acrocentric chromosomes with NF=236 (Kiliç Demirok and Ünlü, 2001).
Günther (1899) points out that this species shows considerable morphological variation, even in fish caught at the same place and time. The mouth can vary from straight to a gentle crescent to a
distinct crescent, e.g. in three fish from the Nazlu Chai. There are also variations in dorsal fin spine development and the crown of the head can be flattened or convex.
Meristics for Iranian material: ?
Sexual dimorphism
The snout in males has 2-4 rows of tubercles and tubercles are present on scales and the rays of the anal fin. Abdurakhamanov (1962) reports that caudal peduncle length and lower caudal lobe length are
longer in males while anal fin height, pelvic-anal fin distance, postorbital distance and interorbital width are greater in females.
Colour
The back is dark grey or green to brownish and the flanks light, silvery or silvery-grey, or yellowish. There may be several large black spots or blotches on the flank. The belly and lower head
surface are pearly-white to dirty yellow. Scales are darkly pigmented. The operculum has a broad, yellow-gold spot. The iris is silvery, somewhat darker or yellow-golden above, or golden overall with
traces of grey. The front of the dorsal fin and the margin of the caudal fin are black, and the rest of these fins are grey or yellowish-grey with some pink. The black margin to the caudal fin may be
best developed on the upper and lower lobes compared to the posterior margin. The pectoral, pelvic and anal fins are grey with some pink or may be an overall pale pinkish. The peritoneum is black.
Size
Reaches 38 cm standard length and 3.5 kg (Amanov, 1970). This species reaches 43 cm in the Aras River basin of Iran (possibly C. capoeta sevangi, see above; A. Abdoli, pers. comm., 1995), 55
cm in Lake Sevan as C. capoeta sevangi (Bănărescu in Bănărescu, 1999) and 43.5 cm fork length and 1.23 kg in Çıır Lake, Turkey as C. capoeta capoeta.
Distribution
The type species is found in the Kura River basin of Azerbaijan with some Aras River basin fishes very similar. It is not known if the fishes from the Aras River basin in Iran belong to the type
species or to gracilis. The subspecies Capoeta capoeta gracilis (known to Russian ichthyologists as the Lenkoran khramulya) was described from near Esfahan and is recognised as the one
found over much of Iran including the Caspian Sea basin from the Astara to the Atrak including the
Atrak, Gorgan, Gharasu, Tajan, Babol, Haraz, Sardab, Aras, Tonekabon, Pol-e Rud
and Safid rivers, the Anzali Talab and Gorgan Bay (Derzhavin, 1934; Bianco and Banarescu, 1982; Holčík and Oláh, 1992;
Kiabi et al., 1994; Roshan Tabari, 1997; Shamsi et al., 1997; Abbasi et al., 1999; Kiabi et al., 1999; Abdoli, 2000;
Abdoli and Naderi, 2009), the Lake Orumiyeh basin including the Urmi River or Shaher
Chai, Nazlu Chai and Talkheh, Tatavi and Zarrineh rivers (Günther, 1899; Abdoli, 2000) and Mahabad Dam (Abdi, 1999; www.mondialvet99.com, downloaded 31 May 2000),
Marmisho Lake, Azerbaijan (Shiri et al., 2009), the Dasht-e Kavir basin (Saadati, 1977), the
Gulf basin in the Zohreh, Shapur, Dalaki, Helleh, Shur rivers and the upper and lower Mand River as Capoeta capoeta intermedia ((Gh. Izadpanahi, pers. comm., 1995;
M. Rabbaniha
(pers. comm., 1995; Abdoli, 2000), the Esfahan basin including the Dopolan River, Gav Khuni marsh (Keyserling, 1861), the Tigris River basin in the Regab River near Kermanshah, Nashad River in the
Divadarreh region, Kordestan and near Borujerd, Lorestan (records need verification by specimens), the Tedzhen River basin including the Jam and Kashaf rivers (Berg, 1949).
This variable species is also recorded from the Dasht-e Kavir, Bejestan. Sirjan
and Namak Lake basins (Esmaeili et al., 2011?).
The subspecies Capoeta capoeta heratensis (the Transcaspian khramulya) is found in the Tedzhen or Harirud basin of Iran and eastwards including the Kashaf River (Abdoli, 2000). This
subspecies is also recorded from the Karakum Canal and Kopetdag Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) and may eventually reach Iranian waters in the Caspian Sea basin.
Zoogeography
Saadati (1977) suggests that this species entered the
Dasht-e Kavir basin from either the Tigris River basin, the Hari River basin or the Caspian Sea basin. See also above under genus.
Habitat
The habitat of this species in the Surkhandar'ya of Uzbekistan is backwaters and channels with weak current and silt beds as well as reservoirs (Amanov, 1970). In Iran, it is one of two most abundant
species in Caspian rivers along with Alburnoides bipunctatus (=
eichwaldii) (Iranian Fisheries Research and Training Organization Newsletter, 19:4, 1998).
Ghasemi and Mustafayev (2008) found this species in the Aras River was the most
dispersed and had the highest frequency (56.6%) of 17 species collected. It was
found to be resistant to environmental changes, such as flooding, in the
Madarsoo River in Golestan (Rezaei et al., 2008). Günther (1899) found that Capoeta capoeta
placed in saline Lake Orumiyeh water died in 3.5 minutes.
Age and growth
Life span is over 8 years and catches in Uzbekistan are dominated by fish 3-4 or 4-5 years old. Growth is fastest in the first two years of life (Amanov, 1970). Life span in Azerbaijan is over 6
years (Abdurakhmanov, 1962), in northern Anatolia 6 years where vertebrae followed by scales were the best structures for aging (Polat and Işik, 1995), while in Georgia life span exceeds 9 years
(Elanidze, 1983), and in Lake Gotchka, Armenia 10 years but only 4+ years in Lenkoran (Bănărescu in Bănărescu, 1999). Günther (1899) reports on a male fish from Ula in the Lake
Orumiyeh basin which was only 12.5 cm long yet a sexually mature male, perhaps an instance of a dwarf form. Canbolat et al. (1999) found life span to be over 9 years in Çıldır Lake,
Turkey for Capoeta capoeta capoeta. Fish aged 6 years dominated at 31.5% and 61.7% of the sample was female.
In Madarsoo Stream of Golestan National Park, this species had age groups 0-10 years and growth parameters were Lt = 229.67 mm and K = 0.54 for males, 327.95 mm and 0.18 for females
(Koohestan Eskandari, 2003). Rezaei et al. (2007) also examined this fish
population in the Madarsoo after two floods in 2001-2002. Growth parameters were
L∞ = 249 mm, K= 0.22 and t0 =
-0.30 for males and L∞ =
306 mm, K= 0.21 and t0 = -0.38 for females. Length-weight
relationships were W = -4.48 + 3.03TL for males and W = -4.59 + 3.0551TL in
females, showing good feeding condition and positive isometric growth. Males
were smaller than females as they matured earlier. Male to female ratio was
1.5:1, significantly different. Age range was 1+ to 5+ for males and 2+ to 8+
years for females. The dominant age was 2+ years. Length was greatly
decreased compared to previous studies and the population was younger,
attributed to the floods.
Gholizadeh et al.
(2009) studied a population in the Zarrin-Gol Stream in Golestan amd found age
group 0+ was the most common at 59% and age groups 3+ and 4+ were the least
common at 1%. Instantaneous growth of fish at age 3+ was much lower than younger
age groups. The length-weight relationship was W = 0.00003xL2.822 and
the von Bertalanffy equation was Lt = 223.8 (1-exp[0.185(t+1.8)].
Maku Dam lake in West Azarbayjan has an estimated 9.4-10.7 tonnes of this species with a maximum sustainable yield of 4.5-4.8 t (Saiad Bourani and Ghaninejad,
2004). Average length of this population was 23.9 cm, weight was 1626.8 g and age was 2.6 years. Most fish were 3+ years old and 5+ fish were at a minimum. Infinite length and the growth coefficient
were computed as 35.6 cm and 0.39 per year. Total mortality was 0.74, natural mortality 0.37 and fishing mortality 0.37.
The Yasalegh Stream in the Gorgan River basin had a male to female ratio of
1:0.54, a maximum weight of 71.2 g for males and 119.4 g for females, and age
range of 0-3 years, von Bertalanffy growth equations of Lt =
190(1-exp{-0.462 [t+1]} for males and Lt = 230(1-exp{-0.472
[t+0.742]} for females, and weight growth was isometric (b = 3.052 for males and
3.050 for females). Tilabad River fish had an age structure of 0-4 years and the
Talar River 2-4 years, similar to Yasalegh Stream but differing from the
Madarsoo Stream. The fish in the latter stream had better living conditions in a
national park, no pollution, no fishing, no competition from exotic carps, no
other human disturbances, no environmental stress and no food shortages. Patimar
et al. (2009) found b values ranging from 2.647 (male at Chekchai) to
2.964 (females at Madarsu) indicating negative allometric growth for fish in the
Gorgan River basin. They interpreted this variation to the species' response to
different habitat conditions.
Food
Food is mainly detritus and ooze, with some higher plants and small amounts of blue-green algae, which is digested in an intestine almost 7 times longer than the body (Amanov, 1970). Small benthic
invertebrates may also be included, such as chironomids and molluscs (Bănărescu in Bănărescu, 1999). In Maku Dam lake, this species is a detritivore consuming Chrysophyta
from the phytoplankton and Cyclotella a diatom, from the benthos as well as Chironomidae and Ephemeroptera (Valipour, 2004).
In the Talar and Yasalegh rivers of the eastern Caspian Sea basin, 27 genera of
phytoplankton were identified in the diet, with Chrysophyta being dominant, but
with some differences between older and younger fish in the species consumed (Mostafavi
and Abdoli, 2005).
Reproduction
Sexual maturity in Uzbekistan is at ages 2-4 years and lengths of 15-20 cm or in some populations at 10-14 cm. Some fish mature as dwarfs before age 1 and Berg (1948-1949) reports males 8.4 cm long of
the subspecies gracilis can be mature. Spawning may take place at any time during the period from March to September (Berg,1948-1949) and is intermittent with the first spawning accounting for
up to 85% of the eggs and the subsequent two spawnings for the remainder. The
yellow eggs have a diameter up to 2.2 mm in the first spawning, up to 0.75 mm in the second and 0.65 mm in the third.
Fecundity is up to 86,800 eggs. Eggs are laid at 50-60 cm on sand and stone beds and in water temperatures of 16-23°C (Amanov, 1970). Fecundity in the Kura River may reach 93,861 at 36-40
cm but this is for C. capoeta capoeta and fecundity for C. capoeta sevangi is less (Bănărescu in Bănărescu, 1999). Eggs are shed in running water and on lake shores,
and eggs are covered by sand or small stones.
Rezaei et al. (2007) found no change in
reproductive characteristics after floods in the Madarsoo Stream population.
Mean fecundity was 3116 eggs and the maximum gonadosomatic index was in June.
Parasites and predators
Williams et al. (1980) report the helminths Khawia armeniaca (a cestode) and Acanthocephalorhynchoides cholodkowskyi (an acanthocephalan) from this species in the
Zayandeh River at Esfahan. Molnár and Jalali (1992) record for this species the monogeneans Dactylogyrus chramulii, D. gracilis and D. lenkorani in the Safid Rud, D.
chramulii and D. lenkorani in the Beshar River of the Persian Gulf drainage, D. gracilis and D. lenkorani in the Zayandeh Rud, D. lenkorani in the Tonekabon and Tajan
rivers of the Caspian Sea drainage and the Kor River drainage of Fars, and D. pulcher from the Safid, Tajan, Tonekabon and "Ghasemlu" rivers of the Caspian Sea basin and the Jajrud
of the Namak Lake basin. Shamsi et al. (1997) report Clinostomum complanatum, a parasite causing laryngo-pharyngitis in humans, from this species, the highest rate in 9 species examined.
Malek (1993) and Malek and Mobedi (2001) report Clinostomum complanatum from this species in Mazandaran, the Shiroud. Up to 60 parasites per fish are recorded, with female fish
having the highest infestation (the later study showing no difference between male and female fish), infestation decreasing with increase in body length, and parasites being concentrated in the gill
cavity and pharynx.
Masoumian and Pazooki (1998) surveyed myxosporeans in this species in Gilan and Mazandaran provinces, finding Myxobolus musayevi and M. samgoricus. The crustacean parasite
Tracheliastes polycolpus is reported from the fins of this species in the Mahabad Dam reservoir (Abdi, 1999; www.mondialvet99.com, downloaded 31 May 2000).
Jafari et al. (2001) isolated the acanthocephalan Dendronucleata
dogieli from fish in the Zarrineh River, West Azerbaijan. Masoumian et al. (2002)
investigated parasites from this fish in the Aras and Mahabad dams in northwest Iran and found the protozoan Myxobolus musayevi which is also recorded from this fish in the Tajan River in
Mazandaran. Mokhayer et al. (2002) report Acanthocephalorhynchoides cholodkowskyi (Quadrigyridae) from the midgut and Tracheliastes polycolpus (Lernaeopodidae) on the fins of this
fish in Golestan National Park, with more parasites on male fish and differences by season and station. Naem et al. (2002) found the following parasites on the gills of this species from the
western branch of the Safid River, namely the protozoan Trichodina sp. and the monogenean trematode Dactylogyrus lenkorani.
Mirhasheminasab and Pazooki (2003) list Ergasilus peregrinus,
Tracheliastes polycolpus and Lernaea cyprinacea from this species in
Mahabad Reservoir, the latter being the most dangerous parasite. Rohei Aminjan and Malek (2004) found 9 parasite species
in fish from the Shiroud, namely the trematodes Clinostomum complanatum, Diplostomum spathaceum, Posthodiplostomum cuticola, Allocreadium sp., the monogeneans
Dactylogyrus pulcher, D. lenkorani, Gyrodactylus mutabilitas and the nematodes Rhabdochona fortunatowi and Capillaria sp. Masoumian et al. (2005) recorded the
protozoan parasites Ichthyophthirius multifilis, Trichodina perforata, Chilodonella, sp., Amphileptus branchiarum, Tetrahymena pyriformis, Apiosoma
sp., and Vorticella sp. from this species in water bodies in West Azarbayjan. Araghi Soureh and Jalali Jafari (2005) recorded Dactylogyrus gracilis, D. charmulii,
D. lenkorani and D. kendalanicus from this species in the Mahabad River of the Lake Orumiyeh basin,
the latter species being a new record for Iran. Pazooki et al. (2007) recorded various parasites from localities in West
Azarbayjan Province, namely Diplostomum spathaceum, Ligula intestinalis, Digrama sp., Rhabdochona hellichi, Argulus foliaceus, Allocreadium isoporum,
Lamprolegna compacta, Myxobolus cristatus and M. musajevi. Pazooki et al. (2005) record Tracheliastes longicollis, Lamprolegna compacta, Neoechinorhynchus rutili,
Capillaria sp., Myxobolus musajevi, M. cristatus, Trichodina perforata, Chilodonella piscicola, Ichthyophthirius multifilis and Ichthyobodo necatrix
from this species in waterbodies of Zanjan Province. Pazooki et al. (2006) record the monogeneans Dactylogyrus chramuli, D. gracilis, D. lamellatus, D. lenkorani, D. pulcher
and Gyrodactylus sp. from this fish in Zanjan Province. Masoumian et al. (2007) record the myxosporean parasite Myxobolus musajevi from this species in the Zayandeh River.
Miar et al. (2008) examined fish in Valasht Lake and the Chalus River,
Mazandaran and found the metazoan Myxobolus saidovi.
Maleki and Malek (2007) examined fish from the Shirud in the Caspian Sea basin
and recorded the digeneans Posthodiplostomum cuticola, Diplostomum
spathaceum, Clinostomum complanatum and Allocreadium sp.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Argulus foliaceus,
Ergasilus sp., Lamproglena compacta, Lernaea sp., Tracheliastes longicollis
and Tracheliastes polycolpus on this species.
Economic importan
The subspecies Capoeta capoeta heratensis is a food fish in Uzbekistan (Amanov, 1970) and C. capoeta sevangi and C. capoeta capoeta are commercially important in Lake Gotchka,
Armenia and eastern Georgia and Azerbaijan respectively (Bănărescu in Bănărescu, 1999).
It is also used in sport fishing in Iran (Samaee et al., 2006).
Shiri et al. (2009) report a case of ichthyotoxism after eating fried
eggs of this species. Nausea resulted after one minute, and the victim was
hospitalised with severe chest pains. No vomiting occurred as this was the only
food eaten and symptoms appeared rapidly. Raw consumption should be avoided and
even cooked fish or inadequately cleaned fish can be dangerous.
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include sport fishing, abundant in numbers, habitat
destruction, widespread range (75% of water bodies), present in other water bodies in Iran, and present outside the Caspian Sea basin.
Further work
The relationships of the various subspecies need study to determine if they are in fact good species.
Sources
Type material: See above, Capoeta gibbosa (ZISP 11104).
Iranian material: CMNFI 1970-0512, 3, mm standard length, Gilan, Shalman River (37º08'N, 50º15'E);
CMNFI 1970-0514, 17, ? mm standard length, Gilan, Shafa River estuary (37º55'N, 49º09'E);
CMNFI 1970-0516, 6, ? mm standard length, Gilan, Lemir River (38º14'N, 48º52'30"E);
CMNFI 1970-0519, 2, ? mm standard length, Gilan, Chelvand River (ca. 38º18'N, ca. 48º52'E);
CMNFI 1970-0520, 2 ?7 on data sheet, 94.0-100.9 mm standard length, Gilan, Astara River (ca. 38º25'N, ca. 48º52'E);
CMNFI 1970-0521, 3, ? mm standard length, Gilan, Safid River near Lulaman (no other locality data);
CMNFI 1970-0522, 10, ? mm standard length, Gilan, Safid River at Astaneh bridge (37º16'30"N, 49º56'E);
CMNFI 1970-0525, 5, 92.8-146.1 mm standard length, Gilan, Safid River near Mohsenabad (ca. 37º22'N, ca. 49º57'E); ?see data sheets 78, 22.1-160.4
CMNFI 1970-0526, 8, ? mm standard length, Gilan, Safid River 6 km below Astaneh bridge (37º19'N, 49º57'30"E);
CMNFI 1970-0531, 7, 60.2-84.6 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1970-0536, 2, ?5 on data sheets 101.4-125.4 mm standard length, Gilan, Siah River estuary (36º53'N, 49º32'E);
CMNFI 1970-0538, 1, ?5 on data sheet 95.4 mm standard length, Gilan, Qezel Owzan River near Manjil (36º44'N, 49º24'E);
CMNFI 1970-0557, 3, ? mm standard length, Azarbayjan-e Bakhtari, Shaher Chay (no other locality data);
CMNFI 1970-0559, 6, 83.9-125.4 mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay (ca. 37º25'N, ca. 45º10'E);
CMNFI 1970-0568, 9, ? mm standard length, Gilan, Caspian Sea at Kazian beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0577, , mm standard length, Gilan, Caspian Sea at Astara (ca. 38º26'N, ca. 48º53'E);
CMNFI 1970-0583, 8, 34.1-93.9 mm standard length, Gilan, Nahang Roga (37º28'N, 49º28'E);
CMNFI 1979-0589, , mm standard length, Gilan, Safid River opposite Kisom (37º12'N, 49º54'E);
CMNFI 1979-0242, 27, 25.6-107.0 mm standard length, Fars, river at Izadkhvast (31º31'N, 52º07'E); check ID?
CMNFI 1979-0249, 33, 66.4-114.2 mm standard length, Esfahan, stream at Dizaj (31º55'N, 51º30'E); check ID?
CMNFI 1979-0429, 1, ? mm standard length, Mazandaran, Chalus River (36º34'N, 51º23'E);
CMNFI 1979-0432, 1, ? mm standard length, Mazandaran, Sardab River branch (36º41'N, 51º22'E);
CMNFI 1979-0434, 1, ? mm standard length, Mazandaran, Shir River (36º51'N, 50º49'E);
CMNFI 1979-0435, 1, ? mm standard length, Gilan, stream 10 km west of Ramsar (36º57'N, 50º37'E);
CMNFI 1979-0433, 1, 115.2 mm standard length, Mazandaran, stream 18 km west of Chalus (36º42'N, 51º15'E);
CMNFI 1979-0438, 2, 142.4-144.8 mm standard length, Gilan, Gholab Ghir River (37º27'N, 49º37'E);
CMNFI 1979-0441, 1, 121.9 mm standard length, Gilan, river 14 km south of Hashtpar (37º42'N, 48º58'E);
CMNFI 1979-0443, 1, ? mm standard length, Gilan, river 34 km west of Hashtpar (38º06'N, 48º53'E);
CMNFI 1979-0444, 1, ? mm standard length, Gilan, Chubar River (38º11'N, 48º52'30"E);
CMNFI 1979-0446, 1, ? mm standard length, Gilan, Astara River (38º26'30"N, 48º51'E);
CMNFI 1979-0449, 2, ? mm standard length, Azarbayjan-e Khavari, river 18 km from Khalkhal (ca. 37º42'N, ca. 48º27'E);
CMNFI 1979-0451, 30, 35.8-97.3 mm standard length, Azarbayjan-e Khavari, Qezel Owzan River (ca. 37º30'N, ca. 47º57'E);
CMNFI 1979-0452, 1, 79.7 mm standard length, Azarbayjan-e Khavari, Qezel Owzan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0453, 24, 36.1-111.1 mm standard length, Zanjan, Zanjan River (37º06'N, 47º56'E);
CMNFI 1979-0469, 2, 56.6-76.2 mm standard length, Mazandaran, river 36 km west of Alamdeh (36º37'30"N, 51º35'E);
CMNFI 1979-0474, 1, ? mm standard length, Mazandaran, Tajan River (36º34'N, 53º05'E);
CMNFI 1979-0475, 1, 86.4 mm standard length, Mazandaran, stream on road to Bandar-e Shah (36º46'N, 54º00'E);
CMNFI 1979-0480, 2, ? mm standard length, Mazandaran, Gorgan River at Gonbad-e Kavus (37º15'30'N, 55º09'E);
CMNFI 1979-0481, 3, 101.9-188.0 mm standard length, Mazandaran, stream 3 km west of Ghalahleekesh (37º18'30"N, 55º31'E);
CMNFI 1979-0482, 2, ? mm standard length, Mazandaran, river 2km west of Ghalahleekesh (37º19'30'N, 55º31'E);
CMNFI 1979-0483, 4, 121.6-160.5 mm standard length, Mazandaran, river 28 km west of Dasht (37º23'30"N, 55º51'30"E);
CMNFI 1979-0485, 3, 71.2-99.1 mm standard length, Khorasan, stream 28 km west of Bojnurd (37º33'N, 57º04'E);
CMNFI 1979-0486, 66, 17.5-97.8 mm standard length, Mazandaran, stream in Atrak River draiange (37º44'N, 56º18'E);
CMNFI 1979-0487, 20, ? mm standard length, Mazandaran, spring 2 km from Maraveh Tappeh (37º54'N, 55º58'E);
CMNFI 1979-0488, 9, 29.7-140.4 mm standard length, Mazandaran, Atrak River at Maraveh Tappeh (37º55'N, 55º57'30"E);
CMNFI 1979-0489, 78, ? mm standard length, Mazandaran, stream 13 km from Maraveh Tappeh (37º50'N, 55º53'E);
CMNFI 1979-0490, 14, 21.0-108.4 mm standard length, Mazandaran, stream in Gorgan River drainage (ca. 37º39'N, ca. 55º42'E);
CMNFI 1979-0491 2, ? mm standard length, Mazandaran, Gorgan River northeast of Kalaleh (ca. 37º33'N, ca. 55º44'E);
CMNFI 1979-0492, 25? check jar, 9.3-183.4 mm standard length, Mazandaran, river in Gorgan River drainage (37º05'N, 55º15'E);
CMNFI 1979-0695, 13, ? mm standard length, Gilan, Safid River at Manjil Bridge (36º46'N, 49º24'E);
CMNFI 1997-0003, , mm standard length, ();
CMNFI 1980-0116, 1, ? mm standard length, Gilan, Safid River at Astaneh (37º16'30"N, 49º56'E);
CMNFI 1980-0120, , mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1980-0121, , mm standard length, Gilan, Shafa River estuary (37º35'N, 49º09'E);
CMNFI 1980-0123, , mm standard length, Gilan, Safid River (ca. 37º22'N, ca. 49º57'E);
CMNFI 1980-0141, , mm standard length, Gilan, Lisar River estuary (37º59'N, 48º56'E);
CMNFI 1991-0163, , mm standard length, Mazandaran, Ramian River (36º58'N, 55º07'E);
CMNFI 1993-0138, 1, mm standard length, Khorasan, Bazangan Lake (36º18'N, 60º27'E);
CMNFI 2007-0014, 4, 39.4-99.1 mm standard length, Khorasan, pool in Kuh-e Sang Park, Mashhad (ca. 36º18'N, ca. 59º36'E);
CMNFI 2007-0086, 6, ? mm standard length, Azarbayjan-e Khavari, Qareh Su basin near Nir (ca. 38º02'N, ca. 48º00'E);
CMNFI 2007-0087, 1, ? mm standard length, Azarbayjan-e Khavari, Qareh Su north of Ardebil (38º22'N, 48º19'E); CMNFI 2007-0088, 5, ? mm standard length, Azarbayjan-e Khavari, Qareh Su east of Lari (38º30'N, 48º03'E);
CMNFI 2007-0089, 4, ? mm standard length, Azarbayjan-e Khavari, Ahar Chay at Ahar (38º28'N, 47º03'E);
CMNFI 2007-0093, 13, ? mm standard length, Azarbayjan-e Bakhtari, Qotur River south of Khvoy (38º30'N, 44º58'E);
CMNFI 2007-0094, 6, ? mm standard length, Azarbayjan-e Bakhtari, Nazlu River north of Reza'iyeh (ca. 37º42'N, ca. 45º04'E); checkID?
CMNFI 2007-0095, 2, ? mm standard length, Azarbayjan-e Bakhtari, Shahr Chay southwest of Reza'iyeh (ca. 37º27'N, ca. 44º56'E); checkID?
CMNFI 2007-0096, 5, ? mm standard length, Azarbayjan-e Bakhtari, Qasemul River in Baranduz Chay basin (ca. 37º25'N, ca. 45º10'E); checkID?
CMNFI 2007-0098, 2, ? mm standard length, Azarbayjan-e Bakhtari, river south of Mahabad (ca. 36º42'N, ca. 45º41'E);
CMNFI 2007-0099, 1, ? mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay west of Mahabad (ca. 36º35'N, ca. 45º25'E); checkID?
CMNFI 2007-0101, 1, ? mm standard length, Azarbayjan-e Bakhtari, Tata'u River south of Miandow Ab (ca. 36º54'N, ca. 46º07'E);
CMNFI 2007-0102, 4, ? mm standard length, Azarbayjan-e Bakhtari, Zarineh River near Miandow Ab (ca. 37º00'N, ca. 46º07'E);
CMNFI 2007-0103, 9, ? mm standard length, Kordestan, Zarineh River basin north of Saqqez (ca. 36º18'N, ca. 46º16'E);
CMNFI 2007-0104, 4, ? mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º12'N, ca. 46º18'E);
CMNFI 2007-0105, 7, ? mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º06'N, ca. 46º20'E);
CMNFI 2007-0106, 9, ? mm standard length, Kordestan, Qezel Owzan River basin near Divandarreh (ca. 35º52'N, ca. 47º05'E);
CMNFI 2007-0107, 10, ? mm standard length, Kordestan, Qezel Owzan River basin near Bijar (ca. 35º54'N, ca. 47º20'E);
ZSM 24500, 6, 24.2-31.0 mm standard length, Khorasan, stream near Bojnurd (no other locality data);
uncatalogued, 2, 75.8-85.5 mm standard length, Khorasan, Hari River at Sarakhs (36º32'N, 61º11'E).
Capoeta damascina
(Valenciennes, 1842)
Common names
sardeh (= cold one, probably zardeh = yellow one, is more correct and appropriate based on yellow-tinged flank) or سياه ماهي (= siah mahi, meaning black
fish) in the Caspian basin; siah mahi damascina; tu'ini (meaning unknown) or gel cheragh (= mud-eater, mud-grazer) or tu'ini gelkhorak (= mud-eater, mud-grazer) in Khuzestan; qezel ala
(red spots) in Chahar Mahall but mistakenly.
[twena, toyoueni or toueni, bertin or bartin, tin, zardah masih, tela shami,
tela Damascus; kollur, kellur, kollur hadjiari (= the pilgrim or migrating kollur), kellur dischileki (= the strawberry-coloured kollur),
kollur achmar (= the red kollur) and kollur aschkar (= the brown kollur), all at Aleppo; all in Arabic; Mesopotamian barb].
Systematics
Gobio damascinus was described from the "fleuve de Damas" (= river of Damascus, Syria).
Synonyms are Scaphiodon capoeta Heckel, 1843 (non sensu Güldenstädt, 1773) described from "Aleppo", Scaphiodon fratercula Heckel, 1843 described from "Gewässern von
Damascus", possibly Scaphiodon Umbla Heckel, 1843 described from the "Tigris bei Mossul", Scaphiodon socialis Heckel, 1843 described from "Um Damascus" (=
around Damascus) (Heckel, 1843b) and later more completely from the "Orontes" (Heckel, 1846-1849a) (placed in Scaphiodon Capoëta of Heckel by Steindachner (1864)), Scaphiodon
peregrinorum Heckel, 1843 described from "Aleppo" and later from "Fluss Kueik bei Aleppo", Chondrostoma syriacum Valenciennes, 1844 from
Abraham's River at the foot of Mount Sinai, Egypt (the correct locality is probably in the Jordan River basin (Coad and Krupp, 1994)), Scaphiodon Amir Heckel, 1849 described from the
"Araxes" (= Kor River, Fars), Scaphiodon niger Heckel, 1849 described from the "Araxes oder Benth-Amir" (= Kor River, also known as the Bandamir River), Scaphiodon Saadii
Heckel, 1849 described from the "Quellen des Saadi" (Sa`di at 29°37'N, 52°35'E, now within the city of Shiraz) and the "Nähe von Persepolis" (= probably the Pulvar (= Sivan) River
near Persepolis, Fars), Scaphiodon chebisiensis Keyserling, 1861 from "Wasserleitung in Chebis" (= canal in Chebis, probably Khabis or Shahdad at 30°25'N, 57°42'E in Kerman),
Scaphiodon rostratus Keyserling, 1861 from "Wasserleitungun in der Umgegend von Jezd. Das abgebildete Exemplar stammte aus Meibut" (= canals in the vicinity of Yazd. The specimen drawn
originated from Meibut, probably Meybod at 32°14'N, 54°01'E), Barbus belayewi Menon, 1960 (Menon and Yazdani (1968) date this species as 1960, presumably the 1956 edition of the journal was
delayed) from the "Tigris, Baghdad, Iraq", and Capoeta capoeta intermedia Bianco and Banarescu, 1982 (non Capoeta intermedia Temminck and Schlegel, 1846 = Acheilognathus
lanceolata (Temminck and Schlegel, 1846) (see Boeseman, 1947)) described from the "Mand River near Akbar, southern Iran".
The synonymy of Barbus belayewi is suggested by F. Krupp (in litt., 1986) and W. Rainboth (pers. comm., 1986). The synonymy of S. fratercula is pointed out by Berg (1949) since
the species was founded on low lateral line scale counts, a variable character in C. damascina, and on a larger orbit but Heckel's comparison was between fish of greatly differing size and no
allowance was made for allometry.
Karaman (1969) places damascina in Capoeta capoeta as a subspecies and umbla as another subspecies. Berg (1949) and Saadati (1977) recognise umbla as a distinct species.
The latter is distinguished from the former by a higher scale count (87-99), higher dorsal fin branched rays (9-10), longer dorsal fin, longer caudal fin (shorter than or equal to head length in C.
damascina), a markedly transverse mouth, and a weaker dorsal fin spine. Saadati (1977) considers fratercula to be a distinct species from the Tigris and Mand rivers in Iran based on scale count
(58-66), more gill rakers (20-22), and a more serrated dorsal fin spine; or a subspecies of Capoeta capoeta based on a close similarity in scale counts, average number of gill rakers, and the
dorsal fin origin being anterior to that of the pelvic fins. He also considers that Scaphiodon niger from the Kor River of Fars is possibly a synonym of fratercula. Krupp (1985c)
considers the synonymy of C. damascina and C. capoeta as extremely doubtful after examining topotypic material.
Bianco and Banarescu (1982) recognise Capoeta saadi as a distinct species based on an arched mouth rather than transverse as in most subspecies of Capoeta capoeta, with a lightly
developed horny cover on the lip, a feebly ossified dorsal fin spine, 13-17 gill rakers, modally 8 dorsal fin branched rays, 53-76 lateral line scales and 24-28 scales around the caudal peduncle.
However they do point out the extreme variability in scale counts, for example, from fish taken in the same locality and even between opposite sides of the same fish (5 more scales on one side than
the other!). Designation of subspecies on such variable characters is difficult and would require very large series and multivariate analysis techniques. Bianco and Banarescu (1982) regard C.
c. intermedia as intermediate between C. c. umbla and their C. c. macrolepis on the basis of scale counts, gill raker counts, smaller transverse mouth than in umbla
and a rather light colouration.
Capoeta damascina, with a wide distribution and wide variation in morphology, must be regarded as a species complex until detailed analyses can be carried out. Final resolution of the species
composition of this complex may well require extensive material for molecular analyses, as well as re-examination of types and topotypic material over the whole range of the taxon.
Samaee and Patzner (2011) examined fish from 6 river systems in Iran
morphometrically and were able to distinguish distinct groups. However, as they
point out, much more work needs to be done to determine if this variation is
genetic differentiation or phenotypic plasticity, or a combination of the two.
The syntypes of Gobio damascinus are in the Muséum national d'Histoire naturelle, Paris (MNHN 4494, 2 specimens, 169-179 mm standard length, Damascus, Syria, Bové, MNHN 3948, 1, 289 mm standard
length, Nahr Barada, Syria and MNHN A.3947, 1, 169 mm standard length, Syria) (Krupp, 1985c). Bertin and Estève (1948) give 200-210 mm total length for MNHN 4494 and 330-390 mm total length for MNHN 3947,
3948 and A.789. Eschmeyer et al. (1996) list MNHN 4494 as the lectotype (as designated by Krupp and Schneider (1989) although this collection comprises two fish) with MNHN 3947 (1, dry) and MNHN
3948 (1, dry) and possibly MNHN A.789 (1) as paralectotypes. The latter is listed as a syntype in Bertin and Estève (1948) although the localities listed in this article "Fl. Jourdain, à Damas
(Syrie)" is obviously an error on geographical grounds.
Syntypes of Scaphiodon capoeta are in the Naturhistorisches Museum Wien under NMW 51650 (1 fish), NMW 51831 (1), and NMW 55845-55846 (2). Heckel (1843) lists 2 specimens in his description.
The holotype of Chondrostoma syriacum is in the Muséum national d'Histoire naturelle, Paris under MNHN 1945 (Eschmeyer et al., 1996).
The holotype of Capoeta capoeta intermedia is in the Istituto di Zoologia dell'Universitá di L'Aquila, Italy (IZA 7892) and is 92.5 mm standard length, collected by P. Bianco and S Zerunian,
27/5/1976. There are 62 paratypes (IZA 7893) from the same collection as the holotype measuring 36-87 mm standard length and 13 paratypes uncatalogued in the Institutul de Stiinte Biologice, Bucuresti,
Romania (ISBB) measuring 68-86 mm standard length (Bianco and Banarescu, 1982).
Another paratype under IZA 7894 measures 105.5 mm standard length was examined
by me. A paratype of Capoeta capoeta intermedia from the Mand River in Fars is in the Zoologischen Instituts und
Zoologischen Museums der Universität Hamburg (ZMH 6090, 83.2 mm standard length) (Wilkens and Dohse, 1993; examined by me), one paratype from the Mand is in the California Academy of Sciences, San
Francisco (CAS 48113), one paratype from the Mand is in the United States National Museum, Washington (USNM 227935), and 6 paratypes are in the Canadian Museum of Nature, Ottawa under CMNFI 1982-0367
(formerly IZA 7893).
The holotype of Scaphiodon fratercula was taken from "Gewässern von Damascus", the syntypes of Scaphiodon umbla from the "Tigris bei Mossul", the types of
Scaphiodon socialis from "Um Damascus" (but listed as "Orontes" in the catalogue in Vienna, possibly in confusion as this part of the catalogue has been overwritten), and
the types of Scaphiodon peregrinorum from "Um Aleppo" according to Heckel (1843b) and "Fluss Kueik bei Aleppo" according to Heckel (1846-1849a).
Two syntypes of Scaphiodon niger are in the Naturhistorisches Museum Wien under NMW 51655 with standard lengths of 140.4 and 188.5 mm (another syntype is under NMW 51654
(232.7 mm), and a fourth under NMW 51656 as seen by me; all 4 are listed as syntypes in the 1997 Vienna card index). Eight syntypes of Scaphiodon amir are under NMW 61472 and measure 42-59 mm
standard length and there are also 6 fish under NMW 46081 (138.1-282.3 mm standard length); however the card index in 1997 lists only NMW 46081 (6) and 16508 (1, dried). Fifteen syntypes in the catalogue
(18 seen by me and in the Vienna card index in 1997) of Scaphiodon saadii from Sa`di are under NMW 51666 (Eschmeyer et al. (1996) have 52666, apparently in error) and measure 58-123
mm standard length (18.3-123.8 mm standard length when measured by me) with a further 4 syntypes from Persepolis under NMW 55900 measuring 84-114 mm standard length (Kähsbauer, 1964; not in the
1997 card index). There is also 1 syntype (RMNH 3166) in the Rijksmuseum van Natuurlijke Historie, Leiden from NMW (Eschmeyer et al., 1996).
The catalogue in Vienna lists no fish opposite the name S. niger, 6 and 2 fish in one column and 5 in the adjacent column for Scaphiodon amir (cf. above), 10 fish in one column and
10 in the adjacent column for S. saadii (cf. above).
A dried syntype of C. umbla is in the Senckenberg Museum Frankfurt (SMF 6777, formerly NMW) (F. Krupp, pers. comm., 1985; ca. 262.3 m standard length), 2 syntypes are in Naturhistorisches
Museum Wien (NMW 55932-55933) and another syntype is under NMW 55934. Eschmeyer et al. (1996) also lists NMW 79373-74, both dried. The catalogue in Vienna lists 2 fish in spirits and 2 fish
stuffed and the card index in 1997 lists as syntypes NMW 55932-33 and 79373-74 (dried).
Eschmeyer et al. (1996) note that there are no types of Scaphiodon fratercula in the Naturhistorisches Museum Wien.
Two fish are labelled as syntypes of Scaphiodon socialis in the Naturhistorisches Museum Wien (NMW 55855) which agrees with Heckel's text although the catalogue lists only 1 specimen.
Eschmeyer et al. (1996) state that there are no types at NMW presumably after Krupp and Schneider (1989) who state that NMW 55670 (1 fish), 55843 (2) and 55855 (2) are not types.
The types of Scaphiodon peregrinorum number 6 according to the catalogue in the Naturhistorisches Museum Wien and may comprise all or part of NMW 51658 (1), NMW 51659 (1), NMW 51660 (1),
NMW 51661 (1), NMW 51662 (1), NMW 51663 (1) NMW 51664 (3), and NMW 51665 (1), all labelled as from "Kueik" and possibly RMNH 2681 (3) in the Rijksmuseum van Natuurlijke Historie, Leiden
from NMW (Eschmeyer et al., 1996).
The types of Barbus belayewi are in the Zoological Survey of India, Calcutta, the holotype being ZSI F1046/2 and a paratype ZSI F1047/2 (Menon, 1960; Menon and Yazdani, 1968).
Types of Scaphiodon chebisiensis and Scaphiodon rostratus were not kept.? phrasing
Key characters
The mode of 9 dorsal fin rays, small scales, and the presence of large black blotches often distinguish this species from other Capoeta in Iran.
Morphology
Dorsal fin with 3-5 unbranched rays and 8-10 branched rays (Krupp (1985c) gives frequency distributions for his material from Turkey, Syria, Lebanon, Israel and Jordan as 8(52), 9(144) and 10(4)),
anal fin with 3 unbranched and 5-6 branched rays (5(179), 6(21) after Krupp (1985c)), pectoral fin branched rays 15-20, and pelvic fin branched rays 8-10. Lateral line scales 60-99. Gill rakers 17-25
(Saadati (1977) gives 9-21!; Krupp (1985c) 12-18 for the lower arm of the arch, Berg (1949) up to 23 on the lower arm). Pharyngeal teeth 2,3,4-4,3,2, often 2,3,5-5,3,2, with spoon-shaped crowns. The
mouth is usually horseshoe-shaped, seldom transverse. The last unbranched dorsal fin ray is moderate to strong with denticles along two-thirds of its length. Heckel (1846-1849b) distinguished his
Scaphiodon amir and S. niger by the dorsal fin denticles being horizontal or perpendicular to the spine, not hooked downward as in related species. Berg (1949) did not attach any
significance to this character, finding it in small fish from the Sarhadd of Baluchestan and from Jordan.
The karyotype for fish in the Tigris River basin of Turkey identified as Capoeta capoeta umbla is 2n=150, possibly hexaploid (Kılıç Demirok and Ünlü, 2001) and of
fish identified as C. damascina from the Wadi Karak, Jordan 2n=148-150, indicating a hexaploid species (Gorshkova et al., 2002).
Body form is highly variable as are scale counts between populations and even within populations when large series are examined (Krupp, 1985c). Subspecific designations can only be valid if very
large series from the whole range of the species are compared.
Meristic values for Iranian specimens are :
IZA7892, 7894 (2 fish) D8, A5, P15-15, V7, ll 62-64, gr 24
Sexual dimorphism
Males develop breeding tubercles around the snout and the posterior body on both sides of the lateral line (Khalaf, 1987).
Colour
The back is dark brown or brownish to olive or blue-grey, the flanks silvery with some yellowish tinges, sometimes golden, or yellow-brown or reddish-brown above the lateral line, silvery below.
The belly is white to yellowish. Cheeks are golden. Dark brown or black spots numbering up to 20 may be scattered irregularly on the flanks. Fins are reddish-brown, yellowish or grey and may be hyaline.
The caudal and pectoral fins may be very dark compared to other fins. The pectoral and pelvic fins may a light pink tinge. The cartilaginous edge to the lower jaw is bright yellow to red-yellow. The
peritoneum is black. Some fish may be very black with only the underside of the head and belly yellowish-white (specimens described by Heckel (1846-1849b) as Scaphiodon niger; however since these
fish "decompose quickly in the commonly used ethyl alcohol concentrations", they may have been poorly preserved and the black colouration resulted from partial decomposition).
Size
Attains 35.3 cm standard length, about 45.0 cm total length and 0.5 kg.
Distribution
Found from Turkey, Syria, Lebanon and Israel to Iran. In Iran, it is reported from the Tigris River including the the Regab River in Kurdistan and the Selakhor River near Borujerd, the Jarrahi,
Marun, Karun, Kuhrang, Bazoft and Khersan rivers, throughout the Dez and Karkheh basins to their uppermost reaches; Lake Zarivar; Esfahan including the Dopolan and Zayandeh rivers,
Dasht-e Kavir including
the Jajarm and upper Kal Shur rivers, Namak Lake including the Karaj, Shur, Abhar, Qareh Su and Qom rivers; Kor River, Lake Maharlu, Gulf including the Zohreh River and its Kheirabad
tributary and the Mand River near Akbar and its Shur (Dasht-e Palang) River tributary,
Kerman-Na'in, Dasht-e Lut, Sirjan, Hormuz including the middle to upper Hasan Langi, Kul and its Shur River tributary, and Hamun-e Jaz
Murian basins (Lovett, 1873; Nikol'skii, 1899; Berg, 1949; Kähsbauer, 1964; Spillman, 1972; Armantrout, 1980; Rainboth, 1981; Bianco and Banarescu, 1982; Abdoli, 2000; Ghorbani Chafi,
2000; R. Mehrani, pers. comm., 2000; Jalali et al., 2005; Esmaeili et
al., 2011?).
Zoogeography
Its relationships with other Capoeta species is generally unclear, as is the status of isolated populations some of which have been named. The larger zoogeographical relationships of this and
other Capoeta species remain uncertain. See also above under genus.
Habitat
Unknown in detail.
Age and growth
All males are mature at 18 cm and all females at 20 cm in Khalaf's (1987) study in the Lebanon. In Lake Kinneret, Israel, Stoumboudi et al. (1993) found that fish longer than 25 cm have
developed gonads, occasionally males mature between 16 and 25 cm as did females between 20 and 25 cm. Khalaf et al. (2002) found 6 age classes (1+ to 6+) in the Nahr el Khalb, a Lebanese stream.
Maximal growth was in July and August and minimal growth between December and February. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 40 Iranian fish measuring
5.23-19.87 cm standard length. The a-value was 0.0282 and the b-value 2.890 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a
b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Khalaf (1985) and Spataru and Gophen (1986) examined the food of this species in Lebanon and Israel respectively. Benthic diatoms and filamentous algae are the main foods. Some other algal species
and some zoobenthic organisms are present along with large quantities of mud. The species is classified as a phytobenthophagous fish, one that takes its food from bottom sediments. Leaf remains have
also been found in gut contents. Abdoli (2000) lists variety of insects: Chironomidae, Formicidae, Epididae, Empididae, Tipulidae, Tabanidae, Simuliidae, Hydroptilidae, Grouvellinus,
Elmis, Hydropsyche, Heptagenia, Baetis and hydracariens.
Reproduction
Al-Rudainy (2008) gives sexual maturity at 2-3 years
in Iraq with spawning in May, absolute fecundity up to 53,000 eggs and relative
fecundity up to 7300 eggs/g body weight, and average egg diameter 1.48 mm.
Khalaf (1987) examined the reproductive cycle in this species for Lebanese waters. Spawning begins in May and ends in July. Eggs number up to 5,138 and egg diameters are up to 2.2 mm. In marked
contrast, Stoumboudi et al. (1993) found that gonad weights are greatest in January in Lake Kinneret, Israel, 4 months earlier. This may be evidence of different temperature regimes or
populational variation. Fishelson et al. (1996) confirm that this species migrates in winter, December to February in the upper Jordan River of Israel, the process being initiated by rainfall
and flooding and a decrease in temperature to 16-18°C. The gonadosomatic index is highest in February and the final months of reproduction are March to May. Lake dwelling fish aggregate and swim up
streams as far as 25 km and altitudes of 400-900 m, fattening and ripening at the spawning site. They can jump rapids on this migration (and in Iran large fish cornered in small streams will jump
over seine nets!). The females excavate a shallow nest in which to deposit adhesive eggs, up to 4.5 mm in diameter. Dozens of nests are found close together and sand and gravel stirred up by the
excavation covers adjacent nests. After spawning the adults return downstream to the stream mouth and lake.
Parasites and predators
Dollfus (1970) describes a new cestode Coelobothrium monodi from this species at "Nasratabad", possibly from the
Dasht-e Lut basin. Jalali et al. (1995) describe two new species of
monogeneans, Dactylogyrus rohdeianus and D. capoetae, from fish caught in the "Chaghalnandi" River, a Karkheh River tributary north of Ahvaz. González-Solís et al. (1997)
report the nematodes Rhabdochona denudata and Rhabdochona fortunatowi from this species in the Mand River, Fars. O. M. Amin (pers. comm., 1998) has identified the acanthocephalan
Acanthocephalorhynchoides cholodkowskyi from specimens collected in the Mand River west of Shiraz, Fars. Jalali et al. (2002) and Jalali and Barzegar (2006) record
Trichodina pediculus, Dogielius molnari, Gyrodactylus sp., Dactylogyrus carassobabrbi and D. lenkorani from this species in Lake Zarivar. Barzegar et al.
(2004) examined this species for parasites in fish from the Beheshtabad river in Chahar Mahall va Bakhtiari Province and found Dactylogyrus lenkorani, Gyrodactylus pulcher, Dactyolgyrus
sp., Allocreadium isoporum and Myxobolus molnari. Mehdipoor et al. (2004) record the monogeneans Dactylogyrus lenkorani and D. pulcher in Zayandeh River fish. Barzegar
and Jalali (2006) report a parasite in this species from Kaftar Lake as Dactylogyrus lenkorani. Masoumian et al. (2007) record the myxosporean parasites Myxobolus
samgoricus and M. varicorhini from this species in the Zayandeh River.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish. Nazari Chamak et al. (2010) found the following myxozoan parasites in the
genus Myxobolus: buckei, cristatus, karelicus, musajevi, samgoricus,
suturalis and varicorhini in fish from the Halil River, Kerman.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea cyprinacea
on this species.
Economic importance
This species is of no economic importance in Iran although in Israel annual catches in Lake Kinneret have been as high as 29 tonnes or 7% of the total fishery (Spataru and Gophen, 1986). Heckel
(1846-1849b) reports that this species was "greatly appreciated as food fish by the local people" in the Kor River basin, Fars (as his Scaphiodon amir).
Samaee and Patzner (2011) mention that it is fished recreationally in Iran.
The eggs are reputedly poisonous and this is said to account for the low population of introduced Oncorhynchus mykiss in Gahaar Lake, Lorestan (R. Mehrani, pers. comm., 2000).
Conservation
A widely distributed species, probably not in need of conservation. Endangered in Turkey (Fricke et al., 2007).
Further work
The relationships of isolated and named taxa under this species, and the relationships of this species to other Capoeta, would benefit from molecular analyses.
Sources
Type material: See above, Capoeta capoeta intermedia (IZA 7892, 7894, CMNFI 1982-0367 (formerly IZA 7893), ZMH 6090) P. G. Bianco.
? check this last speciemne; Scaphiodon amir (NMW 61472, 46081); Scaphiodon niger (NMW 51655, 51654, 51656); Scaphiodon saadii (NMW 51666).
Iranian material:
CMNFI 1993-0154, 1, ? mm standard length, Markazi, Sharra River near Far (34º03'N, 49º20'E);
CMNFI 1995-0020, , mm standard length, ();
CMNFI 1995-0021, , mm standard length, ();
CMNFI 1997-0004, , mm standard length, ();
Comparative material: BM(NH) 1974.2.22:1856, 227.1 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1934.9.5:3-5, 6, 14.8-45.5 mm standard length, Rawanduz River, Razanok.
Capoeta fusca
Nikol'skii, 1897
Common names
سياه ماهي (= siah mahi, meaning black fish).
Systematics
The 2 syntypes, listed in Latin as from "Mondechi in Persia orientali", are in the Zoological Institute, St. Petersburg (ZISP 11108) and measure 121.9-172.9 mm standard length. Berg (1949)
gives the locality in Russian as "Mondekhi, northern periphery of the Bajistan Salt Desert in southeast Khorasan". This locality is possibly Mandehi or Miandehi at 34°53'N, 58°38'E.
Nikol'skii (1897) lists a series of specimens in Latin, presumably all of which he regarded as types, sic:- "11108. Mondechi in Persia orientali. 12.IV.96 (2). 11109. Persia orientalis. 1896.
(6). 11110. Persia orientalis. 1896. (5). 11111. Persia orientalis. 1896. 11112. Kuss in Persia orientali. 6.IV.96.", the last two lacking number of specimens. Berg (1949) gives 20 specimens for
11109, 6 specimens for 11110, and 1 specimen for each of the last two. Catalogue dates in ZISP for all these are 26.IV.96, presumably new style, while Berg (1949) gives new style dates 24.IV.1896 for the
first and 18.IV.1896 for the last (and this last is 26.IV.1896 in the catalogue). Only ZISP 11108 specimens are regarded as syntypes by Berg (1949). Berg (1949) also points out the confusion over the
date when Zarudnyi, the collector, was at "Kuss" (= Khusf at 32°46'N, 58°53'E) given by Nikol'skii as 6.IV.96 old style but on this date Zarudnyi was at "Kiaz-khak" near Asadabad
(35°38'N, 59°21'E) south of Mashhad and only reached Khusf on 8 (or 20 new style).VI.96. This is not particularly critical in this instance but serves to point out the difficulties of reconciling
literature, field notes, catalogues, and jar labels.
Capoeta nudiventris Nikol'skii, 1897 is a synonym. The syntypes are in the Zoological Institute, St. Petersburg (ZISP 11106) according to Berg (1949) and comprise 3 fish 92.4-121.5 mm standard
length. Berg (1949) gives the type locality as "Zeride near Bajistan in southeast Khorasan, 30.IV.1896" (the date in the jar is 26.IV.1896). Nikol'skii (1897) lists 3 collections all from
"Saride in Persia orientali. 18.IV.96." with numbers 11105, 11105 (presumably an error for 11106), and 11107 and 6 (actually 7 in the jar and according to Berg (1949)), 3, and 5 specimens
respectively. Berg (1949) lists the 5 specimens under 11107 as from "Chakhak in the Al'kor region between Bajistan and Birjand. 9.V.1896", presumably at 33°17'N, 58°54'E. These 5 fish are
37.0-55.2 mm standard length, collected on 25.IV.1896 in the ZISP catalogue and not listed as types in the jar, nor in the catalogue, nor in Berg (1949). The 7 fish in ZISP 11105 measure 46.8-75.3 mm
standard length, are from the same locality listed under ZISP 11106 in Berg (1949) and are listed as types in the ZISP catalogue, though not in Berg (1949). Judging from the labels and
catalogue sheets, the types are probably from Sarideh at 34°22'N, 58°14'E and comprise 11105 and 11106.
Rainboth (1981) places both fusca and nudiventris in the genus Schizocypris on the basis of the enlarged scales around the vent and anal fin base, a condition reported on by Berg
(1949) also but not considered by this latter author to warrant inclusion of these fish in Schizocypris.
Key characters
The strong mode of 7 branched dorsal fin rays, distribution, and the relatively low scale count aid in identifying this species.
Morphology
Dorsal fin with 3 unbranched rays and 7-8, strong mode at 7, branched rays, anal fin with 3 unbranched and 5 branched rays, pectoral fin branched rays 14-20 and pelvic fin branched rays 7-9.
Lateral line scales 40-62, mostly 47-56. Scales are found regularly arranged over the whole body and are enlarged around the anus and anal fin base. There is a pelvic axillary scale. Scales are oval
and have a subcentral, markedly anterior focus, numerous radii on all fields and moderate numbers of circuli. Gill rakers 11-20, short and touching the raker below when appressed. The mouth is
horseshoe-shaped. The last unbranched dorsal fin ray is weak with only a few fine denticles along the basal half. The pharyngeal teeth are very spatulate up to the tip but are thick. There is an
occasional trace of a fifth tooth in the major row but all the fish examined had only 4 strongly developed main row teeth. The gut is very elongate with several anterior and posterior loops.
Some populations or individuals may show a very light belly extending up onto the lower flank rendering scales hard to see. Capoeta nudiventris was apparently founded on specimens like this.
Some scales low on the flank are incompletely imbricate and deeply embedded in the skin. Berg (1949) in examining the types of fusca and nudiventris found the extent of the scales
ventrally to be the same and nudiventris is not naked on the lower flank and belly.
Meristics for Iranian specimens:- dorsal fin branched rays 7(77); anal fin branched rays 5(77); pectoral fin branched rays 14(1), 15(1), 16(8), 17(23), 18(26), 19(13) or 20(5); pelvic fin branched
rays 7(8), 8(64) or 9(5); lateral line scales 46(4), 47(6), 48(8), 49(10), 50(10), 51(9), 52(9), 53(9), 54(9), 55(2) or 56(1); total gill rakers 13(1), 14(11), 15(25), 16(26), 17(11), 18(1), or 20(1);
pharyngeal teeth 2,3,4-4,3,2(20); and total vertebrae 40(9), 41(42), 42(20) or 43(4).
Sexual dimorphism
Unknown but males presumably bear large tubercles in the breeding season.
Colour
The back and flanks are dark while below the lateral line the body can be very light. The dorsal, anal and caudal fin membranes are dark. Young fish may have a mid-lateral stripe as wide as the eye
ending in an indistinct dark blotch on the caudal fin base. The peritoneum is dark brown to black.
Size
Reaches 21.5 cm total length (Johari et al.,
2009).
Distribution
This species is found in eastern Iran in the Tedzhen River (including Kashaf River),
Dasht-e Kavir, Bejestan, Dasht-e Lut and Sistan basins in rivers, springs and qanats, some of the latter not easily located on
maps (Nikol'skii, 1899; Berg, 1949; Abdoli, 2000). A record from the "Schalman Rud" presumably in the Caspian Sea basin is most probably an error (Wossughi, 1978).
Johari et al. (2009) record this species from the Ghoorghoori, Asafshad,
Mardan Shah, Gazdmoo and Afin rivers in Qae'nat province and in 44 qanats of
Birjand County in eastern Iran.
--- Zirkhuch may be Zir-e Kuh at 32°48'N, 59°50'E ? check this and if reasonable search for Zir and add in all text - Zirkhuch is in eastern or southeastern Khorasan for sure
Zoogeography
Saadati (1977) considers that this species entered eastern Iran from the west via the Namak Lake basin. See also above under genus.
Habitat
Karaman (1969) considers that this species shows the greatest adaptation among Capoeta species to desert life: an elongate and low body, scaleless belly in many individuals, weak spiny dorsal
fin ray, reduced number of dorsal fin rays, short dorsal fin which can easily lie flat against the body, and the mouth structure.
Johari et al. (2009) studied 10 qanats in Birjand County and found the
following ranges: 3.8-24.9ºC, 0-6.3 p.p.t.
salinity, 7.7-8.5 pH, 3.8-1164 μS, 6.3-13.8
mg/l dissolved oxygen, 0.31-11.5 mg/l nitrate, 0-0.8 mg/l nitrite, 0.04-0.29
mg/l ammonia, 185-750 mg/l total hardness, 2.17-815 total dissolved solids,
25-410 mg/l calcium, 0-100 mg/l magnesium, 0.16-340 mg/l sulphate, 2.3-27 mg/l
potassium, 0.01-0.14 mg/l chlorine and 0.2-0.95 mg/l phosphate. No mortalities
were noted in fish kept in salinities up to 10 p.p.t. for 120 hours, but higher
levels started to show progressive mortalities. As salinity increased, fish
became darker and dead fish were almost black. The fish exhibited schooling
behaviour both in aquaria and in their natural environment.
Age and growth
Johari et al. (2009) found a total
length/weight relationship of body weight = 0.01010 x TL2.9477 for
600 fish from 10 qanats in Birjand County.
Patimar and Mohammadzadeh (2011) examined fish from the Shadmehr qanat in south
Khorasan and found a maximum age of 5+ years, negative allometric growth for
males and isometric for females, males grew faster than females, and von
Bertalanffy growth models Lt=18.74(1-e-0.33(t+0.473)) for
males and Lt=22.35(1-e-0.32(t+0.333)) for females.
Food
Gut contents of the few fish examined contained fragments of large plants including large seeds, filamentous algae and sand grains.
Johari et al. (2009) found this species to be herbivorous based on
relative gut length and to be relatively gluttonous based on gut vacuity index.
Large plants and filamentous algae made up 86.8% of the food but molluscs,
aquatic insects and frog eggs were secondary foods. Feeding was highest in
December and January before spawning and in August and September when presumably
productivity was greatest. In March to May, the spawning season, feeding was
reduced.
Reproduction
Fish caught in April and May have mature eggs along with some immature eggs, indicating that spawning may occur in stages. Fish caught in November have small but obvious and developing eggs.
Johari et al. (2009) found the reproduction period began in March and
lasted until the latter part of May based on the gonadosomatic index.
Patimar and Mohammadzadeh (2011) found a sex ratio of 1:2.42 in favour of
females for their south Khorasan fish, with reproduction in the qanat between
May and August with the gonadosomatic index highest for males in June and for
females in July. egg diameters attained 2.05 mm, maximum fecundity attained
22,773 eggs and relative fecundity up to 583 eggs/g.
Parasites and predators
Black spots on the head and fins (syntypes of nudiventris as noted by Nikol'skii (1897))
were probably encysted larvae of trematodes (Berg, 1949). Johari et al.
(2009) found the trematode Clinostomum in various body parts and their
qanat fishes showed lordosis and scoliosis.
Economic importance
This species has been studied in aquaria for the
toxicity of lead acetate (Omidi et al., 2009). Toxicity decreased with
increase in water hardness, qanat water with a high water hardness (310 mg L-1)
showing low toxicity. It will feed on mosquito larvae under aquarium conditions
and could have been a better candidate for combating malarial mosquitos than the
exotic and deleterious Gambusia holbrooki.
Conservation
A widely distributed species apparently able to survive in a wide range of desert habitats, it may not be in need of conservation.
Further work
Biology in mostly unknown and would help confirm the impression that it is not in need of conservation.
Sources
Type material:
See above, Capoeta fusca (ZISP 11108) and Capoeta nudiventris (ZISP 11105, 11106).
Iranian material: CMNFI 2007-0005, 7, 27.8-84.2 mm standard length, Semnan, spring at Nardin (ca. 37º03'N, ca. 55º47'E); check ID?
CMNFI 2007-0015, 8, 60.1-85.6 mm standard length, Khorasan, qanat at Khalaj (ca. 34º54'N, ca. 58º52'E;
CMNFI 2007-0016, 8, 85.5-171.4 mm standard length, Khorasan, qanat and jube at Bidokht (ca. 34º21'N, ca. 58º46'E);
CMNFI 2007-0017, ?, ? mm standard length, Khorasan, qanat at Dasht-e Bayaz (ca. 34º02'N, ca. 58º47'E);
CMNFI 2007-0018, 15, 21.7-92.4 mm standard length, Khorasan, Shur River (ca. 33º52'N, ca. 59º41'E);
CMNFI 2007-0019, 9, 32.7-141.3 mm standard length, Khorasan, qanat between Esfideh and Abbasabad (ca. 33º29-39'N, ca. 59º38-46'E);
CMNFI 2007-0020, 23, 43.7-115.1 mm standard length, Khorasan, qanats at Marak and Rabi'an (ca. 32º55-58'N, ca. 59º26-27'E);
CMNFI 2007-0021, 16, 24.8-56.3 mm standard length, Khorasan, Shah Abbas qanat in Asadabad (32º55'N, 60º01'E);
CMNFI 2007-0022, 6, 56.7-112.1 mm standard length, Khorasan, qanat pool at Mud-e Dahanab (32º43'N, 59º31'E);
CMNFI 2007-0023, 6, 82.5-113.1 mm standard length, Khorasan, qanat at Sarbisheh (32º34'N, 59º48'E);
BM(NH) 1958.11.7:1-6, 6, 26.1-90.9 mm standard length, Khorasan, near Jajarm (no other locality data).
Capoeta trutta
(Heckel, 1843)
Common names
tu'ini (and variant spellings in transliteration such as touyeni, tuyeni, tuini or too'ini) in Khuzestan (meaning unknown); tu'ini gelkhorak in Khuzestan (see C. damascina for meaning); shir
mahi (= milk fish), barg bidy or barg-e bidi (= willow leaf, perhaps from shape and colour), berzem.
[twena, hemira, tela morqat, tela moraqqat; ethra at Mosul (Heckel (1843b), or takal handscherli (takal = soft or flexible presumably from its small scales, handscherli = armed with a dagger or knife from the
dorsal fin spine) at Aleppo (Heckel, 1843b), all in Arabic; trout barb].
Systematics
Rainboth (1981) places this species in Schizocypris on the basis of enlarged scales forming a split to encompass the urogenital region and a bare to partially bare mid-dorsal strip anterior
to the dorsal fin. However the schizothoracine fishes are quite different (see accounts for Schizothorax, Schizopygopsis and Schizocypris) and this placement is not accepted here.
The type localities of Capoeta Trutta as given by Heckel (1843b) are "Gewässern bei Aleppo" and the "Tigris bei Mossul". The syntypes are in the Naturhistorisches Museum
Wien according to Krupp (1985c) as follows: NMW 55935-37, 55942, 6 specimens 94-274 mm standard length from Mosul, NMW 55926, 55928, 55940-41, 7, 68-192 mm standard length from Aleppo, and in the
Senckenberg Museum Frankfurt (SMF 2567 (formerly NMW), 1, 407 mm standard length, from Mosul and SMF 923 (formerly NMW), 1, 175 mm standard length, from Aleppo. Four other syntypes are under NMW 55939,
1 other syntype under NMW 55938 and a dried syntype under NMW 58875. Eschmeyer et al. (1996) lists similar material with the numbers of fish under each catalogue number detailed thus: NMW 55926
(1), NMW 55928 (2), NMW 55935-37 (2, 2, 1), NMW 55939-42 (4, 1, 3, 1), possibly 1 fish in the Rijksmuseum van Natuurlijke Historie, Leiden (RMNH 3164, formerly NMW), 1 syntype in the Senckenberg Museum
Frankfurt (SMF 923, formerly NMW) and 1 syntype SMF 2567 (formerly NMW), and 1 dried syntype from the Museum für Naturkunde, Universität Humboldt, Berlin (ZMB 8789; not located in February 2006). The
catalogue in Vienna lists only 5 specimens although the card index in 1997 lists NMW fish as syntypes in agreement with Eschmeyer et al. (1996).
Key characters
The combination of small scales, transverse mouth, dorsal and anal fin branched ray counts, the very strong last unbranched dorsal fin ray (longer than head length - usually strong but rarely weak),
and the colour pattern identifies this species.
Morphology
Dorsal fin with 3-5 unbranched rays followed by 7-9, usually 8, branched rays, anal fin with 2-3 unbranched rays followed by 5 branched rays, pectoral fin branched rays 14-18, and branched pelvic
fin rays 5. Hanel et al. (1992) found 23-31 denticles or teeth on the serrated dorsal fin ray, the largest near the centre of the ray length. Scales in lateral line 68-90, scales above lateral
line 15-18 and scales below lateral line 10-17. The back anterior to the dorsal fin is compressed and lacks scales except near the occiput. Scales have a protruding anterior margin but are otherwise
rounded, anterior and posterior radii, fine circuli and a subcentral anterior focus. There is a pelvic axillary scale. Gill rakers 23-33, on the lower arm 18-25 (with lowest counts in smallest fish).
The rakers reach the second raker below when appressed. Pharyngeal teeth 2,3,4-4,3,2. Teeth are broadly spoon-shaped at the tip, with narrow cusps and stems such that they are quite fragile. A
frequency distribution of counts was not taken because of this fragility. Total vertebrae 43-46. The mouth is inferior and transverse with a strong horny cover to the lower jaw. The gut is very
elongate with numerous anterior and posterior loops. The karyotype of fish from the Tigris River of Turkey is 2n=150, possibly hexaploid, with 35 meta-submetacentric chromosomes, 40 pairs of
subtelo-acrocentric chromosomes with NF=220 (Kılıç Demirok and Ünlü, 2001).
Meristics for Iranian specimens:- branched dorsal fin rays 8(34); branched anal fin rays 5(34); branched pectoral fin rays 14(1), 15(8), 16(18), 17(6) or 18(1); branched pelvic fin rays 5(34);
lateral line scales 68(2), 69(1), 70(1), 71(4), 72(5), 73(3), 74(5), 75(2), 76(3), 78(3), 79(1), 80(1), 81(1), 83(1) or 84(1); total gill rakers 22(1), 24(5), 25(4), 26(3), 27(7), 28(8), 29(3), 30(2)
or 31(1); and total vertebrae ?more 43(1), 44(6), 45(3) or 46(2) - NMC 79-269, 367, 384, 269, 268, Behnke 231
done.
Sexual dimorphism
Males bear a single tubercle on each flank scale, sometimes 2 tubercles, positioned about the middle of the exposed scale or nearer the posterior edge. The head has small and widely scattered
tubercles on the top and sides and large tubercles around the snout from eye to eye below the nostril level. Large tubercles occur in single files on the anal and dorsal fin rays, particularly the
posterior rays, becoming apparent on the more anterior rays as tuberculation develops more highly.
Colour
The head and body and the dorsal fin (and sometimes the caudal fin) are covered with small, distinctive black spots, often c- or x-shaped. Spots are apparent through the silver flank colour. Some
fish in Khuzestan lack spots but transitional specimens from fully spotted through weakly spotted to immaculate are found. Colour is brownish to yellowish or olive-green on the back with silvery-white
flanks and the belly lighter, white with silvery tints. Some fish are very pale almost whitish. Upper flank scales in particular are outlined with dark pigment. The eye is orange above or mostly
silvery. Lower fins are orange to yellow at the base and blackish distally, or may be orange to yellow overall. The dorsal and caudal fins are grey or hyaline. The lower rays of the caudal fin have a
slight orange-yellow tint. The peritoneum is dark brown to black.
Size
Attains at least 45.8 cm total length. Heckel (1843b) gives 1 Schuh 8 Zoll, or 52.7 cm.
Distribution
Found in the Quwayq, Orontes and Tigris-Euphrates basins including the Iranian portion of the latter (Berg, 1949; Marammazi, 1995) and the Gulf basin in the Zohreh River.
upper Mand ? to check on maps
Zoogeography
Its relationships with other Capoeta species is generally unclear, as are the larger zoogeographical relationships of this and other Capoeta species. See also above under genus.
Habitat
Marammazi (1994) considers this species to be stenohaline but nonetheless more widely distributed than stenohaline Barbus
(= Mesopotamichthys) sharpeyi in the Zohreh River which drains to the northern Persian Gulf.
Age and growth
The majority of the population studied by Ünlü (1991) in the Tigris River in Turkey are in age groups 2 and 3 although males live to age 7 and females age 10. Females are usually longer and heavier
than males of the same age. Males comprise 41.26% and females 58.74% of this population. In a stream in the Euphrates River drainage of Turkey, Gul et al. (1996) found fish to live for 8 years
with 60-90% of the fish in age groups 1 to 3. Females comprised 53.3% and males 46.7% of the population. Kalkan (2008) studied a population in the Karakaya Dam lake on the Turkish Euphrates River.
Maximum age was 7 years, age groups 4 and 6 were mostly females whereas age group 3 was mostly male, age-length, age-weight and length-weight formulae were given, and the average growth condition factor
was 1.30 for females and 1.28 for males.
Food
Gut contents include diatoms, green algae and large amounts of sand.
Reproduction
Spawning in both the Tigris and Euphrates rivers in Turkey took place in May-June. Males mature at age 2 and females at age 3 in both rivers. Ripe egg size in the Tigris varied between 1.33 and 2.11
mm and egg numbers between 4713 and 18240. Ripe eggs in the Euphrates attained 1.04 mm and the maximum number of eggs per gramme of gonads was 666. Fish from Khuzestan had well-developed eggs on 30
January while adult fish taken on 7 July were not in reproductive condition.
Parasites and predators
Molnár and Jalali (1992) report the monogenean Dactylogyrus pulcher from this species in the Dez River of Khuzestan. Gussev et al. (1993a) describe a new species of monogenean from
this species in the Dez River, Dactylogyrus microcirrus. Baska and Masoumian (1996) describe two new species of Myxosporea from fish caught in the Karun River at Ahvaz, Myxobolus molnari
taken from the gills and Myxobolus mokhayeri taken from between the soft rays of the fins. The latter species is named after Dr. Baba Mokhayer, an internationally renowned Iranian professor.
The new species are of minor pathological importance as the infections are of low intensity and prevalence. Masoumian and Pazooki (1999) list Myxobolus molnari and M. mokhayeri from
this species from localities in Khuzestan.
Peyghan et al. (2001) record Neoechinorhynchus sp. and
Rhabdocona sp. from fish from Khorramabad rivers.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. and Tracheliastes
polycolpus on this species.
Economic importance
Duman and Duman (1996) give the nutritional value of Capoeta trutta from Keban Dam Lake in Turkey but this fish is little used in Iran.
However,
Peyghan et al.
(2001) report that is is an economically important species with a good market
value in the Khorramabad region.
Conservation
This species does not appear in need of conservation but its biology is too poorly known in Iran to be certain.
Kalkan (2008) recommended prohibition of fishing in Turkey during March-August
and fish under 22.62 cm should not be retained.
Further work
The biology of this species and its relationships to other Capoeta species needs work.
Sources
Type material: ?
Iranian material:-
CMNFI 1979-0020, ?, ? mm standard length, ();
CMNFI 1979-0268, 3, 115.7-141.2 mm standard length, Lorestan, between Nowqan and Khorramabad (no other locality data);
CMNFI 1979-0269, 2, 114.1-144.1 mm standard length, Lorestan, between Nowqan and Khorramabad (no other locality data);
CMNFI 1979-0367, 2, 29.7-54.1 mm standard length, Khuzestan, Meymeh River 11 km north of Dehloran (32º44'30"N, 47º09'30"E);
CMNFI 1979-0368, 8, 36.3-67.9 mm standard length Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
CMNFI 1979-0376, 1, 55.2 mm standard length, Khuzestan, river tributary to Karkheh River (32º48'30"N, 48º04'30"E);
CMNFI 1979-0384, 1, 218.4 mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1991-0153, 2, 153.8-217.2 mm standard length, Khuzestan, Zohreh River (no other locality data);
CMNFI 1995-0020, ?, ? mm standard length, ();
CMNFI 1995-0021, ?, ? mm standard length, ();
CMNFI 1995-0030, ?, ? mm standard length, ();
CMNFI 2007-0100, 1, 136.7 mm standard length, Azarbayjan-e Gharbi, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0109, 11, 61.3-167.4 mm standard length, Kordestan, Qeshlaq River basin north of Sanandaj (ca. 35º33'N, ca. 47º08'E);
CMNFI 2007-0110, 3, 96.6-160.3 mm standard length, Kordestan, Yuzidar River basin (ca. 35º05'N, ca. 46º56'E);
CMNFI 2007-0113, 1, 74.8 mm standard length, Kermanshahan, Razavar River 35 km northwest of Kermanahah (ca. 34º25'N, ca. 47º01'E);
CMNFI 2007-0116, 1, 95.9 mm standard length, Kermanshahan, Gav Masiab west of Sahneh (ca. 34º28'N, ca. 47º36'E);
CMNFI 2007-0117, 2, 153.8-217.2 mm standard length, Kermanshahan, Gav Masiab near Sahneh (ca. 34º24'N, ca. 47º40'E);
ZMH 2511, 1, 319.0 mm standard length, Kermanshahan, Karasu-Gamasiab-Seymarreh (no other locality data).
Comparative material: BM(NH) 1931.12.21:8, 1, 113.5 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1968.12.13:376-390, 15, 35.6-123.3 mm standard length, Syria, Euphrates River at Mayadine (?);
BM(NH) 1974.2.22:1374-1377, 4, 66.3-91.2 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1974.2.22:1382, 1, 86.1 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1974.2.22:1388-1389, 2, 259.4-273.3 mm standard length, Iraq, Tigris River at Samarra (?).
Genus Capoetobrama
Berg, 1916
Capoetobrama kuschakewitschi
(Kessler, 1872)
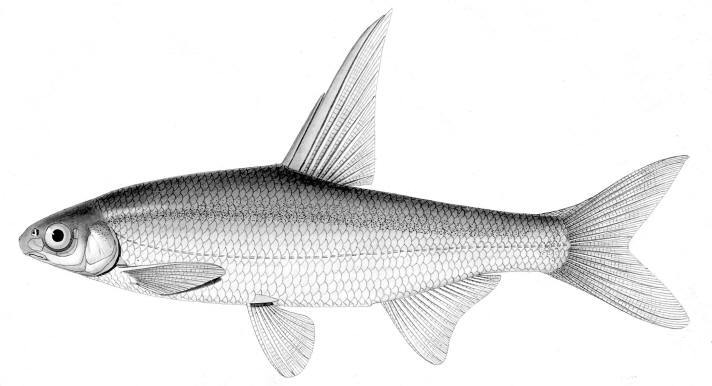
This species is reported from the Karakum Canal of Turkmenistan (Shakirova
and Sukhanova, 1994; Sal'nikov, 1995) and may eventually be found in
the Tedzhen River and Caspian Sea basins of Iran. No Iranian record.
Genus Carasobarbus
Karaman, 1971
?
Some of the past literature on this genus appeared under Barbus (q.v.)
Carasobarbus luteus
(Heckel, 1843)
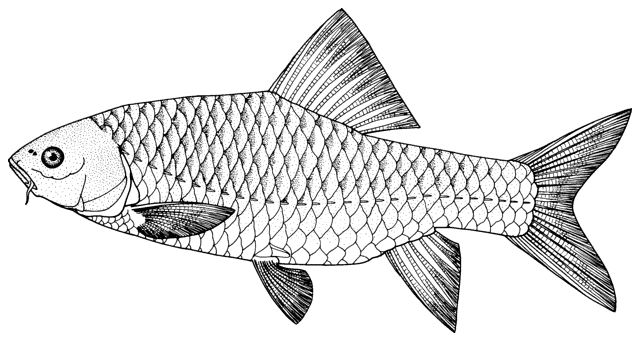
Common names
حمري (= hemri), himri; sangal or zangol (= blackish, used at Kermanshah, J. Valiallahi, pers. comm., 2001); lab matiki (= from lipstick
by professional fishermen at Kermanshah in reference to red lips, from J. Valiallahi, pers. comm., 2001).
[himri, hamria, hamra, binni hamour, binni hamri, bunni himri, binni, binni shifatha, beni asphar (= yellow son), beni abjad (= white son), beni hamra (= red or yellow son), zuri or bartema,
all in Arabic; golden barb, yellow barbel].
Systematics
Heckel (1843b) gives localities for the types of Systomus luteus as "Orontes", and "Tigris", and in the next sentence at "Aleppo" and "Mossul".
Two syntypes were examined in the Naturhistorisches Museum Wien under NMW 54250 (but see below). Krupp (1985c) records a 301 mm standard length syntype from Aleppo formerly in the
Naturhistorisches Museum Wien, now in the Senckenberg Museum Frankfurt as SMF 6784. Eschmeyer et al. (1996) list the following syntypes: NMW 10827 (1 fish), NMW 54247 (2),
NMW 54248 (1), NMW 54249 (1), NMW 54253 (2), NMW 54254 (3), NMW 54255 (2), NMW 54520 (2), NMW 80043 (2) and possibly 2 syntypes in the Rijksmuseum van Natuurlijke Historie, Leiden (RMNH 2463,
formerly NMW) as well as the syntype in Frankfurt. The catalogue in Vienna seems to list 5 specimens but this part of the catalogue is overwritten and difficult to interpret. The
card index in 1997 lists NMW 53680a (1 fish, the lectotype), 53674 (1), 53675 (1) and 53676 (1) as the syntype series.
Systomus albus Heckel, 1843 from the "Tigris" and "Orontes" and Systomus albus var. alpina Heckel, 1849 are synonyms.
Systomus albus var. alpina was described from the "Flusse Kara-Agatsch und den Alpenseen Pire-San und Deria Kaserun" (= Qarah Aqaj River and Lake Famur, Fars;
Pire-San being Parishan and Deria Kaserun being Lake Kazerun, both other names for Lake Famur) (Heckel, 1846-1849b). Krupp (1985c) records 4 syntypes of alpina from
Shiraz (sic), Th. Kotschy as NMW 53679 (2 fish) and NMW 53681 (2). NMW 53678 (5 fish, 27.6-60.8 mm standard length), NMW 53679 (2 fish, 63.8-70.5 mm standard length), and NMW 53681
(2 fish, 79.6-93.3 mm standard length) are from the "Kara Agatsch bei Schiraz"; and NMW 53682 (2 fish, 201.7-203.7 mm standard length) are from the "Alpenseen Pire-san und Deria
Kaserun": all are possibly syntypes of Systomus albus var. alpina although the catalogue in Vienna lists 5 fish under this name in one column and 4
fish in smaller writing in the adjacent column. The card index in 1997 lists syntypes under NMW 53678 (5), 53679 (2), 53681 (2) and 53682 (2, one of which is the lectotype). Eschmeyer et
al. (1996) list 2 fish in the Rijksmuseum van Natuurlijke Historie, Leiden (RMNH 2464) as possible former NMW types of this taxon.
A dried specimen of Systomus albus from Mosul collected by Th. Kotschy may be a syntype (NMW 59485). Eschmeyer et al. (1996) gives the syntypes of this species as NMW 53674 (1),
NMW 53675 (1), NMW 53676 (1), NMW 53677 (1), NMW 53680 (1), NMW 91400 (1, dry) and SMF 812 (1), formerly NMW. Krupp (1985c) records the syntype of albus in the Senckenberg Museum Frankfurt
under SMF 812 as being 84 mm standard length. The Vienna catalogue lists 4 fish under Systomus albus but the card index in 1997 lists the same NMW fish as
Eschmeyer et al. (1996) as above with NMW 53680 as lectotype.
Barbus parieschanica Wossughi, Khoshzahmat and Etemadfar, 1982 is presumably also from Lake Famur or Parishan judging by the name and is a synonym (note that the species name is first
spelt parschanica on page 23 in the abstract in Farsi and on page 44 in the English abstract but in the text species description (page 34) and in the table (page 37) it appears as
parieschanica, and this is presumably the intended correct spelling). The species locality in the text is "Noorabad of Mamasany". ?
Saadati (1977) refers to a new and undescribed Cyprinion species from Lar in southern Iran but the fish are
Carasobarbus luteus.
Günther (1874) placed this species in Barynotus Günther, 1868, a genus with the type species from West Africa. Barynotus is preoccupied in Coleoptera and was replaced by
Barbellion Whitley, 1931 (Eschmeyer, 1990). Most authors place the species in Barbus although Karaman (1971) erected a new genus for it, Carasobarbus;
and Krupp (1985c) also synonymises Carasobarbus with Barbus. Bănărescu (1997) and Ekmekçi and Banarescu (1998) recognise Carasobarbus
as a valid genus however. Borkenhagen et al. (2011) recognise C.
luteus as a single, generalist species tolerating a wide variety of
habitats.
A group of related species share characters with this species (see also under
Kosswigobarbus kosswigi). Carasobarbus may be the generic names for certain members of the group.
Key characters
This species is characterised by a low scale count, smooth last unbranched dorsal fin ray, one or two pairs of barbels, and 10 branched dorsal and 6 branched anal fin rays.
Morphology
Dorsal fin with 4 unbranched rays followed by 9-11, usually and modally 10, branched rays. The last unbranched dorsal fin ray is smooth, thickened, sharp-edged and spine-like. Anal fin with 3
unbranched rays followed by 5-7, usually and modally 6, branched rays. Pectoral fin branched rays 13-17 and pelvic fin branched rays 7-9, usually 8. Lateral line scales 23-36. There is a pelvic axillary
scale. There are moderate to many anterior field radii and many posterior field radii and occasionally few lateral radii. The focus is central to subcentral anterior, the anterior scale margin is wavy
and the exposed part of the scale is coarse. The concealed part of the scale has numerous fine circuli. Total gill rakers 7-14, reaching the adjacent raker when appressed, sometimes forked at the tip and
with spinules on the anterior side. Pharyngeal teeth usually 2,3,5-5,3,2, with the anterior 2-3 teeth rounded and heavier than the posterior teeth. Variants may have 2,3,4 or 1,3,5 (Borkenhagen, 2005).
Posterior teeth are hooked at the tip and the grinding surface below the tip is irregular with a protuberant knob which may be striated. The gut is elongate with both posterior and anterior loops.
The mouth is terminal to subterminal and lips are weakly developed. There is one pair of short and thin barbels at the corner in most descriptions. Number and frequencies for 130 fish are 2 barbels
(47 or 36.2%), 3 barbels with left anterior present (7 or 5.4%), 3 barbels with right anterior present (5 or 3.8%), or 4 barbels (71 or 54.6%). However, this sample is 112 fish or 86.2% from Fars and
Hormozgan. Fish from these provinces, at such localities as the lower Mand River and the Sar Khun oasis north of Bandar Abbas consistently have a high frequency of 4 barbels (58.9%), and with 3 barbel
counts included 68.8%, than fish from the Tigris River basin. Even the 18 fish from the Tigris River basin had 5 fish with 4 barbels so, at least in the eastern part of this species range, 4-barbelled
fish are not rare.
Body form varies with habitat (Ali, 1982a), there being lake and river forms as with many other cyprinid species.
Iranian specimens have the following meristics: dorsal fin branched rays 9(7), 10(102) or 11(7); anal fin branched rays 5(3) or 6(114); pectoral fin branched rays 14(12), 15(44), 16(48) or 17(13);
pelvic fin branched rays 7(9), 8(107) or 9(1); lateral line scales 23(2), 24(10), 25(20), 26(22), 27(28), 28(16), 29(14), 30(4) or 31(1); total gill rakers 8(6), 9(24), 10(40), 11(28), 12(12), 13(3) or
14(2); pharyngeal teeth 2,3,5-5,3,2(19), 2,3,4-5,3,2(4) or 2,3,5-4,3,2(2); and total vertebrae 36(8), 37(53), 38(70), 39(25) or 40(1).
Sexual dimorphism
A 12.7 cm specimen from the Mand River has tubercles on the dorsal, anal, caudal, pectoral and pelvic fins, most strongly on the anal fin rays. Fine tubercles cover the top and sides of the head. A
20 cm fish from the same collection lacked tubercles. Another fish from the lower Mand River (128.5 mm standard length) also has fine tubercles on the upper flank scales as well as the head and fin rays.
Ali (1982) reports no sexual dimorphism for Iraqi fish.
Colour
The back and upper flank is dark brown, greenish black or grey-green fading to a whitish or silvery belly all overlain by an orange to yellowish tinge. On the upper flank, scale bases are black-brown
with a light blue-grey margin. There is a dark stripe along the mid-line of the back and a dark mid-lateral stripe. Fins are greyish to lime-green, reddish-yellow or orange, becoming blackish distally.
The pectoral and pelvic fins tend to be more orange than the anal and caudal fins which are more a faint lime-green. The lips are orange. The eye rim is yellow-green. The peritoneum is black. Small fish
have a collection of melanophores at the mid-base of the caudal fin forming a spot-like structure.
The fish described by Heckel (1846-1849b) as Systomus albus var. alpina were also painted live and had a lead-grey body, light brown at the head and reddish-white on the belly. Each
scale was black-brown at the base and light blue-grey at the margin, particularly on the upper flank. All fins were blackish and the eyes orange-red.
Size
Attains 38 cm calculated maximum length and 501 g (Ahmed, 1982) or 750 g (Borkenhagen, 2005). Heckel (1843b) gives 17 Zoll for Systomus albus (= 44.8 cm).
Distribution
This species is found in the Orontes and Quwayq rivers and the Tigris-Euphrates basin. In Iran, it is found in the Tigris River basin including the Hawr Al Azim marsh, the Gulf basin including the
Helleh, Dalaki, Shapur, Mand and Dasht-e Palang rivers and Lake Famur, the Lake Maharlu basin, the Hormuz basin and the Kor River basin (Wossughi, 1978; Bianco and Banarescu, 1982; Gh. Izadpanahi,
pers. comm., 1995; M. Rabbaniha, pers. comm., 1995; Abdoli, 2000). The record from the Kor River basin (Abdoli, 2000) needs confirmation with specimens.
Zoogeography
Karaman (1971) considers that the closest relatives of this species were to be found in India and southern Asia.
Habitat
van den Eelaart (1954) reports that this species in Iraq is a resident in still water and the slower sections of rivers and is the main fish in canals. In summer it goes to the deeper basins of marshes
and remains in the shade of plants. It tolerates warm water but does not go into open waters. Al-Hassan and Muhsin (1986) record this species from the Khor al Zubair in southern Iraq where annual
temperature range is 12-30°C and annual salinity change is 28-47‰. The fish appear unaffected by these conditions while Heteropneustes fossilis is moribund. Mohamed et al. (1993) report
Barbus (= Carasobarbus) luteus from 2 km southward of Fao, Iraq in a pure marine habitat (temperature 13-35°C and salinity 30-47‰). The fish were caught in April which is the flood season.
Age and growth
Ahmed et al. (1984) studied the reproductive cycle of this species in the Hawr al Hammar in southern Iraq near Basrah. Maturity is attained at a minimum of 11.2 cm for females and 12.2 cm for
males, at age 1+. The largest fish are 26.0 cm and age 6. Barak and Mohamed (1983) also found 6 age groups for fish from the Garma Marshes, Iraq. Ahmed (1982) studied a population in Tharthar Reservoir
about 65 km northwest of Baghdad and found 7 age groups. This study has the fastest growth of Iraqi populations. Khalaf et al. (1988) worked on a population in a flooded gravel pit about 50 km
north of Baghdad in Iraq and found fish up to age group 7+. Growth is greatest in the first year (67 mm) and averaged only 22.5 mm in the following years. Growth is slow in consequence of high salinity
(3-6% (sic)) and poor food resources. Mohamed et al. (1993) report fish up to 7 years of age in a marine setting in Iraq, Epler et al. (1996) up to 5+ years in fresh and salty Iraqi
lakes. Biro et al. (1988) found fish up to age group 8+ in the Diyala River, Iraq. Al Hazzaa and Hussein (2007) describe larval development and growth in the laboratory using fish from a Syrian
hatchery. Gökçek and Akyurt (2008) found fish up to 9 years of age in the
Turkish Orontes River and give growth parameters for this population. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 34 Iranian fish measuring 3.20-16.80 cm standard length. The a-value was 0.0232 and the b-value
3.036 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Naama and Muhsen (1986) examined feeding periodicities in this species in the Hawr al Hammar, Iraq. Food is mainly detritus, aquatic plants and algae taken throughout the night and day. Barak and
Mohamed (1982) studied food habits in the Garma Marshes, near Basrah, Iraq and found this fish to contain principally aquatic plants, the broken and fragmented leaves and stems of Vallisneria in
particular. Diatoms and other algae as well as shrimps, chironomid larvae, gastropods and cladocerans are important foods. Invertebrates are about eight times more important in fish smaller than 30 cm
than in larger fish. Plant parts are more important, almost twice as much, in larger fish than smaller. Mohamed et al. (1993) report plant remains to be dominant and fish eggs in lesser quantities
in a marine setting in Iraq. Epler et al. (1996) found plants to dominate in fish from fresh and salty Iraqi lakes, although not to the same extent as in Barbus
(= Mesopotamichthys) sharpeyi where 95.7-100% of
the diet was plants. Tendipedids, worms, detritus and fish were also found in B. luteus.
Khoshzahmat et al. (1981) found that this species did not eat molluscs in Lake Perishan (= Famur), near Kazerun in Iran and assume its diet is aquatic plants.
Reproduction
Spawning in the Hawr al Hammar starts in April and after July no fish are found in a partially spent phase. Eggs are yellow to orange in colour and testes white. The eggs attain 1.86 mm in diameter
and number up to 38,433 for the oldest fish. Bhatti and Al-Daham (1978) and Al-Daham and Bhatti (1979) report a spawning season of May-July (peak June-July) for a lower Euphrates River, Iraq population,
perhaps as a result of cooler temperatures outside the shallow marshes where warmer temperatures cause an earlier development of gonads. Epler et al. (1996) report spawning in June/July in
freshwater Iraqi lakes, earlier in a saline lake. Iranian fish have well-developed eggs in May.
Parasites and predators
Bykhovski (1949) reports a new species of monogenetic trematode, Dactylogyrus persis, from this species in the Karkheh River, Iran. Ebrahimzadeh and Nabawi (1975) list species in the nematode
genus Philometra, the protozoan genera Myxosoma and Trypanosoma, the trematode genera Dactylogyrus and Gyrodactylus and the nematode species Camallanus lacustris
as well as various unidentified cestodes, trematodes, acanthocephalans and hookworms, from this species in the Karun River. Jalali and Molnár (1990a) records two monogenean species, Dactylogyrus
spp., from this species in the Dez River. Molnár and Jalali (1992) describe a new species of monogenean, Dogielius persicus, from this species in the Dez and Karun rivers of Khuzestan. Gussev
et al. (1993b) describe a new species, Dactylogyrus carassobarbi, from this species in the Dez River, Khuzestan, the specific name being founded on a misspelling of the genus name
Carasobarbus. Masoumian et al. (1994) describe a new species of Myxosporea from the gills of this species in the Karun River, Khuzestan, namely Myxobolus persicus, and later
(Masoumian et al., 1996) another new species of Myxosporea, Myxobolus nodulointestinalis, in the gut lining of this species and also from rivers of southwestern Iran. Molnár et al.
(1996) report additional new species from this fish in Khuzestan, namely Myxobolus iranicus in the spleen and Myxobolus mesopotamiae in connective tissue of the caudal and pectoral fins.
Molnár and Pazooki (1995) record philometrid nematodes from this species in the Karun River, and these are presumed to be a new species.
Masoumian and Pazooki (1999) list Myxobolus persicus, M. karuni, M. sharpeyi, M. nodulointestinalis, M. mesopotamiae and M. iranicus from this species in various localities in Khuzestan.
Jalali et al. (2005) summarise the occurrence of Gyrodactylus species in Iran and record G. sp. from Dez River fish. Farahnak et al. (2002) record Anisakis sp. from
this fish in Khuzestan Province.
González-Solís et al. (1997) report Proleptinae larvae (Nematoda) from this species in the drainage of Lake Maharlu, Fars. The definitive host is a predatory fish, possibly Mastacembelus
mastacembelus, not yet recorded from this basin.
Moghainemi and Abbasi (1992) record a wide range of parasites from this species in the Hawr al-Azim in Khuzestan. Mortazaei et al. (2000) record an infection rate of 1.6% with the worm
Bothriocephalus opsariichthydis in Khuzestan marshes.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Argulus sp., Ergasilus sp., Ergasilus sieboldi and Lernaea
sp. on this
species.
Economic importance
An important food fish in southern Iraq and Iran (Al-Daham and Bhatti, 1979; Ahmed, 1982). Sharma (1980) reports that hamri were the fourth most important fish species at Basrah fish market, accounting
for 267,570 kg from October 1975 to June 1977. Heckel (1846-1849b) reports that they "reach a good size and are very tasty" in Lake Famur, Fars.
In some parts of Southwest Asia this species is regarded as &qut;sacred" kept and bred in special pools where fishing is forbidden (Tortonese, 1934).
The eggs of this species are poisonous (Najafpour and Coad, 2002). A kebab made of about one-quarter of an ovary was eaten. Toxic effects were dizziness, abdominal pain, vomiting, diarrhoea, bitter
taste, dryness of mouth, intense thirst, and faintness. One victim as hospitalised for two days and his stomach pumped while a second victim recovered after one day's rest.
Conservation
Vulnerable in Turkey (Fricke et al., 2007). It is a common species in Iranian freshwaters but no detailed conservation assessment has been made.
Further work
The biology of this species in Iran needs study along with its conservation status. Specimens from Fars show differences in body form from those in Khuzestan and this could be investigated.
Sources
Type material: ?
Iranian material: CMNFI 1979-0023, 17, 58.3-161.4 mm standard length, Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0024, 1, 61.5 mm standard length, Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0026, 2, ? mm standard length, Fars, Shapur River (29º47'N, 51º35'E);
CMNFI 1979-0047, 1, ? mm standard length, Fars, Ab-e Paravan (ca. 29º34'N, ca. 52º42'E);
CMNFI 1979-0076, 1, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0087, 1, ? mm standard length, Khuzestan, Karun River at Ahvaz (31º19'N, 48º42'E);
CMNFI 1979-0125, 1, ? mm standard length, Bushehr, Dalaki River near Dalaki (ca. 29º28'N, ca. 51º21'E);
CMNFI 1979-0129, 26, ? mm standard length, Fars, spring about 2 km from Farrashband (28º54'N, 52º04'E);
CMNFI 1979-0135, 19, ? mm standard length, Fars, Mand River tributary (28º08'N, 53º10'E);
CMNFI 1979-0154B, 3, 160.7-258.6 mm standard length, Fars, stream channels at Koorsiah (28º45'30"N, 54º24'E);
CMNFI 1979-0155, 2, ? mm standard length, Fars, spring at Gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0156, 6, ? mm standard length, Fars, qanat at Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0157, 1, ? mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E);
CMNFI 1979-0160, 2, ? mm standard length, Fars, spring at Arteshkkadeh Pomp (29º09'N, 53º37'E);
CMNFI 1979-0163, 1, 84.9 mm standard length, Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0164, 6, 56.6-91.1 mm standard length, Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0187, 31, ? mm standard length, Hormozgan, stream and pools at Sar Khun oasis (27º23'30"N, 56º26'E);
CMNFI 1979-0206, 3, 24.4-25.1 mm standard length, Fars, qanat near Runiz-e Pa'in (29º12'N, 53º40'E);
CMNFI 1979-0240, 3, ? mm standard length, Fars, Parishan Lake (ca. 29º31'N, ca. 51º50'E);
CMNFI 1979-0304, 5, ? mm standard length, Fars, Parishan Lake (ca. 29º31'N, ca. 51º50'E);
CMNFI 1979-0347, 2, ? mm standard length, Fars, Pol-e Berengie (29º27'30"N, 52º32'E);
CMNFI 1979-0352, 7, ? mm standard length, Khuzestan, marsh in Jarrahi River drainage (30º33'30"N, 48º48'E);
CMNFI 1979-0358, 1, 23.7 mm standard length, Khuzestan, pond southeast of Bostan (31º37'N, 48º07'E);
CMNFI 1979-0360, 8, ? mm standard length, Khuzestan, canal branch of Karkheh River (31º40'N, 48º35'E);
CMNFI 1979-0364, 6, ? mm standard length, Khuzestan, river at Abdolkhan (31º52'30"N, 48º20'30"E);
CMNFI 1979-0371, 7, ? mm standard length, Khuzestan, stream in Karkheh River drainage (32º05'N, 48º19'E);
CMNFI 1979-0687, 7, 124.8-154.1 mm standard length, Fars, Shiraz bazar (no other locality data);
CMNFI 1979-0789, 4, ? mm standard length, Fars, Lake Parishan (29º31'N, 51º48'E);
CMNFI 1991-0154, 1, ? mm standard length, Khuzestan, Hawr al Azim (ca. 31º45'N, ca. 47º55'E);
CMNFI 1993-0126, 1, ? mm standard length, Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E);
CMNFI 1993-0127, 1, ? mm standard length, Kermanshahan, Sarab-e Maran (34º44'N, 46º51'E);
CMNFI 2007-0060, 2, ? mm standard length, Fars, Chashmeh Ab-e Shirin near Lar (ca. 27º41'N, ca. 54º17'E);
CMNFI 2007-0111, 1, ? mm standard length, Kermanshahan, Alvand River near Sar-e Pol-e Zahab (ca. 34º36'N, ca. 45º56'E);
ZSM 21861, 5, 172.0-217.2 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E).
Comparative material: CMNFI 1987-0017, 3, 97.3-143.9 mm standard length, ();
BM(NH) 1934.9.5:6, 1, 117.3 mm standard length, Kurdistan, Ain al Hamra, Shithatha ();
BM(NH) 1973.6.21:194, 1, 203.4 mm standard length, Iraq, Shatt al Arab ();
BM(NH) 1974.2.22:1338, 1, 134.9 mm standard length, Iraq, Najab Bazar ();
BM(NH) 1974.2.22:1346, 1, 108.7 mm standard length, Iraq, Tigris River near Faish Khabour ();
BM(NH) 1986.2.14:4-7, 4, 98.6-146.6 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E).
Genus Carassius
Nilsson, 1832
The goldfishes comprise 2-3 species found in Europe, northern Asia
and the Far East. Eschmeyer (1990) and Kottelat (1997) comment on the
authorship of Carassius. One species is now common in Iran.
These fishes are characterised by a stout and
compressed body, last unbranched dorsal and anal fin rays
finely serrated, long dorsal and short anal fin, mouth small and
terminal, lips thick and fleshy, no barbels, pharyngeal teeth in 1 row
and molariform but compressed, numerous gill rakers, and scales large.
Carassius auratus
(Linnaeus, 1758)
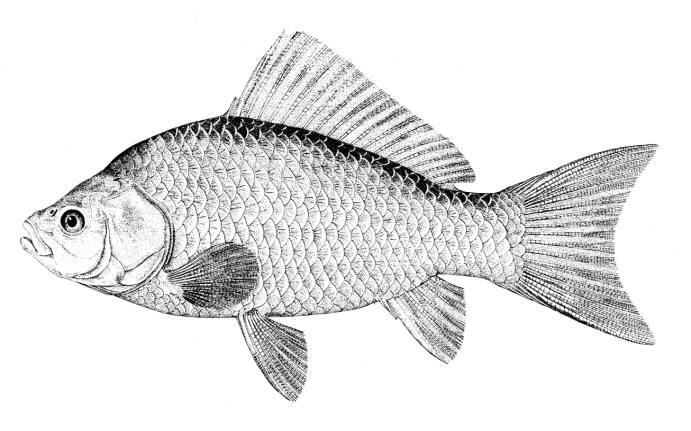


Common names
mahi-ye talaee or mahi-ye talai (= gold fish) or اوشين (ooshin or oushein) in
Khuzestan; kapur safid by anglers in Khuzestan at Ahvaz; kopur-cheh (= small carp) or
كاراس (= karas, karass or karaz) in Mazandaran; kopur cheky (= by the job carp?), kopur
chekeh (= drop carp?); ماهي حوض (= mahi-ye howz
or mahi-e-hoz, meaning pond or pool fish), mahi-ye howz-e
noqrehi (= silvery pond fish, for silvery form), mahi-ye howz-e talaee
(= golden pond fish for orange form).
[samak zahabi, buj-buj in Nasiriyah; samti; yayabash in Basrah; karseen in
Baghdad; carp thahabi, all in Iraqi Arabic; serebryanyi karas or silver crucian carp
in Russian; goldfish for auratus, Prussian carp for gibelio].
Systematics
Cyprinus auratus was originally described from China and Japanese rivers.
Pelz (1987) discusses the scientific name of the goldfish and its
confusion with Carassius carassius. All diploid goldfish of
western Europe are Carassius auratus auratus (from
introductions, presumably including releases and escapes in Iran) and
all triploid goldfish are C. auratus gibelio from eastern
areas. Goldfish do not appear to be native to Iran but Iranian
specimens are sometimes referred to Carassius auratus gibelio
(Bloch, 1782) known as the Prussian carp, European goldfish or silver
crucian carp. Berg (1948-1949) considers the familiar pet
"goldfish" to be a domesticated form of the Prussian carp.
However these fish probably have a number of origins - from aquarium
stock and from China. Kottelat (1997) tentatively recognises Carassius
gibelio (Bloch, 1782) as a species native to eastern Central
Europe, and Kottelat and Freyhof (2007) map gibelio as the introduced
species in the Caspian Sea basin of Iran. Vasil'eva and Vasil'ev (2000) state that fish named in the
literature as Carassius auratus gibelio from Europe, Siberia
and eastern Asia are triploids and are not a valid subspecies of C.
auratus s.s. They consider C. gibelio to be a distinct
species as long as it has a unique and ancient origin rather than
arising de novo, and as long as the type specimens are
triploids. Szczerbowski in Bănărescu
and Paepke (2002) recognises C. a. auratus and C. a. gibelio.
Additionally C. auratus may be a tetraploid derivative of Carassius
carassius. The native distribution of C. carassius is in Europe and
western Asia, reaching northern drainages of the Caspian Sea in the southern
limits of its distribution (Libosvárský, 1962). It differs from C.
auratus in having a slightly convex margin to the dorsal fin (straight or
slightly concave in C. auratus), caudal fin slightly emarginate
(deeply emarginate), usually 6 branched anal rays (always 5), 23-33 gill rakers
(37-53), 31-34 vertebrae, usually 32-33 (28-31, usually 29-30), 28-29 fin
denticles posteriorly on the dorsal fin spine (10-11), peritoneum light (dark),
black spot at the caudal fin base in young and some adults (absent), and a
coppery gold body (silvery, pinkish gold, gold or red) (Szczerbowski in
Bănărescu and Paepke, 2002). Berg (1948-1949) also cites the characters body
rounded, back thick (body angular, back compressed) and scales weakly sculptured
(rough), although his comparison is with C. a. gibelio.
Goldfish commonly hybridise with Cyprinus carpio to further confuse
the identity of these fishes (L. Nico, http://nas.er.usgs.gov/fishes/accounts/cyprinid/ca_aurat.html,
downloaded 24 May 2000). The identity of "goldfish" in Iran has not been
thoroughly surveyed and, along with conflicting views on species and widespread
introductions from many sources, make it simpler to refer to this taxon as C.
auratus for now.
Al-Mukhtar and Al-Hassan (1999) describe a hybrid of this species
and Barbus (= Mesopotamichthys) sharpeyi from Al-Hayei (= Al Ha'i), a seasonal lake
between the Karkheh and Dez rivers in Khuzestan.
Key characters
The combination of spines in both the dorsal and anal fins and the absence of
barbels is unique to this species. Szczerbowski in Bănărescu and Paepke (2002)
distinguishes the subspecies auratus from gibelio by 21-36 lateral line scales (27-35 in gibelio)
and a pink or gold colour (yellowish silver), not very diagnostic. Ilhan et
al. (2005) give gill raker numbers of 34-40 for auratus, 42-56 for
gibelio and 25-32 for C. carassius in Turkish waters (however note
below that counts can increase with growth and see also under C. carassius
for somewhat different counts and other distinguishing characters).
Morphology
Dorsal fin with 3-4 unbranched rays followed by 12-20 branched
rays, anal fin with 2-4, usually 3, unbranched rays followed by 5-6,
usually 5, branched rays, pectoral fin branched rays 11-18, and pelvic
fin branched rays 6-9, usually 8. Dorsal and anal fin spine denticles
coarse and few (about 10-15).
Lateral line scales 21-36. The anterior scale margin is wavy and
there are very few anterior and posterior radii, as few as 3-4. The
focus is slightly subcentral posterior. Circuli on the exposed part of
the scale are more coarse and widely spaced than on the concealed part
of the scale. Gill rakers long with serrated interior margins,
reaching the fifth to eighth raker below when appressed with younger
fish having longer rakers proportionately. Counts are size dependent
in the range 34-54. Total vertebrae 25-34. Pharyngeal teeth 4-4, with
very elongate, narrow, flattened and horizontal cusps arising from a
much narrower stem. The gut is coiled with several loops. This species is
variously reported as only diploid or as a tetraploid (2n=100-104); see above.
There are elongate specimens (morpha humilis, where fish density is high) and
deep-bodied specimens (morpha vovki, where fish density is low) but these names have no taxonomic significance.
Meristic values for Iranian specimens are:- dorsal fin branched rays 16(4),
17(3), 18(3), 19(5) or 20(2); anal fin branched rays 5(17); pectoral
fin branched rays 11(1), 14(1), 15(4), 16(10) or 17(1); pelvic fin
branched rays 7(2) or 8(15); lateral line scales 28(6), 29(9) or
30(2); pharyngeal teeth 4-4(10); and total vertebrae 32(2).
Sexual dimorphism
Breeding males have small nuptial tubercles on the operculum, back and pectoral fin rays.
Colour
The golden or orange colour of artificially bred aquarium goldfish
is distinctive. However populations in the wild, if they breed
successfully, gradually revert to a wild-type of colour, without the appropriate
diet supplement of aquarium fish and, as golden fish,
are readily seen and eaten by birds and other fishes. Yanar and Tekelioğlu (1999) found that
pigmentation increased with fish weight when specimens were fed the carotenoid
zeaxanthin. Wild-type colour is an overall olive-green fading to a white belly. Flanks can be silvery to
almost black. Fins are a dark olive-bronze, the membranes in
particular being heavily pigmented. Young goldfish are usually green,
brown or bronze to almost black and only after about 1 year do they take on the
colour of adult auratus or gibelio. Peritoneum dusky to black.
Young fish at Ahvaz, Khuzestan, however, are a bright silvery overall (more so than Cyprinus carpio of
similar size), the back is grey, the caudal fin is grey on the
proximal half and hyaline distally, and the anal fin rays are white (and thus
partly resemble gibelio).
Prussian carp (subspecies gibelio) is a dark steel colour with dark
blue or greenish dorsally, silver-grey laterally and white ventrally, dorsal and
caudal fins are dark grey and the paired fins and anal fin are light pinkish (Szczerbowski
in Bănărescu and Paepke (2002).
Size
Attains 62.0 cm and about 5.0 kg, the subspecies gibelio being
smaller, up to 45.0 cm and 1.24 kg.
Distribution
The native distribution is in northern Asia and China, reaching
northern drainages of the Caspian Sea in the western limits of its
distribution (Libosvárský, 1962; Plez, 1987). The goldfish has been widely introduced to
garden ponds and released from aquaria in temperate to warm waters world-wide.
In Iran it has been introduced throughout the Caspian Sea basin where it is
reported from the Atrak, Gorgan, Gharasu, Tajan, Babol, Haraz, Sardab, Aras
(including the middle Aras and lower reaches of its tributary the Qareh Chai),
Tonekabon, Pol-e Rud, and Safid rivers, the
Anzali Mordab where it is now the most abundant fish, Gorgan Bay and Alma- and
Ala-Gol (Holčík and Oláh, 1992;
Shamsi et al., 1997; Roshan Tabari, 1997; Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli, 2000; Gasmi and Mirzaei, 2004; Patimar,
2008; Abdoli and Naderi, 2009); the lower Talkheh and
lower Zarrineh rivers in the Orumiyeh basin (Abdoli, 2000); the lower Shur, lower Qareh Chai
and the Latian Reservoir in the Namak Lake basin (Armantrout, 1980; Hosseini,
1987; Abdoli, 2000); the Hamun Kushk and the Sistan Dam as well as throughout the hamuns in
the Sistan basin (Ahmadi and Wossughi, 1988; Mansoori, 1994; J. Holčík,
in litt., 1996; field work in the 1970s); Kerman-Na'in and Dasht-e Lut basins
generally (Abdoli, 2000); lower Kashaf River in the Tedzhen basin (Abdoli,
2000); throughout Khuzestan where now common (N. Najafpour
and M. Al-Mukhtar, pers. comm., 1995; field work 2000, absent in 1970s); middle and lower Hilleh and lower
Mand rivers in the Gulf basin; middle Halil and middle to lower Bampur River (Abdoli,
2000); Dalaki and Shapour rivers (Pazira et al., 2005),
Safid River, Zayandeh River, Zarivar Lake
and the Hamun Lake (Shamsi et al., 2009), and found in garden and park ponds throughout Iran. Some
introductions are probably discarded aquarium fish as goldfish are
sold as pets and for the Now Ruz (= New Year) festivities. They may
also have been introduced accidentally with the commercially important Chinese carps.
This species is also recorded from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov,
1995) and may eventually reach Iranian waters from this source in the Tedzhen (= Hari) River basin.
The Prussian carp (subspecies gibelio) is less widely distributed and its presence and distribution in Iran are not known.
Zoogeography
This species has been introduced to Iran by man. Some are
undoubtedly aquaculture pond escapees or aquarium releases. Goldfish
are kept in aquaria as part of the Now Ruz (New Year) celebrations in
March each year. Tehran television (and the Green Front of Iran, see below) urged people to
release them into local waters rather than killing them after the New
Year (J. Valiallahi, pers. comm., 2000).
Habitat
Goldfish are hardy and can live in winterkill water bodies with
much aquatic vegetation, low oxygen, and high pollution (Gudkov, 1985).
They can also survive several hours out of water (Pelz, 1987) and may bury
themselves in mud, albeit temporarily when scared (Szczerbowski
in Bănărescu and Paepke, 2002). Goldfish appear to favour ponds or pools in streams with aquatic
vegetation but are often introduced into small bodies of water as
ornamental fish. They are tolerant of turbidity, e.g. clay at 225,000 mg/l, pH
from 4.5 to 10.5, very high temperatures (upper lethal limit 41.4°C),
and high salinity (17‰). This species was killed under experimental conditions, when gradually acclimated to increasing
salinity at 28,200 μmho and, by sudden exposure, at 19,200 μmho (Jassim, 1988). This is a greater tolerance than that
shown by Cyprinus carpio, another exotic introduced to Iran. However, Carassius auratus appeared in the Basrah fish market when an
increase in the Tigris River discharge reduced the salinity of the
Shatt al Arab (N. A. Hussain, in litt., 1994).
In Iran it is one of two most abundant species in Caspian wetland
areas along with Gambusia holbrooki (Iranian Fisheries
Research and Training Organization Newsletter, 19:4, 1998).
Age and growth
Maturity is attained at 3-4 years in the Volga Delta for goldfish. Life span is
13 years with most growth in the first 2-4 years to a size of 15-20 cm
(Gudkov, 1985; Kizina, 1986). Life span in captivity in China may
exceed 50 years. Population numbers in confined areas are limited by a
chemical released by the goldfish which represses more spawning. Prussian carp live up to 11 years.
In the Anzali Mordab, Holčík and Oláh (1992) found only 6 age groups (as did Bagirova et al.
(1990) in reservoirs of Azerbaijan while Pipoyan and Rukhkyan (1998)
found 9 age groups in Armenia) with the largest fish 32 cm standard
length owing to intense fishing pressure. Growth in mm increments was
successively 93, 47, 50, 42, 28, and 37. The population is entirely
female (see below). Individual life span is greater in Armenia where
males are scarce or absent than in bisexual populations (Pipoyan and
Rukhkyan, 1998). Sayad Borani et al. (2001) studied this species (as C. auratus gibelio)
in the Anzali Mordab at four localities and found a mean fork length of 19.5 cm
(range 2.5-31.5 cm) and a mean weight of 196.8 g. The mean age was 2.6 years.
The mean length, weight and age were higher in the Sia-Keshim area of the
lagoon. The exploitation rate was 0.47, L∞ was 36.0 cm and K was 0.23 per year.
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 41 Iranian fish measuring 5.65-8.17 cm standard length. The a-value
was 0.0419 and the b-value 2.911 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Patimar (2009) examined fish from the Alma-Gola nd Ala-Gol wetlands in Golestan
from 200 to 2002. Ages ranged from 0+ to 8+ with negative allometric growth in
Alma-Gol, and positive allometric growth in Ala-Gol. The von Bertalanffy growth
curves for mean total lengths were Ltmales = 183.33(1-e-0.31(t+1.05))
and Ltfemales = 245.66(1-e-0.19(t+1.21)) for Alma-Gol and
Ltmales = 224.79(1-e-0.24(t+0.83)) and Ltfemales
= 242.80(1-e-0.23(t+0.80)) for Ala-Gol. The sex ratio was unbalanced
for males:females at 1:10 and 1:12.7 for Alma-Gol and Ala-Gol respectively
because of gynogenesis.
Fish in Buldan Dam Lake, Gediz River basin,
Turkey referred to C. gibelio had a maximum age of 6 years and attained
25.5 cm and 269.1 g (Sarı et al., 2008).
von Bertalanffy growth parameters were L∞
= 31.66 cm, W∞ = 635.91 g, k = 0.146 year-1 and t0
= -2.166 year. Ratios of total, natural and fishing mortality were calculated as
0.632 year-1, 0.456 year-1 and 0.176 year-1.
Food
Food is predominately zooplankton but also includes aquatic
insects, crustaceans, molluscs, worms, detritus, filamentous algae,
macrophytes and young fish, switching from one kind of food to another
as circumstances warrant. Goldfish have a palatal organ on the roof of
the mouth used to taste and touch food and their dense gill rakers aids in
feeding on smaller food items. In the recovering Hawr al Hammar, Iraq, diet is 46.1% algae and 25.5% diatoms, with amounts of plants,
crustaceans, insects, snails and fish being less than 10% each, in the Hawr al Hawizah
36.3% algae, 21.3% diatoms and 17.5% copepods, with amounts of plants, cladocerans, ostracods and insects being less than 10% each, in the
Al Kaba'ish (= Chabaish) Marsh 45.5% algae, 25.2% diatoms, with plants, various crustaceans, insects and
snails at less than 10% each (Hussain et al., 2006).
Reproduction
The fish in the Anzali Mordab are all female, reproducing through gynogenesis.
Sayad Borani et al. (2001) found fish in Anzali Mordab to have a sex
ratio of 99.3 females:0.7 males. Egg development is stimulated by sperm probably from Cyprinus
carpio, Tinca tinca, Blicca bjoerkna or Scardinius
erythrophthalmus. Here fish may mature at 1 year of age, and
coupled with polycyclic ripening of eggs and intermittent spawning,
this has led to the dominance of this species in the fresh waters of
the lagoon (Holčík and Oláh, 1992). In Armenia, maturity appears to be linked with
average annual temperature - at 12.0-13.1°C it occurs at the end of the first year of life while at 8.4-9.0°C
it occurs at the end of third and fourth years (Pipoyan and Rukhkyan, 1998).
Turkish populations in Topçam Dam Lake, Aydın (Şaşı,
2008) and Buldan Dam Lake, Gediz River basin (Sarı et al., 2008) referred
to C. gibelio were 98.84% and 99.44% female. Spawning in the former
locality was from March to August, suggesting multiple spawnings with mean
fecundity ranging from 37,823 in August to 85,159 in March. Egg diameter reached
1.099 mm in June.
Patimar (2009) examined fish from
the Alma-Gola nd Ala-Gol wetlands and found reproduction in February, March and
April. Absolute fecundity reached 13,020 eggs.
Spawning begins in late April to mid-May in the Volga Delta and occurs in
May-June in the Anzali Mordab (Sayad Borani et al., 2001). Eggs
are laid in 2-5 batches over a spawning period extending into July. Up
to 10 batches are laid elsewhere at 8-10 day intervals with up to 4000
greenish-yellow eggs in each batch. Fecundity reaches 253,200 eggs (elsewhere to
685,700 with absolute fecundity reaching 860,000 eggs). The largest eggs are 1.6 mm in diameter (Gudkov, 1985;
Kizina, 1986; Szczerbowski in Bănărescu and Paepke, 2002). Each female is accompanied by 2 or more males and
chases are reported with splashing and shooting through the water near the
surface. The eggs are adhesive and attach to water plants and hatch in 5-8 days.
Parasites and predators
Mokhayer (1976b) records infectious dropsy and swimbladder
inflammation in Iranian goldfish. Saprolegniosis has been reported
from goldfish in Iran (Rahbari and Razavilar, 1982). Growths of the
fungus Saprolegnia parasitica resembled tufts of cotton wool.
Mokhayer (1989) reports metacercariae of the eye fluke, Diplostomum
spathaceum from this species in Iran, which can cause complete
blindness and death in commercially important species. Jalali and
Molnár (1990a) record the monogeneans Dactylogyrus baueri, D.
extensus, D. formosus and D. vastator from this
species in the Safid Rud. Jalali and Molnár (1990b) report a variety
of monogeneans from this species variously in fish farms throughout
Iran, namely Dactylogyrus baueri, D. dulkeiti, D.
formosus, D. vastator and D. vastator forma minor.
Molnár and Jalali (1992) record the monogenean Dactylogyrus
intermedius from this species in a petfish farm near Tehran.
Gussev et al. (1993a) describe a new species of monogenean from
goldfish on a fish farm near Tehran, Dactylogyrus intermedioides.
Shamsi et al. (1997) report Clinostomum complanatum, a
parasite causing laryngo-pharyngitis in humans, from this species. The
helminth Anisakis sp. is recorded from the guts of this species
in the Anzali wetland (Ataee and Eslami, 1999; www.mondialvet99.com,
downloaded 31 May 2000). Mousavi (2003) records the monogeneans Gyrodactylus
sp., G. kabayashi, D. extensus, D. baueri,
Trichodina sp., the ciliates Ichthyophthirius multifilis and Ichthyoboda
sp. and the copepods Lernaea cyprinacea and Argulus foliaceus from
this species in ornamental fish in Iran. Aquarium specimens are often released in the wild at New Year (Now Ruz).
Naem et al. (2002) found the
following parasites on the gills of this species from the western branch of the
Safid River, namely the protozoans Ichthyophthirius multifilis and a
Trichodina species, monogenean trematodes Dactylogyrus anchoratum,
and Gyrodactylus sp.. Jalali et al. (2002) and Jalali and Barzegar (2006) record Diplostomum spathaceum from this species in Lake Zarivar.
Naem (2002) records the monogenean Dactylogyrus
anchoratus from fish in Safid River. Mehdipoor et al. (2004)
record the monogenean Dactylogyrus baueri in this fish in the Zayandeh
River. Masoumian et al. (2005) recorded the protozoan parasite
Ichthyophthirius multifilis from this species in the Aras Dam in West
Azarbayjan (species identified as C. carassius, presumably goldfish).
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. kobayashii and G. sp. in fish from the Safid River.
Khara et al. (2006a) record the eye fluke Diplostomum spathaceum for this fish in the Amirkalayeh Wetland in Gilan.
Sattari et al. (2004, 2005) surveyed this species (as C. carassius) in the Anzali wetland, recording
Raphidascaris acus (and larvae) Eustrongyloides excisus and Camallanus lacustris.
Pazooki et al. (2007) recorded various parasites from localities in West
Azarbayjan Province, and found Eustrongylides excisus.
Sattari et al. (2007) record the nematode Raphidascaris acus, the
digenean Diplostomum spathaceum and the monogeneans Dactylogyrus
extensus,and Gyrodactylus sp. in this species in the Anzali wetland
of the Caspian shore.
Barzegar et al.
(2008) record the digenean eye parasites Diplostomum spathaceum and
Tylodelphys clavata from this fish. Khara et al. (2008) found the eye parasite
Diplostomum spathaceum in this fish from Boojagh Kiashar Wetland in Gilan. Shamsi et
al. (2009) found Dactylogyrus baueri, D. dulikeity, D.
extensus, D. intermedius, D. intermedioides and D. wegeneri in
this species from localities such as fish farms, the Safid River, Zayandeh
River, Zarivar Lake and the Hamun Lake.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Economic importance
This species is raised on Tehran fish farms for the pet trade (Molnár
and Jalali, 1992). It forms part of the Now Ruz (= New Year, usually 21 March)
celebrations in Iran where a bowl with goldfish forms part of the traditional
Haft Sin table setting (so called for seven items that must be present, all
beginning with the letter "S", each having a symbolic meaning, the goldfish is
in addition to these). The goldfish in a bowl represents life within life, and
the sign of Pisces which the sun is leaving.


Haft Sin table, Iranian Embassy, Ottawa, 2009
Ebrahimzadeh Mousavi and Khosravi (2004) report suspected
epizootic ulcerative syndrome from ulcerated goldfish.
In the Anzali Mordab, 62% of the total catch is
goldfish, an accidental introduction (Petr, 1987). The catch in the
mordab in 1990 was 46,472 kg (Holčík and Oláh, 1992). As the salinity of this lagoon increases, the
density of goldfish will decrease. Valeipour and Haghighy (2000)
record the catch for 1992-1996 at 40% of the species taken. Safaei (2005) gives
a goldfish catch figure of 45% of the 313 ton fishery there in 1992. The presence of goldfish in the Anzali Mordab led to a
decline in the native fishery there.
This species is caught by anglers at Ahvaz in Khuzestan using bread or potato as bait.
It is known to control mosquito larvae in Bengal (Chandra et al., 2008).
The peculiar type of reproduction is very successful and affects
the catches of other cyprinid species, being equivalent to a predatory
effect (Holčík and Oláh, 1992).
There is some evidence that this fish disturbs the habitat of
native species, muddying waters, and it may compete for food and
space. Goldfish have destroyed some amphibian populations in other
parts of the world by consuming frog eggs (Coad and Abdoli, 1993b).
The Green Front of Iran recommended the release into pools of mosques,
parks or natural lakes of the estimated 20 million goldfish kept in
aquaria for the Iranian New Year celebrations in March each year. This
would have a deleterious effect on habitats not yet colonised by this
exotic species. A news report in 2005 cites the death of 5 million fish in
transit from the store to the Iranian home at New Year, indicating perhaps that the
numbers that do make it are much higher (www.politicalgateway.com, downloaded 5 August 2005).
Newspaper articles suggested that goldfish should only be released into "pools"
rather than rivers because of all the attendant dangers of this exotic. They are
known to prevent reproduction of native species in Sistan (Iran Daily, 17 March 2005, p. 5).
This species is used in Iran as an experimental organism
and for studies in reproductive biochemistry, e.g. in studying
the effects of anionic detergents (shampoos, a common water pollutant) on blood
parameters, on hepatic and renal pathology and serum biochemical
parameters (Shahsavani et al., 2003; Shahsavani et al., 2004; Shahsavani et al., 2005; Shahsavani and Movassaghi, 2003); the use of phenytoin sodium on skin wounds
(5mg/l showed best healing improvement while zinc oxide was not as effective) (Shahsavani et al.,
2001, 2002, 2002); the formation of lesions and clinical changes in fish exposed
to kerosene (Shahsavani et al., 2003); the
effects of cortisol on testicular apoptosis (Bahmani et al., 2007); the
adverse effects of phenytoin sodium, a drug used for healing skin lesions, on
the gills, liver and kidney (Shahsavani et al., 2007); hormonal GnRHa and
pituitary extract proved more effective on spermatological parameters than the
hormone HCG (Zadmajid et al., 2008); the
effects of seminal plasma indices on sperm motility (Zadmajid and Imanpour,
2009); the effects of hormones on seminal plasma biochemistry (Zadmajid et al.,
2009).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks, in aquaria and
in aquaculture, as bait, as an experimental species and because it has
been introduced outside its natural range. There are numerous, commercial
aquarium forms with particular morphologies and colours that are assigned common
names, e.g. common, veiltail, comet, fans, calicoe, black-moor, telescope-veiltail, lionhead, egg-fish, shubunkin.
Balon (2006) reviews the origin of the species.
Conservation
This species is a successful exotic, in no need of conservation.
Further work
The Carassius species in Iran is generally regarded as C. auratus,
the goldfish of aquaria, as it is used extensively in Now Ruz (New Year)
celebrations and often released into natural waters. This needs confirmation for all major populations.
Sources
Iranian material: CMNFI 1979-0230, 41, 14.7-38.6 mm standard length, Sistan,
Hamun-e Puzak (ca. 31º15'N, ca. 61º42'E); CMNFI 1991-0162, 1, 40.5 mm standard length, Mazandaran, Bagher Tangeh (36º42'N,
52º43'E); CMNFI 1993-0136, 64.0 mm standard length, uncatalogued material, 1, 93.5 mm standard length, Gilan, near
Hendeh Khaleh (ca. 37º23'N, ca. 49º28'E); 1, 52.8 mm standard length, Gilan, near Hendeh Khaleh (ca. 37º23'N, ca. 49º28'E); 4, 16.4-50.3 mm
standard length, Gilan, near Khoshk Bijar (ca. 37º22'N, ca. 49º47'E).
Carassius carassius
(Linnaeus, 1758)

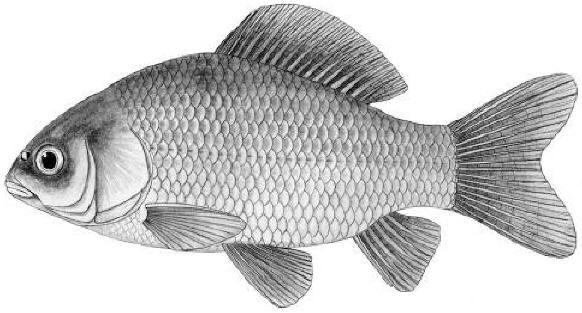


The crucian carp has been reported as introduced to Iran in the Karun
River basin as aquarium releases by Armantrout (1980) without further
details and there are other reports such as in the Gorgan River (Y.
Keivany, in litt., 1992) and Mahabad Dam (Abdi, 1999;
www.mondialvet99.com, downloaded 31 May 2000) but these may be
confusion with Carassius auratus. Specimens are needed to
confirm the presence of this species in Iran. The native distribution
is in Europe and western Asia, reaching northern drainages of the
Caspian Sea in the southern limits of its distribution (Libosvárský, 1962). It
differs from C. auratus in having a slightly convex margin to the
dorsal fin (straight or slightly concave in C. auratus), caudal
fin slightly emarginate (deeply emarginate), usually 6 branched anal rays
(always 5), 23-33 gill rakers (37-53), 31-34 vertebrae, usually 32-33 (28-31,
usually 29-30), 28-29 fin denticles posteriorly on the dorsal fin spine (10-11),
peritoneum light (dark), black spot at the caudal fin base in young and some
adults (absent), and a coppery gold body (silvery, pinkish gold, gold or red) (Szczerbowski
in Bănărescu and Paepke, 2002). Berg (1948-1949) also cites the
characters body rounded, back thick (body angular, back compressed) and scales
weakly sculptured (rough), although his comparison is with C. a. gibelio,
itself recognised as a distinct Carassius gibelio (Bloch, 1782), also of
uncertain occurrence in Iran.

Carassius gibelio from Wikimedia
Commons.
Genus Chondrostoma
Agassiz, 1832
The nases are found from the Iberian Peninsula and France to the
Caspian and Tigris-Euphrates basins. There are about 26 species of
which 2 are known for Iran (Elvira, 1997). Chondrochylus Heckel,
1843 and Chondrochilus Heckel, 1843 are synonyms. Eschmeyer
(1990) gives the year of publication for the genus as 1832 as opposed
to other authors who give 1835 (e.g. Berg, 1948-1949; Reshetnikov et al., 1997).
Doadrio and Carmona (2004) confirm the monophyly of the genus based on the
cytochrome b gene with vicariant events accounting for distribution of
taxa better than a dispersalist model. Middle East taxa belong to a single
lineage with the more differentiated and basal species in the Caucasus and
Mesopotamia, having been isolated in the Upper Miocene-Pliocene.
This genus is characterised by being of moderate size, with a
somewhat compressed body, scales of moderate to small size (44-106 in
the lateral line (Robalo et al. (2007) give a range of 52-78 for their
more restricted genus)), scales squarish with radii in the anterior and
posterior fields and a subcentral anterior focus, no barbels, an
inferior and transverse or crescentic mouth with a cutting edge to the
lower jaw, thin upper lip and no lower lip, pharyngeal teeth knife-like and in 1 row
with a high count (5, 6 or 7, the same number on each arch or one more
on the left), gill rakers short and moderately numerous
(up to 40), short dorsal fin without a thickened ray opposite the
pelvic fins, 7-10 dorsal fin branched rays, a moderately elongate anal
fin with 8-12 branched rays, deeply forked caudal fin and usually
concave dorsal and anal fins, a pelvic axillary process always
present, 42-49 vertebrae, a black peritoneum, and a long, coiled gut.
Elvira (1997) and Robalo et al. (2007) give osteological characters.
Bogutskaya (1997a) places the nominal Iranian species, C. regium
and C. orientalis, in a group characterised by a straight or
only slightly arched mouth cleft, high vertebral counts (total
vertebrae modes 45-47 and abdominal modes 26-28) and often or commonly
4 unbranched rays in the dorsal fin.
Chondrostoma cyri
Kessler, 1877
Common names
shekamsiah-e Aras.
[Kur altagizi in Azerbaijan; chernobryushka or blackbelly, Kurinskii podust or Kura nase, uzkotelii
Kurinskii podust, all in Russian; Kura undermouth, Kura nase].
Systematics
Earlier works by Elvira (1986; 1988; 1991) placed this species as a
subspecies of C. oxyrhynchum but in Elvira (1997), using the
phylogenetic species concept and following the studies of Smirnov
(1992), this taxon is recognised as a species. C. oxyrhynchum
is then found in more northerly rivers of the western Caspian Sea
basin remote from Iranian waters.
C. cyri orientalis (Bianco and Banarescu, 1982 is described from Fars
(see below under C. regium).
Chondrostoma cyri Kessler, 1877 was described from the Kura
River, Tiflis (= Tbilisi), Georgia and Chondrostoma oxyrhynchum
from the Kuma River near Georgiyevsk, Russia in the Caspian Sea basin.
Alburnus alasanicus Kamenskii, 1901 described in part from
the Alasan, Alazan' or Alazani River, a left bank Kura River tributary
in Georgia, Chondrostoma schmidti, Berg, 1910 from the Alazan'
River at Naporiri, and Chondrostoma leptosoma Berg, 1914 from
the Kars-tchai, a tributary of the Aras River in Turkey, the Aras by
Kopri-kei, near Erzurum, Turkey, and the lower Aras at Karadonly and
Dzhulfa in the former U.S.S.R., are synonyms. Subspecies are not
recognised (Elvira, 1991; 1997). C. leptosoma was founded on an
elongate form from the Karasu in the Aras River basin.
Two syntypes of Chondrostoma cyri are also in ZISP (10919)
from "Tiflis" collected by Kessler in September 1875. A
syntype of Chondrostoma oxyrhynchum is in the Zoological
Institute, St. Petersburg (ZISP 2881) from "Fl. Sunsha"
collected in 1830 by Ménétries. According to Elvira (1988), the type
locality is Kuma R. at Georgijewsk and 2 syntypes are under ZISP
10922. Another syntype of Chondrostoma oxyrhynchum is in the
Natural History Museum, London (BM(NH) 1897.7.5:28 (184.8 mm standard length), formerly in ZISP,
as is other syntype of Chondrostoma cyri (BM(NH) 1897.7.5:27 (correctly
numbered 27, 78.4 mm
standard length),
formerly in ZISP)(Elvira, 1988; personal observations).
Five syntypes of Chondrostoma leptosoma are in the
Zoological Institute, St. Petersburg (ZISP 9098) according to (Elvira,
1988) but there are 15 syntypes under this number from the "Reka
Araks", 1888, Warpochowsky as well as additional material listed
as syntypes with numbers ZISP 9107 ("Fl. Araxes", 1888,
Warpochowsky, 12 fish), ZISP 5180 ("Kars-tschai", 1879, Dr.
A. Brandt, 3 fish), ZISP 9099 ("Reka Araks", 1888,
Warpochowsky, 4 fish), ZISP 15264 ("Reka Araks",
20.III.1911, 2 fish), and ZISP 15516 ("Reka Araks near settlement
Djulfa", 17.VI.1911, 13 fish).
Key characters
This species is the only one in its genus in northern Iran and can be recognised by generic characters.
Morphology
Kuru (1981) gives the following meristic characters for 103
specimens from the Aras and Kura river basins in Turkey:- 10-12 dorsal
fin rays, 10-11 anal fin rays, 9-10 pelvic fin rays, 9-15 pectoral fin
rays, 52-62 lateral line scales, 13-18 scales around the caudal
peduncle, 17-32 gill rakers, and 5-6 pharyngeal teeth on each arch
(note that the statistical treatment in this paper is in error and the
conclusion that species of Chondrostoma in Turkey are not
distinct is therefore incorrect). There is clinal variation in scale
numbers, the number increasing from south to north and Elvira (1988;
1991) gives the total range for characters of this species as dorsal
fin branched rays 7-9, usually 8, anal fin branched rays 8-10, usually
9-10, pectoral fin branched rays 13-18, usually 14-16, pelvic fin
branched rays 7-8, usually 8, lateral line scales 50-68 (to 73 in
Kazancheev (1981) and from 48 in Chikova (1967)), scales above the
lateral line 7-10, usually 8-10, scales below the lateral line 3-6,
usually 4-6, pharyngeal teeth 6-5 or 5-5, more rarely 6-6 and mode
6-5, and gill rakers 21-29. Vertebrae number 43-45.
The mouth is arched with a thin horny layer on the lower jaw.
Scales are rounded in overall shape with indentations above and below
a central, rounded protuberance on the anterior margin. The anterior
margin may be wavy. There are few anterior and posterior radii, few
circuli and a subcentral anterior focus. There is a pelvic axillary
scale. The gill rakers are short and reach the one below or just past
it when appressed. Pharyngeal teeth are compressed and thin but deep
with a long, thin and concave grinding surface. Teeth tips may be
slightly hooked. The gut has numerous anterior loops.
Thirteen specimens from Djulfa (presumably in Azerbaijan opposite the Iranian
town across the Aras River) have dorsal fin branched rays 8(12) or 9(1), anal fin branched rays 9(9) or 10(4), and
pharyngeal teeth 6-5(5) or 6-6(1).
Sexual dimorphism
Unknown.
Colour
The flanks are silvery but may have dark pigment spots which may,
or may not, form a stripe. Paired fins are orange to reddish and
median fins grey. The dorsal and caudal fins have dark margins. The
peritoneum is black.
Size
Reaches 80.0 cm and about 5.0 kg.
Distribution
Found in the rivers draining to the western coast of the Caspian
Sea from the Kuma River in the north southward to the Kura and Aras
river basins in the south. Recorded from the Aras River basin of
Iran (Abdoli, 2000).
Zoogeography
This genus has a European and Middle Eastern distribution. Its relationships
to other taxa are poorly known.
Habitat
Unknown.
Found principally in streams and rivers.
Age and growth
Fish are mature at 2 years of age and life span is at least 5 years.
Food
Diet is assumed to consist of bottom organisms including aquatic
insect larvae, detritus and vegetation scraped from the substrate.
Reproduction
Up to 16,217 eggs are produced and maximum diameter is 1.69 mm. The
spawning season is in the spring, peaking in April in the Kura River
basin (Abdurakhmanov, 1962).
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be conservation
dependent, in the south Caspian Sea basin according to IUCN criteria.
Criteria include sport fishing, possibly few in numbers, limited range
(less than 25% of water bodies), absent in other water bodies in Iran,
absent outside the Caspian Sea basin.
Further work
Biology in Iranian waters needs study.
Sources
Morphology based on Bianco and Banarescu (1982), Elvira (1986;
1988), Nelva et al. (1988).
Type material: See above, Chondrostoma cyri (BM(NH) 1897.7.5:25,
formerly in ZISP), Chondrostoma oxyrhynchum (BM(NH) 1897.7.5:28, formerly in ZISP),
and Chondrostoma leptosoma (ZISP 15516).
Iranian material: None.
Comparative material: CMNFI 1980-0812, 2, 101.9-107.9 mm standard length, Turkey, Kars, Selim Çayi (40º28'N, 42º47'E).
Chondrostoma orientale
Bianco and Banarescu, 1982
Chondrostoma
cyri orientalis Bianco and Banarescu, 1982 was originally described from the
"Pulwar River near Persepolis".
The holotype (IZA 8170, 93.7 mm standard length, examined by me) and 19 paratypes (IZA 7833,
51 specimens under this number, 35.4-90.1 mm standard length) of Chondrostoma
cyri orientalis are in the Istituto di Zoologia dell'Universitá
di L'Aquila, Italy (Elvira, 1988). Two paratypes of Chondrostoma
cyri orientalis are stored in the Field Museum of Natural History,
Chicago (FMNH 94519)(Ibarra and Stewart, 1987), 1 paratype is in the
Muséum national d'Histoire naturelle, Paris (1982-1014), 1 paratype
is in the United States National Museum, Washington (USNM 227934), 2
paratypes are in the Academy of Natural Sciences, Philadelphia (ANSP
150985), and 6 paratypes are in the Canadian Museum of Nature, Ottawa
(CMNFI 1982-0365, formerly IZA 7833, 37.8-88.7 mm standard length). The total number of paratypes is 75,
originally under IZA 7833 but some dispersed as noted above, with 10
further fish in the Institutul de Stiinte Biologice, Bucurešti,
Romania (ISBB) but uncatalogued (Bianco and Banarescu, 1982).
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea
cyprinacea on this species (as C. regium).
Barzegar and Jalali
(2006) report parasites in this species from Kaftar Lake as
Unio sp., Lernaea cyprinacea, Ichthyophthirius multifilis
and Diplostomum spathaceum.
Chondrostoma regium
(Heckel, 1843)
Common names
jokhorak, nazok, nazi; heif-e nan (= waste of bread, i.e. valueless)
in Khuzestan; سياه ديم (= siah deem in Behbehan);
سياه دم (= siah dom, meaning blacktail);
كپور پوزه دار (= kapur puzeh dar).
[baloot muluki, pangki; zurri (= the harmful one) at Mosul
(also used for Alburnus mossulensis, Aphanius spp., Gambusia and any small
fishes or large fishes when young); terris or terris achmar meleki (= royal red terris) at Aleppo
(= Haleb, Syria), all in Arabic; based on Heckel (1843b) for zurri and terris;
king nase].
Systematics
Chondrochilus regius Heckel, 1843 was described from the "Orontes"
(= Asi) (but see below) and "Tigris". Elvira (1988;
1991; 1997) considers Chondrostoma orientale to be a valid species while Nelva et al. (1988) retain it as a
subspecies of C. cyri. Bianco and Banarescu (1982) placed orientalis
in C. cyri on the basis of similar dorsal and anal fin ray
counts, scale counts and to a certain degree pharyngeal tooth formula.
Banarescu (1960) regarded C. regium as only a race of a
widespread species, C. nasus (Linnaeus, 1758). C. nasus
has larger scales on average and 6-6 pharyngeal teeth (Berg, 1949);
Heckel (1846-1849c) found 47 C. nasus from the Danube River had
6-6 teeth, 2 had 6-7 and 2 had 5-6 while in 13 C. regium the
count was 7-6 in 12 fish and 6-6 in 1 fish. Krupp (1985c) considers C.
regium to be distinct while recognising the small degree of
morphological variation between species in this genus. Data gathered
for Iran show a wide range in scale and teeth counts (see below).
Ladiges (1960) identified specimens from the same bodies of water in
Turkey as members of both species. The earlier literature on the
systematics of this genus remain confused (see Elvira (1988) for
comments on Ladiges (1966) and Kuru (1981)) and the morphology
summarised here for this species does not adequately resolve the
problem. There may well be significant variation of a clinal nature,
altitude and temperature may be important, and habitat types (lentic
or lotic) may affect body form. Most samples examined previously are
too small in numbers and differences due to size and sex could not be
adequately assessed.
Twelve syntypes of Chondrostoma regium are in the
Naturhistorisches Museum Wien (7 fish as NMW 52532-52535 from the
Quwayq (= Kueik) River near Aleppo and 5 fish as NMW 52536-52538 from
the Tigris River near Mosul)(Elvira, 1988). Krupp (1985c) gives
further details. All material was collected by Th. Kotschy in 1842
from the Quwayq and in 1843 from Mosul and the range in standard
length for the fish from the Quwayq is 102-166 mm and from Mosul
11.9-24.1 cm. The Vienna catalogue lists only 6 fish but the card
catalogue in 1997 lists NMW 52532 (2 fish), 52533 (2), 52534 (2),
52535 (1), 52536 (2), 52537 (1) and 52538 (2) as syntypes. The type
locality "Orontes" (= Asi) in Heckel (1843b) seems to be an error.
Key characters
This species is the only one in its genus in southern Iran and can be
recognised by generic characters.
Morphology
?re-work
The following counts are from literature sources; my counts in the table
below often show a wider range: lateral line scales 56-72 (47-55 for orientalis), scales
above the lateral line 9-13 (8-9 in orientalis), and scales
below the lateral line 5-6 (4-5 in orientalis). Lateral line
scale counts for Iranian fish are as follows: Tigris - 50(1), 51(4),
52(3), 53(12), 54(5), 55(7), 56(4), 57(4), 58(4), 59(8), 60(7), 61(6),
62(5), 63(3), 64(3), 65(1), 66(2), 67(1) or 69(2); Kor (= orientalis)
- 49(1), 50(2), 51(3), 52(8), 53(3), 54(7), 55(3) or 57(4). Despite a
lower range, the counts for the Kor River basin are matched by a sample from
Cheshmeh Javari near Ravansar, Kermanshahan (CMNFI 1979-0287) which have a range
of 50-58, leading to a supposition of altitudinal or habitat variation :-
Dorsal fin branched rays 8-11, mode 9 (note Bogutskaya (1997a)
gives a mode of 10) (7-9, mostly 8 for orientalis), anal fin
branched rays 9-12, mode 11 (note that Bogutskaya (1997a) gives modes
of 11 or 12) (9-10, mode 9 for orientalis), pectoral fin rays
14-18, mostly 15-17 (13-15, mostly 14 in orientalis) and pelvic
fin rays 6-9, mostly 8 (7-8, mostly 8 in orientalis). Gill
rakers 18-36 (probably some lower counts are of rakers on the lower arch
only and ranges in single studies, presumably to a consistent
technique, are 22-34, 24-31, 25-34 and 25-36) (22-28, 22-30 or 28-32
by different authors for orientalis). Counts for the whole arch
on Iranian fish give a wide range of 19-34, highly correlated with
size, larger fish having more (or more discernible) rakers than
smaller fish (r = 0.5049, p<0.001, n = 90).
Scale radii are few and restricted to the posterior field.
Total vertebrae 46-48. Pharyngeal teeth 6-5, 6-6, 6-7, 7-5, 7-6 and
7-7, mode 6-6 or 7-6 (6-5, 6-6, 5-6 and 7-5, mostly 6-6 for orientalis)
but see above. The mouth is straight (= transverse) with a thick horny
layer on the lower jaw.
Esmaeili et al. (2010) give a diploid chromosome number of 2n=52 with 21
pairs of submetacentric and 5 pairs of subtelocentric chromosomes from the
Fahlian River in Fars. The arm number was 58.Other Chondrostoma species
have 2n=50.
Meristics for southern Iranian specimens of
Chondrostoma:
|
Locality/Dorsal Fin Branched Rays |
7 |
8 |
9 |
10 |
x |
S.D. |
|
Tigris River Basin |
|
35 |
46 |
1 |
8.6 |
0.520 |
|
Kor River Basin |
1 |
30 |
|
|
8.0 |
0.180 |
|
Locality/Anal Fin Branched Rays |
8 |
9 |
10 |
11 |
12 |
x |
S.D. |
|
Tigris River Basin |
4 |
45 |
21 |
9 |
3 |
9.5 |
0.892 |
|
Kor River Basin |
|
23 |
8 |
|
|
9.3 |
0.445 |
|
Locality/Pelvic Fin Rays |
7 |
8 |
9 |
x |
S.D. |
|
Tigris River Basin |
3 |
77 |
2 |
8.0 |
0.248 |
|
Kor River Basin |
1 |
29 |
1 |
8.0 |
0.258 |
|
Locality/Lateral Line Scales |
Range |
x |
S.D. |
|
Tigris River Basin |
50-69 |
57.8 |
4.553 |
|
Kor River Basin |
49-57 |
53.2 |
2.131 |
|
Locality/Total Vertebrae |
42 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
x |
S.D. |
|
Tigris River Basin |
1 |
20 |
17 |
9 |
14 |
10 |
9 |
2 |
45.1 |
1.833 |
|
Kor River Basin |
5 |
20 |
5 |
1 |
|
|
|
|
43.1 |
0.680 |
Sexual dimorphism
Unknown.
Colour
The back is olive-brown with bluish tinges and the flanks and belly
are silvery-white. The dorsal and caudal fins are greyish and the
other fins hyaline. Some fish have bright orange fins, the pectorals
paler, the pelvics and anal fins fringed by white. The dorsal and
caudal fins have a black margin, wide on the caudal. These fin colours
give them a flag-like effect (Heckel, 1843b). The caudal fin can be
orange, distally black, with the extreme edge white in freshly dead fish.
Size
Attains 40 cm and 1 kg.
Distribution
Found in the Tigris-Euphrates basin and the
Mediterranean basins of southeastern Turkey and the northern Levant. In Iran
found in the Tigris River basin. Additional localities are springs (sarabs) near Kermanshah, the Marun
River, the Hawr al Azim marsh (Wossughi, 1978; Abdoli, 2000). Ghorbani Chafi
(2000) lists the Bazoft and Kuhrang rivers in the upper Karun River basin and
also possibly the Zayandeh River of the Esfahan basin.
Zoogeography
This genus has a European and Middle Eastern distribution. Its relationships
to other taxa are poorly known.
Habitat
Found in both rivers and lakes (and reservoirs) but habitat requirements have not been studied
in Iran. Ünlü (2006) reports that this species prefers stone grounds and still waters
in rivers and lakes in Turkey.
Age and growth
Khalaf et al. (1986) studied this species in the Diyala River, Iraq.
Maximum age group is 7+ years, males and females show no difference in weight at
the same length and samples from three adjacent areas show no major differences
in growth rates. Length-weight relationship was W = 0.0480 L2.49 (n = 255, r =
0.88). Males mature at 15.0 cm and females at 19.0 cm in the Diyala River at
Rustamiyah in Iraq (Allouse et al., 1986). A population at Al Kadhmia
north of Baghdad in the Tigris River had four age classes dominated by the three
year age class, with all fish being sexually mature during the second year. Fish
smaller than 15 cm for males and 17 cm for females were immature. The disparity
in age structure with the Diyala River population was attributed to pollution in
the Diyala (Daoud and Qasim, 1999).
Polat and Gümüş (1995) aged a population of this species in the Bafra
Altınkaya Dam lake in Turkey using vertebrae, otoliths, scales, opercle and
subopercle. Age reached 5, perhaps 6, years and scales were found to be the best
structure to use. Polat et al. (1999) found a similar age range in the
Suat Uğurlu Dam, Turkey with annulus (hyaline ring) formation in October to
February. Oymak (2000) examined growth characteristics of this species in the
Atatürk Dam on the Turkish Euphrates River. Eight age groups were found and
age-length and age-weight equations given for females and males were Lt
= 38.67[1-e-0.136126(t+3.073799)], Wt = 527.52[1-e-0.136126(t+3.073799)]3.1986
and Lt = 35.01[1-e-0.168137(t+2.754214)], Wt=
724.73[1-e-0.168137(t+2.754214)]3.2779 respectively. The
length-weight relationships were obtained as Log W = -5.4153 + 3.1986 Log FL in
females and Log W = -5.6212 + 3.2779 Log FL in males. The condition factor was
high in age group 7 and high in April and May, lowest in December and January.
Gümüş et al. (2002) found deposition of hyaline rings was synchronous
with decrease in food diversity in autumn in the Suat Uğurlu Dam, Turkey. Aydin
et al. (2004) demonstrated a positive linear relationship between otolith
length and fish length for this species in Keban Dam Lake, Turkey.
Food
This species is omnivorous taking insect larvae and eggs and fry of
other fishes. Gut contents also include diatoms and algae as well as
large quantities of sand. However, Gümüş et al. (2002) examined diet in the Suat
Uğurlu Dam, Turkey and found Navicula, Cymbella and Synedra were the most
frequently consumed organisms. This species feeds mostly on Bacillariophyta in
this dam but also Chlorophyta, Cyanophyta, Xanthophyta, Euglenophyta and
Rotifera. Diet varied with seasonable availability of food items.
Reproduction
Studies on the Diyala River population in Iraq found fish to be mature in
December and by January females lacked eggs. Each female produces up to 6900
eggs and number of eggs increases linearly with length (Allouse et al.
(1986). The breeding season at Al Kadhmia in the Tigris River near Baghdad was
March-May (Daoud and Qasim, 1999). Al-Rudainy (2008) gives sexual maturity as 3
years, 25 cm total length and 250 g weight with spawning in February and March
on gravel beds in shallow water with strong current. for Iraq. Ünlü (2006) reports up to 13,280 eggs for
fish in the Tigris River of Turkey. Beckman (1962) states that this species
probably spawns in May or June in Syria and Oymak (2000) found that condition
factors were highest in April and May in the Atatürk Dam, Turkey.
Parasites and predators
Barzegar et al. (2004) examined this species for parasites in fish
from the Beheshtabad river in Chahar Mahall va Bakhtiari Province and
found Lernaea cyprinacea, Dactylogyrus ergensi,
Ichthyophthirius multifilis and Myxobolus sp. Jalali et al.
(2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sp. from the Dez and Karun rivers in
Chondrostoma nasus, presumably this species.
Barzegar et al.
(2008) record the digenean eye parasites Diplostomum spathaceum and
Tylodelphys clavata from this fish. Riassy et al. (2009) found the digenean eye parasite Tylodelphys
clavata in fish from Choghakhor Lagoon.
Economic importance
This species has been caught and used for food in Khuzestan.
Conservation
This species is relatively common and is not widely used as food; it may not
need conservation.
However it is listed as endangered in Turkey (Fricke et al., 2007).
Further work
Its biology in Iran needs study and this may reveal conservation needs.
Sources
?re-work
Type material: See above, Chondrostoma cyri orientalis (IZA 8170, IZA 7833, CMNFI 1982-0365, formerly IZA 7833).
Iranian material: CMNFI 1979-0025, 16, 22.1-119.0 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0028, 14, 32.2-139.1 mm standard length, Fars, Kor River drainage (no other locality data);
CMNFI 1979-0059, 1, 72.2 mm standard length, Fars, Pulvar River (30º01'30"N, 52º57'E);
CMNFI 1979-0061, 14, 9.5-56.5 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0245, 5, 35.3-47.1 mm standard length, Sharestan-e Bahktiari va Chahar Mahall, stream in Ab-e Shalamzar drainage (32º08'N, 50º51'E);
CMNFI 1979-0247A, 4, 57.2-65.3 mm standard length, Sharestan-e Bakhtiari va Chahar Mahall (31º57'N, 51º01'E);
CMNFI 1979-0248, 2, 39.2-65.2 mm standard length, Sharestan-e Bakhtiari va Chahar Mahall, stream 3 km east of Boldaji (31º55'N, 51º05'E);
CMNFI 1979-0271, 11, 60.0-131.3 mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0272, 1, 58.5 mm standard length, Lorestan, river at Nokhor (ca. 33º40-47'N, ca. 48º28-45'E);
CMNFI 1979-0279, 2, 61.8-134.0 mm standard length, Lorestan, Khorramabad River (33º37'N, 48º18'E);
CMNFI 1979-0280, 1, 114.5 mm standard length, Lorestan, Kashkan River drainage (ca. 33º43-47'N, 48º12-15'E);
CMNFI 1979-0283, 1, 137.0 mm standard length, Kermanshahan, river 15 km before Kermanshah (34º21'N, 47º07'E);
CMNFI 1979-0287, 22, 56.6-112.5 mm standard length, Kermanshahan, Chashmeh Javari near Ravansar (ca. 34º42'N, ca. 46º40'E);
CMNFI 1979-0286, 11, 77.4-100.4 mm standard length, Kermanshahan, Ravansar River at Ravansar (34º43'N, 46º40'E);
CMNFI 1979-0289, 1, 131.5 mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0368, 4, 54.0-84.5 mm standard length, Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
CMNFI 1979-0370, 6, 187.3-221.6 mm standard length, Khuzestan, Karkheh River (32º12'N, 48º14'30"E);
CMNFI 1979-0382, 2, 37.7-62.5 mm standard length, Khuzestan, Karun River at Shushtar (32º03'N, 48º51'E);
CMNFI 1979-0392, 1, 53.7 mm standard length, Khuzestan, Zard River (ca. 31º32'N, ca. 49º48'E);
CMNFI 1979-0421, 5, 114.0-122.0 mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh, stream in Khersan River drainage (30º24'N, 51º47'E);
CMNFI 1979-0499, 1, 113.0 mm standard length, Fars, irrigation ditch 32 km from Kor River bridge (30º04'30"N, 52º36'E);
CMNFI 1979-0500, 7, 94.8-110.5 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 2007-0100, 2, 165.4-165.7 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshar (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0111, 2, 183.3-191.7 mm standard length, Kermanshahan, Alvand River near Sar-e Pol-e Zahab (ca. 34º36'N, ca. 45º56'E);
CMNFI 2007-0113, 2, 106.7-145.0 mm standard length, Kermanshahan, Razavar River, Qareh Su tributary (ca. 34º25'N, ca. 47º01'E);
CMNFI 2007-0115, 3, 72.5-96.5 mm standard length, Kermanshahan, Qareh Su basin north of Kermanshah (ca, 34º34'N, ca. 46º47'E).
Comparative material: BM(NH) 1931.8.12:1-3, 2, 136.0-172.2 mm standard length, Iraq, near Mosul (36º20'N, 43º08'E);
BM(NH) 1971.4.2:6, 1, 147.7 mm standard length, Iraq, River Tigris near Mosul (36º20'N, 43º08'E);
BM(NH) 1974.2.22:81-82, 1, 197.5 mm standard length, Iraq, Great Zab near Eski Kelek and near Bekhne Dam (no other locality data).
Genus Crossocheilus
Kuhl and van Hasselt, 1823
Kottelat (1987) retains the spelling Crossocheilus Kuhl and
van Hasselt in van Hasselt, 1823 as first reviser. The name was spelt Crostocheilus
early in the text but this has never been used again and Crossocheilus
appeared with the description. Crossochilus Günther, 1868 is
an incorrect emendation (Eschmeyer, 1990).
The genus is found chiefly in the Oriental Region but extends into
Iran with one species. There are about 18 species.
The genus is characterised by an elongate body with a rounded
belly; the mouth is inferior and transverse, the crenulated or fringed
upper lip being continuous with the snout (not separated by a groove);
the lower jaw has a horny covering and behind this are several rows
of lobate papillae; 1-2 pairs of barbels; gill membranes attached to
isthmus; dorsal and anal fins are short and spineless; the lateral
line is complete; scales are large to moderate in size; the intestine
is very long; and the peritoneum is black.
The lower surface of the head bears an "adhesive
apparatus", the mechanism of which has been investigated by Singh
(1993) for Crossocheilus latius latius, a subspecies not found
in Iran. The fringed upper lip acts as a food strainer as well as part
of the adhesive apparatus. Both this lip and the area behind the lower
lip are heavily tuberculate with glandular openings and irregularly
arranged hard ridges. Mucus from the glands in conjunction with the
ridges holds the fish to the substrate.
Crossocheilus latius
(Hamilton, 1822)
Common names
None.
[ispigoar or dogra in Pakistan].
Systematics
Cyprinus latius was described from the Tista River in
India/Bangladesh and types are unknown (Eschmeyer et al., 1996).
Bianco and Banarescu (1982) and Bănărescu (1986) consider Discognathus adiscus Annandale, 1919 described
from Sistan (type locality given below) to be a synonym of this
species which is represented in Sistan and Baluchestan by Crossocheilus
latius diplocheilus (Heckel, 1838), originally described from
Kashmir with syntypes in the Naturhistorisches Museum Wien under NMW
48820 (7 fish). Bănărescu (1986) cites 1 fish under NMW 48420 as possibly the holotype but this
specimen is dated 1839 which is anachronistic. Berg (1949) considers Discognathus
adiscus to be a distinct species. I concur with Bianco and
Banarescu (1982) and Bănărescu (1986).
Characters advanced by Berg (1949) for separating the two species
are number of barbels (4 in adiscus, 2 in latius diplocheilus
where mouth angle barbels are absent or rudimentary, not the rostral
ones as implied by Bianco and Banarescu (1982)), upper lip fringe
(barely developed in adiscus, distinctly developed in latius
diplocheilus), the posterior swimbladder (conical in adiscus,
elongate cylinder in latius diplocheilus), and papillae on the
lower lip and chin (rudimental in adiscus and latius diplocheilus
but the latter has almost free lateral edges and an attached posterior
end - this condition is not specified for adiscus). Bianco and
Banarescu (1982) and Bănărescu
(1986) found some latius diplocheilus specimens to have
4 barbels (and this is given too as a character of C. latius latius),
and no difference in development of lip papillae in specimens from the
Indus River basin (actually my reading of Berg (1949) cited above does
not indicate that papillae development differs but that the sucker
area has almost free lateral edges and an attached posterior end; this
occurs in Sistan fish but not in 4 fish from the
Hamun-e Mashkid and Makran
basins of Iran which have a fold in the flesh behind the tuberculate
area - these latter fish are very small however, 20.8-27.8 mm standard
length, and I lack extensive comparative adult material from outside
the Sistan basin in Iran and from neighbouring Pakistan to make an
adequate analysis of nominal adiscus and latius diplocheilus
in this and other characters). My observations of the posterior
swimbladder development indicate a great individual variation in form
for Sistan fish: the swimbladder may be conical, elongate and
tapering, rounded posteriorly, expanded posteriorly, rounded
posteriorly after a constriction, or even a narrow elongate cylinder
supposedly characteristic of latius. Fringe development of the
upper lip is also quite variable and seems to be relatively
well-developed in larger Sistan fish.
Karaman (1971) described a new genus, Hemigarra, for Tylognathus
elegans Günther, 1868 and Discognathus adiscus Annandale,
1919. He places Crossocheilus adiscus as the Sistan subspecies
of his Hemigarra elegans (= Hemigrammocapoeta elegans
here, q.v.) which is found in Mesopotamia. Karaman (1971)
distinguishes the two subspecies by the former having densely arranged
papillae on the chin as opposed to sparse papillae. Bianco and
Banarescu (1982) and Bănărescu
(1986) state that it is not related to Hemigrammocapoeta elegans
but is a typical Crossocheilus species.
The type locality of Discognathus adiscus is Sistan by
implication, as no locality is given for the holotype in Annandale
(1919b). Menon and Yazdani (1968) concur. Distribution is given as
"small watercourses and pools in the plains of Seistan" and
"Nasratabad, irrigation channel in Consulate garden; pool in the
desert 5 miles south of Nasratabad; pools in stream-bed 12 miles north
of Nasratabad; channels in the reed-beds of the Hamun-i-Helmand near
Lab-i-Baring, and channel leading out of the Hamun 12 miles east of
Lab-i-Baring; small watercourse, Lutak, southern Seistan", and
one of these is presumably the type locality.
Twenty syntypes of Discognathus adiscus are in the
Zoological Survey of India, Calcutta (ZSI F9758/1) (Menon and Yazdani,
1968). Annandale (1919b) cites ZSI 9763/1 as the holotype catalogue
number. Three syntypes are in the Zoological Institute, St. Petersburg
(ZISP 25411) from "Nasratabad, Seistan, Indian Museum, Dr. Hora"
and measure 38.0-43.4 mm standard length. Two syntypes (listed as
cotypes) measuring 44.8-45.5 mm standard length from "Jellalabad"
with the annotation "Ind. Mus. Ex. F 9762/1" are in the
Natural History Museum, London (BM(NH) 1919.8.16:7-8; the outside has
1919.3.16:7-8, incorrectly).
Key characters
The characters of the genus, particularly in the mouth region, serve to
identify the only species in Iran.
Morphology
Four short barbels are present, the rostral ones longer than those
at the mouth corner. The upper lip covers the upper jaw, is granular
or tuberculate and has a marginal fringe, variably developed and most
apparent in larger fish. The lower lip is only apparent at the sides
and the exposed lower jaw has a granular or tuberculate pad without a
free posterior margin but with almost completely free edges.
Dorsal fin with 2-3 unbranched and 8-9 branched rays, anal fin with
2-3 unbranched and 5 branched rays, pectoral fin branched rays 14-17, and pelvic
fin branched rays 7-9. Lateral line scales 33-39. Scales
may have short dorsal and ventral projections from the margin at about
one-third of the scale length from the posterior edge. There is a
pelvic axillary scale. Scales have 9-10 radii on the posterior field
and are elongate with a notably anterior focus. Radii in large fish
are parallel rather than divergent. The anus is 4-5 scales in advance
of the anal fin origin. Gill rakers 17-25, small reaching the adjacent
or second raker when appressed. Pharyngeal teeth usually 3,3,5-5,3,3
or 2,4,5-5,4,2, depending on how the crowded teeth are counted; major
row teeth are usually 5 but may be 4 or 6, middle row teeth are 3 or
4, and minor row teeth 2 or 3, more rarely 1 (this difficulty in
assigning teeth to rows is the reason for omitting frequency
distributions below). Supernumerary teeth may be present to further
confuse counts. The crown of major row teeth are flattened, the
anterior tooth may be rounded and some teeth may have a small hooked
tip. The gut is very long and complexly coiled. The chromosome number
is probably 2n=48 (Klinkhardt et al., 1995).
Iranian fish from Sistan and Baluchestan have the following
meristic characters: dorsal fin branched rays 8(81) or 9(1), anal fin
branched rays 5(81), pectoral fin branched rays 14(28), 15(37),
16(16), or 17(1), and pelvic fin branched rays 7(3), 8(76), or 9(3).
Lateral line scales 33(1), 34(2), 35(12), 36(31), 37(32) or 38(4).
Total gill rakers 20-25, but not countable with great accuracy since
the smallest rakers are difficult to detect at the ends of the arch.
Total vertebrae 34(6), 35(20), 36(9) or 37(1).
Sexual dimorphism
Unknown.
Colour
The back is bluish-grey in Sistan fish or brownish to greenish with
irregular spots in other populations and the belly light pink to
yellowish-white or silvery-white. Fins are pink and the dorsal and
caudal fins have a grey tinge. The flank has a bluish, mid-lateral
stripe in Sistan fish and in preserved ones scattered melanophores, or
small blotches of less than scale size, or clumps of melanophores
centred on upper flank scales and more dispersed on the lower flank.
There is a broad stripe along the back mid-line. Fins in preserved
fish from Sistan are mostly immaculate except in the larger fish with
some melanophores lining rays basally. The caudal fin is distinctive
in larger fish from Sistan in having the rays of the lower half of the
fin heavily pigmented while the upper half rays are only lightly
pigmented. Peritoneum is dark brown to black.
Size
Attains 14.6 cm although the largest fish recorded from Sistan was
93.2 mm standard length.
Distribution
Found in submontane areas of Afghanistan, Pakistan and India as
well as eastern and southeastern Iran. The main areas of distribution are Sistan,
the
Hamun-e Mashkid basin including the Simish River and coastal streams of Makran from
the Jagin to middle and upper Nikshahr rivers and the middle and upper Bahu Kalat
River including its Sarbaz River reach. (Nikol'skii, 1899; Annandale, 1919b;
Berg, 1949; Bianco and Banarescu, 1982; J. Holčík, in litt., 1996; Abdoli, 2000).
Zoogeography
This distribution in Iran marks the western limit for the genus and the
relationships of the species lie to the east.
Habitat
Very abundant in small streams, including those with rocky or muddy beds, irrigation ditches, channels in
reed beds and pools in Sistan, less common in Baluchestan streams.
This species is found in large schools in Sistan in still or
slow-flowing water, on the bottom during the day but it may swim at
the surface in the evenings. It is common in the smallest permanent
water channels but Annandale and Hora (1920) reported it to be in
small numbers in the reed beds in winter and these were dead or dying,
perhaps because of low oxygen conditions associated with vegetation
decay. Large numbers die each year in drying stream beds as salt
content increases and the water is fouled by sheep and goats.
Tekrival and Rao (1999) report its aquarium preferences as 18-22°C,
pH 6.5-7.2, algae as food, not too bright lighting, bottom dwelling
with stones, roots and crevices preferred and cave brooding reproduction.
Age and growth
Unknown.
Food
Diet is algae on muddy bottoms. The type subspecies is a bottom
feeding herbivore taking more than 90% plant food such as algae,
diatoms and macrophytes as well as detritus (Sharma, 1984; Singh and
Bahuguna, 1984). Iranian fish contain detritus and some insect
remains, possibly as accidental inclusions.
Reproduction
Iranian adult specimens were caught in May in Sistan and show signs
of developing reproductive organs suggestive of summer spawning.
Parasites and predators
Jalali et al. (2000) describe two new species of monogenean,
Dactylogyrus faridpaki and D. eslamii, from this species
in the Bahu Kalat River of Baluchestan.
Economic importance
This species is of no economic importance although Butt (1995)
suggests it could be cultured as food and as a forage fish in Pakistan.
Conservation
This species does not appear to be under any major threat as it can survive
drying of the Sistan lakes in small ditches and streams.
Further work
The biology of this species needs investigation as does the taxonomic status of Sistan populations.
Sources
Mirza (1972) for colour.
Type material: See above, Discognathus adiscus (ZISP 25411, BM(NH)
1919.8.16:7-8).
Iranian material: CMNFI 1979-0224, 8, 43.6-55.4 mm standard length, Sistan, effluent of Hirmand River (30º53'30"N, 61º27'E);
CMNFI 1979-0226, 277, 29.7-78.8 mm standard length, Sistan, pool near Kuh-e Khajeh (30º57'N, 61º17'E);
CMNFI 1979-0227, 4, 37.0-48.9 mm standard length, Sistan, naizar at Kuh-e Khajeh (30º57'N, 61º16'E);
CMNFI 1979-0228, 1, 42.9 mm standard length, Sistan, ditch 1 km from Zabol (31º02'30"N, 61º31'E);
CMNFI 1979-0229, 5, 52.3-93.2 mm standard length, Sistan, ditch 5 km from Zabol (31º03'N, 61º33'E);
CMNFI 1979-0230, 1, 48.3 mm standard length, Sistan, Hamun-e Puzak (ca. 31º15'N, ca. 61º42'E);
CMNFI 1979-0232, 9, 44.0-65.9 mm standard length, Sistan, ditch 11 km from Zabol (ca. 30º58'30"N, ca, 61º36'E);
CMNFI 1979-0234, 17, 40.4-49.3 mm standard length, Sistan, effluent of Hirmand River (30º54'N, 61º40'E);
CMNFI 1979-0318, 2, 24.0-27.8 mm standard length, Baluchestan, Sarbaz River at Huvar (26º09'N, 61º27'E);
CMNFI 1979-0333, 2, 20.8-21.2 mm standard length, Baluchestan, Mashkid River west of Kuhak (ca. 27º05'N, ca. 63º12'E).
Genus Ctenopharyngodon
Steindachner, 1866
The grass carp genus contains only a single species found in East
Asia but widely introduced for food and its ability to digest macrophytes.
This genus is characterised by a rounded body and broad head, the
eyes are large and positioned at or above the body axis and often
visible from the underside of the head, mouth wide and terminal, no
barbels, moderate-sized scales, a complete lateral line, dorsal and
anal fins short and lacking spines, branchial membranes attached to
the isthmus, short unfused gill rakers, brown to black peritoneum, and
pharyngeal teeth in 2 rows with the crowns strongly compressed and
serrate and with a longitudinal groove on the grinding surface.
Ctenopharyngodon idella
(Valenciennes, 1844)
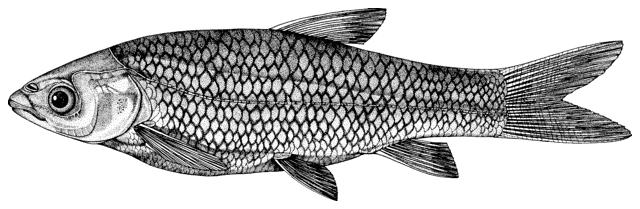
Common names
كپور علفخوار (= kapour-e alaf khaar or alaf khoar
or kopur 'laf khoar, carp grass-eater or grass-eater), آمور (= amur),
سفيد پرورشي (safid parvareshi or mahid safid parvareshi
meaning cultured white fish, from a resemblance to mahi safid, i.e. Rutilus frisii kutum).
[grass carp, white amur].
Systematics
Leuciscus idella was originally described from China.
A hybrid of this carp and Rutilus frisii has been bred at
the Astaneh Ashrafie Fisheries Research Station and named "Samur"
(Iranian Fisheries Research and Training Organization Newsletter, 11:6, 1996).
See under Rutilus frisii for more information.
Key characters
This species is identified by the eyes being low on the side of the
head, the anal fin is far back on the body close to the caudal fin,
and pharyngeal teeth have large, parallel grooves on the grinding surface.
Morphology
Lateral line scales 34-47. Scales have a wavy anterior edge,
central focus and moderate numbers of anterior and posterior radii.
Dorsal fin branched rays 6-8, usually 7, after 3 unbranched rays, anal
fin branched rays 7-9, usually 8, after 3 unbranched rays, pectoral
fin branched rays 13-20 and pelvic fin branched rays 7-8. Gill rakers
number 15-18 and touch the adjacent raker when appressed. Vertebrae
40-47. Pharyngeal teeth are 2,5-5,2, 2,4-5,2, 2,4-4,2, or 1,4-5,2 and
are obviously serrated with a longitudinal grooves. The gut is long
and complexly coiled. The diploid chromosome number is 48, the
triploid 72 (Klinkhardt et al., 1995; Nowruzfashkhami et al., no
date). Serum immunoglobulins have been characterised by Soltani et al. (2003).
Sexual dimorphism
Nuptial tubercles are evident on the male head, upper caudal
peduncle, dorsal and caudal fins and in particular on the pectoral
fins, the first ray of which is thickened, while the female has a
distended belly and a swollen and pinkish vent.
Colour
The back is dark, olive to greenish-brown, the flanks are silvery
but scales are marked with darker pigment on their posterior margin
giving the appearance of a row of spots, and the belly is white to
cream-yellow. Scale centres may reflect golden or yellowish tints.
Upper scales are outlined with dark pigment to give a cross-hatching
effect. The fins are grey-green, or grey to black, except the pelvics
which resemble the belly colour. Peritoneum brownish black.
Size
Reputed to attain 1.6 m and about 50 kg in its native range;
reports of weights up to 180 kg probably being exaggerations. Reaches
80 cm in the Tadjan River near Sari (A. Abdoli, pers. comm., 1995).
Distribution
The native distribution is in East Asia but it has been introduced to Iranian
waters. Also introduced to Afghanistan, Pakistan, and Iraq (Shireman and Smith, 1983).
This species was first introduced in the 1950s according to Armantrout
(1980) in the Anzali Mordab for vegetation control, adults surviving
to the 1960s but no breeding population was established. Also
introduced in 1966 from a hatchery in the Krasnodar region of the
former U.S.S.R. and stocked in the Anzali Mordab (Anonymous, 1970b)
and in October 1970 50,000 fingerlings from the U.S.S.R. were
introduced to the Caspian Sea and Anzali Mordab (Griffiths et al.,
1972). Three large fish (80 cm) were caught in January 1971 and
believed to be from the October introduction and evidence of good
growth although they may have been from an earlier stocking. It is reported
from the Siah-Keshim Protected Region of the Anzali Mordab (Riazi,
1996), presumably recently stocked, and is stocked in a variety of reservoirs
in the provinces of Gilan and Mazandaran but not as widely as silver
carp (Hypophthalmichthys molitrix). It is pen cultured in Gomishan
Reservoir, Mazandaran (Madbaygi, 1993b). Grass carp were introduced to Khuzestan in the 1970s to control
vegetation in irrigation ditches. In April 1974, 1150 fish were
released in the Dez Irrigation Project (Saadati, 1974). It is reported from Mahabad Dam (Abdi, 1999; www.mondialvet99.com,
downloaded 31 May 2000), from the Safid River and Anzali Talab (Abbasi et al.,
1999), from Lake Zaribar, Kordestan (Abzeeyan,
5(5):III, 1994), the Kor River in Fars (A. Alamdari, in
litt., 1997), from the Sistan basin in Hamun Sabari, Hamun Kushk and the
canal flowing into Chahnimeh (Ahmadi and Wossughi, 1988; Mansoori,
1994; J. Holčík, in litt., 1996), from the Haft Barm lakes near Shiraz
in 1984 although these later disappeared, possibly eaten by introduced
Sander lucioperca (Petr, 1987). As escapees from a fish farm, they have
been found in Lake Famur. Also recorded from the Gorgan, Tajan and Safid rivers, and the Anzali Mordab
(Kiabi et al., 1999), and it is mapped from the Kor, Kerman-Na'in, Hormuz,
Dasht-e Lut and Sistan basins without
exact localities; the Kashaf River in the Tedzhen River basin; middle reaches of
the Atrak River, lower reaches of the Gorgan, Neka, Babol, Heraz and Safid
rivers and in the Anzali Mordab, all in the Caspian Sea basin (Abdoli and Naderi,
2009), the middle to
lower Talkheh and lower Zarrineh rivers in the Lake Orumiyeh basin, the middle to lower
Abhar-Shur and Qom River in the Namak Lake basin, the middle to
lower Zayandeh River in the Esfahan basin; the lower Karun and Jarrahi rivers in
the Tigris River basin, and the lower Jovein and middle Kal Shur rivers in the
Dasht-e Kavir basin
(Abdoli, 2000).
It was introduced to the Soviet Caspian Sea in 1970-1974 where small
populations became established in the Terek River and the Volga delta
and to the Karakum Canal and Kopetdag Reservoir of Turkmenistan near
the Iranian border (Baltz, 1991; Shakirova and Sukhanova, 1994;
Sal'nikov, 1995; Opuszynski and Shireman, 1995).
Grass carp could establish breeding populations in the large rivers
of southern Iran and Iraq if the environment proves favourable and
there is enough uninterrupted river flow for eggs to hatch.
Zoogeography
This species is an exotic in Iran and has a native range from the
Amur River basin of Siberia south to southern China. It has been
widely introduced around the world for vegetation control.
Habitat
The natural habitat is large rivers but this species adapts easily
to pond culture. Grass carp can live in the Caspian Sea at salinities
of 5-8‰ although a few are found at 10-12‰. They enter
rivers to spawn (Abdusamodov, 1986). Temperatures in the range 0-41°C
and low oxygen concentrations (0.2 mg/l) are tolerated by this species
as is high turbidity. Fry have an upper lethal temperature range of 33-41°C and
temperatures greater than 38°C are lethal for adults. pH range is 5.0-9.0. Adults prefer densely
vegetated inshore areas with depths of 1-3 m. Adults leave the river
after spawning and feed in lakes, reservoirs and on floodplains,
returning to the river in autumn to overwinter in deep holes separate
from the juveniles. Young hide in vegetation of lakes, reservoirs and
floodplains. Juveniles may migrate as much as 1000 km up- or
downstream from the original spawning site in their native habitat.
Young fish overwinter in deep holes in river beds.
Age and growth
Growth rate in Khuzestan canals was 1.8 g per day while in ponds
growth was 6.6 g per day when fed alfalfa during a 5-month growing
season from April to September (Saadati, 1974; Behnke, 1975a). Males
begin to mature at 4 years and females at 5 years in the Terek River
of Dagestan (Abdusamadov, 1986). Maturity is attained at 6-10 years in
the Amur River, the native habitat, and as early as 10 months in
Malaysia. Life span is over 33 years. Growth rate in this species is
perhaps greater than in any other fish. Growth to 1 kg in the first
year of life and 2-3 kg per year thereafter in temperate areas is very
high; in tropical areas a 20 g fingerling can reach 8.5 kg in 1 year.
Rates of 10-22 g per day have been reported in various areas of the
world depending on local conditions.
Food
Grass carp are herbivores, except for quite small fish (20 mm total
length or less) which consume zooplankton. In Khuzestan, the grass carp
prefers to eat Potamogeton spp. and Alisma gramineum to Chara
and Cladophora (Saadati, 1974). The grass carp can consume
100-150% of its body weight per day of aquatic vegetation. Peak
feeding occurs at 25-30°C but food is taken in the range 15-35°C.
Grass carp stocked in the Anzali Mordab and fish farms of the Caspian
Sea basin consume fresh Azolla, an introduced fern. Grass carp
stocked at 800/ha consume 400-500 kg of Azolla daily gaining
800-1200 g in 5 months. In China this species is known to eat grass,
leaves, small fishes, insects and other items in addition to aquatic
vegetation or when such vegetation is in low supply. About half the
plant food passing through the digestive system is undigested and
large quantities of plant material must be eaten to sustain life. This
consumption rate is the reason for its success at aquatic vegetation
control (Greenfield, 1973). Grass carp overwinter without feeding.
Reproduction
A spawning migration to a large river takes place at about 15-17°C
water temperature. The female swims in the centre of the river at the
surface accompanied by 2-3 males, they roll and rub their bodies
together and often jump out of the water. A male prods the female's
body to stimulate egg release and leans closely to one side. Eggs are
semi-buoyant and require a slow and steady current to keep them off
the bottom (minimum water velocity of 0.23 m/second or more to support
them and allow hatching; this is found in large rivers where the eggs
hatch as they drift downstream; at 20°C
and a not unusual velocity of 1.2 m/second, hatching requires 180 km
of river). Temperatures should be above 20°C and preferably 21-25°C, or 26-30°C
in another source. At these temperatures hatching takes about 40
hours. Flow rates should be 0.7-1.8 m/sec. Spawning occurs after heavy
rain in rising rivers, when turbidity may reduce predatory attacks on
the semi-pelagic eggs (Greenfield, 1973). This regime is also required
for newly hatched fry and such conditions are rare outside their
native habitat. In the Terek River of the Caspian Sea basin, the
spawning migration begins in mid-April at water temperatures of 15-17°C
and continues until August although numbers begin to decrease from the
end of May. Spawning takes place after a sharp rise in water level and
current speed. Eggs are first found in the drift in the second week of
June and hatch 34-70 hours later depending on temperature. Some larvae
reach rice fields and live there until autumn when the fields dry up, some being lost, others migrating. Other larvae are carried into
the Caspian Sea where they are sensitive to the prevailing salinity at
1-1.5 days old (Abdusamadov, 1986). Up to 100,000 eggs are laid at one
time (Greenfield, 1973) and in the Terek River fecundity reaches
1,230,700 eggs (Abdusamadov, 1986). Absolute fecundity may reach 2
million eggs. Eggs are up to 2.5 mm in diameter before fertilisation
and are greyish-blue to bright orange. In water they swell to over 5.3
mm in 2 hours, becoming buoyant in flowing water.
Parasites and predators
Mokhayer (1976b) reports the cestode Bothriocephalus
gowkongensis and the acanthocephalan Pomphorhynchus perforator.
Red-sore disease is reported from fish pond grass carp in Iran by
Razavilar et al. (1981). It is caused by a bacterium Aeromonas
hydrophila and treatment was unsuccessful. Mokhayer (1989) records metacercariae of the eye fluke, Diplostomum spathaceum from
this species in Iran, which can cause complete blindness and death in
commercially important species, as well as shedder scales (sic),
Echinochasmus perfoliatus. Jalali and Molnár (1990b) record
the monogenean Dactylogyrus lamellatus from this species at fish farms in
Iran. Viral haemorrhagic disease has been reported from grass carp in Iran (Iranian
Fisheries Research and Training Organization Newsletter, 6:6,
1994; 9:6, 1995). Pond-cultured grass carp were found to be infected
by the tapeworm Bothriocephalus, with 70-80 parasites causing
intestinal obstruction and lowered haemoglobin, haematocrit and
erythrocyte values (Esmaeli and Abbasi, 1996). Esmaeli and Peighan
(1997) record an Aeromonas-like bacteria from grass carp in
Khuzestan Province. Ebrahimzadeh Mousavi and Khosravi (1999;
www.mondialvet99.com, downloaded 31 May 2000) record the toxigenic
fungi Aspergillus flavus, Alternaria, Penicillium and
Fusarium from this species and the pond water at a fish farm in
northern Iran. The crustacean parasite Lernaea elegans is
reported from this species in the Mahabad Dam reservoir (Abdi, 1999;
www.mondialvet99.com, downloaded 31 May 2000).
The intestinal helminth Bothriocephalus gowkongensis was recorded from
this species on fish farms in West Azarbayjan Province (Azarvandi et al.,
1999). Naem et al. (2002) found the following parasites on the gills of
this species from the western branch of the Safid River, namely the protozoan
Ichthyophthirius multifilis, a copepod crustacean Lernaea sp.,
monogenean trematodes Dactylogyrus lamellatus, D. ctenopharyngodonis,
and Gyrodactylus sp.. Jalali et al. (2002) and Jalali and Barzegar (2006) record Diplostomum spathaceum
and Dactylogyrus lamellatus from this
species in Lake Zarivar. Esmaeili et al. (2005) found a Flavobacterium
columnaris-like bacterium on grass carp form Khuzestan fish ponds, suspected
of either causing a 40% mortality or being a secondary factor in the fish kill.
Pazooki et al. (2005) record Ergasilus peregrinus from this species in
waterbodies of Zanjan Province. Araghi Soureh and Jalali Jafari (2005) recorded Dactylogyrus lamellatus
from this species in the Mahabad River of the Lake Orumiyeh basin. Barzegar and
Jalali (2006) report parasites in this species from Kaftar Lake as Trichodina
sp., Dactylogyrus lamellatus, Lernaea cyprinacea and Diplostomum spathaceum.
Barzegar et al.
(2008) record the digenean eye parasites Diplostomum spathaceum and
Tylodelphys clavata from this fish. Alishahi et al. (2009) examined moribund grass carp from 20 farms in
Khuzestan for bacterial agents but found Aeromonas hydrophila, A.
veroni and A. sohria in only 53 of 300 fish, secondary infections and
not the cause of mortality.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Ergasilus sp.,
Ergasilus peregrinus, Lernaea sp. and Lernaea cyprinacea on this species.
Any piscivore will take this species.
Economic importance
This species has been introduced to Iran to control aquatic weeds
in drainage and irrigation canals as an alternative to using polluting
chemicals or mechanical removal. In some countries at is sought after by
anglers. Grass carp may also help to control
the snail-carried, human disease schistosomiasis, since the vegetation
on which the snails live is severely reduced. They are also a food
fish which relies on food sources not available to native fish (few
fish consume whole plants). Grass carp consume vegetation at a rate of
100:1, i.e. for every 1 kg increase in grass carp biomass 100 kg of
vegetation is consumed. Removal rates may exceed this figure since
leaves are bitten off and branches clipped with not all of it being
consumed. In the Dez Irrigation Project large amounts of this
vegetation were removed daily from screens in test sections. Stocking
in the Dez Irrigation Project in Khuzestan showed a removal rate
approximately the same as mechanical control (Saadati, 1974; Behnke,
1975a). During a 5 month period the grass carp controlled 250 tons of
aquatic vegetation per hectare. The fish preferred plant species which
blocked the canals (Potamogeton spp. and Alisma gramineum)
rather than those which grew close to the substrate (Chara and Cladophora)
and did not interfere with water flow. Shireman and Smith (1983) give
details on artificial propagation of this species.
Esmaeilzadeh et al. (2004) studied the nutrient composition and marinade qualities of this fish in
Iran and compared them to those for safid mahi (Rutilus frisii) and found
them to be preferable according to the organoleptic properties. The marinades
could be stored for 6 months at 10ºC.
Fish farming of this species in Sistan was discontinued as its consumption of vegetation was reducing
food for other species (www.netiran.com, downloaded 28 February 2005).
Holčík and Oláh (1992) report a catch of 315 kg in the Anzali Mordab in
1990. However Iran acounts for almost all the production of grass carp
in the Near East and North Africa (4378 tonnes in 1994) (Food and
Agriculture Organization, Fisheries Department, 1996). The aquaculture
production in 1995 was 3942 tonnes (Bartley and Rana, 1998b). Grass
carp sold for about U.S.$2.00/kg in 1995 (Rana and Bartley, 1998a). Marjan Iran
Company was selling 1500-2000 g fish for U.S.$2.10/kg in August 2003
(http://groups.yahoo.com/groups/hilsa/message/25).
The inland waters of Turkmenistan had catches of 23 to 29.7 tonnes
for the years 1971-1974 and a catch of 76 tonnes in 1970 when a ban on
taking phytophagous fish was lifted.
Greenfield (1973) reviews the advantages and disadvantages of using
this species as a weed control agent in the U.S.A. and Charyev (1984)
in the Kara-kum Canal in Turkmenistan. Destruction of habitat for
fishes and waterfowl, competition with native species and introduction
of exotic diseases and parasites are all problems once this fish
escapes into a main river suitable for reproduction. Their destruction
of plants may interfere with waterfowl management, destroy breeding
grounds for other species and facilitate the attacks of predators.
Ideally triploids, produced by cold or warm shocks or by hydrostatic
pressure on fertilised eggs, should be used initially as they cannot
reproduce (Clugston and Shireman, 1987). However the chromosome number
of each fish must be checked (by electronically measuring the volume
of a red blood cell nucleus) as the process is not 100% effective.
Grass carp are reproducing naturally in the Kara-kum Canal, vegetation
is controlled, fish stocks have increased and some reduction of
mosquitos has been obtained. However the ecosystem has been changed,
spawning grounds of commercial species threatened, undesirable species
have been introduced accidentally, and reduction in vegetation affects
water quality. Grass carp are best used in restricted areas where
improved flow and reduced mosquito populations are required but where
there is no commercial fishery (Charyev, 1984).
The grass carp has a short gut and about half the plant material
eaten is released to enrich the water and promote algal blooms. Oxygen
levels and water clarity are reduced. The removal of plants can remove
food sources for other fishes, shelter and spawning substrate.
Additionally, as noted, the triploid treatment is not always effective and the
species can become established.
Iran has had problems with disease outbreaks and poor survival of
fingerlings which has led to production problems (Shehadeh, 1997).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture, as food and
in textbooks. There are numerous studies on this species as an experimental fish
and in relation to aquaculture. Some Iranian studies include Alboughobish and
Khaksari Mahabadi (2005) on the histology of the liver and pancreas; Pahn et
al. (2005) used electrocardiograms to determine that the anaesthetic
ketamine had no marked effect on heart activities; Morovvati et al.
(2006) on seasonal changes of pronephros lymphoid tissue; Nahavandi et al.
(2006) on the chemiluminescent response to determine the effect of various
concentrations of diazinon, an organophosphate, on phagocytosis in order to
measure immunity after exposure to this toxin; Pourgholam et al.
(2006) and Sharifpour et al. (2006) on the toxicity and histopathological
effects of diazinon; Sharifpour et al. (2006) on the
sub-lethal effects of diazinon on various organs; Pourgholam et al.
(2006) on the toxicity of diazinon and the effects of sub-lethal concentrations
on haematological and biochemical indices;
Rezaei et al.
(2007) on sensory evaluation and lipid quality of fish stored in ice - good to
excellent until the fourth day and good to acceptable to the tenth day; Khajeh
et al. (2008) on haematological parameters in cultured fish and found
some to be lower than in Mesopotamichthys sharpeyi;
etc.
Conservation
No conservation is required for this exotic species. Krasznai (1987) and Petr (1987) give details of fish farms
propagating this species in Iran. For example, 10 million were
produced in the Safid Rud Fish Farm in 1986. 20 million carp, silver
carp and grass carp fingerlings were produced in the Shahid Rajaae
Hatchery in Sari for release across Iran in reservoirs and dams (Abzeeyan,
Tehran, 4(7):VII, 1993). Feeding and growth studies on this species have also
been carried out on this species in the Shaid Rajaee Hatchery (Ahmadi and Rezai, 1998).
Experiments on induction of triploidy have been carried out in Iran using cold
and heat shocks (M. Hassanzadehsaber, M. Pourkazemi, M. R. Nowruzfashkhami and
A. Ghanaatparast (www.meeresschule.com/cgi-bin/abstracts/gastbuch.asp, downloaded 17 January2005).
Further work
Studies on the interactions of this species and native Iranian taxa should be
carried out and introductions carefully controlled and monitored.
Sources
Shireman and Smith (1983) give a summary of the biology of this
species. There is an extensive literature on herbivorous fishes, a
recent book being Opuszynski and Shireman (1995), which has sections
on grass carp. Gholipour (1996) has an account in Farsi.
Comparative material: BC65-381, 2, 95.4-98.8 mm standard length, Singapore, fish ponds (no other locality data).
Genus Cyprinion
Heckel, 1843
Scaphiodon Heckel, 1843 has been used for Cyprinion and Capoeta species in Southwest Asia.
Taki (1975) related members of this genus to a common ancestor with Onychostoma Günther, 1896, a Chinese and southeast Asian genus
although Li et al. (2008) found this lineage to be unsupported on DNA evidence. Howes (1982) synonymises Semiplotus Bleeker, 1859, a
genus found from Nepal to Viet Nam, and Scaphiodonichthys Vinciguerra, 1890, a genus from Indochina, with Cyprinion and refuted Taki's (1975) view using osteological characters, particularly
of the jaws. Howes (1982) considers that Cyprinion cannot be defined on any uniquely derived characters. Krupp (1983) considers Howes' revision as unsatisfactory for the reasons that type specimens
were not examined, relationships are based on jaw anatomy and other characters are largely excluded, variability of osteological characters within a species are largely unknown, and synapomorphies
are not unequivocal. Bănărescu (1992b) and Banarescu and Herzig-Straschil (1995) regard Semiplotus as a distinct genus but probably related to Cyprinion. They comment that
Semiplotus differs sharply from Cyprinion s.s. in the absence of barbels, a higher number of branched dorsal fin rays (20 or more), and in a lower number of branched anal fin rays (5 as in
most related genera rather than the unusual 7 in Cyprinion). Scaphiodonichthys has 2 pairs of barbels (only 1 in Cyprinion), and 5 branched anal fin rays as well as differing from
both Cyprinion and Semiplotus by having the lateral line closer to the ventral margin of the caudal peduncle and divergent rather than parallel striae on the scales.
These latter 2 characters justify generic separation of Scaphiodonichthys. Bănărescu (1997) considers Scaphiodonichthys as valid and not a synonym of Cyprinion. Characters
used by others to define Cyprinion such as expansion of the proximal part of the pelvic fin rays, interpelvic papillate flaps (Banister and Clarke, 1977) and a naked predorsal ridge (Mirza, 1969)
do not occur in all species in this genus. If Semiplotus is included in Cyprinion then several osteological structures, particularly a synarthritic dentary joint, are uniquely derived or
synapomorphic.
In the absence of a detailed revision, I have retained species within Cyprinion as the most familiar name in use in Southwest Asia for these fishes. Cyprinion s.s. is found from the
Indus River basin west to the Arabian Peninsula and the Tigris-Euphrates basin but excluding northern drainages such as the Lake Orumiyeh, Caspian Sea and Hari River basins and excluding the
westernmost edge of Southwest Asia such as the Jordan River basin and coastal drainages of Israel.
The genus Cyprinion is currently under revision by Florian Wicker at the Senckenberg Museum, Frankfurt and the status of the following species may undergo some changes.
Saadati (1977:45) refers to a new and undescribed Cyprinion species from Lar in southern Iran but the fish are
Carasobarbus luteus.
A thorough study of the systematics of this genus in Iran depends to some degree on material from other areas which is not readily available, on large series of well-preserved adult specimens, and
analyses which demonstrate consistency in characters used to define species. These conditions have not been achieved thus far in any studies undertaken and given the wide distribution and individual
variation shown by Cyprinion species an adequate understanding of the species composition is not entirely possible.
This genus is characterised by a moderate sized, compressed body, a thick and blunt snout, an inferior mouth with a straight, crescentic or arched shape and a sharp horny edge to the lower jaw
(which may fall off in preserved specimens), 1 pair of small barbels at the mouth corner, the last dorsal fin unbranched ray is thickened and bears weak to strong serrations (highly variable between
individuals within a species and not a good character in species definitions), the dorsal fin is long (up to 16 branched rays) and the anal fin short (typically 7 branched rays), a ridge in front of
the dorsal fin is formed internally from fused pterygiophores and lacks scales externally, pharyngeal teeth are in 3 rows and are compressed and spoon-shaped, scales large to moderate in size (lateral
line counts (31-45), breast and belly scales may be absent (individually variable and not a good character), scale radii are restricted to the posterior field, peritoneum black, and gut very long
and coiled (several times body length).
Cyprinion kais
Heckel, 1843
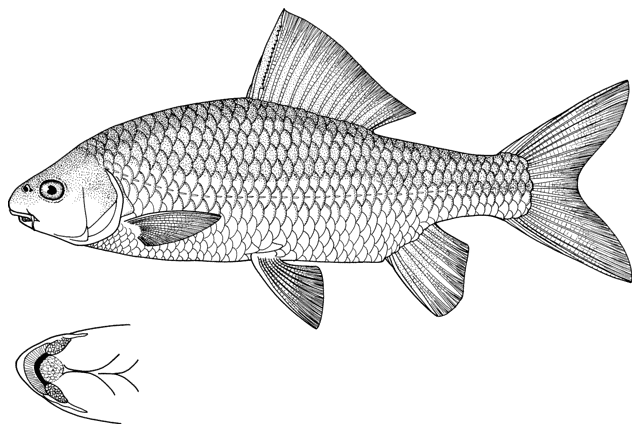

Common names
butak-e dehan kuchek, بوتك (= botak), butak dahan kuchek, butok, لوتك (= lotak), zanbour, زنبور
دهان كوچك (= zanbour dahan kuchek).
[bunni saghir, bnaini; kais at Aleppo (= Haleb, Syria), hence the scientific name, all in Arabic; kais kingfish].
Systematics
Cyprinion Cypris Heckel, 1843 is a synonym, being a juvenile with keratinization of the lower jaw incomplete according to Howes (1982), although he did not examine the types. Krupp (1985c) and
Banarescu and Herzig-Straschil (1995) agree with this synonymy. Berg (1949) placed C. kais (and C. cypris) in C. macrostomum, as the position of the dorsal fin in relation to the
pelvic fins was variable in these fishes and not sufficient to warrant species status as Heckel (1843) stated in describing these species.
The type localities for Cyprinion Kais are "Aleppo" and "Mossul" and for Cyprinion Cypris the "Tigris bei Mossul" (Heckel, 1843b).
The syntypes of C. kais are in the Naturhistorisches Museum Wien comprising 3 fish in NMW 52801 (paralectotypes) and measuring 68.5-97.3 mm standard length, 2 fish in NMW 52802 measuring
120.6-164.3 mm standard length, and 2 fish in NMW 52803 (paralectotypes) measuring 153.4-154.2 mm standard length, the smaller of these being designated as the lectotype by F. Krupp in 1984. Eschmeyer
et al. (1996) list possible syntypes in the Rijksmuseum van Natuurlijke Historie, Leiden under RMNH 2485 (2 fish, formerly NMW) and RMNH 2489 (1), and 1 syntype in the Senckenberg Museum Frankfurt
(SMF 134, formerly NMW). The catalogue in Vienna lists 5 specimens.
A syntype of C. cypris is in the Senckenberg Museum Frankfurt (SMF 849, formerly NMW) (F. Krupp, pers. comm., 1985). Two syntypes, 63.5-106.2 mm standard length are under SMF 849, the larger
one designated as a paralectotype (March 2007). Ten syntypes are in the Naturhistorisches Museum Wien (NMW 52804) measuring 51.2-115.1 mm standard length, the largest being designated as the lectotype
(however Banarescu and Herzig-Straschil (1995) give 44.1-110.0 mm standard length for these 10 fish with one at 99.8 mm standard length as lectotype as selected by F. Krupp in 1984). Another specimen,
110.5 mm standard length, may also be a syntype (NMW 52800); and also NMW 59508, a dried specimen (Eschmeyer et al., 1996). The catalogue in Vienna lists 6 fish in alcohol and 1 fish stuffed.
Key characters
Mouth shape is distinctive. It is small and semicircular with a width about the size of the eye diameter and has large lateral lobes (= lower lips)(Kafuku, 1969). The cartilaginous sheath is thickened
between the corners of the mouth and is rounded posteriorly with a distinct margin. The cartilage can form a tooth-like structure protruding anteriorly from the lower lip. The mouth in
C. macrostomum is wider, arched and lacks the lateral lobes (see also illustrations in Kafuku (1969), Krupp (1985c) and Banarescu and Herzig-Straschil (1995)). These latter authors have the width
of the mouth opening as only 13.5-22.0% of the head length (22.0-27.0% in C. macrostomum) for adult fish and the height of the arch or mouth opening (a line perpendicular from a line between the
mouth corners to the tip of the lower jaw) 48-80% of the mouth width (29-47% in C. macrostomum), i.e. the mouth is narrower and more arched in C. kais. On this character, therefore, the two
species can be distinguished as adults but there is potential for confusion in young fish. A single specimen identified as C. kais on the basis of mouth shape from the Dalaki River of Iran had
values of 23.2% and 47.4% which are arguably C. macrostomum values. This specimen has a protruding tooth-like edge to the lower jaw in a u-shaped mouth with well-developed lips posterior to the
"tooth".
The intestine is shorter and less complexly coiled in this species and the mean number of gill rakers is less in contrast to C. macrostomum (Kafuku, 1969). The back is higher and more curved,
the eyes are larger and the anal fin is more posterior, in addition to the mouth shape (Heckel, 1843b). The dorsal fin origin arises over that of the pelvic fins (Heckel, 1846-1849a). The edge of the
dorsal fin is more notched in C. kais than in C. macrostomum (the length of the fourth branched ray is 48-62% of the length of the first ray as opposed to 55-79% in C. macrostomum,
with extreme values overlapping, according to Banarescu and Herzig-Straschil (1995)).
The form of the pharyngeal teeth is different from C. macrostomum (see Krupp (1985c) for illustrations where kais has hooked tips and macrostomum does not), there are fewer gill
rakers (8-12 on the lower arch in kais, 12-16 in macrostomum), on average there are fewer dorsal fin rays, the last unbranched dorsal fin ray is longer, and interorbital width is smaller.
However sample sizes in some studies are small (in Kafuku (1969) only 5 fish of each species were examined), morphometric characters are notoriously size-dependent, gill raker counts are also size
dependent, and even pharyngeal tooth form varies with age (small macrostomum have hooked tips). C. kais may well be a good species but a wide-ranging comparison of adults and young and of
localities is needed and material from Iran is scarce or equivocal. Further discussion is under C. macrostomum.
Morphology
Dorsal fin with 4 unbranched and 12-16 branched rays, anal fin with 3 unbranched and 7 branched rays. The dorsal fin has the last unbranched developed as
a spine with strong teeth except at the extreme tip which is thin and flexible. Pectoral fin with 14-18 branched rays, and pelvic fin with 8-9 branched rays. Lateral line with 36-43
scales. The belly is scaled. There is a well-developed pelvic axillary scale. Scales have a subcentral anterior focus, fine circuli, few posterior radii and no or very few anterior
radii. Total gill rakers 10-15, short and reaching the raker below when appressed. Rakers are absent on the anterior arch where there are only tubercles. Pharyngeal teeth
2,3,4-4,3,2, with variants 2,3,5-5,3,2 and 2,3,5-4,3,2, spoon-shaped with a small hook at the tip.
Meristics for Iranian material: dorsal fin branched rays 12(2), 13(2), 14(1)
or 15(1); branched anal fin rays 7 (6); branched pectoral fin rays 14(1), 15(2),
16(2) or 17(1); branched pelvic rays 8(4) or 9(2); lateral line scales 38(5) or
39(1); and total gill rakers 12(2), 13(3) or 14(1).
Sexual dimorphism
Tuberculation in a 103.5 mm standard length specimen consisted of ca. 20 tubercles restricted to the area over the lachrymal bone. A specimen 147.5 mm standard length had small to minute tubercles in
front of the eye, under the eye, on the mid-preoperculum and on the mid-operculum. Curiously the individual small tubercles on the operculum were connected by thin lines of horny tissue.
Colour
Overall colour is silvery to yellowish-white with the back grey-brown and the lower surfaces a lemon yellow. The lower jaw margin is a glossy yellow. The fish shown above may represent a spawning
colouration, not seen in all specimens. The pelvic fins are a bright orange-red, the pectorals paler. Some fish have a less strong colour in the pelvic than in the anal fin. The anal fin is yellow, to
orange or greenish, distally black and anteriorly most orange. The caudal fin has light orange to greenish tints. The dorsal fin is black with a yellow-tinged base becoming anteriorly reddish. In
preserved fish, there is some concentration of pigment above and below each lateral line pore, scales on the back and upper flank are outlined with pigment, and there is some concentration of pigment into
a few to moderate number of diffuse spots on the uppermost flank and back midline. The leading edge of the dorsal fin is very dark (but may be light), dorsal fin membranes are dark, anal fin membranes
also dark but to a lesser extent, and the caudal, pectoral and pelvic fins have pigment lining the rays. Peritoneum black.
Size
Attains 21.5 cm total length, or to 25.0 cm total
length in Iraq (Al-Rudainy, 2008).
Distribution
This species is found in the Tigris-Euphrates and Quwaiq basins. Abdoli (2000) maps the Jarrahi, Karun, middle to lower Dez, and Karkheh up to the Simarreh rivers of the Tigris River basin. It is also
found in the Gulf basin, although rare, and specimens from the sugar cane fields of Khuzestan were seen in 2000 (personal observations, B. W. Coad).
Zoogeography
Zoogeographical comments are under the genus above.
Habitat
This species is recorded from a variety of habitats as listed above and is also known to inhabit canals but nothing is known of its environmental
requirements.
Age and growth
Unknown.
Food
Gut contents are filamentous algae in the one specimen examined. Diet may be similar to Cyprinion macrostomus.
Al-Rudainy (2008) gives aquatic insects and detritus for Iraq. Curiously, the mouth structure resembles that of the unrelated cutlips minnow,
Exoglossum maxillingua (Le Sueur, 1817), from North America. This species feeds on insect larvae, with some molluscs and worms. Food is scraped from the bottom or poked out of crevices using the
shovel-like lower jaw. Sand is also taken in and spat out, presumably after food items are extracted. The cutlips also picks out the eyes of other fishes in confined areas (Coad et al., 1995).
Reproduction
Generally unknown.
Ünlü (2006) gives age at first maturity as
2 years in the Turkish Tigris River with spawning over sand, stones and gravel
in May-June.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This minnow appears to be rare, or at least is rarely collected, in Iran. Cyprinion macrostomum is much more common and is taken in most seine hauls in streams and rivers. The distribution and
population numbers are unknown. Endangered in Turkey (Fricke et al., 2007).
Further work
The biology of this species needs to be investigated and the use of the peculiar jaw structure ascertained. Its great rarity, at least in Iran, leads to the suspicion that it may be a developmental
anomaly of Cyprinion macrostomum - the few specimens at hand don't permit a detailed study of characters other than the strikingly different jaw (see comments under C. macrostomum and also
above). Development of pharyngeal teeth, gill raker numbers, complexity of gut coils and morphometric characters are all size dependent and show individual and populational variations not analysable here.
Sources
Type material: See above, Cyprinion kais (NMW 52801, 52802 and 52803) and C. cypris (NMW 52804).
Iranian material: CMNFI 1993-0141, 1, 66.3 mm standard length, Bushehr, Dalaki River (29º28'N, 51º15'E);
CMNFI 2008-0169, 5, 80.4-98.2 mm standard length, Khuzestan, irrigation ditch in sugar cane fields (31º58'42"N, 48º31'07"E);
ZSM 25715, 2, 34.1-65.3 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E).
Comparative material: BM(NH) 1920.3.3:50, 1, 83.6 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1920.3.3:94-115, 40, 65.3-92.4 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1931.12.21:3, 1, 129.8 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1974.2.22:115-120, 5, 90.6-147.9 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1974.2.22:1105, 1, 115.6 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1974.2.22:1106, 1, 101.4 mm standard length, Iraq, Fao (29º58'N, 48º29'E);
BM(NH) 1974.2.22:1214-1255 (in part), Iraq, Khalis (33º49'N, 44º32'E);
BM(NH) 1984.4.18:30, 63.4 mm standard length, Iraq, Kut Hiwa (no other locality data);
FMNH 51229, 1, 103.5 mm standard length, Iraq, Diyala River, 12 miles east of Baghdad (no other locality data);
FMNH 51230, 6, 42.9-60.5 mm standard length, Iraq, Diyala River, 12 miles east of Baghdad (no other locality data);
FMNH 51231, 2, 64.0-64.8 mm standard length, Iraq, Diyala River, 12 miles east of Baghdad (no other locality data);
uncatalogued, 5, 49.1-66.7 mm standard length, Iraq, Shatt al Arab (no other locality data);
uncatalogued, 1, 107.2 mm standard length, Turkey, Euphrates River 20 km west of Erzurum (ca. 41º03'N, ca. 39º55'E).
Cyprinion macrostomum
Heckel, 1843
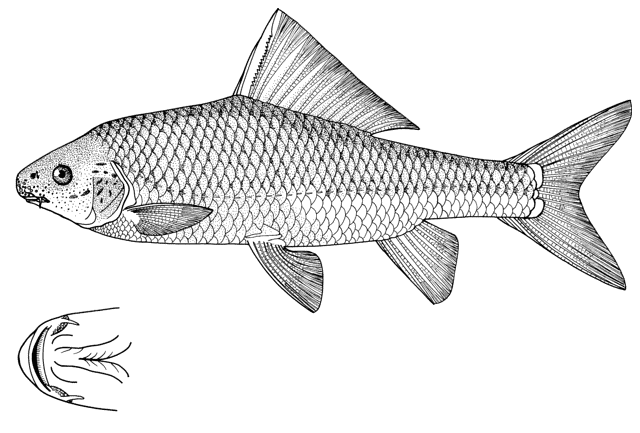
Common names
بوتك (= botak); butok; لوتك (= lotak); butak-e dehan (or dahan) buzorg in Khuzestan; galuk (Mokhayer (1981c); kapour; zanbour (= bee) in Khuzestan and
Boyer Ahmadi-ye Sardsir va Kohkiluyeh provinces; زنبور دهان بزرگ (= zanbour dahan bozorg); ?tumbuek (= hunting horn, possible
name from Heckel (1843b)).
[hmarriya sefra or himriya sefra, surrah masih,
bunni kaper, dunbuk kabir al-fam, benayne; kais at Aleppo (= Haleb, Syria) but see above species (Heckel, 1843b); dombok or dumbek at Mosul (= solid or compact flesh, a good source of food,
according to Heckel (1843b)); all preceding in Arabic; large-mouthed barb, Tigris kingfish].
Systematics
Originally spelt macrostomus but correctly macrostomum (Berg, 1949). Cyprinion neglectus Heckel, 1849 from the "Tigris bei Mossul" is a synonym (Krupp, 1985c; Banarescu
and Herzig-Straschil, 1995). Howes (1982) considered that Cyprinion tenuiradius (q.v.) was only a "variant" of this species but did not examine any material. Berg (1949)
places C. kais (q.v.) in the synonymy of this species along with C. cypris (see C. kais).
The type locality of Cyprinion macrostomus is given by Heckel (1843b) as "Aleppo" and "Mossul". Krupp (1985c) lists 5 syntypes from Aleppo, 81-133 mm standard length in the
Naturhistorisches Museum Wien (NMW 52805), the largest being selected as the lectotype (hence Aleppo is the type locality as designated by the publication of Banarescu and Herzig-Straschil (1995)). One
syntype from Aleppo, 83 mm standard length, is in the Senckenberg Museum Frankfurt (SMF 70, formerly NMW; Eschmeyer et al. (1996) give SMF 870) and 4 syntypes from Mosul, 58-124 mm standard length
are in the Naturhistorisches Museum Wien (NMW 52806). My measurements are 82.1-135.0 mm standard length for NMW 52805 and 59.1-126.2 mm standard length for NMW 52806. Another syntype
is a dried specimen NMW 52503, and the Rijksmuseum van Natuurlijke Historie, Leiden has 1 syntype under RMNH 2487, formerly NMW) and 1 syntype under RMNH 2488, formerly NMW). The catalogue in
Vienna lists 4 specimens.
Seven syntypes of Cyprinion neglectus from Mosul measure 54-131 mm standard length (NMW 52807), the largest being selected as the lectotype (Krupp, 1985c). My measurements are 53.3-131.9 mm
standard length (Banarescu and Herzig-Straschil (1995) have 53.1-128.2 mm standard length). All material was collected by Th. Kotschy in 1842 for Aleppo and 1843 for Mosul. The catalogue in Vienna lists
only 2 specimens under this name.
?Check lengths against data sheets
Key characters
Distinguished from C. kais by mouth and dorsal fin ray characters as described under that species, by having more gill rakers and a longer and more coiled intestine (Kafuku, 1969). The dorsal
fin origin is in front of that of the pelvic fins (Heckel, 1846-1849a). See discussion under C. tenuiradius for distinction from that taxon.
Morphology
Dorsal fin with 4 unbranched and 12-17 branched rays (usually 14-15 according to Banarescu and Herzig-Straschil (1995) but 77% of fish in Iran are 13-14, see below). The last dorsal fin unbranched ray
is strong and serrated to the tip. The anal fin has 3 unbranched and 6-7, usually 7, branched rays. In Iranian specimens, 96.1% of 127 fish have 7 rays, the remainder 6 rays. Pectoral fin branched rays
are 12-17 and pelvic fin branched rays 7-9, usually 8. Lateral line scales 33-45 (usually 41-44 according to Banarescu and Herzig-Straschil, 1995) but a broader range in Iran, see below). The breast is
covered with scales. The pelvic axillary scale is very elongate. Scales are squarish, being deeper than long, often with parallel dorsal and ventral margins (or rounded margins). The anterior margin has
a marked central protuberance and the posterior margin is rounded. Radii are numerous on the posterior field and circuli are fine and numerous. The posterior field circuli break into "bubbles".
The focus is subcentral anterior. Gill rakers 16-17, on the lower arm 12-16, in the literature but a much wider range in total rakers in Iran (see below). Rakers are short and only touch the raker below
or a little further when appressed. Pharyngeal teeth 2,3,5-5,3,2, 2,3,4-4,3,2, and variations on 4 or 5 main row teeth. Teeth are spatulate with broad, flattened crowns. The tips of teeth are slightly
hooked in small fish. The most anterior tooth in the main row may be very small or absent (or incompletely ossified and hard to distinguish). The gut is very elongate with complex coils. In small fish,
the upper lip is not covered with a fold of the snout as in large fish. Also the gut is not as coiled in young fish as in adults. Chromosome number is 2n=48 (Ünlü et al., 1997).
Meristics for Iranian fish from the Tigris River basin: branched dorsal fin rays 12(4), 13(43), 14(52), 15(26) or 16(3)(mean = 13.9, S.D. = 0.861); branched pectoral fin rays 14(3), 15(44), 16(57) or
17(25)(mean = 15.8, S.D. = 0.771); branched pelvic fin rays 7(7), 8(121) or 9(1)( mean = 8.0, S.D. = 0.246); lateral line scales 33(3), 34(1), 35(12), 36(11), 37(3), 38(11), 39(29), 40(31), 41(25), 42(2)
or 45(1)(mean = 38.8, S.D. = 2.211); total gill rakers 13(3), 14(8), 15(15), 16(23), 17(15), 18(24), 19(17), 20(14) or 21(6) (mean = 17.3, S.D. = 2.022); pharyngeal teeth 2,3,5-5,3,2(17), 2,3,4-5,3,2(8),
2,3,5-4,3,2(3), or 2,3,4-4,3,2(2); and total vertebrae ?.
The mouth is usually transverse or slightly arched and usually has a horny covering. Small fish have a crescentic mouth. A wide range of mouth arching is seen in fish of varying sizes and even in fish
of the same size and locality of capture. Banarescu and Herzig-Straschil (1995) note that the syntypes of Cyprinion neglectus have a mouth arch which is more curved and not as wide, somewhat
intermediate between C. macrostomum and C. kais, being closer to the former. This variation is attributed to the material possibly being from some tributary of the Tigris River, or from
isolated ponds, where introgression with C. kais took place. It may well be that variation in mouth shape is more marked than limited sample sizes would indicate. Certainly in smaller fish, e.g.
in 20 specimens of C. macrostomum (38.5-54.0 mm SL) examined by me from Iran, values for mouth width and depth as measured in Banarescu and Herzig-Straschil (1995) are not as clear cut and there
is a variable developmental gradient in mouth shape. Mouth "height" as a % of width was 29.2-53.8 and width as % of head length was 22.1-36.6. Banarescu and Herzig-Straschil (1995) give
"height" as 19-31% of width and width as 26-44% head length for macrostomum and 48-80% and 13.5-22.0% respectively for kais. Large macrostomum and kais (>100 mm
SL) can be distinguished on mouth shape but not smaller specimens which bridge the gap between the two species. The possibility that kais is a developmental anomaly of macrostomum,
retaining juvenile features, should be investigated.
Sexual dimorphism
Mature males have large tubercles on the snout in a broad band below the nostril level, extending back under the eye and breaking up into a few tubercles on the operculum. There is a large tubercle
between the nostril and the eye. Fine tubercles are scattered over the top of the head. Three tubercles are found in rows on the first branched pectoral fin ray and very strong tubercles line each anal
fin branched ray in single file. The anterior pelvic fin rays have the occasional 1-2 tubercles or a row of tubercles. Dorsal and caudal fin rays have fine tubercles, much smaller than those on the anal
fin. Mid and posterior flank scales have 1-3 small tubercles, variably arranged on the exposed scale.
Colour
The back is bluish-grey to bluish-black or brown, flanks silvery or silvery-yellow and the belly whitish with silvery tints. The upper head is light brown. Scales are outlined with dark pigment and
the anterior exposed scale base is darkened. The cleithrum area is pink or orange in some fish with pink or orange spots on up to 5 rows of flank scales but mostly along the anterior lateral line. Fish
from a saline stream in Khuzestan had a pale-pink cleithrum and lateral line spots. There is a reddish-yellow spot at the base of the pectoral and pelvic fins. The pectoral, pelvic, anal and caudal fins
are yellowish to pinkish or orange proximally and blackish distally. The dorsal fin has a narrow, yellow stripe at the base and the rest of the fin is black. The cartilaginous lower jaw is reddish-yellow
to orange. The eye is slightly yellow. Small live fish are silvery overall with a white belly and olive back, the pectoral and pelvic fins slightly orange-yellow and other fins greyish although all fins
may be hyaline. The peritoneum is black.
Small preserved fish have an indistinct blotch at the caudal fin base and a similar blotch on the back at the base of the spine in the dorsal fin. In very small fish these blotches are more distinct
and there are 4-7 irregular blotches on the mid-flank above the lateral line and 3 blotches at the dorsal fin base. Development of blotches is individually variable, some fish being almost immaculate
while in others the blotches extend vertically as bars as far as the back.
Size
Reaches 19.3 cm standard length (Krupp, 1985c).
Distribution
Found in the Orontes, (= Asi), Quwayq and Tigris-Euphrates basins. In Iran, it is found in the Tigris River basin including the Hawr Al Azim, Khersan, Jarrahi and Marun rivers (Berg, 1949; Abdoli,
2000) and the northern Gulf basin in the Shapur, Dalaki and Helleh rivers (Gh. Izadpanahi, pers. comm., 1995), the Zohreh River and possibly Lake Famur - some may be C. tenuiradius. Vossoughi
(1998) reports this species from the western
Hamun-e Jaz Murian basin based on a fishes with 13-15 branched dorsal fin rays, much higher than for C. watsoni, the taxon to be expected in this area.
Zoogeography
Zoogeographical comments are under the genus above.
Habitat
Known from a variety of habitats such as rivers, streams, reservoirs and ponds, as well as canals and gravel pits. Al-Habbib and Al-Habbib (1979) have demonstrated experimentally for
a sample from "Nawaran Spring" north of Mosul, Iraq that this species can survive temperatures up to about 37°C. Akpinar and Aksoylar (1989) and Akpinar (1999) report this species from the
Kangal Thermal Spring, Sivas, Turkey at a constant temperature of 35°C. This is the commonest species in catches in southwestern Iran, followed by Garra rufa. In areas under human influence in
Lorestan, such as the lower reaches of rivers and near cities, it exceeds 80% in numbers in catches.
Age and growth
Maximum age reported for a population in the "Al-Nibaey" Lakes near Baghdad is 7+ years. Growth is slow and there is no difference in growth between males and females, although the habitat
is not considered ideal for these fishes. Females tend to be slightly heavier than males of the same length especially in older fish. The length-weight relationship was W = 0.027 L2.67 (r =
0.78) for both sexes, W = 0.028 L2.65 (r = 0.90) for males and W = 0.020 L2.78 (r = 0.93) for females. Maturity is attained at 10.0-11.1 cm, corresponding to age group 2 (Allouse
et al., 1989). The length-weight equation for commercially caught fish in the Tigris River was log W = 2.884 log L-4.623, condition factor was 1.15-1.47 (mean 1.28) and fish were
immature up to age 2+ (Al-Nasiri, 1991). Haematology of this species from Sarao Subhana Agha near Sulaymaniyah was examined by Al-Mehdi and Khan (1984).
Food
Major food items in the Baghdad study are of plant origin with occasionally some chironomid larvae, copepods and cladocerans. Khan (1988) found for fish from near Sulaimaniyah, Iraq that diatoms and
decayed organic matter are the main foods, with some green algae. Zooplankton are thought to be accidental food items. Guts contain mud and sand, evidence of a bottom feeding habit. Feeding increases at
the start of the breeding season. The horny lower jaw covering is used to scrape algal food off hard bottom objects.
Reproduction
Near Baghdad, most fish are mature by April, the gonads occupying about one-third of the body cavity. Ovaries are orange to yellowish and testes milky white. Spawning occurs principally in May and June,
with some in early July, but by July most fish are spent.
Al-Rudainy (2008) gives a spawning season of May and June in Iraq on gravel beds
in shallow water with fast current. Maturity is attained there at 2-3 years, 15
cm length and 50 g weight.
Iranian material shows minute but developing eggs in a 71.3 mm standard length fish caught on 31 January and specimens caught on 5 July have eggs 1.4 mm in diameter. The 31 January fish has tubercles
on the snout and anal rays so tubercles develop quite early and in small fish. A fish caught on 20 September also shows tubercles around the snout. Small fish caught in January about 20 mm SL are
presumably the young from the previous season and so show slow growth or are evidence of a prolonged or late spawning season.
Parasites and predators
Gussev et al. (1993a) describe a new species of monogenean from C. macrostomum in the Karun River, Dactylogyrus cyprinioni, and Jalali (1992) a new species of monogenean,
Dogielius molnari, in the Dez River, both in Khuzestan. Jalali et al. (1995) describe a new species of monogenean, Dactylogyrus pallicirrus, from fish taken in the Dez River near
Ahvaz.
Economic importance
Al-Mehdi and Khan (1984) report this species to be important in riverine and culture fisheries in northern Iraq. Ündar et al. (1990) identify this species and Garra rufa as the
"doctor fish" of the Kangal hot spring in Turkey (Timur et al., 1983; Warwick and Warwick, 1989; Kürkçüoğlu and Öz, 1989; and various newspaper and television reports). High
water temperatures reduce the amount of plankton available as fish food and the fish nibble away infected skin of humans who bathe in these waters. The fish is known as "striker" (and Garra
rufa as "licker") from its behaviour in the spa pools. The healing properties are linked to the high level of selenium (1.3 p.p.m.) in the water, selenium being beneficial in some
skin diseases, and possibly to UV light. The fish facilitate the action of the selenium and UV light by softening and clearing away psoriatic plaque and scale, exposing the lesions to the water and
sunlight. However, some lesions are made worse and the fish can cause some new ones.
Conservation
This species is widely distributed in southern areas, particularly Khuzestan, and does not appear to be under threat other than that suffered by all species by pollution and water abstraction.
Endangered in Turkey (Fricke et al., 2007).
Further work
See comments above on the need for further work to distinguish this species from C. kais, especially when young and below for distinction from C. tenuiradius.
Sources
Type material: See above, Cyprinion macrostomum (NMW 52805, 52806), C. neglectus (NMW 52807).
Iranian material:
Tigris basin: and presumably macrostomum
CMNFI 1979-0268, 13, 92.2-122.4 mm standard length, Lorestan, Dez or Karkheh drainage between Nowqan and Khorramabad (no other locality data);
CMNFI 1979-0269, 4, 104.7-110.6 mm standard length, Lorestan, Dez or Karkheh drainage between Nowqan and Khorramabad (no other locality data);
CMNFI 1979-0270, 10, 85.5-122.4 mm standard length, Lorestan, Kashkan River drainage (33º26'N, 48º19'E);
CMNFI 1979-0271, 3, 100.7-144.8 mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0273, 9, ? mm standard length, Lorestan, Kashkan River drainage (33º26'N, 48º19'E);
CMNFI 1979-0274, 14, ? mm standard length, Lorestan, Kashkan River drainage (33º27'N, 48º11'E);
CMNFI 1979-0275, 2, 142.4-165.0 mm standard length, Lorestan, Kashkan River drainage (33º25'N, 47º58'E);
CMNFI 1979-0278, 4, 93.5-114.1 mm standard length, Lorestan, Kashkan River drainage (33º34'N, 48º01'E);
CMNFI 1979-0279, 9, 100.3-149.4 mm standard length, Lorestan, Khorramabad River (33º37'N, 48º18'E);
CMNFI 1979-0283, 5, 93.0-144.0 mm standard length, Kermanshahan, Qareh Su drainage (34º21'N, 47º07'E);
CMNFI 1979-0287, 1, 112.6 mm standard length, Kermanshahan, Chashmeh Javari 2 km from Ravansar (ca. 34º42'N, ca. 46º40'E);
CMNFI 1979-0288, 1, 94.3 mm standard length, Ilam and Poshtkuh, Gangir River at Juy Zar (33º50'N, 46º18'E);
CMNFI 1979-0289, 4, ? mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0290, 11, 49.3-133.0 mm standard length, Kermanshahan, Diyala River drainage at Qasr-e Shirin (34º31'N, 45º35'E);
CMNFI 1979-0291, 15, ? mm standard length, Kermanshahan, Diyala River drainage (34º24'N, 45º37'E);
CMNFI 1979-0350, 18, ? mm standard length, Khuzestan, Marun River near Marun (30º39'30"N, 50º02'E);
CMNFI 1979-0355, 1, ? mm standard length, Khuzestan, stream tributary to Karun River at Salmaneh (30º35'N, 48º22'E);
CMNFI 1979-0356, 1, ? mm standard length, Khuzestan, stream at Hoveyzeh (31º27'N, 48º04'E);
CMNFI 1979-0360, 2, ? mm standard length, Khuzestan, canal branch of Karkheh River (31º40'N, 48º35'E);
CMNFI 1979-0361, 3, ? mm standard length, Khuzestan, jube in Karkheh River drainage (31º42'N, 48º33'E);
CMNFI 1979-0363, 1, ? mm standard length, Khuzestan, Karkheh River (31º52'N, 48º20'E);
CMNFI 1979-0364, 2, ? mm standard length, Khuzestan, river at Abdolkhan (31º52'30"N< 48º20'30"E);
CMNFI 1979-0365, 24, ? mm standard length, Khuzestan, stream in Doveyrich River drainage (32º25'N, 47º36'30'E);
CMNFI 1979-0366, 16, ? mm standard length, Khuzestan, stream west of Dehloran (32º45'30"N, 47º05'30"E); ID?
CMNFI 1979-0367, 2, ? mm standard length, Khuzestan, Meymeh River 11 km north of Dehloran (32º44'30"N, 47º09'30"E) ID?
CMNFI 1979-0368, 12, ? mm standard length, Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
CMNFI 1979-0371, 1, ? mm standard length, Khuzestan, stream in Karkheh River drainage (32º05'N, 48º19'E);
CMNFI 1979-0373, 12, ? mm standard length, Khuzestan, Bala River north of Andimeshk (32º35'N, 48º17'E);
CMNFI 1979-0374, 46, ? mm standard length, Khuzestan, stream tributary to Bala River (32º40'N, 48º15'E);
CMNFI 1979-0376, 9, ? mm standard length, Khuzestan, river tributary to Karkheh River (32º48'30"N, 48º04'30"E);
CMNFI 1979-0378, 10, ? mm standard length, Khuzestan, stream tributary to Karkheh River (ca. 32º48'N, ca. 48º04'E);
CMNFI 1979-0379, 11, ? mm standard length, Khuzestan, Dez River (32º12'N, 48º27'E);
CMNFI 1979-0380, 5, ? mm standard length, Khuzestan, stream tributary to Dez River (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0381, 28, ? mm standard length, Khuzestan, stream 40 km west of Shushtar (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0382, 67, ? mm standard length, Khuzestan, Karun River at Shushtar (32º03'N, 48º51'E);
CMNFI 1979-0383, 1, ? mm standard length, Khuzestan, stream in Ab-e Shur drainage (31º59'30"N, 49º06'E);
CMNFI 1979-0384, 7, 86.3-152.2 mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0386, 4, ? mm standard length, Khuzestan, stream 21 km from Haft Gel (ca. 31º34'N, ca. 49º23'E);
CMNFI 1979-0387, 6, ? mm standard length, Khuzestan, stream 12 km from Haft Gel, Jarrahi River drainage (31º25'N, 49º38'E);
CMNFI 1979-0388, 2, ? mm standard length, Khuzestan, Zard River (31º19'N, 49º44'E);
CMNFI 1979-0390B, 23, 36.2-156.2 mm standard length, Khuzestan, stream 3km south of Bagh-e Malek (31º29'N, 49º54'30"E);
CMNFI 1979-0391, 1, 154.5 mm standard length, Khuzestan, stream in Marun River drainage (31º28'N, 49º51'E);
CMNFI 1979-0392, 5, ? mm standard length, Khuzestan, Zard River (ca. 31º32'N, ca. 49º48'E);
CMNFI 1979-0393, 2, 96.9-116.6 mm standard length, Khuzestan, Jarrahi River drainage (31º18'N, 49º37'E);
CMNFI 1979-0394, 1, 130.2 mm standard length, Khuzestan, stream in Marun River drainage (31º01'N, 49º45'E);
CMNFI 1979-0395, 4, ? mm standard length, Khuzestan, stream in Marun River drainage (ca. 30º57'N, ca. 49º51'E);
CMNFI 1979-0396, 1, ? mm standard length, Khuzestan, Kheyrabad River (30º32'N, 50º23'30"E); ID?
CMNFI 1979-0398, 23, ? mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh, stream in Zohreh River drainage (30º24'30"N, 50º37'30"E); ID?
CMNFI 1979-0399, 7, ? mm standard length, Fars, stream in Zohreh River drainage (30º19'30"N, 51º15'E);
CMNFI 1991-0153, 1, 171.3 mm standard length, Khuzestan, Zohreh River (no other locality data);
CMNFI 1991-0154, 1, 109.9 mm standard length, Khuzestan, Hawr al-Azim (ca. 31º45'N, ca. 47º55'E);
CMNFI 1993-0128, 1, 110.7 mm standard length, Kermanshahan, Sarab-e Sabz 'Ali Khan (34º25'N, 46º32'E);
CMNFI 1993-0149, 1, 121.7 mm standard length, Khuzestan, Karun River (no other locality data);
CMNFI 2007-0111, 6, 24.7-173.8 mm standard length, Kermanshahan, Alvand River near Sar-e Pol-e Zahab (ca. 34º36'N, ca. 45º56'E);
CMNFI 2007-0112, 6, 46.5-118.8 mm standard length, Kermanshahan, Kerend River basin near Shahabad-e Gharb (ca. 34º06'N, ca. 46º30'E;
CMNFI 2007-0113, 1, 122.1 mm standard length, Kermanshahan, Razavar River, Qareh Su tributary (ca. 34º25'N, ca. 47º01'E);
CMNFI 2007-0115, 6, 59.7-154.8 mm standard length, Kermanshahan, Qareh Su basin (ca. 34º34'N, ca. 46º47'E);
CMNFI 2007-0116, 12, ?-93.0 mm standard length, Kermanshahan, Gav Masiab basin west of Sahneh (ca. 34º28'N, ca. 47º36'E);
CMNFI 2007-0117, 1, ? mm standard length, Kermanshahan, Gav Masiab basin near Sahneh (ca. 34º24'N, ca. 47º40'E);
BM(NH) 1980.8.28:1, 1, 90.3 mm standard length, Khuzestan, Dezful (32º23'N, 48º24'E);
BWC95-20, 14, ? mm standard length, Khuzestan, Rud Zard at Rud Zard (31º22'N, 49º43'E);
Gulf fish:- ? tenuiradius
CMNFI 1979-0020, 56, ?, mm standard length, Fars, Mand River outside Kavar (29º11'N, 52º41'E);
CMNFI 1979-0054, 14, 37.4-64.1 mm standard length, Fars, Shur River tributary (ca. 28º58-29º03'N, ca, 52º34-35'E);
CMNFI 1979-0075, 123, 21.3-142.4 mm standard length, Fars, Mand River at Pol-e Kavar (29º11'N, 52º41'E);
CMNFI 1979-0109, 5, 63.2-100.2 mm standard length, Fars, Mand River at Shahr-e Khafr (28º56'N, 53º14'E);
CMNFI 1979-0128, 7, 19.2-103.8 mm standard length, Shur River (28º51'N, 52º31'E);
CMNFI 1979-0131, 19, 16.4-41.7 mm standard length, Fars, Ab-Arak River (28º38'N, 52º49'E);
CMNFI 1979-0132, 72?, 15.2-100.1 mm standard length, Fars, Ab-Arak River (28º35'N, 52º58'E);
CMNFI 1979-0133, 50, 45.6-95.5 mm standard length, qanat stream near Qir (28º27'30"N, 53º03'E);
CMNFI 1979-0135, 18, 21.8-49.2 mm standard length, Mand River tributary (28º08'N, 53º10'E);
CMNFI 1979-0157, 4, 23.6-85.4 mm standard length, Fars, qanat stream at Hadiabad (28º52'N, 54º13'E); macrostomum?
CMNFI 1979-0193, 1, 36.3 mm standard length, Fars, river 8 km from Darab (28º45'N, 54º27'30"E); macrostomum?
CMNFI 1979-0195, 1, ? mm standard length, Fars, jube west of Darab (ca. 28º54'N, ca. 53º53'30"E);
CMNFI 1979-0196, 1, 59.9 mm standard length, Fasrs, qanat and pool at Khanehnehrin (28º50'N, 53º31'30"E); not on data sheet check jar?
CMNFI 1979-0197, 1, 51.3 mm standard length, Fars, spring nd stream 33 km from Fasa (28º45'N, 53º25'E);
CMNFI 1979-0198, 23, 22.3-57.7 mm standard length, Fars, stream at Tadovan (28º47'N, 53º24'30"E);
CMNFI 1979-0200, 8, 29.0-46.1 mm standard length, Fars, Mand River tributary (28º36'N, 53º36'30"E);
CMNFI 1979-0202, 12, ? mm standard length, Fars, Mand River (29º01'N, 53º00'E);
CMNFI 1979-0241, 18, 43.8-72.6 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0347, 2, 105.2-106.7 mm standard length, Fasr, Pol-e Berengie (29º27'30"N, 52º32'E);
CMNFI 1979-0348, 4, 52.9-79.1 mm standard length, Fars, stream at Somduldul (ca. 29º28'N, ca. 52º32'E);
CMNFI 1979-0404, 25, 20.2-127.9 mm standard length, Bushehr, stream 33 km south of Kaki (28º08'N, 51º47'E);
CMNFI 1979-0405, 4, 33.5-36.7 mm standard length, Hormozgan, stream about 13 km north of Rostaq (28º29"N, 54º59'E); ID?
CMNFI 1979-0497, 1, 85.6 mm standard length, Fars, Mand River at Band-e Bahman (29º11'N, 52º40'E);
CMNFI 1979-0501, 17, 18.7-91.0 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1979-0504, 6, ?-93.0 mm standard length, Fars, stream at Pol-e Gaz in Lake Maharlu basin (no other locality data);
CMNFI 1979-0789, 1, 164.6 mm standard length, Fars, Lake Parishan (29º45'N, 53º40'E);
CMNFI 1993-0141, 1, 64.4 mm standard length, Bushehr, Dalaki River (29º28'N, 51º15'E); ID?
CMNFI 2007-0061, 2, ? mm standard length, Fars, qanat pool at Ab-e Barik (ca. 27º52'N, ca. 54º09'E);
CMNFI 2007-0063, 6, ? mm standard length, Fars, Mand River outside Jahrom (28º36'N, 53º37'E);
USNM 205890, 2, 46.0-48.7 mm standard length, Fars, Lake Parishan (29º45'N, 53º40'E);
ZSM 25705, 1, 107.0 mm standard length, Fars, Lake Parishan (29º45'N, 53º40'E).
Comparative material:- CMNFI 1980-0811, 2, 82.6-112.4 mm, Turkey, Akziyaret Deresi, Tigris River system (no other locality data);
BM(NH) 1931.12.21:1-2, 2, 69.5-78.5 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1974.2.22:1184, 1, 130.2 mm standard length, Iraq, Sulaimaniyah ();
BM(NH) 1974.2.22:1196, 1, 53.0 mm standard length, Hawiya Canal, Lesser Zab ();
BM(NH) 1974.2.22:1214-1255 (in part), Khalis (33º49'N, 44º32'E).
Cyprinion milesi
(Day, 1880)
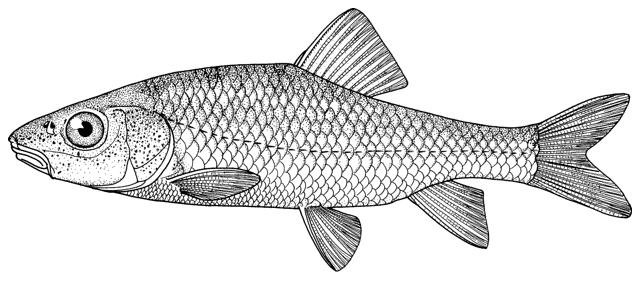
Common names
None.
[sabzug in Pakistan].
Systematics
Barbus milesi was described from "a spring at Tràl", Pakistan.
Berg (1949), Mirza (1969), Mirza et al. (1991) and Howes (1982) recognise this species as valid. If so, synonyms according to Berg (1949), would be Barbus bampurensis Nikol'skii, 1899
described from "Flum. Bampur", Scaphiodon daukesi Zugmayer, 1912 from "Irrigation channels and pools near Panjgur, Baluchistan, Pakistan", and Barbus baschakirdi Holly,
1929 from "Ein Bach bei Guadjik am Wege von Sarzeh in Biabun nach Darpahan in den Bergen von Baschakird, Südostpersien" (= a brook at Guadjik on the way from Sarzeh in Biabun to Darpahan in the
Baschakird Mountains, southeast Persia).
Much of my material from southeastern Iran was assigned by me to C. watsoni. Specimens that resemble C. milesi (lacking a shallowly arched or sector mouth with a horny edge but having an
oblique u-shaped mouth) are found at the same sample localities as typical C. watsoni. The mouth structure of the putative C. milesi resembles that of juvenile C. watsoni, possibly
retained in the adult (paedomorphosis). A Principal Components Analysis does not separate these two forms when the mouth characters are not included in the analysis.
A specimen in the Naturhistorisches Museum Wien under NMW 52736, 34.4 mm standard length, is listed as a syntype under the name Cirrhina milesi but its locality is Gwadur, Hubb River and the
type status may be an error.
Five syntypes of Barbus bampurensis, 32.0-64.8 mm standard length, are in the Zoological Institute, St. Petersburg (ZISP 11715) from "Flum. Bampur, 15-23.VII.1898, Zarudnyi". The jar
label gives a date of 15-19.VII.1898.
The holotype of Barbus baschakirdi, 52.2 mm standard length, is in the Naturhistorisches Museum Wien under NMW 13798 and a cotype (syntype) of Scaphiodon daukesi, 102.8 mm standard
length, is under NMW 19784.
Scaphiodon daukesi types in Munich were destroyed in World War II but one syntype is in the Naturhistorisches Museum Wien under NMW 19784, and two syntypes are in the Zoological Survey of India,
Calcutta under ZSI F8028/1 and 8032/1 ((Menon and Yazdani, 1968; Eschmeyer et al., 1996; Neumann, 2006).
Key characters
The mouth is characteristically oblique, longer in lateral view than C. watsoni.
Morphology
The oblique mouth reaches back to the anterior eye margin in small fish and to the rear of the nostril in larger fish. Dorsal fin with 3 unbranched and 10-13 branched rays, anal fin with 2 unbranched
and 7 branched rays, pectoral fin with 14-16 branched rays and pelvic fin with 7-8 branched rays. Total gill rakers 11-12. The following description is based mostly on Barbus bampurensis types.
Dorsal fin spine strong and serrated, with large teeth in small fish. Lateral line scales 34-39. The scaleless groove before the dorsal fin is weakly expressed. Scales are present on the belly of large
fish, almost absent on small fish. Upper flank scales may be regularly or irregularly arranged. Scales have few to no anterior radii, numerous posterior radii, numerous fine circuli, a subcentral
anterior focus, and an anterior scale margin indented above and below the mid-line. A pelvic axillary scale is present. The head is more massive in relation to the body than for similar size C.
watsoni/kirmanense specimens. The barbel is quite stubby at the base but tapers rapidly to the tip in larger fish. The type series of Barbus bampurensis (= C. milesi) has dorsal fin
branched rays 10(4) or 11 (1), anal fin branched rays 7(5), pectoral fin branched rays 14(1) or 15(3) (one unclear), pelvic fin branched rays 7(1) or 8(4), lateral line scales 34(1), 36(1) and 37(3), and
total gill rakers 11(3) or 12 (2). Two fish from Sib (see below) had dorsal fin branched rays 9(1) or 10(1), anal fin branched rays 7(2), pectoral fin branched rays 15(2), pelvic fin branched rays 6(1)
or 7(1), lateral line scales 35(1) or 37(1), pharyngeal teeth 4,3,2 on the left side, total gill rakers 13(1) or 14 (1), and total vertebrae 38(1) or 39(1). Pharyngeal teeth have a slight hook on the
anteriormost tooth with the rest in the main row with scooped-out crowns.
Sexual dimorphism
Tubercles line the anal fin rays and are apparent on the snout in males.
Colour
Copper-brown on the back and upper flank fading to a pinkish belly. Fins are pink and the lateral line has a bright orange streak along it. The preopercle also has orange-golden spots as does the base
of the pectoral fins. There is a dark blotch at the base of the caudal fin. The caudal fin base bears a spot in small specimens and there are some much smaller, irregular spots on the caudal peduncle.
Peritoneum brown to black.
Size
Attains about 19.0 cm.
Distribution
In Iran, it is recorded from the Sarbaz River of the Makran according to Saadati (1977), the Bampur River of the Hamun-e Jaz Murian basin according to Berg (1949) and the Dozdan River of the Hormuz
basin (H. R. Esmaeili). Also in the Mashkid River basin in Pakistan and in rivers draining to the Indian Ocean.
Zoogeography
See under the genus.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
The distribution, abundance and biology of this species in Iran is poorly known and an assessment for conservation status cannot be given.
Further work
See above.
Sources
Type material: See above, Barbus bampurensis (ZISP 11715),Barbus baschakirdi (NMW 13798) and Scaphiodon daukesi (NMW 19784).
Iranian material: BM(NH)1883.8.2:2-3, 2, 72.2-130.9 mm standard length, Baluchestan, Sib near Dizak (27º15'N, 62º05'E).
BWC97-4 no fish on cat sheet?
Comparative material: BM(NH) 1889.2.1:263-264, 2, 89.3-108.7 mm standard length, Afghanistan (no other locality data).
Cyprinion tenuiradius
Heckel, 1849
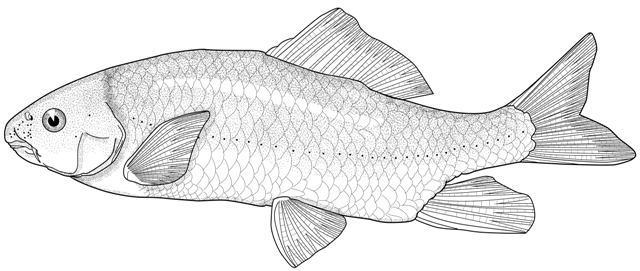
Common names
[Araxes kingfish (Fricke et al., 2007)]
Systematics
The type locality is the "Kara-Agatsch als aus dem Araxes" (= Qarah Aqaj River and the Kor River, Fars). Sometimes spelt tenuiradiatus (e.g. in Rainboth (1981) but this is incorrect).
Syntypes of Cyprinion tenuiradius are in the Naturhistorisches Museum Wien according to Kähsbauer (1964) under NMW 52808 (1 specimen, 116.7 mm standard length), 52809 (2, 52.3-58.0 mm standard
length), 52811 (4, 42.7-47.4 mm standard length), 52815 (1, 77.0 mm standard length) and 52816 (2, 75.5-80.8, although Kähsbauer lists only 1 while Banarescu and Herzig-Straschil
(1995) list 2 as also found by me). Other material marked as syntypes from the "Kara-Agatsch. Th. Kotschy" includes NMW 52810 (2 , 103.7-110.0 mm standard length), NMW 52812 (2, 103.5-104.8 mm
standard length), NMW 52813 (2, 97.7-103.1 mm standard length), NMW 52814 (1, 114.9 mm standard length), and 52817 (1, not examined). The catalogue in Vienna lists 8 specimens in one column and 26 in the
adjacent column. Eschmeyer et al. (1996) add 2 fish from the Araxes River, formerly in NMW, now at the Rijksmuseum van Natuurlijke Historie, Leiden under RMNH 2486. The lectotype as selected by
F. Krupp in 1984 is NMW 52814 and is published by Banarescu and Herzig-Straschil (1995) with NMW 52808, 52809, 52810, 52811, 52812, 52813, 52815 and 52816 as paralectotypes.
Karaman (1971) assigns this taxon as a subspecies of Cyprinion macrostomum and Bianco and Banarescu (1982) suggest it may be a subspecies in a polytypic species. Berg (1949) records it from the
Tigris River where it may be sympatric with C. macrostomum. He considers it to be close to that species, perhaps its southeastern subspecies. Howes (1982) considers tenuiradius to be a
variant of C. macrostomum.
Heckel (1846-1849b) distinguishes this species from C. macrostomum by a lower scale count (35-36 as opposed to 42; Berg (1949) gives 35-38 as opposed to 37-43); Krupp (1985c) gives 34-38
compared to 39-43 in macrostomum; Banarescu and Herzig-Straschil (1995) give 36-38, rarely 35 or 39 in C. tenuiradius compared to 41-44, rarely 40 or 45 in C. macrostomum), slenderer
body, and a much thinner dorsal spine which is soft in its distal third. The mouth is arched and there is some lower lip development at the mouth corner as in C. kais (see illustrations in Krupp
(1985c)). In addition, Berg (1949) gives a branched dorsal fin ray count of 12-13 in C. tenuiradius, 13-15 in C. macrostomum, although Banarescu and Herzig-Straschil (1995) give (12)13-15
for C. tenuiradius from the type locality of Kara-Agasch (sic). Krupp (1985c) states that tenuiradius has a smaller number of scale radii than macrostomum, radii are
divergent and the posterior scale margin is curved. However, data for specimens examined by me show overlaps in meristic characters; although means differ, individual fish would be difficult to
distinguish on counts alone.
The question then arises as to whether tenuiradius is distinct from macrostomum or merely a variant of a wide-ranging, variable species. The only absolute character is a weaker dorsal
fin spine based on examination of type material; other, meristic characters overlap and minor variations in body form are difficult to quantify given a wide range of habitats (lowland rivers
and marshes versus highland streams) which may affect shape. The species tenuiradius is retained here as distinct but would benefit from further analyses using new characters, if available, from
molecular data.
Key characters
Distribution and a weak spine distinguish this taxon. The dorsal fin spine in macrostomum has teeth extending further along the spine, teeth are more well-developed even near the tip. Spine
teeth in tenuiradius are more graded in size as they near the tip and are finer than in macrostomum.
Morphology
Dorsal fin with 4 unbranched and 11-15 branched rays (Berg (1949) has 12-13). The anal fin has 3 unbranched and 6-8 branched rays, usually 7. In 199 Iranian fish, 96.5% have 7 anal fin rays with the
rest having 6 rays and 1, presumably anomalous fish, with 9 rays. Pectoral fin branched rays 13-18, pelvic fin branched rays 7-9. Lateral line scales 32-39. Gill rakers 10-21. Scales on the belly may be
small and skin covered. There is a naked dorsal keel in front of the dorsal fin, although the area behind the occiput may be scaled and the groove begins nearer the dorsal fin. The mouth is transverse to
more or less curved. The dorsal fin spine is weak and serrated only half way or two-thirds of its length. The chromosome number is 2n=50, comprising 13 metacentric, 5 submetacentric and 7 subtelocentric
chromosomes pairs. Arm number is NF=86 (Esmaeili and Piravar, 2006).
Meristics for fish from Persian Gulf drainages of Fars, Bushehr and Hormozgan provinces including the Lake Maharlu endorheic basin:- dorsal fin branched rays 11(4), 12(51), 13(175), 14(74) or 15(9)
(mean = 13.1, S.D. = 0.746); pectoral fin branched rays 13(3), 14(38), 15(117), 16(41), 17(2) or 18(1)(mean = 15.0, S.D. = 0.733); pelvic fin branched rays 7(23), 8(177) or 9(3)(mean = 7.9, S.D. = 0.345);
total gill rakers 10(2), 11(16), 12(27), 13(24), 14(49), 15(35), 16(20), 17(14), 18(8), 19(3) or 21(1)(many counts are based on small specimens and may be low accordingly in comparison with Tigris River
basin fishes; mean = 14.2, S.D. = 2.003); and lateral line scales 32(1), 33(15), 34(28), 35(41), 36(47), 37(56), 38(13) or 39(2)(mean = 35.7, S.D. = 1.431).
Sexual dimorphism
Unknown.
Colour
Overall colour is yellowish-white with a light grey back. Scale bases on the flank above the lateral line are brown. The pectoral and pelvic fins have an orange-yellow spot at their base.
Size
Reaches 16.3 cm (Berg, 1949).
Distribution
This species is found in the Gulf and Lake Maharlu basins in Iran (Bianco and Banarescu, 1982; M. Rabbaniha, pers. comm., 1995; Abdoli, 2000).
Heckel (1849) records this species as from the "Araxes", the modern Kor River in Fars. However, the catalogue sheets in Vienna for the types only list the "Kara Agatsch" (= Mand
River) and no subsequent collections have been made of this species in the internal Kor River basin although Abdoli (2000) also maps it from the middle to lower Kor River, possibly based on Heckel's
report. Berg (1949) records it from the Tigris River basin, perhaps in error, and Fricke et al. (2007) have it in Turkey from the Aras River system of eastern Turkey (presumably a
confusion of the modern Aras or Araxes River with the classical Araxes or Kor River of Fars).
Zoogeography
See under the genus.
Habitat
Unknown in detail but found in springs, streams and rivers of varying descriptions.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 40 fish measuring 5.04-13.49 cm fork length. The a-value was 0.0139 and the b-value 3.063
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
The distribution, abundance and biology of this species in Iran is poorly known and an assessment for conservation status cannot be given. Endangered in Turkey (Fricke et al., 2007) but
probably does not occur there.
Further work
See above.
Sources
Type material: See above, Cyprinion tenuiradius (NMW 52808, 52809, 52810, 52811, 52812, 52813, 52814, 52815, 52816).
Iranian material: ? see above and ID
Cyprinion watsoni
(Day, 1872)
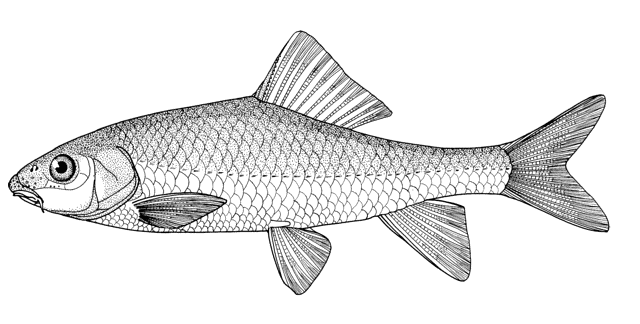
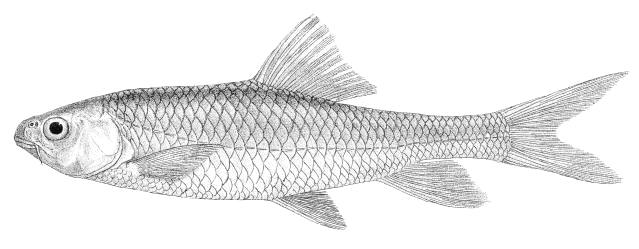
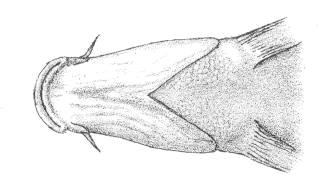
ventral head
Common names
None.
[sehrgoar; sabzug = watsoni and microphthalmum - all in Pakistan].
Systematics
Scaphiodon irregularis Day, 1872 described from "rivers in the Sind hills", India, probably Scaphiodon microphthalmus Day, 1880 from "Quetta", Scaphiodon
muscatensis Boulenger, 1887 from Muscat, Oman, Cirrhina afghana Günther, 1889 from "Nushki (N. Baluchistan)" and "small river at Kushk (N.W. Afghanistan), Badghis",
Cyprinion kirmanense Nikol'skii, 1899 from "Schur-Ab in Kirmano orient.", Cirrhina afghana var. nikolskii Berg, 1905, Scaphiodon macmahoni Regan, 1906,
Scaphiodon baluchiorum Jenkins, 1910 (see below for type locality), Scaphiodon watsoni var. belense Zugmayer, 1912 from the "Purali River, near Las Bela" (in Pakistani
Baluchistan), Scaphiodon readingi Hora, 1923 from the "Salt Range, Punjab", India, and Cyprinion microphthalmum infraspecies nikolskii Berg, 1949 described
originally in part as Cirrhina afghana var. nikolskii Berg, 1905, and Semiplotus dayi Fowler, 1958 are synonyms.
Semiplotus dayi was coined by Fowler to replace Scaphiodon aculeatus, a misidentification by Day (1880) for Chondrostoma aculeatum (= Capoeta aculeata). Fowler thought that
Day's fish represented a new species which he named Semiplotus dayi. Howes (1982) considers Semiplotus dayi to be a synonym of Capoeta capoeta (since Karaman (1969a) synonymises
Scaphiodon aculeata with C. capoeta. Day's Scaphiodon aculeatus is placed in the synonymy of Cyprinion microphthalmum infraspecies nikolskii by Berg (1949).
Syntypes (or at least specimens examined by Day) of Scaphiodon watsoni described from rivers on the Sind Hills and the Salt Range of the Punjab, India are in the Zoological Survey of India,
Calcutta under ZSI 2596 (1), the Natural History Museum, London under BM(NH) 1889.2.1.370-9 (10, but 14 in jar September 2007, 35.6-93.4 mm standard length), the Australian Museum, Sydney under B.7751 (1),
the Zoölogisch Museum, Universiteit van Amsterdam under ZMA 115.924 (2) and ZMA 119.255 (1), the Naturhistorisches Museum Wien under NMW 51671 (1), NMW 51672 (1) and NMW 51673 (1), the Museum für
Naturkunde, Universität Humboldt, Berlin under ZMB 11042 (1)(132.6 mm standard length), the Rijksmuseum van Natuurlijke Historie, Leiden under RMNH 8704 (1) (or possibly 2552), the Zoological Institute,
St. Petersburg under ZISP 8278 (4 but only 2 fish found by me, 63.6-79.6 mm standard length), and the Field Museum of Natural History, Chicago under FMNH 2303
? 2302(4, 34.0-72.5 mm standard length as examined
by me) (Whitehead and Talwar, 1976; Nijssen et al., 1993; Eschmeyer et al., 1996; Ferraris et al., 2000). The 3 fish in the Naturhistorisches Museum Wien measure 86.6, 80.8 and 93.3
mm standard length respectively and are listed there as syntypes.
ZISP 8279 comprising 3 fish, 51.5-52.1 mm standard length, has the same data as ZISP 8278 and may also be types. It is not clear if these are all types, those in ZISP not being marked as types and
those in BM(NH) being marked as "possible types"; they may include material simply collected by Francis Day.
A cotype of Scaphiodon watsoni var. belense (NMW 19833) measures 136.9 mm standard length. Eschmeyer et al. (1996) report 2 fish under NMW 19833 although the Vienna card index in
1997 lists only one syntype under this number. In the Zoological Survey of India, Calcutta there are single syntypes under ZSI F827/1 (a misprint for 8027), ZSI F8029/1, ZSI F8030/1 and ZSI F8031/1 (see
also Menon and Yazdani (1968)). The remainder of 42 syntypes were in the Munich Museum but were destroyed in World War II (Neumann, 2006).
Types of Scaphiodon microphthalmus are probably lost. The species was described from 2 specimens taken at Quetta in Pakistan. One specimen was sent to the Florence Museum but a recent search
failed to locate it and the other specimen has not been located (Whitehead and Talwar, 1976; Banister and Clarke, 1977). A fish measuring 130.1 mm standard length in the Naturhistorisches Museum
Wien is listed as a possible syntype (NMW 55897) and in the 1997 card index as "? holotype" (sic).
Note Howes (1982) and Mirza et al. (1991) consider Cyprinion microphthalmum to be a valid species with muscatensis, afghana, afghana var. nikolskii and
baluchiorum as synonyms. Howes places macmahoni in watsoni rather than microphthalmum as Berg (1949) and Mirza (1969) do. Howes (1982) also includes irregularis,
kirmanense, and readingi in watsoni.
A syntype of Scaphiodon irregularis is in the Australian Museum, Sydney under AMS B.7883 (Ferraris et al., 2000). Syntypes of Scaphiodon muscatensis are in the Natural
History Museum, London under BM(NH) 1885.11.7:35-40 (6, 66.4-89.3 mm standard length) and BM(NH) 1887.11.11:289-291 (3, 72.1-79.3 mm standard length) (Eschmeyer et al., 1996; personal observations).
Syntypes of Scaphiodon readingi are in the Zoological Survey of India, Calcutta under ZSI F10353/1 and ZSI 10354/1 (27) (sic, although the catalogue numbers seem to indicate only 2 fish)
(Menon and Yazdani, 1968) and in the Zoological Museum of Moscow University (ZMMU) (P-1588) (Pavlinov and Borissenko, 2001).
Three syntypes of Scaphiodon baluchiorum (ZSI F9398 to F9400) and one syntype of Scaphiodon macmahoni (ZSI F1239/1) are in the Zoological Survey of India, Calcutta (Menon and Yazdani,
1968). A syntype of Scaphiodon macmahoni measuring 58.6 mm standard length from "Seistan" is in the Natural History Museum, London and was labelled as Cyprinion watsoni (BM(NH)
1905.11.29:27, 58.6 mm standard length). The type locality of Scaphiodon baluchiorum is "Gishtigan (Bampusht); Kalagan, 3,500 feet; Baluchistan". These localities are in Pakistani
Baluchistan; Gishtigan being on the Kulushta River which drains into the Nihing River and then the Dashti River (Jenkins, 1910) (these are near the border of Iranian Baluchestan with the upper reaches
of the Nihing being in Iran) and Kalagan possibly being the Kalugar River with headwaters in Iran and draining to the Hamun-i Mashkel in Pakistan. The type locality of Scaphiodon macmahoni is
"affluents of the Helmand" (Regan, 1906), presumably an error for "effluents" or the delta of the Helmand.
The holotype of Cyprinion kirmanense, 61.6 mm standard length, is in the Zoological Institute, St. Petersburg under ZISP 11712 from "Schur-Ab in Kirmano orient. 27.VI." The 5
syntypes of Cirrhina afghana var. nikolskii are in the Zoological Institute, St. Petersburg (ZISP 11709) and are from the "Bampur River, 27 VII 1898, N. Zarudnyi" according to
Berg (1949) but he mentions 2 additional fish with a somewhat deeper body, presumably also part of the type series. ZISP 11709 does have 7 specimens, 43.0-79.1 mm standard length, with a date
15-27.VII.1898. Four syntypes of Cirrhina afghana measuring 74.6-83.0 mm standard length from "Kushk" annotated Afghan. Boundary Comm. are in the Natural History Museum, London (BM(NH)
1886.9.21:150-154 - note that 150-154 indicates there should be 5 fish) with a further 6 syntypes measuring 44.9-99.5 mm standard length labelled "Nushki" and also annotated Afghan. Boundary
Comm. (BM(NH) 1886.9.21:155-159 - note this indicates there should be 5 fish in this jar and probably one fish has been mixed up). Additional syntypes are in the Zoological Survey of India, Calcutta
under ZSI 11474-11476 (3) and ZSI 11479-11485 (7) (Eschmeyer et al., 1996).
Berg (1949) places Cirrhina afghana var. nikolskii in his Cyprinion microphthalmum infraspecies nikolskii (see also Berg (1933a)). This infraspecies occurs together with
Cyprinion microphthalmum but differs by a stronger osseous ray in the dorsal fin which is serrated almost to the summit (Berg (1949) states that transitions exist). The anterior belly region is
scaleless also. ZISP 11709 fish mostly have their dorsal spines snapped off but one fish has osseous ray teeth between three-quarters and four-fifths along the spine and a second about three-quarters.
ZISP 25406 from a qanat between Kerman and Bandar-e `Abbas comprises 12 fish, 31.0-53.6 mm standard length, belonging to infraspecies nikolskii according to Berg (1949). These fish, of all sizes,
have the last quarter to a third of the osseous spine in the dorsal fin unserrated. The mouth form varies. One large fish has a terminal mouth, moderately oblique in lateral view, and no strong horny
layer on the lower jaw. Others have a u-shaped or horny jaw positioned on the lower head surface so there is no real gape in lateral view. Some small fish are transitional between the two types. Fin
serration, mouth form and development of scales on the anterior belly seem to be widely variable within samples of Cyprinion from a single locality and presumably a single species.
Berg (1949) recognises Cyprinion watsoni belense as a subspecies, rather than a variety as originally described, from Indian Ocean drainages of southeastern Iran and southwestern Pakistan
(Baluchistan). It is distinguished by smaller scales (33-36) from the type form (31-34), hardly a sufficient criterion given the wide distribution range and individual variation shown by these fishes.
This species has not been adequately examined in southeastern Iran and most nominal species are referred to Cyprinion watsoni, the earliest available name for the taxon. C. watsoni is
distinguished from other Iranian Cyprinion by having 9-11 dorsal fin branched rays (macrostomum and tenuiradius have 12-15; C. milesi also has a low dorsal ray count but has
an oblique mouth, not transverse or arched (Berg, 1949)). Bianco and Banarescu (1982) consider that several subspecies may eventually be defined and that some of the names in synonymy here would then be
used.
Berg (1949) also recognises C. irregulare as a distinct species with a low dorsal fin branched ray count as in C. watsoni but usually 37 or more scales in the lateral line, a scaleless
groove on the back before the dorsal fin, and upper scale rows anteriorly arranged irregularly and not imbricate and C. microphthalmum with a low dorsal fin branched ray count as in C.
watsoni but usually 37 or more scales in the lateral line, a scaleless groove on the back before the dorsal fin barely outlined, and upper scale rows anteriorly arranged regularly and imbricate.
C. microphthalmum infraspecies nikolskii is described as having a strong dorsal fin spine with obvious teeth extending to the tip while typical C. microphthalmum has a weak
ray with weak teeth only visible when the skin covering the fin is peeled away.
Berg (1949) later states that no great importance should be attached to the upper row scale arrangement and the groove development - if the groove is well-developed then the upper row scales are
irregular and this phenomenon can be seen in some C. watsoni and C. microphthalmum specimens. Berg then suggests that irregulare could be regarded as an infraspecies of C.
microphthalmum as this type of condition occurs in Capoeta fusca and in Garra rossica. Under the heading C. watsoni Berg also gives mouth shape, scale arrangement, dorsal fin
spine serrations, and body form as characters which can vary greatly. These observations serve to confirm the great variability in characters for these fishes. Large series of adults and young would be
needed to adequately define species and subspecies.
Mirza (1969) reports C. watsoni, C. microphthalmum and C. milesi from western Pakistan and Iran, the former in Makran drainages and the latter two in the Mashkel (= Mashkid) River
basin. The characters used to separate these taxa are an oblique mouth and head length contained less than 4.5 times in total length (= C. milesi), an arched mouth, head length more than 4.5
times in total length, scaleless strip on back conspicuous, and 33-36 lateral line scales (= C. watsoni), and a transverse mouth, head length more than 4.5 times in total length, scaleless strip
on back hardly visible, and 37-40 lateral line scales (= C. microphthalmum). Sample sizes in this study were small (22 fish) and these characters show considerable variation in larger samples and
between fish of different sizes.
Key characters
The arched mouth and and 9-12, usually 10-11, branched dorsal fin rays serve to identify this species.
Morphology
Young fish have a more horseshoe-shaped mouth than larger and older fish where the mouth is a shallow arch, almost straight. The dorsal fin has 3-4 unbranched and 9-12 branched rays, the last
unbranched ray of the dorsal fin being variably serrated and thickened. The extent of serrations appears to vary independently of size, from half to three-quarters or more of the spine length. The anal
fin has 1-3, usually 3, unbranched and 6-8, usually 7 branched rays. In Iranian specimens, 89.7% of 419 fish had 7 branched anal fin rays, the remainder having 6 branched rays. Pectoral fin branched rays
11-18, usually 15-16, and pelvic fin branched rays 6-9, usually 8. Lateral line scales 31-43. Scales have well-developed anterior radii as well as posterior and some lateral radii. The scale focus is
almost central on mid-flank scales. There is a naked median strip on the back in front of the dorsal fin, about one scale wide, in some fish. Some fish may show poor imbrication of scales on the belly
and upper anterior flank. Gill rakers 8-18, reaching to or past the adjacent raker when appressed. Total vertebrae 38. Rarely with a tripartite swimbladder, usually bipartite (Mirza, 1971 - for his
C. microphthalmum). Pharyngeal teeth 2,3,4-4,3,2 or 2,3,5-5,3,2, with spoon-shaped crowns. The back in front of the dorsal fin is naked in the mid-line. Mouth shape variable, from and arch to a
transverse cutting edge. Scales on the belly can be embedded in the skin, obvious or even absent.
Meristics for Iranian specimens:-
|
Locality/Dorsal Fin Rays |
9 |
10 |
11 |
12 |
x |
S.D. |
|
Hamun-e Mashkid |
|
16 |
3 |
|
10.2 |
0.375 |
|
Hamun-e Jaz Murian |
3 |
15 |
5 |
|
10.1 |
0.596 |
|
Dasht-e Lut |
2 |
50 |
4 |
|
10.0 |
0.328 |
|
Makran |
1 |
29 |
3 |
|
10.1 |
0.348 |
|
Hormuz |
2 |
144 |
124 |
7 |
10.5 |
0.562 |
|
Sirjan |
1 |
3 |
7 |
|
10.5 |
0.688 |
|
Locality/Pelvic Fin Rays |
6 |
7 |
8 |
9 |
x |
S.D. |
|
Hamun-e Mashkid |
|
6 |
13 |
|
7.7 |
0.478 |
|
Hamun-e Jaz Murian |
|
1 |
21 |
1 |
8.0 |
0.302 |
|
Dasht-e Lut |
|
1 |
54 |
|
8.0 |
0.135 |
|
Makran |
|
|
33 |
|
8.0 |
0.000 |
|
Hormuz |
2 |
31 |
237 |
8 |
7.9 |
0.400 |
|
Sirjan |
|
2 |
9 |
|
7.8 |
0.405 |
|
Locality/Pectoral Fin Rays |
11 |
13 |
14 |
15 |
16 |
17 |
18 |
x |
S.D. |
|
Hamun-e Mashkid |
|
|
|
9 |
10 |
|
|
15.5 |
0.513 |
|
Hamun-e Jaz Murian |
|
|
1 |
8 |
11 |
2 |
1 |
15.7 |
0.864 |
|
Dasht-e Lut |
1 |
|
2 |
14 |
31 |
8 |
|
14.8 |
0.879 |
|
Makran |
|
|
1 |
14 |
15 |
3 |
|
15.6 |
0.704 |
|
Hormuz |
|
1 |
46 |
111 |
99 |
20 |
1 |
15.3 |
0.863 |
|
Sirjan |
|
|
4 |
4 |
3 |
|
|
14.9 |
0.831 |
|
Locality/Total Gill Rakers |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
x |
S.D. |
|
Hamun-e Mashkid |
|
|
1 |
|
4 |
3 |
4 |
3 |
4 |
|
|
13.8 |
1.718 |
|
Hamun-e Jaz Murian |
|
|
3 |
4 |
10 |
4 |
1 |
|
|
1 |
|
12.0 |
1.492 |
|
Dasht-e Lut |
|
1 |
7 |
8 |
16 |
12 |
10 |
2 |
|
|
|
12.2 |
1.427 |
|
Makran |
|
|
1 |
6 |
9 |
4 |
8 |
3 |
|
1 |
1 |
13.0 |
1.794 |
|
Hormuz |
3 |
7 |
33 |
45 |
64 |
60 |
41 |
10 |
2 |
1 |
|
12.2 |
1.569 |
|
Sirjan |
|
|
2 |
2 |
1 |
5 |
1 |
|
|
|
|
12.1 |
1.375 |
|
Locality/Lateral Line Scales |
33 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
x |
S.D. |
|
Hamun-e
Mashkid |
|
|
1 |
3 |
9 |
|
1 |
1 |
1 |
2 |
1 |
|
|
|
Hamun-e
Jaz Murian |
|
|
1 |
9 |
11 |
1 |
1 |
|
|
|
|
|
|
|
Dasht-e
Lut |
|
|
5 |
12 |
26 |
10 |
2 |
1 |
|
|
|
|
|
|
Makran |
|
|
2 |
1 |
17 |
8 |
4 |
|
1 |
|
|
|
|
|
Hormuz |
1 |
2 |
17 |
57 |
99 |
72 |
20 |
7 |
3 |
|
|
|
|
|
Sirjan |
|
|
|
3 |
4 |
3 |
1 |
|
|
|
|
|
|
? vertebrae
The holotype of Cyprinion kirmanense has 10 dorsal fin branched rays, 7 anal fin branched rays, 15 pectoral fin branched rays, 8 pelvic fin branched rays, 37 lateral line scales and 13 total
gill rakers (not in above tables).
Sexual dimorphism
Males have snout tubercles and tubercles on the anal fin rays (Regan, 1906; Jenkins, 1910; Berg, 1949). Large tubercles are found on the snout in front of the nostrils, the top of the head, and in rows
on the rays of the caudal and anal fin, following the ray branching, in a fish not yet fully mature (40.1 mm standard length). ZMB 11042 (132.6 mm SL) has tubercles thickly present on the snout extending
back to the nostrils and then to the eyes, scattered all over the sides of the head, absent on top of the head (may be lost in this old specimen) and large tubercles on anal fin rays near the tip. There
is a depression in front of the nostrils in adult males.
Colour
The back and upper flank are dark or copper brown, golden or dark olive, light green-brown or brown-grey, sometimes with bluish or orange tinges, fading to a light or yellowish pink on the lower flanks
and belly. The flank scales are silvery and may be outlined in black. Black spots may be present along the flank as may be an orange stripe or a series of 7-9 orange spots above the lateral line
anteriorly. The orange colour may be deep, almost red. Occasionally, there may be an orange spot below the lateral line. There may be a vertical orange line over the cleithrum or a spot at its
postero-ventral corner. In some fish the whole cleithrum area is red-orange. The operculum, preoperculum and cheek can be iridescent blue. The bases of the pectoral and pelvic fins and part of the
operculum may also be pink or orange-coloured. Young have a fine black streak above the lateral line. The dorsal and caudal fins are lead-coloured to black and other fins are pink to yellowish. All the
fins except the paired fins may be hyaline. There can be a black caudal base spot, quite marked in some fish, particularly small ones. The peritoneum is black or dark brown.
Size
Attains 23 cm (Zugmayer, 1912).
Distribution
From southeastern Iran east to India. In Iran, it is recorded from the
Dasht-e Lut, Hormuz, Hamun-e Jaz Murian, Sistan,
Hamun-e Mashkid basins and in the Makran from the Jagin to the Bahu Kalat rivers (Berg, 1949;
Spillman, 1972; Bianco and Banarescu, 1982; Abdoli, 2000; Esmaeili et al.,
2011?).
Zoogeography
The occurrence of this species (as C. microphthalmum in Banister and Clarke (1977)) in Oman across the Straits of Hormuz is a result of the 120 m lowered sea level from 100,000 B.P. to 10,000
B.P. The Tigris-Euphrates then ran down the Gulf and presumably provided ready access across it. Banister and Clarke (1977) comment that it is surprising that only one species made the crossing but
nothing is known of the climate during this 90,000 year period nor of the composition of the Iranian fish fauna. It may well have been quite impoverished. Hora (1956) describes fish paintings on pots
from Nal in Pakistani Baluchistan dating from the third millenium B.C. One of the species not clearly represented is a Cyprinion, now found in that area (not extinct as Banister (1980) would have
it). This potentially shows how the ichthyofauna in Southwest Asia can change over relatively short periods of time; the changes over 100,000 years must have been considerable and not readily traceable.
Dorsal fin branched rays have moderately strong modes of 10 for the
Hamun-e Mashkid,
Hamun-e Jaz Murian, Dasht-e Lut and Makran basins but modes of 10 and/or 11 for Hormuz and Sirjan, the westernmost basins.
C. watsoni may be showing some introgression with Cyprinion species to the west which have higher counts. However, subsamples within the Hormuz basin do not show a clear pattern of
higher modes or means towards the west.
Habitat
Kiabi and Abdoli (2000) found this species to be the commonest and to have the widest range in Hormozgan Province.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 23 Iranian fish measuring 8.34-13.38 cm total length. The a-value was 0.0101 and the b-value
2.952 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Gut contents are primarily herbivorous items including filamentous algae such as Cladophora and Spirogyra, and a wide range of diatoms but some insect material is also found (Mirza,
1969; Farooq et al., 1996).
Reproduction
Spawning takes place in Pakistan at Islamabad from mid- to late March to mid-April (Shaikh and Jalali, 1989, 1991) and near Islamabad (33.3°N, 73.0°E) in April and May (Shaikh and Hafeez, 1993).
Gonads begin to develop in December as photoperiod and temperature rise but a continuing warm temperature is the predominant factor for spawning to occur; a fall in temperature halts spawning.
Eggs are dark yellow when mature, testes creamy when ripe. Spawning occurs once a year. Up to 150 eggs are recorded in fish from the Ab Garm-e Ganow with a diameter of 1.2 mm.
Parasites and predators
Males were reported as having snout tubercles and tubercles on the anal fin rays (Regan, 1906) but these were the encysted glochidia of a unionid mollusc (B. Prashad in Annandale and Hora (1920).
Jalali et al. (1995) describe a new species of monogenean, Dactylogyrus pallicirrus, from fish taken in the Shur River, a Halil River tributary in the
Hamun-e Jaz Murian basin. Jalali et al. (2005) summarise the occurrence of Gyrodactylus species in Iran and record Gyrodactylus sp. for fish from the Minab and Halil rivers.
Economic importance
This species is of no economic importance although Butt (1995) suggests that it could be a food source in Pakistan, occurring in shoals of considerable size in rivers that otherwise support little in
the form of aquatic protein. It could be cultured as food and as a forage fish. This species has been used to study the effects of heavy metals in Pakistan (Shah, 2002). Higher concentrations of copper
and zinc caused lethargy and loss of equilibrium.
Conservation
This species is widely distributed in various basins in southeastern Iran and neighbouring areas and does not appear to under any threat.
Further work
The biology of this species requires study and a thorough review of morphology in relation to named taxa should be carried out.
Sources
Type material: See above and note reservations on type status of some, Cirrhina afghana (BM(NH) 1886.9.21:150-154, BM(NH) 1886.9.21:155-159); Cirrhina afghana var. nikolskii
(ZISP 11709); Cyprinion kirmanense (ZISP 11712); Scaphiodon macmahoni (BM(NH) 1905.11.29:27); Scaphiodon microphthalmus (NMW 55897); Scaphiodon muscatensis
(BM(NH) 1885.11.7:35-40, BM(NH) 1887.11.11:289-291); Scaphiodon watsoni (FMNH 2303, NMW 51671, NMW 51672 and NMW 51673, ZMB 11042, ZISP 8278, ZISP 8279); Scaphiodon watsoni var.
belense (NMW 19833).
Iranian material: CMNFI 1979-0138, 49, ?-66.8 mm standard length, Fars-Hormozgan border, stream in Rasul River drainage (ca. 27º32'N, ca. 54º58'30"E);
CMNFI 1979-0143, 22, ? mm standard length, Hormozgan, marsh in Hasan Langi River drainage (27º21'N, 56º50'30"E);
CMNFI 1979-0144, 76, ? mm standard length, Hormozgan, Minab River at Minab (27º09'30"N, 57º04'E);
CMNFI 1979-0145, 139, ? mm standard length, Hormozgan, Geru River south of Minab (26º55'N, 57º01'30"E);
CMNFI 1979-0149, 54, ?-93.8 mm standard length, Hormozgan, stream north of Bandar Abbas (27º36'N, 56º14'E);
CMNFI 1979-0150, 34, 43.8-101.6 mm standard length, Hormozgan, stream at Gohreh (27º45'N, 56º05'E);
CMNFI 1979-0152, 10, ? mm standard length, Homozgan, Shur River drainage (28º09'N, 55º43'E);
CMNFI 1979-0153, 31 24.5-84.7 mm standard length, Fars, qanat stream and pool at Qaleh-ye Biabani (28º31'N, 54º53'E);
CMNFI 1979-0154B, 49, ? mm standard length, Fars, stream channels at Koorsiah (28º45'30"N, 54º24'E);
CMNFI 1979-0155, 26, ? mm standard length, Fars, springa t gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0156, 12, ? mm standard length, Fars, qanat at Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0167, 25, ? mm standard length, Kerman, qanat at Bam (29º06'N, 58º20'E);
CMNFI 1979-0168, 50, ?-93.8 mm standard length, Kerman, qanat at Shahabad (29º07'N, 58º16'E);
CMNFI 1979-0173, 15, 26.5-84.0 mm standard length, Hormozgan, qanat at Hajjiabad (28º19'N, 55º54'E);
CMNFI 1979-0176, 1, ? mm standard length, Hormozgan, Sarzeh River (27º30'30"N, 56º15'30"E);
CMNFI 1979-0180, 5, ? mm standard length, Hormozgan, stream 3 km east of Essin (27º19'N, 56º17'30"E);
CMNFI 1979-0181, 19, ? mm standard length, Hormozgan, Kul River (27º17'30"N, 56º03'30"E);
CMNFI 1979-0183, 14, ? mm standard length, Hormozgan, stream in Rasul River drainage (27º11'30"N, 55º42'E);
CMNFI 1979-0185, 4, ? mm standard length, Hormozgan, stream in Rasul River drainage (27º06'N, 55º45'E);
CMNFI 1979-0186, 11, ? mm standard length, Hormozgan, strean and pools at Sar Khun oasis (ca. 27º24'30"N, ca. 56º25'E);
CMNFI 1979-0187, 54, 18.9-73.6 mm standard length, Hormozgan, stream and pools at Sar Khun oasis (27º23'30"N, 56º26'E);
CMNFI 1979-0188, 18, ? mm standard length, Hormozgan, jube at Gohreh (27º45'N, 56º05'E);
CMNFI 1979-0189, 25, ? mm standard length, Hormozgan, jube and pool on road to Darab (27º08'30"N, 55º42'E);
CMNFI 1979-0190, 44, ?-83.3 mm standard length, Fars-Hormozgan border, spring and pool at Galah Tuyeh (ca. 28º32'N, ca. 55º14'E);
CMNFI 1979-0191, 35, 36.6-86.5 mm standard length, Fars, stream 10 km east of Furg (ca. 28º16'N, ca. 55º18'E);
CMNFI 1979-0219, 19, 19.1-33.0 mm standard length, Kerman, jube 14 km west of Jiroft (28º37'N, 57º41'E);
CMNFI 1979-0220, 4, 28.0-65.5 mm standard length, Kerman, jube 2 km south of Jiroft (28º39'N, 57º43'E);
CMNFI 1979-0309, 2, 101.2-108.5 mm standard length, Kerman, Fahraj River at Azizabad (28º57'N, 58º42'E);
CMNFI 1979-0310, 1, ? mm standard length, Baluchestan, qanat at Bazman (27º49'N, 60º12'E);
CMNFI 1979-0311, 10, ? mm standard length, Baluchestan, Bampur River at Malakabad (27º11'N, 60º27'E);
CMNFI 1979-0312, 39, ? mm standard length, Baluchestan, dam on Bampur River (27º11'N, 60º36'E);
CMNFI 1979-0313, 68, ? mm standard length, Baluchestan, Bampur River at Bangharabad (27º20'N, 60º46'E);
CMNFI 1979-0314, 10, 25.5-118.4 mm standard length, Baluchestan, qanat at Karavandar (27º50'N, 60º46'E);
CMNFI 1979-0315, 71, ? mm standard length, Baluchestan, Bampur River 2 km north of Karavandar (27º51'N, 60º46'E);
CMNFI 1979-0316, 22, 14.5-69.8 mm standard length, Baluchestan, stream 68 km south of Iranshahr (26º48'N, 61º02'E);
CMNFI 1979-0317, 11, 16.5-118.6 mm standard length, Baluchestan, Sarbaz River at Bondan (26º35'N, 61º13'E);
CMNFI 1979-0318, 11, ? mm standard length, Baluchestan, Sarbaz River at Huvar (26º09'N, 61º27'E);
CMNFI 1979-0323, 6, ? mm standard length, Baluchestan, Sarbaz River (ca. 26º26'N, ca. 61º16'E);
CMNFI 1984-0324, 4, ?39, ?51.9-117.8 mm standard length, Baluchestan, Bampur River at Sa'idabad (27º11'N, 60º22'E);
CMNFI 1979-0325, 7, ? mm standard length, Baluchestan, qanat at Espakeh (26º51'N, 60º14'E);
CMNFI 1979-0326, 10, ? mm standard length, Baluchestan, stream south of Pip (ca. 26º35'N, ca. 60º02'E);
CMNFI 1979-0327, 10, 24.0-62.4 mm standard length, Baluchestan, stream in Geh River drainage (26º32'N, 59º57'E);
CMNFI 1979-0329, 82, ? mm standard length, Baluchestan, stream at Zaminbandan (27º02'N, 61º20'E);
CMNFI 1979-0331, 25, 13.1-50.3 mm standard length, Baluchestan, qanat in Saravan (27º22'N, 62º20'E);
CMNFI 1979-0332, 9, 20.8-33.3 mm standard length, Baluchestan, qanat at Kalapurkan (27º14'N, 62º33'E);
CMNFI 1979-0334, 4, 26.3-38.4 mm standard length, Baluchestan, Mashkid River (27º04'N, 62º54'E);
CMNFI 1979-0335, 2, 66.8-72.2 mm standard length, Baluchestan, qanat at Esfandak (27º07'N, 62º50'E);
CMNFI 1979-0338, 17, ? mm standard length, Baluchestan, Tahlab River drainage 8 km from Mirjaveh (28º58'N, 61º24'E);
CMNFI 1979-0339, 24, 24.5-76.9 mm standard length, Baluchestan, Tahlab River drainage 16 km from Mirjaveh (28º56'30"N, 61º21'E);
CMNFI 1979-0411, 8, ? mm standard length, Hormozgan, Minab River (27º24'N, 57º12'E);
CMNFI 1979-0412, 22, 22.0-122.2 mm standard length, Hormozzgan, spring at Saras (27º30'N, 57º34'E);
CMNFI 1979-0415, 5, ? mm standard length, Hormozgan, stream south of Ab Garm-e Ganow (27º17'30"N, 56º20'E);
CMNFI 1979-0416, 2, 40.1-55.9 mm standard length, Hormozgan, Ab Garm-e Ganow (ca. 27º26'N, ca. 56º20'E);
CMNFI 1979-0418, 5, 58.2-111.2 mm standard length, Hormozgan, river near Kahkom (28º09'N, 55º43'E);
CMNFI 1991-0141, 3, ?, mm standard length, ; see original sheet?
CMNFI 2007-0031, 12, ? mm standard length, Baluchestan, headwater of Bampur River (27º51'N, 60º46'E);
CMNFI 2007-0033, 15, ? mm standard length, Baluchestan, Rusgay qanat in Iranshahr (27º13'N, 60º41'E);
CMNFI 2007-0034, 3, ? mm standard length, Baluchestan, headwater stream on road to Zaboli (ca. 26º58'N, ca. 61º27'E);
CMNFI 2007-0036, 8, ? mm standard length, Baluchestan, qanat at Bazman (27º49'N, 60º12'E);
CMNFI 2007-0037, 7, 62.4-166.3 mm standard length, Kerman, Hosseinabad and Gamatabad qanats at Bam (29º06'N, 58º21'E);
CMNFI 2007-0038, 9, 62.8-101.2 mm standard length, Kerman, Mehtiabad qanat at Bam (29º06'N, 58º21'E);
CMNFI ?, 11, 46.5-69.1 mm standard length, ; 44 ? check, Sirjan on data sheet but no fish at this number in catalogue, check shelf
CMNFI 2007-0049, 11, ? mm standard length, Hormozgan, ditches in upper Kol River basin at Hajjiabad (ca, 28º19'N, ca. 55º55'E); 45
CMNFI 2007-0050, 4, 61.2-92.4 mm standard length, Hormozgan, ditches in upper Kol River basin at Hajjiabad (ca. 28º19'N, ca. 55º55'E); 46
CMNFI 2007-0051, 7, 54.6-84.3 mm standard length, Hormozgan, upper kol River basin at Hajjiabad (28º19'N, 55º55'E); 47
CMNFI 2007-0052, 2, 70.7-92.3 mm standard length, Hormozgan, ditch at Qotbabad (27º46'N, 56º06'E); 48
CMNFI 2007-0055, 15, 24.5-75.3 mm standard length, Hormozgan, headwtaer stream in Minab River basin (ca. 27º47'N, ca. 57º12'E); 51
CMNFI 2007-0056, 14, 30.2-70.4 mm standard length, Kerman, qanat at Kahnuj (27º58'N, 57º45'E); 52
CMNFI 2007-0059, 9, ? mm standard length, Fars, Chashmeh Barashk (ca. 27º24'N, ca. 54º06'E);
CMNFI 2007-0060, 3, 56.2-93.7 mm standard length, Fars, Chashmeh Ab-e Shirin near Lar (ca. 27º41'N, ca. 54º17'E); 57ID?
BM(NH) 1883.8.2:4-9, 5, 42.4-114.9 mm standard length, Baluchestan, Jalq (27º36'N, 62º42'E);
BM(NH) 1883.8.2:20-25, 6, 23.1-85.2 mm standard length, Baluchestan, Sib near Dizak (27º15'N, 62º05'E);
NMW uncatalogued, 19, 18.1-52.9 mm standard length, Hormozgan, Ab Garm-e Ganow (ca. 27º26'N, ca. 56º20'E).
BWC97-4, ?, ? mm standard length, Kerman, Halil River (no other locality data); no fish on cat sheet?
BWC97-5, 1, ? mm standard length, Hormozgan, Hasan Langi River (no other locality data);
Comparative material: CAS 28722, 1, 117.4 mm standard length, India, Punjab, Salt Range, Katas Nallah (no other locality data);
Genus Cyprinus
Linnaeus, 1758
The carp genus is found in Europe and Asia and comprises several
species of which one has been widely introduced as a food fish.
This genus is characterised by a compressed but heavy body, large size,
rounded snout, 2 pairs of barbels, large molar pharyngeal teeth in 3
rows, a very long dorsal fin with the last unbranched ray spine-like
and serrated, the anal fin short but with the last unbranched ray
spine-like and serrated, the gut is moderately long, and the dorsal
and lateral skull bones are sculptured.
Cyprinus carpio
Linnaeus, 1758
Common names
كپور (= kopur, kapur or kapoor (in Gilaki)), kapur-e ainehi (= mirror
carp), كپور معمولي
(kapur-e ma'mouli or mar'mulleh or ma'muli or maamoli (= common carp)), rashti or
كپور رشتي (kapur-e Rashti) in Khuzestan (because their origin was Rasht),
mahi-ye gul (= flower fish, meaning in this sense a good fish).
[car or carp shaeeh in Arabic; caki in Azerbaijan; geitan-tsatsan or dliter, both in Armenia;
sazan for wild and karp for cultured carp in Russian; common carp, European carp, German carp,
wild carp; mirror carp, leather carp, line carp, naked carp (last four referring
to scalation), koi (aquarium variety)].
Systematics
Cyprinus Carpio was originally described from Europe. Cyprinus
carpio var. caspicus Walbaum, 1792 is described from the mouth of the
Volga and Don rivers but is infrasubspecific and the name has not been used in
Iran nor has Cyprinus carpio fluviatilis Pravdin, 1945 described from
floodplain lakes of the Volga River near Saratov. Mousavi Gel Sefid et
al. (2007) give some morphometric and meristic charcters of fish from
the Anzali Lagoon.
Key characters
This species is easily identified by the long dorsal fin, the spine
in both the dorsal and anal fins, and the two pairs of barbels.
Morphology
Dorsal fin with 2-5 unbranched rays followed by 14-23, usually
18-20, branched rays, anal fin with 2-4, usually 3, unbranched rays
followed by 3-7, usually 5, branched rays, pectoral fin branched rays
13-19, and pelvic fin branched rays 5-9, usually 8. The dorsal fin has the last
unbranched ray developed as a toothed spine and the anal fin has a
similar spine. Lateral line scales 26-41, mostly 36-39. Scales may be
absent (leather carp), restricted to a few, enlarged scales (mirror
carp), or only a mid-lateral row of scales (line carp), in cultivated varieties. Wild carp are fully scaled. Individual
scales have a central focus, wavy anterior margin, few radii on the
anterior and posterior fields in young fish, and medium numbers of
radii on fish 12-14 cm standard length. There are numerous fine
circuli and the posterior scale field breaks up into bubble-like
structures. Gill rakers 17-29 (some literature counts may be lower arm
of arch only and there may be size-related variation too) and vertebrae 32-39
(lower counts may not include Weberian vertebrae). Rakers touch the
second raker below when appressed and have a row of knobs on their
medial surface. Pharyngeal teeth 1,1,3-3,1,1 with variants 1,2,3-3,2,1, 1,2,3-3,1,1, 1,1,1,3-3,1,1,
1,1,3-2,1,1 and 1,3-3,1,1. Posterior major row teeth
are large with flattened crowns bearing wavy ridges while more
anterior teeth are a rounded knob, or even concave on top of the knob.
The gut is elongate with several coils. This species is a tetraploid
(2n=98-104)(Al-Sabti, 1986; Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin branched
rays 16(1), 17(5), 18(18), 19(16), 20(10); anal fin branched rays 4(1)
or 5(49); pectoral fin branched rays 14(1), 15(7), 16(25), 17(16) or
18(1); pelvic fin branched rays 6(1), 7(6) or 8(43); lateral line
scales 34(2), 35(6), 36(10), 37(26) or 38(6); total gill rakers 18(1),
20(2), 21(11), 22(11), 23(15), 24(4), 25(4) or 26(2); total vertebrae
36(1), 37(19), 38(26) and 39(6); and pharyngeal teeth ?. Note that these samples may include
individuals which are not native but introduced for fish farming from
stocks outside Iran.
There are morphometric and meristic differences between carp from
the southwestern and southeastern Caspian Sea but these are ecological
not taxonomic. Also, carp from the Anzali Mordab differ from those in
the sea by having a longer head, snout, eye and postorbital region
(although of course some of these are redundant), greater interorbital
width, shorter predorsal and preanal distances, shorter dorsal, anal,
pectoral and caudal fins, and a lower anal fin (A. M. Shukolyukov in
Berg (1948-1949)). Yousefian (2004) found carp from the Caspian Sea in Iran had
a dominant genotype different from those in a fish farm. Meristics (scales and
fin rays) and morphometrics (head length and body width) also differed.
Sexual dimorphism
Females are deeper bodied than males because of their eggs and the
distance between the pectoral and pelvic fins and the pelvic and anal
fins is more. Dorsal and anal fins in males are higher, the anal fin
is longer at the base, the pectoral fin is longer and the lobes of the
caudal fin are longer. This is accounted for by the greater swimming
activity of males during spawning (Kuliyev and Agayarova, 1984).
Breeding males have fine tubercles on the head, particularly on the anterior
operculum and preoperculum and under the eye, above the lateral line and more
frequently below it, and on the fin rays.
Colour
In semi-diadromous carp from the Kura region of the southwest
Caspian Sea, the overall body colour is dark yellow, the flanks being
golden-yellow with dark shading, and the back is black. The belly and
fins are light yellow and the caudal fin is reddish. Lake populations
are darker. Young fish from Iran are silvery on the flanks (but not as
bright as Carassius auratus), greyish on the back, silver-pearl
on the belly, the iris is silvery with grey above and below, the
dorsal fin and upper caudal lobe are pale grey, the lower caudal lobe
and anal fin are orange, the pelvic fin is pale orange, and the
pectoral fin has only traces of orange. The caudal fin may be
yellow-orange with lobe margins red. The freshwater resident form in
the Anzali Mordab is yellowish, the semi-diadromous form dark. The
peritoneum is grey to silvery and may be speckled.
Size
Carp resident in fresh waters are smaller than semi-diadromous
carp. In the 1950s in Iran, carp catches were 20-41 cm long (Farid-Pak,
no date). Fish up to 1.0 m long are caught in the Caspian basin (A.
Abdoli, pers. comm., 1995). Maximum size exceeds 1.2 m and 44.9 kg, possibly to
1.5 m and 69.6 kg.
Distribution
Widely introduced in the Middle East for aquaculture. Found naturally in Iran in the whole Caspian Sea drainage, it is
also widely stocked in the provinces of Gilan and Mazandaran (Petr, 1987; Abbasi et al.,
1999). It is reported from the Atrak, Gorgan, Gharasu, Tajan, Babol, Aras,
and Safid rivers, the Anzali Mordab and the Siah-Keshim Protected Region, Gorgan Bay, the southeast
Caspian Sea, southwest Caspian Sea and south-central Caspian Sea as an
introduced species, and in all these plus the Haraz and Pol-e Rud
rivers as a native species (Riazi, 1996; Kiabi et al., 1999).
It is probably native to the Tedzhen River of Turkmenistan (the
Hari River or Harirud in Iran) (Aliev et al., 1988). This
species is also recorded from the Karakum Canal and Kopetdag Reservoir
in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) and
may eventually reach Iranian waters in the Tedzhen (= Hari) River
basin. These latter stocks may be introduced.
It has been introduced to the Sistan basin to a canal flowing into the Chahnimeh, the Sistan Dam,
Hamun Lake and the Hamun
Kushk (Ahmadi and Wossughi, 1988; J. Holčík, in litt., 1996; Shamsi
et al., 2009)), to the Karaj River in the Namak Lake basin, to the
Mahabad Dam (Abdi, 1999; www.mondialvet99.com, downloaded 31 May 2000), to the Haft Barm lakes near Shiraz in 1984 (Petr,
1987), to the Manjil Reservoir on the Safid Rud (Nümann, 1966), to Mehr Dasht
Lake, Markazi, to the Zayandeh River Dam (Y. Keivany, in litt., 1992; Ghorbani Chafi, 2000), to
Lake Zaribar, Kordestan (Abzeeyan, 5(5):III, 1994), to Lake Famur
from nearby fish farms (Maafi, 1996b), to the Kor River basin in Fars (A.
Alamdari, in litt., 1997; M. Hafezieh, in litt., 1997), throughout Khuzestan
Province (not caught in the 1970s), to the Kol River, Hormozgan (Bagheri et
al., 2010), and to numerous other water bodies and ponds throughout Iran
where it reproduces in the wild. Mirror carp found in the Shadegan
marshes of Khuzestan are escapees from fish farms. Carp in the Bahu Kalat and Sarbaz
rivers of Baluchestan are also escapees from fish farms (A. Mobaraki, pers. comm., June 1999).
Abdoli (2000) records this species as present generally in the
Dasht-e Lut, Sistan,
Dasht-e Kavir and Kerman-Na'in basins; in lower reaches of rivers from the Gorgan to the Astara and along the Caspian coast,
the middle Aras River; the Kashaf River in Khorasan; the middle and lower Talkheh
River and Tatavi River in the Orumiyeh basin; the Qareh Chai
and Qom rivers in the Namak Lake basin; the middle to lower Zayandeh River
in the Esfahan basin; the Aravand, lower Karun, Jarrahi,
lower Karkheh rivers in the Tigris River basin; the middle Halil River in the
Hamun-e Jaz Murian basin; and the middle and upper Kul River including the Shur River
tributary in the Hormuz basin. Jalali et al. (2005) records this species from the Dez, Karun and
Zayandeh rivers, the Vahdat reservoir, and the Zarivar and Kaftar lakes. Abdoli
and Naderi (2009) record it from the Atrak, Gorgan, Ghararasu, Tajan, Babol,
Aras and Safid rivers, the Anzali Talab, Gorgan Bay, and the southwest,
southeast and south-central Caspian Sea.
Zoogeography
The natural distribution of the carp is supposed to be Asia Minor
and the Caspian Sea basin where its origins lie in the late Pliocene.
From this area, modern wild carp spread east and west, perhaps as late
as the last postglacial thermal optimum, and latterly aided by man (Balon,
1974; 1995; Van Damme et al., 2007). Iranian carp may be a mixture of native and introduced
stocks (or species - see Kottelat (1997) - but this remains
unresolved). It is probably not now possible to distinguish the native
stocks morphologically because of admixtures of farmed stocks. All domestic forms
probably originated from native Danube River stocks (Bănărescu, Barus and Peňáz
in Bănărescu and Paepke, 2002). Khalili and Amirkolaie (2010) compared
fish electrophoretically, meristically and morphologically from the Anzali
lagoon, Qareh Su and Bandar Gaz. Morphometric differences between east and west
were attributed to a relatively smaller body size in the east and the influence
of domestic stocks. Electrophoretic differences, particularly obvious at Anzali,
were attributed to the larger number of fish farms in this latter area.
Habitat
This species favours an abundance of soft vegetation in shallow
water, necessary for successful reproduction. Still waters are preferred but
they are found in the lower courses of lowland rivers with moderately flowing
water, and occasionally in water exceeding 2 m/sec. They can often be seen basking at the surface or feeding on
algae and their dorsal fins break the water surface. Large fish often
move into shallows in the afternoon and evening. Carp also leap from
the water but the reason is unknown. They rarely descend below 30 m in
lakes and avoid fast water in streams. Carp overwinter in depressions
in the lower reaches of rivers in the Caspian basin. There are both
freshwater resident populations in the Caspian basin and diadromous
(or semi-diadromous) ones, the latter living in brackish water near
river mouths and only entering fresh water to spawn. The migration up the Ural
River may reach 60 km from the river mouth. Some fish
apparently spawn in the brackish waters of shallow coastal areas in the Caspian.
Knipovich (1921) reports fish in the Iranian Caspian Sea down to
11.9-12.3 m. Riazi (1996) and Karimpour (1998) report that this
species is both native (resident) to, and migrates to, the Siah-Keshim
Protected Region of the Anzali Mordab.
Carp have a salinity tolerance under experimental conditions of up
to 8‰, and for short periods 18.6‰ with acclimation, and
this has significance for survival of carp in the Caspian Sea and in
waters of southern Iran and Iraq where this species is farmed. Hafezamini and
Oryan (2002) and Hafezamini et al. (2003) however found that, under
experimental conditions, all fish in their study died at 18‰ in less than 12
hours. Carp eggs hatch in water up to 10‰, with the favourable level being
up to 6.6‰ (Al-Hamed, 1971).
Low dissolved oxygen concentrations of 3 mg/l are tolerated and levels as low
as 0.5 mg/l can be withstood for 2-3 hours. Normal growth has occurred
in fish kept at 35°C. The immune response of carp following exposure to the
organophosphate malathion has been studied by Soltani et al. (2003).
This species is considered to be a dominant species in the Karun
River along with Barbus (= Tor) grypus (Iranian Fisheries Research
and Training Organization Newsletter, 17:1, 1997).
Introduced to Iraqi waters in 1960 as juveniles, this species
rapidly became established (Ahmed and Taher, 1988). Cyprinus carpio
was caught in large numbers in the Shatt al Arab of Iraq down to the
estuary after an increase in the discharge of the Tigris River reduced
salinity (N. A. Hussain, in litt., 1994).
Age and growth
Fatemi et al. (2009) examined fish from beach
seines along the Caspian shore of Iran for 2006-2007. Ten age groups were
recorded and the catch was dominated by fish aged 4-5 years old. Growth
parameters based on scale reading were FL∞
= 71.52 cm and K = 0.16 per year for the total population, FL∞
= 70.54 cm and K = 0.15 per year for males, and FL∞
= 72.00 cm and K = 0.16 for females. Growth parameters based on
length-frequency analysis gave values of FL∞
= 72.0, 69.3 and 73.0 cm and K = 0.18, 0.15 and 0.18 per year respectively. The
total (Z), natural (M) and fishing (F) mortalities were 0.71, 0.29 and 0.42 per
year respectively for sexes combined. Exploitation (E) was 0.59 for sexes
combined and no further fishing pressure was recommended.
Females are larger and mature a year later than males. Sexual
maturity is attained in the second year of life, and in a few
individuals even by the end of the first year, in the southeastern
Caspian Sea; but in the southwestern Caspian Sea this occurs in the
third and fourth years (Kuliyev and Agayarova, 1984). Resident carp in
Dagestan mature in their third year at about 30 cm and have an average
life span of 6 years whereas the semi-anadromous or semi-diadromous
form matures in its fourth year at 35-36 cm and has an average life
span of 8 years (Shikhshabekov, 1969). Growth is faster in the Kura
River of Azerbaijan than in other populations in the Caspian but
maturity is later at age 4 or more usually at 5 years. Freshwater
residents in the Anzali Mordab are slow-growing compared to the semi-diadromous
form and are less common. Fish taken in the commercial operations in
Iran were 3-7 years old, 31.0-63.0 cm long and weighed 539-3375 g (Razivi
et al., 1972).
Mousavi Gel Sefid et al. (2007) give some growth equations for fish from
the Anzali Lagoon.
This species was stocked in the Dukan and Derbendikhan dams of Iraq in the 1960s
where fish up to 3 years of age were reported by Ciepielewski et al.
(2001). Decreasing growth rates indicate conditions are not too favourable
although growth in the first two years is comparable with that in lakes of
central Iraq. The condition coefficient (K) was higher among smaller fish, e.g.
fish from Dukan at 230 g had a K of 2.32, at 1 kg K was 1.75. Ahmed and Taher
(1989) examined the growth of 0+ carp in Hawr al Hammar, Iraq found the
length-weight relationship to be W = 0.00004627 L2.8022 and derived
the growth equation Lt = 189.87 (1-e-0.0158 (t + 25.662))
with a calculated length at the end of the first year of life of 189.87 mm. This
is relatively larger than for other parts of the world, indicating a successful
introduction of this exotic. Al-Nasiri and Dawood (1991) found the smallest
mature male was 182 mm and the smallest female was 184 mm in Hawr al Hammar, with
sexual maturity achieved in the first year of life. Maximum life span was 8+
years. Epler et al. (2001) found the oldest age groups in Iraqi lakes to be 5+
in Lake Habbaniyah and 3+ in Lake Razzazah. The mean condition factor was 1.47 for Lake
Habbaniyah and 1.50 for Lake Razzazah.
In Sariyar Dam Lake near Ankara in central Anatolia, ages range
from 0 to 18 years (Ekmekci, 1996b). In their first year, fish have an
average fork length of 103 mm and weigh 24 g, in 5 years they average
357 mm and 822 g, and in 10 years 580 mm and 3365 g. In Gölhisar Lake by
contrast, a small water body in western Turkey, age composition was from 1 to 6
years and fish attained a maximum of 494 mm and 1922 g (Alp and Balik, 2000).
Maximum life span for this species is reported as 47 years for domestic fish.
Food
Food is derived from browsing on the substrate at all hours, if the
temperature is favourable. Browsing muddies the water and can inhibit
other species and uproot plants. Mouthfuls of bottom ooze are taken
up, spat out and the food items selected. These include aquatic
insects, crustaceans, worms and molluscs, and more rarely, fish. Plant
material is ground up by the molar pharyngeal teeth and includes
algae, seeds, wild rice, leaves and various aquatic plants. Organic
sewage is also eaten. Some surface feeding on algal mats or insects
will also occur. Feeding almost completely stops in winter and the
fish go into a form of hibernation.
Feeding in the Hawr al Hammar, Iraq is related to
temperature, the peak intensities being July and the minimum in January with
peak activity in September and minimum in January. Feeding does occur year round
and smaller fish (<200 mm) have highest feeding activity in spring while adults
have this in summer (Hussein et al., 2000a). In a study of the recovering
Hawr al Hammar, diet was 25.45% algae, 18.18% snails, 12.73% diatoms and 12.73% copepods, 10.91%
insects with plants, cladocerans and shrimps at less than 10% each, in the
Hawr al Hawizah diet was 27.3% snails, 18.2% insects and 12.1% for algae, plants
and cladocerans with fish, diatoms and copepods at less than 10% each, and in
the Al Kaba'ish (= Chabaish) Marsh 33.3% algae, 20.4% insects, 11.1% snails and diatoms and
plants at 10.2, with various crustaceans at less than 10% each (Hussain
et al., 2006). Hussein et al. (1991) examined diet in the Garma Marshes, Iraq and
found crustaceans, molluscs, aquatic plants and seeds, aquatic insects, oligochaetes
and fish to be dietary items, selection and numbers varying with carp size and season. Some
fish were found to have fed exclusively on only a single, different mollusc
species, presumably as opportunity presented. Al-Shamma'a et al. (1996) examined
the food of this species in Al Qadisiyah Reservoir, Iraq and found plants, their seeds,
molluscs and aquatic insects, all bottom foods. Mangalo and Akbar (1988a, 1988b)
studied the food of carp in a farm pond at Al-Latifiyah, Baghdad where
zooplankton was the principal diet. The gill rakers show an efficient structure
for filtration, indicative of a phytoplanktivorous and omnivorous feeding
(Salman et al., 1993). Salman et al. (1994) report a mixture of
animal and plant foods, with zooplankton a dominant component of all length
groups. The gut is coiled and 3.42 times standard length, indicating omnivory
with plant food being important. Epler et al. (2001) gave the diet in
Lake Habbaniyah, Iraq as 51.7% plants, 15.7% oligochaetes, 15.2% tendipedids, 7.2%
molluscs, 5.2% detritus and 4.1% cladocerans. They also found that where there
is significant competition between autochthonous species, as here, carp become
another strong competitor for food. Dietary coincidence between carp and
shabbout, gattan and himri was 58.5, 68.5 and 54.2 % respectively. Hussein et
al. (2000b; 2000c) examined dietary overlap between this species and three
native carps in the Hawr al Hammar. Overlap with Barbus (=
Mesopotamichthys) sharpeyi was the
weakest as this species is a herbivore but small B. luteus (<200 mm) and
B. xanthopterus showed strong overlaps. This overlap may explain the
decline in some native carps. Hussain and Ali (2006) also examined feeding
relationships among fishes in the Hawr al Hammar and found this species to be a
carnivore, 26.4% of the diet being crustaceans, 12.7% insects and 30.5% molluscs.
Dietary overlap of 84% was found between this species and Barbus
(= Luciobarbus) xanthopterus but the availability of food resources offset possible
competition, contrasting with the conclusions above. Ciepielewski et al.
(2001) found the diet of this species in the Dukan and Derbendikhan reservoirs
in Iraq to be mainly algae, copepods and chironomids.
Reproduction
Under natural conditions, males spend more time on the spawning grounds than females and
spawn several times. More than 7 million eggs up to 1.71 mm in
diameter may be present in a female but only about 500 are laid at a
time. Wild carp in the Atrek River had a fecundity range of 16,000 to 543,000
eggs (Bănărescu, Barus and Peňáz in Bănărescu and Paepke, 2002).
The resident form is less fecund by about half than the semi-anadromous
form (Shikhshabekov, 1969). In the Anzali Mordab a mass spawning run
takes place in April with spawning in April-May. The first migratory
fish are seen as early as January. Shallow weedy areas or the mouths
of rivers are used as spawning sites and adhesive eggs are laid on plants.
Resident populations in Dagestan spawn earlier, by about a month,
than the semi-anadromous population which spawn in early May (Shikhshabekov,
1969). Spawning time variations are governed by temperature and the
most favourable temperature is 18-20°C.
Carp ascend the Kura River of Azerbaijan in spring and autumn. The
spring run begins in mid-March and peaks in April while the weak
autumn run lasts from August to mid-October.
Carp have feeding grounds in the coastal waters of the southeastern
Caspian Sea and enter the Atrak River in winter to spawn between
February and April. Young migrate downstream, this movement ending in
July when the river flow is minimal or ceases. When there is no flood,
spawning does not occur (Petr, 1987).
Fish in Iraqi ponds grew 25-30 cm in the first year of life and matured in
1-2 years. At 16-26ºC they spawned from late February to late April and again in
the autumn (Al-Hamed, 1960). Palm tree fibres were used for egg deposition and
eggs hatched in 4-8 days. Al-Nasiri and Dawood (1991) found a fecundity range of
14,150-1,492,500 eggs in Hawr al Hammar with a mean relative fecundity of 182
eggs/g of body weight, and a egg diameters of 0.90-1.02 mm. The gonadosomatic
index indicated spawning in March and possibly October-November. Epler et al.
(2001) studied reproduction in lakes Tharthar and Habbaniyah and found both sexes
to achieve maturity in the first year of life at 13.5 cm for males and 12.6 cm
for females. Spawning occurred in May and fecundity was 186-531 thousand eggs/kg body mass.
The spawning behaviour involves stimulation of a female while
moving over vegetation and being accompanied by 2-3 males, active
movement and spawning being induced by blows from the male(s). The
eggs adhere to the vegetation or are lost. Most eggs are shed at night
or in the early morning.
Parasites and predators
Eslami and Anwar (1971) record the cestode Caryophyllaeus
fimbriceps from this species on the Caspian coast of Iran.
Mokhayer (1976b) records the cestodes Bothriocephalus gowkongensis,
the nematode larva Anisakis and the acanthocephalan Pomphorhynchus
perforator. Mokhayer (1989) reports metacercariae of the eye
fluke, Diplostomum spathaceum from this species in Iran, which
can cause complete blindness and death in commercially important
species. Jalali and Molnár (1990b) variously record the monogeneans Dactylogyrus
anchoratus, D. extensus, D. sahuensis and D.
vastator from carp on fish farms throughout Iran and Naem (2002) records
D. anchoratus from fish in the Safid River.
Moghainemi and Abbasi (1992) record a wide range of parasites from
this species in the Hawr al-Azim in Khuzestan. Mortazaei et al.
(2000) record an infection rate of 66% (2 of 3 fish) with the worm Bothriocephalus
opsariichthydis in Khuzestan marshes. Sattari and Faramarzi (1997)
record Caryophyllaeus fimbriceps, C. laticeps and C.
brachycollis from 38% of carp in the Anzali lagoon. Akhlagi (1999; 2000)
reports that high temperatures (up to 32°C)
stresses this species and leaves it open to infection with Aeromonas
hydrophila. Safari and Khandagi (1999) record Clostridium
botulinum from 3.8% of fresh and smoked samples of this species in
Mazandaran Province. Mousavi and Khosravi (1999; www.mondialvet99.com,
downloaded 31 May 2000) record the toxigenic fungi Aspergillus
flavus, Alternaria, Penicillium and Fusarium
from this species and the pond water at a fish farm in northern Iran. Farahnak
(2000) records Anisakidae from this species in Khuzestan. Akhondzadeh et al.
(2002) and Akhondzadeh Basti and Zahrae Salehi (2003)
show that the psychotropic pathogen Listeria monocytogenes is found in
market and fish farm samples of this species. Masoumian et al.
(2002) investigated parasites from this fish in the Aras and Mahabad dams in
northwest Iran and found the protozoan Goussia carpelli, also known from
carp in the Safid River. Ebrahimzadeh Mousavi et
al. (2005) isolated the fungus Branchiomyces spp. from gill lesions
of farmed carp in northern Iran. Branchiomycosis or gill rot is a major problem
in commercial fish production.
The intestinal helminth Bothriocephalus gowkongensis was recorded from this species on fish farms
in West Azarbayjan Province (Azarvandi et al., 1999).
Naem et al. (2002) found the following parasites on the gills of this
species from the western branch of the Safid River, namely the protozoans
Ichthyophthirius multifilis and a Trichodina species, a copepod
crustacean Lernaea sp., monogenean trematodes Dactylogyrus anchoratum,
and D. achmerowi. Jalali et al. (2002) and Jalali and Barzegar (2006) record parasites from this species in Lake Zarivar,
namely Trichodina pediculus, Dactylogyrus extensus,
Gyrodactylus stankovici, Diplostomum spathaceum two species of
Argulus, Pseudocapillaria tomentosa and Lernaea
cyprinacea. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. cyprini, G. elegans, G. shulmani,
G. sprostonae, G. stankovici and G. sp. from various
localities for the carp. Farahnak et al. (2002) record Contracaecum sp. and Anisakis
sp. from this fish in Khuzestan Province. Mehdipoor et al. (2004) record
the monogenean Dactylogyrus extensus in Zayandeh River fish. Araghi Soureh and Jalali Jafari (2005) recorded Dactylogyrus extensus
from this species in the Mahabad River of the Lake Orumiyeh basin.
Jalali and Barzegar (2005c) record five
species of monogeneans in the genus Dactylogyrus from both farmed and
native Cyprinus carpio in Iran. These are D. extensus, D.
anchoratus, D. achmerovi, D. vastator and D. sahuensis.
Fry and fingerlings are more sensitive to these parasites and this sensitivity
is increased with crowding in ponds. The paper also deals with gill
histopathology and distribution of the parasites in Iran. Barzegar and Jalali (2006)
report parasites in this species from Kaftar Lake as Lernaea cyprinacea,
Trichodina sp., Dermocystidium sp., Dactylogyrus extensus,
D. anchoratus, Gyrodactylus sp. and Diplostomum spathaceum.
Khara et al. (2006b) record the
cestode Caryophyllaeus fimbriceps from this species in the Boojagh
Wetland of the Caspian coast. Pazooki et al. (2007) recorded various parasites from localities in West
Azarbayjan Province, namely Diplostomum spathaceum, Ligula
intestinalis, Digrama sp. and Argulus foliaceus.
Sattari et al. (2007) record the cestode Caryophyllaeus fimbriceps,
the digenean Diplostomum spathaceum and the monogeneans Dactylogyrus
extensus, Gyrodactylus sp. and Diplozoon sp. in this species
in the Anzali wetland of the Caspian shore
and also mention that the Caryophyllaeus
laticeps is also known from this species in the Iranian Caspian Sea.
Barzegar et al. (2008) record eye parasites from this fish including the
monogenean Gyrodactylus stankovici, the digeneans Diplostomum
spathaceum and Tylodelphys clavata, and the crustacean Lernaea
cyprinacea.
Khara
et al. (2008) found the eye parasite Diplostomum spathaceum in
this fish from Boojagh Kiashar Wetland in Gilan. Shamsi
et al. (2009) found Dactylogyrus achmerovi, D. anchoratus,
D. extensus, D. sahuensis and D. vastator in this species
in fish farms, Safid River and Hamun Lake.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Argulus sp.,
Argulus foliaceus, Ergasilus sieboldi, Lernaea sp. and
Lernaea cyprinacea on this species.
The Caspian seal, Pusa caspica, is a significant predator on
this species (Krylov, 1984). Esox lucius, Sander lucioperca
and Silurus glanis are all predators on small carp. A wide
variety of other fishes and birds eat smaller carp as do predatory
aquatic insects, frogs and toads. Carp eggs are eaten by many fishes.
Adult carp are too large for most predators to take.
Economic importance
Balon (1974; 2006) details domestication of the carp which has been a
cultivated fish for over 2000 years. The carp is an important table
fish in Iraq where details of farming techniques are given by Ahmed
and Taher (1988) and by Mangalo and Akbar (1988a; 1988b). Catches in
former Soviet waters of the Caspian Sea were described by Kuliyev and
Agayarova (1984). In the southwestern part, size range was 21-64 cm in
1960-1961 (92% of fish were 29-41 cm) but in 1972 95% of fish were
31-55 cm. The smallest carp are caught in the southeastern Caspian
where maximum weight was 3.97 kg as opposed to 10.0 kg in the central
Caspian. Spawning fish in the southeastern Caspian were only 0.58-1.0
kg while in the southwestern Caspian carp were 0.25-5.75 kg. About
17-30% of commercial catches in the Volga region are estimated to be
comprised of hatchery production (Petr, 1987).
Nevraev (1929) gives catches for various fishing regions in Iran in
the early twentieth century. For the Safid River region from 1899-1900
to 1917-1918 the catch was 30 to 30,500 individuals with no fish
reported in some years, and in Astrabad (= Gorgan) region from
1900-1901 to 1912-1913 the catch was 14,200 to 851,800 individuals.
The commercial catch in Iran from 1956/1957 to 1961/1962 varied from
3443 kg to 175,295 kg (Vladykov, 1964), from 1965/66 to 1968/69 varied
from 184 to 333 tonnes (Andersskog, 1970) and from 1963 to 1967 from
7.0 to 108.8 t (on a yearly basis 41.1, 9.8, 7.0, 108.8, and 48.1 t respectively) (RaLonde and Walczak, 1970b). Catches from
1933/34 to 1961/62 varied from 34 kg to 1113 t in the Bandar Anzali
region and the total catch of the Northern Shilot (Fisheries Company)
varied from 9.8 t to 333 t from 1963/64 to 1968/69 (RaLonde and
Walczak, 1972). The Food and Agriculture Organization, Rome gave the catches
from Iran for the 6 years 1980 to 1985 as 1032, 2000, 1000, 52, 83, and 100 t respectively. The catch in the Anzali Mordab
in 1990 was 6855 kg and from 1932 to 1964 the annual catch varied from 1.2-638.6
t (Holčík and Oláh, 1992). These wide fluctuations in reporting and in
nominal catches are indicative of the poor fisheries statistics as well as the
inter-year variability of catches in Iran.
March and April are the main fishing months in Iran (Farid-Pak, no
date). It is the main fish in Iranian carp farms, when raised in
polyculture with Chinese carps (Jalali and Molnár, 1990b). Salehi (2004) gives a
figure of 25% as the share of common carp in Iranian polyculture (63% being
silver carp) and gives a review of the economics of carp polyculture.
Kohnehchahry and Heydarpur (1973) outline methods of raising carp
using submerged cages which they believe would be suitable for Iranian
waters. There were about 4000 ha of carp fish ponds (which presumably
included Chinese carps such as Hypophthalmichthys spp.) with an
annual production of 2-3 tonnes/ha (White, 1988). Plans were made in
the 1980s to increase total pond area (including trout which was at 60
ha and produced 1000 t/year) to 35,000 ha over 10 years to yield an
annual harvest of 100,000 t/year. However Bartley and Rana (1998b)
report an aquaculture production of 6561 tonnes in 1995 and Salehi (2004) gives
28,060 t from warm-water fish farming, mostly Chinese carps. More than
700,000 carp fingerlings were released in the small and remote
province of Chahar Mahall va Bakhtiari alone and 20 million carp,
silver carp and grass carp fingerlings were produced in the Shahid
Rajaae Hatchery in Sari for release across Iran in reservoirs and dams
(Abzeeyan, Tehran, 4(7):VII, 1993).
Salehi (1999) states that the marketable size of cultured carp is 1 kg with most
harvested once annually and almost 90% supplied to market in October-March with
a peak in March (the Iranian New Year when fish is a traditional food).
Marjan Iran Company was selling 1500-1800 g fish for U.S.$1.90/kg in August 2003 compared to
$2.10 for Ctenopharyngodon idella (http://groups.yahoo.com/groups/hilsa/message/25).
In Golestan Province, carp cost 1500-1700 tomans/kg in the early 2000s. Salehi
(2006a) analysed the consumer market for this species and its products in Iran.
Shabanpour et al. (2007) investigated preparation of surimi from this
species.
An exotic species in some parts of the world, carp are a nuisance
because they uproot vegetation used by native species for cover, food
and spawning. This activity also increases water turbidity to levels
which many native species cannot tolerate. Stirred up silt may smother
eggs of native species. Carp also compete with other species for food
and eat the eggs of other species.
Various studies have been carried out on its culture in Iran and neighbouring
countries, e.g. see Freshwater Fishes of Iraq, and
its use as an experimental organism, e.g. in Iran, see Mohagheghi and Hedayatifard (Farsi translation of Horváth et al.
(1985a and 1985b); Khazraiinia et al. (2000) on acute ammonia toxicity;
Peyghan et al. (2001) on use of ketamine and xylazine hydrochloride as
anaesthetics; Rostami Bashman et al (2001) on histopathological lesions after exposure
to copper, zinc, mercury and cadmium compounds; Soltani et al. (2001) on
the anti-fungal effectiveness of formalin on hatch rate of eggs; Abtahi et al. (2002) on clove oil having no significant
difference with MS222, another anaesthetic used in fish farms; Arabi and Heydarnejad
(2002) on the deleterious effects of copper and mercury, used in combating
algal blooms and weeds, on the gills of carp; Baradaran Noveyri et al.
(2002) on the effects of cryopreservation on motility of spermatozoa; Peyghan
et al. (2002) on differences between gonads of fishes from two culture
seasons; Dorafshan et al. (2003) on induction of spawning
using pituitary extract and GnRH analogue in combination with
domperidone; Sharifpour et al. (2003) on the anaesthetic effects of clove oil under various pH and temperature
regimes; Vahabzadeh Roodsari et al. (2003) comparing malachite green and
hydrogen peroxide to control fungal infections (the latter is effective and less
dangerous); Akhlaghi et al. (2004) on phagocytosis in relation to
immunostimulants; Ebrahimi (2004) on the deleterious effects of copper, a pollutant, on sperm anatomy; Moini and Basimy
(2004) on production of fish cake according to various recipes and its shelf
life; Sharifpour (2004) on the histology of the response and the circumstances
of wound healing; Soltani et al. (2004) on the effects of clove oil
anaesthesia on haematological parameters, serum enzymes and some tissues, up to
200 p.p.m. being deemed safe; Oryan et al. (2005) on the effects of baclofen on
the pituitary system; Sharifpour et al. (2005) on the highly toxic effects of the pesticide endosulfan (with an
LC50 of less than 0.1 mg/l); Yousefian (2005) on generating
gynogenetic carp through irradiation; Baradaran Noveiri
et al. (2006) on cryopreservation of spermatozoa using different
extenders; Ghiasi and Mirzagar (2006) on lysozyme content in sub-lethal
concentrations of cadmium; Kazemipour and Keivany (2005) on the use of garlic,
mallow and motherwort in healing superficial wounds - garlic reduced recovery by
one week; Kazemipour et al. (2006) on carp kept in aquaria at low
concentrations of garlic (0.1 g/L) which healed superficial wounds more quickly than
controls or aquaria having mallow or motherwort; Kohodabandeh and Abtahi (2006) on the use of sodium
chloride, iodine and formalin to control Saprolegnia sp. on eggs (sodium
chloride was recommended); Naji et al. (2007) on the 96h LC50
value of cobalt chloride at 327-328 mg/l; Naji et al. (2007) on the toxic
effect of zinc sulphate on gill tissues which suffer hypertrophy and
hyperplasia; Darafsh et al. (2008) on the use of scales as an indicator
of heavy metal pollution; Golchinrad et al. (2008) on the effects of
detergent on liver glycogen (decreased) and glucose (increased);
Hasanabadizadeh et al. (2008) on the lack of improvement in wound healing
after vitamin injections; Imnapour and Enayat Gholampour (2008) on the effect of
broodstock migration time on various egg characters; Safari et al. (2008) on changes in muscle chemistry during
maturation; Soleymani et al. (2008) on the effectiveness of vitamin C
injections on survival of juveniles challenged by different doses of the theront
of Ichthyophthirius multifilis; Darvish Bastami and Imanpour (2009) on sperm motility influenced by
high concentration of ions; Baghfalki et al. (2009) on the effect of
broodstock density on survival and growth of larvae and fingerlings in earthen
ponds; Erfani Majd et al. (2009) on evaluating the response of incubated
ovarian follicles to carp pituitary extract and cultivated pituitary cells
secretion; Ghanbari et al. (2009) on the long term effects of changes in
pH on haematological parameters in fingerlings; Imanpour and Safari (2009) on the effect of
maturation stages on gonadal indices and chemical composition of the gonad; Imanpour et al. (2009) on stocking density
and its effect on survival and growth in polyculture, up to 450 fish per
hectare being optimal; Imanpour et al. (2009) on the effects of
broodstock age on various egg dimensions, females age 3-6 years being found
suitable for propagation; Kordjazi et al. (2009) on physicochemical
parameters of water and their correlation with haematocrit indicators, growth
and survival in farm ponds; Rostami Mina and Soltani (2009) on the histopathological effects of
the aquaculture pollutant copper sulphate; Salamat et al. (2009) on
pituitary primary cell culture and its secretion effect on endocrine activity of
incubated ovarian follicles; Sheykhzadeh et al. (2009) on
the effects of Eucalyptus essential oil on immunological variables;
Yehganeh et al. (2009) on seasonal variation in the chemical composition
and fatty acid profile of ovaries, necessary for embryogenesis; Ghiasi et al. (2010) on the effects of low concentrations of cadmium on
the immune response in winter; Salamat et al. (2010) on ovarian
follicular cells and their endocrine activity in cell culture; Soltani et al. (2010) on the immune
responses to Zataria multiflora (Persian thyme) essential oil used as an
anti-fungal for carp eggs; etc.
This species is actively angled for along the Caspian shore of Iran
and in its rivers (e.g. see Noorbakhsh (1993a)), appears regularly in fish
markets of Ahvaz, Khuzestan and is caught by anglers there using bread or potato as bait.
It is characterised as a fatty fish according to a lipid content 9-14% by wet
weight of muscle in autumn (Hantoush et al., 1999). Al-Aswad et al.
(1980) detailed the chemical composition of this species in Dukan Lake, Iraq including
seasonal levels of moisture, fat, protein and ash, and the various types of
fatty acids and amino acids. Hindi et al. (1996a) give the chemical
composition of flesh of this species as 78.87% moisture, 2.46% fat, 17.06%
protein and 1.35% ash, indicating a valuable food fish characterised as lean to
medium fatty. Hindi et al. (1996b) give chemical indices for assessing
fish freshness according to the month of capture and marketing (pH 6.28, total
volatile nitrogen bases 11.07 mg/N/100g fish, thiobarbituric acid 0.47 mg, and
free fatty acids 0.62%).
The roe or eggs of this species have been implicated in poisoning
(Halstead, 1967-1970; Coad, 1979b) and should be avoided (see under
the genus Schizothorax for more information on egg poisoning).
Fish should be carefully cleaned in the spawning season to remove the
eggs and ensure against contamination of flesh. Severe cases of egg
poisoning in other species have resulted in death.
Vaezzadeh et al. (2008) found the levels of the pesticide heptachlor in
fish from Anzali and Ramsar could have a health risk to consumers.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in textbooks, for sport, as an experimental species and
because it has been introduced outside its natural range. Brightly
coloured varieties of carp are known as "koi" and are kept
as ornamental fish. Colours include red, orange, white, black, blue
and yellow in various combinations.
Conservation
Vladykov (1964) recommended that fishing for this species in Mazandaran and Gorgan be prohibited for 5 years because of reduced
stocks. Krasznai (1987) and Petr (1987) give details of fish farms
propagating this species in Iran. For example, 30 million fish were
produced by the Safid Rud Fish Farm in 1986. In 1999-2000, 20 million
juveniles were released into the Caspian Sea (Iranian Fisheries Research
Organization Newsletter, 23:4, 2000). From October to March 2000, 3 million juveniles raised in
the Shahid Ansari aquaculture and breeding centre in Gilan were
released into the Caspian Sea and neighbouring water bodies (Iranian
Fisheries Research Organization Newsletter, 26:2, 2001). Poaching was a problem in the Caspian basin of Iran (Razivi
et al., 1972) and no doubt continues. The Atrak River stocks
are an important fishery for both Iran and Turkmenistan but are
susceptible to loss through the absence of flooding of the spawning
grounds. Fish passes are needed to ensure access to the spawning
grounds, timely release of water from a reservoir to flood the
spawning areas in years of low water flow, enforcement of catch
limits, and continued stocking (Petr, 1987). Yousefian et al. (2009)
examined the normative reproduction values and genetic characters for native
carp in the Caspian Sea. The average weight of sampled fish was 1441.6 g and
standard length was 50.5 cm. Fertilisation rate was 62-79%, absolute fecundity
was 114,347 and relative fecundity 82,007. There was no significant differences
in haplotype distribution between areas in the southern Caspian Sea.
Lelek (1987) considered that the wild form, as opposed to domestic
stocks, was vulnerable to endangered in Europe because of habitat
modifications. Kiabi et al. (1999) consider this species to be
of least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include commercial fishing, sport fishing, abundant
in numbers, habitat destruction, widespread range (75% of water
bodies), present in other water bodies in Iran, and present outside
the Caspian Sea basin. Karami et al. (2005) found river fish had greater
exposure to organophosphorus compounds than Caspian Sea and well-fed fish.
Further work
Where this species is introduced, its interactions with native species should
be carefully studied and monitored in an effort to protect the native fauna.
Sources
A recent summary on this species is Bănărescu, Barus and Peňáz in Bănărescu and Paepke (2002).
Iranian material: CMNFI 1970-0510, 1, 47.8 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1970-0522, 1, 108.5 mm standard length, Gilan, Safid River at Astaneh Bridge (37º16'30"N, 49º56'E);
CMNFI 1970-0563, 3, 28.6-58.6 mm standard length, Gilan, Caspian Sea at Kazian Beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0568, 1, 125.8 mm standard length, Gilan, Caspian Sea at Kazian Beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0582, 3, 63.3- 134.0 mm standard length, Mazandaran, Aliabad Reservoir (36º56'N, 54º50'E);
CMNFI 1970-0587, 2, 47.5-76.7 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1979-0455, 6, 46.9-67.1 mm standard length, Markazi, Manjil Dam (36º45'N, 49º17'E);
CMNFI 1979-0476, 3, 44.1-85.3 mm standard length, Mazandaran, Qareh Su near Kord Kuy (36º51'N, 54º05'E);
CMNFI 1979-0479, 19, 28.0-143.8 mm standard length, Mazandaran, dam on Gorgan River (37º09'30"N, 54º41'30"E);
CMNFI 1979-0685, 2, 31.5-45.7 mm standard length, Gilan, Safid River (ca. 37º22'N, ca. 49º57'E);
CMNFI 1979-0788, 2, 104.2-123.5 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0128, 3, 60.2-75.2 mm standard length, Mazandaran, Qareh Su (36º49'30"N, 54º03'30"E);
CMNFI 1980-0132, 4, 56.0-64.6 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1980-0157, 1, 126.3 mm standard length, Mazandaran, Gorgan River estuary (36º59'N, 53º59'30"E);
CMNFI 1980-0905, 2, 78.1-92.9 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0908, 1, 54.4 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E).
Genus Garra
Hamilton, 1822
The genus Garra is found throughout Southwest Asia and from
Africa to southeast Asia. There are about 73 species and 4 are
recognised from Iran. The genera Discognathus Heckel, 1843 and Discognathichthys
Bleeker, 1859 are synonyms. Hora (1921) presents anatomical arguments
for including Discognathus in Garra.
This genus is characterised by a small to moderate-sized body,
elongate and almost cylindrical, a rounded snout with the mouth
inferior and crescent-shaped, the lower jaw has a horny edge, the
upper lip is usually fringed and continuous with the snout, the lower
lip and chin area modified into a suctorial disc with free posterior
margin (in Iran, elsewhere a some have a callous pad (Stiassny and Getahun,
2007) and the smallest specimens lack full disc development), the anterior disc margin free or adherent (species with the
latter condition were placed in a separate genus, Discognathus
or Discognathichthys), 1 or 2 pairs of short barbels (species
with the former condition were placed in a separate genus, Discognathus
or Discognathichthys), vomero-palatine organ vestigial or regressed, eyes small, usually large scales,
lateral line complete, small dorsal and anal fins without thickened
rays, pectoral and pelvic fins placed horizontally on the body,
first two or more pectoral fin rays prominent and often unbranched, pharyngeal teeth in 3 rows
(typically 2,4,5-5,4,2) with hook-shaped tips and spoon-shaped
crowns, vent may be midway between pelvic and anal fin bases or nearer
the latter, elongate and coiled gut, a black peritoneum, and 2n=50.
The Farsi common name used generally for these fishes is gel
cheragh (= mud-eater, mud-grazer), سنگ ليس (sang lis)
and ماهي سنگي (mahi sangi), not repeated in
each Species Account.
These fishes are found in mountains streams and other flowing
waters, maintaining position with their suctorial disc, reduced
swimbladder, flattened belly and large, splayed and horizontal paired
fins. Also known to occur in slow-moving or stagnant waters. They scrape algae from rocks. These are oily fishes which are
eaten in India (Hora, 1956).
Menon (1954) considers that the members of this genus spread
westwards along the Himalayas as late as the early Pleistocene.
Kosswig (1952) indicates their presence in the Araxes (= Aras) of
Turkey but this seems to an error.
Garra persica
Berg, 1913
Common names
Persian stone lapper.
Systematics
This species is recognised only as a subspecies of Garra rufa
by Bianco and Banarescu (1982) while Menon (1964) and Karaman (1971)
synonymise it with Garra rufa.
Karyotype analysis separates this species from Garra rufa (Esmaeili et
al., 2009).
The syntype specimens are in the Zoological Institute, St.
Petersburg under catalogue numbers ZISP 11707 (6 specimens from the
"River Bampur in Eastern Persia. N. Zarudnyi 1898,
15-27.VII") and 11706 (1 specimen from "Kiabad in Zirkuh
(Eastern Khorassan). N. Zarudnyi 1898, 3.V") according to Berg
(1913) where the original description is founded on these fish,
implying all are types. The latter is also given as "settlement
Kiabad between Zirkuh province and Sistan" in the catalogue (this
locality may be at or near Kuh-e Ziri at 32°48'N, 59°50'E
according to Coad (1981d)). These dates are old style and corrected to
new in Berg (1949) (27.VII-8.VIII and 15.V respectively). In St.
Petersburg under ZISP 11707 there are 10 fish 24.0-46.5 mm standard
length and ZISP 11706 is not listed as a type in the catalogue nor in
Berg (1949). Berg (1949) lists 10 fish in 11707 too. These specimens
were formerly identified as Discognathus lamta by Nikol'skii
(1899) who lists 1 fish in 11706 and 6 in 11707. Three syntypes are in
the Zoological Survey of India, Calcutta (ZSI F11101/1) listed under Garra
rufa obtusa and received from the Zoological Institute, St.
Petersburg, Russia on exchange (Menon and Yazdani, 1968). There are
more apparent types available than those listed by Berg (1913).
Key characters
Two pairs of barbels are present, the adhesive disc is well
developed with a free posterior margin, the dorsal fin has 7 branched
rays, and the caudal fin modally 16 branched rays. The caudal fin ray
count is unique in cyprinids from Iran and very rare elsewhere. Almost all
cyprinids show a strong mode of 17 branched caudal fin rays.
Morphology
Dorsal fin with 4 unbranched and 6-8 branched rays, anal fin with
2-3 unbranched and 4-6 branched rays, branched pectoral fin rays
12-16, pelvic fin branched rays 6-8, caudal fin branched rays number
15-17 with a strong mode at 16, lateral line scales 28-38, total gill
rakers 15-22 with lower counts in smaller fish, pharyngeal teeth
usually 2,4,5-5,4,2, and total vertebrae 34-35.
Scales are regularly arranged and only lacking on the anterior
isthmus in some fish. A pelvic axillary scale is present but is not
always well-developed. Scales have numerous radii on all fields with
the focus broken up into a network of lines. There are 4 short barbels.
Upper lip well-developed, the rostral fold weakly fringed. The
adhesive disc and rostral fold are greatly papillose although the disc
centre is not as papillose as the margins. The gut is very elongate
and greatly coiled. Chromosome number 2n=48 with 15m, 8Sm and 1St (Esmaeili
et al., 2009).
Counts for 12 Iranian topotypic specimens from the Bampur River are
as follows:- dorsal fin with 4(12) unbranched and 7(12) branched rays;
anal fin with 3(12) unbranched and 4(3) or 5(9) branched rays;
branched pectoral fin rays 12(1), 13(6) or 14(4); caudal fin branched
rays number 15(1), 16(8) or 17(1) in the type series and 15(1), 16(10)
or 17(1) in topotypes; lateral line scales 29(2), 30(1), 31(1), 32(2),
33(2), 34(2) or 35(2); total gill rakers 15(6); pharyngeal teeth
2,4,5-5,4,2(5); and total vertebrae 34(9) or 35(3).
Meristic values for topotypes and other material: dorsal fin
branched rays 6(3), 7(115) or 8(4); anal fin branched rays 4(5) or
5(118); branched pectoral fin rays 12(8), 13(40), 14(49), 15(20) or
16(5); branched pelvic fin rays 6(9), 7(100) or 8(13); caudal fin
branched rays number 15(10), 16(113) or 17(9); lateral line scales
28(1), 29(5), 30(5), 31(77), 32(18), 33(28), 34(20), 35(22), 36(11),
37(4) or 38(2); total gill rakers 15(16), 16(13), 17(18), 18(17),
19(11), 20(4) or 21(2); pharyngeal teeth 2,4,5-5,4,2(5); and total
vertebrae 32(4), 33(44), 34(34) or 35(4).
Sexual dimorphism
Males develop numerous breeding tubercles around the snout, between
the nostril and the eye and between the nostrils. There is a
transverse depression anterior to the nostrils on the snout. A
postspawning individual from the Hormuz basin measuring 148.7 mm SL
has small tubercles under the eye running forward onto the snout as a
band, the most evident tuberculation. The swollen snout tip bears no
tubercles. The top of the head has tubercles but these are smaller and
sparser than the band under the eye. Scattered large tubercles are
present on the gill cover. Evident tubercles line the dorsal, anal,
caudal and pectoral fin rays (pelvic fins not present on specimen),
the largest being those on the anal fin.
Colour
The back and flanks are an orange-brown to golden-brown. There is a
blue spot on the flank near the postero-dorsal edge of the operculum
(dark black in preserved fish). The dorsal fin bears elongate blotches
on the posterior half of each fin membrane, usually fading distally,
but in some fish occupying the whole membrane. Proximally there is a
gap between these blotches and 3-5 bars which originate at the
posterior edge of the base of branched ray three and succeeding rays,
and extend distally across the ray and then along the ray and the
membrane to the gap. These bars are much more heavily pigmented than
the dorsal blotches. There is a bluish tinge or spot around the
pectoral fin base, sometimes developed as a bar along the edge of the
gill cleft, becoming dark blue dorsally. There is a dark bar or a roundish,
poorly-delimited spot on the caudal peduncle at the base of
the caudal fin. Fins are generally pink to light orange. The caudal
fin pigmentation is individually variable. Some are blotched
irregularly on both rays and membranes, in others there is a trace of
a band in mid-fin extending from the dorsal to the ventral margin
following the posterior outline of the fin, while others have pigment
heavily concentrated only in the mid-fin clear of the margins. The
pelvic fin has little or no pigment and the anal fin has a very few
irregular light blotches on both rays and membranes. The pectoral fin
is pigmented near the dorsal base with some pigment on anterior rays
and membranes. In live fish the paired fins are a light orange and
other fins show less marked orange tinges. The peritoneum is black.
Size
Attains 7.5 cm total length (Berg, 1913).
Distribution
This species is found in the Hormuz, Makran and
Hamun-e Jaz Murian basins and
possibly the Sistan and Kerman-Na'in basins (Bianco and Banarescu, 1982; Abdoli, 2000).
Localities include springs in the upper Kul River basin near Darab, small
springs in the upper Sarbaz River, and at Jaghin.
Zoogeography
The species of the genus Garra are thought by Menon (1964)
to have colonised Iran from a centre of origin in southern China by a
series of "waves". The earliest wave arrived in the Miocene
and is represented in Iran today by the species rossica and variabilis,
characterised by the primitive condition of a weakly-developed
adhesive disc without a free posterior border, the posterior chamber
of the swimbladder is cylindrical and well-developed, there is no
proboscis on the snout and the vent is close to the anal fin. A second
wave is represented in Iran by rufa (and by implication persica)
characterised by a well-developed disc and a tuberculated snout marked
off by a transverse groove. There were 6 "waves" all told
but only the first two are relevant to Iran. Karaman (1971) criticises
this complex interpretation on two grounds. He maintains that it is
unlikely that fishes would immigrate from southern China to Iran but
leave no extant forms between these two remote places and that the
species assigned to the various waves show no characteristics which
would make them more adaptive and capable of replacing earlier wave
members. The characters of rossica and variabilis (one
pair of barbels, weak disc, reduced squamation) could equally be loss
characters and a more recent specialisation rather than the primitive
condition.
Habitat
Kiabi and Abdoli (2000) found this species to have the widest
altitudinal range in Hormozgan Province.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This species is widely distributed in various smaller water bodies in eastern
Iran and does not appear to be under threat.
Further work
The biology of this species remains unknown and would repay study. It is a
significant component of a variety of water bodies.
Sources
Type material: See above, ZISP 11707 and 11706.
Iranian material: CMNFI 1979-0138, 1, 25.7 mm standard length, Fars-Hormozgan, Rasul River drainage (ca. 27º32'N, ca. 54º58'30"E);
CMNFI 1979-0139, 1, 30.6 mm standard length, Fars-Hormozgan, Rasul River drainage (ca. 27º25'30"N, ca. 54º59'E);
CMNFI 1979-0144, 1, 27.3 mm standard length, Hormozgan, Minab River at Minab (27º09'30"N, 57º04'E);
CMNFI 1979-0145, 4, 14.8-25.4 mm standard length, Hormozgan, Geru River drainage (26º55'N, 57º01'30"E);
CMNFI 1979-0149, 7, 29.0-49.4 mm standard length, Hormozgan, stream north of Bandar Abbas (27º36'N, 56º14'E);
CMNFI 1979-0152, 1, 62.2 mm standard length, Hormozgan, Shur River drainage (28º09'N, 55º43'E);
CMNFI 1979-0178, 23, 25.1-66.9 mm standard length, Hormozgan, Sarzeh River draiange (27º36'N, 56º15'E);
CMNFI 1979-0180, 1, 42.7 mm standard length, Hormozgan, stream 3 km east of Essin (27º19'N, 56º17'30"E);
CMNFI 1979-0181, 1, 44.0 mm standard length, Hormozgan, Kul River drainage (27º17'30"N, 56º03'30"E);
CMNFI 1979-0186, 8, 30.2-64.6 mm standard length, Hormozgan, stream and pools at Sar Khun (ca. 27º24'30"N, ca. 56º25'E);
CMNFI 1979-0187, 9, 32.1-57.9 mm standard length, Hormozgan, stream and pools at Sar Khun (ca. 27º23'30"N, ca. 56º26'E);
CMNFI 1979-0312, 10, 26.6-35.6 mm standard length, Baluchestan, dam on Bampur River (27º11'N, 60º46'E);
CMNFI 1979-0315, 1, 23.8 mm standard length, Baluchestan, Bampur River 2 km north of Karevandar (27º51'N, 60º46'E);
CMNFI 1979-0324, 1, 29.6 mm standard length, Baluchestan, Bampur River at Sa'idabad (27º11'N, 60º22'E);
CMNFI 1979-0329, 2, 25.4-30.8 mm standard length, Baluchestan, stream at Zaminbandan (27º02'N, 61º20'E);
CMNFI 1979-0411, 1, 60.4 mm standard length, Hormozgan, Minab River (27º24'N, 57º12'E);
CMNFI 1979-0412, 9, 22.9-39.3 mm standard length, Hormozgan, spring at Saras (27º30'N, 57º34'E);
CMNFI 1979-0416, 39, 15.1-46.8 mm standard length, Hormozgan, Ab Garm-e Ganow
(ca. 27º26'N, ca. 56º20'E); CMNFI 2007-0051, 10, 29.5-43.7 mm standard length, Hormozgan, upper Kol River basin (28º19'N, 55º55'E);
CMNFI 2007-0055, 5, 30.9-44.6 mm standard length, Hormozgan, Minab River basin (27º47'N, 57º12'E);
CMNFI 2007-0056, 2, 32.1-54.2 mm standard length, Kerman, qanat at Kahnuj (27º58'N, 57º45'E);
CMNFI 2007-0058, 7, 36.7-51.7 mm standard length, Fars, headwaters of Gowdar River (ca. 27º24'N, ca. 54º16'E).
Garra rossica
(Nikol'skii, 1900)
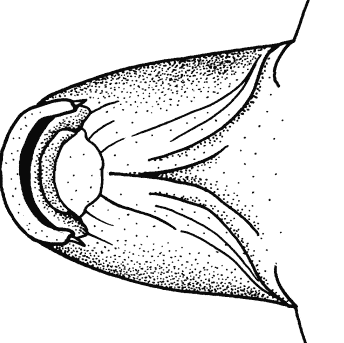
Common names
mahi-e sangi, mahi-e sang lis, anjarak.
[diskognat in Russian; pathar chat or patherchatta in Pakistan].
Systematics
Discognathus phryne Annandale, 1919 described "from
Seistan" is a synonym. Menon and Yazdani (1968) give Nasratabad,
Seistan as the type locality. G. rossica has been placed in the
genera Discognathus Heckel, 1843 and Discognathichthys
Bleeker, 1859, here considered to be synonyms.
Discognathus rossicus var. nudiventris Berg, 1905 was
described from "Schiwar" in Iran for specimens with a naked
abdomen, thoracic region and groove on the back anteriorly. The
distribution of these specimens overlaps with that of the type form
and they are not given independent taxonomic recognition here. Berg
(1949) later placed them as an infraspecies.
The syntypes of Discognathus rossicus are in the Zoological
Institute, St. Petersburg (ZISP 10365), the type locality in Latin on
page 239 being "Flum. Tedschent in prov. Transcasp. Zarudnyi.
1892 (4)" while on p. 240 are the localities "Habitat in flumine Tedshent in provincia Transcaspiensi, nec noc in Persia
orientale ad Kirmanum orientale" (Nikol'skii, 1900), and confirmed
by me. However there were 3 fish in the jar (45.0-54.5 mm standard
length) although 4 are listed in the catalogue and in the type
description. Berg (1905) lists 3 fish but in Berg (1949) lists only 2.
Other materials listed by Nikol'skii (1900) from eastern Iran and
Kerman (ZISP 11113, 11703, 11704, 11705, 11708) are apparently not
types of rossicus although Berg (1949) indicates that 11704
("Neizar in Seistano") and 11705 ("Ljabeab in Seistano")
are part of the type series from Iran. Eschmeyer et al. (1996) also list
ZIL (= ZISP) 10665 (4) as part of the type series, perhaps a misprint
for ZISP 10365.
The types for the var. nudiventris are in ZISP 11113, listed
by Berg (1905) as 2 fish from Schivar, by Berg (1949) as being 4 fish,
not numbered in the ZISP catalogue and with 5 fish in the jar
(45.6-66.2 mm standard length). ZISP 11113 appears to have been
renumbered in part as 11703 and 11708 according to Berg (1949). ZISP
11708 is listed in Berg (1949) as 13 specimens and 11703 seems to be also 13
specimens. The type localities
for var. nudiventris are for ZISP 11113 "Shivar, north of
Nikh (Nekh), north-east Kerman, basin of L. Hamun, 23 VI 1896, N.
Zarudnyi", for ZISP 11708 "Podaghi, 28°08'N,
north-north-east of Bazman, eastern Kerman, 6 VII 1898, N. Zarudnyi"
and 11703 is probably "Neizar in Seistan,
N. Zarudnyi" according to Berg (1949) (the catalogue number 11703 does not
appear under the description of materials in Berg (1949), possibly omitted in
error, and it is deduced here that it should have preceded the locality cited).
This is not critical as the variety or infraspecies has no taxonomic status.
The holotype of Discognathus phryne is in the Zoological
Survey of India, Calcutta (ZSI F9787/1) (Annandale, 1919b; Menon and
Yazdani, 1968), a syntype (listed as a cotype) measuring 42.2 mm
standard length from "Baluchistan" with the annotation
"Ind. Mus. Ex. F 9789/1" is in the Natural History Museum,
London (BM(NH) 1919.8.16:1) with another syntype (cotype) from "Quetta"
measuring 32.2 mm standard length with the annotation "Ind. Mus.
Ex. F 9790/1" (BM(NH) 1919.8.16:2).
Key characters
The single pair of small maxillary barbels (sometimes an anterior
pair), absence of a free anterior margin to a weakly developed
adhesive disc on the lower head surface, gill raker count, and
distribution distinguishes this species. It is separated from the
closely related, but geographically separated G. variabilis by smaller size, head length
longer than caudal peduncle length, head length equal to or longer
than pectoral fin length, distinctly emarginate caudal fin, and dorsal
fin origin mid-way between snout tip and caudal fin base or closer to
caudal fin base (Berg, 1949).
Morphology
Dorsal fin with 2-3 unbranched and 6-7, usually 7, branched rays,
anal fin with 2-3 unbranched and 5 branched rays, pectoral fin with
11-16 branched rays and pelvic fin with 7-8 branched rays. Lateral
line scales 33-46. The mid-line of the back, and the chest and belly
are naked in some populations. Some fish have only 3-4 rows of scales
below the lateral line and this character is extremely variable (Berg,
1949). Scales are a vertical ovoid with an anterior or subcentral
anterior focus. The posterior scale margin is rounded and elongate,
the dorsal and ventral margins are rounded and merge into the
posterior margin and the anterior margin has slight indentations above
and below a shallow, rounded central protuberance. However some scales
may be squarish with rounded corners and scale shape can be very
variable. Circuli are fine. Radii are found on all fields, moderate to
very numerous although this is individually variable. A pelvic
axillary scale may be present or absent. Gill rakers on the lower arm
9-11, 10-12 total (but see below), almost reaching the adjacent raker when appressed.
Pharyngeal teeth 2,4,5-5,4,2, 3,4,5-5,4,3, 2,4,5-5,4,3, 3,4,5-5,4,2,
2,4,5-5,3,2, 2,4,5-5,4,1, 2,4,4-4,4,2, 2,3,5-5,3,2 or 1,3,5-5,3,1.
Teeth are conical to flattened with oblique but flattened crown which
is slightly concave. Rarely crowns are blade-like and lack the
flattened crown. Specimens from the Hari River basin have scales on
the thorax and ridge of the back in front of the dorsal fin while
those from Sistan lack scales in these areas (Annandale and Hora,
1920; Saadati, 1977). The anterior pair of barbels are usually absent
but may be present and minute or even moderately well-developed. Some fish may
lack barbels entirely. The gut is very elongate and coiled.
Meristics for Iranian specimens:- dorsal fin branched rays 6(5) or
7(54); anal fin branched rays 5(59); pectoral fin branched rays 11(2),
12(14), 13(14), 14(15), 15(8) or 16(4); pelvic fin branched rays 7(39)
or 8(14); lateral line scales 34(3), 35(6), 36(17), 37(10), 38(4),
39(7), 40(1), 43(1), 44(1), 45(1) or 46(1); total gill rakers 11(7),
12(18), 13(18), 14(9), 15(3) or 16(2); pharyngeal teeth 2,4,5-5,4,2
(15), 2,4,5-5,3,2(2), 2,4,5-5,4,1(1), 3,4,5-5,4,2(1) or
2,4,5-5,4,3(1); and total vertebrae 34(11), 35(37) or 36(11).
Sexual dimorphism
Males in spawning condition bear small but evident tubercles on the
operculum and the head above the operculum, fine tubercles on top of
and anteriorly on the head, small tubercle son the pectoral fin rays
following the branching of the rays and on the first unbranched ray,
and there are a few minute tubercles on anterior flank scales.
Colour
The upper flanks and back are dark to greyish-brown, greenish-brown
or golden-brown and there may be large spots on the upper flanks. The
body is silvery overall. There is often a dark bar at the base of the
tail. Fins are colourless and the belly and lower head surface are
white to yellowish-white. The belly and lower head may be bright
yellow as are the neighbouring fin bases. The bases of dorsal fin
branched rays 3-5, and sometimes 6, have small dark spots. Young have
a bluish mid-lateral stripe along the flank. The peritoneum is black.
Colour is darker in clear than in muddy water.
Size
Reaches 9.5 cm.
Distribution
This species is found in the Mashkel (= Mashkid) River basin of Pakistan (Mirza,
1992), the Tedzhen and Murgab River drainages of the former U.S.S.R., and the Tedzhen River,
Bejestan, Sistan, Dasht-e Lut, Hamun-e Jaz Murian and Makran basins of Iran (Nikol'skii, 1900; Berg,
1905; Berg, 1949; Menon, 1964; Spillman, 1972; Abdoli, 2000). Localities from
these authors include the following and indicate the wide and common
distribution of the species: Nekh, Shivar north of Nekh basin of Lake Hamun,
Lab-i-ab, Hamun-e Farah in the Sistan basin, Deh Salm in the Lut basin, Laadas,
Sarhad Region near Kuh-i Taftan, Podaghi north-northeast of Bazman, Chanf
between the Makran and the Bampur River, headwaters of the Sarbaz River, Salabad
130 km south of Birjand, Shusef qanat, Khunik Pa'in and Khaneh Sharef on Birjand
to Zahedan highway, and Hormak south of Zabol.
Records of Discognathus variabilis Heckel, 1843 from Sistan
by Nikolskii (1899) and Regan (1906) are G. rossica (Menon, 1964).
Zoogeography
See under Garra persica. G. rossica is related to Garra
variabilis of the Tigris-Euphrates basin.
Habitat
Found in pools and slow-flowing ditches and channels and in reed beds in Sistan.
Age and growth
Sexual maturity is attained at 2-3 years. Females grow more rapidly than males.
Food
Gut contents include green filamentous algae, higher plant fragments and sand grains.
Reproduction
Spawning occurs in the summer and up to 984 eggs are produced. Some
fish still contain undeposited eggs in late July. Egg diameter is up
to 1.06 mm (Nikol'skii, 1945). In southern Iran, fish with 1.1 mm eggs
have been collected on 14 November and on 2 December eggs were 1.3 mm.
On 8 May eggs were 1.2 mm suggesting either early spawning or
prolonged retention of eggs. The most tuberculate males were found in
both November and May.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This species is widely distributed in eastern Iran and does not appear to be
under significant threat.
Further work
Further studies on its biology would clarify details of life history. Its
wide distribution in isolated basins may have led to some divergence and
speciation that is not readily detected with morphological studies and molecular
work on this species might be rewarding.
Sources
Type material: See above, Discognathus rossicus (ZISP 10365), var. nudiventris
(ZISP 11113), and Discognathus phryne (BM(NH) 1919.8.16:1 and BM(NH) 1919.8.16:2).
Iranian material: CMNFI 1979-0091, 5, 24.6-58.3 mm standard length, Khorasan, qanat at Nehbandan (31º32'N, 60º02'E);
CMNFI 1979-0226, 5, 37.2-49.0 mm standard length, Sistan, pool near Kuh-e Khajeh (30º57'N, 61º17'E);
CMNFI 1979-0227, 6, 48.1-61.1 mm standard length, Sistan, naizar at Kuh-E Khajeh (30º57'N, 61º16'E);
CMNFI 1979-0230, 2, 29.2-43.4 mm standard length, Sistan, Jehil-e Puzak at Gohoor-ghoori (ca. 31º15'N, ca. 61º42'E);
CMNFI 1979-0236, 7, 18.3-47.3 mm standard length, Sistan, irrigation ditch 27 km from Zabol (ca. 30º52'N, ca. 61º22'E);
CMNFI 1979-0238, 11, 15.3-29.2 mm standard length, Sistan, irrigation ditch 11 km south of Zabol (30º57'N, 61º27'30"E);
CMNFI 1979-0315, 2, 24.9-50.3 mm standard length, Baluchestan, Bampur River 2 km north of Karevandar (27º51'N, 60º46'E);
CMNFI 1979-0316, 9, 35.7-53.4 mm standard length, Baluchestan, stream 68 km south of Iranshahr (26º48'N, 61º02'E);
CMNFI 1979-0326, 1, 39.0 mm standard length, Baluchestan, Ughin River south of Pip (ca. 26º35'N, ca. 60º02'E);
CMNFI 1979-0327, 5, 37.3-46.7 mm standard length, Baluchestan, stream 26 km south of Pip (26º32'N, 59º57'E);
CMNFI 1979-0330, 68, 14.7-65.1 mm standard length, Baluchestan, stream 22 km west of Qaleh-ye Zaboli (27º02'30"N, 61º26'E);
CMNFI 1979-0336, 30, 22.4-31.6 mm standard length, Baluchestan, qanat 7 km from Khash (28º10'N, 61º15'E);
CMNFI 1979-0339, 3, 40.6-51.0 mm standard length, Baluchestan, Tahlab River drainage 16 km from Mirjaveh (28º56'30"N, 61º21'E);
CMNFI 2007-0025, 8, 36.6-47.1 mm standard length, Khorasan, qanat south of Birjand (ca. 32º24'N, ca. 59º49'E);
CMNFI 2007-0026, 19, 36.3-62.9 mm standard length, Khorasan, qanat at Shusf (31º48'N, 60º01'E);
CMNFI 2007-0027, 13, 31.4-60.7 mm standard length, Khorasan, qanat at Khvansharaf (31º34'N, 60º06'E);
CMNFI 2007-0028, 13, 36.3-58.9 mm standard length, Khorasan, qanat at Khunik-e Pa'in (31º28'N, 60º06'E);
CMNFI 2007-0029, 7, 35.2-60.7 mm standard length, Baluchestan, qanat at Hormak (29º58'N, 60º51'E);
CMNFI 2007-0031, 2, 36.0-45.5 mm standard length, Baluchestan, headwater of Bampur River (27º51'N, 60º46'E);
CMNFI 2007-0035, 9, 28.9-50.1 mm standard length, Baluchestan, stream west of Zaboli (ca. 26º58'N, ca. 61º27'E);
USNM 205905, 6, 30.2-36.9 mm standard length, Baluchestan, small springs in upper Sarbaz River basin (no other locality data).
Garra rufa
(Heckel, 1843)
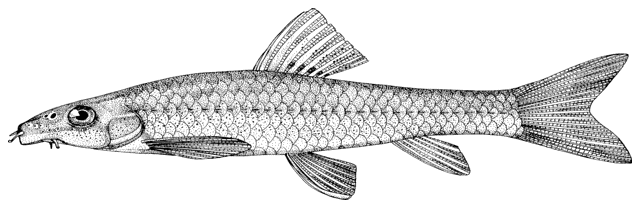


Common names
mahi-e sangi, mahi-e sang lis, shirbot, gel khorok, gel ra (and even gararufa or gara in Farsi).
[djulake; kokur ahmar or karkoor ahmar; garagoor; gassur achmar (= red gassur)
or gassur hadjari (gassur of the pilgrims) at Aleppo (= Haleb, Syria) according to Heckel (1843b), all in Arabic;
red garra].
Systematics
Discognathus obtusus Heckel, 1843 described from
"Aleppo" and "Mossul", Discognathus crenulatus
Heckel, 1849 described from the "Confluenten des Araxes, als aus
den Quellen des Saadi und dem Kara-Agatsch" (= probably includes
the Pulvar (= Sivan) River near Persepolis; Sa`di at 29°37'N, 52°35'E,
now within the city of Shiraz; and the Qarah Aqaj River; all in Fars),
Garra rufa gymnothorax Berg, 1949 from "Kulihan, Karun R.
basin, 6 IV 1904, N. Zarudnyi" and Garra rufa turcica
Karaman (1971) from the Ceyhan River basin in Turkey are synonyms (Krupp,
1985c). Records of Garra lamta (Hamilton, 1822) from
Iran are in error (Menon, 1964).
The types of Discognathus rufus are from "Aleppo"
according to Heckel (1843b). The syntypes of Discognathus rufus
according to Krupp (1985c) are in the Naturhistorisches Museum Wien
under NMW 53240, 8 specimens, 59-108 mm standard length from Aleppo
and 1 syntype is in the Senckenberg Museum Frankfurt under SMF 553
(formerly NMW), 103 mm standard length and also from Aleppo. The
catalogue in Vienna lists 6 specimens. One specimen from NMW 53240, 112.3 mm standard
length (as measured by me), was designated as the lectotype and 7 fish,
60.2-97.5 mm standard length as paralectotypes by F. Krupp, 29 October 1984, and
published in Krupp and Schneider (1989).
Four syntypes of Discognathus obtusus, 46-134 mm standard
length, are under NMW 53238 and 2 syntypes, 65-92 mm standard length,
are under SMF 5408 (formerly NMW and also numbered SMF 447)(65.1-93.1 mm
standard length). A further 10 fish under NMW 53257
and measuring 31.5-106.2 mm standard length are also indicated on the
jar in Vienna as syntypes but this is probably in error as the
catalogue lists 6 fish. Eschmeyer et al. (1996) give only 1
syntype under NMW 53257, 4 syntypes under NMW 53238, 1 dried syntype
under NMW 79372, 1 syntype in the Senckenberg Museum Frankfurt under
SMF 447 and 2 syntypes under SMF 5408 (formerly NMW).
The syntypes of Discognathus crenulatus are in the
Naturhistorisches Museum Wien under NMW 53236 (14 specimens) from the
Qarah Aqaj River and 53237 (6) from Sa`di measuring 33-79 mm standard
length (Kähsbauer, 1964). The 14 specimens under NMW 53236 measure
24.0-75.9 mm standard length and 7 (not 6) specimens under NMW 53237
measure 35.4-56.6 mm standard length according to my observations.
Neither the record of Kähsbauer (1964) nor my own data from jars on
the shelves accord with the catalogue in Vienna which gives 10 or 8
and 6 or 5 specimens respectively for these two syntype localities.
The syntypes of Garra rufa gymnothorax are in the Zoological
Institute, St. Petersburg (ZISP 13214), there being 6 fish in the
catalogue and 6 in the jar although Berg (1949) lists 7 in his
description. They measure 30.5-44.9 mm standard length. The date in
Berg (1949) is 6.VI.1904 while in the catalogue it is 4.III.1904 and
in the jar 24.III.1904, variations not accountable by old and new
styles of dating (13 days apart). A further collection listed by Berg
(1949), (ZISP 24435), is not listed as type material in the text nor
in the jar but the catalogue suggest that they are (Eschmeyer et al.
(1996) list these 10 fish as syntypes). ZISP 24354 is from "Ziaret-Seid-Hasan,
Mesopotamiya". The type series may be only ZISP 13214 as in the
text. Eschmeyer et al. (1996) list ZIL (= ZISP) 13215, 17 fish,
and ZIL 24436, 3 fish, as syntypes also.
Bianco and Banarescu (1982) referred their material from the
Hablehrud and Mand River to Garra rufa crenulata as
these fish had fewer scales (29-32 for Mand and 33-34 for Hablehrud
versus 35-38 from the Tigris-Euphrates) and fewer gill rakers (15-21 versus 25-27
in Tigris-Euphrates specimens). Possible syntypes of crenulata had
intermediate scale counts (31-34) between Mand and Hablehrud fish.
These authors suggest that there may be distinct subspecies in these
two rivers. Their sample sizes are too small in my opinion to warrant
subspecies recognition. Berg (1949) was uncertain of the status of this taxon.
A Principal Components Analysis on 448 fish from the Hormuz, Lake
Maharlu, Gulf and Tigris River basins and Sa`di's Tomb using 20
morphometric and 5 meristic characters did not separate any of these groups.
Note that fish form the Hormuz basin, rivers draining to the Straits of Hormuz,
modally had 7 branched dorsal fin rays (as in G. persica, see table
below) but branched caudal fin rays were modally 17 (not 16 as in G. persica).
There may be some introgression in this region of Iran.
Ghalenoei et al. (2010) examined fish from 13 stations in the Tigris and
Persian Gulf basins and found the Mand River population was separated from the
rest, which overlapped each other.
Menon (1964) and Karaman (1971) consider Garra persica to be
a synonym but this species is regarded here as distinct. Both these
authors refer specimens from the Tigris River basin of Iran (and
therefore all Iranian specimens) to a subspecies, Garra rufa obtusa,
distinguished from the type subspecies in Syria, Lebanon, Israel and
Jordan by having a variable number of dorsal fin branched rays (7-8),
the anal aperture further forward, and the anal fin origin half way
between the pelvic fin base and the caudal fin base as opposed to
closer to the pelvic base. Krupp (1985c) synonymises Garra rufa
obtusa with the type subspecies.
Fish lacking scales on the breast were named by Berg (1949) as Garra
rufa gymnothorax but this is a variable character widespread among
cyprinid fishes in Iran and is unlikely to be of systematic significance.
Key characters
Two pairs of barbels are present, the adhesive disc is well
developed with a free anterior margin, the dorsal fin has 8 branched
rays modally, and the caudal fin 17 branched rays modally.
Morphology
Dorsal fin with 2-3, usually 3, unbranched and 7-9 branched rays
with a very strong mode at 8, anal fin with 2-3 unbranched and 4-6
with a very strong mode at 5 branched rays (99.6% of 534 specimens
from Iran). Pectoral fin branched rays 12-14 (in literature, but see table), pelvic fin branched rays
7-8 (but see table). Lateral line scales 31-38 (but see table), scales from the dorsal fin origin to
the lateral line 3-6, from the lateral line to the pelvic fin origin
2-5, predorsal mid-line scales 9-13, and scales around the caudal
peduncle 12-17 with a strong mode at 16. Pharyngeal tooth formula
2,4,5-5,4,2 or 2,4,4-4,4,2 (3,3,5-5,3,3 in Heckel (1843b)). Teeth are
hooked at the tip. The short gill rakers number 16-21, 12-17 on the
lower arm. In Iranian specimens the range is 14-26 (a range only is
given since rakers are difficult to count on the arch ends with
accuracy and may be related to age). The upper lip is delicately fimbriated. Pharyngeal teeth in Iranian
specimens? Total vertebrae in Iranian specimens 32-37 (see table). The chromosome number is
probably 2n=52 (Klinkhardt et al.,
1995) although Ünlü et al. (1997) give 2n=38 for Turkish specimens with
26 meta- to submetacentric chromosomes and 12 telo- to subtelocentric chromsomes (NF=64)
and Gözükara and Çavaş (2004) gave 2n = 44 for Turkish specimens with 22
metacentric and 20 submetacentric chromosomes and 2 acrocentric ones (NF=85).
Esmaeili and Piravar (2007) examined fish from the Rodbal River in Fars and
found 2n=50 with arm number NF=84 and the karyotype formula of 10 metacentric, 24
sub-metacentric and 16 sub-telocentric chromosomes.
Some fish are very rounded in cross section while others are more terete,
possibly related to habitat as observed in other cyprinid fishes..
Meristics for Iranian specimens:-
Basin/Dorsal fin branched rays |
7 |
8 |
9 |
x |
S.D. |
|
Hormozgan |
27 |
6 |
|
7.2
|
0.39
|
|
Lake Maharlu |
|
90 |
2 |
8.0
|
0.15
|
|
Sa`di's Tomb |
5 |
20 |
|
7.8
|
0.41
|
|
Gulf |
16 |
173 |
1 |
7.9
|
0.29
|
|
Tigris River |
15 |
176 |
3 |
7.9
|
0.30
|
|
Basin/Pectoral fin branched rays |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
18 |
x |
S.D. |
|
Hormozgan |
|
|
1 |
3 |
18 |
10 |
1 |
|
14.2
|
0.78
|
|
Lake Maharlu |
|
1 |
2 |
15 |
50 |
23 |
1 |
|
14.1
|
0.78
|
|
Sa`di's Tomb |
|
|
5 |
16 |
2 |
2 |
|
1 |
13.2
|
1.24
|
|
Gulf |
|
1 |
4 |
57 |
84 |
42 |
1 |
|
13.9
|
0.81
|
|
Tigris River |
1 |
1 |
10 |
54 |
93 |
33 |
2 |
|
13.8
|
0.88
|
|
Basin/Pelvic fin branched rays |
6 |
7 |
8 |
9 |
x |
S.D. |
|
Hormozgan |
|
20 |
12 |
|
7.4
|
0.49
|
|
Lake Maharlu |
|
8 |
84 |
|
7.9
|
0.28
|
|
Sa`di's Tomb |
|
9 |
16 |
|
7.9
|
0.27
|
|
Gulf |
1 |
110 |
79 |
|
7.4
|
0.50
|
|
Tigris River |
2 |
51 |
138 |
3 |
7.7
|
0.50
|
|
Basin/Lateral line scales |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |
40 |
x |
SD |
|
Hormuz |
|
|
|
|
|
|
2 |
7 |
19 |
10 |
1 |
|
|
|
|
33.0
|
0.87
|
|
Lake Maharlu |
|
|
|
|
1 |
4 |
13 |
21 |
32 |
30 |
9 |
|
|
|
|
31.2
|
1.54
|
|
Sa`di's Tomb |
|
|
|
|
|
2 |
|
6 |
6 |
7 |
4 |
|
|
|
|
33.1
|
1.39
|
|
Gulf |
1 |
1 |
5 |
16 |
25 |
24 |
25 |
42 |
36 |
19 |
5 |
|
|
|
|
31.2
|
2.06
|
|
Tigris River |
|
|
|
|
|
2 |
2 |
24 |
44 |
59 |
45 |
9 |
2 |
1 |
1 |
33.9
|
1.34
|
|
Basin/Total vertebrae |
32 |
33 |
34 |
35 |
36 |
37 |
x |
S.D. |
|
Hormozgan |
|
7 |
24 |
7 |
3 |
|
34.2 |
0.78 |
|
Lake Maharlu |
|
6 |
38 |
21 |
3 |
|
34.3 |
0.70 |
|
Sa`di's Tomb |
|
|
14 |
9 |
|
|
34.4 |
0.50 |
|
Gulf |
2 |
15 |
36 |
34 |
9 |
|
34.3 |
0.93 |
|
Tigris River |
|
23 |
52 |
76 |
17 |
2 |
34.5 |
0.92 |
Sexual dimorphism
Large males become heavily tuberculate on the front and sides of
the snout and in a band from the eye to the nostril and across to the
other nostril and eye as illustrated in Fowler and Steinitz (1956) for
a specimen from `Ain Umm Keishik in Iraq. A deep,
tubercle-free groove is apparent between the upper band of tubercles
through the nostrils and the tubercles on the snout above the mouth.
Colour
Overall colour is brownish-olive to dark green with darkly mottled
flanks and a yellowish to whitish belly. The head and flanks may be a
rusty-red, bronze or golden. A dark or bluish-green band runs along
the whole flank ending in a spot on the caudal fin base. Much of the
body may be blackish with only the belly creamy. Others are a light
olive-green with lime-green highlights giving an iridescent effect
especially on upper anterior flank scales. There is a black,
greenish-blue, lime-green or dusky-blue spot behind the upper corner
of the gill opening, sometimes extending as a bar to the pectoral fin
base where the skin is also blue. Fins can be yellowish with darker
margins. The pectoral fins can be orange-pink dorsally, grey-white or
slightly orange-pink ventrally. The pelvic and anal fins may be orange
with the fin rays yellow posteriorly in the anal fin but yellow
mesially in the pelvic fin. The bases of the pectoral, pelvic and anal
fins are orange-red in breeding males and the caudal fin is orange.
The caudal fin can be orange to red ventrally and yellow dorsally.
There is a black spot at the caudal fin base and the upper caudal lobe
may have a few dark grey spots. The dorsal fin is dark green with
reddish pigment at its middle. There is usually a dark spot at the
bases of each of the middle 4-5 dorsal fin rays. In some specimens the
dorsal fin is orange with yellow posterior rays. The pectoral, dorsal
and caudal fin rays may be olive to black rather than yellow or
orange. The iris is bright yellow, orange or red.
There is variation in colouration. Some fish are pale while others
are very dark; the spots on the dorsal fin may extend two-thirds of
the way up the fin rather than being restricted to the base; and the
flanks may not be mottled. Fish from muddy water are a sickly grey
with the body mottled and the lower caudal lobe dark. Their colour
darkens and becomes brighter after immersion in ice water. Fish from
deep in qanats are very pale.
Size
Attains 24 cm total length in the Tigris River in Iraq (Rahemo, 1995).
Reaches 15.9 cm (Krupp, 1985c), over 17 cm according to Heckel
(1843b). Fish up to 18.5 cm total length are known from Khuzestan.
Distribution
Found in the Tigris-Euphrates basin and the Ceyhan, Orontes (= Asi), Quwayq
and Jordan river basins and coastal drainages of the eastern Mediterranean as
well as much of southern Iran.
In Iran it is found in the Tigris River, Gulf, Lake Maharlu, and Kor River
basins and the Hormuz basin (Berg, 1949; Menon, 1964; Bianco and
Banarescu, 1982; Gh. Izadpanahi, pers. comm., 1995; M. Rabbaniha, pers.
comm., 1995; Abdoli, 2000).
Zoogeography
The wide distribution in Southwest Asia and inadequate examination of
variation may mask distinct taxa, although this is not apparent on morphometric
and meristic grounds. If such variation is valid, then taxa may
reflect vicariant events.
Habitat
This is the commonest species in catches in southwestern Iran,
followed by Cyprinion macrostomum. In areas under human
influence in Lorestan, such as the lower reaches of rivers and near
cities, it is more common than in higher, pristine waters. As well as grazing on
exposed rock surfaces in streams, it can be found under pebbles on the stream
bed.
Age and growth
Rahemo (1995) reported fish up to age 7 from the Tigris River, Iraq in a
parasitological study. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 291 Iranian fish measuring 2.28-11.82 cm standard length. The a-value
was 0.0265 and the b-value 2.919 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases)
Abedi et al. (2010) examined the age in a population in the Armand Stream
in Chahar Mahall va Bakhtiari Province. Length and weight were significantly
correlated (W = -5.076 + 3.112 log TL for sexes combined, -5.092 + 3.134 log TL
for males and -5.036 + 3.089 log TL for females), growth was isometric, numbers
of males and females were not significantly different, life span was up to 4
years and most fish were 2-3 years old.
Patimar et al. (2010) examined fish from the Kangir River in Ilam and
found a maximum age of 5+ years, the most frequent size class was 65-70 mm for
both sexes, negative allometric growth in both sexes, a balanced overall sex
ratio but with males predominant at smaller sizes and females at larger sizes,
and von Bertalanffy growth parameters were L∞
= 108.453 mm, K = 0.449 year-1, t0 = 0.192
years for males and L∞ =
115.516 mm, K = 0.420 year-1, t0 = 0.088
years for females. Males grew faster than females. The growth index
ф' was 8.572 for males and 8.631 for
females. Fish were smaller and of lower weight than fish from the Zanjiran
spring stream system in Fars, southern Iran studied by Yazdanpanah (2005), and
this was attributed to severe ecological conditions. The Zanjiran fish has a
similar most frequent size class of 60-70 mm, but growth was positively
allometric and the sex ratio was more balanced.
Food
Gut contents include diatoms, algae and large quantities of sand in fish
examined from Iran. Younis et al. (2001b) found Shatt al Arab fish feeding mainly on
organic detritus, followed by diatoms and algae, with arthropods ranking third.
A study by Yalçin-Özdilek and Ekmekçi (2006) in the Asi (=
Orontes) River in Turkey demonstrated that this species is a grazer on aquatic
plants, mostly consisting of benthic cyanobacteria, chrysophytes and
phytoplankton with included rotifers and protozoans. Both season and location in
a stream affected the composition of the diet with season the most important factor.
Reproduction
Al-Rudainy (2008) gives sexual maturity at 2-3
years, 10 cm in length and a weight of 50 g for Iraqi fish. Spawning took place
in May and June with eggs deposited on vegetation and rocks with a relative
fecundity up to 542 eggs/g. Bardakci, Ozansoy and Koptagel (at www.epress.com/w3jbio/vol5/bardakci/paper.htm,
downloaded 29 March 2001) note depression of vitellogenesis in a hot
spring population in Turkey, perhaps due to temperature and
starvation. A nearby stream population has a higher gonadosomatic
index. Ovaries increased in size and weight from May to July in both
localities although the hot spring had fewer mature oocytes and more
atretic oocytes at various development stages. High temperatures and
poor food conditions in some Iranian habitats may be limiting factors
in reproduction for this species.
The Armand Stream population (Abedi et al.,
2010) had a prolonged and active reproductive season from March to September, an
adaptation to unstable environmental conditions. All mature oocytes are spawned
at once but some may be retained for later spawning. In addition, different
individuals release eggs and sperm at different times. Average egg diameter was
0..67 mm, maximum 1.98 mm, with highest diameter in May and the lowest in
November. Absolute and relative fecundity were 1179.65 and 109.4 respectively on
average. Maximum absolute fecundity reached 3794 eggs. The Kangir River fish
(Patimar et al., 2010) had a maximum fecundity of 13,927 eggs and a
maximum relative fecundity of 2345.72 eggs/g. Egg diameters reached 1.7 mm.
Reproduction occurred in April-May with the highest average gonadosomatic index
for males of 4.21 in April and for females of 7.85 in May. The Zanjiran
population (Yazdanpanah, 2005) had the highest
gonadosomatic index for males in March and for females in April with maximum
averages at 6.49 for males and 16.98 for females. The range and mean of absolute
fecundity for this population were much lower than that in the Kangir River
(184-2396, mean 760 versus 1680-13,927, mean 5806), as was relative fecundity.
Parasites and predators
Jalali and Molnár (1990a) record two monogenean species, Dactylogyrus
spp., from this species in the Dez River. Gussev et al. (1993b)
describe two new species, Dactylogyrus rectotrabus and D.
acinacus, from this species in the Dez River, Khuzestan. Jalali et al. (2005) summarise the occurrence of
Gyrodactylus species in Iran and record G. sp. from fish in the Helleh River.
Eaten by Silurus triostegus at Baghdad (notes on a specimen in the Field Museum of Natural
History, Chicago (FMNH 51251)).
Economic importance
Ündar et al. (1990) identify this species and Cyprinion
macrostomum as the "doctor fish" of the Kangal hot
spring in Turkey (Warwick and Warwick, 1989; Kürkçüoğlu and Öz, 1989;
Bardakci, Ozansoy and Koptagel at www.epress.com/w3jbio/vol5/bardakci/paper.htm,
downloaded 29 March 2001, Bilke, 2004; Anonymous, 2007; and various newspaper and television reports). High
water temperatures around 35°C reduce the amount of plankton available as fish
food and the fish nibble away infected skin of humans who bathe in
these waters. This fish is known as "licker" (and Cyprinion
macostomum as "striker") from its behaviour in the spa pools. The healing properties are linked to the high level of
selenium (1.3 p.p.m.) in the water, selenium being beneficial in some
skin diseases and possibly UV light. The fish facilitate the action of the selenium
and UV light by softening and clearing away psoriatic plaque and scale, exposing the
lesions to the water and sunlight. However some lesions are made worse
and the fish can cause some new ones. Garra rufa is now widely used in
commercial facilities around the world for treating skin diseases and removing
dead skin. Jarvis (2011) gives a biological synopsis of this species, published
in Canada, since it is increasingly being used in private spa facilities and may
escape into the wild.

Conservation
A common species with a wide distribution and not under any specific threat.
Vulnerable in Turkey (Fricke et al., 2007).
Further work
Meristic counts summarised above show overlapping but significant variation
between drainage basins and may reflect recognisable taxa. Molecular studies
would help clarify this situation but is should be noting that variation of this
nature is to be expected in a wide ranging species and does not always warrant
taxonomic distinction.
Sources
Type material: See above, Discognathus rufus (NMW 53240 and SMF 553), Discognathus obtusus (NMW 53238 and SMF 5408), Discognathus crenulatus
(NMW 53236 and 53237), and Garra rufa gymnothorax (ZISP 13214), and see comments on other possible types.
Iranian material: CMNFI 1970-0540, 7, 22.2-41.4 mm standard length, Fars, qanat south of Kazerun (no other locality data);
CMNFI 1979-0018, 48, 21.5-64.9 mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0019, 4, 28.9-35.4 mm standard length, Fars, Barm-e Baba Haji (29º23'N, 52º40'E);
CMNFI 1979-0026, 2, 21.5-22.3 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0027, 1, 50.5 mm standard length, Fars, Chehel Cheshmeh (ca. 29º43'N, ca. 52º04'E);
CMNFI 1979-0033, 34, 23.7-72.0 mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0036, 1, 36.4 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0045, 16, 20.0-59.2 mm standard length, Fars, spring at Sa'adi's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 1979-0046, 1, 43.7 mm standard length, Fars, qanat at Barm-e Dalak (ca. 29º35'N, ca. 52º38'E);
CMNFI 1979-0047, 2, 36.2-67.6 mm standard length, Fars, spring source of Ab-e Paravan marshes (ca. 29º34'N, ca. 52º42'E);
CMNFI 1979-0048, 1, 40.1 mm standard length, Fars, spring and marsh northeast side of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0075, 31, 12.5-57.1 mm standard length, Fars, Mand River at Pol-e Kavar (29º11'N, 52º41'E);
CMNFI 1979-0109, 1, 74.3 mm standard length, Fars, Mand River at Shahr-e Khafr (28º56'N, 53º14'E);
CMNFI 1979-0111, 8, 30.7-62.0 mm standard length, Fars, stream on Shiraz-Bushehr road (29º37'30"N, 52º21'E);
CMNFI 1979-0112, 5, 55.0-77.1 mm standard length, Fars, stream draining Soltanabad Marshes (29º29'N, 52º38'30"E);
CMNFI 1979-0113, 4, 40.8-63.7 mm standard length, Fars, spring at Sa'adi's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 1979-0115, 5, 57.7-66.5 mm standard length, Fars, spring at Sa'adi's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 1979-0120, 5, 27.6-66.3 mm standard length, Bushehr, Dalaki River near Konar Takhteh (29º28'N, 51º21'E);
CMNFI 1979-0125, 2, 99.0-121.4 mm standard length, Bushehr, Dalaki River near Dalaki (ca. 29º28'N, ca. 51º21'E);
CMNFI 1979-0128, 6, 26.6-32.1 mm standard length, Shur River between Atashkadeh and Firuzabad (28º51'N, 52º31'E);
CMNFI 1979-0129, 43, 24.8-46.9 mm standard length, Fars, spring 2 km north of Farrashband (28º54'N, 52º04'E);
CMNFI 1979-0131, 10, 16.9-46.6 mm standard length, Fars, Ab-Arak River 24 km from Qir (28º38'N, 52º49'E);
CMNFI 1979-0132, 11, 23.1-49.5 mm standard length, Fars, Shur River 54 km from Firuzabad (28º35'N, 52º58'E);
CMNFI 1979-0155, 11, 27.1-42.9 mm standard length, Fars, spring at Gavanoo village (28º47'N, 54º22'E);
CMNFI 1979-0156, 20, 33.8-56.7 mm standard length, Fars, qanat at Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0157, 10, 40.6-88.6 mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E);
CMNFI 1979-0158, 13, 35.3-54.2 mm standard length, qanat over Qasook River (28º54'N, 53º53'30"E);
CMNFI 1979-0161, 11, 43.1-92.2 mm standard length, Fars, qanat on Shiraz-Neyriz road (29º10'30"N, 53º41'E);
CMNFI 1979-0193, 3, 22.8-27.1 mm standard length, Fars, river 8 km from Darab (28º45'N, 54º27'30"E);
CMNFI 1979-0195, 3, 44.3-59.4 mm standard length, Fars, jube stream of road to Fasa (ca. 28º54'N, ca. 53º53'30"E);
CMNFI 1979-0199, 4, 37.3-45.0 mm standard length, Fars, qanat 18 km from Jahrom (ca. 28º23-25'N, ca. 53º31-40'E);
CMNFI 1979-0200, 5, 27.6-43.6 mm standard length, Fars, Mand River tributary 13 km from Jahrom (28º36'N, 53º36'30"E);
CMNFI 1979-0202, 9, 19.1-25.1 mm standard length, Fars, Mand River (29º01'N, 53º00'E);
CMNFI 1979-0206, 2, 50.9-51.9 mm standard length, Fars, qanat near Runiz-e Pa'in (29º12'N, 53º40'E);
CMNFI 1979-0241, 6, 35.7-49.2 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0271, 8, 34.7-56.7 mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0273, 15, 40.9-58.2 mm standard length, Lorestan, Kashkan River drainage 5 km from Khorramabad (33º26'N, 48º19'E);
CMNFI 1979-0274, 3, 24.4-32.8 mm standard length, Lorestan, Kashkan River drainage 20 km from Khorramabad (33º27'N, 48º11'E);
CMNFI 1979-0275, 7, 38.6-60.5 mm standard length, Lorestan, Kashkan River near Ma'mulan (33º25'N, 47º58'E);
CMNFI 1979-0276, 10, 42.1-70.0 mm standard length, Lorestan, Chamesk River (ca. 33º19'N, ca. 47º53'30"E);
CMNFI 1979-0277, 1, 128.4 mm standard length, Lorestan, Kashkan River drainage (33º30'N, 47º59'30"E);
CMNFI 1979-0278, 12, 53.9-89.3 mm standard length, Lorestan, Kashkan River draiange, Sarab Dowrah (33º34'N, 48º01'E);
CMNFI 1979-0279, 9, 33.5-117.3 mm standard length, Lorestan, Khorramabad River (33º37'N, 48º18'E);
CMNFI 1979-0288, 43, 22.9-102.3 mm standard length, Ilam and Poshtkuh, Gangir River at Sarab Ewan (33º50'N, 46º18'E);
CMNFI 1979-0289, 5, 57.2-101.9 mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0290, 6, 24.1-54.9 mm standard length, Kermanshahan, Diyala River drainage in Qasr-e Shirin (34º31'N, 45º35'E);
CMNFI 1979-0291, 7, 24.9-66.8 mm standard length, Kermanshahan, Diyala River draiange 15 km from Qasr-e Shirin (34º24'N, 45º37'E);
CMNFI 1979-0293, 8, 92.1-101.3 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1979-0304, 2, 41.6-43.0 mm standard length, Fars, Lake Famur (ca. 29º31'N, ca. 51º50'E);
CMNFI 1979-0350, 4, 28.1-33.5 mm standard length, Khuzestan, Marun River near Marun (30º39'30"N, 50º02'E);
CMNFI 1979-0364, 4, 36.0-50.6 mm standard length, Khuzestan, Karkheh River branch at Abdolkhan (31º52'30"N, 48º20'30"E);
CMNFI 1979-0365, 2, 32.1-32.8 mm standard length, Khuzestan, stream in Doveyrich River drainage (32º25'N, 47º36'30"E);
CMNFI 1979-0366, 2, 22.7-29.2 mm standard length, Khuzestan, stream 17 km west of Dehloran (32º45'30"N, 47º05'30"E);
CMNFI 1979-0367, 1, 38.0 mm standard length, Khuzestan, Meymeh River 11 km north of Dehloran (32º44'30"N, 47º09'30"E);
CMNFI 1979-0368, 4, 25.3-47.7 mm standard length, Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
CMNFI 1979-0369, 4, 24.9-37.7 mm standard length, Khuzestan, Shush River at Shush (32º12'N, 48º14'30"E);
CMNFI 1979-0371, 1, 37.3 mm standard length, Khuzestan, stream in Karkheh River drainage (32º05'N, 48º19'E);
CMNFI 1979-0374, 1, 62.2 mm standard length, Khuzestan, stream tributary to Bala River (32º40'N, 48º15'E);
CMNFI 1979-0375, 10, 37.9-71.1 mm standard length, Khuzestan, stream tributary to Bala River (ca. 32º45'N, ca. 48º14'30"E);
CMNFI 1979-0378, 1, 49.3 mm standard length, Khuzestan, stream tributary to Karkheh River (ca. 32º48'N, ca. 48º04'E);
CMNFI 1979-0379, 1, 63.1 mm standard length, Khuzestan, Dez River (32º12'N, 48º27'E);
CMNFI 1979-0382, 3, 34.7-38.0 mm standard length, Khuzestan, Karun River at Shushtar (32º03'N, 48º51'E);
CMNFI 1979-0383, 5, 31.9-53.0 mm standard length, Khuzestan, stream in Ab-e Shur drainage (31º59'30"N, 49º06'E);
CMNFI 1979-0384, 1, 63.3 mm standard length, Khuzestan, river in Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0385, 2, 44.9-46.8 mm standard length, Khuzestan, stream in Ab-e Shur drainage (ca. 32º01'N, ca. 49º07'30"E);
CMNFI 1979-0387, 2, 48.2-64.5 mm standard length, Khuzestan, stream in Jarrahi River drainage (31º25'N, 49º38'E);
CMNFI 1979-0388, 1, 55.2 mm standard length, Khuzestan, Zard River 21 km north of Ramhormoz (31º19'N, 49º44'E);
CMNFI 1979-0389, 2, 43.8-57.7 mm standard length, Khuzestan, Zard River at Bagh-e Malek (31º31'N, 49º53'30"E);
CMNFI 1979-0390B, 10, 33.8-69.7 mm standard length, Khuzestan, stream tributary to Zard River (31º29'N, 49º54'30"E);
CMNFI 1979-0391, 1, 39.7 mm standard length, Khuzestan, stream in Marun River drainage (31º28'N, 49º51'E);
CMNFI 1979-0392, 1, 42.6 mm standard length, Khuzestan, Zard River 25 km north of Ramhormoz (ca. 31º32'N, ca. 49º48'E);
CMNFI 1979-0396, 3, 22.7-35.1 mm standard length, Kheyrabad River 20 km from Behbehan (30º32'N, 50º23'30"E);
CMNFI 1979-0397, 2, 52.4-59.5 mm standard length, Khuzestan, stream tributary to Kheyrabad River (30º30'N, 50º28'E);
CMNFI 1979-0398, 1, 37.6 mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh, stream in Zohreh River drainage (30º24'30"N, 50º37'30"E);
CMNFI 1979-0399, 1, 41.0 mm standard length, Fars, stream near Basht in Zohreh River drainage (30º19'30"N, 51º15'E);
CMNFI 1979-0497, 2, 52.4-60.3 mm standard length, Fars, Mand River at Band-e Bahman (29º11'N, 52º40'E);
CMNFI 1979-0501, 8, 24.7-48.9 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1987-0217, 5, 35.8-55.6 mm standard length, Khuzestan, Karun River at Kut Abdollah (31º13'N, 48º39'E);
CMNFI 2007-0063, 5, 40.1-65.9 mm standard length, Fars, Mand River tributary near Jahrom (28º36'N, 53º37'E);
CMNFI 2007-0065, 1, 81.2 mm standard length, Fars, Barm-e Dalak (ca. 29º35'N, ca. 52º38'E);
CMNFI 2007-0066, 2, 47.1-55.3 mm standard length, Fars, qanat under Sa'di's Tomb, Shiraz (29º37'N, 52º35'E);
CMNFI 2007-0100, 2, 48.4-54.8 mm standard length, Azarbaijan-e Gharbi, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0109, 10, 54.7-78.3 mm standard length, Kordestan, Qeshlaq River basin (ca. 35º16'N, ca. 47º01'E);
CMNFI 2007-0110, 1, 84.9 mm standard length, Kordestan, Yuzidar River basin (ca. 35º05'N, ca. 46º56'E);
CMNFI 2007-0111, 11, 26.5-64.5 mm standard length, Kermanshahan, Alvand River near Sar-e Pol-e Zahab (ca. 34º36'N, ca. 45º56'E);
CMNFI 2007-0112, 19, 43.1-54.3 mm standard length, Kermanshahan, Kerend River basin near Shahabad-e Gharb (ca. 34º06'N, ca. 46º30'E);
CMNFI 2007-0116, 4, 25.8-31.7 mm standard length, Kermanshahan, Gav Masiab River basin west of Sahneh (ca. 34º28'N, ca. 47º36'E).
Comparative material: BM(NH) 1931.12.21:9-10, 2, 94.9-95.5 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1973.5.21:187-188, 2, 67.6-73.1 mm standard length, Iraq, Tigris River at Jadriyah (no other locality data);
BM(NH) 1974.2.22:1418, 1, 67.4 mm standard length, Iraq, Khalis (33º49'N, 44º32'E);
BM(NH) 1974.2.22:1441-1444, 4, 48.9-86.3 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1986.2.14:2-3, 2, 64.8-91.8 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
NMW 91123, 5, ?, Turkey, Cheilani bei Cizre, Tigris basin (ca. 37º20'N, ca. 42º10'E).
Garra variabilis
(Heckel, 1843)
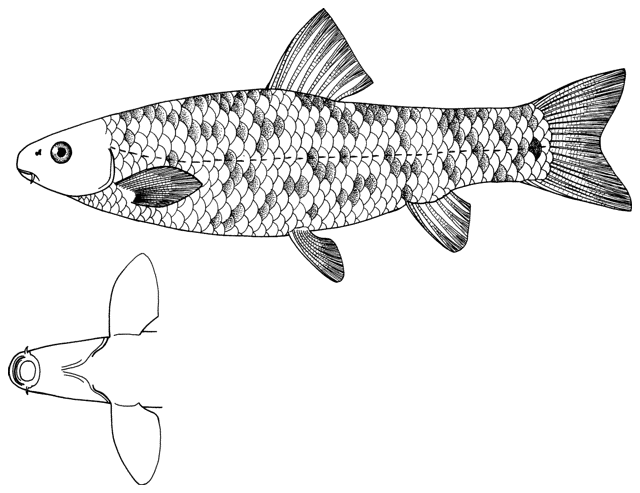
Common names
See under genus account.
[karkoor mit-la'oon, gassur diseileki or gassur isivid (gassur =
colour of strawberries, isivid referring to the spotted, almost black
fish according to Heckel (1843b), all in Arabic; variable garra].
Systematics
Menon (1964) considers this species to be the most primitive in the
genus. It has been placed in the genus Discognathichthys
Bleeker, 1860, e.g. by Berg (1949).
Heckel (1843b) gave the type localities as "Mossul" and
"Aleppo". The syntypes of Discognathus variabilis are
in the Naturhistorisches Museum Wien under NMW 53239, 8 specimens,
38-112 mm standard length and in the Senckenberg Museum Frankfurt
under SMF 403 (formerly NMW), 4, 72-86 mm standard length, all from
Aleppo (Krupp, 1985c). In Vienna I made counts on types as listed below under Sources.
Material under NMW 53238 (3) from Aleppo may also be types. Eschmeyer et al.
(1996) list NMW 532339 (= an error for 53239) (8), NMW 53260-69 (1, 2,
2, 2, 3, 2, 2, 2, 2, 2), NMW 53272 (4), SMF 403 (4) (formerly NMW) and
in the Museum für Naturkunde, Universität Humboldt, Berlin, ZMB 3301 (3)
(formerly NMW; 82.6-99.2 mm standard length measured in February 2006).
Key characters
The single pair of maxillary barbels, absence of a free anterior
margin to a weakly developed adhesive disc on the lower head surface
without papillae on the rear part, scaled back, chest and belly, gill
raker count and distribution distinguishes this species. It is
separated from the closely related but geographically separated G. rossica by larger size,
head length shorter than caudal peduncle length and pectoral fin
length, slightly emarginate caudal fin, and dorsal fin origin closer
to snout tip than the caudal fin base (Berg, 1949).
Morphology
Dorsal fin with 2-3, usually 3, unbranched and 6-8, modally 7,
branched rays, anal fin with 2-3 unbranched and 5 branched rays.
Pectoral fin branched rays 11-14, pelvic fin branched rays 7-8.
Lateral line scales 32-40, scale from the dorsal fin origin to the
lateral line 4-6, scales below the lateral line to the pelvic fin
origin 3-4, scales around the caudal peduncle usually 16, and
predorsal scales in mid-line 12-14. The chest and belly are scaled.
The upper lip is not fimbriate. There may be 2 pairs of barbels in
some larger fish. Pharyngeal tooth formula 2,4,5-5,4,2 (2,3,5-5,3,2 in Berg (1949),
3,3,5-5,3,3 in Heckel (1843b)) and the short gill rakers number 13-20,
on the lower arm of the gill arch only. Gut very elongate and coiled. The
diploid chromosome number was 2n=102 with karyotype formulae being 42m + 18sm +
24st + 18a (FN=186) for females and 41m + 18sm + 24t + 19a (FN=185) for males in
fish from Savur Stream, Turkey (Karahan and Ergene, 2010).
Sexual dimorphism
Specimens from NMW 91121 had the top and sides of the head finely tuberculate
and scales on the back before the dorsal fin with fine tubercles lining the
scale margins. The upper lip, lip sides and sucker (except for a naked central
area) have keratinised tubercles. Tubercles line the dorsal surface of pectoral
fin rays, fading medially and following the ray branching in single rows.
Colour
Overall colour is olivaceous brown or greyish with darker mottlings.
The flanks may have large, irregularly-arranged dark spots. The upper
corner of the operculum may have a black spot. The belly is
reddish-yellow. The middle 3-4 rays of the dorsal fin each have a
small, black spot at their bases. There is a black spot at the caudal
fin base. The lateral line may occasionally have a double row of black
spots as in certain Alburnoides spp. Young fish may have a dark
lateral stripe. Peritoneum black.
Size
Reaches 15.5 cm or according to Heckel (1843b) 5 Zoll (= about 21 cm).
Distribution
Found in the Quwayq (= Kueik), Orontes (= Asi) and Nahr al-Kabir rivers of
the Levant and the Tigris-Euphrates basin (Menon, 1964; Krupp, 1985c). In Iran,
this species is found in the Tigris River basin. Abdoli (2000) maps the
Jarrahi, middle Karun, lower Dez, Karkheh, lower Simarreh, and lower Kashkan rivers.
Keyserling (1861) recorded this species from Sistan but this is a
misidentification. Records of this species (as Discognathus variabilis) from Sistan
by Nikolskii (1899) and Regan (1906) are G. rossica (Menon, 1964).
Zoogeography
This species is related to Garra rossica of eastern Iran.
See also under Garra persica. Krupp (1985c) considers this
species to belong to the indo-asiatic line of Garra.
Habitat
Garra rufa and this species seem to exclude each other, variabilis
being more common in faster water (F. Krupp).
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This species appears to be very rare in Iran and if specific localities are found they should be protected.
Further work
The presence of this species in Iranian waters and its biology there need study.
Sources
Type material: See above, Discognathus variabilis (NMW 53239, SMF 403 and ZMB 3301) and see comments also on other possible types.
Iranian material:- None.
Type material used for counts: NMW 53260, 1, 40.6 mm standard length,
NMW 53261, 2, 87.6-97.8 mm standard length,
NMW 53262, 2, 51.4-52.1 mm standard length,
NMW 53263, 2, 101.8-103.1 mm standard length,
NMW 53264, 3, 52.3-54.2 mm standard length,
NMW 53266, 2, 87.6-92.7 mm standard length,
NMW 53267, 2, 61.5-68.3 mm standard length,
NMW 53268, 7, 43.9- 71.4 mm standard length (dried at some point),
NMW 53269, 2, 100.3-109.1 mm standard length,
NMW 53272, 4, 84.9-92.1 mm standard length, all previous Tigris at Mosul;
NMW 53239, 8 36.9-111.0 mm standard length, Aleppo.
Comparative material: BM(NH) 1935.9.12:27-40, 5, 60.8-69.1 mm standard length, Iraq, Karasu (no other locality data);
BM(NH) 1935.9.12:53, 1, 76.0 mm standard length, Iraq, Tchaiy Su (no other locality data);
BM(NH) 1986.2.14:2-5, 2, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1968.12.13:290-297, 8, 30.7-71.6 mm standard length, Syria, Tigris River at `Ayn Diwar (37º17'N, 42º11'E);
BM(NH) 1968.12.13:298-304, 7, 44.4-66.7 mm standard length, Syria, Quweiq River at Masslemiyeh (no other locality data);
NMW 91121, 10, 71.9-115.4 mm standard length, Turkey, Wadi Mahmedian Tschai (ca. 38°20'N, ca. 40°45'E).
Genus Gobio
Cuvier, 1816
The gudgeon genus includes about 24 species found from the British Isles
through Europe and northern Asia to Korea. There is one species in Iran and two
other species are now in the genus Romanogobio.
The body shape is distinctive, being elongate and fusiform with
moderately large scales (36-51), the throat is naked or scaled, the mouth is inferior or
terminal, horseshoe-shaped and has a barbel at each corner, the lower
lip is thin like the upper lip but is interrupted medially, gill
rakers are short and widely spaced, pharyngeal teeth are in 2 rows
(usually 5 in the main row and 2-3 in the second) and are obviously
hooked at the tips, both dorsal and anal fins are short and spineless,
the gut is short and the peritoneum silvery, and the vent is remote
from the anal and pelvic fin origins.
Gobio gobio
(Linnaeus, 1758)
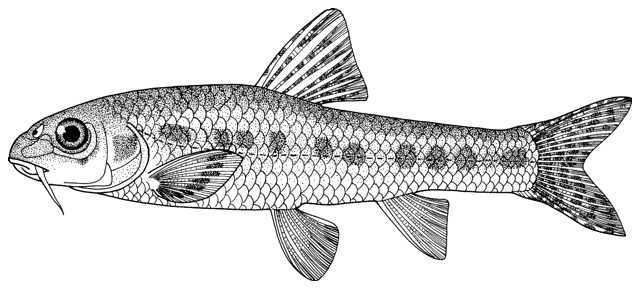
Common names
mahi kopur kafzi (= bottom-dwelling carp fish), گاو ماهي (= gav mahi,
probably in error for Neogobius and related gobies).
[Turkestanskii peskar' or Turkestan gudgeon in Russian].
Systematics
Cyprinus Gobio was originally described from England.
Bungia nigrescens Keyserling, 1861 described from "Fluss
Heri-Rud bei Herat" (the Harirud at Herat in Afghanistan,
formerly part of Persia) is a synonym. Bungia Keyserling, 1861
is a synonym of Gobio Cuvier, 1816 (Eschmeyer, 1990).
This species is represented in Iran by the subspecies Gobio
gobio lepidolaemus Kessler, 1872, originally described as Gobio
fluviatilis var. lepidolaemus from Ak-darja and Chodshaduk
in the Zeravshan River basin, Uzbekistan and the Syr Darya at
Khodzhent, Tajikistan. This subspecies is distinguished from the
typical gudgeon by having a scale-covered throat, deep body, deep and
short caudal peduncle, slightly notched or emarginate caudal fin and
small size (Amanov, 1972). Berg (1948-1949) considers that these
characters would be sufficient to distinguish this taxon as a full
species but there are intermediate forms. Interestingly, Reshetnikov
and Shakirova (1993) list Gobio lepidolaemus as a full species.
Also in the western Caspian Sea basin as Gobio gobio
lepidolaemus natio holurus Berg, 1914 (the Terek gudgeon or
Terskii peskar') but not recorded from Iranian waters and considered
to represent intergrades between G. gobio gobio and G. gobio
lepidolaemus by Bănărescu in Bănărescu (1999).
A syntype from Khodzhent of Gobio gobio lepidolaemus
measuring 49.7 mm standard length is in the Natural History Museum,
London under BM(NH) 1897.7.5:26, formerly in St. Petersburg
University, a syntype is in the Zoological Institute, St. Petersburg
under ZISP 2078 or 2076, and a possible syntype is in the Zoological
Museum of Moscow State University under MMSU P.1052. Svetovidova
(1978) refers to ZISP 2078 as the holotype on page 257 and ZISP 2076
as the holotype on page 262 (Eschmeyer et al., 1996).
Key characters
See above. This is the only gudgeon in eastern Iran and is
separated from other cyprinids by the meristic characters, presence of
barbels, absence of fin spines, mouth not transverse or crescentic but
horseshoe-shaped, and colour pattern. It is separated from the other related species in Iran (Romanogobio
macropterus and R. persus) by having the body and caudal
peduncle compressed (caudal peduncle depth at anal fin insertion
greater than caudal peduncle width) and by well-defined spots on the
dorsal and caudal fins. This subspecies is also characterised by all
members of the population having scales on the breast, absent or
variably developed in other subspecies and populations to the west.
Morphology
Usmanova (1975) found differences between populations of this
species in Uzbekistan associated with habitat. Fish from more stable
habitats have deeper and wider bodies and reduced fin sizes.
Dorsal fin branched rays 6-8, usually 7, after 2-4 unbranched rays,
anal fin branched rays 5-8, usually 5 (Amanov, 1972 - an error, see
below) or 6 (Usmanova, 1975; Banarescu and Nalbant, 1973) after 2-3
unbranched rays, pectoral fin branched rays 13-17 and pelvic fin
branched rays 7-8. Lateral line scales 33-46, usually 37-42 and
averaging less than 40 (Bănărescu in Bănărescu (1999)).
Scales have posterior radii only (or, if present,
very few anterior radii) and the scale focus is subcentral anterior
but not very eccentric in small fish, very eccentric in large fish.
The anterior scale margin is rounded to wavy. There is a pelvic
axillary scale. The anus is separated from the anal fin origin by 5-6 closely
overlapping scales and is near the end of the pelvic fins but
underneath them. Pharyngeal teeth 3,5-5,3 usually but Pipoyan (1998)
found 21 variant counts for 141 Gobio gobio in Armenia with
3,5-5,3 (34.0%), 2,5-5,2(22.7%), 2,5-5,3(10.0%) and other combinations
at about 2% or less. The anterior teeth are blunt with small hooks,
conical and short and are followed by long, thin, strongly hooked
teeth (the description of Bungia nigrescens may be in error in
stating that there is only one row of teeth but Pipoyan (1998) notes
that Gobio gobio in Armenia are exceptionally uniserial). Total
gill rakers 1-7, only developed rakers being counted and anterior
rakers reduced to bumps not included. Developed rakers are stubby and
may or may not touch the adjacent raker when appressed. Total
vertebrae 33-42 (this wide literature range may reflect the wide
species range but could also include specimens counted in varying
ways). The gut is an elongate s-shape with a slight anterior loop. The
chromosome number is 2n=50 (Klinkhardt et al., 1995;
Bănărescu in Bănărescu, 1999).
Meristics in Iranian fish are as follows: branched dorsal fin rays 7(4),
branched anal fin rays 6(4), branched pectoral fin rays 15(2), 16(1) or 17(1), branched pelvic fin rays 7(4);
lateral line scales 37(1), 38(2) or 39(1), scales around caudal peduncle 14(2) or
16(2); total gill rakers 5(1) or 6(2); pharyngeal teeth 3,5-5,3(1),
3,5-4,3(1) or 3,4-5,2 or 3(1); and total vertebrae 38(2) or 39(1).
Sexual dimorphism
Snout length, depth of the head at the occiput, eye diameter,
greatest body depth and thickness, predorsal distance, pectoral-pelvic
fin distance, and barbel length are greater in females than males
while caudal, pectoral and pelvic fin lengths and height of dorsal fin
are greater in males. Females reach larger sizes than males (Usmanova,
1975). Males darken in the spawning season. Fine tubercles develop on
the side and upper surface of male heads, on the upper flank and back anteriorly, and the 8 outer rays of the pectoral fin.
Colour
The top of the head, the back and the flanks above the lateral line
are dark brown and may have a greenish tinge. The lower flanks are
paler and may have a silvery tinge or be a light yellow. The mid-flank
bears a row of 6-13 dark spots which may merge into a line, merge in
pairs or form a lattice. The back may have 4-5 longitudinal dark bands
with a variegated pattern. The dorsal and caudal fins have 3-5 rows of
spots, the pectoral fins have several rows of small spots and the
pelvic and anal fins may also have 2-6 rows of spots but are often
colourless. The peritoneum is silvery.
Colour may vary with habitat, being more uniform and darker on a
monotonous background and more spotted and lighter on a gravelly background.
Size
Attains 20.0 cm total length for the species, 11.1 cm for lepidolaemus.
Distribution
Found from the British Isles and north of the Pyrenees through much
of Europe north of the Alps to Siberia. The subspecies considered here
is found only in the Tedzhen or Hari River basin in Iran (Abdoli, 2000). It is also
found in north flowing rivers of the Kopetdag in Turkmenistan and
eastwards in Central Asia to the Chu River in Kyrgyzstan. The natio holurus
is found in the Kuma, Terek and Sulak rivers of the western Caspian
shore but not as far south as waters neighbouring Iran.
This species is also recorded from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov,
1995) and may eventually reach Iranian waters in the Caspian Sea basin.
Zoogeography
This is a widely distributed and very variable species with many taxa listed
as subspecies, potentially species if further investigated. As part of a gobionine fauna found across Eurasia,
this wide
distribution may be suggestive of further work that could be done to clarify relationships of these fishes.
Habitat
This gudgeon is found in rivers near the bank where there is a sand
or fine gravel bottom and in inlets, backwaters and weed beds of
irrigation canals in the Kashkadar'ya of Uzbekistan (Usmanova, 1975).
It prefers stagnant water in the Surkhandar'ya of Uzbekistan (Amanov,
1972). Gudgeons are generally resistant to pollution and varying
environmental conditions although there is little information on the
eastern Iranian subspecies.
Age and growth
Sexual maturity is reached at the age of 1 year and a length of
3.9-4.3 cm in females from the Kashkadar'ya in Uzbekistan, at 4.6-5.3
cm in the Surkhandar'ya, or at 2-3 years and 3-7 cm in reservoirs in
Uzbekistan; also reported as 2-3 years in the Issyk Kul' and the Chu
River (Usmanova, 1975). Life span is over 4 years (Amanov, 1972).
Food
Food is mainly benthic invertebrates, chiefly insect larvae such as
chironomids in Iranian fish, but varied items may be taken depending
on the water body and food availability. Abdoli (2000) lists Chironomidae,
Ephemeroptera, Plecoptera and Trichoptera. Some insects falling on the
water surface are taken. Remains of terrestrial plants, green algae
and detritus have also been recorded (Usmanova, 1975). Detritus and
vegetation (mostly diatoms) dominate in some gut samples, followed by
insect larvae and benthopelagic crustaceans, and occasionally fish
eggs. Other samples show chironomid larvae to be the main diet item or
cladocerans. Vegetation fragments are apparently seized accidentally
with such foods as chironomids (Amanov, 1972).
Reproduction
Spawning is intermittent and takes place from May to August in
Uzbekistan, May-July in the Issyk Kul' and May-June in the Chu River (Usmanova,
1975). Up to 12,900 eggs are laid on a clay-sand bottom at 18-20°C (Amanov, 1972).
Well-developed eggs are present in Iranian fish caught
on 10 November. Elsewhere gudgeons slap the water surface with the
rear part of the body, and males and females rub their bodies together
while releasing eggs and sperm (Bănărescu in Bănărescu, 1999).
Parasites and predators
None reported from Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and textbooks.
Gudgeons in Europe have been used as bait fish and as food.
Conservation
Lelek (1987) classifies populations of this species in Europe as intermediate to rare.
Further work
The biology of this species in Iran requires study.
Sources
Type material: See above, a syntype of Gobio gobio lepidolaemus (BM(NH) 1897.7.5:26).
Iranian material: CMNFI 2007-0014, 3, 81.5-85.0 mm standard length, Khorasan, Kuh-e Sang
Park, Mashhad (ca. 36º18'N, ca. 59º36'E).
Comparative material: SMF 17137, 5, 61.3-84.1 mm standard length,
Afghanistan, tributary of the Harirud near Herat (34°21'N, 62°14'E).
Genus Hemiculter
Bleeker, 1859
This genus contains several species with a native distribution in
the Amur River basin of the Russian Far East and in China, Taiwan and
Viet Nam. A single species has been accidentally introduced to Iran.
The sawbellies are characterised by an elongate body;
moderate-sized scales with a deeply-decurved lateral line only 1-3
scales above the mid-ventral line; a scaleless keel from the pectoral
fins to the vent; a short dorsal fin with a spine and an elongate anal
fin; pharyngeal teeth in 3 rows; gill rakers short and numerous; and pelagic eggs.
Hemiculter leucisculus
(Basilewsky, 1855)
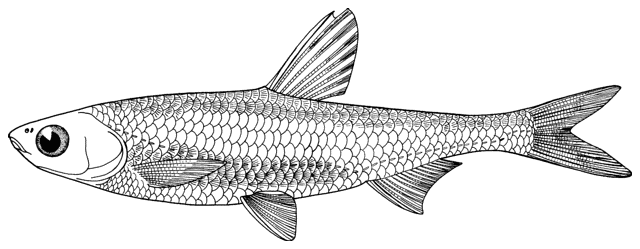
Common names
tizeh kuli or teez-e-kooli (= sharp or spiny fish, kuli being any small fish),
shakam tiz (= ?sharp belly), kuli-e mordab (= lagoon fish).
[vostrobryushka or sharpbelly in Russian; common sawbelly, knifefish].
Systematics
Culter leucisculus was originally described from Peking,
China. Hemiculter eigenmanni (Jordan and Metz, 1913) is a synonym.
Key characters
The sharp keel, lacking scales, extending from the vent or anus to the throat
below the pectoral fin on the mid-ventral surface is distinctive, especially
when combined with the dorsal fin spine and the three rows of pharyngeal teeth.
Morphology
The last dorsal fin unbranched ray is a sharp spine with a flexible tip. The
lateral line curves downward from its origin on the head to the level
of the end of the pectoral fin and then parallels the lower body
margin eventually to curve upward (sometimes sharply) at the end of the anal fin level and
run along the middle part of the caudal peduncle. There is a tubercle
on the lower jaw which fits into a notch on the upper jaw. Dorsal fin
branched rays 6-8, usually 7, after 2-3 unbranched rays and anal fin
branched rays 10-18, mostly 13-14 (but see below), after 3 unbranched rays. Lateral
line scales 43-55, scales above the lateral line 8-11, and scales
below the lateral line to the pelvic fin origin 1-3. Scales bear
numerous fine circuli and a few posterior radii. Total gill rakers
17-29, reaching the second raker below when appressed. Pharyngeal
teeth, 2,4,5-5,4,2, 2,4,5-4,4,2, 2,4,4-5,4,2, 2,4,4-4,3,2, 3,4,5-4,3,2 or
2,3,5-4,3,2. Teeth are hooked at the tip with an elongate and narrow grinding surface.
Total vertebrae 42. The gut is an elongate s-shape. The chromosome number is 2n=48.
Meristic values for Iranian specimens are:- dorsal fin
branched rays 6(1) or 7(6), anal fin branched rays 11(1), 12(5) or
13(1), lateral line scales 47(1), 48(1), 50(1), 52(1), 53(1) or 54(2),
total gill rakers 17(1), 18(4) or 19(2), and pharyngeal teeth
2,4,5-5,4,2(2), 2,4,4-5,4,2(2), 2,4,5-4,4,2(2) or 2,4,4-4,3,2(1).
Sexual dimorphism
Unknown.
Colour
Overall colour dark above, silvery on the flanks and whitish on the
belly. There is a dark stripe along the upper flank. The lips are
dark. The dorsal, caudal and anterior pectoral and anal fin ray edges
and their fin membranes are lightly pigmented with melanophores. The
peritoneum is silvery with some melanophores giving it a brownish
pigmentation in preserved fish.
Size
Reaches 25 cm.
Distribution
The native range of this species is from Maritime Russia south
through China to Korea and Viet Nam. First reported from the Anzali
Mordab by Holčík and Razavi (1992) and apparently not uncommon there. Abbasi et
al., (1999), Kiabi et al. (1999), Abdoli (2000) and Gasmi and Mirzaei
(2004) record this species from the lower Safid River and the Anzali
Talab, and in the middle Aras River. Patimar et al. (2002a; 2002b; 2008) and Patimar (2008) report
it from the International Wetlands of Alma-Gol, Adji-Gol and Ala-Gol and
Esmaeili et al. (2010) from Zarivar Lake. Found in
ab-bandans along the Caspian shore of Iran (Jolodar and Abdoli, 2004). Also reported in the Tedzhen
River, Karakum Canal and Kopetdag Reservoir of Turkmenistan (as H.
eigenmanni)(Aliev et al., 1988; Shakirova and Sukhanova,
1994; Sal'nikov, 1995) and so may eventually be found in the Tedzhen (= Hari) River basin of Iran.
Now recorded from the Hawizah Marsh in southern Iraq (Coad and Hussain, 2007).
Zoogeography
This species is introduced to Iran, probably by accident along with
commercial shipments of Chinese major carps from Central Asia in the
former U.S.S.R. and/or Rumania in 1967. The Chinese major carps in
Central Asia came from the Amur River basin in the Far East and sawbellies were accidentally transferred
with them in the 1950s-1960s (Holčík and Razavi, 1992).
Habitat
Found in rivers, lakes, small ponds and swamps but little appears to be known about its habitat requirements.
Age and growth
Patimar et al. (2002a; 2002b; 2008) report six age groups in the International Wetlands of Alma-Gol, Adji-Gol and Ala-Gol. The smallest
mature specimens there were 2 years old. It is an 'r' strategist, forming dense stunted populations in any new environment.
Food
Macrophytes, fish, crustaceans and insects are eaten by this species and young
fish feed on zooplankton. Iranian fish contain large plant fragments and filamentous algae.
Reproduction
Up to 1,180 eggs are produced. Fish from a swamp near Hendeh Khaleh
in Gilan taken on 9 August contained well-developed eggs but Patimar et al.
(2002a; 2002b; 2008) report a peak spawning in March in the International Wetlands of
Alma-Gol, Adji-Gol and Ala-Gol.
Parasites and predators
Sattari et al. (2007) record the digenean Diplostomum spathaceum
and the monogenean Diplozoon sp. in this species in the Anzali wetland of
the Caspian shore. It is food for Sander lucioperca, Silurus
glanis and Aspius aspius in Turkmenistan (Aliev et al., 1988).
Economic importance
This species has potential as a food fish and is canned in China but this is probably
outweighed by its competition with native species for food and the
possibility of predation on fish eggs and young. The sawbelly is
easily able to switch from one food to another as conditions warrant (Holčík
and Razavi, 1992) and is known to show more rapid growth and higher fecundity
than under native conditions. Welcomme in Courtenay and Stauffer (1984) regards
this species as a pest when introduced.
It is found on the fish market at Bandar Anzali and a catch of 41
kg is reported from the Anzali Mordab in 1990 (Holčík and Oláh, 1992).
Conservation
None required for an introduced species.
Further work
The spread of this species and its effects on native species and habitats should be monitored.
Sources
Based on data in Holčík and Razavi (1992) for 5 Iranian specimens from the Anzali Mordab (134.5-143.4 mm standard length).
Iranian material: Uncatalogued material, 2, 99.6-113.1 mm standard length, Gilan, swamp near Hendeh Khaleh (37°23'N, 49°28'E).
Comparative material: CMNFI 2006-0028, 2, 112.5-123.6 mm standard length, Iraq, Hawizah Marsh (31º38'30"N, 47º35'21"E and 31º36'02"N, 47º33'09"E).
Genus Hemigrammocapoeta
Pellegrin, 1927
This genus comprises 5 species of which 1 is found in Iran. The
genera Tylognathoides Tortonese, 1938, Neotylognathus
Kosswig, 1950 and Hemigarra Karaman, 1971 are synonyms (Krupp,
1985c; Krupp and Schneider, 1989).
The genus is characterised by having a distinct rostral flap
without separate lateral lobes, underslung mouth, horny covering to
the upper and lower jaws, no disc on the chin behind the lower lip but
this area papillose, no or up to 2 pairs of barbels (mouth and lip
structures can be quite variable), short dorsal and anal fins,
complete or incomplete lateral line, and pharyngeal teeth in 3 rows
with somewhat hooked tips and spoon-shaped crowns, count uniquely
2,4,5-5,4,2 (but see below). Most species are found in the Levant. The genus is
closely related to Garra according to Krupp (1985c).
Hemigrammocapoeta elegans
(Günther, 1868)
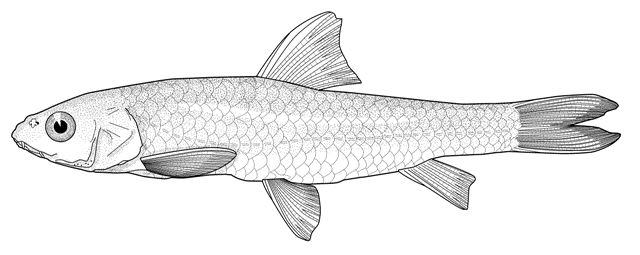
Common names
None.
Systematics
Originally described in the genus Tylognathus Heckel, 1843
and also placed in the genus Hemigarra Karaman, 1971. Tylognathus elegans was described from
"Mesopotamia?" (sic). Six syntypes are in the Natural History Museum, London (BM(NH)
1850.10.21:31-36), one specimen (number 36) being an alizarin preparation. One of the specimens had been designated as a lectotype
but was loose in the jar, and the jar contains a total of 17 fish measuring 36.0-44.5 mm standard length.
Key characters
Characters of the genus serve to identify this species in Iran, particularly
around the mouth region.
Morphology
Dorsal fin with 3 unbranched and 6-8, usually 8 branched rays, anal fin with
3 unbranched and 5 branched rays, pectoral fin with 11-17 branched rays and pelvic fin
with 8 branched rays. Last unbranched dorsal fin ray thickened
although concave posteriorly, tapering distally to a thin ray.
Unbranched ray anterior to the last one also thickened in large fish. Lateral line scales 33-38. Flank scales have a wavy anterior margin,
very fine and numerous circuli, few anterior and more numerous
posterior radii, posterior scale field with tubercles and a subcentral
anterior focus. Pelvic axillary scale present. There is a scale
between the anus and the anal fin origin. Gill rakers short, just reaching the base of the second raker below
when appressed. Barbels 4, thin but moderately long. Snout projecting
over inferior mouth but not strongly folded over the upper lip. Upper
lip free. Lower lip thicker in the middle but no sucker, posterior
edge wavy. Horny edge to lower jaw. Weakly papillose behind the lower lip. Large
nasal flap and large first suborbital bone. Pharyngeal teeth in the major row quite massive and obviously hooked
with a concave space below. The most anterior tooth is the largest and posterior teeth are slender. Minor row teeth are similar but smaller.
Gut complexly coiled. Total vertebrae 46-48.
Meristic values for Iranian specimens are:- dorsal fin branched rays 6(1, ? deformed) or 8(7),
anal fin branched rays 5(8), pectoral fin branched rays 16(2) or 17(6), pelvic fin branched rays 8(8); lateral line
scales 34(1), 36(5), 37(1) or 38(1) and caudal peduncle scales 12(8); total gill rakers 17(1), 18(5), 19(1) or 20(1);
pharyngeal teeth 2,3,5-5,3,2 (5) (sic); and total vertebrae 46(1), 47(5) or 48(2).
Sexual dimorphism
Unknown.
Colour
There is an indistinct silvery stripe along the flank. Dark pigment ends about 1 scale above the lateral line along the flank. Peritoneum
black with some silvery patches.
Size
Reaches 10.9 cm total length.
Distribution
Found in the Tigris-Euphrates basin. Initially recorded from Harmaleh on the Dez River of Khuzestan (ZSM 25716, 25717), Abdoli (2000) maps
distributions from the upper Karun, upper Dez, and upper Karkheh including the Simarreh rivers.
Zoogeography
The relatives of this species are confined to the Levant, a distribution matched by various other unrelated taxa indicating former connections between
these basins (see Krupp (1985c; 1987)).
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
No parasites reported from Iran. One specimen examined was from the gut of a Silurus triostegus taken at Harmaleh on the Dez River.
Economic importance
None.
Conservation
This species is rare in Iran and is poorly known elsewhere. Assuming this is not the result of inappropriate collecting techniques, then the status of this
species should be assessed by field surveys.
Further work
The distribution and biology of this species is very poorly documented and needs attention.
Sources
Type material: See above (BM(NH) 1850.10.21:31-36).
Iranian material: ZSM 21862, 5, 60.5-86.9 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E);
ZSM 25716, 2, 76.4-79.3 mm standard length, same locality as preceding;
ZSM 25717, 1, 78.5 mm standard length, "from the stomach of a wels", same locality as preceding.
Comparative material: BM(NH) 1973.5.21:185, 1, 57.5 mm standard length, Iraq, Tigris River at Jadriyah, Baghdad (no other locality data);
FMNH 51235, 1, 43.9 mm standard length, Iraq, Tigris River at Rustam Farm near Baghdad (no other locality data);
FMNH 51236, 12, 38.7-59.5 mm standard length, same locality as preceding.
Genus Hypophthalmichthys
Bleeker, 1859
The silver carp genus contains 3 species with a native distribution
in eastern Asia. Two species have been widely introduced for food in
aquaculture and for phytoplankton control.
The genus is characterised by an elongate and compressed body, very
small scales (usually over 100 in the lateral line), eyes low on the
head with their lower margin below the mouth corner level, a terminal
mouth, no barbels, gill rakers long and thin, a very long gut,
branchiostegal membranes joined and free of the isthmus, a short
dorsal and elongate anal fin, both spineless, pharyngeal teeth in 1
row, and a ventral keel from the throat or pelvic fins to the anus.
Hypophthalmichthys molitrix
(Valenciennes, 1844)
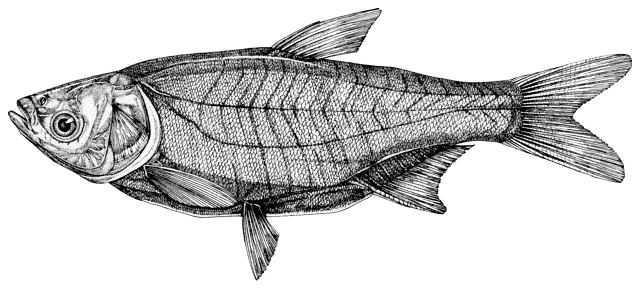

Common names
فيتوفاگ (= fitofag or phytophag, i.e. phytophage or plant eater),
كپور نقره اي
(= kopur-e noqrehi or kapoor-e-noghreie, meaning silver carp), آزاد پرورشي
(= azad-e parvareshi meaning free farmed).
[silver carp, thickforehead, white thickforehead; belyi tolstolobik, tolpyga and maksun in Russian].
Systematics
Leuciscus molitrix was originally described from China. Populations in the Anzali Mordab are hybrids with Hypophthalmichthys
nobilis (J. Holčík, in. litt., 1989).
Key characters
The abdomen has a compressed keel extending from the breast to the vent, the eyes are positioned low such that they are visible from the
underside of the head, and scales are minute. The similar bighead (H. nobilis) can be distinguished by the
long pectoral fins which extend past the origin of the pelvic fins, a shorter keel (pelvic fins to anus), and gill raker structure (free, no
spongy root mass).
Morphology
Dorsal fin with 2-3 unbranched rays followed by 6-7 branched rays,
anal fin with 2-3, usually 3, unbranched rays and 11-15 branched rays,
pectoral fin branched rays 17, and pelvic fin branched rays 7. Lateral
line scales 85-125 Scales are rounded to oval with a posterior focus
and very few posterior radii. Total vertebrae 36-40. Pharyngeal teeth
4-4, well-developed, compressed and with striated grinding surfaces.
Gill rakers exceed 650 and are longer than the gill filaments. The
left and right sides of the gill arches are united by a mucous
membrane to form a continuous band; the gill rakers are
distinguishable distally but the roots form a spongy mass. The gut is
long and complexly coiled. The diploid chromosome number is 48 (Klinkhardt
et al., 1995; Varasteh et al., no date). There were 6 pairs of
metacentric, 14 pairs of submetacentric and 4 pairs of acrocentric chromosomes
in the latter study.
Sexual dimorphism
Unknown.
Colour
Overall colour is silvery, the back bluish to grey-black with upper flanks olivaceous but silver laterally and ventrally, and the fins red or immaculate.
Size
Reaches 1.50 m and 56 kg.
Distribution
The natural distribution is from the Amur River in the former
U.S.S.R. southward to southern China. In Iran, it has been introduced to the Sistan basin
including the Hamun Sabari and the canal flowing into Chahnimeh (Ahmadi and Wossughi, 1988; Mansoori, 1994; J.
Holčík, in litt., 1996), the Voshmgir Reservoir on the Gorgan River
(Petr, 1987) as well as widely stocked in Mazandaran and Gorgan
provinces in reservoirs and lakes by both governmental agencies and
private companies. It is also introduced to the Anzali Mordab and its outlets (Holčík and Oláh, 1992),
the Gorgan, Tajan, Aras, and Safid rivers, and the Anzali Mordab (Kiabi et al., 1999; Abbasi et al.,
1999), Lake Zaribar, Kordestan (Abzeeyan, 5(5):III,
1994), reservoirs and fish farms in Khuzestan, Mahabad Dam (Abdi, 1999; www.mondialvet99.com, downloaded 31 May 2000;
Shamsi et al., 2009).
Abdoli (2000) and Abdoli and Naderi (2009) record this species generally from the Dasht-e Kavir, Kerman-Na'in, Sistan and Hamun-e Jaz Murian basins;
from the middle and lower Kor River and the Pulvar River in the Kor River basin; from the middle and lower Zayandeh River in the
Esfahan basin; from the Khersan River, lower Karun River, the lower Jarrahi river, the lower Dez River the upper
Karkheh River and the lower Kashkan River in the Tigris River basin; the middle and lower Qareh Su and Qom
rivers, the lower Karaj and Shur rivers in the Namak Lake basin; the lower Zarrineh river and the middle and
lower Talkheh River in the Orumiyeh basin; the middle Kashaf River in the Tedzhen basin; the middle and lower Atrak and
Gorgan rivers, the Aras River, the lower Babol, Heraz, Chalus, Tajan, Tonekabon and Safid rivers, the Anzali Talab and the Caspian coast generally in the Caspian Sea basin.
This species is also recorded from the Karakum Canal and Kopetdag Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994;
Sal'nikov, 1995) and may eventually reach Iranian waters in the Tedzhen (= Hari)
River and Caspian Sea basins. It is pond-cultured in Iraq and is known from open
waters such as the Shatt al Arab and Tigris River (Al-Hassan, 1994).
Zoogeography
This species is an exotic, introduced to Iran from a variety of
sources. It may become established in the large river systems of
southern Iran and Iraq from escapees (Al-Hassan, 1994).
Habitat
This species is a riverine fish in its native habitat, or is found in water
bodies connected to rivers, but is extensively cultivated in ponds for food
throughout Asia. Silver carp can live in the Caspian Sea at salinities of 5-8‰
although a few are found at 10-12‰. It enters rivers to spawn
(Abdusamodov, 1986). Temperatures in the range 0-40ºC are tolerated although
26-30ºC or 30-34ºC is preferred in different studies. It is more cold-tolerant
than bighead carp. This species can be difficult to catch as it will jump over
nets, to a height of about 2 m, and when frightened by noise has been known to
jump into boats. Malek Nedjad and Parivar (1993) consider that the level of lead
pollution in the Anzali Mordab (average 0.124 p.p.m. in surface waters, 0.1956
p.p.m. in deeper waters) caused 8% mortality in fertilised eggs and nearly 18%
of eggs are useless for fisheries work.
Age and growth
Terek River silver carp first mature at 4 years for males and 5
years for females. About 15% of females mature at 4 years but 87% of
the females and 85% of the males are in the 5-7 age groups (Abdusamadov,
1986). Maturity varies with locality, at 2-8 years, with males maturing a year
earlier than females. Silver carp can reach 18-23 kg in 4-5 years. Life span
is at least 20 years. Abdolmalaki (2004) examined the fishery
for this carp in the Mahabad Reservoir, Iran and found mean fork length was 51.15 cm
and mean weight was 2272.1 g. The length-weight relationship was W = 0.013L3.04.
The von Bertalanffy growth parameters were L∞ = 150 cm, K = 0.128,
instantaneous rate of total (Z), natural (M) and fishing (F) mortality were
1.68/year, 0.22/year and 1.46/year. The exploitation rate (E) was calculated to
be 0.82. The biomass was 158.5 t with a maximum sustainable yield estimated at
68 t. A decreased fishing effort was recommended as the annual catch in 1998-1999 was 101,123.5 kg.
Food
The gill rakers form a very fine, sponge-like mesh used to filter small
planktonic food, aided by the epibranchial organ that produces mucus to trap
very small particles. This species is a pump filter feeder, taking smaller
particles than bighead carp. Food in Lake Kinneret, Israel is phytoplankton
from February to August and predominately zooplankton from September
to January, a response to a decrease in phytoplankton biomass in
summer-fall (Spataru and Gophen, 1985). Cladocerans and cyclopoid
copepods dominate the biomass of zooplankton taken. The ability to
take cyclopoids is due to the large mouth, strong sucking power and
the high filtration rate when feeding. Food is taken passively rather than selectively.
Mohammadi et al. (2003) found detritus and protozoans to be the main
items in gut contents, with also other algae, diatoms and green algae
respectively.
Reproduction
Silver carp require cool, flowing water to breed. The spawning migration
begins at the end of April in the Terek River of Dagestan at 16-17°C, with a
peak between the middle of May and the beginning of June. Generally spawning
occurs between 18 and 26°C. Fecundity reaches 1,340,500 large, greyish eggs (and
in the Kara-kum Canal 1,525,000 eggs; elsewhere to 5.4 million eggs). Water
hardened eggs are 4.9-5.6 mm, smaller than those of bighead carp. Spawning takes
place after a sharp rise in water level and current speed. Spawners chase each
other near the water surface where eggs and sperm are shed. Eggs are first found
in the drift in the second week of June and hatch 34-70 hours later depending on
temperature. Some larvae reach rice fields and live there until autumn when the
fields dry up where some are lost, others migrating. Other larvae are carried
into the Caspian Sea where they are sensitive to the prevailing salinity at
1-1.5 days old (Abdusamadov, 1986). It does not breed in the wild in Iran.
Parasites and predators
Mokhayer (1989) reports metacercariae of the eye fluke, Diplostomum
spathaceum from this species in Iran, which can cause complete
blindness and death in commercially important species, as well as
black-spot, Posthodiplostomum cuticola. Jalali and Molnár
(1990b) record the monogenean Dactylogyrus hypophthalmichthys
from this species at fish farms in Iran. Masoumian and Pazooki (1998) surveyed
myxosporeans in this species in Gilan and Mazandaran provinces, finding
Myxobolus pavlovskii. Akhlagi (1999) reports that
high temperatures (up to 32°C) stresses this species and leaves it open to infection with Aeromonas
hydrophila. Safari and Khandagi (1999) record Clostridium
botulinum from 1.1% of fresh and smoked samples of this species in
Mazandaran Province. Ebrahimzadeh Mousavi and Khosravi (1999;
www.mondialvet99.com, downloaded 31 May 2000) record the toxigenic
fungi Aspergillus flavus, Alternaria, Penicillium and
Fusarium from this species and the pond water at a fish farm in
northern Iran. Akhondzadeh et al. (2002) and Akhondzadeh Basti and Zahrae Salehi (2003)
show that the psychotropic pathogen Listeria monocytogenes is found in
market and fish farm samples of this species. Naem et al. (2002) found
the following parasites on the gills of this species from the western branch of
the Safid River, namely the protozoans Ichthyophthirius multifilis and a
Trichodina species and the monogenean trematode Dactylogyrus
hypophthalmichthys. Jalali et al. (2002) and Jalali
and Barzegar (2006) record several parasites from this species in Lake Zarivar, namely Dactylogyrus hypophthalmichthys,
D. suchengiaii, Diplostomum spathaceum, two species of Argulus, and myxosporean plasmodia.
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sprostonae from Safid River fish. Araghi Soureh and Jalali Jafari (2005) recorded Dactylogyrus hypophthalmichthys
and D. suchengtaii from this species in the Mahabad River of the Lake Orumiyeh basin,
the latter species being a new record for Iran.
Barzegar et al. (2008) record eye parasites from this fish including the digeneans Diplostomum
spathaceum and Tylodelphys clavata, and the crustacean Lernaea
cyprinacea.
Shamsi et al. (2009)
found Dactylogyrus hypophthalmichthys and D. suchengtaii in this
species from fish farms, and Zarivar Lake, and Mahabad reservoir, respectively.
Barzegar and Jalali (2009) reviewed
crustacean parasites in Iran and found Argulus sp., Argulus foliaceus,
Lernaea sp. and Lernaea foliaceus on this species.
Economic importance
This species is the most productive freshwater fish in the world, with 3.1
million metric tons produced in 1997 (versus 2.2 million mt for Cyprinus
carpio).
Holčík and Oláh (1992) report a total catch of 6585 kg in the Anzali Mordab
in 1990, at 8.8% of the total fish catch being the third largest
catch. This species is used in polyculture with common and grass carps
and comprises 50-63% of the fishes. They are fed through pond
fertilization without supplementary feeding (Emadi, 1993b). Silver carp in oil are packaged in northern Iran
(http://www.netiran.com/business.html, downloaded 31 October 2003). They are
also found in fish stores in Ahvaz, Khuzestan (personal observations,
September 1995). Iran shares with Uzbekistan the most production of
this species among North African and Near Eastern aquaculture; the
Iranian catch increasing from nothing in 1989 to 24,720 tonnes in 1994
(Food and Agriculture Organization, Fisheries Department, 1996).
Bartley and Rana (1998b) however give a production of 15,228 t for
1995. Market price in 1995 was about U.S.$1.00/kg in 1995, lower than
for grass carp at about $2, but silver carp have the higher stocking
ratio (Rana and Bartley, 1998a). Kals and Bartels (2004) give some
recommendations for improving silver carp farming in Iran.
Abdolmalaki (2004) reported that 67.3% of the 150,261 kg of fishes caught in Mahabad Reservoir in
the1998-1999 fishing season were this species. The fishing effort was 69 beach
seine hauls and 2530.7 fishing effort units with 7-15 cm gill nets, each unit
being 100 m of gill net for 24 hours.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and food. The
food of this species being phytoplankton it has been introduced to
areas lacking filter feeders such as Europe and Israel in efforts to
control algal blooms associated with eutrophication. The results in
Israel were controversial and difficult to interpret because of
chemical treatments for nuisance dinoflagellates and introductions of
other fish species (Opuszynski and Shireman, 1995). In some cases the
effects of the introduction were negative because the silver carp fed
from September to January on zooplankton which themselves reduce algal
blooms. There was also competition with a more valuable native species
of cichlid for the same zooplankton resource and the native cichlid
was a more efficient consumer of the nuisance dinoflagellate. Elsewhere,
the consumption of plankton may remove food for native, larval fishes.
This species has been studied extensively as a commercial species in
various parts of the world. Studies specific to Iran include Azari Takami et
al. (2000) on female broodstock selection; Nazifi et al. (2000) on
the effects of the organophosphate trichorofon on serum biochemistry; Nazifi et
al. (2001) on haematological parameters after experimental intoxication with
trichlorofen; Javadian et al.
(2003) on the effect of ice storage on lipid and other chemical changes, moisture
and texture and appearance; Moradian et al. (2003) on the effects of the
hormone thyroxine on the survival of early growth stages (the ratio of hatched
eggs in 0.5 p.p.m. thyroxine was highest); Kashani Sabet et al. (2004) on inducing
ovulation with hormones combined with dopamine antagonists; Peyghan et al.
(2002, 2006) on deleterious effects of malachite green, used to prevent fungal
and parasite infections, on eggs and larvae; Alavi Talab et al. (2007)
on use of skins and fins for gelatin extraction; Moeini et al. (2008) on
producing surimi from this fish; Roshan and Moini (2009) on
washing minced flesh with cold water and brine to increase quality of surimi
(1.5% brine for 10 minutes was best); Shabanpour et al. (2008) on lipid
quality changes of surimi during frozen storage; Alavi Talab et al. (2010) on
optimising encapsulation of oil from this species; Choobkar et al. (2010) on the use
of an essential oil from Persian thyme (Zataria multiflora) to combat
Staphylococcus aureus in salted fillets; etc.
This fish can be dangerous to boaters as it leaps out of the water (Kolar and
Lodge, 2002). Akhondzadeh Basteh et al. (2006) found the bacterial
pathogens Listeria monocytogenes in fresh and smoked H. molitrix,
Staphylococcus aureus in smoked H. molitrix, Escherichia coli
and Salmonella dublin in fresh H. molitrix and Vibrio
haemolyticus in smoked H. molitrix.
The Iranian Fishery Research Institute has made ice cream using this fish.
The product is deoderised so there is no fishy flavour and a protein in the fish
blood is reported to lower the temperature at which ice crystals form meaning
less cream or fat is needed in the product (Iran Daily, 11 February 2009).
Conservation
Krasznai (1987) and Petr (1987) give details of fish farms
propagating this species in Iran. For example, 30 million fish were
produced by the Safid Rud Fish Farm in 1986. 20 million carp, silver
carp and grass carp fingerlings were produced in the Shahid Rajaae
Hatchery in Sari for release across Iran in reservoirs and dams (Abzeeyan,
Tehran, 4(7):VII, 1993). Piri and Ordog (1999) describe the effects of
herbicides and insecticides on this species. These chemicals are used
extensively on rice fields in Gilan where aquaculture is widely developed.
Hybridisation (presumably with H. nobilis) has led to poor
growth and a decline in the fishery (Shehadeh, 1997).
Further work
The biology of this species in Iran needs investigation in relation to its
effects on native fishes and its distribution monitored.
Sources
Opuszynski and Shireman (1995) summarise the biology and culture of this species.
Comparative material: CMNFI 1977-0590, 3, 120.3-135.5 mm standard length, Israel, Kibbutz `En Hamifraz (32º59'N, 35º05'E).
Hypophthalmichthys nobilis
(Richardson, 1844)
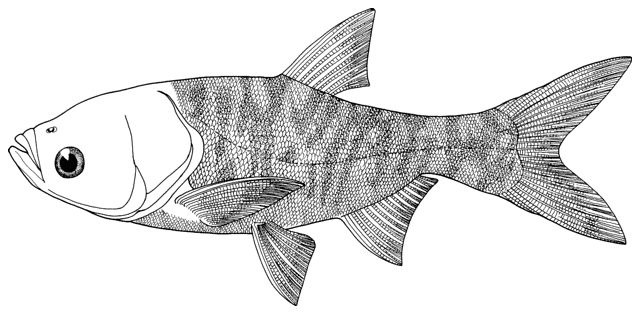

Common names
sar gondeh or sargundeh (= bighead) in the Caspian basin,
كپور سرگنده (= kopur-e sargondeh,
meaning bighead carp), mahi kopur sar gondeh, fitofag.
[pestryi tolstolobik in Russian; bighead, painted thickforehead].
Systematics
Leuciscus nobilis was originally described from Canton,
China. Eschmeyer et al. (1996) give the date of publication as
1845, Reshetnikov et al. (1997) as 1846.
Howes (1981) reaffirms the placement of this species in Hypophthalmichthys
Bleeker, 1860, considering that the characters of abdominal keel
length, pharyngeal dentition and gill raker form are insufficient to
place this species in the distinct genus Aristichthys Oshima,
1919. Other authors disagree (see Eschmeyer, 1990; Reshetnikov et al., 1997).
Populations in the Anzali Mordab are hybrids with Hypophthalmichthys molitrix (J. Holčík, in litt., 1989).
Artificial hybrids with Ctenopharyngodon molitrix have been developed in
Iran and their karyology studied (Dorafshan and Kalbasi, 2007). The F1 hybrids
were triploids with 3n=72 and NF=132.
Key characters
The similar silver carp (H. molitrix) can be distinguished by the short pectoral fins which do not extend past the origin of the
pelvic fins, a longer keel (throat to anus), and gill raker structure (continuous band uniting both sides, roots fused into a spongy mass).
Morphology
Dorsal fin unbranched rays 3 followed by 7 unbranched rays, anal
fin unbranched rays 3 with 11-14 branched rays, pectoral fin branched
rays 16-19, and pelvic fin branched rays 7-9. Lateral line scales
92-115. Scales are a rounded oval with a slightly posterior focus,
very few posterior radii and numerous fine circuli. Total vertebrae
number 36-41. Pharyngeal teeth 4-4, with smooth grinding surfaces.
Gill rakers are very numerous. The gut is elongate and convoluted. The
diploid chromosome number is 48 (Klinkhardt et al., 1995).
Sexual dimorphism
Males have a sharp edge along the dorsal surface of several anterior pectoral fin rays.
Colour
Overall colour is silvery and the body has numerous scattered small black
spots which makes some fish speckled or darker in overall colour. A few larger
blotches may be present. The head is often darker and contrasts with the silvery
body. Fins are greyish and similar to the adjacent body colour with the caudal
fin darkest, sometimes reddish.
Size
Attains 1.57 m in length and 77.5 kg, possibly to 1.95 m.
Distribution
This species is a native of China and was first introduced to Iran in 1966 from a hatchery in the Krasnodar
region of the former U.S.S.R. (Anonymous, 1970b). It is reported from
fish farms in the Caspian Sea basin and is stocked in reservoirs
throughout Iran (Coad and Abdoli, 1993b). Introduced to the Kor River
in Fars (A. Alamdari, in litt., 1997) and reported from the Gorgan,
Tajan, Aras, and Safid rivers, and the Anzali Mordab (Abbasi et al.,
1999; Kiabi et al., 1999; Jalali et al., 2005).
Abdoli (2000) and Abdoli and Naderi (2009) record it generally from the Sistan, Hormuz, Kor, Kerman-Na'in,
Dasht-e Kavir, Esfahan, Namak, Tigris,
and Orumiyeh basins and from the Aras and Safid rivers, lower Gorgan, Neka, Babol, Haraz,
Tajan, Chalus and Tonekabon rivers, the Gorgan and Anzali
mordabs and along the Caspian coast. Also in Lake Zarivar (Shamsi et al.,
2009).
Also reproducing naturally in the Karakum Canal and recorded from
the Kopetdag Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994;
Sal'nikov, 1995) and may eventually reach Iranian waters in the Tedzhen (= Hari) River basin.
Introduced to Iraq for fish farming.
Zoogeography
An exotic introduced to Iran.
Habitat
In their natural habitat, bigheads are found in large rivers and associated
floodplain lakes. They migrate upstream to spawning grounds when water levels
rise, moving to flooded land afterwards, and returning to the river channel as
water levels fall. Bigheads can live in the Caspian Sea at salinities of 5-8
p.p.t. although a few are found at 10-12 p.p.t.. They can adjust gradually to
salinities of 15-20 p.p.t. They enter rivers to spawn (Abdusamodov, 1986) but
are known to spawn in the Karakum Canal of Turkmenistan. Preferred temperatures
for feeding and reproduction are within the general range 20-30°C. Activity
almost ceases at 10°C and the critical thermal maximum is 38.8°C.
Age and growth
Males achieve first maturity at age 5 and males of 5-7 years and
81-90 cm make up 90% of the run in the Terek River. Most females
mature at 6 years and 81% of the females on the spawning run are 6-7
years old and 75-100 cm (Abdusamodov, 1986). Males mature at 2-4 years and
females at 3-5 years (and 10 kg) in Turkmenistan. Sexual maturity varies widely
with environmental conditions, 2-6 years for males and a year later for females.
Life span is up to 16 years. Growth is rapid, attaining 18-23 kg in 4-5 years.
Food
Zooplankton is almost exclusively the food of this species. Phytoplankton and
detritus may be taken when zooplankton biomass is low. Most feeding occurs
during summer and peaks daily in the range 1200 to 2000 hours. This species is
both a pump feeder, using the buccal pump to push food-laden water through the
gill rakers, and a ram feeder, swimming with the mouth open to force water
through the gills, with intermittent gulps. Feeding often occurs at the water
surface, in contrast to silver carp, as well as in the water column and on the bottom.
Reproduction
A spawning migration of this species enters the Terek River in the second
week of May at water temperatures of 18-19°C, numbers increasing until the end
of June. Spawning takes place after a sharp rise in water level and current
speed. Males actively chase females near the water surface, occasionally butting
the female's belly, and sometimes leaping out of the water. Eggs and sperm may
be cast into the air. Fecundity attains 1,860,800 eggs. Unswollen eggs are
1.4-1.5 mm in diameter and water hardened eggs are 5.7-6.2 mm. Eggs are first
found in the drift in the second week of June and hatch 34-70 hours later
depending on temperature. Some larvae reach rice fields and live there until
autumn when the fields dry up where some are lost, others migrating. Other
larvae are carried into the Caspian Sea where they are sensitive to the
prevailing salinity at 1-1.5 days old (Abdusamadov, 1986).
Parasites and predators
Jalali and Molnár (1990b) record the monogeneans Dactylogyrus
aristichthys and D. nobilis from this species in Iranian fish farms.
Naem et al. (2002) found the following parasites on the gills of this
species from the western branch of the Safid River, namely the protozoan
Trichodina sp., monogenean trematodes Dactylogyrus nobilis, D.
aristichthys, and Gyrodactylus sp.. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sprostonae and G. sp. in fish from the Safid River.
Masoumian and Pazooki (1998) surveyed myxosporeans in this species in Gilan and
Mazandaran provinces, finding Myxobolus pavlovskii. Alishahi and Peyghan
(2008) found a heavy infestation with Lernaea cyprinacea from a fish pond
in Tehran. Barzegar
et al. (2008) record the digenean eye parasite Diplostomum spathaceum
from this fish. Shamsi et al.
(2009) found Dactylogyrus aristichthys, D. nobilis and D.
taihuensis in this species from fish farms and Zarivar Lake.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. and
Lernaea cyprinacea on this species.
Economic importance
Holčík and Oláh (1992) report a catch of 466 kg in the Anzali Mordab in
1990. Aquaculture production in 1995 was 1269 tonnes (Bartley and Rana,
1998b). Marjan Iran Company was selling 1500-1800 g fish for U.S.$1.90/kg in August 2003
(http://groups.yahoo.com/groups/hilsa/message/25).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and food.
It ranks fourth on world aquaculture production. This species has been used in Israel to reduce zooplankton populations
in reservoirs in an effort to improve water quality (Opuszynski and
Shireman, 1995). It has a higher net production in culture systems
than H. molitrix and Ctenopharyngodon idella. The
consumption of plankton may remove food for native, larval fishes, and affect
the diet of piscivorous fishes and birds. This species can also cause habitat
alteration, increasing turbidity, and introduce diseases and parasites.
In Iran, it has been used in aquaria for investigations on the effect of lead
nitrate on blood serum electrolytes (Jamili et al., 2006).
Conservation
Krasznai (1987) and Petr (1987) give some details of propagation of
this species in Iran. Some populations are hybrids (see above) and
there is a danger of loss of genetic purity in fish farm stocks should
breeding adults be captured in the wild. As an exotic, there is no need for conservation.
Further work
The biology of this species in relation to native species should be
investigated for Iran.
Sources
Jennings (1988), Opuszynski and Shireman (1995) and Kolar et al. (2005) summarise the biology and culture of this species.
Comparative material: CMNFI 1980-0530, 2, 230.6-255.8 mm standard length, Japan, pond cultured (no other locality data).
Genus Iranocypris
Bruun and Kaiser, 1948
This genus contains a single species found only in Iran and the
characters of the species are therefore the characters of the genus.
This blind cave species is placed in a world-wide context by Proudlove (1997a; 1997b).
Iranocypris typhlops
Bruun and Kaiser, 1948
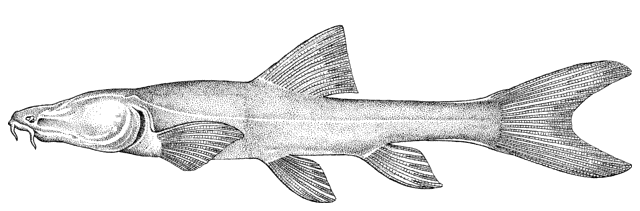
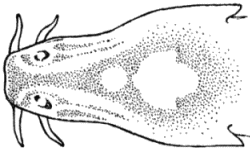
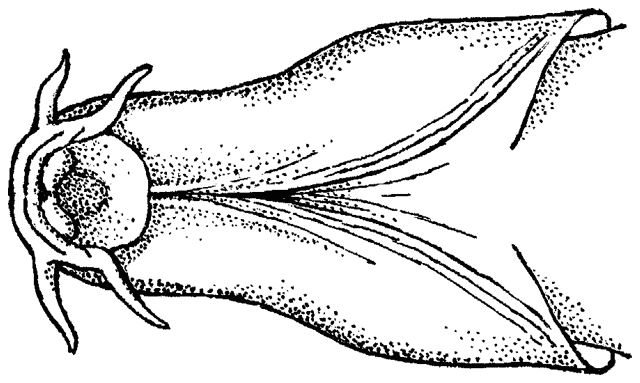
Dorsal view of head
Ventral view of head

Courtesy of R. Mehrani

Courtesy of Amir Hosin Zalaghi, 11-19 May 2010 (all fish returned to cave alive)

Courtesy of Kiavash Golzarianpour
Iranocypris typhlops movie.3gp, courtesy of Kiavash Golzarianpour
Common names
mahi-ye kureghar or mahi-ye kur-e qar (= blind cave fish), kopur mahi kureghar,
ماهي كور (= mahi kur).
[Iran cave barb].
Systematics
The holotype is in the Zoological Museum of Copenhagen (ZMUC P
26475) and measures 46.5 mm total length and 38.5 mm standard length
(Nielsen, 1974; personal observations). The paratypes number 5 (in Nielsen (1974)), or 6 (in
Bruun and Kaiser (1948)) but only 4 were found in ZMUC in December
1999. Paratypes (ZMUC P 26476, 26477, 26478, 26480) measure 19.5-42.0
mm total length and 16.5-34.5 mm standard length according to Bruun and Kaiser
(1948). Two
fish (P 26476 and P 26480) were used in histological studies and one
consists of the body only. The type locality is given below and the
fish were collected by E. Kaiser on 6-5-1937 from "lok 80"
(= locality 80; but no field notes by E. Kaiser are available in ZMUC).
The date of authorship for this species is variously listed as 1943
on an official reprint, as 1944-49 in one set of Contents and
"ready from the press 1944" in another set of contents. Proudlove
(2006) states that is did not appear until 1948 because of World War II.
Bruun and Kaiser (1948) believe this species to be related to the
genus "Barbus", members of which also have two pairs of barbels,
although Saadati (1977) considers this unlikely since most "Barbus"
from the Tigris River basin are large fishes.
Key characters
The only eyeless, depigmented cyprinid species in Iran, it is very
distinctive.
Morphology
The body is compressed and the head somewhat flattened. There are
two pairs of barbels, one pair at the mouth corners and one about half
way along the upper lip. The upper lip has a feebly crenulated edge.
The mouth is subterminal and horseshoe-shaped. A mental disc is developed in
some fish, absent in others. There are significant differences in some
morphometric and meristic characters in fish with, and without, a disc (Sargeran
et al., 2008). There is no visible
trace of eyes in most fish. The skin is naked except for a few rows of scales
behind the pectoral fin base, although some individuals may have more flank
scales. There are about 32 myomeres along the
flank. A lateral line is present. The dorsal fin has 3 unbranched and
7-8 branched rays, the anal fin 3 unbranched and 4-5 branched rays,
the pectoral fin 14-17 branched rays and the pelvic fin 5-7 branched
rays. Pharyngeal teeth in 3 rows, 1 to 3 in the outer row, 3 to 4 in
the middle row and 3-5 in the inner row. Anterior teeth are very
enlarged and conical, appearing as rounded knobs while the posterior
teeth in the main row are flattened and slightly hooked. Smaller fish
have less conical anterior teeth with a tiny hook at the tip and
posterior teeth have a short, flat to slightly concave surface below
the tip. Tooth counts are difficult to make with accuracy as it is not
always clear to which row a tooth belongs. Smaller fish can be
interpreted as 2,3,5-5,3,2 while larger fish may possibly lose a tooth
and have a 2,3,4-4,3,2 count. Abbasi and Gharezi (2003) give a 3,5,5-5,3,3
count. Gill rakers very short, not reaching the
adjacent raker when appressed and numbering 10-13 total. Total
vertebrae 34-36. Gut s-shaped. The morphology and histology of the digestive
tract was examined in detail by
Abbasi and Gharezi
(2003).
Meristics in specimens examined, including the holotype and 2
paratypes: dorsal fin branched rays 7(30) or 8(29); anal fin branched
rays 4(1) or 5(49); pectoral fin branched 12(1), 13(9), 14(8), 15(23) or 16(8);
pelvic fin branched rays 6(18), 7(31) or 8(1); total gill rakers 10(2),
12(4) or 13(1); pharyngeal teeth 2,3,5-5,3,2(1), 3,4,5-5,4,2(1),
2,3,4-5,3,2(1) or 2,3,4-4,3,1(1); total vertebrae 34(3), 35(8) or 36(1).
Sexual dimorphism
None reported.
Colour
This species is almost entirely unpigmented although live fish are
pinkish to red from the blood showing through the skin. The gill
filament area is bright red and some fish give an overall impression
of red like a goldfish. Small, black pigment cells were visible in two
small fish over the brain and just behind it and in these two fish and
three others a very small, black pigment spot deep in the tissues on
the side of the head may indicate a rudimentary but non-functional
eye. Gut contents are visible through a semi-transparent body wall.
Preserved fish are yellowish-white.
Size
Reaches 55.0 mm total length (Kiavash Golzarian, pers comm., 6 April 2008).
Distribution
Found only at "Kaaje-ru" above the garden "Bagh-e
Loveh", "Lowa" or "Levan" (probably Loven at
33°04'N, 48°37'E) which is about 4 km from kilometre 382 on the railway from Bandar
Shapur to Tehran and approximately 12 km north of the railway station
Tang-e Haft. The stream below the cave locality is the "Ab-e
Serum" which runs into the "Ab-e Zezar" which is a
tributary of the Dez River,
in Lorestan Province. Further locality details are given in Bruun and
Kaiser (1948). The locality is at 744 m and 33°04'38.6"N, 48°35'33.1"E
according to the Iranian Fisheries Research and Training
Organization Newsletter, 21:3, 1998 and Kiavash Golzarian, pers comm., 6
April 2008).
Zoogeography
The relationships of cave species, with their reduced characters,
are problematical but the three rows of pharyngeal teeth and mouth structures indicate a
possible relationship with Garra.
Habitat
Known only from a well-like but natural outlet of a subterranean
system. The outlet overflows to form a small stream from January to
May (Smith, 1979) during the snow-melt period in the Zagros Mountains
but in April to June this flow ceases (the precise timing of flow and
its cessation is estimated from villager's comments and scientific
visits and also varies with precipitation). Pictures of show flowing water in
May 2010 are shown in the account of Paracobitis smithi
Nemacheilidae). The well area is about 5
by 3 m and gradually decreases as the year progresses. Divers
descended to a depth of 60 feet (= 18.3 m) in 1977 in the "well" until the
resurgence narrowed (Farr, 1977). A rope was let down by R. Mehrani (pers. comm.,
2000) and reached 23 m before the rope ran out and yet it was not at
the bottom. Smith (1979) reports divers descending to 60-70 feet (18.3- 21.3 m). The
pool shelves deeply under the cliff rearwards but the whole pool
surface is exposed to light. There is no vegetation in the pool except
for some encrusting algae on the rocky sides. The shale fragments
forming the outermost floor of the pool have a thin layer of mud on
them which may contain algae.
It seems probable that a complex of flooded but narrow and
inaccessible passages is the habitat of this species and the well is
merely the surface manifestation of this complex (Bruun and Kaiser,
1948; Smith, 1978; Banister, 1992). There is a smaller pool (about 2 m
across narrowing rapidly inside) and flowing exit stream lower down
the gorge, about 50 m away from the main locality, where a blind fish
was seen but not caught in December 2000 (Smith (1979) also
tentatively reports sighting a fish here). This is assumed to be
evidence of the interconnectivity of subterranean passages. The main
pool was not flowing at this time. The stream from the smaller pool
increases in flow downstream, possibly tapping more groundwater, and
eventually has a moderate flow. No fish were seen in it. The stream
falls over a high waterfall (estimated at 10-15 m high by Smith (1979)
which seems about right) so the well localities are isolated from the
local fishes in the main river. The main river houses Garra rufa
and nemacheilid species. The stream shows evidence of recent higher
flow which tends to confirm overflow from the main well.
More photographs can be seen in the description of Paracobitis smithi (Nemachelidae).
The fish may be seen swimming freely in the well, up to a 20 at a
time may be counted. They can be caught with a dip-net.
Sampling in December 2000 recorded a water temperature of 18.5°C,
pH 7.5 and a conductivity of 334 µS. Aquarium specimens have been maintained at
5-28°C and were very resistant to changes in oxygen levels (R. Mehrani, (pers. comm., 2000).
Amir Hosin Zalaghi recorded the following parameters on 19 May 2010:-
pH 7.3,
18.0ºC, conductivity 506.0 µS/cm, TDS 255.0 mg/l, CO3 0.0 mg/l, HCO3
152.0 mg/l, Cl 35.0 mg/l, SO4 65.0 mg/l, Ca 59.1 mg/l, Mg 27.8 mg/l,
K 0.0 mg/l, Th 250.0 mg/l, turbidity 0.65 NTU, COD 3.0 mg/l. BOD 0.0 mg/l, total
alkalinity 101.0 , alkalinity-f 0.0, DO 7.9 mg/l, TSS 0.5 mg/l, NO3
0.5 mg/l, NO2 0.0 mg/l and Na 12.0 mg/l.


Cave locality with R. Mehrani, 4 December 2000


Cave locality showing friable rock surrounds
Age and growth
Unknown although R. Mehrani (per. comm., December 2000) kept fish in aquaria for 18-24 months.
Food
Unknown but the aquarium specimens referred to above were fed Artemia,
dried and fine-ground Gammarus, zooplankton and phytoplankton. Faecal contents were phytoplankton and one fish was
observed to scrape the aquarium wall. Occasionally aquarium fish will swim
upside down with the snout at the water surface and may be feeding on an algal film.
Fish with a disc can attach to and graze on the substrate; a significantly
longer intestine in such fish may be indicative of a detrital feeding habit (Sargeran et al., 2008).
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks and its status as a cave fish.
Conservation
A fine of 10,000 rials (U.S.$139.94, 15 March 1978) was imposed specifically for illegal
fishing of this species (Anonymous, 1977-1978), now 100,000 rials (U.S.$11.04, 7
April 2008). It is on the 1994 IUCN
Red List of Threatened Animals as one of two rare fish species from
Iran (see also Paracobitis smithi) and is on the 2000 IUCN Red
List and subsequent ones as VU D2 (Vulnerable, acute restriction in its area of occupancy; see also Proudlove (2001)). The habitat is of unusual importance for studies on
evolution in unique environments. Coad (2000a), using 18 criteria, found
this species to be one of the top 4 threatened species of freshwater fishes in Iran.
B. Sandford (in litt., 1979) considered this fish to be
endangered. The cave appeared to be a recently collapsed system and
the network of fissures could be quite small. The main pool is at the
end of a narrow cleft, overhung by a cliff of friable shale. Shale
fragments fall spontaneously and the nearer end of the pool has a floor
of shale fragments. Coupled with recent collecting the number of
extant specimens may be quite low but this is impossible to confirm.
Local informants in December 2000 estimated that 5-6 parties visit
the site each year. The number of specimens taken is unknown but an
estimated 66+, possibly more than 100, have been collected in recent years (from 2000 to 2008). Eight specimens are
referred to in the literature, 4 specimens were caught in 1998 (R. Mehrani, pers. comm., 2000), in the two years 1999-2000 13
specimens were collected by one party, 18 by another in December 2000
(R. Mehrani and IFRO staff, N. Najafpour, IFRO, F. Razi, Darabad
Museum, Tehran and B. W. Coad), 10 specimens by Ali Ebrahimi (pers. comm, 25
January 2006), 11 by Kiavash Golzarian (pers comm., 6 April 2008), and more than 10 by others.
Four fish collected in 1998 survived 2 years in an aquarium (R.
Mehrani, pers. comm., December 2000). They were fed on Artemia,
zooplankton, phytoplankton and fine-ground Gammarus. Water
temperature ranged from 5 to 28°C and resistance to changes in oxygen levels was high. Fish were
sometimes observed to swim upside down at the water surface.
The establishment of a small park or reserve around the site and
education of the local people to maintain a watch on the cave would be
most useful to protect this species, and the other cave species at
this site, from unauthorised collectors. A survey of the local people
and the Department of the Environment files should be made to
determine the numbers of visitors to this remote site.
Further work
This is a small species of fish of unusual appearance and
provenance and could be bred and sold as an aquarium and experimental
species, providing that numbers at the site warrant removal of
breeding stock. If successful, this would ensure survival of the
species. Captures at present appear to be fortuitous and give no real
picture of the population size; removal of more specimens would have
to be carefully planned and monitored. Surveys of groundwater recharge
in the area and a more thorough investigation of the cave system
should be undertaken to assess the status of the habitat.
Sources
Movaghar (1973) is an additional reference, in Farsi, on this species.
Type material: See above (ZMUC P 26475, P 26476, 26477, 26478, 26480).
Iranian material:
CMNFI 2007-0124, 8, 27.3-42.2 mm standard length, Lorestan, type locality as
above.
Genus Kosswigobarbus
Karaman 1971
?
Much of the past literature on this genus appeared under Barbus (q.v.).
Kosswigobarbus kosswigi
(Ladiges, 1960)
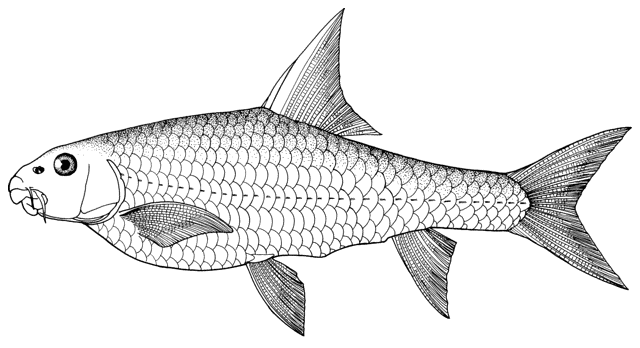
Common names
ابوحنج (abu henej or abu hanaj= father of the hook or spine; possibly abu hanash or abu henesh, father of the snake), shebeh shirbot.
[Kosswig's barbel].
Systematics
This species was described as Cyclocheilichthys kosswigi Ladiges, 1960 from the "Batman suyu" (the holotype is possibly a female, 162.7 mm, Turkey, Siirt Province, Batman suyu (the
Batman stream enters the Tigris River at 37°47.30"N, 41°00'E near Batman), April 1939, C. Kosswig). The holotype is in the Zoologischen Instituts und Zoologischen Museums der Universität Hamburg (ZMH
H1148). The genus Cyclocheilichthys Bleeker, 1860 is found only in Southeast Asia.
A new and monotypic genus, Kosswigobarbus, was erected for this species by Karaman (1971) but this was synonymised with Barbus by Coad (1982f). However Bănărescu (1997) and
Ekmekçi and Banarescu (1998) consider Kosswigobarbus to be valid. Borkenhagen (2005)
considers that kosswigi and sublimus should be placed in Barbus
(Carasobarbus) or Carasobarbus, favouring treating Carasobarbus
as a subgenus until Barbus is revised thoroughly. Borkenhagen et al.
(2011) include the two species in the genus Carasobarbus. Karaman (1971) distinguished the genus on the basis of the fin ray characters, a well-developed rostral flap, numerous fine pores on the head,
and a large lachrymal bone. These characters are
found in other "Barbus" species in Southwest Asia and the whole complex of large-scaled Barbus requires a detailed revision (see also under
Carasobarbus luteus and K. sublimus).
The relationships of this species appear to lie with other Southwest Asian species
formerly in the catchall genus "Barbus" which possess a compressed body, large scales with counts of 38 or less in
the lateral line, a smooth dorsal fin spine, 9 or more branched dorsal fin rays and 6 anal fin branched rays such as Barbus apoensis Banister and Clarke, 1977, B. exulatus Banister and
Clarke, 1977 (both in Southwest Arabia), B. chantrei (Sauvage, 1882) and B. canis Valenciennes, 1842 (both from the Levant),
B. luteus (Heckel, 1843) from
the Tigris-Euphrates and neighbouring basins, and B. sublimus Coad and Najafpour, 1997
from Khuzestan in the Tigris-Euphrates basin of Iran. Borkenhagen (2005) regards these species as a monophyletic group characterised by 6 branched anal fin rays, smooth and ossified last unbranched dorsal fin ray, modally 10 branched dorsal fin rays, less
than 40 scales in the lateral line, medium body size, large, round to shield-shaped scales with numerous parallel radii, pharyngeal teeth usually 2,3,5-5,3,2, gill rakers short, stout and slightly curved,
and barbels short.
Borkenhagen et al. (2011) found the number of
nucleotide differences between individuals of this species to be surprisingly
high, attributing this to rarity and small populations which give rise to
genetic drift and founder effects. Both this species and K. sublimus live
in small mountain streams and were considered to be less likely to migrate
through lowland rivers than, for example, Carasobarbus luteus, a more
widespread and generalist species.
Key characters
This species is characterised by having two pairs of thin barbels, 6 branched anal fin rays, the last unbranched dorsal fin ray strong and sharp-edged but smooth, 9-11 branched dorsal fin rays, large
scales (38 or less in the lateral line), and a deep and compressed body.
Morphology
The rostral flap shows variable development, sometimes overlapping the upper lip to become visible in ventral view and other times not so well developed. Lips are thick, continuous and fleshy and
there is a large median lobe to the lower lip. The mouth is small, ventral and u-shaped. The dorsal fin origin lies over or slightly in advance of the pelvic fin origin. The dorsal fin margin is strongly
concave and the last unbranched dorsal fin ray is a strong spine without teeth. The caudal fin is deeply forked. The anal fin is long and may overlap the caudal fin base.
Scales are regularly arranged over the body. A low sheath of scales is found at the base of the anal and dorsal fins, being most evident anteriorly, and enclosing the anal papilla. There is a pelvic
axillary scale. Anterior scale radii are few (5-11 in five scales from one specimen 126.6 mm SL) while posterior radii are numerous (35-40). There is a scaled keel or ridge before the dorsal fin as the
back narrows dorsally. Pharyngeal tooth formulae 2,3,5-5,3,2, 2,3,5-4,3,2, 2,3,4-5,3,2 and 2,3,4-4,3,2. The teeth are quite small even in the largest specimens. Teeth are hooked at the tip and strongly
recurved there, teeth are conical and have a small, concave to irregular or even rounded grinding surface below the tip. The fifth and most anterior tooth in the main row is small to minute in most fish
and may be absent but this is not size related as both large and small specimens have or lack this tooth. The gut is elongate and coiled.
Meristic data from Iranian and other Tigris-Euphrates specimens: dorsal fin branched rays 9(5), 10(34), 11(1), mean 9.9 after 4 unbranched rays; anal fin branched rays 6(40) (not 7 as in the
original description) after 3 unbranched rays; pectoral fin branched rays 15(2), 16(6), 17(4), 16.1; and pelvic fin branched rays 7(1), 8(11). Lateral line scales 29(1), 31(2), 32(1), 33(2), 34(6),
35(3), 36(1), 37(1), 38(1), 41(1) mean 34.2 (Kuru's (1975) range is 32-36); scales above the lateral line 6(7), 7(10), 8(1), mean 6.7; scales below the lateral line 5(5), 6(13), mean 5.7; scales between
lateral line and pelvic fin 4(11), 5(1), mean 4.1; predorsal scale rows 11(1), 12(2), 13(5), 14(3), 15(1), mean 13.1; and caudal peduncle scales 13(2), 14(2), 15(6), 16(2), mean 14.7. Total gill rakers
10(2), 11(2), 12(3), 13(3), 14(1), mean 11.9. Pharyngeal teeth 2,3,5-5,3,2(4), 2,3,4-4,3,2(3) or 2,3,4-5,3,2(1). Total vertebrae 39(5), 40(4), mean 39.4.
Caudal peduncle length in head length 1.2-1.3, mean 1.3; caudal peduncle depth in caudal peduncle length 1.5-1.7, mean 1.6; pelvic fin length in standard length 4.6-5.4, mean 5.0; pelvic fin length in
pelvic fin origin to anal fin origin distance 1.2-1.6, mean 1.3; dorsal fin spine length in head length 0.8-0.9, mean 0.9; and longest dorsal fin ray in head length 0.9-1.0, mean 0.9.
Sexual dimorphism
Sample sizes are too small to investigate accurately.
Colour
Upper flank scales are outlined by pigment, most evidently anteriorly on each scale. Fins are lightly pigmented with scattered melanophores on both rays and membranes with some concentration on
dorsal fin membranes although the extent varies individually. The peritoneum is black.
Size
Reaches 19.4 cm total length (the holotype).
Distribution
Found in the Tigris-Euphrates basin of Turkey and Iran (Coad, 1982f; Coad and Najafpoiur, 1997; Abdoli, 2000). It may also occur in the Zohreh River (Gh. Izadi, pers. comm., 2001).
Zoogeography
Karaman (1971) considers that the closest relatives of this species are to be found in the Indo-Malayan region.
Habitat
This species is found in large rivers in Iran which, however, in mid-summer are more stream-like in water flow. Collections are from the plains of Khuzestan and from altitudes in excess of 1600 m in
the Zagros Mountains. Temperatures in early July range from 21 to 23°C. One locality was polluted and others were cloudy or muddy. The river beds are composed of stones.
Age and growth
Unknown.
Food
The elongate gut and black peritoneum suggest a plant component to the diet but examination of two gut contents reveal insect remains including chironomid larvae.
Reproduction
Unknown.
Parasites and predators
Sohrabi and Jalali (2002) report the nematode Schulmanella petruchewskii from the liver of this species caught in the Dez River.
Economic importance
This species is too rare in Iran to be of any economic importance.
Conservation
Recommendations are difficult to make since the ecological requirements of this species are unknown. It appears to be rare but this may only be inadequate sampling techniques. Further collections
in addition to the holotype have been made in southern Anatolian Turkey (Kuru, 1978-1979) but it does not seem to be common. Endangered in Turkey
(Fricke et al., 2007).
Further work
Intensive field work utilising a wide variety of techniques should be directed to determining the abundance and distribution of this species. An adequate material base would then enable ecological
studies to be carried out and conservation measures determined.
Sources
Some counts from Kuru (1975) on Turkish material.
Type material: See above, Cyclocheilichthys kosswigi (ZMH H1148).
Iranian material: CMNFI 1979-0275, 1, 126.6 mm standard length, Lorestan, Kashkan River drainage (33º25'N, 47º58'E);
CMNFI 1979-0277, 1, 116.5 mm standard length, Lorestan, Kashkan River drainage (33º30'N, 47º59'E);
CMNFI 1979-0289, 1, 103.5 mm standard length, Kermanshahan, Diyala River drainage (34º28'N, 45º52'E);
CMNFI 1979-0290, 2, 120.1-122.1 mm standard length, Kermanshahan, Diyala River drainage (34º31'N, 45º35'E);
CMNFI 1979-0368, ?, ? mm standard length, Khuzestan, Karkheh River (32º24'30"N, 48º09'E);
uncatalogued, 1, 173.3 mm standard length, Khuzestan, Karkheh River near Shush (no other locality data);
uncatalogued, 3, 140.1-179.0 mm standard length, Khuzestan (no other locality data);
uncatalogued, 1, 160.0 mm standard length, Khuzestan, Karun River basin near Izeh (no other locality data).
Comparative material: BM(NH) 1974.2.22:1281, 1, 31.2 mm standard length, Iraq, Al Hadithah (34º07'N, 42º23'E);
BM(NH) 1974.2.22:1292-1296, 4, 35.5-98.5 mm standard length, Iraq, Al Hadithah (34º07'N, 42º23'E).
Kosswigobarbus sublimus
(Coad and Najafpour, 1997)
Common names
None.
Systematics
The holotype is CMNFI 1995-0009, female, 113.5 mm, Iran, Khuzestan, A'la River at Pol-e Tighen, 31°23.5'N 49°53'E, 20 September 1995, B. W. Coad, N. Najafpour and party. Paratypes are
CMNFI 1995-0009A, 41.9 mm, same locality as the holotype (lost in the mail while on, loan September 2005), CMNFI 1995-0010, female, 115.3 mm, A'la River, 2 km above Pol-e Tighen, 31°23.5'N
49°54'E, 20 September 1995, B. W. Coad, N. Najafpour and party, and CMNFI 1995-0011, 3 females, 90.5-98.6 mm, same locality as holotype, early December 1994, Gh. Eskanderi (one specimen lost in the
mail while on loan, September 2005).
The species was named after its river of capture, the only known locality for this species. A`la means "most high" or "exalted".
Borkenhagen et al. (2011) used limited
molecular data (a small number of base pairs in the cytochrome b gene)
and found evidence of paraphyly of kosswigi with sublimus,
indicating a recent speciation event.
Key characters
A member of the genus Barbus sensu lato characterised by the unique combination of the following characters: large scales (24-27 in the lateral line), 37-38 total vertebrae, 10-11 branched
dorsal fin rays, 6 branched anal fin rays, a relatively short and smooth dorsal fin spine (spine length in head length 1.0-1.1), lower lip with a rounded median lobe and a posterior free flap, a
compressed body (depth 3.3-3.5 in standard length), a short caudal peduncle (length in head length 1.5), long pelvic fins (length in standard length 4.1-4.5), and a short dorsal fin (longest dorsal fin
ray in head length 1.1-1.2).
Morphology
Dorsal fin branched rays 10(5), 11(1), mean 10.2; anal fin branched rays 6(6); pectoral fin branched rays 14(1), 15(5), mean 14.8; and pelvic fin branched rays 8(6). Lateral line scales 24(1), 25(2),
26(2), 27(1), mean 25.5; scales above the lateral line 4(1), 5(5), mean 4.8; scales below the lateral line 4(3), 5(3), mean 4.5; scales between lateral line and pelvic fin 3(6); predorsal scale rows 9(5),
10(1), mean 9.2; and caudal peduncle scales 12(5). Total gill rakers 10(1), 11(1), 12(2), 15(1), mean 12.0. Total vertebrae 37(2), 38(4), mean 37.7. A specimen from the Khersan River had 39 total vertebrae
and one from the Ardal River had 40 total vertebrae; both these fish being unusual in other counts too. Esmaeili et al. (2006) give the following characters for their 6 specimens from Fars: 11
branched dorsal fin rays, 6-8 anal fin branched rays, 16-18 branched pectoral fin rays, 24-28 lateral line scales, and 10-12 total gill rakers.
The body is relatively deep (depth 3.3-3.5 times in standard
length) and compressed. The snout is rounded and overhangs the upper part of the thick upper lip. The extent of overlap varies individually. The lower lip is also thick but has a rounded protuberance at
its centre, visible in lateral view. The protuberance is variably developed as a flap which is free posteriorly and at the rearmost sides. The posterior barbel is longer and thicker than the anterior
barbel. The anus lies just anterior to the anal fin origin.
Scales are regularly arranged over the whole body, there is a pelvic axillary scale, and scales at the anterior base of the anal fin form a small sheath around the bases of the anal rays. Radii are
found on the anterior and posterior fields of each scale, being most numerous posteriorly, about three times as many. Some radii extend into the lateral fields. Circuli are numerous and on the posterior
field break up into bubble-like shapes.
The dorsal fin is slightly to strongly concave on its margin. The spine tapers and is thin and flexible at the tip. The dorsal fin origin lies over the pelvic fin origin. The caudal fin is deeply
forked with the lower lobe more developed and with longer rays than upper lobe. The anal fin reaches or obviously passes the base of the caudal fin rays. This variation in length does not appear to be size
or sex related. The posterior margin of the anal fin is straight to concave. The pelvic fin has a straight to rounded posterior margin. The pectoral fin margin is concave and in some fishes is falcate.
The gut is elongate with anterior and posterior loops. Gill rakers are short and reach to the adjacent raker when appressed. Pharyngeal teeth are rounded with a hooked tip and a flattened area below
the tip. On three specimens counts were 2,3,5-4,3,2, 1,3,5-4,3,2 and 3,3,4-4,3,2.
Sexual dimorphism
Sample size of the type series is too small to document sexual dimorphism.
Colour
The overall live colour of the species is silvery with the back olive-green. Scales are outlined with dark pigment. The pectoral, pelvic, anal and caudal fins are a faintly pigmented with orange to
yellow hues, most apparent when the fin is collapsed. Much of these fins is grey to hyaline. The dorsal fin is grey to hyaline. The eye is silvery with grey-brown pigment at the upper margin. The
peritoneum is silvery with numerous melanophores merging to give an overall dark appearance.
In 70% ethanol the pigmentation pattern is as follows. Upper to mid-flank scales have the margins and bases pigmented with melanophores, outlining the scales. Most pigment is concentrated at
the scale base giving a slight appearance of rows of spots. Larger fish are more fully pigmented so the back and upper flank then appear dark. The dorsal surface of the head is finely speckled black. The
dorsal fin has dark pigment on the membranes, on the distal half or the whole fin, with less pigment on the rays. The caudal fin is mostly hyaline with dark pigment lining the rays. The pectoral and anal
fins have some dark pigment lining or on the anterior rays and, in larger fish, on the membranes. The pelvic fin is hyaline. The smallest specimen has a distinct mid-caudal base spot and another spot on
the back at the anterior dorsal fin base. Fins are more hyaline than in larger fish.
Size
Reaches 115.3 mm standard length. The maximum size is 15.5 cm (Iranian Fisheries Research and Training Organization Newsletter, Tehran, 18:5, 1997).
Distribution
Known from the A'la River in Khuzestan Province in the Tigris River basin and the Fahlian River in Fars (Esmaeili et al., 2006).
Zoogeography
This species is known from the A`la River, which joins with the Rud Zard (rud = river), and emerges from the foothills of the Zagros Mountains onto the Khuzestan plains where it is tributary to
the Jarrahi River. The Jarrahi feeds the Shadegan Marshes and is mostly lost there. In flood times, there may be a connection through the marshes to the Karun River and thence to other large river systems
in the Tigris-Euphrates basin. However, it is suspected that the ecological requirements of this species limit it to fast flowing rivers over hard substrates and the marsh system isolates it from
other river systems. Collections in the Rud Zard at Rud Zard village and Bagh-e Malek on several occasions have not included the new species although the Rud Zard would appear to be a suitable habitat.
The range extension of 380 km southwest of the A`la River to the Fahlian River near Noorabad in Fars places this species in the headwaters of the Zohreh River which drains to the northern Persian Gulf.
This may indicate headwater captures or possibly former interdigitating drainages on the Khuzestan plain.
Habitat
The type habitat is a cloudy river in a wide flood plain at about 800 m. The river bed is stones and pebbles. Water is led off from the river at intervals to irrigate the rice fields of the villages
of Meydavud-e `Olya (31°24'N. 49°52'E) and Meydavud Pa'in (31°23'N. 49°49'E) which extend along the bank of the A`la River. This water abstraction is a potential threat to the well-being of fishes in
this river system. The water demands of rice growing are large and there is little or no rain through the summer months in this area. Air temperatures in September can exceed 40°C
and evaporation from the fields and the river is commensurate.
The fish were caught at the type locality in relatively fast water (0.9 m.s-1) over a one hour fishing period. In September 1995, the river was at the seasonal low water and the type locality
was 10 m wide, 40 cm deep and had a discharge of ca. 2.9 m3.s-1. The water was also cloudy for the collection in December 1994 at the type locality but the river was wider and had
more flow after rain. The second locality had more flow and was deeper and wider than the type locality, to about 30 m and 80 cm. Fishes were caught by
electroshocker and cast-net and were difficult to catch and few in number. Other species captured were the cyprinids
Luciobarbus barbulus, Tor grypus, Barilius mesopotamicus, Capoeta
trutta, Cyprinion macrostomus and Garra rufa, and the sisorid catfish Glyptothorax silviae.
The Fahlian River capture site was shallow, had relatively clear water, a heterogenous bed morphology (sand, gravel, stone, pebbles, rock, etc.), and an absence of aquatic and riparian vegetation
(Esmaeili et al., 2006).
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported.
Economic importance
None reported.
Conservation
Known only from the type series, its conservation status is unknown. It appears to be rare and possibly restricted to areas with running water year round.
Further work
The distribution of this species should be investigated within the A'la River and neighbouring drainages and accurate estimates of its numbers and biology procured as part of a process to determine its
conservation status.
Sources
Type material: See above, CMNFI 1995-0009, CMNFI 1995-0009A, CMNFI 1995-0010, CMNFI 1995-0011.
Genus Labeo
Cuvier, 1816
Labeo dero
(Hamilton, 1822)
Mashkel (= Mashkid) River basin in Pakistan on the southeastern border of Iran (Zugmayer, 1913; Mirza, 1971; 1972; 1974; 1975; 1992).
No Iranian record.
Labeo gedrosicus
Zugmayer, 1912
Mashkel (= Mashkid) River basin in Pakistan on the southeastern border of Iran (Zugmayer, 1912; Mirza, 1971; 1972; 1974; 1975; 1992; Neumann,
2006). No Iranian record.
Labeo macmahoni
Zugmayer, 1912
Makran basin (Dasht River) in Pakistan on the southeastern border of Iran (Zugmayer, 1912; Mirza, 1971; 1972; 1974; 1975; Mirza and
Saboohi, 1990; Neumann, 2006). Placed in a new subgenus, Tariqilabeo by Mirza and Saboohi (1990). Kullander et al. (1999)
and Mirza and Arshad (2008) consider this taxon to be, questionably, a synonym of Crossocheilus diplocheilus (Heckel 1838).
A syntype is in the Naturhistorisches Museum Vienna (NMW 81256).
No Iranian record.
Genus Leucaspius
Heckel and Kner, 1858
The genus Leucaspius has not been recently revised in detail
and its composition remains uncertain. There may be several species in
Europe but only one has a wide distribution and this is found in Iran.
The genus is characterised by a moderately compressed and elongate
body; an incomplete lateral line on up to about 13 scales; moderately
large, easily detached scales; short dorsal and somewhat longer anal
fin; belly without a keel but somewhat compressed; terminal mouth with
lower jaw entering the depression of the upper; pharyngeal teeth
usually in 2 rows; and gill rakers of moderate size and density.
Leucaspius delineatus
(Heckel, 1843)

Common names
mahi-ye riz-e noqrei or mahi-e-rize-noghreie (= small silvery fish).
[gafgaz ustuzani in Azerbaijan; ovsyanka, verkhovka, Kavkazskaya
verkhovka or Caucasian verkhovka in Russian; sunbleak, white aspe,
rain bleak; belica; Moderlieschen in German].
Systematics
Squalius delineatus was originally described from Wien and
Mähren, Austria. The Caspian Sea basin taxon is given by Berg
(1948-1949) as Leucaspius delineatus delineatus natio caucasicus
Berg, 1949, described from Transcaucasia, which is distinguished by a
lower average dorsal fin branched ray count (7-8 rather than 8 or
rarely 9 for the typical form of Europe). This natio has no taxonomic
standing but has been applied as a subspecies by some authors (Arnold and Längert, 1995).
Key characters
The large, rounded papillae around the genital opening are
distinctive in females, and for both sexes the combination of an
incomplete lateral line with moderately large scales is distinctive.
Morphology
Dorsal fin with 2-3, usually 3, unbranched rays followed by 7-10
branched rays (usually 8 in Europe but counts of 7 and 8 are about
equally frequent in the Caucasian populations according to Berg
(1948-1949) but Abdurakhmanov (1962) gives a frequency of 94% for 8
rays and only 6% for 7 rays in fish from Azerbaijan), anal fin with
3-4, usually 3, unbranched rays followed by 9-17 branched rays (10-12
in the Caucasian subspecies), pectoral fin branched rays 11-16 and
pelvic fin branched rays 7-8. Lateral series scales 36-53; lateral line
incomplete with 0-13 pored scales anteriorly. Scales bear few anterior
and posterior radii, have few circuli, a subcentral anterior focus and
are a vertical oval in shape. Gill rakers 10-17 (rarely 20, usually
13-16), reaching the second raker below when appressed. Vertebrae
36-40. Pharyngeal teeth very variable 5-5, 5-4, 4-4, 4-5, 1,5-5,
5-5,1, 1,5-4, 5-4,1, 1,4-4, 4-4,1, 1,5-5,1, 1,5-5,2, 1,4-4,1, 1,4-5,1,
1,5-4,1, 1,5-4,2, 1,4-5,2, 2,5-4,1, 2,5-4,2, 2,5-5,2 and even 2,5-6,1.
The frequency of various counts varies with locality, and even whether
single row counts dominate over two-rowed counts (Arnold and Längert,
1995). Teeth are hooked at the tip and slightly to strongly serrated.
The belly is compressed in the mid-line between the pelvic fins and
the vent but does not form a strong keel. The gut is an elongate
s-shape. The chromosome number is 2n=50 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- branched dorsal fin rays 8(10) or 9(1);
branched anal fin rays 10(4) or 11(7); branched pectoral fin rays 11(7), 12(3) or 13(1);
branched pelvic fin rays 7(10) or 8(1); scales in lateral series 39(3), 40(5), 41(1), 43(1) or 45(1); total
gill rakers 13 (4) or 14(7); and total vertebrae 36(1) or 37(9). One specimen showed fusions of abdominal vertebrae.
Sexual dimorphism
Females have a unique fold of skin in the shape of two, large,
rounded papillae around the genital opening. The male is a little
smaller than the female. Males develop prominent nuptial tubercles on
the dorsal head surface, snout, on the lower jaw in three pairs and on
the upper jaw in two pairs for a total of about 60 tubercles. The male
genital opening is depressed.
Colour
The back is olive-green to brown and the flanks and belly
silvery-white. A steel blue or bluish-green stripe begins at the rear
third of the body and extends back, broadening, to the tail base. Fins
are hyaline or slightly yellowish. The peritoneum is light.
Size
Attains 12 cm total length although only up to 5.6 cm total length in the Caucasian form.
Distribution
Found in western and central Europe from the Rhine and north of the
Alps east to northern drainages of the Black Sea and the western and
northern drainages of the Caspian Sea. Leucaspius delineatus caucasicus is found in the north
Caucasus including the Black Sea parts and in Transcaucasia. In the
southern Caspian Sea basin, it is found in the lower reaches of the Kura River,
Imeni Kirova Bay and the Lenkoran region of Azerbaijan (Kuliev, 1989).
A single specimen from Iran was collected by Mr. Akbar Nasrollazadeh
near Siah Darvishan (which is at 37°22'N, 49°26'E)
in Gilan on 27 May 1993. In June 1996 over 50 specimens were caught in
the Anzali Mordab (= Talab) by K. Abbasi and A. Sarpanah of the Gilan Fisheries
Research Centre (Iranian Fisheries Research and Training
Organization Newsletter, 15:4, 1997). Also reported from the Anzali Talab
by Abbasi et al. (1999) and present in the Safid River and Amirkelayeh
Wetland (Nasrollazadeh, 1999; K. Abbasi, pers. comm., 2001).
Zoogeography
The Caspian shore of Iran has been surveyed in some detail during the 20th
century and it is curious that this species was only discovered towards its end.
It may simply have been confused with other small, silvery minnows although it
should be noted that some of the surveys were carried out by Russian workers
familiar with this species. It may be a recent introduction with other, commercial exotics,
and therefore may not be from a Caucasian population.
Habitat
Found in still or slowly flowing water with vegetated shores in
large schools. It can be found in fish ponds, ditches, gravel pits and
quarries as well as natural habitats. Still water is required for
reproduction. It is tolerant of a wide range of temperatures, pH and
salinity depending on adaptation, e.g. temperature range of 3-32.8°C
(Arnold and Längert, 1995). This small fish is found in large schools
near the water surface. It may appear in small ponds without any
apparent connection to other water bodies, hence the German name
that has been interpreted as "Moderlieschen" or motherless. However,
the German name may more correctly mean mud lover (G. H. Copp, in litt.,
16 June 2004).
Age and growth
Life span is about 4-6 years with growth fairly continuous over this period.
Food
Diet comprises plankton such as cladocerans, copepods and rotifers,
benthic chironomids, flying insects which land on the water surface,
and also some algae and detritus.
Reproduction
There is often a spawning migration against the water flow (up to
2-3 m/sec) to new waters. Eggs are laid in strings which are wound
spirally around plants by the female, aided by the fold of skin around
the genital opening. They may also be laid in a disc-shaped patch on
any flat surface. Several spawnings occur over a few weeks in March to
September in Europe. The eggs are guarded and fanned by the male who
covers them with a bacteriostatic dermal mucus. Up to 485 eggs are
found in females and have diameters up to 0.5 mm in Azerbaijan, up to
3500 eggs and 1.5 mm in Europe. Maximum egg production over two
seasons is about 500-600 (Abdurakhmanov, 1962). Clutch sizes are about
50-350 eggs (Arnold and Längert, 1995). A minimum temperature of 18°C
is required for reproduction.
Parasites and predators
None reported from Iran but eaten by a wide variety of other fishes
in Europe and numerous parasites reported (Arnold and Längert, 1995).
Economic importance
The scales have been used in the production of artificial pearls as
with Alburnus alburnus
(a relative of A. hohenackeri). It has also been used in aquaria and
garden ponds and as bait by anglers.
Conservation
Lelek (1987) classifies this species as rare to vulnerable in
Europe. Kiabi et al. (1999) consider this species to be
conservation dependent in the south Caspian Sea basin according to
IUCN criteria. Criteria include few in numbers, habitat destruction,
limited range (less than 25% of water bodies), absent in other water
bodies in Iran, present outside the Caspian Sea basin.
Further work
More specimens from Iranian waters need to be examined to determine
if this species belongs to a distinct subspecies or is an exotic
population of the European type subspecies. Biology of the Caucasian
subspecies is unknown.
Sources
Arnold and Längert (1995) summarise biology of European populations in detail.
Iranian material: Uncatalogued material, 11, 31.2-37.5 mm standard length,
Gilan, swamp near Hendeh Khaleh (37º23'N, 49º28'E).
Genus Luciobarbus
Heckel, 1843
?
Much of the past literature on this genus appeared
under Barbus (q.v.). Valiallahi (2000)
describes "Barbus persicus", a new species, in his thesis but this has
not been formally described. The name may be preoccupied by Bertinius
longiceps persicus Karaman, 1971.
Seyed Mortezaei et al.
(2008) give details of 11 myxozoan and protozoan parasites of barboid fishes
from rivers, reservoirs and marshes in Khuzestan.
Luciobarbus barbulus
(Heckel, 1849)
Common names
lab pahn (= broad lip), برزم (berzem or barzam); berzem lab pahn in Khuzestan and Iraq to distinguish it from B. pectoralis; boz mahi (= goat fish) or سس
ماهي (= sos or sas mahi in the Dalaki and Shapur river basins); dolenj.
[abu-barattum (= owner or father of lips), abu baratem, abu bratum or nabbash in Arabic; Orontes barbel].
Systematics
Howes (1987) places this species in Barbus sensu stricto. Karaman (1971) places this species in the synonymy of Barbus rajanorum but other authorities consider it to be
Luciobarbus
pectoralis (q.v.). Almaça (1983) placed this species as a subspecies of Barbus mystaceus but later (1984a, 1984b, 1986, 1991) retained barbulus as a full species, known
only from the Levant, despite Heckel's record from both the Qarah Aqaj (= Mand) of Fars, Iran and the Quwayq (= Kueik) River of the Levant. I retain it as a species under Heckel's name for this taxon
until the systematics of this and related species can be worked out as indicated above. It is separated from mystaceus according to Almaça (1983) by having thinner lips, shorter barbels, the last
unbranched dorsal ray weaker and shorter, more dense denticles spread over a shorter length of ray, higher anal fin, gill rakers less numerous and the upper dorsal profile is rectilinear and oblique to
the back.
The type locality of Barbus Barbulus is the "Fluss Kara-Agatsch....bei dem Dorfe Geré" (= Qarah Aqaj or Mand River, Fars; possibly near Kereft, 29°01'N, 52°52'E) and presumably the
"Kueik bei Aleppo" (Heckel, 1846-1849b). J. Valiallahi, pers. comm., 2001 and Edmondson and Lack (2006) suggest Jereh at 29°15'N, 51°58'E but this is in the Hilleh River drainage, a
Dalaki River tributary. In addition, "Geré" takes a hard G in German, not a J. There may be some confusion of names and rivers here.
A possible syntype of barbulus from the Qarah Aqaj was located by Almaça (1983, 1986) in the Naturhistorisches Museum Wien (NMW 53957) and seen by me but is in too poor condition to be of much
value, being mostly bones. Another syntype is listed as NMW 6596 and measures 119.3 mm standard length. In 1997, this was the only syntype recognised and is possibly the same as NMW 53957 re-numbered as
the latter was not located in 2002. The catalogue in Vienna lists only 1 fish, while Heckel's description refers to several fish. NMW 6596 is mostly bones and is dried. The fleshy lip
fold of the original description could not be discerned, teeth are missing and the dorsal fin is broken off short.
"Syntypes" of mystaceus are in the Naturhistorisches Museum Wien from Mosul on the Tigris River (NMW 16472 (1 specimen), NMW 50394 (2), NMW 54384 (2)) and NMW 54385 (2)
but note that authors such as Karaman (1971) and Almaça (1983, 1991) refer the species description to Heckel (1843) in error. These were not marked as being syntypes as observed on a 1997 visit to Vienna.
Key characters
This species is characterised by having two pairs of barbels, a serrated and very strong dorsal fin spine similar to that in Capoeta trutta in its proportions relative to the body, usually 8
dorsal fin rays (never 10), fleshy lips, and 47 or more lateral line scales.
Morphology
The inferior mouth is moderate in size, with moderate to thick lips and with or without a median lower lip lobe. Some fish have very thick lips so a central lobe is apparent. Some show such a degree
of lip development as to appear almost abnormal while fish of similar size or larger lack this hypertrophy. In the latter case, the anterior head may be bluntly rounded and foreshortened rather than
having an almost straight upper margin tapering to a pointed end. Barbels are relatively thin, occasionally quite thick. The anterior barbel does not extend past the anterior eye margin level and the
posterior one not past the posterior eye margin in all sizes of fish. Rarely the anterior barbel extends to mid-eye level and the posterior one almost to the anterior operculum margin.
Dorsal fin with 4 unbranched and 8-9, usually 8, branched rays, anal fin with 3 unbranched and 5 branched rays. The last unbranched dorsal fin ray is usually very strong with a moderate density of
denticles extending along much of the ray but its strength is variably developed. Pectoral fin branched rays 17-19, pelvic fin branched rays 8-9, usually 8. Lateral line scales 47-59. Scale focus
subcentral anterior, many fine circuli, and numerous radii on all fields, curved in the lateral fields. A pelvic axillary scale is present but not strongly developed or apparent. Gill rakers 14-24,
reaching the second raker when appressed. The interior raker surface may be covered with spinules, the internal base is heavily tubercular and the tips may become club-shaped. Pharyngeal teeth 1 or
2,3,4 or 5-5 or 4,3,2 or 1, hooked at the tip but spoon-like below with the fourth tooth of the inner row molariform, with or without a blunt projection (hooked in small fish) and much larger than the
third, and the fifth tooth very small and rounded and sometimes absent apparently independent of size. The gut is elongate and complexly coiled with one anterior and 3 posterior loops. Total vertebrae
44 (Howes, 1987).
Meristics in Iranian fish are as follows: dorsal fin branched rays 8(26); anal fin branched rays 5(26); pectoral fin branched rays 17(6), 18(15), or 19(5); pelvic fin branched rays 8(25) or 9(1);
lateral line scales 47(1), 48(3), 49(4), 50(4), 51(2), 52(5), 53(4), 54(1), 56(1) or 57(1); total gill rakers 15(1), 17(1), 18(4), 19(3), 20(6), 21(5), 22(2), 23(3) or 24(1); pharyngeal teeth
2,3,5-5,3,2(18), 2,3,5-4,3,2(3), 2,3,4-5,3,2(3), 2,3,5-5,3,1(1) or 2,3,4-4,3,2(1); total vertebrae ?.
Sexual dimorphism
Unknown.
Colour
The back and upper flank are brownish, the lower flank yellowish and the belly whitish. Upper flank scales are outlined with pigment, and the anterior edge of the dorsal fin and the caudal fin
margin are black in preserved fish. Small fish have a few spots on the upper to mid-flank or may be profusely speckled in preservative.
Small live fish are silvery overall and have anal and caudal fins orange to bright red, especially the lower caudal fin lobe. The dorsal fin is grey and the pectoral and pelvic fins yellowish. The
operculum is greenish. The lower flank is greenish-golden and the upper flank brown to grey. Large specimens are silvery with clear fins. The belly in small and large fish is white and the back grey
or green to
brown. The iris is silvery. The peritoneum is black.
Size
Reaches 62 cm total length (Atabak Mahjoor Azad, pers. comm., 16 June 2008); J. Valiallahi (pers. comm., 2001) believes this species reaches 1.5 m and 90 kg in the Zagros rivers of western Iran.
Distribution
Found in the Tigris-Euphrates basin, the Orontes River and the Quwayq River. In Iran it is found in the Tigris River basin (Abdoli, 2000; Ghorbani Chafi, 2000), in the Gulf basin from the Zohreh
River and from the Shapur and Dalaki rivers (Gh. Izadpanahi, pers. comm., 1995), the lower Mand River (M. Rabbaniha, pers. comm., 1995), the Helleh, Dozgah, Dasht-e Palang (and its tributary the Shur)
(Abdoli, 2000), in the Kor River basin (Abdoli, 2000), although not confirmed by specimens seen by me, and possibly in the Hormuz basin.
Zoogeography
Almaça (1991) believes that this species originated from a colonisation wave from South Europe.
Habitat
van den Eelaart (1954) records this species from rivers in Iraq, moving into lakes and marshes on the floods but never far from rivers.
Age and growth
Al-Rudainy (2008) gives sexual maturity at 2.8
years, 31.5 cm length and 305 g in Iraq. Hashemi et al. (2010) examined
fish from the southern Karun River in Iran and found a size range of 20-94 cm
and 52-4675 g, growth was isometric, and growth and mortality parameters were L∞
= 132.9, K = 0.17, t0 = -0.66, M = 0.33, F = 1.04, Z = 2.72 and E =
0.76. Relative yield per recruitment (Y'/R) was 0.021, relative biomass per
recruitment (B'/R) was 0.25, exploitation ratio maximum sustainable yield (Emax)
was 0.42, precautionary average target (Fopt) was 0.16 year-1,
and limit (Flimit) as 0.21 year-1. The stock was
overfished and fishing regulations are required.
Food
Diet is benthic organisms including insects. Large plant remains and detritus are also present in gut contents of Iranian fish.
Reproduction
Al-Habbib et al. (1986) report spawning during July and August in fish from the Tigris River at Mosul, Iraq.
Al-Rudainy (2008) cites a major spawning in April and a lesser one in October,
with eggs deposited on gravel beds in fast water. Absolute fecundity is about
100,000 eggs. Reproduction in Iran has not been studied.
Parasites and predators
Peyghan et al. (2001) record the cestode
Bothriocephalus sp. and the nematode Rhabdocona sp. from fish from
Khorramabad rivers. Masoumian et al. (2008) recorded the myxosporeans
Myxobolus karuni and M. persicus from gills of fish captured in the
Karun and Karkeheh rivers and Shadegan Marsh.
Economic importance
This species is a preferred catch of anglers at Ahvaz in Khuzestan, second only to shirbot (Tor grypus).
Peyghan et al.
(2001) report that is is an economically important species with a good market
value in the Khorramabad region.
Conservation
The population numbers of this species have not been well-studied nor has its distribution been well-documented. Since it does appear on fish markets in Khuzestan, is a large species and its
habitats are under threat, it may require protection. Endangered in Turkey (Fricke et al., 2007).
Further work
The biology, distribution and population numbers of this species need investigation in Iranian waters.
Sources
Type material: See above,
Barbus barbulus (NMW 6596), and note comments.
Iranian material: CMNFI 1979-0024, 1, 128.7 mm standard length, Fars, neighbourhood of Shiraz (no other locality data);
CMNFI 1979-0109, 2, 91.1-91.6 mm standard length, Fars, Mand River at Shahr-e Khafr (28º56'N, 53º14'E);
CMNFI 1979-0135, 1, 215.4 mm standard length, Fars, tributary to Mand River (28º08'N, 53º10'E);
CMNFI 1979-0271, 1, 61.8 mm standard length, Lorestan, Kashkan River drainage (33º39'N, 48º32'30"E);
CMNFI 1979-0290, 1, 139.1 mm standard length, Kermanshahan, Diyala River drainage in Qasr-e Shirin (34º31'N, 45º35'E);
CMNFI 1979-0293, 1, 210.8 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1979-0349, 1, 126.0 mm standard length, Fars, Mand River at Kavar (29º11'N, 52º41'E);
CMNFI 1979-0393, 1, 112.1 mm standard length, Khuzestan, Jarrahi River drainage (31º18'N, 49º37'E);
CMNFI 1979-0497, 2, 117.4-134.4 mm standard length, Fars, Mand River at Band-e Bahman (29º11'N, 52º40'E);
CMNFI 1980-0907, 1, ? mm standard length, Iran (no other locality data);
CMNFI 1991-0153, 1, 230.0 mm standard length, Khuzestan, Zohreh River (no other locality data);
CMNFI 2007-0109, 3, 85.1-138.7 mm standard length, Kordestan, Qeshlaq River basin south of Sanandaj (ca. 35º16'N, ca. 47º01'E);
CMNFI 2007-0110, 1, 191.1 mm standard length, Kordestan, Yuzidar River basin (ca. 35º05'N, ca. 46º56'E);
CMNFI 2007-0111, 1, 153.0 mm standard length, Kermanshahan, Alvand River near Sar-e Pol-e Zahab (ca. 34º36'N, ca. 45º56'E);
CMNFI 2007-0113, 2, 123.9-139.6 mm standard length, Kermanshahan, Qareh Su tributary northwest of Kermanshah (ca. 34º25'N, ca. 47º01'E);
CMNFI 2007-0117, 4, 43.4-155.5 mm standard length, Kermanshahan, Gav Masiab near Sahneh (ca. 34º24'N, ca. 47º40'E);
uncatalogued, 1, 60.2 mm standard length, Khuzestan, Rud Zard at Rud Zard (31º22'N, 49º43'E).
Comparative material: BM(NH) 1920.3.3:23-30, 9, 80.2-98.9 mm standard length, ();
BM(NH) 1931.12.21:4, 172.5 mm standard length, ();
BM(NH) 1971.4.2:5, 1, 140.3 mm standard length, Iraq, Tigris near Mosul (36º20'N, 43º08'E);
BM(NH) 1972.3.16:2, 69.4 mm standard length, Iraq, 10 km northwest Qala Dize ();
BM(NH) 1974.2.22:1270, 174.6 mm standard length, ();
BM(NH) 1974.2.22:1271-1272, 2, 91.9-210.2 mm standard length, ();
BM(NH) 1974.2.22:1273-1274, 58.4-62.0 mm standard length, ();
BM(NH) 1974.2.22:1275-1277, 3, 182.4-201.0 mm standard length, ();
BM(NH) 1974.2.22:1278, 81.9 mm standard length, ();
BM(NH) 1974.2.22:1289, 173.3 mm standard length, ();
Luciobarbus brachycephalus
(Kessler, 1872)
Common names
zardek, زرده پر (= zardehpar), سس ماهي (= sos or sas mahi), سس ماهي
خزري (sas mahi khazari), sassmahi-ye Daryaye-Khazar.
[xazar sirbiti or shirbit in Azerbaijan; Kaspiiskii usach or Caspian barbel and korotkogolovyi ustach or short-headed barbel in Russian; Aral barbel; short-headed barbel].
Systematics
Barbus brachycephalus was originally described from the Syr Darya in Uzbekistan.
Howes (1987) considers the generic placement of this species to be problematical. It has slender barbels, 7 branched dorsal fin rays and the cranium is broad and flat, all characters at odds with
Barbus sensu stricto. Doadrio (1990) places it in the subgenus Luciobarbus Heckel, 1843 based on a series of 4 synapomorphic osteological characters, namely the exoccipital contacts the
pterotic "largely" (sic, probably broadly), high medial process of the urohyal, narrow exoccipital apophysis of the pterotic, and wide 4th and 5th infraorbitals.
Barbus obtusirostris (non Valenciennes, 1842) Jakovlev, 1870 (nomen praeoccupatum), described from the Volga River delta, Russia, is a synonym.
A possible syntype of B. brachycephalus from the Aral Sea is in the Naturhistorisches Museum Wien (NMW 53971) (Almaça, 1986). The NMW card index lists this fish plus 2 fish in NMW 53972 and
1 fish in NMW 53973 as syntypes. Syntypes in St. Petersburg, Russia are lost (Bogutskaya in Bănărescu and Bogutskaya, 2003). Syntypes of Barbus brachycephalus caspius are in
the Zoological Institute, Russian Academy of Sciences, St. Petersburg under 2892 (8 fish), Transcaucasia, 3895 (8), Lenkoran, 9076 (22), 9085 (10), 9109(2), 9117(11), 9118(1), 9124(8), 9128(9), all
from the lower Aras River and Lenkoran, 17042(2), 17043(1), 17044(1), all from the Bank Fishery along the lower Kura River. Syntypes under 10619 are apparently lost and a fish under 9108 is
actually a Luciobarbus capito (Bănărescu and Bogutskaya, 2003).
The Caspian Sea basin subspecies is Luciobarbus brachycephalus caspius
(Berg, 1914), described originally from the Caspian Sea basin (Eschmeyer et al., 1996). Karaman (1971), however,
considers differences with the type subspecies of the Aral Sea basin to be minor and not worthy of subspecific recognition. Differences are in body proportions and the Caspian barbel has a smaller eye,
lower dorsal fin, less deep body and head, longer pectoral-pelvic distance, shorter pelvic-anal distance, and dorsal fin further back than in the Aral barbel (Berg, 1948-1949). Fricke et al.
(2007) list this taxon as a full species but also have brachycephalus in the same system in Turkey (Kura-Aras).
Key characters
The 7 branched dorsal fin rays and the predorsal distance shorter than the postdorsal distance distinguishes this species from B. capito, and colour pattern distinguishes it from
B. lacerta, the other Caspian Sea barbels.
?and mursa
Morphology
The mouth is moderate in size and subterminal. Lips are thin to moderate, without a median lobe on the lower lip, and barbels are of moderate thickness. The anterior barbels can reach the level of the
posterior eye margin and the posterior barbels reach or pass the preopercle level but barbel lengths show marked individual variation.
Dorsal fin with 3-5, usually 4, unbranched and 6-8, usually 7, branched rays, anal fin with 2-3, usually 3, unbranched and 5-6, usually 5, branched rays. Pectoral fin branched rays 14-17 and pelvic
fin branched rays 7-8, usually 8. The dorsal fin denticles on the last unbranched ray are usually moderate in number, but may be lost in very large adults, are usually well-developed and extend along
four-fifths of the ray (Karaman, 1971). This ray is very strong. Lateral line scales 62-90, commonly 65-77. Scales are elongate with a central focus and few anterior and posterior radii in young fish.
There is a pelvic axillary scale. Gill rakers 16-25, short and reaching the one below when appressed. Pharyngeal teeth 2,3,5-5,3,2, hooked at the tip with the fourth tooth of the inner row large and
blunt and the first three spatulate, rarely 2,3,4-5,3,2 or 2,3,4-4,3,2. Total vertebrae 45-50, usually 46-49, mode 48. The gut is coiled anteriorly. The chromosome number is 2n=100 (Klinkhardt
et al., 1995).
Iranian specimens have the following meristics: branched dorsal fin rays 7(3), branched anal rays 5(3), branched pectoral fin rays 16(1) or 17(2), and branched pelvic fin rays 8(3). Lateral line
scales 69(2) or 71(1). Total gill rakers 18(1) or 19(2). Pharyngeal teeth 2,3,5-5,3,2(2) or 2,3,4-5,3,2(1). Total vertebrae ?.
Sexual dimorphism
Abdurakhmanov (1962) reports on fish from the Kura River basin where males have a longer dorsal fin base and females have a greater maximum body depth, width and girth. Bogutskaya in
Bănărescu and Bogutskaya (2003) report that males have a shorter head and longer unpaired fins; nuptial tubercles and colouration are absent.
Colour
The back is dark green, flanks and belly lighter, and the two areas may contrast as in
Luciobarbus capito. No dark spots on the body. Fins greyish. Peritoneum brown.
Size
Reaches 22.5 kg (Robins et al., 1991) and 1.2 m.
Distribution
Found in the Caspian and Aral seas and their tributaries. In Iran, it was formerly known from the Anzali Mordab but is probably no longer present (Holčík and Oláh, 1992; but see below) and it
was listed as rare in the Safid Rud (Derzhavin, 1934). Nedoshivin and Iljin (1929) and Nevraev (1929) recorded it from the Gorgan, Astrabad and Enzeli (= Anzali) regions. Recent works place it in rivers
from the Astara to the Neka and Gorgan Bay peninsula, in the Anzali Mordab, and along the whole Caspian Sea coast but these are summaries of past and present distributions (Riazi, 1996; Abbasi
et al., 1999; Kiabi et al., 1999; Abdoli, 2000; Abdoli and Naderi,
2009). This species is now very rare in the Caspian Sea basin of Iran, with only a couple of specimens found in a recent survey (M. Ramin, pers.
comm., 2000).
This species is also recorded from the Karakum Canal and Kopetdag Reservoir in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) and may eventually reach Iranian waters in the Tedzhen
(= Hari) River basin.
Zoogeography
Almaça (1984b) considers this species to be a Sarmatian Sea remnant, a Neogene brackish-water basin.
Habitat
Enters rivers to spawn but does not ascend as high as
Luciobarbus capito. It prefers deep sections of rivers with stony and gravel bottoms. In the Caspian Sea it may be found at 13-25 m depth. On
the Kura River in Azerbaijan there is a spring run and one in August-September. The spring run begins in March and lasts about 50 days; the summer run starts after a short interruption and lasts about
190 days. The water temperature at the start of the spring run is 6.7-11.0°C but the most intensive migration is in summer at 25.2-27.2°C (Bogutskaya in Bănărescu and Bogutskaya, 2003). Spring
run fish spawn in the same year. This species has been recorded at depths of 11.0-11.9 m in the Iranian Caspian Sea (Knipovich, 1921). Riazi (1996) reports that this species migrates into the
Siah-Keshim Protected Region of the Anzali Mordab. Jolodar and Abdoli (2004) state that it is more abundant in Gilan than in Mazandaran coastal waters.
Young females usually enter the sea immediately but males may remain in fresh water for 3-5 years. Spawners return to the sea.
Age and growth
Most fish examined by Razivi et al. (1972) from commercial catches in Iran were 2-7 years old, 38.0-69.0 cm long and weighed 698-4658 g. Low recruitment is attributed to poor spawning success,
a result of water abstraction during its spawning season. Sexual maturity is attained at 6-8 years. Holčík and Oláh (1992) note that the Anzali region catches are dominated by 3-5 year old fish,
38-71 cm fork length, with rapid growth and a weight of 2 kg attained during the fifth year of life. Abdurakhmanov (1962) gives a maximum life span of 13 years in Azerbaijan. Females live longer
than males which only reach 10 years (Bogutskaya in Bănărescu and Bogutskaya, 2003).
Food
No detailed literature reports but gut contents of small specimens from Iran contain crustaceans, and insects such as, curiously, ants, thrips and mosquitos. This fish evidently feeds on insects
taken at the surface and is reported as leaping out of the water to take flying insects (Bogutskaya in Bănărescu and Bogutskaya, 2003). Mayflies and caddisflies are also taken and gut contents
includes detritus. Crustaceans are the main food taken in the Caspian Sea (Abdurakhmanov, 1962) but molluscs are also recorded as well as small fish.
Reproduction
This barbel spawns in swift streams over pebbles or sand during July and August in Iran and the eggs attach to rocks (Razivi et al., 1972). Holčík and Oláh (1992) and Makeeva and Pavlov
(2000) state that eggs are semipelagic, hatching as they drift downstream over 2 days at 25°C. Fry are carried downstream. Up to 1,259,000 bright-yellow eggs are produced of 1.4 mm diameter and the
spawning season on the Kura River begins at the end of April, peaks in June and ends at the end of August. Favoured temperatures are 20-23°C (Abdurakhmanov, 1962). First spawning is at 5-7 years of age
with females taking a year longer to mature than males (Bogutskaya in Bănărescu and Bogutskaya, 2003).
Parasites and predators
Molnár and Jalali (1992) record the monogenean Dactylogyrus affinis from this species in the Safid River.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lamproglena polchella
on this species.
Economic importance
This species is caught as a food fish in Iran. Nevraev (1929) records catches of 37 to 962 individuals from the Anzali region for the years 1914-1915 to 1917-1918. It was abundant in the Anzali Mordab
with total catches for Iran of 54.6 t and 32.9 t in 1969/70 and 1970/71 (28.7 t and 14.4 t for the Anzali region alone) but few fish are captured now (Holčík and Oláh, 1992) (note that these figures
were taken from Appendix 11, on page 10 they are reversed). They are caught in rogas (outflowing rivers from the Anzali Mordab) and inflowing rivers of the mordab (lagoon) in late winter and early
spring. On the Kura River of Azerbaijan average weight in catches was 5.6 kg for females and 3.5 kg for males and the catch from 1920-1944 varied from 0.2 to 3.6 thousand centners.
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use as food and in aquaria.
Conservation
Vulnerable in Turkey (Fricke et al., 2007). Stocks of this species have declined because of poor habitat for spawning and the construction of dams and weirs which restricted
access to spawning grounds. Water abstraction for irrigation during the summer spawning season would have to be balanced against the requirements of the fish. Larvae of spring spawners are lost when they
enter irrigation channels and become stranded in fields (Razivi et al., 1972).
Once known from the Anzali Mordab, it is now absent to rare there and apparently replaced by
Luciobarbus capito (Holčík and Oláh, 1992). Kiabi et al. (1999) consider this species to
be critically endangered in the south Caspian Sea basin according to IUCN criteria. Criteria include commercial fishing, sport fishing, few in number, habitat destruction, limited range (less than 25% of
water bodies), absent in other water bodies in Iran, and absent outside the Caspian sea basin.
This species is regarded as critically endangered through illegal overfishing, pollutants and the destruction of breeding and nursery grounds. Only 2 specimens were caught in the 3 years prior to 2000
during a study of "Barbus"
species in Iran. Additionally, during the 6 month beach seine fishing season (October to April) for the years 1998 and 1999 along the Caspian shore, no specimens were
caught in 138 beach seines used 51,000 times (M. Ramin, pers comm., 2000).
Further work
Detailed surveys, perhaps returning captures alive, need to be carried out to monitor the status of this species in Iran.
Sources
Type material: ?
Iranian material: CMNFI 1970-0553, 2, ? mm standard length, Gilan, Sowsar Roga (37º27'N, 49º30'E);
CMNFI 1980-0120, 1, 115.3 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
Luciobarbus capito
(Güldenstaedt, 1773)
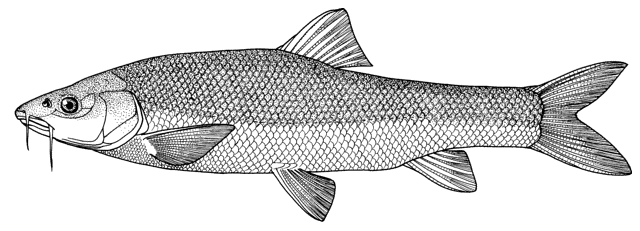

L. capito (above) and L. mursa (below) Aras River, 2 October 1994
Courtesy of Asghar Abdoli
Common names
usach bulatmai, usach chanari; zardi, zardek, zardak, زرده پر (= zardehpar), zard pareh, اورنج (oranj, orenj, orenge or
ourange, possibly from the yellowish fin colour)سس ماهي (= sos, sas or sass mahi), pulad mahi (= steel fish from body colour), ses mahi bozorg (= big ?
fish, ses being a word of unknown meaning).
[zardapar, shirbit, yastibas zardapar for natio platycephalus, all in Azerbaijan; tchanari in Georgian; bulatmai in Turkish; usach (or usatch) bulatmai and usach chanari in
Russian]. Bulatmai is derived from Farsi, bulat = pulad or steel, mai = mahi or fish in reference to the colour on the upper flank].
Systematics
Cyprinus capito was originally described from the Kura River, Transcaucasia. No types are extant.
Howes (1987) places this species in Barbus sensu stricto. Doadrio (1990) places it in the subgenus Luciobarbus Heckel, 1843 based on a series of osteological characters (listed under
Luciobarbus brachycephalus).
Cyprinus bulatmai Hablizl, 1783 (after Berg (1948-1949; Rainboth (1981) has Gmelin, 1774 as the author while Eschmeyer et al. (1996) have Gmelin, 1784 (originally described from Anzali,
Iran), Cyprinus chalybatus Pallas, 1814 (originally described from Anzali, Iran), Cyprinus mystaceus Pallas, 1814 (partim, from Tiflis), Barbus conocephalus Kessler, 1872 described
from the Zeravshan River, Uzbekistan, Barbus lacertoides Kessler, 1872 described from the Syr-Darya in the neighbourhood of Khodzhent (= Leninabad), Tajikistan, Barbus capito var.
tiflissica Kamenskii, 1899 described from the Kura River at Tiflis (= Tbilisi), Georgia, and Barbus bilkewitschi Bulgakov, 1923 (originally described from the "Atrek", i.e. the
Atrak River in Turkmenistan on the northeastern border of Iran; also spelt bilkewitchi on page 236 in Bulgakov but bilkewitschi on the plate), are synonyms. Barbus capito serratus
Sokolinskii, 1927 is a subspecies from the southern Caspian Sea and Barbus capito platycephalus Abdurakhmanov, 1960 is a subspecies or a natio in the lower Kura River basin (see Abdurakhmanov
(1962) for further details). Berg (1948-1949) and Karaman (1971) consider Barbus capito serratus to be a synonym of B. c. capito.
Bianco and Banarescu (1982) record this species from the Hablehrud and the Kul River basin at Darab in Persian Gulf drainages. The 2 specimens have 52 lateral line scales, 8 branched dorsal fin rays
and 18-19 gill rakers. They acknowledge that these 2 fish have fewer scales than
L. capito from the Caspian Sea basin but believe they may represent a new subspecies. These fish are presumed to be
misidentifications as L. capito is restricted to the Caspian Sea basin.
Laloei et al. (2003) using the mitochondrial cytochrome-b gene found no separable populations of this species in 60 samples from the Iranian Caspian Sea coast and rivers.
Key characters
The 8 branched dorsal fin rays and the predorsal distance considerably longer than the postdorsal distance distinguishes this species from
L. brachycephalus, and colour pattern
distinguishes it from Barbus lacerta, the other Caspian Sea barbels.
?mursa
Morphology
There is a rounded keel on the back in front of the dorsal fin. The mouth is moderate in size, inferior and horseshoe-shaped. Lips are fleshy and well-developed with tubercles but there is no free
median lobe on the lower lip. Barbels can be the most developed in thickness in this species among the
Luciobarbus considered here but this can vary. The anterior barbel extends back between the
anterior eye margin level and its middle and the posterior barbel extends to the posterior eye margin level or almost to the preopercle in young and some adults.
Dorsal fin with 3-5, usually 4-5, unbranched rays followed by 7-9, usually 8, branched rays and anal fin with 2-4, usually 3, unbranched and 5-6, usually 5, branched rays. Pectoral fin branched rays
15-19 and pelvic fin branched rays 7-9. The dorsal fin denticles on the last unbranched ray may be lost in very large adults but are evident for two-thirds or more of the spine length in most fish
(Karaman, 1971; Almaça, 1981). The last unbranched ray is moderately strong and the denticles are of moderate density along it. Lateral line scales 51-72, usually 60-66 (Karaman (1971) gives 36-70 but
he includes 8 subspecies over a wide range within his definition of the species). There is no obvious pelvic axillary scale although scales in this region are elongate. The scale focus is slightly
subcentral anterior, there are numerous fine circuli, and there are radii on all fields with those on the lateral fields few and often curved. Gill rakers 12-19, rarely to 22, increasing in number
with the size of the fish, reaching the one below or slightly further when appressed, rounded and knobbed tip, and a large internal rounded extension. Pharyngeal teeth usually 2,3,5-5,3,2 with
minor variants, hooked and spoon-like below with the depression below the crown filled in, the fourth one in the inner row the largest and pointed or blunt and rounded, the fifth smaller and blunt. The
gut is long and complexly coiled with several anterior and posterior loops. Total vertebrae 42-45 (Howes, 1987), 45-47 (Elanidze, 1983), 43-49 (Bogutskaya, Bănărescu and Almaça in
Bănărescu and Bogutskaya, 2003). Chromosome number 2n=100, NF=172 (Pourali Darestani et al., 2006).
Iranian fish have the following meristics: branched dorsal fin rays 7(1) or 8(49), anal fin branched rays 5(50), pectoral fin branched rays 16(3), 17(27), 18(18) or 19(2), pelvic fin rays 7(2) or
8(48); lateral line scales 53(3), 54(4), 55(7), 56(10), 57(6), 58(7), 59(7), 60(5) or 61(1); total gill rakers 13(3), 14(15), 15(18), 16(9), 17(4) or 18(1); pharyngeal teeth 2,3,5-5,3,2(33),
2,3,5-5,3,1(1), 2,3,4-5,3,2(2), 2,3,5-4,3,2(1), 2,3,5-5,2,1(1), 2,2,5-5,3,2(1); and total vertebrae ?
Sexual dimorphism
Unknown, apparently no spawning colouration or breeding tubercles.
Colour
The upper flank and head are steel-grey (hence bulat mahi) and the lower flank and belly are a strongly contrasting pale yellow or pearly-white. Occasionally fish with a uniform coloration are found
and preserved material may be uniform. The steel-grey upper flank may be comprised of dark scale margins surrounding a silvery-grey scale centre. The lateral line may be darkly pigmented. Spots may occur
individually on the body. The iris is silvery with a grey exterior ring and a very narrow interior golden ring. Barbels are white with grey on the inner surface. The dorsal fin is greyish and may have some
dark grey spots. The caudal fin has a greyish or yellowish or slightly orange upper lobe, sometimes with faint dark grey spots, a more strongly coloured and larger yellow-orange to canary-yellow lower lobe
and pink margins. The pectoral fin is whitish with a little or considerable amount of pink or yellow. The pelvic and anal fins are canary-yellow to orange with a white margin. Young fish may be darkly
speckled and mottled on the mid and upper flank rather like Barbus lacerta. Peritoneum dark brown.
Size
Reaches 1.05 m and 15 kg in literature reports. A specimen from the Sardabrud was 85 cm and 5.5 kg (A. Abdoli, pers. comm., 1995).
Distribution
Found in the basins of the Black, Caspian and Aral seas. Karaman (1971) gives a distribution from the Iberian Peninsula and North Africa to Southwest Asia but he includes 8 subspecies within his
definition of
"Barbus" capito.
In Iran, this species is found in the Caspian Sea basin, in rivers from the Aras to the Atrak
includng the Gorgan, Tajan, Babol, Haraz, Sardab, Pol-e Rud and Safid rivers,the Anzali Mordab, the Qezel Owzan and Shahrud in the upper Safid River basin, and the along the sea
coast (Derzhavin, 1934; Bianco and Banarescu, 1982; Almaca 1984a; Aliev et al., 1988; Holčík and Oláh, 1992; Kiabi et al., 1994; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli, 2000; Abdoli and Naderi, 2009). Valiallahi (2000) considers this species to be present in western
Iran, in the Tigris River basin.
Luciobarbus capito conocephalus (Kessler, 1872) is reported from the Karakum Canal, Kopetdag Reservoir and Uzboi lakes in Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) and may
eventually be recorded from the Tedzhen River and Caspian Sea basins in Iran.
Zoogeography
Almaça (1984b) considers this species to be a Sarmatian Sea remnant, a Neogene brackish-water basin, and related to Euro-Mediterranean
"Barbus".
Habitat
This species avoids muddy bottoms (Solak, 1977) although Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya (2003) report that it prefers warm, deep, slowly-flowing
water above gravel, sand or mud and can be found in lacustrine habitats. Spawning migrations in the Kura River of Azerbaijan go as far up as Aragva and generally it ascends to the uppermost tributaries
of rivers it enters. The spawning run in the Kura lasts almost the whole year except for the two coldest months. However the main spawning runs are in September-October and April (Bogutskaya,
Bănărescu and Almaça in Bănărescu and Bogutskaya, 2003). The Caspian Sea form is anadromous but there are also resident forms in the rivers there. Knipovich (1921) reports this
species at depths of 9.15-14.2 m, possibly deeper, in the Iranian Caspian Sea. There are both resident and andromous populations in the Anzali lagoon (Karimpour, 1998).
Age and growth
Solak (1989c) examined a population of this species in the Aras River in Turkey and found a life span of over 4 years, but over 6 years in the Çoruh River of the Black Sea basin of Turkey. In the
Caspian Sea basin fish may live up to 8 years (Abdurakhmanov, 1962). Anadromous fish are heavier than fish of the same length that are river residents. Maturity is attained at 3-5 years with females
mature one year later than males. Spring migrants spawn that summer while summer or autumn migrants overwinter to spawn the following spring or summer (Bogutskaya, Bănărescu and Almaça in
Bănărescu and Bogutskaya, 2003). Shajiee et al. (2002) found a sex ratio of 3:1 for male:female fish in the Caspian Sea off Gilan and a life span of 8 years. Gonadosomatic and
hepatosomatic indices, length-weight relationships and other growth and fecundity indices were given.
Food
Stomach contents consist of insects, crustaceans and worms, and filamentous algae and other plant material with associated invertebrates. Terrestrial insects, small fishes and frogs are also taken.
Abdoli (2000) reports Ephemeroptera, Trichoptera and Chironomidae. One specimen from Iran had fish remains, possibly a small
Luciobarbus capito. Abdurakhmanov (1962) reports grasshoppers and ants,
presumably taken at the surface.
Reproduction
Eggs number up to 193,600 and diameters up to 1.8 mm in Azerbaijan (Abdurakhmanov, 1962). A fish with well-developed testes was caught in the Gorgan River on 7 July, suggesting a spawning season of
late spring and summer, agreeing with egg diameters of fish from Azerbaijan which are largest in June.
Eagderi et al. (2006) studied the reproductive cycle in male fish
migrating to the Safid and Polrud rivers. Spermatogenesis developed rapidly from
late March with the process continuing up to July.
Parasites and predators
Molnár and Jalali (1992) record the monogenean Dactylogyrus linstowi from this species in the Safid Rud. Masoumian and Pazooki (1998) surveyed myxosporeans in this species in Gilan and
Mazandaran provinces, finding Myxobolus musculi. Masoumian et al. (2005) recorded the protozoan parasites Ichthyophthirius multifilis and Trichodina perforata from this
species in water bodies in West Azarbayjan. Masoumian et al. (2003) record Myxobolus musculi while Pazooki et al. (2003) and Pazooki (2006) record Rhabdochona hellichi,
Paradiplozoon homoion and Pseudocapillaria tomentosa, all reports from fishes captured in the Tajan and Zarem rivers of Mazandaran.
Sattari et al. (2002) and Sattari (2004) records the presence of the nematode,
Eustrongylides excisus, in the body cavity. This parasite can damage muscles in commercial species and render them unsuitable for sale. Sattari et al. (2004, 2005) surveyed this species in the
inshore area of the Caspian Sea, recording Eustrongyloides excisus and Anisakis sp. Pazooki et al. (2007) recorded various parasites from localities in West Azarbayjan Province,
including Neoechinorhynchus rutili from this species.
Miar et al. (2008) examined fish in Valasht Lake and the Chalus River,
Mazandaran and found the metazoan Bothriocephalus gowkongensis.
Economic importance
Holčík and Oláh (1992) report a catch of only 9 kg in the Anzali Mordab for 1990. This species had a catch of 17 tonnes in 1997, 28 t in 1998 and 7 t in 1999 during the 6 month beach seine
fishing season (October to April). For the years 1998 and 1999, 138 beach seines were used 51,000 times (M. Ramin, pers. comm., 2000). This species was of minor importance commercially in the
former U.S.S.R. and is a sport fish in Georgia (Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya, 2003). In East Azarbayjan it reaches sizes large enough for sport fishing
and as a commercial species (Ghasemi, 2002).
Conservation
Kiabi et al. (1999) consider this species to be conservation dependent in the south Caspian Sea basin according to IUCN criteria. Criteria include commercial fishing, sport fishing, medium in
numbers, habitat destruction, widespread range (75% of water bodies), absent in other water bodies in Iran, and present outside the Caspian Sea basin. Mostafavi (2007) lists it as conservation
dependent in the Talar River, Mazandaran. Vulnerable in Turkey (Fricke et al., 2007).
Further work
Biology and numbers of this species needs investigation.
Sources
Type material: ?
Iranian material: CMNFI 1970-0521, 7, ?-102.5 mm standard length, Gilan, Safid River near Lulaman (no other locality data);
CMNFI 1970-0525, 5, 111.9-133.4 mm standard length, Gilan, Safid River near Mohsenabad (no other locality data);
CMNFI 1970-0526, 19, ? mm standard length, Gilan, Safid River 6 km below Astaneh Bridge (37º19'N, 49º57'30"E);
CMNFI 1970-0531, 1, 157.3 mm standard length, Mazandaran, Larim River (36º46'N, 52º58'E);
CMNFI 1970-0536, 1, 194.4 mm standard length, Gilan, Siah River estuary near Rudbar (36º53'N, 49º32'E);
CMNFI 1970-0538, 10, 36.7-188.5 mm standard length, Gilan, Qezel Owzan River near Manjil (36º44'N, 49º24'E);
CMNFI 1970-0543A, 1, 170.4 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0546, 10, 39.3-61.8 mm standard length, Gilan, Safid River canal (no other locality data);
CMNFI 1970-0553, 1, 58.1 mm standard length, Gilan, Sowsar Roga (37º27'N, 49º30'E);
CMNFI 1970-0563, 1, 70.1 mm standard length, Gilan, Caspian Sea at Kazian Beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0568, 8, 62.5-132.0 mm standard length, Gilan, Caspian Sea at Kazian Beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0581, 6, 41.3-65.5 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0587, 3, 69.0-91.4 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1979-0431, 2, 240.9-265.5 mm standard length, Mazandaran, bazaar at Now Shahr (no other locality data);
CMNFI 1979-0437, 1, ? mm standard length, Gilan, Safid River 2 km west of Astaneh (37º16'30"N, 49º56'E):
CMNFI 1979-0452, 2, 53.5-56.5 mm standard length, Azarbaijan-e Khavari, Qezel Owzan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0486, 2, 69.2-78.8 mm standard length, Mazandaran, Atrak River draiange (37º44'N, 56º18'E);
CMNFI 1979-0488, 1, 95.8 mm standard length, Mazandaran, Atrak River at Maraveh Tappeh (37º55'N, 55º57'30'E);
CMNFI 1979-0491, 1, 191.5 mm standard length, Mazandaran, Gorgan River 15 km northeast of Kalaleh (ca. 37º33'N, ca. 55º44'E);
CMNFI 1979-0686, 19, ? mm standard length, Gilan, Safid River (37º24'N, 49º58'E):
CMNFI 1979-0695, 4, ? mm standard length, Gilan, Safid River at Manjil Bridge (36º46'N, 49º24'E):
CMNFI 1979-0788, 2, 152.0-202.4 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0116, 8, ?-62.4 mm standard length, Gilan, Safid River at Astaneh Bridge (37º16'30"N, 49º56'E);
CMNFI 1980-0123, 8, ? mm standard length, Gilan, Safid River (ca. 37º22'N, ca. 49º57'E):
CMNFI 1980-0127, 1, ? mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E):
CMNFI 1980-0132, 8, ? mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1980-0138, 2, 132.5-137.6 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E);
CMNFI 1980-0905, 1, 188.9 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0908, 3, 67.2-91.3 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E);
uncatalogued, 2, 245.7-272.8 mm standard length, Markazi, Shah River (no other locality data);
Luciobarbus esocinus
Heckel, 1843
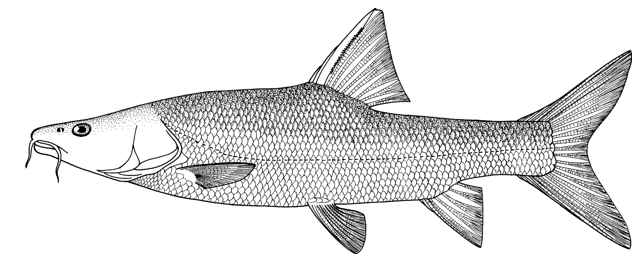

Darreshahr, Simarreh River, April 1987,
photo by N. Atarody; courtesy of B. Kiabi

Kermanshah, Simarreh River,
courtesy of B. Kiabi
more photos
Common names
سونگ (= soong) or بچ ( = bach) in northern Khuzestan and Lorestan, anzeh, anzah, narbach, and anzeh-bach at Ahvaz and in southern Khuzestan (meanings
unknown); بل زرد (= balzard).
[bizz (in Iraq), farkh-el-biz (= cheerful one (Heckel, 1843b) or baby of bizz), farch or mangar in Arabic; "Tigris salmon", "Euphrates salmon", pike barb, pike barbel].
Systematics
Howes (1987) places this species in Barbus sensu stricto. Labeobarbus Euphrati Sauvage, 1882 described from "Biredjik (Euphrates)", Turkey (not "Irak" as in Bertin
and Estève (1948)) is a synonym.
Karaman (1971) places this species in the synonymy of
"Barbus" xanthopterus as he considered the only difference to be scale count and the range of variation for these species is unknown. Almaça
(1983, 1986) agrees that several meristic characters are similar while the main differences are a shorter head and barbels in esocinus and dotted coloration in esocinus as opposed to
uniform in xanthopterus (isn't the reverse true?). He maintains them as separate species because information on variability in characters is lacking.
Examination of the types of L. esocinus (NMW 54088, 2, 58.5-61.5 mm standard length, 54091, 372.4 mm, 54092, 321.3 mm) and
L. xanthopterus (NMW 54841a (a syntype), 216.5 mm, 54786 (not a
type), 292.8 mm) in Vienna showed the following differences. Head size differs in the two taxa in that esocinus postorbital length is very elongate and the head tapers anteriorly in a distinctive
fashion. Head length in standard length is 3.2-3.6, mean 3.4 for esocinus and 4.0-4.2, mean 4.1 for xanthopterus and postorbital length in standard length is 5.9-7.2, mean 6.5 for
esocinus and 7.7-7.8, mean 7.8 for xanthopterus with the higher values for esocinus based on smaller fish which tend to have proportionately larger heads. Total gill raker counts are
8-10, mean 9.3 for esocinus and 12-13, mean 12.5 for xanthopterus. Larger esocinus appear to lose anterior rakers with age but still have fewer than xanthopterus
of similar size. Lateral line scale counts are 63-70, mean 67.3 in esocinus and 57-60, mean 58.5 in xanthopterus. On this limited basis I am maintaining the two species as distinct. An
Iranian specimen, 284.3 mm standard length (ZSM 21830 from the Dez River), falls within the ranges given above. see xanthopterus?
Almaça (1986) records syntypes of Luciobarbus esocinus in the Naturhistorisches Museum Wien from the type locality as given by (Heckel, 1843b) "bei Mossul in Tigris", Iraq (NMW 54088
(2 specimens), NMW 54091 (1), and NMW 54092 (1) but Heckel (1843b) does not specify the number of types). A syntype is in the Senckenberg Museum Frankfurt (SMF 454, formerly NMW; 281.2 mm standard
length) and another syntype is also there but dried (SMF 6785, formerly NMW) (F. Krupp, pers. comm., 1985). The catalogue in Vienna lists 2 fish in spirits and 2 fish stuffed.
The mounted holotype of Labeobarbus euphrati is in the Muséum national d'Histoire naturelle, Paris (MNHN A.6961) and measures 1650 mm total length (Bertin and Estève, 1948). Eschmeyer et
al. (1996) indicate that the catalogue number may be A.6971.
Key characters
This species is characterised by large size, a long, tapering and depressed head (rather pike-like in shape), two pairs of barbels, a serrated dorsal fin spine, lateral line scale count high (63-78),
moderately developed lips, and no large flank spots. Head length in standard length 3.1-3.7, mean ? and postorbital length in standard length 5.9-7.2, mean ? for ? specimens ?-? mm standard length
including the types listed above. (includes SMF454 281.2 mm SL, HL 75.6, postorb 44.1, snout 21.5
Morphology
Dorsal fin with 4 unbranched and 8 branched rays, anal fin with 3 unbranched and 5 branched rays, pectoral fin with 16-18 branched rays and pelvic fin with 8 branched rays. Lateral line scales 62-78.
Scales are regularly arranged, the smallest being on the isthmus anterior to the pectoral fin bases. There is a pelvic axillary scale. Scales have a central focus, numerous fine circuli, a wavy or
rounded anterior margin, and radii on the anterior and posterior fields with a few widely spaced ones on the lateral fields. Gill rakers 8-12, well spaced and just touching the one below when appressed.
Pharyngeal teeth 2,3,5-5,3,2, hooked with the third tooth of the inner row slightly larger than the fourth and the fifth smaller. Heckel (1843b) gives 2,3,4-4,3,2, and teeth from large specimens seen at
Ahvaz in 1995 by me had 2,3,4-4,3,2 and 2,3,5-4,3,2, the anteriormost tooth being small or absent. Even small specimens (85.7 mm standard length) may have the anteriormost tooth absent. Total vertebrae
48 (Howes, 1987) or 48-50 based on comparative materials listed below. The last unbranched dorsal fin ray is very strong, with a low density of denticles but with fine denticles extending over much of
the ray. The mouth is large, terminal and almost horizontal and extends back to the anterior eye margin. Lips are thin to moderate without a median lobe to the interrupted lower lip, and barbels are
thin to very thin. The anterior barbel does not reach past the nostril level and the posterior barbel does not pass the mid-eye to rear eye level. The nostril is elongate and closer to the eye than the
snout tip. The cephalic canals on the suborbital series have numerous branches. The gut is an elongate s-shape with several anterior loops.
Meristics for an Iranian specimen:- dorsal fin branched rays 8; anal fin branched rays 5; pectoral fin branched rays 17; pelvic fin branched rays 8; lateral line scales 69; and total gill rakers 9.
Sexual dimorphism
Unknown.
Colour
The back has numerous scattered, black spots on an olivaceous background, the spots extending onto the base of the dorsal fin. Spots may be weak or absent but this is comparatively rare. Overall colour
is silvery with the anal and caudal fins dark red. The flanks and belly are lighter. The eye is yellowish in colour. Young fish have a yellow tinge or sulphur yellow colour to the fins.
Size
Frequently up to 3 hundredweights (= 152.4 kg) in the Zab River of Iraq southeast of Mosul (Heckel, 1846-1849a); a fish 6'4" (1.93 m) long with a girth of 3'10" (1.17 m) and a weight of 215
lbs (97.6 kg) from the Euphrates River at Hakika (Light, 1917; wrongly identified as
"Barbus" scheich according to the editors in an article by Gudger (1945a)); 69 inches (1.75 m) measured over the
curve of a back with a 38 inch (0.97 m) girth and a weight of 123 lbs (55.8 kg) caught in the Diyala River, Iraq on a light 14-foot rod taking 1½ hours to land (Bagnall, 1919); 96 lb (43.6 kg) fish
caught near Kizil Robat (= As Sa`diyah) in the Diyala River on a lump of atta (a ball of dough)(MacKay, 1919)(Bagnall, a Major, out-doing MacKay, a Brigadier-General); 140 lbs (63.6 kg) Tigris
salmon caught on a 2" spoon at Samarra (Lane, 1920); hundreds of good weight up to 112 lbs (50.8 kg), one caught on a hand-line at 170 lbs (77.2 kg), one netted at 252 lbs (114.4 kg), and reputedly
over 300 lbs (136 kg)(Radcliffe, 1926); up to two yards (1.83 m) as evidenced by a photograph of a specimen draped over a donkey in Iraqi Kurdistan (Hamilton, 1937); 2 m and 150 kg in Iraq (van den
Eelaart, 1954; Herzog, 1967); a 167 lb (75.8 kg) Tigris specimen and a 213 lb (96.7 kg) specimen at Nassiriyah on the Euphrates, called both gattan and "Euphrates salmon" but it was presumably
the latter (Vesey-Fitzgerald and Lamonte (no date)); weights up to 300 lbs (136 kg) and the largest taken on rod-and-line as 220 lb (100 kg) and 7 feet (2.1 m), baits used included atta and dates,
and chicken or sheep liver (Mahdi, 1962). Beck (pers. comm., 2000) reports the largest fish seen in the 1990s along the Syrian Euphrates and its tributaries weighed 198 kg. A fish caught in 2001 on the
Euphrates River near Birecik in Turkey with a net weighed 111 kg and was 2.4 m long (www.fishing-worldrecords.com, downloaded 16 February 2007).
Iranian records of large specimens include one by Mr. Chabok-Savar, a Game Warden or biologist of the Department of the Environment who caught a specimen about 80 kg in the Simareh River in 1973 and
N. Atarody, also a Game Warden or biologist, caught two large specimens in April 1987 from "Tang-e Gheer" on the Simareh near Darreh Shahr (Abzeeyan, Tehran, 3 (August-September):19,
1992). A 1.65 m and 75 kg specimen is reported from the Dez River and a 2.1 m specimen is reported from the market at Ahvaz in 1993 (this last fish may have weighed 150 kg, original report
not seen; J. Valiallahi, pers. comm., 2001). The Gav Masiab, a river in Kordestan, is reputedly named for these large fishes ("river with fishes as large as a cow")(J. Valiallahi,
www.modares.ac.ir, downloaded 4 July 2000). Floor (2003) gives a photograph of a large specimen from the Karun River.
Distribution
This species is found in the Tigris-Euphrates basin including its Iranian portion and the adjacent northern Gulf basin (Marammzai, 1995; Abdoli, 2000). It is reported as common in the Dez Dam
(Gh. Eskandary, pers comm., 2000).
Zoogeography
Almaça (1984b, 1991) considers that the origin of this species lies with a group that migrated southwards in the late Pliocene from the Dacian Lake of the Sarmatian Sea and speciated in Mesopotamia.
Habitat
Found in large rivers and dams but also the more limited environment of palace ponds at Baghdad. Details of environmental requirements unknown.
Age and growth
Life span is at least 10 years (Ahmed, 1982). Fish in Khuzestan were found to have a sex ratio of 4.2:1.0 male:female (Gh. Eskandary, pers. comm., 2000; Eskandari et al., 2004). In the Dez
Dam of northern Khuzestan females had a length range of 156-1350 mm and a weight range of 31.7-26,500 g while for males figures were 183-1065 mm and 48-12,208 g. Males matured faster than females,
annual growth is slow and asymptotic length is more than 2 m. It appears to have a longer reproductive life compared to pre-maturation life (Eskandari et al., 2004).
Food
This species is a predator on other fishes. In the Dez Dam, all samples had fish in their stomachs although the gut to body length ratio indicates omnivory (Eskandari et al., 2004).
Al-Rudainy (2008) cites also crustaceans, aquatic insects and zooplankton in
Iraq.
Reproduction
van den Eelaart (1954) reports spawning in Iraq in March
and Al-Rudainy (2008) gives April to May. Eggs are laid between large stones in the deep part of rivers,
with absolute fecundity in Iraq at 600,000 eggs (Al-Rudainy, 2008). Some fingerlings drift down into lakes and marshes. Eskandari et al. (2004) report a very short spawning season in the Dez Dam in spring after reservoir water levels rise through spring flooding. The fish is a total spawner
with eggs released in upstream areas and shallows of the reservoir over gravel at 24ºC.
Ünlü (2006) gives age at first maturity as 4 years in the Turkish Tigris River
while Al-Rudainy (2008) gives sexual maturity as 10 years in Iraq.
Parasites and predators
Masoumian et al.
(2008) recorded the myxosporeans Myxobolus karuni and M. persicus
from gills of fish captured in the Karun and Karkeheh rivers and Shadegan Marsh.
Economic importance
In Iraqi Kurdistan these fish were caught and tethered by a cord passed through the lips until eaten by the villagers (Elliot, 1977). At Altan Keupri on the Lesser Zab River in Iraq a drugged bait was
used to stupefy the fish so it could be netted and dragged to shore (Hamilton, 1937).
This species was being considered for aquaculture during the year 2000 in Khuzestan although fish larger than 1 m are needed to be ripe adults. Anglers and commercial fishermen seek this fish in the
Iranian Zagros Mountains using ducklings (!) as bait (J. Valiallahi, pers. comm., 2001).
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use in textbooks.
Conservation
This species is under severe threat in the Syria Euphrates and its tributaries. A survey in 1997-1998 caught only a single juvenile and the commercial fisheries had not more than two dozen fish. Blast
fishing and poisoning had led to a decline in age of catches since 1993. Large scale water abstraction, dam building and pollution had destroyed habitats (R. Beck, pers. comm., 2000).
It is listed by Stone (2007) as one of the world's largest freshwater fishes, presumed to be threatened.
A report of fish kill, presumably of this species, in the "Cham Ghorah" River near Mahabad in July 1999 numbering about half a million fish was owing to desiccation of the habitat (J.
Valiallahi, www.modares.ac.ir, downloaded 4 July 2000).
Further work
Distribution and numbers are needed for a conservation assessment.
Sources
Type material: See above, NMW 54088.
Iranian material: ZSM 21830, 1, 284.3 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E). Some specimens observed in the IFRTO laboratory at Ahvaz (pharyngeal arches).
Comparative material: BM(NH) 1892.9.1:30, 1, 197.3 mm standard length, Iraq, Al Faw (29º58'N, 48º29'E);
BM(NH) 1920.3.3:80-82, 3, 85.7-147.0 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1931.8.12:5, 1, 111.8 mm standard length, Iraq, near Mosul (no other locality data);
BM(NH) 1974.2.22:1297, 1, 166.5 mm standard length, Iraq, Diyala River (no other locality data);
BM(NH) 1974.2.22:1810, 1, 220.1 mm standard length, Iraq (no other locality
data).
Luciobarbus kersin
(Heckel, 1843)
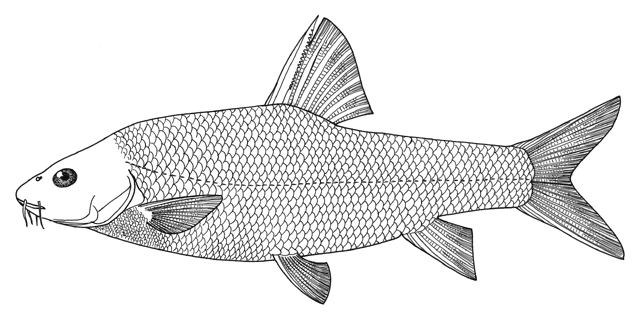
Common names
برزم (= berzem).
[shissan, jassan, gassan, djissan, barsam or bunni, kersin at Aleppo, all in Arabic; kersin barbel].
Systematics
Karaman (1971) places this species as a synonym of his Barbus capito pectoralis. Almaça (1983) suggests that kersin may be only subspecifically distinct from Barbus
(= Luciobarbus) pectoralis
(q.v.) but later (Almaça, 1984b) retains it as a full species until further information becomes available. Krupp (1985c) also synonymises this species with Barbus
(= Luciobarbus) pectoralis.
Syntypes of Barbus Kersin from "Aleppo", the type locality given by Heckel (1843b) or "Gewässern von Aleppo" (Heckel, 1846-1849a), are in the Naturhistorisches Museum Wien
(NMW 54212 and 54215) (Almaça, 1986). Krupp (1985c) lists the following syntypes of B. kersin all from Aleppo and collected by Th. Kotschy: 1 specimen, 141.2 mm standard length as measured by me
(NMW 54212), 4, 89.1-135.1 mm standard length as measured by me (NMW 54213), 1, 166.0 mm standard length as measured by me (NMW 54215) and 1, 152 mm standard length (formerly NMW, now in the Senckenberg
Museum Frankfurt as SMF 610). The card catalogue in 1997 listed NMW 54215 as "? lectotype" and NMW 54213 as "? paralectoptypes" (sic). Eschmeyer et al. (1996) list 1
syntype in the Museum für Naturkunde, Universität Humboldt, Berlin (ZMB 3237, formerly NMW). The catalogue in Vienna lists 6 specimens.
Key characters
This species differs from the similar L. pectoralis by the smaller scales and body depth being greater than head length (equal in pectoralis) (Berg, 1949). ?see BWC95-32 for a kersin
after Valiallahi, its a dark Barbus without the very large D spine and a different colour pattern
Morphology
Dorsal fin unbranched rays 3-4, usually 4, branched rays 7-8, anal fin with 3 branched and 5-6, usually 5, unbranched rays. Pectoral fin branched rays 17, pelvic fin rays 8. Lateral line scales 49-58.
Gill rakers 19. Pharyngeal teeth 2,3,5-5,3,2. The last unbranched dorsal fin ray is strong (as in pectoralis)with a low density of coarse denticles extending over much of the ray. The mouth is
moderate in size and subterminal. The highly rounded snout projects a little. Lips are thin to moderate but not fleshy and lack a median lobe. The upper lip is covered partly by the snout.
Barbels are thin, the anterior barbel not extending back beyond the anterior eye margin and the posterior barbel not beyond the middle of the eye. Body depth is equal to or greater than head length in
the types examined by me.
Sexual dimorphism
Unknown.
Colour
The body lacks distinctive markings and is olive to reddish-brown above, silvery on the flanks and white below. The dorsal and caudal fins have a blackish margin.
Size
Attains 70.1 cm total length (Menon, 1956). Reaches 2 m and over 100 kg (Khalaf, 1961).
Distribution
Found in the Tigris-Euphrates, Quwayq and Orontes River basins. In Iran, it is found in the Tigris River basin in the Karun and Karkheh rivers, and the northern Gulf basin in the Zohreh and Helleh
rivers and questionably the southern Gulf basin (Abdoli, 2000).
Zoogeography
Almaça (1984b, 1991) considers that the origin of this species lies with a group that migrated southwards in the late Pliocene from the Dacian Lake of the Sarmatian Sea and speciated in Mesopotamia.
Habitat
The main habitat of Iraqi fish is rivers, entering marshes and lakes during floods but returning to rivers in June (van den Eelaart, 1954).
Age and growth
Unknown.
Food
This species is said to eat a wide range of food items (Beckman, 1962),
including aquatic insects and plants (Al-Rudainy, 2008).
Reproduction
Eggs are deposited on clay or gravel bottoms during mid-February to early March
in Iraq (van den Eelaart, 1954). Al-Rudainy (2008) gives the spawning season as
March to April in Iraq.
Parasites and predators
Gussev et al. (1993a) describe new species of monogeneans from this species in the Dez River, Khuzestan, namely Dactylogyrus deziensis, D. deziensioides and D. kersini.
Ebrahimzadeh and Kailani (1976) record parasite species in the cestode genera Caryophyllaeus and Isoglaridacris and the protozoan Myxosoma from Barbus
(= Luciobarbus) kersin taken in the
Karun River.
Economic importance
None.
Conservation
Endangered in Turkey (Fricke et al., 2007) but status in Iran unknown.
Further work
The biology of this species in Iran needs study and a molecular comparison with putative synonyms would be of value in clarifying distinctiveness.
Sources
Type material: See above, NMW 54212, NMW 54213 and NMW 54215.
Iranian material: None.
Comparative material: BM(NH)1974.2.22:1324, 186.2 mm standard length ();
BM(NH)1920.3.3:41-50, 12(5 examined), 110.9-165.3 mm standard length ();
BM(NH)1920.3.3:31-40, 10, 141.7-310.9 mm standard length ();
Luciobarbus mursa
(Güldenstaedt, 1773)
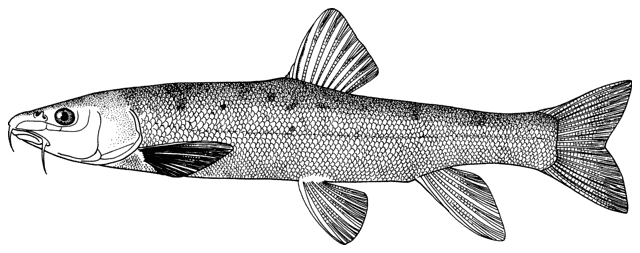

L. capito (above) and L. mursa (below) Aras River, 2 October 1994
Courtesy of Asghar Abdoli
Common names
سس ماهي (= sos, sas or sass mahi), mahi siah (= black fish), zardek-e qalami (= slender or straight yellow one), ses mahi koloft safid rud (= Safid River thick
fish, the meaning of ses, sos or sas being unknown but referring to "Barbus"), sas mahi-ye lab koloft (= thick lip
"Barbus" fish), زرده پر (= zardehpar).
[mursa or shchirbit in Azerbaijan; murtsa or mursa in Georgian; murtsa or Araksinskaya murtsa, both in Russian].
Systematics
Cyprinus mursa was originally described from the Kura River at Tbilisi, Georgia. Syntypes are presumed lost (Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya,
2003)
Barbus miliaris De Filippi, 1863 described from a "fiumicelli presso Teheran" (= a stream near Tehran), Barbus mursoides Kessler, 1877 described from Transcaucasia (presumably
the Kura-Araks basin), Barbus microphthalmus Sauvage, 1882 from "Tiflis" (presumably the Kura River at Tbilisi, Georgia) (and Barbus macrophthalmus and Barbus mycrophtalmus
in Chantre (1882) which are presumably misspellings of this name; the former is in any case preoccupied by B. macrophthalmus Bleeker, 1855 described from Indonesia), Barbus kessleri
Derzhavin, 1929 described in Latin from the "Keredsh flumen" (= Karaj River near Tehran), and Barbus dageti Fowler, 1958 are synonyms. Barbus dageti was coined because Fowler
believed Barbus kessleri was preoccupied by Puntius kessleri Steindachner, 1866; Puntius Hamilton, 1822 is not now considered a synonym of Barbus
sensu lato
(Eschmeyer, 1990) although Eschmeyer et al. (1996) have Barbus kessleri listed as preoccupied by Puntius kessleri. Eschmeyer et al. (1996) record 3 syntypes "whereabouts
unknown" for Barbus dageti, i.e. Derzhavin's Barbus kessleri types. Dadikyan (1986) refers Aras River fish from Armenia to Barbus mursa mursoides.
Three specimens (presumably syntypes)(MZUT N.676) of Barbus miliaris are stored in the Istituto e Museo di Zoologia della R. Università di Torino (Tortonese, 1940).
The holotype of Barbus mursoides is in the Zoological Institute, St. Petersburg (ZISP 2863) from the Caucasus collected by Hohenacker in 1838.
Two syntypes of Barbus microphthalmus, measuring 340 mm total length, are in the Muséum national d'Histoire naturelle, Paris (MNHN A.3923, formerly MNHN 1881-1007 and MNHN 1881-1008)
(Bertin and Estève, 1948).
Howes (1987) considers the generic placement of Barbus miliaris as problematical. It has a series of preanal scales and a prominent genital papilla similar to schizothoracines, and a lachrymal
bone similar to Barbus (= Tor) grypus and B. sharpeyi. Karaman (1971) considers Barbus miliaris from the Namak Lake basin of Iran to be a subspecies of the Caspian Sea basin type subspecies,
differentiated by larger scales (78-92 compared to 85-103), less fleshy lips, an undeveloped lower lip lobe, feebly ossified last dorsal fin spine, and shorter pectoral fins. Derzhavin (1929b) in
describing his Barbus kessleri on fish 121-154 mm total length with well-developed gonads states that the lower lip is clearly trilobate. Berg (1949) recognises miliaris as distinct from
mursa on the basis of a shorter snout, somewhat larger scales, fewer scale rows above the lateral line, smaller dimensions and different colour. Bianco and Banarescu (1982) and Almaça (1984b)
retain it as a full species although Bianco and Banarescu (1982) also suggest that this species may be a subspecies of their wide-ranging taxon Barbus cyclolepis Heckel, 1837. Almaça (1984a)
points out that his conclusion is based in part on small specimens in poor condition and that there is not enough data to take a sound decision (Almaça notes that gill raker counts are low and the lower
lip lobe undeveloped in accordance with Karaman (1971) but these are characters which I believe may be size and age related). Almaça (1992) also distinguishes the two taxa on the shorter barbel in
miliaris (not exceeding the middle of the eye as opposed to not exceeding the rear border of the eye), slope of the dorsal fin oblique in miliaris as opposed to oblique to nearly
perpendicular, and pharyngeal teeth in miliaris 5,3 (or 4),2 as opposed to 4-5,3,2. These characters too may be size dependent or individually variable, as are those of
Berg (1949). I consider that miliaris is at most a subspecies of mursa.
Key characters
The high scale counts are an important character as is the presence, usually, of a fleshy three-lobed lower lip.
Morphology
Dorsal fin unbranched rays 3-5, usually 4, followed by 7-8, usually 8, branched rays, anal fin unbranched rays 3 followed by 5 branched rays. Pectoral fin branched rays 13-17 and pelvic fin branched
rays 6-8, usually 7. Lateral line scales 74-103, often 85 or more. Scales are small, horizontally elongated and almost rectangular, with a anterior margin variably indented, a very anterior focus,
relatively few and well-spaced circuli, and few radii on all fields. A single pelvic axillary scale is not developed but a series of enlarged scales may be separated from other scales by
a fold of skin. Gill rakers 7-18 (10-18 in Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya (2003), lower counts from the literature perhaps not including rakers on the
upper arm of the arch. There may also be differences due to size and, independent of size, the rakers on the lower arch anteriorly are variably developed, sometimes being reduced to bumps which were
counted and sometimes not even bumps are present. The larger rakers reach the second adjacent raker when appressed. Vertebrae 41-45. Pharyngeal teeth 2,3,5-5,3,2, rarely 1,2,3,5-5,3,2,1; or with only
4 teeth in the main row (e.g. see Heckel (1843b)). Teeth are hooked and the fourth inner row tooth is slightly larger or smaller than the third. The fifth tooth is smaller (sometimes minute) than teeth
3 and 4 and may be pointed or blunt. The grinding surface below the tip is short, uneven and concave to rounded. The mouth is moderate in size, inferior, horseshoe-shaped with moderate to thick fleshy
lips and an undeveloped to strongly developed median lower lip lobe (see above). Barbels are thick, the anterior one not extending back beyond the nostril level and the posterior one not exceeding the
middle or posterior eye margin. The last dorsal fin spine is moderate to strong and has many, closely-packed denticles from one-half to four-fifths of the spine length, although denticles are lost in
adults. The gut is elongate with 2-3 anterior loops. Chromosome number 2n = 100, NF 140 (Pourali Darestani et al., 2006).
Meristic variation in Iranian specimens:- dorsal fin branched rays 7(1) or 8(18); anal fin branched rays 5(19); pectoral fin branched rays 14(1), 15(2), 16(13) or 17(3); pelvic fin branched rays 7(13)
or 8(6); lateral line scales 75(1), 80(2), 82(1), 84(1), 85(1), 87(1), 88(3), 89(3), 90(3), 91(1), 92(1) or 95 (1); total gill rakers 9(2), 10(1), 12(1), 13(5), 14(4), 15(2) or 16(3); pharyngeal teeth
2,3,5-5,3,2(7), 2,3,4-5,3,2(5), 2,3,5-4,3,2(2) or 3,4,5-5,3,2; and total vertebrae 43(1), 44(6) or 45(2).
Sexual dimorphism
Etessami (1982) reports an hermaphrodite in this species in the Namak Lake basin. A female specimen, 112.5 mm standard length, caught on 15 July had tubercles on the top and upper sides of the head.
Male tuberculation in large adults has not been reported on.
Colour
Overall colour is a pale grey to olive-grey to brownish, slightly darker over the back, and the belly is white to yellowish-brown. The sides of the head and flanks can have golden tints. The iris is
grey with a narrow rim of silver immediately around the pupil or may be yellow-gold. The dorsal and caudal fins are pale grey to dark reddish-brown. The caudal fin bears several series of small dark
spots. The pectoral and pelvic fins have pale brown rays and transparent membranes but may be pink. The anal fin may be colourless except for a little grey pigment over the last unbranched and first
branched rays to an overall reddish-brown. The margins of the pelvic and anal fins are well-developed and white, while the pectoral fin has a very narrow white margin. Young may have numerous dark spots
on the back and upper flank, lost in adults.
Size
Attains 39.5 cm or 43 cm total length (Jolodar and Abdoli, 2004).
Distribution
This species occurs in the Kura River basin of the southwestern Caspian Sea and in southern tributaries of the Caspian from Iran. In Iran, it is reported in the Caspian Sea basin from the Aras to the
Gorgan rivers including the Tajan, Babol, Haraz, Sardab, Aras, Tonekabon, Pol-e
Rud and Safid rivers (Abbasi et al., 1999; Kiabi et al., 1994; 1999; Abdoli, 2000;
Abdoli and Naderi, 2009), the Namak Lake basin (Wossughi, 1978; Rainboth, 1981; Almaca, 1984a; Bianco and Banarescu, 1982;
Abdoli, 2000), and the Lake Orumiyeh basin in the Arnar Chay, Nowruzlu Chay, Tatavi and Zarrineh rivers (Günther, 1899; Abdoli, 2000).
Zoogeography
This species is possibly a Caspian Sea endemic, depending on the status of populations in the Namak and Orumiyeh Lake basins.
Habitat
Avoids muddy bottoms, preferring streams with rapid water, gravel and sand bottoms and a rich benthos. It may also be found in lacustrine habitats (Solak, 1977; Bogutskaya, Bănărescu and
Almaça in Bănărescu and Bogutskaya, 2003).
Age and growth
Solak (1989b) reports a life span of 6 years in the Aras basin of Turkey. Maturity is attained at 2-3 years (Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya, 2003).
Food
Food items include chironomids, as much as 70-100% of the diet at times, crustaceans such as copepods and ostracods, insects, worms, plankton, vegetation and detritus (Abdurakhmanov, 1962;
Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya, 2003). Iranian fish guts contain plant fragments, aquatic insects such as chironomids and Ephemeroptera
(mayflies), and crustaceans such as amphipods.
Reproduction
Fecundity is up to 25,000 eggs. The spawning season is probably in May and June as noted for Georgian fish in Abdurakhmanov (1962) but may extend from April to August, the peak depending on locale
(Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya, 2003). Fish caught on 6 July in Mazandaran, 64 km west of Dasht had large, possibly atretic, eggs measuring about
1.5 mm although Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya (2003) report a maximum egg diameter of 2.5 mm.
Parasites and predators
Masoumian et al. (2003) record Myxobolus azerbajdzanicus, M. kovali, M. squamae, M. tauricus, M. rutili and M. osmaniae while Pazooki
et al. (2003) and Pazooki (2006) record Rhabdochona hellichi, Bothriocephalus gowkongensis and Paradiplozoon homoion, all reports from fishes captured in the Tajan and Zarem
rivers of Mazandaran.
Economic importance
Said to taste even better than trout (Abdurakhmanov, 1962), it is caught by some anglers but is not commercially important (Bogutskaya, Bănărescu and Almaça in Bănărescu
and Bogutskaya, 2003).
Conservation
Kiabi et al. (1999) consider this species to be near threatened in the south Caspian Sea basin according to IUCN criteria. Criteria include sport fishing, medium in numbers, habitat
destruction, widespread range (75% of water bodies), present in other water bodies in Iran, and absent outside the Caspian Sea basin. Mostafavi (2007) lists it as near threatened in the Talar River,
Mazandaran. Bogutskaya, Bănărescu and Almaça in Bănărescu and Bogutskaya (2003) report that is is extremely rare in Azerbaijan. Endangered in Turkey (Fricke et al., 2007).
Further work
The presence of this species in the Lake Orumiyeh basin and the taxonomic status of Namak Lake basin populations need careful examination.
Sources
Type material: ?
Iranian material: CMNFI 1970-0525, 1, 49.3 mm standard length, Gilan, Safid River near Mohsenabad (ca. 37º22'N, ca. 49º57'E);
CMNFI 1970-0538, 5, 42.5-82.7 mm standard length, Gilan, Qezel Owzan River near Manjil (36º44'N, 49º24'E);
CMNFI 1970-0545, ?, ? mm standard length, ();
CMNFI 1970-0589, 1, 110.0 mm standard length, Gilan, Safid River opposite Kisom (37º12'N, 49º54'E);
CMNFI 1979-0084, 2, 92.5-96.8 mm standard length, Mazandaran, Chalus River (no other locality data);
CMNFI 1979-0253, ?, ? mm standard length, ();
CMNFI 1979-0456, 2, 44.1-50.2 mm standard length, Markazi, Shah River at Lowshan (36º37'30"N, 49º31'E);
CMNFI 1979-0481, 1, 142.7 mm standard length, Mazandaran, stream 3 km west of Ghalahleekesh (37º18'30"N, 55º31'E);
CMNFI 1980-0132, 1, 112.5 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1991-0158, ?, ? mm standard length, ();
CMNFI 1993-0136, 1, 109.9 mm standard length, Mazandaran, Sardabrud (36º39'42'N, 51º22'36'E);
CMNFI 2007-0086, 1, 182.2 mm standard length, Azarbaijan-e Khavari, Qareh Su basin near Nir (ca. 38º02'N, ca. 48º00'E); Behnke 8
Behnke 79 CMNFI 2007-00, ?, ? mm standard length, ();
FMNH 51245, 2, 108.4-128.5 mm standard length, Markazi, Rayy (35º35'N, 51º25'E);
ZMH 2429, 98.1 mm standard length, Markazi, Tehran (no other locality data).
Luciobarbus pectoralis
(Heckel, 1843)
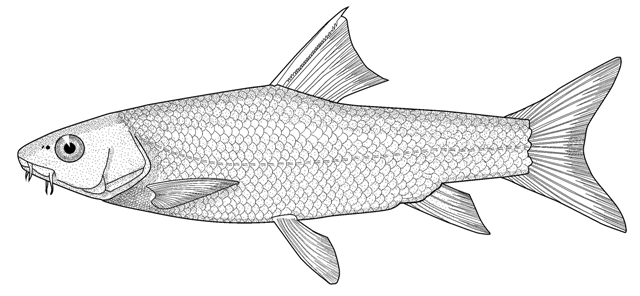
Common names
basan, برزم (berzem or barzam), ? tu'ini.
[nebbash, sheikh san or shabbout pectoralis in Arabic; Heckel's Orontes barbel (Fricke et al., 2007)].
Systematics
Howes (1987) places this species in Barbus sensu stricto. Barbus perniciosus Heckel, 1843 described from "Gewässern bei Damascus", Luciobarbus Schejch Heckel, 1843
described from "Mossul" (also spelt schech on p. 1019 and p. 1098 in Heckel, presumably in error, and sometimes emended to scheich), Labeobarbus Orontis Sauvage, 1882
from the "Canal de l'Oronte à Antioche", Turkey and possibly Barbus
(= Luciobarbus) kersin Heckel, 1843 (q.v.) and possibly Barbus
(= Luciobarbus) barbulus Heckel, 1849 (q.v.) are
synonyms (see Krupp (1985c)). Barbus pectoralis was described from the "Orontes" (Heckel, 1843b) but the catalogue in Vienna reads "Damascus" (possibly in confusion
as this part of the catalogue has been overwritten).
The holotype of Labeobarbus orontis is in the Muséum national d'Histoire naturelle, Paris (MNHN A.3868), with a length of 600 mm (Bertin and Estève, 1948). The catalogue in the
Naturhistorisches Museum Wien appears (the catalogue is overwritten here) to list a single specimen opposite each of the names Barbus perniciosus and Barbus pectoralis, probably the
holotypes. Krupp (1985c) records the holotype of L. pectoralis as being 116 mm standard length (NMW 54474 and the holotype of B. perniciosus as being 105 mm standard length (NMW 54472).
My examination of NMW 54474 showed a length of 117.8 mm standard length.
Karaman (1971) places pectoralis as a subspecies of Barbus
(= Luciobarbus) capito but Almaça (1986) disagrees on several grounds, especially
on the number of pharyngeal teeth (5 in capito and 4 in pectoralis in the main row (yet Heckel (1843b) gives 5 main row teeth for pectoralis, 4 for the synonym
schejch), see also below). Luciobarbus kersin has 5 main row pharyngeal teeth, an indication that it may be distinct. Almaça (1986) points out that the specimen in the
Naturhistorisches Museum Wien (NMW 54475) referred to as the holotype of pectoralis by Karaman (1971) is from the wrong locality and was collected at a later date.
Barbus Rajanorum Heckel, 1843 described from "Aleppo" and later in Heckel (1846-1849a) from "Gewässern von Aleppo" is a hybrid of this species and Capoeta damascina
(F. Krupp, in litt., 1986) and Almaça (1991) also believes it to be founded on a hybrid; see also Almaça (1983; 1991), Berg (1949) and Karaman (1971) for conflicting views).
Valiallahi (2000) also considers this species to be a hybrid, with pectoralis
or barbulus. Almaça (1983) could not
find any specimens attributable to Barbus rajanorum and the holotype housed in the Naturhistorisches Museum Wien is lost. However, the type locality for this taxon is "Aleppo" (Heckel
(1843b) and Krupp (1985c) states that the holotype is NMW 54494, 190 mm standard length, Aleppo, 1842, Th. Kotschy. The catalogue in Vienna lists a single specimen and the card catalogue in 1997 lists
this fish as the holotype.
Karaman (1971) places Barbus (=
Luciobarbus) barbulus, Luciobarbus schejch and fish Heckel (1843) referred to Luciobarbus mystaceus (Pallas, 1814) as synonyms of B. rajanorum. Berg
(1949) also places L. mystaceus of Heckel in B. rajanorum. However mystaceus of Pallas would have priority (authors such as Karaman (1971) and Almaça (1983, 1991) refer the species
description to Heckel (1843) in error) but, as Berg (1949) points out, Pallas's Cyprinus mystaceus is partly Barbus
(= Luciobarbus) mursa and Barbus (= Luciobarbus) capito. Almaça (1983) recognises Barbus
mystaceus with two subspecies, mystaceus from Aleppo, Tigris at Mosul and the Euphrates and barbulus (see above under this latter species). Krupp (1985c) places Barbus
(= Luciobarbus) barbulus
and Heckel's Luciobarbus mystaceus in Barbus pectoralis.
I am uncertain as to the identity of Barbus mystaceus (Pallas, 1814) reported by Heckel (1843b) from the "Tigris bei Mossul", Iraq, in regard to Iranian
Luciobarbus species and do
not assign any Iranian specimens collected by me to it. F. Krupp (in litt., 1987) considers Heckel's mystaceus to be identical with B. barbulus but that Heckel's mystaceus
differs from that of Pallas, as previously noted by Berg (1949). Heckel's B. mystaceus is most probably either B. barbulus or B. pectoralis.
Barbus schejch is recognised as a distinct species by Almaça (1983, 1991) but only one specimen, a syntype from the Tigris (in the Naturhistorisches Museum Wien, NMW 50399), was available to
him. It measures 136.5 mm standard length. Two other specimens identified as syntypes of this this species are under NMW 54520 with standard lengths 175.4 and 270.7 mm. The barbels in the 50399 are very
short, not reaching the eye and about equal in length while in the other two syntypes the posterior barbel reaches the mid-eye and the barbels are subequal. The lips are fleshy, like
Luciobarbus
barbulus, but there is no central lobe in 50399, present in the smaller of the two other syntypes and poorly developed in the larger. The complete dorsal fin spine bears 29 teeth in the 50399 and
29 or 35 in broken spines of the other two fish. Gill rakers number 22 in 50399 and 16 or 18 in the other two fish. Lateral line scales number 52 (or 54 to end of scale row on caudal fin) in the syntype
and 57(58) or 58(60). Main row pharyngeal teeth are 4-4 in 50399, missing in the other two fish. These data are somewhat contradictory and further data are required to resolve the status of this nominal
species.
The catalogue in Vienna lists 4 fish in spirits and 4 fish stuffed.
The synonymy of Luciobarbus barbulus with
L. pectoralis remains uncertain. The putative holotype of B. pectoralis (NMW 54474) was compared with a specimen of similar size from Iran
referred to L. barbulus (CMNFI 1973-0393). The L. pectoralis specimen is partly dried so direct measurement comparisons are not possible. The
L. pectoralis specimen has more teeth in
the dorsal fin spine (27 teeth even though it is broken off, much more than 30 presumably in the intact spine), barbels in pectoralis are shorter, the posterior one reaching the anterior half of
the eye, the anterior one short of the mouth angle, mouths similar in shape but lips appear to be less fleshy, gill rakers number 16, lateral line scales number 44, and 4 main row pharyngeal teeth but
there is a trace of a fifth tooth not fully ossified. ? check counts on NMc fish?
Key characters
The dorsal spine is much stronger than in Luciobarbus barbulus and arises from an elevated base that supports the dorsal fin base. The body is deeper than in
L. barbulus and the lips
usually less fleshy.
Morphology
Dorsal fin with 4 unbranched and 7-9 branched rays (7 in the holotype, usually 8), anal fin with 3 unbranched and 5, rarely 6, branched rays. Pectoral fin branched rays 16, pelvic fin rays 8.
Lateral line scales 42-60 (44 in the holotype; 42 in Barbus perniciosus). Gill rakers 14-17 (to 21 if Barbus schejch is included (Almaça, 1986)). Pharyngeal teeth 2,3,4-4,3,2 in 16 fish,
2,3,5-5,3,2 in 9 fish examined by Krupp (1985c), rarely 2,3,5-4,3,2 or 2,3,4-5,3,2 (1 fish each), spoon-shaped or pointed with the fourth tooth of the inner row large and globose. Larger fish usually
have 4 teeth in the main row and the fourth tooth is globose. Smaller fish with 5 teeth in the main row have cylindrical teeth. All intermediates stages exist (Krupp, 1985c). The mouth is moderate in
size and subterminal. Lips are thin to moderate and the median lobe of the lower lip may be present or absent. Barbels are thin to moderate, the anterior one not extending back beyond the nostril to
anterior eye margin level and the posterior one not beyond the middle to the posterior margin of the eye. The last unbranched dorsal fin ray is moderate to very strong with a low density of denticles
extending along much of the ray. Larger specimens have a lesser extent of denticles along the ray. The body form is extremely variable.
Sexual dimorphism
Nuptial tubercles develop on 88.9% of mature males
(Ghafari and Jamili,
2010).
Colour
The back is brown to bluish-green and the flanks yellowish to silvery-white.
Size
46.9 cm standard length (Krupp, 1985c).
Distribution
Tigris-Euphrates basin and the Orontes and Quwayq rivers. In Iran, it is found in the Tigris River basin in the Hawr Al Azim and the lower Karkheh, Karun and Jarrahi rivers, in the Kor River basin,
and in the Gulf basin in the middle and lower Helleh, middle and lower Mand and Dasht-e Palang rivers (Abdoli, 2000).
Zoogeography
Almaça (1984b) considers that the origin of this species (as Barbus schejch) lies with a group that migrated southwards in the late Pliocene from the Dacian Lake of the Sarmatian Sea and
speciated in Mesopotamia and later (Almaça, 1991) that this species (as Barbus
(= Luciobarbus) pectoralis) originated from a colonisation wave from South Europe.
Habitat
Unknown.
Age and growth
Al-Rudainy (2008) states that in Iraq it reaches
sexual maturity in 4 years at 30 cm length and 350 g weight.
Food
Unknown.
Reproduction
Ghafari and Jamili (2010) sampled fish from the
Karun River and found the breeding season was from January to February at 14.3ºC
and pH 7.75. Eggs
were released in shallow water over gravel or sand in a single batch. Males matured at 3
years with LM50 35-40 cm and females at 4 years with LM50
50-55 cm. Absolute fecundity range was 7144-332,196 eggs and relative fecundity was 3845 to
164,753 eggs/kg. Maximum egg diameter was 2.0 mm in February. Sex ratio was 1:2
in favour of females.
Al-Rudainy (2008) gives
the spawning season in Iraq as March to April on gravel in shallow water with a
strong current.
Parasites and predators
Masoumian et al.
(2008) recorded the
myxosporeans Myxobolus karuni and M. persicus from gills of fish
captured in the Karun and Karkeheh rivers and Shadegan Marsh.
Economic importance
Occasionally caught and used for food.
Ghafari and Jamili
(2010) consider it suitable for aquaculture because of its good taste and
size.
Conservation
Endangered in Turkey (Fricke et al., 2007). Confusion over its identity has made a conservation assessment difficult for Iranian waters.
Further work
The taxonomic status of this species and its presence in Iranian waters should be resolved.
Sources
Type material: See above, Barbus pectoralis (NMW 54474).
Luciobarbus subquincunciatus
(Günther, 1868)
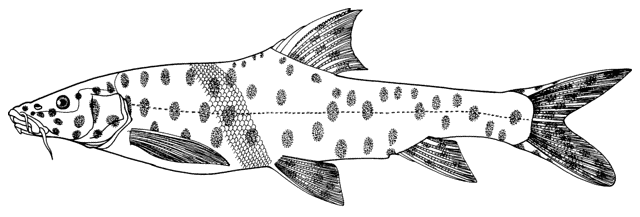
Common names
سليماني (= solimani or soleimani).
[abou khazzama, a'djzan, agzan or adzan, all in Arabic; black spot barb, leopard barbel, Mesopotamian barbel].
Systematics
The type locality of this species is unknown. Günther (1868) gives the following account:- "From the Collection of the East-India Company.- Although no record of the history of this specimen has
been preserved, it is probable that it came from Mesopotamia, as other examples from this country are preserved in precisely the same manner". The type specimen is a "Skin, 15 inches long"
(= 5.9 cm).
Krupp (1985a) removes this species from Bertinius Fang, 1943 since the enlarged molariform pharyngeal teeth on which this genus was erected are due to convergence and are not evidence of
monophyly. Howes (1987) places this species in his Barbus sensu stricto.
Key characters
The numerous, large, dark spots arranged in an almost quincunx pattern are distinctive.
Morphology
Dorsal fin with 3-4 unbranched and 8 branched rays, anal fin with 3 unbranched and 5 branched rays. The last unbranched dorsal fin ray is very strong and bears denticles along almost its whole length
or three-quarters of the length. Pectoral fin branched rays 14-18, pelvic fin rays 7. Lateral line scales 75-88. Scales have few radii on all fields, fine circuli and a focus slightly subcentral
anterior. Total gill rakers about 10-13, broad based and triangular in shape with highly tubercular distal or foliose margin. The longest raker reaches the one below when appressed. Total vertebrae 45
(Howes, 1987). Pharyngeal teeth 2,3,3,-3,3,2, occasionally 2,3,4-4,3,2, the usual number of teeth in the inner row in large specimens being 3 (Krupp, 1985a). The third inner row tooth is
the biggest by far and is molariform. Juveniles have 5 inner row teeth (Krupp, 1985c). Total vertebrae 46. The mouth is horseshoe-shaped, small and inferior. Lips are well-developed and fleshy. The
median lobe of the lower lip is undeveloped. The barbels are thick. The gut has many anterior loops, the number increasing with size.
Meristics for Iranian material:- dorsal fin branched rays 8(1), anal fin branched rays 5(1), pectoral fin branched rays 14(1), and pelvic fin branched rays 5(1). Lateral line scales 83(1). Total gill
rakers ?. Pharyngeal teeth 2,3,4-4,3,2 (1).
?others
Sexual dimorphism
Unknown.
Colour
The whole body, head, fins, barbels, lips and even eyeball are covered with dark spots about the same size as or larger than the eye. Some larger flank spots are 2-3 times the eye diameter. Spots on
fins are elongated along the fin length. These spots are arranged in patterns similar to a quincunx, hence the species name. A quincunx comprises four spots, one at each corner of a square with the fifth
spot in the middle of the square. Sometimes a spot runs into an adjacent one. Some spots below the lateral line may be elongate, three times longer than wide, and arranged vertically. Occasional fish
lack spots on the mid-flank but are still distinctively spotted elsewhere. The overall colour is greenish to brownish-yellow with the belly white. Peritoneum dark brown to black.
Size
Reaches 33.8 cm total length (Menon, 1956), 45.7 cm (Khalaf, 1961) or 60 cm (Sauvage, 1884).
Distribution
Found in the Tigris-Euphrates basin including its Iranian portion in such rivers as the Jarrahi (Wossughi, 1978; Rainboth, 1981; Abdoli, 2000).
Zoogeography
Almaça (1991) believes that this species originated in Mesopotamia.
Habitat
Unknown but recorded from rivers and artificial reservoirs.
Age and growth
Şen et al. (1992) examined 9 fish
in Keban Dam Lake, Turkey and found age groups 3-7, growth rings being best
expressed in sectioned dorsal fin rays. The length-weight relationship was logw
= - 5.78723 + 3.27533 logl and the mean K(TL) was 0.8234. The b value
indicates the habitat is suitable for the species.
Food
The molariform pharyngeal teeth and evidence from gut contents showed this species is an obligate molluscivore (Krupp, 1985a).
However, Al-Rudainy (2008) gives diet in Iraq as insects, as well as detritus
and aquatic plants, but this may apply to younger fish.
Reproduction
Sexual maturity is attained at 3-4 years, 35 cm
length and 500 g weight in Iraq. Spawning takes place in April and May with eggs
deposited on rocks (Al-Rudainy, 2008).
Parasites and predators
None reported from Iran.
Economic importance
This species occasionally occurs in commercial catches in Khuzestan but is not a common food fish compared to other Barbus sensu
lato species. It has been investigated for aquaculture in Khuzestan but
fish are rare and so adults are caught and released.
Conservation
This species is now very rare in Iran and "critically endangered". Reports of 1 fish taken in the Gav Masiab River in 1991, 4 fish from the Karun River in 1995 and 1 fish from the Karun
River at Ahvaz in 1997 were the only records for the 1990s (M. Ramin, pers. comm., 2000). The stock of this species in the Gav Masiab River is severely reduced and during 4 years of collecting in
western Iran only one fish was caught (J. Valiallahi, www.modares.ac.ir, downloaded 4 July 2000; pers. comm., 2001; Valeolahy, 2000).
Syrian populations in the Euphrates River and parts of its tributaries are also in a parlous state (R. Beck, pers. comm., 2000).
Further work
The biology of this distinctive species should be investigated.
Sources
Type material: ?
Iranian material:- CMNFI 1993-0133, ?, ? mm standard length, ():
ZMH 2506, 1, 308.0 mm standard length, Kermanshahan, Karasu-Gamasiab-Seymarreh, Kermanshah ();
FMNH70794,1, ?, Javanarud near Kermanshah ();
and market specimens from Khuzestan.
Comparative material:- CMNFI 1980-1036, 1, 177.5 mm standard length, Turkey, Keban Dam on Murat Nehri near Elazig (38º41'N, 39º14'E);
CMNFI 1986-0676, 1, 283.0 mm standard length, Turkey, Keban Dam on Murat Nehri (no other locality data);
BM(NH) 1874.4.28:15, 1, 415.3 mm standard length, Iraq, Tigris River near Baghdad (33º21'N, 44º25'E);
BM(NH) 1875.1.14:3-5, 3, 377.6-468.2 mm standard length, Iraq, Tigris River (no other locality data);
BM(NH) 1974.2.22:1353, 1, 253.4 mm standard length, Iraq, Sirwan River, Diyala (no other locality data);
Luciobarbus xanthopterus
Heckel, 1843
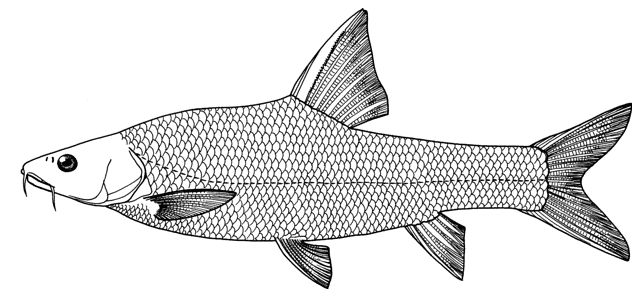
Common names
گطان (gatan or gattan).
[gattan or ghattan, nobbash, or thekar, all in Arabic; yellowfin barbel].
Systematics
Howes (1987) places this species in Barbus sensu stricto. Almaça (1983) briefly reviews the placement of this species in synonymy; most ichthyologists now regard it as a distinct species.
Luciobarbus xanthopterus has been considered as a variant of L. schejch but differs in gill raker count (10-13 in xanthopterus, 21 in schejch) and main row pharyngeal tooth
count (5 in xanthopterus and 4 in schejch) (Almaça, 1983; see also discussion under
Luciobarbus pectoralis; Heckel (1843b), however, gives the main row count for xanthopterus
as 4). It has also been considered as a synonym of esocinus (q.v.) (Almaça, 1986).
Fayazi et al. (2006) used mtDNA to study differentiation between populations of this species in the Karun, Karkheh and Jarrahi rivers in Iran. Diversity was low although the Karun and Karkheh
fish grouped together, leading to the recommendation that fish from the Jarrahi should not be used to stock other river basins.
Almaça (1986) records syntypes of Luciobarbus xanthopterus from the type locality given by Heckel (1843b) "Tigris bei Mossul", Iraq in the Naturhistorisches Museum Wien under NMW
54841 (10 specimens, one large fish at 216.5 mm standard length and 9 smaller fish at 48.6-63.4 mm; one of these was noted as being listed as the lectotype in 1997 (? the largest), and the catalogue
number was 54841a) and NMW 54786 (1 specimen, 292.8 mm, not listed as a type in 1997). Material listed under NMW 1843 (? catalogue number or collection date) may also be syntypes. Eschmeyer et al.
(1996) list 1 dried syntype under NMW 91215. The catalogue in Vienna lists 4 fish in spirits and 2 fish stuffed.
Key characters
This species is characterised by two pairs of barbels, a serrated dorsal fin spine, small scales (57-68 in lateral line), and a subterminal to terminal and oblique mouth. The elongate postorbital
length is also seen in Luciobarbus esocinus but is more marked in the latter (check this?) and scale counts are different (check?).
Morphology
Dorsal fin with 4 unbranched and 7-9, usually 8, branched rays, anal fin with 3 unbranched and 5 branched rays. Pelvic fin branched rays 8. Pectoral fin branched rays 14-18 (Jawad, 1975). Lateral
line scales 57-68. Scales have rounded dorsal, ventral and posterior margins and an anterior margin with a central protuberance and indentations above and below. Circuli are fine and radii are found
on the anterior and posterior fields and sometimes the lateral fields. The focus is subcentral anterior. There is no distinct pelvic axillary scale. Gill rakers 7-13, short and reaching the adjacent
raker when appressed. Pharyngeal teeth 2,3,5-5,3,2, strongly hooked the fourth tooth of the inner row being the largest and anterior teeth being rounded with a small flat or concave grinding surface
below the tip. Qasim and Niazi (1975) gave a tooth formula of 4,3,2-2,3,4, i.e. 2,3,4-4,3,2 as does Heckel (1843b) and teeth were molariform. Total vertebrae 44 (Howes, 1987), 40-42 (Qasim and Niazi,
1975), 42 (Wossughi, 1978) or 46 (BM(NH) 1973.5.21:198). The last unbranched dorsal fin ray is moderately to very strong, has a low denticle density and is serrate along much of its length. Barbels are
thin, the maxillary barbels are longer than rostral barbels but both are short, the rostral ones not extending beyond the level of the nostrils and the maxillary ones not extending back beyond mid-eye to
rear eye level. The gut has one anterior and two posterior loops in an elongate s-shape. The mouth is moderate in size, inferior and an elongate u-shape in young fish and as development progresses becomes
terminal in adults (Karaman, 1971; Almaça, 1984b). Lips are thin to moderate and the lower lip has no median lobe.
Iranian specimens had the following meristics:- dorsal fin branched rays 8(2); anal fin branchd rays 5(2); pectoral fin branched rays 18(2); pelvic fin branched rays 8(2); lateral line scales 57(1) or
68(1); total gill rakers 7(1) or 10(1); pharyngeal teeth 2,3,5-5,3,2(2); and total vertebrae ?.
Sexual dimorphism
Unknown.
Colour
The body is without distinctive marks. The back is brownish to bluish-grey, the flanks silvery to silvery-yellow, and the belly white. The scales are outlined by melanophores. The overall colour
from a marsh habitat is darker than from a riverine habitat, the pigment outlining scales being thicker for example especially at the scale base. The eye is red in marsh specimens, white to yellowish
elsewhere (M. Al-Mukhtar, pers. comm., 1995). All fins are lemon-yellow to orange with some darker melanophores. The unbranched dorsal fin rays and the uppermost caudal fin rays are black.
Two small specimens from Iran have irregular spots and blotches on the flank. The peritoneum is silvery with melanophores developed dorsally.
Size
Al-Hassan et al. (1986) report a specimen 1.5 m total length and 8.6 kg from the Abu Al Khasib area in the Shatt al Arab, Iraq.
Distribution
This species is found in the Tigris-Euphrates basin including its Iranian portion such as the lower Karun River and adjacent lower reaches of the Jarrahi River (Abdoli, 2000), the Qareh Su at
Kermanshah, the Karkheh River and Hawr al Azim.
Zoogeography
Almaça (1984b, 1991) considers that the origin of this species lies with a group that migrated southwards in the late Pliocene from the Dacian Lake of the Sarmatian Sea and speciated in Mesopotamia.
Habitat
van den Eelaart (1954) and Al-Hamed (1966b; 1972) describe the habitat for this species in the Tigris River as distributed in the deep, open waters of lakes and vegetated marshes and to a lesser
extent in the river and its tributaries. Mature fish move upstream to the spawning grounds in February-March and spent fish descend to their original habitat in lakes and marshes. In summer, beginning
in June, under low water level conditions and high temperatures, the smaller fish remain in the deepest depressions of lakes but the large fish (3 kg or more) migrate up rivers and tributaries in search
of cooler water, returning in September and October when temperatures fall to fatten over winter.
In Khuzestan, this species is most abundant in the Karkheh River in March and in the Hawr al-Azim in December, migrating from the wetland to the river in spring. Younger fish are more abundant in the
wetland and older fish in the river (Iranian Fisheries Research Organization Newsletter, 22:3, 2000; Eskandary et al., 2000). Another study showed this species to be most abundant in the
Karkheh River in December, with a migration from wetlands in spring to the main river (Tehran Times, 1 October 2000).
Age and growth
Life span is at least 11 years (Al-Ahmed, 1966a). Al-Hamed (1966b; 1972) working on Tigris River fishes found males to mature at about 43 cm and females at about 48 cm, maturity being attained in the
fourth year of life and spawning occurring at the beginning of the fifth. Some fish mature at age group 3 and some as late as age group 5. Males outnumber females on the spawning grounds, comprising 62%
of the population. Tigris River and Al-Tharthar reservoir fish in Iraq had 7 age groups with growth good in the first three years and slower thereafter (Ali, 1979).
In Keban Dam Lake, Turkey, age determination was best made on sectioned dorsal fin rays (of scales, otoliths, vertebrae and opercula) and up to 9 age groups were detected (Duman and Şen, 1995).
In the Karkheh River, male fish are mature at 151-200 mm (one year old) and females at 501-550 mm (3 years old). The sex ratio is 1:1.31 for males:females but this is not significantly different from
1:1 (Eskandary et al., 2000).
Food
Al-Hassan et al. (1986) report isopods and molluscs. Al-Hamed (1965) considers this species to be an omnivore, consuming filamentous algae, detritus, frogs, molluscs and fishes and even
planktonic organisms. Organic matter is obtained in periods of food shortage by engulfing mud from the pond bottom. van den Eelaart (1954) reports food to be plants, epiphytes and plankton. In cold
winters they take no food. Ali (1979) for Iraqi waters gives insects and plankton as the principle foods.
Al-Shamma'a et al. (2009) found fish from Lake Habbaniyah, Iraq to feed
mostly on animal materials (76.25) including molluscs and insects and their
larvae. Feeding was most active in spring but high feeding intensities were also
observed in autumn.
In Khuzestan it is omnivorous, feeding mainly on insects and vegetation, but also taking
secondarily shrimps, snails and ostracods (Iranian Fisheries Research Organization Newsletter, 22:3, 2000; Eskandari et al., 2003). In the Karkheh River food is insects and vegetation
mainly, with shrimps, gastropods and ostracods secondary food choices (Tehran Times, 1 October 2000). The intestine fullness is greater in fish in the Hawr al Azim, less in the
Karkheh River which is used mainly for spawning (Eskandari et al., 2003).
Reproduction
van den Eelaart (1954) and Al-Hamed (1966b; 1972) studied the reproduction of this species in Iraq. Eggs are deposited on fine gravels overlying a layer of coarse sand in shallow, wide holes
excavated by the fish. Water depth varies from 30 to 150 cm. Egg diameter is 1.0 mm and fecundity up to 340,000 grey eggs. Al-Hassan et al. (1986) record up to 350,000 eggs for their large fish
from the Shatt-al-Arab. The spawning season on the Tigris River between Beled and Tigrit is April and May. Fish appear on the spawning grounds in schools just before dark and remain there until shortly
before midnight, making loud noises by splashing, jumping and chasing.
In Khuzestan, spawning fish are 63.7-80.0 cm total length with a relative fecundity of 18.9-142.5 eggs/g body weight and a minimum and maximum absolute fecundity of 136,924 and 549,211 eggs
(Iranian Fisheries Research Organization Newsletter, 22:3, 2000; Eskandari et al., 2003). In the Karkheh River, spawning took place at surface water temperatures of 25.5-28.65°C
in turbid water after a spring migration from wetlands into the river (Tehran Times, 1 October 2000; Eskandary et al., 2000). Spawning occurs annually in May and June in the Karkheh River
and maximum egg diameter is 2.25 mm (Eskandary et al., 2000).
Parasites and predators
Bykhovski (1949) reports a new species of monogenetic trematode, Dactylogyrus inutilis, from this species in the Karkheh River, Iran. Ebrahimzadeh and Nabawi (1975) list Anisakidae from this
species in the Karun River. Moghainemi and Abbasi (1992) record a wide range of parasites from this species in the Hawr al-Azim in Khuzestan.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Argulus sp., Ergasilus sp. and Lamproglena compacta on this
species.
Economic importance
This species appears regularly in the markets of Ahvaz and Wossughi (1978) states it is of great economic importance. Sharma (1980) reports that gattan is the third most important fish species at
Basrah fish market, accounting for 510,503 kg for the period from October 1975 to June 1977. Petr (1987) reports the annual catch for 1976 in Iraq was 2543 t. This species has been studied for pond
culture in Khuzestan where over 95% of young survived, using hormones to stimulate reproduction (Iranian Fisheries Research Organization Newsletter, 28:3, 2001).
Zadeh et al. (2009) investigated the optimal dietary carbohydrate
to lipid ratio for fingerlings of this species. Mortezavizadeh et al.
(2009) induced reproduction in this species with carp pituitary extract. Sperm
production was increased, 86% of females responded positively, 480 eggs/gram
were produced and mean survival rate was 77.95%. Propagation was best at the
beginning of March at 19.0-24.5°C.
englers in Iraq catch this fish which will reject any bait showing resistance, requiring a fast strike at the first indication that the fish has taken the bait.
Conservation
Several hundred thousand gattan juveniles have been introduced into the Hawr al Azim in Khuzestan in order to restock and protect this resource. The fish were artificially bred from breeders using
hormone treatment (Iranian Fisheries Research Organization Newsletter, 39:3, 2004; Network of Aquaculture Centres in Asia, downloaded 11 January 2007). Endangered in Turkey (Fricke et al.,
2007).
Further work
?
Sources
Type material: ?
Iranian material: ZMH 4071, 1, 151.6 mm standard length, Kermanshahan, Qareh Su at Kermanshah (?).
uncatalogued, 2, 93.7-112.8 mm standard length, Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E);
Comparative material: BM(NH) 1893.6.23:25, 1, 198.0 mm standard length, Iraq, Al Faw (29º58'N, 48º29'E); HL51.5, Postorb 26.6, for body proportions; g.r 13;
BM(NH) 1973.5.21:197, 1, 227.8 mm standard length, Iraq, Shatt al Arab (no other
locality data) HL 60.3, postorb 28.8, g.r. 21; BM(NH) 1973.5.21:198, 1, 143.0 mm standard length, Iraq, Shatt al Arab
(no other locality data). HL 34.6, postorb 15.1, g.r. 18.
Genus Mesopotamichthys
Karaman, 1971
?
Much of the past literature on this genus appeared under Barbus (q.v.)
Mesopotamichthys sharpeyi
(Günther, 1874)
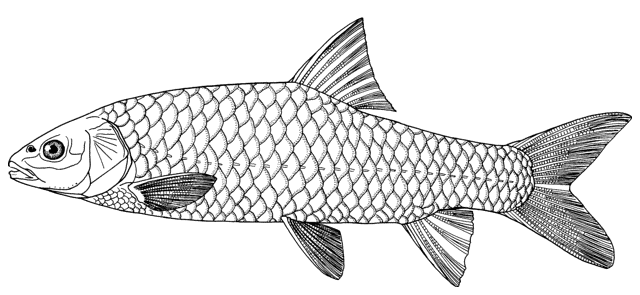
Common names
بني (benni, benny, beni, binni, bini, binny (meaning possibly nose)), سليماني (= solimani or soleimani, meaning unknown).
[binni, bunni, bunnei, bunia or aradah, all in Arabic].
Systematics
Howes (1987) places this species outside the genus Barbus sensu stricto as defined by him because it has the non-elongate lachrymal bone with a sensory canal running along the antero-dorsal
border, a derived condition. Karaman (1971) erected a new genus for this species, Mesopotamichthys, which is not accepted here nor by Krupp (1985c). However Bănărescu (1997) and Ekmekçi
and Banarescu (1998) recognise this genus as valid. The large-scaled "Barbus" of Southwest Asia require a detailed revision probably with additional, molecular characters (see also under
Carasobarbus
luteus and Kosswigobarbus kosswigi).
Barbus faoensis Günther, 1896 described from "Fao (Persian Gulf)", Iraq is a synonym; Karaman (1971) retains it as the subspecies of the lower part of the Tigris-Euphrates basin with
the type subspecies in the upper part of the Tigris River basin.
Barbus sharpeyi was described from "Baghdad". The three syntypes are in the Natural History Museum, London (BM(NH) 1874.4.28:20 labelled "R. Tigris nr. Baghdad. Sharpey",
well sealed in its jar and not measured accurately, and BM(NH) 1874.4.28:27 and BM(NH) 1875.1.14:16 labelled "R. Tigris. Sharpey" and measuring 147.6-178.0 mm standard length). The large
holotype of Barbus faoensis is also there, strongly sealed in its jar, labelled "Persian Gulf. Kurrachee Museum" (BM(NH) 1888.5.17:4).
Al-Mukhtar and Al-Hassan (1999) describe a hybrid of this species and Carassius auratus from Al-Hayei (= Al Ha'i), a seasonal lake between the Karkheh and Dez rivers in Khuzestan.
Key characters
The absence of barbels, the last dorsal fin unbranched ray moderately ossified but lacking teeth, and the low scale count are characteristic.
Morphology
Dorsal fin with 4 unbranched and 7-9, usually 8, branched rays, anal fin with 2-3 unbranched and 4-5, usually 5, branched rays, pectoral rays 13-19, usually 16-17, and pelvic fin branched rays
usually 8. The last third or quarter of the last unbranched dorsal fin ray is thin, flexible and tapering. The gut has several loops, two anteriorly and three posteriorly. Pharyngeal tooth formula is
2,3,5-5,3,2, sometimes with only 4 teeth in the main row but the anterior tooth is missing in both small and large fish and so does not appear to be age related, with teeth hooked at the tip but not
strongly on the posterior teeth which are spoon-shaped with the hollow of the spoon filled in with bone. Total gill rakers number 13-19, reaching the raker below or just beyond when appressed. Total
vertebrae 38-42 (lower values, 38-39, may not include hypural plate). Lateral line scales 29-37. A small pelvic axillary scale may be present or scales in this area may be so weakly developed as not to
be an apparent axillary scale. Scales have a slightly anterior focus, fine concentric circuli, many radii on all fields and the posterior, exposed field bears numerous small tubercles. The mouth is
slightly subterminal. Lips are well-developed but not fleshy and the lower lip is interrupted in the middle. Microscopic studies of the pharynx and oesophagus have been carried out by Alboghobeish and
Moosavi (1998) who confirm that it is adapted for herbivory. Chromosome number 2n=98 (Balasem et al., 1994). Alboghobeish and Hamidian (2006) studied the distribution of alarm cells in the
skin of this species.
Iranian fish have the following meristics: branched dorsal fin rays 8(2), branched anal fin rays 5(2), branched pectoral fin rays 16(2) and branched pelvic fin rays 5(2). Lateral
line scales 30(1) or 31(1). Total gill rakers 16(1) or 18(1). Pharyngeal teeth 2,3,5-5,3,2(2). Total vertebrae 41(1) or 42(2) based on CMNFI 79-0087 (42) and 87-0017 (41 and 42). 41(3) or 42(2) based on
BM(NH) 1920.3.3:71-75, 42 (1973.5.21:195), 41 (1973.5.21:196), 42 (1874.4.28:27), 41 (1875.1.14:16)
Sexual dimorphism
Unknown.
Colour
Overall colour is greenish to light brown or golden brown with the belly white to silvery or yellowish-brown. Scales on the back and uppermost flank have solid dark brown pigment on the exposed part of
the scale. The scale edge is thinner and so appears lighter. The eye is brownish orange, golden or silvery. Fins are darker than the adjacent body, a deep reddish-brown, with melanophores on rays and
membranes in preserved fish. The peritoneum is black.
Size
Attains 55 cm and 4 kg (van den Eelaart, 1954; Al-Hamed, 1966b; 1972). Reaches at least 3.5 kg in Khuzestan (J. Gh. Marammazi, pers. comm., 1995).
Distribution
This species is found in the Tigris-Euphrates River basin including its Iranian portion in such marshes as the Hawr Al Azim and in rivers and in the northern Gulf basin in the Zohreh River (Marammazi,
1995; Abdoli, 2000).
Zoogeography
Karaman (1971) considers that this species originated from the Indian line of the Torini, a tribe of Cyprinidae, in which Karaman includes such genera as Carasobarbus and Kosswigobarbus, Garra, Hemigarra (recognised as Hemigrammocapoeta here) which have Iranian members.
Habitat
van den Eelaart (1954) and Al-Hamed (1966b; 1972) report some movement from lakes and marshes, from the end of February to the beginning of March, to rivers in the Tigris-Euphrates basin of Iraq
during floods for about 3 weeks. There is a return to lakes and marshes for spawning in mid-March to mid-April. However, most fish remain in marshes and lakes for most of the year, in overgrown areas
avoiding open water. Low water levels and high temperatures in the lakes and marshes may cause a migration to their deepest parts or into the lower reaches of the main and more permanent rivers. This
species is less tolerant of low oxygen than Luciobarbus xanthopterus which probably accounts for them not being caught together in any number.
Arzi et al. (2009) compare organochlorine residues in this species in
three cities in Khuzestan.
Marammazi (1994) considers this species to be stenohaline and so restricted in its distribution in the Zohreh River which drains to the northern Persian Gulf. The influence of salinity on growth rate
is examined by Orian et al. (1993).
Age and growth
Al-Hakim et al. (1976) studied some aspects of the biology of this species in Razzaza Lake, Iraq. Females are longer and heavier than males at advanced ages. Life span of females is 9 years and
for males 8 years. Maturity starts in the third year at 32-35 cm total length. Males mature earlier than females. Al-Hamed (1966a; 1966b; 1972) found Tigris River fish in Iraq to mature at 25 cm for
males and 28 cm for females in the second year of life and spawning took place early in the third year. A few matured in age group 1 and some as late as age group 3. Males are somewhat more abundant
than females on the spawning grounds, averaging 57.4% of the fish caught. Maximum age is 6 years. Ali (1982b) found this species to mature in the fourth year of life in Iraq, with growth better in the
marshes than in Tharthar Reservoir. Epler et al. (1996) found fish up to age 6+ years in fresh and salty Iraqi lakes. Nasir et al. (1989) reports on the biology of this species in the
Al-Hammar Marsh, Iraq and found a sex ratio of 1 female:3 males for all months and length groups caught. No explanation for this skewed ratio was found.
Food
Al-Hamed (1965) found this species to be strictly herbivorous, feeding on unicellular Chlorophyceae, diatoms and filamentous algae when young and on higher plants and detritus when older. Nasir et
al. (1989) and Epler et al. (1996) confirm that this species in Iraq is completely herbivorous although some copepods and molluscs are taken, most probably incidental to filamentous algae,
diatoms and detritus. van den Eelaart (1954) reports feeding even in cold winters. In the Karun River, diet includes such plants as Potamogeton, Salvinia, Nuphar and Phragmites
(Annual Bulletin 1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 91-92, 1995).
Reproduction
van den Eelaart (1954) and Al-Hamed (1966b; 1972) studied reproduction in this species on the Tigris River in Iraq and Al-Nasih (1992) in fish ponds. Spawning occurs chiefly in lakes and marshes,
with some spawning in the lower reaches of rivers. Eggs are deposited on submerged, or partially submerged, vegetation, from the surface down to about 1 m depth. Eggs are large, yellow and measure up to
1.7 mm in diameter and number up to 158,000. Epler et al. (1996) give a relative fecundity of 10,021 to 28,471 eggs for fish 4+ to 6+ in age from Iraqi lakes with fish spawning in April in a
freshwater lake and February/March in a saline lake. Al-Nasih (1992) gives details of larval development. The spawning season in Lake Saniyah just north of Amara is March and April, with some ripe fish
caught in May. Fish appear on the spawning grounds about sunset and left before darkness is complete. They return in the early morning and leave again at about 0800 hours.
These fish chase each other, dart about singly or in pairs and sometimes come to the surface and splash.
Al Mukhtar et al. (2006) investigated this species in the Hawizah Marsh
as a source of spawners for aquaculture. Ripe eggs appeared in January and 25%
were running in February and 30% in March. Half of the fish were spent in April.
The spawning migration was lead by males in October and December with females
increasing rapidly in February. Males disappeared in April. Absolute fecundity
reached 236,160 eggs. Al-Rudainy (2008) gives an absolute fecundity of up to
358,343 eggs in Iraq or up to 145 eggs/g body weight and a diameter of 2.0 mm
Petr (1987) reports spawning in Iran at 15-16°C in February in clean water of rivers with sandy bottoms. In the Karun River this species spawned in March-April in river estuaries (Annual
Bulletin 1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 91-92, 1995). A specimen caught in March had well-developed testes.
Spawning in Shadegan Marsh, Khuzestan is in March and in branches of the Karkheh
River in March to April (Al Mukhtar et al., 2006). Shadegan Marsh is one
of the most important spawning areas in Iran (Mohammadi and Marammazi, 2001).
Parasites and predators
Bykhovski (1949) reports a new species of monogenetic trematode, Dactylogyrus pavlovskyi, from this cyprinid in the Karkheh River, Iran. Ebrahimzadeh and Nabawi (1975) list species of the
protozoans Trichodina and Myxosoma and the trematode Dactylogyrus as well as the nematode Camallanus lacustris, from this species in the Karun River. Jalali and Molnár
(1990a) record two monogenean species, Dactylogyrus spp., in the Dez River and Molnár and Jalali (1992) a new species of monogenean, Dogielius persicus, from
this species in the Dez and Karun rivers of Khuzestan. Gussev et al. (1993b) record Dactylogyrus pavlovskyi in the Dez River.
Masoumian et al. (1994) describe a new species of Myxosporea from the gills of this species in the Karun River, namely Myxobolus persicus, and later (Masoumian et al., 1996)
another new species of Myxosporea, Myxobolus nodulointestinalis, in the gut lining of this species and also from rivers of southwestern Iran. Masoumian et al. (1996) describe a new species
of Myxosporea, Myxobolus bulbocordis, from the heart of fish caught at various localities in Khuzestan. Molnár et al. (1996) report additional new
species from this fish in Khuzestan, namely Myxobolus iranicus in the spleen and Myxobolus sharpeyi in the gill cartilage. Myxosporeans are potentially dangerous to fishes such as
Mesopotamichthys
sharpeyi which may be used in fish culture in Khuzestan. Masoumian and Pazooki (1999) list Myxobolus persicus, M. karuni, M. sharpeyi, M. nodulointestinalis, M. bulbocordis and M.
iranicus from this species in various localities in Khuzestan.
Shamsi et al. (2009) found
Dactylogyrus nchoratus in this species from fish farms and the Karun River.
Peyghan (1994) reports ichthyophthiriasis in cultured Barbus
(= Mesopotamichthys) sharpeyi in Khuzestan. This parasite causes severe skin and gill damage and mortality reaches 80%. A combination of formalin and
malachite green with transfer of fish to another pond having a better environment cured the condition.
Molnár and Pazooki (1995) record philometrid nematodes from this species in the Karun River, and these are presumed to be a new species. Pazooki and Molnár (1998) later describe Philometra
karunensis as the new species from the swimbladder and adominal cavity of this fish.
Moghainemi and Abbasi (1992) record a wide range of parasites from this species in the Hawr al-Azim in Khuzestan.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Argulus sp. and Ergasilus sieboldi on this species.
The monogeneans Dactylogyrus povlovskyi, D. barbioides, D. anchoratus, D. carassobarbi and Dogielius persicus are recorded from this species in the
Karun River with heavier infestations in spring and summer than in autumn and winter. These gill parasites caused no serious injuries but were thought to be important in respect of monitoring infestation
levels on fish farms in Khuzestan (www.avz1.8m.com/fulltext.htm, downloaded 28 October 2002). Papahn et al. (2004) record the monogeneans Dactylogyrus pavlovskyi, D. barbioides,
D. anchoratus, D. carassobarbi and Dogielius persicus from this species in the Karun River at Ahvaz.
Mokhayer et al. (2006) collected this species from four sites in Shadegan
Marsh and found Dactylogyrus anchoratus, D. carassobarbi and
Dogelius persicus. The first species had more parasites in the right gill
compared to the left gill, the second had more in the upper holobranch and the
third more in the lower holobranch.
Barzegar et al. (2008) record the digenean eye parasite
Diplostomum spathaceum from this fish.
Economic importance
This species is second in importance after sobour (Tenualosa ilisha) at the Basrah fish market in Iraq with a weight from October 1975 to June 1977 of 772,775 kg. Nasir et al. (1989)
record a total catch for Iraq of 5000 tonnes per year and Petr (1987) for Iraq in 1976 a catch of 4243 t. Young (1976) noted that this species was regarded as the tastiest fish available from the marshes
of Iraq.
Petr (1987) has suggested investigating fish farming of this species in Khuzestan and Al-Nasih (1992) carried out such an investigation for Iraq (see below). The Khuzestan Fisheries Research
Centre at Ahvaz has successfully bred this species in pond and pen culture using hormone stimulation of broodstocks (Emadi, 1993a; Iranian Fisheries Research and Training Organization pamphlet;
Iranian Fisheries Research and Training Organization Newsletter, 5:2, 1994; Annual Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran, p. 49, 1996;
Mohammadian et al., 2009) and in polyculture with Chinese carps such as Ctenopharyngodon idella (Annual Bulletin 1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 93-94, 1995; Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization, Tehran, p. 36-37, 1997). Private companies also culture this species in Khuzestan. In Khuzestan, over 95% of young survived,
using hormones to stimulate reproduction. Khajeh et al. (2008) have
examined haematological parameters in cultured fish and found some to be higher
than in Ctenopharyngodon idella. Mortezazadeh et al. (2009)
studied the effects of propofol as an anaesthetic on cultured fish.
Yazdipour et al. (1991) give a report on propagation of this species in Iran.
Sharifian (2000) gives details of whole body analysis. The highest protein content was in the 30-95 mm and 100-140 mm length groups.
Conservation
Local fishermen in Khuzestan believe numbers of this species declined in the Shadegan marshes after young Hypophthalmichthys molitrix from the Caspian were released. As a food fish, its
population biology should be monitored in Khuzestan. Several hundred thousand juveniles have been introduced into the Hawr al Azim in Khuzestan in order to restock and protect this resource (Network of
Aquaculture Centres in Asia, downloaded 11 January 2007) and Mohammadian et
al. (2009) list production of 1-5 million 1-2 g fry used to restock Hawr al
Azim and Shadegan March annually.
Further work
Al-Nasih (1992) investigated the use of this popular food fish for aquaculture in Iraq. Although its growth rate is slower than in Cyprinus carpio, a popular fish for aquaculture, its plankton
feeding makes it adaptable to pond life without competition with Cyprinus carpio, it has tasty flesh, reaches 2 kg, and has a relatively high fecundity. Hormonal injections with hypophysial
extract from the more readily available Cyprinus carpio induced breeding in this species. Natural production can be increased to 450-600 kg/ha with the use of mineral fertilizers in ponds to
stimulate plankton growth. The biology of this species has been investigated in Khuzestan with a view to aquaculture (Annual Report, 1994-1995, Iranian Fisheries Research
and Training Organization, Tehran, p. 6, 1996).
Sources
Type material: See above, Barbus sharpeyi (BM(NH) 1874.4.28:20, BM(NH) 1874.4.28:27, BM(NH) 1875.1.14:16), Barbus faoensis (BM(NH) 1888.5.17:4).
Iranian material:- CMNFI 1979-0087, 1, 228.0 mm standard length, ? ();
CMNFI 1991-0154, 277.8 mm standard length, ?; material observed on market stalls in Ahvaz, Khuzestan.
Comparative material:- CMNFI 1987-0017, 146.0-175.4 mm standard length, Iraq, vicinity of Basrah (no other locality data);
BM(NH) 1920.3.3:71-75, 17, 58.7-115.0 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1920.3.3:76-77, 1, 261.4 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1922.5.24:1, 1, 113.5 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1973.5.21:195, 1, 185.5 mm standard length, Iraq, Shatt-al-Arab (no other locality data);
BM(NH) 1973.5.21:196, 1, 186.1 mm standard length, Iraq, Shatt-al-Arab (no other locality data).
Genus Mylopharyngodon
Peters, 1881
This genus comprises a single species so the characters of of the genus are the same as for the species.
Mylopharyngodon piceus
(Richardson, 1846)
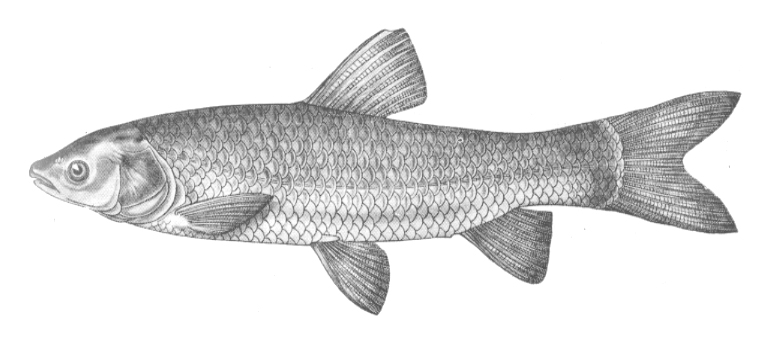
Common names
None.
[black carp, snail carp, black amur, Chinese black carp, Chinese roach, black Chinese roach]
Systematics
This species was originally described from Canton, China.
Key characters
The pharyngeal teeth are strong, grooveless molars in 1-2 series and the overall
colour is black. distinguishing it from the similar grass carp,
Ctenopharyngodon idella.
Morphology
The mouth is terminal to
slightly subterminal with thin lips. Dorsal fin origin slightly in advance of
level of pelvic fin origin. Dorsal fin with 3 unbranched and 7-9 branched rays,
anal fin with 3 unbranched and 7-10 branched rays, pectoral fin branched rays
16, pelvic fin branched rays 8, and lateral line scales 39-46. Gill rakers short
and stout, 14-23. Total vertebrae 36-41. The molar pharyngeal teeth are very
strong and in one or two rows, commonly 1,4-4,1 or 4-5. Intestine short,
1-2 times total length. The chromosome number is 2n = 48. Artificial hybrids
with Ctenopharyngodon idella, Cyprinus carpio,
Hypophthalmichthys molitrix and H. nobilis have been bred.
Meristics on Iranian material from Abbasi (2003): Dorsal fin branched
rays 7(2), anal fin branched rays 8(2), lateral line scales 41(1) and 42(1),
gill rakers 17(1) and 19(1), and pharyngeal teeth 4-5 (1) and 5-5(1).
Sexual dimorphism
Nuptial tubercles are present on the operculum,
interorbitally, pectoral fin rays and on the scales of males.
Colour
The overall colour of the body and fins is black to blackish-brown or blue-grey.
Fins are characteristically dark but may be somewhat lighter than the body, a
blackish-grey, but darker than fins usually are in cyprinids. Scales have dark
edges conferring a cross-hatched appearance. The lower head surface may be
whitish and the belly greyish-white.
Size
Reaches 2.0 m and 106.0
kg.
Distribution
The native distribution is in East Asia from Amur River basin to south China
and perhaps Vietnam but it has been introduced worldwide in suitable waters as a
farm fish. The Food and Agriculture Organization web site "Database on
Introductions of Aquatic Species" lists this species as introduced to Iran for
aquaculture and research from China in 1992. It is reported from the Karakum Canal and the Kopetdag Resevoir in
Turkmenistan (Shakirova and Sukhanova (1994) and Sal'nikov (1995) and
may eventually reach the Tedzhen River basin in Iran. It is recorded from
the Aras River in Armenia (Gabrielyan, 2001) and may eventually be recorded from
the Iranian reach of this river. A large specimen
from Gilan in the Caspian Sea basin shown me in Tehran in December 2000 appeared to be
this species but was frozen solid so certain key characters could not be
examined. The Iranian Fisheries Research and Training Organization Newsletter (27:2,
2001) has an illustration of this specimen which weighed 7.5 kg and was 96 cm long.
Abbasi (2003) gives the length of this fish as 97 cm total length and a
capture date of October 2000 in a beach seine near Bandar Anzali coastal waters.
In March 2001 a fish 80 cm total length and weighing 4.8 kg was caught in the same general area.
Shamsi et al. (2009) record it from fish farms in Gilan.
Zoogeography
An exotic species introduced to Iran.
Habitat
The main habitat is
floodplain lakes and the lower reaches of rivers, down to about 10 m. It may
also be found in canals and reservoirs. Fast flowing water is required for
reproduction. Temperature tolerances are similar to the grass carp,
Ctenopharyngodon idella. Oxygen levels as low as 2 mg/l are tolerated.
Age and growth
Life span is possibly 20 years or more, with maturity
attained at 3-11 years in various habitats. Fish introduced to the Karakum Canal
showed a relatively rapid growth attaining 48 cm and 1.47 kg in their first
year. At six years old fish were 125 cm and 29 kg.
Food
The principal food item of this species is molluscs, snails and clams, although apparently it
does not eat large groups of zebra mussels. A 4-year-old fish can eat 1-2 kg of
molluscs per day. The molar pharyngeal teeth crush the mollusc shells, the soft
tissues are ingested and the broken shells spat out. Young fish eat zooplankton
and aquatic insects and adults may take some crayfishes and other benthic invertebrates.
Reproduction
A stretch of river is required for drifting eggs to mature. before settling in quieter areas of floodplain lakes or
the river itself. The length of river varies with temperature and velocity of
the particular river. Adults migrate up rivers to spawn. Spawning starts at
26-36ºC, and perhaps as low as 18ºC, when rivers begin to rise in flood in
spring and summer. Up to 1,180,000 eggs are produced annually in a single batch,
more in hatchery fish where an average of 1.5-2.1 million eggs is cited. Swollen
eggs have a diameter of 5.6 mm, swelling 4-5 times during hydration. Eggs hatch in 1-2 days.
Parasites and predators
Shamsi et al. (2009) found Dactylogyrus magnihamotus in this
species from fish farms. Various Caspian Sea basin species are reported as
predators including Esox lucius, Silurus glanis, Sander
lucioperca, Aspius aspius, Perca fluviatilis and Lota lota.
Economic importance
None in Iran as not extensively used. This species is widely used on fish
farms elsewhere to control parasite problems occasioned by molluscs acting as
hosts for trematodes. Both diploid and triploid forms are used in farming. It is
also important as a food fish.
Conservation
This species is an exotic and requires no conservation.
Further work
Additional field work is required to determine distribution and abundance in the wild as it can seriously affect
native mollusc populations.
Sources
Iranian material: The specimen noted above.
Nico et al. (2005) summarise the biology of this species on which much of the above is based.
Genus Parabramis
Bleeker, 1865
Parabramis pekinensis
(Basilewsky, 1855)
Reported from the Karakum Canal and Kopetdag Reservoir in
Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) as an
exotic from China. May eventually be found in the Tedzhen (= Hari )
River and Caspian Sea basins of Iran. No Iranian record.
Genus Pelecus
Agassiz, 1835
The sabre carp genus contains only a single species found from the
Baltic to the Black, Caspian and Aral Sea basins including Iran. The
characters of the genus are the same as under the species.
Pelecus cultratus
(Linnaeus, 1758)
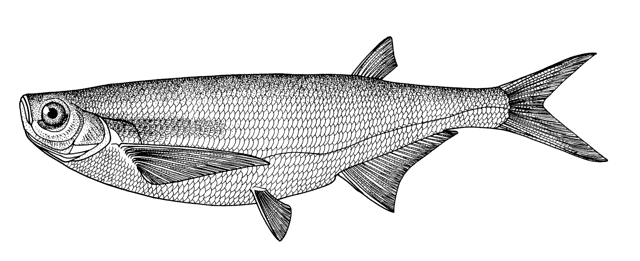
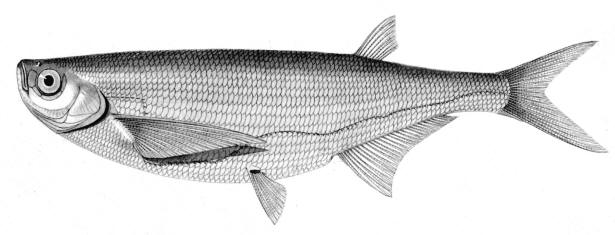

Common names
shamshir mahi (= scimitar or swordfish), shamshir mahi ab shirin (= freshwater swordfish), kuli.
[gilincbalig in Azerbaijan; chekhon' in Russian; sichel, ziege, razorfish, sabre carp, sabrefish].
Systematics
Pelecus cultratus was originally described from the Baltic Sea.
Pelecus cultratus kurensis Smirnov, 1943 is the Kura River
basin subspecies but Berg (1948-1949) considered that other
populations over the range of this species had been insufficiently
studied to validate this subspecies.
Key characters
This species is easily recognised by the scaleless keel extending
from the throat to the anal fin and the decurved and wavy lateral line.
Morphology
The body is elongate and strongly compressed. The mouth is almost
vertical. The pectoral fins are long and curved, used for rapid
manoeuvring when swimming normally and folded against the body when
swimming rapidly. The lower lobe of the caudal fin is larger than the
upper, with more rays and a stiffer ventralmost ray. The lower jaw is
hooked in older specimens and has a tubercle which fits into an upper
jaw notch. Gill openings are very wide with the branchiostegal
membranes attached far forward, under the eye level. Muscles on the
back extend forward to reach the anterior eye margin. Dorsal fin
unbranched rays 2-3, usually 3, followed by 6-10, usually 7, branched
rays, anal fin unbranched rays 2-3, usually 3, and branched rays
23-31, pectoral fin branched rays 13-17, and pelvic fin branched rays
6-8. Lateral line scales 88-120. Scales have extremely fine circuli, a
central to subcentral posterior focus and very few posterior radii.
Gill rakers number 15-26 (reaching the second or third raker below
when appressed) and vertebrae 46-52. Pharyngeal teeth number 2,5-5,2,
are narrow and are very strongly hooked at the tip with obviously
serrate edges. Variants include 2,5-4,2, 2,4-5,2 and 2,5-5,3. The gut
is an elongate s-shape. The chromosome number is 2n=50 (Klinkhardt et al., 1995).
Sexual dimorphism
Unknown.
Colour
The back is greenish and the flanks silvery. Fins are hyaline to
grey, although the paired fins and the anal fin can be a bright yellow.
Size
Reaches 60 cm and 3.5 kg but most fish in the Volga-Caspian region are 80-180 g.
Distribution
Found in the drainages of the Baltic, Black, Caspian and Aral seas. This species is reported as rare in the lower Safid River (Derzhavin, 1934) and
is found in the Anzali Mordab (Abbasi et al., 1999; Kiabi et al., 1999). Recorded by Abdoli (2000) from the middle to lower Safid
River, Anzali Talab and adjacent Caspian coast. Recorded from the Atrak River but not yet from its Iranian reach (Reshetnikov et al.
(1997). Also reported from the Karakum Canal and Kopetdag Reservoir of Turkmenistan (Shakirova
and Sukhanova, 1994; Sal'nikov, 1995) and so may eventually be found in the Tedzhen (= Hari) River basin of Iran.
Zoogeography
A European and western Asian species with its origins in a Danubian or Sarmatian fauna.
Habitat
This species lives primarily in the sea but is also anadromous and
may live permanently in larger tributaries. Jolodar and Abdoli (2004) note two
forms in Iran, one resident in fresh water and a migratory form. It inhabits surface
waters, aided by fin and mouth modifications for this mode of life (Adamicka,
1984). It migrates to the fresh water of large rivers to spawn. In the
sea and larger rivers it occurs in schools.
Age and growth
Sexual maturity in the Volga River is attained at 3-4 years and a
minimum length of 20 cm in males, 4-5 years and 22 cm for females. The
largest immature males are 24 cm long, females 25 cm and the maximum
age of non-spawners of both sexes does not exceed 6 years. The
spawning stock is mostly fish 4-10 years old. Males have a slightly
smaller maximum size than females and life span is at least 16 years (Sil'chenko,
1976). Spawning takes place first at age 2 in the Kura River of
Azerbaijan. Growth is faster than in other populations, the Kura fish
being the same size at age 2 as age 3 fish from the Don River of the
Black Sea and age 4 fish of the Aral Sea.
Food
Food is taken by the vertical mouth from surface waters and
includes insects and spiders. Young fish feed on zooplankton and even
adults will do this if crustaceans are abundant. In the sea, various
crustaceans are taken and these may be pursued near the bottom. Full
grown Pelecus (50-60 cm) will capture fish such as Clupeonella,
gobies (Gobiidae), Cyprinidae and even sticklebacks (Gasterosteidae) (Adamicka, 1984).
Reproduction
Spawning takes place in the latter half of May in the Kyubyshev
Reservoir of the Volga River at 13.5-14.1°C or as high as 18-22°C.
Water depths are 2.0-3.5 m. The main spawning takes place around
sunset over a period of 24 days with a peak period of 10-12 days.
Fecundity reaches 71,400 eggs. Eggs sink to the bottom but swell to an
average diameter of 4.7 mm within an hour of fertilisation. Once
swollen, any slight movement of the water will suspend the eggs in the
water column. Eggs develop pelagically in floodplains, main rivers,
side channels, bays and lakes but all these diverse habitats have a
high oxygen content through flowing water or wind mixing. In the Volga
flow rates are 0.28-1.1 m/sec on a sand-gravel bottom. Spawning may
also take place in brackish water where the eggs float. Spawning in
the Kura River of Azerbaijan takes place at the end of April and in
May. The larvae are phototropic and active swimmers.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks and for food.
The scales contain silvery crystals of guanine which are extracted and
used to make essence d'orient for artificial pearls. In the
period 1909-1913, the catch in the Volga-Caspian region was more than
14 million fish annually. The flesh is fatty and bony and so this species is best smoked.
Conservation
Lelek (1987) classifies this species as intermediate to rare in
Europe but fairly common in the Caspian Sea basin. Kiabi et al.
(1999) consider this species to be critically endangered in the south
Caspian Sea basin according to IUCN criteria. Criteria include sport
fishing, few in numbers, habitat destruction, limited range (less than
25% of water bodies), absent in other water bodies in Iran, and
present outside the Caspian Sea basin.
Further work
The distribution and biology of this species in Iranian waters needs study
although it is probably too rare to be easily captured in numbers.
Sources
Meristics based partly on Wais (1995).
Iranian material: None available.
Comparative material: CMNFI 1971-0825, 1, 232.0 mm standard length, Czechoslovakia, Lake Ozirna (no other locality data);
CMNFI 1987-0220, 2, 96.9-105.7 mm standard length, Rumania, Lake Călăraşi (44º20'N, 27º20'E).
Genus Petroleuciscus
Bogutskaya, 2002
This genus currently comprises 7 species of which 3 are found in
Iran. Some species in this genus were formerly placed in the subgenus Squalius of the genus Leuciscus;
Squalius is now recognised as a distinct genus too.
The genus is distinguished by the small size of adults, the reduced number of vertebrae (modally 34-38 in
total, rarely 39 or 40 ?check esfahani), few sensory cephalic pores (7-10 in the supraorbital
canal, 12-19 in the infraorbital canal and 12-17 in the preoperculo-mandibular
canal), a relatively small supraethmoid-mesethmoid block, narrow infraorbitals, and a deep
neurocranium with a normally developed interorbital septum (Bogutskaya, 2002).
Perea et al. (2010) using mitochondrial and nuclear DNA concluded that
Petroleuciscus is not monophyletic. P. persidis was included with
Abramis, Acanthobrama, Acanthalburnus, Ballerus,
Blicca, Mirogrex and Vimba. The genus is found in the basins of the Aegean, Black, Caspian and Aral seas,
the upper Tigris River , Lake Orumiyeh, and central and south-central Iran.
Petroleuciscus esfahani
Coad and Bogutskaya, 2010

Common names
None.
Systematics
The type locality is Esfahan, stream at Dizaj in the
southern Zayandeh River basin, 31°55’N, 51°30’E (holotype, CMNFI 1979-0249,
female, 106.7 mm SL). Paratypes are from Esfahan, stream 1 km east of Daran,
Pelasegan River tributary in the northern Zayandeh River basin, 32°59’N, 50°26’E
(CMNFI 1979-0251, 134, 22.1–83.7 mm SL). The species is named after the central
Iranian drainage basin in which it was found, itself named for the principal
city of Esfahan, the third largest city in Iran.
Key characters
This species is characterised by having a pharyngeal tooth count of
2,5-4,2,
modally 8 branched dorsal fin rays, modally 10-11 anal fin branched rays, small lateral line scales
numbering 44-54, and in particular a high total vertebrae count of 40-42 (the abdominal + caudal vertebral
formulae being usually 22+19, 22+20 and 21+20).
Morphology
The snout is short, its length equals to or slightly smaller
than the orbit width. The tip of the mouth cleft is on a level with the upper
margin of the pupil in large-sized
individuals such that the mouth is slightly superior or somewhat below, at about
the level of the middle of the
eye, in small-sized ones. The lower jaw projects slightly compared to the upper
jaw, often forming a distinct
chin especially visible in larger specimens.
Dorsal-fin with 3 unbranched rays and 7(22), 8(108) or 9(5)
branched rays; anal fin with 3 unbranched
rays and 9(14), 10(91),
11(25) or 12(5) branched rays; pectoral fin with 14(5), 15(17)
or 16(9) branched rays; pelvic fin with 7(1), 8(28) or 9(2)
branched rays; lateral-line scales to hypural fold 45(3), 46(1), 47(3), 48(8), 49(3), 50(1), 51(4), 52(4), 53(2), 54(1),
or 56(1); total
lateral line scales 45(1), 46(2), 47(4), 48(2), 49(6), 50(14),
51(16), 52(7), 53(5), 54(2),
55(1) or 56(1); among 134 paratypes there are also 8 specimens with
the lateral line widely
interrupted (28–40 pored lateral-line scales from 50–53 scales in lateral
series); scales around caudal peduncle
14(3), 15(12), 16(13), 17(2) or 18(1); predorsal scales 23(12),
24(3), 25(5), 26(10) or 27(1); scales between lateral line and dorsal-fin origin 9(9), 10(17) or 11(5); scales between lateral
line and anal-fin origin 4(6), 5(22) or 6(3); scales between lateral
line and pelvic-fin origin 3(2),
4(17) or 5(12); total gill rakers 12(2), 13(4), 14(8), 15(10), 16(6)
or 17(1); total number of vertebrae 39(2), 40(22), 41(71), 42(35) or 43(5);
abdominal (precaudal) vertebrae including the Weberian and intermediate vertebrae
(those vertebrae that lost
articulation with ribs and differ in the degree of transformation of the
parapophyses into the haemal arch with
the haemal spine) 21(39), 22(87) or 23(9);
caudal vertebrae 18(12), 19(66),
20(53) or 21(4); and the vertebral formula is 22+19(47), 22+20(28),
21+20(23), 21+19(13),
22+18(9), 23+19(6), 22+21(3), 23+20(2), 21+18(2), 23+18(1) or 21+21(1). The
number of predorsal
vertebrae (in front of the first dorsal pterygiophore) is 14(52), 15(76) or
16(7) and the number of intermediate vertebrae is 3(17), 4(69), 5(46) or 6(2)
Pharyngeal teeth 2.5–4.2 (8) or 2.5–5.2(2), hooked at the tip and strongly
serrated below it. The gut is an
elongate s-shape.
The sensory canal system was examined in the holotype and 19 paratypes, so the
numbers of examined
paired canals is 40. The supraorbital canal is not lengthened in its posterior
section and has 8–12, commonly 9
or 10 pores (mean 9.6, standard deviation 1.33), with 3 or 4 (3 in 66%) and 5–8
(counts 6 and 7 found each in
30%) canal openings on the nasal and frontal bones, respectively. The
infraorbital canal has (14, 15)16–18
pores (a mode of 16 found in 38% of canals; mean 16.6, standard deviation 1.01)
with 4 or 5 (in 75%) canal
openings on the first infraorbital. The preoperculo-mandibular canal is
complete, with 15–17 pores (15 in
58%; 15.5, 0.74) with 4 or 5 (in 83%) and (8)9–10 (9 in 50%) canal openings on
the dentary and
preoperculum, respectively. It always communicates with the infraorbital canal
in the pterotic, passing
through the antero-dorsal process of the operculum. The supratemporal canal is
complete (60% of specimens) or incomplete, with 4–6 pores. Other osteological characters
are given in Coad and Bogutskaya (2010).
Sexual dimorphism
Males have a longer dorsal fin base
and females a longer anal fin base, possibly associated with pair spawning.
Tubercles on two male fish, 82.5–83.7 mm SL, are fine and scattered on the upper
head extending down onto the upper half of the operculum but weak to absent on
the snout (Fig. 2). Fine tubercles lining scales are prominent behind the head
dorsally but become less evident posteriorly and ventrally. The pectoral fin
bears fine tubercles on the anterior rays but numbers decrease to none on more
posterior rays.
Colour
There is no distinctive colour pattern in
preserved fish. The flank above the lateral line bears a fine dark
speckling of melanophores and is mostly unpigmented below the lateral line.
Melanophores are present on the
upper part of the operculum and extend down along the posterior edge.
Melanophores on the cheek ring the
lower orbit. Melanophores on the lateral
sides of the head are larger and more evident particularly in smaller fish. The back bears a predorsal and postdorsal stripe. The dorsal and
caudal fins have very fine
melanophores on the rays only. The pectoral fin has similar fine melanophores on
its anterior rays only. The
anal and pelvic fins are almost unpigmented. The peritoneum is silvery to cream
with very few, widely
scattered, melanophores.
Size
Reaches 106.7 mm standard length.
Distribution
This species is found in two streams in the Esfahan
basin, one a right bank tributary of the Zayandeh River and the other a
tributary to the Pelasagan Riveritself a left bank tributary of the Zayandeh
River.
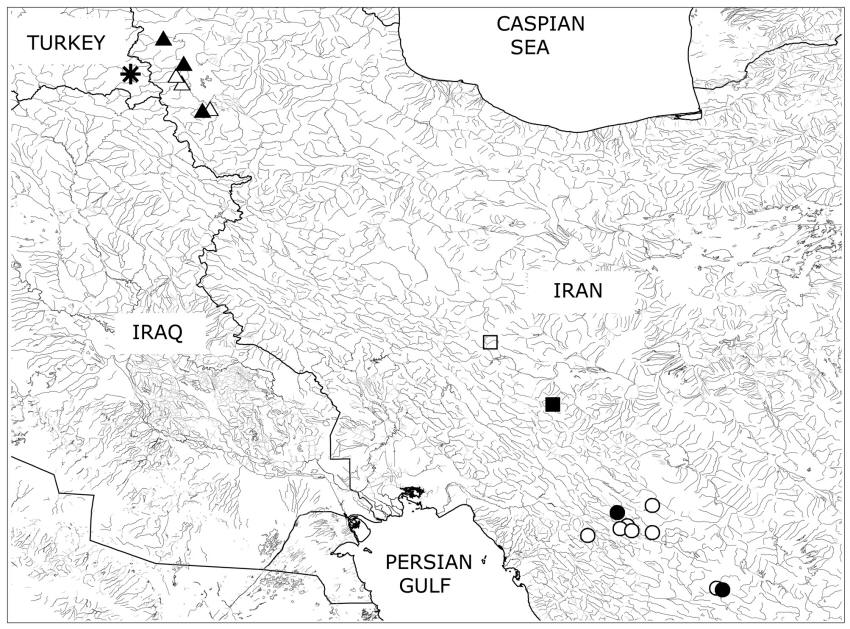
Solid square = holotype of
Petroleuciscus esfahani, open square = paratypes;
solid circles = P. persidis
holotype and paratypes (southern circle) and paratypes (northern circle),
open circles = other material of
P. persidis;
solid triangles = P. ulanus
syntypes (northern triangle);
solid triangles = P. gaderanus
syntypes (other triangles),
open triangles = other material of
P. gaderanus;
star = P. kurui holotype and
paratypes).
Zoogeography
This species is morphologically closest to P.
gaderanus of the Lake Orumiyeh basin.
Habitat
Habitat data is confined to notes made when the type
specimens were collected. The type locality was a fresh, clear stream at an
altitude of 2300 m. Water temperature at 1705 hours on 9 June was 22°C, pH was
6.2, conductivity was 0.455 mS, stream width was 2–8 m, maximum depth was 1 m,
current was slow to moderate, aquatic plant material was of the submergent type,
the shore was grassy, and the stream bottom was a mix of pebbles and mud. A
Capoeta species was also caught. The second locality was a fresh stream with
clear water at an altitude of 2410 m. Water temperature at 1225 hours on 10 June
was 17°C, pH was 6.2, conductivity was 0.4 mS, stream width was 4 m, maximum
depth was 70 cm, current was slow to moderate, aquatic plant material was of the
encrusting type, the shore was grassy, and the stream bottom was a mix of
pebbles, sand and mud.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
This species is known only from two localities from
collections made in 1977. Dam construction on the Zayandeh River may have
altered the ecology of the river system, exacerbated by population growth and
its demands on limited water resources in a desert environment. However, the
species may be relatively secure in higher tributary streams.
Further work
The ecology of this species should be studied, detailed distributional information compiled,
and its conservation status investigated.
Sources
Based on Coad and Bogutskaya (2010).
Type material: See above.
Petroleuciscus persidis
(Coad, 1981)
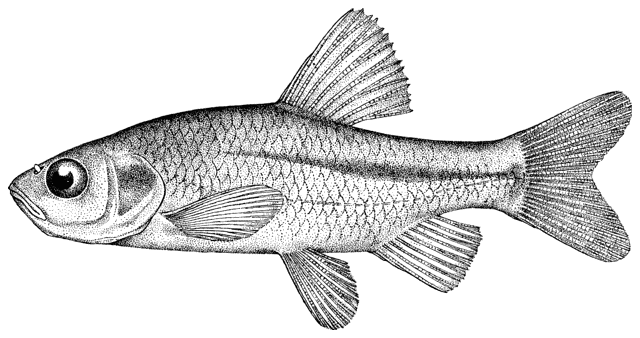
Common names
'rus mahi persidis.
[Persian chub].
Systematics
Trewavas (1972) has discussed the generic nomenclature of Pseudophoxinus
Bleeker, 1859 in which this species was originally described. It is more closely
related to the genus Leuciscus according to N. Bogutskaya (pers.comm.,
1994; 1996). Bogutskaya (1996) placed this species in her Leuciscus
borysthenicus (Kessler, 1859) group which also includes L. ulanus and
deserves subgeneric rank, but later erected a new genus Petroleuciscus.
Perea et al.
(2010) using mitochondrial and nuclear DNA propose placing this species in
Acanthobrama.
Saadati (1977) described, but did not name, a new species in the related
genus Phoxinellus Heckel, 1843 from the Bid Sorkh River in the Simareh
River basin between Sahneh and Kangavar in Kermanshahan (the Bid Sorkh Pass is
at 34°26'N, 47°49'E). I was unable to find this species in his collections and
no further material has come to light. It had 8-9 dorsal fin branched rays, 8-9
branched anal fin rays, 8 branched pelvic fin rays, 47-56 scales in a complete
lateral line, and 20 long and crowded gill rakers.
The type locality of Pseudophoxinus persidis is the "upper Shur
River drainage at "Koorsiah" village, near Darab on Darab-Fasa road,
28°45.5'N, 54°24'E, Fars". The holotype is a 54.7 mm standard length male
held at the Canadian Museum of Nature, Ottawa under CMNFI 1979-0154A (see figure
above). Paratypes comprise 95 fish, 34.7-58.8 mm standard length, from the same
locality as the holotype under CMNFI 1979-0154B, and 5 fish, 74.7-92.4 mm standard
length, under CMNFI 1979-0499 from an "irrigation ditch at village 32 km west
of Kor River bridge on road to Dariush Dam, 30°04.5'N, 52°36'E, Fars".
Paratypes were distributed to the following institutions from CMNFI 1979-0154B: BM(NH)
(2), ROM (2), UAIC (1), UBC (2) and UMMZ (2).
Key characters
This species is characterised by having a pharyngeal tooth count of 1,5-4,1,
modally 7 branched dorsal fin rays, anal fin branched rays 7-9, pelvic fin rays
7-8, pored lateral line scales 35-43 in a complete lateral line, short gill
rakers numbering 10-14 on the whole first arch, total vertebrae 34-37, a light
coloured peritoneum but with numerous melanophores, and flanks with a lateral
stripe evident posteriorly but fading anteriorly and not reaching the head.
Morphology
The mouth is terminal and oblique and the rictus reaches back to a level just
anterior to the anterior eye margin. A pelvic axillary scale is present. The
subcircular to oval scales bear numerous fine circuli and radii on the anterior
and posterior fields (total radii number 23-44). Pharyngeal teeth are strongly
hooked, concave below the hook and serrated along the margins of the concave
surface, particularly on the anterior edge. In the largest fish, the hook may be
much reduced to absent, serrations may be absent, and anterior teeth in the main
row are rounded. Gill rakers reach the adjacent raker when appressed. The gut is
a short and simple s-shape.
Meristic values are as follows: dorsal fin branched rays 6(2) or 7(189) after
3 unbranched rays, anal fin branched rays 7(3), 8(37) or 9(10) after 3
unbranched rays, pectoral fin branched rays 13(10), 14(20) or 15(20), pelvic fin
branched rays 7(31) or 8(19), lateral line scales 35(2), 36(19), 37(4), 38(10),
39(3), 40(6), 41(5) or 43(1), total gill rakers 10(1), 11(9), 12(32), 13(6) or
14(2), pharyngeal teeth 1,5-4,2(1), 1,5-4,1(13) or 1,5-4,0(1), and total
vertebrae 34(1), 35(20), 36(27) or 37(2). Karyotype 2n=50 (Esmaeili and Piravar, 2006a).
Sexual dimorphism
Number of anal fin branched rays is significantly higher in females (mean 8.3
versus 8.0 in males). Pectoral and pelvic fin lengths, longest dorsal and anal
fin rays and caudal peduncle length are shorter in females than in males while
head length, head width and predorsal length are longer in females than in males.
Colour
The back and upper flanks are dark and the belly cream. The straight lateral
stripe extends from a diffuse area on the tail base to a level at or in front of
the dorsal fin origin but does not reach the head. The stripe overlaps the
lateral line on the caudal peduncle but lies above it on the flank, paralleling
the back. Dorsal fin membranes are lightly speckled with melanophores which tend
to be concentrated along the fin ray margins. The caudal and anal fin membranes
are mostly clear with pigment restricted to fin ray margins. The pectoral fin
may be pigmented on the membranes and there is often strong pigment along the
posterior edge of the first unbranched ray. The pelvic fin has little or no
pigmentation. Large fish are much darker overall than small fish, obscuring the
stripe and with more pigment on fin rays and membranes.
Size
Reaches 9.2 cm standard length.
Distribution
This species is found in the Kor River and Hormuz basins (Coad, 1981e; M.
Hafezieh, pers. comm.). Abdoli (2000) reports this species from the
Kor and Pulvar rivers, the Hilleh, middle Mand, Shur (tributary of the Dasht-e
Palang) and the Kul rivers.
Zoogeography
Durand et al. (2000) place this species in a Leuciscus (= Squalius) cephalus
"complex", i.e. descendents of peripheral isolates of a widespread
ancestral species, later re-invaded by Danubian S. cephalus.
Habitat
Habitat knowledge is restricted to field data of collections. This species
was collected in large rivers, streams, springs, irrigation ditches and qanats,
but all these have in common a stream-like environment for much of the year.
They are relatively shallow (20 cm to 2 m), variable width (0.5-75 m), medium to
slow current, some submergent and emergent aquatic vegetation and a bottom
varying from pebbles and gravel to mud. Water temperatures varied from 15 to 23°C
from October to January and presumably would be over 30°C in the summer.
Conductivity ranged from 0.3 to 1.0 mS. Altitude ranged from 980 to 1940 m. The
lower reaches of some of the capture rivers are salty and may not support this species.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 10 fish measuring 4.21-8.33 cm standard length. The a-value
was 0.0178 and the b-value 3.229 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Gut contents include insect remains and fragments of large plants. Diet
presumably consists mostly of aquatic invertebrates.
Reproduction
Reproduction in this species is unknown but egg development in adult fish
collected in winter and young of the year collected in October suggest spring
and early summer as the spawning season.
Parasites and predators
Jalalai et al. (2000) record Dactylogyrus sphyrna, a monogenean,
from the gills of this species in the Kor River basin. Barzegar and Jalali (2006)
report parasites in this species from Kaftar Lake as Lernaea cyprinacea,
Trichodina sp., Ichthyophthirius multifilis and Dactylogyrus
sphyrna.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea foliaceus
on this species.
Economic importance
None.
Conservation
The distribution of this species in various small habitats probably means
that it is not in immediate danger. However water is in short supply in this
part of Iran and abstraction may threaten its survival.
Further work
The ecology of this species should be studied and detailed distributional information compiled.
Sources
Based on Coad (1981e).
Type material: See above, Pseudophoxinus persidis (CMNFI 1979-0154A, 1979-0154B, and
1979-0499).
Iranian material: CMNFI 1979-0025, 11, 19.4-79.6 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0114, 1, 47.1 mm standard length, Fars, Mand River (29º41'N, 52º06'E);
CMNFI 1979-0156, 6, 43.8-64.3 mm standard length, Fars, qanat in Rashidabad (28º47'N, 54º18'E);
CMNFI 1979-0163, 8, 31.1-48.3 mm standard length, Fars (no other locality data);
CMNFI 1979-0164, 15, 28.8-52.6 mm standard length, Fars (no other locality data);
CMNFI 1979-0194, 3, 48.9-58.2 mm standard length, Fars, upper Shur river drainage (28º45'30"N, 54º24'E);
CMNFI 1979-0292, 7, 37.0-54.0 mm standard length, Fars, Lapu'i spring near Zarqan (29º48'N, 52º39'E);
CMNFI 1979-0305, 2, 23.8-25.5 mm standard length, Fars, Pulvar River at Pasargad (30º12'N, 53º12'E);
CMNFI 1979-0342, 33, 23.6-43.2 mm standard length, Fars, Kor River at Band-e Amir (29º46'N, 52º51'E):
CMNFI 1979-0503, 4, 32.2-113.5 mm standard length, Fars (no other locality data);
USNM 258445, 18, 15.5-44.5 mm standard length, Fars, Chasht Khvar (ca. 29º44'N, ca. 53º12'E).
Petroleuciscus ulanus
(Günther, 1899)
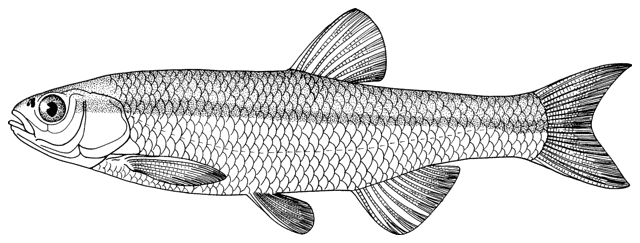
Common names
None.
Systematics
This species was originally described in the genus Leuciscus. Saadati
(1977) considers this species to be in the genus Alburnus but did not
examine the types. Leuciscus gaderanus Günther, 1899 is a synonym, even
Günther (1899) the describer of the two species indicating that this may be the case.
The 2 syntypes of Leuciscus ulanus, 68.0-84.5 mm standard length, are
in the Natural History Museum, London and are "from Ula on the Zola Chai",
Günther (BM(NH) 1984.10.10:1-2). The 3 syntypes of Leuciscus gaderanus,
44.6-73.0 mm standard length, are "from the Gader Chai" which is near
Ocksa (BM(NH) 1899.9.30:113-115). Additionally there are 5 specimens of Leuciscus
gaderanus, 11.0-54.5 mm standard length "from near the mouth of the
Nazlu Chai at Superghan" (BM(NH) 1899.9.30:108-112). Günther (1899) refers
to 3 young specimens from the latter locality as syntypes but of these 5 fish
under this catalogue number and locality given as "Superghan" on the
label only 2 fish are small (11.0-12.5 mm SL) and the other 3 fish are larger
(45.8-54.5 mm SL). Another collection comprising 1 specimen, 27.0 mm standard
length, is from the Urmi River according to the label (BM(NH) 1899.9.30:107).
Its type status is unclear since the locality is wrong but the size is small and
it could be the third of Günther's "three young specimens" since its
catalogue number is in sequence.
Key characters
Distribution and meristic characters identify this species.
Morphology
Dorsal fin unbranched rays 3, branched rays 7-9, anal fin unbranched rays 3,
branched rays 7-11, pectoral fin branched rays 12-14, and pelvic fin branched
rays 7-8. Lateral line scales 36-45. There is a pelvic axillary scale. Scales
with a slightly anterior focus and numerous radii on the anterior and posterior
fields. Total gill rakers 12-16, reaching the first or second raker below when
appressed. Pharyngeal teeth 2,5-4,2, more rarely 2,4-5,2, 2,5-5,2 or 2,4-4,2,
hooked at the tip and strongly serrated below on the larger teeth. Total
vertebrae 37-38. The mouth is oblique and extends back to just behind the front
margin of the eye and the lower jaw protrudes slightly or hardly at all (Günther
(1899) has the upper jaw slightly overlapping the lower). The gut is an elongate
s-shape and may have an anterior loop to the left.
Meristic values for Iranian specimens are:- dorsal fin branched rays 7(5) or 8(15); anal fin branched
rays 7(1), 8(6), 9(11) or 10(2); pectoral fin branched rays 12(5), 13(10), or
14(5); pelvic fin branched rays 7(19) or 8(1); lateral line scales 38(3), 39(6),
40(6), 41(1), 42(1) or 45(2); total gill rakers 12(2), 13(7), 14(6), 15(3) or
16(2); pharyngeal teeth 2,5-4,2(9), 2,4-5,2(2), 2,5-5,2(1) or 2,4-4,2(1); and
total vertebrae 37(4) or 38(1).
Sexual dimorphism
Males bear tubercles on the pectoral fins mostly in a single file,
occasionally two together, branching with the rays. Small tubercles are also
found thickly on the top and sides of the head but no pattern was discernible in
the specimens examined (types of L. ulanus).
Colour
There is a narrow, straight black stripe running from the upper half of the
eye to the end of the lateral line separating the bluish back from the silvery
flanks (Günther, 1899), best developed in preserved specimens posteriorly. Live
fish have a grey-silver flank with some spots, a brown-green back and a light
silver abdomen. Lower fins are pale to light yellow and the dorsal and caudal
fins are light grey (Abbasi and Sabkara, 2004a; 2004b). The
lower half of the operculum below the mid-eye level has few or no spots while
the upper half is heavily pigmented. There is also no pigment below the eye or
it is restricted to a thin line around this lower margin. The flanks are dotted
with minute pigment spots. The back mid-line has a black stripe, most obvious predorsally.
The rays and membranes of the dorsal fin, caudal fin and anterior
pectoral fin bear melanophores; melanophores weak to absent on the anal and
pelvic fins. The peritoneum is silvery brown with scattered melanophores.
Size
Reaches 14.2 cm for length (Abbasi and Sabkara, 2004b).
Distribution
This species is endemic to the Lake Orumiyeh basin (Günther, 1899).
This species was found only in the Gadarchai and Mahabad River and was not
caught in the Zolachai, Nazluchai, Baranduzchai and sampled areas of the
Siminerud and Zarrinerud in recent collections by Abbasi and Sabkara (2004a; 2004b).
Zoogeography
Petroleuciscus kurui (Bogutskaya, 1995) from the upper Tigris River system of
Turkey is the closest relative of this species (Bogutskaya, 1995) although other
members of the Orumiyeh ichthyofauna are related to fishes from the Caspian Sea basin.
Habitat
Essentially unknown, found in stream or river habitats.
Age and growth
Fish caught by Abbasi and Sabkara (2004b) in the Gadarchai and Mahabad River were 34-142 cm in fork length
and 1.4 years old. Males comprised 28.8% and females 71.2% of the total population. Maturity was
attained at 2 years.
Food
Generally unknown but traces of insects and large quantities of filamentous
algae were found in the gut contents of a few specimens. The algae may be an
accidental inclusion as the gut is short and probably cannot digest plant
material. Fish caught by Abbasi and Sabkara (2004a; 2004b) however contained a wide
range of phytoplankton (19 genera) and zooplankton (7 groups), as well as
benthic organisms (4 groups). Daphnia, Chydrus and chironomids
were dominant gut contents. It was considered to be an omnivorous fish
preferring to feed on zooplankton and other mid-water animals.
Reproduction
Spawning of fish in caught by Abbasi and Sabkara (2004b) took place from 1
April until 1 July with absolute fecundity estimated at 1810-16,115, average 6437 eggs.
Parasites and predators
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sp. in fish from the Baranduz and Halaj rivers.
Economic importance
None.
Conservation
This species is known only from the type series and a few other collections.
This suggests that it is quite rare or has a restricted habitat preference.
Further work
The biology, distribution and abundance of this poorly known species should
be investigated to see if it requires protection.
Sources
Type material: See above for L. ulanus (BM(NH) 1984.10.10:1-2) and L. gaderanus
(BM(NH) 1899.9.30:113-115 and BM(NH) 1899.9.30:108-112).
Iranian material: CMNFI 1970-0560, 35,11.4-48.8 mm standard length, Azarbayjan-e Bakhtari, Mamiyand Chay (ca. 36º59'N, ca. 45º39'E);
CMNFI 2007-0096, 6, 38.8-68.5 mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay basin (ca. 37º25'N, ca. 45º10'E);
BM(NH) 1899.9.30:107, 1, 27.0 mm standard length, Azarbayjan-e Bakhtari, Urmi River (no other locality data).
Genus Pimephales
Rafinesque, 1820
This genus is endemic to North America where there are 4 species in
Arctic, Atlantic and Gulf of Mexico drainages. It occurs in Iran as an exotic.
These fishes are small, less than 12 cm standard length, with a
stout to slender body. They are uniquely characterised by the last, unbranched
dorsal fin ray in males being short, blunt and separated from
the succeeding rays by a membrane. There are usually 7 branched dorsal
and 6 branched anal rays. The lateral line is complete to incomplete.
The head and back before the dorsal fin is flattened and the predorsal
scales are small, irregularly arranged and crowded. The intestine
varies from long to short, the peritoneum from black to silvery.
Pharyngeal teeth are in one row. The flank has a dark band often
terminating in a spot at the caudal fin base.
The species of this genus are of some commercial importance as bait or food for sport fishes.
Pimephales promelas
Rafinesque, 1820
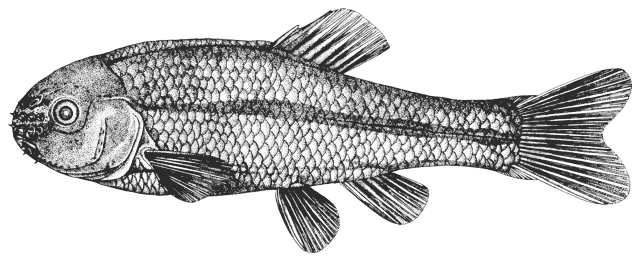
Common names
None.
[fathead minnow].
Systematics
This exotic is unrelated to native Iranian fishes and was described from Lexington, Kentucky, U.S.A..
Key characters
This species is unique in Iran in that the last, unbranched
dorsal fin ray is short and separated by a membrane from the next,
longer ray in males. Other characters include the absence of a barbel,
scales in front of the dorsal fin are much smaller and more crowded
than flank scales, the back is flattened, the lateral line is
incomplete to nearly complete, the mouth is small and terminal, and by
the colour and tubercle patterns described below.
Morphology
Dorsal fin branched rays 7, rarely 8, anal branched rays 6,
sometimes 7, pectoral rays 14-18 and pelvic rays 8-9. Scales in
lateral series 39-56, lateral line pores usually ending before the
dorsal fin origin but rarely may be almost complete. Scales are oval
with a markedly anterior focus, few circuli and moderate numbers of
radii restricted to the posterior field. Vertebrae number 35-38.
Pharyngeal teeth 4-4, slightly hooked at the tip, compressed and
with elongate and concave cutting surfaces. The gut is elongate with
several loops. Gill rakers 12-16, short and touching the adjacent
raker when appressed.
Sexual dimorphism
Large tubercles in males are found on the snout in 3 (rarely 4)
rows with 4-15 in the lower row, and with up to 11 tubercles on the
lower jaws. There are also smaller tubercles on top of the head and
pectoral rays. Tubercles first develop in early April in Canada and
males begin to lose them in late July. By early September only scars
remain. Males also develop a blue-black to grey, spongy, wrinkled pad
on the back between the head and dorsal fin. Fin membranes swell.
Breeding males darken, particularly the head and dorsal fin, and the
stripe is not apparent. The body can be completely black with a white
band at the head-body region and under the dorsal fin. This occurs
only during aggression or sexual activity. The lateral banding
enhances the robust appearance of fish which must maintain a territory
over several weeks without much opportunity to feed. Weight loss is
replaced by water to help maintain the image of a fat and vigorous
male. Very frightened fishes blanch.
Females have a protruding ovipositor.
Colour
The back and upper flank are dark olive-green to brown, silvery to
golden flanks and a whitish belly. There is a mid-flank stripe and a
predorsal stripe. Scales on the upper flank are outlined by pigment. A
spot at the tail base is faint to absent. Peritoneum black.
Size
Reaches 10.2 cm total length but usually much less.
Distribution
This species was reported from a reservoir, probably the Yengi Kand
where bass (Micropterus salmoides) and bluegills (Lepomis
macrochirus) were introduced, south of Tehran at 75 km from
Asia-beg (Andersskog, 1970) (presumably Asia Bak at 35°19'N, 50°30'E
on the Tehran-Esfahan highway). The MMTT catalogue also has a record
for the "Dusadj Reservoir", 90 km west of Saveh, presumably
in Markazi Province and the Namak Lake basin too. These reservoirs may
be the same locality as "Dusadj" or Duzaj, Yang-e Kand and
Yanguikand are all villages in the same general area west of Saveh.
The reservoir is deduced from maps to be at about 35°19'N, 49°55'E.
A report for the Golpayegan Reservoir (Golpayegan is at 33°27'N, 50°18'E)
in the Namak basin by Saadati (1977) was an error (R. J. Behnke, in
litt., 1979).
? Map
Zoogeography
The fathead minnow is native to North America and was introduced to
Iran accidentally in shipments of largemouth bass, Micropterus
salmoides, and bluegills, Lepomis macrochirus. Fathead
minnows are pond-raised in North America as food for bass.
Habitat
Fathead minnows are found in ponds, small lakes and slow-flowing
brooks, often associated with vegetation. They tolerate high
turbidity, high temperatures, low oxygen and high alkalinity and so
are able to survive in desiccating or other conditions unfavourable to most fishes.
Age and growth
Males grow faster and larger than females, typical of nest
defending species. Life span is about 3 years with maturity attained
as early as 1 year, rarely in the year of birth.
Food
Food is bottom sediment for its organic content including plant
material, aquatic insects and zooplankton.
Reproduction
Spawning runs from April to August in North America, once water
temperatures reach 14°C and light-dark hours are 16-8. Males choose a spawning site under a
log, rock, plant stems or even a lily pad or any solid artificial
structure in shallow water. Cavity spawning in mud-bottomed habitats
prevents the eggs from being smothered as well as offering protection
from predators for the relatively few eggs spawned from a small fish.
The male will clean out the cavity, spending up to 10 hours on the
task, using his tubercles to scrape, pulling debris with his mouth and
sweeping with his tail fin. The spongy pad on the back may serve to
test spawning sites and eggs chemically. The pad secretes a mucus
which is smeared on the spawning site perhaps to improve it for egg
survival since mucus protects against disease and parasites. Diseased
eggs are eaten by the male. The mucus may also serve to indicate
ownership of a nest site. Spawning male fatheads lose their ability to
produce alarm or fright chemicals on skin injury, otherwise the
continual pad rubbing would disturb spawning activities by releasing
alarm substance and scaring away females.
Females may enter the spawning site casually, be chased there by a
male or enticed by a face to face encounter and leading to the nest.
The male lifts and presses the female on her side between himself and
the roof of the spawning site and the female rapidly undulates through
an s-shape. Egg deposition is thought to use the same unusual
mechanism as in the bluntnose minnow (Pimephales notatus (Rafinesque,
1820)). As each egg is extruded, it is transferred to the upper side
of the female and the undulation rolls it along between her side and
the nest cavity roof. When the adhesive egg reaches the tail she
presses it against the roof where it sticks. How the female transfers
the egg from her papilla to her side is unknown and the question
remains why this sideways process is used when other roof spawners
simply turn upside down and deposit eggs directly. The process may
serve to roll eggs into a vacant roof space, occupying the roof most
efficiently. Eggs laid on top of others may not attach well or prevent
proper development of the underlying eggs.
Males court females by swimming rapidly up to them and then
freezing at 3-5 cm away, and by leading females with a zig-zag or
straight-line motion from the female to the nest site. Males will also
display to females by erecting their fins for 2-3 seconds and by
jump-swims in which a male swims upwards to a female then rolls on his
side and swims back down. Butting and lateral quivering also occur,
perhaps attempts to assess spawning condition of the female. Males
defend the eggs against other fishes, including female fatheads, by
using the snout tubercles to butt and tail swipes to intimidate by
sending a pressure wave sensed by the lateral line system. Chasing and
biting are common and 2 males may carousel (or circle head-to-tail)
trying to contact each other. Leeches and turtles are also driven
away. Some eggs are lost while the male is distracted chasing away
predators. Repeat spawning may be necessary to replace lost eggs.
Males also aerate and clean the eggs with fin movements. A nest will
contain eggs in various stages of development as the male will spawn
with several females. Females will deposit eggs in several nests.
Orange, mature eggs are up to 1.6 mm in diameter with 12,000 or more
per nest. A female will release up to 10,164 eggs in a season but from
9 to 1136 at a time. Spawning intervals are 2 to 16 days.
Parasites and predators
It is an important food for many other fishes and aquatic birds.
Economic importance
In the U.S.A. fatheads are raised as bait fish and as forage fish
for introduction into bass fishing lakes. They are also used
extensively as a laboratory animal for tests of toxic compounds. They
have even been used to evaluate the biological effects of materials
from the moon.
Conservation
This exotic species may have deleterious effects on the native
fishes and its conservation is of no utility. It is extremely fecund
and could compete with native species for food and habitat. A variety
of parasites have been reported from this species including some which
can have devastating effects on fish populations such as Ligula
intestinalis. The introduction of this fish into Europe led to an
outbreak of enteric redmouth disease (Yersinia ruckeri) which
spread to native and farmed stocks of commercially important fishes
such as trout (Michel et al., 1986; Welcomme, 1988). Native
fishes may be less well adapted to withstand the depredations of
exotic diseases and parasites. Ideally it should be extirpated but, if
this is not possible, its interactions with native fishes should be
studied and attempts made to prevent its spread to other water bodies.
Further work
The status and numbers of this exotic should be checked by field
work. Any data on this exotic species held in files of the Department
of the Environment should be published so that this "grey"
literature is not lost to future students of Iranian fishes.
Sources
Based on North American literature summaries such as Scott and
Crossman (1973) and Becker (1983) as well as personal observations.
The biology of Iranian populations is unknown.
Genus Pseudogobio
Bleeker, 1859
Pseudogobio rivularis
(Basilewsky, 1855)
Reported from the Tedzhen River in Turkmenistan on the northeastern
border of Iran by Aliev et al. (1988) in their text but not in
their distributional table. This exotic species is from China. Also
recorded from the Karakum Canal and Kopetdag Reservoir (Shakirova and
Sukhanova, 1994; Sal'nikov, 1995) and may eventually reach the Caspian
Sea basin. No Iranian record.
Genus Pseudorasbora
Bleeker, 1859
This genus contains 3 species with a native distribution
in eastern Asia including the Amur River basin shared between Russia and China, in Japan,
other parts of China, and in Korea. One species is an exotic now found
in Europe and accidentally introduced in Iran.
The genus is characterised by a small and transverse mouth
positioned at the top of the snout rather than the anterior tip, the
lower jaw has a trenchant edge and projects slightly beyond the upper
jaw, no barbels, pharyngeal teeth are in a single row, the gut is
short, scales are large, gill rakers are rudimentary, dorsal and anal
fins are short and spineless, and there is no keel on the abdomen.
Pseudorasbora parva
(Temminck and Schlegel in Siebold, 1842)
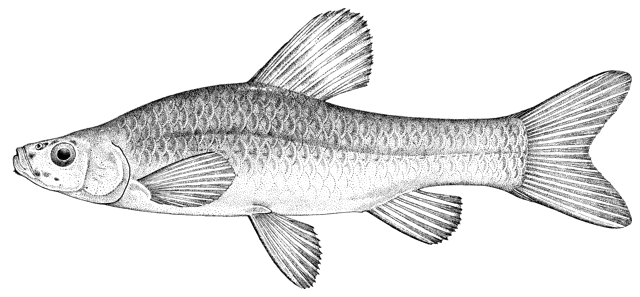

Common names
آمورچه (amurcheh or amorcheh), amoornama, parva.
[stone moroko, topmouth gudgeon, topmouth minnow, false rasbora; chebachek or Amur chebachok in Russian].
Systematics
Leuciscus parvus was originally described from Nagasaki, Japan.
Subspecies have been described in China but exotic introductions
are usually referred to the type subspecies. Reshetnikov et al.
(1997) and Bănărescu in Bănărescu (1999) give the date for this species as 1846.
The phenotype of this species is highly influenced by environmental conditions
and this is suggested to be one of the attributes that make this fish a
successful invasive species (Záhorská et al., 2009).
Key characters
The mouth structure is unique, being very small and lying entirely
before the nostril level, almost vertical, opening antero-dorsally
with the gape entirely visible in dorsal view. The lower jaw protrudes
to form the most anterior part of the head.
Morphology
Dorsal fin unbranched rays 3 followed by 7-8, usually 7, branched
rays, anal fin with 2-3, usually 3, unbranched rays and 5-7 branched
rays, usually 6, pectoral fin branched rays 11-14, and pelvic fin
branched rays 6-8, usually 7. Lateral line scales 30-40, with the
lateral line rarely incomplete. A pelvic axillary scale is present.
The scale radii are restricted to the posterior field. Gill rakers are
rudimentary and are only well-developed at the junction of the upper
and lower arms of the gill arch. These rakers are stubby and rounded,
reaching the adjacent raker when appressed, and bearing fine, fleshy
fimbriae which extend onto the adjacent parts of the gill arch.
Anterior rakers are absent and patches of fimbriae are found. Rakers
number 6-16, usually 9-13. Total vertebrae 31-38 presumably the result of
different counting methods; Naseka (1996) gives 36-38 and fish from Turkey have
34-37, cf. below. Pharyngeal teeth usually 5-5, rarely 6-5, with the tips strongly hooked and the area
below the hook flattened and without ridges or only very weakly ridged
on some teeth. The gut is an elongate s-shape. The chromosome number
is 2n=50 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin
branched rays 7(11) or 8(1), anal fin branched rays 6(12), pectoral
fin branched rays 13(7) or 14(5), pelvic fin branched rays 7(4) or
8(8), lateral line scales 34(4), 35(5) or 36(3), pharyngeal teeth
5-5(9), 6-5(2) or 4-4(1), and total vertebrae 34(2) or 35(10).
Sexual dimorphism
A horny pad develops on the jaws in males and females during
spawning and strong, sharp tubercles in males. One tubercle is found
between the eye and the nostril, one below the nostril (this may be
absent), one next to the upper lip on a line across from the one below
the nostril, 5-8 in a row from the extreme corner of the mouth along
the side of the head over the flesh of the cheek, and 2-3 below the
lower lip from the tip of the lower jaw to the end of the jaw on the
lower head surface. Lower head surface tubercles may coalesce at the
base but each tubercle bears a single rounded cusp. Rarely a tubercle
may have a single base but two cusps.
Males are larger than females and have larger fins. Spawning males
are darker than females and the flank has a metallic violet sheen.
Colour
The head and body has a mid-lateral stripe but this is obscured in
adults by crescentic speckles situated posteriorly on each scale. The
back is light grey, the flanks silvery and the belly whitish. Dorsal
and anal fins are speckled and turn almost black in spawning fish.
Preserved fish have a cream coloured belly with the back much
darker. The head is black dorsally and fades to cream ventrally. The
scales on the back and flanks, but not the belly, carry a broad band
of pigment which follows the scale margin distally. The extreme edge
of the scale is hyaline but the arc of pigment effectively defines the
posterior scale margin and outlines the scale pattern of the back and
flanks. Pigmentation on fins is mostly restricted to the rays and
their margins but is found also on fin membranes to varying extents.
Pigmentation is strongest distally on all fins. The dorsal fin,
particularly in smaller fish, bears patches on the membranes posterior
to branched rays 1 or 2 through 5 or 6, starting on ray 1 or 2 below
the mid-point of the ray length and descending gradually behind
successive rays to lie near the base behind the last ray. These
patches are vertically short and do not touch the succeeding ray. The
leading edge of the dorsal, anal, pectoral and pelvic fins and the
upper and lower edges of the caudal fin are black in large, and some
small, fish. Pigment may be concentrated along the mid-line forming a
thin stripe, only apparent posteriorly in some fish. There is a dark
line along the mid-line of the back. The peritoneum is silvery with
some scattered melanophores.
Size
Attains 12.0 cm (Movchan and Kozlov, 1978).
Distribution
The natural range of this species is in eastern Asia as given above
under the genus. It has been introduced to Iran by accident (Abdoli,
1992; Coad and Abdoli, 1993b). It is now found in ab-bandans at
Avaness, Hasan Tabeeb and Shaeed Ziaee (all about 40-45 km east of Gorgan),
Teer Tash and Lemrask (about 20-25 km east of Behshahr), Lapoo (about
4 km east of Babol Sar) on the Caspian Sea coast, and at Gorgan-Aliabad, Mazandaran, from the Safid River estuary
and neighbouring waters and the Anzali Talab (Iranian
Fisheries Research and Training Organization Newsletter, 6:8,
1994; Anonymous, 1994; Abbasi et al., 1999), from the Atrak, Gorgan, Gharasu,
Tajan, Babol, Haraz, Sardab, Pol-e Rud, and Safid rivers (Kiabi et
al., 1999), from fish ponds at Arak probably inadvertently carried there with carp fingerlings imported
from Gilan on the Caspian shore; at Mashhad in northeastern Iran, in springs near Kermanshah and in Lake Zeribar,
Kordestan (Coad, 1996g); in Sistan at Hamun Kushk, and the canal flowing into the Chahnimeh (J. Holčík,
in litt., 1996), and from the International Wetlands of Alma-Gol, Adji-Gol and Ala-Gol (Patimar et al., 2002a; 2002b;
Patimar, 2008).
Abdoli (2000) records it generally from
the Dasht-e Kavir, Dasht-e Lut, Kerman-Na'in and Sistan basins; the lower Kashaf River in the Tedzhen
River basin; the middle Atrek, lower Neka, Babol, Heraz, Chalus, Tonekabon, and Safid rivers and the
Anzali Talab in the Caspian Sea basin; the middle to lower Talkheh and lower
Zarrineh rivers in the Orumiyeh basin; the lower Shur and middle
and lower Qareh Chai in the Namak Lake basin; the middle and lower Zayandeh
River in the Esfahan basin; and the Simarreh and lower Gav Masiab rivers in the
Tigris River basin. Jolodar and Abdoli (2004) record it from most water bodies
on the Iranian Caspian coast and Abdoli and Naderi (2009) from the Atrak, Gorgan,
Gharasu, Tajan, Babol, Heraz, Sardab, Pol-e Rud and Safid rivers there. Esmaeili
et al. (2010) and Esmaeili et al. (2011) add Lake Zarivar and the Gulf and
Lake Maharlu basins and it is now probably distributed throughout Iran.
It is also recorded in the Karakum Canal, Kopetdag Reservoir and
Tedzhen River of Turkmenistan (Shakirova and Sukhanova, 1994;
Sal'nikov, 1995) so may well reach the Tedzhen (= Hari) River basin of
Iran eventually. Pipoyan (1996a) reports it from the Araks River in Armenia.
Zoogeography
This species was first recorded in western Eurasia in Romania in
1960 as an accidental introduction with Chinese carps from the lower
Yangtze River of China. The species is now widespread in Europe and is
becoming common in western Asia including Kazakhstan, Uzbekistan,
Kyrgyzstan and southern Anatolian Turkey as well as Iran (Wildekamp et al., 1997).
Habitat
This species prefers well-vegetated areas as protection from
predators. It may be found in streams, rivers and ponds, and more
rarely in the shallows of large lakes. It is apparently quite
resistant to pollution (Bănărescu in Bănărescu, 1999) and is
found in waters that freeze over and that attain 30ºC in summer (Boltachev et al., 2006).
Age and growth
Life span is about 5 years with maturity attained at 1-2 years,
usually at 1 year in Europe or the second year of life in the Crimea (Boltachev
et al., 2006). Most fish in a population are 2-3 years
old or 1-2 years in the Crimea. Patimar et al. (2002a; 2002b) report 4 age groups from the
International Wetlands of Alma-Gol, Adji-Gol and Ala-Gol, with the smallest
mature specimens found at 2 years. Esmaeili and Ebrahimi (2006) give a
significant length-weight relationship based on 33 Iranian fish measuring
3.29-5.99 cm standard length. The a-value was 0.0286 and the b-value
2.763 (a b-value < 3 indicating a fish that becomes less rotund as length
increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
This species feeds on benthos but also some zooplankton. Food items
include various aquatic insects such as stone flies, caddis flies,
chironomids, water sawbugs and midge larvae but guts also contain
sponges, bryozoans, Spirogyra, detritus, and fragments of
higher plants (Movchan and Kozlov, 1978). It may also feed on the eggs
and juveniles of native fishes. Young fish take zooplankton (Movchan
and Kozlov, 1978). Bănărescu in Bănărescu
(1999) reports also isopods and aquatic worms and, in fish ponds, artificial food.
Reproduction
Reproduction begins at 16-18°C and lasts two months in its native Amur River basin. Fecundity is
about 5000 elliptical eggs with a diameter of 2.0-2.5 mm, and this
species has intermittent spawning with up to 85 eggs per batch in
introduced populations in Central Asia (Makeyeva and Mokhamed, 1982).
Up to 60 batches may be laid in a spawning season. The spawning site
is cleaned of ooze and plant material. Adhesive eggs are deposited on
the lower surface of stones, and occasionally sticks or empty mollusc
shells, and are protected by the male using the head tubercles to
drive away other fishes. The ellipsoidal eggs are laid in strips,
usually of 5 eggs but as many as 10. Males clean the eggs and remove
dead ones. The spawning season in Central Asia is April to August and
spawning takes place in warm, shallow and calm waters in the morning.
Spawning in the Crimea is in second half of May or in June, late May to July in
the Ukraine and from the end of June to the beginning of August in the Amur (Boltachev
et al., 2006). Female specimens from Iran collected in March, April and May are ripe
and males have well-developed breeding tubercles and Patimar
et al. (2002a; 2002b) report a spawning peak in April in the
International Wetlands of Alma-Gol, Adji-Gol and Ala-Gol of Iran.
Parasites and predators
Malek and Mobedi (2001) report Clinostomum complanatum from this species in
Mazandaran, in the Shiroud. Sander lucioperca and Silurus
glanis are predators in Turkmenistan (Aliev et al. (1988).
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea foliaceus
on this species.
Economic importance
Makeyeva and Mokhamed (1982) and Movchan and Kozlov (1978) report
competition with commercial species and predatory behaviour on carp
larvae if there is insufficient food. Male reproductive aggression may
inhibit breeding of native fishes. Boltachev et al. (2006) report that it
is a facultative parasite of other fishes in enclosed areas including commercial
species such as the silver carp (Hypopthalmichthys molitrix). Areas of
the body are attacked such as behind the dorsal fin and above the anal fin out
of sight of the affected fish. Skin and muscles are eaten away. Welcomme in Courtenay and Stauffer
(1984) regards this species as a pest when introduced. Bănărescu
in Bănărescu (1999) reports this species as a competitor for food with native species in Europe.
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use in textbooks.
Conservation
None required for an introduced species.
Further work
The distribution of his species as an exotic in Iran should be thoroughly documented and its biology and effects on native species studied.
Sources
Iranian material: CMNFI 1991-0160, 3, 47.8-55.8 mm standard length, Mazandaran, Abgeere Avanes (37º03'N, 54º47'E);
CMNFI 1991-0161, 1, 52.5 mm standard length, Mazandaran, Madarso River (37º23'N, 55º47'E);
CMNFI 1993-0134, 7, 45.0-59.1 mm standard length, Mazandaran, Gorgan-Aliabad (37º01'30"N, 54º47'36"E);
uncatalogued material, 1, 86.9 mm standard length, Kermanshahan, sarabs near Kermanshah (no other locality data).
Comparative material: CMNFI 1983-0204, 7, 51.7-76.6 mm standard length, Turkey, Edirne, Meriç River at Ipsala (40º55'N, 26º23'E);
CMNFI 1983-0343, 5, 49.9-83.4 mm standard length, same locality as preceding.
Genus Rhodeus
Agassiz, 1832
The bitterlings comprise about 18 species in Europe, Asia Minor, the
Caspian Sea basin, China, Japan and Korea with 1 species in Iran.
They are small fishes with deep, compressed bodies, an incomplete
lateral line (about 10 pored scales or less), large to moderate-sized
scales, females with an ovipositor, males larger than females (unusual
in cyprinid fishes), brightly coloured and tuberculate when spawning,
pharyngeal teeth in 1 row and not or only slightly serrated, mouth
small, oblique and subterminal, no barbels, dorsal fin short to
moderately long and spineless, anal fin of similar length, gill rakers
short, intestine long and spirally coiled, and peritoneum black.
Rhodeus amarus
(Bloch, 1782)
Common names
ماهي مخرج لوله اي
(= mahi-ye makhraj lulehi or mahi-e-makhraj looleei, meaning tube-like vent fish), rodeus.
[karka in Azerbaijan; gorchak in Russian; bitterling, European bitterling].
Systematics
Holčík and Duyvené de Wit (1964) reviewed the systematic status of Rhodeus
sericeus (Pallas, 1776) in Europe and western Asia (but not Iran) in comparison
to the Chinese populations of this species. They refer European and
western Asian populations to Rhodeus sericeus amarus (Bloch,
1782) and Iranian populations were long regarded as this subspecies. Cyprinus
sericeus was described from Dauriya; Cyprinus amarus was
described from the Elbe basin, Germany and no types are known (Eschmeyer
et al., 1996). Later, Holčík and Jedlička
(1994) consider subspecies not to be warranted as the key characters
used in distinguishing them (pored lateral line scales, scales in
lateral series, gill rakers) showed clinal variation with longitude
and the number of segments was also related to latitude, elevation,
mean annual air temperature, and fish size. Bohlen et al. (2006) consider
Rhodeus from the Vistula to the Volga to belong to the R. amarus
eastern clade based on cytochrome b sequences.
Kottelat (1997) considers Rhodeus amarus to be a distinct
species since it is diagnosable (although differences with Rhodeus
sericeus are slight and largely overlap), and it is separated by
2-4 million years in time and 4000 km in space. However he does admit
that the immense distributional gap may be a source of bias. Holčík in Bănărescu
(1999) considers amarus to be synonymous with sericeus.
Bogutskaya and Komlev (2001) found no characters to clearly confirm the specific
status of R. amarus. The name amarus is retained here as an indication that the taxa
are geographically isolated. Van Damme et al. (2007) refer the Caspian
bitterling to a species as yet undescribed.
This species was described from Lake Müggelsee near Köpenik, Berlin, Germany
and 3 syntypes are in the Museum für Naturkunde, Humboldt-Universität
zu Berlin (or Zoologisches Museum Berlin, ZMB 3393).
Key characters
The presence of an ovipositor in females, the flank stripe and few pored scales are distinctive.
Morphology
Dorsal fin with 2-4, usually 3, unbranched and 7-11, usually 9,
branched rays, anal fin with 2-4, usually 3, unbranched and 6-12,
usually 9, branched rays, pectoral fin branched rays 10-13, and pelvic
fin branched rays 4-8, usually 6-7. Pored lateral line scales 0-11,
usually 4-6, along the flank 28-45, usually about 30-32 in some
reports but 32-38 in Abdurakhmanov (1962), 37-40 in Holčík and Jedlička
(1994), 30-38 in Pipoyan (1996b) and 35-40 in Holčík
in Bănărescu (1999). Gill rakers 9-16, usually 10-14, reaching the raker below when
appressed. Vertebrae 33-39. Pharyngeal teeth 5-5, rarely 5-4, 4-5 or
4-4. Teeth are elongate and narrowly compressed with a slight to
strong hook at the tip and a very long, narrow and concave grinding
surface below the tip. The gut has numerous coils. The chromosome
number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin branched rays 8(4),
9(52), 10(1) or 11(1); anal fin branched rays 8(18), 9(39) or 10(1);
pectoral fin branched rays 10(3), 11(24), 12(29) or 13(2); pelvic fin
branched rays 4(1), 6(3), 7(53) or 8(1); scales in lateral series
32(1), 33(2), 34(22), 35(29) or 36(4); pored lateral line scales 3(1),
4(7), 5(37), 6(11) or 7(2); total gill rakers 10(3), 11(14), 12(27),
13(10) or 14(4); pharyngeal teeth 5-5(20); and total vertebrae 34(9),
35(36), 36(12) or 38(1). Holčík and Jedlička
(1994) give ranges in their Iranian sample of 33-41 for lateral series
scales, 0-6 pored scales and 9-14 for gill rakers, in general
agreement with data here.
Sexual dimorphism
Males develop a triangular or crescent-shaped patch of 7-20
tubercles on each side of the snout and small tubercles are found
above the eyes. The female develops an ovipositor near the genital
opening and it may be longer than the body. The flank stripe is longer
and about one half wider in males compared to females, and male
colouration is distinctive. Males are generally larger than females.
Colour
Males are particularly colourful in the spawning season: the top of
the head and back are olive to bright green, reddish or dark violet,
the iris is bright red, flanks are iridescent with violet and
steel-blue colours most evident, the throat and belly are orange to
blood-red, dorsal and anal fins are bright red and margined with
black, the caudal fin is green at the base and yellow distally, and
pectoral and pelvic fins are yellowish. Females are more yellowish and
less iridescent than males in the spawning season. Outside the
breeding season both sexes are similar in colour with a grey-green
back, silvery flanks and yellowish belly. A grey-green to
greenish-blue stripe originates under the dorsal fin and extends back
to the tail base, broadening posteriorly. The dorsal fin is blackish
and other fins reddish to yellowish. The dorsal, and sometimes the
anal fin, has a dark interrupted stripe. The iris is silvery or
yellowish. The peritoneum is dark.
Size
Reaches a reputed 18.0 cm but usually not more than 7.0-9.0 cm.
Distribution
Found from western Europe north of the Pyrenees and Alps to the
Caspian Sea basin. In Iran, it is recorded from Astara to the Gorgan River
including the Anzali Talab, Gorgan, Tajan, Babol, Haraz, Sardab, and Safid
rivers (Derzhavin, 1934; Holčík and Oláh, 1992; Abbasi et al., 1999;
Kiabi et al., 1999; Abdoli, 2000; Abdoli and Naderi, 2009). Also probably in the Araks River of Armenia (Pipoyan, 1996b).
They may have been introduced into the Zarrineh River of Azarbaijan-e Gharbi
based on photographs by Saber Shiri (pers. comm., 14 June 2008).
Zoogeography
Bohlen et al. (2006) assumed a continuous distribution of Rhodeus
from Europe through Siberia to east Asia during the Pliocene, loss of the
Siberian population in the late Pliocene or early Pleistocene, subsequent
isolation, and later post-glacial expansion from several refugia in the Euro-mediterranean
zoogeographic subregion. The Caspian Sea populations were not examined but seem
to belong to R. amarus although the authors suggest a revisionary study
is required for European bitterlings. Van Damme et al. (2007) consider
this species was restricted to Ponto-Caspian and Aegean regions and its presence
in western and central Europe is associated with the spread of Cyprinus
carpio cultivation, and more recently, anthropogenic alterations to habitats
and temperature changes.
Habitat
This species favours heavily vegetated areas of small lakes, ponds
and slow-moving rivers, rarely in faster water. It is found in the lower reaches
of rivers on the Caspian coast of Iran (Jolodar and Abdoli, 2004). The bottom is usually
a fine sand or a thin layer of mud. Swan mussels (Anodonta) and
freshwater clams (Unio) share this kind of habitat and are
necessary for reproduction. Other genera of clams include Pseudanodonta, Cristaria,
Margaritifera and Dahurinaia (Smith et al., 2004).
Spawning occurs at water temperatures of 12-24°C, although 15-21°C is optimal (Holčík in Bănărescu, 1999),
Van Damme et al. (2007) giving 23°C as optimal. Its distribution is
limited by the 16°C July isotherm (Van Damme et al., 2007).
Age and growth
Maximum life span is 5-8 years, the higher values being uncertain (Holčík in
Bănărescu, 1999). Maturity is attained during the second or third year, the life
span of most individuals. Rarely some fish may mature as early as
before the age of 1 year or as late as the fourth year. Growth is
faster in ponds than in rivers. Males outnumber females at a ratio of
1.2-1.5:1, especially in spawning areas, although females apparently
outlive males (Holčík in Bănărescu, 1999).
Food
Food consists of diatoms and detritus with aquatic insects and
crustaceans being mostly accidental inclusions. They also take eggs of
Rutilus rutilus, Cyprinus carpio and Rutilus frisii,
and also their own eggs which fail to be deposited in the clam or mussel. Most
gut contents in Iranian fish examined were plant fragments,
filamentous algae, detritus and sand grains.
Reproduction
Reproduction takes place mainly in April and May, but can run from
the end of February (in Azerbaijan) to August. The female develops an
ovipositor from the genital opening, up to 6 cm long. In Iranian fish
the best developed ovipositor was seen in a fish collected on 12 May
when it extended back two-thirds along the caudal fin length. Eggs in
this fish were 2.2 mm. Fish taken in September, October, November and
January had short ovipositors progressively increasing in length with
time. A fish taken on 4 July had an ovipositor extending only half way
along the anal fin but no large eggs. Spring spawning is indicated but
an ovipositor can be seen in varying degrees of development year round
in adult females. Holčík in Bănărescu (1999) reports
ovipositor length up to 126.5% of standard length in the Anzali Mordab in April.
The ovipositor lays eggs inside freshwater clams
and mussels, using the excurrent siphon as the entry route. Apparently the
flow of water and a high oxygen content out of this siphon encourage egg laying. Before egg
laying, the female nudges the clam repeatedly to accustom the
mollusc to stimulus so that it does not close up its shell. In the
absence of clams, the bitterling does not become sexually mature.
Clams and mussels with high numbers of bitterling larvae or filled with
glochidia are avoided, as is Anodonta cygnaea, a mussel which is able to
eject eggs and larvae and has low oxygen levels in its excurrent siphon (Kottelat
and Freyhof, 2007). Males select and defend a particular clam against other males. They may headbutt
each other or strike flanks, dislodging scales. Sneaking occurs despite this. The
female deposits eggs 1-2 at a time and the male sheds sperm which are
sucked into the clam on its feeding current. This process may be
repeated with the same or different females. The female deposits about
40-100 eggs at each spawning and can spawn at least 5 times a day. The movement of the ovoid eggs through
the ovipositor can be clearly seen as they are somewhat larger than
the distensible ovipositor. Spawning bouts last 1-3 days with intervals of 5-7
days, variable with feeding conditions and temperature (Smith et al.,
2004). Fecundity is up to 22,136 eggs and maximum
egg diameter to 3.1 mm with width up to 1.52 mm. Eggs hatch 2-5 weeks
later and the young leave the clam after 2 days when the yolk sac
has been absorbed. Young fish leave the clam singly or in pairs. The
fish gain the advantage that the eggs and young are protected inside
the clam, even should the shore area dry out since the clam will
move to deeper water. The clam is unharmed and is able to disperse
its own young or glochidia by their attachment to the fins of the adult fish.
Bitterlings seem however to have an immunological response to glochidia, having
far fewer than other fishes that are less intimately associated with clams.
Circumstantial evidence laid out by Smith et al. (2004) involving inhibition of
free water circulation by the fish embryos, damage to clam gills, and increased
consumption of oxygen by clams, indicate the relationships is a parasitic rather than a commensal one.
Smith et al. (2004) give a detailed account of reproduction, behaviour and development in this species.
Parasites and predators
None reported from Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks and aquaria and
because it has been introduced outside its natural range.
The species is used as a model in behavioural studies. Van Damme et al. (2007) regard it as a parasite on freshwater mussel
populations in western and central Europe outside its native range, escaping
glochidia infection and having low egg ejection rates.
Conservation
Lelek (1987) classifies this species as rare to vulnerable in
Europe. Vulnerable in Turkey (Fricke et al., 2007).Kiabi et al. (1999) consider this species to be of
least concern in the south Caspian Sea basin according to IUCN
criteria. Criteria include medium in numbers, widespread range (75% of
water bodies), absent in other water bodies in Iran, and present outside the Caspian Sea basin.
Further work
Although well-studied elsewhere, the biology of this species in Iran has not been investigated in detail.
Sources
Iranian material: CMNFI 1970-0510, 21, 34.5-47.5 mm standard length,
Gilan, Golshan River (37º26'N, 49º40'E); CMNFI 1970-0512, 25, 26.6-43.9 mm standard length,
Gilan, Shalman River (37º08'N, 50º15'E); CMNFI 1970-0514, 2, 36.6-38.3 mm
standard length, Gilan, Shafa River estuary (37º35'N, 49º09'E); CMNFI 1970-0520,
7, 28.9-45.0 mm standard length, Gilan, Astara River (ca. 38º25'N, ca. 48º52'E);
CMNFI 1970-0526, 4, 30.7-43.2 mm standard length, Gilan, Safid River below
Astaneh Bridge (37º19'N, 49º57'30"E); CMNFI 1970-0579, 8, 32.6-49.0 mm standard
length, Gilan, Old Safid River estuary (37º23'N, 50º11'E); CMNFI 1970-0580, 5,
35.8-53.8 mm standard length, Mazandaran, river near Iz Deh (36º36'N, 52º07'E);
CMNFI 1979-0265, 9, 16.1-47.4 mm standard length, Gilan, Anzali Mordab at
Abkenar (37º28'N, 49º20'E); CMNFI 1979-0472, 6, 37.7-47.7 mm standard length,
Mazandaran, stream west of Mahmudabad (36º37'N, 52º12'E); CMNFI 1980-0117, 3,
42.2-47.8 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E); CMNFI
1980-0127,9, 32.6-43.0 mm standard length, Gilan, Caspian Sea near Hasan
Kiadeh (37º24'N, 49º58'E).
Rhodeus ocellatus
(Kner, 1866)
-m%20cleaned.jpg)
Reported from the Karakum Canal and Kopetdag Reservoir in
Turkmenistan (Shakirova and Sukhanova, 1994; Sal'nikov, 1995) as an
exotic from China. May eventually be found in the Tedzhen (= Hari)
River and Caspian Sea basins of Iran. No Iranian record.
Romanogobio
Bănărescu, 1961
There are about 17 species in the genus found from Europe to China with two
species recorded from Iran. Members of the genus have a shallower body than the
related Gobio, an elongated, usually cylindrical, caudal peduncle, epithelial keels on dorsal scales, an anus
placed closer to ventral fins, absolute or average prevalence of caudal
vertebrae over abdominal ones, and a higher number of preanal vertebrae (Naseka, 1996). Bănărescu
in Bănărescu and Paepke (2002) notes that he considers Romanogobio as a subgenus and
further work is needed to resolve this difference of opinion. Most literature is under the genus Gobio.
Romanogobio macropterus
(Kamensky, 1901)
Common names
گاو ماهي (= gav mahi,
probably in error for Neogobius and related gobies).
[Kur gumlagcisi in Azerbaijan; Kurinskii peskar' or Kura gudgeon
in Russian].
Systematics
Gobio macropterus Kamenskii,
1901 was originally described from the Caucasus. The 14 specimens in the type
series were deposited in the Georgian State Museum,
Zoological Section, Tbilisi (ZMT) and in Kharkov University, Ukraine.
Naseka et al. in Bănărescu (1999) cites types of Gobio macropterus in the Museum of the
Caucasus (Tbilisi, presumably the Georgian State Museum) under numbers
128a (1 fish from Alazan) and 129 (4 fish from Kars-tschai) and 1-2
fish from the Kura (catalogue number unknown) in Khar'kov University. See below
under R. persus for its changing status.
Key characters
The 7 branched dorsal fin rays are characteristic and this is the
only Romanogobio species in the Caspian Sea basin of Iran (see also below
under R. persus). It is separated from the related species in Iran (Gobio gobio) by having the body only
slightly compressed and the caudal peduncle being cylindrical (caudal
peduncle depth at anal fin insertion less than or about equal to
caudal peduncle width) and by faint spots on the dorsal and caudal fins.
Morphology
Sexual dimorphism
Colour
Size
Distribution
Found in the Kura and Aras river basins of Armenia, Azerbaijan and Iran. Abdoli (2000) maps the middle Aras
River and its lower Qara Chai tributary.
Zoogeography
Habitat
Age and growth
Food
The chitinous remains of aquatic insects comprised 30% of the gut
contents from samples in the Kura River basin, caddisflies 21%,
mayflies 14%, chironomids 12%, sevryuga eggs 6%, fish scales 3%, and
much of the remainder was detritus at 20% (Abdurakhmanov, 1962).
Reproduction
Spawning takes place in May in Azerbaijan (Abdurakhmanov, 1962) and
each female may spawn several times in a season. Eggs
number up to 15,840 and are up to 1.62 mm in diameter. Water
temperatures of 12-18°C are recorded for Georgia in April to June
(Naseka et al. in Bănărescu, 1999).
Parasites and predators
Economic
importance
Conservation
Vulnerable in
Turkey (Fricke et al., 2007).
Further work
Sources
?check
Iranian material: CMNFI 1980-0155, 3, 45.9-69.3 mm standard length, Azarbayjan-e Khavari, Qareh
Su near Ardebil (ca. 38º15'N, ca. 48º18'E).
Comparative material: CMNFI 1980-0806, 1, 93.4 mm standard length, Turkey, Kars
Çayi, Kars (40º37'N, 43º05'E); CMNFI 1986-0007, 4, 49.4-84.0 mm standard length,
Turkey, Kars, Kars Çayi north of Kars (41º00'N, 43º00'E).
Romanogobio persus
(Günther, 1899)
Common names
گاو ماهي (= gav mahi,
probably in error for Neogobius and related gobies).
[Persian gudgeon].
Systematics
This species was once thought to belong to the genus Rheogobio Bănărescu,
1961 variously regarded as a synonym of Gobio, a subgenus or a
distinct genus. P. M. Bănărescu (in litt., 1984) and A. Naseka (pers. comm., 1994; in litt.,
1995) place Gobio persus in the subgenus Romanogobius
sensu Bănărescu (1961; 1992a) and Naseka (1996) elevates Romanogobio
to a genus.
A. Naseka (pers. comm., 1994; in litt., 1995; Naseka et
al. in Bănărescu, 1999) has studied R. persus from the Orumiyeh basin and from
the Kars, Kura and Aras rivers of Turkey, Georgia and Azerbaijan. He
distinguished two subspecies, R. p. persus from the Orumiyeh
basin and R. p. macropterus from the Kura River
basin which includes the Kars River in Turkey and the Aras River on
the border of Iran and Azerbaijan. Gobio macropterus Kamensky, 1901 is
now regarded as a
distinct species (Naseka and Freyhof, 2004).
The type series of Gobio persa consists of 7 specimens,
53.1-65.9 mm standard length, in the Natural History Museum, London
from "Ocksa in the Gader Chai" in the description and "Ockra.
NW Persia. Günther" in the jar, the former being more accurate (BM(NH)
1899:30:90-96).
Key characters
The 7 branched dorsal fin rays are characteristic and this is the
only Romanogobio species in the Orumiyeh basin. The number of lateral
line scales is 40 to 42 with modes of 40 or 41 (41 to 45 with modes of 42 and 43
in R. macropterus), total vertebrae are 37 to 40 with modes of 38 and 39
(38 to 42 with modes of 40 and 41), and the connection between the supraorbital and infraorbital head canals
is usually absent (present). The Orumiyeh fish also have a shorter caudal peduncle, a shorter
snout, a shorter barbel, and a longer predorsal distance.
It is separated from the related species in Iran (Gobio gobio) by having the body only
slightly compressed and the caudal peduncle being cylindrical (caudal
peduncle depth at anal fin insertion less than or about equal to
caudal peduncle width) and by faint spots on the dorsal and caudal fins.
Morphology
The barbel is broad and fleshy, the mouth subterminal and
horseshoe-shaped with thick, papillose lips. The lower head surface
between the jaws and the sides of the head are papillose in a mature female.
Dorsal fin with 3, rarely 4, unbranched and 7, rarely 8, branched
rays, anal fin with 3, rarely 2, unbranched and 5-7, usually 6,
branched rays, pectoral fin branched rays 11-16, and pelvic fin
branched rays 6-8. Lateral line scales 39-45. A pelvic axillary scale
is present. The throat, breast and anterior belly are naked. Dorsal
scales bear epithelial keels, usually one central keel and one to
several lateral ones. Scales have a very anterior focus and few
posterior radii. Gill rakers 0-6, small and irregularly spaced.
Vertebrae 36-42. Pharyngeal teeth 2,5-5,2 or 3,5-5,3, or more rarely
2,5-5,1, 2,5-5,3, 2,4-5,3, 2,4-6,3, and 3,5-5,2. Teeth are strongly
hooked at the tip, broadly concave or flattened below the tip, the
surface sloping medially. The gut is s-shaped with a slight anterior
loop to the left. The anus lies between the pelvic fins, remote from
the anal fin origin. The karyotype is 2n=50.
Meristic counts in Iranian fish are: dorsal fin branched rays
7(22); anal fin branched rays 6(21) or 7(1); pectoral fin branched
rays 11(1), 12(1), 13(6), 14(7), 15(6) or 16(1); pelvic fin branched
rays 7(22); lateral line scales 39(3), 40(7), 41(3), 42(2) or 43(2);
pharyngeal teeth 3,5-5,3(5); and total vertebrae 37(1), 38(7), 39(10),
40(4), 42(1).
Sexual dimorphism
Males and females bear irregular-shaped, elongate tubercles on the
head. In a female specimen (and presumably males too), the pectoral
and pelvic fin rays and adjacent membranes bear tubercles both
dorsally and ventrally although the latter are less well developed.
Males have longer pectoral and pelvic fins than females, reaching the
pelvic fin and anal fin origin respectively in males. The snout to
anus distance is more than half body length in females and about equal
in males, head width is less than head depth at nape in females and
equal in males, and snout length is longer than postorbital distance
in females and about equal in males.
Colour
The upper flank is a light yellow-grey to brown with each scale
outlined with, or partially filled in with, dark pigment, fading to a
yellowish colour below. The mid-flank has 6-12 elongate to rectangular
black spots. The lateral line pores may have small dark spots above
and below reminiscent of Alburnoides species. The back also
bears vague dark spots. Dorsal, caudal and pectoral fins bear up to 5
thin to broad discontinuous bars, the pigment being on the rays.
Scales above the lateral line in mature males have longitudinal
streaks. Peritoneum silvery with scattered melanophores.
Size
Reaches ?
Distribution
Found in the Lake Orumiyeh basin (Günther, 1899). Abdoli (2000) maps the middle and lower Talkheh, lower Tatavi and
Zarrineh rivers in the Lake Orumiyeh basin.
Zoogeography
This species is part of a gobionine fauna found across Eurasia and this wide
distribution may be suggestive of further work that could be done to clarify relationships of these fishes.
Habitat
Presumed to be similar to its relative.
Age and growth
Presumed to be similar to its relative.
Food
The principal food is aquatic insects and crustaceans but detritus and vegetation are
also taken and presumably, though rarely, the eggs and fry of other fishes. Abdoli (2000)
lists Trichoptera, Ephemeroptera and Chironomidae as food items.
Reproduction
Presumed to be similar to its related species. An Iranian specimen had
relatively large eggs (1.2 mm) when captured on 23 June.
Parasites and predators
None reported from Iran.
Economic importance
None, although the recorded habit of feeding on fish eggs by its relative may
be true of this species and
make it important in conservation of other species.
Conservation
Endemic to the Lake Orumiyeh basin where few specimens have been caught and
deposited in museums. The numbers of this species in the wild are unknown.
Further work
The biology of this species, and its conservation status, have not been thoroughly investigated.
Sources
check?
Type material: See above, BM(NH) 1899:30:90-96.
Iranian material: CMNFI 2007-0101, 12, 28.8-64.3 mm standard length, Azarbayjan-e Bakhtari, Tat'u River
(ca. 36º54'N, ca. 46º07'E).
Genus Rutilus
Rafinesque, 1820
The roaches are found in Europe and western Asia where there are
about 15 species (Bogutskaya and Iliadou, 2006). Three species are found in Iran.
The genus is characterised by having pharyngeal teeth in one row,
usually 6-5, more rarely 6-6 or 5-5, with conical crowns on the
anterior teeth and posterior teeth slightly hooked and truncated,
scales are large to moderate in size, numbering 33-68 and with
numerous fine circuli and radii on all fields, few and short gill
rakers (17 or less), short gut, usually a light peritoneum, few to
moderate numbers of dorsal and anal fin rays (7-13), the dorsal fin commonly
having 4-5 unbranched rays, abdomen behind
the pelvic fins rounded or with a slight but scaled keel, and various
osteological characters (Bogutskaya and Iliadou, 2006).
Rutilus caspicus
(Yakovlev, 1870)
?
Caspian Sea
species, R. rutilus freshwater residenth
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish.
Rutilus kutum
Kamenskii, 1901
Common names
ماهي سفيد (= mahi safid or safid mahi, meaning white fish),
sifid mahyi and asbalan mahi (in Gilaki), mahi safid daryacheh khazar or mahisephid-e-daryaye khazar (= Caspian Sea white fish), talaji (in
Mazanderani).
[kutum, ak-balyk (= white fish) or ziyad in Azerbaijanian; akbalyk or kutum in Turlmenian; kutum in Russian; Southern Caspian roach; pearl roach;
Caspian kutum].
Systematics
Leuciscus Frisii was originally described from the market in
Odessa and the Danube, Dniester, South Bug, Dnieper and Don rivers,
draining to the Black Sea.
Rework?
Rutilus frisii kutum Kamenskii, 1901 is the generally
accepted Caspian Sea basin subspecies although J. Holčík
(pers. comm., 1994) considers that this is not a good taxon (see also Holčík and Jedlička,
1994). It was originally described as Leuciscus Frisii
var. kutum from the Caspian Sea, essentially in the southern
part, spawning in the rivers and streams of Transcaucasia and Persia (Kura,
Araxes streams of the Lenkoran district) and in lesser numbers
elsewhere. This subspecies is distinguished from the type subspecies
from the Black Sea by having fewer lateral line scales (about 55-58
versus about 60-66), shallower body (depth equal to or less than head
length versus exceeding head length), anal fin longer than high versus
shorter than high, lower lobe of caudal fin usually shorter than head
versus longer (young R. f. kutum have this lobe as long as
head), and dorsal fin as high as long versus higher than long (young R.
f. kutum have dorsal fin higher than long).
Kotlík et al. (2008) discuss
divergence and gene flow between Black and Caspian Sea populations, the majority
of the migrations occurring during the Pleistocene (ca. 10,000-1,800,000 years
ago). The refugial populations in the Black and Caspian seas have diverged
despite periods of migrations between them. Kavan et al. (2009)
investigated the population genetic structure of Iranian and Azerbaijani fish
using microsatellite markers. Most of the variation was within rather than
between locations. Abdolhay et al. (2010) found independent populations
existed in the Lemir, Safid, Shirud and Tajan rivers based on morphometric data.
This has implications for the extensive stocking programmes for this species.
Leuciscus frisii caspius Lönnberg, 1900 described
"from the Volga delta" has priority over Rutilus frisii
kutum which may be a nomen nudum as it is listed in Radde (1899)
as "Leuciscus Frisii Nordm. var. Kutum Kam."
without a description, the description only appearing in Kamenskii
(1901). However, the name caspius has not been used while kutum
appears widely in the literature as well as being the common Russian
name of the fish. Additionally, as mentioned above, there may be no
need of a subspecific name. Kottelat (1997) considers kutum to
be a distinct species as do Bogutskaya and Iliadou (2006) and Fricke et al.
(2007).
Leuciscus friesii Kessler, 1870 from the Volga delta is presumably a misspelling.
Ghasemi et al. (2009) found the spring and
autumn races of this species to be independent populations using microsatellite
markers.
A hybrid of this species and Ctenopharyngodon idella has
been bred at the Astaneh Ashrafie Fisheries Research Station (Sefidrud
Research Station) and named "Samur" (Iranian Fisheries
Research and Training Organization Newsletter, Tehran, 11:6, 1996;
18:6, 1997; Khara et al., 2002; Nouruz Fashkhami et al., 2002). Gynogenesis may have
occurred. Artificial hybrids with Rutilus rutilus (possibly including
R. caspicus) and Abramis
brama have been bred in Iran (Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 39-40, 1996).
Key characters
This species is distinguished from the related Rutilus rutilus
and R. caspicus
by the higher scale count (usually >50) and the posterior part of
the swimbladder being elongate and conical or pointed rather than rounded.
Morphology
Dorsal fin with 3 unbranched and 8-10, usually 9, branched rays,
anal fin with 3 unbranched and 9-12, usually 10, branched rays,
pectoral fin branched rays 16-19 and pelvic branched rays 8-9. Lateral
line scales 47-68, mostly 55-58. Scales are regularly arranged over
the body. A pelvic axillary scale is present. Scales have numerous
circuli, numerous posterior radii, few but distinctively crowded
anterior radii (more than in Rutilus rutilus and R. caspicus) and an almost
central focus which is broken up into a network of lines. The
posterior scale margin is crenulate and the anterior margin is wavy to
crenulate but scale margins vary greatly between individual scales.
Total gill rakers number 7-12 and are very short, hardly reaching the
one below when appressed. Pharyngeal teeth usually 6-5, crowns rounded
above a slender stalk, posterior teeth with a weakly hooked tip,
posteriormost tooth margin may be serrated. The gut is an elongate
s-shape. Chromosome number 2n=50 (Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 43, 1996;
Noruz Fashkhani and Kosroshahi, 1995; Klinkhardt et al., 1995).
Adibmoradi and Sheibani (2002) carried out a histological study of the brain of
this species and concluded, in part, that vision was sharp and the fish were
good chasers of food.
Meristic values for Iranian specimens are:- dorsal fin branched
rays 8(1), 9(58) or 10(1); anal fin branched rays 9(24), 10(35) or
12(1); pectoral fin branched rays 16(15), 17(30), 18(14) or 19(1);
pelvic fin branched rays 8(49) or 9(11); lateral line scales 47(1),
49(1), 50(1), 51(1), 52(7), 53(8), 54(6), 55(5), 56(11), 57(7), 58(7)
or 59(5); total gill rakers 8(1), 9(21), 10(25) or 11(13); pharyngeal teeth
6-5(23), 5-5(1) or 5-4(1); and total vertebrae 40(1), 41(7), 42(34), 43(3) or 44(2).
Sexual dimorphism
Females are larger than males. The breeding tubercles are evident in males
and may develop as early as the end of summer before the spring spawning season in the
following year. Large tubercles are found on the top of the head above
the eye level, behind the eye, between the eye and the mouth and on
the snout as well as on upper flank scales. Tubercles are white in
contrast to the dark head. Imanpour and Shirmohammadli (2008) found that fish
from the Gorgan River showed correlations between increase in number of
tubercles and total length, sperm volume, and gonad weight but not with sperm
motility, spermatocrit, sperm density and gonadosomatic index.
Colour
Small specimens up to a year old are silvery on the flanks and
belly and the back is steel-grey to pale brown or pale olive. In
adults, back scales are circled with black and there is a strong
contrast between the back and flank. The anterior part of each flank
scale, particularly those of the lateral line, is darkly pigmented.
Lateral line scales may have two dots, one above and one below the
opening as in some Alburnoides spp. but not as pronounced.
The sides of the head are silvery with some yellow and darker pigment,
the latter particularly in front of the eye. Adults are a bright
silvery on the flank. The belly is pearly-white. The iris is silvery,
with some spots, and with a marked dark spot above. The dorsal and
caudal fins have some grey and a faint orange tint while the pectoral,
pelvic and anal fins are colourless to lightly pigmented with black.
The pectoral fin may be orange.
Size
Attains 66 cm body length and 4.065 kg in Iran (Farid-Pak, 1968a).
Ouseley (1819-1823) ate one almost 3 feet long (ca. 0.9 m). In Iran
during the 1950s, catches were 36-67 cm long (Farid-Pak, no date).
Reaches 7.0 kg in weight.
Distribution
Found in drainages of the Caspian Sea. In Iran, it is found along the
whole Caspian coast, entering almost all the rivers to spawn including Atrak,
Gorgan, Gharasu, Tajan, Babol, Haraz, Sardab, Aras, Tonekabon, Pol-e Rud and
Safid rivers, the Anzali Talab, Gorgan Bay, and in the southeast, southwest and
south-central Caspian Sea (Nedoshivin and Il'in, 1929; Kozhin, 1957; Holčík and Oláh, 1992; Riazi,
1996; Oryan et al., 1998; Abbasi et al., 1999; Kiabi et al., 1999; Abdoli, 2000;
Lasheidani et al., 2008; Abdoli and Naderi, 2009).
It is also found in Valasht Lake, Mazandaran (landlocked and introduced about 1850 to provide food for
the royal family which had a summer residence there) where it possibly
hybridises with native Alburnus chalcoides (Armantrout, 1980).
von den Driesch and Dockner (2002) record this species as faunal
remains in an excavation at the Great Mosque in medieval Siraf on the Persian
Gulf coast. This may well be a misidentification but, if not, indicates that a
fondness for this species has a long history considering that a preserved fish
or its remains had to be transported all the way from the Caspian Sea.
Zoogeography
A European and western Asian species with its origins in a Danubian or Sarmatian fauna.
Habitat
This species is migratory, spawning in rivers in March-April and
returning to the Caspian Sea. Rezavi (1997) found three populations in Iran, one
autumn and two spring populations. Il'in (1927a) records schools of fish entering the
Anzali Bay as numbering in the several tens of thousands. On one fishing ground
next to the Djifrud in February 1914, 12,000 fish were taken simultaneously. However, while the majority of fish
migrate into lowland rivers not far from the sea to spawn among
bulrushes and cattails, some migrate far upstream into the mountains
of Tavalesh (= Talish) and Gilan where spawning conditions are very
different. These fish may well overwinter in the river (Derzhavin,
1934). Such fish reached altitudes of about 1000 m before
environmental changes inhibited the migration. Young fish migrate
downstream, the migration ending in August, and in Azerbaijan enter
the sea within 20-50 days. Holčík (1994; 1995) stated that Iranian
young from the Anzali Mordab never entered the sea but remained in
fresh or brackish water for 1-2 years. Riazi (1996) reports that this
species migrates into the Siah-Keshim Protected Region of the Anzali
Mordab. Young fish descend the Atrak River and feed in the sea at
depths of 4.0-8.5 m. They winter in Iranian waters (Savenkova, 1985).
Some fish are reported at depths of 36.6-53.0 m in the Iranian Caspian
Sea (Knipovich, 1921).
Age and growth
Azari Takami et al. (1990) described the biology of this
species in Iran. Males normally mature between their third and fourth
year, sometimes earlier, females during their fourth year. Life span
is at least 9 years in Dagestan (Shikhshabekov, 1979) and 8 years in
Iran (Holčík and Oláh, 1992) and maximally about 12 years (Afraei
Bandpei et al., 2010). Spawners are 3-8 years old and the principal age
groups are 4-5 years for males and 5-6 years for females in the Anzali
Mordab (Holčík and Oláh, 1992; Holčík, 1995), older than the 3-4 years of fish reported in 1970-1971.
However, recently males are maturing at age 2 and females at age 4
with most spawners at age 3 and 4 years respectively (Holčík
and Oláh, 1992). The average size of mature females (700 g) has been
decreasing (Bartley and Rana, 1998b). Males grow faster than females
until about the third year of life and then more slowly. Males are
smaller and have a shorter life span than females (Holčík and Oláh, 1992).
An annual increase in length of 17% over the first
four years was observed in tagged fish, decreasing to 4.7% annually in
subsequent years (for weight the figures are 61.2% and 25.7%). Sex
ratios in spring were 3.08 males:1 female and in autumn 0.78 males:1
female (Annual Report, 1995-1996, Iranian Fisheries Research and
Training Organization, Tehran, p. 55, 1997).
A series of studies on this species, apparently
sampling in somewhat different areas or from different commercial fish catches
give several growth parameters as follows.
Abdolmaleki and
Ghaninejad (2007) examined the commercial catch in 2003 -2004 and found mean
fork length was 36.7 cm (range 21-69 cm) and mean age was 3.82 years (range 1-8
years). Age groups 3-5 years comprised 87% of the total catch age composition.
von Bertalanffy growth parameters were L∞
= 70.1 cm, K = 0.138/year, t0 = -1.557 years, total mortality =
1.1/year, natural mortality 0.28/year and fishing mortality 0.83/year.
Abdolmaleki et al. (2007) sampling the commercial catch found the total
catch of kutum, including poaching, was 6612.5 t of 43.3% of all commercial bony
fishes in the 2004-2005 season. Mean fork length was 38.2 cm, age range was 1-8
years and mean age was 4.2 years. Age groups 3-5 years made up 85.5% of the age
composition. von Bertanffy growth parameters were
L∞ = 60.7, K = 0.15 year-1, t0
= -1.75 years. Instantaneous total mortality coefficient (Z) was estimated as
0.88 year-1, instantaneous natural mortality coefficient (M) was 0.31
year-1, and annual fishing mortality coefficient (F) was 0.52 year-1.
Growth rate has decreased in comparison to previous years probably due to
non-selective artificial breeding over more than 20 years.
Afraei Bandpei et al. (2008) surveyed biological characteristics in
samples from Iranian coastal waters. Average fish size was 11.73 cm fork length,
range 8.9-29.0 cm, average weight was 22.74 g and range 8.8-321.2 g. Females
were larger than males (12.27 cm and 32.26 g on average compared to 11.03 cm
and17.07 g). Sex ratio was 1.3:1 male:female. Overall condition factor was 1.23.
Afraei Bandpei et al. (2010) also recorded features of fish from Iranian
coastal waters but found a range in size of 21 to 58 cm and 148 to 2450 g,
average fish size was 39.0 cm fork length and 830.3 g for females and 38.2
cm and 719.1 g for males, sex ratio was 0.65:1 in favour of females, and
condition factor varied from 1.2 to 1.3. The von Bertalanffy growth parameters
were L∞ = 63.00, K = 0.21 year-1, t0 = -0.88
years for both sexes,
L∞ =
62.03, K = 0.21 year-1, t0 = -0.80 years for
females, and L∞ = 54.52, K = 0.27 year-1, t0 =
-0.75 years. the growth performance index was 2.89 for both sexes. Life span was
9 years in females ands 8 years in males with a mean age of 3.99 years overall
and four-year-old fish dominating in the sample. The length-weight relationship
value b was 3.02 indicating growth is isometric. Growth was seen to be
rapid in this southern Caspian Sea population, attributed to warm temperatures,
available food resources and the brackish water environment. Historically there
has been a decrease in size of fish over three decades as length and weight used
to be 67 cm and 7 kg. Afraei Bandpei et al. (2010), in another study,
investigated the population dynamics in Iranian fish during the fishing season
October to April, and found the von Bertalanffy growth parameters to be
L∞
= 59.85 cm fork length (FL), K = 0.27 year-1, C = 0.25, WP (winter
point) = 0.40, instantaneous total mortality coefficient (Z) was estimated as
1.28 year-1, instantaneous natural mortality coefficient (M) was 0.46
year-1, instantaneous fishing mortality coefficient (F) was 0.82 year-1,
current exploitation rate (E) was 0.64, mean length at first capture (Lc)
was 36.8 cm FL, maximum exploitation rate (Emax) was 0.76, and the
current exploitation rate (E) was less than
Emax.
The
highest growth oscillation occurred in December and was attributed to active
feeding before wintering and migration to deeper waters. These figures indicate
that the fish population is moderately exploited and agree in general with other
studies in the Caspian Sea. Variations in growth performance could be due to
food availability, ecological conditions, geographical changes and genetic
variability.
Fish may spend 1 or 2 years in fresh water after hatching
(Holčík and Oláh, 1992) and this affects growth, 1 year fish growing more
quickly than those spending 2 years in fresh water, maturing earlier
and having a shorter lifespan. Growth in the Anzali Mordab is slower
than it was 20 years prior to the report of Holčík
and Oláh (1992), probably owing to a higher population density and
more competition for food resources. The commercial catch in Iran was
3-7 years old, 39.0-57.1 cm long and weighed 613-2525 g (Razivi et al., 1972).
Growth in Iranian fish farms is up to 1.5 g in 8 weeks after a hatching rate of 75% (Aslaanparviz, 1994).
Food
Zarbalieva (1987) provides data on feeding of this species in the
Caspian Sea off Azerbaijan. Fish concentrate on a sandy-shellrock
bottom and remain there for most of the year to feed. The crab Rhithropanopeus
harrisii dominates in the diet, 67.9-93.7% by weight. Molluscs,
mainly Cerastoderma lamarckii, comprise 30% by weight of the food
of fish 30-40 cm long. Fish larger than 40 cm seldom take molluscs but
occasionally Clupeonella spp. Molluscs used to be the main diet
item of this fish. Juveniles in the Anzali Mordab of Iran feed mostly
on phytoplankton in contrast to the zooplankton reported for
Azerbaijan fish (Holčík, 1995). This is a consequence of the poor productivity of this lagoon.
Small Iranian specimens from the Caspian Sea had bivalve shell
remains, plant remains and in one case a worm. Oryan et al.
(1998) state that Cardium is the main food of this species on
the eastern and western coasts of Bandar Anzali. Crabs and Balanus
are also important. The hepatosomatic index is highest in February and
March, the prespawning period. Afraei Bandpei et al. (2008) found diet in
the southern Caspian Sea was Mytilaster (48.46%), Gastropoda (31.84%),
Cerastoderma (19.65%) and Balanus (0.05%).Afraei Bandpei et al.
(2009) sampled fish from the commercial catch caught from October to April and
found the diet was dominated by bivalves (59%) with Cerastoderma lamarcki
the dominant prey (57%).Other foods were cirripeds (21%), gastropods (13%),
malacostracans (3%), fish eggs (2%), amphipods (1%) and filamentous algae (1%).
Fish fed on a wider variety of food groups in November compared to other months
and the lowest feeding activity was in January and April, the latter being the
peak of the spawning season.
The hybrid with Ctenopharyngodon idella fed principally on macrophytes
with phytoplankton as a secondary food in pond sin Gilan (Khara et al., 2002).
Reproduction
Fish spawn annually according to an Iranian study by Azari Takami et
al. (1990) and probably return to their river of birth.
Temperature, and probably river flow, are the factors determining the
entrance of fish into the rivers on the spawning migration. Male fish
run before females. Adeli Mosabbab and Piri (2005) report spawning in
Iranian rivers from the end of March to the middle of April, beginning at 10°C. A large female, 60 cm long, 3.24 kg and 7 years
old contained 124,712 eggs but some large females may have over
250,000 eggs (see also Farid-Pak (1968a) where fish 38-40 cm long have
a mean value of 53,900 eggs, ranging up to 174,400 for fish 62-64 cm
long; Yousefian and Mosavi (2008) give March to May as the spawning season.
Aminian Fatideh et al. (2008, 2009) determined stages of sexual maturity of
migrating fish, 87% achieving stage V in April and 94% stage V in June. Abdurakhmanov (1962) gives a fecundity of 290,000 eggs for fish
in Azerbaijan). Maximum egg diameter is 2.0 mm (Farid-Pak, 1968a).
There are two spawning migrations, winter and spring, and so two forms
or stocks of this species (Azari Takami et al., 1990). There
may be three stocks based on electrophoretic studies of blood proteins
associated with the two spawning migrations (Annual Report,
1994-1995, Iranian Fisheries Research and Training Organization,
Tehran, p. 41, 1996). The winter form enters rivers with emergent
or submerged aquatic plants at the end of autumn or the beginning of
winter. Eggs are deposited on these plants. The principal rivers were
the Nahang Roga, Pir Bazar Roga, Sowsar Roga, and Anzali Roga (all draining
the Anzali Mordab) but the stock has declined with overfishing and
destruction of the habitat. The Safid River is listed as the main spawning
ground by the Caspian Sea Biodiversity Database (www.caspianenvironment.org).
The spring form enters rivers at the end
of winter or the beginning of spring and spawns on gravel or sand.
Adults return downstream after spawning, in the early morning. The run
occurs both by day and night but increasing water clarity near the end
of the spawning season limits runs to the night.
The migration into the "Dinachal" (? = Denya Chal) River
occurred on 1 November in 1975, when sexually immature fish were
caught. By 11 February, 5 spawners were caught and the peak migration
occurred on 26 March when 3,845 fish were caught at a water temperature of 10.5°C.
The migration was continuous until 11 May. Water temperatures between
28 March and 23 April in 1976 and 1977 varied between 9.1°C and 13.5°C
when peak migrations occurred in the "Havyg" (= ? Haviq)
River. Here the spring form of this species started to migrate at 6°C
and the optimum water temperature appears to be between 11 and 13°C. Yousefian
and Mosavi (2008) found a spawning temperature range of 5 to 21°C centred on
11°C. Fertilisation rate in 4 rivers was 75-92% and hatchability was high.
Rivers with high turbidity had low hatchability because of egg damage. Further
details of natural and artificial reproduction are given by these two authors.
The main migration (99%) in the Anzali Mordab began in February and
lasted 3 months at 8-10°C with a much smaller run (only about 1%) in November-December. The
Mordab is now overgrown with reeds or has a silty, vegetated bottom
and is no longer a spawning site (Holčík and Oláh, 1992), an economic and poetic loss.
Holčík and Oláh (1992) translate Il'in (1927a) on Anzali Mordab mahi safid
"During this time the reeds rustle as if of wind as the fish rubs
pushing their flanks to the reed stalks or pushing forward among reeds
standing in rows".
Khaval (1998) reports a spawning migration into the Safid River
despite construction, sand removal and pollution. The migration peaks
in the second half of March at a water temperature of 11°C.
Each female is flanked by two males at spawning in shallow water.
Sometimes the backs of the fish protrude from the water. Females
sharply rub their lower abdomen and pectoral area on the gravel
bottom, males and females convulse, and eggs are shed and fertilised.
Eggs are adhesive and hatch in 20 days at 10-14°C.
Embryonic development in glass incubators at 14-16°C
is described by Parivar et al. (1993). Cleavage and
gastrulation begin within 24-30 hours, hatching at 216 hours (10 days
after fertilisation) and resorption of yolk is complete at day 16.
Some males and females are injured through contact with the gravel
stream bed. Embryonic development takes 10-15 days at 8-16°C
and 5-6 days at 20°C (Aslaanparviz, 1994). Young fish go down to the sea in summer
according to some works although Holčík and Oláh (1992) believe that they stay in fresh water or brackish
river mouths for 1-2 years before entering the sea.
Kousha et al. (2007) examined some aspects of reproductive
endocrinology in this species, specifically on sex steroid-binding protein in plasma.
Najafipour et al. (2008) examined 17-αhydroxy progesterone hormone
levels, egg diameters and liver weight in fish caught at sea and in the Chalvand
River near Astara. These factors were used to indicate physiological indices of
reproduction, hormone levels and egg diameters increasing in fish caught in the
river, while liver weight decreased. Egg diameters increased from 1.55 to 2.14
mm.
Sabet et al. (2009) studied gonad steroid levels in fish from the Safid
River and found estradiol and testosterone reached their highest levels in April
and were closely correlated to ovarian development and the gonadosomatic index.
Parasites and predators
Mokhayer (1976b) records the digenetic trematodes Aspidogaster
limacoides and Asymphylodora macracetabulum. Eslami and
Kohneshahri (1978) report various helminths from this species in
Iranian waters. These are the monogenean Diplozoon paradoxum,
the aspidogastrean Aspidogaster limacoides, the digenean Asymphylodora
kubanicum and larvae of the nematode Anisakis sp. This fish
therefore is potentially a source for human infection with Anisakis.
Mokhayer (1989) reports metacercariae of the eye fluke, Diplostomum
spathaceum from this species in Iran, which can cause complete
blindness and death in commercially important species. Jalali and
Molnár (1990a) record the monogeneans Dactylogyrus frisii from
this species in the Khosk (= ? Khoshk) River (Caspian basin) and D.
rarissimus and two monogenean species, Dactylogyrus spp.,
from the Safid Rud. Jalali and Molnár (1990b) record the monogeneans Dactylogyrus
frisii and D. rarissimus at fish farms in Iran. Molnár and
Jalali (1992) report the monogeneans Dactylogyrus suecicus, D.
haplogonus and D. turaliensis from this species in the
Safid Rud. Gussev et al. (1993b) record the monogenean Dactylogyrus
haplogonus from this species in the Safid Rud. Masoumian and Pazooki (1998)
surveyed myxosporeans in this species in Gilan and Mazandaran provinces, finding
Myxobolus bramae. Safari and Khandagi
(1999) record Clostridium botulinum from 2.2% of fresh and
smoked samples of this species in Mazandaran Province.
Shamsi and Jalali (2001b; 2001c) detail monogenean parasites for Safid Rud
and Caspian Sea samples, including 6 species of Dactylogyrus.
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. prostae and G. sp. in fish from the Safid River.
Khara et al. (2008) found the eye parasite Diplostomum spathaceum
in this fish from Boojagh Kiashar Wetland in Gilan.
Barzegar et al.
(2008) also record the digenean eye parasite Diplostomum spathaceum from
this fish.
Amini (2006) records Gyrodactylus sp., nematodes,
Diplostomum sp., Trichodina sp., Aeromonas sp. and
Hydrophila sp. in fingerlings from hatcheries in Iran. The latter two
parasites caused a heavy mortality in the Shahid Rajaii hatchery ponds.
Tavassoli and Moghir (20020 describe a squamous cell carcinoma in the oral
cavity of this species, the first record for the Caspian Sea.
Economic importance
Safid mahi is the most popular fish in Iran with the highest
economic value (Azari Takami et al., 1990) and Afraei Bandpei et al.
(2008) record 10,773 fishermen making a living from its capture. More than 70% of bony fish
caught in the coastal Caspian Sea of Iran are this fish (Yousefian and Mosavi,
2008) or 78% and 76.6% of the income The average annual catch from 1991 to 2001
was about 9600 t
(Afraei Bandpei
et al., 2010).
It may be available out of
season as mahi qachaq or bootleg fish, an indication of its popularity. Batmanglij (1999)
notes that this fish is baked and served with herbed rice. A stuffing
consists of garlic, parsley, tarragon, scallions, coriander, mint,
ground walnuts, barberries, raisins, lime juice, salt and pepper,
sautéed in butter (he also incorrectly refers to mahi-e safid as
striped bass). In 1996 a news report stated that "In the port city
of Anzali, one small white fish sells for $10" (http://www.iran-e-azad.org/english/boi/03400201.96).
In Rasht, 60% of the inhabitants consume no other fish and "86%
of ladies in Rasht can only cook one kind of seafood" (Khairkhah,
1994). Ouseley (1819-1823) reported that this species "seemed
most abundant, and was found in all the great rivers of this country
near the sea; for several days it had furnished the principal dish of
my dinners and often of my breakfasts". Holmes (1845) recorded
catches with cast-nets in the Anzali Mordab over 150 years ago which
were even then worth £1400-1500. O'Donovan (1882) reported that a small stream
in the Atrak River drainage was so crowded with this species that individuals
could only move by floundering and jumping over one another, and the horses in
crossing the stream, trod them to death by scores. Lönnberg (1900) reported an annual
catch of some millions in Persian waters.
It is caught in rivers and lagoons and by large, mechanically hauled beach nets in the Caspian
Sea. Sea nets can be 1500 m long and 18 m deep. The fishing season
begins in October and reaches a maximum between 20 February and 10
March, ready for the "Now Ruz" or New Year celebrations when
many Iranians eat this fish with rice (Emadi, 1979).
The roe of this species is also eaten, salted or unsalted (the fish are
called asbalan mahi and the roe shur-e asbal in Gilaki, the local dialect of
Gilan). Whole female fish are soaked for one year in a mixture of salt and
madder in special clay jars. The jars are traditionally buried in the ground and
hermetically sealed but a modern technique has the roe removed from the fish
after 20 days. Esmaeilzadeh et al. (2004) studied the nutrient
composition and marinade qualities of this fish and compared them to those for
grass carp (Ctenopharyngodon idella), the latter being preferable
according to the organoleptic properties. The marinades could be stored for 6 months at 10ºC.
Alipour et al. (2009) studied teh effects of different brine
concentrations and temperatures in traditional smoking of this fish, finding a
brine concentration of 26% gave the better quality and taste.
The average weight of fish caught in the Anzali Mordab in the early years of this
century was 2 kg, the catch in the 1914-1915 season was 47,000 fish
and in 1913-1915 123,000 fish. On 26 February 1914 a single haul in
the sea near Anzali took 41,045 fish (Il'in, 1927a; Berg, 1948-1949). Il'in
(1927a) estimated a yearly catch in Anzali Bay to be 3-4 million fish, a figure
conflicting with these other reports.
Nevraev (1929) gives catches for various fishing regions in Iran in
the early twentieth century. For the period 1901-1902 to 1913-1914 the
catch in the Astara region was 0 to 29,053 individuals, for 1901-1902
to 1917-1918 the catch in the Anzali region was 2565 to 124,195
individuals, in the Safid River region from 1904-1905 to 1917-1918 the
catch was 100 to 31,799 individuals, and in Astrabad (= Gorgan) region
from 1900-1901 to 1912-1913 the catch was 4000 to 323,500 individuals.
The total catch for Iran in the 1914-1915 season was 443,000 fish. The
catch in Iran from 1956/1957 to 1961/1962 varied from 197,884 kg to
2,066,580 kg (Vladykov, 1964), from 1965/66 to 1968/69 it varied
between 159 and 1252 tonnes (Andersskog, 1970), from 1963/64 to
1968/69 it varied between 121.3 and 1252 t (RaLonde and Walczak,
1970b; 1972), from 1987 to 1991 it varied between 3500 t and
8855 t (Holčík and Oláh, 1992), and between 1989 and 1998 it varied
between 11,792 kg and 14,336 kg (Caspian Environmental Programme, 2001a) - catch
figures are at variance with each other. Holčík and Oláh (1992) report a catch of 3107 kg in the Anzali Mordab for
1990 and for 1932-1964 a range of 95.1-3488.9 t. They are also
caught in rogas and inflowing rivers of the mordab in late winter and
early spring. Moghim et al. (1994) estimate that coastal areas
of the southern Caspian Sea have a total biomass of 24,000 t with
a maximum sustainable yield of 7000 t. In 1993-1994 the total
catch of this species, including the illegal catch, was 11,175 t with
the total stock estimated at 25,400 t and a maximum sustainable yield
of 9300 t. More than 25% of the catch was young fish indicative of
non-standard methods being used (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 19-20, 1997).
In 1994-1995, the biomass of this species in Iran was 241,000 t (sic,
probably 24,000 t) and the maximum sustainable yield was 9000 t (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 37, 1996). About 62% of the bony fish
catch in the Caspian Sea of Iran in 1993-1994 was this species with
the mullet Liza aurata second at 22% (Annual Bulletin
1993-94, Iranian Fisheries Research and Training Organization, Tehran, p. 83, 1995).
The catch from beach seine cooperatives along the Iranian coast was 8477 t in
in 2004-2005, a 2500 t decrease over the previous year, and 45.5% of the total
bony fish catch. Catch per unit effort was 93.3kg/set. The biomass of this
species in Iranian coastal waters for 2003-2004 was estimated at about 25,000
t (Abdolmalaki, 2006a;
Abdolmaleki and
Ghaninezhad, 2007)).
Bartley and Rana (1998a; 1998b) comment that the fishery collapsed
in 1980 but has risen from 500 tonnes in 1981 to around 10,000 t in
1996 after restocking from around 400,000 fingerlings/year in 1981 to
around 142 million/year in 1997. Rana and Bartley (1998a) note that 7
million 1 g fingerlings were released into the Caspian Sea in 1997
which contradicts their earlier report. There is only a state
supported stocking programme but ERM-Lahmeyer International GmbH, DHI Water & Environment and GOPA
Consultants (2001a) note that this fish is being successfully managed in Iran
while it has been fished almost to extinction in waters off Daghestan and Azerbaijan.
Market price is high at about U.S.$5.00/kg, five times the price for silver carp. Export prices are higher,
700 g of smoked "mahi-sefid" cost £21.00 in 2004
(www.superhormuz.com, downloaded 19 January 2004). However this
species is difficult to culture beyond the 40-50 g stage and growth is slower than carp.
The conflicting ranges seen in Andersskog and RaLonde and Walczak
are typical of the wide variations in reports; figures can only be
taken as general guides for many Iranian fisheries. Abdolmalaki (2006b) states
that fluctuations are due to destruction of spawning grounds, overfishing and
release of fingerlings. The mean catch sizes were 3110 tonnes in 1937-1947, 990
t in 1967-1977 and 8505 t in 1987-1997. A minimum catch of 121 t was taken in
1964 and a maximum of 11,175 t in 1994. Catch-per-unit-effort also showed high
variation,. The calculated stock biomass was 1300 t in 1071 and between
18,489 and 25,400 t between 1990 and 2000. The mean biomass in the past 10 years
was 22,750 t, a 17-fold increase over the year 1971. The catch in the previous
10 years was 35-46% of the annual stock. A decrease in stock size during
1998-1999 was attributed to an exploitation rate being more than maximum
sustainable yield, a decreased mean weight of released fingerlings and a lowered
return rate.
Catches in the Bandar-e Anzali area have been as high as 5,480 t in
1939-1940 but fell to 85 t in 1961-1962 (Vladykov, 1964; RaLonde and
Walczak, 1972) but have risen again as indicated above. This is
attributed to a massive stocking effort with 170 million fingerlings
released in Iran in 1991 (cf. Emadi (1993a) below), about a quarter in
the Anzali Mordab (Holčík and Oláh, 1992). The Sari hatchery produced 400 million "white
fish" over the previous 10 years (Tehran Times, 30 May 1998).
Fingerling production in 1996 was 142.1 million (Bartley and Sana,
1998a). Sea ranching increased the yearly catch to 8500 t in
1991, the highest recorded catch in the past being 5850 t in 1940
(Emadi, 1993a). Natural stocks in the past were very high. Migrating
fish were so dense at the Mordab mouth that they were caught in
buckets and jumping fish literally fell into boats.
Savenkova (1990) comments on the marketing of this species in southwestern
Turkmenistan. Salehi (2003) analysed the economics of production of finglerings
in Iran. The cost of labour was 50%, feed and fertiliser 20%, maintenance 10%,
and harvesting, handling and releasing 6%. The average cost per fingerlings was
37 rials in 2001 (rising to 123 rials in 2003 (Salehi, 2008)). As the average rate of fingerling return was 8.3% and the
average weight and age of commercially caught fish was 815 g and 3.7 years, it
was expected that 19,257,494 individuals weighing 16,000 t would be harvested
over the Iranian year 2004-2005. The value would be an estimated 345 billion rials at the 2001 price (8050 rials to a U.S. dollar in 2001; $42.86 million).
Salehi (2008) estimated the wholesale price of the catch as 505 billion rials in
2004 and reviewed the comparative economics of fingerling production for
2001-2003.
Experimental culture of triploid mahi safid has been carried out at
the Gilan Fisheries Research Centre (Iranian Fisheries Research and
Training Organization Newsletter, 2:2, 1993) to increase fish
weight for exploitation but apparently was unsuccessful although the
techniques worked with grass carp, Ctenopharyngodon idella (Annual
Bulletin 1993-94, Iranian Fisheries Research and Training
Organization, Tehran, p. 70-71, 1995). In addition, monoculture and polyculture of this species has been investigated, the latter with
grass carp (Ctenopharyngodon idella) and silver carp (Hypophthalmichthys
molitrix) (Iranian Fisheries Research and Training Organization
Annual Report, 1992-93; Danesh Khoshashi, 1997). From an average
initial weight of 7 g, monocultured fish weighed 158 or 177 g on
average at the end of each of two one-year periods, polycultured fish
weighed 158 or 168 g, and maximum weight attained was 250 or 300 g (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 38, 1996). Danesh Khoshasi (1997) gives
figures of 177 g for mono-cultured fish after 6 months in ponds,
poly-cultured fish weighed 185 g (with a maximum of 300 g) while
stocking with 7 g fish gave 168 g and 192 g for mono- and poly-culture
fish respectively (maximum weight 250 g)(these two reports on the same
experiment have confusing figures). The fish were fed on pellets
especially made for this species, nitrate and phosphate fertiliser and
cow and chicken manure were added to the ponds, and water temperature
was 15.0-27.64°C.
Experimental studies have been carried out on
different lipid levels in food fed to cultured fry, a level of 12% increasing
growth levels (Novirian et al., 2007). Ghanbari et al. (2009)
investigated production of a bacteriocin or anti-bacterial from intestinal
bacteria of this fish species. This bacteriocin has potential for use as a
biopreservative for food.
This species has been used in Iran for experimental studies
and analysed for pollutants, e.g. on the
acute LC50 and bioconcentration of mercuric chloride (Gharaei et
al., 2006; Gharaei and Esmaili-Sari, 2008); on the toxicity and LC50 of phenol and 1-naphthol (Shariati
et al., 2004); the effects of anionic detergents and diazinon on the LC50,
both separately and in combination (Tehranifard et al., 2002; Tehranifard
et al., 2007); Razavilar and Tavakoli (2006) on the prevalence of human
toxigenic Clostridium botulinum and the need for food safety control
measures; Foroughi et al. (2007) on mercury content which
varied between tissues, was not significantly different between sexes and was
lower than permissible limits; Vaezzadeh et al. (2008) found the levels
of the pesticide heptachlor in fish from Hashtpar and dieldrin in fish from
Kiashahr could have a health risk to consumers; etc.
The hybrid with grass carp, Ctenopharyngodon idella, reached an average
weight of 100 g and a length of 22 cm after 5 months in the first
report cited above and 6.7 g and 9.17 cm after 4 months in the second
report. Larvae were fed live food twice a day and hybrids larger than
fingerling size ate grass. The hybrid phenotype resembled the safid
mahi but was a herbivore. It was expected that this hybrid would be
used as a new culturable "species".
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food.
Conservation
Azari Takami et al. (1990) list the following reasons for a
decline in the commercial catch of this species:- a) regression of the
Caspian Sea which decreased the surface area of the Anzali Lagoon and
increased the growth rate of aquatic vegetation, b) mechanisation of
farming and a consequent increased demand for irrigation water leading
to reduced river flows during the spawning migration, c) use of
fertilisers and pesticides in rivers draining into the Anzali Mordab
(and pesticides and herbicides such as Diazinon, Malathion, Machete
and Saturn have a highly toxic effect on fingerlings of this species (Piri
et al., 1999); and the herbicide butachlor reduces sperm volume and
increases abnormal sperm (Lasheidani et al., 2008)), d) pumping of river water for irrigation
causing mass mortalities of fry, e) industrial development increasing
the pollution load, and f) excessive catches of adults to the extent
that all spawners in a river were taken. Emadi (1979) added such
factors as road construction and the removal of sand from banks and
river beds, erosion caused by felling trees and shrubs along river
banks and in the mountains, construction of bridges and raising of
their substructures which formed barriers to migration, and climatic
changes. Illegal fishing and non-standard nets threaten the stocks
while fingerling release (120-140 million) and improvement of natural
spawning areas through rises in water level have contributed to stock
increases (Annual Report, 1995-1996, Iranian Fisheries Research and
Training Organization, Tehran, p. 19-20, 1997). Three million fingerlings
weighing 3-5 g were released into the Anzali Lagoon (Iranian Fisheries
Research Organization Newsletter, 49:4, 2006).). Poaching is
rampant, even on the spawning grounds, and is also a significant
factor in decline of this species (RaLonde and Walczak, 1972).
Savenkova (1990) lists several reasons for reductions in catches in
the Atrak River stock of Turkmenistan on the border with Iran. These
include ineffective fish passes, poor marketing strategies, and the
low quality of fish reaching the spawning grounds.
In the 1970s, rivers were rented to fishermen to exploit to an unlimited
degree such that all spawning fish were taken and there was no recruitment for 4
years (Carl Bond Archives, Oregon State University, Corvallis).
The catch declined from 5854 t in 1918 to 172 t in 1937. Actual
catches are about 20-30% larger because of local sale and consumption
(Emadi, 1979). RaLonde and Walczak (1970b) note a decline from 1556
t in 1957 to 162.1 t in 1967 so the catch does not decline
evenly. Emadi (1979) also pointed out that intensive sea fishing and
fishing in rivers hindered spawning of the winter form, and led to a
situation where only the spring form spawned. Leasing of rivers to
fishermen was discontinued in an attempt to alleviate the decline in
this species. The migration distance up rivers has decreased from
about 25 km to about 8 km in recent years because of water abstraction
and dams for agriculture (Bartley and Rana, 1998b). However, Yousefian and
Mosavi (2008) report that artificial stocking has increased catches from 1000 t
in 1981 to 10,000 t in 2002.
Ghasemi et al.
(2009) report a catch of 17,000t in 2008.
A further problem is the restocking programme only takes spring-run
fish; the fall-run stock may no longer exist (but see below). In addition, stocks from
various rivers are mixed and may result in outbreeding depression, the
loss of adaptations to specific rivers in terms of migration patterns,
spawning time, behaviour and other factors (Bartley and Rana, 1998b).
Ghasemi et al.
(2009) note that the spring run comprised over 98% of the catch and that the
autumn run stock has declined from deterioration of spawning grounds,
overfishing and other factors.
Measures to combat loss of this valued fish included a ban on
fishing in the Anzali Mordab and its tributary rivers (catches here
were up to 1000 t per year (Emadi, 1979)), effective control of
illegal fishing and artificial spawning experiments (although the
latter was insufficient to replace stocks). For several years starting
in 1925, artificial breeding raised larvae for release in the ten most
important rivers. In 1976-1977, fingerlings were raised to increase the
stock in the Caspian Sea. Over 5 million fry were produced by the
"Havyg" (? = Haviq) Hatchery alone in 1977. Mesh size of nets used to
catch this species are apparently not harmful to younger fish as these have a
chance to escape from the wings, with a wider mesh, before the bag net, with a
smaller mesh, comes into play
(Afraei Bandpei
et al., 2010).
Emadi (1979) recorded releases of 28-44 million fingerlings
and 250 million larvae "in recent years". A farm in the Siah
Kal region of Gilan Province near Rasht was expected to produce 40
million white fish "roe", presumably fry, in 1985 (Kayhan
International, 20 May 1984). The Dr. Beheshti Hatchery near Rasht
expected to release more than 60 million fingerlings in the Iranian
year 1993-1994 (Abzeeyan, Tehran, 4(5):VI, 1993). This hatchery
had a peak production for the period 1973-1993 of over 140 million
fingerlings in 1989, rising from the low period of 1973-1982 with less
than 10 million fingerlings, and with subsequent decreases to about 50
million fingerlings in 1993 (Abzeeyan, Tehran, 5(3 &
4):IX-X, 1994). The number of fingerlings released from 1986 to 1991
climbed from 38 million to 170 million (Holčík and Oláh, 1992).
Krasznai (1987) also refers to propagation of this
species at "Sad-e Sangar" (Dr. Beheshti) and Siah Kal Fish
Farms near Rasht in Gilan. Emadi (1993a) gives fingerling production
at government hatcheries as follows: 25.3 million in 1983, 28.3
million in 1984, 38.0 million 1985, 51.7 million in 1986, 72.0 million
in 1987, 84.3 million in 1988, 140.2 million in 1989, 156.3 million in
1990, 110.0 million in 1991, and 145.0 million in 1992. The Shahid
Rajaee Hatchery in Sari released 358 million fingerlings, possibly in
a single year (Abzeeyan, Tehran, 4(7):VII, 1993), although 70
million are reported as released annually to Mazandaran rivers in 1995
(Abzeeyan, Tehran, 6(8):III, 1995) and production
of 70 million kutum fingerlings annually is reported in 2001 (Iranian
Fisheries Research Organization Newsletter, 28:3, 2001). In 1997, 142 million
fingerlings were produced for restocking (Bartley and Rana, 1998b). In
1999-2000, 150 million juveniles were released into the Caspian Sea (Iranian
Fisheries Research Organization Newsletter, 23:4, 2000). From October to March 2000, 80 million
juveniles raised in the Shahid Ansari aquaculture and breeding centre
in Gilan were released into the Caspian Sea and neighbouring water
bodies (Iranian Fisheries Research Organization Newsletter, 26:2, 2001). Billard and Cosson (2002) cite a mean of 100
million alevins released per year, reaching 140 million in some years, and give
a brief overview of production facilities. Mosabbab and Piri (2005) record the
release of fry into the Gorgan River of the southeast Caspian Sea as 16,663
million in 2000, 19,119 in 2001, 12,263 million in 2002, 11,931 in 2003 and
10,413 in 2004, the decrease being due to a fall in capture of brood fish. Amini
(2006) reports release of fingerlings into the Larim, Goharbaran, Shirood,
Tonekabon, Sardabrud, Mirud, Babol, Asbuchin and Sorkhrud rivers in 2000-2001
numbering 53.7 million and 62.5 million. Fork length was 36.7 mm and 43.3 mm and
condition factor 1.13 and 1.2 0 respectively by year. In 2006, a report has more than 150
million juveniles being released into the Caspian Sea every year (www.iranfisheries.net,
downloaded 28 July 2006). Sources obviously conflict on exact
numbers, nevertheless marked variations in production are evident over
short periods. The South Caspian Fisheries of Azerbaijan also released
larvae in Soviet waters, more than 150 million in 1983 for example (Zarbalieva, 1987).
The Inland Water Aquaculture Research centre in Anzali has artificially
propagated the autumn or fall-run stock (www.iranfisheries.net, downloaded 28 July 2006).
Three million fish were released in July 2006, probably at 3-4 g with more to be
released at a heavier weight. Paykhan Heyrati and Dorafshan (2007) compared the
effectiveness of two kinds of dopamine antagonist combined with a
gonadotropin releasing hormone analogue on ovulation and fertilisation success
under hatchery conditions. Spawning induction techniques using hormones provide
high quality gametes in quantity for aquaculture programmes.
Azari Takami et al. (1990), Woynarovich (1985) and Bartley and Rana (1998b) detail
the technique for artificial spawning, incubation and raising of
fingerling mahi safid. Adults are caught as they enter rivers on the
spawning migration by blocking the river with wooden tripods
interlaced with twigs as screens and using a meshed net. The Shaeed
Ansari Fish Farm (of Shilat, the Iranian Fisheries Company) takes fish
from 5 main rivers, forming a brood stock of 10,000 females and 20,000
males (Bartley and Rana, 1998b). Almost 100% of migrating fish are
caught. Ripe fish are stripped and the eggs fertilised with sperm.
Eggs from 2 females are pooled and mixed with milt from 2 males. The
adhesive layer is washed away with water and continuous stirring. Once
the eggs are completely separated from each other they are placed in
incubator jars. Eggs may be placed in net-bottomed trays in the river
for 2 days before being sent to a hatchery. Egg development takes a
minimum of 7 days but larval development only takes 1-2 days. Larvae
are fed with a mixture of milk and eggs until they are 5 days old and
then put in the earthern rearing ponds. In the Shaeed Ansari Fish
Farm, fingerlings are released in a river mouth at 1 g and most enter
the Caspian Sea within 3 days (Bartley and Rana, 1998b). Fingerlings
are grown in ponds to a weight of 2-3 g and then released in the Safid
River which carries them down to the sea (Petr, 1987). Production of
1-2 g fingerlings attains 3 tonnes per hectare in fish farms (Emadi, 1993a).
Farabi et al. (2007) studied brood stocks and fingerlings in the Shirod,
Tonekabon, Tajan and Goharbaran rivers from March 2004 to March 2005. The mean
length, weight and condition factor for broodstock females and males were 43.75
and 36.5 cm, 1189.5 and 678.13 g and 1.42 and 1.38 respectively. Eggs collected
over a 62 day period weighed 4931 kg, mostly at the end of March and beginning
of April. Percentage of survival of eggs in the four rivers listed above was
94.5, 95.1, 87.7 and 96.9 respectively. Newly hatched larvae averaged a total
length of 6±2 mm and a weight of 2±0.2 g. The number of fish produced at less
than 1g was 16,942,454 representing 19.9% of the total released in Mazandaran in
2004. Fish in the 1.0-1.5 g weight class, suitable for release, numbered
62,905,247. Parasites of fry were Diplostomum, Dactylogyrus,
Butriocephalus, nematodes, Trichodina and Epistelis.
Studies on the best methods of rearing fingerlings and fry have been carried
out in Iranian hatcheries and in rivers. The use of rotifers (Brachionis plicatilis) as food is more effective in
larval growth than concentrated food preparations; after 40 days the rotifer-fed
fish reached 440 mg and 31.36 mm on average and those fed concentrated food
reached 17.2 mg and 14.75 mm (Fallahi et al., 2004; Kapourchali, 2006).
Neverian et al. (2005) studied variation in protein levels fed to
advanced fry at an isocaloric digestable energy of 3400 kcal/kg. The highest
treatment at protein level 35% showed increased body protein, decreased fat and
improved flesh quality in the carcass. Trichlorfon, an organophosphate pesticide is used to protect zooplankton such
as rotiferans, food for larval safid mahi, against predators. However it does
slow growth of the fish and the larvae must only be added to treated water 72
hours after the last application of the chemical (N. Chookbar, www.netiran.com, downloaded 18 April 2005;
Chobkar et al., 2005; 2008). The sex steroid binding protein has been
investigated as a biomarker for sexual behaviour in this species (Kousha et
al., 2006). Spawning induction studies have been carried out on broodfish in
Iran using carp pituitary extract and GnRH analogue, the latter alone or
combined with metoclopramide. The latter treatment gave 90% ovulated females,
71.3% ovulation index and 68.4% fertilisation success, significantly higher than
values for carp pituitary extract. However, the latency period was longer.
Paykhan Heyrati et al. (2006) and Paykhan Heyrati and Dorafshan (2007) induced spawning with a gonadotropin
releasing hormone analogue, both alone and in combination with the dopamine
antagonists domperidone and metoclopramide, the combination with both
antagonists being equally successful. Bahmani et al. (2007) found greater
spermatozoan activity and quantity in fish that were captured further upriver on
the spawning migration thus suggesting better sampling areas for broodstock;
Ghara et al. (2007) found growth and survival of larvae in polyethylene
tanks was better than fibreglass and glass aquaria; Nikou et al. (2007)
studied levels of various hormones during transport of spawners and found clear
indications of stress; Amiri et al. (2008) found that the optimum
salinity for growth and survival of 1 g fingerlings was 8-10 p.p.t.; Keyvan et al. (2008) examined the
effect of frozen storage time on the deterioration of the product.
Neverian et al. (2008) determined the optimum level of vitamin premix for
the diet of advanced fry. Noverian et al. (2008) studied the
effect of dietary digestible energy level on growth indices of advanced fry.
Body protein and fat improved when the dietary energy was raised from 2500 to
2800 kcal kg-1. Haghighi et al. (2009) investigated the
effects of dietary lipid levels on fry, growth, survival, protein and energy
retention decreasing with an increase of dietary lipid level; a lipid level of
8% and 4314 kcal/kg bringing about the best growth performance. Akbarian et
al. (2009) found that mid to high light intensities and long photoperiods
could be useful in hatcheries where no particular light regime had been used.
Kapoorchali et al. (2009) found increased growth of larvae when cow
manure was used to increase abundance of zooplankton on a fish farm.
Kousha et al.
(2009) examined the fluctuation in sex steroid hormones during the spawning
migration and annually, levels of some varying with variations in sea and river
temperatures. Gholami (2010) found that Daphnia magna enriched with cod
liver oil optimised nutrition of fry. Nikoo et al. (2010) studied
physiological stress responses in fish caught on their upstream migration.
Yasemi and Nikoo (2010) studied the impact of captivity on fertilisation and
stress levels in broodstock and found short-term stress did not affect gamete
quality levels.
A fine of 1,500 rials was imposed specifically for illegal angling
of this species (Anonymous, 1977-1978).
Kiabi et al. (1999) consider this species to be of least
concern in the south Caspian Sea basin according to IUCN criteria.
Criteria include commercial fishing, sport fishing, abundant in
numbers, habitat destruction, widespread range (75% of water bodies),
absent in other water bodies in Iran, and present outside the Caspian
Sea basin. The 2000 IUCN Red List lists this species as DD (Data
Deficient). Coad (2000a), using 18 criteria, found this species to be one
of the top 4 threatened species of freshwater fishes in Iran. Extinct in Turkey
(Fricke et al., 2007).
Further work
The heavy demand for this fish as a traditional food warrants extensive
management and conservation of stocks.
Sources
Iranian material: CMNFI 1970-0513, 9, 51.1-66.1 mm standard length, Gilan, Shafa River estuary (37º35'N, 49º09'E);
CMNFI 1970-0516, 27, 42.3-61.0 mm standard length, Gilan, Lomir River (38º14'N, 48º52'30");
CMNFI 1970-0518, 9, 43.4-60.7 mm standard length, Gilan, Haviq River estuary (38º10'N, 48º54'E);
CMNFI 1970-0520, 19, 46.7-73.9 mm standard length, Gilan, Astara River (ca. 38º25'N, ca. 48º52'E);
CMNFI 1970-0563, 24, 40.8-58.1 mm standard length, Gilan Caspian Sea, Kazian Beach (ca. 37º29'N, ca. 49º29'E):
CMNFI 1979-0088, 1, 104.3 mm standard length, Gilan, Safid River (no other locality data);
CMNFI 1979-0471, 1, 106.0 mm standard length, Mazandaran, Caspian Sea west of Alamdeh (36º35'N, 51º48'E);
CMNFI 1979-0686, 30, 42.8-52.2 mm standard length, Gilan, Safid River above ferry (37º24'N, 49º58'E);
CMNFI 1980-0159, 1, 117.0 mm standard length, Gilan, Caspian Sea at Kazian Bridge (37º28'30"N, 49º28'E);
CMNFI 1980-0160, 1, 88.5 mm standard length, Iran, Caspian Sea basin (no other locality data);
CMNFI 1993-0140, 2, 64.5-71.5 mm standard length, Mazandaran, Tirom River, Ramsar (36º51'48"N, 50º48'E).
Comparative material: BM(NH) 1879.11.14:31-32, 2, 410.0-430.0 mm standard length, Russia, Astrakhan (ca. 46º24'N, ca. 48º05'E).
Rutilus rutilus
(Linnaeus, 1758)
need to separate out caspicus information?
Common names
كلمه (= kolme, kollme, kolmeh or koolmeh, meaning unknown), mahi kolme, kolme Gorgan, telagi or
talaji (in Gilaki and Mazandarani, meaning unknown, latter three used for R. r.
caspius natio knipowitschi), mahi cheshm qermez (= redeye
fish), kolme Kura or kolme Anzali for R. r. caspius natio kurensis,
kolme Gorgan or kolme Turmenistan for R. r. caspius natio knipowitschi
[kulma, kyumen, xazar kulmasi and Kur kulmasi in Azerbaijan;
kasli akcapagy in Turkmenian; Armyanskaya plotva or Armenian roach, karmraki and last for schelkovnikovi,
all in Armenia; vobla, severo-kaspiiskaya vobla or North Caspian vobla,
Astrakhanskaya vobla or Astrakhan vobla, Kurinskaya vobla or Kura
vobla, Astrabadskaya vobla or Astrabad vobla, Turkmenskaya vobla or
Turkmenian vobla, all in Russian; roach being the English equivalent of vobla].
Systematics
Cyprinus Rutilus was originally described from lakes in Europe, the type being from Sweden.
Holčík and Skořepa (1971) revised the roach and found no reason to maintain subspecies,
of which they list 12. Rutilus rutilus caspicus (Yakovlev,
1870) (North Caspian or Astrakhan vobla) was the subspecies found in
the Caspian Sea basin with natio knipowitschi Pravdin, 1927 (Astrabad
or Turkmenian vobla) in Gorgan (= Astrabad) and Gasan-kuli bays and
the Gorgan, Atrak and Qareh Su rivers and natio kurensis Berg,
1932 (Kura vobla) in Kyzylagach Bay, the Kura River, Astara and rarely
the Anzali Mordab. The Kura vobla was said to differ from the Astrabad
vobla by a greater body depth, smaller eye and more rapid growth. R.
r. caspicus was distinguished from other subspecies by having
modally 9 branched dorsal fin rays, darker fins, more inferior mouth,
higher dorsal and anal fins, longer pectoral and pelvic fins, a deeper
head, and larger eyes (see Berg (1948-1949) for more details). R.
r. caspicus was originally described as Leuciscus rutilus
var. caspicus from the Volga River delta, Russia. The types are
unknown (Eschmeyer et al., 1996). Eschmeyer et al.
(1996) also list Leuciscus rutilus var. wobla Grimm,
1896 described from the North Caspian Sea and mouths of rivers (Volga,
Ural, Emba, Terek, Kura, Astara) entering the Caspian Sea. This is
presumably a synonym of Rutilus rutilus judging from the
subspecific name which is the Russian word for this species (wobla =
vobla or roach). Kottelat (1997) lists it as a nomen nudum.
Holčík and Skořepa (1971) recognize the Caspian Sea populations as the morph or morpha migratorius,
perhaps a first step towards differentiation and speciation.
Mironovskii and Kas'yanov (1986; 1987) however retained Rutilus
rutilus caspicus as a distinct subspecies in the Caspian Sea based
on a multivariate analysis of 12 meristic and 7 morphometric
characters. Mironovskii (1991; 1992) also demonstrated differences
between Turkmen and Azerbaijan samples. Kuliyev (1984) considered
these variations to be responses to different and changing conditions
of the environment. Naddafi et al. (2002) also demonstrated differences
in meristic (anal fin rays, predorsal scale number and total body vertebrae of
12 characters studied) and morphometric characters (7 measurements of 28
studied) for fish from the Anzali Mordab and the Gorgan River estuary of Iran.
Keyvanshokooh and Kalbassi (2006) however found the value of Nei's genetic
distance (d = 0.04) to be small between these two populations using DNA.
The populations had similar levels of polymorphism.
Keyvanshokooh et al. (2007) compared populations in the Anzali Wetland
and Gorgan Bay using microsatellite markers and found differences between both
populations were not significantly different for average number of alleles per
locus nor for observed heterozygosities. Parafkandeh Haghighi and Rezvani (2005) and Parafkandeh Haghighi (2006) used the
trace element content in otoliths to demonstrate the presence of two
different populations in the southern Caspian Sea, the Anzali-Kura and the
Gorgan-Turkmen, as noted above on meristic and morphometric values.
Patimar et al. (2005) found morphological differences between fish from
the Gomishan Wetland and those from the Adji-gol and Alma-gol wetlands in
southeast Iran, and between the latter two wetlands. Inter- and intra-population
variation was higher in the Gomishan fish. Rezvani et al. (2006) used
mitochondrial DNA to compare Anzali and Turkmen roach and found the former to be
more genetically variable, attributing this to there being more rivers, and
therefore more spawning populations, in that area. Reyhani et al. (2010)
used microsatellite markers and found higher heterozygosity in Anzali Wetland
fish compared to those from Gorgan Bay. However, gene flow was high and
population differentiation was non-significant.
Various body forms of this species have been given
names as morpha, sometimes taken from species names regarded as
synonyms, although as morpha these have no taxonomic significance,
e.g. prasinus from Leuciscus prasinus Agassiz, 1835 (= elongata
from Leuciscus rutilus var. elongata Fatio, 1882) for an
elongate body and rutiloides from Leuciscus rutiloides
Selys-Lonchamps, 1842 (= elata from Leuciscus rutilus
var. elata Fatio, 1882) for a wide-backed body. A golden-orange
or red aberration was known as aurata from Leuciscus rutilus
var. aurata Fatio, 1882.
Bogutskaya and Naseka (2004) and Kottelat and
Freyhof (2007) recognise
Rutilus caspicus (Yakovlev, 1870) as the semi-anadromous species in the Caspian Sea
with R. rutilus in rivers and lakes.
Curiously, R. caspicus and R. frisii showed low genetic divergence
in Ketmaier et al. (2008), a study using DNA but sample sizes were low.
Three "types" of Rutilus rutilus caspicus have
been reported from Iran (Annual Report, 1995-1996, Iranian
Fisheries Research and Training Organization, Tehran, p. 54-55, 1997).
One type lives in the Anzali Mordab and is relatively small, the
second is larger and migrates between Anzali and the Kura River of
Azerbaijan and the largest migrates between the northern and southern coasts of the Caspian Sea.
Rutilus rutilus schelkovnikovi Derzhavin, 1926 was described
from the Karasu which falls into the Aras River 5 km above the mouth
of the Zanga River (Razdan River), Echmiadzin District, Armenia, and Rutilus
rutilus uzboicus Berg, 1932 from lakes in the Uzboi River Valley
(with the type from Lake Yaskhan) in Turkmenistan north of the Iranian
border. Rutilus rutilus var. fluviatilis (Jakovlev,
1870) described from the delta of the Volga River is another synonym.
Hybrids with Blicca bjoerkna are reported from the Aras
River basin in Armenia, which is shared with Iran. Artificial hybrids
with Rutilus frisii kutum and Abramis brama have been
bred in Iran (Annual Report, 1994-1995, Iranian Fisheries Research
and Training Organization, Tehran, p. 39-40, 1996).
Key characters
This species is distinguished from the related Rutilus frisii
by the lower scale count (< 50) and the posterior part of the
swimbladder being rounded rather than pointed.
Morphology
Dwarf and large forms are recognised in Dagestan by Shikhshabekov
(1969) with different life histories and distinct spawning stocks are
known from the north Caspian Sea.
Lateral line scales 39-48, mostly 42-47. Scales have fine circuli
around a central focus. Radii may be found on all fields but fish
often lack radii on lateral fields. Usually there are very few
anterior and posterior radii (e.g. in a fish 150.7 mm standard length
there are 2 anterior and 4 posterior radii; but counts are quite
variable, at least 7-14 radii total). Scales have a wavy anterior
margin and a finely crenulated posterior margin. There is a pelvic
axillary scale and a scaled keel behind the pelvic fins. Dorsal fin
branched rays 8-12, usually 9 in the Caspian Sea (but see below),
after 2-5 unbranched rays, anal rays 8-12, mostly 9-10, after 2-4
unbranched rays, pectoral fin with 14-18 branched rays, and pelvic fin
with 7-9 branched rays. Gill rakers 9-17, usually 10-14, short and
stubby and reaching the adjacent raker when appressed. Vertebrae
38-43. Lake populations generally have more vertebrae than river
populations and the number of vertebrae is genetically fixed,
generally following the maternal genotype (Izyumov and Kas'yanov,
1995). Pharyngeal teeth 6-5 or 5-5, rarely 6-6, 4-5, 5-4, or 5-6 with
the grinding surface slightly folded to form a long, hollow crown for
the largest teeth. There is a rounded and hooked tip to teeth 1-4. The
tooth margin in the posteriormost 2 teeth is serrated. The
anteriormost 2 teeth are rounded. Teeth are more serrated and hooked
in smaller fish. The gut is s-shaped with a small anterior loop. The
chromosome number is 2n=50 (Klinkhardt et al., 1995).
Meristics for Iranian specimens:- dorsal fin branched rays 9(22) or
10(18); anal fin branched rays 9(16), 10(23) or 11(1); pectoral fin
branched rays 14(1), 15(4), 16(22), 17(12) or 18(1); pelvic fin
branched rays 7(4), 8(33) or 9(3); lateral line scales 40(1), 41(4),
42(16), 43(11), 44(5) or 45(3); total gill rakers 11(4), 12(15),
13(18) or 14(3); pharyngeal teeth 6-5(22), 6-6(1), 6-4(1) or 5-5(1);
and total vertebrae 37(1), 39(2), 40(9), 41(64) or 42(19).
Sexual dimorphism
In a male caught on 26 April, moderate-sized tubercles are evenly
distributed over the top and sides of the head, with fewer tubercles
on the undersurface. Scales bear one strong tubercle in the exposed
mid-scale, anterior and dorsal scales have smaller tubercles on the
scale margin and belly scales have minute tubercles on the margin and
exposed scale base. Fine tubercles follow the branching fin rays in
single file on all fins, both dorsally and ventrally on the pectoral
and pelvic fins, and in 1-2 rows on the last unbranched ray of each
fin. Some fish have some scales with 1-3 tubercles, usually 2, one
above the other. When there are 3 tubercles these are variably
arranged, e.g. in an L-shape or a reversed L. Females also bear
tubercles on the head and scales but these are less well-developed and
are not present on fins.
Colour
Overall colour is silvery. The back is grey and the sides of the
head golden. The belly is pearly-white. The iris is silvery with a
black spot above the pupil, some gold on the upper part, and may be
orange to red. Pectoral, pelvic and anal fins light grey with a darker
band on the edge. The middle of the pelvic and anal fins may be
transparent, orange to pink or red. The peritoneum is silvery with
moderately dispersed but evident melanophores.
Size
Attains 56.0 cm and 3.13 kg. Larger fish may be
hybrids with Rutilus frisii. In Iran during the 1950s, catches
were 16-35 cm long (Farid-Pak, no date).
Distribution
Found from the British Isles and France eastwards to Siberia, north
of the Alps; in the Black Sea basin but not in Turkey, except the
northwestern corner and the Yesilirmak River basin, and on all shores
of the Caspian Sea basin including that of Iran, its rivers and bays (Nedoshivin
and Il'in, 1929; Berg, 1948-1949; Kozhin, 1957; Riazi, 1996; Abbasi et al., 1999; Kiabi et al., 1999).
Abdoli (2000) and Abdoli and Naderi (2009) have natio knipowitschi in Gorgan Bay and the
neighbouring Caspian coast and in the Gorgan, Gharasu and Atrak rivers, and natio kurensis in the lower Safid River,
Anzali Talab and neighbouring Caspian coast to Astara and the middle Aras River.
Rutilus rutilus aralensis Berg, 1916 (but see above on
subspecies status) is reported from the Karakum Canal and Kopetdag
Reservoir in Turkmenistan (Shakirova nd Sukhanova (1994; Sal'nikov,
1995) and may eventually be found in the Tedzhen (= Hari) River basin of Iran.
Zoogeography
A European and western Asian species with its origins in a Danubian or Sarmatian fauna.
Habitat
This species is eurytopic, living in rivers, streams, lakes,
reservoirs, and fresher parts of seas. It occurs in schools close to
vegetation in fresh waters.
This species gathers in front of the Volga River delta in the north
of the Caspian Sea in large shoals in autumn when water levels
decrease. At water temperatures of 5°C
they descend to the sea floor and "lie in winter sleep" (Holčík and Skořepa,
1971). It is not known if this occurs off the warmer Iranian shore.
Generally the vobla lives in the sea itself and migrates to rivers for
spawning. Although most spawning takes place in fresh water, some
occurs at a salinity of 6.5‰. This species is the most tolerant
of the semi-anadromous fishes, the lethal salinity level being 15-16‰. In the north Caspian Sea,
this fish are found mostly shallower than 7.5 m but Knipovich (1921) reports this species at 36.6-53.0 m in
the Iranian Caspian Sea.
A mass migration of the Kura vobla (natio kurensis) into the
Anzali Mordab occurred between 21 and 28 February 1915, only to
disappear after 4 April. Riazi (1996) reports that this species
migrates into the Siah-Keshim Protected Region of the Anzali Mordab. Males
migrate into the Anzali and Gomishan wetlands of Iran earlier than females and
the larger fish enter first (Naddafi et al., 2005).
Natio knipowitschi lives in the sea in Gorgan and Gasan-kuli
bays and enters rivers there. Savenkova (1994) reported as many as
2000 million juveniles descending the Atrak River in May and June in
high productivity years and as few as 83 million in low productivity
years. The sea in July and August is as warm as 28-30°C
off the Iran-Turkmenistan border and the juvenile fish from the Atrak
descend to 11-12 m. Savenkova (1994) gives further details of
movements in relation to temperature, e.g. when waters cool to 8-12°C
off Turkmenistan, young fish move to warmer Iranian waters to
overwinter. ERM-Lahmeyer International GmbH, DHI Water & Environment and GOPA
Consultants (2001a) report that the Gomishan Lagoon at 37º11N, 53º57'E is an
important staging area for this species.
Age and growth
Generally growth is a function of type of feeding (a diet favouring
molluscs gives higher growth than herbivory) and temperature. High
growth rate is shown by semi-migratory fish but is also shared by some
populations resident in lakes and rivers. Kas'yanov et al. (
1995) review the complex relationships of environmental factors
affecting growth in this species.
A life span up to 19 years has been reported although most fish are
10 years or less. Growth is fastest in the second and third years of
life when most fish mature sexually (Strubalina and Chernyavskiy, 1992).
Some fish may mature at one year of age and a length of 9-11 cm in
the Atrak River population (Petr, 1987). Growth in the Atrak
population juveniles is heavily dependent on temperature regimes in
the sea, the timing of descent to it, and stock abundance. Fish which
descend earlier because of low flow and have a longer feeding season,
find a favourable, warm regime and few competitors will have faster
growth (Savenkova, 1994).
Young-of-the-year Turkmen fish reach 4.9-5.8 cm in July while in
the northern Caspian they only attain 3.3-4.1 cm, although the latter
have a higher condition factor, possibly because of higher benthic
biomass and heterogeneity (Savenkova, 1994). The dwarf form in
Dagestan is 11 cm and 31 g at 3 years of age while the large form is
20.3 cm and 130 g. Both are sexually mature (Shikhshabekov, 1969).
The commercial catch in Iran during 1971/72 was 3-6 years old,
20.4-26.0 cm long and weighed 146-300 g (Razivi et al., 1972).
The average weight in 1995 was 200-300 g (Abzeeyan, Tehran, 5(9):V, 1995).
In Gorgan Bay, the age composition is from 1+ to 6+ with age group
3+ and 4+ each accounting for about 30%. Growth rate for all south
Caspian populations is higher than the north Caspian populations,
two-year-olds being the same length as three-year-olds. Average weight
is 247 g with larger fish at 30 cm weighing 583 g. Numerous fish
entered Gorgan Bay in mid-January in the early years of this century
and by the end of that month ascended all the tributary rivers, in
particular the Qareh Su (= Karasu).
Growth rate in the Anzali Mordab was higher than in the Gomishan Wetland of
the southeastern Caspian Sea, limited by higher salinity in the latter (Naddafi
et al., 2002a, 2002b, 2005; Paghe et al., 2005). The population density was higher in Gomishan as Anzali
suffers more from pollution, overfishing, illegal fishing and other human
impacts. Mean condition factors for the Gomishan population were 1.84 for males
and 2.09 for females while in the Anzali population these were 2.03 and 2.2
respectively. The most abundant age groups in the commercial fishery of both
areas were 3+, 4+ and 5+. Maximum age at Gomishan was 8+ years. Growth was rapid in the first year with a sharp
decline in the second year and a steady, less rapid decline in subsequent years.
Females were longer than males at in each age class. Sex ratios were did not
differ significantly from 1:1. The Anzali population had longer body lengths
than fish from other studies, perhaps due to a longer growing season and higher
water temperatures in the south Caspian Sea, better food supply and genetic factors.
Food
The young feed first on phytoplankton and then switch to
zooplankton and benthic insect larvae with growth. Adults eat benthic
animals and plants with molluscs an important diet item where present
as in the southern Caspian Sea. A mollusc diet occasions significant
wear on the pharyngeal teeth (Holčík and Skořepa,
1971). There has been a long-term variation in the diet of roach in
the Caspian Sea, molluscs declining and being replaced by crustaceans
and plants (Strubalina and Chernyavskiy, 1992). Plant foods in Europe
include water thyme, milfoil, duckweed, stonewart and algae scraped
from reeds and rocks (Muus and Dahlstrøm, 1999). There is little
feeding during spawning in rivers. Young-of-the-year in the Atrak
delta feed on the polychaete worm Nereis (up to 89.5%) and with
growth the mollusc Cerastoderma lamarckii (up to 70.8%), and to
a lesser extent the exotic mollusc Abra ovata (5.2%) since this
abundant species is partially buried and is not readily available.
Polychaetes and molluscs have high biomasses in the silty sediments of
around 30-50 g/sq m. Sometimes protozoa and crustaceans figure
prominently in the summer diet but diet varies considerably between
months and years (Savenkova, 1994).
Reproduction
A spawning migration runs up rivers in the Caspian Sea basin in
spring, the larger fish first. Spawning grounds are mostly flooded meadows that
warm up easily (Caspian Sea Biodiversity Database, www.caspianenvironment.org). Immature fish only migrate in autumn
after their gonads have matured, spending the summer in deeper water.
The fish return to the sea after spawning in the same sequence as the
upriver migration. Young remain in the river until early summer when
they too go down to the sea. However some populations of this species
are exclusively freshwater and do not migrate.
Spawning is continuous and takes about 5-6 hours. Fecundity reaches
about 202,000 eggs and egg diameters 1.6 mm. In the Volga delta this
species spawns in the same areas as Abramis brama. However, it spawns earlier at temperatures of 7-16°C
on last years dead vegetation while the bream spawns later at 15-23°C
on new growth. Spawning can occur on stony bottoms in the absence of vegetation.
Spawning is a noisy process which can be heard from
some distance. Each female is accompanied by 2-3 males who push on her
belly, sometimes lifting her part way out of the water, as they swim
in circles. With many fish engaged in this process, the water foams.
The fish gradually approach the shore where the eggs are shed in water
only 5-20 cm deep as the female is squeezed by two males. Other males
take part in the process, as many as 10 at a time. Incubation takes 4
days at 17-20°C. Young actively move downstream once they reach 20-25 mm.
The breeding migration in the Atrak River starts in
January-February and continues until April, the fish traveling 70-80
km upriver (Petr, 1987). Nümann (1969) states that the spawning
migration takes place from November to December in Iranian waters. The
migration in the Atrak peaks at 10-12°C
and lasts 20-25 days. Spawning peaks at 15-18°C
in March on the lower Atrak floodplains (in 1908 spawning in the lower
Atrak was observed in mid-April). In the absence of flooding, no
spawning occurs. After spawning, females migrate back to the sea
first. In the Qareh Su of Gorgan Bay, spawning took place 20-25 km
upriver from the mouth in 1907 and 1908. It started in mid-February,
peaked in the second half of February and the first half of March, and
ended between 20 and 30 March. Males with running milt first appeared
between 14 and 20 February in 1915 and ripe females a week later. Mass
spawning took place at the beginning of March (Nedoshivin and Il'in,
1929). The main spawning rivers in Iran are the Qareh Su, Goharbaran,
Larim, Siah-Darvishan and Shafa River (Iranian Fisheries
Research and Training Organization Newsletter, 9:5, 1995).
Fecundity in the Gomishan Wetland (4262-98,804 eggs) was significantly higher than in the
Anzali Mordab (6035-32,141) for a given body size. Egg diameters ranged from 0.9
to 1.45 mm and were not significantly different between the two wetlands. The
peaks for the gonadosomatic index (GSI) curves were early March for males and
mid-March to early April for females. The GSI was higher in Gomishan (Naddafi
et al., 2005).
Parasites and predators
The main predator of this species in the north Caspian Sea is Sander
lucioperca, accounting for 65% of its food. Silurus glanis
and Esox lucius and various birds such as the pelican are also
important predators (Kushnarenko, 1978). The Caspian seal, Pusa
caspica, is a significant predator on this species (Krylov, 1984).
On the spawning grounds, this fish can be picked up by hand and falls
easy prey to birds and other predators.
Mokhayer (1976b) records the cestode larva Diagramma. Jalali
and Molnár (1990a) record the monogenean Dactylogyrus turaliensis from
this species in the Safid River. Masoumian et al. (2002) recorded
parasites from fish captured form the coast of the southeast Caspian Sea as
Anisakis larvae, Aspidogaster limacoides, Bothriocephalus
gowkongensis, Dactylogyrus turaliensis and Diplostomum spathaceum.
Youssefi et al. (2005) record the pleroceroid larvae of Ligula intestinalis in fish from the Aras Dam. A
toxin produced by this parasite can cause infertility and weight loss in the
fish and can be harmful to humans. Khara et al. (2006a) record the eye
fluke Diplostomum spathaceum for this fish in the Amirkalayeh Wetland in
Gilan, having the highest infection rate in 7 species examined.
Khara et al. (2006b) record the
cestode Caryophyllaeus fimbriceps from this species in the Boojagh
Wetland of the Caspian coast. Khara et al. (2008) found the eye parasite
Diplostomum spathaceum in this fish from Boojagh Kiashar Wetland in Gilan.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Economic importance
This species is one of the most important commercial species in the
Caspian Sea with a catch in the north Caspian and lower reaches of
rivers in 1930 being 2,591,000 centners (15,000,000,000 fish) or 45.5%
of the total catch of fishes in the Caspian Sea or nearly 20% of the
whole Soviet fish catch. Archaeological studies on the eastern Caspian
shore in the former Soviet Union has shown this species to have been
fished for 5000 years ago (Tsepkin, 1986). It is a significant food for the
sturgeon Huso huso (Keyvanshokooh and Kalbassi, 2006).
The main fishing season in Iran is February and March (Farid-Pak, no date). Nevraev (1929) gives catches for various fishing regions in Iran in
the early twentieth century. For the Anzali region from 1914-1915 to
1917-1918 the catch was 6000 to 244,800 individuals (the catch
declining steadily over this period), and in Astrabad (= Gorgan)
region from 1909-1910 to 1912-1913 the catch was 8,348,800 to
21,790,000 individuals. The catch in Iran from 1956/1957 to 1961/1962
varied from 2989 kg to 1,092,719 kg (Vladykov, 1964), from 1965/66 to
1968/69 it varied from 32 to 74 tonnes (36, 74, 32 and 35 t respectively) (Andersskog, 1970) and from 1963 to 1967 from 2.4 to 47.0
t (23.1, 2.4, 3.7, 30.7, 47.0 t respectively) (RaLonde and
Walczak, 1970b). Vladykov (1964) reports catches from the Anzali
region for the period 1933/1934 to 1961/1962 to vary from 57 to
716,974 kg, with none reported in some years. Holčík and Oláh (1992)
report an annual catch in the Anzali Mordab from
1932-1964 of 0.8-449.7 t. The total catch in 1989 (or 1990) was
only 26 t but increased to 120 t in 1995 because of the
rising Caspian Sea level which provided more spawning grounds (Abzeeyan,
Tehran, 5(9):V, 1995; Iranian Fisheries Research and Training
Organization Newsletter, 9:5, 1995). Conflicting values from
reports where they overlap are typical of fisheries statistics from
Iran which can only be taken as general trends rather than absolute
values (see below also). The Food and Agriculture Organization, Rome
gave the catch for Rutilus spp., presumably this species, for
the years 1980-1985 as 0, 0, 0, 121, 347, and 350 t respectively. The catch in Gorgan Bay 20-30 years ago according to Petr
(1987) was about 4000 tonnes per year but is now negligible. This
figure conflicts with the ones cited above. In the 1914-1915 fishing
season in Gorgan (= Astrabad) Bay, 24.2 million fish were taken, of
which 21.9 million were taken in the Qareh Su. Petr (1987) cites
catches of 830 t in 1978 for the southeastern Caspian Sea and for 1979
only 120 t, probably including Soviet catches. Recruitment falls when
the Atrak River, a major spawning ground, fails to flood adequately.
The dwarf form in Dagestan is of no commercial value and may have
arisen with changing environmental conditions (Shikhshabekov, 1969).
Moeini and Daneshnuran (20010 and Moini et al. (2005) have investigated the production of marinades using this
fish using various recipes, examining their chemical composition over time and
their comparative tastes.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food,
in sport and in textbooks and because it has been introduced outside its natural range.
Golpour and Imanpour (2010) consider this species to be threatened in Iran from
overfishing and deterioration of spawning grounds. Everard (2006) is one book of many describing the biology and importance of the roach
as Rutilus rutilus.
Conservation
Holčík and Oláh (1992) report a catch of only 5 kg in the Anzali Mordab for
1990. This species has been cultured at "Sad-e Sangar" Fish
Farm near Rasht (3 million larvae a year), at Astara and at Anzali on
the Caspian among other localities (Mokhayer, 1972b). In 1999-2000, 30
million juveniles were released into the Caspian Sea (Iranian Fisheries
Research Organization Newsletter, 23:4, 2000). Noroozi
et al. (2006) detail capture of brood stock from the Gorgan River estuary
for release into 2 ha earthen ponds enriched with manure and fertiliser. The
optimum temperature for spawning brood stocks is 12-17ºC, found in mid-March to
late April. Pine branches were placed in the ponds as spawning sites. Ponds were
stocked with 700 females averaging 150 g and 350 males averaging 100 g. Eggs
hatched on the sixth day. Larvae were fed on natural zooplankton (Rotatoria and
Daphnia) and artificial food.
The Atrak River population can be conserved by regulating the
discharge so that the spawning grounds flood at the appropriate time
(Petr, 1987). Beach seines with mesh sizes of 28 mm, 33 mm and 36 mm
caught fish of ages 2.8 years, 2.9 years and 3.3 years respectively.
This data indicates that 58.3%, 50.5% and 3.6% of the total catch is
non-standard respectively. Seines with a cod end of at least 36 mm are
recommended for stock protection (Iranian Fisheries Research and
Training Organization Newsletter, 6:8, 1994). Catches are
increasing with the rise of the Caspian Sea water level as noted
above, indicating how natural events beyond human control can have a
significant effect on stocks.
Imanpour et al.
(2009) studied the relationship between fish size and various spermatological
parameters. Sperm volume did not increase with body length although gonadal
weight did. Spermatocrit, sperm motility, sperm density, gonadosomatic index and
hematocrit were not influenced by fish body size but sperm density decreased
significantly with increasing gonadal weight.
Golpour and
Imanpour (2010) studied relationships between seminal and blood plasma
composition during the reproductive season, as part of improving artificial
fertilisation.
There have been few studies on the effects of
environmental factors on this species in Iran. Gerve'ei et al. (2008)
determined the LC50 and gill tissue lesions caused by aluminium
sulphate under laboratory conditions and Keramati et al. (2010) studied
the effect of the agricultural
organophosphate diazinon on enzyme activity, also under laboratory
conditions.
Further work
As an important food fish, this species should be effectively managed and conserved.
Sources
Wossugh-Zamani (1991d) and Akbari-Pasand (1996) give accounts of this species in Farsi.
Iranian material: CMNFI 1970-0509, 3, 73.4-82.9 mm standard length, Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0510, 4, 58.6-83.8 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1970-0531, 14, 65.5-115.8 mm standard length, Mazandaran, Larim River (36º46'N, 52º56'E);
CMNFI 1970-0532, 1, 74.9 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 1, 119.4 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0549, 1, 109.7 mm standard length, Mazandaran, Qareh Su near Alm Imamzadeh (no other locality data);
CMNFI 1970-0563, 5, 40.3-112.2 mm standard length, Gilan Caspian Sea, Kazian Beach (ca. 37º29'N, ca. 49º29'E):
CMNFI 1970-0583, 6, 35.7-84.9 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1979-0431, 1, 157.0 mm standard length, Mazandaran, fish bazaar at Now Shahr (no other locality data);
CMNFI 1979-0788, 2, 64.9-66.6 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0126, 4, 162.2-178.3 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0136, 4, 72.1-109.7 mm standard length, Mazandaran, Fereydun Kenar River estuary (36º41'N, 52º29'E);
CMNFI 1980-0148, 1, 85.4 mm standard length, Gilan, Pir Bazar Roga River (37º21'N, 49º33'E);
CMNFI 1980-0157, 2, 73.1-132.0 mm standard length, Mazandaran, Gorgan River estuary (36º59'N, 53º59'30"E);
CMNFI 1980-0905, 9, 98.5-203.0 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E).
Genus Scardinius
Bonaparte, 1837
Howes (1981) placed this genus in Rutilus Rafinesque, 1820
on osteological grounds. Bogutskaya (1988) disagrees. Scardinius
is usually separated from Rutilus by having 2,
as opposed to 1, rows of pharyngeal teeth and a ventral keel on the
body. Howes (1981) considers pharyngeal teeth to be of only species
importance and the keel is variously developed in Rutilus.
The genus contains perhaps 6 species and is found from the British
Isles and the Iberian Peninsula throughout Europe to the Caspian and Aral Sea basins,
with one species in Iran.
The genus is characterised by a pharyngeal tooth count of 3,5-5,3
with major row crowns laterally compressed and bearing 5-8 serrations,
scales moderate in size in a complete lateral line, few, short gill rakers, a keel on the belly behind the pelvic fins covered with
scales, a short gut and light peritoneum, dorsal and anal fins of
moderate length, dorsal fin origin well behind the origin of the
pelvic fins, terminal and oblique mouth,
Scardinius erythrophthalmus
(Linnaeus, 1758)



Common names
sorkh pareh or sorkh par (= red fin), mahi sorkh baleh, ماهي چشم
قرمز (= mahi chesm ghermez).
[giziluzkac in Azerbaijan; krasnoperka in Russian; rudd, redeye, redfin, pearl roach].
Systematics
See above under the genus. Cyprinus Erythrophthalmus was
originally described from northern Europe. This species is widely
known to spawn with other cyprinid fishes making hybrids a common occurrence.
Some Iranian material appears to be hybrids of this species and another, unknown
parental species but this has not been investigated.
Key characters
This species is often confused with Rutilus rutilus and R. caspicus but can
be distinguished by the posterior position of the dorsal fin (in
relation to the pelvic fins), the belly keel, the upturned mouth, and
the serrated pharyngeal teeth in 2 rows.
Morphology
Dorsal fin with 2-4 unbranched and 7-10, usually 8 branched rays,
anal fin with 3-4 unbranched followed by 9-13, usually 11, branched
rays (Abdurakhmanov (1962) initially gives 8-9 anal fin branched rays
for Azerbaijan fish but this may be a misprint as a subsequent table
lists 9-11 rays), pectoral fin with 13-16 branched rays, and pelvic
fin with 7-9 branched rays. Lateral line scales 36-45. There is a
pelvic axillary scale. Scales are squarish in shape, with sharp dorsal
and ventral anterior corners, a wavy anterior margin, central focus,
fine circuli which are coarser on the posterior field, and very few
anterior and posterior radii (e.g. 2 anterior and 3 posterior primary
radii reaching the focus from the margin). The scale margin is
indented where radii terminate and the thick posterior radii are
visible on the flank. Gill rakers short and widely spaced, touching the
adjacent one when appressed, and numbering 8-13. Vertebrae 37-42.
Pharyngeal teeth mostly 3,5-5,3 with variants 3,5-5,2, 3,5-5,1,
3,5-4,3, 2,5-5,3 and 2,5-5,2, narrow and elongate, slightly hooked and
with about 5-8 strong serrations on each tooth. There is a
strongly-developed, scaled keel between the vent and the pelvic fin
base. The gut is s-shaped with an anterior loop. The chromosome number
is 2n=48-50 (Klinkhardt et al., 1995).
A single Iranian specimen had the following meristics:- dorsal fin
branched rays 8; anal fin branched rays 10; pectoral fin branched rays
16; pelvic fin branched rays 8; lateral line scales 37; total gill
rakers 10; pharyngeal teeth 3,5-5,3, and total vertebrae 39..
Sexual dimorphism
Males develop breeding tubercles on the head and body.
Colour
The back is blue-black to greenish- or olive-brown, the flanks are
brassy and the belly silvery-white. Upper flank scales have dark
bases. The tips of the caudal, anal and pelvic fins are a bright,
blood red in the spawning season and the dorsal fin is black
proximally and red distally. The iris is yellow to orange, or gold,
with a red spot at the top. Peritoneum silvery with scattered
melanophores. Young are much less brightly coloured than adults.
Size
Attains 62.0 cm and 3.01 kg.
Distribution
Found from the British Isles and north of the Pyrenees east to the
Caspian and Aral sea basins. It is recorded from the Lenkoran in
Azerbaijan and, in Iran, from the Aras River, Anzali Mordab, Bojaagh wetland, Safid River, Haraz River and
Babol River (Derzhavin, 1934; Holčík and Oláh, 1992; Abbasi et al., 1999; Abdoli, 2000;
Jolodar and Abdoli, 2004; K. Abbasi, pers. comm., 21 February 2005; Patimar
et al., 2010).
Zoogeography
This species is part of a European and West Asian fauna whose origins may lie
in a Danubian or Sarmatian fauna.
Habitat
Rudd can favour heavily overgrown areas (Shikhshabekov, 1979) and
are generally found in shallow warm lakes or slow moving rivers. They
are usually inhabitants of midwater or near the surface but they
overwinter in deep water. They are regarded as fairly hardy and are
adapted to eutrophic, and presumably therefore, polluted waters. In Iranian
waters its density is highest in the Anzali Mordab (K. Abbasi, pers. comm., 21 February 2005)
and the population there is stunted through poor habitat quality
(Patimar et al.,
2010).
Age and growth
Sexual maturity is attained at 3-4 years in Dagestan at lengths of
17-29 cm and 80-530 g. A stunted form is found in rice paddies at an
age of 2 years, 7.5-11.0 cm and 10-23 g (Shikhshabekov, 1979).
Elsewhere life span is at least 17 years.
Patimar et al. (2010) studied a stunted
population in the Anzali Lagoon and found maximum total length was 146 mm and
maximum weight was 46.93 g. Males reached 4+ years and females 5+ years with
92.5 % of fish age 2+ to 3+ years. Females were longer and heavier than males.
Growth was isometric. There was no significant difference in condition
coefficients between sexes and no temporal differences when considered
separately for each date of sampling. The condition coefficient was highest in
early May for males and mid-May for females and was lowest in early June for
both sexes. Females dominated in older age classes and males in the younger.
Food
Food is aquatic macrophytes as well as insect larvae, crustaceans,
molluscs and more rarely fish eggs and fry. The young feed on zooplankton.
Reproduction
Spawning takes place at water temperatures of at least 18-20°C
in June-July in Dagestan. Each female can be accompanied by two males,
one on each side. Two batches of eggs may be spawned in this period (Shikhshabekov,
1979). In the Volga Delta, spawning takes place from April until the
end of June. Eggs attach to water plants. The young remain attached to
vegetation until the yolk-sac is absorbed. Fecundity is up to 232,000
eggs with a diameter of 1.5 mm. Hatching takes 3 days at 20-22°C.
The Iranian study of
Patimar et al.
(2010) found a reproductive period of mid-April to late May and showed that the
gonadosomatric index peaked in mid-May for both sexes and then decreased
sharply. Egg diameters reached 1.23 mm, maximum fecundity reached 59,620 eggs
and relative fecundity 1737.69 eggs/g.
Parasites and predators
Masoumian and Pazooki (1998) surveyed myxosporeans in this species in Gilan
and Mazandaran provinces, finding Myxobolus pfeifferi. Sattari et al.
(2004) record the nematode Raphidascaroides acus larvae from this
species in Gilan. Khara et al.
(2006b) record the nematode Raphidascaris acus from this species in the
Boojagh Wetland of the Caspian coast. The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
Holčík and Oláh (1992) report a catch of 98 kg in the Anzali Mordab in 1990.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in sport and in textbooks and because it has been introduced
outside its natural range.
Conservation
Lelek (1987) classifies this species as vulnerable in Europe. Kiabi
et al. (1999) consider this species to be conservation
dependent in the south Caspian Sea basin according to IUCN criteria.
Criteria include sport fishing, medium numbers, habitat destruction,
limited range (less than 25% of water bodies), absent in other water
bodies in Iran, present outside the Caspian Sea basin. Near threatened in Turkey
(Fricke et al., 2007).
Further work
The distribution and biology of this species and its potential for
hybridisation with other cyprinids requires study in Iran.
Sources
Iranian material: Uncatalogued, 1, 103.7 mm standard length, Gilan, swamp near Hendeh Khaleh (37°23'N, 49°28'E).
Genus Schizocypris
Regan, 1914
This genus of medium-sized snow trouts contains only 2 species
found in Pakistan, Afghanistan and Iran. Coad and Keyzer-de Ville (2005) revised the genus.
It is characterised by a rounded and moderately elongate body,
scales small but larger near the shoulder region, belly scaleless, a
wide and transverse mouth with the snout projecting, no barbels or
barbels vestigial, pharyngeal teeth with a flat tip unlike Schizothorax
and a formula of 2,3,4-4,3,2 rather than 2,3,5-5,3,2 as in Schizothorax,
dorsal and anal fins short but 6 branched anal fin rays not 5 as in
related genera in the same area, dorsal fin with a strongly serrated spine,
scales in the vent region are split and enlarged to flank the urogenital region, and radii are on all scale fields.
Rainboth (1981) includes Capoeta trutta, Capoeta fusca
and Capoeta nudiventris (= C. fusca) in this genus but this is incorrect.
These species show some enlargement of scales around the anus and anal
fin region but it is not as marked and definitive as in true Schizocypris
and other characters of the genus are absent.
Schizocypris altidorsalis
Bianco and Banarescu, 1982
Common names
gorgak (= small wolf), anjak or khaju (A. A. Pasand, pers. comm.,
5 November 2000 but see under Schizothorax zarudnyi and S. pelzami).
Systematics
Schizocypris brucei, non Regan, 1914 (Annandale and
Hora, 1920) is a synonym. Schizocypris brucei Regan, 1914 is
thus restricted to the Gomal River drainage in the Indus River basin
of Pakistan (originally described from the Wana Toi, a Gomal River
tributary). A third species in the genus Schizocypris is S.
ladigesi Karaman, 1969 from the Kankai River, also in the Indus River basin.
Kähsbauer (1964) reports a hybrid between Schizothorax
schumacheri and Schizopygopsis stoliczkae from Sistan which
may in fact be this species.
The holotype of Schizocypris altidorsalis is in the Istituto
di Zoologia dell'Universitá di L'Aquila, Italy under IZA 8169 and is
73.7 mm standard length (my measurment) (Bianco and Banarescu, 1982). The type locality
is "Nahr-Taheri near Zabol, Seistan". Paratypes include 5
fish from the type locality under IZA 7841, 35-65 mm standard length
(4 fish seen by me, 35.4-62.0 mm standard length) with further specimens in the Institutul de Stiinte Biologice,
Bucuresti, Romania (ISBB 3136). Three paratypes, 68-73 mm standard
length, from "Rud-Sistan, 8 km from Zabol, Seistan" are
under IZA 7844 (69.9-74.3 mm standard length measured by me) with further specimens under ISSB 3137. Two paratypes
from the Nahr Taheri are in the Zoologischen Instituts und
Zoologischen Museums der Universität Hamburg (ZMH 6091, 77.2-81.9 mm standard
length) (Wilkens and Dohse, 1993; examined by me), 2 paratypes are in the American Museum of Natural
History, New York (AMNH 40952), 1 paratype is in the Muséum national
d'Histoire naturelle, Paris (1982-1018), 1 paratype is in the United
States National Museum, Washington (USNM 227928), 2 paratypes are in
the Academy of Natural Sciences, Philadelphia (ANSP 150977), and 2
paratypes are in CMNFI 1982-0368 (formerly IZA 7841).
Syntypes of Schizocypris brucei are in the Natural History
Museum, London under BM(NH) 1913.4.15:100-109 (10 fish) and in the
Zoological Survey of India, Calcutta under ZSI F9832/1 (1) (Menon and
Yazdani, 1968; Eschmeyer et al., 1996).
Berg (1949) advanced the possibility that this species is the
juvenile of Schizothorax zarudnyi since barbels and scale cover
develop with age. However the tooth formula is very distinctive as is the anal
fin branched ray count and lack of barbel development at all sizes in S.
altidorsalis (Coad and Keyzer-de Ville, 2005).
Key characters
This species is characterised by a very high dorsal fin with a
strongly denticulated spine. The spine is longer than the head and the
denticles easily catch the skin when the fish are handled. This is
particularly true of small fish, larger ones are not so snaggly.
Bianco and Banarescu (1982) give values for spine length of 24.4-29.8%
of standard length in altidorsalis, 19.4-20.4% in brucei,
and 14.7-19.5% in ladigesi. For 35 altidorsalis
66.1-175.1 mm standard length examined here, spine length is
23.3-31.3% of standard length and 14.0-18.5% for 20 brucei
102.3-170.8 mm standard length. Dorsal spine length in head length is
0.7-0.9, mean 0.9 for altidorsalis, 1.2-1.6, mean 1.4 for brucei
(see also below). Scales in the lateral line are 87-96 (brucei has 74-81 and ladigesi
78-88) according to Bianco and Banarescu (1982). Specimens examined by
me have lateral line counts of 81-95, mean 87.4 for 60 altidorsalis and
73-91, mean 79.6 for 56 brucei, showing some overlap but scales are
definitely smaller on average in altidorsalis.
Bianco and Banarescu (1982) describe the body as mostly scaled
except on the anterior part of the breast (scaled on the mid-line of
the back in front of the dorsal fin as in ladigesi, naked in brucei),
and scales embedded on most of the body except the caudal peduncle and
the lateral line; but see below.
Morphology
Dorsal fin unbranched rays 4, branched rays 7-8, anal fin
unbranched rays 3, branched rays 5-6, pectoral fin rays 14-20, pelvic
fin rays 7-9, lateral line scales 82-96, total gill rakers 24-30,
reaching the third raker below when appressed in large fish but only
one raker below in small fish, pharyngeal teeth usually 2,3,4-4,3,2(16) with
variants 2,3,4-4,3,3(1), 2,3,5-4,3,2(2) and 2,3,5-4,4,2(1),
and total vertebrae 43-45. Since spines are often broken off, the height of the
dorsal fin can be measured as the longest branched ray. For this species it is
16.5-28.8% standard length (mean 24.3, 60 fish) while in S. brucei, the
taxon fish in Sistan were formerly assigned to, it is 11.6-20.0% (mean 16.8, 56
fish), clearly distinguishing these species.
Scales are regularly arranged over most of the body. In some fish,
scales near the tail are difficult to distinguish. Shoulder scales are
moderately large anteriorly above the lateral line and decrease in
size posteriorly. The back is naked in a narrow band for a short
distance anterior to the dorsal fin (not so according to Bianco and
Banarescu (1982) but visible in fish examined by me). Flank scales are small but those at the dorsal fin
base are a little larger. Lateral line scales are larger than those on
the flank but only on the anterior lateral line. The breast is scaleless
according to Bianco and Banarescu (1982) but is scaled on
the breast in large specimens and some small ones too. There is a
pelvic axillary scale. The scale focus is anterior with radii on all
fields and in overall shape is oval to rounded. Radii
are found on all fields but are few in number (15).
The mouth is inferior, transverse, and slightly arched. The lower
lip is developed only laterally. The lower jaw is covered by a horny
sheath in some specimens, lost in others. There are usually no barbels
(n = 105) although one fish, 70.1 mm standard length, had a minute
pair of barbels hidden in the lip grooves and two other fish of
similar size had respectively a single right and a single left minute
barbel. S. brucei has small but protruding barbels on both
sides in 15 fish, a left barbel only in 3 fish, a right barbel only in
5 fish and no barbels in 8 fish.
The gut is very elongate and complexly coiled. The anterior main
row pharyngeal tooth is peg-like while the rest are spatulate with a
deep central groove and crowns flared on each side of the groove.
Meristics for Iranian specimens are:- dorsal fin branched rays 7(2)
or 8(38); branched anal fin rays 5(1) or 6(39); pectoral fin branched
rays 14(1), 16(11), 17(23), 18(4) or 20(1); pelvic fin branched rays
7(1), 8(35) or 9(4); lateral line scales 81(1), 82(2), 83(3), 84(4), 85(6),
86(8), 87(10), 88(7), 89(5), 90(4), 91(3), 92(3), 93(3) or 95(1); total
gill rakers 24(1), 25(4), 26(5), 27(10), 28(12), 29(6) or 30(2);
pharyngeal teeth 2,3,4-4,3,2(16), 2,3,4-4,3,3(1), 2,3,5-4,3,2(2), or
2,3,5-4,4,2(1); and total vertebrae 43(16), 44(33) or 45(11).
Sexual dimorphism
Unknown.
Colour
The back is bluish and the flanks and belly are silvery. The flanks may have a few small to numerous black spots.
Size
Reaches 17.5 cm standard length.
Distribution
This species is endemic to the Sistan basin of Iran, and presumably adjacent Afghanistan (Bianco
and Banarescu, 1982; J. Holčík, in litt., 1996). The distribution of this species (as S. brucei) in Khorasan
and Gorgan reported by Wossughi (1978) is incorrect.
Zoogeography
A relative of other schizothroacine species found along the mountain chain
from Iran to China. See also the genus Schizothorax.
Habitat
Occurs in a wide range of habitats from ditches to hamuns. Reported from pools in dry river beds and still, reedy channels in Sistan. E.
Penning (pers. comm., 28 July 2005) states that this was the dominant fish species in Hamun-e Puzak and Hamun-e
Saberi in April 2005 after a dry period when the hamuns flooded at the end of
February. The fish enter the flooding hamuns from the upstream parts of rivers.
In July, water levels fell from1-2 m to less than 1 m and this species was
absent, presumably having returned to the more permanent rivers. They were also
observed swimming up a fish staircase at the Sistan Dam.
Age and growth
Unknown.
Food
The principal food is aufwuchs and detritus as evidenced by the
sectorial mouth and elongate gut. Gut contents are a fine mush. E. Penning (pers.
comm., 28 July 2005) observed filamentous algae in the gut.
Reproduction
Most fish examined by me were young and no data on reproduction is
available. E. Penning (pers. comm., 28 July 2005) noted that fish at 20-30 cm caught in April 2005 had eggs 1 mm in diameter and
were approaching or ready for spawning.
Parasites and predators
Jalali et al. (1995) describe a new species of monogenean, Dactylogyrus
schizocypris, from fish taken in the Chahnimeh water reservoir near the Hamun Lake in Sistan.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lamproglena compacta
and Lernaea sp. on this species.
Economic importance
Individual fishermen caught 5-10 kg per day of this species in 2005 although
they considered individuals as small and catches very low. Fish were 10-20 cm
long with some larger ones at 20-30 cm (E. Penning, pers. comm., 28 July 2005).
Rahimabadi et al. (2009) assessed the lipid quality of this species and
found it to be less than that of Schizothorax zarudnyi.
Conservation
Further knowledge of biology is needed to assess conservation status.
Further work
The biology of this species needs study.
Sources
Type material: See above, Schizocypris altidorsalis (IZA 8169, IZA 7841, IZA 7844, ZMH 6091 and CMNFI 1982-0368).
Iranian material: CMNFI 1979-0072, 2, 120.8-162.9 mm standard length, Sistan, river near Zabol (ca. 30º58'N, ca. 61º28'E);
CMNFI 1979-0223, 1, 19.9 mm standard length, Sistan, ditch south of Lutak (30º45'N, 61º24'E);
CMNFI 1979-0224, 13, 48.2-77.5 mm standard length, Sistan, Hirmand River effluent (30º53'N, 61º27'E);
CMNFI 1979-0225, 1, 147.7 mm standard length, Sistan, Hirmand River effluent (30º58'N, 61º28'E);
CMNFI 1979-0226, 107, 60.0-82.5 mm standard length, Sistan, pool east of Kuh-e Khajeh (30º57'N, 61º17'E);
CMNFI 1979-0228, 16, 18.1-73.8 mm standard length, Sistan, ditch 1 km from Zabol (31º02'N, 61º31'E);
CMNFI 1979-0229, 11, 61.0-84.9 mm standard length, Sistan, ditch 5 km from Zabol (31º03'N, 61º33'E);
CMNFI 1979-0231, 2, 17.5-19.9 mm standard length, Sistan, irrigation jube 3 km from Zabol (31º01'N, 61º32'E);
CMNFI 1979-0232, 23, 41.8-78.8 mm standard length, Sistan, irrigation ditch 11 km from Zabol (30º58'N, 61º36'E);
CMNFI 1979-0233, 2, 66.0-71.5 mm standard length, Sistan, irrigation ditch 15 km from Zabol (ca. 30º57'N, ca. 61º38'E);
CMNFI 1979-0234, 15, 13.9-82.4 mm standard length, Sistan effluent of the Hirmand River near Zahak (30º54'N, 61º40'E);
CMNFI 1979-0236, 1, 14.4 mm standard length, Sistan, irrigation ditch 27 km from Zabol (ca. 30º52'N, ca. 61º22'E);
CMNFI 1979-0237, 5, 21.4-72.1 mm standard length, Sistan, irrigation ditch 18 km south of Zabol (30º53'N, 61º27'E).
Comparative material: USNM 182276, 5, 95.7-153.1 mm standard length, Afghanistan, Arghandab Reservoir (ca. 31º51'N, 65º55'E);
USNM 182277, 1, 159.7 mm standard length, Afghanistan, Arghandab River at Kandahar (ca. 31º35'N, 65º45'E);
USNM 182281, 3, 71.2-79.9 mm standard length, Afghanistan, Lashka-dah, Helmand River (ca. 31º35'N, ca. 64º21'E);
USNM 182282, 5, 142.1-159.4 mm standard length, Afghanistan, Laskha-dah area (ca. 31º35'N, ca. 64º21'E);
ZMUC 261629-34, 6, 131.4-203.7 mm standard length, Afghanistan, Sistan, Feyzabad (31º28'N, 61º31'E).
Material of Schizocypris brucei and S. ladigesi, nominal species not found in Iran, are listed in Coad and Keyzer-de Ville (2005)
Genus Schizopygopsis
Steindachner, 1866
This genus contains about 8 species distributed from Iran along
the Himalayas to China. There is a single species in Iran.
The osmans are characterised by an elongate and almost cylindrical
body, almost scaleless, scales being restricted to the complete
lateral line, the flank above the pectoral fin and as enlarged scales
around the vent and anal fin base, the mouth is terminal to inferior,
the lower jaw is sometimes covered by a horny sheath, lips are present
but may only be developed at the mouth corners, no barbels, pharyngeal
teeth in 2 rows, spatulate or cochleariform, short dorsal and anal
fins, dorsal fin last unbranched ray thickened (but not strongly) and
serrated or denticulate (denticles lost with age), peritoneum black,
and gut very elongate.
Schizopygopsis stoliczkai
Steindachner, 1866

Common names
kopur-e barfi (= snow trout).
[lozhnyi osman or false osman in Russian; Pamir snowcarp].
Systematics
The type locality of this species is a stream near Hanle Monastery,
Ladakh, India. Syntypes are in the Naturhistorisches Museum Wien under
NMW 9255 (1), NMW 9256 (1), NMW 51472 (9) and NMW 51473 (2) (Eschmeyer
et al., 1996; 1997 Vienna card catalogue).
The species name is spelt Stoličkai by Steindachner (1866) which becomes correctly stoliczkai since
accents are not used in Latin nor capitals for the scientific species
name. It is often spelt stoliczkae in the general literature.
Schizopygopsis stoliczkae infraspecies sewerzowi
Herzenstein, 1890, originally described from the Bulun Kul and Karasu,
Amu Darya basin in the Pamir Mountains has no taxonomic validity (it
occurs together with the typical form in the upper Amu Darya) but it
was used to characterise the form in the Sistan basin of Iran (Berg,
1948-1949). However, Annandale and Hora (1920) report both this and
the stoliczkae form, without intermediates, in Sistan. The sewerzowi
form differs from the typical form by having a larger eye (1.2-1.3
times in interorbital width as opposed to 1.5-1.7 times and 3.8-4.4
times in head length as opposed to 4.8-5.7 times), a spotted rather
than monotone body, smaller size (much less than half that of the
typical form), somewhat deeper body, and more oblique mouth with apex
at the lower eye level. Data presented below under Food
indicates that this species can be very plastic in its characters so
subspecies designations would require extensive study of both
characters and ecology. The Iranian fish are poorly represented by
specimens and are referred here simply to the species.
Syntypes of sewerzowi (dated 1891 in Eschmeyer et al.
(1996)) are in the Zoological Institute, St. Petersburg under ZISP
8747 (2), ZISP 8748 (2), ZISP 8749 (2), ZISP 8780 (1), ZISP 8901 (2) (Eschmeyer
et al., 1996).
Kähsbauer (1964) reports a hybrid between Schizothorax
schumacheri and Schizopygopsis stoliczkae from Sistan which
may in fact be Schizocypris altidorsalis.
Key characters
The anal fin base has a sheath of enlarged scales and there are a
few scales in the shoulder region and along the lateral line but the
body is nearly naked, the anal fin has 5 branched rays, barbels are
absent, and pharyngeal teeth are in 2 rows.
Morphology
Dorsal fin branched rays 7-9, usually 8, after 3-4 unbranched rays,
anal fin branched rays 5-6, usually 5, after 2-3 unbranched rays,
pectoral fin branched rays 12-20, and pelvic fin branched rays 8-10.
Total vertebrae 48 (Howes, 1987). The lateral line is complete, a
pelvic axillary scale is present and the anus and anal fin base are
sheathed in enlarged scales. Scales are also present on the anterior
lateral line and between there and the anterior pectoral fin base. A
double row of scales extends forward from the anal sheath to the
pelvic fin bases. Lateral line scales 96-120.
The dorsal fin spine is weakly developed and lacks denticles in
large fish while young have many well-developed denticles. The gut is
very elongate and coiled. The lower jaw is strong, with a dark brown,
horny plate. The pharyngeal teeth number 3,4-4,3 and have a rounded
base becoming spatulate distally with a rounded, hooked tip. Some
teeth have a weak, flat cusp with a bump posteriorly below a rounded tip.
Meristic values for Iranian specimens are:- dorsal fin branched rays 8(3); anal fin
branched rays 5(3); pectoral fin branched rays 17(1), 18(1) or 19(1);
pelvic fin branched rays 9(3); lateral line scales 91(1) or 92(1);
total gill rakers 11(3), reaching the raker below when appressed; and
total vertebrae 48(1) or 50(2).
Sexual dimorphism
Tubercles are present on the anal fin of two male specimens from
Sistan in a single file on the last unbranched and first 3 branched
rays. They number up to 8 and are often widely spaced. Tubercles are
found low on the flank above the anal fin and anus and on the upper
flank behind the dorsal fin level. They also line the posterior
lateral line. There are also a very few tubercles on the top of the
head, side of the snout, below the eye and on the operculum, widely
scattered and small.
Colour
Dark or yellowish with small dark brown or blackish spots extending
onto the fins, or olive with large grey spots, or bluish-grey. Spots
can be so numerous as to give the appearance of an overall blackish
colour. Tilak (1987) however states that blackish spots are absent in
both old and freshly preserved material. Both spotted and non-spotted
forms occur in Sistan. The belly is whitish. Fins are pink. Peritoneum
dark brown to black.
Size
Reaches 75.4 cm but the Sistan form is only up to 22.0 cm and is
regarded as a dwarf form by Annandale and Hora (1920).
Distribution
Found in the mountainous areas of Afghanistan, Pakistan, India and
western China. In Iran, restricted to the lowland Sistan basin where reported
from the Hirmand River delta and 8 miles east of Lab-e Baring (Annandale, 1921; Vijayalakshmanan, 1950).
Zoogeography
The presence of this species in Sistan is an example of a riverine highway
enabling species usually found at higher altitudes to penetrate into lowlands.
See also the genus Schizothorax.
Habitat
Outside Iran the adults favour the main river while young are found in shallow streams and pools.
Age and growth
Savvaitova et al. (1989) cite a life span up to 22 years.
Maturity in the Sistan form is attained at 18.0 cm. Berg (1949)
reports a male fish 14 cm long with fairly well-developed testes and
tubercles on the anal fin which bears out the dwarf nature of the Sistan fish.
Food
Savvaitova et al. (1989) examined feeding in certain lakes
of the Pamirs at altitudes over 3220 m. This is much higher than the
Sistan populations and the data may not be relevant. However the false
osman is a poorly studied species so such information gives a basis
for comparison and future research. The false osman is a very plastic
species and can adapt to a variety of conditions. The osmans of the
Pamirs were divisible into four groups: herbivores, detritivores,
molluscivores and predators reflected in the structure of the gut and its
length, the number of gill rakers, eye diameter, length and position of
fins, and the shape of the horny plate on the lower jaw. Food includes
higher aquatic plants, aquatic insects, diatoms and blue-green algae,
detritus, molluscs and fish. In herbivores consuming periphyton, the
horny plate on the lower jaw is rasp-shaped with the pointed end aimed
anteriorly. In detritivores, the plate is sharper, and in predators, it
is larger with the pointed edge directed upwards to grasp prey.
Predatory behaviour only develops in fish over 30 cm, until which they eat plants.
Chaudhary et al. (1991) indicate that in a Pakistani
population gut length increases with age and diet changes gradually
from an omnivorous to a herbivorous one.
Reproduction
Spawning in Asia occurs generally in June and July. Eggs are large at 2.0 mm diameter.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This species is rarely collected in Sistan and may require conservation measures.
Further work
Detailed surveys are needed to document the occurrence of this species in
Sistan and to study its biology in a lowland habitat. Comparative studies
with samples of the species from other, remote localities may demonstrate
some level of taxonomic distinction as is seen in other schizothoracines of Sistan.
Sources
Iranian material: BM(NH) 1905.4.7:1-2, 2, 113.0-116.5 mm standard length,
Sistan (no other locality data); ZISP 25854, 1, 111.5 mm SL, Sistan (no other locality data).
Comparative material: BM(NH) 1931.10.26:2-4, 2, 85.5-138.6 mm standard
length, India, Ladkah, stream into Pangong Lake (no other locality data).
Genus Schizothorax
Heckel, 1838
The snow trouts, mountain barbels or Indian trouts are found from
Iran to China, favouring mountainous areas but occasionally in lowlands. There
are about 56 species (more if some other genera are included), with 3 in Iran.
The genera Racoma McClelland and Griffith in McClelland,
1842 and Aspiostoma Nikol'skii, 1897 are synonyms of Schizothorax Heckel, 1838 (Eschmeyer, 1990).
Schizothorax intermedius, and other species, have been placed
in the genus Schizothoraichthys Misra, 1962 (e.g. in Tilak
(1987). However Schizothoraichthys is regarded as a synonym of Schizopyge
Heckel, 1847 by Jayaram (1981) or of Schizothorax (see Talwar,
1978; Eschmeyer, 1990). Schizopyge is itself regarded as a
synonym of Schizothorax by some authors, e.g. Talwar (1978).
Talwar (1978) separates the genus Oreinus McClelland, 1839 from
Schizothorax by the margin of the lower jaw having a firm and
hard horny covering which is thickest internally and a thick lower lip
with a free posterior edge forming a sucker. Schizothorax has a
non-suctorial lower lip and a lower labial fold interrupted or entire
in the middle. However Talwar and Jhingran (1991) contradict this view
and use Schizothoraichthys for Schizothorax and Schizothorax
for Oreinus. Tilak (1987) recognises the name Schizothorax
for fishes with strip of papillated tissue on the chin and Oreinus
as a synonym; Schizothoraichthys is used then for fishes
without the papillated chin. Oreinus is regarded as a synonym
of Schizothorax by Jayaram (1981) and Eschmeyer (1990). Mirza
(1991a; 1991b) recognises a tribe Schizothoracini, with the genera Schizothorax,
Schizopyge, Racoma McClelland (and Schizocypris).
I have retained Schizothorax here as the oldest name in view of
these conflicting opinions.
There are various records of nominal Schizothorax species
from the Helmand River basin in Afghanistan summarised in Coad
(1981d); they have not been reported from the Sistan lowlands of Iran.
This genus is characterised by an elongate and almost cylindrical
body, very small scales, over 100 in the series next to the lateral
line, scales in complete lateral line somewhat larger, the vent and
anal fin base are sheathed in enlarged scales and there may be
enlarged scales near the pectoral fin and edge of the gill opening,
dorsal and anal fins are short, dorsal fin with a thickened last
unbranched ray bearing denticles (denticles lost with age), pharyngeal
teeth in 3 rows and hooked at the tip, 4 barbels (rostral and
maxillary), mouth inferior or subterminal, lower jaw may have a horny
sheath, a papillated area on the chin may be present or absent, the
lower labial fold may be interrupted or not in the middle, elongate
gut and black peritoneum, and poisonous eggs. Members of the
Schizothoracinae tribe are of polyploid origin with 2n=98 and 3n=148.
The ancestors of the schizothoracines in general were barbinines in the
eastern part of the Qinghai-Xizang Plateau as it rose and water temperatures
decreased in the late Miocene to early Pliocene (Sizhong, 1995). Primitive
genera like Schizothorax migrated westwards earlier and further than more
specialised genera such as Schizopygopsis (although both reach their
westernmost distribution in Iran).
These fishes generally prefer rapids and pools of the larger
streams at temperatures of 8-22°C although some occur in lakes with inflowing streams (Sharma, 1988).
They are found in streams above 3000 m. Food varies from detritus to
insects, plankton and fish depending on the species. The spawning
season may be in late summer and early fall or in spring. Egg counts
vary from a few hundred to over 50,000 and egg diameters may attain
3.6 mm. Some species show a spawning migration from warm lakes to cold streams.
Deaths have occurred from eating poisonous eggs of members of this
genus but none are reported from Iran (see under S. zarudnyi).
Symptoms include abdominal pain, nausea, vomiting, diarrhoea,
dizziness, headache, fever, bitter taste, dryness of the mouth,
intense thirst, sensation of chest constriction, cold sweats, rapid
irregular weak pulse, low blood pressure, cyanosis, pupillary
dilatation, syncope, chills, dysphagia and tinnitus. Severe cases show
muscular cramps, paralysis, convulsions, coma, and death. Victims
generally recover within 3-5 days with supportive treatment but it may
take longer. Treatment is symptomatic and there is no known antidote
or therapeutic data available. The patients' stomach should be
evacuated as soon as possible after ingestion of eggs (Halstead,
1967-1970; Coad 1979b). Fish eaten during the breeding season should
be cleaned with care to remove all traces of the eggs to avoid
contamination of the flesh as cooking does not destroy the toxin.
Schizothorax esocinus
(Heckel, 1838)
Reported from the Helmand River drainage of Afghanistan; records
summarised in Coad (1981d). No Iranian record.
Schizothorax intermedius
McClelland, 1842
Variation in ventral head structure, above and below
Common names
mawda (in Sistan), marinka.
[marinka obyknovennaya or common marinka in Russian.
Systematics
Schizothorax intermedius was described from the "Cabul
river at Jullalabad. Tarnuck River" in the Indus River basin.
Oreinus plagiostomus McClelland, 1842 described from the
"Helmund river at Girdun Dewar" in Afghanistan, Racoma
brevis McClelland, 1842 described from the "Helmund
River", Racoma labiatus McClelland, 1842 described from
"Pushut, Koonar river near Jullalabad" in the Indus River
basin but also reported from the Helmand River basin by Annandale and
Hora (1920) and Schizothorax ritchieana McClelland, 1842
described from "Affghanistan. In the Helmund there is a variety
of this species ..... which will probably prove to be distinct."
are probably synonyms (Berg, 1949).
Berg (1948-1949) reviews three morphae or forms of this species
which indicate the great variation in this taxon. He also notes how
barbel length varies independently of morpha and how the lips may be
very strongly developed. The forms are as follows and the latter two
were originally described as distinct species and are recognised as such by some
authors: typica, eurystomus Kessler, 1872 and fedtschenkoi
Kessler, 1872. Eurystomus has a transverse jaw covered by horny padding and a strong or weak
dorsal fin spine. Fedtschenkoi has a lower jaw without the
horny pad and the lower lip continuous or interrupted (the form with
an interrupted lip is called morpha irregularis Day, 1896; the date is
incorrect and should be 1877) and
the dorsal fin spine is weak with 10-22 denticles extending to the
mid-point or two-thirds along the ray. The forma typica has a
crescent-shaped lower jaw without horny padding, the dorsal fin spine
is well-developed with 12-32 denticles extending distally beyond the
mid-point, and the lower lip is interrupted.
Schizothorax schumacheri Fowler and Steinitz, 1956 described
from "Zabol, Eastern Iran" is also a synonym (Saadati,
1977). The holotype of Schizothorax schumacheri (ANSP 71950) at
244 mm total length and a paratype (ANSP 71951)
at 130 mm to the end of the broken caudal fin, are stored in the
Academy of Natural Sciences of Philadelphia (Böhlke, 1984).
Kähsbauer (1964) reports a hybrid between Schizothorax
schumacheri and Schizopygopsis stoliczkae from Sistan which
may in fact be Schizocypris altidorsalis.
Tilak (1987) reports Schizothorax richardsonii (Gray, 1832)
from Sistan based on 2 fish in the Zoological Survey of India,
Calcutta (F. 1226-1227/1), which I have not seen, and this may be the
correct name for this fish.
Key characters
Gill raker counts and distribution separate this Schizothorax from
others in Iran.
Morphology
This is a very variable species, depending on habitat (Mirzaev, 1998). Dorsal fin with 2-4
unbranched and 5-9, usually 8 (Kullander et al. (1999) give 6-7 for their
Kashmir specimens of S. curvifrons), branched rays, anal fin with 1-3
unbranched and 4-7, usually 5, branched rays, pectoral fin branched rays 14-19
and pelvic fin branched rays 7-10, usually 8. Lateral line scales 85-121, scale
series next to the lateral line 115-165. Pharyngeal teeth 2,3,5-5,3,2. Gill
rakers 10-17 (Kullander et al. (1999) give 21-28 for their Kashmir
specimens of S. curvifrons). Total vertebrae 48 (Howes, 1987) or 40-43
(2000a) – presumably excluding 4 Weberian vertebrae). There is considerable
variation in lower jaw form in specimens attributed to this species. The lower
jaw may be crescent-shaped with or without a sharp horny sheath, or covered with
a deciduous horny layer, or transverse and covered by a horny sheath. Lips may
be interrupted medially or continuous, and can be very strongly developed. The
dorsal fin spine may be well-developed with numerous denticles or
weakly-developed with denticles not beyond the middle of the spine. Various
morpha or infraspecies have been described to refer to these forms (see Berg,
1948-1949). Barbel length is highly variable. Young about 30 mm long have a
naked body and no barbels. The karyotype is 2n=98-100.
Usually silvery and occasionally with minute black spots on the upper half of the body
but usually without spots. The head is olive-green. In preservative a pale
greyish brown above, flanks and lower surfaces brilliant silvery or light
yellow. Fins greyish to pale olive with lower ones whitish. Iris bright silvery white.
SizeReaches 60.0 cm (Solijonov, 2007).
Sexual dimorphism
Unknown.
Colour
Usually silvery and occasionally with minute black spots on the
upper half of the body. In preservative a pale greyish brown above,
flanks and lower surfaces brilliant silvery. Fins greyish to pale
olive with lower ones whitish. Iris bright silvery white.
Size
Reaches 50 cm.
Distribution
Found in the basins of the Indus, the Amu Darya and Syr Darya rivers, the Tarim basin and
the Helmand (= Hirmand) of Afghanistan and Iran.
Zoogeography
See under genus description.
Habitat
Reported from both lotic and lentic environments but little is known of
its environmental requirements.
Age and growth
Life span is at least 8 years. Solijonov (2007), in a study of fishes in the Pamir-Alai Transboundary Conservation Area,
found this species as being most active in the evening and so is seldom caught
in the daytime when it hides in refuges among rocks. It maintains station in
fast water of Pamir-Alai mountain streams behind rocks and in whirlpools. Males
mature here at 2-3 years and females at 3-4 years and spawning takes place in
mid-May.
Food
Food is small aquatic fauna, vegetation and detritus. Akhrorov and Kondur (1981) found
macrophytes, detritus and molluscs to be important foods in a Pamir lake,
varying with the year of sampling such that molluscs dominated in one year and
macrophtyes in another.
Reproduction
Spawning takes place between May and September, depending on locality,
and up to 8678-59,895 eggs are produced in fish 21.5-37.1 cm and 211-913 g (Mitrofanov
et al., 1988). Spawning is probably non-annual in some areas (Maksunov,
1971).
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
This species is not well-documented in Iran and no assessment of conversation
needs can be made. It may be a stray from higher latitudes in Afghanistan.
Further work
Surveys should be done to confirm its presence in Iran.
Sources
Iranian material: None.
Schizothorax labiatus
(McClelland, 1842)
Reported from the Helmand River drainage of Afghanistan; records
summarised in Coad (1981d). May be a synonym of Schizothorax
intermedius (McClelland, 1842). No Iranian record.
Schizothorax pelzami
Kessler, 1870
Common names
shir mahi.
[Zakaspiiskaya marinka or Transcaspian marinka, and forel, in Russian].
Systematics
Schizothorax raulinsii Günther, 1889 described from a skin
from the "Hari-rud River, near Khusan" and Schizothorax
pelzami iranicus Karaman, 1969 are synonyms. Wossughi (1978)
considers Schizothorax pelzami iranicus to be only a large
specimen of S. p. pelzami and Coad and Keyzer de Ville (2004) concur.
The holotype of Schizothorax Poelzami is in the Zoological
Institute, St. Petersburg (ZISP 8036, 265 mm total length) and is from "Fl. Schach-rud.
accursus fl. Sefid-rud in Persia. 1889. Univ. Petropol.". The
Shah Rud or Shah River is a tributary of the Safid Rud of the Caspian
Sea basin but this species does not occur there. Berg (1948-1949)
cites A. N. Derzhavin who suggests that this Shah-rud is south of
Astrabad (= Gorgan). There is a Shahr Now River in the Tedzhen or Hari
Rud basin where this species is found (shahr is the Farsi for
city and may have been a general term for a major river of
northeastern Iran as it flows through a city). However, the type
probably came from the environs of the city of Shahrud (= Emamshahr)
in the Damghan basin, a sub-basin of the Dasht-e Kavir basin (see below).
Another specimen listed as a type from the "Schah-Roude.
Persia. Pelzame. St. Petersburg University" measuring 78.6 mm
standard length is in the Natural History Museum, London (BM(NH)
1897.7.5:24). Kessler (1870) and Eschmeyer (1998) list 4 syntypes so two appear
to be lost but Coad and Keyzer de Ville (2004) point out a disparity in size
range (120-180 mm for the syntypes according to Kessler (1870)) while the London
fish is too small and may not be a type despite its label.
The type of Schizothorax raulinsii is from
the Hari-rud River, near Khusan, Afghanistan. BM(NH) 1886.9.21:181, is a skin marked as a syntype, 312.8 mm standard length,
Hari-rud River, near Khusan, presumably the skin referred to by Eschmeyer et al. (1996). The skin is also marked
Schizothorax “aitchisonii” (= S. raulinsii, a synonym of S. pelzami); Albert Günther of the British Museum apparently confused the names Aitchison
and Raulins, collectors of specimens on the Afghan Delimitation Commission;
aitchisonii was never used and the skin is a syntype of raulinsii.
Eschmeyer lists a skin and 2 other syntypes in the Natural History Museum, London and 2
syntypes in the Zoological Survey of India, Calcutta (ZSI F11477-78). These 4
additional specimens are presumably the 4 smaller fishes mentioned by Günther
(1889) in his original description as being collected at Bezd on the Jam River in Iran.
BM(NH) 1886.9.21:171-172, 2, 87.8-100.9 mm standard length, Bezd, are not
currently listed as syntypes in the British Museum however.
Schizothorax pelzami iranicus Karaman, 1969 is described
from "Teheran in Quelle" (= Tehran in a spring) based on a
single specimen. Schizothorax pelzami does not occur in the
Namak Lake basin in which Tehran lies and the subspecies may have come
from the Damghan part of the Dasht-e Kavir basin. The subspecies differs from
the type subspecies by having a weakly ossified spiny ray in the
dorsal fin (only the first half with small teeth), smaller eyes,
longer snout and an overall brown to blackish-grey body colour with
all fins, lips and barbels dark-coloured as opposed to the sharp
boundary between the dark brown dorsal side and the light ventral side
(Karaman, 1969). The Damghan part of the Dasht-e Kavir basin is the type
locality of the species and S. p. iranicus is a
synonym (Coad and Keyzer de Ville, 2004).
The holotype of S. p. iranicus is in the Zoologischen
Instituts und Zoologischen Museums der Universität Hamburg (ZMH
4116, 327.5 mm standard length).
Starostin (1936) reports a hybrid of this species and Capoeta
heratensis (= Capoeta capoeta) from Turkmenistan.
Key characters
This is the only schizothoracine species in northeast Iran and is easily
recognised by its high lateral line scale count and the enlarged scales around
the anus and anal fin.
Morphology
Dorsal fin branched rays 7-8 after 3-4 unbranched rays, anal fin
branched rays 5-6, usually 5, after 3 unbranched rays. Pectoral fin branched
rays 16-21, pelvic fin branched rays 7-9. The dorsal fin
spine is very strong and thick with well-developed and widely-spaced
teeth. Lateral line scales 84-108, lateral series scales 155-170,
about 32 between the dorsal fin spine and the lateral line and about
27 between the lateral line and pelvic fin. The belly is scaled up to
the isthmus. There is a scaled pelvic axillary process. The anal
papilla and anal fin lie in a groove formed by enlarged scales, the
groove extending about one third to half way between the anal fin
origin and pelvic base. Scales are oval and obliquely inserted into
scale pockets on mid-flank, sloping backwards postero-dorsally. The
focus is subcentral anterior and radii are present on all fields.
Circuli are few in these small scales. Pharyngeal teeth usually
2,3,5-5,3,2. Teeth are rounded with an evident hooked tip and
posterior teeth have a short to medium flat grinding surface below the
tip. Teeth may also be spatulate or have a spatulate shape with the
hollow filled in. Gill rakers 9-15, relatively short and reaching the
adjacent raker or slightly beyond when appressed. Occasional rakers
are forked. The mouth is inferior. The lower lip is interrupted
medially. The lower jaw may have a sharp horny sheath but this is
mostly lacking. Mouth shape varies from a u-shape to a sector mouth (a
gentle arch), the latter with a horny edge. The anterior barbels
extend back to the anterior eye margin or the mid-eye while the
posterior barbels extend to the rear eye margin or beyond. Barbel size
is variable and not obviously related to size. The gut is very elongate and coiled.
Meristic values for Iranian specimens are:- dorsal fin branched rays 7(11) or
8(22); anal fin branched rays 5(33); pectoral fin branched rays 16(2),
17(4), 18(12), 19(6), 20(8) or 21(1); pelvic fin branched rays 7(2),
8(30) or 9(1); lateral line scales 85(2), 86(2), 88(2), 89(1), 90(2),
91(2), 92(2), 93(2), 95(2), 98(2), 99(7), 100(3), 102(1), 104(1), 105(1) or 108(1); total gill rakers
9(1), 10(5), 11(6), 12(7), 13(8), 14(1), 15(2), 16(1), 17(1) or 18(1); pharyngeal teeth
2,3,5-5,3,2(15), 2,3,4-5,3,2(2), 2,3,5-4,3,2(1), 2,2,5-5,3,2(1),
1,3,5-5,3,2(1); and total vertebrae 43(6), 44(10), 45(1), 46(2), 47(2), or 49(1).
Sexual dimorphism
A male specimen, 123.9 mm standard length, caught on 6 April had
small to moderate sized tubercles on the top and sides of the head but
these were not fully developed. The largest tubercles are found
between the nostrils and the upper lip on the snout. No tubercles were noted on
the fins. A fish taken on 5 November also had small but distinctive tubercles.
Colour
The overall coloration is silvery without any pattern but the back
and upper flank are blackish to olive or brassy and the belly is
whitish in small fish to a strong yellow in large fish. The back may
be iridescent blue-green. The lateral line may be lighter than the
surrounding flank, appearing as a thin, whitish line. The lips,
pectoral, pelvic and anal fins are yellow. Fins bases are bright
orange, the gill slit has a bright orange streak and the isthmus is
bright orange. All fins may be flushed with red in freshly caught
material and the lower flank and belly can have pinkish tinges. The
iris is red dorsally. The peritoneum is a dark brown.
Preserved fish have a uniform brown colour with faint to dark
speckles arranged irregularly on the flank. There are no obvious
patterns on the fins although they are darkened by melanophores on
both rays and membranes.
Günther (1889) reports the caudal fin as black, but this is
possibly a dried or otherwise abnormal specimen. The colouration of
the iranicus nominal subspecies in the original description
(cited above) is not borne out by specimens from Damghan, the presumed
locality of the type specimen, and again may be an artefact of
preservation or simply a variation.
Size
Attains 54 cm (Muhomedieva, 1967) and reputedly 3 kg in qanat
specimens (R. J. Behnke, in litt., 1981).
Distribution
Found in the Tedzhen and Murgab rivers of Afghanistan and
Turkmenistan including Iranian drainages of the former known as the Hari River
in its Iranian reach (Aliev et al., 1988). It is recorded from the
Jam River, the Sharak River, the Akhland River near Mashhad, the Kashaf Riverand
various smaller water bodies in Khorasan, the upper Kal Shur, Jajarm and Jovein
rivers in the Dasht-e Kavir basin, as well as Cheshmeh Ali at Damghan and Cheshmah Badash near
Shahrud further west, the westernmost distribution of the schizothoracine fishes
(Günther, 1889; Nikol'skii, 1897; 1899; Abdoli, 2000; Coad and Abdoli, 2000b).
Wossughi (1978) records this species from the Hamun-See (= Sistan) but this is an error.
Zoogeography
Saadati (1977) found slight differences between fish from the Dasht-e Kavir
basin and from the Hari River basin, in raker counts and caudal
peduncle depth. He concludes that isolation in the Dasht-e Kavir basin is
relatively recent and migration has occurred westwards in the past
15-25,000 years. The occurrence of this species in the western Dasht-e Kavir basin (the
Damghan basin) is the westernmost distribution of the schizothoracine fishes.
See also the genus Schizothorax.
Habitat
Found in springs, streams, rivers and qanats, the principal habitats of
northeast Iran but environmental requirements are unknown.
Age and growth
Life span exceeds 7 years (Muhomedieva, 1967). Abdoli et al. (2007)
found males reached 23.3 cm and 145.6 g and females 34.0 cm and 428 g in the
Laiinsoo River. The sex ratio was male:female 2.5:1.
Food
The diet is 93-99% fishes including Cyprinus carpio (Muhomedieva,
1967). Other foods include small Capoeta capoeta, Cyprinus
carpio, chironomids, caddis flies, dragonflies, other aquatic
insects, and plant material (Aliev et al., 1988). Crustaceans,
plant fragments and filamentous algae, and possibly fish eggs, may
also be found in gut contents. Abdoli (2000) lists Plecoptera, Ceratopogonidae,
Trichoptera, Ephemeroptera, Chironomidae and Simuliidae. Abdoli et al.
(2007) found fish in the Laiinsoo River had a diet dominated by chrironomid
pupae and simuliid larvae, with 9 other benthic invertebrate food items taken.
Reproduction
Egg diameter reaches 2.0 mm and numbers reach 36,300 eggs (Aliev et
al., 1988). Iranian fish caught on 6 April had developing eggs
suggesting a spring or early summer spawning period.
Parasites and predators
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish from the Hamun Lake, Sistan (sic - misidentification of the fish
species or locality confusion).
Economic importance
None.
Conservation
The numbers and details of distribution need to be examined before an assessment can be made.
Further work
The biology of this species in Iran is unknown in detail.
Sources
Type material: See above, S. pelzami (ZISP 8036) (and possibly (BM(NH) 1897.7.5:24)) and S. p. iranicus (ZMH 4116).
Iranian material:
CMNFI 1993-0124A, 1, 118.7 mm standard length, Semnan, Cheshmeh Ali-Damghan (36º17'N, 54º05'E);
CMNFI 2007-0003, 8, 94.3-188.1 mm standard length, Semnan, Cheshmeh Ali (ca. 36º17'N, ca. 54º46'E);
CMNFI 2007-0004, 6, 75.6-109.5 mm standard length, Semnan, Cheshmeh Bedasht (ca. 36º35'N, ca. 55º03'E);
CMNFI 2007-0012, 2, 77.8-127.6 mm standard length, Khorasan, qanat at Bagh-e Jan (ca. 36º00'N, 58º38'E);
CMNFI 2007-0013, 2, 55.9-80.4 mm standard length, Khorasan, qanat 5 km north of Boghai (ca. 36º02'N, ca. 59º31'E);
CMNFI 2007-0014, 3, 88.7-98.1 mm standard length, Khorasan, Kuh-e Sang Park, Mashhad (ca. 36º18'N, ca. 59º36'E);
BM(NH) 1914.1.1:16-17, 2, 98.0-163.9 mm standard length, Khorasan, Kashaf River, Mashhad (no other locality data);
BM(NH) 1914.1.1:18-20, 3, 138.5-242.0 mm standard length, Khorasan, small stream near Mashhad (no other locality data);
BM(NH) 1914.1.1:21-23, 2, 151.2-165.7 mm standard length, Khorasan, Cheshmeh-e Saby (no other locality data);
BM(NH) 1914.1.1:24-29, 5, 147.6-188.8 mm standard length, Khorasan, Langar, Jam River (35º23'N. ca. 60º25'E).
Schizothorax plagiostomus
Heckel, 1838
Described from Kashmir; records summarised in Coad (1981d) for the
Helmand River basin in Afghanistan but these may refer to Oreinus
plagiostomus, a probable synonym of Schizothorax intermedius
(q.v.). No Iranian record.
Schizothorax zarudnyi
(Nikol'skii, 1897
Common names
hamun mahi (= hamun or lake fish), ماهي خواجو (= mahi khvaju or khaju, after the historic
island in Lake Hamun), sefidak (=white fish) or vatani (A. A. Pasand, pers. comm.,
5 November 2000), shir mahi (= milk fish), anjak (Fowler and Steinitz
(1956) report three kinds of fish being caught by fishermen in Sistan
named anjaq (hence Oreinus anjac, see below), mawda (? unknown)
and mahrmahé (= mar mahi, snake fish, probably a nemacheilid)).
Systematics
The holotype of Aspiostoma zarudnyi is in poor condition
with the tail detached and the body impaled on a wooden spike when
examined by me. Nikol'skii (1897) in his original description states
in Latin "Specimen valde destructum". It is in the
Zoological Institute, St. Petersburg (ZISP 11195a) and measures about
265 mm standard length. Nikol'skii (1897) gives the catalogue number
as 11115 (sic, incorrectly according to Berg (1949)) and the
type locality as "Palus Neizar in Seistan. 3.VI.96.". Berg
(1949) gives the collection locality as "Neizar near the southern
tip of Lake Hamun-i-Farah, western edge of the Helmand delta in
northwestern Seistan" based on Zarudnyi (1901).
This species was originally described in the genus Aspiostoma
Nikol'skii, 1897, a synonym of Schizothorax Heckel, 1838 (Eschmeyer,
1990). Bianco and Banarescu (1982) place this species as Schizopyge
zarudny, the species name being a mis-spelling. Schizopyge
Heckel, 1847 is regarded by these authors as the correct name for snow
trouts without a suctorial disc on the chin (synonym Schizothoraichthys
Misra, 1962 (see discussion above under the genus Schizothorax).
Barbus microlepis Keyserling, 1861 described from "Flüsschen
bei Anardareh, zwischen Herat und Lasch" is a synonym and
is pre-occupied by Barbus microlepis Bleeker, 1851. Oreinus
anjac Fowler and Steinitz, 1956 from "Zabol, Eastern
Iran" is also a synonym as suggested by Saadati (1977) although it may be a hybrid.
The holotype of Oreinus anjac (ANSP 71949) at 281 mm total
length is stored in the Academy of Natural
Sciences of Philadelphia (Böhlke, 1984).
This species is closely related to Schizothorax intermedius
but is distinguished by much smaller paired fins, longer and narrower
branchial isthmus, and the scales slightly enlarged at the base of all
fins, especially the dorsal and anal (Annandale and Hora, 1920).
Key characters
The only common Schizothorax species in Sistan, is is recognised by
the large barbels and enlarged scales around the anal fin.
Morphology
Dorsal fin with 4 unbranched and 7-8 branched rays, anal fin with 3
unbranched and 5 branched rays. The last unbranched dorsal fin ray is
moderately strong and serrated, the serrations being proportionately
longer and spinier in young. Pectoral fin branched rays 18 and pelvic
fin branched rays 9. Lateral line scales 97-114, at least 190 in the
scale series immediately above the lateral line. The breast is naked
or sparsely scaled. There is a pelvic axillary process. The scale
sheath around the anal papilla and anal fin extends about one third to
half way between the anal fin origin and the pelvic fin base. Scales
are very small, horizontally ovoid and have an almost central focus.
Scales are obliquely inserted in the scale pockets on the mid-flank
above the lateral line and below the dorsal fin. Scales on the nape
are none to minimally imnbricate. Radii are found on all fields and
are numerous. Gill rakers on the lower arm 30-39 (Nikol'skii's (1897)
count of 25 is incorrect (Berg, 1949); but see below for wider variation). Gill rakers are long, reaching the third
to the sixth adjacent raker when appressed. The interior margin of
each raker is serrated. Pharyngeal teeth usually 2,3,5-5,3,2,
spoon-shaped with a slightly hooked tip. Anterior teeth are more
rounded and thicker. There are 2 pairs of barbels, the anterior ones
long to rudimentary in literature sources. The barbels are subequal in
length, the anterior ones not reaching the eye and the poterior ones
not reaching beyond the eye. The mouth is usually slightly subterminal
but can be terminal or have the lower jaw projecting slightly. The gut
is elongate and coiled. The chromosome number is 2n=96, NF=142, comprising 9
pairs of metacentric, 14 pairs of submetacentric and 25 pairs of acrotelocentric
chromosomes, and the fish is a tetraploid (Hosseini and Kalbasi, 2003; Kalbassi
et al., 2008).
Meristics for Iranian specimens: dorsal fin branched rays 7(2) or
8(34); anal fin branched rays 5(35); pectoral fin branched rays 16(1), 17(7),
18(10) or 19(16); pelvic fin branched rays 9(30) or 10(5); lateral line
scales 93(1), 97(1), 98(2), 99(3), 100(4), 101(5), 103(4), 105(2), 106(5),
107(2), 108(1), 109(2), 113(1) or 114(1); total gill rakers 24(1), 25(1),
26(1), 27(1), 28(1), 29(1), 31(5), 34(6), 35(2), 36(8), 37(1), 38(2),
40(1) or 41(1); pharyngeal teeth 2,3,5-5,3,2(14), 2,3,5-5,3,3 (1),
2,3,5-5,2,1(1), 2,3,5-5,3,1(1), 2,2,5-5,3,2 (1), 2,2,5-4,2,2(1), 2,3,4-5,3,2(2),
2,3,4-4,3,2(1) or
1,2,5-5,3,2(1); and total vertebrae 47(11), 48(11) or 49(4).
Sexual dimorphism
Males develop prominent nuptial tubercles on the snout and on the scales.
Females have a soft and distended belly during the breeding season (CIRSPE, 2006b).
Colour
Overall colour is silvery, the back and head darker with indistinct
fine dots. The flanks may be spotted with black and some small areas
may be more lightly pigmented and appear as indistinct spots or
blotches. There are melanophores on the fin rays and membranes. Adult
males may have reddish fins and dull red specks on the dorsal surface.
Young are more silvery than adults. Generally there is no distinctive
pattern on the body and fins.
Colour varies with the environment. In muddy water, the back and
fins are pale olive-green, the flanks tinged with green or pale yellow and the belly
pure white while in the yellow water of the reed beds the back and
flanks are much darker, almost black, and even the belly is darkish.
The peritoneum is brown to black.
Size
Reaches 62.1 cm total length and and over 2.2 kg. Ahmadi and Wossughi (1988) give
average weights of 300 to 2000 g in commercial catches while fish more than 12
kg are reported recently (Iranian Fisheries Research Organization Newsletter, 30-31:5, 2002).
Distribution
This species is restricted to the Sistan basin including the Chahnimeh (Nikol'skii,
1897; Bianco and Banarescu, 1982; J. Holčík, in litt., 1996.
Ghanbari and Jami (2011a) report it to be present only in the Chahnimeh
Reservoir as the hamun dried out; presumably it recolonises the hamuns from
rivers and reservoirs in wetter seasons.
Zoogeography
See under the genus description.
Habitat
Found in the open lake, in reed beds and in pools in Sistan
(Annandale, 1921). It is the only species in Sistan common in the open
lake in winter. Young probably make their way up upstream in the flood
season as only adults are found in the lake in winter. The species is
extremely abundant in pools left in stream beds when the floods
recede. Spawning may occur in rivers as fry have not been found in the lakes
(Iranian Fisheries Research Organization Newsletter, 30-31:5, 2002).
Zabihi (2006) characterises it as a potamodromous species and notes that in
March and April, if there is no flow in the rivers and thus no migration from
the lake is possible, female gonads are reabsorbed.
Ghanbari and Jami
(2011a) note that mature fish migrate during April-May from rivers and lakes to
cold and well-oxygenated streams for spawning, and migrate back leaving the
young behind .
Age and growth
Sexual maturity may only be attained after 4 years. Zabihi (2006) examined 697
specimens with a length and weight range of 24.5-62.1 cm and 137-2204 g. Half the
male fishes were mature at 29-31 cm and for females at 38-40 cm. Males mature a
month earlier than females
(Ghanbari and Jami,
2011a)
Food
The diet comprises almost exclusively small fishes.
Reproduction
Eggs in fish caught in spring by me were developing but very small. Specimens with
mature, yellow eggs have been caught in December. However this species is mature in
Esfand (20 February-20 March), incubation is 6-7 days at 16.20-17.75ºC,
maximum egg diameter is 3.8 mm when washed and the yolk sac is absorbed at 6-7
days (M. Abedi, Islamic Azad University of Savad Kooh, abstract). Zabihy et al. (2004) and
Zabihi (2006) found maximum oocyte size in March and April at 14-18ºC when the
gonadosomatic index was highest at 7.9-9.6. The mean absolute and relative fecundities for fish 460-1380 g was 26,256 and 34,418 eggs
respectively. The species was a total spawner showing a synchronous ovary.
Eggs are adhesive to prevent them being washed away by strong currents (CIRSPE, 2006b).
Parasites and predators
Datta (1937) describes the male of the acanthocephalan Eosentis rigidus from the intestine of this species.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Economic importance
Annandale in Annandale and Hora (1920) described the fishery for
this species in the early years of the 20th century. Annandale
commented that the flesh tasted like trout but was bony. The bones can
be softened by cooking in vinegar. This fish is still caught and eaten
and appears in local markets. Ahmadi and Wossughi (1988) cite an annual catch
of 300-500 t while Iran Daily (24 August 2006) gives 700-1000 t before the drought.
Rahimabadi et al.
(2009) assessed the lipid quality of this species and found it to have a higher
nutritional value than Schizocypris altidorsalis.
Annandale and his assistants became sick from eating the eggs of
this species but he maintained that the local fishermen ate it without
any deleterious effects. Fish were caught in a net about 4 feet (1.22
m) deep and 100 feet (30.5 m) or more long anchored at each end by a
tamarisk stick stuck into the lake bottom and with the bottom of the
net on the lake bed and the top of the net slightly above the surface.
The net was positioned in relation to the wind, and therefore the
prevailing currents, so it formed a semi-circle. The net was arranged
in a gap in the reed beds or just outside the reeds in the open lake
if the weather was exceptionally calm. Pools in the reeds were kept
open to facilitate the fishing. The fishermen riding their tutins
(reed boats) would drive the fish into the net by beating the water
with poles and ululating. The two ends of the net were then lifted out
of the water by the men in the two end boats such that the net formed
a bag. The net was drawn into the two boats as rapidly as possible. A
similar but shorter net was used to drag small channels while the men
using it waded. A small-meshed bag net attached to one horizontal and
two upright poles was also used in pools of dry stream-beds. The net
was dragged by ropes, the men wading through the water. Some large
fish were killed in the flooded swamps by striking at them with
swords. Another net consisted of a bag about 7 feet (2.14 m) long and
6 feet (1.83 m) by 2 feet (0.61 m) at the mouth. The mouth was held
open by poles tied together at one end to make a fork. The fork
pivoted on a post on the bank. The mouth of the net had fine lines
across it, the lead string of which was held by the fishermen to warn
him that a fish had entered the net so he could pull the net out of
the water to retrieve the fish. The net was placed along a bank where
the current swirled forming a backwater, at the mouth of a small
canal, or as the focus of a line of stakes blocking a channel. The
season for this type of net began as early as August or not until
October. It lasted several weeks. March and April could also be a
favourable time if the river was not very full but the fish did not
move as actively. The large fish caught were attracted to feed on the
numerous small fish which migrated up river along the shore and were
checked where backwaters met the main flow.
A more recent description of fishing in Sistan is found in Fowler
and Steinitz (1956). Fishing takes place in fall on rivers and in the
lake, preferably the early evening or morning when it is cool. River
fishing is preferred to lake fishing and some fishermen never go out
onto the lake. Lake fishing using boats may last up to 3-5 days at a
time. River fishing is carried out with a cotton-thread seine weighted
by stones at the bottom and with gourds as floats. The seine is tied
to sticks at each end and the sticks to ropes leading to shore. Four
to ten men can own a seine and the catch is divided among those
fishing that day. Fishing is done in teams of 4-7 men or often in two
teams with two nets. Half the men are on one side of the river and
half on the other, pulling each other's nets in and out of the water.
Only large, fat fish are kept, the others being thrown back. One net
catches 30-40 fish in a day which are then sold in Zabol. Women never
fish nor are present during fishing as they bring bad luck. Fish are
always cooked before eating as eating raw fish is reputedly fatal.
Conservation
Ahmadi and Wossughi (1988) state that introductions of various
fishes such as Cyprinus carpio, Carassius auratus,
Ctenopharyngodon idella and Hypophthalmichtys molitrix are
a possible source of competition for native species such as this
schizothoracine since they are voracious, take spawning sites and
carry diseases and parasites. Native catches have decreased in favour
of introduced species. M. Abedi, Islamic Azad University of Savad Kooh, has studied artificial reproduction in this species
including larval development. CIRSPE (2006b) and Iran Daily (24 August 2006)
give details on artificial reproduction of this species. Males
over 600 g and females over 900 g were found to be suitable for breeding, ideal
water temperature range was 18-22ºC, fecundity range was 24,300-37,640 eggs for fish 0.8-1.1 kg, egg size was 1.54 mm
(presumably not water hardened; see above), and survival from egg to 11 mm fingerling was about 10%.
Ghanbari and Jami
(2011a) class this species as endangered.
Further work
The molecular relationships of this species to other Schizothorax species and related genera could be revealing.
Sources
Type material: See above, Aspiostoma zarudnyi (ZISP 11195a).
Iranian material:- CMNFI 1979-0072, 1, 199.1 mm standard length, Sistan, Hirmand River near Zabol (30º58'N, 61º28'E);
CMNFI 1979-0223, 1, 34.4 mm standard length, Sistan, irrigation jube 1 km south of Lutak (30º45'N, 61º24'E);
CMNFI 1979-0225, 11, 182.2-225.3 mm standard length, Sistan, effluent of Hirmand River (30º58'N, 61º28'E);
CMNFI 1979-0226, 2, 146.8-167.2 mm standard length, Sistan, pool near Kuh-e Khajeh (30º57'N, 61º17'E);
CMNFI 1979-0231, 2, 23.1-25.8 mm standard length, Sistan, irrigation jube 3 km from Zabol (31º01'N, 61º32'E);
CMNFI 1979-0232, 2, 26.3-26.5 mm standard length, Sistan, irrigation jube 11 km from Zabol (ca. 30º58'30"N, ca. 61º36'E);
CMNFI 1979-0235, 13, 148.4-193.7 mm standard length, Sistan, effluent of Hirmand (30º54'30"N, 61º41'E);
CMNFI 1979-0237, 8, 24.4-45.3 mm standard length, Sistan, irrigation jube 18 km south of Zabol (30º53'N, 61º27'30"E);
BM(NH) 1920.1.20:35, 1, 232.5 mm standard length, Sistan, near Lab-e Baring (ca. 31º07'N, ca. 61º12'E);
ZMH 5902, 2, 372.3-369.7 mm standard length, Sistan, Hamun See (no other locality data);
ZMH 5903, 3, 274.2-298.7 mm standard length, Sistan, Hamun See (no other locality data);
ZMH 6088, 2, 48.3-136.1 mm standard length, Sistan, Rud Sistan (no other locality data).
Genus Squalius
Bonaparte, 1837
The members of this genus were formerly placed in the dace genus Leuciscus
Cuvier, 1816.
Some subgenera have been elevated to generic rank and vice versa. The genus Leuciscus is not monophyletic based on allozyme
data for a limited number of European taxa (Hänfling and Brandl,
2000). There may be about 22 species in the genus Squalius
with 3 species reported from Iran. It has been suggested that species formerly considered to belong to the subgenus Squalius
should be simply regarded as part of a Leuciscus cephalus
complex characterised by serrated pharyngeal teeth in 2 rows (2,5-5,2)
and an almost straight or convex anal fin margin. This complex would
include L. cephalus, L. gaderanus (= L. ulanus)
and L. lepidus among Iranian species (Bogutskaya, 1994). Bogutskaya
(2002), however, places L. persidis and L. ulanus in a new
genus Petroleuciscus, q.v. and places L. cephalus
and L. lepidus in the genus Squalius, and this is followed
here.
Bogutskaya (2002) gives the characters
of Squalius as numerous total vertebrae (commonly more than 40, up to
48); increased number of sensory cephalic pores (up to 12-20 in the supraorbital
canal) in most species; often fused and very expanded fourth and fifth infraorbitals;
and depressed neurocranium with a reduced interorbital septum. Other characters
are a somewhat compressed body, moderate to
large scales, a complete lateral line, no barbels, mouth terminal or
subterminal, no notch in the upper jaw accommodating a tubercle on the
lower jaw, thin lips with the lower one interrupted medially, a short
dorsal fin without a thickened ray, a moderately long anal fin, long
and hooked pharyngeal teeth in 2 rows (2,5-4,2, 2,5-5,2 or 3,5-5,3
modally) usually with hooked tips and spoon-shaped crowns, short gut,
no keel on the belly, and short and relatively few gill rakers.
Rückert-Ülkümen (2000) and Rückert-Ülkümen and Matzke-Karasz (2000) document Lower Miocene fossils of
Leuciscus, presumably Squalius, from western Turkey.
Squalius cephalus
(Linnaeus, 1758)
Common names
mahi safid (= white fish) in the Caspian basin and in central Iran;
mahi-ye safid rudkhanehi or mahi-e-sephid-e-roodkhaneie (= literally white river, presumably in the
sense of white river fish) in Khuzestan; kuli; 'rus mahi or aroos mahi.
[enlibas or gafgaz enlibasi, nour enlibasi for natio kaznakovi,
all in Azerbaijan; tepug in Armenia; bir-aan siphaloos, baeaan, barayan, or berak (=
breast perhaps in allusion to the broad and fleshy chin (Heckel,
1843b)) at Aleppo, all in Arabic; Kavkazskii golavl' or Caucasian
chub in Russian; European chub].
Systematics
Cyprinus Cephalus was originally described from southern Europe.
Leuciscus orientalis Nordmann, 1840 described from Abkhazia,
Georgia, Squalius Berak Heckel, 1843 described from
"Aleppo" (earlier in the same work - p. 1041 - spelled Berag
without a description, presumably an error), Squalius cephalopsis
Heckel, 1843 described from "Aleppo", Squalius orientalis
Heckel, 1849 described from "Flusse Kueik bei Aleppo", Squalius
turcicus De Filippi, 1865 described from "Dell' Arasse presso
Erzerum" (Aras River at Erzerum in Turkey), and Squalius
agdamicus Kamenskii, 1901 from Agdam in the Kura River basin, are synonyms.
Several natio and varieties within what is now Squalius cephalus have been described from Iran or contiguous drainages and
are listed as follows. They have no taxonomic validity but the names
may reoccur. Leuciscus cephalus orientalis natio kaznakovi
Berg, 1912 was described from Lake Nour near Vandam, Nukha District,
Azerbaijan (in the Tur'yan-chai basin of the Kura River basin but not
connected to it). Squalius turcicus var. platycephala
Kamenskii, 1897 was described from Lake Taparavani (= Lake Paravani at
41°26'N, 43°48'E) and the Kyrchbulach River in the upper Kura River basin, Georgia. Leuciscus
cephalus orientalis natio aralychensis Barach, 1934 was
described in Latin from "Turcia, Aralych. fl. Kara-su", and
in Russian "Reka Kara-su u vblizi Aralykha podnoshchiya Ararata"
(= Kara-su River at the foot of Ararat in the vicinity of Aralykha). Leuciscus
cephalus orientalis natio zangicus Barach, 1934 was
described in Russian from "R. Zanga v Armenii" (i.e. at
Erivan). Leuciscus cephalus orientalis natio ardebilicus
Barach, 1934 was described in Latin from "Ardebil, fl.
Balyk-tchai in systemate fl. Arax. inf." and in Russian "R.
Balyk-chaya, vblizi Ardebilya v Persii" (i.e. Balyk River or
Balyk-chai) in the upper Aras River basin of Iran.
Iranian populations were usually recognised as Leusciscus cephalus
orientalis (Nordmann, 1840). Bianco and Banarescu (1982) correctly
point out that there has been no critical revision of the subspecies
and that differences are slight. Recognition of subspecies is
disputable according to Reshetnikov et al. (1997).
Turan et al. (2007) examined morphological variation in Turkish
populations from the Black, Caspian, Aegean, Mediterranean and Tigris-Euphrates
basins and found three groups, but no discrete taxa.
The nominal Iranian subspecies, L. c. orientalis, differs
from the European subspecies, L. c. cephalus, by having a more
elongate body, a dark stripe behind the operculum and on average fewer
scales and anal fin branched rays (Berg, 1948-1949; 1949).
The syntypes of Cyprinus cephalus are in the Naturhistoriska
Riksmuseet, Stockholm under LP 81 (1 fish) and in the Zoologiska
Museet, Uppsala Universitet, Uppsala under ZMUU Linnaeus Collection
213 (1) (Eschmeyer et al., 1996).
Three syntypes of Squalius orientalis, 111-146 mm
standard length from Aleppo are in the Naturhistorisches Museum Wien
under NMW 49438 (Krupp, 1985c). Two more fish from Aleppo are under
NMW 49440 and measure 130.0-168.3 mm standard length.
Six syntypes of Squalius berak are in the Naturhistorisches
Museum Wien under NMW 48915 and 3 syntypes are in the Senckenberg
Museum Frankfurt under SMF 469, formerly in NMW, and 3 syntypes of Squalius
cephalopsis are under NMW 49438 and 2 other syntypes are under NMW
49440 (Eschmeyer et al., 1996). The Vienna catalogue lists 6
specimens as Squalius berak and 2 specimens as Squalius
cephalopsis but the Vienna card index in 1997 agrees with
Eschmeyer et al. (1996) for the NMW specimens.
Natio ardebilicus syntypes are in the Georgian State Museum,
Zoological Section, Tbilisi under ZMT 136h, natio aralychensis
syntypes are under ZMT 22-11 (10), and natio kaznakovi syntypes
are in the Zoological Institute, St. Petersburg under ZISP 12089 (5) (Eschmeyer
et al., 1996).
A hybrid between Squalius cephalus and Chalcalburnus
(= Alburnus) chalcoides is reported from Turkey (Ünver and Erk'akan, 2005).
Key characters
The relatively large scales outlined by pigment give this fish a
distinctive appearance and, combined with large body size, fin ray
counts, rounded belly and the absence of barbels, separates it from
other Iranian cyprinids.
Morphology
Dorsal fin branched rays 7-9, usually 8, after 2-3, usually 3,
unbranched rays, anal fin branched rays 7-10, usually 8 after 3
unbranched rays, pectoral fin branched rays 14-19, and pelvic fin
branched rays 6-9, usually 8. Lateral line scales 38-48. Scales have
few to moderate numbers of radii on the anterior and posterior fields.
The focus is central to subcentral anterior and circuli are fine. On
the posterior field, circuli break up into "bubbles" and are
coarser than on other fields. The anterior scale margin is wavy,
sometimes irregular and others with a small central protuberance and
indentations above and below. Gill rakers short, 7-12, touching the
adjacent raker when appressed. Vertebrae 40-46. Pharyngeal teeth
2,5-5,2, with variants 2,5-4,2, 2,4-4,2, 2,5-5,1, 1,5-5,2, 1,5-5,1,
2,5-5,3, 2,6-5,2, or even 1,5-5,1,2 and 1,2,5-5,2,2. Teeth are very
narrow, strongly hooked at the tip and strongly serrated so that there
is no obvious flat surface. The serrations on the teeth of orientalis
are stronger than in the typical subspecies. The gut is an elongate
s-shape. Chromosome number is 2n=50 (Al-Sabti, 1986; Klinkhardt et al., 1995).
Meristics for Iranian specimens: dorsal fin branched rays 7(3),
8(103) or 9(1); anal fin branched rays 7(3), 8(60) or 9(44); pectoral
fin branched rays 14(3), 15(13), 16(39), 17(46), 18(5) or 19(1);
pelvic fin branched rays 6(1), 7(1), 6(100) or 9(5); lateral line
scales 40(2), 41(20), 42(45), 43(30), 44(9) or 46(1); total gill
rakers 7(2), 8(14), 9(58), 10(31) or 11(2); pharyngeal teeth ?; and
total vertebrae 40(6), 41(29), 42(45) or 43(6).
Sexual dimorphism
Abdurakhmanov (1962) reports that head length, eye diameter, caudal
peduncle length, dorsal and anal fin heights, pectoral and pelvic fin
lengths and lower caudal fin lobe length are all greater in males
while postorbital length, interorbital width, head depth and
pectoral-pelvic fin distance are greater in females.
Colour
Overall colour is silvery to grey. Scales of the lateral line and
upper back have a strong dark pigmentation along the posterior margin
and a distinct dark spot anteriorly. Scales may have some gold in
their centre. The back is dark brown or reddish-brown to blue-grey and
the belly and lower head are pearly-white to a silvery yellow. The
operculum is a strong copper-yellow colour and the opercular opening
is dark, nearly black. The iris is silvery and has very little gold,
or is golden with a lot of dark grey pigment. There may be an upper
spot of dark grey on the iris. The dorsal and anal fins are grey and
may have some pink. The dorsal fin is blackish distally. The pectoral
fin has a little pale grey pigment and there may be a yellow or
yellow-pink spot at its upper base with this colour extending onto the
first 2-3 rays. The pelvic and anal fins are pink to red-pink with
somewhat colourless posterior margins, especially in spawning males.
The caudal fin is a pale pink to dirty reddish with some dark
pigmentation to the posterior margin.
Size
Possibly as long as 80 cm although 45 cm is a more likely
maximum (Berg, 1948-1949) and a weight of 6-8 kg. Reaches 43 cm
total length in the Madar Su of Golestan National Park (A. Abdoli, pers. comm., 1995).
Reaches 39.2 cm and 870 g in Taham Dam in northern Iran (Iranian Fisheries
Research Organization Newsletter, 54 & 55:4, 2008). Attains 39.0 cm fork length in Turkey (Yerli
et al., 1999).
Maximum size is possibly 85.0 cm and 10.0 kg.
Distribution
Found from the British Isles and the Iberian Peninsula eastwards in
its southern distribution to Turkey and Iraq, the northern half of Iran, and
the whole Caspian and Aral seas drainages. In Iran, it is recorded from
rivers along the Caspian Sea coast including the Anzali Mordab (Holčík and Oláh, 1992; Roshan Tabari, 1997;
Shamsi et al., 1997; Abbasi et al., 1999; Kiabi et al., 1999) but also the Aras River, the Namak Lake basin (Wossughi,
1978; Bianco and Banarescu, 1982), the Lake Orumiyeh basin (Günther, 1899); Lake Zaribar, Kordestan (Abzeeyan, 5(5):III,
1994), and the Esfahan basin and the Tigris River basin (Berg, 1949). Abdoli (2000)
and Abdoli and Naderi (2009) map this species from the middle Atrak and Gorgan, middle and upper Neka, middle
and lower Babol and Heraz, Chalus, Tajan, Tonekabon, Sardab, Pole-e Rud, Safid, Shahrud and Qezel Owzan,
middle Aras and its tributary Qareh Chai, all in the the Caspian Sea basin, the middle and lower Talkheh, Zarrineh and
Tatavi in the Lake Orumiyeh basin, the Karaj, Shur, Abhar, Qareh Chai and Qom
rivers in the Namak Lake basin, and the Marun, upper Karun and middle Khersan, Dez,
middle and upper Karkheh, Simarreh, Kashkan and lower Gav Masiab rivers in the Tigris River basin.
Zoogeography
This widely distributed species has been split into a number of
subspecies but the principal one from the Middle East or Southwest
Asia is recognised as S. c. orientalis. This has not been
examined in detail recently using classical techniques and the relationships between isolated
populations are unknown. Durand et al. (1999), working on the cytochome b
of European populations, found that a lineage from a Ponto-Caspian refugium
recolonised the Baltic area in the Holocene after glaciation. Durand et al.
(2000), again examining cytochrome b, consider that S. cephalus may have
originated from Mesopotamia and, in the late Pliocene, used the large inland
lake of Anatolia existing at that time for dispersion. Uplift of the Anatolian
Plateau, climatic changes and river isolation was probably the main vicariant
event leading to a quick radiation in these chubs.
Habitat
In the Caspian Sea basin, it is found mainly in upstream waters (Jolodar and Abdoli, 2004).
Age and growth
Öztaş (1988; 1989), Öztaş and Solak (1988) and Türkmen et al. (1999)
studied the chub in the Aras River of Turkey and
found its condition factor to be higher in summer and autumn and to
vary between age groups. Life span there is over 8 years, compared
with 7 years in Lake Aksehir, Anatolia (Altindag, 1996) and in the
Savur stream in the Tigris River basin of southeast Anatolia (Ünlü
and Balcı, 1993a; 1993b), over 8 years in the Euphrates River in Turkey (Özdemir
and Şen, 1986), and 10 years in Sariyar Dam Lake near Ankara (Ekmekci, 1996a).
In the Aras, females grow faster than males and there are more
sexually mature females in the older age groups. Sexual maturity is
attained at age 2-3 in males and ages 3-4 for females. Mean condition factor for
Aras males was 1.326 and for females 1.333. In the Savur
stream in the Tigris River basin, 80% of females and 75% of males
reaching sexual maturity at their third (14.6 cm fork length) and
second years (13.0 cm) respectively. Females grow larger than males
and live about 2 years longer as they do in Lake Aksehir. Sexual
maturity is attained at ages 3-4 in Sariyar Dam Lake and these fish at
age 1 have a mean fork length of 90 mm, mean weight of 9.4 g and a
condition factor of 1.263 which by age 10 can reach 370 mm, 910 g and
1.805. Aksehir Lake fish reached 44 cm forked length and 1766 g for
females and 31 cm and 557 g for males. In Lake Tödürge, Turkey, Ünver (1998)
found that males reached maturity in their second or third year of life while
females were in their third or fourth year. Over 68% of the catch was
female and life span attained 7 years. Yerli et al. (1999) invetsigated
growth in Lake Çıldır, Turkey and also found 8 age
groups, dominated by those in age group 3 at 67.3% and by males at 73% and with
maximum fork length at 390 mm and maximum weight at 720 g. Maximum life span is
up to 15 years. The weight class 150-450 g dominated in Taham Dam in Iran
(Iranian Fisheries Research Organization Newsletter, 54 & 55:4, 2008).
Food
Food items include mayfly and caddisfly larvae, other small
organisms such as molluscs, and crayfishes, small fishes and frogs.
Large fish feed mainly on other fish. Reputedly even fruit fallen in the water will be eaten. Trout eggs and
fry are also eaten. Guts of Iranian specimens contained a wide variety
of organisms including ants (presumably taken at the water surface),
aquatic insects such as chironomids among others, crustaceans,
filamentous algae, higher plant fragments, scales of cyprinids and the
remains of a Paracobitis malapterura. Abdoli (2000) reports
Ephemeroptera, Chironomidae and Trichoptera. In Taham Dam in Iran algae, higher
plants, bivalve and insects were found (Iranian Fisheries Research
Organization Newsletter, 54 & 55:4, 2008).
Reproduction
Öztaş (1989) examined reproduction of this species in a stream tributary to
the Aras River in Turkey. Spawning begins at the end of May although most fish
spawn in June. Water temperatures at this time are 12-18°C. Fecundity is up to
61,808 eggs and maximum egg diameter is 1.39 mm. Türkmen et al . (1999)
found spawning between May and July in the Aras River proper with fecundity up
to 17,187 eggs. Ünlü and Balcı (1993a; 1993b) found spawning to take place from
May to late June in southeast Anatolia in a tributary of the Tigris River.
Fecundity reaches 20,140 eggs and egg diameter 1.5 mm. Equations for the
relationship between fecundity (F) and length (FL), weight (W) and ovary weight
(GW) were given as F = 0.0458 FL2.3680 (r = 0.745), F = 270.96 W0.7912
(r = 0.761) and F = 1888.86 GW0.8163 (r = 0.775). In Sariyar Dam Lake
near Ankara, spawning takes place between April and June (Ekmekci, 1996a). Ünver
(1998) found fish in Lake Tödürge, Turkey spawning between May and July with a
mean egg diameter of 0.65 mm (highest 1.04 mm) and a mean fecundity of 14,500
eggs (highest 28,664 eggs).
Iraqi fish attained sexual maturity a in 3 years at
20 cm length and 700 g weight, spawning in March and April and depositing eggs
in shallow water on gravel (Al-Rudainy, 2008). In Azerbaijan, Abdurakhmanov (1962) gives spawning temperatures as 12-21°C,
maximum fecundity as 118,000 eggs and maximum egg diameter as 1.8 mm. Spawning in Iran appears to take place in spring judging from egg development.
Around 40% of the fish in Taham Dam in Iran spawned in early June and around
30% did not spawn at all (Iranian Fisheries Research Organization Newsletter,
54 & 55:4, 2008).
Parasites and predators
Ergens and Gusev (1965) report the monogenean helminth Dactylogyrus
prostae in this species from Bandar-e Shah (= Bandar-e Torkeman)
on the Caspian Sea coast. The monogeneans Diplozoon paradoxum
and D. megan are recorded from this species in the Tajan River, Mazandaran (Iranian Fisheries Research and Training Organization
Newsletter, 6:7, 1994). Shamsi et al. (1997) report Clinostomum
complanatum, a parasite causing laryngo-pharyngitis in humans,
from this species. Mirhasheminasab and Pazooki (2003) list Ergasilus
peregrinus, Tracheliastes polycolpus and Lernaea cyprinacea
from this species in Mahabad Reservoir, the latter being the most dangerous
parasite. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. sp. from fish in the Safid River. Masoumian
and Pazooki (1998) surveyed myxosporeans in this species in Gilan and Mazandaran
provinces, finding Myxobolus minutus and M. muelleri. Pazooki et
al. (2005) record Lamprolegna compacta, Ergasilus peregrinus and
Lernaea cyprinacea from this species in waterbodies of Zanjan Province.
Miar et al. (2008) examined fish in Valasht Lake and the Chalus River,
Mazandaran and found the protozoan Chilodonella hexastica.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Ergasilus peregrinus,
Ergasilus sp., Lamproglena compacta, Lernaea sp.,
Tracheliastes longicollis and Tracheliastes polycolpus on this
species.
Economic importance
Listed as economically important in the Turkish Euphrates River (Özdemir and Şen, 1986).
Conservation
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks and for sport.
Kiabi et al. (1999) consider this species to be of least
concern in the south Caspian Sea basin according to IUCN criteria.
Criteria include sport fishing, medium numbers, habitat destruction,
widespread range (75% of water bodies), present in other water bodies
in Iran, and present outside the Caspian Sea basin.
Ekmekci (1996a) recommends a minimum fishing size of 28 cm and a prohibition of fishing during the April-June spawning season for this
Anatolian population.
Further work
The distribution and biology of this species in Iran needs study, as does the
systematics of isolated taxa.
Sources
Iranian material: CMNFI 1970-0506, 6, 42.5-116.3 mm standard length, Gilan, Shalman River (37º08'N, 50º15'E);
CMNFI 1970-0536, 3, 89.0-223.0 mm standard length, Gilan, Siah River estuary near Rudbar (36º53'N, 49º32'E);
CMNFI 1970-0538, 7, 40.8-137.2 mm standard length, Gilan, Qezel Owzan River (36º44'N, 49º24'E);
CMNFI 1970-0583, 6, 41.4-123.8 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1970-0589, 1, 142.0 mm standard length, Gilan, Safid River (37º12'N, 49º54'E);
CMNFI 1979-0253, 33, 46.4-100.4 mm standard length, Markazi, Qareh Su River drainage (34º52'N, 50º49'E);
CMNFI 1979-0255, 2, 90.7-90.8 mm standard length, Markazi, Bar River drainage (33º51'30"N, 50º23'E);
CMNFI 1979-0276, 1, 59.7 mm standard length, Lorestan, Chamesk River (ca. 33º19'N, ca. 47º53'30"E);
CMNFI 1979-0462, 12, 50.7-104.5 mm standard length, Markazi, Mazdaqan River (35º06'30"N, 49º40'30"E);
CMNFI 1979-0469, 6, 86.0-127.5 mm standard length, Mazandaran, river west of Alamdeh (36º37'30"N, 51º35'E);
CMNFI 1979-0474, 6, 71.3-80.6 mm standard length, Mazandaran, Tajan River (36º34'N, 53º05'E);
CMNFI 1979-0482, 2, 112.2-160.1 mm standard length, Mazandaran, river between Minudasht and Dowlatabad (37º19'30"N, 55º31'E);
CMNFI 1979-0493, 7, 85.2-114.5 mm standard length, Mazandaran, Tajan River drainage (36º19'N, 53º23'E);
CMNFI 1979-0494, 12, 9.1-92.6 mm standard length, Mazandaran, Talar River tributray (36º21'N, 52º51'30"E);
CMNFI 1980-0120, 2, 41.8-118.1 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 2007-0092, 5, 25.5-45.9 mm standard length, Azarbayjan-e Khavari, Zilber Chay (38º42'N, 45º16'E);
CMNFI 2007-0100, 4, 41.2-171.8 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0101, 1, 162.0 mm standard length, Azarbayjan-e Bahktari, Tata'u River (ca. 36º54'N, ca. 46º07'E);
CMNFI 2007-0105, 1, 96.9 mm standard length, Kordestan, Zarineh River basin (ca. 36º06'N, ca. 46º20'E);
CMNFI 2007-0109, 1, 101.1mm standard length, Kordestan, Qeshlaq River basin (ca. 35º16'N, ca. 47º01'E);
CMNFI 2007-0113, 1, 99.5 mm standard length, Kermanshahan, Qareh Su River tributary (ca. 34º25'N, ca. 47º01'E).
Comparative material: BM(NH) 1974.2.22:71-72, 2, 68.0-93.1 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1974.2.22:74, 1, 152.1 mm standard length, Iraq, Greater Zab near Aski Kalak (ca. 36º16'N, 43º39'E);
BM(NH) 1974.2.22:75, 1, 154.0 mm standard length, Iraq, Qizilja River, Lesser Zab (no other locality data).
in Leuciscus?
Squalius latus
Keyserling, 1861
Flank scale
Common names
None.
[Zakaspiiskii elets or Transcaspian dace in Russian; Murgab dace].
Systematics
Squalius latus was described from the "Fluss. Heri-Rud
bei Herat" (now in Afghanistan, then in Persia; Herat is at °'N, °'E).
Squalius transcaspiensis Berg, 1898 from "Habitat in
flum. Tedschent, prope Aschabat in provincia Transcaspica" is a
synonym. The type locality is presumably the Tedzhen River in
Turkmenistan (not Iran as given in Eschmeyer et al. (1996))
although Ashkhabad is not on the Tedzhen River. Many syntypes are in
the Zoological Museum of Moscow State University (MMSU) according to
Eschmeyer et al. (1996). Possibly a subspecies of Squalius lehmanni
(Brandt, 1852) according to Nikol'skii (1938) and
Svetovidova (1967) or of Leuciscus leuciscus (Linnaeus, 1758)
according to V. V. Kafanova (cited in Bogutskaya, 1994). Bogutskaya
(1994) considers S. latus to be distinct.
Key characters
Dorsal and anal fin ray counts, both 7-9, lateral line scale counts under 48,
and distribution identify this species in northeastern Iran.
Morphology
Dorsal fin branched rays 7-9, after 3 unbranched rays, anal fin
branched rays 7-9 after 3 unbranched rays, pectoral fin branched rays
14-16, and pelvic fin branched rays 7-8. Pharyngeal teeth number
2,5-5,2; 2,5-5,1; 1,5-5,1; 3,4-4,2; 3,5-5,2; or 2,5-4,2. In specimens
from the Tedzhen (= Hari) River, part of which is the Iran-Afghanistan
border:- dorsal fin branched rays 7(12), anal fin branched rays 7(1),
8(6) or 9(5), pectoral fin branched rays 15(6) or 16(6), and pelvic
fin branched rays 7(1) or 8(11). Lateral line scales 39(1), 40(1),
41(2), 42(2), 43(1), 44(2), 45(1), 46(1), or 47(1). A pelvic axillary
scale is present. Scales bear a few anterior and posterior radii.
Total gill rakers number 8(2), 9(1), 10(5) or 11(4) and reach the
raker below when appressed. Total vertebrae 39(4), 40(6) or 41(3) (includes data
from x-rays of ZISP 18361 and 11047).
Pharyngeal teeth number 2,5-5,2(3) and have narrow cutting edges on
each side of a shallow groove below a hooked tip and in larger fish
some teeth are serrated below the tip. The mouth is oblique and
extends back to the level of the nostrils, the lower jaw protruding slightly.
Sexual dimorphism
Unknown.
Colour
The back and upper flank are reddish-brown, the flanks yellowish
and the belly silver. The dorsal and caudal fins are yellowish-brown
and the other fins are reddish. The base of all fins becomes bright
orange during spawning.
Size
Reaches 26.7 cm.
Distribution
This species is found in the Tedzhen River (= Harirud in Iran) and the Murgab River to the east
in Afghanistan and Turkmenistan.
Zoogeography
This species is part of a complex of species in the genus Squalius and
the related Leuciscus found from western Europe to Siberia. Its origins
may lie in a Ponto-Caspian-Aralian refugium, becoming isolated and speciating in
the eastern part of this area.
Habitat
Unknown in detail.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Numbers and habitat requirements are unknown and so a conservation assessment cannot be made.
Further work
The biology of this species needs study as well as it relationships to other Squalius.
Sources
Type material: There are no type specimens.
Iranian material: None.
Comparative material: ZISP 10357a, 1, 63.2 mm standard length, Turkmenistan or Afghanistan, Tedzhen River (no other locality data);
ZISP 10359, 4, 93.4-130.5 mm standard length, Turkmenistan or Afghanistan, Tedzhen River (no other locality data);
ZISP 10360, 6, 58.4-122.0 mm standard length, Turkmenistan or Afghanistan, Tedzhen River (no other locality data);
ZISP 10419, 1, 107.6 mm standard length, Turkmenistan Iolotan near Merv (37º18'N, 62º21'E).
Squalius lepidus
Heckel, 1843
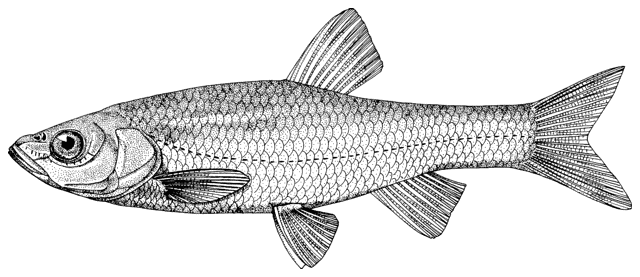
Common names
kavar or kawar.
[bara'an or bir-aan abiadh in Arabic; Tigris dace].
Systematics
Squalius lepidus was originally described from the "Tigris bei Mossul" (Heckel, 1843b).
Alburnus doriae De Filippi, 1865 and Alburnus maculatus
Keyserling, 1861 are synonyms according to Coad (1982d; 1985h) but
these are errors. Note also that Alburnus maculatus Kessler,
1859 from the Salghir River at Simferopol, Crimea, Ukraine preoccupies
Alburnus maculatus Keyserling, 1861 and is a distinct species of
Alburnoides (Bogutskaya and Coad, 2009).
The syntypes of Squalius lepidus are in the
Naturhistorisches Museum Wien according to Krupp (1985c) under NMW 49342, 2 specimens,
229.6-245.5 mm standard length as measured by me and NMW 49343, 2, 88.0-107.8 mm standard length, collected
by Th. Kotschy in 1843. Bogutskaya (1994), however,
lists a lectotype (237.0 mm standard length) and 5 paralectotypes
under NMW 49342, 2 paralectotypes under NMW 49343, and 1 paralectotype
under SMF 847 (the paralectotypes measure 90-8-233.8 mm standard
length). Eschmeyer et al. (1996) also lists 1 syntype under NMW
49344 and this specimen (164.8 mm standard length) appears in the 1997 Vienna card
catalogue as a syntype along with the other 4 fish mentioned by Krupp (1985c). One
syntype is in the Senckenberg Museum Frankfurt (SMF 847, formerly NMW)
(F. Krupp, pers. comm., 1985). The catalogue in Vienna lists 6 fish in spirits and 1 fish stuffed.
Key characters
This species is distinguished from other Squalius by the
elongate and pointed head with a projecting lower jaw, an adaptation
for piscivory which develops early and is not evident in other Squalius
even when these feed on fish as adults.
Morphology
Dorsal fin branched rays 8-10, usually 8 (Krupp, 1985c) or 9 (Bogutskaya,
1994)(8 in four syntypes, 9 in one), after 3 unbranched rays, anal fin branched rays 9-11, usually 9, after 3
unbranched rays, pectoral fin branched rays 14-18, and pelvic fin branched rays
8. Lateral line scales 42-50. Scales have few radii on the anterior and
posterior fields. Gill rakers 7-12, reaching the one below when
appressed (near junction of upper and lower arches) and sometimes curled.
Pharyngeal teeth 2,5-5,2 or 2,5-4,2, strongly hooked and serrated.
Total vertebrae 42-46. The projecting lower jaw fits into a notch in the upper jaw.
Sexual dimorphism
Unknown.
Colour
The back and upper flank are brown to bluish-brown or blackish, the
flanks generally silvery, and the belly silvery-yellow. The upper
flank, head and fins may be sprinkled with black spots. Flank scales have a spot
at their base. All fins are reddish, with the pectoral and anal fins the brightest. The dorsal fin
has a black margin. The caudal fin is blue-grey and its margin is blackish.
Size
Reaches 54.2 cm (Karabatak, 1997).
Distribution
Found in the Orontes (= Asi), Quwayq and Tigris-Euphrates basins as well as
some lakes in central Anatolia (Beyşehir, Akşehir)
and the rivers Ceyhan and Şeyhan in southern Anatolia.
Records from Iran are based on literature and include Lake Zaribar
in Kordestan, Kermanshah, the Marakeh River, Luristan, and Dez River below Dez
Dam (= formerly Mohammad Reza Shah Pahlavi Dam) (Wossughi, 1978). Also recorded from Mendeli
on the Iran-Iraq border (Berg, 1949). Abdoli (2000) records this species from
rivers of Khuzestan such as the Jarrahi, Marun, Karun, middle and lower Dez and
middle Karkheh; and the Qom, Shur and Zayandeh rivers probably in error. A
record from a canal near Gaz a few miles from Esfahan is probably an error (Keyserling, 1861).
Zoogeography
Durand et al. (2000) using cytochrome b suggest that this
species is fully introgressed with L. cephalus mtDNA (= S.
cephalus) and so question the taxonomic validity of this species. Morphological data contradicts
this conclusion. Durand et al. (2000) conclude that their data does
indicate that "L. lepidus and L. cephalus might
have had different dispersion histories over the same geographical range"
and that "L. lepidus introgression by the chub (L. cephalus)
is ancient, explaining the complete sorting of the lepidus lineage".
Habitat
Generally unknown but found in rivers and lakes.
Age and growth
Karabatak (1997) studied fishes identified as this species from Lake Beyşehir
in Turkey and recorded condition coefficients of 1.30-1.45 pre-spawning,
1.13-1.24 spawning and 1.24-130 post-spawning, with maximum values in the
pre-spawning period coincident with maximum gonad development. Males and females
showed similar length-weight relationships although females were slightly heavier because of their eggs.
Çolak (1983) studied the age of this species in the Keban Dam, Turkey and found
a maximum age of 8 years, that females grew faster and were longer than males,
and growth was rapid until five years of age when it fell by almost half in each
succeeding year. von Bertalanffy formulae, sexes combined, for two years were Lt
= 87.92 (1-e-0.112(t+1.464)) and Lt = 65.64 (1-e-0.214(t+0.878)).
Food
Plant remains and fish scales have been found in gut contents. Fish
appear to be the main diet item even in young from about 10 cm in length (Bogutskaya, 1994)
although Al-Rudainy (2008) cites fish fry and aquatic insects for Iraqi fish,
presumably smaller ones.
Reproduction
Karabatak (1997) studied fishes identified as this species from Lake Beyşehir
in Turkey and found a peak spawning in mid to late June at 20.0-21.5°C
with the highest gonadosomatic index on 15 May at 16.0-17.2°C. The spawning
season could extend from late May to early July. Ünlü (2006) gives age at first
maturity as 2-3 years in the Turkish Tigris River with spawning over sand, stone
and gravel. Al-Rudainy (2008) cites sexual maturity at 3-4 years, 30 cm length
and 3 kg weight in Iraq with spawning in March and April with eggs deposited in
shallow water on gravel beds.
Parasites and predators
Williams et al. (1980) report the digenean helminth Allocreadium
isoporum from this species in the Zayandeh River at Esfahan but
the fish species was most probably misidentified.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish.
Economic importance
Economically important in Turkey in Lake Beyşehir (Karabatak, 1997).
Conservation
The biology and distribution of this species in Iran need elucidation so a conservation assessment can be made.
Further work
The biology of this species needs study along with its systematic
relationships with other Squalius species as morphology and molecular
analyses are contradictory.
Sources
Some counts are taken from Bogutskaya (1994).
Type material: See above, Squalius lepidus (NMW 49342, NMW 49343 and NMW 49344).
Iranian material: None.
Comparative material: NMW 87786, 2, 329.0-381.5 mm standard length, Turkey, Karaviran at Konya (ca. 37º52'N, ca. 32º31'E);
NMW 91149, 11, 69.6-115.9 mm standard length, Syria, Euphrates River at Jarabulus (36º49'N, 38º01'E);
NMW 91620, 2, 91.6-95.6 mm standard length, Syria, Euphrates River at Jarabulus (36º49'N, 38º01'E);
NMW 94441, 1, 175.7 mm standard length, Turkey, Palu, Murat River (38º42'N, 39º57'E).
Genus Tinca
Cuvier, 1816
The tench genus contains a single species found from Europe to
Siberia including the Caspian Sea basin.
The characters of this genus include very small, elongate scales
deeply embedded in slimy skin, pharyngeal teeth in a single row, all
fins rounded and without spines, a short barbel at the terminal mouth
corner, no keel on the belly, moderately long gill rakers, and short
dorsal and anal fins.
Tinca tinca
(Linnaeus, 1758)
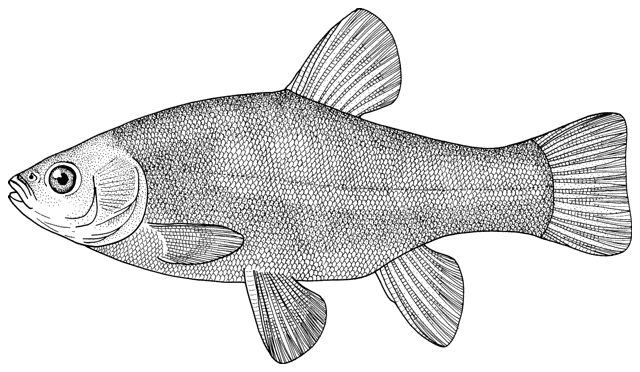

CMNFI 1979-0439, Gilan, Anzali Mordab
Common names
لاي ماهي (= lie, lai, lay or laay mahi, meaning bottom-dwelling or mud fish);
nazi; tilkhos; saboni (= soapy?); hashtarkhan kapur.
[lil baligi in Azerbaijan; sew-zukgná in Armenia; lin' in Russian;
tench, common tench, green tench].
Systematics
Cyprinus Tinca was described originally from European lakes. Ljabner
et al. (2010) found the western and eastern phylogroups of tench were a
single species under the biological species concept, although they were separate
phylogenetic species. Tench from a western European and a Ponto-Caspian refugium
came into contact by postglacial expansion after a separation of 750,000 years,
and showed free interbreeding.
Key characters
The dark coloration, rounded fins and slimy body are characteristic of this species.
Morphology
Dorsal fin unbranched rays 2-4, usually 3-4 followed by 6-9,
usually 8, branched rays, anal fin unbranched rays 3-4 and branched
rays 5-9, usually 6-7, pectoral fin branched rays 13-18, and pelvic
fin branched rays 7-10. Lateral line scales 70-120, gill rakers 10-16,
usually 11-15, and vertebrae 35-44, usually 39-43. Anterior gill
rakers may be difficult to distinguish from throat tubercles. Central
rakers touch the one below when appresssed. Scales are very elongate
ovals with a very anterior focus near the anterior scale margin. Radii
are numerous on all fields. Pharyngeal teeth 4-5, 5-4, 5-3 or 4-4,
more rarely 5-5, slightly to strongly hooked and expanded at the tip
into a head supported on a narrow stalk. The oblique head has rounded
edges and is concave in the middle. The gut is s-shaped with a small
anterior loop. The chromosome number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin branched rays 8(28);
anal fin branched rays 5(1), 6(11), 7(15) or 8(1); pectoral fin
branched rays 15(10), 16(13) or 17(5); pelvic fin branched rays 9(27)
or 10(1); lateral line scales very embedded and obscured by skin and
mucus, range about 82-95; total gill rakers 11(4), 12(7), 13(8), 14(7)
or 15(2); pharyngeal teeth 5-4(5), 4-5(2) or 4-4(1); and total
vertebrae 37(1), 38(7), 39(14) or 40(1).
Sexual dimorphism
The second pelvic fin ray in males is thickened and is accompanied
by a muscular protuberance from the flank. Pelvic fins reach the anal fin in males.
Colour
The overall colour is a blackish-green, green or dark brown to
bronze. Colour varies with habitat and is lighter in areas with less
vegetation. The iris is red and the lips are yellowish-orange. Fins
are grey to greenish or blackish. Golden, red and orange forms, some
with black spots, may be bred in garden ponds and aquaria. Preserved
fish have a dark body and fins with a lighter belly.
Size
Reaches 84.0 cm total length and about 8.5kg, possibly 10.0 kg.
Distribution
Found from the British Isles and the Iberian Peninsula across
Europe to Siberia including southern drainages of the Black and
Caspian seas. In Iran it is reported from the Anzali Mordab and Safid River, neighbouring rivers to the Gorgan River,
and the Tajan and Babol rivers (Holčík and Oláh, 1992; Riazi, 1996; Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli, 2000; Daghigh Roohi and Mokhayer, no date;
Abdoli and Naderi, 2009). Khara et al.
(2005) record it from the Amirkelayeh Wetland near Lahijan.
Zoogeography
This widespread species has no close relatives among Eurasian cyprinids.
Habitat
Tench inhabit bays, small and shallow lakes, slow rivers and
estuarine areas rich in vegetation. They are confined to the lower
reaches of rivers in Iran and do not penetrate upstream (Berg,
1948-1949). Riazi (1996) reports that this species is native
(resident) to the Siah-Keshim Protected Region of the Anzali Mordab.
They are essentially inactive in winter. Tench are reported to bury
themselves in mud during severe winters, lying dormant until spring.
They can tolerate weak brackish water (to 12‰), acidic waters,
low oxygen conditions and temperatures as high as 37°C
for short periods. Preferred temperatures are 15-23.5°C (Brylińska,
Bryliński and Bănărescu in Bănărescu,
1999). They remain alive for long periods when removed from the water.
In Iran, adults have been caught in gill nets at 1-2 m (Annual
Bulletin 1993-94, Iranian Fisheries Research and Training
Organization, Tehran, p. 78-79, 1995).
Age and growth
Nezami Balouchi
et al.
(2004) studied of tench in the Amirkelayeh Lagoon and found age
groups 1+ to 7+ years. Vetlugina (1992) studied the biology of tench in the Volga Delta
using sections of dorsal fin rays for aging. Life span is 13 years or
more. Females grow faster than males and comprise 90-100% of older
fish. Growth rate is similar to fish from Dagestan, nearer Iranian
waters. Tench begin to mature at 3 years (30-40% of fish are sexually
mature) and by age 4-5, 60% are mature. In Dagestan they mature at 3-4
years (Shikhshabekov, 1977). Life span is up to 30 years elsewhere. Turkish
populations in lakes formed by dams range up to 5 or 6 years of age (Altindağ et al., 1998;
2002; Benzer et al., 2009). These authors give age-length and age-weight relationships and condition factors.
There is a substantial European literature on this fish.
Food
Diet comprises insect larvae, crustaceans, small molluscs, aquatic
worms, vegetation and detritus. Chironomids may constitute as much as
91.8% of the diet in the second year of life while older fish favour
snails and algae and macrophytes. This fish is probably a carnivore,
switching to vegetation when animals are not available. Food may be
picked out of vegetation or nosed out of mud as much as 13 cm deep
using the snout (Brylińska, Bryliński and Bănărescu
in Bănărescu, 1999). Nezami Balouchi et al. (2004) studied the diet of tench in the
Amirkelayeh Lagoon and found 17 food groups including Odonata, snails, water
plants, Trichoptera, Chironomidae, Hemiptera, Ephemeroptera, Perca
fluviatilis, Diptera, Gammarus, Tubifex, plant seeds,
Simulium, water bugs, water ticks, zooplankton and phytoplankton.
Phytoplankton, snails and Hemiptera had the highest frequenceis at 68.5%, 65.7%
and 34.0% respectively. This fish is an omnivore and diet varied with season age and sex.
Reproduction
Spawning occurs in shallow water with little current and abundant
vegetation. Spawning aggregations can be seen in April in the Volga
Delta and spawning takes place in May and June (Vetlugina, 1992).
Tench in the Anzali Mordab have well-developed, 1.0 mm eggs in early
June. Water temperatures at peak spawning are 20-26°C.
In Dagestan, spawning takes place in June-July at water temperatures
no lower than 19-20°C (Shikhshabekov, 1977). Each female is accompanied by 2-3 males and
different males will fertilise the egg batches as they are released.
Tench spawn 2 or 3 times at intervals of about 20-30 days, shedding
eggs onto surface vegetation. Eggs are greenish, adhesive and up to
1.57 mm in diameter. Fecundity is up to 863,000 eggs (or 124,850 per
kg of body weight) and increases with age, length and weight. Larvae
have attachment organs which enable them to hang onto plants for the
first few days of life. Maximum fecundity is 1,241,200 eggs in eastern Europe.
Parasites and predators
Daghigh Roohi and Mokhayer (no date) record the trematode Asymphylodora
tincae from this fish in the Anzali Lagoon, the first record of this
parasite in Iran. Khara et al. (2005) found
parasites of this species in the Amirkelayeh Wetland were Raphidascaris acus,
Camallanus lacustris, Asymphylodora tincae, Diplostomum
spathaceum, Dactylogyrus sp., Caryophyllaeus fimbriceps,
Lernaea sp. and Trichodina sp. Khara et al. (2006a) record the eye
fluke Diplostomum spathaceum for this fish in the Amirkelayeh Wetland in
Gilan, although it had the lowest abundance of 7 species examined.
Sattari et al. (2004, 2005) surveyed this species in the Anzali
and Amirkelayeh wetlands, recording Raphidascaris acus larvae and Camallanus lacustris.
Khara et al. (2006b) record the trematode Asymphylodora tincae
from this species in the Boojagh Wetland of the Caspian coast.
Barzegar et al.
(2008) record the digenean eye parasite Diplostomum spathaceum from this
fish.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Economic importance
Commercial catches are made in the Volga Delta where there are
spring (April) and fall (September-October) fishing seasons. Up to
6,300 tonnes are taken using fyke nets, trap nets and seines. Holčík and
Oláh (1992) report a catch of 540 kg in the Anzali Mordab in 1990.
The tench is a sought-after sport fish in Europe, putting on a strong fight for its
weight (personal experience). There is an ornamental form,
orange-yellow or reddish, which is kept in park ponds.
The roe or eggs of this species are particularly poisonous and it
should be cleaned with care to avoid contamination of the flesh
although it is not a common market fish in Iran (Halstead, 1967-1970;
Coad, 1979b). Probable symptoms and treatment are given under the
genus Schizothorax.
The tench is reputedly a "doctor fish" and other species
are said to rub against its slimy body as a cure for injuries. Rubbed
on humans, it is said to cure fever, headache, toothache and jaundice. In Iran,
this species has been proposed as a component of polyculture as it feeds on
snails, a host for diplostomiasis-causing parasites. A decrease in snail
frequency varied from 43 to 82% in experiments using adult and juvenile tench
(Iranian Fisheries Research Organization Newsletter, 37:3, 2003).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, in textbooks and
because it has been introduced outside its natural range.
Conservation
Lelek (1987) classifies this species as intermediate in Europe
(liable to be transferred to vulnerable or rare categories if their
habitat deteriorates further). Kiabi et al. (1999) consider
this species to be of least concern in the south Caspian Sea basin
according to IUCN criteria. Criteria include commercial fishing, sport
fishing, abundant in numbers, habitat destruction, widespread range
(75% of water bodies), absent in other water bodies in Iran, and
present outside the Caspian Sea basin.
There have been attempts to breed this species artificially in Iran
to increase the recruitment rate and control loss (Annual Bulletin
1993-94, Iranian Fisheries Research and Training Organization, Tehran, p.
78-79, 1995; Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 39, 1996; Sirang,
1997). Brood fish weighed 0.2-0.45 kg and were kept in earthen ponds.
Carp culture techniques were used with injection of gonadotropic
hormones from carp pituitary at 3-8 mg per kg body weight applied in
two doses. Water temperatures were 20-24°C,
eggs were placed in saline carbamide solution after fertilisation to
remove stickiness and washed in pure water. Swelled eggs measured
0.65-0.8 mm and were bright yellow to greenish-yellow. The number of
eggs per spawner was 10,848-17,710 and 80-350 eggs per 1 g body
weight. Fertilisation rate was 70-85%. Eggs were incubated for 2.0-2.5
days with a survival rate of hatched fry at 85-95% and size of newly
hatched larvae at 4.5-5.0 mm.
Haematological changes were investigated after
exposure to mercury, cadmium and lead by Lal Shah (2010), examining fish from
Mogan Lake near Ankara, Turkey. Impaired haematological parameters resulted in
hyperactivity, increased breathing, accelerated ventilation, surfacing and
sinking, erratic swimming, inactivity, lethargy and convulsions.
Further work
The biology of this species has been well-studied elsewhere in its extensive
range but not under Iranian conditions.
Sources
Iranian material: CMNFI 1970-0514, 2, 63.9-106.9 mm standard length, Gilan, Shafa River estuary (37º35'N, 49º09'E);
CMNFI 1970-0553, 1, 70.9 mm standard length, Gilan, Sowsar Roga River (37º27'N, 49º30'E);
CMNFI 1970-0580, 3, 28.0-36.2 mm standard length, Mazandaran, river near Iz Deh (36º36'N, 52º07'E);
CMNFI 1979-0439, 1, 170.9 mm standard length, Gilan, Anzali Mordab (ca. 3727'N, ca. 4925'E);
CMNFI 1980-0148, 14, 33.9-49.7 mm standard length, Gilan, Pir Bazar Roga River (37º21'N, 49º33'E);
CMNFI 1980-0916, 6, 38.2-51.1 mm standard length, Gilan, Nahang Roga River (no other locality data);
CMNFI 1993-0137, 1, 150.4 mm standard length, Mazandaran, Sari (36º34'N, 53º04'E).
Genus Tor
Gray1834
?
Much of the past
literature on this genus appeared under Barbus (q.v.)
Tor grypus
(Heckel, 1843)
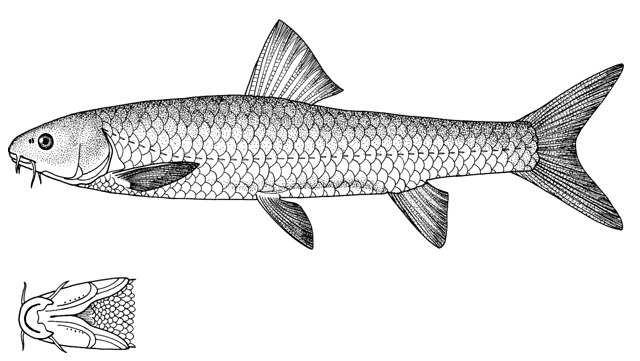

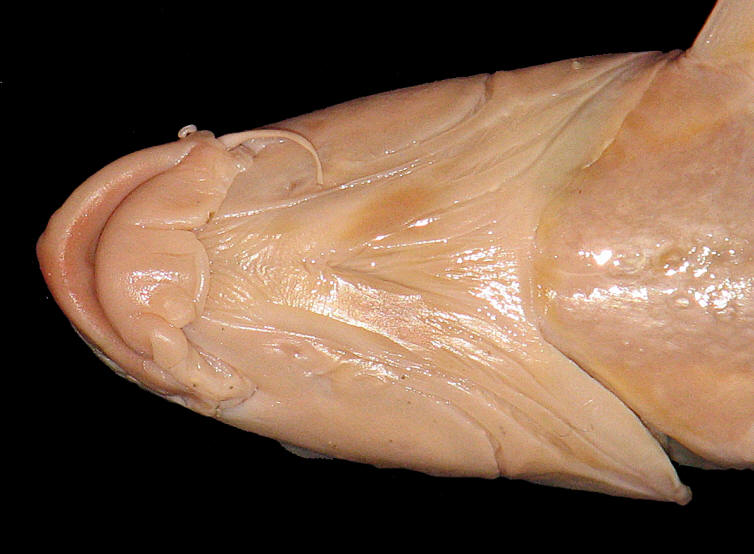

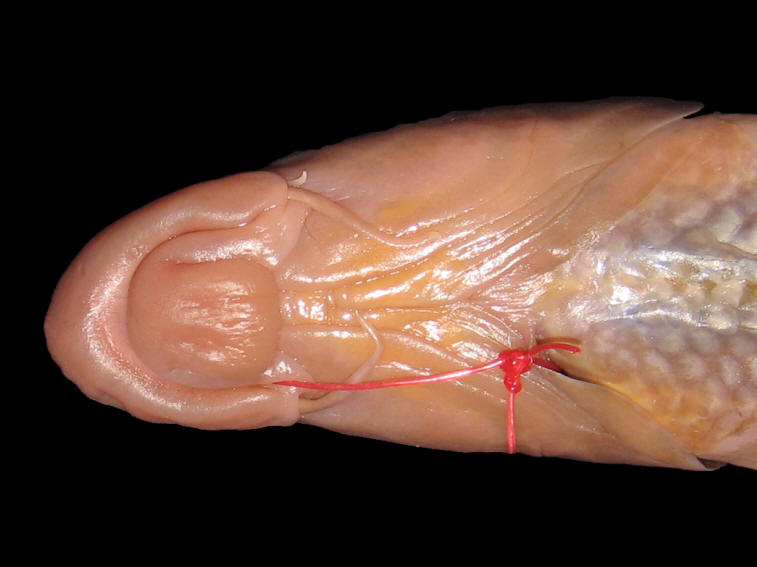
Above photographs showing fleshy lips courtesy of Atabak Mahjoor Azad
Common names
شيربت (= shirbot, shirbod or shilbot), shaboot, سس ماهي (= sos or sas mahi), rumi, shebhe shirbot, سرخه
(sorkheh, meaning= reddish, a local name in the Zohreh River - J. Gh. Marammazi, pers. comm., 1995), rumi (not heard of in Khuzestan).
[shabout or hamrawi in Arabic; large scaled barb, Tigris barbel].
Systematics
Howes (1987) places this species outside the genus Barbus sensu stricto as defined by him because it has the non-elongate lachrymal bone with a sensory canal running along the antero-dorsal
border, a derived condition. Karaman (1971) and Ekmekçi and Banarescu (1998) placed it in the genus Tor (see
Barbus) and it may belong in Naziritor Mirza and Javed, 1985 (M. R.
Mirza, pers. comm., 6 December 2003). Al-Hassan (1984) looked at several "Barbus" species and found the electropherogram of this species to be distinctive, perhaps indicating that molecular studies
could resolve the relationships of this species. This distinction was reiterated by Jawad (2003).
The type locality of Barbus Grypus is "Tigris bei Mossul" (Heckel, 1843b) and Krupp (1985c) records a syntype (dried) from the Naturhistorisches Museum Wien (formerly NMW) now in
the Senckenberg Museum Frankfurt under SMF 2613, 375 mm standard length. One syntype is in the Museum für Naturkunde, Universität Humboldt, Berlin (ZMB 8788, not located February 2006). One syntype is in
Vienna under NMW 54160, 2 are under NMW 54161 (280.9-318.9 mm total length as
measured by me), and 1 is under NMW 91023 (Eschmeyer et al., 1996). The catalogue in Vienna lists 3 fish in spirits and 2 fish stuffed.
Labeobarbus Kotschyi Heckel, 1843 described from the "Tigris bei Mossul"
has long been regarded as a synonym although Valiallahi (2000) resurrects this
species. Krupp (1985c) records a syntype from the Naturhistorisches Museum Wien under NMW 49729, Th.
Kotschy (188.8 mm standard length as measured by me). A dried specimen (NMW 59462) is also a syntype. Eschmeyer et al. (1996) also lists another syntype, NMW 91022. The catalogue in Vienna lists
1 fish in spirits and 2 fish stuffed.
Key characters
This species is identified by having two pairs of barbels, a strong, smooth spine in the dorsal fin, and less than 44 scales in the lateral line.
Morphology
The forehead is more rounded than in type material of kotschyi, although kotschyi types are smaller than grypus types which may account for this distinction. The mouth is
inferior, horseshoe-shaped and has fleshy lips. The median lobe of the lower lip is well-developed in some individuals (such specimens were described as kotschyi - this form is rare
in Khuzestan according to N. Najafpour, pers. comm., 1995) and intermediates can be seen) but not in others (grypus) (Karaman, 1971). The median lobe may extend back almost as far as the level of
the rear margin of the lower lip or be distinctive with free lateral and rear margins but only extend back one third of this distance. The much fleshier lip structure in kotschyi (the upper lip
can be reflexed for example) may be a form of hypertrophy seen in other cyprinid fishes
(see Roberts and Khaironizam (2008) for further discussions on this feature)). The last unbranched dorsal fin ray is smooth and spine-like, with sharp edges but no serrations although serrations
are weakly developed in young fish. Barbels are about equal in length. The gut has two anterior and two posterior loops.
Dorsal fin unbranched rays 4, branched rays 7-9, usually 8, anal fin unbranched rays 3, branched rays 5, pectoral fin branched rays 14-18 (including counts from Jawad (1975)), and pelvic
fin branched rays 7-8, usually 8. Total gill rakers 16-22 (including counts by Jawad (1975)). Krupp (1985c) cites 13-17 gill rakers, presumably lower arch ones only. Gill rakers reach the second raker
below or beyond when appressed, with large tubercles or branches on the inner surface in 2 rows alternating left and right. Lateral line scales 32-43. A pelvic axillary scale is present. Scales have a
subcentral anterior, almost central, focus, numerous fine circuli and many radii on all fields with the exposed part of the scale tubercular. Pharyngeal teeth 2,3,4-4,3,2 in the literature,
but see below, anterior teeth rounded, the most anterior one small and blunt, posterior ones spatulate with hooked tips. Total vertebrae 44-47 (Howes, 1987), 43-45 (Jawad, 1975) or 47(8), 48(7), 49(5)
or 50(1) in fish seen by me (BM(NH) 1974.2.22:1283-1284, 1299-1315, 1317, 1323; 1920.3.3:1-18, 1874.4.28:24-6).
Ali et al. (1981) found differences in morphology for fish from Al-Therthar Reservoir and the Tigris River in Iraq, not by locality but by habitat type.
Iranian specimens have the following meristic data: dorsal fin branched rays 7(1), 8(20) or 9(1); anal fin branched rays 5(22); pectoral fin branched rays 15(2), 16(15), 17(4) or 18(1), and pelvic
fin branched rays 7(1) or 8(21). Lateral line scales 32(4), 33(4), 34(4), 35(3), 36(3) or 37(3). Total gill rakers 16(4), 17(2), 18(3), 19(6), 20(4) or 21(2), with some evidence of higher counts in larger
fish. Pharyngeal teeth usually 2,3,5-5,3,2(14) with variants 2,3,5-4,3,2(2), 2,3,4-5,3,2(2), 2,2,5-5,3,2(1) and 1,2,5-4,3,2(1), in contrast to literature reports of 4 main row teeth being typical.
Sexual dimorphism
Ali et al. (1981) found no sexual dimorphism in their Iraqi samples.
Colour
Overall colour has a pale rose to light orange effect, usually without other markings. The back is a dark olive-brown to blackish-green with the flanks pale rose, light orange to yellowish to silvery
and belly silvery to milk-white. There may be an indistinct stripe along the mid-flank. Large fish have the upper flank darkened from the overall orange colour of the mid-flank and the
lower flank scales are rimmed in white so they stand out. Lips are pale red. The operculum is golden. The pectoral, pelvic, anal and caudal fins are bright orange or pink at the base (perhaps white after
preservation), distally blackish. Pectoral and pelvic fins may be dark overall with a reddish to reddish-brown tinge, and the leading edge of the pelvic fin pink. The anal fin may be a bluish-black
distally. In some fish the caudal fin is black proximally and reddish distally. In large fish the pectoral, pelvic, anal and caudal are progressively darker in this order. The anal and pelvic
fins, the pectoral fins less so, may be heavily pigmented with melanophores on rays and membranes so as to appear black in preserved fish. The dorsal fin is hyaline. The eye rim is yellow-green to
lime-green. Young fish may have some scales darkened, giving a mottled effect and are more silvery on the flank than large fish. Their pectoral and pelvic fins are more orange and the anal and caudal fins
are only slightly tinged with colour. The caudal fin carries a lot of grey. The smallest fish have a very faint fin colouration. Peritoneum black.
Size
Attains 96.0 cm and 9.7 kg in Dukan Reservoir, Iraq and 96.0 cm and 11.0 kg
in Atatürk Dam Lake on the Euphrates River in
Turkey (Al-Hakim et al., 1981; Oymak et al., 2008). Gruvel (1931) cited 1.5 m and 30 kg for Syria. Banister (1980) gives nearly 2 m and 100 kg but this may be confusion
with B. esocinus although Krupp (1992) also cites almost 2 m. Reputedly reaches 60 kg in Lorestan (S. Nazeeri, pers. comm., 2000) and Ghofleh Marammazi (2004) found fish up to 20 kg in Khuzestan.
Specimens reach 3 kg even in the small canals of the sugar-cane fields of Khuzestan.
Distribution
This species is found in the Tigris-Euphrates basin and the Orontes River basin. In Iran it is found in the Tigris River basin up to the Simarreh including marshes such as the Hawr Al Azim, in the
Gulf basin in the Zohreh, Shapur, Helleh, Dalaki, Dasht-e Palang, Shur, Dozgah and Mand rivers and Lake Famur (= Perishan), although rare in the latter, and in the Hormuz basin in the Hasan Langi and
Kul rivers (Berg, 1949; Gh. Izadpanahi, pers. comm., 1995; Marammazi, 1995; M. Rabbaniha, pers. comm., 1995; Maafi, 1996b; H. R. Alizadeh, pers comm., 2000; Abdoli, 2000).
Zoogeography
Karaman (1971) considers this species to have an Indian line of descent, placing it in the genus Tor which most subsequent authors restrict to the Indian subcontinent and southeast Asia.
Habitat
van den Eelaart (1954) and Al-Hamed (1966b; 1972) describe the habitat for this species in the Tigris River as distributed throughout the river and its tributaries. It is a strong swimmer.
Al-Rudainy (2008) states that it can be found in the mid-water column in high
current. Mature fish
move upstream to the spawning grounds and spent fish descend to their original habitat. In summer under low water level conditions and high temperatures, the smaller fish remain in the lower reaches of
rivers but the larger fish migrate up rivers and tributaries, returning in September and October when temperatures fall. This species may enter marshes on floods, favouring areas where there is fresh
river water, but returns to rivers as it requires a higher oxygen concentration than most marsh residents. Heydarpour (1978) gives a temperature range of 9-31ºC for this species under culture conditions
in Khuzestan.
Marammazi (1994) considers this species to be versatile in its habitats in the Zohreh River which drains to the northern Persian Gulf. It was found throughout the river in contrast to
Mesopotamichthys
sharpeyi which, being stenohaline, was restricted in its distribution. The form with a well-developed median lobe is said to occur in rocky habitats. This species is considered to be the dominant
fish in the Karun and Zohreh rivers (Annual Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran, p. 48, 1996; Iranian Fisheries Research and Training Organization
Newsletter, 17:1, 1997). Ghofleh Marammazi (2004) found it in almost all water bodies in Khuzestan where it occurred under a wide range of temperatures and salinities. However its
presence on the Khuzestan plain was for feeding while for reproduction it required more northerly areas with sandy or gravel substrates, high water flow, low temperatures and high oxygen content (Ghofleh
Marammazi, 2000). Ramin (2009) records this species as the most abundant in the
Karkheh River out of 37 species and subspecies.
Age and growth
Dorostghoal et al. (2009) found mean body
lengths were 36.5-43.5 cm and mean body weights were 835.0-1012.0 g for their
Karun River samples. Hashemi et al. (2010) examined fish from the
southern Karun River in Iran and found a size range of 20-76 cm and 52-11170 g,
growth was isometric, and growth and mortality parameters were L∞ =
86.64, K = 0.27, t0 = -0.46, M = 0.5, F = 1.22, Z = 1.78 and E =
0.71. Relative yield per recruitment (Y'/R) was 0.037, relative biomass per
recruitment (B'/R) was 0.29, exploitation ratio maximum sustainable yield (Emax)
was 0.44, precautionary average target (Fopt) was 0.25 year-1,
and limit (Flimit) as 0.331 year-1. The stock was
overfished and fishing regulations are required.
Al-Hakim et al. (1976) studied some aspects of the biology of this species in Razzaza Lake, Iraq. Males are longer than females before maturation and shorter thereafter. Females reach 13 years
and males 8 years of age and fish mature at 45-48 cm total length in their fifth year. Males mature earlier than females. Ali et al. (1981) found this species to mature at 3-5 years of age and
40-50 cm in the Al-Therthar Reservoir (about 65 km northwest of Baghdad) and the Tigris River (Kut Dam) in Iraq. Jiad et al. (1984) studied this species in the Al-Hindiya Dam in Iraq and found
similar results to the studies cited above.
Al-Hakim et al. (1981) studied this species in the Dukan Reservoir, west of Sulaimaniyah, Iraq. Life span is 17 years for females and 11 years for
males. Growth slows with age, and especially after maturity, and is fastest in the first year of life. 30% of males mature at age group 3 (39 cm) and all were mature at age group 6 (48
cm). Al-Hamed (1966a; 1966b; 1972) working with Tigris River populations in Iraq, found males to mature at about 45 cm and females at about 50 cm, with most fish mature in their fourth year and
spawning at the beginning of their fifth year of life. Some fish mature in age group 3 and some as late as age group 5. Maximum age observed was 12 years. Males outnumber females, being two thirds of
the fish on the spawning grounds. Al-Hakim et al. (1981) found all females are mature at 51 cm (age group 7) but only 10% at 42 cm. Males mature earlier than females and may grow faster and die
younger.
Ali et al. (1981) found growth to be better in fish from Al-Therthar Reservoir compared to those from the Tigris River in Iraq. The fishing methods used, both commercially and experimentally,
caught mature fish of 40-50 cm and 3-5 years of age.
Oymak et al. (2008) examined age and growth in the Atatürk
Dam Lake on the Euphrates River in Turkey. Fourteen age classes were found with
age classes 4-6 for females and 2-4 for males dominant. von Bertalanffy
growth equations were given for males and females.
Growth in a polluted section of the Diyala River, Iraq is poor compared to other populations (Khalaf et al., 1984; Khalaf et al., 1985).
Food
Al-Hamed (1965) found this species to be a herbivore taking filamentous algae and higher plant parts. Incidental food items taken while feeding on plants include fish tissue and scales. Fallen ripe
fruits from trees overhanging the water are also consumed as are cereal grains from loading docks. It may also take some small fishes. Iranian specimens contain filamentous algae, plant fragments and
associated invertebrates. Ghofleh Marammazi (2000) considers it to be an omnivore.
Reproduction
In Dukan Reservoir, Iraq spawning takes place from the beginning of May until the end of June. van den Eelaart (1954) and Al-Hamed (1966b; 1972) studied the reproduction of this species in Iraq. Eggs
are deposited on fine gravels overlying a layer of coarse sand in shallow, wide holes. Water depth varies from 30 to 150 cm. Egg diameter is 1.5 mm and fecundity up to 147,000. The spawning season on the
Tigris River between Beled and Tigrit is late May to late June after an upriver migration in April. Fish appear on the spawning grounds in schools just before dark and remain there until shortly before
midnight, making loud noises by splashing, jumping and chasing. After spawning, the fish return downriver but do not enter marshes as these are now too warm.
Oymak et al. (2008) examined reproduction in the Atatürk
Dam Lake on the Euphrates River in Turkey and found a sex ratio of 1:1.34
(females/males), with a spawning period in May to July, a fecundity up to
235,764 eggs and a mean egg diameter of 2.183 mm.
Its presence in areas of the Khuzestan plain is mainly for feeding while reproduction occurs in the northern parts of this province where there are sandy and gravel substrates, fast current, low
temperatures and high oxygen content (Ghofleh Marammazi, 2004). A prolonged
spawning season in fish from the Karun River, late April to early August with
the highest gonadosomatic index June-July, was determined by Dorostghoal et
al. (2009) using macroscopic and microscopic techniques. They also note that
the fish migrate upstream for spawning in May.
Parasites and predators
Bykhovski (1949) report a new species of monogenetic trematode, Dactylogyrus pavlovskyi, from this species in the Karkheh River, Iran. Molnár and Jalali (1992) describe a new species of
monogenean, Dogielius persicus, from this species in the Dez and Karun rivers of Khuzestan. Masoumian et al. (1994) describe two new species of Myxosporea from the gills of this species in
the Karun River, Khuzestan, namely Myxobolus karuni and Myxobolus persicus. Molnár et al. (1996) report additional new species from this fish in Khuzestan, namely Myxobolus
iranicus in the spleen and Myxobolus mesopotamiae in connective tissue of the caudal and pectoral fins. The latter myxosporean is also reported from Barbus rajanorum
as is a new species Myxobolus shadgani infecting the gills - the identity of the host fish is unknown as Barbus rajanorum is not a distinct species (see under
Luciobarbus pectoralis).
Myxosporeans are potentially dangerous to fishes such as Tor grypus which may be used in fish culture in Khuzestan.
The monogeneans Dactylogyrus povlovskyi, D. barbioides, Dogielius persicus, Gyrodactylus sprostonae and Paradiplozoon sp. are recorded from this species in
the Karun River with heavier infestations in spring and summer than in autumn and winter. These gill parasites caused no serious injuries but were thought to be important in respect of monitoring
infestation levels on fish farms in Khuzestan (www.avz1.8m.com/fulltext.htm, downloaded 28 October 2002).
Ebrahimzadeh and Nabawi (1975) list a nematode species Philometra and Ascaridae from this species in the Karun River. Ebrahimzadeh and Kailani (1976) record parasites in the genera
Myxosoma (protozoan) and Isoglaridacris (cestode) and also a nematode from Barbus
(= Tor) grypus taken in the Karun River.
Masoumian and Pazooki (1999) list Myxobolus persicus, M. karuni, M. mesopotamiae and M. iranicus from this species in various localities in Khuzestan. Moghainemi and Abbasi (1992) record
a wide range of parasites from this species in the Hawr al-Azim in Khuzestan. Farahnak et al. (2002) record Contracaecum sp. and Anisakis sp. from this fish in Khuzestan Province.
Peyghan et al. (2001) record Neoechinorhynchus sp. from fish from
Khorramabad rivers. Peyghan et al. (2001)
record Myxobolus karuni in 86.7% of fish in the Karun River at Ahvaz and Papahn et al. (2004) record the monogeneans Dactylogyrus pavlovskyi, D. barbioides, Dogielius persicus, Gyrodactylus sprostonae and Paradiplozoon sp. from the
same locality, the latter two being first records for the host in Iran.
Barzegar et al. (2008) record the digenean eye parasite Tylodelphys
clavata from this fish. Barzegar and Jalali (2009) reviewed crustacean
parasites in Iran and found Argulus sp. and Ergasilus sp. on this
species.
Economic importance
An important food fish, with desirable taste
(Al-Rudainy, 2008), comprising 23% of the total fish production in Iraq for example and forming the most important commercial fish there (Al-Hakim et al., 1981). Petr (1987) reports the
catch for all Iraq in 1976 as 519 t. The weight at the Basrah fish market from October 1975 to June 1977 was only 3,330 kg however (Sharma, 1980) and Khalaf et al. (1984) rank it third in the
inland wholesale trade of Iraq for the period 1967-1970.
This species is the preferred catch of anglers at Ahvaz in Khuzestan, with bread or potato as bait. There is a good demand for this species in local markets of Khuzestan (Ghofleh Marammazi, 2004).
Peyghan et al. (2001) report that is is an economically important species
with a good market value in the Khorramabad region.
Petr (1987) has suggested that this species be investigated for fish farming in Khuzestan. The Khuzestan Fisheries Research Centre at Ahvaz has experimented with this species in pond culture (Emadi,
1993a; Annual Report, 1994-1995, Iranian Fisheries Research and Training Organization, Tehran, p. 6, 1996).
Conservation
The stock of this species in the Gav Masiab River is severely reduced and only 3 fish were caught in western Iran in the Zagros rivers during a 4-year survey (J. Valiallahi, www.modares.ac.ir,
downloaded 4 July 2000; pers. comm., 2001). Shirbot were considered to be on the verge of extinction in the Gav Masiab River of the Tigris River basin, through pollution, overfishing, dam
building, aquaculture, and introduction of exotics (IranMania.com, 29 December 2006).
Dadelahi Sohrab et al. (2009) found lead and cadmium levels in fish from
the Arvand River were higher than acceptable by international standards. Dorostghoal et al. (2009) note that the species is caught on the spawning
migration and while spawning and this accounts for its decline. Probably in decline in Turkey
also (Fricke et al., 2007).
Further work
The biology of this species in Iran needs investigation as does its putative relationship to South Asian fishes in the genus Tor.
Sources
Type material: See above, Barbus grypus (NMW 54161) and Labeobarbus kotschyi (NMW 49729).
Iranian material: CMNFI 1979-0155, 1, 42.8 mm standard length, Fars, spring at Gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0291, 1, 31.4 mm standard length, Kermanshahan, Diyala River drainage (34º24'N, 45º37'E);
CMNFI 1979-0356, 5, 22.5-40.7 mm standard length, Khuzestan, Karkheh River drainage at Hoveyzeh (31º27'N, 48º04'E);
CMNFI 1979-0360, 1, 375.5 mm standard length, Khuzestan, canal branch of Karkheh River (31º40'N, 48º35'E);
CMNFI 1979-0364, 1, 22.3 mm standard length, Khuzestan, river at Abdolkhan (31º52'30"N, 48º20'30"E);
CMNFI 1979-0384, 2, 202.2-222.2 mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0391, 1, 220.5 mm standard length, Khuzestan, stream in Marun River drainage (31º28'N, 49º51'E);
CMNFI 1979-0392, 1, 62.4 mm standard length, Khuzestan, Zard River (ca. 31º32'N, ca. 49º48'E);
CMNFI 1979-0395, 2, 32.7-38.0 mm standard length, Khuzestan, stream in Marun River drainage (ca. 30º57'N, ca. 49º51'E);
CMNFI 1979-0402, 1, 80.7 mm standard length, Bushehr, Mand River 12 km north of Kaki (ca. 28º25'N, ca. 51º32'E);
CMNFI 1991-0153, 1, 253.5 mm standard length, Khuzestan, Zohreh River (no other locality data);
CMNFI 1993-0141, 1, 80.2 mm standard length, Bushehr, Dalaki River (29º28'N, 51º15'E);
CMNFI 1995-009A, 4, Khuzestan, A'la River at Pol-e Tighen (31º23'30"N, 49º53'E);
BM(NH) 1980.8.28:7, 1, 72.0 mm standard length, Khuzestan, Dezful (32º23'N, 48º24'E);
ZMH 2508, 1, 343.3 mm standard length, Khuzestan, Karun River at Ahvaz (31º19'N, 48º42'E);
ZSM 21864, 1, 157.7 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E);
Comparative material:- BM(NH) 1874.4.28:24-26, 3, 231.2-254.7 mm standard length, Iraq, Tigris River at Baghdad (33º21'N, 44º25'E);
BM(NH) 1920.3.3:1-18, 5, 104.8-196.0 mm standard length, Iraq, Basrah (30º30'N,
47º47'E); BM(NH) 1973.5.21:191, 1, 205.4 mm standard length, Iraq, Shatt-al-Arab;
BM(NH) 1973.5.21:192, 1, 139.5 mm standard length, Iraq, Shatt-al-Arab;
BM(NH) 1974.2.22:1283-1284, 2, 121.0-164.5 mm standard length, Iraq, Khalis (no
other locality data); BM(NH) 1974.2.22:1299-1315, 9, 64.9-101.5 mm standard length, Iraq, branch of
Khalis River (no other locality data); BM(NH) 1974.2.22:1317, 93.9 mm standard
length, Iraq, branch of Khalis River (no other locality data); BM(NH) 1974.2.22:1323, 1, 160.1 mm
standard length, Iraq, Basrah (30º30'N, 47º47'E); BM(NH) 1974.2.22:1328, 1, 161.9 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
KU 10516, 1, 124.1 mm standard length, Iraq, Basrah (30º30'N, 47º47'E).
Genus Vimba
Fitzinger, 1873
This genus is found in the basins of the Baltic,
Black and Caspian seas and has a single species.
It is characterised by a compressed, moderately deep
body with an inferior, crescentic mouth, a scaleless keel between the
pelvic and anal fins, a scaleless groove in front of the dorsal fin
and an evident keel behind it, pharyngeal teeth in a single row, short
dorsal and long anal fin, gill rakers short, and scales moderate in size.
Bogutskaya (1986) using skull morphology reaffirms
the generic separation of Vimba Fitzinger, 1873 from Abramis
Cuvier, 1816 although Howes (1981) considers it to be a synonym.
Vimba persa
(Pallas, 1814)
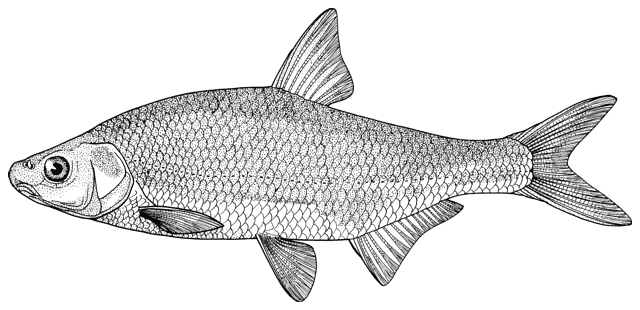

CMNFI 1979-0435, Gilan, stream 10 km west of Ramsar
Common names
سياه كولي (= siah kuli or
siahkooli, meaning black fish, cooli, couli, coli, kooli or kuli being any small fish;
in Gilaki), mahi siah kuli.
[garasol in Azerbaijan; chernospinka, Kaspiiskii rybets or Caspian
vimba, both in Russian; southern white-eye, vimba].
Systematics
Cyprinus persa Gmelin, 1774 is a nomen nudum - see Kottelat
(1997) - and was later made available by Pallas. It was described originally from "Persa; in lacubus ad Cyrum",
i.e. the southern coast of the Caspian Sea in lakes of the Kura River
system in Azerbaijan. It is distinguished by larger scales and usually fewer anal rays from
V. vimba (Linnaeus, 1758) which it was formerly synonymised with or
recognised as a subspecies of. Cyprinus Vimba was originally described from lakes of Sweden.
Hänfling et al. (2009) found phylogenetically
distinct mtDNA sequences for Caspian Sea basin samples, the Caspian clade having
diverged from a western or Pontic clade 1-2 MYA at the beginning of the
Pleistocene. Caspian populations could then rank as a separate species or subspecies
although they consider further work involving western Caucasian populations is needed to
support one conclusion over the other. Naseka and Bogutskaya (2009) recognise
V. persa as a species.
Rahmani and Abdoli (2008)
compared populations from the Gorgan River, Shirud and Anzali Lagoon and found
morphometric and meristic differences between them.
A hybrid with Alburnus chalcoides was reported from the
Safid River (Petrov, 1926).
Key characters
The snout projects over the lower jaw and in large fish is quite
bulbous, there is a keel on the belly and on the back, and fin ray
counts are distinctive.
Morphology
Western and southeastern populations in the Caspian Sea can be
distinguished morphometrically, and represent different stocks.
Dorsal fin with 2-3 unbranched rays (always 2 in the subspecies persa (Berg,
1948-1949) but the first unbranched ray is minute and visible in
x-rays in Iranian specimens) and 7-9, usually 8, branched
rays, anal fin with 3 unbranched and 12-22 branched rays (16-18 in persa
but see below), pectoral fin branched rays 11-18 and pelvic fin
branched rays 7-10. Lateral line scales 47-64, 48-54 in persa
(but see below). The lateral line runs below the midline of the caudal
peduncle. Predorsal scales are small and crowded. A pelvic axillary
scale is present. The naked ventral keel begins 0-3 scales behind the
pelvic fin bases. Scales at the anal fin base form a sheath. The
anterior scale margin is wavy and the posterior margin is crenualte.
There is a central focus, numerous fine circuli and few anterior and
posterior radii. Gill rakers 12-20, small and reaching the raker below
when appressed. Pharyngeal teeth usually 5-5, with the largest teeth
having long and narrow, flat to slightly concave crowns, and tips
recurved or very slightly hooked. Vertebrae 38-45. Gill rakers 14-19.
The gut is s-shaped. The chromosome number is 2n=50 (Klinkhardt et
al., 1995) or 2n=52 (Reshetnikov, 2002).
Meristic values for Iranian specimens are:- branched dorsal fin rays 7(1) or
8(39); branched anal fin rays 16(3), 17(13), 18(18) or 19(6); branched
pectoral fin rays 14(8), 15(22), 16(7) or 17(3); branched pelvic fin
rays 8(10) or 9(30); lateral line scales 47(1), 48(4), 49(7), 50(10),
51(14), 52(1), 53(1), 54(1) or 55(1); total gill rakers 15(1), 16(2),
17(12), 18(14), 19(9) or 20(2); pharyngeal teeth 5-5(15), 5-4(4) or
4-5(1); and total vertebrae 41(3), 42(9), 43(24) or 44(3). Abbasi et al. (2004)
found 149 Safid River fish to have mean values of 50.83 lateral line scales,
branched dorsal fin rays 7.96 and branched anal fin rays 17.58.
Sexual dimorphism
Females are slightly larger than males of the same age and differ
morphometrically on account of the eggs distorting body shape. The
males become black on the back, reddish on the belly, their fins
become red and the tips of the dorsal and caudal fins become dark, and
they develop minute tubercles on the body during the spawning season (Kuliev,
1988; Abbasi et al., 2004). Females may also develop tubercles but to a lesser extent.
Safid River fish showed differences in 2 meristic and 16 morphometric
characters, especially body depth and lengths of dorsal, pectoral, pelvic and
anal fins (Abbasi et al., 2004).
Iranian specimens have small tubercles lining the scale margins and
larger tubercles over the whole head but particularly on the dorsal
surface and upper sides. Fin rays bear small tubercles in files
following the branching of the rays. The pelvic fin has weakly
developed tubercles on its ventral surface as well as dorsally. The
unbranched pectoral and pelvic rays bear several rows of tubercles.
Colour
The back is a reddish-brown to grey-blue, flanks are silvery and
the belly yellowish. Paired fins are red at the base, pink distally.
The anal fin base is red while other fins are grey to hyaline.
Spawning fish develop black stripes along the dorsal and ventral body.
Size
Reaches 60.0 cm and 3 kg. The subspecies persa is smaller, to 30 cm.
Distribution
Found from central Europe to the Caspian Sea basin. In Iran it is
recorded from the Aras to the Atrak rivers in the Caspian Sea basin including
the Manjil Reservoir on the Safid River, the Anzali Mordab and Gorgan Bay, the
Gorgan, Gharasu, Tajan, Babol, Haraz, Sardab, Tonekabon, Pol-e Rud and Safid
rivers, and the southeast, southwest and south-central Caspian Sea (Kozhin, 1957; Nümann, 1966; Holčík
and Oláh, 1992; Riazi, 1996; Abbasi et al., 1999; Kiabi et al.,
1999; Abdoli, 2000; Abdoli and Naderi, 2009). It has also been introduced to Sistan.
Zoogeography
This species reaches its most south-easterly occurrence in Iran and has been
given subspecific status.
Its relationships lie with European taxa (see Abramis).
Habitat
Caspian vimba have a sparse distribution in the sea and are not
fished there commercially. It is more common in Gilan than Mazandaran and
Golestan coastal waters (Jolodar and Abdoli, 2004). The semi-migratory form enters fresh water
or brackish water only for reproduction in spring. After spawning, it
migrates to river mouths to feed until the next reproductive season (Kuliev,
1988). Riazi (1996) reports that this species is native (resident) to
the Siah-Keshim Protected Region of the Anzali Mordab. S. Bazari Moghaddam
(www.meeresschule.com/cgi-bin/abstracts/gastbuch.asp,
downloaded 17 January 2005) records a migration into the Safid River in
spring for reproduction. Feeding continues on this migration. Knipovich
(1921) reports this species from depths of 36.6-53.0 m in the Iranian
Caspian Sea. In fresh water it occurs in schools in the lower reaches
of rivers, in deep water over stone and gravel bottoms. It may also
occur in lakes over mud bottoms.
Age and growth
Maturity is attained in the second or third year of life, males
maturing at age 2 in the Anzali region. In the Safid River migrating fish were
2-4 years old, predominately 3-year-old fish (S. Bazari Moghaddam,
www.meeresschule.com/cgi-bin/abstracts/gastbuch.asp,
downloaded 17 January2005). Four-year-olds predominate in
the spawning population in Kyzylagach or Imeni Kirova Bay, Azerbaijan.
Most spawning females are 16-23 cm (46%) and males 13-19 (42%). Large
fish spawn first and the number of smaller fish spawning increases
towards the end of the reproductive season (Kuliev, 1988;
Shikhshabekov, 1979). Most fish on the spawning migration into the Anzali Mordab
are 170-250 mm and ages 3-4 years (Holčik
and Oláh, 1992). Fish on the spawning migration of the Safid River had a fork
length of 116-208 mm and a weight of 21.1-116.1 g in males and 122-222 mm and
23.1-170.0 g in females. Spawning males were 2-6 years old and females 3-7 years
(Abbasi et al., 2005). Maximum life span is about 15 years.
Food
Diet is aquatic insects, crustaceans, snails, worms and algae on
muddy bottoms. Iranian specimens had zebra mussels and insect remains.
S. Bazari Moghaddam (www.meeresschule.com/cgi-bin/abstracts/gastbuch.asp, downloaded 17
January 2005) reports oligochaetes, chironomids and Odonata in fish from the Safid River.
Reproduction
Kuliev (1988) and Shikhshabekov (1979) studied reproduction in the
Kyzylagach Bay of the southwestern Caspian Sea and the waters of
Dagestan respectively. The spawning migration begins in March or April
at 10-13°C and spawning takes place at the end of April at 16-20°C, continuing
until the end of May or into June.
Fish enter the Anzali Mordab of Iran in mid-January at a water temperature of 8-9°C,
peaking from 21 April-10 May at 19-21°C (Holčík and Oláh, 1992). Khaval (1998) reports a spawning migration into the
Safid River despite construction, sand removal and pollution. Fish
from the Shafa River estuary in Iran caught on 10 April had highly
developed eggs measuring 1.3 mm. Fecundity is up to 89,200 eggs per
female, increasing with age and body size (elsewhere in Europe to
200,000 eggs with a diameter of 1.4 mm). Spawning is non-intermittent,
in contrast to Black Sea vimbas. Eggs are deposited on gravel or
stones where there is a current of 0.6-0.9 m/second. Concrete
structures and flooded fields may be used as long as there is some current (Holčík and
Oláh, 1992). The eggs may form a layer up to 10 cm thick. Initially
attached to plants or stones, the eggs are later washed down between
the plants or stones. Other fishes eat these eggs and mortality is
high. Some fish deposit eggs in sandy shallows of bays or on the roots
of reeds and bulrushes. The young migrate to the coastal zone of the
Caspian Sea for the summer, moving to greater depths as winter
approaches. At temperatures of 17-22°C, eggs incubate for 70-77 hours (Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 37-38, 1996).
Abbasi et al. (2005) found that the Safid River population started the
spawning migration in March and this continued until July, peaking in mid-April
to late May. Gonad weight for females increased with distance from the estuary.
Spawning occurred from late May to late June, peaking in May at 18-29ºC
water temperatures. Eggs were shed on pebble and gravel grounds 25-75 km from
the estuary. The Disaam tributary was the major spawning site.
Parasites and predators
Jalali and Molnár (1990a) record the monogeneans Dactylogyrus
cornoides and D. haplogonus from this species in the Safid
River and Pazooki and Aghlmani (no date) record the trematode Asymphylodora
kubanicum from Iranian specimens. Sattari et al. (2007) record the cestodes Caryophyllaeus laticeps
and Caryophyllaeus fimbriceps, the digenean Diplostomum spathaceum
and the monogenean Dactylogyrus extensus in this species in the Anzali
wetland of the Caspian shore.
Economic importance
The vimba catch over the whole Caspian Sea basin was less than 100
tonnes per year in the 1980s (Kuliev, 1988). The catch by local
fishermen in the Anzali Mordab region in 1990-1991 was 823 kg or about
8400 fish (Holčík and Oláh, 1992). They are caught in rogas and inflowing rivers of the
mordab in late winter and early spring. In 1994-1995, the population
of this species was noted as declining in recent years (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 37-38, 1996).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food and in aquaculture.
Conservation
Weirs are a problem for this species in Iran as they block the
spawning migration, the fish massing below the obstruction, and
causing re-absorption of eggs and sperm (Holčík
and Oláh, 1992). Aquaculture of this species has been investigated in
Iran; it can be bred semi-artificially using methods similar to that
for Rutilus species (Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 38, 1996).
Lelek (1987) classifies this species as intermediate to rare in
northwestern Europe.
Kiabi et al. (1999) consider this species to be near
threatened in the south Caspian Sea basin according to IUCN criteria.
Criteria include commercial fishing, sport fishing, abundant in
numbers, habitat destruction, widespread range (75% of water bodies),
absent in other water bodies in Iran, and absent outside the Caspian
Sea basin. Mostafavi (2007) lists it as near
threatened in the Talar River, Mazandaran.
Further work
The relationships of the Caspian Sea taxon to other Vimba needs
investigation as do certain aspects of its biology in Iranian waters as it is
under some threat.
Sources
Iranian material: CMNFI 1970-0531, 8, 44.4-70.5 mm standard length, Mazandaran, Larim River (36º46'N, 52º56'E);
CMNFI 1970-0543A, 9, 38.9-87.4 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0544, 1, 162.2 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1979-0431, 1, 145.0 mm standard length, Mazandaran, fish bazaar at Now Shahr (no other locality data);
CMNFI 1979-0435, 1, 141.1 mm standard length, Gilan, stream 10 km west of Ramsar (36º57'N, 50º37'E);
CMNFI 1979-0436, 5, 115.1-156.5 mm standard length, Gilan, stream 26 km west of Ramsar (37º02'30"N, 50º27'E);
CMNFI 1979-0437, 1, 155.2 mm standard length, Gilan, Safid River, 2km west of Astaneh (37º16'30"N, 49º56'E);
CMNFI 1979-0438, 2, 139.5-152.6 mm standard length, Gilan, Gholab Ghir River (37º27'N, 49º37'E);
CMNFI 1979-0788, 2, 28.5-50.7 mm standard length, Mazandaran, Gorgan River (37º00'N, 54º07'E);
CMNFI 1980-0120, 7, 47.8-65.5 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1980-0121, 3, 139.5-152.2 mm standard length, Gilan, Shafa River estuary (37º35'N, 49º09'E);
CMNFI 1980-0126, 1, 170.8 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0127, 2, 180.3-182.1 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0129, 1, 51.9 mm standard length, Mazandaran, Tajan River (36º49'N, 53º06'30"E);
CMNFI 1980-0138, 10, 28.1-105.0 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E);
CMNFI 1980-0142, 1, 180.1 mm standard length, Gilan, Nahang Roga River (no other locality data);
CMNFI 1980-0908, 4, 87.8-135.7 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E).
Cobitidae
This family of loaches, sometimes called
sting-loaches, is found in Eurasia and Morocco and has about 26 genera
with about 177 species (Berra, 2001; Nelson, 2006). Berra (2001) does not indicate
the more southern distribution of this genus in Khuzestan and Fars
provinces of Iran. Four species are recorded from Iran.
Anonymous (1988a) places Cobitidae on the Official
List of Family-Group Names in Zoology (rather than the grammatically
correct but unused Cobitididae) and Cobitis taenia is
designated as the type species for the genus Cobitis (see also
Kottelat (1986) for further information).
The body form is fusiform to rounded or elongate;
the mouth is subterminal and has 3-6 pairs of barbels; the mental
lobes of the lower lip have two parts: the anterior which is usually
short and sometimes divided into lobules, and the posterior which is
flap-like and longer and sometimes divided into 2 or more barbel-like
extensions; there is 1 row of pharyngeal teeth; and there is an
erectile spine in a groove below the eye (anterior in a non-Iranian
genus). Iranian species have one pair of rostral barbels and a rounded
or slightly emarginate caudal fin and belong to the subfamily
Cobitinae. Menon (1992) considers that structural details of the bony
covering of the swimbladder and the nature of the scales are only of
use at the generic level. Lip structures, fin positions relative to
one another and secondary sexual characteristics in males are
important characters in differentiating species in India. Economidis
and Nalbant (1996) discuss characters used in the study of these
fishes and consider scales to be characteristic of each species along
with colour pattern, sexual dimorphism, suborbital spine morphology,
barbel and mental lobe morphology, and others. Black spots at the caudal fin
base and four longitudinal pigment zones on the flank (Z1-4 or Gambetta's zones
1-4) are important in distinguishing and describing species. Males have 1-2
laminar projections on the dorsal surface of the anterior pectoral fin rays
known as the laminae circularis or Canestrini's scales. Hybrid lineages are
known, produced by gynogenesis and are nearly all-female. Males of bisexual
lineages are sperm donors but the sperm only induces egg development and
contributes no genetic material. The all-female lineages are therefore sperm
parasites and have to occur in sympatry with one of the parental species in a
hybrid complex. As a result, hybridogenous individuals in the complex are
difficult to distinguish from the bisexual parent species on external
characters. An unbalanced sex ratio in a population with more females than males
is usually evidence that hybridogenous lineages are present (Kottelat and
Freyhof, 2007). Maximum size is about
40 cm but most are much smaller.
The origins of this group of loaches may well lie
at the end of the Eocene or in the early Oligocene in South China,
spreading along a northern route through Europe and Siberia during the
Oligocene-Miocene-Pliocene period and then later southwards into
Southwest Asia (Sawada, 1982; Menon, 1987; 1992; Bănărescu and Nalbant, 1998;
Šlechtová et al., 2008; Tang et al., 2008). A early Oligocene route also existed between the
Anatolian landmass and Central Asia (Tang et al., 2008) and cobitids may
have invaded the Euro-Mediterranean zoogeographic subregion at least five times
independently based on cytochrome b data.
Some members of this family can live in oxygen-poor
waters. They take in air at the surface, and pass it through the
intestine where the mucosa absorbs the oxygen and carbon dioxide waste
is released through the vent. As a consequence, they may be very sensitive to air
pressure changes and become restless when it falls and can be used
to predict the weather. Foods are mostly small insects, worms and
crustaceans detected by the aid of the barbels on the habitat bottom.
Some eat algal films or mats. Most species bury themselves in sand or
mud during the day, emerging to feed at night. Movement is by
undulations of the body, particularly marked in the more elongate
species. A consequence of this form of movement is a reduction in fin
size and variation. Reproduction involves the male chasing the female, entering
vegetation and wrapping around the female as eggs are released and fertilised.
Eggs swell and reach as large as 3.5 mm in diameter and as a result are retained
in the vegetation although they are not adhesive.
A number of species are popular aquarium fishes,
including the coolie or kuhli loaches and the weatherfish. None of the
Iranian fishes are used in this fashion but they can be quite
colourful. Cobitis taenia is a potential fishing bait for
predatory fishes such as Sander lucioperca and has been
examined experimentally for this purpose in Turkey (Kuşat et al., 1995).
Genus Cobitis
Linnaeus, 1758
These fishes are found in Europe, North Africa and
Asia. There are 2 species in Iran. They are known generally as سگ ماهي (sag mahi
meaning dog fish) in Farsi, the equivalent of loach in English. This name is not
repeated under each Species Account. Also called لوچ (= louch in Farsi, from loach).
This genus is characterised by an elongate and
compressed body, a usually bifid, erectile spine below the eye
(sometimes hidden under the skin), 3 pairs of short barbels (4 at the
snout tip and 2 at the mouth corners), minute scales cover the body
(as many as 200 but they are seldom counted accurately), lateral line
faint or indistinct, dorsal and anal fins small, caudal fin rounded or
truncate, and swimbladder in a bony capsule with a free portion
visible. Males have bony extensions of their pectoral fin rays, known as lamina
circularis or scale of Canestrini, and no swellings of their body sides.
Polyploid unisexual, bisexual-unisexual complexes
and gynogenetic forms of Cobitis exist in the basins of the
Baltic, Black, Caspian and Mediterranean seas (Vasil'ev and Vasil'eva,
1996; Kottelat, 1997; Vasil'eva and Vasil'ev, 1998; Vasil'ev et al.,
1999; Bohlen, 2001). The species are morpohologically undifferentiated and therefore
require detailed study to resolve taxonomic and systematic problems.
The composition of Iranian species has not been investigated.
The earliest fossil record is from the middle Miocene about 15MYA with divergence of
Sabanejewia and Cobitis at 12-13MYA (Ludwig et al., 2001).
These loaches often bury themselves in mud to
overwinter or escape predators. The spine under the eye when erected
is an anti-predator device, discouraging swallowing by other fishes
and birds. The fish is said to actively swing the head from side to side to prick predators.
Cobitis linea
(Heckel, 1849)
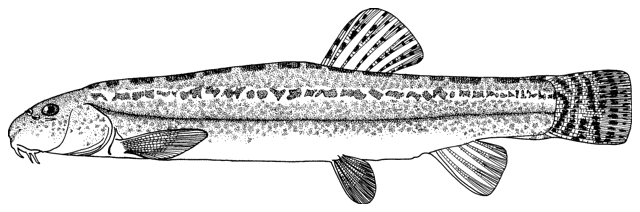
Common names
sagmahi-ye jonubi (= southern dogfish).
[southern spined loach]
Systematics
The type locality of Acanthopsis linea is "Bäche um
Persepolis" according to Heckel (1846-1849b). Persepolis is at 29°57'N,
52°52'E in Fars.
The holotype described by Bănărescu
and Nalbant (1966) is one of 7 specimens assumed to be syntypes. The
catalogue in Vienna lists 1 specimen in one column and 6 in the
adjacent column. The type series is in poor condition being dried with
scales lost and colour mostly faded except for one specimen. Bianco
and Nalbant (1980) cite a specimen as the holotype, a female, 67.0 mm
standard length, housed in the Naturhistorisches Museum Wien (NMW
48560 - this includes all 7 fish) and the type locality as Persepolis,
presumably following Bănărescu
and Nalbant (1966). The dried specimens measure 41.2-62.9 mm standard
length and the undried one 61.3 mm standard length. My measurement is
at variance with these authors.
Placed in the subgenus Bicanestrinia Băcescu,
1962 by Bianco and Nalbant (1980) and originally described in the
genus Acanthopsis Agassiz, 1832 (see Eschmeyer (1990) for
further details on this genus). Bohlen et al. (2006) place this species
in their Bicanestrinia lineage I using the cytochrome b gene.
Bicanestrinia has a derived character state in the duplication of the
Canestrini scale or lamina circularis on the pectoral fin of males, the
primitive condition being a single lamina circularis as in, e.g. Cobitis
s.s. The separation between Bicanestrinia and Cobitis
s.s. occurred 12-17 MYA when the land connection between Central
Europe and Anatolia broke.
Material identified as Cobitis turcica (Hanko, 1924)
in Nalbant and Bianco (1998) is C. linea based on my examination of IZA 7829-30.
Fricke et al. (2007) have it in the Tor (sic, meaning Kor) River
system of Iran, the same error.
Key characters
This species is distinguished by the dark brown lateral spots being
reduced or absent, males have 2 Canestrini's scales at the upper bases
of the unbranched and first branched pectoral fin rays, and the
laterocaudal branch of the suborbital spine is reduced or absent (although the
spine itself is detectable by touch in even the smallest fish).
Morphology
Dorsal fin unbranched rays 2 and branched rays 6-7, anal fin
unbranched rays 2-3 and branched rays 5, pectoral fin branched rays
7-9, and pelvic fin branched rays 5-6.
Meristic values for specimens examined by me are:- dorsal fin branched rays 6(16), anal fin branched rays 5(16), pectoral fin
branched rays 8(6), pelvic fin branched rays 6(6), and
total vertebrae 41(1).
Scales are embedded and have a reduced and eccentric focus. Johal et al.
(2006) and Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales. The
lateral line does not pass the end of the pectoral fin. The swimbladder capsule is globular but has ventral indentations
anterolaterally on both sides. There are small keels on the upper and
lower caudal peduncle. The anterior nostril is a short tube. The upper
lip has fine furrows, the lower lip is thick and folded with a pair of medial lobes.
Sexual dimorphism
Males have Canestrini's scales as detailed above.
Colour
Overall colour is a yellowish-white with a golden iridescence
especially on the operculum. Spots, blotches and dots are blue-grey or
brown. The flanks are variably spotted and striped, the variability
not related to sexual dimorphism. In some fish there is an upper flank,
uniform stripe closely following the line of the back, below it a
longitudinal series of irregular spots and blotches, a third stripe
incompletely developed, and a fourth mid-lateral stripe of spots and
blotches. In others the third and fourth stripes are an
indistinguishable row of speckles. The mid-line of the back has a row
of rounded spots numbering 7-11 (usually 8) predorsally, 0-3 (usually
2) at the dorsal fin base, and 5-11 (usually 8) postdorsally. The upper
head is covered with many minute dots, extending onto the snout and upper head
sides. The iris is golden-yellow.
There is a blackish band from the eye to the mouth corner. There is a
minute black spot on the caudal fin base. Fins are translucent. There are
numerous dots scattered on the rays and membranes of the dorsal and caudal fins
forming up to 9 irregular rows on the former and 8 more regular rows or bars on
the latter, but still not very clearly defined. Spots on the dorsal edge of the
caudal fin are clearly defined and regular; there are no spots on the ventral
edge. The pelvic and anal fins are immaculate while the pectoral fin has some
minor and irregular spots.
Size
Reaches 89.6 mm standard length.
Distribution
This species is found in the Kor River basin and the upper Kul River drainage
of the Hormuz basin (Bănărescu and Nalbant, 1966; Bianco and Nalbant, 1980).
Zoogeography
This species may be related to Cobitis simplicispina Hankó, 1925 from
Anatolia and other nominal species, all placed in the subgenus Bicanestrinia
characterised by two Canestrini's scales. Members of this complex of
species possibly reached western Asia from eastern Asia in the early
Miocene. The disjunct distributions seen today were probably produced
by Pleistocene climatic changes (Bianco and Nalbant, 1980).
Habitat
This species favours muddy bottoms. Specimens were caught in about
5-6 cm of mud, or at the foot of a muddy bank, with aquatic vegetation
in the form of reeds.
Age and growth
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 108 fish measuring 3.35-8.96 cm standard length. The a-value was
0.0089 and the b-value 3.060 (a b-value < 3 indicating a fish that
becomes less rotund as length increases and a b-value >3 indicating a
fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Adult females described by Bianco and Nalbant (1980) measuring
80.0-88.6 mm standard length have ripe eggs 0.8-1.1 mm in diameter. A
specimen from the Lapui spring caught on 30 June carries eggs of this size.
Parasites and predators
Unknown.
Economic importance
This species is too small and rare to be of direct economic importance.
Conservation
Since this species is known only from a limited number of specimens, further
studies should be undertaken to ascertain its abundance and
distribution. It seems to favour muddy habitats and may have a
restricted distribution in the rocky streams of Fars on this account.
Further work
See above.
Sources
Type material: See above (NMW 48560).
Iranian material: CMNFI
1979-0292, 1, 69.8 mm standard length, Fars, Lapui Spring in the Kor River
basin (29º48'N, 52º39'E); IZA 7829, 4, 41.9-85.5 mm standard length, Fars,
Pulvar River 15 km north of Persepolis (29º59'N, 52º59'E); IZA 7830, 1, 27.1 mm
standard length, Fars, springs of Kul River near Darab (no other locality data).
Cobitis taenia
Linnaeus, 1758
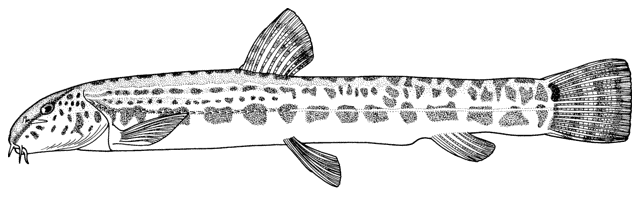
Common names
mahi roshtegar or roftegar (= dustman, cleaner or sweeper fish,
presumably from the bottom-dwelling habit); loch or louch in Khuzestan
(presumably from the English name, but also louch means a person with
a squint); gel khorak in Khuzestan; sagmahi-ye sangi (= stone loach), sagmahi-ye juibari or
sagmahi-e-jooibari, mar mahi (= snake fish).
[lakh mukhattat in Arabic; zagafgaziya iliskani in Azerbaijan;
shchipovka or shchipovka zakavkazskaya or Transcaucasian spiny loach
in Russian; spined or spiny loach, stone loach, spiny stone loach, weatherfish, spotted
weatherfish, Siberian loach].
Systematics
Cobitis Taenia was originally described from Lake Mälaren, Sweden (Kottelat, 1997).
Cobitis taenia satunini Gladkov, 1935 may be the subspecies
of the southern Caspian Sea basin distinguished by a truncate snout
rather than gradually tapering to the tip as in European spined
loaches as well as other characters listed by Gladkov (1935) including
head depth comprising 63.5% of head length on average and 12.27% of
standard length, and caudal peduncle shorter than the head (on average
16.27% of standard (?) length). However, it was described from western
Transcaucasia, outside the Caspian Sea basin, and material from the
Caspian coast of Iran has not been examined and compared with it. Berg
(1948-1949) referred specimens from the upper Kura River basin to this
subspecies but lacked material from the lower Kura, Lenkoran and the
southern Caspian Sea coast.
Kottelat and Freyhof (2007) consider that the taxon in the southern Caspian
Sea basin is not taenia, a northern European species, and this is
followed by Mousavi Sabet et al. (2011) who refer to it as Cobitis
sp.
The holotype of Cobitis taenia satunini described from
"Kavkaz" and "nizov'ya r. Kintrish" (= lower
Kintrish stream in the Caucasus; the Kintrish stream mouth is at 41°48'N, 41°46'E)
is in the Zoological Museum of Moscow State University under MMSU
P.2852 with a cotype (= paratype) under MMSU P.2317 (Eschmeyer et
al. (1996) has MMSU P.2251, quoting Svetovidova (1978), which
disagrees with Gladkov (1935)). The Zoological Museum of Moscow University (ZMMU; their acronym) has P-2852 as the
holotype plus P-2313 as 2 paratypes (Pavlinov and Borissenko, 2001).
Key characters
Distinguished from other Cobitis and related taxa in northern Iran by having
modally 14 branched caudal fin rays, large dark and obvious spots
along the mid-flank, and above them speckles which tend to form a
stripe and above these a row of spots (absent in S. aurata).
Morphology
Dorsal fin unbranched rays 2-3, branched rays 6-8, anal fin
unbranched rays 2-3, branched rays 4-7, usually 5, pectoral fin
branched rays 5-9, and pelvic fin branched rays 5-7. Vertebrae 40-46,
in the south Caspian Sea 40. Some specimens are elongate while others
are deeper bodied. Chromosome number is 2n=48-50 (Klinkhardt et al.,
1995) but triploids and tetraploids are also known.
Meristics for Iranian specimens:- dorsal fin branched rays 6(29) or
7(51); anal fin branched rays 4(1), 5(78) or 7(1); pectoral fin
branched rays 6(4), 7(35), 8(38) or 9(3); pelvic fin branched rays
5(6), 6(70) or 7(4); caudal fin branched rays 10(1, but deformed),
12(2), 13(7), 14(68), 15(1) or 16(1); total vertebrae ?.
Sexual dimorphism
The second ray of the male pectoral fin is thickened and there is
an enlarged scale at the base (Canestrini scale). The body in front of
the dorsal fin of males is not distended as in S. aurata.
Colour
The back is light brown. The flanks are pale yellow with a series
of 10-20, usually 16-18, dark brown blotches or spots and the back bears a more or
less distinct series of 12-21 dark spots. A small stripe of brown
spots on the upper flank lies between the 2 series of dark spots. The
spaces between these stripes are finely speckled with brown. The belly
and lower head are yellowish white. The head is mottled with brown
dots and there is usually a band from the eye to the snout tip. The
head can have lime-green iridescent tints. Females have yellow-orange
dorsal and caudal fins bearing dark spots while in males these fins
are almost orange with dark spots. There is a distinctive dark spot or
two at the upper base of the caudal fin, often of a crescent shape,
although this spot may be absent occasionally. Dorsal and caudal fins have 3-4
rows of brown spots. The pectoral, pelvic
and anal fins are whitish without dark pigment. The iris is silvery,
slightly golden or orange.
Size
Reaches 15.0 cm.
Distribution
Found from England and the Iberian Peninsula across Eurasia to
Japan. However, Kottelat and Freyhof (2007) give a more restricted distribution
and exclude the Caspian Sea basin of Iran in their map. In the Caspian Sea basin of Iran, it is found from Astara to Gorgan
Bay including the Anzali Mordab, the Aras, Safid, Pol-e Rud, Sardab, Takar,
Nessa, Haraz,
Babol. Chowbar, Qasemabad, Qa'emshahr, Tonekabon, Shirud, Tajan and Gharasu rivers, and from the upper Karkheh,
the middle Dez, Kashkan, Qareh Chai, Simarreh and lower Gav Masiab rivers in the Tigris basin
(Saadati, 1977; Roshan Tabari, 1997; Abbasi et al., 1999; Kiabi et al., 1999; Abdoli, 2000;
Abdoli and Naderi, 2009).
An anecdotal report from the Qareh Chai near Hamadan in the Namak Lake basin
needs specimens for confirmation.
Zoogeography
Records from the Tigris River basin of Iran are based on literature
(Saadati, 1977) and a single specimen (CMNFI 1979-0285). Mid-flank spots are less distinct than in C. taenia
from the Caspian Sea basin of Iran of similar size being smaller and
more numerous and the stripe on the centre of the back is continuous
rather than spots as in Caspian fish. Identification is tentative.
Cobitis taenia survived glaciations in at least three refuges in the
Ponto-Caspian area, one remaining near its Black Sea refuge. C. taenia
lineages may have a late Pleistocene origin (Culling et al., 2006).
Habitat
This species remains buried in sand, mud which is not too thick, or
dense weed growths during the day, being active at night, and is
mostly solitary. Swimming is by undulating motions over short
distances. When concealed, the body is bent into an arch so only the
head and tail protrude. It prefers cool, clear running waters.
Along the Caspian shore it is found in the lower reaches of rivers (Jolodar and
Abdoli, 2004).
Age and growth
This loach lives up to 5 years and is mature in its second year of life.
Females dominated the population in the Safid River and growth was positively
allometric (Patimar et al., 2010).
Mousavi Sabet et al. (2011) examined fish from the Babol River and found
the percentage of females was significantly higher than males, and mature
females were longer than 45 mm total length and 2+ years old and mature males
were longer than 35 mm and 1+ years old. Life span was 5+ years for females and
3+ years for males.
Food
Diet is small crustaceans such as ostracods, copepods and rotifers
in the bottom mud or sand. A mouthful of mud or sand is taken in,
chewed, food items extracted, and the residue expelled convulsively
through the gill openings.
Reproduction
Fecundity reaches 5072 eggs, perhaps as high as 10,000 (Palicka,
1996), and egg diameter reaches 1.58 mm. Spawning takes place from
April to June in slow to still water. Eggs are laid on sand, stones
and vegetation in several batches. Eggs may be deposited on the roots
of water plants cleared of debris by males rooting among them. Males
use their enlarged pectoral fins to turn the female during spawning.
The population in the Safid River had egg diameters up to 1.02 mm, absolute
fecundity reached 8111 eggs and relative fecundity up to 383 eggs/g (Patimar
et al., 2010). Mousavi Sabet et al. (2011) found Babol River
fish spawning from the beginning of May to late July at water temperatures of
19.1-24.6ºC. Average absolute and relative fecundities were2172 and 590 eggs
with ranges of 734-3562 and 347 to 945 eggs, respectively. Egg diameters were up
to 1.4 mm with an average of 0.58 mm.
Parasites and predators
This species is infected with Clinostomum complanatum, a
parasite that can cause laryngo-pharyngitis in humans, in the Shirud
of western Mazandaran Province (Shamsi et al., 1997).
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks and in aquaria.
Palicka (1996) gives a short account of its aquarium care.
Conservation
Lelek (1987) classifies this species as rare in Europe. It was
commonly caught in Iranian streams along the Caspian coast. Kiabi et
al. (1999) consider this species to be of least concern in the
south Caspian Sea basin according to IUCN criteria. Criteria include
abundant in numbers, habitat destruction, widespread range (75% of
water bodies), present in other water bodies in Iran, and present
outside the Caspian Sea basin.
Further work
The biology of this species in Iranian waters has not been studied.
Sources
Iranian material:
Cobitis turcica
(Hanko, 1924)
Nalbant and Bianco (1998) record this species from the "River
Kor near Persepolis" but I identify this material as C. linea (q.v.).
The species was described originally from Eregli in Anatolian Turkey and its presence in Iran needs
confirmation. Erkakan et al. (1999) review the Turkish species of Cobitis
but do not mention the occurrence of C. turcica in Iran.
Misgurnus
Lacepède, 1803
Misgurnus anguillicaudatus
(Cantor, 1842)
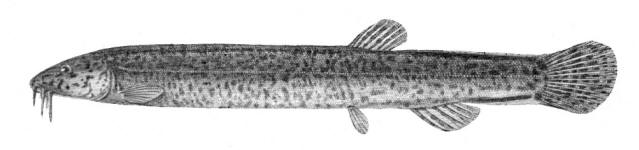
Recorded from the Karakum Canal and Kopetdag Reservoir of
Turkmenistan by Shakirova and Sukhanova (1994) and Sal'nikov (1995),
this exotic species from China may eventually reach the Tedzhen River
and Caspian Sea basins of Iran. No Iranian record.
Sabanejewia
Vladykov, 1929
Sabanejewia is distinguished from Cobitis
Linnaeus, 1758 by having 12 rather than 14 branched caudal fin rays, a
stronger suborbital spine, developed mental lobes which may be
unfringed or well fringed, large imbricated or unimbricated scales
with a relatively large and central focus, and males have a
protuberance on each side of the body in front of the dorsal and
pelvic fins and lack the lamina circularis (a bony process at
the base of the second pectoral fin ray)(see Vladykov (1929), Nalbant
(1963, 1994), Sawada (1982), Vasil'yeva (1995b) and Perdices and
Doadrio (1997, 2001) for further details). Krupp (1985c) does not consider Sabanejewia
to be a distinct genus. Perdices et al. ((2003) using mtDNA demonstrate
that Caucasian-Caspian lineages are the sister group of a Danubian-Balkan lineage.
Tang et al. (2008) using the cytochrome b gene found that
ancestral Sabanejewia might have been the first cobitids to cross Siberia
and invade the Euro-Mediterranean zoogeographic subregion.
Sabanejewia aurata
(De Filippi, 1863)
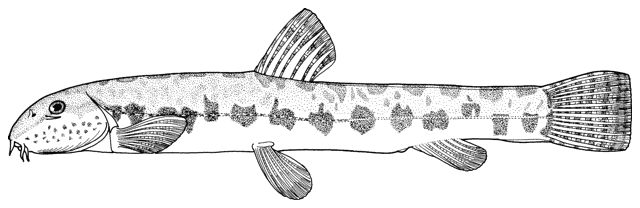
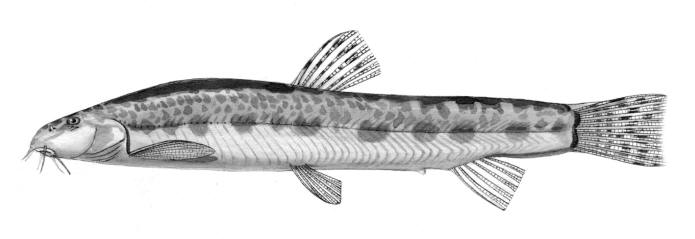
Further illustrations
Sabaneajewia
aurata; Sabaneajewia aurata; both after Berg (1948-1949)
Common names
sagmahi-ye khardar (= spiny loach), sagmahi-e-jooibari.
[gizili iliskan in Azerbaijan; opi-dzug in Armenia;
peredneaziatskaya shchipovka or Hither Asia spined loach, zolotistaya
shchipovka or golden spined (spiny) loach, both in Russian].
Systematics
Kottelat (1997) tentatively considers that this species is restricted
to Iran and possibly adjacent waters and does not occur in Europe.
The lectotype of Cobitis aurata (MZUT N.674), designated by
Tortonese (1961), is stored in the Istituto e Museo di Zoologia della
R. Università di Torino and 2 paralectotypes (MSNG N.365) from the
Collezione di Universitá di Genova are in the Museo Civico di Storia
Naturale di Genova (Tortonese, 1940; Tortonese, 1961). Eschmeyer et
al. (1996) give the paralectotypes' catalogue number as ?MSNG
42727 (ex Univ. Genoa 365).
The type locality is "un fiumicello presso Sartschem" (De
Filippi, 1863; not 1865 as in Berg (1948-1949), Banarescu and Nalbant
(1964) and Reshetnikov et al. (1997) and not 1862 as in
Tortonese (1940) and Eschmeyer et al. (1996)). The type
locality is possibly Sarcham-e Sofla (37°07'N, 47°54'E)
in the Qezel Owzan River drainage of the Caspian Sea basin in Iran.
Cobitis Hohenackeri Brandt in Kessler, 1877 from the Kura
River basin of Transcaucasia (Azerbaijan) is a synonym with 6 syntypes
in the Zoological Institute, St. Petersburg (ZISP).
Key characters
Distinguished from other cobitids in northern Iran by having
modally 12 branched caudal fin rays, large dark spots along the flank,
and above them speckles which do not tend to form a stripe.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 5-8, predominately 6
in the Caspian basin, anal fin unbranched rays 2-3, branched rays 4-8,
usually 5, pectoral fin branched rays 5-9, predominately 7 in the
Caspian basin (but see below), pelvic fin branched rays
4-8, usually 5 (but see below), and vertebrae 39-43.
Meristics for Iranian specimens:- ? check dorsal rays on x-rays, dorsal
fin branched rays 6(11) or 7(4); anal fin branched rays 5(15);
pectoral fin branched rays 7(1) or 8(14); pelvic fin branched rays
5(1) or 6(14); caudal fin branched rays 11(2) or 12(13); and total
vertebrae ?.
Scales minute but visible to the naked eye, ca. 170-200. Dermal
crest or adipose fins are variably developed behind the dorsal and
anal fins. Barbels are longer than in C. taenia, the mouth
corner barbels reaching back to the posterior eye margin. Karyotype is
2n=50 (Klinkhardt et al., 1995).
Sexual dimorphism
The second pectoral fin ray in males is not enlarged as in C.
taenia but there is a lateral distension of the body in front of
the dorsal fin in mature males.
Colour
The back is a brownish olive-green with darker marbling. The flanks
are golden-brown with a row of dark brown spots, less conspicuous than
those in C. taenia. These number 9-18, modally 11-13, in the
Caspian basin. The upper row of spots is absent in contrast to C.
taenia. The number of spots may be related to habitat, those fish
from calm waters having few large spots. The back has several dark
blotches along its mid-line. In the Caspian basin there are 7-16 back
blotches, modally 9-10. There is a bar at the caudal base, sometimes
with a small central gap. Fins are slightly pink.
Size
Attains 13.8 cm, but most fish are less than 10.0 cm.
Distribution
Found in the basins of the Baltic, Aegean, Black and Caspian seas
and in the Tedzhen and Murgab rivers of Afghanistan and Turkmenistan
according to most authors (Aliev et al., 1988; Nalbant and
Bianco, 1998). A distinct subspecies is found in the Aral Sea basin
(see below). Kottelat (1997) however considers that this species is
restricted to Iran and possibly adjacent waters. It is found along the Caspian
Sea coast of Iran including the Anzali Mordab and its tributaries, the Safid River
at Kisom, lower Tonekabon, Chalus, Haraz and Babol rivers, Nakhurde (= ? Noqreh
Deh), and in the Kashaf
River of the Tedzhen River basin (Holčik and Oláh, 1992; Abbasi et al., 1999; Abdoli, 2000;
Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
The record of this species in the Tigris-Euphrates basin at Basrah,
Iraq (BM(NH) 1920.3.5:9) in Nalbant (1963) and Bănărescu
and Nalbant (1966), and repeated in Banister (1980), is probably an
error of labeling (Bănărescu, 1973).
Zoogeography
The subspecies, Cobitis aurata aralensis Kessler, 1877, is
reported from the Karakum Canal and the Uzboi lakes in Turkmenistan by
Shakirova and Sukhanova (1994) and Sal'nikov (1995) and may well enter
both the Tedzhen River and Caspian Sea basins of Iran eventually.
Habitat
This is a nocturnal species, hiding during the day under gravel and
boulders of flowing rivers. If exposed, it will make jerking motions
to the nearest cover. Some authors state that it also hides in sand.
It prefers shallow and clear water. It occurs with C. taenia
but is commoner in faster water in the upper and middle reaches of
rivers, from 5 to 150 cm water depth. However it may form populations
in still water left behind after floods.
Age and growth
Males tend to be slightly smaller than females.
Food
Bottom-dwelling invertebrates are the main food items including
larval insects such as mayflies, dragonflies and caddisflies as well
as nematodes, copepods, chironomids, fish eggs, algae and detritus.
Reproduction
Fecundity reaches 14,700 eggs and egg diameter 0.85 mm. Eggs are
shed over plants from April to August and this species may spawn in batches.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Lelek (1987) lists this species as rare to vulnerable in Europe.
Kiabi et al. (1999) consider this species to be conservation
dependent in the south Caspian Sea basin according to IUCN criteria.
Criteria include few in numbers, habitat destruction, limited range
(less than 25% of water bodies), absent in other water bodies in Iran,
and present outside the Caspian Sea basin. The 2000 IUCN Red List
lists this species as DD (Data Deficient).
Further work
The biology of this species in Iran has not been examined and its systematics
needs clarification.
Sources
Vasil'eva and Vasil'ev (1988) give details on variation in
meristics and colour of this species.
Iranian material: CMNFI 1970-0508, 4, 39.2-57.1 mm standard length;
Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0545, 1, 33.9
mm standard length, Gilan, Safid River (ca. 37º01'N, ca. 49º38'E), CMNFI
1979-0448, 1, 70.1 mm standard length, Azarbayjan-e Khavari, Ahar Chay
(38º18'30"N, 48º22'E); CMNFI 1980-0131, 1, 57.4 mm standard length, Iran,
Caspian Sea basin (no other locality data); CMNFI 1980-0132, 1, 49.8 mm standard
length, Gilan, Safid River at Kisom (37º12'N, 49º54'E); CMNFI 1980-0155, 7,
38.5-63.5 mm standard length, Azarbayjan-e Khavari, Qareh Su at Ardabil (ca.
38º15'N, ca. 48º18'E).
Sabanejewia caspia
(Eichwald, 1838)
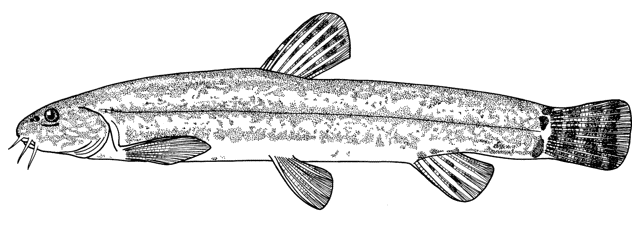
Further illustrations
Sabanejewia caspia; Sabanejewia caspia and
suborbital spine; Sabanejewia caspia; all after Berg
(1948-1949)
Common names
mahi roshtegar talaee (= golden dustman fish), rofteghar mahi, sagmahi-ye Khazari (= Caspian loach),
sag mahi khardar (= spiny loach), mar mahi (= snake fish), sagmahi-e-jooibari.
[xazar iliskani in Azerbaijan; Kaspiiskaya shchipovka or Caspian
spined (spiny) loach in Russian].
Systematics
This species was described from "in sinu mardofiensi prope
castellum Lencoranicum" (i.e. the Caspian Sea in the ?
Mardofiensi Gulf near the Lenkoran fortress). The type specimen is
apparently lost (Vasil'yeva, 1995b).
Key characters
Distinguished from other cobitids in northern Iran by having
modally 12 branched caudal fin rays, no large dark spots along the
flank but a stripe, and above this stripe speckles which do not tend
to form a stripe.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 6-7, anal fin unbranched rays 1-2, branched rays 5-6, pectoral fin branched rays 6-7
(but see below) and pelvic fin branched rays 4-6. Vertebrae 41-42. A crest is well
developed on the lower caudal peduncle but only posteriorly on the
upper edge. Barbels are shorter than in C. taenia, the
posterior ones reaching beyond the posterior eye margin. Karyotype is
2n=50 (Klinkhardt et al., 1995).
Meristics for Iranian specimens:- dorsal fin branched rays 6(83) or
7(1); anal fin branched rays 5(80) or 6(4); pectoral fin branched rays
7(6), 8(72) or 9(6); pelvic fin branched rays 4(3), 5(67) or 6(14);
caudal fin branched rays 7(1, but deformed), 9(1), 10(3), 11(4),
12(74) or 13(1); total vertebrae ?.
Sexual dimorphism
The second pectoral fin ray in males is not enlarged as in C.
taenia but there is a lateral distension of the body in front of
the dorsal fin an mature males.
Colour
Live specimens, especially young, are almost transparent. The adult
has a pronounced dark line along mid-flank indicating the separation
of the upper and lower muscle masses. Above this line the upper flank
is yellowish with irregular dark grey pigment or brown speckles near
the back. The lower flank has irregular grey pigment or brown
speckles. The mid-line of the back has a more or less pronounced dark
line. The belly and lower head surface are pale yellowish without grey
pigment. The dorsal and caudal fins have yellow-orange rays bearing
3-4 series of dark grey spots. The base of the caudal fin has 2 dark
dots although these are not as marked as in Cobitis taenia. The
pectoral, pelvic and anal fins are transparent though larger fish may
have elongate grey spots along the rays. The iris is golden.
Note that Vasil'yeva (1995b), based on original data and that of
Derzhavin (1934), found that the dark longitudinal band and the dark
caudal fin base may not be pronounced.
Size
Reaches 9.2 cm standard length.
Distribution
Principally found in the southern Caspian Sea basin. Records from the
northern Caspian Sea are apparently in error and the range is from the
Kura to the Babol rivers including the Anzali Mordab at "Khalkai" for example,
the Safid, Chowbar, Tonekabon, Chalus, Shahzadeh, "Laidschana", Meshedessera (=
? Babol Sar), Haraz, (Holčik and Oláh, 1992;
Vasil'yeva, 1995b; Abbasi et al., 1999; Abdoli,
2000; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
Zoogeography
This species is endemic to the Caspian Sea basin (Nalbant and Bianco, 1998).
Habitat
This species is found in both fresh and brackish waters, in slow or
still water with aquatic vegetation, in the lower reaches of rivers
and near river mouths, and in brackish bays.
Age and growth
Unnown.
Food
Unknown.
Reproduction
Spawning takes place in April in the brackish lagoons of the
Lenkoran, Azerbaijan. Up to 955 eggs of up to 0.85 mm diameter are
carried by each female.
Parasites and predators
None reported for Iran.
Economic importance
None.
Conservation
Holčík and Oláh (1992) report the loss of this species from the Anzali
Mordab where it was once found. Reasons for this loss are unknown.
Kiabi et al. (1999) consider this species to be conservation
dependent in the south Caspian Sea basin according to IUCN criteria.
Criteria include possibly few in numbers, habitat destruction, limited
range (less than 25% of water bodies), absent in other water bodies in
Iran, and absent outside the Caspian Sea basin. Vulnerable in Turkey (Fricke
et al., 2007).
Further work
The biology of this species in Iran requires study.
Sources
Iranian material: ?
Sabanejewia caucasica
(Berg, 1906)
? to write up
Reported from the Anzali Mordab and lower reaches of the Safid, Toenkabon,
Chalus, Heraz and Babol rivers in Iran (Abdoli, 2000) and mapped from the
Caspian coast of Iran at Babol by Kottelat and Freyhof (2007). Berg (1948-1949) notes that this species
is found in the northern Transcaucasus and is not reported from the
Kura River in the southwestern corner of the Caspian Sea. Its presence
in Iran needs confirmation by specimens. Formerly in the genus Cobitis.
Nemacheilidae
Nemacheilidae, paracobitis, barbatula, schistura, triplophysa genus descriptions
? and search barbatula etc and change as needed to new genusMaterials to ID and add to Sources
Nemacheilid spp.
CMNFI 1970-0521, 1, ?, mm standard length, Gilan, Safid River near Lulaman (no other locality data) ?bergiana;
CMNFI 1970-0545, 2, 34.7-35.6 mm standard length, Gilan, Safid River from Emamzadeh Hashem to Lulaman (ca. 37º01'N, ca. 49º38'E) ?bergiana;
CMNFI 1970-0540, 1, 49.5 mm stanmdard length, ?;
CMNFI 1970-0558, 4, ?mm standard length, Ghasemlou Chay ()?brandtii;
CMNFI 1970-0559, 3, ?, mm standard length, Azarbaijan-e Bakhtari, Barunduz Chay (ca. 37º25'N, ca. 45º10'E);
CMNFI 1970-0560, 3, ?, mm standard length, Azarbaijan-e Bakhtari, Mamiyand Chay near Mamiyand (ca. 36º59'N, ca. 45º39'E); CMNFI 1971-0341, 13, 28.6-44.3 mm standard length, ? ;
CMNFI 1979-0026, 8, 22.8-46.6 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0027, 9, 31.0-41.3 mm standard length, Fars, Chehel Chashmeh (ca. 29º43'N, ca. 52º04'E);
CMNFI 1979-0028, 5, 48.3-55.6 mm standard length, Fars, ?;
CMNFI 1979-0059, 28, 32.8-57.0 mm standard length, Fars, Pulvar River 8 km south of Sivand (30º01'30"N, 52º57'E)?distinct taxonand se other fars fish;
CMNFI 1979-0061, 3, 50.2-63.7 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0067, 1, 55.8 mm standard length, Fars, qanat at Zarqan (ca. 29º46'N, ca. 52º43'E);
CMNFI 1979-0070, 2, 38.8-39.9 mm standard length, Fars, Pulvar River at Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0073, 10, 31.1-56.1 mm standard length, Fars, Mand River (ca. 29º42'30"N, ca. 52º01'30"E);
CMNFI 1979-0111, 5, 43.2-52.7 mm standard length, Fars, stream on Shiraz to Bushehr road (29º37'30"N, 52º21'E);
CMNFI 1979-0117, 2, 42.1-50.6 mm standard length, Fars, Pulvar River on road to Naqsh-e Rostam (29º59'N, 52º54'E);
CMNFI 1979-0155, 2, 30.7-30.9 mm standard length, Fars, spring at Gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0157, 3, 34.8-37.7 mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E);
CMNFI 1979-0167, 41, 20.6-48.3 mm standard length, Kerman, qanat at Bam (29º06'N, 58º20'E);
CMNFI 1979-0168, 1, 47.2 mm standard length, Kerman, qanat at Shahabad (29º07'N, 58º16'E);
CMNFI 1979-0169, 11, ? mm standard length, Kerman, qanat 10 km from Mahan (30º08'30"N, 57º17'E);
CMNFI 1979-0170, 1, 45.0 mm standard length, Kerman, qanat at Baghin (30º12'N, 56º48'E);
CMNFI 1979-0172, 18, 33.8-45.8 mm standard length, Kerman, qanat on Kerman to Bandar Abbas road (29º51'N, 56º14'E);
CMNFI 1979-0184, 2, 27.7-28.4 mm satndard length, ?;
CMNFI 1979-0186, 2, ? mm standard length, Hormozgan, stream at Sar Khun (ca. 27º24'30"N, ca. 56º25'E);
CMNFI 1979-0192, 7, ? mm standard length, Fars, qanat 2 km east of Rostaq (28º26'30"N, 55º04'E);
CMNFI 1979-0193, 1, ? mm standard length, Fars, river 8 km from Darab (28º45'N, 54º27'30"E);
CMNFI 1979-0194, 2, 37.6-45.6 mm standard length, Fars, jube 15 km from Darab (28º45'30"N, 54º24'E);
CMNFI 1979-0206, 3, 24.9-36.1 mm standard length, Fars, qanat 1 km from Runiz-e Pa'in (29º12'N, 53º40'E);
CMNFI 1979-0208, 15, 31.1-58.8 mm standard length, Fars, qanat on road to Qatru (ca. 29º11'N, ca. 54º40'E);
CMNFI 1979-0213, 7, ? mm standard length, Kerman, stream in Kharan River drainage (29º15'N, 56º25'E);
CMNFI 1979-0219, 13, ? mm standard length, Kerman, jube 14 km west of Jiroft (28º37'N, 57º41'E);
CMNFI 1979-0253, 7, 31.2-49.5 mm standard length, Markazi, Qareh Chay west of Baqerabad (34º52'N, 50º49'E);
CMNFI 1979-0276, 14, 36.3-45.8 mm standard length, Lorestan, Chamesk River (ca. 33º19'N, ca. 47º53'30"E);
CMNFI 1979-0284, 3, 38.7-41.9 mm standard length, Kermanshahan, Marek River at Mahidasht (34º16'N, 46º48'30"E);
CMNFI 1979-0288, 1, 32.4 mm standard length, Ilam and Poshtkuh, Gangir River at Juy-e Zar Eivan (33º50'N, 46º18'E);
CMNFI 1979-0292, 8, 29.0-45.6 mm standard length, Fars, Lapu'i spring (29º48'N, 52º39'E) ?distinct taxon;
CMNFI 1979-0306, 11, 20.0-49.3 mm standard length, Kerman, qanat 33 km from Sirjan (29º13'N, 54º33'E);
CMNFI 1979-0307, 4, 31.0-42.3 mm standard length, Kerman, river at Sartal (ca. 29º17'N, ca. 56º38'E)? distinct species;
CMNFI 1979-0316, 1, ? mm standard length, Baluchestan, stream on road to Chah Bahar (26º48'N, 61º02'E);
CMNFI 1979-0341, 10, 25.5-45.6 mm standard length, Kerman, Tahrud west of Bam (29º23'N, 57º52'E);
CMNFI 1979-0365, 7, 32.0-40.5 mm standard length, Khuzestan, stream in Doveyrich drainage (32º25"N, 47º36'30"E);
CMNFI 1979-0366, 1, 36.0 mm standard length, Khuzestan, stream 17 km west of Dehloran (32º45'30"N, 47º05'30"E);
CMNFI 1979-0367, 1, 41.3 mm standard length, Khuzestan, Meymeh River 11 km north of Dehloran (32º44'30"N, 47º09'30"E);
CMNFI 1979-0371, 2, 44.5-55.6 mm standard length, Khuzestan, stream in Karkheh River drainage (32º05'N, 48º19'E);
CMNFI 1979-0374, 2, 41.3-41.7 mm standard length, Khuzestan, stream tributary to Bala River (32º40'N, 48º15'E);
CMNFI 1979-0389, 1, 32.4 mm standard length, Khuzestan, Zard River 1 km south of Bagh-e Malek (31º31'N, 49º53'30"E);
CMNFI 1979-0390B, 3, 29.1-39.4 mm standard length, Khuzestan, stream 3km south of Bagh-e Malek (31º29'N, 49º54'30"E);
CMNFI 1979-0395, 1, 30.7 mm standard length, Khuzestan, stream in Marun River drainage (ca. 30º57'N, ca. 49º51'E);
CMNFI 1979-0399, 1, 26.2 mm standard length, Fars, stream near Basht (30º19'30"N, 51º15'E);
CMNFI 1979-0411, 3, 21.9-25.9 mm standard length, Hormozgan, Minab River near Rudan (27º24'N, 57º12'E)?cf bampurensis;
CMNFI 1979-0419, 19, 32.4-58.7 mm standard length, Fars, stream 7 km from Rostaq (28º29'N, 55º01'E);
CMNFI 1979-0423, 4, 43.2-45.7 mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh-Fars border, river in Beshar River drainage (30º31'N, 51º31'E);
CMNFI 1979-0447, 2, 51.2-59.8 mm standard length, Gilan, stream 7 km east of Namin (38º23'N, 48º28'E);
CMNFI 1979-0448, 9, 31.1-62.0 mm standard length, Azarbayjan-e Khavari, Ahar Chay 8 km from Ardebil (38º18'30N, 48º22'E);
CMNFI 1979-0450, 3, 38.7-40.9 mm standard length, Azarbayjan-e Khavari, stream near Kivi (ca. 37º37'N, ca. 48º17'E);
CMNFI 1979-0452, 2, 42.1-42.8 mm standard length, Azarbayjan-e Khavari, Qezel Owzan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0453, 1, 34.2 mm standard length, Zanjan, Zanjan River (37º06'N, 47º56'E);
CMNFI 1979-0458, 1, 48.2 mm standard length, Markazi, Khar River 6 km north of Ab-Garm (35º47'N, 49º20'E);
CMNFI 1979-0459, 4, ? mm standard length, Hamadan, stream 2 km south of Kazan (35º22'N, 49º02'E);
CMNFI 1979-0462, 4, 32.0-45.6 mm standard length, Markazi, Mazdaqan River (35º06'30"N, 49º40'30"E);
CMNFI 1979-0463, 2, 41.0-51.6 mm standard length, Markazi, Qareh Chay (34º53'N, 50º26'E);
CMNFI 1979-0465, 1, 37.1 mm standard length, Markazi, Qom River at Neizar (34º18'30"N, 50º32'E);
CMNFI 1991-0155, 1, 41.7 mm standard length, Hamadan, Gav Masiab River (34º12'N, 48º20'E);
CMNFI 1991-0156, 1, ? mm standard length, Hamadan, Gav Masiab River (34º16'N, 48º10'E);
CMNFI 1993-0125, 1, 47.8 mm standard length, Kermanshahan, Sarab-e Nilufar (34º24'N, 46º52'E);
CMNFI 1993-0128, 1, 58.3 mm standard length, Kermanshahan, Sarab-e Sabz 'Ali Khan (34º25'N, 46º32'E) ?kermanshahensis q.v.;
CMNFI 2007-0021, 11, 25.5-34.6 mm standard length, ?;
CMNFI 2007-0037, 5, 45.2-54.9 mm standard length, Kerman, Hosseinabad and Gamatabad qanats at Bam (29º06'N, 58º21'E);
CMNFI 2007-0038, 1, 39.0 mm standard length, Kerman, Mehtiabad qanat at Bam (29º06'N, 58º21'E);
CMNFI 2007-0039, 6, 32.4-41.7 mm standard length, Kerman, Tahrud River (ca. 29º23'N, ca. 57º63'E);
CMNFI 2007-0043, 9, 33.0-56.7 mm standard length, Kerman, qanat at Emamzadeh Sultan (ca. 29º40'N, ca. 56º45'E);
CMNFI 2007-0047, 2, 36.8-58.2 mm standard length, Kerman, qanat at Hoshum (29º14'N, 56º19'E);
CMNFI 2007-0051, 5, 28.6-36.3 mm standard length, Hormozgan, upper Kol River basin (28º19'N, 55º55'E);
CMNFI 2007-0055, 3, 36.1-40.3 mm standard length, Hormozgan, stream in Minab River basin (27º47'N, 57º12'E);
CMNFI 2007-0074, 10, 25.6-50.8 mm standard length, Markazi, Qareh Su 32 km west of Arak (34º03'N, 49º21'E);
CMNFI 2007-0075, 6, 41.3-47.9 mm standard length, Hamadan, Malayer River 5 km from Malayer (ca. 34º17'N, ca. 48º47'E);
CMNFI 2007-0081, 10, ? mm standard length, Zanjan, Zanjan River basin near Soltaniyeh (ca. 36º27'N, ca. 48º45'E);
CMNFI 2007-0082, 7, ? mm standard length, Zanjan, Zanjan River basin near Zanjan (ca. 36º36'N, ca. 48º32'E);
CMNFI 2007-0083, 2, ? mm standard length, Azarbayjan-e Khavari, Qaranqu River basin west of Sar Eskand Khan (ca. 37º25'N, ca. 46º55'E);
CMNFI 2007-0084, 2, ? mm standard length, Azarbayjan-e Khavari, Talkheh River basin west of Sarab (ca. 37º56'N, ca. 47º19'E);
CMNFI 2007-0085, 12, ? mm standard length, Azarbayjan-e Khavari, Talkheh River basin east of Sarab (ca. 37º56'N, ca. 47º41'E);
CMNFI 2007-0086, 3, ? mm standard length, Azarbayjan-e Khavari, Qareh Su basin near Nir (ca. 38º02'N, ca. 48º00'E);
CMNFI 2007-0089, 7, ? mm standard length, Azarbayjan-e Khavari, Ahar Chay at Ahar (38º28'N, 47º03'E);
CMNFI 2007-0091, 13, ? mm standard length, Azarbayjan-e Khavari, Zilber Chay west of Marand (38º30'N, 45º23'E);
CMNFI 2007-0093, 8, ? mm standard length, Azarbayjan-e Bakhtari, Qotur River south of Khvoy (38º30'N, 44º58'E);
CMNFI 2007-0095, 2, ? mm standard length, Azarbayjan-e Bakhtari, Shahr Chay southwest of Reza'iyeh (ca. 37º27'N, ca. 44º56'E);
CMNFI 2007-0096, 6, ? mm standard length, Azarbayjan-e Bakhtari, Qasemul River south of Reza'iyeh (ca. 37º25'N, ca. 45º10'E);
CMNFI 2007-0097, 4, ? mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay basin south of Reza'iyeh (ca. 37º16'N, ca. 45º08'E);
CMNFI 2007-0099, 9, 24.6-43.8 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay west of Mahabad (ca. 36º35'N, ca. 45º25'E);
CMNFI 2007-0100, 4, 44.2-58.4 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0103, 3, ? mm standard length, Kordestan, Zarineh River basin north of Saqqez (36º18'N, ca. 46º16'E);
CMNFI 2007-0105, 13, ? mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º06'N, ca. 46º20'E);
CMNFI 2007-0106, 17, ? mm standard length, Kordestan, Qezel Owzan River basin near Divandarreh (ca. 35º52'N, ca. 47º05'E);
CMNFI 2007-0107, 8, ? mm standard length, Kordestan, Qezel Owzan River basin near Bijar (ca. 35º54'N, ca. 47º20'E);
CMNFI 2007-0115, 1, 41.5 mm standard length, Kermanshahan, Qareh Su basin (ca. 34º34'N, ca. 46º47'E);
CMNFI 2007-0115, 2, 49.7-54.7 mm standard length, Kermanshahan, Qareh Su basin (ca. 34º34'N, ca. 46º47'E);
CMNFI 2007-0116, 2, 40.6-44.8 mm standard length, Kermanshahan, Gav Masiab River basin west of Sahneh (ca. 34º28;'N, ca. 47º36'E);
CMNFI 2007-0118, 15, 24.7-58.8 mm standard length, Kermanshahan, Bid Sorkh River between Sahneh and Kangavar (ca. 34º23'N, ca. 47º52'E);
CMNFI 2007-0119, 8, 26.9-37.9 mm standard length, Kermanshahan, Gav Masiab River basin near Kangavar (ca. 34º31'N, ca. 48º03'E);
CMNFI 2007-0121, 6, 32.0-54.1 mm standard length, Hamadan, Qareh Su basin north of Razan (ca. 35º25'N, ca. 49º02'E);
BWC 1995-0002, 1, 41.6 mm standard length, ?;
BWC 1995-0017, 3, 48.4-51.2 mm standard length, ?;
BWC 1995-0020,
BWC 1995-0030, 1, 22.8 mm standard length, ?;
BWC 1997-0005,
BWC 2000-0001,
BWC 2000-0002,
BWC 2000-0004,
BWC 2000-0008,
BWC 2000-0011,
BWC 2000-0016,
BM(NH) 1899.9.30:138-140, 4, 41.8-53.4 mm standard length, ?, Ula on the Zola Chai
BM(NH) 1899.9.30:131-137, 7 (4 examined), 33.3-35.8 mm standard length, ?, Elinja Chai
The hillstream, mountain or river loach family has had various family names - see Menon (1987), Mirza (1989b), Hieronimus (1990; 1991), Ng and Lim (1991), Kottelat (1988, 1991), Nelson (1991),
Thorne (1992), Anonymous (1993a) and Bănărescu and Nalbant (1995) for discussions. The Iranian species were classified in Cobitidae in older works and more recently in Homalopteridae
and Balitoridae. Mirza (1989b),
Nalbant (1998), Tang et al. (2006) and Šlechtová et al. (2007) consider that the correct name for this family is Noemacheilidae (in Mirza) or Nemacheilidae. Nemacheilids are aligned with the main stem of cobitoid fishes rather than homalopterids as proposed by Sawada (1982) on osteological grounds.
Nalbant (1998) considers the similarities observed by Sawada between nemacheilids and homalopterids to be homoplasies. Homalopterids are more closely related to cyprinids and psilorhynchids.
The family is found throughout Eurasia with a single species in northeast Africa. There are about 60 genera and about 590 species (Berra, 2001; Nelson, 2006), with more being described regularly.
Iranian species belong to the subfamily Nemacheilinae.
Conway et al. (2010) point out that although there are no morphological
synapomorphies for the family, molecular data does support monophyly. Prokofiev
(2010) gives osteological characters for the subfamily Nemacheilinae.
Most species in Iran were placed in the genus Nemacheilus Bleeker, 1863 but this name contains only species from Southeast Asia (Kottelat, 1997). Noemacheilus Kuhl and van Hasselt in
van Hasselt, 1823 is a nomen nudum since there are no taxonomic characters accompanying the original description. The next available name is Nemacheilus Bleeker, 1863 (see Kottelat, 1987).
Nemachilus Günther, 1868 is an incorrect spelling. Much of the earlier literature on Iranian members can be found under the name Nemacheilus or its variant spellings.
These loaches have been placed in several genera or subgenera including the following relevant to Iran: Orthrias Jordan and Fowler, 1903 (see Banarescu, Nalbant and Balik (1978); Orthrias
= Barbatula Linck, 1790 of authors, see Bănărescu and Nalbant (1995) for reasons advocating the later name over the earlier one; Kottelat (1997) gives Linck as 1789), Adiposia
Annandale and Hora, 1920 (see Annandale and Hora (1920); see also Bănărescu and Nalbant (1995) where it is synonymised with Paracobitis), Triplophysa Rendahl, 1933 and
Hedinichthys Rendahl, 1933 (see Bănărescu and Nalbant (1966); see also Bănărescu and Nalbant (1995) where the latter is synonymised with the former), Oxynoemacheilus
Bănărescu and Nalbant, 1966 (see Bănărescu and Nalbant, 1966; synonymised with Orthrias in Bănărescu and Nalbant (1995) and with Barbatula in Bogutskaya and
Naseka (2004)), Paracobitis Bleeker, 1863 (see Bănărescu and Nalbant, 1966), Schistura McClelland, 1839 (see Mirza, Nalbant and Banarescu, 1981; regarded as polyphyletic by
Bănărescu and Nalbant (1995)), and Seminemacheilus Bănărescu and Nalbant, 1995 (q.v.). Views on the generic validity of these names conflict between authors and
between the same author at different dates (see Krupp, 1985c; Eschmeyer, 1990; Eschmeyer et al., 1996; Eschmeyer's "Catalog of Fishes"). Kottelat (1984) retained Nemacheilus until
a revision of all species was complete but there has been a tendency to use the above genera and this is followed here.
Prokofiev (2004, 2007, 2009, 2010) has revised some of the Nemacheilidae, in
particular the group of nemacheiline loaches that lack the preethmoid I in the
skull, using osteology and morphology. This includes most of the species found
in Iran and is now being followed by such authors as Esmaeili et al.
(2010).
The table below summarises some of the various allocations:-
?re-do
|
Species |
Original genus^ |
Other generic allocations^ |
Current genus |
|
angorae |
Nemacheilus |
Orthrias, Barbatula |
Oxynoemacheilus |
|
araxensis |
? |
? |
Oxynoemacheilus |
|
bampurensis |
Nemacheilus |
Schistura |
Paraschistura |
|
bergianus*check original ending |
Nemachilus |
Orthrias,
Barbatula |
Oxynoemacheilus |
|
brandtii |
Nemachilus |
Orthrias
Barbatula |
Oxynoemacheilus |
|
cristatus*check
ending |
Nemacheilus |
Paracobitis,
Schistura |
Metaschistura |
|
farsicus ?original spelling |
? |
|
Oxynoemacheilus |
|
frenatus*check ending |
Cobitis |
Nemacheilus, Orthrias,
Barbatula |
|
|
iranica* |
Paracobitis |
- |
Paracobitis |
|
kermanshahensis |
Noemacheilus |
Orthrias,
Barbatula |
Oxynoemacheilus |
|
kessleri |
Nemachilus |
- |
Paraschistura |
|
kosswigi |
Turcinoemacheilus |
- |
Turcinoemacheilus |
|
longicauda*check
ending |
Cobitis |
Nemacheilus, Adiposia |
Paracobitis |
|
longipinnis |
Ilamnemacheilus |
- |
Ilamnemacheilus |
|
malapterura*check
ending |
Cobitis |
Nemacheilus |
Paracobitis |
|
nielseni |
Schistura |
Schistura |
Paraschistura |
|
persa*check
ending |
Cobitis |
Nemacheilus, Oxynoemacheilus, Orthrias,
Barbatula |
Oxynoemacheilus |
|
rhadinea*check ending |
Nemachilus |
Adiposia |
Paracobitis |
|
sargadensis |
Nemacheilus |
Schistura |
Paraschistura |
|
smithi |
Noemacheilus |
- |
Paracobitis |
|
stoliczkai |
Cobitis |
Nemacheilus |
Triplophysa |
|
tongiorgii |
Seminemacheilus |
- |
Seminemacheilus |
|
vignai |
Paracobitis |
- |
Paracobitis |
* original suffix which may change if genus changes to masculine or to feminine; ^ Nemacheilus sometimes spelled Noemacheilus or Nemachilus by various authors
A number of nemacheilid species have been described from waters confluent with Iran, particularly from the Helmand River basin in Afghanistan. They have no Iranian records but are listed here as
they may be relevant to revisionary studies.
The dating of the paper by Bănărescu and Nalbant as 1967 in various
works may follow Nalbant and Bianco (1998). The Bănărescu and Nalbant
paper states in Danish on page 186 "Reprints released the 31 December 1966" and
this is presumably the correct date, as Art. 21.2 of the Code of Zoological
Nomenclature states "Date specified. The date of publication specified in
a work is to be adopted as correct in the absence of evidence to the contrary",
and no evidence to the contrary has been presented (N. G. Bogutskaya, pers
comm., 28 April 2011). This changing of dates here has no nomenclatural
significance.
Members of this family in Iran are characterised by an elongate and weakly compressed and almost cylindrical body, head not compressed but rounded, scaleless or body covered in minute scales (too
small for scale counts to be commonly or easily made), lateral line complete or incomplete, a small and inferior mouth, lips thick, fleshy and papillose, lower lip interrupted in the middle, 2 pairs of
barbels on the snout and 1 pair at the mouth corners (8 pairs in some non-Iranian species), no collapsible spine under the eye (sometimes present in non-Iranian species but distinguishes
members of the related Cobitidae in Iran), eyes small to minute, usually not visible from the underside of the head, reduced gill opening, short to moderate dorsal fin without spines, short anal fins,
vent a short distance in front of anal fin origin, swimbladder enclosed partially or entirely in a bony capsule, certain osteological characters such as the shape of bones in the
Weberian apparatus used in sound transmission from the swimbladder to the ear, gut short or long, a dermal crest or adipose fin may be present, caudal fin truncate, rounded or slightly forked, and
often distinctive colour patterns of bars, stripes and blotches. Iranian species may lack scales, may have an adipose fin, and have a single unbranched ray leading the pectoral and pelvic fins.
Krupp (1985c) reviews prior works by P. M. Bănărescu and co-authors on Levantine
nemacheilids and regards them as unsatisfactory. This calls into question works on Iranian species by this
author. Krupp (1985c) lists characters important in studying Nemacheilus sensu lato and those which are individually variable or develop independently, much in contrast to characters
favoured by Bănărescu. Morphometric characters can vary with nutritional status and ecological factors. Stable characters were head length, interorbital width, caudal peduncle length and depth,
and predorsal length. Fin lengths are dependent on sex in some species, less so in others. Allometry is a problem in fin positions and measurements involving such characters can only be used when
comparing fish of equal size. Mouth width and digestive tract shape are good characters but lip shape and development of the processus dentiformis are not. The swimbladder capsule form, including the
presence or absence of a continuous collar between the two hemispheres and the shape of laminae, is an important character. Reduced laminae and wide recesses on the hemispheres are
derived characters. The dorsal adipose fin development is stable in some species, variable in others, and is independently derived in different phyletic lines, thus being of limited value. The shortening
and deepening of the caudal peduncle is derived in one Levantine species. Scale characters such as size and position of the focus and general scale structure are very variable and not characters easily
quantified. Only specimens of the same size are comparable and numerous scales must be examined because aberrant ones are common. The lateral line length is a good character, although juvenile fish may
have a shortened one. A reduced lateral line is a derived character. Colour patterns are subject to variation and both spotted and striped forms can be found within one species. Nevertheless, patterns can
be important in distinguishing species. Thickening of pectoral fin rays is a derived feature but absence of this character is a symplesiomorph condition and cannot be used to relate species.
The species in this family are often difficult to identify and many literature reports are undoubtedly mis-identifications. While some of these may be corrected based on material deposited in museums,
others have no voucher material and cannot be re-identified. Identification is problematical because scale counts are not available (too minute), fin ray counts are often very similar, and
unique structures uncommon. Colour patterns can be used but are notoriously variable and many types in museums are decoloured making comparisons difficult. Morphometric characters require good series of
adult fish of both sexes, from various localities in the species range, preferably even from the same locality taken over several years to allow for local variations in habitat which may conceivably affect
shape. Menon (1987) considers that many species in this genus are from very similar habitats, the stressful one of running water, and have been constantly selected to fit this niche. Valid species
resemble one another closely. Such characters as position of the anal opening, the dorsal fin origin and barbel length have been used in species definitions but Menon (1987) found these to vary with
growth. Swimbladder structure depends on the habitat where the fish live and scale coverage on the physico-chemical nature of the water. Menon (1987) found lateral line character, number of branched
dorsal fin rays, caudal fin shape, secondary sexual characters in males and, despite the above, body colour and anal opening position to be useful.
Prokofiev (2010) reviewed the morphological classification of loaches and points
out that changes in morphology occur with growth and species with wide ranges
show large variations in morphology associated with the various biotopes. He
gives extensive reviews of characters and their importance in defining genera
and species, e.g. scale cover and scale characters are not considered important
in phylogeny and are of only accessory import for genus and species diagnosis;
colour, sexual dimorphism, adipose keel presence and extent, fin ray numbers,
head and body shapes, abdominal axillary lobe presence and size, variations in
the seismosensory system, nostril size and position, anal opening position,
intestine shape, swimbladder and its capsule structure, and general osteology
are all important characters.
Examination of Iranian species, where good series of fresh material was available, tend to confirm the observations of Krupp and Menon on characters. Position of the dorsal fin origin is variable
within a species among morphometric characters used as distinctive, extent of the lateral line is also variable, there is marked sexual dimorphism, and colour patterns can be useful but also vary with
the individual, the habitat and the temperature (fish kept in ice water have strong colour patterns while those preserved immediately from murky waters have faint patterns).
Two main problems exist in identifying certain Iranian loaches. These are determining appropriate characters which are not individually variable and which are apomorphic, and applying existing names
to fresh material in comparison with poorly-preserved types.
A number of species remain to be described and are currently under study. Some named species are probably distinct taxa, e.g.
"Nemacheilus" tigris (Heckel,
1843) (sagmahi-ye Dajleh) is recorded from the Karun River basin in Khuzestan (ZISP 24098) by Berg (1949) but specimens from this part of Iran differ from Heckel's types. The colour pattern on the fish
figured by Berg (1949) from the Karun River in Iran is atypical according to Bănărescu and Nalbant (1966) - it has only 4-5 bars on the posterior part of the body. The type locality of Cobitis
Tigris is "Flüsschen Kueik bei Aleppo" (Haleb, Syria) according to Heckel (1843b).
Prokofiev 92009) places Cobitis tigris in the genus Paracobitis.
Sawada (1982) thought that this family dispersed by two routes from Southeast Asia, one through Siberia and one through South Asia to reach what is now Iran. Menon (1987) considers that the land mass
between East Africa and the west coast of India has submerged only recently, probably simultaneous with the birth of the Ganges and Indus. Connections of the Pleistocene fore-deep of the Himalayas with
the Tigris-Euphrates basin in what is now the Persian Gulf could have existed, allowing movement of "Nemacheilus" species along a continuous route from Yunnan to Anatolia. Menon (1987)
further suggests a series of waves, spreading "Nemacheilus" westwards into Southwest Asia from a South China origin. The Triplophysa wave is the first wave of evolution, in which
the earliest stock from Yunnan spread through Tibet in the late Miocene and early Pliocene before the major rise of the Himalayas. By the end of the Tertiary, particularly in the Pleistocene, the Tibetan
Plateau had risen causing a dry and cold climate with increased solar radiation and torrential rivers. This change in the environment caused rapid evolution, leading to such taxa as Triplophysa
and Hedinichthys. The rupecola wave took place in the late Pliocene along the southern face of the Himalayas through Iran to Anatolia and even northeast Africa. Some of the criticisms listed
under the cyprinid genus Garra, whose distribution Menon (1964) also attributes to waves, may be apposite here too.
These are small fishes, up to about 200 mm in size although one species (not
in Iran) reaches 482 mm (Prokofiev, 2010). They are quite secretive, hiding under stones or in mud. This common and stressful habitat may have led to a general similarity in body form among the various species. Some are known only
from caves, including one Iranian species. Despite their small size, they are regarded as a delicacy in India (Hora, 1956). Barbatula angorae (and presumably other species) is a potential fishing
bait for predatory fishes such as Sander lucioperca and has been examined experimentally for this purpose in Turkey (Kuşat et al., 1995). They are generally known as سگ
ماهي (sag mahi meaning dog fish, but this is presumably the equivalent of loach in English), لوچ (= louch meaning loach) or mar mahi (= snake fish, presumably
in reference to the elongate shape) in Farsi. These general names are not repeated below.
Genus Barbatula
Linck, 1790
Species in this genus were formerly placed in Orthrias (see Eschmeyer's "Catalog of Fishes" to track conflicting views). There are at least 18 species found mainly in western Asia
with a few in Europe. The body is elongate, thick, and rounded or slightly compressed. The head is slightly depressed or compressed. Eyes are small and widely spaced. The caudal peduncle is relatively
deep. The body is covered by scales at least on the posterior part of the body, the lateral line is complete or at least passes the middle of the body length, and the lateral line tubes do
not penetrate the scales. There are no nasal barbels. The lower lips are moderately furrowed. The dorsal fin has 7-9, rarely 10, branched rays. The pelvic fins are inserted slightly behind the dorsal fin
origin. The caudal fin is slightly emarginated to deeply forked. There is no dorsal crest on the caudal peduncle. The processus dentiformis (a tooth-like projection at the symphysis of the upper jaw) is
weakly or moderately developed and lacks a corresponding gap in the lower jaw. The gut is short. Colour is variable, being barred, striped, irregular spots and blotches, or more or less regular rows of
spots. The pectoral fin of males is broadened and thickened, and covered by tubercles in the spawning season. Tubercles also develop on the sides of the head.
Genus Ilamnemacheilus
Coad and Nalbant, 2005
This genus is characterised by a high, laterally compressed body; large head with small eyes and mouth; anterior lip lacking an interruption in the middle; posterior lip with widened mental
lobes, small round papillae covering only the mental lobes, the rest of the lips being unfurrowed; the processus dentiformes absent; lateral line complete and terminating slightly before the posterior
margin of the caudal peduncle; scales small with a quite large and eccentric focus, sparsely present on the rear half of the body; stomach syphonal and intestine straight without loops; gas bladder
with two encapsulated chambers united by a short encapsulated duct; paired fins very long; dorsal fin long; and caudal fin well forked.
The type species is Ilamnemacheilus longipinnis by original designation and monotypy.
Ilamnemacheilus longipinnis
Coad and Nalbant, 2005
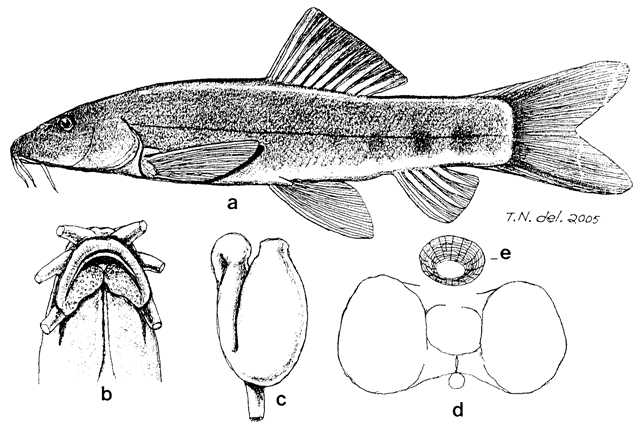
Common names
None.
Systematics
The holotype and only known specimen is CMNFI 1979-0366 (79-966 is a lapsus), 36.0 mm standard length, Iran, Meymeh River, formerly a tributary of the Tigris River, 17 km west of Dehloran and about
21 km east of the Iraqi border, 32º45'30"N, 47º05'30"E, 28 January 1978, B. W. Coad and S. Coad.
Key characters
Characters are those of the genus.
Morphology
Dorsal fin with 3 unbranched and 10 branched rays, anal fin with 2 unbranched and 5 branched rays, pectoral fin with 9 branched rays and pelvic fin with 5 branched rays. Total vertebrae 28 or 29
including the ural centrum (vertebral fusions present), and some centra have two neural and haemal arches. ?check vertebral counts against other loaches - very low ? fusions Other
characters are listed above under the genus and Coad and Nalbant (2005) give some measurements.
Sexual dimorphism
Unknown.
Colour
The sole preserved specimen is a overall a pale brown with 3-4 indistinct greyish blotches in the middle of the second half of the body. All fins are pale but the caudal fin has faded greyish lines
along the marginal rays. In life it was an olive-green overall with orange fins.
Size
Reaches 36.0 mm standard length.
Distribution
Endemic to Iran and found in Tigris River basin at a single locality (see above).
Zoogeography
An endemic genus in the Tigris-Euphrates basin (along with Turcinoemacheilus, not in Iran). This species may be related to an undescribed species from the Orontes River basin in Syria.
Habitat
The sample site was a small stream, 20 m wide with a maximum depth of 1 m. Altitude was 210 m. Capture depth was 30 cm in a medium current. The bottom was a mix of pebbles and mud with some encrusting
algae. Water temperature was 14ºC, pH was 6.0 and conductivity was 1.65 mS. The cyprinids Cyprinion macrostomum and Garra rufa were caught with the loach.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Known only from a single specimen, abundance and wider distribution unknown.
Further work
More collections are needed to record information on biology and distribution and provide a more detailed description.
Sources
The holotype and sole known specimen (see above).
Genus Metaschistura
Prokofiev 2009
This genus has a single, small species and the characters of the species are
those of the genus. Prokofiev (2009) gives further osteological details.
Metaschistura cristata
(Berg, 1898)
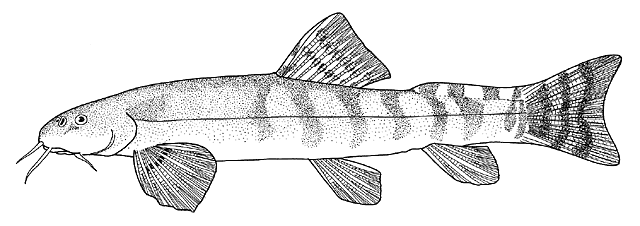
Common names
sagmahi-ye Torkomani
or Turkmeny (= Turkmenian crested loach), sagmahi-ye kakoldar-e Torkomani.
[Turkmenskii grebenchatyi golets or Turkmenian crested loach in Russian].
Systematics
This species was described under Nemacheilus in Latin from "Habitat in flum. Tedschent, prope Aschabad, in provincia Transcaspica". The type locality is presumably the Tedzhen River
in Turkmenistan although Ashkhabad is not on the Tedzhen River. Berg (1948-1949) notes "not in the Tedzhen!". Syntypes are reported to be in the Zoological Museum of Moscow State University
(MMSU) by Eschmeyer et al. (1996).
Placed in the taxon Paracobitis by Bănărescu and Nalbant (1966) and later in Schistura by Bănărescu and Nalbant (1995) and by Nalbant and Bianco (1998).
Key characters
The dorsal fin branched ray count of 8 distinguishes this crested loach from other crested loaches in northeast Iran and adjacent regions. In addition the crest, or adipose fin, is shorter and thicker
than in P. malapterura.
Morphology
Dorsal fin unbranched rays 2-6 (assumed to be usually 3) and branched rays 7-9, modally 8, anal fin unbranched rays 2-4, usually 3, and branched rays 5, pectoral fin branched rays 8-11,
usually 9-10, and
pelvic rays 6-8, usually 7. The dorsal fin origin lies midway between the snout tip and caudal base or nearer the caudal base. There is a fleshy pelvic axillary process
but it does not extend beyond the end of the pelvic fin base. Caudal fin slightly emarginate in
Turkmenistan but Hari Rud fish have a conspicuous fork. The two lobes of the caudal fin may be equal or either may be longer. The lobes are pointed or rounded. The dorsal adipose fin or crest begins
shortly in front of the anal fin origin level and reaches the root of the caudal fin. There is a small gap between the dorsal fin insertion and the origin of the crest, and the depressed dorsal fin
partially occupies this gap and the slight rise of the crest origin. It is strongly developed, high and thick.
The crest is supported by 22-25 procurrent rays of the caudal fin. Large fish have scales posteriorly on the body. Scales are small, oblong and widely separated.
Bănărescu and Nalbant (1966) did not find scales on fish from the Harirud (confirmed for these fish (ZMUC P 2798, 2799, 27100, a fourth specimen P 2797 is lost; these catalogue numbers corrected
from Bănărescu and Nalbant (1966) where incorrect; 63.1-68.3 mm standard length) from Obeh, Afghanistan; and not found on 3 fish, 38.1-60.9 mm standard length from Sarakhs on the Hari Rud) and
this population may be distinct. Scales may be completely absent or only on the
crest (Prokofiev, 2009). The lateral line extends nearly to the caudal base. Caudal peduncle short, 5-6 times in standard length (6.1-6.9 for 3 Iranian fish, 38.1-60.9 mm standard length). Lips
are thick and furrowed, the upper lip with a slight median gap and the lower lip widely interrupted. The dentiform process on the upper jaw is weakly developed. The anterior nostril has a large flap
developed posteriorly and postero-dorsally. Large fish have bulging cheeks. The posterior part of the intestine is straight (Bănărescu and Nalbant, 1966) or with a single loop (Bănărescu
and Nalbant, 1995).
Meristics for Iranian specimens:- dorsal fin branched rays 8(4), anal fin branched rays 5(4), pectoral fin branched rays 9(2), 10(1) or 11(1); and pelvic fin branched rays 7(4).
Sexual dimorphism
There is no sexual dimnorphism.
Colour
Somewhat similar to P. malapterura but with a dark spot at the anterior base of the dorsal fin, on the unbranched and first branched rays. A similar spot may be present at the anterior base of
the anal fin on the first two rays. The anterior part of the body is dark and the posterior half
(from the dorsal fin insertion to the tail base excluding any bar on the caudal
fin rays) has 4-8 broad, brownish bands, separated by narrower light bands and reaching close to or to the ventral
part of the body. Some smaller fish have anterior bands on the flank, the total number of bands reaching
as high as 17. Bands vary in size from equal to twice the width of the pale interspaces. The last 3-4 bands
extend onto the adipose fin. There is also a much darker and narrower band at the caudal base. Bănărescu and Nalbant (1995: Fig. 11A) illustrate a fish where this last band is only developed on
the lower half of the caudal peduncle. The bar may also be composed of two blotches above and below the flank midline plus blotches on the bases of the outermost caudal rays both dorsally and ventrally.
All fins have thin bands comprised of spots irregularly lined up on the fin rays. The caudal fin may have three such bands while this fin and others may have no clear bands. Some fish have pigment on rays
of the dorsal fin almost forming a single broad band extending from mid-fin half way towards the margin. There may be a second smaller spot on the last unbranched dorsal fin ray above the
one on the anterior base.
Size
Attains 8 cm.
Distribution
Found in streams on the northern slope of the Kopetdag, Turkmenistan. Some of these streams presumably have headwaters in Iran and the species may be found there although this has yet to be
confirmed by specimens. Also reported from the drainage of the Tedzhen River in Afghanistan which forms part of the Iran-Afghanistan border as the Hari Rud or Hari River (Bănărescu
and Nalbant, 1966). This species is confirmed for Iran from Sarakhs on the Hari Rud, and probably the Kashaf River, a Hari River tributary (Abdoli, 2000).
A record from Lake Topiatan in the Uzboi River drainage in Turkmenistan north of the Iranian border mentioned in Nikol'skii (1947) is housed at the Zoological Institute, St. Petersburg (ZISP
25788). Berg (1948-1949) corrects the locality as the Sumbar River, an Atrak River tributary in the Caspian Sea basin. This specimen was examined by me and it is not this species but P. malapterura.
Zoogeography
Bănărescu and Nalbant (1966) consider that the closest relative of this species is Nemacheilus tigris (sic) from the Tigris-Euphrates basin rather than other
crested loaches such as P. malapterura and P. rhadinaea found in neighbouring river systems. Note that
Paracobitis tigris was described from Aleppo (= Haleb, Syria) and
its occurrence in the Tigris-Euphrates basin is in question (see family account).
Habitat
A rheophilic species but further details of habitat preferences are unknown.
Age and growth
Unknown.
Food
Gut contents of one fish, 60.9 mm standard length, from the Hari Rud are chironomid larvae.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population numbers or trends are unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Iranian material: BWC 97-3, 3, 38.1-60.9 mm standard length, Khorasan, Hari River at Sarakhs (?);
BM(NH) 1914.1.1:32, 1, 54.5 mm standard length, Khorasan, Kashaf River (no other locality data).
Comparative material: SMF 17133, 2, 34.4-98.3 mm standard length, Afghanistan, Hari River near Herat (34º21'N, 62º14'E);
BM(NH) 1897.7.5:40, 51.4 mm standard length, Turkmenistan, Ashkhabad (no other
locality data).
see also keys in Prokofiev for generic characters?
Genus Oxynoemacheilus
Bănăraescu and Nalbant, 1967
?cf with barbatula diagnosis
Members of this genus are found from Bulgaria east to Iran.
There are 28 or more species with 7 recorded from Iran.
These small loaches are scaled, have a body with spots which
tend to form bands or a stripe along the lateral line and there is no dark black
spot on the base of the anterior dorsal fin rays. There is no caudal peduncle
crest and no pelvic fin axillary lobe. Males do not develop inflated or muscular
cheeks in the spawning season but tubercles are found on the sides of the head
and on the pectoral fins but not as fine brush-like aggregations. Nostrils are
closely spaced or slightly separated, the mouth is semilunar with simple lips
which are almost smooth to plicate and the dentiform process is weak to moderate
but sometimes absent (O. angorae). The lateral line is usually complete
but if incomplete as in O. kermanshahensis it reaches beyond the level of
the dorsal fin origin. The dorsal fin 7-8 branched rays and the anal fin 5
branched rays. Prokofiev (2009) lists various osteological characters such as 4
radial bones in the pectoral fin, usually 6 hypurals in the caudal fin support (O.
brandtii has 5), the epural bone is present in the caudal fin support, the
manubrium in the swimbladder is well-developed and the bony capsule is full
divided by it, no preethmoid I, the supratemporal commissure is open from above,
the fork of cst and cio is not fused to the skull, and others.
Oxynoemacheilus angorae
(Steindachner, 1897)
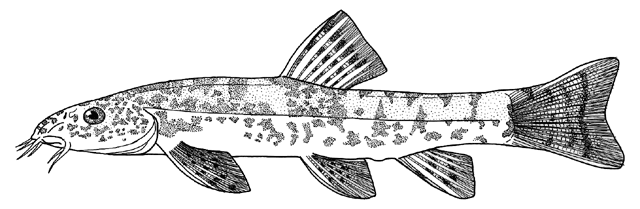
Common names
sagmahi-ye Angora, sagmahi-e-jooibari.
[lakh angorae in Arabic; Angor cilpaxcasi (or cilpagcasi), Lankaran cilpaxcasi (or cilpagcasi), both in Azerbaijan; Angorskii golets or Angora loach, Lenkoranskii golets or Lenkoran loach, both in
Russian; otsidzug in Armenia; stone loach].
Systematics
Nemacheilus angorae was originally described from Angora (= Ankara, Turkey). Nemacheilus bergi Gratsianov, 1907 and possibly Nemacheilus bergianus Derzhavin, 1934 are synonyms.
Nemacheilus angorae lenkoranensis Abdurakhamanov, 1962 (incorrectly lenkoranica in Bănărescu and Nalbant (1966)) is described from "rivers of the Lenkoran
coast; Lenkoranchai, Vilyazhchai, Kumbashichai, Tangyaru, Astarinka" in the southern Caspian Sea basin.
Orthrias angorae araxensis Banarescu and Nalbant, 1978 in Banarescu, Nalbant and Balik (1978), is described from the Aras River basin of Turkey (type locality given below) (this subspecies was
formerly referred to as Nemacheilus angorae bureschi (Drensky, 1928) by Banarescu and Nalbant (1964) and Banarescu (1968)). Nalbant and Bianco (1998) and Fricke et al. (2007) elevate this
taxon to a species.
Nemachilus angorae alasanicus Elanidze, 1983 was described from the upper reaches of the Alazani River at Alvani village, Georgia, a left bank Kura River tributary. It is here attributed to
Elanidze (1983) since it is the only taxon listed in that book without an author.
The lower Kura River basin in Azerbaijan (of which the Aras is part) may have another subspecies in which about 40% of the fish have 7 branched dorsal fin rays, a shallower caudal peduncle than
B. a. araxensis, and a colour pattern similar to B. angorae angorae (Banarescu, Nalbant and Balik (1978); see also description in Abdurakhmanov (1962) for measurements).
B. bergiana from Iran may be another subspecies of B. angorae. The resolution of the systematics of B. angorae and its subspecies or related species depends on obtaining large
numbers of specimens of mature material from the whole range of this species complex. There are few, if any, good characters in this complex which can be used to separate the taxa; body proportions are
especially prone to locally induced variation and even the number of dorsal fin rays is at least as variable within samples as between species.
Sixteen syntypes of Nemacheilus angorae from Tabakane-Sir and
Tschibuk-Tschai, Turkey are in the Naturhistorisches Museum Wien according to Eschmeyer et al. (1996).
The holotype of Orthrias angorae araxensis, 62.0 mm standard length, from the "Kandili Karassu, oberes Araxes-Becken, Osttürkei" is in the Zoologischen Instituts und Zoologischen
Museums der Universität Hamburg (ZMH 4827). Four paratypes, 45.0-65.2 mm standard length, from the same locality are under ZMH 5951 and 2 other paratypes, 51.2-59.3 mm standard length, also from the same
locality are in the Institutul de Stiinte Biologice, Bucuresti, Romania (ISBB 2617). Five paratypes, 38.8-52.0 mm standard length, are from the "Oberlauf des Araxes Flusses bei Aras-Nehri,
Hasankale" under ZMH 4826 and 3 paratypes, 41.0-45.0 mm standard length, from the same locality are under ISBB 2618 (Banarescu et al., 1978; Wilkens and Dohse, 1993).
A series of 18 fish in the Zoological Institute, St. Petersburg catalogued as ZISP 35701 from "Reka Lenkoran, Azerbaidzhan SSR" collected by Yu. Abdurakhmanov, 22.IX.1954 are probably the
type series of Nemacheilus angorae lenkoranensis although they are not marked as such on the jar. One fish is a cobitid, 31.0 mm standard length (probably Cobitis taenia), while the rest
measure 24.9-33.3 mm standard length.
Bănărescu and Nalbant (1966) follow Günther (1899) and describe specimens from the Lake Orumiyeh basin as Nemacheilus (=
Oxynoemacheilus) persa (q.v.) but
Abdurakhmanov (1962) and Saadati (1977) consider them to be close to B. angorae.
Key characters
Abdurakhmanov (1962) distinguishes two subspecies in Azerbaijan by the following key:-
1(2) Caudal peduncle depth in length 2.5-3.3, mean 2.9; dorsal
fin with 7-8 branched rays; length to 80 mm (Kura and Aras river
basins) B. angorae
2(1) Caudal peduncle depth in length 2.0-2.6, mean 2.3; dorsal
fin with 8-9 branched rays; length to 60 mm (rivers of Lenkoran) B.
angorae lenkoranensis
Morphology
Dorsal fin with 2-4, usually 2 according to Banarescu and Nalbant (1964) and Dadikyan (1986), unbranched and 6-9, usually 7-8, branched rays (strong mode of 7 in populations of B. angorae angorae
from Anatolia while the mode for B. angorae araxensis is 8 according to Banarescu, Nalbant and Balik (1978) and for B. angorae lenkoranensis the range is 7-9 usually 8-9 according to
Abdurakhmanov (1962)), anal fin with 2-3 unbranched and 4-6 branched rays, usually 5, pectoral fin with 8-12 branched rays, and pelvic fin branched rays 6-8. The dentiform process on the upper jaw may be
present or absent. The lower lip is more fimbriate than the upper and is interrupted in the middle. Weakly developed median flaps are present. Scales are minute. The lateral line is almost complete
(almost to the caudal fin base). Gut without a posterior loop. Caudal fin almost truncate (deeply forked in Lake Orumiyeh material examined by Bănărescu and Nalbant (1966) - other characters of
this small sample of 3 fish include a long and shallow caudal peduncle, vent just in front of the anal fin, pelvic fin origin under that of dorsal fin, dorsal fin origin much nearer caudal fin base than
snout tip, large eyes, lateral line almost complete ending just before the caudal fin, scales are distinct, especially in the posterior half of the body, and cover the whole body including the ventral
part, the upper lip is nearly smooth and has a narrow median interruption, the lower lip is more furrowed and has a wider interruption, the posterior part of the intestine is straight, males have
thickened pectoral fin rays with tubercles, flanks are spotted to reticulate, and there is a band at the caudal fin base). The subspecies B. angorae araxensis has body proportions practically the same
as the type subspecies from Anatolia according to Banarescu, Nalbant and Balik (1978) but the caudal fin margin is almost straight and colour is different.
Sexual dimorphism
Mature males have a dense covering of tubercles on the head, body and fins. Pectoral fin rays 2-4, and to some extent 1 and 5, are thickened, widened and densely tuberculate. Pectoral and pelvic fins
are longer in males than females.
Colour
Colour is variable but overall is greyish to yellow with orange tints in particular on the head. There are 5-6 spots along the mid-line of the back and about 20 brown to blackish spots along
mid-flank, forming almost a stripe, especially in young. The dorsal spots may also form a stripe. The base of the caudal fin has a black bar. The dorsal and caudal fins have brown bars composed of small
spots. The pectoral fin may also have bars, particularly in males. The other fins are hyaline. The subspecies B. angorae araxensis is darker in colour than B. angorae angorae, the flanks
being covered in nearly black spots and fine dots almost as low as the belly. The spots are irregular or follow the lateral line almost to the caudal fin but there is no stripe as in the other subspecies.
Size
Reaches 8.5 cm.
Distribution
Found in the Black Sea basin, Aegean Sea basin and the Caspian Sea basin. In Iran, it is recorded from the the Anzali Mordab
and the Masouleh River in that basin, upper Safid River basin (Qezel Owzan and Shahrud), the Qareh Su of the
Aras River basin in the Caspian Sea basin, southern and eastern tributaries of Lake Orumiyeh (Zarineh and Tata'u, and Talkheh rivers), rivers of the Namak Lake basin (Karaj, upper Shur or Abhar,
middle and upper Qareh Chai rivers, Jajrud), and possibly in the eastern Dasht-e
Kavir basin (Bănărescu and Nalbant, 1966; Saadati, 1977; Holčík and Oláh, 1992; Abbasi et al., 1999; Abdoli,
2000; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009). Specimens from Lake Orumiyeh identified as Nemacheilus persa by Günther (1899) are probably this species.
Iranian Caspian Sea and Namak Lake basin fish are probably O. bergianus. If
O. angorae is restricted to Turkey, then the species for the western Caspian is lenkoranensis and for the
Kura-Aras basin is araxensis.
Zoogeography
see (Bănărescu and Nalbant, 1966) for discussion, p. 155
Habitat
This species is found in both streams and lakes. In shallow running water it lives among stones and vegetation.
Age and growth
Heydarnejad (2009) gave the length-weight relationship for an Iranian sample
as W = 0.0078TL3.055.
Food
Food items include chironomids, corixids and diatoms as well as the eggs of other fishes such as Alburnus
hohenackeri. Abdoli (2000) lists Trichoptera, Ephemeroptera and Chironomidae.
Reproduction
Fecundity reaches 7000 eggs and egg diameter 0.84 mm. Spawning takes place from May to June.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
None.
Further work
The systematics of this species complex needs to be resolved based on additional material and perhaps modern molecular and genetic techniques.
Sources
Oxynoemacheilus araxensis
(Banarescu and Nalbant, 1978)
The presence of this taxon in Iran needs confirmation. type
locality??
?OxynoemacheilusBarbatula argyrogramma
(Heckel, 1849)
Reported from the Tigris-Euphrates basin in Iraq but no Iranian record except by Saadati (1977). Its validity is doubtful (see Freshwater Fishes of Iraq for more
information).
Oxynoemacheilus bergianus
(Derzhavin, 1934)
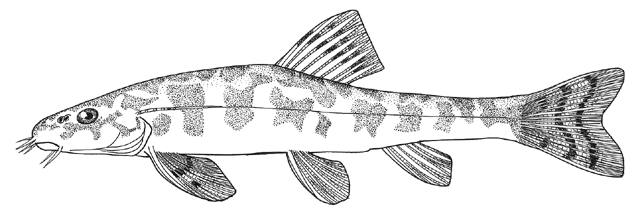
Common names
agmahi-ye Safidruds, sehkhareh (?), sagmahi-e-jooibari.
[Sefidrudskii golets or Sefidrud loach in Russian; Safidrud stone loach].
these names to brandtii?
Systematics
The type locality of this species in Latin from Derzhavin (1934) is "Systema fluminis Sefidrud" (= Safid River system). Berg (1948-1949) gives "Sefid-rud basin: Kisum village; Shah-rud
R., falling into the Sefid-rud". The former is at Kisom at 37°14'N, 49°51'E or 37°12'N, 49°54'E in a gazetteer.
Bănărescu and Nalbant (1966) and Banarescu, Nalbant and Balik (1978) place this species as a subspecies of B. angorae. Nalbant and Bianco (1998) consider it to be a distinct species
in Orthrias. Abdurakhmanov (1962) compares fish from Lenkoran (B. angorae lenkoranensis) with B. bergiana. Head length and depth, predorsal distance, body depth, caudal peduncle depth,
dorsal fin height, and pectoral and pelvic fin lengths are all greater in lenkoranensis while caudal peduncle length, pectoral-pelvic fin distance, interorbital width and eye diameter are all
greater in bergianus.
The problems of the systematics of such a widespread and variable taxon as B. angorae are briefly alluded to under that species and I have retained B. bergiana as a distinct species until
the problem has received a full study.
The holotype of Nemacheilus bergianus is 41.6 mm standard length and is in the Zoological Institute, St. Petersburg (ZISP 25433) and is from "Basin R. Sefid-Rud, c. Kissum, River Shahrud,
tributary Sefid-Rud", collected by A. N. Derzhavin, 20.V.1922. The specimen is faded but there is a figure in Berg (1948-1949, Fig. 619).
Key characters
This species differs from B. angorae by having a less deep caudal peduncle, 3.3 in caudal peduncle length according to ? (but my measurement is 2.6 for the holotype). Saadati (1977) states that the
caudal peduncle is deeper in B. bergiana.
|
Character/Taxon |
angorae |
araxensis |
bergiana |
brandtii |
lenkoranensis |
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
Morphology
Dorsal fin with 3 unbranched and 8 branched rays, anal fin with 3 unbranched and 5 branched rays, pectoral fin with 9 branched rays and pelvic fin with 6-7 branched rays. Vertebrae 31. The pelvic fins
are separated by almost the width of a pelvic fin base. Caudal fin slightly emarginate. The upper lip is indented at the mid-point with a bony projection underneath. The lower lip is interrupted and also
has a bony projection underneath. There are no adipose fins. The flank is minutely scaled and the lateral line is well-developed along the whole flank (not as in Berg's drawing - ?check this).
Meristics for Iranian fish: dorsal fin branched rays 8(1), anal fin branched
rays 5(1), pectoral fin branched rays 9(1) and pelvic fin branched rays 7(1) -
based on type ?see my material and add
Sexual dimorphism
Unknown.
Colour
The flank has several irregular dark grey blotches and the back has transverse, dark, squarish spots. The back and upper flank have a pinkish tinge. The lower flank is pale yellowish or whitish. The
belly and lower head are white. The iris is silvery on the lower part and golden on the upper, strikingly so. The dorsal, pectoral and caudal fins are pinkish with 2-3 rows of elongate, dark grey spots
along the rays. The caudal fin spots are horizontal rather than forming bands as in
Oxynoemacheilus brandtii. The upper base of the caudal fin is yellowish rather than dark as in B. brandtii.
The pelvic and anal fins are colourless or slightly yellowish-pink without spots. The barbels are typically without pigment except occasionally at the base of the second pair.
Size
Reaches 6.8 cm with Jolodar and Abdoli (2004) giving 7 cm total length.
Distribution
This species is found in the middle Aras River and its tributary the Qareh Chai
(?or is araxensis), the Safid, Shafa, Siyah, Shahrud and lower Qezel Owzan rivers of the Caspian Sea basin, and the Karaj, Abhar, upper Qareh Chai and
upper Qom rivers of the Namak Lake basin (Saadati, 1977; Abbasi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004;
Abdoli and Naderi, 2009)
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Females are mature at 5.0 cm.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include few in numbers, habitat destruction, limited range
(less than 25% of water bodies), present in other water bodies in Iran (sic), present outside the Caspian Sea basin (sic).
Further work
The biology and systematics of this species needs study.
Sources
Type material: Holotype of Nemacheilus bergianus (ZISP 25433), see above.
Iranian material: CMNFI 1970-0527, 8, 33.1-42.7 mm standard length, Gilan, Safid River near Kisom (37º12'N, 49º54'E);
CMNFI 1970-0537, 24, 33.3-50.4 mm standard length, Markazi, Shah River near Manjil (36º44'N, 49º24'E);
CMNFI 1970-0545, 1, 44.4 mm standard length, Gilan, Safid River (37º01'N, 49º38'E);
CMNFI 1980-0132, 26, 19.4-44.8 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1980-0154, 26, ? mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1980-0156, 80, 29.9-50.8 mm standard length, Markazi, Karaj River near village (35º47'N, 50º58'E).
Oxynoemacheilus brandtii
(Kessler, 1877)
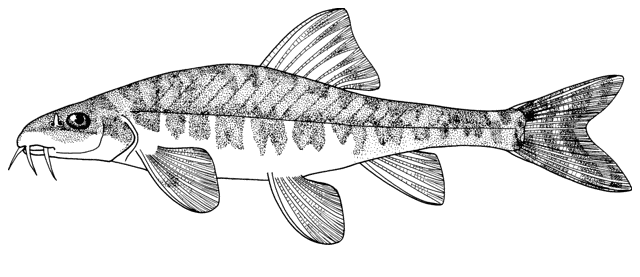
Common names
sagmahi-ye Kura.
[Kur cilpagcasi in Azerbaijan; Kurinskii golets or Kura loach in Russian].
Systematics
A syntype of Nemacheilus Brandtii from the upper Kura River at Tbilisi (= Tiflis), Georgia is in the Natural History Museum, London (BM(NH) 1897.7.5:39, 19.6 mm standard length, small and
decoloured), formerly in ZISP; other syntypes are in the Zoological Institute, St. Petersburg (ZISP) (Eschmeyer et al., 1996).
Nemachilus brandtii gibbusnazus is a subspecies with the author given as Elanidze in Elanidze (1983). The only apparently new taxon in Elanidze (1983) is Nemacheilus (=
Oxynoemacheilus)
angorae alasanicus (q.v.). This taxon is unique in this book as a species without an author name after it. On this basis it was attributed to Elanidze (1983). The date of N. b.
gibbusnazus may also be 1983 but it may have been described in an earlier paper by Elanidze not yet located by me. Both these subspecies are not mentioned in Eschmeyer et al. (1996) nor in the
online version (downloaded 26 August 2007). The distribution of N. b. gibbusnazus is given as "in fact found before in the R. Khrami (1947), then in the R. Kura (1962), in its lower course at
Kukheti, in the upper reaches at Vardziya - Toloshi, in the R. Alazani - in the upper reaches at Alvani", all in Georgia and the drainage of the Kura River.
Placed in the genus Orthrias by Nalbant and Bianco (1998).
Key characters
This species is distinguished from B. angorae by having 3 unbranched and 8 branched dorsal fin rays (as opposed to 2 unbranched and 7-8 (usually 7) branched in B. angorae), longer and
shallower caudal peduncle (caudal peduncle depth 2.2-3.0 in caudal peduncle length instead of less than 2.0 as in B. angorae), and a more forked caudal fin with more pointed lobes. The caudal
peduncle is said to be somewhat shorter and much lower than in B. angorae araxensis (with which species it occurs in the Aras River basin): length of caudal peduncle 18.4-23.4% and depth 8.0-9.4%
of standard length in brandtii, 18.1-21.8% and 10.2-12.7% in araxensis respectively. Other body proportions are given in Banarescu, Nalbant and Balik (1978). The colour pattern is much
darker and the caudal fin margin is almost vertical in B. a. araxensis.
Morphology
Dorsal fin unbranched rays 3-4 and branched rays 7-9, usually 8, anal fin unbranched rays 2-3 and branched rays 5, pectoral fin branched rays 9-12, and pelvic fin branched rays 6-8. Scales minute. A
weakly developed dentiform process on the upper jaw.
Sexual dimorphism
Unknown.
Colour
Typical fish from the Kura-Aras basin lack a stripe on the flank, having brown spots which are either large, more or less triangular and fairly well-defined or broken into many small speckles forming a
reticulate pattern. Dorsal spots are better defined than in B. angorae especially behind the dorsal fin where they fuse completely or incompletely with the lateral flank spots to form bands.
All spots are brown, never blackish as in B. angorae araxensis. There are several rows of speckles on the caudal fin and two rows on the dorsal fin.
Size
Reaches 8.5 cm.
Distribution
Found in the upper and middle Kura and Aras River basins, and presumably in the Iranian reaches of the latter. It is also reported from the Namak Lake basin by Saadati (1977) citing a
manuscript report by V. D. Vladykov, ? confusion with bergianus and from the Arnar Chay, Azarbayjan.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Age at maturity is about 2 years.
Food
Eggs of other fishes may be a food item as well as aquatic insects.
Reproduction
Fecundity reaches 17,409 eggs and egg diameter 0.95 mm.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Endangered in Turkey (Fricke et al., 2007).
Further work
The biology and systematics of this species need study.
Sources
Type material: Syntype of Nemacheilus Brandtii ((BM(NH) 1897.7.5:39),
see above.
Oxynoemacheilus farsicus
(Nalbant and Bianco, 1998)
Common names
None.
Systematics
Originally described in the genus Orthrias. The species is named for Fars Province. The holotype is in the Department of Zoology, Naples University under IZA 7823. It measures 45.8 mm standard
length (51.8 mm standard length when measured by me) and was collected from the "River Kor near Persepolis" in Fars on 30 May 1976 by P. G. Bianco (note that IZA
is the acronym for Dipartimento di Scienze Ambientali, Universita' Degli Studi
Dell'Aquila
L'Aquila, Italy,
where the material was previously stored). Paratypes were all collected by P. G. Bianco and include 64 specimens, 39.0-57.0 mm standard length from the same locality as the holotype (IZA 7824), 61
specimens, 38.5-60.0 mm standard length from the same locality as the holotype but taken on 7 July 1975 (IZA 7825), 7 specimens, 42.0-51.3 mm standard length, same locality as the holotype but taken on 7
July 1975 stored in the Institutul Stiinte Biologice, Bucharest (ISSB 3451), 31 specimens, 43.2-56.3 mm standard length from the River Shur, tributary of the River Mand, near Dasht-e-Arzhan
(Shiraz) 25 May 1976 (IZA 7826), 7 specimens, 47.0-57.0 mm standard length from the latter locality (ISBB 3446), 7 specimens, 47.0-58.0 mm standard length from River Qom, Qom Town 5 May 1975 (IZA 7845)
and 7 specimens, 36.2-47.3 mm standard length from the latter locality (ISBB 3442).
CMNFI material?
Key characters
This fish has long paired fins and a short, deep and compressed caudal peduncle. The flanks and back have roundish brown spots on a marbled background and the head and body are a pale yellow ventrally.
Dorsal and caudal fins have rows of brown spots. The peritoneum is a dull brown. The dorsal fin has 3 unbranched and 8 branched rays, the anal fin 2 unbranched and 5 branched rays, the pectoral fin 2
unbranched and 9-11 branched rays and the pelvic fin 1 unbranched and 6-7 branched rays. It is related to B. brandtii but differs in in the deeper caudal peduncle and longer fins. Other
species outside Iran have differing colour patterns.
Morphology
Dorsal fin branched rays 7-9 (90% with 8 rays in the type description, n=38), anal fin branched rays 4-5 (95% with 5 rays), pectoral fin branched rays 9-11 (69% with 10 rays) and pelvic fin branched
rays 6-7 (65% with 7 rays). The lateral line is straight, on the mid-flank and ends just anterior to the caudal fin base. The cephalic canal system is well-developed laterally. The rear half
of the body is scaled completely and the anterior half has scalation well-developed but not as closely spaced as posterior scales. Scales are oval with a large focus. Esmaeili and Niknejad (2006-2007)
give scanning electron micrographs of the scales along with a description. The head is conical and the eye is small and centrally placed. The nostrils are just anterior to the eyes. The mouth has a
moderate arch with long barbels. Lips and barbels have small tubercles and fine furrows. The upper lip has a median incision and the lower lip lacks mental lobes. The processus dentiformis in the upper
jaw is well-developed but lacks a gap in the lower jaw.
Sexual dimorphism
Males have numerous fine tubercles on the dorsal surface of the pectoral fin rays in bands, and the anterior fn rays are expanded. There are fewer rays and less expansion when progressing proximally.
There is a groove running anteriorly at a slight downward angle from the eye to the antero-ventral corner below the nostril, fading out under the anterior nostril. Near the eye and dorsal to this
groove, fine tubercles form a narrow band. The sides and top of the head are finely tuberculate. The pelvic and dorsal fin rays have tubercles following the rays (not bands as in the pectoral fin). Fine
tubercles line the margin of the scales.
Colour
The dorsal head is speckled brown and there is a dark-brown stripe from the eye to the snout tip, sometimes extending beyond the eye. The back has 7-11, modally 9, rounded dark-brown saddles. Similar
blotches occur on the flank. The dorsal fin has 4 rows of elongate spots, the pectoral fin has up to 6 rows and the caudal fin has 4-5 rows. The anal and pelvic fins are immaculate.
Size
Reaches 67.7 mm standard length.
Distribution
Endemic to Iran and found in the basins of the Namak Lake basin (Qom River at
Qom), Kor River basin (Kor River near Persepolis) and Gulf basin (Shur River
near Dasht-e Arzhan in the Mand River drainage).
Zoogeography
Found in both endorheic and exorheic basins in central and southern Iran.
Habitat
Details are unknown.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 34 fish measuring 3.15-6.77 cm standard length. The a-value was 0.0126 and the b-value 3.084
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Type material: The holotype (IZA 7823) and paratypes (IZA 7824, 7825, 7845) of Orthrias farsicus, see above.
?CMNFI material IZA 7824, 3 as gift, 36.5- , IZA 7826, 3 as gift, 34.0- the measurements are the smallest fish in these samples)
Oxynoemacheilus frenatus
(Heckel, 1843)
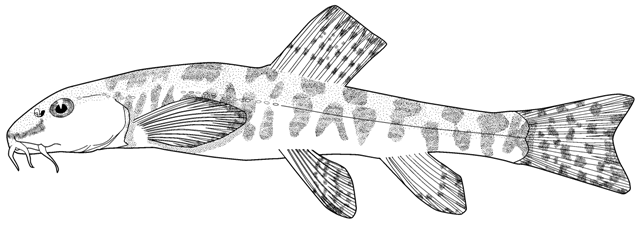
Common names
See genus account.
[lakh or telay (= bowed head according to Heckel (1843b) at Mosul), both in Arabic; banded Tigris loach].
Systematics
The type locality of Cobitis frenata is "Tigris", presumably at Mosul (Heckel, 1843b). Five syntypes are in the Naturhistorisches Museum Wien (NMW 48552) although the catalogue lists
only 4 specimens. A lectotype designated by F. Krupp in 1984 is 70.0 mm standard length, the remaining specimens being small, 27.2-43.4 mm standard length.
Nemacheilus frenatus afrenatus Battalgil, 1942 described from "un petit ruisseau à Diyarbakir" is also from the Tigris River basin but in Turkey. This subspecies lacks the "frein
à la bouche" (presumably the band across the snout) of B. f. frenata and its dorsal fin is higher than long (as measured at the base) while in B. f. frenata it is as high as long.
Bănărescu and Nalbant (1995) place this species in the genus Orthrias. Bănărescu and Nalbant (1966) consider this taxon to be a "doubtful species" but illustrate
it in Bănărescu and Nalbant (1995: Fig. 20).
Key characters
The colour pattern is distinctive and there is no dermal crest or adipose fin behind the dorsal fin.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 7-8, anal fin unbranched rays 2, branched 5, pectoral fin branched rays 10-13,
and pelvic fin branched rays 6-7. Scales are present over the whole body
but not readily visible without magnification. The anterior pectoral fin rays are thickened. Caudal peduncle thick (depth 80-90% of length) according to Saadati (1977). The bulb of the swimbladder
capsule has large ovoid to circular perforations and the anterior and posterior lamina or wings are only moderately developed (Krupp, 1985c). Lips are not strongly plicate and the dentiform process is
well-developed. The gut has a posterior loop.
Meristics for Iranian specimens: dorsal fin branched rays 8(1), anal fin
branched rays 5(1), pectoral fin branched rays 10(1) and pelvic fin branched
rays 6(1) based on lectotype.? add my material
Sexual dimorphism
Bands of tubercles are found on the pectoral fin rays of males, including the first, declining in breadth and extent on the smaller rays. Tubercles are also present on the pelvic and anal fin rays but
are much less well developed. The head is covered in fine tubercles. Flank scales, particularly anterior ones, are lined anteriorly with tubercles. There is an elongate tuberculate swelling anterior to
the lower eye margin on the snout.
Colour
Overall colour is yellowish, mottled with fine but irregular brown or black dots or blotches, some flank blotches being quite large. The rear of the body and the caudal fin in particular are mottled
with brown, tending to form bars. A black band is continuous from the front of one eye, across the snout and round to the other eye. It may be diffuse on the snout or well-defined in fish from the
same locality. The dorsal and caudal fins have thin but irregular bands made up of spots on the rays (up to 3 on the dorsal and 4 on the caudal fin), bands are faintly present on the anal and pelvic fins,
and only a few are visible on the pectoral fins. The dorsal fin has an anterior basal spot at its origin, variably developed in individuals. There are distinct, dark spots at the base of the caudal fin
above and below the body mid-line.
Size
Reaches about 9.2 cm (Heckel, 1843b).
Distribution
Found in the Quwayq and Tigris-Euphrates rivers. Abdoli (2000) records it from the upper Karun, middle and lower Dez, Kashkan and Simarreh rivers in the Tigris River basin of Iran.
Zoogeography
See family account.
Habitat
Known to inhabit both rivers and lakes, the environmental requirements of this species are unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
The biology of this species, its distribution, numbers and habitat requirements are unknown so no comments on conservation can be made. Near threatened in Turkey (Fricke et al., 2007).
Further work
The biology and conservation status of this species need investigation.
Sources
Type material: Syntypes of Cobitis frenata (NMW 48552), see above.
Iranian material: BWC 95-30, mm standard length, .
Comparative material: BM(NH) 1974.2.22:1449-1477, 30, 37.7-55.5 mm standard length, Iraq, branch of Khalis River ();
BM(NH) 1974.2.22:1478-1757, ca. 279, 19.7-53.3 mm standard length, Iraq, Khalis ();
BM(NH) 1974.2.22:1758-1772, 15, 27.2-34.1 mm standard length, Iraq, Khalis ();
BM(NH) 1972.2.22:1773-1776, 4, 28.3-34.0 mm standard length, Iraq, Khalis ();
BM(NH) 1974.2.22:1777-1778, 2, 27.5-31.4 mm standard length, Iraq, Khalis ();.
Oxynoemacheilus kermanshahensis
(Bănărescu and Nalbant, 1966)
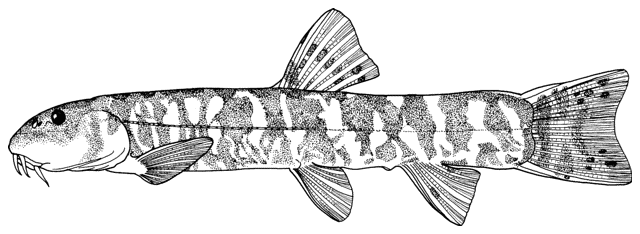
Common names
sagmahi-ye Kermanshah.
[Kermanshah loach].
Systematics
This species was tentatively placed in the subgenus Orthrias Jordan and Fowler, 1903 but is regarded as "aberrant" by Bănărescu and Nalbant (1966).
However, Nalbant and Bianco (1998) later place it in Orthrias.
The holotype of Noemacheilus kermanshahensis (ZMUC P 2787, 46.4 mm standard length) and the 7 paratypes (ZMUC P 2788-94, 25.5-61.8 mm standard length) are stored in
the Zoological Museum of Copenhagen (Nielsen, 1974). The type locality is "Kermanshah in the drainage of the Karun River, a tributary of the lower Euphrates, Western Iran" (Bănărescu
and Nalbant, 1966). The type series was collected on 5 February 1937 by E. Kaiser. The type locality is poorly defined - the city of Kermanshah is on the Qarasu River and lies in the drainage of the
Karkheh River which drains towards the Tigris River. Nalbant and Bianco (1998) correct their original type locality description to the Karkheh River drainage and "Quareh Su, Kermanshah, River
Shimarek", probably referring to the Qareh Su-Simareh drainage of the upper Karkheh River.
Key characters
Bănărescu and Nalbant (1966) consider that this species differs from most South and West-Asiatic loaches by the longitudinal pigment pattern on the flank. Most other species have bars,
vertical patches of pigment. The longitudinal arrangement is found in B. angorae but kermanshahensis has smaller eyes, a shorter lateral line (almost complete in angorae), a
more anteriorly placed vent, and scale shape comprising an almost central focus, vertical sub-oval outline and radii on all fields widely and evenly spaced. Characteristically the caudal peduncle is
short and deep, depth being 92.9-114.3% of length (Saadati, 1977).
Morphology
Dorsal fin with 3 unbranched and 7-8 branched rays, anal fin with 2 unbranched and 5-6 branched rays, and pelvic fin with 6-7 branched rays. Scales only on the posterior part of the body,
well-developed on the caudal peduncle. The lateral line reaches the level of the middle or posterior half of the dorsal fin or over the front half of the anal fin. The origin of the dorsal fin is variable
in relation to the snout tip and caudal fin base (Bănărescu and Nalbant, 1966). The caudal peduncle is short and deep and lacks a crest. The anus is somewhat anterior to the anal fin origin.
Lips are thick and fringed, the lower lip being interrupted in the middle. The intestine is simple with the posterior part straight.
The type series counts are dorsal fin branched rays 7(8), anal fin branched rays 5(8), pectoral fin branched rays 8(3), 9(1), or 10(3), pelvic fin branched rays 6(3) or 7(6); vertebrae 38(6), 39(2) or
40(1).
Sexual dimorphism
Check my fish ? No sexual dimorphism was noted by Bănărescu and Nalbant (1966) as their large specimen is female and others immature.
Colour
The overall colour is yellowish with about 12, dark brown bars on the flank my fish - check this,
see my fish?. A stripe on the mid-line of the back breaks up into spots posteriorly.
The colour pattern in alcohol-preserved specimens dating from 1937 is yellowish with 3 wide, brownish stripes along the flank. The dorsal stripe is continuous anteriorly but breaks up into spots
posteriorly in most specimens. The central, mid-flank stripe is the widest and is continuous although width is variable. The ventral "stripe" (my quotation marks) is absent from smaller
specimens and consists of small and irregular spots. The upper parts of the head have small, irregular brownish spots. The caudal fin has 3 rows of spots and the dorsal fin 2 rows of spots, apparently
concentrated on the fin rays (Bănărescu and Nalbant, 1966).
Size
Reaches 6.3 cm standard length.
Distribution
Known only from Tigris River basin drainages of Iran (see above). Abdoli (2000) reports it from the Marun, upper Karun and lower Khersan, Dez, Simarreh, Qareh Su and Gav Masiab rivers.
Zoogeography
Relationships may lie with B. angorae according to Bănărescu and Nalbant (1966) based on colour pattern and general body shape, or with other species not found in Iran having stripes
along the flank. This seems insufficient evidence on which to base relationships.
Habitat
my collection data ?
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
No measures are being undertaken for this poorly known species.
Further work
Studies should be carried out to determine the numbers of this species, its distribution and its ecological requirements in Iran to ascertain if conservation measures should be taken.
Sources
Type material: The holotype (ZMUC P 2787) and paratypes (ZMUC P 2788-94) of Noemacheilus kermanshahensis, see above.
Iranian material: CMNFI 1979-0285, 20, 32.0-52.6 mm standard length, Kermanshahan, Marek River (34º26'N, 46º37'E)checkID?; CMNFI 1979-0286, 1,
54.2 mm standard length, Kermanshahan, Ravansar River at Ravansar (34º43'N, 46º40'E)checkID?; CMNFI 1993-0128, 1,
? mm standard length, Kermanshahan, Sarab-e Sabz 'Ali Khan (34º25'N, 46º32'E);
BWC 95-17, ? mm standard length, .
? genus(Kessler, 1877)
Reported from the Karakum Canal of Turkmenistan by Shakirova and Sukhanova (1994) and Sal'nikov (1995), this species may eventually reach the Tedzhen River and Caspian Sea basins of Iran. Originally
described in the genus Nemacheilus. No Iranian record.
Oxynoemacheilus persus
(Heckel, 1849)
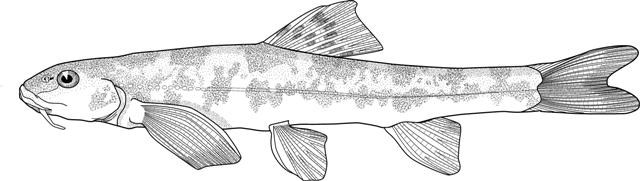
Common names
sagmahi-ye Fars (= Persian loach).
[Persian loach].
Systematics
The type locality for Cobitis Persa is "Quellen um Persepolis" according to Heckel (1846-1849b). Kähsbauer (1964) reports a syntype of this species in the Naturhistorisches Museum Wien
under NMW 48567. It measures 47.6 mm standard length and is probably the holotype as the Vienna catalogue lists only 1 specimen (and the Vienna card catalogue examined in 1997 concurs). This specimen is in
poor condition and not readily comparable to fresh material. It is scaled although scales are not imbricate, the caudal fin is broken off, the body is collapsed so its shape cannot be determined, and it
is decoloured.
Kessler (1877) refers to a Heckel species Nemachilus persicus, presumably this taxon judging from Kessler's page number reference.
Günther (1899) recorded Nemacheilus persa from the Lake Orumiyeh basin ("Zola Chai near Ula") and the upper Aras River basin ("Elinja Chai") but these fish were probably
B. angorae. Berg (1948-1949), Bănărescu and Nalbant (1966) and Nalbant and Bianco (1998) also refer Günther's material to Nemacheilus persa but Abdurakhmanov (1962) suggests that
these fish are Nemacheilus angorae. Saadati (1977) places Lake Orumiyeh fish close to Nemacheilus angorae which makes more sense geographically.
? check my specimens and compare ? The dorsal fin origin is closer to caudal base than the snout tip in the Lake Orumiyeh fish, interorbital width is greater,
the head is longer, the caudal fin less emarginate, and there are more and darker spots on the body (Saadati, 1977). Evidently new material from the Kor River basin wherein lies Persepolis, the type
locality for Cobitis persa, and the Lake Orumiyeh basin are required to resolve this problem - the Lake Orumiyeh sample examined by Bănărescu and Nalbant (1966) comprised only 3 males.
Bănărescu and Nalbant (1966) place this species in their subgenus Oxynoemacheilus but later (Bănărescu and Nalbant (1995); and also Nalbant and Bianco (1998)) place it in
Orthrias.
Key characters
?
Morphology
Dorsal fin with 2-3 unbranched and 8 branched rays, anal fin with 2 unbranched and 5 branched rays, pectoral fin with 8 branched rays, and pelvic fin with 6-7 branched rays. Lateral line almost
complete. Scales are present on the anterior and posterior flank according to Banarescu and Nalbant (1964). Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales along with a
description. The caudal fin is slightly emarginate to forked. The barbels are hair-like thin (Heckel, 1846-1849b).
Meristics for Iranian specimens: dorsal fin branched rays 8(1), anal fin
branched rays 5(1), pectoral fin branched rays 9(1) and pelvic fin branched rays
6(1) based on type ?add my material
Sexual dimorphism
Banarescu and Nalbant (1964) report that pectoral fin rays 2-5 are widened and thickened in males. The appearance of a subocular pad in Bănărescu and Nalbant (1966:Fig. 3) is an error and
was apparently meant to represent a small groove (Bănărescu and Nalbant, 1995).
Colour
The overall colour is yellowish with some brown blotches and 5-7 brown bars on the posterior body and irregular brown blotches on the anterior body. The dorsal and caudal fins are barred.
Size
Reaches 63.2 mm standard length.
Distribution
Found in the Kor River and Lake Maharlu basins (Esmaeili et al.,
2011?) and, according to Bănărescu and Nalbant (1966), also in the Lake Orumiyeh basin and probably the whole of western and central Iran. Abdoli (2000) maps the Kor
and Pulvar rivers.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 32 fish measuring 2.94-6.32 cm standard length. The a-value was 0.0211 and the b-value 2.784
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
?
Further work
?
Sources
Type material: The holotype of Cobitis persa (NMW 48567), see above.
Iranian material: CMNFI 1979-0019, 2, 28.9-33.7 mm standard length, Fars, Barm-e Baba Hajji (29º23'N, 52º40'E);
CMNFI 2004-0004, ? mm standard length.
Genus Paracobitis
Bleeker, 1863
This genus
comprises about 17, comparatively large species, some over 28 cm, in western
Asia. Six species are reported from Iran.
The body
is elongate, thick anteriorly and posteriorly compressed. The head is strongly
depressed. Nostrils are closely spaced or weakly separated. The caudal peduncle is long, low and bears an elongate crest dorsally
from the dorsal fin to the caudal fin and, often, a more or less well-developed
ventral adipose crest. However, the crest is not supported by the procurrent
rays of the caudal fin unlike in Metaschistura. The caudal fin is
slightly emarginate or truncate, rarely rounded. There is a well-developed
pelvic axillary lobe reaching beyond the vertical of the posterior edge of the
pelvic fin base. Scales are present or absent anteriorly and the lateral line is complete.
The processus dentiformis is usually strongly developed (weak to absent in P.
vignai - ?check) but its notch on the
lower jaw is reduced. Lips are smooth or grooved. The gut is short with a single
loop. The colour pattern is variable comprising bars or irregular spotting.
There is no intense black spot at the base of the anterior dorsal fin rays.
Males and females show no external differences except males have more muscular
cheeks. Prokofiev (2009) gives further osteological details of this genus
including cst having a bony roof, the fork of cst and cio
is fused to the skull in adults (the
cst is the supratemporal commissure running across the top of the head at
the rear and cio is the temporal part of the infraorbital head canal),
the free part of the swimbladder is reduced or absent, right and left lobes of
the bony capsule are fully divided by the manubrium, and the fifth trunk vertebra
does not take part in support of the capsule. There are 4 radials in the
pectoral fin skeleton and the caudal fin support has 6 hypurals and an epural is
present. There is no preethmoid I bone in
the skull.
Paracobitis boutanensis
(McClelland, 1842)
Probably described from the neighbourhood of the Bolan Pass, Helmand River drainage of Afghanistan in the Sistan basin according to Hora (1929) and Bănărescu and Nalbant (1966). No Iranian
record.
Paracobitis cyri
(Berg, 1910)
Nemacheilus tigris cyri Berg, 1910 is described from "Fontes fl. Cyrus, Caucasus". This species is placed in the subgenus or genus
Paracobitis by Banarescu (1968) and Prokofiev (2009) while Fricke et al. (2007) recognise the subspecies as a distinct species in
Orthrias. Six syntypes of N. tigris cyri (31.4-51.5 mm standard length) are in the Zoological Institute, St. Petersburg
(ZISP 13291) from "Okam village Gel'skaya Plain, Karka Oblast", K. Satunin, 6.IX.1901. This is in the upper Kura River basin of Turkey. No Iranian record.
Paracobitis ghazniensis
(Bănărescu and Nalbant, 1966)
Described from "Ghazni, on the Ghazni River, tributary of the Ab-i-Istadah Lake, Helmand drainage; East Afghanistan" (Bănărescu and Nalbant, 1966). Ghazni is at 33°33'N, 68°26'E in
the Sistan basin. No Iranian record.
Paracobitis iranica
Nalbant and Bianco, 1998
Common names
sagmahi-ye irani.
[Iranian loach].
Systematics
The species is named for Iran. The holotype is 79.8 mm standard length (66.0 mm standard length when measured by me) and is from "River Qom near the town of Qom" collected on 6 May 1976 by
P. G. Bianco and stored in the Department of Zoology, University of Naples (IZA 7831). The locality in the jar is "Qom River near Qom (a little salt river 3.5 p.p.t.) near Qom at the bridge,
950 m, 5 June 1976", the date being at variance with the published date. Paratypes number 5 specimens, 59.0-92.0 mm standard length (IZA 7832)(only two present in 2002 visit by me), and 4 specimens,
47.0-72.0 mm standard length (Institutul Stiinte Biologice, Bucharest, ISBB uncatologued). It may be related to P. malapterura and P. longicauda
but differs in colour and the larger caudal peduncle keel.
Key characters
The body is elongate with a compressed posterior region and a depressed head. The caudal peduncle is elongate and has a long dorsal adipose crest. The body is scaled. The overall colour is
yellowish-white with a row of dark grey spots on the mid-back and on mid-flank. Spots extend onto the adipose crest. A row of dark irregular spots is found on the posterior part of the ventral region.
The holotype has a dorsal fin with 3 unbranched and 7 branched rays, an anal fin with 2 unbranched and 5 branched rays, a pectoral fin with 2 unbranched and 9 branched rays and a pelvic fin with
1 unbranched and 5 branched rays.
Morphology
The dorsal fin has 7-8 branched rays (90% with 7 rays, n=9), the anal fin has 5 branched rays in all specimens examined in the type description, the pectoral fin has 9 branched rays in all specimens
and the pelvic fin has 6-7 branched rays (78% with 7 rays, n=9). The lateral line is straight and extends to the caudal fin base. The body is minutely scaled with a relatively eccentric and
large focal zone. The head is conical with eyes in the anterior half. The nostrils are just in front of the eyes. The mouth has a strong arch with short barbels and well-furrowed lips interrupted in the
middle. The processus dentiformis is well-developed. The stomach is syphonal and the intestine straight. The gas bladder capsule has a relatively short duct.
Sexual dimorphism
Unknown.
Colour
The head is covered by dark grey dots and all fins have small grey dots. See
also Key characters.
Size
Attains 92.0 mm standard length.
Distribution
Known only from the Qom River near Qom in the Namak Lake basin, an Iranian endemic.
Zoogeography
This species and similar ones are widely distributed in Iran, although poorly collected, and their systematics and zoogeographical relationships are unknown.
Habitat
Details are unknown. The type series was collected in a small salt river (3.5‰).
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Type material: The holotype (IZA 7831) and paratypes (IZA 7832) of Paracobitis iranica, see above.
Paracobitis longicauda
(Kessler, 1872)
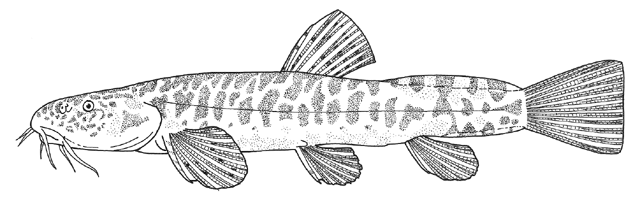

Common names
sagmahi-ye kakoldar-e sharqi (= eastern crested loach).
[vostochnyi grebenchatyi golets or eastern crested loach in Russian].
Systematics
May be a subspecies or synonym of P. malapterura. Has been placed in the genus Adiposia Annandale and Hora, 1920. Banarescu and Nalbant (1964) restrict P. longicauda to basins
in Central Asia (e.g. the Amu Darya) and not Iran. Cobitis longicauda was originally described from the Ak-Darya in the Zeravshan River basin of Uzbekistan and the holotype is in the
Zoological Institute, St. Petersburg under ZISP 2686 (Eschmeyer et al., 1996).
Bănărescu and Nalbant (1995) place this species in Paracobitis.
Key characters
This species is distinguished from P. malapterura by distinct scales and larger size.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 7, anal fin unbranched rays 2-3, branched rays 5, pectoral fin branched rays 9-10,and pelvic fin branched rays 7. Caudal fin truncate. Large fish are
scaled on the flanks, dorsal crest and belly, scales being visible to the naked eye in contrast to the few scales needing a microscope to be visible in P. malapterura and in P. rhadinaea.
Caudal peduncle long, about 4.5-4.6 in standard length.
Sexual dimorphism
Colour
Colour is very similar to P. malapterura with spots sometimes loop-shaped and sometimes rounded.
Size
Reaches 20.0 cm.
Distribution
Found in the Tedzhen and Murgab rivers of Afghanistan and Turkmenistan and in the Aral Sea basin, and in the Tedzhen
(= Hari) River basin of Iran.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
This species is too poorly known in terms of biology and numbers for an effective conservation assessment.
Further work
The biology of this species in Iran needs study.
Sources
Comparative material: ZISP 2686, 1, 140.8 mm standard length, ?, Samarkand ();
ZISP 4482, 1, 145.7 mm standard length, Zeravshan (?);
ZISP 4483, 4, 107.2- 125.8 mm standard length, ?, Zeravshan ();
ZISP 4484, 8, 62.2-96.7 mm standard length, ?, Zeravshan ();
ZISP 10362, 4, 31.8-112.1 mm standard length, ?, Tedzhen ();
ZISP 10963, 1, 93.7 mm standard length, ?, Samarkand ();
ZISP 13300-13301, 3, 93.7-157.6 mm standard length, Pul-e Khatun (?);
ZISP 14510, 3, 119.4-173.4 mm standard length, ?, Samarkand ();
ZISP 33105, 9, 30.4-98.6 mm standard length, ?, Ak-Darya ().
Paracobitis malapterura
(Valenciennes, 1846)
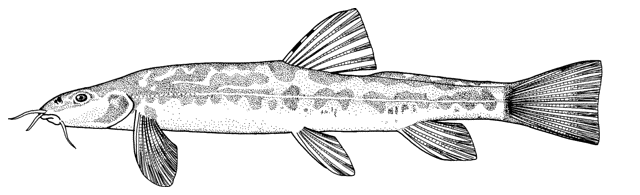
Common names
sagmahi-ye kakoldar-e gharbi (= western crested loach), sagmahi-ye juibari or sagmahi-e-jooibari.
[lakh in Arabic; zapadnyi grebenchatyi golets or western crested loach in Russian].
Systematics
The type locality of Cobitis malapterura is given as "Syrie" in Cuvier and Valenciennes (1846) but has not been found there since (Berg, 1948-1949); it does occur in the
Tigris-Euphrates basin however (Coad, 1991b). The specimen was collected by Aucher-Éloy who visited Iran and the specimen may in fact be from there. It is possible that the type locality is in the
Caspian Sea basin of Iran although Banarescu and Nalbant (1964) give the Tigris-Euphrates basin which extends though Syria for the distribution of P. malapterura malapterura.
Bănărescu and Nalbant (1995) places this species Paracobitis.
Cobitis longicauda Kessler, 1872 is possibly a synonym. Banarescu and Nalbant (1964) restrict P. longicauda (q.v.) to basins in Central Asia (e.g. the Amu Darya) and not
Iran. Nemacheilus macmahoni Chaudhuri, 1909 has been advanced as a synonym or subspecies (Nikol'skii, 1947; Berg, 1948-1949; 1949; Banarescu and Nalbant, 1964) but see the review in
Bănărescu and Nalbant (1966) and below under P. rhadinaea. Banarescu and Nalbant (1964) consider fish from Sistan and the Caspian Sea basin of Iran to be P. malapterura macmahoni.
Filippi's (1865) record of Cobitis merga (Krynicki, 1840) from "fiumicelli di Sartschem e di Sainkalé" was P. malapterura; these localities being in the Safid River basin near
the falling of the Zanjan River into the Qezel Owzan of Safid Rud (Berg, 1948-1949) presumably at Sarcham-e Sofla (37°07'N, 47°54'E) and possibly Sa'in Qaleh (36°18'N, 49°04'E) in the Namak Lake basin.
Two syntypes are in the Muséum national d'Histoire naturelle, Paris under MNHN 3962 and B.3070 (formerly MNHN 3962) (Eschmeyer et al., 1996) and measure 125-145 mm total length (Bertin and
Estève, 1948).
Key characters
The colour pattern is distinctive and there is a well-developed dermal crest or adipose fin behind the dorsal fin to the caudal fin base.
Morphology
Dorsal fin with 2-3 unbranched and 6-8 branched rays, anal fin with 2-3 unbranched and 5 branched rays, pectoral fin branched rays 10-12 (Nikol'skii (1947) gives 7-10) and pelvic fin branched rays 5-7.
Caudal fin slightly emarginate. There is a well-developed dermal crest or adipose fin behind the dorsal fin to the caudal fin base. Scales are scattered on the posterior body in large fish but need
magnification to be seen. The lateral line extends almost to the caudal fin. The dentiform process of the upper jaw is well-developed and fits in a lower jaw groove. The lips, especially the lower one,
are strongly plicate. The eyes are small and widely spaced. Caudal peduncle short, 5.6-6.3 times in standard length. The gut is straight posteriorly.
Sexual dimorphism
The cheeks are distended in some fish and this is believed to be a sexual character.
Colour
The top and sides of the body and head are mottled or reticulated with grey and some yellowish pigment. The reticulations may be very fine, giving a more mottled appearance. The belly and lower head
surface are white. The flank reticulations extend onto the caudal peduncle crest. When touching the dorsal margin of the crest, the reticulations make the crest there dark, otherwise the crest is a light
creamy colour along the margin and, in some fish without reticulations reaching the margin, the whole edge is light. The lateral line is white, sometimes in marked contrast to the rest of the flank. The
dorsal fin has darkly pigmented rays, sometimes broken into series of spots. The caudal fin has a series of 4-5 small spots elongated along the rays, the middle series being the blackest. The dorsal margin
of the caudal fin may have 2-4 isolated spots. The pectoral fin has dark pigment along the rays or 2-3 series of small spots. The pelvic and anal fins have 1-2 series of grey spots and the pelvic fin may
have only 1-2 spots. Pelvic and anal fins may be immaculate. At the front or along the base of the anal fin there is a broad pigmented band. There may be a dark, zig-zag bar at the caudal base, merging
dorsally and/or ventrally with flank botches. The barbel bases are all darkly pigmented especially the second pair. The iris is silvery. Young fish have a less reticulated pattern with more
blotches on the flank.
Size
Reaches 15.0 cm.
Distribution
This species is found in the Caspian Sea basin of Iran in rivers from the Safid to the Atrak including the
Qezel Owzan, Sardab, Haraz, Ramian, Neka, Chalus, Tonekabon, Shahrud, Babol, Tajan, Karasu,
Madar Su, Gorgan
rivers, Sarchem near the Zanjan-Safid river junction, Bandar Gaz in Gorgan Bay (Laptev, 1934; Nikol'skii, 1947; Kiabi
et al., 1994; 1999; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009; A.
Abdoli, pers. comm.). Material from the Kor River and
Esfahan basins may be this species.
Need to be checked and re-mapped?
Zoogeography
See family account.
Habitat
This species appears to favour deeper water and stronger current than other sympatric loaches.
Age and growth
Tabiee and Abdoli (2005) found a sex ratio of 4:1 (male:female) in the Zarringol River of Golestan Province. Average total length was 59.07 mm for males and 82.42 mm for females. Condition factor was
2.6 for females and 1.97 for males. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 48 Iranian fish measuring 2.67-7.43 cm standard length. The a-value was 0.0126
and the b-value 2.628 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length
increases). Another study in the Zarringol River by Patimar et al. (2009)
found maximum ages of 3+ years for males and 4+ years for females, length-weight
relationship was W = 0.020L2.62 for males, 0.002L3.81 for
females and 0 .008L3.08 for the population, growth was positively
allometric, and sex ratio was 1:1.27 in favour of females (cf. Tabiee and Abdoli,
2005). The difference with Tabiee and Abdoli (2005) who found negative
allometric growth was attributed to environmental conditions changing between
years of sampling.
Food
The Zarringol fish were carnivorous with chironomids making up 65.3% of the diet
(Tabiee and Abdoli, 2005) or had a diet dominated by Plecoptera (72.4% and
Trichoptera (20.8%) (Patimar et al., 2009).
Reproduction
The Zarringol River examined by Patimar et al. (2009) reproduced in
April-June with highest GSI in May. Spawning was prolonged, over about 2 months.
Absolute fecundity reached 1180 eggs with a maximum diameter of 2.8 mm.
Parasites and predators
A Diplostomum species is recorded from the muscles of this nemacheilid in the Shirud of Mazandaran (Alghmandi and Dalimi Asi, 2000).
Economic importance
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include medium in numbers, habitat destruction, widespread
range (75% of water bodies), present in other water bodies in Iran, and present outside the Caspian Sea basin.
Conservation
Little is known of the biology and population numbers so no conservation assessment can be made.
Further work
The systematics of this species and its relatives need further study.
Sources
?check for newer species from Bianco and Nalbant
Iranian material: CMNFI 1977-0510A, 2, 74.2-81.5 mm standard length, ?;
CMNFI 1979-0252, 1, 53.7 mm standard length, Markazi, jube at Baqerabad (34º55'N, 50º50'E);
CMNFI 1979-0253, 1, 58.9 mm standard length, Markazi, Qareh Chay west of Baqerabad (34º52'N, 50º49'E);
CMNFI 1979-0481, 1, 77.9 mm standard length, Mazandaran, stream 3 km west of Ghalahleekesh (37º18'30"N, 55º31'E);
CMNFI 1979-0486, 18, 12.7-63.2 mm standard length, Mazandaran, stream in Atrak River drainage (37º44'N, 56º18'E);
CMNFI 1979-0489, 2, 27.4-45.3 mm standard length, Mazandaran, stream in Atrak River drainage (37º50'N, 55º53'E);
CMNFI 1980-0154, 8, 45.8-80.8 mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1980-0156, 24, 28.4-63.6 mm standard length, Markazi, Karaj River near village (35º47'N, 50º58'E);
CMNFI 1980-0160, 3, 35.4-40.0 m standard length, ?
CMNFI 1991-0157, 2, 70.4-73.0 mm standard length, Mazandaran, Ramian River (36º58'N, 55º07'E);
CMNFI 1993-0145, 2, ? mm standard length, Mazandaran, Qareh Su (no other locality data);
CMNFI 1993-0155, 2, 53.4-58.7 mm standard length, Markazi, Sharra River near Khosbijan (34º07'N, 49º23'E);
CMNFI 2007-0121, 5, 45.3-98.3 mm standard length, Hamadan, Qareh Su basin north of Razan (ca. 35º25'N, ca. 49º02'E);
USNM 205921-22, 6, 25.9-34.6 mm standard length, Markazi, Baragon River (ca. 36º00'N, ca. 50º50'E);
BWC 1995-0009, 2, 83.4-83.9 mm standard length, Gorgan River from Kiabi?
Comparative material: ZISP 25788, 1, 62.2 mm standard length, ?, Sumbar River at Aidere (?).
Paracobitis rhadinaea
(Regan, 1906)
Common names
See genus account. Fowler and Steinitz (1956) refer to a fish from Sistan known locally as mahrmahé (sic, presumably mar mahi, snake fish) and this may refer to this species which has an
elongate body.
Systematics
Bănărescu and Nalbant (1995) and Nalbant and Bianco (1998) place this species in Paracobitis.
Nemacheilus macmahoni Chaudhuri, 1909 described from the "affluents (= delta, an error for effluents) of the Helmand" is a synonym according to Bănărescu and Nalbant (1966)
who refute the opinions of Nikol'skii (1947) and Berg (1948-1949; 1949) who consider macmahoni to be identical to P. malapterura. Earlier Banarescu and Nalbant (1964) placed fish from Sistan
and the Caspian Sea basin of Iran as Nemacheilus malapterurus macmahoni. P. malapterura has both lips strongly furrowed, pelvic fin origin under the dorsal fin origin rather than behind,
better developed scales which are also present on mid-flank, and a colour pattern of numerous oblique bands.
P. rhadinaea is distinguished from macmahoni by Annandale and Hora (1920) in having an extremely short posterior diverticulum and minute vesicle in the swimbladder, by the absence of
scales, a more elongate body, smaller, narrower and less flattened head, and by differences in the profile of the body.
A syntype of Nemacheilus rhadinæus (ZSI F1240/1) is in the Zoological Survey of India, Calcutta under the name Adiposia rhadinaea and the holotype of Nemacheilus macmahoni (ZSI
F1222/1) is also there under the name Adiposia macmahoni (Menon and Yazdani, 1968). Two syntypes listed as Nemacheilus rhadinaeus from "Sistan" are in the Natural History Museum,
London (BM(NH) 1905.11.29:28-29, 2, 137.8-209.1 mm standard length).
A specimen in the Zoological Institute, St. Petersburg (ZISP 24413, 76.5 mm standard length) is from "Helmand delta, northwest of Jalalabad, Seistan, XI 1918, Indian Mus. (Dr. Hora)"
according to Berg (1949) and could be a syntype of Nemacheilus macmahoni (Eschmeyer's "Catalog of Fishes", downloaded 20 May 2008). However, this taxon was described from a single
specimen ? . The jar bears a label on the outside reading "N. malapterurus XI 1918 Indian Museum S. L. Hora Delta of Helmand near Jalabad" and another label reads "Adiposia
macmahoni Randa stream 4 mls N.W. of Jalabad Seistan. N. Annandale coll."
Adiposia Annandale and Hora, 1920 (type species Nemachilus macmahoni Chaudhuri, 1909, and including Nemacheilus longicaudus and N. rhadinaeus according to Annandale and Hora
(1920)) is a synonym of Paracobitis.
Key characters
?
Morphology
macmahoni:- ? check The head is broad and flattened and the small eyes are dorsal. The dorsal profile is slightly convex behind the head but the dorsal and ventral profiles soon become straight
and nearly parallel, or thicker in front and tapering behind the dorsal fin. Body depth 5.8 in total length. Caudal fin rounded. Oval scales are found on the posterior part of the body in adults (not in
rhadinaeus in Regan (1906) and Annandale and Hora (1920)) or scales very small and deciduous all over the body. Nostrils are nearer to the eye than the snout tip. The two anterior pairs of barbels
reach back to the nostril level and the posterior pair to the anterior or to middle or to the rear of the eye. The upper lip is minutely tubercular and the lower lip is widely interrupted in the middle.
Scale radii are few and widely spaced, on all fields. Material identified originally as macmahoni has the pelvic origin behind that of the dorsal fin origin level, the caudal fin is slightly
emarginate, the dorsal fin edge is straight, the anus is some distance in front of the anal fin, a well-developed adipose dorsal ridge runs from the dorsal fin to the caudal fin base, the lateral line is
almost complete, scales are restricted to the last third of the body, being small, rounded and far apart, lips are almost smooth, and the intestine has a single loop posteriorly. The
description of rhadinaeus is short. The snout is longer than the postorbital distance, body depth is 7-10 times in body length, head length 5.0-5.5 times in body length, the mouth cleft extends to
below the nostrils, lower lip interrupted medially, outer rostral barbel as long as maxillary barbel reaching back to or beyond nostrils, no scales, dorsal fin origin nearer tip of snout than caudal
fin base, caudal fin slightly emarginate, caudal peduncle 2.0-2.75 as long as deep, 5.0-5.3 in length of fish.
Dorsal fin with 2-3 unbranched and 7 branched rays. Anal fin with 2-3 unbranched and 5 branched rays. Pectoral fin branched rays 10 and pelvic fin branched rays 6-8. Scales are highly deciduous and not
always present on old preserved material. The dorsal fin rounded. There is a well-developed post-dorsal fin crest and a slight ventral crest on the caudal peduncle. The pelvic fin origin lies just in front
of the mid-point of the dorsal fin base. There is an adipose tissue flap at the pelvic fin base. The anterior nostril is a tube followed immediately by a horizontal slit. The bony upper jaw has a slight
protuberance and the lower jaw is curved and not indented.
Meristic values for Iranian specimens including types and macmahoni are:- dorsal fin branched rays 7(33) or 8(1), anal fin branched rays 5(34), pectoral fin branched rays 9(2) or 10(32) and pelvic
fin branched rays 6(2) or 7(32).
Sexual dimorphism
None reported, the pectoral fin being identical in both sexes in material identified as macmahoni (Bănărescu and Nalbant, 1966).
Colour
Living fish identified as macmahoni are pale olivaceous fading to silvery-white on the belly. The head and upper part of the body are irregularly spotted and darker. Some fish are pale yellowish
without markings or with faint marbling. All fins are tinged a dull red, most evidently on the caudal fin, and are obscurely marked with small dark spots. There is a dark band at the caudal base. Preserved
material identified originally as macmahoni is whitish with 9-21 irregular, brownish spots along the flank, other spots are present dorsally and smaller ones between the dorsal and lateral rows.
There are 3 rows of minute spots on the dorsal and caudal fins and 2 rows on the pectoral fin (Bănărescu and Nalbant, 1966). Types of macmahoni are brown all over,
darker dorsally, barbels a lighter brown, dorsal and caudal fins with darker bands, pectoral also slightly banded but anal and caudal uniform light brown.
P. rhadinaea has large oblong or rounded
dark spots on the back and sides, dorsal and caudal fins with small spots and red tinged, lower fins pale and immaculate although pectoral, and to a lesser extent pelvic, fins may be red tinged.
Size
Attains 28.8 cm as macmahoni.
Distribution
This species is probably restricted to the Sistan basin of Iran and presumably Afghanistan. Bănărescu and Nalbant (1966) place this species in the Atrak and Safid rivers of the Caspian Sea
basin, the Abkhar River of central Iran, probably most of Iran, the Helmand drainage and the Tedzhen River, evidently confusing it with P. malapterura and P. iranica. Abdoli
(2000) lists as questionably from the Bejestan, Kerman-Na'in and Dasht-e Lut basins, from the middle and lower Halil and Bampur rivers of the Hamun-e Jaz Murian basin, and from the Simish and the river to its north in
the Mashkid River basin.
Zoogeography
The closest relatives of this species are P. malapterura and P. longicauda (q.v.) in Iran (Bănărescu and Nalbant, 1966).
Habitat
Annandale and Hora (1920) note that Adiposia macmahoni was healthy buried some inches in mud when cyprinids died in foul water above.
Age and growth
Unknown.
Food
Stomach contents include cyprinid fish remains and mayfly larvae (Annandale, 1921).
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus rhadinæus (BM(NH) 1909.11.29:28-29), see above.
Iranian material: CMNFI 1979-0222, 11, 16.8-28.8 mm standard length, Sistan, jube 2 km south of Lutak (30º46'30"N, 61º24'E);
CMNFI 1979-0223, 1, 23.9 mm standard length, Sistan, ditch 1 km south of Lutak (30º45'N, 61º24'E);
CMNFI 1979-0228, 3, 55.1-119.8 mm standard length, Sistan, ditch 1 km from Zabol (31º02'30"N, 61º31'E);
CMNFI 1979-0229, 3, 87.4-115.5 mm standard length, Sistan, ditch 5 km from Zabol (31º03'N, 61º33'E);
CMNFI 1979-0231, 1, 22.6 mm standard length, Sistan, jube 3 km from Zabol (31º01'N, 61º32'E);
CMNFI 1979-0232, 2, 82.5-95.6 mm standard length, Sistan, jube 11 km from Zabol (ca. 30º58'30"N, ca. 61º36'E);
CMNFI 1979-0233, 1, 68.7 mm standard length, Sistan, ditch at Deh Vazi (ca. 30º57'N, ca. 61º38'E);
CMNFI 1979-0234, 1, 85.5 mm standard length, Sistan, effluent of Hirmand River near Zahak (30º54'N, 61º40'E);
CMNFI 1979-0237, 2, 17.5-25.2 mm standard length, Sistan, ditch 18 km south of Zabol (30º53'N, 61º27'30"E);
CMNFI 1979-0238, 2, 23.6-27.7 mm standard length, Sistan, ditch 11 km south of Zabol (30º57'N, 61º27'30"E);
BM(NH) 1920.1.20:31-34, 4, 79.6-109.5 mm standard length, Sistan, northwest of Jalalabad (no other locality data);
ZISP 24413, 1, 76.5 mm standard length, Sistan, Randa stream 4 miles northwest of Jalalabad (?).
Paracobitis smithi
(Greenwood, 1976)
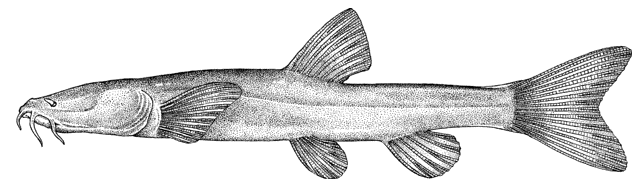
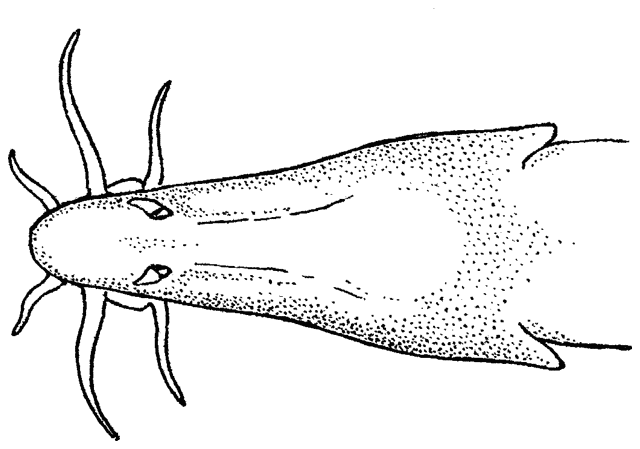
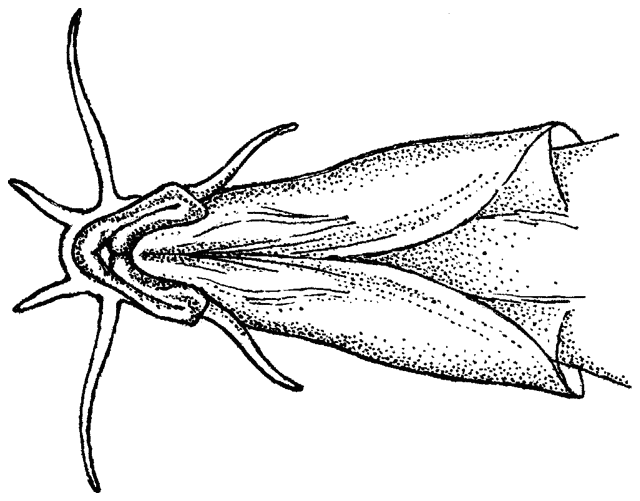
Dorsal view of head
Ventral view of head



Photographs courtesy of Amir Hosin Zalaghi, 11-19 May 2010
Common names
sagmahi-ye gharzi (= cave loach), mahi kurghar (= cave fish).
[blind loach].
Systematics
This species is named for Anthony Smith who collected the holotype. Accounts of Smith's searches for cave fishes in Iran are given in his two books (Smith, 1953; 1979). The holotype is in the Natural
History Museum, London under BM(NH) 1976.6.28:1, was collected in April 1976, is apparently an immature male and is 35.5 mm standard length. Locality data is given below.
Greenwood (1976) placed this species in the catchall genus Noemacheilus (correctly
spelled Nemacheilus) pending an adequate revision of the subfamily Nemacheilinae and since little purpose would
be served by erecting a new genus for a fish with such features as eyelessness and depigmentation found in common with other unrelated cave-dwelling fishes. Greenwood (1976) considers its relationship to
lie with species in the subgenus or genus Paracobitis as does Nalbant and Bianco (1998).
Key characters
The only eyeless, depigmented nemacheilid reported from Iran.
Morphology
Dorsal fin with 3 unbranched and 7 branched rays, anal fin with 3 unbranched and 5 branched rays, pectoral fin with 10 branched rays and pelvic fin with 5-6 branched rays. In specimens seen by me
dorsal fin with 7(5) branched rays, anal fin with 5(5) branched rays, pectoral fin with 10(5) branched rays and pelvic fin with 5(5) branched rays. The caudal fin is unusual in having only 14(5) branched
rays. Vertebrae 37-38. check on my fish? The barbels are short with the middle pair the longest. There are no scales. The lateral line is interrupted irregularly and there are more pores on the posterior
half of the body. A long adipose fin is present dorsally, most obvious in young, and a weaker ventral ridge is present. The mouth is subterminal, lips are weakly to moderately papillose, and there is a
horny ridge on the dentaries and on the upper jaw. The middle pair of barbels is the longest. The internarial flap is long. Gill membranes are broadly attached to the isthmus. The gut is short with a
single transverse loop.
Sexual dimorphism
Unknown.
Colour
A dead white with the red of blood visible as a pale pink cast and a deeper red at the gills visible through the gill cover. Small, straw-coloured fat globules are visible under the skin of formalin
preserved fish over the whole head and body with concentrations in the orbits and the fin bases, particularly the dorsal and anal bases. The viscera are visible through the body wall. There is no
peritoneal pigment.
Size
Attains 64.5 mm total length.
Distribution
Found only at "Kaaje-ru" above the garden "Bagh-e Loveh", "Lowa" or "Levan" (probably Loven at 33°04'N, 48°37'E) which is about 4 km from kilometre 382 on the
railway from Bandar Shapur and approximately 12 km north of the railway station Tang-e Haft. The stream below the cave locality is the "Ab-e Serum" which runs into the "Ab-e Zezar"
which is a tributary of the Dez River. The locality is at 33°04'38.6"N, 48°35'33.1"E in Lorestan Province. Further locality details are given in Bruun and Kaiser (1948) and under the cyprinid
Iranocypris typhlops.
Zoogeography
Endemic to Iran, its relationships to other species are unknown. It shares the cave habitat with another eyeless species, Iranocypris typhlops, a member of the family Cyprinidae. This blind cave
species is placed in a world-wide context by Proudlove (1997a; 1997b).
Habitat
Known only from a well-like but natural outlet of a subterranean
system. The outlet overflows to form a small stream from January to
May (Smith, 1979) during the snow-melt period in the Zagros Mountains
but in April to June this flow ceases (the precise timing of flow and
its cessation is estimated from villager's comments and scientific
visits and also varies with precipitation). The pictures above show flowing
water in May 2010. The well area is about 5
by 3 m and gradually decreases as the year progresses. Divers
descended to a depth of 60 feet (= 18.3 m) in 1977 in the "well" until the
resurgence narrowed (Farr, 1977). A rope was let down by R. Mehrani (pers. comm.,
2000) and reached 23 m before the rope ran out and yet it was not at
the bottom. Smith (1979) reports divers descending to 60-70 feet (18.3- 21.3 m). The
pool shelves deeply under the cliff rearwards but the whole pool
surface is exposed to light. There is no vegetation in the pool except
for some encrusting algae on the rocky sides. The shale fragments
forming the outermost floor of the pool have a thin layer of mud on
them which may contain algae.
It seems probable that a complex of flooded but narrow and
inaccessible passages is the habitat of this species and the well is
merely the surface manifestation of this complex (Bruun and Kaiser,
1948; Smith, 1978; Banister, 1992). There is a smaller pool (about 2 m
across narrowing rapidly inside) and flowing exit stream lower down
the gorge, about 50 m away from the main locality, where an Iranocypris
typhlops
was seen but not caught in December 2000 (Smith (1979) also
tentatively reports sighting a fish here). This is assumed to be
evidence of the interconnectivity of subterranean passages. The main
pool was not flowing at this time. The stream from the smaller pool
increases in flow downstream, possibly tapping more groundwater, and
eventually has a moderate flow. No fish were seen in it. The stream
falls over a high waterfall (estimated at 10-15 m high by Smith (1979)
which seems about right) so the well localities are isolated from the
local fishes in the main river. The main river houses Garra rufa
and Nemacheilus species s.l.. The stream shows evidence of recent higher
flow which tends to confirm overflow from the main well.
Sampling in December 2000 recorded a water temperature of 18.5°C, pH 7.5 and
a conductivity of 334 µS. More photographs of the habitat can be seen under the
account of Iranocypris typhlops (Cyprinidae).
A captive specimen attempted to climb out of a glass tank, almost its whole body being out of the water before it slid back. It did not hide from or
react to light and spent most of the time resting on the tank bottom, moving along the bottom or more often swimming actively around the tank (Greenwood, 1976).
Amir Hosin Zalaghi reports that this species swims faster than Iranocypis
tyhplops (pers. comm., 10 August 2010).


Photographs courtesy of Amir Hosin Zalaghi, 11-19 May 2010
Age and growth
Unknown.
Food
Unknown but a captive specimen was fed on mosquito larvae.
Reproduction
Unknown.
Parasites and predators
Unknown but there are probably no predators in the cave environment.
Economic importance
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use in textbooks.
Conservation
A fine of 10,000 rials is imposed specifically for illegal fishing of this species (Anonymous, 1977-1978), more recently 100,000 rials (N. Najfpour pers. comm., 2008). It is on the IUCN 1994 Red List
of Threatened Animals as one of two rare fish species from Iran (see also Iranocypris typhlops) and is on the 2000 IUCN Red List as VU D2 (Vulnerable, acute restriction in its area of occupancy,
also on subsequent Red Lists; see also Proudlove (2001)). Coad (2000a), using 18 criteria, found this species to be one of the top 4 threatened species of freshwater fishes in Iran.
B. Sandford (in litt., 1979) considered this fish to be endangered. The cave appeared to be a recently collapsed system and the network of fissures could be quite small. Coupled with recent
collecting the number of extant specimens may be quite low.
This species is much rarer than the co-occurring Iranocypris typhlops, by at least an order of magnitude. Including the holotype, about 14 specimens are known to have been collected
(see below; plus 2 in Muze-ye Melli-ye Tarikh-e Tabi'i, Tehran (MMTT 1227-1228) and 6 collected by students of A. Abdoli, Shahid Beheshti University, Tehran in 2001).
Further work
?
Sources
Type material: Holotype of Noemacheilus smithi (BM(NH) 1976.6.28:1), see above.
Iranian material: CMNFI 2007-0123, 5, 24.1-52.9 mm standard length, type locality, 28 January 1977.
Further information on the habitat is in the account of Iranocypris typhlops.
Paracobitis vignai
Nalbant and Bianco, 1998
Common names
sagmahi-ye Sistan.
[Sistan loach].
Systematics
The species is named for Professor Augusto Vigna Taglianti, La Sapienza University, Rome. The holotype is 89.0 mm standard length (86.5 mm standard length when measured by me) collected from
"Nahr Taheri, Zabol, Seistan" on 9 October 1977 by A. Vigna and is deposited in the (Department of Zoology, University of Naples (IZA 7838). Paratypes are from the same locality and number 24
specimens, 35.0-78.0 mm standard length (IZA 7839) and 7 specimens, 44.0-49.0 mm standard length (Institutul Stiinte Biologice, Bucharest, ISBB uncatalogued). One specimen is in the American Museum of
Natural History (AMNH 40946), presumably a paratype from one of the preceding series.
CMN fish?
Key characters
This species is scaleless and has a deeply forked caudal fin. The dorsal adipose crest or keel on the caudal peduncle is well-developed. The anus is placed well anterior to the anal fin origin. The
dorsal fin has 3 unbranched and 7 branched rays, the anal fin has 2 unbranched and 5 branched rays, the pectoral fin has 1 unbranched and 9 branched rays and the pelvic fin has 1 unbranched and 7 branched
rays.
Morphology
Dorsal fin branched rays 6-8 (80% with 7 rays in original description, n = 20), anal fin with 5-6 branched rays (95% with 5 rays), pectoral fin with 8-10 branched rays (85% with 9 rays) and pelvic fin
with 6-7 branched rays (95% with 7 branched rays). The lateral line extends to the base of the caudal fin. The body is slender and compressed, particularly posteriorly. The head is long and the eyes are
small. The dorsal fin origin is at mid-body (snout tip to caudal fin base). The mouth is arched with strongly furrowed lips that have few papillae. Mental lobes are reduced. The stomach is syphonal and the
intestine is straight. The gas bladder capsule has globular chambers and a short duct.
Sexual dimorphism
Unknown.
Colour
The head and body are greyish, lighter ventrally. Dark spots along the back and flank may be occasionally fused. Fine spots are scattered in a reticulated pattern on the body. The dorsal and caudal
fins have dots in rows while other fins are colourless. The caudal fin base has a distinct blackish bar.
Size
Reaches 89.0 mm standard length.
Distribution
Endemic to Sistan.
Zoogeography
Nalbant and Bianco (1998) consider this species to be an epigean form of the blind cave fish P. smithi. The differences are in head shape and mouth and lip morphology, apart from the eyes and
pigment loss typical of ipogean P. smithi.
Habitat
Details are unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Type material: The holotype ((IZA 7838)) and paratypes (IZA 7839) of Paracobitis vignai, see above. + ?CMNFI paratypes
Genus Paraschistura
Prokofiev, 2009
This genus is recently described and not all species have been examined and
ascribed to it or related genera. Many were formerly included in the genus
Schistura McClelland, 1838.
The species in this genus are small and are distributed from interior water
bodies of Turkmenistan and from Iranian Baluchestan east to the upper reaches of
the Indus River in Afghanistan and Pakistan. Four species are reported from
Iran.
The genus is characterised by an elongate body with dorsal and ventral
profiles almost parallel. The belly is not rounded. The body is compressed
anteriorly and the head is compressed or depressed. The caudal peduncle is short
and moderately deep. The snout is usually blunt. The upper lip is furrowed and
is continuous or has a slight median interruption. Lips are simple, being
plicate to almost smooth. The processus dentiformis on the upper jaw is
well-developed in many species and there is a corresponding notch on the lower
jaw but the processus can be absent. Nostrils are closely spaced. The dorsal fin
usually has 7 branched rays and the anal fin 5 branched rays. The caudal fin is
slightly to deeply emarginated. There is no adipose crest or, if present, it is
weakly-developed near the caudal fin and not supported by procurrent rays of the
caudal fin. The lateral line is incomplete, may be very short and rarely reaches
the end of the anal fin level. Scales are absent or rarely present but weakly
developed on the caudal peduncle. A pelvic axillary lobe is present but is short
and does not usually extend beyond the pelvic fin base.
Prokofiev (2009) lists various osteological characters that help diagnose the
genus including the absence of a bony bridge between the parietal and pterotic
bones which separates the species from Schistura sensu lato. There is no
preethmoid I bone in the skull. Externally, there is no bony roof to cst,
the fork of cst-cio is not ossified and it is not fused to the
skull. The cst is the supratemporal commissure running across the top of
the head at the rear and cio is the temporal part of the infraorbital
head canal. The free part of the swimbladder is absent, the right and left lobes
of its bony capsule are fully divided by the manubrium, and the fifth trunk
vertebra does not take part in support of the capsule.
There is no sexual dimorphism. Colour pattern is distinctive with dark bars
of varying number and form being present. The bar at the caudal fin base is
usually much darker than anterior bars, and is never in the form of a spot.
There is no stripe along the flank although a dark line is rarely present. A
dark black spot or strip is always present at the base of the anterior dorsal
fin rays.
Paraschistura alta
(Nalbant and Bianco, 1998)
Described from "Afghanistan, Kajkai, Helmand river drainage, north east of Girisk". No Iranian record.
?genus as sexually dimoprhic see ProkofievSchistura baluchiorum
(Zugmayer, 1912)
Possibly a synonym of S. bampurensis. The type locality is Panjgur on the Rakhshan River of the Hamun-i Mashkel basin in Pakistani Baluchistan and it is also recorded from Afghanistan
in the Helmand River drainage. A cotype is in the Naturhistorisches Museum Wien
under NMW 19851 and is 46.0 mm standard length. Not recorded from Iran by specimens although Abdoli (2000) reports it from the Simish River and a river just north of it, both in the Mashkel basin.
Paraschistura bampurensis
(Nikol'skii, 1899)
Common names
sagmahi-ye Bampur.
[Bampur loach]
Systematics
Publication date is given as 1900 in Berg (1949) and Bănărescu and Nalbant (1966). This may be correct if the volume appeared late as the volume of the publication is for the year 1899; but
note that a footnote and the plate both bear the year 1899.
Nemacheilus baluchiorum Zugmayer, 1912 is possibly a synonym (Berg (1949) places this species in synonymy with Nemacheilus montanus - see below). Bănărescu and Nalbant (1966)
and Nalbant and Bianco (1998) place S. bampurensis in the subgenus or genus Schistura.? read description carefully and comment
Berg (1949) placed S. bampurensis in Nemacheilus montanus (McClelland, 1839) but Bănărescu and Nalbant (1966) recognise bampurensis as distinct on its incomplete lateral
line, focal zone of scales larger, dorsal fin positioned more anteriorly, deeper body, and colour pattern without regular bands (Bănărescu and Nalbant (1966: fig 12) for three pattern varieties).
Observations were made by me on the faded syntypes of N. montanus from Simla, 62.8-68.7 mm standard length (BM(NH) 1860.3.19:118-119)
as well as ZISP 8298, 9 specimens 25.3-51.1 mm standard length. Additional characters which distinguish bampurensis and
montanus are as follows. The preorbital process is very strongly developed in N. montanus, being almost as deep as the eye and extending almost an eye length below the lower orbit margin,
extending almost twice the distance of the process in bampurensis types
and being less curved and close under the eye. N. montanus has a slight but obvious keel on the back before the caudal fin while bampurensis are humped there but usually not keeled. The
caudal fin is dark at the base in montanus, not so in bampurensis or not as solid, wide and dark. The flank bars are oblique with the top forward in contrast to
bampurensis, and are less numerous than in bampurensis (5-10
versus 11-22 total, from under dorsal fin insertion to caudal fin but
excluding any caudal fin base bar). N. montanus types have faded bars but
are clearly fewer.
The syntypes of Nemacheilus bampurensis are in the Zoological Institute, St. Petersburg under catalogue numbers ZISP 11698 and 11699 at the localities, dates and number of specimens in Latin as
follows respectively:- "Kjagur prope urb. Bazman. 4. VII (6)" and "Kaskin prope urb. Bazman. 6.VII (4)" (Nikol'skii, 1899). Berg (1949) gives both these localities as between Bazman
and Bampur. However, ZISP 11698 comprises 9 specimens, 35.1-44.6 mm standard length and ZISP 11699 comprises 4 specimens not listed as types on the jar, 36.8-44.5 mm standard length.
Key characters
The male has a characteristic, moveable protuberance directed downward on the preorbital bone at the antero-ventral corner of the eye. It lies close to the eye, extends slightly below the lower orbit,
and curves partly around the orbit.
Morphology
Dorsal fin with 2-3 unbranched and 6-7 branched rays, anal fin with 2-3 unbranched and 4-5 branched rays. Pelvic fin branched rays 6-7. Lateral line incomplete, ending in front of the dorsal fin level.
Scales well-developed but the anterior third to a quarter of the body is scaleless. The posterior nostril is ovoid, slanting postero-dorsally. Barbels are large and the third pair is usually the largest
although in some fish the second pair is the largest (Saadati, 1977). The lower lip is divided, lips are corrugated, and the upper jaw process is rounded, overlapping the lower one. There is a fleshy
pelvic axillary process. Caudal fin slightly emarginate.
The type series (ZISP 11698) has dorsal fin branched rays 6(3) or 7(6); anal fin branched rays 4(1) or 5(8); pectoral fin branched rays 9(8) or 10(1); pelvic fin branched rays 6(2) or 7(7); and flank
bars 14(2), 15(3), 17(1), 19(2) or 22(1).
Meristic counts for Iranian material:- Dorsal fin branched rays 6(3) or 7(73), anal fin branched rays 4(1) or usually 5, pectoral fin branched rays 8(2), 9(59) or 10 (10), pelvic fin branched rays 6(7),
7(51) or 8(2), and flank bars 10(1), 11(7), 12(6), 13(12), 14(6), 15(8), 16(3), 17(1), 18(2), 19(2) and 22(1).
Sexual dimorphism
See above under Key characters. Males also have tubercles on the pectoral fin rays and on the operculum (see figure in Berg (1949)). The first branched pectoral fin ray is greatly expanded,
2-3 times broader than the second ray, which itself may be expanded a little.
Colour
Body yellowish to a light olive-green with 9-18 dark, brownish or chestnut-brown bands. The caudal fin has 3-4 dark wavy bars and a bar at its base, the dorsal fin 2-3 dark bars and a black spot at the
anterior fin base. Fins are a light orange or pinkish, particularly the caudal fin.
Size
Reaches 5.3 cm.
Distribution
Berg (1949) and Abdoli (2000) record this species from the Dasht-e Lut basin without specific localities, questionably from the Tigris River basin (presumably based on Bănărescu
and Nalbant (1966), see below), the middle and lower Bampur and Halil rivers of the
Hamun-e Jaz Murian basin, and the Sarbaz and Nikshahr rivers of the eastern Makran.
Specific localities include Kjagur and Kaskin near Bazman (Nikol'skii, 1899),
both between Bazman and Bampur (Berg, 1949), Shur Ab near Kuh-i Birk (Berg
(1949) and the Bampur River near Bazman.
Bănărescu and Nalbant (1966) report this species from Shapur, 12 km north of Kazerun and from Shah Bazan on a tributary of the Ab-i-Diz, and "probably most of Iran". This
seemed inherently unlikely as the type locality is in Baluchestan and the material
was later described as a new species, S. nielseni q.v.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
A female measuring 4.8 cm and caught in late February carried fairly well-developed eggs (Berg, 1949).
Parasites and predators
Unknown.
Econmic importance
None.
Conservation
This species is reported from several localities in Baluchestan, including those remote from human influence, and does not seem to be in any danger.
Further work
The biology of this species needs to be studied.
Sources
Type material: Syntypes of Nemacheilus bampurensis (ZISP 11698 and 11699), see above.
Iranian material: CMNFI 1979-0312, 14, 24.9-41.5 mm standard length, Baluchestan, Bampur River 8 km west of Iranshahr (27º11'N, 60º36'E);
CMNFI 1979-0313, 5, 31.1-39.9 mm standard length, Baluchestan, Bampur River at Bangharabad (27º20'N, 60º46'E);
CMNFI 1979-0315, 17, 20.0-44.8 mm standard length, Baluchestan, Bampur River 2 km north of Karevandar (27º51'N, 60º46'E);
CMNFI 1979-0317, 28, 19.9-45.8 mm standard length, Baluchestan, Sarbaz River at Bondan (26º35'N, 61º13'E);
CMNFI 1979-0318, 1, 23.8 mm standard length, Baluchestan, Sarbaz River at Huvar (26º09'N, 61º27'E);
CMNFI 1979-0323, 5, 20.7-31.4 mm standard length, Baluchestan, Sarbaz River (ca. 26º26'N, ca. 61º16'E);
CMNFI 1979-0326, 12, 19.3-35.0 mm standard length, Baluchestan, stream in Oghin River drainage (ca. 26º35'N, ca. 60º02'E);
CMNFI 1979-0327, 10, 21.2-37.4 mm standard length, Baluchestan, stream in Nikshahr River drainage (26º32'N, 59º57'E);
CMNFI 1979-0329, 14, 21.1-33.4 mm standard length, Baluchestan, stream at Zaminbandan (27º02'N, 61º20'E);
CMNFI 2007-0045, 6, 28.8-39.2 mm standard length, Kerman, Kharan River drainage at Baft (29º14'N, 56º38'E)checkID
?Schistura chrysicristinae
Nalbant, 1998
Described from the Tigris River basin in Turkey but no Iranian record.
Paraschistura kessleri
(Günther, 1889)
Common names
sagmahi-ye Bejestan, sagmahi-ye Kessler.
[golets kesslera or Kessler's loach in Russian; sundali in Pakistan].
Systematics
Nemacheilus prashari Hora, 1933 is possibly a synonym, along with its subspecies (q.v.). Banarescu and Mirza (1965) have comparisons of Nemacheilus lindbergi (which is later
regarded as a subspecies of Nemacheilus (= Parachistura) prashari, q.v.) with
P. kessleri where most characters overlap in varying degrees. Bănărescu
and Nalbant (1966) place P. kessleri in the subgenus Schistura and Nalbant and Bianco (1998) place it in the genus Schistura. Berg (1949) refers
P. prashari to a subspecies of
P. kessleri.
Nemacheilus kessleri turcomanus Nikol'skii, 1947 is reported from Turkmenistan east of the Iranian border in the Murgab River drainage (Kushk River near Kushk). Bănărescu and Nalbant
(1966) consider that the differences are too slight to warrant a separate subspecies.
Berg (1948-1949) considered S. kessleri to be close to S. sargadensis, differing in having the snout slanting steeply in front of the eyes (rounded in sargadensis - this is
generally true but exceptions occur with sharply downturned snouts in sargadensis examined by me), dorsal origin midway between the snout tip and the caudal fin base (further back in
sargadensis - but see below, same in both species; and in a series of sargadensis of varying size small fish have a longer predorsal distance than dorsal origin to caudal base distance, in
large fish a shorter predorsal distance, and in intermediate-sized fish an about equal predorsal distance, so this character appears to be highly variable and unreliable for separating
these species), rudimentary caudal peduncle crest (weak to clearly pronounced crest - ?check on my sargadensis), fewer and less sharply pronounced flank bars (10-12 total, broad bars in kessleri
seen by me while sargadensis has 13 or more thinner bars - ?check on my sargadensis), longer intestine (?), and pelvic fin origin just behind the level of the dorsal fin origin (under dorsal fin
origin or slightly in advance - kessleri types seen by me had the pelvic fin origin almost exactly under the dorsal fin origin). Generally S. sargadensis agrees with the description of
S. kessleri except for pigmentation (?I need to run type measurements I have against sargadensis noting that Ghazni fish may be a different species and comparing gut length/shape and bladder
capsule (see Mirza et al. (1981)).
Nemacheilus kessleri was described from Nushki in the Pishin Lora River basin of Afghanistan (Menon, 1987). Four syntypes are in the Natural History Museum, London (BM(NH) 1886.9.21:177-180,
38.3-48.4 mm standard length) and 4 syntypes (now 3) are in the Zoological Survey of India, Calcutta (ZSI 11487-11490) (Eschmeyer et al., 1996). The holotype of Nemacheilus kessleri turcomanus
is in the Zoological Museum of Moscow State University (MMSU P.5734) with apparently a paratype under MMSU P.5734 and another paratype under MMSU P.5735 (Eschmeyer et al., 1996). The
Zoological Museum of Moscow University (ZMMU; their acronym) has 3 specimens under P-5734 and 1 specimen under P-5735, all syntypes (Pavlinov and Borissenko, 2001).
Key characters
Characterised by Menon (1987) as a member of the subgenus Schistura with 7 branched dorsal fin rays, no scales, forked caudal fin, short lateral line not reaching mid-body and no sexual
dimorphism, and additionally by and Mirza et al. (1981) and Bănărescu and Nalbant (1995) as a member of the genus Schistura part of the kessleri-lindbergi group with the
above characters and also small size, rather compressed head, emarginate or slightly forked caudal fin and a reduced dentiform process.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 7, anal fin unbranched rays 2-3, branched rays 5, pectoral fin branched rays 8-11, pelvic fin branched rays 6-7,
usually 7. Dorsal fin origin midway between the
nostrils and the caudal fin base according to Berg (1948-1949) but the types examined by me clearly have the dorsal fin origin closer to the snout (predorsal distance when stepped back from dorsal fin
origin overlaps the rays of the caudal fin). Scales are absent. The lateral line ends under the dorsal fin (above the tip of the pectoral fin in Menon (1987) ? check on fish, Menon quote is correct). No
adipose fin but a rudimentary crest may be evident. Caudal fin slightly emarginate to quite deeply forked. Pelvic fin origin somewhat behind the level of the dorsal fin origin. The snout is blunt; in the
types it falls gently at the nostrils in 3 fish, abruptly in 1 fish; and in 3 fish from Ghazni, Afghanistan abruptly in 2 fish and less so in the third, all apparently independent of size. Both lips are
fringed, the upper with a slight interruption, the lower with a wide interruption. The dentiform process is reduced (Berg (1949), well-developed in Menon (1987) reduced in Ban and Nal 1995 ? check on
fish). Barbels are variably developed or absent in some individuals, a condition illustrated by Hora (1933). The posterior part of the intestine has a single loop.
Sexual dimorphism
There is no sexual dimorphism.
Colour
The back is crossed by 10-12 irregular, brownish bands. There is a dark spot at the base of the 3 anteriormost dorsal fin rays and there are 1-2 bands composed of small spots on the distal part of the
fin. The anal fin may have an anterior basal spot. There is a narrow blackish band at the caudal fin base and 2 narrower bands on the fin itself. The sides of the head are minutely spotted.
Bănărescu and Nalbant (1966) consider that the fish reported by Berg (1949: fig. 54) from Kelat Margh in Neh-i-Bendan do not have the typical colour pattern of this species. Berg (1949) describes
6-8 chestnut-brown bars on the posterior part of the body, none anteriorly.
Size
Reaches 8.7 cm total length (Patimar et al., 2010).
Distribution
Found from the Lora River basin of Pakistan through Afghanistan to the Murgab River basin of Turkmenistan and in eastern Iran
at such localities as Keljate-Marg in Zirckuch region and the Neh-i Bendan
district (?Berg check localities for modern spelling). For Iran, Abdoli (2000) lists middle and upper Kashaf River of the Tedzhen
River basin, questionably in the Bejestan basin, and in the Simish and Mashkid rivers, and river north of them in the Mashkid River basin. Nalbant and Bianco (1998) also cite the Mashkel Lake, northern
Sistan.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Patimar et al. (2010) examined fish from the Zanglanlou River in
northeast Iran and found maximum age for both sexes was 4+ years. Growth
was positively allometric and sex ratio was 1:1.2 in favour of females.
Food
Unknown.
Reproduction
Patimar et al. (2010) found reproduction occurred in April-May with
the highest GSI in April. Maximum egg diameter was 1.46 mm, aboslute fecundity
reached 1246 eggs and up to 1285.71 eggs in relation to total weight.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus kessleri (BM(NH) 1886.9.21:177-180), see above.
Comparative material: BM(NH) 1944.4.1:7-9, 3, 42.0-57.6 mm standard length, Afghanistan, Ghazni (?).
Schistura lindbergi ?genus
(Banarescu and Mirza, 1965)
Found in the Mashkel (= Mashkid) River basin of Pakistan on the southeastern border of Iran (see S. prashari for further discussion). This species was described from a rivulet connected to the
drainage of the Farah River, Siaw, between Farah and Dilaram, Afghanistan. No Iranian record although Nalbant and Bianco (1998) record a specimen from near the Iranian border in the "Mashkel Lake
system". Originally described in the genus Noemacheilus.
Schistura namiri ?genus see Tigris/Euphrates list
(Krupp and Schneider, 1991)
Recorded from the Tigris River basin in Turkey by Nalbant (1998) but no Iranian record. Originally described in the genus Nemacheilus.
Paraschistura nielseni
(Nalbant and Bianco, 1998)
Common names
sagmahi-ye Nielsen.
[Nielsen's loach].
Systematics
Note that P 27110 (33.7 mm SL, my measurement here and below) "Shah Bazan, W. Iran, 16. IV. 1937. E. Kaiser" is listed as the holotype in the Zoological Museum, University of Copenhagen
(ZMUC), not 27109 as in the original, published description (observed in 1999). The Shah Bazar locality in the description is presumably a misprint for Shah Bazan. Paratypes are 27109 (25.8 mm SL) from
"Shah Bazan..." etc as above (probably reversed with the holotype), and 27105 "lok 44" (30.7 mm SL), 27106 "lok 54" (12.7 mm SL), 27107 "lok 54" (29.5 mm SL),
27117 "lok 54" (37.3 mm SL) from "Shapur, 15.03.1937. E. Kaiser. W. Iran". The published description gives the locality of Shapur as "12 km north-west of Kazerun".
The size of one paratype (12.7 mm) is smaller than any cited in the type description. The catalogue number of one fish in a drawing in the original description (i.e. 27108) is not in that description
nor in jars in ZMUC. There has evidently been some confusion over the type series.
Named for Dr. Jorgen G. Nielsen, Department of Ichthyology, Zoological Museum, University of Copenhagen.
This species was previously reported as Nemacheilus bampurensis by Bănărescu and Nalbant (1966) based on this type series.
Key characters
This species has a relatively short body with a short head and blunt snout. The lateral line is incomplete ending just anterior to the dorsal fin origin. Scales have a large, eccentric focus. The
caudal fin is distinctly emarginate. The body is yellowish with 7-16 dusky brown bars. The dorsal fin has 2 unbranched and 7 branched rays, the anal fin 2 unbranched and 5 branched rays, the pectoral fin
has 1 unbranched and 9 branched rays and the pelvic fin has 1 unbranched and 7 branched rays.
Morphology
Dorsal fin branched rays 7(20), anal fin branched rays 5(20), pectoral fin branched rays 7(19) or 8(1) and pelvic fin rays 7(1), 8(3), 9(15) or 10(1) based on my collection. Meristics from type series
dorsal fin branched rays 7(5), anal fin branched rays 5(5), pectoral fin branched rays 9(4), 10(1), pelvic fin branched rays 6(1), 7(4), and vertebrae 33(1), 34(4), 35(1). The body is compressed,
especially posteriorly. The eyes are small and positioned in the middle or anterior portion of the head. The mouth is slightly arched and the upper jaw has a reduced processus dentiformis. Lips are
slightly furrowed and the mental lobes are reduced. The maxillo-mandibular barbels are the longest. The nostrils are near the eyes with a relatively long tube on the anterior opening. The dorsal fin
origin is at the middle of the body or slightly posterior. The pelvic fin insertion is just below the dorsal fin origin. The caudal fin is emarginate or slightly forked with rounded lobes. The anterior
part of the body is scaleless. The stomach is syphonal and there is a single intestine loop. The gas bladder capsule has two globular chambers connected by a relatively long duct.
See BWC00-8 for more details on colour and sexual dimorphism and general description ?
Sexual dimorphism
A 37.3 mm standard length specimen from the type series (ZMUC 27117) has the first pectoral fin branched ray broadened with wide band of tubercles and rays 2-4 decreasingly tuberculate. This male has
a preorbital prolongation. Paratype ZMUC 27105 has tubercles on up to 6 branched rays of the pectoral fin in broad bands, gradually decreasing in extent on more medial rays.
Colour
The head and body are yellowish, darker and more intense along the upper flank. Bars on the body are sometimes irregular or interrupted and number 7-17. The caudal fin has a bar at its base. The
anterior dorsal fin base has a round dark spot.
Size
Reaches 54.9 mm total length and 45.0 mm standard length.
Distribution
This species is endemic to Iran, known from the Tigris River and Gulf basins as in the type description cited above (and my fish?)
Zoogeography
Nalbant and Bianco (1998) regard this species as the westernmost member of the genus Schistura.
See also family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Type material: The holotype (ZMUC P 27110) and paratypes (ZMUC P 27105, 27106, 27107, 27109, 27117) of Schistura nielseni, see above and note possible confusion in numbers.
Iranian material: BWC 00-8, 54, 27.5-45.0 mm standard length, ?
All fish are badly faded and can't compare with drawings in original description.
Compare measurements of snout, interorbital and HL from Bănărescu and Nalbant (1966) and Nalbant and Bianco (1998) with my Khuzestan fish - snout and interorbital appear different from my
cf. tigris fish NEED to check again with my 2000 material from Khuzestan - see counts e.g.
?genus and search prashari here Schistura prashari
(Hora, 1933)
Reported from the Mashkel (= Mashkid) River basin of Pakistan on the southeastern border of Iran and from the Farah River basin in the Sistan drainage of Afghanistan as Nemacheilus prashari
lindbergi Banarescu and Mirza, 1965 (or as a distinct species, lindbergi, in Schistura or Nemacheilus in Mirza, Nalbant and Banarescu (1981) and Menon (1987)) and from the
Helmand River basin in the Sistan drainage of Afghanistan as Nemacheilus prashari haarlovi Bănărescu and Nalbant, 1966 (Banarescu and Mirza, 1965; Bănărescu and Nalbant, 1966;
Mirza, Bănărescu and Nalbant, 1969; Coad, 1981c; Mirza, Nalbant and Banarescu, 1981). Possibly a synonym of S. kessleri as suggested by Berg (1949). No Iranian record.
Paraschistura sargadensis
(Nikol'skii, 1899)
Common names
sagmahi-ye Sarhad.
[Turkmenskii golets or Turkmen loach in Russian; perhaps Sarhadd loach should be used in English, see below].
Systematics
The syntypes of Nemacheilus sargadensis are in the Zoological Institute, St. Petersburg under catalogue number ZISP 11700 with locality data in Latin as "Sija-Rischan in sargado. 20.VIII
(6)" (Nikol'skii, 1899) and in Russian in Berg (1949) "Zia-rishan, Province of Sarhad in Kerman, S.W. Iran, border with Beluchistan, not far from Kuh-e Taftan volcano, 1 IX 1898, N.
Zarudnyi". The catalogue data in ZISP give the date as 20.VIII. There are 31 fish, 31.0-53.7 mm standard length, in rather poor shape, some stained dark brown, others uniformally decoloured or drab.
Sarhad is the "frontier" or "land at the upper boundary", an area of plains or broad valleys and isolated mountains around Khash, for example, and a term used generally for the
mountainous plateau of Baluchestan: Saadati's (1977) statement that the Sarhad is unlocatable is in error.
Nemachilus turcmenicus Berg, 1932 is described from "einem Bache unweit von der Eisenbahnstation Gjaurs in Turkmenistan (Transkaspien)" near the Iranian border
and alternatively from the Kelte-chinar River (Cherokh River) near Gyaurs
(37°47'N, 58°44'E), Turkmenistan (Catalog of Fishes). Three syntypes, 31.7-33.8 mm
standard length, are in ZISP 11064. Berg (1948-1949) synonymises turcmenicus with sargadensis but Bănărescu
and Nalbant (1966) consider it to be a valid subspecies, adding a third, paludani, from the Kabul River drainage of Afghanistan. Later Mirza, Nalbant and Banarescu (1981) separate paludani
as a distinct species in a different species group from S. sargadensis, since S. sargadensis has scales, an almost forked caudal fin, a feeble dentiform process and irregular bars on the
flank, all in contrast to S. paludani.
Berg (1948-1949; 1949) and Saadati (1977) consider this species to be close to S. kessleri (q.v.), possibly a synonym. Nalbant and Bianco (1998) place this species in the genus
Schistura.
Key characters
The caudal peduncle is deeper than in Schistura bampurensis and it
lacks the protuberance under the eye in males.
Morphology
Dorsal fin with 2-5 unbranched and 6-8 branched rays, anal fin with 2-3 unbranched and 4-6, usually 5, branched rays, pectoral fin branched rays 7-10, and pelvic fin branched rays 6-7 for S.
sargadensis turcmenica (Berg, 1932a; 1948-1949; Nikolaev, 1993). Body scaleless (scaled according to Mirza, Nalbant and Banarescu (1981) based on fish from the Kul River, Hormozgan ? check on my fish
as this would distinguish from kessleri). Caudal fin moderately emarginate (deeply emarginate, almost forked in Mirza, Nalbant and Banarescu (1981)). Caudal peduncle depth 1.6-2.2 in caudal peduncle
length. Dorsal fin origin nearer caudal base than snout tip and slightly behind the pelvic fin origin. A dorsal adipose fin may be present or absent. Dentiform process on upper jaw, if present,
rudimentary.
Meristics for Iranian specimens:- branched dorsal fin rays 6(1) or 7(105), anal fin branched rays 5(106), pectoral fin branched rays 9(11), 10(84) or 11(11), pelvic fin branched rays 6(1), 7(94) and
8(11), and flank bars 11(12), 12(8), 13(13), 14(11), 15(8), 16(5), 17(5) or 18(5).
Sexual dimorphism
Unknown.
Colour
Body colour is brown backed with greenish tinges or grey, with 13-14 dark bands, in some fish hardly visible (and irregular in form according to Mirza, Nalbant and Banarescu (1981)). The bands are
broad, slightly broader than the interspaces. A dark stripe runs along the midline of the back. The caudal fin has 2 dark bars and the dorsal fin an anterior dark spot at its base. A dark band is present
at the caudal base. Young fish have a row of dark speckles along the lateral line.
Size
Reaches 6.5 cm.
Distribution
Found in the streams of the northern slope of the Kopetdag in Turkmenistan as Nemacheilus sargadensis turcmenicus, possibly extending into northern Iran. In southern Iran it is found in the
Hormuz, Hamun-e Jaz Murian, Hamun-e Mashkid and Makran basins (Nalbant and Bianco, 1998)
including at Tangeh Sarreh, Baluchestan, Shah Abbas qanat at Assadabad and the
Kul River near Darab.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Maximum age in the subspecies turcmenica does not exceed 1.5 years (Nikolaev, 1993).
Food
Important food items in the subspecies turcmenica are larval mayflies, caddis flies, chironomids, dragonflies and detritus (Nikolaev, 1993).
Reproduction
Eggs in the subspecies turcmenica reach 1.24 mm in diameter (Nikolaev, 1993).
Parasites and predators
Unknown.
Economic importance
None.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus sargadensis (ZISP 11700), see above.
Iranian material: CMNFI 1979-0210, 37, 21.0-35.4 mm standard length, Kerman, river west of Istor (29º21'N, 56º08'E);
CMNFI 1979-0215, 14, 27.7-51.7 mm standard length, Kerman, Kharan River drainage at Baft (29º14'N, 56º37'E);
CMNFI 1979-0217, 6, 30.1-41.8 mm standard length, Kerman, Kharan River drainage (ca. 28º59'30"N, ca. 56º51'30"E);
CMNFI 1979-0218, 8, 32.8-47.8 mm standard length, Kerman, Kharan River drainage (28º53'N, 56º55'E);
CMNFI 1979-0220, 1, 37.3 mm standard length, Kerman, jube 2 km south of Jiroft (28º39'N, 57º43'E);
CMNFI 1979-0221, 19, 24.3-46.8 mm standard length, Kerman, river in Halil River drainage (28º51'N, 57º52'E);
CMNFI 1979-0308, 11, 22.2-40.1 mm standard length, Kerman, river 44 km from Baft (29º02'N, 56º50'E);
CMNFI 1979-0338, 68, ? mm standard length, Baluchestan, Tahlab River drainage 8 km from Mirjaveh (28º58'N, 61º24'E);
CMNFI 1979-0339, 5, ? mm standard length, Baluchestan, Tahlab River drainage 16 km from Mirjaveh (28º56'30"N, 61º21'E);
CMNFI 1979-0340, 25, ? mm standard length, Baluchestan, jube at Ladiz (28º55'N, 61º18'E);
OSU 4270 ?
Genus Seminemacheilus
Banarescu and Nalbant, 1995
This genus has 3 species in Turkey and Iran, with one of these species and
Iranian endemic.
The body is deep, more so than in other Iranian
genera. The snout is blunt and the eyes are small. Nostrils are widely spaced and equal in size.
The processus dentiformis is weak to absent. A
crest on the caudal peduncle is rudimentary near the caudal fin base. The
lateral line is short and does not reach beyond the end of the pectoral fin tip
level. There are no scales and no pelvic axillary lobe. There are 7, rarely 8, branched
dorsal fin rays and 5 branched anal fin rays. The pelvic fin insertion is
opposite the dorsal fin origin. Sexual dimorphism consists of the
anterior rays in the pectoral fin having isolated and small tubercles (not
patches) and male pectoral fins are larger than in females. Prokofiev (209)
lists various osteological features including the manubrium in the swimbladder
being absent or weakly developed and the bony capsule swollen. 4
elongated-cylindrical radial bones in the pectoral fin, no preethmoid I
bone in the skull and 5 hypurals but no epural in the caudal fin support, among
others.
Seminemacheilus tongiorgii
Nalbant and Bianco, 1998
Common names
sagmahi-ye Hormuz.
[Hormuz loach].
Systematics
This species is named for Professor Paolo Tongiorgi, University of Modena. The holotype measures 23.7 mm standard length and is from " large water spring near Darab town, Kul river
basin", collected on 2 June 1976 by P. G. Bianco and is stored in the Department of Zoology, University of Naples (IZA 801 but not located in a 2002 visit by me). One paratype, 20.0 mm standard
length, is from the same locality (Institutul Stiinte Biologice, Bucharest, ISBB uncatalogued). It differs from the Anatolian species S. lendlii (Hankó 1925) mostly by colour pattern and possession
of scales.
Key characters
This species has a short and deep body. The mid-flank has a row of small scales. Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales along with a description.The
processus dentiformis is absent. The lips are furrowed, particularly the lower lip. The gas bladder capsules are strongly united. The dorsal fin has 3 unbranched and 8 branched rays, the anal fin has 3 unbranched and 5 branched rays, the pectoral fin has 2 unbranched and 9 branched rays and the pelvic fin has 1 unbranched and 6 branched rays.
Morphology
The head is wide with large eyes and nostrils just anterior to the eyes. The mouth is arched and the lower lip has large lobules but the mental lobes are reduced. The gut has a short oesophagus and the
stomach and intestine have one loop. The gas bladder capsule valves are proximal. The lateral line is short and does not extend posterior to the level of the pectoral fin. The body is almost
entirely scaleless except for minute scales on mid-flank.
Sexual dimorphism
Unknown.
Colour
The overall colour is yellowish-white. There is a row of grey blotches along the mid-flank and a row of grey dots on the posterior half of the belly. All fins have grey dots except the whitish pelvics.
The peritoneum is silvery-white with scattered melanophores.
Size
Attains 59.3 mm standard length.
Distribution
Known from Iran in the upper reaches of the Hormuz basin near Darab, in
the Kor River basin and in northwestern Iran (Nalbant et al., 2010; Esmaeili et al., 2011?).
Zoogeography
The limited distribution of this endemic and lack of information on relationships preclude zoogeographical analysis. See family account.
Habitat
Details are unknown.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 45 fish measuring 2.95-5.93 cm standard length. The a-value was 0.0264 and the b-value 2.904
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Nalbant and Bianco (1998).
Genus Triplophysa
Rendahl, 1933
This genus contains loaches of the High Asian region numbering over 110 species.
The region centres on the Tibetan plateau with some species found in north China
to the east and in Central Asia to the west. The largest nemacheilines are found
in this genus. One species is reported from Iran.
The body is elongate with a rounded belly and the caudal peduncle is slightly
compressed or highly depressed, and is often very elongate or whip-like. The
upper lip is deeply furrowed and there is no processus dentiformis on the upper
jaw. The dorsal fin has 6-9 branched rays and is emarginate. There is no adipose
crest on the caudal peduncle although a small ridge may be present in some
species. The caudal fin is slightly to deeply forked, or is truncate. There is
no pelvic axillary lobe. Scales are absent and the lateral line is usually
complete. The swimbladder is reduced to two lateral parts and a posterior
chamber which is rudimentary or with a secondarily developed bladder lying free
in the abdominal cavity. A reduced swimbladder is an advantage in fast water,
reducing buoyancy. The gut is short or long, a simple s-shape or a spiral. The
colour pattern is usually mottled rather than banded. Males have a raised
tuberculate area below the nostrils extending from the lip corner to the
anterior eye margin, separated by a groove from a lower tuberculate area.
Pectoral fin rays have thickened and tuberculate pads on their dorsal surface.
Prokofiev (2009) summarises osteological characters in his key and these
include possession of 3 pectoral fin radials, if 4 are present then the 2
external ones are more or less dilated, flattened and at least partially overlie
each other, and the presence of the preethmoid I bone in the skull. Prokofiev
(2010) adds other osteological characters.
Triplohysa farwelli
(Hora, 1935)
Described from the "Helmand river, Afghanistan" in the Sistan basin but without any definite locality; it may be remote from the Sistan lowlands of Iran (Hora, 1935). Originally described
under Nemacheilus. No Iranian record.
Triplophysa griffithii
(Günther, 1868)
Described erroneously as from Assam but the type locality is the Arghandab River near Kandahar in the Helmand River drainage of the Sistan basin (Hora, 1929; 1935; Bănărescu and Nalbant,
1966). Originally described in Nemachilus (sic). No Iranian record but see T. stoliczkai.
Triplophysa stoliczkai
(Steindachner, 1866)


Common names
sagmahi-ye Pamir.
[Tibetskii golets or Tibetan loach, Pamirskii golets or Pamirs loach, both in Russian; singhat in Pakistan; Stoliczka's loach].
Systematics
Often spelt stoliczkae, (e.g. in Annandale and Hora (1920)), the original spelling is Stoličkai, which becomes stoliczkai in Latin where accents on letters are not used.
This species is placed in the genus Triplophysa by Menon (1987) and Bănărescu and Nalbant (1995).
Nemacheilus akhtari Vijayalakshmanan, 1950 described from "Farakhollum, about 10 miles South of Gardan Diwar, Helmund river" (Farakhulm at 34°31'N, 68°08'E),
Afghanistan in the Sistan basin may be a synonym of Nemacheilus (= Triplophysa) griffithii (Günther, 1868) according to Bănărescu and Nalbant (1966)
or T. stoliczkai according to Prokofiev (2007). There is no Iranian record.
This is a highly variable species originally described from streams around Lake Tsumureri in Rupshu Province of western Tibet and also found in the upper reaches of such rivers as the Hwang,
Brahmaputra and Yangtse. It is reported further west from the Aral Sea basin, the upper Indus River basin, the upper Helmand River basin in Afghanistan and probably in Sistan in Iran. Prokofiev
(2007) considers the systematics of the loaches in the stoliczkai group to be the most complicated in High Asian species. Berg (1948-1949) gives a series of figures which plainly show the
variability in body form and pigmentation of this species. Such a wide distribution and variability may be indicative of several taxa being confused under this name.
Regan (1906) refers specimens from Sistan to Nemacheilus stenurus Herzenstein, 1888. Annandale and Hora (1920) consider stenurus not to be a distinct species but Hora (1922)
compares stenurus with Nemacheilus tenuis (see below) and notes that stenurus has a continuous and entire lower lip (slightly indented in ZISP 7355 examined by me -
wide interruption and greatly plicated in tenuis) and the dorsal fin origin is closer to the snout than the caudal fin base (equidistant or nearer the caudal base in tenuis).
Specimens from Sistan labelled as Nemacheilus stenurus by Regan (1906) are then misidentified T. stoliczkai according to Annandale and Hora (1920). Since N. stenurus was
described from the sources of the Yangtse Kiang in China and is a more recent name, it is probably not relevant to Iranian loaches.
Berg (1948-1949) places Nemacheilus tenuis Day, 1877 (publication dated 1876, apparently published 1877 according to Eschmeyer et al. (1996)) in stoliczkai as var. tenuis
but notes that it occurs together with the type form in the Gunt River of the Pamirs in Tadjikistan as well as in Sistan, and thereby the implication is that this variety is not a subspecies (since
subspecies do not occur together). Berg (1948-1949) also has the subspecies Nemacheilus stenurus uranoscopus Kessler, 1872 described from the Zeravshan River in Uzbekistan as possibly occurring in
the Helmand River basin, presumably the upper reaches, but he includes Nemacheilus tenuis Day under this subspecies too.
Annandale and Hora (1920) initially place Sistan specimens in Nemacheilus stoliczkai but later Hora (1922) places them in Nemacheilus tenuis. Hora (1922) restricts the name
stoliczkai to fish from the Indus River basin and considers that the various wide-ranging reports of stoliczkai refer to various other species. See also Vijayalakshmanan (1950) for
comparisons with some nominal Afghan species in the Helmand basin.
The fish from Sistan examined by Annandale and Hora (1920) and assigned to T. stoliczkai are in two forms - one with a thin caudal peduncle, the stenurus type which they do not recognise
as specifically distinct, and one with a thick caudal peduncle which they place in var. leptosoma Herzenstein, 1888 (note that varieties are not recognised by the International Code of Zoological
Nomenclature). The former has a minimum caudal peduncle depth in caudal peduncle length of 5.3-7.8, mean 6.8 based on 3 fish from a table in Annandale and Hora (1920) and the one fish seen by me, and
the latter has values of 2.9-3.6, mean 3.1 from the table in Annandale and Hora (1920). Annandale and Hora (1920) point out that this character is dimorphic but is neither related to sex nor
"race" and that gut loop development also varies but independently of the caudal peduncle character. The specimen I examined from Sistan has a stenurus form of caudal peduncle
(value 7.6), the dorsal fin origin is about equidistant between the snout tip and caudal base and so agrees with neither stenurus nor stoliczkai (see table in Vijayalakshmanan, 1950)), the
head length in standard length (4.5) falls within the limits of stoliczkai (4.2-4.8) and not stenurus (4.9-5.5) (see table in Vijayalakshmanan, 1950)), and the lip grooves resemble another
species, Nemacheilus akhtari Vijayalakshmanan, 1950, described from the Helmand River at Farakhollum about 10 miles south of Gardan Diwar in Afghanistan. The conclusion I reach here is that an
individual fish in this group of species or forms can have a mix of characters used by authors to separate and define species.
The resolution of this problem, the correct name for Sistan loaches, other than the distinctive P. rhadinaea and P. vignai, requires extensive material from the whole range of the nominal
species involved for dissection and comparison of gut shape, for analysis of sexual dimorphism and individual variability in such morphometric characters as caudal peduncle breadth and thickness and
position of the dorsal fin, as well as in determining new and, hopefully, definitive characters. Conservatively, I refer Sistan fish to stoliczkai, the oldest available name, while recognising that
tenuis may be a distinct species or subspecies and be the fish found in Sistan, or even that the fish in Sistan are an unnamed taxon or taxa. The Iranian and other material available to me does not
permit a resolution of this wide ranging problem.
Syntypes of Cobitis stoliczkai are in the Naturhistorisches Museum Wien under NMW 48436 (5 fish), NMW 48439 (1) and NMW 50477 (4).
A syntype, or at least material examined by Day, of Nemacheilus tenuis is in the Naturhistorisches Museum Wien under NMW 48477 (Eschmeyer et al., 1996).
A syntype of Nemacheilus stenurus, 135.2 mm standard length, is in the Zoological Institute, St. Petersburg, Russia under ZISP 7355. Other types are ZISP 7256 (4), 7354 (3, now 2), 7355 (1),
and BM(NH) 1891.10.7.33 (1, 64.9 mm standard length) (formerly in ZISP).
SEE Prokofiev (2007) for tenuis and stoliczkai and other data ?
Key characters
The elongate yet rounded caudal peduncle without an adipose fin distinguishes this species from P. rhadinaea and P. vignai the only other
nemacheilids recognised in Sistan. The depth of the
caudal peduncle just behind the end of the anal fin is the same as the width.
Morphology
Dorsal fin with 2-3 unbranched and 6-9, usually 7-8, branched rays, anal fin with 2-3 unbranched and 5 branched rays, pectoral fin with 8-12 branched rays, and pelvic fin with 6-8 branched rays. Lateral
line complete and distinctive. Scales are absent. Caudal peduncle long and slender or short and deep (see above). Head length in standard length 4.0-4.8. Eye diameter in head length 4.8-5.5, mean 5.2 in
Annandale and Hora (1922) for Sistan fish (note that Day (1876; 1878) gives eye diameter as 8 in head length but Steindachner (1866) in his original description gives 5.5). Snout blunt. Barbels are
reported as the maxillary ones reaching behind the eye, the rostral ones shorter; the specimen from Sistan had the maxillary and longest rostral barbel about equal in length, the maxillary reaching back
to about mid-eye level. Lips are rugose, deeply incised and may be fimbriated. The lower lip is fimbriate and interrupted in the middle. There is a narrow, elongate post-labial groove with well-marked
ridges on each side extending back on the mid-line of the lower head (in one specimen seen by me). The pectoral fin tip is formed by the third and fourth branched rays. Dorsal fin origin nearer the snout
tip than the caudal base in Iranian specimens or equidistant while elsewhere in the range of this species it is nearer the caudal base (but dorsal origin is individually quite variable in
Nemacheilidae).
Caudal fin slightly emarginate, lower lobe longer than upper. A well-marked groove between the anus and the anal fin is absent. The gut length is slightly longer than the fish itself with two loops.
Chromosome number is 2n=50 for fish from Kyrgyzstan (Klinkhardt et al., 1995).
Sexual dimorphism
Mature males have the first 3-6 pectoral fin rays thickened and tuberculate. A raised tuberculate area below the nostrils extends from the lips to the eye and is separated by a groove from a tuberculate
area below (see Fig. 2B in Bănărescu and Nalbant (1995)).
Colour
The flank bears irregular dark spots which may coalesce into a stripe, or is marbled dark green or black. There are dark brown saddles over the back. Fins have rows of pigment, best developed on
the dorsal and caudal.
Size
Reaches 16.5 cm.
Distribution
Found from Tibet to northern Kashmir, Turkestan and Sistan. In Iran, it is reported from the Hirmand River delta of Sistan (Annandale, 1921).
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Biology and numbers are poorly known so an assessment of conservation status
cannot be made.
Further work
The biology of this species needs study.
Sources
Type material: Syntypes of Nemacheilus stenurus (ZISP 7355 and BM(NH) 1891.10.7.33), see above.
Comparative material: BM(NH) 1905.4.7:3, 1, 87.7 mm standard length, Sistan, Helmand River, collected by H. McMahon and from the Indian Museum, Calcutta (the size of this fish is at variance with the
6 specimens measured by Annandale and Hora (1920:181) but appears to be from the collections made by the Seistan Arbitration Commission of 1902-1904 and is possibly the seventh specimen referred
to in the text. This specimen has been identified as Triplophysa griffithii by S. O. Kullander and has old labels as Nemacheilus stenurus and Nemacheilus tenuis).
Genus Turcinoemacheilus
Banarescu and Nalbant, 1964
This genus contains a single species and is only recently
recorded from Iran. The characters of the genus are the same as the species.
Turcinoemacheilus kosswigi
Banarescu and Nalbant, 1964
Common names
None.
Systematics
Originally described from Kapozik Kadun, Hakkari, Turkey. The holotype is ZMH
H1884 and 6 paratypes are ZMH H1885.
Key characters
The pelvic fin origin lies in front of the dorsal fin origin and the anus
lies closer to the pelvic fins base than the anal fin base, distinguishing this
species from all other Iranian nemacheilids.
Morphology
The body is of uniform depth and almost cylindrical. Dorsal fin with 2-4 unbranched rays and 7 branched rays, anal fin with 1-4
unbranched rays and 5 branched rays, pectoral fin with 7-9 branched rays, and
pelvic fin with 5-7 branched rays. The lateral line is short with 18-19 pores,
ending before the dorsal fin origin level. The lateral line pores are larger
than in other species in Iran. The body is scaleless. Eyes are small
and far apart. There is no processus dentiformis. All fins are
small and more or less rounded. The dorsal fin origin is behind the level of the
posterior margin of the pelvic fins. The caudal fin is weakly emarginate. The lips are simple and smooth, the
head short and flat and there is no keel on the caudal peduncle. The greater
distance between dorsal and ventral fin, longer snout length, lower body depth
and narrow body width are viewed as adaptations to fast flowing streamlets (Golzarianpour
et al., 2009).
The pectoral fins are large and horizontal and act as vacuum suckers (and
possibly the pelvic fins too) (Breil and Bohlen, 2001).
There is a fairly large fleshy pelvic axillary lobe.
Sexual dimorphism
None.
Colour
Overall colour is brown to yellowish. The back has a brown line. There are a series of irregular flank blotches (ca. 12)
extending from the back to the mid- or lower flank but not the belly. These are weakly
expressed anteriorly or merge into the general dark pigmentation there. There is a bar
or arc of dark pigmentation at the caudal fin base.
Fins are not spotted.
Size
Attains 55.3 mm total length.
Distribution
Reported from the Sezar River, a tributary of the Dez River at 33°28'N,
49°03'E by Golzarianpour et al. (2009) and in the northern Gulf basin (Nalbant
et al., 2010). Also found in the Tigris River
and its tributaries and the upper Euphrates River basin including the Göksu
River in Turkey (Breil and Bohlen, 2001; Golzarianpour et al., 2009).
Zoogeography
An endemic species in the Tigris-Euphrates basin, its relationships with
other nemacheilids is uncertain.
Habitat
Gravel beds with a strong current are favoured, the slender body allowing
this fish to exploit spaces between the gravel. Capture sites in Turkey had
clear to muddy water. Aquarium specimens showed thigmotaxis, fixing themselves
in place by erecting all fins and pressing against opposing structures. In a
strong current, they attached to the substrate with the pectoral fins using
suction. Fish even left the aquarium water, a few centimetres above the
waterline, attached to the glass with only the tail tip in the water. Splashing
with water moved the head and body but the pectoral fins remained attached (Breil
and Bohlen, 2001).The Iranian specimens were
caught at 1456 m altitude.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Biology and numbers are poorly known so an assessment of conservation status
cannot be made.
It is widely distributed and probably more common than current knowledge
indicates. Golzarianpour et al. (2009) point out that dam construction
and human activities may threaten the species.
Further work
The biology of this species needs study.
Sources
Based on Golzarianpour et al. (2009).
Bagridae
The bagrid catfishes are found in fresh waters of Africa and Asia.
Some species attain 2 m in length. There are about 18 genera and about
170 species (Nelson, 2006). Only one species is known from Iran.
This family is characterised by a scaleless body; a depressed head
and rounded to compressed elongate body; short dorsal fin with a
strong, often serrated spine; a strong, serrated spine in the pectoral
fin; a short to long adipose fin; anal fin short to long; caudal fin
forked or deeply emarginate; a free margin to the gill membranes over
the isthmus; the anterior and posterior nostrils are well separated;
anterior nostrils tubular; mouth ventral and transverse or arched; 4
pairs of barbels with the nostril barbel on the posterior nostril,
maxillary barbels can be very long, other barbels are mandibular and
mental (chin); teeth on the prevomer, premaxillaries and mandible; and
eyes often covered by skin. Maximum size is about 2 m.
These catfishes are generally nocturnal. Certain species are
important food fishes and others are kept as pets in aquaria.
Genus Mystus
Scopoli, 1777
This is a catchall genus comprising numerous species in Asia.
Roberts (1994) restricts the genus to 8 closely related species (see also Grant
(2004)). Only 1 species is known from Southwest Asia including Iran. A history and
usage of the name Mystus is given by Jayaram and Anuradha (1984).
This genus is characterised by an elongate body, rounded anteriorly
and compressed posteriorly, a short and moderately depressed head,
head smooth or rugose, an elongate cranial fontanelle extending
posteriorly to the base of the occipital process and divided into
anterior and posterior portions of nearly equal length by an
epiphyseal bar (Roberts, 1994), small to moderate eyes set high and
not visible from the ventral surface of the head, a free circular
eyelid, a wide transverse, usually subterminal mouth, maxillary
barbels very long, jaw teeth are in villiform patches, on the lower
jaw as a curved or angular band interrupted at the mid-point,
continuous and curved slightly in the upper jaw, total gill rakers
11-30, gill openings very wide and free from the isthmus, adipose fin
high and very long, caudal fin deeply forked, upper caudal lobe often
much larger than lower, 37-46 vertebrae about equally divided between
abdominal and caudal ones, and branchiostegal rays 6-12.
Mystus pelusius
(Solander, 1794)
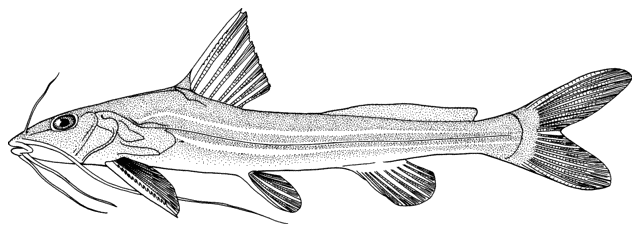
Common names
ابوزمير (abu-zummair or abu zumir, an Arabic name used in Khuzestan), mahi nish dor
(an Abadani name, from www.abadan.net/abadanidictionary.html, downloaded 4 December 2003), sag mahi (= dog fish),
گربه ماهي (= gorbeh mahi, meaning catfish), chamu.
[abu-zummair, abouz-zoumeir, abu-al-zamir, abu'l-zoumeir, zugzug in Aleppo, jahudi in Mosul, all in Arabic;
Tigris mystus (Fricke et al., 2007)].
Systematics
Synonyms are Bagrus halepensis Valenciennes in Cuvier and
Valenciennes, 1840 from the "Couiac, qui est la rivière d'Alep"
(= Quwayq at Aleppo, Syria) (see Bailey (1951) on the publication
date), Macrones aleppensis Günther, 1864, Macrones
colvillii Günther, 1874 from "Bagdad", and Mystus
misrai Anuradha, 1986 described from "Lake Antioche,
Syria". Hypselobagrus Aleppensis is a new combination by
Lortet (1883). Günther (1864) proposed Macrones aleppensis on
page 75 but withdrew the name on page 431 when he realised the species
was the Silurus pelusius of Solander.
No types of Silurus pelusius described from the "River
Kowick" (= Quwayq) are known (Eschmeyer et al., 1996). A
syntype of Macrones aleppensis, 127.8 mm standard length, from
the "R. Coic, Aleppo, Syria" is in the Natural History
Museum, London (BM(NH) 1955.6.25:1). Six syntypes of Macrones
colvillii are also in London, ca. 175-234 mm standard length, from
"R. Tigris nr. Baghdad" collected by Colville (BM(NH)
1874.4.28:6-8, 1875.1.14:19-21; Roberts (1994) agrees with my
observations although Eschmeyer et al. (1996) give BM(NH)
1875.1.14:19-20 for the latter, i.e. 2 fish only).
The holotype of Mystus misrai is in the Muséum d'Histoire
Naturelle, Geneva under MHNG 603.95, measuring 123.1 mm standard
length, with 1 paratype under MHNG 2231.84 measuring 117.6 mm standard
length, 1 paratype in the Zoological Survey of India, Calcutta under
FF2315 and 1 specimen missing (Eschmeyer et al., 1996).
Roberts (1994) indicates that more material should be examined to
compare fish with short barbels, weakly serrate dorsal spine, short
adipose fin and a highland distribution (= pelusius) while the
contrasting fish are colvillii.
Key characters
The 4 pairs of barbels, a strong spine in both the dorsal and
pectoral fins, elongate and strong adipose fin are distinctive. The
head tapers but is not as flattened as in Heteropneustes fossilis,
and the mouth is subterminal.
Morphology
The dorsal fin spine is smooth on most of the
outer edge and rough on the inner edge. Serrae can be weakly developed
or absent, or may be well developed. The dorsal fin spine has 1-4 serrae or
notches at the anterior tip and 5-9 along the rear margin, apparently not
related to fish size. The pectoral spine
is stronger than the dorsal spine and is serrated with 14-23 antrorse
teeth on the inner margin, the number increasing with size. The maxillary barbel extends back to the
pectoral fin origin or, rarely, as far as beyond the anal fin.
Dorsal fin with 1-2 spines and 7-8, usually 7, branched rays, anal
fin with 6-10 branched rays, pectoral fin with 1 spine and 7-9
branched rays and the pelvic fin with 5-6, usually 5, branched rays.
Al-Hassan and Hassan (1993) have shown asymmetry in pectoral ray and gill raker counts in samples of this species from the Shatt
al Arab, Iraq, possibly due to environmental stress. Total gill rakers 10-18 (Roberts (1994) gives 12(1), 13(7), 14(11),
15(11), 16(8), 17(4) and 18(1), reaching the second raker or further when appressed. Total vertebrae 42-46
(Roberts (1994) gives 42(10), 43(16), 44(3), 45(2) and 46(5). The gut has a large stomach followed by an
intestine with about 4 loops.
In specimens examined
by me dorsal fin with 2(11) spines and 6(1) or 7(10) branched rays, anal
fin with rays difficult to separate into branched and unbranched
(perhaps 4-6 unbranched and 6-10 branched rays), pectoral fin with 1(11) spine
and 7(3), 8(5), 9(2) branched rays and the pelvic fin with 5(11) branched rays. Total gill rakers 12(1), 13(2), 14(2),
16(2). One specimen with only 7 gill rakers, was possibly abnormal. Total
vertebrae 42(1), 43(1), 44(2) and 45(1).
Sexual dimorphism
Unknown.
Colour
Pale brown to olivaceous overall with fins and belly lighter, on a
predominant dark silver. Some fish may be silvery-grey overall when
fresh. A dark shoulder spot is present. There may be a black spot at the base of
the dorsal fin. The dorsal and anal fins have
melanophores on the rays and membranes and so are darker than the
other fins. The margin of the adipose fin is narrowly black. The
caudal fin has a black margin. There may be 3 (sometimes 2), narrow,
white stripes on the flank, one along and one each above and below the
lateral line. The stripe below the dorsal and adipose fins is narrower than the
others. Barbels are whitish, somewhat darker dorsally.
Peritoneum silvery to light brown. Jayaram and Sanyal (2003) report an albino
specimen from Baghdad.
Size
Reaches 171.2 mm standard length or 22.9 cm total length (Günther,
1874) but possibly to 30 cm total length (Firouz, 2005) or 33.0 cm (Al-Rudainy,
2008).
Distribution
Found in the Orontes, Quwayq, and Tigris-Euphrates basins. In Iran this fish
is found in the lower reaches of rivers in the Tigris River basin of Iran such
as the Karun, Karkheh and Jarrahi rivers,
including too the Zohreh River of the northern Gulf basin (Abdoli, 2000).
Also recorded from Fars near Darab in the Rudbal River drainage which flows to
the Straits of Hormuz, possibly an accidental introduction, although Esmaeili
and Coad (2005) point out that there is no evidence of fish introductions from
Khuzestan to Fars. It may simply be very rare outside the Tigris-Euphrates basin in Iran.
Zoogeography
Jayaram and Sanyal (2003) consider that Mystus is derived from an
African Bagrus-like ancestor and the genus spread from west to east.
Habitat
Niazi (1976) observed this species in rivers, marshes and brackish
waters in Iraq although summer kill resulted from very low water levels and increased salinity.
Age and growth
Al-Hassan et al. (1991) aged this species using eye lenses and
vertebrae for a population from the Qarmat Ali River north of Basrah, Iraq. Fish
up to 20 cm total length were examined and three age groups were determined,
with considerable overlap of lengths for each group. Al-Shami (1998) however
found 7 age groups (0+-6+) for the same river using vertebrae to age fish 54-223
mm total length. The highest growth was found in the first year and no
significant differences were found between males and females in growth rate. The
L∞ was 225.75 mm and the length-weight relationship log W = -4.7516 +
2.8173 log L. The relative condition ranged from 0.94 in December to 1.22 in
May. Heydarnejad (2009) gave the length-weight relationship for an
Iranian sample as W = 0.0277TL2.999.
Food
Roberts (1994) found eggs in the branchial chamber and stomach
apparently identical with those from the ovary. Other stomach items
were fish fin pieces and cyprinid fish scales. Aquatic insects,
crustaceans, detritus and plant remains are also found in stomach contents of
fish examined by me and Al-Rudainy (2008) also mentions fish. Al-Shami (1998) found mean feeding activity and
intensity in the Qarmat Ali River, Iraq to be higher in spring and summer, declining
in autumn and winter. This fish was carnivorous, taking mainly crustaceans but
also insects, fishes, molluscs and aquatic plants. Hussein and Al-Shami (2001)
also reported that fish in the Garma Canal, Iraq had a diet dominated by crustaceans
(the isopod Sphaeroma annandalei, amphipods, the decapod Elamenopsis
kempi, and the prawns Metapenaeus affinis and Atyaephyra
desmaresti), followed by aquatic insects (chironomids, corixids and
dytiscids), fish (Alburnus sp. and Aphanius dispar), molluscs (the
gastropod Lymnaea tenera euphratica) and aquatic plants. Feeding occurred
year round with a peak in May and a lowest value in November. Al-Shamma'a (2005)
found shrimp and insects to form 47% by volume of the diet of this fish at
Al-Fuhoud, Hawr al Hammar, Iraq.
Reproduction
The Qarmat Akli River fish attained maturity in the first year of life with
the smallest mature male 92 mm long and the smallest female 72 mm. Eggs were
laid in May and June with a fecundity range of 1156-25,833 eggs for fish 105-180
mm total length and 11.88-49.29 g in weight. Relative fecundity was 97.3-524.1
eggs/g (Al-Shami, 1998). Al-Rudainy (2008) gives a relative fecundity of up to
541 eggs/g in Iraq
Parasites and predators
None reported.
Economic importance
This species is of no economic importance. Anglers may catch it on
hook and line in Khuzestan but, being scaleless, it is not eaten.
Conservation
This species appears to be relatively common, although not often caught in
large numbers, and its conservation status has not been assessed.
Further work
See under Systematics.
Sources
Description also based on Anuradha and Jayaram (1985) and Anuradha (1986).
Type material: See above under Macrones aleppensis (BM(NH) 1955.6.25:1) and Macrones colvillii (BM(NH) 1874.4.28:6-8, 1875.1.14:19-21).
Iranian material:- CMNFI 1979-0087, 1, 162.2 mm standard length, Khuzestan, Karun River at Ahvaz (31º19'N, 48º42'E);
CMNFI 1979-0368, 1, 75.6 mm standard length, Khuzestan, Karkheh River (32<º24'30"N, 48º09'E);
CMNFI 1991-0153, 1, 123.3 mm standard length, Khuzestan, Zohreh River (no other locality data);
CMNFI 1993-0133, 1, 152.6 mm standard length, Khuzestan (no other locality data);
BM(NH) 1905.10.14:57, 1, 140.1 mm standard length, Bushehr, Jarrahi River 140 miles northwest of Bushehr (no other locality data);
ZMH 2524, 1, 137.1 mm standard length, Kermanshah, Karasu-Gamasiab-Seymarreh (no other locality data);
ZMH 4339, 2, 100.5-100.6 mm standard length, Khuzestan, Karun River (no other locality data);
uncatalogued material, 1, 57.7 mm standard length, Fars, Cheshmeh Golabi, 15 km west of Darab (28º47'N, 54º22'E)(Esmaeili and Coad, 2005).
Comparative material:- CMNFI 1980-1036, 2, 161.0-167.8 mm standard length, Turkey, Elazig, Keban Dam near Elazig (38º41'N, 39º14'E);
CMNFI 1987-0017, 1, 156.8 mm standard length, Iraq, Hawr al Hammar (no other locality data);
BM(NH) 1912.5.2:7, 1, 172.5 m standard length, Iraq, Shatt al Arab (no other locality data);
BM(NH) 1920.3.5:5-6, 2, 95.4-101.0 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1936.3.10:3, 1, 56.1 mm standard length, Iraq, Euphrates River at Nasiriyah (31º02'N, 46º16'E);
BM(NH) 1974.2.22:1781-2, 1, 52.3 mm standard length, Iraq, Khalis (33º49'N, 44º32'E);
BM(NH) 1974.2.22:1783-4, 1, 31.0 mm standard length, Iraq, Khalis (33º49'N, 44º32'E);
BM(NH) 1975.5.16:6, 1, 155.2 mm standard length, Turkey, Elazig, Euphrates River, Keban Dam Lake (no other locality data).
Siluridae
The sheatfishes are found in Europe and Asia. There are about 11
genera and about 97 species (Nelson, 2006) with 2 reported from Iran. The phylogenetic
relationships within the family are examined by Bornbusch (1995).
This family is characterised by a scaleless and elongate body; a
moderately compressed head; a non-protractile mouth; teeth on the jaws
and palate; 1-4 pairs of barbels (nasal barbels usually absent;
maxillary barbels 1-2 pairs, sometimes vestigial or absent); nostrils
separate, anterior ones tubular; 4-21 branchiostegal rays; gill
openings very wide; dorsal fin short and spineless (usually fewer than
7 rays and sometimes absent); anal fin very long (41 or more rays) and
may be confluent with the caudal fin; adipose fin absent; pectoral fin
with a spine, often serrated; and pelvic fins small to absent. The
largest species is found in Iran (Silurus glanis).
Genus Silurus
Linnaeus, 1758
These catfishes comprise about 5 species found from Europe to China and India.
This genus is characterised by an elongate body, rounded anteriorly
but compressed posteriorly; a depressed head; 2-3 pairs of barbels,
the maxillary barbels well-developed and often as long as, or longer
than, the head; a large and terminal or superior mouth; teeth in bands
on the jaws and roof of the mouth; nostrils well separated; eyes small
and not visible from the underside of the head; a very short and
spineless dorsal fin; no adipose fin; anal fin very long and united to
the rounded caudal fin; pectoral fin with a strong serrated spine; and branchiostegal
rays 12-15. The genus has been revised by Kobayakawa (1989).
Krieg et al. (1999) isolated microsatellite
loci in both S. glanis and S. triostegus and found that the
species diverged less than 20MYA and/or high levels of genomic
conservation. Krieg et al. (2000) investigated mitochondrial DNA in
S. glanis but found no consistent pattern of geographic structuring in
European populations, evidence that gene flow and migration between populations
were possible until quite recently. Their study also included S. triostegus
and the data was diagnostic for the two species.
A general Farsi name for these fishes is گربه ماهي (= gorbeh mahi, meaning cat fish).
Silurus glanis
Linnaeus, 1758
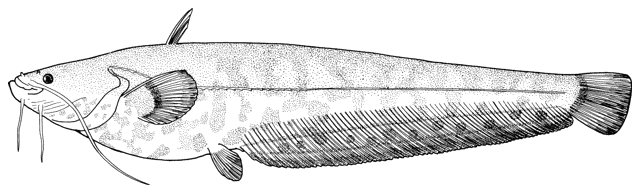

Aras River Dam, courtesy of Asghar Abdoli (measuring fish)
2.25 m, ca. 90 kg, 5 October 1994

Safid River, 2 km west of Astaneh, 4 June 1978, caught in a large dip-net
Common names
mahi-e sebili, esbele or esbeleh in Gilaki (probably
derived from sibil meaning moustache in reference to the barbels),
esbele-ye orupaiye, Urupai or Europaiye; ispek (Floor, 2003), گربه ماهي روگاهي
or gorbeh mahi rogahee, nake or naque in the Lake Orumiyeh basin, where
they are known as "whales".
[naxa, nagka or nakki in Azerbaijan; loko in Armenia; som in Russian; European catfish, sheatfish, wels,
wels catfish, Danube catfish].
Systematics
Silurus Glanis was originally described from lakes of
Europe, Sweden. A skin is a syntype in the Natural History Museum,
London under BM(NH) 1853.11.12:168 (Eschmeyer et al., 1996). An image of
this type is available at http://acsi.acnatsci.org/base/image_show_wrapper.html?target=230943. Silurus chantrei Sauvage, 1882
is possibly a synonym (see under Silurus triostegus).
Key characters
This species differs from S. triostegus by having
weaker and shorter teeth, the upper and lower jaws meet at an antero-dorsal
position (dorsal and superior position in S. triostegus),
a less serrate pectoral fin spine posteriorly, and a darker colour. Maxillary
barbel length is much longer on average, although there is some overlap. Anterior mandibular barbels are alwayslonger than
posterior mandibular barbels while in S. triostegus the posterior mandibular barbels are always longer
(Ünlü and Bozkurt, 1996).
Morphology
The lower jaw is longer than the upper. Adults have one pair of maxillary barbels and two pairs of
mandibular barbels, for a total of 6 barbels. The maxillary barbel is
much longer than the head (equal to head length in Silurus
triostegus). The pectoral fin spine is finely serrated or smooth
on its inner surface and smooth on its outer surface. Vomerine teeth
form a single broad patch, not two as in S. triostegus (Kobayakawa, 1989).
Dorsal fin branched rays 3-5, usually 4, anal fin branched rays 70-108,
pectoral rays 12-18 with 1 spine (generally higher on average than in S.
triostegus but still overlapping), and pelvic rays 1 unbranched
followed by 9-14 branched rays (Coad and Holčík,
2000; Reshetnikov, 2002). Vertebrae 70-76 and total gill rakers 9-17 (counts of 9 and 10 may be
lower arch rakers only). Total vertebrae 67-74. In specimens examined by me dorsal fin branched rays 3-4, anal
fin branched rays 83-87, pectoral rays 15-16 with 1 spine, and pelvic
rays 1 unbranched followed by 11-12 branched rays. Vertebrae 18-19 +
54-56 = 72-74. Total gill rakers 12, reaching the raker below when
appressed. The gut has a large stomach and an intestine with 3 about
loops. Chromosome number is 2n=60 (Ráb et al., 1994; Klinkhardt et al., 1995).
Sexual dimorphism
Abdurakhmanov (1962) reports that females have longer maxillary barbels, a longer postorbital length and a greater caudal peduncle
depth than males in Azerbaijan.
Colour
The body is mottled with brown, green or dark grey, even ventrally,
over the base colour. The back is dark, from olive-brown to a
blue-black, the sides lighter and the belly greyish-white with bluish
speckles. Fins are a dark red-brown to brown-violet. Paired fins have
a yellowish streak in the middle. Iris yellowish with black speckles.
This species can blend its colour with any bottom on which it lies in
wait for prey (Fortunatova, 1961).
Size
Reaches legendary sizes of 5.0 m and 367 kg ( and possibly over 500 kg) but most are much
smaller. In the Volga Delta females reach 1.75 m and 31 kg and males
1.95 m and 41 kg (Orlova, 1988); in Dagestan specimens up to 1.93 m
and 41.3 kg are recorded although fish weighing 3.2-4.8 kg predominate (Shikhshabekov, 1978).
In the Caspian Sea commercial fishery of Iran, this species ranges
in size from 41 to 186 cm and 0.6 to 42 kg (Farid-Pak, no date).
Sohrabi (1996a) reports the larger fish in Iranian waters usually
weigh 10-40 kg and depicts two fish from Gilan. One caught in 1995
weighed 27 kg, the other caught in 1994 weighed 62 kg. The record fish
from Iran, caught by a Mr. Haratonian weighed 120 kg and was 2.2 m
long. Eastwick (1864) bought a specimen 4.5 feet long (1.37 m) in the
Safid River which had a 7 lb (3.2 kg) fish in its stomach. De
Mecquenem (1908) reported that they reached 2 m in the Lake Orumiyeh
basin and Anonymous (1977) reports fish from there at 400 lbs (= ca.
182 kg). Asghar Abdoli of the Agricultural and Natural Resources
University, Gorgan kindly sent me a photograph of a specimen from the
Aras Dam caught 5 October 1994 which was 2.25 m long and weighed about 90 kg.
Distribution
This species is found in Europe, Central Asia and Southwest Asia. In Iran it
is found from the Aras River and Dam, along the whole Caspian coast from the Astara to the Atrak rivers
including the Anzali Mordab and its Siah Keshim Protected Region, the Safid
River, the Manjil Dam on the Safid River, the Amirkelayee Lagoon near Lahijan,
Gorgan Bay, and from the southwest Caspian Sea and south-central Caspian Sea;
and in the Lake Orumiyeh basin including the Nowruzlu Dam, Shapur-e Avval
Reservoir, Mahabad, Gader Chai at Ocksa (Günther, 1899; Nedoshivin and Iljin, 1929;
Berg, 1936; Nümann, 1966; Holčík and Oláh, 1992; Nejatsanatee, 1994; Riazi, 1996; Abbasi et al., 1999;
Kiabi et al., 1999; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009). Also reported from the Karakum Canal and Kopetdag Reservoir in
Turkmenistan (Aliev et al., 1988; Shakirova and Sukhanova, 1994; Sal'nikov, 1995) and may
enter Iranian waters of the Tedzhen (= Hari) River basin.
Abdoli (2000) maps this species from lower Gorgan, Neka, Babol, Heraz, Chalus,
Tonekabon, and Safid rivers, the Anzali Talab, along the Caspian coast, and in
the middle Aras River, and in the lower Talkheh and Zarrineh rivers of the Lake Orumiyeh basin.
Also said to occur in the Tigris-Euphrates basin in Iraq but needs specimens
for confirmation (see Freshwater Fishes of Iraq).
Zoogeography
The isolated populations of this wide-ranging species have not been studied.
It may well have an origin in the Ponto-Caspian basin from which it has dispersed.
Habitat
This large species is found in the larger water bodies over soft
bottoms and can tolerate brackish water (even for spawning) and
moderately low oxygen levels. Warm, deep waters with slow current are
preferred. It is active at night. Adults are solitary and found under
overhanging banks or submerged trees. This catfish overwinters in
aggregations on river beds. Resumed activity in spring depends on the
local water temperature regime, probably as early as March in Iran.
Riazi (1996) reports that this species is native (resident) to the
Siah-Keshim Protected Region of the Anzali Mordab. Knipovich (1921)
reports this species from depths of 23.8-25.6 m in the Iranian Caspian Sea.
Khodabandeh and Shahriari Moghadam (2007) examined the ultrastructure of
ionocyte cells which play an active role in osmotic regulation in this fish.
Movements, such as hunting for food, are stimulated by such
environmental factors as temperature, solar radiation, air pressure
and turbidity after rain.
It is sensitive to extra-aquatic sounds. The head canal system is very sensitive
and can track prey over distances up to 55 times the length of the prey and
follow signals up to 10 seconds old (Kottelat and Freyhof, 2007).
Age and growth
Life span is 22 years for males and 16 years for females in the
Volga Delta. Growth is most intensive in the first years of life.
After maturity the annual increase in length is 5-7 cm. Relative
weight increase is very high (30%) while length increase is 6-10%.
June-July is the period of greatest length increase while weight
increase takes place mainly in autumn. Maturity begins at 3-4 years,
57-66 cm and 1.3-2.3 kg but may be as early as 2 years, 51-52 cm and
1.2-2.2 kg or as late as the sixth year (Orlova, 1988). Maximum life span is 80
years.
Abbasi and Valipour (2005) found 9 age groups in the Anzali Lagoon with
females making up 68.5% of fish caught. Total length of 95 fish was 19.8-186.5
cm and weight was 47.7-30,000 g. Nezami Balouchi et al. (2007) found age
groups 1+ to 7+ in the Amirkelayeh Wetland with an average total length of 50.6
cm (range 32.5-73.0 cm) and an average weight of 944.2 g (range 200-3000 g).
Food
Food in the Volga Delta includes such fishes as Cyprinus carpio,
Abramis brama, Scardinius erythrophthalmus and, prior to
the regulation of the Volga, Rutilus rutilus (andéor R. caspicus) and herrings (Clupeidae).
Crustaceans now form part of the diet there (Orlova, 1988). At one
time this catfish in the Volga delta ate 62-68% of its annual
food in one month in spring when the Caspian roach (Rutilus rutilus -
this may be R. caspicus)
arrived on its spawning run. In the Kura region, commercial fishes
such as Cyprinus carpio, Abramis brama, Rutilus
rutilus (andéor R. caspicus), Aspius aspius, Sander lucioperca, Chalcalburnus
(= Alburnus) chalcoides, Barbus (= Luciobarbus) brachycephalus or Barbus
(= Luciobarbus) capito,
and Silurus glanis make up 30.27% by frequency and 20.18% by
weight, non-commercial species such as Scardinius erythrophthalmus,
Tinca tinca, Cobitis spp., Rhodeus amarus, Pungitius
platygaster, Atherina boyeri (= caspia) Alburnus alburnus
(= hohenackeri), Blicca
bjoerkna, and Caspiomyzon wagneri make up 50.68% and 33.79%
respectively, marine fishes such as Clupeidae, Mugilidae, and Gobiidae
make up 12.90% and 8.60% respectively, and crustaceans 25.64% and
17.09% respectively (Mamedov and Abbasov, 1990). In Azerbaijan,
Abdurakhmanov (1962) reports Gobio gobio to comprise 22.4% of
the diet, Caspiomyzon wagneri 15.7% and eggs 2.2%, Chalcalburnus
(= Alburnus) chalcoides 10.2%, Alburnus alburnus
(= hohenackeri) 10.2%, Cobitis
taenia 9%, Barbus lacerta 7.8%, Barbus (= Luciobarbus) capito 6.7%, Chondrostoma
oxyrhynchum 5.6%, Capoeta capoeta 3.4%, Blicca bjoerkna 2.2%, and loaches 4.5%.
In the Anzali Lagoon of Iran according to Abbasi and Valipour (2005), this
species ate 78.6% bony fishes, 15.8% crustaceans, 4.13% insects, 0.9% amphibians
and 0.5% bivalves. Carassius auratus dominated at 33.9%, followed by the
crustacean Macrobrachium spp., at 14.22%, Neogobius kessleri (=
Ponticola gorlap) at
4.59% and Proterorhinus marmoratus (= nasalis) at 2.75%. C. auratus dominated
in spring and autumn, Macrobrachium spp. in summer and N. kessleri
in winter. Consumption of fish increased with size, being 44.2% at 20-55 cm and
94.5% at 91-125 cm, Cannibalism was not observed and commercial fish stocks were
not consumed. Catfish in the Amirkkelayeh Wetland fed principally on Tinca
tinca and amphipods each at a frequency of 36.3%, along with minor amounts
of Blicca bjoerkna, Pungitius platygaster, Perca fluviatilis,
Proterorhinus marmoratus (= nasalis), Carassius auratus, Cobitis taenia,
frogs, rats, birds, water bugs and beetles, plecopterans and odonatans (Nezami
Balouchi et al., 2007). They were also cannibals.
Generally a wide variety of fishes is taken along with crayfish,
frogs and even birds and small aquatic mammals. It is a voracious
predator but stories of attacks on dogs and small children are more
legendary than factual although human remains may be scavenged (Gudger,
1945b). Active feeding occurs at water temperatures above 8°C
so winter feeding is minimal or absent. While feeding often occurs at
night, catfish can be heard feeding in the evenings by the snapping of
the mouth and tail strikes on the water. In cloudy water conditions
they come into shallow water to take earthworms, grasshoppers and
frogs washed in from nearby fields (Mihálik, 1982).
Young catfish feed on plankton, particularly Cladocera such as Daphnia,
Chydorus, Alona and Bosmina among others. Later
the diet involves mosquito larvae, larger crustaceans, organisms
associated with the river bank, worms, snails and young fishes.
Cannibalism occurs if food is short (Mihálik, 1982).
Reproduction
Behmanesh et al. (2009) found the maximum mean gonadosomatic index for
Anzali Lagoon fish was in May and June, decreasing to September. Maximum values
for gonad maturity were in April-May at water temperatures of 19.3-22.7°C.
Spawning was from April to mid-June.
Non-intermittent spawning in Dagestan takes place in late May
and continues to the middle of July when the eggs of females mature at
a water temperature of 20-22°C. Spawning rarely occurs below 20°C.
Males may actually have running milt 30-40 days earlier than this and
also later, a longer potential spawning season. Adhesive yellow eggs
are laid in depressions in weed beds, formed by the male pressing on
the plants. Fecundity is up to 285,000 eggs with diameters around 2-3
mm (to 467,000 elsewhere). Elsewhere spawning may be intermittent (Shikhshabekov, 1978).
The male guards the incubating eggs, even during the day, moving
his tail fin every 3-5 minutes to ensure adequate oxygen supplies.
Nests in Europe may be on the fine roots of plants which hang freely
in the water. The nest is in shallow, 40-60 cm, water. Males pursue
females just under the water surface, an indication spawning will
occur the same evening or the next day. Spawning usually occurs in the
evening, often before a thunderstorm on warm and stifling days. The
male nudges the female in the anal region, swims under her and may
lift her so that her back is above water, the male wraps himself
around the female for 10-12 seconds, the male and female separate and
the female sinks slowly to the bottom and discharges eggs, the male
following to release milt. This process can be repeated several times
over 1.5-2.0 hours and the water around the nest is milky from sexual
products. Eggs hatch after 2.5-3.0 days at 23-25°C.
Larvae are light sensitive and die in direct sunlight ands also if
water temperature falls below 13-14°C (Mihálik, 1982).
Parasites and predators
Mokhayer (1976b) records the digenetic trematodes Aphanurus
stossichi and Bunocotyle cingulata, the nematode larvae Anisakis
sp. and the nematode adults Cucullanus sphaerocephala, and the
acanthocephalan Corynosoma caspicum. Ataee and Eslami (1999,
www.mondialvet99.com, downloaded 31 May 2000) report the helminth Mazocea
alaosa from the gastro-intetsinal tract of fish from the Anzali
wetland. Masoumian et al. (2005) recorded the protozoan parasite
Trichodina perforata from this species in the Aras Dam in West Azarbayjan. Khara et al. (2006a) record the eye
fluke Diplostomum spathaceum for this fish in the Amirkalayeh Wetland in
Gilan. Sattari et al. (2004; 2005) surveyed this species in the Anzali and Amirkelayeh wetlands, recording
Raphidascaris acus, Raphidascaroides sp. and Eustrongyloides excisus.
Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Cyprinacea sp. and
Ergasilus sp. on this species.
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
There is some opportunity for sport fishing for this species in the
Anzali Mordab and Lake Orumiyeh basin where it will take spinners and
spoons as well as frog live bait. It reputedly puts up a tremendous
fight (Anonymous, 1977).
Nevraev (1929) reports on catches in various regions of Iran in the
early years of the twentieth century. There were no evident trends of
increase or decrease. In the Astara region from 1901-1902 to 1913-1914
the catch varied irregularly from 699 to 4031 fish, in the Anzali
region from 1901-1902 to 1918-1919 the catch varied from 18,177 to
206,485 fish, in the Safid River region from 1899-1900 to 1917-1918
the catch varied from 3290 to 43,835 fish, in the Mazandaran region
from 1906-1907 to 1913-1914 the catch varied from 5282 to 11,283 fish,
and in the Astrabad region from 1902-1903 to 1912-1913 the catch
varied from 3500 to 26,200 fish. The commercial catch in Iran from
1956/1957 to 1961/1962 varied between 4,913 kg and 37,630 kg (Vladykov,
1964) and from 1965/66 to 1968/69 varied from 11 to 31 tonnes (Andersskog,
1970) but in the 6 years from 1980 to 1985 catches were recorded by
the Food and Agriculture Organization, Rome as respectively 2, 2, 0,
2, 3, and 0 tonnes. The catch has been as high as 107,593 kg for the
Anzali region alone in 1934/1935 (Vladykov, 1964). Holčík
and Oláh (1992) report a catch of 2663 kg in the Anzali Mordab in
1990, at 3.6% of the catch the sixth most important fish there, and
from 1932-1964 reported catches varied from none to 12.6 tonnes
annually. The Iranian fishery peaks in October and in April (Farid-Pak,
no date). This species is of great commercial importance in Dagestan (Shikhshabekov,
1978). The roe has been used as a form of caviar and glue has been
made from the swimbladder and bones. In the Lake Orumiyeh basin this
fish was used as fertiliser since, being scaleless, it could not be
eaten for religious reasons (De Mecquenem, 1908). Its export value is recognised
and studies have been carried out on this species such as the effect of ascorbic
acid and citric
acid on the lipid stability and rancidity inhibition of fillets in frozen storage (Pourashouri et al.,
2008a. 2008b; Pourashouri et al., 2009).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use as food, for industrial
processes (such as fertilizers, fish meal, pet food, novelty products
although not specified for this species), in sport, and in textbooks.
The swimbladders were used for isinglass in the Caspian Sea basin (Lönnberg, 1900a).
This species has been implicated in ichthyootoxism, the symptoms of
which are summarised under the genus Schizothorax. The presence
of venom associated with the pectoral fins (Coad, 1979b) needs
definitive examination.
Conservation
Lelek (1987) classifies this species as rare to vulnerable in
Europe because of habitat changes and angling pressure. Kiabi et al.
(1999) consider this species to be of least concern in the south
Caspian Sea basin according to IUCN criteria. Criteria include
commercial fishing, sport fishing, medium numbers, habitat
destruction, widespread range (75% of water bodies), present in other
water bodies in Iran, and present outside the Caspian Sea basin. Vulnerable in
Turkey (Fricke et al., 2007).
Further work
Population numbers need to be monitored carefully as it is fished for
although it lacks scales. Populations in isolated basins have not been examined
in detail for their relationships to see if they are distinct.
Sources
Kobayakawa (1989) revised the genus Silurus and his data are
incorporated here. Mihálik (1982) reviewed the biology of this catfish.
Iranian material: CMNFI 1970-0509, 1, 131.2 mm standard length, Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1979-0685, 2, 63.9-107.7 mm standard length, Gilan, Safid River below Dehcha (ca. 37º22'N, ca. 50º06'E);
CMNFI 1979-0788, 2, 293.5-335.6 mm standard length, Mazandaran, Gorgan River at Khvajeh Nafas (37º00'N, 54º07'E);
CMNFI 1979-1236, 1, 269.0 mm standard length, Mazandaran, Gorgan River at Khvajeh Nafas (37º00'N, 54º07'E);
CMNFI 1980-0123, 1, 225.3 mm standard length, Gilan, Safid River around Dehcha (37º22'N, 50º06'E);
CMNFI 1980-0905, 1, 208.0 mm standard length, Mazandaran, Gorgan River at Khvajeh Nafas (37º00'N, 54º07'E);
OSU 4278, 2, 227.2-280.2 mm standard length, Azarbayjan-e Bakhtari, Zarineh River (no other locality data).
Silurus triostegus
Heckel, 1843
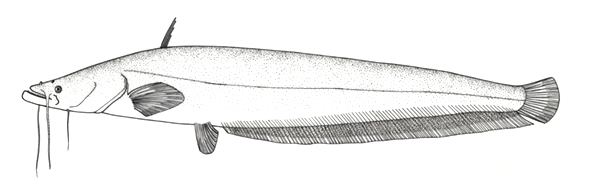
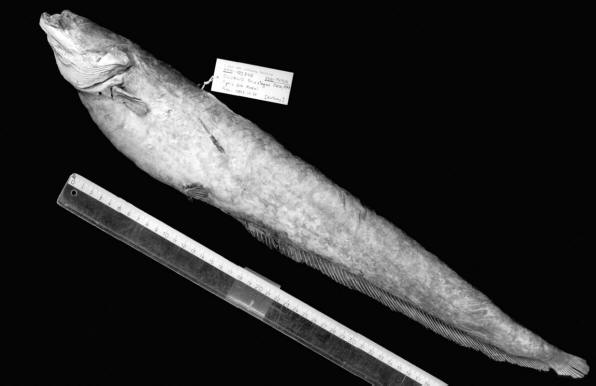
NMW 92345, syntype, Tigris River near Mosul, ca. 582 mm standard length
Common names
esbele, and jirri, yeri, yery, yari or iry (= eel), esbele-ye
beinolnahrein (= Mesopotamian catfish); sag mahi (= dog fish) in
Khuzestan; gorbeh mahi (= catfish).
[djirri, jirri, girri or yerri (= eel) in Arabic; Mesopotamian catfish,
Tigris catfish, Asian djirri].
Systematics
Silurus chantrei Sauvage, 1882, a species with 4 barbels,
was described from the "Fleuve Koura à Tiflis (= Kura River at
Tbilisi, Georgia) but was possibly based on material from the
Tigris-Euphrates basin (Berg, 1948-49; Haig, 1952) and may well be a
synonym of this species. Two syntypes of Silurus chantrei,
160-170 mm total length, are in the Muséum national d'Histoire
naturelle, Paris under MNHN A.3932 (Eschmeyer et al., 1996).
Günther (1899), Banister (1980) and Hora and Misra (1943) considered that S. triostegus may not be
distinct from S. glanis but Coad and Holčík (2000) detail differences.
The type locality of Silurus triostegus is the "Tigris
bei Mossul" according to Heckel (1843) and the description was
based on 4 specimens although the catalogue in Vienna lists 1 specimen
in spirits and 2 stuffed specimens. The card index in Vienna in 1997
lists only NMW 92345 as a dried syntype. Coad and Holčík (2000) found only
a single stuffed type, ca. 582 mm standard length. Eschmeyer et al. (1996) list 1 dried syntype of triostegus
in the Senckenberg Museum Frankfurt (SMF 2623, formerly NMW) and this has a
standard length of ca. 710 mm. An image of S. triostegus (SMF 2623) is available
at http://acsi.acnatsci.org/base/image_show_wrapper.html?target=221708.
Key characters
This species differs from S. glanis by having robust and longer teeth
(snaggly, catching on flesh), the upper and lower jaws meet at a dorsal and
superior position (antero-dorsal in S. glanis), a distinctly and coarsely
serrate pectoral fin spine posteriorly, and a lighter colour. Maxillary barbel
length is about equal to head length while in S. glanis it is much longer
on average, although there is some overlap. Anterior mandibular barbels (when present) are always shorter than
posterior mandibular barbels while in Silurus glanis the
anterior mandibular barbels are always longer (Ünlü and Bozkurt, 1996).
Morphology
Bears 4 barbels in some specimens as opposed to the usual 6 in S.
glanis but there is evidence that the four-barbelled Silurus
(sometimes placed in a distinct genus Parasilurus Bleeker,
1862, e.g. in Berg (1949), now synonymised with Silurus - see
Eschmeyer (1990)) have 6 barbels when young and one pair of mandibular
barbels is reabsorbed (see Haig, 1952). Specimens with one pair and
with two pairs of mandibular barbels have been reported (Kobayakawa,
1989). Ünlü and Bozkurt (1996) record 4 mandibular barbels (2 pairs)
in 3 specimens, 3 barbels in 1 specimen and 1 pair in another specimen
for Turkish Euphrates fish. In Silurus triostegus, the adults
apparently lose one pair of barbels (F. Krupp, in litt., 1992). Coad
and Holčík (2000) found all S. glanis in their study had 4 mandibular barbels (2
pairs) while in S. triostegus 11 fish had 4 mandibular barbels and 12
fish lacked the posterior mandibular pair. There was no apparent trend in barbel
loss associated with increase in body size.
The pectoral fin spine is strongly serrated on its inner surface and smooth
on its outer surface. Vomerine teeth are in two patches, not one as in
Silurus glanis (Kobayakawa, 1989), although Ünlü and Bozkurt
(1996) record some specimens of S. triostegus with only one
patch. The maxillary barbel reaches only to the end of the head, not
much longer as in S. glanis. However, Ünlü and Bozkurt (1996)
report that maxillary barbels are longer than the head and later that
they reach the end of the head; it is suggested here that barbel
length varies individually. The lower jaw is longer than the upper
jaw. Teeth in both jaws are recurved, the band of teeth is wider than
in S. glanis, and the teeth are stronger and longer. The eye is
larger than in S. glanis.
Dorsal fin branched rays 3-4 (counts of 3 rays are more common than in S.
glanis; 3 in ten fish and 4 in thirteen fish examined by Coad and Holčík
(2000)), pectoral fin branched rays 11-14 with
1 spine, pelvic fin branched rays 8-13 after 1 unbranched ray, anal
fin rays 77-94, vertebrae 16-17 + 52-53 = 69-70, and total gill rakers
12-17, reaching the one below when appressed (Coad and Holčík,
2000). The stomach is large, and apparently more elonagte than in S. glanis although this
may be distortion due to food content. The intestine has about 3 loops.
Sexual dimorphism
Unknown.
Colour
The upper body is mottled pale yellow-brown and black. Overall colour may
appear dark or light and yellowish. Generally much
lighter than Silurus glanis. The belly
and lower head are white with the belly having black spots. Maxillary
barbels and margin of the lower jaw very dark brown.
Size
Reaches 1.5 m
in the Syrian Euphrates (Gruvel, 1931) and to more than 2.0 m (Krupp, 1992) and
more than 50.0 kg.
Distribution
This species is found in the Tigris-Euphrates basin including its Iranian
portion in Khuzestan and such rivers as the Arvand, Bahmanshir, and Jarrahi, and
the lower Karun, Karkheh and Dez (Marammazi, 1995; Abdoli, 2000).
Zoogeography
This species is presumably a relative of S. glanis but its closest
affinities may lie with species to the east. Differentiation of the two species
may have occurred around the Middle to late Miocene but this requires further
study (Coad and Holčík, 2000).
Habitat
van den Eelaart (1954) reports this species from open and vegetated
lakes and marshes and rivers in Iraq. The larger fish are mostly
confined to rivers, entering marshes and lakes only on floods. The
young have a greater tolerance of high temperatures and low oxygen.
Age and growth
Al-Abood (1989) found age groups 2 to 7 years for fish from a marsh
area north of Basrah, Iraq in 6 weight groups from 300 to 3900 g. Al-Hassan
and Al-Sayab (1994) examined 600 specimens from the Al-Hammar Marsh
north of Basrah, Iraq for age using vertebrae and eye lens diameter
and found 6 age groups. Oymak et al. (2001) describe 11 age groups
in Atatürk Dam Lake, Turkey with females having higher L∞ (202.85 cm versus
113.98) and lower K (0.046871 versus 0.101972) values than males. Males matured
at age 3 and females at age 4.
Food
Fish are an important food including Liza abu and Acanthobrama
marmid in the Iraqi marshes and presumably those across the border in Iran (Al-Shamma'a and Jasim, 1993; Ünlü and Bozkurt, 1996;
Dawood, 1997; personal
observations). Aquatic insects are also taken but fish predominate. The food in Hawr al Hammar, Iraq was predominately fish (Liza abu,
Aphanius spp., Aspius vorax, Thryssa spp., Acanthobrama
marmid, Silurus triostegus (young less than 16 cm were eaten by
adults longer than 35 cm), Barbus (= Mesopotamichthys) sharpeyi, Heteropneustes fossilis
and Cyprinus carpio) followed by shrimps (mainly Metapenaeus affinis),
frogs (Rana esculenta) and crabs (mainly Sesarma boulengeri) with
relative importance indices of 70.8, 16.3, 6.4 and 4.9 respectively (Al-Daham
and Al-Seyab, 2000). Liza abu was the most important fish through most of
the year (except July and August when absent)(relative importance index 42.0,
followed by C. carpio at 11.5), in numerical abundance and total weight.
During July the prey was B. sharpeyi and C. carpio and in August
prey was restricted to C. carpio, presumably opportunistic feeding. Other
fish species were mostly young of the year and of minor importance. The diet at
Al-Fuhoud in the Hawr al Hammar was 70.7% fish by volume (Al-Shamma'a, 2005).
Aquatic insects are also taken but fish predominated. Dawood (1997) also studied
diet in the southern Hammar Marsh and found fish to be the most important prey
year round while shrimps (Metapenaeus affinis) and molluscs were
important in certain months (mostly absent April-August). The
disappearance of shrimps probably relates to their migratory pattern. Aquatic
insects were found mostly in the spring. There is a reverse relationship between
the presence of fish and shrimps. Fish and shrimps increased in the diet with
increase in size while aquatic insects, molluscs and small crustaceans decreased
with size. Fish species eaten were Liza abu, Barbus (=
Carasobarbus) luteus,
Alburnus sp., Cyprinus carpio, Thryssa hamiltoni,
Heteropneustes fossilis, Aphanius sp., Gambusia holbrooki and
Silurus triostegus. Frogs, detritus and aquatic plants were also found in
the gut contents. Feeding occurred more at night and with another peak in late
afternoon. The index of fullness values increased in April-September when water
temperatures and metabolic rate rose.
Reproduction
Spawning takes place in March in Iraq (van den Eelaart, 1954; Al-Hassan et
al., 1990) to May and June in Turkey (Oymak et al.,
2001). The highest condition factors were found in
April in Atatürk Dam Lake, Turkey, the mean egg diameter was greatest in May at
1.937 mm and fecundity attained 120,300 eggs (Oymak et al., 2001).
Al-Rudainy (2008) gives sexual maturity in Iraq at 3 years, 50 cm total length
and 1 kg weight, spawning in May and continuing for a few months, eggs being
deposited on vegetation and egg diameters up to 4.2 mm.
Parasites and predators
Mortazaei et al. (2000) report an infection rate of 33.3%
(22 of 6 fish) with the parasitic worms Proteocephalus sp. in
this species from Khuzestan marshes.
Economic importance
This species forms 8.5% of the total catch in Iraq (Das et al.,
1978), the total catch in 1976 being 691 t (Petr, 1987), but it is not
a popular food. As a scaleless fish it is not eaten by Shi'a Muslims.
It was found to have potential for use as a protein concentrate.
Conservation
This species is not commonly collected in Iran but this may be a consequence
of habitats sampled and gear used. It may be under some threat as it is fished
for, at least in neighbouring Iraqi waters.
Further work
Population numbers have not been examined for fish in Iran nor are its biology
or relationships well known.
Sources
Kobayakawa (1989) revised the genus Silurus and his data are
incorporated here as is the data of Ünlü and Bozkurt (1996) for Turkish Euphrates specimens.
Type material: See above, Silurus triostegus (NMW 92345).
Iranian material: CMNFI 1993-0133, 1, 192.9 mm standard length, Khuzestan, probably Karun River at Ahvaz (31º19'N, 48º42'E);
SNM-YR 6421, 1, 325.6 mm standard length, Khuzestan, Karun River at Ahvaz (31º19'N, 48º42'E);
ZSM 21832, 1, 406.0 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E);
ZSM 21833, 1, 425.4 mm standard length, Khuzestan, Dez River at Harmaleh (31º57'N, 48º34'E);
uncatalogued, 1, 317.7 mm standard length, Khuzestan, probably Karun River at Ahvaz (31º19'N, 48º42'E).
Comparative material: BM(NH) 1874.4.28:3-5 and 1875.1.14:8 (same jar), 4, 236.7-511.5 mm standard length, Iraq, Tigris River near Baghdad (ca. 33º21'N, ca. 44º25'E);
BM(NH) 1888.5.17:1, 1, 336.2 mm standard length, Iraq, Fao, presumably a fish market (no other locality data);
BM(NH) 1893.6.23:26-28, 3, 254.3-425.7 mm standard length, Iraq, Fao (no other locality data);
BM(NH) 1892.9.1:26, 1, 270.8 mm standard length, Iraq, Fao (no other locality data);
BM(NH) 1920.3.3:167-176, 13, 123.1-490.9 mm standard length, Iraq, Basrah (30°30'N, 47°47'E).
Sisoridae
The sisorid or sucker catfishes are found in Asia as far east as
Borneo. There are about 17 genera with about 112 species (Nelson, 2006). They are
mostly small (as small as 2 cm) although some are very large (2 m).
Five nominal species are reported from the Tigris-Euphrates basin in
Southwest Asia but the diversity there is very limited, compared to
India for example. A single specimen of a sisorid catfish has been
caught in the Yeşil Irmak of Anatolian Turkey at Taşova (40°46'N, 36°20'E).
This Black Sea drainage specimen calls into question the utility of
characters used in identifying and defining species in Southwest Asia.
Its characters are a mixture of features shared by G. armeniacus
and G. silviae. A wide range of specimens of both sexes,
various age groups and from various localities is not available to assess
variation and resolve the species composition of the Southwest
Asian fauna (Coad and Delmastro, 1985)).
The sisorid catfish family is characterised by a rounded to
compressed body and a flattened head; short dorsal fin positioned
anterior to the level of the pelvic fins; a spine in the dorsal fin
(absent in some non-Iranian species) and in the pectoral fin; a
well-developed adipose fin; a short anal fin; paired fins horizontal;
gill membranes generally united to the isthmus; anterior and posterior
nostrils close together; distinct nasal barbel present; 4-6 pairs of
barbels; body with small tubercles (unculi); and a distinct thoracic
adhesive apparatus in Iranian species but absent in some other species.
These catfishes are found in mountain streams where they use the
adhesive apparatus to maintain position in the current. In Iran they
are reputed to lie on their backs in the water to take a rest! They
are also very resistant and can live for 6 hours wrapped in wet cloth,
reviving when placed in an aquarium (R. Mehrani, pers. comm., 2000).
Genus Glyptothorax
Blyth, 1860
This genus is characterised by a flattened head, an adipose fin of
moderate length, a short dorsal fin with a strong spine, the spine
serrated anteriorly or posteriorly, or smooth, pectoral fin spine
serrated posteriorly and in some with plicate skin ventrally, 4 pairs
of barbels, maxillary barbels broadly based, an inferior and
transverse mouth, villiform teeth on the roof of the mouth in two
patches, eyes small and partly obscured by skin, gill openings wide,
gill membranes joined to the isthmus, and an adhesive apparatus on the
chest formed from plaits or folds of skin, often with a central depression.
The validity of the described Glyptothorax species in the
Tigris-Euphrates has not been adequately resolved. Three other nominal
species occur in addition to the two reported here from Iran, namely G.
armeniacus (Berg, 1918), G. cous (Linnaeus, 1766), and G.
steindachneri (Pietschmann, 1913) (see Coad and Delmastro (1985)
for a partial discussion of this problem). Resolution of the taxa
found in Iran awaits more extensive material for a better
understanding of individual and species variation.
Aglyptosternon Bleeker, 1862 (and such mispellings as Aclyptosteron,
Enclyptosternum and variants- see Eschmeyer (1990) for details)
are synonyms of Glyptothorax (Li, 1986; Eschmeyer, 1990).
Confusion over the family placement of these fishes in various
literature sources is reviewed in Banister (1980).
They are known generally as گربه ماهي
(= gorbeh mahi, meaning cat fish) or arteshi in
Farsi. Arteshi (meaning soldier-like) may be from their tank-like appearance
or for their pigmentation which is said to resemble camouflage. Another general
name is سگ ماهي (sag mahi, meaning dog fish).
General names are not repeated below.
Menon (1954) considered that the members of this genus had spread
westwards along the Himalayas as late as the early Pleistocene. A
centre of origin in western Yunnan and the southern slopes of the
Himalayas is advocated by Li (1986). This author suggests that a
Pliocene movement occurred westwards and that, as well, the
distribution of Glyptothorax was influenced by Pleistocene
glaciations. Their entry route into the Tigris-Euphrates basin is
given as along the Amu Darya system.
Glyptothorax armeniacus
(Berg, 1918)
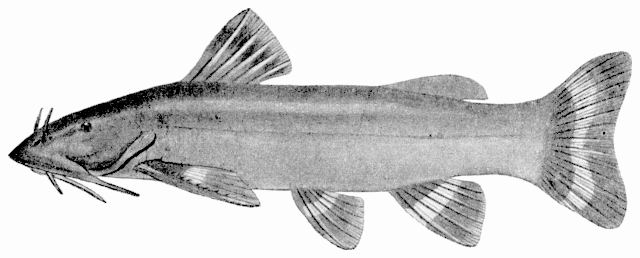
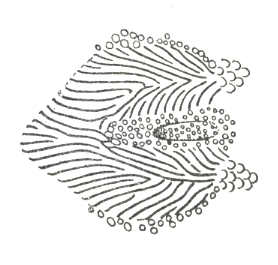
Found in the Euphrates River basin southeast of Erzurum in Turkey but no Iranian record.
Glyptothorax cous
(Linnaeus, 1766)
Reported from the Tigris River basin in Iraq but no Iranian record.
Species identity in the Tigris needs confirmation by specimens. See Banister (1980) for a brief history of the confusion surrounding
the name of this species in the literature.
Glyptothorax kurdistanicus
(Berg, 1931)
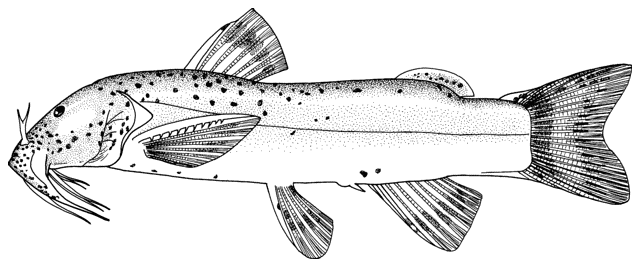
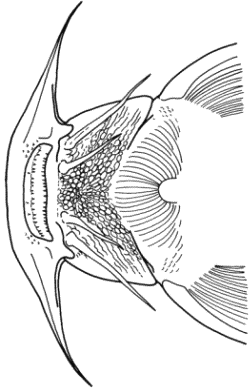
Common names
gorbehmahi-ye Kordestan.
[Kordestan sisorid; Iran cat (Fricke et al., 2007)].
Systematics
This species was originally described in the genus Glyptosternum,
an unjustified emendation of Glyptosternon McClelland, 1842 by
Berg (1931a). See Eschmeyer (1990) for further details on this genus name.
The holotype of this species is in the Zoological Institute, St.
Petersburg (ZISP 20780) and is in poor condition, the pectoral spines
being damaged for example. It is an adult male. The collection date in
Berg (1931a) is 10 July 1914 (or 27 June old style). The type locality
is "in Kurdistan, at the village Germau (or Germav), at the
height of 1500 m, during the works of the Turko-Persian delimitation
commission. Germau (or Germav, Germaw) is situated in latitude 36°N
south-east of Serdesht, on the western slope of the Sur-kei Range, in
the basin of the river Bané, tributary to the Little Zab, which is
tributary to the Tigris R.". Berg (1949) gives the variants
Germab and Sar Dasht for the localities and Bané is probably Baneh.
The village Germab could not be located in gazetteers or on maps but
Sar Dasht (36°09'N, 45°28'E) and Baneh (35°59'N, 45°53'E)
are evident and the locality is between them.
Key characters
Berg (1931a) separates this species from G. armeniacus by
the broader than long adhesive apparatus which does not have pinnate
lateral branches and these characters also contrast with G. silviae.
The caudal peduncle is short (5.9-6.0 in standard length compared to
4.7-5.2 in G. silviae).
Morphology
Dorsal fin with 1 spine followed by 5-7 branched rays, anal fin
with 2 unbranched rays followed by 7-9 branched rays (note that these
fin ray counts in Berg (1931a) do not agree with his figure). Pectoral
fin with 1 spine and 7-9 branched rays. Total gill rakers 7-9,
moderately long and reaching beyond the base of the second raker below
when appressed. The adipose fin is short, much shorter than the
distance between the dorsal and adipose fins. There are oblique osseus
striae under the skin of the upper surface of the first pectoral ray.
Head and body covered with minute, elongate tubercles oriented
longitudinally but without striae. Tubercles are also present on the
base of the caudal fin rays, adipose fin, base of the dorsal and
pectoral fins, on the pectoral spine along its whole length both
dorsally and ventrally, a few on the base of the pelvic fin rays and
few to none on the belly particularly anterior to the pelvic fins.
Tubercles on the side of the head are more rounded. Berg (1931a)
states that the upper jaw tooth patch has well-developed lateral rami,
but these do not extend markedly from the main patch. The nasal barbel
is short and does not extend back to the eye. The maxillary barbel is
shorter than head length and the mandibular and mental barbels are
progressively shorter. The gut is an elongate s-shape after a muscular stomach.
Sexual dimorphism
Unknown.
Colour
Overall colour grey to brown with large, obvious, round, black
spots and blotches on the sides or with small round black spots about
eye size. All fins with broad black central band and variably
developed basal bar. Basal bar most evident on the caudal fin. Adipose
mostly covered with a large dark spot but dorsal and posterior edges hyaline.
Size
Attains 267.2 mm total length.
Distribution
Found in the Tigris-Euphrates basin including that part in Iran. Abdoli (2000) has
mapped it in the upper Karun, middle and lower Dez, middle and upper
Karkheh, Kashkan, Simarreh and lower Gav Masiab rivers. Jawad et al.
(2009) record it from the Garaf River of the lower Tigris River basin in Iraq
Zoogeography
The relationships of this species, as with other members of the genus, is
presumably with the more diverse fauna to the east.
Habitat
Unknown in detail but it is assumed to be in rocky and gravelly rivers which provide hiding places.
Age and growth
Unknown.
Food
Unknown for Iran but Turkish specimens contained fish remains in the stomach.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
This species is not of any direct economic importance.
Conservation
This species is poorly known in Iran and may be rare enough to warrant conservation efforts.
Further work
See under G. silviae.
Sources
Type material: See above, Glyptosternum armeniacum (ZISP 20780).
Iranian material: None.
Comparative material:- BM(NH) 1974.2.22:1789, 346.6 mm standard length, Iraq (no
other locality data); BM(NH) 1968.12.13:465-470, 4, 53.7-76.8 mm standard length, Syria, Euphrates at
Mayadine (35º01'N, 40º27'E); SMF 23676, 4, 229.0-297.7 mm standard length, Syria,
bei al-Hasaka (36º30'N, 40º44'E); SMF 23677, 2,
65.7-122.0 mm standard length, Syria, Wadi Furati (36º26'N, 40º52'E); ZMH 4430, 2, 129.8-133.3 mm standard length, Turkey, Kemaliye
Karasu (no other locality data).
Glyptothorax silviae
Coad, 1981
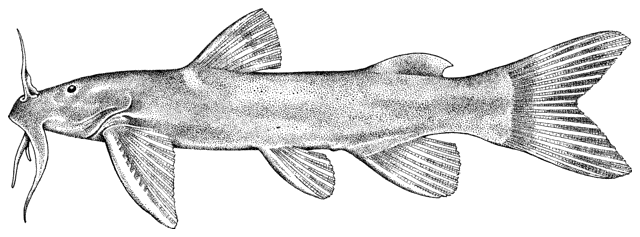
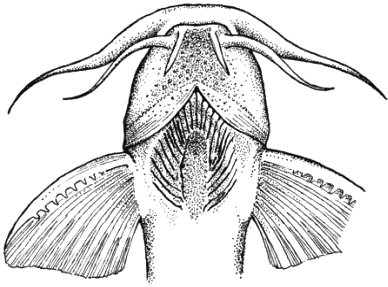
Common names
gorbehmahi-ye jonubi or jonub (= southern catfish).
[Sylvie's sisorid, southern sisorid].
Systematics
The holotype is in the Canadian Museum of Nature, Ottawa under CMNFI 1979-0390A and measures 67.6 mm standard length. It is from "Khuzestan,
stream 3 km south of Bagh-e Malek, tributary to Rud-e Zard or Ab-e Ala
in the drainage of the Jarrahi River, 31°29'N, 49°54'30"E".
Two paratypes from the same locality measure 44.0-51.5 mm standard
length and are under CMNFI 1979-0390B, a third paratype measuring 42.3 mm
standard length under CMNFI 1979-0389 is from a "stream tributary to
Rud-e Zard or Ab-e Ala, 1 km south of Bagh-e Malek, in the drainage of
the Jarrahi River, 31°31'N, 49°53'30"E",
and a fourth paratype measuring 134.8 mm standard length under CMNFI 1979-0280 is from "Lorestan, river at "Pol-e Chubee" in
Kashkan River drainage on Khorramabad to Kermanshah road via Nurabad
(either Kaka Reza River at 33°43'N, 48°15'E or its tributary at 33°47'N,
48°12'E)".
Key characters
The head and body dorso-laterally lack striated or elongate
tubercles (present in G. kurdistanicus). The thoracic adhesive
apparatus is longer than wide (the reverse in G. kurdistanicus).
The caudal peduncle is long (4.7-5.2 in standard length, 5.9-6.0 in G. kurdistanicus).
Morphology
Dorsal fin spines are smooth and number 2, branched rays 6; anal
fin branched rays 8; pectoral fin branched rays 7-9; pelvic fin
branched rays 5; total gill rakers 6-9; retrorse pectoral fin spine
teeth 7-16, the number increasing with size of the fish; and total
vertebrae 35-38. The adipose fin is long, its length being about equal
to the distance between the dorsal fin insertion and the adipose
origin (0.9-1.1). The pectoral fin is short and does not extend back
to the pelvic fin origin. The caudal peduncle is deep (depth 47-62% of
caudal peduncle length). The "sucker" or thoracic adhesive
apparatus has pinnate lateral branches and is markedly longer than
wide with a wide and long central depression. The head and body are
finely papillose, particularly on the ventral surface. Anterior to the
adhesive apparatus the ventral head surface is strongly papillose,
becoming less developed laterally. The upper lip is much more strongly
papillose than the weakly papillose lower lip. The diploid chromosome number is
2n=52 and the karyotype consists of 9 pairs of metacentric, 14 pairs of
submetacentric and 3 pairs of subtelocentric chromosomes. The arm number is 98
(Esmaeili et al., 2009).
Sexual dimorphism
Unknown.
Colour
The body is nearly immaculate but in live fish is mottled light
lime- green, grey-green, brown or grey. All fins have a central black
bar on a salmon-pink, peach or yellow coloured background. The
thoracic adhesive apparatus is pink due to an underlying vascular
supply. The eye is red.
Preserved fish may have very few scattered brown or black spots
dorsally and laterally on an otherwise immaculate body. The overall
body colour is brown becoming pale brown or cream on the belly. The
base of the caudal fin has a wide black bar separated from a second
distal bar by an unpigmented section of the fin rays. The central-most
4 rays of the caudal fin are variably black in the otherwise
unpigmented bar. The postero-dorsal and postero-ventral corners of the
caudal lobes are not pigmented but the margins of the lobes are black.
The central portion of the adipose fin is black with the margins
unpigmented in smaller specimens. There is no black pigment on the
basal part of this fin. The paired fins and the anal fin are
unpigmented distally but become yellowish with fleshy tissue
proximally and then brown at their bases. A central band is not
well-defined in small specimens. A light patch is found on the back at
the dorsal fin origin and at the dorsal fin insertion. The dorsal fin
is darkly pigmented and a central black band is apparent although
poorly defined. The adult female is generally darker than the smaller
male fish in the type series such that the caudal fin bars are not as
well defined, The adipose fin is dark brown and the light patches at
the dorsal fin are not distinctive. However the bars on the dorsal,
anal and paired fins are more obvious.
Size
Reaches 13.5 cm standard length.
Distribution
Known only from rivers draining to the Persian Gulf in southwestern
Iran. Abdoli (2000) has also mapped it in the upper Karun and middle to lower Khersan,
and middle to lower Dez rivers in the Tigris River basin and in the Mand and Shur
rivers of the Gulf basin (the latter tributary to the Dasht-e Palang River).
Zoogeography
This species is known from waters in southern Khuzestan and from a
single specimen from the Gulf basin. This latter is probably a relict of the
late Pleistocene when the Tigris-Euphrates flowed down a drained Gulf receiving
tributaries now isolated by the post-Pleistocene rise in sea level (Coad and Krupp, 1983).
Habitat
Their ability to use their sucker for clinging to objects can be
seen in plastic jars where small specimens will adhere to the sides out of the water.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 10 fish measuring 5.60-10.66 cm fork length. The a-value
was 0.0164 and the b-value 2.975 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
The largest specimen in the type series is a female bearing eggs
and was caught on 6 July. The breeding season is probably the summer months.
Parasites and predators
Unknown.
Economic importance
This species is not of any direct economic importance.
Conservation
Collections with an electroshocker in 1995 showed this species to be common in the Rud-e Zard.
Further work
The distribution of this and related species should be determined
by further field work using electroshocking equipment to extract
specimens from under rocks. The validity of the nominal
Tigris-Euphrates basin species, including this one, needs examination
using large series of adult and young which are not yet available in
collections. Variation in critical characters is poorly known because
of this shortage of specimens. Molecular and chromosomal techniques
may provide additional characters.
Sources
Type material: See above, Glyptothorax silviae (CMNFI 1979-0390A, CMNFI
1979-0390B, CMNFI 1979-0280 and CMNFI 1979-0389).
Iranian material: Type material.
Glyptothorax steindachneri
(Pietschmann, 1913)
Reported from the Tigris River basin at Mosul in Iraq. The two
syntypes in the Naturhistorisches Museum Wien have not been located
(1997 visit) and the brief description is without figures or details of the
thoracic adhesive apparatus. Its validity is in question. Not reported from Iran.
Heteropneustidae
The stinging or airsac catfishes comprise a single genus with about three
species found naturally from Pakistan through India to Thailand.
The family is characterised by an elongate and compressed body with
a flattened head; the mouth is small and transverse with fleshy, papillated lips; villiform teeth
present on the jaws and vomer; 4 pairs of barbels present (nasal, maxillary and 2 mandibular); the anterior
nostril is tubular and the posterior nostril a slit; gill openings
wide and gill membranes free from the isthmus; air sacs are present
(see below); swimbladder very small; the dorsal fin is short and
spineless; no evident adipose fin; very long anal fin confluent with
the caudal or separated from it by a notch; pectoral fin with a strong
and venomous spine; skin scaleless; and branchiostegal rays 7.
These fishes can live in stagnant water by breathing air. They are
dangerous to man since the pectoral spine harbours a strong venom.
Stinging catfishes nonetheless are an important food in the native range.
Genus Heteropneustes
Müller, 1840
The only genus in the family, its characters are given above.
Heteropneustes fossilis
(Bloch, 1794)
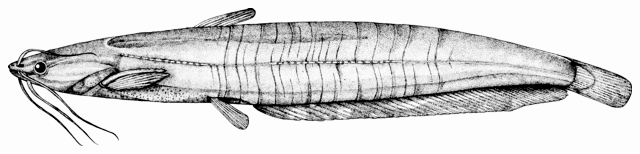
Common names
eshlambo or abu shalambo (note variants on this word are used for
catfishes and mudskippers); dudeh, doodeh or dood in Khuzestan (=
smoke, perhaps because it is blackish); bu shalambo in Khuzestan;
گربه ماهي نيش زن
(= gorbeh mahi-e nishzan); گربه ماهي هندي
(= gorbeh mahi hendi, meaning Indian catfish).
[samaka, abu-al-hukum, abu al-hakim, abu-alhaka, samma, samaka samma, djirri lasseye or jamhoori (latter at Baghdad in reference to the then new republic or jamhooria
(F. Kedairy, in litt., 21 December 2005)) in
Arabic; singhi in Pakistan; Indian stinging catfish].
Systematics
Silurus fossilis was originally described from India.
A syntype of this species is in the Museum für Naturkunde,
Universität Humboldt, Berlin under ZMB 3074 (Eschmeyer et al., 1996).
Key characters
The 4 pairs of barbels, short and spineless dorsal fin, absence of
an adipose fin, and the long anal fin are distinctive. The head is
small and very flattened and tapers both dorsally and ventrally to a
terminal mouth.
Morphology
There are two, tubular air sacs extending from the gill cavity
almost to the caudal peduncle, enabling this catfish to breathe air.
On capture, air from these sacs may escape and cause a peculiar
squawking sound. The anatomy and function of these organs was reviewed
by Datta Munshi (1993).
Dorsal fin with 6-8 rays but no spine, anal fin rays 60-79,
pectoral fin branched rays 7-8 after a strong spine serrated on its
inner margin, and pelvic fin branched rays 5-6. Fin rays are difficult
to count without dissection or x-rays because of the fleshy and
heavily pigmented nature of the fins. Iranian specimens generally fall
within the ranges cited above from literature sources as far as can be
determined. Spine serrations are more notch-like than toothed. Al-Hassan
et al. (1990) have demonstrated that the level of asymmetry in
pectoral fin ray and total gill raker counts increases with fish
length. Gill rakers are elongate, reaching adjacent raker 5-7 when
appressed and number about 25. Barbels are elongate, the snout barbel
being the shortest at about head length, the inner mandibular barbel
being head length or longer, and the mouth corner and outer mandibular
barbel being much longer than the head. The gut is elongate with
several posterior coils.
Zakaria (1964) gives details of the pectoral fin spine anatomy. Singhkohli and Goswami (1987) and Kaul and Rishi (1987) describe
abnormalities in this species including an upturned tail, a forked
tail and forked barbels.
Sexual dimorphism
Unknown.
Colour
Yellow or leaden to dark green, grey-brown, rust-brown or even
black, occasionally with two yellowish stripes. The flanks may also be
spotted. The barbels are darker than the adjacent body. The eye is
yellow. Young specimens are reddish and have a pale belly with
numerous melanophores.
Size
Males reach 24.2 cm, females 34.4 cm in India (Datta Munshi and Choudhary, 1996).
Distribution
First recorded from Iraq for 1960 by Khalaf (1961) and Zakaria
(1964) when a strange fish was reported to have inflicted a
"painful bite" on several victims. The species spread
northward and also eastward into Iran from southern Iraq. One collection from
Dezful (see below) is dated February 1960 so the spread into Iran must have been
very rapid or the original Iraqi introduction some years earlier than
documented. It is now known from the Turkish Tigris River near Diyarbakır,
presumably dispersing upstream from southern Iraq (Ünlü et al., 2010).
Found from
Turkey and Iraq eastward but not continuously through South Asia to
Vietnam. It is
common in rivers and marshes of Khuzestan including the Dasht-e Azadegan and the
Arvand Dez, Karkheh, Karun and Jarrahi rivers (Abdoli, 2000). Berra (2001) omits their Middle Eastern
distribution as they are thought to be introduced.
Zoogeography
An Iraqi biologist told me that this species was introduced to Iraq
for mosquito control (sic) by local authorities although no one
would later admit to it. A more reasonable assumption is that it was
introduced to eat the snail Bulinus truncatus, a vector for the
human parasite causing schistosomiasis. It was ineffective in this
regard (L. A. J. Al-Hassan, in litt., 1995; Jawad, 2003). There has probably
been no natural, large scale migration from Pakistan as envisaged by
Banister and Clarke (1977) and Banister (1980). Some Sumerian names
may refer to this species but this is by no means certain (Sahrhage, 1999).
Habitat
It is common in rivers, marshes, ponds and canals and is found in both fresh and slightly
brackish waters. Al-Daham and Bhatti (1977) found this species to suffer a 25% mortality over 72
hours at 10.25‰ sea water. It was most abundant in polluted and stagnant
areas in the lower Diyala River where it dominated catches or was the only fish
present (Khalaf et al., 1986; Biro et al., 1988). It is common in
swamps and can survive temperatures up to 39.8°C (Pethiyagoda, 1991). Specimens
survived 3-6 hours in air. It air
breathes every 3-5 minutes but the frequency varies with time of day and weather
conditions. On hot and calm days it visits the surface more frequently than
during a heavy shower. S. Cowton (pers. comm., 23 August 2005) has observed
schools of this species gaping at the surface in the artificial lake around Al
Faw Palace in Baghdad, presumably in response to high temperatures and low
oxygen. Individuals were also seen rapidly swimming straight up to the surface,
gulping and diving straight back down again. On especially sultry days it may
float or swim near the surface. In the dry season of India it can live in
semi-liquid mud or at the bottom of fissures where the mud has cracked (Jayaram,
1980). It makes nest holes in the sides of ponds about 1 foot below the water
surface in the form of anastomosing tubes with several exits. Up to 364 fish can
be found in one complex of holes (Datta Munshi and Choudhary, 1996). Tekrival
and Rao (1999) report its aquarium preferences and habits as 22-25°C, pH
7.5-8.5, alkaline water, predator, not too bright lighting, bottom dwelling with
stones, roots and crevices preferred and cave brooding reproduction. Zakaria
(1964) noted aquarium specimens producing audible squeaks when excited and
preferred the darker side of an aquarium.
Age and growth
Khalaf et al. (1987) gave length-weight relationships for Diyala River,
Iraq
fish in autumn as W = 9.12 L2.95 (r = 0.98, n = 58) and in spring W =
0.11 L2.15 (r = 0.84, n = 66) and condition factor was 0.0012 in
autumn and 0.08 in spring. Islam et al. (1982) give the following
length-weight relationships for fish from the Ashar Canal, Basrah, Iraq:- log W =
-6.35211 + 3.53226 log TL (r = 0.93543), log W = -5.96765 + 3.42353 log SL (r =
0.93687), and log W = -1.35223 + 2.04705 log GL (r = 0.87876) (TL = total
length, SL = standard length, GL = girth length). Tabrez Nasar (1993) studied
populations in India and gives length-weight relationships, log W = 1.7661 +
3.035 log L for one population and log W = 1.805 + 2.615log L for another. The
coefficient of condition varied from 1.582 to 2.151, mean 1.89. A pond
population did not grow as well as a natural one. Life span was up to 4 years in
Iraq at Qarmat Ali using ocular lens diameter and vertebral rings (Al-Hassan
et al., 1992) and may be 4+ years in India (Datta Munshi and Choudhary,
1996).
Food
Al-Daham et al. (1977) studied the diel feeding of this
species in the Ashar Canal, Basrah, Iraq. Two feeding peaks were
observed - at 0500 hours and 1700 hours, dawn and dusk, but stomachs
examined around the clock had food in them. Aquatic plants and
detritus are the bulk of the diet, followed by entomostracans and
aquatic insect larvae. Also present are fish parts, molluscs and
non-aquatic organisms. Cannibalism is reported in India for young fish
(Jayaram, 1980). Khalaf et al. (1987) studied this species in
the Diyala River, Iraq and found young fish to take chironomids and
worms while larger ones ate fish. However all sizes take aquatic
insects in spring. There is some competition with Barbus (=
Mesopotamichthys) sharpeyi, a
commercial species (Jawad, 2003).
In a study of the recovering Hawr al Hammar, diet was 47.2% insects, 22.1% shrimps
and 20.8% fish, in the Hawr al Hawizah 33.9% shrimps, 25.8% fish, 20.8% insects
and 19.2% snails and in the Al Kaba'ish (= Chabaish) Marsh 51.2% shrimps, 26.4% fish, 12.0%
insects and 10.4% snails (Hussain et al., 2006).
Chili macaroni, corned beef casserole, mixed vegetables and salad are dietary
items at Camp Liberty, a former palace in Baghdad, where American soldiers feed
leftovers from a distinguished visitors dining hall to catfishes, apparently
this species (www.estripes.com, downloaded 7 September 2006).
Small Iranian specimens contained insects in their guts including
Notonectidae and Diptera larvae. Abdoli (2000) lists diatoms, Chlorophyceaae,
fish remains, Corixidae, Hemiptera, cyclopoid Copepoda, termites, Isopoda,
Chironomidae, Oligochaeta and Rotifera.
Reproduction
Sexual maturity in India is reached when fish are about 1 year old,
at 8.5 cm for males and 12.0 cm for females.
Fecundity reaches 12,000 eggs (Haniffa et al., 2008). Al-Rudainy (2008)
reported sexual maturity at 2-3 years, 12 cm length and 40 g weight in Iraq.
Spawning there took place continuously from June to August with eggs deposited
in batches on vegetation. Females take care of the fry.
Eggs are laid in a
shallow depression excavated by both the male and female in mud or
sand. Eggs are light green. They hatch in about 2 days in Sri Lanka.
The parents guard the eggs and young until the young fish are about a
month old and able to look after themselves. Singh Kohli and Goswami (1987)
describe spawning behaviour in aquaria after hypophysation using
pituitary glands of Indian major carps. A pair of males circled each
other in a figure 8 pattern until one established dominance. The
dominant male chased the female, swimming underneath her or
obstructing her path, and touching barbels. The male tried to bite the
female in the chases and shivered its whole body while making lateral
passes. The male arcs its body into a u-shape, the female touches the
male's genital papilla and the pair remain motionless for 2-5 seconds.
The female jerks and separates from the male releasing the eggs which
settle to the bottom. The pair rest before mating agian. About 40-200
eggs are released after each mating. Mating acts number 20-100 and
always occur near the surface of the aquarium. Spawning is more
complete and egg fertilisation is better when there is one female and
two males, the other male acting as a stimulator, with the spawning
male quarrelling with the non-spawning male between mating with the
female. Datta Munshi and Choudhary (1996) report similar behaviour. The male nudges
the genital region of the female with his head, occasionally
shaking it from side to side. Eventually the female is aroused and
nudges the male genital region. This
female action was necessary for mating to occur. Mating did not
happen when spawners were of different sizes. Once
the female has her snout below the male genitalia, the male twists his
body to place his snout below her genitalia. The fish remain
motionless for 2 seconds, then the male vibrates his body and the
female convulses and releases eggs. This can happen 30-50 times for
each couple at 2-3 minute and then later 5-10 minutes or longer
intervals. About 100-150 eggs are extruded, the number decreasing over
time. If more than one male is present, mating only occurs after one
establishes dominance. Males may eat eggs. Spawners mate in the water
column or near the surface.
Parasites and predators
Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. fossilis in fish from the Karun River.
Economic importance
An important food fish in India and Sri Lanka, where its flesh is
reported to have invigorating qualities. Some fish are exported from
Sri Lanka for the aquarium trade. V. D. Vladykov reported (in litt.,
22 July 1963) that he had seen this species in pet shops in Tehran, on
sale at about $2.00 each.
The pectoral spine can cause a serious wound because of the toxin
content of the epidermal cells covering the spine. The histology of
the pectoral spines was described by Bhimachar (1944) based on Indian
material and the toxin was found to have both neurotoxic and
haemolytic effects. The toxin is fatal to frogs (within 15-20 minutes
of subcutaneous injection of glycerinated venom) and to other fishes.
Zakaria (1964) reported severe swelling involving the whole arm
from a hand sting in Iraq. The swelling and pain recede after about a
day but the puncture wound can take about two weeks to heal and some
pain can be felt when applying pressure to the wound site up to six
weeks later. Caras (1964) (probably based on a report in Farsi in Game
and Nature, Tehran, ca. 1961) recorded a diminutive black fish
found in the Shatt al Arab which reputedly killed 28 people with a
venomous bite (sic). Death was said to be swift. This
was presumably a garbled report on this species. Verbal and newspaper
reports from Tehran (V. D. Vladykov, in litt., 26 August 1961)
maintain that this species could cause death to cattle and humans
although Vladykov (in litt., 30 September 1963) considered
fatal cases "not well proved". I was stung in the thumb by
this fish in Iran with no effect (although I did devote considerable
time and effort into squeezing and sucking blood from the puncture
site!). Freshly caught or netted fish swing the head from side to side
and thus are active envenomators (despite knowing this I was still
stung). Treatment is symptomatic and some relief can be obtained by
immersing the sting site in water as hot as can be withstood and
applying a meat tenderiser. These treatments serve to coagulate the
protein toxin. The wound should be cleaned to avoid secondary
infections such as tetanus (Halstead, 1967-1970; Coad, 1979b).
R. Beck (pers. comm., 2000) reports that this fish is now
present in the Syrian Euphrates, its tributaries, and in irrigation
canals. Incredible numbers occur near town sewage outlets and in weed beds. It
is known to consume eggs of
Tor grypus, a preferred food species
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture, as food, in
textbooks and because of its venomous nature.
Conservation
This is an exotic species and requires no conservation
although it is is listed as Vulnerable in its natural habitats (Haniffa et al., 2008a).
Further work
The distribution of this species should be mapped as it is potentially hazardous to humans.
Sources
Iranian material:- CMNFI 1979-0087, 1, 189.0 mm standard length, Khuzestan,
Karun River at Ahvaz (31º19'N, 48º42'E), CMNFI 1979-0359, 5, 96.9-114.2 mm
standard length, Khuzestan, Karkheh River at Hamidiyeh (31º29'N, 48º26'E); CMNFI
1980-0909, 4, 113.2-165.0 mm standard length, ? Khuzestan (no other locality
data); BM(NH) 1980.8.28:4-5, 2, 91.7-98.0 mm standard length, Khuzestan, Dezful
(32º23'N, 48º24'E); ZSM
27369, 3, 111.9-139.0 mm standard length, Khuzestan, Karun River near Ahvaz (ca.
31º19'N, ca. 48º42'E).
Comparative material: BM(NH) 1962.7.26:80-83, 4, 127.8-189.0, Iraq, Baghdad
(33º21'N, 44º25'E); BM(NH) 1974.2.22:1785,
1, 89.8 mm standard length, Iraq (no other locality data); BM(NH)
1974.2.22:1786-1788, 3, 102.3-165.3 mm standard length, Iraq (no other locality
data); ZSM 19455-56, 2, 123.2-127.7 mm standard length, Iraq, Tigris River near Amara
(ca. 31º43'N, ca. 47º06'E).
Salmonidae
This family comprises the salmons, trouts, charrs and whitefishes
and contains 11 genera and about 66 species found in cooler water in the Northern
Hemisphere (Nelson, 2006). The distribution map in Berra (2001) is too extensive in
the south of Iran. There are only two species native to Iran but several
others have been introduced with varying degrees of success.
Whitefishes may be placed in their own family, Coregonidae, but views
differ (see Nelson, 1994; 2006).
Members of this family are characterised by numerous, deeply
embedded scales on the body but not the head; an adipose fin; a
lateral line with relatively numerous and often quite small scales; 7-20 branchiostegal rays; 3 upturned vertebrae at the tail
fin; a large swimbladder; usually numerous pyloric caeca (11-210);
gill membranes free from the isthmus; a small dorsal fin with few rays
(less than 17); a pelvic axillary process; young usually with parr
marks (bars along the flank); and a tetraploid karyotype.
Family members are important in fish culture in all the cooler
waters world-wide.
Genus Coregonus
Linnaeus, 1758
This genus is characterised by small, almost toothless mouth, 115
or fewer scales in the lateral line, gill rakers often long and slender, there
is a single flap between the nostrils, and no bright and complex colour patterns
in adults and no parr marks in young, forked caudal fin. Numerous named species
in North America and Eurasia (FishBase gives 217 in Coregonus alone,
August 2007), variously recognised as full species or not.
Coregonus lavaretus
(Linnaeus, 1758)
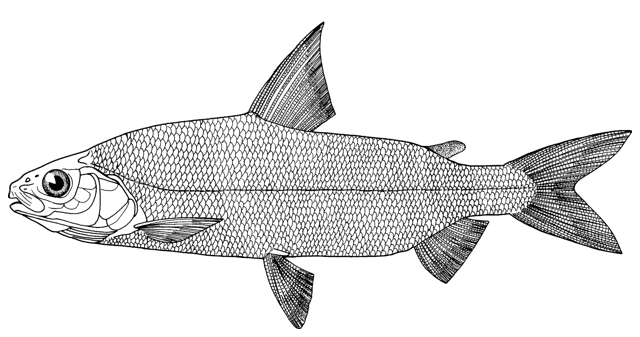
Common names
safid mahi (= white fish at Karaj Dam), safid mahi juibarye (=
brook or rivulet white fish), koregon or coregon.
[European whitefish, powan, houting, skelly, gwyniad, lavaret, pollan].
Systematics
Salmo lavaretus was originally described from Lake Bourget,
France (Kottelat, 1997). There are numerous subspecies and
infrasubspecific names for populations of this fish in northern Europe
(Berg, 1948-1949). The origin of the Iranian specimens is unknown so
it is not clear which subspecies or species they belong to and accordingly
information on biology is of a general nature.
Key characters
This species is the only one in its genus reported from Iran and is
recognisable by the generic characters above.
Morphology
The mouth is small, inferior and lacks evident teeth. Lateral line
scales are 84-105. The dorsal fin has 3-5 unbranched and 8-13 branched
rays, the anal fin has 3-5 unbranched and 11-15 branched rays and the
caudal fin has a strong fork. Gill rakers are sparse and short or long
and dense, denticulated or not, and number 21-56, with means and form
varying between subspecies. Vertebrae number 56-64. The chromosome
number is 2n=80 (Klinkhardt et al., 1995).
Sexual dimorphism
Males may bear rows of elongate tubercles on the scales.
Colour
The back and upper head is a dark bluish-grey, blue-green or dark
green, flanks are greenish-grey to silvery and the belly is silvery to
yellowish-white. Fine speckles may be apparent on the body. The snout
is black. The iris is white. The dorsal, anal, adipose and caudal fins
are dark while the pectoral and pelvic fins are only dark at the tips.
Size
Attains 97.0 cm and 10.0 kg.
Distribution
The native distribution of this species is in northern Europe and
Siberia, in particular the drainages of the Baltic, North, Barents and
White seas with isolated populations in central Europe in mountainous areas.
Fingerlings were released into the Karaj and Latian reservoirs near
Tehran from 1965-1968 after hatching from eggs imported from Europe (Armantrout,
1980). Walczak (1972) reports some existing still in the Karaj
Reservoir but none were found in the Latian. It was also introduced to
the Manjil Dam on the Safid River (Griffiths et al., 1972) but
this reservoir is drained to remove excess silt and no fishery exists
(J. Holčík, pers. comm., 1992). There is no evidence of reproduction. Saadati
(1977) states that they were established in both reservoirs. Also
reported from the Farahnaz Reservoir, Markazi. Abdoli (2000)
depicts the Karaj River and the Abhar River as harbouring this species.
Zoogeography
An exotic species not naturally occurring in Iran.
Habitat
This species is typically an inhabitant of large and deep lakes
where its oxygen requirments are quite high. However it is tolerant of
warm water and even a measure of pollution. Spawning may occur in
rivers tributary to the lake. Anadromous forms occur but are rare in
full seawater.
In lakes during the day, it is found at depths of 20-30 m or on the
bottom if water is shallower. At night it may rise as far as the
surface following the migrating plankton or to the waters edge in the
shallows. Schools form in sub-littoral areas during the spawning
season and strandings may occur.
Age and growth
Maximum age attained is 20+ years although in some populations most
fish are 1+ to 3+ years old while in others most fish are 7+ to 10+
years old. Under good conditions young can attain 10-12 cm after one
year and 15-20 cm after two years of life. Maturity is attained at 3-5
years and 25-35 cm.
Food
Plankton is the principal food item, with benthic crustaceans being
taken in brackish water and crustaceans, molluscs and insect larvae in
rivers. This species cannibalises its own eggs and eats the eggs of
other fishes. Feeding may vary through the year, planktonic
crustaceans being taken in summer and benthic invertebrates in winter.
Reproduction
There is a spawning migration which may occur in summer but more
usually peaks in autumn. Spawning takes place in summer, autumn or
winter, varying with the subspecies or form. Summer spawning takes
place in deeper water than winter spawning. In lakes, the adult males
enter the spawning grounds at dusk, these grounds being gravelly
shoals off headlands or on offshore reefs. Some populations spawn over
sand or even mud. Females move in each night as they ripen. The yellow
eggs are 2-3 mm in diameter and slightly adhesive. They stick to
gravel and are protected from predators by falling in crevices between
the gravel. Up to 82,250 eggs may be laid. Eggs incubate for 90-100
days during winter at an optimum temperature of 6°C or less.
Parasites and predators
None reported from Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food
in Europe and Russia. It has been widely introduced to reservoirs.
Conservation
No conservation of this exotic species is advisable.
Further work
The survival of this species in Iran should be verified.
Sources
Based on general European literature.
Iranian material: None.
Comparative material: BM(NH) 1986.11.14:1-3, 3, ca. 312-330 mm standard
length, England, Cumbria, Lake Haweswater (no other locality data).
Genus Oncorhynchus
Suckley, 1861
Members of this genus are found naturally in the Northern Pacific
Ocean and its drainages, migrating between fresh and salt water. Some
live permanently in fresh water. There are about 12 species and two have
been successfully introduced to Iran.
This genus is characterised by a large mouth, in adults extending
back to the level of the posterior eye margin or further, scales are small, ca.
100 or more in the lateral line, lateral line scales are as large or larger than
scales in adjacent rows and overlap with scales in front and behind, evident
teeth in adults, teeth are present on the head and shaft of the vomer bone in
the roof of the mouth, usually dark parr marks in young but adults without
snow-white leading edge to lower fins, and usually black spots on the body or
caudal fin or both.
These salmons spawn once and then die. They are important commercially as food and as sport fishes.
Oncorhynchus gorbuscha
(Walbaum, 1792)
Introduced to the former Soviet Caspian Sea basin in 1963 but not observed subsequently (Baltz, 1991). No Iranian record.
Oncorhynchus keta
(Walbaum, 1792)
Common names
azad mahi keta (= keta free fish or keta salmon, azad mahi being
the Farsi for trout or salmon), mahi-ye azad-e chum.
[chum salmon, dog salmon, keta, summer chum].
Systematics
Salmo keta was originally described from rivers of Kamchatka, Russia.
Key characters
This species is distinguished by having 124-153 lateral line
scales, 13-17 principal anal fin rays, flanks and caudal fin without
distinct black spots, short, stout gill rakers 18-26 and pyloric caeca 135-249.
Morphology
Dorsal fin with 10-14 principal rays, pectoral rays 14-16 and
pelvic rays 10-11. Scales are horizontal ovals with a central focus
and no radii. Circuli are fine but are lost or only partly developed
on the flesh-covered exposed part of the scale. The gill rakers reach
just beyond the first to just beyond the second raker when appressed.
The pelvic axillary scale is very elongate. The gut is s-shaped. The
chromosome number is 2n=74 (Klinkhardt et al., 1995).
Sexual dimorphism
Breeding males develop hooked jaws and large teeth and a slight
hump in front of the dorsal fin.
Colour
Marine fish are steel-blue on the back and upper flank, silvery on
the flank and silvery-white on the belly. The upper flank and back may
have fine black speckles but no spots. The pectoral, pelvic, anal and
caudal fins have dark edges. Spawning males in fresh water are dark
olive to black above, greyish-red to brick-red on the flank with
greenish to purplish bars or blotches and a dark grey belly. Calico is
the characteristic colour of dominant males. The anal and pelvic fins
are often tipped with white. Females are less strongly marked. Young
chum are iridescent, mottled green on the back and silvery iridescent
green on the flanks and belly. There are 6-14 parr marks which do not
descend much below the lateral line and are narrower than the space
between them. Fins are clear to white.
Size
Reaches 120.0 cm and 20.8 kg.
Distribution
Found from Alaska to California on the Pacific coast of North
America and also in eastern Arctic Siberia, the Beaufort Sea and south
to the Sea of Japan, the widest distribution of any Pacific salmon.
This species was introduced to the former Soviet Caspian Sea in 1962-1970
in an attempt to offset losses of Salmo caspius which
was cut off from its spawning grounds by dams. It was considered as
ideal because it spawns in the lower reaches of rivers and dams would
not affect its spawning migration, it returns to spawn after 2 or 3
years and so some year-classes could escape detrimental conditions in
any one year, and because it produces downstream migrants at a smaller
size and age than the native salmonid (Magomedov, 1970; 1978; Baltz,
1991). 7.5 million fertilised eggs were transported from the Amur
River and Sakhalin Island in the former Soviet Far East and incubated at the Samur Fish Farm, Dagestan. 5,850,000 fry were released.
Walczak (1972) reported capture of a specimen in Iranian waters in
1971, presumably a stray from the former Soviet stocking programmes. Holčík and
Razavi (1992) record only two specimens from Iran, one in 1964 and
one in 1972, taken between Bandar Anzali and the Astara River on the
Iranian coast of the Caspian Sea. They also seem to indicate another
specimen taken after 1972. Jolodar and Abdoli (2004) report it from Astara and
the Anzali regions, indicating no large scale spread.
Zoogeography
A species introduced to Iranian waters by man.
Habitat
In North America, chum salmon enter streams on the spawning
migration and often travel less than 150 km, stopping at the first
barrier as they are not strong jumpers. Some fish even spawn in tidal
areas. However some rivers, like the Yukon, have a run which travels
about 3200 km and takes from early June to the end of September. A
migratory speed of up to 115 km/day has been recorded with bursts of
speed to 4.6 m/s. Most runs are in the fall but some are in the
summer. Very rarely a chum will become trapped in a lake if an outlet
stream dries up but normally adult chum are only found in fresh waters
on the spawning run.
Age and growth
Reproduction occurs at ages 1+ to 2+, with spawning fish at 2+
predominating in Caspian populations from former Soviet waters. In
their native streams in the Far East fish are in their fourth and
fifth years of life. 1500 adults were caught returning to one river to
spawn in the Caspian in 1966, considered impressive by former Soviet
biologists. Accelerated growth and maturity in transplanted fish is
well documented (Magomedov, 1970). There is a 15C°
difference between the Caspian and native water temperatures for
spawning and egg development. Life span in Canada is about 7 years,
perhaps 9 years, with fish in British Columbia maturing at 1-6 years
with age 3 fish dominant. They spend 2-7 years at sea before returning
to spawn. Males grow faster and larger than females.
Food
Food in the sea includes crustaceans, worms, molluscs, squids,
jellyfish and fishes. Young fish in the sea take zooplankton. Adults
on the spawning run do not feed. Young fish in fresh water eat aquatic
insects, such as chironomids, mayflies and caddisflies, as well as
crustaceans, worms and terrestrial insects.
Reproduction
Transplantations into the former Soviet Caspian Sea basin were not very
successful because of a lack of suitable spawning streams (McNeil,
1979). Spawning runs into some former Soviet streams were recorded from the
first half of September to the end of October, the same periods as in
the native habitat (Magomedov, 1970). Males arrived first, the mass
run was composed more of females and there were more males at the end
of the run. Since a single male will spawn with several females, early
male arrival on the spawning grounds may promote successful
fertilisation. The absolute fecundity of a 3+ salmon was 2739 eggs in
the Caspian.
Females excavate a redd by lying on their sides and lashing the
tail. The redd is a trough up to about half a metre deep and up to 3.2
m long and 2.1 m wide bordered by a ridge of gravel at the downstream
end. In some cases no redd is excavated and eggs are shed over and
between boulders. Females may excavate more than one redd and males
may spawn with more than one female. A female and one dominant male
lie in the redd, gape their mouths, vibrate and release eggs and
sperm. The dominant male may have several accessory males accompanying
him. The female dislodges gravel at the upstream end of the redd to
cover the eggs. The orange eggs are up to 7.8 mm in diameter (perhaps
9.5 mm when fertilised) and each female may shed up to 7779 in the
Pacific basin. The adult fish die after spawning and may live only a
week after first entering fresh water. The fry emerge from the gravel
in March-May in North America, some remaining for several weeks in
fresh water or immediately migrating to the sea.
Parasites and predators
Various insects, fish, birds and mammals prey on both young and adult chum.
Economic importance
It is too rare to have any economic importance in Iran. Total
catches in the North Pacific Ocean have been as high as 69.2 million
fish annually. In Japan it is very important in an ocean ranch industry.
Conservation
The presence of this species in Iranian waters is the result of
former Soviet attempts to acclimatise it to the Caspian Sea as a potential
replacement for declining stocks of Salmo caspius. The
stocking programme lasted from 1962 to 1979 and rivers along the
Dagestan coast showed mass spawning but this became rare. Pollution by
wood from forestry operations in Dagestan, absence of suitable gravel
beds, low salinity in the Caspian Sea and heavy surf on the Dagestan
coast may be responsible for the heavy mortality (Magomedov, 1978; Holčík
and Razavi, 1992). As an exotic species, there is no need for
conservation of chum salmon.
Further work
Reports of this species in Iranian waters should be documented.
Sources
Bakkala (1970) summarised the biology of this species in North
American waters and Salo in Groot and Margolis (1991) over its whole range.
Iranian material: None.
Oncorhynchus kisutch
(Walbaum, 1792)
Introduced to the Caspian Sea basin but not subsequently observed and no Iranian records (Holčík and Razavi, 1992).
Oncorhynchus mykiss
(Walbaum, 1792)

Lorestan fish farm specimen, north of Aleshtar (golden form), 3 December 2000
Common names
قزل آل (= gazalala, ghezel ala, qezel ala or kizil ala; probably in confusion with native Salmo
caspius,
these words meaning literally red spots or red spot fish but used for trout and salmon species in Iran),
قزل آلاي رنگين كمان (qezel ala-ye ranginkhaman,
meaning rainbow trout).
[mykiss in Russian; rainbow trout, steelhead trout].
Systematics
Salmo mykiss was originally described from Kamchatka, Russia.
Formerly known as Salmo gairdneri Richardson, 1836 (see
Bailey and Robins, 1988; Smith and Stearley, 1989). Placed in the
genus Parasalmo Vladykov, 1972 by Reshetnikov et al.
(1997) and Mednikov et al. (1999) but Osinov (1999) has
reservations since allozyme data show specific level differences with Oncorhynchus
although further studies were deemed necessary.
Key characters
This species is distinguished by having 100-161 lateral line
scales, 8-12 principal anal fin rays, the vomer bone in the roof of
the mouth has teeth on its head and shaft, no red spots on the body
but only small dark ones and radiating rows of black spots on the
dorsal and caudal fins, and no teeth at the tongue base.
Morphology
Dorsal fin principal rays 10-12, pectoral rays 11-17 and pelvic
rays 9-10. Gill rakers 16-22. Pyloric caeca 27-80. The chromosome
number is 2n=58-62 (Klinkhardt et al., 1995).
Sexual dimorphism
Breeding males have an elongated snout, the lower jaw is hooked and the roof of the mouth is white.
Colour
Overall colour is very variable. Stream fish are darker and more
colourful (rainbows) than lighter, silvery lake or sea fish
(steelhead). Some sea-run and lake fish have small orange to red marks
below the lower jaw. The back and upper flank are steel-blue,
greenish, silvery-olive or even brown, flanks and belly are silvery,
grey, white or yellow-green. The side of the head and the flank are
characteristically pink. The flank has a broad pink to red or lilac
stripe with small black spots. The adipose fin has a black margin and
a few spots. Pectoral, pelvic and anal fins may have a few spots and
are dusky without any strong markings. Pectoral and pelvic fins are
often orange-red. Spawning fish are very dark and the flank stripe is
dark red or purple. The young have 5-13 dark, oval parr marks centred
on the lateral line with the spaces between the marks wider than the
marks. There are 5-10 parr marks on the back in front of the dorsal
fin. The upper flank has some dark spots. The dorsal fin is tipped
white or orange and has a dark leading edge, sometimes broken up into
spots. The adipose fin has a black margin. The anal fin has a white or
orange tip. Some adults in streams do not lose their parr marks. A
golden form occurs rarely on fish farms in Lorestan and elsewhere is
farmed specifically.
Size
Reaches 122.0 cm, possibly 150 cm, and 35.0 kg as sea-run or lake fish but smaller in streams.
Distribution
Found in western Canada and from Japan and Alaska to Mexico. Widely
introduced outside this natural range world-wide in suitable waters. They have been introduced to the
former Soviet part of the Caspian
Sea in 1973 and several hundred returning adults were reported in 1975 (McNeil, 1979; Baltz, 1991).
Rainbow trout were introduced to Iran about 1966 for hatchery
production as a commercial product (MacCrimmon, 1971; 1972). They are
now widespread in Iran, stocks being maintained by hatchery
introductions and sometimes natural reproduction, wherever temperature
regimes are suitable in the higher reaches of the Alborz and Zagros
mountains (Walczak, 1972; Anonymous, 1977; B. Sandford, in litt.,
1979; Y. Keivany, in litt., 1992; Ghorbani Chafi, 2000; personal visits to fish farms).
B. Sandford considered few populations became established because of competition with large
populations of omnivorous cyprinids which also take fish eggs,
presumably of trout too. Sandford cited viable populations in the Madar Su, a small stream in the former Mohammad Reza Shah
Park (see below), Tar Lake in the Alborz (also recorded from Tar Lake by Riahi (1996)),
Gahar Lake in the Zagros and the Qara Chai west of Hamadan.
Introductions to Neuer or Neur Lake near Ardabil, Ghorighol Lake,
Rebeshahr and the Ab-e Bazuft were all failures (although Walczak
(1972), Saadati (1977) and R. Mehrani (pers. comm., 2000) report several successful stockings
including the Ab-e Bazuft, Gahar Lake, Namrud, Dez River and Jajehrud).
Also reported from the Gorgan and Haraz rivers and Gorgan Bay (the latter escapees
from cages)(Kiabi et al., 1999), Madar Su, Golestan National Park (Kiabi et al., 1994),
from the Farahnaz Reservoir, Markazi, Namrud in Semnan, Golestan
River, Karaj River, Amir Kabir Dam (www.iran-doe.org/Special/Alborz.htm), Shah
Abbas Dam west of Esfahan, Darius-e Kabir Dam north of Shiraz, Karaj River,
Zayandeh near Esfahan, Rebeshahr southeast of Yasuj (Anonymous, 1977; Y. Keivany, in litt.,
1992). An attempt to culture this species in cages in the Avan River, 7 km from
Alamut, in 1994 failed and fish escaped (Nialmir, 2001). Also in the Sardab Rud (Jalali et al., 2005).
An attempt to establish a run of steelhead, the migratory form, in
the Chalus River from the Caspian Sea was apparently unsuccessful (Armantrout, 1980).
This species was stocked in the 1970s by the Iranian Department of the Environment in
the Doogh or Madar Su, head stream of the Gorgan River in Golestan
National Park and "Neur" Lake in East Azarbayjan near Ardabil in the
Caspian Sea basin; in Ghorigol Lake (east of Tabriz) and the Liqvan Chay in the
Lake Orumiyeh basin; the Karaj River, the Jajrud, the Namrud, Tar Lake northeast of Tehran, Lake Lasem
near Tehran, the Lar River, probably in the Chashmeh Do Barare, Ab Kharsang,
Varangarud and Baragon near Tehran, all in the Namak Lake basin; the Karun
River, Mohammad Reza Shah Dam, Rebeshahr River southeast of Yasuj, the Ab-e Bazuft in 1975 (established), Gahar
Lake in the headwaters of the Dez River and its outlet stream (established, caught by A. Abdoli in
1995), and an isolated section of the Dez River formerly fishless (established),
all in the Tigris River basin; the Shah Abbas Dam west of Esfahan, in the Zianrud
(presumably the Zayandeh River of the Esfahan basin) where a reproducing population
was established; in the Dorudzan (Dariush-e Kabir) Dam near Shiraz (104,000 in Esfand
1350)(Surber, 1969; Anonymous, 1977; Armantrout, 1980; Petr, 1987; Abdoli, 1993b).
Qanats in Markazi Province were stocked with 930,000 trout (presumably this
species) in 2006 (www.iranfisheries.net, downloaded 28 July 2006).The qanats
were in Shazand (2 qanats), Arak (4), Delijan (1) and Khomein (1).
Abdoli (2000) lists the Dasht-e Kavir and Kerman-Na'in basins generally, the upper Kashaf River in the
Tedzhen River basin, the Gorgan, lower Neka, middle and lower Babol, and Heraz
rivers in the Caspian Sea basin; the upper Talkheh,
Tatavi and Zarrineh rivers in the Lake Orumiyeh basin, the Abhar stretch of the Shur
and the upper and middle Karaj rivers of the Namak Lake basin; and the Khersan,
upper Dez and Kashkan rivers of the Tigris River basin, and the upper and middle Zayandeh
River of the Esfahan basin.
Zoogeography
An exotic species introduced as a food fish.
Habitat
Rainbows are found in rivers or streams where there are pools and
riffles. Some live in lakes and are called Kamloops trout in Canada
while others run to sea and are called steelheads. They can tolerate
temperatures up to 24°C, warm for a trout, but prefer temperatures below 20°C.
Sea-run fish spend about 1-4 years usually in inshore waters at middle
to surface depths after 1-4 years in fresh water. Some fish (half-pounders)
return to streams after a few months at sea. Summer steelhead have
spent one winter at sea and return in summer (April-October) to spawn
next spring while winter steelhead are larger and return from December
to April peaking in January to spawn in March and April. Aquaculture of trout in
Iran is affected by drought conditions reducing water flow to farms. Production
was higher in 1999 compared to the previous year but this was because 82 new
farms were opened; average production per farm fell (Foghi, 2004).
Age and growth
Life span varies with habitat, up to 11 years in some lake fish but
only 3-4 years in many streams and small lakes. Growth varies with
habitat including such factors as length of sea, stream or lake life,
years before spawning, available food supplies, latitude, altitude,
temperature regime, competition with other salmonids, and so on.
Ageing these fish may be difficult because of the complicated life
history pattern of stream and lake residency. Maturity is also
variable with habitat. Some males mature at 9 months in fish
introduced to warm southern waters and some females only at 8 years,
but generally maturity is reached at 3-5 years in Canada, for example,
with males maturing a year earlier than females. Neur Lake fish had a
growth rate of 17 lbs (7.72 kg) in 3 years, more than doubling in
size, because of the excellent food supply of shrimp. In Tar Lake,
where food resources were poor, mostly surface insects, fish grew to
only 3-5 lbs (1.4-2.3 kg) in 2 years (Anonymous, 1977). In the Karaj
Reservoir, one-year-old fish with a length of 8-10 cm grew to 25 cm in
one year although the available food was only plankton.
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 111 Iranian fish measuring 27.5-57.0 cm standard length. The a-value
was 0.0161 and the b-value 3.044 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Food includes plankton, crustaceans, aquatic and terrestrial
insects, snails, leeches, salmon eggs, and other fishes. The fish
eaten enhance growth and the species taken depends on what is
available. In Lorestan, this species thrives in rivers where Gammarus
is numerous, e.g. near Dow Ab-e Aleshtar. Fish and squid are taken at
sea along with some crustaceans. Abdoli (2000) lists Trichoptera, Plecoptera,
Chironomidae, Ephemeroptera, Ceratopogonidae, Odonata, Simuliidae, Coleoptera,
Decapoda and Amphipoda as food in Iran.
Reproduction
Spawning takes place from March to August but is usually in spring.
North American Great Lakes fish may spawn from late December to late
April. Water temperature for spawning usually exceeds 10°C
but may be 5-13°C. A female excavates a redd by lying on her side and thrashing her tail.
Redd excavation occurs during the day and night and dimensions are
usually longer and deeper than the female's body. A male courts a
female by rubbing his snout and body against her, by vibrating, by
swimming over her in the redd and by pressing against her. Several
males are found around each female but one male is dominant. The
spawning act lasts 5-8 seconds with the pair parallel in the redd
pressed together, both fish gape, arch and vibrate. Other males may
shed sperm. The female covers the eggs with gravel by dislodging it
from the upstream end of the redd. Most spawning takes place in the
morning and evening and nests may be abandoned during the day. Females
construct several redds and may spawn with several males. Eggs are
orange or pink, 5.0 mm in diameter and up to 12,749 in number.
However, egg numbers are usually a few hundreds to thousands per
female. The eggs hatch in about 8 weeks and fry generally emerge in
June to August from spring spawnings. Repeat spawning can occur for up
to 5 years.
Nematollahi and Azari Takami (2002) studied the quality and quantity of
seminal fluid in cultured male broodstock specimens of this species at the Karaj
Fish Farm. Differences were found between stocks in sperm motility and concentration.
Parasites and predators
A wide variety of other fishes and birds feed on this species and
there have been extensive studies on parasites and diseases of this
commercially important species. Akhlagi (1999, www.mondialvet99.com,
downloaded 31 May 2000) reports infectious pancreatic and
haemotopoitic necrosis in fish from Bovir Ahmadi va Kohkiluyeh Province but not in Fars Province. Liver and intestinal submucosa
degeneration and kidney necrosis were observed in diseased fish.
Prearo and Ghittino (1993) record a case of lipid liver degeneration in cultured
trout. Asadzadeh Mangili (2000) records ichthyobodosis in 3 cm fry from a
farm in Bovir Ahmadi va Kohkiluyeh. The mortality rate was
40% and infection rate with Ichthyobodo was 100%. Rainbow trout
fed on raw marine fishmeal powder in Fars Province were found to be
more exposed to vibiosis (pathogenic Vibrio anguillarum)(Ghazi
and Akhlaghi, 1998). Diplostomiasis (infection with Diplostomum
spathaceum) is reported from cultured trout in West Azarbayjan (Asadzadeh
Manjili and Ghorbanzad, 1999). Zorriehzahra (2002) reports on diseases in farmed
fry and Zorriehzahra et al. (2002) record enteric redmouth disease in
farmed fish around Tehran. Akhondzadeh et al. (2002) and Akhondzadeh Basti and Zahrae Salehi (2003) show that the
psychotropic pathogen Listeria monocytogenes is found in market and fish
farm samples of this species. Soltani and Rostami (1997) record a mortality level of about 16% for farmed
trout in northern Iran infected with a Cytophaga/Flexibacter-like
bacterium. Soltani et al. 1999) report a yersinosis-like infection in
farmed Iranian rainbow trout. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. derjavini from fish in the Sardab-rud.
Asadzadeh Mangili (2001) describes gas bubble disease from fish farms in
Kermanshah Province. Mortality occurred mostly in fish at 100-200g and symptoms
included inappetition, skin darkening, aggregation of fish at inlets and outlets
in the ponds, and swimming near the surface. In 20% of the samples, unilateral
or bilateral exophthalmia was observed. High day-night fluctuations in the
oxygen and carbon dioxide content of the pond water was observed through
excessive plant growth. Abdi et al. (2006) recorded the first mortality
from acanthocephalosis with Pomphorhynchus laevis in fish farms in Maku,
West Azarbayjan. Ebrahimzadeh Mousavi et al. (2007) isolated 12 species
of fungi from eggs in Mazandaran hatcheries.
Barzegar et al. (2008) record eye parasites from this fish including the
protozoan Ichthyophthirius multifilis and the digenean Diplostomum
spathaceum.
Miar et al. (2008) examined fish in Valasht Lake and the Chalus River,
Mazandaran and found the protozoans Trichodina trutta,
Ichthyophthirius multifilis and Chilodonella hexastica as well as the
metazoan Gyrodactylus derjavini. Khosravi et al. (2010) found 5
species of Saprolegniaceae fungi on eggs in two hatcheries in Kermanshah
Province, three species for the first time in Iran.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Economic importance
This species is commercially farmed in Iran for local sale.
Experimental studies in the 220 ha "Neur", "Neuer"
or "Noor" Lake near Ardabil have shown very good production
of this species. A growth rate of 17 lbs (7.72 kg) in 3 years was
reported (Anonymous, 1977). A yield of 160 kg/ha was obtained and a
catch of 35,000 kg reported (Saadati, 1977) but this was unusual in
that there were no other fish and food supplies were extensive. The
lake is subject to winter-kill and requires stocking and/or helixers
to maintain open water in winter (Bullock, 1971; Nehring, 1973b;
1973c; 1973d; 1974b; 1974c; 1974d; 1974e; 1974f; 1975b; 1975c; 1975d;
1975e; 1975f; 1975g; Nehring et al., 1974; Abbasi, 1974; 1975;
Boettcher, 1974a; 1974b; Sanford, 1975; Harrington, 1976).
Kohnehchahry and Heydarpur (1973) outline methods of raising trout
using submerged cages which they believe would be suitable for Iranian
waters. Cage culture has been tried in Gorgan Bay, the fish escaping
during several storms and numbering in the millions (B. Kiabi, pers.
comm., 1994; www.ramsar.org/ram_rpt_37e.htm, downloaded 28 July 2000).
They have also been cage cultured in Valasht Lake, Mazandaran where 12
cages were expected to produce 22 tonnes of fish (Abzeeyan,
Tehran, 5(5):IV, 1994). Nezami et al. (2000) give a
total production of fish farms in Gilan, Mazandaran and Golestan provinces
(along the Caspian Sea) as 851 t.
The "Kelardasht" or Kalerdasht" Fish Farm (= Shaheed
Bahonar Trout Farm of Shilat, the Iranian Fisheries Company, near
Kelardasht) has an annual production capacity of 2,500,000 fingerlings
of 2 g. Various other fish farms in Iran produce rainbow trout for
stocking (Surber, 1969; Krasznai, 1987; Petr, 1987). More recently,
Bartley and Rana (1998b) give a figure for the Kelardasht Farm of 3
million fingerlings from a pool of 2000 brood fish at a cost of 600
million rials. White (1988) reported that 60 ha of trout ponds and
raceways produced 1000 t a year (see also under Cyprinus
carpio). The largest fish farm in Lorestan produced 500 t a year
in 2000. Edwards (1989) reported production of 1750 t from 20 licensed
farms with a further 1000 t from unlicensed farms. Fingerling
"trout" production (probably rainbow trout) in government
hatcheries was 0.57 million in 1984, 1.81 million in 1985, 1.57
million in 1986, 3.02 million in 1987, 0.50 million in 1988, 4.23
million in 1989, 4.34 million in 1990, 1.90 million in 1991, and 2.00
million in 1992 (Emadi, 1993a). Fingerling production in 1996 was 6
million (Bartley and Rana, 1998a). Production of this trout increased
27% per year betwen 1991 and 1996, the raceway area for trout
increased from 80,000 to 166,000 sq m between 1992 and 1996 and
production increased from 775 to 1900 t (Rana and Bartley,
1998a). For comparison, the figures for carps (probably Chinese carps)
exceeded 100 million and for mahi safid (Rutilus frisii kutum)
it was 145 million. However there are also private commercial
hatcheries producing about 750 t of trout each year. The Jajrud
Hatchery near Tehran produced 1 million fingerlings as early as 1968
as well as several thousand marketable sized fish (Surber, 1969); and
there has been a considerable expansion of hatchery capacity in Iran
since that time. Rainbow trout production is only about 5% of total
aquaculture production although value per metric tonne is U.S.$14,000
while for carp it is U.S.$10,000. Trout production in Ilam Province was
estimated at 3.3 million fish based on 21 farms (Tehran Times, 8 August 2005,
downloaded 20 January 2006).
Rana and Bartley (1998a; 1998b) outline trout aquaculture methods
and problems in Iran. Most culture of this trout takes place in the
Alborz and Zagros mountains which are cooler because of altitude.
Farms are small, producing less than 50 tonnes per year. Raceways are
used for breeding, rearing of larvae and growing out. Eyed eggs may be
imported from Scotland and Norway but this may be banned in future
because of disease risks. Increased Iranian expertise and costs may
also favour production of eyed eggs in Iran. Survival to the eyed
stage attains 80% and to the alevin stage 70%. The private sector
produced 10 million fingerlings in 1996. The Manzanarbad Trout Farm
near Kelardasht sold 2 million fingerlings in 1997 and produced 25 t
for local consumption from 2000 sq m of concrete raceways (Bartley and
Rana, 1998b). The Mahi Sera Fish Farm at Karaj produces market sized
fish from 20,000 brood stock (Bartley and Rana, 1998b). These are
replaced every 2-3 years to maintain growth rates. About 5000 fish are
saved each year as next year's brood stock based on size, form and
colour. Milt from 4 males is used to fertilise eggs from one female.
In 1997, the farm produced 185 t of fish each weighing about 250
g (= 800,000 fish). Sale price is 10,000-12,000 rials/kg. The
production cycle is 9-10 months at 13°C.
Trout are fed commercial pellets with a conversion efficiency of
1:1.1-1.4 (wet:dry weight). Growth to marketable size (about 30 cm and
at least 225 g) takes 9-14 months depending on temperature (2-13°C)
at different localities. Feed deficiencies, poor genetic stocks and
low temperatures contribute to slow growth in some farms. Production
in raceways is nevertheless about 15kg/cu m, a good efficiency. Cost
of fingerlings to grow out farms is 120 rials for a 1 g fry, 250 rials
for a 5 g fry and 15 rials/g for heavier fish (Bartley and Rana, 1998b).
There is no consistent monitoring of released fish so the success
of stocking is unknown. The Dorudzan Reservoir about 100 km from
Shiraz has received 400,000 fry in 1970, 2 million in 1972, 300,000
from Australia in 1974, 100,000 in 1984 and 100,000 in 1985 (Petr,
1987). Experimental fishing in 1976 gave a poor return, perhaps
through competition for space with native Capoeta species,
heavy poaching during migration into the inflowing river, and lowering
of the reservoir level. Eggs are no longer imported and the supplies
are spawned in Iran (Emadi, 1993b).
Artificial breeding of this species at the Shahid Motahary Fish
Hatchery in Yasuj has been carried out 4-5 months earlier than normal
using changes in light regime and hormones. The object of this work
was to produce fingerlings throughout the year, increase production
and save on production costs (Abzeeyan, Tehran, 7(5):IV, 1996).
Dehghani and Akbari (1997) have successfully carried out gonadectomies
on this species under field conditions.
A production of 5.5-8.0 tonnes of rainbow trout in rice paddies
during winter has been recorded (Iranian Fisheries Research
Organization Newsletter, 22:2, 2000).
Production in Ilam Province was estimated at to be 3.3 million fish by the end
of Iranian year 2005 (http://agriculturenews.faorne.net,
downloaded 11 April 2006). However, some farms are not economical in Ilam (Rezaei
and Darvishi, 2007).
Culture of this species in earthern ponds at Bafgh in Yazd Province
used running, saline ground water unsuitable for drinking or
agriculture. Production was 2-4 tonnes/ha in 0.40-0.75 ha ponds with
salinity at 10-15‰, well water temperature 24ºC
reduced to usually less than 20ºC, with a water replacement of 5-10% per day for
a period of 5-6 months in autumn and winter. The highest growth performance was
from fish fed 35% protein, 430 Kcal/100g energy diet and 20.6% lipid with P/E
ratio of 81.4 mg protein/Kcal energy. Aeration had no significant effects on
growth (Iranian Fisheries Research Oraganization Newsletter, 25:4, 2000;
54 & 55:4, 2009; Ahmadi and Alizadeh, 2004; Bahabadi, 2006).
A total of 500,000 fingerlings in fish ponds were released in a two month period in 2005, expected to result in 220 t
of product (www.growfish.org/Iranianreport.html).
Gonad development was found to be accelerated in brackish water, males maturing
two months earlier than in fresh water and ovarian development also being
accelerated in Yazd fish (Fahlati Marvast et al., 2003; Fahlati Marvast et al., 2006).
Edwards (1989) gives details on culturing this species and the
problems to overcome. Abtahi et al. (2002) found clove oil had no
significant difference with MS222, another anaesthetic used in fish farms. Sajedi et al. (2003) have examined mtDNA in Iranian
hatchery stocks to measure inter- and intra-populational diversity as an aid in
fishery management by selection of the best broodstocks. Studies on the best
foods and conditions for this trout in Iran have been undertaken by various
authors. Kenari and Mirzakhani (2005) examined the effects of using Artemia
urmiana from Lake Urmia (= Orumiyeh) enriched with n-3 HUFA, a fatty acid not present in the brine
shrimp, as a larval food. Growth and survival was better than with non-enriched
shrimp or with artificial feed. All-female diploid and triploid rainbow trout
have been produced in Iran, such trout being unable to reproduce if escaping
from fish farms (Johari, 2005). Ahmadi et al. (2005) used the Caspian Sea
amphipod, Pontogammarus maeoticus, in rainbow trout food as a means of
improving muscle pigmentation, the reddish colour from carotenoids in this
dietary item enhancing market value. Carotenoid deposition was higher in males
(R. Khodarahami in 5th International Symposium on Sturgeon, Iranian Fisheries
Research Organization, 9-13 May 2005, Ramsar). Alavi Yeganeh et al. (2004) studied the use
of gammarid powder as a dietary item and its effects in reducing stress in trout
larvae, finding a 10% supplement to be effective. Supplementary levels of 2 and
4% of the amphipod were effective in improving flesh colour. Fani (2006a) found
fishmeal to have higher digestible and metabolizable energy than soybean meal as
feed. Fani (2006b) examined the use of oil by-product from soybeans on weight
gain, feed conversion ratio and flesh chemical composition, recommending 10% oil
by-product be added to the diet. Shadnoush (2006) showed that corn meal could be
used as a nutrient in trout diet, improving weight and length. Kalbassi et al.
(2004) induced tetraploidy using heat shock. Johari et al. (2006)
investigated production of all-female trout using sex-reversed males and found
this to be one of the best methods. Zamani et al. (2006) showed that
chromosome manipulation (triploidy) had no effects on digestive enzyme activity.
The decline in caviar production has led to a project in Iran to use trout eggs,
test being made on egg tenderness, colour, smell taste and shelf life (Iranian Fisheries Research
Organization Newsletter, 58 & 59:1, 2009).
In addition to these reports, there have been a wide variety of studies in
Iran on methods and treatments relevant to culturing rainbow trout, briefly
summarised here. Others may be searched for in the Bibliography by common or
scientific name of the fish. Sharifian (1998) studied increases in cortisol hormone levels
in trout as a measure of stress caused by handling and induced spawning;
Akhlaghi (2000c) on immunology of suspected viral diseases in cultured trout,
namely infectious haematopoitic and pancreatic necrosis; Akhlagi
et al. (2000) on using powdered clove tree as an anaesthetic; Mirvaghefi et al. (2000) on hydrogen peroxide
for fungal control on eggs; Alizadeh et al. (2001) on the effects of
dietary energy levels on growth and carcass composition in trout maintained in
brackish water; Nafisi Behabadi et al. (2001) on using poultry by-product
meal as a dietary replacement for fish meal fed to trout maintained in brackish
water; Soltani et al. (2001) on the anaesthetic effect of clove flower
extract under various temperatures and pH; Vahdati et al. (2001) on optimal conditions
for phagocytic activity of granulocytes; Vahdati et al. (2001) on
crowding stress and haematological parameters; Vahdati et al. (2001) on the
effect of cortisol on phagocytic activity of macrophages; Akhlagi (2002) on vaccination
against streptococcosis; Azari Takami et al. (2002)
and Dorafshan et al. (2006) examined induction of gynogenesis by UV
radiation; Dorafshan et al. (2002) induced spawning by use of a GnRH
analogue; Soltani et al. (2002) on fry mortality syndrome in farmed fish
in Tehran and Lorestan provinces; Yakhchali (2002) on the abundance of the parasite
Ichthyophthirius multifilis on coldwater fish farms (which produce trout
mostly); Fallahi et al. (2003) on isolating and identifying infectious
haematopoietic necrosis virus from farmed fish; Farhangi et al. (2003) on the use of natural zeolites for reducing
ammonia toxicity; Johari et al. (2003) on sperm traits and fertilisation performance of
normal males and neomales; Ebrahimi (2004; 2005) on the deleterious effects of copper, cadmium and
zinc, pollutants, on sperm anatomy and motility; Lorestany et al. (2004) on activating
solutions and their effects on sperm motility and fertilisation rate; Sharifrohani (2004) on the use
of plant essential oils as an antifungal agent for fish eggs in aquaculture; Sheikhi
Moghaddam et al. (2004) on the use of alvita (sodium di-acetate) as a
fungicide and bactericide; Bazyar Lakeh et al. (2005) on the effect of dietary astaxanthin on fertilisation rate; Azari
Takami et al. (2005) on the nutritional effects on trout larvae of vitamin
C-enriched Artemia urmiana nauplii in relation to growth, survival and
environmental stress; Esmaeili et al. (2006) induced meiotic gynogenesisi
by thermal shocks, the yield being best using shock 35 and 50 minutes after
fertilisation; Fallahi et al. (2006) on the serological diagnosis
of infectious haematopoietic necrosis disease in fry; Gholipour et al. (2006) found a density of 62 fish
per square metre was optimal in concrete ponds for weight gain and feed
conversion; Hosseini Najd Geramei and Irani (2006) studied the effects of
photoperiod on growth, survival and and feeding of larvae; Johari and Kalbassi (2006) on alterations in the red blood cells of
triploid trout; Mirzakhani et al. (2006) studied the feeding of n-3HUFA enriched
Artemia nauplii as food for larvae used to increase resistance to
environmental stress from pH and temperature; Mirzakhani and Mahmood Abad (2006) found feeding n-3HUFA enriched Artemia
nauplii to larvae increased resistance to environmental stress (pH and
temperature); Niksirat et al. (2006) found that unfertilised eggs can be
held in coelomic fluid outside the body for at least 48 hours without any other
treatment; Rohani et al. (2006) on using Geranium herbarum
essence to control fungi on eggs, Safari and Boldaji (2006) examined use of canola meal as a partial
replacement for fishmeal to reduce cost of diets; Sear et al. (2006)
evaluated sperm in relation to weight, length and condition factor of the fish; Tala et al. (2006) on
gonad morphology after administration of 17α-methyl testosterone, female trout
becoming functional males; Tala et al. (2006) on histological changes to
the gonads after application of 17α-methyl testosterone, ranging from functional
gonads to intersexes and sterility; Yarahmadi and Moghadasi (2006)
on use of decapsulated cysts of Artemia urmiana in the larval diet; Zorriehzahra (2006) on fry
mortality syndrome from 52 fish farms and hatcheries across Iran; Emtiazjou and Sheykhi Moghadam (2007) on
the use of Alvita (sodium di-acetate) for removing fungi from eggs as an
alternative to the carcinogen malachite green; Geramy et al. (2007) on photoperiod on growth, survival and feeding
parameters in larvae; Hosseinie Najd et al.
(2007) on initial different foods on subsequent growth of larvae; Kalbasi and
Lorestani (2007) investigated the effect of various solutions on the duration of
sperm motility, coelomic fluid without blood being the best; Khajeh and Peyghan
(2007) evaluated blood serum parameters in fish cultured in earthen ponds and
showed age-related changes; Latifi et al. (2007) found that citric acid
retarded deterioration of trout during refrigerated storage; Lorestani et al.
(2007) correlated various sperm quality measures of broodstocks with fish of
different ages; Meshkat Rouhani et al. (2007) found that a lower protein
content (40%) when the carbohydrate to lipid ratio in diet is 0.5 gave the most
favourable conditions for growth; Mohagheghi Samarin et al. (2007) on the effect of
temperature on oocyte quality and therefore stripping time; Naem et al.
(2007) on treatment of diplostomiasis using praziquantel; Nafisi Bahabadi (2007)
found that high energy feeds for trout reared in brackish water were more
effective and commercial; Rezaei et al. (2007) on bacterial changes and the biogenic amines found in
farmed trout stored on ice, Soltani et al. (2007, 2007) on vaccination against,
and immunological responses to,
Streptococcus iniae infection; Naji et al. (2007) on the effects of 17ß-estradiol
valerat on sex reversal on sac fry larvae; Rahmati Leeshei et al. (2007)
on the effect of phytase enzyme on digestibility of four vegetative
foods; Safari and Boldaji (2007) on dietary lipid level effects on growth, feed
utilisation and composition; Tabandeh and Akhlaghi (2007) on efficacy of conventional disinfectants on
isolated Streptococcus iniae from diseased fish, sodium iodide and sodium
hypochlorite being most efficient although biofilms lowered efficiency;
Varirizadeh et al. (2007) on using the gonadotropin releasing
hormone agonist (GnRHa) in emulsified form to stimulate onset of ovulation;
Yousefian and Rezvani (2007) found that abnormalities were not significantly
higher in brother-sister matings than controls; Zamani et al. (2007) on the activity of various digestive enzymes at
initiation of exogenous feeding in comparison to Salmo caspius, activity
being higher in rainbow trout; Zargarian et al. (2007) on using 2000
units/kg diets of phytase with at least 35% replacement of fish meal by soybean
meal in farming trout; Akhlaghi and Sharifi Yazdi (2008) identified
Yersinia ruckeri, the causative agent of enteric redmouth disease, in
cultured fish from Fars Province; Alevi Yaganeh et al. (2008) on
use of Gammarus powder as a dietary supplement for larvae; Alishahi
(2008) on vaccinating trout against the ciliate Ichthyophthirius multifilis; Faghani et al.
(2008) on the immunostimulatory effects of alginic acid and anti-streptococcus
vaccine on growth rate, feed conversion ratio, condition factor and
survival rate; Johari et
al. (2008) on red blood cell alterations in triploids; Karbassi et
al. (2008) on the environmental management of aquaculture in raceways at
Sarab Gerdu; Manouchehri and Akrami (2008) on a new automatic sorter for large
amounts of fish; Mohagheghi Samarin et al. (2008) on eyeing and hatching
rates under cold temperatures; Motalebi et al. (2008) on production of
aflatoxins by molds in improperly stored fish meal used as food for trout; Nafisi Bahabadi and Soltani (2008) on dietary energy levels and feeding
rates in fingerlings; Safari and Boldaji (2008) on replacing soybean meal with
canola meal in diets; Shadnoush et al. (2008) on using acorn meal as a
pellet binder and stabiliser; Shafaeipour et al. (2008) on the effects of dietary canola meal
on physiology and biochemistry as it can be used to replace substantial levels
of fish meal in food; Yousefian (2008) on genetic parameters of
growth; Yousefian and Nejati (2008) on inbreeding depression shown as variations
in percentage egg hatchability, survival of fry and weights; Zargar et al.
(2008) on the distribution of infectious haematopoietic necrosis in five
provinces; Iranian Fisheries Research
Organization Newsletter (56:42, 2008) on use of the growth hormone
somatotropin to increase body weight, daily weight gain and length; Akbari et
al. (2009) on feeding larvae with HUFA and vitamin C enriched Artemia
urmiana nauplii; Fazaeili et al. (2009) on deposition of pigments
from plants in various tissues (feed conversion ratio and specific growth rate
were affected by the experimental diets); Gashti (2009)
determined that machine trimming of fillets produced less waste than hand
trimming, although hand trimming was faster; Heydarnezhad and Purser (2009)
assessed food anticipatory behaviour in fish hand-fed in small raceways; Imani et al. (2009) on
feeding deprivation and re-feeding, showing that fish could tolerate and
compensate for starvation; Jafarian et al. (2009) on the increase in
growth performance and feeding efficiency in larvae fed probiotic larvae with
Daphnia magna meal; Lorestani et al. (2009) on
the interaction of male broodstock age and different dilutions on the production
rate of eyed eggs; Mahmoudzadeh et al. (2009) on
the effect of synthetic and alfalfa pigments on tissue pigmentation (feed
conversion ratio and specific growth rate were affected by the experimental
diets); Mousavi et al. (2009) on the antifungal activity on trout eggs of
a combination of essential oils from various herbs; Mousavi et al. (2009)
on identification of 7 species of Saprolegniaceae fungi from infected trout
eggs; Omran and Hedayatifard (2009) on fatty acid composition; Pooramini et
al. (2009) on use of yeast as a probiotic and its affect on growth, survival
and carcass quality; Rafaei et al. (2009) on the bioaccumulation of
copper, zinc iron and manganese in eggs during incubation, found to be lower
than EPA standards; Shafaeipour et al. (2009) on effects of canola meal
on growth, body composition and biochemical parameters and its use to replace
fish meal in diets; Soltani et al. (2009) on the use of Zataria
multiflora (Persian thyme) essential oil and its effects on egg hatchability
and survival of larvae compared with hydrogen peroxide and malachite green used
as anti-fungal agents; Soltani et al. (2009) on the experimental
pathology of Streptococcus sp.; Iranian Fisheries Research
Organization Newsletter (56:2, 2008 and 58 & 59:2, 2009) on culturing trout
using Silo tanks which are easier to maintain and have higher production than
cement tanks; Tavakoli and Akhlaghi (2009) on changes in serum and blood factors
after infection with the pathogen Aeromonas hydrophila; Akbary et al. (2010) comparing live food and an artificial
diet on survival, growth and body composition in larvae, the former two factors
being better with live food; Dadgar et al. (2010) on cottonseed meal as a
total replacement for soybean meal; Haghparast et al. (2010) on the
antioxidant properties of various sodium salts on refrigerated sticks; Moogouei et al. (2010) on the effects of physcio-chemical
parameters on growth in a raceway culture system; Zorriehzahra et al.
(2010) on hematological and biochemical parameters of fry in western Mazandaran,
helpful in diagnosing some diseases; etc.
The Department of the Environment has arranged fishing matches for
this trout in the Lar Dam since 1994 (www.iran-doe.org/Special/LAR.htm,
downloaded 29 December 2000).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria,
as food, in sport and in textbooks. Rainbows have been used
extensively as research animals as their requirements are well known
and they are readily available from hatcheries. Hatchery fish often
have reduced or absent fins and deformed mouths. Farmed fish are sold
frozen world-wide, the most important trout in this regard. Rainbow
trout are one of the top few sport fishes in North America and of
great commercial importance because of the money spent on gear,
accommodation, transport, etc. by anglers in pursuit of this fish.
Many books and articles have been written on the methods and joy of
catching this trout. The flesh is excellent eating fresh or smoked and
may be red if food is mostly invertebrates or white if food is fishes.
Conservation
Alborz trout have escaped and made their way to the Caspian Sea but
the numbers and impact are unknown (Bartley and Rana, 1998b). This
species is an exotic and does not need conservation but escapees may
affect survival of native species.
Further work
Bartley and Rana (1998b) make various recommendations for the
aquaculture of this species in Iran. The establishment of reproducing
populations in the wild should be carefully monitored because of the
dangers for native species posed by this exotic. Triploid all-female
trout should be considered.
Sources
Sources are summaries of biology in North America such as Scott and
Crossman (1973). Observed on trout farms in Iran but there is little data on escaped Iranian populations.
Iranian material: no preserved material.
Genus Salmo
Linnaeus, 1758
Members of this genus comprise about 25 nominal species found in North
America and Eurasia including the Black, Caspian and Aral seas basins
and the upper, and cooler, reaches of neighbouring basins in Iran and
Turkey including the Euphrates River, and the Orumiyeh and Namak lakes
(Berg, 1948-1949). There is a single described species, native to Iran. These
fishes have teeth on the shaft and head of the vomer bone in the roof of the
mouth (may be lost with age), the jaw is long, reaching to the posterior eye
margin or beyond, scales are small usually more than 100 in the lateral line,
colour is pale silvery with dark markings or spottings, anal fin rays (all
counted) number 15 or less (16 or more in Oncorhynchus), and there are
various unique osteological characters.
These are famous sport fishes and were once caught in commercial quantities. Their biology has been studied extensively
and numerous books and papers have been written about them.
Salmo salar
Linnaeus, 1758
Introduced to the Caspian Sea (Mamaev, 2002). No Iranian record.
Salmo caspius
Kessler, 1877

Stuffed specimen, Bandar-e Anzali

Liqvan Chay trout, ca, 27.0 cm total length, 4 October 1994, courtesy of Asghar Abdoli

Liqvan Chay trout, ca, 27.0 cm total length, 4 October 1994, courtesy of Asghar Abdoli
Common names
آزاد ماهي (azad mahi or mahi-ye azad = free fish, used in Farsi
for trout and salmon), ماهي آزاد (= mahi azad), azad mahi qezelala-ye daryacheh khazar (= Caspian Sea
trout), mahi azad Darya-ye Khazar, mahi azad setareh-i Darya-ye Khazar,
all for the subspecies caspius; قزل آلا (= gazalala, ghezel ala or
kizil ala, meaning red spots), قزل آلاي خال قرمز
(= qezelala-ye khalqermez; the Turkic qezel ala is
used in Farsi for trout and hence this last means "trout with red
spots" although literally it is a tautonym, "red spots with
red spots"), mahi azad-ye kezel ala-ye ilbumi (= native salmon
trout), qezel ala khalqermez yabumi, qezel ala-ye juibary (= brook
trout), all apparently for freshwater residents; mahi azade khal sorkh; qezel ala-ye
Liqvanchai (for trout in the Lake Urmia basin).
[gizilxalli or ala-balyk for freshwater residents and gizil balig
or kizil-balyk for sea-run fish in Azerbaijanian; Kaspi azatmahysy or kumja in
Turkmenian; ala-balukh Armenia; Kaspiiskii losos' or Caspian salmon for sea-run fish, and ruch'evaya
forel or brook trout and pestrushka for freshwater residents in
Russian; alabalik in Turkish; massialé in Kurdish; brown trout, brook trout, river trout, Caspian trout].
Systematics
Salmo Trutta was originally described from European rivers. Salmo
caspius Kessler, 1877 was described from the Bozh'em-Promysl (or
Bozhii Promysel) fishing grounds on the Kura River, Azerbaijan with 3
syntypes in the Zoological Institute, St. Petersburg (ZISP). Salmo
spurius Pallas, 1814 from the Terek River in the Caspian Sea
basin, Russia and Salmo lacustris var. Romanovi
Kavraiskii, 1896 from Lake Tabiszchuri in Transcaucasia are usually
regarded as synonyms of Salmo trutta. Salmo trutta ezenami
Berg, 1948 is a subspecies from Lake Ezenam in Dagestan (ZISP 28356)(Dorofeyeva
and Salmanov (2001)) though it may be deserving of species rank (Reshetnikov et al.,
1997). Salmo trutta ciscaucasicus Dorofeyeva, 1967 (see also
Dorofeyeva (1997) and Dorofeyeva and Salmanov (2001)) is described from the Keyranchay (ZISP 26244) and
is found in drainages of the western shore of the Caspian Sea, except
for the Kura River and, presumably, excepting Iranian rivers.
The native brown trout (freshwater populations) and Caspian salmon
(sea-run populations) of Iran were both usually referred to Salmo
trutta caspius Kessler, 1877 in the Caspian Sea and Namak Lake
basins (Saadati, 1977). Derzhavin (1929b) examined two small fish from
the Karaj River of the Namak Lake basin and recognised them as typical
"brook trout" (Salmo trutta). Nümann (1969)
considers that southern Alborz trout originate from Caspian stocks.
Some authors call the species Salmo fario caspius Kessler,
1877, e.g. Dadikyan (1986). Fricke et
al. (2007) and Naseka and Bogutskaya (2009) regard caspius as a full species.
Tortonese (1954), Kuderskii (1974) and Kottelat (1997) discuss the
confused nomenclature of this species. Boulenger (1896), Berg
(1948-1949) and Derzhavin (1929b) consider Namak Lake basin trout to
be Salmo trutta macrostigma (Dumeril, 1858), originally
described from Algeria as a distinct species. The Namak trout were
supposedly derived from Mediterranean populations via Persian Gulf
drainages but Saadati (1977) dismisses this on the grounds of a close
similarity between Namak and Caspian trout and the apparent absence of
trout from the Zagros Mountains and Persian Gulf drainages of Iran.
Osinov (1988) could not determine a Mediterranean route based on
electrophoretic evidence.
In the Lake Orumiyeh basin, apparently restricted to the "Lighvanchai"
(probably the Liqvan Chay, the town of Liqvan being at 37°50'N, 46°26'E),
is a population of brown trout which has been referred to as a
distinct subspecies (Anonymous, 1977). It has not been formally
described. Saadati (1977) considers all Iranian trout to be S. t.
caspius, with those in the Namak Lake basin being a distinct
"race", with some slight differences to typical S. t.
caspius (see below), and those trout in the Lake Orumiyeh basin
could be a new subspecies.
Earlier authors consider the Caspian basin species to be the same
as the Atlantic salmon, Salmo salar Linnaeus, 1758, but blood
serum electrophoresis (Ostroumova, 1970) support its position as Salmo
trutta (and presumably caspius) as does karyology and osteology (Dorofeyeva, 1965; 1967)
and enzyme electrophoresis (Osinov, 1984; 1988). Osinov and Bernatchez
(1996) consider trout from the Caspian Sea basin to be part of their
"Danubian" grouping, brown trout from the Black, Caspian and
Aral sea basins as opposed to an "Atlantic" grouping from
the Baltic, Barents and White sea basins (and also a tributary of the
upper Volga River in the Caspian Sea basin), based on allozyme and
mtDNA studies. Vera et al. (2011) concur. Novikov et al. (2008) examined allozyme variability in
trout populations from Iran and found them to be similar but diverging
significantly from other populations in the Caspian Sea basin. Turan et al.
(2009) described two new species of trouts from northern Anatolian streams, one
resident and the other migratory. A similar situation may occur in Caspian
streams where there are the two life history types, although the authors caution
that each situation should be investigated individually. They advocate a more
cautious treatment of trout taxonomy as such results have important implications
for management and conservation. Vera et al. (2011) found two main
clusters, using microsatellite data, connected by gene flow among river basins,
presumably by anadromous fish.
A artificial hybrid of Salmo trutta caspius with Oncorhynchus
mykiss is reported on by Pourgholam and Noruzy Moghadam (1996).
Key characters
The dark-spotted back, light halos around some of the dark-coloured flank
spots, caudal fin not or only weakly spotted, teeth on the vomer shaft, and only
9-15 total anal fin rays are distinctive.
? from S. trutta?
Morphology
Salmanov (1990) has demonstrated that brook, lake and sea forms of
trout from the Caspian Sea basin differ in head and body proportions
and in fin shapes although no populations in Iran were considered or
given taxonomic status. Osinov (1988) maintains that migratory and
non-migratory populations are the same species.
In the Caspian Sea basin dorsal fin with 3-5 unbranched and 7-12
branched rays, anal fin with 2-5 unbranched and 6-10 branched rays,
pectoral fin with 10-14 branched rays, and pelvic fin with 6-9
branched rays. Lateral line scales 111-132. Gill rakers 16-23. Pyloric
caeca 26-61. Vertebrae 55-61. Scales in lateral series for S. caspius 140-160, vertebrae 59-61 (Derzhavin, 1929b; Behnke, 1965).
See also below under Zoogeography. The chromosome number is
2n=78-84 (Klinkhardt et al., 1995), 2n=80 (Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization,
Tehran, p. 61, 1997; Kalbassi et al., 2006) or 2n=80-82 (Dorofeeva, 1998).
The chromosome formula is 14M + 10SM + 56T with NF=104 (Kalbassi et al., 2006).
Meristic values for Iranian specimens are:- dorsal fin branched rays 9(1), 10(5) or 11(1), anal fin
branched rays 8(7), pectoral fin branched rays 12(7), pelvic fin branched
rays 7(1) or 8(6), and total gill rakers 17(3), 18(3) or 19(1).
The Liqvan Chay fish are similar to S. caspius in all
except colour, gill rakers being somewhat higher (18-22, mean 20.2)
and lateral series scales being higher (132-154, mean 142) but sample sizes were small.
Sexual dimorphism
Abdurakhmanov (1962) reports on two populations form Azerbaijan
where he found head length, predorsal distance, snout length and lower
jaw length to be greater in males and postorbital length greater in
females for both populations, while body depth, dorsal and anal fin
heights, and pectoral and pelvic fin lengths are greater in males and
eye diameter, pelvic-anal fin distance, head depth and interorbital
width are greater in females for one population.
Colour
A specimen from the Anzali Mordab had a dark brown-grey back and
top of the head with some round black spots, silvery-yellowish sides
with 3-4 longitudinal rows of small red spots with whitish halos, a
large black spot on the preoperculum and several smaller spots on the
operculum. The belly and lower head surface were white with minute
dark melanophores. The dorsal fin was a pale grey with 3-4 horizontal
rows of black spots and 1-2 series of 4-8 ellipsoid red spots
vertically. The adipose fin was overall yellowish with some grey
pigment while the upper half of the fin was more orange in colour. The
pectoral, pelvic and anal fins were a pale yellowish or nearly
colourless with grey pigment. The anterior 2-3 rays were coloured
orange. The caudal fin was greyish with the upper and lower marginal
rays dark orange. All fins lacked spots except one red spot observed
on the dorsal fin of a larger fish (19.8 cm total length). Smaller
fish (13.7-16.2 cm fork length) had 12-14 parr marks. The red flank
spots are recognised in the name "kizil ala". Generally in
the sea, this species has numerous, dark, x-shaped spots on silvery
flanks although some individuals have almost none. The fish caught by
Holmes (1845) mentioned below had a dirty olive-green back, dark brown
and red spots on the flanks, a roseate tint to the flanks, and a
golden belly. The migratory form was dark blue on the back, silvery on
the flanks and belly, and had dark spots on the flanks.
Prosek (2003) illustrates in colour a trout from the Tigris River in Turkey,
from the Caspian Sea basin in Turkey and Armenia, and from the Liqvan Chay.
The Liqvan Chay population has 54-400 red to orange spots along its
flank (compared to only 30-50 generally in European brown trout)
giving it a ruby-red sheen (Anonymous, 1977). Only one Liqvan Chay
fish had less than 100 red spots based on 13 fish examined by Saadati
(1977) compared to a range of 27-134 for 13 S. t. caspius from
the Shah Neshin River near Ardabil, where only 2 fish had more than
100 spots. The spotting pattern consists of profuse black and red
spots and is distinct from all other Salmo trutta sensu lato which have
fewer and larger spots (Saadati, 1977). Colour photographs, courtesy
of Asghar Abdoli of the Agricultural and Natural Resources University,
Gorgan, of 3 fish from the Liqvan Chay dated 4 October 1994 with a
total length of 27.0 cm showed two fish with about 87 red spots while
the other had about 200 (accurate counts not possible because of body
curvature and silvery reflections). There was considerable variation
in spot size among the three fish, the one with most spots having
generally smaller spots which give the sheen referred to above, while
the other two fish have more discrete and larger spots. It is unknown
whether the Liqvan Chay population has been contaminated with other
stocks of Salmo trutta.
Size
The sea-run Caspian trout attains a larger size than freshwater
populations. Attains 51 kg and 1.5 m but most seen in Iran were 10-15 kg (Walczak,
1972). Length reaches 118 cm and weight 21 kg in Iranian samples but
such large fish are rare in Iran (Farid Pak, 1968b). Most Iranian
catches are 55-105 cm and 1.8-12.7 kg (Farid-Pak, no date). Holmes
(1845) reported one fish caught at the mouth of the "Mazzur"
River weighing 16 lbs (7.3 kg) and measuring 38.5 inches total length
(0.94 m). Nümann (1969) reports fish almost 1 m long in the Karaj
Reservoir in 1967. A fish 78 cm long and weighing 8 kg was worthy of
note in 1996 (Anonymous, 1996c).
Distribution
The Caspian salmon is found in the Caspian Sea and enters Iranian
rivers to spawn as well as being resident in Gilan, Mazandaran and Golestan
provinces (Sheil, 1856; Nedoshivin and Iljin, 1929; Kozhin, 1957; Nümann, 1966;
R. J. Behnke files; Armantrout, 1980; Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 28, 1996; Abbasi et al.,
1999; Abdoli, 2000). Formerly known from the
Anzali Mordab and its tributaries (Holčík and Oláh, 1992).
Reported prolifically from the Seh Hazar, Chalus and Babul
rivers (Fortescue, 1920). Found in the Safid River, Kargan River near Hashtpar,
Chalus and Babol rivers. Rare in the Aras (Berg, 1948-1949). Abundant
in the upper reaches of the Gorgan (G. S. Karelin cited in Berg
(1948-1949) although Karelin only visited the lower Gorgan himself).
Reported as S. t. caspius from the Sardab and Tonekabon rivers,
Gorgan Bay, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea and as S. t. fario from the Tajan,
Babol, Haraz, Sardab, Tonekabon, Pol-e Rud, and Safid rivers (Kiabi et al., 1999;
Abdoli and Naderi, 2009). Jolodar and Abdoli (2004) give its distribution in the Caspian basin as in
upstream waters from the Aras to the Tajan, absent in the Atrak and Gorgan
rivers.
Also found in the Jajrud (Ouseley, 1819-1823), in the Karaj River (Fortescue,
1924; Derzhavin, 1929b; Berg, 1949), and from the vicinity of Damavand (? Damavand
River) (Fraser, 1825), and numerous other localities in the Namak Lake basin
(see Armantrout (1980) for localities from the 1970s and Abdoli (2000) for more
recent general distributions). However Nümann
(1969) seems to indicate that fish in the Karaj River were stocked
about 100 years previously from Caspian Sea rivers. Reports from the
Zagros Mountains in Iran are uncertain and those from mountains near
Kerman even more so! (Walczak, 1972). These last two records have not
been confirmed by specimens and with introduction of exotic material
may now be impossible to verify. Fraser (1834) reports that "Trouts
are found in several of the streams of Azerbijan and Kurdistan".
It may be worth noting that Heckel (1843b:995) reports that the
collector Theodor Kotschy "did not find trout....in the mountains
of Kurdistan. Since we know our collector's diligence, we doubt that
trout occurs there" but in (1846-1849a:254) Heckel states
"we must add one trout (Salmo), which tastes excellently
according to the report presented by our traveller; that trout occurs
relatively frequently in the mountains of Kurdistan, but has not been
seen by us". This is merely confusing: did Kotschy later find
trout or change his account, or did Heckel recall Kotschy's accounts incorrectly?
The Liqvan Chai in the Lake Orumiyeh basin contains a distinctive
trout and Liqvan Chay trout were stocked in the Ab-e Bazuft of the upper Karun
River (Tigris River) basin in the autumn of 1975 but this
failed (B. Sandford, in litt., 1979; Prosek, 2003).
Zoogeography
The origin of Iranian trout is probably from the north via glacial
lakes during the last or earlier ice ages and via the Volga River
system or, possibly, from the west via the Black and Mediterranean sea
basins (Tortonese, 1954; Nümann, 1969; Kuderskii, 1974; Osinov, 1984;
Farid-Pak, 1991). The initial colonisation of the Caspian Sea by this
trout apparently occurred much earlier than the late glacial period on
electrophoretic evidence (Osinov, 1988). The question of a northern
origin remains open however on present biochemical evidence (Osinov
and Bernatchez, 1996). The division between their Atlantic and
Danubian groupings occurred about 0.5-0.9 million years ago based on
their data, perhaps 0.7-2.0 million years ago when the division of
European and North American groups of salmonids are taken into
account. These authors note that populations within the Caspian Sea
basin have unique gene pools, suggestive of reproductive isolation,
and these fish are not simply the subspecies S. trutta caspius.
Bernatchez (2001) considers all Caspian trout to belong to the Danubian or Ponto-Caspian
lineage, one of five lineages, and that the centre of origin was probably from
drainages associated with the Cauacasus region of the Black Sea. The major
demographic expansion of this lineage probably occurred about 270,000-290,000
years ago. Bernatchez (2001) considers that populations of each sea basin
occupied by the Danubian lineage should be recognised as distinct evolutionary
lineages but he does not give them names.
Trout in the Zagros Mountains may be Salmo trutta macrostigma
(Dumeril, 1858), if this is a valid subspecies, while some authors
consider Zagros trout to be S. t. caspius on zoogeographical
grounds but pure samples of this population have never been examined
systematically (Behnke, 1965). Boulenger (1896) and Berg (1948-1949;
1949) consider trout in the upper Euphrates and in the Namak Lake
basin to be S. t. macrostigma. Namak Lake trout are then
Pliocene relicts of this Mediterranean subspecies from an invasion via
the Persian Gulf region. However, the Namak Lake fishes generally show
evident affinities with the Caspian Sea basin ichthyofauna and the
trout themselves are very similar in appearance.
The Namak Lake basin trout are generally held in Iran to have been
planted by the royal family in the nineteenth century (Saadati, 1977).
The royal family had sporting camps on the Lar River and the
"Varang-e Rud", a tributary of the Karaj River. Saadati
(1977) compares 30 specimens from each of these two localities and
found characters to be similar except for pyloric caeca (45-58, mean
51 in the Karaj sample, 35-46, mean 39 in the Lar sample) and in
scales in lateral series (131-146, mean 138) and 121-134, mean 127
respectively). He concluded that Karaj River fish are native, of
relatively recent origin from the Caspian Sea basin, and only slightly divergent.
Saadati (1977) also reports on a population of trout in the Mordugh
Chai (probably Mordaq Chay) in the Lake Orumiyeh basin. This
population was typical S. caspius with only 17-38 red spots
on the flank. It may have been of recent origin via a headwater
transfer as the tributaries to the Caspian Sea basin are found nearby.
A human agency may have been involved, always a complicating factor in
the distribution of fish which are of sporting or commercial interest.
Habitat
Mahi azad live in the Caspian Sea proper and migrate up rivers to
spawn. Moosari (1996) describes migration into the Tonekabon River of
Iran. Young mahi azad stay in rivers for 2 years. There are also
land-locked populations such as that of the Lar River near Damavand
Mountain and this species may also be found in cool to cold lakes.
Areas with clean gravel are required for reproduction. Temperatures
above 15°C cause egg mortality. Adults can survive up to 29°C,
18-24°C is the optimum range and 12.4-17.6°C the preferred range. Atai Mehr
et al. (2006) found juveniles to survive salinities of 0 to 12.5 g/l over
120 hours, indicative of their adaptability to changing environments. The best
weight and water salinity for release of cultured juveniles was 10 g and 8.0-12.5 g/l.
In the sea, they inhabit coastal areas at 40-50 m with distinct
stocks centred on the basins of the major rivers. Migrations occur from Iranian
shores to Dagestan (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
The Liqvan Chay population females only begin to mature in their
third year and only 50% of males are mature in their second year. This
population appears to be in good condition compared to other
populations, e.g. in the Lar River, and these figures probably
represent more natural values. A population estimate was 1246 fish per
km compared to 11,380 per km in part of the Lar River where fish had
concentrated to avoid severe drought. The life span in the Lar is 6
years (Nehring, 1975a).
There are stocks of dwarf males in Caspian rivers which do not
descend to the sea.
Farid Pak (1968b) found the smallest sexually mature fish in the
Caspian Sea was 53 cm long and the largest 105 cm. Most fish in the
commercial catch of the Caspian Sea were 5-9 years old in the 1950s
(Farid-Pak, no date). Caspian Sea fish may mature as early as 1 year for males
under riverine conditions but most fish mature at 3-9 years, depending on the
river (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Afraei et al. (2000) examined 190 fish from the Tonekabon River and found
age groups 0+ to 4+. Most fish were in the 2+ age group. Maximum size was 175 mm
and 84.5 g. Condition factors were 1.268 for males and 1.257 for females.
See also below under Reproduction.
Niksirat and Abdoli (2009) and Abdoli et al. (2011) found a decline in length from 77.6 cm in 1947 to
59.8 cm in 2007 (22.4%), in weight from 4880.2 g to 2486.8 g (49.5%), in
absolute fecundity from 7041.8 in 1947 to 4526.1 in 1973 and 2941.2 in 1986, and
in relative fecundity from 1451.4 per kg body weight in 1947 to 1372.6 in 1973
and 1199.8 in 1986 (only latter value significantly different in relative
fecundity). Size classes over 75 cm have disappeared from the population over
the past 60 years and mature samples were less than 50 cm. Various factors cause
such declines, including fishing mortality and overfishing of prey such as
Clupeonella spp. (and their loss from the exotic comb jelly), an important
food which helps produce more eggs. Kheyrandish et al. (2010) examined
fish from six rivers in Mazandaran and found five age classes (0+ to 4+) with
the most frequent being 1+ and 2+. Condition factor ranged from 0.58 to 1.47.
Females dominated over males in 5 of 6 rivers, the highest sex ratio being 6.7
females:1 male in the Pajimiane River and 0.8:1 in the Khojirood. There were
significant correlations between fish length and depth and fish length and width
of rivers surveyed.
Nümann (1969) found Alborz trout to reach 26 cm at the end of 3
years, corresponding to the growth rate for central European fish.
Food
Freshwater populations are known to eat mayfly larvae in Iran as
well as other aquatic insects and crustaceans and terrestrial
insects taken at the surface. Larger specimens elsewhere are known to
take fish and crayfish, and more rarely frogs, salamanders and rodents
and this probably occurs in Iran too. Tonekabon River fish (Afraei et al., 2000) fed most
intensively in spring and least intensively in autumn. The main prey was
Simulium, Plecoptera and Ephemeroptera.
In the sea, young feed on amphipods, mysids and shrimps while
adults take common and anchovy kilkas, silversides and shads.
Reproduction
This fish runs up rivers in October-November and March-May in Iran
(Roux, 1961b). Holčík
and Oláh (1992) record migrations into the Anzali Mordab and a run up
the Pasikhan and Siahdarvishan rivers from late September to the end
of December. A second run occurred in April-May but was much smaller.
Holmes (1845) reports "salmon" in rivers of Gilan from June to the end
of October. Bartley and Rana (1998b) report the spawning run on the
Tonekabon River to be from September to November and spawning to be
from November to December. Spawning occurs on riffles of sand and gravel beds. Spawners are 8-12 years old and are mature
at 700 g. About 1500 eggs are produced for each kilogramme of female. The fish
examined from the Tonekabon River (Afraei et al., 2000) had an
absolute fecundity of 168-379 eggs, average 268 eggs.
Maximum egg diameter is 6.1 mm, number of eggs per gram reaches an
average of 14.7 and absolute fertility reaches 13,468 eggs in Iranian
fish which are considerably smaller than Kura River fish in Azerbaijan (Farid Pak, 1968b).
Alborz trout are ripe at 2-3 years while Caspian Sea fish spend 2-3
years in rivers, then 2 years in the sea before re-entering rivers to
spawn at 60-70 cm. Spawning can occur more than once in a life span (Nümann, 1969).
In the Kura River of Azerbaijan there are two races. One enters the
river in October and travels no higher than the middle reaches 600-700
km from the mouth, is sexually mature and does not weigh more than 12
kg, and spawns in the same year. The second begins to enter the Kura
in October but most fish run in November and December, this race spawns in the upper
reaches about one year later (8-11 months) after a long migration of over 1000
km, and attains 51 kg. There are two races also in Iranian rivers (Berg,
1959). The Kura salmon may have up to 45,000 eggs of up to 6.5 mm
diameter. Kura salmon spawn only once between ages 5 and 9 years. Some
Kura salmon become smolts and migrate to sea in the first year of life
but most descend in their second year.
Parasites and predators
Mokhayer (1976b) records the protozoan Trichodina from the
gills of trout (? this species) in the Karaj River, the cestodes Eubothrium
crassum and E. rugosum, the acanthocephalan Corynosoma
caspicum, and the annelid Piscicola geometra.
Jalali et al. (2005) and Malmberg et al. (2007) summarise the occurrence of Gyrodactylus
species in Iran and record G. derjavini from fish in the Sardab-rud.
Sattari et al. (2005) surveyed this species in the
Chesli and Khorma rivers, recording Cystidicoloides ephemeridarum.
Sattari et al. (2004) record the nematode Cystidicoloides
ephemeridarum sp. from this species in Gilan.
Economic importance
In a survey of the Lar River in June, RaLonde and Walczak (1970b)
found 22 fishermen had caught 222 trout with an average length of
19.85 cm, comparable with an earlier survey by Nümann (1964). The
largest fish was 27 cm long. The catch was 2.56 fish per fisherman per
hour, a good rate of success (but see older records below). This rate
and the production of trout in this river was expected to decline
drastically with construction of a dam on the Lar. Sport fishermen
took 50,000 trout from the Lar River in 1967 (Surber, 1969).
Malek-Eizadi (1993) gives a recent account, in Farsi, of the Lar trout
and apparently confirms its decline. Floor (2003) cites older records of fishing
in the Lar River.
Azad mahi were caught in weirs, nets and with long, 3-pronged forks year
round but the principal seasons were spring and autumn (Holmes, 1845; Floor,
2003).
The catch in the Safid River region was largest from February to
April, with a maximum in the middle of March. In 1912-1913, a total of
1180 fish were caught in the Safid River, Anzali and Astara regions.
Weights reached 10-12 kg, average 7.5 kg (Nedoshivin and Iljin (1929)
cited in Berg (1948-1949)). Fortescue (1920) reported 1500-2500
"salmon" from the Seh Hazar at Shahsevar, averaging 8-10 lbs
(3.63-4.54 kg) each and seldom exceeding 18 lbs (8.17 kg). Nevraev (1929) reports on catches in
various regions of Iran in the early years of the twentieth century.
In the Astara region from 1901-1902 to 1913-1914, the catch ranged in
numbers from 236 to 1563 and in the Safid River region from 1916-1917
to 1917-1918 the range was 185-663 fish. Yearly catches of mahi azad
in the Anzali region alone have reached as high as 3,037 kg in
1939/1940 and as low as 90 kg in 1946/1947 before commercial fishing
was prohibited (Vladykov, 1964; Walczak, 1972). The Food and
Agriculture Organization, Rome reported only 1 tonne of
"salmonoids" caught in Iran for each of the years 1983
to 1985, presumably the Caspian salmon. Occasional catches are taken in
sturgeon nets of fish 1-4 kg in the Anzali region and elsewhere along
the Iranian coast (Holčík and Oláh, 1992). Many fish are taken by poachers in traps and nets
used to block spawning streams. Salehi (2008a) notes a decline in catches of this
species from 13 t in 1995 to less than 3 t in 2005 and most of the catch is
based on stocking rather than natural reproduction.
There is a state supported stocking programme but growth rate is slow. High summer temperatures
(28°C) in the Caspian Sea would affect cage culture and survival (Rana and Bartley, 1998a).
Salmo trutta is reported
to be ichthyootoxic although there are no reports for S. caspius (Coad,
1979b).
Conservation
Pietro delle Valle, who traveled in Iran from 1616 for 7 years,
reported trout from rivulets in Ardabil in the Caspian Sea basin (see
Pinkerton, 1758-1826, volume IX:84), a city not now noted for so
salubrious an environment for salmonids.
Ouseley (1819-1823) dined while at Tehran on dried and salted azad
mahi two feet long (0.61 m) from the Caspian Sea for breakfast one day
in November 1811 and on fresh trout (later referred to as
"kizl-áleh") from the Jajrud for dinner.
Vigne (1842) recorded catching six or seven dozen a day in the Lar
River near Tehran, Charles Murray reported in 1858 that the stream
"abounds so much in trout that I frequently kill 50 in an hour
with a fly" (recorded in Wright (1977)), Valentine Baker (cited in Prosek
(2003)) caught 50-60 trout a day up to 4 lb in weight, and Mounsey (1872)
caught 450 fish up to 1.5 lbs (0.68 kg) in 3 days using flies.
Anderson (1880) found that the Lar River "abounded in
trout". They were still numerous there in the 1970s (Nehring,
1975a). However this population was unusual in that growth rates and
population densities fluctuated drastically from year to year,
probably because spawning habitat and nursery areas were the only
adequate features of this river. Features lacking were feeding areas,
stable banks and stream bed, good plant cover along the banks to
supplement productivity, good physical and chemical water quality and
water levels, and presence of predators as controls on excess
reproduction. As a result, recruitment of young-of-the-year was high
and led to interspecific competition, earlier sexual maturity, and
stunting. Overgrazing in the Lar Protected Area (formerly a National
Park) has affected the survival of trout in this region since this
reduces the availability of insect food, and in 2000 this situation
was exacerbated by the prolonged drought (Imamai, 2000).
Numbers of this species have declined drastically as evidenced by
catch records. The catch decreased 100 times since 1959 to the early 1970s and smaller fish taken
in beach seines were used by fishermen to eat as their market value was low.
In the 1970s, the Shahsavar River was assessed as the best in Iran for this
species as the river was in good condition. However, even this river was blocked
in at least two areas for capturing fish and almost all adults were taken for
"culture" by Shilat. A take of 300 fish provide as many eggs as 20
should because of poor treatment (15°C, Saprolegnia infections). The
Anzali Mordab was surveyed half a mile from a guard station and 11 gill nets
were found, all illegal, in a half mile stretch in half an hour (Carl Bond Archives, Oregon State
University, Corvallis). Niksirat and Abdoli (2009) report gill nets used in
estuaries catch migrating fish illegally and they are also caught as bycatch in
the Rutilus frisii kutum fishery, but not released. About 2-3 times the
the legal fishery for artificial reproduction is taken by poaching.
Attempts to increase natural reproduction by raising
young to a size more likely to survive have been attempted in Iran
(Andersskog, 1970). The "Kelardasht" (or Shaeed Bahonar)
Fish Farm (part of Shilat, the Iranian Fisheries Company) on the
"Sandar" River has a production capacity of 100,000
fingerlings of 15-20 g but this has never been achieved (Woynarovich,
1985; Krasznai, 1987). It takes about 2 years before fingerlings reach
15-20 g and 10-15 cm, large enough to release. Brood stock are
collected from the Tonekabon River during the September to November
spawning migration. Spawners are 8-12 years old and mature at 700 g.
New brood stock are collected each year although stock from previous
years are kept as backup. In 1997, 500 fish were caught in a 3:1 ratio
of males to females. Eggs from two females are mixed with milt from
two males. Egg yield is about 1500 eggs/kg. Growth rate is slow in
this species, compounded by low rearing temperatures of 2-17°C.
Fingerlings take 10 months to reach 10 g (Bartley and Rana, 1998b). In
a study of the Tonekabon River migration pattern, the following
conditions are found to be favourable for release of cultured fish:
release size should be at least 15 g, release point 500-700 m away
from the estuary, release time March and October, and for one-year-old
fish smolt release is favoured over parr as the former would migrate
to the sea (Annual Report, 1994-1995, Iranian Fisheries Research
and Training Organization, Tehran, p. 28, 1996). Fingerling
production by government hatcheries was 0.01 million in 1986, 0.05
million in 1988, 0.10 million in 1989, 0.16 million in 1990, and 0.20
million in 1991 and in 1992 (Emadi, 1993a). Fingerling production in
1995 was 0.8 million and in 1996 was 0.42 million (Bartley and Rana,
1998a; 1998b). In 2002 and 2003, 344,000 and 325,000 fingerlings were released
at a cost of 2125 and 2288 million rials respectively (Salehi, 2008a). The "Gharasoo" Research Station in Sari is
using cage culture of this species (Madbaygi, 1993b). Smolts weighing
25 g on average were released into cages and fed on locally prepared
food (Iranian Fisheries Research and Training Organization
Newsletter, 4:5, 1994). Culture of a rapid-growing triploid sea
trout has been studied in Iran (Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 32, 1996;
Annual Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 67, 1997).
Sayyad Borani et al. (2006) studied the effects of weight on the
osmoregulatory ability of juveniles in an attempt to determine the ideal weight
for release (see also above under Habitat). Weight classes were 5, 10, 15 and 20 g and the three larger classes
were capable of osmoregulation in Caspian Sea water. Mojazi Amiri et al.
(2005) detailed the histology of the digestive tract from hatching to parr stage
in order to better understand the digestive system capabilities and enhance
rearing. Asaeian et al. (2006) studied brood fish taken from the early
mid and late migrations, finding those from the early period were more suitable
for offspring production, passing through smoltification more rapidly. However,
they noted that fish from all periods should be used to maintain genetic
diversity. Bahrkazemi et al. (2006) traced the histochemical changes in
the digestive tract from hatching to parr stage. Javaheri Baboli et al.
(2006) examined the effects of n-3 HUFA enriched Artemia nauplii as a
starter food and showed it was no better than newly hatched Artemia. Sarvi et al. (2006), Hatef et al. (2007), Niksirat
et al. (2007) and Sarvi Moghanloo et al. (2007), have studied cryopreservation of sperm, sperm and seminal
plasma composition, and in vitro storage of unfertilized ova in Iran as
means to help conserve wild populations. Research into osmotic regulation
determined that fingerlings 10 g or above have a higher chance of survival (Iranian Fisheries Research Organization
Newsletter, 53:4, 2008). Ataei Mehr et al. (2007) studied the effects
of weight and salinity on the number and size of Malphigian bodies in juvenile
kidneys which has relevance for release of cultured fish in fresh and salt
water. Saber et al. (2007) found that for larvae a
diet with 50% protein and 4600cal/g energy was appropriate. Jamalzadeh et al.
(2008) established haematological and serum biochemical indices for smolts,
juveniles and breeders. Zamani et al. (2007) found differences in
activity of some digestive enzymes in the stomach, pyloric caeca and intestine
of parr and of smolt. Bahrekazemi et al. (2009) studied the
relation between egg viability, egg and ovarian fluid composition and time of
stripping in fish at the Kelardasht Hatchery. Eyeing and hatching rate declined
with over-ripening time although larval abnormalities remained constant.
The best time to take eggs was up to 10 days post-ovulation. Mahmoudi et al.
(2009) fed different levels of dietary nucleotides showed positive effects on
growth performance and a decrease in liver demolition. Ramezani (2009) fed
fish experimental diets and found a better feed conversion ratio and specific
growth rate at lower protein levels, 50% protein supporting maximum growth.
Bahre Kazemi et al. (2010) investigated the use of biochemical and
histological parameters as egg quality biomarkers. Hajirezaee et al.
(2010a; 2010b) examined the chemical properties of seminal fluid and its effect
on sperm motility, and effects on semen characteristics of various stripping
frequencies.
Salehi (2008a) carried out a cost
factor analysis for fingerling production at the Kelardasht Hatchery in Iran and
found labour costs to be 52% and feed 16%. The average cost of production of a
fingerling was U.S.$0.84 (6269-7123 rials), more than that for sturgeon
(1753-2028) and kutum (54-121). Higher costs are probably associated with lack
of broodstock, lower production quantities and the scale of the hatchery. The
rate of return at age 3-4 years was estimated at 0.5% of those released
annually.
RaLonde and Walczak (1970b) and Walczak (1972) lists the following
reasons for decline of this species: lowering of the Caspian Sea level
making spawning migrations difficult, habitat changes including
siltation, pollution, irrigation dams, and unscreened irrigation
intake canals, and poaching. Both adults and freshly released
fingerlings are heavily poached (Petr, 1987). In the Lar and Karaj
rivers about 1960 professional fishermen took trout using nets,
chemicals and explosives until laws regulating seasons and equipment
were passed and game wardens hired to enforce them (Surber, 1969).
Streams running into the Caspian have their lower 1-3 km dried up by
May through irrigation demands and trout are unable to reach the sea.
Stronger enforcement of laws by game guards, education of the public,
particularly local people, habitat protection and improvement, and a
rational exploitation system are required to protect this species.
Firouz (1974; 1976) reported that the sea-run form was a major
commercial species 25 years prior to his account but the deteriorating
environment coupled with dynamiting, netting and trapping severely
reduced populations. This fish is now completely absent from the
Anzali Mordab (Holčík and Oláh, 1992). Waters where populations survived were designated
"Protected Rivers" in an effort to manage this species
effectively. There are special licence requirements, bag limits and
seasons which anglers must observe. A fine of 10,000 rials is imposed
specifically for illegal fishing of this species (Anonymous, 1977-1978).
The Liqvan Chay population has been confined to this single river
by, it is believed, destruction of habitat through agriculture and
domestication of sheep and goats (Anonymous, 1977). De Mecquenem
(1908) said trout were abundant in the upper reaches of rivers in the
Lake Orumiyeh basin. In 1975, 500 specimens of this subspecies were
transplanted into the Ab-e Bazoft near Shiraz in an effort to conserve
it (Anonymous, 1977).
Caspian salmon used to enter the Kura River basin, with 20% running
up the Aras River on the Iranian border. Most spawning beds on the
Aras became inaccessible in the 1950s with the construction of the
Bagramtapinskaya Dam. The Mingechaurskaya Dam on the Kura cut off 80%
of that rivers spawning beds in 1951. The former Soviets built salmon
hatcheries to offset the losses but these proved to have a low
efficiency. The young fish had a high mortality because of release at
unsuitable high temperatures, release in poor feeding areas, and
exposure to water intakes. In addition, the released fish were too
small or too few to undergo significant smoltification and migration
to ensure subsequent reproduction. Under natural conditions only a
small proportion of juveniles undergo smoltification (Bakshtanskii et al., 1973).
Kiabi et al. (1999) and Jalali et al. (2009) consider S. caspius to be
critically endangered in the south Caspian Sea basin according to IUCN
criteria. Criteria include sport fishing, few in numbers, habitat
destruction, medium range (25-75% of water bodies), absent in other
water bodies in Iran, and absent outside the Caspian Sea basin. Kiabi et al. (1999) also consider S. t. fario to be vulnerable in the south Caspian
Sea basin according to IUCN criteria. Criteria include sport fishing,
medium numbers, habitat destruction, medium range (25-75% of water
bodies), present in other water bodies in Iran, and present outside
the Caspian Sea basin. Coad (2000a), using 18 criteria, found this species
to be one of the top 4 threatened species of freshwater fishes in Iran. Nezami
et al. (2000) consider this species to be endangered because of
overfishing, habitat destruction and spawning ground degradation. Mostafavi
(2007) lists it as vulnerable in the Talar River, Mazandaran.
Critically endangered in Turkey (Fricke
et al., 2007). Jalali et al. (2009) recommend reduction in pollution,
waters where fish are released should be designated Protected Rivers, cage
culture should be enhanced, spawners from different populations should be used
to enhance genetic diversity in restocked populations, and gamete storage
protocols could be applied to commercial aquaculture or to a biological
conservation programme..
Osinov and Bernatchez (1996) note that populations within the
Caspian Sea basin have unique gene pools, suggestive of reproductive
isolation, and these fish cannot be managed as a single subspecies, S.
trutta caspius. Re-introductions and artificial maintenance of
native populations could lead to loss of diversity.
Further work
Togan et al. (1995) show that two Turkish populations of
brown trout are genetically distinct, at a level often found between
species. Genetical analysis of suspected surviving pure populations of
Iranian Salmo caspius should be carried out to determine which
stocks should be receive special attention for conservation.
Sources
Iranian material:- CMNFI 1970-0551, 5, 109.9-166.3 mm standard length, Gilan, Ghaleh River near Fowman (37º13'N, 49º19'E);
CMNFI 1979-0086, 2, 76.5-145.5 mm standard length, Mazandaran, Hasanabad River tributary to Chalus River (no other locality data);
CMNFI 1980-0133, 3, 116.8-139.2 mm standard length, Markazi, Karaj fish hatchery (no other locality data);
CMNFI 1980-0158, 1, 93.3 mm standard length, Markazi, Polurd River at Damavand Mountain after Ab-e Ali (35º51'N, 52º04'E);
CMNFI 2007-0125, 3, 91.2-169.3 mm standard length, Markazi, Luniz River in Karaj River basin (no other locality data);
CMNFI 2007-0126, 7, 95.2-158.3 mm standard length, Azarbayjan-e Khavari, Aldarvish River, Sabalan Mountain (no other locality data);
BM(NH) 1877.7.5:1, 7, 168.1-194.7 mm standard length, Tehran (no other locality data); BM(NH) 1908.8.7:27-28, 2, 129.0-145.7 mm standard length, north slope of Elburz
Mountains near Tehran (no other locality data).
Salmo trutta
Linnaeus, 1758
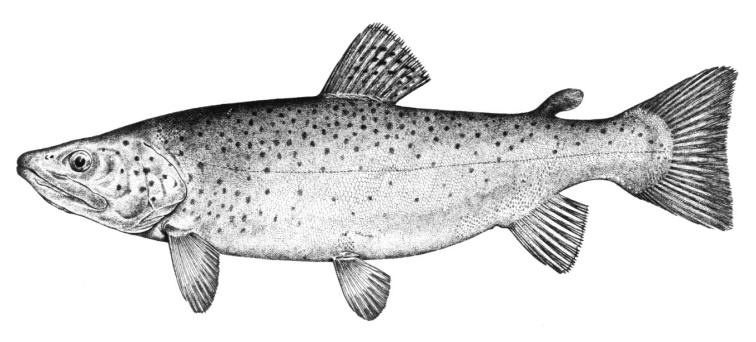

Common names
See above under Salmo caspius where general names for trout will
encompass this species.
[Brown trout, German brown trout, European brown trout, sea trout, brownie, Loch Leven
trout, Von Behr trout, spotted
trout and liberty trout].
Systematics
See above under Salmo caspius.
Key Characters
?This species is a relative of the Atlantic Salmon and is distinguished from it by the upper jaw extending beyond the eye in adults
(and below rear half of the eye in young rather than the centre in young), the gill cover has many spots, dorsal fin principal rays
usually 10-14, and orange to rusty-red spots are often present on adult flanks.
Morphology
Principal anal fin rays 9-12, pectoral rays 13-14 and pelvic rays 9-10. Lateral line scales 110-136 and pyloric caeca 30-60. Males
develop a hooked lower jaw when spawning and the brown colour becomes more intense and golden.
Sexual dimorphism
Males develop a hooked lower jaw when spawning and
the brown colour becomes more intense and golden.
Colour
Overall colour is a light to golden brown with silvery flanks and a white to yellowish belly. The back, flanks, side of the head and
dorsal and adipose fins bear black or dark brown spots, often with a lighter halo of orange, pink or red, and the flank has pink or red
spots. Only brown trout have both light and black spots on the flanks. The caudal fin may have spots restricted to its upper lobe but
lacks the overall radiating spots of rainbow trout. The adipose fin is orange to orange-red, the only family member with this colouration. Sea run or lake fish are more silvery, obscuring some of the spots. Spots may be x- or y-shaped. The red flank spots
usually have blue halos. Young have 7-14, narrow parr marks and a few red spots
along the lateral line. The adipose fin is orange with a light margin.
Size
Reaches 1.4 m and reputedly 50 kg.
Distribution
The trout is found throughout Europe, in North Africa and east to
the Aral Sea basin. It is also recorded from the upper Euphrates basin (Berg, 1949).
Brown trout were artificially planted in Gahar Lake of the upper
Dez River of the Tigris River basin where viable populations
existed in the 1970s in both the upper and lower lake, and more recently (B. Sandford, in
litt., 1979; R. Mehrani, pers. comm., 2000). European brown trout
were planted in the Caspian Sea and Namak Lake
basin and established south of Dorud in the Zagros, and in the Zayandeh
River dam but their origin is unknown (Coad and Abdoli, 1993). Trout were also
introduced to the Karun River basin and the Zayandeh River Dam (Y. Keivany, in litt., 1992).
Trout are also recorded from the the Lake Orumiyeh basin in the upper Talkheh,
Zarreineh and Tatavi rivers (Abdoli, 2000) but whether these are introduced is
not certain.
Zoogeography
This species is introduced in Iran.
Habitat
Brown trout are mostly stream and river dwellers although some are in lakes and ponds. They can tolerate warmer and more turbid
waters than brook trout and only the tainbow trout is more tolerant among salmonids. Brown
trout may survive in areas no longer
suitable for Brook Trout because deforestation has increased stream temperatures and agriculture and industry have increased pollution
and turbidity. Ideally there should be overhanging and submerged vegetation, coarse gravel and cover such as logs, boulders and
undercut banks, growth is better in alkaline waters (20-200 mg L-1, total dissolved solids 800 mg L-1 for
culturing this species), a depth of 7-58 cm is needed for spawning, a winter flow of less than 15 cm sec-1 is required,
preferred temperatures are 10.0-17.6°C (other reports give 21.1°C and temperatures up to 24°C are tolerated) and for spawning 6.7-8.9°C,
dissolved oxygen is optimal at 7-9 mg L-1 and pH range tolerated is 5.0-9.5 although the optimal range is 6.8-7.8.
Age and Growth
Life span is over 18 years with maturity attained at 2-4 years.
Çetinkaya (1999)
found the fish in the Çatak stream, of the upper Tigris River, Turkey to have an
age range of 1-8 years, a fork length of 8.4-39.0 cm, a weight of 6.7-756 g, and
a sex ratio 2.45:1 male:female. This population may not be the same taxon as
introduced populations in Iran.
Food
Food is aquatic and terrestrial insects, crustaceans, molluscs, various fishes, frogs, salamanders and even small mammals. Most
food is taken as drift, the trout positioned in slower water behind a rock and darting out into faster current to seize prey.
Predators include other fishes, birds, water-snakes and otters. Çetinkaya (1999)
found the diet of fish in the Çatak stream, of the upper Tigris River, Turkey to
be Trichoptera, Ephemeroptera, Gammarus, chironomid pupae and larvae, and
Plecoptera. This population may not be the same taxon as introduced populations
in Iran.
Reproduction
Spawning occurs from October to January depending on locality, usually at
7-9°C but as low as 2°C or as high as 14°C. It usually
takes place in gravel stream riffles but may occur on rocky shores of lakes. The female excavates a redd into which the spawning pair
deposit eggs and sperm while gaping and quivering over a 4 second period. The female covers the redd with gravel after spawning to
protect the eggs. Subsequent spawning occurs, usually after a 10 hour interval. Occasionally a community redd is excavated by a number
of fish spawning close together. Eggs are up to 5.0 mm in diameter and each female can contain 20,865. Young may spend about 2 years
in their natal stream before going to a lake but some fish remain permanently in streams.
Çetinkaya (1999)
found the fish in the Çatak stream, of the upper Tigris River, Turkey, to be
sexually mature at age 4 and 16-17 cm fork length with an individual fecundity
of 2349 eggs.This population may not be the same taxon as introduced populations
in Iran.
Parasites and predators
None known in Iran.
Economic importance
This trout is a valuable and popular sport fish in Europe, The benefits
of stocking brown trout include its difficulty of capture (and thus appeal to anglers), its longer contribution to the fishery than
brook trout and rainbow trout, better survival and growth than other trouts, and its tolerance of warmer waters. There is an extensive
European literature on angling methods and it is reputed to be a wilier fish than
brook trout, for example. They tend to bite best in the late evening or at dawn, especially
when large. These trout are caught on worms, crayfish, lures and flies. Brown trout have white to pink flaky flesh and are excellent
eating. Flesh colour changes with age, to pink, and is related to diet.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in sport, in textbooks and because it has been widely
introduced outside its natural range. Salmo trutta is reported
to be ichthyootoxic although there are no reports for Iran or for S. caspius (Coad, 1979b; see also under the genus Schizothorax
for symptoms of this egg poisoning).
Conservation
None required fro an exotic species.
Further work
The distribution and biology of this trout in Iran needs detailed investigation
to separate it from native taxa.
Sources
Based on general accounts of
the species.
Genus Salvelinus
Richardson, 1836
The charr genus comprises many species in Europe, northern Asia and
North America whose systematics have not been fully worked out. FishBase lists
83 nominal species (August 2007). The charrs have numerous small scales 105 or
more in lateral series), teeth on the jaws, palatines and tongue, teeth are
present on the head but not the shaft of the vomer bone in the roof of the
mouth, scales in the lateral line are smaller than surrounding scales and have
little or no overlap with scales before and behind, the body has light pink, red
or cream spots, and the lower fins have snow-white leading edges.
Salvelinus fontinalis
(Mitchill, 1814)
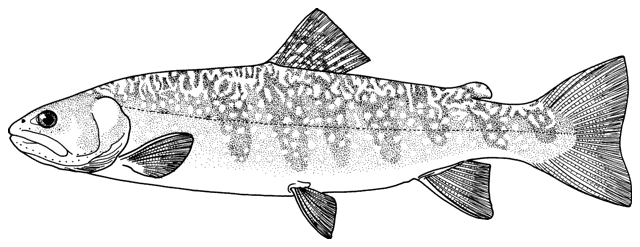
Common names
ماهي آزاد چشمه اي (= mahi azad
cheshmehi or azad mahi cheshmehi, meaning spring free fish, i.e. spring salmon, free
fish being used for salmon and trout species in Farsi), qezel ala-ye juibary (= brook trout).
[brook trout, brook charr, speckled trout].
Systematics
Salmo fontinalis was originally described from the vicinity of New York city.
Key characters
This species is characterised by 109-132 lateral line scales, 8-13
anal fin principal rays, light-coloured spots on the body, teeth on
the head of the vomer bone in the roof of the mouth, pectoral, pelvic
and anal fins with a white leading edge followed by contrasting black,
truncate caudal fin, dorsal and caudal fins have wavy, dark lines and
blotches and the back has dark or light green or cream, worm-track
markings (vermiculations).
Morphology
Dorsal fin principal rays 9-14, pectoral rays 10-15 and pelvic rays
7-10. Scales are minute, horizontal and irregular ovals with a central
focus, few circuli and no radii. The pelvic axillary scale is
well-developed. Gill rakers 13-22, reaching the second adjacent raker
whem appressed. Pyloric caeca 20-55. The gut is s-shaped. The
chromosome number is 2n=84 (Klinkhardt et al., 1995).
Sexual dimorphism
Spawning males develop a hooked lower jaw or kype.
Colour
The back is olive-green to dark brown or blackish fading to a
silvery-white belly. Flanks have a red to yellow tint. Flank spots are
pale but there are also small, red spots with blue halos. The
pectoral, pelvic and anal fins are yellow, orange, or reddish behind
the white and black leading edges. Sea-run fish have a blue-green back
and silvery flanks with a purplish tinge. Spots are obscured except
for the red ones. Brook trout in large lakes are also more silvery
than stream resident fish. The jaw tips and the roof of the mouth are
blackish. Spawning males are much brighter in overall colour and have
an orange-red lower flank and upper belly, bordered below by black on
each side which delimits the white belly. Young have 6-12 brown parr
marks, the widest equal to eye diameter, and small red, yellow or blue
flank spots. The white leading edge to the lower fins is apparent.
Size
Attains a reputed 86.0 cm and 8.0 kg, possibly 9.39 kg, but most are smaller.
Distribution
In North America this species is found from the shores of Hudson
Bay and Labrador south in marine waters to Cape Cod in the east and
Georgia in the Appalachian Mountains, west through all the Maritime
provinces, Québec and Ontario (except the extreme west) to northeast
Manitoba. Widely introduced to western Canadian provinces, the U.S.A.,
South America, Europe, Asia and Australasia.
A private hatchery on the Jajrud imported over 1 million brook
trout eggs which were raised to fingerling size only for most to be
lost in a flood in 1968. Some were planted in the Jajrud and in the
Latian Reservoir in the Namak Lake basin. Survival remains unknown. Also
recorded from the Sardab and Chalus rivers of the Caspian Sea basin (Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 26, 1996) but this
is possibly a misidentification for Oncorhynchus mykiss.
Zoogeography
An exotic species from North America, not closely related to native Iranian salmonids.
Habitat
Brook trout are found in cool waters of streams and lakes, usually less than 20°C,
adults preferring 14-16°C. Spawning requires temperatures below 15°C, and below 9°C
for optimal hatching. The upper lethal limit is 25°C for adults and 20°C
for newly hatched fish. pH range is 4.1-9.5. Pools, underneath banks,
under overhanging bushes or behind rocks are favoured spots. During
summer months they retreat to deeper water, to about 8 m, in lakes.
Some populations run to sea in Hudson Bay and Atlantic Canada. They
stay in coastal waters, not moving more than a few kilometres from
their natal stream. Populations in the North American Great Lakes live
and feed mostly in the lake and run up natal streams to spawn.
Age and growth
Maximum life span is over 20 years but most reach only 5 years in
North America. Maturity is attained at 2-3 years, some males at 1
year. Stunting is common in small streams while sea run fish grow
faster than freshwater ones. Optimum growth temperatures are 10-19°C.
Food
Food in North America includes aquatic and terrestrial insects,
molluscs, various fishes, frogs, and even snakes, mice, voles and
shrews. Stream-dwelling fish feed heavily on drifting aquatic
organisms during spring run-off but in summer as drift decreases
surface insects become important. Most feeding occurs in the early
morning and late evening although some food is taken throughout the
day. Diet shifts in response to competition with other species. Sea
run fish take various marine invertebrates and fishes. Sea run adults
in spring and summer eat crustaceans and fish in lower estuarine areas
while young are in the upper estuary eating crustaceans and insects.
During the fall in the river adults eat little and during winter back
in the estuary consumed mostly crustaceans. There is thus a division
of food resources between young and adults.
Reproduction
Spawning in North America takes place from August to December,
earlier in the north and later in the south. Sea-run charr enter their
natal stream in spring and summer even though spawning occurs in fall.
Each year they spend 1.5-3 months feeding in the sea. The spawning
ground is usually gravelly streams but may be lake shoals if there is
some current or spring outflow to keep eggs oxygenated. Spring flows
are preferred even in streams. Males arrive on the spawning ground
first and defend a territory. Both sexes will rush at other fish
entering the redd area. The female cleans a redd of debris by turning
on her side and lashing her tail. Redd depth between stones is tested
by inserting the anal fin. Redd construction may take up to 2 days
with work being carried out both by day and night. Courtship involves
gentle pushes, touches and strokes of the female by the male. The
female is ready to spawn when she crouches in the redd with her
genital area between the stones. The male arches his body and may
press the female against the redd bottom, both fish vibrate and eggs
and sperm are shed. Accessory or sneaky males may rush in to shed
sperm. The female lashes her tail to push eggs into the gravel and
then dislodges gravel with her anal fin to cover the eggs to depths as
great as 20 cm. Yellow-orange eggs are up to 5.0 mm in diameter and
number up to perhaps 17,000 per female although averages range from
the low hundreds to a few thousand. Both sexes may spawn again with
other fish. The eggs develop over winter, taking 165 days at 2.8°C
but only 47 days at 10°C. Temperatures above 11°C will kill the eggs.
Parasites and predators
Brook trout are cannibals on their eggs and young and are eaten
themselves by other fishes, water snakes, turtles, various birds and otters.
Economic importance
Brook trout are very popular sport fish in North America caught on
lures, live baits and flies. These trout are easier to catch than Salmo
trutta sensu lato and take a wider range of lures. They fight well but are
often quite small and do not leap spectacularly like some other
members of the salmon family.
The ready availability of this salmonid has made it a useful
experimental fish for various physiological, biochemical,
toxicological and other studies. Some reared stocks however show
deformed or lost fins and distorted mouths.
Conservation
This introduced species is not in need of conservation but in fresh
water their preference for cool and clear water makes them susceptible
to loss if waters are dammed, channelised and polluted or if banks are
eroded deforested and overgrazed, conditions not uncommon in Iran.
Further work
The survival of stocks of this species in Iran should be verified
and the effects of this exotic on native species researched.
Sources
Based on general summaries on North American populations such as
those in Scott and Crossman (1973) and Becker (1983) as no Iranian material to
hand. Iranian
populations have not been studied.
Genus Stenodus
Richardson, 1836
The inconnu genus contains a single species found in waters
draining to the Arctic Ocean in Eurasia and North America and in the Caspian Sea.
Its characters are essentially those in the species description; a
small bone lacking a head canal on the outer side of the lower jaw at
the joint of the articular and dentary is distinctive. Branchiostegals number 8-11.
Stenodus leucichthys
(Güldenstaedt, 1772)
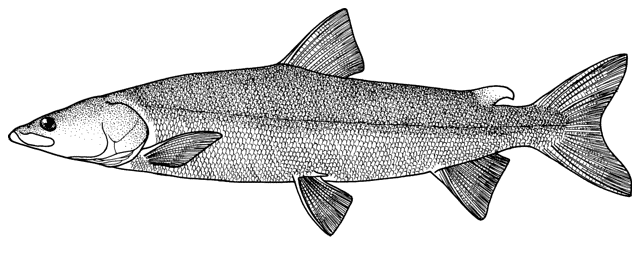
Common names
safid mahi (= white fish), آزاد ماهي (azad mahi or free fish), azad
mahi Volga (= Volga free fish, or
Volga salmon, free fish being used in Farsi for salmon), زيبا (= ziba), mahi-ye ziba or
ziba mahi (= beautiful fish, in northwest Gilan, e.g. at Astara),
mahi-e-azad-e-ziba, inkonu.
[belorybitsa in Russian; nelma or azatmahy in Turkmenian; inconnu, conny, white salmon, Caspian inconnu].
Systematics
Salmo leucichthys was originally described from the Volga
and Ural rivers, Caspian Sea and Kamchatcka, Russia.
The type subspecies in the Caspian Sea basin is the one found in
Iran. The subspecies Stenodus leucichthys nelma (Pallas, 1773)
of White Sea, Siberian and northwestern North American drainages is more common.
Key characters
The characters of the species are those of the genus.
Morphology
Dorsal fin branched rays 8-13 after 2-6 unbranched rays and anal
fin branched rays 9-16 after 2-5 unbranched rays. Lateral line scales
88-121, scales above the lateral line 8-13, and scales below the
lateral line 10-12. There is a pelvic axillary scale. Scales bear
numerous fine circuli, the focus is slightly subcentral posterior, the
anterior scale margin is wavy but irregular in outline and the scale
is generally rounded. There are no radii. There is a single flap between the
nostrils.Gill rakers number 17-27 and
reach the fourth adjacent raker when appressed. Pyloric caeca number
191-193 and the gut is s-shaped. Vertebrae number 64-70.
Sexual dimorphism
Males develop tubercles on the head and sides of the abdomen during spawning.
Colour
Overall colour is silvery without spots.
Size
Reaches 1.5 m and 28 kg, rarely 40 kg, possibly to 48 kg. On the coast of Dagestan the
average size was 7.6-10.8 kg in the 1920s (Berg, 1948-1949).
Distribution
The subspecies is restricted to the Caspian Sea and its drainage.
Rare in Iranian waters with 5 specimens caught and preserved from 5-7
km east of Bandar-e Anzali in the Caspian Sea from 1984-1990. Several
dozen fish have been caught each year in Iran, starting in the late
1960s, all from between the mouths of the Safid and Astara rivers (Holčík
and Razavi, 1992). Reported from the southeast Caspian Sea, southwest
Caspian Sea and south-central Caspian Sea (Kiabi et al., 1999; Abdoli and
Naderi, 2009).
Zoogeography
This species is found naturally in the Caspian Sea but was
generally believed to inhabit only the northern, and rarely the
western, parts to the north of 40°N.
Rare specimens were reported by Kazancheev (1981) from the coasts of
Dagestan and Azerbaijan in early summer. It probably penetrated into
the Caspian Sea from the Arctic Ocean basin during the last glacial
period via ponded lakes.
Habitat
Inconnu live in the Caspian Sea proper at depths less than 60-65 m (optimally
25-45 m) and migrate up rivers to
spawn, in the Volga for 3000 km. Fat content of fish at Astrakhan at
the Volga mouth was 26% of total weight but by the time fish reached
the spawning grounds in the Ufa River this had fallen to 14%, and
after spawning to 1.5% for females and 1.6% for males. Off Iran they
are caught mostly above depths of 25 m from October until the end of
December. Some are caught in beach seines in shallow water (Holčík
and Razavi, 1992) but elsewhere are known from depths down to 46 m. It
is said to move into the central and southern Caspian in summer and
returns north when water temperatures fall below 8-10°C
in the first half of September (Berg, 1948-1949). This contrasts with
the catch records for Iran. Fingerlings tolerate temperatures up to 25ºC
in culture ponds but in the sea it is found at temperatures below 20ºC
(Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
Life span is at least 12 years in the Volga population for females
and 8 years for males. Iranian fish were up to 6 years old (Holčík
and Razavi, 1992). Spawning females are predominately 6-8 years and
males 5-6 years old (Letichevskiy, 1981). Females on the Volga
spawning grounds are 85-105 cm, 5.5-9.5 kg while males are 75-100 cm, 3.5-7.5 kg.
Food
There is no feeding on the spawning migration, the fish relying
solely on their stored reserves of fat. Fat declined in one study from
21% before, to 2% after, spawning. In the Caspian Sea it feeds on
fishes such as gobies (Gobiidae), Rutilus rutilus (presumably R.
caspicus), Atherina
boyeri (= caspia), young Sander, common and anchovy kilkas, and shads. In
the sea, adults feed on kilka and silversides (97-99%)(Caspian
Sea Biodiversity Database, www.caspianenvironment.org).
Young feed on zooplankton 7-10 days after hatching but are predatory
in their second month. Fry descend to the Caspian Sea to feed until
they are sexually mature.
Reproduction
Spawning takes place in October and November at 0.2-6.0°C
after an ascent up north Caspian rivers. The Volga River run peaks in
December and March. Fecundity reaches 390,000 eggs of up to 2.4 mm
diameter. Eggs average 26% of the female's weight. The eggs are
slightly adhesive and shed on the river bottom. Spawning occurs only
once or twice in the life cycle of most fish although exceptional
females may spawn 3 times. Spawning occurs at intervals of 2-3 years.
Incubation takes 180-200 days and hatching takes place in spring. The
young immediately descend to the sea.
Parasites and predators
Eggs are eaten by Gobio species and by Lota lota.
Economic importance
This species has no economic importance in Iran but was a
significant member of former Soviet fisheries, taken both in the sea and on
its spawning migration. Up to 110,000 fish were taken annually in the
Volga-Caspian region. It has a rich, delicate flesh with a fat content of 18-26% and is a favoured
smoked product (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Conservation
This species was listed as threatened in Southwest Asia by Holloway
(1976) and endangered in the Caspian Sea by
Fricke et al. (2007).
Kottelat and Freyhof (2007) state that it is maintained only by stocking and is
on the brink of extinction. The population of this species, spawning principally in the
Volga, declined drastically after regulation of this river. In the
1960s, the population was estimated to be only 2000 fish but it has
recovered to 17,000 fish through artificial rearing (Mina, 1992). In
the two decades 1961-1980, fish hatcheries in the Volga Delta raised
72.4 million inconnu. Gravel spawning grounds were also constructed
(Letichevskiy, 1981). It has been proposed for inclusion in the
"Red Book of the U.S.S.R." which forms the basis for
measures to protect species (Pavlov et al., 1985). Lelek (1987)
classified it as endangered. Kiabi et al. (1999) consider this
species to be data deficient in the south Caspian Sea basin according
to IUCN criteria. Criteria include commercial fishing, sport fishing,
few in numbers, bodies), limited range (less than 25% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin.
Further work
Further records of this species should be recorded to document the presence
and spread of this fish in Iranian waters.
Sources
Morphology based on Holčík and Razavi (1992) for Iranian specimens.
Iranian material: None.
Comparative material: BM(NH) 1878.12.26:23, 1,
199.5 mm standard length, Bremen (no other locality data); BM(NH) 1899.7.25:22,
1, 215.5 mm standard length, Russia, River Obi (no other locality data).
Esocidae
The pikes, pickerels and muskellunge are found in fresh waters of
the Northern Hemisphere. They are moderate to large-sized fishes, up
to 1.4 m. There are only 5 species with 1 reported in Iran.
The family is characterised by a flattened, elongate, duck-billed
snout; dorsal and anal fins far back on the body near the tail; no
adipose fin; teeth on the tongue and on the basibranchial bones behind
the tongue are small; jaws have large teeth; branchiostegal rays
10-20; nasal bones are present; the swimbladder is connected to the
gut by a duct; intermuscular bones are forked or y-shaped; no fin
spines; pelvic fins are abdominal; cycloid scales; the infraorbital
sensory canal on the head has 8 or more pores; gill rakers are present
as sharp denticles in patches; no pyloric caeca; the lateral line is
complete; and the forked caudal fin has mostly 17 branched rays.
Pikes are predators on other fishes aided by the posterior dorsal
and anal fins which facilitate rapid darts forward. They are important
sport fishes, much sought after by anglers for their fighting ability,
but are not very good eating because of the intermuscular bones.
Genus Esox
Linnaeus, 1758
The characters of this genus have been outlined above under the family.
Esox lucius
Linnaeus, 1758
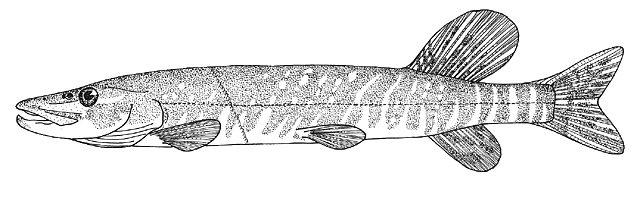
Common names
ordak mahi (= duck fish from the snout shape), shok, shuk or shook (in
Gilaki), shook chehkhab, chekab.
[durnabaligi in Azerbaijan; shchuka in Russian; pike; northern pike].
Systematics
Esox Lucius was originally described from Europe.
Key characters
The broad and flat snout and the dorsal and anal fins set far back on the body are distinctive.
Morphology
Dorsal fin principal rays 15-19, about 6-10 unbranched and 13-18
branched, principal anal rays 12-16, about 4-8 unbranched and 10-15
branched, pectoral rays 11-17 and pelvic rays 7-13. The number of
branched rays may be size-related as in smaller fish more anterior
rays in the dorsal and anal fins are not branched. Lateral line scales
105-148, pored scales 42-56, but difficult to count accurately. Each
scale on mid-flank is a rounded rectangle. The anterior margin is
indented where 1-2 radii terminate. The radii split the scale so that
the segments overlap. Circuli are very fine and the focus is
posterior. Gill rakers are broad and spinulose, embedded in the arch
skin with the tips of the spinules protruding. There are 9-11 pores on
the lower jaws (usually 5 on each jaw). Vertebrae 56-65. The
chromosome number is 2n=50 (Klinkhardt et al., 1995). The gut
is an elongate s-shape.
Meristic values for Iranian specimens are:- dorsal fin branched
rays 14(7) or 15(9); anal fin branched rays 11(1), 12(8) or 13(7);
pectoral fin branched rays 14(9), 15(5) or 16(1); pelvic fin branched
rays 8(1), 9(7), 10(6), 12(1) or 13(1); pores on each lower jaw 5(22).
Sexual dimorphism
There is no obvious sexual dimorphism. Attempts have been made to
sex pike by characters of the urogenital region, but these are
hampered by seasonal variations. Abdurakhmanov (1962) reports on fish
from Azerbaijan where head length is greater in males while predorsal
distance and interorbital width are greater in females.
Colour
The overall colour is dark with light spots, although there is
variation over the vast range of this species in the details. The back
and upper flank are dark green, olive-green or brownish, fading to a
whitish belly. The flank has 7-9 rows of greenish, yellow to whitish
blotches along it. Scales have a golden tip. The head sides have wavy,
golden or yellow blotches and lines and the eyes are bright yellow to
golden. The dorsal, anal and caudal fins are green, yellow, orange or
pale red, blotched and barred irregularly with black. The pectoral and
pelvic fins are dusky to orange. Young have 8-12, wavy, white or
yellow bars which become the bean-shaped blotches in adults as they
gradually break up. There is a gold to green stripe along the middle
of the back in some fish but others are completely dark green. There
is a stripe below the eye. The peritoneum is silvery.
Size
Attains 1.75 m and about 48.0 kg, possibly to 2.13 m and 65.78 kg, despite legends of pike up to 5.0 m (Tsepkin, 1986).
Distribution
Across northern Eurasia and northern North America. Iranian
populations are found in the Caspian Sea basin, from the Anzali Mordab and the
Sia Keshim Protected Region to Gorgan Bay and its tributaries such as the Karasu, with other rivers including the Safid, Tajan, Babol and Haraz, and the Atrak River basin,
and the Amirkelaye Lagoon near Lahijan (Derzhavin, 1934; Berg, 1936; Armantrout,
1980; Holčík and Oláh, 1992; Nejatsanatee, 1994; Riazi, 1996; Abbasi et al., 1999;
Kiabi et al., 1999; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
However, Abdoli and Naderi (2009) do not report it from the Atrak River.
This species has been introduced to Valasht Lake near Marzanabad, Avan River
and Evan Lake northeast of Qazvin, the upper Karaj and middle Shur (Abhar stretch) rivers of the Namak
lake basin, the Avan River near Alamut (in 1956), Ghorigol Lake near Tabriz, Marivan Lake in Kordestan, and
the Haft Barm Lakes west of Shiraz (Anonymous, 1977; Petr, 1987; Niamir, 2001).
Across northern Eurasia and
northern North America. Iranian populations are at the southern edge of the
range for this species. Atrak River basin (Berg, 1936). Geluga, Anzali Mordab,
Gorgan Mordab (Armantrout, 1980 and USNM 205941 at 37 30'N, 49 20"E). Anzali
Mordab, its outlets and in the "Khalkai" (Hol…ik and Oláh, 1992). Gorgan Bay
incl. Karasu and other tributaries (Derzhavin, 1934). Siah-Keshim Protected
Region of the Anzali Mordab (Riazi, 1996). Introduced to Valasht Lake near
Marzanabad, Evan Lake northeast of Qazvin, Ghorigol Lake near Tabriz, Marivan
Lake in Kordestan and the Haft Barm Lakes west of Shiraz (Anonymous, 1977; Petr,
1987). Ameerkalaye Lagoon near Lahijan (Nejatsanatee, 1994) ?? not checked in
gazeteer??Introduced to the Avan River 7 km from Alamut in 1956 (Nialmir, 2001).
Reported from the Tajan,
Babol, Haraz, and Safid rivers, and the Anzali Mordab (Kiabi et al., 1999).
Anzali Talab and Safid River (Abbasi et al. (1999).
Abdoli (2000) records from
lower Babol, Heraz, Chalus, Tonekabon and Safid rivers; upper Karaj and middle
Shur (Abhar stretch).
Zoogeography
This widely-distributed species reaches its southern range limit in Iran.
Habitat
Pike are solitary and are found in lakes and rivers where the water
is still or flowing slowly as well as marshes and ponds. They are
found only in the lower reaches of rivers along the Iranian shore and
do not penetrate upstream (Berg, 1948-1949). O'Donovan (1882) reported that a
small stream in the Atrak River drainage had many large pike lurking under
bushes, stupefied by foul water, and that the Cossacks in his escort caught many of them by striking with
the point of their sabres or simply whisked them out of the water by the tail.
Large numbers of Rutilus frisii were seen here too and presumably the
pike thrived on this food source. Riazi (1996) reports that
this species is native (resident) to the Siah-Keshim Protected Region
of the Anzali Mordab. They are found in the more brackish areas of the
Caspian Sea, at least in Kizlyar Bay of the north Caspian, at
salinities up to 4‰ under the influence of fresh water from the
Volga River. Here they are found in thickets of soft and rigid
vegetation, as solitary predators but also feeding beyond the
vegetation limit in the sea. They aggregate only in spring for
spawning and in autumn prior to wintering on the bottom (Stolyarov and Abusheva, 1997).
In fresh water, vegetation is heavy and the water warm but they
usually retire to deeper, cooler water at the height of summer.
Temperatures above about 30°C are usually fatal to pike. However pike are active in winter, as
anglers can testify, and at this time can tolerate dissolved oxygen
concentrations lower than 0.1 mg/l. pH range is 5.0-9.5 although they
have been recorded as spawning at 4.2-4.4 but embryos are then
malformed (Mann, 1996). Summer distribution is usually within 300 m of
shore and less than 4 m deep. On windy days, pike retreat offshore in
surface waters. Pike tolerate brackish water, up to about 7‰ for
reproduction and 10‰ for feeding and growth. Reproduction
requires living or dead vegetation (either aquatic or flooded
terrestrial vegetation) in shallow, still waters protected from strong
winds. Vegetation is also important for recruitment of young pike. Pike deposit
their faeces at specific locations far from their usual feeding area as the
faeces contain alarm pheromones recognised, and avoided by, prey species.
Age and growth
Life span is up to about 26 years but is less than half this in
fast-growing southern populations. Some aquarium fish have lived 75
years. Maturity, like growth, varies with latitude and habitat, and
also with quality of food. Higher temperatures may inhibit growth.
Males mature at 1-6 years (30-46 cm) and females usually at 2-6 years,
rarely at 1 year (31-63 cm). Females grow larger, faster and live
longer than males. Growth is best at 19-21°C and is very efficient.
Nezami Balochy et al. (2005) examined this species in the
Zibakenar-Kiashar Bojagh lagoon on the Caspian coast of Iran. The 122 fish were
17.7-74.0 cm long, average total length being 33 cm, and weight was 38-1100 g
(average 307.3 g). The age groups were 0-9 years. Nezami et al. (2004; 2006)
examined this species in the Amirkelayeh Lagoon and found ages 1+ to 6+
years, average total length 44.8 cm (range 15.6-63.0 cm) and average weight
717.9 g (24-1700 g). Valipour (1998) investigated the pike in four areas of the
Anzali Lagoon and found relatively fast growth with fish above 2 years of age
mature with an average length of 32 cm.
In the Kizlyar Bay of the north Caspian Sea, males mostly mature at
age 2+ with a body length of 36-40 cm while females are 3+ and 45-50
cm. Some males are mature in the first year of life at 26-30 cm and
some females in the second year of life at 33-36 cm. Maximum age is 11
years in this population (Stolyarov and Abusheva, 1997). In Lake Aksehir, Turkey most males and females are mature at 2
years of age (Karabatak, 1988). Altindağ et al. (1999)
give growth features for a population in a Kesikköprü Dam
lake, Turkey where females reached age 5 and males age 4.
Food
Food is initially zooplankton and aquatic insects but fish begin to
predominate at 5.0 cm after about 1 month's growth. Over 90% of the
diet of adults is fish, but frogs, crayfish, mice, muskrats and
ducklings are taken. Both sexes fast during spawning but females have
rations 1.5-2.3 times as much as males in summer and winter. The daily
ration was high from May to August with a June peak and very low in
winter in North America.
Pike have a highly mobile eye which enables them to spot prey in
almost any direction and have sighting grooves along the snout to
facilitate their judgement of depth and distance. Food is seized after
a rapid dart from concealment. Cylindrical fish like perch are
preferred over more deep-bodied species as being easier to swallow.
The prey capture process can be summarised as follows: eye movements
towards prey, turning of body towards prey, stalking, darting,
capture, rotating prey head first in mouth, and swallowing. Prey is sucked
into the mouth which is opened just as the pike reaches its prey. Prey
size is usually about one-third to one-half the length of the pike.
Valipour (1998) found feeding intensity and growth coefficient of this
species in the Anzali Lagoon decreased with age. Fish in the 0+ age group fed
mainly on zooplanktonic mysids while older fish took Carassius auratus,
Hemiculter leucisculus, Rhodeus amarus and larvae of Alburnus
chalcoides. Apparently, the pike is not restricting populations of
commercial species in the lagoon but has an important role in controlling
non-commercial exotics such as Carassius auratus and Hemiculter
leucisculus. Abdoli (2000) lists Gambusia holbrooki, Carassius auratus, Hemiculter
leucisculus, Liza saliens, Atherina boyeri (= caspia) and Alburnus
charusini (= Alburnus hohenackeri) as food items for Iranian pike.
The Zibakenar-Kiashar Bojagh lagoon fish had Odonata (14%) as the most frequent
food, followed closely by Syngnathus caspius (13.8%) and Ponticola
gorlap (13.4%). Other fish eaten included Gambusia holbrooki and pike.
Nezami et al. (2004) and Nezami Balouchi
. (2006) examined this species in the Amirkelayeh
Lagoon and found the diet to be 24% Tinca tinca, 16% Proterorhinus
marmoratus (= nasalis), with Esox lucius, plecopterans, frogs and water beetles
at 8%, and Syngnathus caspius, Carassius auratus and Gammarus
at 4%. Diet varied with season, and age and sex of the pike.
In the Kyzylagach or Imeni Kirova Bay of Azerbaijan Abramis
brama, Cyprinus carpio, Rutilus rutilus (presumably R.
caspicus), Rutilus
frisii, Vimba vimba (= V. persa), Mugilidae, and Atherina boyeri
(= caspia)
are taken (Kuliev, 1989). Pike are cannibals when food is short. They
compete with other piscivorous fishes, such as Sander lucioperca
and Perca fluviatilis, for food.
In the Kizlyar Bay of the north Caspian, the food of 2-month-old
pike 3-4 cm long is mainly Rutilus rutilus (presumably R. caspicus) fry, of pike 9-15 cm
long this species plus fry of Cyprinus carpio and Scardinius
erythrophthalmus, and of adults mainly Cyprinus carpio, Rutilus
rutilus (presumably R. caspicus) and Clupeonella cultriventris
(= caspia)
(during its spawning
migration in April and May), Scardinius erythrophthalmus, Tinca
tinca, Blicca bjoerkna, Alburnus alburnus
(= hohenackeri), Cobitis
sp., some gobies, frogs and crayfish. Sturgeon fry were once an
important diet item but this declined with the decline in sturgeon
numbers (Stolyarov and Abusheva, 1997).
Reproduction
The spring spawning migration in Dagestan begins at ice melt and
spawning takes place from mid-March to the beginning of April when
water temperatures reach 5-7°C.
One batch of eggs is laid over a period of 10-15 days (Shikhshabekov,
1978). Spawning runs occur in the late evening and early night,
several days before spawning occurs. Spawning takes place during the
day, often in mid-afternoon, in shallow bays or flooded fields just
after ice melt, generally in late March to May. Water temperatures on
the spawning run are as low as 1.1°C.
Spawning itself takes place at temperatures several degrees warmer
than this, up to 17.2°C. Each female is accompanied by 1-2 males as she swims over vegetation in the shallow
water. Both sexes roll to bring their genital regions close together,
vibrate and release 5-60 eggs and the sperm. Tail sweeps scatter the
eggs. This 3-10 second process is repeated many times each day. Eggs
are amber, up to 3.4 mm in diameter after fertilisation, adhesive and
each female can produce up to 595,000, although usually much less. In
the Kizlyar Bay of the north Caspian Sea, the oldest females produce
365,000 eggs (Stolyarov and Abusheva, 1997). Eggs hatch 12-14 days
later and the fry attach to vegetation by an adhesive head gland until
the yolk sac is absorbed 6-10 days later.
The Kizlyar Bay population begins to spawn in late February or
early March, sometimes under ice or immediately after ice melt at 4-6°C.
Eggs are laid at depths of about 0.5 m and may dry out during water
surges caused by northwestern winds although the increase in sea level
has lessened this. Eggs adhere to vegetation but fall off after 2-3
days but do not die because of the low water temperatures and
favourable oxygen conditions. Eggs hatch in 7-18 days depending on
water temperature (Stolyarov and Abusheva, 1997).
Parasites and predators
Eslami et al. (1972) found helminths in 78.9% of 109 pike
examined from Iran, a very high rate of infestation. The species
encountered were Triaenophorus crassus, Raphidascaris acus
and Contracaecum osculatum baicalensis. This latter parasite
can infest man if fish is eaten smoked, salted or fried at
temperatures below 50°C. Mokhayer (1976b) records the digenetic trematode Rhipidocotyle
illense, the nematode larva Eustrongylides excisus, and the
acanthocephalan Acanthocephalus lucii. Molnár and Jalali
(1992) report the monogenean Tetraonchus monenteron from pike
in Lake "Sama" in the Alborz Mountains. Ataee and Eslami
(1999, www.mondialvet99.com, downloaded 31 May 2000) report Asymphylodora
tinca from the gastro-intestinal tract of fish from the Anzali
wetland. Naem et al. (2002) found the monogenean trematode Tetraonchus
monenteron on the gills of this species from the western branch of the Safid
River. Khara et al. (2004) looked at pike from the Amir Kelaieh Lagoon
and recorded Raphidascaris acus, Camallanus lacustris,
Eustrongylides excisus, Triaenophorus crassus, Trichodina sp.,
Tetraonchus monenteron, Diplostomum spatheceum, Lernaea
sp., Argulus sp. and Piscicola sp. and atrributed the diversity to
its piscivorous diet. Khara et al. (2006a) record the eye fluke Diplostomum
spathaceum for this fish in the Amirkalayeh Wetland in Gilan.
Sattari et al. (2002) and Sattari (2004)
records the presence of the nematode, Eustrongylides excisus. This
parasite can damage muscles in commercial species and render them unsuitable for
sale. Sattari et al. (2005) surveyed this species in the Anzali,
Amirkelayeh and Boojagh wetlands, recording Raphidascaris acus,
Eustrongyloides excisus and Camallanus lacustris.
Khara et al. (2006b) record the nematode Raphidascaris acus from
this species in the Boojagh Wetland of the Caspian coast. Khara et al.
(2007) examined fish from the Chamkhaleh River and recorded Raphidoscaris
acus, Camallanus lacustris, Diplostomum spathaceum, Tetraonchus
monenteron, Triaenophorus crassus, Corynosoma strumosum and
Lernaea sp. Sattari et al.
(2004; 2007) record the nematodes Camallanus lacustris, Raphidascaris acus and Eustrongylides
excisus, the digeneans Rhipidocotyle illense and Diplostomum
spathaceum and the monogenean Tetraonchus monenteron in this species
in the Anzali wetland of the Caspian shore. Khara et al. (2004) recorded
parasites from fish in the Amir Kelayeh Lagoon including Raphidascaris acus,
Camallanus lacustris, Eustrongylides excisus, Triaenophorus
crassus, Trichodina sp., Tetraonchus monenteron,
Diplostomum spathaceum, Argulus sp., Piscicola sp., and
Lernaea sp., a diversity governed by its piscivorous diet. Barzegar
et al. (2008) record the digenean eye parasite Diplostomum spathaceum
from this fish. Khara et al. (2008) found the
eye parasite Diplostomum spathaceum in this fish from Boojagh Kiashar
Wetland in Gilan. Miar et al. (2008) examined
fish in Valasht Lake and the Chalus River, Mazandaran and found the metazoans
Rhaphidoscaris acus and Tetraonchus menonteron.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp.
on this species.
Young pike are eaten by various other fishes including adult pike,
birds and even large aquatic insects.
Economic importance
Nevraev (1929) reports a catch for the 1901-1902 to 1913-1914
period in the Anzali region was 1150 to 20,529 fish.
Holčík and Oláh (1992) report a catch of 5836 kg in the Anzali Mordab in
1990, at 7.8% of the catch the fourth most important fish there, while
annual reported catches from 1932-1964 varied from none to 98 tonnes.
However it is not a favoured food fish in Iran (Vladykov, 1964).
There is some opportunity for sport fishing for this species in the
Anzali Mordab and potentially in various lakes around the country
where it has been introduced (Anonymous, 1977). Anglers catch this
species in rivers along the Caspian shore such as in the Shazdeh River
at Babol Sar (Noorbakhsh, 1993b). It is an important sport and
commercial fish in other parts of its range. The catch in Turkey in
1981 was 796 tonnes and for the inland waters of the former U.S.S.R.
in 1975 it was 16,101 tonnes. Stolyarov and Abusheva (1997) report a
commercial stock of 2500 t with a recommended catch of 800-850 t in
Kizlyar Bay in the north Caspian in the early 1990s.
The eggs or roe of this species are very poisonous as fresh
extracts injected intravenously into rabbits have caused respiratory
distress, convulsion and death within one hour (Halstead, 1967-1970).
Curiously, Anzali lagoon "perk" eggs have been studied as a replacement for
caviar (Iranian Fisheries Research
Organization Newsletter, 58 & 59:1, 2009).
Conservation
Raat (1988) gives details of conditions which should exist to
facilitate pike reproduction and growth, including such factors as
vegetation, water levels, eutrophication, pollution, prey
availability, intra- and inter-specific interactions, fishery,
stocking, concentration of dissolved solids, pH levels, and
temperature regimes. Vladykov (1964) noted a fish kill in the Anzali
Mordab on 11 June 1962 where water chestnut (Trappa natans) had
caused an oxygen deficiency in the shallow water.
Gilan Fisheries Research Station has cultured pike in earthern
ponds. Pike caught in autumn and winter were injected with
gonadotropic hormone from carp (4-7 mg/kg). Eggs were stripped after
48-72 hours and incubated for 10 days at 8-11°C
and 7-8 days at 10-15°C. Absorption of the larval yolk sac took approximately twice as long as
the incubation period.
Ramin (1999) reports on a project involving artificial spawning and
raising of fry in earthen ponds. Spawning temperatures were 8-15°C
from 4 February to 20 March. Males and females were a minimum of 3
years old and a maximum of 5 and 6 years respectively and weighed
0.75-4.0 kg. Eggs composed 10-20% of body weight, incubation lasted
120 degree days and yolk sac absorption 160-180 degree days. The rate
of fertilization was 45-85% and swelled eggs were 2.5-3.5 mm. Absolute
fecundity was 22,400-112,000. Survival from larvae to fingerlings in
chicken manure enriched ponds was 20-22% over 50 days with growth to
7.4 g and 8.5 cm on average.
Pike from the Anzali Lagoon have been cultured with Chinese carps and were
found to be mature in less than a year (Iranian Fisheries Research
Organization Newsletter, 56:2, 2008).
Kiabi et al. (1999) consider this species to be conservation
dependent in the south Caspian Sea basin according to IUCN criteria.
Criteria include commercial fishing, sport fishing, medium numbers,
habitat destruction, medium range (25-75% of water bodies), present in
other water bodies in Iran, and present outside the Caspian Sea basin.
Mostafavi (2007) lists it as conservation dependent in the Talar River,
Mazandaran. Endangered in Turkey (Fricke et al., 2007).
Further work
The distribution and population numbers of of this species needs documentation in Iran.
Sources
Crossman and Casselman (1987) give a bibliography and Raat (1988)
and Craig (1996) give synopses of biological data on this extensively
studied species. General biology and characters are based on
world-wide data. Sohrabi (1996b) gives an account of this species in Farsi.
Iranian material: CMNFI 1970-0510, 1, 250.3 mm standard length, Gilan,
Golshan River (37°26'N, 49°40'E); CMNFI 1970-0535A, 1, 329.6 mm standard length,
Gilan, Pir Bazar Roga (37°21'N, 49°33'E); CMNFI 1970-0542, 1, 39.0, mm standard
length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0543A, 1.
136.2 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37°24'N, 49°58'E);
CMNFI 1970-0553, 1, 136.7 mm standard length, Gilan, Sosar Roga (37°27'N,
49°30'E); CMNFI 1970-0579, 9, 71.9-111.2 mm standard length, Gilan, Old Safid
River estuary (37°23'N, 50°11'E); CMNFI 1971-0343, 3, 63.8-74.7 mm standard
length, Gilan, Langarud at Chamkhaleh (37°13'N, 50°16'E); CMNFI 1979-0685, 1,
149.2 mm standard length, Gilan, Safid River around Mohsenabad below Dehcha (no
other locality data); CMNFI 1980-0123, 2, 126.3-209.3 mm standard length, Gilan,
Safid River around Dehcha above Mohsenabad (no other locality data); CMNFI
1980-0138, 2, 148.2-164.3 mm standard length, Gilan, Safid River estuary (ca.
37°28'N, ca. 49°54'E); CMNFI 1993-0147, 1, 106.0 mm standard length, Iran (no
other locality data).
Lotidae
The Lotidae or cuskfishes may be included within the cod family Gadidae. The
family is found in temperate to cold marine waters in the Northern Hemisphere, with only one species always resident in fresh water.
There are about 5 species in the family. Maximum size exceeds 1.5 m.
Cuskfishes ods are recognised by the single barbel usually present under the
chin; 1-2 dorsal fins and 1 anal fin; the caudal fin usually
extends around the dorsal and ventral tip of the caudal peduncle and is rounded; no
fin spines; wide gill openings with the branchiostegal membranes free
or narrowly attached to the isthmus; 6-8 branchiostegal rays; vomer
bone in the roof of the mouth toothed; swimbladder not connected to the auditory
capsules and with 2 slender, anterior processes; scales small and cycloid; an obvious lateral line;
and the egg with an oil globule. Eggs and larvae are usually pelagic.
Genus Lota
Oken, 1817
This genus has a single species found in North America and Eurasia,
rarely in Iran. The characters of the species are therefore the same
as for the genus.
Lota lota
(Linnaeus, 1758)
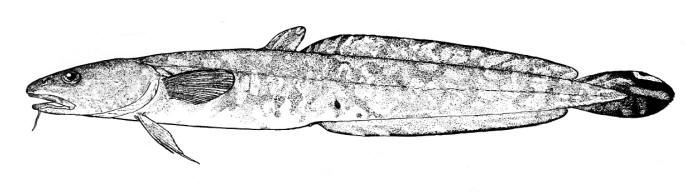
Common names
mahi charb (= fat or greasy fish), lot.
[nalim in Russian; burbot, eelpout].
Systematics
Gadus Lota was originally described from European lakes.
Iranian specimens are presumed to be the type subspecies with a long
and low caudal peduncle and high meristic counts (Pivnička, 1970).
Subspecies defined on numbers of pyloric caeca are not accepted
since there is clinal and size variation (Kottelat, 1997).
Key characters
The chin barbel and 2 dorsal fins, both lacking spines, are distinctive.
Morphology
First dorsal fin rays 7-16, second dorsal fin rays 60-94, anal fin
rays 58-86, pectoral fin rays 15-24, pelvic fin rays 5-10, gill rakers
4-12, short, rounded and spinulose, discrete or almost reaching the
base of the adjacent raker when appressed, pyloric caeca 21-67, and
vertebrae 50-72. The head is flattened, the anterior body rounded and
the posterior body compressed. Scales on the body are minute, cycloid,
embedded, with few circuli and no radii, and extend onto fin bases and
gill covers, and to the level of the nostrils on the head. The second
pelvic fin ray is elongated. The anterior nostril is a barbel-like
tube. The gut has numerous pyloric caeca, and anterior and posterior
loops. The chromosome number is 2n=48 (Klinkhardt et al., 1995).
Sexual dimorphism
Unknown.
Colour
Overall colour is a yellowish to olive-green to dark brown and may
be black, with large light blotches on the head, body and vertical
fins. The belly is yellow to white and may be finely spotted. All fins
are strongly mottled. Mottling is most evident in younger fish; adults
tend to become uniformly dark.
Size
Reaches 1.52 m and 34 kg.
Distribution
Found across northern Eurasia and northern North America. Recorded from the lower
Safid River in 1921 (Derzhavin, 1934; Berg, 1948-1949) and possibly waters in Gilan but
apparently rare in Iran. Berra (1981) omits the distribution in Iran
and the southern Caspian Sea basin of Azerbaijan.
Zoogeography
Populations in the Kura River of Azerbaijan may be disjunct from
northern populations but their characters have not been thoroughly
examined. Abbasov (1980) did not report this species from the Aras
River on the northern border of Iran so the Iranian fish, if not
strays, may be disjunct even from the southern populations in the Kura River.
Habitat
This species favours cool, clear rivers and lakes where it is most
active at night. It hides under rocks and plant roots or in holes in
river banks during the day. Young fish remain in shallow weeded areas or rocky streams.
It is found only in the lower reaches of rivers along the Iranian
shore and does not penetrate upstream (Berg, 1948-1949). This species can
tolerate 6‰ and so may enter the Caspian Sea near river mouths. Embryos of
this species suffer a 50% mortality if pH rises to 8.0 (Mann, 1996).
Fat reserves in the liver are used up in summer when this species is less active.
Age and growth
Maturity is attained at age 2-7 years, varying with habitat, rarely
at 1 year for males. Growth is faster in southern populations and
maturity is earlier than for northern populations. Maximum life span is 22 years.
Food
Food when young is mostly aquatic insects, crustaceans and molluscs
while older burbot feed voraciously on fishes and their eggs and on
frogs. Adults do not feed during the spawning period. Most feeding
occurs at night. It is reported to be cannibalistic. Glycogene and fat are
stored in the liver during spring and autumn feeding periods, allowing the fish
to cope with high water temperatures in summer when the fish is less active and
feeding is low, even enabling some growth and gonad development (Kottelat and
Freyhof, 2007).
Reproduction
Spawning takes place from November to March, even under ice, at
temperatures of 0.5-4.0°C on gravel, sand or hard bottoms. Successive matings occur with the
male and female swimming head down along the bottom until the male
rotates and pushes his belly against hers, releasing eggs and sperm in
a cloud. The female beats her tail, mixing the gametes and dispersing
the fertilised eggs. One or two females may be surrounded by many males in a
spawning ball. The eggs are easily moved by water currents
because of a large oil globule in the yolk but gradually sink and lodge in
interstices in gravel and sand. Up to 5 million eggs are
produced with a diameter about 1.9 mm. About 80 "day
degrees" are needed for the eggs to hatch and since they are laid
in winter this takes some weeks, e.g. about 6 weeks at 2°C.
Parasites and predators
None reported from Iran.
Economic importance
This species is of economic importance in the former U.S.S.R. and
the flesh is said to be excellent as is the liver. The eggs have been
used as caviar but are also reported as toxic (Halstead, 1967-1970;
Coad, 1979b; see under the genus Schizothorax for symptoms of
ichthyootoxism). However it is too rare in Iran to be a food fish and
a potential health hazard.
Conservation
Classified as rare to intermediate by Lelek (1987) for Europe.
Abdurakhmanov (1962) gives data on only one specimen from the southern
Caspian Sea basin, indicative of its rarity. It may be extirpated from Iranian waters.
Further work
Anglers and commercial fishermen should report any captures of this
rare species in Iranian waters. Specimens should be preserved for
comparison with northern populations.
Sources
Counts are taken from Pivnička (1970).
Mugilidae
The mullets or grey mullets are found world-wide in temperate to
tropical coastal waters readily entering estuaries and even resident
in freshwaters. There are about 17 genera and 72 species but only 3 species are
native to Iran in fresh and brackish waters and a further 2 species
have been successfully introduced (the latter not mapped in Berra
(2001) because they are exotics). Other species are recorded as
entering the rivers of southern Iran from the Persian Gulf and Sea of Oman although identification is not
always certain (see Marine List in Checklists in the Introduction). Maximum size is about 0.9 m.
This family is characterised by a compressed to subcylindrical body
with a somewhat flattened head; moderate sized scales which may be
cycloid but are ctenoid in most adults and extend onto the top and
sides of the head; faint or no lateral line along the flank but pits
or grooves on scales contain the sense organs; the eye may have an
adipose or fatty eyelid forming a vertical, slit-like opening;
vertebrae usually 24, rarely as high as 26; wide gill openings; gill rakers long and slender and increasing in number with growth; upper elements of
the gill arch are specialised as a pharyngobranchial organ,;5-6 branchiostegal rays; a spiny and short first dorsal fin (4 spines),
the second dorsal with 1 unbranched ray or spine and 6-10, usually 8,
branched rays; anal fin with 2-3 spines and 8-12 branched rays; an
abdominal pelvic fin with 1 spine and 5 soft rays; pelvic bones
connected to the postcleithrum by a ligament; mouth transverse
and small; teeth on jaws short, weak and flexible, and the lower jaw may
be toothless; the stomach wall is strongly muscled (gizzard-like); and
the gut is very long and coiled.
Mullets are schooling fish which feed on microscopic algae and the
minute animals associated with the algae. They grub, gulp or suck
(hence "mugil") bottom deposits, spitting out some of the
debris and extracting nutrient from the remainder. The long gill
rakers filter the food, the strong gizzard-like stomach crushes it and
the long intestine (about 7 times body length) aids in digestion.
Their bottom feeding leaves long patches of disturbed sediment readily
visible from a distance. Eggs, larvae and young mullet are pelagic.
Adults are found in large schools in coastal waters or on tidal flats.
These fishes are very important economically as food eaten fresh,
smoked or canned, as bait, and as cultured fish in ponds. The flesh is
oily and rich but has few bones. Mugilid species in Khuzestan are thought
to be the intermediate hosts of Heterophyidae flukes found in humans and
carnivores (Massoud et al., 1981).
Recent studies have been carried out on culturing Mugil cephalus and Liza aurata in brackish
water, in inland pools and salt and freshwater culture ponds in the
northern and central parts of Iran (Emadi, 1993a; Iranian Fisheries
Research and Training Organization Newsletter, 11:6, 1996; Azari Takami et
al., 1997a). In April 1994, 20,000 Mugil cephalus fry weighing 0.5 g were
imported from Hong Kong as part of this programme (Iranian
Fisheries Research and Training Organization Newsletter, 4:8,
1994). Azari Takami et al. (1997a) successfully raised fry of Liza
aurata and L. saliens at Bafgh-Yazd in central Iran in earthen ponds
supplied with brackish well water. Fry in the 3-10 g range were cultivated for
100 days at 2200 fry/ha with pond fertilisation and with fertilisation and
complementary feeding, giving 100 g and 120 g fish respectively. Experiments
with different stocking densities and mixes of the two species were also carried
out. Second year L. saliens reached 165 g and third year L. aurata
reached 630 g and sexual maturity.
Experiments have been carried out on fishing methods for these
fishes in Iran, comparing encircling gill nets with fixed gill nets
and with purse seines (Annual Report 1992-93, Iranian Fisheries
Research and Training Organization, Tehran; Annual Report
1993-94, Iranian Fisheries Research and Training Organization, Tehran).
In 1994-1995, the total mullet catch was 4145 t, 85% of which was L.
aurata. Catch per unit effort decreased by 50% over the preceding
three years (Annual Report, 1994-1995, Iranian Fisheries Research
and Training Organization, Tehran, p. 37, 1996).
Soltani and Rahanandeh (2001) describe a case of gastric bloat in Mugil
capito in the Caspian Sea. Mugil capito Cuvier, 1829 is a synonym of
Liza ramada (Risso, 1827), a species not known from the Caspian Sea.
The general name for mullets in Farsi is kefal or kafal.
Pillay (1972) gave a bibliography on mullets and Thomson (1997)
reviewed the family, giving further details of anatomy than summarised here.
Genus Liza
Jordan and Swain, 1884
This genus is characterised by thin to moderately thick, terminal
upper lip without papillae, the lower lip is directed forwards and is
thin-edged, teeth are setiform, ciliiform or absent in the upper lip,
ciliiform or absent in the lower lip, there is a symphysial knob to
the lower jaw and the lower jaws meet at a 90º
angle or more, the maxilla is bent down over the premaxilla and is
either uniformly curved or is s-shaped, the maxilla end is visible
when the mouth is closed, the anteroventral edge of the preorbital bone is
serrate, weakly concave or kinked and ventrally it is broad and squarish, an adipose eyelid is present sometimes but
is not well-developed being a narrow rim around the eye at all ages,
the pharyngobranchial organ has two valves, pyloric caeca number 2-14, predorsal scales are unicaniculate, and the
pectoral axillary scale is weak or absent. Thomson (1997) lists
further characters but the genus is distinguished easily from Mugil
(q.v.), the only other genus from Iran.
Liza abu
(Heckel, 1843)
Further illustrations
Liza abu, lateral view of head of above; Liza abu, dorsal view of head of above; both after Berg (1949)
Common names
بياح (biah), biah zury, zuri, kafal, shochy, do'kelki in Khuzestan (= with two
sails, presumably referring to the two dorsal fins), ? derbak.
[maid, khishni, hishni, abu-khraiza, hosoon, hashsoun, abu-khraiza, or abu sukkanejn (=
father of two anchors in allusion to the toothed suborbital bone
according to Heckel (1843b)), all in Arabic; minghaj in Pakistan; abu mullet, freshwater mullet].
Systematics
Mugil pseudotelestes Pietschmann, 1912, described from the
"Schatt el Arab bei Basra (Aschar)" (Ashar is at 30°31'N, 47°50'E),
is probably a synonym (Coad, 1991b) based on the original description.
Mugil hishni Misra in Hora and Misra, 1943 described from
"Rivers and Hors, Iraq" (hors or hawrs are marshes) is also
a synonym based on the description, an opinion concurred in with
Ingham (no date) and Özdilek (2003). Thomson (1997) considers hishni to be a
synonym and possibly pseudotelestes although he considers the
description of this species insufficiently detailed. Mugil abu
zarudnyi Berg, 1949 was described from "Ser-i-pul, 30 km from
Malamir, between Deh-i-dez and Malamir, region of the Bakhtiars and
Lurs, upper course of the Karun R., south-western Ian, 17 III 1904, N.
Zarudnyi". Thomson (1997) considers this subspecies to be of
doubtful validity given the variability of this species. Different stocks exist
in the Tigris, Euphrates and Orontes rivers as evidenced by morphology (Turan et al., 2004).
Randall (1995b) places this species in the genus Chelon
Rose, 1793; Thomson (1997) does not.
Material of Mugil abu, housed in the Naturhistorisches
Museum Wien, is under NMW 9224-9230 (7 fish) and 67868 (2) and are syntypes. The type
locality is the "Tigris bei Mossul" according to Heckel
(1843b) and the catalogue lists 4 specimens.
Mugil (Liza) abu zarudnyi Berg, 1949 was described from
Iranian tributaries of the Tigris River basin. The 5 syntypes of zarudnyi
are in the Zoological Institute, St. Petersburg (ZISP 24336), measure
69.1-80.7 mm standard length. The date in the catalogue is 4.II.1904 (old style).
The holotype of Mugil hishni is in the Zoological Survey of
India, Calcutta under ZSI F13626/1 with 1 paratype under ZSI F13627/1
(Menon and Yazdani, 1963; Eschmeyer et al., 1996).
Key characters
The high lateral scale count, long pectoral fins reaching almost
level with the first dorsal fin origin when folded back (note fin tips
often frayed, especially in preserved material, so not as apparent),
short pectoral axillary scale, thin lips, 3 anal fin spines and 8
branched rays, relatively strong spines in the first dorsal and anal
fins, and peg-like or setiform teeth (not tricuspid) in the upper jaw
only, distinguish this species from other species in the genus Liza
and from other mullets.
Morphology
The eye has fatty tissue covering as far as part of the iris. Lips
are thin and the lower lip has a pronounced knob. Peg-like teeth are
obviously present only on the upper lip, the lower lip having
scattered ciliate teeth. The end of the maxilla has an s-shaped bend
and its tip is exposed. The anterior edge of the denticulate
preorbital is angular, bending down at the corner of the mouth. The
pectoral axillary scale is weakly developed. Occasional antero-dorsal
scales have double canals. Pectoral fin length is 75-78% of head
length and this fin extends back to a level almost at the first dorsal
fin origin. First dorsal fin spines 3-4, usually 4, second dorsal fin
soft rays 5-10, usually 8 after 1-2 spines, anal fin spines 3 followed
by 5-10, usually 8 soft rays, pectoral fin branched rays 13-17 and
pelvic fin branched rays 5-6, usually 5. Vertebrae 21-25. Fusions and minor
vertebral anomalies were described by Al-Hassan (1987)
and Al-Hassan and Naama (1986b) from Iraqi fish. Lateral
scales 39-53, usually 44-50. Scales are strongly ctenoid on the
exposed part and the fish feels rough to touch when rubbed from tail
to head. The embedded part of the scale has fine circuli. The focus is
very posterior. Scale shape is rectangular with the anterior margin
vertical or somewhat wavy. The anterior upper and lower corners are
square or even pointed. The dorsal and ventral scale margins are
straight and the posterior margin is rounded. There are a few radii
(as few as 4) from the focus to the anterior margin and, where these
intersect the margin, it may be slightly indented to form a wavy edge.
There are 4 pyloric caeca and the gut is elongate and coiled. Gill
rakers are short than the gill filaments and number 44-55. They bear
teeth on the internal edge. Islam and Al-Nasiri (1978) give
morphometric characters. Hoda (1978) describes the larva (as Mugil hishni)
in Iraq. 2n = 50 (Balasem et al. (2000). Thomson (1997) notes that this is the most
variable species in the family.
Al-Hassan (1984b) showed differences in counts of vertebrae and dorsal fin
rays between fish from Basrah and the Karkheh River in Iran. Turan et al.
(2004) also noted differences in morphometric data for stocks from the Orontes
(= Asi), Tigris and Euphrates rivers of Turkey, attributing this to phenotypic adaptation
as the stocks were genetically homogenous. Thomson (1997) notes that this is the
most variable species in the family. Jawad (2004b) studied asymmetry in this
species from the Shatt al Arab at Basrah and suggested pollution could be a cause.
Jawad and Öktener (2007) record lordosis (axial spinal curvature) in this
species in the Atatürk Dam Lake, Turkey. Jawad et al. (2010) describe a
specimen from Karkheh River branch near Susangerd with a malformed caudal fin.
The nominal subspecies zarudnyi has 3-4 first dorsal fin
spines, 1-2 spines and 5-7 soft rays in the second dorsal fin, the
anal fin has 3 spines and 5-9 soft rays, usually 9, and scales in
lateral series 41-46. The larger scales distinguish it from the
typical form at Mosul in Iraq (Berg, 1949). My observations on the
types show 4(4) first dorsal fin spines, 1(2) or 2(2) second dorsal
fin spines and 7(3) or 8(1) soft rays, 7(1), 8(1) or 9(2) anal fin
soft rays, 5(4) pelvic fin rays, 13(1) or 14(3) branched pectoral fin
rays, and 39(1), 43(1), 44(1) or 45(1) scales in lateral series. One
specimen is unusual in having a mix of abnormally low counts and
normal counts: 3 first dorsal fin spines, 5 second dorsal fin soft
rays, 5 anal fin soft rays, 5 pelvic fin rays, 15 pectoral fin rays,
and 43 lateral series scales.
Meristic values for Iranian specimens, excluding the above: first dorsal
fin spines 4(36); second dorsal fin soft rays 7(4), 8(31) or 9(1);
anal fin soft rays 8(28) or 9(8); pectoral fin branched rays 13(1),
14(17), 15(13) or 16(4); pelvic fin branched rays 5(35) or 6(1); lateral series
scales 44(5), 45(5), 46(5), 47(8), 48(2), 49(4), 50(1) or 51(1);
and total vertebrae ?.
Sexual dimorphism
Unknown.
Colour
The back is a light brown or greyish or olive-green with the flanks
and belly silvery or yellowish-silver. There may be an indistinct
silvery stripe or 2 grey stripes along the flank. Generally the upper
flank is dark, the lower flank pale, and the two clearly demarcated.
The upper part of the lower flank may bear alternating light and dark
stripes but these are sometimes poorly expressed. The second dorsal
and caudal fins are dusky to brownish and lower fins are
yellowish-white to hyaline. Scale margins are covered in small black
spots. The top of the eye is lime-green and reddish-brown just below
but still silvery around the pupil generally. Young fish have a
reddish colour at the base of the pelvic, anal and caudal fins and
have an evident mid-lateral stripe against a pale body.
Size
Attains 26 cm total length and 0.15 kg.
Distribution
Found in the Tigris-Euphrates and Orontes (= Asi) (Özdilek, 2003) river basins including the Tigris River basin in Iran
in rivers and marshes such as Dez, Karkeh and Karun rivers and Shadegan Marsh, in lower reaches of rivers draining to the Persian Gulf, and in Pakistan.
In Iran it is also recorded from Parishan (= Famur) Lake but it was not captured in a 1995 survey.
A record from the Barm-e Shur near Shiraz is probably an introduction as this
species was not caught there in the 1970s by me (Gh. Izadi, pers. comm.,
2001).
Abdoli (2000) maps this species in the lower Hasan Langi, Kul, Gowdar and Mehran
rivers at the Straits of Hormuz in the Hormuz basin;
the lower Mand River and the lower Zohreh (= Hendijan) rivers in the Gulf basin,
the Arvand, lower Karun, lower Karkeheh and the Simarreh rivers in the Tigris
River basin. It is also reported from the upper Karun River as M. a. zarudnyi.
Zoogeography
As a freshwater member of a genus with many species in the sea, its origins
lie with these marine relatives.
Habitat
This mullet is a freshwater species found in rivers, streams, channels,
canals and drains, lakes, reservoirs and ponds, on fish farms of Iraq and neighbouring countries, occasionally entering estuaries.
It usually occurs in schools. Epler et al.
(2001) found it to be the dominant species of fish in lakes Habbaniyah, Tharthar
and Razzazah, Iraq, comprising 72% of all fish collected. This was one of the most
abundant species in the recovering marshes of southern Iraq in 2005-2006 (at
almost 36% of 16,199 fishes collected)(Hussain et al., 2006) and in the
marshes in the 1980's (Hussain and Ali, 2006). Nasir and Na'ama (1988) and Hussain and Naama (1989) report it
from the Khawr az Zubayr in a marine environment, probably a consequence of
human-induced environmental changes. It is the most abundant species in autumn
in the Karun River, Iran, comprising 44.2% of the catch (Mokhayer, 1981c).
van den Eelaart (1954) reporting on Iraqi populations found this mullet in
the surface waters and submerged vegetation of lakes and marshes, preferably
where there is a gentle water flow. In December-January it enters rivers and
deeper waters, especially in very cold winters. Ahmad et al. (1983; 1985)
have shown this species can withstand abrupt increases of temperature up to 30°C
and salinity up to 10‰ for 24 hours under experimental conditions.
Mortality in water at 15‰ is low. Salinities up to 30‰ and
temperatures up to 35°C are tolerated, presumably if increases are gradual.
Ahmed et al. (2002) stated that it prefers salinities not exceeding 2‰
(later 5.6‰) but can survive abrupt transfer to 15‰. S. Cowton (pers. comm., 23 August 2005) has observed
schools of this species gaping at the surface in the artificial lake around Al
Faw Palace in Baghdad, presumably in response to high temperatures and low
oxygen. It is often the dominant species left in small pools when marshes dry
up, as in the Shadegan Marshes of Iran observed by B. W. Coad.
Age and growth
Al-Nasiri and Islam (1978) studied the age and growth of this species from
commercial catches in the Shatt al Arab, Iraq. Specimens were aged by examining
the otoliths and three age groups determined with 0+ fish being 8.9-10.9 cm, 1+ fish
being 9.9-14.8 cm and 2+ fish being 13.6-18.2 cm total length. Length-otolith
formulae were given. Al-Yamour et al. (1988) examined a population of
this species in the Al-Daoodi Drain, Baghdad and found an age span up to 7 years
using scales to age fish. Older age groups (5 and 6) grow more slowly than
Diyala River fish, perhaps because of higher salinity in the Drain and the
enriched conditions in the Diyala River due to sewage. The length-weight
relationship was W = 0.034 L2.6, for males W = 0.062 L2.396,and
for females W = 0.031 L2.642. The condition factor was 6.308 for
males and 3.155 for females. Khalaf et al. (1986) studied a population in
the Diyala River, Iraq which was polluted with sewage from the Rustamiyah treatment
plant. Khishni is the second dominant species from December to March after
Chondrostoma regium and in August the dominant species in catches. Fish were
heavier and in better condition in the more polluted areas, but probably not as
good for human consumption! Growth declines after age group 5 with few fish in
age group 6. Length-weight relationships were W = -3.39 L2.64 for
males and W = -2.96 L2.50 for females. Mhaisen and Yousif (1989)
examined a population of khishni in Mehaijeran Creek, a side branch of the Shatt al Arab
south of Basrah, Iraq. Only 3 age groups (0+ to 2+) are reported since the creek water
level is affected by the tide and older fish tend to move into the deeper waters
of the adjacent Shatt al Arab. Growth is faster in the creek than in other Iraqi
locations. The length-weight relationship was log W = 2.794 log TL - 1.64840 for
males and log W = 3.337 log TL - 2.25728 for females. Mhaisen and Al-Jaffery
(1989) report 6 age groups for fish from Babylon Fish Farm west of Hilla, Iraq with a
total length-weight relationship log W = 2.96057 log L - 11.19599 (and for
standard length log W = 2.87574 log SL -10.24769). Fish were in good condition
as they were taking food meant for cultured carp. The relative condition factor
was 0.98-1.04, similar to values obtained by other authors. The von Bertalanffy
growth equation was TLt = 409.6 {1 - exp [-0.06314 (t - 3.13868)]}.
Al-Shamma'a et al. (1995) examined fish from the Tigris River at Al-Za'faraniyah,
Iraq and found log total weight = -2.033 + 3.119 log total length (r= 0.955). Epler
et al. (2001) found the oldest age groups to be 4+ in Iraqi lakes Habbaniyah and
Razzazah and 5+ in Lake Tharthar. The mean condition factor was 0.97, 1.05 and
1.09 in lakes Habbaniyah, Tharthar and Razzazah respectively. Syzpuła et al.
(2001), studying age and growth in the lakes Habbaniyah, Tharthar and Razzazah in
1981 and 1982, found this species grew fastest in Lake Tharthar. A decrease in
length growth was noted in the second year of life and a decrease in body mass
in the last years of life.
The effect of starvation over 35 days on the proximate chemical composition
of this species was studied by Yesser et al. (1999). They found a sharp
decline in lipid content (4.38 to 0.98%), a slight drop in protein and an
increase in both moisture and ash. Both condition factor and viscera somatic
index declined gradually, 1.2 to 0.83 and 10.18 to 3.63% respectively. Body
weight fell by 30.59%. Lipid reserves in muscle were therefore an important form
of energy storage.
Ünlü et al. (2000) report age groups of 1+ to 4+ in the Turkish Tigris
River. Hussain et al. (1987) found condition factor to range from 1.12 to
1.64 for different length groups for Karkheh River fish from Iran near the Iraq
border and Hawr al Hawizah.
Food
Al-Nasiri et al. (1977) studied the feeding ecology of this species in
the Shatt al Arab, Iraq. Aquatic plant parts and phytoplankton are food items
but the vast bulk of material is sand grains and organic detritus, the former
presumably ingested while searching for detrital food. Phytoplankton is made up
of diatoms (50%), green algae (36%) and blue-green algae (14%), with diatoms the
most abundant numerically. Plant parts are fragments of Vallisneria
leaves and rarely leaves and stems of Potamogeton and Polygonum.
Islam et al. (1981) reported on the seasonal patterns of feeding in
Rashdiyah Reservoir, Baghdad. However it should be noted that only 10 fish were
examined for each month. Aquatic plant parts, organic debris and phytoplankton
are dominant foods followed by zooplankton and aquatic insects. The diet is
diverse, 48 species of Chlorophyceae, 60 species of Bacillariophyceae, 9 species
of Myxophyceae and 3 of Euglenophyceae. Heavy feeding occurs in late winter,
spring and fall with peaks in April and November. Empty stomachs are more common
in summer. Islam and Khalaf (1983) studied diel feeding patterns in the same
reservoir and found peaks at 0600 and 2100 hours. Naama and Muhsen (1986)
studied the feeding periodicity of this species in the Hawr al Hammar, Iraq and
found the main food to be algae, mixed with incidental sand grains. There is a
single feeding peak each day at 1730 hours and feeding stops at 0200 hours. This
species is a day feeder. In another study of the recovering Hawr al Hammar, diet
was 76.49% algae and 20.3% diatoms with amounts of crustaceans and plants being less than 10%
each, in the Hawr al Hawizah 74.3% algae and 22.5% diatoms, with amounts of plants
and crustaceans being less than 10% each, and in the Al Kaba'ish (= Chabaish) Marsh 75.8% algae
and 20.0% diatoms with plants and crustaceans at less than 10% each (Hussain et al.,
2006). Ahmed and Hussain (1982) examined the food of young
fish near Basrah, Iraq and found organic detritus to be an important food
followed by phytoplankton. The smallest fish are consuming eggs in large
quantities. Al-Shamma'a and Jasim (1993) studied feeding in the Hawr al Hammar
in Iraq during the flood period and reports small quantities of adult insects,
chironomid larvae, trichopteran larvae, other insect larvae, molluscs and worms,
while copepods, cladocerans, and rotifers are important foods. This variation
from the diet studies reported above may be due to the flood not allowing
diatoms and algae to settle out and become available as food. Typical foods and
gut contents such as organic detritus, algae, diatoms, plant tissues and sand
grains are also present. Sand grains may help in trituration of food and
therefore are not an accidental inclusion. Al-Shamma'a et al. (1995)
examined fish from the Tigris River at Za'faraniyah and found phytoplankton
ranked first in the diet followed by detritus. Debris comprised more than 37% of
stomach contents on the points method. Epler et al. (1996) found detritus
to be the main food in three Iraqi lakes. Epler et al. (2001) found
detritus to be 83.7% of the diet in Lake Tharthar, mineral parts (presumably
sand) 12.7% and plants 3.6%. In Lake Habbaniyah detritus was 73.0%, mineral parts
16.5% and plants 8.4%. In Lake Razzazah detritus was 84.4%, mineral parts 14.7%
and plants 0.9%. Detritus was the principal food year round with no seasonal
variations. Hussain and Ali (2006) examined feeding relationships among fishes
in the Hawr al Hammar and found this species to be a herbivore, 39.6% of the
diet being plants and algae, with 18% detritus.
Hussain et al. (1987) examined the food of khishni in the Karkheh
River, Khuzestan, where it is the dominant species in terms of numbers
caught, by two orders of magnitude. However capture techniques would affect this
assessment. Food was similar to that reported from Iraqi fish. Phytoplankton is
the most important food for fish 3.0-3.9 cm long, followed by organic detritus,
while in larger fish this order is reversed. Silt is an important content of the
gut in all fishes studied. Eighteen species of Bacillariophyceae, 6
Chlorophyceae and 4 Cyanophyceae species were recorded from gut analyses. Abdoli
(2000) reports Daphnia, Hemiptera, Navicula, Nitzschia, Amphora
and Cymbella species for Iranian specimens.
Reproduction
Marammazi (1994) considered it likely that this species spawns
twice each year in the Zohreh River, Iran draining to the northern Persian Gulf.
Chelemal et al. (2009) studied reproduction in fish from Dasht-e Azadegan
west of Ahvaz. Females predominated over males (2.7:1) and maximum gonadosomatic
indices were found in March for both sexes with a minimum in August, suggesting
spawning in April with a spawning season extending from February to June.
Details were given on histological development of the ovaries with spawning
females having eggs 470.3μ in diameter.
van den Eelaart (1954) gave the spawning season in Iraq as end of February to
mid-March. In the Al-Daoodi Drain at Baghdad and the Hawr al Hammar of southern
Iraq spawning starts in March and continued through May (Al-Yamour et al.,
1988). Mean number of eggs reaches 59,793, increasing with size and weight. In
the study by Khalaf et al. (1986), all age group 1 fish are mature and
females outnumber males in age groups 2 to 6 although this may be due to
sampling bias. Ünlü et al. (2000) report maturity at age 1 in the Turkish
Tigris River based on gonad development, with no spawning in the period August
to February. Mhaisen and Yousif (1988) examined a population of khishni in
Mehaijeran Creek, a side branch of the Shatt al Arab south of Basrah. Fish are
ripe from January to March and females partly spent in April and spent in May.
The fish spawn once. All males and females were mature at 16 cm in the creek
while females mature at 13 cm in the Hawr al Hammar. Some males matured at 10.5
cm and some females at 10.6 cm in the creek. Naama et al. (1986) studied
reproduction in the Hawr al Hammar and found a prolonged breeding period from
November to April during which two batches of eggs could be shed. Maturity is
attained at the end of the first year of life for both sexes when they are about
10 cm total length, with all males mature at 16 cm and all females at 13 cm. Egg
diameter is up to 0.75 mm. Epler et al. (1996) record a relative
fecundity of 359,873 to 756,118 eggs for fish of age groups 1+ to 3+ from three
lakes in Iraq. Mature eggs were found from January to May in one lake and from
November to March in another, maturity being two months earlier in saltier
water. Daoud et al. (1998) examined reproductive biology in fish from the
Tigris River at Al-Khadhmia north of Baghdad. They found a sex ratio of 1:1.45
(male:female), significantly different from 1:1, a breeding season from December
to March, sexual maturity in all fish longer than 10 cm total length, smallest
mature male 10.4 cm, smallest mature female 10.6 cm, absolute fecundity
12,033-63,836 eggs and relative fecundity 1237 eggs/g. Epler et al.
(2001) studied reproduction in lakes Tharthar and Habbaniyah and found both sexes
to achieve maturity in the first year of life at 14.2 cm. Spawning occurred in
May and fecundity was 652-791 thousand eggs/kg body mass. Eggs are shed on, and
adhere to, vegetation.
Rashid (19940 gives details of the histological changes in the ovaries for
fish from the Qarmat Ali canal, along with several indices. The gonosomatic
index showed no significant change from October to December but increased from
3.03 in December to 9.43 in January and 16.4 in February, decreasing
subsequently suggesting a gradual spawning took place in March and into April.
The hepatosomatic index showed a significant increase in February to 3.34 and a
significant decrease in March to 1.32, suggesting the liver was involved in
vitellogenin synthesis necessary to oocyte development. The somatic index
increased from 0.65 in October to 1.15 in December as the fish lay down
reserves, decreasing to 0.53 in February and rising throughout March and April.
Parasites and predators
Bykhovski (1949) reports a new species of monogenetic trematode, Ancyrocephalus
fluviatilis, from this species in the Karkheh River, Iran.
Mokhayer (1981c) reports a high infestation rate with larvae of the
nematode Contracaecum in Karun River fish. This has health
consequences for humans if fish are eaten raw, or are inadequately
salted, smoked or cooked. There has been no report yet of this
parasite from humans in Iran.
Moghainemi and Abbasi (1992) record a wide range of parasites from
this species in the Hawr al-Azim in Khuzestan. Mortazaei et al.
(2000) report the worm Neoechinorhynchus tylosuri in 54.8% of
fish examined from Khuzestan marshes. Farahnak (2000) and Farahnak et
al. (2002) record Anisakidae from this species in Khuzestan.
Barzegar et al. (2008) record the digenean eye parasites Diplostomum
spathaceum and Tylodelphys clavata from this fish.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and
found Caligus lacustris, Lamproglena sp. and Ergasilus sp.
on this species.
Economic importance
It appears in stores as a regular food fish in Ahvaz, Khuzestan, and is an
important food fish in southern Iraq (Al-Nasiri and Islam, 1978) with
212,850 kg marketed at Basrah from October 1975 to June 1977 (Sharma, 1980). It
forms about 29% of the total annual catches of freshwater fishes in southern
Iraq and about 7.4% of the total annual catch for the whole country despite its
small individual size (Mhaisen and Yousif, 1989; Mhaisen and Al-Jaffery, 1989).
Its caloric value is 162.42 Kcal/100g (Mahdi et al., 2005). Salim (1962)
records this species jumping into boats in Iraq when attracted by lights. It is
important on fish farms in Iraq where it competes for food with cultured carp
(Al-Shamma'a and Jasim, 1993) and Mhaisen and Al-Jaffery (1989) believed it
could be farmed successfully because of its high growth rate. Taken also in the
subsistence fisheries of the Indus River in Pakistan.
van den Eelaart (1954) gave the fishing season for this species as
December-January (peaking in January) in rivers, and December-March (January)
for lakes and marshes in Iraq.
Balasem et al. (2000) showed this species to be more sensitive to
pollution than other Iraqi species tested, namely Barbus (=
Carasobarbus) luteus,
Cyprinus carpio, Carassius carassius (sic, probably C.
auratus) and Gambusia affinis (sic, probably G. holbrooki).
The LC50 of L. abu to copper after 72 hours exposure was 0.4
p.p.m. while the other species showed no mortality at this level. L. abu
was also more sensitive to arsenic than these species and also Barbus (=
Mesopotamichthys) sharpeyi, Barbus (= Luciobarbus) xanthopterus, Barbus
(= Tor) grypus and Ctenopharyngodon
idella (Ali et al., 1999). Wahab (1999) also tested the toxicity of
four organophosphate insecticides to young fish, namely Dursban, Superacid,
Malathion and Nogos with LC50 at 0.102. 0.125, 0.875 and 1.2 5 mg/L
respectively. Salinity has a significant effect on the toxicity. This mullet has
also been used to assess the impact of gas oil from oil spills in the Shatt al
Arab on ionic regulation, on the chloride cells and on the gills (Faddagh et
al., 2004). It was found to be sensitive to this form of pollution and
suitable as an indicator species.
Conservation
Mhaisen and Yousif (1988) recommend banning fishing from
mid-January to mid-May during the spawning season and instituting an
increase in fishing net mesh size to 23.2 mm.
Further work
Variation in this species should be re-examined to determine if nominal
species and subspecies are valid. Biology and stock assessments for Iranian
waters need to be carried out.
Sources
Type material: Liza abu zarudnyi (ZISP 24336).
Iranian material: CMNFI 1979-0240, 3, 57.1-70.7 mm standard length, Fars, Lake Famur (ca. 29º31'N, ca. 51º50'E);
CMNFI 1979-0304, 12, 21.5-50.0 mm standard length, Fars, Lake Famur (ca. 29º31'N, ca. 51º50'E);
CMNFI 1979-0352, 5, 11.4-22.6 mm standard length, Khuzestan, marsh 57 km east of Abadan (30º33'30"N, 48º48'E);
CMNFI 1979-0353, 3, 15.7-18.3 mm standard length, Khuzestan, Karun River (30º22'30"N, 48º17'E);
CMNFI 1979-0354, 6, 18.4-25.2 mm standard length, Khuzestan, Karun River tributary (30º31'N, 48º19'E);
CMNFI 1979-0355, 3, 16.7-29.4 mm standard length, Khuzestan, Karun River tributary (30º35'N, 48º22'E);
CMNFI 1979-0360, 12, 17.7-35.8 mm standard length, Khuzestan, canal branch of Karkheh River (31º40'N, 48º35'E);
CMNFI 1982-0369, 11, 39.3-57.9 mm standard length, Khuzestan, Karkheh River (no other locality data);
CMNFI 1993-0149, 2, 106.0-142.2 mm standard length, Khuzestan, Karun River (no other locality data);
ZSM 25387, 4, 56.6-90.9 mm standard length, Khuzestan, Karun River at Ahvaz (31º19'N, 48º42'E);
ZSM 25700, 6, 41.0-57.4 mm standard length, Khuzestan, fish pond near Ahvaz (no other locality data);
uncatalogued 1, 169.8 mm standard length, Khuzestan (no other locality data).
Comparative material: BM(NH) 1971.4.2:1, 1, 130.2 mm standard length, Iraq, pond 25 km
from Mosul (no other locality data); BM(NH) 1973.5.21:179-184, 6, 41.4-51.4 mm
standard length, Iraq, Tigris River at Jadriyah, Baghdad (no other locality data); BM(NH) 1974.2.22:10-13,
4, 107.3-119.5 mm standard length, Iraq, Najab Bazar (no other locality data).
Liza aurata
(Risso, 1810)
Common names
kefal-e auratus, mahi kefal-e talaee (= golden mullet fish), kefal-e zarin, kafal.
[gizili kefal in Azerbaijanian; gatykelle, singil, orsbalyk in Turkmenian; singil' in Russian; golden mullet,
golden grey mullet, long-finned mullet].
Systematics
Mugil Auratus was originally described from Nice, France. No major relevant synonyms.
Key characters
This species is distinguished from its relative in the Caspian Sea
by each head and antero-dorsal flank scale having only one pit or
groove (occasionally double), 6-11 pyloric caeca of about equal length
or gradually becoming longer from ventral to dorsal, scales on the
snout ending anteriorly as a single row of small scales, and the oral
edge of the preorbital bone is moderately concave. The upper jaw
reaches posteriorly to a level with the posterior nostril, the only
species in the genus with this character. Young have two vertical dark lines at
the origin of the caudal fin rays and a herring bone pattern on the flanks
(Harrison in Miller, 2003).
Morphology
The first dorsal fin has 4 strong spines, the last weakly developed
compared to the first three. The second dorsal fin has 1-2 weak spines
(more resembling unbranched rays than spines) and 6-9, usually 7 or 8
soft rays (the second unbranched ray or spine may be branched near the
tip - the mode of 8 may include this ray in literature sources). The
anal fin has 3 spines and 7-10, usually 9 branched soft rays. The
pectoral fin has 2 unbranched rays and 13-17 branched rays and the
pelvic fin has 1 spine and 5 branched rays. Scales number 40-49 in
lateral series. There is no pectoral axillary scale. There is an
elongate first dorsal fin axillary scale and a pelvic axillary scale.
Scales have an almost vertical anterior margin with the anterior
dorsal and ventral corners square to pointed. This margin is slightly
indented where radii intersect it. The dorsal and ventral scale
margins are parallel and the posterior margin is rounded. The focus is
posterior and the posterior field has weak ctenii. Circuli are fine
and numerous and radii are limited to the the anterior field,
numbering up to 13 in fish examined here but probably size dependent.
The adipose membrane of the eye is rudimentary. The gill rakers are
fine and numerous, apparently finer and more numerous than in Liza
saliens, exceeding 100 in the larger fish examined here. Raker
number is probably size dependent. Rakers are slender, compressed and
serrated medially. When appressed, a raker reaches the thirteenth
raker below. The gut is elongate with several anterior and posterior loops
after a muscular stomach. The chromosome number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- first dorsal fin spines 4(30);
second dorsal fin with 1(3) or 2(27) "spines" or unbranched
rays and 6(1), 7(27), 8(1) or 9(1) branched rays; pectoral fin
unbranched rays 2(30) and branched rays 15(7), 16(20) or 17(3); pelvic
fin branched rays 5(30); lateral line scales 41(2), 42(4), 43(8),
44(3), 45(8), 46(4) or 47(1); and total vertebrae ?.
Sexual dimorphism
Unknown.
Colour
The body is a dusky grey to blue-grey with a silvery flank and
belly. The back and upper flank have a series of dark to golden stripes. There
is a golden blotch on the operculum. The peritoneum is dark brown to black.
See also Key characters.
Size
Commercial catches in Iran during the 1950s weighed 0.3-0.8 kg,
sometimes as much as 1.5-1.8 kg (Farid-Pak, no date). Reaches 59 cm and 2.5 kg on
the coast of Turkmenistan. The Fisheries Research Centre in Mazandaran
caught an exceptional specimen of this species weighing 1.5 kg and
measuring 54 cm long (Iranian Fisheries and Research Training
Organization Newsletter, 3, 1994).
Distribution
Found in the Mediterranean and Black seas and from the British
Isles south to South Africa.
First reported from Iranian waters in 1933 by Shukolyukov (1937a)
and by Dmitriev (1946), this species and Liza saliens became
acclimated to the Caspian Sea over a period of 30-35 years and are
regarded as naturalized from the latter half of the 1960s onward in
Soviet waters (Marti, 1940; 1941; Khoroshko, 1982). It was first
introduced to the Soviet Caspian Sea in 1930-1931 (Baltz, 1991).
Kaplin in Mandych (1995) indicates that these grey mullets entered
from the Black Sea during a Caspian transgression but this is incorrect.
In Iran, this species is found in the lower reaches of rivers along the
Caspian coast, the Anzali Mordab and its outlets where numbers will
probably increase with increasing salinity (Holčík and Oláh, 1992), the Safid River, Gorgan Bay, the
southeast Caspian Sea, southwest Caspian Sea and south-central Caspian
Sea (Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
Zoogeography
This species is an exotic in Iran.
Habitat
This mullet inhabits the sea and enters the lower reaches of
rivers, and is occasionally found in nearby lakes. It is recorded from depths of
5-700 m (Caspian Sea Biodiversity Database, www.caspianenvironment.org). Eggs develop in the
open sea, larvae migrate to the coast and young feed along the shore
and in bays. It winters along the Iranian shore in coastal waters, has a feeding
migration in surface waters beginning in March to shallow areas of the middle
Caspian and, by September-October, mature fish migrate over deeper water
(300-700 m) of the middle and south Caspian to spawn. Feeding ceases at 6-8ºC
and death occurs below 1.5ºC. The optimum temperature is 23-25ºC but young can be
found in shallow water at 37.5ºC. It occurs from fresh water to salinities of 57‰ with
mass mortalities recorded at 65‰ (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Amini Ranjbar and Sotoudebnia (2005) assessed heavy metal (Zn, Cu, Pb and Cd)
concentrations in fish from the Caspian Sea in Mazandaran and found higher than
normal levels of Pb and Cd in muscle tissues.
Age and growth
Life span is up to 12 years in the Caspian Sea (Khoroshko, 1982).
Maturity is attained at 38-45 cm and an age of 3-4 years for males and
5-6 years for females (RaLonde and Walczak, 1972). Fish taken from
commercial catches in Iran are mostly 3-7 years old, 30.2-49.1 cm long
and weigh 354-1266 g (Razivi et al., 1972).
Fazli (1998) studied this species in the southeastern Caspian Sea of Iran and
found that both scales and opercula could be used in ageing, although the
latter were better. Maximum growth occurred at 3 years of age. The b value in
the relation between length and weight was 3.019 and sex ratio was 1:1.3 with
females dominating.
Ghaninejad et al. (2010) sampled fish from along the Iranian coast
captured in 2007-2008. The overall male/female sex ratio at 1:1.22 differed
significantly from 1:1 although only fish from Golestan were significantly
different, size range was 19.0-50.2 cm, mean 32.7 cm, weight was 67 g to 1475 g,
mean 411 g, age range was 2 to 10 years with mean age 4.42 years, age groups 3,4
and 5 years constituted 62% of the age composition, peak spawning was in October
off Gilan but in November off Mazandaran and Golestan, average absolute
fecundity was 700,881 eggs with a range of 200,112 to 2,282,862 eggs, and length
of females at 50% maturity was estimated at 28.4 cm.
Food
This species is reported to enter the Anzali Mordab to feed from
January to March (Holčík and Oláh, 1992), a result of the increasing salinity of this lagoon.
Food items are small benthic invertebrates and detritus with some
insects and plankton. Most stomach contents contain numerous sand
grains. Adults scrape periphyton from rocks, silt and artificial structures. The
predominant food items found by Ghadirnejad and Ryland (1996) from fish taken in
the southern Caspian Sea were bivalves, foraminiferans and calanoid copoepods.
Other food items were ostracods, eggs, nematodes, Nereis and cyclopoid
copepods. Juveniles fed on ostracods and copepods while adults preferred
bivalves, foraminferans and Nereis. This fish consumed more foraminferans
than its relative and less copepods.
Reproduction
This mullet spawns in more northerly waters of the Caspian than its
relative Liza saliens. Spawning begins in the middle of July in
the middle Caspian and ends in the south Caspian from the middle to
the end of October. Intensive spawning in the middle Caspian takes
place in August-September at 20-22°C
and a transparency of 7-11 m. Spawning depth is in the upper 0.5 m. The main spawning areas are at least
50-60 miles (80-96 km) from the coast (Avanesov, 1972) and this
prevents the spawning area being easily exploited. Fecundity exceeds
4.4 million eggs in the Caspian Sea (Khoroshko, 1982); in Mazandaran
average absolute fecundity reaches 772,350 eggs (Abdoli et al.,
1996). The eggs are pelagic and have a diameter of 0.9 mm. In Iran, Gorgan Bay is believed to be a
very important nursery and this is the major species caught by
fishermen in winter (www.ramsar.org/ram_rpt_37e.htm, downloaded 28 July 2000).
Shabanipour (1995) and Shabanipour and Heidari (2004) carried out histomorphological studies on the
ovary of this species in Iran. Fazli (1998) found populations in the
southeastern Caspian Sea of Iran spawned in September-November based on the
gonadsomatic index, with most spawning in October. Males are ready to reproduce
earlier than females. Absolute fecundity was 586,165 eggs. Fazli et al.
(2008), again examining populations in the southeastern Caspian Sea, report a
reproductive season of October to December, a male:female ratio of 1:1.42,
sexual maturity at 26.0 cm fork length, mature gonads in 20% of fish at age 3,
63% at age 4, 88% at age 5 and 97% at age 6, individual absolute fecundity from
113,386 to 1.47 million eggs with a mean of 451,963. They concluded that the
overlap between the fishing and spawning seasons required a delay of one month
in the former to protect stocks. Heidari et al. (2009) studied late
oogenesis in fish from Anzali. Recently spawned oocytes were lemon in colour and
up to 0.8 mm in diameter.
Parasites and predators
Mokhayer (1976b) records the trematode Saccocoelium obesum
from this species on the Iranian coast. This is a parasite of the
intestine, with contamination rates of 90.4%, 88.6%, 81% and 34% for
autumn, winter, spring and summer respectively. Gills carry Microcotyle
mugilis at a rate of 2.0-2.3% and fish are also infected with
Ancylodiscoides (sic) at a rate of 19.6%, 43%, 72% and 43.3%
for autumn, winter, spring and summer respectively (Annual Report,
1994-1995, Iranian Fisheries Research and Training Organization,
Tehran, p. 27-28, 1996; Annual Report 1993-94, Iranian
Fisheries Research and Training Organization, Tehran, p. 49-50, 1995).
Naem et al. (2002) found species in the monogenean treamtode genus
Ligophorus on the gills of this species from the western branch of the Safid
River.
This species is eaten by Sander spp., Silurus glanis and Huso huso.
At Ziba-kenar beach, Gilan in February 2004, this species showed
erratic swimming and belly-up posture. A post-mortem showed gas accumulation and
distension in the swimbladder, a yellowish liver, liquefaction of the gall
bladder, excess micro-sand in the the caecum and hyperaemia of the intestine.
The cause is thought to be an infectious agent closely related to a betanovirus
or nodavirus (Iranian Fisheries Research Organization Newsletter, 38:3, 2004; Zorriehzahra et
al., 2005).
Economic importance
The catch in Iran for this species and Liza saliens from
1956/1957 to 1961/1962 varied from 166,197 kg to 960,282 kg (Vladykov,
1964) and from 1965/66 to 1968/69 varied from 490 to 1916 tonnes (Andersskog,
1970). Vladykov (1964) and RaLonde and Walczak (1972) cite figures for
the Bandar-e Anzali region for Liza aurata from 1937/38 to
1961/62 of 14 kg to about 113 t and for 1969/70 a catch of 1,085 t was
recorded, while for the whole Northern Shilot (Fisheries Company) in
the period 1965/66 to 1968/69 catches varied between 548 t and 1916 t.
Holčík and Oláh (1992) report a catch of only 3 kg in the Anzali Mordab in
1990. The Food and Agriculture Organization, Rome records the
following catches for the 6 years from 1980 to 1985 for mullets in
Iran, presumably from the Caspian Sea:- 150, 400, 1500, 2733, 2135,
and 2200 t respectively. Moghim et al. (1994) give a total
biomass of 2400 tonnes for this species in the southern coastal area
of the Caspian Sea with a maximum sustainable yield of 960 tonnes
(figures for Liza saliens are 7000 tonnes and 2900 tonnes
respectively). Caspian Sea catches of this species in Iran for the
year 1993-1994 was 22% of the bony fish catch with Liza saliens
comprising only 4% (the major part of the catch was safid mahi, Rutilus
frisii, at 62%)(Annual Report 1993-94, Iranian Fisheries
Research and Training Organization, Tehran, p. 83, 1995). Catches in former Soviet waters of the Caspian Sea are about three-quarters or
more this species and one-quarter Liza saliens (Khoroshko,
1982). The main season for fishing in the Caspian Sea off Iran is from
December to February, peaking in January (Farid-Pak, no date). Abdoli et
al. (1996) note that beach seiner cooperatives started fishing a
month earlier than usual in 1993, coinciding with the spawning season
in October. Annual catch variations occur in Iranian waters, this
species having an abundance of 86.5% in 1994 decreasing to 38% in 1995
(Iranian Fisheries Research and Training Organization Newsletter,
9:5, 1995). The catch of this species declined from 76.9% to 41.2% of
the total mullet catch from 1993-1994 to 1994-1995 through overfishing
of juveniles (Iranian Fisheries Research and Training Organization
Newsletter, 11:7, 1996). Abdolmalaki (2001) records a catch per unit effort for beach seining as 93
kg in 1991, falling to 33 kg in 1997 through overfishing. The mean length of the
catch in Guilan decreased from 1991 to 1997 while in Mazandaran it increased.
Fazli and Ghaninejad (2004) give the year 2001 as that of the maximum mullet
catch in their survey of the years 1993-2001, the catch increasing through this
period. Catch per unit effort in each seine declined from 114 kg in 1993 to 43
kg in 1996, increasing to 66.4 kg and 78.4 kg in 2000 and 2001. For the years
2000 and 2001, L. aurata had a mean fork length of 32.7 and 32.3 cm, a
weight of 418.3 and 419.8 g, and a K value of 0.61 and 0.93. They concluded that
the harvest of this species is at a sustainable level. Heydatifard et al. (2002) state that its catch is greater than that of
other bony fishes (presumably excluding kilka) in the southern Caspian Sea. S.
M. E. J. Zorriehzahra (pers comm., 19 January 2011) noted a decline in catches
from 6442 mt in 2002 to 2400 mt in 2010.
Hosseini et al. (2004) determined the shelf life of this species on
ice in Iran was 10 days. Mazandaran fish have an average fat composition of
9.25%, 72% being unsaturated. Omega-3 fatty acids constitute 18.7% of the total
fatty acids with a significant ratio to omega-6 fatty acids of 1.97 (Bedayatifard
et al., 2002). Akhondzadeh Basteh et al. (2006) found the
bacterial pathogens Listeria monocytogenes and Staphylococcus aureus
in salted Liza aurata and Vibrio haemolyticus in fresh and salted L. auratus.
Hedayatifard and Yousefian (2009; 2010) determined the comparative fatty acid
composition in filleted fish that were fresh or dry-salted and found this fish
to be one of the best sources for omega-3 essential fatty acids compared to
other fishes in the Caspian Sea basin.
Razavilar and
Tavakoli (2006) studied the the prevalence of human toxigenic Clostridium
botulinum and the need for food safety control measures.
Conservation
The successful introduction of this species and Liza saliens
in the Caspian Sea has apparently led to a decline in various clupeid
species (Alosa) and of Sander marinum (Baltz, 1991). Beach seines with mesh sizes of 28 mm, 33 mm and 36 mm caught
non-standard fish as 43.3%, 35% and 2.2% of the total catch
respectively. Seines with a cod end of at least 36 mm are recommended
for stock protection (Iranian Fisheries Research and Training
Organization Newsletter, 6:8, 1994) (note the report names the
fish as Mugil cephalus but the catch was probably a mix of Liza
aurata and L. saliens).
Contamination of this species in Iranian waters with Pb, Ni and Zn was examined
by Fazeli et al. (2005). The highest contamination was in the southwest
part of the Caspian followed by the south centre and the southeast. The highest
concentrations observed were 17.51 mg/kg for lead, 6.23 mg/kg for nickel and
647.28 mg/kg for zinc. Pazooki et al. (2009) found the heavy metals
cadmium and chromium in fish from Bandar Anzali, more in the skin than in muscle
and more in females than males, but not enough to constitute a health hazard.
Further work
As an important fisheries species, population numbers and biology should be
carefully monitored on an ongoing basis.
Sources
Distinguishing characters based on Trewavas and Ingham (1972);
Sakri (1993) gives an account in Farsi on the spawning and catch of
mullets in the Iranian Caspian.
Type material: None.
Iranian material: CMNFI 1970-0509, 1, 124.2 mm standard length, Gilan, Safid River (37º24'N, 49º58'E);
CMNFI 1970-0535, 1, 137.2 mm standard length, Gilan, Shafa River (37º35'N, 49º09'E);
CMNFI 1970-0543, 3, 33.5-38.5 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0543A, 4, 33.1-60.5 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0563, 19, 52.8-87.0 mm standard length, Gilan, Caspian Sea, Kazian Beach (ca. 37º29'N, ca. 49º29'E);
CMNFI 1970-0565, 4, 52.2-93.0 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E);
CMNFI 1970-0581, 2, 147.7-151.5 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0587, 1, 78.9 mm standard length, Mazandaran, Babol River (36º43'N, 53º39'E);
CMNFI 1971-0326A, 20, 30.0-46.0 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1979-0430, 1, 42.0 mm standard length, Mazandaran, river 1 km east of Now Shahr (36º39'N, 51º31'E);
CMNFI 1979-0470, 19, 29.4-57.4 mm standard length, Mazandaran, stream 21 km west of Alamdeh (36º35'N, 51º43'E);
CMNFI 1979-0471, 3, 56.7-65.8 mm standard length, Mazandaran, Caspian Sea 14 km west of Alamdeh (36º35'N, 51º48'E);
CMNFI 1979-0686, 2, 59.1-87.3 mm standard length, Gilan, Safid River near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0117, 2, 99.7-114.5 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1980-0127, 6, 29.5-36.9 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0146, 1, 135.7 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak(36º50'N, 53º56'E);
CMNFI 1980-0149, 1, 38.4 mm standard length, Gilan, Chabak River (37º21'N, 49º50'E).
Liza saliens
(Risso, 1810)
Common names
kefal-e saliens, kafal-e poozehbarik, kafal.
[sivriburun kefal in Azerbaijanian; vitibutum or gatykelle in Turkmenian; ostronosik in Russian; sharpnose
mullet, small mullet, grey mullet, leaping mullet].
Systematics
Mugil Saliens was originally described from Nice, France. No major relevant synonyms.
Key characters
This species is distinguished from its relative in the Caspian Sea
by each head and antero-dorsal flank scale having 2-7 or more grooves
(mostly 2 grooves), pyloric caeca in two groups, 3-5 short and 3-4
long (total 6-9), scales on the snout ending anteriorly as numerous
rows of small scales, and the oral edge of the preorbital bone is
deeply notched. Young lack the vertical dark lines at the origin of the caudal
fin rays and the herring bone flank pattern seen in L. aurata (Harrison in Miller, 2003).
Morphology
The first dorsal fin has 4 spines, the last one weakly developed
compared to the first three. The second dorsal fin has 1-3, usually 1or 2,
spines (more resembling unbranched rays than true spines) and 6-9,
usually 8 or 9 soft rays (the second unbranched ray or spine may be
branched near the tip - modes of 8 or 9 in literature may include this ray). The anal fin has 3-4, usually 3, spines and 7-10,
usually 9, soft rays. The pectoral fin has 2 unbranched rays and 14-17
branched rays and the pelvic fin has 1 spine and 5 branched rays.
Scales number 42-50 in lateral series. There is no pectoral axillary
scale. There is an elongate first dorsal fin axillary scale and a
pelvic axillary scale. Scales have an almost vertical anterior margin
with the anterior dorsal and ventral corners square to pointed. This
margin is slightly indented where radii intersect it. The dorsal and
ventral scale margins are parallel and the posterior margin is
rounded. The focus is posterior and the posterior field has weak
ctenii. Circuli are fine and numerous and radii are limited to the the
anterior field, numbering up to 10 in fish examined here but probably
size dependent. The gill rakers are fine and numerous, but not as fine
and numerous as in Liza aurata, exceeding 70 in the larger fish
examined here, and probably size dependent. When appressed, a raker reaches raker
6 or 7 below. Rakers have a serrated medial edge. Vertebrae
23-25. The gut is elongate with 2 posterior loops after a muscular stomach. The
chromosome number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- first dorsal fin spines 4(30);
second dorsal fin with 1(6), 2(22) or 3(2) "spines" or unbranched rays and 6(1), 7(20) or 8(9) branched rays; pectoral fin
unbranched rays 2(30) and branched rays 14(2), 15(27) or 16(1); pelvic
fin branched rays 5(30); lateral line scales 42(2), 44(6), 46(6),
47(8), 48(4) or 49(4); and total vertebrae ?.
Sexual dimorphism
Males are smaller than females.
Colour
The back is dark grey to blue-grey or grey-brown, the belly pale to silvery
or yellowish, and the silvery-grey flanks have about 7 bluish stripes. There is a golden blotch on the
operculum. The peritoneum is brown to black.
Size
Commercial catches in Iran during the 1950s weighed 0.3-0.8 kg,
sometimes as much as 1.5-1.8 kg (Farid-Pak, no date). Reaches 40.0 cm.
Distribution
Found in the Mediterranean and Black seas and along the Atlantic coast to South Africa.
First reported from Iranian waters by Dmitriev (1946), this species
was introduced to the Soviet Caspian Sea in 1930-1931 (Baltz, 1991). In Iran it
is reported from the lower reaches of such rivers as the Gorgan, Gharasu, Tajan,
Talar, Babol, Haraz, Sardab, Pol-e Rud, and Safid, the Anzali Mordab and Gorgan Bay, the southeast
Caspian Sea, southwest Caspian Sea and south-central Caspian Sea (Roshan tabari,
1987; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
Zoogeography
This species is an exotic in Iran.
Habitat
This is a pelagic species found mostly in inshore areas and entering lagoons and rivers.
It is found in surface waters over depths of 5-700 m at salinities of 4-13‰.
It migrates to the southern Caspian in autumn when water temperatures fall (Caspian Sea Biodiversity Database,
www.caspianenvironment.org). Generally it tolerates fluctuations of 5-27Cº and 11-28‰ although
fry need time to adjust to lower salinities (Harrison in Miller, 2003).
Age and growth
Life span is up to 10 years in the Caspian Sea (Khoroshko, 1982).
Males mature at 3 years and females at 4 years (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
The maximum growth rate in fish sampled between Babolsar and Bandar-e
Torkeman in the Iranian Caspian was observed in fish age 3 years. The
operculum gave better ages than scales (Fazli, 2000). Patimar (2008a) examined
fish in Gorgan Bay and found maximum ages of 6+ for males and 7+ for females.
Both sexes grew allometrically (b = 2.478 for males, 2.545 for females) and
rapidly during their first year of life, reaching more than 40% of their growth.
Age and growth were in contrast to other populations in the southeast Caspian
Sea. The von Bertalanffy growth functions were Lt = 30.415(1-e-0.275(t+0.645))
for males and Lt = 34.832(1-e-0.211(t+1.009)) for females.
Males predominated at smaller sizes and females at larger sizes but see below.
Food
Diet is very similar to that of Liza aurata, comprising
detritus, periphyton and small benthic organisms
Stomach contents contain sand grains in Iranian fish.
The predominant food items found by
Ghadirnejad and Ryland (1996) from fish taken in the southern Caspian Sea were
bivalves, foraminiferans and calanoid copoepods. Other food items were ostracods,
eggs, nematodes, Nereis and cyclopoid copepods. Juveniles fed on
ostracods and copepods while adults preferred bivalves, foraminferans and
Nereis.
Reproduction
Spawning starts at the end of May and the beginning of June in the
southern Caspian Sea and continues until the end of September and the
beginning of October, most intensively between June and August off the
Turkmenistan coast according to Avanesov (1972). Iranian fish spawned
in July-August and males ripen earlier than females (Fazli, 2000; Yousefian et al., 2003).
Yousefian et al. (2003) give details of oogenesis, detailing the stages in gonad
development. Spawning occurs over depth ranges of 5-700 m but most
spawning in the southeastern Caspian is at 5-7 m. Temperatures are between 17 and 29°C
with the most intensive spawning when sea surface temperature is 25-29°C.
Water transparency is 1-9 m. The greatest aggregations of eggs are
found 5-7 miles (8-11 km) from shore in the eastern Caspian. Fecundity
exceeds 2.1 million eggs in the Caspian Sea (Khoroshko, 1982). Egg diameter is
up to 0.8 mm. Females predominate in Iranian samples examined by Fazli (2000) at a ratio of 1:3.14.
Patimar's (2008a)
Gorgan Bay fish reproduced in May-July with an average fecundity reaching
389,790 eggs in 7+ fish and relative fecundity 4281 eggs/g. The low absolute
fecundity contrasted with other populations in the southeast Caspian Sea.
Parasites and predators
This species has a worm parasite in the intestine, Saccocoelium
obesum, with contamination rates of 62%, 10.7%, 12% and 22.2% for
autumn, winter, spring and summer respectively, lower than in Liza
aurata. Gills carry Microcotyle mugilis at a rate of
2.0-2.3% and fish are also infected with Ancylodiscoides (sic)
at a rate of none, none, 1.6% and 4% for autumn, winter, spring and
summer respectively, much lower than in Liza aurata (Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 27-28, 1996; Annual Report 1993-94,
Iranian Fisheries Research and Training Organization, Tehran, p. 49-50, 1995).
Economic importance
The main season for fishing in the Caspian Sea off Iran is from
December to February, peaking in January (Farid-Pak, no date). Some
catch information is summarised above under Liza aurata which
is 5-6 times more abundant in catches. However a report on the
1994-1995 catch showed a severe reduction of 2500 tonnes, as Liza
saliens replaced L. aurata as the main part of the catch. L.
aurata declined from 76.9% of the mullet catch in 1993-1994 to
41.2% in 1994-1995, the cause being overfishing of juveniles (Iranian
Fisheries Research and Training Organization Newsletter, 11:7,
1996). Moghim et al. (1994) give a total biomass of 7000 t
for this species in the southern coastal area of the Caspian Sea with
a maximum sustainable yield of 2900 t (figures for Liza aurata
are 2400 tonnes and 960 tonnes respectively). Annual catch variations
occur in Iranian waters, this species having an abundance of 13.5% in
1994 increasing to 62% in 1995 (Iranian Fisheries Research and
Training Organization Newsletter, 9:5, 1995).
Abdolmalaki (2001) records a catch per unit effort for beach seining as 78
kg in 1991, falling to 15 kg in 1997 through overfishing. The mean length of the
catch in Gilan and Mazandaran did not decrease from 1991 to 1997, the former in
contrast to Liza aurata. Fazli and Ghaninejad (2004) give the year 2001
as that of the maximum mullet catch in their survey of the years 1993-2001, the
catch increasing through this period. Catch per unit effort in each seine
declined from 114 kg in 1993 to 43 kg in 1996, increasing to 66.4 kg and 78.4 kg
in 2000 and 2001. For the years 2000 and 2001, L. saliens had a mean fork
length of 27.5 and 25.1 cm, a weight of 224.7 and 179.1 g, and a K value of 0.91 and 0.71.
Conservation
See above under Liza aurata.
Mercury levels at 0.0102-0.108 mg kg -1 w.w. in this species from the
Iranian shore were lower than the WHO guideline of 0.5 mg kg-1 w.w. (Agah
et al., 2007).
Further work
As an important fisheries species, population numbers and biology should be
carefully monitored on an ongoing basis.
Sources
Distinguishing characters based on Trewavas and Ingham (1972).
Type material: None.
Iranian material: CMNFI 1970-0510, 34, 38.1-72.8 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1970-0507, 9, 23.6-46.8 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0508, 1, 50.6 mm standard length, Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0509, 4, 31.9-66.8 mm standard length, Gilan, Safid River at Hasan Kiadeh(37º24'N, 49º58'E);
CMNFI 1970-0528, 16, 43.6-64.4 mm standard length, Mazandaran, Tajan River estuary (36º49'N, 53º06'30"E);
CMNFI 1970-0535, 1, 153.3 mm standard length, Gilan, Shafa River (37º35'N, 49º09'E);
CMNFI 1970-0539, 43, 25.1-102.0 mm standard length, Gilan, Caspian Sea off Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1970-0543A, 2, 76.7-79.9 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0549, 1, 48.1 mm standard length, Gilan, Qareh Su near Alb Imamzadeh (no other locality data);
CMNFI 1970-0565, 3, 91.8-108.8 mm standard length, Gilan, Safid River estuary (ca. 37º28'N, ca. 49º54'E);
CMNFI 1970-0577, 13, 22.1-38.8 mm standard length, Gilan, Caspian Sea near Astara (ca. 38º26'N, ca. 48º53'E);
CMNFI 1970-0581, 4, 41.0-71.0 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1970-0586, 8, 27.3-50.4 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak(36º50'N, 53º56'E);
CMNFI 1970-0587, 165, 15.7-47.9 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1970-0590, 11, 28.6-54.0 mm standard length, Mazandaran, Shesh Deh River near Babol Sar (ca. 36º43'N, ca.52º39'E;
CMNFI 1979-0470, 17, 16.6-43.4 mm standard length, Mazandaran, stream 21 km west of Alamdeh (36º35'N, 51º43'E);
CMNFI 1979-0471, 1, 59.1 mm standard length, Mazandaran, Caspian Sea 14 km west of Alamdeh (36º35'N, 51º48'E);
CMNFI 1979-0477, 1, 16.7 mm standard length, Mazandaran, Gorgan Mordab at Bandar-e Shah (36º54'N, 54º02'30"E);
CMNFI 1979-0788, 1, 22.7 mm standard length, Mazandaran, Gorgan River at Khadje Nafas (37º00'N, 54º07'E);
CMNFI 1980-0120, 9, 48.8-73.2 mm standard length, Mazandaran, Babol River at Babol Sar (36º43'N, 52º39'E);
CMNFI 1980-0126, 2, 217.5-244.2 mm standard length, Gilan, Caspian Sea near Bandar-e Anzali (37º28'N, 49º27'E);
CMNFI 1980-0127, 5, 22.8-52.8 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0129, 18, 28.3-64.2 mm standard length, Mazandaran, Tajan River estuary (36º49'N, 53º06'30"E);
CMNFI 1980-0136, 13, 35.9-76.8 mm standard length, Mazandaran, Fereydun Kenar River estuary (36º41'N, 52º29'E);
CMNFI 1980-0146, 2, 88.8-96.6 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak(36º50'N, 53º56'E);
CMNFI 1980-0149, 10, 36.0-83.2 mm standard length, Gilan, Chabak River (37º21'N, 49º50'E);
CMNFI 1980-0157, 77, 25.7-50.1 mm standard length, Mazandaran, Gorgan River estuary (36º59'N, 53º59'30"E);
CMNFI 1993-0144, 2, 78.4-92.2 mm standard length, Mazandaran, Neka Power Plant (36º51'48'N, 53º23'24"E).
Liza subviridis
(Valenciennes, 1836)

Common names
biah or biah arabi at Bushehr, garriz at Bandar Abbas; meid or maid
(for small specimens); kefal posht sabz.
[biag, meid or maid, biah akhtar, beyah akhtar in Arabic; greenback mullet, greenback grey mullet, silver mullet].
Systematics
Originally described from Malabar, India. Synonyms are Mugil dussumieri Valenciennes in Cuvier and
Valenciennes, 1836 and Mugil jerdoni Day, 1876. Placed in the
genus Chelon Rose, 1793 in Randall (1995b).
Key characters
The preorbital bone does not occupy the whole space between the eye
and upper lip as in all other mullets.
Morphology
The head is wide and flattened with head length 23-27% of standard
length. There is a well-developed adipose eyelid, covering about half
of the iris, but this is absent in young fish. The lower lip has a
weak to marked symphysial knob (authors differ) and both lips are
thin. The upper jaw extends back on a level with the anterior nostril
to the anterior eye rim. Upper jaw teeth are in several rows and
ciliiform lower jaw teeth in one row. The preorbital bone is strongly
notched anteriorly and only occupies three-quarters of the space
between the eye and upper lip. Scales are regularly arranged and are
dense on the second dorsal and anal fins. The second dorsal fin origin
lies over the anterior half of the anal fin base. The pectoral
axillary scale is rudimentary or absent. The pectoral fin does not
reach back on a level with the first dorsal fin origin and is 74-76% of head length.
The first dorsal fin has 4 spines, the second dorsal fin has 1
spine and 8-9, usually 8, soft rays and the anal fin has 3 spines and
8-9, usually 9, soft rays. Scales in lateral series 27-32. Transverse
scales 10-11, usually 11. Pyloric caeca 4-5.
Ahmed and Hussain (2003) describe the larvae of this species from Khawr az Zubayr
and the Shatt al Arab estuary. Al-Hassan and Madhi (1989) studied enzyme polymorphisms in populations from
the Shatt al Arab, Khawr az Zubayr and Kuwait but found no evidence of
sub-population differentiation. A single stock is found in the northern Persian Gulf.
Sexual Dimorphism
Unknown.
Colour
The back is dark to light green or greyish-green, the flanks
silvery to white and the belly white. There may be 3-7 blackish
stripes along the flank but these may not always be obvious. The
caudal fin is bluish and has a black margin, other fins are hyaline.
Size
Attains 39.5 cm total length and 270 g (Al-Hassan and Al-Seyab, 1992).
Distribution
Found in the Persian Gulf and eastwards to India, China, northern
Australia and Polynesia.
Reported from the Shatt al Arab and Hawr al Hammar, Iraq near
Iranian Khuzestan by Al-Hassan et al. (1989), Al-Hassan and Al-Seyab
(1992) and Hussain et al. (1997; 2001).
Records of Liza dussumieri from the Tigris River at Amara
and Baghdad, the Euphrates River and the Shatt al Arab by Kennedy
(1937), Khalaf (1961), Mahdi (1962), Al-Nasiri and Hoda (1976) and
Hussain et al. (1989), if correctly identified, are referred to
this species (Thomson and Luther in Fischer and Bianchi, 1984).
Recorded from the lower Mand River in Bushehr Province at 28°14'N,
51°51'E, 28°11'N, 51°32'E and 28°07'N, 51°24'E by
M. Rabbaniha (pers. comm., 1995) and from Khuzestan in the Zohreh and
Bahmanshir rivers and the Hawr al Azim by Najafpour (1997). Records
from Iran could be L. vaigiensis (q.v.). No specimens have been
examined by me to confirm these anecdotal and literature records.
Zoogeography
A widely distributed marine species, this mullet also enters and is
resident in fresh waters but is dependent on reproduction of marine
populations to maintain the freshwater ones.
Habitat
This species lives in the sea, particularly in coastal waters,
lagoons and estuaries, but regularly enters fresh water.
At a freshwater station on the Shatt al Basrah Canal, Iraq with salinities up to 3.5‰, Al-Daham and Yousif (1990)
found this species to be dominant, comprising 59.6% by number and 40.0% by
weight. Eggs of this species and L. klunzingeri were most abundant in the
inner part of the Shatt al Arab estuary in February with larvae most
abundant in February and April. The temperature range for several sites was
12.5-22ºC and salinity 32-38‰. Temperature is the most important factor
influencing spawning (Ahmed and Hussain, 2000). This was one of the more
abundant species in the recovering Hawr al Hammar of southern Iraq in 2005-2006
and in the marshes in the 1980's (Hussain and Ali, 2006).
Age and Growth
In the Shatt al Basrah Canal of southern Iraq, populations of this species
are comprised of age groups 1 to 6 but most fish are in age groups 1 and 2
(Al-Daham and Wahab, 1991). Age 2 dominates from February to April and age 1
from June to January. Females are slightly longer in each age group and have a
longer life span than males. The oldest females are age 6 and 303 mm and the
oldest males age 4 and 251 mm. Males are less numerous than females with a sex
ratio 1.0:1.4. Maturity is attained at age 1 and the smallest mature male is 137
mm and the smallest mature female is 142 mm. Ali et al. (1999) examined
the stock of this species in Khawr Abdullah and found the infinity length (L∞)
to be 34.3 cm total length (TL), the growth coefficient (K) 0.31, annual total
mortality (Z) 1.06, natural mortality (M) 0.70 and fishing mortality (F) 0.366.
The exploitation rate (E) was 0.34 indicating that the stock was slightly
exploited during September 1989-August 1990. The length-weight relationship was
W = 0.0000208 L2.985. Mohamed et al. (1998) found, for fish
from the northwest Arabian (= Persian) Gulf, a length-weight relationship of W =
1.34 x 10-5 L2.961, condition factor ranged from 0.98 to
1.21 and the growth model was Lt = 308 (1-e-0.225 (t + 0.618)).
Ali et al. (1999) summarise annual growth of this species from their
own and other studies as follows, attributing differences to differing
methodologies:-
|
Author |
sex |
L∞ |
K |
1 |
2 |
3 |
4 |
5 |
6 |
|
Wahab (1986) (Shatt al Basrah) |
♂
♀ |
29.4
40.2 |
0.39
0.151 |
14.6
15.5 |
19.3
20.5 |
22.1
23.0 |
24.0
25.2 |
-
27.3 |
-
29.0 |
|
Al-Hisnawi (1990) (Khawr az Zubayr) |
♂
♀ |
25.2
48.9 |
0.474
0.141 |
11.4
11.35 |
16.55
16.78 |
19.84
20.92 |
21.85
24.31 |
-
27.82 |
-
30.5 |
|
Mohammed et al. (1998) (Northwest Arabian
Gulf) |
♂ + ♀ |
30.8 |
0.225 |
9.4 |
13.6 |
17.0 |
19.7 |
21.9 |
23.7 |
|
Ali et al. (1999) (Khawr
Abdullah) |
♂ + ♀ |
34.3 |
0.31 |
8.9 |
15.5 |
20.37 |
23.97 |
26.65 |
28.64 |
Muhsin (1988) determined protein, lipid and ash content of fish taken monthly
from the marine Khawr az Zubayr and found fluctuations to follow the reproductive
cycle. Lipid content of the liver increased in winter and of the carcass in
winter and spring, the pre-spawning period, along with increases in the
gonadosomatic and hepatosomatic indices. The latter indices peaked in March and
decreased sharply until June. Total protein decreased during winter and spring
from the carcass and in spring from liver and ovaries as they were used for
energy and to support maturation of the ovaries.
In Malaysia, maturity is attained at 95-115 mm for males and 105-115 mm for
females (Chan and Chua, 1980).
Food
Food items are algae, diatoms and detritus extracted from ingested mud and
sand. Al-Hassan and Madhi (1987) include higher plants as part of the diet and
Al-Hassan and Al-Seyab (1992) copepods for Iraqi waters. Mohamed et al.
(1998) found this species to be a detritivore in the northwest
Persian Gulf. Hussain and Ali (2006) examined feeding relationships among
fishes in the Hawr al Hammar, Iraq and found this species to be a herbivore, 75.2% of
the diet being plants and algae. In another study of the recovering Hawr al Hammar, diet
was 80.84% algae and 15.66% diatoms with amounts of crustaceans and plants being
less than 10% each (Hussain et al., 2006).
In Malaysian estuaries and coastal areas, Chan and Chua (1979) working in
Malaysia found this species to feed only on zooplankton when less than 12 mm
standard length, becoming bottom feeding at 16-20 mm on zooplankton, diatoms and
detritus and by 24 mm zooplankton was absent but filamentous algae was added
where available. The proportion of the various food items varied with the locality.
Reproduction
Spawning occurs in the sea, with all eggs released at once in a Malaysian
population (Chan and Chua, 1980). Al-Daham and Wahab (1991) were unable to
locate eggs and larvae in fresh waters of Iraq. They believe that adults leave
the Shatt al Basrah Canal to spawn in the sea. However, Al-Hassan and Madhi
(1987) give a reproductive season of February through April in the Shatt al Arab,
Iraq and Ahmed and Hussain (2003) give March to June in the offshore area near the
mouth of the Khawr az Zubayr and Shatt al Arab. Muhsin (1988) determined
April-June to be the spawning season, with mature ova released in May, in the
Khawr az Zubayr (see above). Male and female gonads are best developed in
February to March and spawning is deduced to have occurred from March to May,
possibly offshore in the Persian Gulf. In Malaysia, spawning can
occur from June to November and in the off-season (Chan and Chua, 1980). In
Iraq, fry 27-40 mm in length are captured from April to June in fresh water. The
adult mullet return to fresh water after spawning. Fecundity was 133,224-295,065
eggs for fish 182-243 mm total length, this being higher than in Malaysia at
40,000-145,000. Fecundity is somewhat lower than in other mullets but may be
attributed to year round spawning at some localities (Chen and Chua, 1980).
Al-Hassan and Madhi (1987) give a fecundity of 549,278 eggs with egg diameter up
to 0.65 mm and Al-Hassan and Al-Seyab (1992) give 580-590,000 eggs for their
large specimen from Hawr al Hammar.
Parasites and predators
Unknown.
Economic importance
This species forms part of coastal fisheries in shallow waters and
is caught with a variety of nets, particularly seines, stationary
traps, gill nets and cast nets. It forms a substantial part of
brackish water fisheries in southern Iraq, being the most abundant
species in estuaries, and economically the most important mullet (Al-Daham
and Wahab, 1991). Ali et al. (1999) gave a
catch of 35 t in Khawr Abdullah, Iraq, almost 6% of the marine catch. Mohamed et al.
(1998) gave a value of 3.4% of the commercial catch for this species with catch
rates in the northwest Arabian (= Persian) Gulf of 0.15 kg/h in June to 0.75
kg/h in October. This species is potentially important in fish
culture as it is herbivorous. Consequently ponds can be fertilised
inexpensively and plant food supplements such as rice bran and peanut
meal can be given. Chan and Chua (1979) recommend that fish for
stocking should be at least 30 mm standard length as their bottom
feeding habit is then most suitable for pond culture.
The proximate chemical composition measured in fish from the Basrah fish
market was moisture 76.16%, protein 16.82%, fat 5.1% and ash 1.82% and the
general condition was a maximum of 1.78 (Ali et al., 2004). It is
characterised as a fatty fish according to a lipid content 9-14% by wet weight
of muscle in autumn (Hantoush et al., 1999).
Conservation
Population numbers and trends are unknown so conservation
requirements cannot be ascertained.
Further work
Materials of freshwater mullets should be collected in Iranian
waters and studied for their identity and biology.
Sources
Description based on literature sources, particularly Blegvad and
Løppenthin (1944), Fischer and Bianchi (1984), Talwar and Kacker
(1984) and Thomson (1997).
Liza vaigiensis
(Quoy and Gaimard, 1824)
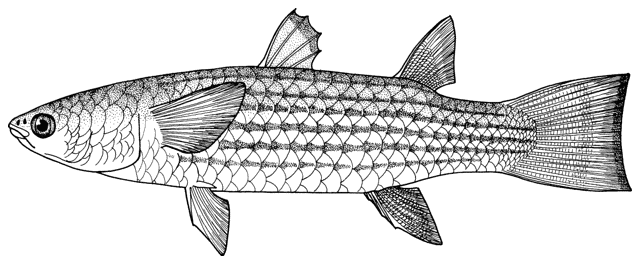
Common names
[massiyat or biah in Iraq; square-tail mullet, diamond-scaled mullet, diamond-scale grey mullet, brown-banded mullet].
Systematics
Originally described from Waigeo, Indonesia. Placed in the genus Ellochelon Whitley, 1930 in Randall (1995b).
Key characters
This species is uniquely characterised in this area of the northen
Indian Ocean by the caudal fin being only slightly emarginate. All
other local mullets have an obviously forked tail fin.
Morphology
The preorbital bone is notched anteriorly and is wide, filling the
space between the eye and the upper lip. The head is broad and
flattened on top, with head length 24-30% of standard length. The eye
has adipose tissue around its margin. Lips are thin but the lower lip
has a high symphysial knob. There are ciliate teeth on the lips, in
1-2 rows on the upper lip and 1 row on the lower lip. Larger fish lose
the upper lip teeth at >250 mm standard length and the lower lip
teeth at ca. 100 mm standard length. About half of the anal fin base
lies anterior to the level of the second dorsal fin origin. The
pectoral axillary scale is rudimentary or absent. The pectoral fin
does not reach back on a level with the first dorsal fin origin. The
second dorsal and anal fins are densely scaled. Hoda (1978) describes the larva in Iraq.
First dorsal fin spines 4, second dorsal fin with 1 spine and 7-9
soft rays, anal fin with 3 spines and 8 soft rays, pectoral fin with
15-18 branched rays, lateral series scales 24-29, transverse scales
(from first dorsal fin origin to pelvic fin origin) 9-11, and
predorsal scales 15-16. Gill rakers 40-61.
Sexual Dimorphism
Unknown.
Colour
The back is olive brown to pale olive, the flanks silvery and the
belly white with yellowish tinges. Upper flank scales have
characteristic brown blotches which appear to run together to form
about 5 stripes. The iris has yellow patches. Fin margins are dusky to
black but the body of each fin is yellowish. Young fish have black
pectoral fins but in adults the lower half is yellow. The caudal, anal
and pelvic fins are a bright yellow in young fish while the second
dorsal fin is dark on its distal half.
Size
Reaches 63 cm.
Distribution
This species is widespread in the tropical Indo-Pacific Ocean from
Africa to Japan and Tahiti including the Persian Gulf. It also enters
fresh waters. Reported from the Shatt al Arab, Iraq near Iranian
Khuzestan by Al-Nasiri and Hoda (1976). A single specimen from
Khuzestan, Iran confirms its presence in fresh water. Akbari (1998) records it
from coastal and estuarine waters in Hormozgan. Other records
from Iranian rivers draining to the Persian Gulf (e.g. Dez, Karun,
Dalaki and Mand rivers) may be this species but could also be L. subviridis.
Zoogeography
A widely distributed marine species, this mullet also enters and is
resident in fresh waters but is dependent on reproduction of marine
populations to maintain the freshwater ones.
Habitat
This species is found in coastal waters over sand, in lagoons and
over reef flats and commonly enters fresh waters. It apparently enters
fresh water during the rainy season elsewhere in its range, ascending
only to tidal limits.
Age and Growth
Unknown.
Food
Food items are algae, diatoms and detritus whih are ingested along
with associated sand and mud.
Reproduction
Fry are known to occur along the sea coast.
Parasites and predators
None reported from Iran.
Economic importance
This mullet is common in commercial catches along the west coast of
India. It is caught in castnets, stakenets and beach seines.
Conservation
Population numbers and trends are unknown so conservation
requirements cannot be ascertained.
Further work
Materials of freshwater mullets should be collected in Iranian
waters and studied for their identity and biology.
Sources
Description based on literature sources, particularly Fischer and
Bianchi (1984), Talwar and Kacker (1984) and Thomson (1997).
A single specimen from Khuzestan, Iran was examined measuring ?.
Genus Mugil
Linnaeus, 1758
This genus is characterised by a strongly flattened head, a
transverse mouth with the lateral cleft short, thin lips, lower jaw
with a symphysial knob and lower jaws meeting at symphysis with an acute angle, the maxilla end is not visible when the mouth
is closed, the anteroventral edge of the preorbital is serrate and straight and
the ventral edge is slender and pointed, no true teeth in the jaws, a very well-developed adipose
eyelid reaching to, or nearly to, the pupil, two pyloric caeca,
pharyngobranchial organ with a single valve, no vomerine or palatine
teeth, scales cycloid or feebly ctenoid, and pectoral axillary scale
long and pointed. Thomson (1997) and Harrison in Miller (2003) give a wide range of generic
characters but since only one species is found in Iranian waters, and
that rarely, the species description suffices here.
Mugil cephalus
Linnaeus, 1758
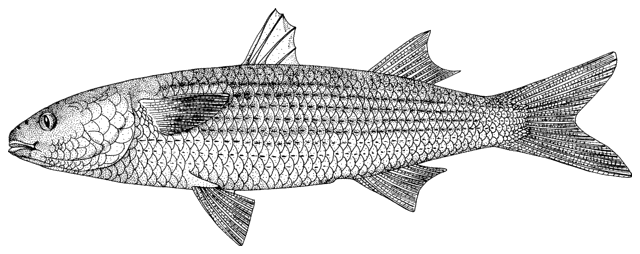
Common names
kefal or kafal, biah-e sarpahn (= flathead mullet), كفال
خاكستري (= kafal-e khakestari).
[biah, biyah, biaha Ramadiyah, anubah or asfatiya in Arabic;
striped mullet, loban, common, grey, jumping, flathead grey, sea, or
river mullet; mighach, minghaj or karul in Pakistan].
Systematics
Mugil Cephalus was originally described from the Atlantic
Ocean at the shores of Europe, entering rivers. No major relevant synonyms.
Key characters
This species is distinguished from Liza species by having
only 2 pyloric caeca, the posterior end of the maxilla is straight
rather than curved down behind the premaxilla and visible behind the
corner of the closed mouth, adipose tissue on the eye reaches the
pupil in adults, and the tip of the jaw end is on the line of the gape.
Morphology
There is a prominent adipose eyelid which leaves only a narrow slit
over the pupil and extends twice as far behind the eye as in front of it.
First dorsal fin with 4 spines, second dorsal fin with 1-2 spines
and 6-9, usually 8or 9, soft rays, anal fin with 3 spines and 7-9, usually
8, soft rays (below 5 cm there are 2 spines and 9 soft rays), pectoral
fin branched rays 14-17, and pelvic fin branched rays 5.
Lateral series scales 36-46 with 3-5 on the caudal fin, transverse
scale rows 14-15, predorsal scales 24-26 and cheek scales in 3-4 rows.
Scales on the head extend forward to the tip of the snout. Scales are
found on the caudal fin rays and at the bases of the anterior dorsal
and anal fin rays. The pectoral axillary scale is very long, about
one-third the length of the fin, and there is a pelvic axillary scale.
Scales bear an anterior mid-margin notch, the anterior corners are
very square and sharp, upper and lower margins are parallel and the
posterior margin is rounded. There are few radii (as few as 5) running
from a subcentral posterior focus to the notch. Circuli are numerous
and fine and the exposed scale surface is coarse. Gill rakers 24-80,
long, fine and crowded, count increasing with growth of fish. Pyloric caeca number 2 and the gut is long
with numerous loops, complexly coiled. The chromosome number is 2n=48
(Klinkhardt et al., 1995).
There is a distinct axillary scale at the pectoral fin, 33-36% of
pectoral fin length. The pectoral fin reaches the tenth postopercular
scale but not the level of the first dorsal fin origin; folded forward
it reaches the eye but not the pupil.
A single Iranian specimen has 4 first dorsal fin spines, 8 soft
second dorsal fin rays, 8 anal fin soft rays, 15 pectoral fin branched
rays, 5 branched pelvic fin rays, 43 scales in lateral series, and 24
total vertebrae.
Sexual dimorphism
Females grow larger and weigh more than males.
Colour
Back bluish-grey to olive-green or greyish-bown, flanks silvery and belly silvery
to white. Each scale has a dark spot which line up to give the
impression of 6-12 indistinct brownish or grey stripes. The upper rear
corner of the operculum has a golden spot. There is dark purple blotch
at the pectoral fin base. The dorsal and pectoral fins are a dark
blue-grey while the anal and caudal fins are a yellowish-green. The
dorsal and caudal fins have dusky margins. Young are a bright,
iridescent silver. Estuarine and freshwater fish may be duller overall than
marine fish.
Size
Attains 1.2 m and 7 kg.
Distribution
Found world-wide between about 51°N and 42°S. Introduced into the Caspian Sea in 1902, it has not been observed
there since and there appear to be no valid Caspian records for Iran (Dmitriev,
1946; Baltz, 1991). The introduction probably failed because the
pelagic eggs were not buoyant at the low Caspian salinities (Baltz,
1991) although Harrison in Miller (2003) notes spawning has been induced in
fresh water. However Kiabi et al. (1999) report it from the southeast
Caspian Sea and the species is being cultured at Sari on the Iranian coast of
the Caspian Sea (Iranian Fisheries Research Organization Newsletter, 40 & 41:3,
2004; and see below). Kolodar and Abdoli (2004) report it from the Gomishan Lagoon but only in farms
there.
Reported from the Shatt al Arab, Iraq near Iranian Khuzestan by Al-Nasiri
and Hoda (1975b). Akbari (2002) records this species from creeks and coastal
waters of Hormozgan. It enters rivers of southern Iran from the Persian Gulf
and Sea of Oman, notably the Sarbaz River.
Zoogeography
A widely distributed marine species, this mullet also enters and is
resident in fresh waters but is dependent on reproduction of marine
populations to maintain the freshwater ones.
Habitat
This mullet is found in surface waters of the sea and frequently
ascends rivers. It is common in coastal and estuarine waters. It
favours marine waters where the average monthly temperature is above
16°C and where summer temperatures rise above 18°C. Temperature ranges of 5-37°C are tolerated.
It can live in salinities up to 126‰. It is a fast swimmer and may leap out of the water.
Age and growth
Age at maturity for both sexes varies with water temperatures, at 1
year in the warmest waters, such as Florida, and as late as 6-8 years
in the Black Sea for example. Black Sea fish were 31-37 cm long at
maturity while in the Gulf of Mexico females were 25.8 cm and males
24.0 cm and larger. Life span lies between 11 and 21 years. Growth
rate varies with locality and ceases in warm temperate waters during mid-winter.
Food
Diet is comprised of microscopic organic matter taken in with
mouthfuls of sand. The sand probably helps grind up food in the
gizzard-like stomach. The food items are diatoms, blue-green and green
algae, foraminifers, small crustaceans and detritus, and sometimes
plankton. Rarely, swarming marine worms are eaten rather than
microscopic items, perhaps as an additional energy source prior to
spawning. Juveniles eat invertebrates.
Reproduction
In the sea, spawning occurs in surface waters near the edge of the
continental shelf over deep water after a migration from rivers. In
the Sea of Marmara, Turkey, this takes place in October and in southern India
in October-May. Eggs and larvae drift with ocean currents until they
are 20-30 mm about 2-3 months after hatching. Fry make their way into
estuaries and juveniles live in estuaries and lower reaches of rivers.
Each female is attended by several males during spawning. The males
nudge and press against the female's abdomen. The fish may quiver and
cease swimming momentarily, sometimes rising to the surface.
Fertilisation is external. Spawning occurs once yearly and some
females only spawn in alternate years after first maturity. Fecundity
is up to 4,800,000 non-adhesive, straw-coloured eggs and egg diameter
reaches 1.08 mm (some reports cite 7 million eggs). Hatching occurs in about 2 days.
Parasites and predators
Eslami and Anwar (1971) record the cestode Caryophyllaeus
fimbriceps from this species on the Caspian coast of Iran. In the
absence of valid records for this fish in Iranian Caspian waters, the
host may have been another mullet species.
Economic importance
Fisheries take place around coasts world-wide as the mullet merge
into migratory schools. In Hormozgan, is is caught in coastal waters by cast net
and stake-net but only at 1.33% by weight and 0.19% by frequency compared to
other edible fishes and with a frequency of 5% compared to other mugilids.
World-wide, it is sold fresh, frozen or salted and the roe
is made into a type of caviar. This species is also widely cultured,
for example in Israel and India, with yields up to 2434 fish per
hectare. It has been reported as being ciguatoxic (intermittently
poisonous through feeding on toxic food) (Bagnis et al., 1970)
and as being ichthyoallyeinotoxic (hallucinogenic fish poisoning)
(Halstead, 1967-1970; 1978). The latter is a sporadic and mild form of
poisoning. This species is rare in Iranian freshwaters and unlikely to
be health hazard there (Coad, 1979b).
Fingerlings from Taiwan, Egypt and Hong Kong have been cultured in
enclosed ponds in Mazandaran and the Gomishan Wetland (A. Matinfar,
pers. comm., 1995; Annual Report 1993-94, Iranian Fisheries
Research and Training Organization, Tehran, p. 43-44, 1995; Annual
Report, 1994-1995, Iranian Fisheries Research and Training
Organization, Tehran, p. 31, 1996; Annual Report, 1995-1996,
Iranian Fisheries Research and Training Organization, Tehran, p.
39, 42, 1997; Iranian Fisheries Research and Training Organization
Newsletter, Tehran, 18:5, 1997; Iranian Fisheries Research Organization
Newsletter, 40 & 41:3, 2004; Iranian Fisheries Research Organization
Newsletter, 53:3, 2008). 20,000 fingerlings, weighing about 0.5 g,
were imported to the Caspian Sea Ecology Research Center in Sari in 1993. Fry were cultured for 21 days on
plankton grown by manuring ponds with wheat and rice bran and soya.
Growth in salt water averaged 235 g and 28.4 cm in the first year and
544 g and 40 cm in the second year. Growth in fresh water averaged 216
g and 28.5 cm in the first year and 668 g and 38 cm in the second
year. Experiments on the mass production of Nannochloropsis oculata algae
used in the culture of rotifers and Mugil cephalus larvae
have been carried out in Golestan Province (Iranian Fisheries Research
Organization Newsletter, 37:3, 2003).
Mirhashemi Rostami et al. (2008) has examined mortality factors in fry
from 9 year old broodstock in Iran.
Sharifpour et al. (2003), Ghelichi et al. (2004), Yeganeh et al. (2005), Mirhashem
Rostamy et al. (2006) and Tehrani (2006) give further details on
culturing this mullet in Iran. Peak spawning was mid to late January with the
best time for artificial breeding using hormones being December. Up to 2.6
million eggs could be produced from a female with fertilisation varying from 10
to 95% and hatching rate between 0.008 and 88.9%. Ghelichi and Jorjani (2004)
state that this species is expected to play an important future role in fish
culture in Iran. They studied induced spawning in this species and found
17-α hydroxyprogesterone to be effective.
Gelichi et al. (2007) studied oocyte maturation after stimulation with
hormones and also found 17-α
hydroxyprogesterone to be the most effective steroids in final maturation.
Conservation
Population numbers and trends are unknown so conservation requirements cannot be ascertained.
Further work
The biology and frequency of occurrence of this species in fresh waters of Iran is unknown.
Sources
Thomson (1963) gives a synopsis of the biology of this species and
the above account is based mostly on his work. Distinguishing
characters are based on Trewavas and Ingham (1972).
Iranian material: OSU 4281, 1, 234.3 mm standard length, Baluchestan, Kalani, Sarbaz River (25º17'N, 61º24'E).
Atherinidae
The silversides or sand smelts are found in coastal areas of temperate to tropical
seas and in fresh water. There are about 60 species (Nelson, 2006) with only one
reported from the Caspian Sea and Iran. Most are small fishes with a
maximum length of 60 cm.
These small, silvery fishes have a moderately elongate body;
usually cycloid and moderately large scales; no lateral line but
sometimes a pit on each scale; small teeth in the jaws and sometimes
on the roof of the terminal, upwardly-directed mouth; wide gill
openings with branchiostegal membranes free of the isthmus; 5-6
branchiostegal rays; gill rakers usually long and slender; two
well-separated dorsal fins, the first with 3-10 unbranched but flexible
rays, and the second with 1-2 unbranched and the rest branched rays;
anal fin long; pectoral fins high on the flank; no pyloric caeca; egg
membranes with filamentous outgrowths; back bluish to greenish with
small melanophores but translucent; and a silvery stripe along the
flank, often distinctively outlined with black.
Silversides can occur in vast schools in inshore waters and are an
important item in the diet of other fishes. They have been used as
bait but are too bony to be much used as food. Their food is plankton.
Eggs are large and greenish. The sticky egg filaments entangle with
plants, rocks or sand as anchors until hatching.
Genus Atherina
Linnaeus, 1758
Members of this speciose genus are found in fresh and brackish
waters with a single representative in Iran. The record of Atherina
hepsetus Linnaeus, 1758 in the Caspian Sea by Quignard and Pras in
Whitehead et al. (1984-1986) is an error (Vasil'eva, 1994).
The body is compressed with a rounded belly, the mouth large and
terminal, jaws large, reaching back to the anterior eye level or
beyond, sides of the upper jaw are straight and premaxillaries
protractile, the dentary bone has a high central portion, a
preopercular notch is absent, rows of setiform teeth on the jaws,
vomer and palatines, cycloid scales extending onto the head, short
pectoral fins, vent nearer the pelvic fin origin than the anal fin,
numerous gill rakers, 5-10 flexible rays in the first dorsal fin, and the second
dorsal fin is similar in length to the anal fin with its origin above the anal
fin.
Atherina caspia
Eichwald, 1831
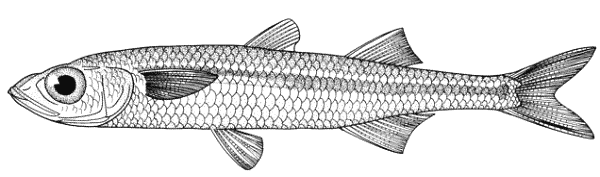
Common names
shisheh mahi (= glass fish), گل آذين ماهي (gol azin mahi, meaning flower decoration
fish), atrinka (from the Russian).
[aterinka or xazar aterini in Azerbaijan; kaspi aterinasy in Turkmenian; Kaspiiskaya aterinka or
Caspian silverside in Russian; Caspian sand smelt, Boyer's sand
smelt, big-scale sand smelt].
Systematics
Formerly called Atherina mochon pontica natio caspia
Eichwald, 1831 or Atherina mochon caspia in Caspian Sea literature. Atherina
mochon Cuvier, 1829 was described originally from Ivasa and Atherina
presbyter pontica Eichwald, 1831 from near Odessa, Ukraine. The Caspian Sea
taxon was also known as Atherina boyeri Risso, 1810. Atherina Boyeri was originally described from the sea shore
and lower course of rivers in the Département du Var, France.
Reshetnikov et al. (1997) give the date for this species as
1826. Atherina presbyter var. caspia Eichwald, 1831
described from "in Caspii maris littore australiore, sinu
balchanensi" (i.e. southern shore of the Caspian Sea, Balkhan
Bay) was considered a synonym. No types are known. Naseka and Bogutskaya (2009)
consider caspia to be a full species.
Syntypes of A. boyeri are in the Muséum national d'Histoire naturelle, Paris under MNHN
A-4342 (2) and MNHN B-860 (1) (Eschmeyer's "Catalog of Fishes", downloaded 29
August 2007).
Bamber and Henderson (1985) demonstrated how the morphology of this
species around Britain varies according to temperature and salinity
during embryonic development. Isolation of populations results in
local selection and random genetic drift and thus recognisable
morphologies but these were not recognised as distinct. Kottelat
(1997) reviews literature reports of variation in this species in the
Mediterranean and Black seas and neighbouring fresh waters and
concludes that lacustrine populations should be called A. boyeri
while marine populations are A. mochon. The status of Caspian
populations is not commented on.
The silverside in the Caspian Sea was referred to as Atherina
boyeri caspia by Savenkova and Asanov (1991) and by Vasil'eva
(1994; 1996b) and this seemed a reasonable step as the population is
isolated from other populations in the Atlantic Ocean and
Mediterranean Sea. The subspecies was characterised by a reduced number
of infraorbital bones (3 bones in Caspian Sea fish as opposed to 4 in
Black Sea fish; apparently infraorbital bones 2 and 3 fuse), a reduced
number of gill rakers (generally 19-27 as opposed to 27-37), and the
form of the maxilla (the inferior margin in Caspian Sea fish is always
smooth while in the Black Sea and Sea of Azov a "wing" is
usually developed). However Kiener and Spillman (1972) allocated
Caspian populations to this subspecies on the basis of larger size,
hardly an adequate criterion. Vasil'eva (1994) makes the allocation
based on a reduced number of gill rakers, reduction of the number of
bones around the eye to three (preorbital, infraorbital and
postorbital) and a smooth lower margin to the maxilla without a wing protuberance.
Dobrovolov and Ivanova (1999) studied two non-enzymatic proteins and 11
enzymes for putative Atherina boyeri and A. mochon pontica. They
concluded that these are distinct species and indeed the Black Sea fish are a
distinct species, A. pontica. They diverged 2.316MYA. These authors did
not examine Caspian Sea material. Miller in Miller (2003) followed a conservative approach, regarding the various
named and wide-ranging populations as representing a single polymorphic species.
Key characters
The two dorsal fins, cycloid scales, pectoral fin high on the flank
and the vent remote from the anal fin are characteristic.
Morphology
First dorsal fin spines 5-10; second dorsal fin with 1-2 spines and
8-15 soft rays; anal fin with 1-2 spines and 9-18 soft rays; pectoral
branched rays 10-17, usually 12-15; pelvic fin with 1 spine 5-7,
usually 5, branched rays; lateral series scales 37-53, possibly to 61,
see Vasil'eva (1994); total gill rakers 19-27 in the Caspian Sea, to
39 elsewhere, spinulose on the interior surface, and long and reaching
about 7 rakers along the arch when appressed; and vertebrae 39-52.
The anus is 4-5 scales in advance of the anal
fin. Scales are higher than broad, with slight indentations on the
otherwise straight dorsal and ventral margins, a rounded posterior
margin, and a wavy to rounded anterior margin with a protuberant
central point. There are no radii. Circuli are restricted to the
anterior third of the scale with a central and vertical roughened area
posterior to the circuli, presumably made up of fragmented circuli.
The focus is central. The lower jaw symphysis fits into a notch in the
upper jaws. The haemal arches of the anterior 4-7 caudal vertebrae are
expanded around the swimbladder. Gut s-shaped. The chromosome number
is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are: first dorsal fin spines 8(28), 9(5) or 10(1);
second dorsal fin soft rays 11(10), 12(21), 13(2) or 14(1); anal fin
soft rays 13(7), 14(15), 15(9) or 16(3); pectoral fin branched rays
12(1), 13(12), 14(14) or 15(6); pelvic fin branched rays 5 (34);
caudal fin branched rays 13(1), 15(30), 16(2) or 17(1); lateral series
scales 50(1), 51(10), 52(5), 53(3), 54(8), 55(3), 56(3) or 57(1); predorsal scales 17(1), 18(2), 19(12), 20(8), 21(3), 22(3), 23(1),
24(3) or 26(1); caudal peduncle scales 12(31), 13(2) or 14(1);
transverse scales from anal fin antero-dorsally 10(2), 11(6), 12(23)
or 13(3); total gill rakers 24(3), 25(4), 26(14), 27(8), 28(4) or
29(1); total vertebrae 45(1), 46(19), 47(7) or 48(7).
Sexual dimorphism
Females tend to be larger than males and there are some differences in
morphometrics and meristics (Ghoorbanalidoost et al., 2003).
Colour
General colour is given in the family account. The lateral band is strongly
developed and bright in Caspian Sea specimens. The belly is white, fins are pale
to translucent grey. Lagoon specimens are brownish or grey-brown on the back. The peritoneum is brown to black,
eggs being encased in a black peritoneum while the abdomen wall is a light brown.
Size
Reaches 11.8 cm and 17.6 g in the Atrak River (Savenkova and
Asanaov, 1991), to 14.5 cm standard length in the Caspian Sea basin
generally, and elsewhere to 19.0 cm standard length (Henderson and
Bamber, 1987). Kiener and Spillman (1972) found a maximum of 15.4 cm
in their Caspian Sea sample.
Distribution
Found from the shores of Europe to the Black and Caspian seas, and in the
Uzboi Valley of Turkmenistan. In Iran, it is reported
along the whole Caspian Sea coast, and from the the Safid River, Talar River, Anzali Mordab, and
its outlet the Sowsar Roga seasonally, the Bandar Anzali breakwater and beach, Farahbad, Mazandaran, and Gorgan
Bay (Abbasi et al., 1999; Holčík and Oláh, 1992; Roshan Tabari, 1997; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
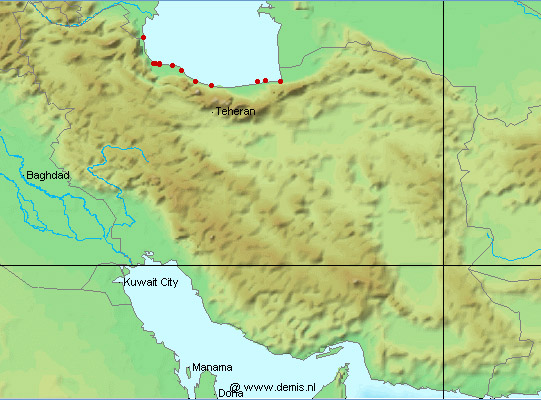
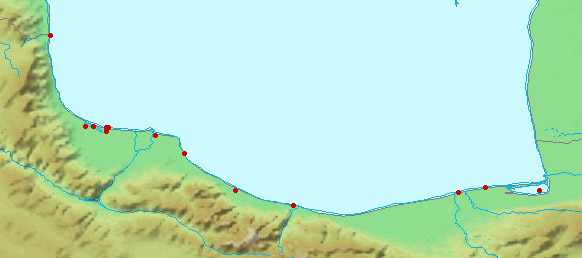
Zoogeography
This species probably entered the Caspian Sea from the Black Sea
during Khvalyn transgression (10-70,000 years B.P.) via the
Kumo-Manych Depression (Kosarev and Yablonskaya, 1994). Berg
(1948-1949) contends that it entered the Caspian from the Black Sea in
post-glacial times while most other Caspian fishes are relicts of
earlier transgressions or migrants from northern waters.
Habitat
The Caspian silverside is a schooling fish found at depths
exceeding 100 m but is concentrated at 10-20 m. Salinity tolerance is
high, up to 77‰ (Miller in Miller, 2003), up to
60‰ in the Atrak River (Savenkova and Asanov, 1991). Reproduction
can occur up to 42‰> while preferred levels are 3-12‰. A wide temperature
range is tolerated, 0-31ºC (Miller in Miller, 2003). It is also found in lagoons and river mouths, and enters
rivers to spawn, against currents up to 1.2 m/sec. It is the dominant species in
the Gomishan Wetland in spring, summer and autumn (Patimar et al., 2009). Holčík and Oláh (1992) report its apparent recent occurrence in the Anzali
Mordab in response to increased salinity there. It is also known from fresh water in Lenkoran.
Age and growth
Maturity is attained in the first year and life span varies from 1
to 5 years, 4-5 years usually in the Caspian Sea basin (Henderson and
Bamber, 1987). Miller in Miller (2003) summarises growth and population dynamics
from populations outside the Caspian Sea. Ghoorbanalidoost et al. (2003)
found populations in the south Caspian Sea to be 73.82 mm long on average and
3.15 g in weight, with age groups 1-3 years, average 1.7 years, total
length and length-weight relationship W = 0.00000615L3.02 and a sex
ratio of male:female = 0.47:0.53. Heydarnejad (2009) gave the length-weight
relationship for an Iranian sample as W = 0.0326TL3.033.
Food
Savenkova and Asanov (1991) report plankton, eggs and juvenile
fishes to be food items in the Atrak River of Turkmenistan. Some
populations also eat benthic organisms such as amphipods, worms and
molluscs. Iranian specimens contained encysted cladocerans and beetles
in their guts. It has a trophic plasticity, adapting to whatever conditions
obtain (Miller in Miller, 2003).
Reproduction
Spawning in this species is intermittent and occurs along the coast
from May to July, peaking in mid-May to mid-June (Savenkova and Asanov,
1991) or from April to August (Henderson and Bamber, 1987). Preserved
Iranian fish samples have relatively large eggs from at April to
September, e.g. 1.4 mm on 27 April, 1.6 mm on 14 May. Savenkova and
Asanov (1991) also studied the annual spawning migration into the
Atrak River (lower reaches in Turkmenistan, upper reaches shared with
Iran). Fish are caught in a fish ladder 18-19 km from the sea and in
the river. The first schools appear in the Atrek mouth as early as
mid-February at water temperatures of 8-10°C but the mass migration takes
place in mid to late March and the first half of April at 14-22°C.
Spawning occurs in March, April and May but most intensively in April
at flood water temperatures of 13.7-23.0°C.
There is a larger migration in high-water years. Sex ratio is about
1:1. A female may be able to spawn 5-6 times in one season so that egg
numbers and diameters vary within each individual for different
generations of eggs. Egg deposition in the Caspian Sea is associated
with the alga Cladophora to which the eggs are attached or
entangled by long filaments (up to 15) and this plant is present in
the Atrak. Egg diameters are up to 2.0 mm (3.5 mm in Europe, but possibly these
are water hardened) and the light yellow eggs number up to 568 (Gon and Ben-Tuvia, 1983)
although the Caspian Sea Biodiversity Database (www.caspianenvironment.org)
gives 5500 eggs per female (presumably total seasonal fecundity). Only one gonad develops.
Larvae are pelagic but may school close to shore.
Henderson and Bamber (1987) and Gon and Ben-Tuvia (1983) review the
reproductive biology of this sand smelt. It can rapidly adjust its
life history to a range of environments, from fresh water to coastal water.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984) as are the larger fishes such as Stenodus
leucichthys and Alosa saposchnikowii (Lönnberg, 1900a).
Economic importance
This species is food for a number of other fishes including such
economic species as sturgeons, Sander and the predacious shads.
It has been caught as a bycatch in kilka seine nets and used in fishmeal
production. In Europe it has been sold fresh or canned.
Conservation
Kiabi et al. (1999) consider this species to be of least
concern in the south Caspian Sea basin according to IUCN criteria.
Criteria include abundance in numbers, widespread range (75% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin.
Further work
The biology of this species in Iranian waters has been investigated by
Patimar (1995) (not seen).
Sources
Some meristics are taken from Altun (1999) and Trabelsi et al.
(2002).
Iranian material: CMNFI 1970-0507, 11, 22.2-56.3 mm standard length, Gilan,
Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0509, 5, 49.5-101.7 mm standard length, Gilan, pond at Hasan Kiadeh
(37º24'N, 49º58'E); CMNFI 1970-0543A, 7, 52.9-83.1 mm standard length, Gilan, Caspian Sea at Hasan
Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0563, 18, 39.8-112.5 mm standard length, Gilan, Kazian Beach
(ca. 37º29'N,
ca. 49º29'E); CMNFI 1970-0581, 5, 47.6-54.8 mm standard length, Caspian Sea near Hasan Kiadeh
(37º24'N,
49º58'E); CMNFI 1971-0343, 2, 65.9-72.5 mm standard length, Gilan, Langarud at Chamkhaleh
(37º13'N,
50º16'E); CMNFI 1979-0081, 3, 74.1-77.3 mm standard length, Mazandaran, Caspian Sea, 3 km west of Chalus
(36º41'N,
51º24'E); CMNFI 1980-0146, 4, 92.1-103.6 mm standard length, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak
(36º50'N,
53º56'E); and CMNFI 1980-0160, 2, 49.2-73.8 mm standard length, Iran, Caspian Sea basin (no other locality data).
Adrianichthyidae
Genus
Oryzias
Jordan and Snyder, 1906
Oryzias latipes
(Temminck and Schlegel, 1846)
Introduced to the Tedzhen River basin and Karakum Canal in
Turkmenistan (Aliev et al., 1988; Sal'nikov, 1995) but no
confirmed Iranian record. It is reported as reproducing in Iran by the
Food and Agriculture Organization, Rome (www.fao.org/scripts/acqintro/query/retrive.ide,
downloaded 28 July 1999) but this record is presumably in error and
needs confirmation by specimens.
Cyprinodontidae
The tooth-carps, killifishes or pupfishes are small fishes found in fresh, brackish and sea water. There are 9 genera and about 105 species found in tropical to warm temperate climates almost
world-wide. Iran has 7 described species, with more to be discovered.
This family is characterised by a moderately elongate and compressed body, rather cyprinid-like but with jaw teeth (hence the common and scientific names), body and head covered with scales, no
spines in the fins, no barbels, the mouth is very protractile and armed with comb-like teeth, the lower jaw is strong and robust as the dentary is expanded medially, 4-6 branchiostegal rays, gill
membranes free from the isthmus, lateral line absent or reduced to points, caudal fin rounded or truncate, pectoral fins set low on the body (in contrast to Poeciliidae), dorsal and anal fins short, no
adipose fin, no pyloric caeca, and no gonopodium (egg layers, in contrast to the livebearer subfamily in the Poeciliidae).
Wildekamp (1993) gives a general account of cyprinodontid species. These fish can be maintained in aquaria, and are very popular with aquarists. Specific requirements where known are given under each
species. Moderate to hard fresh water is used and for water with a total hardness less than 10dGH, sea salt can be added (about one teaspoon per 10 litres). Summer temperatures of 20-25°C are
good and although higher temperatures make the fish more active, they also age the fish more rapidly. Winter temperatures below 20°C imitate nature and are recommended. Weekly partial water changes are
suggested and live and frozen fish foods and even flake foods are eaten. Breeding occurs throughout the year in aquaria kept above 15°C, eggs are deposited on fine-leaved plants (thread
algae, Java moss or yarn mops) or even on aquarium filter sponges and gravel. Egg development takes 8-20 days, fry take about 2 days to absorb their yolk sac and will then consume baby brine shrimp.
These fish can be kept in outdoor ponds in milder climates (http://ark.aka.org/Eurasian.htm, downloaded 1 December 2003).
Genus Aphanius
Nardo, 1827
These tooth-carps are found around the shores of the Mediterranean, in Southwest Asia and as far as northeastern India and Somalia. There are about 23 known species.
Sethi (1960) advocated placing Aphanius in a separate family, Aphaniidae, but this did not find general acceptance.
The genus Aphanius Nardo, 1827 has been used for these tooth-carps for many years. However, Lazara (1995) designated Lebias fasciata Valenciennes, 1821 as the type species for
Lebias Goldfuss, 1820, making Lebias a subjective senior synonym of Aphanius. On this basis Lebias must be used rather than Aphanius but Lazara's type species
designation is invalid (Kottelat and Wheeler, 2001). Since Lazara (1995) could have chosen another species as type species, the change involves a large number of species and these species are
threatened and listed in various legislation, a petition is before the International Commission on Zoological Nomenclature to suppress Lazara's designation (Kottelat, 1997; Kottelat and Wheeler, 2001;
Wildekamp, 2001; Villwock et al., 2002). Wildekamp et al. (1999) present evidence that Lebias is a synonym of Cyprinodon.
Literature on these Mediterranean and Southwest Asian fishes may appear under either of these names or under Cyprinodon Lacepède, 1803, the latter now restricted to American species.
Bănărescu (1995) disagrees with Parenti's (1981) relationship of Aphanius to South American Orestias (see also Parker and Kornfield (1995), Stevenson (1997) and Costa (1997)
for conflicting views).
This genus is characterised by a thick oval body, large to moderate cycloid scales, the head flattened on top, a small, superior mouth with tricuspid teeth, the upper jaw bordered by the premaxillaries
only, lateral line system present only on the head, dorsal fin positioned somewhat posteriorly with1-2 unbranched rays and 7-13 branched rays, anal fin rays 1-2 unbranched and 7-14 branched, dorsal and
anal fins larger in males than in females, dorsal fin inserted opposite the anal fin origin (in contrast to Gambusia), and colouration of males and females distinct. Seyfali et al. (2002)
has 2n=48 for fish from the Damghan basin which Coad and Abdoli (2000b) suggest may be a distinct taxon.
Kamal et al. (2009, 2009) studied some biological characteristics of the Damghan population.
Aphanius is the only genus in the family currently recognised in Iran. However Parenti (1981) distinguishes derived members of the genus Aphanius as `Aphanius' without
formally describing a new genus. One of the distinguishing features of `Aphanius' is the reduction of cephalic sensory pores to neuromasts, a character found in A. mento, A. sophiae
and A. vladykovi in Iran.
Wildekamp et al. (1999) review the Turkish species which show scale reductions not seen in Iranian populations. Hrbek et al. (2002) outline the historical biogeography of the species
complex in central Anatolia, Turkey using mtDNA, testing the hypothesis of geographic speciation driven by early Pliocene orogenic events that may well be paralleled in Iran. Hrbek and Meyer (2003)
studied the phylogeny of Eurasian tooth-carps using mtDNA and observed a western Tethys Sea clade (all species except those listed below) with a middle Oligocene divergence into Iberian
Peninsula and Atlas Mountains, and Turkey and Iran sections. Late Miocene orogenic events were correlated with a large amount of genetic differentiation in Turkey (and presumably Iran). An eastern Tethys
Sea clade (dispar, ginaonis, mento, sirhani) had an Oligocene divergence into a freshwater clade inhabiting the Arabian Peninsula and neighbouring areas and a euhaline
clade inhabiting coastal areas from Pakistan to Somalia. Speciation is predominately vicariant-based but ecological factors played a significant role.
These fishes are known generally as gour-e khar in Farsi (= literally "striped donkey" which means zebra here although usually in Iran this term refers to the wild ass or onager),
kapurdandandar or kopurdandandar (= tooth-carp), or even آفانيوس (= Aphanius). Saefali (1999) is a recent study of these fishes in Farsi.
Iranian Aphanius away from coastal drainages are thought to be relicts of the Tethys Sea (Kosswig, 1955a; 1967; Bianco, 1995), having been trapped by the rising Iranian Plateau, rather than
invaders from the coast (Krinsley, 1970). Fossil tooth-carps, Aphanius kirgisicus and A. longipinnis have been described from Kyrgyzstan in Miocene deposits, far inland from the distribution
of living species (Yakovlev, 1959). Priem (1908) describes a fossil cyprinodont from the Miocene of Iran.
The systematics of the Iranian populations would repay careful study using biochemical and genetic techniques coupled with aquarium studies on behaviour and cross breeding. Certain populations,
isolated from others in discrete drainage basins, appear to be different on the basis of colour patterns yet show little morphological variation, e.g. the Damghan population, see Coad and Abdoli (2000b),
Saefali (1999) and Seyfali et al. (2002), the most northerly and easterly population in Iran characterised by large spots in females; populations in Fars outside the Lake Maharlu and Kor River
basins; and populations in the Namak Lake basin - all yet to be investigated in detail (Coad, 2000b). Since these patterns are used in mate recognition, the
populations could be distinct taxa. Huber (1996) has noted in cyprinodonts from Africa that many taxa can only be separated on the basis of colour in life, other characters overlapping. He suggests a
cyprinodont species definition which includes possession of at least one stable phenotypic characteristic, and this could be colour of the male fish. He does recommend that genetic isolation be
demonstrated where possible by karylogy, biochemical techniques or breeding experiments.
Seifali et al. (2003) compare A. sophiae and A. vladykovi
meristically and morphometrically. Saifali et al. (2004) found a suite of morphometric characters to be different between samples of A. vladykovi and fish from Qomy-Abad in Tehran Province, the Namak Lake basin.
The mosquitofish (Gambusia) is a niche competitor and may well eliminate these tooth-carps. Generally, Aphanius prefers springs, lakes, marshes, sea shores and hot springs. Flowing
water is usually avoided but they can be found near the source of springs flowing into salt lakes, constrained by hypersaline waters downstream. However they are tolerant of high salinities. They occur
in schools but males are aggressive to other males. Males are brightly coloured, often striped, while females are subdued with spots or faint bars.
A related Mediterranean species is reputed to be ichthyootoxic but this has not been demonstrated for Iranian species (Coad, 1979b). Symptoms of this egg poisoning are summarised under the genus
Schizothorax.
These small fishes are used in the aquarium trade as they tolerate a wide range of temperatures and salinities and are particularly colourful. As early as 1912, J. P. Arnold in Germany noted the
survival of imported Iranian Aphanius (possibly A. persicus) in aquaria at temperatures of 14-27°C, with a variety of plants such as Myriophyllum, Nitella, Riccia,
Cabomba, Salvinia and filamentous algae, with daphnia, cyclops, enchytraeids and red mosquito larvae as food, and egg laying apparently preferred on the Salvinia with 8-12 days for
hatching at 27°C.
Aphanius sp. from the Namak Lake is recorded as having the parasite Clinsotomum complanatum (Hosseinie, 1987).
Aphanius from Damghan were infected with the trematode Tetracotyle
sp. (Gholami et al., 2011).
Kamal et al. (2007; 2009) and Bakhtiyari et al. (2011) give some life history data on the Damghan and Shour
River of Eshtehard (west of Tehran) populations.
Food in the Shour River was mostly chironomids while crustaceans including
Daphnia were eaten at Damghan. Stable environmental conditions at Damghan
allowed more prey selection and a higher condition factor.
The Damghan fish lived longer and had a higher egg diameter while Shour
River fish had a higher L∞ and a shorter
reproductive period, again probably reflecting environmental constraints..
Aphanius dispar
(Rüppell, 1829)
Male
Common names
gour-e khar, kopurdandandar-e balehbolband (= tooth-carp with striped fin), kapurdandan-e balmband.
[harsun. batrikh or batrikh motakayer in Arabic; nambal in Pakistan; high-finned pupfish, Arabian killifish, mother of pearl fish].
Systematics
A detailed synonymy is given by Villwock et al., (1983), Wildekamp et al. (1986) and Wildekamp (1993). Hoedeman (1951) proposed a new genus for this species, Aphaniops, based on
the absence of a dermal sheath or genital pouch around the anterior anal fin rays, only 8-9 dorsal fin rays in contrast to 10-14 rays in Aphanius and 7-8 pelvic fin rays in contrast 5-7 rays. It
has not found general acceptance.
The type subspecies is found in all Iranian drainages (Krupp, 1983) although Aphanius dispar stoliczkanus (Day, 1872) has been recorded in earlier literature as the subspecies of these drainages
except the Tigris River basin for which Aphanius dispar richardsoni (Boulenger, 1907) is reported (Berg, 1949). Aphanius dispar richardsoni is limited to the Dead Sea valley of Israel and
western Jordan by Villwock et al. (1983) and Wildekamp (1993). Cyprinodon stoliczkanus Day, 1872 is regarded as a synonym of Aphanius dispar dispar by Krupp (1983) while Berg (1949)
places Bampur River, Baluchestan fish in this subspecies as a southern representative of Aphanius dispar with 6-9, rarely 10 dorsal fin rays as opposed to 9-10 rays in Aphanius d. dispar.
Hrbek and Meyer (2003) note that, based on their mtDNA study, the monophyly of A. dispar is strongly rejected and it does not constitute a species in terms of the phylogenetic species concept.
Reichenbacher et al. (2009) found that isolated populations in Saudi
Arabia may be capable of evolving into new species in a short period of time,
based on otolith morphology - evidently there is still much to be learned about
this nominal species.
Hybrids with Aphanius dispar richardsoni are reported from Israel (Goren and Rychwalski, 1978).
The lectotype of Lebias dispar as designated by Villwock et al. (1983) is in the Senckenberg Museum Frankfurt under SMF 821 with paralectotypes SMF 1988 (10). Further paralectotypes in the
Natural History Museum, London under BM(NH) 1860.11.9:152 listed as from Abyssinia (3 fish, 44.1-49.6 mm standard length) (Eschmeyer et al., 1996) but a label in the jar states that "status
as types doubtful, types in SMF range from 26.1-35.8 mm SL, locality in description and types given as 'Red Sea'".
Types of Cyprinodon stoliczkanus Day, 1872 are in the Zoological Survey of India, Calcutta (ZSI 1477, ZSI 1478), the Natural History Museum, London (BM(NH) 1889.2.1.2065-74, originally 21
fish, now 14 fish, 14.4-29.4 mm standard length when examined in September 2007) and the Australian Museum, Sydney (AMS B.7730-7731) (Whitehead and Talwar, 1976; Eschmeyer et al.,
1996; Ferraris et al., 2000). This species was originally described from a "stream at the village of Joorun, and also at Lodai, along the edge of the Rann" (= the salt water Rann of
Cutch, Sind, India).
The lectotype of Cyprinodon richardsoni as designated by Krupp and Schneider (1989) is in the Natural History Museum, London under BM(NH) 1856.5.2:4 with paralectotypes under BM(NH) 1856.5.2:5
(8 fish) (12.9-25.0 mm standard length, the largest being the lectotype) (Eschmeyer et al., 1996; personal observations).
Krupp (1983) did not find the variation in teeth numbers between different populations and subspecies observed by Berg (1949).
Key characters
The colour pattern is distinctive.
Morphology
Scales along the flank 24-35. Scales are squarish with an almost vertical anterior margin and protruding anterior corners, parallel dorsal and ventral margins and a rounded posterior margin with tiny
teeth. The anterior margin has a small central protuberance with shallow indentations above and below or almost straight margins to each anterior corner. Radii are present on the anterior field,
numbering 10-20 but mostly 14-16, and are almost horizontal and parallel. The exposed part of the scale has dimples rather than circuli. Circuli are few. The focus is subcentral posterior. There is
no pelvic axillary scale. Total dorsal fin rays 7-11, usually 9-10, total anal fin rays 8-12, usually 10-11 (the number of unbranched rays in the dorsal and anal fins varies from 1-3), total pectoral rays
12-18 and total pelvic rays 6-7, usually 7. Vertebrae 24-29. Al-Hassan (1982b) reports on abnormalities in the vertebrae including loss of the posterior part of the centrum and fusions. Teeth are
tricuspid with the central cusp concave at its tip and only slightly longer than the lateral cusps (Goren and Rychwalski, 1978), although in some fish seen by me the tip is rounded. There are 12-20 teeth
per jaw (Krupp, 1983). Gill rakers 11-20, modally 13-16, reaching the second adjacent raker when appressed. Some gill raker counts are difficult to make accurately as those at the anterior arch end are
minute and those at the dorsal end are partially concealed in flesh. The gut is coiled ?see my ginaonis paper. Reichenbacher et al. (2007) give a description of otolith morphology. Chromosomes
number 2n=48 (Klinkhardt et al., 1995; Esmaeili et al., 2008).
Karyotype 16Sm + 32St and arm number 32 (Esmaeili et al., 2008).).
Meristic values for Iranian specimens:- total dorsal fin rays 8(1), 9(39) or 10(10); total anal fin rays 9(2), 10(42) or 11(6); total pectoral fin rays 14(2), 15(16), 16(28), 17(3) or 18(1); total
pelvic fin rays 6(6) or 7(44); lateral series scales 24(2), 25(5), 26(11), 27(17), 28(7), 29(2), 30(1), 31(2), 33(2) or 35(1); total gill rakers 15(13), 16(16), 17(16) or 18(3); and total vertebrae ?.
Sexual dimorphism
Males have longer fins than females and are more brightly coloured. The dorsal fin is twice as long in the male and reaches the caudal fin when appressed. When expanded it is widely flared and
distinctive as is the enlarged anal fin.
Colour
Breeding males are brown-grey, grey or black-brown with iridescent blue-white flank spots and white and brown to light orange or light blue, irregular, narrow bars. The head has blue and orange tinges
and in particular there is an orange spot on the operculum postero-dorsally. Lips are blue-white. The flank over the pectoral fin has electric blue spots. The anterior belly becomes blue with pearl spots.
Scales have a dark margin. The dorsal fin is spotted light blue on a bright orange background and is barred. Barring may be irregular and in overall view in breeding males appears as speckling. Pectoral,
anal and pelvic fins are lemon-yellow. The anal fin has barring on the posterior 4 rays in breeding males. The caudal fin has 2-3 dark and light blue alternating bars, the last bar being yellow. The bars
are crescent-shaped, with concave side posterior. Males outside the breeding season are less brightly coloured with silvery on the flanks with a grey or black-brown back and irregular flank bars. Male
specimens from the Bampur River basin in Iran have a body speckled silvery-white to light blue, weak tail bars, blue and orange tinges to the head, an orange spot on the operculum and an orange-yellow
edge to the anal fin.
Young have pale brown flank bars on the caudal peduncle. Some young may have a few spots on the flank rather than bars. Females are brown-grey to silvery with 8-20 narrow flank bars and a dark-brown
back. The flank bars are dark brown and may have a tinge of orange, the interspaces silvery. Flank bars may number as few as 7 (Talwar and Jhingran, 1991). Females also have the orange operculum spot and
the blue spots over the pectoral fin. Female fins are hyaline. The peritoneum is brown to black.
Ross (1980) notes that Arabian specimens are brighter in waters with algae and plants; those found over sand and mud are duller, an effective camouflage. He also records variant colour patterns with
blue tails, or an overall gold colour, or with green or turquoise flank spots.
Size
Reaches 8.0 cm, but this may be an error as Krupp (1983) points out. He found fish only to 5.2 cm standard length although in Carpenter et al. (1997) 8 cm is given as a maximum. Wildekamp
(1993) records fish to 7.0 cm for males and 6.5 cm for females in total length. Fish from Ain Al Adhari, Bahrein reach 7.3 cm total length. Generally fish in fresh water are smaller than marine specimens.
Distribution
Found from Egypt, eastern Sudan and Ethiopia, and in the Red Sea through southern Southwest Asia and east to Rajasthan. Also in the eastern Mediterranean Sea (Kornfield and Nevo, 1976). The
subspecies Aphanius dispar richardsoni is found in the Dead Sea rift valley.
A specimen in the Naturhistorisches Museum Wien from the hot spring at Ginao (NMW 13799) may be mislabelled as several other collections from this hot spring are all clearly A. ginaonis.
This species is found in Iranian fresh waters from the Tigris basin to the Makran basin in water bodies draining to the Persian Gulf and Sea of Oman, islands in the Gulf such as Qeshm (OSU
8120), and in the endorheic
Hamun-e Jaz Murian basin. It is reported from the Shapur and Dalaki rivers (Gh. Izadpanahi, pers. comm., 1995),
Gudar River east of Anveh, Mehran River below Bastak, Sarageh River north of
Bandar Abbas, Galashkerd Lake near Minab, and the Kol. Shur, Hasan Langi and
Ghale-gazii rivers in Hormozdgan. Abdoli (2000) illustrates distributions in the Arvand, lower Zohreh (=
Hendijan), lower Hilleh, Mand, Kul, Hasan Langi and Minab rivers, lower reaches of rivers in the Makran basin from the Jagin to Bahu Kalat, the lower Halil and Bampur Rivers in the
Hamun-e Jaz Murian
basin, and the endorheic Mashkid River.
Zoogeography
Villwock et al. (1983) contend that an ancestor of A. dispar was present in the middle Miocene Transgression connecting the Mediterranean Sea to the Indian Ocean. The mesohaline
conditions, which developed in the Mesopotamian basin, were a good prerequisite for the development of A. dispar since, apart from some euryhaline species, there would be no competitors.
A. dispar spread from Mesopotamia through the Persian Gulf to India and the Red Sea. During the Pleistocene, it colonised inland waters.
Habitat
Highly tolerant of salinity, this species is found from the sea to fresh water habitats, including landlocked basins. Slightly saline waters in aquaria seem to discourage parasites (Andrews, 1983).
Salinities as high as 145‰ are tolerated (Lotan, 1971) and this fish can be moved abruptly from fresh to hypersaline water. Adaptation to high salinity waters involves a reduced osmotic
permeability of the gills and an increase in transportation of sodium chloride by the intestine (Skadhauge and Lotan, (1974). Lotan (1969, 1971, 1973) and Skadhauge and Lotan (1974) describe sodium,
chloride and water balance, and osmoregulation in this species. Plaut (1999) found that salinities above ~250% seawater seem to decrease swimming capabilities and routine activity rate and a
decrease in resting metabolic rate indicated stress. This species has been reported to survive in waters 38.4°C in Oman and only began to die at 45.1-46.0°C (Haas, 1982). Fish survived up to 40°C in
aquaria without acclimation (Lewis, 1970). Populations survive in isolated sections of streams with little food because of histological changes in the stomach (Ba-Omar et al. (1998).
Aphanius dispar occurs in shallow water and among vegetation over sand, rock or soft detritus bottoms.
Frenkel (1995) describes the effects of environmental factors on growth and reproduction in this species in Israel. Frenkel and Goren (1997) showed fish to be tolerant of a wide range of environmental
variables as measured against the gonado-somatic index (GSI) and ovary maturation stages. The GSI was not affected by temperature over a range of 18 to 37°C while maturation increased from 18 to
27°C but remained the same between 27 and 37°C. For salinities between 0‰ and 56‰ differences were only found in mean GSIs at the two extremes and at 0‰ ovaries contained only primordial germ cells.
GSIs and maturation stages only differed in feeding experiments when fish were deprived of food or fed at equal or greater than 1% of body weight per day. A decrease in photoperiod from 14L:10D (14
hours light, 10 hours dark) to 10L:14D caused a decrease in oocyte maturation stages and ovaries were no longer suitable for spawning. In aquaria, a pH of 7.5-8.5, temperatures above 27°C and water
hardness greater than 200 p.p.m. and the addition 4 heaped tablespoons of kosher salt per ten gallons of water (38 litres) favoured reproduction (Mackowiak, 1988). Alkahem (1989) found 50% mortality in
96 hours when fish were exposed to pH levels of 3.5-3.9.
Feulner (1998) reports that United Arab Emirate fish "hover" in the water column with the tail slightly curved to one side, presumably a feeding behaviour. Males and females in aquaria
swam in separate schools (Al-Daham et al., 1977).
Age and growth
Sexual maturity in ideal conditions may be attained as early as 7 months but full size takes a year. The length-weight relationship in southern Iraq was W = 1.2301 x 10-4
L2.4544 for males and W = 2.8357 x 10-6 L3.5112 for females. The average condition factor for males was 1.8900 and for females 1.6952. For a given length group males are
heavier than females and condition factors are higher for males (Huq et al. 1977). Muhsin (1987) studied the effect of differing food supply on the weight and chemical composition in females of
this species taken from the Al-Asafiah River, Basrah, Iraq but examined in tanks. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 41 Iranian fish measuring 2.37-3.56
cm total length. The a-value was 0.0012 and the b-value 3.214 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Iranian specimens have guts packed with filamentous algae and detrital remains. Al-Daham et al. (1977) found food in southern Iraq to be predominately filamentous algae and diatoms, with some
copepods, rotifers and, in one instance, a small winged insect. In contrast, Shafi and Shalli (1986) report a diet of beetles, ephemeropteran nymphs, rotifers, filamentous algae and plants
in southern Iraq. Younis et al. (2001b) found Shatt al Arab, Iraq fish fed on amphipods and copepods (29.9%), organic detritus (11.6%), and algae and diatoms (7.1%). In aquaria, Al-Daham et
al. (1977) found this species to prefer animal foods in contrast to Aphanius mento which preferred plant foods. Al-Akel et al. (1987) have observed the selective feeding behaviour of
this species at Al-Khurj, Saudi Arabia. Certain species of filamentous algae are selected over others, e.g. the blue-green alga Oscillatoria, the green algae Ulothrix, Spirogyra and
Crucigenia, the desmid Cosmarium, and the diatoms Cocconeis, Diatoma, Navicula and Rhoicosphania, and algal spores and zygotes. Haas (1982) observed this species in Oman to pick
at rocks and other substrates, take items at the surface, chase small fish and eat insect larvae. Gut contents are mainly desmids and diatoms, some filamentous algae and rarely insect larvae and one
snail. This species is an omnivorous surface feeder (Carpenter et al., 1997).
Reproduction
A female in spawning condition approached a male and appeared to feed on a plant or plant-encrusted rock in fish studied by Haas (1982) in Oman. Feulner (1998) reports fluttering of the tail by male
fish. The male places his chin on her nape and 2-3 eggs are spawned rapidly. The female presses her vent against the plant or rock vertical surface, the male arches his back into an s-shape and presses
his body and anal fin against hers and clasps her with his dorsal fin. Eggs are released singly with a quiver. The time elapsed from chin on nape to egg release is as short as 1-2 seconds. Reproduction
occurs throughout the day and throughout the year in Oman with 69% of females having ripe eggs (up to 41) in April-June. Peak spawning in southern Iraq is April to June when only a small proportion of
eggs in the single ovary are fully developed eggs (Al-Daham et al., 1977). Shafi and Shalli (1986) record up to 73 mature eggs in southern Iraq specimens with a diameter of 220 microns. Breeding
season was May-July. In Saudi Arabia mature and developing oocytes are observed in fish during the whole year. The egg cycle may be more than one year with spawning in March and April (El-Hawawi and
Al-Imam, 1984). On the Mediterranean coast of Egypt, spawning occurs from March to September with a peak in July and August. Maximum egg diameter is 2.2 mm and size at maturity for females is 30 mm total
length (Lotan and Ben-Tuvia, 1996). Iranian specimens have large eggs (2.0 mm) on 16 March but young fish (8.9 mm standard length) were caught on 26 November suggesting reproduction is almost year round.
Spawning areas have some water flow and vertical surfaces of plants or algae-encrusted rocks. Males defend a territory about 30 cm wide against other males and will display with erect fins if
approached. Parallel swimming, head to head confrontations, rapid circling, and flank bites were observed by Haas (1982). Two males will swim and chase each other in circles until one swims away with
fins depressed. Males will change their territory after one day defending it. In Israel, males of Aphanius dispar richardsoni do not show any territorial behaviour (Goren and Rychwalski, 1978).
This fish will spawn in a few months of birth in aquaria (Ross, 1987). Three pairs of fish yielded about 40 eggs per day in aquaria and these eggs hatched in two weeks at 27°C (Mackowiak, 1988).
Parasites and predators
This species is reported to be heavily infested with metacercariae of the trematode Clinostomum complanatum in eastern Saudi Arabia, up to 86.6% incidence with up to 41 metacercariae per fish,
the infestation being higher in females than males (Kalantan et al., 1987).
Economic importance
Al-Akel et al. (1987) suggest the use of this species as a control agent for filamentous algae.
Steven (1913) reported that this fish was responsible for the absence of mosquito larvae from streams in Karachi which otherwise appeared to be good breeding places. Christophers and Shortt
(1920-1921) thought that tooth-carps in southern Mesopotamia were a significant factor in lowering the malaria rate, presumably mostly this species. Sharma and Al-Daham (1979) compared the efficiency of
this tooth-carp with the mosquitofish for mosquito control under experimental conditions. The tooth-carp is more efficient when males and females were together than mosquitofish since males of the latter
expend time and energy defending territory. Mosquitofish are more active in pursuit of mosquito larvae than the tooth-carp when filamentous algae was present. Cloudy conditions and lower temperatures
slow consumption and both species prefer pupae to larvae of mosquitos, with females taking more pupae than males. The tooth-carp could consume nearly twice the amount of mosquitos than the mosquitofish
over a given period of time. Overall the tooth-carp compares favourably with the mosquitofish as a destroyer of the malaria-carrying mosquito. Homski et al. (1994) under laboratory conditions
found that Aphanius dispar is more successful than the mosquitofish in preying on the third and fourth instars and on pupal stages of mosquitos while the mosquitofish, with a smaller relative mouth
size, is more successful on the first two instars. Small fish of both species eat first instars exclusively. However, Aphanius dispar eats more second instar larvae under a vegetation cover since
the larger Aphanius are more capable of penetrating shallow water than mosquitofish. Aphanius dispar occurs in shallower water and among more vegetation than the open water mosquitofish.
The two species can be used to complement each other in control of mosquitos.
Ataur-Rahim (1981) found that this species will eat mosquito larvae in water storage tanks in Riyadh, Saudi Arabia. Fifteen fish consumed all larvae at a mean density of 5/100 sq cm water surface area
in a tank 3 by 2 m in a day. One fish, 5.2 cm long, ate 61 mosquito larvae. Haas (1982) fed this species mosquito larvae in the presence of abundant algae as an alternative food. An average of 96 larvae
are eaten each day. Etemadfar et al. (1983) studied the potential use of this species for malaria control in southern Fars, Bushehr and Hormozgan provinces. Louis et al. (1988) detail a
control strategy against malaria using this species in Djibouti as do Fletcher et al. (1992) in Ethiopia and Fareed and Baban (1995) in Yemen. Fletcher et al. (1992) showed a decrease in
sites harbouring mosquito larvae from 34% without the fish to 1.6% with the fish stocked.
Chandra et al. (2008) briefly reviews the use of this species in
biocontrol.
The studies of Frenkel and Goren (1997) in Israel show that the reproduction of this species can be controlled such that it has potential for mass production and could be used in mosquito control.
Carpenter et al. (1997) state that this species is used in mosquito control. Ross (1987) gives details of aquarium care and breeding of this species.
Conservation
Al-Johany and Yousuf (1993) show that Gambusia affinis has a wider range of temperature tolerance than this species and is better suited for a desert habitat in Saudi Arabia. The exotic
Gambusia is therefore a threat to A. dispar.
Al-Kaheh-Al-Balawi et al. (2008) found that Saudi Arabian populations are
declining from reduced availability of food, habitat degradation, chemical
contamination, introduction of exotics and exploitation and some of these
factors doubtless obtain in Iran.
Further work
This widespread species has been reported from a number of springs in southern Iran and, like A. ginaonis, may be distinct at some level. Molecular work would be useful in determining
the taxa involved and evolution in hot springs. The utility of this species in mosquito control in smaller water bodies of Iran where it also occurs naturally could be explored. Al-Daham et al.
(1977) found that this species in aquaria ate mosquito larvae "eagerly" compared to "very eagerly" for Gambusia holbrooki and "not eaten" for Aphanius mento.
Sources
Type material:
Iranian material: NMW 13799, 1, ?;
CMNFI 1979-0129, 1, 31.3 mm standard length, Fars, spring about 2 km north of Farrashband (28º54'N, 52º04'E);
CMNFI 1979-0137, 106, 8.9-37.7 mm standard length, Fars, stream outside Lar (27º40'N, 54º32'30"E);
CMNFI 1979-0138, 1, ? mm standard length, Fars-Hormozgan border, Rasul River drainage (ca. 27º32'N, ca. 54º58'30"E);
CMNFI 1979-0139, 2, ? mm standard length, Fars-Hormozgan border, Rasul River drainage (ca. 27º25'30"N, ca. 54º59'E);
CMNFI 1979-0140, 3, ? mm standard length, Hormozgan, Kul River drainage (27º14'N, 55º46'30"E);
CMNFI 1979-0141, 6, ? mm standard length, Hormozgan, Kul River at road bridge (27º17'30"N, 56º03'30"E);
CMNFI 1979-0142, 10, ? mm standard length, Hormozgan, Baghu River at Baghu (27º17'N, 56º28'E);
CMNFI 1979-0143, 118, ? mm standard length, Hormozgan, Hasan Langi River drainage (27º21'N, 56º50'30"E);
CMNFI 1979-0145, 1, ? mm standard length, Hormozgan, Geru River (26º55'N, 57º01'30"E);
CMNFI 1979-0148, 21, ? mm standard length, Hormozgan, Sarzeh River (27º30'30"N, 56º15'30"E);
CMNFI 1979-0151, 45, ? mm standard length, Hormozgan, Shur River drainage near Kahkom (28º02'N, 55º53'E);
CMNFI 1979-0177, 37, ? mm standard length, Hormozgan, Sarzeh River drainage (27º33'N, 56º14'E);
CMNFI 1979-0179, 13, ? mm standard length, Hormozgan, Sarzeh River drainage (27º36'N, 56º15'E);
CMNFI 1979-0181, 9, ? mm standard length, Hormozgan, Kul River drainage (27º17'30"N, 56º03'30"E);
CMNFI 1979-0182, 31, ? mm standard length, Hormozgan, stream on road to Bandar-e Lengeh (27º14'N, 55º46'30"E);
CMNFI 1979-0185, 24, ? mm standard length, Hormizgan, stream in Rasul Rievr drainage (27º06'N, 55º45'E);
CMNFI 1979-0187, 2, 26.2-33.2 mm standard length, Hormozgan, Sar Khun oasis (27º23'30"N, 56º26'E);
CMNFI 1979-0312, 7, ? mm standard length, Baluchestan, dam on Bampur River (27º11'N, 60º36'E);
CMNFI 1979-0313, 1, ? mm standard length, Baluchestan, Bampur River at Bangharabad (27º20'N, 60º46'E);
CMNFI 1979-0328, 18, ? mm standard length, Baluchestan, jube 2 km south of Bampur River near Bampur (27º10'30"N, 60º21'E);
CMNFI 1979-0352, 2, ? mm standard length, Khuzestan, marsh in Jarrahi River drainage (30º33'30"N, 48º48'E);
CMNFI 1979-0354, 8, ? mm standard length, Khuzestan, Karun River tributary (30º31'N, 48º19'E);
CMNFI 1979-0355, 1, ? mm standard length, Khuzestan, Karun River tributary (30º35'N, 48º22'E);
CMNFI 1979-0362, 2, ? mm standard length, Khuzestan, jube in Karkheh River drainage (31º42'N, 48º33'E);
CMNFI 1979-0379, 4, ? mm standard length, Khuzestan, Dez River (32º12'N, 48º27'E);
CMNFI 1979-0381, 1, ? mm standard length, Khuzestan, stream west of Shushtar (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0384, 7, ? mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0403, 25, check size range ?19.7-37.0 mm standard length, Bushehr, stream 23 km south of Kaki (ca. 28º11'N, ca. 51º43'E);
CMNFI 1979-0406, 48, 15.6-35.8 mm standard length, Hormozgan, stream near Bandar-e Charak (26º48'N, 54º18'E);
CMNFI 1979-0407, 3, ? mm standard length, Hormozgan, stream 30 km from Barvedun (26º51'N, 54º37'E);
CMNFI 1979-0408, 106, 14.6-48.4 mm standard length, Hormozgan, Mehran River (27º04'N, 54º35'E);
CMNFI 1979-0410, 10, ? mm standard length, Hormozgan, Mehran River (26º53'N, 55º17'E);
CMNFI 1979-0414, 12, ? mm standard length, Hormozgan, pool on road to Tiab (27º05'N, 57º02'E);
CMNFI 1979-0415, 1, ? mm standard length, Hormozgan, stream 18 km south of Genu (27º17'30"N, 56º20'E);
CMNFI 2007-0053, 4, ? mm standard length, Hormozgan, Sarzeh River (ca. 27º36'N, ca. 56º15'E);
CMNFI 2007-0057, 16, ? mm standard length, Hormozgan, Mehran River 4 km below Bastak (ca. 27º05'N, ca. 54º05'E);
CMNFI 2007-0058, 3, ? mm standard length, Fars, headwaters of Gowdar River (ca. 27º24'N, ca. 54º16'E);
OSU 8129, 1, 54.5 mm standard length, Hormozgan, Qeshm Island salt spring (no other locality data);
BWC95-30, uncatalogued, 3, ?, Khuzestan, Kupal (31º15'N, 49º10'E);
BWC98-6, uncatalogued, 24, ?, Hormozgan, Ab-e Garm-e Khamir (ca. 26º59'N, ca. 55º35'E);
BWC2000-6, uncatalogued, ?, ?, Khuzestan, Dez River (32º14.663'N, 48º20.112'E);
BWC2000-15, uncatalogued, ?, ?, Khuzestan, stream south of Shushtar (31º41.867'N, 48º59.581'E).
Comparative material:- BM(NH) 1920.3.3:193-202, 12, 21.0-30.5 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1920.3.6:9-10, 3, 17.1-26.8 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1949.7.21:1-11, 12, 18.4-42.4 mm standard length, Iraq, Bahr-el-Milk (32º35'N, 43º50'E);
BM(NH) 1974.5.22:30, 1, 35.2 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1974.5.22:31-32, 2, 37.4-44.4 mm standard length, Iraq, Al Faw ().
Aphanius ginaonis
(Holly, 1929)
Male
Common names
gour-e khar, kopurdandan-e Genu or kapurdandan-e Geno (= Genow tooth-carp).
[Holly's pupfish, Genow pupfish, Gheno pupfish].
Systematics
Cyprinodon ginaonis was originally described from "Hei$e Quelle vom Djebel Ginao nordlich von Bender Abbas, südöstliches Persien". This is the Ab Garm-e Ganow at 27°26-28'N,
56°18-20'E, north of the Iranian port of Bandar Abbas at the Straits of Hormuz.
Berg (1949) places this species in the synonymy of Aphanius dispar stoliczkanus (see A. dispar above) and Villwock et al. (1983) regard it tentatively as a synonym of Aphanius
dispar. Wildekamp (1993) is of the opinion that it may be a subspecies of Aphanius dispar. Hrbek and Meyer (2003) using mtDNA found this species to be deeply nested within the A. dispar
clade. Villwock (2004) has used cross-breeding experiments that demonstrate this taxon and A. dispar are comparable to intraspecific crosses in other taxa. However, fin ray counts are
non-overlapping and Reichenbacher et al. (2007; 2009) demonstrate differences in otolith morphology
and affirm its validity as a species. Holly (1929b) reports a specimen of Cyprinodon dispar from the same spring. I was unable to
verify the co-occurrence of two species in the hot spring through my own samples. Holly's specimen of A. dispar is in the Naturhistorisches Museum Wien (NMW 13799) but there are no field notes to
confirm its locality with accuracy. It may have been collected in an adjacent stream. A. ginaonis is regarded here as a good species on morphological grounds (see below).
Four syntypes are in the Naturhistorisches Museum Wien (NMW 13800-13803). Holly (1929a) in his original description refers to 3 syntypes. One of these 4 fish was selected as a lectotype by F. Krupp
(NMW 13800) and measures 21.8 mm standard length while the remainder measure 17.8-23.2 mm standard length.
Key characters
This species is distinguished from Aphanius dispar, the only other tooth-carp in this part of southern Iran by its locality and by having fewer total dorsal fin rays (5-7 as opposed to 8-11,
mostly 9-10 in A. dispar from neighbouring drainages).
Morphology
Total dorsal fin rays 5(16), 6(38) or 7(6). Gut coiling is highly variable between individuals and is complex (illustrated in Coad (1980b)). Reichenbacher et al. (2007) give a description of
otolith morphology. Chromosome number 2n=48, karyotype 14Sm + 34St and arm
number 31 (Esmaeili et al., 2008). Esmaeili and Gholami (2007) give
details of scale ultrastructure.
Saefali (1999) has D4-7, Pelvic5-7, Pect 13-17, A6-10, ll25-32,vert 26-30, bars10-22, gr13-21 SEE DATA SHEETS
Sexual dimorphism
Females have a higher mean numbers of anal fin rays, total vertebrae and flank bars, and longer pelvic fins (Coad, 1980b). Seifali and Sheidai (2001) found rostrum to pelvic fin distance, rostrum to
anal fin distance, weight, total distance (? total length), head depth, head distance (? length) and body depth to differ between the sexes.
Esmaeili and Gholami (2007) found that female scales were larger than those in
males.
Colour
Males have a brighter colouration than females although colouration is individually variable within sexes. The back is mottled black. The dorsal, anal, pectoral and pelvic fins are a light orange.
Flank bars are alternately black tinged with orange and grey-white, light blue or light orange. The side of the head anteriorly is iridescent light blue to green and the opercle is dorsally orange,
postero-ventrally blue. The iris is iridescent green and the anterior ventral head surface is light blue. The peritoneum is black.
Preserved specimens have two bars on the male caudal fin with the fin margin hyaline. These bars are usually straight. Some fish have 1-3 bars or a forked bar. Females lack these bars and their caudal
fin rays are lightly speckled. The male dorsal fin has 4-5 horizontal rows of spots, fading dorsally. These are not always well-developed and then consist of irregular, black pigmentation on the rays and
membranes. The dorsal fin pigmentation in females is weak. The anal and pelvic fins have little or no pigment. The pectoral fins of males carry pigment concentrated on the rays, weakly speckled in females.
Flank bars in females vary from weak to obvious, the light intermediate bars not as highly coloured as in males nor as well defined.
Size
Reaches 4.5 cm total length.
Distribution
This species was described by Holly (1929a) from the Ab Garm-e Ganow at 27°26-28'N, 56°18-20'E (various reports give differing latitude-longitudes for this well-known locality, hence the range). Ab
Garm literally means "hot water" in Farsi and is used to denote a hot spring. Ganow means "foul water". The spring lies at an altitude of about 135 m (Moghadam, 1974) on the slopes of
the Ganow Mountain (= transliterated as Ginao in German), hence its scientific name.
Zoogeography
The occurrence of sympatric A. dispar would confirm the species distinction of this species.
Habitat
The species is common within the bounds of the hot spring stream, numbering in the thousands in the 1970s, but no population trends have been recorded (but see below). Fish are found particularly along
the stream margin and in many minor subsidiary springs which emerge a few metres from the main spring. Some fish are found in water as shallow as 1 cm but temperatures are 37-40°C (when shade air
temperature was 20°C at 1400 hours on 27 January). The main spring itself issues from the ground at 30 litres second-1 and 41°C and drains as a stream about 10-15 m wide. A fault, over which
the spring stream pours, isolates the fish from those downstream. Coad (1980b) summarises water chemistry
and Reichenbcher et al. (2009) give a recent description of the habitat. The water is clear and colourless but there is a strong sulphur odour. The stream bed is
composed of stones and pebbles covered by lime-green to dark blue-green algae. Moghadam (1974) gives details of the algae species.
Age and growth
Essentially unknown although fish are larger later in the year (11.8-31.4 mm standard length in January and 15.6-34.7 mm in March). Esmaeili and Ebrahimi (2006) give a significant length-weight
relationship based on 33 fish measuring 1.70-3.17 cm standard length. The a-value was 0.0210 and the b-value 3.375 (a b-value < 3 indicating a fish that becomes less rotund as
length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Gut contents are the lime green algae typical of the hot spring and chironomids.
Reproduction
Temperature is constant and daylength may then be important in triggering reproduction, although fishes caught on 27 January and 21 March have mature eggs of similar size (ca. 1.6-1.7 mm) suggesting
an extended reproductive period.
Parasites and predators
Unknown.
Economic importance
None except related species are important in the aquarium trade and this is a potential economic use.
Conservation
Coad (1980b) listed this species as rare although it should be classed as vulnerable under the revised IUCN Red List Categories (IUCN, 1996) according to Coad (1998a).
The site is easily accessible by an asphalt road and is close to the large city of Bandar Abbas. Two hammams (bath houses) are in operation, one for men and one for women, the spring water is drained
off to irrigate date palms, and local people also use the site for bathing, washing kitchen utensils and food containers, and washing clothes so there is input of soap and food debris (personal
observations; A. R. Zinaei and H. R. Esmaeili, Payam-e Noor University, Bandar Abbas, 6 August 1997). The area is not under direct management by environment officials although in 1999 press reports
referred to planned research studies aimed at preventing extinction of this taxon (IRNA, 29 September 1999) and the spring does lie in the Genu Protected Area (Biosphere Reserve) described by
Zehzad et al. (1997).
The habitat could deteriorate if too much soap and food debris enter the stream. A single catastrophic event, such as a chemical spill, water diversion or construction activity, could eliminate the
species. Recent construction has severely affected the population with only 1-2 specimens being caught and the population being replaced by A. dispar (H. R. Esmaeili, pers. comm., September 2007).
Reichenbcher et al. (2009) suggest that A. dispar specimens
have been added to the spring by local people to increase the population size.
Otolith differences between samples taken in 201 and 2009 suggest hybridisation.
Local people using the spring should be educated on its ecological significance and alternative facilities for bathing and washing established. Roadside access to the spring should be prevented. The
population structure and reproductive regime of the fishes could be analyzed to determine if they are susceptible to abrupt changes.
Further work
This species differs from A. dispar in a number of characters but this may merely be a consequence of the unusual environment of the hot spring. Cross-breeding experiments would be very
revealing and would demonstrate or disprove reproductive isolation and hybrid fertility. The population is very important as the only species in a unique habitat, lending itself to studies of speciation,
adaptation and variation in response to high temperatures.
Sources
Type material: see above, NMW 13800-13803.
Topotypes: CMNFI 1979-0175, 77, 11.8-31.4 mm standard length. check size range;
CMNFI 1979-0416, 7, ? mm standard length, Hormozgan, Ab Garm-e Ganow below falls (ca. 27º26'N, ca. 56º20'E);
CMNFI 1979-0417, 44, ? mm standard length, Hormozgan, Ab Garm-e Ganow, side springs and edge of mainstream (ca. 27º26'N, ca. 56º20'E); CMNFI 2007-0054, 13, ? mm standard length, Hormozgan, Ab Garm-e Ganow (ca. 27º26'N, ca. 56º20'E);
Aphanius isfahanensis
Hrbek, Keivany and Coad, 2007
Common names
كپوردندان اصفهان (= kapurdandan-e Esfahan or Esfahan tooth-carp).
Systematics
The holotype is in the Canadian Museum of Nature, Ottawa under CMNFI 2004-0001, male, 25.0 mm SL, Esfahan Province, Zayandeh Rud (Zayandeh River) at Varzaneh Bridge, 32º25'32"N, 52º39'14"E,
1 July 2002, Y. Keivany and S. Asadollah and paratpes are under CMNFI 2004-0002, 18 males, 20.8–30.9 mm SL, 18 females, 22.0–38.4 mm SL, of 49 total (13 not used in meristic and morphometric
analyses, total range in size 12.1-38.4 mm standard length), same locality as holotype; AMNH 233639, 1 male, 25.2 mm SL, 1 female, 21.6 mm SL, same locality as holotype; MRAC 2004-05-P-01-02, 1 male, 25.2
mm SL, 1 female, 21.6 mm SL, same locality as holotype; GenBank accession numbers AY593488, AY593489, AY593497, and AY593498.
Key characters
Distribution and colour pattern identify this species. Males can be distinguished from those of all other Iranian species by having distinct black edge on the dorsal, anal, and pelvic fins. The dorsal
fin is covered with a high density of black blotches. Females can be distinguished from females of A. sophiae and A. vladykovi by having flank-bars rather than spots. It can also be
distinguished from A. persicus by less well-defined bars terminating at a mid-flank stripe and a relatively light gray stripe at the caudal-fin base rather than a black spot or blotch. Flank-bars
are also characteristic of females of Aphanius dispar, however.
Morphology
Morphometric data are given by Hrbek et al. (2007) but these characters cannot be used for a simple distinction from other Aphanius in Iran but only in multivariate space. This species
is clearly distinguished at the genetic level from all other species of Aphanius. It has 82 molecular apomorphies – 19 transversions, two transversions/transitions (depending on comparison), and
61 transitions – that show fixed character state differences to homologous characters analyzed in A. sophiae, A. persicus, and A. vladykovi from Iran. Thirty-seven of these character
states are also apomorphies when compared to A. anatoliae, A. danfordii, A. villwocki, A. asquamatus, and A. fasciatus from Turkey.
Dorsal fin rays 11-14, anal fin rays 10-13, pectoral fin rays 13-16, pelvic fin rays 4-6, lateral line scales 25-29, total gill rakers 10-13, precaudal vertebrae 10-13 and caudal vertebrae 15-18.
Chromosome number 2n=48, karyotype 12Sm + 36St and arm number 30 (Esmaeili et
al., 2008).
Sexual dimorphism
See colour below.
Colour
Male flank bars number 8-12, mean 10.2, significantly less than in A. sophiae at 10-21, mean 14.4. The bars are broad with interspaces about equal or slightly narrower. The bars extend from
behind the head to the tail. Anterior bars fade on the belly, whereas, posteriorly on the caudal peduncle, they encircle the body. Dorsally, the head is dark gray and the body is lighter but still
heavily pigmented with melanophores; the belly lacks pigmentation. The sides of the head are densely speckled with melanophores, more thinly on the ventral side; in most specimens the chin is darker than
the rest of the ventral head surface. The eye is bounded ventrally and postero-ventrally by a thin line of black pigment. The defining male coloration is the black margins of the pelvic, anal, and dorsal
fins. The dorsal and anal fins may present a halo effect, the margins being so dark in relation to the rest of these fins. The tips and outer margin of the pelvic fin are blackish. The anal
fin has a broad, blackish margin with the rest of the fin light cream-colored. The dorsal fin has the blackest margin. The rest of the dorsal fin is variably blotched, the blotches being much lighter than
the fin margin. Most specimens have a contrasting pigmentless area just below the fin margin. The pectoral fin has sparse pigmentation along rays and ventrally on the interradial membranes
but lacks the concentrated black pigmentation seen on the pelvic fins. The caudal fin rays and membranes are sparsely pigmented, and the whole margin may be blackish but in most fish pigment is restricted
to the upper and lower margins, the lower margin only, or is absent. Large females have a grayish dorsal surface to the head, a lighter back and upper flank covered with scattered melanophores, and a
mid-flank stripe terminating on the caudal peduncle in a blackish, short stretch covering up to three scales (Fig. 2b). This short stretch of pigment is present in all females, faint in some, rarely
forming a blotch and in some small fish tapering anteriorly. The flank stripe may be broken into a series of blotches in some smaller females, or it may be continuous as in large fish. Starting anterior
to the belly there is a ventrolateral series of thin bars (up to ten) separated by cream colored interspaces 1–3 bars wide. These are absent in some smaller females, which may only have blotches at various
levels present in this region. Even some large females have faint flank pigmentation so that bars and the stripe are weakly expressed. At about the origin of the anal fin, the flank bars may continue onto
the caudal peduncle in regular form or become irregular, breaking up into blotches. Anteriorly the bars terminate ventrally at about the level of the lower edge of the pectoral fin. The belly and lower
head have sparse pigment although the chin and sides of head are speckled with melanophores. The eye is bounded ventrally and posteroventrally by a thin line of black pigment. Fins lack any distinctive
pigment pattern. Fin rays are outlined with melanophores, and interradial membranes of the caudal and anal fins have melanophores at varying degrees of density. The dorsal fin has the most interradial
pigmentation, particularly near, but clear of the fin base.
Size
Attains 38.4 mm standard length.
Distribution
The type locality is near the town of Varzaneh on the lower reaches of the Zayanadeh River, about 30 km upriver from the terminal sump, the Gav Khuni marsh.
Zoogeography
An estimate of a 4.8 MYA divergence of A. isfahanensis and A. sophiae + A. persicus is given by Hrbek et al. (2007). This divergence time is in accord with the hypothesis
of a near-simultaneous diversification of ~5 MYA of organisms occupying different geological units of central Iran.
Habitat
The type locality had a water temperature of 27ºC, pH was 6.7, the water was brackish, conductivity was 10.9 mS, dissolved solids were 5450 ppm, dissolved oxygen was 12.3 mg/L, river width was 50 m,
and capture depth was 0.5 m. Current was slow, and there was no cover. Gambusia holbrooki was captured at the same locality.
Bleher (20110 notes that the type locality was dry at Varzaneh Bridge and
only found specimens in an artificial circulating spring channel 50 m long in
southern Esfahan.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Gholami et al. (2009) record the trematode Diplostomum spathaceum
from the eyes of this fish.
Economic importance
None at present but a potential aquarium species. Breeding and aquarium conditions are similar to A. mento although it is reputedly difficult to breed.
Conservation
Known only from the type locality, which is not protected, this species needs a conservation assessment.
Further work
The biology of this species requires study.
Sources
Type material: See above, CMNFI 2004-0001, CMNFI 2004-0002, AMNH 233639, MRAC 2004-05-P-01-02.
Aphanius mento
(Heckel, 1843)
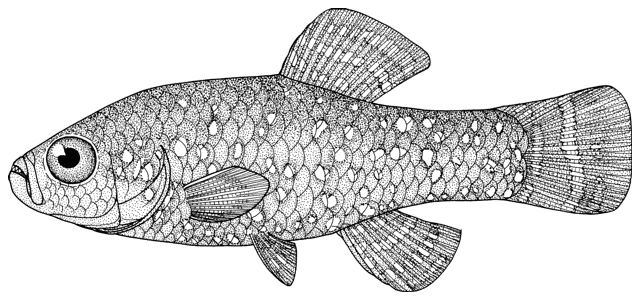
Male
Common names
gour-e khar, kopurdandandar-e Arvand (= Arvand River tooth-carp), kopurdand-e Irani.
[batrikh qabras in Arabic; Persian pupfish, Persian minnow, Black Persian minnow, Persian killie, Arvand pupfish, chin killifish, pearl-spotted killifish].
Systematics
Lebias Cypris Heckel, 1843 is a synonym. Krupp (1984a) gives a detailed synonymy of this species but considered cypris to have priority. Klee (1967) and Lazara (1989) point out that
Garman (1895), in a work dated in July of that year, as first revisor placed cypris in mento. This decision has priority over Gaillard (1895), a work with no exact date of publication, in
which mento was placed in cypris, according to the Zoological Code of Nomenclature (Ride et al., 1985). The type locality for both Lebias mento and Lebias cypris is
"Mossul" according to Heckel (1843b).
Hybrids with Aphanius dispar richardsoni are reported from Israel (Goren and Rychwalski, 1978).
Parenti (1981; 1984) places this species in the genus "Aphanius", i.e. distinct from true Aphanius without defining and naming a new genus. This genus is more closely related
to the Anatolian Kosswigichthys Sözer, 1942 and the South American Orestias Valenciennes in Cuvier and Valenciennes, 1846 than true Aphanius. Hrbek and Meyer (2003) note that, based
on their mtDNA study, this species diverged from the ancestor of the A. dispar clade at an early date, 26.87±1.31 MYA. They speculate that the ancestor of mento invaded the northern
perimeter of the Arabian plate as it was abutting Laurasia some time after the separation of their eastern and western clades of Aphanius (see above) before the closing of the Tethys Sea. It later
spread through the present-day Tigris-Euphrates and into the Levant and southern Turkey.
The 5 syntypes of Lebias cypris are in the Naturhistorisches Museum Wien (NMW 59598) with 1 specimen selected as a lectotype by Krupp and Schneider (1989), the other 4 being paralectotypes. The
syntypes of Lebias mento are (NMW 62105 (4 fish), 59042 (5) and 22624-22632 (9). Eschmeyer et al. (1996) list NMW 21699-704 (6) and NMW 59832 (21) as possible syntypes. The Vienna catalogue
lists 5 specimens of Lebias cypris and 8 of Lebias mento.
Key characters
The adult colour pattern is distinctive and dorsal fin ray counts are usually higher than in A. dispar.
Morphology
Scales along side of body 23-28, total dorsal fin rays 9-14, usually 12-14, total anal fin rays 9-13, pectoral rays 12-16, and pelvic rays 4-6, usually 5. Frontal scalation is E-type (Hoedemann, 1958).
Flank scales are squarish to a vertical oval. The former shape has a vertical anterior margin which may be wavy or slightly convex, upper and lower anterior corners rounded but square-cut, dorsal and
ventral margins parallel, and the posterior margin rounded. The oval scales have all margins rounded. The focus is central to subcentral posterior, circuli are fine although coarser on the posterior field,
radii are restricted to the anterior field and are almost horizontal and parallel. There is no pelvic axillary scale. Total gill rakers 11-15, rakers being spinulose and reaching beyond the adjacent raker
when appressed. Vertebrae 25. Teeth are tricuspid with a long and pointed central cusp (Goren and Rychwalski, 1978). There are 14-16 teeth on the lower and 10-12 on the upper jaw (Berg, 1949). The gut is
s-shaped. Reichenbacher et al. (2007) give a description of otolith morphology. Chromosome number 2n=44 or 48 (Wildekamp, 1993; Klinkhardt et al., 1995).
Sexual dimorphism
See below under Colour.
Colour
Adult, breeding males are a dark blue-black to dark brown or almost black with iridescent blue-white to silvery spots regularly-arranged on the fins as curved lines, and irregularly on the body
(sometimes as irregular vertical bars and sometimes the spots are vertically elongate) (Richter, 1989; preserved material from Iraq; Goren and Rychwalski, 1978). The edges of the gill cover are
orange-red (Wildekamp et al. (1999). Male colour fades in low light conditions and in winter males have silvery flanks with a dark brown back. Spots are silvery-blue on the upper flank and
are not as numerous as in the spawning male. Females are grey-brown or grey-white to silvery with large golden blotches or silvery to blue spots and dark dots. Scales along the mid-flank usually have a
dark border (Wildekamp et al. (1999). Fins in females are hyaline. Body colour is reportedly heightened in brackish water (Grimes, 1974). The peritoneum is silvery with dense but fine melanophores.
Size
Reaches 8.4 cm total length or more (Güçlü and Küçük, 2008).
Distribution
Found in the Orontes (= Asi) and Tigris-Euphrates basins, the Levant in coastal and Dead Sea basins, western Jordan, and in southern Turkey in Mediterranean basins as well as in central Turkey.
Tigris-Euphrates records are relatively rare (see map in Wildekamp (1993)). The species was described from Mosul in northern Iraq and there are records from the region of Basrah in southern Iraq near the
border with Khuzestan. Wildekamp (1993) has one record apparently mapped in southern Iran near the border with Iraq. This is the only record from Iran although Abdoli (2000) indicates the Arvand River on
a map.
Zoogeography
See Systematics above and under the genus.
Habitat
This species has been reported to survive in waters up to 38°C and as low as 0°C. pH at 7.0-8.5 has been reported as ideal for aquaria maintenance. Aquarists report it as a robust species favouring
hard and alkaline water with some salt added (Thiermann, 1978; Kostich, 1979; Phillips, 1985). Grimes (1974) considers it to be quarrelsome in aquaria like other Aphanius and Allen
(1988) also noted that males are very aggressive. It inhabits fresh or slightly brackish water of springs, streams and lakes, usually near shore where males establish territories in vegetation. It
generally prefers habitats dense in vegetation.
Age and growth
Life span is about 3 years with maturity in aquaria at 4-6 months. The length-weight relationship in southern Iraq was W = 6.0001 x 10-5 L2.6403 for males and W = 2.3028 x
10-4 L2.235 for females. The average condition factor for males was 2.0499 and for females 2.3121. The condition factor increases with increase in length in females and decreases in
males (Huq et al. 1977).
Güçlü and Küçük (2008) examined a population of this species in a spring in the
Mediterranean Sea basin of Turkey and found fish in age groups 0 to 7. They also
give length-weight relationships and a von Bertalanffy growth equation.
Food
Al-Daham et al. (1977) found filamentous algae and diatoms were the most important foods in southern Iraq. Shafi and Shalli (1986) report a diet of beetles, ephemeropteran nymphs and algae in
southern Iraq. Aquarium specimens prefer meaty foods such as brine shrimps over flake foods (Grimes, 1974) but flake food is taken (Allen, 1988). Curiously, Al-Daham et al. (1977) for their
southern Iraqi fish in aquaria found this species to prefer plant food. Males approached food items individually and prevented other fish from taking the food. Females were also solitary though less
aggressive than the males. However Gambusia holbrooki out-competed male A. mento for food, on one occasion even snatching a Gambusia embryo from the mouth of an A. mento.
Güçlü and Küçük (2008) found their Turkish fish to feed principally on
Gammarus sp and Palaemon sp.
Reproduction
Anderson (1966), Lutman (1982) and Richter (1989) observed spawning in aquaria. There is a dominant male which is more deeply and richly coloured than other males. The dominant male defends a spawning
territory, fighting other males. Males will defend separate spawning mops if these are made available in aquaria. The dominant male will pursue a female and places his larger dorsal fin over the her back.
Several spawnings will follow and females will spawn with more than one male. Spawning can occur near the surface. These fish can spawn at six months of age. Peak spawning in southern Iraq is April to
June when only a small proportion of eggs in the single ovary contained fully developed eggs (Al-Daham et al., 1977). However, Shafi and Shalli (1986) state that it breeds in southern Iraq in
May-July. There are up to 71 highly-adhesive, eggs of 170 microns diameter. Aquarium spawned eggs hatch in 7-14 days, depending on temperature, 10 days at 25°, 3 days in the 80s°F, or 6-7 days in the low
70s°F (accounts vary). Allen (1988) found egg production to be higher in aquaria when the equivalent of seven tablespoons of salt was added per gallon at a water temperature of 25°C. A minimum of 20 eggs
per day were produced.
Byniak (1979) describes the effects of day length and temperature on the reproductive cycle of females in this species in Israel.
Parasites and predators
Unknown.
Economic importance
An aquarium fish with breeding details given by Zipay (1961), Anderson (1966), Lutman (1982), Richter (1989), Semeit (1999) and www.killi-data.org/ (downloaded 21 September 2007). The species is
hardy and fry are easily raised, given large enough tanks and an easily defensible area for the male to guard eggs. However, populations stop breeding in aquaria after 6-8 generations
(www.killi-data.org/, downloaded 21 September 2007). Blaustein and Byard (1993) show that this species will prey on mosquito larvae in a laboratory situation and has potential in control of this vector
of malaria, at least in vegetated habitats.
Conservation
The presence of this species in Iran needs confirmation by field work and until this is done and its distribution, population numbers and biology worked out, its conservation status cannot be assessed.
Endangered in Turkey (Fricke et al., 2007).
Further work
See above.
Sources
Iranian material: None.
Comparative material: BM(NH) 1920.3.3:203-222, 22, 24.9-36.0 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1949.7.21:12, 24.4 mm standard length, Iraq, Bahr-el-Milh Lake east of Karbala (32º35'N, 43º50'E);
BM(NH) 1981.10.6:19-23, 5, 26.6-31.8 mm standard length, Iraq, Qarmat Ali, Basra (30º30'N, 47º47'E);
BM(NH) no catalogue number, 4, 19.7-31.7 mm standard length, Iraq, Basra Liwa (no other locality data);
uncatalogued, 1, 31.2 mm standard length, Iraq, Khawr az Zubayr (no other locality data).
?Iraq fish from Hussain
Aphanius mesopotamicus
Coad, 2009


Male paratype
21.7 mm SL above, female holotype below
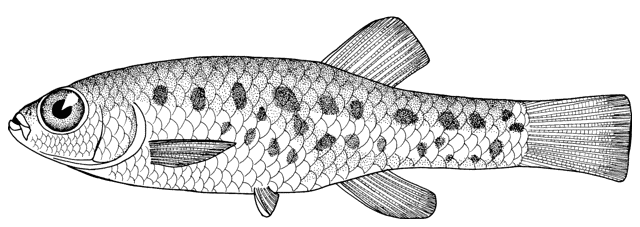
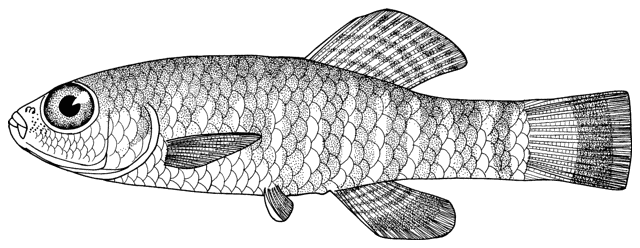
From Basrah, Iraq (ZISP
25393) after Berg (1949). Female (above), 30 mm, and male, 29 mm.
Common names
None.
[Mesopotamian tooth-carp].
Systematics
The
holotype is a female, 29.3 mm SL from Khuzestan, canal branch of Karkheh
River, 31º40’N, 48º35’E (CMNFI 1979-0360A) and paratypes are
37 specimens, 14.6-29.1 mm SL, same locality as above (CMNFI 1979-0360B) and 6
specimens, 15.1-20.5 mm SL, from Khuzestan, Karkheh River branch at Abdolkhan,
31º52’30”N, 48º20’30”E (CMNFI 1979-0364). The species
is named for Mesopotamia (the land between the rivers) referring to the
Tigris-Euphrates basin where the species is found.
Key characters
This species is distinguished by distribution and by
pigmentation, males having clear margins to the unpaired fins, no bars on the
caudal fin and 10-15 clearly defined flank bars, females bear irregular blotches
or spots on the flank
Morphology
Total dorsal fin rays 11-13, total anal fin rays 10-12, branched pectoral fin
rays13-15, branched pelvic fin rays5-6, lateral series scales 26-29, total gill
rakers 10-14, precaudal vertebrae 11-13 and caudal vertebrae 14-17.
Sexual dimorphism
See colour description.
Colour
Males have clear margins to the unpaired
fins, no bars on the caudal fin and have 10-15 clearly defined flank bars. The
dorsal surface of the head and the upper flank are more heavily pigmented with
melanophores than more ventral areas. The belly and lower head are unpigmented.
The chin and snout have dense melanophores and a rim of melanophores underscores
the eye in both sexes. The dorsal, anal and caudal fins in males have
wide clear margins. This is also seen in the material from Basrah, Iraq (BM(NH)
1982.9.2:326-328). The material from Basrah figured by Berg (1949) has
distinct light margins in some printed versions of the figures. The caudal
fin is darker just proximal to the clear margin, lighter in mid-fin and dark
again at the base. The dorsal fin has irregular pigmentation on the membranes
and, to a lesser extent, on the rays. The pigmentation may involve an overall
darker colour in contrast to the light margin or may have some pattern to it.
The pattern is often elongate and short blotches with no regular arrangement and
sometimes may appear as up to 5 wavy and oblique bands. Dark pigmentation is
found just behind the first ray on the fin membrane. The anal fin is darkest
just proximal to the clear margin. Up to the last 6 membranes of the anal fin
are dark and this pigment may be broken up in as many as 4 elongate bars along
each membrane. A similar pattern is found in some dorsal fins and the general
effect on both fins is that the postero-dorsal (anal fin) and postero-ventral
(dorsal fin) parts of these fins are the darkest. The dorsal, anal and caudal
fins generally have more pigment on the membranes than the rays and in some this
is quite distinctive, making the rays stand out.
The pectoral and pelvic fins in males
are generally clear or somewhat milky and opaque and lack melanophores. The
distal parts of the membranes between the last 5 rays of the pectoral fin and
the small membrane area of the pelvic fins can be pigmented. Males have flank
bars circling the caudal peduncle and reaching the anal fin base but fading
ventrally on the lower part of the anterior flank, not reaching the ventral
margin of the belly and becoming progressively shorter and less distinct the
more anterior they are. Bars are 2-5 times broader than the pale interspaces.
Females have a similar head and dorsal
and ventral body pigmentation but it is much lighter than in males. Fins have
little or no pigmentation. The proximal third to half of the dorsal fin rays and
membranes, particularly the anterior ones, have pigment in some fish but this is
weakly expressed compared to the condition in males. Some fish have a few faint
melanophores lining the anal fin rays.
The most distinctive feature in females is a spot, oval to lozenge-shaped,
at the central base of the caudal fin. Its concentration of melanophores is much
higher than any other feature. The flank in females
can have up to 14 thin, dark, wavy and irregular vertical patches of pigment.
These patches may be interrupted in their vertical extent and are weakly
expressed anteriorly. They fade ventrally, ending above the lower edge of the
caudal peduncle and above the anal fin base, and are absent on the lower
anterior flank. The patches are light and not as contrasting with the lighter
interspaces as the bars found in males. Patches are thin, half to one third of
the width of the interspaces. Very often the patches are broken up into spots
and elongate blotches of various sizes, and a regular barred appearance is not
usual. The spots and blotches are all smaller than the eye size by at least
half. Material from Basrah, Iraq figured by Berg (1949) has the spots emphasised
but material from Basrah (BM(NH) 1982.9.2:326-328) examined for this study has a
more blotchy appearance and spots are not well-defined.
Size
Attains
29.3 mm standard length.
Distribution
Found in the southern Karkheh River basin of Iran and at Qarmt 'Ali at the
northern part of Basrah on the Shatt al Arab, southern Iraq. Possibly also at
Shalili-ye Bala or Shalili-ye Pa'in (ca. 31º58'N,
48º53'E), near Shushtar, Karun River basin
of Iran from a record in Berg (1949) from Shellali (ZISP 25446).
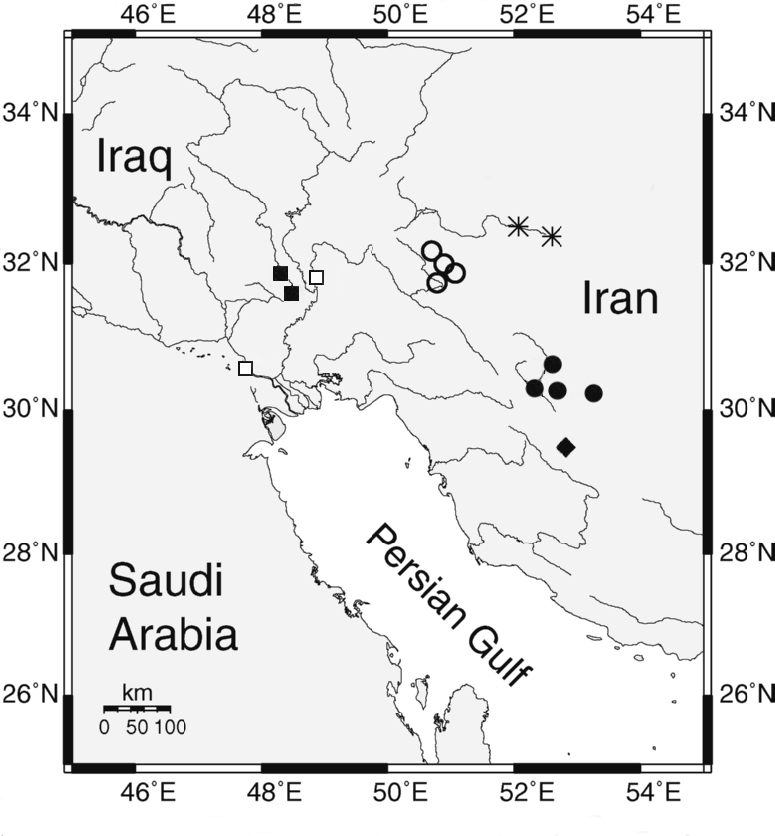
Distribution map of Aphanius in Mesopotamia and adjacent areas. Solid
squares type series of A. mesopotamicus (lower one CMNFI 1979-0360A and
B, upper 1979-0364), open squares additional, non-type material of A.
mesopotamicus (BM(NH) 1982.9.2:326-328 and ZISP 25393 (lower left) and ZISP
25446 (adjacent to type series)), open circles A. vladykovi, stars A.
isfahanensis, solid circles A. sophiae, and diamond A. persicus
(map modified after Hrbek et al. (2006)).
Zoogeography
This species is found in the Tigris River basin and its morphology shows
evidence of relationship with species of internal basins in Iran.
Habitat
The habitat of the new species is known
only from field notes made at the time of capture in Iran on the Khuzestan
plains. The two localities are a river and a canal branching from that river.
The 25 m wide river had a water temperature of 22°C at 1205 hours, pH 6.0,
conductivity 1.0 milliSiemens, a mud bottom
and the principal plant materials were rushes and reeds. The 30 m wide canal had
a water temperature of 15°C at 0930 hours, pH 6.0, conductivity 1.8 milliSiemens,
a mud bottom and the principal plant material was filamentous green algae.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None recorded in Iran.
Economic importance
None at present in Iran but a possibly a species of interest to aquarists.
Conservation
This species is known only from museum collections and recent efforts to
capture fresh material were unsuccessful. These attempts should be repeated and
the numbers and biology of the species assessed.
Further work
The biology and continued presence of this species in Iranian waters requires study.
Sources
Based on Coad (2009).
Type material: See above.
Comparative material:
BM(NH) 1982.9.2:326-328, 4, 20.2-25.3 mm SL, Iraq, Qarmat `Ali, Basrah (30°34’N,
47°46’E).
Aphanius persicus
(Jenkins, 1910)
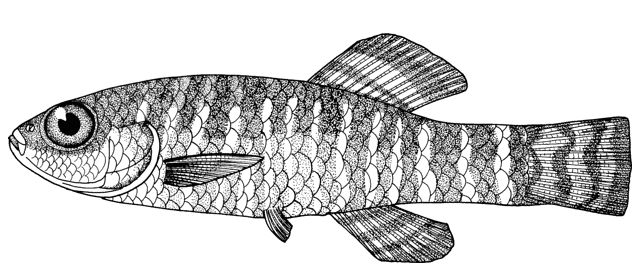
Male
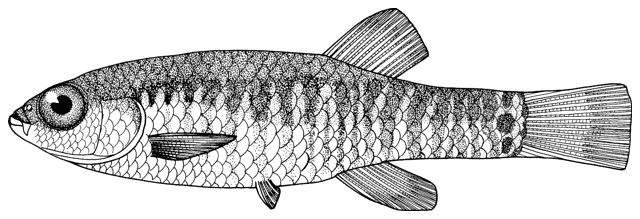
Female
check male has bars on tail!
Common names
gour-e khar, kopurdandan-e Irani (= Iranian tooth-carp).
[Persian pupfish or toothcarp, but see also A. sophiae].
Systematics
Cyprinodon blanfordii Jenkins, 1910 and Cyprinodon pluristriatus Jenkins, 1910 are potentially synonyms. All are synonymised with A. sophiae by authors (see Coad, 1996a). Hanko
(1924) placed Cyprinodon blanfordi and C. persicus in Cyprinodon chantrei Gaillard, 1895 but this is an error.
Jenkins (1910) described Cyprinodon blanfordii ("East of Shiraz, South Persia"), C. persicus ("Spring on the edge of Shiraz Lake, Southern Persia") and C.
pluristriatus ("East of Shiraz, stream running to Fussa, Southern Persia, 5,000 feet"). The first nominal species has so generalised a locality as to be of very uncertain provenance. It
could conceivably be in the Shiraz valley, the Kor River valley or outside either of these (see below under A. sophiae where it is argued that is is a synonym of this species). The second nominal
species is clearly from the valley of Shiraz and its salt lake Maharlu, an endorheic basin. The last nominal species was found near Fussa (= ? Fasa) and, based on maps, is probably in the drainage of the
Mand River which flows to the Persian Gulf.
The valid name for Lake Maharlu basin Aphanius is given here as persicus. The types in Calcutta have not been examined by me. Cyprinodon blanfordii has page priority but the
locality is vague and the fish have a pigment pattern description and illustration which is spotted on the flank with a lozenge-shaped spot at the caudal fin base, thus appearing to be a female A.
sophiae. Cyprinodon pluristriatus appears last in the descriptions and may be an available name for Aphanius outside the Lake Maharlu basin in rivers draining to the Persian Gulf should
these prove distinct.
Material described and illustrated by Villwock (1960) as A. sophiae are this species.
Three syntypes of Cyprinodon blanfordi (ZSI F9416 to ZSI F9418), two syntypes of Cyprinodon persicus (ZSI F9403 and ZSI F9404) and four syntypes of Cyprinodon pluristriatus
(ZSI F9408 to ZSI F9411) are in the Zoological Survey of India, Calcutta (Menon and Yazdani, 1968), although Jenkins (1910) has ZSI F9408-9412 for C. pluristriatus.
Key characters
Females of this species are barred where all other Aphanius species in this area of southern Iran have spotted females. The distribution is apparently limited to the Lake Maharlu basin near
Shiraz.
Morphology
Although populations are isolated in springs and streams around Lake Maharlu, Coad (1996a) found them to be relatively homogenous.
Lateral line scales 24(3), 25(34), 26(69), 27(176), 28(100) or 29(22). Scales above the lateral line 4-6, scales between lateral line and the anal fin 4-8, scales between the lateral line and the
pelvic fin 5-9, and scales around the caudal peduncle 12-17. Esmaeili et al. (2007) determined a chromosome number of 2n=48 with 11 pairs of submetacentric and 13 pairs of subtelocentric
chromosomes and an arm number of NF=70. Karyotype 16Sm + 32St and arm number 32
(Esmaeili et al., 2008).
Total dorsal fin rays 8(1), 10(8), 11(97), 12(204), 13(89), or 14(7); total anal fin rays 9(3), 10(111), 11(225), 12(64) or 13(3); total pectoral fin rays 13(9), 14(82), 15(211), 16(94), 17(9) or
18(1); total pelvic fin rays 4(11), 5(181) or 6(213); total gill rakers 9(5), 10(51), 11(220), 12(108), 13(19) or 14(3); abdominal vertebrae 9(1), 10(1), 11(27), 12(355), or 13(22); and caudal
vertebrae 12(1), 14(16), 15(285) or 16(104).
Sexual dimorphism
Most apparent in colour and pigmentation detailed below. Females are longer and heavier than males (Esmaeili and Shiva, 2006).
Colour
"The basic colouration is bright shining silvery white. The entire body, with the exception of the dorsal crest and the belly, is tigered with small brown spots; in some specimens these spots
seem to be arranged in three irregular longitudinal rows of which the uppermost extends as a band across the operculum and to the eyes; all fins are uniformly yellow with a reddish border. In males the
spots are more dense, in females they are paler and scarcer, only the 2-3 at the base of the caudal fin are dark brown" (Heckel (1846-1849b) on A. sophiae). "The colour of this small
fish with its enviable crystal teeth is uniformly brown, at least it is now in ethyl alcohol, with a silvery-white throat and belly; laterally on the tail there are a few scattered, darker spots, and a
deeper black spot is located on the last scales anteriorly of the caudal fin. All fins are blackish without any markings" (Heckel (1846-1949b) on A. crystallodon). "Silvery, brownish on
back, brown on top of the head, with numerous small spots of brownish, arranged in three or more, more or less irregular, longitudinal series, the median of which, on the lateral line, commonly ends in a
black spot on the middle base of the caudal. On some this median series is entirely composed of blackish spots. Rarely the spots are somewhat confluent into longitudinal bands. Brownish specimens show a
longitudinal band of silvery along the middle of the flank. The form described as A. crystallodon has spots only towards the base of the caudal, around the black spot usually ending the median
series" (Garman, 1895).
The flank in females bears numerous alternating light and dark bars, the light bars varying in width from about one half to twice that of dark bars. The bars gradually merge with background
pigmentation anteriorly on the flank and while clearly defined on the rear flank are difficult to distinguish anteriorly.
The caudal fin spot in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally, single, smaller, dark, subsidiary spots may be found antero-dorsally and
antero-ventrally to the basal spot or scattered spots may be found irregularly before and behind the basal spot.
Males have a pigmentation very similar to that of A. sophiae and the description here is identical. Some minor observed variation is attributed to variation in size and maturity of the fish
compared. Males have light flank bars half the width or much narrower than alternating dark bars. The margins of the dorsal, anal and caudal fins are clear while the rest of these fins is dark. Some fish
have up to 3, but usually 2, thin, light bars on the basal half of the caudal fin; these are generally larger fish. The margin of the lower half of the pectoral fin has concentrations of pigment on the
membranes such that this area is darker than the rest of the fin. The anal fin is darkest posteriorly where pigment is concentrated on membranes. The distal third of the fin is pigmented to form a dark
band, becoming lighter proximally. The dorsal fin is the darkest fin (except for the clear margin) and the anterior base is the darkest part of the fin. Bands are not always evident but pigment spots are
large proximally. Some fish have 2, sometimes 3, thin light bars at the base separated by thin dark bars and paralleling the body profile while others have none. The dorsal fin base may have instead a
series of lighter spots, sometimes irregular and not paralleling the body profile.
Size
Reaches 59.5 mm standard length (Esmaeili and Shiva, 2006).
Distribution
This species is restricted to the Lake Maharlu basin near Shiraz.
Zoogeography
This species is found only within the endorheic Lake Maharlu basin, Coad (1996a) suggests that tooth-carps in inland waters may have risen with the post-Pliocene uplift of the Zagros Mountains rather
than being the result of relatively recent inter-basin dispersal.
Habitat
This species is found in fresh streams and springs and in springs of varying saline content or saline influence from hypersaline chloride Lake Maharlu. The lake at 124‰ is an impassable barrier
to dispersal but is very shallow and dries out periodically, e.g. in 1967 (Cornwallis, 1968a). The larger springs and their streams can then meet and transfer fishes on the dried-out lake bed. Some springs
are 27 km apart along the lake margin and have probably had no contact or fish exchange over many generations. This is especially the case for the smaller springs which emerge from the ground with a
diameter of only 1 m and restricted flow. When lake water rises, lower springs are inundated with hypersaline water and the fishes are killed. The spring is presumably recolonised from a higher spring
when lake level falls.
Fish placed in pure lake water die within minutes. Springs discharging directly into the lake contain tooth-carps but the fish do not venture into the lake. Some fish, if disturbed or deliberately
harassed, will swim out for a short distance into the hypersaline lake water but they blanch and rapidly retreat to fresher water (Coad, 1996a).
Esmaeili and Shiva (2006) found the bottom of these water bodies to be muddy but the water was clear and slow-running. Conditions in October at three different sites were 16.9-19.0ºC, pH 6.70-6.74,
dissolved oxygen 3.96-6.11 mg/l, nitrate 0.9-1.6 mg/l, nitrite 0.029-0.062 mg/l, phosphate 0.35-0.65 mg/l and ammonium 1.55-2.60 mg/l.
Age and growth
In the studies of Esmaeili and Shiva (2006), the sex ratio was 1.67:1 for females:males, highly significant, the age groups were 0+ to 3+ years, and positive allometric growth was determined (b
value significantly greater than 3).The condition factor for females was highest in February and March and lowest in September while in males it was lowest in December and increased until February.
Fishes smaller than 25 mm had an equal sex ratio, suggesting selective predation on males, or better survival or longevity of females. Esmaeili and Ebrahimi (2006) give a significant length-weight
relationship based on 62 fish measuring 1.86-4.27 cm standard length. The a-value was 0.0222 and the b-value 3.395 (a b-value < 3 indicating a fish that becomes less rotund as
length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown but presumably similar to other tooth-carps.
Reproduction
Esmaeili and Shiva (2006) found the gonadosomatic index in females to increase from November to June, decreasing slowly from late June to November. The reproductive period lasted six months. Males had
two peaks, in April and August. Egg diameters reached 1.71 mm, absolute fecundity ranged from 45 to 250 eggs (average 115.7 eggs) and relative fecundity was 21.6 to 244.1 with a mean of 90.01 per gram
body weight. Even small fish had hydrated eggs and, with the extended reproductive period, shows adaptation to an unstable habitat.
Monsefi et al. (2009) found that this species is a batch spawner in the
Barm-e Shur spring with a spawning period from April to November. This species
lives in unstable environments such as temporary lagoons or very small pools and
batch spawning of relatively large eggs gives a greater chance of survival.
Parasites and predators
Mokhayer (1989) reported metacercariae of the eye fluke, Diplostomum spathaceum from Aphanius sophiae, probably this species, in Iran. The fluke can cause complete blindness and death in
commercially important species of fish. This fish was also infested with yellow grub, Clinostomum complanatum. González-Solís et al. (1997) report Contracaecum sp. larvae from this
species in the Lake Maharlu drainage, Fars.
Economic importance
This species has no current economic importance although it would make an excellent aquarium fish. Villwock (1959) gives details of maintaining what seems to be this species under aquarium conditions,
gradually transferring them from a mixture of salty and magnesium sulphate water to stagnant tap water. They prefer some admixture of sodium chloride and magnesium sulphate which matches their natural
habitat (2% synthetic sea water plus a tablespoon of epsom salts per 25 litres water). Aquatic plants such as Myriophyllum and Cabomba are also necessary for breeding as the eggs are
attached to them but artificial substitutes can be used. Yellowish transparent eggs are 1.5 mm and are deposited usually individually, rarely in small groups.
Larvae feed on Artemia salina nauplii and adults on daphnia, red mosquito larvae and tubifex. Male-male interactions include mock fights with spread fins and close swimming. The loser hides in the
algae and is not strongly pursued by the victor. These interactions, and those between sexes, are not strongly developed and aquaria can be stocked at 15-20 fish per 25 litres. Y. Keivany notes (pers.
comm., 2004) that this species is difficult to maintain in aquaria.
Conservation
The populations are found in a variety of springs and streams around Lake Maharlu. Some of these localities are threatened by water abstraction, road construction and pollution but a number of
localities are small and/or saline and not under threat.
Further work
Studies on aquaria maintenance would enable it to become established in the aquarium trade and help its conservation.
Sources
Type material: None seen.
Iranian material: CMNFI 1979-0017, ?47, ?17.9-35.5 mm standard length, Fars, stream at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 1979-0018, 75, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0033, 1, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0034, ?124, 14.8-38.7 mm standard length, Fars, spring on shore of Lake Maharlu (29º27'N, 52º44'E);
CMNFI 1979-0035, 17, ? mm standard length, Fars, spring south of Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0038, 16, ? mm standard length, Fars, spring near Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0039, 91, 15.9-37.9 mm standard length, Fars, spring on shore of Lake Maharlu (29º27'N, 52º44'E);
CMNFI 1979-0041, 61, 20.8-41.6 mm standard length, Fars, spring on shore of Lake Maharlu (29º23'N, 52º48'E);
CMNFI 1979-0042, 28, ? mm standard length, Fars, spring on shore of Lake Maharlu (29º23'N, 52º48'E);
CMNFI 1979-0046, 5, 18.1-32.2 mm standard length, Fars, qanat at Barm-e Dalak (ca. 29º35'N, ca. 52º38'E);
CMNFI 1979-0047, 41, 11.3-32.1 mm standard length, Fars, spring source of Ab-e Paravan marshes (ca. 29º34'N, ca. 52º42'E);
CMNFI 1979-0048, ?45, ?15.3-39.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0049, 12, 17.2-38.5 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0050, 27, 18.3-28.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'30"N, ca. 52º49'30"E);
CMNFI 1979-0051, 3, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º30'N, ca. 52º52'E);
CMNFI 1979-0052, 16, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º30'N, ca. 52º52'E);
CMNFI 1979-0064, 10, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'30'N, ca. 52º49'30"E);
CMNFI 1979-0065, 8, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'N, ca. 52º50'E);
CMNFI 1979-0066, ?31, 15.6-42.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0110, 2, ? mm standard length, Fars, lake in Park-e Shahr, Shiraz (29º38'N, 52º32'E);
CMNFI 1979-0112, 24, 11.9-45.9 mm standard length, Fars, stream at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 1979-0118, 12, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0119, 75, ? mm standard length, Fars, steram at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 2004-0006 (GenBank AY593484, AY593493), 15, ?22.2-41.8 mm standard length, Fars, Nasrabad Spring near Imamzadeh Ibrahim (29º35'09"N, 52º39'08"E);
Aphanius sophiae
(Heckel, 1849)
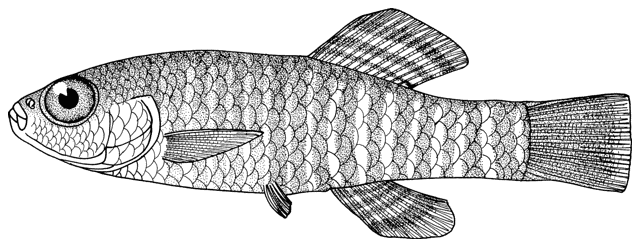
Male
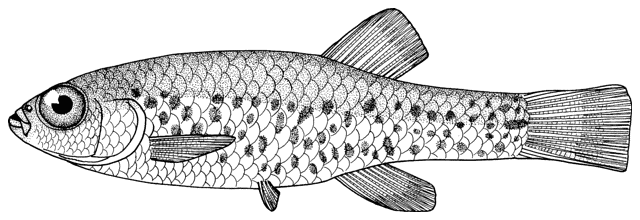
Female
Common names
gour-e khar, kopurdandan-e safiyeh or kopurdandan-e sufieh (= sufi or sophy tooth-carp).
[batrikh sophiae in Arabic; Persian minnow, Cypris pupfish, but this seems inapt].
Systematics
The type locality of Lebias Sophiae is in "lauen Salzquellen bei Persepolis" according to Heckel (1846-1849b). Lebias punctatus Heckel, 1849 listed from "Nemek-Deria oder
Salzsee, in welchen sich unter Schiraz die Quellen des Saadi ergiessen" or "the salty Maharlu Lake below Shiraz into which the Saadi springs flow" (Heckel, 1846-1849b) and Lebias
crystallodon from "grossen Salzsee Nemek-Deria, unter Schiraz" (Heckel, 1846-1849b) are synonyms (Günther, 1859-70; Garman, 1895; Gaillard, 1895; Hanko, 1924; Berg, 1949; Coad, 1996a). The
original catalogue handwritten by Heckel in Vienna gives the type locality as "Salzquellen bei Persepolis" which is in agreement with Heckel's publication. Lebias punctatus and Lebias
crystallodon are described from localities in the Shiraz valley wherein lies the salt lake Maharlu. The catalogue is more equivocal for these two nominal species, listing only "Nemek Deria".
The labels in jars containing L. crystallodon and L. punctatus were written under Franz Steindachner's curatorship and are not original (H. Ahnelt, in litt., 1987). Nemek Deria (=
salt lake in Farsi) is a general term employed for the type of terminal water body seen in both the Maharlu and Kor River basins. The situation was further confused by Berg (1949) who listed the locality
of punctatus as "Lake Niriz in Shiraz". Lake Niriz (= Neyriz or Bakhtegan) is the terminal sump for the Kor River.
All material of Aphanius from the Kor River basin examined fresh by me comprises fish with bars along the flank and fish with spots. The barred fish are males and the spotted fish females as
confirmed by dissection. Males and females do not segregate in the field and it seems very unlikely that the material examined by Heckel from several collections would be comprised solely of males (I found
sample sizes as low as 5 to contain both males and females). The males and females may have been separated and allocated different collection localities. The material described as L. punctatus is
then female L. sophiae and I strongly suspect that it was misunderstood as coming from the Shiraz valley and the locality in Heckel (1846-1849b) is incorrect. All female Aphanius from the
Shiraz valley are barred and the name punctatus is singularly inappropriate and would not be applied to them (see illustrations in Villwock (1960; 1977)). L. crystallodon could be a female
L. sophiae with weak spotting on the flanks, again mislabelled as from the Shiraz valley. Günther (1866) considers crystallodon to be a post-spawning female of L. sophiae and
Gaillard (1895) also considers it to be a female L. sophiae. Berg (1949) places L. sophiae as the male and L. punctatus and L. crystallodon the females of L. sophiae.
Cyprinodon blanfordii Jenkins, 1910 may well be a synonym of A. sophiae (see above under A. persicus for other views). The locality is vague, "East of Shiraz, South
Persia" and could lie in the Lake Maharlu basin (occupied only by A. persicus, the females of which are barred on the flank), the Kor River basin (A. sophiae only), or outside either of
these, in rivers draining to the Persian Gulf. The illustration shows a finely-spotted fish with a lozenge-shaped larger spot at the tail base, pigmentation found in A. sophiae. Cyprinodon
pluristriatus Jenkins, 1910 is often referred to the synonmy of A. sophiae but is found "East of Shiraz, stream running to Fussa, Southern Persia, 5,000 feet". Assuming this to be
Fasa and using topographic maps, the locality is in the Mand River basin draining to the Persian Gulf. The illustration and description refer to male fish and fall within the parameters of A.
sophiae, a species thought to be restricted to an endorheic basin. It may be an available name for a taxon occurring outside the endorheic Kor River basin where A. sophiae is endemic.
Parenti (1981; 1984) places Aphanius sophiae in the genus "Aphanius", i.e. distinct from true Aphanius without defining and naming a new genus. This genus is more closely
related to the Anatolian Kosswigichthys and the South American Orestias than true Aphanius.
The syntypes of Lebias sophiae are in the Naturhistorisches Museum Wien and include a wide range of material of which the following is part:-
Lectotype and paralectotypes (as labelled in 1997): NMW 14496 (6 fish measuring 25.8-30.4 mm standard length, one of which measuring 29.3 mm standard length is the lectotype as designated by F. Krupp,
24 October 1984 according to a jar label), NMW 22616-22623 (8, 26.1-29.0 mm standard length), NMW 60327 (8, 25.2-31.1 mm standard length), NMW 68283 (8, 18.9-27.6 mm standard length, partially dried and
distorted), NMW 75067 (7, 24.7-27.4 mm standard length), and probable syntypes NMW 14760 (67) and NMW 15056 (7). The catalogue in Vienna lists 40 fish in one column and 12 fish in the adjacent column, the
latter possibly meant to be the type series as it is written more boldly but which fish these were cannot now be ascertained. All these fish are males.
Eschmeyer et al., (1996) list syntypes under NMW 14496 (6), NMW 33616-23 (8) (not noted above), NMW 60327 (8), NMW 68283 (8), NMW 75067 (7) and also 9 fish in the Museum für Naturkunde,
Universität Humboldt, Berlin under ZMB 31377 (not noted above). The Berlin material is listed as ex 16140, is all male and 20.3-26.7 mm standard length.
Syntypes of Lebias punctatus comprise the following material:-
Lectotype and paralectotypes (as labelled in 1997): NMW 76509 (7 fish, measuring 29.9-36.0 mm standard length, one of which measuring 36.0 mm standard length is the lectotype as designated by F. Krupp,
24 October 1984 according to a jar label), NMW 15070 (5, ca. 29.9-35.6 mm standard length, dried and distorted), NMW 15156 (14, 19.2-32.1 mm standard length), NMW 59837 (5, 26.6-35.7 mm standard length),
and probable syntypes NMW 59609 (6, ca. 21.4-34.7 mm standard length, smallest dried). The Vienna catalogue lists only 12 fish opposite this name. These fish are all females.
The holotype of Lebias crystallodon measuring 40.1 mm standard length is NMW 15175 as the description and catalogue agree there is only one fish. The specimen is decoloured and in poor condition.
Key characters
The large, dark, usually lozenge-shaped spot at the caudal fin base in females with fine spotting on the flanks is distinctive for tooth-carps in this area of southern Iran.
Morphology
Lateral line scales 25(1), 27(7), 28(14), 29(17), 30(12) or 31(2). Scales above the lateral line 4-7, scales between lateral line and the anal fin 5-8, scales between the lateral line and the pelvic
fin 6-9, and scales around the caudal peduncle 15-20.
Total dorsal fin rays 11(1), 12(11), 13(27), 14(12) or 15(2); total anal fin rays 10(1), 11(18), 12(27) or 13(7); total pectoral fin rays 14(1), 15(7), 16(31), 17(10), 18(3) or 19(1); total pelvic fin
rays 5(9) or 6(44); total gill rakers 10(2), 11(28), 12(21) or 13(2); abdominal vertebrae 11(11), 12(37), or 13(5); caudal vertebrae 15(3), 16(33) or 17(17); and total vertebrae 27(8), 28(29) or 29(16).
Cephalic sensory pores are reduced to a series of neuromasts. Older literature on chromosome number was 2n=48 but is based on fish from outside the Kor River basin to which the species is restricted
here (Klinkhardt et al., 1995). Esmaeili et al. (2007) determined a chromosome number of 2n=48 with 14 pairs of submetacentric and 10 pairs of subtelocentric chromosomes and an arm number
of NF=76. Karyotype 8Sm + 40St and arm number 280 (Esmaeili et al.,
2008).
Sexual dimorphism
Males have more bands on the flank and are darker and more intensely coloured than females. Male dorsal, anal and caudal fins are a deep black with narrow, silvery-white borders and the anal fin has a
few silvery spots at its base. Females have brown dorsal, anal and caudal fins with 3-4 rows of black spots and the anal fin also has 2 cross rows of white silvery spots near the base. The caudal fin spot
in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally, single, smaller, dark, subsidiary spots may be found antero-dorsally and antero-ventrally to
the basal spot or scattered spots may be found irregularly before and behind the basal spot. Male flank bars 10(4), 11(1), 12(2), 13(3), 14(5), 15(4), 16(6), 17(3), 18(1) or 21(1).
Eleven of 21 morphometric characters are significantly different between the sexes including head length, head and body depths, fin lengths, and caudal peduncle shape (Coad, 1998i).
Colour
"The body colouration is basically dark brown; the body, with the exception of the forward dorsal portion and the belly, is marked by 12-17 white, silvery shining vertical lines or narrow bands.
Those individuals which have the most bands and are also darker and more intensively coloured seem to be males; their vertical fins are deep black with narrow silvery-white borders, and only the anal fin
has a few silvery spots at the base. The lighter individuals which are also slightly higher and wider, we believe to be the females; they have brown vertical fins with 3-4 cross rows of black spots;
their anal fins are marked with 2 cross rows of white silvery spots at the base" (Heckel, 1846-1849b).
The caudal fin spot in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally single, smaller, dark, subsidiary spots may be found antero-dorsally and
antero-ventrally to the basal spot or scattered spots may be found irregularly before and behind the basal spot.
The flank spots in females are much lighter than the caudal base spot and are from a half to much less in size, usually much smaller than half size. Spots are usually rounded but can be oval and
vertically elongate. Spots are independent of scale arrangement on the lower half of the flank, particularly posteriorly and may form 2-3 poorly defined to distinct longitudinal rows. Upper flank scales
have a crescent of pigment in mid-scale, leaving the margin and anterior base mostly free of pigment, or pigment may fill each exposed scale surface almost entriely except for the posterior margin, and
thus appear as spots. Females have purplish tints on mid-flank and yellowish tints on the lower flank, upper flank, head top and chin.
Males have a pigmentation very similar to that of A. persicus and the description here is identical. Some minor observed variation is attributed to variation in size and maturity of the fish
compared. Males have light flank bars half the width or much narrower than alternating dark bars. The margins of the dorsal, anal and caudal fins are clear while the rest of these fins is dark. Some fish
have up to 3, but usually 2, thin, light bars on the basal half of the caudal fin; these are generally larger fish. The margin of the lower half of the pectoral fin has concentrations of pigment on the
membranes such that this area is darker than the rest of the fin. The anal fin is darkest posteriorly where pigment is concentrated on membranes. The distal third of the fin is pigmented to form a dark
band, becoming lighter proximally. The dorsal fin is the darkest fin (except for the clear margin) and the anterior base is the darkest part of the fin.
Bands are not always evident but pigment spots are large proximally. Some fish have 2, sometimes 3, thin light bars at the base separated by thin dark bars and paralleling the body profile while others
have none. The dorsal fin base may have instead a series of lighter spots, sometimes irregular and not paralleling the body profile.
Size
Reaches 5.6 cm total length.
Distribution
This species is restricted to the Kor River basin in Fars.
Zoogeography
As noted in the genus account, Aphanius species in Southwest Asia are regarded as relicts of the Tethys Sea. The mountain populations of A. sophiae (and A. vladykovi) may well
have risen with the post-Pliocene uplift of the Zagros Mountains. Since Aphanius species have a low dispersal ability (Kosswig, 1967) it is considered unlikely that these small fishes dispersed
from lowland populations into an existing, high mountain range represented today by the Zagros.
Bobek (1963) suggests that there may have been an outflow from the Kor River basin to the Persian Gulf at the south-east corner which was cut off at the end of the Pleistocene by alluvial fans.
Krinsley (1970) maintains that any outlet was closed by the late Pliocene. Evidence for the isolation time of Kor River basin tooth-carps is equivocal.
Habitat
Tooth-carps identified as this species has been reported to survive in waters up to 39°C and salinities as high as 130‰. In aquaria lives at 5-37°C. It is found in springs, streams and small pools
and lakes in fresh and saline waters.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 64 fish measuring 2.18-4.64 cm standard length. The a-value was 0.0343 and the b-value 2.787
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown in detail.
Reproduction
Unknown. Incubation is said to be 10 days at 25°C but this is a general citation for Aphanius spp. in aquaria.
Parasites and predators
Unknown.
Economic importance
This species has importance as an aquarium species, being colourful and hardy.
Conservation
Construction of irrigation canals and drying of some springs because of man-induced changes in the water table may threaten populations of this species.
Further work
The distribution of this species and its population numbers should be documented by field work. Molecular and chromosomal techniques would prove useful information for relating this species with others
from neighbouring basins and throughout central Iran. Its biology is poorly known under natural conditions.
Sources
Type material: See above, Lebias sophiae (NMW 14496, 22616-22623, 60327, 68283, 75067, 14760 and 15056, Lebias crystallodon (NMW 15175), and Lebias punctatus
(NMW 76509, 15070, 15156, 59837 and 59609).
Iranian material:- CMNFI 1979-0025, 1, 31.6 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0059, 30, 18.1-39.3 mm standard length, Fars, Pulvar River 8 km south of Sivand (30º01'30"N, 52º57'E);
CMNFI 1979-0061, 3, 23.2-32.1 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0062, 6, 24.0-42.0 mm standard length, Fars, spring 17 km south of Sa'adatabad (30º05'N, 53º00'E);
CMNFI 1979-0067, 1, 25.7 mm standard length, Fars, qanat at Zarqan (ca. 29º46'N, ca. 52º43'E);
CMNFI 1979-0071, 6, ? mm standard length, Fars, qanat stream on road to Ramjerdi (ca. 30º00'N, ca. 52º38'E);
CMNFI 1979-0091, 3, 23.2-32.1 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0117, , mm standard length, ; fish?
CMNFI 1979-0155, 2, ? mm standard length, Fars, spring at Gavonoo (28º47'N, 54º22'E);ID?
CMNFI 1979-0292, 6, 26.5-34.4 mm standard length, Fars, Lapu'i spring near Zarqan (29º48'N, 52º39'E);
CMNFI 1979-0342, 1, 39.4 mm standard length, Fars, Kor River at Band-e Amir (29º49'N, 52º51'E);
CMNFI 1979-0498, 6, 17.4-25.2 mm standard length, Fars, spring in Kor River basin (30º05'N, 52º27'E);
CMNFI 2004-0003 (GenBank AY593483, AY593492), 47, ?20.7-28.8 mm standard length, Fars, Abdolmahdi Spring (30º06'12"N, 52º58'38"E);
CMNFI 2004-0004 (GenBank AY593482, AY593492), 38, ?19.5-34.0 mm standard length, Fars, Malasskuh Spring (29º52'04"N, 52º29'20"E);
uncatalogued (GenBank AY593481, AY593490), 6, 25.7-37.0 mm standard length, Fars, Dolatabad Spring (29º43'05"N, 52º50'11"E);
Aphanius vladykovi
Coad, 1988
Male
Female
Common names
gour-e khar, mahi-e gour-e khari (= "striped donkey" or zebra fish), kopurdandan-e Zagros (= Zagros tooth-carp), mahi parchami (= flag fish).
[Zagros pupfish].
Systematics
The type locality is in the Shahrestan-e Bakhtiari va Chahar Mahall in a large pool, 3 km west of Boldaji at 31°57'N, 51°01'E. The male holotype, 36.6 mm standard length, is in the Canadian Museum
of Nature, Ottawa under CMNFI 1979-0247 with 35 male and 16 female paratypes from the same locality under CMNFI 1979-0247A and 1 male paratype from a stream 3 km east of Boldaji at 31°55'N, 51°05'E
under CMNFI 1979-0248. Villwock (2004) has used cross-breeding experiments that demonstrate this species, A. sophiae and A. dispar are genetically isolated and distinct species.
Key characters
This species is distinguished from all other Aphanius in Iran and Southwest Asia by the higher number of normal-sized lateral line scales (36-47). Other members of Aphanius, and the
related Kosswigichthys, have 35, usually 30, or less in the lateral series. The high number of scales is matched only by the genus Anatolichthys Kosswig and Sozer, 1945 of southwest
Anatolia. Anatolichthys is polymorphic for scale counts which reach as high as 55 and shows scale loss, perhaps associated with the bitter, salty lakes in which it lives (Coad, 1988i). Male dorsal
fin colour is much darker and the anal fin is light compared to A. sophiae, which most closely resembles A. vladykovi. Females lack the typical large spot at the base of the caudal fin
found in A. sophiae.
Morphology
Scales are relatively small and numerous over the whole body as exemplified by scale counts. Scales are regularly arranged, embedded and imbricate. Anterior flank scales are a vertical oval with
circuli and radii restricted to the posterior field. Numbers of circuli and radii are body and scale dependent and so are fewer in this species than in A. sophiae. There are up to 21 teeth in the
upper jaw and teeth are in a single row in each jaw. Gill rakers are short, just reaching the adjacent raker when appressed. All fins are rounded distally and the anal fin is enclosed by a fleshy sheath,
most apparent in large females but is also present in males. The gut has a single large loop. Cephalic sensory pores are reduced to a series of neuromasts. Keivany (2003) gives details of cephalic
osteology. Pazooki et al. (2008) and Mardani Karani et al. (2008) examined interlocality morphological
variation, finding one of four populations to be more distinctive and 55 of 72
characters different respectively. Esmaeili et al. (2009) give a
chromosome number of 2n = 48 and a karyotype of 8 submetacentric and 40
subtelocentric chromosomes with an arm number of 28.
Lateral line scales 36(2), 37(8), 38(15), 39(6), 40(10), 41(6), 42(3), 43(1), 46(1) or 47 (1). Scales above the lateral line 6-9, scales between lateral line and the anal fin 8-12, scales between the
lateral line and the pelvic fin 11-15, and scales around the caudal peduncle 20-25.
Total dorsal fin rays 11(4), 12(21), 13(27) or 14(1), total anal fin rays 11(13), 12(25) or 13(15), total pectoral fin rays 14(4), 15(18), 16(25) or 17(6), total pelvic fin rays 5(9), 6(42), 7(1) or
8(1), total gill rakers 10(8), 11(41), or 12(4), and total vertebrae 27(2), 28(24), 29(26) or 30(1). Saefali (1999) records 8-11 gill rakers, 9-14 anal rays, and 9-15 dorsal rays.
Sexual dimorphism
Colour is sexually dimorphic and is outlined below. Ten of 21 morphometric characters are significantly different between the sexes including head length, head and body depths, fin lengths, and caudal
peduncle shape (Coad, 1998i). Females attain larger sizes than males (76 mm versus 58 mm total length (Keivany and Soofiani, 2004a; b)).
Colour
Live adult males bear creamy bars on yellowish flanks. The pelvic fins and the edge of the caudal fin are yellow, the anal fin has an orange-yellow edge and the pectoral fins are orange-yellow. The
dorsal fin is white with a wide blue-black band in the centre and a narrow blue-black band at the base. In some males the basal band is absent. Live adult females have a slight bluish tinge to their
flanks and flank spots are brown.
In preserved specimens, the dorsal fin bands are dark and remain darker in preservative than the pigmentation seen in Aphanius sophiae which is more diffuse, tending to several horizontal rows
of speckles, and which is not formed into a strongly distinct band or bands. Other fins are lightly pigmented, being finely speckled with melanophores with the dorsal and pectoral fins the darkest. The
anal fin is fleshy and not hyaline but pigmentation is light and not dark as in A. sophiae. There is some tendency to formation of one or two narrow bars on the anal fin, paralleling the base, but
these are weakly developed or absent in most fish. A mid-dorsal stripe is not apparent, perhaps because the dorsal flanks and back are darker than in females. Flank bars are light alternating with darker
broad bars. The lighter bars become broader posteriorly. There is a light, wide bar at the base of the caudal fin which is not included in the count of male flank bars. Male flank bars 9(3), 10(8), 11(8),
12(10), 13(3), or 14(5). The sides of the head and upper and lower jaws are pigmented with scattered melanophores while the underside of the head is not. The belly is also free of extensive melanophores.
The most characteristic feature of females is the large number of scattered spots on the flank, extending from behind the head to the base of the tail. These spots do not extend to the dorsal flank or
the back. The distinctive, large and often lozenge-shaped spot on the central caudal fin base of A. sophiae is absent. The upper back and flank are more heavily pigmented than the flanks (except
for the flank spots). The lower surface of the head and the belly are melanophore free as in males and the sides of the head and the upper and lower jaws are heavily pigmented. The dorsal fin is finely
pigmented on the rays and membranes and is generally darker than the caudal fin which is also finely pigmented. The other fins have very little pigment but all are fleshy and not hyaline. There is some
suggestion of a thin horizontal stripe near the base of the dorsal fin in the largest specimens but this is never well developed.
Teeth are hyaline or discoloured with brown in patches. The peritoneum is dark brown.
Size
Reaches 7.6 cm (Keivany and Soofiani, 2004b).
Distribution
This species is found near Boldaji and in the Chaghakor Wetland in the upper reaches of the Karun River basin. Abdoli (2000) indicates its presence on a map in the upper Marun River and the upper
Khersan River (the latter in the Karun basin).
Zoogeography
The origins of this mountain species have been discussed above under the account for A. sophiae.
It is found only in the uppermost reaches of the Karun River basin which drains to the head of the Persian Gulf. In this locality it is isolated from populations of Aphanius in the lowlands by a
series of tangs which block gene flow. Oberlander (1965) in studying the origin of Zagros drainage patterns found that streams did not follow the line of least resistance but often incise the
higher parts of mountain ridges which are anticlines composed of Miocene Asmari limestone. This situation arises because the Zagros ridges were eroded by a superposed drainage pattern already antecedent
to the exhumation of overlying sediments of later Miocene marls and evaporites. The trend of the Zagros ridges is in a NW-SE direction and streams often cross this direction at right angles. This has
resulted in the development of the tangs, narrow clefts in the Zagros ridges with very swift waters, which may be up to 2400 m deep with vertical sides rising from the water surface for 300 m,
effective barriers to small fishes.
Habitat
This species is found high in the Zagros Mountains (the type locality lies at an altitude of about 2380 m). It is found in small pools, streams and marshes, in fresh water. The type locality is an
artificially dammed pool about 300 m wide with cloudy water and a mud and pebble bottom. Capture depth was 40 cm. The shore was grassy and the pool contained large amounts of Myriophyllum and
marginal rushes. At 1515 hours on a warm, windy and sunny day, water temperature was 29°C, pH was 6.5 and conductivity was 0.2 mS. A second locality in Coad (1988i) was a mud-bottomed stream 3-4 m wide
with pools up to 1 m deep. At 1545 hours water temperature was 22°C, pH was 6.2 and conductivity was 0.45 mS. Current was slow and the stream had moderate amounts of Myriophyllum.
Keivany and Soofiani (2004b) reporting on the habitat at the Madar-Dokhtar spring near Gandoman, found water conductivity of 240-280 μS, pH 6.9-8.5 and total dissolved solids125-138 mg/l. The bottom
was muddy, interspersed with pebbles, and water is clear (but cloudy in the river). Myriophyllum and Potamogeton were used for spawning. Fish survive 30‰ and 30°C in aquaria.
Pazooki et al. (2008) found females to be more numerous than males,
and the sexes lived in mixed schools. Occasionally larger males would chase
smaller males but then both would rejoin the school. Males are territorial and
defended against fish and beetles up to twice their size. The species preferred
areas free from vegetation. These authors found the species easily tolerated to
33°C in aquaria for long periods, and the optimum temperature for the species is
probably 21-24°C.
A mass mortality of this species was observed by A. Abdoli in late March or early April 1994 at Chagh-khor wetland near Boldaji.
Bagheri (1999) describes the macrofauna and environment of the Chaghakhour Lagoon where this species occurs.
Age and growth
Keivany and Soofiani (2004b) show maximum age is 2+ years. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 319 fish measuring 1.46-4.64 cm standard length. The
a-value was 0.0309 and the b-value 3.062 (a b-value < 3 indicating a fish that becomh increaseand a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Abdoli (2000) reports Daphnia and Gammaridae as food. The main food in the Madar-Dokhtar spring is Gammarus, but a specimen from a river also had some algae, Cyclops sp., snail
eggs and fish larvae. Mouth structure would lead to the conclusion that this species is a surface feeder but it concentrated on the dominant crustacean in the Madar-Dokhtar spring (Keivany and
Soofiani, 2004b). Pazooki et al. (2008) found chironomids,
dipterans, Diaptomus, Daphnia, diatoms and filamentous algae in
guts and considered this species to prefer an animal diet.
Reproduction
Mature males chase the female in aquaria, pushing her into a corner, and showing pectoral fin flipping and shivering. Eggs were laid in a batch of 3-10. Absolute fecundity in the Madar-Dokhtar spring
examined by Keivany and Soofiani (2004b) was 220-650 eggs, relative fecundity was 45-155 per gram body mass and working fecundity (free and ripe eggs) was 110 eggs absolute and 73 relative.
Size of ripe eggs was 0.8-1.2 mm. Eggs are adhesive and attch to plants in small patches, from 3 to 30. Early April was the spawning peak. Eggs hatched at 9-13 days at 21-22ºC. Young reached 11.0-27.2
mm after three months in an aquarium.
Parasites and predators
Barzegar et al. (2004) examined this species for parasites in fish from the Beheshtabad river in Chahar Mahall va Bakhtiari Province and found Lernaea cyprinacea. Keivany and Soofiani
(2004b) found it to be susceptible to trichodiniasis and ichthyophthiriasis in aquaria.
Barzegar et al. (2008) record eye
parasites from this fish including the digeneans Diplostomum spathaceum,
Tylodelphys clavata and Ornithodiplostomum sp.
Raissy (2008) also investigated parasites in this species from the Gandoman
Lagoon.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea cyprinacea
on this species.
Economic importance
This pretty little fish has great potential as an aquarium species (Coad and Keivany, 1998; Keivany and Soofiani, 2004b). It has been maintained in an aquarium for several months, tolerating
temperatures of 30°C but probably favouring 20-22°C. pH values were 8-9. Water changes with their associated variations in conditions had no affect on the fish. The pupfish survived 3-4 hours longer
than a Capoeta species when the latter was treated with formalin in a shared tank for a Saprolegnia infection and the tank was inadvertently left for 6-8 hours without a water change.
Male pupfish are very aggressive and frequently attack other fishes of the same size or even larger. They ate all the coexisting guppy larvae in the aquarium, even those aged 2-3 weeks. The pupfish was
fed crushed, dried freshwater shrimp and sometimes live shrimp although they had difficulty handling live shrimp. They were also observed biting and destroying vegetation in the aquarium, although it is
unclear whether they actually ate any. It is now available in aquarium stores in Germany (Oliver Lucanus, pers. comm., 23 January 2004) and is kept during summer months in garden ponds, reproducing there
successfully (Thomas Schulz, pers. comm., 14 January 2005).
Conservation
The distribution of the species is apparently quite limited, in waters neighbouring the towns of Boldaji and Shahr-e Kord. This should be confirmed by extensive field work in this poorly explored
area of the Zagros Mountains. A limited distribution renders this species liable to significant loss or even extinction if habitats are disturbed or destroyed. The type locality, it should be noted,
is an artificially dammed pond.
Significantly, a mass mortality of this species was observed by A. Abdoli in late March or early April 1994 at Chagha Khur wetland although the cause is unknown.
M. Raissy (pers. comm., 1 July 2009) reports parasitic infections (7 species,
including the digenean Ornithodiplostomum in most organs) as a cause for
depopulation in Choghakhor (Chagha Kur) lagoon.
An additional threat is escapes of cultured rainbow trout (Oncorhynchus mykiss). Trout culture is being rapidly developed in this region and the trout are known predators on native fishes.
Further work
The distribution of this species in the Zagros needs to be clarified.
Sources
Type material: See above, CMNFI 1979-0247, CMNFI 1979-0247A and CMNFI 1979-0248.
Iranian material: CMNFI 2004-0005, ?, ?, mm standard length, ;
uncatalogued (GenBank AY593486, AY593495), 4, 25.6-37.0 mm standard length, Chahar Mahall va Bakhtiari, Ebrahimabad (31º52'30"N, 51º10'10"E);
uncatalogued (GenBank AY593487, AY593496), 2, 21.5-28.7 mm standard length, Chahar Mahall va Bakhtiari, Taqanak Bridge (32º12'35"N, 50º49'29"E);
uncatalogued (GenBank AY593485, AY593494), 5, 31.0-49.8 mm standard length, Chahar Mahall va Bakhtiari, Madar-Dokhtar spring (31º52'12"N, 51º08'29"E).
Poeciliidae
Poeciliids, including the livebearers, are found in fresh and
brackish waters from the eastern United States south through the
Caribbean to northeastern Argentina and in Africa. There are about 37 genera
with about 304
species (Nelson, 2006) and one or two species of livebearer has been widely introduced
around the world as a control agent for malaria and is found in Iran, along with
a released aquarium species.
This family is characterised by an anteriorly rounded and
posteriorly compressed body and a depressed head; large cycloid scales
on the head and body; supraorbital pores with neuromasts in fleshy
grooves; a small, terminal mouth with the lower jaw projecting; teeth
incisor-like or villiform on the jaws; gill membranes free from the
isthmus; 4-6 branchiostegals; no spiny fin rays; a small dorsal fin;
pectoral fins set high on the side (in contrast to Cyprinodontidae
anterior pelvic fins; no adipose fin; and the anal fin in males is
developed into a copulatory organ with rays 3-5 modified while females
have a rounded anal fin (in livebearers only, the species found in
Iran, in contrast to Cyprinodontidae).
The gonopodium is used for transferring sperm packets into the
female. It is moveable to the side or forward to allow copulation to
occur. The sperm packets release sperm when placed in the female and
some can be stored for future use. Details of gonopodium anatomy are
important in identifying and classifying species. The gonopodium is
composed primarily of the third, fourth and fifth anal rays with
various hooks, serrae and spines. Young are born alive, a condition
known as ovoviviparity where the eggs develop and hatch in the mother.
Livebearers are found in habitats from mountain streams to brackish
coastal marshes and river mouths. Food is mostly encrusting algae and
the associated, small invertebrates. Males tend to be smaller than
females and more brightly coloured. These colours are best seen during
the courtship display.
Some species are important in the aquarium business, such as the
guppy and swordtail, while the mosquitofish has been used world-wide
as a predator on aquatic mosquito larvae, the adult fly being a
carrier of malaria. They have also been used extensively in genetics
research, research on tumours and in immunology. Some livebearers are
all-female species and egg development is stimulated by spermatozoa
from another species, without any genetic contribution. Young are
identical to the mother. This unusual form of reproduction is called
gynogenesis. Others have superfetation where eggs are at different
developmental stages within the mother and are born over a period of
several days rather than all at once.
Genus Gambusia
Poey, 1854
This genus is characterised by large scales, short dorsal and anal
fins, the anal fin in males anteriorly placed and modified into a long
intromittent organ (or gonopodium), dorsal fin inserted behind the
anal fin origin (in contrast to Aphanius, Cyprinodontidae), and dorsal and
caudal fins are spotted.
Gambusia affinis
(Baird and Girard, 1853)
Gambusia holbrooki was considered to be a
subspecies of this species. Iranian mosquitofish were G. holbrooki
but it is possible that some populations consist of G. affinis
although none were seen by me or by Holčík and Oláh (1992). Shakirova and Sukhanova (1994) report
this species from the Atrek River in Turkmenistan, which flows into
Iran, as well as Kopetdag streams on the northern border of Iran. However
identification of these taxa may be confused. The source of most (? all) Middle
East mosquitofish is Europe and Kottelat and Freyhof (2007) record that there is
no confirmed presence of G. affinis in Europe.
Gambusia holbrooki
Girard, 1859
Common names
گامبوزيا (= gambusia).
[gambuzi, zoory, or zurri in Arabic; gambuziya in Azerbaijan; gambuziya in Russian;
mosquitofish; eastern mosquitofish (for holbrooki, western
mosquitofish for affinis); plague minnow, in reference to its ecological impact].
Systematics
Gambusia holbrooki was originally described from eastern
Florida and South Carolina, U.S.A. Wooten et al. (1988)
distinguish the eastern and western mosquitofish in the U.S.A. based
on morphology, biochemistry and distribution. However, extensive
hybridization occurs in the native habitat and the composition of all
the introduced Iranian populations has not been studied. Introduced
populations world-wide are generally referred to the taxon holbrooki.
Key characters
Males are easily recognised by the anal fin rays 3-5 being
specially modified into an elongate gonopodium for intromittent
fertilisation. G. holbrooki has 8 dorsal and 11 anal total fin
rays while G. affinis has 7 dorsal and 10 anal total fin rays
(Walters and Freeman, 2000).
Morphology
Dorsal fin with 5-9 rays, usually 7 (with the last two counted as one),
anal fin with 7-11 rays, usually 10, and pectoral fin with 11-14
rays. Lateral scale rows number 26-33. In males, the posterior edge of
the joints of the first elongate ray is serrated (smooth in affinis),
the cusps of the posterior branch of the second elongate ray are short
and almost straight (long and curved in affinis), and the
apical hook of the posterior branch of this ray is short with 2-3
joints (very long with 4-6 joints). Vertebrae 28-34. The karyotype is 2n=48.
Detailed counts on Iranian specimens were not made; examination of cleared and
stained material indicates that the species in Iran and Iraq is G. holbrooki.
Sexual dimorphism
Males reach a smaller adult size than females (see below). Males
have the end of the anal fin base well ahead of the beginning of the
dorsal fin. Females lack a gonopodium and the end of the anal fin is
under the beginning of the dorsal fin. In addition various
morphometric characters differ widely (see Abdurakhmanov (1962) for details).
Colour
Males are translucent grey to light olive with a blue, green or
purplish sheen on the sides and opercle. The back is olive-brown to
yellowish-brown and the belly silvery or yellowish. A dark bar passes
through the eye. The iris has a purple sheen. The flanks may appear
spotted as pigment margins the scales to form a diamond pattern on the
body. Dorsal and caudal fins are spotted and a dusky, light tan but
other fins are clear. Adult females have a large, triangular,
bluish-purple blotch on the lower flank behind the pectoral fin
(called the gravid spot). The black peritoneum can be seen through the body wall.
Size
Reaches 63.0 mm in females and 45.4 mm in males (Tabibzadeh et
al., 1970a; Vargas and de Sostoa, 1996), perhaps to 8 cm in the largest
females (Reshetnikov, 2002).
Distribution
The natural distribution is from New Jersey southward to northern
Mexico but it has been introduced to all continents except Antarctica. This species was
first introduced to the Ghazian marshes of the
Caspian littoral of Iran in 1922-1930. Some mosquitofish may have spread from the Lenkoran
District of Azerbaijan, reaching the Safid Rud in 1937 (Shukolyukov, 1949). From the Ghazian stock, additional samples
were collected in 1966 for introduction around the country via raising
ponds to over 3000 permanent water bodies (Tabibzadeh et al.,
1970a, 1970b; Spillman, 1972; Emadi, 1996c). Many of these contained
fishes but some were previously fishless or at least now appear to
contain only mosquitofish, e.g. springs at Kahurak and Hormak in
Sistan and Baluchestan Province. Over 1.5 million fish were
distributed in 1969 alone (Tabibzadeh et al., 1970b). This species is now
the most widely distributed fish in Iran (Abbasi et al., 1999; Kiabi et
al., 1999; Abdoli, 2000).
Zoogeography
This species is an exotic, originating in eastern and southern
North America and introduced to Iran from stocks introduced to Italy
and from Baku in Azerbaijan.
Habitat
Mosquitofish are normally inhabitants of clear and weedy streams
and ditches, weedy margins of large rivers and lakes, marshes and
brackish coastal lagoons, usually over mud or sand bottoms. They
prefer more open waters with less vegetation than Aphanius dispar,
for example. Males and females in
aquaria swam together in a mixed school in contrast to the sexually segregated
schools of Aphanius dispar (Al-Daham et al., 1977). In the Anzali Mordab, dense schools are found in surface
waters of areas covered by submerged and floating vegetation while
deeper water is fishless (Holčík and Oláh, 1992). In Iran, it is one of two most abundant species in
Caspian wetland areas along with Carassius auratus (Iranian
Fisheries Research and Training Organization Newsletter, 19:4, 1998).
Al-Daham and Bhatti (1977) found G. affinis, probably this
species, to have a complete tolerance of 10.25‰ sea water and
90% of fish survived 20.5‰ for 24 hours and even as high as 58‰.
This species has been reported to survive in waters up to 42°C
and as low as 0.5°C. Summer air temperatures of 45°C
and winter temperatures several degrees below zero are survived as
long as the water is deep enough (Tabibzadeh et al., 1970a).
Upper and lower thermal tolerance increases with body size (Al-Habbib
and Yacoob, 1993). At temperatures below 15-18°C, mosquitofish grow but
do not mature or breed. They prefer a temperature of 31°C.
Muddy and polluted conditions, acid to alkaline water, and
dissolved oxygen less than 1 mg/l are survived by this species. pH
range survived is 4.46-10.2 and dissolved oxygen levels as low as
0.2-0.4 mg/l as long as water surface access is available. They can take
advantage of the oxygen rich surface layer as they position their bodies with
flattened head and back immediately adjacent to t\he air-water interface (Lewis, 1970).
Their success in Iran and other waters world-wide is attributed to
the following factors according to Meffe and Snelson (1989):- 1)
abundant in original range, 2) polyphagous, 3) short generation time,
4) a single female can colonise since livebearer, 5) broad
physiological tolerances, 6) closely associated with man, 7) high
genetic variability, 8) specialized reproduction with moderate numbers
of advanced young several times a year, and 9) high aggression levels.
Age and growth
Numbers of each sex in a population vary between localities, mostly
females predominate over males. Males are more sensitive to
temperature extremes, starving and overcrowding and this will affect
such things as size and age at maturity. Males do not grow much after
sexual maturity is attained but females have indeterminate growth,
although growth slows as energy is put into egg production. The average condition factor for males was 1.2369 and for females 1.0906 in
southern Iraq. The condition factor slowly decreases as length increases in the
male and vice versa for females (Huq et al. 1977). Sexual
maturity can be reached in only 2-3 months but such fish die before
winter. Minimal size for maturity is 16-28 mm total length. In the
laboratory under ideal conditions sexual maturity can be attained in 3
weeks. Life span seldom exceeds 15 months, although the range is 6-24+
months depending on when in the spawning season the fish was born. In
Spain, maximum ages are 2+ for females and 0+ for males (Vargas and de Sostoa, 1996).
Fish born late in the year may not mature until the following year.
Growth to maturity can be so rapid that a 50-fold increase in
numbers can occur over a 10-week period. Densities may reach over half
a million fish per hectare. Under ideal conditions it is theoretically
possible for 10 pregnant females to produce 5 million fish in 6 months.
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 35 Iranian fish measuring 1.98-3.27 cm standard length. The a-value
was 0.0190 and the b-value 3.214 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Generally this species is regarded as an opportunistic omnivore.
Swanson et al. (1996) refer to this fish as an aggressive
predator and Al-Daham et al. (1970) for southern Iraqi fish considered it to be a carnivore,
feeding on aquatic and terrestrial insects including spiders, ants, beetles and
mites, and also filamentous algae. Females are cannibals. Food includes diatoms, algae, worms,
crustaceans including zooplankton, insects, snails, other fishes and
amphibian larvae. The mosquitofish is dependent on sight to detect and
attack prey. Selection of zooplankton can lead to an increase in
phytoplankton numbers (and thus affect water quality) and to changes in size
frequencies of zooplankton populations.
Food is taken at or near the surface and by grazing on plants and
rocks. Tabibzadeh et al. (1970a, 1970b) record diet as molluscs, aquatic
insects and various forms of mosquito larvae. They report up to 94 pupae or 104
mosquito fourth-stage larvae eaten per day by this fish and effective clearance
of springs and marshes. Large-sized mosquitofish took more than 500 second instar mosquito larvae per
day and medium- and large-sized mosquitofish consumed 22-64 fourth instar larvae
per day in an Iraqi study under laboratory conditions (Mohsen et al.,
1989). Abdoli (2000) lists Diptera and Chironomidae as
food items. Meffe and Snelson (1989) report
predatory behaviour on the young of other species of fish and females
attack other fishes, shredding fins and sometimes causing mortality.
Reproduction
Na'ama and Al-Hassan (1989) report G. affinis (presumably G.
holbrooki) to have a brood size up to 48 from Baghdad specimens and 51 from
the Basrah area, Iraq. The smallest reproductive female was 1.7 cm total length. Weight is the most
accurate predictor of brood size. In Iran, Tabibzadeh et al. (1970a) found that females mature and
reproduce within 2 months at temperatures above 15°C.
In less favourable conditions this may take 8-10 months. A minimum
photoperiod of 12.5-13 hours is necessary to stimulate reproduction
although year-round temperatures above 20°C
can offset this light requirement. About 17 days after fertilisation,
the female gives birth to as many as 428 live fish over a period of
about 1 month after 3-8 weeks gestation. Each fertilisation can give
2-3 broods and each female can produce up to 9 broods during her life,
although 2-5 is more usual.
The breeding season extends from April to November in favourable
conditions, May to September in more temperate conditions. Several
months pass between successive spawnings. Eggs are up to 1.8 mm in diameter when
mature and embryos are about 6-8 mm at birth. The young are protected
within the female and are independent when born, with resulting low
mortality. Environmental breeding requirements are simple and with low
mortality results in the successful spread of this exotic.
The small male approaches the female from behind and with a rapid
motion inserts his gonopodium tip into the female. The gravid spot is
a releasing stimulus. It indicates a receptive female and also is a
target for the male gonopodium. Sperm are transferred in a
spermatophore. During mating the gonopodium is angled forward at 140-150°.
Most males copulate without courtship. When courtship occurs it
involves the male assuming an s-shaped position with its body and
vibrating in front of, or at the side of, the female. Males are
aggressive and dominate smaller males to restrict access to females.
Females may be inseminated by several males and sperm can be stored to
be used up to 10 months later. A single transplanted female can
populate a new habitat.
Parasites and predators
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species (as G. affinis).
Economic importance
Introduced widely to combat malaria by consuming the aquatic larvae
and pupae of the carrier mosquito. However to be effective, this fish
must have aquatic plants cleared from its habitat. The mosquitofish is
also a predator on the eggs and young of other, native fishes and a
competitor for food, and it alters ecosystems by greatly reducing rotifer,
crustacean and insect populations allowing phytoplankton to increase
dramatically (Myers, 1965; Hurlbert et al., 1972; Tabibzadeh et al., 1970a;
Edrissian, 2006; Chandra et al., 2008).
Native species might well be more effective predators on mosquito
larvae (see account of Aphanius dispar and Ahmed et al. (1988) and Beidas and White (1982)). Muhaisen et al. (1985) studied control of this
species in Iraq as larvae on fish farms and found potassium permanganate, copper sulphate, acriflavine and malachite green to be effective.
Haas and Pal (1984) point out that Gambusia may favour schistosomiasis, a human parasite, by altering the ecology of
freshwaters, perhaps by reducing the numbers of natural predators on vectors of this parasite. They also note that it is considered a pest by fish farmers.
Tabibzadeh et al. (1970a, 1970b), Motabar (1978) and Swanson et al. (1996) give methods for raising and distributing
mosquitofish. Also used as a research species and sometimes seen in aquaria. Al-Nasiri and Sharma (1978) used this species in Iraq to study the toxicity of
the agricultural insecticide Ekatin 25 to fish and Mohsen et al. (1989) studied the predatory efficiency and tolerance to mosquito larvicides.
Conservation
The most widespread fish in Iran and, as an exotic, not in need of
any protection. However this distribution undoubtedly affects the
conservation status of native species since mosquitofish have a strong
impact in small and non-diverse habitats such as the small springs
found throughout Iran. Muus and Dahlstrøm (1999) mention that it
tends to oust native toothcarps.
Further work
The effects of this species in competition with native fishes
should be investigated although it is so widespread that it could
never be eradicated.
Sources
Meffe and Snelson (1989) give the ecology and evolution of the
family and Rauchenberger (1989) the systematics of the genus. Swanson et
al. (1996) and Pyke (2005, 2008) review biology, culture and mosquito control and are a
source for general information above.
Iranian material: The following is a sub-sample of Iranian material as this
species is very common and material extensive (see map for distributional
information): CMNFI 1970-0515, 2, 20.6-30.2 mm standard length, Gilan, Shafa River near estuary (37º35'N, 49º09'E);
CMNFI 1970-0519, 5, 14.1-19.5 mm standard length, Gilan, Chelvand River (ca. 38º18'N, ca. 48º52'E);
CMNFI 1970-0590, 3, 25.1-29.2 mm standard length, Mazandaran, Shesh Deh River near Babol Sar (ca. 36º43'N, ca. 52º39'E);
CMNFI 1971-0343, 1, 26.0 mm standard length, Gilan, Langarud at Chemkhaleh (37º13'N, 50º16'E);
CMNFI 1979-0019, 26, 15.4-29.0 mm standard length, Fars, Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 1979-0025, 2, 28.7-33.8 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0026, 1, 21.6 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E);
CMNFI 1979-0126, 53, 14.9-33.1 mm standard length, Fars, Dasht-e Arjan springs (29º37'N, 51º58'E);
CMNFI 1979-0173, 13, 24.7-39.6 mm standard length, Hormozgan, qanat at Hajjiabad (28º19'N, 55º54'E);
CMNFI 1979-0192, 2, 24.9-26.4 mm standard length, Fars, qanat 2 km east of Rostaq (28º26'30"N, 55º04'E);
CMNFI 1979-0226, 65, 23.1-39.2 mm standard length, Sistan, 3 km east of Kuh-e Khajeh (30º57'N, 61º17'E);
CMNFI 1979-0230, 36, 7.2-44.4 mm standard length, Sistan, Jehil-e Puzak (ca. 31º15'N, ca. 61º42'E);
CMNFI 1979-0283, 1, 35.9 mm standard length, Kermanshahan, Qareh Su basin (34º21'N, 47º07'E);
CMNFI 1979-0288, 7, 26.8-29.4 mm standard length, Ilam and Poshtkuh, Gangir River (33º50'N, 46º18'E);
CMNFI 1979-0309, 5, 15.4-32.8 mm standard length, Kerman, Fahraj River (28º57'N, 58º42'E);
CMNFI 1979-0328, 2, 24.3-24.8 mm standard length, Baluchestan, jube near Bampur (27º10'30"N, 60º21'E);
CMNFI 1979-0360, 13, 17.8-25.9 mm standard length, Khuzestan, Karkheh River canal (31º40'N, 48º35'E);
CMNFI 1979-0478, 2, 17.8-28.2 mm standard length, Mazandaran, ditch 29 km from Eimar (37º09'N, 54º36'E);
CMNFI 1979-0498, 2, 22.1-35.8 mm standard length, Fars, spring on road to Dariush Dam (30º05'N, 52º27'E);
CMNFI 1980-0122, 25, 14.3-26.8 mm standard length, Mazandaran, Nerissi River (36º38'N, 52º16'E);
CMNFI 1980-0128, 7, 23.6-35.6 mm standard length, Mazandaran, Qareh Su (36º49'30"N, 54º03'30"E).
CMNFI 1980-0132, 5, 18.4-30.2 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E);
CMNFI 1980-0145, 75, 32.6-40.4 mm standard length, Gilan, Bandar-e Anzali (37º28'N, 49º27'E).
Comparative material: BM(NH) 1974.5.2:38-40, 3, 17.3-25.1 mm standard length,
Iraq, Baghdad (33º21'N, 44º25'E); BM(NH) no catalogue number, 6, 10.7-37.5 mm standard length,
Iraq, Baghdad Liwa (no other locality data); BM(NH) no catalogue number, 4, 28.3-38.8
mm standard length, Iraq, Baghdad Liwa (no other locality data); BM(NH) no catalogue
number, 8, 16.8-32.5 mm standard length, Iraq, Kut Liwa (no other locality data);
BM(NH) no catalogue number, 2, 20.3-21.9 mm standard length, Iraq, Hilla Liwa (no
other locality data).
Genus Xiphophorus
Heckel, 1848
This genus comprises the platyfishes and swordtails
and is found in Mexico and central America with 25 or more species. these are
small fishes, 14 cm or less, with several members and hybrids important in the
aquarium trade. They are also used in genetic studies. They have a gonopodium in
males as in other poeciliids with a unique structure in detail (Rosen, 1960).
Male swordtails may have a characteristic elongate lower caudal fin lobe, the
"sword" but this is not always developed in all species and all individuals.
Xiphophorus hellerii
Heckel, 1848


Male and female specimens from Jahrom
Common names
dom shamshiri.
[swordtail, green swordtail]
Systematics
The trivial name is often spelled helleri.
Key characters
The swordtail is characteristic along with details of the gonopodium.
Morphology
Dorsal fin branched rays 11-15, anal fin branched rays 8-10, pectoral fin
branched rays 12-13 and pelvic fin branched rays 6. Lateral line scales 26-30.
Vertebrae usually 30-31.
The swordtail is medium to large with a long straight caudal appendage. The
terminal segment of gonopodial ray 3 is produced into a crescent-shaped hook and
blade, pointed distally Ray 4a curves strongly backward over the blade at
an angle greater than 90°. Distal serrae of ray 4p are reduced in size and
number, and proximal serrae are rather slender; The terminal segment of ray 5a
is produced into a claw, several times larger than the distal serrae of ray 4p.
scale rows along the middle of the body are green to blue; belly is white and
there is a s in all populations (regional forms). In a few variants, there can
be one or two additional lines above and one or two below this line. In almost
all populations, the dorsal fin has spots or flecks of red. This coloration can
also appear in the caudal fin. The sword of males is bright yellow and edged
more broadly in black below than above. Old fish (3 to 4 years old) can have a
totally black sword from the caudal fin all the way to the tip of the sword.
Sexual dimorphism
The male sword may not always develop in aquarium specimens but usually
appears at 3-4 weeks of age. Mature females have a gravid spot in front of the
anal fin.
Colour
The overall colour is olive-grey to olive-green. There is a light red, dark
red dark, violet or dusky brown longitudinal line or rarely a zig-zag stripe on the
greenish-yellow flank, and there may be 1-2 reddish stripes above and 1-2
reddish stripes below. Scale rows along the mid-flank are green to blue, edged
with brown and net-like in appearance. The dorsal fin is spotted red or
brownish in median and below the margin rows. Fins are generally a yellow-green. The belly is white. The sword is a
bright yellow or orange-yellow edged more broadly in black below than above. The
black lower edge is carried forward onto the caudal peduncle. Fish 3 to 4 years
old can have a totally black sword. Aquarium specimens may be red, green, black
and albino, and various combinations of these.
Size
Attains
16.0 cm total length, females larger than males.
Distribution
The native distribution is from southern Mexico to Guatemala, Honduras and
Belize. Introduced specimens were caught in Iran at Jahrom (28º29'N,
53º31'E) and Kashan 34º04'N,
51º31'E) (Esmaeili et al., 2010).
Zoogeography
An exotic species presumably released from aquaria.
Habitat
In its native habitat this species is found in streams and rivers but can
also occur in canals, ponds, lagoons, ditches and in warm springs. The Iranian
specimens were caught in a small pool, part of a qanat system, and in a spring. Vegetation
can be none to common. The water may be clear to polluted and current none
to moderate. Adults prefer current in streams while the young remain in quieter
shore areas. It occurs at both low and relatively high elevations, up to ca.
1500 m. In aquaria this species prefers slightly alkaline, medium hard water and
temperatures in the 22-28ºC range.
Age and growth
Sexual maturity is reached after eight to twelve months in some reports,
three months in others. Life span exceeds 4 years.
Food
Food is crustaceans, insects, worms and plant matter.
Reproduction
This species is a livebearer and is easy to breed in aquaria. Females can
produce more than 180 young at one time, at intervals of about a month. Adult
males can be aggressive to other fishes. A well-developed male sword stimulates
females to mature and inhibits maturity in younger males. The female swordtail can undergo sex reversal,
becoming male under certain environmental conditions.
Parasites and predators
None recorded in Iran.
Economic importance
None at present in Iran but a possible source of aquarium specimens. It is a
popular aquarium species, easy to maintain and is sold world-wide and it has
been used in genetic studies. There are various cultivated forms of this
species, hybrids with related species. It does adversely affect native species
when introduced.
Conservation
None required as an exotic.
Further work
The biology of this species in Iranian waters, and its effects on native species, requires study.
It should be extirpated, if possible.
Sources
General aquarium literature and Esmaeili et al. (2010).
Gasterosteidae
Sticklebacks are found in marine and fresh waters of the cooler
parts of the Northern Hemisphere. There are about 8 species of which
one is native to Iran and another has been introduced.
This family of small fishes is characterised by a compressed,
fusiform body; teeth in bands in each jaw but none on the tongue or
palate; a protractile mouth; 3 branchiostegal rays; no postcleithrum; no scales but a
series of plates along the flank variably developed, sometimes absent;
2 or more (usually 3-16) isolated spines in front of a soft dorsal fin
(usually 6-14 rays); and a pelvic fin with a strong spine and only 0-2
soft rays.
The species in this family have been studied extensively (Coad,
1981f; Paepke, 1983; Wootton; 1976; 1984; Paepke in Bănărescu
and Paepke, 2002). There is variation in
colour, body form, spine numbers and development, and plate numbers.
These variations in anatomy are matched by variations in biology such
as habitat, feeding and reproduction. Variation in behaviour, biology
and in speciation makes these small fishes, which have no commercial
value, particularly important. Some of the variation is owing to
environmental factors while some has a genetic basis. Several books
have been devoted to them and thousands of scientific studies.
Sticklebacks make excellent aquarium fishes. Their reproductive
behaviour is complex, involving courtship and nest building. Some
populations are anadromous and enter fresh water to breed.
Genus Gasterosteus
Linnaeus, 1758
This genus is characterised by having a modal count of 3 isolated
strong spines in front of the soft dorsal fin, the body usually has
strong bony scutes along the flank developed as a keel on each side of
the caudal peduncle, a strong spine in the pelvic fin, and a complex
reproductive behaviour.
The threespine stickleback, and related populations which act as
good species but have not been formally named, have been studied
extensively for their reproductive and other behaviours and for the
insights that morphological variation and genetics throw on evolution.
Gasterosteus aculeatus
Linnaeus, 1758
Common names
سه خار seh khar (= threespine), سه خاره
(= seh khareh or sehkhareh), mahi seh khareh.
[trekhiglaya kolyushka or threespine stickleback in Russian; threespine stickleback].
Systematics
Gasterosteus aculeatus was originally described from Europe. The complex systematics of sticklebacks
are not reviewed here. The Iranian specimens appear to be of a "marine" form with
well-developed scutes on the flanks but their origin is not known.
Key characters
The 3 strong dorsal spines, strong pelvic fin spine with a single
cusp at the base and the row of plates along the flank are distinctive.
Morphology
First dorsal fin comprised of usually 3 spines (2-4), second dorsal fin
with 1 spine and 7-14 soft rays, anal fin with 1 spine and 6-13 soft
rays, pectoral fin with 8-11, usually 10, branched rays and pelvic fin
with 1 spine and 1 soft ray. Gill rakers 14-27, elongate and reaching
as far as the fourth adjacent raker when appressed. Vertebrae 27-36.
Plates along the flank 30-37; the Iranian specimens have a complete
row of flank plates and a well-developed caudal peduncle keel, other
populations may have an incomplete row or no plates at all. This
species is very widespread and extremely variable over this range in
the characters listed above and may well exceed the limits cited, e.g.
dorsal fin spines are absent in some fish but the Iranian fish are of
the typical marine form. The chromosome number is 2n=42 (Klinkhardt et
al., 1995). The gut is short and s-shaped.
An Iranian specimen had 3 dorsal fin spines and 10 soft rays, 1
anal fin spine and 7 soft rays, 10 pectoral in rays and 1 spine and 1
soft ray in the pelvic fin. Scutes, including those forming the caudal
peduncle keel, numbered 32. Total gill rakers numbered 19.
Sexual dimorphism
Breeding males develop a red belly and throat, blue sides, light blue back and have
bright blue or turquoise eyes.
Colour
Marine populations are more silvery on the flanks than freshwater
ones which are more olive. Generally the back is green-brown, olive or
grey to blue-black, flanks olive to silvery and the belly
silvery-white. Fins are generally clear. The peritoneum is silvery
with large, widely spaced melanophores. Stream-dwellers have an irregular
pattern of dark spots on the flanks and the back is an iridescent dark green.
Size
Reaches 11.0 cm.
Distribution
Found around the Northern Hemisphere in fresh and marine waters.
Reported as an introduction to Iran by Abdoli (1993a, 1993c) and Coad
and Abdoli (1993b), this species is found at the Neka Power Plant in
Mazandaran near Behshahr, the Tajan River, the lower Gorgan River and Gorgan Bay,
Bandar-e Torkeman, Gomishan Lagoon, the lower Babol, Heraz, Chalus, Tonekabon, Langarud, Polarud and Safid rivers, the Anzali Talab and the
southeast Caspian Sea, the middle to upper Kashaf River in Khorasanin the
Tedzhen (= Hari) River basin, and the upper Kal
Shur and upper Jomein.rivers in the Dasht-e Kavir basin (Abbasi et al., 1999;
Kiabi et al., 1999; Abdoli, 2000; K. Abbasi, pers. comm., 2001; Jolodar and Abdoli, 2004;
Niksirat et al., 2006; Abdoli and Naderi, 2009).
It is also caught by herring fishery ships on the coast of Iran (K. Abbasi, pers. comm., 2001).
Zoogeography
The exotic origin of the Iranian population is not known but the
complete row of plates suggests a marine population. Sal'nikov (1995)
reports this species from the southeastern Caspian Sea and moving into
the Atrek River in Turkmenistan in large numbers. He refers to it as
the Black Sea-Azov three-spined stickleback but it is unclear whether
by this is meant its origin, its dispersal route or simply the nearest
natural habitat for the species.
Habitat
These sticklebacks inhabit inshore coastal waters, lakes, ponds,
rivers and streams, and may be anadromous. Marine and lake fish can be pelagic. They are
often found among algae. In Iran, this species is caught at 30-100 m by
herring fishery ships (K. Abbasi, pers. comm., 2001).
Age and growth
Maximum life span is a little over 3 years although some fish
probably live only 1 year and a few months, dying after they spawn. Paepke in
Bănărescu and Paepke (2002) is a recent review of literature.
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 500 Iranian fish measuring 3.60-7.20 cm standard length. The a-value
was 0.0090 and the b-value 3.428 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Patimar et al. (2010) studied this exotic in the Gomishan Wetland of the
southeast Caspian Sea. Maximum ages were 2+ years for males and 3+ years for
females, and the length-weight relationship was W = 0.0042TL3.711 for
immature fish, 0.0095TL3.1328 for males and 0.0075TL3.4678
for females. The sex ratio was 1:2.63 in favour of females.
Food
Food is various crustaceans, aquatic and terrestrial insects,
snails, worms, fish eggs and fry including their own species, and a
wide variety of other available organisms taken both on the bottom or
pelagically. Copepods and chironomids tend to dominate as food items. Again
Paepke in Bănărescu and Paepke (2002) is a recent review of literature.
Niksirat et al. (2010) found stomach contents in the southeast Caspian
Sea comprised gammarids, Nereis sp., chironomids, fishes, oligochaetes,
fish eggs and hirudineans. Gammarids dominated in December and January and were
replaced by Nereis in February. The preference shifted to chironomids in
July and to fishes in October.
Reproduction
Spawning occurs from April to October, varying with locality over
the wide range of this species. The male parental cycle at one site in
Canada lasts 9-15 days with female interspawning intervals of 19 days.
Males and females only complete one spawning here, though laboratory
studies show males capable of 5 reproductive cycles and females of
producing a clutch of eggs every 3-4 days. Harsh physical conditions
are probably the cause. The male builds a barrel-shaped nest in
shallow, sandy areas from plant fragments glued together on the bottom
with kidney secretions. The nest is in an open area but near
vegetation. The nest has an opening at each end. The male has a
complex courtship dance with zig-zag motions and a leading motion to
the nest. A responsive female adopts a submissive head up position,
which also reveals the egg-swollen belly. The male pokes his snout at
the nest to indicate its position to the female, tipping his head
sideways to display the bright red throat. The male jabs the female
with his snout through the nest wall after she enters to stimulate egg
release. He then follows the female through the nest to fertilize the
eggs and drives the female away. Several females may spawn in one nest
which can contain up to 1026, yellowish 1.8 mm diameter eggs. The male
guards and fans the eggs and guards the fry.
Patimar et al. (2010) in their Gomishan Wetland study found
reproduction to occur in March-June, peaking in April. Large yolk-filled eggs
with a mean diameter of 1.317 mm (maximum 1.96 mm) numbered 128-885. Small white
opaque eggs were also found in the ovaries with a mean diameter of 0.549 mm and
numbered 311-4709. The mean absolute fecundity was 1241.69 eggs and mean
relative fecundity was 535.24 eggs/g body weight. Niksirat et al. (2010)
found the breeding season in the southeast Caspian Sea began in March and lasted
5 months. Condition factor somatic condition factor and hepatosomatic index
increased at the beginning of the spawning season and decreased as it
progressed.
Parasites and predators
Many fishes and birds, and even snakes, seals and small mammals,
feed on sticklebacks despite their protective spines which are locked
erect when they are disturbed.
Niksirat et al. (2006) record Corynosoma strumosum in this species
from the Gomishan Lagoon.
Economic importance
The threespine stickleback has received much attention for its
evolutionary interest, behaviour and utility as an experimental fish. They may
be predators on fish eggs and larvae, and competitors for food, of more
commercially important species. The Baltic Sea area has had sufficient numbers
to support an oil extraction industry (Paepke in Bănărescu and Paepke, 2002).
In the Iranian parts of the Caspian Sea this exotic may compete with juvenile
sturgeons for food and its spread may account for the disappearance of the
native Pungitius platygaster (Niksirat et al., 2010).
Conservation
Lelek (1987) classifies this species as intermediate to rare,
locally vulnerable in Europe. As a recently recorded exotic species in
Iran it is still comparatively rare but should not be accorded any status.
Further work
The spread of this exotic species should be monitored and its
effects on native fishes observed.
Sources
Iranian material: CMNFI 1993-0144, 1, 57.3 mm standard length, Mazandaran, Neka Power Plant
(36º51'48"N, 53º23'24"E).
Genus Pungitius
Coste, 1848
This genus of sticklebacks is found in North America and Eurasia
with about 3-5 species (opinions vary) but only 1 in Iran (Paepke in Bănărescu
and Paepke, 2002).
It is distinguished by a series of 7-12 small spines (much smaller than
in the genus Gasterosteus) in front of the dorsal fin, arranged
alternately to the left and right, the pelvic fin has 1 spine and 0-1
soft rays, and the skin is naked or has small bony plates in a row along mid-flank.
Pungitius platygaster
(Kessler, 1859)
Common names
nohkhar or nokhareh (= ninespine), mahi seh khareh nama (= threespine-like fish), mahi khardar (= spiny fish).
[tikan balig in Azerbaijan; malaya yuzhnaya kolyushka or southern ninespine stickleback in Russian;
Ukrainian stickleback].
Systematics
Gasterosteus platygaster was originally described from
Odessa and Aleshki on the Dnieper in the Ukraine.
Gasterosteus pungitius var. Kessleri Yakovlev, 1870
and Gasterosteus pungitius var. niger Yakovlev, 1870 both from lakes near
Astrakhan, Volga River delta, Russia, Gasterosteus platygaster
var. caucasicus Kessler, 1877 from Transcaucasia, and Pygosteus
platygaster nuda Berg, 1905 from Lake Charkhal in the Ural River
valley, are synonyms. Syntypes of the latter are in the Zoological
Institute, St. Petersburg (ZISP 10613) (Eschmeyer et al., 1996; Kottelat, 1997).
Münzing (1969) retains this taxon as a distinct species but
suggests that it may only be a subspecies of Pungitius pungitius
(Linnaeus, 1758). Ziuganov and Gomeluk (1985) studied hybridisation of
this species and P. pungitius under experimental conditions and
consider that they are at most subspecifically distinct. They found 9
out of 19 morphological characters distinguish this species from P.
pungitius as well as a differing ecology. However there were no
ethological, nor presumably genetical, isolating mechanisms since F3
hybrids were fertile. Ziuganov (1991), Keivany (1996), Keivany and
Nelson (1997) and Keivany et al. (1997) recognise P.
platygaster as a distinct species since it is 100% distinguishable
from other species in the genus. However, Keivany and Nelson (2004) found that
P. platygaster lay within a polychotomy with subspecies of P.
pungitius. P. platygaster is retained as
a full species until the two taxa come into contact naturally, which
will test the hypothesis of Ziuganov and Gomeluk (1985).
Key characters
The small spines usually alternating left and right in front of the
dorsal fin are distinctive.
Morphology
Characterised by 22-34 bony plates along the flank, perhaps a
response to predators (Ziuganov and Gomeluk, 1985). The first 4-12
plates are large (Keivany and Nelson, 1998). There is no caudal
peduncle keel. The plates in young fish are minute fragments of bone
and difficult to see, especially posteriorly. The body form is less
elongate than the related Pungitius pungitius. Dorsal fin
spines slightly curved, 7-11 (in Iranian specimens 8(7), 9(11)) and
branched rays 6-11, anal branched rays 6-10, pectoral fin rays 9-11
(usually 10), and pelvic fin with 1 serrate spine and 0-2 soft rays,
usually 1. Rarely up to 4 dorsal fin spines may follow the midline and
not alternate markedly in Iranian fish. The last spine in the series
is usually slightly longer than the others. Vertebrae number 29-31 (in
Iranian specimens 29(9), 30(9)) and gill rakers 7-13, reaching the
base of the second raker below when appressed. The gut is short and
s-shaped. The chromosome number is 2n=42 (Klinkhardt et al., 1995).
Sexual dimorphism
Colour at breeding is the most evident sexual dimorphism.
Colour
Females are olive with greenish-brown stripes, patches and mottling
even during spawning. Males are jet black and develop a blue-white
colour on the posterior side of the ventral spines as a stimulus for
leading the females. The specimens described as var. kessleri were
light grey and var. niger were black, perhaps non-spawners and
spawners. The peritoneum is silvery.
Size
Attains 7.0 cm standard length.
Distribution
Found in the Black, Caspian and Aral seas and their basins.
Reported from the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea, the Anzali Mordab, Ameerkalaye Lagoon near Lahijan,
and lower reaches of rivers such as the Safid, Shakhzaderud (lower Babol),
lower Karasu and Gorgan (Derzhavin, 1934; Holčik
and Oláh, 1992; Nejatsanatee, 1994; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
Keivany (1996) considers it to be rare on the southern Caspian Sea coast. Jolodar and Abdoli (2004) report it from brackish portions of the Caspian Sea
and rivers falling into it, being more numerous in the Gomishan Lagoon.
Zoogeography
Pungitius platygaster aralensis (Kessler, 1877) is reported
from the western Uzboi lakes in Turkmenistan (Shakirova and Sukhanova,
1994) and with canal construction projects may ultimately reach the Caspian Sea basin.
Habitat
Described as a benthivore living a cryptic life in weed beds (Ziuganov
and Gomeluk, 1985). It can be found in fresh and brackish waters.
Age and growth
In the Volga delta, mature adults are 3.2-5.8 cm long (mean 4.0 cm)
from April to July and young-of-the-year are 1.2-2.8 cm long (mean 2.3
cm). Life span appears not to exceed 2 years as only sexually mature
adults are caught in spring (Fortunatova, 1961) although a few fish may
reach 3 years and maturity may be attained shortly before reaching 1 year (Paepke
in Bănărescu and Paepke, 2002).
Food
Diet is small invertebrates including chironomids. Eggs of
commercially important fishes may be eaten.
Reproduction
Reproduction can occur at high temperatures, e.g. 28°C,
at which eggs of P. pungitius die. Spawning begins at 17°C.
In the Volga delta spawning begins in late April and continues to the
middle of June but is most intensive in the middle of May. Dense
schools are formed which disperse after spawning with the fish
becoming secluded in the heavy vegetation which develops at this time
(Fortunatova, 1961). Fecundity is up to 90 eggs. Males build nests out
of the remains of the previous year's vegetation glued together with
kidney secretions (Fortunatova, 1961). A female indicates readiness to
spawn by a head-up posture near the nest. The male performs a zig-zag
dance and leads the female to the nest entrance by angling his head
down at 60°, flashing his blue-white pelvic spines and moving by short jerks. The female
enters the nest, the male taps her caudal peduncle to stimulate egg
deposition, she leaves and the male enters the nest to fertilize the
eggs. The leading part takes only 10 seconds.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this
species (Krylov, 1984) as are such fishes as Perca fluviatilis,
Esox lucius, Silurus glanis, Aspius aspius and Sander
lucioperca (Fortunatova, 1961) and Neogobius melanostomus,
water snakes, pond turtles, frogs and fish-eating birds (Ziuganov, 1991).
Economic importance
None; although Paepke in Bănărescu and Paepke
(2002) consider it important as a predator on mosquito larvae (and hence
a biological control for malaria) in smaller water bodies.
Conservation
Holčík and Oláh (1992) report the loss of this species from the Anzali
Mordab, where it was once abundant, through a change in the environment
to fresh water from brackish, siltation, oxygen depletion and loss of
spawning grounds. Keivany (1966) considers it to be rare on the
southern Caspian Sea coast while Kiabi et al. (1999) consider
this species to be of least concern in the south Caspian Sea basin
according to IUCN criteria. Criteria include medium numbers,
widespread range (75% of water bodies), absent in other water bodies
in Iran, and present outside the Caspian Sea basin.
Further work
The distribution and biology of this species in Iranian waters needs further investigation.
Sources
Descriptions of breeding and some meristic characters are taken
from Münzing (1969) and Ziuganov and Gomeluk (1985).
Iranian material: CMNFI 1970-0552, 1, 16.0 mm standard length, Gilan, Sowsar
Roga (37º27'N, 49º30'E); CMNFI
1970-0554, 12, 17.5-22.0 mm standard length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1970-0567,
2, 17.7-21.4 mm standard length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1970-0591, 2, 15.8-17.4 mm standard
length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1979-1215, 1, 22.3 mm standard length, Gilan,
Sowsar Roga (37º27'N, 49º30'E).
Syngnathidae
The pipefishes and seahorses are found world-wide, mostly in
tropical and warm-temperate waters, with some brackish and freshwater
species. There are 52 genera and about 232 species (Nelson, 2006) but only one species of pipefish
is reported for Iranian fresh waters. Maximum length is about 65 cm.
The body of pipefishes is characteristic being very thin and very
elongate, and enclosed in bony rings as a form of armour. The body is
divided into a trunk and a tail, the tail being prehensile in
seahorses. The first trunk ring has the pectoral fin base and the last
has the anus in it. The snout is elongate with a small toothless mouth
at the tip. Gills are tufted and the gill opening is small. There are
1-3 branchiostegal rays. There is a single dorsal fin without spines,
the pelvic fins are absent and the pectoral, anal and caudal fins may
be absent too. The caudal is always small when present. The anal fin
is always small with only 2-6 rays. Those without a caudal fin may
have the tail prehensile, able to grasp and hold onto objects.
Pipefishes and seahorses have only one kidney, on the right side.
Males usually have a pouch on the belly formed from two skin folds
which meet at the mid-line. The female attaches eggs to the male belly
or places them in a pouch (or marsupium). Here the eggs develop and
eventually leave after about 4-6 weeks through a slit or pore so the
male "gives birth".
Pipefishes and seahorses are found mostly in shallow coastal areas
and in estuaries but also occur down to about 400 m and in the open
ocean often associated with floating seaweed. They are slow-moving
because of their armour and easily picked up by hand. Propulsion is by
undulating the dorsal and pectoral fins. Since they cannot outswim
most predators, they are often very well camouflaged by colour, body
form and by appendages which disrupt the body outline. Their food is
small crustaceans sucked into the tube-like snout by a sudden
expansion of the buccal cavity. Many species have a life span of only about 2 years.
Dried seahorses and pipefishes are commonly sold as curios and some
family members have been used as medicines or aphrodisiacs in the
East. They are popular aquarium fishes. Certain species are endangered
by this collecting for curios, medicines and aquaria.
Genus Syngnathus
Linnaeus, 1758
Members of this genus are found in all seas and there are about 31
species. A single species is found in the Caspian Sea and Iranian waters.
The genus is characterised by a brood pouch in the caudal area of
males, running forward to about the origin of the dorsal fin. The eggs
develop in isolation from the sea, oxygen diffusing from the
"pregnant" male. Dorsal, caudal, anal and pectoral fins are
present. The caudal and anal fins are very small.
Syngnathus caspius
Eichwald 1831

Common names
ney mahi or naymahi (= reed fish, i.e. as thin as a reed), ney mahi darya-ye Khazar (=
reed fish from the Caspian Sea), سوزن ماهي (= suzan mahi, meaning needle fish).
[xazar iynabaligi in Azerbaijan; Kaspiiskaya igla-ryba or Caspian pipefish in Russian; short-snouted pipefish,
blackstripe pipefish].
Systematics
The Caspian pipefish has appeared under several scientific names. Lueken (1967) demonstrated that Syngnathus nigrolineatus
caspius Eichwald, 1831, the taxon generally referred to in the
literature as occurring in the Caspian Sea, is a synonym of Syngnathus abaster.
Syngnathus abaster was originally described from Nice, France.
Other taxa of relevance are Syngnathus ponticus Pallas, 1814 described in part from
mouths of rivers falling into the Caspian Sea, Syngnathus
nigrolineatus Eichwald, 1831 described from "Hab. in sinu
Bacuensi, Murdofiensi Caspii maris", i.e. Baku Bay, and also at Odessa on the
Black Sea, Syngnathus caspius Eichwald, 1831
described from "Hab. in sinu balchanensi Caspii maris" (Balkhan
Bay in the Caspian Sea), and Syngnathus bucculentus Rathke,
1837 described from Sevastopol and Feodosiya on the Black Sea, all considered as
synonyms of S. abaster. However, Naseka and Bogutskaya (2009) retain
S. caspius as the species in the Caspian Sea.
Key characters
The extremely thin, elongate body encased in bony rings is unique in Iran.
Morphology
Dorsal fin rays 22-43, the count increasing with age; anal fin rays
3, pectoral fin rays 9-15, and total caudal fin rays 8-11. Trunk rings
14-18; tail rings 28-42, and rings under the dorsal fin 5-12. A dorsal
ridge ends near the rear of the dorsal fin and a continuation of the mid-lateral ridge becomes the dorsal ridge posteriorly. A mid-belly
ridge extends back to the anus where it terminates. As a result the
anterior part of the body from the dorsal fin forward has 7
longitudinal ridges, reduced to 4 posteriorly.
Meristic values for Iranian specimens are:- 30(3), 31(6), 32(9), 33(18),
34(13), 35(7), 36(1), 37(1), 39(2), 40(4), 41(3), 43(1); anal fin rays
uniformly 3; pectoral fin rays 11(14), 12(22), 13(10), 14(8), 15(1);
total caudal fin rays 9(1), 10(51), 11(2). Trunk rings 15(46), 16(18),
17(4); tail rings 33(3), 34(7), 35(28), 36(13), 37(5), 38(6), 39(4),
40(2), rings under the dorsal fin 6(1), 7(28), 8(24), 9(2); and brood
pouch rings 15(1), 17(2), 18(3), 19(1), 20(3), 21(1), 22(2), 23(1). Total vertebrae
52(3), 53(2), 54(2), 55(4), 58(1), 59(1).
Sexual dimorphism
Males have a brood pouch under the tail.
Colour
Overall colour is brown to green with dark or light spots and bars
arranged in a reticulate fashion on each ring. Pigment is best
developed on the dorsal half of the head and body. The ventral part of
the tail becomes darkest posteriorly near the tail fin. The reticulate
pattern of darker pigment can form a consistent pattern of arcs
bounding the plate junctions along both the upper and lower flank such
that a double row of dark ovals with light centres is apparent. Behind
the dorsal fin where there is no central ridge on the flank, there is
a single dark oval at each plate junction. While some fish have this
regular pattern of dark ovals, others are more irregular with the
ovals filled in with pigment leaving only very small clear patches in
the centre. Various other patches of clearer or lighter pigmentation
form part of the general reticulate pattern. Additionally, large dark
spots or pigment concentrations may be present along the edge of the
uppermost ridge anteriorly and small dark spots may be scattered along
the back and flanks.
The dorsal fin base can be darkly spotted or striped. The fin
itself may be almost immaculate but a few melanophores line the rays.
The pectoral fin base has a dark line of merged spots and the rest of
the fin is almost immaculate although the rays can be very lightly
lined with melanophores. The anal fin is immaculate. The caudal fin is
dark on the rays and membranes, in particular on the central part.
Size
Reaches 23 cm total length.
Distribution
Found in the Mediterranean, Black and Caspian seas and the west
coast of the Iberian Peninsula. In Iran, it is recorded from the Anzali
Mordab and neighbouring rivers such as the Sowsar Roga and at Ab Kenar village,
at Bandar-e Anzali and Anzali beach, the Amirkelayee Lagoon near Lahijan, Gorgan Bay, lower reaches of Caspian Sea rivers
such as the Safid, Rud-e Sera, Lalarud and Babol,
the southeast Caspian Sea, southwest Caspian Sea and south-central
Caspian Sea, including the deltas of some rivers (Derzhavin, 1934; Holčík
and Oláh, 1992; Paateemaar, 1993; Nejatsanatee, 1994; Kiabi et al., 1999;
Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
Zoogeography
Berg (1948-1949) contends that this species entered the Caspian
from the Black Sea in post-glacial times while most other Caspian
fishes are relicts of earlier transgressions or migrants from northern waters.
Habitat
This pipefish can live and reproduce in water at a salinity of 59.5‰ (Zenkevitch, 1963) and is euryhaline. It is found over sandy or
muddy bottoms usually where there is vegetation or detritus, down to
about 5 m. Campolmi et al. (1996) consider this species to be a
major component of nearshore meadows in Sicily. Juveniles have a benthic
distribution for at least 4 weeks after release from the marsupium in Portugal
(Silva et al., 2006).
Age and growth
Growth is faster in the first year of life as in the second year
energy is used for reproduction. Life span is slightly over one year
in northwest Sicily (Campolmi et al., 1994; 1996) and 17 months
in the Po River Delta, Italy (Franzoi et al., 1993), or up to 4 years
generally. Male:female sex ratio is 1:2.18 in Sicily (Campolmi et al.,
1994; 1996) but about 50:50 in the Po Delta (Franzoi et al.,
1993). Maturity in the Mauguio Lagoon in southern France is reached as
early as 3-4 months and almost all individuals are mature after their
first winter (Tomasini et al., 1991).
Heydarnejad (2009) gave the length-weight relationship for an Iranian sample as
W = 0.0099TL3.543.
Food
Campolmi et al. (1994; 1996) found their northwest Sicily
population to feed mainly on zoobenthos, 70% being harpacticoids, ca.
18% or less gammarids and caprellids, and ca. 10% or less isopods.
Prey hidden in vegetation was taken on account of the short and
conical snout which was less effective than the longer snout of other
species which feed on more active pelagic prey. Franzoi et al.
(1993) in their study found harpacticoids associated with algae to be
the most important food item, with up to 87.5% of the diet comprised
of only 3 species in 1 genus, Tisbe.
Reproduction
Broods are found from April to October with the first breeding
males appearing in March in Sicily, the Po Delta and southern France (Campolmi
et al., 1994; 1996; Franzoi et al., 1993; Tomasini et al., 1991).
Reproductive behaviour starts with mutual flickering movements in fish
observed in Portugal. This comprised rapid and vigorous bends of the body with
both males and females approaching the opposite sex (Silva et al., 2006).
If the opposite flickered in response, the next phase was rapid side-by-side
movements while swimming more or less parallel. Flickering increased in
frequency and the female's genital papilla protruded. The distended genital
papilla is placed in the anterior area of the marsupium of the male, the folds
in this area being separated and swollen. The mated pair then ascend slowly in
the water column, vibrating the dorsal fin and rotating a few times. Eggs are
transferred and the female retreats to the substratum while the male
continues swimming with violent body contractions - these help pack the eggs in
the posterior end of the marsupium. Later, spawning occurs again up to three
times by the same pair although females may mate with up to 3 different males in
less than 30 minutes.
Egg numbers in the Po Delta are 104±40 in females and 109±27 in
males. Eggs are yellowish to bright orange and translucent with diameters usually
1.0-1.4 mm, up to 1.8 mm (Tomasini et al., 1991) or 1.09-2.06 mm in
Portugal (Silva et al., 2006). Young first
appear first in May and are present until August in the Po Delta. This
species is a batch spawner in northwest Sicily and southern France and
males can incubate several broods during the breeding season since
incubation lasts about 1 month (Tomasini et al., 1991; Franzoi et
al., 1993; Campolmi et al., 1994; 1996). Females in the Po
Delta probably breed only once but the female may parcel out the same
egg batch among many males. A male may have several batches of eggs in
different stages of development in the brood pouch at any one time.
This distribution among several males increases survival (Tomasini et
al., 1991). Incubation at 20-21°C
in aquaria is about 15 days and a new mating occurs 8 days after the
litter (Tomasini et al., 1991). In Portugal, development lasted 24-32
days at 18-19ºC or 21 days at 21-22ºC (Silva et al., 2006). The female potential fecundity
per breeding act in southern France averages 21-23 ovocytes while the
male brood pouch can accommodate 36-52 eggs. As a consequence the
female matures rapidly between two breedings and the species is
polygynous (Tomasini et al., 1991). Males carry 10-64 eggs in Portugal
(Silva et al., 2006). New-borns are about 13.5 mm
long in the Po Delta (Franzoi et al., 1993).
Broods are found in males from Iran collected on 4 May, the same
sample having males with eggs and males with minute young pipefish. A
sample from 22 September has eggs just about to hatch while males
taken on 3 August, 28 September and 17 December contain neither eggs
nor young. Females with large eggs have been caught in Iran on 17
April and 7 July while on 3 August, 19 September, and 12 and 17
December females had only small eggs. The 17 April sample contained
eggs of dissimilar sizes. Iranian fish appear to have a similar
breeding season to those from other localities and probably have batch
spawning too.
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984).
Economic importance
None.
Conservation
The ecology of this species has not been studied in detail in
Iranian waters and its population is unknown rendering an assessment
of its conservation status difficult. Kiabi et al. (1999)
consider this species to be of least concern in the south Caspian Sea
basin according to IUCN criteria. Criteria include abundant in
numbers, widespread range (75% of water bodies), absent in other water
bodies in Iran, and present outside the Caspian Sea basin.
Further work
The significance of this species in the ecology of the Iranian coastal region
and its biology have not been determined.
Sources
Paateemaar (1993) gives an account of this species in Iran in Farsi. Data
were taken from Lueken (1967) and Dawson in Whitehead et
al. (1984-1986).
Iranian material:
CMNFI 1970-0507, 1, 91.3 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh
(37º24'N, 49º58'E); CMNFI 1970-0508, 1, 89.4 mm
standard length, Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0531, 1, 108.1 mm standard length,
Mazandaran, Larim River mordab (36º46'N, 52º58'E); CMNFI 1979-0535, 1, 126.3 mm standard length, Gilan, Shara River estuary (37º35'N,
49º09'E); CMNFI 1970-0543A, 5, 104.6-144.8 mm standard length, Gilan, Caspian Sea at Hasan Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0554,
13, 40.4-95.6 mm standard length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1970-0563, 1, 134.9 mm standard length, Gilan, Caspian Sea, Kazian Beach
(ca. 37º29'N, ca. 49º29'E); CMNFI 1970-0567, 7, 52.9-99.5 mm standard length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1970-0575, 1, 94.3 mm standard length,
Gilan, Pir Bazar Roga (37º21'N, 49º33'E); CMNFI 1970-0587, 3, 131.6-140.0 mm standard length, Mazandaran, Babol River near
Babolsar (36º43'N, 52º39'E); CMNFI 1971-0343, 3, 104.6-109.0 mm standard length, Langarud at Chamkhaleh (37º13'N, 50º16'E); CMNFI 1979-0077, 8,
128.4-149.2 mm standard length, Mazandaran, Caspian Sea beach at Now Shahr (36º39'N, 51º31'E); CMNFI 1980-0130, 9, 59.3-97.8 mm standard length, Mazandaran,
river near Iz Deh (36º36'N, 52º07'E); CMNFI 1980-0136, 16, 76.6-100.5 mm standard length, Mazandaran, Fereydun Kenar River estuary (36º41'N, 52º29'E).
Mastacembelidae
The spiny eel family is found principally in fresh waters of tropical Africa
and eastwards to Korea and Malaysia. The distribution mapped by Berra
(2001) extends too far into central Iran. There are about 73 species.
Maximum length is less than 1 m.
The family is characterised by an very elongate, compressed or
sub-cylindrical body; minute cycloid scales or body naked; a
non-protractile mouth; gill opening a slit; an elongate snout with a
sensitive tip flanked by tubular anterior nostrils, the posterior
nostrils are hence far to the rear; long dorsal and anal soft fins
(30-131 rays), dorsal soft fin preceded by usually numerous isolated
spines (9-42); anal fin preceded by 2-3 spines; pectoral fin present
with 12-27 rays but pelvic fin absent; caudal fin short and confluent
with the dorsal and anal fins or only separated narrowly; 60-110
vertebrae; no pseudobranchiae; 2 pyloric caeca; and swimbladder present.
Some species are food fishes and they regularly appear in the
aquarium trade as they are brightly coloured with distinctive
patterns. They may burrow in mud and even survive some drying in ponds
through their air-breathing ability. Their eel-like shape is reflected
in a wriggling behaviour when handled and some are known to move
backwards to impale the hand. They are found at high altitudes as well
as in lowlands, in both still and running waters, often in rocky
crevices or in vegetation near banks, and they emerge particularly at
night to feed on prey. The rostral appendage is used to detect the
prey by touch and the prey is rapidly inhaled.
Genus Mastacembelus
Scopoli, 1777
This spiny eel genus is found from the Levant to Southeast Asia.
There is 1 species in Southwest Asia. Heckel (1846-1849a) misspells
the genus name Mastacacemblus.
The genus is characterised by a moderate to large size, an elongate
and compressed body, inferior mouth with a narrow cleft, small eyes,
the absence of eye spots on the soft dorsal fin, the absence of
striations under the snout, 6 branchiostegal rays, preopercle spiny or
not at its postero-ventral corner, preorbital spine present, scales
minute and cycloid, elongate swimbladder, lateral line present, minute
jaw and palate teeth, and a rounded caudal fin.
Mastacembelus mastacembelus
(Banks and Solander, 1794)
Common names
مارماهي (= mar mahi, meaning snake fish), marmahi-ye khardar (= snake fish with
spines or spiny snakefish), مارماهي
شاخدار (= mar mahi shakhdar).
[salbouh abu-el-sian, salbu-al-sayan, saebouh abou siyan; abu salmabah; simack, englisi or englese at Aleppo, marmaritch
or marmarij at Mosul, all in Arabic; Mesopotamian spiny eel, Euphrates spiny eel].
Systematics
Ophidium Mastacembelus was originally described from
"Aleppo" and from the "River Kowick" (= Quwayq) in the book
by Russell (1794).
Ophidium Simack Walbaum, 1792 (non-binomial), Rhynchobdella
haleppensis Bloch and Schneider, 1801 and Mastacembelus
syriacus Gronow in Gray, 1854 are synonyms. Mastacembelus
aleppensis Günther, 1861 is an unjustified emendation of haleppensis
(Eschmeyer et al., 1996). Wheeler (1956) and Sufi (1957)
discuss the names of this species more fully.
Three syntypes of Ophidium Mastacembelus from
"Aleppo" are in the Natural History Museum, London under
BM(NH) 1955.6.25:4-6, measuring 289.5-544.0 mm standard length.
Çakmak and Alp (2010) found morphological differences between river and
reservoir populations in Turkey, but none for meristic traits. Some differences
appear to be associated with habitat type, a thicker and longer caudal peduncle
in river populations may enhance swimming ability in faster water and longer
lower jaws in river fish could be a feeding adaptation, for example.
Key characters
The eel-like body with 30-35 short, sharp dorsal spines, long soft
dorsal and anal fins and the unique flexible snout flanked by tubular
nostrils distinguish this species from all other Iranian fishes.
Morphology
The mouth gape extends back as far as the anterior eye margin or
somewhat forward of this point. The posterior nostril is slit-like.
The structure of the elongated eye, typical of streamlined forms, has been
described by Jasim (1998) based on Iraqi specimens. The regular, mosaic pattern
of the retina is associated with fishes that search for their food and the
double cone structure may be associated with detection of moving prey.
Soft dorsal rays 68-90, soft anal rays 70-90 after 3 spines,
pectoral rays 18-24 and total vertebrae 85-88. The penultimate spine in the dorsal fin is the
longest and the central anal spine is the longest. Iranian specimens
had 31(2), 32(6) or 33(4) dorsal fin spines, 19(5) or 20(3) pectoral
fin branched rays, and total vertebrae 86(1), 87(1). The diploid chromosome
number is 2n=48, arms number NF=88 and there are 11 metacentric, 9
submetacentric and 4 subtelocentric chromosome pairs (Esmaeili et al., 2006).
Scales minute but covering the whole body, under the eye, below the
posterior nostril and between this nostril and the maxilla. Each scale
has a central to anterior focus with radii on all fields and an oval
shape. There is a strong preorbital spine under the eye, present in
some fish but concealed under the skin or absent in others. Teeth form
broad bands in both jaws with the outermost teeth the largest. There
are no gill rakers but spinulose patches lying flat on the arch. The
gut is an elongate s-shape.
Sexual dimorphism
Unknown.
Colour
The body is blotched and barred, often forming a reticulate
pattern, or a series of mid-flank blotches most evident posteriorly
and sometimes running together as a stripe anteriorly. Flank blotches
may form up to 17 bars running from the dorsal to the anal fin across
the flank. The back is blackish to brown, olive, greyish or blue-grey,
the lower flank is spotted yellow or is yellow overall and the belly
is white to yellowish. A series of about 20-24 black to
blackish-brown, oval spots ringed with a lighter brown follow a dark,
broad but irregular stripe on the head and anterior back in the
mid-line. Dorsal, anal and caudal fins are yellowish with the dorsal
and caudal fins finely barred and the anal fin continuing the pattern
on the adjacent body. The anal fin may be almost immaculate. The soft
dorsal fin may have vermiculations rather than bars. The pectoral fins
are yellowish and are finely barred. The peritoneum is brownish, with
numerous fine melanophores. Small fish (about 7.7 cm total length) can
be an almost uniform grey-brown to brown-green, with yellowish brown
on the fins and the tail region, and fin spots are dark to absent.
There is a thin bar extending vertically down or obliquely back from the eye.
Size
Reaches 76.9 cm total length (Gümüş et al., 2010), probably larger to almost 1 m.
Distribution
Found in the Quwayq, Orontes and Tigris-Euphrates basins. In Iran its
is recorded from the Tigris River, Gulf and Kor River basins, including lakes
Zaribar and Marivan, and the Fasa, Dez and Dalaki rivers (Löffler, 1957; Abzeeyan, 5(5):III, 1994; Gh. Izadpanahi, pers. comm., 1995).
The record of Mastacembelus armatus (Lacepède, 1800)
reported by Mokhayer (1981b) from the Kor River basin is probably a
mis-identified Mastacembelus mastacembelus.
Abdoli (2000) maps this species from the Kor and Pulvar rivers of the Kor River basin; the middle and lower Shur
River tributary of the Dasht-e Palang, the upper Mand and Qara Agaj, the middle and upper
Hilleh and the upper Zohreh rivers of the Gulf basin; the Jarrahi, lower Karun, middle and lower Dez, Karkheh, Simarreh and lower
Kashkan rivers of the Tigris River basin.
Zoogeography
This species is now known to occur outside the Tigris-Euphrates and
Quwayq basins in Southwest Asia in contrast to Banister's assertion
(1980). The distribution of this species is not, however, continuous
across Iran as shown in a figure by Travers (1984b) mis-quoting Coad
(1979, actually 1980a). It appears to be absent from the saline rivers
draining to the Straits of Hormuz and from Baluchestan.
Habitat
This species is known from both lotic and lentic environments (Pazira et al.,
2005). Sufi (1957) described mastacembelids as usually lurking in rock
crevices or among stumps of plants near the bank and I have observed
them at Lake Parishan (= Famur) inhabiting crevices of a submerged
rock wall. They may be able to survive desiccation by burying
themselves in mud. They are not commonly caught with nets and may be
mostly nocturnal in habits. In the Marun River below Behbehan,
Khuzestan this species is very common, commoner then any other site
sampled in this province, possibly the result of pollution-enriched
water (field sample; H. R. Alizadeh, pers. comm., 2000). In
areas under human influence in Lorestan, such as the lower reaches of
rivers and near cities, it is more common than in higher, pristine waters.
Age and growth
In the Helleh and Dalaki river basins of southern Iran, the condition factor
of this species was 0.162-0.458 (mean 0.296) for females and 0.162-0.386 (mean
0.289) for males. Condition factor = 0.374 - 0.004 total length. Life span was
up to 6 years although most fish were 3 years or younger. Females grew rapidly
to age 3, after which annual growth decreased. von Bertalanffy length-at-age
equations were Lt = 873.4 (1-exp{-0.082[t + 1.488]}) for females and Lt = 923
(1-exp{-0.081[t + 1.464]}) for males (Pazira et al., 2005).
Eroğlu and Şen (2007) examined fish in Karakaya Dam Lake, Malatya, Turkey and
found 9 age groups with males in age group 4 forming the majority of the
population. Males generally outnumbered females in all age groups. Gümüş
et al. (2010)
found males up to 21 years and females to 9 years in fish from Atatürk Dam Lake
on the Turkish Euphrates River. The length and weight frequency distributions
were significantly different between sexes in this population and other growth
parameters were given. It was suggested that differences in life span between
Iranian and Turkish fish could be due to habitat differences (lotic and lentic
habitats) or fishing pressure.
Food
The flexible snout is used for sniffing out food but the eye structure
suggests a visually feeding fish also. Food is assumed to include invertebrates
but two fish from Iran contained fish scales and fish skeletal remains. Other
species are known to eat fish eggs and fry. Food in the Hawr al Hawizah, Iraq in 2005-2006
was 55.0% shrimps and 45.0% fish and in the Al Kaba'ish (= Chabaish) Marsh entirely fish (Hussain
et al., 2006). Pala et al. (2010) found fish from Karakaya Dam
Lake, Turkey to contain plant material (predominately 16 taxa of
Bacillariophyta), cladocerans, copepods, and fish.
Reproduction
Fish taken on 26 November have small but developing eggs, suggestive of spring spawning.
Al-Rudainy (2008) states that Iraqi fish reach sexual maturity at 2 years, 25 cm
length and 125 g in weight, and spawn in June and July in shallow muddy water
among rocks. Şahinöz
et al. (2006) give details of development of embryos and artificial
breeding, based on fish taken from Ataturk Dam Lake in Turkey.
They obtained a fertilisation rate of 80%, egg diameters reached 2.015 mm, and
hatched larvae were observed at 85 h after insemination. Eroğlu and Şen (2007)
in their Turkish population found males to mature at age 2, females matured at
age 1 (although in most fish females mature later because of the demands of egg
production), spawning took place mostly in June-July and eggs numbered up to
27,944 and reached 2.1 mm in diameter.
Parasites and predators
Predators might find this species difficult to swallow. The row of
dorsal spines are very sharp and can severely lacerate the hand when
this fish is picked up carelessly. Mokhayer (1981b) records a heavy
infestation with Contracaecum larvae in Lake Parishan near Kazerun.
Akhalaghi (2001d) found the nematode Anguillicola crassus in swimbladders
of this fish from Parishan Lake. Jalali et al. (2002) and Jalali and Barzegar (2006) record Diplostomum spathaceum,
Trichodina pediculus, Ichthyophthirius multifilis, Mastacembelocleidus
heteranchorus, two species of Argulus, Lernaea cyprinacea, a Polyonchobothrium species and an
Ancyrocephalus sp. from this species in Lake Zarivar. Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Jalali et al.
(2008) examined this fish in lakes Zerivar and Parishan and the Helleh River and
found the protozoans Trichodina pediculus and Ichthyophthirius
multifilis (Zarivar), the monogenean Mastacembelocleidus heteranchorus
(all three localities), the digenean Diplostomum spatheceum (Zarivar),
the crustaceans Argulus foliaceus, Argulus sp., Lernaea
cyprinacea and Lernaea sp. (all Zarivar), the cestode
Polyonchobothrium sp. (Zarivar), and the nematode Contracaecum sp. (Parishan).
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Lernaea sp. , Lernaea cyprinacea, Argulus foliaceus and
Argulus sp. on this species.
Economic importance
Russell (1794) reported that this fish was "found in great
abundance" and "esteemed a lighter and more delicate
food" than the eel at Aleppo but it does not seem to be so common
in Iran or used generally as food. Heckel (1846-1849a) gives the
common name "englisi" because it was highly prized by Europeans at Aleppo.
Foreign soldiers have caught this species by angling at Baghdad (www.carpecapio.com, downloaded
26 August 2005). Harlioğlu and Yilmaz (2011) note its local
commercial value in Turkey, analyse its chemical composition, and
recommend it for human consumption as a good source of nutrition.
Conservation
This species appears to thrive in polluted areas and is not commonly caught
or utilised. Its conservation status has not been assessed in field studies but
it is probably not under any threat. Critically endangered in Turkey (Fricke
et al., 2007).
Further work
The biology of this species needs further investigation.
An assessment of its conservation status could be carried out as its somewhat
cryptic nature makes its numbers difficult to determine.
Sources
Type material:- See above (BM(NH) 1955.6.25:4-6).
Iranian material: CMNFI 1979-0029, 1, 148.0 mm standard length, Fars, Dalaki River (no other locality data);
CMNFI 1979-0075, 1, 252.0 mm standard length, Fars, Mand River at Pol-e Kavar (29º11'N, 52º41'E);
CMNFI 1979-0124, 1, 436.0 mm standard length, Fars, Mand River at Shahr-e Khafr (28º56'N, 53º14'E);
ZSM 21831, 1, 584.5 mm standard length, Khuzestan, Harmaleh on Dez River (31º57'N, 48º34'E);
ZSM 26629, 1, 154.4 mm standard length, Kordestan, Lake Zaribar (35º32'N, 46º08'E);
uncatalogued: 1, 227.5 mm standard length, Fars, Mand River outside Jahrom (28º36'N, 53º37'E);
1, 219.0 mm standard length, Khuzestan, Rud Zard, (31º22'N, 49º43'E);
3, 105.0- ca. 420.0 mm standard length, Khuzestan (no other locality data).
Comparative material:- BM(NH) 1875.1.14:7, 2, 341.8-569.1 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1912.5.2:8, 1, 314.3 mm standard length, Iraq, Shatt-al-Arab (no further locality data);
BM(NH) 1920.3.3:297-300, 4, 134.5-289.6 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1920.3.5:7, 1, 103.8 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1922.3.25:1, 1, 424.0 mm standard length, Iraq, Baghdad (33º21'N, 44º25'E);
BM(NH) 1936.3.10:4, 1, 40.0 mm standard length, Iraq, Nasiriyah (31º02'N, 46º16'E);
BM(NH) 1974.2.22:1798, 1, 380.5 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1974.2.22:1807-1808, 2, 132.5-414.7 mm standard length, Iraq, Mosul (36º20'N, 43º08'E);
BM(NH) 1968.12.13:440-442, 3, 178.9-351.2 mm standard length, Syria, Euphrates River at Mayadine (35º01'N, 40º27'E).
Percichthyidae
Genus Morone
Mitchill, 1814
Morone saxatilis
(Walbaum, 1792)
Introduced to the Caspian Sea basin by Soviet authorities (McNeil, 1979) but no Iranian record.
Centrarchidae
Genus Lepomis
Rafinesque, 1819
Lepomis macrochirus
Rafinesque, 1819
The bluegill sunfish, a North American species, was introduced to the Yengi Kand Reservoir of
the Namak Lake basin in 1969 from the U.S.A. (Andersskog, 1970;
Anonymous, 1970a; Saadati, 1977; see also under Pimephales promelas)
but the population perished through winterkill (R. J. Behnke, in litt.,
1979). The locality mentioned in Saadati (1977), the Golpayegan
Reservoir, is an error (R. J. Behnke, in litt., 1979). Hoffman
(1970) mentions that a disease-free certification was required for
importation of this species to Iran, indicating that the introduction was deliberate.
Genus Micropterus
Lacepède, 1802
Micropterus salmoides
(Lacèpede, 1802)
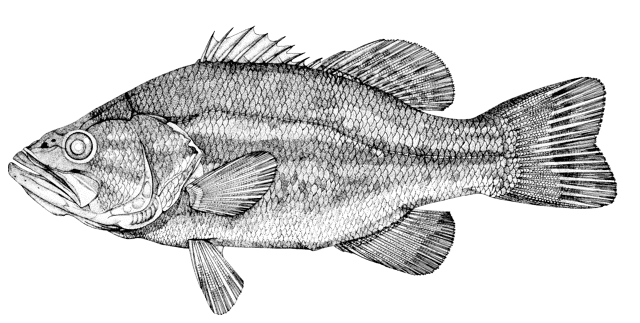
This North American species was introduced to the Yengi Kand
Reservoir in the Namak Lake basin and possibly the Tigris River basin
in the Ardakan-Yasuj area (Saadati, 1977; A. Shiralipour, pers. comm.,
1978; see also under Pimephales promelas). The Namak Lake basin
population died because of winterkill (R. J. Behnke, in litt.,
1979) and the survival of the Ardakan-Yasuj population is not known.
Its Farsi name is khorshid mahi baleh kuchek (= small-finned sunfish)
or bas dehan or dahan bozorg (= largemouth bass). Heidinger (1976)
summarises the biology of this species.
Percidae
The perch, darters, pike-perches and their relatives comprise a
family of mostly freshwater species found across the Northern
Hemisphere. There are 10 genera and about 201 species total with about 187 in North
America alone (Nelson, 2006). Maximum size approaches 1 m but many species (the darters
particularly) are small.
They are characterised by ctenoid scales; a dorsal fin with an
anterior spiny portion and a soft rayed posterior portion; an anal fin
with 1-2 spines (rather than 3 as in related families) and a few soft
rays; pelvic fins thoracic in position, with 1 spine and 5 soft rays; branchiostegal membranes not attached to the isthmus; branchiostegal
rays 5-8; teeth on the jaws, vomer and palatines in patches, sometimes with canine teeth; and the operculum has a sharp spine.
Perches are found in warm southern waters to subarctic ones, in
both flowing and still water. Some larger species are commercially
important while smaller species make attractive aquarium fishes. The
small darters of North America rival coral reef fishes for colour when
in breeding condition. Perches have a variety of reproductive
strategies which include broadcasting, stranding, burying, attaching,
clumping and clustering. During the breeding season tubercles develop,
particularly on the male. These may be on the body, fins or head and
are used to maintain contact and enhance grip between males and
females during the spawning act.
Genus Perca
Linnaeus, 1758
This genus comprises 2 species, one found in North American and
one in Eurasian, fresh waters.
The body is compressed, scales are small and ctenoid, cheeks and
gill covers are scaled, the opercular bone carries a single flat
spine, the preopercle is serrated posteriorly and has spikes
ventrally, there are no canine teeth, branchiostegal rays 7, the lateral line
does not continue onto the caudal fin, and the body usually has strong bars.
Perca fluviatilis
Linnaeus, 1758
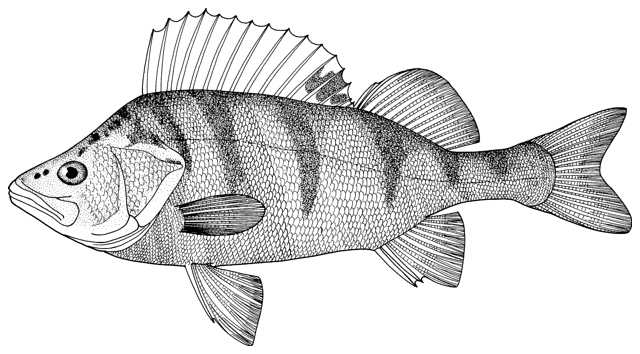


Common names
mahi-ye khardar, bacheh suf (= baby suf), mahi suf rudkhanehi Astrakhan (= Astrakhan river suf fish, presumably an
old name at this Russian locality), سوف حاجي طرخان
(= suf-e Haji Tarkhan meaning Astrakhan suf, an old name no longer in use), suf-e rudkhaneh'i (= river suf),
hashtarkhan suf.
[xanibaligi in Azerbaijan; okun' in Russian; perch, European perch, Eurasian perch, river perch].
Systematics
Perca fluviatilis was originally described from Europe.
Collette and Bănărescu (1977) refute earlier workers who maintain that this species and the
North American yellow perch (Perca flavescens (Mitchill, 1814))
were the same or at best subspecies, e.g. see Svetovidov and Dorofeeva
(1963) and Čihar (1975) for opposing views. Collette and Bănărescu (1977) base their
conclusion on the observation that the predorsal bone is anterior to
the first neural spine in fluviatilis rather than extending
between the first and second neural spines as in flavescens.
Other characters also exist to separate the two species. Data on the
North American perch cannot therefore be used uncritically as a
summary of biology for the Iranian perch.
Key characters
Characters of the genus serve to identify the single, distinctive species.
Morphology
Lateral line scales 40-78; scale rows above lateral line 7-10; rows
below lateral line 12-22; and predorsal scales 10-21. Scales have very fine circuli, few anterior radii, a posterior focus and a markedly
incised anterior margin where about 5-7 radii terminate. The exposed
part of the scale is coarse and is the base for ctenii, best developed on the
margin. Dorsal fin spines 12-18; dorsal soft rays 8-17, usually 12-15, after 0-5, usually
2-3 spines; anal fin soft rays 6-11 after 1-3 spines; pectoral rays
9-17; and pelvic rays 4-6, usually 5 after 1 spine. Gill rakers 23-25, reaching between the third and fourth rakers below when
appressed usually but variable in length with diet, shortest when
feeding on fish, longer when food is zooplankton. The gut is s-shaped
with a large anterior loop and there are 3 pyloric caeca. Vertebrae 38-44,
and gill rakers 14-29. The chromosome number is 2n=48 (Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- lateral line scales 59(3), 60(2), 61(2); scale
rows above lateral line 9(7); rows below lateral line 17(4), 18(2),
19(1); scales between lateral line and pelvic fin 6(2), 7(5); predorsal scales
10(1), 11(2), 12(1), 13(2), 14(1); and caudal peduncle scales 24(1), 25(1),
26(1), 27(3), 28(1); dorsal fin spines 14(2), 15(1), 16(4); dorsal soft rays
13(2), 14(5) after 2 spines; anal fin soft rays 8(3), 9(3), 10(1) after 2
spines; pectoral rays 11(2), 12(2), 13(3); pelvic rays 5(7) after 1 spine;
vertebrae 40(2), 41(3), 42(1).
Sexual dimorphism
Males have longer paired fins than females and are brighter in
colour. Females are larger than males of the same age.
Colour
Colour can be affected by diet, especially in the fins which are
reddest when feeding on certain crustaceans, and by habitat depth but
generally the colour is stable. Fish from along the shore in weedy
habitats are greenest, those in open water a pale yellow, and at depth
are darker. The body is an overall greenish-yellow with 5-9 black bars
on the flanks. The first dorsal fin is grey with black markings on the
membranes. The first spine is often black and deep black membranes are
evident between spines 1 and 2 and the last 4 to 5 spines. The second
dorsal fin is greenish-yellow with melanophores on the rays and
membranes, the pectoral fin yellowish and other fins pinkish to yellow
to silvery-white. Paired and caudal fins have much sparser
melanophores than the second dorsal fin. The lower part of the caudal
fin is orange to red. Peritoneum silvery and speckled with melanophores.
Size
Reaches 68.0 cm and possibly 10.4 kg but most are much smaller than this.
Distribution
Found from the British Isles across northern Eurasia to eastern
Siberia. Their presence in the Caspian Sea basin of Iran is their most
southerly natural distribution. Also introduced to South Africa, Australia and New Zealand.
In Iran it has been reported from the Anzali Mordab (= Talab) and its outlets,
Ab Kenar and Siah Keshim Protected Region in the Anzali Talab, at Bandar Anzali,
Bandar Anzali beach, the lower Safid River and the Caspian Sea near Bandar Anzali (Derzhavin, 1934;
Holčík and Oláh, 1992; Riazi, 1996; Abbasi et al., 1999; Abdoli, 2000;
Abdoli and Naderi, 2009). Jolodar
and Abdoli (2004) restrict its presence to the Anzali lagoon and rivers draining
into it. Anderson (1880) reports perch to be abundant in the Lar River near Tehran, but this
is probably a misunderstanding at this early date.
Zoogeography
Its closest relative is found in North America and they were once thought to be the same species on both continents.
Habitat
Distribution is limited by an inability to survive a temperature of 31°C for
more than a few hours, by an inability to tolerate salinities above
about 10-12‰ and by avoidance of waters with an oxygen level of
less than 3 ml/l. The upper lethal temperature is 33.5°C (Collette et al., 1977).
It is not found in the Caspian Sea proper because of
the latter limitation. Fresh water is required for spawning. Riazi
(1996) reports that this species is native (resident) to the
Siah-Keshim Protected Region of the Anzali Mordab and it is also reported from
swamps near Hendeh Khaleh in Gilan at about 37°23'N, 49°28'E.
Optimal conditions are large, weed-free, moderately deep,
mesotrophic waters with food fish such as Rutilus rutilus
readily available. Turbidity is a limiting factor for this species
which depends on sight to feed. It is found only in the lower reaches
of rivers along the Iranian shore and does not penetrate upstream
(Berg, 1948-1949). Nevertheless it can be found in both running and
still water and in both small and large water bodies.
Perch are a schooling fish, arranged by size and age. Schools form
in the morning and disperse as dusk falls. Schools usually number
about 50-200 fish but schools in the thousands are reported. There is
a nocturnal resting area and perch move from it to a diurnal active
area. The perch may move short distances within a lake and in large
water bodies over 90 km but show strong homing tendencies. Seasonal
movements are between feeding, spawning and overwintering areas.
Different morphs are found in some areas, depending on habitats:
one small, slow- growing, dark and gregarious, feeding on small
crustaceans, and found in reed beds, the other large, fast-growing,
light and solitary, feeding on fish, and found in open waters.
Populations in the Safid River were supposedly increased after
construction of the dam which reduced water flow and raised temperatures.
Age and growth
Maturity in males is usually attained in the second year of life
(at only 5-12 cm long) with females maturing 1-2 years later (at 12-18
cm or larger). However some males may mature during their first year
or as late as their third. Females grow slightly faster than males
after the first 1-2 years. Eutrophication may reduce the age of first
maturity because of increased growth rates. Perch in different
habitats within the same water body, e.g. weeds beds as opposed to
open water, will show different growth rates and body forms. Growth
over the whole range of the species varies markedly. Generally fish at
age 2 which are greater than 20 cm total length are characterised as
having very good growth, moderate growth would be fish at age 3
greater than 16 cm total length while a very poor growth would be
evidenced by all fish in the population being less than 16 cm total
length. Life span is up to at least 21 years and under artificial
conditions up to 27 years, perhaps even 50 years. A maximum age of 11
years is given for a Volga delta population examined by Makarova (1986).
Nezami et al. (2004) found fish in the Amirkelayeh Lagoon on the
Caspian coast of Iran were in age groups 1+ to 6+, a total length of 9.5-33.5 cm
and a weight of 10.5-350.0 g. Heydarnejad (2009) gave the length-weight
relationship for an Iranian sample as W = 0.0145TL3.011.
Food
Food for small perch is zooplankton such as rotifers, switching to
insect larvae, crustaceans, molluscs and leeches with growth (larger
than about 20 mm). Growth is enhanced if fish and crayfish are
available. Fish predominate in the diet at a range of sizes between 10
and 25 cm. In the Caspian basin, Rutilus rutilus, Blicca
bjoerkna, Pungitius platygaster and gobies (Gobiidae) are
eaten (Makarova, 1986). Some slow-growing perch may feed on plankton
until 2-3 years old. Cannibalism is common. Maximum feeding levels
occur in summer and by autumn has fallen to a maintenance level (Collette
et al., 1977; Popova and Sytina, 1977). Feeding is a daylight
and highly visual activity. Feeding is more effective in shoals as the
perch attempts to seize other fish by the head and, if an individual
perch misses, then other members of the shoal have an opportunity to
seize the prey. Large perch lie in wait for passing prey items and
then dart out to seize them. Unlike northern pike, perch will pursue a
prey item if it tries to escape.
In the Amirkelayeh Lagoon diet varied according to age, season and sex, and
comprised a wide variety of organisms such as water bugs, odonates, gammarids,
plant materials, chironomids, Tinca tinca, hemipterans, Perca
fluviatilis, snails, Syngnathus caspius, Gambusia holbrooki,
Pungitius platygaster, dipterans, branchiopods, trichopterans, tubifex,
frogs and shrimps. The species is an omnivore here and a cannibal.
Reproduction
Spawning occurs in the spring in shallow water, 0.5-3.0 m deep, end
of March to early June in the Volga Delta (Makarova, 1986). In
Dagestan spawning is from the end of March to the beginning of April
and lasts 10-15 days (Shikhshabekov, 1978), elsewhere only 2-3 days.
Water temperatures are around 11°C on the Volga (Lönnberg, 1900b) and above 8°C
in Dagestan (Shikhshabekov, 1978) but can occur under ice at 4°C.
Up to 80% of the spawning population in the Volga Delta is female (Makarova,
1986). There is a spawning migration from deepwater resting areas to
shallow spawning areas. Males precede females onto the spawning ground
by days or weeks and remain behind after spawning. Brackish-water
populations migrate into fresh water. Spawning itself can occur by day or by night.
Although temperature is the major factor affecting the timing of
spawning, the occurrence of spring floods is significant in some
populations as it gives access to inundation zones of large rivers.
Eggs are twisted around plants, roots and logs in an egg-strand, a
cylindrical, hollow, twisted structure up to 3.75 m long, 3.8 cm thick
and 8 cm wide. This structure offers protection from predators, fungal
infections, desiccation, mechanical damage and smothering in the mud.
Egg diameters reach 2.5 mm and fecundity 300,000 eggs. Fecundity
increases with age and depends on food supply as in most fish species.
As many as 15-25 males queue up to fertilize the egg-strand, following
the female as she twists around the logs and plants, rubbing against
the plants to void the eggs. The female drives the males away from the
egg-strand after fertilisation and may guard the egg-strand for some hours.
Parasites and predators
Sander lucioperca, Esox lucius and Lota lota
are predators on perch in the Caspian basin and doubtless other large
fishes and birds take this species. Cannibalism begins as early as the
fry stage when fish only 2.1 cm long eat smaller fry.
Mokhayer (1976b) records the protozoan Trypanosoma percae from this species in
the Caspian Sea basin, the nematode larva Eustrongylides excisus
and the annelid Piscicola geometra.
Khara et al. (2006a) record the eye fluke Diplostomum spathaceum for
this fish in the Amirkalayeh Wetland in Gilan.
Sattari et al. (2002) and Sattari (2004)
records the presence of the nematode, Eustrongylides excisus. This
parasite can damage muscles in commercial species and render them unsuitable for
sale. Sattari et al. (2004; 2005) surveyed this species in the Anzali and Amirkelayeh
wetlands, recording Raphidascaris acus, Eustrongyloides excisus and Camallanus lacustris.
Khara et al. (2005) examined this species in the Amirkalayeh Wetland and
found its diversity of parasites to be less than other predatory species such as
Esox lucius. Parasites recorded were Camallanus lacustris,
Diplostomum spathaceum, Lemaea sp., Argulus sp., and Dactylogyrus sp.
Sattari et al. (2007) record the nematode Eustrongylides excisus
and the digenean Diplostomum spathaceum in this species in the Anzali
wetland of the Caspian shore. Barzegar et al. (2008) record the digenean
eye parasite Diplostomum
spathaceum from this fish.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Achtheres percarum on this species.
Economic importance
Holčík and Oláh (1992) report a catch of only 15 kg in the Anzali Mordab in
1990. This species has been studied for aquaculture in Iran.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in sport, in textbooks, in experiments and because it has
been widely introduced outside its natural range. It has been
implicated in ichthyootoxism (Coad, 1979b), the symptoms of egg
poisoning being summarised under the genus Schizothorax.
Conservation
Illegal fishing and non-standard nets threaten stocks of this
species (Annual Report, 1995-1996, Iranian Fisheries Research and
Training Organization, Tehran, p. 55, 1997). Kiabi et al.
(1999) consider this species to be vulnerable in the south Caspian Sea
basin according to IUCN criteria. Criteria include commercial fishing,
sport fishing, medium numbers, habitat destruction, limited range
(less than 25% of water bodies), absent in other water bodies in Iran,
and present outside the Caspian Sea basin.
Further work
The status of populations of this species in Iran should be carefully
monitored in view of the threats outlined above.
Sources
General biology is based on Thorpe (1977) and
Craig (1987), and there is extensive European angling literature on this species.
Iranian material: CMNFI 1970-0510, 1, 156.8 mm standard length, Gilan, Golshan River (37º26'N, 49º40'E);
CMNFI 1979-0685, 1, 71.5 mm standard length, Gilan, Safid River around Mohsenabad below Dehcha (no other locality data);
CMNFI 1980-0123, 1, 68.9 mm standard length, Gilan, Safid River around Dehcha above Mohsenabad (no other locality data);
CMNFI 1980-0127, 1, 152.8 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0148, 2, 118.2-142.1 mm standard length, Gilan, Pir Bazar Roga (37º21'N, 49º33'E).
Genus Sander
Oken, 1817
This genus is found in both North America and Eurasia and contains
5 species. There are 3 species in the Caspian Sea basin, 2 of which
are reported from Iran. The Shahid Beheshti hatchery on the Safid River breeds the third species, S.
volgense (Gmelin, 1789), a northern Caspian Sea species, according to
Raymakers (2002) but it is unclear if these have been released and have
established populations in the southern Caspian Sea.
The genera Stizostedion Rafinesque, 1820 and Lucioperca
Schinz in Cuvier, 1822 are junior synonyms of Sander (see Kottelat, 1997).
The pike-perches are elongate and compressed, have large jaws
reaching back beyond mid-eye level, canine teeth on the jaws and
palatines, the preopercle is serrated posteriorly and has spines
ventrally, the opercle has a weak, flat spine at its postero-dorsal
corner, cheeks are naked or scaled only dorsally, there are 7-8
branchiostegal rays, adult gill rakers are densely denticulated
tubercles, in young denticulated rods, and the lateral line continues
onto the caudal fin with accessory lateral lines on the upper and lower caudal lobes.
Divergence between North American and Eurasian members of this
genus may have occurred in the middle to late Miocene or in the
Pliocene (Billington et al., 1990; 1991; Faber and Stepien, 1998).
Sander lucioperca
(Linnaeus, 1758)
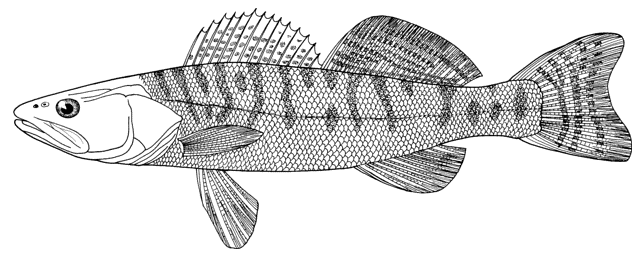
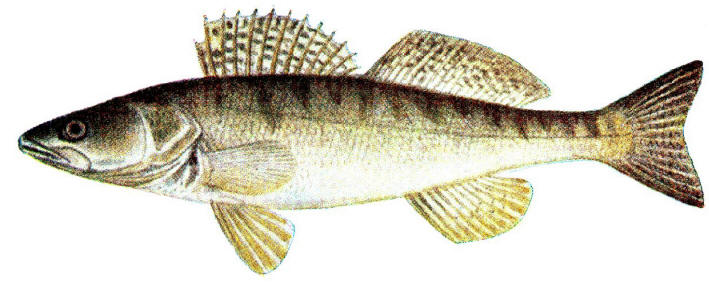

Common names
سوف (= suf), سوف معمولي
(= suf-e ma'muli or soof-e-maamooli, meaning common suf), sibey(ak) in Gilaki, ? sevideh.
[sif, caysifi or quaysifi in Azerbaijanian; adaty silebalyk in Turkmenian; sudak in Russian; pike-perch, European
pikeperch, zander].
Systematics
Perca Lucioperca was originally described from European lakes.
This may be the fish referred to as sevideh from the
Langarud Mordab by Holmes (1845). However the word may be sepideh
which means "the white one" and may in fact refer to Rutilus
frisii, the safid mahi. Akbarzadeh et al. (2007, 2009) identified three
distinct populations in the southern Caspian Sea using a truss analysis with morphometric characters (body heights and caudal peduncle measures) showing
better discrimination than meristic characters. Gharibkhani et al. (2009)
examined the population genetic structure of fish from the Talesh coast, Anzali
Wetland and Chaboksar coast and found them to be genetically differentiated,
with consequences for conservation.
Key characters
This species is separated from S. marinum by the dorsal fins
being close together, obviously much less than the eye diameter apart,
the anal fin spines are not closely joined to the soft rays, the
interorbital width is less than, or equal to, the vertical eye
diameter in adults, the upper jaw extends rearwards on a level behind
the posterior eye margin in adults (under the rear of the eye in
young), there are usually more than 18 soft rays in the dorsal fin,
and the spiny dorsal fin bears large spots.
Morphology
Lateral line scales 75-150 (this wide range from various literature
sources presumably includes counts of scales in the lateral line, and
other counts which are of smaller scales to one side of the lateral
line; Berg (1948-1949) gives a count of 80-97 for example, which is
inherently more reasonable). Scales above the lateral line 12-17 and
scales below the lateral line 16-24. Scales are incised on the
anterior margin where about 6-9 radii terminate, although not as
incised as in Perca fluviatilis. The focus is posterior. Ctenii
are well-developed. First dorsal fin spines 11-17; second dorsal
spines 1-4 and soft rays 16-27, usually 19-24. Anal fin spines 1-3,
soft rays 9-14. Pectoral fin branched rays 11-18 and pelvic fin
branched rays 5. Stubby, spinulose gill rakers number 10-17 and
vertebrae 40-48, usually 45-48, mode 46. There are 4-9 pyloric caeca.
The gut is relatively short but has a long anterior loop. The
chromosome number is 2n=48 (Klinkhardt et al., 1995; Miri Nargesi et
al., 2007) although the latter authors found a different karyotype formula
in Iranian fish compared to other populations (2n=1m + 13sm + 4st + 6a).
Meristic values for Iranian specimens are:- lateral line scales 82(1), 86(1), 89(1),
90(1), 91(3); scales above the lateral line 13(1), 14(4), 15(1);
scales below the lateral line 21(2), 22(1), 24(2), 25(1); caudal
peduncle scales 33(3), 34(3); first dorsal fin spines 13(3), 14(4);
second dorsal soft rays 20(2), 21(2), 22(1), 23(2); anal fin soft rays
11(4), 12(1) 13(2); pectoral fin branched rays 13(2), 14(2), 15(3);
pelvic fin branched rays 5(7); and vertebrae 45(3) or 46(3).
Sexual dimorphism
Females have a much lighter, whitish belly than that of males which
is marbled blueish in the spawning season. The genital papilla of females protrudes.
Colour
The back and flanks are green to blue-grey to brown-black, the
belly is white to bluish and fins are yellow-grey. The dorsal and
caudal fins have rows of black spots on the membranes, largest and
most distinctive on the spiny dorsal fin. Other fins are pale yellow.
The eye is silvery because of reflection from the tapetum lucidum.
Young have 8-13 brown to blackish-brown bars but these usually fade
with maturity. The peritoneum is silvery to brownish in preserved fish.
Size
Up to 1.5 m and 20.0 kg. The Iranian commercial catch in the 1950s
was 24-60 cm long and weighed 1.6-2.7 kg, declining to 0.6 kg (Farid-Pak, no date).
Distribution
Found in the basins of the Baltic, Aegean, Black, Caspian and Aral
seas. In Iran reported in the Aras River and along the whole Caspian coastal plain from
Astara to the Atrak River including the Ameerkalaye Lagoon near Lahijan, the
Gorgan, Qareh Su, Neka, Babol, Haraz, Safid and middle Aras rivers, as well as in the southeast Caspian Sea, southwest Caspian
Sea and south-central Caspian Sea, Gorgan Bay, Anzali Mordab and Siah Keshim
Protected Region (Nedoshivin and
Iljin, 1929; Derzhavin, 1934;
Kozhin, 1957; Griffiths et al., 1972; Holčík and Oláh, 1992; Nejatsanatee, 1994; Riazi, 1996; Abbasi et al.,
1999; Kiabi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004; Abdoli
and Naderi, 2009).
This species has also been stocked in the Zarreinerud, 70 km upstream of Miandoab in 1971
(Griffiths et al. (1972), to Valasht Lake near Marzanabad, Evan Lake northeast of
Qazvin, Ghorigol Lake near Tabriz, Marivan Lake in Kordestan and the
Haft Barm Lakes west of Shiraz (Anonymous, 1977). Although this species has been
introduced to the Manjil Reservoir on the Safid River (Griffiths et al. (1972), this reservoir is
drained to remove excess silt and no fishery exists (J. Holčík, pers. comm., 1992). Introductions to reservoirs in Khuzestan did not
survive (M. Al-Mukhtar, pers. comm., 1995).
It is also found in the Karakum Canal and Kopetdag Reservoir of Turkmenistan (Sal'nikov,
1995) and may eventually reach the Iranian Tedzhen (= Hari) River basin.
Zoogeography
The relationships of this species are discussed under the genus.
Habitat
Generally this species is found in small schools near sandy and
stony bottoms in deeper water of rivers. Ideally there should be some
concealment. It can also live in reservoirs.
This species has both freshwater and semi-anadromous forms in the Caspian Sea
basin. It has a limited migratory behaviour such that morphologically distinct
stocks may exist in larger water bodies. The population of the Anzali
Mordab represents a separate stock (Hydrorybproject, 1965). Riazi
(1996) and Karimpour (1998) report that this species migrates into the
Siah-Keshim Protected Region of the Anzali Mordab and is also resident there.
Most movements of this species are within 10-20 km although
distances up to 300 km have been recorded in the Volga River. Suf show strong homing
tendencies. The upper lethal temperature is 35°C.
High turbidity levels are preferred. During the day, suf shelter from
strong light by descending in the water column (Collette et al.,
1977; Marshall, 1977). Knipovich (1921) reports this species from
depths of 11.0-11.9 m, possibly deeper, in the Iranian Caspian Sea. In
Dagestan this species prefers areas where there is flowing water
well-supplied with oxygen (Shikhshabekov, 1978) and avoids vegetation
and therefore competition with Esox lucius. Suf will tolerate
low salinities and can be found around river mouths in the Caspian Sea
basin but the sea itself is too saline.
Age and growth
Males mature at 2-6 years (32 cm) and females at 3-6 years (42-44
cm). Life span may exceed 19 years, although in Lake Eğirdir,
Turkey only 7 age groups were recorded (Becer and Ikiz, 1999a). Optimum growth occurs at 28-30°C
(Marshall, 1977).
During 1932-1933 in the Anzali Mordab, 5-7 year old
fish dominated in catches and weighed 2.6-7.4 kg but by the 1960s this
had declined to 2-5 years and 1.6-2.7 kg (Hydrorybproject, 1965).
Catches in 1971/72 in the commercial fishery of Iran were 3-7 years
old, 33.0-55.0 cm long and weighed 370-2100 g (Razivi et al., 1972).
Abdolmalaki (2005a) found age groups in the Iranian Caspian to be 2-5 years with
2-3 year olds forming 78.5% of the catch. The von Bertalanffy growth equation
was Lt = 52.5*[1-exp-0.158*(t + 1.852)]. The instantaneous rate of
total, natural and fishing mortality was 0.95 year-1, 0.31 year-1
and 0.64 year-1 respectively. The calculated exploitation ratio was
0.67, the estimated biomass was 31.56 tons, the minimum sustainable yield was
13.89 tons (lower than the total catch), and the fishery return coefficient was 2.87%.
Abdolmalaki and Psuty (2007) report 6 age groups for coastal waters of the
southern Caspian Sea, the length-weight relationship was w = -0.020606L2.85,
and the von Bertalanffy parameters were L∞ = 55.05 cm fork length
(substantially less than in the Volga River delta at 79.0 cm and Aras Lake in
Iran at 73.3 cm), K = 0.15, t0 = -2.59 and M = 0.31. More than 90% of
the beach-seine caught fish were smaller than the minimum legal length. These
authors also provide details of recruitment and fishing mortality for this
population which is enhanced by introduction of fingerlings.
Rahimibashar et al. (2008) found that the fish in the Aras Dam lake had
allometric growth and age classes caught with cast nets and gill nets were 2+ to
5+ years.
Food
The suf is an ambush-pursuit predator. Feeding on fish begins at a
length of 5-10 cm (2-3 months of age) depending on the relative
abundance of zooplankton, invertebrates and forage fish. In the Volga Delta, spawning
Rutilus rutilus (presumably R. caspicus) in April-May is the most important food, up to
80% of the annual ration. In the 1960s and 1970s when the population of suf was
7 million fish, they ate 53,000 tonnes of Rutilus rutilus (presumably
R. caspicus) (Caspian Sea
Biodiversity Database, www.caspianenvironment.org). Adults are solitary but young fish feed in
schools on nauplii, copepods and some rotifers. Some adults are
cannibals (Collette et al., 1977; Marshall, 1977; Popova and
Sytina, 1977) and Balik (1999) reports that in Lake Beyşehir, Turkey, a suf can eat its own species with a
mean size of 35.9% of its length. Apparently many prey fish are seized and swallowed tail
first. One Iranian specimen contained a Neogobius melanostomus
and gobies are an important food item generally in the Caspian Sea,
17.8% of the diet compared to 59.9% for Rutilus rutilus (presumably R.
caspicus).
Rahimibashar et al. (2008) found that the fish in the Aras Dam lake
were carnivorous, almost gluttonous, feeding principally on bony fishes (92%
food preference) with some crab larvae and aquatic insects.
Reproduction
The spawning migration begins in late March-early April in Dagestan
with spawning in early to mid-April (Shikhshabekov, 1978). In Eğirdir
Lake, Turkey spawning took place from April to June and Becer and Ikiz (1999b)
give details of fecundity, egg diameters, and the relationships between length,
weight and gonad weight and fecundity for fish that mature as young as ages 1-2. The
spawning season over the range of this species is late February to
late July, usually April-May at 12°C (range 6-22°C)
as deep as 17 m. There are distinct spawning stocks.
In the Anzali Mordab, the main spawning area in the southern Caspian, the spawning
run usually starts in the first 10 days of March at water temperatures
of 8.0-9.5°C, ending at 12-14°C (Hydrorybproject, 1965; Razivi et al., 1972).
Apparently, natural spawning has stopped completely in the mordab and this
lagoon is stocked with fingerlings from spawners held at Aras, a border
reservoir lying between Iran and Azerbaijan (Abdolmalaki and Psuty, 2007). Males build nests
in depths of 30 cm to several metres on hard bottoms usually in turbid
water. Each nest is a flat pit edged by gravel or shell. Plant roots
are often exposed as a spawning substrate on which eggs are laid
individually. The nest is guarded by the male and eggs are fanned. The
female is driven away after spawning. Male suf are so devoted to
protecting the nest that they will remain on site even if water levels
fall and their backs stick out of the water. In addition they will try
to bite humans if they approach the nest. Spawning is intermittent
over several days and usually takes place at dawn. Maximum fecundity
is 2.5 million eggs and egg diameters are up to 1.5 mm. Hatching
occurs from 4 to 26.5 days, depending on temperature (Collette et
al., 1977; Marshall, 1977). Females descend to the sea first from
the Anzali Mordab after spawning and fry there are 19-33 mm long by
the end of May (Hydrorybproject, 1965).
Parasites and predators
Eslami and Mokhayer (1977) examined 100 specimens of suf and found 20% to
be infested with larvae of the nematode Anisakis. Ataee and Eslami
(1999, www.mondialvet99.com, downloaded 31 May 2000) report the
helminth Anisakis from the gastro-intestinal tract of fish from
the Anzali wetland. This parasite can infest man if fish is eaten smoked, salted or fried at
temperatures below 50°C. Mokhayer (1976b) records the acanthocephalan Corynosoma caspicum.
Jalali and Molnár (1990a) record the monogenean Ancyrocephalus
paradoxus from this species in the Safid River. Masoumian et al. (2005) recorded the protozoan
parasite Trichodina perforata from this species in the Aras Dam in West Azarbayjan. Pazooki et al. (2007) recorded various parasites from localities in West
Azarbayjan Province, including Diplostomum spathaceum and Argulus foliaceus from this species.
Barzegar et al. (2008) record the digenean eye parasite Diplostomum
spathaceum from this fish.
Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found
Achtheres percarum on this species.
The Caspian seal, Pusa caspica, is a significant predator on
this species (Krylov, 1984) as are predatory fishes such as Esox
lucius, Perca fluviatilis and Silurus glanis. Adult suf have few predators.
Economic importance
There is some opportunity for sport fishing for this species in the
Anzali Mordab and potentially in various lakes around the country
where it has been introduced (Anonymous, 1977). It is a very popular
food fish in Iran (Razivi et al., 1972). It has also been
studied in Iran as a control species for undesirable fishes (Annual
Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 80, 1997).
Nevraev (1929) reports on catches in various regions of Iran in the
early years of the twentieth century. There were no evident trends of
increase or decrease. In the Astara region from 1901-1902 to 1913-1914
the catch varied irregularly from 154 to 31,931 fish, in the Anzali
region from 1901-1902 to 1918-1919 the catch varied from 608,300 to
3,367,000 fish, in the Safid River region from 1899-1900 to 1917-1918
the catch varied from 9983 to 125,182 fish, and in the Astrabad region
from 1900-1901 to 1912-1913 the catch varied from 1400 to 22,900 fish.
Stocks of this species are known to fluctuate in Iran, as obviously do the
catch statistics. Most fish are caught in beach seines although some are caught
in gillnets, both legally and illegally (see below). The main fishing ground is
coastal waters in the Anzali region. Catches in the 1920s were at 3000-4000 tonnes for the coastal
zone of the southern Caspian Sea but declined drastically afterward (Razavi,
1999). The commercial catch in Iran from 1956/1957 to 1961/1962 varied from
206 kg to 20,945 kg (Vladykov, 1964; RaLonde and Walczak, 1972), from 1965/66 to 1968/69 it
varied from 7 to 77 tonnes (Andersskog, 1970) and from 1963 to 1967
ranged from 0 to 14.6 t (RaLonde and Walczak, 1970b). In the 6 years from 1980
to 1985 catches were recorded by the Food and Agriculture Organization, Rome as
respectively 0, 0, 0, 12, 13 and 10 t. Catches in 1990 were
about 5-10 t and in 1996 about 35-40 t (Bartley and Rana, 1998b). In 2000-2001,
the catch was 18 t or 11% of the total commercial catch in the Iranian
Caspian Sea basin. 12 t were caught by beach seine along the coast, 3 t
were taken in the Anzali Mordab and the rest was an estimated amount of
unlicensed captures (Abdolmalaki, 2005a). In 2003-2004 the catch was 38 t, a
decrease in comparison to the previous year, with 15 t of this from
beach seine cooperatives. Most fish were immature and undersized and the catch
was based on release of fingerlings (Abdolmalaki, 2006).
Summaries of catches of this species in the coastal southern Caspian Sea over
8 decadal periods is given in Fisheries.
Recent catches from Abdolmalaki
and Psuty (2007) are as follows:-
|
Year |
Total catch (t) |
Catch by beach seine (t) |
Number of beach seine cooperatives |
Beach seine efforts (hauls) |
Number of illegal gillnets confiscated |
|
1990 |
4.0 |
4.0 |
68 |
20.975 |
No data |
|
1991 |
12.3 |
12.3 |
81 |
27,200 |
104,828 |
|
1992 |
10.0 |
8.7 |
88 |
30,239 |
109,446 |
|
1993 |
12.3 |
7.3 |
93 |
33,986 |
138,026 |
|
1994 |
40.2 |
22.6 |
91 |
27,868 |
215,381 |
|
1995 |
10.1 |
4.0 |
101 |
34,055 |
204,831 |
|
1996 |
8.0 |
2.8 |
109 |
42,847 |
270,727 |
|
1997 |
8.1 |
2.9 |
111 |
45,263 |
205,999 |
|
1998 |
95.0 |
54.8 |
125 |
52,574 |
222,897 |
|
1999 |
17.5 |
11.5 |
139 |
50,953 |
130,849 |
|
2000 |
18.0 |
12.0 |
147 |
56,913 |
82,678 |
|
2001 |
26.0 |
21.5 |
150 |
60,006 |
113,729 |
|
2002 |
30.0 |
20.3 |
150 |
57,310 |
141,506 |
|
2003 |
23.8 |
15.0 |
151 |
53,846 |
179,656 |
|
2004 |
22.5 |
14.4 |
151 |
49,809 |
261,875 |
In the period from 1933/34 to 1961/62 in the Bandar-e Anzali region catches varied from
about 3483 t at the earlier date to 33 kg at the later one, with large
variations between years. Holčík
and Oláh (1992) report a catch of only 22 kg in the Anzali Mordab in
1990, and from 1932-1964 reported catches varied from 1 to 2581 t annually.
Hedayatifard and Jamali (2008) showed that this fish is a good source of
polyunsaturated fatty acids and one of the best sources for omega-3 fatty acids,
useful in preventing cardiovascular disease.
Khaval (2007) investigated
polyculture of this species with silver, bighead, grass and common carp at the
Safidrud Fisheries Research Station. Carp density was 3000 fish per hectare with
60% silver carp, 20% grass carp and 10% each for common and bighead carp. Suf
weighed 2.1 g and were stocked at 250 fish per hectare. Survival rates were
93.33% for the suf and 83.77% for the Chinese carps. Over the period April to
November, suf fingerlings reached 54.4 g on average and production was 4446.66
kg per hectare compared to 3212.8 kg without polyculture. Ghafouri Salah et
al. (2008) studied physiological stress in this species and its effects on
cortisol and muscle compounds.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in sport, in textbooks, and because it has been widely
introduced outside its natural range. The suf or pike-perch is also
fish-farmed in Europe for stocking purposes as eggs or young.
Conservation
The decline in catches from about 3000-4000 tonnes to about 30 t noted above
has never been adequately explained. Overfishing, degradation of spawning
grounds, fluctuating water levels and salinity variations in coastal waters, the
volume of freshwater input, oxygen concentrations and input of organic material
to estuaries and the sea, are all possible factors (Abdolmalaki and Psuty,
2007). The stocks of this species in Iranian waters also declined in the 1960s
(Walczak and RaLonde, 1970; RaLonde and Walczak, 1972). Causes were
reduction in estuarine habitat needed for spawning, man-made habitat
changes and overfishing of the younger age classes and first year
spawners. The catch declined from 125 tonnes annually in the 1940s to
14.6 t in 1967. Vladykov (1964) considered stocks all along the
Iranian shore to be at dangerously low levels. Griffiths et al.
(1972) suggested that stocks in Iran were on the verge of extinction
and recommended a three-year ban on catching this species. Artificial
raising of this species is difficult but more than 6 million
fingerlings were raised and released into the Anzali Mordab in the
Iranian year 1991-1992, a 100% increase over the previous year (Abzeeyan,
Tehran, 4(2):VI, 1993). Fingerling production rose from 0.12 million
in 1990, to 1.50 million in 1991 and 2.50 million in 1992 (Emadi,
1993a)(note that Matinfar and Nikouyan (1995) give 1.63 and 2.44 million
fingerlings for 1991 and 1992). Fingerling production in 1995 was 2.269 million, in 1996 2.4
million and for 1996-1997 8 million (Bartley and Rana, 1998a; 1998b).
The Sturgeon International Research Institute, which opened in 1994
near Rasht, released 5-8 million fry in 1996-1997 (Bartley and Rana,
1998b). The release in 1999 numbered 5 million "juveniles" (I.F.R.O.
Newsletter, 23:4, 2000). Billard and Cosson (2002) cite an annual production
of 5-10 million, mostly released in the Anzali lagoon and Moghaddam (2006) gives
a figure of 5.13 million fingerlings for 2002. The highest number of fingerlings
released in the Anzali Lagoon was 6,604,000 in 2003 with the lowest being
1,160,000 in 1993 for the period 1991-2003 according to Abdolmalaki and Psuty
(2007). The latter authors also note that the beach seines used in Iran (see
Fisheries in Introduction) do not protect young
fish. There is a heavy mortality of discarded fish even when legal landing size
is enforced and resources are inadequate to manage the fishery effectively. The
minimum mesh size of the cod-end of the seines should be increased and its use
monitored.
Ramin (1996) has studied semi-artificial propagation and rearing of
fry of this species in Iran. Broodstock spawning occurs in March-April
at 12-14°C on artificial nests of green wool bunches on wooden frames placed in
ponds at 5 m intervals. Nests close to the bottom are preferred and
eggs are dropped on them with an average fertilization of 30-90%. The
nests with eggs on them are kept in a mist chamber and the eggs
collected and placed in jars. Eyed eggs appear on day 3 or 4 of
incubation. Yolk-sac absorption lasts 9-13 days and exogenous feeding
fry measure 4-6 mm.
Lelek (1987) classifies this species as intermediate to vulnerable
in Europe. Kiabi et al. (1999) consider this species to be
vulnerable in the south Caspian Sea basin according to IUCN criteria.
Criteria include commercial fishing, sport fishing, few in numbers,
limited range (less than 25% of water bodies), absent in other water
bodies in Iran, and present outside the Caspian Sea basin. Nezami
et al. (2000) consider this species to be endangered because of
overfishing, habitat destruction and spawning ground degradation.
Further work
The numbers of this species should be actively surveyed because of threats outlined above.
Sources
Deelder and Willemsen (1964) reviewed the biology of this species
as did Craig (1987). Robins (1970) gave a bibliography of the genus Stizostedion (= Sander).
Iranian material: CMNFI 1970-0532, 1, 209.3 mm standard length, Gilan, Caspian Sea near Bandar Anzali (37º28'N, 49º27'E);
CMNFI 1979-0431, 2, 211.5-241.0 mm standard length, Mazandaran, Now Shahr fish bazaar (no other locality data);
CMNFI 1979-0455, 1, 68.5 mm standard length, Markazi, Manjil Dam (36º45'N, 49º17'E);
CMNFI 1980-0127, 1, 266.1 mm standard length, Gilan, Caspian Sea near Hasan Kiadeh (37º24'N, 49º58'E);
CMNFI 1980-0130, 1, 197.4 mm standard length, Mazandaran, river near Iz Deh (36º36'N, 52º07'E);
CMNFI 1980-0150, 1, 280.8 mm standard length, Gilan, Caspian Sea at Safid River estuary (37º24'N, 49º58'E).
Sander marinus
(Cuvier, 1828)
Common names
suf-e daryai (= sea suf), souf-e-daryaee.
[daniz sifi in Azerbaijan; morskoi sudak or sea pike-perch,
erroneously bërsh by fishermen in the Caspian Sea, both in Russian; sea zander, estuarine perch].
Systematics
Lucioperca marina was originally described from the Black Sea at Feodosiya. No types are known (Eschmeyer et al., 1996).
Key characters
This species is distinguished from S. lucioperca by the
dorsal fins being well-separated, but by a distance less than eye
diameter, anal fin spines are weak and closely joined to the soft
rays, interorbital width in adults is much greater than eye diameter,
the upper jaw extends back level with the posterior pupil edge or
almost to the posterior eye edge in adults, the dorsal fin soft rays
are 18 or less, and the spiny dorsal fin lacks large spots.
Morphology
First dorsal fin spines 12-14, second dorsal fin spines 1-4
followed by 12-18 soft rays, anal fin spines 2-4 followed by 9-12 soft
rays, pectoral fin with 1 spine and usually 15 soft rays, and pelvic
fin with 1 spine and 5 soft rays. Lateral line scales 75-88, scales
above the lateral line 9-13 and caudal peduncle scales 34-36. Scales
have few anterior radii, a crenulate anterior margin and a posterior
focus. Gill rakers on the upper arch number 12-20, on the lower arch
11-17, reaching the second raker below when appressed, elongate and spinulose. Vertebrae
number 38-44. There are 5-7 pyloric caeca. Cheeks are scaleless or
almost scaleless. There are canines on the jaws and palatines. These
counts are combined for several literature sources and include eastern
and western populations of the southern Caspian Sea which show evident
differences indicating distinct stocks. The gut is short and s-shaped.
Meristic values for Iranian specimens are:- first dorsal fin spines 13(2) or 14(1),
second dorsal fin spines 2 (3), soft rays 16 (3); anal fin spines 2(3), soft rays 12(3); soft pectoral
rays 15(3), pelvic fin with 1(3) spine and 5(3) soft rays, lateral line scales 79(2) or 82(1),
scales above lateral line 10(1), 12(1) or 13(1), caudal peduncle scales 34(2) or 36(1);
and total gill rakers 12(1), 13(1) or 15(1).
Sexual dimorphism
Unknown.
Colour
The back is light grey and the flanks have 12-15 dark bars, which
are sometimes indistinct and may be absent, e.g. in females from the
eastern Caspian which had small, irregular, dark-brownish speckles.
Some fish are almost black and lack bars. The first dorsal fin lacks
the strong spots seen in Sander lucioperca and is dark grey to
black with patches of concentrated melanophores and clearer areas
forming irregular and incomplete stripes, or darkly fringed and with a
dark spot at the posterior base. The second dorsal fin and the caudal
are finely spotted. Other fins are grey with some melanophores on
rays. Eyes are silvery due to the tapetum lucidum. The peritoneum is brownish.
Size
Attains 62 cm (65 cm total length in Jolodar and Abdoli (2004)) and 2.2 kg.
Distribution
Found only in the northwestern Black Sea and the Caspian Sea.
Reported from near Gasan-kuli in Turkmenistan (Berg, 1948-1949) and from the southeast
Caspian Sea in Iran (Kiabi et al.,
1999). Jolodar and Abdoli (2004) and Abdoli and Naderi (2009) record it from the central, southwestern
and southeastern regions of the Caspian Sea including in Astara. An old record is cited below under Habitat.
Zoogeography
The relationships of this species are discussed under the genus.
Habitat
This species lives in the Caspian Sea proper and rarely enters
rivers. De Filippi (1865), however, did record "Un molto bello e
grosso individuo....in un canale di Murdab, ove l'aqua era del pari
sensibilmente dolce". It favours areas with rocky bottoms and
does not migrate. In winter, part of the population moves into deeper
water at depths of 30 m, rarely 100 m, while another part remains near
the shore. The major concentration of this pike-perch is found near
the shores of Turkmenistan, and secondly of Azerbaijan.
Age and growth
Sexual maturity is attained at 3-4 years for most fish with a few
fish maturing at 2 years (Guseva, 1975). Life span is at least 12
years. Growth is slightly faster in females up to age 5, evens out
later and males become larger (Kuliyev, 1981). Males on the spawning
grounds average 41.2 cm and females 42.9 cm, with an average weight of
0.94 kg (Berg, 1948-1949).
Food
The principal foods are gobies (Gobiidae), young herring and
tyulkas (Clupeidae), silversides (Atherinidae), and crayfish.
Reproduction
The male prepares a nest site and protects the eggs. Spawning takes
place at 3-12 m on open, stony bottoms or in "nest-caves"
and eggs are laid in a continuous layer. The male constructs and
guards the nest. Spawning usually begins in the second half of April
and ends in mid-May at temperatures of 10-17°C,
and is most intense at 13-15°C (Gusev, 1974a). Up to 126,000 adhesive eggs are laid (Guseva, 1974a;
1975; Kuliyev, 1981) and are larger than in S. lucioperca.
Fertilised eggs are 2.6-3.8 mm and at water temperatures of 14.7°C,
incubation takes 12-17 days (Gusev, 1974a).
Parasites and predators
None reported from Iran.
Economic importance
The sea pike-perch was commercially fished off the Turkmenistan
coast in the 1930s and 1940s with catches of 19 thousand centners (1
centner = 100 kg) or 2,271,000 fish. In 1927-1929 the annual average
on the shores of Azerbaijan was 7000 centners and in 1930-1932 10,3000
centners. In 1930 the catch for the whole Caspian Sea was 909 thousand
centners (Zenkevitch, 1963). The development of offshore oil deposits
has drastically reduced stocks throughout the Caspian Sea (Guseva,
1974b; 1975; Kuliyev, 1981).
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in textbooks.
Conservation
This species has been proposed for inclusion in the "Red Book
of the U.S.S.R." which forms the basis for measures to protect
species (Pavlov et al., 1985). Kiabi et al. (1999)
consider this species to be data deficient in the south Caspian Sea
basin according to IUCN criteria. Criteria include possibly few in
numbers, limited range (less than 25% of water bodies), absent in
other water bodies in Iran, and present outside the Caspian Sea basin.
The 2000 IUCN Red List lists this species as DD (Data Deficient).
Further work
The distribution and abundance of this species in Iranian waters
needs to be examined to determine its conservation status.
Sources
Morphology based in part on Svetovidov and Dorofeeva (1963) and Kuliyev (1981).
Iranian material: CMNFI 1970-0532, 3, 156.3-165.5 mm standard length, Gilan, Caspian Sea near Bandar Anzali (37º28'N, 49º27'E).
Sparidae
The sea breams or porgies are found in the Atlantic, Indian and
Pacific oceans and comprise 33 genera and about 115 species (Nelson, 2006). Some commonly enter
estuaries and penetrate up rivers. Maximum length is about 1.2 m.
This family is characterised by a groove in the distal end of the
premaxilla which accommodates the maxilla; the body is oblong to ovate
and is compressed; the head is large with a steep upper profile; the preopercle margin is smooth; scales are weakly
ctenoid, moderate in size and extend on to the cheeks and operculum;
teeth are conical to incisiform and molar teeth are found in some at
the rear of the jaw; there are no teeth on the vomer, palatines or
tongue; the dorsal fin is continuous with an anterior spiny portion
and a soft-rayed posterior portion about equal in size; with 10-13
spines and 10-15 soft rays respectively; spines fold into a groove;
the anal fin has 3 spines (the second the largest) and 8-14 soft rays;
branchiostegal rays 5-6; branchiostegal rays 5-6; and lateral line not continued onto the
caudal fin but with enlarged scales near the head.
Many species in this family are hermaphrodites with male and female sex
organs developing simultaneously, changing sex from male to female (protandry),
or from female to male (protogyny). These fishes are often important as food or
sought by anglers. Young fish may very different in colour to adults, usually
being more vividly coloured with distinctive patterns. Most species are marine (see
Marine List in Checklists in the Introduction)
but a few enter fresh water and penetrate a considerable distance from
the sea including one species in Iran.
Genus Acanthopagrus
Peters, 1855
Members of this genus have a compressed and moderately deep body,
4-6 enlarged incisiform teeth at the front of the jaws followed by 3-4
rows of molars, the second anal fin spine is longer than the third, there is a
scaly sheath at the base of the dorsal and anal soft fins, and moderate-sized scales.
Iwatsuki and Heemstra (2010) revised the genus in the western Indian Ocean.
Acanthopagrus latus
(Houttuyn, 1782)
Common names
شانك (= shanak), شانك زردباله
(= shanak-e zardbaleh or yellowfin shanak).
[shanak, shaghoom, shaam, sha'm, shaem, sheim or sha-om in Arabic; yellow-finned porgy or
seabream, yellow-finned black porgy, Japanese silver bream].
Systematics
Sparus latus was originally described from Japan.
Al-Hassan (1990) found differences in two meristic characters
(pectoral and dorsal fin ray counts) but no differences in
electrophoretic characters between populations from the Shatt al Arab
and Khor al Zubair areas of southern Iraq. He concludes that there is
only one stock of this species in southern Iraq as meristic variation
may reflect environmental conditions.
Key characters
This species is the only sparid member recorded from Iranian
freshwaters and is recognised by the dorsal fin spines alternately
thick and thin and the colour pattern.
Morphology
Upper profile of head steep and convex back to above the posterior eye
margin. The head bulges over the eye. Dorsal fin spines 11-13, soft rays 9-13. Anal fin with 3 spines,
the second much stronger and wider than the third, and 8-9 soft rays.
Pectoral fin branched rays 10-16. There is a strong spine in the
pelvic fin and a well-developed axillary scale. Lateral line scales
41-46, or 48-50, or up to 55 depending probably on differing counting
methods. The scales are vertical ovals with the anterior margin wavy
where radii intersect. They have very fine circuli, moderate numbers
of posterior radii, a subcentral posterior focus, and ctenii on the
central part of the posterior margin extending inwards towards the
focus. Four or five series of preopercular scales. The first pelvic fin ray is elongated as a small filament.
There is a strong pelvic axillary scale. There are 3-4 scale rows sheathing the dorsal and anal fin bases. There are 4-6 compressed
teeth in front of each jaw followed by 3-5 rows of molar teeth. The chromosome number is 2n=48 (Klinkhardt et
al., 1995). The gut is an elongate s-shape.
Meristic values for Iranian specimens are:- dorsal fin spines 12, soft rays 10, anal fin spines 3, soft
rays 8, pectoral fin branched rays 13, scales mostly lost.
Sexual dimorphism
This species is a protandrous hermaphrodite, being male early in
its life and then becoming female later. Catches will include males, females and
hermaphrodites, e.g. in Abu-Hakima's (1984a) study in Kuwait, there were 326
males, 343 females and 41 hermaphrodites.
Colour
Overall colour is a silvery-grey or silvery-white with the back darker
and the belly yellowish. Scales each have a dark, brownish to golden
spot at the base which line up to form apparent stripes along the flank. There is a dark
blotch at the upper corner of the gill opening, on both the body and
gill cover. There is a dark band over the head between the eyes and
the edge of the operculum is dark. Dorsal fin spines are white and the
membranes are grey, with dark margins between the spine tips. The soft
dorsal fin is dark grey with a light orange tinge. There is a small
back spot at the pectoral fin base and the fin is mostly hyaline with
a light orange tinge. The anal and pelvic fins are a light
yellowish-brown. The caudal fin is dark grey on the upper lobe and
yellow on the lower with a black margin. The peritoneum is silvery
brown in preserved fish with widely scattered melanophores.
Size
Reaches over 50 cm total length.
Distribution
Found from the Persian Gulf to Japan and north Australia. Recorded
from the Helleh River in Bushehr Province, Iran (J. Holčík,
pers comm., 1995) and from the Bahmanshir River (Marammazi, 1995; Eskandary
et al., 1999).
Also from the Shatt al Arab in Iraq on the border with Khuzestan in
Iran (Al-Hassan et al., 1989; Hussain et al., 1989).
Zoogeography
This marine species enters rivers in southern Iran and presumably freshwater
stocks are maintained from this marine gene pool.
Habitat
The usual habitat is over sand and rock bottoms in the sea down to about 50
m, but young fish may enter estuaries and may penetrate considerable distances
inland, although some fish remain at sea permanently. The frequency of
penetration into Iranian rivers along the Persian Gulf coast is not known. Larger specimens are known
to penetrate the Shatt al Arab in autumn, October to December, and this water
body is an important nursery for this species, found there year round as young.
Adults emigrate from January to March (Al-Hassan, 1990; Hussain et al.,
1987). At a freshwater station on the Shatt al Basrah Canal with salinities up
to 3.5‰, Al-Daham and Yousif (1990) found this species to be the second most
dominant after Liza subviridis, comprising 7.1% by number and 10.9% by
weight. Al-Daham et al. (1993) found young fish in the Shatt al Basrah
mostly from April to October. Cage-cultured fish are reared at 14-31°C in Kuwait
Bay (Abou-Seedo et al., 2003). Temperatures in Kuwait Bay during spawning
are 14-18ºC during the winter months, a situation mirrored by Liza
klunzingeri but not other species which begin to spawn in April (Abou-Seedo
and Dadzie, 2004).
Age and growth
A fast growing and hardy fish. Life in Iranian fresh waters has not been
studied. A study of fish in the Shatt al Basrah canal,
a man-made estuary of southern Iraq, was based on mostly small and immature fish
(49-181 mm standard length) caught mostly in April-October. The length-weight
relationship was W = 0.0511 L2.893, the dominant age group was 1+
fish, the maximum age was 3+ years, fish grew to 95, 155 and 215 mm total length
in their first three years of life, and mortality (Z) was 2.23 (Al-Daham et
al., 1993).
In an aquaculture experiment in Kuwait, fish more than doubled in weight over
a 6 week period (Jafri et al., 1981). Males mature at a smaller size
(12.3-14.2 cm) than females (24.3-26.2 cm) in cage-culture in Kuwait Bay
(Abou-Seedo et al., 2003). However fish in the Shatt al Arab are usually
less than 20 cm long and most are immature, in age groups 0 and 1. The
length-weight relationship for both sexes was W = 0.0916 L2.6601. The
lowest condition factors were found in April and May, possibly because fish were
spent after spawning or were in lower condition after the winter (Hussain et
al., 1987). In Kuwait, ages up to 14 years have been reported with ranges in
von Bertalanffy growth parameters for the years 1981-1985 of L (cm) =
38.3-52.29, K = 0.169-0.298, and t0 -0.213- to -2.237. Total
mortality values (Z) were 0.432-0.709, this range suggesting that, even for
relatively large samples (92-314 fish), may be too small to provide reliable
estimates of Z in species with a large overlap in age groups and where old fish
are sampled only with difficulty. The length-weight relationship was total
weight = 0.02874 x total length2.79198. Growth and mortality
estimates based on all data were L∞ = 40.48 cm, K = 0.258, t0
= -0.965, tmax = 14 years and Z = 0.600 (Samuel and Mathews, 1987).
Morgan (1985) gave values of L∞ = 43, K = 0.20 and Z = 0.97 but his
study excluded fish over 30 cm and below 19 cm which accounts for the difference
in mortality; and Samuel and Mathews (1987) had a value about 2.0 for Z when
only fish 20-30 cm long were analysed.
Heydarnejad (2009) gave the length-weight relationship for an Iranian sample
as W = 0.0451TL3.091.
Food
Freshwater food habits not known
for Iran in detail but the one specimen examined contained plant
fragments and scales of a cyprinid.
In a study of the recovering Hawr al Hammar, diet was 60% shrimps and 40%
insects (Hussain et al., 2006). Feeds on echinoderms, worms, crustaceans, insects, bivalve molluscs and
plants in the sea (Nasir, 2000; Al-Daham et al., 1993). Hussain et al.
(1987) found crabs and bivalves to be the most important items by percentage in
the Shatt al Arab Fish, shrimps and aquatic insects were also taken and there
was significant seasonal variation, with shrimps and aquatic insects more
important in December and spring. Hussain et al (1994) found bivalves in
91% of fish by number and weight in the Khawr az Zubayr. Al-Daham et al.
(1993) found fish in the Shatt al Basrah fed on, in order of occurrence,
crustaceans (decapods, amphipods, isopods, mysids, cladocerans and cyclopoids),
fishes (Liza spp., Barbus (= Carasobarbus) luteus, Thryssa purava, eggs and
scales), molluscs (Corbicula, Lymnaea, Tryonia and
Sphaeriidae), algae (Oscillatoria, Syndera, Fragillaria and
Cladophora), higher plants (Vallisneria, Ceratophyllum,
seeds and roots) and aquatic insects (Corixidae, Hemiptera, Odonata and
Coleoptera). Crustaceans were most important during July and November, molluscs
in May and fishes during August. Hosseini (1998) examined food in coastal waters
of Bushehr, Delvar and Rostami in the northern Persian Gulf of Iran and found
36.4% to contain crabs, 34.0% other fish and 13.4% shrimps. Snails and sea
urchins were also eaten.
Reproduction
Al-Hassan et al. (1993) have shown for fish
from the Shatt al Arab near Basrah, Iraq that haematological parameters vary
with reproductive phase and between sexes. Cage-reared fish in Kuwait Bay have a
prolonged spawning season from February to April. Fecundity there is up to
3,837,000 eggs. Spawning in the Shatt al Arab estuary is reported for April
(Hussain and Ahmed, 1995) and Al-Daham et al. (1993) record a spawning
season for the northwestern Arabian (= Persian) Gulf as January to April with a
peak in February and March. Abu-Hakima (1984a) found the spawning period in
Kuwait waters to be January to March with fecundity up to 2,152,993 eggs. This
species is a protandrous hermaphrodite with males dominating in smaller size
groups (22.3-24.2 cm) while females dominate in larger groups (24.3-26.2 cm)
(Abou-Seedo et al., 2003). Samuel and Mathews (1987) give a spawning date
of 1 December for their Kuwait sample. The gonadosomatic index was highest in
February-March in Hosseini's (1998) study in coastal waters of the northern
Persian Gulf.
The average absolute fecundity in coastal waters near Bushehr in Iran was
1,842,700, sex ratio was 1:1, and the spawning peak was January-February (Hossini
and Savari, 2004).
Parasites and predators
None recorded.
Economic importance
A good food fish seen in bazaars along the Persian Gulf coast and in the
Shatt al Arab. It was selling at U.S.$3.5-5.5 per kg in Kuwait about 1980, with 213 tons landed in
1995 for a value of U.S.$1,769,407 (Abou-Seedo et al., 2003). Experiments there indicate that
this species can be farmed (Jafri et al., 1981; Abou-Seedo et al., 2003) and this has been proposed for
Iranian waters in the Persian Gulf (Regunathan and Kitto, 2005). Experimental culture has been
tried at Qeshm Island where a million larvae were produced in March with
100,000 larvae 2.0-3.5 cm long surviving in May (www.shilat.com, downloaded 7
June 2007). It is caught by trawls, handlines, in hadra (fixed stake nets) and gargoor (fish pots) and in
the sport fishery in the Arabian (= Persian) Gulf (Samuel and Mathews, 1987;
Carpenter et al., 1997).
Conservation
This marine species is fished commercially in the sea and populations there
may be under some threat as a consequence. The status of freshwater populations
is unclear as they appear quite rare and are presumably derived from marine
populations at intervals.
Further work
The frequency of occurrence, detailed distribution and biology of this
species in Iranian fresh waters needs study.
Sources
Iranian material: uncatalogued, 1, 62.9 mm standard length, Bushehr, about15
km above mouth in Helleh River (ca. 29º13'N, ca.
50º43'E).
Comparative material: BM(NH) 1974.2.22:1859, 1, 76.4 mm standard length, Iraq, Basrah (30º30'N, 47º47'E); BM(NH)1974.2.22:1858,
1, 76.3 mm standard length, Iraq, Beree (no other locality data).
Mullidae
Genus Mullus
Linnaeus, 1758
Mullus barbatus
Linnaeus, 1758
Introduced to the Soviet Caspian Sea basin in 1931-1934 but not subsequently observed (Baltz, 1991). No Iranian record.
Cichlidae
Cichlids are found in fresh and brackish waters of Central and
South America, Africa, Madagascar, the Levant, southern India, Sri
Lanka and southern Iran. There are over 1300 species (with several hundred more
to be described) (Nelson, 2006) but only 1 is
found in Iran. Maximum length is about 80 cm.
Cichlids are distinguished by having only a single nostril on each
side; practically all other fishes have two nostrils. The lateral line
is in 2 parts, an anterior and higher portion ending under the soft
dorsal fin and a lower, mid-flank, posterior part beginning below
where the first part ends and continuing to the tail base, usually
7-25 spines in the dorsal fin followed by 5-30 soft rays, anal fin
usually with 3 spines, but some species have 4-9 or 12-15 spines,
followed by usually 4-15 soft rays, scales ctenoid or cycloid,
extending onto the head, a specialised pharyngeal bone in the throat
breaks up food by pressing it against a hard pad on the skull base,
mouth dentition highly specialised in relation to diet with scraping
(for algae on rocks), pointed (to seize fish), crushing (for
hard-shelled molluscs), winkling (for removing snails from their shell
or picking eyes) or reduced and embedded (for egg eating). Body form
varies greatly between species and many are colourful and highly
prized as aquarium fishes. They are often highly territorial,
defending a breeding area against all invaders. They are most common
in still waters where there are branches, rocks or plants.
Some cichlids are important food fishes but they
have attracted scientific attention for their elaborate breeding
behaviour and evolutionary history. Certain cichlids, for example, are
mouthbrooders, carrying eggs and fry in their mouths to protect them,
while others spawn on the substrate, build nests or nourish young from
a skin secretion. African lakes contain rich species flocks of
cichlids which show various feeding behaviours. How these species
arose and adapted to different ways of life have been important to
scientists in understanding the mechanisms of evolution and adaptation.
Murray (2001) reviews the fossil record and the
biogeography of the family and suggests an origin less than 65 MYA in
the Early Tertiary in contrast to other studies that give an origin
over 130 million years ago. Their salinity tolerance has enabled them
to cross marine barriers.
Genus Iranocichla
Coad, 1982
The genus is monotypic so its description is subsumed under the Species Account
below.
Iranocichla hormuzensis
Coad, 1982



Colour photographs of males (females in background) courtesy
of Thomas Schulz
Common names
mahi-e karoo, siklid Irani, siklid-e Hormuz, cichlid-e Hormuz.
[Hormuz cichlid, Iranian cichlid]
Systematics
Trewavas (1983) relates this species to Danakilia Thys van
den Audenaerde, 1968, a genus with two species (Stiassny et al., 2010) from northeast Africa, on
the basis of the low gill raker count, lower pharyngeal bone and teeth
morphology, and morphometric characters such as a deep preorbital
depth, long snout, head length and the small eye. Trewavas (1983)
places Iranocichla in Danakilia but also agrees with
Coad (1982a) that Iranocichla (and Danakilia) may be
related to species in the genus Tristramella Trewavas, 1942 of
the Jordan River basin and that this requires further investigation. Schwarzer
et al. (2009) found molecular evidence for the relationship of
Iranocichla and Tristramella (DNA material of Danakilia was
lacking). I
retain Iranocichla as a distinct genus until these
relationships are examined more closely as some of the characters used
to relate Danakilia and Iranocichla may be common
responses to temperature and salinity extremes. In addition, Trewavas
(1983) suggests a possible relationship of Danakilia with Oreochromis
alcalicus (Hilgendorf, 1905) of the African Rift Valley. This
species too is found in waters of high temperature and mineral
content. Klett and Meyer (2002) group this genus with Sarotherodon,
Oreochromis and Tristramella on mitochondrial NADH dehydrogenase
subunit 2 gene sequences.
The type locality is the "Mehran River at 27°04'N, 54°35'E,
Hormozdgan Province" (Coad, 1982a). The holotype, 94.2 mm
standard length, is a female with eggs in the mouth held in the
Canadian Museum of Nature, Ottawa under CMNFI 1979-0408A (see figure above). Paratypes are
CMNFI 1979-0408B, 15, 24.3-86.5 mm standard length, same locality as the
holotype and CMNFI 1979-0139, 35, 29.6-95.2 mm standard length, stream in Rasul River
drainage between Chahar Berkeh and Tang-e Dalan, ca. 27°25.5'N,
54°59'E, Fars-Hormozgan border. Paratypes were deposited in the British Museum
(Natural History), London under BM(NH) 1981.1.12:1-2 (2 specimens),
Muséum national d'Histoire naturelle, Paris under MNHN 1981-107, 108
(2), California Academy of Sciences, San Francisco under CAS 47324
(2), the Royal Ontario Museum, Toronto under ROM 36389 (1) and the
University of British Columbia, Vancouver under BC 81-1 (1).
Key characters
This is the only cichlid species in Iran, easily recognised by the
single nostril opening on each side of the head.
Morphology
This cichlid is uniquely characterised by a nearly circular dental
field on the lower pharyngeal bone, the teeth there being of uniform
size and not enlarged medially and by cheek, operculum, belly, isthmus
and area between the pectoral and pelvic fin bases naked or poorly
scaled. Other significant characters are the posteriorly rounded
dorsal and anal fins, short pectoral fins not reaching the vent,
cycloid scales with granular posterior circuli bearing rounded or
irregular protuberances, inferior apophyses for support of the
swimbladder centred around the fourth vertebra (figured in Coad
(1982a)), mesethmoid not meeting the vomer, modal vertebral count 29,
median length of lower pharyngeal bone 31.8-40.9% (mean 35%) length of
head, and pharyngeal blade/median length toothed area 0.6-1.0, mean 0.8.
Scales are regularly arranged on the flanks except that in some
large specimens the regular scale rows are interspersed with
irregularly distributed smaller scales, particularly on the upper
flank. Scales may be absent entirely from the head, sparse above the
lateral line anteriorly and on the belly posterior to the pelvic fins,
absent from the dorsal and anal fin bases, absent from between the
pectoral and pelvic fin bases and on the belly and isthmus anterior to
the pelvic fins. However, in other specimens the head may be scaled
dorsally to above the eyes, with scales variably imbricate, there may
be 2-3 rows containing 4-7 minimally or non-imbricate scales on the
cheek which is never completely scaled. The dorsal border of the
opercle may have two large scales next to each other and a single
scale may be present over the centre of the subopercular bone. Scales
may be present on the whole belly, isthmus and between the pectoral
and pelvic fin bases, but they are minute, embedded, and
non-imbricate. Their extent and number varies between individuals.
Small to minute scales, numbering up to about 20, are present on the
caudal fin base, extending distally onto the fin membranes for more
than half the fin ray length in some specimens.
Flank scales below the mid-point of the spiny dorsal fin and
beneath the upper lateral line are cycloid or very weakly ctenoid. The
focus is central and there are 9-14, mean 12.4, radii on the anterior
field based on 5 scales from 7 adult specimens 59.2-87.1 mm standard
length. Posterior circuli are granular so the exposed scale surface
has rows of rounded or irregular protuberances.
The gut is a tightly coiled spiral with its apex ventral. Gut
length in 5 specimens (59.2-90.5 mm standard length) is 6.8-8.3, mean
7.6, times the standard length. Gill rakers are short and rounded, reaching
the adjacent raker or a little further when appressed.
The pharyngeal apophysis is of the Tilapia type (Greenwood,
1978). The mesethmoid does not meet the vomer, the intervening space
being cartilaginous. Pores at the openings of the cephalic lateral
line canals on the preorbital and preoperculum are single not
multiple. The inferior apophyses for support of the anterior end of
the swimbladder involve vertebrae 2 to 5, the fourth vertebra being
involved in 8 out of 10 fish examined.
Teeth in the jaws are often irregularly arranged so that 4 rows are
found in some places in both jaws. In some individual fish where teeth
are regularly arranged there are 3 rows in the upper jaw and 4 rows in
the lower jaw. Number of rows decreases laterally to one at the rictus.
The outer row teeth are bicuspid with the lateral cusp the smaller,
while inner row teeth are tricuspid, with the central cusp the most
prominent. The upper jaw has more teeth than the lower jaw.
The diploid chromosome number is 2n=44, comprising 25 submetacentic, 18
subtelocentric and 1 metacentric chromosomes with an arm number of 70. The
chromosome count may indicate a relationship to the Levantine Tristramella
(Esmaeili et al., 2006).
Scales in upper lateral line 17(1), 18(1), 19(2), 20(8), 21(9),
22(10), 23(7), 24(4), 25(2), 26(1) or 29(1); scales in lower lateral
line 9(6), 10(17), 11(14) or 12(9); total scales in lateral series
28(1), 29(2), 30(4), 31(9), 32(13), 33(5), 34(4), 35(4), 36(3) or
40(1); scales around caudal peduncle 16(15), 17(13), 18(15), 19(2) or
20(1); precaudal vertebrae 14(2), 15(53) or 16(11); caudal vertebrae
13(26), 14(35) or 15(5); total vertebrae 28(19), 29(40) or 30(7).
Dorsal fin spines 14(6), 15(46) or 16(14); dorsal fin branched rays
9(2), 10(36) or 11(28); anal fin branched rays 6(7), 7(20), 8(38) or
9(1); pectoral fin branched rays 11(42) or 12(24); and total gill
rakers 14(6), 15(9), 16(24), 17(19), 18(6) or 19(1).
Sexual dimorphism
Head length is greater in females while pelvic fin length is
smaller in females compared to males. Interorbital width is greater in
males. Dorsal and anal fins are larger in males when expressed in
terms of longest ray length in head length (Coad, 1982a). Colour differs as
described below.
Colour
Live specimens are brightly coloured in spawning
condition (based on aquarium photographs in Schulz (2004)). The male is brick-red
on the lower sides and underside of the head with black on the dorsal head
surface. The underside of the head may also be black. The belly anterior to the pelvics
is black. The chin is white. The sides off the head have a few, scattered white
spots but the body, dorsal and caudal fins are densely covered with white spots
and blotches. Those on the dorsal fin are arranged in oblique rows and those on
the caudal fin in bars. The anal fin has white spots also but these are not
present distally. The pectoral fin has darkened rays but lacks spots. The pelvic
fin has white spots proximally but less than the anal fin but is overall a dark
black. Other reports and photographs (Svardal (2006) and Svardal and Svardal
(2006)) show dominant spawning males to be black
with brilliant turquoises blotches on the body but especially so on the fins.
The female has an overall silvery colour with up to 9 faint to moderate
flank bars. Fins are yellowish. The dorsal fin has a black tilapia-mark on the
posterior dorsal fin.
Overall body colour outside the spawning season is a
light lime green, with an iridescent tinge to the posterior edge of the
operculum and on the back. The dorsal fin has light, lime-green, oblique bars,
the last one or two black-edged and spot-like. The peritoneum is black.
Preserved specimens have the following pigmentation. Young fish
have a distinct tilapia-mark, a spot on the rays of the soft dorsal
fin typical of these cichlid fishes. The spot is black and is
surrounded by a hyaline ring. Occasionally a second spot is found
posterior to the first spot. The principal spot is often retained in
adult fish. Young also have 7-11 bars along the flank which are also
retained by adults but are then less distinct. In adults the dorsal
fin rays and membranes are covered with melanophores interspersed with
hyaline spots and irregular blotches. Wavy, oblique bars are found
posteriorly on the soft dorsal fin in some specimens. The caudal fin
has a series of about 7 narrow bars in some male specimens while
females are uniformly grey. The anal fin is narrowly barred with up to
6 vertical to oblique bars in some specimens, in others uniformly
pigmented grey proximally fading to hyaline distally. Pectoral and
pelvic fins are not barred and are lightly pigmented, the pelvics
being the darker. The head and body, including the belly, are more
heavily pigmented to give an overall brown colour, lightest on the
belly anterior to the pelvic fins in females. Scales are not pigmented
on their free margins, which are pale.
Some specimens may be quite dark, particularly the back and fins and strikingly the lips.
Size
Attains 11.09 cm standard length or 12.95 cm total length (Esmaeili and
Ebrahimi, 2006). Lamboj et al. (2006) give 13 cm, presumably total length.
Distribution
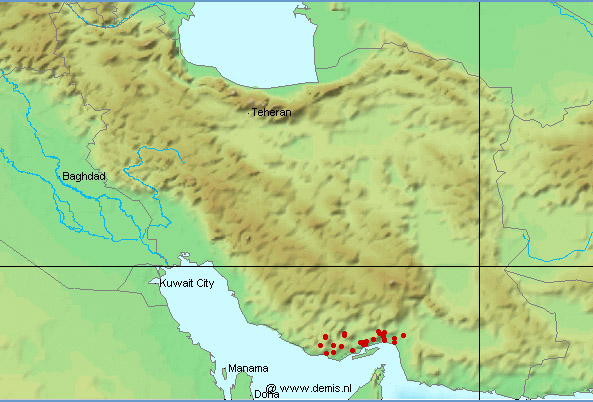
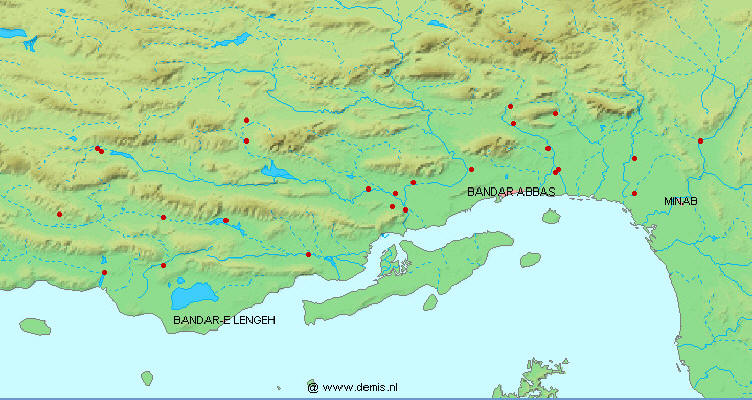
The cichlid is restricted to rivers draining to the Straits of
Hormuz in southern Iran (Coad, 1982a; Abdoli, 2000). Svardal and Svardal (2006)
also map this species at 27.770°N, 54.999°E, slightly to the north of samples
mapped here. The distribution mapped by Stiassny in
Keenleyside (1991) following Berra (1981) is too far north. The map in Berra (2001) is more accurate.
Abdoli (2000) records this species from the lower Minab basin, lower Hasan Langi, middle to lower Kul, Gowdar and middle to lower Mehran
rivers.
Specimens kindly sent to me by H. R. Esmaeili in 1997 are from the Dozdan River at 27°26'N,
57°10'E, an eastwards extension into the Minab River basin. The cichlid was not
collected there in the 1970s. The new record may simply be filling in
a collecting gap, a natural range extension or possibly the result of an introduction.
Bleher (2011) found only 2 of the 22 sites recorded by Coad (1982) to still
have water, although I have observed river stretches drying up and re-connecting
and not always the same stretches.
Zoogeography
Trewavas (1983) suggests that the ancestor of this cichlid was
distributed across the Arabian Peninsula in the Late Pliocene/early
Pleistocene when this area was more humid. Desiccation in the
Pleistocene and Recent Periods then led to the extinction of the
ancestor. A Miocene-Oligocene fossil "Tilapia" was
reported from Jisan in southwest Saudi Arabia where cichlids are not
now native (Brown, 1970) and would help support a southern
distribution of cichlids as the origin of Iranocichla. However
Trewavas (1983) reports that this fossil cannot be identified as a
cichlid. Micklich and Roscher (1990) and Lippitsch and Micklich (1998)
also report three species of what are presumably cichlids from
southwest Saudi Arabia in the Baid Formation of Oligocene age at Ad
Darb, Tihamat Asir. They belong to the basal grade of cichlids and to
two different clades within the African assemblage. Whybrow and
Clements (1999) record unidentified Cichlidae from the Early Oligocene
from the coastal trip of Dhofar, Sultanate of Oman with a date of 33
MYA. Murray (2001) reviews these and other cichlid fossil material and
the identity of Omani material as cichlids appears questionable.
Southwest Saudi Arabian material is more clearly cichlid but does not
point to a continuous distribution eastwards across the Arabian
Peninsula. However, Bănărescu
(1992b) considers that a common ancestor to Iranocichla
and the Levantine Tristramella evolved in the Arabian Peninsula
from African forebears in the Miocene, the latter lineage extending
its range northwards to the Levant and the former eastwards to Oman
and southern Iran, the Straits of Hormuz not then being in existence.
Murray (2001) gives an earliest date for colonisation of Iranocichla
ancestors to be the Middle Miocene when southern Iran rose above sea
level. She does not consider a coastwise dispersal through brackish
waters of Arabia to be a possible route as cichlids are not found
there today but indicates a route through the Tethys Sea/Indian Ocean
could be possible.
Coad (1982a) suggests another hypothesis. Iranocichla could be a
relict of a once wider distribution across the Tigris-Euphrates basin
in a northern arc rather than directly across the Arabian Peninsula.
The absence of cichlids from southern Arabia today warrant this
alternative hypothesis. Warm streams have probably been continually
present in southern Arabia and support a limited fish fauna today.
There is no apparent reason why cichlids should have become extinct
there. Murray (2001) points out that Iranocichla lives at
40-400 m above sea level and is limited by mountains north of the
present distribution, and so it must have arrived before the mountains
attained their current height, to support Coad's hypothesis.
The headwaters of the Tigris-Euphrates basin are narrowly separated
from the Levant Rift Valley today and at times in the past may have
had direct exchanges of faunas (Kosswig, 1965; 1973; Krupp, 1987). The
modern absence of cichlids from the Tigris-Euphrates basin may be
explained by low temperatures. The effects of low temperature on Iranocichla
have not been investigated but fingerlings of Tilapia aurea,
which occurs naturally in the southern Levant Rift Valley, begin to die at 11°C
and cease all motion at temperatures below 10°C (Chervinski and Lahav, 1976).
Most of Syria, northern Iraq and the northern Arabian Peninsula have temperatures below 10°C
in winter (Beaumont et al., 1976). Spot temperatures from
southern Iran at the head of the Persian Gulf are about 12-13°C
in January compared to 18-20°C around the Straits of Hormuz where Iranocichla occurs.
Additionally the death rate of Tilapia aurea in fresh water is
twice that in salt water at low temperatures: it may be pertinent that
Iranocichla is found mainly in saline streams. This hypothesis
can only be confirmed by fossil discoveries.
Habitat
The streams in which this species lives are subject to desiccation
with continuous flow breaking up into isolated pools. The survival of
cichlid populations in these pools varies between years and some pools
may be fishless in one year and populated in another.
The area around the Straits of Hormuz is rich in salt domes and
consequently most surface streams are saline, up to 80 mS. Cichlids
are found in these streams but also in the Sar Khun oasis which is
fresh with a conductivity of 1.6 mS. Apparently they can be transported at 10 mS
as this is less stressful. Stream waters are cloudy to clear
and colourless. Water temperatures in winter (November to March) range
from 15 to 33°C and would be considerably higher in summer when air temperatures reach
45°C with no riparian shade and low water levels. Lamboj et al. (2006)
and Svardal (2006) give water
temperatures of 33-40°C and conductivity of 45-75 mS.
Streams are 1 to 50 m wide and consist of alternating riffles and
pools with occasional backwaters. The bottom is pebbles, sand or mud.
Aquatic vegetation is restricted to encrusting algae.
Kiabi and Abdoli (2000) found this species to be, with Cyprinion
watsoni, the commonest in Hormozgan Province.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 379 fish measuring 2.74-11.09 cm standard length. The a-value was
0.0349 and the b-value 3.047 (a b-value < 3 indicating a fish that
becomes less rotund as length increases and a b-value >3 indicating a
fish that becomes more rotund as length increases).
Food
Gut contents of 5 specimens (41.2-90.5 mm standard length) included
only algae and diatoms suggesting food is scraped from rocks and from
bottom deposits. This is consistent with an elongate gut and black peritoneum.
Aquarium specimens eat algal tabs but also appreciate insects and fish remains.
Reproduction
This species is a mouth brooder. A breeding female and a male were
caught in a backwater on March 18 of the Mehran River (the type series).
This backwater was 1-5 m wide, maximum depth was 40 cm over a mud
bottom, the water was cloudy and highly saline (40mS) and temperature
ranged from 26°C at the mouth of the backwater to 33°C
at its head. Eggs in fish taken in November and January are small so
the breeding season is deduced to be around March. Five eggs ranged in
length from 3.2 to 3.8 mm, mean 3.6 mm and in width from 2.4 to 2.7,
mean 2.5 mm. Total number of eggs from 2 females, 59.0-59.2 mm
standard length was 36 and 38 respectively. Eggs are yellow-orange in
preserved fish.
A female 116.9 mm standard length from the Mehran River had 153 larvae in her
mouth, ranging in length from 9.6 to 10.9 mm (H. R. Esmaeili, pers. comm., 6 October 2005;
Esmaeili et al., 2009). Esmaeili et al. (2009) found a sex ratio
biased towards males in May and June, presumably because males were defending
nests and easily caught, or possibly differential survival of the sexes. They
suggest that the breeding season begins in March and lasts until the end of June
with a peak in May. Eggs attained 3.76 mm and fecundity reached 151 eggs with a
relative fecundity of 5.4 eggs per gram body weight. Esmaeili et al. (2010)
detail gonad morphology and histology and confirmed peak spawning in May.
Schulz (2004) observed fish in the field and found each male occupying a
territory defending a nest about 1 m from each neighbouring nest. The nests were
made on light grey, fine sand and consisted of a pit approximately 15 cm in
diameter. The pit was black because of anoxic conditions below the sand surface.
The actual nest was about the same as the body length of the fish (8-10 cm) and
lay at the centre of the pit. The pit was surrounded by a rim about 1.5 cm high
with an internally indented margin. Simpler pits are built where building
materials are unavailable. Females were present in schools in deeper water in
the river centre. Individual females swam purposefully to the nest defended by
the male. The male directed the female to the nest centre with folded up fins
while the female spread her fins and showed radiating colour changes. Spawning
occurred immediately and neighbouring males intervened continuously at a speed
that did not allow full analysis of the movements. A defending male would chase
away an intruding male allowing another male into the unprotected nest to mate
with the female. A clutch of eggs was always inseminated by a whole group of males.



Spawning site photographs courtesy of Thomas Schulz
Parasites and predators
Unknown although piscivorous birds have been observed along the
streams where the cichlid is found.
Economic importance
Saadati (1977) suggests that this salt-tolerant species could be a
valuable resource if introduced into the saline and fishless waters of
internal basins. However this is not advisable since the native fauna,
evolved in a fishless environment, could be devastated before it has
even been documented. Esmaeili et al. (2009) note that it is eaten by
local people when available in large numbers in spring. It is now an aquarium fish in Germany (Schulz, 2002;
2004a; 2004b; Oliver Lucanus, pers. comm., 23
January 2004; Lamboj et al., 2006; Svardal, 2006; Svardal and Svardal,
2006).
Articles in aquarium magazines give photographs in spawning condition, including
mouth-brooding, and details for their maintenance, including
water with a conductivity of 50-70mS/cm NaCl or sea salt mixture, tank water
changed once a week, vegetarian food tabs (containing the blue-green alga
Spirulina), and a temperature of 20-35°C, optimally 27°C. It has been noted
that males, in continually defending a nest and courting, "wear out" earlier
than females (Thomas Schulz, in litt., 12 October 2006).
Conservation
Axelrod (1993) states that "as pollution chokes the waters of
southern Iran, we can expect this fish to disappear very quickly"
and "Because of the restricted range of this fish and the
continual warfare and oil pollution in the area, Iranocichla
hormuzensis may well be on its way to extinction - if it is not
gone already". Bailey (2006) apparently repeats this. However its habitat is mostly saline streams which
cannot readily be used for agriculture or industry. The surrounding
area is not industrialised, nor likely to be, and was never a war zone
so pollution is not a problem for this species.
Flash floods are probably a significant problem as water drains
rapidly off vegetation barren land. The scouring action may well
displace or strand cichlids. Mouth brooding offers protection against
floods and against associated fishes.
Svardal (2006) and Svardal and Svardal (2006) give details of capture,
transport and aquarium care of this species.
Further work
Further investigations into the biology of this species are needed.
Sources
Rabbaniha (1993a, 1993b, 1994) gives Farsi accounts of this species
and cichlids in general. The account is based principally on Coad
(1982a).
Type material: See above, CMNFI 1979-0408A, CMNFI 1979-0408B, CMNFI 1979-0139, BM(NH) 1981.1.12:1-2, MNHN 1981-107, 108, CAS 47324, ROM 36389 and BC 81-1.
Iranian material:
CMNFI 1979-0138, 17, 25.6-97.3 mm standard length, Fars-Hormozgan border, stream
in Rasul River drainage (ca. 27º32'N, ca. 54º58'30"E); CMNFI 1979-0140, 48,
24.2-59.4 mm standard length, Hormozgan, stream east of Kichal, Kul River
drainage (27º14'N, 55º46'30"E); CMNFI 1979-0141, 5, 15.0-21.0 mm standard
length, Hormozgan, Kul River (27º17'30"N, 56º03'30"E); CMNFI 1979-0142, 65,
21.3-82.8 mm standard length, Hormozgan, Baghu River (27º17'N, 56º28'E); CMNFI
1979-0143, 3, 25.0-26.7 mm standard length, Hormozgan, marsh in Hasan Langi
River drainage (27º21'N, 56º50'30"E); CMNFI 1979-0148, 5, 33.2-92.7 mm standard
length, Hormozgan, Sarzeh River (27º30'30"N, 56º15'30"E); CMNFI 1979-0181,
Hormozgan, Kul River (27º17'30"N, 56º03'30"E); CMNFI 1979-0184, 12, 31.4-50.4 mm
standard length, Hormozgan, effluent of Rasul River (27º11'N, 55º42'E); CMNFI
1979-0185, 7, 26.1-59.8 mm standard length, Hormozgan, stream in Rasul River
drainage (27º06'N, 55º45'E); CMNFI 1979-0187, 2, 63.9-64.6 mm standard length,
Hormozgan, stream at Sar Khun (27º23'30"N, 56º26'E); CMNFI 1979-0406, 5,
33.4-59.2 mm standard length, Hormozgan, stream north of Bandar-e Charak turnoff
(26º48'N, 54º18'E);CMNFI 2007-0053, 17, 28.0-59.9 mm standard length, Hormozgan,
Sarzeh River (ca. 27º36'N, 56º15'E); CMNFI 2007-0057, 14, 30.2-85.5 mm standard length, Hormozgan, Mehran River below Bastak (ca. 27º05'N, ca. 54º05'E);
CMNFI 2007-0058, 2, 64.8-83.0 mm
standard length, Fars, headwaters of Gowdar River (ca. 27º24'N, ca. 54º16'E)
Genus Oreochromis
Günther, 1889
Oreochromis niloticus
(Linnaeus, 1758)
Introduced to the Tigris River basin in Iraq but did not apparently
survive winterkill (Herzog, 1969). Mutlak and Al-Faisal ( 2009), however, record
O. aureus (possibly O. niloticus) from Basrah in southern Iraq and
this species could easily become established in Iran. No Iranian record
confirmed as yet.
Red tilapias (Oreochromis sp.) have been studied in aquaponic systems in
Iran so there is a potential for an exotic release (Rafiee and Saad, 2005).
Fingerlings from Indonesia have been reared using saline waters at Bafgh, Yazd
Province in 3 ton fibreglass tanks. Larvae were successfully grown to 2.0 kg at
28±1ºC (Iranian Fisheries Research Organization Newsletter, 57:2, 2009).
Genus Tilapia
Smith, 1840
Tilapia zilli
(Gervais, 1848)
Introduced to the Tigris River basin in Iraq but did not apparently
survive (Job, 1967). Redbelly tilapias are established in the Syrian
Euphrates (R. Beck, pers. comm., 2000) and a recent report by Beshar Abd Al-Hussain
Al-Saadi (in litt., 10 October 2006) of a cichlid at Al Musayyib on the
Euphrates River in Iraq may well be this species. Mutlak and Al-Faisal ( 2009),
record this species from Basrah in southern Iraq and these could spread to Iranian waters. No Iranian record
as yet.
The Farsi name is تيلاپيا (= tilapia).
Gobiidae
The gobies are a world-wide family found mostly in warmer marine waters although some species enter fresh water and others live there
permanently (see also Marine List in Checklists in
the Introduction). The number of species is high and this may be the most
speciose fish family in the world with about 210 genera and an estimated 2000 species, perhaps more. A
diversity of gobies occurs in the Caspian Sea basin. Not all Caspian gobies have valid Iranian records
but most will probably be found there. Several
gobies penetrate southern waters of Iran from the Persian Gulf and Sea of Oman and are described here. Others will probably be discovered
when more detailed surveys are made.
Gobies are easily distinguished by their pelvic fins being united
as an adhesive or sucking disk or cup. Body form and coloration are diverse. The
pattern of head canals, canal pores and neuromasts is distinctive and
used in identifying and relating species (except in "Neogobius" (Pinchuk,
1991)). However the neuromasts may be sunken in narrow furrows or pits
and completely covered by epithelium so they do not preserve well and
this can lead to confusion in identifications (Zambriborshch, 1968).
There is usually a short spiny dorsal fin (2-8 flexible spines)
separated from, but close to, a soft dorsal fin. The soft dorsal fin and anal
fin are longer than the caudal peduncle. Scales may be
cycloid, ctenoid or rarely absent. No obvious lateral line. There are
5 branchiostegal rays. Gill membranes are connected to the isthmus and
gill openings are moderate to wide, or very restricted in the
mudskippers. The head is usually blunt and the mouth is usually large.
Teeth are usually small and conical in one to several rows in both
jaws. Miller in Miller (2003) gives a suite of osteological characters defining the family.
Most gobies are quite small (5-10 cm) and they are often very
abundant. Maximum size is about 50 cm. Some of the world's smallest
vertebrates are gobies from the Indian Ocean, mature at 8 mm. Others,
however, are large and form part of fisheries in both the Caspian Sea
and the Indian Ocean. They are not significant food fishes in Iran. Gobies tend
to rest on the bottom and move in sudden, characteristic dashes. The male goby
guards a nest. Food is crustaceans, worms, molluscs and small fishes. Many
gobies are important in the aquarium trade since they are beautifully coloured,
small and tough.
They are known generally as gav mahi (= cow fish) or sag mahi (=
dog fish) or contain the word gel (= mud) in Iran. A general review in
Farsi of the Caspian gobies is given by Aslaanparviz (1991).
The males of some Caspian species become black during the spawning
season, their fins elongate, head shape alters and some even become
naked. Loss of tubercles in adult male gobies of the genus Benthophilus
makes it possible to identify only juveniles and females. The males
build nests and guard the eggs. Life span of certain Caspian
species is said to be as short as one year, e.g. some species of Benthophilus,
Knipowitschia, and Proterorhinus.
Neogobius pallasi, N. melanostomus and Ponticola gorlap
consume the invasive ctenophore, Mnemiopsis leidyi, as much as 10-15% of
total biomass in some areas (Mamedov, 2006).
The Black and Caspian Sea basins contain an endemic Sarmatian
fauna of gobies. There are two main clades, the gobiine-benthophilines (or transverse gobiids) and the
pomatoschistines (or sand gobies), that have probably been distinct for at least 40 million
years. Miller (2001) and Miller in Miller (2003) reviews the evolutionary history of these two clades
and their anatomical differences based on head papillae and osteology. The
transverse gobiids include Mesogobius, Neogobius,
Proterorhinus, Chasar, Anatirostrum, Benthophiloides,
Benthophilus and Caspiosoma while the sand gobies include
Knipowitschia and Hyrcanogobius.
The Sarmatian fauna was separated from the Atlantic-Mediterranean fauna with the
isolation of the Paratethys during the late Miocene Messinian salinity crisis as
the Mediterranean dried. Partial flooding of the Mediterranean from the
Paratethys in the early Pliocene allowed Sarmatian gobies to spread westwards.
Within the Ponto-Caspian basin, evolution of species flocks was favoured by
basin sub-divisions and rejoinings. The benthophilines may be a monophyletic
group from these events.
Ahnelt and Duchkowitsch (2004) give information
on the neogobiine stock. About 12-13 million years ago in the Middle Miocene,
the Ponto-Caspian endemic and ancestral neogobiine stock may have differentiated
from an Atlantic-Mediterranean gobiine stock. At this time the Paratethys was a
sea with reduced salinity and a high level of endemism. The Neogobius-Proterorhinus
stock developed independently from the recent Gobius stock that invaded
the Mediterranean basin after that sea was restored about 5 million years ago in
the Late Miocene.
Neilson and Stepien (2009a) using mitochondrial and nuclear
genes detail the evolution and biogeography of the subfamily Benthophilinae
restricting Neogbius to caspius, melanostomus and
pallasi with other known Iranian species formerly in Neogobius placed
in Ponticola. The Benthophilinae includes Babka, Mesogobius,
Neogobius, Ponticola and Proterorhinus as well as the
tadpole gobies (Anatirsotrum, Benthophiloides, Benthophilus
and Caspiosoma). The origin of the genera Babka, Benthophilus,
Caspiosoma, Mesogobius, Neogobius, Ponticola and
Proterorhinus ranges from 4.29 to 6.25 MYA and the paper gives divergence
times for major lineages in relation to geological events in the Ponto-Caspian.
These events include connections with, and isolation from, the World Ocean and
salinity changes in a range of 1-30 p.p.t. over the last 5 million years. Most
genera diversified about 5 MYA when the Black and Caspian seas separated.
More recent separation events occurred during the Pleistocene glaciations
Proterorhinus diverged from Mesogobius about 6.18 MYA, the
Ponticola clade diverged about 4.51-4.86 MYA, and freshwater
Proterorhinus emerged 1.18 MYA.
The principal recent works on the systematics of Caspian gobies are
by V. I. Pinchuk, D. B. Ragimov, Ye. D. Vasil'yeva, H. Ahnelt and P. J. Miller.
Earlier works are by B. S. Iljin (also spelled Il'in or Ilyin). Later molecular
studies are cited above.
Other gobies in Iran are the familiar tropical mudskippers which
can move quickly over land, using the muscular-based paired fins to
row across mud, and some can even clasp and climb mangroves. They can live out
of water because the gill openings are small to prevent desiccation of
the gills, oxygen can be taken into the chamber and absorbed through
the gills and chamber wall, and they can also absorb oxygen through
their skin. They often rest with the tail immersed in water for this
purpose or roll around in shallow water to moisten themselves. They
may live entirely in water, or will come onto land even when there is
enough oxygen in the water. Their eyes are high on the head,
protruding and able to revolve independently, and have a movable lower
lid. The eyes are retracted periodically into small cups below the
head to moisten them. Such eyes are very effective as a means to watch
for potential enemies on land but their vision under water is blurred.
Mudskippers have elaborate reproductive behaviour which involves tail
standing, flip-flops, and fin displays. They are very territorial and
defend their territory against other mudskippers and crabs. They can
deliver a skin-breaking bite to humans even though they are only about 15 cm long!
Genus Anatirostrum
Iljin, 1930
This genus comprises only a single species and so its characters are those of
the species. The snout is very distinctive and details of neuromasts are not
given here as they are not needed in identification, although of importance in
relating the genus. The genus is closely related to the tadpole goby clade
comprising Benthophiloides-Caspiosoma-Benthophilus and details are give
in Miller in Miller (2004). This author also gives an alternative terminology
for the arrangement of neuromasts than that of Ahnelt et al. (2000).
Anatirostrum profundorum
(Berg, 1927)
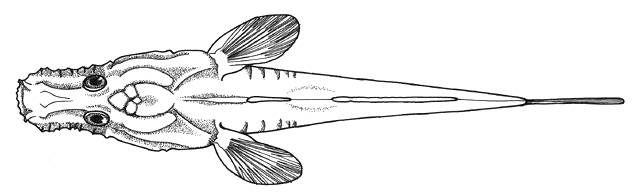
Dorsal view
Common names
gavmahi nuk-ordaki (= duckbill goby), gav
mahi-ye poozehderaz.
[duckbill tadpole goby].
Systematics
This species was originally described in the genus Benthophilus
Eichwald, 1831 by Berg (1927) but later Iljin (1930) erected a new genus because
of its unusual and distinctive morphology. The type locality is the Caspian Sea
at 37°58'N, 52°22'E at a depth of 294 m (but see below).
There are 14 fish under ZISP 23134 recognised as syntypes in Ahnelt et al.
(2000) although Berg (1927) mentions 15 fish in his description. Ragimov (1985)
states that Berg described this species from a single young specimen and also
visually observed 15 others for a total of 16 in the type series.
Key characters
The duckbill tadpole goby is characterised by the elongate and flattened head
which is similar to a duck's bill. Unlike gobies of the genus Benthophilus
the body has minute platelets and granules, there are 8 rather than 6, vertical,
suborbital series of pit organs, no postorbital occipital series, and no chin
barbel or cheek flaps behind the jaw angle (Ahnelt et al., 2000).
Morphology
First dorsal fin with 3-4 spines, usually 4, second dorsal fin with 1 spine
followed by 8-11, usually 10, soft rays. Anal fin with 1 spine followed by 8-11 soft rays. Pectoral fin rays 14-16. Gill rakers on the posterior part
of the arch are very short and anteriorly are minute. Pit organs on the side of
the head are papilliform and clearly visible with the naked eye. Further details
of anatomy are given by Ahnelt et al. (2000).
Iranian specimens had the following meristics:- first dorsal fin with 4(4)
spines; second dorsal fin with 1(4) spine followed by 10(4) soft rays;
pectoral fin rays 14(1), 15(2) or 16(1); anal fin with 1(4) spine followed by
11(4) soft rays; and total vertebrae 29(4).
Sexual dimorphism
Females may retain dermal granules when mature.
Colour
Overall, colour is a light grey or pale fawn fading to a whitish grey on the
belly. Various speckles and melanophores are found on the back and upper flank.
The dorsal, caudal and pectoral fins have dark grey speckles. The head sides
from the snout to the cheek are dark with transversal suborbital papillae series
whitish giving the impression of narrow light stripes below the eye and on the
cheek. The peritoneum is black or densely covered in fine speckles.
Size
Reaches 11.2 cm, or 13 cm total length (Jolodar and Abdoli, 2004). Females may be larger than males (mean total length 84 mm
versus 77 mm).
Distribution
Found in the southern Caspian Sea including Iranian waters (Berg, 1927; Ahnelt et al., 2000;
Abdoli and Naderi, 2009).
Zoogeography
Known only from the Caspian Sea and one of the endemic Sarmatian fauna (see Family Account).
Habitat
Found to a depth of 294 m on white silt bottoms according to Berg (1927) but
the data in ZISP states 244 sazhems (= 446.5 m). Recent Iranian material is from
45-80 m, at 9.7-16.4°C at 50 m (Ahnelt et al., 2000) and Jolodar and
Abdoli (2004) state it lives mainly at 50-100 m depths in the south Caspian Sea.
Age and growth
Unknown.
Food
Unknown but the duck bill may be an adaptation for feeding on silt bottoms (Ragimov, 1986).
Reproduction
Apparently mature eggs reach 1.9 mm in diameter in the Iranian specimen.
Parasites and predators
Unknown.
Economic importance
This species is too rare to be of any economic importance.
Conservation
Conservation requirements are unknown. Kiabi et al. (1999) consider
this species to be data deficient in the south Caspian Sea basin according to IUCN criteria.
Further work
More specimens need to be caught to assess its distribution, numbers, variation and biology.
Sources
Type material: See above (ZISP 23134).
Iranian material: CMNFI 1999-0023, 4, 76.1-79.1 mm standard length, Gilan, Caspian Sea off Astara (38º00'N, 49º30'E to 38º20'N, 50º00'E).
Genus Babka
Iljin, 1927
Babka gymnotrachelus
(Kessler, 1857)
Caspian Sea basin but no Iranian record although Kottelat and Freyhof (2007) map it from the Iranian shore. Gobius macropus De Filippi, 1863 described from Lake Palestrom near Poti, Georgia is
a synonym.
Babka
macrophthalma
(Kessler, 1877)
Reported from the south Caspian Sea
by Naseka and Bogutskaya but no confirmed specimen from Iran.
Genus Benthophiloides
Beling and
Iljin, 1927
Benthophiloides brauneri
Beling and Iljin, 1927
Originally described from the lower Dnieper River between Kherson and
Kakhovka and the south Bug River between Novaya Odessa and Nikolayev, Ukraine
and also recorded from the Caspian Sea basin but no Iranian record. Ragimov
(1998c) and Miller (2004) give recent descriptions.
Pinchuk and Miller in Miller (2004) question the validity of the Caspian Sea
record for this species.
Benthophiloides turcomanus
(Iljin, 1941)
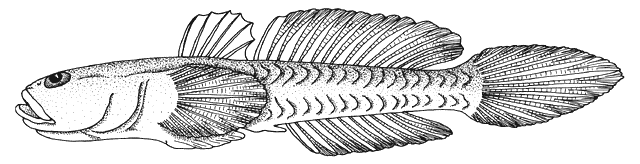
Caspian Sea basin, described from off Chikishlar at 37°45.5'N, 53°47'E
and southwest of Ulsky Bank at 38°05'N, 52°34'E,
Turkmenistan, but no Iranian record. Known only from the two type
specimens, now lost (Reshetnikov et al., 1997).
Miller in Miler (2004) places this species in Benthophiloides; formerly
it was in the genus Asra Iljin, 1941. Miller in Miler (2004) gives a
general map that encompasses the southeastern Caspian Sea including Iranian
waters but the only records are not in Iran.
Genus Benthophilus
Eichwald, 1831
Recheck Benthophilus illustrations in this account for source (correct species)
The tadpole gobies are found in the Black and Caspian seas where there are about 20 species, 16 endemic to the Caspian (Pinchuk and Miller in Miller, 2004; Boldyrev and Bogutskaya, 2007). The general
Farsi name for fishes in this genus is گاو ماهي (gav mahi) or سگ ماهي (sag mahi), not repeated under each species description.
Members of this genus are characterised by the broad and flattened head, dorsal muscles not extending to the eyes, no sensory canals or pores, first dorsal fin with 2-4 rays, well-separated form the
second dorsal fin, the caudal fin has only 2 rows of papillae, head and body scaleless but with spinulose bony granules, scutes and platelets except in adult males which are naked (and cannot therefore
be readily identified), anterior nostrils developed as small tubes overlying the upper lip, no swimbladder, a longitudinal dermal fold usually present on each side behind the mouth corner, and presence
of a chin barbel. There is no pelagic larval stage and eggs are large and oligoplasmatic. Ahnelt (2003) discuss the unique specialisations in the postcranial skeleton of benthophiline gobies (which also
includes Anatirostrum and Benthophiloides) including reductions in vertebral numbers and arrangement of pterygiophores. Pinchuk and Miller in Miller (2004) review other characters that show
relationships of this specialised group within a larger tadpole-goby clade, the broad and flattened head being the most obvious.
Pinchuk and Miller in Miller (2004) give details of the head neuromast organs that characterise this genus, namely 6 transverse infraorbital rows, 1-4 before and 5s and 6s
well above the level of the hyomandibular row b; row a is absent; row 5s is distant from row 4 which does not extend above the level of row b; rows 5i and
6i are arranged successively but row 5i is mostly below the level of row 6i separating the latter from the posterior end of row d; dorsal supraorbital rows o
very short and well-separated in the dorsal midline; and supraorbital series p present in interorbit as 3 pairs of short transverse rows.
These fishes are found in brackish waters with a salinity up to about 20‰, in deeper waters, estuaries and coastal waters. Life span of these tadpole gobies is only a year, some individuals maturing
at 6-7 months, called ephemery. Fish die after spawning, females earlier than males by 3-4 weeks (Boldyrev and Bogutskaya, 2004; 2007).
Pinchuk and Miller in Miller (2004) and Boldyrev and Bogutskaya (2004; 2007) give descriptions and keys for all Caspian Sea species. The 5 species cited here are the ones with records from Iran although
it is likely other species will eventually be found along the southern Caspian Sea coast. Boldyrev and Bogutskaya (2007) gives keys and descriptions to all the species.
Benthophilus abdurahmanovi
Ragimov, 1978
Endemic to the Caspian Sea, found in the North Caspian and reported from the
south Caspian Sea by Naseka and Bogutskaya (2009). No Iranian record.
Benthophilus baeri
Kessler, 1877
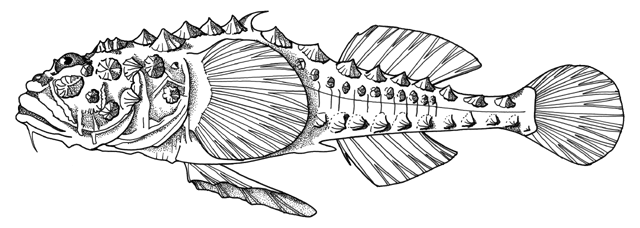
Common names
gavmahi-ye Baer, gavmahi-ye tokmehsar.
[Ber comcaxulu in Azerbaijan; Baer pugolovka or Baer tadpole goby in Russian].
Systematics
Benthophilus Baeri was described from the Caspian Sea at Mangyshlak Peninsula, Kazakhstan and in the South Caspian.
A specimen listed as a syntype is in the Natural History Museum, London under BM(NH) 1897.7.5:16 (32.5 mm standard length) from St. Petersburg University (sic, ZISP?) with others in the
Zoological Institute, St. Petersburg (ZISP 2239, lectotype and ZISP 53665 (ex 2239) (4)) and SPU 395 (463/410) (1) (Eschmeyer et al., 1996; Pinchuk et al., 2004).
Lectotype: ZISP 2239. Paralectotypes: (11) BMNH 1897.7.5.16 [ex St. Petersberg Univ.] (1), SPU 395 [463/410] (1), ZIN 53665 [ex 2239] (4).
Key characters
This species has very large and high, conical tubercles arranged in rows, not all tubercles are spinous, dorsal row tubercles usually 11-17 (lower than other Benthophilus), ventral row tubercles
9-14 (lower than other Benthophilus), granules are mostly absent between body tubercles rows, interorbit with a shallow median groove, a chin barbel is present, there are 1-2 dermal barbels behind
jaw angle, the first dorsal fin has 1-2 spines, and dark bands are absent.
Morphology
First dorsal fin with 0-2 spines, usually 1 (Ragimov (1985) and Boldyrev and Bogutskaya (2007) but 2 according to Pinchuk et al. in Miller (2004)), second dorsal fin with 1 spine followed by
6-9, usually 7, soft rays. Anal fin with 1 spine followed by 5-9, usually 6-7, soft rays. Dorsal row tubercles 11-17, usually 13-15, lateral row 1-12, usually 7-9 (but sometimes completely absent), and
abdominal row 9-14, usually 11-12. Total vertebrae 24-27. Tubercles on the head and flanks are large and can bear 1-4 sharp spines but only some tubercles retain spines. The dorsal tubercle row
continues onto the head ending near the eyes while the lateral row is short, extending from a level between the dorsal fins and ending at the rear of the second dorsal fin. There may be a few small
granules scattered between the major row tubercles and on the head. There is a long chin barbel, about equal to eye diameter. There is one neuromast in row 7 behind the eye.
A single Iranian specimen had ?
Sexual dimorphism
The second dorsal and anal fins of large males are enlarged.
Colour
The back, upper flank and upper head are pale grey to pale brown with dark grey speckles, and often minute dark dots form dark areas. Some bands may be weakly expressed on the back and flank, more
obvious in immature specimens. The lower body and belly are whitish. The pectoral, second dorsal and caudal fins have dark grey speckles and bars while the other fins are pale.
Size
Reaches 8.6 cm for males and 6.9 cm for females.
Distribution
Found in the central and southern Caspian Sea, rarely in the northwest Caspian. Reported from Gorgan Bay, the southeast Caspian Sea, southwest Caspian Sea and south-central Caspian Sea in
Iran (Kiabi et al., 1999; map in Reshetnikov, 2002; Pinchuk et al. in Miller, 2004;
Abdoli and Naderi, 2009).
Zoogeography
A Caspian Sea endemic, part of a unique fauna poorly studied and collected in Iranian waters, as are all its relatives below.
Habitat
Found in depths of 5 to 81 m in littoral areas and also known to enter rivers in Russia (Reshetnikov et al., 1997). Found at 0.5-30.0 m in warm seasons and migrates to deeper water at 100 m in
winter (Boldyrev and Bogutskaya ,2007). During the spawning season they are absent from shallow waters (5-10 m) and deeper waters (30-50 m)(Pinchuk et al. in Miller, 2004).
Age and growth
Up to 4 age groups are known (Reshetnikov, 2002) although Pinchuk et al. in Miller (2004) indicate life-span may be only 18 months.
Food
Corophiid crustaceans and some molluscs such as gastropods are principal diet items. Cumaceans, amphipods and Nereis worms are also important food items (Pinchuk et al. in Miller, 2004).
Reproduction
Mature males measure 8 cm while a mature female from the same collection was 6 cm (Berg, 1948-1949) while Pinchuk et al. in Miller (2004) state that both sexes are mature by 4.0 cm, in the first
year of life. Eggs have a maximum diameter of 1.2-1.8 mm and a minimum diameter of 0.3-0.5 mm and fish up to 5.2 cm long produce up to 1860 eggs (Reshetnikov, 2002). Spawning takes place from the second
half of May to August and rarely into September with a second batch of eggs laid in July (Pinchuk et al. in Miller, 2004). Eggs are laid on silt and silt-sand bottoms, probably using shells, at
10-25 m (Pinchuk et al. in Miller, 2004).
Parasites and predators
None recorded from Iran.
Economic importance
This species is food for sturgeon and Sander species (Pinchuk et al. in Miller, 2004).
Conservation
Kiabi et al. (1999) consider this species to be data deficient in the south Caspian Sea basin according to IUCN criteria. Criteria include possibly few in numbers, limited range (less than 25%
of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
Type material: See above (BM(NH) 1897.7.5:16).
Iranian material:
Benthophilus casachicus
Ragimov, 1978
Endemic to the Caspian Sea and found on the eastern Caspian coast, the Volga delta area,
and from
the south Caspian Sea by Naseka and Bogutskaya (2009). No Iranian record.
Benthophilus ctenolepidus
Kessler, 1877
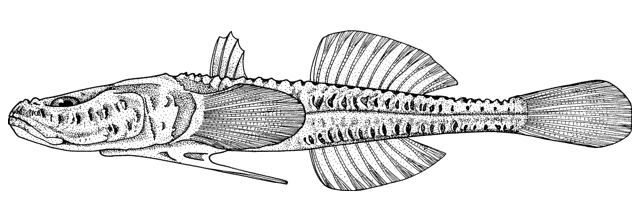
Common names
gavmahi-ye shafaf or shaffaf (= transparent or clear goby); gavmahi-ye Lankaran or gavmahi-ye Lenkoran.
[prozrachnaya pugolovka or transparent tadpole goby, shipogolovaya pugolovka or spiny-headed (?) tadpole goby in Russian; spiny-scaled tadpole goby; Lenkoranskaya pugolovka or Lenkoran tadpole goby in
Russian for the subspecies in the southern Caspian Sea].
Systematics
A subspecies reported from off the Iranian coast was Benthophilus ctenolepidus pinchuki Ragimov, 1982 but this is now recognised as a distinct species by Pinchuk and Miller in Miller (2004)
(see below). The lectotype of Benthophilus ctenolepidus as designated by Ragimov (1982) is in the Zoological Institute, St. Petersburg under ZISP 10897 with 3 paralectotypes lost and 1
paralectotype from ZISP in the Natural History Museum, London under BM(NH) 1897.7.5:13 (66.9 mm standard length) (Eschmeyer et al., 1996). The type locality is "yuzhnoi i srednei chastyakh'
Kaspiiskago morya" (= southern and middle Caspian Sea), or Caspian Sea, 40°08'N, 0°26'E off Baku, Azerbaijan (in Eschmeyer's "Catalog of Fishes" online, downloaded 22 August
2007). Records of B. magistri Iljin, 1927 in the Caspian Sea, as B. m. lencoranicus Ragimov, 1982, are this species (Boldyrev and Bogutskaya, 2007). The taxon Benthophilus magistri
lencoranicus Ragimov, 1982 was described from "raione protib Zelenogo bugra (38°10' s. sh.)" (= area opposite Green Hill, Turkmenistan). The holotype of lencoranicus
is in the Caspian Biological Station, Institute of Zoology, Azerbaijan under CBSIZA 546 (no. 2), paratypes under 546 (20) and additional non-type material in the Zoological Institute, St. Petersburg
under ZISP 23131 and 41912 (9) (Eschmeyer et al., 1996). Boldyrev and Bogutskaya (2007) were unable to locate IZA 546.
Key characters
The narrow head has a trough-shaped depression or groove on the occiput, the large tubercles are vertically elongate, curved and crest-like and have their rear edges fringed with a comb of spines,
dorsal row tubercles usually 30-33, abdominal row tubercles usually 24-26, temporal and occipital region without large tubercles but having granules, the body has tiny granules anteriorly interspersed
among the large tubercles, dermal filaments present or absent, a small but well-developed chin barbel is present, the dermal process behind jaw angle is lobed, narrow, with an acute protuberance, first
dorsal fin spines 3-4 (rarely 2), and back without brown bands.
Morphology
First dorsal fin with 3-4 spines, second dorsal fin with 1 spine followed by 8-11, usually 9-10, soft rays. Anal fin with 1 spine followed by 7-11, usually 8, soft rays. Total vertebrae 29-230. Dorsal
row tubercles 27-33, usually 30-33 (?) or 22-24 (Boldyrev and Bogutskaya, 2007), lateral row 11-22, usually 17-19, abdominal 20-26, usually 24-26 (22-24 in Boldyrev and Bogutskaya (2007)). Tubercles on
the head (when present) and flanks are large and bear sharp spines, the flank tubercles being angular in shape. The dorsal row of tubercles is incomplete, usually beginning below the first dorsal fin.
The first 3-4, and as many as 7, dorsal row tubercles are markedly smaller than the succeeding ones. The dermal plate or fold behind the mouth corner is large (1.2 times eye diameter),
oval, and without a wavy margin.
Sexual dimorphism
Undocumented.
Colour
Mostly transparent as the name suggests, a light to ash grey. The body bears dark lines and speckles but no strong dark bands, spots or blotches. The belly is yellowish-white. The dorsal and pectoral
fins have thin banding and the pelvic and and anal fins are pale.
Size
Reaches 10.7 cm.
Distribution
Found in the central, southwestern and southeastern Caspian Sea and in adjacent Iranian waters (Ragimov, 1965;
Abdoli and Naderi, 2009). Records are from Astara on the Azerbaijan-Iran border to Hasan Kuli in Turkmenistan and
adjacent waters.
Zoogeography
A Caspian Sea endemic.
Habitat
Found in depths of 0.5-74 m in littoral areas but deeper than B. magistri and B. macrocephalus. It feeds and spawns in 0.5-10.0 m and migrates to deeper water in winter. Occurs in higher
salinity areas at 12.4-13.0‰. Jolodar and Abdoli (2004) place it at 30-200 m depths in the south Caspian Sea basin. The pelvic fin is less developed, shorter and not as dilated than in these shallow
water species.
Age and growth
Life span is probably not more than 18 months with maturity at one year (Pinchuk and Miller in Miller, 2004).
Food
Food in northern Caspian specimens was found to include mostly corophiid amphipods, with some gammarids, other crustaceans, and gastropods (Pinchuk and Miller in Miller, 2004).
Reproduction
Spawning is thought to occur from spring to autumn and into winter (Pinchuk and Miller in Miller, 2004).
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be data deficient in the south Caspian Sea basin according to IUCN criteria. Criteria include possibly few in numbers, limited range (less than 25%
of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
Based on Pinchuk and Miller in Miller (2004).
Type material: See above under B. ctenolepidus (BM(NH) 1897.7.5:13).
Benthophilus granulosus
Kessler, 1877
Endemic to the Caspian Sea and found in coastal areas of the North and eastern and western Middle Caspian. No Iranian record although Kottelat and Freyhof (2007) map this species from the Iranian shore.
Benthophilus grimmi
Kessler, 1877
A Caspian Sea endemic found in the western part of the Middle Caspian and the northern part of the South Caspian south to about 39º40'N,
and from the
south Caspian Sea by Naseka and Bogutskaya (2009). No Iranian record.
Benthophilus kessleri
Berg, 1927
A Caspian Sea endemic found in coastal waters of the eastern Middle Caspian,
and from the
south Caspian Sea by Naseka and Bogutskaya (2009). No Iranian record.
Benthophilus leobergius
Berg, 1949
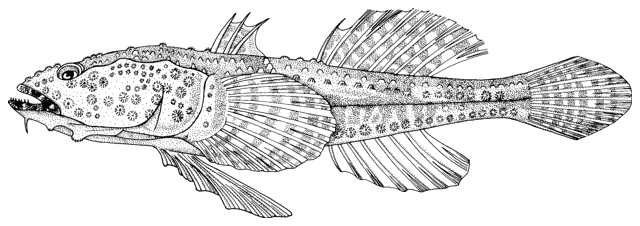
Common names
gavmahi-ye akhtari (= stellate or starry goby), gav mahi bacheh qurbaqei, gavmahi-ye bacheghoorbagheie, sebele.
[ulduzlu comcaxul or khazar julduzlu chomchakhulu in Azerbaijan; zvezdchataya pugolovka or stellate tadpole goby in Russian; Caspian starry goby, Caspian stellate goby].
Systematics
This taxon was originally described as a subspecies of Benthophilus stellatus (Sauvage, 1874). B. stellatus is now restricted to fish from the Black Sea. Note that views do conflict on
the status of this taxon and more studies remain to be done (Pinchuk et al. in Miller, 2004).
The subspecies leobergius (sic; leobergi has been used, see below) was first proposed by
Iljin (1949) and was thought not be available as no distinguishing features were given in
the description (Eschmeyer et al., 1996) although Berg (1948-1949) gives a description referring to "higher development of spiny tubercles" and colouration "less bright"
than then Black Sea form in his volume III dated 1949 (not 1948 as given in Eschmeyer et al. (1996)). Eschmeyer et al. (1996) consider that Berg may be the author of this taxon and Pinchuk
et al. in Miller (2004) concur. The holotype is probably in the Zoological Institute, St. Petersburg under ZISP 10891 (Eschmeyer et al., 1996). Berg (1948-1949) notes that the taxon is found
"in the freshened regions throughout the Caspian Sea, south to Astrabad Bay (No. 10891, 5 August 1874, 62 mm)". This may be the type locality, i.e. Gorgan (= Astrabad) Bay,
Iran. However this specimen may be merely recording the southernmost distribution of the taxon and a larger fish at 103 mm under ZISP 23128 labeled as from the Caspian Sea could be the holotype as it is
depicted in two views (Figure 858, p. 1115 in the Russian text and p. 196 in the English text, and Figure 859, p. 1116 Russian and p. 197 English). Both these fish may be syntypes.
Iljin (1949) simply
referred to "Kaspii", i.e. the Caspian Sea, as the type locality. Boldyrev and Bogutskaya (2007) selected ZISP 23128 as the lectotype.
Benthophilus macrocephalus variety b (large-scaled)
Kessler, 1877 is a synonym of B. s. leobergius according to Berg (1948-1949). Benthophilus aculeatus Baer in Lukina, 1984 is possibly a synonym, with no types known (Boldyrev and Bogutskaya, 2007).
Kottelat (1997) considers, tentatively, that Benthophilus stellatus is restricted to the Black Sea basin. Furthermore, Kottelat points out that perhaps the Caspian Sea species is Benthophilus
leobergi Ragimov, 1978, leobergi since the species is founded on a personal name and should be emended to this spelling and Ragimov, 1978 since the name is a nomen nudum in Iljin (1949) (no
description or diagnostic characters (but see above)) until Ragimov (1978) makes the name available by listing characters distinguishing it from B. s. casachicus. Benthophilus casachicus
Ragimov, 1978 is then another Caspian Sea species.
Key characters
Distinguished by stellate tubercles, mostly spinous at the tip, tubercles rows on body well-defined but scattered on head, dorsal tubercles 25-30, abdominal tubercles 21-25, granules present
predorsally but mostly absent from head and body, interorbit without a median groove, chin barbel present but small (eye diameter or less), dermal process behind jaw angle obvious, 3-4 first dorsal fin
spines, and first dorsal fin patterned. and dark bands present.
Morphology
The snout is very long, 30-33% of head length, and the upper jaw projects. The head is wider than long. There are 3-4 neuromasts in row 7 (also in B. macrocephalus, usually 1 in other species).
First dorsal fin spines 2-6, usually 3-4, second dorsal fin with 1 spine and 6-11 soft rays, usually 8-10, anal fin with 1 spine and 7-9 soft rays. Pectoral fin rays 15-18. The gill arch is without
evident rakers and is irregularly tuberculate. Vertebrae number 28-31. There are 10-11, often 11, precaudal vertebrae (commonly 9 in other Benthophilus). There is a flap behind the jaw angle. The
gut is moderately long with several convolutions. The body bears 3 rows of very spinulose tubercles; the upper row numbering 25-30, usually 26-28 or 27-29 (authors vary), the lower 21-25, usually 23-25
or 22-24 (authors vary), and the dorso-lateral row 15-24, usually 18-20. The ventro-lateral row is usually absent.There are numerous small tubercles between the main rows on the flanks, particularly the
dorsal and lateral rows. The head surface has numerous small and large tubercles, arranged irregularly. Small tubercles or granules are even found on the medial half of the eyeball. There are 2
well-developed tubercles on the midline between the eyes (in 80% of specimens) as opposed to one or none in other Benthophilus.
The chromosome number of B. leobergi is 2n=44 with 46-48 chromosomal arms (Grigoryan and Vasil'ev, 1993; Vasil'yev and Grigoryan, 1993) or 2n=46 (Klinkhardt et al., 1995).
Iranian specimens had the following meristics: first dorsal fin spines 3(1) or 5(1), second dorsal fin with 1 spine and 9 soft rays (2), anal fin with 1 spine and 8 soft rays (2), and pectoral fin with
17(2) branched rays. The extent of tubercle rows is difficult to distinguish since their size gradually decreases posteriorly and their arrangement is irregular anteriorly. Dorsal row tubercles 31(2),
lateral row tubercles ca. 19(2), and lower row tubercles 23(1) or 24(1).
Sexual dimorphism
The pectoral fin is longer than the pelvic fins in males when spawning. Females have a very small chin barbel.
Colour
Overall colour is brownish to whitish-grey. The head surface and upper flank have large, dark brown spots and small dark speckles. The body has about 5 transverse bands which circle the first dorsal
fin, rear of the second dorsal fin and caudal fin base when viewed from above. Rows of speckles are found on the second dorsal, pectoral and caudal fins.
Size
Reaches 10.7 cm.
Distribution
Found in the Caspian Sea. In Iran, it is reported from Gorgan Bay (Abdoli, 1994a). the Safid River (Abbasi et al., 1999), the southeast Caspian Sea, southwest Caspian Sea and south-central
Caspian Sea (Kiabi et al., 1999;
Abdoli and Naderi, 2009). Jolodar and Abdoli (2004) consider it to be widely distributed along the Iranian coast but more abundant in Gomishan Wetland and Gorgan Bay. It is also recorded
between Kultuk and Astara in Azerbaijan, near the Iranian border (Ragimov, 1965).
Zoogeography
Endemic to the Caspian Sea.
Habitat
This species is found in the Caspian Sea and in brackish and fresh waters, and is widespread in coastal waters over sand and shell debris bottoms down to at least 100 m. Said to prefer salinities
below 9‰. Feeding and spawning occur in shallow water at 0.5-10.0 m, retreating to deeper water in winter (Boldyrev and Bogutskaya, 2007). It is said not to enter fresh waters unlike its relative
B. stellatus but is known from fresh water in the Volga delta. In spring and autumn it can be found in coastal zone at 1-20 m. It prefers warmer waters at 18-26ºC (Caspian Sea Biodiversity
Database, www.caspianenvironment.org).
e
Age and growth
Young fish reach 1.83 cm in January and early February after spawning in autumn and reach their maximum size in July-August of the following year at 8.5-9.4 cm and 21.6-24.7 g. The spring spawners
reach 1.9-2.0 cm and 0.17-0.24 g in May-June and 3.5-5.9 cm and 1.1-5.4 g in July-August. With two spawning seasons, there are always fish of markedly different size and maturation stage
in any population. Life span is about 18 months (Ragimov, 1985a). Abdoli et
al. (2006) examined fish from the Gomishan and Miankaleh wetlands and found
a length (L)-weight (W) relationship of W = 0.0499L2.5 for males.
Food
Diet is dominated by molluscs (bivalves and gastropods) but includes crustaceans, worms, insect larvae and small fishes. An Iranian specimen contained the remains of a crab.
Reproduction
In the southern Caspian spawning occurs at depths of 30-35 m and in October-December and March-April (in the northern Caspian it is in shallow water (1-5 m) and during summer months). Batch spawning
occurs and eggs number up to 2136 large and 1284 small with diameters 1.5-2.1 mm and 0.3-0.7 respectively (Ragimov, 1985a). Fecundity may reach 3500 eggs and maximum diameter 4.5 mm (Reshetnikov, 2002).
Males protect the nests; unprotected nests have their eggs eaten by crustaceans within 2-3 hours (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Parasites and predators
Sattari et al. (2004) record the nematode Anisakis sp. from
this species in Gilan. This fish is eaten by other fishes and by seals.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include medium numbers, medium range (25-75% of water bodies),
absent in other water bodies in Iran, and present outside the Caspian Sea basin.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
Iranian material: CMNFI 1971-0326A, mm standard length, ():
CMNFI 1970-0543, ();
3, 90.4-90.9 mm standard length, Mazandaran, Gorgan Bay and the Caspian Sea.
Benthophilus leptocephalus
Kessler, 1877
A Caspian Sea, deep-water endemic found in the western Middle Caspian south to 39º41'N and the eastern South Caspian south to Hasan Kuli in Turkmenistan. No Iranian record.
Benthophilus leptorhynchus
Kessler, 1877
A Caspian Sea, deep-water endemic known from the western coast of the Middle Caspian and the north of the South Caspian south to Baku. No Iranian record.
Benthophilus macrocephalus
(Pallas, 1788)
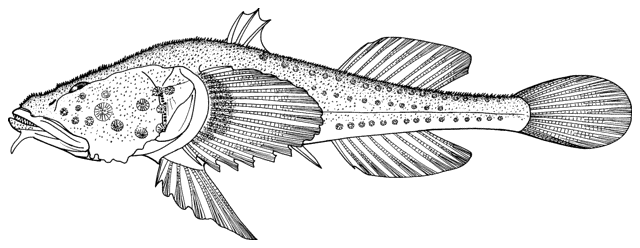
Common names
gavmahi-ye vazaghi (= tadpole goby).
[iribas comcaxul in Azerbaijan; Kaspiiskaya pugolovka or Caspian tadpole goby in Russian; bighead goby].
Systematics
Eschmeyer et al. (1996) gave the publication date as 1788 (later 1787), Berg (1948-1948) and Pinchuk and Miller in Miller (2004) as 1787. The original description is dated 15. Mars. 1787 but
was probably published in the following year (not verified). The type locality is the Caspian Sea near the mouths of rivers and streams, and in small inlets, although the type of Gobius
macrocephalus was probably from the Volga delta. No type specimens are known.
Key characters
Distinguished by having moderate, stellate tubercles, spinous at their apex with usually 22-23 (but see below) in the dorsal row and 19-24 in the abdominal row, numerous granules over the head and body,
head wide, width less than to slightly more than length, large snout tubercle, two rows of tubercles from dorsal rows extend onto temporal and occipital areas, no occipital groove, chin barbel present,
dermal process behind jaw angle very large (base length 20-26% head length), rounded or scalloped in larger fish, first dorsal fin spines 3-4, no dark annular bands.
Morphology
There are 3-4 neuromasts in row 7 (shared with B. leobergius, usually one in other Benthophilus). The head is very wide , 103-111% head length (only B. leobergius has similar
measurements). First dorsal fin with 2-4 spines, usually 3, second dorsal fin with 1-2 spines, usually 1, followed by 6-10 soft rays, mostly 8-9. Anal fin with 1 spine followed by 6-9, usually 7, soft
rays. There are no evident gill rakers on the arch but rounded tubercles are present. Total vertebrae number 27-28, with 17-19 caudal vertebrae, usually 18. The head and body are covered with
minute granules with larger stellate scutes or tubercles bearing sharp spines on the body and on the dorsal and lateral head surfaces. Granules extend onto the medial half of the eyeball and onto the
pectoral fin base. Scutes are few and scattered on top of the head, strongly developed and close together on the side of the head. There are three longitudinal rows of tubercles on the body with
19-27, usually 22-23 (or 23-27, modally 25, authors differ) tubercles in the dorsal row, 8-16, usually 10-13, laterally, and 19-24 abdominally, usually 21-22. The ventro-lateral row is
usually absent. The belly is naked. The gut is moderately elongate with several convolutions.
Iranian specimens had the following meristics: first dorsal fin spines 3(3), second dorsal fin with 1 spine and 8 soft rays (4), anal fin with 1 spine and 7 soft rays (4), and pectoral fin with 16(1)
or 17(3) branched rays. The extent of tubercle rows is difficult to distinguish since their size gradually decreases posteriorly and their arrangement is irregular anteriorly. Dorsal row tubercles
25(4), lateral row tubercles 9(1), 13(2) or 14(1), and lower row tubercles 21(1), 22(2) or 23(1).
Sexual dimorphism
Mature males lose the granules, scutes and tubercles, their dorsal and anal fins enlarge and their cheeks become more muscular or inflated.
Colour
Overall colour is ash-grey with scattered melanophores on the upper flank and dorsal surface. Melanophores line the pectoral and caudal fin rays being best developed on the more dorsal rays.
Size
Reaches 13 cm.
Distribution
This species is reported from the Caspian Sea including the south from the Anzali Mordab and Gorgan Bay in Iran and between Kultuk and Astara in Azerbaijan (Ragimov, 1965).
Zoogeography
A Caspian Sea endemic.
Habitat
Found mostly in the sea over muddy bottoms but may approach river mouths. Commonly at 0.5-10.0 m, migrating to deeper areas at 20-25 m in winter. De Filipii (1865) reports this species from the Murdab
(= Anzali Mordab presumably) in relatively fresh water.
Age and growth
Young fish taken in early June are 2.4-3.5 cm and 0.35-1.00 g and by the end of November they are 7.0-8.0 cm and 10.15 g, the maximum values for the species. By February-March they are nearly mature
(Ragimov, 1985a). There are up to 4 age groups (Reshetnikov, 2002) although Pinchuk et al. in Miller (2004) suggest 18 months.
Food
Food includes molluscs (80%) crustaceans, fish, worms and chironomids (Zenkevitch, 1963). Iranian fish contained clams, crustaceans and a polychaete in gut contents.
Reproduction
Spawning takes place in April-May and rarely up to mid-June near the shore on silt or silt-sand bottoms with a mixture of shells. The eggs are laid in large, empty mollusc shells. Egg laying occurs in
two batches as females carry eggs of two sizes. Fecundity of the larger eggs is up to 3180 and the smaller 1403. Their diameters are 2.0-3.0 mm and 0.3-0.6 mm respectively. Young are caught in
early June (Ragimov, 1985a). Males probably die after spawning. An Iranian fish, 77.9 mm standard length, caught on 13 March had minute but developing eggs.
Parasites and predators
None reported from Iran.
Economic importance
None.
Conservation
Numbers in Iranian waters and threats to this species need to be assessed.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
Iranian material: CMNFI 1970-0543, CMNFI 1970-0544, mmm standard length, ();
CMNFI 4, 55.3-82.8 mm standard length, Gilan, Caspian Sea near Bandar Anzali ().
Benthophilus mahmudbejovi
Ragimov, 1976
Endemic to the Caspian Sea and found on the western coasts of the Middle and South Caspian and the Volga delta region. No Iranian record.
Benthophilus pinchuki
Ragimov, 1982
Common names
None.
[Pugolovka Pinchuka or Pinchuk's tadpole goby in Russian]
Systematics
Originally described as a subspecies of B. ctenolepidus, Pinchuk and Miller in Miller (2004) recognise it provisionally as a distinct species. This taxon was described from "raione Belogo
bugra y vostochnogo poberezh'ya Yuzhnogo Kaspiya" (= area of the White Hill (? locality not ascertained further by me) near the eastern coast of the southern Caspian Sea). A specimen listed as a
syntype is in the Natural History Museum, London (no number, noted in October 1982). The holotype of pinchuki is in the Caspian Biological Station, Institute of Zoology, Baku, Azerbaijan under
CBSIZA 52 (later in ZISP 53569, ex IZA), paratypes are under CBSIZA 304 (24, later 8 fish and ZISP 53660 9ex IZA 304) (6)). Additional material is in the Zoological Institute, St. Petersburg under ZISP
33143 (6) and 44346 (1) (Eschmeyer et al., 1996; on-line version downloaded 22 August 2007).
Key characters
Tubercles are vertically elongated, curved and crest-like, with a spiny fringe on their rear edge, dorsal row tubercles complete and 30-33, ventral row tubercles 23-27, granules are absent
postorbitally on the head and on the body, temporal and occipital region without large tubercles, head narrow, the interorbit has a shallow median groove, dermal filaments present or absent, chin barbel
usually present, dermal process behind jaw angle narrow (less than eye diameter) and barbel-like, first dorsal fin spines 3-4, and colour without dark bands.
Morphology
The first dorsal fin has 3-4 spines, usually 4, the second dorsal fin 1 spine and 9-11 soft rays, and the anal fin 1 spine and 8-11 soft rays (Ragimov, 1982; note the holotype has a much lower count
of anal fin rays than figure in table). Dorsal row tubercles 30-33, usually 31-32 (other species having 30 or less usually except B. ragimovi), ventral row tubercles 23-27, and dorso-lateral row
tubercles 10-23. Total vertebrae 30-32 and caudal vertebrae 21-23 (26-31 and and 17-21 in most other Benthophilus). The pelvic fin extends to the anus or beyond. Chin barbel less than half eye
diameter. One neuromast in row 7.
Sexual dimorphism
Males may have longer pectoral fins than females and grow larger than females.
Colour
Not described except for the absence of dark bands, blotches and spots. Probably an overall greyish.
Size
Reaches 8.9 cm in males.
Distribution
Found throughout the Caspian Sea.
Zoogeography
Endemic to the Caspian Sea.
Habitat
Known down to 282-294 m, usually 50-100 m but as shallow as 20 m, and a salinity of 12.4-13.2‰.
Age and growth
Life span is probably 18 months with maturity at 1 year.
Food
Unknown.
Reproduction
Spawning probably occurs in spring as fry have been caught in late April.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Numbers in Iranian waters and threats to this species need to be assessed. Boldyrev and Bogutskaya (2007) call it a rare deepwater species.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
No specimens examined, based on Pinchuk and Miller in Miller (2004).
Benthophilus ragimovi
Boldyrev and Bogutskaya, 2004
Endemic to the Caspian Sea from the western coast of the Middle and South Caspian, south to Astara. No Iranian record.
Benthophilus spinosus
Kessler, 1877
Endemic to the Caspian Sea from the eastern and western coast of the Middle Caspian , the southeastern North Caspian and southeastern South Caspian. No Iranian record.
Benthophilus svetovidovi
Pinchuk and Ragimov, 1979
A Caspian Sea endemic found on the eastern coast of the Middle Caspian, and
from the
south Caspian Sea by Naseka and Bogutskaya (2009). No Iranian record.
Genus Boleophthalmus
Valenciennes, 1837
This genus of gobies comprises several species in the Indian Ocean including one reported from Iran.
The genus is characterised by a very elongate, compressed body, a
rounded head, greatly thickened head and nape epidermis (as protection against
drying when out of water), scales cycloid, moderate to minute in size, eyes high on the head
and close together, lower eyelid or dermal cup well-developed, mouth slightly
oblique, teeth in a single row in each jaw, upper jaw teeth conical
with some canines, lower jaw teeth flattened, obliquely notched and
almost horizontal, tongue truncate and nearly all adnate to mouth
floor, gill opening small and oblique, and second dorsal and anal fins long (23 or more rays).
Boleophthalmus dussumieri
Valenciennes, 1837
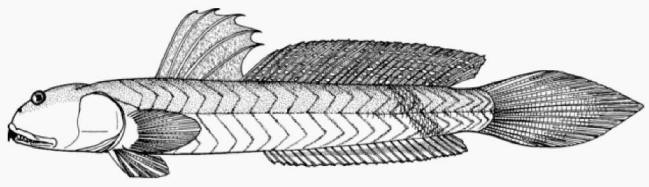
Common names
eshlambo, neeshlambo, gel khorak.
[kelb-el-daw, abou-shlembo, abu-shelamboo or triton in Arabic; gullo in Pakistan; Dussumier's mudskipper].
Systematics
Boleophthalmus Dussumieri was originally described from
Bombay, India and the holotype is in the Muséum national d'Histoire
naturelle, Paris under MNHN A.1468 (Bauchot et al., 1991).
Synonyms are Boleophthalmus dentatus Valenciennes in Cuvier
and Valenciennes, 1837, described from Bombay and reported from the
Shatt al Arab, Iraq by Iraqi authors, and Boleophthalmus chamiri
Holly, 1929 from "Chamir an der Straße von Clarence gegenüber der Insel Tawilah in der Meerenge von Hormus,
südöstliches Persien" (Holly, 1929a; Berg, 1949) (Khamir is at 26°57'N, 55°36'E in
Khowran Strait (= Clarence Strait) opposite Qeshm Island (= Tawilah or
Tavileh Island). Boleophthalmus boddarti (Pallas, 1770) as
reported by Relyea (1981) does not occur in the Persian Gulf region (Murdy, 1989).
Mirza et al. (1996) separate B. dentatus and B.
dussumieri on the basis of first dorsal fin height, body depth,
number of teeth on the jaws and the membrane between the two dorsal
fins. Murdy (1989) defines the genus Boleophthalmus in part by
the dorsal fins being separate, but does not give first dorsal fin
height, body depth and tooth counts for B. dussumieri or its synonyms.
A syntype of Boleophthalmus chamiri is in the Naturhistorisches Museum Wien (NMW 13804).
Key characters
The anal fin base and second dorsal fin base are 34% or more of
standard length and there are 2 canine teeth internal to the lower jaw
symphysis in contrast to Periophthalmus, the other mudskipper of southern Iran.
Morphology
First dorsal fin spines 4-5, usually 5, second dorsal fin with 1
spine and 24-28 soft rays, anal fin with 1 spine and 23-27 soft rays,
and pectoral fin branched rays 16-20. Scales are minute and embedded,
103-185 in longitudinal series; predorsal scales 48-56. Each scale is
rounded with radii around a posterior focus. Circuli are few. There
are 3 canine teeth on each side of the upper jaw (1-2 may be lost),
overlapping it when the mouth is closed and obviously protruding in
some preserved fish, and 39-76 upper jaw teeth and 43-71 notched lower
jaw teeth, the latter projecting almost horizontally from the jaw. The
number of teeth increases with age and B. chamiri was founded
on juveniles with fewer teeth (Berg, 1949). Gill rakers are partly
embedded in a membrane and not always easy to count; the range is
12-14 for Iranian specimens. Upper arm rakers are long and pointed,
less developed on the lower arm. When appressed, each raker touches
the adjacent one. Gut very elongate and coiled. Muscle blocks may be
evident in preserved fish.
Meristic values for Iranian specimens are:- first dorsal fin with 4(2) or
5(20) spines, second dorsal fin soft rays 24(3), 25(5), 26(9), 27(3),
or 28(2), anal fin soft rays 23(3), 24(4), 25(13), 26(1) or 27(1), and
pectoral fin rays 17(3), 18(8) or 19(11). Total vertebrae 26(10).
Sexual dimorphism
The male has a higher dorsal fin and larger canine teeth than the
female. Females were described as B. dussumieri while males
were described as B. dentatus (Berg, 1949).
Colour
Overall colour a greyish violet or light blue. The eye is light
yellow to brown around the rim. The opercle, pectoral fin base and
area just above the pectoral base have small green spots. There are 3
greenish-brown blotches on the nape, 3 more at the first dorsal fin
base and 2 others at the anterior second dorsal fin base. The first
dorsal fin is purple to light blue with small black or brown spots, or
with greenish spots at the base and wavy white lines and bluish spots
over the whole fin. The second dorsal fin has 2-6 horizontal rows of
oblong blue (or white in preservative) spots on the fin membranes on a
purplish background. Large white spots are found on the base of
interradial membranes 7, 10, 14, 17, and 22 on a specimen examined by
Murdy (1989) but this varies markedly between individuals seen by me.
The margin of this fin is black, edged with orange (white in
preservative). The caudal fin is black distally and bluish near the
base. The anal fin is transparent, the pectoral fin bluish and the
pelvic fin whitish.
In preservative, larger fish are darker than small ones. The body
is speckled darker above than below but this is still much lighter
than the dark dorsal fins. The first dorsal fin is very dark, almost
black, because the small pigment spots are so dense. The second dorsal
fin is lighter with alternate horizontal light and dark rows on the
membranes. Rays are somewhat lighter or equally dark. The second
dorsal fin margin is transparent but some fish may lack this feature
and be dark interradially (because of a dark row) and light at ray tips. The
caudal fin rays are dark and the membranes are dark next to the rays
but light in the centre. The anal fin is immaculate and the pectoral
and pelvic fins are very light with some pigment lining the rays. Some
pectoral fin pigment appears to be extensive vascularisation.
Peritoneum dark brown to black.
Young fish have several vague bars posteriorly on the upper flank
above the anal fin level. The anterior flank and side of the head is
covered with small spots.
Size
Attains 24 cm and 35.4 g.
Distribution
Found from Iraq to Pakistan and the west coast of India. Reported
from the Shatt al Arab, Iraq near Iranian Khuzestan by Menon (1956),
Khalaf (1961), and Al-Nasiri and Hoda (1976). In Iran it is recorded from the
Sarbaz River at Bahu Kalat in the Makran, and the Karun River near Mohammerah
(Berg, 1949) and in the Gulf, Hormuz and Makran basins.
Abdoli (2000) maps this species from the lower Karun and Arvand rivers in the Tigris River basin,
the lower Hilleh and Mand rivers in the Gulf basin, the Mehran, Kul and Minab
rivers in the Hormuz basin and lower reaches of Makran rivers from the Jagin to Bahu Kalat.
Zoogeography
This species has ready access to marine waters and can easily move between rivers via the sea.
Habitat
This species is active on mud flats in streams under and above
tidal influence but most information on behaviour and biology is based
on studies of fish on marine tidal flats (as recounted below). In
southern Iranian rivers, this species can be seen swimming with its
head out of the water and pectoral fins splayed, presumably on the
look-out for danger. Populations are very numerous on mudflats on
lower reaches of rivers in Khuzestan. It hides in mud burrows when
approached and a plume of dark muddy water can be seen emerging from
the burrow, or it may bounce over the water surface. They are
difficult to catch with a seine when approached from the shore because
of this behaviour, scurrying rapidly from the land and shallows into
deeper water, muddying the water and obscuring vision, and thus
disappearing from view. However they can be caught in shallower rivers
by using a seine net from mid-river to shore, after waiting for them
to re-emerge and enter the shallows. Their reaction is still flight to
water but the movement from water to land confuses them and they can
be scooped up in their panic. In Khuzestan, local people whip them out
of the water with palm fronds.
Both sexes of this species construct U-shaped, vertical burrows in
sand to a depth of about 1 m in marine situations. Clayton and Vaughan (1986)
illustrate burrow shapes, outline construction methods and experimented on
territoriality by removal of fish. A polygonal, mostly pentagonal but
significant numbers of hexagonal and quadrangular, dam of mud is built up around
the burrow hole using the mouth, again by both sexes, and is used to reduce
aggression between neighbours (Clayton
and Vaughan, 1982; Clayton and Wright, 1989). This dam serves to keep water in the burrow at low tide
although not all populations maintain this dam as completely. The burrows are
used as shelter from low temperatures during winter and high temperatures in
summer, at high tides, and when danger threatens. Air temperatures in Kuwait
reach 51.1°C in summer and fall to -4°C in winter, conditions mirrored in Iraq.
Polygonal dams were not seen in rivers, perhaps because of river flow and tidal
fluctuations. Fish are active in the 13-40°C range and daily variations may be
as much as 20C°. Fish tend to be inactive in winter in the northern Gulf, with
feeding the main activity, and are easy prey for migratory birds feeding on the mudflats.
At low tide the fish emerges and raises its head, scanning the
surrounding surface and recognising humans as threats at 5-7 m.
Movement is with the aid of the muscular pectoral fins and the gill
covers are puffed out, presumably to obtain water or to maintain a
moist atmosphere for the gills. It does not usually travel further
than the mud walls of its territory in contrast to Periophthalmus
waltoni and is exposed to air for much shorter periods being
submerged in water or watery mud most of the time. The goby basks when
recently emerged from its burrow in order to raise body temperature to
14°C before moving out onto the mud. The minimum temperature for emergence
is 10°C. Some larger fish will make rapid and short sorties from the burrow to
test air temperatures. Evaporative loss of body fluids is used to cool
the fish in summer and the body is kept wet by immersion in pools and
burrows. Body temperatures do not exceed 33°C even when air temperatures were over 40°C.
There can be up to 52 fish in 100 sq m in Kuwait (Tytler and Vaughan
(1983) and Clayton and Vaughan (1988) on B. boddarti in Kuwait
marine waters, identified as this species by Murdy (1989)). At the Khowr-e Mussa
in Iran, each fish defends a dammed territory of about 1 sq m, this area
containing the epiphytobenthic food it requires (Höpner and Kazem Maraschi, 1999).
The skin has large numbers of mucous cells which prevent desiccation on the mud
flat and lubricate the fish during burrowing (Salih and Al-Jaffery
(1980) on B. dentatus from Al Faw (= Fao), assumed to be this species). Gas
exchange in air and water takes place through the gill, inner
operculum, nasal, body and outer operculum skin in that order, as
adaptations to low oxygen conditions in estuarine areas and to life
out of water (Al-Kodhomiy and Hughes (1988) on B. boddarti but
presumed to be this species). In addition to the territorial
individuals, there are also smaller, non-territorial fish which roam
more freely and are called "errant" (Clayton and Vaughan,
1988). Clayton and Vaughan (1988) describe in detail a series of body
postures and movements associated with being stationary, moving,
feeding, maintaining moisture levels, construction, interacting with
other individuals, avoiding predators, and courtship. Males raise
their dorsal fins when they meet, gape widely and fight mouth to mouth.
Age and growth
On the Karachi coast of Pakistan, juveniles and young of the year
are found from September to December, growing from 45 mm standard
length to 60 mm over three months (Hoda and Akhtar, 1985). In Gujarat,
India three age groups are reported (Soni and George, 1986).
Food
Diet includes diatoms (Pankow and Huq (1979) recorded 114 species at Al Faw (= Fao)),
blue-green algae, filamentous algae, insects, crustaceans, nematodes and teleost
fish eggs in marine situations, the polygonal enclosure acting as a
"farm" promoting growth of diatoms (Sarker et al., 1980;
Clayton and Vaughan, 1988). It grazes on surface films of yellow-green algae at
Fao (Pankow and Huq, 1979) and brown algae in Kuwait. The grazing action,
with side-to-side head movements, is distinctive for this species
among other mudskippers in the Gulf (Clayton and Vaughan, 1988). The
fish leave their burrows on feeding excursions. Young are found in
non-territorial groups in shallow pools, where in winter they take
some time to warm up before leaving to feed. The pools offer a
protective environment for the young.
These mudskippers are fed on by gulls, terns, bitterns, egrets and
herons as well as a wide variety of other fishes and sea snakes (Clayton and Vaughan, 1988).
Reproduction
Spawning in the marine environment of the Khawr az Zubayr, Iraq takes place in June,
the release of larvae being timed to take advantage of phytoplankton and
zooplankton blooms (Hussain and Ahmed, 1999).
On the Karachi coast of Pakistan, 50% maturity (= first maturity)
is given as 69 mm standard length in males and 72 mm in females (Hoda
and Akhtar, 1985) while maturation starts at 60-65 mm standard length
in males and 70-75 mm in females according to Hoda (1987). Spawning
there occurs twice each year during April-May and July-September (Hoda,
1986) or April-May and July-August (Hoda and Akhtar, 1985). Fecundity
range is 986-4912 eggs for fish 74-110 mm long and maximum egg
diameter is 1.05 mm (Hoda, 1986). Hoda and Akhtar (1985) give a range
of 970 eggs at 75 mm standard length to 4113 eggs at 110 mm, mean 2371 eggs.
Clayton and Vaughan (1988) give the start of the breeding season in
Kuwait as March but the majority of males are only displaying by
mid-April. Courtship continues until August and most territories have
breached walls where fish have crossed over into other territories to
find mates. Larvae appear as early as mid-July although most appear
later than this. Territories are repaired in October and November.
Courtship involves competition between males for neighbouring
females. Each male has on average 5 neighbours but only half will be
females. Males identify themselves and try to attract females by
elevating their fins and leaping from a lateral prone position with
about two-thirds of the fish clearing the ground. Other signaling
devices are pectoral fin waves, lateral tail-beats, and quivers. The
female advances and the male leads her into his burrow where spawning
presumably occurs. While errant females may be mated with territorial
males, errant males having no territory do not show the leaping behaviour.
Parasites and predators
Migrating birds are heavy predators of this fish on mud flats (Tytler
and Vaughan, 1983). Indian pond herons are known to eat this fish in
Iran. E. Kahrom in the Crocodile Specialist Group Newsletter reports that
muggers (marsh crocodile, Crocodylus palustris) eats a Periophthalmus
in the Sarbaz River of Iranian Baluchestan (www.flmnh.ufl.edu/natsci/herpetology/newsletter/news173b.htm,
downloaded 15 February 2002). However this may well be Boleophthalmus dussumieri.
Economic importance
None, although children play with them in the Karun River
(www.abadan.net/abadanidictionary.html, downloaded 4 December 2003).
Conservation
There has been no assessment of the conservation status of this species but
it is common on mudflats in the lower reaches of rivers Iran.
Further work
The biology of this species in Iranian fresh waters has not been investigated.
Sources
Iranian material: CMNFI 1979-0141, 1, 86.2 mm standard length, Hormozgan, Rud-e Kul (27º17'30"N, 56º03'30"E);
CMNFI 1979-0142, 5, 74.6-137.5 mm standard length, Hormozgan, Baghu River (27º17'N, 56º28'E);
CMNFI 1979-0145, 10, 42.6-136.6 mm standard length, Hormozgan, Geru River (26º55'N, 57º01'30"E);
CMNFI 1979-0322, 5, 57.1-111.8 mm standard length, Baluchestan, Bahu Kalat River (ca. 25º45'N, ca. 61º26'E);
OSU 8121, 1, 61.4 mm standard length, Baluchestan, Bahu Kalat (no other locality data).
Comparative material:- CMNFI 1985-0182, 3, 44.7-68.1 mm standard length, Iraq, Shatt al Arab (no other locality data);
BM(NH) 1976.12.8:4-7, 4, 103.9-128.2 mm standard length, Iraq, Basrah (30º30'N, 47º47'E);
BM(NH) 1954.11.10:3-7, 6, 79.9-147.5 mm standard length, Iraq, Fao (29º58'N, 48º29'E).
Genus Caspiosoma
Iljin, 1927
Caspiosoma caspium
(Kessler, 1877)
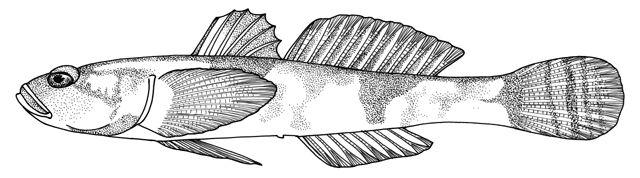
Caspian Sea basin, described and recorded from the northern and
middle Caspian Sea, but no Iranian record. Ragimov (1998c), Miller (2004) and
Reshetnikov (2002) give recent descriptions.
Genus Chasar
Vasil'eva, 1996
This genus has a single species in the
Caspian Sea. The characters of the genus are covered in more detail by Miller in
Miller (2004) and are the characters of the species below. It is recognised as
distinct from the related Neogobius by head sensory papillae patterns
(especially an additional transverse row before row b) and modally 7
first dorsal fin rays rather than 6. The number of the transverse suborbital neuromast rows of the cephalic lateral
line system is an important diagnostic feature for the monotypic genus Chasar
according to Miller (2004). Pinchuk and Ragimov (1985) count these rows consecutively from anterior to posterior as
1 – 8 while Miller (2004) postulates an additional transverse row anterior to
the longitudinal suborbital row b labelling the posteriormost row as row
7 (Pinchuk et al. in Miller, 2004). A Farsi common name for Neogobius
and related gobies is گاو ماهي (gav mahi).
Chasar bathybius
(Kessler, 1877)

Common names
[bychok-glubokovodny or deepwater goby]
Systematics
Described from Svinoi Island south of Baku, Azerbaijan. No types known.
Key characters
Nape with cycloid scales anteriorly, not quite reaching orbit level, head
depth at eyes about equal or slightly less than width between upper origins of
opercles, interorbital distance equal to or slightly less than orbit, angle of
jaw below pupil of eye, snout longer than eye, up to 1.8 times orbit diameter,
upper lip not swollen at angle, pelvic fin not reaching anal fin, except in
young, anterior pelvic fin membrane without well-developed lateral lobes, caudal
peduncle depth 0.3-0.5 length, first dorsal fin with 7 rays and little higher
than second dorsal fin (which is lower posteriorly), overall colour semi-transparent,
and lateral series scales mostly 55-60.
Morphology
First dorsal fin with 6-8 spines, usually 7, second dorsal fin with 1 spine
and 14-16 soft rays, and anal fin with 1 spine and 11-14 soft rays, lateral series scales 55-65.
Scales are overall ctenoid but cycloid on the nape, breast, opercle, abdomen and
and lobe of the pectoral fin. The pectoral fin is long reaching back well beyond
the level of the second dorsal fin origin. Lateral line
system usually with eight or nine transversal suborbital neuromast rows, two
rows ventral to the longitudinal suborbital row b. Four neuromast rows on the
opercle. Lateral line system with eight suborbital neuromast rows arranged
transversally, five before, three above and two below the longitudinal row b.
Four rows of free neuromasts on the opercle instead of the characteristic three
rows of all other neogobiines.
Meristics for Iranian specimens: first dorsal fin with 6(1),
7(14) or 8(1) spines, second dorsal fin with 14(4), 15(9) or 16(3) soft rays,
anal fin with 11(3), 12(9) or 13(4) soft rays, pectoral fin with 18(10) or 19(6)
rays, scales in lateral series 55(1), 56(3), 57(6), 59(1) or 60(1), and
transverse rows of scales 16(8) or 17(4).
Sexual dimorphism
Males grow larger than females.
Colour
The body is semi-transparent with reddish or yellowish-brown colour. The myotomes are
visible but scales are difficult to see. The back is darker than the flank and
belly. Preserved fish have 4 faint saddles and faint spots along the lateral
midline, the first dorsal fin has two brownish bands, and young have a
stripe-like spot posteriorly on the first dorsal fin.
Size
Attains 29.0 cm.
Distribution
Caspian Sea basin, recorded between Kultuk and Astara in Azerbaijan (Ragimov,
1965). Kiabi et al. (1999) report this species from the southeast Caspian Sea, southwest Caspian
Sea and south-central Caspian Sea in Iran. Miller in Miller (2004) maps this species
from the Safid River westwards and from Gorgan Bay but there are no text records
to go with the map. Recently recorded from Iran (Ahnelt, et al., 2007). South
coast of the Caspian Sea especially in Gorgan Bay (Jolodar and Abdoli, 2004).
Zoogeography
Endemic to the Caspian Sea but related to the speciose genera Neogobius
and Ponticola.
Habitat
A marine species, not entering fresh waters, found down to 75 m and perhaps
deeper, especially in colder conditions. It is found on sandy and shelly bottoms
and in smaller numbers on firm silt.
Age and growth
Life span is estimated to be 4 years, with maturity in the second year and spawning every year.
Abdoli et al. (2006) examined fish from the Gomishan and Miankaleh
wetlands and found a length (L)-weight (W) relationship of W = 0.0777L2.44
for sexes combined.
Food
Fish are an important food for larger specimens, smaller fish take
crustaceans. Fish food includes Knipowitschia iljini and crustaceans
include the decapod Palaemon, corophiids, mysids and some gammarids. A
small number of worms (Nereis) are also eaten.
Reproduction
Eggs of only one size have been found in this species, suggesting a single
spawning each year. Up to 2979 eggs have been recorded with a maximum diameter
of 2.6 mm. Spawning in the southern part of the Middle Caspian Sea takes place
from the second half of June until July. Males approach the coast in the first
half of March or April while females remain at 20-30 m. Females enter shallow
water in late April and in greater numbers in May and June. After spawning, fish
retreat to areas deeper than 10-15 m.
Parasites and predators
Eaten by sturgeons and the Caspian seal. Sattari et al. (2004; 2005) surveyed this species in the inshore area of the Caspian Sea, recording
Anisakis sp. and Dichelyne minutus.
Economic importance
None in Iran but caught in Lenkoran, Azerbaijan with fixed nets and in northern
Azerbaijan with cast nets. The fish are oily but pleasant tasting (Pinchuk et al. in Miller, 2004).
Conservation
Kiabi et al. (1999) consider this species to be data deficient in the south Caspian Sea
basin according to IUCN criteria. Criteria include medium numbers, medium range
(25-75% of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin.
Further work
The distribution of this species in Iranian waters needs clarifictaion.
Sources
Based on Pinchuk et al. in Miller (2004) and Ahnelt et al. (2007).
Iranian material: CMNFI 1971-0352, 8, 141.7-195.8 mm standard length, Gilan, Caspian Sea + Shalman River (37°28’N, 49°27'E, 37°08’N, 50°15’E);
CMNFI 2006-0022, 3, 142.9-195.4 mm standard length, Gilan, southwest Caspian Sea off Astara (38°00’N, 49°30’E to 38°20’N, 50°00'E);
CMNFI 2006-0023, 1, 99. 4 mm standard length, Gilan, Chaboksar (36°58’N, 50°34’E);
CMNFI 2006-0024, 2, 160.4-183.1 mm standard length, Gilan, Talesh (37°48’N, 48°55’E);
CMNFI 2006-0025, 2, 174.7-187.9 mm standard length, Gilan, Talesh (37°48’N, 48°55’E);
CMNFI 2006-0026, 2, 76.7-81.3 mm standard length, Gilan, southwest Caspian Sea off Bandar Kiashahr (37°25’N, 49°57’E).
Genus Glossogobius
Gill, 1859
The gobies of this genus are found in fresh, brackish and marine waters in
the Indo-West Pacific area. Most species are freshwater residents and there are
about 24 species with one in Iran. They are characterised by an elongate body,
anteriorly rounded and posteriorly compressed, head depressed, snout
elongate, scales on the body ctenoid and on the head cycloid, head
scaled behind eyes, cheeks and operculum scaleless, anterior nostril a
short tube, lower jaw prominent, tongue bilobed, teeth in several rows
in each jaw, gill openings wide, isthmus narrow, no flaps on the inner
edge of the shoulder girdle, and pectoral fin without free rays and base scaled.
Glossogobius giuris
(Hamilton, 1822)

Sarbaz River, courtesy of Asghar Mobaraki.
Common names
gel-ye mahi cheshm navari (= band- or bar-eyed goby) or gavmahi-ye cheshmnavari.
[guloo in Pakistan; bar-eyed goby, tank goby, white goby, flathead goby, crocodile goby].
Systematics
Gobius giuris was originally described from the Ganges River, India.
Key characters
As the sole representative of the genus in Iran, the characters of the genus
serve to identify it.
Morphology
The first dorsal fin has 6 spines, the second dorsal fin has 1
spine followed by 7-9 soft rays, the anal fin has 1 spine and 7-9 soft
rays and the pectoral fin has 16-21 branched rays. Lateral series
scales 28-36. Scales on the head are mostly cycloid while body scales
are ctenoid. The exposed portion of flank scales are diamond-shaped.
Scales are rectangular in overall shape with a rounded anterior margin
which may be slightly indented above and below the mid-point, parallel
dorsal and ventral margins, and a posterior margin with two straight
edges meeting at a rounded central tip. The anterior dorsal and
ventral corners are sharp. There are fine ctenii on the posterior
margin. Numerous radii radiate anteriorly from the rounded central tip
of the posterior margin. Circuli are fine. Esmaeili et al. (2009) give
details of scale morphology using scanning electron microscopy. Gill rakers are short and
reach the one below when appressed. The cheek has 3-5 longitudinal
mucus canals. The gut is short and s-shaped.
The chromosome number is 2n=46, with 46 acrocentric chromosomes and
46 chromosomal arms (Subrahmanyam, 1969; Ráb, 1985; Vasil'ev and
Grigoryan, 1993; Klinkhardt et al., 1995).
Meristics on Iranian specimens: first dorsal fin with 6(3) spines,
second dorsal fin soft rays 9(3), anal fin soft rays 8(3), and
pectoral fin rays 19(1) or 20(2). Scales in lateral series 32(1) or
36(1), total gill rakers 12(1) or 14(1) and total vertebrae 27(1).
Sexual dimorphism
Males are larger than females. Male dorsal fins are longer and
their overall colour is brighter (Pethiyagoda, 1991).
Colour
Olive-green to blackish-green or yellowish to sandy brown on the
back with flanks similar but lighter, often a greenish-yellow. The
belly is cream coloured. The head and body are darkly blotched and
spotted. The flank may have 5 regularly spaced blotches. The sides of
the head have irregular dark to violet spots. The fins have thin bars
appearing as rows of spots on each fin ray, in some concentrated at
the base of the second spine of the first dorsal fin. Fins are a
yellowish-green. The first dorsal fin may have a dark spot posteriorly
and the anal fin a black margin. The pectoral fin has a dark spot
above the base. Small preserved fish are immaculate. The peritoneum is
silvery with a few small and scattered melanophores.
Size
Attains about 50 cm.
Distribution
Found in the Indo-West Pacific entering rivers. Abdoli (2000) maps this
species as questionably present in the lower Karun and Arvand rivers of the
Tigris River basin, questionably in the Gulf
and Hormuz basins; and in lower reaches of Makran rivers from the Jagin to
the Bahu Kalat or Sarbaz.
It is recorded from the Hormuz and Makran basins by specimens.
Zoogeography
The marine abilities of some members of this family would aid in dispersal
between river systems connected to the sea.
Habitat
Sand and mud substrates are preferred over rock in Sri Lanka (Pethiyagoda,
1991). The fish lies embedded in the sand with only the eyes
protruding and rarely swim freely. Young fish may form schools or may rest close
to shore in shallow water where they can be caught by hand. Tekrival
and Rao (1999) report its aquarium preferences and habits as 22-25°C,
pH 6.5-7.2, predator, almost dark lighting, bottom dwelling with
stones preferred and a pair or more can be kept.
Age and Growth
Hossain et al. (2009) found a value close to isometric for this fish
in the Ganges River of northwestern Bangladesh (b = 3.068 for total length and
combined sexes, 2.954 for males and 3.293 for females)
Food
Diet includes small fishes seized from its concealment in the sand
by a sudden lunge; this species being a visual ambush predator. It may
browse on the substrate and take living food. An Iranian specimen had
crustacean remains.
Reproduction
Eggs are green and when laid become strongly attached to any
submerged structure by a tube 3-8 mm long. The eggs are 0.5 mm in
diameter. Freshwater populations may migrate to the sea to breed as
there is a marine larval stage although some are reported to be
land-locked in South Asia and hence freshwater breeders (Pethiyagoda,
1991). The spawning period is June to September, peaking in July to
August (Saksena, 1980).
Parasites and predators
None reported from Iran.
Economic importance
This goby is a food fish in South Asia and can be caught on light
angling gear. It has been reported as being ciguatoxic (intermittently
poisonous through feeding on toxic food) (Bagnis et al., 1970).
Conservation
The distribution and abundance of this species in Iranian waters has not been examined and no conservation assessment can be made.
Further work
This species seems to have a fairly restricted distribution in Iran and this should be investigated further.
Sources
Iranian material: CMNFI 1979-0322, 2, 20.7-53.6 mm standard length, Baluchestan, Sarbaz River (ca. 25º45'N, ca. 61º26'E);
OSU 8124, 1, 162.0 mm standard length, Baluchestan, Bahu Kalat, Sarbaz River (25º43'N, 61º25'E).
Genus Hyrcanogobius
Iljin, 1928
Hyrcanogobius bergi
Iljin, 1928
This
species was described from the north Caspian Sea near the mouths of the
rivers Volga, Ural and Emba. Ragimov (1977) reports this species from off Gasan
Kuli at 37°30'N in Turkmenistan near Iranian waters. Shakirova and Sukhanova (1994) record this
species from the Atrek lakes of Turkmenistan, the Atrek River flowing into Iran
for part of its course.
Gobius longecaudatus var. c Kessler, 1877 described from Baku Bay
is a synonym.
Miller and Pinchuk in Miller (2004) map this species from
Iranian waters (Gorgan Bay) but this is not confirmed by specimens.
Miller and Pinchuk in Miller (2004) give a recent description of this species.
Genus Knipowitschia
Iljin, 1927
This genus of sand gobies has about 13 species in the Caspian, Black, Aegean and
Adriatic seas and adjacent fresh waters with 2 recorded from Iran.
Later spelt Knipovitschia, incorrectly, in the English summary
of Iljin's 1928 description. The original description is apparently in Iljin (1927d) (as is that of Caspiosoma) but was repeated in
Iljin (1928). Hyrcanogobius Iljin, 1928 and Bubyr Iljin,
1930 are synonyms (Economidis and Miller, 1990) although Miller in Miller (2004)
separates Hyrcanogobius.
The body is fusiform without a strongly depressed head, the dorsal
profile anteriorly being straight. The mouth angle does not reach past
the anterior part of the eye. The mouth is oblique. The eyes are
moderate in size and may be lateral or directed upwards. The body
usually bears imbricate ctenoid scales but the head is naked as can be
the anterior part of the back as far as the second dorsal fin and the
middle of the abdomen to the anal fin. There may be axillary scales
and scales on the belly midline. Some members lack scales except for the
axillary region and the caudal peduncle. Lateral series scales less than 40.
There are no barbels and the anterior nostrils are not an extended tube-like
structure. The posterior nostrils are pore-like. The
nape lacks a bony crown and only the posterior part of the cranium is
covered with dorsal muscles. The preoperculum lacks teeth or
projections. The isthmus is broad and the branchiostegal membrane is
attached to its entire lateral margin. Jaw teeth are conical,
caniniform and in several rows forming a band. The outer row is
slightly enlarged but there are no strongly enlarged teeth. The vomer
lacks teeth. The tongue is truncated or slightly rounded. The gill
rakers are small and broadly spaced apart. The pectoral fins lack
fine, silk-like rays. The anterior edge of the pectoral girdle lacks
dermal flaps. The pelvic fin disc reaches to, or near to, the anus.
The pelvic fin anterior membrane is well-developed but without obvious
lateral lobes. The caudal peduncle is slender and longer than the second dorsal
fin base. The upper angle of the caudal fin is nearly rectangular and the caudal
fin is rounded and shorter than the head. The head canals are variably developed
or absent, and may be damaged by abrasion in poorly preserved material. The
first dorsal fin modally has 6 or 7 rays and vertebral modes are 30-33. The
swimbladder is present or reduced. There is no perianal organ. Colour
is light. Postlarvae are planktonic.
Miller in Miller (2004) also defines this genus by
characters of the head lateral line system, namely having the infraorbital
longitudinal row a present with typically at
least 2 transverse rows from this row; transverse infraorbital rows between
levels of rows b and d along cheek, with rearmost descending
behind posterior end of row d; transverse row behind jaw angle not
descending below level of row d; row b short, to below rear
eye; anterior row tra distant from row b; anterior dorsal row g
distant from row n; snout rows r and s linear; uniserial
row of papillae along upper edge of each orbit when canals are absent but never
with transverse rows; anterior oculoscapular canal (if present) ending
anteriorly in interorbit, dividing from pore κ to end at double pores β;
posterior oculoscapular present or absent; and preopercular canal when present
always lacks middl;e pore δ.
Vasil'eva and Kuga (2001) propose that the
separation of Knipowitschia from Pomatoschistus is doubtful based
on similar cranial osteology and the variation in the degree of development of
head canals seen in Knipowitschia.
However Miller in Miller (2004) disagrees and presents arguments for a
"sand-goby" group of 5 distinct genera, of which only Knipowitschia and
Hyrcanogbius are known from Iran (Pomatoschistus, Gobiusculus
and Economidichthys being the others).
Miller (1990) states that the majority of the members of this genus
evolved after the draining of the Western Paratethys basin into the
Adriatic Sea basin in the late Miocene about 6.0-5.5MYA.
.Members of this genus are called gav mahi and سگ ماهي (sag mahi) in
Farsi and this not repeated under each Species Account.
Knipowitschia caucasica
(Berg, 1916)
Common names
gel-ye mahi qafqazi (= Caucasian goby) or gavmahi-ye qafqazi.
[gafgaz xulu in Azerbaijan; bubyr kavkazskii in Russian; Caucasus bald goby;
Caucasian dwarf goby].
Systematics
Pomatoschistus caucasicus was originally described from
Batumi/Temirgoe, a station south of the Sulak mouth on the Black Sea.
The name of this species first appeared in Kavraiskii in Radde
(1899) as a museum name without a description ( a nomen nudum)
and was subsequently made available by L. S. Berg in 1916 with the
type locality being a swamp near Batum and Lake Inkit near Pitzunda,
Georgia (see Eschmeyer (1990) and Eschmeyer et al. (1996) for details).
Gobius lenkoranicus Kessler, 1877 described from "pribrezhnom'
bolote, po blizosti Lenkorana" (= a coastal marsh near Lenkoran,
Azerbaijan) is a synonym, tentatively so according to Economidis and
Miller (1990). This name was suppressed by the International
Commission on Zoological Nomenclature (see Kottelat, 1997; Miller et al.
in Miller, 2004)). This earlier name for what could be caucasica was suppressed as a nomen
dubium because of an inadequate original description in its
details and the small and poorly-preserved holotype which could not be
readily compared with caucasica. The name could also have been
suppressed as a nomen oblitum as it had not been used for 50
years (Economidis and Miller, 1990).
Formerly placed in the genus Pomatoschistus Gill, 1863 and
in the genus Bubyr Iljin, 1930.
Three syntypes from Lake Temirgorje, Georgia (formerly in the
Tiflis Museum) are in the Natural History Museum, London under BM(NH)
1896.3.28:26-28 (22.1-25.2 mm standard length) (Eschmeyer et al., 1996;
personal observations). Economidis and Miller
(1990) list these three specimens (as evidenced by the catalogue
numbers 26-28) as two males (22.8-24.0 mm standard length) and two
females but give the length of only one female (24.5 mm). Miller et al.
in Miller (2004) consider the material used by L. S. Berg (see above) to be the
type series (ZISP 15343 from Lake Inkit and ZISP 15321 a swamp near Batum).
Key characters
The anterior oculoscapular lateral line head canals unite in the posterior
interorbit, with a single median pore κ, and canals
extend anteriorly to pores λ. The preopercular canal is present (Miller in Miller, 2004).
Morphology
First dorsal fin with 5-8, usually 6, spines (rarely 4 as an
anomaly, e.g. in Berg (1948-1949, Fig. 784 (Iljin, 1956)), second
dorsal fin with 1 spine and 6-10 branched rays, usually 7-8, anal fin
with 1 spine and 5-10 branched rays, usually 7-8, and pectoral fin
with 13-18 rays. Lateral scales 29-38. Vertebrae 30-33. The back in
front of the first dorsal fin and the belly in front of the anal fin
are usually scaleless. The caudal peduncle is completely scaled and
there is a complete row of scales anteriorly along the lateral
midline, although other flank scales may be missing. The anterior oculoscapular
head lateral line canals join in the posterior interorbit region, with one pore κ,
and paired canals in the interorbit each to
a pore λ and single pore α. The posterior oculoscapular canal is typically
present, the preopercular canal is present and there are no additional pores on
the horizontal anterior oculoscapular canal behind the eyes (Miller et
al. in Miller, 2004). The subopercular canal is variably present which has led to different generic
assignments for this species. The larva has been described by Daoulas et al. (1993).
Meristic values for Iranian specimens are:- first dorsal fin spines 6(4), second dorsal fin rays after a spine
6(1) or 8(3), anal fin rays after a spine 8(2) or 9(2), lateral scales 33(1), 34(1), 36(1), 39(1), and total gill rakers
9(3) or 10(1). Rakers are very short, not reaching half way to the adjacent one when appressed.
Sexual dimorphism
See under colour. Males are larger than females. There are various
morphometric differences (Miller et al. in Miller, 2004).
Colour
Overall colour is grey to fawn with olive-green tints. Fish in hypersaline
habitats are darker. Males are generally darker than females and have distinct dark bars
on the flanks below the two dorsal fins and on the caudal peduncle
while females have irregular spots or blotches along the flank
mid-line. Nuptial males have 4 prominent bars. However some males may lack bars and have spots and some
females lack spots (Ahnelt et al., 1995). The head in males is
densely pigmented, including ventrally. The caudal peduncle is
completely pigmented, usually including the ventral surface. The flank
bar under the first dorsal fin in males is long and usually runs from
near the dorsal mid-line to the belly. The male first dorsal fin has
three oblique dark bands and a dark spot medially on rays 5 and 6
extending posteriorly on the fin membrane. The distal edge of this
dorsal fin is dark. The second dorsal fin has three bands also and a
dark edge. Males have dark pigment on the pelvic fins, head and
breast, and a silver hue on the anal fin during the breeding season.
The male pectoral fin has a short and oblique dark band on the upper
rays. The anal fin in males is normally dark with a pale margin while
in females it is pale overall. The caudal fin has thin dark bands.
Females are a pale fawn overall. The female has a densely spotted
first dorsal fin while the male has a blue blotch at the fin rear when
in breeding condition. The first dorsal fin in females has a
distinctive middle band but the others are faint. The second dorsal
fin in females is only faintly banded. Breeding females have 2
intensely black areas on the first dorsal fin, a black spot on the
lower jaw, 2 rows of melanophores running obliquely from the eyes to
the upper jaw, and a yellow abdominal region (Economou et al.,
1994). The back has fine melanophores extending to the rear of the
second dorsal fin. There are light saddles at the origins of the first
and second dorsal fins, the end of the second dorsal fin and on the
caudal peduncle. The mid-flank has several blotches, some slightly
deeper than wide. The rest of the body below these blotches is pale
except for some short vertical groups of melanophores below the
lateral midline and melanophores along the base of the anal fin. The
breast is pale as opposed to dark in males.
Large and minute pigment blotches and spots variably developed are
scattered over the whole head and body in both sexes of an Iranian
sample. The peritoneum has large pigment spots ventrally or is almost immaculate.
Size
Reaches 48.6 mm, perhaps 69 mm, total length. Fish in Gorgan Bay
reached 46.0 mm (Afraei and Hassannia, 1999).
Distribution
Found in the Aegean, Black and Caspian Sea basins. Introduced to
the Aral Sea. Caspian localities include Imeni Kirova or Kyzylagach
Bay near the northwestern Iranian border, the lower Safid River and lower Babol
River, near Anzali and Ashuradeh (Derzhavin, 1934). It is reported
from Gorgan Bay, the delta of the Karasu, the Atrak River, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea (Kiabi et al., 1999; Jolodar and Abdoli, 2004; Miller
et al. in Miller, 2004; Abdoli and Naderi, 2009).
Zoogeography
This species is part of marine fauna in the eastern Mediterranean and
the Black-Caspian-Aral seas basins and its relationships are outlined under the genus description above.
Habitat
This species lives and reproduces in salinities as high as 59.5‰ and up to 83‰ (Zenkevitch, 1963; Miller et al. in Miller,
2004) but also enters fresh water. Preferred
conditions in the Aegean area are 15-20‰ (Ahnelt et al.,
1995; Miller et al. in Miller, 2004). Juveniles prefer sand, mud or gravel bottoms near shore in
Greece (Daoulas et al., 1993) but weedy shallows are also
favoured (Economidis and Miller, 1990). They may overwinter in deeper
water at 1-2 m in the Volga delta (Economidis and Miller, 1990) and migrate into
shallow water in April-May prior to spawning (Miller et al. in Miller,
2004). The Atrek River population moves downstream (Miller et al. in Miller, 2004).
Afraei et al. (2001) found this species to be most frequent in Gorgan Bay
in January and least frequent in March.
Age and growth
Life span may be only 1 year and as a consequence numbers can
fluctuate widely in any given year depending on environmental
conditions. In the Evros Delta of the north Aegean Sea, fish grow
rapidly during the summer after hatching, breed from the end of April
to the end of July in the following summer and grow rapidly again from
July to September. Older males die the following year after February
but some females will survive to spawn a second time at the end of
April and the beginning of May and then die. Fish may be mature at
14.5 mm total length (Kevrekedis et al., 1990).
In the spawning season, females comprise as much as 85.5% of the
population (Miller et al. in Miller, 2004).
A study of this species in Gorgan Bay, Golestan (Iranian Fisheries
Research Organization Newsletter, 23:2, 2000) showed that the maximum size obtained was
46 mm with average lengths of 33 and 37 mm for males and females
respectively. The sex ratio was 1:1.1 for males to females and growth is
positive allometric (see also Afraei and Hassannia (1999) and Afraei et al.
(2001)).
In the Volga delta spawning males were 33.3 mm and females 30.2 mm on average
(Miller et al. in Miller, 2004).
Abdoli et al. (2006) examined fish
from the Gomishan and Miankaleh wetlands and found a length (L)-weight (W)
relationship of W = 0.135L2.72 for males, 0.0377L1.97 for
females and 0.0182L2.51 for sexes combined.
Food
Diet was dominated by polychaete worms and chironomids (88%) in one
study but crustaceans such as amphipods, copepods, ostracods, cladocerans,
insects such as choronomids, bivalve mollusc larvae, and
fish are also taken and items may be both benthic and planktonic.
Reproduction
Females may be carrying eggs at 20-24 mm and 8-10 months of age.
Fecundity reaches 1389 eggs but can be as low as 60 eggs. Fecundity in
the Caspian Sea ranges from 209 to 786 eggs with a mean of 423, from
209 to 382, mean 285 and from 527 to 863, mean 715.6 for various
reports (Kevrekedis et al., 1990). Batches consist of 80-100
eggs. Eggs are cylindrical, 2.6 x 0.97 mm. Larvae are pelagic and as
small as 4.1 mm standard length. Relative fecundity in the Gorgan Bay
study in Iran was 290-550, mean 395.5 eggs (Afraei et al., 2001).
There is a pre-spawning migration into shallow water in April in
the Caspian Sea. The male approaches the female from below, touching
her lower jaw with his snout and leading her to a reed nest. Such
behaviour occupies several hours to more than a day. The female enters
the reed nest, inverts and deposits eggs on the roof of the nest. The
underside of bivalve shells or gravel may also be used. The male then
fertilises the eggs, inverting to do so. The eggs are laid in a line
and the pair will form other lines. Egg patches may eventually contain
as many as 3000 eggs at different developmental stages, which
indicates males may spawn with several females. Spawning takes about
1.5 hours and the female gradually loses the brightness to her
breeding colours and almost all breeding pigment is lost within a day.
The pair mated 4 times over a period of 35 days (28 March, 6 April, 14
April and 2 May in an aquarium with fish from Lake Trichonis, Greece).
The male remains mostly inside the reed nest, aerating the eggs and
defending them against other gobies. At irregular intervals (8-60
minutes), the male would leave the nest for 1-5 minutes, occasionally
feeding but always remaining near the nest.
Spawning in the pre-estuary area of the Volga River in the Caspian
Sea takes place from the middle of April to the end of May, rarely to
June. Spawning in the Caspian takes place in shallow water with some
current at a depth of 0.15 to 1.5 m, a temperature of 15-27°C
and over a wide range of salinities (Kevrekedis et al., 1990).
Parasites and predators
None reported for Iran.
Economic importance
None.
Conservation
This species seems to be rare in Iran with only Derzhavin's (1934)
report and one other record. Its status cannot be assessed until more
is known about its distribution. Kiabi et al. (1999) consider
this species to be data deficient in the south Caspian Sea basin
according to IUCN criteria. Criteria include abundant in numbers,
habitat destruction, limited range (less than 25% of water bodies),
absent in other water bodies in Iran, absent outside the Caspian Sea
basin (sic). Vulnerable in Turkey (Fricke et al., 2007).
Further work
Further collecting will need to be carried out to determine its
abundance and distribution in Iranian waters. Its small size may have
led to it being ignored or confused with other gobies as young and it
may be more widely distributed than current knowledge indicates.
Sources
Iranian material: Uncatalogued, 4, 22.2-35.3
mm standard length, Mazandaran, Gorgan Bay (no other locality data).
Knipowitschia iljini
Berg, 1931
Common names
gavmahi-ye Iljin, gel-ye mahi.
[Iljin's bychok in Russian, Iljin xulu in Azerbaijan].
Systematics
At least 4 syntypes are in the Zoological Institute, St. Petersburg
(ZISP 22052) along with ZISP 24370 and possibly ZISP 24424 (Miller and Pinchuk
in Miller, 2004). Although Berg (1931b) states "many specimens
caught" this may refer to abundance while only 4 fish are
illustrated and may be the type series. This species was described
from "in the middle part of the Caspian Sea".
Key characters
The anterior oculoscapular lateral line head canals are more or less separate
in the midline of the posterior interorbit, with double or separate pores
κ, and canals extend anteriorly through interorbit
of variable extent. The preopercular canal is absent or present (
Miller in Miller, 2004).
Morphology
The first dorsal fin has 6-8 spines, usually 7, the second dorsal fin has 1
spine followed by 8-10 soft rays, the anal fin has 1 spine and 7-10, usually 9,
soft rays, the pectoral fin has 15-18 rays. Lateral series scales 28-38.
Vertebrae 31-33. Scales are ctenoid. The belly,
head and the dorsal surface back to the second dorsal fin are all
naked. There is no small, straight canal extending posteriorly from
the junction of the orbital canals. The caudal fin is rounded and
symmetrical. The anterior oculoscapular lateral line head canals do not unite in
the posterior interorbit. Canals anterior to pores κ are typically absent and pore κ is double.
The posterior oculoscapular canal is absent, the preopercular canal absent or
present. Pore "a" is single or double with sometimes an additional pore or two
on horizontal anterior oculoscapular canal behind eye.
Sexual dimorphism
Males have darker overall colouration and darker fins than the
females (see below). The first dorsal fin in males has free tips and
usually a dark spot at the rear margin. Males have a pointed genital
papilla while females have a distally bifurcated papilla. The pelvic
fins reach the genital papilla in males while in females they do not
reach the vent. Females have a more slender caudal peduncle and the
snout is more produced than in males.
Colour
The body overall has a brownish reticulate pattern. The back and flanks bear about 6-10 dark bands in males while in
females bands are faint and restricted to the posterior flank. The male has a
darkly edged caudal fin and the pectoral fin is dark ventrally. The
dorsal fins have dark stripes and the anal fin has a dark stripe along
its margin in males while females lack stripes. Females bear small
dark spots irregularly distributed on the back and anterior flanks.
Size
Attains about 4.7 cm for males and 5.0 cm for females. Ragimov (2003) gives a maximum length of 5.2 cm.
Distribution
Ragimov (1965) reports this species between Kultuk and Astara in
Azerbaijan and generally in the central and southern Caspian Sea, and mapped
around the coast of the whole Caspian by Miller and Pinchuk in Miller (2004).
Zoogeography
This species is part of a marine fauna in the
eastern Mediterranean and the Black-Caspian-Aral seas basins and its
relationships are outlined under the genus description above.
Habitat
Found benthically and in the water column with adults in shallow coastal
areas at 20-70 m with juveniles offshore as deep as 500 m Not recorded from fresh water.
Age and growth
Life span is estimated at one year (Miller and Pinchuk in Miller, 2004).
Food
Only mysids have been recorded from 8 fish examined (Miller and Pinchuk in Miller, 2004).
Reproduction
Gonad maturity begins in late August to early September, many fish are ripe
by the end of January and most by the end of February. Spawning occurs from
early April to possibly as late as September with a peak in May-June (Miller and
Pinchuk in Miller, 2004). Fecundity reaches 2240 eggs and egg diameter 0.8 mm
(Miller and Pinchuk in Miller, 2004).
Parasites and predators
None reported from Iran but sturgeon and Sander species eat this fish (Miller and Pinchuk in Miller, 2004).
Economic importance
None.
Conservation
Apparently rare in Iran but this may reflect collecting effort for this small
species. Insufficient work has been devoted to this species to assess its status.
Further work
Further collecting will need to be carried out to determine its
abundance and distribution in Iranian waters. Its small size may have
led to it being ignored or confused with other gobies as young and it
may be more widely distributed than current knowledge indicates.
Sources
Iranian material: None.
Knipowitschia longecaudata
(Kessler, 1877)
Black and Caspian seas, described from the southern and middle Caspian
Sea, but no Iranian record. Ragimov (1965) reports this species
between Kultuk and Astara in Azerbaijan. Sometimes spelt longicaudata
erroneously in the literature.
Genus Mesogobius
Bleeker, 1874
This is an endemic Ponto-Caspian genus with 3-4
species, with one species being of uncertain status (see below). It is distinguished by
having three (rather than the more usual two) transverse head papillae rows
between rows b and d. Other characters include suborbital papillae in transverse rows, no row a,
snout with longitudinal rows s1 and s2, no perianal organ; row 5i not below level of row 6i, 6i at or opposite
end of row d, scales normal, anterior nostril elongate but not
overhanging lip, and pelvic fins without lobules. A detailed, recent description is given by Miller in Miller (2004).
Mesogobius nigronotatus
(Kessler, 1877)
Caspian Sea basin, described from Fort Aleksandrov (= Shevchenko)
on the Caspian Sea coast of Kazakhstan, and from the
south Caspian Sea by Naseka and Bogutskaya (2009),
but no Iranian record. The
only specimen was lost (Reshetnikov et al., 1997).
M. nonultimus may be a synonym (Pinchuk and Miller in Miller, 2004).
Mesogobius nonultimus
(Iljin, 1936)

Common names
[bychok neposledniy or seriy bychok-martovic in Russian; Caspian toad goby]
Systematics
Described from 24 miles southwest of Ulsky Bank, Caspian Sea, Turkmenistan.
The type is lost. May be a subspecies of Mesogobius
batrachocephalus (Pallas, 1814) although Miller revises his opinion in
Pinchuk and Miller in Miller (2004) and recognises it as a distinct
species. See also under M. nigronotatus above.
Key characters
It is distinguished by having three (rather than the more usual two)
transverse head papillae rows between rows b and d. Nape scales
weakly ctenoid, almost reaching median pore κ,
snout less than twice eye diameter, lower jaw not protruding, and males
with distinct breeding colours.
Morphology
First dorsal fin rays 6-7, second dorsal fin with 1 spine and 16-19 (usually
17-18) soft rays, and anal fin with 1 spine and 15-18 soft rays. Scales ctenoid
except for a few, small cycloid scales on the opercle. The breast is naked. Lateral line
scales 73-83. Lateral line system with 8 transverse suborbital neuromast rows.
Three rows of free neuromasts on the opercle. The height of the second dorsal fin diminishes posteriorly. Head
width 1.25 depth.
Meristics for an Iranian specimen: first dorsal fin with 6 spines, second
dorsal fin 1 spine and 19 soft rays, anal fin with 17 soft rays and pectoral fin
with 16 branched rays. Lateral series scales 80 and transverse scale rows 20.
Sexual dimorphism
Male spawning colour is distinctive and males are larger than females.
Colour
Overall colour is pale grey with dark bands, up to 6 on the body. The first
band is in front of or below the first dorsal fin and is visible from above.
Intensity of bands varies with ones below the middle of the second dorsal fin
and before the caudal fin origin being the most intense. The first dorsal fin
has two bands, the dorsal, pectoral and caudal fins are dark while the pelvic
and anal fins are pale. Males have a dark, bluish-black spawning colouration
with spots and bands on the upper body becoming indistinct.
Size
Reaches 17.4 cm.
Distribution
Probably the whole Caspian Sea basin. Known from the Turkmenistan and Daghestan coasts and
from recorded between Kultuk and Astara in Azerbaijan (Ragimov, 1965). Pinchuk and Miller in Miller (2004) map this species from the
entire Caspian Sea including the Iranian shore but only recently has it been
recorded from Iran (H. Ahnelt, et al., 2007).
Zoogeography
This species is related to Neogobius and Ponticola, more speciose genera of Ponto-Caspian gobies.
Habitat
This species has been collected down to 50 m, perhaps deeper, but little else is known of its
habits. It is suspected to feed, winter and spawn in deeper waters without an onshore migration.
Age and growth
Maturity is attained in the second year of life.
Food
Unknown.
Reproduction
Fecundity reaches 1544 eggs, probably laid in a single batch. The spawning
season off the Lenkoran coast is late March and early April, the earliest of all
Caspian Sea gobies.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Numbers in Iranian waters and threats to this species need to be assessed.
Further work
The biology and distribution of this species in Iranian waters needs to be studied.
Sources
Based in part on Pinchuk and Miller in Miller (2004).
Iranian material: CMNFI 2006-0022, 1, 114.2 mm standard length, Gilan, southwest Caspian Sea (38º00'N, 49º30'E to 38º20'N, 50º00'E) (Ahnelt et al., 2007).
Genus Neogobius
Iljin, 1927
This genus of gobies is found in the Black and Caspian seas where there are about 14 species, some large enough to be the
object of commercial fisheries. The general Farsi name for fishes in this genus
is گاوماهي (gav mahi), سگ ماهي (sag mahi) or gel-ye mahi, not repeated under each species.
Berg (1948-1949) and various papers by Pinchuk (1976, 1977, 1991) recognise Neogobius as a genus but Vasil'yeva (1989) considers that the separation of Neogobius, even as a subgenus, is
doubtful and refers the species to Gobius. Simonovic (1999) briefly reviews the literature on Neogobius and its distinction from Gobius. Presence or absence of a swimbladder, egg size,
and presence or absence of pelagic larvae are characters which have been used to recognise genera or subgenera but these may be phylogenetically independent (Svetovidov, 1964b). Various subgenera may be
recognised within Gobius such as Gobius Linnaeus, 1758, Neogobius
Iljin, 1927 (type species N. fluviatilis), Apollonia Iljin, 1927 (monotypic for N. melanostomus
according to Pinchuk (1991) although Vasil'yeva (1989) and Dobrovolov et al. in Charlebois et al. (1997) add the species fluviatilis on the basis of osteology and electrophoretic
studies; and then later she (Vasil'eva, 1996a) places both these species in the subgenus Neogobius, again on osteological grounds), Ponticola
Iljin, 1927 including such species as
ratan (type species), and Eichwaldia for caspius (type species)(Eichwaldia is preoccupied by Eichwaldia Billings 1858 in fossil brachiopods, replaced by and objective
synonym of Eichwaldiella Whitley, 1930). Miller and Vasil'eva in
Miller (2003 - Vasil'eva's assignments) give the following subgenera, with definitions (these subgenera may eventually be elevated to genus once a thorough analysis is complete (see below); note
assignments conflict in part with the above:- Neogobius (with one species, caspius), Apollonia (fluviatilis and melanostomus), Babka (gymnotrachelus, not
in the Caspian Sea), Ponticola (the remainder - including species not in the Caspian Sea). Miller in Miller (2004) place Neogobius bathybius in the genus Chasar and this is followed
here. Neilsen and Stepien (2006) and Stepien and Tumeo (2006) used mtDNA cytochrome b sequence data and consider Apollonia (N. melanostomus and N. fluviatilis) to be highly
divergent from all other neogobiins, Mesogobius is the sister genus of Proterorhinus and a clade of these two genera is sister to Neogobius.
Neilsen and Stepien (2009a) using two mitochondrial and two nuclear genes
conclude that Neogobius, or in a restricted form Apollonia,
consists of fluviatilis, melanostomus and caspius, Babka
is monotypic (species gymnotrachelus), and Ponticola includes
cyrius, gorlap, ratan and syrman among others.
Mesogobius and Proterorhinus are sister groups. Tribes are also
delineated. Apollonia is synonymised with Neogobius.
Pinchuk (1991) states that many of the characters used to define genera are composed for convenience in identification rather than indicating natural relationships, i.e. the characters are simply those
used in keys. Ahnelt and Holčík (1996) call the taxonomy of this genus "variable, unstable and frequently uncertain" since variation between Black and Caspian Sea populations is usually very
small and since there are migrating and resident marine and freshwater populations of the same species. Miller and Vasil'eva in Miller (2003) state that the genus is paraphyletic (included species do not
share an autapomorphy) but retain it for convenience on morphological grounds, a practice followed here until a cladistic revision is performed.
These gobies are characterised by having an elongate body, compressed posteriorly, mouth of moderate size, 6 first dorsal fin rays, more than 10 rays in the second dorsal fin (large number of rays
in the unpaired fins according to Pinchuk (1991) compared to Gobius sensu stricto), anterior nostrils tubular but not very elongate, posterior nostrils near eye, ctenoid scales of moderate
size, a scaled nape and predorsal area, cheek naked, the pelvic fin anterior membrane may have obvious lateral lobes, no barbels, upper rays of pectoral fin may be free and filament-like (Pinchuk (1991)
states that they are always within the fin membrane or joined), teeth conical and in a few series, back muscles extend forward almost to the eyes, tongue not notched or only slightly notched, no
swimbladder in adults, 32-35 vertebrae modally (Gobius has only 28 and Proterorhinus 30-32 (Simonovič et al. (1996)), dorsal pterygiophore formula (3)22110, anal pterygiophores
before first caudal haemal spine 2 or 3, large, oligoplasmatic roe, and no pelagic larvae. Miller and Vasil'eva in Miller (2003) give details of neuromast organs as follows:- infraorbital neuromasts
typically in 7 transverse rows, 4 before and 3 above hyomandibular row b, and no row a. Row 7 is made up of a few to several papillae in a row descending postero-ventrally from just in front
of the anterior oculoscapular pore α. Rows 5i and 6i are separated with 5i well behind the anterior end of hyomandibular row b and with 6i reaching or falling short
of row b. Dorsal supraorbital rows o are separate in the dorsal midline. Hyomandibular row z ends near pore χ. Supratemporal accessory line x1
ending anteriorly behind pore β. Anterior oculoscapuler, posterior oculoscapular, and preopercular canals present. These have the pores σ,λ,κ,ω,α,β and ρ; θ
and τ; and χ, δ and ε. Anterior oculoscapular pore ρ and posterior oculoscapular pore θ well separated.
Neogobius is distinguished from the closely related genus Mesogobius Bleeker, 1874 by having a smaller number of infraorbital transverse rows of pit organs and the transverse parietal
posterior rows of pit organs are separated by a wide interspace (Pinchuk, 1991).
Simonovič et al. (1996) consider this genus of gobies to be a young one as evidenced by the distribution of species in the Caspian and Aral seas which closed recently. Simonovic (1999)
concludes that "Neogobius" (and Proterorhinus) evolved from a common ancestor in the Dacian Basin of the eastern Paratethys (now the Black Sea) or its tributaries during the early to
mid-Pliocene, as evidenced by their absence from Adriatic, Ionian and Aegean basins. Divergence probably occurred in freshwater, riverine habitats as development is direct without a pelagic larval stage
as found in marine gobiids. The hypersalinity of the isolated Black Sea in the late Miocene to early Pliocene was the causal event that led to rapid speciation of
"Neogobius" and the genus retained
direct development when it returned to the Black and Caspian seas. Dillon and Stepien (2001) using mitochondrial DNA suggest that the genera
"Neogobius" and Proterorhinus diverged about
5.2±1.0 MYA in the late Miocene/early Pliocene from a common ancestor shared with Gobius during the isolation of the Paratethys Basin from the Mediterranean Tethys Sea. This contradicts
suggestions that the neogobiins diverged from Gobius during the Quaternary interglacial connections of the Black and Mediterranean seas.
Masoumian and Aghl Mandi (2009) record Neogobius spp (presumably
including Ponticola spp.) infected with Ceratomyxa caspia, a
myxosporean, from the Iranian shore of the Caspian Sea.
Neogobius caspius
(Eichwald, 1831)

Common names
gel-ye mahi Khazari (= Caspian goby), gavmahi-ye Khazari, gavmahi-ye Daryaye-e Khazar.
[xval xulu in Azerbaijan; Khvalynskii bychok or Hyrcanian goby in Russian; Caspian goby].
Systematics
No major synonyms and no types known. Described in Latin from "Hab. in Caspio mari, in sinu Bacuensi" (in the Caspian Sea, in Baku Bay, Azerbaijan).
Key characters
This species is separated from other Caspian gobies in Iran by the rear nostril being slit-like and far from the eye margin. It has a comparatively small mouth.
Morphology
Head canals and free neuromasts on the body are typical of a Neogobius. First dorsal fin spines 5-7, usually 6, second dorsal fin with 1 spine followed by 14-17 soft rays. The first ray of the
second dorsal fin may be elongated and branched at its tip and in such cases was counted as a "spine". Anal fin with 1-2 spines, usually 1, and 10-14 soft rays. Lateral series scales 57-71.
Predorsal area and nape scaled, anteriorly reaching to the orbit (postorbital section of the supraorbital canal of the cephalic lateral line system). Anteriormost scales cycloid. Scales have a
diamond-shaped pattern on the flank. Individual scales are squarish with the upper and lower margins straight to slightly rounded or, more rarely, each scale has an overall rounded shape. The posterior
margin has two straight edges meeting at the posterior centre. The posterior margin bears ctenii. The anterior margin is wavy where the radii terminate at indentations and there is a central protuberance.
Moderately numerous radii radiate anteriorly from the central point of the posterior margin, occupying the entire anterior field and spilling into the lateral fields. Circuli are fine. There is a dermal
papilla on the posterior edge of the gill chamber, just under the gill cover. The gill rakers are short anteriorly and posteriorly but vary in length and the longer central rakers reach the second adjacent
raker when appressed. Rakers are commonly forked or even antler-like. Dorsally the smallest rakers are difficult to distinguish from mouth cavity tubercles and only definite rakers were included in the
count. The pelvic fin anterior membrane has pointed lobes. The gut is an elongate s-shape with convolutions or twists along its length. The genital papilla is bifurcated at the tip.
Meristics for Iranian specimens: first dorsal fin spines 5(1), 6(37) or 7(2); second dorsal fin soft rays 14(2), 15(10), 16(27) or 17(1); anal fin soft rays 11(2), 12(25) or 13(13); pectoral fin rays
17(6), 18(29) or 19(5); lateral series scales 60(2), 61(4), 62(6), 63(8), 64(10), 65(6), 66(1), 67(1), 68(1) or 70(1); total gill rakers 8(1), 9(3), 10(17), 11(14), 12(4) or 13(1); and total vertebrae ?.
Sexual dimorphism
Colour differences are given below.
Colour
Overall colour is a dark grey or grey-brown with a clearly contrasting white to pearly white belly and lower head surface. The back may have 7-8 dark brown or grey saddles. The flanks have 6-10
obscure dark spots. The first dorsal fin has 2-4 dark brown stripes and a black spot over the last three rays. The black spot may not be very evident and merges with the general background pigmentation.
There are no clearly defined spots on the fins although the rays are brown in all but the pelvic and anal fins. The anal and pelvic fins are pearly-white. The caudal fin has a narrow whitish transparent
edge. The iris has a narrow golden ring. The genital papilla is white or grey. The peritoneum is silvery-brown in preserved specimens.
Breeding males are black all over, often including the margin of the first dorsal fin. First dorsal fin ray tips may be yellowish. The belly is whitish to pale grey in front of the pelvic fins. There
is a dark spot at the end of the first dorsal fin. The second dorsal fin has a narrow colourless margin but fin tips are not extended into free ends as obviously as in some Neogobius species. All
fins have a fringe effect although this is not well developed in the first dorsal fin. The caudal fin has a colourless or whitish margin. The dorsal fin spot is lost temporarily after spawning.
Size
Reaches 20.2 cm.
Distribution
This species is found in the Caspian Sea and is recorded between Kultuk and Astara in Azerbaijan (Ragimov, 1965). In Iran records are from both the southwestern,
south-central and southeastern Caspian Sea, and Gorgan Bay (Abdoli
and Naderi, 2009).
Zoogeography
Endemic to the Caspian Sea.
Habitat
Found throughout the Caspian Sea but does not enter fresh water. It is found on sand or pebble bottoms, less often on stones and rarely on soft mud. They tend to remain inshore until the winter when
temperatures fall although high summer temperatures may drive them into deeper and cooler water for a short period (Pinchuk and Miller in Miller, 2003).
Age and growth
Sarpanah Sarkohi et al. (2010) found fish to reach age 5 years in the
Iranian Caspian Sea sample sites listed below.
Food
Gut contents include polychaetes, crustaceans, small bivalve molluscs and fish. In some areas molluscs predominate, in others crustaceans.
The study by Sarpanah Sarkohi et al. (2010) of fish taken in bottom
trawlers from three stations at Astara, Anzali and Chaboksar found a relative
gut length of less than one suggesting this fish is carnivorous. Intensity of
fullness of guts was highest from April to October. The main food items were
molluscs (principally Cardium, Didacna, Mytilaster, Abra),
worms (principally Nereis, oligochaetes) and crustaceans (principally
Niphargoides, Petrcuma (sic), Paramysis) with food
preferences of 100%, 89% and 74% respectively. Some chironomids and gobiids were
also eaten.
Reproduction
Egg sizes in Iranian specimens reach 2.1 mm and fish taken on 14 May have eggs 1.9 mm in diameter suggesting a spring to summer spawning season. This species approaches coastal areas in the southern
Caspian Sea at the end of March and the beginning of April, later than P. syrman and
P. gorlap. Females may have up to 1007 large eggs and up to 1985 small eggs, indicating
repeat spawning (Pinchuk and Miller in Miller, 2003). Sarpanah Sourkouhi et
al. (2008) examined fish form the Gilan coast and found the highest
gonadosomatic indices in June, suggested spawning occurs several times, found a
sex ratio of 1.47:1 in favour of females, an absolute fecundity of 212-1234
eggs, a relative fecundity of 18.73-29.89 eggs/g body weight and an egg diameter
range of 0.06-0.23 mm.
Parasites and predators
Sattari et al. (2002) and Sattari (2004) records the presence of the nematode, Eustrongylides excisus, in this species. This parasite can damage muscles in commercial species and render them unsuitable for sale. This
species is eaten by the Caspian seal and by sturgeons. Daghigh Roohi and Sattari
(2004) record Eustrongylides excisus, and Dichelyne minutus from
this species in the southwestern Caspian Sea of Iran. Sattari et al. (2004; 2005) surveyed this species in the inshore area of the Caspian Sea, recording Eustrongyloides excisus and Dichelyne
minutus.
Economic importance
Not reported as of economic importance in Iran, it has been caught commercially in the former Soviet Union and on rod and line. It does form part of the diet of sturgeons (Pinchuk and Miller in Miller,
2003).
Conservation
Trends in numbers of individuals of this species and threats to it have not been examined so its conservation status is unknown.
Further work
The biology of this species needs investigation.
Sources
Pinchuk and Miller in Miller (2003) is the most recent summary on this species.
Iranian material: CMNFI 2006-0022, 1,124.8 mm standard length, Gilan, south-west Caspian Sea off Astara (38°00’N, 49°30’E to 38°20’N, 50°00'E);
CMNFI 2006-0027, 2, 113.4-121.1 mm standard length, Gilan, Talesh (37°48’N, 48°55’E);
NMW 80605, 1, 116.1 mm standard length, Mazandaran, Now Shahr beach (36°39’N, 51°31’E);
NMW 95073, 1, 92.8 mm standard length, Mazandaran, Gomishan lagoon near estuary of Gorgan River (36°59’N, 54°00’E).
Neogobius melanostomus
(Pallas, 1814)
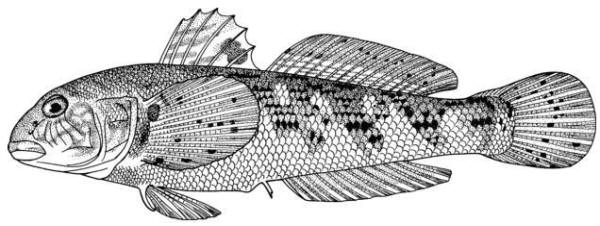


Common names
gavmahi-ye gerd (= round goby), gel-ye mahi gerd
(= round goby), gavmahi domgerd, gavmahi-e-domguerd, sebele.
[xazar kirda xulu in Azerbaijan; Kaspiiskii bychok-kruglyak or Caspian round goby in Russian; black spotted goby].
Systematics
Gobius melanostomus was originally described from Sevastopol and Balaklava, Ukraine, on the Black Sea. No types known.
Gobius affinis Eichwald, 1831 described in Latin from "Hab. in Caspii maris sinu bacuensi, balchanensi" (Baku and Balkhan bays in the Caspian Sea, no types known) and Gobius
sulcatus Eichwald, 1831 described in Latin from "Hab. in Caspii maris sinu balchanensi" (Balkhan Bay in the Caspian Sea, no types known), are synonyms. The Caspian Sea
population was referred to Neogobius melanostomus affinis (Eichwald, 1831) but subspecies are no longer recognised (Iljin, 1956; Pinchuk, 1976).
However, Brown and Stepien (2008) conclude on DNA evidence that Black and
Caspian Sea populations diverged ca. 350,000 years ago and this supports
subspecies separation.
Key characters
Pelvic fin anterior membrane with rounded and shallow lateral lobes; lobes not more than one-sixth width of anterior edge of membrane, or lacking entirely; nape scaled and at least anterior nape scales
cycloid; first dorsal fin with large dark spot at rear; and lateral series scales usually 49-55.
Morphology
This species is separated from other Caspian gobies in Iran by having a small mouth, rounded, shallow, poorly-expressed lobes to the pelvic anterior membrane, scales in lateral series 41-59 (usually
47-55), generally at the low end of this range, mid- and anterior nape scales are cycloid as are most of those on the gill cover, throat, pectoral in bases and part of the abdomen, the pelvic disc is
0.6-0.8 pelvic base to anal fin length, the jaw angle is below the level of the anterior part of the eye, the posterior nostril is close to the eye margin, and the first dorsal fin
has an obvious dark spot posteriorly.
First dorsal fin spines 5-8, usually 6, second dorsal fin with 1 spine and 11-18 soft rays, anal fin with 1 spine and 9-14 soft rays, and pectoral fin with 16-20 branched rays. Scales appear as
diamond-shaped on the flank since the posterior margin has two straight edges coming to a central point. However scales can be quite rounded even adjacent to those forming a diamond pattern. The
posterior margin bears ctenii. Dorsal and ventral margins are rounded and the anterior margin is indented above and below a central protuberance. Radii radiate anteriorly from the centre of the
posterior margin. Circuli are fine. Gill rakers 9-13, presumably on lower arch only, see below. Gill rakers may reach the second adjacent raker when appressed and although usually
regular can be branched and variable in size along the arch. Vertebrae 31-34.
Head depth at the eyes is 0.9-1.2 head width between the upper origins of the opercles. The interorbital width is 0.8 to slightly less than eye diameter. The snout is longer than the eye, 1.1-1.4
times. The upper lip narrow slightly rearward and is about half the lateral preorbital area (between lip and eye). Caudal peduncle depth is two-thirds its length. The tongue is truncate or slightly
notched. The posterior teeth on the dentary bone are smaller than the anterior teeth and all are blunt. Pharyngeal teeth are molariform, adapted to crushing molluscs. The anal papilla is bifurcated at
the tip. The gut is short and roughly s-shaped with some convolutions, particularly on the posterior section.
The chromosome number for "N. m. affinis" is 2n=46 with 46 chromosomal arms (Ráb, 1985; Vasil'ev and Grigoryan, 1993; Simonovic, 1999).
Meristics for Iranian fish:- first dorsal fin spines 5(6) or 6(34); second dorsal fin soft rays 12(1), 13(2), 14(18), 15(18) or 16(1); anal fin soft rays 10(3), 11(17), 12(19) or 13(1); total pectoral
fin rays 17(11), 18(28) or 19(1); lateral series scales 48(1), 49(3), 50(8), 51(4), 52(8), 53(4), 54(5), 55(4), 56(2) or 57(1); total gill rakers 11(5), 12(15), 13(11), 14(7) or 15(2); total vertebrae ?.
Sexual dimorphism
Females are smaller than the male. Mature males have larger dorsal, pectoral and anal fins, swollen cheeks, and colour is different (see below).
Colour
Overall colour is yellowish-grey or brownish-grey (fawn) with 8-9 large, dark brown, blue-grey to black flank blotches. There are 4 saddles across the back. The first dorsal fin has a black blotch
posteriorly over the last two rays (and sometimes on the interradial membrane between rays 4 and 5), and the blotch or spot may have a white border in juveniles. The spot can be absent as reported by
Jude (1997) for fish introduced to the North American Lake Erie. The sides of the head have oblique, curved bands or a reticulate pattern of a rusty to gingery tint on a bluish-grey background. The
pattern is darker on the opercle and snout. The first dorsal fin may be tinged green and has 3 rusty-brown bands and a narrow margin may be a rusty or yellowish colour. The second dorsal and caudal fins
may have a light margin even in non-spawning individuals. The second dorsal fin has 3-4 rows of small rusty spots. The caudal fin also bears rusty spots anteriorly but not in very definite rows. The anal
fin is grey to dark brown. The pectoral fin lacks spots and is almost colourless but may be tinged with yellow. The pelvic disc is grey with a rusty colour in the centre. The genital papilla is grey or
unpigmented. The iris is yellow to orange. The peritoneum is a silvery brown.
Colour varies with habitat. In bays with algal growth fish are darker, blue-grey tints are lost, there are more yellowish-brown colours and there is an increase in number and size of dark-brown spots.
Others may have an olive-green tint.
When spawning, males are charcoal black overall, sometimes with a bluish tint, and with indistinct yellow spots. The dorsal, caudal and anal fins are white-edged or yellow, although the first dorsal
margin can be black and there may be practically no margin to the anal fin. The black spot on the first dorsal fin can still be seen against the light. There are no free tips to fin rays according to
literature reports but Iranian specimens have all fin tips separated to form a fringe.
The young are a slate grey overall with a mottled and blotched flank. The mid-flank blotches may form a series of about 6. There is a spot at the upper base of the pectoral fin. The dorsal fin spot is
centred on the membrane between the last two rays but spills over onto the adjacent membranes, particularly the posterior one. Fins have pigment on the rays and membranes with no clear pattern. The dorsal
fins are darker than the anal which is darker than the pectoral or pelvic. There is a bar from the lower eye margin to the upper lip just anterior to the mouth corner.
Size
Reaches 29.0 cm total length. Freshwater fish are smaller than marine ones.
Distribution
Found from the Aegean to the Black and Caspian seas. Introduced to the Aral Sea in the 1950s but increasing salinity in the 1980s eliminated the population. Now in the Baltic Sea via rivers and canals
or in ship ballast. Also present by introduction in the Great Lakes of North America where first reported in 1990, apparently from several sources (Brown and Stepien, 2006).
Reported from a wide range of rivers along the Caspian coast of Iran
including the Gorgan, Tonekabon, Safid, Dyusan, Shimrud, Chemkhaleh, Rud-e Sere,
Kargan, Navarus, Chalus, Babol and Haraz rivers, the Anzali Mordab, Gorgan Bay, the southeast Caspian Sea, southwest Caspian Sea and south-central Caspian Sea (Holčík and Oláh,
1992; Abbasi et al., 1999; Kiabi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004;
Abdoli and Naderi, 2009). Apparently absent from the Atrak River in Iran
and of uncertain presence in the Aras River (Abdoli
and Naderi, 2009).
Zoogeography
See genus account above.
Habitat
This species is found in inshore waters at depths to about 20 m, sometimes to 70 m or even 200 m in deeper sea areas in winter, on rock, gravel, shell, sand or silt bottoms. Aquarium specimens are seen
to hide in crevices and under rocks. In the Caspian Sea, it may be found among eelgrass, Zostera. It also enters rivers. Larvae live near the bottom. The species prefers littoral areas where wave
action keeps oxygen levels high and decaying material is reduced. It leads a sedentary life (Moskal'kova, 1996). It may be found from fresh waters to salinities of 40.5‰ from -1 to 32°C, and respiring
through the skin can tolerate low oxygen conditions from 0.3-0.9 ml/l (Moskal'kova, 1996; Jude, 1997).
In the North American Great Lakes basin, where it is an exotic, this species prefers rocky habitats and is more active during the day than the night. The mean density in one river was approximately 7
gobies/sq m and can reach as high as 90 gobies/sq m since they aggregate. This goby has a high site fidelity in mark-recapture studies (Ray, 1997; Ray and Corkum, 1997a).
The gobies can tolerate a flow of 0.34 m/s for 3-4 minutes but at higher levels they retreat to the bottom and brace themselves against the current using the pectoral fins.
The onshore spawning migration in the southern Caspian Sea occurs in spring, later than
P. syrman and P. gorlap. A spawning area is chosen on the basis of stones being present rather than
depth. Larger males approach the shore first. There is an offshore movement as winter begins and in the hottest part of summer.
Age and growth
Maturity is attained in the second year by females and in the third year by males but may be as early as 1 year. Life span can exceed 5 years for females. Males die after spawning. Faster growth
results in a shorter life span. At the end of the first year of life they can reach 5.5-6.0 cm (Moskal'kova, 1996). In North America, populations of this exotic are dominated by age one fish (MacInnis
and Corkum, 1997a). Growth is slower in North America and maximum size is less than in its native habitat, probably because temperatures are lower than in Europe (Jude, 1997).
Abdoli et al. (2006) examined fish from the Gomishan and Miankaleh
wetlands and found a length (L)-weight (W) relationship of W = 0.0091L3.17
for males, 0.0249L2.7 for females and 0.0112L3.08 for
sexes combined.
Food
Molluscs predominate with significant amounts of crustaceans in the north Caspian Sea but this can vary annually with crustaceans becoming predominant. They feed most heavily in the post-spawning
period in July and August (Opalatenko, 1979). Other important foods are bivalves, such as zebra mussels, snails, polychaetes, chironomids, other aquatic insects, goby eggs and small fishes. Attached
clams and mussels are bitten off the substrate by the large jaw teeth and crushed by the pharyngeal teeth (Moskal'ova, 1996). Molluscivory is also documented for Danube River populations (Simonovič
et al., 2001) and for Baltic Sea populations (Skora and Rzeznik, 2001) and molluscs appear to be the preferred diet wherever this species is found. An exception may be the southern Caspian Sea
where one study found crustaceans to predominate (Pinchuk et al. in Miller, 2003).
In the Caspian Sea, introduced species, such as crabs, the worm Nereis diversicolor and the mollusc Abra ovata, are also eaten (Kosarev and Yablonskaya, 1994). Iranian specimens contain
plant fragments, mollusc shells, shrimps, fish remains, aquatic insects and polychaetes.
In North America, immature round gobies are the most successful predators on round goby eggs. Round gobies are said to be aggressive and will take a baited hook. However this goby may need to be
stationary to detect prey. Nocturnal feeding occurs. The blunt teeth are indicative of a primarily mollusc diet.
Experimental studies in North America have shown this species to prefer individual and clumped zebra mussels to other foods such as sphaeriid clams. Feeding rates vary between 36 and 47 mussels
(4.5-12.5 mm long) per day for fish 6-10 cm long, over 100 mussels per day when the mussels are smaller than 4 mm (Ghedotti et al., 1995). Mussels can remain in the mouth and pharynx for less than
one hour to over 12 hours; shells are then split and ejected. However some are crushed and passed through the digestive tract. In the upper Detroit River, Canada, round gobies eat zebra mussels (58%),
snails (6%) and other invertebrates (36%) such as aquatic insects, softshelled crayfish and zooplankton. In laboratory studies, round gobies ate an average of 1 g of mussels in 24 hours, smaller mussels
being preferred (Ray and Corkum, 1997b). However, field studies in Lake Michigan show that round gobies are unlikely to remove zebra mussels (also a pest in North America) from a habitat (Djuricich and
Janssen, 2001). These gobies are also predators on lake trout eggs under laboratory conditions in North America (Chotkowski and Marsden, 1999). They are also reported to eat sturgeon eggs in North America.
Reproduction
There is a movement inshore to waters about 0.2-1.5 m deep for spawning, males preceding females. Spawning takes place at the end of April to September in near shore areas of the north Caspian Sea and
over a similar period in the Baltic Sea. Iranian specimens have well-developed testes on 10 April and spawning may occur earlier than in the North Caspian. There may be repeat spawning, up to 6 times
every 18-20 days, as indicated by captive specimens. Peak spawning occurs at 15°C (range 10-30°C). Eggs are attached to or under rocks, in cavities, under logs or in such objects as cans and are
aggressively guarded by the male. Early maturity, a very long spawning season and aggressive defense contribute to high egg, larvae and fry survival. The male prepares the nest site with a secretion from
a cement gland which is coated over the site. As the female lays each egg, it is glued to the underside of the nest cavity roof in single layer rows. Egg clutches can be transported on the hulls of ships
(Ahnelt et al., 1998). Fecundity reaches 6,177 eggs. Eggs are pale yellow, orange or pink, with red pigmentation. the eggs are ovoid with a sharp apex and measure 3.9 by 2.2 mm. Details of
development are given by Moskal'kova (1996).
In North America, the species has been shown to be a multiple spawner with an extended reproductive season and male guarding of eggs (MacInnes, 1997). Here maturity occurs as early as year one and 43
mm standard length, a year earlier than in their natural habitat. Spawning extends from May to early August. Mean fecundity is 198 eggs. Each nest is utilised by large numbers of females since as many as
an estimated 9462 eggs were found in each artificial nest site (MacInnis and Corkum, 1997b). Such a nest site can lose 50-70% of the eggs to predators while smaller clutches lose few eggs.
Jude (1997) notes that in North America some males do not defend a nest, the reason being unknown. This may well be due to such factors as availability of suitable spawning sites, large size or
aggressiveness. He reports that after reaching a large size, males spawn once and then die, although females spawn up to six times through spring and summer, about every 20 days. Elsewhere males guard
the nest from predators and fan the eggs to oxygenate them and to reduce siltation and fungal infection. Males will eat infected and unfertilised eggs. Males defend the nest by flaring the gills,
spitting sand, darting at nearby intruders, biting, and growling for 1.0-1.5 seconds loud enough to be heard 10 m away. Nest sites in North America may be as deep as 7 m (Corkum and Wickett, 1998).
Males make several sounds including one attracting females to nest sites and another to intimidate other males. Females respond to male calls by a quieter sound (Jude, 1997).
Parasites and predators
Fil mahi (Huso huso), chalbash (Acipenser gueldenstaedtii), uzun burun (Acipenser stellatus), mash mahi (Aspius aspius), ordak mahi (Esox lucius), suf
(Sander lucioperca) and seals are predators. Daghigh Roohi and Sattari
(2004) record Dichelyne minutus and Corynosoma strumosum from this
species in the southwestern Caspian Sea of Iran. Sattari et al. (2004; 2005) surveyed this species in the inshore area of the Caspian Sea, recording Dichelyne minutus.
Sattari et al. (2007) report the nematode Eustrongylides excisus
for this species in Iranian waters.
Economic importance
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use in aquaria, as food and because it has been introduced outside its natural range. It is
caught by anglers in some parts of the Caspian Sea basin.
It is present in the North American Great Lakes and is expanding (Ray and Corkum, 2001; Clapp et al., 2001; Jude et al., 1992; Jude, 2001). It is an introduction from the
ballast waters of ships from multiple sources, perhaps including the Black Sea (Dillon and Stepien, 2001). Round gobies may displace native fish, compete for food and eat their eggs and young, and are the
target of major efforts to study and contain them (Arrigoni and Berg, 2001; Charlebois et al., 2001; Diers et al., 2001; French and Jude, 2001; Janssen and Jude, 2001; Kaur et al.,
2001; Schreier et al., 2001; Wolfe, 2001). They may also accumulate and pass on large concentrations of such chemicals as PCBs to sport fishes in North America (Jude, 1997). An extensive literature
is now available on the Great Lakes populations.
It is an important food species in the Black Sea marketed fresh and formerly canned in tomato sauce. In the Sea of Azov in 1950-1960, gobies made up the bulk in local fisheries and round gobies
comprised almost 90% of a catch exceeding 90,000 t (Moskal'kova, 1996). Commercial catches in the Caspian Sea have comprised up to 88.2% of goby catches but pollution has severely depleted stocks
(Pinchuk et al., 2003).
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include medium numbers, habitat destruction, widespread range
(75% of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin (as N. m. affinis).
Further work
Biology in Iran has not been studied as well as elsewhere.
Sources
Charlebois et al. (1997) and Pinchuk et al. (2003) give general reviews of biology and are excerpted briefly above.
Iranian material:
Neogobius pallasi
(Berg, 1916)
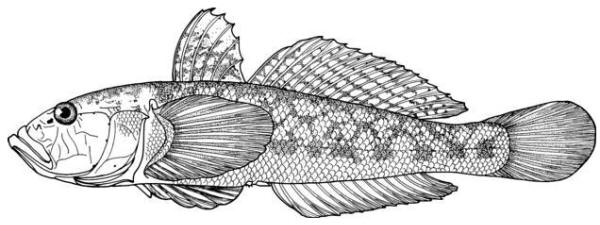
Common names
gavmahi-ye tireh (= dark goby), gel-ye mahi
rudkhanehai (= river goby), gavmahi-ye sheni, sebele.
[gumlur xulu in Azerbaijan; Kaspiiskii bychok-pesochnik or Caspian sand goby, rastrepka (= a person with untidy hair in reference to the fringed ends of the fin rays), chernysh (= darkie), khylak (when
emaciated), all in Russian; Caspian monkey goby].
Systematics
Gobius fluviatilis was originally described in part from near the mouths of rivers falling into the Black Sea and similarly the Caspian Sea. No types are known.
Neogobius fluviatilis pallasi (Berg, 1916) was the subspecies of the Caspian Sea basin. Kottelat and Freyhof (2007) recognise pallasi as the Caspian Sea species and restrict fluviatilis
to the Black Sea basin. The type locality is the Caspian Sea, mouths of the Ural and Volga and syntypes are in the Zoological Institute, St. Petersburg under ZISP 2195, 2204, 23137, 30729, 30736, 30738,
30919, 30920, 30924-26. 33182, 34276, 34277 (Eschmeyer's "Catalog of Fishes", downloaded 23 August 2007).
Abd Elahpour Biria et al. (2009) examined fish from 4 localities on
the Gilan coast using morphometric characters and found 3 different populations
in Astara, Anzali and Chamkhaleh and Chaboksar.
Key characters
Pelvic fin anterior membrane with rounded and shallow lateral lobes. less than one-fifth rear edge width; pelvic fin disc 0.9 to more than distance to anal fin origin; nape with ctenoid scales; first
dorsal fin without large dark spot (except in juveniles); lateral series scales usually 55-70; pelvic fin membrane anterior lobes small, rounded; angle of jaw anterior to orbit; and two transverse
infraorbital papillae rows below longitudinal hyomandibular row b.
Morphology
The head width (between upper origins of opercles) is about equal to head depth (at eyes). The interorbital distance is not more than 0.75 eye diameter. The top of the head and the occiput are scaled
with ctenoid scales. The upper lip is expanded towards the mouth corners, sometimes narrowing at the very end. The angle of the jaws reaches back at a level between the eye and the posterior nostril. The
snout is longer than the eye diameter (1.6 times). The upper lip is not swollen at the angle, and measures 0.4-0.6 lateral preorbital area (between lip and eye). The first dorsal fin is high with an acute
anterior profile, the first branched ray about twice as long as the penultimate ray. The pelvic fin has small, rounded lateral lobes (< 0.2 rear edge width) and almost reaches the anal fin (0.9
distance) or extends beyond the anal fin origin. The caudal peduncle depth is 0.5-0.6 length.
First dorsal fin spines 5-7, usually 6, second dorsal fin with 1 spine followed by 11-18, usually 15-17, soft rays, anal fin with 1 spine and 11-17 soft rays, usually 13-15, and pectoral fin rays 16-19.
Lateral line scales 49-69, mostly 52-65. Gill rakers 6-10. Vertebrae 31-35. The chromosome number is 2n=46, with 46 acrocentric chromosomes and 46 chromosomal arms (Grigoryan and Vasil'ev, 1993; Klinkhardt
et al., 1995; Simonovic, 1999).
N. pallasii is distinguished by the dorsal fin spot in young fish and an average number of lateral series scales being 53 as opposed to 62 for Black Sea fish.
Sexual dimorphism
See under colour. Males become slimmer at spawning and their are morphometric differences between the sexes (Pinchuk et al. in Miller, 2003).
Colour
Overall colour is pale and sandy, in cantrast to other gobies. On the flanks and over the back there is a series of 8-9 brownish elongate spots or blotches. The belly, lower head and genital papilla
are pearly white. A dark bar runs from the eye antero-ventrally to the lip. The first dorsal fin has a dark spot posteriorly, although it may be absent in some adults, and is characteristic of the
former subspecies pallasi, and found in small fish in the southern Caspian, persisting in adults in the northern Caspian (Pinchuk et al. in Miller, 2003).
The first dorsal fin has 1-2 rows of grey to black spots and the second dorsal fin has 1-3 rows of pale grey spots. The caudal, anal, pectoral and pelvic fins are mostly grey on the rays and immaculate.
Breeding males become black or blue-black with the dorsal, caudal and anal fins bearing a yellow-orange margin. Some fish have a very narrow colourless margin to the pectoral and pelvic fins. The second
dorsal fin may only be yellowish on its first ray, the remainder being white and the caudal margin may be colourless. Rays in the second dorsal fin of breeding males become extended into free tips.
Size
Reaches 24 cm total length although in the Caspian the largest fish are 16.0
cm males (Pinchuk et al. in Miller, 2003).
Distribution
Found in the Caspian Sea and its basin. In Iran, it is found in rivers along the Caspian coast including some upper reaches, the
Aras River middle reaches and its tributary the Qareh Chai, the Anzali Mordab, Gorgan Bay,
at Bandar Gaz, in the Gorgan, Madarso (or Madar Su), Tajan, Babol, Haraz, Sardab,
Tonekabon, Pol-e Rud and Safid rivers (including their middle to upper reaches
such as the lower Qezel Owzan and Shahrud of the Safid River basin), the southeast Caspian Sea, the southwest Caspian Sea and the south-central Caspian Sea (Shamsi et al., 1997; Abbasi et al., 1999; Kiabi et
al., 1994; 1999; Abdoli, 2000;
Abdoli and Naderi, 2009).
Note that Abdoli (2000) records it from the upper Atrak River in contrast to
earlier authors.
Zoogeography
The southern Caspian Sea and its tributary rivers is inhabited by a distinct subspecies, presumably on account of isolation from populations in the Black Sea. See genus account above.
Habitat
This species is found in inshore brackish waters and enters rivers. Salinities up to 46.9‰ are tolerated in marine bays. It is found down to 25 m although depths of 0.5-10 m are preferred, shallower
in spring (0.5-5.0 m), slightly deeper in summer and deeper still in fall (5-10 m)(Pinchuk et al. in Miller, 2003). It is found over sand, mud and sandy-shelly bottoms. In the Madarsoo stream of
Golestan National Park, abundance ranged from 0.1 to 0.75 fish per sq m and biomass from 0.21 to 4.88 g/m2 (Abdoli et al., 2002). Floods in this stream reduced the population, presumably
through destruction of the substratum and its food supply. Smaller fish were caught in riffles while larger fish lived close to to the river bank in depths up to 0.5 m. Abdoli and Rahmani (2001) found this
species to represent 95% of the goby catch in the Madarsoo stream, with N. melanostomus occupying the remainder.
Age and growth
Sexual maturity is attained at 2 years of age (9-12 cm for males, 8.5-10.5 cm for females) but may be as early as 1+ year (5.5 cm), particularly in the Caspian. Spawning males near Bandar-e Anzali were
12.4-15.2 cm long. Life span is 6 years but perhaps only 2+ years in the Caspian (Pinchuk et al. in Miller, 2003). Abdoli et al. (2002) found a sex ratio of 1 male to 3 females in the
Madarsoo stream. Patimar et al. (2008) reported 5 age groups in fish from
the Zarrin-Gol River in the eastern Alborz Mountains. Maximum total lengths were
12.1 cm in males and 10.8 cm in females, both 4+ years old. Fish aged 2+ and 3+
years were the most frequent in the population. sex ratio was equal and growth
was negative allometric. Condition factor was highest in early September. Males
showed a higher growth rate than females after age 1+ years. Abdoli et
al. (2006) examined fish from the Gomishan and Miankaleh wetlands and found
a length (L)-weight (W) relationship of W = 0.0059L3.291 for males,
0.0128L2.92 for females and 0.0057L3.29 for sexes
combined.
Food
Diet items include crustaceans with lesser amounts of molluscs, polychaetes, chironomids and small gobies in the north Caspian Sea. They feed most heavily in the post-spawning period in July and August
(Opalatenko, 1979). The quantity of amphipods eaten increases with age while the amount of mysids decreases. Introduced species, such as crabs, the worm Nereis and the mollusc Abra, are also
eaten (Kosarev and Yablonskaya, 1994). In the Madarsoo stream of Golestan National Park in Iran, Ephemeroptera and Chironomidae are the most important food items but also included Trichoptera, other
aquatic and terrestrial insects, crustaceans (including freshwater crabs), arachnids, worms and snails (Abdoli and Rahmani, 1999; Abdoli and Rahmani, 2001; Abdoli et al., 2002).
Alavi Yeganeh and Kalbasi (2006) found fish in shallow water at Noor fed on
amphipods, fish larvae, barnacles, palaemonid shrimps, mytilid bivalves, xanthid
crabs, cardid bivalves, fish eggs and oligochaete worms, in order of preference.
Diet varied with season, males feeding less during spawning for example Patimar et al. (2008) found the most frequent food items of fish in the
Zarrin-Gol River were trichopterans, molluscs, chironomids and gammarids.
Reproduction
Spawning can take place from late April to September, varying with locality. Peak spawning in the Volga River delta is in May. Spawning is recorded at 18-26ºC, 20-28‰ and 0.5-1.3 m (Pinchuk
et al. in Miller, 2003). In the Madarsoo stream of Iran, spawning peaked in March with an absolute fecundity of up to 532 eggs (Abdoli et al., 2002). Fecundity reaches 1025 eggs in the
southern Caspian (perhaps 12,800 eggs elsewhere) and egg diameter 2.1 mm. Yellow-amber or pale pink eggs are laid on or under stones and plants. Nests are excavated by the male under natural or artificial
objects. The male defends the nest and larger males are more successful. However, the stress associated with constant defense against predators and competing males leads to extreme emaciation. Nests
contain more eggs than fecundity would indicate suggesting that more than one female lays eggs in a nest (Pinchuk et al. in Miller, 2003).
Patimar et al. (2008) found the Zarrin-Gol River population to have an
average absolute and relative fecundity of 508.47 eggs and 61.27 eggs/g. The
reproductive season was March to April.
Parasites and predators
Pazooki and Aghlmandi (1998) found the nematode Dichelyne minutus infecting the intestine of 6.6% of fish examined from the Tajan River in Mazandaran.
and Sattari et al. (2002) and Sattari (2004) records the presence of the
nematode, Eustrongylides excisus, in this species. This parasite can damage muscles in commercial species and render them unsuitable for sale. Fil mahi (Huso huso), chalbash (Acipenser
gueldenstaedtii) and suf (Sander lucioperca) are predators. Sattari et al. (2004; 2005) surveyed this species in the inshore area of the Caspian Sea, recording Eustrongyloides excisus
and Dichelyne minutus.
Daghigh Roohi and Sattari (2004) record Eustrongylides excisus,
Dichelyne minutus and Corynosoma strumosum from this species in the
southwestern Caspian Sea of Iran.
Economic importance
This is the most economically important goby in the north Caspian Sea according to Berg (1948-1949).
Iljin (1956) considered this an error; it is the most numerous species but commercial fishing does
not occur there. Pinchuk et al. in Miller (2003) state however that it comprised 54% of the entire goby catch, although only 7.7% in southern Dagestan. It is food for sturgeons
and Sander lucioperca.
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include abundant in numbers, habitat destruction, widespread
range (75% of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin (as N. f. pallasi).
Further work
On of the better known gobies in Iran but could still use work on its biology.
Sources
Genus Periophthalmus
Bloch and Schneider, 1801
This mudskipper genus is found in the eastern Atlantic, Indian and western
Pacific oceans. There are 12 species in the genus as yet undefined by a
synapomorphy (Murdy, 1989). The pelvic fins are only partially united or may be
totally separate. There are usually 12-14 pectoral fin rays, 4-17 first dorsal
fin spines, and 8-13 second dorsal and anal fin rays. Scales are cycloid and
cover the whole body and head except for the snout, isthmus and interorbital
region in most species. Caninoid teeth are present in both jaws as a single row,
with the anterior ones larger and pointed. The eyes are erectile and have a
dermal cup covering the lower portion. There is a median fleshy ridge anterior to the eyes.
Mudskippers are found on muddy shores associated with mangroves but
also venture into rivers. They spend much time out of the water.
Periophthalmus waltoni
Koumans, 1955
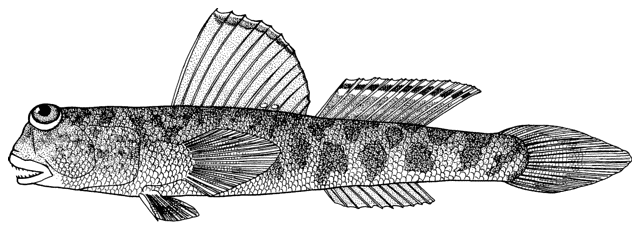
Common names
gel-cheragh (= mud-eater, mud-grazer), mahi-sag (= dog fish), gel khorak.
[shelambo, abou-shlembo or abu-shelamboo in Arabic; gullo in
Pakistan; dark-blotched mudskipper, Walton's mudskipper, spotted mudskipper].
Systematics
This species was originally described from Iraq and Pakistan. The
type locality is unclear as the information given by Koumans (1955)
gives more than one locality, as follows:-
"Habitat.-Mesopotamia, Karachi.
I have seen 2♂ and 2♀. These were from the following localities:-
Indian Museum Collection.
Fao estuary of the Shatt-el-Arab, Mesopotamia, ♀ H. J. Walton.
Karachi ♂ and ♀
W. D. Cumming.
Mouth of Shatt-el-Arab ♂
W. T. Blanford.".
The type locality could be Fao and the holotype is the female specimen caught by H. J. Walton and named for him, the remainder being paratypes
- by implication. Alternatively and probably correctly, these are all syntypes as no holotype was formally designated. The date of publication of
Koumans work is 1955, not 1941 as cited by Murdy (1989).
This species has been confused with Periophthalmus koelreuteri
(Pallas, 1770), itself a synonym of Periophthalmus barbarus (Linnaeus,
1766) according to Murdy (1989). P. barbarus does not occur in
the Persian Gulf region. P. waltoni has been placed in the
synonymy of P. koelreuteri in works on Persian Gulf fishes,
e.g. by Relyea (1981), but the revision by Murdy (1989) is followed
here. Another mudskipper species listed in Coad (1991b), Periophthalmus
weberi Eggert, 1935, should be deleted from the list as Murdy
(1989) does not report it from the Persian Gulf.
Two syntypes of Periophthalmus waltoni are in the
Rijksmuseum van Natuurlijke Historie, Leiden (RMNH 17004) and 4
syntypes are in the Zoological Survey of India, Calcutta (possibly lost) (Murdy, 1989).
However, this is more fish than listed in Koumans (1955).
Key characters
The pelvic fins are totally united into a sucker with a
well-developed basal membrane or frenum (absent in P. barbarus).
The anal fin base and second dorsal fin base are 27% or less of
standard length and there are no canine teeth internal to the lower
jaw symphysis in contrast to Boleophthalmus, the other mudskipper of southern Iran.
Morphology
First dorsal fin spines 10-14, second dorsal fin with 1 spine and
12-14 soft rays. Anal fin with 10-13 soft rays. Pectoral fin rays
13-15. Scales in lateral series 90-121, predorsal rows 27-37. The
upper jaw has 4-5 anterior teeth on each side much larger than lateral
teeth. Upper jaw teeth 19-28, lower jaw teeth 17-23. Gill rakers 9-16,
mean 11, on each arch, triangular in shape with 3-4 spines at the top
of each raker (Barak et al., 1994). The first dorsal fin lacks
elongate spines and is slightly rounded. The dorsal fins are not
connected by a membrane. The gut shortens with growth in terms of body
length indicative of a carnivorous habit (Barak et al., 1994).
Sexual dimorphism
Females have a lozenge-shaped first dorsal fin (i.e. anterior 6
spines equal in height followed by abruptly shortened ones) with a
height less than body depth; in males this fin equals body depth.
Colour
The overall colour is a light grey-brown. The flank bears blotches
which, below the soft dorsal fin, take on the form of 8 short bars and
there is a scattering of small silvery or white dots. Ventrally the
spots form roughly transverse silvery stripes. The head top is also
blotched and the sides bear a few small white dots. There are 7
irregular black blotches along the back. The first dorsal fin is dusky
brown to light grey and its margin is yellowish between spines 1 to 8
and dark just below this except for the posteriormost 3-4 spines.
There is a series of light spots on each interray membrane on the
basal half of the fin. The second dorsal fin is dusky brown, with a
pale margin, followed by a black stripe, and then with a white spot at
the middle of each membrane thus forming a horizontal dotted line
along the mid-fin. The caudal fin is a dusky brown, darker basally.
There are 3 black blotches on the caudal peduncle. The pectoral fins
are a light dusky brown on the rays with the membranes on the upper
half pale with dark, brownish streaks in mid-fin, and a whitish margin
is present. The pelvic fins are blackish with a reddish-brown or pale margin.
Size
Reaches 20.0 cm total length (Mhaisen and Al-Maliki, 1996).
Distribution
Found from the Persian Gulf to Pakistan. Reported from the Shatt al
Arab, Iraq near Iranian Khuzestan by Khalaf (1961), Al-Nasiri and Hoda
(1975b, 1976), Al-Hassan et al. (1989), and Hussain et al.
(1989; 1997). Specimens believed to be this species have been reported
from Iranian fresh waters but no specimens have been examined. Holly
(1929b) records this species from the type locality of Barbus
baschakirdi in the Bashakird Mountains (see Cyprinion watsoni). The
Museum of Comparative Zoology, Harvard has a Periophthalmus species
recorded from Manyuhi on the Shatt al Arab in irrigation ditches (MCZ 149602) but
the identity of this specimen needs to be confirmed.
Abdoli (2000) maps this species from the Arvand River in the Tigris River
basin, the lower Zohreh (= Hendijan), lower Helleh and Mand rivers in the Gulf
basin, the Kul and Mehran rivers of the Hormuz basin, and the lower reaches of rivers of
the Makran from the Jagin to the Bahu Kalat.
Zoogeography
This species has ready access to the sea and can colonise rivers along the Iranian coast.
Habitat
The habitat and behaviour of this species is similar to Boleophthalmus
dussumieri (q.v.; Tytler and Vaughan (1983) review behaviour of this
species in Kuwait, their Periophthalmus koelreuteri being this species
according to Murdy (1989)). However it favours areas of mud which are drier on
the upper and middle regions of the shore. The burrow is Y-shaped with two
entrances and a depth of 30 cm in mud. Fish occupy several burrow systems within
a 2-3 m home range (Clayton and Snowden, 2000). Young first appear on mud flats
in April but stay in wet mud or shallow pools. They move onto drier areas by May
after some growth (1.5-2.5 cm). There are up to 30 fish per 100 sq m on Kuwait
mud flats. The activity range of body temperatures is 14-20°C in January and
26-34.5°C in June. Body temperatures do not exceed 35°C even when air
temperatures exceed 40°C because of evaporative cooling. In tidal pools of a few
centimetres depth in the Khawr az Zubayr, water temperatures ranged from 16 to
44ºC, pH from 7.6 to 8.7, dissolved oxygen from 5.2 to 8.8 mg/l and salinity
from 10.2 to 20.2‰ (Al-Daham and Al-Noor, 2000). Holes from 50 to 80 cm depth,
with two openings separated by not more than 50 cm, are constructed as refuge
from extreme temperatures, high winds and predators. The mud is carried to the
outside of the hole in the fish's mouth, and forms a dam-like structure around
the shelter not exceeding 8 cm in height. The fish spends most of the ebb tide
outside the shelter.
As well as being found on mudflats of the sea shore, this
mudskipper is found in the Shatt al Arab and other rivers of the Persian Gulf.
Age and growth
Barak et al. (1994) studied this species in the Khawr az Zubayr, Iraq and
found growth to be allometric with the regression coefficient significantly
(p<0.05) greater than 3. (W = 1.74x10-4 L2.35 (r = 0.99)).
Al-Daham and Al-Noor (2000) found the smallest mature males were 7.3 cm and
females 7.4 cm in the Khawr az Zubayr with maturity at age one year.
Food
Fiddler crabs are stalked as the main prey of this mudskipper in Kuwait
(Tytler and Vaughan, 1983) but shrimps and other invertebrates are also taken.
High wind speeds and surface temperatures below 15ºC inhibited hunting. Hunts
were spatially well separated because of the long time period before crabs
returned to the surface after a hunt in their vicinity (Clayton and Snowden,
2000). On the intertidal mudflats of Iraq at the head of the Persian
Gulf, this mudskipper feeds predominately on fish when larger than 90 mm
(frequency 78.9%, occurrence 80.0%) with shrimps (14.1%, 20.0%), insects (3.2%,
10.0%), copepods 2.1%, 5.0%) and Daphnia sp. (1.7%, 5.0%). Fish smaller
than 80 mm had values much lower for fish (6.7%, 8.3%) and higher for copepods
(75.1%, 83.3%) with Daphnia sp. at 13.3% and 16.6%, insects at 2.7% and
4.1% and shrimps at 2.2% and 4.1%. Barak et al. (1994) found this species
to be cannibalistic and piscivorous in the Khawr az Zubayr based on gill raker
gap, gut-length/body length ratios decreasing with size, and stomach contents.
Copepods were the major food item of smaller mudskippers (<70 mm) while
mudskippers larger than this took fish. Mhaisen and Al-Maliki (1996) report food
items as percentage of occurrence from fish caught in the Khawr az Zubayr estuary
of Iraq to be 67.2% crustaceans (shrimps, crabs, barnacles and others), 34.5%
snails, 3.4% insects and 1.7% fishes. Some fish consume plant material in winter
months only, presumably in the absence of animal food.
Reproduction
Spawning in the marine environment of the Khawr az Zubayr takes place in
March, the release of larvae being timed to take advantage of phytoplankton and
zooplankton blooms (Hussain and Ahmed, 1999). Al-Daham and Al-Noor (2000) give
the spawning period as March to April in the Khawr az Zubayr with a fecundity of
11,560-35,005 eggs for fish 7.4-12.9 cm long. A water temperature over 18ºC was
the primary factor for spawning stimulation.
Parasites and predators
Mhaisen and Al-Maliki (1996) give information for Iraqi fish. E. Kahrom in
the Crocodile Specialist Group Newsletter reports that muggers (marsh crocodile,
Crocodylus palustris) eats a Periophthalmus in the Sarbaz River of
Iranian Baluchestan (www.flmnh.ufl.edu/natsci/herpetology/newsletter/news173b.htm,
downloaded 15 February 2002). However this may well be Boleophthalmus dussumieri.
Economic importance
None.
Conservation
No study of the numbers of this species in Iranian waters has been carried out so its status cannot be assessed.
Further work
The biology and population numbers of this species in Iran needs study.
Sources
The colour description is based on Kuronuma and Abe (1986).
Iranian material: None seen.
Comparative material: CMNFI 1985-0183, 2, 35.1-40.7 mm standard length, Iraq, Shatt al Arab (no other locality data);
BM(NH) 1954.11.10:1-2, 2, 112.6-119.0 mm standard length, Iraq, Fao (29º58'N,
48º29'E); BM(NH) 1976.12.8:1-2, 76.1-84.1 mm standard length, Iraq, Basrah (30º30'N, 47"47'E);
BM(NH) 1981.3.19:7-10, 4, 91.3-104.3 mm standard length, Kuwait mudflats (no
other locality data).
Ponticola
Iljin, 1927
Ponticola cyrius
(Kessler, 1874)
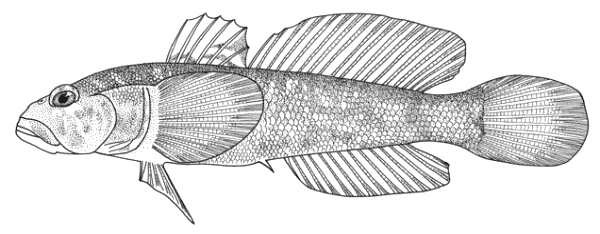
Common names
gav mahi rud-e Kura (= Kura River goby) or gavmahi-ye Rud-e Kurosh.
[Kura goby, Kura River goby].
Systematics
Studies by Vasil'eva (1995a) and Vasil'yeva and Vasil'yev (1995) indicate that gobies from the upper and middle Kura River described as Gobius cyrius Kessler, 1874 and Gobius Weidemanni
Kessler, 1874, and identified by various authors as Gobius platyrostris, Gobius constructor, Gobius platyrostris cyrius, Gobius cephalarges constructor, Neogobius
cephalarges constructor and Neogobius platyrostris constructor should all be referred to
Neogobius (now Ponticola) cyrius.
Gobius cyrius was described from the Kura River near Borzhom, Georgia and 3 syntypes are in the Zoological Institute, St. Petersburg under ZISP 2235 (Eschmeyer et al., 1996). One of
these, a male 103 mm total length and 84 mm standard length, is designated as the lectotype by Vasil'yeva and Vasil'ev (1995). Two syntypes of Gobius Weidemanni described from Transcaucasia are
under ZISP 2224.
Key characters
The pelvic fin anterior membrane has large, angular lateral lobes, at least one-fifth width of rear edge and pelvic fin less than nine-tenths distance to anal fin; the posterior nostril is near the
edge of the orbit; lateral series scales usually 54-76; upper lip moderately swollen, width about 0.75 to more than length lateral preorbit; and interorbital distance 0.4-0.8 eye diameter.
Morphology
The head width is much greater than its depth. The interorbital distance is not more than half the eye diameter. The top of the head and the occiput are scaled with cycloid scales. The upper lip is
expanded towards the mouth corners, sometimes narrowing at the very end. The lower jaw protrudes and the angle of the jaws reaches back below the anterior half of the eye. The snout is longer than the
eye diameter (1.3-14 times). The upper lip is swollen at the angle. The second dorsal fin is of equal height along its length or rising slightly higher at its middle or end. The pelvic fin has a
well-developed membrane with pointed lobes and does not extend to the anal opening. The caudal peduncle is compressed, depth about 0.30-0.35 length. The caudal fin is usually rounded but may be pointed,
truncated or scalloped. The predorsal area is marbled and the first dorsal fin has a distal dark band.
First dorsal fin with 6-8, usually 6, spines, second dorsal fin with 1 spine and 15-20, usually 18-19, soft rays, and anal fin with 10-16, usually 14, soft rays (but see below), pectoral fin rays 17-19,
and lateral line scales 53-78. Vertebrae 33-34. The chromosome number is 2n=36-42 (Klinkhardt et al., 1995).
Meristics in Iranian material: first dorsal fin spines 6(38), second dorsal fin with 15(7), 16(28) or 17(3) soft rays, anal fin with 10(1), 11(8), 12(26) or 13(3) soft rays, and pectoral fin rays 17(3),
18(28) or 19(7). Lateral line scales 54(2), 55(5), 56(7), 57(11), 58(6), or 59(4).
Diploid chromosome number 36-42, chromosome arms 46, polymorphism seen in a pair of subteleocentric chromosomes, the number of which varies from 0 to 2.
Sexual dimorphism
Males may not become black during the breeding season (Vasil'yeva and Vasil'yev, 1995). There are various differences in morphometric characters, summarised in Vasil'eva and Vasil'ev in Miller (2003).
Colour
The body is marked by a reticulate or marbled pattern in dark fawn, by scattered light spots and by 8-10 elongate dark spots along the lateral line. The back anterior to the dorsal fin is marbled. The
belly is paler than the flank. The first dorsal fin is dark, particularly in the upper part where the darkening may form a band, with a transparent margin and a conspicuous black spot anteriorly
below the tip of the fin. The first dorsal, caudal and anal fins have light margins but the fins are mostly dark. The light edge is obvious in the anterior parts of the first dorsal and anal fins. Only the
second dorsal fin ray tips are light. The pectoral and pelvic fins are both uniformly dark.
Size
Attains 101.3 mm standard length, 13.0 cm total length.
Distribution
This species is found in the Caspian Sea basin including the Anzali Mordab and tributary rivers,
the Massuleh River at Siahbar, and the Pasikhan River just above confluence with
Pirbazar River and a tributary near Islamabad (Ahnelt and Holčík, 1996;
Abdoli and Naderi, 2009) and the upper and Middle Kura River and upper Aras River of the former U.S.S.R. (Berg, 1948-1949; Vasil'eva and Vasil'ev in Miller, 2003).
Zoogeography
This species is distributed in rivers of the Caspian Sea basin in Azerbaijan and Iran while Neogobius constructor, with which it has been confused, is found in the Black Sea basin. The absence
of P. cyrius from the lower Kura River could have been prevented by the prior penetration of
P. gorlap according to Vasil'eva and Vasilev (1995).
Vasil'eva and Vasilev (1995; 1996a) consider that Sarmatian marine ancestors of modern
P. cyrius and N. constructor most probably moved into fresh water during the Miocene, about 5 MYA.
Mountain building in the Caucasus isolated the Kura and other rivers which once flowed to the Black Sea and their course became oriented to the Caspian Sea. Isolation of the gobies resulted in speciation.
Ahnelt and Holčík (1996) present an alternative view. They place the origin of this species in the Quaternary when fresh water from melting glaciers connected the Black and Caspian seas via the
Manych Channel allowing freshwater fishes to disperse. This occurred four times during the Pleistocene, the last time about 14-12,000 years BP. A goby species was able to colonise both fresh and brackish
water and particular stocks began to favour certain habitats. Those stocks which favoured headwaters evolved into
P. cyrius while those which favoured lower regions and coastal areas evolved into
P. gorlap. Ahnelt and Holčík (1996) point out that there is an analogous situation in the Black Sea with N. constructor and the species N. rhodioni
Vasil'yeva and Vasil'yev, 1995. Additionally all west Caucasian and Caspian riverine gobies are very similar morphologically as evidenced by the difficulty in constructing reliable keys, suggestive of
recent differentiation about 11,000-9,000 years BP after the isolation of the Black and Caspian seas.
Habitat
This small goby is found only in freshwater in mountain and foothill zones in contrast to
P. gorlap. It is found in shallow (3 m or less) streams with a high gradient and current speed (1.6-4.9
m/sec on the bottom) in Iran (Ahnelt and Holčík, 1996). However, Vasil'yeva and Vasil'yev (1995) record it as absent in areas of strong current in the Kura River, preferring slow current and
muddy bottoms. This type of habitat is limited in Iranian rivers which are much shorter and smaller than those of the Caucasus where an extensive lowland course is found. In Iran, the stream bed is
composed of stones and boulders with some pebbles in the upper reaches and pebbles and gravel in the lower reaches. Small body size is an advantage for hiding under rocks to avoid predators and to escape
the rapid current.
Age and growth
Life span is up to 3 years with maturity attained in the second or third year (Vasil'eva and Vasil'ev in Miller, 2003).
Food
Vasil'eva and Vasil'ev in Miller (2003) report feeding on insect larvae and parts of plants.
Reproduction
This species may contain eggs in three size groups, the largest being 2.81 mm in diameter, indicative of batch spawning. Total fecundity reached 1060 eggs. Spawning occurs from May to August at 11-20ºC,
in some localities reported only for April-May (Vasil'eva and Vasil'ev in Miller, 2003).
Parasites and predators
Unknown.
Economic importance
None reported although in Azerbaijan some are caught by anglers.
Conservation
Further field work is necessary to assess numbers and status of this species.
Further work
The biology, distribution and conservation status of this species, recently recorded from Iran, remain to be examined in detail.
Sources
Counts and data are based on Ahnelt and Holčík (1996).
Ponticola goebelii
(Kessler, 1874)
Common names
gavmahi-ye ratan, gel-ye mahi ratan. ?name cahnge
[Kaspiiskii bychok-rotan or Caspian ratan or rotan goby in Russian].
Systematics
The Caspian Sea basin subspecies was usually referred to Neogobius ratan goebelii (Kessler, 1874). Gobius Goebelii
Kessler, 1874 was described from Baku, Azerbaijan and Gobius ratan was originally described from Odessa on the Black Sea.
Gobius Bogdanowi Kessler, 1874 described from the Caspian Sea at Makhachkala (Petrovsk) is a synonym (the male of N. ratan goebeli according to
Iljin (1956) although Pinchuk (1991) and
Ragimov (1998a) consider it to be a subspecies of N. ratan). Pinchuk (1976) notes that fish
from the Azerbaijan and Iranian coast differ from those from Dagestan and Turkmenistan, the first having fewer scales, but the number of specimens were insufficient to define taxa.
Gobius Goebelii has a syntype in the Naturhistorisches Museum Wien under NMW 33910 and others possibly in the Zoological Institute, St.
Petersburg (ZISP 2229-30) (Eschmeyer et al., 1996; Pinchuk et al., 2003). Two probable syntypes of Gobius ratan are in the Muséum national d'Histoire naturelle, Paris under MNHN A.1125 and a syntype is in the Zoologisches Museum Berlin (Museum für Naturkunde,
Universität Humboldt, Berlin) under ZMB 2098 (Bauchot et al., 1991).
Two syntypes of Gobius Bogdanowi are possibly in the Zoological Institute, St. Petersburg (ZISP 10902) (Eschmeyer et al., 1996; Pinchuk et al., 2003).
Key characters
Pelvic fin anterior membrane with angular lateral lobes, not more than one-fifth width of rear edge; pelvic fin almost reaches the anal fin (0.9 distance) or extends beyond the anal fin origin;
posterior nostril near edge of orbit; anterior nape with cycloid scales; rear of first dorsal fin without a dark spot; lateral series scales usually 49-54; and upper lip width 0.4-0.67 lateral preorbital
width (lip to orbit).
Morphology
Pelvic fin anterior membrane with angular lateral lobes, rather than rounded; lobes small, not more than 0.2 width of rear edge; posterior nostril near edge of orbit; angle of jaw below pupil of eye;
upper lip slightly swollen posteriorly, 0.5-0.67 lateral preorbital area (between lip and eye); first dorsal fin with sub-horizontal upper profile; rear of first dorsal fin without a dark spot; head depth
at eyes slightly less to somewhat greater than width between upper origins of opercles; snout length about 0.9-1.2 orbit; lateral series scales usually 52-61; interorbital width 0.4-0.6 eye diameter; nape
scales cycloid; pelvic fin almost reaches the anal fin (0.9 distance) or extends beyond the anal fin origin; the caudal peduncle depth almost equals its length, the posterior nostril is close to the eye
margin, and colour dark brown with few spots (spawning males black). The two subspecies listed above are distinguished partly by scale counts, 63-66 in bogdanowi and 63 or less in goebeli.
First dorsal fin with 5-7, usually 6, spines, second dorsal fin with 1 spine and 15-19 soft rays, anal fin with 1 spine and 11-15 soft rays, and pectoral rays 17-21. Range in lateral series scales
49-70. Vertebrae 32-34.
Sexual dimorphism
Male colouration is distinctive and they have a higher dorsal fin.
Colour
Overall colour is dark brown with a few, discrete, rounded pale spots and, along the mid-flank, a series of small horizontal bars. Pale saddles cross the back. The first dorsal fin has a yellow or
intense orange margin above a black bar (white in preserved fish). This fin also has a dark blotch, often with a bluish tint, on the first and second and second and third interradial membranes, and
sometime son the third and fourth interradial membrane. The second dorsal fin is greyish with 3-4 rows of brown spots proximally, and it may have a pale margin. The pectoral and caudal fins have rows of
rusty-brown spots proximally. The pelvic fin disc and the anal fin are grey with a broad pale margin. The belly is grey. Breeding males become black with a bluish tint.
Size
Reaches 23 cm.
Distribution
Found in the Black and Caspian seas including Iranian waters of the latter.
Zoogeography
See genus account above.
Habitat
This species lives in inshore waters over rocks, gravel and stones, although a spawning male was caught over silt near the Kura River, and a female was caught at 11 m at Krasnovodsk (Pinchuk
et al. in Miller, 2003). It rarely enters fresh waters, at least in the Caspian.
Age and growth
Sexual maturity is attained at 2 years.
Food
The main food items are crustaceans, with smaller quantities of worms, molluscs and fish.
Reproduction
Spawning takes place from the end of March to the end of May, with a single repeat spawning. Eggs are deposited on and between stones. Females contain 22-360 ripened eggs (Pinchuk et al. in
Miller, 2003).
Parasites and predators
None reported from Iran.
Economic importance
None as it is disliked by commercial and sport fishermen and is not common in the Caspian Sea (Pinchuk et al. in Miller, 2003).
Conservation
No data for Iran. Endangered in Turkey (Fricke et al., 2007).
Further work
The biology of this species in Iranian waters requires study.
Sources
Iranian material:
Ponticola gorlap
(Iljin, 1949)
Common names
mahi kafzi (= bottom-dwelling or benthic fish), gel-ye mahi sar bozorg (= bighead goby), gavmahi-ye sarbozorg, gav mahi sar gondeh, sebele, gorzak.
[Names once used for Neogobius kessleri gorlap may be applied to this species: iribogaz xul for Azerbaijan; bychok-golovach or bighead goby in Russian; gorlap goby, Caspian bighead goby]
Systematics
The Caspian Sea basin was thought to contain the subspecies Neogobius
(now Ponticola) kessleri gorlap (Iljin, 1949). Iljin (1956) and Vasil'yeva (1991) considered
P. k. gorlap to
be a distinct species while Berg (1948-1949) and Pinchuk (1977) on external morphological grounds considered it to be a subspecies. Karyotype data (Vasil'ev and Grigoryan, 1993) shows
P. k. kessleri
from the Black Sea basin to have 29 chromosomes in males and 30 in females and the number of biarmed chromosomes in 2n=29 individuals is 17 and in 2n=30 individuals it is 16. Nominal
P. k. gorlap
from the Caspian Sea have 2n=46 in both sexes (Vasil'yev and Vasil'yeva, 1992). Vasil'ev and Grigoryan (1993), Vasil'yev and Vasil'yeva (1992), Vasil'yeva et al. (1994) and Vasil'yeva and Vasil'yev
(1994) referred the Caspian Sea basin populations to Neogobius gorlap, but indicated that this taxon required redescription and this was carried out in 1996. Vasil'eva and Vasil'ev (1996) consider
that Gobius gorlap Iljin is unpublished and nonvalid, i.e. a nomen nudum (Iljin's 1941 manuscript is lost) and give the taxon the name Neogobius iljini with a type locality in the Mangyshlak
region of the Caspian Sea (holotype in the Zoological Museum of Moscow State University under ZMMGU P-19726 with paratypes ZMMGU P-46453 (3 specimens); note that the Zoological Museum of Moscow University
(ZMMU; their acronym) has P-19726 and P-4643 (sic)(Pavlinov and Borissenko, 2001)). Kottelat (1997) points out that Berg (1948-1949; volume 3 including gobies dated 1949) had access to and used some
of Iljin's data, thus making the name gorlap available. Indeed, any of the subsequent papers by Vasil'eva and Vasil'ev and others using the name with descriptive details would have made the name
available. Neogobius iljini is therefore a junior synonym of Ponticola gorlap
(Iljin in Berg, 1949). There is no type series for P. gorlap as it is based on
Iljin's data as outlined
above.
Key characters
Pelvic fin anterior membrane with angular lateral lobes, lobes about one-sixth to almost one-half width of anterior edge of membrane; pelvic fin less than nine-tenths distance to anal fin; posterior
nostril near edge of orbit; anterior nape with ctenoid or cycloid scales; rear of first dorsal fin without a dark spot; lateral series scales usually 54-76; lateral lobes of pelvic fin anterior membrane
large, at least one-fifth width of rear edge; upper lip not markedly swollen, width at least 0.6 lateral preorbital width (lip to orbit), if less than 0.75, then nape scales ctenoid; interorbital distance
0.8-0.9 eye diameter; and caudal peduncle depth 0.67-0.75 length.
Morphology
This species is separated from other Caspian gobies in Iran by having a completely scaled nape, scales usually cycloid on the sinciput (anteriorly on the head just behind the eyes) (70-100%
cycloid; 0-30% uniseriate ctenoid in Kura River fish) and cycloid or ctenoid posteriorly over the operculum (60-100% ctenoid in Kura River fish, 0-50% ctenoid in others); scales on the middle of the upper
third of the operculum usually cycloid (70-100%); head depth at the eyes is slightly less or about equal to head width between the upper origins of the opercles; a large mouth with the jaw angle below the
anterior part of the eye; the snout is much longer than the eye, 1.5-1.6 times; upper lip not expanded, or only slightly, less than lateral preorbital area (between lip and eye); interorbital distance less
than 0.85 eye diameter; first dorsal fin not higher than second dorsal fin; moderately large, angular or pointed rather than rounded lateral lobes to the anterior pelvic membrane (rather small and obtuse
lobes according to Ahnelt and Holčík (1996)), the membrane is 0.2-0.3 the width of the rear edge as opposed to not more than one-sixth; pelvic disc length is about 0.8 length of pelvic origin to anus
length; scales in lateral series 53-79; caudal peduncle depth is 0.6-0.75 its length; the posterior nostril is close to the eye margin; and overall colour is yellowish and marbled.
The head in fish from the Kura River and possibly Iran have a rather subcylindrical shape (43.8-60.0%), while marine and north and middle Caspian Sea basin fish have a depressed and wide head, the width
being markedly greater than the depth. The caudal fin is rounded, oval, or rarely elongated.
First dorsal fin spines 5-7, usually 6, second dorsal fin with 1 spine followed by 15-20, usually 18, soft rays, anal fin with 1 spine and 11-16, usually 13-15 (but see below), soft rays, pectoral fin
rays 17-22, and pelvic fin rays 8-9. Marine specimens and those from the lower Volga River and Yashan Lake in the Uzboi River system of Turkmenistan have less than 15 anal fin branched rays while
freshwater populations (in the Kura River at least, perhaps Iran) have 30-60% of all fish with 15 rays. Scale counts in the southern Caspian Sea are less (53-74) on average than for other populations
(61-79) (Vasil'eva and Vasil'ev, 1996). Gill rakers 5-8. Vertebrae 33-35. The chromosome number for
P. gorlap is distinctive with 2n=46 with 46 chromosomal arms (Vasil'ev and Grigoryan, 1993;
Esmaily and Kalbassi, 2008).
Meristic values for Iranian specimens are: first dorsal fin spines 6(16), second dorsal fin soft rays 16(4), 17(11) or 18(1), anal fin soft rays 12(13) or 13(3), pectoral fin rays 18(8) or 19(8) and
lateral line scales 63(5), 64(2), 65(2), 66(2), 67(2) or 68(2).
Sexual dimorphism
Abdurakhmanov (1962) reports on fish from Azerbaijan that head width, predorsal distance, second dorsal fin and anal fin bases and length of third branched anal fin ray are all greater in males while
pelvic fin length and eye diameter are greater in females. In sea populations, females are larger than males.
Colour
A strongly yellow to reddish-yellow coloration distinguishes this species (Berg, 1948-1949; Vasil'eva and Vasil'ev, 1996) although Gavlena (1977) describes Volgograd Reservoir specimens as brown and
others agree fish may be dark brown to dark grey or greenish. There are 4-8 elongated dark brown spots along the mid-flank in freshwater specimens, the last forming a horizontal "T" with the
cross-bar on the caudal fin base. The flanks are usually marbled dark brown with light brown and yellowish strips, sometimes in a cellular design. There are light spots, darkly rimmed, along the sides of
the head and near the pectoral fin bases, the latter also being likened to a network, reticulate pattern or cellular design. Cheeks with reticulate or roundish-cellular design. Short, longitudinal stripes
often present below the eyes. The belly is light grey or light yellowish.
Dorsal, caudal and pectoral fins yellowish to light brownish. Second dorsal fin branched ray tips white. The fins typically have rows of small dark, orange-grey spots, although the pelvic fin has only
a little dark grey or yellowish pigment with some brownish strips or many minute spots. The first dorsal fin lacks a large spot but has 3-4 series of spots; the second dorsal fin has 2-6, usually 3-4,
series of spots; the anal fin is yellow to grey with occasional spots; the pectoral fin has 8-10 series of spots; and the caudal fin has 7-8 series of spots. The ends of the second dorsal fin branched rays
are white. The anal fin has a light border as does the pelvic disc, although the latter sometimes has a black centre. The caudal fin has many minute spots. The genital papilla is dark grey. Young fish may
appear spotted but have a more reticulate pattern on the flank than N.
goebelii and are generally darker.
The dorsal fins in breeding males have a wide yellowish or white margin and the second dorsal fin rays have free tips. The margin is rusty in a female. The head can be a dark bluish-grey and the flanks
are dark with some paler markings. Fin membranes become dark.
Size
Reaches 22.5 cm. Freshwater populations have fish much smaller than those in the sea (Vasil'eva and Vasil'ev in Miller, 2003).
Distribution
Found in the inshore Caspian Sea and tributary rivers. It is reported from between Kultuk and Astara in Azerbaijan (Ragimov, 1965) and from the Uzboi Lakes in Turkmenistan. In Iran, it is reported
from a wide range of rivers from Astara to the Gorgan and probably the Atrak,
including the Siazan, Safid, Duyusan, Shimrud, Khoshkerud, Palarud, Naviq,
Shasdehrud, Shafarud, Kargan, Gharasu, Tajan, Babol, Haraz, Pol-e Rud and Safid, the Aras River, the Anzali Mordab
and Bahambar, Siah Darvishan, Pasikhan, Massouleh and Pir Bazar river, Gorgan Bay, the southeast Caspian Sea, southwest Caspian Sea and south-central Caspian
Sea (Holčík and Oláh, 1992; Ahnelt and Holčík, 1996; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
Abdoli and Naderi (2009)
consider it to be absent from the Atrak, Sardab, Tonekabon and Aras rivers.
Zoogeography
This species is thought to have evolved from populations which settled in the lower reaches of rivers and in coastal areas while those populations which penetrated headwaters evolved into
P.
cyrius (q.v.) (Ahnelt and Holčík, 1996). See also genus account above.
Habitat
This species is found in fresh and brackish waters in rivers and lagoons and in inshore waters of the Caspian Sea. In the sea, it is found down to 10 m, rarely to 20 m, on rocky and dense sand bottoms.
It moves inshore in the sea for spawning and retreats to deeper water (6-12 m) to overwinter (Vasil'eva and Vasil'ev in Miller, 2003). It may be found among reeds. It can live at river sites with
a current velocity at 4.5 m/sec on the bottom (Holčík and Oláh, 1992). This species is allopatrically distributed with
Ponticola cyrius (q.v.). Ahnelt and Holčík (1996) rate
this species as a eurytope, inhabiting both fresh and brackish waters, slow and rapid currents, and clay and pebble and gravel bottoms.
Kirilenko and Shemonaev (2010) found fish in the Kuibyshev Reservoir in Russia
to prefer rocky, sandy-slimy grounds rich in higher aquatic vegetation and are
sometimes found among cane roots.
Age and growth
Brackish water populations grow larger than freshwater ones (Ahnelt and Holčík, 1996). Males attain 3 years, females 2 years, and males grow faster than females. Maturity is reached at
1+ for most females and 2+ years for males (Vasil'eva and Vasil'ev in Miller, 2003).
Kirilenko and Shemonaev (2010) found a female in the Kuibyshev Reservoir that
was 4 years old while the oldest fish in the 3+ class were all males, fish
matured at 1+ years for both sexes. Abdoli et al. (2006) examined fish
from the Gomishan and Miankaleh wetlands and found a length (L)-weight (W)
relationship of W = 0.1L2.39 for males, 0.0595L2.588 for
females and 0.1L2.4 for sexes combined.
Food
The principal food in the sea is fishes, mostly gobies and Atherina, but also includes crustaceans. Benthic food means this species competes with commercial fishes. In fresh water, fishes eaten
include Nemacheilidae and the cyprinid Alburnoides bipunctatus (=
eichwaldii), as well as crustaceans such as gammarids (e.g. Dikerogammarus haemobaphes), gobies such as its own species and N.
melanostomus, insects and clams (Gavlena, 1977). Kirilenko and Shemonaev
(2010) found food in the Kuibyshev Reservoir to be mainly crustaceans and
chironomids.
Reproduction
Spawning takes place in June in the Volgograd Reservoir and each female may contain up to 576 eggs (Gavlena, 1977). In the Caspian Sea, fish have as many as 3506 large eggs and 3399 smaller ones.
Spawning takes place on rock and shingle bottoms with eggs laid on various objects. The males of this species are nest guarders. Spawning in the southern Caspian is in April and May with two batches of
eggs deposited. In the lower Volga River, spawning is from the end of April to July (Vavsil'eva and Vasil'ev in Miller, 2003).
Parasites and predators
The Caspian seal, Pusa caspica, is a predator on this species (Krylov, 1984). Pazooki and Aghlmandi (1998) found the nematode Dichelyne minutus infecting the intestine of 100% of
fish examined from the south Caspian Sea in Iran. and Sattari et al.
(2002; 2004) and Sattari (2004) record the presence of the nematode, Eustrongylides excisus, in this species. This parasite can damage muscles in commercial
species and render them unsuitable for sale. Dichelyne minutus was also
recorded. Daghigh Roohi and Sattari (2004)
record Eustrongylides excisus, Dichelyne minutus and Corynosoma
strumosum from this species in the southwestern Caspian Sea of Iran. Sattari et al. (2005) surveyed this species in the inshore area of the Caspian Sea, recording Eustrongyloides excisus and Dichelyne
minutus.
Economic importance
None in Iran although it has formed a small part of the goby catches in the former Soviet Union. It is also caught by anglers (Vasil'eva and Vasil'ev in Miller, 2003).
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include abundant in numbers, widespread range (75% of water
bodies), absent in other water bodies in Iran, and present outside the Caspian Sea basin.
Further work
The biology of this specie sin Iran needs work.
Sources
Meristics and colour description based in part on Gavlena (1977) describing fish from the Volgograd Reservoir, identified as N. kessleri. Counts and data for Iranian fish are based on Ahnelt and
Holčík (1996).
Iranian material:
Ponticola syrman
(Nordmann, 1840)

Common names
None (other than general names listed under genus above).
[shirman in the Ukraine, syrman goby, Kaspiiskii shirman or Caspian syrman goby]
Systematics
Gobius syrman is described from Odessa, Dniester estuary, in the former U.S.S.R., now Ukraine). Gobius Trautvetteri Kessler, 1859 described from the lower Bug and Dniester rivers in the
former U.S.S.R. and Gobius eurystomus Kessler, 1877 described from the southern Caspian Sea are synonyms. Berg (1948-1949) has eurystomus as a valid subspecies of Neogobius syrman but this
is not accepted now (Reshetnikov et al., 1997). Presumed syntypes of G. syrman are in the Natural History Museum, London (BM(NH) 1872.5.30:35, 105.0 mm standard length), Muséum national
d'Histoire naturelle, Paris (MNHN A. 1126) and in the Naturhistorisches Museum Wien (MNMW 30099. Syntypes of G. eurystomus are under BM(NH) 1897.7.5.8-9 (2, 73.6-88.6 mm standard length),
in the Zoological Institute, St. Petersburg (ZISP 10904-05), and perhaps NMW 29176. (Pinchuk et al. in Miller, 2003).
Key characters
Three transverse suborbital neuromasts rows occur ventral to the longitudinal suborbital row b while all other Neogobius have typically two rows.
Additionally, pelvic fin anterior membrane with rounded and shallow lateral lobes, lobes not more than one-sixth width of anterior edge of membrane, or lacking entirely; pelvic fin disc two-thirds
distance to anal fin origin; nape with ctenoid scales; first dorsal fin without large dark spot; lateral series scales usually 55-70; and angle of jaw below pupil of eye.
Morphology
First dorsal fin with 5-7, usually 6, spines, second dorsal fin with 1 spine and 15-19, usually16-18 soft rays, anal fin with 1 spine and 10-15, usually 12-14, soft rays, and pectoral rays 17-21.
Range in lateral series scales 56-79. Vertebrae 34-35. Pelvic fin anterior membrane with very shallow, rounded lateral lobes. Posterior nostril near edge of orbit. Angle of jaw below anterior part of
pupil, upper lip with uniform width, about half lateral preorbital area (between lip and eye). First dorsal fin relatively high, with rounded upper profile, without an upper anterior dark blotch. Second
dorsal fin highest in middle. Head depth at eyes 1.1-1.2 width between upper origins of opercles. Interorbital width less than or equal to eye diameter. Snout length 1.3-1.4 orbit. Lateral series scales
usually 59-67. Nape and predorsal area scaled completely. Breast, base of pectoral fin and upper part of opercle scaled, cycloid scales on ventral part of abdomen, opercle and anterior part of nape.
Pelvic fin about two-thirds distance to anal fin origin. Caudal peduncle depth about half length. Posterior nostril close to the eye margin. The head canals and the free neuromasts on the body are
typically arranged for a neogobiine. Three transverse suborbital neuromasts rows occur ventral to the longitudinal suborbital row b.
Sexual dimorphism
Males may have a more intensely dark distal band and body spots during spawning. Male median fin rays are slightly elongated during spawning. Females are usually smaller than males.
Colour
Overall colour pale grey, brownish-grey or more rarely yellowish-brown (perhaps fish parasitised with the strigeid nematode Neascus), with pale saddles across the back extending onto the flank
and sometimes meeting dark blotches along the mid-flank. The body has large grey-brown markings arranged in a chequer-like pattern. The head has brown markings laterally and a dark band antero-ventrally
from the eye to the upper lip. The first dorsal fin has a pale yellowish margin set off by a dark stripe above two brown stripes proximally. The second dorsal fin bands are less distinct and the anal fin
has an indistinct, bluish band and a white margin. The pectoral fins are yellowish and the pelvic disc is pale. Belly pale. Males do not become dark during spawning (Pinchuk et al. in Miller, 2003).
Size
Attains 29.2 cm.
Distribution
Black and Caspian Sea basins including the Imeni Kirova or Kyzylagach Bay near the northwestern Iranian border (Kuliev, 1989) and between Kultuk and Astara in Azerbaijan (Ragimov, 1965) and recently
recorded from Iran (Ahnelt et al., 2007). Reported from the Anzali Mordab and Gomishan Lagoon,
Gorgan Bay, and the southwestern, southeastern and south-central Caspian Sea (Jolodar and Abdoli, 2004;
Abdoli and Naderi, 2009). Introduced to the Aral Sea.
Zoogeography
See genus account above.
Habitat
This goby is found principally in the sea, with one report from fresh water in the Caspian Sea basin (Emba River, Kazakhstan). It is unusual in being found on mud and silt bottoms and can tolerate low
oxygen concentrations (to 20% saturation). It is rare offshore at 6-20 m in the Caspian Sea. It is found in the Caspian at 1-10 m, retreating in summer to slightly deeper water, reappearing as autumn
cooling occurs, and then spending the winter at 30-50 m. It approaches shores earlier than other gobies for spawning, as early as the latter half of February for males with females in the second half of
March (Pinchuk et al. in Miller, 2003).
Age and growth
Maximum age may be 6 years although most fish probably live only half this time. Maturity may be attained shortly before age 1 year and by 2 years for most fish.
Abdoli et al. (2006) examined fish from the Gomishan and Miankaleh
wetlands and found a length (L)-weight (W) relationship of W = 0.0033L3.41
for males, 0.0033L3.5 for females and 0.003L3.5 for sexes
combined.
Food
Fish, crustaceans and Nereis worms are the main diet items in Caspian Sea fish examined. Fish included Knipowitschia and fry of Neogobius
pallasi and N. melanostomus as
well as clupeids and its own species. Smaller individuals take crustaceans and larger ones favour fish (Pinchuk et al. in Miller, 2003).
Reproduction
The spawning season is from the beginning of April into May in the southern Caspian Sea. The eggs are found in two sizes, indicative of batch spawning. Up to 8474 ripe eggs are found in each female.
Eggs are 4.2 by 1.9 mm and are laid under and between stones, on any available object or even muddy bottoms.
Parasites and predators
This species is eaten by Sander lucioperca and fry are eaten by sturgeons.
Economic importance
This species has been fished in former Soviet waters of the Caspian Sea because of its large size but the proportion in catches varies from 1-50%. It is important as food for commercial species.
Conservation
Only recently recorded from Iran, its conservation status in unknown.
Further work
The distribution and abundance of this species in Iranian waters needs to be surveyed as it is an important food for commercial fishes.
Sources
Pinchuk et al. in Miller (2003) is a recent summary of data on this species.
Iranian material: CMNFI 1970-0544, 1, 141.7 mm standard length, Gilan, near Bandar-e Anzali, (37°28’N, 49°27’E);
CMNFI 1979-0689, 9, 87.1-124.0 mm standard length, Gilan, Safid River at Hasan Kiadeh (37°24’N, 49°58’E);
CMNFI 1970-0586, 1, 100.1 mm, Mazandaran, Gorgan Mordab at Ashuradeh-ye Kuchak (36°50’N, 53°56’E);
NMW 95074, 2, 100.1–130.2 mm, Mazandaran, south-east Caspian Sea near estuary of Gorgan River (36°59’N, 53°59’E);
CMNFI 1979-0788, 2, 135.3–146.2 mm standard length, Mazandaran, Gorgan River (37°00’N, 54°07’E);
NMW 95075, 1, 186.6 mm standard length, Mazandaran, south-east Caspian Sea, Mian Kaleh near Behshahr (36°53’N, 53°32’E).
Genus Proterorhinus
Smitt, 1900
This genus is characterised by having 6 first dorsal fin rays, a
pelvic disc without obvious lateral lobes and, uniquely in Ponto-Caspian gobies, a very elongate
anterior nostril which hangs over the lip. Miller in Miller (2004) give
additional characters, in particular the distribution of neuromasts on the head. It contains only a single
species found in the Black and Caspian seas and their tributary rivers
and in the northern Aegean Sea in Greek fresh waters. There may be two species
if P. semilunaris is recognised (see below). Simonovič (1999) considered this genus to be a
subgenus of Neogobius but Ahnelt and Duchkowitsch (2001) provisionally
consider Proterorhinus to be distinct based on their study of the lateral
line system and consider it distinct based on osteology (Ahnelt and Duchkowitsch,
2004). Miller in Miller (2004) recognises the genus as distinct as do Stepien
and Tumeo (2000) based on mtDNA. Simonovič et al. (1996)
consider this genus of gobies to be a young one as evidenced by the distribution of the species in the Caspian Sea which closed recently.
Reshetnikov et al. (1997) and Miller in Miller (2004) have the date for this genus as
1899 but Eschmeyer's "Catalog of Fishes" states that it was published in 1900 although dated 1899.
Proterorhinus nasalis
(De Filippi, 1863)
Common names
gav mahi, sag mahi, gel-ye mahi marmari, gavmahi-ye marmari, sebele.
[marmar xul in Azerbaijan; bychok-tsutsik or tubenose goby,
marmornyi bychok or marbled goby, both in Russian; mottled goby].
Systematics
The Caspian Sea tubenose goby has generally been referred to Proterorhinus marmoratus
(Pallas, 18l4). Gobius marmoratus was originally described from Sevastopol, Ukraine, on the Black Sea.
Types are lost (Miller in Miller, 2004).
Gobius nasalis De Filippi, 1863 described in Latin from the
"Mar Caspio presso Baku" and Gobius blennioides
Kessler, 1877 described from "Bakinskoi bukhte" (= Baku
Bay), were regarded as synonyms. The meristic characters reported by De Filippi
(1863) for nasalis are probably in error according to Berg
(1948-1949) but the only character falling outside the ranges compiled
below is for soft dorsal rays given as 21.
Stepien and Tumeo (2006) used mtDNA cytochrome b sequence data and
consider there are two species in this genus. P. marmoratus is a marine
species in the Black Sea and P. semilunaris (Heckel, 1837) is a
freshwater species in other Eurasian habitats. The type locality of the latter
is "Maritza [Marizza] R., near Plovdiv, e. Rumelia, Balkan region of Bulgaria".
Caspian Sea basin Proterorhinus were not investigated. Kottelat and Freyhof (2007), Freyhof and Naseka (2007) and
Sorokin et al. (2011) restrict marmoratus to brackish waters in
Sevastopol and consider nasalis to be the name for Iranian Caspian Sea
populations.
Neilson and Stepien (2009b) examined mtDNA and nuclear DNA and confirmed the
presence of a distinct taxon in the Caspian Sea basin. They advocated P. semipellucidus
as the name for the Caspian Sea basin taxon. Gobius semipellucidus
Kessler, 1877 was
described from a single specimen taken at the "ust'e rechki Kara-su,
vpadayushchei vb Astrabadskii zalivb" (= mouth of the Karasu
River falling into Astrabad Bay (Qareh Su, Gorgan Bay) and the
holotype is in the Zoological Institute, St. Petersburg (ZISP) (Eschmeyer
et al., 1996). P. semipellucidus is given as a valid species in Eschmeyer's "Catalog of
Fishes" but is presumably a synonym of P. nasalis. The relationship
between marine Caspian Sea (brackish) populations (nasalis) and true
freshwater populations (possibly semipellucidus) remains to be resolved.
Neilson and Stepien (2009b) examined only freshwater and brackish (0-6 p.p.t.)
specimens from the Caspian Sea basin. Sorokin et al. (2011) consider
nasalis to be the taxon widely distributed in the Caspian and Sea of Azov
basins with pellucidus a synonym, although further work is needed to
determine if pellucidus is the freshwater taxon.
The syntypes of Gobius nasalis are in the Istituto e Museo
di Zoologia della R. Università di Torino under MZUT N.672 (7
specimens), the Natural History Museum, London under BM(NH)
1869.3.4:34 (1, 43.4 mm standard length), in the Museo Civico di Storia Naturale di Genova
under MSNG 12655 (2) and MSNG 36228 (3), in the Naturhistorisches
Museum Wien under NMW 33894 (1), NMW 33895 (1) and NMW 33896 (1), and
in the Museum für Naturkunde, Universität Humboldt, Berlin under ZMB
5015 (3) (Eschmeyer et al., 1996; Miller in Miller, 2004; 43.3-48.4 mm
standard length, measured in February 2006). The holotype of Gobius semipellucidus
is lost. Ahnelt and Mikschi (2008)
give details on the types of Gobius semilunaris.
Key characters
The combination of the pelvic fins forming a disc, the head not
being flattened, and the anterior nostrils forming a tube overhanging
the upper lip are distinctive. The origin of the first dorsal fin is displaced
anteriorly in comparison to other Ponto-Caspian and Atlantic-Mediterranean
gobioids, and this reflects the differing postcranial osteology detailed in Ahnelt and Duchkowitsch (2004).
Morphology
First dorsal fin spines 5-7, usually 6, second dorsal fin with 1
spine and 12-20, usually 16-17, soft rays, anal fin with 1-2 spines and 11-17,
usually 13-14, soft rays, and pectoral fin with 14-16 branched rays. Scales in lateral
series 34-53. Scales are a vertical oval with the anterior margin
rounded to slightly wavy, the upper and lower margins rounded and the
posterior margin with almost straight upper and lower elements coming
almost to a central point. This causes the exposed portion of the
scales to be delimited by almost straight edges and flank scales have
a diamond pattern. Circuli are numerous and fine. Radii are numerous
and radiate back from a focus almost at the posterior margin. The
posterior scale margin bears fine ctenii. Gill rakers 3-6, short and
concentrated around the angle of the gill arch. A membrane partly
closes off and narrows the gill opening at the gill raker level and
may partially encompass a raker making counts difficult. Vertebrae
30-33. The chromosome number is 2n=46, with 46 acrocentric chromosomes
and 46 chromosomal arms (Ráb, 1985; Grigoryan and Vasil'ev, 1993; Simonovič, 1999).
The predorsal area on the back and the nape are scaled. The cheek has
infraorbital neuromast organs in 7 transverse rows from the lower orbit border
without uniserial papillae between the rows. The preopercular canal
has three pores, the anterior oculoscapular canal has 7 pores
and the posterior oculoscapular canal has 2 pores. Ahnelt
and Duchkowitsch (2001) give a detailed description of the lateral line
system on the head. The gut is short and s-shaped. The genital papilla has a two-lobed tip.
Freyhof and Naseka (2007) note that Caspian tubenose gobies are most similar
to P. semilunaris (Heckel, 1837) of the Aegean and Black seas basins and
are distinguished by having a shorter posterior membrane to the first dorsal
fin, not reaching the origin of the second dorsal fin (versus reaching), a
longer anterior naris reaching to the middle or to the lower margin of the lower
lip when folded down (versus to the upper lip or to the upppermost margin of the
lower lip), and a smaller eye (diameter 16-21% head length versus 20-28%).
Meristic values for Iranian specimens are:- first dorsal fin spines 5(1),
6(28) or 7(1); second dorsal fin soft rays 13(1), 14(6), 15(17) or
16(6); anal fin soft rays 12(13), 13(14), 14(2) or 15(1); total
pectoral fin rays 14(18) or 15(12); lateral series scales 36(1),
38(4), 39(7), 40(7), 41(4), 42(5), or 44(2); total gill rakers 3(18),
4(9) or 5(3), although accuracy is doubtful because of the membrane;
and total vertebrae 33(10), 34(16) or 35(3).
Sexual dimorphism
Males are overall darker than females, e.g. one at 60.1 mm standard
length had pigment on both rays and membranes of fins, and all fin
margins frilly and pale or orange-yellow. Cheeks are swollen in spawning males
and fins are longer than in females.
Colour
Overall colour is brownish or yellowish-brown to olive or yellowish-green with about
5 oblique and irregular bars or blotches across the flank. Colour varies with
the background, being darker on mud bottoms and a bright reddish-brown on sand. The head
has brown-grey bands over the top and sides on a pale background. The
first dorsal fin may have a pinkish tinge and irregular grey blotches.
The second dorsal fin has several series of brown spots with the upper
2-3 greenish-yellow. The caudal fin is grey interrupted by up to 9
narrow bands of greenish-yellow. There is a triangular black spot at
the caudal fin base, flanked by two white spots. The pectoral fin is
similarly greyish with 5-7 narrow bands of pale green or yellow. The
pelvic fin has 3-4 yellow bands and the anal fin 6-7 yellowish and
oblique bands over a grey background. The iris has a narrow golden
ring. Spawning males have all fins dark, although the pectoral fin is
lighter than the rest and the caudal fin has an obvious white margin.
The genital papilla is grey. Breeding females have a colour similar to
that of both sexes in winter. Young (16-22 mm) have an unpigmented
median band, wider than the eye, from the head to the first dorsal fin
and even to the caudal fin. In front of the first dorsal fin is a dark
saddle. The peritoneum is light brown to silvery with varying amounts of melanophores.
Size
Attains 15.0 cm although Caspian specimens reputedly reach only 7.6 cm.
Distribution
Found in the Caspian Sea basin and the Uzboi Valley of Turkmenistan. It is found in a wide range of rivers on the Caspian coast of Iran
including the Qareh Su falling into Gorgan Bay, Kargan River estuary near
Hashtpar, lower Safid, Rud-e Sera and Babol, in the Anzali Mordab and
Kolesar River, the southeast Caspian Sea,
the southwest Caspian Sea and south-central Caspian Sea (Derzhavin, 1934; Holčík and Oláh,
1992; Abbasi et al., 1999; Kiabi et al., 1999;
Abdoli and Naderi, 2009).
Zoogeography
The genus Proterorhinus diverged from the related
genus Neogobius about 5.2MYA during the late Miocene/early
Pliocene according to mitochondrial DNA evidence (Dillon and Stepien, 2001).
Freshwater and marine/brackish populations apparently diverged 3.82-4.30 MYA and
freshwater lineages in the Black and Caspian seas diverged 0.92-1.03 MYA
(Neilson and Stepien, 2009b).
Habitat
This species occurs in both salt and brackish waters and enters the
fresh water of rivers. There are some permanent freshwater populations
in Europe. The usual habitat is shallow sea bays, offshore banks or flowing water
of streams but it may also be found
in ponds and canals overgrown with vegetation. Where current is strong it hides
under boulders. Some fish are found below 3 m depths in the sea. It is often found under
stones or among weed, to which it can retreat rapidly if disturbed.
Most activity takes place at night.
Age and growth
Life span is variably reported as 2 or about 5 years with maturity attained at 1-3 years.
Young-of-the-year can mature by autumn at 5.5 cm in the Caspian Sea (Miller in Miller, 2004).
Food
Kuliev (1989) records insect remains, gammarids, daphnia,
chironomids, molluscs and fish eggs in the guts of this species in
Kirova Bay of Azerbaijan. For the Caspian Sea as a whole, amphipods
predominate at 45.7% followed by mysids at 21.8%, cumaceans at 16.4%
and decapods at 7.2%. Molluscs make up 5.6% (Kosarev and Yablonskaya,
1994). Iranian specimens contained crustacean and insect remains. Elsewhere
polychaete worms and small fish have been found in stomachs of this species
(Miller in Miller, 2004).
Reproduction
Both sexes produce sounds during the breeding season. Eggs are laid from shallow water (0.5 m) down to 20 m in
bowl-shaped nests, under rocks, in empty mollusc shells, and on roots
and stems of plants. Empty cans may be used. Two females may lay eggs in the
same nest. Spawning begins in mid-April in the southern Caspian and may continue as late as the
first half of August. Some fish may be mature in the middle of March
in Iranian waters. Fish presumed to be young-of-the-year at 9.8 mm
standard length have been collected on 26 April, by 10-11 June fish
are 11.2-11.6 mm (mean 11.4 mm), on 20 June the 5 smallest fish of a
sample were 12.5-14.1 mm, mean 13.6 mm while the 5 largest were
21.0-25.2 mm, mean 23.4 mm, and on 12 October the 5 smallest were
16.6-19.9 mm, mean 17.7 mm while the 5 largest were 26.4-33.3 mm, mean
29.6 mm. By 15 March, eggs in a 49.7 mm standard length fish are 1.3
mm in diameter and well-developed. This data suggests an extended
spawning season in Iran. However young are first found in mid-June
according to Ragimov (1987). Eggs are deposited in 2-3 batches and are
guarded. Females contain up to 1335 eggs of diameter 3.3-3.6 by 1.4-1.5 mm.
Elsewhere up to 2500 eggs may be laid. Egg clutches can be transported
on the hulls of ships (Ahnelt et al., 1998). Males are nest guarders.
Parasites and predators
Unknown for Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and because it
has been introduced outside its natural range (Jude, 2001).
Conservation
Lelek (1987) classifies this species as vulnerable in Europe on
account of water level changes. Kiabi et al. (1999) consider
this species to be data deficient in the south Caspian Sea basin
according to IUCN criteria. Criteria include few in numbers, medium
range (25-75% of water bodies), absent in other water bodies in Iran,
and present outside the Caspian Sea basin.
Further work
The biology of this species in Iran is not well known.
Sources
Type material: See above for Gobius nasalis (ZMB 5015).
Iranian material: CMNFI 1970-0530, 66, 41.6-64.5 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1970-0542, 5, 48.0-59.6 mm standard length, Gilan, Old Safid River estuary (37º23'N, 50º11'E);
CMNFI 1970-0585, 2, 52.6-55.9 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E);
CMNFI 1970-0590, 5, 39.5-47.7 mm standard length, Mazandaran, Shesh Deh River near Babol Sar (no other locality data);
CMNFI 1979-0787, 3, 52.7-59.1 mm standard length, Gilan, Nahang Roga River (37º28'N, 49º28'E).
Genus Rhinogobius
Gill, 1859
Members of this genus are characterised by an elongate body which is
compressed posteriorly, a depressed head, a long snout, usually small scales on
the occiput, short soft dorsal and anal fins both with 9-10 rays, the first
dorsal fin with 6 spines, scales are ctenoid and number 23-40 in lateral series,
anterior nostrils are tubular, the tongue is not notched, and teeth are simple.
There are about 48 species in Japan, Korea, Taiwan, China and the Amur River
basin of eastern Asia. One species, presumably from the Amur River basin in the
former Soviet Union, has been introduced to Turkmenistan and has reached Iranian waters.
Rhinogobius similis
Gill, 1859
Common names
gel-ye mahi, gavmahi-ye talabi (= pond or lake goby).
[lake goby]
Systematics
Rhinogobius similis was originally described from Shimoda, Japan
Rhinogobius similis lindbergi Berg, 1933 described from the Amur and
Ussuri rivers, Russia is the subspecies of the Amur River basin according to
Pinchuk (1978) but Eschmeyer (1998) records it as a synonym of the type
subspecies. The Iranian specimens probably had their ultimate origin in the Amur River basin.
Vasil'eva (2007) and Vasil'eva and Kuga (2008) consider that the Rhinogobius introduced to Central
Asia are R. cheni (Nichols, 1931), a Chinese species of the Yangtze
River, and this could be the species found in Iran. However, the large number of
species in the genus, the poor state of knowledge on these fishes and the
source presumably being the Amur River, leaves this
identification as open.
Key characters
The only member of its genus in Iran, its key characters are those outlined in the genus.
Morphology
Dorsal fin spines 5-8, dorsal soft rays 7-10 after an initial unbranched ray,
anal fin soft rays 6-10 after an initial unbranched ray, lateral series scales
28-36. Scales can be absent from the midline of the nape and the parietal and
occiptal regions but this is variable (the nominal subspecies lindbergi
lacks scales). Adult fish have the dorsal spines elongated (but not in lindbergi,
not even in males). Scales in small Iranian specimens are a vertical oval, the
posterior margin is fringed with ctenii, circuli in these small specimens are
moderately devloped, radii radiate from the central point of the posterior
margin to the anterior margin, the posterior margin has almost straight upper
and lower margins coming to a central point so flank scales form a diamond
pattern. The gut is a short s-shape. Gill rakers number 8-12 and are short and
touch the adjacent one when appressed. Total vertebrae number 24-28.
Meristic values for Iranian specimens are:- first dorsal fin spines 6(3); second dorsal
fin soft rays 9(3); pectoral fin rays 18(2) or 19(1); lateral series scales
31(3); total gill rakers 10(1) or 11(2); and total vertebrae 27(2).
Sexual dimorphism
Unknown.
Colour
Overall colour is a pale yellow or light brown with 6 brownish blotches on
the flank and 6 blotches on the back, their position alternating. A dark band
runs through the eye to the posterior edge of the mouth. The upper head is
speckled brown. The belly is white. There is a dark spot between the first and
second dorsal fin spines in the nominal lindbergi subspecies. There are 2
rows of spots on the first dorsal fin and 3-4 more distinctive rows on the
second dorsal fin. The caudal fin has 2-5 rows of spots. The anal fin bears
pigment distally on the membranes. The pectoral and pelvic fins are greyish. The
pigmentation of the type subspecies is given by Pinchuk (1974) but may differ in
detail from the nominal lindbergi subspecies so is not given here.
Young fish have 6 blotches along the mid-flank and membranes of fins are
either strongly pigmented overall or bear up to 6 bars on the first dorsal fin,
second dorsal fin and caudal fin with pectoral, pelvic and anal fins much
lighter, almost immaculate. There is a small blotch between the first and second
first dorsal fin rays near the fin base. The distal margin of the anal fin is
yellowish-orange. The peritoneum is silvery but has a strong development of
melanophores dorsally.
Size
Attains 100 mm.
Distribution
Aliev et al. (1988) refer to an exotic species of goby occurring in
the Tedzhen River of Turkmenistan in the text of their work but not in the table
of distributions. A goby is also reported by Shakirova and Sukhanova (1994) and
Sal'nikov (1995) from the Karakum Canal and the Kopetdag Reservoir in
Turkmenistan on the northern borders of Iran. Specimens have been collected from
Sarakhs, Iran in the Tedzhen (= Hari) River (Coad and Abdoli, 2000a; Abdoli et
al., 2000) and Abdoli (2000) records it from the Kashaf and Hari rivers in
the Tedzhen River basin.
Zoogeography
An exotic or alien species from eastern Asia, introduced to Turkmenistan from
where it presumably entered Iranian waters. The genus Rhinogobius is in
need of revision and the correct name for the taxon in Iran may change.
Habitat
The Iranian specimens were caught in the Hari River which had a depth of
35-120 cm , a width of 12-27 m, a turbidity reading of 15 cm, a water
temperature of 12°C and a coarse gravel bed. It eastern Asia it is found in
both rivers and lakes (hence the common name of lake goby as used in Japan). It
is found on shallow sandy bottoms and on the upper surface of large stones in
shallow water. Water temperatures reach 28°C (Kopylets and Dukravets, 1981).
Age and growth
Life span exceeds 4 years (Kopylets and Dukravets, 1981).
Food
Unknown.
Reproduction
Egg production reaches 1862 eggs in total (Kopylets and Dukravets, 1981).
Parasites and predators
Unknown for Iran.
Economic importance
Welcomme in Courtenay and Stauffer (1984) reports this species to be a pest when introduced.
Conservation
As an introduced species this fish requires no conservation.
Further work
The spread of this species in Iranian waters and its effects on native fauna should be documented.
Sources
Iranian material: Uncatalogued material, 5, 42.4-47.6 mm standard length, Khorasan, Hari River near Sarakhs (36º30'N, 61º10'E).
Channidae
This family is known as the snakeheads or serpent-heads because of the
characteristic broad head with large scales and a large oblique mouth.
They are found from Africa to eastern Siberia and Southeast Asia and
comprise about 29 species but the family is need of a revision. Maximum size is about 1.2 m.
Snakeheads are characterised by paired accessory organs or suprabranchial organs in the upper
gill chamber (above and behind the gills) which enable these fishes to breathe air, survive low
oxygen conditions and even reputedly travel overland; an elongate
rounded body becoming compressed posteriorly; the dorsal and anal fins
are long and of even height, and spineless; pelvic fins are present or
absent; the mouth is large and the lower jaw protrudes; there are
teeth on the jaws, vomer and palatines; gill openings are wide and the
gill membranes are united but free from the isthmus; branchiostegal
rays number 5; the caudal fin is rounded; scales are small and cycloid
or ctenoid; and colour is highly variable, rapidly changing to suit the surroundings.
Snakeheads are ambush predators, living in still waters although
some inhabit the larger rivers. Most species build a bubble nest in
vegetation, laying and fertilising the eggs below it so that they
float up into the bubbles. Others are mouth brooders. One or both
adults guard the nest and young and will attack intruders savagely,
including humans according to folklore. Many species are known to
aestivate in summer when the habitat dries. They can be carried alive
wrapped in wet cloths or vegetation and may be introduced into areas
outside their natural distribution.
A number of species grow large enough to be an important food in
Southeast Asia but are also pests, eating more valuable species. Some
species are popular in the aquarium trade. Juveniles may be called,
incorrectly, "larvae" in the aquarium trade and are brighter
in colour than adults.
Genus Channa
Scopoli, 1777
Channa Scopoli, 1777 has priority over Ophicephalus
Bloch, 1793 (Eschmeyer, 1990). Ophiocephalus is an incorrect
emendation. See also Myers and Shapovalov (1931-1932), DeWitt (1960),
Ettrich and Schmidt (1989) and Pethiyagoda (1991) for discussions on
the taxonomy of this genus and species.
The characters listed under the family above obtain for the genus
which has only one confirmed Iranian species.
Channa argus
(Cantor, 1842)
Reported as Channa argus warpachowskii (Berg, 1909) from the
Karakum Canal and Kopetdag Reservoir in Turkmenistan as an exotic from
China (Shakirova and Sukhanova, 1994; Sal'nikov, 1995). This canal
connects with the Tedzhen River basin shared with Iran and ultimately
may connect with the Caspian Sea basin. No Iranian record.
Channa gachua
(Hamilton, 1822)
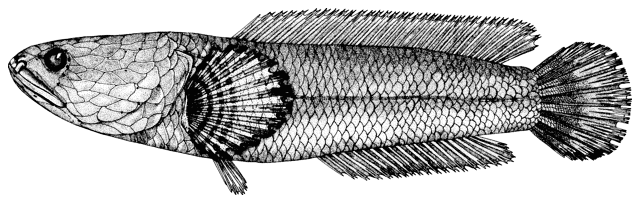
Common names
mahi-ye sarmari (= snakeheaded fish).
[tond, dolli or dauli in Pakistan; dwarf snakehead, frog snakehead, brown snakehead, oriental snakehead,
smooth-breasted snakehead].
Systematics
Ophicephalus gachua was originally described from Bengal, India.
Possible syntypes are in the Natural History Museum, London under BM(NH)
1858.8.15.54 (1) and BM(NH) 1858.8.15.144 (1) (Eschmeyer's "Catalog of Fishes",
downloaded 29 August 2007).
Key characters
The large head scales and elongate dorsal and anal fins are distinctive.
Morphology
Dorsal fin rays 30-37 (the last 2 rays counted as 1 where close
together at base), anal fin rays 20-24, pectoral rays 13-16, and
lateral line scales 39-47. Pelvic fins may be present or absent. The lateral line is displaced down one row under, or
just beyond, the posterior end of the pectoral fin. Scales have a vertical anterior margin,
parallel dorsal and ventral margins and a rounded posterior margin.
The anterior dorsal and ventral corners are square cut, rounded to
sharp. Circuli are fine and numerous on the anterior field but on the
posterior field become coarser and are parallel to the horizontal
axis. Radii are numerous in the anterior field and radiate from a
central focus. Gill rakers are minute. The gut is short and s-shaped. The
anterior nostril is tube-shaped and hangs over the upper lip to the mouth. The
chromosome number is 2n=78 (Banerjee et al., 1988; Klinkhardt et al., 1995).
Meristic values for Iranian specimens are:- dorsal fin rays 33(1), 34(3) or
35(1) (the last 2 rays counted as 1 where close together at base); anal fin rays
22(5); pectoral rays 14(3), 15(1) or 16(1); and lateral line scales 41(2), 43(2) and 44(1).
Sexual dimorphism
Females have a dark eye-spot at the posterior part of the dorsal fin (Pethiyagoda, 1991).
Colour
Colour varies with the habitat. The back is usually greenish-grey
to brownish with bluish tints and the flank is crossed by irregular
oblique bars, less obvious in adults than young. The background colour of the
flank is dove grey with a violet sheen in males. The dorsal, anal and
caudal fins are slate-coloured and have characteristic, narrow and
strong orange margins (white in preservative). The membranes of these
fins may be an iridescent green. The dorsal fin may have a blue or
bluish-green stripe with a vertical extent from the fin mid-point
distally about half-way to the fin margin on the membranes. The caudal
fin has blue and green rays. Females have a dark eye-spot at the end
of the dorsal fin. The pectoral fin base is dark blue and there are
4-5 orange and blue bands on the fin, with the margin orange. Bars on the
pectoral fin are very distinctive in preservative, alternating dark and light. The iris is
reddish. The peritoneum is silvery.
Size
Attains 33 cm (Day, 1875-1878) although Courtenay and Williams (2004) give 17
cm, probably more accurate given taxonomic confusion in the past.
Distribution
Reported from the Dasht and Rakshan rivers, the Makran
coast and the Mashkel (= Mashkid) River basin in Pakistan (Zugmayer,
1913). Iranian records were limited to 4 specimens collected by N. A. Zarudnyi in the Bampur river (upper or middle course) during 15-23 July 1898 (27
June-4 July in Berg (1949)) until 1 specimen was found in the Halil River basin near Sabzeveran at 28°39'N,
57°45'E, over 300 km northwest of the Bampur River at Bampur (Coad, 1979a). Abdoli (2000)
reports this species from the Halil and questionably from the Iranian Makran.
Ebrahimi (2001) maps two localities in the Halil River. The map in Berra (2001) has this species too close to the Straits of Hormuz and
the map in Courtenay and Williams (2004) does not extend far enough into Iran.
Zoogeography
The distribution of this species in southeastern Iran is confirmed
in Coad (1979a), a record not noted by Bănărescu
(1992b). It is the westernmost occurrence of the species in Asia. It
is found eastwards to Indonesia (Ettrich and Schmidt, 1989).
Habitat
This species can survive in turbid and poorly oxygenated water
because of its ability to breathe air. Air breathing is so well
developed that this snakehead can travel overland between water bodies, using a hopping motion.
Two pharyngeal suprabranchial cavities extend along the body to the
caudal peduncle and are lined with vascularized mucous membranes.
Mountain streams, rivers, lakes, reservoirs, ponds, rice paddies and
even hot springs are recorded as habitats for this species in South
Asia. Clear water in shallow streams and swamps in forested areas are preferred
(Courtenay and Willimas, 2004) while Pethiyagoda (1991) states that flowing
water is preferred. It is found in mud among emergent vegetation in Sri Lanka (De Silva, 1991). It may be largely
nocturnal. This species can survive temperatures as low as 13°C,
a pH range of 3.1-9.6, brackish water, and waters low in calcium, low
in mineral content and pronounced anion excess (Lee and Ng, 1994). Low
temperatures may be a limiting factor in its distribution in Iran.
Age and growth
Some populations in mountain streams are mature at about 13 cm
while in lower areas maturity is reached at about 10.2 cm at 20
months. The population examined in Sri Lanka has 90% of the
individuals less than 24 months old and 99% less than 38 months.
Longevity is about 6 years (De Silva, 1991).
Food
This species is recorded as a nocturnal predator on other fishes
and on frogs but most diet studies indicate that insects and
crustaceans are the main foods (De Silva, 1991; Pethiyagoda, 1991).
Young fish feed on unfertilised eggs from the mother for about 4
weeks. These eggs are released and fall in the water (while those
which hatch float). The young stimulate egg release through close body
contact with the mother, who swims in a circle while releasing the
eggs. In aquaria, a female will take Artemia nauplii into its
mouth and swim over to the male which releases the young to feed on
the nauplii as they are emitted from behind the gill cover (Ettrich
and Schmidt, 1989). An Iranian specimen had only sand grains in its gut.
Reproduction
Spawning takes place over silt or gravel bottoms or in areas of
cleared vegetation forming a "nest". Vegetation is cleared
by fin movements and can be 15 cm across (Ettrich and Schmidt, 1989).
Some reports have the female swimming belly up and the male then
fertilises the eggs as they are released by swimming diagonally over
the female's vent. Ettrich and Schmidt (1989) state that the male
forms a loop around the belly region of the female, an intensive and
long-lasting process. This occurs after pair-bonding lasting several
weeks which serves to synchronise reproduction, necessary since all
the eggs are released at once. The male picks up the eggs in his mouth
and keeps them there for 4-5 days until hatching (Ettrich, 1989; Pethiyagoda, 1991),
as the eggs are oily and slowly float to the surface. The fry may also
be protected in the male's mouth for up to three days before releasing
them, but retain the fry behind the gill cover when danger threatens
or night descends (Ettrich and Schmidt, 1989). Egg numbers vary
between 20 and 200 per spawning (Lee and Ng, 1994). Fecundity ranges from 389
and 7194 ((De Silva, 1991; Courtenay and Williams, 2004). Brood size (97-343 larvae)
is smaller than the number of mature
eggs reported for females according to De Silva (1991) and many eggs
either fail to develop or are lost to predators despite parental care.
An Iranian fish caught on 6 May contained small and possibly atretic
eggs. Egg diameter is 2.6 mm and the eggs are a golden yellow.
Ettrich and Schmidt (1989) report that 6 days after being released
from the mouth, the fry ascend to the water surface and draw air. A
foam nest is produced under which the young fish hide.
De Silva (1991) found breeding to take place throughout the year in
Sri Lanka, with enhanced breeding in May to July and October to
December. In the Karnataka State, India this species breeds from May
to August. Individuals appear to spawn once in each rainy season in Sri Lanka.
Parasites and predators
None reported from Iran.
Economic importance
This species is too rare in Iran to be of any economic importance
but in Sri Lanka it consumes pests in rice paddies (De Silva, 1991) and in
Singapore in 1990 sold for up to Singaporean $60 (Courtenay and Willimas, 2004).
Conservation
This species would be difficult to conserve in Iran as it is rare and at its
extreme westernmost distribution. Survivability may be marginal and numbers low.
Further work
Surveys to determine the distribution of this species, its numbers and its
environmental requirements may enable conservation to be effective.
Sources
Iranian material: ZISP 11714, 4, 42.9-70.2 mm standard length, Baluchestan, Bampur River (no other locality data);
CMNFI 1979-0220, 1, 110.9 mm standard length, Kerman, irrigation ditch, 2 km south of Jiroft (= Sabzeveran) (28°39'N, 57°45'E).
Comparative material: BC 66-32, 2, 63.8-71.5 mm standard length, East Pakistan, Chittagong Hill Tracts, Karnaphuli Reservoir tributary (no other locality data);
BC 66-38, 23, 79.5-124.9 mm standard length, East Pakistan, Dacca, Bellabor Fish Market (no other locality data);
BC 66-44, 2, 109-6-122.3 mm standard length, East Pakistan, Tipperali, Chandpur (no other locality data).
Scophthalmidae
Genus Psetta
Swainson, 1839
Psetta maxima
(Linnaeus, 1758)
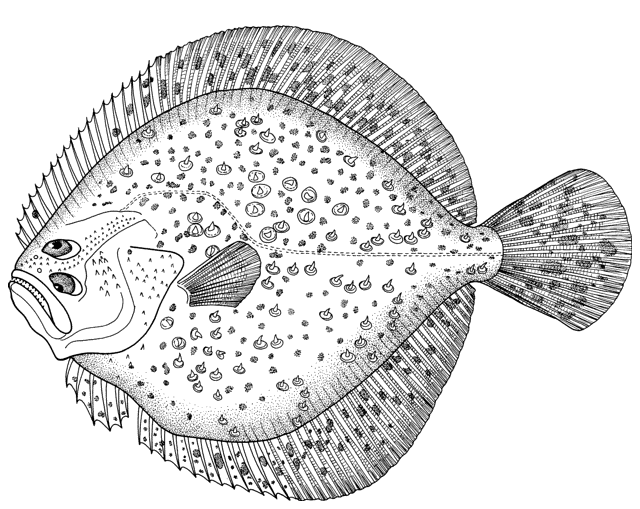
Introduced to the Soviet Caspian Sea basin as the subspecies Psetta maxima maeotica (Pallas, 1811) in 1930-1931 but not subsequently
observed. The introduction probably failed because the pelagic eggs were not buoyant at the low Caspian salinities (Baltz, 1991). No Iranian record.
Pleuronectidae
Genus Platichthys
Girard, 1854
Platichthys flesus
(Linnaeus, 1758)
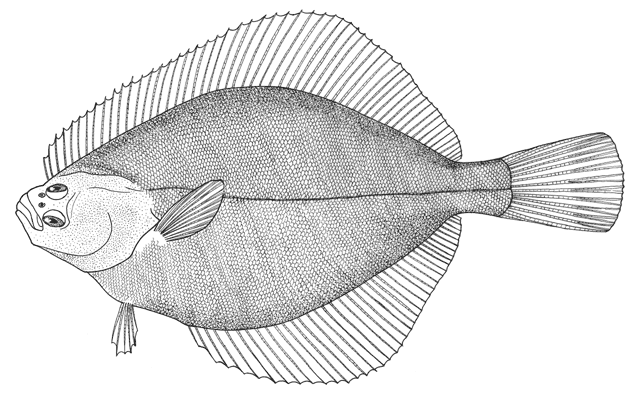
Introduced to the Soviet Caspian Sea basin in 1902 and 1930-1931
but not subsequently observed (Shukolyukov, 1937a). The introduction probably failed because the pelagic eggs were not buoyant at the low
Caspian salinities (Baltz, 1991). Voronina (1999) is a recent systematic review of this species. No modern Iranian record although
some were caught in the Anzali Mordab outlets in the 1930s (Holčík and Oláh, 1992). The Farsi name is pahn mahi.
Bibliography
1711-2010
This section contains all the papers and books referred to in the text of the web site on Freshwater Fishes of
Iran but also attempts to be a bibliography on these fishes and their environment. Entries ceased at the end of 2010 except for taxonomic and systematic works. The
volume of literature on Iranian fishes, and the ease of search and access through the internet, render additions tedious and nugatory.
A partial version of this bibliography was first posted on 10 September 1996 and maintained by Yazdan Keivany, then at the University of Alberta, Edmonton. There has been a rapid
increase in studies on fishes of Iran, starting in the 1990s. Prior to 1900, this bibliography lists less than 100 publications relevant to this work, many not strictly on Iranian fishes. On a
decadal basis, it is only in the 1960s that publications exceed 100 and by the 1990s are an order of magnitude larger.
The large number of articles listed here, the length of many, their limited availability, and costs involved make it impossible to loan xeroxes except in restricted cases. Please use your interlibrary
loan service.
The website http://www.sid.ir (Scientific Information Database or SID, Tehran) appears in Farsi and English and lists publications in Iranian
journals, with abstracts in English and Farsi. One journal given is listed as "Virtual" sometimes without date or pagination and is presumably some form of preliminary listing.
Students of Iranian fishes should also consult the Freshwater Fishes of Iraq website for its
Bibliography as this includes additional and complementary studies on species shared with Iran.
There is a moderately extensive grey literature on Iran, mostly manuscript reports of the Department of the Environment, Tehran. Some of these are in English and were written by Peace Corps workers in
the 1960s and 1970s. I have cited all those I came across but the listings are by no means exhaustive. Additionally, magazines and newspapers in Iran carry articles on fishes
and numerous conference presentations appear as titles, abstracts and even lengthy articles. Regretfully, a complete survey of these was not possible
although all those found are included.
The internet now carries English translations of Iranian newspapers and press releases. The URL (Universal Resource Locator) for these and
for websites having information on Iran are cited directly in the text and not in this bibliography. Note that links often change or are no longer functional and these textual references serve only to
document a source. Generally the date when they were
downloaded is given. They may still be accessible but are not hot linked in the text. Some papers cited in this Bibliography have only been seen in their online version and pagination of the original,
printed version is unknown. Others are known only from abstracts as costs prevent obtaining all papers when these are marginal to the main purpose of this website - describing the fishes
and their general biology. Wherever possible the language of the article is indicated by the title (for European languages including Turkish) or by adding the language at the end. In
some cases, particularly for articles published in Iran and seen only in English abstract, the language could be Farsi or English as journals may have articles in both languages.
Transliteration of personal names into English from other languages, notably Farsi, often gives variant spellings. Iranians may spell their name differently in different papers (you know who you
are). When I am aware that the same person is meant, the original journal spelling is given followed by (sic) to indicate that the author may appear under another spelling. The same (sic) is
also used to denote misspellings in titles (if these are egregious). Note that family names in Farsi may appear in two forms, a single name or a double-barrelled name (separated or hyphenated), and these
are variously used in papers. Personal names may be abbreviated to a single letter when appearing in English but sometimes appears as two letters to accord with the Persian alphabet (e.g. there are single
letters s and sh).
The "Iranian Journal of Fisheries Sciences" (from 7(2) 1998 onwards) also appeared earlier as the "Iranian Fisheries Scientific Journal" (3(2) 1994 to 7(1)
1998), and earlier still as "Iranian Fisheries Journal", "Iranian Fisheries Scientific Bulletin" and "Iranian Fisheries Bulletin", is published in Farsi
with English abstracts. It now appears as the "Iranian Scientific Fisheries Journal" in Iranian abstracting journals and websites. The "Iranian Journal of Fisheries
Sciences" (starting in January 1999 with volume 1(1)) is published in English with Farsi abstracts. The Iranian Fisheries Research Organization (formerly Iranian Fisheries Research
and Training Organization) publishes these journals and has a website with lists of publications.
Note that articles published in the "Journal of Ichthyology" are translations from the original Russian journal "Voprosy Ikhtiologii" although pagination (and sometimes issue number) are
not the same. Only "Journal of Ichthyology" pagination is cited here but readers may wish to avail themselves of the Russian versions of these articles (however some earlier issues of "Voprosy
Ikhtiologii" are only available in Russian). Prior to 1970 the English translation was called "Problems of Ichthyology". Year of publication as marked on the cover of each issue was the one
cited here although internal evidence indicates translated issues were often published in the following year, a delay presumably because of translation time required. The date thus used is the Russian
date of publication which is the relevant one for the few taxon descriptions appearing in this journal.
The Journal of the Faculty of Veterinary Medicine, University of Tehran contains many articles on aquaculture in Iran and other articles on Iranian fishes generally. It sometimes appears in abstracting
services, and in this bibliography, as Journal of Veterinary Research.
Some articles may be identified by journal name and a DOI but not pagination. DOI is the Digital Object Identifier and is found, in these cases, on articles that appear on-line but are
not yet in print. Eventually volume, issue and pages replace the DOI herein. Occasionally, some articles seem to languish as a DOI but for most the journal pagination follows within a few months.
A
Abaee, S. 2001. Rapid assessment of point sources pollution in Iranian part of Caspian Sea area using GIWA methodology. Caspian Environment Programme, Baku, Azerbaijan and Tehran. 8 pp., 3 figs.,
appendices A-B.
Abakumov, V. A. 1960. The damage done by lampreys to fish stocks. Fisheries Research Board of Canada Translation Series, 274:1-2.
Abassali Zadeh, A. 2003. Effects of ablading of sensory barbels on growth of Huso huso (young stage). Iranian Journal of Fisheries Sciences, 12(3):193-200. In Farsi.
Abaychi, J. and Al-Saad, H. T. 1988. Trace elements in fish from the Arabian Gulf and the Shatt al-Arab River, Iraq. Bulletin of Environmental Contamination and Toxicology, 40:226-232.
Abaychi, J. K., Darmoian, S. A. and DouAbul, A. A. Z. 1988. The Shatt al-Arab River: A nutrient salt and organic matter source to the Arabian Gulf. Hydrobiologia, 166:217-224
Abbasi, H. 1974. Neur Lake experimental commercial fishery, 27 Mehr - 16 Aban. L-6-53, Job Progress Report, Department of the Environment, Tehran. 9 pp. MS.
Abbasi, H. 1975. Neur Lake commercial fishery, 29 Mehr - 19 Aban 1354. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of the Environment, Tehran. MS (Abstract).
Abbasi, K. 2003. First record of black carp, Mylopharyngodon piceus from southern Caspian Sea (Iran). Iranian Journal of Fisheries Sciences, 12(2):139-146. In Farsi.
Abbasi, K. 2005. Studying alien fishes and macrocrustaceans distribution and their effects on rivers and wetlands of the Iranian basin of Caspian Sea. Borok II,
International Workshop, Invasions of Alien Species in Holarctic, 27 September-1 October 2005, Borok (abstract).
Abbasi, K. 2006a. Identification and distribution of fish fauna in Shafarud River, Guilan Province. Iranian Journal of Fisheries Sciences, 15(2):73-86. In Farsi.
Abbasi, K. 2006b. Identification and distribution of fish fauna in Hevigh River (Guilan Province). Iranian Journal of Biology, 18(4):370-382. In Farsi.
Abbasi, K., Keyvan, A. and Ahmadi, M. R. 2004. Morphometric and meristic characteristics of Vimba vimba persa in Sefidrud River. Iranian Journal of Fisheries Sciences, 13(1):61-76. In Farsi.
Abbasi, K., Keyvan, A. and Ahmadi, M. R. 2005. Studying natural reproduction, spawning grounds and spawning period of Vimba vimba persa population in Sefid-Roud
River, Guilan Province in north Iran. Iranian Journal of Fisheries Sciences, 14(3):113-126. In Farsi.
Abbasi, K., Moradkhah, S. and Sarpanah, A. N. 2007. Identification and distribution of fish fauna in Siahdarvishan
River (Anzali Wetland basin). Pajouhesh va Sazandegi, 19(1)(74):27-39. In Farsi.
Abbasi, K. and Sabkara, J. 2004a. Studying food items of Leuciscus ulanus, an endemic fish species in Iran. The 1st Congress on Animal and Aquatic
Sciences, University of Tehran, 31 August - 2 September 2004, p. 492-495.
Abbasi, K. and Sabkara, J. 2004b. Studying some biological characteristics of Leuciscus ulanus (Cyprindae), an endemic fish and little-known in Iran.
2nd International Conference on Applied Biology, Mashad, 29-30 September 2004, p. 29.
Abbasi, K. and Sabkara, J. 2004c. Studying Alosa caspia caspia (Caspian shad) diet in southeastern Caspian Sea (Mazandaran and Golestan provinces). Iranian Journal of Biology, 17(3):272-290.
In Farsi.
Abbasi, K., Salavatian, S. M. and Abdollapoor, H. 2005. Investigating fish diversity and distribution in the Mahabad-Chai River of the Lake Urmia basin, north-western Iran.
Iranian Journal of Fisheries Sciences, 13(4):75-94. In Farsi.
Abbasi, K. and Sarpanah, A. 2001. Fish fauna investigation in Aras Reservoir and its Iranian tributaries. Iranian Journal of Fisheries Sciences, 10(2):41-62. In Farsi.
Abbasi, K., Valipour, A., Talebi Haghighi, D., Sarpanah, A. and Nezami, Sh. 1999. Atlas of Iranian Fishes. Gilan Inland Waters. Gilan Fisheries Research Centre, Rasht. vi + 113 pp. In Farsi.
Abbasi, K. and Valipour, A. R. 2005. Studying the Silurus glanis Linnaeus, 1758 food items in Anzali lagoon. Pajouhesh va Sazandegi,
17(1)(66):14-24. In Farsi.
Abbasi, M. and Gharezi, A. 2008. Morphology and histology of the digestive tract of Iranian blind cave fish (Iranocypris typhlops). Iranian Veterinary Journal, 4(2)(19):60-69. In Farsi.
Abbasian, G-R. 1997. Fisheries of Iran: Development possibilities. INFOFISH International, Kuala Lumpur, 2:14-18.
Abbasov, G. S. 1980. The ichthyofauna of the main freshwater bodies of Azerbaidzhan. Journal of Ichthyology, 20(4):140-142.
Abbaspour, M. and Sabetraftar, A. 2005. Review of cycles of drought and indices of drought and their effect on water resources, ecological, biological,
agricultural, social and economical issues in Iran. International Journal of Environmental Studies, 62(6):709-724.
Abd, I. M., Rubec, C. and Coad, B. W. 2009. Key biodiversity areas - Rapid assessment of fish fauna in southern Iraq, p. 161-171. In: Krupp, F., Musselman, J. L., Kotb, M. M. A. and Weidig, I.
(Eds.). Environment, Biodiversity and Conservation in the Middle East, Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20-23 October 2008. BioRisk 3(Special
Issue):219 pp. ISSN 1313-2652 (online), ISSN 1313-2644 (print). Pensoft Publishers, Sofia-Moscow (http://pensoftonline.net/biorisk/index.php/journal/article/view/15/27).
Abd Almaleki, Sh and Ghaninezhad, D. 2007. Stock assessment of the Caspian Sea kutum (Rutilus frisii kutum) in Iranian coastal waters of the Caspian Sea. Iranian Scientific Fisheries
Journal, 16(1):103-114. In Farsi.
Abd Elahpour Biria, H., Abbasi, K., Sarpanah, A. N. and Pourgholami Moghadam, A. 2009. Population of sand goby (Neogobius fluviatilis pallasi) in southwest coasts of the Caspian Sea using
morphological characteristics. Iranian Scientific Fisheries Journal, 18(2):81-90. In Farsi.
Abdi, K. 1999a. Survey on the four new species of parasitic leeches of Mahabad's Lake Dam in Iran. In: 26th World Veterinary Congress, Lyon, France, 23-26 September 1999 (abstract).
Abdi, K. 1999b. Survey on the crustacean parasites of fishes of Mahabad's reservoir dam with new records of Tracheliastes polycolpus in Iran. In: 26th World Veterinary Congress, Lyon, France,
23-26 September 1999 (abstract).
Abdi, K., Jalalai, B. Moobedi, I. and Naem, S. 1995. Identification of crustacean parasites in Mahabad Reservoir. Pajouhesh va Sazandegi, 36:128-132. In Farsi.
Abdi, K., Mobedi, I. and Rostamzad, R. 2006. The first outbreak of acanthocephalosis in rainbow trout fish farms of Iran. Pajouhesh va Sazandegi,
18(2)(71):9-12. In Farsi.
Abdolhay, H. 1996. Aquaculture status and development in the Islamic Republic of Iran. Paper presented to the Working Group on Aquaculture, Indian Ocean Fishery
Commission Committee for the Development and Management of the Fishery Resources of the Gulf, Cairo, Egypt, 1-3 October, 1996.
Abdolhay, H. 1997a. Artificial reproduction of fish for stock enhancement in the Caspian Sea. Seventh Conference of Shilat, Responsible Fisheries, 17-18 February 1997, Tehran. p. 187-207. In Farsi.
Abdolhay, H. 1997b. Fingerling production and stock enhancement of sturgeon in south of Caspian Sea. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts
(poster).
Abdolhay, H. and Baradaran Tahori, H. 1998. Review of reproduction of sturgeon from 1991-1997 in Iranian hatchery. First National Symposium on Sturgeon, Rasht, 15-16 December 1998, p. 107-124. In Farsi.
Abdolhay, H. and Baradaran Tahori, H. 1999. Fingerling production and stock enhancement of sturgeon in south of Caspian Sea. Journal of Applied Ichthyology, 15(4-5):298.
Abdolhay, H. and Baradaran Tahori, H. 2006. Fingerling production and release for stock enhancement of sturgeon in the southern Caspian Sea: an overview. Journal of Applied Ichthyology, 22(s1):125-131.
Abdolhay, H., Baradaran Tahouri, H. and Amini, R. 2006. An audit of sturgeon reproduction in Iran over the years 1998-2002. Iranian Scientific Fisheries Journal, 14(4):97-112. In Farsi.
Abdolhay, H. A., Daud, S. K., Pourkazemi, M., Siraj, S. S., Rezvani, S., Mostafa, K. A. S. and Hosseinzadeh Sahafi, H. 2010. Morphometric studies of mahisefid (Rutilus frisii kutum,
Kamensky, 1901) from selected rivers in the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 9(1):1-18.
Abdoli, A. 1992. A new fish species in Mazandaran ab bandans. Abzeeyan, Tehran, 2(8):54-55. In Farsi.
Abdoli, A. 1993a. Threespine stickleback in the Caspian Sea. Abzeeyan, Tehran, 3(10 & 11):45. In Farsi.
Abdoli, A. 1993b. Fishes of Golestan National Park's river. Abzeeyan, Tehran, 4(3):32-33, XIII. In Farsi.
Abdoli, A. 1993c. Three-spined stickleback in southern basins of the Caspian Sea. Abzeeyan, Tehran, 4(5):10-13, III. In Farsi.
Abdoli, A. 1994a. Tadpole goby Benthophilus stellatus leobergius in Gorgan Bay. Abzeeyan, Tehran, 4(12):58-59. In Farsi.
Abdoli, A. 1994b. Ecological study of fish populations in Sardabrud River and Chalus River, Mazandaran, Iran. M.Sc. Thesis, University of Tehran. iv + 94 pp, 28 figs, 20 tables.
Abdoli, A. 1998a. An investigation of some biological characteristics and distribution of Pseudorasbora parva in freshwaters of Iran. Journal of Agricultural Sciences and Natural Resources,
8(1):33-40. In Farsi.
Abdoli, A. 1998b. Fish species status in southern Caspian Sea. First International Student Seminar of Coastal University of the Caspian Sea, Astrakhan, Russia (title).
Abdoli, A. 2000. The Inland Water Fishes of Iran. Iranian Museum of Nature and Wildlife, Tehran. 378 pp. In Farsi.
Abdoli, A., Allahyari, S., Kiabi, B. H., Patimar, R., Ghelichi, A., Mostafavi, H., Aghili, S. M. and Rasooli, P. 2009. Length-weight relationships for seven Gobiid fish species in the
southeastern Caspian Sea basin, Iran. Journal of Applied Ichthyology 25(6):785-786.
Abdoli, A., Coad, B. and Naderi, M. 2000. First record of Rhinogobius similis, Gill 1859 in Iran. Iranian Journal of Fisheries Sciences, 9(1):73-76, 9. In Farsi.
Abdoli, A. Ghorbani, S., Niksirat, H., Mashhadi Ahmadi, A. and Golzarianpour, K. 2010. Iranian Wild Nature, Brown Trout. Plan for the Land, Tehran (pamphlet) In Farsi.
Abdoli, A., Golzarianpoor, K., Kiabi, K. H. and Patimar, R. 2010. Status of the endemic loaches of Iran. International Loach Conference 2010, 31 August to 3 September 2010, Prague,
Czech Republic (abstract).
Abdoli, A., Kiabi, B., Patimar, R. and Allahyari, S. 2008. Study of species diversity of fishes of Gomishan wetland in south east of Caspian Sea. International Conference on Biodiversity Conservation and
Management, University of Science and Technology, Cochin, Kerala, India, 3-6 February 2008 (abstract).
Abdoli, A. and Naderi, M. 2009. Biodiversity of Fishes of the Southern Basin of the Caspian Sea. Abzian Scientific Publications, Tehran. 243 pp. In Farsi.
Abdoli, A., Naderi, M., Aboo, M., Fazli, H. and Afraee, M. A. 1996. Spawning time and fecundity of the mullet, Liza auratus (sic) in south eastern part
of the Caspian Sea. Abzeeyan, Tehran, 7(2):24-26, III. In Farsi.
Abdoli, A., Niksirat, H. and Patimar, R. 2011. Variation of some biological characteristics of Caspian brown trout in southern Caspian Sea. The 4th International Symposium on Stock Enhancement and
Sea Ranching, 9th Asian Fisheries and Aquaculture Forum, Shanghai Ocean University, April 21 to 23, 2011 (abstract).
Abdoli, A., Patimar, R. Mostafavi, H., Kiabi, B. H. and Rasooli, P. 2007. Feasibility of Le Cren Method for population estimation of freshwater fish in Iran. Fish Stock Assessment Methods for Lakes
and Reservoirs: Towards the True Picture of Fish Stock, 11-15 September 2007, Ceske Budejovice, Czech Republic (poster).
Abdoli, A and Rahmani, H. 1999. The populations, food consumption and reproduction of Neogobius fluviatilis in a small stream of Madarso, Golestan National Park, North East of Iran. Seventh
International Symposium on the Ecology of Fluvial Fishes, 10-13 May 1999, Lodz, Poland (poster).
Abdoli, A. and Rahmani, H. 2001. Food habits of two species of Gobiidae Neogobius fluviatilis, Neogobius melanostomus in the
Madarsoo stream, Golestan National Park. Journal of Agricultural Sciences and Natural Resources, 8(1):3-15, 191. In Farsi.
Abdoli, A., Rahmani, H. and Rasooli, P. 2002. On the occurrence, diet and reproduction of Neogobius fluviatilis in Madarsoo stream, Golestan National Park, (north eastern Iran). Zoology in the
Middle East, 26:123-128.
Abdoli, A., Rasooli, P. and Mostafavi, H. 2008. Length-weight relationships of Capoeta capoeta capoeta (Gueldenstaedt, 1772) in the Gorganrud River,
south Caspian basin. Journal of Applied Ichthyology, 24(1):96-98.
Abdoli, A., Rasooli, P. and Soltaninasab, S. 2008. A contribution to the biology of Acanthalburnus urmianus (Günther, 1899) (Osteichthyes: Cyprinidae): an endemic fish of Iran. Zoology in the
Middle East, 43:111-112.
Abdoli, A., Rasooli, P., Yazdandad Bibalan, H. and Abdoli, L. 2007. A study on some ecological aspects of snow trout (Schizothorax pelzami) from Laiinsoo River in northeastern Iran.
Environmental Sciences, 4(3):69-76.
Abdoli, A. and Skandari, S. K. 1999. Reproductive biology of khramulya (Capoeta capoeta gracilis) in Madarso stream in Golestan National Park. Journal of
Agricultural Sciences and Natural Resources, 6(3):38-51. In Farsi.
Abdollapour Bereya, H., Abbasi, K., Sabkara, J. and Keyvan, A. 2007. Studying Caspian shad (Alosa caspia caspia) diet in southwest coastal area of the Caspian Sea, Guilan Province
waters. Iranian Scientific Fisheries Journal, 16(1):115-128. In Farsi.
Abdolmalaki (= Abd Almaleki), Sh. 2000. Survey of parasites of fishes of Makoo Reservoir. Department of Fisheries and Sciences, Iran. 21 pp. In Farsi.
Abdolmalaki, Sh. 2001. Catch statistic review of mullet fishery in Iranian coastal waters of the Caspian Sea. Iranian Journal of Fisheries Sciences, 9(4):39-52. In Farsi.
Abdolmalaki, Sh. 2004. Fishery status and stock assessment of silver carp (Hypophthalmichthys molitrix) in Mahabad Reservoir in fishing season
of 1998-1999. Iranian Journal of Fisheries Sciences, 13(1):77-96. In Farsi.
Abdolmalaki, Sh. 2005a. Stock assessment of the pick (sic) perch (Stizostedion lucioperca) in the southern coastal waters of the Caspian Sea. Iranian
Journal of Fisheries Sciences, 13(4):95-110. In Farsi.
Abdolmalaki, Sh. 2005b. Population dynamics of Caspian sea bream (Abramis brama orientalis) in Iranian coast of the Caspian Sea in 2000-2001. Pajouhesh va Sazandegi,
18(3)(68):85-92. In Farsi.
Abdolmalaki, Sh. 2006a. Stock assessment of bonyfishes in the Iranian coastal water of the Caspian Sea. Iranian Fisheries Research Organization, Agriculture Research
and Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Abdolmalaki, Sh. 2006b. Trends in stock fluctuations of Rutilus frisii kutum in the Caspian Sea. Iranian Journal of Fisheries Sciences, 15(2):87-100. In Farsi.
Abdolmalaki, Sh., Amirkhani, A., Borani, M., Ghaninejad, D., Rastin, R., Porgholami, A. and Moradkhah, S. 1998. A survey on catch status and sexual maturity of mullets in Iranian coastal water
of the Caspian Sea (Gilan Province) in October 1998. Research Report, Fisheries Research Center of Gilan Province, Bandar Anzali. 13 pp. In Farsi.
Abdolmalaki, Sh. and Ghaninezhad, D. 1999. A survey on releasing fingerlings and catching pikeperch (Stizostedion lucioperca) in Iranian coastal
waters of the Caspian Sea. Proceedings of the First National Congress on Caspian Sea Bony Fish, 21-23 January 1999, Bandar Anzali, Gilan. In Farsi.
Abdolmalaki, Sh. and Ghaninezhad, D. 2007a. Stock assessment of the Caspian kutum Rutilus frisii kutum in the Iranian coastal waters of the Caspian. Sea. Iranian Journal of Fisheries
Sciences, 16(1):103-114.
Abdolmalaki, Sh. and Ghaninezhad, D. 2007b. Releasing fry of kutum (Rutilus frisii kutum) for restocking in Iranian waters of the Caspian Sea. Abzeeyan, 36:8-13. In Farsi.
Abdolmalaki, Sh. and Psuty, I. 2007. The effects of stock enhancement of pikeperch (Sander lucioperca) in Iranian coastal waters of the Caspian Sea. ICES Journal of Marine Sciences, 64:8 pp.
(advance copy).
Abdolmalaki, Sh., Sadat, H. A. and Nahrevar, R. 2007. Catch status and population structure of kutum (Rutilus frisii kutum) in Iranian coastal water of the Caspian Sea. Journal of Marine
Sciences and Technology, 6(3-4):51-62.
Abdolmaleki, S. 1994. An investigation on the bentic (sic) macrofauna of the Anzali Lagoon. Iranian Fisheries Scientific Bulletin, (5):27-38, 4. In Farsi.
Abdurakhmanov, Yu. A. 1962. Ryby Presnykh vod Azerbaidzhana [Freshwater Fishes of Azerbaidzhan]. Akademii Nauk Azerbaidzhanskoi SSR, Institut Zoologii, Baku. 407 pp.
Abdurrahmanov, Y. A. 1966. Azarbaycanin Faunasi. VII Cild. Baliglar (Pisces). [The Fauna of Azerbaidzhan. VII. Fishes (Pisces)]. Zoologiya Institutu, Azarbaycan SSR Elmlar Akademiyasi, Baki. 224 pp.
In Azerbaijani.
Abdurakhmanov, Yu. A. 1975. Transformation of the diadromous Kura shemaya Chalcalburnus chalcoides into a land-locked population in the Mingechaur Reservoir. Journal of Ichthyology,
15(2):189-196.
Abdurakhmanov, Yu. A., Kuliev, Z. M. and Agayarova, A. E. 1968. Materialy po Biologii i Raspredeleniyu Ryb u Azerbaidzhanskogo Poberezh'ya Srednego i Yuzhnogo Kaspiya
[Materials on the biology and distribution of fishes at the Azerbaidzhan coast of the Central and Southern Caspian]. In: The Biology of the Central and Southern Caspian. Moscow. pp. 113-146.
Abdurakhmanov, Yu. I. and Kuliyev, Z. M. 1968. European eel in Azerbaidzhan waters. Problems of Ichthyology, 8:592-594. In English.
Abdusamadov, A. S. 1986. Biology of white amur, Ctenopharyngodon idella, silver carp, Hypophthalmichthys molitrix, and bighead,
Aristichthys nobilis, acclimatized in the Terek region of the Caspian basin. Journal of Ichthyology, 26(4):41-49.
Abedi, M., Shiva, A. H., Mohammadi, H. and Malekpour, R. 2010. Reproductive biology and age determination of Garra rufa Heckel, 1843 (Actinopterygii: Cyprinidae) in central Iran. Turkish
Journal of Zoology, 35(3):317-323.
Abedian Kennari, A. M., Oveisipour, M. R. and Nazari, R. M. 2007. Effects of n3-HUFA enriched Daphnia magna on growth, survival, stress resistance,
and fatty acid composition of larvae of Persian sturgeon (Acipenser persicus). Iranian Journal of Fisheries Sciences, 7(1):1-14.
Abell, R., Thieme, M. L., Revenga, C., Bryer, M., Kottelat, M., Bogutskaya, N., Coad, B., Mandrak, N., Contreras Balderas, S., Bussing, W., Stiassny, M. L. J., Skelton, P., Allen, G. R., Unmack, P.,
Naseka, A., Ng, R., Sindorf, N., Robertson, J., Armijo, E., Higgins, J. V., Heibel, T. J., Wikramanayake, E., Olson, D., López, H. L., Reis, R. E., Lundberg, J. G., Sabaj
Pérez, M. H. and Petry, P. 2008. Freshwater Ecoregions of the World: A new map of biogeographic units for freshwater biodiversity conservation. BioScience, 58(5):403-413.
Abich, H. 1856. Vergleichende Chemische Untersuchungen der Wasser des Caspischen Meeres, Urmia- und Van-See's. Mémoires de l'Académie Impériale des sciences de
Saint-Pétersbourg, sixième série, Sciences mathématiques et physiques, 7:57 pp., 2 plates.
Abou-Seedo, F., Dadzie, S., Al-Kanaan, K. and Sukumaran, J. V. 2003. Aspects of reproductive biology of the hermaphroditic yellowfin seabream, Acanthopagrus
latus (Hottuyn (sic), 1782), in cages in Kuwait Bay. Zoology in the Middle East, 29:51-58.
Abtahi B., Esmaili Sari, A., Khodabandeh, S. and Seifabadi J. 2001. Ctenophores of the Caspian Sea and their socio-economic impacts. The Second International
Iran and Russia Conference (Agriculture & Natural Resources), Moscow. Abstract, p. 164.
Abtahi, B., Faghiehzadeh, S., Omidbeigi, R., Shariefpour, A., Rassoli, A., Nazari, R. M. and Aghajanpour, M. 2002. The comparison of LC50 of clove essence
and MS222 in Acipenser persicus, Oncorhynchus mykiss and Cyprinus carpio. Iranian Scientific Fisheries Journal, 11(3):1-12. In Farsi.
Abtahi B., Ghodrati Shojaei, M., Esmaeili Sari, A., Rahnama, M., Sharifpour, I., Bahmani, M., Kazemi, R. A. and Halajian, A. 2007. Concentration of some heavy metals in tissues of stellate sturgeon
(Acipenser stellatus) in the south Caspian Sea. Environmental Sciences,
4(3):77-84.
Abtahi, B.. Nazari, R. M.. Rasouli, A. and Shafiezade Samakosh, P. 2005. Comparison of antifungal therapeutic indices of formalin, malachite green and potassium permanganate on Persian
sturgeon in farming conditions of north of Iran (Sari). Pajouhesh va Sazandegi, 18(2)(67):42-49. In Farsi.
Abtahi, B., Soltani, M., Mohammadi Arani, M., Amin, G. and Khoshbavar-Rostami, H. 2003. Anaesthetic effects of clove essence (Eugenia caryophyllata) on
fingerlings of Persian sturgeon (Acipenser persicus). Journal of Agricultural Sciences and Natural Resources, 10(1):161-172. In Farsi.
Abtahi, B., Taghavi, H., Fazli, H. and Usefian, M. 2002. Fecundity and some biometrical characteristics of common kilka (Clupeonella delicatula) in
Mazandaran Province. Iranian Journal of Marine Sciences, 1(2):1-9, 88. In Farsi.
Abtahi, B., Taghavi Jolodar, H., Yousefian, M. and Fazi, H. 2004. Anatomical and histological study of ovary maturity stages of common kilka (Clupeonella delicatula). Pajouhesh va Sazandegi,
17(2)(63):47-54. In Farsi.
Abuzyarov, Z. K. 1999. Problems of the Caspian Sea level predictions. TACIS (Technical Assistance to the Commonwealth of Independent States, European Union), Caspian Environment Programme, Baku,
Azerbaijan. 17 pp.
Abzigostar. 1996. Iran fisheries sector study. Report by Abzigostar Consulting Engineering Company to Shilat, Tehran. 190 pp.
Abzigostar. 2004a. Report on fisheries activity in Zarivar Lake. Report by Abzigostar Consulting Engineering Company to Fisheries General Directorate, Kurdistan Province. 68 pp.
Abzigostar. 2004b. Report on environment in Zarivar Lake. Report by Abzigostar Consulting Engineering Company to Fisheries General Directorate, Kurdistan Province. 95 pp.
Abzigostar. 2004c. Report on limnology in Zarivar Lake. Report by Abzigostar Consulting Engineering Company to Fisheries General Directorate, Kurdistan Province. 100 pp.
Adamicka, P. 1984. On the construction of the Pontic cyprinid Pelecus cultratus (L.). Archiv für Hydrobiologie, 101(1/2):9-19.
Adams, C. G., Bayliss, D. D. and Whittaker, J. E. 1999. The terminal Tethyan event: A critical review of the conflicting age determinations for the disconnection of the
Mediterranean from the Indian Ocean, p. 477-484. In: Whybrow, P. J. and Hill, A. (Eds.). Fossil Vertebrates of Arabia with Emphasis on the Late Miocene Faunas, Geology, and
Palaeoenvironments of the Emirate of Abu Dhabi, United Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Adams, R. M. 1962. Agriculture and urban life in early southwestern Iran. Science, 136(3511):109-122.
Adeli, A., Hasangholipour, T., Hossaini, S. A., Salehi, H. and Shabanpour, B. 2010. Tehranish household preference for farmed fish consumption. Research Journal of Fisheries and Hydrobiology,
5(2):129-136.
Adeli, A. and Shaabanpour, B. 2007. Study on Tehran citizens behaviour change in consumption of the aquatic products. Iranian Scientific Fisheries Journal, 16(2):117-126. In Farsi.
Adeli Mosabbab, Y. and Piri, Kh. 2005. Monitoring of release rnies (sic) of juvenile vobla (Rutilus rutilus) at the Iranian coast of the Caspian
Sea with the purpose of restoring of its stocks, p. 15. In: Proceedings XIII International Conference on Fisheries Oceanology, Svetlogorsk, Kanliningrad region, September 12-17, 2005.
Adibmoradi, M and Sheibani, M. T. 2002. The histological study of mesencephal and cerebellum in Rutilus frisii kutum. Journal of the Faculty of Veterinary Medicine,
University of Tehran, 57(2):15-18. In Farsi.
Adler, K. 1989. Contributions to the History of Herpetology. Society for the Study of Amphibians and Reptiles, Contributions to Herpetology, Oxford,
Ohio, 5:1-202.
Admiralty Naval Staff. 1918. Geology of Mesopotamia and its borderlands. Intelligence Department, Admiralty Naval Staff, HMSO, London (I. D. 1177). 116 pp.
Afifi, H. 1966. A summary of method analysis used in the Zayandeh Rud river discharge study, pp. 213-226. In: Symposium on Hydrology and Water Resources
Development held in Ankara, Turkey February 7 to 12, 1966, Central Treaty Organization, Ankara. 484 pp.
Afkhami, A. A. 1998. Disease and water supply: the case of cholera in 19th century Iran. Yale School of Forestry & Environment Studies Bulletin, 103:206-220.
Afkhami, M., Shariat, M., Jaafarzadeh, N., Ghadiri, H. and Nabizadeh, R. 2007. Regional water quality management for the Karun-Dez River basin, Iran. Water and Environment Journal, 21(3):192-199.
Afraei, M. A. 2006. Identifcation and appointment distribution Clupeonella (genus Alosa) in the South
Caspian Sea coastal. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Afraei, M. A., Fazli, H. and Moslemi, M. 2000. Some biological characteristics of the brown trout Salmo trutta fario (Linnaeus, 1758) in Tonekabon River. Iranian Journal of Fisheries
Sciences, 9(3):21-34. In Farsi.
Afraei, M. A. and Hassannia, M. R. 1999. Some biological aspects of the smallest goby fish (Knipowitschia caucasica Berg, 1916) in Gorgan Bay, Caspian Sea.
Iranian Journal of Fisheries Sciences, 8(2):59-68, 5. In Farsi.
Afraei, M. A., Hassannia, M. R. and Rostamian, M. T. 2001. Some biological characteristics and distribution of the goby (Knipowitschia caucasica) in Gorgan. Pajouhesh va Sazandegi,
13(4)(49):99-101. In Farsi.
Afraei, M. and Lalooie, F. 2000. Fishes distribution assay in Tonekabon River. Iranian Journal of Fisheries Sciences, 9(1):1-14, 1. In Farsi.
Afraei, M. A., Safari, R. and Salmani, A. 2006. Distribution and density of juvenile Acipenser persicus at the lower 10 meter depth of the southern Caspian Sea. Journal of Applied Ichthyology,
22(s1):108-110.
Afraei Bandpei, M. A., Abdolmaleki, S., Kymaram, F., Ghasemi, S., Parafkande, F., Janbaz, A., Kor, D., Daryanabard, G., Taleshian, H. and Larijani, M. 2007. Age, growth, feeding regime, and
reproduction of kutum (Rutilus frisii kutum) in the south of the Caspian Sea in survey, 2006-2007. Final Report, Iranian Fisheries Research Organization,
Tehran. 97 pp. In Farsi.
Afraei Bandpei, M. A., Mashhor, M., Abdolmalaki, S. and El-Sayed, M. A.-F. 2009. Food and feeding habits of the Caspian kutum, Rutlius frisii kutum (Cyprinidae) in Iranian waters of the Caspian
Sea. Cybium, 33(3):193-198.
Afraei Bandpei, M. A., Mashhor, M., Abdolmalaki, S. and El-Sayed, A.-F. M. 2010. Population dynamics of Caspian kutum, Rutilus frisii kutum (Cyprinidae) in southern Caspian Sea, Iran. Cybium,
34(3):285-292.
Afraei Bandpei, M. A., Mashhor, M., Abdolmalaki, S., Keymaram, S., Isa, M. M. and Janbaz, A. A. 2010. Age and growth of kutum (Rutilus frisii kutum, Kamensky, 1901) in southern Caspian
Sea. International Aquatic Research, 2:25-33.
Afraei Bandpei, M. A., Mashhor, M., Khoo, K. H., Abdolmalaki, S., Fazli, H. and Janbaz, A. A. 2008. Some biological characteristics of the Caspian kutum, Rutilus frisii kutum (Cyprinidae) of the
southern Caspian Sea. International Conference on Environmental Research and Technology (ICERT 2008), Penang, Malaysia, 28 to 30 May 2008, p. 494-497.
Afraei Bandpyi, M. A., Salmani, A., Khoshbavar Rostami, H. R. and Paratkandeh Hagbighy, F. 2004. Biosystematic study of (Clupeidae) Alosa in Mazandaran and
Golestan Province coastal waters. Iranian Journal of Fisheries Sciences, 13(3):13-26. In Farsi.
Afrasiabi, K. L. 2003. The environmental movement in Iran: perspectives from below and above. Middle East Journal, 57(3):432-448.
Afraz, A. 1996. Classification of Anzali Lagoon rivers. Iranian Fisheries Scientific Journal, 5(1):1-17, 1. In Farsi.
Afshin, I. 1994. Rivers of Iran. Ministry of Energy of Iran Publications. 575 pp. In Farsi.
Agah, H., Leermakers, M., Elskens, M., Fatemi, S. M. R. and Baeyens, W. 2007. Total mercury and methyl mercury concentrations in fish from the Persian Gulf
and the Caspian Sea. Water, Air, & Soil Pollution, 181(1-4):95-105.
Aghaei Moghadam, A. A. and Aslan Parviz, H. 2006. The study on the nutrition of the juvenile sturgeon (Acipenser persicus) in fish ponds of Shahid
Rajaie's centre (1999). Pajouhesh va Sazandegi, 60:77-83. In Farsi.
Aghili, S. M., Rasouli P. and Abdoli, L. 2008. Possible impacts of the Alamut Dam construction on the fish fauna of Alamut and Taleghan streams (Sefid-Rud
River basin). Environmental Sciences, 5(3):75-83.
Ahmad, M. F. and Niazi, M. S. 1988. Important Edible Fishes of Pakistan. Zoological Survey Department, Government of Pakistan, Karachi. ix + 31 pp.
Ahmad, T., Slaman (sic), N. A. and Hussain, N. A. 1983. Effects of some experimental conditions on the behaviour and survival of Liza abu (Heckel) from Basrah, Iraq. Journal of the
Faculty of Marine Science, Jeddah, 3:111-117.
Ahmad, T. A. and Hussain, N. A. 1982. Observations on the food of young Liza abu (Heckel) from Salihyia river (Basrah, Iraq). Iraqi Journal of Marine Science, Basrah, 1(1):79-88.
Ahmad, T. A., Salman, N. A. and Hussain, N. A. 1985. Effect of some laboratory controlled conditions on the survival of Liza abu (Heckel). Kuwait Bulletin of Marine Science, 1985(6):125-131.
Ahmadi, M. R. 1988. The basic principles of breeding in fish culture. Journal of the Veterinary Faculty of the University of Tehran, 44(1):1-20 In Farsi.
Ahmadi, M. R. 1993. Aquaculture industry in Iran, p. 69-79. In: I Chiu Liao, Chung-Zen Shyu and Nai-Hsien Chao. (Eds.). Aquaculture in Asia. Proceedings of the 1990
Asian Productivity Organization Symposium on Aquaculture, Keelung, Taiwan, 5-13 September, 1990.
Ahmadi, M. R. and Alizadeh, M. 2004. Effects of dietary protein and energy levels on rainbow trout (Oncorhynchus mykiss) reared in brackish water. Iranian Journal of Fisheries Sciences,
4(1):77-88, 123.
Ahmadi, M. R. and Rezai, M. 1998. Comparison of feeding preference and growth of grass carp (Ctenopharyngodon idella) fed different plant with emphasis on chemical
composition. Journal of the Faculty of Veterinary Medicine, University of Tehran, 53(1-2):1-5. In Farsi.
Ahmadi, M. R., Safi, Sh., Bjerkeng, B., Gerami, A., Ranaii Siadat, S. O. and Khodarahmi, R. 2005. Caspian Sea gammarus (Pontogammarus maeoticus)
as a carotenoid source for muscle pigmentation of rainbow trout (Oncorhynchus mykiss). Iranian Journal of Fisheries Sciences, 5(1):1-12.
Ahmadi, M. R. und Wossughi, Gh. 1988. Ein Beitrag zur fischereilichen Bedeutung des Hamun-Sees, Iran. Österreichs Fischerei, 41(10):209-216.
Ahmadifar E., Jalali M. A., Soudagar, M., Azari Takami, Gh and Mohammadi Zarjabad, A. A. 2009. Effects of aquavac Ergosan on growth performance, survival and haematological factors in beluga
(Huso huso) juvenile. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A). In Farsi.
Ahmed, A. H. and Taher, M. M. 1988. Observations on the extensive and intensive cultured carp, Cyprinus carpio L. in Basrah, Iraq. Basrah Journal of Agricultural Science, 1:35-47.
Ahmed, H. A. 1982. Crowth (sic) of the cyprinid fish, Barbus luteus (Heckel) in Tharthar Reservoir, Iraq. Bulletin of the Basrah Natural History Museum, 5:3-15.
Ahmed, H. A., Al-Mukhtar, M. A. and Al-Adhub, A. H. Y. 1984. The reproductive biology of Carasobarbus luteus (Pisces, Cyprinidae) in Al-Hammar Marsh, Iraq. Cybium, 8(4):69-80.
Ahmed, S. S., Linden, A. L. and Cech, J. J. 1988. A rating system and annotated bibliography for the selection of appropriate, indigenous fish species for mosquito and
weed control. Bulletin of the Society for Vector Ecology, 13(1):1-59.
Ahnelt, H. 2003. The postcranial skeleton of the benthophiline gobiids Anatirostrum and Benthophilus (Teleostei: Gobiidae). Folia Zoologica, Prague, 52(2):213-221.
Ahnelt, H., Abdoli, A., Naderi, M. and Coad, B. W. 2000. Anatirostrum profundorum: a rare deep-water gobiid species from the Caspian Sea. Cybium, 24(2):139-159.
Ahnelt, H., Bănărescu, P., Spolwind, R., Harka, Á. and Waidbacher, H. 1998. Occurrence and distribution of three gobiid species (Pisces, Gobiidae) in the
middle and upper Danube region - examples of different dispersal patterns? Biologia, Bratislava, 53(5):665-678.
Ahnelt, H., Bianco, P. G. and Schwammer, H. 1995. Systematics and zoogeography of Knipowitschia caucasica (Teleostei: Gobiidae) based on new records from the
Aegean Anatolian area. Ichthyological Exploration of Freshwaters, 6(1):49-60.
Ahnelt, H. and Duchkowitsch, M. 2001. The lateral line system of two Ponto-Caspian gobiid species (Gobiidae, Teleostei): a comparison. Folia Zoologica, Prague, 50(3):217-230.
Ahnelt, H. and Duchkowitsch, M. 2004. The postcranial skeleton of Proterorhinus marmoratus with remarks on the relationships of the genus Proterorhinus
(Teleostei: Gobiidae). Journal of Natural History, 38(7):913-924.
Ahnelt, H. and Holčík, J. 1996. Distribution of two species of the genus Neogobius (Pisces: Gobiidae) in the catchment area of the southern Caspian Sea.
Acta Universitatis Carolinae Biologica, 40(1-2):99-114.
Ahnelt, H. and Mikschi, E. 2008. The type of Gobius semilunaris Heckel, 1837 (Teleostei: Gobiidae). Annalen des Naturhistorischen Museums in Wien, B, 109:67-72.
Aisenstein, B. 1947. The "kahrez", an ancient system of artificial springs. Journal of the Association of Engineers and Architects in Palestine, 8:2-3.
Aitchison, J. E. T. 1889. The Zoology of the Afghan Delimitation Commission. Introduction. Transactions of the Linnaean Society of London, Second Series, Zoology, 5:53-55, 2 maps.
Akbari, H. 1998. Mullets found in Hormozgan moshtas in the Persian Gulf. Iranian Journal of Fisheries Sciences, 7(3):1-12, 1. In Farsi.
Akbari, H. 2002. Frequency of Mugil cephalus in catch composition of stake-nets in the Hormozgan Province waters. Iranian Journal of Fisheries Sciences, 11(1):1-8. In Farsi.
Akbari, P., Baghfalaki, M. and Imanpour, M. R. 2009. The study of effects of some extenders to prolong sperm motility in Huso huso (Linnaeus, 1758). Iranian Journal of Biology,
21(5):829-836. In Farsi.
Akbari, P., Hosseini Seyed, A., Imanpour, M. R., Soudagar, M. and Makhdoumi, N. M. 2009. The effect of N-3HUFA and vitamin C enriched Artemia urmiana nauplii on growth and survival in
rainbow trout (Oncorhynchus mykiss) larvae. Journal of Agricultural Sciences and Natural Resources, 16(1):43-53. In Farsi.
Akbari-Pasand, A. 1996. Biological study of the Caspian roach. Abzeeyan, Tehran, 7(8):14-16. In Farsi.
Akbari-Pasand, A. 1998. Ecological study of fishes of Gorganroud River in the Golestan National Park. M.Sc. Thesis, Tarbiat Modarres University, Gorgan. 71 pp. In Farsi.
Akbary, P., Hosseini, S. A., Imanpoor, M., Sudagar, M. and Makhdomi, N. M. 2010. Comparison between live food and artificial diet on survival rate, growth and body chemical composition of
Oncorhynchus mykiss larvae. Iranian Journal of Fisheries Sciences, 9(1):19-32.
Akbarian, M. A., Hosseinzadeh, H., Seyfabadi, J. and Sarafraz, S. 2009. Effect of light intensity and photoperiod on growth and survival of kutum roach early stages. Annual Meeting of the American
Society of Ichthyologists and Herpetologists, 22-27 July 2009, Portland, Oregon (abstract).
Akbarzadeh, A. 2006. A comparative study of the morphometric and meristic characters and some biological features of pikeperch Sander lucioperca in
southern shores of Caspian Sea and Aras reservoir. University of Tehran, Karaj. 113 pp. In Farsi.
Akbarzadeh, A., Farahmand, H., Shabani, A. A., Karami, M., Kaboli, M., Abbasi, K. and Rafiee, G. R. 2009. Morphological variation of the pikeperch Sander lucioperca (L.) in the southern
Caspian Sea, using a truss system. Journal of Applied Ichthyology, 25(5):576-582.
Akbarzadeh, A., Karami, M., Nezami, Sh. A., Jodari, S., Bakhtiari, M. and Khara, H. 2007. Analysis of population structure of pikeperch (Sander lucioperca), in Iranian waters of Caspian Sea and
Anzali Wetland using truss system. Iranian Journal of Natural Resources, 60(1):127-139. In Farsi.
Akhlagi, M. 1999a. Effect of stress factors in the occurrence of Aeromonas hydrophila infection in cultured carp. Iranian Journal of Fisheries Sciences, 7(4):1-8, 1. In Farsi.
Akhlaghi, M. 1999b. Immunological survey of viral diseases of cultured salmonids in Iran. In: 26th World Veterinary Congress, Lyon, France, 23-26 Sep 1999 (abstract).
Akhlaghi, M. 2000a. Immunogenicity of Aeromonas hydrophila in common carp (Cyprinus carpio, L). Journal of the Faculty of Veterinary Medicine, University of Tehran, 55(1):62-67.
In Farsi.
Akhlaghi, M. 2001b. Bacterial diseases of cultured carp in Iran. In: 6th Asian Fisheries Forum Book of Abstracts, Kaosiung, Taiwan, 25-30 November 2001. p. 5.
Akhlaghi, M. 2000c. Immunological study of suspected viral diseases, infectious
haematopoietic and pancreatic necrosis in cultured rainbow trout (Oncorhynchus mykiss). Iranian Journal of
Veterinary Research, 1(2):85-95.
Akhlaghi, M. 2001d. A report on infestation of Parishan Lake eels to the nematode Anguillicola crassus. Pajouhesh va Sazandegi, 14(1)(50):26-27. In Farsi.
Akhlaghi, M. 2002. Vaccination against streptococcosis in Fars rainbow trout farms. Book of Abstracts, 27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Akhlagi, M. and Anharaki Motlagh, M. 2004. Phagocytic changes in common carp (Cyprinus carpio) following administration of immunostimulants Quil-A and
levamisole. Iranian Journal of Fisheries Sciences, 13(3):1-12. In Farsi.
Akhlagi, M. and Mirab Borujerdi, M. 2000. Anesthetic effect of clove tree and LC50 determination in rainbow trout (Oncorhynchys mykiss). Journal of the Faculty of Veterinary Medicine,
University of Tehran, 54(2):49-52. In Farsi.
Akhlaghi, M. and Sharifi Yazdi, H. 2008. Detection and identification of virulent Yersinia ruckeri: the causative agent of enteric redmouth disease in rainbow trout (Oncorhynchus
mykiss) cultured in Fars Province, Iran. Iranian Journal of Veterinary Research, Shiraz University, 9(4)(25):347-352.
Akhondzadeh, A., Iravani, A. A., Bokaei, S. and Zahrahi, T. S. 2000. The incidence of Listeria monocytogenes in rainbow trout in Tehran fish markets. The Third World Fisheries Congress,
Beijing, 31 Oct - 2 Nov 2000 (title).
Akhondzadeh, A., Zahraie Salehi, T. and Misaghi, A. 2002. The survey of Listeria monocytogenes in fresh and smoke fish and ice used in fish markets for retaining the freshness of fish in Tehran
and Gilan. Journal of the Faculty of Veterinary Medicine University of Tehran, 57(4):9-12. In Farsi.
Akhondzadeh Basti, A. 2004. The study of bacterial pathogens and fungi in fresh fish and heavy salted fish in Gilan. The 4th International Iran and Russia Conference
"Agriculture and Natural Resources", 8-10 September 2004, Shahr-e Kord, Iran (title).
Akhondzadeh Basti, A., Misaghi, A., Zahraei Salehi, T. and Kamkar, A. 2006. Bacterial pathogens in fresh, smoked and salted Iranian fish. Food Control, 17(3):183-188.
Akhondzadeh Basti, A., Misaghi, A. and Zahrae Salehi, T. 2003. The study of fungi and bacterial pathogens in fresh and salted cold smoked fish in Iran. AquaEuro 2003,
European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Akhondzadeh Basti, A. and Zahrae Salehi, T. 2003. Incidence of Listeria moncytogenes in fresh and smoked fish in Iran. American Fisheries Society Symposium, 38:163-165.
Akhondzadeh Basti, A., Zahrae Salehi, T. and Misaghi, A. 2003.The incidence of Listeria monocytogenes in fresh and smoked fish in Iran. European Aquaculture
Society, Aquaculture Europe 2002, Parallel Session VII: Quality aspects, safety and public image of aquaculture products, p. 117.
Akhrorov, F. and Kondur, L. V. 1981. The diet of commercial fish in Pamir lakes. Izvestiya Akademii Nauk Tadzhikskoi SSR (Otdelenie Biologicheskhikh Nauk), 1981(1):48-53. In Russian.
Akpınar, M. A. 1999. Besinsel Yağ Asitlerinin ve Açlığın Cyprinion macrostomus Heckel, 1843'un Kas Dokusu Yağ Asidi Bileşimine
Etkisi [Effect of dietary fatty acids and starvation on the fatty acid composition in the muscle tissue of Cyprinion macrostomus Heckel, 1843]. Turkish Journal of Biology, 23(3):309-317.
Akpınar, M. A. and Aksoytlar, M. Y. 1989. Cyprinion macrostomus Heckel, 1843'un Kas Dokusu Yağ Asidi Bileşiminin Sı Caklı
Kla Ilişkişi [Relation to temperature of fatty acid composition in muscle tissue of Cyprinion macrostomus Heckel, 1843]. Doğa Türk Biyoloji Dergisi, Ankara, 13(2):57-62.
Akrami, R., Hajimoradlou, A. A., Matinfar, A., Abedian Kenari, A. A. M. and Alimohammadi, S. A. 2008. Effect of different dietary prebiotic inulin on growth performance, nutrition factor, survival and
body composition of cultured juvenile beluga (Huso huso Linnaeus, 1758). Journal of Agricultural Sciences and Natural Resources, 15(5):55-67. In Farsi.
Akrami, R., Shabani, A. and Kheyrabadi, V. 2005. Investigation on the feeding habits of Huso huso and Acipenser guldenstaedti from stage of fry
to fingerling in earthen pond. Journal of Agricultural Sciences and Natural Resources, 12(5):109-118. In Farsi.
Akşiray, F. 1952. Genetical contributions to the systematical relationship of Anatolian cyprinodont fishes. İstanbul Üniversitesi Fen Fakültesi Hidrobiologi
Araştırma Enstitüsü Yayınlarından, B, 1(1):33-83.
Akşiray, F. 1955. Beiträge zur Kenntnis des Formenkreises Aphanius dispar (Rüppel). İstanbul Üniversitesi Fen Fakültesi Hidrobiologi Araştirma Enstitüsü Mecmuasi, B, 3:63-74, Taf. I-IV.
Al-Abood, A. Y. 1989. Studies on the blood haemoglobin and haematocrit of the silurid fish, Silurus triostegus in relation to weight. Rivista di Idrobiologia, 28(3):255-259.
Al-Akel, A. S., Shamsi, M. J. K. and Al-Kahem, H. F. 1987. Selective feeding behaviour of the Arabian freshwater fish, Aphanius dispar. Pakistan Journal of Zoology, 19(3):211-215.
A'lam, H. 1999a. (Fish). ii. Salt water fishes, p. 668-671. In: Yarshater, E. (Ed.). Encyclopædia Iranica. Bibliotheca Persica Press, New York. Volume IX, Fascicle 6. Festivals VIII-Fish.
A'lam, H. 1999b. (Fish). iii. In pre-Islamic Persian lore, p. 671-672. In: Yarshater, E. (Ed.). Encyclopædia Iranica. Bibliotheca Persica Press, New York. Volume IX, Fascicle 6. Festivals VIII-Fish.
Alam, H. No date. Fish and fisheries, 14 pp. In: Yarshater, E. (Ed.). Encyclopædia Iranica. Bibliotheca Persica Press, New York. www.bibliothecapersica.com/, downloaded 13 December 2006.
Alamouti, A. M. 1966. Groundwater resources of Iran, pp. 415-430. In: Symposium on Hydrology and Water Resources Development held in Ankara, Turkey February 7 to 12, 1966, Central Treaty Organization,
Ankara. 484 pp.
Al-Asfour, T. 1978. The marine terraces of Kuwait, pp. 245-254. In: Brice, W. C. (Ed.). The Environmental History of the Near and Middle East Since the Last Ice Age. Academic Press, London. xx + 384 pp.
Alavi, S. M. H. 2002. Threatened fish species in the southern part of the Caspian Sea. Program of the 26th Annual Larval Fish Conference (22-26 July 2002, Bergen, Norway). 1 p.
Alavi, S. M. H. 2003. Comparative study on motility and fertilizing ability of Acipenser persicus spermatozoa between freshwater and saline solution. M. Sc. Thesis, University of Tehran,
Tehran. 105 pp.
Alavi, S. M. H., Cosson, J., Karami, M., Amiri, B. M. and Akhoundzadeh, M. A. 2004. Spermatazoa motility in the Persian sturgeon, Acipenser persicus: effects of pH, dilution rate, ions and
osmolality. Reproduction, 128:819-828.
Alavi, S. M. H., Karami, M., Abdoulhay, H. and Ghadirnejad, H. 2005. Length, weight, and age relationships of the Persian sturgeon, Acipenser persicus,
in the southeast of the Caspian Sea: a case study at Turkaman Station during summer catching. Iranian Journal of Natural Resources, 58(3):603-614. In Farsi.
Alavi, S. M. H., Mojazi Amiri, B., Cosson, J., Pourkazemi, M. and Karami, M. 2002. A preliminary investigation on motility of Acipenser persicus spermatozoa: A
comparative study between freshwater and saline solutions at different dilution rate. The Second National-Regional Symposium on Sturgeon, Rasht, pp. 128-130.
Alavi, S. M. H., Mojazi Amiri, B., Cosson, J., Karami, M., Abdoulhay, H. A., Pourkazemi, M. and Akhoundzadeh, M. A. 2006. Determination of some seminal plasma indices,
sperm density and sperm motility in the Persian sturgeon, Acipenser persicus. Iranian Journal of Fisheries Science, 5(2):1-18.
Alavi, S. M. H., Mojazi Amiri B., Cosson, J., Karami, M. and Pourkazemi, M. 2007. Seminal plasma composition in Acipenser persicus: effect of stripping frequency on ionic content and osmolality.
Iranian Journal of Natural Resources, 59(4):865-874. In Farsi.
Alavi Talab, H., Tavvakoli Pour, H. and Ghoroghi, A. 2007. Investigation and comparison of quality of fitofague's (sic) skins and fins acidic and alkaline gelatin with another source.
Pajouhesh va Sazandegi, 19(3)(72):50-57. In Farsi.
Alavi Talab, H., Ardjmand, M., Motallebi, A. A. and Pourgholam, R. 2010. Optimization of morphology and geometry of encapsulated Hypophthalmichthys molitrix oil. Iranian Journal of
Fisheries Sciences, 9(2):199-208.
Alavi Yeganeh, M. S., Abedian Kenari, A., Rezaee, M. and Mohammadi Azarm, H. 2004. Resistance enhancement to environmental stress (pH and temperature) in rainbow trout
larvae (Oncorhynchus mykiss) by feeding supplementary gammarid powder. Iranian Journal of Marine Sciences, 3(1):57-66. In Farsi.
Alavi Yeganeh, M. S., Abedian Kenari, A. A. M. and Rezaee, M. 2008. Effect of
Gammarus powder as a supplement on growth and survival of rainbow trout larvae (Oncorhynchus mykiss).
Pajouhesh va Sazandegi, 20(4)(77):113-123. In Farsi.
Alavi Yeganeh, M. S. and Kalbasi, M. R. 2006. Study on diet composition of Caspian sand goby, Neogobius fluviatilis pallasi (Berg, 1916), in south of Caspian Sea (Noor Beach). Iranian Journal
of Biology, 19(3):180-190.
Al-Baharna, W. S. 1986. Fishes of Bahrain Directorate of Fisheries, Ministry of Commerce and Agriculture, Bahrain. 294 pp.
Alboghobeish, N. and Hamidian, Gh. R. 2006. Alarm cells and their distribution in different regions of Barbus sharpeyi skin. Iranian Journal of Fisheries Sciences, 15(2):1-8. In Farsi.
Alboghobeish, N. and Moosavi, S. M. 1998. Light and electron microscopic studies of phyarynx (sic) and osophagus (sic) of barbus (sic) sharpeyi
fish. Journal of the Faculty of Veterinary Medicine, University of Tehran, 53(1-2):75-90.
Alboughobish (sic), N. and Khaksari Mahabadi, M. 2005. Histologocal study of liver and pancreas in Ctenopharyngodon idella. Iranian Veterinary Journal, 9(11):25-34. In Farsi.
Alboughobish, N., Peyghan, R. and Hamidian, Gh. R. 2006. Mucous secreting chief cells and their distribution in different regions of epidermis in Barbus sharpeyi skin.
Iranian Veterinary Journal, 2(2)(13):102-111. In Farsi.
Al-Daham, N. K. 1977. Fishes of Iraq and the Arabian Gulf. Volume 1. Squaliformes to Atheriniformes. Publication 9, Centre for Arab Gulf Studies, University of Basrah, Iraq. 546 pp. In Arabic.
Al-Daham, N. K. 1979. Fishes of Iraq and the Arabian Gulf. Volume 2. Order Beryciformes - Order Perciformes (Suborder Percoidei). University of Basrah Press, Basrah, Iraq. 406 pp In Arabic.
Al-Daham, N. K. 1982. The ichthyofauna of Iraq and the Arab Gulf: A check-list. Publications of the Basrah Natural History Museum, 4:1-102.
Al-Daham, N. K. and Bhatti, M. N. 1977. Salinity tolerance of Gambusia affinis (Baird & Girard) and Heteropneustes fossilis (Bloch). Journal of Fish Biology, 11:309-313.
Al-Daham, N. K. and Bhatti, M. N. 1979. Annual changes in the ovarian activity of the freshwater teleost, Barbus luteus (Heckel) from Southern Iraq. Journal of Fish Biology, 14:381-387.
Al-Daham, N. K., Huq, M. F. and Sharma, K. P. 1977. Notes on the ecology of the genus Aphanius and Gambusia affinis in southern Iraq. Freshwater Biology, 7:245-251.
Al-Daham, N. K., Sarker, A. L. and Bhatti, M. N. 1977. Diel patterns of feeding in Heteropneustes fossilis from southern Iraq. Transactions of the American Fisheries Society, 106(6):614-616.
Al-Daham, N. K., Sarker, A. L. and Al-Nasiri, S. K. 1981. Industrial pollution of inland waters in Iraq - A fishery problem. The Arab Gulf, Basrah, 13(1):17-25.
Al-Daham, N. K. and Wahab, N. K. 1991. Age, growth and reproduction of the greenback mullet, Liza subviridis (Valenciennes), in an estuary in southern Iraq. Journal of Fish Biology, 38(1):81-88.
Al-Daham, N.K. and Yousif, A. Y. 1990. Composition, seasonality and abundance of fishes in the Shatt Al-Basrah Canal, an estuary in Southern Iraq. Estuarine, Coastal and Shelf Science, 31:411-421.
Alghmandi, F. and Dalimi Asi, A. 2000. A report on the isolation of Diplostomum sp. from Nemachilus melapterus (sic) fish in Shirud
River of Mazandaran. Pajouhesh va Sazandegi, 13(2)(47):114-115. In Farsi.
Al-Habbib, O. A. M. 1981. Acclimation to temperature and death at changing lethal temperatures in the freshwater fish Chalcalburnus chalcoides. Journal of Thermal Biology, 6:365-371.
Al-Habbib, O. A. M. and Al-Habbib, W. M. S. 1979. Acclimation to temperature and death at high changing lethal temperatures in Cyprinion macrostomus (Heckel). Journal of Thermal Biology, 4:75-77.
Al-Habbib, O. A. M., Salih, W. A. and Hamed, K. M. 1986. Seasonal variation in the biochemical composition of the skeletal muscle of the freshwater fish Barbus barbulus
(Heckle)(sic). Journal of Biological Sciences Research, Baghdad, 17(1):219-225.
Al-Habbib, O. A. M. and Yacoob, M. P. 1993. Effects of acclimation and experience to changing heat and cold shock temperature on lethal temperature and thermal tolerance
of Gambusia affinis (Baird & Girard) (Poeciliidae). Cybium, 17(4):265-272.
Al-Hakim, A. W. H., Al-Mehdi, M. I. A. and Al-Salman, A. H. J. 1981. Determination of age, growth and sexual maturity of Barbus grypus in the Dukan reservoir of Iraq. Journal of Fish Biology,
18:299-308.
Al-Hakim, A. W. H., Niazi, A. D. and Al-Ani, B. J. 1976. A study on the morphological characters and determination of sexual maturity in fishes, Barbus sharpeyi Gunther and Barbus grypus
Heckel. Bulletin of the Natural History Research Centre, Baghdad, 7(1):177-179.
Al-Hamed, M. I. 1965. On the morphology of the alimentary tract of three cyprinid fishes of Iraq. Bulletin of the Iraq Natural History Museum, Baghdad, 3(4):1-25.
Al-Hamed, M. I. 1966a. On the age and growth of three cyprinid fishes of Iraq. Ministry of Agriculture, Baghdad, Technical Bulletin, 135:1-70.
Al-Hamed, M. I. 1966b. On the reproduction of three cyprinid fishes of Iraq. Ministry of Agriculture, Baghdad, Technical Bulletin, 136:1-26.
Al-Hamed, M. I. 1966c. Limnological studies on the inland waters of Iraq. Bulletin of the Iraq Natural History Museum, Baghdad, 3(5):1-22.
Al-Hamed, M. I. 1971. Salinity tolerance of common carp (Cyprinus carpio, L.). Bulletin of the Iraq Natural History Museum, Baghdad, 5(1):1-7.
Al-Hamed, M. I. 1972. On the reproduction of three cyprinid fishes of Iraq. Freshwater Biology, 2:65-76.
Al-Hamed, M. I. 1976. Limnological investigations of Dokan Reservoir. Bulletin of the Natural History Research Centre, Baghdad, 7(1):91-109.
Al-Hashimi, A. 1987. Pollution of a river: How to prevent it. International Journal of Environmental Studies, 29:307-313.
Al-Hassan, L. A. J. 1982. The use of electrophoresis in the identification of fish stock, and its future application in the Arabian Gulf. Journal of the Faculty of Marine Science, Jeddah, 2:81-84.
Al-Hassan, L. A. J. 1984a. Comparative electrophoretic studies of muscle and eye lens proteins in freshwater fish of Iraq. Biochemical Systematics and Ecology, 12(2):205-208.
Al-Hassan, L. A. J. 1984b. Meristic comparison of Liza abu from Basrah, Iraq and Karkhah River, Arabistan, Iran. Cybium, 8(3): 107-108.
Al-Hassan, L. A. J. 1985. Phosphoglucose isomerase and phosphoglucose mutase isozymes in some freshwater fishes from Basrah, Iraq. Biochemical Systematics and Ecology, 13(3):357-364.
Al-Hassan, L. A. J. 1988. Genetic variation and population structure in Nematalosa nasus and Thryssa hamiltonii from Iraqi waters. Bolletino del Museo Regionale di Scienze Naturali,
Torino, 6(2):639-646.
Al-Hassan, L. A. J. 1990. Genetic and morphological variation in Acanthopagrus latus (Sparidae) in Iraq. Asian Fisheries Science, 3(2):269-273.
Al-Hassan, L. A. J. 1993. Additional synopsis of biological data on Tenualosa ilisha. Marina Mesopotamica, supplement, 2:1-23 (in Arabic, pp. 1-13).
Al-Hassan, L. A. J. 1994. The silver carp, Hypophthalmichthys molitrix (Cyprinidae) in the Shatt Al-Arab River, Iraq. Cybium, 18(2):204.
Al-Hassan, L. A. J. 1999. Shad of the Shatt Al-Arab River in Iraq. Shad Journal, 4(2):4 pp. (www.cqs.washington.edu/~hinrich/shad/JOURNAL4.2/vol4n2.htm, downloaded 29 May 2000).
Al-Hassan, L. A. J., Ahmed, H. K. and Majeed, S. A. 1993. Some haematological parameters in relation to the biology of the fish Acanthopagrus latus. Journal of Environmental
Science and Health, Part A, Environmental Science and Engineering, 28(7):1599-1611.
Al-Hassan, L. A. J., Al-Abood, A. Y. and Al-Seyab, A. A. 1990. Seasonal variations in the haemoglobin concentration and haematocrit values of Silurus triostegus. Acta Ichthyologica
et Piscatoria, 20(1):99-103.
Al-Hassan, L. A. J., Al-Daham, N. K. and Hassan, S. S. 1991. Eye lens as an age indicator in Mystus pelusius (Bagridae). Cybium, 15(2):171-172.
Al-Hassan, L. A. J., Al-Dubaikel, A. Y. and Wahab, N. K. 1992. Ocular lens diameter as an age indicator in two teleost fishes collected from Basrah waters (Iraq). Acta Hydrobiologica, 34:275-279.
Al-Hassan, L. A. J., Al-Dubaikel, A. Y., Wahab, N. K. and Al-Daham, N. K. 1990. Asymmetry analysis in the catfish, Heteropneustes fossilis collected from Shatt Al-Arab River, Basrah, Iraq.
Rivista di Idrobiologia, 29(3):775-780.
Al-Hassan, L. A. J., Al-Saboonchi, A. A. and Binayan, L. A. A. 1986. A record-size cyprinid fish, Barbus xanthopterus (Heckel) from Shatt Al-Arab river, Iraq. Cybium, 10(2):204.
Al-Hassan, L. A. J. and Al-Sayab, A.A. 1994. Eye lens diameter as an age indicator in the catfish, Silurus triostegus. Pakistan Journal of Zoology, 26(1):81-82.
Al-Hassan, L. A. J. and Al-Seyab, A. A. 1992. A record size mugilid fish, Liza subviridis (Valenciennes, 1836) from the marsh area, southern part of Iraq. Pakistan Journal of Zoology,
24(4):354-355.
Al-Hassan, L. A. J. and Elias, N. H. 1987. Isoenzymes of cyprinids from the vicinity of Basrah in relation to classification. Biochemical Systematics and Ecology, 16(2):223-226.
Al-Hassan, L. A. J. and Hassan, S.S. 1994. Asymmetry study in Mystus pelusius collected from Shatt Al-Arab River, Basrah, Iraq. Pakistan Journal of Zoology, 26(3):276-278.
Al-Hassan, L. A. J. and Hussain, N. A. 1985. Hydrological parameters influencing the penetration of Arabian Gulf fishes into the Shatt al Arab River, Iraq. Cybium, 9(1):7-16
Al-Hassan, L. A. J., Hussain, N. A. and Soud, K. D. 1989. A preliminary, annotated check-list of the fishes of Shatt Al-Arab River, Basrah, Iraq. Polskie Archiwum Hydrobiologii, 36(2):283-288.
Al-Hassan, L. A. J. and Madhi, A. A. 1987. Enzyme polymorphisms in the mullet, Liza dussumeiri (sic) from Shatt al-Arab river, Khor al-Zubair and the Arabian Gulf. Biochemical
Systematics and Ecology, 15(2):269-271.
Al-Hassan, L. A. J. and Muhsin, K. A. 1986. The presence of Barbus luteus and Heteropneustes fossilis in the Khor al Zubair, in the North-West of the Arabian
Gulf. Zoology in the Middle East, 1:116-118.
Al Hazzaa, R. 2005. Some biological aspects of the himri barbel, Barbus luteus, in the intermediate reaches of the Euphrates River. Turkish Journal of Zoology, 29(4):311-315.
Al Hazzaa, R. and Hussein, A. 2003a. Stickiness elimination of himri barbel (Barbus lutes (sic), Hecekel) eggs. Turkish Journal of Fisheries and Aquatic Sciences, 3:47-50.
Al-Hazzaa, R. and Hussein, A. 2003b. Initial observations in himri (Barbus lutes, (sic) Heckel) propagation. Turkish Journal of Fisheries and Aquatic Sciences, 3:41-45.
Al Hazzaa, R. and Hussein, A. 2003c. Propagation attempts in himri barbel (Barbus luteus, Heckel). Proceedings of the International Conference on Fish Welfare and Food Security in Arab
and Muslim Countries, 22-24 October, Azhar University, Cairo, Egypt (title).
Al Hazzaa, R. and Hussein, A. 2007. Larval development of himri, Barbus luteus (Cyprinidae: Cypriniformes) reared in the laboratory. Turkish Journal of Zoology, 31:27-33.
Ali, A. A. 1979. Study of morphological and biological characteristics of gattan (Barbus xanthopterus) from river Tigris and Al-Tharthar reservoir. The Arab Gulf, 11(1):181-197.
Ali, A. M. 1969. Morphometric features of the gattana (Barbus xanthopterus (Heck.)). Journal of the Arab Gulf, Basrah, 11(1):3-11. In Arabic.
Ali, A. M. 1974. Fecundity and histological description of the gonads of the female asp (Aspius aspius). Journal of Ichthyology, 14(6):896-903.
Ali, A. M. 1979. Study of morphological and biological characteristics of gattan Barbus xanthopterus from Tigris River and Al-Tharthar water reservoir. The Arab Gulf, 11(1):181-197. In Arabic.
Ali, A. M. 1982a. Morphological characteristics of himri (Barbus luteus G. (sic) from the reservoir of Tharthar and the River Tigris. Programme of the Fourth Congress of European
Ichthyologists, 20.-24. 9. 1982, Hamburg, West Germany. 1 p. (Abstract).
Ali, A. M. 1982b. On the biology of bunni (Barbus sharpeyi) from Mesopotamia. Programme of the Fourth Congress of European Ichthyologists, 20.-24. 9. 1982, Hamburg, West Germany. 1 p.
(Abstract).
Ali, A. M. 1985. Morphometric characteristics of the khimri, Barbus luteus (Cyprinidae), from Tartar Reservoir and the Tigris River (Iraq). Journal of Ichthyology, 25(5):168-171.
Ali, A. M., Tertar, T. M. and Tomas, N. K. 1981. Morphological and activity characteristics of the fish Barbus grypus in Al-Therthar Reservoir and Tigris River. Journal of the Arab Gulf,
Basrah, 13(3):117-128. In Arabic.
Ali Kehkha, A. O. and Cacho, O. 2005. The Hirmand River: conflicts and resolutions. Program & Abstracts, 8th International Riversymposium 2005, 6-9 September, Brisbane, Australia (abstract).
Alibekov, L. A. 1994. Iskysstvo drevnikh gidrotekhnikov i pust'ne [Art of ancient hydrotechnicians in deserts]. Priroda, 9(949):93-99.
Aliev, D. S., Sukhanova, A. I. and Shakirova, F. M. 1987. Redkie i ischezayushchie vidy ryb Turkmenistana [Rare and endangered species of fish in Turkmenistan]. Izvestiya Akademii Nauk Turkmenskoi
SSR, Seriya Biologicheskikh Nauk, 1987(1):70-71.
Aliev, D. S., Sukhanova, A. I. and Shakirova, F. M. 1988. Ryby vnutrennikh vodoemov Turkmenistana [Fishes of the inland waters of Turkmenistan]. Ylym, Ashkhabad. 142 pp., 11 pls.
Alipour, A., Baradaran Noveiri, S., Nowruzfashkhami, M. R. and Pourkazemi, M. 2009. Fertilizing ability of cryopreserved spermataozoa in the Persian sturgeon (Acipenser persicus) and stellate
sturgeon (A. stellatus). Iranian Journal of Fisheries Sciences, 8(1):1-12.
Alipour, Gh. H., Shaabanpour B., Shaabani, A. and Imanpour, M. R. 2009. Effects of brine concentration and temperature on quality of mahi sefid (Rutilus frisii kutum) smoked by traditional
method. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A). In Farsi.
Alipour, H. K., Shabanpour, B., Shabani, A. and Mahoonak, A. S. 2010. Effects of cooking methods on physico-chemical and nutritional properties of Persian sturgeon Acipenser persicus
fillet. International Aquaculture Research, 2:15-23.
Alishahi, M. 2006. Survey of the effects of protecting bacteria antigens of Streptococcus iniae in rainbow trout. Ph.D. Thesis, Tehran University. 105 pp. In Farsi.
Alishahi, M. 2008. The effect of intraperitoneal injection of Ichthyophthirius multifilis theront on serum antibodies in rainbow trout. Iranian Veterinary Journal 4(1)(18):68-77. In Farsi.
Alishahi, M., Mesbah, M., Najafzadeh, M. and Ghorbanpoor, M. 2009. Effects of Silybum marianum on resistance against Aeromonas hydrophila infection in Cyprinus carpio.
13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Alishahi, M. and Peyghan, R. 2008. Report of a severe and scarce infection to Lernaea cyprinacea in bighead Hypophthalmichthys nobilis. Iranian Veterinary Journal, 4(2)(19):122-128.
In Farsi.
Alishahi, M., Soltani, M. and Zargar, A. 2009. Bacteriological study of grass carp (Ctenopharyngodon idella) mortality in Khuzestan Province. Iranian Veterinary Journal, 4(5)(22):25-34. In Farsi.
Alizadeh, M., Ahmadi, M. R. and Farkhoy, M. 2001. Effects of dietary energy levels on growth performance and carcass composition of rainbow trout in brackish water. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 56(2):71-75. In Farsi.
Alizadeh, M., Sepahdari, A. A. F., Sarsangi, H. and Hedayati, S. A. A. 2009. Effects of different dietary energy levels on growth performance and sexual gonads development of beluga (Huso huso)
reared in brackish water. Iranian Scientific Fisheries Journal, 18(2):91-104. In Farsi.
Alizadeh Sabet, H. R. 2003a. A limnology study on Ghalehroodkhan River, Guilan Province, Iran. World Aquaculture 2003, 20-23 May, Salvador, Brazil (title).
Alizadeh Sabet, H. R. 2003b. Identification of Jarahee River fishes in Kohguilouye & Boyerahmad and Khouzestan provinces. Iranian Scientific Fisheries Journal, 12(1):63-76, 5. In Farsi.
Al-Johany, A. M. and Yousuf, M. 1993. Thermal ecology of two fresh water fishes Aphanius dispar and Gambusia affinis from Central Saudi Arabia. Arab Gulf Journal of Scientific
Research, 11(2):241-251.
Al-Kadhomiy, N. K. and Hughes, G. M. 1988. Histological study of different regions of the skin and gills in the mudskipper, Boleophthalmus boddarti with respect to their respiratory function.
Journal of the Marine Biological Association of the United Kingdom, 68:413-422.
Al-Kahem, H. F., Al-Balawi, K. A., Al-Ghanim, Z., Ahmad, Z., Temraz, T. A., Al-Akel, A. S., Al-Misned, F. and Annazri, H. 2008. A threatened fish species (Aphanius dispar) in Saudi Arabia, a case
study. Pakistan Journal of Biological Sciences, 11(19):2300-2307.
Alkahem, H. F. 1991. Studies on the variations in meristic characters of Aphanius dispar collected from three localities in Saudi Arabia. Pakistan Journal of Zoology, 23(1):95-96.
Alkahem, H. F. and Behnke, R. J. 1983. Freshwater Fishes of Saudi Arabia. Fauna of Saudi Arabia, 5:545-567.
Al-Khashab, W. H. 1958. The water budget of the Tigris and Euphrates basin. University of Chicago, Department of Geography, Research Paper, 54:ix + 105 pp., 3 maps.
Allahyari, M. S. 2010. Social sustainability assessment of fishery cooperatives in Guilan Province, Iran. Journal of Fisheries and Aquatic Sciences, 5(3):216-222.
Allameh, S. K. 2003. Use of oil by product (wastage oil) in diet for rainbow trout (Oncorhynchus mykiss). AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Allen, B. 1988. Aphanius mento: A Mediterranean jewel. Journal of the American Killifish Association, 21(1-2):75-76.
Allouse, S. B., Khalaf, A. N. and Al-Jafary, A. 1986. Some biological aspects of Chondrostoma regius (sic)(Heckel) - (Cyprinidae: Cypriniformes) in Diyala River
(Baghdad-Iraq). Journal of Biological Sciences Research, Baghdad, 17(1):227-239.
Almaça, C. 1981. La collection de Barbus d'Europe du Muséum national d'Histoire naturelle (Cyprinidae, Pisces). Bulletin du Muséum national d'Histoire naturelle, Paris,
4e série, 3, section A, numéro 1:277-307.
Almaça, C. 1983. Remarks on some Heckel's species of Barbus from western Asia. Arquivos do Museu Bocage, B, II(12):95-102.
Almaça, C. 1984a. Notes on some species of western Palearctic Barbus (Cyprinidae, Pisces). Arquivos do Museu Bocage, C, II(1):1-76.
Almaça, C. 1984b. Form relationships among western Palearctic species of Barbus (Cyprinidae, Pisces). Arquivos do Museu Bocage, A, II(12):207-248.
Almaça, C. 1985. Morphological relationship and evolutionary rate of taxonomic characters in Euro-Mediterranean Barbus (Cyprinidae, Pisces). Arquivos do Museu Bocage, B, II(16):129-136.
Almaça, C. 1986. On some BARBUS species from Western Asia (Cyprinidae, Pisces). Annalen des Naturhistorischen Museums in Wien, B, 87:5-30.
Almaça, C. 1988. Remarks on the biogeography of Euro-Mediterranean Barbus (Cyprinidae, Pisces). Bulletin d'Ecologie, 19(2-3):159-162.
Almaça, C. 1990. Neogene circum-Mediterranean paleogeography and Euro-Mediterranean Barbus biogeography. Arquivos do Museu Bocage, nova série, 1(41):585-611.
Almaça, C. 1991. Evolutionary, biogeographical, and taxonomical remarks on Mesopotamian species of Barbus s.s. Arquivos do Museu Bocage, nova série, 2(4):63-78.
Almaça, C. 1992. A tentative key to the species of Euro-Mediterranean Barbus (Cyprinidae, Pisces). Garcia de Orta, Série de Zoologia, Lisboa, 16(1-2)(1989):25-30.
Almaça, C. 1994. Studies on taxonomy, biogeography, and speciation in Euro-Mediterranean Barbus (Barbus s.s.). Arquivos do Museu Bocage, nova série, 2(28):455-462.
Al-Mehdi, M. I. A. and Khan, A. A. 1984. Haematology of a freshwater carp Cyprinion macrostomus from northern Iraq. Environment & Ecology, 2(3):222-226.
Al-Mukhtar, M. A. and Al-Hassan, L. A. J. 1999. A natural intergeneric hybrid of Barbus sharpeyi (Günther, 1874) and Carassius auratus (L.) from a small water body in
Khoozestan (Iran) Acta Zoologica Bulgarica, 51(1):35-41.
Al-Nasih, M. H. 1992. Preliminary observations related to the culture of Barbus sharpeyi (bunni). Journal of Aquaculture in the Tropics, 7(1):69-78.
Al-Nasiri, S. K. and Al-Mukhtar, M. A. 1988a. On the biology of sbour Hilsa ilisha (Hamilton)(Pisces, Clupeidae) from Ashar Canal, Basrah. Iraqi Journal of Agricultural Sciences
"Zanco", 6(1):97-106.
Al-Nasiri, S. K. and Al-Mukhtar, M. A. 1988b. On the biology of sbour Hilsa ilisha (Hamilton)(Pisces, Glupeidae (sic)) from Ashar Canal, Basrah. Pakistan Journal of Zoology, 20(4):321-328.
Al-Nasiri, S. K. and Hoda, S. M. S. 1975a. Some notes on the larvae and juveniles of half-beak, Hemirhamphus xanthopterus (Val) in Iraqi waters. Bulletin of the Basrah Natural History
Museum, 2:3-12.
Al-Nasiri, S. K. and Hoda, S. M. S. 1975b. Survey of fish fauna of Shatt-Al-Arab (from Abu-al-Khasib to Karmat Ali). Bulletin of the Basrah Natural History Museum, 2:36-46.
Al-Nasiri, S. K. and Hoda, S. M. S. 1976. A guide to the freshwater fishes of Iraq. Basrah Natural History Museum Publication, 1: xii + 124 pp.
Al-Nasiri, S. K., Jawad, L. A. J., Al-Salami, M. A. and Marina, B. A. 1975. Biometric studies on Aspius vorax Heckel from Basrah waters. Bulletin of the Basrah Natural History Museum, 2:59-67.
Al-Nasiri, S. K. and Salman, N. A. 1977. Length-weight relationship and condition of Acanthobrama marmid Heckel, in the Lesser Zab River. Iraqi Journal of Agricultural Science, 12:186-194.
Al-Nasiri, S. K., Sarker, A. L. and Hoda, S. M. S. 1977. Feeding ecology of a mugilid fish, Liza abu (Heckel) in Basrah, Iraq. Bulletin of the Basrah Natural History Museum, 4:27-40.
Al-Nasiri, S. K. and Sirajul Islam, A. K. M. 1978. Age and growth of Liza abu (Heckel) (Perciformes: Mugilidae) from Southern Iraq. Bangladesh Journal of Zoology, 6(2):85-89.
Alp, A. and Balik, S. 2000. Growth conditions and stock analysis of the carp (Cyprinus carpio, Linnaeus 1758) population in Gölhisar Lake. Turkish Journal of Zoology, 24(3):291-304.
Al-Rawi, A. H., Al-Obaidi, S. and Jawdat, S. Z. 1978. A list of fishes collected from the Little Zab River in Iraq: 1. Bulletin of the Biological Research Centre, Baghdad, 10:3-12.
Al-Rudainy, A. J. 2008. Atlas of Iraqi Fresh Water Fishes. Ministry of the Environment, Baghdad. 107 pp. In English and Arabic.
Al-Saadi, H. A. and Arndt, E. A. 1973. Some investigations about the hydrographical situation in the lower reaches of Shatt Al-Arab and the Arabian Gulf. Wissenschaftliche Zeitschrift der
Universität Rostock Mathematisch-Naturwissenschaftliche, 22(10):1169-1174.
Al-Saadi, H. A., Arndt, E. A. and Hussain, N. A. 1975. A preliminary report on the basic hydrographical data in the Shatt Al-Arab estuary and the Arabian Gulf. Wissenschaftliche Zeitschrift der
Universität Rostock Mathematisch-Naturwissenschaftliche, 24(6):797-802.
Al-Saadi, H. A., Hadi, R. A. M., Schiewer, U. and Al-Mousawi, A. H. 1989. On the influence of the sewage drainage from Basrah city on the phytoplankton and related nutrients in the Shatt al-Arab
estuary, Iraq. Archiv für Hydrobiologie, 114(3):443-452.
Al-Sabti, K. 1986. Karyotypes of Cyprinus carpio and Leuciscus cephalus. Cytobios, 47:19-25.
Al-Sahaf, M. 1975. Chemical composition of the water resources of Iraq. Vodnye Resursy, 4:173-185. In Russian. (Water Resources, 4(1976):677-687. In English).
Al-Sayari, S. S. and Zötl, J. G. (Eds.). 1978. Quaternary Period in Saudi Arabia. Volume 1: Sedimentological, hydrogeological, hydrochemical, geomorphological, and climatological
investigations in central and eastern Saudi Arabia. Springer-Verlag, Wien. xi + 335 pp.
Al-Shamma'a, A. A., Al-Mashhadany, A. J., Alasha'ab, M. H. and Nasser, E. N. 2009. Natural feeding of fish from Habbanyia Lake 3 - Kattan (Barbus (Luciobarbus) xanthopterus
(Heckel 1843). Iraqi Journal of Agriculture (Special Issue), 14(1):170-176. In Arabic.
Al-Shamma'a, A. A. and Jasim, Z. M. 1993. The natural food of Liza abu during the flood in Al-Hammar Marsh, South Iraq. Zoology in the Middle East, 9:59-64.
Al-Shamma'a, A. A., Mohammad, M. A., Shalash, F. J. and Nashaat, M. R. 2009. Seasonal variation in the natural diet of khisni Liza abu (Heckel, 1843) from Tigris River, Iraq. Alanbar
University Journal for Pure Science, 3(3):66-73. In Arabic.
Al-Shamma'a, A. A., Shawardy, A. O., Hassan, A. F. and Nashaat, M. R. 2009. Natural diet of four fish species from the Euphrates River at Ash-Shamiyah, Iraq. Alanbar University Journal for
Pure Science, 3(3):74-82. In Arabic.
Altindağ, A. 1996. Some population feature, growth and condition of the chub (Leuciscus cephalus L., 1758) in Aksehir Lake (Konya). Turkish Journal of Zoology, 20(Supplement):53-65.
Altindağ, A., Yiğit, S., Ahiska, S. and Özkurt, Ş. 1999. Kesikköprü Baraj Gölündeki Turna (Esox lucius L., 1758) Balığının Büyüme Özellikleri
[The growth feature of pike (Esox lucius L., 1758) in Dam Lake Kesikköprü]. Turkish Journal of Zoology, 22(4):311-318.
Altindağ, A., Yiğit, S., Ahiska, S. and Özkurt, Ş. 2002. The growth features of tench (Tinca tinca L., 1758) in Kesikköprü Dam lake, Ankara, Turkey. Turkish Journal of
Zoology, 22(4):311-318.
Altindağ, A., Shaha, S. L. and Yiğit, S. 2002. The growth features of tench (Tinca tinca L., 1758) in Bayındır Dam lake, Ankara, Turkey. Turkish Journal of Zoology,
26(4):385-391.
Altug, G. and Bayrak, Y. 2003. Microbiological analysis of caviar from Russia and Iran. Food Microbiology, 20(1):83-86.
Altun, O. 1999. Gümüşbalığı (Atherina boyeri Risso, 1810) Populasyonlarında Gözlemlenen Morfolojik Varyasyonlar [Morphological variations
observed on the sand smelt (Atherina boyeri Risso, 1810) populations]. Turkish Journal of Zoology, 23(3):911-918.
Al-Yamour, K. Y., Allose, S. B. and Al-Jafary, A. R. 1988. Some biological aspects of Liza abu (Heckel) Mugilidae, in Al-Daoodi Drain. (Baghdad, Iraq). Journal
of Environmental Science and Health, Part A, Environmental Science and Engineering, 23(5):497-508.
Alyan, F. 2010. Iran's northern fishing industry wanes. http://iwpr.net, downloaded 2 June 2011.
Amanov, A. A. 1970. Morphology and ecology of the Samarkand khramulya [Varicorhinus capoëta heratensis (Kessl.)] of the Surkhan Dariya River basin. Journal of
Ichthyology, 10(4):475-481.
Amanov, A. A. 1972. Morphology and mode of life of the Turkestan gudgeon [Gobio gobio lepidolaemus (Kessler)] from the Surkhandar'ya Basin. Journal of Ichthyology, 12(4):601-608.
Amini, K. 2005. A review on the situation of Persian sturgeon (Acipenser persicus) stock recruitment activities in south part of the Caspian Sea, p. 338-339. In: Proceedings XIII
International Conference on Fisheries Oceanology, Svetlogorsk, Kanliningrad region, September 12-17, 2005.
Amini Rad, A. 2001. Community level survey to assess the importance of fisheries in a socio-economic context in Bandar Anzali. Caspian Environment Programme, Baku, Azerbaijan. 24 pp.
Amini, K. 2006. Study on quantity and quality of released kutum fingerling into the Mazandaran rivers. Iranian Fisheries Research Organization, Agriculture Research and Education
Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Amini Ranjbar, Gh. 1994. Heavy metal concentration in surficial sediments from Anzali wetland. Iranian Fisheries Scientific Journal, 3(3):5-26, 2. In Farsi.
Amini Ranjbar, Gh. 1998. Heavy metal concentration in surficial sediments from Anzali Wetland, Iran. Water, Air, and Soil Pollution, 104(3-4):305-312.
Amini Ranjbar, Gh., Bahmani, M., Farsh, C. P. and Shariat, F. 2003. Coefficient correlation of heavy metals (Cu, Cd, Zn, Ob) in Persian sturgeons liver and kidney (Acipenser persicus)
and in the sediments of the southern coast of the Caspian Sea. Journal of Environmental Science and Technology, 17:61-84.
Amini Ranjbar, Gh. and Shariat, F. 2006. Trace metals (Cu, Cd, Zn, and Pb) in the liver and the kidney of Acipenser persicus with relation to their concentrations in the surficial
sediments of the west coast of the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 5(2):19-40.
Amini Ranjbar, Gh. and Sotoudebnia, F. 2005. Investigation of heavy metals accumulation in muscle tissue of Mugil auratus in relation to standard
length, weight, age and sex. Iranian Journal of Fisheries Sciences, 14(3):1-18. In Farsi.
Aminian Fatideh, B., Hosseinzadeh Sahafi, H., Shaabani, A. and Yaghmaei, F. 2008. Reproduction characteristics of Rutilus frisii kutum in the southern coast of Caspian Sea. Pajouhesh va
Sazandegi, 79:144-152. In Farsi.
Aminian Fatideh, B., Hosseinzadeh Sahafi, H., Shaabani, A., Yaghmaei, F. and Shafiei Sabet, S. 2008. Determination of stages of sex maturity of Rutilus frisii kutum Kamenskii, 1901 in
the Caspian Sea using biological indexes. Journal of Science, Teacher Training University, 8(2):107-120. In Farsi.
Aminipouri, B. 2002. Water policy development in I.R. of Iran. MS, 39 pp.
Amiri, A. M. 1999. EKG on sturgeon juvenile (Acipenser guldenstadti Brandt). In: Fourth Symposium on Diseases in Asian Aquaculture: Aquatic Animal Health for Sustainability, Asian Fisheries
Society, Manila, Fish Health Section, November 22-26, 1999, Cebu International Convention Center, Waterfront Cebu City Hotel, Cebu City, Philippines. Book of Abstracts. [unpag.].
Amiri, S. A., Sayad Bourani M., Moradi, M. and Pourgholami, A. 2008. The effect of water salinity on growth and survival of Rutilus frisii kutum fingerlings. Iranian Scientific Fisheries
Journal, 17(1):23-30. In Farsi.
Amirkolaie, A. K. 2008. Environmental impact of nutrient discharged by aquaculture waste water on the Haraz River. Journal of Fisheries and Aquatic Science, 3(5):275-279.
Anderson, R. 1966. Aphanius mento. Journal of the American Killifish Association, 3(2):24-25.
Anderson, S. C. 2002. An introduction to the literature of the vertebrate zoology of Iran. Zoology in the Middle East, 26:15-28.
Anderson, T. S. 1880. My Wanderings in Persia. James Blackwood & Co., London. xii + 364 pp., map.
Andersskog, B. 1970. Report to the Government of Iran on fisheries activities in Iran with particular reference to the northern fisheries company. Food and Agriculture Organization, Rome,
United Nations Development Programme, Technical Assistance, UNDP (TA) 2878:v + 18 pp.
Andrews, D. A. 1983. Aphanius dipar (sic) a brackish water killie. Journal of the American Killifish Association, KN Features, 16(1):24-26.
Annandale, N. 1919a. Geographical introduction. Records of the Indian Museum, 18:3-16, pl. I-II.
Annandale, N. 1919b. Notes on the fish of the genus Discognathus from India and Persia. Records of the Indian Museum, 18:65-78, pl. IX-XI.
Annandale, N. 1921. The aquatic fauna of Seistan. A summary. Records of the Indian Museum, 18:235-253.
Annandale, N. and Hora, S. L. 1920. The fish of Seistan. Records of the Indian Museum, 18:151-203, pls. XV-XVII (includes:- Appendix. Note on the fisheries of the delta of the Helmand and
on the use of shaped rafts of bulrushes in India and Seistan, by N. Annandale).
Annandale, N. and Hora, S. L. 1921. The fish of Seistan. Proceedings of the Seventh Indian Science Congress, Calcutta, p. lxxxiii.
Anonymous. 1877. Persian fisheries. Forest and Stream, 9:249-250.
Anonymous. 1905a. Eminent living geologists: William Thomas Blanford. Geological Magazine, New Series, Decade V, 2(1):1-15, 1 pl.
Anonymous. 1905b. William Thomas Blanford, C.I.E., LL.D., F.R.S., and Lieut. Sutton Aylmer Davies. The Ibis, 5(Eighth Series):643-647.
Anonymous. 1925. Obituary. Nelson Annandale, 1876-1924. Journal of the Bombay Natural History Society, 30(1 & 2):213-214.
Anonymous. 1961a. Slow death faces Russian caviar trade. Business Week, 22 July 1961, p. 122-124.
Anonymous. 1961b. Preserving the surplus Persian sturgeon. New Scientist, 9(233):459.
Anonymous. 1961c. Iran. Salient features, pp. 19-39. In: Multiple-purpose river basin development. Part 2D. Water resources development in Afghanistan, Iran, Republic of Korea and Nepal.
United Nations Economic Commission for Asia and the Far East, Bangkok.
Anonymous. 1963. Decrease in water in the Caspian Sea and the means to overcome this. Game and Nature, Tehran, 43:8-14. In Farsi.
Anonymous. 1970a. Introductions of fish and shrimps. Food and Agriculture Organization, Rome, Fish Culture Bulletin, 2(3):16.
Anonymous. 1970b. Chinese carps in Iran. Food and Agriculture Organization, Rome, Aquaculture Bulletin, 3(1):15.
Anonymous. 1970c. Oil in the caviar. Nature, London, 226(5245):486-487.
Anonymous. 1971. The Wetlands and Waterfowl of Iran. The Game and Fish Department of Iran, Tehran. 43 pp., 3 figs.
Anonymous. 1976. The blind white fish of Persia. Nature, London, 264:113-114.
Anonymous. 1977a. Sport fishing in Iran.... Homa, Iran Air In-Flight Magazine, September-October, p. 18-21.
Anonymous. 1977-1978. A survey of game laws and regulations in Iran. Department of the Environment, Tehran. 22 pp.
Anonymous. 1988a. OPINION 1500. Cobitis Linnaeus, 1758 (Osteichthyes, Cypriniformes): Cobitis taenia Linnaeus, 1758 designated as the type species, and the original
spelling of the family-group name cobitidae Swainson, 1839 confirmed. Bulletin of Zoological Nomenclature, 45(2):178-179.
Anonymous. 1988b. National Parks on the Threatened List. Species, Newsletter of the Species Survival Commission, IUCN, 10:25.
Anonymous. 1988c. The lingering death of the Caspian Sea. New Scientist, 118(1611):26.
Anonymous. 1989. Mullah fish. The Economist, 313(7631):86.
Anonymous. 1991a. The one that didn't get away. Seafood Business, 10(1):36.
Anonymous. 1991b. Plans of the Iranian Fisheries Company regarding utilization of the aquatic resources of the country. Abzeeyan, Tehran, 2:37-39. In Farsi.
Anonymous. 1991c. Introduction to Lurestan Fish Culture Company. Abzeeyan, Tehran, 2:58-59. In Farsi.
Anonymous. 1992a. Iran 21 years on. Newsletter of the Convention on Wetlands, 12:1.
Anonymous. 1992b. Status of fish culture in Chahar Mahal va Bakhtiari Province. Abzeeyan, Tehran, 2:52-53. In Farsi.
Anonymous. 1992c. Iranian fisheries in a glance. Abzeeyan, Tehran, 3:52-55. In Farsi.
Anonymous. 1993a. OPINION 1715. homalopteridae Bleeker, 1859 (Osteichthyes, Cypriniformes): not given precedence over
balitoridae Swainson, 1839. Bulletin of Zoological Nomenclature, 50(1):92-93.
Anonymous. 1993b. A river in the desert. Discover, 14(7):10.
Anonymous. 1993c. Opening day of the first closed system fish culture plan in Iran. Abzeeyan, Tehran, 4(9):52-53. In Farsi.
Anonymous. 1994. Report of a new cyprinid fish in Gilan rivers. Scientific Newsletter of the Iranian Fisheries Research and Training Organization, pp. 12-13. In Farsi.
Anonymous. 1995. Dangerous condition of caviar industry in the Caspian Sea. Abzeeyan, Tehran, 6(1):34-35. In Farsi.
Anonymous. 1996a. Threat to Caspian sturgeon. Oryx, 30(2):93.
Anonymous. 1996b. Inland fisheries in Kermanshah: a natural favourable capacity for development. Abzeeyan, Tehran, 7(1):11-13. In Farsi.
Anonymous. 1996c. Hidden treasures in the Iranian rivers. Shekar-o Tabiat, Tehran, (42):31. In Farsi.
Anonymous, 1996d. Annual report of aquaculture production in Iran. Shilat, Tehran. 120 pp. In Farsi.
Anonymous. 1997a. Islamic Republic of Iran. FAO Aquaculture Newsletter, 17:23.
Anonymous. 1997b. Caviar trade endangers Caspian Sea sturgeons. Fisheries, 22(1):47-48.
Anonymous. 1997c. Iran sees caviar output at 140 tonnes by year end. WorldFish Report, 49:SP/2.
Anonymous. 1997d. Iranian minister urges transfer of fisheries to private sector. WorldFish Report, 51:FS/5.
Anonymous. 1997e. The Ramsar Commission on Wetlands. Ramsar Advisory Missions: Report No. 37, Islamic Republic of Iran. 28 pp.
Anonymous. 1997f. Iran develops farm skills to meet fishing needs. Fish Farming International, 24(5):26-27.
Anonymous. 1997g. Iran goes private! - Moving fisheries out of state control. Fish Farming International, 24(5):38.
Anonymous. 1997h. Annual report of aquaculture production in Iran. Shilat, Tehran. 135 pp. In Farsi.
Anonymous. 1998a. Illegal caviar threatens sturgeon survival. Marine Pollution Bulletin, 36(4):254-255.
Anonymous. 1998b. Iranian caviar exports up. WorldFish Report, 62:SP/2.
Anonymous. 1998c. Iranian exports. Seafood International, 13(11):7.
Anonymous. 1998d. Annual report of aquaculture production in Iran. Shilat, Tehran. 168 pp. In Farsi.
Anonymous. 2000. National Report of Islamic Republic of Iran for the Convention on Biological Diversity, Draft. Department of Environment, Tehran. 40 pp.
(www.abdnet.com/doe/Projects/NBSAP/C_Report/country%20Report(Rev33).htm. downloaded 7 June 2000).
Anonymous. 2001a. Caviar House s'installe en Iran. Aqua Revue, 101:15.
Anonymous. 2001b. Drought in Iran. Careless in a dry country. The Economist, July 28th-August 3rd, 2001, p. 48.
Anonymous. 2001c. Report of aquaculture production in Iran. Shilat, Tehran. 8 pp. In Farsi.
Anonymous. 2002. Report of aquaculture production in Iran. Shilat, Tehran. 98 pp. In Farsi.
Anonymous. 2003. Water crisis threatens the capital. Gostaresh-e Sannat, Tehran, 7:7 pp. (www.netiran.com/Htdocs/WeeklyJournal/Social/wj01503.html, downloaded 23 September 2003).
Anonymous. 2007. Doctor fish. New Scientist, 195(2612):52.
Anoosheh, K. S. and Aliasghar, K. 2009. Caspian Sea fisheries of Iran. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 81 (abstract).
Antoine, S. E. 1983. Limnological investigation in the polluted Rabat Canal and the Shatt al-Arab river, Basrah, Iraq. Nova Hedwigia, 38:497-518.
Antoine, S. E. and Al-Saadi, H. A. 1982. Limnological studies on the polluted Ashar Canal and Shatt al-Arab River at Basrah (Iraq). Internationale Revue der Gesamten Hydrobiologie, 67(3):405-418.
Anuradha, S. 1986. Contributions to the study of Bagrid fishes. 19. Systematic position of Macrones halepensis colvillii Hora & Misra, 1943, with description of a
new species (Siluriformes, Bagridae). Revue Suisse de Zoologie, 93(2):291-296.
Anuradha, S. and Jayaram, K. C. 1985. Contributions to the study of bagrid fishes. 18. Redescription of Mystus pelusius Solander, the type species of the genus Mystus Scopoli.
Bulletin of the Zoological Survey of India, 7(1):107-111.
AnvariFar, H., Khyabani, A., Farahmand, H., Vatandoust, S., AnvariFar, H. and Jahageerdar, S. 2011. Detection of morphometric differentiation between isolated up- and downstream populations of Siah
Mahi (Capoeta capoeta gracilis) (Pisces: Cyprinidae) in the Tajan River, Iran. Hydrobiologia, 673(1):41-52.
Aqrawi, A. A. M. 2001. Stratigraphic signatures of climatic change during the Holocene evolution of the Tigris-Euphrates delta, lower Mesopotamia. Global and Planetary Change, 28(1-4):267-283.
Arabi, M. and Heydarnejad, M. S. 2002. Relative impact of metal ion contamination on functional capacity of gill cells in carp (Cyprinus carpio L.). Book of Abstracts, 27th World
Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Araghi Soureh, A. and Jalali Jafari, B. 2005. A study on monogenean parasites of fishes gills in Mahabad's river with introducing two new species for parasitic fauna of Iran. Pajouhesh va Sazandegi,
17(1)(66):39-45. In Farsi.
Arani, M. M., Allameh, S. K., Esteky, A. A. and Danialy, S. R. 2003. Evaluation of particles abundance and digestion in silver carp Hypophthalmichthys molitrix gut.
AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Arjagh, M. M. 2003. An overview of fisheries and research in Iran. Hook, Line and Thinker, 2003(4):10.
Arjmandi, R., Karbassi, A. and Moogouie, R. 2007. Environmental effects of aquaculture in Iran. Journal of Environmental Science and Technology, 9(2)(33):19-28. In Farsi.
Armantrout, N. B. 1966. Report on observations on streams along the Caspian Sea. Report to the Game and Fish Department, Tehran. MS.
Armantrout, N. B. 1967. Summary of salmon surveys. Report to the Game and Fish Department, Tehran. MS.
Armantrout, N. B. 1967-1968. Recommendations on management of the Caspian salmon. Report to the Game and Fish Department, Tehran. MS.
Armantrout, N. B. 1968a. Seistan-Baluchistan trip report, 9-18 March, 1968. Report to the Game and Fish Department, Tehran. MS.
Armantrout, N. B. 1968b. Observations and recommendations on the fisheries of Iran. Final Report to the Game and Fish Department and South Shilot, Iran. MS.
Armantrout, N. B. 1969. The fishes of Iran, a preliminary checklist. Bander Enzeli, Iran. MS, 39 pp.
Armantrout, N. B. 1980. The freshwater fishes of Iran. Ph.D. Thesis, Oregon State University, Corvallis, Oregon. xx + 472 pp.
Armantrout, N. B. 1990. Fish distribution as influenced by aquatic habitat alteration and species introductions, pp. 199-206. In: Daniel, J. C. and Serrao, J. S. (Eds.). Conservation in developing
countries: problems and prospects. Proceedings of the centenary seminar of the Bombay Natural History Society. Bombay Natural History Society / Oxford University Press, Bombay, Delhi, Calcutta,
Madras. ix + 656 pp.
Armantrout, N. B. and Hosseinzadeh, H. 1967a. Report on trip to Caspian. Report to the Game and Fish Department, Tehran, 2 July MS; 5 August MS.
Armantrout, N. B. and Hosseinzadeh, H. 1967b. Report on trip to Mordab. Report to the Game and Fish Department, Tehran. MS.
Arndt, E. A. and Al-Saadi, H. A. 1975. Some hydrographical characteristics of the Shatt Al-Arab and adjacent areas. Wissenschaftliche Zeitschrift der Universität Rostock
Mathematisch-Naturwissenschaftliche, 24(6):789-796.
Arnold, A. and Längert, H. 1995. Das Moderlieschen. Leucaspius delineatus Biologie, Haltung und Artenschutz. Die Neue Brehm-Bücherei (Band 623), Westarp Wissenschaften, Magdeburg und
Spektrum Akademischer Verlag, Heidelberg, 623:121 pp.
Arnold, J. P. 1912. Cyprinodon (Lebias) spec.? aus Persien und seine Zucht im Zimmeraquarium. Wochenschrift für Aquarien- und Terrarienkunde, 9(45):665-668.
Arrigoni, H. M. and Berg, M. B. 2001. Comparing the effects of the round goby (Neogobius melanostomus) and mottled sculpin (Cottus bairdi) on benthic invertebrate community
structure. International Association for Great Lakes Research, Abstracts from the 44th Conference on Great Lakes Research June 10-14, 2001, Great Lakes Science: Making it Relevant.
University of Wisconsin - Green Bay, Wisconsin Sea Grant Institute, Green Bay, Wisconsin, Part 2:2.
Arshad, U., Aliakbar, A., Sadeghi, M., Jamalzad, F. and Chubian, F. 2006. Pesticide (Diazinon and Butachlor) monitoring in waters of the Shahid Beheshti Sturgeon Hatchery, Rasht, Iran. Journal of
Applied Ichthyology, 22(s1):231-233.
Artyukhin, E., Barannikova, I. A. and Romanov, A. G. 1999. Russian strategies for conservation and reproduction or rare and common Caspian sturgeon species. Journal of Applied Ichthyology,
15(4-5):191-192.
Artyukhin, E. N. 1995. On biogeography and relationships within the genus Acipenser. The Sturgeon Quarterly, 3(2):6-8.
Artyukhin, E. N. 2006. Morphological phylogeny of the Order Acipenseriformes. Journal of Applied Ichthyology, 22(supplement S1):66-69.
Artyukhin, E. N. and Zarkua, Z. G. 1986. Taxonomic status of sturgeon of the Rioni River (Black Sea Basin). Journal of Ichthyology, 26(2):29-36.
Arzi, L., Khorasgani, Z. N. and Arzi, A. 2009. Comparison of the organochlorine pesticide residue levels among benni fish of Shadegan, Mahshahr and Susangerd cities, Khouzestan Province, Iran.
Toxicology Letters, 189(supplement 1):S207.
Asadi, F., Hallajian, A., Asadian, P., Shahriari, A. and Pourkabir, M. 2009. Serum lipid, free fatty acid, and proteins in juvenile sturgeons: Acipenser persicus and Acipenser stellatus.
Comparative Clinical Pathology, 18(3):287-289.
Asadi, F., Masoudifard, M., Vajhi, A., Lee, K., Pourkabir, M. and Khazraeinia, P. 2006. Serum biochemical parameters of Acipenser persicus. Fish Physiology and Biochemistry, 32(1):43-47.
Asadollah, S., Soofiani, N. M., Keivany, Y. and Shadkhast, M. 2011. Reproduction of Capoeta damascina (Valenciennes, 1842), a cyprinid fish, in Zayandeh-Roud River, Iran. Journal of Applied
Ichthyology, 27(4):1061-1066.
Asadollahi, M. 1995. A review of fisheries activities regarding the preservation of sturgeon resources. Abzeeyan, Tehran, 5(7):45-49, III. In Farsi.
Asadzadeh Manjili (sic), A. and Ghorbanzad, A. 1999. Diplostomiasis in cultured rainbow trout (Onchorhynchus (sic) mykiss) in Iran. Iranian Journal of Fisheries Sciences,
7(4):103-110, 8. In Farsi.
Asadzadeh Mangili (sic), A. 2000. Ichthyobodosis in Oncorhynchus mykiss fry. Iranian Journal of Fisheries Sciences, 8(4):89-92, 8. In Farsi.
Asadzadeh Mangili (sic), A., Mokhayer, B. and Jajali, B. 2000. Health assessment of external parasites of cultured Cyprinidae in pen culture of Anzali Lagoon. Pajouhesh va Sazandegi,
13(3)(47):96-101. In Farsi.
Asaeian, H., Mousavi, M., Haghighi, M. and Rezvani, M. 2006. Investigation of survival ratio and growth rate in offspring of Salmo trutta caspius.
Iranian Journal of Fisheries Sciences, 15(3):109-118. In Farsi.
Asadzadeh Mangili (sic), A. 2000. Occurrence of gas-bubble disease in rainbow trout. Iranian Journal of Fisheries Sciences, 10(3):101-103, 8. In Farsi.
Ashtiani-Zarandi, M. A. 1990. The current status of waterfowl and wetland conservation in the Islamic Republic of Iran, pp. 81-83. In: Matthews, G. V. T. (Ed.). Managing Waterfowl Populations.
Proceedings of the International Waterfowl and Wetlands Research Bureau (IWRB) Symposium, Astrakhan 1989, IWRB Special Publication, Slimbridge, 12.
Askarian, F. and Kousha, A. 2006. Investigation on effect of photoperiod on growth indices in beluga Huso huso. World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Askarian, F. and Kousha, A. 2006. An overview on use of enzyme in processing of fish with emphasize (sic) on fish sauce. World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy
(abstract).
Askarian, F. and Kousha, A. 2008. The influence of feeding ration on the acute stress response of beluga (Huso huso). Journal of Fisheries and Aquatic Science, 3(6):366-374.
Askarian, F. and Kousha, A. 2009. The influence of photoperiod in farming beluga sturgeon (Huso huso): evaluation by growth and health parameters in serum. Journal of Fisheries and Aquatic
Science, 4(1):41-49.
Askarian, F., Kousha, A. and Bahmani, M. 2006. Serum osmoregulatory parameters on beluga sturgeon Huso huso (Pisces: Acipenseriformes): effect of different light regimes. World Aquaculture
Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Askarian, F., Kousha, A., Khorshidi, K. J. and Matinfar, A. 2006. Future of applying yeast as probiotics in culture of Acipenseridea (sic) family. World Aquaculture Society, Aqua 2006,
May 9-13. Florence, Italy (abstract).
Askarian, F., Matinfar, A., Kousha, A., Bahmani, M., Khorshidi, K., Shenavar, A. and Ringø, E. 2008. Diversity of lactic acid bacteria in the gastrointestinal tracts of reared beluga (Huso huso)
and Persian sturgeon (Acipenser persicus): A comparative study. Journal of Fisheries and Aquatic Science, 3(5):302-311.
Askerov, F. S., Zaytsev, Y. Y., Kasumov, R. Y. and Kuliyev, Z. 2001. Bioraznoobrazie: Chudesnye Ryby Kaspiya/Biodiversity: Amazing Caspian Fishes. Print Studio, Baku. 164 pp. In Russian and English.
Aslaanparviz, H. 1991. Gobiid fishes of the Caspian Sea (Gobiidae). Abzeeyan, Tehran, 2(November):36-39. In Farsi.
Aslaanparviz, H. 1992. Survival and growth of sturgeon fry released in the Caspian Sea. Abzeeyan, Tehran, 2:4-9. In Farsi.
Aslaanparveez, H. 1993. Iranian acetra and the future prospects for its propagation in Volga hatcheries. Abzeeyan, Tehran, 4(8):52-55, III. In Farsi.
Aslaanparviz, H. 1994. Artificial spawning of Caspian white fish, Rutilus frissii (sic) kutum. Abzeeyan, Tehran, 4(12):28-29. In Farsi.
Aslan Parviz, H. and Aghaei Moghadam, A. A. 2003. The study on the nutrition of the juvenile sturgeon (Acipenser persicus) in fish ponds of Shahid Rajaie's Centre (1999).
Pajouhesh-va-Sazandegi, 16(6)(60):77-83. In Farsi.
Assadi, H. and Dehqani Posterudi, R. 1997. Atlas-e Mahian-e Khalij-e Fars o Dary-ye Oman/Atlas of the Persian Gulf & the Sea of Oman Fishes. Iranian Fisheries Research and Training
Organization, Tehran. 10 + 226 + 23 pp. In Farsi and English.
Ataei Mehr, B., Mojazi Amiri, B., Abd Elahi, H. and Mirvaghefi, A. R. 2007. Study on the effect of weight and salinity on changes in number and size of kidney Malpighian bodies of juvenile Caspian
Sea brown trout, Salmo trutta caspius. Iranian Journal of Natural Resources, 60(2):577-585. In Farsi.
Ataei Mehr, B., Mojazi Amiri, B., Mirvaghefi, A. R. and Abdolhay, H. 2006. Changes in gill histology and mortality rate of juvenile Caspian Sea brown trout
(Salmo trutta caspius Kessler, 1877) in different weights and water salinities. Iranian Journal of Fisheries Sciences, 15(3):119-128. In Farsi.
Ataur-Rahim, M. 1981. Observations on Aphanius dispar (Rüppell, 1828), a mosquito larvivorous fish in Riyadh, Saudi Arabia. Annals of Tropical Medicine and Parasitology, 75(3):359-362.
Avakh-Kismi, M. 1996. The first Iranian fish propagation plant replaced by a sand/silt producer plant. Abzeeyan, Tehran, 7(10):36-38. In Farsi.
Avanesov, E. M. 1972. Present spawning conditions of mullets (genus Mugil) in the Caspian Sea. Journal of Ichthyology, 12(3):419-425.
Averintsov, S. V. and Sych, N. O. 1930. O metodike opredeleniya ras (populyatsii) u otdel'nykh podvidov kaspiiskikh sel'dei [Methods of identifying races (populations) in various subspecies of
Caspian herrings]. Trudy Astrakhanskoi nauchnoi rybokhozyaistvennoi stantsii, 7(3):5-15 (also Zoologischer Anzeiger, 85(1929):99-105).
Axelrod, H. R. 1993. The Most Complete Colored Lexicon of Cichlids. Every known cichlid illustrated in colour. Tropical Fish Hobbyist Publications Inc., Neptune, New Jersey. 864 pp.
(Second Edition, 1996).
Ayati, B. 2003. Investigation of sanitary and industrial wastewater effects on Anzali Reserved Wetland (Final Report). Report presented to MAB-UNESCO by Environmental Engineering Division,
Civil Engineering Department, Tarbiat Modarres University, Tehran. 52 pp.
Ayazi, M. 1961. Drainage and reclamation problems in the Garmsar area, pp. 285-290. In: Salinity Problems in the Arid Zones. Proceedings of the Teheran Symposium, UNESCO, Rome. 395 pp.
Aydin, R., Calta, M., Sen, D. and Coban, M. Z. 2004. Relationships between fish lengths and otolith length in the population of Chondrostoma regium
(Heckel, 1843) inhabiting Keban Dam Lake. Pakistan Journal of Biological Sciences, 7(9):1550-1553.
Azari-Takami, Gh. 1977. A study on the unsuitable conditions caused by Apus sp. in sturgeon fish pond culture. In: Proceedings of the XX Congress of the Societas Internationalis
Limnologiae, Copenhagen, August, 1977.
Azari Takami, G. 1975. Culture of Artemia salina for nutrition of small fishes. Journal of the Iranian Veterinary Medical Association, 5(1):10-16.
Azari Takami, G. 1979. Fecundity of Rutilus frisii kutum (Kamensky). Journal of the Veterinary Faculty of the University of Tehran, 35(1-2):66-78. In Farsi.
Azari Takami, G. 1984. The principles of fish propagation and farming. Shilat, Tehran. 152 pp. In Farsi.
Azari Takami, G. 1987. The use of Artemia from Ormia Lake (Iran) as food for sturgeon fry, p. 467-468. In: Sorgeloos, P., Bengston, D. A., Declair, W. and Jaspers, E.
(Eds.). Artemia Research and its Applications, Volume 3. Ecology, Culturing, Use in Aquaculture. Universa Press, Wetteren, Belgium. 556 pp.
Azari Takami, G. 1990. The nutritional value of Artemia in feeding acipenserids. In: The Collection of Papers on Proper Exploitation of Caspian Fish Reserves, National Conferences
in Iranian Shilat Company, pp. 509-523. In Farsi.
Azari Takami, G. 1993. Uromieh lake as a valuable source of Artemia for feeding sturgeon fry. Journal of the Veterinary Faculty of the University of Tehran, 47(3 & 4):2-14. In Farsi.
Azari Takami, G. 1997. Some new data on artificial propagation and source of broodstocks of beluga (Huso huso) in Iran. Third International Symposium on Sturgeon, Piacenza, Italy,
8-11 July 1997, Book of Abstracts (poster).
Azari Takami, G. 1999. New data on propagation and source of beluga (Huso huso) broodstock in Iran. Journal of Applied Ichthyology, 15(4-5):299.
Azari Takami, Gh. 2001. Possibilities of sturgeon culture and problems of broodstocks in southern part of Caspian Sea, Iran. 4th International Symposium on Sturgeon, Oshkosh,
Wisconsin, 8-13 July 2001 (abstract).
Azari Takami, Gh., Amini, F. and Kalbasi, M. 1997. Induction of triploidy in rainbow trout (Oncorhynchus mykiss) by thermic shocks. Journal of the Faculty of Veterinary Medicine,
University of Tehran, 52(2):60-70. In Farsi.
Azari Takami, G., Amini, F. Ranjbar, T. and Khodaii, A. 1997a. Experimental survey of Caspian Sea mullets culture in brackish water of salt desert region of Iran. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 51(1-2):59-69. In Farsi.
Azari Takami, G., Amini, F. Ranjbar, T. and Khodaii, A. 1997b. The historical development of the caviar trade and the caviar industry. Journal of the Faculty of Veterinary Medicine,
University of Tehran, 51(1-2):70-79.
Azari Takami, Gh., Etessami, S. and Saremi, A. 1980. Régime alimentaire des esturgeons (Acipenseridés) sur les côtes sud de la mer Caspienne. Cybium, 3e série (10):65-72.
Azari Takami, Gh., Farahmand, H. and Bahrami Kamangar, B. 2002. Induction of gynogenesis in rainbow trout (Oncorhynchus mykiss) by UV-radiation. Iranian Journal of Natural Resources,
54(4):369-382. In Farsi.
Azari Takami, G. and Khairandish, M. B.1981. A study on the unsuitable conditions caused by Apus sp. and Leptestheria sp. in sturgeon fish pond culture.
Journal of the Veterinary Faculty, University of Tehran, 36(2):1-20. In Farsi.
Azari Takami, G., Meshkini, S., Rassouli, A. and Amini, F. 2005. The nutritional effects of vitamin C-enriched Artemia urmiana nauplii on growth, survival rate and resistance to environmental
stresses in rainbow trout (Oncorhynchus myskiss) larvae. Pajouhesh va Sazandegi,
17(1)(66):25-32. In Farsi.
Azari Takami, G., Pousty, I. and Ebrahimi, I. 1997. Study on determination of reproductive potential in Iranian sturgeon broodstocks (Acipenser persicus Borodin, 1897).
Journal of the Faculty of Veterinary Medicine, University of Tehran, 51(3-4):97-112.
Azari Takami, G., Razavi, B. and Hosseinpoor, N. 1990. A study on artificial propagation and culturing of the white fish Rutilus frisii kutum (Kamensky) (sic)
in Iran. Journal of the Veterinary Faculty of the University of Tehran, 45(1):69-90. In English.
Azari Takami, G., Shahidi, H. and Adeli, A. 2000. Estimation of female silver carp broodstocks "Hypophthalmichthys molitrix" for selection breeding. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 55(2):45-50. In Farsi.
Azarvandi, A.R., Dalimi Asi, A., Ghamari, Z. and Ghebleh, F. 1999. A study on intestinal helminths of pond cultured fish in west Azerbaijan province. Pajouhesh va Sazandegi Journal,
43:42-43 (In Farsi).
B
Babaev, A. G., Ammaniyazov, K. N. and Muradov, Ch. 1997. Kolevanie uroviya kaspiiskogo morya i ego ekologicheskie posledstviya [Fluctuation in the Caspian Sea level and its ecological
consequences]. Problemy Osvoeniya Pustyn', 6(1996):3-11
Babaii, A. 1995. Examining the fish consumption market development in Iran (Tehran). M.Sc. Thesis, University of Tehran. 232 pp. In Farsi.
Babaii, M. 2002. Effective parameters on farmed fish consumption. M.Sc. Thesis, University of Tehran. 231 pp. In Farsi.
Babushkin, N. Ya. 1942. K sistematike kaspiiskoi belugi [On the systematics of Caspian beluga]. Izvestiya Azerbaidzhanskoi Nauchno-Issledovatel'skoi Rybokhozyaistvennoi Stantsii, Baku, 7:115-132.
Babushkin, N. Ya. 1964. Biology and catch of the Caspian beluga. Trudy VNIRO, 52:183-258. In Russian.
Băcescu, M. 1962. Contribution à la systématique du genre Cobitis. Description d'une espèce nouvelle, Cobitis calderoni, provenant de l'Espagne.
Revue de Biologie, Bucarest, 6(4)(1961):435-448.
Backiel, T. and Zawisza, J. 1968. Synopsis of biological data on the bream Abramis brama (Linnaeus, 1758) Food and Agriculture Organization, Rome, Fisheries Synopsis, 36:iv + 110 pp.
Badalov, F. G. 1972. The food of Clupeonella delicatula (Nordm.) and C. engrauliformis (Borodin) in Bol'shoy Kyzylagach Bay in the Caspian.
Journal of Ichthyology, 12(6):1027-1030.
Badiei, N. H. 2002. Identification and study of qanat ichthyofauna of
Bedjestan basin. M.Sc. Thesis, Ferdowsi University, Mashhad. 167 pp. In Farsi.
Bagarinao, T. 1994. Systematics, distribution, genetics and life history of milkfish, Chanos chanos. Environmental Biology of Fishes, 39:23-41.
Bagarinao, T. U. 1991. Biology of milkfish (Chanos chanos Forsskal). Aquaculture Department, Southeast Asian Fisheries Development Center, Tigbauan, Iloilo, Philippines. vii + 94 pp.
Bagheri, A., Kamrani, E. and Esmaeili, H. R. 2010. Idenitified fishes of Kol River in Hormuzgan Province. Iranian Scientific Fisheries Journal, 19(2):143-148. In Farsi.
Bagheri, N. 2002. In an interview with Dr. Masumeh Ebtekar, Vice President in Charge of the Department of Environment. Nowruz, Tehran, 2(361):10 pp.
Bagheri, S. 1999. An ecological investigation on macrobenthic assemblage of Choghakhour Lagoon, Iran. Iranian Journal of Fisheries Sciences, 5(3):37-52, 4. In Farsi.
Bagheri, S. 2005. Distribution and abundance of Mnemiopsis leidyi in the southern coasts of Caspian Sea (Guilan Province). Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Bagheri, S. 2006. An investigation on abundance and distribution of Mnemiopsis leidyi in Guilan waters, southwest Caspian Sea. Iranian Journal of Fisheries Sciences, 14(4):1-16. In Farsi.
Bagheri, S., Kideys, A. and Mirzajani, A. 2005. Effect of Mnemiopsis leidyi invasion on zooplankton in the Iranian coasts of the Caspian Sea. Borok II, International Workshop, Invasions of
Alien Species in Holarctic, 27 September-1 October 2005, Borok (abstract).
Bagherian, A. and Rahmani, H. 2007. Morphological differentiation between two populations of the shemaya, Chalcalburnus chalcoides: a geometrical morphometric approach. Zoology in the
Middle East, 40:53-62.
Bagherian, A. and Rahmani, H. 2009. Morphological discrimination between two populations of shemaya, Chalcalburnus chalcoides (Actinopterygii, Cyprinidae) using a truss network. Animal
Biodiversity and Conservation, 32(1):1-8.
Baghfalaki, M., Hosseini, S. A., Imanpour, M. R., Soudagar, M., Shalouei, F. and Iri, M. 2009. Effect of Cyprinus carpio wild (sea) broodstock stocking density on survival and some growth
parameters of larvae and fingerlings in earthen ponds. Journal of Agricultural Sciences and Natural Resources, 16(3):39-47. In Farsi.
Baghfalaki, M., Shalouei, F. and Imanpour, M. R. 2009. The relationships between some spermatological and biochemical parameters of beluga (Huso huso Linnaeus 1758) semen in the
south-eastern Caspian Sea. Iranian Journal of Biology, 22(2):312-320. In Farsi.
Bagirova, Sh. M., Askerova, Kh. M., Agayarova, A. E. and Agaeva, S. A. 2003. Dannye o biologicheskoi kharakteristike lenkoranskoi khramuli i kurinskogo usacha v p. Lenkoranchai [Data on the
biological characteristics of the Lenkoran khramulya and the Kura barbel in the Lenkoranchai River]. Azerbaycan Milli Elmler Akademiyasinin Xeberleri, Biologiya Elmleri Seriyasi, 2003(3-4):128-132.
In Azerbaijanian.
Bagirova, Sh. M., Kuliev, Z. M. and Askerova, Kh. M. 1990. Morfobiologicheskaya kharakteristika karasya - Carassius auratus gibelio (Bloch) v vodoemakh Azerbaidzhana [Morphobiological
characteristics of crucian carp - Carassius auratus gibelio (Bloch) in the reservoirs of Azerbaijan]. Izvestiya Akademii Nauk Azerbaidzhanskoi SSR, Seriya Biologischeskikh Nauk, 1990(1):57-64.
Bagley, F. R. C. 1976. A bright future after oil: dams and agro-industry in Khuzistan. The Middle East Journal, 30(1):25-35.
Bagnall, R. 1919. Large carp from Mesopotamia. Journal of the Bombay Natural History Society, 26:679-680.
Bagnis, R., Berglund, F., Elias, P. S., van Esch, G. J., Halstead, B. W. and Kojima, K. 1970. Problems of toxicants in marine food products. 1. Marine biotoxins. Bulletin
of the World Health Organization, 42:69-88.
Bahabadi, M. N. 2006. Intensive culture of rainbow trout (O. mykiss) in earth pond brackish water with aeration system. Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Yazd-Bafgh. http://en.ifro.ir (abstract).
Bahmani, M. 1998. Phylogenic (sic) and systematic study on sturgeons. Iranian Journal of Fisheries Sciences, 7(2):9-30, 2-3. In Farsi.
Bahmani, M. 1999. Study of the ecophysiological stress effects on HPG and HPI axes, immunity system and reproduction processes in Persian sturgeon, Acipenser persicus. Ph.D. Thesis,
Islamic Azad University, Tehran. 277 pp.
Bahmani, M. 2006. Qualitative assessment of sturgeon. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Rasht.
http://en.ifro.ir (abstract).
Bahmani, M. and Kazemi, R. 1998. Histological study on the gonads of reared juvenile sturgeon. Iranian Fisheries Scientific Journal, 7(1):1-16, 1-2. In Farsi.
Bahmani, M., Kazemi, R. and Donskaya, P. 1999. Comparative study of biochemical and hematological features in reared sturgeons. Iranian Journal of Fisheries Sciences, 1(2):61-73, 82.
Bahmani, M., Kazemi, R. and Donskaya, P. 2001. A comparative study of some hematological features in young reared sturgeons (Acipenser persicus and Huso huso).
Fish Physiology and Biochemistry, 24(2):135-140.
Bahmani, M., Kazemi, R., Pour Dehghani, M., Halajian, A., Mohseni, M., Dejhandian, S., Vahabi, Y., Malekzad, R. and Mohamadi Parshkouhi, H. 2006. A new method for artificial breeding of
Acipenser stellatus. Iranian Journal of Fisheries Sciences, 14(4):31-48. In Farsi.
Bahmani, M. and Oryan, Sh. 2004. Study of catch, transport and confinement effects of stress on blood leukocytes index (BLI) levels of Persian sturgeon, Acipenser persicus in south Caspian Sea.
Journal of Sciences, Islamic Azad University, 13(50):4117-4130. In Farsi.
Bahmani, M., Oryan, S., Pourkazemi, M. and Vosoughi, G. 2000b. Morpho-physiological studies in female sturgeon, Acipenser persicus broodfishes in the south Caspian
Sea. Paper presented at the First Iranian Congress of Veterinary Basic Sciences, Tehran University.
Bahmani, M., Oryan, S., Pourkazemi, M. amd Vosoughi, G. 2000b. Ecophysiological indicators of stress in female Persian sturgeon, Acipenser persicus. Iranian Journal of Fisheries Sciences,
2(1):37-52, 110.
Bahmani, M., Oryan, S., Pourkazemi, M. amd Vosoughi, G. 2001. Ecophysiological effects of stress (HPI axis) on levels of sex steroids (HPG axis) during artificial breeding in female Persian sturgeon,
Acipenser persicus. International Union of Physiological Sciences, 2001 abstracts, 1 p.
Bahmani, M., Pirasteh, S., Baradaran Noveyri, Sh., Kazemi, R. A. and Kouchinian, P. 2007. Water osmolarity effect on spermatocrit and its relations to spermatozoan count in male breeders of
Rutilus frisii kutum in the south-western Caspian Sea. Iranian Scientific Fisheries Journal, 16(2):11-18. In Farsi.
Bahmani, M., Yousefi Jourdehi, A., Kazemi, R. A., Pourdehghani, M., Hallajian, A., Dezhandian, S. and Jalailpour, J. 2009. Seasonal fluctuations of sex steroids (testosterone, 17 >α-hydroxy
progesterone and 17 β-estradiol) with sexual development in farmed stellate sturgeon (Acipenser stellatus). Iranian Scientific Fisheries Journal, 17(4):7-16. In Farsi.
Bahrami Kamangar, B., Mojazi Amiri, B., Javad Rassaie, M., Abtahi, B. and
Bahmani, M. 2006. Insulin-like growth factor-1 can induce oocyte maturation in Persian sturgeon (Acipenser persicus), in vitro. Iranian Journal of Natural Resources, 59(1):151-165. In Farsi.
Bahramian, B. 2001. The role Shahid Bahonar Cold Water Fishes Center at Salmo trutta caspius stocking. Mazandaran
Fisheries Research Center Report, 59 pp. In Farsi.
Bahrekazemi, M., Matinfar, A., Soltani, M., Abtahi, B., Pusti, I. and Mohagheghi, A. 2009. The relation between egg viability, selected aspects of egg and ovarian fluid composition and time of
stripping in endangered Caspian brown trout (Salmo trutta caspius). Journal of Fisheries and Aquatic Science, 4(6):306-315.
Bahrekazemi, M., Mojazi Amiri, B., Pousti, I. and Vilaki, A. S. 2004. Histochemical study on the development of the digestive tract of the Caspian salmon, Salmo trutta caspius, from hatching
to parr stage. Proceedings of the First Congress on Animal and Aquatic Sciences, University of Tehran, pp. 1032-1034.
Bahre Kazemi (sic), M., Soltani, M., Matinfar, A., Abtahi, B., Pusti, I., Mohagheghi Samarin, A. and Mojazi Amiri, B. 2010. Biochemical and histological studies of over-ripened oocyte in
the Caspian brown trout (Salmo trutta caspius) to determine biomarkers for egg quality. Iranian Journal of Fisheries Sciences, 9(1):33-48.
Bahrkazemi (sic), M., Mojazi Amiri, B., Pousti, I. and Vilaki, A. S. 2006. A histochemical study of the digestive tract of Caspian salmon (Salmo trutta caspius) from hatching to parr
stage. Iranian Journal of Natural Resources, 59(3):639-648. In Farsi.
Bailey, M. 2007. Back from the dead - Iranocichla hormuzensis Coad, 1982. Cichlidae, 27(3):17.
Bailey, R. M. 1951. The authorship of names proposed in Cuvier and Valenciennes' "Histoire Naturelle des Poissons". Copeia, 1951(3):249-251.
Bailey, R. M. and Robins, C. R. 1988. Changes in North American fish names, especially as related to the International Code of Zoological Nomenclature, 1985. Bulletin of Zoological Nomenclature,
45(2):92-103.
Baird, S. F. 1873. The fish of the Caspian Sea. Annual Record of Science and Industry, New York, 1873-1875:459.
Ba Kader, A. B. A., Al Sabbagh, A. L. T. El S., Al Glenid, M. Al S. and Izzidien, M. Y. S. 1983. Basic paper on the Islamic principles for the conservation of the natural
environment. International Union for Conservation of Nature and Natural Resources, Gland. 25 pp.
Bakhshizod-Mahmoodi, A. 1996. Different methods of fishing in the inland waters of Gilan Province. Abzeeyan, Tehran, 7(10):39-41. In Farsi.
Bakhtiarian, H., Abdoli, A. and Pazooki, J. 2008. Inter-population variation of Capoeta buhsei in central basin of Iran. International Conference on Biodiversity Conservation and Management,
University of Science and Technology, Cochin, Kerala, India, 3-6 February 2008 (abstract).
Bakhtiyari, M., Kamal, S., Abdoli, A., Esmaeili, H. R. and Ebrahimi, M. 2011. Comparison of the feeding behaviour and strategy of the killifish, Aphanius sophiae Heckel, 1847, at two different
localities in Iran (Actinopterygii: Cyprinodontidae). Zoology in the Middle East, 52:47-56.
Bakkala, R. G. 1970. Synopsis of biological data on the chum salmon, Oncorhynchus keta (Walbaum) 1792. Food and Agricultue Organization, Rome, Species Synopsis, 41:iii + 89 pp.
Bakshtanskii, E. L., Rimsh, E. Y. and Kyasimov, I. B. 1973. Efficiency of salmon hatcheries in the Kura River basin, and ecological characteristics of trout and sea trout.
Fisheries Research Board of Canada Translation Series, 2370:64 pp.
Balasem, A. N., Dalli, F. A. and Mutar, A. J. 1994. Karyotyping of Barbus sharpeyi. Cytobios, 78(314):177-180.
Balik, I. 1999. The feeding features of the pike-perch (Stizostedion lucioperca) population in Lake Beyşehir. Turkish Journal of Zoology, 23(2):189-194.
Balık, S., Ustaoğlu, M. R., Sarı, H. M. and Özbek, M. 1996. Kuş Gölündeki (Bandırma) Tatlısu Kolyozu (Chalcalburnus chalcoides Güldenstaedt, 1772)
Populasyonunun Biyolojik Özelliklerinin İncelenmesi [Investigations on biological characteristics of the Danube bleak (Chalcalburnus chalcoides Güldenstaedt, 1772) population in Lake
Kuş (Bandırma)]. Su Ürünleri Dergisi, 13(1-2):171-182.
Balletto, E. and Spanò, S. 1977. Ciprinidi del genere Garra Hamilton 1822, raccolti nello Yeman dal Prof. Giuseppe Scortecci. Annali del Museo Civico di Storia Naturale "Giacomo
Doria", Genova, 81:246-287.
Balon, E. K. 1974. Domestication of the carp Cyprinus carpio L. Royal Ontario Museum Life Sciences Miscellaneous Publication, 37 pp.
Balon, E. K. 1995. The common carp, Cyprinus carpio: its wild origin, domestication in aquaculture, and selection as colored nishikigoi. Guelph Ichthyology Reviews, 3:1-55.
Balon, E. K. 2006. The oldest domesticated fishes, and the consequences of an epigenetic dichotomy in fish culture. Aqua: Journal of Ichthyology and Aquatic Biology, 11(2):47-86.
Baltz, D. M. 1991. Introduced fishes in marine systems and inland seas. Biological Conservation, 56:151-177.
Bamber, R. N. and Henderson, P. A. 1985. Morphological variation in British atherinids, and the status of Atherina presbyter Cuvier (Pisces: Atherinidae). Biological Journal of the Linnaean
Society, 25:61-76.
Banagar, G. R., Kiabi, B. H., Homayoonnezhad, I., Piri, I. and Amirian, P. 2008. Biodiversity of fish species in Haraz River (an ecological approach). World Applied Sciences Journal, 5(1):5-11.
Banagar, Gh. R., Karami, M., Hasanzadeh Kiabi, B. and Ghasempouri, S. M. 2009. Distribution and biodiversity of fish species in Haraz River in Mazandaran
Province. Environmental Sciences, 6(2):21-31.
Banarescu, P. 1960. Einige Fragen zur Herkunft und Verbreitung der Süβwasserfischfauna der europäisch-mediterranen Unterregion. Archiv für Hydrobiologie, 57(1/2):16-134.
Banarescu, P. 1961. Weitere systematische Studien über die Gattung Gobio (Pisces, Cyprinidae) insbesondere im Danaubecken. Vĕstník československé Společnosti zoologické, 25:318-416.
Banarescu, P. 1968. Süßwasserfische der Türkei. Ergänzende Angaben zu Teil 2: Cobitidae. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 65:353-356, Taf. 1-3.
Bănărescu, P. 1969. Characterisation of world freshwater fish faunas according to Mayr (1965)'s schema. Revue Roumaine de Biologie, Zoologie, Bucarest, 14(1):9-15.
Bănărescu, P. 1970. Die Fische des pontokaspischen potamophilen Artenkomplexes und die karparto-kaukasische Disjunktion. Hidrobiologia, Bucarest, 11:135-141.
Bănărescu, P. 1973. Some reconsiderations on the zoogeography of the Euro-Mediterranean fresh-water fish fauna. Revue Roumaine de Biologie, Zoologie, Bucarest, 18(4):257-264.
Banarescu, P. 1976. Position zoogéographique de l'ichthyofaune d'eau douce de l'Asie occidentale. Revue des Travaux de l'Institut des Pêches maritimes, 40(3 et 4):486-487.
Banarescu, P. 1977. Position zoogéographique de l'ichthyofaune d'eau douce d'Asie occidentale. Cybium, 3(2):35-55.
Bănărescu, P. M. 1986. A review of the species of Crossocheilus, Epalzeorhynchos and Paracrossocheilus (Pisces, Cyprinidae). Travaux du Muséum d'Histoire naturelle
"Grigore Antipa", Bucarest, 28:141-161.
Banarescu, P. M. 1989. Vicariant patterns and dispersal in European freshwater fishes. Spixiana, 12(1):91-103.
Bănărescu, P. M. 1991. The subspecies in freshwater ichthyology. Evolution and Adaptation, Cluj, 4:183-190.
Bănărescu, P. M. 1992a. A critical updated checklist of Gobioninae (Pisces, Cyprinidae). Travaux du Muséum d'Histoire naturelle "Grigore Antipa", Bucarest, 32:303-330.
Bănărescu, P. 1992b. Zoogeography of Fresh Waters. Volume 2. Distribution and Dispersal of Freshwater Animals in North America and Eurasia. AULA-Verlag, Wiesbaden. pp. 519-1091.
Bănărescu, P. 1995. Zoogeography of Fresh Waters. Volume 3. Distribution and Dispersal of Freshwater Animals in Africa, Pacific Areas and South America. AULA-Verlag, Wiesbaden. pp. 1092-1617.
Bănărescu, P. M. 1997. The status of some nominal genera of Eurasian Cyprinidae (Osteichthyes, Cypriniformes). Revue Roumaine de Biologie, Série de Biologie Animale, 42(1):19-30.
Bănărescu, P. M. (Ed.). 1999. The Freshwater Fishes of Europe. Volume 5. Cyprinidae 2. Part I: Rhodeus to Capoeta. AULA-Verlag, Wiebelsheim. xviii + 427 pp.
Bănărescu, P. M. and Bogutskaya, N. G. (Eds.). 2003. The Freshwater Fishes of Europe. Volume 5/II. Cyprinidae 2. Part II: Barbus. AULA-Verlag, Wiebelsheim. x + 454 pp.
Bănărescu, P. and Coad, B. W. 1991. Cyprinids of Eurasia, Chapter Five, pp. 127-155, 1 figure, 1 table. In: Winfield, I. J. and Nelson, J. S. Cyprinid Fishes. Systematics, biology and
exploitation. Chapman & Hall, London. 667 pp., 132 illustrations.
(Fish and Fisheries Series, 3).
Banarescu, P. M. and Herzig-Straschil, B. 1995. A revision of the species of the Cyprinion macrostomus-group (Pisces: Cyprinidae). Annalen des naturhistorischen Museums in Wien, 97B:411-420
Banarescu, P. and Mirza, M. R. 1965. Noemacheilus lindbergi n. sp., a new loach from Afghanistan and West Pakistan. Senckenbergiana biologica, 46(4):265-269.
Banarescu, P. und Nalbant, T. 1964. Süßwasserfische der Türkei. 2. Teil Cobitidae. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 61:159-201.
Bănărescu, P. and Nalbant, T. 1966. The 3rd Danish Expedition to Central Asia. Zoological Results 34. Cobitidae (Pisces) from Afghanistan and Iran.
Videnskabelige Meddelelser fra Dansk naturhistorisk Forening, 129:149-186, pl. XIX-XXI.
Banarescu, P. and Nalbant, T. 1973. Pisces, Teleostei, Cyprinidae (Gobioninae). Das Tierreich, Berlin, 93:vii + 304 pp.
Bănărescu, P. M. and Nalbant, T. T. 1995. A generical classification of Nemacheilinae with description of two new genera (Teleostei: Cypriniformes: Cobitidae). Travaux du Muséum d'Histoire
Naturelle Grigore Antipa, Bucurešti, 35:429-496.
Bănărescu, P. M. and Nalbant, T. T. 1998. Cobitis elongata Heckel and Kner, 1858 (Pisces: Ostariophysi: Cobitidae): distribution, relationships, geographical variation and
protection status. Revue Roumaine de Biologie, Série de Biologie Animale, 43(1-2):57-65.
Banarescu, P., Nalbant, T. T. und Balik, S. 1978. Süßwasserfische der Türkei 11. Teil. Die Gattung Orthrias in der Türkei und in Südbulgarien (Pisces, Cobitidae, Noemacheilinae).
Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 75:225-266, 1 Taf.
Banarescu, P., Nalbant, T. T. and Goren, M. 1982. The Noemacheiline loaches from Israel (Pisces: Cobitidae: Noemacheilinae). Israel Journal of Zoology, 31:1-25.
Bănărescu, P. M. and Paepke, H-J. (Eds.). 2002. The Freshwater Fishes of Europe. Volume 5/III. Cyprinidae 2. Part III: Carassius to Cyprinus. Gasterosteidae. AULA-Verlag,
Wiebelsheim. xi + 305 pp.
Bandani, G. 2006a. Studying quality and quantity of fingerling of some bony fish. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Bandani, G. 2006b. Survey of biological condition of fingerlings released into Gorgan-rood. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry
Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Banerjee, S. K., Misra, K. K., Banerjee, S. and Ray-Chaudhuri, S. P. 1988. Chromosome numbers, genome sizes, cell volumes and evolution of snake-head fish (family Channidae).
Journal of Fish Biology, 33:781-789.
Bani, A., Zad Bahger, F. and Jadidi, N. 2008. Brain morphology in Acipenser stellatus and Acipenser persicus. Iranian Journal of Biology, 21(2):341-350. In Farsi.
Banister, K. E. 1980. The fishes of the Tigris and Euphrates rivers, pp. 95-108. In: Rzóska, J. Euphrates and Tigris, Mesopotamian ecology and destiny. Monographiae Biologicae, 38:x + 122 pp.
Banister, K. 1992. Blind cave fishes. Aqua Geõ-graphia, 2:65-73.
Banister, K. E. and Bunni, M. K. 1980. A new blind cyprinid fish from Iraq. Bulletin of the British Museum (Natural History) Zoology, 38(3):151-158.
Banister, K. E. and Clarke, M. A. 1977. The freshwater fishes of the Arabian Peninsula. Journal of Oman Studies, Special Report, 1:111-154, 1 map.
Bankehsaz, Z. 1996. Study of the fluctuation in the fat content of anchovy (Cl. engrauliformis) during one year. Abzeeyan, Tehran, 7(9):9-11. In Farsi.
Bankehsaz, Z. 2009. Quality control (total volume nitrogen and peroxide value) of Persian caviar during processing and cold storage. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009,
Basrah, p. 112 (abstract).
Ba-Omar, T. A. and Prentis, P. F. 1992. An ultrastructural study of Sertoli cells in two geographically isolated populations of Aphanius dispar, p. 548-549.
In: Bailey, G. W., Bentley, J. and Small, J. A. (Eds.). Proceedings of the 50th Annual Meeting of the Electron Microscopy Society of America, Part 1. Boston.
Baqeri, O. 2000. Chaghakhor Wetland and its general characteristics. Moj-e Sabz, Tehran, 1:36-38. In Farsi (English translation at www.netiran.com/Htdocs/Clippings/Social/201209XXSO01.html, downloaded
15 December 2000).
Barach, G. P. 1934. K sistematike i geograficheskomu rasprostraneniyu kavkazskikh golavlei. 1. Istoricheskii ocherk isucheniya kavkazskikh golavlei [On the systematics and geographical distribution of
the Caucasian chubs. 1. Historical sketch of the Caucasian chubs studied]. Trudy Zoologicheskogo Sektora, Akademiya Nauk SSSR, Zakavzkazskii Filial Gruzinskoe Otdelenie, 1:91-133.
Barach, G. P. 1939. Ryby presnykh vod Abkhazii [Freshwater fishes of Abkhazia]. Materialy po fauna Abkhazii, izdanie Gruzinskogo filiala Akademii Nauk, Tbilisi, 1939:45-114.
Barach, G. P. 1940. Ryby Armenii [The fishes of Armenia]. Trudy Sevanskoi Gidrobiologicheskoi Stantsii, 6:5-70.
Barach, G. P. 1941. Fauna Gruzii, T. I. Ryby presnykh vod [Fauna of Georgia, Volume 1. Freshwater Fishes]. Izvestiya Akademii Nauk Gruzinskoi SSR, Tbilisi. vii + 288 pp.
Baradaran Tahouri, H. 1994. Effect of pond fertilization on growth in Acipenser stellatus. M.Sc. Thesis, Islamic Azad University, Tehran. 87 pp. In Farsi.
Baradaran Tahouri, H. and Abtahee, B. 1998. Comparison of sturgeon fingerling production between south and north of Caspian Sea. First National Symposium on Sturgeon, Rasht, 15-16 December 1998,
p. 62-71. In Farsi.
Baradarn Noveyri (sic), Sh., Alipour, A. R. and Pourkazemi, M. 2007. Morphological characteristics, sperm density and spermatocrit of Persian sturgeon (Acipenser persicus) along the
west southern part of the Caspian Sea. Pajouhesh va Sazandegi, 20(2)(75):138-144. In Farsi.
Baradaran Noveiri, Sh., Pourkazemi, M., Kouchinian, P. and Yavari, V. 2002. The effects of cryopresrvation on movement patterns of carp (Cyprinus carpio) spermatozoa. Pajouhesh va Sazandegi,
15(2)(55):6-10. In Farsi.
Baradaran Noveiri, Sh., Pourkazemi, M., Kouchinian, P. and Yavari, V. 2006. Cryopreservation of carp spermatozoa by different extenders. Iranian Journal of Fisheries Sciences, 14(4):17-30. In Farsi.
Barak, N. A. A. and Mohamed, A-R. M. 1982. Food habits of cyprinid fish, Barbus luteus (Heckel). Iraqi Journal of Marine Science, Basrah, 1(1):59-66.
Barak, N. A. A. and Mohamed, A-R. M. 1983. Biological study of the cyprinid fish, Barbus luteus (Heckel) in Garma Marshes. Journal of Biological Sciences Research, Baghdad, 14(2):53-70.
Barak, N. A.-E., Salman, N. A. and Ahmad, S. M. 1994. The piscivorous feeding of mudskipper Periophthalmus waltoni Koumaus (sic) from Khor Al-Zubair, northwest Arabian Gulf.
Pakistan Journal of Zoology, 26(3):280-283.
Barannikova, J. A. 1972. Functional basis for migrations of the Acipenseridae. Fisheries Research Board of Canada Translation Series, Halifax, 2777:70 pp.
Barannikova, I. A. 1987. Review of sturgeon farming in the Soviet Union. Journal of Ichthyology, 27(6):62-71.
Barannikova, I. A., Burtsev, I. A., Vlasenko, A. D., Gershanovich, A. D. Makarov, E. V. and Chebanov, M. S. 1995. Sturgeon fisheries in Russia, p. 124-136.
In: Gershanovich, A. D. and Smith, T. I. J. (Eds.). Proceedings of the International Symposium on Sturgeons, 6-11 September 1993. VNIRO, Moskva.
Barimani, A. 1960-1961. Fishes of the Caspian Sea. Game and Nature, Tehran, 5:60-65; 6:70-75; 7:58-62; 8:72-77; 9:56-64; 10:68-70; 11:8-9; 12:58-61; 13:64-66; 15:64-67; 18:52-57. In Farsi.
Barimani, A. 1961a. The history of Shilot Fishing Company and its fishes in the Mazanderan Sea. Game and Nature, Tehran, 14:64-68. In Farsi.
Barimani, A. 1961b. The fish foods. Game and Nature, Tehran, 19:50-55 In Farsi.
Barimani, A. 1966. Ichthyology and fisheries. Tehran University Press, Tehran. In Farsi.
Barimani, A. 1977. The Caspian Sea. University of Tehran, Tehran. Volume I. 302 pp. In Farsi.
Barrois, T. 1894. Contribution à l'étude de quelques lacs de Syrie. Revue biologique du nord de la France, 6(1893-1894):224-312.
Barthold, W. 1984. An Historical Geography of Iran. Princeton University Press, Princeton. xv + 285 pp.
Bartley, D. M. and Rana, K. 1998a. Stocking inland waters of the Islamic Republic of Iran. FAO Aquaculture Newsletter, 18:16-19.
Bartley, D. M. and Rana, K. 1998b. Evaluation of artificial rehabilitation of the Caspian Sea fisheries and genetic resource management in aquaculture and fisheries of the Islamic
Republic of Iran. Report prepared for Shilat (Fisheries Department), Ministry of Jihad-e-Sazandagi, Islamic Republic of Iran, Food and Agriculture Organization, Rome, 35 pp.
Barzegar, M. 2005. Crustacean parasites of cultured and wild fishes of Iran and Caspian Sea: a review. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28
October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Barzegar, M., Asadollah, S., Hemmat-Zadeh, A., Jalali, B. and Rahnama, R. 2004. Parasites of fishes in Behesht-Abad River (Chaharmahal-o-Bakhtiary). Iranian Journal of Veterinary Science, 1(1):67-74.
In Farsi.
Barzegar, M. and Jalali, B. 2002. Parasites of Kaftar Lake fishes, their geographical distribution and economical importance. Science Journal of the School of Veterinary Medicine, Shahid Chamran
University, Ahvaz, 3(5):52-64. In Farsi.
Barzegar, M. and Jalali, B. 2006. Helminthes, Acanthocephala and crustacean parasites of fishes in Vahdat Resevoir. Iranian Journal of Veterinary Science, 3:229-2234. In Farsi.
Barzegar, M. and Jalali, B. 2009. Crustacean parasites of fresh and brackish (Caspian Sea) water fishes of Iran. Journal of Agricultural Science and Technology, 11:161-171.
Barzegar, M., Raeisi, M., Bozorgnia, A. and Jalali, B. 2008. Parasites of eyes of fresh and brackish water fishes in Iran. Iranian Journal of Veterinary Research, Shiraz University, 9(3)(24):256-261.
Baska, F. and Masoumian, M. 1996. Myxobolus molnari sp. n. and M. mokhayeri sp. n. (Myxosporea, Myxozoa) infecting a Mesopotamian fish, Capoeta trutta Heckel, 1843. Acta
Protozoologica, 35(2):151-156.
Başusta, N. and Çiçek, E. 2006. Length-weight relationships for some teleost fishes caught in Atatürk dam lake on (sic) southeastern Anatolia, Turkey. Journal of Applied Ichthyology,
22(4):279-280.
Batmanglij, N. 1990. Food of Life. A Book of Ancient Persian and Modern Iranian Cooking and Ceremonies. Mage Publishers, Inc., Washington, D.C. 247 pp.
Batmanglij, N. 1999. (Fish). iv. Fish as food, p. 672. In: Yarshater, E. (Ed.). Encyclopædia Iranica. Bibliotheca Persica Press, New York. Volume IX, Fascicle 6. Festivals VIII-Fish.
Battalgil, F. 1942. Contribution à la connaissance des poissons des eaux douces de la Turquie. Istanbul Üniversitesi Fen Fakültesi Mecmuasi, B, 7:287-306.
Battalgil, F. 1944. Nouveaux poissons des eaux douces de la Turquie. Istanbul Üniversitesi Fen Fakültesi Mecmuasi, B, 9(2):126-133.
Bauchot, M.-L., Daget, J. et Bauchot, R. 1990. L'ichtyologie en France au début du XIXe siècle. L'Histoire naturelle des Poissons de CUVIER et VALENCIENNES. Bulletin du Muséum national
d'Histoire naturelle, Paris, 4e série, 12, section A, numéro 1, supplément:3-142.
Bauchot, M. L., Desoutter, M., Hoese, D. F. and Larson, H. K. 1991. Catalogue critique des types de poissons du Muséum national d'Histoire naturelle (Suite) Sous-ordre des Gobioidei. Bulletin
du Muséum national d'Histoire naturelle, Paris, 4e série, 13, section A, numéros 1-2, supplément:1-82.
Bayrami, A., Abtahi, B., Farajzadeh, M. A., Mohammadi, M., Rahnema, M. and Haghdoust, M. 2003. Salinity and major ions concentration in Noor area and Gorgan Bay of south Caspian Sea. Iranian Journal
of Marine Sciences, 2(2-3):21-28, In Farsi.
Bazari Moghaddam, S., Mokhayer, B., Masoumian, M., Shenavar Masouleh, A., Jalilpour, J., Masoumzadeh, M. and Alizadeh, M. 2010. Parasitic infection among larvae and fingerlings of the Persian
sturgeon (Acipenser persicus) in Vniro tanks and earthen ponds. Iranian Journal of Fisheries Sciences, 9(3):342-351.
Bazyar Lakeh, A. A., Ahmadi, M. R. and Majazi Amiri, B. 2005. The effect of different astaxanthin concentrations on its retention in ovule and consequently fertilization rate in rainbow trout
(Oncorhynchus mykiss). Iranian Journal of Natural Resources, 58(1):113-124. In Farsi.
Beaumont, P. 1968. Qanats on the Varamin Plain, Iran. Transactions of the Institute of British Geographers, 45:169-179.
Beaumont, P. 1971. Qanat systems in Iran. Bulletin of the International Association of Scientific Hydrology, 16(1):39-50.
Beaumont, P. 1973a. A traditional method of ground-water utilization in the Middle East. Ground Water, 11(5):23-30.
Beaumont, P. 1973b. River regimes in Iran. Occasional Papers (New Series, Department of Geography, University of Durham, 1:29 pp.
Beaumont, P. 1974. Water resource development in Iran. Geographical Journal, 140:418-431.
Beaumont, P. 1981. Water resources and their management in the Middle East, pp. 40-72. In: Clarke, J. I. and Bowen-Jones, H. (Eds.). Change and development in the Middle East. Essays in Honour of
W. B. Fisher. Methuen, London. xiii + 322 pp.
Beaumont, P. 1998. Restructuring of water usage in the Tigris-Euphrates basin: the impact of modern water management policies. Yale School of Forestry & Environment Studies Bulletin, 103:168-186.
Beaumont, P., Blake, G. H. and Wagstaff, J. M. 1976. The Middle East: A Geographical Study. John Wiley and Sons, London. xvii + 572 pp.
Beaumont, P., Bonine, M. and McLachlan, K. (Eds.). 1989. Qanats, Kariz, and Khattara: Traditional Water Systems in the Middle East and North Africa. The Middle East Centre, School of Oriental and
African Studies, University of London and Middle East & North Africa Studies Press, Wisbech. xxii + 305 pp.
Beaumont, P. and Neville, J. H. 1968. Rice cultivation in Iran's Caspian lowlands. World Crops, December 1968, pp. 70-73.
Becer, Z. A. and İkiz, R. 1999a. Eğirdir Gölü sudak (Stizostedion lucioperca L., 1758) populasyonunun Büyüme Özellikleri [Growth features of pikeperch (Stizostedion lucioperca
L., 1758) population in Eğirdir Lake]. Turkish Journal of Zoology, 23 (supplement 1):215-224.
Becer, Z. A. and İkiz, R. 1999b. Eğirdir Gölündeki sudak (Stizostedion lucioperca (L., 1758))'in Üreme Özellikleri [Reproductive characteristics of pikeperch (Stizostedion
lucioperca (L., 1758)) in Eğirdir Lake]. Turkish Journal of Zoology, 23 (supplement 3):919-926.
Becker, G. C. 1983. Fishes of Wisconsin. The University of Wisconsin Press, Madison. xii + 1052 pp.
Beckett, P. H. T. 1951. Waters of Persia. Geographical Magazine, 24:230-240.
Beckett, P. 1952. Qanāts in Persia. Journal of the Iran Society, London, 1(4):125-133.
Beckett, P. 1953. Qanats around Kirman. Royal Central Asian Journal, 40:47-56.
Beckett, P. 1966. The city of Kerman, Iran. Erdkunde, 20:119-125.
Beckman, W. C. 1961. Inland fisheries biology (Caspian Sea). Food and Agriculture Organization, Rome, Technical Assistance Project Summary, 277-54:11 pp.
Beckman, W. C. 1962. The freshwater fishes of Syria and their general biology and management. Food and Agriculture Organization, Rome, Fisheries Biology Technical Paper, 8:v + 297 pp.
Behmanesh, Sh. 2002. Comparison study on different synthetic hormones used for artificial propagation of stellate sturgeon Acipenser stellatus stellatus Berg, 1932. Iranian Journal
of Fisheries Sciences, 11(1):9-24. In Farsi.
Behmanesh, Sh., Kamali, A., Bahmani, M. and Borrani, M. 2009. Some biological characters of Silurus glanis L., 1758, for reproduction management in Anzali Lagoon. The 6th Scientific Conference of
Fisheries Resources, 3-4 March 2009, Basrah, p. 118 (abstract).
Behnia, A. 1988. Qanat: Construction and maintenance. Centre for University Publications, Tehran. In Farsi. (not seen).
Behnke, R. J. 1965. A systematic study of the family Salmonidae with special reference to the genus
Salmo. Ph.D. Thesis, University of California, Berkeley. viii + 237 pp.
Behnke, R. J. 1969. Süßwasserfische der Türkei. 6. Teil. A new subgenus and species of trout, Salmo (Platysalmo) platycephalus, from south-central Turkey, with comments on the classifikation
(sic) of the sub-family Salmoninae. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 66:1-15.
Behnke, R. J. 1975a. Efficacy of the grass carp for vegetation control and the potential environmental impact of their introduction. MS, 7 pp.
Behnke, R. J. 1975b. Fishes from the qanats of Iran. Abstract, 55th Annual Meeting, American Society of Ichthyologists and Herpetologists, Williamsburg, Virginia, 8-14 June 1975. p. 75.
Behrouzi Rad, B. 2004. Fish-eating bird's diversity of south coast of Caspian Sea. The 4th International Iran and Russia Conference "Agriculture and Natural Resources", 8-10 September 2004,
Shahr-e Kord, Iran (title).
Behrouzirad, B. 2007. Identification of fish-eating birds. International Journal of Environmental Research, 1(2):88-95.
Beidas, M. F. and White, G. B. 1982. Comparison of three types of larvivorous fish (Aphanius Ruppell, Gambusia Girard, Poecilia Peters) for predation on anopheline versus
culicine larvae. Progress Reports on Mosquito Studies, London School of Hygiene and Tropical Medicine 43:23-24.
Beling, D. und Iljin, B. 1927. Benthophiloides brauneri n.g., n.sp., ein für das Schwarzmeerbassin neuer Vertreter der Familie der Gobiidae. Travaux de la Station Biologique du Dnièpre,
Kief, 2:309-325, 2 pl.
Bell, A. (Director). 1977. The world about us: a fish called Smith is alive and well and living in Persia. BBC2 Bristol, film transmitted17 July 1977.
Belousov, A. 1929. K Voprosu ob Ikryanosti Krasnoi Ryby v Dovoennykh i Sovremennykh Ulobakh Kaspiiskogo Morya [To the question about caviar in sturgeons in pre-war and contemporary catches in the
Caspian Sea]. Rybnoe Khozyaistvo, (7-8):31-33.
Beloussov, A. 1931? Rapport du Laboratoire d'Ichthyologie de Pahlavi sur la pêche de l'Esturgeon de Güldenstädt dans le secteur du Sefidroud en 1929/30 et 1930/31. MS. In Russian. (not seen).
Belyaev, V. N. 1929. Abramis sapa bergi subs. nova. Podvid beloglazki iz yuzhnogo Kaspiya [Abramis sapa bergi subs. nova. A new subspecies of the
white-eyed bream from the south Caspian]. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii, Baku, 2(2):80-98.
Bemis, W. E. and Findeis, E. K. 1994. The sturgeons' plight. Nature, London, 370(6491):602.
Bémont, F. 1961. L'irrigation en Iran. Annales de Géographie, 70(377):597-620.
Ben-Yami, M. 1976. Fishing with Light. FAO Fishing Manuals. Fishing News Books, Farnham. x + 121 pp.
Benzer, S. Ş., Gül, A. and Yılmaz, M. 2009. Growth properties of tench (Tinca tinca L., 1758) living in Hirfanlı Reservoir (Kırşehir, Turkey). Iranian Journal of
Fisheries Sciences, 8(2):219-224.
Berberian, M. and King, G. C. P. 1981a. Towards a paleogeography and tectonic evolution of Iran. Canadian Journal of Earth Sciences, 18:210-265.
Berberian, M. and King, G. C. P. 1981b. Towards a paleogeography and tectonic evolution of Iran: Reply. Canadian Journal of Earth Sciences, 18:1764-1766.
Berg, L. S. 1898. K ikhtiofaune Aziatskoi Rossii. Ryby zakaspisskoi oblasti, sovrannye P. A. Varenstovym [On the ichthyofauna of Asiatic Russia. Fishes of the Transcaspian Province, collected by
P. A. Varentsov]. Izvestiya Imperatorskago Obshchestva Lyubitelei Estestvoznaniya, Antropolgii i Etnografii pri Imperatorskom Moskovskom Universitete, Moskva, 86(2)(7):14-20.
Berg, L. S. 1905. Ryby Turkestana [Fishes of Turkestan]. Izvestiya Turkestanskogo Otdela Imperatorskogo Russkogo Geograficheskogo Obshchestva, 4:xvi + 261 pp., 6 pls.
Berg, L. S. 1906. Beschreibung einiger kaukasischer Fische. Bulletin de l`Académie Impériale des Sciences de St.-Pétersbourg, V, 24(1-2):35-39.
Berg, L. S. 1911-1914. Fauna Rossii i sopredel'nykh stran. Ryby. [Fauna of Russia and adjacent countries. Fishes]. Volume I. Marsipobranchii, Selachii and Chondrostei. iii + 337
pp., pl. I-VIII; Volume III. Ostariophysi. Book 1:336 pp., pl. I-II, Book 2:337-704, pl. III-VI. Izdatel'stvo Akademii Nauk, St. Petersburg/Petrograd.
Berg, L. S. 1913. Description of a new species of Garra (= Discognathus) from eastern Persia. Ezhegodnik Zoologicheskago Imperatorskoi Muzeya Akademii Nauk, St. Petersburg, 18:61. In English.
Berg, L. S. 1916. Ryby presnykh vod Rossiiskoi imperii [Freshwater Fishes of the Russian Empire]. Moskva. xxvii + 563 pp.
Berg, L. S. 1918. Novyi vid somika, Glyptosternum armeniacum, iz basseina Evfrata [On a new siluroid fish, Glyptosternum armeniacum n. sp., from the upper Euphrates]. Izvestiya
kavkazkago Muzeya, Tiflis, 11(3-4):145-148.
Berg, L. S. 1925. Opisanie novogo vida roda Alburnus (Pisces) iz basseina Oz. Urmii [Description of a new species of the genus Alburnus (Pisces) from the basin of Lake Urmia]. Ezhegodnik
Zoologischekogo Instituta Akademii Nauk SSSR, 26:213-214.
Berg, L. S. 1927. Zametki o kaspiiskikh Benthophilus (Gobiidae) [Notes on the Caspian Benthophilus (Gobiidae)]. Sbornik v chest' Professora Nikolaya Mikhailovicha Knipovicha 1885-1925, Moskva,
1927:331-344.
Berg, L. 1931a. Description of a new siluroid fish, Glyptosternum kurdistanicum from the basin of the Tigris river. Izvestiya Akademii Nauk SSSR, 7:1267-1270. In English.
Berg, L. S. 1931b. Description of a new gobioid fish, Knipowitschia iljini, from the Caspian Sea. Izvestiya Akademii Nauk SSSR, 7:1271-1273 In English.
Berg, L. S. 1932a. Zwei neue Bartgrundeln (Nemachilus, Pisces) aus Turkestan. Zoologischer Anzeiger, 98:149-150.
Berg, L. S. 1932b. Eine neue Barilius-Art (Pisces, Cyprinidae) aus Mesopotamien. Zoologischer Anzeiger, 100:332-334.
Berg, L. S. 1933a. Note on Cirrhina afghana Günther (Pisces-Cyprinidae). Records of the Indian Museum, 35:193-196.
Berg, L. S. 1933b. Übersicht der Verbreitung der Süβwasserfische Europas. Zoogeographia, 1(2):107-208, 1 Karte.
Berg, L. S. 1934a. Acipenser güldenstädti persicus, a sturgeon from the south Caspian Sea. The Annals and Magazine of Natural History, 10(13):317-318.
Berg, L. S. 1934b. Razdelenie Palearktiki na zoogeograficheskie oblasti na osnovanii rasprostraneniya presnovodnykh ryb [Zoogeographic divisions of the Palaearctic based on the distribution of
freshwater fishes]. Trudy vsesoyuznogo Geograficheskogo, 1933(3):3-10, map.
Berg, L. S. 1936. Ryby basseina Atreka [Fishes of the Atreck basin]. Akademiya Nauk SSSR Trudy Soveta po Izucheniyu Proizvoditel'nykh Sil, Seriya Turkmenskaya, 6:241-253.
Berg, L. S. 1940. Zoogeografiya presnovodnykh ryb Perednei Azii [Zoogeography of freshwater fish of the Near East]. Uchenye Zapiski leningradskogo gosudarstvennogo Universiteta Seriya
Geograficheskikh Nauk, 3:3-31.
Berg, L. S. 1948-1949. Freshwater fishes of the USSR and adjacent countries. Israel Program for Scientific Translations, Jerusalem (1962-1965). 3 volumes.
Berg, L. S. 1949. Presnovodnye ryby Irana i sopredel'nykh stran [Freshwater fishes of Iran and adjacent countries]. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 8:783-858.
Berg, L. S. 1959. Vernal and hiemal races among anadromous fishes. Journal of the Fisheries Research Board of Canada, 16(4):515-537.
Berka, R. 1990. Inland capture fisheries of the USSR. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 311:vi + 143 pp.
Bernatchez, L. 2001. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution,
55(2):351-379.
Berra, T. M. 1981. An Atlas of Distribution of the Freshwater Fish Families of the World. University of Nebraska Press, Lincoln. xxix + 197 pp.
Berra, T. M. 2001. Freshwater Fish Distribution. Academic Press, San Diego. xxxviii + 604 pp.
Berrebi, P. 1995. Speciation of the genus Barbus in the north Mediterranean basin: recent advances from biochemical genetics. Biological Conservation, 72(2):237-249.
Berrebi, P., Kotlík, P. and Tsigenopoulos, C. S. 2001. Phylogeny of the polyphyletic genus Barbus (Cyprinidae): mtDNA to solve (nuclear) polyploid phyla organization. The 10th European
Congress of Ichthyology ECI X, Programme & Book of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 26.
Berrebi, P., Kottelat, M., Skelton, P. and Ráb, P. 1996. Systematics of Barbus: state of the art and heuristic comments. Folia Zoologica, Prague, 45 (Supplement 1):5-12.
Bertin, L. 1956. Eels. A Biological Study. Cleaver-Hune, London. viii + 192 pp.
Bertin, L. et Estève, R. 1948. Catalogue des types de poissons du Muséum national d'Histoire naturelle, Paris, 4e Partie. Ostariophysaires (Cypriniformes). Paris. 117 pp.
Bertin, L. et Estève, R. 1950. Catalogue des types de poissons du Muséum national d'Histoire naturelle, Paris, 5e partie. Ostariophysaires (Siluriformes). Paris. 85 pp.
Bertram, G. C. L. 1944. A statement on the fisheries of Iran. Food and Agriculture Organization, Rome, FAO/53/1/976 and FAO 53/1/976 (A report made to the Middle East Supply Centre, mimeographed
as pp. 87-90 of "The Fisheries of the Middle East. Reports dated: 1943 to 1949". (not seen).
Besharat, K. and Khatib, S. 1993. Determination of the commercial catch region in Iranian coastal zone, 1990-1991. Final Report, Mazandaran Fisheries Research Center, Sari. 181 pp. In Farsi.
Besharat, K. and Rezvani Gilkolaei, S. 2006. Status of aquaculture in Islamic Republic of Iran. Country Report to the workshop on a regional approach for responsible development of marine farming
in the Asia-Pacific Region, 6-11 March 2006, China PR. 11 pp.
Bhattacharjeee, P. C. and Dasgupta, M. 1988. Some observations on the biology of Aspidoparia morar (Ham.) from River Brahmaputra, Assam (India). Arquivos do Museu Bocage, nova série, 1(2):197-203.
Bhatti, M. N. and Al-Daham, N. K. 1978. Annual cyclical changes in the testicular activity of a freshwater teloest, Barbus luteus (Heckel) from Shatt-Al-Arab, Iraq. Journal of Fish Biology,
13:321-326.
Bhatti, M. N. and Mirza, M. R. 1995. Pakistan ki Nachhlian aur Mahi Parvari [Fish and Fisheries of Pakistan]. Part II. Ferozsons, Lahore. 309 pp. In Urdu.
Bhimachar, B. S. 1944. Poison glands in the pectoral spines of two catfishes - Heteropneustes fossilis (Bloch) and Plotosus arab (Forsk), with remarks on the nature
of their venom. Proceedings of the Indian Academy of Sciences, B, 19:65-70.
Bianco, P. G. 1990. Potential role of the palaeohistory of the Mediterranean and Paratethys basins on the early dispersal of Euro-Mediterranean freshwater fishes.
Ichthyological Exploration of Freshwaters, 1(2):167-184.
Bianco, P. G. 1995a. A revision of the Italian Barbus species (Cypriniformes: Cyprinidae). Ichthyological Exploration of Freshwaters, 6(4):305-324.
Bianco, P. G. 1995b. Factors affecting the distribution of freshwater fishes especially in Italy. Cybium, 19(3):241-259.
Bianco, P. G. and Banarescu, P. 1982. A contribution to the knowledge of the Cyprinidae of Iran (Pisces, Cypriniformes). Cybium, 6(2):75-96.
Bianco, P. G. and Nalbant, T. T. 1980. Redescription of Cobitis linea, with some remarks on the subgenus Bicanestrinia (Cypriniformes: Cobitidae). Copeia, 1980(4):903-906.
Bickham, J. W. 1996. Ecotoxicology in Azerbaijan. Quarterdeck, College Station, Texas, 4(3):7 pp. (internet version).
Bilio, M. and Niermann, U. 2004. Is the comb jelly really to blame for it all? Mnemiopsis leidyi and the ecological concerns about the Caspian Sea. Marine Ecology Progress Series, 269:173-183.
Bilke, E. H. 2004. Eine Bilanz: Saugbarben im therapeutischen Einsatz. Die Aquarien und Terrarien-Zeitschrift, 57(5):20-21.
Billard, R. (Ed.). 1995. Carp: biology and culture. Springer-Praxis Series in Aquaculture & Fisheries, Praxis Publishing, Chichester. xiii + 342 pp.
Billard, R. 2000. Biology and control of reproduction of sturgeons in fish farm. Iranian Journal of Fisheries Sciences, 2(2):1-20, 100.
Billard, R. (Ed.). 2002. Esturgeons et Caviar. Editions TEC & DOC, Londres. xiv + 298 pp.
Billard, R. and Berni, P. 2004. Trends in cyprinid polyculture. Cybium, 28(3):255-261.
Billard, R. et Cosson, J. 2002. Le repeuplement de Rutilus frisii kutum dans la partie iranienne de la mer Caspienne. Bulletin de Liaison de la Société Française d'Ichtyologie, 22-23:4-5.
Billington, N., Danzmann, R. G., Hebert, P. D. N. and Ward, R. D. 1991. Phylogenetic relationships among four members of Stizostedion (Percidae) determined by mitochondrial DNA and allozyme
analyses. Journal of Fish Biology, 39 (Supplement A):251-258.
Billington, N., Hebert, P. D. N. and Ward, R. D. 1990. Allozyme and mitochondrial DNA variation among three species of Stizostedion (Percidae): phylogenetic and zoogeographical
implications. Canadian Journal of Fisheries and Aquatic Sciences, 47(6):1093-1102.
Bilqees, F. M., Ataur-Rahim, (M.) and Khatoon, N. 1995. History of Ichthyology in Pakistan. Biological Research Journal, Karachi, 2:77-141.
Birks, J. S. 1984. The falaj: modern problems and some possible solutions. Waterlines, 2(4):28-31.
Biro, P., Al-Jafery, A. R. and Sadek, S. E. 1988. On stunted growth of Barbus luteus (Heckel) in river Diyala, Iraq. Journal of Biological Sciences Research, Baghdad, 19(1):129-147.
Birstein, V. J. 1993. Sturgeons and paddlefishes: threatened fishes in need of conservation. Conservation Biology, 7(4):773-787.
Birstein, V. 1996. Sturgeons may soon disappear from the Caspian Sea. Russian Conservation News, 7:15-16.
Birstein, V. J. and DeSalle, R. 1998. Molecular phylogeny of Acipenserinae. Molecular Phylogenetics and Evolution, 9(1):141-155.
Birstein, V. J., Doukakis, P., Sorkin, B. and DeSalle, R. 1998. Population aggregation analysis of three caviar-producing species of sturgeons and implications for the species identification
of black caviar. Conservation Biology, 12(4):766-775.
Birstein, V. J. and Ruban, G. 2004. A comment on the Siberian, Acipenser baerii, and Russian, Acipenser gueldenstaedtii, sturgeons. Environmental Biology of Fishes, 70(1):91-92.
Birstein, V. J., Ruban, G., Ludwig, A., Doukakis, P. and DeSalle, R. 2005. The enigmatic Caspian Sea Russian sturgeon: how many cryptic forms does it contain? Systematics and Biodiversity,
3(2):203-218.
Birstein, V. J., Waldman, J. R. and Bemis, W. E. (Eds.). 1997. Sturgeon biodiversity and conservation. Environmental Biology of Fishes, 48(1-4):1-440.
Bishop, J. M. 2003. History and current checklist of Kuwait's ichthyofauna. Journal of Arid Environments, 54(1):237-256.
Biswas, A. K. (Ed.). 1994. International Waters of the Middle East from Euphrates-Tigris to Nile. Oxford University Press, Bombay. xvii + 221 pp.
Blaber, S. J. M., Milton, D. A. and Brewer, D. T. 1997. Life history strategies of tropical estuarine shads genus Tenualosa (Clupeidae). Ninth International Congress of European
Ichthyologists (CEI9) "Fish Biodiversity". Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 14.
Blanco, J. L., Hrbek, T. and Doadrio, I. 2006. A new species of the genus Aphanius (Nardo, 1832)(Actinopterygii, Cyprinodontidae) from Algeria. Zootaxa, 1158:39-53.
Blanford, W. T. 1876. Eastern Persia. An Account of the Journeys of the Persian Boundary Commission 1870-71-72. Vol. II. The Zoology and Geology. Macmillan and Co., London. viii + 516 pp.
Blaustein, L. and Byard, R. 1993. Predation by a cyprinodontid fish, Aphanius mento, on Culex pipiens: effects of alternative prey and vegetation. Journal of the American Mosquito
Control Association,9(3):356-358.
Blegvad, H. and Løppenthin, B. 1944. Fishes of the Iranian Gulf. Einar Munksgaard, Copenhagen. 247 pp., XII pls. (1999 translation into Farsi by E. Etemad and B. Mokayyer with supplement, Tehran
University Publications No. 1744:26 + 416 pp., XI pls.).
Bleher, H. 2011. Biotopes of Iran: On the very brink. Practical Fishkeeping (www.practicalfishkeeping.co.uk/content.php?sid=3797, downloaded 12 April 2011), 8 pp.
Bless, R. 1996. Reproduction and habitat preference of the threatened spirlin (Alburnoides bipunctatus Bloch) and soufie (Leuciscus souffia Risso) under laboratory conditions
(Teleostei: Cyprinidae), p. 249-258. In: Kirchhofer, A. and Hefti, D. Conservation of Endangered Freshwater Fish in Europe. Birkhäuser Verlag, Basel. xii + 341 pp.
Bobek, H. 1952. Beiträge zur Klima-Ökologischen Gliederung Irans. Erdkunde, 6(2/3):65-84.
Bobek, H. 1959. Features and formation of the Great Kawir and Masileh. Arid Zone Research Center, University of Tehran, 2:63 pp.
Bobek, H. 1963. Nature and implications of Quaternary climatic changes in Iran, pp. 403-413. In: Arid Zone Research - XX. Changes of Climate. Proceedings of the Rome Symposium organized by UNESCO
and WHO, 1963.
Bodenheimer, F.-S. 1934. Contribution à l'étude de la zoogéographie des poissons du sud Paléarctique. Bulletin de la Société centrale d'Aquiculture, Clermont, 4-6:33-42.
Boeseman, M. 1947. Revision of the fishes collected by Burger and von Siebold in Japan. E. J. Brill, Leiden. viii + 242 pp., 5 pls.
Boettcher, J. 1974a. Neur Lake stocking report/amphipod population estimation. L-6-53. Job Progress Report, Fisheries and Aquatic Ecology Group, Department of the Environment, Tehran. 9 pp.
Boettcher, J. 1974b. Neur Lake limnological investigation II. 6-11 Mordad 1353. L-6-53. Job Progress Report, Fisheries and Aquatic Ecology Group, Department of the Environment, Tehran.
7 pp., 5 appendices, 5 figs.
Bogutskaya, N. G. 1986. Relationships between species of Abramis, Blicca and Vimba (Cyprinidae). Journal of Ichthyology, 26(5):1-9.
Bogutskaya, N. G. 1988. Morphological characters of some groups of genera of the subfamily Leuciscinae. Journal of Ichthyology, 28(3):26-34.
Bogutskaya, N. G. 1990. Morphological fundamentals in classification of the subfamily Leuciscinae (Leuciscinae, Cyprinidae). Communication 1. Journal of Ichthyology, 30(3):63-77.
Bogutskaya, N. G. 1992. A revision of the genus Pseudophoxinus (Leuciscinae, Cyprinidae) from Asia Minor. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 89:261-290.
Bogutskaya, N. G. 1994. A description of Leuciscus lepidus (HECKEL, 1843) with comments on Leuciscus and leuciscine - aspinine relationships (Pisces: Cyprinidae).
Annalen des naturhistorischen Museums in Wien, 96B:599-620.
Bogutskaya, N. G. 1995. Leuciscus kurui, a new cyprinid fish from the upper Tigris (Dicle) system. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 92:149-154.
Bogutskaya, N. G. 1996. Contribution to the knowledge of leuciscine fishes of Asia Minor. Part 1. Morphology and taxonomic relationships of Leuciscus borysthenicus (Kessler, 1859),
Leuciscus smyrnaeus Boulenger, 1896 and Ladigesocypris ghigii (Gianferrari, 1927) (Cyprinidae, Pisces). Publicaciones Especiales Instituto Español de Oceanografía, 21:25-44.
Bogutskaya, N. G. 1997a. Chondrostoma beysehirense, a new cyprinid fish from Beysehir Lake, Central Turkey. Ichthyological Exploration of Freshwaters, 8(2):151-158.
Bogutskaya, N. G. 1997b. Contribution to the knowledge of leuciscine fishes of Asia Minor. Part 2. An annotated check-list of leuciscine fishes (Leuciscinae, Cyprinidae) of Turkey with descriptions
of a new species and two new subspecies. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 94:161-186.
Bogutskaya, N. G. 2002. Petroleuciscus, a new genus for the Leuciscus borysthenicus species group (Teleostei: Cyprinidae). Zoosystematica Rossica, 11(1):235-237.
Bogutskaya, N. G. and Coad, B. W. 2009. A review of vertebral and fin-ray counts in the genus Alburnoides (Teleostei: Cyprinidae) with a description of six new species. Zoosystematica
Rossica, 18(1):126-173.
Bogutskaya, N. G. and Iliadou, K. 2006. Rutilus panosi, a new roach from western Greece (Teleostei: Cyprinidae). Zoosystematica Rossica, 14(2):293-298.
Bogutskaya, N. G. and Komlev, A. M. 2001. Some new data to morphology of Rhodeus sericeus (Cyprinidae: Acheilognathinae) and a description of a new species, Rhodeus colchicus, from
west Transcaucasia. Proceedings of the Zoological Institute, St. Petersburg, 287:81-97.
Bogutskaya, N. G., Kucuk, F. and Unlu, E. 2000. Alburnus baliki, a new species of cyprinid fish from the Manavgat River system, Turkey. Ichthyological
Exploration of Freshwaters, 11(1):55-64.
Bogutskaya, N. G. and Naseka, A.M. 2004. Katalog beschelyustnykh i ryb presnykh i solonovatykh vod Rossii c nomenklaturnymi i taksonomicheskimi kommentariyami [Catalogue of Agnathans and Fishes of
Fresh and Brackish Waters of Russia with comments on nomenclature and taxonomy]. Zoological Institute, Russian Academy of Sciences and KMK Scientific Press Ltd, Moscow. 389 pp., CD.
Bogutskaya, N. G., Naseka, A. M. and Komlev, A. M. 2001. Freshwater Fishes of Russia: preliminary results of the fauna revision. Proceedings of the Zoological Institute, Russian Academy of Sciences,
Zoological Sessions (Annual Reports 2000), 289:39-50.
Bogutskaya, N. G., Naseka, A. M. and Tikhonov, P. A. 2008. A brief history of the study of fishes of the Caspian Sea and scientific results of the Caspian Expedition of 1904 headed by N. M. Knipovich.
Aqua, International Journal of Ichthyology, 14(1):1-26.
Bogutskaya, N. G. and Zupančič, P. 1999. A re-description of Leuciscus zrmanjae (Karaman, 1928) and new data on the taxonomy of Leuciscus illyricus, L.
svallize and L. cephalus (Pisces: Cyprinidae) in the West Balkans. Annalen des naturhistorischen Museums in Wien, 101B:509-529.
Bohlen, J. 2001. Biology and diversity of spined loaches of the genus Cobitis - state of the art and open questions. The 10th European Congress of Ichthyology ECI X, Programme &
Book of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 26.
Bohlen, J. 2004. Vielfalt und aquaristische Pflege von Goldsteinbeiβern, Sabanejewia. Aquaristik Fachmagazin und Aquarien Heute, 36(1)(175):36-39.
Bohlen, J., Perdices, A., Doadrio, I. and Economidis, P. S. 2006. Vicariance, colonisation, and fast local speciation in Asia Minor and the Balkans as revealed from the phylogeny of spined
loaches (Osteichthyes; Cobitidae). Molecular Phylogenetics and Evolution, 39(2):552-561.
Bohlen, J., Šlechtová, V., Bogutskaya, N. and Freyhof, J. 2006. Across Siberia and over Europe: phylogenetic relationships of the freshwater fish genus Rhodeus in Europe and the phylogenetic
position of R. sericeus from the River Amur. Molecular Phylogenetics and Evolution, 40(3):856-865.
Böhlke, E. B. 1984. Catalog of type specimens in the Ichthyological Collection of the Academy of Natural Sciences of Philadelphia. Special Publication of the Academy of Natural Sciences of
Philadelphia, 14:viii + 246 pp.
Boldyrev, V. S. and Bogutskaya, N. G. 2004. Description of two new species of tadpole-gobies (Teleostei: Gobiide: Benthophilus). Zoosystematica Rossica, 13(1):129-135.
Boldyrev, V. S. and Bogutskaya, N. G. 2007. Revision of the tadpole-gobies of the genus Benthophilus (Teleostei: Gobiidae). Ichthyological Exploration of Freshwaters, 18(1):31-96.
Bolourchi, M. 1997. Caviar, the perfect pearls of nature. http://www.caviarclub.com/pearls.html. 8 pp.
Boltachev, A. R., Danilyuk, O. N., Pakhorukov, N. P. and Bondarev, V. A. 2006. Distribution and certain features of the morphology and biology of the stone moroko Pseudorasbora parva
(Cypriniformes, Cyprinidae) in the waters of Crimea. Journal of Ichthyology, 46(1):58-63.
Bonine, M. E. 1980. The Urbanization of the Persian Gulf Nations, pp. 225-278. In: Cottrell, A. J. The Persian Gulf States. A General Survey. The Johns Hopkins University Press, Baltimore and London.
xxxiv + 695 pp.
Bonine, M. E. 1982. From qanāt to kort: traditional irrigation terminology and practices in Central Iran. Iran, 20:145-159.
Booth, B. 1977. The making of Iran. Geographical Magazine, 49(4):243-249.
Borkenhagen, K. 2005. Systematik und Zoogeographie der "groβschuppigen Barben" des vorderen Orients. Diplomarbeit an der Mathematisch-Naturwissenschaftlichen Fakultät der
Christian-Albrechts-Universität zu Kiel. 121 + x pp.
Borkenhagen, K. 2010. Range extension of the saddleback silver-biddy Gerres limbatus Cuvier, 1830 (Teleostei: Gerreidae) with a new record from an Iranian tributary to the Persian Gulf.
Journal of Applied Ichthyology, doi: 10.1111/j.1439-0426.2010.01614.x.
Borkenhagen, K., Esmaeili, H. R., Mohsenzadeh, S., Shahryari, F. and
Gholamifard, A. 2011. The molecular
systematics of the Carasobarbus species from Iran and adjacent areas,
with comments on Carasobarbus albus (Heckel, 1843). Environmental Biology
of Fishes, 91(3);327-335.
Bornbusch, A. H. 1995. Phylogenetic relationships within the Eurasian catfish family Siluridae (Pisces: Siluriformes), with comments on generic validities and biogeography.
Zoological Journal of the Linnaean Society, 115(1):1-46.
Borodin, N. 1904a. Izsliedovanie obraza zhizmi i razmnozheniya kaspiiskikh sel'dei [Studies on the life history and propagation of the Caspian herrings]. Vestnik Rybopromyshlennosti, St. Petersburg,
19(3):167-198.
Borodin, N. 1904b. Novyi vid kaspiiskoi sel'di (Clupea engrauliformis n. sp.) [A new species of Caspian herring, Clupea engrauliformis n. sp.] Vestnik Rybopromyshlennosti, St.
Petersburg, 19(6):335. (not seen).
Borodin, N. 1926. Acipenser persicus, a sturgeon from the Caspian Sea. The Annals and Magazine of Natural History, (9)20:26-28.
Borodin, N. A. 1930. Russian fisheries The Russian Student, February 1930:15-18.
Borodin, N. and Suvorov, E. 1908. Kaspiiskie sel'di i ikh promysel [Caspian herrings and their commercial exploitation]. Trudy Kaspiiskoi Expeditsii, II. iv + 382 pp., 1 map.
Borowicka, H. 1958. Report to the Government of Iran on the design of irrigation projects. Food and Agriculture Organization, Rome, Expanded Technical Assistance Program, Report No. 800:iv + 69 pp., 17 figs.
Borzenko, M. P. 1942. Caspian sevruga (systematics, biology and catch). Izvestiya Azerbaidzhanskoi Nauchno-Issledovatel'skoi Rybokhozyaistvennoi Stantsii, Baku, 7:3-114. In Russian.
Bosch, M., Kinunen, W., Bahrami, -. and Ahdami, A. 1970. Wetland survey and Mordab observations. Progress Report for July, 1970, Iran Game and Fish Department, Tehran, MS, 4 pp.
Boulenger, G. A. 1892. On Lucioperca marina, C. & V. Proceedings of the Zoological Society, London, 1892:411-413, pl. XXV.
Boulenger, G. A. 1895. Catalogue of the perciform fishes in the British Museum. Second Edition. London. xix + 394 pp., XV pl.
Boulenger, G. A. 1896. On freshwater fishes from Smyrna. The Annals and Magazine of Natural History, 6(18):153-154.
Bourguignon, G. 1989. The flesh and caviar world markets of sturgeon. Publications du Departement d'Halieutique, Ecole nationale superieure agronomique de Rennes, 12:76 pp.
Boyce, M. 1977. A Persian stronghold of Zoroastrianism based on the Ratanbai Katrak Lectures, 1975. Clarendon Press, Oxford. xv + 284 pp.
Boyle, R. 1994. The cost of caviar. The Amicus Journal, Spring:22-27.
Brainard, J. 1998. DNA fingerprinting to track caviar. Science News, 154(8):116.
Brandt, A. 1880. Von den armenischen Alpenseen. Zoologischer Anzeiger, 3(50):111-115.
Braun, C. 1974. Teheran, Marrakesch und Madrid. Ihre Wasserversorgung mit Hilfe von Qanaten. Eine stadtgeographische Konvergenz auf kulturhistorischer Grundlage. Bonner geographische Abhandlungen,
52:133 pp., 44 figs., 3 maps.
Breckle, S. W. 1983. Temperate deserts and semi-deserts of Afghanistan and Iran, pp. 271-319. In: West, N. E. (Ed.). Ecosystems of the World 5. Temperate Deserts and Semi-Deserts. Elsevier,
Amsterdam. xi + 522 pp.
Breil, M. and Bohlen, J. 2001. First record of the loach fish Turcinoemacheilus kosswigi in the basin of Euphrates River, with first observations on habitat and behaviour. Zoology of the Middle
East, 23:71-76.
Brice, W. C. (Ed.). 1978. The Environmental History of the Near and Middle East Since the Last Ice Age. Academic Press, London. xx + 384 pp.
Briggs, J. C. 1987. Biogeography and plate tectonics. Developments in Palaeontology and Stratigraphy, Elsevier, Amsterdam, 10:x + 204 pp.
Brooks, C. E. P. 1949. Climate through the Ages: A study of the climatic factors and their variations. McGraw-Hill, New York. 395 pp.
Brooks, I. A. 1989. The physical geography, geomorphology and Late Quaternary history of the Mahidasht Project Area, Qara Su Basin, Central West Iran. Royal Ontario Museum, Toronto, Mahidasht
Project, 1:xi + 48 pp., pls. I-V.
Brown, G. F. 1970. Eastern margin of the Red Sea and the coastal structures in Saudi Arabia. In: A discussion on the structure and evolution of the Red Sea, Gulf of Aden and Ethiopian rift junction.
Philosophical Transactions of the Royal Society, A, 267:75-89, pl. 8.
Brown, J. E. and Stepien, C. A. 2006. Round goby population genetics: comparisons between Eurasia and North America. International Association for Great Lakes research, 49th Annual Conference, Great
Lakes in a Changing Environment, May 22-26, 2006, University of Windsor, Windsor, Ontario (abstract).
Brown, J. E. and Stepien, C. A. 2008. Ancient divisions, recent expansions: phylogeography and population genetics of the round goby, Apollonia melanostoma. Molecular Ecology, 17(11):2598-2615.
Bruun, A. F. and Kaiser, E. W. 1948. Iranocypris typhlops n. g., n. sp., the first true cave fish from Asia. Danish Scientific Investigations in Iran, Copenhagen, 4(1944):1-8.
Buckingham, J. S. 1829. Travels in Assyria, Media, and Persia, including a Journey from Bagdad by Mount Zagros, to Hamadan, the Ancient Ecbatana, Researches in Ispahan and the Ruins of Persepolis,
and Journey from thence by Shiraz and Shapoor to the Sea-shore. Description of Bussorah, Bushire, Bahrein, Ormuz, and Muscat, Narrative of an Expedition against the Pirates of the Persian Gulf, with
Illustrations of the Voyage of Nearchus, and Passage by the Arabian Sea to Bombay. Henry Colburn, London. xvi + 545 pp., map.
Bulgakov, G. P. 1923. K ikhtiofaune Turkestana [To the ichthyofauna of Turkestan]. Trudy Turkmenskogo Nauchnogo Obshchestva, 1:225-238, 2 plates.
Bulgakov, G. P. 1926. K poznaniyu kaspiiskikh sel'dei. Opisanie novogo vida roda Caspialosa [To the knowledge of Caspian herrings. Description of a new species of the genus Caspialosa]. Byulleten'
Sredneaziatskii Gosudarstvennyi Universitet, Taschkent, 13:27-39, 1 plate.
Bullock, S. 1970. Winter fishery survey. Division of Research and Development, Iran Game and Fish Department. MS.
Bullock, S. 1971. Neur Lake Survey (Lisar Protected Region) S-15-50. Iran Game and Fish Department, Tehran. MS.
Bullock, S. and Kinunen, W. 1970. Namrud and Delichay River Survey. Report, Division of Research and Development, Iran Game and Fish Department, Tehran. MS.
Bullock, S. and RaLonde, R. 1971a. Fall fishery survey Karaj Reservoir. Division of Research and Development, Iran Game and Fish Department, Tehran. MS.
Bullock, S. and RaLonde, R. 1971b. Fishery inventory survey coastal streams, 11-16 June 1971. Progress Report, Division of Research and Development, Iran Game and Fish Department, Tehran.7 pp., 1 fig.
Buringh, P. 1957. Living conditions in the lower Mesopotamian plain in ancient times. Sumer, 13(1 & 2):30-46, figs. 1-10.
Butler, M. A. 1933. Irrigation in Persia by kanáts. Civil Engineering, 3(2):69-73.
Butt, J. A. 1995. On the riverine piscine culture in N.W.F.P. and adjoining areas of Pakistan. Proceedings of Seminar on Aquaculture Development in Pakistan
held at Fisheries Research & Training Institute, Manawan, Lahore, Pakistan, October, 17-18, 1993:105-111.
Butz, K. 1978. Fischabgabe und Feldabgabe in Fischen und Vögeln an den Nanna-Tempel in Ur in altbabylonischer Zeit? Ein Versuch. Archiv für Orientforschung, 26:30-44.
Butzer, K. W. 1957. Late glacial and postglacial climatic variation in the Near East. Erdkunde, 11:21-35.
Butzer, K. W. 1958a. The Near East during the last glaciation: a palaeogeographical sketch. Geographical Journal, 124(3):367-369.
Butzer, K. W. 1958b. Quaternary stratigraphy and climate in the Near East. Bonner Geographische Abhandlungen, 24:1-157, 16 figs.
Butzer, K. W. 1961. Climatic change in arid regions since the Pliocene, pp. 31-56. In: Stamp, L. D. Arid Zone Research - XVII. A history of land use in arid regions. UNESCO, Paris. 388 pp.
Butzer, K. W. 1975. Patterns of environmental change in the Near East during Late Pleistocene and Early Holocene times, pp. 389-410. In: Wendorf, F. and Marks, A. E. (Eds.). Problems in
Prehistory: North Africa and the Levant. Southern Methodist University Press, Dallas. 462 pp.
Butzer, K. W. 1978. The late prehistoric environmental history of the Near East, pp. 5-12. In: Brice, W. C. (Ed.). The Environmental History of the Near and Middle East Since the Last Ice Age.
Academic Press, London. xx + 384 pp.
Bybordi, M. 1974. Ghanats of Iran: drainage of sloping aquifer. Journal of the Irrigation and Drainage Division, American Society of Civil Engineers, 100(IR3)(10785):245-253.
Bykhovski, B. E. 1949. Monogeneticheskie Sosal'shchiki Nekotorykh Ryb Irana, Sobrannye Akad. E. N. Pavlovskim [Monogenetic trematodes of some fishes in Iran, collected by Acad. E.
N. Pavlovski]. Trudy Zoologicheskogo Instituta Akademii Nauk SSR, 8:870-878.
Byniak, E. 1979. The effect of day length and temperature on the reproductive cycle of the female Aphanius mento (Heckel), (Cyprinodontiformes, Teleostei). Ph.D. Thesis, Zoology Department,
Hebrew University, Jerusalem. 69 pp. In Hebrew.
C
Caddy, J. F. 1984. Observations on prospects and constraints of development of Iranian fisheries. Food and Agriculture Organization, Rome, FI:TCP/IRA/2301 (Mf) Field Document 1:iv + 30 pp.
Çakmak, E. and Alp, A. 2010. Morphological differences among the Mesopotamian spiny eel, Mastacembelus mastacembelus (Banks & Solander 1794),
populations. Turkish Journal of Fisheries and Aquatic Sciences, 10(1):87-92.
Çalişkan, M., Yerli, S. V. and Canbolat, A. F. 1999. Çıldır Gölü (Ardahan) Barbus plebejus Heckel, 1843 Populasyonunun Büyüme Parametreleri
[The growth parameters of Barbus plebejus Heckel, 1843 in Çıldır Lake-Ardahan]. Turkish Journal of Zoology, 23 (supplement 1):233-239.
Campolmi, M., Franzoi, P. and Mazzola, V. 1994. Observations on pipefhises (sic) (Syngnathidae) biology in the Stagnone Lagoon (Nort-west (sic) Sicily).
VIII Congress Societas Europaea Ichthyologorum, Oviedo, Spain, September 26 to October 2, 1994, p. 108-109 (abstract).
Campolmi, M., Franzoi, P. and Mazzola, A. 1996. Observations on pipefish (Syngnathidae) biology in the Stagnone lagoon (west Sicily). Publicaciones Especiales Instituto Español de Oceanografía, 21:205-209.
Canbolat, A. F., Yerli, S. V. and Çalişkan, M. 1999. Çıldır Gölü'ndeki (Ardahan) Capoeta capoeta capoeta (GULDENSTANT (sic), 1773)'nın Büyüme Özelliklerinin
İncelenmesi [The investigation of growth parameters of Capoeta capoeta capoeta (GULDENSTANT (sic), 1773) in Çıldır Lake (Ardahan, Kars)]. Turkish Journal of Zoology, 23
(supplement 1):225-232.
Caras, R. A. 1964. Dangerous to Man. Chilton Books, Philadelphia. xix + 433 pp.
Carp, E. (Ed.). 1972. Proceedings of the International Conference on the Conservation of Wetlands and Waterfowl, Ramsar, Iran, 30 January- 3 February 1971.
International Wildfowl Research Bureau, Slimbridge. 303 pp.
Carpenter, K. E., Krupp, F., Jones, D. A. and Zajonz, U. 1997. Living Marine Resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates.
FAO Species Identification Field Guide for Fishery Purposes, Food and Agriculture Organization, Rome. viii + 293 pp., XVII plates.
Carre, F. 1978. The Caspian Sea fisheries. Ann. George., 479:1-39.
Caspian Environmental Programme. 1998. Caspian Sea Environment National Report, Islamic Republic of Iran. Baku, Azerbaijan. pagination various.
Caspian Environmental Programme. 2000. Biodiversity of the Caspian Sea. Baku, Azerbaijan. 122 pp.
Caspian Environmental Programme. 2001a. Islamic Republic of Iran National Coastal Profile. Caspian Regional Thematic Center (CRTC) for Integrated Transboundary Coastal
Area Management and Planning (ITCAMP), Tehran for the Caspian Environmental Programme, Baku, Azerbaijan. 219 pp.
Caspian Environmental Programme. 2001b. Habitat & biodiversity of Caspian Sea I. R. Iran. Baku, Azerbaijan. 70 pp.
Caspian Environmental Programme. 2001c. Emergency response data collection study, I. R. Iran. Caspian Regional Thematic Centre, Emergency Response (CRTC ER), Caspian
Environmental Programme, Baku, Azerbaijan. 43 pp.
Caspian Environmental Programme. 2002a. Caspian Sea biodiversity strategy and action plan. Final draft. Prepared by Fauna and Flora International for the Caspian
Environmental Programme, Baku, Azerbaijan. 96 pp.
Caspian Environmental Programme. 2002b. Islamic Republic of Iran. National Caspian Action Plan (NCAP). Caspian Environmental Programme, Baku, Azerbaijan. 102 pp.
Çetinkaya, O. 1999. Çatak Çayi (Dicle Nehri) Dağ Alabaliklarinin (Salmo trutta macrostigma, Dum., 1858) Bazi Biyolojik Ozelliklerinin Incelenmesi [Investigations of some biological
properties of brown trout (Salmo trutta macrostigma, Dum., 1858) living in Çatak Stream (Tigris River, Turkey)]. Istanbul Üniversitesi Su Ürünleri Dergisi, 9-13(1995-1999)(1-10):111-122.
Chakrabarty, P. 2004. Cichlid biogeography: comment and review. Fish and Fisheries, 5:97-119.
Chan, E. H. and Chua, T. E. 1979. The food and feeding habits of greenback grey mullet, Liza subviridis (Valenciennes), from different habitats and at various
stages of growth. Journal of Fish Biology, 15(2):165-171.
Chan, E. H. and Chua, T. E. 1980. Reproduction in the greenback grey mullet, Liza subviridis (Valenciennes, 1836). Journal of Fish Biology, 16(5):505-519.
Chandra, G., Bhattacharjee, I., Chatterjee, S. N. and Ghosh, A. 2008. Mosquito control by larvivorous fish. Indian Journal of Medical Research, 127(1):13-27.
Chang, K. K. 1972. Composition of spawning stocks of the big-eyed shad [Alosa saposhnikovi (Grimm)] in the north Caspian. Journal of Ichthyology, 12(1):46-54.
Chantre, E. 1882. Rapport sur une Mission scientifique dans l'Asie occidentale et spécialement dans les régions de l'Ararat et du Caucase. Archives des Missions
scientifiques et litteraires, Série III(10):199-263, pl. I-XIV.
Chapman, R. W. 1971. Climatic changes and the evolution of landforms in the Eastern Province of Saudi Arabia. Bulletin of the Geological Society of America, 82:2713-2728.
Charamlambous, A. 2001. Industrial survey & urban wastewater assessment of pollution loads in I.R. Iran. TACIS (Technical Assistance to the Commonwealth of
Independent States, European Union), Caspian Environment Programme Phase 2, Baku, Azerbaijan. ii + 45 pp.
Charlebois, P. M., Corkum, L. D., Jude, D. J. and Knight, C. 2001. The round goby (Neogobius melanostomus) invasion: current research and future needs. Journal of Great Lakes Research,
27(3):263-266.
Charlebois, P. M., Marsden, J. E., Goettel, R. G., Wolfe, R. K., Jude, D. J. and Rudnika, S. 1997. The round goby, Neogobius melanostomus (Pallas), a review of
European and North American literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey, INHS Special Publication, 20:iv + 76 pp.
Charyev, R. 1984. Some consequences of the introduction and acclimatization of grass carp, Ctenopharyngodon idella (Cyprinidae), in the Kara Kum Canal. Journal of Ichthyology, 24(3):1-8.
Chaudhary, A. E., Mirza, M. R. and Nazneen, S. 1991. A contribution to the anatomy of the alimentary canal and the food of some snow-carps (Pisces, Cyprinidae) of
Pakistan. First Symposium on Fish and Fisheries of Pakistan, October, 19-20, 1991, Department of Zoology, Government College, Lahore, p. 27-28 (abstract).
Chaudhuri, B. L. 1909. Descriptions of new species of Botia and Nemachilus. Records of the Indian Museum, 3:339-342.
Chavoshian, S. A., Takeuchi, K. and Funada, S. 2005. An overview to transboundary and shared water resources management in Iran, technical challenges and solutions.
Role of Water Sciences in Transboundary River Basin Management, Thailand, p. 189-195.
Chavoshian, S. A., Takeuchi, K. and Magome, J. 2005. An overview of transboundary and shared water resources management in Iran: technical challenges and solutions.
Program & Abstracts, 8th International Riversymposium 2005, 6-9 September, Brisbane, Australia (abstract).
Cheatsazan, H., Mahjoorazad, A., Rabani, V. and Zehzad, B. 2005. Identification of the fish fauna of Hajilar-Chai River (Northern Azerbaijan). International Congress of Biological Sciences, November
205, Karaj Azad University, Karaj (title).
Chelemal M., Jamili S. and Sharifpour, I. 2009. Reproductive biology and histological studies in abu mullet, Liza abu in the water of the Khozestan Province. Journal of Fisheries and Aquatic
Science, 4(1):1-11.
Chen, W-J. and Mayden, R. L. 2009. Molecular systematics of the Cyprinoidea (Teleostei: Cypriniformes), the world's largest clade of freshwater fishes: Further evidence
from six nuclear genes. Molecular Phylogenetics and Evolution, 52(2):544-549.
Chenciner, R. 1998. Dying for caviar, p. 69-74. In: Walker, H. (Ed.). Fish. Food from the Waters. Proceedings of the Oxford Symposium on Food and Cookery 1997. Prospect Books, Totnes, Devon. 335 pp.
Chepalyga, A. L. 1984. Inland Sea Basins, pp. 229-247. In: Velichko, A. A. (Ed.) (Wright, H. E. and Barnosky, C. W. (Editors of the English-Language Edition)). Late
Quaternary Environments of the Soviet Union. University of Minnesota Press, Minneapolis. xxvii + 327 pp.
Cheraghi, M., Khorasani, N. A., Jafari, M. and Mansouri Seyed, M. 2007. Analysis of pollution sources in Meymeh River. Iranian Journal of Natural Resources, 60(1):193-201. In Farsi.
Chervinski, J. and Lahav, M. 1976. The effect of exposure to low temperature on fingerlings of local tilapia (Tilapia aurea)(Steindachner) and imported tilapia
(Tilapia vulcani)(Trewavas) and Tilapia nilotica. Bamidgeh, 28:25-29.
Chicca, M., Suciu, R., Ene, C., Lanfredi, M., Congiu, L., Leis, M., Tagliavini, J., Rossi, R. and Fontana, F. 2002. Karyotype characterization of the stellate sturgeon,
Acipenser stellatus by chromosome banding and fluorescent in situ hybridization. Journal of Applied Ichthyology, 18(4-6):298-300.
Chikova, V. M. 1967. O sistematike golyavlya Leuciscus cephalus orientalis Nordm. i podusta Chondrostoma cyri Kessler Arpilichskogo vodokhranilishcha [On the systematics
of golyavlya Leuciscus cephalus orientalis Nordm. and podusta Chondrostoma cyri Kessler in the Arpilichskogo reservoir]. Voprosy Ikhtiologii, 7, 1(42):18-22.
Chobkar, N., Emadi, H. and Negarestan, H. 2005. Effects of application trichlorfon toxin with different concentrations on growth of larvae and fry of Caspian kutum
Rutilus frisii kutum. Journal of Environmental Science and Technology, 23:33-44. In Farsi.
Choobkar, N., Emadi, H. and Tlooei Gilani, M. H. 2008. The effects of trichlorfon with different concentrations on the survival of larvae and fry of Caspian kutum, Rutilus frisii kutum Kamensky
1901. Journal of Environmental Science and Technology, 36(special issue).
Choobkar, N., Soltani, M., Ebrahimzadeh Mousavi, H. A., Akhonzadeh Basti, A. and Matinfar, A. 2010. Effect of Zataria multiflora Boiss essential oil on the growth of Staphylococcuis aureus
in the light salted fillets of silver carp (Hypophthalmichthys molitrix). Iranian Journal of Fisheries Sciences, 9(3):352-359.
Chotkowski, M. A. and Marsden, J. E. 1999. Round goby and mottled sculpin predation on lake trout eggs and fry: field predictions from laboratory experiments. Journal of Great Lakes Research,
25(1):26-35.
Christie, T. 1995. Caviar still spells luxury. Seafood International, December 1995:21, 23.
Christophers, S. R. and Shortt, H. E. 1920-1921. Malaria in Mesopotamia. Indian Journal of Medical Research, 8:508-552.
Chubian, F., Rofchaei, R., Arshad, U., Sadeghi Rad, M., Haddadi Moghadam, K., Pajand, Z. and Nikoein, A. R. 2005. Comparison of plankton and benthic organisms
diversity and density in sturgeon hatcheries and assessing their effects on condition factor in sturgeon fingerlings. Iranian Journal of Fisheries Sciences, 14(1):51-64. In Farsi.
Chugani, U. 2000. Azerbaijan's black gold. Impressions, October/November:16-18.
Churchill (Consul). 1877. Persian fisheries. Forest and Stream, London, 9:249-250.
Ciepielewski, W., Martyniak, A. and Szczerbowski, J. A. 2001. Ichthyofauna in the Dokan and Derbendikhan reservoirs. Archives of Polish Fisheries, 9(supplement 1):157-170.
Čihar, J. 1975. Geographical and ecologic variability of perch [Perca fluviatilis (Linnaeus)] and history of its distribution from Eurasia to North America. Sborník Národního Muzea v Praze,
31B(1-2):57-89.
CIRSPE (Italian Research and Study Centre for the Fishery). 2006a. Aquaculture Development in Sistan-Baluchestan 2005-2008. Technical Report, The
Hamun Lake, Preliminary Analysis. CIRSPE (Italian Research and Study Centre for the Fishery), Rome. 70 pp. (www.iranitaly.net).
CIRSPE (Italian Research and Study Centre for the Fishery). 2006b. Aquaculture Development in Sistan-Baluchestan 2005-2008. Technical Report, Artificial
reproduction of Schizothorax zarudny (sic). Methodological approach, Zabol/Zahedan March-June 2006. CIRSPE (Italian Research and Study
Centre for the Fishery), Rome. 48 pp., figs. (www.iranitaly.net).
CITES. 2001. CITES Identification Guide - Sturgeons and Paddlefishes. Guide to the Identification of Sturgeon and Paddlefish Species Controlled under the Convention on International Trade in Endangered
Species of Wild Fauna and Flora. Environment Canada, Ottawa. Pagination various.
Civil, M. 1961. The home of the fish. A new Sumerian literary composition. Iraq, 23:154-175.
Clapp, D. F., Schneeberger, P. J., Jude, D. J., Madison, G. and Pistis, C. 2001. Monitoring round goby (Neogobius melanostomus) population expansion in eastern
and northern Lake Michigan. Journal of Great Lakes Research, 27(3):335-341.
Clark, R. B. 1986. Marine Pollution. Clarendon Press, Oxford. xiii + 215 pp.
Clay, C. H. 1977. Report on a mission to Iran to investigate the potential for fish production from man-made lakes. Food and Agriculture Organization, Rome. MS. (not seen).
Clayton, D. A. and Vaughan, T. C. 1988. Ethogram of Boleophthalmus boddarti (Pallas)(Teleostei, Gobiidae), a mudskipper found on the mudflats of Kuwait.
Journal of the University of Kuwait (Sciences), 15:115-138.
Clugston, J. P. and Shireman, J. V. 1987. Triploid grass carp for aquatic plant control. Fish and Wildlife Service, Washington, Fish and Wildlife Leaflet, 8:3 pp.
Coad, B. W. 1979a. Range extension for the snakehead Ophiocephalus gachua Hamilton-Buchanan (Osteichthyes: Channidae) in Iran. Journal of the Bombay Natural History Society, 75(2):(1978):500-501.
Coad, B. W. 1979b. Poisonous and venomous freshwater fishes of Iran. Pahlavi Medical Journal, 9(4):388-407.
Coad, B. W. 1980a. A provisional, annotated check-list of the freshwater fishes of Iran. Journal of the Bombay Natural History Society, 76(1)(1979):86-105.
Coad, B. W. 1980b. A re-description of Aphanius ginaonis (Holly, 1929) from southern Iran (Osteichthyes: Cyprinodontiformes). Journal of Natural History, 14(1):33-40.
Coad, B. W. 1980c. Environmental change and its impact on the freshwater fishes of Iran. Biological Conservation, 19(1):51-80.
Coad, B. W. 1981a. Glyptothorax silviae, a new species of sisorid catfish from southwestern Iran. Japanese Journal of Ichthyology, 27(4):291-295.
Coad, B. W. 1981b. First record of the milkfish, Chanos chanos (Forskal, 1775) from Iran and the Persian Gulf. Journal of the Bombay Natural History Society, 77(3)(1980):522-524.
Coad, B. W. 1981c. A new genus and species of cichlid endemic to southern Iran. Program of the American Society of Ichthyologists and Herpetologists, 61st Annual Meeting,
21-26 June 1981, Corvallis, Oregon. 1 p. (Abstract).
Coad, B. W. 1981d. Fishes of Afghanistan, an annotated checklist. Publications in Zoology, National Museums of Canada, 14:v + 26 pp.
Coad, B. W. 1981e. Pseudophoxinus persidis, a new cyprinid fish from Fars, southern Iran. Canadian Journal of Zoology, 59(11):2058-2063.
Coad, B. W. 1981f. A bibliography of the sticklebacks (Gasterosteidae: Osteichthyes). Syllogeus, Ottawa, 35:1-142.
Coad, B. W. 1982a. A new genus and species of cichlid endemic to southern Iran. Copeia, 1982(1):28-37.
Coad, B. W. 1982b. Review of "Euphrates and Tigris, Mesopotamian Ecology and Destiny" by Julian Rozska (sic) with contributions by J. F. Talling, F. R.
S. and Dr. K. E. Bainster (sic). Monographiae Biologicae, Volume 38, Dr. W. Junk by (sic) Publishers, The Hague, 1980. x + 122 pp., 36 figs., map.-U.S. $33.31.
Matsya, Bulletin of the Indian Society of Ichthyologists, 7(1981):102-104.
Coad, B. W. 1982c. Garra persica Berg, 1913, a valid species of cyprinid fish from southern Iran. Cybium, 6(2):97-100.
Coad, B. W. 1982d. The identity of Alburnus maculatus Keyserling, a cyprinid fish from Esfahan Province, Iran. Japanese Journal of Ichthyology, 29(2):227-228.
Coad, B. W. 1982e. Studies on the systematics and zoogeography of the freshwater fishes of Iran. Programme of the Fourth Congress of European Ichthyologists,
20.-24. 9. 1982, Hamburg, West Germany. 1 p. (Abstract).
Coad, B. W. 1982f. A re-description and generic re-assignment of Kosswigobarbus kosswigi (Ladiges, 1960), a cyprinid fish from Turkey and Iran.
Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 79:263-265.
Coad, B. W. 1983. Review of "The Freshwater Fishes of India, Pakistan, Bangladesh, Burma and Sri Lanka - A Handbook". By K. C. Jayaram. 1981. Zoological
Survey of India, Calcutta. xxii + 475 pp., 208 figs., 13 plates (2 color). Rs 100.00 or $22.50. Copeia, 1983(1):280-282.
Coad, B. W. 1984. Acanthobrama centisquama Heckel and the validity of the genus Mirogrex Goren, Fishelson and Trewavas (Osteichthyes: Cyprinidae). Hydrobiologia, 109(3):275-278.
Coad, B. W. 1985. Alburnus doriae De Filippi, 1864 a synonym of Leuciscus lepidus (Heckel, 1843)(Osteichthyes: Cyprinidae). Matsya, 9-10(1983-1984):173-175.
Coad, B. W. 1987a. The tooth-carps of Iran. Program and Abstracts, American Society of Ichthyologists and Herpetologists, 67th Annual Meeting, 21-26 June 1987, Albany, New York. p. 39 (Abstract).
Coad, B. W. 1987b. Zoogeography of the Freshwater Fishes of Iran, pp. 213-228, 1 figure, 2 tables. In: Krupp, F., Schneider, W. and Kinzelbach, R. (Eds.).
Proceedings of the Symposium on the Fauna and Zoogeography of the Middle East, Mainz, 1985. Beihefte zum Tübinger Atlas des Vorderen Orients, Reihe A (Naturwissenschaften),
28, Dr. Ludwig Reichert Verlag, Wiesbaden, 338 pp.
Coad, B. W. 1988. Aphanius vladykovi, a new species of tooth-carp from the Zagros Mountains of Iran (Osteichthyes: Cyprinodontidae). Environmental Biology of
Fishes, 23(1-2):115-125. (Reprinted with same pagination in McAllister, D. E. and Kott, E. (Eds.). On lampreys and fishes: a memorial anthology in honor of Vadim D. Vladykov.
Developments in Environmental Biology of Fishes, 8:1-162, Kluwer Academic Publishers, Dordrecht/Boston/London).
Coad, B. 1989. Tooth-carps and Tangs or Orogeny and Speciation in the Zagros Mountains of Iran. Research Results Conference, National Museum of Natural Sciences, Ottawa, 2-3 October 1989. 2 pp.
(Abstract).
Coad, B. W. 1991a. The qanat ichthyofauna of Iran. Research Results CONFERENCE des résultats de recherche 1991, Canadian Museum of Nature / Musée canadien de la nature,
Ottawa, March 21, 1991 / le 21 mars 1991. p. 27 (Abstract).
Coad, B. W. 1991b. Fishes of the Tigris-Euphrates Basin: A Critical Checklist. Syllogeus, Ottawa, 68:1-49.
Coad, B. W. 1992a. Marine Fishes of the Persian Gulf and Sea of Oman. Checklist and Bibliography. Syllogeus, Ottawa, MS. 214 pp.
Coad, B. W. 1992b. Out of Africa? The large-scale barbs of Southwest Asia. Collections and Research CONFERENCE des collections et recherche 1992, Canadian Museum of
Nature / Musée canadien de la nature, Ottawa, February 18, 1992 / le 18 février 1992. p. 28. (Abstract).
Coad, B. W. 1992c. Zoogeography of the fishes of Mesopotamia. Collections and Research CONFERENCE des collections et recherche 1992, Canadian Museum of Nature / Musée
canadien de la nature, Ottawa, February 18, 1992 / le 18 février 1992. p. 30. (Abstract).
Coad, B. W. 1992d. Environmental change and its impact on the freshwater fishes of Iran, pp. 103-104. In: Trussell, D. (Ed.). The Social and Environmental Effects of
Large Dams. Volume III. A Review of Literature. Wadebridge Ecological Centre, Camelford, U.K. xiv + 243 pp. (abstract of Coad (1980c)).
Coad, B. W. 1993. Expedition Field Techniques. Fishes. Expedition Advisory Centre, Royal Geographical Society, London. iv + 82 pp., 25 illustrations.
Coad, B. W. 1994a. Exotic and transplanted fishes in Southwest Asia. VIII Congress Societas Europaea Ichthyologorum, Oviedo, Spain, September 26 to October 2, 1994, p. 20 (abstract).
Coad, B. W. 1994b. Fishes from the qanats of Iran. VIII Congress Societas Europaea Ichthyologorum, Oviedo, Spain, September 26 to October 2, 1994, p. 75 (abstract).
Coad, B. W. 1994c. Criteria for assessing the conservation status of taxa. Science Forum Scientifique, Canadian Museum of Nature / Musée canadien de la nature, Ottawa, November 21 Novembre
1994, p. 35 (abstract).
Coad, B. W. 1994d. Exotic and transplanted fishes in Southwest Asia. Science Forum Scientifique, Canadian Museum of Nature / Musée canadien de la nature, Ottawa, November 21 Novembre 1994, p. 36
(abstract).
Coad, B. W. 1995a. Freshwater Fishes of Iran. Acta Scientiarum Naturalium Academiae Scientiarum Bohemicae, Brno, 29(1):1-64.
Coad, B. W. 1995b. Expedition Field Techniques: Fishes. Expedition Advisory Centre, Royal Geographical Society, London. 2nd Edition. v + 97 pp., 27 illustrations.
Coad, B. W. 1996a. Systematics of the tooth-carp genus Aphanius Nardo, 1827 (Actinopterygii: Cyprinodontidae) in Fars Province, southern Iran. Biologia, Bratislava, 51(2):163-172.
Coad, B. W. 1996b. Systematics of the shah mahi, Chalcalburnus chalcoides (Güldenstädt, 1772), in the southern Caspian Sea basin (Actinopterygii: Cyprinidae). Zoology in the Middle East,
12:65-70.
Coad, B. W. 1996c. Threatened fishes of the world: Iranocypris typhlops Bruun and Kaiser, 1944 (Cyprinidae). Environmental Biology of Fishes, 46(4):374.
Coad, B. W. 1996d. Freshwater fishes of Iranian and Pakistani Baluchistan. Second Symposium on Fish and Fisheries of Pakistan, November, 25, 26, 1996, Department of Zoology, Government
College, Lahore, p. 25-26 (abstract).
Coad, B. W. 1996e. Zoogeography of the fishes of the Tigris-Euphrates Basin. Zoology in the Middle East, 13:51-70.
Coad, B. W. 1996f. Exotic fish species in the Tigris-Euphrates basin. Zoology in the Middle East, 13:71-83.
Coad, B. W. 1996g. Fishes from the qanats of Iran. Publicaciones Especiales Instituto Español de Oceanografía, 21:63-79.
Coad, B. W. 1996h. Exotic and transplanted fishes in Southwest Asia. Publicaciones Especiales Instituto Español de Oceanografía, 21:81-106.
Coad, B. W. 1997a. Status of shad in Iranian waters? Shad Journal, 2(2):3.
Coad, B. W. 1997b. Systematic biodiversity in the freshwater fishes of Iran. Ninth International Congress of European Ichthyologists (CEI9)
"Fish Biodiversity". Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 25.
Coad, B. W. 1997c. Freshwater fishes of Iranian and Pakistani Baluchistan. Biologia, Lahore, 42(1 & 2)(1996):1-18.
Coad, B. W. 1997d. Systematic biodiversity in the freshwater fishes of Iran. Science FORUM scientifique, Canadian Museum of Nature / Musée canadien de la nature, Ottawa, November 20 novembre,
1997, p. 27 (abstract).
Coad, B. 1997e. Shad in Iranian waters. The Shad Journal, Seattle, 2(4):4-8.
Coad, B. W. 1998a. Threatened fishes of the world: Lebias ginaonis (Holly, 1929)(Cyprinodontidae). Environmental Biology of Fishes, 51(3):284.
Coad, B. W. 1998b. Lebias ginaonis (Holly, 1929). Holly's pupfish. Killi Kontakt, Wommelgem, 4 pp., 1 figure.
Coad, B. W. 1998c. Description of Barbus sublimus Coad and Najafpour, 1997, 2 pp., 2 figures. In: Berrebi, P. (Ed.). Atlas Barbus on the Internet,
Université Montpellier, France (http://162.38.181.13/webbarbus/).
Coad, B. W. 1998d. Description of Barbus grypus Heckel, 1843, 2 pp., 2 figures. In: Berrebi, P. (Ed.). Atlas Barbus on the Internet,
Université Montpellier, France (http://162.38.181.13/webbarbus/).
Coad, B. W. 1998e. Systematic biodiversity in the freshwater fishes of Iran. Italian Journal of Zoology, 65 (Supplement):101-108. (Proceedings of the Ninth Congress of European Ichthyologists (CEI-9)
"Fish Biodiversity" organised in Naples at the University Federico II and held in Trieste - Italy, 24-30 August 1997).
Coad, B. W. 1998f. Expedition Field Techniques: Fishes. Expedition Advisory Centre, Royal Geographical Society, London. 2nd Revised Edition. v + 97 pp., 27 illustrations. ISBN 0-907649-71-8.
Coad, B. W. 1999a. Researchs and scientists. Brian W. Coad. Iran Nature and Wildlife Magazine, Farvadin 1378(3):2 pp., 2 figures. In Farsi and English.
Coad, B. W. 1999b. (Fish). i. Freshwater fishes, p. 655-669. In: Yarshater, E. (Ed.). Encyclopædia Iranica. Bibliotheca Persica Press, New York. Volume IX, Fascicle 6. Festivals VIII-Fish.
Coad, B. W. 1999c. Verspreiding van Aphanius-soorten in Iran. Killi-Kontakt, Wommelgem, 27(4):102-110. In Flemish.
Coad, B. W. 2000a. Criteria for assessing the conservation status of taxa (as applied to Iranian freshwater fishes). Biologia, Bratislava, 55(5):539-557.
Coad, B. W. 2000b. Distribution of Aphanius species in Iran. Journal of the American Killifish Association, 33(6):183-191.
Coad, B. W. 2000c. Aphanius ginaonis (Holly, 1929). Holly's pupfish. Journal of the American Killifish Association, 33(6):192-194.
Coad, B. W. 2003. Freshwater fishes, 15 pp., 20 figures, 2 tables. In: Yarshater E. (Ed.). Encyclopædia Iranica (Daneshnameh-ye Iranika). Volume IX, Fascicule 6. Festivals VIII - Fish. Bibliotheca
Persica Press, New York. (on-line version of Coad (1999b) at http://www.iranica.com/articles/v9f6/v9f641a.html, downloaded 30 July 2003).
Coad, B. W. 2004. Review of "Caviar: The Strange History and Uncertain Future of the World’s Most Coveted Delicacy” by Inga Saffron. 2002. Broadway Books, New York. xv + 270 pp. U.S.
$35.95. Canadian Field-Naturalist, 117(2)(2003):324-325.
Coad, B. W. 2006. Endemicity in the freshwater fishes of Iran. Iranian Journal of Animal Biosystematics, 1(1)(2005):1-13.
Coad, B. W. 2007. Review of “The Complete Fauna of Iran” by Eskandar Firouz. 2005. I. B. Tauris. London, New York. xiv + 322 pp. US$90.00. Canadian Field-Naturalist, 120(1)(2006):114-116.
Coad, B. W. 2008a. Fishes of Tehran Province and adjacent areas. Shabpareh Publications, Tehran. 244 pp., 38 figures. ISBN 978-600-5038-02-6. (40,000 rials).
Coad, B. W. 2008b. Biodiversity in Iranian tooth-carps (Cyprinodontidae). International Congress on Documenting, Analysing and Managing Biodiversity in the Middle East 20-23 October 2008,
Intercontinental Hotel, Aqaba, Jordan (abstract).
Coad, B. W. 2009a. Threatened fishes of the world: Luciobarbus subquincunciatus (Günther, 1868) (Cyprinidae). Environmental Biology of Fishes, 86(2):323.
Coad, B. W. 2009b. A new species of tooth-carp, Aphanius mesopotamicus, from Iran and Iraq (Actinopterygii, Cyprinodontidae), p. 149-163., In: Neubert, E., Amr, Z., Taiti, S. and Gümüs, B.
(Eds.).Animal Biodiversity in the Middle East. Proceedings of the First Middle Eastern International Congress, Aqaba, Jordan, 20-23 October 2008, Aqaba, Jordan. ZooKeys, 31 (Special Issue):252 pp.
Pensoft Publishers, Sofia-Moscow.
Coad, B. W. 2009c. Alburnus zagrosensis n. sp., a new species of fish from the Zagros Mountains of Iran (Actinopterygii: Cyprinidae). Zoology in the Middle East, 48:63-70.
Coad, B. W. 2010. Freshwater Fishes of Iraq. Pensoft Publishers, Sofia-Moscow. 294 pp.,16 colour plates. ISBN 978-954-642-530-0, Pensoft Series Faunistica, 93, ISSN 1312-0174.
Coad, B. W. (Continuing). Bibliography on the Freshwater Fishes of Iran. http://gause.biology.ualberta.ca/Keivany/irbiblcoad.html. First posted 10 September 1996. Page originally maintained by
Yazdan Keivany at the University of Alberta, Edmonton and now replaced by this website at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Ottawa, Ontario, Canada.
Coad, B. W. (Continuing). Freshwater Fishes of Iran. First posted 26 February 2002 at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Ottawa, Ontario, Canada.
Coad, B. W. (Continuing). Freshwater Fishes of Iraq. First posted 28 September 2005 at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Ottawa, Ontario, Canada.
Coad, B. W. and Abdoli, A. 1993a. Exotic fish species in the fresh waters of Iran. Science Forum scientifique, Canadian Museum of Nature / Musée canadien de la nature, Ottawa, November 17 novembre
1993. p. 40. (Abstract).
Coad, B. W. and Abdoli, A. 1993b. Exotic fish species in the fresh waters of Iran. Zoology in the Middle East, 9:65-80.
Coad, B. W. and Abdoli, A. 1996. Biodiversity of Iranian freshwater fishes. Abzeeyan, Tehran, 7(1):4-10, IV. In Farsi, English abstract.
Coad, B. W. and Abdoli, A. 2000a. Rhinogobius cf. similis Gill, 1859, a goby new to the fish fauna of Iran and the problem of alien invasions. Zoology in the Middle East, 20:55-59.
Coad, B. W. and Abdoli, A. 2000b. Systematics of an isolated population of tooth-carp from northern Iran (Actinopterygii: Cyprinodontidae). Zoology in the Middle East, 21:87-102.
Coad, B. W. and Al-Hassan, L. A. J. 1989a. A Bibliography of the Fishes of the Tigris-Euphrates Basin / Bibliographie der Fische des Euphrat-Tigris-Beckens. Max Kasparek Verlag, Heidelberg.
56 pp. ISBN 3-925064-05-2. DM 19.80. (dated 1988).
Coad, B. W. and Al-Hassan, L. A. J. 1989b. Freshwater shark attacks at Basrah, Iraq. Zoology in the Middle East, 3:49-53.
Coad, B. W., Alkahem, H. F. and Behnke, R. J. 1983. Acanthobrama hadiyahensis, a new species of cyprinid fish from Saudi Arabia. Publications in Natural Sciences, National Museums of Canada,
2:v + 6 pp.
Coad, B. W., Atz, J. W. and Keivany, Y. 2000. Fish imagery in art 82: Jonah and the fish. Environmental Biology of Fishes, 57(1):9.
Coad, B. W. and Bogutskaya, N. G. 2009. Alburnoides qanati, a new species of cyprinid fish from southern Iran (Actinopterygii, Cyprinidae). ZooKeys, 13:67-77.
Coad, B. W. and Bogutskaya, N. G. 2010. Petroleuciscus esfahani, a new species of fish from central Iran (Actinopterygii: Cyprinidae). Zootaxa, 2534:37-47.
Coad, B. W. and Delmastro, G. B. 1985. Notes on a sisorid catfish from the Black Sea drainage of Turkey. Cybium, 9(3):221-224.
Coad, B. W. and Esmaeili, H. R. 2009a. Desert Fishes of Iran. Desert Fishes Council 41st Annual Meeting, Furnace Creek Ranch, Death Valley National Park, California, 19 to 22 November, 2009 (abstract).
Coad, B. W. and Esmaeili, H. R. 2009b. Desert Fishes of Iran. Videotape of Coad and Esmaeili (2009a) presented at the Native Aquatic Species Restoration Webinar CSP3901, National Conservation Training
Center, Shepherdstown, West Virginia, 10 December 2009.
Coad, B. W. and Holčík, J. 1999. Systematics of the cyprinid fish Chalcalburnus atropatenae (Berg, 1925) from the Lake Orumiyeh basin in northwest Iran. Biologia, Bratislava, 54(2):179-186.
Coad, B. W. and Holčík, J. 2000. On Silurus species from Iran (Actinopterygii: Siluridae). Folia Zoologica, Prague, 49(2):139-148.
Coad, B. W. and Hussain, N. A. 2007. First record of the exotic species Hemiculter leucisculus (Actinopterygii: Cyprinidae) in Iraq. Zoology in the Middle East, 40:107-109.
Coad, B. W., Hussain, N. A., Ali, T. S. and Limburg, K. E. 2003. Middle Eastern shads. In: Limburg, K. E. and Waldman, J. R. (Eds.). Biodiversity, Status, and Conservation of the World’s Shads.
American Fisheries Society Symposium, 35:59-67 (ISBN 1-888569-51-4, ISSN 0892-2284).
Coad, B. W. and Keivany, Y. 1998b. Lebias vladykovi (Coad, 1988). Killi-Kontakt, Wommelgem, 26(3):43-48. In Flemish.
Coad, B. W. and Keivany, Y. 2000. Aphanius vladykovi Coad, 1988. Zagros pupfish, mahi-e gour-e khari. Journal of the American Killifish Association, 33(6):195-198.
Coad, B. W. and Keivany, Y. 2002a. Aphanius vladykovi Coad, 1988 "Zagros pubfish" (sic) mahi-e gour-e khari. Skandinaviska Killi Sällskapet Killibladet, 2002(2):3-6, 1 figure.
In Swedish (translation of Coad and Keivany, 2000).
Coad, B. W. and Keivany, Y. 2002b. Reviews of "Atlas of Iranian Fishes: Gilan Inland Waters. K. Abbasi, A. Valipour, D. Talebi Haghighi, A. Sarpanah, and Sh. Nezami. 1999. Gilan Fisheries
Research Centre, Rasht. vi + 113 p. 35,000 Rls ($4.38)(paperback). The Inland Water Fishes of Iran. A. Abdoli. 2000. Iranian Museum of Nature and Wildlife, Tehran. ISBN: 964-6902-01-4. 378 p. 75,000
Rls ($9.38)(hardbound). A Guide to the Fauna of Iran. E. Firouz. 2000. Iran University Press (University Publication Centre), Tehran. ISBN: 964-01-0956-8. vi + 491 p. 45,000 Rls ($5.63)(hardbound).
Freshwater Fishes of Iran. H. Mohammadian. 1999. Sepehr Publications Centre, Tehran. ISBN: 946-6123-18.X. vii + 178 p. 12,000 Rls ($1.50)(paperback)". Copeia, 2002(4):1164-1166.
Coad, B. W. and Keyzer-de Ville, N. 2004. On the systematics and distribution of the snow trout, Schizothorax pelzami Kessler, 1870, in Iran (Actinopterygii:
Cyprinidae). Zoology in the Middle East, 32:57-62.
Coad, B. W. and Keyzer-de Ville, N. 2005. On the validity of the species in the snow-trout genus Schizocypris Regan, 1914 (Cyprinidae: Actinopterygii). Zoology in the Middle East, 35:35-42.
Coad, B. W. and Krupp, F. 1983. Redescription of Barilius mesopotamicus Berg, 1932 a poorly known cyprinid fish from the Tigris-Euphrates basin. Cybium, 7(1):47-56.
Coad, B. W. and Krupp, F. 1994. Capoeta aculeata (Valenciennes in Cuv. & Val., 1844), a valid species of cyprinid fish from Iran (Teleostei: Cyprinidae). Zoology in the Middle East,
10:63-72.
Coad, B. W. und Kuru, M. 1986. Bibliographie der Fische der Türkei/A Bibliography of the Fishes of Turkey, pp. 15-77. In: Kasparek, M. (Ed.). Zoologische Bibliographie der Türkei. Zoological
Bibliography of Turkey. Pisces, Amphibia, Reptilia. Max Kasparek Verlag, Heidelberg. 118 pp. ISBN 3-925064-01-X. DM 24.50.
Coad, B. W. and McAllister, D. E. (Continuing). Dictionary of Ichthyology. First posted 4 September 2002 at www.briancoad.com, maintained by Brian W. Coad and Nicholas P. Coad, Pure Throttle
Technologies Inc., Ottawa, Ontario.
Coad, B. W., Mehrani, R. and Najafpour, N. 2009. Threatened fishes of the world: Paracobitis smithi (Greenwood, 1976) (Balitoridae). Environmental Biology of Fishes, 84(3):323.
Coad, B. W. and Najafpour, N. 1997. Barbus sublimus, a new species of cyprinid fish from Khuzestan Province, Iran. Ichthyological Exploration of Freshwaters, 7(3):273-278.
Coad, B. W. and Nalbant, T. T. 2005. A new genus and a new species of a remarkable nemacheilid fish from Iran (Pisces: Ostariophysi: Nemacheilidae). Travaux du Muséum National d’Histoire
Naturelle "Grigore Antipa", Bucharest, 48:303-308.
Coad B. W. and Nalbant T. T. 2006. On the systematic position of the recently described genus and species Ilamnemacheilus longipinnis from Iran (Pisces: Ostariophysi: Nemacheilidae).
3rd International Conference Loaches of the Genus Cobitis and Related Genera. Biology, Systematics, Genetics, Distribution, Ecology, Conservation. Sibenik, Croatia, 24-29 September 2006 (title).
Coad, B. W. and Papahn, F. 1988. Shark attacks in the rivers of southern Iran. Environmental Biology of Fishes, 23(1-2):131-134.
(Reprinted with same pagination in McAllister, D. E. and Kott, E. (Eds.). On lampreys and fishes: a memorial anthology in honor of Vadim D. Vladykov. Developments in Environmental Biology of Fishes,
8:1-162, Kluwer Academic Publishers, Dordrecht/Boston/London).
Coad, B. W. and Sarieyyüpoğlu, M. 1988. Cobitis elazigensis, a new species of cobitidid fish from Anatolia, Turkey. Japanese Journal of Ichthyology, 34(4):426-430.
Coad, B. W. and Vilenkin, B. Ya. 2004. Co-occurrence and zoogeography of the freshwater fishes of Iran. Zoology in the Middle East, 31:53-61.
Coad, B. W. with Waszczuk, H. and Labignan, I. 1995. Encyclopedia of Canadian Fishes. Canadian Museum of Nature, Ottawa and Canadian Sportfishing Productions, Waterdown. viii + 928 pp.
COFREPECHE. 1997. Iranian Fisheries Sector Study. Parts 1-4. Report prepared for Shilat (Fisheries Department), Jihad-e Sazandagi, Islamic Republic of Iran.
Collares-Pereira, M. J. 1994. The karyology of barbins and the possible plesiomorphic condition of polyploïdy in Cyprinidae. Bulletin français de la pêche et de la pisciculture, 334:191-199.
Collette, B. B. 1982. Rediscovery of Hyporhamphus xanthopterus, a half beak endemic to Vembanad Lake, Kerala, southern India. Matsya, 7(1981):29-40.
Collette, B. B., Ali, M. A., Hokanson, K. E. F., Nagieć, M., Smirnov, S. A., Thorpe, J. E., Weatherly, A. H. and Willemsen, J. 1977. Biology of Percids. Journal of the Fisheries Research Board
of Canada, 34:1890-1899.
Collette, B. B. and Bănărescu, P. 1977. Systematics and zoogeography of the fishes of the Family Percidae. Journal of the Fisheries Research Board of Canada, 34:1450-1463.
Compagno, L. J. V. 1984. FAO Species Catalogue. Volume 4. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Part 1 - Hexanchiformes to Lamniformes, Part 2 -
Carcharhiniformes. Food and Agriculture Organization, Rome, Fisheries Synopsis, 125(4), part 1:viii + 1-249; part 2:x + 251-655.
Compagno, L. J. V. 1988. Sharks of the Order Carcharhiniformes. Princeton University Press, Princeton, New Jersey. xxii + 486 pp., 21 figs., 35 plates.
Conrad, G. et Conrad, J. 1970. L'évolution quaternaire de la dépression du Lout (Iran oriental). Comptes Rendus de l'Académie des Sciences, Paris, 270, série D, 9:1672-1674.
Cook, J. M. 1985. The rise of the Achaemenids and establishment of their empire, pp. 200-291. In: Gershevitch, I. (Ed.). The Cambridge History of Iran. Volume 2. The Median and Achaemenian Periods.
Cambridge University Press, Cambridge. 946 pp.
Corkum, L. D. and Wickett, R. G. 1998. Nest defense by the exotic fish, round goby (Neogobius melanostomus), on a shipwreck in western lake Erie. American Society of Ichthyologists and
Herpetologists' 78th Annual Meeting, July 16 to July 22, 1998, University of Guelph, Guelph, Ontario, Canada. 1 p. (abstract).
Cornwallis, L. 1968a. A report on the wetlands and waterfowl of Fars, S.W. Iran. MS Report, Department of Biology, Pahlavi University, Shiraz, Iran. 32 pp., 7 maps.
Cornwallis, L. 1968b. Some notes on the wetlands of the Niriz basin in S. W. Iran. Proceedings of a Technical Meeting on Wetland Conservation, Ankara-Bursa-Istanbul, 9 to 16 October 1967,
International Union for Conservation of Nature and Natural Resources Publication, new series, 12:152-160.
Cornwallis, L. 1976. The impact of the Mahabad Project on Lake Rezaiyeh and its southern satellite wetlands together with proposals for the conservation of the latter. Division of Nature Conservation,
Department of the Environment, Tehran, Report Number W-1-35-2, 11 pp.
Costa, W. J. E. M. 1997. Phylogeny and classification of the Cyprinodontidae revisited (Teleostei: Cyprinodontiformes): Are Andean and Anatolian killifishes sister taxa? Journal of Comparative
Biology, 2(1):1-17.
Costantini, L. and Tosi, M. 1978. The environment of southern Sistan in the Third Millennium B.C., and its exploitation by the proto-urban Hilmand civilization, pp. 165-183. In: Brice, W. C. (Ed.).
The Environmental History of the Near and Middle East Since the Last Ice Age. Academic Press, London. xx + 384 pp.
Courtenay, W. R. and Stauffer, J. R. (Eds.). 1984. Distribution, Biology, and Management of Exotic Fishes. Johns Hopkins University Press, Baltimore. xiv + 430 pp.
Courtenay, W. R. and Williams, J. D. 2004. Snakeheads (Pisces, Channidae) - a biological synopsis and risk assessment. U. S. Geological Survey Circular, 1251:v + 143 pp.
Craig, J. 1987. The Biology of Perch and Related Fish. Croom Helm, London. 333 pp.
Craig, J. F. 1996. (Ed.). Pike. Biology and Exploitation. Chapman & Hall, London. xxii + 298 pp. (Fish and Fisheries Series, 19).
Crawford, S. S. and Muir, A. M. 2008. Global introductions of salmon and trout in the genus Oncorhynchus: 1870-2007. Reviews in Fish Biology and Fisheries, 18(3):313-344.
Cressey, G. B. 1958a. The Shatt al-Arab Basin. Middle East Journal, 12(4):448-460.
Cressey, G. B. 1958b. Qanats, karez, and foggaras. Geographical Review, 48: 27-44.
Crockett, J. 1996. Project formulation for fisheries training and extension. Islamic Republic of Iran, Fisheries Training Service, Food and Agriculture Organization, Rome, Technical Cooperation
Programme, Rome. 25 pp.
Crossman, E. J. and Casselman, J. M. 1987. An Annotated Bibliography of the Pike, Esox lucius (Osteichthyes: Salmoniformes). Life Sciences Miscellaneous Publication, Royal Ontario Museum,
Toronto. xix + 386 pp.
Cudmore, B. and Mandrak, N. E. 2004. Biological synopsis of grass carp (Ctenopharyngodon idella). Canadian Manuscript Report of Fisheries and Aquatic Sciences, 2705:v + 44 pp.
Culling, M. A., Janko, K., Boroń, A., Vasil`ev, V. P., Côté, I. M. and Hewitt, G. M. 2006. European colonization by the spined loach (Cobitis taenia) from Ponto-Caspian refugia based on
mitochondrial DNA variation. Molecular Ecology, 15(1):173-190.
Cuvier, G. et Valenciennes, A. 1828-1849. Histoire naturelle des poissons. 22 volumes. Paris. (Reprint A. Asher & Co., Amsterdam).
Cyrus, A., Gholam Reza, E. G., Elham, J., Reza, M. S., Afsane, T., Rahim, O. and Soroor, E. 2009. Genetic survey on some important Barbus species in south-west of Iran. The 6th Scientific
Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 66 (abstract).
Czeczuga, B., Muszyñska, E., Wossughi, Gh., Kamaly, A. and Kiziewicz, B. 1995. Aquatic fungi growing on the eggs of several species of acipenserid fishes. Acta Ichthyologica et Piscatoria,
Szczecin, 25(2):71-79.
D
Dadaye Ghandi, A., Khodaparast, S. K. and Esmaeili Sari, A. 2005. Assessing water contamination with anionic surfactants in Anzali Lagoon. Iranian Journal of Fisheries Sciences, 14(3):60-68. In Farsi.
Dadelahi Sohrab A., Nabavi S. M. B. and Khirvar, N. 2009. The relationship between biometric characters of Barbus grypus with heavy metals levels in tissues (muscle and gills) from Arvand River,
Iran. Iranian Scientific Fisheries Journal, 17(4):27-34. In Farsi.
Dadgar, S., Bin Saad, C. R., Alimon, A. R., Kamarudin, M. S. and Nafisi Bahabadi, M. 2010. Comparison of soybean meal and cottonseed meal variety Pak (CSMP) on growth and feed using in rainbow
trout (Oncorhynchus mykiss). Iranian Journal of Fisheries Sciences, 9(1):49-60.
Dadikyan, M. G. 1970. A new subspecies of white bream [Blicca bjoerkna derjavini, subsp. N.] from the Sevdzhur River. Journal of Ichthyology, 10(4):550-552.
Dadikyan, M. G. 1972. A new subspecies of the European riffle minnow Alburnoides bipunctatus armeniensis subsp. nov. Journal of Ichthyology, 12(3):519-522.
Dadikyan, M. G. 1973. Variability of the Armenian riffle minnow (Alburnoides bipunctatus eichwaldi
(Filippi)) in relation to the altitude at which it occurs. Journal of Ichthyology, 13(1):68-78.
Dadikyan, M. G. 1986. Ryby Armenii [Fishes of Armenia]. Izdatel'stvo Akademiya Nauk Armyanskoi SSR, Yerevan. 245 pp.
Daei S., Jamili S. Mashinchian, A. and Ramin, M. 2009. Effect of Pb and Cd on the iron solute in blood (Chalcalburnus chalcoides). Journal of Fisheries and Aquatic Science, 4(6):323-329.
Daghighi Roohi, J. and Mokhayer, B. ?. Infection of Tinea tinea (sic) with Asymphylodora tineae Modeer, 1790 (Trematoda) in Anzali
Lagoon. Virtual, 1(1). In Farsi.
Daghighi Roohi, J. and Sattari, M. 2004. Occurrence and intensity of parasites in some gobiids (Perciformes: Gobiidae) from the southwest of the Caspian Sea. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 59(1):17-22. In Farsi.
Danesh Khoosh Asl, A. 1998. The role of stock enhancement of kutum on quality and quantity of resources in the Caspian Sea. The Seventh National Fisheries
Conference, Responsible Fisheries. Shilat Company, Tehran. pp. 207-219. In Farsi.
Danesh-e-Khoshasi, A. 1997. Fish culture experiments with Abramis brama orientalis in earthen pond to diversify aquaculture fish composition in Iran.
Iranian Fisheries Scientific Journal, 5(4):49-62, 5-6. In Farsi.
Danesh Khoshasi (sic), A. 1997. Culturing of Rutilus frisii kutum, a fish species of the Caspian Sea, to marketable size. Iranian
Fisheries Scientific Journal, 6(3):49-58, 6-7. In Farsi.
Danesh-e-Khoshasi (sic), A. 1998. Semi-natural breeding of Chinese carps. Iranian Fisheries Scientific Journal, 6(4):19-34, 3. In Farsi.
Daniels, R. J. R. 2002. India - A Lifescape. Freshwater Fishes of Peninsular India. Universities Press, Delhi. viii + 288 pp., 75 colour figs.
Daoud, A. 1978. Contribution à la connaissance de la biologie de Barbus xanthopterus, poisson, cyprinidé du lac Dokan.
Doctoral Thesis, Academie Montpellière, France. 88 pp. (not seen).
Daoulas, Ch., Economu, A. N., Psarras, Th. and Barbieri-Tseliki, R. 1993. Reproductive strategies and early development of three freshwater gobies. Journal of Fish Biology, 42:749-776.
Darabi, A. 1999. Investigation on age and growth of bleak Chalcalburnus chalcoides and Caspian vimba Vimba vimba persa. B.Sc. Project, Gorgan
University of Agricultural Sciences and Natural Resources, Gorgan. 72 pp. In Farsi.
Darafsh, F., Mashinchian, A., Fatemi, M. and Jamili, S. 2008. Study of the application of fish scale as bioindicator of heavy metal pollution (Pb, Zn) in the Cyprinus carpio of the
Caspian Sea. Research Journal of Environmental Sciences, 2(6):438-444.
Darvish Bastami, K. and Imanpour, M. R. 2009. Survey of effects of different concentrations of electrolytes and pH on characterization of sperm motility in wild carp (Cyprinus carpio). Iranian
Scientific Fisheries Journal, 18(2):27-34. In Farsi.
Darvish Bastami, K., Soudagar, M., Imanpour, M. R. and Taheri, S. A. 2009. Effects of different levels of Daphnia and Artemia extracts on food acceptability and growth factor rates of beluga
(Huso huso Linnaeus, 1758). Iranian Scientific Fisheries Journal, 17(4):35-44. In Farsi.
Darvishi, F., Esmaili Sari, A. and Farshchi, P. 2004. Dietary overlap between invasion Ctenophora (M. leidyi) and anchovy (C. engrauliformis) in
the southern pars of Caspian Sea. International Journal of Environmental Science and Technology, 1(2):89-96.
Das, K., Shukri, N. A. and Al-Nasiri, S. K. 1978. Production of protein concentrate from catfish. Iraqi Journal of Agricultural Science, 13:3-11.
Daskalov, G. M. and Mamedov, E. V. 2007. Integrated fisheries assessment and possible causes for the collapse of anchovy kilka in the Caspian Sea. ICES Journal of Marine Science, 64(3):503-511.
Datta, M. N. 1937. Description of the male of Eosentis rigidus Van Cleave occurring in the
intestine of Scizothorax (sic) zarudnyi from Seistan. Records of the Indian Museum, 39:303-304.
Datta Munshi, J. S. 1993. Structure and function of the air-breathing organs of Heteropneustes fossilis. Advances in Fish Research, 1:99-138.
Datta Munshi, J. S. and Choudhary, S. 1996. Ecology of Heteropneustes Fossilis (sic)(Bloch). An Air-breathing Catfish of South East Asia.
Narendra Publishing House, New Delhi. x + 174 pp.
Davarzani, M. 1995. Leather from sturgeon skin. Iranian Fisheries Scientific Journal, 3(4):45-58, 6. In Farsi.
Davoudzadeh, M. 1997. Iran, p. 384-405. In: Moores, E. M. and Fairbridge, R. W. (Eds.). Encyclopedia of European and Asian Regional Geology. Chapman and Hall, London.
Day, F. 1872a. Monograph of Indian Cyprinidae, (Part VI). Journal of the Asiatic Society of Bengal, 41(2):318-327.
Day, F. 1872b. Notes on fish, collected by Dr. Stoliczka in Kachh. Journal of the Asiatic Society of Bengal, 41(3):258-260.
Day, F. 1875-1878. The Fishes of India; being a natural history of the fishes known to inhabit the seas and fresh waters of India, Burma and Ceylon.
London. Volume 1, Text: xx + 778 pp. Volume 2, Atlas - containing 198 plates.
Day, F. 1876. On the fishes of Yarkand. Proceedings of the Zoological Society, London, 1876:781-807.
Day, F. 1878. Scientific Results of the Second Yarkand Mission; based upon the collections and notes of the late Ferdinand Stoliczka, Ph.D. Ichthyology.
Superintendent of Government Printing, Calcutta. 25 pp., plates I-V.
Day, F. 1880. On the fishes of Afghanistan. Proceedings of the Zoological Society, London, 1880:224-232.
Day, F. 1888. Supplement to the Fishes of India; being a natural history of the fishes known to inhabit the seas and fresh waters of India, Burma and Ceylon. Williams and Norgate, London. pp. 779-816.
Day, F. 1889. The Fauna of British India including Ceylon and Burma. Fishes. Taylor and Francis, London. 2 volumes, xx + 548 pp., xiv + 509 pp.
De Bruyn, C. 1711. Reysen over Muscovien door Persien en Indien. Amsterdam (another Dutch edition published in 1714; French
editions (under Corneille Lebrun) at Amsterdam in 1718 as:- "Voyages par la Moscovie, en Perse et aux Indes Orientales,
ouvrage enrichi de plus de 300 tailles douces des plus curieuses representent les animaux, les oiseaux, les poissons et les plantes,"....
with 320 plates, and at Rouen in 1725 in 5 volumes; English edition at London in 1737 as:- "Travels into Muscovy, Persia, and part of the East Indies", in 2 volumes).
Deelder, C. L. 1984. Synopsis of biological data on the eel Anguilla anguilla (Linnaeus, 1758). Food and Agriculture Organization, Rome, Fisheries Synopsis, 80, Revision 1:vii + 73 pp.
Deelder, C. L. and Willemsen, J. 1964. Synopsis of biological data on pike-perch Lucioperca lucioperca (Linnaeus) 1758. Food and Agriculture Organization, Rome, Fisheries Synopsis, 28:iv + 51 pp.
De Filippi, F. 1863. Nuove o poco note specie di animali vertebrati raccolte in un viaggio in Persia nell'estate dell'anno 1862. Archivio per la Zoologia, l'Anatomia e la Fisiologia, Modena, 2(2):377-394.
De Filippi, F. 1864. Riassunto del catalogo degli animali vertebrati delle provincie caucasiche e della Persia occidentale. Atti della Societa Italiana di Scienze Naturale di Milano, 7:184-186.
De Filippi, F. 1865. Note de un viaggio in Persia nei 1862. Pesci, pp. 3, 63, 141, 357-360. G. Daelli & C. Editori, Milano. viii + 396 pp.
Dehgan Madiseh, S., Savary, A., Kochinian, P. and Marammazy, J. 2000. Abundance, diversity and distribution of ichthyoplanktons in creeks and coastal
waters of Khouzestan Province. Iranian Journal of Fisheries Sciences, 9(2):41-60. In Farsi.
Dehghani, S. and Akbari, S. 1997. Experimental gonadectomy in rainbow trout (Oncorhynchus mykiss). Iranian Fisheries Scientific Journal, 6(1):17-22, 3. In Farsi.
Dehghani, S. N., Maleki, M. and Akbari, S. 1998. The clinical study of wound healing after laparatomy (sic) in Cyprinus carpio. Iranian Fisheries Scientific Journal, 6(4):87-94, 8-9.
In Farsi.
De Mecquenem, R. 1908. Le lac d'Ourmiah. Annales de Géographie, Paris, 17:128-144.
De Menasce, J. P. 1966. Textes Pehlevis sut les qanats. Acta Orientalis, 30:167-175.
De Meulenaer, T. and Raymakers, C. 1996. Sturgeons of the Caspian Sea and the international trade in caviar. TRAFFIC International, Cambridge. iv + 71 pp.
de Moor, J. 1998. In the beginning was fish. Fish in the ancient Near East, p. 84-93. In: Walker, H. (Ed.). Fish. Food from the Waters. Proceedings
of the Oxford Symposium on Food and Cookery 1997. Prospect Books, Totnes, Devon. 335 pp.
De Pinna, M. C. C. 1996. A phylogenetic analysis of the Asian catfish families Sisoridae, Akysidae, and Amblycipitidae, with a hypothesis on the relationships
of the Neotropical Aspredinidae (Teleostei, Ostariophysi). Fieldiana Zoology, new series 84 (1478):iv + 83 pp.
Derakhshandeh Ghazi Mahale, R. 1997. Determining ideal stocking density for Acipenser persicus larvae in vniro tanks using aeration. M.Sc. Thesis, Islamic Azad University, Tehran. 74 pp. In Farsi.
Derzhavin, A. N. 1922. Stellate sturgeon. A biological essay. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii, 1:1-204. In Russian.
Derzhavin, A. 1923. Iskusstvennoe pazvedenne osetra na Sefid-Rude [Artificial sturgeon breeding on the Sefid-Rud]. Russkii Gidrobiologicheskii Zhurnal, 2(11-12):250.
Derzhavin, A. N. 1926. Ryby reki Kara-su [Fishes of the Kara-su River]. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii, 2(1):161-184.
Derzhavin, A. N. 1929a. Kurinskoe rybnoe khozyastvo i melioratsiya mugani [The fisheries of the Kura and the melioration of the Mugan]. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii, 2(2):1-68.
Derzhavin, A. N. 1929b. Zametka o rybakh reki Keredzh (sev. Persiya) [A note on fishes of the River Karaj (North Persia)]. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii, 2(2):69-79.
Derzhavin, A. N. 1934. Presnovodnye ryby yuzhnogo poberezh'ya Kaspiya. Vstuplenie [Freshwater fishes of the southern shore of the Caspian Sea. Introduction].
Trudy Azerbaidzhanskogo Otdeleniya Zakavkazskogo Filiala Akademii Nauk SSSR, Sektor Zoologii, Baku, 7:91-126.
DeSalle, R. and Birstein, V. J. 1996. PCR identification of black caviar. Nature, London, 381(6579):197-198.
De Silva, K. H. G. M. 1991. Population ecology of the paddy field-dwelling fish Channa gachua (Günther)(Perciformes, Channidae) in Sri Lanka. Journal of Fish Biology, 38:497-508.
De Tchihatcheff, P. 1856. Asie Mineure. Part ii. Climatologie et zoologie. Paris. 2 volumes, xix + 842 pp, 4 pls.
De Villiers, M. 1999. Water. Stoddart, Toronto. xvi + 413 pp.
Dettlafff, T. A., Ginsburg, A. S. and Schmalhausen, O. I. 1993. Sturgeon Fishes. Developmental Biology and Aquaculture. Springer-Verlag, Berlin. xiii + 300 pp.
DeWitt, H. H. 1960. A contribution to the ichthyology of Nepal. Stanford Ichthyological Bulletin, 7(4):63-88.
Dezfouli, I. B. 1996. Iran. Report on land and water laws and institutions. Food and Agriculture Organization, Rome, Technical Cooperation Programme, TCP/IRA/4452:28 pp.
Diagomanolin, V., Farhang, M., Ghazi-Khansari, M. and Jafarzadeh, N. 2004. Heavy metals (Ni, Cr, Cu) in the Karoon waterway river, Iran. Toxicology Letters, 151(1):63-68.
Diemel, A. 1926. Fisch-Texte der Zeit Urukaginas. Orientalia, 21:40-83.
Diers, J. A., Fynn-Aikins, K. and Diggins, T. P. 2001. Experimental analysis of native sport fish predation on the round goby (Neogobius
melanostomus). International Association for Great Lakes Research, Abstracts from the 44th Conference on Great Lakes Research
June 10-14, 2001, Great Lakes Science: Making it Relevant. University of Wisconsin - Green Bay, Wisconsin Sea Grant Institute, Green Bay, Wisconsin, Part 2:27.
Diester-Haass, L. 1973. Holocene climate in the Persian Gulf as deduced from grain-size and pteropod distribution. Marine Geology, 14:207-223.
Dillon, A. K. and Stepien, C. A. 2001. Genetic and biogeographic relationships of the invasive round (Neogobius melanostomus) and tubenose (Proterorhinus marmoratus)
gobies in the Great Lakes versus Eurasian populations. Journal of Great Lakes Research, 27(3):267-280.
Dimand, M. S. 1934. Persian miniatures of the Fourteenth Century. Bulletin of the Metropolitan Museum of Art, New York, 29(4):58-60.
Djuricich, P. and Janssen, J. 2001. Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan. Journal of Great Lakes Research, 27(3):312-318.
Dmitriev, N. A. 1946. Kefal' v iranskikh vodakh Kaspiya [Mullet in the Iranian waters of the Caspian]. Priroda, 1946(12):74-75.
Dmitriev, N. A. 1947. Nekotorye dannye po biologii pekhleviiskogo (enzeliiskogo) puzanka (Caspialosa caspia knipowitschi Iljin)[Some biological data on the Pahlevi (Enzeli)
shad (Caspialosa caspia knipowitschi Iljin)]. Zoologicheskii Zhurnal, 26(6):559-560.
Doadrio, I. 1990. Phylogenetic relationships and classification of western palaearctic species of the genus Barbus (Osteichthyes, Cyprinidae). Aquatic Living Resources, 3(4):265-282.
Doadrio, I. and Carmona, J. A. 2004. Phylogenetic relationships and biogeography of the genus Chondrostoma inferred from mitochondrial DNA
sequences. Molecular Phylogenetics and Evolution, 23:802-815.
Dobrovolov, I. S. and Ivanaova, P. P. 1999. Biochemical comparison of the Atherina boyeri and Atherina mochon pontica (Pisces, Atherinidae). Folia Zoologica, Prague, 48(1):55-60.
Dollfus, R. Ph. 1970. D'un cestode ptychobothrien parasite de cyprinide en Iran. Mission C.N.R.S. (Théodore Monod), février 1969). Bulletin du Muséum national d'Histoire naturelle,
Paris, série 2, 41(6):1517-1521.
Dor, M. 1984. CLOFRES. Checklist of the Fishes of the Red Sea. Israel Academy of Sciences and Humanities, Jerusalem. xxii + 1-437.
Dor, M. and Goren, M. 1994. An Updated Checklist of the Fishes of the Red Sea. Israel Academy of Sciences and Humanities, Jerusalem. 132 pp., 2 maps.
Dorafshan, S. 2004. The Caspian salmon, Salmo trutta caspius. Eslahenejad, 12:8-11. In Farsi.
Dorafshan, S., Amiri, B. M., Peykan Heyrati, F., Hajeezadeh, A. and Mostafavi, H. 2002. Induced spawning in female rainbow trout Oncorhynchus mykiss by
GnRH analog. Iranian Journal of Fisheries Sciences, 11(3):23-38. In Farsi.
Dorafshan, S. and Heyrati, F. P. 2006. Spawning induction in kutum Rutilus frisii kutum (Kamenskii, 1901) using carp pituitary extract or GnRH analogue
combined with metoclopramide. Aquaculture Research, 37(8):751-845.
Dorafshan, S. and Kalbasi, M. R. 2007. Karyological study of Ctenopharyngodon idella ♀ Hypophthalmichthys nobilis ♂ F1 hybrids. Iranian Journal of Biology, 20(2):277-285.
In Farsi.
Dorafshan, S., Kalbassi, M. R., Pourkazemi, M. and Mojazi Amiri, B. 2006. Optimization of UV irradiation for production of gynogenetic rainbow trout, Oncorhynchus mykiss:
emphasising Hertwig effect and photoreactivation. Iranian Journal of Fisheries Sciences, 6(1):19-34.
Dorafshan, S., Mostafavi, H. and Mojazi Amiri, B. 2003. Induction of spawning in common carp (Cyprinus carpio), using pituitary extract and GnRH analogue in combination with
domperidone. Iranian Journal of Biotechnology, 1:213-217.
Dorafshan, S., Mostafavi, H., Mojazi Amiri, B. and Habibi, H. 2002. Induced spawning by injection of GnRH in female rainbow trout. Iranian Journal of Fisheries Sciences, 11:23-38. In Farsi.
Dorofeyeva, Ye. A. 1965. Kariologicheskoe obosnovanie sistematicheskogo polozheniya Kaspiiskogo i Chernomorskogo
losoei (Salmo trutta caspius Kessler, Salmo trutta labrax Pallas) [A karyological demonstration of the taxonomic position of the Caspian
and Black Sea salmon (Salmo trutta caspius Kessler, Salmo trutta labrax Pallas)]. Voprosy Ikhtiologii, 5, 1(34):38-45.
Dorofeyeva, Ye. A. 1967. Sravnitel'no-morfologicheskie osnovy sistematiki vostochnoevropeiskikh lososei [Comparative morphological principles of taxonomy of East European salmons].
Voprosy Ikhtiologii, 7(1):3-17.
Dorofeyeva, E. A. 1997. The trouts Salmo trutta, of the Mediterranean, Black and Caspian Sea basins with comments on classification of the genus Salmo (Salmonidae). Ninth International
Congress of European Ichthyologists (CEI9) "Fish Biodiversity". Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 30.
Dorofeeva, E. A. 1998. Systematics and distribution history of European salmonid fishes of the genus Salmo. Journal of Ichthyology, 38(6):419-429.
Dorofeyeva, E. A. and Salmanov, A. V. 2001. Comparative osteology of trouts (Salmonidae) with comments on their taxonomy. The 10th
European Congress of Ichthyology ECI X, Programme & Book of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 170.
Dorostghoal, M., Peyghan, R., Papan, F. and Khalili, L. 2009. Macroscopic and microscopic studies of annual ovarian maturation cycle of Shirbot Barbus grypus in Karoon
River of Iran. Iranian
Journal of Veterinary Research, Shiraz University, 10(2)(27):172-179.
DouAbul, A. A. Z., Al-Omar, M., Al-Obaidy, S. and Al-Ogaily, N. 1987. Organochlorine pesticide residues in fish from the Shatt al-Arab River, Iraq. Bulletin of Environmental Contamination and
Toxicology, 38:674-680.
DouAbul, A. A. Z., Al-Saad, H. T. and Al-Rekabi, H. N. 1987. Residues of organochlorine pesticides in environmental samples from the Shatt al-Arab River, Iraq. Environmental Pollution, 43:175-187.
DouAbul, A. A. Z., Al-Saad, H. T., Al-Timari, A. A. K. and Al-Rekabi, H. N. 1988. Tigris-Euphrates
delta: a major source of pesticides to the Shatt al-Arab River (Iraq). Archives of Environmental Contamination and Toxicology, 17:405-418.
Dragomirescu, L., Constantinescu, V. and Bănărescu, P. M. 1985. A numerical taxonomic method adequate to the biological thinking. Applications: Watanabe's example and the Acanthobrama
genus (Pisces, Cyprinidae). Travaux du Muséum d'Histoire Naturelle "Grigore Antipa", Bucurešti, 27:243-265.
Dresch, J. 1975. Bassins arides iraniens. Bulletin de l'Association de Géographes Français, 430:337-351.
Dugan, P. (Ed.). 1993. Wetlands in Danger. A World Conservation Atlas. Oxford University Press, New York. 193 pp.
Duman, E and Duman, M. 1996. Keban Baraj Gülü'nden Avlanan Capoeta trutta Heckel, 1843 Ile Barbus rajanorum mystaceus Heckel, 1843'ün et Verimi Besin Değerleri [Meat yield and
nutritional value of Capoeta trutta Heckel, 1843 and Barbus rajanorum mystaceus Heckel, 1843 caught from Keban Dam Lake]. Su Ürünleri Dergisi, 13 (1-2):83-88.
Duman, E. and Şen, D. 1995. Keban Baraj Gölü'nde Yaşayan Barbus xanthopterus (Heckel, 1843)'da Karşılaştırmalı
Yaş Tayini [Comparative age determination of Barbus xanthopterus (Heckel, 1843) living in Keban Dam Lake. Su Ürünleri Dergisi, 12(3-4):293-297.
Dumont, H. 1995. Ecocide in the Caspian Sea. Nature, London, 377(6551):673-674.
Dumont, H. J. 1998. The Caspian Lake: history, biota, structure, and function. Limnology and Oceanography, 43(1):44-52.
Dumont, H. J. 2002. Possible consequences of the Mnemiopsis invasion for the biodiversity of the Caspian Sea. First International
Meeting "The invasion of the Caspian Sea by the comb jelly Mnemiopsis - problems, perspectives, need for action", Baku, Azerbaijan, 24-26 April 2001. Caspian Environment Programme, Baku,
Azerbaijan. 1 p.
Dunin-Barkovsky, L. V. 1977. Integrated historical-archeological and paleogeographical studies of drainless lakes in the USSR. In: Greer, D. C. (Ed.). Desertic Terminal Lakes. Utah State University.
pp. 91-94.
Durand, J. D., Persat, H. and Bouvet, Y. 1999. Phylogeography and postglacial dispersion of the chub (Leuciscus cephalus) in Europe. Molecular Ecology, 8:989-997.
Durand, J.-D., Tsigenopoulos, C. S., Ünlü, E. and Berrebi, P. 2002. Phylogeny and biogeography of the Family Cyprinidae in the Middle
East inferred from cytochrome b DNA - evolutionary significance of this region. Molecular Phylogenetics and Evolution, 22(1):91-100.
Durand, J.-D., Tsigenopoulos, C. S., Ünlü, E. and Berrebi, P. 2002. Erratum to "Phylogeny and biogeography of the Family Cyprinidae in the Middle
East inferred from cytochrome b DNA - evolutionary significance of this region" [Mol. Phylogenet. Evol. 22(2002) 91-100]. Molecular Phylogenetics and Evolution, 25(1):218.
Durand, J. D., Ünlü, E., Doadrio, I., Pipoyan, S. and Templeton, A. R. 2000. Origin, radiation, dispersion and allopatric hybridization in the chub Leuciscus cephalus.
Proceedings of the Royal Society of London, Series B, Biological Sciences, 267(1453):1687-1697.
Dürkoop, A., Leimbach, F. and Wilde, S. 1979. Bibliographie der geologischen Literatur des Iran bis 1978. Bochumer geologische und geotechnische Arbeiten, 2:iii + 149 pp.
E
Eadoollah, A. 1992. Rivers of Iran. Ministry of Energy, Jamab Company, Tehran. 2 volumes. In Farsi.
Eagderi, S., Majazi Amiri, B. and Mirvaghefi A. R. 2006. A histological study of testis structure and reproductive cycle in male bulatmai barbel (Barbus capito), migratory to Sefidrood and
Polrood rivers. Iranian Journal of Natural Resources, 59(1):139-149. In Farsi.
Eastwick, E. B. 1864. Journal of a Diplomate's Three Years' Residence in Persia. Smith, Elder and Co., London. 2 volumes, viii + 333 pp., vi + 339 pp.
Ebadi, A. G. and Shokrzadeh, M. 2006. A survey and measurement of residues of lindane (organochlorine pesticides) in four species of the most consumed fish in
the Caspian Sea (Iran). Toxicology and Industrial Health, 22(1):53-58.
Ebrahimi, D. 1994. Study on fertility in Persian sturgeon, Acipenser persicus broodfish. M.Sc. Thesis, College of Natural Resources, Tarbiat Modarres University, Noor. 180 pp.
Ebrahimi, E., Pourreza, J., Panamariov, S. V., Kamali, A. and Hosaini, A. 2004. Effects of different levels of protein and fat on growth and chemical
composition of fingerlings of Iranian sturgeon (Acipenser persicus). Journal of Agricultural Sciences and Natural Resources, 11(3):141-151. In Farsi.
Ebrahimi, M. 1999. 17, 20 dihydroxy progesterone (17, 20 DPH) production by different tissue in common carp
(Cyprinus carpio) and gold fish (Carasius (sic) auratus). Iranian Journal of Fisheries Sciences, 8(2):1-14, I. In Farsi.
Ebrahimi, M. 2001. Identification of freshwater fishes in some permanent rivers of Kerman Province. Iranian Scientific Fisheries Journal, 10(3):1-12, 1. In Farsi.
Ebrahimi, M. 2003.An enzyme linked immunosorbant assay (ELISA) for 11-ketotestosterone hormone using acetylcholinesterase as tracer: application to sex determination of cultured Caspian
sea sturgeon. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Ebrahimi, M. 2004. Morphological changes of fish sperm affected by copper using (SEM). Iranian Journal of Fisheries Sciences, 13(2):1-10. In Farsi.
Ebrahimi, M. 2005a. Using computer assisted sperm analysis (CASA) to monitoring the effects of zinc and cadmium pollution on fish sperm. Iranian Journal of Fisheries Sciences, 4(2):81-100, 119.
Ebrahimi, M. 2005b. 17α-hydroxy-4-pregenen-3-one (17αP) assay, using acetylcholinesterase enzyme as tracer. Iranian Journal of Fisheries Sciences, 5(1):13-28.
Ebrahimi, M. 2007. Qanat fishes in Markazi and Sirjan basins of Kerman Province, Iran. Iranian Scientific Fisheries Journal, 16(2):153-160. In Farsi.
Ebrahimi, M. and Malek, M. 200. Study of helminth paarsites of Acipenser persicus and A. stellatus (Chondrostei: Acipenseriformes) from the southern coast of Caspian Sea. Journal
of Science, University of Tehran, 32(4)(2):361-368.
Ebrahimi, M. and Taherianfard, M. 2010a. Concentration of four heavy metals (cadmium, lead, mercury and arsenic) in organs of two cyprinid fish (Cyprinus carpio and Capoeta sp.) from the
Kor River (Iran). Environmental Monitoring and Assessment, 168(1-4):575-585.
Ebrahimi, M. and Taherianfard, M. 2010b. Are the fish captured from Kor River, Fars (Iran), safe to eat? Aquatic Ecosystem and Health Management, 13(1):94-98.
Ebrahimi, M., Taherianfard, M. and Rafiee, M. M. 2008. Lead contamination in fishes of the Kor River. Iranian Journal of Fisheries Sciences, 7(2):1-14.
Ebrahimi, S. and Moshari, M. 2006. Evaluation of Choghakhor Wetland status with the emphasis on environmental management problems. Publications of the
Institute of Geophysics, Polish Academy of Sciences, Water Resources, E-6(390):8 pp.
Ebrahimzadeh, H. A. 1999. Evaluation of toxigenic fungi at Cyprinid Farm in Northern Iran. In: 26th World Veterinary Congress, Lyon, France, 23-26 September 1999 (abstract).
Ebrahimzadeh, A. and Kailani, R. 1976. Survey on the parasites of digestive organs, gills and muscles of Karun River fishes. Chamran University Publication, Ahvaz, 110:1-22. In Farsi.
Ebrahimzadeh, A. and Kilany-Damawandy, R. 1977. Parasitic infections in the fish or (sic) Karoon River in Province Khouzestan (Southwest
Iran), p. 130. Summaries of the First Mediterranean Conference on Parasitology, Izmir, Turkey, 5-10 October 1977.
Ebrahimzadeh, A. and Nabawi, L. 1975. Survey of the parasites in the fishes of Karun River. Chamran University Publication, Ahvaz, 81:1-35. In Farsi.
Ebrahimzadeh, H. and Karami, M. 2002. Biological education and research in Iran: past and present. Zoology in the Middle East, 26:11-14.
Ebrahimzadeh, M., Kalbassi, M. R., Nazari, R. M. and Behrozi, S. H. 2003. Comparison of some biological parameters between grass carp and female grass
carp x male bighead carp hybrid. Iranian Journal of Marine Sciences, 2(2-3):1-10. In Farsi.
Ebrahimzadeh, M. A., Karami, M. and Golrokh, M. 2004. Measurements of ache activity in the brain of sea, well- and river-fed white fish (Rutilus frisi
(sic) as a health index. Shahrekord University of Medical Sciences Journal, 6(3):33-38. In Farsi.
Ebrahimzadeh Mousavi, H. A. 2000. Occurrence of Vibrio parahaemolyticus in freshwater fish farms (Iran). The Third World Fisheries Congress, Beijing, 31 Oct - 3 Nov 2000 (title).
Ebrahimzadeh Mousavi, H. A., Hoosseinifard, S. M., Khosravi, A. R., Soltani, M. and Yosefian, M. 2007. Isolation and identification of parasite and saprophyte fungi from fungal affected eggs of the
rainbow trout (Oncorhynchus mykiss) in Mazandaran Province. Journal of Veterinary Research, 62(3):163-168. In Farsi.
Ebrahimzadeh Mousavi, H. A. and Khosravi, A. R. 2001. Isolation of toxigenic fungi at cyprinids farms in northern Iran. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(3):47-49.
In Farsi.
Ebrahimzadeh Mousavi, H. A. and Khosravi, A. R. 2002. Isolation of toxigenic fungi at cyprinids farms in Northern Iran, p. 222. In: Aquaculture Europe - 2002, "Seafarming today
and tomorrow", October 16-19, 2002, Trieste.
Ebrahimzadeh Mousavi, H. A. and Khosravi, A. R. 2004. Report of suspected epizootic ulcerative syndrome in goldfish (Carassius auratus). Journal of the Faculty of Veterinary Medicine,
University of Tehran, 59(1):29-31. In Farsi.
Ebrahimzadeh Mousavi, H. A., Khosravi, A. R. and Azari Takami, G. 2000. A survey of fungal flora of cultivated cyprinids in Sefid Rood
Fish Farmed Center. Journal of the Faculty of Veterinary Medicine, University of Tehran, 55(3):57-60. In Farsi.
Ebrahimzadeh Mousavi, H. A., Khosravi, A. R., Firouzbakhsh, F., Mokhayer, B., Sasani, F., Mirzagar, S. S. and Rostami, M. 2005. A case report of
Branchiomyces infection in common carp (Cyprinus carpio) from Iran. Iranian Journal of Fisheries Sciences, 5(1):105-112.
Ebrahimzadeh Mousavi, H. A., Khosravi, A. R., Mokhayer, B. and Khakhal, R. 2001. Fungal flora of two species of acipenserid fishes (Acipenser persicus and Acipenser stellatus)
in Acipenser centre in northern Iran. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Economidis, P. S. and Miller, P. J. 1990. Systematics of freshwater gobies from Greece (Teleostei: Gobiidae). Journal of Zoology, London, 221(1):125-170.
Economidis, P. S. and Nalbant, T. T. 1996. A study of the loaches of the genera Cobitis and Sabanejewia (Pisces, Cobitidae) of
Greece, with description of six new taxa. Travaux du Muséum national d'Histoire naturelle "Grigore Antipa", Bucurešti, 36:295-347.
Economou, A. N., Daoulas, C. Psarras, T. and Barbieri-Tseliki, R. 1994. Further data on the reproduction and larval development of Knipowitschia
caucasica (Gobiidae). Journal of Fish Biology, 45(2):360-362.
Edmondson, J. R. and Lack, H. W. 2006. Karl Georg Theodor Kotschy's itinerary in southern Iran, 1841-42. Willdenowia, 36:579-588.
Edrissian, Gh. H. 2006. Malaria in Iran: past and present situation. Iranian Journal of Parasitology, 1(1):1-14.
Edwards, D. (Ed.). 1989. Training Course in Coldwater Fish Culture. Lectures delivered at Kalerdasht salmonid hatchery, Iran 18 January - 3 March 1988. Food and Agriculture Organization,
Rome, Technical Cooperation Programme, FI:TCP/IRA/6755, Field Document 3:v + 145 pp.
Edwards, M. 1971. Water from the desert's bed. The qanats of Iran. Country Life, 149(3849):612-614.
Edwards, M. 1994. Pollution in the former U.S.S.R. Lethal legacy. National Geographic, 186(2):70-99.
Efron, S. 1993. Caviar crisis in the Caspian Sea. Abzeeyan, Tehran, 4(9):4-7. In Farsi.
Egglishaw, H. J. 1980. Benthic invertebrates of streams on the Alburz Mountain range near Tehran, Iran. Hydrobiologia, 69(1-2):49-55.
Ehlers, E. 1971. Südkaspisches Tiefland (Nordiran) und Kaspisches Meer. Beiträge zu ihrer Entwicklungsgeschichte im Jung- und Postpleistozän. Tübinger Geographische Studien,
44(5):184 pp., 29 foto.
Ehlers, E. 1980. Iran Grundzüge einer Geographischen Landeskunde. Wissenschaftliche Buchgesellschaft, Darmstadt, 18:xxxii + 596 pp., 11 Karten, 8 Bildtaf.
Eichwald, E. 1838. Faunae Caspii Maris Primitiae. Byulleten' Moskovskogo Obshchestva Ispytatelei Prirody, 11:125-174. In Latin.
Eichwald, E. 1841. Fauna caspio-caucasia nonnullis observationibus novis. IV. De Piscibus, pp. 163-220, XXXII-XXXV. Petropoli. iv + 233 pp. In Latin.
Eimanifar, A. and Mohebbi, F. 2007. Urmia Lake (Northwest Iran): a brief review. Saline Systems, 3:8 pp.
Eiselt, J. 1971. Forschungsarbeit des Naturhistorischen Museums Wien im und für den Iran. Österreiche Zeitschrift für Kultur, Politik und Wirtschaft des Islamischer Länder, 11-12(4):29-33.
Ekman, S. 1953. Zoogeography of the Sea. Sidgwick and Jackson, London. xiv + 417 pp.
Ekmekci, F. G. 1996a. Growth and reproduction properties of chub (Leuciscus cephalus Linnaeus, 1758) in Sariyar Dam Lake. Turkish Journal of Zoology, 20(Supplement):95-106. (original not seen).
Ekmekci, F. G. 1996b. Growth properties of carp (Cyprinus carpio L., 1758) population in Sariyar Dam Lake (Ankara). Turkish Journal of Zoology, 20(Supplement):107-115. (original not seen).
Elahimanesh, P. 2001. Fisheries of Iran in the 3rd millennium. In: 131st American Fisheries Society Annual Meeting, Phoenix, Arizona, 19-23 August 2001 (abstract).
Elanidze, M., Demetrashvili, M., Burchuladze, O. and Kurashvili, B. 1970. Ryby Presnykh vod Gruzii Atlas [Atlas of the freshwater fishes of
Georgia]. Akademiya Nauk Gruzinskoi SSR, Institut Zoologii, Tbilisi. 115 pp. In Russian and Georgian.
Elanidze, R. F. 1983. Ikhtiofauna rek i ozer Gruzii [Ichthyofauna of the rivers and lakes of Georgia]. Akademiya Nauk Gruzinskoi SSR, Institut Zoologii "Metsniereba", Tbilisi. 319 pp., map.
El-Hawawi, A. S. N. and Al-Imam, M. S. E. 1984. Oöcyte differentiation and yolk formation in the Arabian barred fish Aphanius dispar. Journal of the College of Science, King Saud University,
15(2):443-452.
El Kholy, F. H. 1952. Hydrology of River Tigris. Directorate General of Irrigation, Iraq. 183 pp., map.
Elliot, G. F. 1977. Untitled letter on Kurdistan fishes. Proceedings of the Geological Association, 87(4):430.
Elvira, B. 1986. Revisión taxonómica y distribución geográfica del género Chondrostoma Agassiz, 1835 (Pisces,
Cyprinidae). Tesis Doctorales, Instituto Nacional de Investigaciones Agrarias, Madrid. 530 pp.
Elvira, B. 1988. Taxonomic revision of the genus Chondrostoma Agassiz, 1835 (Pisces, Cyprinidae). Cybium, 11(2)(1987):111-140.
Elvira, B. 1991. Further studies on the taxonomy of the genus Chondrostoma (Osteichthyes, Cyprinidae): species from eastern Europe. Cybium, 15(2):147-150.
Elvira, B. 1997. Taxonomy of the genus Chondrostoma (Osteichthyes, Cyprinidae): an updated review. Folia Zoologica, Prague, 46(Supplement 1):1-14.
Emadi, H. 1979. The state of the fishing and reproduction of the kutum, Rutilus frisii kutum, in the Caspian Sea of Iran. Journal of Ichthyology, 19(4):151-154.
Emadi, H. 1989. The beluga, Huso huso, and its past and present conditions in the Iranian part of Caspian Sea. First International Symposium on Sturgeon, Bordeaux, 3-6 October 1989, p. 63
(abstract).
Emadi, H. 1990. Aquaculture in Iran. In: World Aquaculture 90, Halifax, N.S., Canada, 10-14 June 1990 (abstract).
Emadi, H. 1991. Biology and identification of Caspian kilka. Abzeeyan, Tehran, 2:8-12. In Farsi.
Emadi, H. 1993a. The status of aquaculture in Iran. Abzeeyan, Tehran, 4(2):VII-IIX. In English.
Emadi, H. 1993b. Nutrient requirements of warm water fishes. Abzeeyan, Tehran, 4(4):2-7, II. In Farsi.
Emadi, H. 1993c. Nutrient requirements for cultured salmonids. Abzeeyan, Tehran, 4(5):2-6, II. In Farsi.
Emadi, H. 1994. Caviar. Abzeeyan, Tehran, 4(12):2-5. In Farsi.
Emadi, H. 1996a. The situation of catching and the reason of decreasing the sturgeon stocks. Abzeeyan, Tehran, 5:16-18. In Farsi.
Emadi, H. 1996b. No more Caspian caviar in future, if poaching continues over Caspian sturgeon. Abzeeyan, Tehran, 7(1):II-III.
Emadi, H. 1996c. The origin of mosquitofish in southern regions. Abzeeyan, Tehran, 7(5):2-3. In Farsi.
Emadi, H. and Amini, R. 2001. Classification index and determination of oocyte maturation in beluga, Huso huso, in the coast of Iran. 4th International Symposium on Sturgeon,
Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Emami, A., Bahmani, M. and Oryan, S. 2001. Determination of parameters in reproduction biology as indicators of artificial breeding in Persian sturgeon. 4th International Symposium on Sturgeon,
Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Emami, R. and Hosseini, S. J. F. 2004. Assessing the factors that affect the participation of fishery cooperatives in city of Sari in protecting the fish reserves in Caspian Sea. Journal of
Agricultural Sciences, 9(4):93-116. In Farsi.
Emami, S. H. 1974. Neur Lake winter survey I -1352. Progress Report, Department of Environmental Conservation, Tehran. MS, 5 pp. In Farsi.
Emtiaziou, M. and Sheykhi Moghadam, L. 2007. Using of Alvita for removing fouling fungus during incubation phases of rainbow trout (Oncorhyunchus mykiss) eggs. Journal of Science (Teacher
Training University), 7(1-2):835-846. In Farsi.
Encyclopaedia Brittanica. 1974. Encyclopaedia Brittanica. Chicago, 15th Edition. 30 volumes.
English, P. W. 1966. City and village in Iran: Settlement and economy in the Kirman Basin. The University of Wisconsin Press, Madison. xx + 204 pp.
English, P. W. 1968. The origin and spread of qanats in the Old World. Proceedings of the American Philosophical Society, 112(3):170-181.
English, P. W. 1998. Qanats and lifeworlds in Iranian Plateau villages. Yale School of Forestry & Environment Studies Bulletin Series, 103:187-205.
Epler, P., Bartel, R., Chyb, J. and Szczerbowski, J. A. 2001. Diet of selected fish species from the Iraqi lakes
Tharthar, Habbaniya and Razzazah. Archives of Polish Fisheries, 9(supplement 1):211-223.
Epler, P., Bartel, R., Szczerbowksi, J. A. and Szypuła, J. 2001. The ichthyofauna of lakes Habbaniya, Tharthar and Razzazah. Archives of Polish Fisheries, 9(supplement 1):171-184.
Epler, P., Sokołowska-Mikołajczyk, M., Popek, W., Bieniarz, K., Bartel, R. and Szczerbowski, J. A. 2001.
Reproductive biology of selected fish species from lakes Tharthar and Habbaniya in Iraq. Archives of Polish Fisheries, 9(supplement 1):199-209.
Epler, P., Sokołowska-Mikołajczyk, M., Popek, W., Bieniarz, K., Kime, D. E. and Bartel, R. 1996. Gonadal development
and spawning of Barbus sharpei (sic), Barbus luteus and Mugil hishni in fresh and saltwater lakes of Iraq. Archiwum Rybactwa Polskiego, 4(1):113-124.
Erençin, Z., Baran,İ. ve Ergüven, H. 1971.Doğu Anadoluda Bodur Yayın (Ameiurus nebolosus (sic)
Le Sueur, 1890) (sic). Ankara Üniversitesi Veteriner Fakültesi Dergisi, 18(2):214-218.
Erfani Majid, N., Salamat, N. and Hashemi Tabar, M. 2009. Evaluation of incubated carp ovarian follicles response (Cyprinus carpio) to carp pituitary extract (CPE) and cultivated pituitary
cells secretion (CPS) in vitro. Iranian Veterinary Journal, 5(3)(24):24-30. In Farsi.
Ergene, S. 1993. Karasu'da Yaşayan Chalcalburnus mossulensis (Heckel, 1843), (Pisces, Cyprinidae)'in Büyüme
Oranları[The growth rates of Chalcalburnus mossulensis (Heckel, 1843), (Pisces, Cyprinidae) in Karasu].Turkish Journal of Zoology, 17(4):367-377.
Ergens, R. und Gusev, A. V. 1965. Dactylogyrus prostae Molnár, 1964 (Monogenoidea) aus den Kiemen von Leuciscus cephalus (L.) und Leuciscus
cephalus orientalis Nordmann. Ceskoslovenská Parazitologie, 12:323-325.
ERM-Lahmeyer International GmbH, DHI Water & Environment and GOPA Consultants. 2001a. State and Challenges of the Marine and Coastal
Environment of the Caspian Sea (Transboundary Diagnostic Analysis). Caspian Environment Programme: Phase 2, European Tacis Programme. 162 pp., annexes A and B.
ERM-Lahmeyer International GmbH, DHI Water & Environment and GOPA Consultants. 2001b. Proposals for Establishment of a Caspian Fisheries Commission.
Caspian Environment Programme: Phase 2, European Tacis Programme. 56 pp.
Ermolchev, V. A., Ermolchev, M. V. and Besharat, K. 1997. Results of the Russian-Iranian hydroacoustic survey of kilka stocks in the Southern Caspian to
the south of the Astara-Gasan-Kuli line in 1995. PNIRO (Polar Research Institute of Marine Fisheries and Oceanography), Murmansk.
Eroğlu, M. and Şen, D. 2007. Reproduction biology of Mastacembelus simack (Walbaum, 1792) inhabiting Karakaya Dam Lake (Malatya, Turkey). International
Journal of Natural and Engineering Sciences, 1(2):69-73.
Eschmeyer, W. N. 1990. Catalog of the Genera of Recent Fishes. California Academy of Sciences, San Francisco. 697 pp.
Eschmeyer, W. N. (Ed.). 1998. Catalog of Fishes. California Academy of Sciences, San Francisco. 3 volumes, CD-ROM.
Eschmeyer, W. N., Ferraris, C. J., Hoang, M. D. and Long, D. J. 1996. A Catalog of the Species of Fishes. Preliminary version (Sept. 1996). http://www.calacademy.org/research/ichthyology/species.
Eskandari, Gh., Dehghan, S. and Nikpay, M. 2004. The reproduction biology of Barbus esocinus in Dez Dam reservoir. Iranian Journal
of Fisheries Sciences, 13(1):1-24. In Farsi.
Eskandari, Gh., Sabz Alizadeh, S. Dehgan Madiseh, S. and Mayahi, Y. 2007. Fish populations structures in the Dez Dam
Lake. Pajouhesh va Sazandegi, 19(1)(74):123-129. In Farsi.
Eskandari, Gh., Safeikhani, H., Dehghan, S. and Esmaeli, F. 2003. Fecundity and feeding of Barbus xanthopterus in Karkheh
River and Hoor-al-Azim Marsh. Iranian Scientific Fisheries Journal, 12(1):21-42, 2. In Farsi.
Eskandary, Gh., Safeikhani, H. and Ghofleh Maramazi, J. 1999. Ichthyofauna and some biological indices in Karoon, Dez and Bahmanshir rivers (southwest
of Iran). Iranian Journal of Fisheries Sciences, 8(3):23-36, 2-3. In Farsi.
Eskandari, Gh., Safikhani, H., Dehghan, S., Esmaeily, F. and Amiriney, A. S. 2000. Abundance, spawning season and type of gattan (Barbus xanthopterus
Heckel, 1843) in south of Karkheh River and Hour-al-Azim Marsh (Khouzestan Province). Iranian Scientific Fisheries Journal, 9(2):1-26. In Farsi.
Eslami, A. H. and Anwar, M. 1971. Occurrence and intensity of the infestation by Caryophyllaeus fimbriceps in carp and mullet (new host) in Iran. Rivista italiana di Piscicoltura e Ittiopatologia,
6(1):21-24.
Eslami, A. H., Anwar, M. and Khatiby, Sh. 1972. Incidence and intensity of helminthoses in pike (Esox lucius) of Caspian Sea (Northern Iran). Rivista italiana di Piscicoltura e Ittiopatologia, 7:11-14.
Eslami, A. and Kohneshahri, M. 1978. Study on helminthiasis of Rutilus frisii katum (sic) from the south Caspian Sea. Acta Zoologica et Pathologica Antverpiensia, 70:153-155.
Eslami, A. and Mokhayer, B. 1977. Nematode larvae of medical importance found in market fish in Iran. Pahlavi Medical Journal, 8:345-348.
Eslami Ali, M. B. 2005. Analyzing the prevalence of parasitic infection in salmon. Ph.D. Thesis, Veterinary Faculty, Islamic Azad University, Tabriz. In Farsi.
Esmaeili, A., Farshchi, P. and Darvishi, F. 2003. The impacts of Mnemiopsis leidyi's invasion in the Caspian Sea. Journal of Environmental Science and Technology, 2003(18):35-53. In Farsi.
Esmaeili, A. H., Kalbasi M. R. and Pousti, I. 2006. Induction of meiotic gynogenesis in rainbow trout (Oncorhynchus mykiss) with emphasis on time determination of second polar body extrusion.
Journal of Agricultural Sciences and Natural Resources, 13(4):120-127. In Farsi.
Esmaeili, F., Sharifpour, I. and Soltani, M. 2005. Isolation and identification of Flavobacterium columnaris-like organisms from grass
carp (Ctenopharyngodon idella) and assessment of its histo-pathological effects in Khouzestan Province, southern Iran. Iranian Journal of Fisheries Sciences, 14(2):1-12. In Farsi.
Esmaeli, F. and Abbasi, S. 1996. The effect of Bothriocephalus invasion on blood parameters of grass carp. Iranian Fisheries Scientific Journal, 5(3):19-26, 2. In Farsi.
Esmaili (sic), F. and Peighan, R. 1997. Isolation of Aeromonas-like bacteria from the grass carp (Ctenopharyngodon idella). Iranian
Fisheries Scientific Journal, 6(2):1-8, 1-2. In Farsi.
Esmaeili, H. R. 2006. Freshwater fish diversity in the Gulf drainage basins of Iran. The 1st International Conference on the State of the Gulf Ecosystem:
Future and Trends. 5-7 March 2006, Rotana Hotel, Al Ain, United Arab Emirates University (abstract).
Esmaeili, H. R., Baghbani, S., Zareian, H. and Shahryari, F. 2009. Scale morphology of tank goby Glossogobius giuris (Hamilton-Buchanan, 1822) (Perciformes:
Gobiidae) using scanning electron microscope. Journal of Biological Sciences, 9(8):899-903.
Esmaeili, H. R. and Coad, B. W. 2005. Range extension for Mystus pelusius (Solander in Russell, 1794) (Actinopterygii: Bagridae) in southern Iran. Zoology in the Middle East, 34:112-114.
Esmaeili, H. R., Coad, B. W., Gholamifard, A., Nazari, N. and Teimory, A. 2011. Annotated checklist of the freshwater fishes of Iran. Zoosystematica Rossica, 19(2)(2010):361-386.
Esmaeili, H. R. and Ebrahimi, M. 2006. Length-weight relationships of some freshwater fishes of Iran. Journal of Applied Ichthyology, 22:328-329.
Esmaeili, H. R., Ebrahimi, M., Ansari, T. H., Teimory, A. and Gholamhosseini, G. 2009. Karyotype analysis of Persian stone lapper, Garra persica Berg, 1913 (Actinopterygii: Cyprinidae) from Iran.
Current Science, 96(7):959-962.
Esmaeili, H. R., Ebrahimi, M. and Rahmati, M. 2002. Loaches of Iran. II International Conference, Loaches of the Genus Cobitis and Related Genera. Biology, Systematics, Genetics,
Distribution, Ecology, Conservation. September 09-13, 2002, Olsztyn, Poland (title).
Esmaeili, H. R., Ebrahmi, M., Piaravar, Z. and Teimory, A. 2006. First report on karyotype of spiny eel Mastacembelus mastacembelus (Banks and
Solander in Russel, 1794) from south of Iran. Journal of Science, Al-Zahra University, Tehran, 18(3):48-54. In Farsi.
Esmaeili, H. R., Ebrahimi, M. and Saifali, M. 2008. Karyological analysis of five tooth-carps (Actinopterygii: Cyprinodontiae) from Iran. Micron, 39:95-100.
Esmaeili, H. R., Ebrahimi, M., Teimory, A. and Ansary, T. H. 2008. First karyological analysis of an endemic fish, Isfahan tooth-carp, Aphanius isfahanensis (Actinopterygii: Cyprinodntidae)
from Iran. Journal of Applied Animal Research, 33:73-76.
Esmaeili, H. R., Ebrahimi, M., Teimori, (sic) A. and Ansari (sic), T. H. 2009. First karyological analysis of an endemic fish, Zagros tooth-carp, Aphanius vladykovi Coad,
1988 (Actinopterygii: Cyprinodntidae) from Iran. Iranian Journal of Science and Technology, Transaction A, 33(A4):349-354.
Esmaeili, H. R., Ganjali, Z. and Monsefi, M. 2009. Reproductive biology of the endemic Iranian cichlid, Iranocichla hormuzensis Coad, 1982 from Mehran River, southern Iran. Environmental Biology
of Fishes, 84(1):141-145.
Esmaeili, H. R., Ganjali, Z. and Monsefi, M. 2010. Gonad morphology and histology of the endemic Hormuz cichlid, Iranocichla hormuzensis Coad, 1982 from Mehran River, southern Iran.
IUFS Journal of Biology, 69(1):1-12.
Esmaeili, H. R. and Gholami, Z. 2007. Investigations on the surface ultrastructure of scale of Geno tooth-carp, Aphanius ginaonis (Holly, 1929) (Actinopterygii: Cyprinodontidae) using scanning
electron microscope. Iranian Journal of Biology, 20(2):307-314. In Farsi.
Esmaeili, H. R., Gholami, Z., Nazari, N., Gholamifard, A., Shahryari, F., Baghbani, S. and Ebrahimi, M. 2009. Karyotype analysis of an endemic sucker catfish, Glyptothorax silviae Coad, 1981
(Actinopterygii: Sisoridae), from Iran. Turkish Journal of Zoology, 33(4):409-412.
Esmaeili, H. R., Gholamifard, A. and Freyhof, J. 2011. Ichthyofauna of Zarivar Lake (Iran) with the first records of Hemiculter leucisculus and Alburnus hohenackeri in the Tigris drainage.
Electronic Journal of Ichthyology, 7(1):1-6.
Esmaeili, H. R., Gholamifard, A., Teimori, A., Baghbani, S. and Coad, B. W. 2010. Xiphophorus hellerii Heckel, 1848 (Cyprinodontiformes, Poeciliidae), a newly introduced fish recorded
from natural freshwaters of Iran. Journal of Applied Ichthyology, 26(6):937-938.
Esmaeili, H. R., Hagmoradlo, A. and Niksirat, H. 2003. Exotic fishes of Iran with special reference to Gasterosteus aculeatus. World Aquaculture
2003, 20-23 May, Salvador, Brazil (title).
Esmaeili H. R. and Niknejad, V. 2006. Preliminary study of southern spined loach Cobitis linea (Heckel, 1849) (Pisces; Cobitidae) in Fars province,
Southwest Iran. 3rd International Conference Loaches of the Genus Cobitis and Related Genera. Biology, Systematics, Genetics, Distribution, Ecology, Conservation.
Sibenik, Croatia, 24-29 September 2006 (title).
Esmaeili H. R. and Niknejad, V. 2006-2007. Scale morphological studies of 4 loaches (Actinopterygii: Cypriniformes) in Fars Province. Journal of
Science, Al-Zahra University, 19(2):1-10, 67. In Farsi.
Esmaeili, H. R. and Piravar, Z. 2006a. Karyotype of Persian chub, Petroleuciscus persidis (Coad, 1981) (Actinopterygii: Cyprinidae) from southern Iran. Turkish Journal of Zoology, 30:1-3.
Esmaeili, H. R. and Piravar, Z. 2006b. On the karyotype of Cyprinion tenuiradius Heckel, 1849 (Pisces: Cyprinidae) from the southwest of Iran. Zoology in the Middle East, 39:75-80.
Esmaeili, H. R. and Piravar, Z. 2007. Karyotype analysis of Garra rufa (Heckel, 1843) (Actionopterygii: Cyprinidae) in Fars Province. Iranian Scientific Fisheries Journal, 16(3):11-18. In Farsi.
Esmaeili, H. R., Piravar, Z. and Ebrahimi, M. 2006. Karyological analysis of Iranian cichlid fish, Iranocichla hormuzensis Coad, 1982 (Perciformes, Cichlidae)
from southern Iran. Journal of Applied Animal Research, 30:77-79.
Esmaeili, H. R., Piravar, Z. and Shiva, A. H. 2007. Karyological analysis of two endemic tooth-carps, Aphanius persicus and Aphanius sophiae (Pisces:
Cyprinodontidae) from southwest Iran. Turkish Journal of Zoology, 31:69-74.
Esmaeili, H. R. and Shiva, A. H. 2006. Reproductive biology of the Persian tooth-carp, Aphanius persicus (Jenkins, 1910)(Cyprinodontidae), in southern Iran. Zoology in the Middle East, 37:39-46.
Esmaeili, H. R. and Teimori, A. 2009. Exotic and introduced fish species of Iran and their impact on native fishes. 13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda,
Lithuania (abstract).
Esmaeili, H. R., Teimory, A. and Coad, B. W. 2008. Qanat systems as fish refuge. International Congress on Documenting, Analysing and Managing Biodiversity in the Middle East 20-23 October 2008,
Intercontinental Hotel, Aqaba, Jordan (title).
Esmaeili, H. R., Teimory, A., Coad, B. W. and Gholami, Z. 2008. Threatened fishes of the world: Cobitis linea (Heckel, 1849). Environmental Biology of Fishes, 83(4):407-408.
Esmaeili, H. R., Teimory, A., Coad, B. W. and Gholami, Z. 2009. Threatened fishes of the world: Seminemacheilus tongiorgii Nalbant and Bianco, 1998 (Balitoridae). Environmental Biology of
Fishes, 84(4):375.
Esmaeili, H. R., Teimory, A., Gholami, Z. and Hosseinie, F. 2006. Range extension of Barbus sublimus Coad and Najafpour, 1997 (Actinopterygii:
Cyprinidae) and its sympatric species in southwest of Iran. Iranian Journal of Animal Biosystematics, 2(1):19-24.
Esmaeili, H. R., Teimory, A. and Khosravi, A. R. 2007. A note on biodiversity of Ghadamghah spring-stream system in Fars province, southwest Iran. Iranian Journal of Animal Biosystematics, 3(1):15-23.
Esmaeili, H. R., Teimory, A. and Piravar, Z. 2009. Otolith morphology of some freshwater fishes of Iran. Iranian Scientific Fisheries Journal, 17(4):163-168. In Farsi.
Esmaeili, H. R. and Timoorei, A. 2006. Morphology of urohyal bone and its importance in taxonomy of some freshwater fishes of Iran. Iranian Scientific Fisheries Journal, 15(3):1-8. In Farsi.
Esmaeili, H. R., Yazdanpanah, M. and Monsefi, M. 2005. Reproductive biology of doctor fish, Garra rufa (Cyprinidae: Garrinae), in southwest of Iran. Journal of Fish Biology, 67(Supplement B):282.
Esmaeili, H. R., Zareian, H., Gholamhosseini, A., Ebrahimi, M., Gholami, Z.
Teimori, A. and Ansari, T. A. 2010. Karyotype analysis of the king nase fish, Chondrostoma regium (Heckel, 1843) (Actinopterygii: Cyprinidae) from Iran. Turkish Journal of Fisheries and
Aquatic Sciences, 10(4):477-481.
Esmaeilzadeh, K. R., Sahari, M. A. and Hamidi, E. Z. 2004. Comparative study on nutrient compositions of kutum (Rutilus frisii kutum) and grass carp (Ctenopharyngodon idella) and their
marinade qualities. Iranian Journal of Fisheries Sciences, 12(4):13-28. In Farsi.
Esmaili, A., Ryahi, A. and Savari, A. 1999. Determination of heavy metals (Cd, Co, Pb, Ni, Zn and Cu) in water, sediments and fish from the Karun
River. Iranian Journal of Natural Resources, 52(2):37-46. In Farsi.
Esmaili Sari, A. 2001. Acute toxicity of butachlor and diazinon in fingerlings of Acipenser stellatus and Acipenser persicus. 4th International Symposium on Sturgeon, 2001, Oshkosh, Wisconsin (title).
Esmaili Sari A., Abtahi, B., Seyfabadi, J. and Khodabandeh, S. 2001. Invasive comb-jelly and future of the Caspian Sea. Naghshe Mehr Publication, Tehran, p. 144. In Farsi.
Esmaili Sari A., Abtahi, B., Seifabadi, J. Khodabandeh, S. Ershad, H. 2001. A study on the density and distribution of comb-jelly, Mnemiopsis leidyi, in the Caspian Sea.
Daneshvar, 31:145-148. In Farsi.
Esmaili Sari A., Abtahi, B., Talaei, R and Sifabadi, J. 2002. Recherche sur la distribution et la densite du cnidaire Mnemiopsis leidyi, le longe de cote meridionale de la Mer
Caspienne. 126eme Congres la Societe Zoologique de France du 16 au 18 septembre 2002, Plouzane-Brest, France (ttle).
Esmaili Sari A., Abtahi, B., Seifabadi J., Khodabandeh, S. and Ershad, H. 2001. Occurrence of ctenophores in the Caspian Sea and its environmental consequences. In: The Fifth Iranian Symposium on
Marine and Atmospheric Science and Technology, Bandar Abbas.
Esmaili Sari, A., Farshchi, P and Darvishi, F. 2002. Feeding competition between invasive comb-jelly (M. leidyi) and anchovy kilka (C.
engrauliformis) in south coast of the Caspian Sea. Iranian Journal of Marine Sciences, 1(4):25-42, 143. In Farsi.
Esmaili Sari, A., Imandel, K. A. and Georgez, Sh. 2001. The valuation of the damages resulting from oil material on Shadegan Wetland environment. Journal of Environmental Science and Technology,
2001(9):21-30. In Farsi.
Esmaili Sari, A., Khodabandeh, S., Abtahi, B., Seyfabadi, J. and Ershad, H. 1999. The first report on the occurrence of a comb jelly in the Caspian
Sea. Journal of Environmental Science and Technology, 1999(3):63-68. In Farsi.
Esmaili Sari A., Seifabadi, J., Khodabandeh, S., Abtahi, B. 2001. Feeding regime of comb-jelly, Mnemiopsis leidyi, from the Caspian Sea. Daneshvar, 31:139-144. In Farsi.
Esmaili Sari A., Saifabadi J, Khodabandeh S., Abtahi B. and Talayi R. 2001. Feeding regime of comb-jelly (Mnemiopsis leidyi) from Caspian Sea. Daneshvar Medicine, 8(31):139-144. In Farsi.
Esmailzadeh, K. R., Sahari M. A. and Hamidi, E. Z. 2004. Comparative study on nutrient compositions of kutum (Rutilus frisii kutum) and grass carp (Ctenopharyngodon idella)
and their marinade qualities. Iranian Scientific Fisheries Journal, 12:13-28, 2-3. In Farsi.
Esmaily, A. H. and Kalbassi, M. R. 2008. Karyological study on bighead goby (Neogobius kessleri) from southern part of the Caspian Sea. Iranian Journal of Fisheries Sciences, 7(2):15-26.
Esteky, A. A. 2001. Conditions of pH and ionic composition of water in a macrophyte dominated reservoir (Hanna Reservoir - Isfahan Province), Iran. Iranian Journal of Fisheries Sciences,
3(1):23-38, 90.
Esteky, A. A. 2006a. Determination of best condition for fermentation of organic manure for utilization in aquaculture. Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Esfahan. http://en.ifro.ir (abstract).
Esteky, A. A. 2006b. Hydrological and hydrobiological survey for determination of fish production potential in eastern
area of Esfahan. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Esfahan. http://en.ifro.ir (abstract).
Etemadfar, A. R., Motabar, M. and Wossughi, Gh. 1983. A biological and ecological survey on the geographical distribution of Aphanius dispar as
larvivorous fish ar (sic) the southern parts of Iran. Journal of the Veterinary Faculty, University of Tehran, 38(2-4):1-12. In Farsi.
Etessami, S. 1982. L'histologie des gonades chez deux cyprinides Alburnoides bipunctatus eichwaldi (Filipi, 1863) (sic) et Barbus mursa
miliaris (Karaman, 1972) (sic) avec la description d'un cas d'hermaphrodisme chez ce dernier. Cybium, 6(2):5-13.
Ettrich, G. 1989. Breeding the green snakehead - it's a mouthbrooder. Tropical Fish Hobbyist, 37(10):34-36.
Ettrich, G. und Schmidt, J. 1989. Channa gachua aus Südostasien und Channa orientalis von Sri Lanka - zwei gute Arten. Die Aquarien und Terrarien-Zeitschrift, 42(8):465-467.
Everard, M. 2006. The Complete Book of the Roach. Medlar Press, Ellesmere. 436 pp.
F
Faal, Z. 2009. Investigation of physicochemical factors of water in Bahmanshir River, Khouzestan Province, Iran. Iranian Scientific Fisheries Journal, 18(1):167-172. In Farsi.
Faber, J. E. and Stepien, C. A. 1998. Tandemly repeated sequences in the mitochondrial DNA control region and phylogeography of the pike-perches
Stizostedion. Molecular Phylogenetics and Evolution, 10(3):310-322.
Faber, R. 1994. Moscow symposium studies sturgeon. Fish Farmer, 8(1):20-22.
Fabricant, F. 2000. The dearest eggs since Faberge, Iranian caviar returns. New York Times, 4 October 2000, Section F, p. 3.
Fadaee, B., Pourkazemi, M., Tavakoli, M., Joushideh, H., Khoshghalb, M. R. B., Hosseini, M. R. and Abdulhay, H. 2006. Tagging and tracking juvenile sturgeons in shallow waters of the Caspian Sea (less
than 10 m depth) using CWT (Coded Wire Tags) and barbel incision. Journal of Applied Ichthyology, 22(s1):160-165.
Fadaei Fard, F., Mokhayer, B. and Ghorbani, H. 2001. Study of fish parasites in the lagoon of Choghakhor, Chaharmahal-va-Bakhtiari, Iran. Journal of the Faculty of Veterinary Medicine, University of
Tehran, 56(3):109-114. In Farsi.
Fadayee, B. 1997. Study of fingerling release in Sefidrud River to reach the sea in in Iran. Iranian Fisheries Research Organization, Tehran. 235 pp. In Farsi.
Fadayee, B. 2002. The survey of probability role of beach seine net on sturgeon resources. International Sturgeon Research Institute, Rasht. 25 pp. In Farsi.
Fadayee, B., Pourkazemi, M., Nezami, Sh., Bahmani, M., Nowei, M. R., Parandavar, H., Imanpour, J. and Jooshedeh, H. 1999. Study on probable
natural reproduction in sturgeon of the south Caspian Sea in the Sefidrud River. Iranian Journal of Fisheries Sciences, 8(2):69-82, 6-7. In Farsi.
Faeed, M., Mozafari, N. A. and Shojaee Arany, A. 2006. Isolation and identification of bacteria and fungi spoilage in kilka meal production in Guilan
Province. Iranian Journal of Fisheries Sciences, 14(4):127-138. In Farsi.
Faghani, T., Kousha, A., Azari Takami, G. H. and Faghani, S. 2008. Study on growth performance, survival rate, hematological parameters in rainbow trout (Oncorhynchus mykiss) in Mazandaran
Province of Iran. Journal of Fisheries and Aquatic Science, 3(6):398-403.
Falahati Marvast, A., Mojazi Amiri, B., Abtahi, B. and Alizadeh, M. 2003. Comparative study on ovarian development of rinbow (sic) trout,
Oncorhynchus mikiss (sic), reared in fresh and brackish water. Iranian Journal of Marine Sciences, 2(1):47-58. In Farsi.
Falahati Marvast, A., Mojazi Amiri, B., Alizadeh, M. and Abtahi, B. 2006. Comparative study on gonad development in rainbow trout (Oncorhynchus mykiss) reared in fresh
and brackish water in the Yazd Province. Iranian Journal of Fisheries Sciences, 14(4):113-126. In Farsi.
Falahatkar, B. 1999. Determination of palatable aquatic plants in nutrition of grass carp, Ctenopharyngodon idella. Agriculture and Industry, 5:28-29. In Farsi.
Falahatkar, B. 2005. The effect of dietary vitamin C on some hematologic biochemistry and growth indexes of great sturgeon (Huso huso). Ph.D. Thesis, Tarbiat Modarres University,
Tehran. In Farsi.
Falahatkar, B. 2006. Biological characteristics of the Persian sturgeon, Acipenser persicus Borodin, 1897 in the southern Caspian Sea. Zoology in the Middle East, 38:113-114.
Falahatkar, B. 2007. Ascorbic acid biosynthesis by 3 species of Acipenseriformes and its role on quantitative growth parameters. Iranian Journal of Biology, 20(1):128-137.
Falahatkar, B. and Amini, K. 2003. Determination of artificial propagation normatives in Acipenser gueldenstaedti. Iranian Journal of Fisheries Sciences, 12(1):77-92, 6. In Farsi.
Falahatkar, B., Pourkazemi, M. and Soltani, M. 2001. A study on relationship between haematological factors and broodstock quality in Russian sturgeon (Acipenser
gueldenstaedtii). 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Falahatkar, B., Pourkazemi, M. and Soltani, M. 2002. A study on relationship between haematological factors and broodstock
quality in Russian sturgeon, Acipenser gueldenstaedti. Pajouhesh-va-Sazandegi,
4(53):26-31. In Farsi.
Falahatkar, B., Pourkazemi, M., Soltani, M. and Amini, K. 2002b Selection of Russian sturgeon, Acipenser gueldenstaedti,
broodstocks for artificial propagation based on blood parameters. Second National and Regional Symposium on Sturgeon, Iran, 26-28 October 2002, p. 131-134.
Falahatkar, B., Soltani, M., Abtahi, B., Kalbassi, M. R., Pourkazemi, M. R.
and Yasemi, M. 2006. Effects of vitamin C on some growth parameters, survival and hepatosomatic
index in juvenile cultured beluga, Huso huso. Pajouhesh va Sazandegi,
19(3)(72):98-103. In Farsi.
Falahatkar, B., Soltani, M., Alishahi, M. and Zargar, A. 2009. Effects of different levels of ascorbic acid on some variables of juvenile great sturgeon (Huso huso) immunity indices. Journal of
Veterinary Research, 63(5):337-343. In Farsi.
Falahatkar, B. and Yasemi, M. 2001. Stocks condition Aspius aspius taeniatus in the south coastline of the Caspian sea. Veterinarian 2(14):44-47.
Falahatkar B. and Yasemi, M. 2003. An overview on length, weight, age and sex characteristics of Persian sturgeon, Acipenser persicus, to assess the broodstocks for artificial propagation in
the south of the Caspian Sea.AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Falcon, N. L. 1974. Southern Iran: Zagros Mountains, pp. 199-211. In: Spencer, A. M. (Ed.). Mesozoic-Cenozoic Orogenic Belts. Data for Orogenic
Studies. Special Publication, Geological Society, London, 4:xvi + 809 pp.
Fallahi, M. 1993. Plankton survey in the southern part of the Caspian Sea. Iranian Fisheries Bulletin, (4):19-38, 3. In Farsi.
Fallahi, M. 1998. Distribution and biomass of the Cladocera in the western basin of the Anzali Lagoon (Abkenar). Iranian Fisheries Scientific Journal, 6(4):59-74, 6. In Farsi.
Fallahi, M., Daghigh Roohi, J., Nahrevar, M. R., Moradi Chafi, M and Sarpanah, A. 2004. The role of rotifer (Brachionus plicatilis) in increasing
survival of Rutilus frisii kutum larvae and its comparison by concentrated food. Pajouhesh va Sazandegi,
17(2)(63):66-71. In Farsi.
Fallahi, R., Soltani, M., Zorriehzahra, M. E. J. and Hemmatzadeh, E. 2006. Serological diagnosis of infectious haematopoietic necrosis disease (IHN) in rainbow trout (Oncorhynchus mykiss) using
indirect fluorescence antibody test. Journal of the Faculty of Veterinary Medicine, University of Tehran, 61(1):19-22. In Farsi.
Fallahi, R., Soltani, M., Kargar, R., Zorriehzahra, M. E. J., Shchelkunov, I., Hemmatzadeh, F. and Nouri, A. 2003. Isolation and identification of the infectious haematopoietic necrosis virus
(IHNV)-like agent from farmed rainbow trout (Oncorhynchus mykiss) in Iran. Archives of the Razi Institute 56:37-45.
Fang, P.-W. 1942. Sur certains types peu connus de Cyprinidés des collections du Muséum de Paris. Bulletin du Muséum national d'Histoire naturelle, Paris, (2)14:169-172.
Fani, S. K. A. 2006a. Determination of digestible and metabolizable energy from protein feedstuffs (fishmeal and
soybean meal) on rainbow trout. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Esfahan. http://en.ifro.ir (abstract).
Fani, S. K. A. 2006b. Use of oil by-product in rainbow trout diet. Iranian Fisheries Research Organization,
Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Esfahan. http://en.ifro.ir (abstract).
Farabi, S. M. V., Hajimoradloo, A. and Bahmani, M. 2007. Study on salinity tolerance and some physiological indicators of ion-osmoregulatory system in juvenile beluga, Huso huso (Linnaeus,
1758) in the south Caspian Sea: Effect of age and size. Iranian Journal of Fisheries Sciences, 6(2):15-32.
Farabi, S. M. V., Khoshbavar Rostami, H., Ghaneei Tehrani, M., Ghiasi, M., Azari, A., Behrouzi, S., Mosavi, H., Firozkandian, S. and Habibi, F. 2007. The
investigation of status brood stocks and releasing fingerlings of Rutilus frisii kutum (Kaminski, 1901) in the south of Caspian sea (Mazandaran
province, 2004). Pajouhesh va Sazandegi, 74:156-166. In Farsi.
Farabai, S. M. V., Taheri, S. A. and Nadri, H. 2007. The comparison efficiency incubator of sturgeon fish eggs: Yushchenko Incubator of Russian model with Azarakhsh Incubator of Iranian model and
emphasis on Acipenser persicus (Borodin, 1897) (sic). Pajouhesh va Sazandegi, 19(1)(74):9-18. In Farsi.
Farabi, S. M. V., Youefian, M. and Hajimoradloo, A. 2006. Aflatoxicosis in juvenile Huso huso fed a contaminated diet. Journal of Applied Ichthyology, 22(s1):234-237.
Farahnak, A. 2000a. Fish Anisakidae in Khuzestan province of South West Iran. In: Ecological Parasitology on the Turn of the Millennium, St. Petersburg, Russia, 1-7 July 2000 (abstract).
Farahnak, A. 2000b. Fish Anisakidae in Khuzestan province of South West Iran. Bulletin of the Scandinavian Society for Parasitology, 10(2):71.
Farahnak, A., Mobedi, I. and Tabibi, R. 2002. Fish Anisakidae helminthes in Khuzestan Province, south west of Iran. Iranian Journal of Public Health, 31(3-4);129-132.
Fareed, K. H. and Baban, M. R. 1995. Aphanius dispar, a larvivorous fish as biocontrol of malarial mosquito in Al-Hudaidah Province, Yemen. Asia Life Sciences, 4(2):147-150.
Farhadian, O. 1999. Investigations into the methods of fertilization and determination of degree-days for the egg incubation in rainbow trout in Chaharmahal and Bahktiari, Iran. M.Sc. Thesis,
Tarbiat Modares University, Tehran.
Farhangi, M., Kamali, A. and Hajimoradloo, A. 2003. Study of natural zeolites efficiency for reduction of ammonia toxicity in rainbow trout (Oncorhynchus
mykiss). Journal of Agricultural Sciences and Natural Resources, 10(2):195-208. In Farsi.
Farid-Pak, F. 1957. Fishes of the Caspian Sea on the north borders of Iran. Journal of Veterinary Medicine, Tehran, 4:12-24. In Farsi.
Farid-Pak, F. 1963. Société Anonyme des Pêcheries Iraniens. Keyhan Press, Tehran.
Farid-Pak, F. 1965. Caspian Sea fishes. Fisheries Research Institute, Bandar Pahlavi, Iran. In Farsi.
Farid-Pak, F. 1966. Fishes of the Caspian Sea area and the northern shores of Iran. Leaflet No. 6, Shilot Industrial Fish Study Institute of Iran, Bandar Pahlavi. In Farsi.
Ferid-Pak (Farid-Pak), F. 1968a. Fertility of the kutum Rutilus frisi kutum (Kamensky). Problems of Ichthyology, 8(1):61-68.
Farid Pak, (F.). 1968b. Fertility of the salmon [Salmo trutta caspius Kessl.] from the Iranian coast of the Caspian. Problems of Ichthyology, 8(2):215-222.
Farid-Pak, F. 1991. How and when trout entered the Caspian, Black and Aral seas. Abzeeyan, Tehran, 7:22-23. In Farsi.
Farid-Pak, F. No date. Société anonyme des Pècheries (sic) iraniennes. Bank Melli Iran Press, Tehran. 10 pp.
Farooq, G., Mirza, M. R. and Masud-ul-Hasan. 1996. A contribution to the gut contents of Cyprinion watsoni (Day) (Pisces: Cyprinidae)
from Pakistan. Second Symposium on Fish and Fisheries of Pakistan, November, 25, 26, 1996, Department of Zoology, Government College, Lahore, p. 1-2 (abstract).
Farquharson, F. A. K., Meigh, J. R. and Sutcliffe, J. V. 1992. Regional flood frequency analysis in arid and semi-arid areas. Journal of Hydrology, 138(3-4):487-501.
Farr, M. J. 1977. Karue ru insurgence, Zagros Mts., Iran. Cave Diving Group Newsletter, U.K., 44:18-19.
Farr, M. J. 1984. The great caving adventure. Oxford Illustrated Press, Yeovil. 229 pp.
Farrand, W. R. 1979. Blank on the Pleistocene map. Geographical Magazine, 51(8):548-554.
Fatemi, M., Kaymaram, F., Parafkandeh Haghighi, F., Vosoughi, G. H. and Taghavi Motlagh, S. A. 2009. Validation of back-calculation methods using otoliths to determine the length of anchovy
kilka (Clupeonella engrauliformis). Iranian Journal of Fisheries Sciences, 8(2):115-126.
Fatemi, S. M., Kaymaram, F., Jamili, S., Taghavi Motlagh, S. A,. and Ghasemi, S. 2009. Estimation of growth parameters and mortality rate of common carp (Cyprinus carpio, Linnaeus 1758)
population in the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 8(2):127-140.
Fathiazad, F., Afshar, J. and Maham, M. 2002. Study and comparison of anesthetic effect of clove oil and tricaine in Cyprinus carpio,
Hypophthalmichthys molitrix and Ctenopharyngodon idella. Pharmaceutical Sciences, 1:61-68.
Fayazi, J., Moradi, M., Rahimi, G., Ashtyani, R. and Galledari, H. 2006. Genetic differentiation and phylogenetic
relationships among Barbus xanthopterus (Cyprinidae) populations in southwest of Iran using mitochondrial DNA markers. Pakistan Journal of Biological Sciences, 9(12):2249-2254.
Fazaeili, H., Mehrabi, Y. and Mahmoudzadeh, H. 2009. Comparing the pigment sources on the tissue pigmentation of male rainbow trout. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009,
Basrah, p. 70 (abstract).
Fazeli, M. S., Abtahi, B. and Sabbagh Kashani, A. 2005. Assessing Pb, Ni and Zn accumulation in the tissues of Liza
aurata in the south Caspian Sea. Iranian Journal of Fisheries Sciences, 14(1):65-78. In Farsi.
Fazlei, H. ?. Study on quantity and quality of released sturgeon larvae into the Mazandaran and Golestan provinces rivers in 1999. Virtual, 1(1):91-102. In Farsi.
Fazli, H. 1990. Biology of genus Clupeonella in the Caspian Sea. National Conference on Fisheries Resources
Management of the Caspian Sea, Babolsar. Iran Fisheries Organization, Sari. p. 81-97. In Farsi.
Fazli, H. 1998. Some biological characteristics of Liza aurata in the southern coasts of the Caspian Sea. Iranian Journal of Fisheries Sciences, 7(3):41-56, 5. In Farsi.
Fazli, H. 2000. Study on some biological characteristics of Liza saliens in southern part of the Caspian Sea. Iranian Journal of Fisheries Sciences, 8(4):29-42, 3-4. In Farsi.
Fazli, H. 2006a. Monitoring (biology and catch) of kilka in Iranian commercial catch in 2001-2002. Iranian Fisheries
Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Sari. http://en.ifro.ir (abstract).
Fazli, H. 2006b. The biological characteristics of Clupeonella cultriventris caspia in southern coast and the impacts of Mnemiopsis leidyi invasion on the Caspian
ecosystem. Pajouhesh va Sazandegi, 69:87-96. In Farsi.
Fazli, H. 2007. Population dynamics and stock assessment of kilka (genus Clupeonella) in the Iranian waters of the Caspian Sea. Ph.D. Thesis, Pukyong National University, Busan, South Korea.
145 pp.
Fazli, H. ?. The study of catch and CPUE of kilkafishes in Mazandaran Province (1996-2000). Virtual, 1(1). In Farsi.
Fazli, H. and Besharat, K. 1998. Kilka stock assessment using hydro-acoustic method and commercial catch monitoring, 1976-1977. Final report, Mazandaran Fisheries Research Center, Sari. 105 pp. In Farsi.
Fazli, H., Borani, M. S. and Janbaz, A. A. 2005. Assessing the biological characteristics of Clupeonella grimi in Iranian commercial catch during 1997-2001 in the Caspian Sea.
Iranian Journal of Fisheries Sciences, 13(4):125-138. In Farsi.
Fazli, H., Borani, M. S. and Janbaz, A. A. 2006. The biological characteristics of Clupeonella cultriventris caspia in southern coast and
the impacts of Mnemiopsis leidyi invasion on the Caspian ecosystem. Pajouhesh va Sazandegi,
18(69):87-96. In Farsi.
Fazli, H. and Ghaninejad, D. 2004. Some biological aspects and catch trend of mullets in the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 13(1):97-114. In Farsi.
Fazli, H., Janbaz, A. A., Taleshian, H. and Bagherzadeh, F. 2008. Maturity and fecundity of golden grey mullet (Liza aurata Risso, 1810) in Iranian waters of the Caspian Sea. Journal of Applied
Ichthyology, 24(5):610-613.
Fazli, H. and Roohi, A. 2002. The impacts of Mnemiopsis leidyi on kilka resources in the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 11(1):63-72. In Farsi.
Fazli, H., Zhang, C. I., Hay, D. E. and Lee, C. W. 2009a. Multi-species approach for stock management of kilka fish (genus: Clupeonella) in Iranian waters. Iranian Journal of Fisheries
Sciences, 8(2):141-154.
Fazli, H., Zhang, C. I., Hay, D. E. and Lee, C. W. 2009b. Fishery biological characteristics and changes in the annual biomass of bigeye kilka (Clupeonella grimmi) in the Caspian Sea. Asian
Fisheries Science, 22(3):943-960.
Fazli, H., Zhang, C. I., Hay, D. E., Lee, C. W., Janbaz, A. A. and Bourani, M. S. 2007. Population dynamics and stock assessment of common kilka (Clupeonella cultriventris caspia) in
Iranian waters of the Caspian Sea. Iranian Journal of Fisheries Sciences, 7(1):47-70.
Fazli, H., Zhang, C-I., Hay, D. E., Lee, C-W., Janbaz, A-A. and Sayad Borani, M. 2007. Population ecological parameters and biomass of anchovy kilka
Clupeonella engrauliformis in the Caspian Sea. Fisheries Science, 73(2):285-294.
Federal Register (United Sattes). 2005. Endangered and Threatened Wildlife and Plants: Notice of suspension of trade in threatened beluga sturgeon (Huso
huso) from the Black Sea basin. Federal Register, 70(208):62135-62137.
Ferdouse, F. 1997. Iranian sturgeon: A fish fit for kings. INFOFISH International, Kuala Lumpur, 5:14-16.
Ferguson, J. 1994. Caviar. Rotunda, Toronto, 27(3):5-7.
Ferraris, C. J., McGrouther, M. A. and Parkinson, K. L. 2000. A critical review of the types and putative types of southern Asian marine and
freshwater fish species in the Australian Museum named by Francis Day. Records of the Australian Museum, 52(3):289-306.
Ferrier, R. W. 1996. A Journey to Persia. Jean Chardin's Portrait of a Seventeenth-century Empire. I. B. Tauris Publishers, London. xiv + 193 pp.
Feulner, G. R. 1998. Wadi fish of the UAE. Tribulus, 8(2):16-22.
Feylessoufi, E. 1959. Eaux souterraines, kanats et puits profonds en Iran. University Press, Tehran. 13 pp., 3 figs.
Feyzbakhsh, H., Nazari, H., Ghorbani, R., Hosseini, A. and Taheri, A. 2006. Possibility of spawning of Persian sturgeon (Acipenser persicus)
broodstocks by oviduct incision (microcesarean) and comparison of usual method. Journal of Agricultural Sciences and Natural Resources, 13(5):183-190. In Farsi.
Fiedler, P. L. and Jain, S. K. (Eds.). 1992. Conservation Biology. The Theory and Practice of Nature Conservation and Management. Chapman and Hall, New York and London. xxix + 507 pp.
Filippov, G. M. 1976. Some data on the biology of the beluga Huso huso from the south-eastern part of the Caspian Sea. Journal of Ichthyology, 16(4):566-574.
Filizadeh, Y. 2002. An ecological investigation into the excessive growth of Azolla in the Anzali Wetland and its control. Iranian Journal of Natural Resources, 55(1):65-80. In Farsi.
Filizadeh, Y. and Khodaparast, S. H. 2005. Investigating effects of the excessive growth of aquatic plants on water quality in Anzali Lagoon,
south-western Caspian Sea. Iranian Journal of Fisheries Sciences, 13(4):139-150. In Farsi.
Firouz, E. 1968a. The sport fisheries of Iran. Paper presented at a Fishery Seminar, Tehran, 4-13 June 1968. MS. (not seen).
Firouz, E. 1968b. The mordab of Pahlavi. Proceedings of a Technical Meeting on Wetland Conservation, Ankara-Bursa-Istanbul, 9 to 16 October
1967, International Union for Conservation of Nature and Natural Resources Publication, new series, 12:239-241.
Firouz, E. 1974. Environment Iran. The National Society for the Conservation of Natural Resources and Human Environment, Tehran. v + 55 pp.
Firouz, E. 1976. Environmental and nature conservation in Iran. Environmental Conservation, 3(1):33-42.
Firouz, E. 2000. A Guide to the Fauna of Iran. Iran University Press, Tehran. vi + 491 pp. In Farsi.
Firouz, E. 2005. The Complete Fauna of Iran. I. B. Tauris, London. xiv + 322 pp.
Firouz, E. and Harrington, F. A. 1976. Iran: Concepts of biotic community reservation. In: Proceedings of an International Meeting on ecological
guidelines for the use of natural resources in the Middle East and South West Asia held at Persepolis, Iran 24-30 May 1975. International Union
for the Conservation of Nature and Natural Resources Publications, new series, 34:147-169.
Firouz, E., Hassinger, J. D. and Ferguson, D. A. 1970. The Wildlife Parks and Protected Regions of Iran. Biological Conservation, 3(1):37-45.
Firouzbakhsh, F., Ebrahimzadeh Mousavi, H. A. and Khosravi, A. R. 2005. Isolation and identification of pathogenic and saprophytic fungi from gill lesions in cultivated cyprinids (common carp, silver
carp and grass carp). Journal of the Faculty of Veterinary Medicine, University of Tehran, 60(1):15-19. In Farsi.
Firouzbakhsh, F., Kazemi, R. A., Kazemi, M., Khosravi, A. R., Jalaipour, J. and Ebrahimzadeh Mousavi H. A. 2010. Identification of flora in cultivated and natural Caspian Sea Acipenser persicus.
Journal of Veterinary Research, 64(4):291-295.
In Farsi.
Fischer, W. and Bianchi, G. (Eds.). 1984. FAO Species Identification Sheets for Fishery Purposes. Western Indian Ocean (Fishing Area 51). Food and Agriculture Organization, Rome. 6 volumes.
Fishelson, L., Goren, M., van Vuren, J. and Manelis, R. 1996. Some aspects of the reproductive biology of Barbus spp., Capoeta damascina
and their hybrids (Cyprinidae, Teleostei) in Israel. Hydrobiologia, 317(1):79-88.
Fisher, B. 1928. Irrigation systems of Persia. Geographical Review, 1928:302-306.
Fisher, W. B. (Ed.). 1968. The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press, Cambridge. xix + 784 pp.
Fitt, R. L. 1953. Irrigation development in central Persia. Journal of the Royal Central Asian Society, 40(2):124-133.
Fletcher, M., Teklehaimanot, A. and Yemane, G. 1992. Control of mosquito larvae in the port city of Assab by an indigenous larvivorous fish, Aphanius dispar. Acta Tropica, 52(2-3):155-166.
Floor, W. 2003. Agriculture in Qajar Iran. Mage, Washington. 692 pp.
Floor, W. 2007. Travels through northern Persia 1770-1774 by Samuel Gottlieb Gmelin. Translated and Annotated by Willem Floor. Mage Publishers, Washington, D.C. xxvii + 381 pp.
Foghi, M. 2004. The impact of drought on agriculture and fisheries in Iran, p. 79-85. In: Petr, T. (Ed.).
Fisheries in irrigation systems of arid Asia. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 430:ix + 150 pp.
Foltz, R. C. 2001. Environmental initiatives in contemporary Iran. Central Asian Survey, 20(2):155-165.
Folyz, R. C. 2002. Iran's water crisis: cultural, political, and ethical dimensions. Journal of Agriculture and Environmental Ethics, 15(4):357-380.
Food and Agriculture Organization. 1996. National Training Course on Aquaculture Nutrition and Feed Technology held in the International Sturgeon Institute, Rasht, Gilan, the Islamic Republic of Iran,
1-10 September 1996, Food and Agriculture Organization, Rome, Reg. Off. for the Near East, Cairo. 30 pp.
Food and Agriculture Organization. 1992. The Islamic Republic of Iran: Report of the aquaculture fact finding mission. Food and Agriculture Organization, Rome, Field Document, TCP/IRA/2251(F).
Food and Agriculture Organization, Fisheries Department. 1996. Fisheries and aquaculture in the Near East and North Africa: situation and outlook
in 1996. Food and Agriculture Organization, Rome, Fisheries Circular, 919:37 pp.
Food and Agriculture Organization, Fisheries Department. 2006. State of world aquaculture 2006. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 500:134 pp.
Food and Agriculture Organization, Fisheries Department, Rome, Italy and Network of Aquaculture Centres in Asia-Pacific, Bangkok, Thailand.
1997. Survey and analysis of aquaculture development research priorities and capacities in Asia. Food and Agriculture Organization, Rome, Fisheries Circular, 930:viii + 263 pp.
Food and Agriculture Organization, Inland Water Resources and Aquaculture Service, Fishery Resources Division. 1995a. Review of the state of world
fishery resources: inland capture fisheries. Food and Agriculture Organization, Rome, Fisheries Circular, 885:iii + 63 pp.
Food and Agriculture Organization, Inland Water Resources and Aquaculture Service, Fishery Resources Division. 1995b. Review of the state of world
fishery resources: aquaculture. Food and Agriculture Organization, Rome, Fisheries Circular, 886:v + 127 pp.
Forey, P. L. and Young, S. V. T. 1999. Late Miocene fishes of the Emirate of Abu Dhabi, United Arab Emirates, p. 120-135. In: Whybrow, P. J. and
Hill, A. (Eds.). Fossil Vertebrates of Arabia with Emphasis on the Late Miocene Faunas, Geology, and Palaeoenvironments of the Emirate of Abu Dhabi,
United Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Foroughi, R., Esmaeili Sari, A. and Ghasempour, S. M. 2007. Correlation of length and weight with mercury concentration in different tissues of kutum roach (Rutilus frisii kutum) in central
south of Caspian Sea. Iranian Scientific Fisheries Journal, 15(4):97-102. In Farsi.
Foroughifard (sic), H. A. 2001. Some ecological effects of milkfish (Chanos chanos) on Indian white shrimp (Penaeus indicus) farms. Pajouhesh va Sazandegi, 14(1)(50):38-43.
In Farsi.
Förster, H. 1976. Continental drift in Iran in relation to the Afar structures, p. 182-190. In: Pilger, A. and Rosler, A. (Eds.). Proceedings
of an International Symposium on the Afar Region and Related Rift Problems, held in Bad Bergzabern, F. R. Germany, 1-6 April 1974. Volume 2, Afar between
continental and oceanic rifting. E. Schweizerbartsche Verlagsbuchhandlung, Stuttgart.
Fortescue, L. S. 1922. Military Report on Tehrān and adjacent Provinces of North-West Persia (including the Caspian Littoral). Superintendent Government Printing, Calcutta. iii + 553 pp.,
17 pls., map.
Fortescue, L. S. 1924. The western Elburz and Persian Azerbaijan. Geographical Journal, 63:301-318.
Fortunatova, K. P. 1961. Availability of sticklebacks as food for the predacious fishes of the Volga delta. Fisheries Research Board of Canada Translation Series, 331:1-18.
Forughi-e-Fard, H. and Gharibnia, M. 1998. Culturing milkfish Chanos chanos (Forsskal, 1775) in earthen ponds (Tiyab). Iranian Fisheries Scientific Journal, 6(4):11-18, 2. In Farsi.
Fourooghi-e-Fard (sic), H. 2000. Culturing milkfish, Chanos chanos (Forsskal, 1775) in concrete tanks. Iranian Journal of Fisheries Sciences, 9(3):81-92. In Farsi
Fowler, H. W. 1956. Archaeological fishbones collected by Carleton S. Coon at Hotu, Iran. Bulletin of the Research Council of Israel, 5B:293-297.
Fowler, H. W. 1958. Some new taxonomic names of fishlike vertebrates. Notulae Naturae of the Academy of Natural Sciences of Philadelphia, 310:1-16.
Fowler, H. W. and Steinitz, H. 1956. Fishes from Cyprus, Iran, Iraq, Israel and Oman. Bulletin of the Research Council of Israel, 5B:260-292.
Franzoi, P., Berera, R., Maio, G. and Rossi, R. 1997. Some taxonomic features of Italian Syngnathus species (Syngnathidae). Ninth International
Congress of European Ichthyologists (CEI9) "Fish Biodiversity".Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 37.
Franzoi, P., Maccagnani, R., Rossi, R. and Ceccherelli, V. U. 1993. Life cycles and feeding habits of Syngnathus taenionotus and S.
abaster (Pisces, Syngnathidae) in a brackish bay of the Po River Delta (Adriatic Sea). Marine Ecology Progress Series, 97(1):71-81.
Fraser, J. B. 1825. Narrative of a Journey into Khorasan, in the years 1821 and 1822, including some account of the countries to the North-east
of Persia; with remarks upon the national character, government, and resources of that kingdom. Longman, Hurst, Rees, Orme, Brown, and Green, London. xxvi + 623 pp., + 148 pp., map.
Fraser, J. B. 1834. An Historical and Descriptive Account of Persia, from the earliest ages to the present time: with a detailed view of its
resources, government, population, natural history, and the character of its inhabitants, particularly of the wandering tribes; including a description
of Afghanistan and Beloochistan. Oliver & Boyd, Edinburgh. 472 pp., 13 plates, map.
French, J. R. P. and Jude, D. J. Diets and diet overlap of nonindigenous gobies and small benthic native
fishes co-inhabiting the St. Clair River, Michigan. Journal of Great Lakes Research, 27(3):300-311.
Frenkel, V. and Goren, M. 1997. Some environmental factors affecting the reproduction of Aphanius dispar (Rüppell, 1828). Hydrobiologia, 347:197-207.
Frenkel, V. 1995. The effects of environmental factors on the growth and reproduction of the fish Aphanius dispar (Rüppell, 1828).
M.Sc. Thesis, Zoology Department, Tel Aviv University, Tel Aviv. 88 pp.
Frenkel, V. 1995. The effects of environmental factors on the growth and reproduction of the fish Aphanius dispar (Rüppell, 1828).
M.Sc. Thesis, Zoology Department, Tel Aviv University, Tel Aviv. 88 pp.
Freyhof, J., Erk'akan, F., Özerhen, C., Özulug, M., Abdoli, A. and Perdices, A. 2010. Diversity of nemacheilid loaches in the Middle East. International Loach
Conference 2010, 31 August to 3 September 2010, Prague, Czech Republic (abstract).
Freyhof, J. and Naseka, A. M. 2007. Proterorhinus tataricus, a new tubenose goby from Crimea, Ukraine (Teleostei: Gobiidae). Ichthyological Exploration of Freshwaters, 18(4):325-334.
Fricke, R., Bilecenoglu, M and Sari, H. M. 2007. Annotated checklist of fish and lamprey species (Gnathostomata and Petromyzontomorphi) of Turkey, including
a Red List of threatened and declining species. Stuttgarter Beiträge zur Naturkunde, Serie A (Biologie), 706:169 pp.
Friedland, S. R. 1986. Caviar. Charles Scribner's Books, New York. viii + 152 pp.
Friend, P. F. 1999. Rivers of the Lower Baynunah Formation, Emirate of Abu Dhabi, United Arab Emirates, p. 38-49.In: Whybrow, P. J. and Hill,
A. (Eds.). Fossil Vertebrates of Arabia with Emphasis on the Late Miocene Faunas, Geology, and Palaeoenvironments of the Emirate of Abu Dhabi, United
Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Friese, E. 1967. Cyprinodont summaries. Journal of the American Killifish Association, 4(2):31-34.
Froese, R. and Pauly, D. (Eds.). 1996. FishBase 96. Concepts, design and data sources. International Center for Living Aquatic Resources Management, Manila. xi + 179 pp., CD-ROM.
G
Gabriel, A. 1938. The southern Lut and Iranian Baluchistan. Geographical Journal, 92(3):193-210.
Gabrielyan, B. K. 2001. An annotated checklist of freshwater fishes of Armenia. Naga, The ICLARM Quarterly, 24(3 & 4):23-29.
Gafarian, H., Soltani, M. and Abedian, A. M. 2007. The influence of some of the probiotic bacillus on feeding efficiency and nutrient body composition of beluga (Huso huso) larvae.
Journal of Agricultural Sciences and Natural Resources, (Special Issue Natural Resources), 14(Supplement):60-71. In Farsi.
Gaffaroglu, M., Yuksel, E. and Ráb, P. 2006. Note on the karyotype and NOR phenotype of leuciscine fish Acanthobrama marmid (Osteichthyes, Cyprinidae). Biologia, Bratislava, 61(2):207-209.
Gaillard, C. 1895. Notes sur quelques espèces de Cyprinodons de l'Asie Mineure et de la Syrie. Archives du Muséum d'Histoire Naturelle de Lyon, 6(2):1-15.
Gall, C. 2004. Seven-year drought. New York Times, 12 December 2004 (www.nytimes.com, downloaded 13 December 2004).
Ganji, M. H. 1960. The climates of Iran. Bulletin de la Société de Géographie d'Égypte, 28:195-299.
Ganji, M. H. 1968. Climate, pp. 212-249. In: Fisher, W. B. (Ed.). The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press, Cambridge. xix + 784 pp.
Ganji, M. H. 1978. Post-glacial climatic changes on the Iranian Plateau, pp. 149-163. In: Brice, W. C. (Ed.). The Environmental History of the Near
and Middle East Since the Last Ice Age. Academic Press, London. xx + 384 pp.
Ganjian, A., Hosseini, S. A., Kyhan-e-Sani, A. and Khousravi, M. 1998. The density and distribution of the dominant phytoplankton in the southern
Caspian Sea. Iranian Journal of Fisheries Sciences, 7(2):95-107, 10. In Farsi.
Ganjidoust, H., Ayati, B., Khara, H., Khodaparast, S. H., Akbarzadeh, A., Ahmadzadeh Layeghi, T., Nezami, Sh. A. and Zolfinezhad, K. 2009. Investigation of environmental pollution in Shiah Keshim
Wetland. Environmental Sciences, 6(3):117-122.
Ganjidoust, H., Rahimi, V., Khodadadi, A. and Yong, R. 2002. Modeling Sefidrood River of Iran in term of fishery. Second International Symposium
on GIS/Spatial Analyses in Fishery and Aquatic Sciences, The University of Sussex, Brighton, 3rd-6th September, 2002 (title).
Garakouei, M. Y., Pajand, Z., Tatina, M. and Khara, H. 2009. Medial lethal concentration (LC50) for suspended sediments in two sturgeon species Acipenser persicus and
Acipenser stellatus fingerlings. Journal of Fisheries and Aquatic Science, 4(6):285-295.
Garman, S. 1895. The Cyprinodonts. Memoirs of the Museum of Comparative Zoology at Harvard College, 19(1):179 pp., 12 pls.
Garrick, J. A. F. 1982. Sharks of the genus Carcharhinus. National Oceanic and Atmospheric Administration Technical Report, National Marine Fisheries Service Circular, 445:vii + 194 pp.
Gashti, G. Z. 2009. Evaluation of hand and machinery trimming used in the production of silver carp fillets. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 87
(abstract).
Gasmi, H. and Mirzaei, M. 2004. Sargi Azarbaycan Yerli Balixlari Araz va Gzluzan Zirhovzasinda. The Joint Agriculture and Natural Resources Symposium, Tabriz-Ganja, May 14-16, 2004. 3 pp.
Gäsowska, M. 1968. The biometric comparison of the bream Abramis brama (Linnaeus)(Teleostei, Cyprinidae) from Polish water with the bream from other European countries.
Vĕstník československé Společnosti zoologické, 32(4):319-336.
Gäsowska, M. 1974. Biometric and ecological studies on the bleak, Alburnus alburnus (LINNAEUS) (Pisces, Cyprinidae) from different bodies of water in
Poland, in connexion with the geographic variability of this species. Annales Zoologici, Warszawa, 31(4):373-405.
Gavlena, F. K. 1977. The "bighead goby", Neogobius kessleri, in Volgograd Reservoir. Journal of Ichthyology, 17(2):318-319.
Geldiay, R. and Balık, S. 1996.Türkiye Tatlısu Balıkları. (Ders Kitabı). II. Baskı.Ege Üniversitesı SuÜrünleri
Fakültesi Yayınları No: 46, Ders Kitabı Dizini No: 16, Ege Üniversitesı Basımevı, Bornova, Izmir.xviii + 532 pp., map.
Gelodar, A. S. 2006. Hygienic evaluation of caviar processing plan included EC code in Mazandaran. Iranian Fisheries Research Organization, Agriculture Research and Education
Organization, Ministry of Jihad-e-Agriculture, Sari. http://en.ifro.ir (abstract).
Georgacas, D. J. 1978. Ichthyological terms for the sturgeon and etymology of the international terms botargo, caviar and congeners (a
linguistic, philological, and culture-historical study). Pragmateiai Ths Akademias Athenon, 43:330 pp.
Georgieveskiy, V. Y. 2001. A review and a critical analysis of the methods and results of the Caspian Sea level forecasts, developed for 5 to 10 years and 20 to 100 years time
horizons. Caspian Environment Programme, TACIS (Technical Assistance to the Commonwealth of Independent States, European Union). 18 pp.
Geramy, E. H. N. and Irani, A. J. 2007. The effects of photoperiod on the growth, survival and feeding parameters in the rainbow trout (Oncorhynchus
mykiss) larvae. Pajouhesh va Sazandegi, 72:2-5. In Farsi.
Gerasimov, I. P. 1978a. Ancient rivers in the deserts of Soviet Central Asia, pp. 319-334. In: Brice, W. C. (Ed.). The Environmental History of the Near and Middle East Since the Last Ice Age. Academic
Press, London. xx + 384 pp.
Gerasimov, I. P. 1978b. The past and future of the Aral and the Caspian seas, pp. 335-349. In: Brice, W. C. (Ed.). The Environmental History of the Near and Middle East Since the Last Ice Age. Academic
Press, London. xx + 384 pp.
Gerbilskii, N. L. 1955. Biological Races of Caspian Sturgeons. Systematic Zoology, 4:83-92.
Gerson, R. 1982. The Middle East: landforms of a planetary desert through environmental changes. Striae, Uppsala, 17:52-78.
Gerve'ei, H., Jamili, S., Mashinchian, A. and Rostami, M. 2008. Determination of LC50 and tissue lesions caused by aluminum sulfate at acidic pH on gills of roach (Rutilus rutilus).
Journal of Environmental Science and Technology, 36(special issue).
GESAMP (IMO/FAO/UNESCO-IOC/WMO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). 1997. Opportunistic
settlers and the problem of the ctenophore Mnemiopsis leidyi invasion in the Black Sea. GESAMP Reports and Studies, 58:x + 84 pp.
Gessner, J., Würtz, S., Kirschbaum, F. and Wirth, M. 2008. Biochemical composition of caviar as a tool to discriminate between aquaculture and wild origin. Journal of Applied Ichthyology, 24(s1):52-56.
Ghadiri, H. and Ghadiri, M. 2005. Marshlands of Mesopotamia and the rivers which feed them. Program & Abstracts, 8th International Riversymposium 2005, 6-9
September, Brisbane, Australia (abstract).
Ghadirnejad, H. 1996. Population dynamics of grey mullet species (Liza aurata and L. saliens) in the southern Caspian Sea. Ph.D. Thesis, University of Wales, Swansea. 207 pp.
Ghadirnejad, H. 2003. Impact of the invasive ctenophore Mnemiopsis leidyi on the kilka fisheries Clupeonella engrauliformis in the
southern Caspian Sea. Australian Society for Fish Biology Annual Conference, New Zealand (abstract)(www.asfb.org.au/pubs/2003/2003nz-51.htm, downloaded 12 January 2004).
Ghadirnejad, H. 2000. Sturgeon fisheries in the Caspian Sea: past. present and future. The Third World Fisheries Congress, Beijing, 31 Oct - 2 Nov 2000 (title).
Ghadirnejad, H. and Rezvani Gilkolaei, S. 2001. Present status of sturgeon stocks in the southern Caspian Sea. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Ghadirnejad, H. and Ryland, J. S. 1996. A study of food and feeding of grey mullets in the southern Caspian Sea, p. 137-144. In: GUTSHOP '96, Feeding ecology and nutrition in fish: symposium and
proceedings, American Fisheries Society, Physiology Section, Bethesda, Md.214 pp.
Ghadirnejad, H., Zhu, B. and Chang, J. 2008. Study on the population genetics of the great sturgeon (Huso huso) in the southern part of the Caspian Sea using microsatellite loci.
Journal of Applied Biological Sciences, 2(2):93-97.
Ghadri, V., Esmaili Sari, A., Saifabadi, S. and Owfi, F. 2006. Mercury assessment concentration in the habitat and muscle of the mudskipper, Boleophthalmus dussumieri, in
Mahshahr estuary, Iran. The 1st International Conference on the State of the Gulf Ecosystem: Future and Trends. 5-7 March 2006, Rotana Hotel, Al Ain, United Arab Emirates University (abstract).
Ghadri, V., Malek, M,. and Nasrolahi, A. 2006. Bioaccumulation of nickel and cadmium in the muscle and gill of mudskipper, Boleophthalmus dussumieri in Mahshahr estuary, Iran. The
1st International Conference on the State of the Gulf Ecosystem: Future and Trends. 5-7 March 2006, Rotana Hotel, Al Ain, United Arab Emirates University (abstract).
Ghaemi, E. O., Ghazesaeed, K., Nasab, F. F., Hashemzade, Z., Vatani, S., Mohamedi, M. and Mansourian, A. R. 2006. Mycobacterium marinum infection
in caviar fishes and fishermen in a Caspian Sea province in north of Iran. Journal of Biological Sciences, 6(6):1145-1147. In Farsi.
Ghafar Zadeh, H. R. and Honar Bakhsh, N. 2008. Economic consequences of lack of action against invasive Mnemiopsis leidyi in the Caspian Sea coasts. Journal of Environmental Science and
Technology, 9(4)(35):117-127. In Farsi.
Ghafari, S. M. and Jamili S. 2010. Certain aspects of the reproductive biology of berzem (Barbus pectoralis) in Karoon River. Journal of Fisheries and Aquatic Science, 5(1):33-41.
Ghafleh Marammazi, J., Eskandari, G. R., Al-Mukhtar, M. A. and Kiabi, B. H. 2004. Study of spawning season and spawning ground of soboor (Tenualosa ilisha, Ham. Bunch.,
(sic) 1822) during its migration in Khuzestan rivers. Iranian Journal of Fisheries Sciences, 4(1):89-102, 124.
Ghafouri Saleh, S., Jamili, S. and Abbasi, F. 2008. Study of effect of physiological stress on cortisol and muscle compounds in Stizostedion lucioperca. Pajouhesh va Sazandegi, 79:87-94.
In Farsi.
Ghahaeri, M. 2003. Saving the Caspian Sea, first version: Iranian share, p. 76 (abstract). Global Threats to Large Lakes, 46th Conference on Great Lakes Research, 10th World Lake Conference,
June 22-26, 2003, DePaul University, Chicago. iv + 304 pp.
Ghaheri, M., Baghal-Vayjooee, M. H. and Naziri, J. 1999. Lake Urmia, Iran: A summary review. International Journal of Salt Lake Research, 8(1):19-22.
Ghahraman, A. and Atar, F. 2003. Anzali Wetland in danger of death (an ecologic-floristic research). Journal of Environmental Studies, 28(Special issue):1-38. In Farsi.
Ghahraman, F. 1958. The right of use and economics of irrigation water in Iran. Ph.D. Thesis, University of Minnesota. xviii + 209 pp.
p>Ghanbari, M., Jamai, M., Naghdi, M. and Shahriari Moghadam, M. 2009. The investgation of long-term effects of water pH changes on haematological parameters in fingerlings of common carp
(Cyprinus carpio L.). Iranian Journal of Biology, 22(1):143-150.
Ghalenoei, M., Pazooki, J., Abdoli, A., Hassanzadeh Kiabi, B. and Golzarian, K. 2010. Morphometric and meristic study of Garra rufa populations in Tigris and Persian Gulf basins.
Iranian Scientific Fisheries Journal, 19(3):107-118. In Farsi.
Ghanbari, M. and Jami, M. 2011a. Threatened fishes of the world: Schizothorax zarudnyi Nikolskii, 1897. The 4th International Symposium on Stock Enhancement and Sea Ranching, 9th
Asian Fisheries and Aquaculture Forum, Shanghai Ocean University, April 21 to 23, 2011 (abstract).
Ghanbari, M. and Jami, M. 2011b. Isolation and characterization of Lactobacillus species from intestinal contents of Caspian Sea sturgeon. The 4th International Symposium on
Stock Enhancement and Sea Ranching, 9th Asian Fisheries and Aquaculture Forum, Shanghai Ocean University, April 21 to 23, 2011 (abstract).
Ghanbari, M., Rezaei, M., Jami, M. and Nazari, R. M. 2009. Isolation and characterization of Lactobacillus species from intestinal contents of beluga (Huso huso) and Persian
sturgeon (Acipenser persicus). Iranian Journal of Veterinary Research, Shiraz University, 10(2)(27):152-157.
Ghanbari, M., Rezaei, M., Soltani, M. and Shahhosseini, Gh. R. 2009. Production of bacteriocin by a novel Bacillus sp. strain RF 140, an intestinal bacterium of Caspian frisian roach
(Rutilus frisii kutum). Iranian Journal of Veterinary Research, Shiraz University, 10(3)(28):267-272.
Ghaninejad, D. 1997. On some biological aspects of Acipenser stellatus in the south west of the Caspian Sea in 1994. Iranian Fisheries Scientific Journal, 6(1):49-64, 6. In Farsi.
Ghaninezhad (sic), D. and Abd Almalaki, Sh. 2009. Sustainable exploitation of the bony fishes in Iranian coastal waters of the Caspian Sea: necessities and requirements. Iranian Scientific
Fisheries Journal, 18(2):105-118. In Farsi.
Ghaninejad, D., Abdolmalaki, S. and Kuilyev, Z. M. 2010. Reproductive biology of the golden grey mullet, Liza aurata in the Iranian coastal waters of the Caspian Sea. Iranian Journal of
Fisheries Sciences, 9(3):402-411.
Ghaninejad, D., Moghim, M., Sayyad Bourani, M. and Abdulmaleki, S. 2001. Stock enhancement evaluation. Bony Fishes Research Station, Rasht. 98 pp. In Farsi.
Ghara, K., Moradi, M., Ghorbani, S. A., Amiri, A., and Parvaneh Moghadam, D. 2007. An investigation on the growth and survival of Rutilus frisii kutum in geomembrane tanks. Iranian Scientific
Fisheries Journal, 16(3):155-158. In Farsi.
Gharaei, A. and Esmaili-Sari, A. 2008. LC50 and bioaccumulation of mercury chloride (HgCl2) in Rutilus frisii (Nordmann, 1840). Journal of Applied Ichthyology, 24(6):705-706.
Gharaei, A., Esmaili Sari, A., Shahriari Moghadam, M., Nazari, R. M. and Karami, R. 2006. Acute LC50 and bioconcentration of mercury chloride
in Rutilus frisii kutum. Iranian Journal of Fisheries Sciences, 15(2):101-108. In Farsi.
Gharaei, A., Pourkazemi, M., Rezvani, S and Mojazi Amiri, B. 2005. Genetic difference and resemblance between Acipenser persicus and Acipenser
gueldenstaedtii by means of RAPD technique. Iranian Scientific Fisheries Journal, 14(2):91-102. In Farsi.
Gharibkani M., Pourkazemi, M., Soltani, M., Rezvani, S. and Azizzadeh L. 2009. Population genetic structure of pikeperch (Sander lucioperca Linnaeus, 1758) in the southwest Caspian Sea using
microsatellite markers. Journal of Fisheries and Aquatic Science, 4(3):161-168.
Ghasemi, A., Keyvanshokooh, S., Shahriari-Moghadam, M., Khara, H. and Sourinejad, I. 2007. Genetic comparison of Iranian and Azeri populations of the oriental bream Abramis brama orientalis
(Berg) using microsatellites. Aquaculture Research, 38(16):1742-1746.
Ghasemi, F. C., Pourkazemi, M., Zamini, A., Yarmohammadi, M., Noveiri, S. B., Saber, M. H., Rezvani, S. and Azizzadeh, l. 2009. Genetic analysis of spring and autumn races of Caspian Sea kutum
(Rutilus frisii kutum) using microsatellite markers. Iranian Journal of Animal Biosystematics, 5(1):1-8.
Ghasemi, H. 2002. Identification of Barbus species in the east Azarbaijan rivers. Iranian Journal of Fisheries Sciences, 11(3):81-90. In Farsi.
Ghasemi, H and Mustafayev, Q. T.2008. Identification of fishes and some ecological parameters of Aras River (eastern side of Azerbaijan Province). Iranian Scientific Fisheries Journal,
17(3):121-132. In Farsi.
Ghasempouri, S. M. 1993. The Caspian Sea lamprey. Abzeeyan, Tehran, 4(7):18-21, II. In Farsi.
Ghasempouri, S. M. 1999. Systematics, distribution and ecology of Caspian sea lamprey (Caspiomyzon wagneri) in south region. Scientific and Research Quarterly of Jihad-e Sazandegi, 44. In Farsi.
Ghasempouri, S. M. and Esmaili Sari, A. 2002. Determination of total hydrocarbons in water and sediment of Gomishan Wetland. Iranian Journal of Marine Sciences, 1(1):61-67, 80. In Farsi.
Ghassemi, F. 1979. Mathematical model application in ground-water studies of Iran. Ground Water, 17(4):359-365.
Ghassemzadeh, F. 2004. Limnological studies of Bazangan Lake, northeast Iran. Ecological Society of America, 2004 Annual Meeting, Portland, Oregon (abstract).
Ghassemzadeh, F. and Madjdzadeh, S. M. 1996. Identification of fishes of Radkan and Frizy rivers in Khorasan province, p. 52 (abstarct). The Fifth Iranian Biology Conference, Tabriz University.
Ghayoumi, R., Malek, M., Jamili, S., Nabavi, M. and Motallebi, A. 2009. Epizootiology of intestinal helminth parasites of Clupeonella spp. (Osteichthyes: Clupeidae) from the Caspian Sea, Iran.
Bulletin of the European Association of Fish Pathologists, 29(4):109-117.
Ghazi, S. and Akhlaghi, M. 1998. Serum anitibody against Vibrio anguillarum in cultured rainbow trout (Oncorhynchus mykiss). Iranian Fisheries Scientific Journal, 6(4):47-58, 5. In Farsi.
Ghazi Saidi, K. and Mohammadi, M. 1994. Environmental mycobacteria isolated from fish breeding pools in the north of Iran. In: 28th World Conference of the International Union Against
Tuberculosis and Lung Disease /UICTMR, Mainz, Germany, 14-17 June 1994 (abstract).
Ghedotti, M. J., Smihula, J. C. and Smith, G. R. 1995. Zebra mussel predation by round gobies in the laboratory. Journal of Great Lakes Research, 21(4):665-669.
Ghelichi, A. Hajimoradlou, A. A. and Jorjani, S. 2007. 17α hydroxyprogesterone profiles during induced spawning of the grey mullet (Mugil cephalus). Journal of Agricultural Sciences and
Natural Resources, 14(2):88-97. In Farsi.
Ghelichi, A. and Jorjani, S. 2004. Sex steroid profiles and ovarian development during induced spawning of grey mullet (Mugil cephalus) by CPH and hCG. Biology in Asia International Conference,
Singapore, 7-10 December 2004 (abstract).
Ghelichi, A., Oryan, S., Ahmadi, M. R., Kazemi, R. A. and Halajian, A. 2004. The histological study of ovarian development of grey mullet (Mugil cephalus)
in Gomishan fish and shrimp rearing center. Journal of Agricultural Sciences and Natural Resources, 10(4):115-124. In Farsi.
Ghenaat Parast, A. 1993. Mass production of Caspian bream fingerlings. Abzeeyan, Tehran, 4(8):13-16, II. In Farsi.
Ghiasi, F. 2006. Effect of sub-lethal concentration of cadmium on the level of lysozyme in serum of common carp Cyprinus carpio. World Aquaculture
Society, Aqua 2006, May 9-13, Florence, Italy (abstract).
Ghiasi, F. and Mirzargar, S. S. 2005. Effect of sub-lethal concentration on the level of lysozyme in serum of common carp (Cyprinus carpio). Sixth Symposium on Diseases in Asian
Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Ghiasi, F. and Mirzagar, S. S. 2006. Effect of sub-lethal concentration of cadmium on the level of lysozyme in serum of common carp Cyprinus carpio. World Aquaculture Society,
Aqua 2006, May 9-13. Florence, Italy (abstract).
Ghiasi, F., Mirzagar, S. S., Badakhshan, H. and Shamsi S. 2010. Effects of low concentration of cadmium on the levels of lysozyme in serum, leukocyte count and phagocytic index in
Cyprinus carpio under the wintering conditions. Journal of Fisheries and Aquatic Sciences 5(2):13-119.
Ghiasi, M., Zahedi A. and Rostami, H. K. 2000. The epidemia expression of streptococcosis in Mazandarn Province rainbow trout. The First Meeting of Health and Disease of Iran Aquatics, Ahwaz
University Publications, 59 pp. In Farsi.
Ghodrati, A. R., Sobh Zahedi, Sh. and Dadashi, M. A. 2007. Investigation on industrial pollution of Zarjub River - Rasht City - Guilan Province. Iranian Journal of Natural Resources, 60(1):213-224.
In Farsi.
Ghods, F., Malekghassemi, B., Stachelberger, H., Abedinsahde, A. und Tehrani, N. 1977. Die Bestimmung einiger Inhaltsstoffe in iranischen Kaviar. Ernährung, 1(2):11-12.
Ghofleh Marammazi, J. 2000. Feeding and spawning characters of Barbus grypus Heckel, 1843 in Khouzestan water bodies. Iranian Journal of Fisheries Sciences, 9(3):67-80. In Farsi.
Gholami, M. 2010. Effects of n-3 HUFA enriched Daphnia magna on growth, survival, stress resistance and fatty acid composition of white fish fry
(Rutilus frisii kutum). Journal of Fisheries and Aquatic Science, 5(1):49-55.
Gholami, Z., Akhlaghi, M. and Esmaeili, H. R. 2009. Infection of the Isfahan tooth-carp, Aphanius isfahanensis (Actinopterygii: Cyprinodontidae) with Diplostomum spathaceum
(Trematoda: Diplostomidae): a case report. International Journal of Veterinary Research, 3(2):129-131.
Gholami, Z., Akhlaghi, M., Mobedi, I. and Esmaeili, H. R. 2011. Infection of Aphanius sophiae (Actinoptrygii (sic): Cyprinodontidae) with Tetracotyle sp. Veterinary Research Forum,
2(1):65-68.
Gholipoor, F. 1996. Survey of growth of grass carp in relation to aquatic plant consumption. Abzeeyan, Tehran, 7(1):36-38. In Farsi.
Gholipour, F., Allameh, S. K., Mohammadi Arani, M. and Nasr Esfahani, M. 2006. Effect of density on growth and feed conversion ratio in rainbow trout (Oncorhynchus mykiss).
Pajouhesh va Sazandegi, 19(1)(70):23-27. In Farsi.
Gholizadeh, M., Ghorbani, R., Salman Mahini, A. A. R., Haji Moradlou, A. A., Rahmani, H. and Molaei, M. 2009. A investigation on morphology, age and growth of Capoeta capoeta gracilis in
Zarrin-Gol stream, Golestan Province of Iran. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A). In Farsi.
Gholizadeh, M. B. 1963. Paper No. 6. Tidal irrigation in the delta of the Karun and Shatt-al-Arab rivers with complications from increased salinity of water, pp. 187-193. In:
Proceedings of the Regional Symposium on Flood Control, Reclamation, Utilization and Development of Deltaic Areas (Held at Bangkok, Thailand, 2 to 9 July 1963), United Nations, New York, Water
Resources Series, 25.
Gholizadeh, M. B. and Fatemi, M. R. 1969. A study of a change from tidal irrigation to gravity irrigation on Abadan Island in the delta of the Arvandrud
and Karun rivers, pp. 177-194. In: Proceedings of the Second Symposium on the Development of Deltaic Areas (Held at Tokyo, Japan, 4 to 13 July 1969), United Nations, New York, Water Resources Series, 39.
Ghomi, M. R., Esmaili, A., Vosoughi, G., Keyvan, A. and Nazari, R. M. 2003. Effect of ozone and physical treatment on hatching rate of Iranian sturgeon (Acipenser
persicus). Iranian Journal of Marine Sciences, 2(2-3):59-68. In Farsi.
Ghomi, M. R., Esmaili, A., Vossughi, G., Keyvan, A. and Nazari, R. M. 2005. Effect of ozone and physical treatment to control Saprolegnia on Iranian sturgeon (Acipenser persicus)
eggs. European Association of Fish Pathologists, 12th International Conference, "Diseases of Fish and Shellfish', 11-16 September 2005, Copenhagen (title).
Ghomi Marzdashti, M. R. and Azari Takami, G. 2004. Effect of species combination on growth and yield of cultured carps. Iranian Journal of Natural Resources, 5(1):145-156. In Farsi.
Ghoorbanalidoost, Gh., Kayvan, A. and Ramin, M. 2003. Morphology and population structure of Atherina boyeri in the southern Caspian Sea. Iranian Journal
of Fisheries Sciences, 12(2):63-78. In Farsi.
Ghorbani, R. 2000. The study of morphologic, age, growth, diet and parasites of juvenile Acipenser persicus and A. gueldenstaedtii in southeast of Caspian Sea. M.Sc. Thesis,
Gorgan University of Agricultural Science and Natural Resources, Gorgan. 99 pp. In Farsi.
Ghorbani, R. and Hajimoradloo, A. A. 2002. A comparative morphological analysis of juvenile Acipenser persicus and A. guldenstaedti in southeast of Caspian Sea. Journal of
Agricultural Sciences and Natural Resources, 8(4):191-204. In Farsi.
Ghorbani, R., Hajimoradlou, A. A., Agh, N., Soltani, M., Nouri, F. and Irani, A. A. J. 2007. Enrichment and excretion of oxolinic acid in Artemia urmiana nauplii and Acipenser persicus
larvae. Journal of Agricultural Sciences and Natural Resources, 14(2):117-126. In Farsi.
Ghorbani, R., Kamali, A., Nevalennyy, A. N., Hajimoradloo, M. and Pourkazemi, M. 2004. Influence of some microelements on the level of proteolytic enzymes and alkaline phosphatase activity
in digestive system of juvenile beluga (Huso huso). Iranian Journal of Fisheries Sciences, 12(4):111-126. In Farsi.
Ghorbani, R., Nevale, A., Kamali, A., Hajimoradlou, A. A. and Pourkazemi, M. 2003. Influence of microelements zinc, nickel, cobalt, manganese, iron and copper on the level of alfa-amylase activity in
intestinal mucosa, pyloric caecae and pancreas in beluga juveniles (Huso huso). Pajouhesh va Sazandegi, 15(3-4)(56-57):86-96. In Farsi.
Ghorbani Chafi, H. 2000. Identification of different fish species in Koohrang, Bazoft and Zayandeh Rood River in Chahar Mahal-e-Bakhtiary Province. Iranian Journal of Fisheries Sciences, 8(4):43-56,
5. In Farsi.
Ghorbani Vaghei, R. and Ahmadi, M. R. 2007. The comparison of diversity and abundance of macrozoobenthos in three farms of Chinese carps in Guilan Province. Pajouhesh va Sazandegi, 19(1)(74):58-66.
In Farsi.
Ghoroghi, A. 1996. Survey of Diplostomiasis in sturgeon fingerling. Iranian Fisheries Scientific Journal, 5(2):11-22, 5. In Farsi.
Gilles, A., Lecointre, G., Miquelis, A., Loerstcher, M., Chappaz, R. and Brun, G. 2001. Partial combination applied to phylogeny of European cyprinids using the mitochondrial control region.
Molecular Phylogenetics and Evolution, 19(1):22-33.
Ginzburg, Ya. I. 1936a. Opisaniye lichinok kaspiiskoi minogi Caspiomyzon wagneri (Kessler) iz rechki Cheshmkelya (Iran) [Description of larvae of the Caspian lamprey (Caspiomyzon
wagneri (Kessler)) from the Cheshmkelya stream (Iran)]. Izvestiya Azerbaidzhanskogo Filiala Akademii Nauk SSSR, 1936(1):47-50.
Ginzburg, Ya. I. 1936b. K sistematike i biologii shemai Chalcalburnus chalcoides (Güldenstädt) iranskogo poberezh'ya Kaspiiskogo Morya [A taxonomic and biological study of
the shemaya Chalcalburnus chalcoides (Güldenstädt) from the Iranian coast of the Caspian Sea]. Izvestiya Azerbaidzhanskogo Filiala Akademii Nauk SSSR, 1936(1):53-62.
Ginzburg, Ya. I. 1939. Materialy po sistematike molodi osetrovykh yuzhnogo Kaspiya i opredelitel'ikh lichinok i mal'kov [Taxonomic data on the young of the South Caspian Acipenserids and
identification key to their larvae and fry]. Akademiya Nauk SSSR, Azerbaidzhanskii Filial, Trudy Zoologicheskogo Instituta, Baku, 10:77-106.
Ginzburg, Ya. I. 1969. The spawning population of the Caspian lamprey [Caspiomyzon wagneri (Kessler)] after regulation of the Volga River by the dam of the Volgograd Power Station.
Problems of Ichthyology, 9(6):821-828.
Ginzburg, Ya. I. 1970. Reproduction of the lamprey [Caspiomyzon wagneri (Kessler)] below the Volgograd Dam and the development of its larvae. Journal of Ichthyology, 10(4):485-493.
Gladkov, N. A. 1935. Materialy po izmenchivosti shchipovki (Cobtitis taenia L.) [Materials on the variability of the loach (Cobitis taenia L.)].
Sbornik Trudov Gosudarstvennogo Zoologicheskogo Muzeya, Moskva, 2:69-74.
Glantz, M. H. and Zonn, I. S. (Eds.). 1997. Scientific, Environmental, and Political Issues in the Circum-Caspian Region. Kluwer Academic Publishers, Dordrecht/Boston/London. xvi + 312 pp.
Gleick, P. H. (Ed.). 1993. Water in Crisis. A Guide to the World's Fresh Water Resources. Oxford University Press, New York. xxiv + 473 pp.
Gmelin, S. G. 2007. Travels through northern Persia 1770-1774 by Samuel Gottlieb Gmelin. Translated and Annotated by Willem Floor. Mage Publishers, Washington, D.C. xxvii + 381 pp.
Goblot, H. 1963. Dans l'ancien Iran, les techniques de l'eau et la grande histoire. Annales Économies, Sociétés, Civilisations, 18:499-519.
Goblot, H. 1979. Les qanats. Une technique d'acquisition de l'eau. École des Hautes Études en Sciences Sociales, Centre de Recherches Historiques,
Industrie et artisanat, 9, Mouton, Paris. 236 pp., 14 plates.
Gökçek, C. K. and Akyurt, I. 2008. Age and growth characteristics of himri barbel (Barbus luteus Heckel, 1843) in Orontes River, Turkey. Turkish Journal of Zoology, 32(4):461-467.
Golaghaie, M., Yusefian, M. and Kalbasi, M. R. 1999. Comparative study on some biochemical characters in propagation of sturgeon broodstocks. First National Sturgeon Symposium, Rasht, 1999 (abstract).
Golani, D. 1997. Handbook of the Fishes of Israel. Keter Publishing House, Jerusalem. 269 pp. In Hebrew.
Golbaz Hagh, F. and Wossughi, Gh. 1985. The study of microscopic anatomy of barbels in Acipenser nudiventris, Acipenser stellatus,
Silurus glanis, Cyprinus carpio, Capoeta barroisi and Tor grypus fishes. Journal of the Veterinary Faculty of the University
of Tehran, 40(2, 3 and 4):27-44. In Farsi.
Golchinrad, A., Askari Hosni, M., Naser Alavi, M. Gh. and Atabati, M. 2008. An investigation on the effects of anionic detergents on liver glycogen and glucose in common carp (Cyprinus carpio).
Iranian Scientific Fisheries Journal, 17(3):161-164. In Farsi.
Golden, F. 1982. Making rivers run backward. Time, June 14:88.
Goldsmith, E. and Hildyard, N. (Eds.). 1984. The Social and Environmental Effects of Large Dams. Volume One: Overview, 346 pp., 2 appendices, references;
Volume Two: Case Studies, 331 pp.; Volume III: A Review of the Literature (Trussell, D. (Ed.), xiv + 243 pp. Wadebridge Ecological Centre, Camelford, U.K.
Golpour, A. and Imanpoor, M. R. 2010. Relationships between compositions of seminal plasma and blood plasma parameters in Caspian roach (Rutilus rutilus caspicus) during the reproductive season.
Research Journal of Fisheries and Hydrobiology, 5(2):105-110.
Golub, R. 1992. The Caspian/Khazar Sea. Environmental Policy Review, 6:1-10.
Golubev, G. N. 1996. Caspian and Aral Seas: two different paths of environmental degradation. Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 26:159-166.
Golvan, Y. J. and Mokhayer, B. 1973. Acanthocéphales des esturgeons de la mer Caspienne. Annales de Parasitologie Humaine et Comparée, 48(4):597-602.
Golzarianpour, K., Abdoli, A., Kiabi, B. H. and Freyhof, J. 2009. First record of the freshwater loach, Turcinoemacheilus kosswigi (Bănărescu and Nalbant, 1964), from Iran (Karoun
drainage). Zoology in the Middle East, 47:57-62.
Gon, O. and Ben-Tuvia, A. 1983. The biology of Boyer's sand smelt, Atherina boyeri Risso in the Bardawil Lagoon on the Mediterranean coast of Sinai. Journal of Fish Biology, 22:537-547.
González-Solís, D., Moravec, F. and Coad, B. W. 1997. Some nematode parasites of fishes from southwestern Iran. Zoology in the Middle East, 15:113-119.
Goren, M. 1974. The freshwater fishes of Israel. Israel Journal of Zoology, 23:67-118.
Goren, M., Fishelson, L. and Trewavas, E. 1973. The cyprinid fishes of Acanthobrama Heckel and related genera. Bulletin of the British Museum (Natural History) Zoology, 24(6):293-315.
Goren, M. and Ortal, R. 1999. Biogeography, diversity and conservation of the inland water fish communities in Israel. Biological Conservation, 89:1-9.
Goren, M. and Rychwalski, E. M. 1978. Hybrids of Aphanius dispar and Aphanius mento (Cyprinodontidae: Pisces).Zoological Journal of the Linnaean Society, 63:259-264.
Gorji-Bandpy, M. and Hooman, K. 2004. Caspian Sea level fluctuations. Caspian Journal of Environmental Sciences, 2(1):1-8.
Gorjipoor, A., Asadi, M. and Hasanpoor, B. 2007. Limnological investigation of Zohreh River. Pajouhesh va Sazandegi,
19(1)(74):105-110. In Farsi.
Gorogi, A. 1996a. Identification of blood and intestinal parasites of A. persicus in southern part of Caspian Sea. Iranian Journal of Fisheries Science, 1:35-39. In Farsi.
Gorogi, A. 1996b. Identification of blood and intestinal parasites of H. huso in southern part of Caspian Sea. Iranian Journal of Fisheries Science, 4:43-47. In Farsi.
Gorshkova, G., Gorshkov, S. and Golani, D. 2002. Karyotypes of Barbus canis and Capeota damascina (Pisces, Cyprinidae) from the Middle
East. Italian Journal of Zoology, 69(3):191-194.
Goryunova, B. B., Shagaeva, V. G. and Nikol'skaya, M. P. 2000. Analysis of anomalies in the structure of larval and young Acipenseridae in the Volga-Caspian basin under artificial reproduction.
Journal of Ichthyology, 40(9):762-766.
Gould, R. G. and Atz, J. W. 1996. The trouble with "jewfish" or what's in a name? Tropical Fish Hobbyist, 44(12)(486):172-182.
Grant, S. 2004. The striped catfishes of the genus Mystsus Scopoli, 1777 (Siluriformes: Bagridae). Cat Chat, The Journal of the Catfish Study Group, 5(2):5-17.
Green, A. 1986. A note on the Assyrian "goat-fish", "fish-man" and "fish-woman". Iraq, 48:25-30, pl. V-X.
Greenfield, D. W. 1973. An evaluation of the advisability of the release of the grass carp, Ctenopharyngodon idella, into the natural waters
of the United States. Transactions of the Illinois State Academy of Science, 66(1-2):47-53.
Greenspan, D. 1989. Caviar emptor. Connoisseur, 219:144-147, 157.
Greenwood, P. H. 1976. A new and eyeless cobitid fish (Pisces, Cypriniformes) from the Zagros Mountains, Iran. Journal of Zoology, London, 180:129-137.
Greenwood, P. H. 1978. A review of the pharyngeal apophysis and its significance in the classification of African cichlid fishes. Bulletin of the British Museum of Natural History (Zoology), 33:297-323.
Gribbin, J. 1979. Climatic impact of Soviet river diversions. New Scientist, 84(1184):762-765.
Griffiths, F., RaLonde, R. and Walczak, P. 1972. Review of Fisheries and Aquatic Biology Projects, 1971-1972. Fisheries Research Institute,
Bandar Pahlavi, Iran. MS, 16 pp. + 6 pp. on reports written by RaLonde and Walczak, 1969-1972.
Griffiths, H. I., Schwalb, A. and Stevens, L. R. 2001. Environmental change in southwestern Iran: the Holocene ostracod fauna of Lake Mirabad. Holocene, 11(6):757-764.
Grigorovich, I. A., Therriault, T. W. and MacIsaac, H. J. 2003. History of aquatic invertebrate invasions in the Caspian Sea. Biological Invasions, 5(1-2):103-115.
Grigoryan, K. A. and Vasil'ev, V. P. 1993. Karyotypes of five species of goby (Gobiidae) from the basins of the Black and Caspian seas. Journal of Ichthyology, 33(1):137-143.
Grimes, C. 1974. Observations on Aphanius mento. Journal of the American Killifish Association, 7(11):395-396.
Grimm, H. 1979. Eigröße und Eizahl in der Zahnkarpfengattung Aphanius (Pisces, Cyprinodontidae). Meeresforschung, 27:186-197.
Groombridge, B. (Ed.). 1992. Fishes, p. 116-135. In: Global Biodiversity. Status of the Earth's Living Resources. A report compiled by the World
Conservation Monitoring Centre. Chapman & Hall, London. xx + 585 pp.
Groot, C. and Margolis, L. (Eds.). 1991. Pacific Salmon Life Histories. UBC Press, Vancouver. xv + 564 pp.
Gruvel, A. 1931. Les États de Syrie. Richesse marines et fluviales. Exploitation actuelle - Avenir. Bibliothèque de la Faune des Colonies Françaises, Paris.
Güçlü, S. S. and Küçük, F. 2008. Population age, sex structure, growth and diet of Aphanius mento
Heckel in: Russegger, 1843 (Cyprinodontidae: Teleostei), at Kırkgöz Spring, Antalya-Türkiye. Turkish Journal of Fisheries and Aquatic Sciences, 8(2):269-274.
Gudger, E. W. 1924. The sources of the material for Hamilton-Buchanan's Fishes of the Ganges, the fate of his collections, drawings and notes, and the use
made of his data. Journal and Proceedings of the Asiatic Society of Bengal, 19:121-136.
Gudger, E. W. 1945a. The giant freshwater fishes of Asia. Journal of the Bombay Natural History Society, 45(3):374-390.
Gudger, E. W. 1945b. Is the giant catfish, Silurus glanis, a predator on man? The Scientific Monthly, 61(6):451-454.
Gudkov, P. K. 1985. Biology of goldfish, Carassius auratus gibelio, from the Volga Delta. Journal of Ichthyology, 25(4):157-160.
Gueldenstaedt, A. I. 1772. Salmo leucichthys et Cyprinus chalcoïdes descripti. Novi Commentarii Academiae Scientiarum Imperialis Petropolitanae, 16(1771):531-547. In Latin.
Gueldenstaedt, A. I. 1773. Cyprinus capoeta et Cyprinus mursa. Novi Commentarii Academiae Scientiarum Imperialis Petropolitanae, 17(1772):507-521, pls. VIII-IX. In Latin.
Gul, A., Yilmaz, M. and Solak, K. 1996. Growth characteristics of Capoeta trutta (Heckel, 1843) living in Tohma stream of Firat River. Turkish
Journal of Zoology, 20(Supplement):177-185.
Gül, S., Çolak, A. and Sezgin, I. 2000. Gümüş Balığı'nda (Chalcalburnus mossulensis Heckel, 1843) Karyotip Analizi [Karyotype
analysis in Chalcalburnus mossulensis (Heckel, 1843)]. Turkish Journal of Biology, 24(3):657-662.
Güldenstaedt, A. I. 1778. Cyprinus barbus et Cyprinus capito descripti. Acta Academiae Scientiarum Imperialis Petropolitanae, 2:239-260, pls. VIII-X. In Latin.
Gümüş, A., Sahinöz, E., Doğu, Z. and Polat, N. 2010. Age and growth of the Mesopotamian spiny eel, Mastacembelus mastacembelus (Banks & Solender, 1794), from southeastern Anatolia.
Turkish Journal of Zoology, 34(3):399-407.
Gümüş, A., Yılmaz, M. and Polat, N. 2002. Relative importance of food items in feeding of Chondrostoma regium Heckel, 1843, and its relation with the time of annulus formation.
Turkish Journal of Zoology, 26(3):271-278.
Günther, A. 1859-1870. Catalogue of the fishes in the British Museum. London. 8 volumes.
Günther, A. 1869. The fishes of the Holy Land. The Student and Intellectual Observer of Science, Literature and Art, 3:409-417.
Günther, A. 1874. A contribution to the fauna of the River Tigris. The Annals and Magazine of Natural History, 4(14):36-38, pl. VIII-IX.
Günther, A. 1889. Fishes, pp. 106-109, pl. XII. In: Aitchison, J. E. T. The Zoology of the Afghan Delimitation Commission. Transactions of the Linnaean Society of London, Second Series, 5.
Günther, A. 1896. Description of two new species of fishes (Mastacembelus and Barbus). The Annals and Magazine of Natural History, 6(17):397.
Günther, A. 1899. Fishes, pp. 381-391, pl. 23-24. In: Günther, R. T. Contributions to the natural history of Lake Urmi, N. W. Persia, and
its neighbourhood. Journal of Zoology of the Linnaean Society, 27:345-453, pl. 21-30.
Günther, R. T. 1899. Contributions to the natural history of Lake Urmi, N. W. Persia, and its neighbourhood. Journal of Zoology of the Linnaean Society, 27:345-453, pl. 21-30.
Guseva, T. V. 1974a. Ob ekologii neresta i embrional'nom razvitii morskogo sudaka - Lucioperca marina Cuv. [About the ecology of spawning and embryonal development of the marine pikeperch
- Lucioperca marina Cuv.]. Izvestiya Akademii Nauk Turkmenskoi SSR, Seriya Biogicheskikh Nauk, 2:87-91.
Guseva, T. V. 1974b. K voprosu o rasprostranenii i migratsiyakh morskogo sudaka v yugo-vostochnom Kaspii [On the matter of site conditions and migration
of the marine pikeperch in the south-eastern Caspian]. Izvestiya Akademii Nauk Turkmenskoi SSR, Seriya Biogicheskikh Nauk, 5:34-38.
Guseva, T. V. 1975. Postembryonic development of the sea pike-perch Lucioperca marina of the Caspian Sea. Journal of Ichthyology, 15(2):245-256.
Gussev, A. V., Jalali, B. and Molnár, K. 1993a. Six new species of the genus Dactylogyrus (Monogenea: Dactylogyridae) from Iranian freshwater fishes. Zoosystematica Rossica, 2(1):29-35.
Gussev, A. V., Jalali, B. and Molnár, K. 1993b. New and known species of Dactylogyrus Diesing, 1850 (Monogenea, Dactylogyridae) from Iranian freshwater cyprinid fishes.
Systematic Parasitology, 25(3):221-228.
H
Haas, R. 1982. Notes on the ecology of Aphanius dispar (Pisces, Cyprinodontidae) in the Sultanate of Oman. Freshwater Biology, 12:89-95.
Haas, R. and Pal, R. 1984. Mosquito larvivorous fishes. Bulletin of the Entomological Society of America, 30(1):17-25.
Habibnejaad, H. 1993. No more gill nets in the Caspian Sea. Abzeeyan, Tehran, 4(4):38-43, II. In Farsi.
Haddadi Moghaddam, K. 2006. Study on the usage of rotifer (Brachionus plicatilis) in A. persicus in larval stage. Iranian Fisheries Research
Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Haddadi Moghaddam, K., Ahmadi, M. R. and Kayvan, A. 2001. The study of effective zooplanktons in nutrition of Acipenser stellatus fingerlings in
earthen ponds. Iranian Journal of Fisheries Sciences, 10(2):1-14. In Farsi.
Haddadi Moghaddam, K. and Negaresten, H. 2003. Macrobenthos feeding and abundance of immatured sturgeons in the regions under 10 m depth of southern
part of Caspian Sea. 5th International Conference on Environmental Future, 23-27 March 2003, Zurich, Switzerland. 1 p.
Haddadi Moghadam, K., Parandavar, H., Pajand, Z. and Chubian, F. 2005. Feeding habits of sturgeon fishes in shallow coastal waters of Guilan Province, southern
Caspian Sea. Iranian Scientific Fisheries Journal, 14(3):37-48. In Farsi.
Haddadi Moghadam, K., Tavakoli, M., Pazhand, Z. A., Roufchahi, R., Choubian, F. and Parand Avar, H. 2009. Summer and winter feeding behaviour in Acipenser persicus and Acipenser stellatus
in the south Caspian Sea. Iranian Scientific Fisheries Journal, 18(2):13-26. In Farsi.
Hadgmohammadi, M. R. and Ghaziaskar, H. S. 1989. Quantitative and qualitative determination of heavy and toxic elements in the fishes grown in treated wastewater and their comparison
with other fishes. Iranian Journal of Chemistry and Chemical Engineering, 12, 20-31.
Hadi Alavi, S. M., Mojazi Amiri, B. and Pourkazemi, M. 2004. Total period of motility of Acipenser persicus spermatozoa in freshwater and saline
solution. Iranian Journal of Fisheries Sciences, 4(1):67-76, 122.
Hafezamini, P. and Oryan, Sh. 2002. The effects of NaCl on hematocrit and hemoglobin of Cyprinus carpio blood. Iranian Journal of Fisheries Sciences, 11(3):13-22. In Farsi.
Hafezamini, P., Oryan, Sh. and Parivar, K. 2003. Effects of NaCl on blood glucose and cortisol in common carp (Cyprinus carpio). Iranian Journal of Fisheries Sciences, 12(3):35-42. In Farsi.
Hafezieh M., Kamarudin M. S., Saad, C. R. B., Sattar, M. K. A., Agh, N. and Hosseinpour, H. 2009. Effect of enriched Artemia urmiana on growth, survival and composition of larval Persian
sturgeon. Turkish Journal of Fisheries and Aquatic Sciences, 9(2):201-207.
Hafezieh M., Kamarudin M. S., Saad, C. R. B., Sattar, M. K. A., Agh, N., Valinassab, T., Sharifian, M. and Hosseinpour, H. 2010. Effects of enriched Artemia urmiana with HUFA on growth,
survival, and fatty acids composition of the Pesrian sturgeon larvae (Acipenser persicus). Iranian Journal of Fisheries Sciences, 9(1):61-72.
Hagh-Panah, V. 1992. General values of the Anzali Bay. Abzeeyan, Tehran, 2:16-21. In Farsi.
Haghighi, D. T., Saad, C. R., Hosainzadeh Sahafi, H. and Mansouri, D. 2009. The effect of dietary lipid level on the growth of kutum fry (Rutilus frisii kutum). Iranian Journal of Fisheries
Sciences, 8(1):13-24.
Haghighi, F. P. 2006a. Investigation of by-catch of sturgeon fishery in the Caspian Sea. Iranian Fisheries Research Organization, Agriculture Research and Education
Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Haghighi, F. P. 2006b. Stock discrimination of Rutilus rutilus by using otoliths. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Haghighi, S. and Arabi, H. 2010. Water exploitation of Karoon River for fish culturing through monitoring and simulation systems. Iranian Journal of Fisheries Sciences, 9(2):209-218.
Haghparast, S., Aghaei Moghadam, A. A., Haji Moradlou, A. A., Pahlevani, M. H., Amini, K., Taheri, A. and Mohammadkhani, H. 2007. Prevalence of helminth parasites in digestive tract of Persian sturgeon
(Acipenser persicus) broodstocks southeast of the Caspian Sea. Iranian Scientific Fisheries Journal, 16(3):55-64. In Farsi.
Haghparast, S., Kashiri, H., Shabanpour, B. and Pahlavani, M. H. 2010. Antioxidant properties of sodium acetate sodium citrate and sodium lactate on lipid oxidation in rainbow trout
(Oncorhynchus mykiss) sticks during refrigerated storage (4ºC). Iranian Journal of Fisheries Sciences, 9(1):73-86.
Haig, J. 1952. Studies on the classification of the catfishes of the Oriental and Palaearctic Family Siluridae. Records of the Indian Museum, 48(1950):59-116.
Haji Hassani, N., Hasani, A. R., Farahnak Ghazani, M. and Kasebi, N. 2004. Heavy metals concentrations in the largest watershed basin of east Urumieh Lake,
Iran. The Joint Agriculture and Natural Resources Symposium, Tabriz-Ganja, May 14-16, 2004. 5 pp.
Hajimohamadi, R. 2002. Presentation of improvement methods for fishery management in order to improve product, quality, distribution and aquatics' consumption in the largest cities of Iran. M.Sc.
Thesis, Amirkabir University of Technology, Tehran. 73 pp. In Farsi.
Hajimoradiou (sic), A., Sargeran, P. and Moghanlou, K. S. 2002. The study of morphometric and meristic characteristics of Nemacheilus smithi, blind cave fish
in Iran. II International Conference, Loaches of the Genus Cobitis and Related Genera. Biology, Systematics, Genetics, Distribution, Ecology, Conservation. September 09-13, 2002, Olsztyn, Poland
(title).
Hajimoradloo (sic), A. A. 2002. The prevalence of Cystoopsis acipenseris in sturgeon (Chondrostei: Acipenseridae) from east-southern part of the Caspian
Sea. Journal of Agricultural Sciences and Natural Resources, 8(4):181-190. In Farsi.
Hajimoradloo, A., Abdoli, A. and Ghorbani Nasrabadi, R. 2002. The study of juveniles of Acipenser persicus Borodin 1897 and A. guldenstaedti Brandt 1833
diets in southeast of the Caspian Sea, Golestan Province., Iran. Journal of Agricultural Sciences and Natural Resources, 9(2):139-151. In Farsi.
Hajimoradloo, A., Abdoli, A. and Ghorbani Nasrabadi, R. 2002. The study of juveniles Acipenser persicus Borodin 1897 and A. guldenstaedti Brandt 1833 diets in
southeast of Caspian Sea, Golestan Province, Iran. 37th European Marien Bioology Symposium, Reykjavik, Iceland, 5-9 August 2002 (title).
Hajimoradlou (sic), A. A. and Agh, N. Bioencapsulation of oxolinic acid in Artemia urmiana as a means of prevention and treatment of bacterial infectious disease
(Aeromonas hydrophila) of Acipenser persicus larvae. Journal of Agricultural Sciences and Natural Resources, 13(2):155-164. In Farsi.
Hajimoradloo, A. and Ghorbani Nasrabadi, R. 2003. The prevalence of metazoan parasites of juvenile Acipenser persicus and Acipenser guldenstaedti
from southeast of Caspian Sea. Journal of Agricultural Sciences and Natural Resources, 10(1):151-160. In Farsi.
Hajirezaee, S., Mojazi Amiri, B. and Mirvaghefi, A. R. 2010a. Relationships between the chemical properties of seminal fluid and sperm motility characteristics of Caspian brown trout,
Salmo trutta caspius (a critically endangered salmonid fish). Research Journal of Fisheries and Hydrobiology, 5(1):27-31.
Hajirezaee, S., Mojazi Amiri, B. and Mirvaghefi, A. R. 2010b. Assessment of ability of endangered Caspian brown trout, Salmo trutta caspius (Salmonidae) in production of semen with desirable
quality. Journal of Ichthyology, 50(11):1070-1076.
Hajirezaee, S., Rafiee, G. R., Hushangi, R., Rahimi, R., Niksirat, H. and Kazemi, R. 2010. Germinal vesicle breakdown rates in oocytes and steroid levels in blood and ovarian fluid of the
Persian sturgeon Acipenser persicus. International Aquatic Research, 2:71-75.
Haajian (sic), A., Kazemi, R. A., Mohseni, M., Bahmani, M. and Yousefi, A. 2007. Determination of sex and sexual maturation stages in cultured Acipenser nudiventris using biopsy method.
Iranian Scientific Fisheries Journal, 16(3):65-72. In Farsi.
Halajian, A., Pourkazemi, M., Kalbasi, M. R. and Amini, K. 1999.An investigation on the number of micropyle contained in the ovum
of sturgeon in the south coast of Caspian Sea. Iranian Journal of Fisheries Sciences, 8(1):35-48, 4-5. In Farsi.
Hallajiyan, A. 1998. Study of the micropyle number and condition in Caspian Sea sturgeon eggs. M.Sc. Thesis, College of Marine Sciences, Tarbiat Modarres University, Noor. 233 pp.
Hallajiyan, A. and Pourkazemi, M. 2001. Comparison of micropyle number in Persian sturgeon, Acipenser persicus and stellate sturgeon, Acipenser
stellatus in the south Caspian Sea. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Hallajiyan, A., Pourkazemi, M. and Kalbasi, M. R. 1999. Determination and micropile number of Iranian sturgeon of Caspian Sea. First National Sturgeon Symposium, Rasht, 1999 (abstract).
Hallajiyan, A., Pourkazemi, M., Kalbasi, M. and Amini, K. 1999. An investigation on the micropyle number in the ova of the sturgeon species in Caspian Sea.
Iranian Journal of Fisheries Sciences, 1(2):49-59, 81.
Halstead, B. W. 1967-1970. Poisonous and Venomous Marine Animals of the World. Volume Two - Vertebrates. xxxi + 1070 pp., Volume Three - Vertebrates
continued. xxv + 1006 pp. United States Government Printing Office, Washington, D.C.
Halstead, B. W. 1978. Poisonous and Venomous Marine Animals of the World (Revised Edition). The Darwin Press, Princeton, New Jersey. xlvi + 1043 pp., 283 pages of plates.
Hamblin, D. J. 1987. Has the Garden of Eden been located at last? Smithsonian, 18(2):127-135.
Hamid, I. W., Agopian, T. and Tigranian, E. A. 1977. Ryby vnutrennych vod Iraka [Inland water fishes of Iraq]. Baghdad. 89 pp. (not seen).
Hamilton, A. 2002. The beluga blues. Time, 160(26):34-35.
Hamilton, A. M. 1937. Road through Kurdistan. The Narrative of an Engineer in Iraq. Faber & Faber Ltd., London. 331 pp., 2 maps.
Hanel, L., Oliva, O. and Ali, A. M. 1992. Note on the morphometry of Varicorhinus trutta (Pisces, Cypriniformes) from Iraq. Acta Societatis Zoologicae Bohemoslovacae, 56(2):115-119, photos 1-2.
Hanel, L., Oliva, O. and Atallah, M. A. 1993. Morphometric note on Barbus sharpeyi (Pisces: Cypriniformes: Cyprinidae) from Iraq. Acta Societatis Zoologicae Bohemicae, 57(2):101-103.
Hänfling, B. and Brandl, R. 2000. Phylogenetics of European cyprinids: insights from allozymes. Journal of Fish Biology, 57(2):265-276.
Hänfling, B., Dümpelmann, C., Bogutskaya, N. G., Brandl, R. and Brändle, M. 2009. Shallow phylogeographic structuring of Vimba vimba across Europe suggest two distinct refugia
during the last glaciation. Journal of Fish Biology 75(9):2269-2286.
Haniffa, M. A., Dhanaraj, M., Ramakrishnan, C. M., Sethuramalingam, T. A., Singh, S. V. A., Kumar, Y. A. and Manju, R. A. 2008a. Threatened fishes of the world: Heteropneustes fossilis
(Bloch, 1794)(Cypriniformes: Saccobranchidae). Environmental Biology of Fishes, 82(2):203-204.
Haniffa, M. A., Dhanaraj, M., Ramakrishnan, C. M., Sethuramalingam, T. A., Singh, S. V. A., Kumar, Y. A. and Manju, R. A. 2008b. Threatened fishes of the world: Heteropneustes fossilis (Bloch,
1794)(Siluriformes: Heteropneustidae). Environmental Biology of Fishes, 82(2):205.
Hanko, B. 1924. Fische aus Kleinasien. Annales Musei nationalis Hungarici, 21:137-158, Taf. iii.
Hansman, J. F. 1978. The Mesopotamian delta in the First Millennium, BC. Geographical Journal, 144:49-61.
Harbison, R. 2002. The use of butterfish, Peprilus triacanthus, as a biological control agent in the Caspian. Firste International Meeting "The invasion of the Caspian
Sea by the comb jelly Mnemiopsis - problems, perspectives, need for action", Baku, Azerbaijan, 24-26 April 2001. Caspian Environment
Programme, Baku, Azerbaijan. Unpaginated PowerPoint presentation.
Hariri, D. 1966. A study of inter-relationship between surface water and ground water in the Karaj River basin, pp. 227-241. In: Symposium on
Hydrology and Water Resources Development held in Ankara, Turkey February 7 to 12, 1966, Central Treaty Organization, Ankara. 484 pp.
Harlioğlu, A. G. and Yilmaz, Ö. 2011. Fatty acid composition, cholesterol and fat-soluble vitamins of wild-caught freshwater spiny eel, Mastacembelus simack (Walbaum, 1792). Journal of
Applied Ichthyology, 27(4):1123-1127.
Harrington, F. A. 1976. Iran: Wildlife research as a basis for management. In: Proceedings of an International Meeting on ecological guidelines for
the use of natural resources in the Middle East and South West Asia held at Persepolis, Iran 24-30 May 1975. International Union for the Conservation
of Nature and Natural Resources Publications, new series, 34:114-132.
Harrison, F. 2005. Lean times for Iran caviar fishermen. BBC News/Middle East, 14 November 2005, 3 pp. (http://news.bbc.co.uk, downloaded 11 January 2007).
Harrison, J. V. 1937. The history of the river system of South Iran. International Geological Congress, XVII Session, Moscow and Leningrad, p. 227-228.
Harrison, J. V. 1941. The Jaz Murian depression, Persian Baluchistan. Geographical Journal, 101:206-225.
Harrison, J. V. 1942. The Shatt-el-Arab. Journal of the Royal Central Asian Society, 29(1):43-51.
Harrison, J. V. 1968. Geology, pp. 111-185. In: Fisher, W. B. (Ed.). The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press, Cambridge. ix + 784 pp.
Harrison, J. V., Sherwin-White, A. N. and Mason, K. 1945. Persia. Geographical Handbook Series, Naval Intelligence Division, London. xix + 638 pp., 1 map.
Hartl, M. 1979. Das Najafabadtal: Geographische Untersuchung einer Kanatlandschaft im Zagrosgebirge (Iran). Regensburger Geographische Schriften, 12:172 pp., 24 Fotos, 10 Karte.
Harwit, M. 1990. From qanats to qantas. Air and Space, August/September, 1990:4.
Hasanabadizadeh, Z., Hajimoradlou, A. A., Ghorbani, R., Khoshbavar Rostami, H. A. and Soleymani, N. 2008. The effects of vitamins injection (A, C, A+C and AD3E) on wound healing process and
some hematological response in common carp, Cyprinus carpio. Journal of Agricultural Sciences and Natural Resources, 15(6):117-124. In Farsi.
Hasanzadeh Kiabi, B., Madjnonian H., Goshtab Meygouni, H. and Mansori, J. 2004. Criteria for assessing the conservation status of Iranian wetland. Journal of Environmental Studies, 30(33):74-89.
In Farsi.
Hashemi, H. S. 2001. Caspian Phoca (seal), Green Wave, Tehran, 5:14-17. In Farsi (In English at www.netiran.com/Htdocs/WeeklyJournal/Social/wj00685.html, downloaded 5 November 2001).
Hashemi, S., Mohammadi, G. and Eskandary, Gh. 2010. Population dynamics and stock assessment of hilsa shad, (Tenualosa ilisha Hamilton-Buchanan, 1822) in coastal waters of Iran
(northwest of Persian Gulf). Australian Journal of Basic and Applied Sciences, 4(12):5780-5786.
Hashemi, S., Mortezavi, A. and Kashi, M. 2010. Population dynamics and assessment of Barbus grypus (Heckel, 1843) and Barbus barbulus (Heckel, 1847) in Karoon River. Research Journal of
Fisheries and Hydrobiology, 5(2):119-128.
Hashemi, S. A. R., Mohammadi, G. Safikhani, H. and Kashi, M. 2009. Population dynamics and stock assessment of Hilsa shad and Tenualosa ilisha in Khuzestan Province coast (northwest of Gulf).
The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 121 (abstract).
Hashemian, A. 1996. Food and feeding habits of Huso huso, caught by gillnet in southern part of the Caspian Sea. Iranian Fisheries Scientific Journal, 5(3):61-70, 6. In Farsi.
Hashemyan, A., Khoshbavar Rostami, H. A. and Taleshian, H. 2005. A comparative analysis of feeding habits of sturgeon fish in shallow coastal waters of Mazandaran and Golestan Provinces,
south Caspian Sea. Iranian Scientific Fisheries Journal, 14(3):157-166. In Farsi.
Hashemy-Tonkabony, S. E. and Asadi Langaroodi, F. 1976. Detection and determination of chlorinated pesticide residues in Caspian Sea fish by gas-liquid chromatography. Environmental Research,
12(3):275-280.
Hassan Nia, M. R. 1995. Fishing information analysis of Acipenser stellatus by use of chronological series. Iranian Fisheries Scientific Journal, 4(1):3-14, 2. In Farsi.
Hasselquist, F. 1757. Iter Palaestinum eller Resa til Heliga Landet Förrättad ifrån År 1749 till 1752 Utgiven av Carl
Linnaeus (Iter Palaestinum or A Voyage to the Holy Land Undertaken from the Year 1749 to 1752 Published by Carl Linnaeus in 1757). Rediviva Publishing House, Stockholm (1969 Reprint).
Hatami, H. 1998. Age structure and growth of Capoeta capoeta gracilis in Tilabad River. B.Sc. Seminar, Agricultural Sciences and Natural Resources University, Gorgan. 85 pp. In Farsi.
Hatef, A., Niksirat, H., Mojazi Amiri, B., Alavi, S. M. H. and Karami, M. 2007. Sperm density, seminal plasma composition and their physiological
relationship in the endangered Caspian brown trout (Salmo trutta caspius). Aquaculture Research, 38:1175-1181.
Haynes, S. J. 1981. Towards a paleogeography and tectonic evolution of Iran: Discussion. Canadian Journal of Earth Sciences, 18:1763-1764.
Heckel, J. J. 1843a. Abbildungen und Beschreibungen der Fische Syriens nebst einer neuen Classification und Characteristik sämmtlicher Gattungen der Cyprinen. Stuttgart, 109 pp.
Heckel, J. J. 1843b. Ichthyologie.In: Russegger, J. Reisen in Europa, Asien und Afrika, mit besonderer Rücksicht auf die naturwissenschaftlichen
Verhältnisse der betreffenden Länder, unternommen in den Jahren 1835 bis 1841 von Joseph Russegger. Schweitzerbart'sche Verlagsbuchhandlung,
Stuttgart, 1(2):991-1099, Taf. II-XIII (separate but identical version of the above).
Heckel, J. J. 1846-1849a. Naturhistorischer Anhang. In: Russegger, J.Reisen in Europa, Asien und Afrika, mit besonderer Rücksicht auf die naturwissenschaftlichen
Verhältnisse der betreffenden Länder, unternommen in den Jahren 1835 bis 1841 von Joseph Russegger. Schweitzerbart'sche Verlagsbuchhandlung, Stuttgart, 2(3):207-254, Taf. XIV-XXI.
Heckel, J. J. 1846-1849b. Anhang. Die Fische Persiens gesammelt von Theodor Kotschy. In: Russegger, J.Reisen in Europa, Asien und Afrika, mit
besonderer Rücksicht auf die naturwissenschaftlichen Verhältnisse der betreffenden Länder, unternommen in den Jahren 1835 bis 1841 von
Joseph Russegger.Schweitzerbart'sche Verlagsbuchhandlung, Stuttgart, 2(3):255-272, Taf. XXII.
Heckel, J. J. 1846-1849c. Nachtrag zur Charakteristik und Classifikation der Cyprineen-Gattungen. In: Russegger, J. Reisen in Europa, Asien und
Afrika, mit besonderer Rücksicht auf die naturwissenschaftlichen Verhältnisse der betreffenden Länder, unternommen in den Jahren 1835 bis 1841 von
Joseph Russegger. Schweitzerbart'sche Verlagsbuchhandlung, Stuttgart, 2(3):273-290.
Heckel, J. J. 1846-1849d. Index. Addenda et Corrigenda. In: Russegger, J. Reisen in Europa, Asien und Afrika, mit besonderer Rücksicht auf
die naturwissenschaftlichen Verhältnisse der betreffenden Länder, unternommen in den Jahren 1835 bis 1841 von Joseph Russegger. Schweitzerbart'sche Verlagsbuchhandlung, Stuttgart, 2(3):347-360.
Heckel, J. J. und Kner, R. 1858. Die Süsswasserfische der Oesterreichischen Monarchie mit Rücksicht auf die angrenzenden Länder. Leipzig. 388 pp., 204 figs.
Heydarnezhad, M. S. and Purser, G. J. 2009. Agonistic acts as possible indicator of food anticipatory activity (FAA) in rainbow trout (Oncorhynchus mykiss). Iranian Journal of Veterinary
Research, Shiraz University, 10(2)(27):137-145.
Hedayati, S. A. A., Bagheri, T., Yavari, V., Bahmani, M. and Alizadeh, M. 2008. Examination of some biochemical factors of blood serum in beluga (Huso huso) cultured in brackish water. Iranian
Journal of Biology, 21(4):658-666.
Hedayati, S. A. A., Yavari, V. and Bagheri, T. 2009. The examination of effect of somatic growth indices on gonadic growth indices in immature great sturgeon (Huso huso). The 6th Scientific
Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 117 (abstract).
Hedayatifard, M. 2003. Fish and Shrimp Processing Technology. Persia Fishing Industries Company, Tehran. 120 pp.
Hedayatifard, M. and Aroujalian, A. R. 20101. Improvement of shelflife for stellate sturgeon fillet, Acipenser stellatus, under Modified Atmosphere Packaging (MAP) and vacuum conditions.
Iranian Scientific Fisheries Journal, 19(3):127-140. In Farsi.
Hedayatifard, M. and Jamali, Z. 2008. Evaluation of omega-3 fatty acids composition in Caspian Sea pike perch (Sander lucioperca). International Journal of Agriculture and Biology, 10(2):235-237.
Hedayatifard, M. and Keyvan, A. 2009. Changes of fatty acid composition of Persian sturgeon Acipenser persicus affected by freezing and long term cold storage. The 6th International
Symposium on Sturgeon, Wuhan, Hubei Province, China, 25-30 October, 2009. 3 pp.
Hedayatifard, M. and Moeini, S. 2004. Quantitative and qualitative identification of the fatty acids in Persian sturgeon tissue (Acipenser
persicus) and effect of long term freezing on them. Agricultural Science, 14(23):123-132. In Farsi.
Hedayatifard, M. and Moeini, S. 2007. Loss of omega-3 fatty acids of sturgeon (Acipenser stellatus) during cold storage. International Journal of Agriculture and Biology, 9(4):598-601.
Hedayatifard, M., Moeini, S., Keyvan, A. and Yousefian, M. 2002. Quantitative and qualitative identification of fatty acids in muscle of golden mullet (Liza aurata). Iranian Journal of
Marine Sciences, 1(2):73-78. In Farsi.
Hedayatifard, M., Moeini, S., Keyvan, A. and Yousefian, M. 2003. The variation of fatty acids composition of sevruga tissues in cold storage condition. Pajouhesh va Sazandegi, 15(3-4)(55-57):72-75.
In Farsi.
Hedayatifard, M. and Ramezani, H. 2007. Applied Ichthyology. Islamic Azad University, Ghaemshahr. xii + 206 pp. In Farsi.
Hedayatifard, M. and Yousefian, M. 1997. Research on the effects of environmental factors on the development of sturgeon gonads. Iranian Fisheries Research Organization, Sari. 28 pp.
Hedayatifard, M. and Yousefian, M. 2001. Oogenesis studies of southern Caspian Sea sturgeon. Fourth International Symposium of Sturgeon, 7-13 July 2001, Oshkosh, Wisconsin (abstract).
Hedayatifard, M. and Yousefian, M. 2007. Investigation on shelf life and changes of lipid and fatty acid composition of sturgeon (Acipenser stellatus) in frozen storage. Fishery
Technology, 44(2):193-198.
Hedayatifard, M. and Yousefian, M. 2009a. Composition of fatty acid in golden mullet (Liza aurata) fillet affected by dry-salting. The 6th Scientific Conference of Fisheries Resources,
3-4 March 2009, Basrah, p. 106 (abstract).
Hedayatifard, M. and Yousefian, M. 2009b. The fatty acid composition of golden mullet fillet Liza aurata as affected by dry-salting. Journal of Fisheries and
Aquatic Sciences 5(3):208-215.
Hedayatifard, M. and Yousefian, M. and Ahmadi, M. R. 2009. Investigation of oogenesis of the Russian sturgeon from Caspian Sea Acipenser guldenstaedti. The 6th International Symposium
on Sturgeon, Wuhan, Hubei Province, China, 25-30 October, 2009. 3 pp.
Hedayatifard, M. and Yousefian, M. and Amini, R. 2004. Surveying of two different dietary effects on the growth of cultured beluga larvae (Huso huso). European Aquaculture 2004, 20-23
October 2004, Barcelona (abstract).
Hedayatifard, M., Yousefian, M. and Jamali, Z. 2006. Feeding of cultured great sturgeon larva Huso huso by compound food. Proceedings of the Third International Congress on Aquaculture,
Fisheries Technology and Environmental Management, 3-4 November 2006, AquaMedit 2006, Athens (abstract).
Heidari, B., Shabanipour, N., Yavari, L. and Totani, M. M. 2009. Histomorphological investigation of Liza aurata (Risso, 1810) (Mugilidae) ovary in the late oogenesis in the Caspian Sea. Iranian
Journal of Fisheries Sciences, 8(1):25-36.
Heidinger, R. C. 1976. Synopsis of biological data on the largemouth bass Micropterus salmoides (Lacepède) 1802. Food and Agriculture Organization, Rome, Fisheries Synopsis,
115:viii + 85 pp.
Henderson, P. A. and Bamber, R. N. 1987. On the reproductive biology of the sand smelt Atherina boyeri Risso (Pisces: Atherinidae) and
its evolutionary potential. Biological Journal of the Linnaean Society, 32:395-415.
Hensel, K. 1978. Morphology of lateral-line canal system of the genera Abramis, Blicca and Vimba with regard to their ecology and systematic position.
Acta Universitatis Carolinae, Biologica, 1975-1976:105-149, 2 figs.
Herzenstein, S. 1888-1891. Nauchnye rezul'taty puteshestvii N. M. Przheval'skogo po Tsentral'noi Azii. III (2) Ryby. [Scientific Results of N. M. Przeheval'skii's
Travels in Central Asia. III(2) Fishes]. Akademia Nauk, Sankt-Peterburg, 262 pp.
Herzig-Straschil, B. 1997. Franz Steindachner (1834-1919) and other prime contributors to the Ichthyological Collection of the Naturhistorisches
Museum Wien, pp. 101-108. In: Pietsch, T. W. and Anderson, W. D. (Eds.). Collection Building in Ichthyology and Herpetology. American Society of Ichthyologists and Herpetologists, Special Publication,
3:xiii + 593 pp.
Herzog, P. 1967. Die Binnenfischerei im Irak. Die Fischwirt, 17(10):249-255.
Herzog, P. 1969. Untersuchungen über die Parasiten der Süßwasserfische des Irak. Archiv für Fischereiwissenschaft, 20:132-147.
Heydarnejad, M. S. 2009. Length-weight relationships for six freshwater fish species in Iran. Chinese Journal of Oceanology and Limnology, 27(1):61-62.
Heydarpour, E 1978. Erste Erfahrung mit dem Aufbau einer Karpfenteichwirtschaft im Iran. Österreichs Fischerei, 31(2-3), 46-48.
Hieronimus, H. 1990. Homalopteridae Bleeker, 1859 (Osteichthyes, Cypriniformes): proposed precedence over
balitoridae Swainson, 1839. Bulletin of Zoological Nomenclature, 47(4):277-279.
Hieronimus, H. 1991. Comment on the proposed precedence of homalopteridae Bleeker, 1859 overbalitoridae
Swainson, 1839 (Osteichthyes, Cypriniformes). Bulletin of Zoological Nomenclature, 48(3):253.
Higgins, R. P. 1973. Survey of pesticide residues and heavy metals in Caspian Sea biota from Bandarpahlavie, Iran. Oceanography and Limnology
Program, Office of Environmental Sciences, Smithsonian Institution, Washington, D.C. Mimeo, 11 pp., including Winnett, G. Levels of DDT and related compounds
in sturgeon, sturgeon caviar and other fish from the Caspian Sea near Bandarpahlavie, Iran. March and August, 1971. Attachment 1:i-iv and Straat, P. Attachment 2 (latter not seen).
Hill, A. and Whybrow, P. J. 1999. Summary and overview of the Baynunah fauna, Emirate of Abu Dhabi, and its context, p. 7-14. In: Whybrow, P.
J. and Hill, A. (Eds.). Fossil Vertebrates of Arabia with Emphasis on the Late Miocene Faunas, Geology, and Palaeoenvironments of the Emirate of
Abu Dhabi, United Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Hochleithner, M. 2010. Aale (Anguillidae) Biologie und Kultur. AquaTech Publications, Kitzbühel. 156 pp.
Hochleithner, M. and Gessner, J. 1999. The Sturgeons and Paddlefishes (Acipenseriformes) of the World. Biology and Aquaculture. AquaTech Publications, Kitzbuehel. 166 pp.
Hoda, S. M. S. 1986. Maturation and fecundity of the mudskipper Boleophthalmus dussumieri Cuv. & Val. from the Karachi coast. Mahasagar, Bulletin of the National Institute of Oceanography,
19(1):73-78.
Hoda, S. M. S. 1987. Relative growths of body parts and length-weight relationships in Boleophthalmus dussumieri and B. dentatus of Karachi coast. Indian Journal of Fisheries,
34(1):120-127.
Hoda, S. M. S. and Akhtar, Y. 1985. Maturation and fecundity of the mudskipper Boleophthalmus dentatus in the northern Arabian Sea. Indian Journal of Fisheries, 32:64-74.
Hoedemann, J. J. 1951. Rediagnosis of the Old World cyprinodont genus Aphanius. Beaufortia, 1:1-6.
Hoedemann, J. J. 1958. The frontal scalation pattern in some groups of toothcarps (Pisces, Cyprinodontiformes). Bulletin of Aquatic Biology, 1:23–28.
Hoestlandt, H. (Ed.). 1991. The Freshwater Fishes of Europe. Volume 2. Clupeidae, Anguillidae. AULA-Verlag, Wiesbaden. 448 pp.
Hoffman, G. L. 1970. International control of parasitic diseases of fish. Journal of Parasitology, 56(4)(Section II, Part I):151-152.
Hoghooghi-Rad, N., Maraghi, S. and Narenj-Zadeh, A. 1987. Capillaria philippinesis infection in Khoozestan Province, Iran: case report. American
Journal of Tropical Medicine and Hygiene, 37(1):135-137.
Holčík, J. (Ed.). 1986. The Freshwater Fishes of Europe. Volume 1, Part I. Petromyzontiformes. AULA-Verlag, Wiesbaden. 313 pp.
Holčík, J. (Ed.). 1989. The Freshwater Fishes of Europe. Volume 1, Part II. General Introduction to Fishes. Acipenseriformes. AULA-Verlag, Wiesbaden. 469 pp.
Holcik, J. 1992. New data from the ecology of Rutilus frisii kutum (Kamenskij 1901) juveniles. Proceedings of the Scientific Conference on Fish Reproduction '92, Vodnany,
Czechoslovakia, 2-4 March, 1992, pp. 139-140.
Holcik, J. 1993. Iranian acetra, Acipenser persicus Borodin. Abzeeyan, Tehran, 4(8):26-32, II. In Farsi.
Holcik, J. 1994. New data on the ecology of kutum Rutilus frisii Nordmann, 1840 from the Caspian Sea.VIII Congress Societas Europaea Ichthyologorum,
Oviedo, Spain, September 26 to October 2, 1994, p. 117-1118 (abstract).
Holčík, J. 1995. New data on the ecology of kutum, Rutilus frisii (Nordmann, 1840) from the Caspian Sea. Ecology of Freshwater Fish, 4(4):175-179.
Holčík, J. and Duyvené de Wit, J. J. 1964. Systematic status of the bitterlings from Asia Minor and notes on the geographical variability of
Rhodeus sericeus (Pallas, 1776) in the area of its distribution. Zeitschrift für Wissenschaftliche Zoologie, Leipzig, 169(3/4):396-412.
Holčík, J. and Jedlička, L. 1994. Geographical variation of some taxonomically important characters
in fishes: the case of the bitterling Rhodeus sericeus. Environmental Biology of Fishes, 41(1-4):147-170.
Holcik, J. and Olah, J. 1990. Iran. Anzali Lagoon productivity and fish stock investigations. Progress Report from the Joint Mission (7 August
to 14 September 1990). Food and Agriculture Organization, Rome, IRA/88/001, Working Document 3:9 pp., Appendix 1:1 p., Appendix 2:5 pp., Appendix 3:2 pp.
Holčík, J. and Oláh, J. 1992. Fish, fisheries and water quality in Anzali Lagoon and its watershed. Report prepared for the project - Anzali
Lagoon productivity and fish stock investigations. Food and Agriculture Organization, Rome, FI:UNDP/IRA/88/001 Field Document 2:x + 109 pp.
Holčík, J. and Razavi, B. A. 1991. New species of freshwater fish from the Iranian coast of the Caspian Sea. Report prepared for the Anzali Lagoon
productivity and fish stocks investigation project. Food and Agriculture Organization, Rome, FI/UNDP/IRA/88/001 Field Document 1. En; en., 14 pp.
Holčík, J. and Razavi, B. A. 1992. On some new or little known freshwater fishes from the Iranian coast of the Caspian Sea. Folia Zoologica, Prague, 41(3):271-280.
Holčík, J. and Skořepa, V. 1971. Revision of the roach Rutilus rutilus (Linnaeus, 1758), with regard to its subspecies. Annotationes Zoologicae et Botanicae, Bratislava, 64:1-60.
Hollis, G. E. 1978. The falling levels of the Caspian and Aral seas. Geographical Journal, 144:62-80.
Holloway, C. W. 1976. Conservation of threatened vertebrates and plant communities in the Middle East and South West Asia. In: Proceedings of
an International Meeting on ecological guidelines for the use of natural resources in the Middle East and South West Asia held at Persepolis, Iran
24-30 May 1975. International Union for the Conservation of Nature and Natural Resources Publications, new series, 34:179-188.
Holly, M. 1929a. Drei neue Fischformen aus Persien. Anzeiger der Oesterreichischen Akademie der Wissenschaften Mathematisch-Naturwissenschaftliche Klasse, Wien, 66:62-64.
Holly, M. 1929b. Beiträge zur Kenntnis der Fischfauna Persiens. Zoologischer Anzeiger, 85:183-185.
Holmes, W. R. 1845. Sketches on the Shores of the Caspian, descriptive and pictorial. Richard Bentley, London. xii + 412 pp.
Homski, D., Goren, M. and Gasith, A. 1994. Comparative evaluation of the larvivorous fish Gambusia affinis and Aphanius dispar as mosquito control agents. Hydrobiologia, 284(2):137-146.
Honary, M. 1980. Qanat and Human Ecosystems in Iran. Unpublished Thesis, University of Edinburgh.
Hoosini Najd Gerami, E. and Hajimoradlu, A. 2005. Effect of temperature on the artificial propagation of Persian sturgeon (Acipenser persicus).
Journal of Science (Teacher Training University), 4(3-4):421-426. In Farsi.
Höpner, T. and Kazem Maraschi, S. M. 1999. Intertidal treasure Khowr-e Mussa - unraised. Wadden Sea Newsletter, 1:3-6.
Hora, S. L. 1921. Indian cyprinoid fishes belonging to the genus Garra, with notes on related species from other countries. Records of the Indian Museum, 22:633-687, pl. XXIV-XXVI.
Hora, S. L. 1922. Notes on fishes in the Indian Museum. III. On fishes belonging to the family Cobitidae from high altitudes in Central Asia. Records of the Indian Museum, 24:63-83.
Hora, S. L. 1929. The habitat and systematic position of two imperfectly known loaches from Afghanistan. Journal and Proceedings of the Asiatic Society of Bengal, 24(1928):481-484.
Hora, S. L. 1933a. Notes on fishes in the Indian Museum. XX. Loaches of the genus Nemachilus from Baluchistan. Records of the Indian Museum, 35:183-191, pl. V.
Hora, S. L. 1933b. Fish of Afghanistan. Journal of the Bombay Natural History Society, 36:688-706.
Hora, S. L. 1935. On a collection of fish from Afghanistan. Journal of the Bombay Natural History Society, 37:784-802.
Hora, S. L. 1937. Geographical distribution of Indian freshwater fishes and its bearing on the probable land connections between India and the adjacent countries. Current Science, Bangalore, 5:351-356.
Hora, S. L. 1956. Fish paintings of the third millennium B.C. from Nal (Baluchistan) and their zoogeographical significance. Memoirs of the Indian Museum, 14(2):73-86, pls. IV-VI.
Hora, S. L. and Misra, K. S. 1943. On a small collection of fish from Iraq. Journal of the Asiatic Society of Bengal, Science, 9(1):1-15.
Horváth, L., G. Tamás and A.G. Coche, 1985a. Common carp. Part. 1. Mass production of eggs and early fry., FAO Training Series 8, Food and Agriculture Organization of the United Nations, Rome. 87 pp.
Translated into Farsi by A. S. Mohagheghi and M. Hedayatifard, Islamic Azad University, Ghaemshahr, 2010.
Horváth, L., G. Tamás and A.G. Coche, 1985b. Common carp. Part. 2. Mass production of advanced fry and fingerlings in ponds. FAO Training Series 9, Food and Agriculture Organization of the United
Nations, Rome. 85 pp. Translated into Farsi by A. S. Mohagheghi and M. Hedayatifard, Islamic Azad University, Ghaemshahr, 2010.
Hoseinie, S. E. 1995. Artificial propagation of (Aspius aspius) (sic). Iranian Fisheries Scientific Journal, 3(4):23-31, 4. In Farsi.
Hossain, M. Y., Ohtomi, J., Ahmed, Z. F., Ibrahim, A. H. M. and Jasmine, S. 2009. Length-weight and morphometric relationships of the tank goby Glossogobius giuris (Hamilton, 1822)
(Perciformes: Gobiidae) in the Ganges of northwestern Bangladesh. Asian Fisheries Science, 22(3):961-969.
Hosseini, A. 1998. An investigation on food habits and spawning season of Acanthopagrus latus (Sparidae) in the Persian Gulf. Iranian Journal of Fisheries Sciences, 7(3):13-20, 2. In Farsi.
Hosseini, M. 1998. The end of unresponsible sturgeon fishing. The Seventh National Fisheries Conference, Responsible Fisheries. Shilat Company, Tehran. pp. 115-128. In Farsi.
Hosseini, S. H. 1987. The study of infection of fish with Clinostomum sp. in the lake south of Tehran. Ph.D. Thesis, Department of Veterinary Medicine, University of Tehran. In Farsi.
Hosseinie, M. R. 1996. Iran - producer of the best caviar in the world. Abzeeyan, Tehran, 7(8):50-52. In Farsi (translation of a Mail on Sunday, London article, 4 August 1996).
Hosseinie, S. A. 1997. Reasons for deformation of rainbow trout in hatcheries with emphasis on intraspecific breeding. Ph.D. Thesis, Islamic Azad University, North Tehran Division. 105 pp. In Farsi.
Hosseinie, S. N., Seyfabadi, S. J., Kalbasi, M. R. and Vilaki, A. S. 2002. Effect of L-carnitine supplement on early growth and body composition of rainbow trout (Oncorhynchus mykiss).
Iranian Journal of Marine Sciences, 1(2):41-45, 85. In Farsi.
Hosseini(e), S. V., Behroooz, R. D., Esmaili-Sari, A., Bahramifar, N., Hosseini, S. M., Tahergorabi, R., Hosseini, S. F. and Feás, X. 2008. Contamination by organochlorine compounds in the
edible tissue of four sturgeon species from the Caspian Sea (Iran). Chemosphere, 73(6):972-979.
Hosseinie, S. V. and Kalbasi, M. R. 2003. Karyological study on snow trout, Schizothorax zarudnyi, in Zahak of Sistan-Balochestan Province - Iran.
Iranian Journal of Marine Sciences, 2(1):13-22. In Farsi.
Hosseinie, S. V., Rezaee, M., Sahari, M. A. and Hosseini, H. 2004. The effect of ice storage on lipid quality and sensory evaluation of golden muillet (Liza aurata).
Iranian Journal of Marine Sciences, 3(1):31-40. In Farsi.
Hosseine Najd, G. E., Manaffar, F., Meshkini, S. and Salimi Behnam, S. 2007. An investigation on the effect of initial feeding on growth rate of trout (Oncorhynchus mykiss) larval stage.
Journal of Science (Teacher Training University), 7(1-2):874-854. In Farsi.
Hosseini Najde, G. E. and Hajimoradloo, A. 2002. Morphophysiologic changes in Persian sturgeon female broodstock (Acipenser
persicus ) in the Southeast Caspian Sea. The Second National – Regional Symposium on Sturgeon, Rasht, October 26-28, 2002 (abstract).
Hosseini Najd, G. E. and Haji Moradlou, A. A. 2004. Effect of temperature on the artificial propagation of Persian sturgeon (Acipenser persicus). Journal of Science, Teacher Training University,
4(3-4):421-426. In Farsi.
Hosseini Najd Geramei (sic), E. and Irani, A. A. J. 2006. The effects of photoperiod on the growth, survival and feeding parameters in the rainbow trout (Oncorhynchus mykiss) larvae.
Pajouhesh va Sazandegi, 19(3)(72):2-5. In Farsi.
Hosseini Najdegrami, I., Hajimoradlou, A. A., Kamali, A. G. and Karimabadi, A. 2005. Reproductive conditions of the Iranian sturgeon (Acipenser persicus) in the southeast of the Caspian Sea,
Golestan Province, Iran. Journal of Agricultural Sciences and Natural Resources, 11(4):141-150. In Farsi.
Hosseini Seyed, S., Kavousi Kelashemi, M. and Darijani, A. 2008. The comparative advantages and ranking export goal markets of Iran caviar. Journal of Agricultural Sciences and Natural Resources,
15(3):1-8. In Farsi.
Hosseinpour, N. 1995. Survey on macro-zoobenthic resources in Siahdarvishan and Pasikhan Rivers, Potamon Zone (Quantitative). Iranian Fisheries Scientific Journal, 4(3):8-21, 3. In Farsi.
Hosseinzadeh, H. 2000. Status of aquaculture in Iran: Past, present, future. The Third World Fisheries Congress, Beijing, 31 Oct - 2 Nov 2000 (title).
Hosseinzadeh, H. 2003. Status of aquaculture in Iran: Past, present, future. American Fisheries Society Symposium, 38:9-17.
Hossini, A. and Savari, A. 2004. Some aspects of reproductive biology of yellowfin seabream Acanthopagrus latus in the coastal waters of Busher
(Persian Gulf). Iranian Journal of Marine Sciences, 3(1):41-50. In Farsi.
Hourcade, B., Mazurek, H., Papoli-Yazdi, M.-H. and Taleghani, M. 1998. Atlas d'Iran. Reclus - La Documentation Française, Montpellier. 192 pp.
Houtum-Schindler, A. 1891. Note on the Kur River in Fârs, its sources and dams, and the districts it irrigates. Proceedings of the Royal Geographical Society, 13:287-291.
Howes, G. J. 1980. The anatomy, phylogeny and classification of bariliine cyprinid fishes. Bulletin of the British Museum (Natural History) Zoology, 37(3):129-198.
Howes, G. 1981. Anatomy and phylogeny of the Chinese major carps Ctenopharyngodon Steind., (sic) 1866 and Hypophthalmichthys Blkr.,
1860. Bulletin of the British Museum (Natural History) Zoology, 41(1):1-52.
Howes, G. 1982. Anatomy and evolution of the jaws in the semiplotine carps with a review of the genus Cyprinion Heckel, 1843 (Teleostei:
Cyprinidae). Bulletin of the British Museum (Natural History) Zoology, 42(4):299-335.
Howes, G. 1984. Phyletics and biogeography of the aspinine cyprinid fishes. Bulletin of the British Museum (Natural History) Zoology, 47(5):283-303.
Howes, G. J. 1987. The phylogenetic position of the Yugoslavian cyprinid fish genus Aulopyge Heckel, 1841, with an appraisal of the genus
Barbus Cuvier & Cloquet, 1816 and the subfamily Cyprininae. Bulletin of the British Museum (Natural History), Zoology, 52(5):165-196.
Hrbek, T., Keivany, Y. and Coad, B. W. 2006. New species of Aphanius (Teleostei, Cyprinodontidae) from Isfahan Province of Iran and a reanalysis of other Iranian species. Copeia, 2006(2):244-255.
Hrbek, T, Küçük, F., Frickey, T., Stölting, K. N., Wildekamp, R. H. and Meyer, A. 2002. Molecular phylogeny and historical biogeography of the Aphanius (Pisces,
Cyprinodontiformes) species complex of central Anatolia, Turkey. Molecular Phylogenetics and Evolution, 25:125-137
Hrbek, T. and Meyer, A. 2001. Closing of the Tethys and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae).
The 10th European Congress of Ichthyology ECI X, Programme & Book of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 172-173.
Hrbek, T. and Meyer, A. 2003. Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae). Journal of Evolutionary Biology, 16(1):17-36.
Hrbek, T. and Wildekamp, R. H. 2003. Aphanius villwocki, a new species from the Sakarya River basin of central Anatolian plain, Turkey (Teleostei:
Cyprinodontiformes). Ichthyological Exploration of Freshwaters, 14(2):137-144.
Hsü, K. J. 1978. When the Black Sea was drained. Scientific American, 238(5):52-63.
Hubbs, C. L. and Lagler, K. F. 1958. Fishes of the Great Lakes Region. University of Michigan Press, Ann Arbor. xv + 213 pp.
Huber, J. H. 1996. Killi-Data 1996. Updated checklist of taxonomic names, collecting localities & bibliographic references of oviparous Cyprinodont
Fishes (ATHERINOMORPHA, PISCES). First Edition, Société Française d'Ichtyologie, Paris. 399 pp.
Hunt, R. S. 1951. The sharks of Ahwaz. Journal of the Royal Army Medical Corps, 97(1):79-85.
Huntington, E. 1905a. The Basin of Eastern Persia and Sistan, pp. 217-316. In: Explorations in Turkestan with an account of the Basin of Eastern Persia and Sistan. Carnegie Institution of Washington
Publication, 26:324 pp.
Huntington, E. 1905b. The depression of Sistan in eastern Persia. Bulletin of the American Geographical Society, New York, 37(5):271-281.
Huntington, E. 1907. The historic fluctuations of the Caspian Sea. Bulletin of the American Geographical Society, 39(10):577-596.
Huq, M. F., Al-Saadi, H. A. and Hameed, H. A. 1981. Studies on the primary production of the river Shatt Al-Arab at Basrah, Iraq. Hydrobiologia, 77:25-29.
Hurlbert, S., Zeidler, J. and Fairbanks, D. 1972. Ecosystem alteration by mosquito fish (Gambusia affinis) predation. Science, 175:639-641.
Hussain, N. A. 1997. Hilsa shad invade estuary in the Arabian Gulf. Shad Journal, Seattle, 2(4):2.
Hussain, N. A., Ali, T. S. and Coad, Brian. 2001. Middle Eastern shads. Paper presented at Shad 2001: A Conference on the Status and Conservation of Shads Worldwide, 20-23
May, 2001, National Aquarium, Baltimore, Maryland (abstract).
Hussain, N. A., Ali, T. S. and Saud, K. D. 1989. Seasonal fluctuations and composition of fish assemblage in the Shatt Al-Arab at Basrah, Iraq.
Journal of Biological Sciences Research, Baghdad, 20(1):139-150.
Hussain, N. A., Al-Najar, H. H. K., Al-Saad, H. T., Yousif, O. H. and Al-Saboonchi, A. A. 1991. Shatt al-Arab River: Basic scientific studies.
Marine Science Centre Publication, Basrah, 10:391 pp. In Arabic.
Hussain, N. A., Al-Saboonchi, A. A. and Hamza, H. A. 1987. Aspects of abundance and the food habit of Liza abu (Heckel) in the Karkheh River
(Arabistan). Journal of Biological Sciences Research, Baghdad, 18(1):145-161.
Hussain, S. A., Al-Mukhtar, M. A. and Al-Daham, N. K. 1991. Preliminary investigation on fishery and some biological aspects of sbour, Hilsa
ilisha, from Shatt al-Arab River, Iraq. Basrah Journal of Agricultural Science, 4(1 & 2):141-151.
Hussain, N. A., Younis, K. H. and Yousif, U. H. 1997. The composition of small fish assemblages in the River Shatt Al-Arab near Basrah (Iraq). Acta Hydrobiologica, 39(1/2):29-37.
Hussain, N. A., Younis, K. H. and Yousif, U. H. 2001. Evaluation of environmental degradation in the fish assemblage of Shatt Al-Arab River. Pakistan Journal of Zoology, 33(2):93-98.
Hutchinson, G. E. and Cowgill, U. M. 1963. Chemical examination of a core from Lake Zeribar, Iran. Science, 140:67-69.
Hydrorybproject. 1965. Fish-cultural reclamation of the Pahlavi (Mordab) Bay. State Industrial Fisheries Committee, U.S.S.R., State Design Institute
on Hydrotechnical, Fish-Cultural Reclamation and Land Construction, Moscow. MS, 60 pp.
I
Ibarra, M. and Stewart, D. J. 1987. Catalogue of type specimens of recent fishes in Field Museum of Natural History. Fieldiana Zoology, 35:iii + 112 pp.
Ibrahimzadeh, I. 1995. Hamoon Lake and its vital role. Water and the Environment, Tehran, 12 & 13:47-52. In Farsi.
Iezadi, Gh. 2000. Preliminary step of study on Barbus species in Fars Province. First National Symposium on Barbus fishes of Iran, Ahvaz, January, 10-11 (abstract).
Iezadi, Gh. 2002. Study on possibility of introduction of sturgeon fishes to inland water of Fars province. Second National and Regional Symposium on Sturgeon, Rasht, October, 26-28 (abstract).
Iljin (= Il'in or Ilyin), B. S. 1927a. Rybolovstvo v vodakh severnoi Persii, sdavaemykh v arendu mestnomu naseleniyu (iz rabot Kaspiiskoi Ekspeditsii
1914-1915 g) [Fisheries in the waters of northern Persia, leased to the local tenant population (from the work of the Caspian Expedition 1914-1915)].
Izvestiya Otdela Prikladnoi Ikhtiologii, Leningrad, 7(1):101-134.
Iljin, B. S. 1927b. Novyi vid sel'di iz yuzhnogo Kaspiya (iz rabot Kaspiiskoi Ekspeditsii 1914-15 gg) [A new species of herring from the Southern Caspian
(from the work of the Caspian Expedition 1914-15)]. Sbornik v chest' Professora Nikolaya Mikhailovicha Knipovicha 1885-1925, Moskva, 1927:69-75.
Iljin, B. S. 1927c. Zametka o bychkakh (Gobiidae) Zoologicheskogo muzeya Leningradskogo universiteta [A note on the gobies (Gobiidae) of the Zoological
Museum of the Leningrad University]. Trudy Leningradskogo Obshchestva Estestvoispytatelei, 57(1):73-75.
Iljin, B. S. 1927d. Opredelitel' bychkov (Fam. Gobiidae) Azovskogo i Chernogo Morei, pp. 128-143, 2 figs. In: Trudy Azovsko-Chernomorskoi Nauchno-Promyslovoi
Ekspeditsii isdavaemye pod redakstiei nachal'nika Ekspeditsii Professora N. M. Knipovicha, II, Leningrad. 143 pp.
Iljin, B. S. 1928. Dva novykh roda i novyi vid bychkov (Gobiidae) iz Kaspiiskogo morya [Two new genera and a new species of gobies (Gobiidae)
from the Caspian Sea]. Trudy Astrakhanskoi Nauchnoi Rybokhozyaistvennoi Stantsii, 6(3):40-51.
Iljin, B. S. 1930. Le système des Gobiidés. Instituto Español de Oceanografia, Madrid, Trabajos, 2:63 pp.
Iljin, B. S. 1936. Novyi bychok iz Kaspiiskogo morya Gobius nonultimus sp. n. (Pisces, Gobiidae) [A new goby from the Caspian Sea Gobius nonultimus
sp. n. (Pisces, Gobiidae)]. Doklady Akademii Nauk SSSR (Comptes Rendus de l'Académie des Sciences de l'URSS, 4(13), 7(111):337-339. In English.
Iljin, B. S. 1938. Bychki (Gobiidae) po materialam ekspeditsii Akademii Nauk SSSR v Mertvyi Kultuk i Kaidak [Gobies (Gobiidae) collected by the
U.S.S.R. Academy of Sciences Expedition to Mertvyi Kultuk and Kaidak]. Trudy Kaspiiskoi komissii, 2:111-130.
Iljin, B. S. 1941. Asra turcomanus gen. nov., sp. nov., novyi rod i vid bychkov (Gobiidae) iz Kaspiiskogo morya [Asra turcomanus gen. nov.,
sp. nov., a new genus and species of goby (Gobiidae) from the Caspian Sea]. Izvestiya Akademii Nauk SSSR, Otdel Biologicheskikh Nauk, 1941(3):385-390.
Iljin, B. S. 1949. Kratkii obzor chernomorskikh bychkov (Pisces, Gobiidae). [Brief review of the Black Sea gobies (Pisces, Gobiidae)].
Byulleten' Moskovskogo Obshchestva Ispytatelei Prirody, Otdel Biologicheskii, 54(3):16-30.
Iljin, B. S. 1956. Zamechaniya i popravki k podotryadu Gobioidei v knige L. S. Berga "Ryby presnykh vod SSSR i sopredel'nykh stran", Izd. 4, 1948-1949,
str. 1055-1125 [Observations on and corrections to the suborder Gobioidei in L. S. Berg's book: "Freshwater fishes of the USSR and adjoining countries",
4th Ed., 1948-1949, pp. 1055-1125]. Voprosy Ikhtiologii, 7:185-192.
Ilhan, A., Balık, S., Sarı, H. M. and Ustaoğlu, M. R. 2005. Batı ve Orta Anadolu, Güney Marmara, Trakya ve Batı Karadeniz
Bölgeleri Içsularındaki Carassius (Cyprindiae, Pisces) Türleri ve Dagılımları. Ege Űniversitesi Su Űrünleri Dergisi, 22(3-4):343-346.
Imami, S. M. H. 2000. Lar National Park, a beautiful and unique region in Iran. Hunting and Nature Lovers, Tehran, 2:4-7. In Farsi (English at
www.netiran.com/Htdocs/Clippings/Social/200917XXSO02.html, downloaded 15 December 2000).
Imandel, K., Razeghi, N. and Samar, P. 1978. Tehran ground water pollution by detergents. Water, Air, and Soil Pollution, 9:119-122.
Imani, A., Farhangi, M., Yazdanparast, R., Bakhtiyari, M., Shokouh Saljoughi, Z. and Mojazi Amiri, B. 2009. Feeding and growth eficiency indices of rainbow trout (Oncorhynchus mykiss) during
deprivation and re-feeding periods. Iranian Scientific Fisheries Journal, 18(2):1-12. In Farsi.
Imanpour, M. R., Ahmadi, A. R. and Kordiazi, M. 2009. Effects of stocking density on survival and growth indices of common carp (Cyprinus carpio). Iranian Scientific Fisheries Journal,
18(3):1-10. In Farsi.
Imanpoor, M. R., Alavi, S. H. and Cosson, J. 2009. Relationship between biological characteristics of eggs and female brood stocks of Persian sturgeon Acipenser persicus: a comparison
with other fish species. Fish Physiology and Biochemistry, 35(4):701-707.
Imanpour, M. R., Bagheri, T. and Hedayati, S. A. A. 2010. The anesthetic effects of clove essence in Persian sturgeon, Acipenser persicus. World Journal of Fish and Marine Sciences,
2(1):29-36.
Imnapour, M. R. and Enayat Gholampour, T. 2008. Effects of reproductive migration time on some biological characters of eggs in Gorgan River wild common carp (Cyprinus carpio). Journal of
Agricultural Sciences and Natural Resources, 15(6):125-131. In Farsi.
Imanpour, M. R. and Enayat Gholampour, T. 2009. The effects of broodstock age on some biological characters of wild common carp Cyprinus carpio eggs in Gorganrud River. Journal of
Agricultural Sciences and Natural Resources, 16(2):?. In Farsi.
Imanpour, M. R., Fendereski, F. and Kordjazi, M. 2009. The relationship between fish size and sperm volume, hematocrit index, gonadal characteristics and spermatological parameters in
Rutilus rutilus caspicus. Journal of Agricultural Sciences and Natural Resources, 16(3):60-66. In Farsi.
Imanpour, M. R. and Safari, R. 2009. Effect of maturation stages on gonadal indices and chemical composition of gonad in Cyprinus carpio (Cyprinidae). Journal of Agricultural Sciences
and Natural Resources, 16(1):35-41. In Farsi.
Imanpour, M. R. and Shirmohammadli, R. 2008. Effects of fish size and breeding tubercles on gonadosomatic index and some spermatological parameters in the mahisefid Rutilus frisii kutum
Kamensky, 1901. Journal of Agricultural Sciences and Natural Resources, 15(6):139-144. In Farsi.
Ingham, S. E. No date. The biology and taxonomy of the Mugilidae. Zoology Library, British Museum (Natural History), London. 276 pp., + 5 pp., 73 figs.
Innal, D. and Erk'akan, F. 2006. Effects of exotic and translocated fish species in the inland waters of Turkey. Reviews in Fish Biology and Fisheries, 16(1):39-50.
Ionides, M. G. 1937. The régime of the rivers Euphrates and Tigris. E. & F. N. Spon, London. vii + 278 pp.
Ionides, M. G. 1954. The geographical history of the Mesopotamian Plains. Geographical Journal, 120:394-397 (with comments by S. Smith and by G. M. Lees and N. L. Falcon).
Irani, A. J. 2001. Investigation of age, growth and maturity of mullets in the Gomishan Wetland. M.Sc. Thesis, Gorgan University. In Farsi.
Iranian Fisheries Company. 1976. In greeting the National Ceremony of the Fiftieth Anniversary of the Pahlavi Kingdom. Iranian Fisheries Company
booklet, 18 pp. In Farsi (brief overview of fisheries in the Iranian Caspian Sea).
Iranian Fisheries Company. 1977. Congratulations for the Ceremony greeting the 100th Birthday of King Reza the Great. Iranian Fisheries Company booklet,
17 pp. In Farsi (brief overview of fisheries in the Iranian Caspian Sea; essentially the same as the above).
Iranian Fisheries Research and Training Organization (IFRTO), Tehran. 1995. Aquaculture status and development requirement in the Islamic Republic of Iran. Iranian
Fisheries Research and Training Organization (IFRTO), Tehran. 34 pp.
Iranian Fisheries Research and Training Organization (IFRTO), Tehran. 1997. Survey and analysis of aquaculture development research priorities
in Iran. Paper presented at the FAO/NACA Workshop on Aquaculture Development Research Priorities and Capacities in Asia, 21-23 May 1997, Bangkok, Thailand.
Network of Aquaculture Centres in Asia-Pacific, Bangkok.
Islam, A. K. M. S. and Al-Nasiri, S. K. 1978. Some morphometric studies on khishni (Liza abu) from Basrah, Iraq. Zanco, A, 4/3:129-140.
Islam, A. K. M. S. and Khalaf, A. N. 1983. Diel patterns of feeding of khishni Liza abu (Heckel) in Rashdiyah Reservoir in Baghdad,
Iraq. Indian Journal of Fisheries, 29(1 and 2)(1982):223-228.
Islam, A. K. M. S., Khalaf, A. N., Jafery, A. R. and Sadek, S. E. 1981. Seasonal patterns of feeding of khishni, Liza abu (Heckel) in Rashdiyah
Reservoir, Baghdad, Iraq. Bangladesh Journal of Zoology, 9(2):151-158.
Islam, B. N. and Talbot, G. B. 1968. Fluvial migration, spawning, and fecundity of Indus River hilsa, Hilsa ilisha. Transactions of the American Fisheries Society, 97(4):350-355.
Issar, A. 1967. Hydrogeology of the Central Plateau of Iran. Israel Journal of Earth-Sciences, 16(1):38-39.
IUCN. 1996. 1996 IUCN Red List of Threatened Animals. IUCN, Gland, Switzerland. 70 + 368 pp., annex 10 pp.
Ivanov, A. P. 1971. A programme for development of inland fisheries in Iran. Food and Agriculture Organization, Rome, United Nations Development Programme, Technical Assistance, UNDP (TA) 3628:57 pp.
Ivanov, V. P. 2000. Biologicheskie resursy Kaspiiskogo Morya [Biological Resources of the Caspian Sea]. Kasp NIRKH, Astrakhan. 100 pp. in Russian, 96 pp. in English, 8 pls.
Ivanov, V. P., Belyaeva, V. N. and Vlasenko, A. D. 1995. Regional'noe raspredelenie promyslovykh resursov Kaspiiskogo Morya [Regional distribution
of commercial resources of the Caspian Sea]. Rybnoe Khozyaistvo, 2:18-21.
Ivanov, V. P. and Katunin, D. N. 2001. Report on the results of the Caspian Marine Expedition. Caspian Regional Thematic Center for Management of
Bioresources (CRTC MB), Caspian Environmental Programme, Baku, Azerbaijan. 2 volumes.
Ivanov, V. P., Vlasenko, A. D. and Khodorevskaya, R. P. 1995. Puti sokhraneniya osetrovykh [How to preserve sturgeons]. Rybnoe Khozyaistvo, 2:24-26.
Ivanov, V. P., Vlasenko, A. D., Khodorevskaya, R. P. and Raspopov, V. M. 1999. Contemporary status of Caspian sturgeon (Acipenseridae) stock
and its conservation. Journal of Applied Ichthyology, 15(4-5):103-105.
Iwatsuki, Y. and Heemstra, P. C. 2010. Taxonomic review of the western Indian Ocean species of the genus Acanthopagrus Peters, 1855 (Perciformes: Sparidae), with description of a new
species from Oman. Copeia, 2010(1):123-136.
Izyumov, Yu. G. and Kas'yanov, A. N. 1995. Hereditary factors affecting the number of vertebrae in the roach, Rutilus rutilus. Journal of Ichthyology, 35(9):20-26.
J
Jabbarzadeh Shiadeh, S. M., Mojazi Amiri, B., Abtahi, B. and Nazari, R. M. 2000. Study on the changes of some physiological factors during osmoregulation of juvenile Persian sturgeons
(Acipenser persicus). Iranian Journal of Fisheries Sciences, 2(1):61-74, 112.
Jabir, M. K. and Faris, A. A. 1989. Fecundity of sbour, Tenualosa ilisha (Hamilton-Buchanan, 1822) in the Shatt al-Arab River, Basrah. Marina Mesopotamica, 4(2):281-296.
Jackson, J. and Wood, R. M. 1980. The earth flexes its muscles. New Scientist, 88(1231):717-720.
Jacobsen, T. 1960. The waters of Ur. Iraq, 22:174-185.
Jacobsen, T. and Adams, R. M. 1958. Salt and silt in ancient Mesopotamian agriculture. Science, 128(3334):1251-1258.
Jado, A. R. and Zötl, J. G. (Eds.). 1984. Quaternary Period in Saudi Arabia. Volume 2: Sedimentological, hydrogeological, hydrochemical, geomorphological, geochronological and climatological
investigations in western Saudi Arabia. Springer-Verlag, Wien. xii + 360 pp.
Jafari, M., Dalimiasi, A. A. H. and Azarvandi, A. R. 2001. First report of isolation of Dendronucleata dogieli from freshwater fish in Iran. Pajouhesh va Sazandegi, 14(1)(50):44-46. In Farsi.
Jafari Salim, B., Nabi Bidhendi, Gh. R., Salemi, A. Taherioun, M. and Ardestani, M. 2009. Water quality assessment of Gheshlagh
River using water quality indices. Environmental Sciences 6(4):19-28.
Jafari Shamushaki, V. A., Abtahi, B., Kasumyan, A. O., Abedian Kenari, A. and Ghiorbani, R. 2008. Taste attractiveness of free amino acids for juveniles of Persian sturgeon Acipenser
persicus. Journal of Ichthyology, 48(1):124-133.
Jafarian, H. 1990. A preliminary investigation on Nemacheilus malapterurus in Qareh-chai River. B.Sc. Project, Gorgan University, Gorgan. 48 pp. In Farsi.
Jafarian, H. A., Azari Takami, Gh., Kamali, A. A., Soltani, M. and Habibi
Rezaei, M. 2007. The use of probiotic bacillus bioencapsulated with Artemia urmiana nauplii for the growth and survival in Acipenser persicus larvae. Journal of Agricultural Sciences and
Natural Resources, 14(2):77-87. In Farsi.
Jafarian, H. A., Shahi, Gh. A. and Yazdani, A. R. 2009. The effect of probiotics on the feeding efficiency and larval growth of three species of Caspian sturgeon. Journal of Agricultural
Sciences and Natural Resources, 16(2):?. In Farsi.
Jafarian, H., Taati Kelei, M. and Nazarpour, A. R. 2009. The study effect of probiotic bacillus on growth of rainbow trout (Oncorhynchus mykiss) larvae via supplementation with meal of
Daphnia magna. Journal of Agricultural Sciences and Natural Resources, 16(3):39-47. In Farsi.
Jafarzadeh-Haghiehi, N., Tavasoli, M. H. and Barootkoob, A. 2005. Investigation of Karoon River water quality variation using QUA12E Program. Iran Water Resources Research, 1(2):85-96.
Jaferian, A. 2009. Biological control of aquatic plants by fish. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 99 (abstract).
Jaffary, M., Dalimi, A. and Azarvandi, A. 2001. First report of isolation of Dendronucleata dogieli from freshwater fish in Iran. Pajouhesh va Sazandegi Journal, 50:44-46 (In Farsi).
Jafri, A. K., Al-Judaimi, M. and George, K. A. 1981. Preliminary investigations feeding yellow-finned black porgy, Acanthopagrus latus (Houttuyn), with artificial feed. Aquaculture,
22(1,2):117-124.
Jafri, S. I. H. 1994. The anadromous shad, Tenualosa ilisha: an overview of its fishery in Pakistan. Proceedings of First Symposium on Fish and Fisheries of Pakistan held at Department
of Zoology, Government College, Lahore, Pakistan on October 19-20, 1991, p. 33-42.
Jalali, B. 1987. Lernaensis in cyprinid cultured fish in Iran. M.Sc. Thesis, University of Godolo, Hungary.
Jalali, B. 1992. Description of Dogielius molnari n. sp. (Monogenea: Dactylogyridae) from the gills of an Iranian freshwater fish, Cyprinion macrostomum (Heckel). Acta
Veterinaria Hungarica, 40(4):239-242.
Jalali, B. 1995a. Monogenean parasites of freshwater fishes of Iran. Ph.D. Thesis, Veterinary Medical Research, Hungarian Academy of Sciences. 105 pp.
Jalali, B. 1995b. Current parasites and parasitic diseases of freshwater fishes in Iran. Proceedings of the Fourth International Symposium of Ichthyology, 3-7 October, Munich, Germany (abstract).
Jalali, B. 1998. Parasites and parasitic diseases of freshwater fishes of Iran. Fisheries Company of Iran,
Tehran. 564 pp. In Farsi.
Jalali, B. and Barzegar, M. 2005a. Dactylogirid (sic) infection in cultured fish in lake and reservoir in Iran an their epidemiological peculiarities. Sixth Symposium on Diseases in Asian
Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Jalali, B. and Barzegar, M. 2005b. A survey on parasites of gills of fishes of Vahdat Reservoir. Iranian Journal of Veterinary Science, 3:41-50. In Farsi.
Jalali, B. and Barzegar, M. 2005c. Dactylogyrids (Dactylogyridae: Monogenea)
on common carp (Cyprinus carpio L.) in freshwaters of Iran and description of the pathogenicity of D. sahuensis. Journal of Agricultural Science and Trechnology, 7:9-16.
Jalali, B. and Barzegar, M. 2006. Fish parasites in Zarivar Lake. Journal of Agricultural Science and Technology, 8(1):47-58.
Jalali, B., Barzegar, M. and Nezamabadi, H. 2008. Parasitic fauna of the spiny eel Mastacembelus mastacembelus Banks et Solander (Teleostei: Mastacembelidae) in Iran.
Iranian Journal of
Veterinary Research, Shiraz University, 9(2)(23):158-161.
Jalali, B., Barzegar, M. and Shamsi, Sh. 2000. Occurrence and description of Dactylogyrus sphyrna Linstow, 1878 (Monogenea: Dactylogyridae) on the gills of an Iranian endemic fish
Leuciscus persidis Coad, 1981 (sic) as a new host. Iranian Journal of Fisheries Sciences, 2(2):71-76, 104.
Jalali, B. and Molnár, K. 1990a. Occurrence of monogeneans on the freshwater fishes of Iran: Dactylogyridae from fish of natural waters and description of Dogielius mokhayeri sp.n.
Parasitologia Hungarica, 23:27-32.
Jalali, B. and Molnár, K. 1990b. Occurrence of monogeneans on freshwater fishes in Iran: Dactylogyrus spp. on cultured Iranian fishes. Acta Veterinaria Hungarica, 38(4):239-242.
Jalali, B., Papp, M. and Molnár, K. 1995. Four new Dactylogyrus species (Monogenea: Dactylogyridae) from Iranian fishes. Folia Parasitologica, 42(2):97-101.
Jalali, B. and Rohani, M. 1997. Monogeneans parasites of the southeastern part of Iran and their zoogeographical peculiarities. Third International Symposium of Monogenea, 25-30 August 1997, Czech
Republic, Papers and Abstracts, p. 75.
Jalali, B. and Shamsi, Sh. 2005a. Government approaches and research priorities for monogenean infections in aquaculture. 5th International Symposium on Monogenea, 8-12 August 2005, Zhongshan
University, Guangzhou, China (title).
Jalali, B. and Shamsi, Sh. 2005b. Dactylogyrus infection of introduced fishes (common carp and other major species of Chinese cyprinids) in Iran. 5th International Symposium on Monogenea,
8-12 August 2005, Zhongshan University, Guangzhou, China (title).
Jalali, B., Shamsi, Sh. and Barzegar, M. 2005. Occurrence of Gyrodactylus spp. (Monogenea: Gyrodactylidae) from Iranian freshwater fishes. Iranian Journal of Fisheries Sciences, 4(2):19-30, 114.
Jalali, B., Shamsi, S. and Imanzadeh, F. 2001a. Specific composition and morphological peculiarities of endemic monogenean parasites of freshwater fishes of Iran. In: ISM 4: 4th International
Symposium on Monogenea, Brisbane, Australia, 9-13 July 2001, p. 97 (abstract).
Jalali, B., Shamsi, Sh. and Imanzadeh, F. 2001b. Specific composition and morphological peculiarities of endemic monogenean parasites of freshwater fishes of Iran. Iranian Journal of Fisheries
Sciences, 3(1):13-22, 89.
Jalali, B., Shamsi, S. and Molnár, K. 2000. New Dactylogyrus species (Monogenea, Dactylogyridae) from cyprinid fishes of the Bahu-Kalat River in southeast Iran. Acta Parasitologia,
45(4):289-294.
Jalali, J. B., Barzegar, M. and Sohrabi, H. I. 2002. Preliminary survey on the parasites of some fishes in Zarivar Lake. Iranian Journal of Marine Sciences, 1(2):27-40, 86. In Farsi.
Jalali, M. A. and Amiri, B. M. 2009. Threatened fishes of the world: Salmo trutta caspius (Kessler, 1877) (Salmoniformes: Salmonidae). Environmental Biology of Fishes, 86(3):375-376.
Jalali, M. A., Hosseini Seyed, A. and Imanpour, M. R. 2009. Effects of Artemia urmiana nauplii enriched with vitamin C in combination with HUFA on growth, survival and stress resistance
of beluga (Huso huso) larvae. Journal of Agricultural Sciences and Natural Resources, 16(2):?. In Farsi.
Jalali, M. A., Mohammadi Zarjabad, A. A. Imanpour, M. R. and Borami, A. 2009. Influences of temperature on hatching time, exogenous feeding onset, growth performance and survival of
Acipenser persicus larvae. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A). In Farsi.
Jalilpour, J., Khosravi, A. R., Ebrahimzadeh Mousavi, H. A. and Shenavar
Masouleh, A. R. 2009. Identification and abundance of fungal flora in egg, and larvae, of Acipenser persicus in Shahid Beheshti sturgeon rearing center (2007). Iranian Scientific Fisheries Journal
18(3):35-42. In Farsi.
Jamali, Z., Jozi, S. A. and Hedayatifard, M. 2009. Hydrochemical study of Kooseh-Abband Wetland for warm water fish culture, northern Iran. The 6th Scientific Conference of Fisheries Resources, 3-4
March 2009, Basrah, p. 123 (abstract).
Jamalpour, M. 1998. Biodiversity of fish species in Helleh River (an ecological approach). M.Sc. Thesis, Tarbiat Modarres University. In Farsi.
Jamalzadeh, H. R., Keyvan, A., Oryan, Sh. and Ghomi, M. R. 2008. An assessment of hematological and serum biochemical indices in Salmo trutta caspius. Iranian Scientific Fisheries Journal,
17(3):47-54. In Farsi.
Jamalzadeh, H. R., Keyvan, A., Oryan, Sh., Jamili, Sh. and Saeidi, A. A. 2002. Study on some hematological factors of Salmo trutta caspius. Iranian Journal
of Fisheries Sciences, 11(1):25-34. In Farsi.
Jamili, S., Kousha, A. and Askarian, F. 2006. In vivo investigation on effect of lead nitrate on blood serum electrolyte of bighead carp Aristischtis (sic) nobilis.
World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Jamshidi, Sh., Kalbassi, M. R. and Daliri, M. H. 2009. Application of D-loop and cytochrome b genes for detection of genetically differences (sic) between two migratory forms of
endangered Salmo trutta caspius Kessler, 1870) assessed by DNA sequencing and RFLP analysis. 13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Jamshidi, S., Kalbassi, M. R. and Sadeghizadeh, M. 2009. Inheritance of RAPD marker in female grass carp (Ctenopharyngodon idella), male bighead carp (Hypophthalmichthys nobliis) and
their F1 hybrids. Iranian Journal of Fisheries Sciences, 8(1):47-56.
Jamshidi, Sh., Kalbassi, M. R., Shirangi, A. and Daliri, M. H. 2009. Genetic relationships between two seasonal migratory forms of endangered Caspian salmon Salmo trutta caspius Kessler, 1870
based on RAPD markers. 13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Janssen, J. and Jude, D. J. 2001. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius
melanostomus. Journal of Great Lakes Research, 27(3):319-328.
Jarvis, P. L. 2011. Biological synopsis of Garra rufa. Canadian Manuscript Report of Fisheries and Aquatic Sciences, 2946:vi + 14 pp.
Jaubert, M. le Comte. 1843. Relations de Voyages en Orient de 1830 à 1838, par Aucher-Éloy, revues et annotées. Première Partie. Librairie Encyclopédique de Roret,
Paris. xxxi + 364 pp.
Javadian, R., Rezaei, M., Sahari, M. A. and Hosseni, S. V. 2003. Effect of ice storage on lipid changes in silver carp (Hypophthalmichthys molitrix). Iranian Journal of Marine Sciences,
2(4):19-26. In Farsi.
Javaheri, M. 1998. Age structure and growth of Capoeta capoeta gracilis in Zaringol River. B.Sc. Seminar, Agricultural Sciences and Natural Resources University, Gorgan. 30 pp. In Farsi.
Javahrei Baboli, M., Matinfar, A. and Agh, N. 2006. A survey of subsistence effects of N-3HUFA enriched Artemia nauplii as a start feeding for Caspian salmon (Salmo trutta caspius) larvae.
Environmental Sciences, 3(11):55-63.
Javanmard, M. and Taghavi, K. 2002. Study of microbiological and chemical characteristics of Iranian sturgeon fish products exported to EU. Book of Abstracts, 27th World Veterinary
Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Javanmard, M. and Taghavi, K. 2003. Comparative study of two handling methods of raw fish on the fishmeal composition and T.V.N.. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway,
August 8-12, 2003 (title).
Javanshir A., Shapoori, M. and Jamili, S. 2008. Diversity of benthic invertebrates fauna and secondary production in southern Caspian Sea basin case study on Tajan River estuary. Journal; of Fisheries
and Aquatic Science, 3(6):353-365.
Jawad, L. A. 2003. Impact of environmental change on the freshwater fish fauna of Iraq. International Journal of Environmental Studies, 60(6):581-593.
Jawad, L. A. J. 1975. Biometric studies on three Barbus species from Basrah waters, Basrah, Iraq. The Arabian Gulf Journal, University of Basrah, 3:212-247.
Jawad, L. A. 2003. Biochemical approaches: their present usage and future application in the systematic problems of the freshwater fishes of Mesopotamia. Anales de Biologia, 25:199-208.
Jawad, L. A. J. 2004a. Asymmetry analysis in the mullet, Liza abu collected from Shatt al-Arab River, Basrah, Iraq. Bollettino del Museo Regionale di Scienze Naturali di Torino,
21(1):145-150.
Jawad, L. A. J. 2004b. Preliminary study on the use of eye lens diameter and weight as an age indicator in two cyprinid fishes collected from Basrah, Iraq. Bollettino del Museo Regionale di
Scienze Naturali di Torino, 21(1):151-158.
Jawad, L. A. 2010. Book review of "Freshwater Fishes of Iraq". Pensoft Series
Faunistica No. 93. By B. Coad. 274 pp. Published by Pensoft Publishers, Moscow.
2010. Price €48.00. ISBN: 978-954-642-530-0.
Journal of Fish Biology, 77(4):1041-1042.
Jawad, L. A., Al-Mukhtar, M. A. and Ahmed, H. K. 2004. The relationship between haematocrit and some biological parameters of the Indian shad,
Tenualosa ilisha (Family Clupeidae). Animal Biodiversity and Conservation, 27(2):47-52.
Jawad, L. A., Hussein, S. A. and Fahad, K. K. 2009. Glyptothorax kurdistanicus (Berg, 1931) (Pisces, Siluriformes, Sisoridae) in the lower reaches of the Tigris River, Iraq. Journal of Applied
Ichthyology, 25(6):779-781.
Jawad, L. A., McKenzie, A. and Al-Noor, S. S. 2009. Relationship between opercular girth, maximum girth and total length of fishes caught in gillnets in the estuarine and lower river sections
of Shatt al-Arab River (Basrah Province, Iraq). Journal of Applied Ichthyology, 25(4):470-473.
Jawad, L. A. and Öktener, A. 2007. Incidence of lordosis in the freshwater mullet, Liza abu (Heckel, 1843) collected from Atatürk Dam Lake, Turkey. Anales de Biologia, 29:105-113.
Jawad L. A., Sadighzadeh Z. and Valinassab, T. 2010. Malformation of the caudal fin in the freshwater mullet, Liza abu (Actinopterygii: Mugilidae) collected from Karkhe River Iran.
Anales de Biologia, 32:11-14.
Jayaram, K. C. 1955. A preliminary review of the genera of the family Bagridae (Pisces: Siluroidea). Proceedings of the National Institute of Sciences of India, 21B(3):120-128.
Jayaram, K. C. 1959. Systematic position of fishes described under Bagrus by Valenciennes, 1839. Records of the Indian Museum, 54(1-2):53-59.
Jayaram, K. C. 1980. Aid to the identification of siluroid fishes of India, Burma, Sri Lanka, Pakistan and Bangladesh 4. Clariidae, Heteropneustidae, Chacidae and Olyridae. Records of the
Zoological Survey of India, Miscellaneous Publications, Occasional Paper, 23:1-23.
Jayaram, K. C. 1981. The Freshwater Fishes of India, Pakistan, Bangladesh, Burma and Sri Lanka - A Handbook. The Director, Zoological Survey of India, Calcutta. xxii + 475 pp., 13 pls.
Jayaram, K. C 1999. The Freshwater Fishes of the Indian Region. Narendra Publishing House, Delhi. xxvii + 551 pp., 18 pls.
Jayaram, K. C. and Anuradha, C. 1984. Contributions to the study of bagrid fishes. 17. The history and usage of the name "Mystus". Bulletin of the Zoological Survey of India, 6(1-3):289-293.
Jayaram, K. C. and Sanyal, A. 2003. A taxonomic revision of the fishes of the genus Mystus Scopoli (Family Bagridae). Records of the Zoological Survey of India, Occasional Paper,
207:iv + 136 pp., 5 plates.
Jazebi Zadeh, M. K. and Shirin Abadi, M. 2007. Identification and estimation of the population of fishes in Namroud River. Journal of Environmental Science and Technology, 9(3)(34):103-111. In Farsi.
Jazebi Zadeh, M. K. and Shirin Abadi, M. 2008. Identification and estimation of the population of fishes in Namroud River. Journal of Environmental Science and Technology, 10(1)(36):127-136. In Farsi.
Jazebizadeh, K. 1983. Study on parasitic diseases of fishes of Zarivar Lake. Environmental Protection Organization of Iran Publication, Tehran. 19 pp. In Farsi.
Jazebizadeh, M. K. and Yekta, A. A. 2008. Identification and estimation of Namrud River's fisher above the Tehran Gezel Aquaculture Farm by electroshocker. Journal of Environmental Science and
Technology, 36(special issue).
Jehbez, O. and Zare, M. 1995. Evaluation and determination of design flood considering socio-economic aspects. WRM '95. Proceedings of the Regional Conference on Water Resources
Management, Conference Secretariat, Isfahan University of Technology, Isfahan, pp. 379-388.
Jeitteles, L. H. 1861. Zoologische Mittheilungen. Ueber zwei die Fauna Ungarns neue Fische: Lucioperca volgensis Cuv. Val. und Alburnus maculatus Kessler. Verhandlungen der
Zoologisch-Botanischen Gesellschaft in Wien, 11:324-325.
Jeney, Z. 1984. Consultancy report in Fish Health Management. Iranian Fisheries Organisation, Tehran, Iran. 60 pp.
Jenkins, J. T. 1910. Notes on fish from India and Persia, with descriptions of new species. 1. On a collection of fishes made by W. T. Blanford in 1872 in Persia and Baluchistan. Records of the
Indian Museum, 5:123-128, pl. VI.
Jenkins, R. W. G. 2001. Sustainable sturgeon fisheries in the Caspian Sea. Will a trade ban contribute to achieving this objective? IWMC - World Conservation Trust, 8 pp. (www.iwmc.org/).
Jennings, D. P. 1988. Bighead carp (Hypophthalmichthys nobilis): a biological synopsis. U.S. Fish and Wildlife Service Biological Report, 88(29):vii + 47 pp.
Jentsch, C. 1970. Die Kareze in Afghanistan. Erdkunde, 24(2):112-120.
Jiad, J. H., Hameed, A-H. M., and Al-Faisal, A-H. M. 1984. Study of age, growth and blood contents of Barbus grypus Heckel in Al-Hindiya dam. Journal of Biological Sciences Research,
Baghdad, 15(2):29-48.
Job, T. J. 1960a. Fish crops from multipurpose dams in the Middle East. FAO in the Near East, "A Quarterly Review", Food and Agriculture Organization, Cairo, 1960(1):2-14.
Job, T. J. 1960b. Travel report on a visit to Iran from 18 September to 5 October 1960. Food and Agriculture Organization, Rome, NEFi/T/60:12 pp.
Job, T. J. 1961a. Fisheries notes. FAO in the Near East, "A Quarterly Review", Food and Agriculture Organization, Cairo, 3(2):23.
Job, T. J. 1961b. Iran - background information. Food and Agriculture Organization, Rome, NEFi - Iran Inf., 26 pp., appendix (mimeo).
Job, T. J. 1965. Travel report on a visit to Iran from 20 July to 2 August 1965. Food and Agriculture Organization, Rome, NECFi-T/65-2, 10 pp., 3 appendices (mimeo).
Job, T. J. 1967. Status of fish culture in the Near East region, pp. 54-69. In: Pillay, T. V. R. (Ed.). Proceedings of the FAO World Symposium on warm-water pond fish culture. Food and Agriculture
Organization, Rome, Fisheries Report, 44, volume 2:174 pp.
Johal, M. S., Esmaeili, H. R. and Sharma, M. L. 2006. Scale structure of a cobitid fish, Cobitis linea (Heckel, 1849) using different modes of SEM. Current Science, 91(11):146-1466.
Johari, S. A. 2005. Production and rearing of all-female diploid and triploid rainbow trout (Oncorhynchus mykiss). M.Sc. Thesis, Tarbiat Modarres University, Tehran.
Johari, S. A. and Asghari, S. 2009. Identification and distribution of fish fauna in qanats and standing rivers of Qae'nat County (South Khorasan). 13th European Congress of Ichthyology,
6-12 September 2009, Klaipeda, Lithuania (abstract).
Johari, S. A., Coad, B. W. and Asghari, S. 2009. Biological and morphometric characteristics of siah mahi, Capoeta fusca a cyprinid fish living in the qanats of Birjand County (South
Khorasan Province, Islamic Republic of Iran). 13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Johari, S. A., Coad, B. W., Mazloomi, S., Kheyri, M. and Asghari, S. 2009. Biological and morphometric characteristics of Capoeta fusca, a cyprinid fish living in the qanats of south
Khorasan, Iran (Osteichthyes: Cyprinidae). Zoology in the Middle East, 47:63-70.
Johari, S. A. and Kalbassi, M. R. 2006. Investigation of red blood cell alterations in triploid rainbow trout (Oncorhynchus mykiss). Iranian Journal of Biology, 19(4):492-495. In Farsi.
Johari, S. A., Kalbassi, M. R., Amiri, B. M. and Hallajian, A. 2006. Production of all-female rainbow trout (Oncorhynchus mykiss) using
sex-reversed males and investigation of their growth parameters in the first year of culture. Iranian Journal of Fisheries Sciences, 15(3):45-54. In Farsi.
Johari, S. A., Kalbassi, M. R., Sourinezhad, I. and Wlasow, T. 2008. Observation of red blood cell alterations in triploid rainbow trout (Oncorhynchus mykiss). Acat Scientatiarum Polonorum
Piscaria, 7(1-4):49-52.
Johari, S. A., Kalbassi, M. R., Veylaky, A. S. and Takla, M. 2003. Comparison of sperm traits and fertilization performance of normal male and neomale rainbow trout (Oncorhynchus mykiss).
Iranian Journal of Marine Sciences, 2(4):27-38. In Farsi.
Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. 1997. Opportunistic settlers and the problems of the ctenophore Mnemiopsis leidyi in the Black Sea.
GESAMP Reports and Studies, 58:x + 84 pp.
Jolodar, M. N. and Abdoli, A. 2004. Fish Species Atlas of South Caspian Sea Basin (Iranian Waters). Iranian Fisheries Research Organization, Teheran.110 pp. In Farsi and English.
Jonasson, G. 1987. Islamic Republic of Iran. Development of the artisanal and industrial fisheries. A report prepared for the Fisheries Development Project, Food and Agriculture Organization, Rome. 8 pp.
Joneidi, M. J., Khodabandeh, A., Ghafouri, M. R. and Chariat, M. 1971?. Lee (sic) eaux minerales (sic) de L'Iran. University of Tehran Environmental Studies, 4:149-156.
Jorfi, E., Amini, F., Ghoreyshi, S. A. and Mortezaei, S. R. S. 2008. A study on population genetics of hilsa shad, Tenualosa ilisha, in Khoozestan, Iran using molecular method (RAPD). Iranian
Scientific Fisheries Journal, 17(1):31-44. In Farsi.
Jorfi, E., Farhad, A. and Reza, M. S. 2009. A comparison between sobour (Tenualosa ilisha) of Iran and Iraq by molecular method. The 6th Scientific Conference of Fisheries Resources, 3-4 March
2009, Basrah, p. 155 (abstract).
Josupeit, H. 1994. World trade of caviar and sturgeon. Food and Agriculture Organization, Rome. 100 pp.
Jude, D. J. 1997. Round gobies: cyberfish of the Third Millennium. Great Lakes Research Review, 3(1):27-34.
Jude, D. J. 2001. Round and tubenose gobies: 10 years with the latest Great Lakes phantom menace. Dreissena! The Digest of the National Aquatic Nuisance Clearinghouse, 1(4)1:1-14.
Jude, D. J., Reider, R. H. and Smith, G. R. 1992. Establishment of Gobiidae in the Great Lakes basin. Canadian Journal of Fisheries and Aquatic Sciences, 49(2):416-421.
Justin, J. B. and Taleghani, K. 1955. Iran develops its rivers. Civil Engineering, 25:49-53 (or 153-157).
K
Kafilzadeh, F., Kargar, M. and Kadivar, E. 2007. The study of cadmium, copper, iron and nickel concentration in Khoshk River (Shiraz) and some products of the neigbouring (sic). Journal of
Environmental Science and Technology, 8(4)(31):67-75. In Farsi.
Kafuku, T. 1969. Morphological differentiation of Cyprinion in Iraq. Bulletin of the Freshwater Fisheries Research Laboratory, Tokyo, 19(2):155-159.
Kahrom, E. 2000. Wildlife conservation in Iran. Asian Affairs, 31(1):49-56.
Kähsbauer, P. 1957. Viktor Pietschmann. Annalen des Naturhistorischen Museums in Wien, 61:1-3, Tafel 1.
Kähsbauer, P. 1959. Intendant Dr. Franz Steindachner, sein Leben und Werk. Annalen des naturhistorischen Museums in Wien, 63:1-30, Tafel 1.
Kähsbauer, P. 1963. Zur Kenntnis der Ichthyofauna von Iran. Annalen des naturhistorischen Museums in Wien, 66:317-355.
Kähsbauer, P. 1964. Zur Kenntnis der Ichthyofauna von Iran (II. Teil). Annalen des naturhistorischen Museums in Wien, 67:453-475, 1 Taf.
Kajiwara, N., Ueno, D., Monirith, I., Tanabe, S., Pourkazemi, M. and Aubrey, D. G. 2003. Contamination by organochlorine compounds in sturgeons from
Caspian Sea during 2001 and 2002. Marine Pollution Bulletin, 46(6):741-747.
Kalantan, A. M. N., Arfin, M. and Nizami, W. A. 1987. Seasonal incidence and pathogenicity of the metacercariae of Clinostomum complanatum
in Aphanius dispar. Japanese Journal of Parasitology, 36(1):17-23.
Kalbasi, M. and Mousavi, S. F. 1995. Seven-year study of fluctuations in N, P, EC and pH of the Zayandehrud River. WRM '95. Proceedings of the Regional Conference on
Water Resources Management, Conference Secretariat, Isfahan University of Technology, Isfahan, p. 151.
Kalbasi, M. R. 1995. Induction of triploidy in rainbow trout. Seventh Congress of Iranian Marine Organisations, Noor, Iran, p. 35 (abstract).
Kalbasi, M. R., Azari Takami, G. and Amini, F. 1997. Induction of triploidy in rainbow trout stocks. Journal of the Veterinary Faculty of the University of Tehran, 52:51-61.
Kalbasi, M. R., Soltani, M. and Porbakhsh, A. 1999. Immune response of Iranian sturgeons to four different Aeromonas antigens. Ninth Congress of the European Association of Fish
Pathologists, Greece, 19-24 September 1999 (abstract).
Kalbassi, M. R., Bagheri, A. Pourkazemi, M. and Abdolhay, H. 2004. Induction of tetraploidy in rainbow trout (Oncorhynchus mykiss) by heat shock.
Iranian Journal of Fisheries Sciences, 12(4):143-152. In Farsi.
Kalbassi, M. R., Dorafshan, S., Tavakolian, T., Khazab, M. and Abdolhay, H. 2006. Karyological analysis of endangered Caspian salmon, Salmo trutta caspius (Kessler,
1877). Aquaculture Research, 37(13):1341-1347.
Kalbassi, M. R., Hosseini, S. V. and Tahergorabi, R. 2008. Karyotype analysis in Schizothroax zarudnyi from Hamoon Lake, Iran. Turkish Journal of
Fisheries and Aquatic Sciences, 8(2):335-340.
Kalbassi, M. R. and Keyvanshokoh, S. 2004. Current status of aquaculture genetics research in Iran. Proceedings of the Seventh Asian Fisheries Forum, 3-4 November 2004, Penang, Malaysia.
Kalbassi, M. R. and Lorestani, R. 2007. Effect of different diluent solutions on the duration of sperm motility in rainbow trout (Oncorhynchus mykiss). Journal of Agricultural Sciences and
Natural Resources, 13(6):132-142. In Farsi.
Kalbassi, M. R., Mostafaei, A. and Majidi, J. 2003. Purification and partial characterization of serum immunoglobulin from Persian sturgeon (Acipenser persicus). Iranian
Scientific Fisheries Journal, 12(3):141-152. In Farsi.
Kalbassi, M. R., Panahi Sahebi, H. and Nazari, R. M. 2002. Crossbreeding of female grass carp (Ctenopharyngodon idella)*male bighead carp (Hypophthalmichthys molitrix) and
observation on (F1) hybrids. Iranian Journal of Marine Sciences, 1(3):35-44, 76. In Farsi.
Kalbassi, M. R., Soltani, M., Pourbakhsh, S. A. and Adams, A. 2000. Humoral immune response of cultured Persian sturgeon (Acipenser persicus) to four
different Aeromonas hydrophila antigens. Archives of the Razi Institute, 51:75-83.
Kalkan, E. 2008. Growth and reproduction properties of Capoeta trutta (Heckel, 1843) in Karakaya Dam Lake. Turkish Journal of Zoology, 32:1-10.
Kals, J. 2006. Improving the utilization of silver carp (Hypothalmichthys molitrix) (sic) and other under-utilized fish species, especially fresh water bream (Abramis
brama) 2005-2006: possibilities for value adding supply chains and international trade of silver carp (Hypothalmichthys molitrix) (sic)
in the Islamic Republic of Iran. Research Report, Netherlands Institute for Fisheries Research, IJmuiden, RIVO C006B/06, 25 pp.
Kals, J. and Bartels, P. V. 2004. Improving utilization of silver carp (Hypopthalmichthys Molitrix (sic)) and other under-utilized fish species. Fact finding and goal
establishing mission to the Islamic Republic of Iran (31 January - 5 February 2004). Wageningen University and Research Centre Report DWK, 404:1-30.
Kalkan, E. 2008. Growth and reproduction properties of Capoeta trutta (Heckel, 1843) in Karakaya Dam Lake. Turkish Journal of Zoology, 32:1-10.
Kamal, S. 2006. The study of systematic and some biological factors in killifish (Aphanius sophiae) in Chehsme Ali-Damghan. M.Sc. Thesis, University of Tehran, Karaj. In Farsi.
Kamal, S., Abdoli, A., Bakhtiari, M. and Karami, M. 2009. Some biological characteristics of killifish (Aphanius sophiae) in Cheshme Ali Damghan.
Environmental Sciences, 6(3):52-62.
Kamal, S., Bakhtiari, M. and Abdoli, A. 2007. Comparison of biological factors of Aphanius sophiae in Cheshmeh-Ali of Damghan and Shour Rivers of Eshtehard. Iranian Scientific Fisheries
Journal, 16(3):113-122. In Farsi.
Kamal, S., Bakhtiari, M., Abdoli, A., Eagderi, S. and Karami, M. 2009. Life-history variations of killifish (Aphanius sophiae) populations in two environmentally different habitats in central
Iran. Journal of Applied Ichthyology, 25(4):474-478.
Kamali, A. and Farabi, S. M. V. 2005. Effect of initial body weight of beluga (Huso huso Linnaeus, 1758) juveniles on adapting to concentrated feed, reared in fibreglass's basins.
Journal of Agricultural Sciences and Natural Resources, 12(5):82-90. In Farsi.
Kamali, A. and Imanpoor, R. 2002. Nutritonal status of released Acipenser persicus fingerlings in Gorgan-Roud. Journal of Agricultural Sciences and Natural Resources, 9(3):129-139. In Farsi.
Kamali, A. and Shabanpour, B. 2004. Effects of Daphnia magna and Artemia nauplii on growth performance in Persian sturgeon Acipenser persicus larvae. Iranian Journal of
Fisheries Sciences, 4(1):103-115, 125.
Kamali-Far, R., Amiri-Moghaddam, J. and Maniei, F. 2009. Induction of spawning in Capoeta aculeata (Valenciennes in Cuv. & Val., 1844) (Teleostei, Cyprinidae), using carp pituitary extract.
Animal Biology & Animal Husbandry (ABAH) Bioflux, 1(1):27-31.
Kamaly, A. 1991. Effect of cold weather on the growth of mixed carp species in ponds. Bulletin Zoölogisch Museum Universiteit van Amsterdam, Special Issue, August 1991, Abstracts of
Presentations, Seventh International Ichthyology Congress of the European Ichthyological Union, The Hague, The Netherlands 26-30 August, 1991:35.
Kamenskii, S. N. 1899-1901. Karpovye (Cyprinidae) Kavkaza i Zakavkaz'ya [Die Cypriniden der Kaukasusländer und ihrer angrenzenden Meere]. Tiflis. viii + 157 pp., Taf. I-VI, ii + 192 pp.,
Taf. VII-XII. In Russian and German.
Kami, H. Gh. and Aghaei Moghadam, A. A. 2005. An introduction to nutrition and reproduction of pond turtle Emys orbicularis in culturing ponds of sturgeon fishes of Gorgan Centre,
Voshemegir Dam of Golestan Province. Journal of Agricultural Sciences and Natural Resources, 12(5):91-99. In Farsi.
Kamiar, M. 1983. The qanat system in Iran. Ekistics, 50(303):467-472.
Kamilov, B. G. 1994. Fecundity of eastern bream, Abramis brama orientalis Berg, from reservoirs in southern Uzbekistan. Acta Hydrobiologica, 36(2):245-253.
Kamran, A. 2006. Survey on bony fish catches in Gorgan Gulf. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture.
http://en.ifro.ir (abstract).
Kanazawa, R. H. 1955. Fishes of Iraq and Iran. American Documentation Institute, ADIM 4612:43-46.
Kapourchali, M. F. 2006. The role of Brachionus plicatillis (Rotifers) by increasing survivor of Rutilus frisii kutum larvae and its comparison by concentrate food. Iranian
Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Kapoorchali, M. F., Fatemi, S. M. R., Vosoghy, G., Matinfar M. and Sharifian, M. 2009. Increasing in growth of Rutilus frisii kutum larvae with using slurry (fermented organic manure) in
Yosefpoor Propagation and Rearing Center (Iran). Journal of Fisheries and Aquatic Science, 4(1):22-31.
Karabatak, M. 1997. The time of spawning and seasonal variations in the length-weight relationship and condition of chub, Leuciscus cephalus (Heckel, 1843) in Lake Beyşehir
(Turkey). Acta Hydrobiologica, 39(1/2):39-46.
Karabatak, M. 1988. The reproduction of pike, Esox lucius L. 1758, in Lake Aksehir, Konya. First International Symposium on Aquaculture, Istanbul (Turkey). Journal of Aquatic Products,
2:205-223.
Karahan, A. and Ergene, S. 2010. Cytogenetic analysis of Garra variabilis (Heckel, 1843) (Pisces, Cyprinidae) from Savur Stream (Mardin). Turkey. Turkish Journal of Fisheries and Aquatic
Sciences, 10(4):483-489.
Karaman, M. S. 1969a. Süßwasserfische der Türkei. 7. Teil. Revision der kleinasiatischen und vorderasiatischen Arten des Genus Capoeta (Varicorhinus, Partim).
Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 66:17-54.
Karaman, M. S. 1969b. Zwei neue Süßwasserfische aus Afghanistan und Iran. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 66:55-58.
Karaman, M. S. 1971. Süßwasserfische der Türkei. 8. Teil. Revision der Barben Europas, Vorderasiens und Nordafrikas. Mitteilungen aus dem hamburgischen Zoologischen Museum und
Institut, 67:175-254.
Karaman, M. S. 1972. Süßwasserfische der Türkei. 9. Revision einiger kleinwüchsiger Cyprinidengattungen Phoxinellus, Leucaspius,
Acanthobrama usw. aus Südeuropa, Kleinasiens, Vorder-Asien und Nordafrika. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 69:115-155.
Karami, M. 1992. Nature Reserves in the Islamic Republic of Iran. Species, Newsletter of the Species Survival Commission, IUCN, 19:11.
Karami, M., Ebrahimzadeh, M. A. and Masror, Y. 2005. Measurement of ache activity in the brain of sea, well and river-fed Cyprinus carpio as a health index. Scientific Journal of Kurdistan
University of Medical Sciences, 9(2)(34):13-19. In Farsi.
Karami, M., Kasmani, M. E. and Alamesh. A. A. 2001. Plants of Hashilan Wetland, Kermanshah, Iran. Journal of Sciences, Islamic Republic of Iran, 12(3):201-207.
Karamouz, M., Kerachian, R., Zahraie, B. and Jafarzadeh, N. 2005. Development of a master plan for river water pollution control: a case study of Karoon-Dez River system. Iran Water Resources
Journal, 1(1):12-28.
Karayev, R. A. 2006. Modelling Caspian sturgeon population dynamics: a new paradigm and new technology. ICES Journal of Marine Science, 63(6):980-994.
Karbassi, A., Bidhendi, G. N., Pejman, A. and Bidhendi, M. E. 2010. Environmental impacts of desalination on the ecology of Lake Urmia. Journal of Great Lakes Research, 36(3):419-424.
Karbassi, A., Monavari, M. and Moogouei, R. 2008. Environmental management of aquaculture in the area of Sarab Gerdu. Journal of Environmental Science and Technology, 36(special issue).
Kardovani, P. 1995. Iranian marine ecosytem (The Persian Gulf and the Caspian Sea). Ghomes, Tehran. 2 volumes.
Karemi, S., Massoud, M. R., Mehdizadeh Fanid, L. and Hajirostamlo, M. 2006. Procercoid of Ligula intestinalis from copepods in Satarkhan Dam, East Azerbaijan
Province, north-west Iran. Iranian Journal of Fisheries Sciences, 15(2):151-154. In Farsi.
Karim Koshteh, M. H., Akbari, A. and Rezazadebahi, M. M. 2000. Economical efficiency of fishery units in Sistan area. The Third World Fisheries Congress, Beijing, 31 Oct - 2 Nov 2000 (title).
Karimpour, M. 1993. Back calculation and growth studies in fishes. Abzeeyan, Tehran, 4(6):22-25, V. In Farsi.
Karimpour, M. 1998. The ichthyofauna of Anzali Lagoon. Iranian Journal of Fisheries Sciences, 7(2):83-94, 9. In Farsi.
Karimpour, M. and Hosseinpour, N. 1988. Caspian salmon. Iranian Fisheries Research Organization, Tehran. 134 pp. In Farsi.
Karimpour, M., Hosseinpour, N. and Haghighi, D. 1993. Biological survey of Chalcalburnus chalcoides guldenstadti (sic) spawning migration in Anzali Lagoon. Iranian Fisheries Bulletin,
(4):39-52, 4. In Farsi.
Karimzadeh, G., Gabrielyan, B. and Fazli, H. 2010. Population dynamics and biological characteristics of kilka species (Pisces: Clupeidae) in the southeast coast of the Caspian Sea. Iranian
Journal of Fisheries Sciences, 9(3):422-433.
Karimzadeh, K., Mostafaie, A., Esmaeli, A., Poorkazemi, M. and Zahmatkesh, A. 2005. Induction of cytochrome P4501A1 by beta-naphthoflavone and determination of enzyme properties in Huso huso.
Iranian Journal of Fisheries Sciences, 14(2):115-126. In Farsi.
Karimzadeh, K., Mostafaie, A., Esmaili Sarii, A., Pourkazemi, M. and Zahmatkesh, A. 2006. Induction and purification of cytochrome P4501A1 by β-naphthoflavon-treated beluga Huso huso.
Journal of Applied Ichthyology, 22(s1):215-225.
Karimzadeh, K., Mostafaie, A., Esmaeli Sari, A., Pourkazemi, M. and Zahmatkesh, A. 2003. Effects of 3-methyl cholantheren on liver microsomal mixed function oxidase activities of Acipenser
persicus. Iranian Journal of Marine Sciences, 2(4):71-80. In Farsi.
Karpevich, A. F. and Lukonina, N. K. 1970. Transplantations of fishes and aquatic invertebrates in 1966. Journal of Ichthyology, 10(3):404-420.
Karpevich, A. F. and Lukonina, N. K. 1971. Transplantations of fishes and aquatic invertebrates in 1967. Journal of Ichthyology, 11(1):98-112.
Karpevich, A. F. and Lukonina, N. K. 1972. Transplantations of fishes and aquatic invertebrates in 1968. Journal of Ichthyology, 12(2):325-341.
Karpinsky, M. G. 1992. Aspects of the Caspian Sea benthic ecosystem. Marine Pollution Bulletin, 24(8):384-389.
Karrer, C., Whitehead, P. J. P. and Paepke, H-J. 1994. BLOCH & SCHNEIDER's Systema Ichthyologiae, 1801: History and authorship of fish names. Mitteilungen aus dem Zoologischen Museum in Berlin,
70(1):99-111.
Kashani Sabet, A. R., Oryan, Sh. and Bahmani, M. 2004. Ovulation induced in brood fishes of Hypophthalmichthys molitrix by using LHRH-A hormone and its combination with dopamine antagonists.
Iranian Journal of Fisheries Sciences, 13(3):129-144. In Farsi.
Kashefi Alasl, M. and Zaeimdar, M. 2009. The need for Jajrood River quality management. Journal of Environmental Science and Technology, 11(2)(41):119-129. In Farsi.
Kashfi, M. S. 1976. Plate tectonics and structural evolution of the Zagros geosyncline, southwestern Iran. Bulletin of the Geological Society of America, 87:1486-1490.
Kashi, M., Hashemi, S. and Al-Mukhtar, M. 2009. Some biological aspects of keeled mullet, Liza klunzingeri in the east coastal of Khuzestan, north-west of the Gulf. The 6th Scientific
Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 82 (abstract).
Kask, J. L. 1970. Report to the Government of Iran on general fisheries development. Food and Agriculture Organization, Rome, United Nations Development Programme, Technical Assistance, UNDP (TA)
2887:v + 10 pp.
Kassim, T. I. and Al-Saadi, H. A. 1995. Seasonal variation of epiphytic algae in a marsh area (southern Iraq). Acta Hydrobiologica, 37(3):153-161.
Kassler, P. 1973. The structural and geomorphic evolution of the Persian Gulf, pp. 11-32. In: Purser, B. H. (Ed.). The Persian Gulf. Holocene carbonate sedimentation and diagenesis in a shallow
epicontinental sea. Springer-Verlag, New York. vi + 471 pp.
Kas'yanov, A. N., Izyumov, Yu. G. and Kas'yanova, N. V. 1995. Growth of roach, Rutilus rutilus, in Russia and adjacent countries. Journal of Ichthyology, 35(9):256-272.
Kasymov, A. and Rogers, L. 1996. Ecological description of the southern Caspian Sea in the oil-field region of "Guneshly". Polish Ecological Studies, 22(3-4):83-93.
Kasymov, A. G. 1982. The role of Azov-Black Sea invaders in the productivity of the Caspian Sea benthos. Internationale Revue der Gesamten Hydrobiologie, 67(4):533-541.
Katunin, D. N. 2000. Transboundary diagnostic analysis of relevantly important commercial bioresources. TACIS (Technical Assistance to the Commonwealth of Independent States, European Union),
The Caspian Scientific and Research Institute of Fishery (Kaspnirkh), The Caspian Regional Thematic Center on Fish and other Commercial Bioresources Management (CRTC MB). 49 pp.
Kaul, M. and Rishi, K. K. 1987. A case of forked caudal fin inHeteropneustes fossilis (Bloch). Matsya, 12 & 13:183-184.
Kaur, J., Diggins, T. P., Chakraborti, R. K. and Depinto, J. V. 2001. Diet choice of round goby (Neogobius melanostomus) as a function of prey motility and environmental complexity.
International Association for Great Lakes Research, Abstracts from the 44th Conference on Great Lakes Research June 10-14, 2001, Great Lakes Science: Making it Relevant. University of
Wisconsin - Green Bay, Wisconsin Sea Grant Institute, Green Bay, Wisconsin, Part 2:66.
Kavan, L. S., Gilkolaei, S. R., Vossoughi, G., Fatemi S. M. R., Safari, R. and Jamili, S. 2009. Population genetic study of Rutilus frisii kutum (Kamensky 1901) from the Caspian Sea; Iran and
Azerbaijan regions, using microsatellite markers. Journal of Fisheries and Aquatic Science, 4(6):316-322.
Kavraiskii, F. F. 1896-1897. Lososevye (Salmonidae) Kavkaza i Zakavkaz'ya [Die Lachse der Kaukasusländer und ihrer angrenzenden Meere]. Tiflis. No. 1:89 pp., Taf. I-IX; No. 2:79 pp., Taf. X-XIV.
In Russian and German.
Kavraiskii, F. F. 1897. Zametki o rybakh Kavkaza. 1. Ukleiki (g. Alburnus) [Notes on fishes of the Caucasus. 1. Bleaks (Genus Alburnus)]. Izvestiya Kavkazskago Muzeya, Tiflis, 1(1):1-18
(Vestnik Rybopromyshlennosti, St. Petersburg, 1896:405-418). Bemerkungen über Kaukasische Fische I. Die Ucklei-Arten (g. Alburnus). Mittheilungen des Kaukasischen Museums, 1(1):1-20.
Kavraiskii, F. F. 1901. Otchet o komandirovke dlya izucheniya r. Kury i ozer Tiflisskoi gubernii i Karskoi oblasti [Report of the Expedition for Exploration of the Kura River and lakes of the Tiflis
Province and the Kars Region]. St. Petersburg. 70 pp.
Kavraiskii, F. F. 1906. Osetrovye (Acipenseridae) Kavkaza i Zakavkaz'ya [Die Störarten des Kaukasusländer und ihrer angrenzenden Meere]. Izdanie Kavkazskogo Muzeya, Tiflis. 2 + xii +
78 pp.; 2 + xii + 52 pp., 14 Tafs. In Russian and German.
Kay, P. A. and Johnson, D. L. 1981. Estimation of Tigris-Euphrates streamflow from regional paleoenvironmental proxy data. Climatic Change, 3:251-163.
Kazancheev, E. N. 1981. Ryby Kaspiiskogo Morya [Fishes of the Caspian Sea]. Legkaya i Pischchevaya Promyshlennost, Moskva. 167 pp.
Kazemi, R., Bahmani, M., Hallajian, A., Pourkazemi, M., and Dezhandian, S. 2006. Investigation of blood osmo- and ion-regulation of mature and reared juvenile Acipenser persicus. Journal of
Applied Ichthyology, 22(s1):188-192.
Kazemi, R., Bahmani, M., Hallajian, A., Pourkazemi, M.,, Dezhandian, S. and Mojazi Amiri, B. 2005. Determination of optional weight and length of Persian sturgeon's fingerling Acipenser
persicus on the salinity index in the southern part of Caspian Sea. Iranian Scientific Fisheries Journal, 14(3):127-140. In Farsi.
Kazemi, R., Bahmani, M., Hallajian, A. and Yarmohammdi, M. 2006. Blood serum osmotic and ionic regulation of wild adults and reared juvenile Persian
sturgeon, Acipenser persicus. Iranian Journal of Fisheries Sciences, 6(1):43-56.
Kazemi, R., Bahmani, M., Krayushkina, L. S. and Mojazi Amiri, B. 2002. Study on osmoregulation in Persian sturgeon (Acipenser persicus). Ministry of Jihad-e Agriculture Fisheries Company.
77 pp. In Farsi.
Kazemi, R., Bahmani, M., Krayushkina, L. S. and Pourkazemi, M. 2001. Percentage survival and LC50 in Persian sturgeon, Acipenser persicus, fingerlings at different salinities to determine
suitable releasing time. Extended Abstracts of 4th International Symposium on Sturgeon, Wisconsin. 236 pp.
Kazemi, R., Bahmani, M., Krayushkina, L. S., Pourkazemi, M. and Ogorzalek, A. 2003. Changes in blood serum osmolarity and ultrastructure of gill chloride cells in young Persian sturgeon Acipenser
persicus (Borodin) of different sizes during adaptation to sea water. Zoologica Poloniae, 48(1-4):5-30.
Kazemi, R., Bahmani, M. and Romanof, A. 2003. Histological study of different layers of oocyte in stellate sturgeon (Acipenser stellatus). Iranian Journal of Fisheries Sciences, 12(1):93-102,
7. In Farsi.
Kazemipour, Y., Rezaei, M. and Keivany, Y. 2005. Qualitative comparison of effects of garlic and mallow and motherwort extracts in healing of superficial wounds in the common carp (Cyprinus
carpio). Pajouhesh-va-Sazandegi, 17(1)(66):93-97. In Farsi.
Kazemzadeh Khajuie, L., Esmaili S. A. and Ghasempouri, S. M. 2002. Assessment of Haraz River pollution from a rainbow trout farm. Iranian Journal of Marine Sciences, 1(3):27-34, 77. In Farsi.
Kazeraani, B. 1994. Barbels of Iran. Abzeeyan, Tehran, 5(1 & 2):24-25, III. In Farsi.
Kazerooni Monfared, M. 1995. 1995. Rearing Acipenser persicus fingerlings using earthworms. M.Sc. Thesis, Tehran University. 87 pp. In Farsi.
Keddie, W. H. 1971. Fish and futility in Iranian development. Journal of Developing Areas, 6(1):9-28.
Keenleyside, M. H. A. (Ed.). 1991. Cichlid Fishes. Behaviour, ecology and evolution. Chapman & Hall, London. xxi + 378 pp.
Keivan, A., Askarian, F. and Kousha, A. 2006. Investigation on tradition and industrial catching fish methods with emphasis on fish processing effects in southern Iran. World Aquaculture Society,
Aqua 2006, May 9-13. Florence, Italy (abstract).
Keivany, Y. 1996. Taxonomic revision of the genus Pungitius with emphasis on P. hellenicus. M.Sc. Thesis, University of Alberta, Edmonton. 98 pp.
Keivany, Y. 2002. Review of the book "Southern fishes of Iran". Journal of Agricultural Sciences and Natural Resources, 9(3):185-188. In Farsi.
Keivany, Y. 2003a. Review of "Atlas of the Persian Gulf & Sea of Oman Fishes. Vol. 1" and "Morphological and biological characteristics of the southern
Iranian fishes (Persian Gulf and Oman Sea)". Ichthyological Research, 50(2):201-202.
Keivany, Y. 2003b. Superfacial skull bones in Zagros toothcarp, Aphanius valdykovi (Cyprinodontidae). Iranian Journal of Biology, 15(1):25-30.
In Farsi.
Keivany, Y. and Nasrollahzadeh, G. A. 1990. A report on fishing and fishery industries in Iran. Gorgan University College, Gorgan, Iran. MS, 22 pp. (English abstract, 1 p.). In Farsi.
Keivany, Y., Nasrollahzadeh, G. and Saadati, M. 1990. A preliminary study on the Gorgan-Rud aquatic life - a limnological approach (from Gonbad to Gorgan Dam). A Project Submitted for the B.Sc.
(Eng.) Degree, Gorgan University College of Agricultue and Natural Resources, Gorgan, Iran (English abstract, 2 pp.). In Farsi.
Keivany, Y. and Nelson, J. S. 1997. Validity of the Greek ninespine stickleback, Pungitius hellenicus, with comments on the other species. American Society of Ichthyologists and Herpetologists,
77th Annual Meeting, June 26-July 2, 1997, University of Washington, Seattle, Washington. p. 182 (Abstract).
Keivany, Y. and Nelson, J. S. 1998. Comparative osteology of the Greek ninespine stickleback, Pungitius hellenicus (Teleostei, Gasterosteidae). Journal of Ichthyology, 38(6):430-440.
Keivany, Y. and Nelson, J. S. 2004. Phylogenetic relationships of sticklebacks (Gasterosteidae), with emphasis on ninespine sticklebacks (Pungitius spp.). Behaviour, 141(11-12):1485-1497.
Keivany, Y., Nelson, J. S. and Economidis, P. S. 1997. Validity of Pungitius hellenicus Stephanidis, 1971 (Teleostei, Gasterosteidae), a stickleback fish from Greece. Copeia, 1997(3):558-564.
Keivany, Y. and Soofiani, N. M. 2002. Preliminary study of biodiversity in Iranian toothcarps. Abstracts of the First Iranian Conference on Animal Sciences and Biodiversity, University of Kerman,
Iran, p. 30.
Keivany, Y. and Soofiani, N. M. 2003. Biology of the Zagros toothcarp, Aphanius vladykovi (Cyprinodontidae). 11th Iranian Biology Conference, Urmia University, Iran (title).
Keivany, Y. and Soofiani, N. M. 2004a. Biology of a pupfish, Aphanius vladykovi, from Central Iran. Program of the XIXth International Congress of Zoology, August 23-27, 2004,
Beijing, China (title).
Keivany, Y. and Soofiani, N. M. 2004b. Contribution to the biology of Zagros tooth-carp, Aphanius vladykovi (Cyprinodontidae) in central Iran. Environmental Biology of Fishes, 71(2):165-169.
Keivany, Y. and Soofiani, N. M. 2004c. Preliminary study of biodiversity in Iranian toothcarps. Frst Iranian Conference on Animal Sciences and Biodiversity, University of Kerman (abstract).
Keivany, Y. et al. 1992. The suitable springs for trout culture in Chahar Mahal va Bakhtiary Province. Iranian Fishery Company. MS, 173 pp. (English abstract 2 pp.). In Farsi.
Kenari, A. A. and Mirzakhani, M. K. 2005. Effects of using Artemia urmiana enriched with n-3 HUFA in first feeding of rainbow trout (Oncorhynchus
mykiss) larvae. Caspian Journal of Environmental Sciences, 3(2):123-129.
Kell, V. and Saad, M. A. H. 1975. Untersuchungen über das Phytoplankton und einige Umweltparameter des Shatt al-Arab (Irak). Internationale Revue der Gesamten Hydrobiologie, 60(3):409-421.
Kelts, K. and Shahrabi, M. 1986. Holocene sedimentology of hypersaline Lake Urmia, northwestern Iran. Palaeogeography, Palaeoclimatology and Paleoecology, 54:105-130.
Kemp, S., Prashad, B., Hora, S. L., Chopra, B. N. and Rao, H. S. 1925. Nelson Annandale 1876-1924. Records of the Indian Museum, 27:1-28, pl. 1. (S. Kemp is assumed to be the author of the obituary
on the basis of the initials S. K. at the end of this part of the article; the remaining authors compiled the bibliography).
Kennedy, W. P. 1937. Some additions to the fauna of Iraq. Journal of the Bombay Natural History Society, 39:745-749.
Kent, P. E. 1979. The emergent Hormuz salt plugs of southern Iran. Journal of Petroleum Geology, 2(2):117-144.
Keramati, V., Jamili, S. and Ramin, M. 2010. Effect of diazinon on catalase antioxidant enzyme activity in liver tissue of Rutilus rutilus. Journal of Fisheries and Aquatic Science, 5(5):368-376.
Kessler, K. 1870a. Volzhskaya minoga (Petromyzon wagneri n. sp.) [Volga lamprey (Petromyzon wagneri n. sp.)]. Trudy Sankt-Peterburgskogo Obshchestva Estestvoispytatelei, 1:207-214.
Kessler, K. F. 1870b. Opisanie novago vida ryb iz semeistva karpo vykh, Cyprinoidei [Description of a new fish species from the Family Cyprinoidei]. Trudy Sankt-Peterburgskago Obshchestva
Estestvoispytatelei, 1(1):320-323, pl. III.
Kessler, K. F. 1872. Ikhtiologicheskaya fauna Turkestana [Ichthyological fauna of Turkestan]. Izvestiya Obshchestva Lyubitelei Estestvoznaniya, Antropologii i Etnografii, Moskva, 10(1):47-79,
pls. VI-XII.
Kessler, K. 1874a. Opisanie ryb, prinadlezhashchikh k semeistvam, obshchim Chernomu i Kaspiiskomu moryam [Fishes of the Black and Caspian Seas compared and described]. Trudy Sankt-Peterburgskogo
Obshchestva Estestvoispytatelei, 5:191-320, 1 pl.
Kessler, K. F. 1874b. Puteshestvie A. P. Fedchenko v Turkestan. Ryby [A. P. Fedchenko's Travel to Turkestan. Fishes]. Izvestiya Obshchestva Lyubitelei Estestvoznaniya, Antropologii i Etnografii,
Moskva, 11(3):1-63, pls. I-VIII.
Kessler, K. F. 1877. Ryby, vodyashchiesya i vstrechayushchiesya v Aralo-kaspiisko-pontiiskoi ikhtiologicheskoi oblasti [Fishes of the Aral-Caspian-Pontic Ichthyological Region]. Trudy
Aralo-kaspiiskoi ekspeditsii, Sankt- Peterburg, 4:1-360, pls. I-VIII.
Ketmaier, V., Bianco, P. G. and Durand, J.-D. 2008. Molecular systematics, phylogeny and biogeography of roaches (Rutilus, Teleostei, Cyprinidae).
Molecular Phylogenetics and Evolution, 49(1):362-367.
Kevrekidis, T., Kokkinakis, A. K. and Koukouras, A. 1990. Some aspects of the biology and ecology of Knipowitschia caucasica (Teleostei: Gobiidae) in the Evros Delta (North Aegean Sea).
Helgoländer Meeresuntersuchungen, 44:173-187.
Keyserling, E. 1861. Neue Cypriniden aus Persien. Zeitschrift für die Gesammten Naturwissenschaften, Halle, 17(1):1-24, Taf. I-IX.
Keyserling, E. 1863. Neue Cypriniden.... Nouveaux cyprinides de la Perse. Revue et Magazine de Zoologie, 15:419-422. (In French without plates).
Keyvan, A. 2002. Introduction to biotechnology of cultured sturgeon. Azad University, Lahijan. 270 pp. In Farsi.
Keyvan, A. 2003. Iranian sturgeons in the Caspian Sea (Systematic, biology, artificial propagation, biomass evaluation and conservation, fishing and production of caviar). Iranian Fisheries
Company, Tehran. 400 pp. In Farsi.
Keyvan, A. and Khanipour, A. 1997. The result of using trammel net in Iranian coastal waters of Caspian Sea to catch intact healthy productive specimens for artificial culturing. Third International
Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (poster).
Keyvan, A., Moini, S., Ghaemi, N., Haghdoost, A. A., Jalili, S. and Pourkabir, M. 2008. Effect of frozen storage time on the lipid deterioration and protein denaturation during Caspian Sea white fish
(Rutilus frisii kutum). Journal of Fisheries and Aquatic Sciences, 3(6):404-409.
Keyvan, A. and Nasrichari, A. 1997. The result of biometrical and biochemical studies in Iranian coastal waters of Caspian Sea on the independence of Acipenser persicus from
Acipenser gueldenstaedtii. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (poster).
Keyvan Shokoo, S., Pourkazemi, M. and Kalbassi, M. R. 2004. The possibility of sex identification in beluga (Huso huso) by using PCR-RAPD technique. Iranian Journal
of Fisheries Sciences, 13(1):149-162. In Farsi.
Keyvanfar, A. 1954. Caviar et l'art de sa fabrication. Revue de la Faculté de Médecine vétérinaire de Téhéran, 14(2-4):2-18. In Farsi. Résumé 1 pp.
Keyvanfar, A. 1955. Caviar. Tehran. In Farsi.
Keyvanfar, A. 1981. Technologie du Caviar des Esturgeons de la mer Caspienne. Bulletin de l'Institut de Nutrition et d'Alimentation, Téhéran. 3ième Edition, 27:55 pp. In
Farsi. Résumé 9 pp. In French.
Keyvanfar, A. 1984. Absence de polymorphisme génétique au niveau des érythrocytes de quatre espèces d'Acipenseridae de la mer Caspienne, capturées sur les
côtes d'Iran. Comptes Rendus de l'Académie des Sciences, Paris, 299, série III, 12:503-506.
Keyvanfar, A. 1986. Existence d'un polymorphisme de la transferrine chez une espèce d'esturgeon de la mer Caspienne: Acipenser stellatus. Comptes Rendus de l'Académie des Sciences,
Paris, 302, série III, 11:401-404.
Keyvanfar, A. 1988. Étude comparative des protéines sériques et cellulaires de quatre espèces d'esturgeons anadromes de la mer Caspienne. Annales de l'Institut
océanographique, Paris, 64(1):25-64.
Keyvanfar, A. and Khanipour, A. 1999. Using trammel net in coastal waters of Iran to catch healthy broodstock. Journal of Applied Ichthyology, 15(4-5):301.
Keyvanfar, A. and Nasrichari, A. 1999. Biometric and biotechnical studies of the independence of Acipenser persicus from Acipenser gueldenstaedtii in Iranian coastal waters of the
Caspian Sea. Journal of Applied Ichthyology, 15(4-5):281.
Keyvanfar, A., Rochu, D., Marneux, M., Herance, N. et Fine, J.-M. 1987. Différenciation par focalisation isoélectrique des protéines de caviar de quatre espèces et
d'une sous-espèce d'Esturgeons anadromes de la mer Caspienne. Comptes Rendus de l'Académie des Sciences, Paris, 304, série III, 9:191-193.
Keyvanshokooh, S. and Kalbassi, M. R. 2006. Genetic variation of Rutilus rutilus caspicus (Jakowlew 1870) populations in Iran based on random amplified polymorphic DNA markers: a
preliminary study. Aquaculture Research, 37(14):1437-1440.
Keyvanshokooh, S., Ghasemi, A., Shahriari-Moghadam, M., Nazari, R. M. and Rahimpour, M. 2007. Genetic analysis of Rutilus rutilus caspicus (Jakowlew
1870) populations in Iran by microsatellite markers. Aquaculture Research, 38(9):953-956.
Keyvanshokooh, S., Pourkazemi, M. and Kalbassi, M. R. 2007. The RAPD technique failed to identify sex-specific sequences in beluga (Huso huso). Journal of Applied Ichthyology, 23(1):1-2.
Khadjeh, G. H. 2005. Study of some biochemical parameters of rainbow trout (Oncorhynchus mykiss) in earthen pond of Abadan region, Iran. Sixth Symposium on Diseases in Asian Aquaculture
(DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Khairkhah, A. 1994. Seafood consumption in Rasht. Abzeeyan, Tehran, 5(3 & 4):23, IV. In Farsi.
Khajeh, Gh. H. and Peyghan, R. 2007. Evaluation of some blood serum biochemical parameters of rainbow trout (Oncorhynchus mykiss) cultured in earthen ponds. Journal of Veterinary Rresearch,
62(3):197-203. In Farsi.
Khajeh, Gh. H., Peyghan, R., Mesbah, M. and Rasekh, A. A. R. 2008. A comparative study on haematological parameters of culturing benni (Barbus sharpeyi) and grass carp (Ctenopharyngodon
idella). Iranian Veterinary Journal, 4(1)(18):24-36. In Farsi.
Khajeh, M. and Alaghi, K. H. 1998. A study of age, growth and reproduction of roach, Rutilus rutilus caspicus, in Gomishan Wetland. B.Sc. Project, University of Gorgan, Gorgan. In Farsi.
Khajehpour-Khouei, B. 2000. Survey of Iran's economic interests in the Caspian, p. 75-86. In: Amirahmadi, H. (Ed.). The Caspian Region at a Crossroad. Challenges of a New Frontier of Energy and
Development. St. Martin's Press, New York. xii + 299 pp.
Khalaf, A. N., Al-Jafery, A., Allouse, S. B. and Sadek, S. E. 1986. Observations on the age and growth of Chondrostoma regius (sic) (Heckel) in Diyala River. Journal of Biological
Sciences Research, Baghdad, 17(2):83-98.
Khalaf, A. N., Al-Jafery, A. R., Khalid, B. Y., Elias, S. S. and Ishaq, M. W. 1985. The patterns of accumulation of some heavy metals in Barbus grypus (Heckel) from a polluted river. Journal of
Biological Sciences Research, Baghdad, 16(2):51-75.
Khalaf, A. N., Allouse, S. B., Al-Yamour, K. Y., Al-Jafary, A. R. and Sadek, S. E. 1987. Food and feeding of the catfish Heteropneustes fossilis (Bloch) from a polluted river. Journal
of Environmental Science and Health, A22(5):397-410.
Khalaf, A. N., Al-Yamour, K. Y., Allouse, S. B., Al-Jafary, A. and Sadek, S. E. 1986. Age, growth, length-weight relationships, and distribution of khishni Liza abu (Heckel)
(Mugilidae) in a polluted habitat. Journal of Biological Sciences Research, Baghdad, 17(2):63-81.
Khalaf, A. N., Mansoor, K. Y., Al-Jafery, A. R., Allouse, S. B., Penaz, M. and Sadek, S. E. 1988. Growth pattern of the Barbus luteus population inhabiting a flooded Iraqi gravelpit
near Al-Nibaey. Journal of Biological Sciences Research, Baghdad, 19(3):681-692.
Khalaf, A. N., Shafi, M., Islam, A. K. M. S., Al-Jafery, A. R. and Sadek, S. E. 1984. Age and growth of Barbus grypus Heckel from a polluted river. Environmental Pollution (Series A), 35:83-95.
Khalaf, G. 1985. Etude de quelques aspects du régime alimentaire de Capoeta damascina (Cyprinidae) dans les cours d'eau libanais. Verhandlungen der internationalen Vereinigung
für theoretische und angewandte Limnologie, 22:2631-2635.
Khalaf, G. 1987. Le cycle sexuel de Capoeta damascina (Cyprinidae) dans les cours d'eau libanais. Cybium, 11(4):395-401.
Khalaf, G., Kraïem, M. M. and Nakhlé, K. 2002. Dynamique de populations de Capoeta damascina (poisson, Cyprinidae) dans un cours d'eau Libanais. Lebanese Science Journal, 3(1):17-26.
Khalaf, K. T. 1961. The marine and freshwater fishes of Iraq. Ar-Rabitta Press, Baghdad. 164 pp.
Khalaji-Pirbalouty, V. and Sari, A. 2004. Biogeography of amphipods (Crustacea: Amphipoda: Gammaridae) from the central Zagros Mountains, Iran, with descriptions of two new species. Journal of
Natural History, 38(19):2425-2445.
Khalili, K. J. and Amirkolaie, A. K. 2010. Comparison of carp (Cyprinus carpio L.) morphological and electrophoretic characteristics in the southern coast of the Caspian Sea. Journal of
Fisheries and Aquatic Sciences 5(3):200-207.
Khalili, S. M. 1994. A study of causes and the prevention of pollution in the Caspian Sea. Bandar va Darya, Tehran, 49-50:26-28. In Farsi.
Khan, A. A. 1988. Food and feeding habits of a freshwater carp, Cyprinion macrostomus (Heckel) from Sulaimaniyah, Iraq. Indian Journal of Fisheries, 35(4):323-326.
Khan, A. A., Scott, D. A. and Smart, M. 1992. Wetlands of the Seistan Basin, South Caspian and Fars, Islamic Republic of Iran. Ramsar Convention Monitoring Procedure, Ramsar Convention Bureau,
Gland, Report No. 26:37 pp., 1 table, 3 figures, 6 appendices.
Khancheh-Sepehrredin, K. 2000. Survey on Diplostomum spathaceum in warm water fish cultured and natural water resources in Sanandaj-Kurdistan. DVM Thesis, Urmia Islamic Azad University, No. 394.
In Farsi.
Khara, H. 1994. The Protected Region of Siah-Kesheem. Abzeeyan, Tehran, 5(3 & 4):10-17, III. In Farsi.
Khara, H., Bagherzadeh, D., Yousefi, M., Sattari, M., Nezami Balochi, Sh. and Mirhasheminasab, S. F. 2005. Occurrence and intensity of parasites from tench (Tinca tinca L., 1758) in Amirkelayeh
wetland of Lahijan. Iranian Journal of Biology, 18(3):180-190. In Farsi.
Khara, H., Keyvan, A., Nezami, Sh., Mehdinejad, K. and Moahmmadjani, T. 2002. Diet of Rutilus frisii kutum x Ctenopharyngodon idella hybrid. Iranian Journal
of Fisheries Sciences, 11(2):31-42. In Farsi.
Khara, H., Keyvan, A., Pourkazemi, M., Vosoughi, Gh. H., Rezvani, S., Nezami, Sh. A., Hasanzadeh, M., Ghasemi, S. A., Ahmadnezhad, M. and Ghanaatparast, A. 2009. A survey of genetic diversity
of bream (Abramis brama orientalis) in Anzali Wetland, south coast of Caspian Sea (Iran) and southwest coast of Caspian Sea (Azerbaijan Republic). Iranian Journal of Biology, 21(5):849-856.
In Farsi.
Khara, H., Keyvan, A., Vosoughi, G., Pourkazemi , M., Rezvani, S., Nezami , S. A., Ramin, M., Sarpanah, A. N. and Ghanaatparast, A. 2007. Comparison of morphometric and meristic of bream
(Abramis brama orientalis Berg 1905), in Caspian Sea and Anzali wetland. Pajouhesh va Sazandegi,
19(4)(73):177-187. In Farsi.
Khara, H., Keyvan, A., Vosoughi, Gh. H., Pourkazemi, M., Rezvani, S., Nezami, Sh. A., Ramin, M., Sarpanah, A. N. and Ahmadnezhad, M. 2007. Comparison of morphometric and meristic attributes of bream
(Abramis brama orientalis) in Caspian Sea and Aras Dam reservoir. Iranian Scientific Fisheries Journal, 15(4):33-48. In Farsi.
Khara, H., Nezami, A., Jafarzadeh, A., Ajang, B., Satari, M. and Mosavi, S. A. 2004. Occurrence and intensity of parasites from pike (Esox lucius Linnaeus, 1758) in Amir Kelaieh Lagoon.
Journal of Veterinary Research, 59(4):333-339. In Farsi.
Khara, H., Nezami, A., Satari, M., Mousavi, A. Mousapour, M. S. and Hajipour, A. 2005. Occurrence and intensity of parasites from perch (Perca fluviatilis, L.1785)(sic) in Amirkelaieh
Wetland of Lahijan (Caspian basin - Iran). Pajouhesh va Sazandegi, 18(2)(67):92-103. In Farsi.
Khara, H., Nezami, S. and Sakari, M. 2004. Fish diversity in Boojagh marine and land National Park in the southern part of the Caspian Sea of Iran. Biology in Asia International Conference, Singapore,
7-10 December 2004 (abstract).
Khara, H., Nezami, Sh. A., Sattari, M., Mirhasheminasab, S. F. and Mousavi, S. A. 2006a. An investigation on fish infection with Diplostomum spathecum in Amirkalayeh
Wetland. Iranian Journal of Fisheries Sciences, 14(4):49-66. In Farsi.
Khara, H., Nezami, Sh. A., Sattari, M., Mirhasheminasab, S. F. and Mousavi, S. A. 2006b. An investigation on digestive parasites of fishes in Boojagh Wetland, north Iran. Iranian Journal
of Fisheries Sciences, 15(2):9-18. In Farsi.
Khara, H., Nezami, Sh. A., Sattari, M., Mirhasheminasab, S. F. and Mousavi Seyed, A. 2008. An investigation on fish infection with Diplostomum spatheceum (Rudolphi, 1891) in Boojgah Wetland.
Iranian Journal of Biology, 20(4):418-429.
Khara, H., Nezami, Sh., Sattari, M., Mousavi Seyed, A., Kousari, A., Daneshvar, S. and Ali Nia, M. R. 2007. Occurrence and intensity of parasites in pike (Esox lucius) in river of Chamkhaleh.
Iranian Scientific Fisheries Journal, 16(2):37-50. In Farsi.
Khara, H. and Nezami Baluchie, Sh. 2005. Studying fish biodiversity and abundance in Boujagh Wetland of Kiashar, south-western Caspian Sea. Iranian Scientific
Fisheries Journal, 13(4):41-54. In Farsi.
Khara, H., Sattari, M. and Nezami, S. 2005. Occurrence and intensity of some parasites in Rutilus rutilus caspicus (Cypriniformes: Cyprinidae) from the south-west of the Caspian Sea.
Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Khara, H., Sattari, M., Nezami, S., Mirhasheminasab, S. F. and Mousavi, S. A. 2005. Parasites of some bonyfishes in Amirkelayeh wetland from the southwest of the Caspian Sea. 12th EAFP International
Conference on Diseases of Fish and Shellfish, Copenhagen (title).
Khara, H., Sattari, M., Nezami, S. A., Mosavi, S. A., Jafarzadeh, A. and Ajang, H. 2004. Occurrence and intensity of parasites from pike (Esox lucius Linnaeus, 1758) in Amir Kelaieh Lagoon.
Journal of the Faculty of Veterinary Medicine, University of Tehran, 59(4):333-339. In Farsi.
Khatami, S. H. and Shayegan, S. J. 2003. Classification of springs in Kermanshah Province (Iran) based on water quality for sustainable sarabs utilization.
Germany-Iran Alumni Network, Ist International Symposium cum-Workshop "Sustainable Resource Utilization: Local Structures vs Globalization, September 14-18, Tehran University, pp. 311-320.
Khatib Haghighi, S. 1996. Effect of coloured gillnet on sturgeon fishing in the southwest of the Caspian Sea. Iranian Fisheries Scientific Journal, 5(3):51-60, 5. In Farsi.
Khatoonabadai, A. and Dehcheshmeh, A. R. M. 2006. Oil pollution in the Caspian Sea coastal waters. International Journal of Environment and Pollution, 26(4):347-363.
Khaval, A. 1998. The migration of Rutilus frissi (sic) kutum, Vimba vimba persa and Calcalburnus (sic) chalcoides to the Sefidrud River. Iranian Fisheries Scientific Journal,
6(4):75-86, 7. In Farsi.
Khaval, A. 2007. Experiments on polyculture of Sander lucioperca with Chinese carp. Iranian Scientific Fisheries Journal, 16(1):39-48. In Farsi.
Khazraiinia, P., Payghan, R. and Azari Takami, Gh. 2001. Studies on the effect of experimental acute ammonia toxicity on serum enzymes, urea and cholesterol in common carp. Journal of the Faculty of
Veterinary Medicine, University of Tehran, 55(3):29-32. In Farsi.
Kheyrandish, A., Abdoli, A., Mostafvi, H., Niksirat, H., Naderi, M. and Vatandoost, S. 2010. Age and growth of brown trout (Salmo trutta) in six rivers of the southern part of Caspian basin.
American Journal of Animal and Veterinary Sciences, 5(1):8-12.
Khlofe Nilsaz, M. 1996. Limnological study of Karoon River (Gotevand to Band-e-Ghir). Iranian Fisheries Scientific Journal, 4(4):25-32, 3. In Farsi.
Khodabandeh, S. and Abtahi, B. 2006. Effects of sodium chloride, formalin and iodine on the hatching success of common carp, Cyprinus carpio. Journal of Applied Ichthyology, 22(1):54-56.
Khodabandeh S., Esmaili Sari, A., Abtahi, B. and Seifabadi, J. 2001. Distribution and density of ctenophores along the southern reach of the Caspian Sea. The Second International Iran and Russia
Conference (Agriculture & Natural Resources), Moscow. Abstract, p. 167.
Khodabandeh, S, Esmaili Sari, A., Talaei, R. and Sifabadi, B. 2002. Regime alimentaire d'un cnidaire envahissant, Mnemiopsis leidyi, le long de la cote iranienne de la Mer Caspienne.
Laboratoire d'Ecophysiologie des Invertebres, Université Montpellier, Brest (title).
Khodabandeh, S., Mosafer, S., Khoshnood, Z. and Tolouei, M. 2007. Salinity tolerance capacity in Persian sturgeon, Acipenser persicus, fry. Comparative Biochemistry and Physiology,
Part A: Molecular and Integrative Physiology, 146(4):S93-S94.
Khodabandeh, S. and Shahriari Moghadam, M. 2007. Ultra-structure of ionocyte cells in gills of Silurus glanis of Mahabad Dam, Kordestan Province. Iranian Scientific Fisheries Journal,
16(2):51-62. In Farsi.
Khodabandeh, S. and Taghizadeh, Z. 2006. Immunolocalization of Na+, K+, ATPase and ionocytes in ills of catfish, Silurus glanis. Yakhteh, 8(1):45-52. In Farsi.
Khodjeini, A. V. and Mohamed, A. 1975. Etude du debit solide et de la sedimentation du barrage de Shah-Banou Farah. Bulletin des Sciences Hydrologiques, 20:223-231.
Khodorevskaya, R. P. 1999. Formation of commercial stock of Huso huso in the Volga-Caspian region by hatchery reproduction. Journal of Ichthyology, 39(9):807-810.
Khodorevskaya, R. P., Dovgopol, G. F., Zhuravleva, O. L. and Vlasenko, A. D. 1997. Present status of commercial stocks of sturgeons in the Caspian Sea. Environmental Biology of Fishes,
48(1-4):209-219.
Khodorevskaya, R. P. and Krasikov, Ye. V. 1999. Sturgeon abundance and distribution in the Caspian Sea. Journal of Applied Ichthyology, 15(4-5):106-113.
Khodorevskaya, R. P., Krasikov, E. V., Fedin, A. A., Fedorov, V. A. and Shvedov, V. V. 2001. Abundance and distribution of the Russian sturgeon Acipenser gueldenstaedtii in the Caspian Sea.
Journal of Ichthyology, 41(5):368-375.
Khodorevskaya, R. P., Krasikov, E. V., Dovgopol, G. F. and Zhuravlev, O. L. 2000. Formation of the stock of Caspian acipenserids under present-day conditions. Journal of Ichthyology, 40(8):602-609.
Khodorevskaya, R. P. and Novikova, A. S. 1995. Status of beluga sturgeon, Huso huso, in the Caspian Sea. Journal of Ichthyology, 35(9):59-68.
Khojeini, A. V. 2003. Fluctuations of the Caspian Sea p. 77 (abstract). Global Threats to Large Lakes, 46th Conference on Great Lakes Research, 10th World Lake Conference, June 22-26, 2003, DePaul
University, Chicago. iv + 304 pp.
Khorasani, N. 2001. An environmental study of Jajrood River fauna and flora. Iranian Journal of Natural Resources, 54(1):31-4. In Farsi.
Khorasani, N. A., Farshchi, P., Monavari, M. and Jabari, H. 2004. Determination of the environment consequences of Shahr Chai Dam on natural environment of the region. Journal of Environmental Science
and Technology, 2004(19):39-50. In Farsi.
Khorasani, N. A. and Rokni, M. A. 2001. Environmental study on floodgates of "Seyed Mahale" and Lapoye Zarrinkola". Journal of Environmental Science and Technology, 2001(7-8):91-101.
In Farsi.
Khorashadizadeh, M. A., Abtahi, B., Kazemi, R. and Fazli, H. 2006. Anatomical and histological study of ovary development stages of big-eye kilka (Clupeonella
grimmi) in Babolsar area, Iran. Iranian Journal of Fisheries Sciences, 15(3):61-74. In Farsi.
Khoroshko, A. I. 1982. Population abundance and structure in the long-finned mullet (genus Liza, Mugilidae) during acclimation in the Caspian Sea. Journal of Ichthyology, 22(6):62-69.
Khorshidi, K. J., Askarian, F. and Kousha, A. 2006. Review of use of glucan as immunistimulant in diet of fish. World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Khosh Kholgh, M. R. 1994. Studying Oligochaeta density and distribution in the southern coasts of the Caspian Sea. Iranian Fisheries Scientific Journal, 3(3):39-51, 4. In Farsi.
Khoshbavar-Rostami, H. A. 2006. Toxicity, hematological changes and chemiluminescence response of great sturgeon Huso huso following exposure
to polyaromatic hydrocarbons (PAHs). World Aquaculture Society, Aqua 2006, May 9-13, Florence, Italy (abstract).
Khoshbavar Rostami, H. A. and Soltani, M. 2005. The effects of diazinon on haematological indices and LC50 (96h) of Acipenser nudiventris. Iranian Journal
of Fisheries Sciences, 14(3):49-60. In Farsi.
Khoshbavar Rostami, H. A., Soltani, M. and Hassan, H. M. D. 2004. Acute toxicity and some haematological and biochemical changes in giant sturgeon (Huso huso) exposed to diazinon. Bulletin
of the European Association of Fish Pathologists, 24(2):92-99.
Khoshbavar Rostami, H. A., Soltani, M. and Hassan, H. M. D. 2006. Some haematological and biochemical changes in blood serum of beluga (Huso huso) after chronic exposure to diazinon. Iranian
Journal of Fisheries Science, 5(2):53-66.
Khoshbavar Rostami, H. A., Soltani, M. and Hassan, H. M. D. 2007. Immune response of great sturgeon Huso huso to Aeromonas hydrophila bacterin. Journal of Fish Biology,
70(6):1931-1938.
Khoshbavar-Rostami, H. A., Soltani, M. and Mokarami, A. 2006. Toxicity, hematological changes and chemiluminescnce response of great sturgeon Huso huso following exposure to polyaromatic
hydrocarbons (PAHs). World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Khoshbavar-Rostami, H. A., Soltani, M. and Yelghi, S. 2005. Effects of diazinon on the haematological profiles of Acipenser stellatus and determination of LC50. Journal of
Agricultural Sciences and Natural Resources, 12(5):100-108. In Farsi.
Khoshkholgh, M. 2007. Genetic population structure of Persian sturgeon (Acipenser persicus) and Russian sturgeon (Acipenser guldenstadti) in south of Caspian Sea using microsatellite
markers. Ph.D. Thesis, Gorgan University of Agricultural Science and Natural Resources, Gorgan. 145 pp. In Farsi.
Khoshzahmat, A., Tadjalli-pour, M. and Gafari Sadeghi, M. 1981. The study of the molluscs in the Parishan Lake and fish nutrition from molluscs point of view. Journal of the Veterinary Faculty
of the University of Tehran, 37(3):23-36. In Farsi.
Khoushnoud, Z., Khodabandeh, S. and Mosafer Khourjestan, S. 2009. Immunolocalization of Na+, K+-ATPase enzyme and gill chloride cells in fries of Persian sturgeon,
Acipenser persicus. Iranian Scientific Fisheries Journal, 17(4):17-26. In Farsi.
Kiabi, B. H. 2004. Biodiversity of fish species in inland waters of north-east parts of Iran. Program of the XIXth International Congress of Zoology, August 23-27, 2004, Beijing, China (title).
Kiabi, B. H. and Abdoli, A. 1999. A preliminary study of inland water fish distribution and abundance in Hormozgan Province (with emphasis on endemic species in rivers). Seventh International
Symposium on the Ecology of Fluvial Fishes, 10-13 May 1999, Lodz, Poland (title).
Kiabi, B. H. and Abdoli, A. 2000. Fish distribution and abundance in the inland waters of Hormuzgan Province, Iran, with particular reference to endemic species in rivers. Polskie Archiwum
Hydrobiologii, 47(1):87-98.
Kiabi, B. H., Abdoli, A. and Naderi, M. 1999. Status of the fish fauna in the South Caspian Basin of Iran. Zoology in the Middle East, 18:57-65.
Kiabi, B. H., Ghaemi, R. and Abdoli, A. 1999. Wetland and riverain ecosystems of Golestan province. Department of the Environment, Golestan Provincial Office of Department of the Environment, Gorgan.
182 pp. In Farsi.
Kiabi, B. H., Majnoonian, H. G. and Mansoori, J. 2004. Suggested criteria to evaluate the conservation status of Iran wetlands. Journal of Environmental Studies, 33:74-89. In Farsi.
Kiabi, B, H.-Z., Zehzad, B., Darreh-Shoori, B. F., Majnounian, H. and Meigouni, H. G. 1994. Golestan National Park. Department of the Environment, Tehran. 203 pp. In Farsi.
Kideys, A., Finenko, G. A., Anninsky, B. E., Shiganova, T. A., Roohi, A., Tababri, M. R., Youseffyan, M., Rostamian, M. T., Rostami, H. and Negarestan, H. 2004. Physiological characteristics of the
ctenophore Beroe ovata in Caspian Sea water. Marine Ecology Progress Series, 266:111-121.
Kideys, A. E. 2002a. Fall and rise of the Black Sea ecosystem. Science, 297(5586):1482-1484.
Kideys, A. E. 2002b. The invasive ctenophore Mnemiopsis problem in the Black and Caspian Seas. Biomare Newsletter, 3:5-6 pp. (www.biomareweb.org/3.3.html).
Kideys, A. E. and Moghim, M. 2003. Distribution of the alien ctenophore Mnemiopsis leidyi in the Caspian Sea in August 2001. Marine Biology, 142:163-171.
Kiener, A. et Spillmann, C. J. 1972. Note complémentaire à l'étude systématique et écologique d'Atherina boyeri Risso (Poissons, Cyprinidae) dans sa zone
de dispersion actuelle. Bulletin du Muséum national d'Histoire naturelle, Zoologie, Paris, série 3, numéro 55, 41:563-580.
Kılıç Demirok, N. and Ünlü, E. 2001. Karyotypes of cyprinid fish Capoeta trutta and Capoeta capoeta umbla (Cyprinidae) from the Tigris River. Turkish Journal of
Zoology, 25(4):389-393.
Kimball, K. D. 1973. A preliminary investigation of Caspian Sea water penetration into the effluent rivers of the Pahlavi marsh. Technical Report, Fisheries Research Institute, Bandar Pahlavi,
Iran. MS, 5 pp.
Kimball, K. D. and Kimball, S. F. 1974. The limnology of the Pahlavi Mordab, Iran: A study of eutrophication problems. Human Environment Division, Department of the Environment, Iran. MS, 43 pp.
Kimball, K. D. and Shayegan, H. 1973. A limnological study of the Pahlavi marsh and its effluent river system during the year (1971-1972) 1350. Technical Report, Fisheries Research Institute,
Bandar Pahlavi, Iran. MS, 40 pp.
Kingsford, R. (ed.). 2006. Ecology of Desert Rivers. Cambridge University Press. xiv + 354 pp.
Kinneir, J. M. 1813. A Geographical Memoir of the Persian Empire, accompanied by a map. John Murray, London. viii + 486 pp.
Kinunen, W. 1970. Caspian salmon. Progress Report 1970, Iran Game and Fish Department, Tehran, pp. 5-6.
Kinunen, W. 1972. Lar River creel census. Report No. S-2-51-1, Department of Environmental Conservation, Tehran. MS.
Kinunen, W., Bullock, S. and Bosch, M. 1970. Nam-rud and Dali-chay Survey. Progress Report 1970, Iran Game and Fish Department, Tehran, pp. 9-10.
Kirilenko, E. V. and Shemonaev, E. V. 2010. Some features of biology of big-headed goby Neogobius gorlap (Perciformes, Gobiidae) in waters of the Kuibyshev Reservoir. Journal of Ichthyology,
50(8):627-631.
Kiselevich, K. A. 1923. Kaspiisko-volzhskie sel'di. Chast' I. Sistematika [Volga-Caspian herrings. Part I. Systematics]. Trudy Astrakhanskoi nauchno-promyslovoi ekspeditsii 1914-1915 gg.,
2(1):147 pp., + 55 pp.
Kizina, L. P. 1986. Some data on the biology of the genus Carassius from the lower reaches of the Volga Delta. Journal of Ichthyology, 26(4):31-40.
Klee, A. J. 1967. Letters to the Editor. Journal of the American Killifish Association, 4(2):30-31.
Klett, V. and Meyer, A. 2002. What, if anything, is a Tilapia? - mitochondrial ND2 phylogeny of tilapiines and the evolution of parental care systems in the African cichlid fishes. Molecular
Biology and Evolution, 19(6):865-883.
Klige, R. K. and Myagkov, M. S. 1992. Changes in the water regime of the Caspian Sea. GeoJournal, 27(3):299-307.
Klinkhardt, M., Tesche, M. and Greven, H. 1995. Database of Fish Chromosomes. Westarp Wissenschaften, Magdeburg. 237 pp.
Klykov, A. A., Morozov, A. V., Smetanin, K. A. and Bulgakov, G. P. 1929. Rybnoe khozyaistvo Turkmenistana (Kaspiiskoe poberezh'e) [Fisheries of Turkmenistan. The Caspian Coast]. Trudy Nauchnogo
Instituta Rybnogo Khozyaistva, 5(1):216 pp.
Knipovich, N. M. 1921. Gidrologicheskie issledovaniya v Kaspiiiskom more v 1914-1915 g. [Hydrological investigations in the Caspian Sea in the years 1914-1915]. Trudy Kaspiiskoi Ekspeditsii,
1914-1915 gg., Petrograd, 1:xxviii + 943 pp.
Kobayakawa, M. 1989. Systematic revision of the catfish genus Silurus, with description of a new species from Thailand and Burma. Japanese Journal of Ichthyology, 36(2):155-186.
Kobori, I. and Glantz, M. H. (Eds.). 1998. Central Asian Water Crisis: Caspian, Aral, and Dead Seas. United Nations University Press (www.unu.edu/unupress/unupbooks/uu18ce/uu18ce00.htm,
downloaded 28 August 2002).
Kochakian Sabour, A. 1996. Providing frozen minced fish meat. Iranian Fisheries Scientific Journal, 5(1):18-24, 2-3. In Farsi.
Kohnechahry (sic), M. et Heydarpur, E. 1973. Élevage de truites et de carpes dans l'eau immobill (sic) des lacs à l'interieur de nasses immergées. Journal of the
Veterinary Faculty of the University of Tehran, 29(2):21-29. In Farsi.
Kohneshahri, M. and Azari Takami, G. 1974. Artificial Propagation of Sturgeons. University of Tehran Publications 1451, Tehran. 298 pp. In Farsi.
Kokoza, A. A., Kamolikova, L. I. and Izmailova, N. A. 1995. [Artificial breeding of sturgeons]. Rybnoe Khozyaistvo, 2:27-28.
Kolar, C. S., Chapman, D. C., Courtenay, W. R., Housel, C. M., Williams, J. D. and Jennings, D. P. 2005. Asian carps of the genus Hypophthalmichthys (Pisces, Cyprinidae) - a biological
synopsis and environmental risk assessment. Report to the U.S. Fish and Wildlife Service per Interagency Agreement 94400-3-0128. vi + 175 pp. (www.asiancarp.org/risks.asp).
Kolar, C. S. and Lodge, D. M. 2002. Ecological predictions and risk assessment for alien fishes in North America. Science, 298:1233-1236.
Kompani-Zare, M. and Moore, F. 2001. Chemical thermometry and origin of the Dalaki mineral springs, Bushehr Province, Iran. Journal of Hydrology, New Zealand, 40(2):189-204.
Konar, V., Canpolat, A., Yılmaz, Ö. and Gürsu, F. 1999. Capoeta trutta ve Barbus rajanorum mystaceus'un Kas Dokularındaki Total Lipit ve Yağ Asidi Miktar ve
Bileşimlerinin Üreme Periyodu Süresince Değişimi [Variation of total lipid and fatty acid amount and compositions in muscles of Capoeta
trutta and Barbus rajanorum mystaceus during reproduction period]. Turkish Journal of Biology, 23(3):319-330.
Koocheki, A. 1996. Qanat, a sustainable ancient system for exploitation of underground water in Iran. 11th IFOAM (International Federation of Organic Agriculture Movements) Scientific Conference,
11-15 August 1996, Copenhagen, ifoam '96, Book of Abstracts. S18.
Koochekian, A., Ghorban, Z. and Yousefi, A. 2006. Production of isin glass from the swim bladder of sturgeons. Journal of Applied Ichthyology, 22(supplement S1):419-421.
Koochekian Sabour, A. and Moini, S. 2009. Producing fish sauce from Caspian kilka. Iranian Journal of Fisheries Sciences, 8(2):155-162.
Koohestan Eskandari, S. 2003. Population dynamic of Capoeta capoeta gracilis in Madarsoo Stream in Golestan National Park. Iranian Journal of Marine Sciences, 2(2-3):11-20. In Farsi.
Köpp, H. and Yachkaschi, A. 1978. Development and status of protected areas in Iran. Parks, 2(4):11-13.
Kopylets, S. K. and Dukravets, G. M. 1981. Morfometricheskaya i biologicheskaya kharakteristika bychka Rhinogobius similis Gill., sluchainogo vselentsa v bassein reki Ili [Morphological and
biological characteristics of the goby Rhinogobius similis Gill., an adventitious species from the Ili River]. Voprosii Ikhtiologii, 21(4):600-607.
Kor, D., Fatemi, S. M. R. and Keymaram, F. 2008. The abundance and species composition of sturgeon in the northern Caspian Sea. Iranian Scientific Fisheries Journal, 17(3):165-170. In Farsi.
Kor, D., Keymaram, F., Moghim, M., Janbaz, A. A., Darya Nabard, Gh. R. and Bagherzadeh Afrouzi, F. 2007. Population structure of sturgeons in the coastal waters of Mazandaran Province, south Caspian
Sea. Iranian Scientific Fisheries Journal, 16(3):91-102. In Farsi.
Kordjazi, M., Imanpour, M. R. and Shabanpour B. 2009. The correlation between some water ionic and nonionic parameters with haematocrit indicator growth and survival of common carp in culturative
ponds. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A). In Farsi.
Kordjazi, Z., Kamali, A., Yaghmaii, F. and Mohammad Nazari, R. 2005. Larval behaviour and melanin plug status in the spiral intestine of Persian sturgeon (Acipenser persicus)
larvae in the timing of initial feeding. Journal of Agricultural Sciences and Natural Resources, 12(3):155-164. In Farsi.
Kordjazi, Z., Kamali, A., Yaghmaei, F. and Nazari, R. M. 2004. Effect of the timing of initial feeding on growth and survival of Persian sturgeon (Acipenser persicus) larvae. Iranian
Scientific Fisheries Journal, 13(1):115-128. In Farsi.
Kornfield, I. L. and Nevo, E. 1976. Likely pre-Suez occurrence of Red Sea fish Aphanius dispar in the Mediterranean. Nature, London, 264:289-291.
Korki, M. 1992. Hawr-al-Azim or Hawr-al-Hoveyzeh (a nature inside the nature). Abzeeyan, Tehran, 3:82-83. In Farsi.
Kosarev, A. N. and Yablonskaya, E. A. 1994. The Caspian Sea. SPB Academic Publishing, The Hague. xiii + 259 pp.
Koshara, A. V. and Izyumov, Yu. G. 1991. The intraspecific systematics of the bream, Abramis brama (Cypriniformes, Cyprinidae). Journal of Ichthyology, 31(8):29-41.
Kosswig, C. 1951. Contributions to the knowledge of the zoogeographical situation in the Near and Middle East. Experientia, 7(2):401-406.
Kosswig, C. 1952. Die Zoogeographie der türkischen Süßwasserfische. Istanbul Üniversitesi Fen Fakültesi Hidrobiologi Araştirma Enstitüsü
Yayınlarından, Seri B, 1(2):85-101.
Kosswig, C. 1955a. Zoogeography of the Near East. Systematic Zoology, 4:49-73, 96.
Kosswig, C. 1955b. Contributions to the historical zoogeography of African freshwater fishes. Istanbul Üniversitesi Fen Fakültesi Hidrobiologi Araştırma Enstitüsü
Yayınlarından, Seri B, 2(2/3):83-91.
Kosswig, C. 1965. Zur historischen Zoogeographie der Ichthyofauna im Süsswasser des sudlichen Kleinasiens. Zoologische Jahrbücher für Systematik, 92:83-90.
Kosswig, C. 1967. Tethys and its relation to the peri-Mediterranean faunas of fresh-water fishes, pp. 313-324. In: Adams, C. G. and Ager, D. V. (Eds.). Aspects of Tethyan Biogeography. Systematics
Association Publication No. 7. vi + 336 pp.
Kosswig, C. 1973. Über die Ausbreitungswege sogenannter perimediterraner Süßwasserfische. Bonner Zoologische Beiträge, 24:165-177.
Kostianoy, A. G. and Kosarev, A. N. (Eds.). 2005. The Caspian Sea Environment. Springer. xiv + 271 pp.
Kostich, J. 1979. In the beginning... Aphanius mento (Heckel, 1843). Journal of the American Killifish Association, 12(6):186-187.
Kotlík, P., Marková, S., Choleva, L., Bogutskaya, N. G., Ekmekçi, F. G. and Ivanova, P. P. 2008. Divergence with gene flow between Ponto-Caspian refugia in an
anadromous cyprinid Rutilus frisii revealed by multiple gene phylogeography. Molecular Ecology, 17(4):1076-1088.
Kottelat, M. 1984. Revision of the Indonesian and Malaysian loaches of the subfamily Noemacheilinae. Japanese Journal of Ichthyology, 31(3):225-260.
Kottelat, M. 1986. Cobitis Linnaeus, 1758 (Osteichthyes, Cypriniformes): proposed designation of Cobitis taenia Linnaeus, 1758 as type species and request for a ruling on the stem of
the family-group name Cobitididae Swainson, 1839. Z.N.(S.) 2566. Bulletin of Zoological Nomenclature, 43(4):360-362.
Kottelat, M. 1987. Nomenclatural status of the fish names created by J. C. van Hasselt (1823) and of some cobitoid genera. Japanese Journal of Ichthyology, 33(4):368-375.
Kottelat, M. 1988. Indian and Indochinese species of Balitora (Osteichthyes: Cypriniformes) with descriptions of two new species and comments on the family-group names Balitoridae and
Homalopteridae. Revue Suisse de Zoologie, 95(2):487-504.
Kottelat, M. 1990. Indochinese nemacheilines. A revision of nemacheiline loaches (Pisces: Cypriniformes) of Thailand, Burma, Laos, Cambodia and southern Viet Nam. Verlag Dr. Friedrich Pfeil,
München. 262 pp.
Kottelat, M. 1991. Comments on the proposed precedence of homalopteridae Bleeker, 1859 overbalitoridae
Swainson, 1839 (Osteichthyes, Cypriniformes). Bulletin of Zoological Nomenclature, 48(4):333-335.
Kottelat, M. 1997. European freshwater fishes. An heuristic checklist of the freshwater fishes of Europe (exclusive of former USSR), with an introduction for non-systematists and comments on
nomenclature and conservation. Biologia, Bratislava, 52 (Supplement 5):1-271.
Kottelat, M. and Freyhof, J. 2007. Handbook of European Freshwater Fishes. Kottelat, Cornol, Switzerland and Freyhof, Berlin, Germany. xiii + 646 pp.
Kottelat, M., Nielsen, J. G. and Nijssen, H. 1993. Survey of ichthyological resources in European museums and collections. Verlag Dr. Friedrich Pfeil, Munich. 24 pp.
Kottelat, M. and Wheeler, A. 2001. Aphanius Nardo, 1827 (Osteichthyes, Cyprinodontiformes): proposed placement on the Official List. Bulletin of Zoological Nomenclature, 58(2):110-115.
Kouchakian Sabour, A., Zare Gashti, Gh., Sababi, H. and Moeini, S. 2001. A study on production of gelatin from sturgeon fish skin. Iranian Journal of Fisheries Sciences, 3(1):85-87.
Kouchoukos, N., Smith, R., Gleason, A., Thenkabail, P., Hole, F., Barkoudah, Y., Albert, J., Gluhosky, P. and Foster, J. 1998. Monitoring the distribution, use, and regeneration of natural
resources in semi-arid Southwest Asia. Yale School of Forestry & Environment Studies Bulletin, 103:467-491.
Kouhetsan-Eskandari, S. 1998. Investigation of some biological and ecological parasitological characteristics of khramulya Capoeta capoeta gracilis in the river of Golestan National Park.
M.Sc. Thesis, Tarbiat Modarres University. 111 pp. In Farsi.
Koumans, F. P. 1955. Gobioid fishes of India. Memoirs of the Indian Museum, 13(1938-1942):205-330.
Kousha, A. and Askarian, F. 2006a. SBP as a biomarker in kutum blood. 93rd Indian Science Congress Association (ISCA), Section of Fisheries, Hyderabad, India (title).
Kouisha, A. and Askarian, F. 2006b. Role of packaging in quality improve (sic) of fish processing for consumers in Iran market. World Aquaculture Society, Aqua 2006, May 9-13. Florence,
Italy (abstract).
Kousha, A. and Askarian, F. 2006c. Introduce (sic) of rainbow trout Onchorhinchus (sic) mykiss as a new salting and smoking product for home preparation in Iran. World
Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Kousha, A., Askarian, F., Ghate, H. V. and Ghole, V. S. 2006. Use of biotechnology on food processing and quality of fish products in Iran. World Aquaculture Society, Aqua 2006, May 9-13. Florence,
Italy (abstract).
Kousha, A., Askarian, F., Ghate, H. V. and Ghole, V. S. 2007. Study on sex steroid-binding proteins (with emphasize (sic) on 17β-estradiol) in plasma of female and juvenile
kutum (Rutilus frisii kutum). Iranian Journal of Fisheries Sciences, 6(2):77-92.
Kousha, A., Askarian, F., Ghate, H. V., Yousefian, M. and Ghole, V. S. 2006. Investigation on sex steroid binding protein (SBP) as a biomarker for sexual behaviour of kutum (Rutilus frissi
(sic) kutum). World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Kousha, A., Askarian, F., Yousefian, M., Ghate, H. V. and Ghole, V. S. 2009.
Annual fluctuation of sex steroid hormones in pre-spawning female kutum (Rutilus
frissi (sic) kutum). World Journal of Fish and Marine
Sciences, 1(1):65-73.
Kousha, A., Yousefian, M., Ghate, H. V. and Ghole, V. S. 2003. Study on monoculture breeding of trout in Mazandaran province. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway,
August 8-12, 2003 (title).
Kovda, V. A. 1961. Land use development in the arid regions of the Russian Plain, the Caucasus and Central Asia. In: Stamp, L. D. Arid Zone Research - XVII. A history of land use in arid regions.
UNESCO, Paris. 388 pp.
Kozhara, A. V. 2005. Structural traits of the axial skeleton in some groups of genera of Leuciscinae (Cyprinidae). Journal of Ichthyology, 45(8):566-576.
Kozhara, A. V., Izyumov, Yu. G., Kasyanov, A. N. and Zelenetskii, N. M. 1999. Dependence of vertebral numbers on water body type in freshwater fish. Journal of Ichthyology, 39(3):209-217.
Kozhara, A. V. and Mironovskiy, A. N. 1988. Species structure, variability and some aspects of the microphylogeny of the bream, Abramis brama. Journal of Ichthyology, 28(1):108-121.
Kozhin, N. I. 1957. Materialy po ikhtiofaune iranskogo poberezh'ya Kaspiya [Material on the ichthyofauna of the Iranian coast of the Caspian Sea]. Voprosy Ikhtiologii, 8:8-18.
Krasznai, Z. L. 1987. Mission to Iran to assess the needs in coldwater fish culture 14 August - 2 September 1987. A report prepared for the project Training Course in Coldwater Fisheries. Food
and Agriculture Organization, Rome, TCP/IRA/6755(T), Field Document 1, iii + 23 pp.
Krieg, F., Estoup, A., Triantafyllidis, A. and Guyomard, R. 1999. Isolation of microsatellite loci in European catfish, Silurus glanis. Molecular Ecology, 8(11):1964-1966.
Krieg, F., Triantafyllidis, A. and Guyomard, R. 2000. Mitochondrial DNA variation in European populations of Silurus glanis. Journal of Fish Biology, 56(3):713-724.
Krieger, J., Hett, A. K., Fuerst, P. A., Artyukhin, E. and Ludwig, A. 2008. The molecular phylogeny of the order Acipenseriformes revisited. Journal of Applied Ichthyology, 24(s1):36-45.
Krinsley, D. B. 1970. A geomorphological and paleoclimatological study of the playas of Iran. Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. Part I:xxiv + 329 pp.,
Part II:4 plates, 155 figures, 17 tables, 4 appendices.
Krupp, F. 1983. Fishes of Saudi Arabia. Freshwater Fishes of Saudi Arabia and Adjacent Regions of the Arabian Peninsula. Fauna of Saudi Arabia, 5:568-636.
Krupp, F. 1984a. Aphanius cypris (Heckel, 1843) versus Aphanius mento (Heckel, 1843)(Pisces: Cyprinodontidae). Cybium, 8(2):63-69.
Krupp, F. 1984b. Recent changes in the distribution of Syrian freshwater fishes. Roczniki Nauk Rolniczych, seria H, 100(3)(1983):79-88.
Krupp, F. 1985a. Rehabilitation of Barbus lorteti Sauvage, 1882, and comments on the validity of the generic names Bertinius Fang, 1943, and Bertinichthys Whitley, 1953
(Pisces: Cyprinidae). Hydrobiologia, 120:63-68.
Krupp, F. 1985b. Barbus chantrei (SAUVAGE 1882), a valid species of cyrinid fish from the northern Levant (Pisces: Osteichthyes: Cyprinidae). Senckenbergiana biologica, 66(1/3):17-25.
Krupp, F. 1985c. Systematik und Zoogeographie der Süßwasserfische des levantinischen Grabenbruchsystems und der Ostküste des Mittelmeeres. Dissertation zur Erlangung des Grades
"Doktor der Naturwissenschaften" am Fachbereich Biologie der Johannes Gutenberg - Universität in Mainz. 215 pp., Anhang: Abbildungen, Karten, Tabellen, 169 pp.
Krupp, F. 1985d. A new species of Chondrostoma from the Orontes river drainage basin of Turkey and Syria (Pisces: Osteichthyes: Cyprinidae). Senckenbergiana biologica, 66(1/3):27-33.
Krupp, F. 1987. Freshwater ichthyogeography of the Levant, pp. 229-237. In: Krupp, F., Schneider, W. and Kinzelbach, R. (Eds.). Proceedings of the Symposium on the Fauna and Zoogeography of the
Middle East, Mainz, 1985. Beihefte zum Tübinger Atlas des Vorderen Orients, Reihe A (Naturwissenschaften), 28, Dr. Ludwig Reichert Verlag, Wiesbaden, 338 pp.
Krupp, F. 1992. The karst springs of Ra's Al-Ain. Aqua Geõ-graphia, 1(1):26-33.
Krupp, F., Al-Hassan, L. A. J. and Ziegler, T. 1992. A possible natural hybrid of Acanthobrama marmid and Alburnus mossulensis from Haur al-Hammar, southern Iraq. Senckenbergiana
Biologica, 72(4/6):219-223.
Krupp, F. and Schneider, W. 1989. The fishes of the Jordan River drainage basin and Azraq Oasis. Fauna of Saudi Arabia, 10:347-416.
Krupp, F. and Schneider, W. 1991. Two new species of Nemacheilus Bleeker 1863 from the Orontes River drainage basin of Lebanon, Syria and Turkey. Senckenbergiana biologica, 71(1/3)(1990):23-34.
Krylov, V. I. 1984. An estimate of the effect of the Caspian seal (Pusa caspica) on fish populations. Canadian Translation of Fisheries and Aquatic Sciences, 5066:15 pp.
Krysanov, E. Yu. 1999. Karyotypes of Varicorhinus capoeta and Barbus goktschaicus (Cypriniformes) from Lake Sevan, Armenia. Journal of Ichthyology, 39(2):187-189.
Kuchekian, A. 1989. Fish and Fishery in Iran. Parvaneh Publications, Tehran. 45 pp. In Farsi.
Kuderskii, L. A. 1974. O proiskhozhdenii lososei i forelei (Salmo trutta L.) v basseinakh Aral'skogo, Kaspiiskogo i Chernogo morei. [The origin of the salmon and trout (Salmo
trutta L.) in the basins of the Aral, Caspian and Black Seas]. Izvestiya Gosudarstvennogo Nauchno-Issledovatel'skogo Instituta Ozernogo i Rechnogo Rybnogo Khozyaistva, 97:187-216. (Translation
series number 3773, Fisheries and Marine Service, Arctic Biological Station, Ste. Anne de Bellevue, Québec, Canada).
Kuliev, Z. M. 1988. Morphometric and ecological characteristics of Caspian vimba, Vimba vimba persa. Journal of Ichthyology, 28(2):56-64.
Kuliev, Z. M. 1989. Ryby Zaliva Kirova Kaspiiskogo Morya (sistematika, biologiya, promysel) [Fishes of Kirova Bay Caspian Sea (systematics, biology, fisheries)]. Akademiya Nauk Azerbaidzhanskoi
SSR, Instituta Zoologii, Baku. 183 pp.
Kuliyev, Z. M. 1981. Morphobiological characteristics of the sea zander Schizostedion (sic) marinum Cuvier from the Caspian Sea. Journal of Ichthyology, 21(5):36-42.
Kuliyev, Z. M. 1984. Variation of morphometric indices in Caspian vobla, Rutilus rutilus caspicus. Journal of Ichthyology, 24(6):139-148.
Kuliyev, Z. M. and Agayarova, A. E. 1984. Ecological-morphometrical characteristics of wild carp, Cyprinus carpio (Cyprinidae), of the Central and Southern Caspian. Journal of Ichthyology, 24(3):9-17.
Kullander, S. O., Fang, F., Delling, B. and Åhlander, E. 1999. The fishes of the Kashmir Valley, pp. 99-167. In: Nyman, L. (Ed.). River Jhelum, Kashmir Valley. Impacts on the aquatic environment.
Swedmar, Göteborg.
Kurdistani, S. M. and Bajestan, M. S. 2004. Rehabilitation for exist (sic) fishway of Jaeezan diversion dam and find a possibility for installing a fishway at exist (sic) Behbahan
diversion dam in Iran. World Water Congress 2004, Critical Transitions in Water and Environmental Resources Management, World Water and Environmental Resources Congress 2004, 27 June - 1 July Salt Lake
City, ASCE (American Society of Civil Engineers), Reston, Virginia. 7 pp.
Kürkçüoğlu, N. and Öz, G. 1989. Psoriasis and the doctor fish. The Lancet, 2(8676):1394.
Kuronoma, K. and Abe, Y. 1986. Fishes of the Arabian Gulf. Kuwait Institute for Scientific Research, State of Kuwait. xiii + 357 pp., 30 plates.
Kuros, G-R. 1943. Irans Kampf um Wasser. Die Vergangenheit und ihre Lehren, die Zukunft und ihre Aufgaben in der iranischen Wasserwirtschaft. Springer-Verlag, Berlin. viii + 93 pp.
Kuru, M. 1971. The fresh-water fish fauna of eastern Anatolia. Istanbul Üniversitesi Fen Fakültesi Mecmuasi, B, 36(3-4):137-147.
Kuru, M. 1975.Dicle-Fırat Kura-Aras, Van Gölü ve Karadeniz Havzası tatlısularında yaşayan Balıkların
(Pisces) Sistematik ve Zoocoğrafik Yönden İncelenmese.Docentliktezi-Basilmamis, Atatürk Üniversitesi, Erzurum.
Kuru, M. 1978-1979. The fresh water fish of south-eastern Turkey - 2 (Euphrates-Tigris system). Hacettepe Bulletin of Natural Sciences and Engineering, 7-8:105-114.
Kuru, M. 1980a. Key to the inland water fishes of Turkey. Parts I-III. Hacettepe Bulletin of Natural Sciences and Engineering, 9:103-112; 113-122; 123-133.
Kuru, M. 1980b.Türkiye Tatlısu Balıkları Kataloğu.Hacettepe Üniversitesi Fen Fakültesi Yayınları Yardımcı Kitapler Dizisi, 1:vii + 73 pp.
(Seri:12, Bölüm:1, Sayı:1).
Kuru, M. 1981. Revision of Chondrostoma species of Turkey. Hacettepe Bulletin of Natural Sciences and Engineering, 10:111-112.
Kuru, M. 1987. Vertebrate Animals. Atatürk Űniversitesi Yayınları, Erzurum. 435 pp. In Turkish.
Kuşat, M., Bolat, Y. and Tokgözlü, A. 1995.Sudak Avcılığında Kullanilan Çöpçü (Neomacheilus (sic) angorae,
Steindachner, 1897) ve Taşyiyen (Cobitis taenia L. 1758) Balıklarının Paraketa İğnesinde Canlı Kalma Süreleri [The survival times on the longline
hook of the Angora loach (Nemacheilus angorae Steindachner, 1897) and spined loach (Cobitis taenia L., 1758) used in pikeperch fishing]. Su Ürünleri Dergisi,İzmir-Bornova,
12 (1-2):61-67.
Kushnarenko, A. I. 1978. The minimum commercial size and natural mortality of the north Caspian roach, Rutilus rutilus caspicus. Journal of Ichthyology, 18(6):886-895.
Kushnarenko, A. L. 1986. Ecological basis of the fishery for Caspian clupeids. Journal of Ichthyology, 26(2):16-23.
L
Ladiges, W. 1960. Süßwasserfische der Türkei, 1. Teil Cyprinidae. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 58:105-150.
Ladiges, W. 1964. Süßwasserfische der Türkei, 3. Teil restliche Gruppen. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 61:203-220.
Ladiges, W. 1966. Süßwasserfische der Türkei, 4. Teil: Die Gattung Chondrostoma (Cyprinidae) in der Türkei. Mitteilungen aus dem hamburgischen
Zoologischen Museum und Institut, 63:101-109.
Ladiges, W. und Vogt, D. 1979. Die Süßwasserfische Europas bis zum Ural und Kaspischen Meer. Verlag Paul Parey, Hamburg und Berlin. 299 pp.
Laessøe, J. 1951. The irrigation system at Ulhu, 8th century B.C. Journal of Cuneiform Studies, 5:21-32.
Lagounov, I. I., Podsevalov, V. N. and Skvortsov, I. K. 1933?. Matériaux de l'Expédition iranienne de recherches scientifiques de la pêche en 1932-1933. MS. In Russian. (not seen).
Lajbner, Z. Kohlmann, K., Linhart, O. and Kotlík, P. 2010. Lack of reproductive isolation between the Western and Eastern phylogroups of the tench. Reviews in Fish Biology and Fisheries, 20:289-300.
Lal Shah, S. 2010. Hematological changes in Tinca tinca after exposure to lethal and sublethal does of mercury, cadmiun and lead. Iranian Journal of Fisheries Sciences, 9(3):434-443.
Laloei, F. 2006. Hydrology and hydrobiology and environmental pollutions in lower than 10 meters depth of Caspian Sea. Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Sari. http://en.ifro.ir (abstract).
Laloie, F., Nirani, M., Taghavi, M. J and Rezvani Gilkolaei, S. 2006. The PCR-RFLP investigation of Clupeonella cultriventris from the south
Caspian Sea. Iranian Journal of Fisheries Sciences, 15(2):119-128. In Farsi.
Laloie, F., Resvani Gilkolaei, S. and Pourkazemi, M. 2003. Genetic variation of Barbus capito in the southern Caspian Sea by PCR-RFLP method. Iranian
Journal of Fisheries Sciences, 12(1):117-130, 9. In Farsi.
Lalouie (sic), F. 1993. Hydrobiological survey of Gorgan-Bay. Iranian Fisheries Bulletin, (4):53-68, 5. In Farsi.
Laluie (sic), F. 1996. The study of bio-environmental effects of Neka Power Plant. Iranian Fisheries Scientific Journal, 4(4):14-24, 2. In Farsi.
Lalooei (sic), F. 1997. Studying the manner of sturgeon fishes migration to Gorganrud and Tajan rivers. Iranian Fisheries Scientific Journal, 5(4):17-30, 2. In Farsi.
Lamb, H. H. 1977. Climate, present, past and future. Volume 2. Climatic history and the future. Methuen, London. xxx + 835 pp.
Lamb, H. H. 1982. Climate, history and the modern world. Methuen, London. xix + 387 pp.
Lambeck, K. 1996. Shoreline reconstructions for the Persian Gulf since the last glacial maximum. Earth and Planetary Science Letters, 142(1):43-57.
Lamboj, A., Svardal, H. and Svardal, K. 2006. Erste Erfahrungen zur Pflege und Zucht von Iranocichla hormuzensis Coad, 1982. Deutsche Cichliden-Gesellschaft Informationen, 37(6):123-129.
Lambton, A. K. S. 1953. Landlord and Peasant in Persia. A Study of Land Tenure and Land Revenue Administration. Oxford University Press, London. xxxi + 459 pp., map.
Lambton, A. K. S. 1969. Landlord and peasant in Persia. A study of land tenure and land revenue administration. Oxford University Press, Oxford. xxxvii + 459 pp.
Landsberger, B. 1962. Materialien zum Sumerischen Lexikon. MSL VIII/2. The Fauna of Ancient Mesopotamia. Second Part. HAR-ra=hubullu Tablets XIV and XVIII. Pontificium Institutum Biblicum, Roma.
x + 180 pp.
Lane, F. B. 1920. Large carp from Mesopotamia. Journal of the Bombay Natural History Society, 27:176-177.
Laptev, M. K. 1934. Materialy k poznaniyu fauny pozvonochnykh Turkmenistana (Bol'shie Balkhany i zapadnyi Kopet-Dag) [Materials to the knowledge of
the vertebrate fauna of Turkmenistan (Great Balkhan and western Kopet-Dag)]. Izvestiya Turkmenskogo mezhduvedomstvennogo Komiteta po okhrane prirody
i razvitiyu prirodnykh bogatstv, Ashkhabad, 1:154-165.
Larsen, C. E. 1975. The Mesopotamian delta region: a reconsideration of Lees and Falcon. Journal of the American Oriental Society, 95(1):43-57.
Larsen, C. E. and Evans, G. 1978. The Holocene geological history of the Tigris-Euphrates-Karun Delta, pp. 227-244.In: Brice, W. C. (Ed.). The
Environmental History of the Near and Middle East Since the Last Ice Age. Academic Press, London. xx + 384 pp.
Lasheidani, M. F., Balouchi, S. N., Keyvan, A., Jamili, S. and Falakrou, K. 2008. Effects of butachlor on density, volume and number of abnormal sperms in Caspian kutum (Rultilus frisii kutum
Kamenskii 1901). Research Journal of Environmental Sciences, 2(6):474-482.
Latifi, A., Moeini, S., Yarmand, S. and Yousefian, M. 2007. Studying the effect of citric acid on preserving the quality of rainbow trout in cold storage. Iranian Journal of Natural Resources,
60(1):151-159. In Farsi.
Lazara, K. 1988. Taxonomic and nomenclatural notes on the genera Aphanius, Kosswigichthys, and Valencia. Journal of the American Killifish Association, 21(1-2):5-15.
Lazara, K. J. 1989. Aphanius mento (Heckel, 1843) - ein älteres Synonym zu Aphanius cypris (Heckel, 1843). Deutsche Killifische Gemeinschaft Journal, Köln, 21(6):87-90.
Lazara, K. J. 1995. History of the genera Lebia Oken 1817 and Lebias Goldfuss 1820 (Teleostei: Cyprinodontiformes: Cyprinodontidae)
with designation of a type species for Lebias. Copeia, 1995(2):501-503.
Lazara, K. J. (Ed.). 2001. The Killifishes: An annotated checklist, synonymy and bibliography of recent oviparous cyprinodontiform fishes. The
Killifish Master Index, 4th Edition. xviii + 624 pp., appendices A-C.
Lazur, A. M. and Pouder, D. B. 2001. Status and potential of sturgeon aquaculture for meat and caviar. Aquaculture, Book of Abstracts. p. 356.
Lee, P. G. and Ng, P. K. L. 1994. The systematics and ecology of snakeheads (Pisces: Channidae) in Peninsular Malaysia and Singapore. Hydrobiologia, 285:59-74.
Lees, G. M. and Falcon, N. L. 1952. The geographical history of the Mesopotamian Plains. Geographical Journal, 118:24-39.
Legeza, M. I. 1972. The role of abiotic factors in the distribution of sturgeons (Pisces, Acipenseridae) in the Caspian Sea. Journal of Ichthyology, 12(1):10-20.
Legeza, M. I. 1973. The present distribution of sturgeons (Acipenseridae) in the Caspian Sea. Journal of Ichthyology, 13(6):840-847.
Lehner, E. 1944. The Persian salt formations. Proceedings of the National Academy of Sciences India, 14(6), Section B:249-258.
Lelek, A. 1981. The occurrence, taxonomy and future of trouts in North East Turkey. European Committee for the Conservation of Nature and Natural Resources, Council of Europe, Strasbourg. 110 pp.
Lelek, A. 1987. The Freshwater Fishes of Europe. Volume 9. Threatened Fishes of Europe. AULA-Verlag, Wiesbaden. 343 pp.
Letichevskiy, M. A. 1981. Prospects of increasing the artificial and natural reproduction of the inconnu, Stenodus leucichthys, in the
lower course and delta of the Volga. Journal of Ichthyology, 21(5):103-107.
Lever, C. 1996. Naturalized Fishes of the World. Academic Press, London. xxiv + 408 pp.
Levin, A. V. 1997. The distribution and migration of sturgeons in the Caspian Sea. Occasional Papers of the IUCN Species Survival Commission (SSC), 17:13-19.
Levin, B. A. 2004. Phenetic relationship of barbels of the Caucasian region and their status in the system of the genus Barbus sensu stricto (Cyprinidae). Journal of Ichthyology, 44(6):444-449.
Levin, B. A., Rubenyan, A. R. and Salnikov, V. B. 2005. Phenetic diversity of khramulya Capoeta capoeta (Ostariophysi, Cyprinidae). Journal of Ichthyology, 45(9):754-767.
Leviton, A. E., Gibbs, R. H., Heal, E. and Dawson, C. E. 1985. Standards in herpetology and ichthyology: Part I. Standard symbolic codes for institutional
resource collections in herpetology and ichthyology. Copeia, 1985(3):802-832.
Lewis, F. 1980. The black market in Iranian caviar. New York Times, CXXIX, No. 44,471:C1, C3 (23 January 1980).
Li, J., Wang, X., Kong, X., Zhao, K., He, S. and Mayden, R. L. 2008. Variation patterns of the mitochondrial 16S rRNA gene with secondary structure constraints and their application to phylogeny of
cyprinine fishes (Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution, 47(2):472-487.
Li, S. 1986. Systematics, distribution and evolution of Glyptothorax (Siluriformes: Sisoridae). In: Uyeno, T., Arai, R., Taniuchi, T. and Matsuura,
K. (Eds.).Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, Tokyo. pp. 521-528.
Liao, T-Y., Ünlü, E. and Kullander, S. O. 2011. Western boundary of the subfamily Danioninae in Asia (Teleostei, Cyprinidae): derived from the systematic position of Barilius mesopotamicus
based on molecular and morphological data. Zootaxa, 2880:31-40.
Liaqati, M. B. 1997. A passing glance at the appearance and extension of Quanats (aqueducts). Farhang-e Jihad (Construction Crusade Culture Quarterly),
Spring and Summer, 1997(3 & 4) (www.netiran.com/Htdocs/Clippings/Social/970700XXSO01.html).
Libosvárský, J. 1962. Zur palaeoborealen Verbreitung der Gattung Carassius Jarocki 1822. Zoologische Jahrbücher Abteilung
für Systematik Öekologie und Geographie der Tiere, Jena, 90:197-210.
Light, W. A. 1917. A large carp from the Euphrates River. Journal of the Bombay Natural History Society, 25:308-309.
Lindberg, G. U. and Heard, A. S. 1972. Slovar nazvanii presnovodnikh ryb SSSR na yazikakh narodov SSSR i evropeiskikh stran [Dictionary of the
Names of Freshwater Fishes of the USSR on the Languages of the Peoples of the USSR and European Countries]. Izdatel'stvo Nauka, Leningrad. 367 pp.
Lindberg, O. 1994. Black market turns importer to Iran for caviar. International Management, June 1994:12-13.
Lippitsch, E. and Micklich, N. 1998. Cichlid fish biodiversity in an Oligocene lake. Italian Journal of Zoology, 65 (Supplement):185-188. (Proceedings
of the Ninth Congress of European Ichthyologists (CEI-9) "Fish Biodiversity" organised in Naples at the University Federico II and held in Trieste - Italy, 24-30 August 1997).
Liška, L. und Pivnička, K. 1985. Morphologische Variabilität von drei Arten der Ukeleie (Alburnus alburnus, A. albidus, A. charusini)(Pisces: Cyprinidae).
Vĕstník československé Společnosti zoologické, 49:111-122.
Löffler, H. 1956. Ergebnisse der Österreichischen Iranexpedition 1940/50: Limnologische Beobachtung an Iranischen Binnengewässern. Hydrobiologia, 8:201-278.
Löffler, H. 1957. Limnological investigations in Southern Persia especially concerning the shallow salt lakes of the country. Year Book of the American Philosophical Society, 1957:242-244.
Löffler, H. 1959. Beiträge zur Kenntnis der Iranischen Binnengewässer. I. Der Nirizsee und sein Einzugsgebiet. Internationale Revue der Gesamten Hydrobiologie, 44:227-276.
Löffler, H. 1961. Beiträge zur Kenntnis der Iranischen Binnengewässer. II. Regional-limnologische Studie mit besonderer Berücksichtigung
der Crustaceenfauna. Internationale Revue der Gesamten Hydrobiologie, 46(3):309-406.
Löffler, H. 1968. The hydrobiology of Lake Niriz, Iran. Proceedings of a Technical Meeting on Wetland Conservation, Ankara-Bursa-Istanbul, 9 to 16 October 1967, International
Union for Conservation of Nature and Natural Resources Publication, new series, 12:141-151.
Löffler, H. 1981. The winter condition of Lake Niriz in Southern Iran. Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 21:528-534.
Löffler, H. 1993. The future of large lakes in the third world. Memorie dell'Istituto Italiano di Idrobiologia, 52:27-38 (In: de Bernardi, R., Pagnotta, R. and Pugnetti, A. (Eds.).
5th International ILEC Conference, Stresa '93 "Strategies for lake ecosystems beyond 2000" Selected papers. 400 pp.).
Lohnechahri, M., Farkhondeh, A. et Maleki, M. 1972. Etude sur la contamination par les Salmonella des poissons d'eau douce en Iran. Receuil de
Médecine Vétérinaire, CXLVIII:1259-1262.
Loiselle, P. V. 1994. The Cichlid Aquarium. Tetra-Press, Melle. 447 pp. + index 24 pp., 517 figs.
Lönnberg, E. 1900a. Contributions to the ichthyology of the Caspian Sea. Kunglica Svenska Vetenskapsakademiens Handlingar, 26, IV(8):3-38.
Vestnik Rybopromyshlennosti, St. Petersburg, 16:30-39.
Lönnberg, E. 1900b. Short notes on Caspian fishes. Revue Internationale de Pêche et de Pisciculture, St. Petersbourg, 2(2):3-6.
Lorestani (sic), R., Ahmadi, M. R. and Kalbasi, M. R. 2007. Correlation of sperm quality of different rainbow trout (Oncorhynchus mykiss) brood stocks in propagation process. Iranian
Journal of Natural Resources, 60(1):141-150. In Farsi.
Lorestany, R., Ahmadi, M,. R. and Kalbassi, M. R. 2004. Effect of activating solutions on propagation performances in rainbow trout (Oncorhynchus mykiss).
Iranian Journal of Marine Sciences, 3(1):67-74. In Farsi.
Lorestany, R., Ahmadi, M,. R. and Kalbassi, M. R. 2009. Interaction of male broodstock age and different dilution on eyed eggs rate in rainbow trout (Oncorhynchus mykiss). Journal of Veterinary
Research, 63(5):345-350. In Farsi.
Lortet, L. 1883. Études zoologiques sur la Faune du Lac de Tibériade suivies d'un aperçu sur la faune des lacs d'Antioche et de Homs.
I. Poissons et reptiles du lac de Tibériade et de quelques autres parties de la Syrie, p. 99-180. Poissons de Syrie recueillis par M. Ernest
Chantre en 1881 pendant son mission scientifique en Mésopotamie, dans l'Armenie et le Kurdistan, p. 181-182. Archives du Museum d'Histoire naturelle de Lyon, 3:99-182, plates vi-xviii.
Lotan, R. 1969. Sodium, chloride and water balance in the euryhaline teleost Aphanius dispar (Rüppell) (Cyprinodontidae). Zeitschrift für vergleichende Physiologie, 65:455-462.
Lotan, R. 1971. Osmotic adjustment in the euryhaline teleost Aphanius dispar (Cyprinodontidae). Zeitschrift für vergleichende Physiologie, 75(3):383-387.
Lotan, R. 1973. Osmoregulation during adaptation to fresh water in the euryhaline teleost Aphanius dispar Rüppell (Cyprinodontidae, Pisces). Journal of Comparative Physiology, 87:339-349.
Lotan, R. and Ben-Tuvia, A. 1996. Distribution and reproduction of killifish Aphanius dispar and A. fasciatus and their hybrids in the
Bardawil Lagoon on the Mediterranean coast of Sinai, Egypt. Israel Journal of Zoology, 42:203-213.
Louis, J. P., Albert, J. P. and Moussa, A. M. 1988. Le paludisme en Republique de Djibouti. Strategie de controle par la lutte antilarvaire
biologique: poissons larvivores autochthones (Aphanius dispar) et toxines bacteriennes. Médecine tropicale, 48(2):127-131.
Ludwig, A. 2008. Identification of Acipenseriformes species in trade. Journal of Applied Ichthyology, 24(s1):2-19.
Ludwig, A., Bohlen, J., Wolter, C. and Pitra, C. 2001. Phylogenetic relationships and historical biogeography of spined loaches (Cobitidae, Cobitis and Sabanejewia) as
indicated by variability of mitochondrial DNA. Zoological Journal of the Linnaean society, 131(3):381-392.
Lueken, W. 1967. Süßwasserfische der Türkei 5. Teil Syngnathidae. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 64:127-146.
Lukyanenko, V. I. 1992. Kak spasti kaspiiskikh osetrov? [How to save the Caspian sturgeons?]. Vestnik Rossiiskoi Akademii Nauk, 1992(2):55-74.
Luk'yanenko, V. I., Umyerov, Zh. G. and Karateva, B. B. 1974. Yuzhnokaspiiskii osetr - samostoyatel'nyi vid roda Acipenser [The south Caspian sturgeon
- an independent species of the genus Acipenser]. Izvestiya Akademii Nauk SSSR, Seriya Biologicheskaya, (1974)5:736-739.
Luk'yanenko, V. I., Vasil'ev, A. S., Luk'yanenko, V. V. and Khabarov, M. V. 1999. On the increasing threat of extermination of the unique Caspian
sturgeon populations and the urgent measures required to save them. Journal of Applied Ichthyology, 15(4-5):99-102.
Lutman, C. A. 1982. Aphanius mento. Journal of the American Killifish Association, 15(4):129-131.
Lyovin, A. V. 1981. Distribution and behavior of young Russian sturgeon, Acipenser guldenstadti (Acipenseridae), in the Caspian Sea. Journal of Ichthyology, 21(2):138-144.
M
Maafi, A. 1996a. Parishan Lake, second registered Iranian Lagoon in Ramsar Convention. Abzeeyan, Tehran, 7(4):14-16, V. In Farsi.
Moafi (Maafi), A. 1996b. Parishan Lake, second registered Iranian lagoon in Ramsar Convention. Abzeeyan, Tehran, 7(5):15-17, III. In Farsi.
Maafi, S. A. 1996c. Parishan Lake, and human role on its ecological balance. Abzeeyan, Tehran, 7(6):7-9, 17, III. In Farsi.
MacCrimmon, H. R. 1971. World distribution of rainbow trout (Salmo gairdneri). Journal of the Fisheries Research Board of Canada, 8:663-704.
MacCrimmon, H. R. 1972. World distribution of rainbow trout (Salmo gairdneri): further observations. Journal of the Fisheries Research Board of Canada, 29(12):1788-1791.
MacCrimmon, H. R. and Marshall, T. L. 1968. World distribution of brown trout, Salmo trutta. Journal of the Fisheries Research Board of Canada, 25(12):2527-2548.
MacCrimmon, H. R., Marshall, T. L. and Gots, B. L. 1970. World distribution of brown trout, Salmo trutta: further observations. Journal of the
Fisheries Research Board of Canada, 27(4):811-818.
MacFadyen, W. A. 1938. Water Supplies in Iraq. Ministry of Economics and Communications, Government Press, Baghdad and Iraq Geological Department Publication, 1:206 pp., + viii, XIX plates.
MacFadyen, W. A. and Vita-Finzi, C. 1978. Mesopotamia: the Tigris-Euphrates delta and its Holocene Hammar Fauna. Geological Magazine, 115(4):287-300.
MacFarquhar, N. 2001. Drought chokes off Iran's water and its economy. The New York Times on the Web, 3 pp. (http://www.climateark.org/articles/2001/3rd/drchoffi.htm,
downloaded 9 March 2003).
Machordom, A. and Doadrio, I. 2001. Evidence of a Cenozoic Betic-Kabilian connection based on freshwater fish phylogeography (Luciobarbus,
Cyprinidae). Molecular Phylogenetics and Evolution, 18(2):252-263.
MacInnis, A. J. 1997. Reproductive biology of the exotic Great Lakes fish Neogobius melanostomus. American Society of Ichthyologists and Herpetologists, 77th Annual Meeting,
June 26-July 2, 1997, University of Washington, Seattle, Washington. p. 201-202 (abstract).
MacInnis, A. J. and Corkum, L. D. 1997a. Age and growth of the round goby, Neogobius melanostomus (Gobiidae), in the Detroit River, Canada.
American Society of Ichthyologists and Herpetologists, 77th Annual Meeting, June 26-July 2, 1997, University of Washington, Seattle, Washington. p. 202 (abstract).
MacInnis, A. J. and Corkum, L. D. 1997b. Life history aspects of the round goby. International Association for Great Lakes Research. Program
& Abstracts, 40th Conference, June 1-5, 1997, Buffalo State College & University of Buffalo, New York, p. 81 (abstract).
Mackay, H. 1919. Large carp from Mesopotamia. Journal of the Bombay Natural History Society, 26:680.
Mackowiak, L. 1988. Aphanius dispar. a Middle Eastern favorite. Journal of the American Killifish Association, 21(1-2):51-53.
MacMahon, H. 1906. Recent survey and exploration in Seistan. Geographical Journal, 28(3):209-228, 333-352, map p. 312.
Madbaygi, S. 1992. A review of fisheries in Mazandaran. Abzeeyan, Tehran, 2:40-43. In Farsi.
Madbaygi, S. 1993a. A brief review of the Mollasani Research Centre for Freshwater Fish Culture. Abzeeyan, Tehran, 4(11):39-41. In Farsi.
Madbaygi, S. 1993b. Gharasoo Research Station, pioneer in fisheries applied research projects. Abzeeyan, Teghran, 4(5):38-42, III. In Farsi.
Mageramov, Ch. M. and Zarbalieva, T. S. 1989. Pitanie belugi v zapadnoi chasti srednego Kaspiya [Beluga feeding in the western part of the southern
Caspian]. Izvestiya Akademii Nauk Azerbaidzhanskoi SSR, Seriya Biologicheskikh Nauk, 1989(1):49-52.
Magomedov, G. M. 1970. Some data on the chum salmon [Oncorhynchus keta (Walb.)] acclimatized in the Caspian Sea. Journal of Ichthyology, 10(4):552-555.
Magomedov, G. M. 1978. Some data on the biological basis for acclimatization of the chum salmon, Oncorhynchus keta, in the Caspian Sea. Journal of Ichthyology, 18(2):318-323.
Mahdavi, M. and Anderson, E. W. 1983. The water-supply system in the margin of Dasht-e-Kawir (Central Iran). Bulletin of the British Society for Middle Eastern Studies, 10(2):131-145.
Mahdi, N. 1962. Fishes of Iraq. Ministry of Education, Baghdad. 82 pp.
Mahdi, N. and Georg, P. V. 1969. A systematic list of the vertebrates of Iraq. Iraq Natural History Museum Publication, Baghdad, 26:1-104.
Mahdipoor, M., Barzegar, M. and Jalali, B. 2004. Monogenean parasites of fishes in Zayandeh-rud River. Iranian Journal of Veterinary Science, 1(2):19-28.
Mahjoorazad, A. and Coad, B. W. 2009. A new cave fish locality for Iran. Electronic Journal of Ichthyology, 5(2):30-33.
Mahjoorazad, A., Pazooki, J. and Sheidai, M. 2006. Intrapopulational study of genus Aphanius in hotsprings of Hormozgan province. 14th National and 2nd International Conference of Biology,
29-31 August 2006, Tarbiat Modares University, Tehran (title).
Mahmoudi, N. A., Abedian Kenari, A. A. M. and Soltani, M. 2009. Effects of different levels of dietary nucleotide on growth performance, survival and liver enzyme activity of Caspian salmon
(Salmo trutta caspius Kessler, 1877) juveniles. Iranian Scientific Fisheries Journal, 17(4):123-132. In Farsi.
Mahmoudzadeh, H., Mehrabi, Y. and Fazaeli, H. 2009. Effect of synthetic and alfalfa pigments on the tissue pigmentation of female rainbow trout. The 6th Scientific Conference of Fisheries Resources,
3-4 March 2009, Basrah, p. 68 (abstract).
Mahtab, E. 2002. Identification of freshwater fishes in some permanent rivers of Kerman Province. International Congress on the Biology of Fish, Congress 2002: Vancouver, Canada. p. 51-54.
Maitland, P. S. 1991. Conservation of threatened freshwater fish in Europe. Convention on the Conservation of European Wildlife and Natural
Habitats, Nature and Environment Series, Council of Europe Press, Strasbourg, 46:1-76.
Majdzadeh, M. 1987. Collection and identification of fishes in Radkan and Frizy tributries: Unpublished Honours B.Sc. Thesis, Biology Department, Mashhad University, Mashhad, Iran.
Majnunian, H. 1985. National Parks and Natural Reserves. Environmental Studies, Tehran University Press, 12:10-14. In Farsi.
Makaraova, N. P. 1986. Changes in some morphoecological parameters of perch, Perca fluviatilis, in the lower reaches of the Volga Delta. Journal of Ichthyology, 26(3):157-163.
Makeeva (sic), A. P. and Pavlov, D. S. 2000. Morphological characteristics and main features for the identification of pelagic eggs of freshwater
fish of Russia. Journal of Ichthyology, 40(9):740-750.
Makeyeva (sic), A. P. and Mokhamed, M. I. Z. 1982. The reproduction and development of Pseudorasbora parva in Soviet Central Asia. Journal of Ichthyology, 22(1):69-82.
Makhmudbekov, A. 1940. K sistematike kaspiiskoi shemai [On the systematics of the Caspian shemaya]. Izvestiya Azerbaidzhanskoi rybokhozyaistvennoi stantsii, 1940(5):3-16.
Maksunov, V. A. 1971. Non-annual spawning of some fishes of Soviet Central Asia. Journal of Ichthyology, 11:192-198.
Malek, M. 1993. Investigation on the infection of Capoeta capoeta with Clinonstomum (sic) and it's life cycle in the Shirrod
River. Iranian Fisheries Bulletin, (3):45-65, 6-7. In Farsi.
Malek, M. and Mobedi, I. 2001. Occurrence of Clinostomum Complanatum (sic) (Rudolphi, 1819) (Digenea: Clinostomatidae) in Capoeta capoeta gracilis (Osteichthys
(sic): Cyprinidae) from Shiroud River, Iran. Iranian Journal of Public Health, 30(3-4):95-98.
Malek, M. and Shamsi, Sh. 1994. Infection with Clinostomum complanatum in Capoeta capoeta in Shiroud River and its life cycle.
Proceedings of Seminar of Fisheries Researches, Tehran University, Tehran, Iran, September 1994, p. 31-32.
Malek-Eizadi, A. 1993. In memory of the Lar River: not a good memento. Abzeeyan, Tehran, 4(5):22-23. In Farsi.
Malek Nedjad, M. R. and Parivar, K. 1993. Influence of lead nitrate upon organogenesis of hypophthalmichthys (sic) molitrix. Iranian Fisheries Bulletin (1):31-46, 6-7. In Farsi.
Maleki, L. and Malek, M. 2007. Digenean parasites of Capoeta capoeta and Chalcalburnus chalcoides (Osteichthyes: Cyprinidae) in Shiroud River. Journal of Science, University of
Tehran, 32(4)(2):369-373.
Malhotra, Y. R. and Munshi, S. 1985. First feeding and survival of Aspidoparia morar larvae (Cyprinidae). Transactions of the American Fisheries Society, 114:286-290.
Mallowan, M. E. L. 1964. Noah's flood reconsidered. Iraq, 26:62-82, pls. XVI-XX.
Malmberg, G., Collins, C. M., Cunningham, C. O. and Jalali, B. J. 2007. Gyrodactylus derjavinoides sp. nov. (Monogenea, Platyhelminthes) on Salmo
trutta trutta L. and G. derjavini Mikailov, 1975 on S. t. caspius Kessler, two different species of Gyrodactylus - combined morphological
and molecular investigations. Acta Parasitologica, 52(2):89-103.
Maltby, E. (Ed.). 1994. An Environmental and Ecological Study of the Marshlands of Mesopotamia. Wetland Ecosystems Research Group, University of Exeter and the AMAR Appeal Trust. 228 pp.
Mamaev, V. 2002. Europe's biodiversity - biogeographical regions and seas. Seas around Europe. The Caspian Sea - enclosed and with many endemic species. European
Environment Agency, 25 pp. (http://reports.eea.eu.int/report_2002_0524_154909/en/CaspianSea.pdf, downloaded 11 December 2002).
Ma'manpoush, A. R. 1995. Environmental effects of hydraulic structures on aquatic life. WRM '95. Proceedings of the Regional Conference on Water Resources Management, Conference Secretariat,
Isfahan University of Technology, Isfahan, p. 153.
Mamedov, E. V. 2006. The biology and abundance of kilka (Clupeonella spp.) along the coast of Azerbaijan, Caspian Sea. ICES Journal of Marine Science, 63(9):1665-1673.
Mamedov, A. L. and Abbasov, G. S. 1990. Pitanie soma v prikurinskom raione yuzhnogo kaspiya [Feeding of catfish in the pre-Kura region of the southern Caspian Sea]. Izvestiya Akademii Nauk
Azerbaidzhanskoi SSR, Seriya Biologischeskikh Nauk, 1990(1):65-67.
Mamedov, A. V. 1997. The late Pleistocene-Holocene history of the Caspian Sea. Quaternary International, 41/42:161-166.
Mandel, S. 1975. Plain of Ghazvin, Iran, pp. 157-160. In: Ground-water storage and artificial recharge. Natural Resources / Water Series No. 2,
Department of Economic and Social Affairs, United Nations, New York.
Mandych, A. F. (Ed.). 1995. Enclosed seas and large lakes of eastern Europe and middle Asia. SPB Academic Publishing, Amsterdam. viii + 273 pp.
Mangalo, H. H. and Akbar, M. M. 1988a. Limnological investigations on the Al-Latifiyah common carp (Cyprinus carpio) pond (Baghdad - Iraq). I- Environmental conditions and zooplankton
abundance. Journal of Environmental Science and Health, A23(4):335-349.
Mangalo, H. H. and Akbar, M. M. 1988b. Limnological investigation on the Al-Latifiyah common carp (Cyprinus carpio) pond (Baghdad - Iraq). II Food and
feeding habits of Cyprinus carpio L. Journal of Environmental Science and Health, A23(6):513-524.
Mann, R. H. K. 1996. Environmental requirements of European non-salmonid fish in rivers. Hydrobiologia, 323(3):223-235.
Manouchehri, G. R. and Mahmoodian, S. A. 2002. Environmental impacts of dams constructed in Iran. Water Resources Development, 18(1):179-182.
Manouchehri, H. and Akrami, R. 2008. Constructing a new automatic sorter for trout culture farms in Iran. Iranian Scientific Fisheries Journal, 17(1):117-122. In Farsi.
Manouchehri, H., Emadi, H. and Salehi, H. 2005. Constructing a fish egg counter device. Iranian Journal of Fisheries Sciences, 14(2):127-144. In Farsi.
Mansoori, J. 1994. The Hamoun Wildlife Refuge. Max Kasparek Verlag, Heidelberg. 53 pp., 2 plates.
Marammazi, Gh. (sic). 1995. Survey of the biology of Tenualosa ilisha, southern Iran. Aquaculture Fishery Research Centre, Iranian Fisheries Research and Training Organization,
Ahvaz. 212 pp. In Farsi.
Marammazi, J., Al-Mukhtar, M. A., Dehgan, S., Maraashi, S. Z., Eskandari, G., Shakiba, G. and Mayiahi, Y. 1995. Biology of soboor Tenualosa ilisha
(phase 1), final report. Iranian Fisheries Research Organization, Tehran. 194 pp. In Farsi.
Marammazi, J., Al-Mukhtar, M. A. and Kiabi, B. H. 1998. Comparison of morphometric and meristic traits and length frequency in soboor (Tenualosa ilisha Ham. Buch. 1822) of the
migration pathways in Khuzestan province. Pajouhesj-va-Sazandagi, 11(39):13-120. In Farsi.
Marammazi, J., Parsamanesh, A., Dehgan, S., Najafpour, N. and Maraashi, S. Z. 1993. Limnological study of Zohreh River. Iranian Fisheries Research Organization, Tehran, pp. 72-95. In Farsi.
Marammazi, J. Gh. 1994. Preliminary ecological investigation of six fish species in Zohre River. Iranian Fisheries Scientific Journal, 3(2):57-70, 6. In Farsi.
Marammazi, J. Gh. 1995. A study of identification and some biological aspects of sobour Tenualosa ilisha (Ham. Buch 1822) in Bahmanshir River, Khuzestan, Iran. M.Sc. Thesis, Tarbiat Modarres
University. 78 pp. In Farsi.
Marammazi, J. G. H., Kiabi, B. O. and Al-Mukhtar, M. A. 1995. Morphometric and meristic characteristics of sobur Tenualosa ilisha (Ham. Buch 1822) in Bahmanshir River - Khuzestan - IRAN.
Iranian Fisheries Scientific Journal, 4(3):40-55, 7. In Farsi.
Marammazi, J. Gh., Al-Mukhtar, M. and Eskandari, Gh. R. 1998. Fecundity estimation of sobour (Tenualosa ilisha, Ham. Buch. 1822) in Khouzestan
Province's rivers. Iranian Fisheries Scientific Journal, 7(1):69-82, 7. In Farsi.
Marammazi, J. Gh., Al-Mukhtar, M. A., Eskandari, Gh. R. and Dehghan, S. 1996. Biological study of sobour Tenualosa ilisha (Ham. Buch. 1822) in migratory passways in southwest of
Iran. Khuzestan Fisheries Research Centre, Ahaz, Iran. XIII + 214 pp. In Farsi.
Marammazi, J. Gh., Mustafa, A. and Al-Mukhtar, M. A. 2001. The occurrence, feeding and reproduction of three Barbus spp. in Shadegan Marsh. The First National Symposium on
Barbus fishes of Iran, 9-10 January 2001, Khuzestan Fisheries Research Centre, Ahvaz. 50 pp. In Farsi.
Mar'ashi, A. 1984. Vaza-nama-ye guyesh-e gilaki.... Rasht.
Mardani Karani, M., Sheydaei, M. and Pazouki, J. 2008. Morphometric and meristic study of zebra fish (Aphanius vladykovi) in Charmahal and Bakhteyari Province. Iranian Journal of Biology,
20(4):447-457. In Farsi.
Markarova, I. A. and Alekperov, A. P. 1989. Age composition of sturgeons (Acipenseridae) occurring along the western shores of the south Caspian. Journal of Ichthyology, 29(2):72-76.
Markarova, I. A., Alekperov, A. P. and Zarbalina, T. S. 1991. Present status of the spawning run of sheap sturgeon, Acipenser nudiventris,
in the Kura River. Journal of Ichthyology, 31(5):17-22.
Markham, A. (Ed.). 1989. Painful death of the Caspian Sea. World Wildlife Fund Special Report, 1:1 p.
Markun, M. I. 1929. Materialy po sistematike shamai (Alburnus chalcoides) [Material on the systematics of shemaya (Alburnus chalcoides)].
Izvestiya Otdela Prikladnoi Ikhtiologii i Nauchno-promyslovykh Issledovanii, 9(2):179-190.
Marshall, T. R. 1977. Morphological, physiological, and ethological differences between walleye (Stizostedion vitreum vitreum) and pikeperch (S. lucioperca). Journal of the
Fisheries Research Board of Canada, 34:1515-1523.
Marti, V. Yu. 1940. Akklimatizatsiya kefali v Kaspiiskom more [Acclimatization of the grey mullet in the Caspian Sea]. Priroda, 1940(1):88-90.
Marti, V. Yu. 1941. Novoe ob akklimatizatsii kefali v Kaspiiskom more [New data on the acclimatization of the grey mullet in the Caspian Sea]. Priroda, 1941(3):85-86.
Marti, Yu. Yu. 1972. Problems in developing the sturgeon culture in the Caspian Sea. Fisheries Research Board of Canada Translation Series, Halifax, 2745:79 pp.
Marwick, R. and Germond, J. P. 1975a. The River Lar multipurpose project in Iran. Part One. Water Power & Dam Construction, 27:133-141.
Marwick, R. and Germond, J. P. 1975b. The River Lar multipurpose project in Iran. Part Two. Water Power & Dam Construction, 27:178-183.
Mashai, M. A. and Peyghan, R. 1998. Health and culture of warmwater fishes. Noorbaksh, Tehran. 118 pp.
Mason, K., Sherwin-White, A. N. and Hyamson, A. M. 1944. Iraq and the Persian Gulf. Naval Intelligence Division, Geographical Handbook Series, B.R. 524, London. xviii + 681 pp, map.
Masoumian, M. 1997a. Myxosporean parasites of freshwater fishes from south-western part of Iran. In: 8th International Conference of the European Association of Fish Pathologists:
Diseases of Fish and Shellfish, Edinburgh, 14-19 September 1997 (abstract).
Masoumian, M. 1997b. Myxosporean parasites of freshwater fishes from south-western part of Iran. In: 8th International Conference of the European Association of Fish Pathologists:
Diseases of Fish and Shellfish, Edinburgh, 14-19 September 1997 (abstract).
Masoumian, M. and Aghl Mandi, F. 2007. Infection of Neogobius spp. with Ceratomyxa caspia in the south Caspian S, Mazandaran Province. Iranian Scientific Fisheries Journal,
15(4):155-158. In Farsi.
Masoumian, M., Barzegar, M., Mehdipoor, M., Asadollah, S. and Jalali, B. 2007. Myxobolus spp. (Myxosporea: Myxobolidea) from fishes of the Zayandeh-rud River (Esfahan, Iran); new hosts
and locality record. Iranian Journal of Fisheries Sciences, 7(1):89-100.
Masoumian, M., Baska, F. and Molnár, K. 1994. Description of Myxobolus karuni sp. n. and Myxobolus persicus sp. n. (Myxosporea,
Myxozoa) from Barbus grypus of the River Karun, Iran. Parasitologia hungarica, 27:21-26.
Masoumian, M., Baska, F. and Molnár, K. 1996a. Description of Myxobolus bulbocordis sp. nov. (Myxosporea: Myxobolidae) from the heart of Barbus sharpeyi (Günther)
and histopathological changes produced by the parasite. Journal of Fish Diseases, 19(1):15-21.
Masoumian, M., Baska, F. and Molnár, K. 1996b. Myxobolus nodulointestinalis sp. n. (Myxosporea, Myxobolidae), a parasite of the intestine of Barbus
sharpeyi. Diseases of Aquatic Organisms, 24(1):35-39.
Masoumian, M., Choubchian, M., Pazouki, J., Sharifpour, I. and Jalali, B. 2008. Histozoic developmental stages of Myxobolus karuni and Myxobolus persicus and introducing three new hosts.
Journal of Veterinary Research, 63(3):117-122. In Farsi.
Masoumian, M., Mehdizadeh, A. and Yahyazadeh, M. 2002. Protozoans (Coccidia and Myxosporea) infections in some fishes of Aras and Mahabad Dams (northwest of
Iran). Iranian Journal of Fisheries Sciences, 11(2):79-90. In Farsi.
Masoumian, M. and Pazooki, J. 1998. Survey on myxosporean parasites in the Mazandaran and Guilan provinces (southern part of Caspian Sea). Iranian Journal of Fisheries Sciences, 7(3):57-74, 6. In Farsi.
Masoumian, M. and Pazooki, J. 1999a. Myxosporean parasites from freshwater fishes of Guilan and Mazandaran provinces. Iranian Journal of Fisheries Sciences, 7(3):47-57. In Farsi.
Masoumian, M. and Pazooki, J. 1999b. Myxosporean parasites from Mesopotamian part of Iran. Iranian Journal of Fisheries Sciences, 1(1):35-46.
Masoumian, M. and Pazooki, J. 2001. Cryptobia acipenseris and Haemogregarina acipenseris in Persian and Russian sturgeons in the Caspian
Sea. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Masoumian, M., Pazooki, J. and Ghasemi, R. 2003. Myxobolus infection in three Barbus spp. from the southern part of the Caspian Sea. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 58(4):329-334.
In Farsi.
Masoumian, M., Pazooki, J., Yahyazadeh, M. and Teymornezhad, A. 2005. Protozoan (sic) from fresh water fishes of north west Iran. Iranian Journal of Fisheries Sciences, 4(2):31-42, 115.
Masoumian, M., Setareh, J. and Mokhayer, B. 2002. Parasitological investigations on Rutilus rutilus caspicus from south-east of the Caspian Sea (Iran).
Iranian Journal of Fisheries Sciences, 10(4):61-74. In Farsi.
Masoumzadeh, H., Shenavar Masouleh, A. R., Jalilpour, J., Bazari Moghadam, S., Masoumian, M. and Satari, M. 2007. Prevalence of infection of Acipenser persicus broodstocks with internal
parasites in eths south-west Caspian Sea. Iranian Scientific Fisheries Journal, 15(4):129-134. In Farsi.
Massoud, J., Jalali, H. and Reza, M. 1981. Studies on trematodes of the family Heterophyidae (Odhner, 1914) in Iran: 1. Preliminary epidemiological surveys in man and carnivores in
Khuzestan. Journal of Helminthology, 55(4):255-260.
Mathews, C. P., Peacock, N. and Gilkolei, R. 2006. Theoretical scenarios for research and development needs to save dwindling sturgeon populations in the Caspian Sea. Journal of Applied Ichthyology,
22(s1):132-138.
Mathias, P. 1921. Étude du genre Chondrostoma dans l'Europe occidentale et la région circumméditerranéanne. Mémoires
de la Société zoologique de France, 28(1/2):1-52, pl. I-II.
Matinfar, A. 2001. Diversification of aquaculture in Iran. In: Kjørsvik, E. and Stead, S. (Eds.). Aquaculture Europe 2001. Abstracts of contributions
presented at the International Conference Aquaculture Europe 2001, Trondheim, Norway, 4-7 August 2001. EAS Special Publication, 29:xiv + 286 pp.
Matinfar, A. 2003. Endangered fish and shellfish species of Iran. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Matinfar, A. and Nikouyan, A. 1995. Islamic Republic of Iran, pp. 207-226. In: Report on a regional study and workshop on the environmental assessment
and management of aquaculture development. Network of Aquaculture Centres in Asia-Pacific, Bangkok. 491 pp.
Matthews, O. 1998. Bye, beluga, later, sevruga. Newsweek, 131(25):42.
Maulood, B. K., Hadi, R. A. M., Saadalla, H. A. A., Kassim, T. I. and Al-Lami, A. A. 1993. Check list of algae in Iraq. Marina Mesopotamica, Supplement 1:1-128.
Maulood, B. K., Hinton, G. C. F., Kamees, H. S., Saleh, F. A. K., Shaban, A. A. and Al Shahwani, S. M. H. 1979. An ecological survey of some aquatic
ecosystems in southern Iraq. Tropical Ecology, 20(1):27-40.
Maulood, B. K., Hinton, G. C. F., Whitton, B. A. and Al-Saadi, H. A. 1981. On the algal ecology of the lowland Iraqi marshes. Hydrobiologia, 80:269-276,
Mayland, H. J. 1995. Cichliden. Landbuch, Hannover. 596 pp.
Mazlomi, M. 2003. Streptocccosis Antrococcosis. Important Economic Disease in Fish Culture. Navid Shiraz Publication, Iran. 94 pp. In Farsi.
McClelland, J. 1842. On the fresh-water fishes collected by William Griffith in his travels from 1835-1842. Journal of Natural History, Calcutta, 2:560-589.
McClure, H. A. 1976. Radiocarbon chronology of late Quaternary lakes in the Arabian Desert. Nature, London, 263(5572):755-756.
McCord, M. E. and Lamberth, S. J. 2009. Catching and tracking the world's largest Zambezi (bull) shark Carcharhinus leucas in the Breede Estuary, South Africa: the first 43 hours. African
Journal of Marine Science, 31(1):107-111.
McKay, S. I. and Miller, P. J. 1997. The affinities of European sand gobies (Teleostei: Gobiidae). Journal of Natural History, 31:1457-1482.
McKinnon, M. and Vine, P. 1992. Tides of War. Eco-Disaster in the Gulf. Immel Publishing, London. 192 pp.
McLachlan, K. 1988. The Neglected Garden: The Politics and Ecology of Agriculture in Iran. I. B. Tauris & Co. Ltd., London. xxi + 303 pp.
McLachlan, K. and Schofield, R. N. 1987. A Bibliography of the Iran-Iraq Borderland. Middle East & North African Studies Press Limited, Wisbech. xii + 383 pp.
McNeil, W. J. 1979. Review of transplantation and artificial recruitment of anadromous species, p. 547-554. In: Pillay, T. V. R. and Dill W. M. (Eds.). Advances in Aquaculture.
Papers presented at the FAO Technical Conference on Aquaculture Kyoto, Japan, 26 May - 2 June 1976. Fishing News Books, Farnham, Surrey. xviii + 653 pp.
Mearns, B. and Mearns, R. 1988. Biographies for Birdwatchers. The Lives of Those Commemorated in Western Palearctic Bird Names. Academic Press, New York. xx + 490 pp.
Mednikov, B. M., Shubina, E. A., Melnikova, M. H. and Savvaitova, K. A. 1999. The genus status problem in Pacific salmons and trouts: a genetic systematics investigation.
Journal of Ichthyology, 39(1):10-17.
Meffe, G. K. and Snelson, F. F. (Eds.). 1989. Ecology and Evolution of Livebearing Fishes (Poeciliidae). Prentice Hall, Englewood Cliffs, New Jersey. xxv + 453 pp.
Mehdipoor, M., Barzegar, M. and Jalali, B. 2004. A survey on monogenean parasites of gills of fishes in Zayandeh-rud River. Iranian Journal of Veterinary Science, 2:19-28. In Farsi.
Mehdipoor, M. and Esteki, A. A. 2006. Use of oil by-product in rainbow trout diet. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture, Esfahan. http://en.ifro.ir (abstract).
Mehdizadeh, M. R. 1993. Body malformations in sturgeons. Abzeeyan, Tehran, 4(7):25, II-III. In Farsi.
Mehraban, N. 1996. Income effect on fish demand in Iran (1971-1994). Shilat (Fisheries), Tehran. 42 pp. In Farsi.
Mehrabi, H. 2004. Gav-Khooni Pond. Jam-e-Jam, Tehran, 1181:17 (6 July 2004).
Mehrabi, Y. 2002. Cold water aquaculture in Iran, 9 pp. In: Petr, T. and Swar, D. B. (Eds.). Cold water fisheries in the Trans-Himalayan countries. Food and Agriculture Organization, Fisheries
Technical Paper, 431 (www.fao.org/DOCREP/005/Y3994E/y3994e08.htm).
Mehrtabor, R. R. 1960. Fishes of Iran. Tehran. 123 pp. In Farsi.
Meigolinezhad, A. 2000. The effective parameters of fish consumption in land-locked cities and the method of increasing fishes by using Delphi Method. M.Sc. Thesis, Amirkabir University of Technology,
Tehran. 88 pp. In Farsi.
Meijer, K. 2006. Integrated water resources management for the Sistan closed inland delta, Iran. Annex E. Socio-economic valuation of allocating water to the hamouns and to agriculture. WL Delft
Hydraulics, The Netherlands. v + 89 pp.
Mellat-Parast, A. 1992. Temperature and water fluctuations in the southern Caspian Sea. Iranian Fisheries Bulletin, 1:28-41, 7. In Farsi.
Melville, C. 1984. Meteorological hazards and disasters in Iran: a preliminary survey. Iran (Journal of the British Institute of Persian Studies), 22:113-150.
Ménétries, E. 1832. Catalogue raisonné des objets de zoologie recueillis dans un voyage au Caucase et jusqu'aux frontières actuelles de la Perse. St. Pétersbourg.
300 pp.
Menon, A. G. K. 1954. Fish geography of the Himalayas. Proceedings of the National Institute of Science of India, 20(4):467-493.
Menon, A. G. K. 1957. The external relationships of the Indian freshwater fishes, with special reference to the countries bordering on the Indian Ocean. Journal of the Asiatic Society, Science,
21(1)(1955):31-38.
Menon, A. G. K. 1964. Monograph of the cyprinid fishes of the genus Garra Hamilton. Memoirs of the Indian Museum, 14(4):173-260, pl. VIII-XIII.
Menon, A. G. K. 1987. The Fauna of India and the Adjacent Countries. Pisces. Vol. IV. Teleostei - Cobitoidea. Part 1. Homalopteridae. Zoological Survey of India, Calcutta. x + 259 pp., pls. I-XVI.
Menon, A. G. K. 1992. The Fauna of India and the Adjacent Countries. Pisces. Vol. IV. Teleostei - Cobitoidea. Part 2. Cobitidae. Zoological Survey of India, Calcutta. 113 pp., pls. 1-10.
Menon, A. G. K. and Yazdani, G. M. 1968. Catalogue of type-specimens in the Zoological Survey of India. Part 2.-Fishes. Records of the Zoological Survey of India, 61(1 & 2):1-190.
Menon, M. A. S. 1960. On a third collection of fish from Iraq. Records of the Indian Museum, 54(1956):139-157, pl. 2.
Merchant, N. M. and Ronaghy, H. A. 1976. Water resources and pollution control planning in Iran. Journal of Environmental Health, 38(6):406-409.
Meriç, N., Eryılmaz, L. and Özuluğ, M. 2007. A catalogue of the fishes held in the Istanbul University, Science Faculty, Hydrobiology Museum. Zootaxa, 1472:29-54.
Meshgi, B., Eslami, A. and Yazdani, H. 2006. Study on parasitic infections of aquarium fishes around Tehran. Journal of the Veterinary Faculty of the University of Tehran, 61(1):1-5.
Meshkat Rouhani, A., Abedia Kenari, A. A. M. and Shariatmadari, F. 2007. Effects of dietary carbohydrate to lipid ratios at two levels of protein on growth performance, body composition and
hepatosomatic index of rainbow trout (Oncorhynchus mykiss). Iranian Journal of Natural Resources, 60(1):161-175. In Farsi.
Meshkov, M. M. 1941. K sistematike ryb semeistva Atherinidae Chernogo i Kaspiiskogo Morei [On the systematics of the fish of the family Atherinidae
from the Black and Caspian seas]. Izvestiya Akademii Nauk SSSR, Otdel Biologicheskikh Nauk, 3:400-406.
Mhaisen, F. T. 1980. Fish Parasitology in Iraq. Basrah Natural History Museum Publication, 3:1-36, IX plates.
Mhaisen, F. T. and Al-Jaffery, A. R. 1989. Determination of age and growth of the mugilid fish Liza abu (Heckel) inhabiting Babylon Fish
Farm, Hilla, Iraq. Journal of Biological Sciences Research, Baghdad, 20(3):547-554.
Mhaisen, F. T. and Al-Maliki, N. S. 1996. Parasites, diseases and food of the dark-blotched mudskipper Periophthalmus waltoni (Perciformes:
Gobiidae) in the Khor Al-Zubair estuary (Iraq). Zoology in the Middle East, 13:85-87.
Mhaisen, F. T. and Yousif, U. H. 1988. Reproductive biology of the mugilid fish Liza abu (Heckel) from Mehaijeran Creek, Basrah. Basrah Journal of Agricultural Science, 1:48-55.
Mhaisen, F. T. and Yousif, U. H. 1989. Age and growth of the mugilid fish Liza abu (Heckel) from Mehaijeran Creek, Basrah, Iraq. Bulletin of the Iraq Natural History Museum, 8(2):147-155.
Miar, A., Bozorgnia, A., Pazooki, J., Barzegar, M., Masoumian, M. and Jalali, B. 2008. Identification of parasitic fauna of fishes in
Valasht Lake and Chalus River. Iranian Scientific Fisheries Journal,
17(1):133-138. In Farsi.
Michel, A. A. 1973. The impact of modern irrigation technology in the Indus and Helmand basins of southwest Asia, pp. 257-275. In: Farvar, M.
T. and Milton, J. P. (Eds.). The Careless Technology. Ecology and International Development. Tom Stacey, London. xxix + 1030 pp.
Michel, C., Faivre, B. and de Kinkelin, P. 1986. A clinical case of enteric redmouth in minnows (Pimephales promelas) imported in Europe as bait-fish. Bulletin of the European Association of
Fish Pathologists, 6(4):97-99.
Micklich, N. und Roscher, B. 1990. Neue Fischfunde aus der Baid-Formation (Oligozän; Tihamat Asir, SW Saudi-Arabien). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 180(2):139-175.
Micklin, P. P. 1979. Disciplinary plans for USSR rivers. Geographical Magazine, 51(10):701-706.
Micklin, P. P. 1986. Soviet river diversion plans: their possible environmental impact, pp. 91-106. In: Goldsmith, E. and Hildyard, N. (Eds.). The Social
and Environmental Effects of Large Dams. Volume Two: Case Studies. Wadebridge Ecological Centre, Camelford, U.K. 331 pp.
Mietle, P. L. 1967. The fisheries of the Near East region. Fisheries Circular, Food and Agriculture Organization, 112:140 pp.
Mihálik, J. 1982. Der Wels. Silurus glanis. A. Ziemsen Verlag, Wittenberg. 71 pp. (Die Neue Brehm-Bücherei, 209, 1995 Reprint).
Mikaeili, A. R., Abdoli, A. and Amini Nasab, S. M. 2005. Physical structure of the Madar-sou stream in Golestan National Park. Journal of Agricultural Sciences and Natural Resources, 12(3):100-111.
In Farsi.
Mikailov, T. K. 1975. Parazity ryb vodoemov Azerbaidzhana (sistematika, dinamika, proiskhozhdenie) [Fish parasites of the waters of Azerbaidzhan
(systematics, dynamics, origin]. Akademiya Nauk Azerbaidzhanskoi SSR, Institut Zoologii, Baku. 299 pp.
Mikailov, T. K. and Ibragimov, Sh. P. 1980. Ekologiya i zoogeografiya parazitov ryb vodoemov Lenkoranskoi pripodnoi oblasti [Ecology and zoogeography
of the fish parasites of the natural waters of the Lenkoran region]. Akademiya Nauk Azerbaidzhanskoi SSR, Institut Zoologii, Baku. 115 pp.
Mikhailovskaya, A. A. 1941. On form-building in the Caspian herring Caspialosa brashnikovi (Borodin). Comptes Rendus (Doklady) de l'Académie
des Sciences de l'URSS, nouvelle série, 30(6):563-566.
Miller, N. F. 1985. Paleoethnobotanical evidence for deforestation in ancient Iran: a case study of urban Malyan. Journal of Ethnobiology, 5(1):1-19.
Miller, P. J. 1990. The endurance of endemism: the Mediterranean freshwater gobies and their prospects for survival. Journal of Fish Biology, 37(supplement A):145-156.
Miller, P. J. 2001. The phylogeny of Ponto-Caspian gobiid fishes. The 10th European Congress of Ichthyology ECI X, Programme & Book
of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 172-173.
Miller, P. J. (Ed.). 2003. The Freshwater Fishes of Europe. Volume 8/I. Mugilidae, Atherinidae, Atherinopsidae, Blenniidae, Odontobutidae, Gobiidae 1. AULA-Verlag, Wiebelsheim. xii + 404 pp.
Mil'shtein, V. V. 1957. Razvedenie Osetrovykh (Raising of Sturgeons). Pishchepromizdat, Moscow. 67 pp.
Mil'shtein, V. V. 1972. Osetrovododstvo (Sturgeon rearing). Izdatel'stvo "Pishchevaya Promyshlennost', Moscow. 129 pp.
Mina, M. V. 1992. Problems of protection of fish faunas in the USSR. Netherlands Journal of Zoology, 42(2-3):200-213.
Mina, M. V., Mironovsky, A. N. and Golani, D. 2001. Consequences and modes of morphological diversification of East African and Eurasian barbins (genera Barbus,
Varicorhinus and Capoeta) with particular reference to Barbus intermedius complex. Environmental Biology of Fishes, 61(3):241-252.
Mirbagheri, S. A. 1995. Best management practices in erosion control and sediment production from the watershed area of a dam. WRM '95.
Proceedings of the Regional Conference on Water Resources Management, Conference Secretariat, Isfahan University of Technology, Isfahan, pp. 467-476.
Mirfendereski, G. 2000. Evolution of Iranian sovereignty in the Caspian Sea, p. 227-262. In: Amirahmadi, H. (Ed.). The Caspian Region at a Crossroad. Challenges of a New Frontier of
Energy and Development. St. Martin's Press, New York. xii + 299 pp.
Mirhashemi Rostami, A. 2000. Effect of coconut oil edible film on quality and shelf life of Acipenser persicus during frozen stoarge. M.Sc. Thesis, University of Tehran. 120 pp. In Farsi.
Mirhashemi Rostami, S. A., Amini, K., Jorjani, M., Piri, H., Hami Tabari, A., Iri, Y., Shafe, A. A. Gh. Saghali, M. and Poursoufi, T. 2008. Mortality factors in artificially reproduced grey mullet
(Mugil cephalus L.) fries. Iranian Scientific Fisheries Journal, 17(3):153-160. In Farsi.
Mirhashemi Rostamy (sic), S. A., Joorjani, M., Amini, K., Shafaee, A. and Ghazel, H. Gh. 2006. An investigation on artificial reproduction of Mugil cephalus. Iranian
Journal of Fisheries Sciences, 14(4):181-196. In Farsi.
Mirhasheminasab, S. F. and Pazooki, J. 2003. Identification of crustacean parasites in some fishes of Mahabad Reservoir. Iranian Journal of Fisheries Sciences, 11(4):133-148. In Farsi.
Miri Nargesi, M. S., Pourkazemi M. and Nourouz Fashkhami, M. R. 2007. Karyology of pikeperch (Sander lucioperca) in the south Caspian Sea. Iranian Scientific Fisheries Journal, 16(2):137-144.
In Farsi.
Mirkou, N. 2001. Environmental audit of agro-chemical usage along the Caspian shore of I. R. Iran. Caspian Environment Programme, Baku, Azerbaijan. 31 pp.
Mironovskii, A. N. 1991. Variability and population structure of some cyprinid fishes in the Volga-Caspian and adjacent regions. 1. Population subdivision. Journal of Ichthyology, 31(8):96-105.
Mironovskii, A. N. 1992. Variability and population structure of some cyprinid fishes in the Volga-Caspian and adjacent regions. 2. Analysis of character variability. Journal of Ichthyology,
32(1):77-87.
Mironovskii, A. N. and Kas'yanov, A. N. 1986. Struktura vida Rutilus rutilus v basseine Kaspiiskogo Morya [The Rutilus rutilus species structure in the Caspian Sea basin].
Zoologicheskii Zhurnal, 65(7):1024-1031.
Mironovskii, A. N. and Kas'yanov, A. N. 1987. Mnogomernyi analiz morfologicheskoi izmenchivosti plotvy - Rutilus rutilus (Cyprinidae) iz vodoemov
SSSR [Multivariate analysis of morphological variations of the roach, Rutilus rutilus (Cyprinidae), from waters of the USSR]. Zoologicheskii Zhurnal, 66(3):393-401.
Mirvaghefi, A. R., Azari Takami, G and Jafarpour, S. A. 2006. A comparative study of the effect of hydrogen peroxide as against malachite green used for
prevention and control of fungal infection during hatching period in rainbow trout (Oncorhynchus mykiss) eggs. Iranian Journal of Natural Resources, 58(4):853-860. In Farsi.
Mirza, M. R. 1969. Fishes of the genus Cyprinion Heckel (Cyprinidae, Osteichthyes) from West Pakistan. Pakistan Journal of Zoology, 1:141-150.
Mirza, M. R. 1971. Freshwater fishes of Makran, with a note on the swim-bladder of Cyprinion microphthalmum (Day). Pakistan Journal of Zoology, 3(2):240-242.
Mirza, M. R. 1972. Freshwater fishes of Baluchistan Province, Pakistan. Biologia, Lahore, 18:153-190.
Mirza, M. R. 1974. Freshwater fishes and ichthyogeography of Baluchistan and adjoining areas of the Indus Plain, Pakistan. Biologia, Lahore, 20:67-82.
Mirza, M. R. 1975. Freshwater fishes and zoogeography of Pakistan. Bijdragen tot de Dierkunde, 45(2):143-180.
Mirza, M. R. 1978. History of ichthyology in Pakistan. Biologia, Lahore, 24(2):305-348.
Mirza, M. R. 1980. The systematics and zoogeography of the freshwater fishes of Pakistan and Azad Kashmir. Proceedings of the 1st Pakistan Congress of Zoology, 1980:1-41.
Mirza, M. R. 1983. A review of natural history research in the Muslim World. National Science Council, Islamabad. 144 pp.
Mirza, M. R. 1986. Ichthyogeography of Afghanistan and adjoining areas. Pakistan Journal of Zoology, 18(4):331-339.
Mirza, M. R. 1989a. Ichthyogeography of Pakistan and adjoining areas. Science International, Lahore, 1(3):199-207.
Mirza, M. R. 1989b. A note on the classification of Cobitoidea with the description of a new family Noemacheilidae (Pisces, Cypriniformes). Science International, Lahore, 1(5):319-320.
Mirza, M. R. 1990a. Pakistan main taza pani ki macchalian [Freshwater Fishes of Pakistan]. Urdu Science Board, Lahore. 128 pp. In Urdu.
Mirza, M. R. 1990b. Ichthyogeographical regions of Eurasia with a plea for the retention of name Oriental Region instead of Indian or Sino-Indian or Indoasiatic Region. Pakistan Journal of Zoology,
22(4):403-404.
Mirza, M. R. 1991a. A contribution to the systematics and biology of the snow-carps (Pisces, Cyprinidae, Schizothoracinae) of Pakistan. First
Symposium on Fish and Fisheries of Pakistan, October, 19 - 20, 1991, Department of Zoology, Government College, Lahore, p. 29 (abstract).
Mirza, M. R. 1991b. A contribution to the systematics of the Schizothoracine fishes (Pisces: Cyprinidae) with the description of three new tribes. Pakistan Journal of Zoology, 23(4):339-341.
Mirza, M. R. 1992. A note on the fishes of the River Rakhshan with the record of Garra rossica (Nikolsky)(Pisces: Cyprinidae). Pakistan Journal of Zoology, 24(1):79.
Mirza, M. R. 1993. Pakistan ki Nachhlian aur Mahi Parvari [Fish and Fisheries of Pakistan]. 80 pp., colour plates.
Mirza, M. R. 1994a. A note on the fishes of the River Hingol, Pakistan. Pakistan Journal of Zoology, 26(2):181.
Mirza, M. R. 1994b. Geographical distribution of freshwater fishes in Pakistan: a review. Punjab University Journal of Zoology, 9:93-108.
Mirza, M. R. 1995. Distribution of freshwater fishes in Pakistan and Kashmir. Proceedings of Seminar on Aquaculture Development in Pakistan
held at Fisheries Research & Training Institute, Manawan, Lahore, Pakistan, October, 17-18, 1993:1-15.
Mirza, M. R. 2000. A contribution to the fishes of the River Kurram with proposal of a new subfamily Aspidoparinae (Cyprinidae). Science International, Lahore, 12(4):355-357.
Mirza, M. R. 2003. Checklist of freshwater fishes of Pakistan. Pakistan Journal of Zoology Supplement Series, 3:1-30.
Mirza, M. R. 2006. Revised ichthyogeography of Pakistan and adjoining areas. Biologia, Lahore, 52(2):105-115.
Mirza, M. R. and Afridi, R. 2002. A note on the distribution of the snow-carps (Pisces: Cyprinidae: Schizothoracinae) in Pakistan and Kashmir. Pakistan Journal of Zoology, 34(2):171-173.
Mirza, M. R. and Ahmed, M. S. 2004. Distribution of freshwater fishes in Balochistan Province of Pakistan. Punjab University Journal of Zoology, 19:103-113.
Mirza, M. R. and Alam, M. K. 1994. A checklist of the freshwater fishes of Pakistan and Azad Kashmir. Science International, Lahore, 6(2):187-189.
Mirza, M. R. and Arshad, M. 2008 Status of Labeo macmahoni Zugmayer (Pisces: Cyprinidae). Pakistan Journal of Zoology, 40(6):465-466.
Mirza, M. R., Awan, K. M. and Javed, M. N. 1991. A contribution to the systematics of the fishes of the genus Cyprinion Heckel from Pakistan. First Symposium on Fish and Fisheries of Pakistan,
October, 19 - 20, 1991, Department of Zoology, Government College, Lahore, p. 12 (abstract).
Mirza, M. R., Bănărescu, P. and Nalbant, T. T. 1969. Two new loaches of the genus Noemacheilus from West Pakistan. Pakistan Journal of Zoology, 1:87-90.
Mirza, M. R., Banarescu, P. and Nalbant, T. T. 1970. A little-known and three new loaches of the genus Noemacheilus (Pisces, Cobitidae) from West Pakistan. Biologia, Lahore, 16(1):47-58.
Mirza, M. R. and Bhatti, M. N. 1993. Pakistan ki Machhlian aur Mahi Parvari [Fish and Fisheries of Pakistan]. Part I. Ferozsons, Lahore. 184 pp. In Urdu.
Mirza, M. R., Hameed, S. and Naeem Javed, M. 1996. Fishes of Shadi Khoar (Baluchistan). Second Symposium on Fish and Fisheries of Pakistan, November, 25, 26, 1996, Department of Zoology, Government
College, Lahore, p. 8-9 (abstract).
Mirza, M. R. and Naeem Javed, M. 1985. A note on the mahseers of Pakistan with the description of Naziritor, new subgenus (Pisces: Cyprinidae).
Pakistan Journal of Zoology, 17(3):225-227.
Mirza, M. R. and Naeem Javed, M. 1986. A contribution to the fishes of the genus Tor Gray (Pisces, Cyprinidae) from Pakistan and Azad Kashmir. Biologia, Lahore, Special Supplement, 1986:71-82.
Mirza, M. R., Nalbant, T. T. and Banarescu, P. M. 1981. A review of the genus Schistura in Pakistan with description of new species and subspecies (Pisces, Noemacheilinae).
Bijdragen tot de Dierkunde, 51(1):105-130.
Mirza, M. R. and Omer, T. 1984. A key to the identification of the freshwater fishes of Baluchistan. Biologia, Lahore, 30(1):73-91.
Mirza, M. R. and Saboohi, N. 1990. A note on the freshwater fishes of the River Dasht with the description of Tariqilabeo new subgenus (Pisces, Cyprinidae). Pakistan Journal of Zoology,
22(4):405-406.
Mirzaei, R., Krami, M., Danehkar, A. Abdoli, A. and Conroy, J. 2010. Prey size selection of the Eurasian otter, Lutra lutra (Linnaeus, 1758), at the Jajrood River, Iran. Zoology in the Middle
East, 50:19-25.
Mirzajani, A., Yosefzadeh, A. and Ganea, A. 1999. Zoobenthic invertebrate of Anzali Lagoon and their relation with organic matter of the bottom.
Iranian Journal of Fisheries Sciences, 7(4):83-102, 7. In Farsi.
Mirzajani, A. R. 2006. Controlling methods investigation of the Caspian Sea invasive Ctenophora (Mnemiopsis leidyi). Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Mirzajani, A. R., Ghaninezhad, D. and Ghane Sasan Saraei, A 2005. The relation between fish catch values and macrobenthic biomass in Caspian Sea of Guilan province. Pajouhesh va Sazandegi,
18(3)(68):2-9. In Farsi.
Mirzajani, A. R., Khoda Parast, H., Babaei, H., Abedini, A. and Dadi Ghandi, A. 2010. Eutrophication trend of Anzali Wetland based on 1992-2002 data. Journal of Environmental Studies,
35(52):19-21. In Farsi.
Mirzajani, A. R., Shiganaova, T., Finenko, G., Bagheri, S., Kideys, A. E. and Roohi, A. 2007. Reproduction of the ctenophore, Beroe ovata, in the Caspian Sea water. Iranian Journal of Fisheries
Sciences, 6(2):93-104.
Mirzakhani, M. K. and Abad, M. 2006. Increasing the resistance to environmental stress (pH and temperature) in larvae of Oncorhynchus mykiss
by feeding of n-3HUFA enriched Artemia nauplii. Pajouhesh va Sazandegi, 68:2-9. In Farsi.
Mirzakhani, M. K., Azari Takami, Gh., Abedian Kenari, A. and Banavreh, A. 2006. Increasing the resistance to environmental stress (pH and temperature) in larvae of Oncorhynchus
mykiss by feeding of n-3HUFA enriched Artemia nauplii. Pajouhesh va Sazandegi,
18(69):47-52. In Farsi.
Mirzargar, S. S., Soltani, M. and Rostami, M. 2000. Assessment of residues of some antibiotics in serum and tissues of common carp and rainbow
trout using thin layer chromatography-bioautography (TLC-B). The Third World Fisheries Congress, Beijing, 31 Oct - 2 Nov 2000 (title).
Misaghi A., Akhondzadeh Basti, A. and Zahrae Salehi, T. 2003. The study of fungi and bacterial pathogens in salted fish in Iran. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway,
August 8-12, 2003 (title).
Misra, K. S. 1949. On a second collection of fish from Iraq. Records of the Indian Museum, 45(1947):115-127.
Mitchell, K. and Staff. 1980. Appendix L. Agriculture, Fishing, and Pearls, pp. 652-666. In: Cottrell, A. J. The Persian Gulf States. A General Survey. The Johns Hopkins University Press, Baltimore
and London. xxxiv + 695 pp.
Mitrofanov, I. V. 2003. Identification tables (keys) for Caspian gobies. Tethys Aqua Zoological Research, 2:171-176. In Russian.
Mitrofanov, V. P., Dukravets, G. M., Mel’nikov, V. A. and Baimbetov, A. A. 1988. Fishes of Kazakhstan. Volume 3, Carps. Nauka, Alma Ata. 303 pp. In Russian.
Mobayen, Gh. H. 1968. La pêche, le caviar et la perle en Iran. Thèse présentée à la Faculté des Lettres de l'Université de Neuchâtel, Neuchâtel. 157 pp.
Moeini, S. 1989. Fisheries Products Industries. Iranian Fisheries Research and Training Organization, Tehran. 212 pp.
Moeini, S. 2002. Investigation on the method of production of sausages from kilka. Iranian Journal of Marine Science, 1(4):111-120, 136. In Farsi.
Moeini, S. and Daneshnuran, B. 2001. Production of cold marinade from roach (Rutilus rutilus). Iranian Journal of Natural Resources, 54(1):63-73. In Farsi.
Moeini, S., Delroushan, M., Kouchakian Sabour, A. and Asgari Sari, A. A. F. 2008. A comparative assessment of the effects of fresh and saltwater on soluble proteins and surimi made from
Hypophthalmichthys molitrix. Iranian Scientific Fisheries Journal, 17(3):143-152. In Farsi.
Moeini, S. and Hedayatifard, M. 2007. Variation of fatty acids composition in fresh and frozen Persian sturgeon tissues Acipenser persicus. Iranian Journal of Natural Resources, 59(4):875-884.
In Farsi.
Moeini S., Sabetian, M., Khaleghi Gorj, A. and Farhangi, M. 2009. An investigation on relationships of chemical indices of kilka (Clupeoenlla engrauliformis) with weight loss during cold storage
at -18ºC. Iranian Scientific Fisheries Journal, 18(2):129-140. In Farsi.
Moghadam, F. 1974. The blue-green algae of Geno Hot Spring (Iran). Bulletin of the Faculty of Science, Tehran University, 6(2):78-83.
Moghadam, F. 1976. Diatoms as indicator of pollution Zayandeh River, Iran. Proceedings of the Academy of Natural Sciences, Philadelphia, 127(19):281-297.
Moghadam, H. N. 2006. The possibility study of preservation and freezing sperm of two sturgeon species Acipenser persicus , Acipenser stellatus. Iranian Fisheries Research
Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Moghadam, K. H. 2006. Study of the usage of rotifer (Brachionus plicatilis) in A. persicus in larval stage. Iranian Fisheries Research Organization,
Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Moghadamm, A. K. 2006. Qualitative and quantitative survey of bonyfishes fingerlings being released as stocking material in southern Caspian Sea Guilan Province in 2002. Iranian Fisheries
Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Bandar Anzali. http://en.ifro.ir (abstract).
Moghadamnia, A. A., Mirhosseini, N., Abadi, M. H., Omranirad, A.S. and Omidvar, S. 2010. Effect of Clupeenonella grimmi (anchovy/kilka) fish oil on dysmenorrhoea. Eastern Mediterranean Health
Journal, 16(4):408-413.
Moghainemi, R. S. and Abbasi, S. 1992. Parasitic fauna of economically important fishes from Hoor-Elazim Marsh. Iranian Fisheries Research and
Training Organization Publication, Tehran. iv + 107 pp. In Farsi.
Moghiem, M. 2003. Stock assessment and population dynamics of Acipenser persicus in the southern Caspian Sea (Iranian waters). Iranian Journal of Fisheries Sciences, 11(4):97-118. In Farsi.
Moghim, M. 1994. Sturgeon fishing in common waters of the Caspian Sea. Iranian Fisheries Journal, 3(1):53-70, 5. In Farsi.
Moghim, M. 1999. Final report of statistics and biologic of sturgeon fish. Mazandaran Research Centre. 98 pp. In Farsi.
Moghim, M. 2004a. Evolution of Russian sturgeon stocks (Acipenser gueldenstaedtii) in the southern Caspian Sea, Iran. Iranian Journal of Fisheries Sciences, 12(4):173-192. In Farsi.
Moghim, M. 2004b. Evolution of ship stocks (Acipenser nudiventris) in the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 13(1):171-190. In Farsi.
Moghim, M., Fazli, H. and Khoshbavar Rostami, H. A. ?. By-catch of sturgeon juveniles in beach seine fishing method in Mazandaran Province, northeast Iran. Virtual, 1(1):190. In Farsi.
Moghim, M., Ghani Nezhad, G., Hoseinnia, M., Fazly, A. and Amirkany, I. 1996. Investigation of statistics and biology of capture fisheries of sturgeons in Iran. Iranian Fisheries
Research Organization, Tehran. 107 pp. In Farsi.
Moghim, M., Ghani Nezhad, G. and Fazli, F. 1994. Stock assessment of the Caspian Sea bony fishes. Iranian Fisheries Journal, 3(1):35-52, 4. In Farsi.
Moghim, M. and Hassannia, M. 1997. Study on starred sturgeon (Acipenser stellatus) stocks in the southern coast of the Caspian Sea. Iranian
Fisheries Scientific Journal, 6(3):81-100, 10. In Farsi.
Moghim, M., Heist, E. J., Tan, S. G., Pourkazemi, M., Siraj, S. S. and Panadam, J. M. 2009. Amplification of microsatellites in the Persian sturgeon (Acipenser persicus). Iranian Journal of
Fisheries Sciences, 8(1):97-102.
Moghim, M., Kor, D., Bagherzadeh, F., Taleshian, H and Tavakoli M. 2008. Sex composition of beluga (Huso huso Linnaeus, 1758) along the Iranian coastal waters, Caspian Sea. Iranian Scientific
Fisheries Journal, 17(1):155-160. In Farsi.
Moghim, M., Kor, D., Tavakolieshkalak, M. and Khoshghalb, M. B. 2006. Stock status of Persian sturgeon (Acipenser persicus Borodin, 1897) along the Iranian coast of the Caspian Sea.
Journal of Applied Ichthyology, 22(s1):99-107.
Moghim, M. and Rouhi, A. A. Gh. 2009. Distribution and abundance of the invasive ctenophore Mnemiopsis leidyi in the littoral waters of the Caspian Sea, Iran. Iranian Scientific Fisheries
Journal, 17(4):169-173. In Farsi.
Moghim, M., Vajhi, A. R., Veshkini, A. and Masoudi Fard, M. 2000. Determination of sex and maturity in Acipenser stellatus by using ultrasonography. Iranian Journal of
Fisheries Sciences, 2(2):89-99, 106.
Moghim, M., Vajhi, A. R., Veshkini, A. and Masoudifard, M. 2000. Sex and maturity determination of Persian sturgeon by using ultrasonography methods. Iranian Journal of Fisheries
Sciences, 10(3):71-88, 5-6. In Farsi.
Moghim, M., Vajhi, A. R., Veshkini, A. and Masoudifard, M. 2002. Determination of sex and maturity in Acipenser stellatus by using ultrasonography. Journal of Applied Ichthyology,
18(4-6):325-328.
Moghime, M. 2006a. Sex determination of Huso huso, Acipenser guldenstadti, A. stellatus and A. nudiventris by using ultrasonography. Iranian
Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Moghime, M. 2006b. Study on sturgeon fishes with statistical and biological data. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Mohagheghi Samarin, A., Ahmadi, M. R., Azuma, T., Rafiee, G. R., Mojazi Amiri, B. and Naghavi, M. R. 2008. Influence of the time to egg stripping on eyeing and hatching rates in rainbow trout
(Oncorhynchus mykiss) under cold temperatures. Aquaculture, 278(1-4):195-198.
Mohagheghi Samarin, A., Rafiee, G. R., Ahmadi, M. R., Mojazi Amiri, B. and Vilaki, A. S. 2007. Effect of temperature on post ovulated oocyte quality
maintenance in female rainbow trout, Oncorhynchus mykiss. Journal of Veterinary Research, 62(5):263-268. In Farsi.
Mohamed, A. R. M., Hussain, N. A., Al-Noor, S. S., Mutlak, F. M., Al-Sudani, I. M., Mojer, A. M., Toman, A. J. and Abdad, M. A. 2008. Fish assemblage of restored Al-Hawizeh marsh, southern Iraq.
Ecohydrology and Hydrobiology, 8(2-4):375-384.
Mohamed, A. R. M., Al-Hassan, L. A. J. and Ali, T. S. 1993. The presence of a cyprinid fish, Barbus luteus in marine waters of Iraq. Arquivos do Museu Bocage, nova série, 2(25):415-416.
Mohamed, A. R. M. and Barak, N. A. 1988. Seasonal variations in some limnological features of the Garma Marshes. Basrah Journal of Agricultural Sciences, 1(1):56-63.
Mohamed, M. B. M. 1965. Preliminary observations on some chemico-physical features of the Shatt Al-Arab estuary. Proceedings of the Iraqi Science Society, 6:34-40.
Mohamed, M-B. M. 1966. Further observations on some environmental conditions of Shatt Al-Arab. Bulletin of the Biological Research Centre, Baghdad, 1(1965):71-79.
Mohammad Nazari, M., Yosefian, M., Rezaeian, M. and Gholamipour, S. 2002. Study on the relationship between biochemical composition of egg and fertilization rate in Persian sturgeon
(Acipenser persicus). Pajouhesh va Sazandegi, 15(2)(55):84-91. In Farsi.
Mohammadi, G. and Marammazi, J. Gh. 2000. Comparison of biomass of Cyprinidae and Mugilidae in the fish community of Shadegan Marsh. The First National Symposium on Barbus fishes of
Iran, 9-10 January 2001, Khuzestan Fisheries Research Centre, Ahvaz. 50 pp. In Farsi.
Mohammadi, M., Abedian, A. M., Shariatmadari, F. and Mohseni, M. 2002. Effect of dietary protein levels on the growth performances and body composition of
juvenile beluga (Huso huso). Iranian Journal of Marine Science, 1(4):99-110, 137. In Farsi.
Mohammadi Arani, M., Staki, A. A., Allameh, S. K. and Daniali, S. R. 2003. Evaluation of particles abundance and digestion in silver carp. Pajouhesh va Sazandegi, 16(1)(58):84-86. In Farsi.
Mohammadian, H. 1999. Freshwater Fishes of Iran. Sepehr Publications Center, Tehran. vii + 178 pp. In Farsi.
Mohammadian, T., Kouchenin, P., Nikou, S., Sheykh Aleslami, M., Bita, S., Eskandari, Gh. R. and Abhari Seh Gonbad, H. 2009. Comparison of the effectiveness GnRHa hormone (ova-fact) and carp pituitary
extract on reproduction indexes of Barbus sharpeyi. Iranian Veterinary Journal, 5(2)(23):70-80. In Farsi.
Mohsen-Zadeh, A. and Bahadori, Z. 2001. Investigation on age and growth of bleak Chalcalburnus chalcoides. B.Sc. Project, Gorgan University of Agricultural Sciences and Natural Resources,
Gorgan. 66 pp. In Farsi.
Mohseini, M., Pourali Fashtomi, H. and Arshad, U. 2001. Determining the best stocking density for commercial culture of great sturgeon
Huso huso. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Mohseni, M. 1998. Study on effects of environmental factors like increase in density of sturgeon eggs and larvae produced through artificial breeding on the appearance of morphological
features. Ph.D. Thesis, University of Tehran, Tehran. 125 pp. In Farsi.
Mohseni, M. 2006. Determining embryogenetic deformities during incubation and rearing in A. persicus and A. stellatus fry. Iranian Fisheries Research Organization,
Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Mohseni, M., Bahmani, M., Pourkazemi, M., Pourali, H. R. and Arshad, A. 2006. Determination of the best feeding ratio in Huso huso meat production
cultured in fiber glass tanks. Iranian Journal of Fisheries Sciences, 14(4):165-180. In Farsi.
Mohseni, M., Pourali, H. R., Aghtoman, V. and Sajadi, M. M. 2006. Determination of the best stocking density for rearing Huso huso. Iranian Journal of Fisheries Sciences,
15(3):129-138. In Farsi.
Mohseni, M., Pourkazemi, M., Bahmani, M., Pourali, H., Kazemi, R. and Salehpour, M. 2004. Effects of feeding frequency on growth in hatchery reared beluga sturgeon (Huso huso).
Iranian Journal of Fisheries Sciences, 13(3):145-158. In Farsi.
Mohseni, M., Pourkazemi, M., Bahmani, M., Pourali, H. and Sajjadi, M. M. 2007. Effects of different dietary protein to energy ratio (P/E) on growth performance and body composition of farmed Persian
sturgeon (Acipenser persicus). Iranian Scientific Fisheries Journal, 16(1):129-140. In Farsi.
Mohseni, M., Pourkazemi, M., Bahmani, M., Salehpour, M., Pourali, H. R. and Haddadi Moghadam, K. 2005. Rearing Huso huso in earthen ponds and fiberglass tanks. Iranian Journal
of Fisheries Sciences, 14(1):119-132. In Farsi.
Mohseni, M., Pourkazemi, M., Mojazi Amiri, B., Kazemi, R., Norooz Foshkhomi, M. R. and Kaladkova, L. N. 2000. A study on the effects of stocking density of eggs and larvae on the
survival and frequency of morphological deformities in Persian sturgeon, great sturgeon and stellate sturgeon. Iranian Journal of Fisheries Science, 2(1):75-90, 114.
Mohseni, M., Sajjadi, M. and Pourkazemi, M. 2007. Growth performance and body composition of sub-yearling Persian sturgeon, (Acipenser persicus,
Borodin, 1897), fed different dietary proteins. Journal of Applied Ichthyology, 23(3):204-208.
Mohseni, M., Seyfabadi, J., Pourali, H., Pourkazemi, M. and Bahmani, M. 2008. Effects of supplementary dietary L-carnitine on growth and body composition of
beluga (Huso huso) juveniles. Iranian Journal of Fisheries Sciences, 7(2 supplementary):157-170.
Moini, S. and Basimy, B. 2004. Production of fish cake from carp and its shelf life in cold store at -18ºC. Iranian Journal of Fisheries Sciences, 13(1):163-170. In Farsi.
Moini, S. and Koochekian, A. 2003. Production of fish sauce from Caspian sea kilka, with use of traditional, microbial and enzymatic methods. Iranian Journal
of Fisheries Sciences, 12(2):79-94. In Farsi.
Moini, S., Sobhanipour, N. and Moini, S. 2005. Production of fried marinade from roach (Rutilus rutilus caspius). Iranian Journal of Fisheries Sciences, 14(1):133-146. In Farsi.
Moiseev, P. A. 1971. The living resources of the world ocean. Israel Program for Scientific Translations, Jerusalem. 334 pp.
Mojabi, A., Safi, Sh., Azari Takami, Gh. and Nowrouzian, I. 1997. Comparative study of classification index and hormonal measurement to determine
the time of sexual maturity in Acipenser persicus. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (poster).
Mojabi, A., Safi, Sh., Azari Takami, Gh. and Nowrouzian, I. 1999. Comparative study of classification index and hormonal measurement to determine the
time of sexual maturity in Acipenser persicus. Journal of Applied Ichthyology, 15(4-5):318.
Mojazi Amiri, B., Bahrekazemi, M., Pousti, I. and Vilaki, A. S. 2005. A histological study on the development of the digestive tract of Caspian salmon,
Salmo trutta caspius (Kessleri),(sic) from hatching to parr stage. Iranian Journal of Fisheries Sciences, 5(1):63-84.
Mojjabavi, H. R. 1994. Aquaculture needs and strategies. Abzeeyan, Tehran, 4(10):44-47. In Farsi.
Mojtahedzadeh, P. 2001. Hamoon Lake catastrophe, the most similar to Oral (sic) Lake catastrophe. Osveh, Teheran, 1:34-49. In Farsi
(www.netiran.com/HtDocs/Clippings/Social/010130XXSO03.html).
Mokaremi Rostami, A., Jamili, Sh., Khoshbavar Rostami, H. A., Vosoughi, Gh. and Ospooei, H. 2007. The effects of creosote on mortality rate and blood biochemical factors of stellate sturgooen
(Acipenser stellatus). Iranian Scientific Fisheries Journal, 16(1):141-150. In Farsi.
Mokhayer, B. 1972a. Recherches sur le parasitsme des Esturgeons de la mer Caspienne Mériodionale. Thèse, Université de Paris.
Mokhayer, B. 1972b. La piscicoltura in Iran. Rivista italiana di Piscicoltura e Ittiopatologia, 7(1):7-9.
Mokhayer, B. 1973a. La liste des parasites des esturgeons Iraniens. Journal of the Veterinary Faculty of the University of Tehran, 29(1):1-12. In Farsi.
Mokhayer, B. 1973b. Study on the parasitism of Mugil auratus Risso (sic) from the out (sic, south) Caspian Sea.
Rivista italiana di Piscicoltura e Ittiopatologia, 8(2):53-54.
Mokhayer, B. 1974a. Aspect écologique du parasitisme des esturgeons Iraniens. Journal of the Veterinary Faculty of the University of Tehran, 30(1):38-48. In Farsi.
Mokhayer, b. 1974b. A check list on parasites of sturgeons (Acipenseridae) of Iran. Veterinary Letter, 1:1-13.
Mokhayer, B. 1975. Study on the parasitic infection of fishes in river Sefid. Veterinary School Bulletin, Tehran, 4:36. In Farsi.
Mokhayer, B. 1976a. Treatment of bothriocephalosis in grass carp. Rivista italiana di Piscicoltura e Ittiopatologia, 11:119-121.
Mokhayer, B. 1976b. Fish diseases in Iran. Rivista italiana di Piscicoltura e Ittiopatologia, 11(4):123-128.
Mokhayer, B. 1981a. Parasites des poissons du bassin de Sefid-Roude. Journal of the Veterinary Faculty of the University of Tehran, 36:61-75. In Farsi.
Mokhayer, B. 1981b. Habitat of spiny eels in Iranian water with a note on their helminthic infection. Journal of the Veterinary Faculty of the University of Tehran, 36(3):35-47. In Farsi.
Mokhayer, B. 1981c. Infection of Karoun River and Arvandroud mullet with Contracaecum larvae. Journal of the Veterinary Faculty of the University of Tehran, 37(1):91-102. In Farsi.
Mokhayer, B. 1985. Diseases of Cultivated Fishes. Tehran University Publications, Tehran.
318 pp. In Farsi. (not seen).
Mokhayer, B. 1989. Fish Diplostomiasis in Iran. Journal of the Veterinary Faculty of the University of Tehran, 44(2):11-18.
Mokhayer, B. 1993. Surgery in Iranian sturgeons. Iranian Fisheries Bulletin, (2):3-10, 1. In Farsi.
Mokhayer, B. 2000. Parasitic helminths of Caspian roach (larval stage). The Third World Fisheries Congress, Beijing, 31 Oct - 3 Nov 2000 (title).
Mokhayer, B. and Anwar, M. 1973. Effet pathogène des parasites de l'esturgeon dans le milieu naturel et artificiel. Rivista italiana di Piscicoltura e Ittiopatologia, 8(4):111-115.
Mokhayer, B., Kohneshahri, M. and Malaki, M. 1975. Occurrence of Trypanosoma percae in perches of Southern Caspian Sea. Third International Wildlife
Disease Conference, Munich. August 1975.
Mokhayer, B., Kyabee, B. and Kohestani-Skandari, S. 2000. Investigation on the infection of khramulya (Capoeta capoeta gracilis) with Acanthocephalorhynchoides and Tracheliastes.
Journal of the Faculty of Veterinary Medicine University of Tehran, 55(2):65-66.
Mokhayer, B., Mesbah, M., Peyghan, R. and Jalali, B. 2006. Study of monogenetic trematodes infestation in benni, Barbus sharpeyi from Shadegan Marsh and mode of their attachment to gills.
Iranian Veterinary Journal, 2(2)(13):48-57. In Farsi.
Mokhayer, B. and Setareh, J. 2000. Parasitic helminths of Caspian roach from southern Caspian Sea. The Third World Fisheries Congress, Beijing, 31 Oct - 3 Nov 2000 (title).
Molnár, K. and Baska, F. 1993. Scientific report on intensive training course on parasites and parasitic diseases of freshwater fishes of Iran, 15-25 November
1993, Fisheries Company of Iran. 15 pp.
Molnár, K. and Jalali, B. 1992. Further monogeneans from Iranian freshwater fishes. Acta Veterinaria Hungarica, 40(1-2):55-61.
Molnar, K. and Jalali, B. 1993. Occurrence of monogeneans on common carp of Iran and description of pathogenicity of D. sahuensis (Ling 1965) in infected
common carp. In: Symposium on the Carp, Budapest, Hungary, 6-9 September 1993 (abstract).
Molnár, K., Masoumian, M. and Abasi, S. 1996. Four new Myxobolus spp. (Myxosporea: Myxobolidae) from Iranian barboid fishes. Archiv für Protistenkunde, 147(1):115-123.
Molnár, K. and Pazooki, J. 1995. Occurrence of philometrid nematodes in barboid fishes of River Karun, Iran. Parasitologia hungarica, 28:57-62.
Monavari, S. M. and Mardani, N. 2007. Environmental effects of fish culture ponds on Jajrood River, Tehran Province. Iranian Scientific Fisheries Journal, 16(1):169-176. In Farsi.
Monawari, S. M. 1990. Ecological situation of the Anzali Marsh. Rasht. 227 pp. In Farsi.
Monsefi, M., Shiva, A. H. and Esmaeili, H. R. 2009. Gonad histology of the Persian tooth-carp Aphanius persicus (Jenkins, 1910)(Cyprinodontidae) in
southern Iran. Turkish Journal of Zoology, 33(1):27-33.
Moogouei, R., Karbassi, A. R., Monavari, S. M., Rabani, M. and Taheri Mirghaed, A. 2010. Effect of the selected physico-chemical parameters on growth of rainbow trout (Oncorhynchus mykiss)
in raceway system in Iran. Iranian Journal of Fisheries Sciences, 9(2):245-254.
Moore, M. J., Mitrofanov, I. V., Valentini, S. S., Volkov, V. V., Kurbskiy, A. V., Zhimbey, E. N., Eglinton, L. B. and Stegeman, J. J. 2003.
Cytochrome P4501A expression, chemical contaminants and histopathology in roach, goby and sturgeon and chemical contaminants in sediments from the Caspian Sea,
Lake Balkhash and the Ily River Delta, Kazakhstan. Marine Pollution Bulletin, 46(1):107-119.
Moosari, M. 1996. Studying the process of migration of trout smolts (Salmo trutta caspius) into Tonekabon River. Abzeeyan, Tehran, 7(10):10-12. In Farsi.
Moradian, F., Jamili, Sh., Bahmani, M., Toloei, M. H. and Mohammadi, Gh. 2003. The effect of thyroxine on percentage of hatching (Hypophthalmichthys
molitrix). Iranian Journal of Fisheries Sciences, 12(3):167-174. In Farsi.
Moravec, F. and Amin, A. 1978. Some helminth parasites, excluding Monogenea, from fishes of Afghanistan. Acta Scientiarum Naturalium Academiae Scientiarum Bohemoslovace, Brno, 12(6):1-45.
Morovvati, H. and Alboghobeish, N. 2006. Seasonal changes of grass carp (Ctenopharingodon (sic) idella) lymphoid organs. XXVI Congress of the European
Association of Veterinary Anatomists, 19-22 July 2006, Messina, Italy (title).
Morovvati, H., Alboghobeish, N., Noori, A. and Rasekh, A. 2006. Seasonal changes of pronephros lymphoid tissue in grass carp (Ctenopharingodon (sic)
idella): a histometrical and histological study. Iranian Journal of Veterinary Research,
Shiraz University, 7(3)(16):42-49.
Morris, J. 1997. New initiative for sturgeon. Oryx, 31(1):1.
Morris, M. E. 1992. Poisoned wells: the politics of water in the Middle East. Middle East Insight, 8(2):35-39.
Mortazavi Tabrizi, J., Pazooki, J. and Javanmard, A. 2005. Infection of the fishes with Ligula intestinalis and Bothriocephalus acheilognathi
in Sattarkhan Dam, northwestern Iran. Iranian Journal of Fisheries Sciences, 13(4):161-168. In Farsi.
Mortezaei, S., Mobedi, I. and Farahnak, A. 2000. Infection of some species of fresh water fishes to parasitic worms in Khouzestan Province, Iran. Iranian Journal of Fisheries Sciences, 9(1):25-36, 4.
In Farsi.
Mortezavizadeh (sic), S. A., Moazedi, J. and Jorfi, E. 2009. The first attempt to artificial propagation of gattan (Barbus xanthopterus) by hypophysis extraction. The 6th Scientific
Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 51 (abstract).
Mortezazadeh, S. A., Payghan, R. and Jalaly, E. R. 2009. Study on effect of the use of propofol anesthesia on some biochemical factors of blood serum of benni (Barbus sharpeyi). The 6th
Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 72 (abstract).
Moshkani, M. and Pourkasmani, M. 2004. Identification of fishes in qanats of Birjand. Iranian Scientific Fisheries Journal, 12(4):163-172, 17. In Farsi.
Moskal'kova, K. I. 1996. Ecological and morphophysiological prerequisites to range extention (sic) in the round goby Neogobius melanostomus
under conditions of anthropogenic pollution. Journal of Ichthyology, 36(8):584-590.
Mossanen, D. L. 2002. Harem. Scribner Paperback Fiction, New York. 378 pp.
Mostafavi, H. 1998. Investigation and distribution of fish species in Talar River, Mazandaran, Iran. Seminar, Agricultural Sciences and Natural Resources University, Gorgan. 85 pp. In Farsi.
Mostafavi, H. 2007. Fish biodiversity in Talar River, Mazandaran Province. Journal of Environmental Studies, 32(40):127-135. In Farsi.
Mostafavi, H. and Abdoli, A. 2003. A study of fish species diversity in Talar River in Mazandaran.
Environmental Sciences, 1(1):20-29.
Mostafavi, H. and Abdoli, A. 2005. A preliminary survey on diet of Capoeta capoeta gracilis in Talar and Yasalegh
rivers from the southern basin of Caspian Sea. Environmental Sciences,
2(7):53-62.
Mostafavi, H. and Abdoli, A. 2006. Fish species diversity, distribution and abundance in Kesseliian Stream, Mazandaran, Iran. Journal of Environmental Sciences, 12(6): 25-32.
Motabar, M. 1978. Larvivorous fish, Gambusia affinis - a review. World Health Organization, Vector Biology and Control, WHO/VBC/78.703:5 pp.
Motalebi, A. A., Ardalani, K. and Jamili, S. 2008. Effect of temperature on the produced aflatoxins in the rainbow trout feed in West Azerbaijan Province. Journal of Fisheries and Aquatic Science,
3(6):392-397.
Motalebi, A. A., Hasanzati Rostami, A., Khanipour, A. A. and Soltani, M. 2010. Impacts of whey protein edible coating on chemical and microbial factors of gutted kilka during frozen storage.
Iranian Journal of Fisheries Sciences, 9(2):255-264.
Motamedzadegan, A., Shahidi, F., Mortazavi, S. A., Pourazarang, H., Hamzeh, Sh., Shahidi Yasaghi, S. A., Ghorbani Hasan Saraei, A. and Khanipour, E. 2009. Effect of kilka fish myofibrillar protein
hydrolysis by papain on peptide chain length and degree of hydrolysis. Journal of Agricultural Sciences and Natural Resources, 16(3):172-181. In Farsi.
Mousavi, H. A. E. 2000. Occurence of Vibrio parahaemolyticus in fresh water fish Farms (Iran). In: The 3rd World Fisheries Congress, Beijing, China, 31 Oct-2 Nov 2000 (abstract).
Mousavi, H. A. E., Soltani, M., Khosravi A., Mood, S. M. and Hosseinifard, M. 2009. Isolation and characterization of Saprolegniaceae from rainbow trout (Oncorhynchus mykiss) eggs in Iran.
Journal of Fisheries and Aquatic Science, 4(6):330-333.
Mousavi, H. A. E. 2003. Parasites of ornamental fish in Iran. Bulletin of the European Association of Fish Pathologists, 23(6):297-300.
Mousavi, S. F. and Samadi-Boroujeni, H. 1998. Evaluation of sedimentation in small dam reservoirs in Chaharmahal-Bakhtiary region. Iranian Journal of Science and
Technology, Transactions A: Science, 24(4):421-430.
Mousavi, S. M., Mirzagar S. S., Mousavi, H. E. Z., Baigi, R. O., Khosravi, A., Bahonar, A. and Ahmadi, M. R. 2009. Evaluation of antifungal activity of new combined essential oils in comparison with
malachite green on hatching rate in rainbow trout (Oncorhynchus mykiss) eggs. Journal of Fisheries and Aquatic Science, 4(2):103-110.
Mousavi Gel Sefid, S. A., Keyvan, A. and Piri, M. 2007. Morphometric analysis of the common carp (Cyprinus carpio Linnaeus, 1758) in Anzali Lagoon, south west Caspian Sea. Iranian
Scientific Fisheries Journal, 15(4):141-154. In Farsi.
Mousavi Sabet, H., Kamali, A., Soltani, M., Bani, A., Esmaeili, H. R.,
Rostami, H., Vatandoust, S. and Moradkhani, Z. 2011. Age, reproduction, and
fecundity of a population of Cobitis sp. (Actinopterygii:
Cypriniformes: Cobitidae) from the Babolrud River in the southern Caspian Sea
basin. Acta Ichthyologica et Piscatoria, 41(2):117-122.
Moussavi, M. and Saber, I. N. H. 1999. Mercury pollution in Kor River. Iranian Journal of Science and Technology, Transaction B: Technology, 23(3):165-172.
Mousavi Nadoushan, R., Fatemi, M. R., Esmaeili Sari, A. and Vosoughi, Gh. H. 2008. Determination of trophic status and potential of fish production in Lake Choghakhor. Journal of Fisheries,
2(2):71-74.
Mounsey, A. H. 1872. A Journey through the Caucasus and the Interior of Persia. Smith, Elder & Co., London. xi + 336 pp.
Movaghar, S. 1973. Iranocypris typhlops Kaiser the blind cave fish from Iran. Journal of the Veterinary Faculty of the University of Tehran, 29:43-50. In Farsi.
Movahedinia, A., Abtahi, B., Falahatti, M. A., Heydari, B. and Salari, A. M. 2009. Gill histopathology of Caspian Sea sturgeons from natural environments. The 6th Scientific Conference of Fisheries
Resources, 3-4 March 2009, Basrah, p. 46 (abstract).
Movahedinia, A., Esmaeili Sari, A., Abtahi, B. and Ershad Langarudi, H. 2002. Seasonal changes in biomass and density of Mnemiopsis leidyi
from Noor coastal waters of the Caspian Sea. Iranian Journal of Marine Sciences, 1(3):57-64, 74. In Farsi.
Movchan, Yu. V. and Kozlov, V. I. 1978. Morphological characters and certain ecological characteristics of the Chinese chebachok Pseudorasbora
parva in Ukrainian reservoirs. Hydrobiological Journal, 14(5):36-41.
Muhomedieva, F. D. 1967. Morfometriya i ekologiya zakaspiiskoi marinki Schizothorax pelzami Kessler pervogo Tedzhenskogo vodokhranilishcha
[Morphometry and ecology of the Transcaspian marinka Schizothorax pelzami Kessler from the first Tedzhen water reservoir]. Voprosy Ikhtiologii, 7(6)(47):1060-1065.
Munro, D. C. and Touron, H. 1997. The estimation of marshland degradation in southern Iraq using mulitemporal Landsat TM images. International Journal of Remote Sensing, 18(7):1597-1606.
Münzing, J. 1969. Variabilität, Verbreitung und Systematik der Arten und Unterarten in der Gattung Pungitius Coste, 1848 (Pisces,
Gasterosteidae). Zeitschrift für zoologische Systematik und Evolutionsforschung, 7:208-233.
Murdy, E. O. 1989. A taxonomic revision and cladistic analysis of the Oxudercine gobies (Gobiidae: Oxudercinae). Records of the Australian Museum, Supplement 11:1-93.
Murray, A. M. 2001. The fossil record and biogeography of the Cichlidae (Actinopterygii: Labroidei). Biological Journal of the Linnaean Society, 74:517-532.
Mutlak, F. M. and Al-Faisal, A. J. 2009. A new record of two exotic cichlids fish Oreochromis aureus (Steindachner, 1864) and Tilapia zilli (Gervais, 1848) from south of the main
outfall drain in Basrah city. Mesopotamian Journal of Marine Science, 24(2):160-170. In Arabic.
Muus, B. J. and Dahlstrøm, P. 1999. Freshwater Fish. Scandinavian Fishing Yearbook, Hedehusene, Denmark. New Edition, Re-edited by C. Coyne-Boragine,
C. Frimodt, S. Skydsgaard, S. Weiss and E. Mogensen. 224 pp.
Myers, G. S. 1960. Preface to any future classification of the cyprinid fishes of the genus Barbus. Stanford Ichthyological Bulletin, 7(4):212-215.
Myers, G. S. 1965. Gambusia, the fish destroyer. Tropical Fish Hobbyist, 13(5):31-32, 53-54.
Myers, G. S. and Shapovalov, L. 1931-1932. On the identity of Ophicephalus and Channa, two genera of labyrinth fishes. Peking Natural History Bulletin, 6(2):33-37.
N
Naama, A. K., Ahmed, H. A. and Al-Adhub, A. H. Y. 1986. Aspects of reproduction of the mullet Liza abu (Heckel)(Pisces, Mugilidae) in Al-Hammar Marsh, Iraq. Cybium, 10(1):47-55.
Na'ama, A. K. and Al-Hassan, L. A. J. 1989. Note on the potential brood size of mosquito fish, Gambusia affinis (Baird & Girard) collected from Iraq and Egypt (Pisces).
Bolletino del Museo Regionale di Scienze Naturali Torino, 7(1):117-123.
Naama, A. K. and Muhsen, K. A. 1986. Feeding periodicites (sic) of the mugilid Liza abu (Heckel) and cyprinid Carasobarbus luteus
(Heckel) from Al-Hammar Marsh, southern Iraq. Indian Journal of Fisheries, 33:347-350.
Naddafi, R., Abdoli, A., Kiabi, B. H., Amiri, B. M. and Karami, M. 2005. Age, growth and reproduction of the Caspian roach (Rutilius rutilus caspicus)
in the Anzali and Gomishan wetlands, North Iran. Journal of Applied Ichthyology, 21(6):492-497.
Naddafi, R., Amiri, B. M., Kiabi, B. H. and Abdoli, A. 2002. A comparative study of morphometric and meristic characters of the Caspian roach,
Rutilus rutilus caspicus, in Gorgan-Rud estuary and Anzali Wetland, Iran. Iranian Journal of Natural Resources, 54(4):383-399. In Farsi.
Naddafi, R., Amiri, B. M., Karami, M., Kiabi, B. H. and Abdoli, A. 2002a. A study of some ecological and biological characters of roach, Rutilus rutilus caspicus, in Anzali wetland.
Iranian Journal of Natural Resources, 55(2):225-241. In Farsi.
Naddafi, R., Amiri, B. M., Karami, M., Kiabi, B. H. and Abdoli, A. 2002b. A study of some biological characters of roach, Rutilus rutilus caspicus,
in Gomishan wetland. Iranian Journal of Fisheries Sciences, 11(3):103-126. In Farsi.
Nader, I. A. and Jawdat, S. 1977a. First records of fishes from Iraqi waters. Bulletin of the Faculty of Science of Riyadh University, 8:429-443.
Nader, I. A. and Jawdat, S. Z. 1977b. New records of fishes from Iraq. Bulletin of the Biological Research Centre, Baghdad, 8:73-87.
Naderi, J. M. and Abdoli, A. 2004. Fish Species Atlas of South Caspian Sea Basin (Iranian Waters). Iranian Fisheries Research Organization, Tehran. 80 pp. + 10 pp. + 12 pp. In Farsi and English.
Naderi, M., Fazli, H., Afraee, M. and Ganjian, A. 1997. The study of reproduction, fecundity and diet in 3 species of kilka in the southern
part of the Caspian Sea (Babolsar region). Iranian Fisheries Scientific Journal, 6(1):65-78, 7. In Farsi.
Naderi Jeloudar, M., Esmaeili Sari, A., Ahmadi, M. R., Seyfabadi, S. J. and Abdoli, A. 2007. The effects of trout farm effluents on the water quality parameters of Haraz
River. Environmental Sciences, 4(2):21-36.
Nadim, A. 1977. Study on the mercury content of fish and sea food in Iran. Iranian Journal of Public Health, 6(4):166-178. In Farsi.
Nadim, F., Bagtzoglou, A. C. and Iranmahboob, J. 2006. Management of coastal areas in the Caspian Sea region: environmental issues and political challenges. Coastal Management, 34(2):153-165.
Nadji, M. 1970. Die Kanat-Abflüsse im Iran als Basis eines neuen Verfahrens zur Berechnung der Grundwasserbilanz. Geologische Mitteilungen, 10:333-362.
Nadji, M. 1972a. Kanate in der Ebene von Kashan, Iran. Die Erde, Berlin, 102(314):209-215.
Nadji, M. 1972b. Geologie und Hydrogeologie des Gebietes von Kashan/Iran. Geologische Mitteilungen, 11:275-362.
Naem, S. 1997a. A survey on the 4 species of parasitic leeches of Mahabad dam lake in Iran. Pajouhesh-va-Sazandegi, 36:124-127. In Farsi.
Naem, S. 1997b. A survey on the crustacean parasites of fishes of Mahabad reservoir dam lake with new records of Tracheliastes polycolpus in Iran. Pajouhesh-va-Sazandegi, 36:128-133. In Farsi.
Naem, S. 2002. The recognising of bronchial monogene parasite of fishes, in Sepidroud River in Guilan Province by
introducing a new genus and four species for parasitic fauna Book of Abstracts, 27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Naem, S. and Meshkini, S. 2002. A survey on prevalence rate of diplostome spateceum (sic) metacercaria in Cyprinus fishes of Babol-Rood River, Iran. Book of Abstracts,
27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Naem, S., Mobedi, I., Khomirani, R. and Abolghasemi, S. J. 2002. A survey on gill parasites in cultured and wild fishes of western branch of Sepid-Rood
river in Gilan province, Iran and introducing new species. Iranian Journal of Veterinary Research,
Shiraz University, 3(2):118-128. In Farsi.
Naff, T. and Matson, R. C. (Eds.). 1984. Water in the Middle East. Conflict or Cooperation? Westview Press, Boulder and London. xvii + 236 pp.
Nafisi Bahabadi, M. 2007. Effects of feed energy on growth and body composition of rainbow trout (Oncorhynchus mykiss) reared in brackish water. Journal of Agricultural Sciences and Natural
Resources, 14(3):21-30. In Farsi.
Nafisi Bahabadi, M. and Soltani, M. 2008. Effect of dietary energy levels and feeding rates on growth and body composition of fingerling rainbow trout (Oncorhynchus
mykiss). Iranian Journal of Fisheries Sciences, 7(2 supplementary):171-186.
Nafisi Bahabadi, M., Mahmoudzadeh, H., Soltani, M. and Ahmadi, M. R. 2001. Replacing poultry by-product meal with fish meal in diets for rainbow trout (Oncorhynchus mykiss) rearing in brackish
water. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(2):33-40. In Farsi.
Naghili, H. 2001. Survey on Diplostomum spathaceum in cold water fishes in West Azarbaijan Province. DVM Thesis, Urmia Azad University, No. 497. In Farsi.
Nahavandi, R., Amini, F. and Rezvani, S. 2001. Karyology of Abramis brama in the southern waters of Caspian Sea. Iranian Scientific Fisheries Journal, 10(3):89-100, 7. In Farsi.
Nahavandi, R., Pourgholam, R., Hassan, M. D., Soltani, M., Ghoroghi, A. and Pourgholam, H. 2006. Chemiluminescent response of grass carp (Ctenopharyngodon
idella) following exposure to sublethal concentrations of diazinon. Iranian Journal of Fisheries Sciences, 6(1):57-68.
Naik, I. U. 1985. WAPDA Fisheries Gazetteer. Directorate of Fisheries, Water and Power Development Authority, Lahore, Pakistan, Publication No. 1/Fish/85:61 pp., 11 illustrations, 5 maps.
Najafpoor, A. A., Vojoudi, Z., Dehgani, M. H., Changani, F. and Alidadai, H. 2007. Quality assessment of the Kashaf River in North East of Iran in 1996-2005.
Journal of Applied Sciences 7(2):253-257.
Najafpour, N. 1994. Be familiar with fishes of Khuzestan. 2 Mahi hemri Barbus luteus (Heckel, 1843). Gozaresh Elmi (Scientific Report of the Iranian Fisheries Research and Training
Organization), 1(2):7. In Farsi.
Najafpour, N. 1997. Identification of some freshwater fishes of Khuzestan Province. Fisheries Research Centre of Khuzestan Province, Iranian Fisheries Research and Training Organisation,
Tehran. vi + 96 pp., 4 plates. In Farsi.
Najafpour, N. 2001. Diversity of fresh-water fishes of Khuzestan. The 10th European Congress of Ichthyology ECI X, Programme & Book of Abstracts, Prague, Czech Republic, September 3-7, 2001. p. 40.
Najafpour, N. 2002. Freshwater fishes of Khuzestan Province, I. R. Iran. Freshwater Biological Association Annual Scientific Meeting & Training Seminar, 4-6 September 2002, University
of Durham, "Freshwater in the Landscape" (title).
Najafpour, N. 2003. Freshwater fishes of Khuzestan Province (Part II). Fisheries Research Centre of Khuzestan Province, Iranian Fisheries Research Organisation, Tehran.49 pp.
Najafpour, N. and Coad, B. W. 2002. Ichthyootoxism in Barbus luteus from Iran (Actinopterygii: Cyprinidae). Zoology in the Middle East, 26:129-131.
Najafpour, N., Eskandary, G., Nikpay, M. and Al-Mukhtar, M. 1997. Fresh water fishes of Khuzestan area. Ninth International Congress of European Ichthyologists (CEI9) "Fish Biodiversity".
Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 64.
Najafpour, N. and Sabet, H. R. A. 2002. Distribution of Cobitidae family in Iran. II International Conference, Loaches of the Genus Cobitis and Related Genera. Biology,
Systematics, Genetics, Distribution, Ecology, Conservation. September 09-13, 2002, Olsztyn, Poland (title).
Najafpour, S., Alkarkhi, A. F. M., Rahman, N. N. N. A. and Golamipour, S. 2005. Organochlorine pesticides residues of kelthane, DDE and ß-BHC in freshwater of Tajan River (southern part
of Caspian Sea). International Conference on Innovations and Technologies in Oceanography for Sustainable Development, 26-29 September 2005, Istana Hotel, Kuala Lumpur (title).
Najafpour, S., Alkarkhi, A. F. M., Rahman, N. N. N. A. and Nasrollahzadeh, H. S. 2005. Determination of chlorinated pesticides residues of DDT, lindane and a-BHC
in freshwater of Tonekabon River (southern part of Caspian Sea). International Conference on Innovations and Technologies in Oceanography for
Sustainable Development, 26-29 September 2005, Istana Hotel, Kuala Lumpur (title).
Najafpour, S., Alkarkhi, A. F. M., Rahman, N. N. N. A. and Rostami, H. A. Kh. 2005. Study of chlorinated pesticides residues of kelthane, ß-BHC and DDE in freshwater of Shiroud River
(southern part of Caspian Sea). International Conference on Innovations and Technologies in Oceanography for Sustainable Development, 26-29 September 2005, Istana Hotel, Kuala Lumpur (title).
Najafipour, S., Bahmani, M., Oryan, S. and Nejatkhah, P. 2008. Determining the levels of 17-αhydroxy progesterone hormone and its relationship with some biological indexes in female brood kutum
roaches (Rutilus frisii kutum) in west of Gilan. Journal of Environmental Science and Technology, 36(special issue).
Naji, T., Nejatkhah Manavi, P. and Shirin Abadi, M. 2008. Effects of 17ß-estradiol valerat on gonadal sex differentiation of rainbow trout (Oncorhynchus mykiss).
Journal of Environmental Science and Technology, 10(2)(37):106-113. In Farsi.
Naji, T., Oryan, S. and Karami, S. 2007. Determining LC50 of cobalt chloride of Cyprinus carpio. Journal of Environmental Science and Technology, 8(4)(31)):51-57. In Farsi.
Naji, T., Safaeian, Sh., Rostami, M. and Sabrjou, M. 2007. Toxic effect of zinc sulfate on gill tissues of common carp (Cyprinus carpio). Journal of Environmental Science and Technology,
9(2)(33):29-36. In Farsi.
Nalbant, T. 1963. A study of the genera of Botiinae and Cobitinae (Pisces, Ostariophysi, Cobitidae). Travaux du Muséum d'Histoire naturelle "Grigore Antipa", Bucurešti, 4:343-379.
Nalbant, T. T. 1993. Some problems in the systematics of the genus Cobitis and its relatives (Pisces. Ostariophysi, Cobitidae). Revue Roumaine de Biologie, Serie de Biologie
Animale, 38(2):101-110.
Nalbant, T. T. 1994. Studies on loaches (Pisces: Ostariophysi: Cobitidae). I. An evaluation of the valid genera of Cobitinae. Travaux du Muséum d'Histoire naturelle "Grigore Antipa",
Bucurešti, 34:375-380.
Nalbant, T. T. 1998. The presence of the genus Schistura (Pisces: Ostariophysi: Nemacheilidae) in Tigris drainage, western Asia. The description of a new species. Travaux du
Muséum d'Histoire naturelle "Grigore Antipa", Bucurešti, 40:371-375.
Nalbant, T. and Bianco, P. G. 1997. The loaches of Iran (Cobitidae). Ninth International Congress of European Ichthyologists (CEI9) "Fish Biodiversity".
Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 64.
Nalbant, T. T. and Bianco, P. G. 1998. The loaches of Iran and adjacent regions with description of six new species (Cobitoidea). Italian Journal of Zoology, 65 (Supplement):109-123.
(Proceedings of the Ninth Congress of European Ichthyologists (CEI-9) "Fish Biodiversity" organised in Naples at the University Federico II and held in Trieste - Italy, 24-30 August 1997).
Nalbant, T. T., Coad, B. W. and Lajbner, Z. 2010. Note on loach distribution in Iran. International Loach Conference 2010, 31 August to 3 September 2010, Prague,
Czech Republic (poster).
Namazi, S. 2000. The Caspian's environmental woes, p. 120-136. In: Amirahmadi, H. (Ed.). The Caspian Region at a Crossroad. Challenges of a New Frontier of Energy and Development.
St. Martin's Press, New York. xii + 299 pp.
Namin, J. I. 1998. Larviculture of sturgeon (Acipenser persicus) using nauplii of fairy shrimps (Streptocephalus proboscideus). Iranian First National Symposium on
Sturgeon, 1998, Rasht, Iran (abstract).
Namin, J. A. 2002. Persian sturgeon (Acipenser persicus Borodin, 1897) of the Caspian Sea and its stock rehabilitation activities in Iran. The 5th Czech Conference of Ichthyology, 25-26
September 2002, Brno (abstract).
Narjespour, F. and Oloomee, Y. 1990. Artificial reproduction of roach, Rutilus rutilus caspicus. B.Sc. Project, University of Gorgan, Gorgan. In Farsi.
Naseka, A. M. 1996. Comparative study on the vertebral column in the Gobioninae (Cyprinidae, Pisces) with special reference to its systematics.
Publicaciones Especiales Instituto Español de Oceanografía, 21:149-167.
Naseka, A. M. 2010. Zoogeographical freshwater divisions of the Caucasus as a part of the West Asian Transitional Region. Proceedings of the Zoological Institute, Russian Academy of Sciences,
314(4):469-492.
Naseka, A. M. and Freyhof, J. 2004. Romanogobio parvus, a new gudgeon from River Kuban, southern Russia (Cyprinidae, Gobioninae). Ichthyological Exploration of Freshwaters, 15(1):17-23.
Nash, C. 1997a. Iran develops farm skills to meet fishing needs. Fish Farming International, 24(4):26-28.
Nash, C. 1997b. Iran goes private! - moving fisheries out of state control. Fish Farming International, 24(5):38-39.
Nasir, N. A. 2000. The food and feeding relationships of the fish communities in the inshore waters of Khor Al-Zubair, North-west Arabian Gulf. Cybium, 24(1):89-99.
Nasir, N. A., Naama, A. K. and Al-Saboonchi, A. 1989. The distribution, length-weight relationships, food and feeding of the cyprinid fish Barbus
sharpeyi from Al-Hammar Marsh, Iraq. Fisheries Research, 7:175-181.
Nasrallah, H. A. and Balling, R. C. 1993. Spatial and temporal analysis of Middle Eastern temperature changes. Climatic Change, 25(2):153-161.
Nasrollahzadeh, A. 1999. Zur Süßwasserfauna des Gilan (Iran). Zoology in the Middle East, 17:91-98.
Nasrollahzadeh, A. 2010. Caspian Sea and its ecological challenges. Caspian Journal of Environmental Science, 8(1):97-104.
Nasrollahzadeh, H. S., Rezvani, S., Rostami, H. A., Unesipour, H., Laloei, F., Pardakhti, A. R., Zinatizadeh, A. A., Zahed, M. A. and Najafpour, S. H.
2005. Studies on the accumulation of petroleum-derived polycyclic aromatic hydrocarbons (PAHs) in muscles of two bony fish in Caspian Sea, Iran.
International Conference on Innovations and Technologies in Oceanography for Sustainable Development, 26-29 September 2005, Istana Hotel, Kuala Lumpur (title).
Nasrolazadeh Saravi, H. 2001. Determination of oil pollution rate in southern Caspian Sea (from Tonekabon to Bandar Torkaman). Iranian Journal of Fisheries Sciences, 10(1):91-102. In Farsi.
Nasri-Chaari, A. 1993a. Status of aquaculture in Mazanadaran. Abzeeyan, Tehran, 4(3):34-37, XIII. In Farsi.
Nasri-Chaari, A. 1993b. Study of morphobiology between Russian sturgeon and Persian sturgeon. M.Sc. Thesis, University of Tehran. 150 pp. In Farsi.
Nasri-Chaari, A. 1994. Prevention of eventual destruction of river ecosystems in northern regions of Iran. Abzeeyan, Tehran, 5(3 & 4):31-33, IV. In Farsi.
Nazari, H. 2007. Evaluation of some population parameters of Caspian lamprey (Caspiomyzon wagneri) during the migratory season in the Shirud
and Talar rivers southern Caspian Sea. Dissertation, Islamic Azad University, Tehran. In Farsi.
Nazari, H. and Abdoli, A. 2010. Some reproductive characteristics of endangered Caspian lamprey (Caspiomyzon wagneri
Kessler, 1870) in the Shirud River southern Caspian Sea, Iran. Environmental Biology of Fishes, 88(1):87-96.
Nazari, H., Abdoli, A., Vosoughi, G. H. and Keymaram, F. 2009. Investigation of morphometric and meristic characters of the Caspian lamprey (Caspiomyzon wagneri) during migration season
in Shirud and Talar rivers. Iranian Scientific Fisheries Journal, 17(4):147-162. In Farsi.
Nazari, K. 2002. Identification of different fish species in Kargan-Rood River on Guilan Province, 1994. Iranian Scientific Fisheries Journal, 11(1):73-84. In Farsi.
Nazari, H., Abdoli, A., Vossoughi, G. H. and Kaymaram, F. 2010. Evaluation of some growth parameters of Caspian lamprey (Caspiomyzon wagneri) during the migratory season in the Shirud and
Talar rivers (Mazandaran Province). Journal of Marine Science and Technology, 9(2):55-67, 87. In Farsi.
Nazari, R. M. 1996. Biology and propagation of silver carp. Ranghineh-Afrouz, Tehran. 94 pp. In Farsi.
Nazari, R. M. 2001. Investigation of the relation between some biochemical ingredients of ova and blood serum levels with sex stage of maturation in Persian sturgeon (Acipenser persicus).
Ph.D. Thesis, Tarbiat Modarres University, Iran. 84 pp. In Farsi.
Nazari, R. M. 2010. Plasma sex steroid hormones of Persian sturgeon Acipenser persicus as influenced by gonad development stages and season. International Aquatic Research, 2:49-54.
Nazari, R. M., Abd Alhay, H., Modanlou Kordkolaei, M., Kalantarian, H., Sohrabnezhad, M. and Oveysipour, M. R. 2009. Effects of egg size on length, weight, growth and survival of prelarval and
early feeding stage of Persian sturgeon (Acipenser persicus). Iranian Scientific Fisheries Journal, 18(1):137-150. In Farsi.
Nazari, R. M., Abdolhy, H., Taghikhahnia, E., Sohrabnezhad, M. and Nouri, H. 2005. Study on the relationship between sperm density and fertilization rate in Persian sturgeon (Acipenser
persicus Borodin 1897). Journal of Agricultural Sciences and Natural Resources, 12(1):17-25. In Farsi.
Nazari, R. M., Sohrabnejad, M., Ghomi, M. R., Modanloo, M., Ovissipour, M. and Kalantarian, H. 2009. Correlations between egg size and dependent variables related to larval stage in Persian sturgeon
Acipenser persicus. Marine and Freshwater Behaviour and Physiology, 42(2):147-155.
Nazari, R. M., Yousefian, M., Abdolhy, H., Majazi, A. B., Soltani, M. and Moghadasi, M. 2001. Study on the dynamic of sex steroids and its relation with stage of gonad maturity in Persian
sturgeon. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Nazari, R. M. Yousefian, M., Mojazi Amiri, B. and Soltani, M. 2002. Study on the changes of sex steroids and its relation with oocyte development in Persian sturgeon (Acipenser persicus).
Pajouhesh va Sazandegi, 14(4)(53):89-94. In Farsi.
Nazari, S., Pourkazemi, M. and Rebelo Porto, J. I. 2009. Comparative karyotype analysis of two Iranian cyprinids, Alburnoides bipunctatus and Alburnus filippii (Cypriniformes,
Cyprinidae). Iranian Journal of Animal Biosystematics, 5(2):23-32.
Nazari Chamak, F., Pazouki J, Ebrahimi, M. and Masoumian, M. 2010. Capoeta damascina of Halil-Rud River, a new host for Myxozoan parasites. Journal of Veterinary Research, 64(4):323-327.
Nazifi, S., Firoozbakhsh, F. and Bolouki, M. 2000. Evaluation of serum biochemical parametere in experimental intoxication with trichlorofan in silver carp (Hypophthalmichthys molitrix
Valenciennes). Journal of the Faculty of Veterinary Medicine, University of Tehran, 55(3):55-60. In Farsi.
Nazifi, S., Firozbakhsh, F. and Ghazizadeh, M. 2001. Evaluation of hematological parameters in experimental intoxication with trichlorofen in the silver carp. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 56(2):23-27. In Farsi.
Nechat, H. A. et Mokhayer, B. 1974. Parasites de l'appareil genital des esturgeons. Revue de Médecine Vétérinaire, 125(3):355-360.
Nedoshivin, A. Ya. and Iljin, B. S. 1927. Ulovy krasnoi ryby v vodakh yuzhnogo poberezh'ya Kaspiya v 1913-1915 g.g. (Iz rabot Kaspiiskoi ekspeditsii 1914-1915 g.g.)
[Ganoid fish catches in the waters of the southern coast of the Caspian Sea in 1913-1915 (from the works of the Caspian Expedition 1914-1915)]. Izvestiya Otdela prikladnoi
ikhtiologii i nauchno-promyslovykh issledovanii, Leningrad, 7(1):5-100.
Nedoshivin, A. Ya. and Iljin, B. S. 1929. Rybolovstvo v vodakh yuzhnogo Kaspiya (arendyemykh firmoi "Naslednikhi Lianozova") [Fisheries
in the southern waters of the Caspian Sea (held by the firm "Lianozov Heirs"]. Trudy Instituta Rybnogo Khozyaistva i Promyslovykh Issledovanii, Leningradskoe Otdelenie, 1:5-142.
Negarestan, H., Hoseini, S., Rouhi, A., Bagheri, S., Pajand, Z. and Ghasemi, S. 2002. Presence of the ctenophore Mnemiopisis leidyi in south Caspian Sea. First International
Meeting "The invasion of the Caspian Sea by the comb jelly Mnemiopsis - problems, perspectives, need for action", Baku, Azerbaijan, 24-26 April 2001.
Caspian Environment Programme, Baku, Azerbaijan. 4 p.
Nehring, R. B. 1973a. Lar River population estimations. Job Progress Report, Department of Environmental Conservation, Tehran. 18 pp.
Nehring, R. B. 1973b. Neur Lake Survey I (Lisar Protected Region). S-29-52 WP 1. Job Progress Report, Environment Sector, Department of Environmental Conservation, Tehran. MS, 7 pp.
Nehring, R. B. 1973c. Neur Lake Winter Survey II, 3 Bahman 1352. Job Completion Report, Human Environment Division, Tehran. MS, 3 pp.
Nehring, R. B. 1973d. Neur Lake Limnological Survey, 30-31 Tir 1352. Job Progress Report, Environment Sector, Department of Environmental Conservation, Tehran. MS, 2 pp.
Nehring, R. B. 1974a. Lar River valley population estimations - 1353. Job Progress Report, Department of Environmental Conservation, Tehran. 32 pp.
Nehring, R. B. 1974b. Neur Lake Winter Survey II, 3 Bahman 1352. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of Environmental Conservation, Tehran. MS, 5 pp.
Nehring, R. B. 1974c. Neur Lake Winter Survey II - 1353, 19-28 Bahman 1353. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of Environmental Conservation,
Tehran. MS, 7 pp., 2 tabs.
Nehring, B. 1974d. Neur Lake Limnological Survey I-1353, 3-6 Ordibehest 1353. Job Progress Report, Human Environment Division, Tehran. MS, 4 pp.
Nehring, B. 1974e. Neur Lake Winter Survey III - 1353, 20-25 Esfand 1353. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of the Environment, Tehran. MS, 5 pp., 1 fig.
Nehring, B. 1974f. Neur Lake Limnological Survey III 1353 and Installation of Neur Lake Aeration Equipment- 1353, 30 Sharivar-12 Mehr 1353 L-6-53. Job Progress Report, Fisheries and Aquatic
Ecology Section, Department of the Environment, Tehran. MS, 13 pp., appendix, 6 figs.
Nehring, R. B. 1974g. The ecology and population dynamics of the Neuer Lake amphipod population and Aban amphipod population estimation. L-6-53, Job Progress Report, Department of the
Environment, Tehran. MS, 14 pp.
Nehring, B. 1975a. Lar, Karadj & Lighvanchai brown trout population estimates - 1354, 9-19 Sharivar 1354. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of
the Environment, Tehran. 30 pp.
Nehring, B. 1975b. Neur Lake amphipod population dynamics & ecology in the presence and absence of predation by rainbow trout. Job Progress Report, Fisheries and Aquatic Ecology
Section, Department of the Environment, Tehran. 19 pp., 24 figs., tabs.
Nehring, B. 1975c. Neur Lake Aeration System Activation and Winter Survey I -1353, 16-21 Mehr 1353. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of the Environment,
Tehran. MS, 8 pp., appendix, 1 tab., 1 fig.
Nehring, R. B. 1975d. Neur Lake amphipod population dynamics and ecology in the presence and absence of predation by rainbow trout. Job Progress Report, Fisheries and Aquatic Ecology
Section, Department of the Environment, Tehran. MS, 18 pp., appendices, 36 tabs., 28 figs.
Nehring, B. 1975e. Neur Lake Limnological Survey I - 1354, 13-20 Ordibehesht 1354, L-7-54. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of the Environment,
Tehran. MS, 8 pp., appendix, 10 tabs., 1 fig.
Nehring, B. 1975f. Neur Lake Limnological Survey III (1354) and Modification of Helixor Aeration System Configuration, 20 -27 Mehr 1354. Job Progress Report, Fisheries and Aquatic Ecology
Section, Department of the Environment, Tehran. MS, 5 pp., 2 tabs., 1 fig.
Nehring, B. 1975g. The ecology and population dynamics of the Neur Lake amphipod population and Aban amphipod population estimation. Job Progress Report, Fisheries and Aquatic Ecology
Section, Department of the Environment, Tehran. MS.
Nehring, B., Boettcher, J. and Soltanpour, A. 1974. Neur Lake amphipod populations estimates IV - 1353. Job Progress Report, Fisheries and Aqautic Ecology Section, Department of the
Environment, Tehran. 9 pp., appendix, 3 figs.
Neilson, M. E. and Stepien, C. A. 2006. Molecular systematics and invasive pattern of the neogobiin fishes in the Great Lakes and Europe. International Association for Great Lakes Research,
49th Annual Conference, Great Lakes in a Changing Environment, May 22-26, 2006, University of Windsor, Windsor, Ontario (abstract).
Neilson, M. E. and Stepien, C. A. 2009a. Escape from the Ponto-Caspian: Evolution and biogeography of an endemic goby species flock (Benthophilinae; Gobiidae; Teleostei). Molecular Phylogenetics and
Evolution, 52(1):84-102.
Neilson, M. E. and Stepien, C. A. 2009b. Evolution and phylogeography of the tubenose goby genus Proterorhinus (Gobiidae: Teleostei): evidence for new cryptic species. Biological Journal of the
Linnaean Society, 96(3):664-684.
Nejad, M. C. D., Hajimoradloo, A. and Makhdomi, N. 2006. Reproductive performance of Acipenser persicus females in hatcheries after being captured in the southeastern Caspian Sea (Golestan
Province, Iran). Journal of Applied Ichthyology, 22(s1):248-251.
Nejatkhah, O., Oryan, Sh., Rostaeian, A. Naghshineh, R. and Fatemi, M. R. 2003. Bloom of phytoplanktons in Anzali Lagoon and identification of poisonous
algae. Iranian Journal of Fisheries Sciences, 12(2):95-110. In Farsi.
Nejatsanatee, A. R. 1994. Ameerkalaye Lagoon in Lahijan, Abzeeyan, Tehran, 5(1 & 2):2-4, II-III. In Farsi.
Nekoee Fard, A. and Dini Talatapeh, H. 2000. Survey on disease caused by Diplostomum spathaceum and Ichthyophthirius multifilis in cultured rainbow trout hatchery in West Azarbaijan
Province. First Symposium of Health and Disease of Aquatic Animals, Ahvaz, 14-16 February 2000. In Farsi.
Nelson, G. and McCarthy, L. 1995. Two new species of gizzard shads of the genus Nematalosa (Teleosti, Clupeidae, Dorosomatinae) from the Persian/Arabian Gulf. Japanese Journal of
Ichthyology, 41(4):379-383.
Nelson, J. S. 1991. Comments on the proposed precedence of homalopteridae Bleeker, 1859 over
balitoridae Swainson, 1839 (Osteichthyes, Cypriniformes). Bulletin of Zoological Nomenclature, 48(4):335.
Nelson J. S. 1994. Fishes of the World. 3rd Edition. John Wiley & Sons, New York. xvii + 600 pp.
Nelson, J. S. 2006. Fishes of the World. Fourth Edition. John Wiley & Sons, New York. xix + 601 pp.
Nelva, A. Collares-Pereira, M-J. et Coelho, M. 1988. Systematique et repartition du genre Chondrostoma Agassiz, 1835 (Pisces, Cyprinidae). Archiv für Hydrobiologie, 113(1):93-112.
Nematollahi, M. A. 2001. Study on populations of rainbow trout in Iran for improvement of generation and selection. Scientific Report, Fisheries Company, Tehran. 25 pp.
Nematollahi, M. A. and Azari Takami, G. 1993. Comparative study of semen in populations of rainbow trout in Iran. Tehran University Press, Tehran. 205 pp.
Nematollahi, M. A. and Azari Takami, G. 2002. Comparative study of quality and quantity of seminal fluid in cultured salmonids of Iran. International Congress on the Biology of Fish,
Congress 2002: Vancouver, Canada, p. 79-82.
Nesbitt, A. P. and Bawa, K. S. 1960. "Ghanat" water collection system. The Military Engineer, 350:463-466.
Nesbitt, A. P. and Bawa, K. S. 1961. "Ghanat" water collection system. Mines Magazine, Golden, Colorado, 1961:27-30.
Neumann, D. 2006. Type catalogue of the Ichthyological Collection of the Zoologische Staatssammlung München. Part I: Historic type material from the "Old Collection",
destroyed in the night 24/25 April 1944. Spixiana, 29(3):259-285.
Neumann, H. 1953. Die physisch-geographischen Grundlagen der künstlichen Bewässerung des Iran und Irak. Wissenschaftliche Veröffentlichungen des Deutschen Instituts für
Länderkunde, Leipzig, 12:4-46.
Neumann, J. 1993. Climatic changes in Europe and the Near East in the second millenium BC. Climatic Change, 23:231-245.
Neumann, J. and Sigrist, R. M. 1978. Harvest dates in ancient Mesopotamia as possible indicators of climatic variations. Climatic Change, 1:239-252; Addendum, pp. 253-256.
Neverian, H. A., Mostafazadeh, S. S. and Toluei Gilani, M. H. 2005. A study on various protein levels on growth indices (SR, WG, RGR, FCR and PER) of Rutilus frisii kutum,
Kamenskii 1901 (advanced fry). Pajouhesh va Sazandegi, 18(3)(68):61-68. In Farsi.
Neverian (sic), H., Zahmatkesh, A., Zamani-Kyasajmahalleh, H. A. and Ghanaatparast, A. 2008. Determination of optimum level of vitamin premix for the diet of Caspian frisii kutum (advanced fry).
Pajouhesh va Sazandegi, 79:166-174. In Farsi.
Nevraev, A. F. 1929. Yuzhnoe poberezh'e Kaspiya (raion persidskikh vod) [South Caspian Coast (Region of Persian Waters)]. Trudy Nauchnogo Instituta Rybnogo Khozyaistvo, 4:350-399.
Nezami, B. Sh. and Khodaparast, S. H. 1996. Survey on organic matter accumulation in the Anzali Lagoon. Iranian Fisheries Scientific Journal, 5(2):1-10, 1. In Farsi.
Nezami, S., Khara, K., Sakari, M., Foroozan, M. and Bakhtazma, N. 2004. Feeding biology of pike (Esox lucius) in Amirkelayeh Wetlands, north of Iran. Biology in Asia International Conference,
Singapore, 7-10 December 2004 (abstract).
Nezami, S., Khara, H. and Sattari, M. 2005. Occurrence and intensity of some parasites in Perca fluviatilis (Perciformes: Percidae) from the
south-west of the Caspian Sea. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Nezami, S., Sattari, M. and Khara, H. 2005. Parasites of some bonyfishes in Boojagh wetland from the southwest of the Caspian Sea. 12th EAFP International
Conference on Diseases of Fish and Shellfish, Copenhagen (title).
Nezami, S. A., Savari, A., Sakari, M. and Alizadeh, M. 2000. National Report of Biodiversity in Caspian Coastal Zone. Research Department, Gilan Provincial Office, Department of the
Environment Conservation, Iran (TACIS (Technical Assistance to the Commonwealth of Independent States, European Union), Caspian Environmental Programme). vi + 97 pp., 63 tables.
Nezami, Sh., Khara, H., Afsordeh, A. and Padjand, Z. 2005. Determining the lethal concentration (LC50 96h) of phenol and I-naftol for Acipenser persicus fingerlings.
Iranian Journal of Fisheries Sciences, 14(1):147-160. In Farsi.
Nezami, Sh. A. and Khara, H. 2004. Species composition and abundance of fishes in Amirkelayeh Wetland. Iranian Journal of Fisheries Sciences, 12(4):193-206. In Farsi.
Nezami, Sh. A., Khara, H. and Pavand, P. 2004. Feeding behaviour of Perca fluviatilis in Amirkelayeh Lagoon. Iranian Journal of Fisheries Sciences, 13(1):201-220. In Farsi.
Nezami, Sh. A., Pajand, Z. A., Khara, H. and Keshvar Doost, F. 2004. Determination of LC50 96h on Saturn (herbicide) and Malathion (insecticide) on
Persian sturgeon (Acipenser persicus). Journal of Environmental Science and Technology, 20:1-11. In Farsi.
Nezami Abadi, H. and Abdi, K. 2002. Review of diplostomiasis in fishes. Final report. Aquatic Health and Disease Office, Veterinary Organization of Iran. 44 pp. In Farsi.
Nezami Balouchi, Sh. A., Khara, H., Bakhtazman, N. and Forouzan, M. 2005. Diet study of pike (Esox lucius) in Lahijan Amirkelayeh Lagoon. Pajouhesh va Sazandegi,
18(3)(68):46-55. In Farsi.
Nezami Balouchi, Sh. A., Khara, H., Rashidi, Sh. and Arefi, N. 2007. Diet of wels, Silurus glanis of Amirkelayeh Wetland of Lahijan. Iranian Journal of Biology, 20(2):295-306. In Farsi.
Nezami Balouchi, Sh. A., Khara, H., Sabkara, J., Soltanzadeh, M. and Damshenas, Z. 2004. Study of tench (Tinca tinca) diet of Lahijan Amirkelayeh Wetland. Pajouhesh va Sazandegi,
16(4)(618):81-91. In Farsi.
Nezamy Balochy, S. A., Khara, H., Nikokerdar, L. and Mirmosavy, M. 2005. The diet survey Zibakenar-Kiashar Bojagh Lagoon pike (Esox lucius). Journal of Agricultural Sciences and Natural
Resources, 11(4):175-186. In Farsi.
Ng, P. K. L. and Lim, K. K. P. 1991. Comment on the proposed precedence of homalopteridae Bleeker,
1859 over balitoridae Swainson, 1839 (Osteichthyes, Cypriniformes). Bulletin of Zoological Nomenclature, 48(2):148-150.
Niak, N., Kohnehshahri, M. and Azari, G. 1970. Infestation of Trichodina sp. in sturgeons of Caspian Sea. Journal of the Veterinary Faculty of the University of Tehran, 26(4):51-54. In Farsi.
Niamir, A. 2001. Avan River. Moj-e Sabz; Social-Cultural Quarterly, (4):16-18. In Farsi (In English at www.netiran.com/Htdocs/WeeklyJournal/Social/wj00666.html).
Niazi, A. D. 1976. The Weberian system of Mystus pelusius (Solander) and Mystus colvilli (Gunther), Siluriformes. Bulletin of the Natural History Research Center, Baghdad, 7(1):42-90.
Niazi, A. D., Qasim, H. H. and Shalli, M. R. 1976. The comparative osteology of the osteocranium of Garra rufa (Heckel) & G. variabilis (Heckel). Bulletin of the Natural History
Research Center, Baghdad, 7(1):110-126.
Nico, L. G., Williams, J. D. and Jelks, H. L. 2005. Black Carp. Biological Synopsis and Risk Assessment of an Introduced Fish. American Fisheries Society Special Publication, 32:xx + 337 pp.
Nielsen, J. G. 1974. Fish Types in the Zoological Museum of Copenhagen. Zoological Museum, University of Copenhagen. 115 pp.
Nielsen, J. G. 1993. Peter Forsskål - a pioneer in Red Sea ichthyology. Israel Journal of Zoology, 39(4):283-286.
Nijssen, H., van Tuijl, L. and Isbrücker, I. J. H. 1982. A catalogue of the type-specimens of recent fishes in the Institute of Taxonomic Zoology (Zoölogisch Museum),
University of Amsterdam, The Netherlands. Verslagen en Technische Gegevens, Institut voor Taxonomische Zoölogie (Zoölogisch Museum), Universiteit van Amsterdam. 173 pp.
Nijssen, H., van Tuijl, L. and Isbrücker, I. J. H. 1993. Revised catalogue of the type specimens of recent fishes in the Institute of Taxonomic Zoology (Zoölogisch Museum),
University of Amsterdam, The Netherlands. Bulletin Zoölogisch Museum Universiteit van Amsterdam, 13(18):211-262.
Nikolaev, A. A. 1993. Morfoekologicheskaya kharakteristika turkmenskogo gol'tsa (Noemacheilus sargadensis turcmenicus Berg, 1933) [Morphoecological
characteristics of the Turkmen loach (Noemacheilus sargadensis turcmenicus Berg, 1933)]. Izvestiya Akademii Nauk Turkmenistana Seriya Biologicheskikh Nauk, 2:42-49.
Nikol'skii, A. M. 1897. Presmykayushchiyasya, amfibii i ryby, sobrannyya N. A. Zarudnym vostochnoi Persii [Reptiles, amphibians and fishes,
collected by N. A. Zarudnyi in eastern Persia]. Ezhegodnik Zoologicheskogo Muzeya Imperatorskoi Akademii Nauk, St. Petersburg, 2:306-348, pl. XVII-XIX.
Nikol'skii, A. M. 1899. Presmykayushchiyasya, amfibii i ryby vtorogo puteshestviya N. A. Zarudnego v Persiyu v 1898 g [Reptiles, amphibians and fishes collected on the second expedition
of N. A. Zarudnyi to Persia in 1898]. Ezhegodnik Zoologicheskago Muzeya Imperatorskoi Akademii Nauk, St. Petersburg, 4:375-417, pl. XX.
Nikol'skii, A. M. 1900. Novyi vid Discognathus iz Rossii (Pisces, Cyprinidae) [A new species of Discognathus from Russia]. Ezhegodnik Zoologicheskago Muzeya Imperatorskoi Akademii
Nauk, St. Petersburg, 5:239-241. In Latin and Russian.
Nikol'skii, G. V. 1937. Materialy po sistematike bystryanok Alburnoides (Pisces, Cyprinidae) Srednei Asii [Material on the systematics of bystryanok Alburnoides (Pisces, Cyprinidae) of
Central Asia. Pamyati Menzbira, Moskva (M. A. Menzbier Memorial Volume), 1937: 306-316.
Nikol'skii, G. V. 1938. Ryby Tadzhikistana [Fishes of Tadzhikistan]. Izdatel'stvo Akademii Nauk SSSR, Leningrad. 217 pp.
Nikol'skii, G. V. 1945. Materialy po sistematike i obrazu zhizni Discognathichthys rossicus (Nik) (Pisces, Cyprinidae) [Material on the
systematics and mode of life of Discognathichthys rossicus (Nik) (Pisces, Cyprinidae)]. Uchenye Zapiski Moskovskogo Gosudarstvennogo Universiteta, Novaya Seriya, 85:127-131.
Nikol'skii, G. V. 1947. Gol'tsy besstochnykh vodoemov Turkmenii [The loaches of the inland waters of Turkmenia]. Byulleten' Moskovskogo Obshchestva Ispytatelei Prirody, Otdel Biologii, 52(3):29-34.
Nikonorov, I. V. 1964. Pump fishing with light and electric current, pp. 577-579. In: Modern Fishing Gear of the World 2: Fishing News (Books), London. xvi + 603 pp.
Nikoo, M., Falahatkar, B., Alekhorshid, M., Haghi, B. N., Asadollahpour, A., Dangsareki, M. Z. and Langaroudi, H. F. 2010. Physiological stress responses in kutum Rutilus frisii kutum
subjected to captivity. International Aquatic Research, 2:55-60.
Nikou, M., Saeidi, A. A., Yasemi, M., Jafari, A. and Alekhourshid, M. 2007. Changes in levels of cortisol, glucose and sex hormones during transportation of southern Caspian kutum (Rutilus frisii
kutum) spawners. Iranian Scientific Fisheries Journal, 16(3):147-154. In Farsi.
Nikpay, M., Marashi, S. Z. and Moazedi, J. 1992. Survey of the biological features of Barbus sharpeyi and B. grypus. Iranian Fisheries Research and Training Organization
Publications, Tehran. ix + 124 pp. In Farsi.
Nikravesh, S. 1997. An analysis of the status of water resources in Iran. Ab va Mohitezist (Water and Environment), Tehran, (1997,
December), 25:44-51 (www.netiran.com/Htdocs/Clippings/DEconomy/971200XXDE01.html).
Niksirat, H. and Abdoli, A. 2009. On the status of the critically endangered Caspian brown trout, Salmo trutta caspius, during recent decades in the southern Caspian Sea basin (Osteichthyes:
Salmonidae). Zoology in the Middle East, 46:55-60.
Niksirat, H., Hatef, A. and Abdoli, A. 2010. Life cycle and feeding habits of the threespine stickleback Gasterosteus aculeatus (Linnaeus, 1758): an alien species in the southeast
Caspian Sea. International Aquatic Research, 2:97-104.
Niksirat, H., Hatef, A., Nikoo, M., Hajimoradloo, A. and Ghorbani, R. 2006. Infection of three-spined stickleback Gasterosteus acuoleatus (L.) with
Corynosoma strumosum in Gomishan Lagoon. Iranian Journal of Fisheries Sciences, 15(2):155-160. In Farsi.
Niksirat, H., Sarvi Moghanlou, K., Mojazi Amiri, B., Hatef, A., Pashazanoosi, A. and Mirnaimi, M. 2006. Storage of unfertilized eggs of rainbow trout (Oncorhynchus mykiss) in ovarian fluid
media. Iranian Journal of Natural Resources, 59(1):189-197. In Farsi.
Niksirat, H., Sarvi, K., Mojazi Amiri, B., Karami, M. and Hatef, A. 2007. In vitro storage of unfertilized ova of endangered Caspian brown
trout (Salmo trutta caspius) in artificial media. Animal Reproduction Science, 100:356-363.
Nilsaz, M., Najafpour, N., Sabz-Alizadeh, S., Safikhani, H., Khodadadi, M. and Davoodi, F. 1993. Limnological study of Karun River (Gotvand to Bandeghir). Iranian Fsheries Research Organization,
Tehran. 64 pp. In Farsi.
Noel, E. 1944. Qanats. Royal Central Asian Society Journal, 31:191-202.
Noorbakhsh, A. 1993a. Carp sport fishing in the north of Iran. Abzeeyan, Tehran, 4(3):24-30. In Farsi.
Noorbakhsh, A. 1993b. Reminding (sic) a river and its fishes (for the sport fishermen). Abzeeyan, Tehran, 4(4):16-19, II. In Farsi.
Noorbakhsh, H. 1993. Hamoon Lake and Zabol's fishermen. Abzeeyan, Tehran, 4(3):18-22. In Farsi.
Noorbaksh, H. 1995. Fish in Iranian poetry and idioms. Abzeeyan, Tehran, 5(9):21-24, III. In Farsi.
Noori, F., Azari Takami Gh. and Sorgeloos, P. 2002. Enrichment of Artemia with essential fatty acids, lipid emulsions and vitamin C and its effect on the performance of Acipenser persicus
larvae under effect of salinity stress. Extended Abstracts Aquaculture, 5th ISS, Ramsar, 2005:54-55.
Noori, M. 1990. Caspian lamprey (Caspiomyzon wagneri). In: National Conference on Suitable Beneficiary Resources of the Caspian Sea Fisheries, pp. 100-115. In Farsi.
Noori, M. and Lalouie, F. 1994. Studying Mugil sp fishing period from the management point of view. Iranian Fisheries Scientific Journal, 3(2):71-77, 7. In Farsi.
Noori, M. G. 1966. Hydrology of surface water in Iran, pp. 199-211. In: Symposium on Hydrology and Water Resources Development held in Ankara,
Turkey February 7 to 12, 1966, Central Treaty Organization, Ankara. 484 pp.
Noroozi, M., Akrami, R. and Matinfar, A. 2006. Seminatural propagation and rearing of roach (Rutilus rutilus caspicus). Iranian Journal of Fisheries Sciences, 15(3):165-170. In Farsi.
Noroozi, M., Oryan, Sh. and Bahmani, M. 2006. The effects of glycerate hypophysis injection on the fluctuation of steroid hormones on Persian sturgeon
(Acipenser persicus). Iranian Scientific Fisheries Journal, 14(4):197-214. In Farsi.
Norouzi, M. 2007. Population structure of A. stellatus from Caspian Sea using microsatellite. Ph.D. Thesis, Islamic Azad University, Tehran. 150 pp. In Farsi.
Norouzi, M. and Pourkazemi, M. 2009a. Applying of microsatellite markers to identify population genetic structure of the stellate sturgeon, Acipenser stellatus Pallas, 1771, in the north (Volga
and Ural rivers) and south Caspian (Sefidrud drainage). 13th European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Norouzi, M. and Pourkazemi, M. 2009b. Genetic structure of Caspian populations of stellate sturgeon, Acipenser stellatus (Pallas, 1771), using microsatellite markers. International
Aquatic Research, 1:61-65.
Norouzi, M., Pourkazemi, M. and Fatemi, M. 2009. Application of microsatellite markers to study the genetic structure of stellate sturgeon populations (Acipenser stellatus Palls, 1771) in the
south Caspian Sea. Iranian Journal of Fisheries Sciences, 8(1):73-84.
Nourouzi, M., Pourkazemi, M., Keyvan, A., Fatemi, S. M. R. and Kazemi, B. 2008. Population genetic structure of stellate sturgeon (Acipenser stellatus Pallas, 1771) in the south Caspian Sea
using microsatellite markers. Journal of Fisheries and Aquatic Science, 3(3):158-166.
North, A. 1993. Saddam's water war. Geographical Magazine,July 1993:10-14.
Noruz Fashkhami, M. R. 1995. Dtermining (sic) karyotype of sturgeons. Iranian Fisheries Scientific Journal, 4(2):63-71, 7. In Farsi.
Noruz Fashkhami, M. R. and Khosroshahi, M. 1995. Karyology (chromosomal study) of the Caspian Sea kutum roach by white blood cells culture. Iranian Fisheries Scientific Journal, 4(1):64-71, 6. In Farsi.
Nouri, J., Gharagozlou, A., Nourifard, A. and Tehrani, S. M. 2005. Environmental impact assessment of the largest man made lake of Iran. Pakistan Journal of Biological Science, 8(12):1672-1677.
Nouruz Fashkami (sic), M. R., Pourkazemi, M. and Kalbassi, M. R. 2002. Progeny karyotyping of female Rutilus frisii kutum x male Ctenopharyngodon
idella hybrid. Iranian Journal of Marine Sciences, 1(1):69-74. 79. In Farsi.
Norouz Fashkhami, M. R., Safaeian, Sh., Bahmani, M. and Choubian, F. 2009. Mitogenic effects of phytohemagglutinin (PHA-P) and lipopolysaccharide extracted from E. coli on cultured lymphocyte
of the the Caspian Sea ship sturgeon (Acipenser nudiventris). Iranian Scientific Fisheries Journal, 18(3):157-162. In Farsi.
Noverian, H. A., Shabanipour, N., Khoshkholgh, M. R. and Hosseini, M. R. 2008. Effect of dietary digestible energy level on growth indices of kutum (Rutilus
frisii kutum Kamenskii, 1901). Iranian Journal of Fisheries Sciences, 7(2 supplementary):205-214.
Novirian (sic), H., Shaabanipour, N., Zaman Kiasaj Mahaleh, H. A. and Khadem, H. 2007. The effect of different level of lipids on growth index of Caspian frisian kutum (fry stage)
(Rutilus frisii kutum Kamneskii, 1901) utilizing semi-purified diets. Pajouhesh va Sazandegi, 20(3)(76):35-42. In Farsi.
Novikov, G. G., Semenova, A. V., Stroganov, A. N. and Tagizade, V. 2008. Allozyme variability in populations of trout (Salmo trutta)
from the rivers of Iran. Journal of Ichthyology, 48(4):356-360.
Novikova, A. S. 1994. Current status of natural reproduction of beluga, Huso huso, in the lower Volga. Journal of Ichthyology, 34(1):68-75.
Nowruz Fashkhami, M. R. 1996. On the karyotypes of Acipenser persicus, A. stellatus, and Huso huso from the Iranian waters of the Caspian Sea. The Sturgeon Quarterly, 4(3):7.
Nowruz Fashkhami, M. R. and Khosroshahi, M. 1997. Karyotype study on stellate and great sturgeon by leucocyte culture. Third International Symposium on Sturgeon, Piacenza,
Italy, 8-11 July 1997, Book of Abstracts (poster).
Nowruz Fashkhami, M. R. and Khosroshahi, M. 1999. Karyotype study on stellate and great sturgeon by leukocyte culture. Journal of Applied Ichthyology, 15(4-5):283.
Nowruzfashkhami, M. R., Pourkazemi, M. and Baradarannoveiri, S. 2000. Chromosome study of Persian sturgeon Acipenser persicus B. Cytologia, 65(2):197-202.
Nowruzfashkhami, M. R., Pourkazemi, M. and Kalbasi, M. R. ?. Karyotypic characterization of the grass carp, Ctenopharyngodon idella by leukocyte culture. Virtual, 1(1):137-142. In Farsi.
Nowruzfashkhami, M. R., Safaiian, S., Bahmani, M. and Chubian, F. 2006. Karyotype analysis in ship sturgeon Acipenser nudiventris in the south Caspian Sea using leukocyte culture.
Journal of Applied Ichthyology, 22(s1):97-98.
Nowrouzi, S. and Valavi, H. 2011. Effects of environmental factors on phytoplankton abundance and diversity in Kaftar Lake. Journal of Fisheries and Aquatic Science 6(2):130-140.
Nuhi, A and Khorasani, Y. 1981. Bacterial pollution indicators in the intestinal tract of various fish species living in Amir-Kolayeh Lagoon. Zentralblatt für Bakteriologie,
Parasitenkunde und Infektionskrankheiten und Hygiene, 2:566-571.
Nümann, W. 1963. Report on the possibilities to manage the fishery in Karaj Reservoir. Report to the Game and Fish Department, Tehran. MS.
Nümann, W. 1964. Report on the possibilities of managing the fishery in Iranian rivers and reservoirs. Report to the Game and Fish Department, Tehran. MS.
Nümann, W. 1966. Limnologische Vorstudien zur fischereilichen Bewirtschaftung iranischer Stauseen und Fließgewässer.
Zeitschrift für Fischerei und deren Hilfswissenschaften, 14(5/6):433-478.
Nümann, W. 1969. Untersuchungen zur fischereilichen Bewirtschaftung iranischer Stauseen und Fließgewässer. Verhandlungen der internationalen Vereinigung
für theoretische und angewandte Limnologie, 17:705-713.
Nümann, W. and Hosseinzadeh, H. 1968. A study of some waters of Iran for increasing fish and fish culture. Report to the Game and Fish Department, Tehran. MS, 28 pp.
Nupky, M. 1996. Survey of the biology of Barbus grypus and Barbus sharpeyi in Khuzestan Province. Southern Iran Aquaculture Fishery Research Centre, Ahvaz. 120 pp. In Farsi.
Nuttall, N. 1996. Caviare could be off the menu as poachers threaten sturgeon. The Times, London, Internet Edition, 13 November 1996, 2 pp.
Nützel, W. 1975. The formation of the Arabian Gulf from 14000 B.C. I. II. The postglacial climatic optimum and the possible flooding
of south Mesopotamia. Sumer, 31(1 & 2):101-110.
Nützel, W. 1976. The climate changes of Mesopotamia and bordering areas 14 000 to 2 000 B.C. Sumer, 32:11-25.
Nyrop, R. F. 1978. Iran, a country study. 3rd Edition, The American University, Washington, D.C. xxviii + 492 pp.
O
Oberlander, T. 1965. The Zagros Streams. A New Interpretation of Transverse Drainage in an Orogenic Zone. Syracuse Geographical Series, Syracuse University, New York, 1:xii + 168 pp.
Oberlander, T. M. 1968a. The origin of the Zagros defiles, pp. 195-211. In: Fisher, W. B. (Ed.). The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press,
Cambridge. xix + 784 pp.
Oberlander, T. M. 1968b. Hydrography, pp. 264-279. In: Fisher, W. B. (Ed.). The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press, Cambridge. xix + 784 pp.
O'Donovan, E. 1882. The Merv Oasis: Travels and Adventures East of the Caspian During the Years 1879-81. Smith Elder, London, 2 volumes.
Oliva, O. and Holčík, J. 1977. The hundredth anniversary of the birth of Academician L. S. Berg (1876-1950). Folia Zoologica, Prague, 26(1):93-95.
Oliva, O. and Šafránek, V. 1962. To systematics of the European bleak, Alburnus alburnus (Linnaeus). Vĕstník československé Společnosti
zoologické, 26(4):324-328.
Oliver, C. 2003. Iran battles to revive stocks of caviar sturgeon. Environmental News Network, 19 November 2003 (www.enn.com/news/2003-11-19/s_10544.asp, downloaded 3 August 2004).
Oloomi, Y. 2001. Biological study on Gorganrood River. Iranian Journal of Fisheries Sciences, 10(1):55-72. In Farsi.
Omidi, A., Mazloomi, S. and Farhangfar, H. 2009. Preservative effect of quanats water to reduce lead acetate toxicity (LC50 96h) on Capoeta fusca. Journal of Fisheries and Aquatic
Science, 4(1):50-56.
Omidi, S. 2006. A survey on effects of aquaculture on the coastal waters of Bushehr (Helleh and Mond). Iranian Fisheries Research Organization, Agriculture Research and Education
Organization, Ministry of Jihad-e-Agriculture, Bushehr. http://en.ifro.ir (abstract).
Omidkar, H. 2001. A study of the fishes in subterranean channels (ghanats) around the city Sabzevar. Ph. D. Thesis, Veterinary Faculty, University of Tehran, Tehran. (not seen).
Omran, R. Gh. and Hedayatifard, M. 2009. Evaluation of fatty acid composition in rainbow trout (Oncorhynchus mykiss) during growth. The 6th Scientific Conference of Fisheries Resources, 3-4
March 2009, Basrah, p. 135 (abstract).
Oosterbroek, P. and Arntzen, J. W. 1992. Area-cladograms of circum-Mediterranean taxa in relation to Mediterranean palaeogeography. Journal of Biogeography, 19:3-20.
Opalatenko, L. K. 1979. Feeding and food relationships of Neogobius fluviatilis pallasi and N. melanostomus affinis in the
North Caspian. Hydrobiological Journal, 15(2):72-73.
Opuszynski, K. and Shireman, J. V. 1995. Herbivorous fishes. Culture and Use for Weed Management. CRC Press, Boca Raton. 223 pp.
Orian, Sh., Seifabady, J. and Jamily, Sh. 1993. Influence of salinity upon growth rate and tolerance of Barbus sharpeyi. Iranian Fisheries Bulletin, (2):45-55, 5-6. In Farsi.
Orlova, E. L. 1988. Peculiarities of growth and maturation of the catfish, Silurus glanis, in the Volga Delta under regulated flow conditions. Journal of Ichthyology, 28(3):35-45.
Orlova, E. L. and Popova, O. A. 1976. The feeding of predatory fish, the sheatfish, Silurus glanis, and the pike, Esox lucius,
in the Volga Delta following regulation of the discharge of the river. Journal of Ichthyology, 16(1):75-87.
Oryan, S., Parivar, K. and Rasekhi, K. 2005. The effects of baclofen (GABA-B receptor agonist) administration alone and accompanied with LRH-A and metoclopramid on GTH-I release in carp (Cyprinus
carpio L.). Journal of the Faculty of Veterinary Medicine, University of Tehran, 60(3):265-271. In Farsi.
Oryan, S., Vosoughi, Gh. R. and Zarrin Kamar, H. 1998. The role of some physiological changes in the feeding of Rutilus frisii kutums (sic)
(within Anzali port province). Journal of the Faculty of Veterinary Medicine, University of Tehran, 53(1-2):14-18.
Osinov, A. G. 1984. Zoogeographical origins of brown trout, Salmo trutta (Salmonidae): data from biochemical genetic markers. Journal of Ichthyology, 24(1):10-23.
Osinov, A. G. 1988. Kumzha (Salmo trutta L., Salmonidae) Basseinov Chernogo i Kaspiiskogo Morei: Populyatsionno-geneticheskii Analiz [Brown trout (Salmo
trutta L., Salmonidae) of Black and Caspian Sea basins: Population-genetic analysis]. Genetika, 24(12):2172-2186.
Osinov, A. G. 1999. Salmonid fish of the genera Salmo, Parasalmo, and Oncorhynchus: genetic divergence, phylogeny, and classification. Journal of Ichthyology, 39(8):571-587.
Osinov, A. G. 2009. Iranian samples of brown trout Salmo trutta or rainbow trout Oncorhynchus mykiss: comparison of evidence from allozyme and mitochondrial DNA control region sequence
analysis. Journal of Ichthyology, 49(9):825-828.
Osinov, A. G. and Bernatchez, L. 1996. "Atlantic" and "Danubian" phylogenetic groupings of brown trout Salmo trutta complex: genetic divergence, evolution, and conservation.
Journal of Ichthyology, 36(9):723-746.
Osipov, V. V. and Kiyashko, V. I. 2008. Method of age determination of Ponto-Caspian kilka Clupeonella cultriventris (Clupeiformes, Clupeoidei) using scales
and otoliths. Journal of Ichthyology, 48(8):637-643.
Ostroumova, I. N. 1970. Protein composition of the blood serum of the Caspian salmon in connection with its taxonomic position. Journal of Ichthyology, 10(3):338-341.
Otero, O. 2001. The oldest-known cyprinid fish of the Afro-Arabic Plate, and its paleobiogeographical implications. Journal of Vertebrate Paleontology, 21(2):386-388.
Ouseley, W. 1819-1823. Travels in various countries of the East; more particularly Persia. A work wherein the Author has described, as far as his own Observations extended, the State of
those Countries in 1810, 1811, and 1812; and has endeavoured to illustrate many subjects of Antiquarian Research, History, Geography, Philology and Miscellaneous Literature, with
extracts from rare and valuable Oriental Manuscripts. Rodwell and Martin, London. Volume I(1819):455 pp., plates, maps; volume II(1821):544 pp.,
plates, maps; volume III(1823):600 pp., plates, maps.
Oymak, S. A. 2000. Atatürk Baraj Golü'nde Yasayan Chondrostoma regium (Heckel, 1843)'un Büyüme Ozellikleri (The growth characteristics of Chondrostoma
regium (Heckel, 1843) in Atatürk Dam Lake (Turkey). Turkish Journal of Zoology, 24(supplement):41-50.
Oymak, S. A., Dogan, N. and Uysal, E. 2008. Age, growth and reproduction of the shabut Barbus grypus (Cyprinidae) in Atatürk Dam Lake (Euphrates River), Turkey. Cybium, 32(2):145-152.
Oymak, S. A., Solak, K. and Ünlü, E. 2001. Some biological characteristics of Silurus triostegus Heckel, 1843 from Atatürk Dam lake (Turkey). Turkish Journal of Zoology, 25(2):139-148.
Özdemir, N. and Şen, D. 1986. Age determination by scale, vertebra and operculum of Leuciscus cephalus orientalis (Nordmann, 1840) in the Euphrates. Journal of Fırat
University, Elazığ, 1(1):101-111.
Özdilek, Ş. Y. 2003. Occurrence of the abu mullet, Liza abu (Heckel, 1843)(Pisces, Mugilidae), in the Orontes River. Zoology in the Middle East, 30:111-113.
Öztaş, H.1988. Müceldi Suyu'nda (Doğu Anadolu) Yaşayan Tatlısu Kefali [Leuciscus cephalus (L., 1758)] Populasyonunda Mevsimsel
Kondüsyon Faktörü Değişimleri Üzerine Araştırmalar (Studies on the seasonal condition factor variations of chub (Leuciscus
cephalus (L., 1758) population in Müceldi stream in east Anatolien (sic)).Doğa Türk Zooloji Dergisi, 12(3):256-261.
Öztaş, H. 1989.A study the reproduction biology of chub (Leuciscus cephalus L., 1758)) in Müceldi stream in east Anatolien (sic).Doğa Türk Veterinerlik
Hayvancılık Dergisi, 13(2):171-179.
Öztaş, H. and Solak, K. 1988.Müceldi Suyu'nda (Doğu Anadolu) Yaşayan Tatlısu Kefalinin [Leuciscus Cephalus (sic) (L., 1758)] Büyüme
Özellikleri ve Eşem Oranları (The growth and sexual rates of chub [Leuciscus cephalus (L., 1758)] living in the Müceldi stream in east Anatolien (sic)).
Doğa Türk Zooloji Dergisi, 12(3):262-271.
Özuluğ, M. and Freyhof, G. 2008. Capoeta turani, a new species of barbel from River Seyhan, Turkey (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters, 19(4):289-296.
P
Paateemaar, R. 1993. Pipefish of the Caspian Sea. Abzeeyan, Tehran, 4(8):20-23. In Farsi.
PADECO Co. Ltd. 2002. Ecotoxicological study: investigation into toxic contaminant accumulation and related pathology in the Caspian sturgeon, seal and
bony fish (ECOTOX Study). Final Workshop Report, Baku, Azerbaijan, 28-29 March 2002, The World Bank. 17 pp.
Padjand, Z., Esmaieili, S. A. and Piri, M. 2005. Determining the lethal concentration LC50 96h) of butachlor herbicide for Acipenser
persicus and Acipenser stellatus fingerlings. Iranian Scientific Fisheries Journal, 14(1):41-50. In Farsi.
Paepke, H-J. 1983. Die Stichling Gasterosteidae. A. Ziemsen Verlag, Wittenberg Lutherstadt. 144 pp.
Paepke, H.-.J. 1999. Bloch's Fish Collection in the Museum für Naturkunde der Humboldt Universität zu Berlin. An Illustrated Catalog and Historical Account.
A. R. G. Gantner Verlag KG, Ruggell, Liechtenstein. 216 pp., 32 pls.
Paghe, E., Maghsoudlu, T. and Abdoli, A. 2005. Study of age and growth of Caspian roach (Rutilus rutilus caspicus) in Gomishan Wetland
(southeastern Caspian Sea). Journal of Agricultural Sciences and Natural Resources, 11(4):151-162. In Farsi.
Pagheh, A. and Maghsoodlu, T. 2000. A study of age. growth and reproduction of roach, Rutilus rutilus caspicus, in Gomishan Wetland. B.Sc. Project, University of Gorgan, Gorgan. In Farsi.
Pahlavan Yali, M., Mojazi Amiri, B., Poosti, A. and Bahmani, M. 2004. Histological study of Acipenser persicus alimentary canal during early life stages. Iranian
Scientific Fisheries Journal, 13(2):33-50. In Farsi.
Pala, C. 1994. Caviar: A health hazard? Condé Nast Traveller, 29(10):48.
Pala, C. 2005. Ban on beluga caviar points to sturgeon's worldwide decline. Science, 310:37.
Pala, G., Tellioğlu, A., Eroğlu, M. and Şen, D. 2010. The digestive system content of Mastacembelus mastacembelus (Banks
& Solander, 11794) inhabiting in Karakaya Dam Lake (Malatya-Turkey). Turkish Journal of Fisheries and Aquatic Sciences, 10(2):229-233.
Palicka, J. 1996. The spined loach, Cobitis taenia. Tropical Fish Hobbyist, 45(4)(490):82, 84.
Pallas, P. S. 1771. Reise durch verschiedene Provinzen des russischen Reiches, 1768 und 1769sten Jahre. St. Petersburg. Volume 1.
Pallas, P. S. 1776. Reise durch verschiedene Provinzen des russischen Reiches, 1768 und 1769sten Jahre. St. Petersburg. Volume 3.
Pallas, P. S. 1787. Piscium novae species descriptio. Nova Acta Academiae Petropolitanae, 1 (Hist.):258.
Pallas, P. S. 1814. Zoographia Rosso-Asiatica. Vol. 3, Animalia Monocardia seu Frigida Sanguinis Imperii Rosso-Asiatici. Petropolis, 428 pp., + atlas.
Paphan, A. A., Peyghan, R. and Modaresi, Sh. 2005. Electrocardiograph changes in anesthesia with ketamine in grass carp. Journal of Veterinary Research, 60(1):59-64. In Farsi.
Papahn, F., Valinejad Zavaregh, A. A. and Hoghooghirad, N. 2004. Identification of monogeneans and their population density impact on Barbus
grypus and B. sharpeyi in Karoon River in Ahwaz. Journal of the Faculty of Veterinary Medicine, 59(3):283-288. In Farsi.
Parafkandeh, F. and Jamalzad, F. 1997. A biological investigation into anchovy kilka (Clupeonella engrauliformis) in Anzali fishing ground. Iranian Fisheries
Scientific Journal, 5(4):31-42, 3. In Farsi.
Parafkandeh Haghigi, F. 1996. A look at kilka catch in 1995. Abzeeyan, Tehran, 24-27, II. In Farsi.
Parafkandeh Haghighi, F. and Rezvani, S. 2005. Discrimination of populations of Caspian roach (Rutilus rutilus) using trace element content in
otoliths. Iranian Journal of Fisheries Sciences, 14(3):19-36. In Farsi.
Parandavar, H. 2006. Study the biotechnique of broodfish formation from sturgeon broodfish from natural stock and those reared in captivity. Iranian Fisheries Research
Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Parandavar, H., Kazemi, R., Pourali, H. R., Vahabi, Y. and Pourkazemi, M. 2006. Potential for egg extraction from female sturgeon spawners through key-hole surgery. Journal of Applied Ichthyology,
22(s1):252-256.
Parandavar, H., Tavakoli, M. and Touraji, M. R. 2008. Effects of photoperiod on ship sturgeon (Acipenser nudiventris), feeding on Daphnia at laboratory condition.
Pajouhesh va Sazandegi, 20(4)(77):192-199. In Farsi.
Parenti, L. R. 1981. A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). Bulletin of the American Museum of Natural History, 168(4):335-557.
Parenti, L. R. 1984. A taxonomic revision of the Andean killifish genus Orestias (Cyprinodontiformes, Cyprinodontidae). Bulletin of the American Museum of Natural History, 178(2):107-214.
Parin, N. V. 2001. An annotated catalog of fishlike vertebrates and fishes of the seas of Russia and adjacent countries. Part 1. Orders Myxiniformes
- Gasterosteiformes. Journal of Ichthyology, 41(supplement 1):S51-S131.
Parivar, K., Behzadi, S. and Razavi, B. 1993. The chronological development of embryo in Rutilus frisii kutum (Kamensky). Iranian Fisheries Bulletin, (1):3-24, 3-4.In Farsi.
Parker, A. and Kornfield, I. 1995. Molecular perspective on evolution and zoogeography of cyprinodontid killifishes (Teleostei; Atherinomorpha). Copeia, 1995(1):8-21.
Parks, Y. P. and Smith, P. J. 1983. Factors affecting the flow of aflaj in Oman: A modelling study. Journal of Hydrology, 65(4):293-312.
Parin, N. V., Collette, B. B. and Shcherbachev, Yu. N. 1980. Predvaritel'nyi obzor morskikh polurylov (Hemiramphidae, Beloniformes) tropicheskoi indo-vest-patsifiki
[Preliminary review of the marine halfbeaks (Hemiramphidae, Beloniformes) of the tropical Indo-West Pacific]. Trudy Instituta Okeanologii im. P. P. Shirshova, Akademiya Nauk SSSR, 97:7-173.
Parsa, S. 1999. On the biosystematic and population dynamics of Nemacheilus spp. in Jajeroud and Gorgan-roud rivers. M.Sc. Thesis,
University of Tehran. 325 pp. In Farsi.
Parsamanesh, A., Eskandari, G., Kashi, M. T. and Shalbaf, M. R. 2001. Stock assessment of Khuzestan marine fishes (final report). Iranian Fisheries Research Organization, Tehran. 72 pp. In Farsi.
Partow, H. 2001. The Mesopotamian Marshlands: Demise of an Ecosystem. Early Warning and Assessment Technical Report, Division of Early Warning and Assessment, United
Nations Environment Programme, Nairobi, UNEP/DEWA/TR.01-3 Rev.1:x + 47 pp. (www.grid.unep.ch/activities/sustainable/tigris/marshlands).
Parvaneh, S. A. and Parivar, K. 1996. Survey on Acipenser persicus populations by use of the electrophoretic technique and morphological characteristics. Iranian
Fisheries Scientific Journal, 5(3):41-50, 4. In Farsi.
Patimar, R. 1995. The biology of Boyer's sand smelt population Atherina boyeri Risso 1810, in Gomishan Lagoon. M. Sc. Thesis, Tarbiat Moddaress University, Tehran. In Farsi.
Patimar R. 2007. None-indigenous (sic) fishes of Alma-Gol, Adji-Gol and Ala-Gol Wetlands (Golestan Province): implications for conservation and management programs of wetlands.
Environmental Sciences, 4(3):1-8.
Patimar R. 2008a. Some biological aspects of the sharpnose mullet Liza saliens (Risso, 1810) in Gorgan Bay-Miankaleh Wildlife Refuge (the southeast Caspian Sea). Turkish Journal of Fisheries
and Aquatic Sciences, 8(2):225-232.
Patimar R. 2008b. Fish species diversity in the lakes of Alma-Gol, Adji-Gol, and Ala-Gol, Golestan Province, northern Iran. Journal of Ichthyology, 48(10):911-917.
Patimar, R. 2009. Some biological parameters of silver crucian carp, Carassius auratus, in the international wetlands of Alma-Gol and Ala-Gol (Golestan Province, Iran). Iranian Journal of
Fisheries Sciences, 8(2):163-174.
Patimar R. and Abdoli A. 2011. Allometry variation in sturgeon fishes in southeastern Caspian Sea, its biological implications. The 4th International Symposium on Stock Enhancement and Sea
Ranching, 9th Asian Fisheries and Aquaculture Forum, Shanghai Ocean University, April 21 to 23, 2011 (abstract).
Patimar R., Abdoli A., Hasanzadeh Kiabi, B., Alahyari, S. and Naderi Jeloudar, M. 2009. Fish species diversity of the coastal areas in Gomishan Wetland. Journal of Agricultural Sciences and
Natural Resources, 16(Special Issue 1-A).
Patimar R., Abdoli A. and Kiabi, B. H.. 2008. Biological characteristics of the introduced sawbelly, Hemiculter leucisculus (Basilewski, 1855), in three wetlands of northern Iran: Alma-Gol,
Adji-Gol and Ala-Gol. Journal of Applied Ichthyology, 24(5):617-620.
Patimar R., Abdoli A. and Pesaraklou A. 2006. Feeding habits of Paracobitis malapterurus in some streams of eastern Alborz Mountain, Golestan Province, Iran.
3rd International Conference Loaches of the Genus Cobitis and Related Genera. Biology, Systematics, Genetics, Distribution, Ecology, Conservation. Sibenik,
Croatia, 24-29 September 2006 (title).
Patimar, R., Adineh, H. and Mahdavi, H. J. 2009. Life history of the western crested loach Paracobitis malapterura in the Zarrin-Gol River, east of
the Elburz mountains (northern Iran). Biologia (Section Zoology), Bratislava, 64(2):350-355.
Patimar, R., Amouei, M and Langaroudi, S. M. M.-A. 2010. Some life history pattern of northern spined loach Cobitis taenia in southern Caspian basin - Iran.
International Loach Conference 2010, 31 August to 3 September 2010, Prague, Czech Republic (poster).
Patimar, R., Chalanchi, M. G., Chamanara, V. and Naderi, L. 2010. Some life history aspects of Garra rufa (Heckel, 1843) in the Kangir River, western Iran. Zoology in the Middle East, 51:57-66.
Patimar, R., Ezzati, M. and Sarli, J. 2010. Life-history aspects of Caspian shemaya Alburnus chalcoides in two south
Caspian rivers (Siahroud and Gorganroud). Turkish Journal of Fisheries and Aquatic Sciences, 10(2):277-285.
Patimar, R., Igdrei, S., Hamid-Sales, B and Ba-Ensaf, S. 2002a. Two alien species in the International Wetlands, Alma-Gol, Adji-Gol and Ala-Gol,
of Golestan Province, Iran. World Congress on Aquatic Protected Areas, Cairns, 14-17 August 2002 (abstract).
Patimar, R., Igdrei, S., Hamid-Sales, B and Ba-Ensaf, S. 2002b. Two alien species in the International Wetlands, Alma-Gol, Adji-Gol and Ala-Gol, of Golestan Province, Iran
(a new record).Australian Society for Fishery Biology Annual Conference, 2002 (abstract).
Patimar, R. and Kiabi, B. H. 2005. Alien fish species, implications for conservation and management programmes; a case study of wetlands of Golestan
province (north of Iran). Borok II, International Workshop, Invasions of Alien Species in Holarctic, 27 September-1 October 2005, Borok (abstract).
Patimar, R., Kiabi, B., Salnikov, N. and Kamali, A. G. 2005. Univariate and multivariate analysis of the morphological variability among roach populations (Rutilus
rutilus caspicus) from Gomishan, Adji-Gol and Alma-Gol wetlands. Journal of Agricultural Sciences and Natural Resources, 11(4):163-174. In Farsi.
Patimar, R. and Kiabi, B. H. 2002a. Fish species diversity and abundances in the International Wetlands, Alma-Gol, Adji-Gol and Ala-Gol of Golestan Province, Iran. World
Congress on Aquatic Protected Areas, Cairns, 14-17 August 2002 (abstract).
Patimar, R. and Kiabi, B. H. 2002b. Fish species diversity and abundances in the International Wetlands, Alma-Gol, Adji-Gol and Ala-Gol of
Golestan Province, Iran. Australian Society for Fishery Biology Annual Conference, 2002 (abstract).
Patimar, R. and Kiabi, B. H. 2003. Fish species diversity in the International Wetlands, Alma-Gol, Adji-Gol, and Ala-Gol of Golestan Province, North of Iran. 3rd Annual
Conference 2003 Ecology Society,Halle/Saale, Germany, September 8 - 12, 2003 (title).
Patimar, R. and Kiabi, B. H. 2004a. Fish species diversity in the international wetlands, Alma-Gol, Adji-Gol and Ala-Gol, of Golestan Province, north of Iran. Societas
Internationalis Limnologiae (SIL) XXIX Congress, Lahti, Finland, 8-14 August 2004 (title).
Patimar, R. and Kiabi, B. H. 2004b. Fish species diversity in the international wetlands, Alma-Gol, Adji-Gol and Ala-Gol, of Golestan Province, north of Iran. The XIXth
International Congress of Zoology, August 23-27, 2004, Beijing (title).
Patimar, R., Mahdavi, M. J. and Adineh, H. 2008. Biology of sand goby Neogobius fluviatilis pallasi (Berg 1916) in Zarrin-Gol River (east Alborz Mountain). Journal of Agricultural Sciences
and Natural Resources, 15(1):54-64. In Farsi.
Patimar, R. and Mohammadzadeh, B. 2011. On the biological characteristics of Capoeta fusca Nikolskii, 1897 in eastern Iran. Journal of Applied Ichthyology, 27(3):873-878.
Patimar, R., Mortazaei, R. K. and Mollamohammad, Z. S. 2010. On the biological characteristics of a Kessler's loach Schistura kessleri (Günther, 1889)
in the northeastern Iran. International Loach Conference 2010, 31 August to 3 September 2010, Prague, Czech Republic (abstract).
Patimar, R., Nadjafypour, E., Yaghouby, M. and Nadjafy, M. 2010. Reproduction characteristics of a stunted population of rudd, Scardinius erythrophthalmus (Linnaeus, 1758) living in the Anzali
Lagoon (the southwest Caspian Sea, Iran). Journal of Ichthyology, 50(11):1060-1065.
Patimar, R., Najafabadi, M. H. and Souraki, M. G. 2010. Life history features of the nonindigenous three-spined stickleback (Gasterosteus aculeatus Linnaeus, 1758) in the Gomishan wetland
(southeast Caspian Sea, Iran). Turkish Journal of Zoology, 34(4):461-470.
Patimar, R., Ownagh, E., Jafari, N. and Hosseini, M. 2009. Intrabasin variation in allometry coefficients of Lenkoran Capoeta capoeta gracilis (Keyserling, 1861) in
the Gorganroud basin, southeast Caspian Sea. Journal of Applied Ichthyology, 25(6):776-778.
Patimar, R., Rishkhori, K. M., Zamani, S. M. 2010. On the biological characteristics of a Kessler’s loach Schistura kessleri (Günther, 1889) in the northeastern Iran. International Loach
Conference 2010, 31 August to 3 September 2010, Prague, Czech Republic (abstract).
Pavlinov, I. Ya. and Borissenko, A. V. (Eds.). 2001. Types of Vertebrates in the Zoological Museum of Moscow University. Sbornik Trudov Zoologicheskogo Muzeya MGU (Archives
of the Zoological Museum of Moscow State University), 41:1-250 (In Russian and English).
Pavlov, D. S., Reshetnikov, Yu. S., Shatunovskiy, M. I. and Shilin, N. I. 1985. Rare and disappearing fishes in the USSR and the principles of their inclusion in the "Red Book".
Journal of Ichthyology, 25(1):88-99.
Pavlov, D. S., Ruban, G. I. and Sokolov, L. I. 2001. On types of spawning migrations in sturgeon fishes (Acipenseriformes) of the world fauna. Journal of Ichthyology, 41(supplement 2):S225-S236.
Pavlov, D. S., Savvaitova, K. A., Sokolov, L. I. and Alekseev, S.S. 1994. Redkie i Ischezayushchie Zhivotnye. Ryby [Rare and vanishing animals. Fishes]. Vysshaya Shkola, Moskva. 334 pp., 16 pls.
Pavlov, D. S. and Vilenkin, B. Ya. 1989. Present state of the environment, biota, and fisheries of the Volga River, p. 504-514. In: Dodge, D. P. (Ed.).
Proceedings of the International Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Sciences, 106:v + 629 pp.
Payanda Langarudi, M. 1987. Farhang-e Gil o Deylam: Farsi ba Gilaki. Tehran.
Paykan Heyrati, F. and Dorafshan, S. 2007. Study on the potency of Domperidone and Metoclopramide for spawning induction in kutum (Rutilus
frisii kutum). Iranian Journal of Fisheries Sciences, 6(2):105-118.
Paykan Heyrati, F., Mostafavi, H., Mojazi Amiri, B., Hajizadeh, A. and Dorafshan, S. 2001. Induced spermiation of rainbow trout, Oncorhynchus mykiss,
using a GnRH analogue. Iranian Journal of Fisheries Sciences, 3(2):95-108, 128-129.
Paykan Heyrati, F., Mostafavi, H., Toloee, H. and Dorafshan, S. 2007. Induced spawning of kutum, Rutilus frisii kutum (Kamenskii, 1901) using (D-ala6,
Pro9-NEt) GnRHa combined with domperidone. Aquaculture, 265(1-4):288-293.
Pazhand, Z. A., Esmaeili Sari, A and Piri Zirkouhi, M. 2003. Effect of diazinon in the Persian sturgeon and stellate sturgeon. Pajouhesh va Sazandegi, 16(1)(58):64-67. In Farsi.
Pazira, A., Abdoli, A., Kouhgardi, E. and Yousefifard, P. 2005. Age structure and growth of the Mesopotamian spiny eel, Mastacembelus mastacembelus (Banks &
Solander in Russell, 1974) (sic) (Mastacembelidae), in southern Iran. Zoology in the Middle East, 35:43-47.
Pazooki, J. 1996. A faunistical survey and histopathological studies on freshwater fish nematodes in Iran and Hungary. Ph.D. Thesis, Veterinary Medical Research Institute,
Hungarian Academy of Sciences. 111 pp.
Pazooki, J. 2006. Infections of three species of Barbus fishes by helminth parasites in Tadjan and Zaremrood rivers, Mazandaran province, Iran. Pajouhesh va Sazandegi, 59:80-85. In Farsi.
Pazooki, J., Abtahi, B. and Rezaei, F. 2009. Determination of heavy metals (Cd, Cr) in the muscle and skin of Liza aurata from the Caspian Sea (Bandar Anzali).
Environmental Sciences, 7(1):21-31.
Pazooki, J. and Aghlmandi, F. 1998. Dichelyne minutus Rudolphi, 1819 (Nematoda: Cucullanidae) infection in Neogobius fluviatilis and Neogobius kessleri from southern part
of Caspian Sea. Iranian Journal of Fisheries Sciences, 7(2):31-38, 4. In Farsi.
Pazooki, J. and Aghlmandi, F. ?. Occurrence of Asymphylodora kubanicum in Vimba vimba (southern Caspian Sea, Iran). Virtual, 1(1). In Farsi.
Pazooki, J., Ghasemi, R. and Masoumian, M. 2003. Infections of three species of Barbus fishes by helminth parasites in Tadjan and Zaremrood Rivers, Mazandaran Province,
Iran. Pajouhesh-va-Sazandegi, 16(2)(59):80-85. In Farsi.
Pazooki, J., Ghobadian, M. and Masoumian, M. 2005. Identification of some parasites of freshwater fishes of Zanjan Province, northwest Iran. Iranian Scientific Fisheries Journal, 14(1):23-40. In Farsi.
Pazooki, J., Jalali Jafari, B. and Ghobadian, M. 2006. Monogenean species from freshwater fishes of Zanjan province, Iran. Iranian Journal of Fisheries Sciences, 6(1):103-112.
Pazooki, J. and Masoumian, M. 2001. Parasitic problems on sturgeons farmed in Iran. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Pazooki, J. and Masoumian, M. 2004. Cryptobia acipenseris and Haemogregarina acipenseris infections in Acipenser guldenstadti and A. persicus
in the southern part of the Caspian Sea. Journal of Agricultural Science and Technology, 6(3-4):95-101. In Farsi.
Pazooki, J., Masoumian, M. and Ghobadian, M. 2005a. Parasitological study on fresh water fishes of Zanjan Province, Iran. European Association of Fish Pathologists, 12th
International Conference, "Diseases of Fish and Shellfish', 11-16 September 2005, Copenhagen (title).
Pazooki, J., Masoumian, M. and Ghobadian, M. 2005b. Identification of parasites of some fishes in water resources of Zanjan Province. Iranian Scientific Fisheries Journal, 1:23-35.
Pazooki, J., Masoumian, M., Yahyazadeh, M. and Abbasi, J. 2007. Metazoan parasites from freshwater fishes of northwest Iran. Journal of Agricultural Science and Technology, 9(1):25-33.
Pazooki, J., Masoumian, M., Yahyazadeh, M. Y., Sadri Mehrabad, Gh. R. and Jalali, B. 2008. Monogenean parasites from fresh water fishes of northwestern Iran. Pajouhesh va Sazandegi, 77(4):17-25.
In Farsi.
Pazooki, J. and Molnár, K. 1998. Philometra karunensis sp. n. (Nematoda: Philometridae) from Barbus sharpeyi (Pisces) in
freshwaters of southwest Iran. Acta Veterinaria Hungarica, 46(4):465-472.
Pazooki, J., Sheidai, M. and Korani, M. M. 2008. A systematic and ecological study of Aphanius vladykovi Coad, 1988 (Actinopterygii: Cyprinodontidae) in Iran. Zoology in the Middle East,
43:85-89.
Pazwash, H. 1983. Iran's mode of modernization: Greening the desert, deserting the greenery. Civil Engineering, 53(3):48-51.
Pearce, F. 1984. Fall and rise of the Caspian Sea. New Scientist, 104(1433):10-11.
Pearce, F. 1993. Draining life from Iraq's marshes. New Scientist, 138(1869):11-12.
Pearce, F. 1995. How the Soviet seas were lost. New Scientist, 148(2003):38-42.
Pearce, F. 1997. Changing the game. New Scientist, 154(2085):14-15.
Pearce, F. 2001. Iraqi wetlands face total destruction. New Scientist, 170(2291):4-5.
Pearce, F. 2003. A high price to pay for caviar. New Scientist, 179(2413):6-7.
Pearce, F. 2004. Russia revives epic river plan. New Scientist, 181(2433):8-9.
Pearse, C. K. 1973. Qanats in the Old World: horizontal wells in the New. Journal of Range Management, 26(5):320-321.
Pellegrin, J. 1928. Les poissons des eaux douces d'Asie-Mineure. J.-B. Baillière et Fils, Paris (Extrait du tome second et dernier du Voyage
zoologique d'Henri Gadeau de Kerville en Asie-Mineure (Avril-Mai 1912)). 134 pp., 2 pls.
Pelz, G. R. 1987. Der Giebel: Carassius auratus gibelio oder Carassius auratus auratus? Natur und Museum, 117(4):118-129.
Peng, Z., Ludwig, A., Wang, D., Diogo, R., Wei, Q. and He, S. 2007. Age and biogeography of major clades in sturgeons and paddlefishes (Pisces:
Acipenseriformes). Molecular Phylogenetics and Evolution, 42(3):854-862.
Perdices, A. and Doadrio, I. 1997. Phylogenetic relationships and classification of the genera Cobitis and Sabanejewia (Cobitidae) based on
allozyme data. Ninth International Congress of European Ichthyologists (CEI9) "Fish Biodiversity". Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 71.
Perdices, A. and Doadrio, I. 2001. The molecular systematics and biogeography of the European cobitids based on mitochondrial DNA
sequences. Molecular Phylogenetics and Evolution, 19(3):468-478.
Perdices, A., Doadrio, I., Economidis, P. S., Bohlen, J. and Bănărescu, P. 2003. Pleistocene effects on the European freshwater fish fauna:
double origin of the cobitid genus Sabanejewia in the Danube basin (Osteichthyes: Cobitidae). Molecular Phylogenetics and Evolution, 26(2):289-299.
Perea, S., Böhme, M., Zupančič, P., Freyhof, J., Šanda, R., Özuluğ, M., Abdoli, A. and Doadrio, I. 2010. Phylogenetic relationships and
biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both miotochondrial and nuclear data. BMC Evolutionary
Biology, 10:265 (doi:10.1186/1471-2148-10-265).
Perera, J. 1989. Abandoned canal scheme saves the Caspian Sea. New Scientist, 121(1655):30.
Peritore, N. P. 1999. Third World Environmentalism. Case Studies from the Global South. University Press of Florida, Gainesville. xii + 328 pp.
Perkins, S. 2002. Once upon a lake. Science News, 162(18):283-284.
Perry, C. 1998. Medieval Arab fish: fresh, dried and dyed, p. 229-233. In: Walker, H. (Ed.). Fish. Food from the Waters. Proceedings of the Oxford
Symposium on Food and Cookery 1997. Prospect Books, Totnes, Devon. 335 pp.
Pesaraklou, A. R. 2006. Investigation of feeding habits of Nemacheilus malapterurus in the Chelchai River. B.Sc. Thesis, Gorgan University, Gorgan. 56 pp. In Farsi.
Peterson, D., Vecsei, P. and Hochleithner, M. 2009. Threatened fishes of the world: Acipenser ruthenus Linnaeus, 1758 (Acipenseridae). Environmental Biology of Fishes, DOI 10.1007/s10641-006-6659-1.
Pethiyagoda, R. 1991. Freshwater fishes of Sri Lanka. Wildlife Heritage Trust of Sri Lanka, Colombo. xiii + 362 pp.
Petr, T. 1987. Observations on prospects for further inland fisheries development in Iran. A Mission report for the project Training in Coldwater
Fish Culture. Technical Cooperative Programme, FAO, FI:TCP/IRA/6675, Field Document 2:vi + 71 pp.
Petr, T. 1999. Coldwater fish and fisheries in Afghanistan, p. 138-148. In: Petr, T. (Ed.). Fish and fisheries at higher altitudes. Asia. Food
and Agriculture Organization, Rome, Fisheries Technical Paper, 385:v + 304 pp.
Petr, T. and Marmulla, G. 2002. Report of the FAO expert consultation on the use of irrigation systems for sustainable fish production in arid
countries of Asia, Almaty, Kazakhstan, 25-29 September 2001. Food and Agriculture Organization, Rome, Fisheries Report, 679:ix + 22 pp.
Petrov, V. V. 1926. K poznaniyu kavkazskikh ukleyek (genus Alburnus Heck.). [To the knowledge of the Caucasian bleaks (genus Alburnus Heck)]. Izvestiya Bakinskoi Ikhtiologicheskoi Laboratorii,
2(1):133-160.
Petrov, V. V. 1930. Die geographische Variabilität von Alburnus alburnus L. Zoologischer Anzeiger, 88:141-150.
Peyghan, R. 1994. Treatment of ichthyophthiriasis in Barbus sharpeyi in Khouzestan Province. Iranian Fisheries Scientific Journal, 3(2):17-24, 2. In Farsi.
Peyghan, R. 2003. Effects of intraperitoneal and intramuscular injection of killed Aeromonas hydrophila on common carp lymphocytes. Aquarama, The 8th International Aquarium Fish
& Accessories Exhibition & Conference, Singapore, 30 Oct to 2 Nov 2003 (title).
Peyghan, R., Alboughobish, N., Pourmahdi Brojeni, M. and Rasekh, A. A. R. 2002. Study of some biological and histological factors of common carp, Cyprinus carpio, gonad in two culture season.
Pajouhesh va Sazandegi, 15(2)(55):16-23. In Farsi.
Peyghan, R. and Azary Takami, G. 2002. Histopathological, serum enzyme, cholesterol and urea changes in experimental acute toxicity of ammonia in common
carp Cyprinus carpio and use of natural zeolite for prevention. Aquaculture International, 10(4):317-325.
Peyghan, R., Baniadam, A. and Amirdad, S. H. 2001. Using ketamine and xylazine hydrochloride for anaesthesia of common carp (Cyprinus carpio). Pajouhesh va Sazandegi, 14(3)(52):18-19.
In Farsi.
Peyghan, R., Mesbah, M., Rasekh, A. and Mohammadi Dehchashmeh, M. 2002. The investigation on deleterious effect of malachite green on hatching eggs of silver carp (Hypophthalmichthys
molitrix). Iranian Veterinary Journal, 5(8):67-73. In Farsi.
Peyghan, R., Mesbah, M. A., Mohammadi Dehchashmeh, M. and Rasekh, A. 2006. Teratologic and gross pathological effect of malachite green bath on the eggs and larvae of silver carp
(Hypophthalmichthys molitrix). Shahid Chamran University Journal of Science, 15(B):27-34. In Farsi.
Peyghan, R., Nabavi, L. and Hosseini, M. R. 2001. The prevalence of helminth parasites in intestines of Capoeta trutta, Barbus barbulus and Barbus grypus in Khorramabad
rivers. Iranian Veterinary Journal, 4(7):55-66. In Farsi.
Peyghan, R., Parvar, N., Esmaeilzadeh, S. and Hoghoughirad, N. 2001. Histopathological survey of infection of Barbus grypus gills by Myxobolus karuni in Karun Rriver. Iranian Journal
of Veterinary Research, 2(1):63-70.
Peyghan, R., Raazi Jalali, M. and Dastour Nejad, F. 2003. Study of normal
serum enzymes (ALT, AST, ALP, LDH) levels in common carp, grass carp and silver
carp. Pajouhesh va Sazandegi, 16(1)(58):90-93. In Farsi.
Phillips, T. 1985. My experience with Aphanius mento. Journal of the American Killifish Association, 18(2):52.
Pietschmann, V. 1912. Eine neue Mugil-Art aus dem Schatt el Arab. Anzeiger der Akademie der Wissenschaften, Wien, Mathematische-naturwissenschaftliche Klasse, 49(17):268-270.
Pietschmann, V. 1913. Eine neue Glyptosternum-Art aus dem Tigris. Anzeiger der Akademie der Wissenschaften, Wien, Mathematische-naturwissenschaftliche Klasse, 50(8):93-95.
Pietschmann, V. 1940. Durch Kurdische Berge und Armenische Städte. Tagebuch der Armenischen Expedition 1914. Wien.
Pikitch, E.K. 2001. Give sturgeon a break. Caviar emptor - let the connoisseur beware. Wildlife Conservation, 104(1):10.
Pillay, S. R. 1972. A bibliography of the grey mullets Family Mugilidae. Food and Agriculture Organization Fisheries Technical Paper, 19:v + 99 pp.
Pillay, S. R. and Rosa, H. 1963. Synopsis of biological data on hilsa Hilsa ilisha (Hamilton) 1822. Food and Agriculture Organization, Rome, Fisheries Biology Synopsis, 25:iv + 61 pp.
Pinchuk, V. I. 1968. Novye dannye po sistematike bychkov grunny Ponticola Iljin v svyazi s problemoi vnyutrividovoi izmenchivosti i vidoobrazovaniya
[New data on the systematics of gobies of the Ponticola Iljin group in connection with the problem of intraspecific variability and species formation]. Voprosy Ikhtiologii, 8(4)(51):619-627.
Pinchuk, V. I. 1974. New data on Rhinogobius pflaumi and Rhinogobius similis similis in the Maritime Territory. Journal of Ichthyology, 14(3):373-377.
Pinchuk, V. I. 1976. Systematics of the goby genera Gobius Linné (native species), Neogobius Iljinu and Mesogobius Bleeker. Journal of Ichthyology, 16(4):543-552.
Pinchuk, V. I. 1977. The systematics of gobies of the genera Gobius Linné (native species), Neogobius Iljin and Mesogobius Bleeker. Journal of Ichthyology, 17(4):517-525.
Pinchuk, V. I. 1978. Notes and supplements to the Family Gobiidae in the book by Lindberg and Krasyukova "Fish of the Sea of Japan and the neighbouring
parts of the Sea of Okhotsk and the Yellow Sea", Part 4, 1975 with a description of a new species Chaenogobius taranetzi. Journal of Ichthyology, 18(1):1-14.
Pinchuk, V. I. 1980. The lateral-line system of Caspiosoma caspium (Kessler, 1877) and the systematic status of Asra turcomanus Iljin, 1941. Journal of Fish Biology, 17:231-235.
Pinchuk, V. I. and Rahimov, D. B. 1979. Novyi vid pugolovki - Benthophilus svetovidovi Pinchuk et Rahimov, sp. n. (Pisces, Gobiidae) iz kaspiiskogo
morya i opredelitel'naya tablitsa vidov roda Benthophilus [Benthophilus svetovidovi Pinchuk et Rahimov sp. n. (Pisces, Gobiidae) from the Caspian
Sea and a key to the species of the genus Benthophilus]. Zoologicheskii Zhurnal, 58(1):515-519.
Pinchuk, V. I. and Ragimov, D. B. 1985. Sistema bokovoi linii dvukh endemichnykh vidov bychkov kaspiiskogo morya (Perciformes, Gobiidae) [The
lateral line system in two endemic species of gobies of the Caspian Sea]. Zoologicheskii Zhurnal, 64(4):562-567.
Pinchuk, V. P. (sic). 1991. Species groupings in the genus Neogobius (Perciformes). Journal of Ichthyology, 31(7):1-15.
Pinkerton, J. (Ed.). 1758-1826. A General Collection of the Best and Most Interesting Voyages and Travels in All Parts of the World; many of
which are now first translated into English, digested on a new plan. Longman, Hurst, Rees, Orme, and Brown, and Cadell and Davies, London. 17 volumes.
Pipoyan, S. Kh. 1996a. Pseudorasbora parva (Cypriniformes, Cyprinidae) in water bodies of the Ararat Plain (Armenia). Journal of Ichthyology, 36(7):536-538.
Pipoyan, S. Kh. 1996b. Bitterling Rodeus (sic) sericeus amarus, a new species in the fauna of Armenia. Journal of Ichthyology, 36(8):676-678.
Pipoyan, S. Kh. 1998. Gobio gobio (L.) (Cyprinidae), a new species of fauna in Armenia. Journal of Ichthyology, 38(6):441-446.
Pipoyan, S. Kh. and Arakelyan, M. S. 1999. Acanthalburnus microlepis in water bodies of Armenia. Journal of Ichthyology, 39(9):726-731.
Pipoyan, S. Kh. and Kirakosyan, L. A. 1998. The Azerbaijan bleak (Alburnus charustini (sic) hohenackeri Kessler) (Cyprinidae) in Armenian waters. Journal of Ichthyology, 38(7):547-551.
Pipoyan, S. Kh. and Rukhkyan, R. G. 1998. Reproduction and development of Carassius auratus gibelio in water bodies of Armenia. Journal of Ichthyology, 38(5):374-379.
Pipoyan, S. Kh. and Tigranyan, E. A. 2002. Recent ichthyofauna of Armenia. Journal of Ichthyology, 42(8):575-578.
Piri, H., Keyvan, A., Piri, M. and Yelghi, S. 2007. Identification and biological characteristics of Gobiidae in coastal area of the southern Caspian Sea (Guilan Province). Iranian Scientific Fisheries
Journal, 16(2):19-28. In Farsi.
Piri, M. and Ordog, V. 1999. Effects of chemical herbicides and insecticides on mortality and feeding of silver carp (Hypophthalmichthys molitrix).
Iranian Journal of Fisheries Sciences, 1(2):11-21, 78.
Piri, M., Nezami, Sh. and Ordog, V. 1999. Effects of Diazinon, Malathion, Machete and Saturn on mortality of fingerling of Rutilus frisii kutum.
Iranian Journal of Fisheries Sciences, 7(4):9-18, 2. In Farsi.
Pirnia, H. 1951. Experience in integrated development of a river basin. Excerpt from an economic report on the conservation and utilization of
the natural resources of Iran (with reference to the Iranian seven-year plan for reconstruction and development), pp. 162-165. Proceedings of the
United Nations Scientific Conference on the Conservation and Utilization of Resources. 17 August - 6 September 1949, Lake Success, New York. Department
of Economic Affairs, United Nations, New York. 466 pp.
Piroozi, F. and Tavakoli, M. 1996. Survey of the ecosystem of Pol-e Dohktar wetlands. Abzeeyan, Tehran, 7(9):12-16. In Farsi.
Pivnička, K. 1970. Morphological variation in the burbot (Lota lota) and recognition of the subspecies: A review. Journal of the Fisheries Research Board of Canada, 27(10):1757-1765.
Platt, A. 1995. Reversing our path to destruction (Goodbye to the black pearls of the Caspian?). People & the Planet, 4(2):6-9.
Plattner, F. 1955. Über den Salzgehalt des Urmia-Sees. Petermanns Geographische Mitteilungen, 99:276-278.
Plaut, I. 1999. Effects of salinity on swimming performance, routine activity and resting metabolic rate on the euryhaline killifish, Aphanius dispar. Poster presented at the
Fifth International Congress of Comparative Physiology and Biochemistry, Calgary, Alberta, 23-28 August 1999.
Podsevalov, V. N. 1935. Khimicheskii sostav otdel'nykh chastei tela ryb yuzhnogo poberezh'ya Kaspiya [Chemical composition of various parts
of fish off the South Caspian coast]. Izvestiya Astrakhanskogo Otdeleniya Nauchno-Issledovatel'skogo Instituta Rybnoi Promyshlennosti, 2:241-260. (not seen).
Pohlman, J. W. and Naismith, J. M. 1996. Geographic information systems in action. Quarterdeck, College Station, Texas, 4(3):6 pp. (internet version).
Polat, N. and Gümüş, A. (K.). 1995. Age determination and evaluation of precision using five bony structures of the brond-snout (sic) (Chondrostoma regium
Heckel, 1843). Turkish Journal of Zoology, 19(4):331-335.
Polat, N., Gümüş, A. and Kandemir, S. 1999. Kababurun baliğı (Chondrostoma regium (Heckel, 1843))'nda yaş halkasıoluşumu [Annulus formation
in Tigris-nase (Chondrostoma regium (Heckel, 1843))]. Turkish Journal of Zoology, 23, supplement 3:959-964.
Polat, N. and Işik, K. 1995.Altınkaya Baraj Gölündeki Siraz Balığı (Capoeta capoeta Guldenstaedt, 1773)'nin
Yaş Belirleme Yöntemleri ile Büyüme Özellikleri [Ageing methods and growth rates of siraz balığı (Capoeta capoeta Guldenstaedt, 1773) in
Altınkaya Dam Lake]. Turkish Journal of Zoology, 19(3):265-271.
Polyaninova, A. A., Kashentseva, L. N., Malinovskaya, L. V., Molodtsova, A. I. and Smirnova, L. V. 1999. Feeding conditions for sturgeons (Acipenseridae)
in the Caspian Sea. Journal of Applied Ichthyology, 15(4-5):293-294.
Pooramini, M., Kamali, A., Hajimoradloo, A., Alizadeh, M. and Ghorbani, R. 2009. Effect of using yeast (Saccharomyces cerevisiae) as probiotic on growth parameters, survival and carcass
quality in rainbow trout Oncorhynchus mykiss fry. International Aquatic Journal, 1:39-44.
Poorgholam, R., Sedov, V., Yermalchev, V., Besharat, K. and Fazli, H. 1996. Stock assessment of kilka fishes by hydro-acoustic method 1994-95. Final Report,
Mazandaran Fisheries Research Center, Sari. In Farsi.
Popova, O. A. and Sytina, L. A. 1977. Food and feeding relations of Eurasian perch (Perca fluviatilis) and pikeperch (Stizostedion
lucioperca) in various waters of the USSR. Journal of the Fisheries Research Board of Canada, 34:1559-1570.
Por, F. D. 1975. An outline of the zoogeography of the Levant. Zoologica Scripta, 4:5-20.
Por, F. D. 1987. The Levantine landbridge: historical and present patterns, p. 23-28. In: Krupp, F., Schneider, W. and Kinzelbach, R. (Eds.). Proceedings of the Symposium on the Fauna
and Zoogeography of the Middle East, Mainz, 1985. Beihefte zum Tübinger Atlas des Vorderen Orients, Reihe A (Naturwissenschaften), 28, Dr. Ludwig Reichert Verlag, Wiesbaden, 338 pp.
Por, F. D. and Dimentman, Ch. 1989. The Legacy of Tethys. An Aquatic Biogeography of the Levant. Monographiae Biologicae, 63:xi + 214 pp.
Pouralfashtomi, H. 2006. Rearing of beluga (Huso huso) in brackish water of the southern coastals of Caspian Sea. Iranian Fisheries Research Organization, Agriculture Research and Education
Organization, Ministry of Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Pourali, S., Khazab, M., Kiabi, B. and Sheidai, M. 2000. Karyological study of two populations of Capoeta capoeta from north Iran. Cytologia, 65(3):231-234.
Pourali Darestani, S., Bazyar Lakeh, A. A. and Hassan Zadeh Kiabi, B. 2006. A karyological study of Barbus capito, Barbus mursa and two
populations of Capoeta capoeta from northern Iran. Iranian Journal of Natural Resources, 58(4):831-842. In Farsi.
Pourali Fashtomi, H. R. and Mohseni, M. 2006. Survival and growth of larval and juvenile Persian sturgeon (Acipenser persicus) using formulated diets
and live food. Journal of Applied Ichthyology, 22(supplement S1):303-306.
Pourang, N. 1995. Heavy metal bioaccumulation in different tissues of two fish species with regards to their feeding habits and trophic levels.
Environmental Monitoring and Assessment, 35(3):207-219.
Pourang, N. 1996. Heavy metal concentrations in surficial sediments and benthic macroinvertebrates from Anzali wetland, Iran. Hydrobiologia, 331(1-3):53-61.
Pourang, N., Tanabe, S., Rezvani, S. and Dennis, J. H. 2005. Trace elements accumulations in edible tissues of five sturgeon species from the Caspian Sea.
Environmental Monitoring and Assessment, 100(1-3):89-108.
Pourashouri, P., Shabanpour, B., Auburg, P., Daghigh Rohi, J. and Shabani, A. 2009. An investigation of rancidity inhibition during frozen storage of wels catfish (Silurus glanis)
fillets by previous ascorbic and citric acid treatment. International Journal of Food Science and Technology, 44(8):1503-1509.
Pourashouri, P., Shabanpour, B., Daghigh Rohi, J. and Shabani, A. 2008a. Inhibitory effect of citric acid on rancidity of frozen catfish (Silurus glanis) cutlets. Iranian Journal of Fisheries
Sciences, 7(2 supplementary):215-228.
Pourashouri, P., Shabanpour, B., Daghigh Rohi, J. and Shabani, A. 2008b. Oxidative and hydrolytic rancidity of lipid on catfish (Silurus glanis) during frozen storage. Journal of Agricultural
Sciences and Natural Resources, 15(4):107-114. In Farsi.
Pourgholam, R. 1994. Surveying infection of sturgeons with Polypodium Hydriform (sic). Iranian Fisheries Scientific Bulletin, (5):13-20, 2. In Farsi.
Pourgholam, R., Malek, M. and Shamsi, Sh. 1995. Infection with Clinostomum complanatum in Capoeta capoeta in Shiroud River.
Proceedings of the 7th Congress on Food Industries, Tehran University, Tehran, Iran, 23-25 January 1995, p. 54-55.
Pourgholam, R. and Noruzy Moghadam, H. 1996. Hybridization in Salmonidae. Iranian Fisheries Scientific Journal, 4(4):46-54, 5. In Farsi.
Pourgholam, R. and Saeidi, A. 2000. Evaluation of some haematological variables of Acipenser persicus and Acipenser stellatus at different water temperatures. Iranian Journal of
Fisheries Sciences, 2(1). In Farsi.
Pourgholam, R., Sedov, V., Yermalchev, V., Besharat, K. and Fazli, H. 1996. Stock assessment of kilka fishes by hydro acoustic method, 1994-1995. Final
Report, Mazandaran Fisheries Research Centre. 125 pp. In Farsi.
Pourgholam, R., Soltani, M., Hassan, D. M., Esmaeili, F., Farhoomand, H. and Usefi, P. 2001. Evaluation of blood characteristics of grass carp (Ctenopharyngodon
idella) after exposure to organophospahte, diazinon. Iranian Journal of Fisheries Sciences, 3(2):1-18, 120-121.
Pourgholam, R., Soltani, M., Hassan, M. D., Ghoroghi, A., Nahavandi, R. and Pourgholam, H. 2006. Determination of diazinon LC50 in grass carp (Ctenopharyngodon
idella) and the effect of sublethal concentration of toxin on some hematological
and biochemical indices. Iranian Journal of Fisheries Sciences, 5(2):67-82.
Pourkazemi, M. 1996. Molecular and biochemical genetic analysis of sturgeon stocks from the South Caspian Sea. Ph. D. Thesis, University of Wales, Swansea. 260 pp.
Pourkazemi, M. 1997. Stock assessment and conservation of sturgeon stocks of the Caspian Sea. Iranian Fisheries Scientific Journal, 6(3):13-22, 3. In Farsi.
Pourkazemi, M. 2004. Management options to support endangered sturgeon stocks in enclosed seas. Program of the XIXth International Congress of Zoology, August 23-27, 2004, Beijing, China (title).
Pourkazemi, M. 2006a. Physiological principles in selecting suitable brood fish (male and female) for artificial reproduction in Acipenser stellatus. Iranian Fisheries Research
Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Pourkazemi, M. 2006b. Caspian Sea sturgeon conservation and fisheries: past present and future. Journal of Applied Ichthyology, 22(supplement S1):12-16.
Pourkazemi M., Asadian, H., Pazouki, J., Masoumian, M. and Ebrahimzadeh Mousavi, H. A. 2007. Introducing molecular markers for Lernaea cyprinacea and Lernaea ctenopharyngodoni using RAPD
technique. Iranian Scientific Fisheries Journal, 16(3):19-28. In Farsi.
Pourkazemi, M., Nazari, S. and Bakhshalizadeh, S. 2010. Karyotype analysis in white bream (Blicca bjoerkna transcaucasica) from north coast of Iran. Iranian Journal of Fisheries Sciences,
9(3):454-463.
Pourkazemi, M. and Skibinski, D. O. F. 2001. Allozyme and mtDNA study on population structure of stellate sturgeon (Acipenser stellatus) in Iran. 4th International Symposium on Sturgeon,
Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Pourkazemi, M., Skibinski, D. O. F. and Beardmore, J. A. 1997. Application of media d-loop region for the study of Russian sturgeon population
structure from Iranian coastline of the Caspian Sea. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (abstract).
Pourkazemi, M., Skibinski, D. O. F. and Beardmore, J. A. 1999. Application of mtDNA d-loop region for the study of Russian sturgeon population structure from Iranian coastline of the Caspian Sea.
Journal of Applied Ichthyology, 15(4-5):23-28.
Pourkazemi, M., Skibinski, D. O. F. and Beardmore, J. A. 2000. A preliminary study on phylogenetic relationship between five sturgeon species in the Iranian coastline of the Caspian Sea.
Iranian Journal of Fisheries Science, 2(1):1-12, 107-108.
Pousti, I and Seddigh Marvasti, A. 2000. Fish Histological Atlas. Tehran University Publishing. In Farsi.
Pravdin, I. F. 1927. Rutilus rutilus caspicus natio knipowitschi. Vobla iz Astrabadskogo zaliva [Rutilus rutilus caspicus natio knipowitschi.
The roach of the Astrabad Lagoon]. Sbornik v chest' Professora Nikolaya Mikhailovicha Knipovicha 1885-1925, Moskva, 1927:83-88. (not seen).
Prearo, M. I. and Ghittino, C. 1993. Case of Lipoid Liver Degeneration in rainbow trout cultured in Iran: evaluation of the different histological methods to detect ceroid.
Bollettino Societa Italiana di Patologia Ittica 5(12):46-53.
Pretzmann, G. 1973. Bericht über eine Sammelreise nach Iran und Anatolien 1970. Annalen des naturhistorischen Museums in Wien, 77:321-329.
Pretzmann, G. 1974. Bericht über eine Sammelreise nach Iran im Frühjahr 1972. Annalen des naturhistorischen Museums in Wien, 78:453-455.
Priem, F. 1908. Poissons fossiles de Perse (Mission de Morgan). In: Annales publiées sous la direction de J. Morgan, Paris, pp. 1-25, pl. I-III.
Prikhod'ko, B. I. 1979a. Relationship between abundance of young Caspian "anchovy kilka", Clupeonella engrauliformis, and water temperature. Journal of Ichthyology, 19(2):86-95.
Prikhod'ko, B. I. 1979b. Ecological features of the Caspian kilka (genus Clupeonella). Journal of Ichthyology, 19(5):27-37.
Prikhod'ko, B. I. 1981. Selective fishing for the Caspian "anchovy kilka", Clupeonella engrauliformis, using electric light. Journal of Ichthyology, 21(1):104-110.
Proffitt, F. 2004. U.N. stalls on sturgeon, to critics' dismay. Science, 303(5666):1955-1956.
Prokofiev, A. M. 2004. Phylogenetic relationships of the nemacheiline loaches (Teleostei: Balitoridae: Nemacheilinae). Integrated Scientific Journal, 8(100):71-74.
Prokofiev, A. M. 2007. Materials towards the revision of the genus Triplophysa Rendahl, 1933 (Cobitoidea: Balitoridae: Nemacheilinae): a revision of nominal taxa of Herzenstein
(1888) described within the species "Nemacheilus" stoliczkae and "N." dorsonotatus, with the description of the new species T.
scapanognatha sp. nova. Journal of Ichthyology, 47(1):1-20.
Prokofiev, A. M. 2009. Problems of the classification and phylogeny of Nemacheiline loaches of the group lacking the preethmoid I (Cypriniformes: Balitoridae: Nemacheilinae). Journal of Ichthyology,
49(10):874-898.
Prokofiev, A. M. 2010. Morphological classification of loaches (Nemacheilinae). Journal of Ichthyology, 50(10):827-913.
Prosek, J. 2003. Trout of the World. Stewart, Tabori and Chang, New York. 223 pp.
Proudlove, G. S. 1997a. A synopsis of the hypogean fishes of the world. Proceedings of the 12th International Congress of Speleology, La Chaux de Fonds, Switzerland, August 1997, 3:351-354.
Proudlove, G. S. 1997b. The conservation status of hypogean fishes. Proceedings of the 12th International Congress of Speleology, La Chaux de Fonds, Switzerland, August 1997, 3:355-358.
Proudlove, G. S. 2001. The conservation status of hypogean fishes. Environmental Biology of Fishes, 62(1-3):201-213.
Proudlove, G. S. 2006. Subterranean Fishes of the World. An account of the subterranean (hypogean) fishes described up to 2003 with a bibliography
1541-2004. International Society for Subterranean Biology, Moulis. xviii + 300 pp.
Pushbarnek, E. B. 1987. Characteristics of the spawning population of Caspian shad, Alosa caspia caspia, from the Dagestan Coast during 1980-1984. Journal of Ichthyology,
27(5):107-112.
Pyka, J., Bartel, R., Szczerbowksi, J. A. and Epler, P. 2001. Reproduction of gattan (Barbus xanthopterus Heckel), shabbout (Barbus grypus Heckel) and bunni (Barbus sharpeyi
Gunther) and rearing stocking material of these species. Archives of Polish Fisheries, 9(supplement 1):235-246.
Pyke, G. H. 2005. A review of the biology of Gambusia affinis and G. holbrooki. Reviews in Fish Biology and Fisheries, 15:339-365.
Pyke, G. H. 2008. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution, and Systematics, 39:171-191.
Q
Qasemi, A., Pourkazemi, M. and Kalbassi, M. R. 2006. Comparison of genetic variation of ship sturgeon (Acipenser nudiventris) in the southern
Caspian Sea and Ural River using PCR-RFLP. Iranian Journal of Fisheries Sciences, 14(4):151-164. In Farsi.
Qasim, H. H. and Niazi, A. D. 1975. The osteology of Barbus xanthopterus & B. sharpeyi with special reference to their lateral-line system
(Cyprinidae). Bulletin of the Natural History Research Centre, Baghdad, 6(1):73-77.
Qin, B. and Yu, G. 1998. Implications of lake level variations at 6 ka and 18 ka in mainland Asia. Global and Planetary Change, 18:59-72.
Quliyev, Z. M. 2006. Azәrbaycanda әmtәә balıqçılığı. Baku. 214 pp. In Azerbaijanian.
Qureshi, M. R. 1965. Common Freshwater Fishes of Pakistan. Government of Pakistan Press, Karachi. viii + 61 pp.
R
Raat, A. J. P. 1988. Synopsis of biological data on the northern pike Esox lucius Linnaeus, 1758. Food and Agriculture Organization, Rome, Fisheries Synopsis, 30, Rev. 2:ix + 178 pp.
Ráb, P. 1985. Karyotype of the Danube goby, Proterorhinus marmoratus (Pisces, Gobiidae). Folia Zoologia, Prague, 34(4):329-334.
Ráb, P., Karakousis, Y. and Peios, C. 1994. Karyotype of Silurus aristotelis with reference to the cytotaxonomy of the genus Silurus (Pisces, Siluridae). Folia Zoologia, Prague, 43(1):75-81.
Rabani, M. and Nourouzi, B. 2002. Study on the quality of output water from the Neka Power Station for using in carp culture. Pajouhesh va Sazandegi, 15(1)(54):29-33. In Farsi.
Rabbaniha, M. 1993a. Study of Cichlidae and introduction of a new genus and a new species Iranocichla hormuziensis (sic) (Cichlidae). Abzeeyan, Tehran, 4(4):20-29. In Farsi.
Rabbaniha, M. 1993b. Study of Cichlidae and introduction of a new genus and a new species from Iran Iranocichla hormuziensis (sic) (Cichlidae). Abzeeyan, Tehran, 4(5):24-27. In Farsi.
Rabbaniha, M. 1994. Introduction of a new species and genus of chichlids (sic) of Iran. Abzeeyan, Tehran, 5(1 & 2):54-65, IV. In Farsi.
Rabbaniha, M., Seyfabadi, S. J., Sharifpour, I. and Owfi, F. 2003. Abundance and diversity of fish larvae in the Farakeh creek-estuary area of Bushehr
Province. Iranian Journal of Marine Sciences, 2(4):39-48. In Farsi.
Radcliffe, W. 1926. Fishing from the Earliest Times. Second Edition. E. P. Dutton, New York. xxi + 494 pp.
Radda, A. 1972. Die Gattung Aphanius NARDO - eine Synopsis. Aquaria, St. Gallen, 19(1):125-129; (6):141-150.
Radde, G. 1899. Die Sammlungen des kaukasichen Museums im Vereine mit Special-Gelehrten Bearbeitet und Herausgegeben. Band I. Zoologie. Museum Caucasicum, Tiflis. I:520 pp., 24 pls.
Radmehr, B. and Wossughi, G. 1982. The osteological study of the scull (sic) of some fishes in Iran (Pomadasys, Lucioperca and Schizothorax).
Journal of the Veterinary Faculty of the University of Tehran, 38(4):51-69. In Farsi.
Rafiee, Gh. R and Saad, Ch. R. 2005. Effects of dietary zeolite on the growth of red tilapia (Oreochromis sp.) and lettuce (Lactuca sativa) in an aquaponic system. Iranian Journal of
Natural Resources, 58(2):363-371. In Farsi.
Rafiei (sic), Gh. R., Mirvaghefi, A. R., Rezaei Tavabe, K., Mohazi Amiri, B and Abd Alhay, H. 2009. The study of heavy metals copper, zinc, iron and manganese bioaccumulation in the eggs of
rainbow trout (Oncorhyunchus mykiss) during incubation period. Iranian Scientific Fisheries Journal, 17(4):57-66. In Farsi.
Ragimov, D. B. 1965. O rasprostranenii bychkov u zapadnogo poberezh'ya srednego i yuzhnogo Kaspiya [Concerning the distribution of gobies near
the western coast of the central and southern Caspian]. Doklady Akademii Nauk Azerbaidzhanskoi SSR, Baku, 21(12):47-50.
Ragimov, D. B. 1976. Benthophilus mahmudbejovi sp. n. (Pisces, Gobiidae) iz kaspiiskogo morya [Benthophilus mahmudbejovi sp. n.
(Pisces, Gobiidae) from the Caspian Sea]. Zoologicheskii Zhurnal, 55(8):1196-1200.
Ragimov, D. B. 1977. O rasprostranenii i chislennosti nekotorykh bychkovykh ryb u vostochnogo poberezh'ya srednego i yuzhnogo Kaspiya [Concerning the
distribution and population numbers of some gobies along the eastern coast of the central and southern Caspian]. Izvestiya Akademii Nauk Azerbaidzhanskoi SSR, 4:87-92.
Ragimov, D. B. 1978. On the systematic status of some species of the genus Benthophilus (Family Gobiidae) from the Caspian Sea and the Sea of Azov. Journal of Ichthyology, 18(5):701-708.
Ragimov, D. B. 1982. Novye podvidy Kaspiiskikh pugolovok (Gobiidae, Benthophilus) [New subspecies of Caspian gobies (Gobiidae, Benthophilus). Zoologicheskii Zhurnal, 61(1):47-55.
Ragimov, D. B. 1985a. Material on the reproduction of pugolovka goby, species of the genus Benthophilus, from the Caspian Sea. Journal of Ichthyology, 25(3):66-72.
Ragimov, D. B. 1985b. Some Caspian species of the genus Benthophilus (Gobiidae). Journal of Ichthyology, 25(6):153-161.
Ragimov, D. B. 1987. Reproduction of small goby species (Gobiidae) of the Caspian Sea. Journal of Ichthyology, 27(1):58-65.
Ragimov, D. B. 1995. Vnutrividovaya differentsiatsiya pugolovki Benthophilus grimmi Kessler iz Kaspiiskogo morya [Intraspeific forms of the pugolovki Benthophilus grimmi Kessler
in the Caspian Sea]. Izvestiya Akademii Nauk Azerbaidzhana, Seriya Biologicheskie Nauki, 1995(1-6):102-109.
Ragimov, D. B. 1997. Materialy po sistematike nekotorykh malochislennykh i redkikh vidov pugolovok Kaspiya [Materials on the systematics of some less known and rare species of bullheads
from the Caspian]. Izvestiya Akademii Nauk Azerbaidzhana, Seriya Biologicheskie Nauki, 1997 (1-6):68-75.
Ragimov, D. B. 1998a. Material po sistematike Kaspiiskikh podvidov bychka-ratana [Material on the systematics of the Caspian subspecies of ratan
gobies]. Izvestiya Akademii Nauk Azerbaidzhana, Seriya Biologicheskie Nauki, 1998(1-6):47-51.
Ragimov, D. B. 1998b. Dopolnenie k sistematike nekotorykh malochislennykh i redkikh vidov bychkov Kaspiya [Additions to the systematics of some rare and little known Caspian goby
species]. Izvestiya Akademii Nauk Azerbaidzhana, Seriya Biologicheskie Nauki, 1998(1-6):82-87.
Ragimov, D. B. 2003. Material po sistematike Kaspiiskikh vidov bychkov iz roda Knipowitschia. [Materials on the systematics of Caspian species of gobies
from the genus Knipowitschia]. Azerbaycan Milli Elmler Akademiyasinin Xeberleri, Biologiya Elmleri Seriyasi, 2003(3-4):100-105. In Azerbaijanian.
Rahanandeh, M. 2006. Reports on diplostomiasis in Gilan Province. Veterinary Department, Sciences and Research Branch, Islamic Azad University. 27 pp. In Farsi.
Rahbari, S. and Razavilar, V. 1982. Saprolegniosis of goldfish (Carassius auratus) in Iran. Journal of the Veterinary Faculty of the University of Tehran, 38(1):55-62. In Farsi.
Rahimabadi, E. Z., Mirdar, J., Elahi, M. Y., Arshadi, A. and Haidari, M. R. 2009. The lipid quality assessment of Shizothorax zarudnyi and Schizocypris altidorsalis by fatty acid
analysis. Pakistan Journal of Biological Sciences, 12(15):1090-1093.
Rahimibashar, M. R., Alipoor, V. Danesh, M. and Alinia, M. R. 2008. Survival of biometrical characteristics, diet, gonad and liver index of Sander lucioperca in the lake of Aras Dam. Pajouhesh
va Sazandegi, 79:58-65. In Farsi.
Rahimi, E. and Raeisi, M. 2009. Determination of lead and cadmium residual in meat of fishes caught from Choghakhor Lagoon in Chaharmahal and Bakhtiary Province. Iranian Veterinary Journal,
4(4)(21):79-83. In Farsi.
Rahmani, A. R. and Ehsani, H. R. 2006. Application of ion exchange and air stripping methods to remove ammonium in recirculation fish culture system effluents. Iranian Journal of Fisheries Sciences,
15(2):19-28. In Farsi.
Rahmani, H. 2006. Population dynamics and genetic variation of shemaya, Chalcalburnus chalcoides (Guldenstadt, 1772) in Haraz, Shirud and Gazafrud
rivers. Ph.D. Thesis, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan. 102 pp. In Farsi.
Rahmani, H. 2008. A study on populations of endangered species, shemaya, Chalcalburnus chalcoides in the Haraz and Shirud rivers. Journal of Environmental Studies, 34(46):129-138. In Farsi.
Rahmani, H. and Abdoli, A. 2008. Inter-population morphological diversity in Vimba vimba persa (Pallas, 1815) in Gorganrud River, Shirud river and Anzali Lagoon. Journal of Agricultural
Sciences and Natural Resources, 15(1):28-37. In Farsi.
Rahmani, H. and Hasanzadeh Kiabi, B. 2006. Inter-population morphological diversity in Chalcalburnus chalcoides (Gueldenstaedt, 1772) in Haraz and Gazafrud
rivers. Environmental Sciences, 3(10):21-33.
Rahmani, H., Hasanzadeh Kiabi, B., Kamali, A. and Abdoli, A. 2007. A study of morphological analysis of Chalcalburnus chalcoides (Gueldenstaedt, 1772) in Haraz River and Shirud River. Journal
of Agricultural Sciences and Natural Resources, 14(3):40-50. In Farsi.
Rahmani, H., Kazemi, B. Pourkazemi, M., Bandehpour, M., Naderi, J. M., Seyed, N. and Ataei, F. 2009. Genetic diversity of the shemaya (Chalcalburnus
chalcoides) populations in Haraz, Shirud and Gazafrud rivers using 18S RRNA gene and PCR-RFLP
method. Environmental Sciences, 6(3):43-52.
Rahmani, H., Kiabi, B., Kamali, A. A. and Abdoli, A. 2009. Some biological characteristics of shemaya Chalcalburnus chalcoides in Shirud River. Journal of Agricultural Sciences and Natural
Resources, 16(3):67-76. In Farsi.
Rahmani, I. and Golmohamaddi, G. H. 1991. A study of absolute fecundity of roach, Rutilus rutilus caspicus, in Gorgan-Rud River. B.Sc. Project, University of Gorgan, Gorgan. In Farsi.
Rahmati Leeshei, M., Abedian, A. A. M. and Nazari, R. M. 2007. The effect of phytase enzyme on apparent digestibility of four vegetative foods in rainbow trout (Oncorhynchus mykiss). Iranian
Scientific Fisheries Journal, 16(1):49-62. In Farsi.
Raikova, E. V. 2002. Polypodium hydriforme infection in the eggs of acipenserifrom fishes. Journal of Applied Ichthyology, 18(4-6):405-415.
Rainboth, W. J. 1981. Systematics of the Asiatic barbins (Pisces, Cyprinidae). Ph.D. Thesis, University of Michigan. ix + 253 pp.
Raiss-Tousi, A. 1999. Caspian Sea ecology at risk, Iranian expert warns. Sea Wind, 13(4):21-22.
Raissy, M. 2008. Parasites of Aphanius vladykovi (Coad 1988) in Gandoman Lagoon, Iran. 29th World Veterinary Congress, Vancouver, British Columbia, 27-31 July 2008 (title).
Raissy, M., Ansari, M., Lashkari, A. and Jalali, B. 2010. Occurrence of parasites in selected fish species in Gandoman Lagoon, Iran. Iranian Journal of Fisheries Sciences, 9(3):464-471.
Raissy, M., Ansari, M. and Jalali, B. 2010. Parasites of three species of Capoeta spp from Beheshtabad River, Iran. Journal of Veterinary Pathobiology, 1(1):38-43. In Farsi.
Raissy, M., Barzegar, M. and Jalai, B. 2007. Parasites of native and introduced fishes in Choghakhor Lagoon, Iran. World Aquaculture Society, San Antonio, Texas, February 26 - March 2, 2007 (abstract).
Raissy, M., Barzegar, M., Jalali, B. and Alimardani, K. 2006. A survey on monogenean parasites of gills of fishes in Chaghakhor Lake
and introducing Dactylogyrus spiralis. Iranian Journal of Veterinary Science, 1(3):411-418.
Raissy, M., Doosti, A. and Ansari, M. 2009. Cloning and sequencing of Its-1 region of the parasite Tylodelphys clavata (Von Nordman 1832). World Applied Sciences Journal, 7(3):355-357.
Raissy, M., Fadaeifard, F., Ansari, M., Tajizadegan, H. and Hosseini, R. 2010. Parasites of fish in Sooleghan Lagoon. Veterinary Journal of Islamic Azad University, Garmsar Branch, 5(1):143-148.
In Farsi.
Riassy, M., Rahimi, E., Ansari, M. and Ebadi, A. G. 2010. Determination of mercury and arsenic levels in fish caught in the Beheshtabad River, Chaharmahal and Bakhtiari Province, Iran.
Toxicological and Environmental Chemistry, 92(9):1627-1631.
Rajahpour, M., Malek, M., MacKenzie, K. and Aghlmandi, F. 2008. Helminth parasites of stellate sturgeon Acipenser stellatus Pallas, 1771 and Persian sturgeon Acipenser persicus Borodin,
1897 (Pisces: Acipenseridae) from the South-East Caspian Sea. Parasitology Research, 102(5):1089-1091.
RaLonde, R. 1970a. Report of the Nahang Roga migration study. Report of the Fisheries Research Institute, Bandar Pahlavi. MS.
RaLonde, R. 1970b. A short summary of sturgeon biology. Report of the Fisheries Research Institute, Bandar Pahlavi. MS.
RaLonde, R., Kinunen, W. and Walczak, P. 1970. Sardab River survey. Division of Research and Development, Iran Game and Fish Department, Tehran. MS.
RaLonde, R. and Razavi, B. 1972. The growth of the whitefish Rutilus frisii kutum of the southern Caspian Sea. Report of the Fisheries Research Institute, Bandar Pahlavi. MS.
(not seen).
RaLonde, R. and Walczak, P. 1970a. Stock assessment and composition of the commercial bony fishes of the southern Caspian Sea. Report of the Fisheries Research Institute, Bandar Pahlavi. 40 pp.
(not seen).
RaLonde, R. and Walczak, P. 1970b. Summary of the fisheries of Iran. Report of the Fisheries Research Institute, Bandar Pahlavi. MS, 9 pp., 5 tabs.
RaLonde, R. and Walczak, P. 1970c. Report on Lar River survey. Report of the Fisheries Research Institute, Bandar Pahlavi. MS, 11 pp., 5 tabs., 3 figs.
RaLonde, R. and Walczak, P. 1970d. Summary of the fisheries of Iran. Project 1. Commercial Fish Statistics. Fisheries Research Institute, Bandar
Pahlavi, Iran. MS, 5 pp., 5 tables, refs., 2 unnumbered pages. Revised 1971.
RaLonde, R. and Walczak, P. 1971a. Stock assessment and composition of the commercial bony fishes of the southern Caspian Sea. Report of the Fisheries Research Institute, Bandar Pahlavi. 76 pp.
(not seen).
RaLonde, R. and Walczak, P. 1971b. A short report on net selection of the Shilot beach seines. Report of the Fisheries Research Institute, Bandar Pahlavi. MS.
RaLonde, R. and Walczak, P. 1971c. A research proposal for studying the tyulka (Clupeonella sp.) populations of the southern Caspian Sea with notes on progress to date.
Report of the Fisheries Research Institute, Bandar Pahlavi. MS, 6 pp.
RaLonde, R. and Walczak, P. 1972. A review of Shilot's bony fishing policy from 1929-1972. Report of the Fisheries Research Institute, Bandar Pahlavi. MS, 10 pp., 3 tabs., 9 figs.
Ramezani, H. 2003. A survey of staying period and migration process of the Persian sturgeon, Acipenser persicus, fry after releasing into Tajan
River. Iranian Journal of Marine Sciences, 2(4):49-58. In Farsi.
Ramezani, R. 1998. Investigation on feeding habits of Nemacheilus malapterurus in the Madarsou stream-Golestan National Park. B.Sc. Thesis, Gorgan University, Gorgan. 56 pp. In Farsi.
Ramezani, R. 2009. Effects of different protein and energy levels on growth performance of Caspian brown trout, Salmo trutta caspius (Kessler, 1877). Journal of Fisheries and Aquatic Science,
4(4):203-209.
Ramin, M. 1996. Semiartificial propagation of Stizostedion lucioperca and rearing them to fingerling size.Iranian Fisheries Scientific Journal, 5(1):25-36, 4. In Farsi.
Ramin, M. 1997a. Artificial breeding of Abramis brama orientalis. Iranian Fisheries Scientific Journal, 6(1):23-32, 4. In Farsi.
Ramin, M. 1997b. Indentification (sic) of Babolrud River's fish fauna. Iranian Fisheries Scientific Journal, 6(3):59-72, 8. In Farsi.
Ramin, M. 1998. An investigation on sturgeon fish migration in Sepid Rud River. Iranian Journal of Fisheries Sciences, 7(3):21-32, 3. In Farsi.
Ramin, M. 1999. Artificial spawning of Esox lucius (pike), cultured fingerling size fish, providing bio-normative. Iranian Journal of Fisheries Sciences, 8(1):49-57, 6-7. In Farsi.
Ramin, M. 2009. A survey on ichthyofauna of Karkheh River basin. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 150 (abstract).
Rana, K. and Bartley, D. M. 1998. Iran promotes aquaculture development. FAO Aquaculture Newsletter, 19:26-30.
Randall, J. E. 1986. Sharks of Arabia. IMMEL Publishing, London. 148 pp.
Randall, J. E. 1995a. Ilisha compressa, a new species of clupeid fish from the Persian Gulf. Raffles Bulletin of Zoology, 42(4)(1994):893-899.
Randall, J. E. 1995b. Coastal Fishes of Oman. University of Hawai'i Press, Honolulu. xvi + 439 pp.
Randall, J.E., Allen, G.R. and Smith-Vaniz, W.F. 1978. Illustrated Identification Guide to Commercial Fishes. Food and Agricultural Organization, Rome, Regional Fishery Survey and
Development Project, Bahrain, Iran, Iraq, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates. FI: DP/RAB/71/278/3:v + 1-221.
Rasoli, -. 1992. Sturgeon, a suitable fish for fish culture. Abzeeyan, Tehran, 2:36-39. In Farsi.
Raspopov, V. M. 1987. Fecundity of beluga sturgeon, Huso huso, of the Caspian Sea. Journal of Ichthyology, 27(3):141-150.
Raspopov, V. M. 1993a. Age structure and population dynamics of the beluga, Huso huso, migrating into the Volga. Journal of Ichthyology, 33(3):105-112.
Raspopov, V. M. 1993b. Growth rate of Caspian Sea beluga. Journal of Ichthyology, 33(9):72-84.
Raspopov, V. M. and Dubinin, V. I. 1990. Fecundity of the winter and spring races of the Volga-Caspian beluga, Huso huso. Journal of Ichthyology, 30(4):152-159.
Rastorguev, S., Mugue, N., Volkov, A. and Barmintsev, V. 2008. Complete mitochondrial DNA sequence analysis of Ponto-Caspian sturgeon species. Journal of Applied Ichthyology, 24(supplement 1):46-49.
Rauchenberger, M. 1989. Systematics and biogeography of the genus Gambusia (Cyprinodontiformes: Poecilidae). American Museum Novitates, 2951:74 pp.
Rawi, A. H. A., Obaidi, S. and Zaki, S. 1975. Preliminary list of fishes from the Little Zab River. Iraqi Science Conference, pp. 1-7 (abstract).
Ray, W. J. 1997. Dispersal and habitat preference of the exotic round goby (Neogobius melanostomus). American Society of Ichthyologists and Herpetologists, 77th Annual Meeting,
June 26-July 2, 1997, University of Washington, Seattle, Washington. p. 247 (abstract).
Ray, W. J. and Corkum, L. D. 1997a. Habitat preference of the exotic round goby (Neogobius melanostomus) in the Great Lakes. International
Association for Great Lakes Research. Program & Abstracts, 40th Conference, June 1-5, 1997, Buffalo State College & University of Buffalo, New York, p. 102 (abstract).
Ray, W. J. and Corkum, L. D. 1997b. Predation of zebra mussels by round gobies, Neogobius melanostomus. Environmental Biology of Fishes, 50(3):267-273.
Ray, W. J. and Corkum, L. D. 2001.Habitat and site affinity of the round goby. Journal of Great Lakes Research, 27(3):329-334.
Raymakers, C. 2002. Study on the social and economic aspects of illegal fishing in the Caspian Sea. Caspian Environmental Programme, Baku, Azerbaijan. 47 pp., 17 annexes.
Raymakers, C. 2006. CITES, the Convention on International Trade in Endangered Species of Wild Fauna and Flora; its role in the conservation of Acipenseriformes. Journal of Applied Ichthyology,
22(s1):53-65.
Razavi, B. and Pour, N. H. 1979. Artificial spawning and culturing of Rutilus frisii kutum (Kamensky) in the year (1976-1977) 2535. Report of the Seminar on Fishery Technology Education July 11 -
August ?, Tehran. (not seen).
Razavi, B. A. 1999. Introduction to the Ecology of the Caspian Sea. Iranian Fisheries Research Organization, Tehran. 110 pp. In Farsi.
Razavi, S. 1991. Agrarian change in two regions of Kerman. Iran (Journal of the British Institute of Persian Studies), 29:161-179.
Razavi Sayad, B. 1993a. Morphometric and electrophoresis genetic markers with blood serum in mahisefid (Rutilus frisii kutum). M.Sc. Thesis, Islamic Azad University, Tehran. 115 pp. In
Farsi.
Razavi Sayad, B. 1993b. On kilka fishing status in the Caspian Sea. Iranian Fisheries Bulletin, 2:11-25, 2-3. In Farsi.
Razavi Sayad, B. 1995. Kutum fish. Iranian Fisheries Research Organization, Tehran. 165 pp. In Farsi.
Razavilar, V., Shodjai, A. H., Safari, R., Salmani, A. and Rostami, H. 2001. Study of microbial hazard analysis of Persian granular caviar during processing, storage and the processing environment in
Mazandaran region. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(1):81-87. In Farsi.
Razavilar, V., Tabatabayi, A. H., Gharagozlou, M. J. and Djalali, B. 1981. The study of red-sore disease in grass carp (Ctenopharyngodon idella)
in Iran. Journal of the Veterinary Faculty of the University of Tehran, 37(3):11-22. In Farsi.
Razavilar, V. and Tavakoli, H. R. 2006. A prevalence study of human toxigenic types of Clostridium botulinum (A, B, E) in some sea water fishes of northern (Rutilus frisii kutum and
Mugil auratus Risso) and southern (Otolithes ruber and Stromateus niger) regions of Iran. Journal of Veterinary Research, 61(1):39-42. In Farsi.
Razivi, B., RaLonde R. and Walczak, P. 1972. 1972 report on stock assessment and composition of the commercial bony fishes of the Southern Caspian Sea.
Report of the Fisheries Research Institute, Bandar Pahlavi. 32 pp.
Redding, T. A. and Midlen, A. B. 1991. Fish production in irrigation canals. A review. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 317:vii + 111 pp.
Regan, C. T. 1906. Two new cyprinoid fishes from the Helmand basin. Journal of the Asiatic Society of Bengal, new series, 2(1):8-9.
Regunathan, C. and Kitto, M. R. 2005. Persian Gulf fish culture in Iran - pointers for success. Aquaculture Asia Magazine, April-June 2005, pp. 40-42.
Rehbein, H. 1985. Caviare: Proximate composition, amino acid content and identification of fish species. Zeitschrift für Lebensmitteluntersuchung und -Forschung A, 180(6):457-462.
Rehbein, H., Molkentin, J., Schubring, R., Lieckfeldt, D. and Ludwig, A. 2008. Development of advanced analytical tools to determine the origin of caviar. Journal of Applied Ichthyology, 24(s1):65-70.
Reichenbacher, B., Feulner, G. R. and Schulz-Mirbach, T. 2009. Geographic variation in otolith morphology among freshwater populations of Aphanius dispar (Teleostei, Cyprinodontiformes)
from the southeastern Arabian Peninsula. Journal of Morphology, 270(4):469-84.
Reichenbacher, B., Kamrani, E., Esmaeili, H. R. and Teimori, A. 2009. The endangered cyprinodont Aphanius ginaonis (Holly, 1929) from southern Iran is a valid species: evidence from otolith
morphology. Environmental Biology of Fishes, 86(4):507-521.
Reichenbacher, B., Sienknecht, U., Küchenoff, H. and Fenske, N. 2007. Combined otolith morphology and morphometry for assessing taxonomy and diversity in
fossil and extant killifish (Aphanius, †Prolebias). Journal of Morphology, 268:898-915.
Reid, G. Mc. 1982. The form, function and phylogenetic significance of the vomero-palatine organ in cyprinid fishes. Journal of Natural History, 16(4):497-510.
Relyea, K. 1981. Inshore Fishes of the Arabian Gulf. George Allen & Unwin, London. 149 pp.
Renaud, C. B. 1982. Food and feeding habits of the Caspian lamprey after metamorphosis. Fourth Congress of European Ichthyologists, 20.-24.9.1982, Hamburg (abstract number 252).
Renaud, C. B., Gill, H. S. and Potter, I. C. 2009. Relationships between the diets and characteristics of the dentition, buccal glands and velar tentacles of the adults of the parasitic species of
lampreys. Journal of Zoology, 278(3):231-242.
Reshetnikov, Yu. S. 2002. Atlas presnovodnykh ryb rossii [Atlas of Russian Freshwater Fishes]. Nauka, Moscow. Volume 1:1-379, Volume 2:1-253.
Reshetnikov, Yu. S., Bogutskaya, N. G., Vasil'eva, E. D., Dorofeeva, E. A., Naseka, A. M., Popova, O. A., Savvaitova, K. A., Sideleva, V. G. and Sokolov, L. I. 1997. An annotated
check-list of the freshwater fishes of Russia. Journal of Ichthyology, 37(9):687-736.
Reshetnikov, Yu. S. and Shakirova, F. M. 1993. Zoogeograficheskii analiz ikhtiofauny srednei Asii po spiskam presnovodnykh ryb [Zoogeographical analysis of the ichthyofauna of Central
Asia by lists of freshwater fish]. Voprosy Ikhtiologii, 33(1):37-45.
Reyhani, S., Rezvani Gilkolaee, S., Mostafavi, P. G., Fazli, H., Porkazemi, M. and Golestani N. 2010. Microsatellite markers indicate a different structure among three populations of the Caspian roach,
Rutilus rutilus caspicus (Jakowlew, 1870), in the Caspian Sea. Zoology in the Middle East 50:67-73.
Reza Mojtabavi, H. 1996. Sustainable development and fisheries management in Islamic Republic of Iran. In: Second World Fisheries Conference, Brisbane, Australia, 28 July-2
August 1996 (abstract).
Rezaei, J. and Darvishi, B. 2007. Economic evaluation of trout fish farming in Ilam Province. Pajouhesh va Sazandegi,
20(3)(76):150-160. In Farsi.
Rezaei, M. 1996. Research on feeding of grass carp (Ctenopharyngodon idella) with emphasis on chemical composition of plants. M.Sc. Thesis of Fishery Department, College of Marine Science,
Tarbiat-e Modares University, Tehran. 94 pp. In Farsi.
Rezaei, M. 1998b. Determination of Artemia nauplii food conversion in Acipenser stellatus larvae culture. First National Symposium on Sturgeon. Rasht, Iran. (abstract). In Farsi.
Rezaei, M. and Ahmadi. M. R. 2000. Comparison of food value of different plants in grass carp (Ctenopharyngodon idella) culture.
The First Iranian Congress of Fish Health and Diseases, Ahvaz, Iran (abstract). In Farsi.
Rezaei, M., Javadian Seyed, R. E. and Sahari, M. A. 2007. Sensory evaluation and lipid quality of grass carp (Ctenopharyngodon idella) stored in ice. Iranian Journal of Natural Resources,
60(2):545-553. In Farsi.
Rezaei, M., Montazeri, N., Langrudi, H. E., Mokhayer, B., Parviz, M. and Nazarinia, A. 2007. The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss)
stored in ice. Food Chemistry, 103(1):150-154.
Rezaei, M., Nazari, R. M. and Kalbassi, M. R. 1998. Identification of Artemia (nauplii), nutritional comparison and food conversion
rate of Acipenser stellatus larvaea culture. Fishery Department, College of Marine Science, Tarbiat-e Modares University, Tehran. 49 pp. In Farsi.
Rezaei, M., Nazari, R. M. and Kalbassi, M. R. 2000. Application and nutrition value of Artemia parthnogenitica nauplii in feeding of fry of sturgeons (Acipenser stellatus). Journal of
Pajouhesh Va-Sazadegi, 13(2):120-123. In Farsi.
Rezaei, M., Nikfetrat, E. and Khaleghi, R. 1993a. Role of aeration in intensive carp culture. B.Sc. Thesis of College of Natural Resources, Tehran University, Tehran. 148 pp. In Farsi.
Rezaei, M., Nikfetrat, E. and Khaleghi, R. 1993b. Role of aeration in intensive carp culture. Fishery Organization, Ministry of Jihad-e-Sazendegi, Tehran. 148 pp. In Farsi.
Rezaei, M., Sahari, M. A., Moini, S., Safari, M. and Ghafari, F. 2003. Comparative study on lipid quality on anchovy kilka (Clupeonella
engrauliformis) under two temporary chilled transport storage methods. Iranian Journal of Fisheries Sciences, 12(3):97-108. In Farsi.
Rezaei, M., Sahari, M. A., Moini, S., Safari, M., Rezaiean, M. and Ghafari, F. 2002. Some qualitative characteristics of lipid in anchovy kilka, (Clupeonella
engrauliformis), during frozen storage. Iranian Journal of Marine Sciences, 1(4):55-64, 141. In Farsi.
Rezaei, M. M., Kamali, A. A., Hasanzadeh Kiabi, B, and Shaabani, A. 2007. A survey of age, growth and reproduction of Capoeta capoeta gracilis of the Madarsoo River, Golestan National Park,
north-east Iran. Iranian Scientific Fisheries Journal, 16(2):63-74. In Farsi.
Rezaei, M. M., Kamali, A. A., Kiabi, B. and Rahman, H. 2008. Distribution, diversity and abundance of fish species in the Madarsoo River, Golestan National Park, Iran. Iranian Scientific
Fisheries Journal, 17(3):65-76. In Farsi.
Rezaei Tavabai, K., Zare Chahouki, M. A., Yazdanpanah, A. and Vazirzadeh, A. 2009. Limnological and pollution study of Shahdadroud River, Kerman Province. Biaban, Tehran, 14(1):21-26. In Farsi.
Rezavi, S. et al. 1997. Breeding and rearing of the Black Sea roach in the Islamic Republic of Iran. The First Congress of Ichthyologists of Russia, Book of Abstracts, VNIRO Press, Moscow, p. 452.
Rezvani, S., Eimanifar, A., Aghili, R. and Laloei, F. 2006. PCR-RFLP analysis of mitochondrial DNA for identification of Rutilus rutilus caspicus
populations on the southern coast of the Caspian Sea, Iran. Journal of the Marine Biological Association, U.K., 86(6):1463-1467.
Rezvani Gilkolaei, S. 1997. Molecular population genetic studies of sturgeon species in the South Caspian Sea. Ph.D. Thesis, University of Wales, Swansea. 196 pp.
Rezvani Gilkolaei, S. 2000a. Study of mtDNA variation of Russian sturgeon population from the South Caspian Sea using RFLP analysis of PCR amplified ND5/6 gene regions. Iranian Journal
of Fisheries Sciences, 2(1):13-36, 109.
Rezvani Gilkolaei, S. 2000b. Persian sturgeon as the candidate for sturgeon fisheries and aquaculture development in Iran. The Third World Fisheries Congress, Beijing, 31 Oct - 3 Nov 2000 (title).
Rezvani Gilkolaei, S. 2002. DNA PCR amplification for species diagnosis of caviar from Caspian Sea sturgeon. Journal of Agricultural Science and Technology, 4:40-48.
Rezvani Gilkolaei, S. 2007. Aquaculture development in Sistan-Baluchistan. Visit of fish rearing sector report Zabol-Zahedan 18th to 20th September 2007. Project
financed by Italian Cooperation, Italian Ministry of Foreign Affairs. 17 pp.
Rezvani Gilkolaei, S., Javanshir, A., Roushan Tabari, M., Roohi, A. and Negarestan, H. 2005. Ecologic comprehensive studies of invasive comb jelly (Mnemiopsis leidyi),
possibility of biologic control through introducing Beroe ovata and mesocosm experiment. Borok II, International Workshop, Invasions of Alien Species in Holarctic, 27 September-1 October
2005, Borok (abstract).
Rezvani Gilkolaei, S., Lalouei, F., Aghili, R. and Ebrahimzadeh Mousavi, H. A. 2007. Introducing genetic markers to identify and distinguish five species of Cyprinidae in the Caspian Sea using
PCR-RFLP. Iranian Scientific Fisheries Journal, 15(5):49-58. In Farsi.
Rezvani Gilkolaei, S. and Skibinski, D. O. F. 1999. Polymerase chain reaction (PCR) and direct sequencing of mtDNA from the ND5/6 gene region in Persian sturgeon Acipenser persicus
from the southern Caspian Sea. Iranian Journal of Fisheries Sciences, 1(1):23-34.
Rezvanullah K., Bahmani, M., Krayushkina, L. and Pourkazemi, M. 2001. Percentage survival and LC50 in Persian sturgeon Acipenser persicus fingerlings at different salinity
releasing time. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Riahi, M. 1996. Tar, the silent lake. Shekar-o Tabiat, Tehran, (42):52-53. In Farsi.
Riahi Bakhtiari, A. 2001. Determination of the budget and changes of lead and cadmium in the tissues of different fish species in Haraze River (N. Iran). Journal of Environmental Studies,
27(Special Issue):15-22. In Farsi.
Riahi Bakhtiyari, A. 2001. Determination of the level and changes of lead and cadmium in the tissues of different fish species in Harraze River (Amol - Sorkhrood). Daneshvar Medical, 8(31):12-134.
Riahi Bakhtiyari, A. 2002. Concentrations and fluctuations of lead and cadmium in the tissues of different fish species of the Haraz River. Iranian Journal of Marine Sciences, 1(1):37-45, 83. In Farsi.
Riazi, B. 1993. Bahoo-Kalat River basin. Department of the Environment, Tehran. 88 pp. In Farsi.
Riazi, B. 1996. Siah-Keshim. The Protected Area of Anzali Wetland. Department of the Environment, Tehran. 101 pp. In Farsi.
Rich, V. 1982. Caspian water level drops. Nature, London, 299:384.
Rich, V. 1983. Whose finger in the dyke? Nature, London, 302:98.
Rich, V. 1991. Floods threaten cities around the Caspian Sea. New Scientist, 130(1773):16.
Richter, H-J. 1989. Breeding the Persian pupfish, Aphanius mento. Tropical Fish Hobbyist, 38(4)(406):10-25.
Ricker, W. E. 1970. Some Fishery Research Stations in the USSR, 1969. Fisheries Research Board of Canada Manuscript Report Series, 1091:109 pp.
Ride, W. D. L., Sabrosky, C. W., Bernardi, G. and Melville, R. V. (Eds.). 1985. International Code of Zoological Nomenclature. Third Edition. International
Trust for Zoological Nomenclature, London. xx + 338 pp.
Rieben, E. 1954. Report to the Government of Iran on the ground water resources of the Teheran alluvial plain. Food and Agriculture Organization, Rome, Report No. 168(a):24 pp.
(English version of FAO Report No. 168 (1953) "Les Ressources en Eaux Souterraines de la Plaine Alluviale de Teheran").
Rives, J. H. 1968. Letter on qanats. Scientific American, 219:8, 10, 16.
Robalo, J. I., Almada, V. C., Levy, A. and Doadrio, I. 2007. Re-examination and phylogeny of the genus Chondrostoma based on mitochondrial and nuclear data and the definition of 5
new genera. Molecular Phylogenetics and Evolution, 42(2):362-372.
Roberts, N. and Wright, H. E. 1993. Vegetational, lake-level, and climatic history of the Near East and Southwest Asia, pp. 194-220. In: Wright, H. E., Kutzbach, J. E., Webb, T., Ruddiman,
W. F., Street-Perrott, F. A. and Bartlein, P. J. (Eds.). Global Climates since the Last Glacial Maximum. University of Minnesota Press, Minneapolis. viii + 569 pp.
Roberts, T. R. 1984. Cobitidae. In: Daget, J., Gosse, J.-P. and Thys van den Audenaerde, D. F. E. (Eds.). Check-list of the Freshwater Fishes of Africa. Paris. 343 pp.
Roberts, T. R. 1994. Systematic revision of Asian bagrid catfishes of the genus Mystus sensu stricto, with a new species from Thailand and Cambodia. Ichthyological Exploration of
Freshwaters, 5(3):241-256.
Roberts, T. R. and Khaironizam, M. Z. 2008. Trophic polymorphism in the Malaysian fish Neolissochilus soroides and other Old World barbs (Teleostei, Cyprinidae). Natural History Bulletin
of the Siam Society, 56(1):25-53.
Robins, C. R., Bailey, R. M., Bond, C. E., Brooker, J. R., Lachner, E. A., Lea, R. N. and Scott, W. B. 1991. World Fishes Important to North Americans Exclusive of Species from the Continental
Waters of the United States and Canada. American Fisheries Society Special Publication, 21:viii + 243 pp.
Robins, G. L. 1970. A Bibliography of the Pike Perch of the genus Stizostedion (including the genus known as Lucioperca. Fisheries Research Board
of Canada Technical Report, 161:iii + 67 pp.
Rochard, E., Castelnaud, G. and LePage, M. 1990. Sturgeons (Pisces: Acipenseridae); threats and prospects. Journal of Fish Biology, 37(Supplement A):123-132.
Rochard, E., Williot, P., Castelnaud, G. et LePage, M. 1991. Elements de systematique et de biologie des populations sauvages d'esturgeons, pp. 475-507. In: Williot, P. (Ed.). Acipenser.
Actes du premier colloque international sur l'esturgeon. Bordeaux 3-6 octobre 1989, CEMAGREF (Centre National du Machinisme Agricole du Génie Rural des Eaux et des Forêts,
Bordeaux). Paris. 519 pp.
Rodionov, S. N. 1994. Global and regional climate interaction: the Caspian Sea experience. Kluwer Academic Publishers, Dordrecht/Boston/London. vii + 241 pp.
Rodler, A. 1887. Der Urmia-See und das nordwestliche Persien. Vereine zur Verbreitung naturwissenschaftlicher Kenntnis in Wien, 27:535-575.
Rögl, F. 1998. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene). Annalen des naturhistorischen Museums in Wien, 90A:279-310.
Rögl, F. 1999. Oligocene and Miocene palaeogeography and stratigraphy of the circum-Mediterranean region, p. 475-500. In: Whybrow, P. J. and Hill, A. (Eds.). Fossil Vertebrates of Arabia
with Emphasis on the Late Miocene Faunas, Geology, and Palaeoenvironments of the Emirate of Abu Dhabi, United Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Rögl, F. and Steininger, F. F. 1984. Neogene Paratethys, Mediterranean and Indo-pacific seaways. Implications for the paleobiogeography of marine and terrestrial biotas, pp. 171-200.
In: Brenchley, P. J. (Ed.). Fossils and Climate. John Wiley & Sons, New York. xv + 352 pp.
Rohani, M. S., Ebrahimzadeh Mousavi, H. A., Khosravi, B., Mokhayer, B., MirZagar, S. S. and Mehrabi, Y.
E. 2006. Evaluation of Geranium herbarum essence application in control of fungal
contamination in rainbow trout eggs. Journal of Veterinary Research, 61(3):269-272.
In Farsi.
Rohani, M. S., Khosravi, A. R., Ebrahimzadh (sic) Moosavi, H. and Mehrabi, Y. 2005. The feasibility study of "Malachite green" substitution with
herbal essence. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Rohani, N. 2004. Kavir park, a forgotten region!! Sharq (Persian Morning Daily), 135:3 pp. (www.netiran.com/Htdocs/WeeklyJournal/Social/wj01733.html, downloaded 2 March 2004).
Rohbakhshan, A. 1991. Aquatic organisms in the old Persian literature: Naser Khosro and a seven year journey around the civilised world 1000 years ago. Abzeeyan, Tehran, 7:24-25. In Farsi.
Rohei Aminjan, A. and Malek, M. 2004. Ecology of helminthes parasites of Capoeta capoeta gracilis from Shiroud River, Iran. Iranian Journal of Fisheries Sciences, 13(2):73-82. In Farsi.
Rohi Kolagar, A. 1994. The seasonal survey of copepods density and distribution in the southern parts of the Caspian Sea. Iranian Fisheries Scientific Journal, 3(3):53-65, 5. In Farsi.
Romanov, N. S. 1955. Annotated Bibliography on Fisheries of the Southern Basins of the U.S.S.R. (1918-1953). Israel Program for Scientific Translations, Jerusalem (1968). 400 pp.
Romero, A. 2008. Typological thinking strike again. Review of "Proudlove, G. S. 2006. Subterranean fishes of the world. International Society for
Subterranean Biology, Moulis. 287 pp. US$65." Environmental Biology of Fishes, 81(3):359-363.
Romero, A. and Green, S. M. 2005. The end of regressive evolution: examining and interpreting the evidence from cave fishes. Journal of Fish Biology, 67(1):3-32.
Romero, A. and Paulson, K. M. 2001. It's a wonderful hypogean life: a guide to the troglomorphic fishes of the world. Environmental Biology of Fishes, 62(1-3):13-41.
Roohi, A., Kideys, A. and Fazli, H. 2003. Comparative study on lipid quality of distribution and abundance of Mnemiopsis leidyi on the eastern Iranian
coasts of the Caspian Sea. Iranian Scientific Fisheries Journal, 12(3):67-82. In Farsi.
Roomiani, L. and Jamili, S. 2011. Population dynamics and stock assessment of hilsa shad, Tenualosa ilisha in Uran (Khuzestan Province). Journal of Fisheries and Aquatic Science, 6(2):151-160.
Rosen, D. E. 1960. Middle-American poeciliid fishes of the genus Xiphophorus. Bulletin of the Florida State Museum, Biological Sciences, 5(4):57-242.
Rosenberg, S. 2002. Leaks ruin caviar-in-a-coffin plan. BBC News World Edition, 2 pp. (http://news.bbc.co.uk/2/hi/europe/2489831.stm).
Roshan Tabari, M. 1995. Hydrology and hidrobiology (sic) of Tadjan River. Iranian Fisheries Scientific Journal, 3(4):59-72, 7. In Farsi.
Rooshan (sic) Tabari, M. 1996. Tajan River and its ecological situation. Abzeeyan, Tehran, 7(2):16-20, II-III. In Farsi.
Roushan (sic) Tabary, M. 1996. Hydrology and hydrobiology of the Haraz River. Iranian Fisheries Scientific Journal, 5(2):43-63, 5. In Farsi.
Roshan, M. D. and Moini, S. 2009. The effect of washing of water soluble protein on the quality of the surimi produced from silver carp (Hypophthalmichthys
molitrix). The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 133 (abstract).
Roshan Tabari, M. 1997. Hydrological and hydrobiological study in the Syiah-rud River. Iranian Fisheries Scientific Journal, 6(2):27-42, 6. In Farsi.
Roushan (sic) Tabari, M., Khoda Parast, N. B., Vahedi, F. and
Rostamian, M. T. 2009. An investigation on the feeding behaviour of common kilka (Clupeonella cultriventris caspia) in the southern Caspian Sea, Mazandaran Province, Iran. Iranian Scientific
Fisheries Journal, 18(3):79-88. In Farsi.
Roshan Tabari, M., Takmeliyan, K., Najafpoor, Sh., Abdoli,. A. and Fourooghi-e Fard, H. 2001. Hydrology and hydrobiology of Chaloos River. Iranian Journal of Fisheries Sciences, 9(4):1-14. In Farsi.
Ross, W. 1979. Speckled Arabian killifish. The Aquarist, 43:500-501.
Ross, W. 1980. Colour variations of Aphanius dispar. The Aquarist, 44:48-49.
Ross, W. 1987. The Arabian killifish. Tropical Fish Hobbyist, 36(2)(380):16-19.
Ross, W. 1999. De Arabische tandkarper (Aphanius dispar dispar). Killi-Kontakt, Wommelgem, 27(4):99-102. In Flemish.
Rostami, I. 1940. Normes de consommation des matériaux dans la Société des pêcheries du nord de l'Iran, sous la direction
de I. Rostami. Bandar Pahlavi, Iran. MS. In Farsi and Russian.
Rostami, I. 1943. Determination of the quality of products from fish. Bulletin of the Veterinary Faculty, University of Tehran, 1943 (January-March):476-612. In Farsi.
Rostami, I. 1945. Freshwaters of the Caspian basin (in Iran) and the conservation of fish stocks. Bulletin of the Veterinary Faculty, University of Tehran, 1945 (August-November):111-175. In Farsi.
Rostami, I. 1946. Ichthyofauna of Iran and its utilization. Bulletin of the Veterinary Faculty, University of Tehran, 1946 (October-March):89-113. In Farsi.
Rostami, I. 1950. Utility of the expansion of the fishery for certain fishes on the northern coast of Iran. Reviews of the Veterinary Faculty, University of Tehran, 1:68-98. In Farsi.
Rostami, I. 1961a. Acclimation and raising fish in Karadj Reservoir. Studies of the Karadj Water and Power Organization. MS.
Rostami, I. 1961b. Biologie et exploitation des Acipenséridés Caspiens. Thèses présentés à la Faculté
des Sciences de l'Université de Paris pour obtenir le Grade de Docteur ès Sciences Naturelles. Ire Thèse. Imprimerie Comte-Jacquet, Bar-le-Duc (Meuse), France. 210 pp.
Rostami, I. 1963a. Acclimatization of some species of fish in the Karadj River Basin. Gohar Printing, Tehran. 22 pp.
Rostami, I. 1963b. Malaria control in Iran and application of a biological method. Publications of the Ahwaz Agriculture College, 3:1-36. In Farsi.
Rostami, K., Soltani, H. A. and Hassan, M. D. 2005. Purification and partial characterization of serum immunoglobulins from Caspian Sea sturgeons. Sixth
Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Rostami, M. and Soltani, M. 2009. Histopathological effects of copper sulphate chronic doses on common carp (Cyprinus carpio). Journal of Veterinary Research, 64(3):193-198. In Farsi.
Rostami, M. R. 2001. Study of the mix factors of marketing effects on the behavioural mechanism of fishery products consumers in Iran. M.Sc. Thesis, Tarbiat Modarres University, Tehran. 188 pp.
In Farsi.
Rostami Bashman, M., Soltani, M. and Sasani, F. 2001. A survey of copper (Cu), zinc (Zn), mercury (Hg), cadmium (Cd) histopathological lesions in common carp (Cyprinus carpio).
Journal of the Faculty of Veterinary Medicine, University of Tehran, 55(4):1-3. In Farsi.
Rostamian, M. T. 1998. Hybridization of Acipenser nudiventris x Acipenser stellatus and comparing the hybrid species growth to the growth of Acipenser stellatus.
Iranian Journal of Fisheries Sciences, 7(2):39-48, 5. In Farsi.
Rostamzad, H., Shabanpour, B., Kashaninejad, M. and Shabani, A. 2010. Inhibitory impacts of natural antioxidants (ascorbic and citric acid) and vacuum packaging on lipid oxidation in frozen
Persian sturgeon fillets. Iranian Journal of Fisheries Sciences, 9(2):279-292.
Roux, C. 1961a. Quelques aspects de la faune de la Mer Caspienne. Comptes Rendus de la Société de Biogéographie, 38(333):51-53.
Roux, C. 1961b. Rapport sommaire sur une Mission en Iran. Bulletin du Muséum national d'Histoire naturelle, Paris, 2(33):294-295.
Rowe, G. T. 1996. Azerbaijan, oil, and sustainable development in Azerbaijan. Quarterdeck, College Station, Texas, 4(3):10 pp. (internet version).
Rowley, G. 1986. Irrigation systems in the Holy Land: a comment. Transactions of the Institute of British Geographers, new series, 11(3):356-359.
Roy, R. N. 1996. Project formulation for fisheries training and extension. Islamic Republic of Iran. Technical Report, Fisheries Extension Service, FAO Technical Cooperation Programme, Rome. 43 pp.
Rozengurt, M. A. and Hedgpeth, J. W. 1989. The impact of altered river flow on the ecosystem of the Caspian Sea. Reviews in Aquatic Sciences, 1(2):337-362.
Ruban, G. I., Kholodova, M. V., Kalymkov, V. and Sorokin, P. A. 2008. Morphological and molecular genetic study of the Persian sturgeon Acipenser persicus Borodin (Acipenseridae) taxonomic
status. Journal of Ichthyology, 48(10):891-903.
Rückert-Ülkümen, N. and Matzke-Karasz, R. 2000. Taxonomische Untersuchungen an fossilen und rezenten Arten der Gattung Leuciscus Cuvier (Pisces, Teleostei, Cyprinidae).
Mitteilungen der Bayerischen Staatssammlung für Paläontologie und historische Geologie, 40:165-180.
Rudin, Z. N. 1966. K voprosu o sovremennom razvitii rybnogo khozyaistva iranskogo Kaspiya [On the problems concerning contemporary progress in the fisheries economy of the Iranian Caspian].
In: Problemy ekonomiki i istorii stran Blizhnego i Srednego Vostoka. Izdatel'stvo Nauka, Moskva, pp. 139-149.
Ruhi, A. 1998. The biomass and species composition of zooplankton populations in Gorgan Bay. Iranian Fisheries Scientific Journal, 6(4):35-46, 4. In Farsi.
Rüppell, W. P. E. S. 1828-1830. Atlas zu der Reise im nördlichen Afrika. Fische des Rothen Meeres. Frankfurt-am-Main. 141 + 3 pp.
Russel, A. 1756. An account of four undescribed fishes of Aleppo; in a letter to Mr. Peter Collinson, F.R.S. by Alexander Russel, M.D. Philosophical Transactions of the Royal Society,
London, 49:445-449, 1 pl.
Russell, A. 1794. Chap. III. Of Fishes, pp. 207-219, pls. VI-VII. In: Vol. II. The Natural History of Aleppo, containing a description of the city, and the principal natural productions
in its neighbourhood together with an account of the climate, inhabitants and diseases; particularly of the plague. Second Edition by P. Russell. London. Volume I:xxiv + 446 pp., Appendix
(xxiii) + Errata (1 page); Volume II:v + 430 pp., + Appendix (xxxiv) + Index to both volumes (25 pp., not numbered) + Errata (1 page).
Ruttner-Kolisko, A. 1964. Kleingewässer am Ostrand der persischen Salzwüste. Ein Beitrag zur Limnologie arider Gebiete. Verhandlungen
der internationalen Vereinigung für theoretische und angewandte Limnologie, 15:201-208.
Ruttner-Kolisko, A. 1966. The influence of climatic and edaphic factors on small astatic waters in the East Persian salt desert. Verhandlungen
der internationalen Vereinigung für theoretische und angewandte Limnologie, 16:524-531.
Ruttner, A. W. and Ruttner-Kolisko, A. E. 1972. Some data on the hydrology of the Tabas - Shirgesht - Ozbak-kuh area (East Iran). Jahrbuch der Geologischen Bundesanstalt, Wien, 115:1-48, 2 plates.
Ruttner, A. W. and Ruttner-Kolisko, A. E. 1973. The chemistry of springs in relation to the geology in an arid region of the Middle East (Khurasan,
Iran). Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 18:1751-1752.
Ryan, P. R. 1986. Soviets shelve plan on diverting rivers in Arctic regions. Oceanus, 29(1):78-80.
Ryan, S. 1994. Saddam kills off marsh wildlife in eco-disaster. Sunday Times, London, No. 8,852, p. 1.16 (17 April 1994).
Rychagov, G. I. 1997. Holocene oscillations of the Caspian Sea, and forecasts based on palaeogeographical reconstructions. Quaternary International, 41/42:1671-172.
Rzóska, J. 1980. Euphrates and Tigris, Mesopotamian ecology and destiny. Monographiae Biologicae, 38:x + 122 pp.
S
Saad, M. A. H. 1978a. Some limnological studies on the lower reaches of Tigris and Euphrates, Iraq. Acta Hydrochimica et Hydrobiologica, 6(6):529-539.
Saad, M. A. H. 1978b. Seasonal variations of some physico-chemical conditions of Shatt al-Arab estuary, Iraq. Estuarine and Coastal Marine Science, 6:503-513.
Saad, M. A. H. and Antoine, S. E. 1978a. Limnological studies on the River Tigris, Iraq. I. Environmental characteristics. Internationale Revue der Gesamten Hydrobiologie, 63(5):685-704.
Saad, M. A. H. and Antoine, S. E. 1978b. Limnological studies on the River Tigris, Iraq. II. Seasonal variations of nutrients. Internationale Revue der Gesamten Hydrobiologie, 63(5):705-749.
Saad, M. A. H. and Antoine, S. E. 1978c. Limnological studies on the River Tigris, Iraq. III. Phtyoplankton. Internationale Revue der Gesamten Hydrobiologie, 63(6):801-814.
Saad, M. A. H. and Antoine, S. E. 1982. Effect of pollution on phytoplankton in Al-Khandak and Al-Rabat, polluted outlet canals of the Shatt al-Arab estuary at Basrah, Iraq. Internationale
Revue der Gesamten Hydrobiologie, 67(3):419-429.
Saad, M. A. H. and Antoine, S. E. 1983. Effect of pollution on phytoplankton in the Ashar Canal, a highly polluted canal of the Shatt al-Arab estuary
at Basrah, Iraq. Hydrobiologia, 99:189-196.
Saad, M. A. H. and Kell, V. 1975. Observations on some environmental conditions as well as phytoplankton blooms in the lower reaches of Tigris and Euphrates. Wissenschaftliche
Zeitschrift der Universität Rostock Mathematisch-Naturwissenschaftliche, 24(6):781-787.
Saadati, M. 1974. Research proposal for the biological control of aquatic weeds on the Dez Irrigation Project at Andimeshk, Iran. Submitted to Department of the Environment,
Parks and Wildlife Division, Khuzestan Water and Power Authority and Colorado State University, Department of Fishery and Wildlife Biology. MS, 9 pp.
Saadati, M. A. G. 1977. Taxonomy and distribution of the freshwater fishes of Iran. M.S. Thesis, Colorado State University, Fort Collins. xiii + 212 pp.
Sabaj Pérez, M. H. (Ed.). 2010. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an Online Reference Version 1.5 (4 Oct 2010).
www.asih.org. American Society of Ichthyologists and Herpetologists, Washington, D.C. 35 pp.
Saber, A. A. H., Abedian Kenari, A. A. M. and Hayati, F. 2007. Effects of dietary protein and energy levels on growth and body composition of Caspian trout larva (Salmo trutta caspius).
Iranian Scientific Fisheries Journal, 16(1):93-102. In Farsi.
Saberi, H. J. 1998. Fish in Afghanistan, p. 259-263. In: Walker, H. (Ed.). Fish. Food from the Waters. Proceedings of the Oxford Symposium on Food and Cookery 1997. Prospect Books, Totnes,
Devon. 335 pp.
Sabet, S. S., Imanpoor, M. R., Aminian Fatideh, B. and Gorgin, S. 2009. Study on sexual maturity and levels of gonad steroid hormones in female kutum Rutilus frisii kutum (Kamenskii, 1901)
during spawning season from river Sefid-Rood of the southern Caspian sea. Journal of Cell and Animal Biology, 3(11):208-215.
Sabour, A. K. 2006a. Production of isinglass from the swim bladder of sturgeon fishes. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Sabour, A. K. 2006b. Study and production of sauce and gelatin from intestine and skin (remained parts) of sturgeon fish. Iranian Fisheries Research Organization, Agriculture Research and
Education Organization, Ministry of Jihad-e-Agriculture, Rasht. http://en.ifro.ir (abstract).
Sabour, A. K., Zareh Gashti, G., Sababi, H. and Moeini, S. 2001. A study on the production of gelatin from sturgeon fish skin. Iranian Journal of Fisheries Sciences, 3(1):85-87, 94.
Sabzalizadeh, S. 2006. Ecological investigation on Dez Dam lake. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture, Ahvaz. http://en.ifro.ir (abstract).
Sabzalizadeh, S. and Amirineia, S. 2003. Study on some physical and chemical characteristics of Shadegan Marsh. Iranian Journal of Fisheries Sciences, 12(2):47-62. In Farsi.
Sadegh, M., Yeganeh, A., Keivany, Y. and Seyfabadi, J. 2009. Biosystematic of two isolated population of Aphanius sp. from Iran central Plateau.13th European Congress of Ichthyology, 6-12
September 2009, Klaipeda, Lithuania (abstract).
Sadeghi, H. and Agheli, L. A. 2002. Evaluation of fish resources depreciation in Iran. Pajouhesh va Sazandegi, 15(2)(55):11-15. In Farsi.
Sadeghi, M. 1995. Heavy metal concentrations in edible fishes from Anzali Wetland. Iranian Fisheries Research and Training Press, Tehran. 57 pp. In Farsi.
Sadeghi, S. N. 2001. Morphological and Biological Characteristics of Southern Iranian Fishes (The Persian Gulf & Oman Sea). Edited by F. Owfi and T. Valinasab. Naghsh-e Mehr Publishing,
Tehran. xx + 440 pp. In Farsi.
Sadeghi Rad, M. 1997. Heavy metal determination in fish species of Anzali Lagoon. Iranian Fisheries Scientific Journal, 5(4):1-16, 1. In Farsi.
Sadhegi Rad, M. 2002. Heavy metal determination (Zn, Cu, Cd, Pb and Hg) in muscle tissue and caviar in two sturgeon species A. persicus and A. stellatus in the southern shores of
the Caspian Sea. Final report, Iranian Fisheries Research Organization. 81 pp. In Farsi.
Sadeghi Rad, M., Amini Ranjbar, Gh, R., Arshad, A. and Joushideh, H. 2004. Determination of Zn and Cu in muscle tissue and caviar in Persian sturgeon (A. persicus) and stellate sturgeon
(A. stellatus) in the Caspian Sea basin. Pajouhesh va Sazandegi, 16(4)(61):51-55. In Farsi.
Sadeghi Rad, M., Amini Ranjbar, G., Jooshideh, H. and Arshad, U. 2009. Heavy metal concentrations in the selected tissues of the Persian sturgeon, Acipenser persicus, from southern coast
of the Caspian Sea. Iranian Journal of Fisheries Sciences, 8(2):175-184.
Sadhegi Rad, M., Arshad, A., Joshiedeh, H. and Amini Ranjbar, Gh. 2005. Assessing heavy metal content of muscle tissue and caviar of Acipenser persicus and Acipenser stellatus
in southern Caspian Sea. Iranian Scientific Fisheries Journal, 14(3):79-100. In Farsi.
Sadegi, M. M. 2003. Creating Karkheh. International Water Power & Dam Construction, 55(1):36-37.
Sadeq, G. N. 1999. Hamoun with its latent beauty and migrating birds on the verge of destruction. Osveh (Cultural, Political and Social Magazine of Sistan and Baluchestan Province),
1999(7):15 pp. (www.netiran.com/Htdocs/Clippings/Social/991121XXSO01.html, downloaded 3 May 2000).
Sadlayev, K., Novikov, N. and Zanov, S. 1965. Economic and Technical Report of the Production of Fish Stocks in Iranian Waters of the Caspian Sea. Fisheries
Research Centre of Guilan Province, Bandar Anzali. 142 pp. In Farsi.
Saeedi, A. A. 2004. The comparison of some hematological and serum biochemical parameters in healthy and diseased golden grey mullets (Liza auratus(sic)) fishes in southern
coasts of Caspian Sea. The 4th International Iran and Russia Conference "Agriculture and Natural Resources", 8-10 September, Shahr-e Kord, Iran (title).
Saeedi, L. 2002. Rich resources of seas in Iran. Payam-e Darya, Tehran11(109):4 pp. (www.netiran.com/Htdocs/WeeklyJournal/Economy/wj01133.html, downloaded 12 February 2002).
Saefali, M. 1999. Comparative morphological and meristic analyses of Lebias (Aphanius) in Iran. M.Sc. Thesis, Shahid Beheshti University, Tehran. 206 pp. In Farsi.
Saeidi, M., Abesi, A.. and Jamshidi, A. 2010. Assessment of heavy metal and oil pollution of sediments of south eastern Caspian Sea using indices. Journal of Environmental Studies, 36(53):21-38.
In Farsi.
Safaee, S. 2005. Carassius auratus (Linnaeus, 1758) as a (sic) invasive specie in Anzali Lagoon. Borok II, International Workshop, Invasions of Alien
Species in Holarctic, 27 September-1 October 2005, Borok (abstract).
Safahieh, A. and Padash-Barmchi, Z. 2009. The differential leucocyte count of Persian sturgeon fingerlings Acipenser persicus exposed to sublethal concentrations of daizinon. 13th
European Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Safaian, N. A. and Shokri M. 2003. Ab-bandans or ponds of Mazandaran. Journal of Environmental Studies, 29(31):47-70. In Farsi.
Safari, O. and Boldaji, F. 2008. Study of effect of partial substitution of canola meal and soybean meal with fishmeal in the diet of rainbow trout (Oncorhynchus mykiss). Pajouhesh va Sazandegi,
79:45-51. In Farsi.
Safari, O. and Boldaji, F. A. 2008. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella).
Pajouhesh va Sazandegi, 20(3)(76):109-117. In Farsi.
Safari, O., Boldaji, F. A. and Hajimoradlou, A. A. 2007. Effect of glucosinolate in diets containing canola meal on rainbow trout (Oncorhynchus mykiss) blood serum. Pajouhesh
va Sazandegi, 19(4)(73):168-176. In Farsi.
Safari, R. 2005. Genetic structure of ship sturgeon from south Caspian Sea and Ural River using microsatellite. M.Sc. Thesis, University of Agricultural
Sciences and Natural Resources, Gorgan. 102 pp. In Farsi.
Safari, R. 2006. Production of bacterial probiotic for improvement warmwater fish farms emphasized to decreasing of ammonia, nitrate and SH2. Iranian Fisheries Research Organization,
Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Safari, R., Imanpour, M. R. and Shaabanpour, B. 2008. Study of changing of chemical muscle content during maturation in Cyprinus carpio (Linnaeus, 1758)(Cyprinidae). Pajouhesh va Sazandegi,
77(4):63-69. In Farsi.
Safari, R. and Khandagi, A. 1999. Evaluation and isolation of Clostridium botulinum type E from fresh and smoked Cyprinus carpio, Rutilus frisii kutum and Hypophtalmicthys
(sic) molitrix. Iranian Journal of Fisheries Sciences, 7(4):19-26, 3. In Farsi.
Safari, R., Pourkazemi, M., Rezvani, S. and Shabani, A. 2007. Study of genetic variation of ship sturgeon (Acipenser nudiventris) from the south coast of Caspian Sea using microsatellite.
Journal of Agricultural Sciences and Natural Resources, (Special Issue Natural Resources), 14:49-59. In Farsi.
Safari, R., Pourkazemi, M., Rezvani Gilkolaei, S. and Shabani, A. 2008. Population structure of Acipenser nudiventris in the south coast of Caspian Sea and Ural River using microsatellite
method. Iranian Scientific Fisheries Journal, 17(1):99-108. In Farsi.
Safari, R., Pourkazemi, M., Rezvani Gilkolaei, S., Shabani, A. and Bagherian Yazdi, A. 2008. Genetic relationships of Iranian coastal ship sturgeon (Acipenser
nudiventris) samples and Ural population based on microsatellite DNA. Iranian Journal of Fisheries Sciences, 7(2 supplementary):229-241.
Saffron, I. 2002. Caviar: the strange history and uncertain future of the world’s most coveted delicacy. Broadway Books, New York. xv + 270 pp.
Safi, S. Mojabi, A., Azari Takami, Gh., Nowrouzian, I., and Shariati, A. 1997. Evaluation of FSH, LH, progesterone, estradiol and testosterone to determine the most appropriate time for
injection of pituitary extract in Acipenser persicus. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (abstract).
Safi, S. H., Mojabi, A., Azari Takami, G., Nowrouzian, I., Mahmoodi, M. and Bokaei, S. 1999. Evaluation of sturgeon follicle-stimulating hormone, luteinizing hormone, estradiol,
progesterone and testosterone in Acipenser persicus serum to identify fertile broodstock. Journal of Applied Ichthyology, 15(4-5):196-198.
Safikhani, H., Nilsaz, M., Sabz-Alizadeh, S. and Esmaeili, F. 1998. Final report of limnological study of Karun River (Bandeghir to Khorramshahr). Iranian Fisheries Research Organization,
Tehran. 56 pp. In Farsi.
Şahinöz, E., Doğu, Z. and Aral, F. 2006. Development of embryos in Mastacembelus mastacembelus (Bank & Solander, 1794) (Mesopotamian spiny eel) (Mastacembelidae).
Aquaculture Research, 37(16):1611-1616.
Sahrhage, D. 1999. Fischfang und Fischkult im alten Mesoptamien. Peter Lang, Frankfurt am Main. 241 pp.
Sahrhage, D. and Lundbeck, J. 1992. A History of Fishing. Springer-Verlag, Berlin. viii + 348 pp.
Saiad Borani, M. 2001. The role of restocking bream for improvement of the natural stock. Iranian Journal of Fisheries Sciences, 9(4):27-38. In Farsi.
Saiad Bourani, M. and Ghaninejad, D. 2004. Stock assessment of Capoeta capoeta in Mako Reservoir. Iranian Journal of Fisheries Sciences, 13(3):115-128. In Farsi.
Saidov, Yu. S. and Magomedov, G. M. 1989. Sravnitel'no-morfologicheskie osnovy sistematiki forelei i kaspiiskogo lososya [Comparative morphological principles on the systematics of trouts
and Caspian salmon]. Nauka, Moskva. 108 pp.
Saifali, M., Kiabi, B. H. and Azad, A. M. 2004. Systematic study of two species of tooth-carp (Aphanius Nardo, 1827)(Actinopterygii; Cyprinodontidae) from Iran. Journal of
Science, Al-Zahra University, 17(1):34-42. In Farsi.
Sajaadyeh, M. A. 1995. Water and its importance in Iranian new year traditions. Abzeeyan, Tehran, 5(9):10-13, III. In Farsi.
Sajedi, R. H., Aminzadeh, S., Naderi-Manesh, H., Sadeghizadeh, M., Abdolhay, H. and Naderi-Manesh, M. 2003. Genetic variation within and among rainbow trout, Oncorhynchus mykiss,
hatchery populations from Iran assessed by PCR-RFLP analysis of mitochondrial DNA segments. Journal of Food Science, 68(3):870-873.
Sajjadi, M. S. 1982. Qanat/kariz: storia, tecnica construttiva ed evoluzione. Quaderni della Sezione archeologica dell'Istituto italiano di cultura, Teheran, 1:xxiii + 172 pp., 30 plates.
Sakri, M. 1993. Shahrivar (September), the month of spawning and retainment for mullets. Abzeeyan, Tehran, 4(7):11. In Farsi.
Saksena, D. N. 1980. On the pituitary gland and reproductive cycle of Indian fresh water goby, Glossogobius giuris (Ham.). II. Seasonal changes in the proximal pars distalis and
their correlation with the reproductive cycle. Zeitschrift für mikroskopisch-anatomische Forschung, Leipzig, 94(4):705-714.
Salahi Kojoure, A. A. 1997. Environmental aspects of rain quality. XI World Forestry Congress, 13 to 22 October 1997, Antalya, Turkey, 1 p. (abstract).
Salamat, N., Alboughobish, N., Hashemi Tabar, M., Mesbah, M. and Ahangarpour, A. 2009. Pituitary primary cell culture of common carp (Cyprinus carpio)
and evaluation of its secretion effect on endocrine activity of incubated
ovarian follicles. Iranian Journal of Veterinary Research, 10(1)(26):61-65.
Salamat, N., Erfani Majd, N., Hashemitabar, M., Mesbah, M. and Ahangarpoor, A. 2010. Isolation of common carp ovarian follicular cells and evaluation of their endocrine activity in primary cell
culture. Iranian Journal of Fisheries Sciences, 9(2):305-314.
Salaramoli, J. 2005. The influence of heavy metal pollution due to industrial and agricultural activities in fish ponds. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI),
Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Salaramoli, J., Ghiasi, F. and Mirzargar, S. S. 2005. Influence of zeolite (Clinoptilolite) on the level of cadmium and some water quality parameters in common carp (Cyprinus
carpio) pond water exposed to sublethal concentration of cadmium. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel,
Columbo, Sri Lanka (title).
Salari, A. M. A. and Sadough, N. A. 2009. Comparison of heavy metals content of muscle, liver and gill tissues of Tenualosa ilisha in Karoon River. The 6th Scientific Conference of Fisheries
Resources, 3-4 March 2009, Basrah, p. 136 (abstract).
Salehi, A. 1999. Survey on distribution and natural feeding of Chanos chanos fry in eastern part of Hormozgan Province, Iran. Iranian Journal of Fisheries Sciences, 8(3):69-86, 7. In Farsi.
Salehi, H. 1999. A strategic analysis of carp culture development in Iran. Ph.D. Thesis, Institute of Aquaculture, University of Stirling. xix + 328 pp.
Salehi, M. 2001a. Application of adaptive sampling in fishery part 1: adaptive cluster sampling and its strip designs. Iranian Journal of Fisheries Sciences, 3(1):55-76.
Salehi, M. 2001b. Application of adaptive sampling in fishery part 2: truncated adaptive cluster sampling designs. Iranian Journal of Fisheries Sciences, 3(1):77-84.
Salehi, H. 2003a. Carp culture sector in Iran: an economic analysis of farmed production. World Aquaculture 2003, 20-23 May, Salvador, Brazil (title).
Salehi, H. 2003b. Economic analysis of production and releasing of kutum roach, Rutilus frisii kutum, fingerling in Iran. Iranian Journal of Marine Sciences, 2(1):35-46. In Farsi.
Salehi, H. 2003c. The needs of research on aquaculture economics in Iran. Iranian Journal of Fisheries Sciences, 11(4):75-96. In Farsi.
Salehi, H. 2004a. Carp culture in Iran. Aquaculture Asia, 9(2):8-11.
Salehi, H. 2004b. An economic analysis of carp culture production cost in Iran. Iranian Journal of Fisheries Sciences, 4(1):1-24, 119.
Salehi, H. 2005a. An economic analysis of fingerling production of sturgeon in the south Caspian Sea over the years 2002-03. The Fifth International
Symposium on Sturgeon, Ramsar, Iran, 9-13 May 2005, pp. 319-324.
Salehi, H. 2005b. An economic analysis of trout farm production in Iran. World Aquaculture 2005, Bali, Indonesia. 35 pp.
Salehi, H. 2006a. An analysis of the consumer market for carp and carp products in Iran. Iranian Journal of Fisheries Science, 5(2):83-110.
Salehi, H. 2006b. Economic analysis of sturgeon fingerling production in Iran. Iranian Journal of Fisheries Sciences, 14(4):67-80. In Farsi.
Salehi, H. 2006c. A brief analysis on the economics of fingerling production of sturgeons in Iran: a case study for the period 2002-2003. Journal of Applied Ichthyology, 22(s1):257-260.
Salehi, H. 2007. New opportunities for fish market development in Iran. Fish Marketing Conference, 26-29 November 2007, Kish Island, Iran. 45 pp. In Farsi.
Salehi, H. 2008a. Cost factor analysis of Caspian salmon (Salmo trutta caspius Kessleri (sic), 1877) fingerling production and release in Iran. Iranian Journal of Fisheries
Sciences, 7(2):59-72.
Salehi, H. 2008b. Comparative economics of kutum (Rutilus frisii kutum) fingerling production and releasing over 2001-2003 in north of Iran. Pajouhesh va Sazandegi,
20(4)(77):131-140. In Farsi.
Salehi, H. 2008c. Stock enhancement initiative in the Caspian - the Iranian way. INFOFISH International, 5:59-62.
Salehi, H. 2009. Comparative analysis of carp farming costs in Iran, in 1996 and 2001. Iranian Journal of Fisheries Sciences, 8(2):185-200.
Salehi, M. and Mokhtari, A. 2008. An investigation of fish consumption attitude among nutrition experts in Iran. Iranian Scientific Fisheries Journal, 17(1):79-90. In Farsi.
Salehi, H. and Momen Nia, M. 2006. The benefits of fish and rice integrated culture in Iran. Iranian Scientific Fisheries Journal, 15(3):97-108. In Farsi.
Salemi, H. R. and Heydari, N. 2006. Assessment of water supply and use in the Zayandeh-Rud River basin, Iran. Iran Water Resources Research, 2(1):72-76.
Salih, M. S. and Al-Jaffery, A. 1980. Morphological study of Boleophthalmus dentatus Cuvier & Valenciennes, 1837 with reference to its feeding habits. 1: Habitat, external
features and integument. Bulletin of the Biological Research Center, Baghdad, 12(2):11-44.
Salim, S. M. 1962. Marsh Dwellers of the Euphrates Delta. London School of Economics, Monograph on Social Anthropology, 23, University of London, The Athlone Press. x + 157 pp.
Salimi Manshadi, M. A., Amin, S. and Sadeghi, J. M. 1997. The economic feasibility of water conservation by ghanat outflow control in Yazd Province, Iran. Iranian Journal of Science
and Technology, Transactions B: Technology, 21(4):407-416.
Salmani, A. 1995. Usage of potassium sorbate in caviar processing. Iranian Fisheries Scientific Journal, 4(3):30-40, 6. In Farsi.
Salmani, A., Gholamipour, S. and Yousefian, M. 2001. The changes in TVN and histamine of kilka within the different preservation methods. Iranian Journal of Fisheries
Sciences, 10(2):31-40. In Farsi.
Salmani, A., Safari, R., Soltani, M. and Tavakoli, H. R. 2009. Growth and toxigenesis behaviour of Clostridium botulinum type E in Persian sturgeon (Acipenser persicus) caviar
prepared with various preservatives. International Journal of Veterinary Research, 3(1):17-23.
Salmani Joloudar, A., Rezaeian, M., Gholamipour, S., Safari, R. and Pourgholam, R. 2009. Extraction of omega-3 fatty acid from kilka fish oil. Iranian Scientific Fisheries Journal, 17(4):89-100.
In Farsi.
Salmanov, A. V. 1990. Morfometricheskie osobennosti forelei rek i ozer basseina Kaspiiskogo Morya (Salmo, Salmonidae, Pisces) [Morphometric characteristics of the brown trout from rivers
and lakes of the Caspian Sea basin (Salmo, Salmonidae, Pisces)]. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 213:55-74.
Salnikov (sic), V. B. 1994. Formation of the fish population in the artificial hydrographic network of Turkmenistan (the Amudarya River basin), pp. 365-387. In: Fet, V. and
Atamuradov, K. I. (Eds.). Biogeography and Ecology of Turkmenistan. Kluwer Academic Publishers, Dordrecht/Boston/London. viii + 650 pp.
Sal'nikov, V. B. 1995. Possible changes in the composition of the ichthyofauna after completion of the Karakum Canal in Turkmenistan. Journal of Ichthyology, 35(7):108-121.
Sal'nikov, V. B. 1998. Anthropogenic migration of fish in Turkmenistan. Journal of Ichthyology, 38(8):591-602.
Sal'nikov, V. B. 2009. Pervyi sluchai poimki pantsirnoi shchuki Atractosteus sp. (Actinopterygii, Lepsiosteiformes, Lepisosteidae) v Kaspiiskom More u beregov Turkmenistana.
Rossiiskii Zhurnal Biologicheskikh Invazii, 2009(2):23-27. In Russian.
Salonen, A. 1970. Die Fischerei im Alten Mesopotamien nach Sumerisch-Akkadischen Quellen. Eine Lexikalische und Kulturgeschichtliche Untersuchung. Annales Academiæ Scientiarum Fennicæ,
Ser. B, 166:1-314, tafs I-LI.
Samadani, A. A., Javanshir Khouei, A. and Jamili, Sh. 2009. Population distribution of bony fishes in less-than-ten-metre depths of Mazandaran coasts. Journal of Environmental Science and Technology,
11(2)(41):131-142. In Farsi.
Samaee, S. M., Mojazi-Amiri, B. and Hosseini-Mazinani, S. M. 2006. Comparison of Capoeta capoeta gracilis (Cyprinidae, Teleostei) populations in the
south Caspian Sea river basin, using morphometric ratios and genetic markers. Folia Zoologica, Brno, 55(3):323-335.
Samaee, S. -M. and Patzner, R. A. 2011. Morphometric differences among populations of tu'ini, Capoeta damascina (Teleostei: Cyprinidae), in the interior basins of Iran. Journal of Applied
Ichthyology, 27(3):928-933.
Samaee, S. -M., Patzner, R. A. and Mansour, N. 2009. Morphological differentiation within the population of siah , Capoeta capoeta gracilis (Cyprinidae, Teleostei) in a river of the south
Caspian Sea basin: a pilot study. Journal of Applied Ichthyology, 25(5):583-590.
Sanford, B. 1975. Neur Lake limnological survey II - 1354, 19-28 Mordad 1354. Job Progress Report, Fisheries and Aquatic Ecology Section, Department of the Environment, Tehran. 5 pp., 3 tabs., 4 figs.
Sarafraz, J. and Akbarian, M. A. 2005. A Biological Review of Caspian Sturgeons. Tehran. 110 pp., 16 pls. In Farsi.
Sardar, Z. 1979. Iran faces an environmental crisis. Nature, London, 282:441.
Sargeran, P., Bakhtiyari, M., Abdoli, A., Coad, B. W., Sarvi, K., Lishi, M. R. and Hajimoradloo, A. 2008. The endemic Iranian cave-fish, Iranocypris typhlops: two taxa or two forms
based on the mental disc? Zoology in the Middle East, 44:67-74.
Sarı, H. M., Balık, S., Ustaoğlu, M. R. and Ilhan, A. 2008. Population structure, growth and mortality of Carassius gibelio (Bloch, 1782) in Buldan Dam Lake. Turkish
Journal of Fisheries and Aquatic Sciences, 8(1):25-29.
Sarig, S. 1966. Synopsis of biological data on common carp Cyprinus carpio (Linnaeus), 1758 (Near East and Europe). Food and Agriculture Organization, Rome, Fisheries
Synopsis, 31.2:iii + 35 pp.
Sarker, A. L., Al-Nasiri, S. K. and Hussein, S. A. 1980. Diurnal fluctuations in the physico-chemical conditions of the Shatt al-Arab and the Ashar Canal. Proceedings of the Indian Academy
of Sciences (Animal Sciences), 89(2):171-181.
Sarkhosh, A., Oryan, Sh and Yussefian, M. 2002. Effects of xylazine and ketamine as anaesthetics on sturgeon. Iranian Journal of Marine Sciences, 1(1):47-53, 82. In Farsi.
Sarnthein, M. 1972. Sediments and history of the postglacial transgression in the Persian Gulf and northwest Gulf of Oman. Marine Geology, 12:245-266.
Sarpanah, A., Abbasi, K., Rezvani, S. and Sakari, M. 2004. A survey of fish species diversity in the biggest river of the southern part of Caspian Sea, Sefidroud River. Biology in Asia International
Conference, Singapore, 7-10 December 2004 (abstract).
Sarpanah Sarkohi, A., Ghasemzadeh, G. R., Nezami, S. A., Shabani, A., Christianus, A., Shabanpour, B. and Bin Saad, C. R. 2010. Feeding characteristics of Neogobius caspius in the south
west coastline of the Caspian Sea (Gilan Province). Iranian Journal of Fisheries Sciences, 9(1):127-140.
Sarpanah Sourkouhi (sic), A. N., Christianus, A., Shaabani, A., Nezami, Sh. A., Shaabanpour, B. and Cheroos, B. S. 2008. Reproductive characteristics of Neogobius caspius from the
southwest coasts of the Caspian Sea, Guilan Province of Iran. Iranian Scientific Fisheries Journal, 17(3):87-98. In Farsi.
Sartaj, M., Fathollahi, F. and Filizadeh, Y. 2005. An investigation of the evolution of distribution and accumulation of heavy metals (Cr, Ni, Cu, Cd, Zn and Pb) in Anzali Wetland's
sediments. Iranian Journal of Natural Resources, 58(3):623-634. In Farsi.
Sarvi, K., Niksirat, H., Mojazi Amiri, B., Mirtorabi, S. M., Rafiee, G. R. and Bakhtiyari, M. 2006. Cryopreservation of semen from the endangered Caspian
brown trout (Salmo trutta caspius). Aquaculture, 256:564-569.
Sarvi Moghanloo, K., Niksirat, H., Mojazi Amiri, B. and Mirtorabi, S. M. 2007. The effects of extender type, freezing and thawing rates on fertility of the cryopreserved semen of the Caspian
brown trout (Salmo trutta caspius). Iranian Journal of Fisheries Sciences, 7(1):111-128.
Şaşı, H. 2008. The length and weight relations of some reproduction characteristics of Prussian carp, Carassius gibelio (Bloch, 1782) in the South Aegean region
(Aydın-Turkey). Turkish Journal of Fisheries and Aquatic Sciences, 8(1):87-92.
Sattari, M. 1999. Parasites of sturgeons (Chondrostei: Acipenseridae) from the southwest of the Caspian Sea. Ph.D. Thesis, University of Tehran. 280 pp. In Farsi.
Sattari, M. 2004. The occurrence and intensity of Eustrongylides excisus (Nematoda: Dioctophymidae) in some bony fish species of Caspian Sea and its
basin. Caspian Journal of Environmental Sciences, 2(1):9-12.
Sattari, M. and Dick, T. 2001. Parasites of Acipenser persicus (Chondrostei: Acipenseridae) from south-west of Caspian Sea. 4th International Symposium on Sturgeon,
Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Sattari, M. and Faramarzi, N. 1997a. A survey on infection of fishes of Anzali Lagoon with parasites of genus Caryophyllaeus. Iranian Fisheries Scientific Journal, 5(4):63-72, 7. In Farsi.
Sattari, M. and Faramarzi, N. 1997b. A study on infection of fishes of Anzali wetland with parasites of genus Caryophyllaeus. Pajouhesh va Sazandegi, 5(4):763-72. In Farsi.
Sattari, M., Khara, H., Nezami, S., Roohi, J. D. and Shafii, S. 2006. Occurrence and intensity of some nematodes in the bonyfish species of the Caspian Sea and its basin. Bulletin of the
European Association of Fish Pathologists, 25(4):166-178.
Sattari, M., Khara, B., Roohi, J. D. and Shafii, S. 2004. Occurrence and intensity of some nematodes in the bony fish species of the Caspian Sea and its basin. Biology in Asia International Conference,
Singapore, 7-10 December 2004 (abstract).
Sattari, M. and Mokhayer, B. 2005a. Occurrence and intensity of some parasites in five sturgeon species (Chondrostei: Acipenseridae) southwest of Caspian Sea. Current Science, Bangalore,
89(2):259-263.
Sattari, M. and Mokhayer, B. 2005b. Occurrence and intensity of some parasitic worms in Acipenser gueldenstaedti, A. nudiventris and Huso huso (Chondrostei:
Acipenseridae) from the southwest of the Caspian Sea. Turkish Journal of Veterinary and Animal Science, 29:1279-1284.
Sattari, M., Mokhayer, B., Eslami, A. and Bokaei, S. 2000. Parasites of Acipenser persicus (Chondrostei: Acipenseridae) from south-west of Caspian Sea. Journal of the Faculty of Veterinary
Medicine University of Tehran, 55(3):19-24. In Farsi.
Sattari, M., Mokhayer, B. and Hasheminasab, M. F. 2002. Parasites of Acipenser gueldenstaedti, A. nudiventris and Huso huso (Chondrostei: Acipenseridae) from south-west of
Caspian Sea. Journal of the Faculty of Veterinary Medicine, University of Tehran, 57(4):33-38. In Farsi.
Sattari, M., Mokhayer, B., Khara, H., Nezami, S. and Shafii, S. 2007. Occurrence and intensity of parasites in some bonyfish species of Anzali wetland
from the southwest of the Caspian Sea. Bulletin of the European Association of Fish Pathologists, 27(2):54-60.
Sattari, M., Mokhayer, B. and Mirhasheminasab, S. F. A. D. 2001. Parasites of Acipenser stellatus (Chondrostei: Acipenseridae) from south-west of Caspian Sea. Pajouhesh va Sazandegi,
13(4)(49):92-08. In Farsi.
Sattari, M., Mokhayer, B. and Shafii, S. 2006. Parasitic worms of Persian sturgeon (Acipenser persicus Borodin, 1897) from the southwest of the Caspian Sea. Bulletin of the European
Association of Fish Pathologists, 26(3):131-136.
Sattari, M., Roostaei, M. and Shafii, S. 2001. Occurrence and intensity of Raphidascaris acus (Nematoda: Anisakidae) in some fish species of Anzali wetland in the southwest of the Caspian Sea.
Pajouhesh va Sazandegi, 14(3)(52):79-83. In Farsi.
Sattari, M., Shafiei, S., Daghigh Roohi, J. Abdolahpour Biria, H. and Bekhsat, N. 2002. Occurrence and intensity of Eustrongylides excisus (L.) (Nematoda: Dioctophymidae) from some
bony fish species in the Caspian Sea and its basin. Journal of the Faculty of Veterinary Medicine, University of Tehran, 57(1):37-41. In Farsi.
Sauvage, H. E. 1882. Catalogue des poissons recueillis par M. E. Chantre pendant son voyage en Syrie, Haute-Mésopotamie, Kurdistan et Caucase. Bulletin de la Société
philomathique de Paris, 6:163-168.
Sauvage, H. E. 1884. Notice sur la faune ichthyologique de l'ouest de l'Asie et plus particulièrement sur les poissons recueillis par M. Chantre pendant son voyage dans cette region.
Nouvelles Archives du Muséum d'Histoire naturelle, Paris, 2 sèrie, 7:1-42, pl. 1-3.
Savenkova, T. P. 1985. Osobennosti Skata Molodi Turkmenskoi Vobly v Nizov'yakh R. Atrek i Nagula v Yugo-vostochnoi Chasti Kaspiiskogo Morya (Peculiarities of descent of young Turkmen Caspian
roach in the Atrek River lower reaches and graziery in the south-eastern part of the Caspian Sea). Izvestiya Akademii Nauk Turkmenskoi SSR (Seriya Biologicheskikh Nauk), 1985(2):41-47.
Savenkova, T. P. 1990. Dinamika Promyslovykh Ulovov i Struktura Perestovogo Stada Vobly Yugo-vostochnogo Kaspiya (Dynamics of marketable catches and structure of the spawning stock of Caspian
roach of the south-eastern Caspian Sea). Izvestiya Akademii Nauk Turkmenskoi SSR (Seriya Biologicheskikh Nauk), 1990(1):47-54.
Savenkova, T. P. 1994. Distribution and characteristics of the biology of young-of-the-year vobla, Rutilus rutilus caspicus, in the southeastern Caspian Sea. Journal of Ichthyology,
34(3):28-38.
Savenkova, T. P. and Asanov, A Yu. 1988. Nablyudeniya za skatom molodi ryb v nizov'yakh reki Atrek [Observations on the downstream migration of young fish in the lower Atrek River].
Voprosy Ikhtiologii, 28(4):649-656.
Savenkova, T. P. and Asanov, A. Yu. 1991. The Caspian silverside, Atherina boyeri caspia, in the lower reaches of the Atrek River. Journal of Ichthyology, 31(6):134-138.
Savvaitova, K. A., Shanin, A. Yu. and Verigina, I. A. 1989. The formation and population structure of the false osman, Schizopygopsis stoliczkai,
in Pamir. Journal of Ichthyology, 29(1):136-147.
Sawada, Y. 1982. Phylogeny and zoogeography of the superfamily Cobitoidea (Cyprinoidei, Cypriniformes). Memoirs of the Faculty of Fisheries of Hokkaido University, 28(2):65-223.
Sayad Bourani, M. S. 1999. Some biological survey of the Clupeonella engrauliformis (anchovy-like kilka) in Guilan coastal region, the south-west of Caspian Sea, in 1996-97.
Iranian Journal of Fisheries Sciences, 8(1):59-70, 8-9. In Farsi.
Sayad Borani, M., Nezami, Sh. and Hassanzadeh Keyabi, B. 2001. Biological study and population dynamics of Carassius auratus gibelio in Anzali Lagoon. Iranian Journal of Fisheries
Sciences, 10(3):57-70, 4. In Farsi.
Sayyad Borani, M., Daghigh Ruhi, J., Amiri, A., Abtahi, B., Kazemi, R., Dejhandian, S. and Bahmani, M. 2006. Effects of weight on osmoregulatory ability of Salmo trutta caspius juveniles.
Iranian Journal of Fisheries Sciences, 14(4):81-96. In Farsi.
Sayyad Bourani, M. 2003. Situation of kilka fishing in the Iranian part of the Caspian Sea. Iranian Inland Waters Aquaculture Center, Bandar Anzali. 15 pp. In Farsi.
Sayyad Bourani, M., Abdolmalaki, S., Khanipour, A. A., Fazli, H. and Khedmati, K. 2008. Catch composition and fishing trend of kilka in Iranian part of the Caspian Sea. Iranian Journal of
Fisheries Sciences, 7(2):73-86.
Scharlau, K. 1968. Geomorphology, pp. 186-194. In: Fisher, W. B. (Ed.). The Cambridge History of Iran. Volume 1. The Land of Iran. Cambridge University Press, Cambridge. xix + 784 pp.
Scheel, J. J. 1990. Atlas of Killifishes of the Old World. Tropical Fish Hobbyist Publications, Neptune City, New Jersey. 448 pp.
Scheil, V. 1918. Sur le marché aux poissons de Larsa. Revue d'Assyriologie et d'Archéologie Orientale, 15(4):183-194.
Schiewer, U., Al-Saadi, H. A. and Hameed, H. A. 1982. On the diel rhythm of phytoplankton productivity in Shatt al-Arab at Basrah, Iraq. Archiv für Hydrobiologie, 93(2):158-172.
Scholl, A., Corzillius, B. and Villwock, W. 1976. Genetic differentiation in fishes of the tribe Aphaniini (Cyprinodontidæ) as revealed by electrophoretic techniques. Revue des
Travaux de l'Institut des Pêcheries maritimes, 40(3 et 4):742-743.
Scholl, A., Corzillius, B. und Villwock, W. 1978. Beitrag zur Verwandtschaftsanalyse altweltlicher Zahnkarpfen der Tribus Aphaniini (Pisces, Cyprinodontidae) mit Hilfe elektrophoretischer
Untersuchungsmethoden. Zeitschrift für zoologische Systematik und Evolutionsforschung, 16(2):116-132.
Schreier, T. M., Dawson, V. K., Larson, W. J. and Schleis, S. M. 2001. Piscicides as an emergency tool for controlling range expansion of the round goby (Neogobius melanostomus)
in the Illinois Waterway. International Association for Great Lakes Research, Abstracts from the 44th Conference on Great Lakes Research June 10-14, 2001, Great Lakes Science:
Making it Relevant. University of Wisconsin - Green Bay, Wisconsin Sea Grant Institute, Green Bay, Wisconsin, Part 2:120.
Schulz, T. 2002. Buntbarsche aus dem Iran. Die Aquarien- und Terrarienzeitschrift, 55(3):22-25.
Schulz, T. 2004a. Aquarienfische aus dem Iran. Die Aquarien- und Terrarienzeitschrift, 57(2):20.
Schulz, T. 2004b. Iran, der dritte Versuch. Die Aquarien- und Terrarienzeitschrift, 57(9):24-27.
Schultz, L. P. 1957. The generic names Barbus and Puntius. Tropical Fish Hobbyist, 5(4):14-15, 29-31.
Schuster, W. H. 1960. Synopsis of biological data on milkfish Chanos chanos (Forskal), 1775. Food and Agriculture Organization, Rome, Fisheries Biology Synopsis, 4:vi + 58 pp.
Schüz, E. 1959. Die Vogelwelt des Südkaspischen Tieflandes. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart. 199 pp.
Schwarzer, J., Misof, B., Tautz, D. and Schliewen, U. K. 2009. The root of the East African cichlid radiations. BMC Evolutionary Biology, 9:186.
Schweizer, G. 1975. Untersuchungen zur Physiogeographie von Ostanatolien und Nordwestiran. Geomorphologische, klima- und hydrogeographische Studien
im Vansee- und Rezaiyehsee-Gebiet. Tübinger Geographische Studien, 60(9):VIII + 145 pp., 21 Karten, 12 Fotos.
Scott, D. A. 1993. Wetlands of West Asia - A regional overview, pp. 9-22. In: Moser, M. and van Vessem, J. (Eds.). Wetland and Waterfowl Conservation
in South and West Asia. Proceedings of the International Symposium, Karachi, Pakistan 14-20 December 1991, International Waterfowl and Wetlands Research
Bureau (IWRB) Special Publication, Slimbridge, 25, Asian Wetland Bureau (AWB) Publication, Kuala Lumpur, 85.
Scott, D. A. 1995 (Ed.). A Directory of Wetlands in the Middle East. IUCN, Gland, Switzerland and International Waterfowl and Wetlands Research Bureau, Slimbridge, U.K.. xvii + 560 pp.
Scott, D. A., Hamadani, H. M. and Mirhosseyni, A. A. 1975. The Birds of Iran. Department of the Environment, Tehran. 410 pp.
Scott, W. B. and Crossman, E. J. 1973. Freshwater Fishes of Canada. Bulletin of the Fisheries Research Board of Canada, 184:xi + 966 pp.
Sear, L., Azari Takami, Gh., Schahidi, R. and Vosoughi, A. R. 2006. Evaluation of rainbow trout sperm in Namroud Hatchery. Pajouhesh va Sazandegi, 19(3)(72):30-36. In Farsi.
Secor, D. H., Arefjev, V., Nikolaev, A. and Sharov, A. 2000. Restoration of sturgeons: lessons from the Caspian Sea Sturgeon Ranching Programme. Fish and Fisheries,1(3):215-230.
Sedaqat-kish, J. 2000. Marriage with a qanat in Iran. Barzegar, Tehran, 819:64. In Farsi (English translation at www.netiran.com/Htdocs/Clippings/Social/200629XXSO01.html,
downloaded 15 December 2000).
Sedov, S. I. and Rychagova, T. L. 1983. Morphological characteristics of anchovy kilka, Clupeonella engrauliformis (Clupeidae), in winter and spring. Journal of Ichthyology,
23(3):140-143.
Seegers, L. 1997. Killifishes of the World. Old World Killis II. Verlag A. C. S. GmbH, Mörfelden-Walldorf. 112 pp.
Seifabadi J., Khodabandeh, S., Esmaili Sari, A. and Abtahi B. 2001. Some biological features of Caspian Sea ctenophores. The Second International Iran and Russia Conference (Agriculture and Natural
Resources), Moscow, Abstract, pp. 171-172.
Seifali, M., Kiabi, B. and Mahjoorazad, A. 2003. Systematics of two species of tooth carps (genus Aphanius) in Iran. Science Journal of Al-Zahra University, 17(1):43-51. In Farsi.
Seifali, M. and Sheidai, M. 2001. Morphometric and meristic analysis of Aphanius ginaonis (Holly, 1929) from Geno hot spring in Bandar Abbas. Journal of Science, Al-Zahra University,
14(1):42-53. In Farsi.
Seifi, H. 1990. The investigation of aquatic fauna in Talar River. B.Sc. Thesis, Agricultural Sciences and Natural Resources University, Gorgan. 64 pp. In Farsi.
Seifzadeh, M., Shogaei, A. and Amirmozapheri, N. 2009. Microbial quality monitoring of farmed Huso huso fillets packaged by sous vide method. The 6th Scientific Conference of Fisheries
Resources, 3-4 March 2009, Basrah, p. 108 (abstract).
Semeit, A. 1999. Aphanius mento: A jewel of the desert. ARK - Arizona Rivulin Keepers, 3 pp. (http://ark.killi.net/mento.htm, downloaded 9 March 2003).
Şen, D, Duman, E. and Ayvaz, Y. 1992. Age determination and length-weight relationship of Bertinius subquincuncinatus (Günther,1868) in Keban Dam Lake, Ege Üniversitesi Su Ürünleri
Yüksekokulu Su Ürünleri Dergisi, 9(33-36):203-210.
Šengör, A. M. C. 1984. The Cimmeride Orogenic System and the Tectonics of Eurasia. Special Paper of the Geological Society of America, 195:ix + 82 pp., map.
Sepahdari, A., Ebrahimzadeh Mosavi, H. A., Sharifpour, I., Khosravi, A., Motallebi, A. A., Mohseni, M., Kakoolaki, S., Pourali, H. R. and Hallajian, A. 2010. Effects of different dietary levels
of AFB1 on survival rate and growth factors of beluga (Huso huso). Iranian Journal of Fisheries Sciences, 9(1):141-150.
Sepahdari, A. A. F., Ebrahimzadeh Mousavi, H. A., Sharifpour I., Motalebi, A.
A., Khosravi, A. R., Kakoulaki, Sh. Pourali, H. R., Masoumzadeh, M. and Halajian, A. 2009. The study of skin lesions in beluga (Huso huso) fed with different dietary levels of aflotoxin
B1. Iranian Scientific Fisheries Journal, 18(2):35-43. In Farsi.
Sethi, R P. 1960. Osteology and phylogeny of oviparous cyprinodont fishes (order Cyprinodontiformes). Ph.D. Thesis, University of Florida (University Microfilms, Ann Arbor, Michigan, 275 pp.).
Seyed Mortezaei, S. R., Akhlaghi, M. and Abbasi, S. 2002. Comparison of different methods of in vitro culture and maintenance of Ichthyophthirius multifilis, the cause of white spot disease in
fish. Iranian Journal of Veterinary Research, 3(1):65-72.
Seyed Mortezaei, S. R., Pazooki, J. and Masoumian, M. 2008. Nematodes from fresh water fishes of Khouzestan Province. Pajouhesh va Sazandegi, 77(4):2-10. In Farsi.
Seyed Mortezaei, S. R., Pazooki, J., Masoumian, M. and Kor, N. M. 2008. Identification of Myxozoa and Protozoa parasites of barboid fishes of water resources in Khouzestan Province. Iranian Scientific
Fisheries Journal, 17(1):63-78. In Farsi.
Seyfabadi, S. J., Matinfar, A., Jafari Shamushaki, V. and Jorjani, M. 2005. Effect of L-carnitine on fingerlings of Acipenser persicus growth. Journal of Marine Science and
Technological Research, 1(3):1-7. In Farsi.
Seyfabadi, S. J., Matinfar, A. and Khodadoost, D. 2006. Effect of L-carnitine on growth and body protein and fat content of common carp. Journal of Marine Science and Technological Research,
1(1):21-27. In Farsi.
Seyfabadi, S. J., Oraji, H. and Nazari, R. M. 2002. Effect of L-carnitine on growth of kutum roach fry (Rutilus frisii kutum). Iranian Journal of Marine Science, 1(4):77-84, 139. In Farsi.
Seyfali, M., Kiabi, B. and Sheidai, M. 2002. Karyotype of Aphanius sp. from Iran. The Nucleus, 45(3):114-115.
Seyfzadeh, M. and Zare Gashti, Gh. 2007. Comparison of meat quality of farmed Acipenser persicus using two processing methods of dry and mix salting. Iranian Scientific Fisheries Journal,
16(2):93-102. In Farsi.
Shabani, A., Pourkazemi, M. and Rezvani, S. 2006. Study of mtDNA variation of stellate sturgeon (Acipenser stellatus) population from the north (Volga
River) and south (Sefidrud River) Caspian Sea using RELP anlaysis of PCR amplified ND5/6 gene regions. Journal of Agricultural Sciences and Natural Resources, 12(6):195-204. In Farsi.
Shabani, A., Pourkazemi, M., Rezvani, S. and Vitvitskaya, V. 2003. Genetic variation of stellate sturgeon, Acipenser stellatus, from the north
(Volga River) and south (Gorgan River) of Caspian Sea using RFLP analysis of PCR amplified ND 5/6 gene. Iranian Journal of Marine Sciences, 2(4):59-70. In Farsi.
Shabanipour, N. 1995. Histomorphological study on the ovary of Mugil auratus (Risso). Iranian Fisheries Scientific Journal, 4(2):47-62, 6. In Farsi.
Shabanpour, B. 1998. Determination of food conversion ratio of Daphnia sp. and Artemia sp. in larvae of Acipenser persicus. M.Sc. Thesis,
Gorgan University of Agriculture and Natural Resources, Gorgan. 72 pp. In Farsi.
Shabanpour, B., Asgharzadeh Kani, A., Hosseini, H. and Abbasi, M. 2008. Lipid quality changes of silver carp (Hypophthalmichthys molitrix) during frozen storage. Journal of Agricultural
Sciences and Natural Resources, 15(1):38-43. In Farsi.
Shabanpour, B., Kashiri, H., Moloudi, Z. and Hoseininezhad, A. S. 2007. Effects of washing bouts and times on surimi quality prepared from common carp (Cyprinus carpio). Iranian Scientific
Fisheries Journal, 16(1):81-92. In Farsi.
Shabanpour, B., Moini, S., Hamedi, M., Poorkabireh, M. and Seyfabadi, S. J. 2002. Effect of washing condition on organoleptic properties of surimi from kilka anchovy (Clupeonella
engrauliformis). Iranian Journal of Marine Sciences, 1(3):17-25, 78. In Farsi.
Shabanpour, B., Shaabani, A., Moeeni, S. and Poorkabireh, M. 2006. The effect of different washing methods on chemical and gel forming properties of kilka surimi. Pajouhesh va Sazandegi,
19(3)(72):84-92. In Farsi.
Shabanipour, N. and Heidari, B. 2004. A histological study of the zona radiata during late oocyte developmental stages in the Caspian Sea mugilid Liza aurata (Risso 1810).
Brazilian Journal of Morphological Sciences, 21(4):191-195.
Shadnoush, Gh. H. 2006. Acorn meal as a nutrient in rainbow trout (Oncorhynchus mykiss) diet. Iranian Journal of Fisheries Sciences, 15(3):87-96. In Farsi.
Shadnoush Gh. R., Shadnoush, F. and Taheri Mirghaed, A. 2008. Acorn meal as a pellet binder and its effects on chemical carcass characteristics in rainbow trout (Oncorhynchus mykiss). Iranian
Scientific Fisheries Journal, 17(3):99-106. In Farsi.
Shafaeipour, A., Yavari, V., Falahatkar, B., Maremmazi, J. Gh. and Gorjipour, E. 2008. Effects of canola meal on physiological and biochemical parameters in
rainbow trout (Oncorynchus mykiss). Aquaculture Nutrition, 14(2):110-119.
Shafaeipour, A., Yavari, V., Ghofleh Maramazi, J., Falahatkar, B. and Gorjipour, E. A. 2009. Effects of different levels of canola meal on growth, body composition and biochemical parameters in
rainbow trout (Oncorhynchus mykiss). Iranian Scientific Fisheries Journal, 18(1):81-100. In Farsi.
Shafi, M. and Jasim, B. M. 1982. Some aspects of the biology of a cyprinid, Aspius vorax Heckel. Journal of Fish Biology, 20:271-278.
Shafi, M. and Shalli, M. R. 1986. On the taxonomy, distribution and ecology of genus Aphanius Nardo (Cypriniformes (sic): Cyprinodontidae)
of Iraq. Bangladesh Journal of Zoology, 14(2):129-133.
Shafi, M., Shalli, M. R. and Saeed, B. M. 1981. Capoeta pestai (Pietschmann) (Cyprinidae) a new record for Iraqi waters. Bulletin of the Natural History Research Centre,
Baghdad, 7(4):(1980):173-175.
Shafizade, S. S., and Parivar, K. 1997. A study of the embryonic development of Persian sturgeon (Acipenser persicus Borodin).
Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (abstract).
Shafizadeh, S. S. and Vahabi, Y. 1996. Use of LH-RH in reproduction of sturgeon in Shahid Beheshti Hatchery in Iran. Shahid Beheshti Hatchery, Rasht. 155 pp. In Farsi.
Shah, S. L. 2002. Behavioural abnormalities of Cyprinion watsoni on exposure to copper and zinc. Turkish Journal of Zoology, 26(1):137-140.
Shahbazian, N., Ebrahimzadeh Mousavi, H. A., Soltani, M., Khosravi, A. R., Mirzagar, S. and Sharifpour, I. 2010. Fungal contamination in rainbow trout eggs in Kermanshah province propagations
with emphasis on Saprolegniaceae. Iranian Journal of Fisheries Sciences, 9(1):151-160.
Shahifar, R. 2001. Designing and establishing an HACCP system for Iranian caviar (1997-1999). 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Shahifar, R. 2006. Economic comparison between sturgeon fishery yield and releasing efforts of fingerlings in the southern Caspian Sea (1972-2003). Journal of Applied Ichthyology, 22(s1):119-124.
Shahsavani, D. 2002. Determination of some blood parameters fingerling sturgeon (Huso huso) in Guilan Province. Book of Abstracts, 27th World Veterinary Conference, September 25-29,
2002, Tunis-Tunisia.
Shahsavani, D., Farhoudi, M. Movasaghi, A. R. and Keykha, F. 2007. Clinical and pathological study of effects of phenytoin sodium in gill, liver and kidney of goldfish (Carassius auratus).
Pajouhesh va Sazandegi, 19(1)(74):150-155. In Farsi.
Shahsavani, D., Mohri, M. and Mazandarani, M. 2006a. Determination of reference values of some blood serum nonelectrolytes of Acipenser persicus. Pajouhesh va Sazandegi, 71:48-51. In Farsi.
Shahsavani, D., Mohri, M. and Mazandarani, M. 2006b. Determination of some blood serum electrolyte concentrations in Acipenser persicus. Iranian Veterinary Journal, 2(2)(13):112-117.
In Farsi.
Shahsavani, D., Mohri, M. and Nazeri, K. 2004. A study of anionic detergent (shampoo) on blood parameters of goldfish (Carassius auratus). Pajouhesh va Sazandegi,
16(4)(61):99-103. In Farsi.
Shahsavani, D., Mohri, M. and Taghvaeimoghadam, E. 2007. Determination of concentration of some blood serum enzymes of Huso huso. Journal of Veterinary Research, 62(3):127-129. In Farsi.
Shahsavani, D., Mohry, M. and Bijarchi, A. 2005. Effects of anionic detergent (labs) on serum biochemical parameters in Carassius auratus. Iranian
Journal of Fisheries Sciences, 14(2):51-58. In Farsi.
Shahsavani, D. and Movassaghi, A. 2003. Study on hepatic and renal pathology induced by anionic detergent in goldfish (Carassius auratus). Pajouhesh va Sazandegi,
16(2)(59):100-103. In Farsi.
Shahsavani, D., Movassaghi, A. and Sahebi, G. H. 2002. Survey of the healing effects of three drugs phenytoin sodium, znic oxid (sic) and vitamin A ointment on the gold fish
cutaneous lesions. Book of Abstracts, 27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Shahsavani, D., Movassaghi, A. R. and Baghi, L. 2005. Studying clinical and histopathological effects of phenytoin sodium on cutaneous wounds in the goldfish (Carassius auratus). Iranian Journal
of Fisheries Sciences, 13(4):65-74. In Farsi.
Shahsavani, D., Movasaghi (sic), A. R. and Faraji S. H. 2003. Clinical and histopathological study of experimental kerosene poisoning in goldfish (Carassius auratus). Pajouhesh va
Sazandegi, 16(1)(58):8-11. In Farsi.
Shahsavani, D., Movasaghi, A. R., Ghodrati, H. and Sahebi, Gh. H. 2001. Comparative study of the healing effects of zinc oxide and phenytoin sodium on cutaneous lesions of goldfish
(Carassius auratus). Pajouhesh va Sazandegi, 14(3)(52):98-102. In Farsi.
Shahsavani, D., Movassaghi, A. R. and Maghsoudlou, A. 2003. Clinical and histopathological study of the effects of an anionic detergent on the gill of goldfish (Carassius auratus). Journal of
the Faculty of Veterinary Medicine, University of Tehran, 58(1):29-32. In Farsi.
Shahsavani, D., Movasaghi, A. R. and Sahebi, Gh. H. 2002. Comparative histological survey of the healing effects of two drugs vitamin A and phenytion sodium ointment on the gold fish
(Carassius auratus) cutaneous lesion. Journal of Veterinary Research, 57(1):43-46. In Farsi.
Shahsavani, D., Vosoghi (sic), Gh. and Khazraeinia, P. 1998. Determination of some hematological factors in Acipenser stellatus in southeast Caspian Sea coasts. Pajouhesh
va Sazandegi, Tehran, 44(3):126-130. In Farsi.
Shasavani, D. Vosoughi Gh. H. and Khazraeinia, P. 2001. Determination of some blood parameters in fingerling sturgeons (Acipenser stellatus, A. persicus) in Gilan Province.
Pajouhesh va Sazandegi, 14(1)(50):14-18. In Farsi.
Shaikh, S. A. and Hafeez, M. A. 1993. Effects of photoperiod and temperature on gonadal response in the cyprinid fish, Cyprinion watsoni. Pakistan Journal of Zoology, 25(3):233-241.
Shaikh, S. A. and Jalali, S. 1989. Seasonal variation in the testes of the fish, Cyprinion watsoni. Proceedings of the Pakistan Congress of Zoology, 9:47-59.
Shaikh, S. A. and Jalali, S. 1991. Seasonal changes in the ovary of the cyprinid fish Cyprinion watsoni. Pakistan Journal of Zoology, 23(1):19-25.
Shajiee, H., Vossughi, G. H., Oryan, S. and Ramin, M. 2002. Biological characteristics of growth and reproduction in Barbus capito in south coasts of the Caspian Sea - Gilan Province.
Iranian Journal of Marine Science, 1(4):85-98, 138. In Farsi.
Shakirova, F. M. and Sukhanova, A. I. 1994. Iktiofauna Turkmenistana (sostav i rasprostranenie) [Ichthyofauna of Turkmenistan (composition and distribution)]. Izvestiya Akademii Nauk
Turkmenistana, Seriya Biologicheskikh Nauk, 3(1993):35-45.
Shalouie, F. and Imanpour, M. R. 2009. Study of ovarian fluid biochemical parameters and its influence on spermatozoa motility of the ship (Acipenser nudiventris Lovetzky, 1828) in the
south-eastern Caspian Sea. Iranian Scientific Fisheries Journal, 18(1):73-80. In Farsi.
Shalouei, F., Imanpour, M. R., Shaabani, A. and Baghfalaki, M. 2008. Correlation between seminal plasma indices and spermatozoa motility in ship sturgeon (Acipenser nudiventris Lovetzky, 1828).
Journal of Agricultural Sciences and Natural Resources, 15(1):22-27. In Farsi.
Shalouei, F., Shabani, A., Imanpour, M R., Shabanpour, B. and Baghfalaki, M. 2009. Spermatozoa motility in the ship sturgeon (Acipenser nudiventris Lovetzky, 1828): effects of pH, dilution
ratio, ions and osmolality. Journal of Agricultural Sciences and Natural Resources, 16(Special Issue 1-A).
In Farsi.
Shamloufar, M., Kamali, A., Piri, M., Yaghmaei, F. and Makhtoumi, N. M. 2007. The determination of LC50 of diazinon and its sub-lethal effect on haematological indices of
beluga (Huso huso). Iranian Scientific Fisheries Journal, 15(4):69-78. In Farsi.
Shams, M. and Afsharzadeh, S. 2009. Study of seasonal variations in phytoplankton in Zayandeh Rud Dam Lake. Iranian Journal of Biology, 21(5):784-795. In Farsi.
Shamsi, Sh. 1996a. A review on Heterophys heterophys, a parasite from fish to man. Aquatics, 69:55-56. In Farsi.
Shamsi, Sh. 1996b. Human parasitic diseases caused by fishes: Dyctophyma renale. Aquatics, 71:30-31. In Farsi.
Shamsi, Sh. and Dalimi, A. 1996. Identification of Pseudopentagramma symmetrica in Clupeonella spp. in the Caspian Sea. (Pajouhesh va Sazandagi (Research and Reconstruction
Journal), 32:104-105 (In Farsi).
Shamsi, Sh. and Dalimi, A. 2001. Study on parasitic infection of Caspian common kilka. Proceedings of the 5th Symposium of Marine and Atmospheric Sciences, 7-9 March 2001, p. 107. In Farsi.
Shamsi, Sh., Dalimi, A., Jalali, B. and Alizadeh Shabani, A. 1998. Incidence and intensity of helminths in Clupeonella grimmi Kessler 1877 (Clupeidae) from Caspian Sea. The 18th
International Congress of Parasitology,.24-28 August 1998, Makuhari, Chiba, Japan, Parasitology International, 47(supplement):170.
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1996a. Study on helminths of anchovy kilka. Proceedings of the 5th Iranian Biology Conference, University of Tabriz, Tabriz, Iran, 22-24 August 1996 (abstract).
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1996b. Helminths of Clupeonella engrauliformis from south coast of Caspian Sea, p. 50. In: Abstracts VII European Multicolloquium of Parasitology,
2-6 September 1996, Parma, Italy, Parassitologia, 38(1-2):1-479.
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1997a. First record of Pseudopentagramma symmetrica from I. R. Iran. Proceedings of the First Iranian Congress of Zoology, Tehran University
of Teacher Education, Tehran, Iran, 17-18 September 1997, p. 92.
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1997b. Study on helminths of bigeye kilka, Clupeonella grimmi. Proceedings of the 6th Iranian Biology Conference, Shahid Bahonar University
of Kerman, 25-27 August 1997, p.67.
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1998a. Study on zoonotic parasites in kilka fish. Iranian Fisheries Scientific Journal, 7(1):45-58. In Farsi.
Shamsi, Sh., Dalimi, A. and Pourgholam, R. 1998b. Caspian kilka as a new host for some helminths. Proceedings of the 7th Iranian Biology Conference, University of Isfahan, Isfahan, Iran,
22-24 August 1998, p. 138.
Shamsi, Sh., Dalimi, A., Pourgholam, R. an Habibi, F. 1997. Parasitic study on Caspian kilka and its zoonoses. Proceedings of the 3rd Iranian Congress of Veterinary Students, University of
Urmia, Urmia, Iran, 23-26 April 1997, p. 83.
Shamsi, Sh. and Jalali, B. 1997. First record of some freshwater fish parasites (monogeneans) in Iran. Third International Symposium of Monogenea, 25-30 August 1997, Mazaryc University, Brno,
Czech Republic, Papers and Abstracts, p. 76.
Shamsi, Sh. and Jalali, B. 2001a. Monogenean parasites of Barbus fishes of Iran. Proceedings of the First National Symposium on Barbus Fishes of Iran. Khuzestan Fisheries
Research Center, Ahwaz, Iran, 9-10 January 2001, p. 12.
Shamsi, Sh. and Jalali, B. 2001b. Monogenean parasites of the Caspian roach (Rutilus frisii kutum) in Iran. In: ISM 4: 4th International Symposium on Monogenea, Brisbane, Australia,
9-13 July 2001, p. 80 (abstract).
Shamsi, Sh. and Jalali, B. 2001c. Monogenean parasites of Caspian Frisian roach (Rutilus frisii kutum) in Sefid-Rood River and Caspian Sea. Iranian Journal of Fisheries Science, 3(2):19-24.
Shamsi, Sh. and Jalali, B. 2003. Occurrence of Gyrodactylus pp. (Monogenea: Gyrodactylidae) from Iranian freshwater fishes. 45th Annual Scientific Meeting of the Australian Society for
Parasitology, 6-10 July 2003, Darwin (title).
Shamsi, S. (sic), Jalali, B. and Aghazadeh Meshgi, M. 2009. Infection with Dactylogyrus spp. among introduced cyprinid fishes and their geographical distribution in Iran. Iranian Journal
of Veterinary Research, Shiraz University, 10(1)(26):70-74.
Shamsi, Sh. and Pourgholam, R. 2001. Study on helminths of fishes of Shiroud River. Proceedings of the First Iranian Congress of Health and Diseases, University of Ahwaz, Iran, 13-15
February 2001, p. 48.
Shamsi, Sh., Pourgholam, R. and Daleemi-e-Asl, A. 1997. Infection rate of the Shiroud River fishes with Clinostomum complanatum. Iranian Fisheries Scientific Journal, 6(2):53-62, 9. In Farsi.
Shapland, G. 1997. Rivers of Discord. International Water Disputes in the Middle East. St. Martin's Press, New York. xi + 183 pp.
Shargh, S., Shamsaii, M. and Karimi, S. 2008. Distribution of parasitic cestod (sic) "Ligula intestinalis" in Mazandaran region. Iranian Journal of Parasitology, 3(2):26-33.
Shariati, A. 1994. More about Iranian acetra. Abzeeyan, Tehran, 5(7):16-17. In Farsi.
Shariati F. 2004. Status of sturgeon stocks in the south of Caspian Sea. The 4th International Iran and Russia Conference "Agriculture and Natural Resources", 8-10 September,
Shahr-e Kord, Iran (title).
Shariatt, M-T. 2003. Function of libraries and printed materials in Iranian aquaculture information system. World Aquaculture 2003, 20-23 May, Salvador, Brazil (title).
Shariati, F., Esmaeili Sari, A. and Piri, M. 2004. Toxicity and LC50 determination of phenol and 1-naphthol in Caspian kutum and bream fingerlings.
Iranian Journal of Fisheries Sciences, 12(4):57-68. In Farsi.
Shariati, M. T. and Nikfetrat, E. 2005. The attitude of fishermen towards stock enhancement and influencing factors: A case study in Guilan Province of Iran. Iranian Journal of Fisheries
Sciences, 5(1):85-104.
Sharif Rohani, M. 1994. Survey on parasites and parasitic diseases in Sistan region. Proceedings of the 2nd Symposium of Iranian Veterinary Clinics, Tehran.
p. 109. In Farsi.
Sharifi, M. 2006. The pattern of Caspian Sea water penetration into Anzali Wetland: introduction of a salt wedge. Caspian Journal of Environmental Sciences, 4(1):77-81.
Sharifian, M. 1998. Changes of cortisol hormone caused by stressing rainbow trout (Oncorhynchus mykiss). Iranian Journal of Fisheries Sciences, 7(3):33-40, 4. In Farsi.
Sharifian, M. 2006. Determination of feeding benni (Barbus sharpyie)(sic)(first phase: whole body analysis and biological characteristics). Iranian Fisheries Research
Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture, Ahvaz. http://en.ifro.ir (abstract).
Sharifpour, A., Soltani, M., Abdolhai, H. and Ghauomi, R. 2003. Study of anaesthetic effects of clove oil (Eugenia caryophyllata) in common carp (Cyprinus carpio) under various
pH and temperature condition. Iranian Journal of Fisheries Sciences, 11(4):59-74. In Farsi.
Sharifpour, I. 2004. Experimental study on histology of circumstance of wound healing process in common carp (Cyprinus carpio). Iranian Journal of Fisheries Sciences, 13(2):91-116. In Farsi.
Sharifpour, I., Pourgholam, R., Soltani, M., Hassan, M. D., Akbari, S. and Nouri, A. 2006. Light and electron microscope studies of grass carp (Ctenopharyngodon idella) organs
following exposure to various sublethal concentrations of diazinon. Iranian Journal of Fisheries Sciences, 5(2):111-136.
Sharifpour, I., Soltani, M. and Fathollahi, B. 2005. A study of endosulfan caused lethal effects as well as histopathological changes in common carp (Cyprinus carpio). Iranian Journal
of Natural Resources, 58(2):373-382. In Farsi.
Sharifpour, I., Soltani, M. and Javadi, M. 2001. Determination of LC50 and pathological effects due to endosulfan in beluga sturgeon (Huso huso).
4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Sharifpour, I., Soltani, M. and Javadi, M. 2004. Determination of LC50 and histopathological changes due to endosulfan in beluga (Huso huso). Iranian Journal
of Fisheries Sciences, 12(4):69-84. In Farsi.
Sharifpour, I., Yousefian, M. and Khalesi, M. K. 2003. Reproduction cycle and histology of the oocyte maturation in cultured grey mullet (Mugil cephalus). Iranian Journal of Marine
Sciences, 2(1):23-34. In Farsi.
Sharifrohani, M. 2004. Evaluation of antifungal effects of some plants essential oils as a possible replacement with malachite green in rainbow trout
eggs under farm condition. Ph.D. Thesis, University of Tehran. 102 pp. In Farsi.
Sharma, B. P. 1988. Status of Schizothorax species in the Indian-Chinese Sub-Continent. Food and Agriculture Organization Indo-Pacific Fishery Commission, Bangkok (Thailand). Workshop
on the Use of Cyprinids in the Fisheries Management of Larger Inland Water Bodies of the Indo-Pacific. 4. Session of the Indo-Pacific Fishery Commission Working Party of Experts on Inland
Fisheries Commercial Species, Kathmandu (Nepal), 8-10 September 1988, FAO FIRI/405 (Supplement), pp. 90-94.
Sharma, K. P. 1980. Further studies on the fish marketing conditions of Southern Iraq. The Arab Gulf, Basrah, 2(1):223-228.
Sharma, K. P. and Al-Daham, N. K. 1979. Comparative studies on the efficiency of Aphanius dispar (Ruppell) and Gambusia affinis (Baird & Girard) in mosquito control.
Comparative Physiology and Ecology, 4(2):106-109.
Sharma, R. C. 1984. Dynamics of food and feeding habits of Crossocheilus latius (Hamilton) in fluvial ecosystem of Garhwal Himalaya. Comparative Physiology and Biochemistry, 9(4):305-308.
Sharvashidze, V. A. 1984. Ryby vnutrennikh vodoemov Gruzinskoi SSR (Morfo-ekologicheskaya kharakteristika i khozyastvennoe znachenie) [Fishes of the interior reservoirs of Georgia
(Morpho-ecological characteristics and economic significance)]. Izdatel'stvo "Sabchota Sakartvelo", Tbilisi. 214 pp.
Shayegan, J. and Badakhshan, A.1996. Causes and effects of the water-level rise in the Caspian Sea. Lakes and Reservoirs: Research and Management, 2(1-2):97-100.
Shcherbukha, A. Ya. 1973. Primenenie taksonomicheskogo analiza dlya vyyasneniya rodstvennykh svyazei mezhdu predstavitelyami rodov Abramis i Blicca (Pisces, Cyprinidae) [Use of taxonomic
analysis for understanding the relationship between the members of genera Abramis and Blicca (Pisces, Cyprinidae)]. Zoologicheskii Zhurnal, 52(1):225-228.
Shearman, D. J. 1976. The geological evolution of southern Iran. The report of the Iranian Makran Expedition. Geographical Journal, 142:393-410.
Shehadeh, Z. H. 1997. West Asia, 4 pp. In: Review of the state of world aquaculture. Food and Agriculture Organization, Rome, Fisheries Circular, 886, Revision 1:163 pp.
Sheibani, M. T. 2003a. Macroscopic and microscopic study of esophagus, glandular stomach, pyloric part and gizzard of Acipenser stellatus. Pajouhesh va Sazandegi, 16(1)(58):28-32. In Farsi.
Sheibani, M. T. 2003b. Macroscopic and microscopic study of posterior alimentary canal of digestive tract of the Russian sturgeon, Acipenser guldenstadti. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 58(1):45-48. In Farsi.
Sheibani, M. T. 2005. Macroscopic and microscopic study of spleen and gut associated lymphatic tissue (GALT) in Acipenser persicus. Journal of Veterinary Research, 60(1):37-42. In Farsi.
Sheibani, M. T. 2006. Histological and histochemical study of secretory components in distal section of digestive tract of Acipenser stellatus. World Aquaculture Society, Aqua 2006,
May 9-13, Florence, Italy (abstract).
Sheibani, M. T. and Adibmoradi, M. 2002. Histological study of the liver and pancreas and their ducts in Acipenser stellatus. Journal of the Veterinary Faculty of the University of Tehran,
57(1):19-23. In Farsi.
Sheibani, M. T. and Pahlavan, M. 2003. Histological study of larval development of accessory digestive glands of Acipenser persicus from fry to fingerling. Journal of the Faculty of
Veterinary Medicine, University of Tehran, 58(4):341-345. In Farsi.
Sheibani, M. T. and Pahlavan Yali, M. 2006. Histological structures of the accessory glands of the digestive system in adult Caspian Sea beluga (Huso huso). Journal of Applied Ichthyology,
22(s1):193-195.
Sheibani, M. T. and Pousti, I. 2001. Histological study of intestines of Acipenser persicus. Pajouhesh va Sazandegi, 13(4)(49):89-91. In Farsi.
Sheikhi Moghaddam, L., Emtyazjoo, M. and Emadi, H. 2004. Alvita as alternative to malachite green in cold water fish systems. Journal of Environmental Science and Technology, 21:30-44. In Farsi.
Sheil, Lady. 1856. Glimpses of Life and Manners in Persia with Notes on Russia, Koords, Toorkomans, Nestorians, Khiva, and Persia. John Murray, London. xi + 402 pp.
Shenavar Masouleh, A. R., Bazari Moghadam, S., Jalilpour, J., Masoomzadeh, M., Masoumian, M., Sattari, M., Shafiei, Sh., Nooshi Masouleh, N. and Nooshali, M. 2006. Study on the prevalence and
intensity of three parasites in sturgeon fingerlings cultured in earthen ponds. Iranian Journal of Fisheries Sciences, 15(2):55-62. In Farsi.
Shenavar Masouleh, A., Sattari, M., Masoumian, M., Ebrahimzadeh Mousavi, H., Jalilpour J., Masoumzadeh, M. and Bazari Moghaddam, S. 2003. Qualitative and quantitative study of sturgeon fingerlings
(quality control). Final Report, Iranian Fisheries Research Organization. 173 pp. In Farsi.
Shevchenko, U. P., Rogov, M. A. and Komaei, I. E. 1999. Rearing technology for fingerlings of the Sefidrud Russian sturgeon (Acipenser gueldenstaedtii B.) in the Islamic Republic of
Iran. Journal of Applied Ichthyology, 15(4-5):331.
Sheykhzadeh, N., Soltani, M., Ebrahimzadeh Mousavi, H. A., Khosravi, A. R., Bagheri Hadi, F. E. A. and Zargar, A. 2009. Effects of Eucalyptus globules labile essential oils on some immunological
variables of common carp (Cyprinus carpio). Journal of Veterinary Research, 64(1):47-54. In Farsi.
Shiati, K. 1989. The origin and sources of salinity in river basins: A regional case study in southern Iran. International Association of Hydrological Sciences Publication, 182:201-210.
Shikhshabekov, M. M. 1969. Different forms of vobla, bream, and carp in the Arakum waters of Dagestan. Problems of Ichthyology, 9(1):34-38.
Shikhshabekov, M. M. 1977. Annual cycle of ovaries and testes in the tench, Tinca tinca, in the waters of Dagestan. Journal of Ichthyology, 17(4):685-689.
Shikhshabekov, M. M. 1978. The sexual cycles of the catfish, Silurus glanis, the pike, Esox lucius, the perch, Perca fluviatilis,
and the pike-perch, Lucioperca lucioperca. Journal of Ichthyology, 18(3):457-468.
Shikhshabekov, M. M. 1979. The reproductive biology of the "kutum", Rutilus frisii kutum, the asp, Aspius aspius, the vimba, Vimba
vimba persa, and the rudd, Scardinius erythrophthalmus, in the waters of Dagestan. Journal of Ichthyology, 19(3):98-105.
Shilat, Tehran. 1994. Fisheries in Iran (Shilat). Shilat (Fisheries), Tehran. 212 pp. In Farsi (reports in other years too).
Shilat, Tehran. 1996. Consumer behaviour on fishery products in Tehran. Shilat (Fisheries), Tehran. 86 pp. In Farsi.
Shilat, Tehran. 1997a. Shilat Yearbook. Shilat (Fisheries), Tehran. 212 pp. In Farsi.
Shilat, Tehran. 1997b. Fisheries statistics. Comprehensive Development Studies Department, Shilat (Fisheries), Tehran. 63 pp. In Farsi.
Shilat, Tehran. 1997c. Production, imports, exports and per capita fish consumption in Iran. Comprehensive Development Studies Department, Shilat (Fisheries), Tehran. 85 pp. In Farsi.
Shilat, Tehran. 1998a. Annual report of aquaculture production in Iran. Aquaculture Department, Shilat (Fisheries), Tehran. 168 pp. In Farsi. (annual reports in other years too).
Shilat, Tehran. 1998b. Fisheries production report in Iran. Shilat (Fisheries), Tehran. 67 pp. In Farsi.
Shirazinejad, A. R. and Saremi, A. 2000. Biology of Caspian lamprey (Caspiomyson wagneri). Dissertation, Esfahan University of Technology. In Farsi.
Shireman, J. V. and Smith, C. R. 1983. Synopsis of biological data on the grass carp, Ctenophaon idella (Cuvier and Valenciennes, 1844). Food and Agriculture Organization,
Rome, Fisheries Synopsis, 135:iv + 86 pp.
Shiri, S., Seidgar, M. and Najafpour, N. 2009. Ichthyotoxism in Capoeta capoeta from West Azerbaijan of Iran (Actinopterygii: Cyprinidae). 1st International Congress on Aquatic Animal Health Management
and Diseases, 27-28 January 2009, Tehran (abstract).
Shirvani, E. and Jamili, S. 2009. Assessing Cd, Pb accumulation in the tissues of Chalcalburnus chalcoides in Anazali Port. Research Journal of Environmental Sciences, 3(5):522-529.
Shnitnikov, A. V. 1969. On the Holocene history of the Aral. Mitteilungen internationale Vereinigung für theoretische und angewandte Limnologie, 17:400-413.
Shojaei, A. 1998. Producing cracker from kilka. Iranian Fisheries Scientific Journal, 7(1):25-44, 4. In Farsi.
Shojaei, A. H. 1998. Cracker from kilka. Fishery Technology, Society of Fisheries Technologists (India), Kochi, 35(2):90-94.
Shokrzadeh, M. and Ebadi, A. G. 2005. Investigating and measurement of residues of D.D.T. and D.D.E. in four species of fishes in Caspian Sea, Iran. International Journal of Biology and
Biotechnology, 2(1):53-56.
Shokrzadeh, M. and Ebadi, A. G. 2006. Investigating and measurement of residues of chlorobenzilate (organochlorine pesticides) in four species of the most consumed fishes in Caspian Sea (Iran).
Pakistan Journal of Nutrition, 5(1):68-70.
Shokrzadeh, M., Saeedi Saravi, S. S. and Zehtab Yazdi, Y. 2009. Lindane residues in cultivated cucumber and in the most consumed fish in Caspian Sea (Iran). Toxicology and Industrial Health,
25(8):517-523.
Shubina, L. I. 1981. Growth and sexual maturation of the Caspian shad Alosa caspia caspia. Journal of Ichthyology, 21(2):98-108.
Shukolyukov, A. 1937a. K voprosu ob akklimatizatsii cherno-morskikh ryb v Kaspiiskom more [Acclimatization of Black Sea fish in the Caspian Sea]. Rybnoe Khozyaistvo, 6:34-35.
Shukolyukov, A. 1937b. Ob uvelichenii zapasov ryby [On the increase of fish stocks]. Rybnoe Khozyaistvo, 7:29-30.
Shukolyukov, A. M. 1949. Gambuziya v Irane (Gambusia in Iran). Priroda, 1949(5):61.
Shutov, V. A. 1969. O revizii roda Blicca i nekotorykh pokazatelyakh fileticheskikh svyazei mezhdu predstavitelyami roda Abramis (Pisces, Cyprinidae) [Revision of the genus Blicca and
some data concerning phyletic relationships between representatives of the genus Abramis (Pisces, Cyprinidae)]. Zoologicheskii Zhurnal, 48:1105-1107.
Sil'chenko, G. F. 1976. Reproduction of sichel (Pelecus cultratus) stocks in Kuybyshev Reservoir. Journal of Ichthyology, 16(6):931-939.
Silva, K., Monteiro, N. M., Almada, V. C. and Vieira, M. N. 2006. Early life history of Syngnathus abaster. Journal of Fish Biology, 68(1):80-86.
Silva, K., Monteiro, N. M., Vieira, M. N. and Almada, V. C. 2006. Reproductive behaviour of the black-striped pipefish Syngnathus abaster
(Pisces; Syngnathidae). Journal of Fish Biology, 69(6):1860-1869.
Simonovič, P. 1996. Karyological relationships between gobies (Gobiidae: Perciformes) of Euro-Mediterranean and Ponto-Caspian Districts. Ichthyologia, 28(1):25-31.
Simonovič, P., Paunovič, M. and Popovič, S. 2001. Morphology, feeding and reproduction of the round goby, Neogobius melanostomus (Pallas), in the Danube River basin,
Yugoslavia. Journal of Great Lakes Research, 27(3):281-289.
Simonovic P. D. 1999. Phyolgenetic relationships of Ponto-Caspian gobies and their relationship to the Atlantic-Mediterranean Gobiinae. Journal of Fish Biology, 54:533-555.
Simonovič, P. D., Nikolič, V. P. and Skora, K. E. 1996. Vertebral number in Ponto-Caspian gobies: phylogenetic relevance. Journal of Fish Biology, 49(5):1027-1029.
Singh, H. R. and Bahuguna, S. N. 1984. Eco-morphological adaptation of the gill rakers in relation to the food and feeding habits of some hillstream fishes of Garhwal Himalaya.
Vĕstník československé Společnosti zoologické, 48:64-68.
Singh, N. 1993. The scanning electron microscopic structure of the `adhesive apparatus' and mechanism of adhesion in hillstream teleost, Crossocheilus latius latius (Family-Cyprinidae)
from Garhwal Himalaya. Journal of Animal Morphology and Physiology, 40(1 & 2):109-114.
Singh Kohli, M. P. and Goswami, U. C. 1987. Spawning behaviour of a freshwater air-breathing Indian catfish Heteropneustes fossilis (Bloch). Matsya, 12 & 13:180-183.
Singhkohli, M. P. and Goswami, U. C. 1987. Teratological manifestations in an air-breathing catfish Heteropneustes fossilis (Bloch). Matsya, 12 & 13:188-192.
Sinha, V. R. P. and Jones, J. W. 1975. The European Freshwater Eel. Liverpool University Press, Liverpool. xv + 146 pp.
Sirang, H. 1997. Artificial propagation of tench (Tinca tinca) to fingerling size in 1994. Iranian Fisheries Scientific Journal, 6(2):43-52, 7-8. In Farsi.
Sivasubramaniam, K. and Ibrahim, M.A. 1982. Common Fishes of Qatar. Scientific Atlas of Qatar, 1. Doha, Qatar.
Sizhong, L. 1995. On the geographical distribution of the Schizothoracine fishes (Cypriniformes, Cyprinidae). Sinozoologia,12:297-310. In Chinese.
Skadhauge, E. and Lotan, R. 1974. Drinking rate and oxygen consumption in the euryhaline teleost Aphanius dispar in waters of high salinity. Journal of Experimental Biology, 60(2):547-556.
Skandari, S. 1998. Investigation on some biological, ecological and parasitological characteristics of khramulya (Capoeta capoeta gracilis) in the river of Golestan National Park. M.Sc.
Thesis, Tarbiat Modarres University, Tehran. 111 pp. In Farsi.
Skora, K. E. and Rzeznik, J. 2001. Observations on diet composition of Neogobius melanostomus Pallas 1811 (Gobiidae, Pisces) in the Gulf of Gdansk (Baltic Sea). Journal of Great
Lakes Research, 27(3):290-299.
Šlechtová, V., Bohlen, J. and Perdices, A. 2008. Molecular phylogeny of the freshwater fish family Cobitidae (Cypriniformes: Teleostei): Delimitation of genera, mitochondrial introgression and
evolution of sexual dimorphism. Molecular Phylogenetics and Evolution, 47(2):812-831.
Šlechtová, V., Bohlen, J. and Tan, H. H. 2007. Families of Cobitoidea (Teleostei; Cypriniformes) as revealed from nuclear genetic data and the position of the mysterious genera
Barbucca, Psilorhynchus, Sepenticobitis and Vaillantella. Molecular Phylogenetics and Evolution, 44(3):1358-1365.
Smirnov, A. I. 1992. Reviziya taksonov roda podust Chondrostoma Agassiz, 1835 (Pisces, Cyprinidae) vostochnoy Evropy v predyelaj byvshego SSSR [Taxonomic revision of the genus
Chondrostoma Agassiz, 1835 (Pisces, Cyprinidae) from eastern Europe and the former USSR]. Institut Zoologii, Akademii Nauk Ukrainy, Kiev. 63 pp.
Smith, A. 1951.Qanāts. Journal of the Iran Society, London, 1:86-90.
Smith, A. 1953. Blind white fish in Persia. George Allen and Unwin, London. 207 pp. (1966 Edition). Also in French with additional illustrations as "La Perse et ses mystères",
Plon, Paris. x + 274 pp. (1957).
Smith, A. 1977. Blind white fish discovery. The Illustrated London News, February 1977, p. 37, 39.
Smith, A. 1978. The blind fishes of Persia. Nature, London, 271:711.
Smith, A. 1979. A Persian Quarter Century. Hodder and Stoughton, London. 192 pp.
Smith, A. 2004. Letter to the Editor. The Linnaean (Newsletter and Proceedings of the Linnaean Society of London), 20(3):16-17.
Smith, C., Reichard, M., Jurajda, P. and Przybylski, M. 2004. The reproductive ecology of the European bitterling (Rhodeus sericeus). Journal of Zoology, London, 262(2):107-124.
Smith, G. R. and Stearley, R. F. 1989. The classification and scientific names of rainbow and cutthroat trouts. Fisheries, 14(1):4-10.
Snyder, J. A., Wasylik, K., Fritz, S. C. and Wright, H. E. 2001. Diatom-based conductivity reconstruction and palaeoclimatic interpretation of a 40-ka record from Lake Zeribar, Iran.
Holocene, 11(6):737-745.
Sociedad de Estudios Ictiológicos. 2006. Boletín No 0, Mayo de 2006. 56 pp. (www.soesic.org).
Södergren, A., Djirsarai, R., Gharibzadeh, M. and Moinpour, A. 1978. Organochlorine residues in aquatic environments in Iran, 1974. Pesticides Monitoring Journal, 12(2):81-86.
Sohrabi, A. 1996a. Esbeleh, the monster of the wetland. Shekar-o Tabiat, Tehran, (42):28-30. In Farsi.
Sohrabi, A. 1996b. Ordak mahi. Shekar-o Tabiat, Tehran, (43):54-57. In Farsi.
Sohrabi, H. I. and Jalali, J. B. 2002. Observation of a nematod (sic) (Schulmanella petruschewskii, Schulman (sic)) infection in
a barble (sic) (Barbus koswigi (sic)) in Iran. Iranian journal of Marine Sciences, 1(2):47-50, 84. In Farsi.
Solak, K. 1977.Çoruh-Aras Havzası, Caner ve Murzu Balıklarının (Barbus Türleri) Dağılışında Populasyon Dinamiği Üzerine
Araştırmalar [Investigations on the population dynamics of the Barbus species (Caner and Murzu) in the Çoruh-Aras basin].Ege Üniversitesi Fen Fakültesi Dergisi,
Seri B, 1(4):361-374.
Solak, K. 1978.Çoruh ve Aras Havzasında Yaşayan Üç Barbus (Cyprinidae) Türü Barbus plebejus BON., Barbus mursa
(GÜLD.), Barbus capito (GÜLD.).Doğa Bilim Dergisi, 2(3):161-167.
Solak, K. 1989a.Aras Havzasında Yaşayan Barbus plebejus lacerta Heckel, 1843'nin (Cyprinidae, Pisces) Yaş-Boy ve Yaş-Ağırlık Ilişkileri
Üzerine Araştırmalar. Doğa Türk Zooloji Dergisi, 13(1):28-33.
Solak, K. 1989b.Kura ve Aras Havzasışayan Barbus mursa mursa (Güldenstadt, 1773)'nin (Cyprinidae, Pisces) Yaş-Boy ve Yaş-Ağırlık
Ilişkileri Üzerine Araştırmalar [Investigations on the age-length and age-weight relations of Barbus mursa mursa (Güldenstadt, 1773)(Cyprinidae, Pisces)
inhabiting Kura and the Arax basins].Doğa Türk Zooloji Dergisi, 13(1):34-38.
Solak, K. 1989c.Çoruh ve Aras Havzası'nin Bazı Derelerinda Yaşayan Barbus capito capito (Güldenstadt, 1773)'nun Yaş-Ağırlık ve
Yaş-Boy Dağılımı [Age-length and age-weight distribution of Barbus capito capito (Güldenstadt, 1773) inhabiting some streams of the Çoruh and
the Arax basins].Doğa Türk Zooloji Dergisi, 13(2):109-118.
Soleimani, A. 1994. A preliminary study of benthic fauna in southern coastal waters of the Caspian Sea. Iranian Fisheries Scientific Journal, 3(2):41-56. In Farsi.
Soleymani, A. R. and Karimabadi, A. 2009. The temperature effects of the embryonic period of the Persian sturgeon (Acipenser persicus). Iranian Scientific Fisheries Journal, 18(2):171-176.
In Farsi.
Soleymani, N., Hajimoradlou, A. A., Ghorbani, R., Khoushbavar Rostami, H. A. and Hasanabadizadeh, Z. 2008. Effect of different levels of injectable vitamin C on common carp, Cyprinus carpio
survival challenged by different doses of theront of Ichthyophthirius multifilis. Journal of Agricultural Sciences and Natural Resources, 15(6):132-138. In Farsi.
Solijinov, S. 2007. Review on a fish fauna of the Pamir-Alai transboundary conservation area (overview report). Report on the Establishment of Pamir-Alai transboundary conservation area
between Kyrgyzstan and Tajikistan. Dushanbe. 8 pp.
Soltani, M. 2000. Study of lethal toxicity and histopathological effects due to organochlorine pesticide, Endosulfan, in common cap (Cyprinus carpio). The Third World
Fisheries Congress, Beijing, 31 Oct - 2 Nov 2000 (title).
Soltani, M. 2003. The status of aquaculture health management in Iran. AquaEuro 2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Soltani, M. 2005. Streptococcosis caused by Streptococcus iniae in farmed rainbow trout in Iran: epidemiological aspects. European Association of Fish Pathologists, 12th International
Conference, "Diseases of Fish and Shellfish', 11-16 September 2005, Copenhagen (title).
Soltani, M., Alishahi, M., Khazrayeenia, P., Rabani, M. and Sattari, A. 2007. Study of some immunological responses of rainbow trout (Oncorhynchus mykiss)
to some antigens of Streptococcus iniae. Journal of Veterinary Research, 61(1):1-9. In Farsi.
Soltani, M., Alishahi, M., Mirzargar, S. and Nikbakht, Gh. 2007. Vaccination of rainbow trout against Streptococcus iniae infection: comparison of different routes of administration and
different vaccines. Iranian Journal of Fisheries Sciences, 7(1):129-140.
Soltani, M., Esfandiari, M., Khazraeinia, S. and Sajadi, M. M. 2009. Effects of Zataria multiflora essential oil on rainbow trout (Oncorhynchus mykiss) egg hatching and survival of
larvae compared with hydrogen peroxide and malachite green. Journal of Veterinary Research, 64(2):127-134. In Farsi.
Soltani, M., Fadaeifard, F., Sharifpour, I. and Zargar, A. 2009. Experimental pathology of Streptococcus sp. in rainbow trout, Oncorhunchus mykiss. Iranian Scientific Fisheries
Journal, 17(4):81-88. In Farsi.
Soltani, M., Falahatkar, B., Pourkazemi M., Abtahi, B., Kalbasi, M. R. and Mohseni, M. 2008. Effects of dietary L-ascorbyl-2-polyphosphate as a source of vitamin C on growth indices in beluga
sturgeon (Huso huso L.). Iranian Scientific Fisheries Journal, 17(3):107-120. In Farsi.
Soltani, M., Fard, F. F. and Mehrabi, M. R. 1999. First report of a yersinosis-like infection in Iranian farmed rainbow trout. Bulletin of the European Association of Fish Pathologists, 19(4):173-176.
Soltani, M., Ghafari, M., Khazraeinia, P. and Bokaei S. 2004. Effects of clove oil (Eugenia caryophyllata) anesthesia on haematological parameters, certain serum enzymes and some tissues in
common carp (Cyprinus carpio). Journal of Veterinary Research, 59(3):295-299. In Farsi.
Soltani, M. and Kalbassi, M. R. 2001. Protection of Persian sturgeon (Acipenser persicus) fingerling against Aeromonas hydrophila septicemia using three different
antigens. Bulletin of the European Association of Fish Pathologists, 21(6):235-240.
Soltani, M., Kalbassi, M. R., Mohammad Nazar, R. and Mostafvi, H. 2001. Therapeutic effects of formalin on hatch rate of common carp (Cyprinus carpio) eggs under farmed conditions in Shahid
Rajaii Centre, Sari, Iran. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(4):69-71. In Farsi.
Soltani, M. and Khoshbavar-Rostami, H. 2002. Study of diazinon effects on some hematological and biochemical parameters of Acipenser guldenstadti. Iranian Journal of Marine Science,
1(4):65-76, 140. In Farsi.
Soltani, M., Khoshbavar-Rostami, H. A. and Hassan, H. M. D. 2005. Assessment of some immune variables of glucan received giant sturgeon (Huspo huso) following long-term exposure to
sublethal concentration of diazinon. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Soltani, M., Khoshbavar-Rostami, H. A. and Mokarami, A. 2005. Toxicity and hematological changes and chemiluminescence response of Persian sturgeon (Acipenser persicus) following
exposure to polyaromatic hydrocarbons (PAHs). Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme, 25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title).
Soltani, M., Khoshbavar-Rostami, H. A. and Mokarami, A. 2006. Toxicity, hematological changes and chemiluminescence response of Persian sturgeon Acipenser persicus following exposure
to polyaromatic hydrocarbons (PAHs). World Aquaculture Society, Aqua 2006, May 9-13. Florence, Italy (abstract).
Soltani, M., Omidbeigi, R., Rezvani, S., Mehrabi, M. R. and Chitsaz, H. 2001. Study of anaesthetic effects induced by clove flower (Eugina caryophillata) on rainbow trout (Oncorhynchus
mykiss) under various water quality conditions. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(4):85-89. In Farsi.
Soltani, M. and Pourgholam, R. 2007. Lysozyme activity of grass carp (Ctenopharingodon (sic) idella) following exposure to sublethal concentrations of
organophosphate, diazinon. Journal of Veterinary Research, 62(2):49-52.
Soltani, M., Pourgholam, R., Kazemi, B. and Hassan, M. D. 2003. Purification and partial characterization of serum immunoglobulin from grass carp (Ctenopharyngodon idella). Bulletin
of the European Association of Fish Pathologists, 23(3):102-107.
Soltani, M. and Rahanandeh, M. 2001. Report of blout (sic) in Mugil capito in the Caspian Sea. Journal of the Faculty of Veterinary Medicine, University of Tehran, 56(1):106-107.
In Farsi.
Soltani, M. and Rostami, M. 1997a. First report of a flexibacteriosis-like infection in farmed rainbow trout in Iran. In: 8th International Conference of the European Association of Fish
Pathologists: Diseases of Fish and Shellfish, Edinburgh, 14-19 September 1997 (abstract).
Soltani, M. and Rostami, M. 1997b. A Cytophaga/Flexibacter like bacterium (CFLB) infection in farmed rainbow trout in north Iran. Journal of the Faculty of Veterinary Medicine,
University of Tehran, 52(3):14-22. In Farsi.
Soltani, M., Rostami, M., Ebrahimzadeh Mousavi, H. A., Mirzagar, S. S. and Falahi, R. 2002. Study of fry trout mortality syndrome in some farmed rainbow trout in Tehran and Lorestan Provinces.
Pajouhesh va Sazandegi, 14(4)(53):2-5. In Farsi.
Soltani M. and Soflayi, N. 2002. Sanitary microbiological examination of sturgeon fish in freshwater environment, p. 489. In: Aquaculture Europe - 2002, "Seafarming today and
tomorrow", October 16-19, 2002, Trieste.
Soltani, M., Mikryakov, V. R., Lapirova, T. B., Zabotkina, E. A. and Popov, A. V. 2003. Assessment of some immune response variables of immunized common carp (Cyprinus carpio) following
exposure to organophosphate, malathion. Bulletin of the European Association of Fish Pathologists, 23(1):18-24.
Soltani, M., Sheikhzadeh, N., Ebrahimzadeh-Mousavi, H. A. and Zargar, A. 2010. Effects of Zataria multiflora essential oil on innate immune
responses of common carp (Cyprinus carpio). Journal of Fisheries and Aquatic Sciences, 5(3):191-199.
Soni, V. C. and George, B. 1986. Age determination and length-weight relationship in the mudskipper Boleophthalmus dentatus. Indian Journal of Fisheries, 33:231-234.
Sorokin, P. A., Medvedev, D. A., Vasil'ev, V. P. and Vasil'eva, E. D. 2011. Further studies of mitochondrial genome variability in Ponto-Caspian Proterorhinus species (Actinopterygii:
Perciformes: Gobiidae) and their taxonomic implications. Acta Ichthyologica et Piscatoria, 41(2):95-104.
Soulati, N. H. and Falahatkar, B. 2007. Stress response in sub-yearling great sturgeon to the air exposure. Caspian Journal of Environmental Sciences, 5(2):99-103.
Spärck, R. 1962. Anton Frederik Bruun 14. December 1901 - 13. December 1961. ICES Journal of Marine Science, 27:121-123.
Spataru, P. and Gophen, M. 1985. Feeding behaviour of silver carp Hypophthalmichthys molitrix Val. and its impact on the food web in Lake Kinneret, Israel. Hydrobiologia, 120:53-61.
Spataru, P. and Gophen, M. 1986. Food and feeding habits of Capoeta damascina (Cyprinidae) in Lake Kinneret, Israel.Journal of Aquaculture in the Tropics, 1:147-153.
Specter, M. 1994. Pollution threatens world's caviar source. New York Times (late New York Edition), 7 June 1994:C1, C15.
Speer, L., Lauck, L. and Spruill, V. 2000. A petition to list beluga sturgeon (Huso huso) as an endangered species. In the Office of Scientific
Authority, U.S. Fish and Wildlife Service, U. S. Department of Interior, Washington. 26 pp.
Speer, L., Lauck, L., Pikitch, E., Boa, S., Dropkin, L. and Spruill, V. 2000. Roe to ruin: The decline of sturgeon in the Caspian Sea and the road to recovery. National Resources Defense
Council, Wildlife Conservation Society and Sea Web iii + 26 pp.
Spencer, A. M. (Ed.). 1974. Mesozoic-Cenozoic Orogenic Belts. Data for Orogenic Studies. Special Publication, Geological Society, London, 4:xvi + 809 pp.
Spillman, C. J. 1972. Sur une collection de poissons (Cyprinidae, Poeciliidae) recueillis dans le sud-est de 'Iran. Bulletin du Muséum national d'Histoire naturelle, Paris, 41:581-584.
Stakei, A. 1999. Stressful chemical factors in the water bodies of Parishan Lake. Iranian Journal of Fisheries Sciences, 8(2):15-30, 2. In Farsi.
Stakei, A. A. 1999. Determination of nutrients, BOD and COD in manured polyculture ponds. Iranian Journal of Fisheries Sciences, 8(3):1-22, 1. In Farsi.
Starostin, I. V. 1936. Ikhtiofauna rechek severnogo sklona Kopet-Daga [Ichthyofauna of the streams of the northern Kopet-Dag]. Byulleten' Turkmenskoi Zoologicheskoi Stantsii, 1936(1):81-97.
Starostin, I. V. 1992. Fauna vnutrennikh vodoemov Turkmenistana [Fauna of the interior reservoirs of Turkmenistan]. Ylym, Ashgabat. 256 pp. In Russian.
Stchoukine, I. 1936. La peinture iranienne sous les derniers `Abbâsides et les Îl-Khâns. Bruges. 188 pp., XLVI pl.
Stein, G. M. and Bain, D. 1981. Caviar! Caviar! Caviar! Lyle Stuart Inc., Secaucus, New Jersey. 220 pp.
Steindachner, F. 1864. Ichthyologische Mittheilungen. (VII.). I. Scaphiodon Capoëta Heck. Verhandlungen der Zoologisch-Botanischen Gesellschaft, Wien, 14:223-234.
Steindachner, F. 1866. Zur Fischfauna Kaschmirs und der benachbarten Länderstriche. Ichthyologische Mittheilungen IX (VI). Verhandlungen der Zoologisch-Botanischen Gesellschaft,
Wien, 16, 9(6):784-796 Tafs. XIII, XIV, XVI, XVII.
Steinitz, H. 1951. On the distribution and evolution of the cyprinodont fishes of the Mediterranean region and the Near East. Bonner Zoologische Beiträge, 2(1-2):113-124.
Stephenson, J. 1928. The Zoological Section of the Nuzhatu-l-Qulūb of Hamdullāh al-Mustaufī Al-Qazwīnī.Oriental Translation Fund, New Series, 30:xix + 100 pp.
Stepien, C. A. and Tumeo, M. A. 2006. Invasion genetics of Ponto-Caspian gobies in the Great Lakes: a 'cryptic' species, absence of founder effects, and
comparative risk analysis. Biological Invasions, 8:61-78.
Sternin, V. and Doré, I. 1993. Caviar. The Resource Book. Cultura, Moscow. 256 pp. (Le caviar. De la pêche au grain. Editions INRA, 1998. 216 pp).
Stevens, L. R., Wright, H. E. and Ito, E. 2001. Proposed changes in seasonality of climate during the Late glacial and Holocene at Lake Zeribar, Iran. Holocene, 11(6):747-755.
Stevenson, M. 1997. "Aphanius" / "Cyprinodon" and the propinquity of descent. Ninth International Congress of European Ichthyologists (CEI9) "Fish Biodiversity". Italy 1997
(Napoli-Trieste). Book of Abstracts, p. 88-89.
Steyskal, G. C. 1980. The grammar of family-group names as exemplified by those of fishes. Proceedings of the Biological Society of Washington, 93(1):168-177.
Stiassny, M. L. J., de Marchi, G. and Lamboj, A. 2010. A new species of Danakilia (Teleostei, Cichlidae) from Lake Abaeded in the Danakil Depression of Eritrea (East Africa). Zootaxa,
2690:43-52.
Stiassny, M. L. J. and Getahun, A. 2007. An overview of labeonin relationships and the phylogenetic placement of the Afro-Asian genus Garra Hamilton, 1922 (sic) (Teleostei: Cyprinidae),
with the description of five new species of Garra from Ethiopia, and a key to all African species. Zoological Journal of the Linnaean Society, 150(1):41-83.
Stibane, F. A. 1992. Histochemical investigations of the ontogenetical development of acellular teleost bones with special reference of the vertebral column of Aphanius mento (Heckel, 1843)
(Pisces, Cyprinodontidae). Part I: Introduction, historical survey, material and methods, vertebral column and ribs. Zoologisches Jahrbuch für Anatomie, 122:449-477. In German.
Stibane, F. A. 1993a. Histochemical investigations of the ontogenetical development of acellular teleost bones with special reference of the vertebral column of Aphanius mento
(Heckel, 1843)(Pisces, Cyprinodontidae). Part II: Development and ossification of the fins. Zoologisches Jahrbuch für Anatomie, 123:1-18. In German.
Stibane, F. A. 1993b. Histochemical investigations of the ontogenetical development of acellular teleost bones with special reference of the vertebral column of Aphanius mento
(Heckel, 1843)(Pisces, Cyprinodontidae). Part III: General discussion and references. Zoologisches Jahrbuch für Anatomie, 123:125-154. In German.
Stöcklin, J. 1968. Structural history and tectonics of Iran: A review. Bulletin of the American Association of Petroleum Geologists, 52(7):1229-1258.
Stöcklin, J. 1974a. Northern Iran: Alborz Mountains, pp. 213-234. In: Spencer, A. M. (Ed.). Mesozoic-Cenozoic Orogenic Belts: data for Orogenic Studies. Geological Society of London
Special Publication, 4.
Stöcklin, J. 1974b. Possible ancient continental margins in Iran, pp. 873-887. In: Burk, C. A. and Drake, C. L. (Eds.). The Geology of Continental Margins. Springer-Verlag, New York.
xiii + 1009 pp.
Stolyarov, I. A. and Abusheva, K. S. 1997. Pike Esox lucius of Kizlyar Bay of the northern Caspian Sea. Journal of Ichthyology, 37(3):268-271.
Stone, R. 2002a. Scientists deplore OK for sturgeon catch. Science, 295:219.
Stone, R. 2002b. Caspian ecology teeters on the brink. Science, 295(5554):430-433.
Stone, R. 2005a. Attack of the killer jellies. Science, 309:1805-1806.
Stone, R. 2005b. The sturgeons's last stand. Science, 309:1806.
Stone, R. 2007. The last of the leviathans. Science, 316:1684-1688.
Stone, R. and Mervis, J. 2002. One beluga, two beluga. Science, 296:449.
Stoneley, R. 1974. Evolution of the continental margins bounding a former southern Tethys, pp. 889-903. In: urk, C. A. and Drake, C. L. (Eds.). The Geology of Continental Margins.
Springer-Verlag, New York. xiii + 1009 pp.
Storch, G. 1980. Spätglaziale Kleinsäuger der Ali Tepeh-Höhle (Behshar). Zur klima-ökologischen Faunengeschichte in NE-Iran. Senckenberginan biologica,
60(5/6)(1979):285-302.
Stoumboudi, M. Th., Villwock, W., Sela, J. and Abraham, M. 1993. Gonadosomatic index in Barbus longiceps, Capoeta damascina and their natural
hybrid (Pices, Cyprinidae), versus spermatozoan index in the parental males. Journal of Fish Biology, 43:865-875.
Stratil-Sauer, G. 1937. Kanate, Persiens künstliche Bewässerungsanlagen. Die Umschau, Frankfurt, 41(12):271-275.
Street, W. S., Street, J. K. with Sawyer, R. 1986. Iranian Adventure. The First Street Expedition. Field Museum of Natural History, Chicago. x + 305 pp.
Stroganov, N. S. 1968. Acclimatization and farming of sturgeons in ponds. Moscow State University Press, Moscow. 377 pp. In Russian.
Strubalina, N. K. and Chernyavskiy, V. I. 1992. Growth of northern Caspian roach, Rutilus rutilus caspicus, under contemporary environmental conditions. Journal of Ichthyology,
32(6):43-51.
Subbotkin, M. F. and Subbotkina, T. A. 2001. Study of relationships between four species of Acipenser using the antigens of serum proteins. Journal of Ichthyology, 41(8):625-634.
Subrahmanyam, K. 1969. A karyotypic study of the estuarine fish Boleophthalmus boddaerti (Pallas) with calcium treatment. Current Science, Lahore, 38(18):437-439.
Sudagar, M., Azari Takami, Gh., Panomarev, C. A., Mahmoudzadeh, H., Abedian, A. and Hosseini, S. A. 2005. The effects of different dietary levels of betaine
and methionine as attractants on the growth factors and survival rate of juvenile beluga (Huso huso). Iranian Journal of Fisheries Sciences, 14(2):41-50. In Farsi.
Sufi, S. M. K. 1957. Revision of the Oriental fishes of the Family Mastacembelidae. Bulletin of the Raffles Museum, Singapore, 27:93-146.
Surber, E. W. 1969. Report to the Government of Iran on a programme for the development of the Inland Fisheries of Iran. Food and Agriculture Organization, Rome, United Nations Development
Programme, Technical Assistance, UNDP (TA) 2723:viii + 64 pp.
Sutcliffe, J. V. and Carpenter, T. G. 1967. The assessment of runoff from a mountainous and semi-arid area in western Iran. Bulletin of the International Association of Scientific Hydrology,
76:383-394.
Suvorov, E. K. 1907. Etyudy po izucheniyu kaspiiskikh sel'dei [Studies on Caspian herrings]. Trudy Kaspiiskoi Ekspeditsii 1904 g, 1:139-196.
Svardal, K, and Svardal, H. 2006. Der Persiche Buntbarsche - erstmals nachgezüchtet. Die Aquarien- und Terrarienzeitschrift, 59(10):28-32.
Svardal, H. 2006. Iranocichla. Zu Besuch bei den schönen asiatischen Unbekannten. Aquaristik Aktuell, 3-4:50-55.
Svetovidova, A. A. 1978. [List of holotypes, syntypes and paratypes kept in the Department of Ichthyology of the Moscow State University Museum]. Sbornik Trudov Zoologicheskogo Muzeya
MGU, 16:256-263. (not seen; see Pavlinov and Borissenko (2001)).
Svetovidov, A. N. 1945a. On Clupeonella delicatula (Nordmann) of the Caspian and that of the Black and Azov seas. Comptes Rendus de l'Academie des Sciences de l'URSS, 46(5):207-208. In English.
Svetovidov, A. N. 1945b. Chalcalburnus chalcoides iranicus subsp. nova from the Caspian coast of Iran, and some zoogeographical problems of the southern part of this sea. Comptes Rendus de
l'Academie des Sciences de l'URSS, 48(2):142-144. In English.
Svetovidov, A. N. 1949. Ryby Irana po materialam, sobrannym akad. E. N. Pavlovskim [Fishes of Iran from material collected by Acad. E. N. Pavlovskii].
Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 8:859-869.
Svetovidov, A. N. 1952. Fauna of U.S.S.R. (Fauna SSSR). Fishes (Ryby). Vol. II, No. 1, Clupeidae (Sel'devye). Israel Program for Scientific Translations, Jerusalem (1963). iv + 428 pp.
Svetovidov, A. N. 1964a. Systematics of the North American anadromous clupeoid fishes of the genera Alosa, Caspialosa, and Pomolobus. Copeia, 1964(1):118-130.
Svetovidov, A. N. 1964b. Ryby Chernogo Morya [Fishes of the Black Sea]. Akademiya Nauk SSSR, Izdatel'stvo "Nauka", Moskva. 551 pp.
Svetovidov, A. N. 1981. The Pallas fish collection and the Zoographia Rosso-Asiatica: an historical account. Archives of Natural History, 10(1):45-64.
Svetovidov, A. N. and Dorofeeva, E. A. 1963. Systematics, origin, and history of the distribution of the Eurasian and North American perches and pike-perches (genera Perca,
Lucioperca, and Stizostedion). Voprosy Ikhtiologii, 3(4):625-651. (Translation by the Ichthyological Laboratory, Bureau of Commercial Fisheries, U.S. National Museum, Washington, D.C.).
Svetovidova, A. A. 1967. Sistematika sredneaziatskikh el'tsov roda Leuciscus (Cyprinidae Pisces) [Taxonomy of the dace genus Leuciscus (Cyprinidae Pisces) from Central Asia].
Voprosy Ikhtiologii, 7(6)(47):979-989.
Svetovidova, A. A. 1978. [List of holotypes, syntypes and paratypes kept in the Department of Ichthyology of the Moscow State University Museum]. Sbornik Trud. Zool. Muz. MGU, 16:256-263. (not seen).
Swanson, C., Cech, J. J. and Piedrahita, R. H. 1996. Mosquitofish. Biology, Culture, and Use in Mosquito Control. Mosquito and Vector Control Association of California and the University of
California Mosquito Research Program, Davis. 88 pp.
Syed Mortezaii, S. 2002. Protozoan infections of fresh water fishes in Khouzestan Province. Pajouhesh and Sazandegi, 51:86-89. In Farsi.
Sykes, P. M. 1902. Ten thousand miles in Persia; or, eight years in Iran. John Murray, London. 481 pp., map.
Sykes, P. 1927. Sir John Chardin's Travels in Persia. The Argonaut Press, London. xxx + 287 pp.
Szczerbowksi, J. A., Bartel, R. and Epler, P. 2001. Fishing gear selectivity, fish survival rates and resources in lakes Tharthar, Habbaniya and Razzazah. Archives of Polish Fisheries,
9(supplement 1):225-233.
Szypuła, J., Epler, P., Bartel, R. and Szczerbowksi, J. A. 2001. Age and growth of fish in lakes Tharthar, Razzazah and Habbaniya. Archives of Polish Fisheries, 9(supplement 1):185-197.
T
Tabanadeh, M. R. and Akhlaghi M. 2007. Efficacy of conventional disinfectants on isolated Streptococcus iniae from diseased rainbow trout in laboratory and culture tank conditions. Iranian
Scientific Fisheries Journal, 16(3):29-38. In Farsi.
Tabibzadeh, I., Behbehani, G. and Nakhai, R. 1970a. Use of Gambusia fish in the malaria eradication programme of Iran. World Health Organization, WHO/MAL/70.716; WHO/VBC/70.198, 13 pp.
Tabibzadeh, I., Behbehani, G. and Nakhai, R. 1970b. Use of Gambusia fish in the malaria eradication programme of Iran. Bulletin of the World Health Organization, 43(4):623-626.
Tabiee, O. 1998. Investigation of some biological and ecological characteristics of Nemacheilus malapterurus in Zarrin-Gol River. B.Sc. Project, Gorgan University, Gorgan. 35 pp. In Farsi.
Tabiee, O. and Abdoli, A. 2005. A study of some biological aspects of Nemacheilus malapterurus, Zarringol River, Golestan Province. Iranian Journal of Natural Resources, 57(4):715-728. In Farsi.
Tabrez Nasar, S. S. 1993. Length-weight relationship of Heteropneustes fossilis (Bloch). Matsya, 15 & 16:94-99.
TACIS (Technical Assistance to the Commonwealth of Independent States, European Union). 1999. Proposed scheme of fishery resource management. Caspian Environment Programme Facilitating
Thematic Advisory Groups in Azerbaijan, Kazakhstan, Russia & Turkmenistan, Caspian Regional Thematic Center on Management of Fish Resources and other Commercial
Bioresources on a Sustainable Basis (CRTC MB), Baku, Azerbaijan. 25 pp.
TACIS (Technical Assistance to the Commonwealth of Independent States, European Union). 2000a. Country report on desertification Islamic Republic of Iran. Caspian Environment Programme
Facilitating Thematic Advisory Groups in Azerbaijan, Kazakhstan, Russia & Turkmenistan, Baku, Azerbaijan. iii + 23 pp.
TACIS (Technical Assistance to the Commonwealth of Independent States, European Union). 2000b. Proposal for a management system for the transboundary fishery resources of the Caspian Sea.
Caspian Environment Programme Facilitating Thematic Advisory Groups in Azerbaijan, Kazakhstan, Russia & Turkmenistan, Baku, Azerbaijan. v + 76 pp.
TACIS (Technical Assistance to the Commonwealth of Independent States, European Union). 2000c. Assessment of pollution control measures I. R. Iran. Caspian Environment Programme Facilitating
Thematic Advisory Groups in Azerbaijan, Kazakhstan, Russia & Turkmenistan, Baku, Azerbaijan. 22 pp.
TACIS (Technical Assistance to the Commonwealth of Independent States, European Union) and United Nations Development Programme. 2002. Transboundary diagnostic analysis for the Caspian Sea.
Caspian Environment Programme, Baku, Azerbaijan. Volume 1:viii + 26 pp., Volume 2:iv + 128 pp., Volume 3:i + 11 annexes + 13 pp.
Tadajewska, M. 1998. Pharyngeal teeth and shape of the ossa pharyngea inferiora during development of Abramis brama (L.) and Blicca bjoerkna (L.)(Cyprinidae). Cybium,
22(2):123-147.
Taghavi, S. A. 2000a. Age composition and prediction of optimum fishing mortality (Fmsy) for the beluga, stellate sturgeon, Persian sturgeon and Russian sturgeon in the south part of the
Caspian Sea. Iranian Journal of Fisheries Sciences, 8(4):1-28, 1-2. In Farsi.
Taghavi, S. A. 2000b. Fisheries monitoring, control and surveillance in the Islamic Republic of Iran. Country paper 5. In: FAO Report of a Regional Workshop on Fisheries Monitoring, Control and
Surveillance, FAO/Norway Programme of Assistance to Developing Countries for the Implementation of the Code of Conduct for Responsible Fisheries. Food and Agriculture Organization, Rome.
Taghavi-Motlagh, A. 1995. Investigation of food composition of sturgeons from the southern part of the Caspian sea (Iranian coast). Sturgeon Quarterly, 3(4):11.
Taghavi Motlagh, A. 1997. Von Bertalanffy growth parameters of the beluga, stellate sturgeon, Persian sturgeon and Russian sturgeon living in the
south part of the Caspian Sea. Third International Symposium on Sturgeon, Piacenza, Italy, 8-11 July 1997, Book of Abstracts (poster).
Taghavi Motlagh, A. 2001. An estimation of growth parameter, mortality rates and yield-per-recruit for beluga (Huso huso) living in Caspian Sea. Iranian Journal of Fisheries Sciences,
3(2):25-48, 123-124.
Taha, M. F., Harb, S. A., Nagib, M. K. and Tantawy, A. H. 1981. The Climate of the Near East, pp. 183-255. In: Takahashi, K. and Arakawa, H. (Eds.). Climates of Southern and Western Asia. Elsevier,
Amsterdam. xiii + 333 pp.
Taherianfard, M., Ebrahimi, M. and Soodbaksh, S. 2008. Bioaccumulation of
mercury in fishes of Kor River. Australian Journal of Basic and Applied
Sciences, 2(4):904-908.
Tahori, B. H. 1998. Sturgeon hatching in south Caspian Sea. The Seventh National Fisheries Conference, Responsible Fisheries. Shilat Company, Tehran. pp. 221-224. In Farsi.
Tajbakhsh, F., Pazooki, J., Masoumian, M. and Daghigh Rouhi, J. 2010. The first record of Philometra rischta (Nematoda: Philometridae) in Blicca bjoerkna of Anzali wetland, Iran. Iranian
Journal of Fisheries Sciences, 9(3):485-488.
Taki, Y. 1975. Cyprinid fishes of the genera Onychostoma and Scaphiodonichthys from Upper Laos, with remarks on the dispersal of the genera and their allies. Japanese Journal
of Ichthyology, 22(3):143-150.
Takin, M. 1972. Iranian geology and continental drift in the Middle East. Nature, London, 235:147-150.
Tala, M., Amini, F. and Fatemi, M. R. 2006. Morphological study of gonads of rainbow trout (Oncorhynchus mykiss) after administration of 17-α-methyl testosterone via feed and/or immersion.
Journal of the Faculty of Veterinary Medicine, University of Tehran, 61(1):91-99. In Farsi.
Tala, M., Rezaian, M. and Amini, F. 2006. Histological study of rainbow trout (Oncorhynchus mykiss) after administration of 17α-methyl testosterone. Journal of the Faculty of Veterinary
Medicine, University of Tehran, 61(2):187-193. In Farsi.
Talalaev, ?. 1910. Otchet Upravleniya kaspiisko-volzhskoi rybnoi promyshlennosti za 1908 g. [Report of the Volga-Caspian Fisheries Board for 1908]. Astrakhan. (not seen).
Taleban, F. A., Karandish, M. and Ghaffarpour, M. 1998. Effect of consumption of fatty fish from Northern and Southern Iran on serum lipids and blood pressure of healthy young men.
Pejouhandeh, Tehran, 3(2):1 p. (http://www.sbmu.ac.ir/Journal/Pejouhandeh/Summer98/pj9_3.htm, downloaded 20 August 2002).
Talebi, K. 1998. Diazinon residues in the basins of Anzali Lagoon, Iran. Bulletin of Environmental Contamination and Toxicology, 61(4):477-483.
Talwar, P. K. 1978. Identity of the schizothoracid fish genus Schizothorax Heckel, 1838, with considerations of the status of Schizothoraichthys
Misra, 1962. Bulletin of the Zoological Survey of India, 1(1):81-85.
Talwar, P. K. and Jhingran, A. G. 1991. Inland Fishes of India and Adjacent Countries. Oxford & IBH Publishing Company, New Delhi. 2 volumes.
Talwar, P. K. and Kacker, R. K. 1984. Commercial Sea Fishes of India. Handbook No. 4, Zoological Survey of India, Calcutta. lii + 997 pp.
Tang, Q., Freyhof, J., Xiong, B. and Liu, H. 2008. Multiple invasions of Europe by east Asian cobitid loaches (Teleostei: Cobitidae). Hydrobiologia, 605:17-28.
Tang, Q., Liu, H., Mayden, R. and Xiong, B. 2006. Comparison of evolutionary rates in the mitochondrial DNA cytochrome b gene and control region and
their implications for phylogeny of the Cobitoidea (Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution, 39(2):347-357.
Taqvaie, M. and Ramezani, A. 2002. Characteristics of tourism in Chaharmahal-Bakhtiari Province. Environment, Tehran, 37:20-27
(www.netiran.com/Htdocs/WeeklyJournal/Social/wj01140.html, downloaded 12 February 2002).
Tarkan, A. S., Gaygusuz, Ö., Acıpınar, H. and Gürsoy, Ç. 2005. Characteristics of a Eurasian cyprinid, shemaya, Chalcalburnus chalcoides (Güldenstädt, 1772), in a mesotrophic
water reservoir. Zoology in the Middle East, 35:49-60.
Tate, G. P. 1910. Seistan. A memoir on the history, topography, ruins, and people of the country. Superintendent Government Printing, Calcutta. Volume 1:1-271, 5 maps, photos; volume 2:272-378,
index 17 pp.
Tatina M., Bahmani, M., Soltani, M., Abtahi, B. and Gharibkhani, M. 2010. Effects of different levels of dietary vitamins C and E on some hematological
and biochemical parameters of sterlet (Acipenser ruthenus). Journal of Fisheries and Aquatic Science, 5(1):1-11.
Tavakol, S. and Jalali, B. 2005. Parasites of cultured salmonids in Iran. Sixth Symposium on Diseases in Asian Aquaculture (DAA VI), Conference Programme,
25-28 October 2005, Columbo Plaza Hotel, Columbo, Sri Lanka (title)
Tavakoli, H. and Akhlaghi, M. 2009. Study of lysozyme immunoglobulin, blood cell and hematocrit changes following experimental infection with a pathogenic Aeromonas hydrophila in rainbow
trout. Journal of Veterinary Research, 64(2):157-162. In Farsi.
Tavakoli, H. R. and Razavilar, V. 2003a. The toxin detection of proteolytic and non-proteolytic types of Clostridium botulinum in fishes from Southern and Northern Iran. AquaEuro
2003, European Aquaculture Society, Trondheim, Norway, August 8-12, 2003 (title).
Tavakoli, H. R. and Razavilar, V. 2003b. Isolation of Clostridium botiulinum (sic) (types A, B, E) in sediments from coastal areas in the north of Iran. Iranian Journal of Public Health,
32(3):37-40.
Tavakoli, H. R. and Tabatabaei, A. 2005. The distribution study of A, B and E types of Clostridium botulinum in some natural fish species of Iran (Salmo
trutta fario, Sander lucioperca, Pomadasys argenteus and Scomberomorus commerson). Kowsar Medical Journal, 10(1):13-20. In Farsi.
Tavakoli, M., Keymaram, F., Behrouz Khoush Galb, M. R. and Parand Avar, H. 2007. An investigation on the sturgeon stocks in southern Caspian Sea. Iranian Scientific Fisheries Journal, 16(2):29-36.
In Farsi.
Tavassoli, A. and Moghir, B. 2002. Squamous cell carcinoma of oral cavity in Rutilus frisii of Caspian Sea (the first case report).
Book of Abstracts, 27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Tawafi, A. 1994. Red-spotted trout in Shonood River of Gilan. Abzeeyan, Tehran, 5(1 & 2):48. In Farsi.
Taylor, S. 1997. The historical development of the caviar trade and the caviar industry. Occasional Papers of the IUCN Species Survival Commission (SSC), 17:45-53.
Taziekeh, A. 1999. Integrated shrimp culture (Penaeus indicus) with Chanos chanos in the Hormozgan Province, Iran. Iranian Journal of Fisheries Sciences, 8(3):53-68, 5-6. In Farsi.
Tehrani, M. G. 2006. Culturing of mullet fingerlings species Mugil cephalus L. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture, Sari. http://en.ifro.ir (abstract).
Tehranifard, A., Fazeli, M. and Yarrabi, J. 2007. Determination of LC50 of linear anionic detergents and diazinon toxin on Rutilus frisii kutum. Marsh Bulletin, 2(2):147-154.
Tehranifard, A., Sharif Fazeli, M. and Piri, M. 2002. Determination of LC50 of linear anionic detergents and diazinon toxin on Rutilus frisii kutum.
Iranian Journal of Marine Sciences, 1(1):55-59, 81. In Farsi.
Teimori, A., Esmaeili, H. R. and Gholamhosseini, A. 2010. The ichthyofauna of Kor and Helleh River basins in southwest of Iran with reference to taxonomic and zoogeographic features of native fishes.
Iranian Journal of Animal Biosystematics, 6(1):1-8.
Teimori, A., Esmaeili, H. R. and Motamedi, M. 2009. Freshwater ichthyodiversity, threats and conservation status in desert habitats of Iran with emphasis on qanats systems. 13th European
Congress of Ichthyology, 6-12 September 2009, Klaipeda, Lithuania (abstract).
Tekriwal, K. L. and Rao, A A. 1999. Ornamental and Aquarium Fish of India. Kingdon Books, Waterlooville, England.144 pp.
Tesch, F.-W. 1973. Der Aal. Biologie und Fischerei. Verlag Paul Parey, Hamburg. 306 pp.
Teugels, G. G. 1982. A systematic outline of the African species of the genus Clarias (Pisces; Clariidae), with an annotated bibliography. Annales du Musée Royale de l'Afrique centrale,
serie 8, 236:1-249.
Thesiger, W. 1985. Marsh Arabs. Collins, London. 2nd Revised Edition, 236 pp.
Thiermann, A. 1978. Aphaniinae. Journal of the American Killifish Association, 11(3):86-86.
Thinès, G. 1969. L'évolution régressive des poissons cavernicoles et abyssaux. Masson et Cie, Paris. 394 pp.
Thinès, G. 1978. The blind fishes of Persia. Nature, London, 271:305.
Thomas, H., Sen, S., Khan, M., Battail, B. and Ligabue, G. 1982. The Lower Miocene fauna of Al-Sarrar (Eastern province, Saudi Arabia). ATLAL, Journal of Saudi Arabian Archaeology, 5(III):109-136,
pls. 116-117.
Thomas, M. L. H. 1961. Lamprey fisheries, past and present, of North America and Europe. Fisheries Research Board of Canada, Biological Station and Technical Unit, London, Ontario, Circular, 3:4-7.
Thomson, A. W. and Page, L. M. 2006. Genera of the Asian catfish families Sisoridae and Erethistidae (Teleostei: Siluriformes). Zootaxa, 1345:1-96.
Thomson, J. M. 1963. Synopsis of biological data on the grey mullet Mugil cephalus Linnaeus 1758. Commonwealth Scientific and Industrial
Research Organization (CSIRO), Melbourne, Fisheries and Oceanography, Fisheries Synopsis, 1:74 pp.
Thomson, J. M. 1997. The Mugilidae of the world. Memoirs of the Queensland Museum, 41(3):457-562.
Thorne, M. J. 1992. Comment on the citation of names in Zoological Record as evidence of general scientific use. Bulletin of Zoological Nomenclature, 49(2):144.
Thorpe, J. 1977. Synopsis of biological data on the perch Perca fluviatilis Linnaeus, 1758 and Perca flavescens Mitchill,
1814. Food and Agriculture Organization, Rome, Fisheries Synopsis, 113:vii + 138 pp.
Thorpe, J. E. 1977. Morphology, physiology, behavior, and ecology of Perca fluviatilis L. and P. flavescens Mitchill. Journal of the Fisheries Research Board of Canada, 34:1504-1514.
Tidwell, J. 2001a. Sturgeon fishes and caviar dreams. Zoogoer, 30(3):11 pp. (www.fonz.org/zoogoer/zg2001/30(3)sturgeon.htm).
Tidwell, J. 2001b. Caspian calamities. Zoogoer, 30(3):2 pp. (www.fonz.org/zoogoer/zg2001/30(3)caspianside.htm).
Tilak, R. 1987. The Fauna of India and the Adjacent Countries. Pisces (Teleostomi). Sub-Family: Schizothoracinae. Zoological Survey of India, Calcutta. x + 229 pp.
Tilak, R. and Sinha, N. K. 1975. A study of the fishes of the subfamily Schizothoracinae (Pisces, Cyprinidae). I. On the generic status of Schizothorax Heckel,
1838. Annales Zoologici, Warszawa, 32(13):289-297.
Timur, M., Çolak, A. and Marufi, M. 1983.Balıklı Kaplıcadki (Sivas) Balık
Türlerinin Tanımı ve Deri Hastalıı Tedavisindeki Etkisinin Araştirılması [A study on the systematic
identification of the Balıklı thermal spring (Sivas) fish and the curative effects of the fish on dermal diseases].Ankara Üniversitesi Veteriner Fakültesi Dergisi, 30(2):276-282.
Tipper, G. H. 1921. The geology and mineral resources of eastern Persia. Records of the Geological Survey of India, 53:51-80, pl. 4-11.
Togan, I., Fidan, A. Z., Yain, E., Ergüven, A. and Emre, Y. 1995. Genetic structure of two Turkish brown trout populations. Journal of Fish Biology, 47 (Supplement A):164-169.
Tomasini, J.-A., Quignard, J.-P., Capapé, C. et Bouchereau, J.-L. 1991. Facteurs du succès reproductif de Syngnathus abaster
Risso, 1826 (Pisces, Teleostei, Syngnathidae) en milieu lagunaire méditerranéen (lagune de Mauguio, France). Acta Oecologica, 12(3):331-355.
Torcu-Koç, H., Erdogan, Z. and Treer, T. 2006. A review of length-weight relationships of fishes from freshwaters of Turkey. Journal of Applied Ichthyology, 22(4):264-270.
Torrik, A. B. 1994. A survey on the diet of sturgeons in the southern part of the Caspian Sea. Iranian Journal of Fisheries Science, 1:26-29. In Farsi.
Tortonese, E. 1934. Pesci della Persia raccolti dal Marchese Giacomo Doria (1862). Bollettino dei Museo di Zoologia e di Anatomia Comparata della R. Università di Torino, 44, III(49):153-171,
tav. 1.
Tortonese, E. 1937-1938a. Viaggio del dott. Enrico Festa in Palestina e in Siria (1893). Bollettino dei Musei di Zoologia e di Anatomia Comparata della R. Università de Torino, 46,
III(85):1-48, tav. I-II.
Tortonese, E. 1937-1938b. Note di Ittiologia. III. Note sui Ciprinidi. Bollettino dei Musei di Zoologia e di Anatomia Comparata della R. Università de Torino, 46, III(74):81-85.
Tortonese, E. 1940. Elenco dei tipi esistenti nella collezione ittiologica del R. Museo di Torino. Bollettino dei Musei di Zoologia e di Anatomia Comparata
della R. Università de Torino, 48, III(111):133-144.
Tortonese, E. 1951-1952. Ricerche sistematico-faunistiche sui Pesci d'acqua dolce dell'Anatolia. 1. Cobitidae. Bollettino dell'Istituto e Museo di
Zoologia dell'Università di Torino, 3(8):1-14.
Tortonese, E. 1954. The trouts of Asiatic Turkey. Istanbul Üniversitesi Fen Fakültesi Hidrobiologi Araştırma Enstitüsü Yayınlarından, B, 11(1):1-25, pl. I-II.
Tortonese, E. 1961. Catalogo dei tipe di pesci del Museo Civico di Storia Naturale di Genova (Parte I). Annali del Museo Civico di Storia Naturale "Giacomo Doria", Genova, 72:179-191.
Tortonese, E. 1973. The ichthyological collections now existing in Italy. Annali del Museo Civico di Storia Naturale di Genova, 79:108-116.
Touraji, M. R. 2007. Identification and study of fish species in Mazdaghan River. Pajouhesh va Sazandegi,
19(3)(72):19-29. In Farsi.
Tourchi-Moghaddam, M. 2003. An investigation on feeding habitats of Hemiculter leucisculus in the Anzali lagoon. M.Sc. Thesis, Higher Education Institute of Mirza-Kouchakhan, Rasht. 137 pp.
In Farsi.
Trabelsi, M., Quignard, J.-P., Tomasini, J.-A., Boussaid, M., Focant, B. and Maamouri, F. 2002. Discriminative value of the meristic characters of Atherina
boyeri, lagoon populations. Vie et Milieu, 52(2-3):77-84.
Travers, R. A. 1984a. A review of the Mastacembeloidei, a suborder of synbranchiform teleost fishes. Part I: Anatomical description. Bulletin of the
British Museum (Natural History) Zoology, 46(1):1-133.
Travers, R. A. 1984b A review of the Mastacembeloidei, a suborder of synbranchiform teleost fishes. Part II: Phylogenetic analysis. Bulletin of the British Museum (Natural History) Zoology,
47(2):83-150.
Travis, J. 1993. Invader threatens Black, Azov seas. Science, 262(5138):1366-1367.
Trewavas, E. 1972. The type-species of the genera Phoxinellus, Pseudophoxinus and Paraphoxinus (Pisces, Cyprinidae).
Bulletin of the British Museum (Natural History) Zoology, 21(8):359-361.
Trewavas, E. 1977. The sciaenid fishes (croakers or drums) of the Indo-West Pacific. Transactions of the Zoological Society of London, 33(4):253-541.
Trewavas, E. 1983. Tilapiine Fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum (Natural History), London. xi + 583 pp.
Trewavas, E. and Ingham, S. E. 1972. A key to species of Mugilidae (Pisces) in the Northeastern Atlantic and Mediterranean, with explanatory notes. Journal of Zoology, 167:15-29.
Trickey, M. 1995. Poachers, pollution jeopardize delicacy. The Ottawa Citizen, 15 June 1995, p. F14.
Troll, C. 1963. Qanat-Bewässerung in der Alten und Neuen Welt. Mitteilungen der Österreichischen Geographischen Gesellschaft Wien, 105(3):313-330, 6 figs.
Tsepkin, E. A. 1986. Brief review of the freshwater Quaternary ichthyofauna of the Asian part of the USSR. Journal of Ichthyology, 26(3):110-116.
Tsepkin, E. A. 1987. On the maximum sizes of pike Esox lucius. Journal of Ichthyology, 27(1):155-157.
Tsepkin, Ye. A. and Sokolov, L. I. 1971. The maximum size and age of some sturgeons. Journal of Ichthyology, 11(3):444-446.
Tsigenopoulos, C. S. 1999. Phylogéographie du genre (Téléostéens, Cyprinidae) dans les régions péri-méditerranéennes.
Reconstitution des grandes lignées de migration au moyen des marqueurs nucléaires et mitochondriaux. Thèse de Doctorat en Biologie
des Populations et Ecologie, Université Montpellier, Montpellier. 190 pp.
Tsigenopoulos, C. S. and Berrebi, P. 2000. Molecular phylogeny of North Mediterranean freshwater barbs (genus Barbus: Cyprinidae) inferred from cytochrome b sequences: biogeographic
and systematic implications. Molecular Phylogenetics and Evolution, 14(2):165-179.
Tsigenopoulos, C. S., Durand, J. D., Ünlü, E and Berrebi, P. 2003. Rapid radiation of the Mediterranean Luciobarbus species (Cyprinidae) after the Messinian salinity crisis of the
Mediterranean Sea, inferred from mitochondrial phylogenetic analysis. Biological Journal of the Linnean Socity, 80(2):207-222.
Turan, C. 2008. Molecular systematics of the Capoeta (Cypriniformes: Cyprinidae) species complex inferred from mitochondrial 16S rDNA sequence data. Acta Zoologica Cracoviensia,
51A(1-2):1-14.
Turan, C., Ergüden, D., Turan, F. and Gürlek, M. 2004. Genetic and morphologic structure of Liza abu (Heckel, 1843) populations from the rivers Orontes, Euphrates and Tigris. Turkish
Journal of Veterinary and Animal Sciences, 28:729-734.
Turan, D., Kottelat, M. and Engin S. 2009. Two new species of trouts, resident and migratory, sympatric in streams of northern Anatalia (Salmoniformes: Salmonidae). Ichthyological Exploration of
Freshwaters, 20(4):333-364.
Turan, D., Tomovic, L. and Pešić, V. 2007. Morphological variation in a common Turkish cyprinid, Squalius cephalus,
across Turkish water catchment areas. Zoology in the Middle East, 40:63-70.
Türkmen, M. and Akyurt, I. 2000. Karasu İmağı'n ın Aşkale Mevkiinden Yakalanan Gümüş Balığı Chalcalburnus mossullensis, (sic) Heckel,
1843)'nın Populasyon Yapısı ve Büyüme Özellikleri [The population structure and growth properties of Chalcalburnus mossulensis (Heckel, 1843) caught from Aşkale region
of River Karasu]. Turkish Journal of Biology, 24(1):95-111.
Türkmen, M., Erdoğan, O., Haliloğlu, H. I. and Yildirim, A. 1999. Age, growth and reproduction of Acanthalburnus
microlepis Filippi 1863 from the Yağan Region of the Aras River, Turkey. Turkish Journal of Zoology, 25(2):127-133.
Türkmen, M., Haliloğlu, H. I., Erdoğan, O. and Yildirim, A. 1999. The growth and reproduction characteristics
of chub Leuciscus cephlaus orientalis (Nordmann, 1840) living in the River Aras. Turkish Journal of Zoology, 23(4):355-364.
Turnbull, P. F. and Reed, C. A. 1974. The fauna from the Terminal Pleistocene of Palegawra Cave, a Zarzian occupation site in northeastern Iraq. Fieldiana Anthropology, 63(3):81-146.
Tytler, P. and Vaughan, T. 1983. Thermal ecology of the mudskippers, Periophthalmus koelreuteri (Pallas) and Boleophthalmus boddarti (Pallas) of Kuwait Bay. Journal of Fish Biology,
23:327-337.
U
Ündar, L., Akpinar, M. A. and Yanikoğlu, A. 1990. "Doctor fish" and psoriasis. The Lancet, 335:470-471.
UNDP and FAO. 1998. Fish for Life. Creating sustainable livelihoods. UNDP (United Nations Development Programme) and FAO (Food and Agriculture Organisation) working in the Islamic Republic of Iran
with collaboration of Fisheries Organisations of Iran. UNDP, Tehran. In English and Farsi.
UNEP. 2003. Afghanistan. Post-Conflict Environmental Assessment. United Nations Environmental Programme, Switzerland. 176 pp.
Ünlü, E. 1991. Dicle Nehrinde Yaşayan Capoeta trutta (Heckel, 1843)'nin Biyolojik Özellikleri Üzerinde Çalişmalar [Studies on the biological
characteristics of Capoeta trutta (Heckel, 1843) living in Tigris River].Doğa Türk Zooloji Dergisi, 15(1):22-38.
Ünlü, E. 2006. Tigris River ichthyological studies in Turkey. A review with regard to the Ilisu Hydroelectrcic Project. Environmental Impact Assessment Report, Ilisu Environment Group, Hydro
Concepts Engineering, Hydro Québec International, Archéotec Inc. 34 pp.
Ünlü, E. and Balcı, K. 1993a. A study on the reproductive characteristics of Leuciscus cephalus orientalis (Nordmann, 1840) from the Savur Stream. Doğa Türk
Zooloji Dergisi, 17(1):91-102.
Ünlü, E. and Balci, K. 1993b. Observation on the reproduction of Leuciscus cephalus orientalis (Cyprinidae) in Savur Stream (Turkey). Cybium, 17(3):241-250.
Ünlü, E., Balcı, K. and Akbayin, H. 1994. Some biological characteristics of the Acanthobrama marmid Heckel, 1843 in the Tigris River (Turkey). Turkish Journal of Zoology,
18(2):131-139.
Ünlü, E., Balci, K. and Meriç, N. 2000. Aspects of the biology of Liza abu (Mugilidae) in the Tigris River (Turkey). Cybium, 24(1):27-43.
Ünlü, E. and Bozkurt, R. 1996. Notes on the catfish, Silurus triostegus (Siluridae) from the Euphrates River in Turkey. Cybium, 20(3):315-317.
Ünlü, E., Çiçek, T., Değer, D. and Coad, B. W. 2010. Range extension of the exotic Indian stinging catfish, Heteropneustes fossilis (Bloch, 1794) (Heteropneustidae) into the Turkish
part of the Tigris River watershed. Journal of Applied ichthyology, DOI: 10.1111/j.1439-0246.2010.01580.x.
Ünlü, E., Kilic-Demirok, N., Cengiz, E. I. and Karadede, H. 1997. Karyology of Garra rufa (Cyprinidae) in River Tigris (Turkey). Ninth International Congress of European
Ichthyologists (CEI9) "Fish Biodiversity". Italy 1997 (Napoli-Trieste). Book of Abstracts, p. 95.
Ünver, B. 1998. An investigation on the reproduction properties of chub (Leuciscus cephalus L., 1758) in Lake Tödürge (Zara/Sivas). Turkish Journal of Zoology, 22(2):141-147.
Ünver, B. and Erk'akan, F. 2005. A natural hybrid of Leuciscus cephalus (L.) and Chalcaburnus chalcoides (Güldenstädt) (Osteichthyes-Cyprinidae) from Lake Tödürge (Sivas, Turkey).
Journal of Fish Biology, 66(4):899-910.
Ünver, B, Tatlidil, H. and Erka'akan, F. 2008. Biometrical features of intergeneric hybrid between Leuciscus cephalus (L.) and Chalcalburnus chalcoides (G.) (Osteichthyes-Cyprinidae)
distributed in Lake Tödürge (Sivas-Turkey). Turkish Journal of Fisheries and Aquatic Sciences, 8(2):207-213.
Urch, M. 1999. Caviar crisis. Seafood International, London, 14(9):30-31.
Usmanova, R. G. 1975. Ecological variability of the "Turkestan gudgeon" (Gobio gobio lepidolaemus) of the Kashkadar'ya basin. Journal of Ichthyology, 15(5):724-729.
V
Vadrot, C-M. 1990. Le triangle d'or du caviar. Geo Magazine, 142:248-266.
Vaezzadeh, V., Mashinchian Moradi, A. Esmaeili Sari, A. and Fatemi, S. M. R. 2008. Study of organochlorine pesticides in muscle tissue of two commercial fish species (Cyprinus carpio and
Rutilus frisii kuttum) in southwest of Caspian Sea. Environmental
Sciences, 5(3):33-40.
Vahabzadeh, R. H. 2003. Surveying oxygen peroxide and levamizole hydrochloride application treating eggs and frys of Persian sturgeon and Chinese carp. Ph.D. Thesis, University of Tehran. In Farsi.
Vahabzadeh, R. H., Ahamadi, M. R., Keyvan, A. and Masoumian, M. 2005. Evaluation of hydrogen peroxide effectiveness in fungal disinfection of Acipenser persicus eggs. Iranian Scientific
Fisheries Journal, 14(1):161-176. In Farsi.
Vahabzadeh Roodsari, H., Ahmadi, M. R., Keyvan, A., Masumian, M. and Monajemi, B. 2003. Comparison of hydrogen peroxide and malachite green efficacy to control fungal infection in carp,
Cyprinus carpio, artificial propagation. Iranian Journal of Marine Sciences, 2(1):77-86. In Farsi.
Vahdati, A., Asisi Malekabadi, H., Raiati, A. A. and Esteki, A.2001. The effects of crowding stress on haematological parameters of rainbow trout (Oncorhynchus mykiss).
Research Bulletin of Isfahan University (Science), 14(2):47-54. In Farsi.
Vahdati, A., Nekui, N. and Adib, M.2001. Effect of cortisol on phagocytic activity of macrophages in rainbow trout. Iranian Journal of Biology, 10(1-2):12-21.
Vahdati, A., Rahimi, H., Mahdani, H. and Fazeli, A. 2001. Evaluation of optimal conditions for the measuring of phagocytic activity of granulocytes in rainbow trout (Oncorhynchus mykiss).
Journal of Science, Al-Zahra University, 14(1):15-21. In Farsi.
Vahidi, I. 1963. Groundwater investigations in Iran, pp. 107-109. In: The Development of Groundwater Resources with Special Reference to Deltaic Areas (Transactions of the Regional Seminar on the
Development of Groundwater Resources with Special Reference to Deltaic Areas, held at Bangkok, Thailand, from 24 April to 8 May 1962). United Nations Economic Commission for Asia and the Far East,
New York.
Vaisman, A. and Raymakers, C. 2001. The status of sturgeon resources in Russia. Traffic Bulletin, 19(1):33-44.
Vajhi, A. R., Masoudifard, M., Veshkini, A., Akhtarzadeh, M. and Moghim, M. 2001. Normal ultrasonographic findings of the digestive system of Persian sturgeon (Acipenser gueldenstaedtii
persicus). 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Vajhi, A. R., Moghim, M., Veshkini, A. and Masoudifard, M. 2001. Sex and maturity determination by ultrasonography in Persian sturgeon (Acipenser gueldenstaedtii persicus). 4th International
Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Vakili, A. 1995. An overview of water resources development in Iran. WRM '95. Proceedings of the Regional Conference on Water Resources Management, Conference Secretariat,
Isfahan University of Technology, Isfahan, pp. 1-2.
Valeipour, A. and Haghighy, D. 2000. Study on changes in fishing in Anzali Lagoon (1992-1996). Iranian Journal of Fisheries Sciences, 8(4):73-88, 7. In Farsi.
Valeolahy, J. 2000. Uniquely significant fresh water fishes of Iran are exposed to environmental stress. Journal of Environmental Studies, 26(25):29-38. In Farsi.
Valiallahi, J. 2000. A revision of Barbus species of Iran (Cypriniformes: Cyprinidae). Ph.D. Thesis, Faculty of Natural Resources, University of Tarbiat Modares, Tehran.
200 pp. In Farsi.
Valiallahi, J. 2004. Habitats, distribution and notes on Barbus mystaceus and Barbus barbulus, the two Barbus species of Iran. Journal of Environmental Studies, Tehran, 29:27-34, 4. In Farsi.
Valiallahi, J. 2006. Identification of Barbus plebjus (Bonaparte, 1832) a valid species of cyprinid fish from Iran. Iranian Journal of Biology, 19(1):109-116. In Farsi.
Valid, H. and Tigranian, E. A. 1977. Inland water fishes of Iraq. Short key to the species and their characteristics. Manuscript, Inland Water Fisheries Institute, Baghdad. 89 pp. (In Russian)
(not seen).
Valinasab, T., Seyfabadi, S. J., Javadzadeh, N. and Safikhani, H. 2004. Fecundity of Klunzinger mullet, Liza klunzingeri, in coastal waters of Hendijan (Persian Gulf). Iranian
Journal of Marine Sciences, 3(1):75-82. In Farsi.
Valinassab, T., Seyfabadi, S. J., Javadzadeh, N. and Safikhani, H. 2007. Reproduction of Klunzinger mullet, Liza klunzingeri, in coastal waters of Khuzestan Province (Persian Gulf).
Iranian Journal of Fisheries Sciences, 6(2):129-142.
Valipour, A. 1998. An investigation on feeding habits of northern pike of Anzali Lagoon. Iranian Journal of Fisheries Sciences, 7(3):75-90, 7. In Farsi.
Valipour, A. R. 1997. Distribution and abundance of chironomid (sic) larvae in the Anzali Lagoon in 1993. Iranian Fisheries Scientific Journal, 6(2):75-92, 10. In Farsi.
Valipour, A. R. 2004. An investigation of Capoeta capoeta feeding in Makoo Dam Lake. Iranian Journal of Fisheries Sciences, 13(2):163-176. In Farsi.
van Beek, E., Bozorgy, B., Vekerdy, Z. and Meijer, K. 2008. Limits to agricultural growth in the Sistan Closed Inland Delta, Iran. Irrigation and Drainage Systems, 22(2):131-143.
van Buren, E. D. 1948. Fish-offerings in ancient Mesopotamia. Iraq, 10:101-121.
Van Damme, D., Bogutskaya, N., Hoffman, R. C. and Smith, C. 2007. The introduction of the European bitterling (Rhodeus amarus) to west and central Europe. Fish and Fisheries, 8:79-106.
van den Eelaart, A. 1954. Report to the Government of Iraq on the Development of Inland Fisheries. Food and Agriculture Organization, Rome, Report of the Expanded Technical Assistance Program,
270:42 pp.
van der Leeden, F. (Ed.). 1975. Water Resources of the World. Selected Statistics. Water Information Center, Port Washington, New York. xi + 568 pp.
van Stappen, G., Fayazi, G. and Sorgeloos, P. 2001. International study on Artemia LXIII. Field study of the Artemia urmiana (Günther, 1890) population in Lake Urmiah,
Iran. Hydrobiologia, 466:133-143.
van Zeist, W. 1967. Late Quaternary vegetation history of western Iran. Review of Palaeobotany and Palynology, 2:301-311.
van Zeist, W. and Bottema, S. 1977. Palynological investigations in western Iran. Palaeohistoria: Acta et Communicationes, 19:19-85.
van Zeist, W. and Bottema, S. 1982. Reflections on a Holocene subdivision of the Near East. Striae, Uppsala, 16:36-39.
van Zeist, W. and Woldring, H. 1978. A postglacial pollen diagram from Lake Van in east Anatolia. Review of Palaeobotany and Palynology, 26:249-276.
van Zeist, W. and Wright, H. E. 1963. Preliminary pollen studies at Lake Zeribar, Zagros Mountains, southwestern Iran. Science, 140:65-67.
Varasteh, A., Hosseinzadeh Mogaddam, M., Pourkazemi, M. and Norooz Fashkhami, M. R. ? Karyotyping and number of chromosomes of silver carp (Hypophthalmichthys molitrix). Virtual, 1(1).
In Farsi.
Varedi, S. E. and Fazli, H. 2005. Analyzing water quality in Mazandaran Province rivers during release of fish fingerlings. Iranian Journal of Fisheries Sciences, 14(3):167-182. In Farsi.
Varedi, S. E., Vahedi, F., Oloomi, Y., Younesi Pour, H. and Nasrolatabar, A. 2007. A survey of three trout farms phosphorus loading into Haraz River, north Iran. Iranian Scientific Fisheries
Journal, 16(1):151-160. In Farsi.
Vargas, M. J. and de Sostoa, A. 1996. Life history of Gambusia holbrooki (Pisces, Poeciliidae) in the Ebro Delta (NE Iberian peninsula). Hydrobiologia, 341(3):215-224.
Various Authors. 2000. History of qanats (aqueducts). Barzegar, Tehran, 819:54-63. In Farsi (English translation at www.netiran.com/Htdocs/Clippings/Social/200629XXSO01.html, downloaded 15
December 2000).
Varkouhi, Sh. 2007. Biogeochemical evaluation of trace elements in fish liver case study: Khorramabad River basin, Lorestan, Iran. Iranian Journal of Science and Technology, Transaction A,
31(A1):53-61.
Varkouhi, Sh. and Sobhani, E. A. 2005. The study of environmental biogeochemistry of trace elements in fishes of Khorramabad River watershed. Journal of Environmental Science and Technology,
24:55-64. In Farsi.
Varshoie, H., Mobedi, I., Meshgi, M. A. and Jalali, B. 2010. Survey on the digenean and monogenean helminthes of Clupeidae (Teleostes)(sic) from southern part of Caspian Sea.
Research Journal of Parasitology, 5(3):148-153.
Vasil'yev, V. P. and Grigoryan, K. A. 1993. Karyology of the Gobiidae. Journal of Ichthyology, 33(2):1-16.
Vasil'yev, V. P. and Vasil'yeva, Ye. D. 1992. Kariologicheskoe dokazatel'stvo vidovoi obosoblennosti Neogobius kessleri (Günther) i Neogobius gorlap Iljin (Pisces, Gobiidae) [Karyological
evidence of the separate species status of Neogobius kessleri (Günther) and Neogobius gorlap Iljin (Pisces, Gobiidae)]. Doklady Akademii Nauk Rossiya, 324(4):898-900.
Vasilev, V. P. (sic) and Vasil'eva, E. D. (sic). 1996. Biogeography of polyploid forms of European spined loaches from genus Cobitis (Cobitidae, Pisces). American Society of
Ichthyologists and Herpetologists, 76th Annual Meeting, At the Hotel Inter-Continental, June 13-19, 1996, New Orleans. p. 308 (Abstract).
Vasil'ev, V. P., Vinogradov, A. E., Rozanov, Yu. M. and Vasil'eva, E. D. 1999. Cellular DNA content in different forms of the bisexual-unisexual complex of spined loaches of the genus Cobitis
and in Luther's spined loach C. lutheri (Cobitidae). Journal of Ichthyology, 39(5):377-383.
Vasil'yeva, Ye. D. 1984. Osteological analysis of some Caspian gobies in connection with the systematics of the genus Benthophilus (Gobiidae). Journal of Ichthyology, 23(4):14-26.
Vasil'eva, Ye. D. (sic). 1988. Vnutrividovaya izmenchivost' i vidovaya spetsifika cherepa bychka-peschanika Gobius fluviatilis Pallas (Gobiidae, Pisces) [Intraspecific variability
and species specificity of the cranium of the monkey goby Gobius fluviatilis (Gobiidae, Pisces)]. Vestnik Moskovskogo Yuniversiteta, Biologiya, 16(2):30-39.
Vasil'yeva, E. D. (sic). 1989. Morphology of the cranium of Gobius melanostomus and G. syrman in relation with their position in the genus Gobius sensu
lato. Journal of Ichthyology, 29(2):144-154.
Vasil'eva, E. D. (sic). 1991. Morfologiya cherepa bychka gorlapa (Gobius gorlap Iljin) v svyazi s ego polosheniem v rode Gobius sensu lato (Gobiidae, Pisces) [The morphology
of the skull of a goby (Gobius gorlap Iljin) in connection with its place in the genus Gobius sensu lato (Gobiidae, Pisces). Byulleten' Moskovskogo Obshchestva Ispytatelei
Prirody, Otdel Biologicheskii, 96(5):36-45.
Vasil'eva, E. D. (sic). 1994. Skull morphology of atherine fishes of the Black Sea, the Sea of Azov, and the Caspian Sea, and certain problems of the systematics of the genus Atherina
(Atherinidae). Journal of Ichthyology, 34(9):125-140.
Vasil'eva, E. D. (sic). 1995a. Differentiation of Caucasian gobies, presently grouped in the subspecies Neogobius platyrostris constructor (Gobiidae), based on an analysis of museum
collections. Journal of Ichthyology, 35(1):1-20.
Vasil'yeva, Ye. D. 1995b. On the absence of Caspian spiny loach, Sabanejewia caspia (Cobitidae), in the North Caspian Basin. Journal of Ichthyology, 35(9):362-365.
Vasil'eva, E. D. (sic). 1996a. The craniological evidence for the subgeneral structure of Gobius sensu lato (Gobiidae) in the Black-Caspian Sea basin. American Society of
Ichthyologists and Herpetologists, 76th Annual Meeting, At the Hotel Inter-Continental, June 13-19, 1996, New Orleans. p. 308 (Abstract).
Vasil'eva, E. D. (sic). 1996b. Cranial data and some problems in the systematics of the genus Atherina (Atherinidae). Publicaciones Especiales Instituto Español de
Oceanografía, 21:199-204.
Vasil'eva, E. D. (sic). 1996. Morphology of the skull of a deep-water goby Gobius bathybius Kessler in relation to its position within the genus Gobius sensu lato (Gobiidae).
Journal of Ichthyology, 36(7):487-491.
Vasil'eva, E. D (sic). 2000. Craniological analysis of some species of Benthophilus Gobiidae and problems of taxonomy and phylogenetic relations of this group. Journal of Ichthyology,
40(9):724-731.
Vasil'eva, E. D. 2007. Gobies of the genus Rhinogobius (Gobiidae) from Primor'e and water bodies of Central Asia and Kazakhstan: I. Morphological characteristic and taxonomic status.
Journal of Ichthyology, 47(9):691-700.
Vasil'eva, E. D. (sic), Grunina, A. S. and Recoubratsky, A. V. 2001. The pattern of manifestation of some morphological characters in androgenetic nucleo-cytoplasmatic hybrids of the Persian
Acipenser persicus and Russian A. gueldenstaedtii sturgeons in the postlarval period. Journal of Ichthyology, 41(6):454-460.
Vasil'eva, E. D. (sic) and Kuga, T. I. 2001. Cranial differences between Caucasian goby Knipowitschia caucasica and marbled goby Pomatoschistus marmoratus and the taxonomic
problems of "sand gobies" (Gobiidae). Journal of Ichthyology, 41(2):135-142.
Vasil'eva, E. D. and Kuga, T. I. 2008. Gobies of the genus Rhinogobius (Gobiidae) of Primorye and water bodies of Central Asia and Kazakhstan: II. Comparative craniological analysis of
gobies introduced to Central Asia. Journal of Ichthyology, 48(1):29-36.
Vasil'yeva, E. D. (sic) and Ustarbekov, A. K. 1991. Variability in the skull of the bream Abramis brama from the Caspian and Aral Sea basins. Journal of Ichthyology, 31(3):82-99.
Vasil'eva, E. D. (sic) and Vasil'ev, V. P. (sic). 1988. Study of the intraspecific structure of Sabanejewia aurata (Cobitidae) with description of a new subspecies,
S. aurata kubanica. Journal of Ichthyology, 28(6):15-35.
Vasil'yeva, Y. D. (sic) and Vasil'yev, V. P. 1994. On the systematics of Caucasian gobies (Gobiidae): Craniological and karyological analyses and distribution by biotope of some populations
from the Black Sea and Caspian basins. Journal of Ichthyology, 34(6):14-25.
Vasil'yeva, Ye. D. and Vasil'yev, V. P. 1995. Systematics of Caucasian freshwater gobies (Gobiidae) in the light of contemporary data, with a description of a new species, Neogobius rhodioni,
sp. nov. Journal of Ichthyology, 35(2):139-157.
Vasil'eva, E. D. (sic) and Vasilev, V. P. (sic). 1996a. The evolution and dispersal of freshwater caucasian (sic) gobies (Neogobius). American Society of Ichthyologists
and Herpetologists, 76th Annual Meeting, At the Hotel Inter-Continental, June 13-19, 1996, New Orleans. p. 309 (Abstract).
Vasil'eva, E. D. (sic) and Vasil'ev, V. P. (sic). 1996b. New gobiid species from the Black-Caspian Sea basin. American Society of Ichthyologists and Herpetologists, 76th Annual Meeting,
At the Hotel Inter-Continental, June 13-19, 1996, New Orleans. p. 309 (Abstract).
Vasil'eva, E. D. (sic) and Vasil'ev, V. P. (sic). 1996c. The description of Neogobius iljini sp. nov. within former N. kessleri (Gobiidae, Pisces). Acta Universitatis
Carolinae Biologicae, 39(3-4)(1995):261-270.
Vasil'eva, E. D. (sic) and Vasil'ev, V. P. 1998. Sibling species in genus Cobitis (Cobitidae), Cobitis rossomeridionalis sp. nova. Journal of Ichthyology, 38(8):580-590.
Vasil'eva, E. D. (sic) and Vasil'ev, V. P. (sic). 2000. The origin and taxonomic status of the triploid form of the goldfish, Carassius auratus (Cyprinidae). Journal of
Ichthyology, 40(8):553-563.
Vasil'eva, I. (sic) D., Vasil'ev, V. P. (sic) and Pinchuk, V. I. 1993. A craniological analysis of gobies of the subgenus Ponticola Iljin, 1927. 1. A comparative morphological
investigation of N. cephalargoides and other forms of gobies assigned to the species N. platyrostris (Gobiidae). Journal of Ichthyology, 33(5):108-123.
Vasil'yeva, Ye. D., Vasil'yev, V. P. and Pinchuk, V. I. 1994. Craniological analysis of the goby subgenus Ponticola Iljin, 1927. 3. Comparative morphological study of Neogobius kessleri,
N. ratan, and additional findings on N. syrman relevant to the diagnosis and content of the subgenus. Journal of Ichthyology, 34(2):35-47.
Vasil'eva, E. D., Vasil'ev, V. P., Ponomareva, E. N. and Lapukhin, Yu. A. 2010. Triple hybrids obtained by artificial hybridization of the Russian sturgeon Acipenser gueldenstaedtii with the
hybrid of of the starred sturgeon A. stellatus and the great sturgeon A. huso (Acipenseridae): The kind of inheritance of some morphological characters and fertility of the
parental hybrid form. Journal of Ichthyology, 50(8):605-617.
Vasil'eva, E. D., Vasil'ev, V. P., Shedko, S. V. and Novomodny, G. V. 2009. The revision of the validity of genus Huso (Acipenseridae) based on recent morphological and genetic data with
particular reference to the Kaluaga H. duaricus. Journal of Ichthyology, 49(10):861-867.
Vaziri, M. and Borghei, M. 1995. A study of Caspian Sea surface water level fluctuations. Iranian Journal of Science and Technology, Transactions B: Technology, 19(4):399-?.
Vazirizadeh, A., Haji Moradlou, A. A., Esmaeili, H. R. and Akhlaghi, M. 2007. An investiagation on the sustained releasing delivery of GNRHA using Freund's incomplete adjuvant (FIA) in rainbow trout
(Oncorhynchus mykiss). Iranian Scientific Fisheries Journal, 16(2):145=152. In Farsi.
Vecsei, P. 2001. Threatened fishes of the world: Acipenser gueldenstaedtii Brandt & Ratzenburg, 1833 (Acipenseridae). Environmental Biology of Fishes, 60(4):362.
Vecsei, P. and Artyukhin, E. 2001. Threatened fishes of the world: Acipenser persicus Borodin, 1897 (Acipenseridae). Environmental Biology of Fishes, 60(2):160.
Vecsei, P., Artyukhin, E. and Peterson, D. 2002. Threatened fishes of the world: Acipenser nudiventris Lovetsky, 1828 (Acipenseridae). Environmental Biology of Fishes, 65(4):455-456.
Vecsei, P., Peterson, D., Suciu, R. and Artyukhin, E. 2007. Threatened fishes of the world, Acipenser stellatus, (sic) Pallas, 1771 (Acipenseridae). Environmental Biology of Fishes,
78(3):211-212.
Vecsei, P., Sucui, R. and Peterson, D. 2002. Threatened fishes of the world: Huso huso (Linnaeus, 1758)(Acipenseridae). Environmental Biology of Fishes, 65(3):363-365.
Vera, M., Sourinejad, I., Bouza, C., Vilas, R., Pina-Querida, A., Kalbassi, M. R. and Martínez, P. 2011. Phylogeography, genetic structure, and conservation of the endangered Caspian brown trout,
Salmo trutta caspius (Kessler, 1877), from Iran. Hydrobiologia, 664(1):51-67.
Verigina, I. A. 1991. Ichthyological collections of the Zoological Museum of the Lomonosov Moscow State University (On the 200th Anniversary of the Zoological Museum of MSU). Journal of
Ichthyology, 32(2):110-119.
Veshchev, P. V. 1995. Natural reproduction of Volga River stellate sturgeon, Acipenser stellatus, under new fishing regulations. Journal of Ichthyology, 35(9):281-294.
Veshchev, P. V. and Novikova, A. S. 1986. Analysis of the spawning run of osetr, Acipenser gueldenstaedti, in the Volga River. Journal of Ichthyology, 26(3):86-96.
Vesey-Fitzgerald, B. and Lamonte, F. (no date). Game Fish of the World. Harper & Brothers, New York. xvii + 446 pp.
Vesiland, P. J. 1993. The Middle East's water - critical resource. National Geographic, 183(5):38-71.
Vetchanin, V. I. 1984. Feeding of the Astrakhan shad, Alosa brashnikovi (Clupeidae), in the southeastern Caspian Sea. Journal of Ichthyology, 24(5):143-147.
Vetchanin, V. I. 1992. Razmer' i rost Brazhnikovskoi sel'di v yugo-vostochnom Kaspii [Size and growth of Brazhnikov's shad in the south-east Caspian]. Izvestiya Akademii Nauk Turkmenskoi SSR,
Seriya Biologicheskikh Nauk, 1992(4):48-53.
Vetlugina, T. A. 1992. The biology of tench, Tinca tinca, in the Volga Delta. Journal of Ichthyology, 32(5):58-64.
Vigne, G. T. 1842. Travels in Kashmir, Ladak, Iskardo, the countries adjoining the mountain course of the Indus and the Himalaya, north of the Punjab. Sagar Publications, New Delhi. 1981 Reprint.
2 volumes.
Vijayalakshmanan, M. A. 1950. A note on the fishes from the Helmund River in Afghanistan, with the description of a new loach. Records of the Indian Museum, 47:217-224.
Vila-Gispert, A. and Moreno-Amich, R. 2002. Life-history patterns of 25 species from European freshwater fish communities. Environmental Biology of Fishes, 65(4):387-400.
Vilenkin, B. Ya., Chikatunov, V. I., Coad, B. W. and Schileyko, A. A. 2009. A random process may control the number of endemic species. Biologia, Bratislava, 64(1):107-112.
Villwock, W. 1958. Weitere genetische Untersuchungen zur Frage der Verwandtschaftsbeziehungen anatolischer Zahnkarpfen. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 56:81-153,
Tafel 5-6.
Villwock, W. 1959. Über einen seltenen Aquariengast, den echten "Perserkärpfling" Aphanius sophiae (Heckel) 1846. Die Aquarien und Terrarien-Zeitschrift, 12:105-108.
Villwock, W. 1960. Zur Synonymie von Aphanius sophiae (Heckel) 1846. Mitteilungen aus dem hamburgischen Zoologischen Museum und Institut, 58:151-154.
Villwock, W. 1963. Genetische Analyse des Merkmals "Beschuppung" bei anatolischen Zahnkarpfen (Pisces, Cyprinodontidae) im Auflöserversuch. Zoologischer Anzeiger, 170:23-45.
Villwock, W. 1964. Genetische Untersuchungen an altweltlichen Zahnkarpfen der Tribus Aphaniini (Pisces, Cyprinodontidae) nach Gesichtspunkten der Neuen Systematik.
Zeitschrift für zoologische Systematik und Evolutionsforschung, 2:267-382.
Villwock, W. 1966. Isolationsmechanismen und Artbildung bei Fischen, unter besonder Berücksichtigung geographischer Isolationsfaktoren. Zoologischer Anzeiger, 177:84-110.
Villwock, W. 1970. Distribution, ecology and relationship of Near East and Mediterranean Cyprinodonts of the genus Aphanius. Journées Ichthyologies, Rome, Commission Internationale
pour l'Exploration Scientifique de la Mer Méditerranée, pp. 89-92.
Villwock, W. 1976. A contribution to the understanding of the evolution of meristic characters, with special reference to Old World cyprinodontids (Pisces, Cyprinodontidae). Abhandlungen und
Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg, NF 18/19 (Suppl.):11-27.
Villwock, W. 1977. Das Genus Aphanius Nardo, 1827. Deutsche Killifische Gemeinschaft Journal, Köln, 9(11):165-185.
Villwock, W. 1980. Aphanius (Nardo 1827) and Cyprinodon (Lac. 1803), an attempt for a genetic interpretation of speciation. Second International Congress of Systematic and Evolutionary Biology,
Vancouver, Canada, July 17-24, 1980, p. 378 (Abstract).
Villwock, W. 1981. Distribution, ecology and intraspecific variability of some external characters in Aphanius (Pisces: Cyprinodontidae). A contribution to regressive evolution in fish.
Rapport de la Commission Internationale pour l'Exploration scientifique de la Mer Méditerranée, 27(5):119-125.
Villwock, W. 1982. Aphanius (Nardo, 1827) and Cyprinodon (Lac., 1803) (Pisces, Cyprinodontidae), and attempt for a genetic interpretation of speciation. Zeitschrift für zoologische Systematik
und Evolutionsforschung, 20(3):187-197.
Villwock, W. 1984. CURT KOSSWIGs ichthyologische Forschungen - ein Beitrag zur Faunengeschichte Anatoliens und zu Phänomenen der Evolution am Beispiel anatolischer Zahnkarpfen (Pisces:
Cyprinodontidae). Mitteilungen aus dem hamburgischen zoologischen Museum und Institut, 80:19-40.
Villwock, W. 2004. Synopsis of classic and molecular investigations of Old Word (sic) cyprinodontids of the genus Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae), with special
concern of the Anatolian species, their speciation phenomena and their probable historic development. Mitteilungen aus dem hamburgischen zoologischen Museum und Institut, 101:35-46.
Villwock, W., Holčík, J., Wildekamp, R. H. and Doadrio, I. 2002. Comments on the proposed placement of the name Aphanius Nardo, 1827 (Osteichthyes, Cyprinodontiformes) on the Official
List (Case 3028; see BZN 58: 110-115). Bulletin of Zoological Nomenclature, 59(2):133-134 (separate short submissions by these authors grouped here for convenience).
Villwock, W., Scholl, A. und Krupp, F. 1983. Zur Taxonomie, Verbreitung und Speziation des Formenkreises Aphanius dispar (Rüppell, 1828) und Beschreibung von Aphanius sirhani n.
sp. (Pisces: Cyprinodontidae). Mitteilungen aus dem hamburgischen zoologischen Museum und Institut, 80:251-277.
Vita-Finzi, C. 1978. Recent alluvial history in the catchment of the Arabo-Persian Gulf, pp. 255-261. In: Brice, W. C. (Ed.). The Environmental History of the Near and Middle East Since the Last
Ice Age. Academic Press, London. xx + 384 pp.
Vladykov, V. 1929. Sur un nouveau genre de Cobitides: Sabanejewia. Bulletin du Muséum national d'Histoire naturelle, Paris, 2 série, 1(1):85-90.
Vladykov, V. D. 1962. Fisheries survey of Karadj Lake. MS, 9 pp.
Vladykov, V. D. 1964. Report to the Government of Iran on the inland fisheries, especially of the Caspian Sea with special reference to sturgeon. Food and Agriculture Organization, Rome, Report
FAO/EPTA 1818:51 pp.
Vlasenko, A. D. 1995. Current status of sturgeon stocks in the Caspian Sea, urgent needs for preservation. Abzeeyan, Tehran, 5(7):41-44, III. In Farsi.
von den Driesch, A. and Dockner, A. 2002. Animal exploitation in medieval Siraf, Iran, based on the faunal remains from the excavations at the Great Mosque (seasons 1966-1973).
Bonner Zoologische Beiträge, 50(3):227-247.
von Seidlitz, N. 1858. Rundreise um den Urmia-See in Persien, im Jahre 1856. Petermann's Geographische Mittheilungen, 4(6):227-236.
Voronina, E. P. 1999. Morphology and systematics of river flounders of the genus Platichthys. Journal of Ichthyology, 39(8):588-599.
Voropaev, G. and Kosarev, A. 1982. The fall and rise of the Caspian Sea. New Scientist, 94(1300):78-80.
Voropaev, G. V., Krasnozhon, G. F. and Lahijani, K. K. 1998. River runoff and stability of the Iranian Caspian coast. Water Resources, 25(6):689-700.
Voropaev, G. V. and Velikanov, A. L. 1985. Partial southward diversion of northern and Siberian rivers, pp. 67-83. In: Golubev, G. N. and Biswas, A. K. (Eds.). 1985. Large Scale Water Transfers:
Emerging Environmental and Social Experiences. Water Resources Series, 7:viii + 158 pp.
Vossughi, G. (= Wossughi, Gh.). 1985. The identification of freshwater fishes in the Dashti province of Bushehr. Fifth Congress of European Ichthyologists, Stockholm, 12-16 August 1985. p. 158
(abstract).
Vossoughi, G H. (= Wossughi, Gh.). 1998. On a collection of fresh water fishes from west of Jazmurian in southeastern Iran. Indian Journal of Fisheries, 45(2):221-224.
Vossughi, Ch. (= Wossughi, Gh.) and Mostajeer, B. 1994. Freshwater Fishes. Second Edition. Tehran University Publication No. 2132, 317 pp. In Farsi.
W
Wagstaff, J. M. 1985. The evolution of Middle Eastern landscapes. An outline to A.D. 1840. Croom Helm, London. xiii + 304 pp.
Wais, A. 1995. Zur Morphologie des Sichlings Pelecus cultratus (Linnaeus) (Pisces: Cyprinidae). Annalen des naturhistorischen Museums in Wien, 97B:421-435.
Walczak, P. 1972. A brief review of Salmonidae in Iran. Fisheries Research Institute, Bandar Pahlavi, Iran. MS, 7 pp.
Walczak, P. and RaLonde, R. 1970. Lucioperca lucioperca commercial catch data- 1969-1970 fishing season. Report of the Fisheries Research Institute, Bandar Pahlavi. MS, 3 pp., 3 figs.
Waldman, J. R. 1995. Sturgeons and paddlefishes: A convergence of biology, politics, and greed. Fisheries, 20(9):20-21, 49.
Walters, D. M. and Freeman, B. J. 2000. Distribution of Gambusia (Poeciliidae) in a southeastern river system and the use of fin ray counts for species determination. Copeia, 2000(2):555-559.
Wandzel, T. 2000. The fecundity and reproduction of round goby Neogobius melanostomus (Pallas, 1811) in the Puck Bay (Baltic Sea). Bulletin of the Sea Fisheries Institute, 2(150):43-51.
Wardlaw, R. 2001. Kara Bogaz Gol as a regulator of Caspian Sea levels. TACIS (Technical Assistance to the Commonwealth of Independent States, European Union), Caspian
Environment Programme, Centre for Water Level Fluctuations, Almaty (Nr WLF 12E). 11 pp.
Waring, E. S. 1807. A Tour to Sheeraz, by the route of Kazroon and Feerozabad; with various remarks on the manners, customs, laws, language, and literature of the
Persians. To which is added a History of Persia, from the death of Kureem Khan to the subversion of the Zund Dynasty. T. Cadell and W. Davis, London. xiii + 329 pp.
Waring, G. A. 1965. Thermal Springs of the United States and Other Countries of the World - A Summary. United States Government Printing Office, Washington. ix + 383 pp.
Warpachowski, N. A. 1892. Kollektsii ryb na Vserossiiskoi rybopromyshlennoi vystavke [Fish collection at the All-Russian Fisheries Management Exhibition].
Vestnik Rybopromyshlennosti, St. Petersburg, 7(4):145-157.
Warpachowski, N. A. 1895. Neskol'ko dannykh po ikhtiofaune vostochnogo Zakavkaz'ya [Some data on the ichthyofauna of the eastern Transcaucasus]. Russkoye Sudokhodstvo, 158:25-35.
Warwick, D. and Warwick, J. 1989. The doctor fish - a cure for psoriasis? The Lancet, 4 November 1989:1093-1094.
Weber, A., Proudlove, G. S. and Nalbant, T. T. 1998. Morphology, systematic diversity, distribution, and ecology of stygobitic fishes, p. 1177-1213. In: Juberthie, C. and
Decu, V. (Eds.). Encyclopaedia Biospeleologica. Tome II. Société de Biospéologie, Moulis, Bucarest (Academie Roumaine).
Weier, J. 2002. From wetland to wasteland. The destruction of the Hamoun Oasis. Earth Observatory Features, NASA. http://earthobservatory.nasa.gov/Study/hamoun, downloaded 14 February 2003.
Welcomme, R. L. 1981. Register of International Transfers of Inland Fish Species. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 213:vii + 120 pp.
Welcomme, R. L. 1988. International introductions of inland aquatic species. Food and Agriculture Organization, Rome, Fisheries Technical Paper, 294:x + 318 pp.
Welcomme, R. L. and Bartley, D. M. 1998. An evaluation of present techniques for the enhancement of fisheries, p. 1-35. In: Petr, T. (Ed.). Inland fishery enhancements.
Food and Agriculture Organization, Rome, Fisheries Technical Paper, 374:vi + 463 pp.
Werner, F. 1929. Beiträge zur Kenntnis der Fauna von Syrien und Persien. Zoologischer Anzeiger, 81:238-245.
Wessels, J. and Hoogeveen, R. J. A. 2003. Renovation of qanats in Syria. International Network on Water, Environment and Health, United Nations University, Japan. 25 pp.
Wheeler, A. C. 1956. The type species of Mastacembelus and the second edition of Russell's "Natural History of Aleppo". Bulletin of the Raffles Museum, Singapore, 27:91-92.
White, A.W. and Barwani, M.A. 1971. Common Sea Fishes of the Arabian Gulf and Gulf of Oman. Trucial States Council, Dubai. Volume 1:1-166.
White, P. G. 1988. A regional survey of the aquaculture sector in eleven Middle East countries (including Bahrain, Iraq, Islamic Republic of Iran, Jordan, Kuwait,
Oman, Qatar, Saudi Arabia, United Arab Emirates, Yemen Arab Republic and the People's Democratic Republic of Yemen). Food and Agriculture Organization, Rome, Aquaculture
Development and Coordination Programme, ADCP/REP/88/30:v + 50 pp.
White, T. F. 1987. Islamic Republic of Iran. Collection and analysis of fisheries data. A report prepared for the Fisheries Development Project, Food and Agriculture Organization, Rome,
FI/DP/IRA/83/013:1-8.
Whitehead, P. J. P. 1985. FAO Species Catalogue. Volume 7. Clupeid Fishes of the World (Suborder Clupeoidei). An Annotated and Illustrated Catalogue of the Herrings,
Sardines, Pilchards, Sprats, Shads, Anchovies and Wolf-herrings. Part 1 - Chirocentridae, Clupeidae and Pristigasteridae. Food and Agriculture Organization, Rome, Fisheries
Synopsis, 125, volume 7, part 1:x + 1-304.
Whitehead, P. J. P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J. and Tortonese, E. (Eds.). 1984-1986. Fishes of the North-eastern Atlantic and the Mediterranean.
UNESCO, Paris. Volume I:1-510; Volume II:511-1007; Volume III:1008-1473.
Whitehead, P. J. P., Nelson, G. J. and Wongratana, T. 1988. FAO Species Catalogue. Volume 7. Clupeid Fishes of the World (Suborder Clupeoidei). An Annotated and
Illustrated Catalogue of the Herrings, Sardines, Pilchards, Sprats, Shads, Anchovies and Wolf-herrings. Part 2 - Engraulididae. Food and Agriculture Organization, Rome,
Fisheries Synopsis, 125, volume 7, part 2:viii + 305-579.
Whitehead, P. J. P. and Talwar, P. K. 1976. Francis Day (1829-1889) and his collections of Indian fishes. Bulletin of the British Museum (Natural History), Historical Series, 5(1):1-189, 4 pls.
Whitley, A. and Gallagher, K. 1995. Karun River politics. Iran's multi-billion dollar hydroelectric plans lay in the hands of foreign policymakers. Middle East Insight, 11(5):33-35.
Whitley, G. P. 1931. New names for Australian fishes. Australian Zoologist, 6(4):310-334, pls. 25-27.
Whitley, G. P. 1953. Studies in ichthyology No. 16. Records of the Australian Museum, 23:133-138.
Whitney, C. R. 1979. Where caviar comes by the ton. International Wildlife, 9:4-10.
Whybrow, P. J. and Clements, D. 1999. Arabian Tertiary fauna, flora, and localities, p. 460-473. In: Whybrow, P. J. and Hill, A. (Eds.). Fossil Vertebrates of Arabia with
Emphasis on the Late Miocene Faunas, Geology, and Palaeoenvironments of the Emirate of Abu Dhabi, United Arab Emirates. Yale University Press, New Haven and London. xxv + 523 pp., 40 pp.
Whyte, R. O. 1961. Evolution of land use in South-western Asia, pp. 57-118. In: Stamp, L. D. Arid Zone Research - XVII. A history of land use in arid regions. UNESCO, Paris. 388 pp.
Wiepkema, P. R. 1961. Ethological analysis of the reproductive behaviour of the bitterling (Rhodeus amarus Bloch). Archives Néerlandaises de Zoologie, 14(2):103-109.
Wildekamp, R. 2001. Lebias oder Aphanius, dass ist die Frage. DKG-Journal, 33(3):72-75.
Wildekamp, R. H. 1993. A world of killies. Atlas of the oviparous cyprinodontiform fishes of the world. Volume 1. The genera Adamas, Adinia, Aphanius,
Aphyoplatys and Aphyosemion. Edited by B. R. Watters. American Killifish Association, Mishawaka, Indiana. 311 pp.
Wildekamp, R. H., Küçük, F., Ünlüsayin, M. and Neer, W. V. 1999. Species and subspecies of the genus Aphanius Nardo 1897 (Pisces: Cyprinodontidae) in Turkey. Turkish Journal of Zoology,
23(1):23-44.
Wildekamp, R. H., Romand, R. and Scheel, J. J. 1986. Cyprinodontidae, pp.165-169. In: Daget, J., Gosse, J.-P. and Thys van den Audenaerde, D. F. E. (Eds.). Check-list of
the Freshwater Fishes of Africa (CLOFFA). Volume 2:xiv + 520 pp.
Wildekamp, R. H., Van Neer, W., Küçük, F. and Ünlüsayin, M. 1997. First record of the eastern Asiatic gobionid fish Pseudorasbora parva from the Asiatic part of
Turkey. Journal of Fish Biology, 51:858-861.
Wilkens, H. 1977. Die Typen der Ichthyologischen Sammlung des Zoologischen Instituts und Zoologischen Museums der Universität Hamburg (ZMH). Teil III. Mitteilungen aus
dem hamburgischen zoologischen Museum und Institut, 74:155-163.
Wilkens, H. und Dohse, R. 1993. Die Typen der Ichthyologischen Sammlung des Zoologischen Instituts und Zoologischen Museums der Universität Hamburg (ZMH) Teil IV.
Mitteilungen aus dem hamburgischen zoologischen Museum und Institut, 90:401-426.
Wilkinson, J. C. 1974. The Organization of the Falaj Irrigation System in Oman. School of Geography, University of Oxford, Research Paper, 10:1-47.
Wilkinson, J. C. 1977. Water and tribal settlement in south-east Arabia. A study of the aflaj of Oman. Clarendon Press, Oxford. xvi + 276 pp.
Williams, J. S., Gibson, D. I. and Sadighian, A. 1980. Some helminth-parasites of Iranian freshwater fishes. Journal of Natural History, 14(5):685-699.
Williams, W. D. 1996. The largest, highest and lowest lakes of the world: Saline Lakes. Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 26:61-79.
Williams, W. D. 2002. Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environmental Conservation, 29(2):154-167.
Williot, P. et Bourguignon, G. 1991. Production d'esturgeon et de caviar, etat actuel et perspectives, pp. 509-513. In: Williot, P. (Ed.). Acipenser. Actes du premier
colloque international sur l'esturgeon. Bordeaux 3-6 octobre 1989, CEMAGREF (Centre National du Machinisme Agricole du Génie Rural des Eaux et des Forêts, Bordeaux). Paris. 519 pp.
Wilson, A. 1942. S.W. Persia. Letters and Diary of a Young Political Officer 1907-1914. Readers Union Limited and Oxford University Press, London. ix + 305 pp.
Wilson, M. 1995. Fisheries development Iran. Fisheries population dynamics and stock assessment course. Food and Agriculturfe oragnization, Rome, FI-DP/IRA/83/013-field-document:1-25.
Wolfart, R. 1987. Late Cretaceous through Quaternary palaeogeographic evolution of the Middle East, pp. 9-22. In: Krupp, F., Schneider, W. and Kinzelbach, R. (Eds.).
Proceedings of the Symposium on the Fauna and Zoogeography of the Middle East, Mainz, 1985. Beihefte zum Tübinger Atlas des Vorderen Orients,
Reihe A (Naturwissenschaften), 28, Dr. Ludwig Reichert Verlag, Wiesbaden, 338 pp.
Wolfe, R. K. 2001. Nest preference of the round goby (Neogobius melanostomus): birth control in the bedroom. International Association for Great Lakes Research,
Abstracts from the 44th Conference on Great Lakes Research June 10-14, 2001, Great Lakes Science: Making it Relevant. University of Wisconsin - Green Bay,
Wisconsin Sea Grant Institute, Green Bay, Wisconsin, Part 2:151.
Wolski, K. 1965. Les karez (Installations d'irrigation de terrains semi-désertiques) Afghanistan - Baloutchistan. Folia Orientalia, 6:179-204.
Woosley, A.-I. and Hole, Fr. 1978. Pollen evidence of subsistence and environment in ancient Iran. Paléorient, 4:59-70.
Wooten, M. C., Scribner, K. T. and Smith, M. H. 1986. Genetic variability and systematics of Gambusia in the southeastern United States. Copeia, 1988(2):283-289.
Wootton, R. J. 1976. The Biology of the Sticklebacks. Academic Press, London. x + 387 pp.
Wootton, R. J. 1984. A Functional Biology of Sticklebacks. University of California Press, Berkeley. 265 pp.
World Conservation Monitoring Centre. 1990. Directory of Wetlands of International Importance. Sites Designated for the List of Wetlands of International Importance.
Ramsar Convention Bureau, Gland, Switzerland. 782 pp.
Wossughi, G. 1978. Beitrag zur Systematik und Zoogeographie der Cyprinidae (Pisces, Teleostei) des Mittleren Ostens, unter besonderer Berücksichtigung des Irans.
Dissertation zur Erlangung des Doktorgrades des Fachbereichs Biologie der Universität Hamburg. 89 pp.
Wossughi, Gh. 1985. The identification of fresh-water fishes in the "Dashti" province of Bushehr. Fifth Congress of European Ichthyologists, Stockholm 12-16 August 1985, p. 158 (Abstract).
Wossughi, G. H. 1987. Die Sueswasserfische des Hamun Sees. Journal of the Veterinary Faculty, University of Tehran, 41(3-4):83-97. In Farsi.
Wossughi, Gh., Ahmadi, M. R., Kiaghadi, R. und Nikoie, A. 1991. Ein Beitrag zur Fischfauna des Haraz Flusses. Journal of the Veterinary Faculty of the University of Tehran, 45(3):63-82. In Farsi.
Wossughi, Gh., Azari Takami, Gh., Khoschzahmat, A. und Ahmadi, M. R. 1981. Die Suesswasserfische des Parischan Sees sowie der Quellen und Flosse in der Umbegung der Stadt
Kaserun. Journal of the Veterinary Faculty of the University of Tehran, 37(1):103-125. In Farsi.
Wossughi, G. H., Khoshzahmat, A. and Etemadfar, A. 1982. Recognition of fresh-water fishes in the area between Noorabad of Mamasany, Kazeron and Dashtestan.
Journal of the Veterinary Faculty of the University of Tehran, 38(1-3):23-44. In Farsi.
Wossughi, G. H. and Radmehr, B. 1983. An osteological study of the skull of some Iranian fishes (Part II). Journal of the Veterinary Faculty of the University of Tehran, 38(1):37-53. In Farsi.
Wossugh-Zamani, A. 1991a. Tirij [Acipenser stellatus]. Abzeeyan, Tehran, 2:60. In Farsi.
Wossugh-Zamani, A. 1991b. Safid-rud. Abzeeyan, Tehran, 2:28-30. In Farsi.
Wossugh-Zamani, A. 1991c. Fluctuations in the Caspian Sea water level. Abzeeyan, Tehran, 2:10-14. In Farsi.
Wossugh-Zamani, A. 1991d. The Blackwater River and the roach. Abzeeyan, Tehran, 2:24-27. In Farsi.
Woynarovich, E. 1985. Iran consultancy on fisheries development. Food and Agriculture Organization, Rome. IRA/83/013:27 pp.
Wright, D. 1977. The English amongst the Persians during then Qajar Period 1787-1921. Heinemann, London. xv + 218 pp.
Wright, H. E. 1977. Environmental change and the origin of agriculture in the Old and New Worlds, pp. 281-318. In: Reed, C. A. Origins of Agriculture. Mouton, The Hague. xvi + 1013 pp.
Wright, H. E. 1983. Climatic change in the Zagros Mountains - revisited, pp. 505-510. In: Braidwood, L. S., Braidwood, R. J., Howe, B., Reed, C. A. and Watson, P. J.
(Eds.). Prehistoric Archeology along the Zagros Flanks. Oriental Institute of Chicago University, Chicago. ix + 695 pp.
Wright, G. R. H. 1990. Of fishes and men. Fish symbols in ancient religion. Journal of Prehistoric Religion, 3-4:30-44.
Wright, H. E., McAndrews, J. H. and van Zeist, W. 1967. Modern pollen rain in western Iran, and its relation to plant geography and Quaternary vegetational history. Journal of Ecology, 55:415-443.
Wulff, H. E. 1966. The traditional crafts of Persia. Their development, technology, and influence on eastern and western civilizations. The Massachusetts Institute of
Technology Press, Cambridge, Massachusetts. xxiv + 404 pp.
Wulff, H. E. 1968. The qanats of Iran. Scientific American, 218(4):94-105.
Wundsch, H. H. 1973. Barsch und Zander Perca fluviatilis und Lucioperca lucioperca. Die Neue Brehm-Bücherei, 305, A. Ziemsen Verlag, Wittenberg Lutherstadt. 76 pp.
Y
Yachkaschi, A. 1976. Stand und Planung von Natur- und Nationalparken im Iran. Nature and National Parks, 14(53):12-13.
Yakchali (sic), M. 2002. Survey of (Ichthyophtirius (sic) multifilis) abundance (sic) in water cold fish
farms in west Azarbaijan in Iran. Book of Abstracts, 27th World Veterinary Congress, September 25-29, 2002, Tunis-Tunisia (abstract).
Yakhchali, M and Mahmudihesar, R. 2002. Survey of Ichthyophthirius multifilis abundance in cold water fish farms in West Azarbaijan in Iran. Pajouhesh va Sazandegi, 15(2)(55):58-59. In Farsi.
Yakovlev, V. N. 1959. Ryby iz minotsenovykh otlozhenii Kirgizii [Fishes from the Miocene of Kirghizia]. Paleontologicheskii Zhurnal, 1959(3):107-111.
Yalçin-Özdilek, Ş. and Ekmekçi, F. G. 2006. Preliminary data on the diet of Garra rufa (Cyprinidae) in the Asi basin (Orontes), Turkey. Cybium, 30(2):177-182.
Yanar, M. and Tekelioğlu, N. 1999. Balık Büyüklü ğünün Japon Balıklarında (Carassius auratus) Pigmentasyon Üzerine Etkisi
[The effect of fish size on pigmentation in goldfish (Carassius auratus)]. Turkish Journal of Biology, 23(1):101-105.
Yarahmadi, B., Moghadasi, F. and Siavashi, R. 2007. Use of Artemia urmiana decapsulated cyst in rainbow trout larvae diet. Pajouhesh va Sazandegi,
19(4)(73):49-58. In Farsi.
Yarshater, E. (Ed.). (Continuing). Encyclopæedia Iranica. Routledge & Kegan Paul, London.
Yasemi, M. and Nikoo, M. 2010. The impact of captivity on fertilization, cortisol and glucose levels in plasma in kutum broodstock. Iranian Journal of Fisheries Sciences, 9(3):478-484.
Yassaie, M. 1993. Common carp: Its status in present fish culture activities in Iran and its economical importance. In: Symposium on the Carp, Budapest, Hungary, 6-9 September 1993 (abstract).
Yazdanpanah, M. 2005. Reproductive biology of Garra rufa (Heckel, 1843) (Cypriniformes, Cyprinidae) in a spring-stream system, Zanjiran, Fars province. M.Sc. Thesis, Shiraz University.
xiii + 136 pp.
In Farsi.
Yazdipour, A., Marashi, S. Z. and Moazedi, J. 1991. Biotechnological report on artificial propagation of Barbus sharpeyi. Iranian
Fisheries Research and Training Organization Publication, Tehran. 34 pp. In Farsi.
Yelghi, S., Hajimoradlou, A. A., Ghorbani, R. and Kor, A. A. V. 2007. Investigation of some biological parameters of stellate sturgeon, Acipenser stellatus Pallas, 1771 in the south-eastern
part of Caspian Sea and its perspective. Journal of Agricultural Sciences and Natural Resources, 14(2):98-107. In Farsi.
Yerli, S. V., Çalişkan, M. and Canbolat, A. F. 1999. Çıldır Gölü (Ardahan)'ndeki Leuciscus cephalus'un Büyüme Ölçütleri Üzerine İncelemeler
[An investigation on the growth criterias of Leuciscus cephalus in Çıldır Lake-Ardahan]. Turkish Journal of Zoology, 23(supplement 1):271-278.
Yehganeh, S., Mojazi Amiri, B., Yousefian, M. and Nematollahi, M. A. 2005. The influence of extenders on motility duration of gray (sic) mullet (Mugil
cephalus) spermatozoa. Iranian Journal of Natural Resources, 58(2):383-393. In Farsi.
Yehganeh, S., Shabanpour, B., Hosseini, H., Imanpour, M. R., Shaabani, A., Moeini, M. and Motalebi, A. A. 2009. Seasonal variation of chemical composition and fatty acid profile of ovary in wild
common carp (Cyprinus carpio) of southeastern Caspian Sea. Iranian Scientific Fisheries Journal, 18(1):151-160. In Farsi.
Yıldırım, A., Halıloğlu, H. I., Erdoğan, O. and Türkmen, M. 2007. Some reproduction characteristics of Chalcalburnus mossulensis (Heckel,
1843) inhabiting the Karasu River (Erzurum, Turkey). Turkish Journal of Zoology, 31:193-200.
Yılmaz, S. and Polat, N. 2002. Age determination of shad (Alosa pontica Eichwald, 1838) inhabiting the Black Sea. Turkish Journal of Zoology, 26(4):393-398.
Yosefi Garakoei, M., Khara, M., Mohammadnezhad, M., Nezami, Sh. A., Mehdinejad, K. and Pajand, Z. 2006. Determination of maximum allowable concentration and LC50 96h of Sefidroud River
sediments for Persian sturgeon (Acipenser persicus) fingerlings. Iranian Journal of Fisheries Sciences, 15(3):153-160. In Farsi.
Young, G. 1976. Water dwellers in a desert world. National Geographic, 149(6):502-523.
Young, G. 1989. Return to the Marshes. Life with the Marsh Arabs of Iraq. Penguin, London. 192 pp.
Yousefian, M. 2004. Morphometric and electrophoretic comparison of common carp (Cyprinus carpio L.) in north of Iran. Iranian Journal of Fisheries Sciences, 13(3):179-198. In Farsi.
Yousefian, M. 2005. Generating gynogenetic common carp (Cyprinus carpio L.) by gamma ray. Iranian Journal of Fisheries Sciences, 14(3):183-198. In Farsi.
Yousefian, M. 2006. Quantitative, qualitative and hygienic investigation of sturgeon fingerlings in Shahid Rajaii fish farm (2002). Iranian Fisheries Research Organization,
Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Yousefian, M. 2007. Comparison of the survival rate percentage of small sturgeon fishes (Acipenser persicus and Acipenser stellatus) in different concentrations of Sefidrud Dam
deposits. M.Sc. Thesis, Islamic Azad University, Lahijan. In Farsi.
Yousefian, M. 2008. Genetic parameters of growth in rainbow trout, Oncorhynchus mykiss, at early rearing stage. Iranian Journal of Fisheries Sciences, 7(2):121-136.
Yousefian, M. and Farabi, S. M. V. 2007. The propagation efficiency of Acipenser persicus in restocking fish farms of Mazandaran and Golestan Provinces. Iranian Scientific Fisheries Journal,
15(4):159-166. In Farsi.
Yousefian, M., Gezel, H. G. and Hedayatifard, M. 2008. Induction of ovulation in endemic Chalcarburnus (sic) chalcoides, living in the Caspian Sea, using LRH-Aa combined with
metoclopramide. African Journal of Biotechnology, 7(22):4199-4201.
Yousefian, M. and Hedayati, M. 2001. Oogenesis studies of southern Caspian Sea Acipenser. 4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Yousefian, M. and Mosavi, H. 2008. Spawning of south Caspian kutum (Rutilus frisii kutum) in most migratory river of south Caspian Sea. Asian Journal of Animal and Veterinary Advances,
3(6):437-442.
Yousefian, M. and Nejati, A. 2008. Inbreeding depression by family matching in rainbow trout (Oncorhynchus mykiss). Journal of Fisheries and Aquatic Science, 3(6):384-391.
Yousefian, M., Norouzian Amiri, M. B., Magtomi, C. H., Mousavi, H., Hedayatifard, M., Laloei, F. and Farabi, S. M. V.2009. The reproduction normative and genetic characteristic of endemic common carp
Cyprinus carpio of Caspian Sea. The 6th Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 59 (abstract).
Yousefian, M., Oryan, Sh., Farokhi, F. and Asaeian, H. 2003. A study on oogenesis of Liza saliens. Iranian Journal of Fisheries Sciences, 12(1):131-152, 10. In Farsi.
Yousefian, M. and Rezvani, M. 2007. Effect of genetic and environmental factors on malformation in rainbow trout (Oncorhynchus mykiss). Pajouhesh va Sazandegi, 19(1)(74):78-85. In Farsi.
Yousefpour Pirbazari, H., Aghtouman, V. and Mohseini, M. 2000. Determination of optimum feeding rate expressed as percent of body weight in Persian sturgeon,
Acipenser persicus. Iranian Journal of Fisheries Sciences, 2(1):91-104, 113.
Youssefi, M. R., Sefidgar, A. A., Maligi, Gh., Mousavi, J. and Asnaashari, M. Y. 2005. Infection of river whitefishes (Rutilus rutilus) by Ligula
intestinalis parasite in Aras Dam; case series. Journal of Babol University of Medical Sciences, 7(2):80-83. In Farsi.
Yussefian, M. 2002. Biochemical composition of Mnemiopsis leidyi and its relation to population outburst in Caspian Sea. Iranian Journal of Marine Sciences, 1(3):65-70, 73. In Farsi.
Z
Zabihi, M. 2006. Some aspects of reproductive biology of Schizothorax zarudnyi. Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of
Jihad-e-Agriculture, Zabol. http://en.ifro.ir (abstract).
Zabihy, M., Pourkazemi, M., Kazemi, R. and Kamalai, A. 2004. Determination spawning season and changes in reproductive
cycle of Schizothorax zarudnyi and condition factor in Hamoon Lake. Iranian Journal of Fisheries Sciences, 12(4):41-56. In Farsi.
Zadeh, M. K., Maramzy, J. G., Preetha, K., Mansour, N., Ebrahim, R. Z. G. and Vahid, Y. 2009. Optimal dietary carbohydrate to lipid ratio in gattan Barbus xanthopterus fingerlings. The 6th
Scientific Conference of Fisheries Resources, 3-4 March 2009, Basrah, p. 96 (abstract).
Zadmajid, V. and Imanpour, M. R. 2009. The correlation between some biochemical and spermatological parameters in goldfish (Carassius auratus) semen. Journal of Agricultural Sciences and
Natural Resources, 16(1):54-61. In Farsi.
Zadmajid, V., Imanpour, M. R., Soudagar, M. and Shaabani, A. 2008. Comparison of the injection effects of GnRHa, HCG and pituitary extract on spermatological parameters in goldfish
(Carassius auratus). Journal of Agricultural Sciences and Natural Resources 15(1):65-72. In Farsi.
Zadmajid, V., Imanpour, M. R., Soudagar, M. and Shaabani, A. 2009. The effects of GnRHa, HCG and pituitary extract on biochemical parameters of seminal plasma in goldfish (Carassius auratus
gibelio). Iranian Journal of Biology, 22(2):333-342. In Farsi.
Zahmatkesh, A. 1993. On Gammaridae distribution in Caspian Sea bottom sediments. Iranian Fisheries Bulletin, (4):1-10, 1. In Farsi.
Záhorská, E., Kováč, V., Falka, I., Beyer, K., Katina, S., Copp, G. H. and Gozlan, R. E. 2009. Morphological variability of the Asiatic cyprinid, topmouth gudgeon Pseudorasbora parva, in
its introduced European range. Journal of Fish Biology, 74(1):167-185.
Zaidi, I. H. 1981. On the ethics of Man's interaction with the environment: an Islamic approach. Environmental Ethics, 3(1):35-47.
Zaitsev, V. F., Aslan Parviz, H. and Shipulin, S. V. 2001. Improvement of sturgeon fry rearing at the Mardzani fish factory (Iran).
4th International Symposium on Sturgeon, Oshkosh, Wisconsin, 8-13 July 2001 (abstract).
Zakaria, H. 1964. A catfish (Heteropneustes fossilis) of medical importance invades Iraqi waters. Journal of the Faculty of Medicine Baghdad, 6(n.s.)2:48-55.
Zakeri, H. 1997. Water catchment area of the Caspian Sea. Abangan, Student Quarterly of the Water Engineering Faculty of Khajeh Nassir ud-Din Tousi,
1997(12) (www.netiran.com/Htdocs/Clippings/Social/970700XXSO02.html).
Zakharyan, G. B. 1972. The natural reproduction of sturgeons in the Kura River following its regulation. Journal of Ichthyology, 12(2):249-259.
Zakharyan, G. B. and Teruni, V. N. 1979. The influence of light-assisted fishing of the "kilka" of the genus Clupeonella on stocks of the
Caspian shad of the genus Alosa (Family Clupeidae). Journal of Ichthyology, 19(5):129-132.
Zamakhaev, D. F. 1944. K voprosu o sistematicheskom polozhenii prokhodnykh sel'dei Kaspiya [On questions concerning the systematic position of the
Caspian anadromous herrings]. Zoologicheskii Zhurnal, 23(2-3):65-81.
Zamani, A., Hajimoradloo, A., Madani, R., Johari, A., Kalbasi, M. R. and Farhangi, M. 2006. Comparison of digestive enzyme activity in the stomach,
pyloric caeca and intestine in diploid and triploid female of rainbow trout (Oncorhynchus mykiss). Iranian Journal of Fisheries Sciences, 15(2):29-36. In Farsi.
Zamani, A., Hajimoradloo, A. A., Madani, R., Farhangi, M. and Vilaki, A. S. 2007. A measurement and comparison of digestive enzymes activity in larvae of Caspian brown trout and rainbow trout at
initiation of exogenous feeding. Iranian Scientific Fisheries Journal, 16(3):73-80. In Farsi.
Zamani, A., Hajimoradlou, A. A. Madani, R. and Golchinfar, F. 2007. Comparison of some digestive enzymes activity in the stomach, pyloric caecae and intestine of the parr and smolt of Caspian brown
trout (Salmo trutta caspius). Journal of Agricultural Sciences and Natural Resources, 14(3):31-39. In Farsi.
Zambriborshch, F. S. 1968. K sistematike bychkov Chernogo i Azovskogo Morei [On the systematics of the gobies from the Black Sea and the Sea of Azov]. Vestnik Zoologii, (1):37-44.
Zanoosi, A. P. 1993. The importance of site selection for beach seining in the Caspian Sea. Abzeeyan, Tehran, 4(7):44-45. In Farsi.
Zanoosi, A. P. 1994. Prohibited fishing or recruitment. Abzeeyan, Tehran, 4(11):56, III. In Farsi.
Zanusi, A. 1995. Fishing resources in the southeastern corner of the Caspian Sea. Marine Animals, Research and Science, Tehran, August-September:67-73
(internet version: http://netiran.com/press/economy-domestic/html/000000XXDE0062.html).
Zarbalieva, T. S. 1987. Information on the feeding of kutum, Rutilus frisii kutum, along the western coast of the southern Caspian Sea. Journal of Ichthyology, 27(4):170-173.
Zardoya, R. and Doadrio, I. 1999. Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids. Journal of Molecular Evolution, 49:227-237.
Zargar, A., Soltani, M., Hematzadeh Farhid, K. B. and Ebrahimzadeh Mousavi, H. A. 2008. Study on distribution of infectious hematopoietic necrosis (IHN) in five major provinces producing rainbow
trout (Oncorhynchus mykiss) fry in Iran by indirect fluorescence antibody (IFAT) and nested-RTPCR techniques. Journal of Veterinary Research, 63(3):99-105. In Farsi.
Zargarian, P., Abedian Kenari, A. A. M. and Nazari, R. M. 2007. Influence of supplemental phytase on fish meal replacement by soybean and its effects on growth and body composition of rainbow trout
(Oncorhynchus mykiss). Iranian Scientific Fisheries Journal, 16(2):75-84. In Farsi.
Zarin Kamar, H. 1996. Survey of feeding habits of kutum in Bandar Anzali coastal waters. M.Sc. Thesis, Islamic Azad University, Tehran. 150 pp. In Farsi.
Zarra Nezhad, M. and Rezaei, N. 2004. Estimating the production of cold water fishes in Kohgiloyeh and Boirahmad Provinces. Scientific Journal of Agriculture, 27(1):147-160. In Farsi.
Zarudnyi, N. 1896. Marshrut N. A. Zarudnego po vostochnoi Persii v 1896 g. [Itinerary of N. A. Zarudnyi in eastern Persia in 1896]. Ezhegodnik
Zoologicheskogo Imperatorskogo Muzeya Akademii Nauk, St. Petersburg, 1:18-21.
Zarudnyi, N. 1898. Marshrut N. A. Zarudnego po vostochnoi Persii v 1898 g. [Itinerary of N. A. Zarudnyi in eastern Persia in 1898]. Ezhegodnik
Zoologicheskogo Imperatorskogo Muzeya Akademii Nauk, St. Petersburg, 3:5-12.
Zarudnyi, N. 1901. Ekskursiya po vostochnoi Persii [An excursion through eastern Persia]. Zapiski Imperatorskogo Russkogo Geograficheskogo Obshchestva po Obshchei Geografii, St. Petersburg,
36(1):362 pp., map.
Zarudnyi, N. 1902. Marshrut exspeditsii Imper. Russk. Geograf. Obshchestva nachal'stvom N. Zarudnego po vostochnoi Persii v 1900-1901 gg. [Itinerary of the expedition of the Imperial Russian
Geographical Society to eastern Persia in 1900-1901]. Ezhegodnik Zoologicheskogo Imperatorskogo Muzeya Akademii Nauk, St. Petersburg, 7(1-2):1-9.
Zarudnyi, N. 1903. O gadakh i rybakh vostochnoi Persii. Gerpetologicheskie i ikhtiologicheskie resul'taty ekskursii po vostochnoi Persii v 1898 g.
[Reptiles, amphibians and fishes of eastern Persia. Herpetological and ichthyological results of the trip to eastern Persia in 1898]. Zapiski
Imperatorskogo Russkogo Geograficheskogo Obshchestva po Obshchei Geografii, St. Petersburg, 36(3):1-42.
Zarudnyi, N. 1904. Marshrut puteshestviya po zapadnoi Persii v 1903-1904 gg. [Itinerary of the expedition in eastern Persia in 1903-1904]. Ezhegodnik
Zoologicheskogo Imperatorskogo Muzeya Akademii Nauk, St. Petersburg, 9:45-51.
Zarudnyi, N. 1916. Tret'ya ekskursiya po vostochnoi Persii (Khorasan, Seistan i Persidskii Beludzhistan) [Third excursion throughout eastern
Persia (Khorasan, Seistan and Persian Beluchistan)]. Zapiski Imperatorskogo Russkogo Geograficheskogo Obshchestva po Obshchei Geografii, St. Petersburg, 50:448 pp.
Zauderer, J. 1988. The qanats of Yazd. In: Hydrology and Water Resources in Arizona and the Southwest (Proceedings of the 1988 Meetings of the Arizona
Section, American Water Resources Association and the Hydrology Section, Arizona-Nevada Academy of Science), University of Arizona, Tucson, Arizona, 18:97-109.
Zehzad, B. 1991. Preliminary identification of Gorgan Gulf and its fisheries reserves. In: Articles of the National Conference on the Suitable
Exploitation of Marine Reserves of the Caspian Sea, Babolsar. Iranian Fisheries Corporation Publications, Jehad-e Sazandegi, Tehran.
Zehzad, B., Kiabi, B. H. and Madjnoonian, H. 2002. The natural areas and landscape of Iran: an overview. Zoology in the Middle East, 26:7-10.
Zehzad, B., Madjnoonian, H., Darreh Shouri, B. F., Ziaie, H. and Samanpour, M. R. 1997.Geno Protected Area (Biosphere Reserve). Hormozgan Provincial
Office, Department of the Environment and Shahid Beheshti Research Bureau, Tehran. 70 pp. In Farsi.
Zehzad, B., Madjnoonian, H., Darreh Shouri, B. F., Ziaie, H. and Samanpour, M. R. 1998.Hara Protected Area (Biosphere Reserve). Hormozgan Provincial
Office, Department of the Environment and Shahid Beheshti Research Bureau, Tehran. 69 pp. In Farsi.
Zenkevich, L. A. 1957. Caspian and Aral Seas. Geological Society of America Memoir, 67(1):891-916.
Zenkevitch, L. 1963. Biology of the Seas of the U.S.S.R. George Allen & Unwin, London. 956 pp.
Zeynali, F., Tajik, H., Asri Rezaie, S. 2009. Evaluation of copper and zinc levels in muscles of some fish species of Caspian Sea. Iranian Veterinary Journal, 4(4)(21):84-89. In Farsi.
Zeynali, F., Tajik, H., Asri-Rezaie, S. and Meshkini, S. 2009. Determination of copper, zinc and iron levels in edible muscle of three commercial fish species from Iranian coastal waters of the
Caspian Sea. Journal of Animal and Veterinary Advances, 8(7):1285-1288.
Ziai, Z. 1971. Inland fish program for the fifth Development Plan. Iran Game and Fish Department, Tehran. MS. In Farsi.
Zibaee Nezhad M. J., Khosravi M., Baniasadi, N. and Daneshvar Z. 2008. Omega-3 fatty acid content in various tissues of different Persian Gulf fish. Iranian Cardiovascular Research Journal, 2(1):24-31.
Zipay, R. 1964. A Turkish killifish. Journal of the American Killifish Association, 1(1):13-14 (reprinted 1977, 10(2):35).
Ziuganov, V. V. 1991. Fauna SSSR. Ryby. Tom V, Vypusk 1. Semeistvo Kolyushkovykh (Gasterosteidae) Mirovoi Fauny [Fauna of the U.S.S.R. Fishes. Vol. V, No.
1. The Family Gasterosteidae of the World Fish Fauna]. Nauka, Leningrad. 261 pp.
Ziuganov, V. V. and Gomeluk, V. Ye. 1985. Hybridization of two forms of ninespine stickleback, Pungitius pungitius and P. platygaster,
under experimental conditions and an attempt to predict the consequences of their contact in nature. Environmental Biology of Fishes, 13(4):241-251.
Zohary, M. 1963. On the geobotanical structure of Iran. Bulletin of the Research Council of Israel, Botany, 11D, Supplement:1-113 pp., map.
Zolfaghari, Gh., Esmaeili Sari, A. and Ghasempouri, S. M. 2008. Mercury concentration in fishermen's hair and fishes in the south of the Caspian Sea: levels, specific accumulation and risk assessment.
Journal of Environmental Science and Technology, 36(special issue).
Zorriehzahra, M. E. J. 2000. Preliminary study of infectious agents associated with fry mortality syndrome of farmed rainbow trout in some Iranian
provinces. Program of the 26th Annual Larval Fish Conference (22-26 July 2002, Bergen, Norway). 1 p. (title).
Zorriehzahra, M. E. J. 2006. Preliminary study of infectious agents (viral and bacterial) of rainbow trout (Oncorhynchus mykiss) fry mortality syndrome in Iran.
Iranian Fisheries Research Organization, Agriculture Research and Education Organization, Ministry of Jihad-e-Agriculture. http://en.ifro.ir (abstract).
Zorriehzahra, M. J. (sic), Hassan, M. D., Gholizadeh, M. and Saidi, A. A. 2010. Study of some hematological and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fry
in western part of Mazandaran province, Iran. Iranian Journal of Fisheries Sciences, 9(1):185-198.
Zorriehzahra, M. J., Nakai, T., Gomez, D. K., Sharifpour, I., Shau-Chi, C., Soltani, M., Daud, H. H. M., Rohani, M. S. and Saidi, AA. A. 2005. Mortality of
wild golden grey mullet (Liza auratus) (sic) in Iranian waters of Caspian Sea, associated with viral nervous necrosis. Borok II, International Workshop,
Invasions of Alien Species in Holarctic, 27 September-1 October 2005, Borok (abstract).
Zorriehzahra, M. J., Nakai, T., Sharifpour, I., Kaw Gomez, D., Shau-Chi, C., Soltani, M., Mohd Daud, H. Hj., Sharif Rohani, M. and Saidi, A. A. 2005. Mortality of wild golden grey mullet (Liza
auratus) (sic) in Iranian waters of the Caspian Sea, associated with viral nervous necrosis-like agent
(2005). Iranian Journal of Fisheries Sciences, 4(2):43-58, 116-117.
Zorriehzahra, M. E. J., Soltani, M., Fallahi, R., Rezavani, S. and Karegar Moakhar, R. 2002. Observation of a case of entric (sic)
redmouth morbidity in fry of rainbow trout propagation farm (sic) around of Tehran Province in Iran. Program of the 26th Annual Larval Fish Conference (22-26 July 2002, Bergen, Norway).
1 p. (title).
Zorriehzahra, M. E. J., Soltani, M., Sharifpour, L., Saiedi, A. A. and Mehrabi, M. 2005. Preliminary study of infectious agents (viral and bacterial) of rainbow trout (Oncorhynchus mykiss)
fry mortality syndrome. Final Research Report, Iranian Fisheries Research Organization, Tehran, 84/470:290 pp.
In Farsi.
Zorzi, G. D. 1995. The biology of freshwater elasmobranchs: an historical perspective. Journal of Aquariculture and Aquatic Sciences, 7:10-31.
Zugmayer, E. 1912. Eight new fishes from Baluchistan. The Annals and Magazine of Natural History, 8(10):595-599.
Zugmayer, E. 1913. Die Fische von Balutschistan. Mit einleitenden Bemerkungen über die Fauna des Landes. Wissenschaftliche Ergebnisse der Reise
von Dr. Erich Zugmayer in Balutschistan 1911. Abhandlungen der Königlich Bayerischen Akademie der Wissenschaften Mathematisch-physikalische Klasse, 26(6):1-35.
Tomumshud



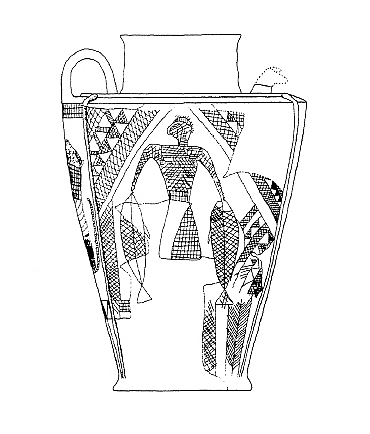




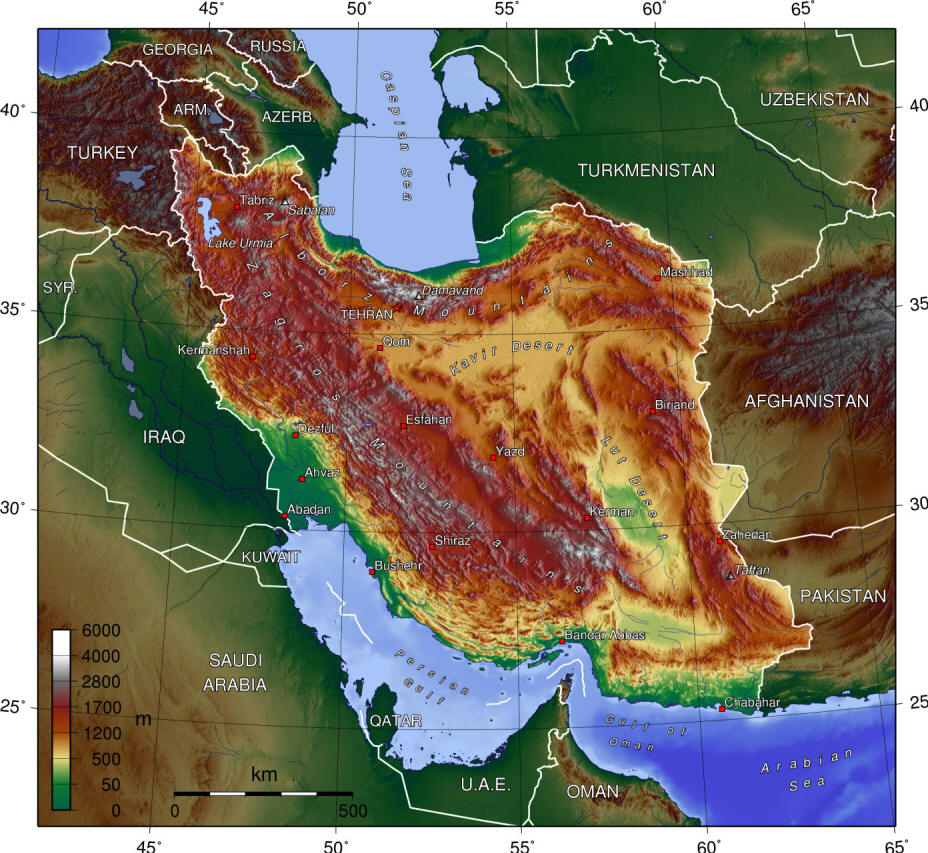

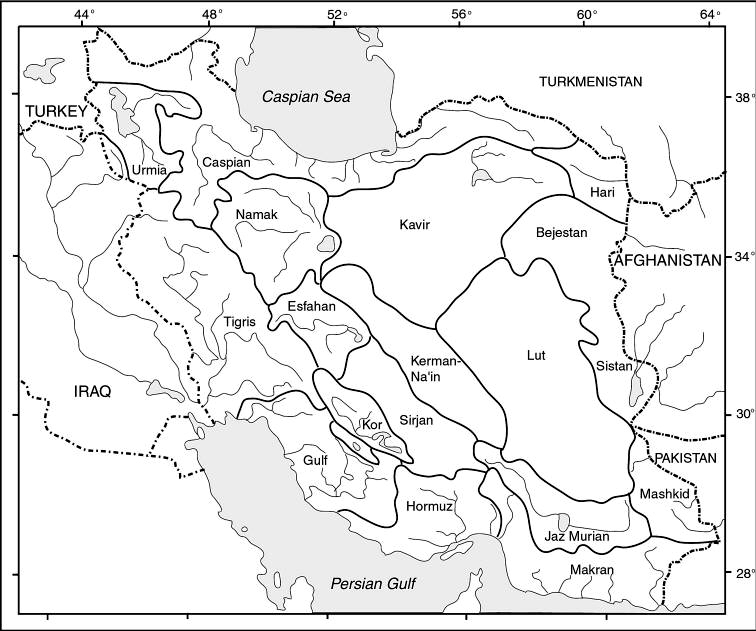




..jpg)











 e.g.
Pungitius platygaster
e.g.
Pungitius platygaster






 e.g.
Liza abu
e.g.
Liza abu e.g.
Lepomis macrochirus
e.g.
Lepomis macrochirus

 Sucker in
Glyptothorax silviae
Sucker in
Glyptothorax silviae

 e.g. Cobitis
taenia
e.g. Cobitis
taenia
 Cobitis taenia suborbital spine (enlarged)
?just top pic
Cobitis taenia suborbital spine (enlarged)
?just top pic e.g.
Oxynoemacheilus kermanshahensis
e.g.
Oxynoemacheilus kermanshahensis e.g.
Salmo caspius
e.g.
Salmo caspius

 e.g.
Alburnus filippii
e.g.
Alburnus filippii e.g.
Alosa braschnikowii
e.g.
Alosa braschnikowii e.g. Gambusia holbrooki
female
e.g. Gambusia holbrooki
female Gambusia
holbrooki male anal fin
Gambusia
holbrooki male anal fin e.g.
Aphanius vladykovi
e.g.
Aphanius vladykovi





















































 ph
teeth? her and elsewhere
ph
teeth? her and elsewhere

















 male
male
 male
?gut pics
male
?gut pics
 male
male
 male
male female
female male
male female
female male
male female
female



 e.g.
Knipowitschia iljini
e.g.
Knipowitschia iljini

















































.jpg)































































































































































-m%20cleaned.jpg)









































































































































































