Freshwater Fishes of Iran
Species Accounts
Cobitidae to Cyprinodontidae
Revised: 04 March 2017
Back to
Contents
This family of loaches, sometimes called sting-loaches, is found in Eurasia and Morocco and has about 28 genera with about 236 species (Berra, 2001; Nelson, 2006; Eschmeyer and Fong, 2011). Berra (2001) does not indicate the more southern distribution of this genus in Khuzestan and Fars provinces of Iran. Five to six species are recorded from Iran.
Anonymous (1988a) places Cobitidae on the Official List of Family-Group Names in Zoology (rather than the grammatically correct but unused Cobitididae) and Cobitis taenia is designated as the type species for the genus Cobitis (see also Kottelat (1986) for further information).
The body form is fusiform to rounded or elongate; the mouth is subterminal and has 3-6 pairs of barbels; the mental lobes of the lower lip have two parts: the anterior which is usually short and sometimes divided into lobules, and the posterior which is flap-like and longer and sometimes divided into 2 or more barbel-like extensions; there is 1 row of pharyngeal teeth; and there is an erectile spine in a groove below the eye (anterior in a non-Iranian genus). Iranian species have one pair of rostral barbels and a rounded or slightly emarginate caudal fin and belong to the subfamily Cobitinae. Menon (1992) considers that structural details of the bony covering of the swimbladder and the nature of the scales are only of use at the generic level. Lip structures, fin positions relative to one another and secondary sexual characteristics in males are important characters in differentiating species in India. Economidis and Nalbant (1996) discuss characters used in the study of these fishes and consider scales to be characteristic of each species along with colour pattern, sexual dimorphism, suborbital spine morphology, barbel and mental lobe morphology, and others. Black spots at the caudal fin base and four longitudinal pigment zones on the flank (Z1-4 or Gambetta's zones 1-4) are important in distinguishing and describing species. Males have 1-2 laminar projections on the dorsal surface of the anterior pectoral fin rays known as the laminae circularis or Canestrini's scales. Hybrid lineages are known, produced by gynogenesis and are nearly all-female. Males of bisexual lineages are sperm donors but the sperm only induces egg development and contributes no genetic material. The all-female lineages are therefore sperm parasites and have to occur in sympatry with one of the parental species in a hybrid complex. As a result, hybridogenous individuals in the complex are difficult to distinguish from the bisexual parent species on external characters. An unbalanced sex ratio in a population with more females than males is usually evidence that hybridogenous lineages are present (Kottelat and Freyhof, 2007). Maximum size is about 40 cm but most are much smaller.
The origins of this group of loaches may well lie at the end of the Eocene or in the early Oligocene in South China, spreading along a northern route through Europe and Siberia during the Oligocene-Miocene-Pliocene period and then later southwards into Southwest Asia (Sawada, 1982; Menon, 1987; 1992; Bănărescu and Nalbant, 1998; Šlechtová et al., 2008; Tang et al., 2008). An early Oligocene route also existed between the Anatolian landmass and Central Asia (Tang et al., 2008) and cobitids may have invaded the Euro-Mediterranean zoogeographic subregion at least five times independently based on cytochrome b data.
Some members of this family can live in oxygen-poor waters. They take in air at the surface, and pass it through the intestine where the mucosa absorbs the oxygen and carbon dioxide waste is released through the vent. As a consequence, they may be very sensitive to air pressure changes and become restless when it falls and can be used to predict the weather. Foods are mostly small insects, worms and crustaceans detected by the aid of the barbels on the habitat bottom. Some eat algal films or mats. Most species bury themselves in sand or mud during the day, emerging to feed at night. Movement is by undulations of the body, particularly marked in the more elongate species. A consequence of this form of movement is a reduction in fin size and variation. Reproduction involves the male chasing the female, entering vegetation and wrapping around the female as eggs are released and fertilised. Eggs swell and reach as large as 3.5 mm in diameter and as a result are retained in the vegetation although they are not adhesive.
A number of species are popular aquarium fishes, including the coolie or kuhli loaches and the weatherfish. None of the Iranian fishes are used in this fashion but they can be quite colourful. Cobitis cf. taenia is a potential fishing bait for predatory fishes such as Sander lucioperca and has been examined experimentally for this purpose in Turkey (Kuşat et al., 1995).
These fishes are found in Europe, North Africa and Asia. There are 3-4 species in Iran. They are known generally as سگ ماهي (sag mahi meaning dog fish) in Farsi, the equivalent of loach in English. This name is not repeated under each Species Account. Also called لوچ (= louch in Farsi, from loach).
This genus is characterised by an elongate and compressed body, a usually bifid, erectile spine below the eye (sometimes hidden under the skin), 3 pairs of short barbels (4 at the snout tip and 2 at the mouth corners), minute scales cover the body (as many as 200 but they are seldom counted accurately), lateral line faint or indistinct, dorsal and anal fins small, caudal fin rounded or truncate, and swimbladder in a bony capsule with a free portion visible. Males have bony extensions of their pectoral fin rays, known as lamina circularis or scale of Canestrini, and no swellings of their body sides.
Polyploid unisexual, bisexual-unisexual complexes and gynogenetic forms of Cobitis exist in the basins of the Baltic, Black, Caspian and Mediterranean seas (Vasil'ev and Vasil'eva, 1996; Kottelat, 1997; Vasil'eva and Vasil'ev, 1998; Vasil'ev et al., 1999; Bohlen, 2001; Vasil'ev et al., 2011). The species are morpohologically undifferentiated and therefore require detailed study to resolve taxonomic and systematic problems. The composition of Iranian species has not been investigated throroughly.
The earliest fossil record is from the middle Miocene about 15MYA with divergence of Sabanejewia and Cobitis at 12-13MYA (Ludwig et al., 2001).
These loaches often bury themselves in mud to overwinter or escape predators. The spine under the eye when erected is an anti-predator device, discouraging swallowing by other fishes and birds. The fish is said to actively swing the head from side to side to prick predators. Vasil'ev et al. (2011) review evolutionary ecology of clonal-bisexual complexes. Clonal forms, for example, are polyploids and have larger eggs and therefore reduced individual fecundity, and hatchlings are larger, perhaps with higher fitness initially in a local environment. The usual population fecundity of clonal forms being twice that of a bisexual species does not apply here because of the larger eggs.
Cobitis amphilekta
Vasil'eva and Vasil'ev, 2012
Described from the Kyzylagach Bay in Azerbaijan and the northeastern Caspian Sea, this taxon has not been recorded from Iran.
Cobitis faridpaki
Mousavi-Sabet, Vasil'eva, Vatandoust and Vasil'ev, 2011
Common names
Faridpak's spine loach. Some Farsi names formerly under C. taenia (see C. keyvani) may apply here as some Caspian Iranian Cobitis may be this species.
Systematics
The holotype is a male, 51.3 mm standard length, Mazandaran, Siahrud River (sic), 36º26'85.05"N, 52º56'70.08"E under GUIC (Ichthyological Museum, Department of Fisheries, Natural Resources Faculty, University of Gilan, Rasht) CC1403MA. Paratypes are GUIC CC1403M, 3 specimens, 54.7-62.3 mm standard length, same locality as holotype, C1404M, 9, 48.5-61.4 mm standard length, same locality as holotype, GUIC CC1405, 10, 49.2-67.4 mm standard length, same locality as holotype, ZMMU (Zoological Museum of Moscow State University) P-22694, 2, 50.6-63.2 mm standard length, same locality as holotype.
Cobitis taenia Linnaeus, 1758 was formerly the species for Iranian Caspian Cobitis (see below under C. keyvani) and would have been used for this taxon in part.
The new species is named for Farhad Faridpak (1911-1996) an Iranian ichthyologist working on Caspian Sea fishes.
Key characters
This species is distinguished from the other members of the genus in Iran by lacking a second lamina Canestrini at the base of the first pectoral fin ray (C. linea) and by lacking large flank spots (C. keyvani).
Morphology
The body is compressed and relatively deep. Subdorsal scales are rounded with a reduced eccentric focus. The single lamina Canestrini is axe-shaped and relatively broad. Dorsal fin with 2-3 unbranched and 6-7, usually 7, branched rays, anal fin with 2-3 unbranched and 5-7, usually 7, branched rays, pectoral fin with 7-9 branched rays, pelvic fin with 6-7 branched rays, and caudal fin with 13-16, usually 14(64%) or 16(32%), branched rays. Total vertebrae 40-41, usually 40 and gill rakers 11-12, usually 11.
Sexual dimorphism
Females are more stout than males and reach a longer body length. Male barbels and pectoral fins are longer and males have lamina Canestrini.
Colour
Flanks are pale yellow in life with 25-30 dark brown spots or blotches, smaller than eye diameter and usually merged. The Gambetta's zones of pigment are nearly complete, the fourth zone composed of the 25-30, small spots. The second Gambetta's zone on the upper flank is a narrow zone of brown spots between the first and third zones both of which are wider and composed of small dots. The lower head and abdomen are yellowish-white. The head is darkly mottled with dots and there is usually a dark stripe from the eye to the snout tip. Males have almost orange dorsal and caudal fins with dark spots. Females have yellow-orange dorsal and caudal fins with 3-4 rows of dark spots. Other fins are whitish without pigmentation. The caudal fin base has a black spot smaller than eye diameter on its upper part (sometimes absent),
Size
Attains 78.2 mm total length.
Distribution
Described from the Siahrud or Siah River in Mazandaran and presumably present also in the Babol River (Mousavi Sabet et al., 2011), if not all the rivers of the Iranian Caspian coast.
Zoogeography
See genus and family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Known only from a restricted area with no data on numbers and threats.
Further work
The biology of this species and its conservation status need study.
Sources
The type description.
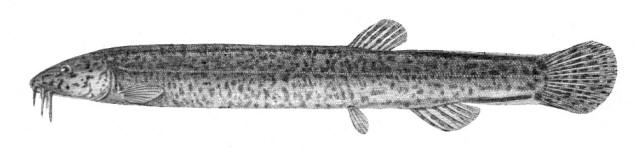
Cobitis keyvani
Mousavi-Sabet, Yerli, Vatandoust, Özeren and Moradkhani, 2012



Common names
mahi roshtegar or roftegar (= dustman, cleaner or sweeper fish, presumably from the bottom-dwelling habit); sagmahi-ye sangi (= stone loach), sagmahi-ye juibari or sagmahi-e-jooibari, mar mahi (= snake fish); loch or louch in Khuzestan (presumably from the English name, but also louch means a person with a squint); gel khorak in Khuzestan (latter two not necessarily this taxon, see below).
[lakh mukhattat in Arabic; zagafgaziya iliskani in Azerbaijan; shchipovka or shchipovka zakavkazskaya or Transcaucasian spiny loach in Russian; spined or spiny loach, stone loach, spiny stone loach, weatherfish, spotted weatherfish, Siberian loach; all these being for C. taenia but the Azerbaijani and Russian names presumably applicable to this species].
Systematics
Kottelat and Freyhof (2007) considered that the taxon in the southern Caspian Sea basin was not Cobitis taenia Linnaeus, 1758 as long used. C. taenia is restricted to northern and eastern Europe. Cobitis Taenia was originally described from Europe although Kottelat (1997) gives Lake Mälaren, Sweden.
Cobitis taenia satunini Gladkov, 1935 has been considered the subspecies or species of some southern Caspian Sea basin Cobitis, distinguished by a truncate snout rather than gradually tapering to the tip as in European spined loaches as well as other characters listed by Gladkov (1935) including head depth comprising 63.5% of head length on average and 12.27% of standard length, and caudal peduncle shorter than the head (on average 16.27% of standard (?) length). However, it was described from western Transcaucasia, outside the Caspian Sea basin, and material from the Caspian coast of Iran has not been examined and compared with it in detail. Berg (1948-1949) referred specimens from the upper Kura River basin to this subspecies but lacked material from the lower Kura, Lenkoran and the southern Caspian Sea coast.
The holotype of Cobitis taenia satunini described from "Kavkaz" and "nizov'ya r. Kintrish" (= lower Kintrish stream in the Caucasus; the Kintrish stream mouth is at 41°48'N, 41°46'E) is in the Zoological Museum of Moscow State University under MMSU P.2852 with a cotype (= paratype) under MMSU P.2317 (Eschmeyer et al. (1996) has MMSU P.2251, quoting Svetovidova (1978), which disagrees with Gladkov (1935)). The Zoological Museum of Moscow University (ZMMU; their acronym) has P-2852 as the holotype plus P-2313 as 2 paratypes (Pavlinov and Borissenko, 2001).
The holotype of Cobitis keyvani is a female, 62.9 mm standard length, "from the Keselian stream, Talar River, southeast of the Caspian Sea basin, Mazandaran province, north of Iran, 36°11’74.09 "N, 53°00’92.01"E", catalogued in the Ichthyology Museum, Department of Fisheries, Faculty of Natural Resources, the University of Gilan as GUIC CC1389MA. Paratypes are GUIC CC1389M, 8,, 37.4-80.3 mm standard length, GUIC CC1390M, 6, 44.4-67.4 mm standard length, and GUIC CC1391, 5, 48.3 and 68.9 mm standard length (sic), all same locality as holotype. The species is named for Prof. Dr. Amin Keyvan (1930-2007), Iranian ichthyologist.
Key characters
This species is distinguished from the C. linea by lacking a second lamina Canestrini at the base of the first pectoral fin ray, and from C. faridpaki by large, dark and obvious spots along the mid-flank.
Morphology
Some specimens are elongate while others are deeper bodied. Dorsal fin unbranched rays 2-3, branched rays 6-7, anal fin unbranched rays 2-3, branched rays 4-7, usually 5, pectoral fin branched rays 6-9, usually 7-8, pelvic fin branched rays 5-7, usually 6, caudal fin branched rays 10-16, usually 14, gill rakers 9-10, and total vertebrae 39-40.
Meristics for Iranian specimens:- dorsal fin branched rays 6(29) or 7(51); anal fin branched rays 4(1), 5(78) or 7(1); pectoral fin branched rays 6(4), 7(35), 8(38) or 9(3); pelvic fin branched rays 5(6), 6(70) or 7(4); caudal fin branched rays 10(1, but deformed), 12(2), 13(7), 14(68), 15(1) or 16(1); total vertebrae ?. Flank spots number 9(1), 10(2), 11(9), 12(17), 13(14), 14(18), 15(16), 16(14), 17(8), 18(11), 19(5), or 20(3) (excluding fish with bars, see below). The type description gives 14-20 (and later 17-24) flank spots. The type material has counts as follows:- dorsal fin branched rays 6(10) or 7(10); anal fin branched rays 5(17) or 7(3); pectoral fin branched rays 6(2), 7(12) or 8(6); pelvic fin branched rays 5(2), 6(13) or 7(5); caudal fin branched rays 13(1), 14(12), 15(1) or 16(6); total vertebrae 39(1) or 40(19); and gill rakers 9(1) or 10(19).
Sexual dimorphism
The second ray of the male pectoral fin is thickened and there is an enlarged scale at the base (Canestrini scale). The body in front of the dorsal fin of males is not distended as in S. aurata. Females are longer than males, and pectoral fins and barbels are longer in males (Mousavi-Sabet et al., 2012).
Colour
The back is light brown, the flanks pale yellow and the belly and lower head are yellowish-white. Five lateral pigmentation zones exist with L1 narrower than L2, L3 with 20-28 obvious dark speckles, L4 with a few small spots and reduced in the postdorsal fin part, L2 and L4 zones with irregular spots, and L5 zone wider than L1 and L3 (Mousavi-Sabet et al., 2012). The flank spots of L5 are usually clear and distinct, usually round but sometimes squarish, and some can be poorly defined, especially on the anterior flank. Anterior flank spots may merge to form short bars although their origin from spots can still be discerned. Rarely a long bar forms extending anteriorly from the mid-dorsal fin level. All fish have several large and distinct spots on the posterior flank. The number of spots can vary between high and low counts within the same sample, and are readily visible on even the smallest fish. The base of the caudal fin rays has a dark spot dorsally, much denser in pigmentation than flank spots. Some fish have a ventral caudal fin spot as well. Large spots along the mid-line of the back may be present or absent. The dorsal and caudal fins have spots or bars of pigment arranged in 3-5 rows. Other fins are whitish. The head is mottled with brown dots. A bar extends from the eye antero-ventrally. A postero-dorsal bar is present in some fish, meeting the one from the opposite side at the midline of the back of the head. This bar can be a row of spots or there may be no discernible pattern in this area. The iris is silvery, slightly golden or orange.
Size
Attains 80.3 mm standard length (Mousavi-Sabet et al., 2012).
Distribution
Found from Astara to Gorgan Bay including the Anzali Mordab, the Aras, Safid, Pol-e Rud, Sardab, Takar, Nessa, Haraz, Talar, Babol. Chowbar, Qasemabad, Qa'emshahr, Tonekabon, Shirud, Tajan and Gharasu rivers, and from the upper Karkheh.
Reports of Cobitis taenia from the middle Dez, Kashkan, Qareh Chai, Simarreh and lower Gav Masiab rivers in the Tigris basin may represent another new species (Saadati, 1977; Roshan Tabari, 1997; Abbasi et al., 1999; Kiabi et al., 1999; Abdoli, 2000; Abdoli and Naderi, 2009). An anecdotal report of a Cobitis from the Qareh Chai near Hamadan in the Namak Lake basin needs specimens for confirmation.
Zoogeography
Records from the Tigris River basin of Iran are based on literature (Saadati, 1977) and a ???single specimen (CMNFI 1979-0285). Mid-flank spots are less distinct than in Cobitis keyvani from the Caspian Sea basin of Iran of similar size being smaller and more numerous and the stripe on the centre of the back is continuous rather than spots as in most Caspian fish. Identification is tentative.
Habitat
This species remains buried in sand, mud which is not too thick, or dense weed growths during the day, being active at night, and is mostly solitary. Swimming is by undulating motions over short distances. When concealed, the body is bent into an arch so only the head and tail protrude. It prefers cool, clear running waters. Along the Caspian shore it is found in the lower reaches of rivers (Jolodar and Abdoli, 2004).
Age and growth
Females dominated the population in the Safid River and growth was positively allometric (Patimar et al., 2010). ??Mousavi Sabet et al. (2011) examined fish from the Babol River and found the percentage of females was significantly higher than males, and mature females were longer than 45 mm total length and 2+ years old and mature males were longer than 35 mm and 1+ years old. Life span was 5+ years for females and 3+ years for males. Mousavi-Sabet et al. (2012) found the population in the Talar River had significantly more females than males, females were mature at lengths longer than 49 mm and were at age 3+ while males matured at lengths longer than 45 mm and were at age 1+, and maximum age was 5+ years.
Food
Diet for related C. taenia is small crustaceans such as ostracods, copepods and rotifers in the bottom mud or sand. A mouthful of mud or sand is taken in, chewed, food items extracted, and the residue expelled convulsively through the gill openings.
Reproduction
Spawning of related C. taenia takes place from April to June in slow to still water. Eggs are laid on sand, stones and vegetation in several batches. Eggs may be deposited on the roots of water plants cleared of debris by males rooting among them. Males use their enlarged pectoral fins to turn the female during spawning.
The population in the Safid River had egg diameters up to 1.02 mm, absolute fecundity reached 8111 eggs and relative fecundity up to 383 eggs/g (Patimar et al., 2010). ??Mousavi Sabet et al. (2011) found Babol River fish spawning from the beginning of May to late July at water temperatures of 19.1-24.6ºC. Average absolute and relative fecundities were2172 and 590 eggs with ranges of 734-3562 and 347 to 945 eggs, respectively. Egg diameters were up to 1.4 mm with an average of 0.58 mm. Mousavi-Sabet et al. (2012) found the population in the Talar River had an average egg diameter of 0.56 mm, a maximum egg diameter of 1.4 mm, spawning took place from May to late July at 18.7-24.0°C, and mean (and maximum) absolute and relative fecundities were 2211 (3319) and 586 (902) eggs respectively.
Parasites and predators
This species (identified as C. taenia) is infected with Clinostomum complanatum, a parasite that can cause laryngo-pharyngitis in humans, in the Shirud of western Mazandaran Province (Shamsi et al., 1997).
Economic importance
None. Palicka (1996) gives a short account of aquarium care for C. taenia which may prove applicable to this species.
Conservation
This species was commonly caught in Iranian streams along the Caspian coast. Kiabi et al. (1999) consider it (as C. taenia) to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include abundant in numbers, habitat destruction, widespread range (75% of water bodies), present in other water bodies in Iran, and present outside the Caspian Sea basin (the latter two criteria no longer applicable).
Further work
The biology of this species needs study.
Sources
Iranian material: CMNFI 1970-0511, 35, 32.0-74.1 mm standard length, Gilan, Shafa River estuary (37°35'N, 49°09'E); CMNFI 1970-0514, 39, 37.6-79.4 mm standard length, Gilan, Shafa River estuary (37°35'N, 49°09'E); CMNFI 1970-515, 22, 38.8-73.7 mm standard length, Gilan, Shafa River estuary (37°35'N, 49°09'E); CMNFI 1970-0516, 2, 44.2-52.1 mm standard length, Gilan, Limir River at Lomir (38°14'N, 48°52'30"E); CMNFI 1970-0518, 4, 32.4-69.1 mm standard length, Gilan, Haviq River estuary (38°10'N, 48°54'E); CMNFI 1970-0519, 5, 29.6-39.2 mm standard length, Gilan, Chelvand River (ca. 38°18'N, ca. 48°52'E); CMNFI 1970-0525, 1, 20.4 mm standard length, Gilan, Safid River near Mohsenabad (ca. 37°22'N, ca. 49°57'E); CMNFI 1970-0526, 3, 32.9-39.1 mm standard length, Gilan, Safid River 5 km below Astaneh bridge (37°19'N, 49°57'30"E); CMNFI 1970-0527, 12, 27.4-69.7 mm standard length, Gilan, ditch near Kisom (37°12'N, 49°54'E); CMNFI 1970-0534, 16, 31.2-51.2 mm standard length, Gilan, Shafa River estuary (37°35'N, 49°09'E); CMNFI 1970-0535A, 3, 29.0-48.3 mm standard length, Gilan, Pir Bazar Roga (37°21'N, 49°33'E); CMNFI 1970-537, 16, 38.6-76.9 mm standard length, Markazi, Shah River near Manjil (36°44'N, 49°24'E); CMNFI 1970-0542, 37, 19.5-70.7 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0545, 14, 41.7-64.8 mm standard length, Gilan, Safid River (ca, 37°01'N, ca, 49°38'E); CMNFI 1970-0548, 33, 36.3-63.8 mm standard length, Mazandaran, Qareh Su (36°49'30"N, 54°03'30"E); CMNFI 1970-0549, 18, 14.5-22.8 mm standard length, Mazandaran, Qareh Su near Alm Emamzadeh (no other locality data); CMNFI 1970-0553, 13, 15.8-35.6 mm standard length, Gilan, Sowsar Roga (37°27'N, 49°30'E); CMNFI 1970-0554, 7. 17.5-33.8 mm standard length, Gilan, Pir Bazar and Sowsar Roga (37°21'N, 49°33'E and 37°27'N, 49°30'E); CMNFI 1970-0562, 1, 20.4 mm standard length, Gilan, Sowsar Roga (37°27'N, 49°30'E); CMNFI 1970-0566, 16, 19.5-39.9 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0567, 53, 15.9-38.3 mm standard length, Gilan, Pir Bazar Roga (37°21'N, 49°33'E); CMNFI 1970-0574, 5, 30.0-50.6 mm standard length, Gilan, Sowsar Roga (37°27'N, 49°30'E); CMNFI 1970-0575, 8, 24.8-32.9 mm standard length, Gilan, Pir Bazar Roga (37°21'N, 49°33'E); CMNFI 1970-0576, 3, 35.8-46.1 mm standard length, Gilan, Shafa River estuary (37°55'N, 49°09'E); CMNFI 1970-0579, 63, 26.2-71.9 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0580, 67, 31.1-48.5 mm standard length, Mazandaran, Iz Deh (36°36'N, 52°07'E); CMNFI 1970-0582, 1, 60.9 mm standard length, Mazandaran, Aliabad Reservoir (36°56'N, 54°50'E); CMNFI 1970-0587, 4, 46.4-53.1 mm standard length, Mazandaran, Babol Sar (36°43'N, 52°39'E); CMNFI 1970-590, 5, 24.5-70.0 mm standard length, Mazandaran, Shesh Deh River near Babol Sar (ca. 36°43'N, ca. 52°39'E); CMNFI 1979-0430, 2, 55.2-76.1 mm standard length, Mazandaran, river 1 km east of Now Shahr (36º39'N, 51º31'E); CMNFI 1979-0435, 1, 62.0 mm standard length, Gilan, stream 10 km west of Ramsar (36°57'N, 50°37'E); CMNFI 1979-0446, 4, 43.1-62.3 mm standard length, Gilan Astara River 2 km from Astara (38°26'30"N, 48°51'E); CMNFI 1979-0470, 8, 32.9-53.9 mm standard length, ; CMNFI 1979-0472, 9, 43.8-56.9 mm standard length, Mazandaran, stream 7 km west of Mahmudabad (36°37'N, 52°12'E); CMNFI 1979-0476, 1, 25.6 mm standard length, Mazandaran, Qareh Su 6 km from Kord Kuy (36°51'N, 54°05'E); CMNFI 1979-0479, 1, 47.6 mm standard length, Mazandaran, dam on Gorgan River 20 km north of Eimar (37°09'30"N, 54°41'30"E); CMNFI 1979-0626, 1, 49.5 mm standard length, Gilan, Safid River (no other locality data); CMNFI 1979-0685, 6, 26.8-44.2 mm standard length, Gilan, Safid River below Dehcha (ca. 37°22'N, ca. 49°57'E); CMNFI 1979-1215B, 5, 21.7-68.8 mm standard length, Gilan, Sowsar Roga (37°27'N, 49°30'E) ; CMNFI 1980-0117, 1, 47.4 mm standard length, Gilan, Golshan River (37°26'N, 49°40'E); CMNFI 1980-0122, 8, 34.4-47.9 mm standard length, Mazandaran, Nevissi River (36°38'N, 52°16'E); CMNFI 1980-0130, 28, 33.2-42.5 mm standard length, Mazandaran, Iz Deh (36°36'N, 52°07'E); CMNFI 1980-0132, 32, 25.8-52.8 mm standard length, Gilan, Safid River at Kisom (37°12'N, 49°54'E); CMNFI 1980-0136, 6, 31.0-65.9 mm standard length, Mazandaran, Fereydun Kenar River estuary (36°41'N, 52°29'E); CMNFI 1980-0138, 1, 31.8 mm standard length, Gilan, Safid River estuary (ca. 37°28'N, ca. 49°54'E); CMNFI 1980-0140, 1, 33.9 mm standard length, Gilan, Astara Mordab (ca. 38°26'N, ca. 48°53'E); CMNFI 1980-0916, 1, 53.3 mm standard length, Gilan, Nahang Roga (no other locality data); CMNFI 1991-0159, 1, 68.5 mm standard length, Mazandaran, Talar River (36°27'N, 52°48'E); CMNFI 1993-0140, 1, 44.3 mm standard length, Mazandaran, Shir River, Ramsar (36°51'48"N, 50°48'E); CMNFI 2008-0111, 1, 62.8 mm standard length, Gilan, Caspian Sea coast near Hendeh Khaleh (37°23'N, 49°28'E); CMNFI 2008-0112, 4, 26.6-51.4 mm standard length, Gilan, Caspian Sea coast near Hendeh Khaleh (37°23'N, 49°28'E); CMNFI 2008-0122, 2, 34.5-62.3 mm standard length, Gilan, Anzali Mordab (no other locality data).
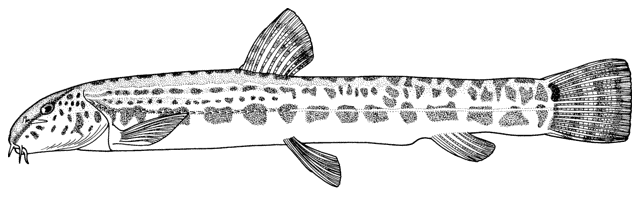
Cobitis linea
(Heckel, 1847)
Common names
sagmahi-ye jonubi (= southern dogfish).
[southern spined loach]
Systematics
The type locality of Acanthopsis linea is "Bäche um Persepolis" according to Heckel (1847b). Persepolis is at 29°57'N, 52°52'E in Fars.
The holotype described by Bănărescu and Nalbant (1966) is one of 7 specimens assumed to be syntypes. The catalogue in Vienna lists 1 specimen in one column and 6 in the adjacent column. The type series is in poor condition being dried with scales lost and colour mostly faded except for one specimen. Bianco and Nalbant (1980) cite a specimen as the holotype, a female, 67.0 mm standard length, housed in the Naturhistorisches Museum Wien (NMW 48560 - this includes all 7 fish) and the type locality as Persepolis, presumably following Bănărescu and Nalbant (1966). The dried specimens measure 41.2-62.9 mm standard length and the undried one 61.3 mm standard length. My measurement is at variance with these authors.
Placed in the subgenus Bicanestrinia Băcescu, 1962 by Bianco and Nalbant (1980) and originally described in the genus Acanthopsis Agassiz, 1832 (see Eschmeyer (1990) for further details on this genus). Bohlen et al. (2006) place this species in their Bicanestrinia lineage I using the cytochrome b gene. Bicanestrinia has a derived character state in the duplication of the Canestrini scale or lamina circularis on the pectoral fin of males, the primitive condition being a single lamina circularis as in, e.g. Cobitis s.s. The separation between Bicanestrinia and Cobitis s.s. occurred 12-17 MYA when the land connection between Central Europe and Anatolia broke.
Material identified as Cobitis turcica (Hanko, 1924) from the "River Kor near Persepolis" in Nalbant and Bianco (1998) is C. linea based on my examination of IZA 7829-30. Fricke et al. (2007) have it in the Tor (sic, meaning Kor) River system of Iran, the same error. The species was described originally from Eregli in Anatolian Turkey and its presence in Iran needs confirmation. Erkakan et al. (1999) review the Turkish species of Cobitis but do not mention the occurrence of C. turcica in Iran.
Key characters
This species is distinguished by the dark brown lateral spots being reduced or absent, males have 2 Canestrini's scales at the upper bases of the unbranched and first branched pectoral fin rays, the laterocaudal branch of the suborbital spine is reduced or absent (although the spine itself is detectable by touch in even the smallest fish), and 14 branched caudal fin rays.
Morphology
Dorsal fin unbranched rays 2 and branched rays 6-7, anal fin unbranched rays 2-3 and branched rays 5, pectoral fin branched rays 7-9, and pelvic fin branched rays 5-6.
Meristic values for specimens examined by me are:- dorsal fin branched rays 6(16), anal fin branched rays 5(16), pectoral fin branched rays 8(6), pelvic fin branched rays 6(6), and total vertebrae 41(1).
Scales are embedded and have a reduced and eccentric focus. Johal et al. (2006) and Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales. The lateral line does not pass the end of the pectoral fin. The swimbladder capsule is globular but has ventral indentations anterolaterally on both sides. There are small keels on the upper and lower caudal peduncle. The anterior nostril is a short tube. The upper lip has fine furrows, the lower lip is thick and folded with a pair of medial lobes.
Sexual dimorphism
Males have Canestrini's scales as detailed above.
Colour
Overall colour is a yellowish-white with a golden iridescence especially on the operculum. Spots, blotches and dots are blue-grey or brown. The flanks are variably spotted and striped, the variability not related to sexual dimorphism. In some fish there is an upper flank, uniform stripe closely following the line of the back, below it a longitudinal series of irregular spots and blotches, a third stripe incompletely developed, and a fourth mid-lateral stripe of spots and blotches. In others the third and fourth stripes are an indistinguishable row of speckles. The mid-line of the back has a row of rounded spots numbering 7-11 (usually 8) predorsally, 0-3 (usually 2) at the dorsal fin base, and 5-11 (usually 8) postdorsally. The upper head is covered with many minute dots, extending onto the snout and upper head sides. The iris is golden-yellow. There is a blackish band from the eye to the mouth corner. There is a minute black spot on the caudal fin base. Fins are translucent. There are numerous dots scattered on the rays and membranes of the dorsal and caudal fins forming up to 9 irregular rows on the former and 8 more regular rows or bars on the latter, but still not very clearly defined. Spots on the dorsal edge of the caudal fin are clearly defined and regular; there are no spots on the ventral edge. The pelvic and anal fins are immaculate while the pectoral fin has some minor and irregular spots.
Size
Reaches 89.6 mm standard length.
Distribution
This species is found in the Kor River basin and the upper Kul River drainage of the Hormuz basin (Bănărescu and Nalbant, 1966; Bianco and Nalbant, 1980).
Zoogeography
This species may be related to Cobitis simplicispina Hankó, 1925 from Anatolia and other nominal species, all placed in the subgenus Bicanestrinia characterised by two Canestrini's scales. Members of this complex of species possibly reached western Asia from eastern Asia in the early Miocene. The disjunct distributions seen today were probably produced by Pleistocene climatic changes (Bianco and Nalbant, 1980).
Habitat
This species favours muddy bottoms. Specimens were caught in about 5-6 cm of mud, or at the foot of a muddy bank, with aquatic vegetation in the form of reeds.
Age and growth
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 108 fish measuring 3.35-8.96 cm standard length. The a-value was 0.0089 and the b-value 3.060 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Adult females described by Bianco and Nalbant (1980) measuring 80.0-88.6 mm standard length have ripe eggs 0.8-1.1 mm in diameter. A specimen from the Lapui spring caught on 30 June carries eggs of this size.
Parasites and predators
Unknown.
Economic importance
This species is too small and rare to be of direct economic importance.
Conservation
Since this species is known only from a limited number of specimens, further studies should be undertaken to ascertain its abundance and distribution. It seems to favour muddy habitats and may have a restricted distribution in the rocky streams of Fars on this account.
Further work
See above.
Sources
Type material: See above (NMW 48560).
Iranian material: CMNFI 1979-0292, 1, 69.8 mm standard length, Fars, Lapui Spring in the Kor River basin (29º48'N, 52º39'E); IZA 7829, 4, 41.9-85.5 mm standard length, Fars, Pulvar River 15 km north of Persepolis (29º59'N, 52º59'E); IZA 7830, 1, 27.1 mm standard length, Fars, springs of Kul River near Darab (no other locality data).
Cobitis sp.
Common names
None.
Systematics
Key characters
Morphology
Dorsal fin unbranched rays ? and branched rays ?, anal fin unbranched rays ? and branched rays ?, pectoral fin branched rays ?, and pelvic fin branched rays ?.
Sexual dimorphism
Males have Canestrini's scales as detailed above.
Colour
Size
Reaches ? standard length.
Distribution
This species is found in the Tigris River basin of Iran and Iraq. Iranian localities include ?
Zoogeography
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
This species is too small and rare to be of direct economic importance.
Conservation
Since this species is known only from a limited number of specimens, further studies should be undertaken to ascertain its abundance and distribution.
Further work
This taxon needs diagnosis and description, including DNA data, to distinguish it from other Cobitis in Southwest Asia.
Source
Iranian material: CMNFI 1979-0285, 1, 44.9 mm standard length, Kermanshahan, Qareh Su drainage (34º26'N, 46º37'E); CMNFI 1993-0127, 1, 50.5 mm standard length, Kermanshahn, Sarab-e Maran (34º44'N, 46º51'E); CMNFI 2008-0102, 1, 54.1 mm standard length, Kermanshahan, sarabs near Kermanshah (34º19'N, 47º04'E).
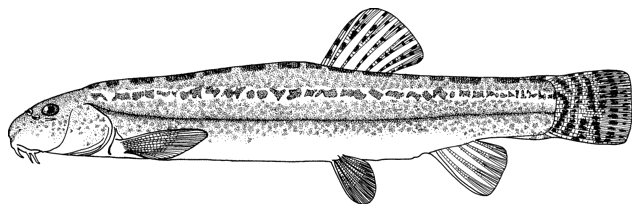
Misgurnus anguillicaudatus
(Cantor, 1842)
Recorded from the Karakum Canal and Kopetdag Reservoir of Turkmenistan by Shakirova and Sukhanova (1994) and Sal'nikov (1995), this exotic species from China may eventually reach the Tedzhen River and Caspian Sea basins of Iran. No Iranian record.
Sabanejewia is distinguished from Cobitis Linnaeus, 1758 by having modally 12 rather than modally 14 branched caudal fin rays, a stronger suborbital spine, developed mental lobes which may be unfringed or well-fringed, large imbricated or unimbricated scales with a relatively large and central focus, and males have a protuberance on each side of the body in front of the dorsal and pelvic fins and lack the lamina circularis (a bony process at the base of the second pectoral fin ray)(see Vladykov (1929), Nalbant (1963, 1994), Sawada (1982), Vasil'yeva (1995b) and Perdices and Doadrio (1997, 2001) for further details). Krupp (1985c) does not consider Sabanejewia to be a distinct genus. Perdices et al. (2003) using mtDNA demonstrate that Caucasian-Caspian lineages are the sister group of a Danubian-Balkan lineage. Tang et al. (2008) using the cytochrome b gene found that ancestral Sabanejewia might have been the first cobitids to cross Siberia and invade the Euro-Mediterranean zoogeographic subregion.
Sabanejewia aurata
(De Filippi, 1863)
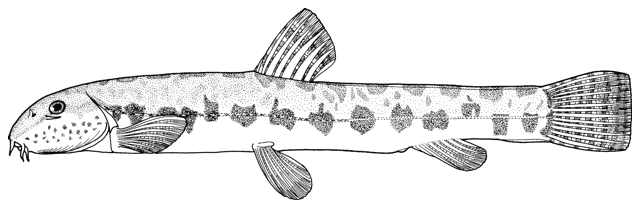
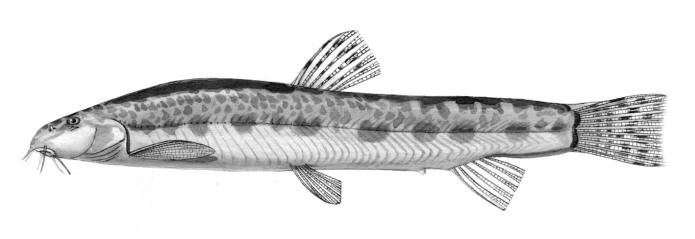

Common names
sagmahi-ye khardar (= spiny loach), sagmahi-e-jooibari.
[gizili iliskan in Azerbaijan; opi-dzug in Armenia; peredneaziatskaya shchipovka or Hither Asia spined loach, zolotistaya shchipovka or golden spined (spiny) loach, both in Russian].
Systematics
Kottelat (1997) tentatively considers that this species is restricted to Iran and possibly adjacent waters and does not occur in Europe.
The lectotype of Cobitis aurata (MZUT N.674), designated by Tortonese (1961), is stored in the Istituto e Museo di Zoologia della R. Università di Torino and 2 paralectotypes (MSNG N.365) from the Collezione di Universitá di Genova are in the Museo Civico di Storia Naturale di Genova (Tortonese, 1940; Tortonese, 1961). Eschmeyer et al. (1996) give the paralectotypes' catalogue number as ?MSNG 42727 (ex Univ. Genoa 365).
The type locality is "un fiumicello presso Sartschem" (De Filippi, 1863; not 1865 as in Berg (1948-1949), Banarescu and Nalbant (1964) and Reshetnikov et al. (1997) and not 1862 as in Tortonese (1940) and Eschmeyer et al. (1996)). The type locality is possibly Sarcham-e Sofla (37°07'N, 47°54'E) in the Qezel Owzan River drainage of the Caspian Sea basin in Iran.
Cobitis hohenackeri Brandt in Kessler, 1877 from the Kura River basin of Transcaucasia (Azerbaijan) is a synonym with 6 syntypes in the Zoological Institute, St. Petersburg (ZISP).
Key characters
Distinguished from other cobitids in northern Iran by having modally 12 branched caudal fin rays, large dark spots along the flank, and above them speckles which do not tend to form a stripe.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 5-8, predominately 6 in the Caspian basin, anal fin unbranched rays 2-3, branched rays 4-8, usually 5, pectoral fin branched rays 5-9, predominately 7 in the Caspian basin (but see below), pelvic fin branched rays 4-8, usually 5 (but see below), and vertebrae 39-43.
Meristics for Iranian specimens:- ? check dorsal rays on x-rays, dorsal fin branched rays 6(11) or 7(4); anal fin branched rays 5(15); pectoral fin branched rays 7(1) or 8(14); pelvic fin branched rays 5(1) or 6(14); caudal fin branched rays 11(2) or 12(13); and total vertebrae ?.
Scales minute but visible to the naked eye, ca. 170-200. Dermal crest or adipose fins are variably developed behind the dorsal and anal fins. Barbels are longer than in C. keyvani, the mouth corner barbels reaching back to the posterior eye margin. Karyotype is 2n=50 (Klinkhardt et al., 1995).
Sexual dimorphism
The second pectoral fin ray in males is not enlarged as in Cobitis but there is a lateral distension of the body in front of the dorsal fin in mature males.
Colour
The back is a brownish olive-green with darker marbling. The flanks are golden-brown with a row of dark brown spots, less conspicuous than those in C. keyvani. These number 9-18, modally 11-13, in the Caspian basin. An upper row of spots is absent. The number of spots may be related to habitat, those fish from calm waters having few large spots. The back has several dark blotches along its mid-line. In the Caspian basin there are 7-16 back blotches, modally 9-10. There is a bar at the caudal base, sometimes with a small central gap. Fins are slightly pink.
Size
Attains 13.8 cm, but most fish are less than 10.0 cm.
Distribution
Found in the basins of the Baltic, Aegean, Black and Caspian seas and in the Tedzhen and Murgab rivers of Afghanistan and Turkmenistan according to most authors (Aliev et al., 1988; Nalbant and Bianco, 1998). A distinct subspecies is found in the Aral Sea basin (see below). Kottelat (1997) however considers that this species is restricted to Iran and possibly adjacent waters. It is found along the Caspian Sea coast of Iran including the Anzali Mordab and its tributaries, the Safid River at Kisom, lower Tonekabon, Chalus, Haraz and Babol rivers, Nakhurde (= ? Noqreh Deh), and in the Kashaf River of the Tedzhen River basin (Holčik and Oláh, 1992; Abbasi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
The record of this species in the Tigris-Euphrates basin at Basrah, Iraq (BM(NH) 1920.3.5:9) in Nalbant (1963) and Bănărescu and Nalbant (1966), and repeated in Banister (1980), is probably an error of labeling (Bănărescu, 1973).
Zoogeography
The subspecies, Cobitis aurata aralensis Kessler, 1877, is reported from the Karakum Canal and the Uzboi lakes in Turkmenistan by Shakirova and Sukhanova (1994) and Sal'nikov (1995) and may well enter both the Tedzhen River and Caspian Sea basins of Iran eventually.
Habitat
This is a nocturnal species, hiding during the day under gravel and boulders of flowing rivers. If exposed, it will make jerking motions to the nearest cover. Some authors state that it also hides in sand. It prefers shallow and clear water. It occurs with C. keyvani but is commoner in faster water in the upper and middle reaches of rivers, from 5 to 150 cm water depth. However it may form populations in still water left behind after floods.
Age and growth
Males tend to be slightly smaller than females.
Food
Bottom-dwelling invertebrates are the main food items including larval insects such as mayflies, dragonflies and caddisflies as well as nematodes, copepods, chironomids, fish eggs, algae and detritus.
Reproduction
Fecundity reaches 14,700 eggs and egg diameter 0.85 mm. Eggs are shed over plants from April to August and this species may spawn in batches.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Lelek (1987) lists this species as rare to vulnerable in Europe. Kiabi et al. (1999) consider this species to be conservation dependent in the south Caspian Sea basin according to IUCN criteria. Criteria include few in numbers, habitat destruction, limited range (less than 25% of water bodies), absent in other water bodies in Iran, and present outside the Caspian Sea basin. The 2000 IUCN Red List lists this species as DD (Data Deficient).
Further work
The biology of this species in Iran has not been examined and its systematics needs clarification.
Sources
Vasil'eva and Vasil'ev (1988) give details on variation in meristics and colour of this species.
Iranian material: CMNFI 1970-0508, 4, 39.2-57.1 mm standard length; Gilan, Safid River at Hasan Kiadeh (37º24'N, 49º58'E); CMNFI 1970-0545, 1, 33.9 mm standard length, Gilan, Safid River (ca. 37º01'N, ca. 49º38'E), CMNFI 1979-0448, 1, 70.1 mm standard length, Azarbayjan-e Khavari, Ahar Chay (38º18'30"N, 48º22'E); CMNFI 1980-0131, 1, 57.4 mm standard length, Iran, Caspian Sea basin (no other locality data); CMNFI 1980-0132, 1, 49.8 mm standard length, Gilan, Safid River at Kisom (37º12'N, 49º54'E); CMNFI 1980-0155, 7, 38.5-63.5 mm standard length, Azarbayjan-e Khavari, Qareh Su at Ardabil (ca. 38º15'N, ca. 48º18'E); CMNFI 2008-0221, 2, 56.0-61.9 mm standard length, Gilan, Safid River (no other locality data).
Sabanejewia caspia
(Eichwald, 1838)
Common names
mahi roshtegar talaee (= golden dustman fish), rofteghar mahi, sagmahi-ye Khazari (= Caspian loach), sag mahi khardar (= spiny loach), mar mahi (= snake fish), sagmahi-e-jooibari.
[xazar iliskani in Azerbaijan; Kaspiiskaya shchipovka or Caspian spined (spiny) loach in Russian].
Systematics
This species was described from "in sinu mardofiensi prope castellum Lencoranicum" (i.e. the Caspian Sea in the ? Mardofiensi Gulf near the Lenkoran fortress). The type specimen is apparently lost (Vasil'yeva, 1995b).
Key characters
Distinguished from other cobitids in northern Iran by having modally 12 branched caudal fin rays, no large dark spots along the flank but an irregular stripe, and above this stripe speckles which do not tend to form a stripe.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 6-7, anal fin unbranched rays 1-2, branched rays 5-6, pectoral fin branched rays 6-7 (but see below) and pelvic fin branched rays 4-6. Vertebrae 41-42. A crest is well developed on the lower caudal peduncle but only posteriorly on the upper edge. Barbels are shorter than in C. keyvani, the posterior ones reaching beyond the posterior eye margin. Karyotype is 2n=50 (Klinkhardt et al., 1995).
Meristics for Iranian specimens:- dorsal fin branched rays 6(83) or 7(1); anal fin branched rays 5(80) or 6(4); pectoral fin branched rays 7(6), 8(72) or 9(6); pelvic fin branched rays 4(3), 5(67) or 6(14); caudal fin branched rays 7(1, but deformed), 9(1), 10(3), 11(4), 12(74) or 13(1); total vertebrae ?.
Sexual dimorphism
The second pectoral fin ray in males is not enlarged as in Cobitis but there is a lateral distension of the body in front of the dorsal fin an mature males.
Colour
Live specimens, especially young, are almost transparent. The adult has a pronounced dark line along mid-flank indicating the separation of the upper and lower muscle masses. Above this line the upper flank is yellowish with irregular dark grey pigment or brown speckles near the back. The lower flank has irregular grey pigment or brown speckles. The mid-line of the back has a more or less pronounced dark line. The belly and lower head surface are pale yellowish without grey pigment. The dorsal and caudal fins have yellow-orange rays bearing 3-4 series of dark grey spots. The base of the caudal fin has 2 dark dots although these are not as marked as in some Cobitis keyvani. The pectoral, pelvic and anal fins are transparent though larger fish may have elongate grey spots along the rays. The iris is golden.
Note that Vasil'yeva (1995b), based on original data and that of Derzhavin (1934), found that the dark longitudinal band and the dark caudal fin base may not be pronounced.
Size
Reaches 9.2 cm standard length.
Distribution
Principally found in the southern Caspian Sea basin. Records from the northern Caspian Sea are apparently in error and the range is from the Kura to the Babol rivers including the Anzali Mordab at "Khalkai" for example, the Safid, Chowbar, Tonekabon, Chalus, Shahzadeh, "Laidschana", Meshedessera (= ? Babol Sar), Haraz, (Holčik and Oláh, 1992; Vasil'yeva, 1995b; Abbasi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009).
Zoogeography
This species is endemic to the Caspian Sea basin (Nalbant and Bianco, 1998).
Habitat
This species is found in both fresh and brackish waters, in slow or still water with aquatic vegetation, in the lower reaches of rivers and near river mouths, and in brackish bays.
Age and growth
Unnown.
Food
Unknown.
Reproduction
Spawning takes place in April in the brackish lagoons of the Lenkoran, Azerbaijan. Up to 955 eggs of up to 0.85 mm diameter are carried by each female.
Parasites and predators
None reported for Iran.
Economic importance
None.
Conservation
Holčík and Oláh (1992) report the loss of this species from the Anzali Mordab where it was once found. Reasons for this loss are unknown. Kiabi et al. (1999) consider this species to be conservation dependent in the south Caspian Sea basin according to IUCN criteria. Criteria include possibly few in numbers, habitat destruction, limited range (less than 25% of water bodies), absent in other water bodies in Iran, and absent outside the Caspian Sea basin. Vulnerable in Turkey (Fricke et al., 2007).
Further work
The biology of this species in Iran requires study.
Sources
Iranian material: CMNFI 1970-0517, 2, 24.4-31.7 mm standard length, Caspian Sea basin (no other locality data); CMNFI 1970-0537, 6, 25.4-53.6 mm standard length, Markazi, Shah River near Manjil (36°44'N, 49°24'E); CMNFI 1970-0542, 16, 25.0-59.1 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0550, 2, 18.7-27.2 mm standard length, Caspian Sea basin (no other locality data); CMNFI 1970-0555, 4, 20.9-38.0 mm standard length, Caspian Sea basin (no other locality data); CMNFI 1970-0566, 40, 26.6-58.7 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0567, 27, 26.3-62.0 mm standard length, Gilan, Pir Bazar Roga (37°21'N, 49°33'E); CMNFI 1970-0570, 2, 19.2-27.1 mm standard length, Gilan, Hasan Kiadeh (37°24'N, 49°58'E); CMNFI 1970-0579, 57, 24.7-60.4 mm standard length, Gilan, Old Safid River estuary (37°23'N, 50°11'E); CMNFI 1970-0580, 1, 63.4 mm standard length, Mazandaran, Iz Deh (36°36'N, 52°07'E); CMNFI 2008-0122, 49.7 mm standard length, Gilan, Anzali Mordab (no other locality data)..
Sabanejewia caucasica
(Berg, 1906)
CMNFI
Reported from the Anzali Mordab and lower reaches of the Safid, Toenkabon,
Chalus, Heraz and Babol rivers in Iran (Abdoli, 2000) and mapped from the
Caspian coast of Iran at Babol by Kottelat and Freyhof (2007). Berg (1948-1949) notes that this species
is found in the northern Transcaucasus and is not reported from the
Kura River in the southwestern corner of the Caspian Sea. Its presence
in Iran needs confirmation by specimens. Formerly in the genus Cobitis.
Nemacheilidae CMNFI 1970-0558, 4, ?mm standard length, Ghasemlou Chay ()?brandtii; CMNFI 1970-0560, 3, ?, mm standard length, Azarbaijan-e Bakhtari, Mamiyand Chay near Mamiyand
(ca. 36º59'N, ca. 45º39'E)?brandtii CMNFI 1979-0026, 8, 22.8-46.6 mm standard length, Fars, Shapur River at Shapur (29º47'N, 51º35'E); CMNFI 1979-0155, 2, 30.7-30.9 mm standard length, Fars, spring at Gavanoo (28º47'N, 54º22'E);
CMNFI 1979-0167, 41, 20.6-48.3 mm standard length, Kerman, qanat at Bam (29º06'N, 58º20'E);
CMNFI 1979-0168, 1, 47.2 mm standard length, Kerman, qanat at Shahabad (29º07'N, 58º16'E);
CMNFI 1979-0169, 11, ? mm standard length, Kerman, qanat 10 km from Mahan (30º08'30"N, 57º17'E);
CMNFI 1979-0170, 1, 45.0 mm standard length, Kerman, qanat at Baghin (30º12'N, 56º48'E);
CMNFI 1979-0172, 18, 33.8-45.8 mm standard length, Kerman, qanat on Kerman to Bandar Abbas road (29º51'N, 56º14'E);
CMNFI 1979-0184, 2, 27.7-28.4 mm satndard length, ?;
CMNFI 1979-0186, 2, ? mm standard length, Hormozgan, stream at Sar Khun (ca. 27º24'30"N, ca. 56º25'E);
CMNFI 1979-0192, 7, ? mm standard length, Fars, qanat 2 km east of Rostaq (28º26'30"N, 55º04'E);
CMNFI 1979-0193, 1, ? mm standard length, Fars, river 8 km from Darab (28º45'N, 54º27'30"E);
CMNFI 1979-0194, 2, 37.6-45.6 mm standard length, Fars, jube 15 km from Darab (28º45'30"N, 54º24'E);
; CMNFI 1979-0208, 15, 31.1-58.8 mm standard length, Fars, qanat on road to Qatru (ca. 29º11'N, ca. 54º40'E);
CMNFI 1979-0213, 7, ? mm standard length, Kerman, stream in Kharan River drainage (29º15'N, 56º25'E);
CMNFI 1979-0219, 13, ? mm standard length, Kerman, jube 14 km west of Jiroft (28º37'N, 57º41'E);
CMNFI 1979-0276, 14, 36.3-45.8 mm standard length, Lorestan, Chamesk River (ca. 33º19'N, ca. 47º53'30"E);
CMNFI 1979-0284, 3, 38.7-41.9 mm standard length, Kermanshahan, Marek River at Mahidasht (34º16'N, 46º48'30"E);
CMNFI 1979-0288, 1, 32.4 mm standard length, Ilam and Poshtkuh, Gangir River at Juy-e Zar Eivan (33º50'N, 46º18'E);
CMNFI 1979-0306, 11, 20.0-49.3 mm standard length, Kerman, qanat 33 km from Sirjan (29º13'N, 54º33'E);
CMNFI 1979-0307, 4, 31.0-42.3 mm standard length, Kerman, river at Sartal (ca. 29º17'N, ca. 56º38'E)? distinct species;
CMNFI 1979-0316, 1, ? mm standard length, Baluchestan, stream on road to Chah Bahar (26º48'N, 61º02'E);
CMNFI 1979-0341, 10, 25.5-45.6 mm standard length, Kerman, Tahrud west of Bam (29º23'N, 57º52'E);
CMNFI 1979-0365, 7, 32.0-40.5 mm standard length, Khuzestan, stream in Doveyrich drainage (32º25"N, 47º36'30"E);
CMNFI 1979-0366, 1, 36.0 mm standard length, Khuzestan, stream 17 km west of Dehloran (32º45'30"N, 47º05'30"E);
CMNFI 1979-0367, 1, 41.3 mm standard length, Khuzestan, Meymeh River 11 km north of Dehloran (32º44'30"N, 47º09'30"E);
CMNFI 1979-0371, 2, 44.5-55.6 mm standard length, Khuzestan, stream in Karkheh River drainage (32º05'N, 48º19'E);
CMNFI 1979-0374, 2, 41.3-41.7 mm standard length, Khuzestan, stream tributary to Bala River (32º40'N, 48º15'E);
CMNFI 1979-0389, 1, 32.4 mm standard length, Khuzestan, Zard River 1 km south of Bagh-e Malek (31º31'N, 49º53'30"E);
CMNFI 1979-0390B, 3, 29.1-39.4 mm standard length, Khuzestan, stream 3km south of Bagh-e Malek (31º29'N, 49º54'30"E);
CMNFI 1979-0395, 1, 30.7 mm standard length, Khuzestan, stream in Marun River drainage (ca. 30º57'N, ca. 49º51'E);
CMNFI 1979-0411, 3, 21.9-25.9 mm standard length, Hormozgan, Minab River near Rudan (27º24'N, 57º12'E)?cf bampurensis;
CMNFI 1979-0419, 19, 32.4-58.7 mm standard length, Fars, stream 7 km from Rostaq (28º29'N, 55º01'E);
CMNFI 1979-0423, 4, 43.2-45.7 mm standard length, Boyer Ahmadi-ye Sardsir va Kohkiluyeh-Fars border, river in Beshar River drainage (30º31'N, 51º31'E);
CMNFI 1979-0459, 4, ? mm standard length, Hamadan, stream 2 km south of Kazan (35º22'N, 49º02'E);
CMNFI 1991-0156, 1, ? mm standard length, Hamadan, Gav Masiab River (34º16'N, 48º10'E);
CMNFI 2007-0037, 5, 45.2-54.9 mm standard length, Kerman, Hosseinabad and Gamatabad qanats at Bam (29º06'N, 58º21'E);
CMNFI 2007-0038, 1, 39.0 mm standard length, Kerman, Mehtiabad qanat at Bam (29º06'N, 58º21'E);
CMNFI 2007-0039, 6, 32.4-41.7 mm standard length, Kerman, Tahrud River (ca. 29º23'N, ca. 57º63'E);
CMNFI 2007-0043, 9, 33.0-56.7 mm standard length, Kerman, qanat at Emamzadeh Sultan (ca. 29º40'N, ca. 56º45'E);
CMNFI 2007-0047, 2, 36.8-58.2 mm standard length, Kerman, qanat at Hoshum (29º14'N, 56º19'E);
CMNFI 2007-0051, 5, 28.6-36.3 mm standard length, Hormozgan, upper Kol River basin (28º19'N, 55º55'E);
CMNFI 2007-0055, 3, 36.1-40.3 mm standard length,-0103 Hormozgan, stream in Minab River basin (27º47'N, 57º12'E);
CMNFI 2007-0075, 6, 41.3-47.9 mm standard length, Hamadan, Malayer River 5 km from Malayer (ca. 34º17'N, ca. 48º47'E); CMNFI 2007-0089, 7, ? mm standard length, Azarbayjan-e Khavari, Ahar Chay at Ahar (38º28'N, 47º03'E);
CMNFI 2007-0093, 8, ? mm standard length, Azarbayjan-e Bakhtari, Qotur River south of Khvoy (38º30'N, 44º58'E);
CMNFI 2007-0100, 4, 44.2-58.4 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay near Piranshahr (ca. 36º44'N, ca. 45º10'E);
CMNFI 2007-0105, 13, ? mm standard length, Kordestan, Zarineh River basin south of Saqqez (ca. 36º06'N, ca. 46º20'E);
CMNFI 2007-0116, 2, 40.6-44.8 mm standard length, Kermanshahan, Gav Masiab River basin west of Sahneh (ca. 34º28;'N, ca. 47º36'E);
CMNFI 2007-0118, 15, 24.7-58.8 mm standard length, Kermanshahan, Bid Sorkh River between Sahneh and Kangavar (ca. 34º23'N, ca. 47 52'E); CMNFI 2007-0119, 8, 26.9-37.9 mm standard length, Kermanshahan, Gav Masiab River basin near Kangavar (ca. 34º31'N, ca. 48º03'E);
CMNFI 2008-0102, 1, 41.6 mm standard length, Kermanshahan, sarabs near
Kermanshah (34º19'N, 47º04'E); CMNFI 2008-0117, 3, 48.4-51.2 mm standard length,
Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E); CMNFI 2008-0120, 1, ? mm
standard length, Khuzestan, Rud Zard at Rud Zard (31º22'N, 49º43'E); CMNFI
2008-0130,1, 22.8 mm standard length, Khuzestan, Kupal (31º15'N, 49º10'E); BWC 1997-0005,
BWC 2000-0001,
CMNFI 2008-0161, 10, ? mm standard length, Khuzestan, A'la River at Pol-e Tighen
(31º23'20"N, 49º52'44"E), CMNFI 2008-0163, 1, ? mm standard length, Khuzestan,
Marun River at Chahar Asiab (30º40'28"N, 50º09'34"E); ?nielseni CMNFI 2008-0167,
54, ? mm standard length, Khuzestan, stream above Diuni Darreh (32º37'42"N,
48º41'40"E); CMNFI 2008-0170, 7, ? mm standard length, Khuzestan, Zard Rud
(31º22'28"N, 49º43'15"E); CMNFI 2008-0175, 8, ? mm standard length, Lorestan,
Kahman River at Dow Ab-e Aleshtar (33º47'N, 48º12'E);
New species: CMNFI 1979-0217, 6, 30.1-41.8 mm standard length, Kerman, Kharan River drainage (ca. 28º59'30"N, ca. 56º51'30"E);
CMNFI 2007-0045, 6, 28.8-39.2 mm standard length, Kerman, Kharan River drainage at Baft (29º14'N, 56º38'E)checkID
ID?persaCMNFI 1970-0540, 1, 49.5 mm standard length, ?;
CMNFI 1979-0019, 2, 28.9-33.7 mm standard length, Fars, Barm-e Baba Hajji (29º23'N, 52º40'E);
CMNFI 1979-0027, 9, 31.0-41.3 mm standard length, Fars, Chehel Chashmeh (ca.
29º43'N, ca. 52º04'E); CMNFI 1979-0073, 10, 31.1-56.1 mm standard length, Fars, Mand River (ca. 29º42'30"N, ca. 52º01'30"E);
CMNFI 1979-0086, ?; CMNFI 1979-0111, 5, 43.2-52.7 mm standard length, Fars, stream on Shiraz to Bushehr road (29º37'30"N, 52º21'E); CMNFI 1979-0157, 3, 34.8-37.7 mm standard length, Fars, qanat at Hadiabad (28º52'N, 54º13'E); CMNFI 1979-0206, 3, 24.9-36.1 mm standard length, Fars, qanat 1 km from Runiz-e Pa'in
(29º12'N, 53º40'E); CMNFI 1979-0399, 1, 26.2 mm standard length, Fars, stream near Basht (30º19'30"N, 51º15'E); The hillstream, mountain or river loach family has had various family names - see Menon (1987), Mirza (1989b), Hieronimus (1990; 1991), Ng and Lim (1991), Kottelat (1988, 1991), Nelson (1991),
Thorne (1992), Anonymous (1993a) and Bănărescu and Nalbant (1995) for discussions. The Iranian species were classified in Cobitidae in older works and more recently in Homalopteridae
and Balitoridae. Mirza (1989b),
Nalbant (1998), Tang et al. (2006) and Šlechtová et al. (2007) consider that the correct name for this family is Noemacheilidae (in Mirza) or Nemacheilidae. Nemacheilids are aligned with the main stem of cobitoid fishes rather than homalopterids as proposed by Sawada (1982) on osteological grounds.
Nalbant (1998) considers the similarities observed by Sawada between nemacheilids and homalopterids to be homoplasies. Homalopterids are more closely related to cyprinids and psilorhynchids.
The family is found throughout Eurasia with a single species in northeast Africa. There are about
72 genera and about 793 species (Berra, 2001; Nelson, 2006; Eschmeyer and Fong,
2011), with more being described regularly.
Iranian species belong to the subfamily Nemacheilinae.
Conway et al. (2010) point out that although there are no morphological
synapomorphies for the family, molecular data does support monophyly. Prokofiev
(2010) gives osteological characters for the subfamily Nemacheilinae. Most species in Iran were placed in the genus Nemacheilus Bleeker, 1863 but this name contains only species from Southeast Asia (Kottelat, 1997). Noemacheilus Kuhl and van Hasselt in
van Hasselt, 1823 is a nomen nudum since there are no taxonomic characters accompanying the original description. The next available name is Nemacheilus Bleeker, 1863 (see Kottelat, 1987).
Nemachilus Günther, 1868 is an incorrect spelling. Much of the earlier literature on Iranian members can be found under the name Nemacheilus or its variant spellings.
These loaches have been placed in several genera or subgenera including the following relevant to Iran: Orthrias Jordan and Fowler, 1903 (see Banarescu, Nalbant and Balik (1978); Orthrias
= Barbatula Linck, 1790 of authors, see Bănărescu and Nalbant (1995) for reasons advocating the later name over the earlier one; Kottelat (1997) gives Linck as 1789), Adiposia
Annandale and Hora, 1920 (see Annandale and Hora (1920); see also Bănărescu and Nalbant (1995) where it is synonymised with Paracobitis), Triplophysa Rendahl, 1933 and
Hedinichthys Rendahl, 1933 (see Bănărescu and Nalbant (1966); see also Bănărescu and Nalbant (1995) where the latter is synonymised with the former), Oxynoemacheilus
Bănărescu and Nalbant, 1966 (see Bănărescu and Nalbant, 1966; synonymised with Orthrias in Bănărescu and Nalbant (1995) and with Barbatula in Bogutskaya and
Naseka (2004)), Paracobitis Bleeker, 1863 (see Bănărescu and Nalbant, 1966), Schistura McClelland, 1839 (see Mirza, Nalbant and Banarescu, 1981; regarded as polyphyletic by
Bănărescu and Nalbant (1995)), and Seminemacheilus Bănărescu and Nalbant, 1995 (q.v.). Views on the generic validity of these names conflict between authors and
between the same author at different dates (see Krupp, 1985c; Eschmeyer, 1990; Eschmeyer et al., 1996; Eschmeyer's "Catalog of Fishes"). Kottelat (1984) retained Nemacheilus until
a revision of all species was complete but there has been a tendency to use the above genera and this is followed here.
Prokofiev (2004, 2007, 2009, 2010) has revised some of the Nemacheilidae, in
particular the group of nemacheiline loaches that lack the preethmoid I in the
skull, using osteology and morphology. This includes most of the species found
in Iran and is now being followed by such authors as Esmaeili et al.
(2010). The table below summarises some of the various allocations and
includes names used in Iran but not necessarily occurring there (see text below
for further details:-
* original trivial name, the suffix of which may change if genus changes to masculine or to feminine; ^ Nemacheilus sometimes spelled Noemacheilus or Nemachilus by various authors
A number of nemacheilid species have been described from waters confluent with Iran, particularly from the Helmand River basin in Afghanistan. They have no Iranian records but are listed here as
they may be relevant to revisionary studies.
The dating of the paper by Bănărescu and Nalbant as 1967 in various
works may follow Nalbant and Bianco (1998). The Bănărescu and Nalbant
paper states in Danish on page 186 "Reprints released the 31 December 1966" and
this is presumably the correct date, as Art. 21.2 of the Code of Zoological
Nomenclature states "Date specified. The date of publication specified in
a work is to be adopted as correct in the absence of evidence to the contrary",
and no evidence to the contrary has been presented (N. G. Bogutskaya, pers
comm., 28 April 2011). This changing of dates here has no nomenclatural
significance. Members of this family in Iran are characterised by an elongate and weakly compressed and almost cylindrical body, head not compressed but rounded, scaleless or body covered in minute scales (too
small for scale counts to be commonly or easily made), lateral line complete or incomplete, a small and inferior mouth, lips thick, fleshy and papillose, lower lip interrupted in the middle, 2 pairs of
barbels on the snout and 1 pair at the mouth corners (8 pairs in some non-Iranian species), no collapsible spine under the eye (sometimes present in non-Iranian species but distinguishes
members of the related Cobitidae in Iran), eyes small to minute, usually not visible from the underside of the head, reduced gill opening, short to moderate dorsal fin without spines, short anal fins,
vent a short distance in front of anal fin origin, swimbladder enclosed partially or entirely in a bony capsule, certain osteological characters such as the shape of bones in the
Weberian apparatus used in sound transmission from the swimbladder to the ear, gut short or long, a dermal crest or adipose fin may be present, caudal fin truncate, rounded or slightly forked, and
often distinctive colour patterns of bars, stripes and blotches. Iranian species may lack scales, may have an adipose fin, and have a single unbranched ray leading the pectoral and pelvic fins.
Krupp (1985c) reviews prior works by P. M. Bănărescu and co-authors on Levantine
nemacheilids and regards them as unsatisfactory. This calls into question works on Iranian species by this
author. Krupp (1985c) lists characters important in studying Nemacheilus sensu lato and those which are individually variable or develop independently, much in contrast to characters
favoured by Bănărescu. Morphometric characters can vary with nutritional status and ecological factors. Stable characters were head length, interorbital width, caudal peduncle length and depth,
and predorsal length. Fin lengths are dependent on sex in some species, less so in others. Allometry is a problem in fin positions and measurements involving such characters can only be used when
comparing fish of equal size. Mouth width and digestive tract shape are good characters but lip shape and development of the processus dentiformis are not. The swimbladder capsule form, including the
presence or absence of a continuous collar between the two hemispheres and the shape of laminae, is an important character. Reduced laminae and wide recesses on the hemispheres are
derived characters. The dorsal adipose fin development is stable in some species, variable in others, and is independently derived in different phyletic lines, thus being of limited value. The shortening
and deepening of the caudal peduncle is derived in one Levantine species. Scale characters such as size and position of the focus and general scale structure are very variable and not characters easily
quantified. Only specimens of the same size are comparable and numerous scales must be examined because aberrant ones are common. The lateral line length is a good character, although juvenile fish may
have a shortened one. A reduced lateral line is a derived character. Colour patterns are subject to variation and both spotted and striped forms can be found within one species. Nevertheless, patterns can
be important in distinguishing species. Thickening of pectoral fin rays is a derived feature but absence of this character is a symplesiomorph condition and cannot be used to relate species.
The species in this family are often difficult to identify and many literature reports are undoubtedly mis-identifications. While some of these may be corrected based on material deposited in museums,
others have no voucher material and cannot be re-identified. Identification is problematical because scale counts are not available (too minute), fin ray counts are often very similar, and
unique structures uncommon. Colour patterns can be used but are notoriously variable and many types in museums are decoloured making comparisons difficult. Morphometric characters require good series of
adult fish of both sexes, from various localities in the species range, preferably even from the same locality taken over several years to allow for local variations in habitat which may conceivably affect
shape. Menon (1987) considers that many species in this genus are from very similar habitats, the stressful one of running water, and have been constantly selected to fit this niche. Valid species
resemble one another closely. Such characters as position of the anal opening, the dorsal fin origin and barbel length have been used in species definitions but Menon (1987) found these to vary with
growth. Swimbladder structure depends on the habitat where the fish live and scale coverage on the physico-chemical nature of the water. Menon (1987) found lateral line character, number of branched
dorsal fin rays, caudal fin shape, secondary sexual characters in males and, despite the above, body colour and anal opening position to be useful.
Prokofiev (2010) reviewed the morphological classification of loaches and points
out that changes in morphology occur with growth and species with wide ranges
show large variations in morphology associated with the various biotopes. He
gives extensive reviews of characters and their importance in defining genera
and species, e.g. scale cover and scale characters are not considered important
in phylogeny and are of only accessory import for genus and species diagnosis;
colour, sexual dimorphism, adipose keel presence and extent, fin ray numbers,
head and body shapes, abdominal axillary lobe presence and size, variations in
the seismosensory system, nostril size and position, anal opening position,
intestine shape, swimbladder and its capsule structure, and general osteology
are all important characters. Examination of Iranian species, where good series of fresh material was available, tend to confirm the observations of Krupp and Menon on characters. Position of the dorsal fin origin is variable
within a species among morphometric characters used as distinctive, extent of the lateral line is also variable, there is marked sexual dimorphism, and colour patterns can be useful but also vary with
the individual, the habitat and the temperature (fish kept in ice water have strong colour patterns while those preserved immediately from murky waters have faint patterns).
Two main problems exist in identifying certain Iranian loaches. These are determining appropriate characters which are not individually variable and which are apomorphic, and applying existing names
to fresh material in comparison with poorly-preserved types.
A number of species remain to be described and are currently under study. Some named species are probably distinct taxa, e.g.
"Nemacheilus" tigris (Heckel,
1843) (sagmahi-ye Dajleh) is recorded from the Karun River basin in Khuzestan (ZISP 24098) by Berg (1949) but specimens from this part of Iran differ from Heckel's types. The colour pattern on the fish
figured by Berg (1949) from the Karun River in Iran is atypical according to Bănărescu and Nalbant (1966) - it has only 4-5 bars on the posterior part of the body. The type locality of Cobitis
Tigris is "Flüsschen Kueik bei Aleppo" (Haleb, Syria) according to Heckel (1843b).
Prokofiev 92009) places Cobitis tigris in the genus Paracobitis. Sawada (1982) thought that this family dispersed by two routes from Southeast Asia, one through Siberia and one through South Asia to reach what is now Iran. Menon (1987) considers that the land mass
between East Africa and the west coast of India has submerged only recently, probably simultaneous with the birth of the Ganges and Indus. Connections of the Pleistocene fore-deep of the Himalayas with
the Tigris-Euphrates basin in what is now the Persian Gulf could have existed, allowing movement of "Nemacheilus" species along a continuous route from Yunnan to Anatolia. Menon (1987)
further suggests a series of waves, spreading "Nemacheilus" westwards into Southwest Asia from a South China origin. The Triplophysa wave is the first wave of evolution, in which
the earliest stock from Yunnan spread through Tibet in the late Miocene and early Pliocene before the major rise of the Himalayas. By the end of the Tertiary, particularly in the Pleistocene, the Tibetan
Plateau had risen causing a dry and cold climate with increased solar radiation and torrential rivers. This change in the environment caused rapid evolution, leading to such taxa as Triplophysa
and Hedinichthys. The rupecola wave took place in the late Pliocene along the southern face of the Himalayas through Iran to Anatolia and even northeast Africa. Some of the criticisms listed
under the cyprinid genus Garra, whose distribution Menon (1964) also attributes to waves, may be apposite here too.
These are small fishes, up to about 200 mm in size although one species (not
in Iran) reaches 482 mm (Prokofiev, 2010). They are quite secretive, hiding under stones or in mud. This common and stressful habitat may have led to a general similarity in body form among the various species. Some are known only
from caves, including one Iranian species. Despite their small size, they are regarded as a delicacy in India (Hora, 1956). Barbatula angorae (and presumably other species) is a potential fishing
bait for predatory fishes such as Sander lucioperca and has been examined experimentally for this purpose in Turkey (Kuşat et al., 1995). They are generally known as سگ
ماهي (sag mahi meaning dog fish, but this is presumably the equivalent of loach in English), لوچ (= louch meaning loach) or mar mahi (= snake fish, presumably
in reference to the elongate shape) in Farsi. These general names are not repeated below.
Genus Ilamnemacheilus This genus is characterised by a high, laterally compressed body; large head with small eyes and mouth; anterior lip lacking an interruption in the middle; posterior lip with widened mental
lobes, small round papillae covering only the mental lobes, the rest of the lips being unfurrowed; the processus dentiformes absent; lateral line complete and terminating slightly before the posterior
margin of the caudal peduncle; scales small with a quite large and eccentric focus, sparsely present on the rear half of the body; stomach syphonal and intestine straight without loops; gas bladder
with two encapsulated chambers united by a short encapsulated duct; paired fins very long; dorsal fin long; and caudal fin well forked.
The type species is Ilamnemacheilus longipinnis by original designation and monotypy.
Ilamnemacheilus longipinnis
Common names
None.
Systematics
The holotype and only known specimen is CMNFI 1979-0366 (79-966 is a lapsus), 36.0 mm standard length, Iran, Meymeh River, formerly a tributary of the Tigris River, 17 km west of Dehloran and about
21 km east of the Iraqi border, 32º45'30"N, 47º05'30"E, 28 January 1978, B. W. Coad and S. Coad.
This species was described by the late T. T. Nalbant on material collected by
me. I first saw the species description when I received reprints and had no
input into this paper. The extensive vertebral fusions account in part for the
unusual body form. Key characters
Characters are those of the genus.
Morphology
Dorsal fin with 3 unbranched and 10 branched rays, anal fin with 2 unbranched and 5 branched rays, pectoral fin with 9 branched rays and pelvic fin with 5 branched rays. Total vertebrae 28 or 29
including the ural centrum (vertebral fusions present), and some centra have two neural and haemal arches. ?check vertebral counts against other loaches - very low ? fusions Other
characters are listed above under the genus and Coad and Nalbant (2005) give some measurements.
Sexual dimorphism
Unknown.
Colour
The sole preserved specimen is a overall a pale brown with 3-4 indistinct greyish blotches in the middle of the second half of the body. All fins are pale but the caudal fin has faded greyish lines
along the marginal rays. In life it was an olive-green overall with orange fins.
Size
Reaches 36.0 mm standard length.
Distribution
Endemic to Iran and found in Tigris River basin at a single locality (see above).
Zoogeography
An endemic genus in the Tigris-Euphrates basin (along with Turcinoemacheilus, not in Iran). This species may be related to an undescribed species from the Orontes River basin in Syria.
Habitat
The sample site was a small stream, 20 m wide with a maximum depth of 1 m. Altitude was 210 m. Capture depth was 30 cm in a medium current. The bottom was a mix of pebbles and mud with some encrusting
algae. Water temperature was 14ºC, pH was 6.0 and conductivity was 1.65 mS. The cyprinids Cyprinion macrostomum and Garra rufa were caught with the loach.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Known only from a single specimen, abundance and wider distribution unknown.
Further work
More collections are needed to record information on biology and distribution and provide a more detailed description.
Sources
The holotype and sole known specimen (see above).
Genus Metaschistura
This genus has a single, small species and the characters of the species are
those of the genus. Prokofiev (2009) gives further osteological details. Metaschistura cristata
Common names
sagmahi-ye Torkomani
or Turkmeny (= Turkmenian crested loach), sagmahi-ye kakoldar-e Torkomani.
[Turkmenskii grebenchatyi golets or Turkmenian crested loach in Russian].
Systematics
This species was described under Nemacheilus in Latin from "Habitat in flum. Tedschent, prope Aschabad, in provincia Transcaspica". The type locality is presumably the Tedzhen River
in Turkmenistan although Ashkhabad is not on the Tedzhen River. Berg (1948-1949) notes "not in the Tedzhen!". Syntypes are reported to be in the Zoological Museum of Moscow State University
(MMSU) by Eschmeyer et al. (1996).
Placed in the taxon Paracobitis by Bănărescu and Nalbant (1966) and later in Schistura by Bănărescu and Nalbant (1995) and by
Nalbant and Bianco (1998).
Key characters
The dorsal fin branched ray count of 8 and unique colour pattern distinguishes this crested loach from other crested loaches in northeast Iran and adjacent regions. In addition the crest, or adipose fin, is shorter and thicker
than in P. malapterura.
Morphology
Dorsal fin unbranched rays 2-6 (several minute, embedded in flesh and only
visible on x-rays) and branched rays 7-9, modally 8, anal fin unbranched rays 2-4
(most anterior minute and embedded in flesh), and branched rays 5, pectoral fin branched rays 8-11,
usually 9-10, and
pelvic rays 6-8, usually 7. The dorsal fin origin lies midway between the snout tip and caudal base or nearer the caudal base. There is a fleshy pelvic axillary process
but it does not extend beyond the end of the pelvic fin base. Caudal fin slightly emarginate in
Turkmenistan but Hari Rud fish have a conspicuous fork. The two lobes of the caudal fin may be equal or either may be longer. The lobes are pointed or rounded. The dorsal adipose fin or crest begins
shortly in front of the anal fin origin level and reaches the root of the caudal fin. There is a small gap between the dorsal fin insertion and the origin of the crest, and the depressed dorsal fin
partially occupies this gap and the slight rise of the crest origin. It is strongly developed, high and thick.
The crest is supported by 22-25 procurrent rays of the caudal fin (only visible
on x-rays). Large fish have scales posteriorly on the body. Scales are small, oblong and widely separated.
Bănărescu and Nalbant (1966) did not find scales on fish from the Harirud (confirmed for these fish (ZMUC P 2798, 2799, 27100, a fourth specimen P 2797 is lost; these catalogue numbers corrected
from Bănărescu and Nalbant (1966) where incorrect; 63.1-68.3 mm standard length) from Obeh, Afghanistan; and not found on 3 fish, 38.1-60.9 mm standard length from Sarakhs on the Hari Rud) and
this population may be distinct. Scales may be completely absent or only on the
crest (Prokofiev, 2009). The lateral line extends nearly to the caudal base. Caudal peduncle short, 5-6 times in standard length (6.1-6.9 for 3 Iranian fish, 38.1-60.9 mm standard length). Lips
are thick and furrowed, the upper lip with a slight median gap and the lower lip widely interrupted. The dentiform process on the upper jaw is weakly developed. The anterior nostril has a large flap
developed posteriorly and postero-dorsally. Large fish have bulging cheeks. The posterior part of the intestine is straight (Bănărescu and Nalbant, 1966) or with a single loop (Bănărescu
and Nalbant, 1995).
Meristics for Iranian specimens:- dorsal fin branched rays 8(4), anal fin branched rays 5(4), pectoral fin branched rays 9(2), 10(1) or 11(1); pelvic fin branched rays 7(4);
and total vertebrae 36(1), 37(1) or 38(1).
Sexual dimorphism
There is no sexual dimnorphism.
Colour
Somewhat similar to P. malapterura but with a dark spot at the anterior base of the dorsal fin, on the unbranched and first branched rays. A similar spot may be present at the anterior base of
the anal fin on the first two rays. The anterior part of the body is dark and the posterior half
has 4-8 broad, brownish bands, separated by narrower light bands and reaching close to or to the ventral
part of the body. Some smaller fish have anterior bands on the flank, the total number of bands reaching
as high as 17. Bands vary in size from equal to twice the width of the pale interspaces. The last 3-4 bands
extend onto the adipose fin. There is also a much darker and narrower band at the caudal base. Bănărescu and Nalbant (1995: Fig. 11A) illustrate a fish where this last band is only developed on
the lower half of the caudal peduncle. The bar may also be composed of two blotches above and below the flank midline plus blotches on the bases of the outermost caudal rays both dorsally and ventrally.
There 3-4 saddles anterior to the dorsal fin. All fins have thin bands comprised of spots irregularly lined up on the fin rays. The caudal fin may have three such bands while this fin and others may have no clear bands. Some fish have pigment on rays
of the dorsal fin almost forming a single broad band extending from mid-fin half way towards the margin. There may be a second smaller spot on the last unbranched dorsal fin ray above the
one on the anterior base.
Size
Attains 8 cm.
Distribution
Found in streams on the northern slope of the Kopetdag, Turkmenistan. Some of these streams presumably have headwaters in Iran and the species may be found there although this has yet to be
confirmed by specimens. Also reported from the drainage of the Tedzhen River in Afghanistan which forms part of the Iran-Afghanistan border as the Hari Rud or Hari River (Bănărescu
and Nalbant, 1966). This species is confirmed for Iran from Sarakhs on the Hari Rud, and probably the Kashaf River, a Hari River tributary (Abdoli, 2000).
A record from Lake Topiatan in the Uzboi River drainage in Turkmenistan north of the Iranian border mentioned in Nikol'skii (1947) is housed at the Zoological Institute, St. Petersburg (ZISP
25788). Berg (1948-1949) corrects the locality as the Sumbar River, an Atrak River tributary in the Caspian Sea basin. This specimen was examined by me and it is not this species but P. malapterura.
Zoogeography
Bănărescu and Nalbant (1966) consider that the closest relative of this species is Nemacheilus tigris (sic) from the Tigris-Euphrates basin rather than other
crested loaches such as P. malapterura and P. rhadinaea found in neighbouring river systems. Note that
Paracobitis tigris was described from Aleppo (= Haleb, Syria) and
its occurrence in the Tigris-Euphrates basin is in question (see family account).
Habitat
A rheophilic species but further details of habitat preferences are unknown.
Age and growth
Patimar et al. (2011) studied fish
from the Zanglanlou River in Khorasan Razavi Province. They found a maximum age
of 4+ years, most fish (44%) were 1+, fish showed positive allometric growth,
and male:female sex ratio was significantly different at 1:1.2, thought to be
due to higher survival rate of adult females. Safai and Naserizadeh
(2013) examined fish in the Radkan River of northeastern Iran. The sex ratio was
1:1, maximum age for both sexes was 6+ years, length-weight relationships were
W=0.005166TL3.225 for males and W=0.006192TL3.125 for
females, von Bertalanffy growth parameters were L∞=354.9 mm,
k=0.0038, t0=-26.82 for males and L∞=339.0
mm, k=0.0043, t0=-24.88 for females, and growth
performance indices were Φ’=6.17 for males and 6.20 for females. Food
Gut contents of one fish, 60.9 mm standard length, from the Hari Rud were chironomid larvae.
Reproduction
Patimar et al. (2011) found the
highest reproductive activity was in March to May, maximum number of eggs was
1246, mean absolute fecundity was 350.8 eggs, relative fecundity reached 1285.71
eggs and the mean was 287.78 eggs, and egg diameter reached 1.46 mm.
Safai and Naserizadeh (2013) found the gonadosomatic index indicated peak
reproduction in April-June with highest average values in May. Oocyte diameter
was 0.09-1.58 mm, mean 0.54 mm. Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population numbers or trends are unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Iranian material: CMNFI 2008-0138, 3, 38.1-60.9 mm standard length, Khorasan, Hari River at Sarakhs (36°32'N,
61°11'E);
BM(NH) 1914.1.1:32, 1, 54.5 mm standard length, Khorasan, Kashaf River (no other locality data).
Comparative material: SMF 17133, 2, 34.4-98.3 mm standard length, Afghanistan, Hari River near Herat (34º21'N, 62º14'E); BM(NH) 1897.7.5:40, 51.4 mm standard length, Turkmenistan, Ashkhabad (no other
locality data).
Genus Oxynoemacheilus Members of this genus are found from Bulgaria east to Iran.
There are 41 or more species with ?8 recorded from Iran. Characters below
are based on Freyhof (2011).
These small loaches are scaled or scaleless, have a body with spots which
tend to form bands or a stripe along the lateral line and there is no dark black
spot on the base of the anterior dorsal fin rays. The dorsal fin origin is in
front of or above the vertical from the pelvic fin origin and the anus is an eye
diameter or less in front of the anal fin. Prominent black spot absent from the
base of the unbranched, and sometimes first and second branched, dorsal fin rays
(a saddle may extend onto the anterior base of the dorsal fin but there is no
distinctive, very dark spot). There is usually no strong caudal peduncle
crest.
A crest,
when present, is best developed at the caudal fin base and supported by
procurrent rays. A small pelvic fin axillary lobe,
variably developed, is present (but see Prokofiev (2009) where it is stated,
erroneously, to be absent - Freyhof et al. (2011)). When a well-developed
crest is present, it is usually highest at the caudal fin base and is supported
by procurrent caudal fin rays. The caudal fin is very slightly emarginate to
deeply emarginate or truncate. A forked caudal fin is found in a species with a
cylindrical body and caudal peduncle. Males do not develop inflated or muscular
cheeks in the spawning season but tubercles are found on the sides of the head
and on the pectoral fins but not as fine brush-like aggregations. A suborbital
flap or groove is present or absent in males, and may vary from present to
absent within species. Nostrils are
closely spaced or slightly separated, the mouth is semi-lunar with simple lips
which are almost smooth to plicate and the dentiform process is weak to moderate
but sometimes absent (O. angorae). The lateral line is usually complete
but if incomplete as in O. kermanshahensis it reaches beyond the level of
the dorsal fin origin or to the dorsal fin level (O. kiabii). The dorsal fin
has 7-9 (11 in one species) branched rays and the anal fin 5
branched rays. Prokofiev (2009) lists various osteological characters such as 4
radial bones in the pectoral fin, usually 6 hypurals in the caudal fin support (O.
brandtii has 5), the epural bone is present in the caudal fin support, the
manubrium in the swimbladder is well-developed and the bony capsule is full
divided by it, no preethmoid I, the supratemporal commissure is open from above,
the fork of cst and cio is not fused to the skull, and others.
Oxynoemacheilus angorae
This taxon has been widely reported from northern Iran and several subspecies
have been described under it. These taxa are now recognised as distinct species
below.
Freyhof et al. (2011) doubt that O. angorae s.s.
occurs in the Caspian Sea basin and it may not be present in Iran at all. If
O. angorae is restricted to Turkey, then the species for the western Caspian is lenkoranensis. Kura-Aras basin
fish would then be araxensis
but see below where this taxon could be in the Tigris-Euphrates basin
(although adjacent headwaters could harbour the same taxon.
Nemacheilus angorae was originally described from Angora (= Ankara, Turkey). Sixteen syntypes of Nemacheilus angorae from Tabakane-Sir and
Tschibuk-Tschai, Turkey are in the Naturhistorisches Museum Wieen according to Eschmeyer et al. (1996).
Nemacheilus bergi Gratsianov, 1907 from the Akstapha (= Akstafa)
River, a right tributary of the Kura River, Azerbaijan is regarded as a synonym (types are
lost) (Kottelat, 2012).
Some taxa or potential taxa peripheral to
Iranian waters have been described namely:- Nemachilus angorae alasanicus Elanidze, 1983
was described from the upper reaches of the Alazani River at Alvani village, Georgia, a left bank Kura River tributary. It is here attributed to
Elanidze (1983) since it is the only taxon listed in that book without an
author; and the lower Kura River basin in Azerbaijan (of which the Aras is part) may have another subspecies in which about 40% of the fish have 7 branched dorsal fin rays, a shallower caudal peduncle than
O. a. araxensis, and a colour pattern similar to O. angorae angorae (Banarescu, Nalbant and Balik (1978); see also description in Abdurakhmanov (1962) for measurements).
N. bergi and N. alasanicus may be synonyms of O. araxensis
or O. lenkoranensis according to Freyhof et al. (2011) although
N. bergi is an earlier name.
Oxynoemacheilus araxensis
Species
Original genus^
Other generic allocations^
Current genus
Current species
angorae
Nemacheilus
Barbatula, Orthrias
Oxynoemacheilus
angorae
araxensis
Orthrias
Barbatula
Oxynoemacheilus
araxensis
bampurensis
Nemacheilus
Schistura
Paraschistura
bampurensis
bergianus
Nemachilus
Barbatula, Orthrias
Oxynoemacheilus
bergianus
brandtii
Nemachilus
Barbatula, Orthrias
Oxynoemacheilus
brandtii
cristatus*
Nemacheilus
Paracobitis,
Schistura
Metaschistura
cristata
frenata*
Cobitis
Barbatula, Nemacheilus, Orthrias
Oxynoemacheilus
frenatus
iranica
Paracobitis
-
Paracobitis
iranica
kermanshahensis
Noemacheilus
Orthrias,
Barbatula
Oxynoemacheilus
kermanshahensis
kessleri
Nemachilus
Schistura
Paraschistura
kessleri
kiabii
Oxynoemacheilus
-
Oxynoemacheilus
kiabii
kosswigi
Turcinoemacheilus
-
Turcinoemacheilus
kosswigi
longicauda
Cobitis
Adiposia, Nemacheilus
Paracobitis
longicauda
longipinnis
Ilamnemacheilus
-
Ilamnemacheilus
longipinnis
malapterura
Cobitis
Nemacheilus
Paracobitis
malapterura
nielseni
Schistura
-
Paraschistura
nielseni
persa
Cobitis
Barbatula, Nemacheilus,
Orthrias
Oxynoemacheilus
persa
rhadinaeus*
Nemachilus
Adiposia
Paracobitis
rhadinaea
sargadensis
Nemacheilus
Schistura
Paraschistura
sargadensis
smithi
Noemacheilus
-
Paracobitis
smithi
stolickai
Cobitis
Nemacheilus
Triplophysa
stolickai
tongiorgii
Seminemacheilus
-
Oxynoemacheilus
tongiorgii
vignai
Paracobitis
-
Paracobitis
vignai
Coad and Nalbant, 2005
Coad and Nalbant, 2005
Prokofiev 2009
(Berg, 1898)
Bănăraescu and Nalbant, 1967
(Steindachner, 1897)
(Banarescu and Nalbant, 1978)
Orthrias angorae araxensis Banarescu and Nalbant, 1978 in Banarescu, Nalbant and Balik (1978), is described from the Aras River basin of Turkey (type locality given below) (this subspecies was formerly referred to as Nemacheilus angorae bureschi (Drensky, 1928) by Banarescu and Nalbant (1964) and Banarescu (1968)). Nalbant and Bianco (1998), Fricke et al. (2007) and Freyhof et al. (2010) elevate this taxon to a species.
The holotype of Orthrias angorae araxensis, 62.0 mm standard length, from the "Kandili Karassu, oberes Araxes-Becken, Osttürkei" is in the Zoologischen Instituts und Zoologischen
Museums der Universität Hamburg (ZMH 4827). Four paratypes, 45.0-65.2 mm standard length, from the same locality are under ZMH 5951 and 2 other paratypes, 51.2-59.3 mm standard length, also from the same
locality are in the Institutul de Stiinte Biologice, Bucuresti, Romania (ISBB 2617). Five paratypes, 38.8-52.0 mm standard length, are from the "Oberlauf des Araxes Flusses bei Aras-Nehri,
Hasankale" under ZMH 4826 and 3 paratypes, 41.0-45.0 mm standard length, from the same locality are under ISBB 2618 (Banarescu et al., 1978; Wilkens and Dohse, 1993).
Note that Kottelat (2012) states that Kandili is a city on the upper Karasu in
the Tigris-Euphrates basin
Oxynoemacheilus argyrogramma Reported from the Tigris-Euphrates basin in Iraq but no Iranian record except by Saadati (1977).
Oxynoemacheilus bergianus
Common names
agmahi-ye Safidruds, sehkhareh (?), sagmahi-e-jooibari.
[Sefidrudskii golets or Sefidrud loach in Russian; Safidrud stone loach]. Systematics
The type locality of Nemachilus bergianus in Latin from Derzhavin (1934) is "Systema fluminis Sefidrud" (= Safid River system). Berg (1948-1949) gives "Sefid-rud basin: Kisum village; Shah-rud
R., falling into the Sefid-rud". The former is at Kisom at 37°14'N, 49°51'E or 37°12'N, 49°54'E in a gazetteer.
Bănărescu and Nalbant (1966) and Banarescu, Nalbant and Balik (1978) place this species as a subspecies of
what is now called Oxynoemacheilus angorae (Steindachner, 1897). Nalbant and Bianco (1998) considered it to be a distinct species
in Orthrias. Abdurakhmanov (1962) compares fish from Lenkoran (his O. angorae lenkoranensis) with
O. bergianus. Head length and depth, predorsal distance, body depth, caudal peduncle depth,
dorsal fin height, and pectoral and pelvic fin lengths are all greater in lenkoranensis while caudal peduncle length, pectoral-pelvic fin distance, interorbital width and eye diameter are all
greater in bergianus.
The problems of the systematics of such a widespread and variable taxon as
O. angorae are too complex to resolve here and I have retained O. bergianus as a distinct species until
the problem has received a full study.
Note also the confusion of some materials of this species with O. persa
dealt with under that species description. The holotype of Nemacheilus bergianus is 41.6 mm standard length and is in the Zoological Institute, St. Petersburg (ZISP 25433) and is from "Basin R. Sefid-Rud, c. Kissum, River Shahrud,
tributary Sefid-Rud", collected by A. N. Derzhavin, 20.V.1922. The specimen is faded but there is a figure in Berg (1948-1949, Fig. 619).
Key characters
This species differs from related taxa by the following combination of
characters (note that this is based mostly on very limited, often old material,
and literature sources (often contradictory) - a thorough comparison of these
fishes needs to be carried out):- Morphology
Dorsal fin with 3-4 unbranched (the anterior 2-3 rays minute and not readily
visible) and 8-9 branched rays, usually 8, anal fin with 3 unbranched and 5 branched rays, pectoral fin with
8-11 branched rays, and pelvic fin with 6-8 branched rays. Total vertebrae
35-37. The pelvic fins
are separated by almost the width of a pelvic fin base. Caudal fin slightly emarginate. The upper lip is indented at the mid-point with a bony projection underneath. The lower lip is interrupted and also
has a bony projection underneath. There are no adipose fins. The flank is minutely scaled and the lateral line is well-developed along the whole flank. Meristics for Iranian fish: dorsal fin branched rays 8(57) or 9(1), anal fin branched
rays 5(58), pectoral fin branched rays 8(1), 9(12), 10(41) or 11(4), pelvic fin branched rays
6(4), 7(48) or 8(6), and total vertebrae 35(10), 36(26) or 37(4). Sexual dimorphism
Males have the anterior 2-3 rays of the pectoral fin expanded, with broad
bands of fine
tubercles overlapping on the rays and membranes, becoming less extensive
medially. Less well-developed tubercles are present on the pelvic fin membranes.
In "high" males fine tubercle are found all over the head and body,
concentrated in some below the lateral line posterior to the first third of the
pectoral fin level back to before the the pelvic fin level, generally lining scale
margins (up to 6 per scale). the flap at the antero-ventral margin of the eye is well-developed in
males, extending to below the nostril and sometimes as a thin band over the
antero-dorsal eye margin. The edge of the flap may have fine tubercles. Colour
The flank has several irregular dark grey to brown blotches. Some mid-flank
blotches centred on the lateral line appear bar-like or form rough squares from
the level of the dorsal fin origin posteriorly, but often they are less
well-defined and much smaller. Posteriorly the flank blotches may give a barred
appearance. The back has
four transverse, dark, squarish spots or saddles behind the dorsal fin. The
saddles may or may not extend to the mid-flank line but when they do the most
posterior saddles are the most evident. The back anterior to the dorsal fin
usually has
no clear pattern although there is a saddle or arc of dark pigment over the back
just in front of the fin. Some fish do bear saddles of pigment in front of the
dorsal fin but these are much less obvious than the very dark ones behind the
dorsal fin. The base of the caudal fin has a dark blotch, sometimes in the
from of a vertical zig-zag pattern. The back and upper flank have a pinkish tinge. The lower flank is pale yellowish or whitish. The
belly and lower head are white. The iris is silvery on the lower part and golden on the upper, strikingly so. The dorsal, pectoral and caudal fins are pinkish with 2-5 rows of elongate, dark grey
to brown spots
along the rays forming thin bars. Pigmentation on the pectoral fin is often
along the rays and not in the form of rows. The leading edge of the dorsal fin may have
elongated bars of dark pigment related to the thin bars on the rays, although
often the most ventral, longest and darkest may have no corresponding fin
bar. The caudal fin spots are horizontal rather than forming bands as in
Oxynoemacheilus brandtii. The upper base of the caudal fin is yellowish rather than dark as in
O. brandtii and has 3-7 spots or elongate patches of pigment along its
length, some of which line up with the thin bars. The pelvic and anal fins are colourless or slightly yellowish-pink without spots,
although in some fish the pelvic fin has one row of spots. The barbels are typically without pigment except occasionally at the base of the second pair.
Size
Reaches 6.8 cm with Jolodar and Abdoli (2004) giving 7 cm total length.
Distribution
This species is found in the middle Aras River and its tributary the Qareh Chai
(?or is araxensis), the Safid, Shafa, Siyah, Anzali Mordab
and the Masouleh River in that basin, upper Safid River basin (Qezel Owzan and Shahrud), and the Karaj,
Jaj, upper Shur or Abhar, middle and upper Qareh Chai and
upper Qom rivers of the Namak Lake basin, southern and eastern tributaries of
Lake Orumiyeh (Zarineh and Tata'u, and Talkheh rivers), and possibly in the
eastern Dasht-e
Kavir basin (Bănărescu and Nalbant, 1966; Saadati, 1977; Holčík and Oláh, 1992; Abbasi et al., 1999; Abdoli, 2000; Jolodar and Abdoli, 2004;
Abdoli and Naderi, 2009) Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Females are mature at 5.0 cm.
Heydarnejad (2009) gave the length-weight relationship for an Iranian sample
?where
as W = 0.0078TL3.055. Golzarianpour et al. (2011) give a
length-weight relationship for fish from the Jaj River identified as O. angorae
?check with a (intercept)
being 0.008 and b (slope) 3.01. Food
Unknown in detail but gut contents include aquatic insects.
Abdoli (2000) lists Trichoptera, Ephemeroptera and Chironomidae for fish
identified as ?. Reproduction
Unknown, although fish with well-developed eggs have been captured as late as
15 July.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include few in numbers, habitat destruction, limited range
(less than 25% of water bodies), present in other water bodies in Iran (sic), present outside the Caspian Sea basin (sic).
Further work
The biology and systematics of this species needs study. It is currently
recognised as present in three basins although other nemacheilids (and
cyprinids) are similarly distributed and are recognised as distinct taxa in
these basins.
A detailed study involving DNA might determine whether these basins harbour
distinct taxa. Sources
Type material: Holotype of Nemacheilus bergianus (ZISP 25433), see above.
Iranian material: Caspian:- CMNFI 1970-0521, 1, 33.4, mm standard length, Gilan, Safid River near Lulaman (no other locality data);
CMNFI 1970-0527, 8, 33.1-42.7 mm standard length, Gilan, Safid River near Kisom (37º12'N, 49º54'E);
CMNFI 1970-0537, 24, 33.3-50.4 mm standard length, Markazi, Shah River near Manjil (36º44'N, 49º24'E);
CMNFI 1970-0545, 3, 34.7-44.4 mm standard length, Gilan, Safid River from Emamzadeh Hashem to Lulaman
(ca. 37º01'N, ca. 49º38'E); CMNFI 1970-0556, 17, 22.6-58.6 mm standard length,
Iran, Caspian Sea basin (no other locality data); CMNFI 1971-0341A, 13, 28.6-44.3 mm standard length, Gilan, Safid River (no other
locality data); CMNFI 1979-0447, 2, 51.2-59.8 mm standard length, Gilan, stream 7 km east of Namin (38º23'N, 48º28'E);
CMNFI 1979-0452, 2, 42.1-42.8 mm standard length, Azarbayjan-e Khavari, Qezel Owzan River 6 km from Mianeh (37º23'N, 47º45'E);
CMNFI 1979-0453, 1, 34.2 mm standard length, Zanjan, Zanjan River (37º06'N, 47º56'E);
CMNFI 1979-0692, 1, 27.7 mm standard length, Iran, Caspian Sea basin (no other
locality data); CMNFI 1980-0132, 26, 18.5-44.1, Gilan, Safid River at Kisom
(37º12'N, 49º54'E); CMNFI 1980-0160, 1, 46.4 mm standard length, Iran, Caspian
Sea basin (no other locality data); CMNFI 2007-0081, 10, 42.2-58.6 mm standard length, Zanjan, Zanjan River basin near Soltaniyeh (ca. 36º27'N, ca. 48º45'E); CMNFI 2007-0082, 7,
31.2-53.8 mm standard length, Zanjan, Zanjan River basin near Zanjan (ca. 36º36'N, ca. 48º32'E);
CMNFI 2007-0106, 17,
42.2-59.9 mm standard length, Kordestan, Qezel Owzan River basin near Divandarreh (ca. 35º52'N, ca. 47º05'E); CMNFI 2007-0107, 8,
34.7-57.0 mm standard length, Kordestan, Qezel Owzan River basin near Bijar (ca.
35º54'N, ca. 47º20'E). Namak:- CMNFI 1979-0253, 7, 31.2-49.5 mm standard length, Markazi, Qareh Chay west of Baqerabad (34º52'N, 50º49'E);
CMNFI 1979-0458, 1, 48.2 mm standard length, Markazi, Khar River 6 km north of Ab-Garm (35º47'N, 49º20'E);
CMNFI 1979-0462, 4, 32.0-45.6 mm standard length, Markazi, Mazdaqan River (35º06'30"N, 49º40'30"E);
CMNFI 1979-0463, 2, 41.0-51.6 mm standard length, Markazi, Qareh Chay (34º53'N, 50º26'E);
CMNFI 1979-0465, 1, 37.1 mm standard length, Markazi, Qom River at Neizar (34º18'30"N, 50º32'E);
CMNFI 1993-0152, 4, 34.3-48.4 mm standard length, Markazi, Sharra River near
Khosbijan (34º07'N, 49º23'E); CMNFI 1993-0157, 2, 48.9-49.2 mm standard length,
Markazi, Sharra River near Far (34º03'N, 49º19'E); CMNFI 1993-0159, 8, 36.8-48.8
mm standard length, Markazi, Sharra River near Pol-e Do Ab (34º03'N, 49º21'E); CMNFI
1993-0160, 3, 34.9-43.2 mm standard length, Markazi, Sharra River at Pol-e Do Ab
(34º03'N, 49º22'E); CMNFI 1980-0154, 28, 10.9-26.8 mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1980-0156, 80, 29.9-50.8 mm standard length, Markazi, Karaj River near
village (35º47'N, 50º58'E); CMNFI 2007-0074, 10, 25.6-50.8 mm standard length, Markazi, Qareh Su 32 km west of Arak (34º03'N, 49º21'E);CMNFI 2007-0121, 6, 32.0-54.1 mm standard length, Hamadan, Qareh Su basin north of Razan (ca. 35º25'N, ca. 49º02'E);
CMNFI 2008-0152, 1, 43.5 mm standard length, Markazi, Namak Lake basin (no other
locality data). Orumiyeh:- CMNFI 1970-0559, 3, 14.2-37.3 mm standard length, Azarbaijan-e Bakhtari, Barunduz Chay (ca. 37º25'N, ca. 45º10'E);
CMNFI 1970-0561, 29, 18.3-30.0 mm standard length, Azarbayjan-e Bakhtari, Cheagi
Chai (no other locality data); CMNFI 2007-0084, 2, 28.5-43.4 mm standard length, Azarbayjan-e Khavari, Talkheh River basin west of Sarab (ca. 37º56'N, ca. 47º19'E); CMNFI 2007-0085, 12,
42.3-56.5 mm standard length, Azarbayjan-e Khavari, Talkheh River basin east of Sarab (ca. 37º56'N, ca. 47º41'E); CMNFI 2007-0091,
9, 36.2-49.9 mm standard length, Azarbayjan-e Khavari, Zilber Chay west of Marand (38º30'N, 45º23'E);
CMNFI 2007-0095, 2, 47.5-53.3 mm standard length, Azarbayjan-e Bakhtari, Shahr Chay southwest of Reza'iyeh (ca. 37º27'N, ca. 44º56'E);
CMNFI 2007-0096, 6, 36.5-51.3 mm standard length, Azarbayjan-e Bakhtari, Qasemul River south of Reza'iyeh (ca. 37º25'N, ca. 45º10'E);
CMNFI 2007-0097, 4, 39.2-47.8 mm standard length, Azarbayjan-e Bakhtari, Baranduz Chay basin south of Reza'iyeh (ca. 37º16'N, ca. 45º08'E);
CMNFI 2007-0099, 9, 24.6-43.8 mm standard length, Azarbayjan-e Bakhtari, Kalwi Chay west of Mahabad (ca. 36º35'N, ca. 45º25'E); CMNFI 2007-0103, 3,
36.4-55.8 mm standard length, Kordestan, Zarineh River basin north of Saqqez (36º18'N, ca. 46º16'E);
CMNFI 2008-0279, 5, 52.3-57.6 mm standard length, Azarbayjan-e Khavari, "Kozem"
or "Rozem" Chai (37º39'N, 45º36'E); BM(NH) 1899.9.30:131-137, 7,
33.3-35.8 mm standard length, Azarbayjan-e Khavari, Elinja Chai (= Alinja Chai, no other
locality data).; BM(NH) 1899.9.30:138-140, 4,
41.8-53.4 mm standard length, Azarbayjan-e Bakhtari, Ula on the Zola Chai (=
Zowla River, ca. 38º11N, ca. 44º51'E).
Oxynoemacheilus brandtii
Common names
sagmahi-ye Kura.
[Kur cilpagcasi in Azerbaijan; Kurinskii golets or Kura loach in Russian].
Systematics
A syntype of Nemacheilus Brandtii from the upper Kura River at Tbilisi (= Tiflis), Georgia is in the Natural History Museum, London (BM(NH) 1897.7.5:39, 19.6 mm standard length,
(small and
decoloured), formerly in ZISP; other syntypes are in the Zoological Institute, St. Petersburg (ZISP) (Eschmeyer et al., 1996).
Nemachilus brandtii gibbusnazus is a subspecies with the author given as Elanidze in Elanidze (1983). The only apparently new taxon in Elanidze (1983) is Nemacheilus (=
Oxynoemacheilus)
angorae alasanicus (q.v.). This taxon is unique in this book as a species without an author name after it. On this basis it was attributed to Elanidze (1983). The date of N. b.
gibbusnazus may also be 1983 but it may have been described in an earlier paper by Elanidze not yet located by me. Both these subspecies are not mentioned in Eschmeyer et al. (1996) nor in the
online version (downloaded 26 August 2007). The distribution of N. b. gibbusnazus is given as "in fact found before in the R. Khrami (1947), then in the R. Kura (1962), in its lower course at
Kukheti, in the upper reaches at Vardziya - Toloshi, in the R. Alazani - in the upper reaches at Alvani", all in Georgia and the drainage of the Kura River.
Placed in the genus Orthrias by Nalbant and Bianco (1998) and in
Oxynoemacheilus by Freyhof et al. (2011).
Key characters
This species is distinguished by a more forked caudal fin with more pointed lobes. The caudal
peduncle is said to be somewhat shorter and much lower than in O. araxensis (with which species it occurs in the Aras River basin): length of caudal peduncle 18.4-23.4% and depth 8.0-9.4%
of standard length in brandtii, 18.1-21.8% and 10.2-12.7% in araxensis respectively. Other body proportions are given in Banarescu, Nalbant and Balik (1978). The colour pattern is much
darker and the caudal fin margin is almost vertical in O. araxensis.
Morphology
Dorsal fin unbranched rays 3-4 and branched rays 7-9, usually 8, anal fin unbranched rays 2-3 and branched rays 5, pectoral fin branched rays 9-12, and pelvic fin branched rays 6-8. Scales minute. A
weakly developed dentiform process on the upper jaw.
Sexual dimorphism
Unknown.
Colour
Typical fish from the Kura-Aras basin lack a stripe on the flank, having brown spots which are either large, more or less triangular and fairly well-defined or broken into many small speckles forming a
reticulate pattern. Dorsal spots are better defined than in B. angorae especially behind the dorsal fin where they fuse completely or incompletely with the lateral flank spots to form bands.
All spots are brown, never blackish as in B. angorae araxensis. There are several rows of speckles on the caudal fin and two rows on the dorsal fin.
Size
Reaches 8.5 cm.
Distribution
Found in the upper and middle Kura and Aras River basins, and presumably in the Iranian reaches of the latter. It is also reported from the Namak Lake basin by Saadati (1977) citing a
manuscript report by V. D. Vladykov, ? confusion with bergianus and from the Arnar Chay, Azarbayjan.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Age at maturity is about 2 years.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Zarrineh River with a (intercept) being 0.009 and b
(slope) 2.97. Food
Eggs of other fishes may be a food item as well as aquatic insects.
Reproduction
Fecundity reaches 17,409 eggs and egg diameter 0.95 mm.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Endangered in Turkey (Fricke et al., 2007).
Further work
The biology and systematics of this species need study.
Sources
Type material: Syntype of Nemacheilus Brandtii ((BM(NH) 1897.7.5:39),
see above.
IDs to check:- CMNFI 1979-0448, 9, 31.1-62.0 mm standard length, Azarbayjan-e Khavari, Ahar Chay 8 km from Ardebil (38º18'30N, 48º22'E);
CMNFI 1979-0450, 3, 38.7-40.9 mm standard length, Azarbayjan-e Khavari, stream near Kivi (ca. 37º37'N, ca. 48º17'E);
CMNFI 2007-0083, 2, 28.7-33.1 mm standard length, Azarbayjan-e Khavari, Qaranqu River basin west of Sar Eskand Khan (ca. 37º25'N, ca. 46º55'E);
CMNFI 2007-0086, 3, 42.5-59.1 mm standard length, Azarbayjan-e Khavari, Qareh Su basin near Nir
(ca. 38º02'N, ca. 48º00'E). Oxynoemacheilus cyri Nemacheilus tigris cyri Berg, 1910 is described from "Fontes fl. Cyrus, Caucasus". This species
was placed in the subgenus or genus
Paracobitis by Banarescu (1968) and Prokofiev (2009) while Fricke et al. (2007) recognise the subspecies as a distinct species in
Orthrias. Freyhof et al. (2011) place it in Oxynoemacheilus. Six syntypes of N. tigris cyri (31.4-51.5 mm standard length) are in the Zoological Institute, St. Petersburg
(ZISP 13291) from "Okam village Gel'skaya Plain, Karka Oblast", K. Satunin, 6.IX.1901. This is in the upper Kura River basin of Turkey. No Iranian record.
Oxynoemacheilus frenatus
Common names
See genus account.
[lakh or telay (= bowed head according to Heckel (1843b) at Mosul), both in Arabic; banded Tigris loach].
Systematics
The type locality of Cobitis frenata is "Tigris", presumably at Mosul (Heckel, 1843b). Five syntypes are in the Naturhistorisches Museum Wien (NMW 48552) although the catalogue lists
only 4 specimens. A lectotype designated by F. Krupp in 1984 is 70.0 mm standard length, the remaining specimens being small, 27.2-43.4 mm standard length.
Nemacheilus frenatus afrenatus Battalgil, 1942 described from "un petit ruisseau à Diyarbakir" is also from the Tigris River basin but in Turkey. This subspecies lacks the "frein
à la bouche" (presumably the band across the snout) of B. f. frenata and its dorsal fin is higher than long (as measured at the base) while in B. f. frenata it is as high as long.
Bănărescu and Nalbant (1995) place this species in the genus Orthrias. Bănărescu and Nalbant (1966) consider this taxon to be a "doubtful species" but illustrate
it in Bănărescu and Nalbant (1995: Fig. 20).
Key characters
The colour pattern is distinctive and there is no dermal crest or adipose fin behind the dorsal fin.
Morphology
Dorsal fin unbranched rays 2-3, branched rays 7-8, anal fin unbranched rays 2, branched 5, pectoral fin branched rays 10-13,
and pelvic fin branched rays 6-7. Scales are present over the whole body
but not readily visible without magnification. The anterior pectoral fin rays are thickened. Caudal peduncle thick (depth 80-90% of length) according to Saadati (1977). The bulb of the swimbladder
capsule has large ovoid to circular perforations and the anterior and posterior lamina or wings are only moderately developed (Krupp, 1985c). Lips are not strongly plicate and the dentiform process is
well-developed. The gut has a posterior loop.
Meristics for Iranian specimens: dorsal fin branched rays 8(1), anal fin
branched rays 5(1), pectoral fin branched rays 10(1) and pelvic fin branched
rays 6(1) based on lectotype.? add my material Sexual dimorphism
Bands of tubercles are found on the pectoral fin rays of males, including the first, declining in breadth and extent on the smaller rays. Tubercles are also present on the pelvic and anal fin rays but
are much less well developed. The head is covered in fine tubercles. Flank scales, particularly anterior ones, are lined anteriorly with tubercles. There is an elongate tuberculate swelling anterior to
the lower eye margin on the snout.
Colour
Overall colour is yellowish, mottled with fine but irregular brown or black dots or blotches, some flank blotches being quite large. The rear of the body and the caudal fin in particular are mottled
with brown, tending to form bars. A black band is continuous from the front of one eye, across the snout and round to the other eye. It may be diffuse on the snout or well-defined in fish from the
same locality. The dorsal and caudal fins have thin but irregular bands made up of spots on the rays (up to 3 on the dorsal and 4 on the caudal fin), bands are faintly present on the anal and pelvic fins,
and only a few are visible on the pectoral fins. The dorsal fin has an anterior basal spot at its origin, variably developed in individuals. There are distinct, dark spots at the base of the caudal fin
above and below the body mid-line.
Size
Reaches about 9.2 cm (Heckel, 1843b).
Distribution
Found in the Quwayq and Tigris-Euphrates rivers. Abdoli (2000) records it from the upper Karun, middle and lower Dez, Kashkan and Simarreh rivers in the Tigris River basin of Iran.
Zoogeography
See family account.
Habitat
Known to inhabit both rivers and lakes, the environmental requirements of this species are unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
The biology of this species, its distribution, numbers and habitat requirements are unknown so no comments on conservation can be made. Near threatened in Turkey (Fricke et al., 2007).
Further work
The biology and conservation status of this species need investigation.
Sources
Type material: Syntypes of Cobitis frenata (NMW 48552), see above.
Iranian material: BWC 95-30, mm standard length, .
Comparative material: BM(NH) 1974.2.22:1449-1477, 30, 37.7-55.5 mm standard length, Iraq, branch of Khalis River ();
BM(NH) 1974.2.22:1478-1757, ca. 279, 19.7-53.3 mm standard length, Iraq, Khalis ();
BM(NH) 1974.2.22:1758-1772, 15, 27.2-34.1 mm standard length, Iraq, Khalis ();
BM(NH) 1972.2.22:1773-1776, 4, 28.3-34.0 mm standard length, Iraq, Khalis ();
BM(NH) 1974.2.22:1777-1778, 2, 27.5-31.4 mm standard length, Iraq, Khalis ().
Oxynoemacheilus kermanshahensis
Common names
sagmahi-ye Kermanshah.
[Kermanshah loach].
Systematics
This species was tentatively placed in the subgenus Orthrias Jordan and Fowler, 1903 but is regarded as "aberrant" by Bănărescu and Nalbant (1966).
However, Nalbant and Bianco (1998) later place it in Orthrias.
The holotype of Noemacheilus kermanshahensis (ZMUC P 2787, 46.4 mm standard length) and the 7 paratypes (ZMUC P 2788-94, 25.5-61.8 mm standard length) are stored in
the Zoological Museum of Copenhagen (Nielsen, 1974). The type locality is "Kermanshah in the drainage of the Karun River, a tributary of the lower Euphrates, Western Iran" (Bănărescu
and Nalbant, 1966). The type series was collected on 5 February 1937 by E. Kaiser. The type locality is poorly defined - the city of Kermanshah is on the Qarasu River and lies in the drainage of the
Karkheh River which drains towards the Tigris River. Nalbant and Bianco (1998) correct their original type locality description to the Karkheh River drainage and "Quareh Su, Kermanshah, River
Shimarek", probably referring to the Qareh Su-Simareh drainage of the upper Karkheh River.
Key characters
Bănărescu and Nalbant (1966) consider that this species differs from most South and West-Asiatic loaches by the longitudinal pigment pattern on the flank
but not all fish have this pattern. Most other species have bars,
vertical patches of pigment. The longitudinal arrangement is found in O. angorae but kermanshahensis has smaller eyes, a shorter lateral line (almost complete in angorae), a
more anteriorly placed vent, and scale shape comprising an almost central focus, vertical sub-oval outline and radii on all fields widely and evenly spaced. Characteristically the caudal peduncle is
short and deep, depth being 92.9-114.3% of length (Saadati, 1977). The dorsal
fin branched ray count of 7 is also a key character. Morphology
Dorsal fin with 3 unbranched and 7 branched rays, anal fin with 2 unbranched and 5 branched rays,
pectoral fin with 8-10 branched rays, and pelvic fin with 6-7 branched rays. Scales only on the posterior part of the body,
well-developed on the caudal peduncle. The lateral line reaches the level of the middle or posterior half of the dorsal fin or over the front half of the anal fin. The origin of the dorsal fin is variable
in relation to the snout tip and caudal fin base (Bănărescu and Nalbant, 1966). The caudal peduncle is short and deep and
often lacks a crest although a weak crest may be developed just anterior to the
caudal fin. The anus is somewhat anterior to the anal fin origin.
Lips are thick and fringed, the lower lip being interrupted in the middle. The intestine is simple with the posterior part straight.
Meristics for Iranian material, including the type series: dorsal fin branched rays 7(10), anal fin branched rays 5(10), pectoral fin branched rays 8(3), 9(2), or 10(4), pelvic fin branched rays 6(3) or 7(7),
and total vertebrae 38(7), 39(2) or
40(1).
Sexual dimorphism
No sexual dimorphism was noted by Bănărescu and Nalbant (1966) as their large specimen is female and others immature.
Freyhof et al. (2011) note the presence of a groove at the antero-ventral
margin. Colour
The overall colour is yellowish with continuous, dark brown blotches on the flank my fish.
A series of connected blotches line the upper flank, most obvious as blotches
postdorsally, and separated from the mid-flank stripe by clear areas. A ventral
stripe composed of blotches is also present and more clearly defined than
indicated below. There is a bar at the caudal fin base. A broad bar is seen in
some fish in the middle of the caudal fin with a less broad bar distally. A stripe
may be present on the mid-line of the back and break into spots posteriorly, or
the back may be uniformly pigmented.
The colour pattern in alcohol-preserved specimens dating from 1937 is yellowish with 3 wide, brownish stripes along the flank. The dorsal stripe is continuous anteriorly but breaks up into spots
posteriorly in most specimens. The central, mid-flank stripe is the widest and is continuous although width is variable. The ventral stripe is absent from smaller
specimens and consists of small and irregular spots. The upper parts of the head have small, irregular brownish spots. The caudal fin has 3 rows of spots and the dorsal fin 2 rows of spots, apparently
concentrated on the fin rays (Bănărescu and Nalbant, 1966).
Size
Reaches 6.3 cm standard length.
Golzarianpour et al. (2011) record a total length of 8.4 cm. Distribution
Known only from Tigris River basin drainages of Iran (see above). Abdoli (2000) reports it from the Marun, upper Karun and lower Khersan, Dez, Simarreh, Qareh Su and Gav Masiab rivers.
Zoogeography
Relationships may lie with B. angorae according to Bănărescu and Nalbant (1966) based on colour pattern and general body shape, or with other species not found in Iran having stripes
along the flank. This seems insufficient evidence on which to base relationships.
Habitat
Unknown. Age and growth
Unknown in detail.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Gamasiab River with a (intercept) being 0.015 and b
(slope) 2.89. Food
Unknown.
Reproduction
Unknown, although fish caught on 11 May had well-developed eggs..
Parasites and predators
Unknown.
Economic importance
None.
Conservation
No measures are being undertaken for this poorly known species.
Further work
Studies should be carried out to determine the numbers of this species, its distribution and its ecological requirements in Iran to ascertain if conservation measures should be taken.
Sources
Type material: The holotype (ZMUC P 2787) and paratypes (ZMUC P 2788-94) of Noemacheilus kermanshahensis, see above.
Iranian material: CMNFI 1993-0128, 1,
50.0 mm standard length, Kermanshahan, Sarab-e Sabz 'Ali Khan (34º25'N, 46º32'E);
CMNFI 2008-0117, 510 mm standard length, Kermanshahan, Sarab-e Yavari (34º28'N, 46º56'E). Oxynoemacheilus kiabii
Common names
None. Systematics
The holotype ZFMK 41847, 54.5 mm standard length, is in the Zoologisches
Forschungsmuseum Alexander Koenig, Bonn and is from Hamadan, Dehnoo stream, 3 km
west of Nahavand, 34º10'N, 48º24'E. Paratypes are FCKG (Fish Collection, K.
Golzarianpour) 8658, 8 specimens, 31.4-50.7 mm standard length, same locality as
holotype, FCKG 8659, 7, 33.6-53.3 mm standard length, same locality as holotype,
and FSJF (Fischsammlung J. Freyhof, Berlin) 3004, 2, 38.4-44.4 mm standard
length, Babarostam, Gamasiab River tributary, 34º10'18"N, 48º21'23"E. The
species is named after Bahram H. Kiabi for his conservation work, especially on
fishes. Key characters
This species is distinguished from other members of the genus by having two,
rarely one or three, lateral pores (on each side) in the supratemporal head
canal and no central pore; no suborbital flap and no groove in males; an
incomplete lateral line reaching the level of the dorsal fin; a slightly
emarginate caudal fin; a short pectoral fin not reaching the pelvic fin origin;
a shallow adipose crest on the caudal peduncle dorsally from the level of the
last dorsal fin ray base to anterior part of caudal fin (looks more extensive on
photographs in type description); a long head (26-30% standard length); and
colour pattern. Morphology
A stout and deep-bodied species with a long and wide head. Many fish have a
distinct hump at the nape. The compressed caudal peduncle is about as deep as
long. Dorsal fin branched rays 8, anal fin branched rays 5, pectoral fin rays 10
and pelvic fin rays 8. The pelvic fin base has a short, triangular axillary lobe
completely attached to the body. The incomplete lateral line has 18-29 pores.
The body is scaled except on the back predorsally and the belly. On fish had
three lateral pores in the supratemporal head canal and two fish had one left
pore and two right pores. Lips are thick and well-furrowed, the lower lip
interrupted medially. Processus dentiformis small and pointed. There is a
shallow ventral crest on the caudal peduncle. Sexual dimorphism
Males have slightly longer pectoral fins than females. Colour
Overall body colour is brown in life and grey in preserved fish. The flank
has 10-19, dark brown or black, large bars or blotches, most evident posterior
to the dorsal fin level, and more irregular anterior to the dorsal fin level.
The flank bars or blotches are not connected to predorsal back blotches.
Blotches below the lateral line are smaller than those above it. A bar at the
tail base may also take the form of two half-moon blotches. Head spotted and
blotched, sometimes marbled. A tesselated pattern on the caudal fin rays forms
2-3 irregular bars and a similar pattern on the dorsal fin forms 3 bands. Other
fins have a few dark brown or black spots on the rays. Size
Reaches 56.5 mm standard length.
Distribution
Found in the Karkheh River drainage at the localities listed above for the
type series.
Zoogeography
See family account.
Habitat
Found in clear waters of streams with a swift current. Age and growth
Unknown. Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Known only from a restricted area with no data on numbers and threats. Further work
The biology of this species and its conservation status need study. Sources
The type description. Oxynoemacheilus lenkoranensis Lankaran cilpaxcasi (or cilpagcasi), both in
Azerbaijan; Lenkoranskii golets or Lenkoran loach, both in Russian. Nemacheilus angorae lenkoranensis Abdurakhamanov, 1962 (incorrectly lenkoranica in Bănărescu and Nalbant (1966)) is described from "rivers of the Lenkoran
coast; Lenkoranchai, Vilyazhchai, Kumbashichai, Tangyaru, Astarinka" in the southern Caspian Sea basin.
A series of 18 fish in the Zoological Institute, St. Petersburg catalogued as ZISP 35701 from "Reka Lenkoran, Azerbaidzhan SSR" collected by Yu. Abdurakhmanov, 22.IX.1954 are probably the
type series of Nemacheilus angorae lenkoranensis although they are not marked as such on the jar. One fish is a cobitid, 31.0 mm standard length (probably Cobitis
keyvani), while the rest
measure 24.9-33.3 mm standard length. Oxynoemacheilus namiri Recorded from the Tigris River basin in Turkey by Nalbant (1998) but no Iranian record. Originally described in the genus Nemacheilus.
Oxynoemacheilus persa
Common names
sagmahi-ye Fars (= Persian loach).
[Persian loach].
Systematics
The type locality for Cobitis Persa is "Quellen um Persepolis" according to Heckel (1847b). Kähsbauer (1964) reports a syntype of this species in the Naturhistorisches Museum Wien
under NMW 48567. It measures 47.6 mm standard length and is probably the holotype as the Vienna catalogue lists only 1 specimen (and the Vienna card catalogue examined in 1997 concurs). This specimen is in
poor condition and not readily comparable to fresh material. It is scaled although scales are not imbricate, the caudal fin is broken off, the body is collapsed so its shape cannot be determined, and it
is decoloured.
Kessler (1877) refers to a Heckel species Nemachilus persicus, presumably this taxon judging from Kessler's page number reference.
The trivial name is spelled persa as it is a noun in apposition (see Catalog of
Fishes; Kottelat (2012)). Kotteleat (2012) gives the publication date as 1848. Günther (1899) recorded Nemacheilus persa from the Lake Orumiyeh basin ("Zola Chai near Ula"
- see materials under O. bergianus) and the upper Aras River basin ("Elinja Chai"
- see materials under O. bergianus) but these fish were possibly
O. angorae or O. bergianus. Berg (1948-1949), Bănărescu and Nalbant (1966) and Nalbant and Bianco (1998) also refer Günther's material to Nemacheilus persa but Abdurakhmanov (1962) suggests that
these fish are O. angorae. Saadati (1977) places Lake Orumiyeh fish close to
O. angorae which makes more sense geographically.
The dorsal fin origin is closer to caudal base than the snout tip in the Lake Orumiyeh fish, interorbital width is greater,
the head is longer, the caudal fin less emarginate, and there are more and darker spots on the body (Saadati, 1977). Evidently new material from the Kor River basin wherein lies Persepolis, the type
locality for Cobitis persa, and the Lake Orumiyeh basin are required to resolve this problem - the Lake Orumiyeh sample examined by Bănărescu and Nalbant (1966) comprised only 3 males.
The loach from the Lake Orumiyeh basin is now referred to O. bergianus,
q.v. Orthrias farsicus Nalbant and Bianco, 1998, named for Fars Province,
collected from the "River Kor near Persepolis" in Fars is a synonym (Freyhof
et al., 2011). The holotype of Orthrias farsicus is in the Department of Zoology, Naples University under IZA 7823. It measures 45.8 mm standard
length (51.8 mm standard length when measured by me) and was collected from the "River Kor near Persepolis" in Fars on 30 May 1976 by P. G. Bianco (note that IZA
is the acronym for Dipartimento di Scienze Ambientali, Universita' Degli Studi
Dell'Aquila
L'Aquila, Italy,
where the material was previously stored). Paratypes were all collected by P. G. Bianco and include 64 specimens, 39.0-57.0 mm standard length from the same locality as the holotype (IZA 7824), 61
specimens, 38.5-60.0 mm standard length from the same locality as the holotype but taken on 7 July 1975 (IZA 7825), 7 specimens, 42.0-51.3 mm standard length, same locality as the holotype but taken on 7
July 1975 stored in the Institutul Stiinte Biologice, Bucharest (ISSB 3451), 31 specimens, 43.2-56.3 mm standard length from the River Shur, tributary of the River Mand, near Dasht-e-Arzhan
(Shiraz) 25 May 1976 (IZA 7826), 7 specimens, 47.0-57.0 mm standard length from the latter locality (ISBB 3446), 7 specimens, 47.0-58.0 mm standard length from River Qom, Qom Town 5 May 1975 (IZA 7845)
and 7 specimens, 36.2-47.3 mm standard length from the latter locality (ISBB 3442).
Three specimens from each of IZA 7824 (40.2-50.5 mm standard length) and 7826
(46.6-50.1 mm standard length) are in the Canadian Museum of Nature, Ottawa (CMNFI
2012-0005 and 2012-0006 respectively).
Bănărescu and Nalbant (1966) place this species in their subgenus Oxynoemacheilus but later (Bănărescu and Nalbant (1995) and also Nalbant and Bianco (1998)) place it in
Orthrias.
Freyhof et al. (2011) place the species in Oxynoemacheilus. Key characters
This is the only known Oxynoemacheilus species from the Kor River
basin and colour pattern and tuberculation are distinctive. Morphology
This fish has long paired fins and a short, deep and compressed caudal
peduncle. Lips and barbels have small tubercles and fine furrows. The upper lip has a median incision and the lower lip lacks mental lobes. The processus dentiformis
in the upper jaw is well-developed but lacks a gap in the lower jaw. Dorsal fin
with 2-4 unbranched (anteriormost very small and only visible under x-rays hence
literature range) and 7-9, usually 8, branched rays, anal fin with 2 unbranched and
4-5, usually 5, branched rays, pectoral fin with 8-12 branched rays, pelvic fin with 6-8 branched rays,
and total vertebrae ?.
The type description of O. farsicus (a synonym) has dorsal fin branched
rays 7-9 (90% with 8 rays in the type description, n=38), anal fin branched rays
4-5 (95% with 5 rays), pectoral fin branched rays 9-11 (69% with 10 rays) and
pelvic fin branched rays 6-7 (65% with 7 rays). The lateral line is straight, on
the mid-flank and ends just anterior to the caudal fin base. The cephalic canal
system is well-developed laterally. Scales are present on the anterior and posterior flank according to Banarescu and Nalbant (1964)
and Nalbant and Bianco (1998) for O. farsicus state that the rear half of
the body is scaled completely and the anterior half has scalation well-developed but not as closely spaced as posterior scales. Scales are oval with a large focus. Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales along with a
description. The caudal fin is slightly emarginate to forked. The barbels are hair-like thin (Heckel, 1847b).
Meristics for Iranian specimens including the holotype of O. persa and
the holotype and 6 paratypes of O. farsicus: dorsal fin branched rays
8(47) or 9(2), anal fin branched rays 5(49), pectoral fin branched rays 9(1),
10(25), 11(21) or 12(1), pelvic fin branched rays 6(13), 7(35) or 8(1), and
total vertebrae 35(2), 36(13), 37(23) or 38(2). Sexual dimorphism Banarescu and Nalbant (1964) report that pectoral fin rays 2-5 are widened and thickened in males. The appearance of a subocular pad in Bănărescu and Nalbant (1966:Fig. 3) is an error and
was apparently meant to represent a small groove (Bănărescu and Nalbant, 1995).
Nalbant and Biamco (1998) describe fish identified as O. farsicus (a
synonym) as follows with additional comments by me in parentheses. Males have numerous fine tubercles on the dorsal surface of the pectoral fin rays in bands
(following the rays but extending onto the membranes), and the anterior fn rays are expanded. There are fewer rays and less expansion when progressing proximally.
There is a groove running anteriorly at a slight downward angle from the eye to the antero-ventral corner below the nostril, fading out under the anterior nostril. Near the eye and dorsal to this
groove, fine tubercles form a narrow band. The sides and top of the head are finely tuberculate. The pelvic and dorsal fin rays have tubercles following the rays (not
in bands as on the pectoral fin). Fine
tubercles line the margin of the scales (and are especially evident on the
anterior lower flank). Colour
The overall colour is yellowish with brown to black blotching. There are 5-7 brown bars
or blotches (shape varies) on the posterior body and irregular brown to black
blotches on the anterior body, numbering up to about 12, being less well-defined
anteriorly. The back has 7-11, modally 9, rounded dark-brown saddles, most
apparent post-dorsally. The dorsal head is speckled brown and there is a dark-brown
stripe from the eye to the snout tip, sometimes extending beyond the eye. The
dorsal fin has 3-4 rows of elongate spots, the pectoral fin has up to 6 rows
(not always in clearly defined rows but extending along the whole ray) and
the caudal fin has 4-5 rows. The rows may be poorly defined with pigment spread
along rays and not on the membranes. The anal and pelvic fins are immaculate
although some fish may have pigment on the pelvic rays but not in rows. The
description of O. farsicus is as follows. The flanks and back have
roundish brown spots on a marbled background and the head and body are a pale
yellow ventrally. Dorsal and caudal fins have rows of brown spots. The
peritoneum is a dull brown.
Size
Reaches 67.7 mm standard length. Golzarianpour et al. (2011) give 7.1 cm total length.
Distribution
Found in the Kor River and Lake Maharlu basins (Esmaeili et al.,
2011?). Abdoli (2000) maps the Kor
and Pulvar rivers. Nalbant and Bianco (1998)
report it (as O. farsicus) from the Namak Lake basin (Qom River at
Qom - this is remote from the type locality of O. persa and needs further
investigation), Kor River basin (Kor River near Persepolis) and Gulf basin (Shur River
near Dasht-e Arzhan in the Mand River drainage). Records by Bănărescu and Nalbant (1966) in the Lake Orumiyeh
basin are in error (see above). Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 32 fish measuring 2.94-6.32 cm standard length. The a-value was 0.0211 and the b-value 2.784
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 34 fish
identified as O. farsicus (a synonym) measuring 3.15-6.77 cm standard length. The a-value was 0.0126 and the b-value 3.084
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Kor River with a (intercept) being 0.012 and b (slope)
2.88, the difference with the previous study attributed to small sample sizes. Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Conservation requirements are unknown but it may be relatively common as a
secretive species. Further work
The abundance and habitat requirements of this species need to be determined. Sources
Type material: The holotype of Cobitis persa (NMW 48567), see above,
and the holotype (IZA 7823) and paratypes (IZA 7824, 7825, 7845) of Orthrias farsicus,
see above. Iranian material: CMNFI 1979-0028, 5, 48.3-55.6 mm standard
length, Fars, Kor River drainage (no other locality data); CMNFI 1979-0059, 28,
32.8-57.0 mm standard length, Fars, Pulvar River 8 km south of Sivand
(30º01.5'N, 52º57'E); CMNFI 1979-0061, 3, 50.2-66.5 mm standard length, Fars,
stream tributary to Pulvar River 14 km south of Sa'adatabad (30º04'N, 53º01'E);
CMNFI 1979-0067, 1, 55.8 mm standard length, Fars, qanat at Zarqan (ca. 29º46'N,
ca. 52º43'E); CMNFI 1979-0070, 2, 38.8-39.9 mm standard length, Fars,
Pulvar River near Naqsh-e Rostam (29º59'N, 52º54'E); CMNFI 1979-0117, 2,
42.1-50.6 mm standard length, Fars, Pulvar River at road bridge to Naqsh-e
Rostam (29º59'N, 52º54'E); CMNFI
2008-0255, 47.9-51.3 mm standard length, Fars, Kor River (30º00'N, 52º45'E).
Oxynoemacheilus tongiorgii Common names
sagmahi-ye Hormuz.
[Hormuz loach].
Systematics
This species is named for Professor Paolo Tongiorgi, University of Modena. The holotype measures 23.7 mm standard length and is from " large water spring near Darab town, Kul river
basin", collected on 2 June 1976 by P. G. Bianco and is stored in the Department of Zoology, University of Naples (IZA 801 but not located in a 2002 visit by me). One paratype, 20.0 mm standard
length, is from the same locality (Institutul Stiinte Biologice, Bucharest, ISBB uncatalogued).
It differs from an Anatolian species, Seminemacheilus lendlii (Hankó
1925), mostly by colour pattern and possession of scales. It was originally
described in the genus Seminemacheilus Banarescu and Nalbant, 1995 but
Freyhof et al. (2011) transfer it to Oxynoemacheilus on
unpublished DNA evidence. Kottelat (2012) gives a publication date of 1999 but
the journal is dated November 1998. Key characters
This species has a short and deep body, deeper than in related species in
Iran, and a deep and short caudal peduncle. Scales are scattered and
non-imbricate. Morphology
The body is stout with a short and deep caudal peduncle. Caudal peduncle
depth in length is 0.9-1.2, mean 1.0 for 23 fish), and larger fish tend to have
a deeper caudal peduncle (this character may be difficult to measure as dorsal
and ventral crests may vary in development and be collapsed). The short caudal
peduncle means the anal fin reaches, or almost reaches, the caudal fin base. The
crest on the caudal peduncle is low and originates at the caudal fin base,
decreasing in height anteriorly and not reaching the dorsal fin insertion. A
small ventral keel is also present. In the original description the body is said
to be almost entirely scaleless except for minute scales on mid-flank
but flanks and back are all scaled in samples seen
here. Scales are scattered and non-imbricate.
The belly anterior to the pelvic fins is scaleless except for some non-imbricate
scales scattered immediately anterior to the fins. Radii are present on all
fields, radiating from an almost central focus on anterior flank scales. The lateral line is short and
may not reach beyond the end of the pectoral fin
tip level but can extend back under the dorsal fin level. Esmaeili and Niknejad (2006-2007) give scanning electron micrographs of the scales along with a description. The
processus dentiformis is given as absent in the original description but is
weakly to well-developed in material seen here. The lips are furrowed, particularly the lower lip. The gas bladder capsules are strongly united. The dorsal fin has 3 unbranched and
7-9, usually 8, branched rays, the anal fin has 3 unbranched and 5-6, usually 5, branched rays, the pectoral fin has 2 unbranched and 9-11 branched rays and the pelvic fin has 1 unbranched and
5-7 branched rays. Total vertebrae 34-35. The pelvic fin insertion is opposite the dorsal fin origin.
The caudal fin is very slightly emarginate. The head is wide with large eyes and nostrils just anterior to the eyes. The
mouth is arched and the lower lip has large lobules but the mental lobes are
reduced. The snout is blunt. Nostrils are widely spaced
and equal in size. The
gut has a short oesophagus and the stomach and intestine have one loop. Prokofiev (2009)
lists various osteological features including the manubrium in the swimbladder
being absent or weakly developed and the bony capsule swollen, 4
elongated-cylindrical radial bones in the pectoral fin, no preethmoid I
bone in the skull and 5 hypurals but no epural in the caudal fin support, among
others. Meristics for Iranian specimens: dorsal fin branched rays 7(1), 8(27)
or 9(2), anal fin branched rays 5(28) or 6(2), pectoral fin branched rays 9(2),
10(22) or 11(6), pelvic fin branched rays 5(2), 6(18) or 7(10), and total
vertebrae 34(11) or 35(10). Sexual dimorphism
A small slit or groove is found at the antero-ventral eye margin. Sexual dimorphism
consists of the anterior 2-3 rays in the pectoral fin being expanded, and
pectoral fins having fine tubercles on rays and membranes, extending to all rays
except the innermost three. Very fine tubercles are found on the dorsal and
lateral head. Male pectoral fins are larger than in females. Colour
The overall colour is yellowish-white to brown. The head is finely mottled
dorsally and laterally. A short bar extends between the eye and snout tip. There is a row of grey to brown or black blotches along the mid-flank
and these may line up vertically to form the appearance of thin to broad bar,
especially on the rear half of the body.
The bars may become weak saddles on the back and keel behind the dorsal fin. The
lateral line anteriorly is white. The
dorsal and caudal fins may have usually 3-4 rows of spots on the rays and membranes but
these do not always line up in neat rows. A fish photographed in Freyhof et
al (2011) has about 7 rows on the caudal fin. The dorsal fin may have pigment on the
rays and patches of pigment proximally on the membranes. The caudal fin can have
to 6 large and darker spots on its dorsal margin and up to 4 spots on the
ventral margin, not necessarily lined up with the fin rows. The pectoral and
pelvic fins bear extended patches of pigment on the rays and to some extent on
the membranes, but not arranged in neat rows. There is an irregular bar at the
caudal fin base.
The peritoneum is silvery-white with scattered melanophores. Size
Attains 59.3 mm standard length.
Distribution
Known from Iran in the upper reaches of the Hormuz basin near Darab, in
the Kor River basin and in northwestern Iran (Nalbant et al., 2010; Esmaeili et al., 2011).
Note that Freyhof et al. (2011), based on collecting expeditions by Hamid
Reza Esmaeili, Shiraz University, consider that the Darab (Kol River) locality
could be in error and Kol and Kor were confused. Zoogeography
The limited distribution of this endemic and lack of information on relationships preclude zoogeographical analysis. See family account.
Habitat
Details are unknown.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 45 fish measuring 2.95-5.93 cm standard length. The a-value was 0.0264 and the b-value 2.904
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Iranian material: CMNFI 1979-0285, 20, 29.6-49.8 mm standard length, Kermanshahan,
Marek River basin (34º26'N, 46º37'E); CMNFI 1979-0286, 1, 51.8 mm standard length, Kermanshahan, Ravansar River at Ravansar (34º43'N, 46º40'E); CMNFI 1979-0292, 8, 29.0-45.6 mm standard length, Fars, Lapu'i
spring (29º48'N, 52º39'E); CMNFI 1991-0155, 1, 41.7 mm standard length, Hamadan, Gav Masiab River (34º12'N, 48º20'E);
CMNFI 1993-0128, 1, 58.3 mm standard length, Kermanshahan, Sarab-e Sabz 'Ali
Khan (34º25'N, 46º32'E); CMNFI 2007-0118B, 17, 23.9-58.0 mm standard length, Kermanshahan, Bid Sorkh River between Sahneh and Kangavar (ca. 34º23'N, ca.
47º52'E). ID?CMNFI 2007-0115, 1, 41.5 mm standard length, Kermanshahan, Qareh Su basin (ca. 34º34'N, ca. 46º47'E);
CMNFI 2007-0115, 2, 49.7-54.7 mm standard length, Kermanshahan, Qareh Su basin (ca. 34º34'N, ca. 46º47'E);
CMNFI 1993-0125, 1, 47.8 mm standard length, Kermanshahan, Sarab-e Nilufar (34º24'N, 46º52'E);
CMNFI 2008-0236, ?
Genus Paracobitis
This genus
comprises about 17, comparatively large species, some over 28 cm, in western
Asia. Six species are reported from Iran but more may exist. One is
mentioned here as specimens exist from the Kor River basin in Fars; a record of
a Paracobitis from Iraq (see Coad, 2010) in the Tigris River basin is not
matched with specimens from adjacent parts of Iran as yet.
The body
is elongate, thick anteriorly and posteriorly compressed. The head is strongly
depressed. Nostrils are closely spaced or weakly separated. The caudal peduncle is long, low and bears a
distinctive, elongate and high crest dorsally
from the dorsal fin to the caudal fin and, often, a more or less well-developed
ventral adipose crest. However, the crest is not supported by the procurrent
rays of the caudal fin unlike in Metaschistura and Oxynoemacheilus.
The crest is not connected to the anterior part of the caudal fin (cf.
Oxynoemacheilus species, when crest present). The caudal fin is
slightly emarginate or truncate, rarely rounded. There is a well-developed
pelvic axillary lobe reaching beyond the vertical of the posterior edge of the
pelvic fin base. Scales are present or absent anteriorly and the lateral line is complete.
The processus dentiformis is usually strongly developed (weak to absent in P.
vignai) but its notch on the
lower jaw is reduced. Lips are smooth or grooved. The gut is short with a single
loop. The colour pattern is variable comprising bars or irregular spotting.
There is no intense black spot at the base of the anterior dorsal fin rays.
Males and females show no external differences except males have more muscular
cheeks. Prokofiev (2009) gives further osteological details of this genus
including cst having a bony roof, the fork of cst and cio
is fused to the skull in adults (the
cst is the supratemporal commissure running across the top of the head at
the rear and cio is the temporal part of the infraorbital head canal),
the free part of the swimbladder is reduced or absent, right and left lobes of
the bony capsule are fully divided by the manubrium, and the fifth trunk vertebra
does not take part in support of the capsule. There are 4 radials in the
pectoral fin skeleton and the caudal fin support has 6 hypurals and an epural is
present. There is no preethmoid I bone in
the skull. Paracobitis boutanensis Probably described from the neighbourhood of the Bolan Pass, Helmand River drainage of Afghanistan in the Sistan basin according to Hora (1929) and Bănărescu and Nalbant (1966). No Iranian
record.
Paracobitis ghazniensis Described from "Ghazni, on the Ghazni River, tributary of the Ab-i-Istadah Lake, Helmand drainage; East Afghanistan" (Bănărescu and Nalbant, 1966). Ghazni is at 33°33'N, 68°26'E in
the Sistan basin. No Iranian record.
Paracobitis iranica Common names
sagmahi-ye irani.
[Iranian loach].
Systematics
The species is named for Iran. The holotype is 79.8 mm standard length (66.0 mm standard length when measured by me) and is from "River Qom near the town of Qom" collected on 6 May 1976 by
P. G. Bianco and stored in the Department of Zoology, University of Naples (IZA 7831). The locality in the jar is "Qom River near Qom (a little salt river 3.5 p.p.t.) near Qom at the bridge,
950 m, 5 June 1976", the date being at variance with the published date. Paratypes number 5 specimens, 59.0-92.0 mm standard length (IZA 7832)(only two present in 2002 visit by me), and 4 specimens,
47.0-72.0 mm standard length (Institutul Stiinte Biologice, Bucharest, ISBB uncatologued).
Kottelat (2012) gives a publication date of 1999 but the journal is dated
November 1998. Key characters
This is the only known Paracobitis species in the Namak Lake basin. No
single character is uniquely definitive (see below) and locality is key. Morphology
The body is elongate with a compressed posterior region and a depressed head.
The caudal peduncle is elongate and has a long dorsal adipose crest. The body is
scaled. The dorsal fin has 7-8 branched rays (90% with 7 rays, n=9 in type
description), the anal fin has 5 branched rays in all specimens examined in the type description, the pectoral fin has 9 branched rays in all specimens
and the pelvic fin has 5-7 branched rays (78% with 7 rays, n=9). The unbranched
dorsal and anal fin rays are often 4, the anterior two minute. The lateral line is straight and extends to the caudal fin base. The body is minutely scaled with a relatively eccentric and
large focal zone. The head is conical with eyes in the anterior half. The nostrils are just in front of the eyes. The mouth has a strong arch with short barbels and well-furrowed lips interrupted in the
middle. The processus dentiformis is well-developed. The stomach is syphonal and the intestine straight. The gas bladder capsule has a relatively short duct.
Multivariate analyses using 21 morphometric and 6 meristic characters to
compare 47 fish from the Namak Lake basin with 19 fish from the Caspian Sea
basin showed no unique character or small suite of unique characters that would
separate these fishes. When all characters were used, a Discriminant Function
Analysis separated 99% of individuals by basin. Nalbant and Bianco (1998)
separate this taxon from P. malapterura by body colour pattern and a
larger adipose crest. However, the colour pattern figured by them is seen in
P. malapterura and Namak Lake basin fish may lack the posterior ventral row
of spots or blotches. The adipose crest development is also mirrored in P.
malapterura. The only field identification character is collection site. Meristics for non-type material: branched dorsal fin rays 6(1) or 7(46),
branched anal fin rays 4(1) or 5(46), branched pectoral fin rays 8(4), 9(35) or
10(8), branched pelvic fin rays 6(8) or 7(38), predorsal fin vertebrae 13(10) or
14 (37), abdominal vertebrae 25(12), 26(29), 27(5) or 28(1), caudal vertebrae
14(2), 15(25), 16(17) or 17(1), and total vertebrae 40(6), 41(28), 42(11) or
43(2). Sexual dimorphism
Unknown.
Colour
The overall colour is yellowish-white with the flank and back marbled or with
blotches of varying shape, often sub-oval and sometimes coalesced. The blotches
extend onto the adipose crest. Blotches are irregularly arranged anteriorly but
may line up in upper, mid and lower flank rows posteriorly The lower row may be
reduced or absent. The head is covered by dark grey dots. The dorsal,
caudal and pectoral fin are strongly pigmented on the rays, sometimes appearing
as 3-4 elongate strips lining up as bands, or as almost continuous strips. The
anal and pelvic fins lack pigment. There is a wide irregular bar at the caudal
fin base. Size
Attains 92.0 mm standard length.
Distribution
Known from the Namak Lake basin, an Iranian endemic.
Zoogeography
This species may have its origins from its Caspian congener as is seen with
other taxa in this family and Cyprinidae.
Habitat
Details are unknown. The type series was collected in a small salt river (3.5‰).
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Molecular studies would help define its distinction from P. malapterura. Sources
Type material: The holotype (IZA 7831) and paratypes (IZA 7832) of Paracobitis iranica,
see above. Other material: CMNFI 1979-0252, 1, 53.7 mm standard length, Markazi, jube at Baqerabad (34º55'N, 50º50'E);
CMNFI 1979-0253, 1, 58.9 mm standard length, Markazi, Qareh Chay west of Baqerabad (34º52'N, 50º49'E);
CMNFI 1980-0154, 8, 45.8-80.8 mm standard length, Markazi, Karaj River below village (35º47'N, 50º58'E);
CMNFI 1980-0156, 24, 28.4-63.6 mm standard length, Markazi, Karaj River near village (35º47'N, 50º58'E);
CMNFI 1993-0155, 2, 53.4-58.7 mm standard length, Markazi, Sharra River near Khosbijan (34º07'N, 49º23'E);
CMNFI 2007-0121, 5, 45.3-98.3 mm standard length, Hamadan, Qareh Su basin north of Razan (ca. 35º25'N, ca. 49º02'E);
CMNFI 2008-0152, 1, 71.9 mm standard length, Namak Lake basin (no other locality
data); USNM 205921-22, 6, 25.9-34.6 mm standard length, Markazi, Baragon River
(ca. 36º00'N, ca. 50º50'E). Paracobitis longicauda
Common names
sagmahi-ye kakoldar-e sharqi (= eastern crested loach).
[vostochnyi grebenchatyi golets or eastern crested loach in Russian].
Systematics
May be a subspecies or synonym of P. malapterura. Has been placed in the genus Adiposia Annandale and Hora, 1920. Banarescu and Nalbant (1964) restrict P. longicauda to basins
in Central Asia (e.g. the Amu Darya) and not Iran. Cobitis longicauda was originally described from the Ak-Darya in the Zeravshan River basin of Uzbekistan and the holotype is in the
Zoological Institute, St. Petersburg under ZISP 2686 (Eschmeyer et al., 1996).
Bănărescu and Nalbant (1995) place this species in Paracobitis.
Key characters
This species is distinguished from P. malapterura by distinct scales and larger size
(including a longer caudal peduncle).
Morphology
Dorsal fin unbranched rays 2-3, branched rays 7, anal fin unbranched rays 2-3, branched rays 5, pectoral fin branched rays 9-10,and pelvic fin branched rays 7.
Total vertebrae 42-44. Caudal fin truncate. Large fish are
scaled on the flanks, dorsal crest and belly, scales being visible to the naked eye in contrast to the few scales needing a microscope to be visible in P. malapterura and in P. rhadinaea.
Caudal peduncle long, about 4.5-4.6 in standard length.
Sexual dimorphism
None reported. Colour
Colour is very similar to P. malapterura with spots sometimes loop-shaped and sometimes rounded.
Size
Reaches 20.0 cm.
Distribution
Found in the Tedzhen and Murgab rivers of Afghanistan and Turkmenistan and in the Aral Sea basin, and in the Tedzhen
(= Hari) River basin of Iran.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
This species is too poorly known in terms of biology and numbers for an effective conservation assessment.
Further work
The biology of this species in Iran needs study.
Sources
Comparative material: ZISP 2686, 1, 140.8 mm standard length, Uzbekistan, Samarkand (); ZISP 4482, 1, 145.7 mm standard length,
Uzbekistan, Zeravshan (?);
ZISP 4483, 4, 107.2- 125.8 mm standard length, Uzbekistan, Zeravshan ();
ZISP 4484, 8, 62.2-96.7 mm standard length, Uzbekistan, Zeravshan ();
ZISP 10362, 4, 31.8-112.1 mm standard length, Turkmenistan, Tedzhen River ();
ZISP 10963, 1, 93.7 mm standard length, Uzbekistan, Samarkand (); ZISP 13300-13301, 3, 93.7-157.6 mm standard length,
Uzbekistan, Pul-e Khatun (?);
ZISP 14510, 3, 119.4-173.4 mm standard length, Uzbekistan, Samarkand (); ZISP 33105, 9, 30.4-98.6 mm standard length, ?, Ak-Darya ().
Paracobitis malapterura
Common names
sagmahi-ye kakoldar-e gharbi (= western crested loach), sagmahi-ye juibari or sagmahi-e-jooibari.
[lakh in Arabic; zapadnyi grebenchatyi golets or western crested loach in Russian].
Systematics
The type locality of Cobitis malapterura is given as "Syrie" in Cuvier and Valenciennes (1846) but has not been found there since (Berg, 1948-1949); it does occur in the
Tigris-Euphrates basin however (Coad, 1991b). The specimen was collected by Aucher-Éloy who visited Iran and the specimen may in fact be from there. It is possible that the type locality is in the
Caspian Sea basin of Iran although Banarescu and Nalbant (1964) give the Tigris-Euphrates basin which extends though Syria for the distribution of P. malapterura malapterura.
Kottelat (2012) notes that the specimens were "sent from Syria" and not
necessarily collected there. Bănărescu and Nalbant (1995) places this species Paracobitis.
Material from the Kor River basin may be this species but given that these
are south of the Namak Lake basin where a distinct species occurs (P.
iranica, q.v.), it seems likely to be distinct. Available
material is limited to three specimens with no material for DNA (see below). Cobitis longicauda Kessler, 1872 is possibly a synonym. Banarescu and Nalbant (1964) restrict P. longicauda (q.v.) to basins in Central Asia (e.g. the Amu Darya) and not
Iran. Nemacheilus macmahoni Chaudhuri, 1909 has been advanced as a synonym or subspecies (Nikol'skii, 1947; Berg, 1948-1949; 1949; Banarescu and Nalbant, 1964) but see the review in
Bănărescu and Nalbant (1966) and below under P. rhadinaea. Banarescu and Nalbant (1964) consider fish from Sistan and the Caspian Sea basin of Iran to be P. malapterura macmahoni.
Filippi's (1865) record of Cobitis merga (Krynicki, 1840) from "fiumicelli di Sartschem e di Sainkalé" was P. malapterura; these localities being in the Safid River basin near
the falling of the Zanjan River into the Qezel Owzan of Safid Rud (Berg, 1948-1949) presumably at Sarcham-e Sofla (37°07'N, 47°54'E) and possibly Sa'in Qaleh (36°18'N, 49°04'E) in the Namak Lake basin.
Two syntypes are in the Muséum national d'Histoire naturelle, Paris under MNHN 3962 and B.3070 (formerly MNHN 3962) (Eschmeyer et al., 1996) and measure 125-145 mm total length (Bertin and
Estève, 1948).
Key characters
This is the only Paracobitis in the Caspian Sea basin and locality is
key (see above under P. iranica). The caudal peduncle is shorter
than in P. longicauda, 5.6-6.3 times in standard length (versus about
4.5-4.6). Morphology
Dorsal fin with 2-3 unbranched and 6-8 branched rays, anal fin with 2-3 unbranched and 5 branched rays, pectoral fin branched rays 10-12 (Nikol'skii (1947) gives 7-10) and pelvic fin branched rays 5-7.
Caudal fin slightly emarginate. There is a well-developed dermal crest or adipose fin behind the dorsal fin to the caudal fin base. Scales are scattered on the posterior body in large fish but need
magnification to be seen. The lateral line extends almost to the caudal fin. The dentiform process of the upper jaw is well-developed and fits in a lower jaw groove. The lips, especially the lower one,
are strongly plicate. The eyes are small and widely spaced. The adipose crest is
of variable height, lower than or similar to that figured in the description of
P. iranica. Caudal peduncle short, 5.6-6.3 times in standard length. The gut is straight posteriorly.
Meristics for Iranian material: branched dorsal fin rays 7(19),
branched anal fin rays 5(19), branched pectoral fin rays 8(4), 9(35) or
10(8), branched pelvic fin rays 6(6) or 7(13), predorsal fin vertebrae 13(3) or
14 (16), abdominal vertebrae 25(4), 26(14) or 27(1), caudal vertebrae
15(9), 16(9) or 17(1), and total vertebrae 40(1), 41(11), 42(5) or
43(2). Sexual dimorphism
The cheeks are distended in some fish and this is believed to be a sexual character.
Colour
The top and sides of the body and head are mottled or reticulated with grey and some yellowish pigment. The reticulations may be very fine, giving a more mottled appearance.
A reticulated pattern is found in larger fish; young fish have a less
reticulated pattern with more blotches on the flank, including in some fish on
the lower posterior flank (cf. P. iranica description). The belly and lower head
surface are white. The flank reticulations or blotches extend onto the caudal peduncle crest. When touching the dorsal margin of the crest, the reticulations make the crest there dark, otherwise the crest is a light
creamy colour along the margin and, in some fish without reticulations reaching the margin, the whole edge is light. The lateral line is white, sometimes in marked contrast to the rest of the flank. The
dorsal fin has darkly pigmented rays, sometimes broken into series of spots, but
in larger fish the whole ray is pigmented, except distally so the fin margin is
white. The caudal fin has a series of 4-5 small spots elongated along the rays, the middle series being the blackest.
Large fish have continuous pigment along the rays, except distally so the margin
of the fin is white. The dorsal margin
of the caudal fin may have 2-4 isolated spots. The pectoral fin has dark pigment along the rays or 2-3 series of small spots. The pelvic and anal fins have 1-2 series of grey spots and the pelvic fin may
have only 1-2 spots. Pelvic and anal fins may be immaculate. At the front or along the base of the anal fin there is a broad pigmented band. There may be a dark,
broad zig-zag bar at the caudal base, merging
dorsally and/or ventrally with flank botches. The barbel bases are all darkly
pigmented especially the second pair. The iris is silvery. Size
Reaches 15.0 cm.
Distribution
This species is found in the Caspian Sea basin of Iran in rivers from the Safid to the Atrak including the
Qezel Owzan, Sardab, Haraz, Ramian, Neka, Chalus, Tonekabon, Shahrud, Babol, Tajan, Karasu,
Madar Su, Gorgan
rivers, Sarchem near the Zanjan-Safid river junction, Bandar Gaz in Gorgan Bay (Laptev, 1934; Nikol'skii, 1947; Kiabi
et al., 1994; 1999; Jolodar and Abdoli, 2004; Abdoli and Naderi, 2009; A.
Abdoli, pers. comm.).
Note that limited material from the southern Kor River basin (three fish, see
below) may represent a distinct species. Zoogeography
See family account.
Habitat
This species appears to favour deeper water and stronger current than other sympatric loaches.
Age and growth
Tabiee and Abdoli (2005) found a sex ratio of 4:1 (male:female) in the Zarringol River of Golestan Province. Average total length was 59.07 mm for males and 82.42 mm for females. Condition factor was
2.6 for females and 1.97 for males. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 48 Iranian fish measuring 2.67-7.43 cm standard length. The a-value was 0.0126
and the b-value 2.628 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length
increases). Another study in the Zarringol River by Patimar et al. (2009)
found maximum ages of 3+ years for males and 4+ years for females, length-weight
relationship was W = 0.020L2.62 for males, 0.002L3.81 for
females and 0 .008L3.08 for the population, growth was positively
allometric, and sex ratio was 1:1.27 in favour of females (cf. Tabiee and Abdoli,
2005). The difference with Tabiee and Abdoli (2005) who found negative
allometric growth was attributed to environmental conditions changing between
years of sampling. Golzarianpour et al. (2011) give a
length-weight relationship for fish identified as this species from the Jaj
River with a (intercept) being 0.007 and b (slope) 3.01 and from
the Gharechay River with a (intercept) being 0.001 and b (slope)
2.819. Food
The Zarringol fish were carnivorous with chironomids making up 65.3% of the diet
(Tabiee and Abdoli, 2005) or had a diet dominated by Plecoptera (72.4% and
Trichoptera (20.8%) (Patimar et al., 2009).
Reproduction
The Zarringol River examined by Patimar et al. (2009) reproduced in
April-June with highest GSI in May. Spawning was prolonged, over about 2 months.
Absolute fecundity reached 1180 eggs with a maximum diameter of 2.8 mm. Parasites and predators
A Diplostomum species is recorded from the muscles of this nemacheilid in the Shirud of Mazandaran (Alghmandi and Dalimi Asi, 2000).
Economic importance
Kiabi et al. (1999) consider this species to be of least concern in the south Caspian Sea basin according to IUCN criteria. Criteria include medium in numbers, habitat destruction, widespread
range (75% of water bodies), present in other water bodies in Iran, and present outside the Caspian Sea basin.
Conservation
Little is known of the biology and population numbers so no conservation assessment can be made.
Further work
The systematics of this species and its relatives need further study.
Sources
Iranian material: CMNFI 1979-0481, 1, 77.9 mm standard length, Mazandaran, stream 3 km west of Ghalahleekesh (37º18'30"N, 55º31'E);
CMNFI 1979-0486, 18, 12.7-63.2 mm standard length, Mazandaran, stream in Atrak River drainage (37º44'N, 56º18'E);
CMNFI 1979-0489, 2, 27.4-45.3 mm standard length, Mazandaran, stream in Atrak River drainage (37º50'N, 55º53'E);
CMNFI 1980-0160, 3, 35.4-40.0 m standard length, Caspian Sea basin (no other
locality data); CMNFI 1991-0157, 2, 70.4-73.0 mm standard length, Mazandaran, Ramian River (36º58'N, 55º07'E);
CMNFI 1993-0145, 2, 53.4-57.0 mm standard length, Mazandaran, Qareh Su (no other locality data);
CMNFI 2008-0109, 2, 83.4-83.9 mm standard length, Mazandaran, Gol-e Ramian 35 km
east of Gorgan (37º01`N, 55º09È). Comparative material: ZISP 25788, 1, 62.2 mm standard
length, ?, Sumbar River at Aidere (?).
Paracobitis rhadinaea
Common names
See genus account. Fowler and Steinitz (1956) refer to a fish from Sistan known locally as mahrmahé (sic, presumably mar mahi, snake fish) and this may refer to this species which has an
elongate body.
Systematics
Bănărescu and Nalbant (1995) and Nalbant and Bianco (1998) place this species in Paracobitis.
The Catalog of Fishes spells the trivial name as rhadinaeus but Kottelat
(2012) notes the name is an adjective. Nemacheilus macmahoni Chaudhuri, 1909 described from the "affluents of the Helmand" is a synonym according to Bănărescu and Nalbant (1966)
who refute the opinions of Nikol'skii (1947) and Berg (1948-1949; 1949) who consider macmahoni to be identical to P. malapterura. Earlier Banarescu and Nalbant (1964) placed fish from Sistan
and the Caspian Sea basin of Iran as Nemacheilus malapterurus macmahoni. P. malapterura has both lips strongly furrowed, pelvic fin origin under the dorsal fin origin rather than behind,
better developed scales which are also present on mid-flank, and a colour pattern of numerous oblique bands.
Affluents (=headwaters) is an error for effluents (= delta) and the species
is not from the upper reaches of the Helmand River basin in Afghanistan as
suggested by Kottelat (2012) but from the Helmand delta in Sistan. P. rhadinaea is distinguished from macmahoni by Annandale and Hora (1920) in having an extremely short posterior diverticulum and minute vesicle in the swimbladder, by the absence of
scales, a more elongate body, smaller, narrower and less flattened head, and by differences in the profile of the body.
A syntype of Nemacheilus rhadinæus (ZSI F1240/1) is in the Zoological Survey of India, Calcutta under the name Adiposia rhadinaea and the holotype of Nemacheilus macmahoni (ZSI
F1222/1) is also there under the name Adiposia macmahoni (Menon and Yazdani, 1968). Two syntypes listed as Nemacheilus rhadinaeus from "Sistan" are in the Natural History Museum,
London (BM(NH) 1905.11.29:28-29, 2, 137.8-209.1 mm standard length).
A specimen in the Zoological Institute, St. Petersburg (ZISP 24413, 76.5 mm standard length) is from "Helmand delta, northwest of Jalalabad, Seistan, XI 1918, Indian Mus. (Dr. Hora)"
according to Berg (1949) and could be a syntype of Nemacheilus macmahoni (Eschmeyer's "Catalog of Fishes", downloaded 20 May 2008). However, this taxon was described from a single
specimen ? . The jar bears a label on the outside reading "N. malapterurus XI 1918 Indian Museum S. L. Hora Delta of Helmand near Jalabad" and another label reads "Adiposia
macmahoni Randa stream 4 mls N.W. of Jalabad Seistan. N. Annandale coll."
Adiposia Annandale and Hora, 1920 (type species Nemachilus macmahoni Chaudhuri, 1909, and including Nemacheilus longicaudus and N. rhadinaeus according to Annandale and Hora
(1920)) is a synonym of Paracobitis. Key characters
The body bears some scales and there are modally 10 branched pectoral fin
rays in contrast to P. vignai. Morphology
macmahoni:- ? check The head is broad and flattened and the small eyes are dorsal. The dorsal profile is slightly convex behind the head but the dorsal and ventral profiles soon become straight
and nearly parallel, or thicker in front and tapering behind the dorsal fin. Body depth 5.8 in total length. Caudal fin rounded. Oval scales are found on the posterior part of the body in adults (not in
rhadinaea in Regan (1906) and Annandale and Hora (1920)) or scales very small and deciduous all over the body. Nostrils are nearer to the eye than the snout tip. The two anterior pairs of barbels
reach back to the nostril level and the posterior pair to the anterior or to middle or to the rear of the eye. The upper lip is minutely tubercular and the lower lip is widely interrupted in the middle.
Scale radii are few and widely spaced, on all fields. Material identified originally as macmahoni has the pelvic origin behind that of the dorsal fin origin level, the caudal fin is slightly
emarginate, the dorsal fin edge is straight, the anus is some distance in front of the anal fin, a well-developed adipose dorsal ridge runs from the dorsal fin to the caudal fin base, the lateral line is
almost complete, scales are restricted to the last third of the body, being small, rounded and far apart, lips are almost smooth, and the intestine has a single loop posteriorly. The
description of rhadinaea is short. The snout is longer than the postorbital distance, body depth is 7-10 times in body length, head length 5.0-5.5 times in body length, the mouth cleft extends to
below the nostrils, lower lip interrupted medially, outer rostral barbel as long as maxillary barbel reaching back to or beyond nostrils, no scales, dorsal fin origin nearer tip of snout than caudal
fin base, caudal fin slightly emarginate, caudal peduncle 2.0-2.75 as long as deep, 5.0-5.3 in length of fish.
Dorsal fin with 2-4 unbranched (first two minute and not readily visible) and 7,
rarely 8, branched rays, anal fin with 2-4 unbranched (first two minute) and 5 branched rays,
pectoral fin branched rays 9-10, usually 10, and pelvic fin branched rays 6-8,
usually 7. Scales are highly deciduous and not
always present on old preserved material. The dorsal fin rounded. There is a well-developed post-dorsal fin crest and a slight ventral crest on the caudal peduncle. The pelvic fin origin lies just in front
of the mid-point of the dorsal fin base. There is an adipose tissue flap at the pelvic fin base. The anterior nostril is a tube followed immediately by a horizontal slit. The bony upper jaw has a slight
protuberance and the lower jaw is curved and not indented.
Meristic values for Iranian specimens including types and macmahoni are:- dorsal fin branched rays 7(33) or 8(1), anal fin branched rays 5(34), pectoral fin branched rays 9(2) or 10(32), pelvic
fin branched rays 6(2) or 7(32), and total vertebrae 41(1), 42(1), 43(4), 44(6),
45(6) or 46(2)..
Sexual dimorphism
None reported, the pectoral fin being identical in both sexes in material identified as macmahoni (Bănărescu and Nalbant, 1966).
Colour
Living fish identified as macmahoni are pale olivaceous fading to silvery-white on the belly. The head and upper part of the body are irregularly spotted and darker. Some fish are pale yellowish
without markings or with faint marbling. All fins are tinged a dull red, most evidently on the caudal fin, and are obscurely marked with small dark spots. There is a dark band at the caudal base. Preserved
material identified originally as macmahoni is whitish with 9-21 irregular, brownish spots along the flank, other spots are present dorsally and smaller ones between the dorsal and lateral rows.
There are 3 rows of minute spots on the dorsal and caudal fins and 2 rows on the pectoral fin (Bănărescu and Nalbant, 1966). Types of macmahoni are brown all over,
darker dorsally, barbels a lighter brown, dorsal and caudal fins with darker bands, pectoral also slightly banded but anal and caudal uniform light brown.
P. rhadinaea has large oblong or rounded
dark spots on the back and sides, dorsal and caudal fins with small spots and red tinged, lower fins pale and immaculate although pectoral, and to a lesser extent pelvic, fins may be red tinged.
Size
Attains 28.8 cm as macmahoni.
Distribution
This species is probably restricted to the Sistan basin of Iran and presumably Afghanistan. Bănărescu and Nalbant (1966) place this species in the Atrak and Safid rivers of the Caspian Sea
basin, the Abkhar River of central Iran, probably most of Iran, the Helmand drainage and the Tedzhen River, evidently confusing it with P. malapterura and P. iranica. Abdoli
(2000) lists as questionably from the Bejestan, Kerman-Na'in and Dasht-e Lut basins, from the middle and lower Halil and Bampur rivers of the Hamun-e Jaz Murian basin, and from the Simish and the river to its north in
the Mashkid River basin.
Zoogeography
The closest relatives of this species are P. malapterura and P. longicauda (q.v.) in Iran (Bănărescu and Nalbant, 1966).
Habitat
Annandale and Hora (1920) note that Adiposia macmahoni was healthy buried some inches in mud when cyprinids died in foul water above.
Age and growth
Unknown.
Food
Stomach contents include cyprinid fish remains and mayfly larvae (Annandale, 1921).
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
Unknown.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus rhadinæus (BM(NH) 1909.11.29:28-29), see above.
Iranian material: CMNFI 1979-0222, 11, 16.8-28.8 mm standard length, Sistan, jube 2 km south of Lutak (30º46'30"N, 61º24'E);
CMNFI 1979-0223, 1, 23.9 mm standard length, Sistan, ditch 1 km south of Lutak (30º45'N, 61º24'E);
CMNFI 1979-0228, 3, 55.1-119.8 mm standard length, Sistan, ditch 1 km from Zabol (31º02'30"N, 61º31'E);
CMNFI 1979-0229, 3, 87.4-115.5 mm standard length, Sistan, ditch 5 km from Zabol (31º03'N, 61º33'E);
CMNFI 1979-0231, 1, 22.6 mm standard length, Sistan, jube 3 km from Zabol (31º01'N, 61º32'E);
CMNFI 1979-0232, 2, 82.5-95.6 mm standard length, Sistan, jube 11 km from Zabol (ca. 30º58'30"N, ca. 61º36'E);
CMNFI 1979-0233, 1, 68.7 mm standard length, Sistan, ditch at Deh Vazi (ca. 30º57'N, ca. 61º38'E);
CMNFI 1979-0234, 1, 85.5 mm standard length, Sistan, effluent of Hirmand River near Zahak (30º54'N, 61º40'E);
CMNFI 1979-0237, 2, 17.5-25.2 mm standard length, Sistan, ditch 18 km south of Zabol (30º53'N, 61º27'30"E);
CMNFI 1979-0238, 2, 23.6-27.7 mm standard length, Sistan, ditch 11 km south of Zabol (30º57'N, 61º27'30"E);
BM(NH) 1920.1.20:31-34, 4, 79.6-109.5 mm standard length, Sistan, northwest of Jalalabad (no other locality data);
ZISP 24413, 1, 76.5 mm standard length, Sistan, Randa stream 4 miles northwest of Jalalabad (?).
Paracobitis smithi
(Heckel, 1847)
(Derzhavin, 1934)
Character/Taxon
araxensis
bergianus
brandtii
lenkoranensis
Distribution
Upper Aras River basin in Turkey
Caspian, Namak and Orumiyeh basins in Iran
Upper and middle Kura River and Aras River, Georgia and Azerbaijan
(and Aras River in Iran?)
Lenkoran, Azerbaijan (and western Caspian Sea basin in
Iran?)
Caudal fin margin
almost straight
truncate, slightly emarginate
deeply emarginate, pointed lobes, strongly forked
deeply emarginate, forked
Caudal peduncle length/depth
2.3 in type examined by me (ZISP 36846)
3.3
(Derzhavin, 1934); 2.6 in type examined by
me (ZISP 25433)
2.2-3.0 (Berg, 1948-49), 2.9-3.2 in Abdurakhmanov (1962); 1.7 in
small type (19.3 mm SL) examined by me (BMNH 1897.7.5:39))
2.0-2.6 (Abdurahkmanov, 1962)
Standard length/body depth
5.1-6.5 (Banarescu et al.,
1978); 6.3 in type examined by me
6.5-8.5
(Derzhavin, 1934); 5.6 in type examined by
me
6.2-6.6 (Berg, 1948-49);
4.9-7.6 (Banarescu et al., 1978);
5.7 in type
examined by me
-
Suborbital flap in males
present
present
present
absent
(Kessler, 1877)
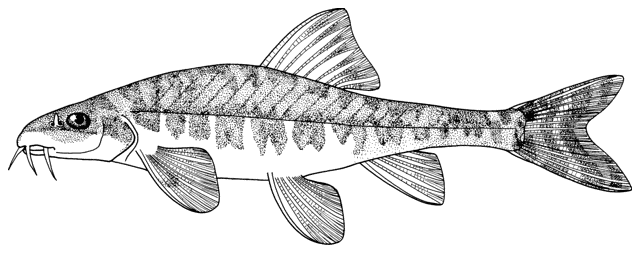

(Berg, 1910)
(Heckel, 1843)
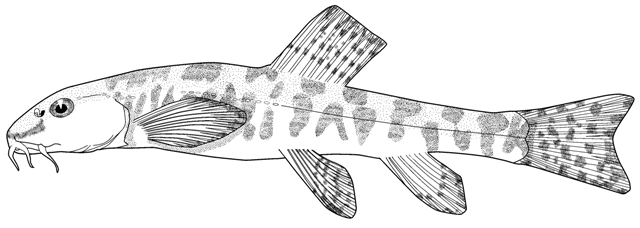
(Bănărescu and Nalbant, 1966)
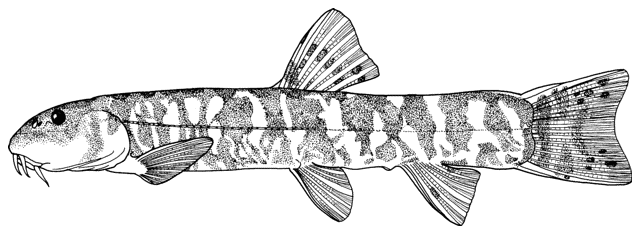

Golzarianpour, Abdoli and Freyhof, 2011
(Abdurakhamanov, 1962)
(Krupp and Schneider, 1991)
(Heckel, 1847)
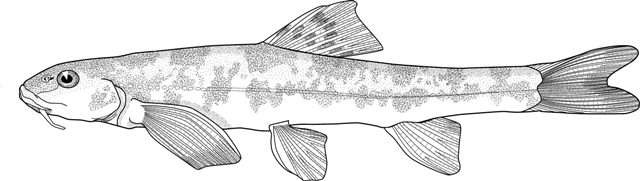

(Nalbant and Bianco, 1998)
Bleeker, 1863
(McClelland, 1842)
(Bănărescu and Nalbant, 1966)
Nalbant and Bianco, 1998
(Kessler, 1872)
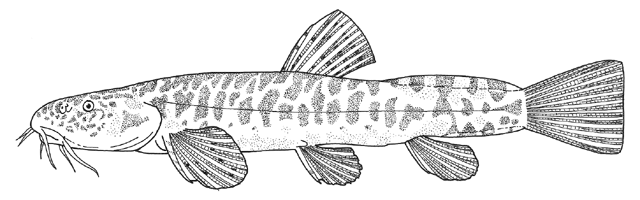

(Valenciennes, 1846)
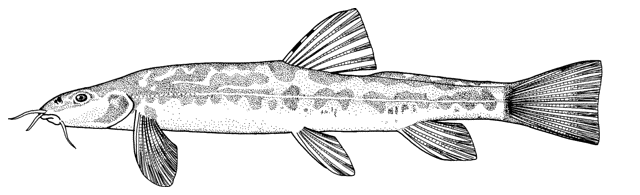
(Regan, 1906)
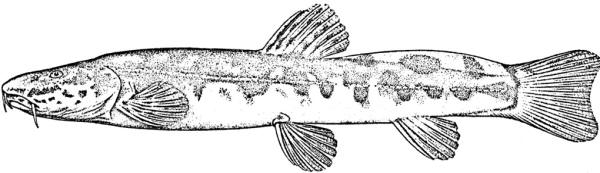
(Greenwood, 1976)
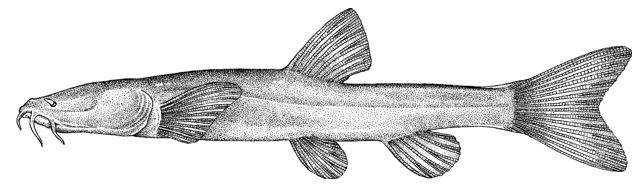
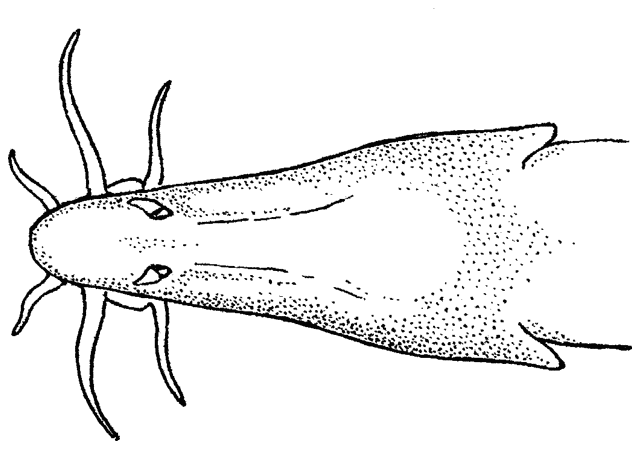
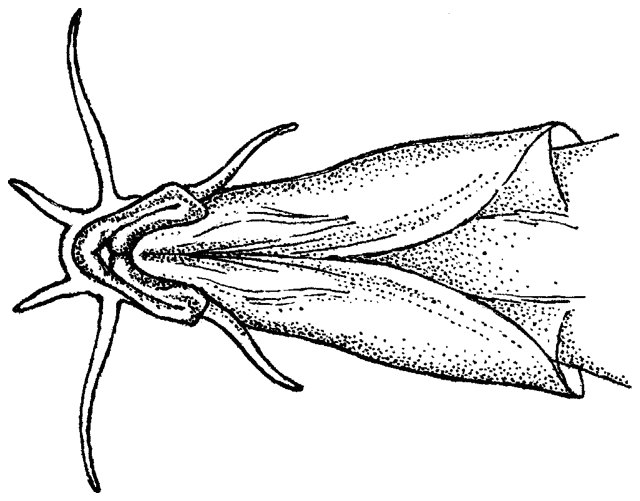
Ventral view of head



Three photographs above courtesy of Amir Hosin Zalaghi, 11-19 May 2010
Common names
sagmahi-ye gharzi (= cave loach), mahi kurghar (= cave fish).
[blind loach].
Systematics
This species is named for Anthony Smith who collected the holotype. Accounts of Smith's searches for cave fishes in Iran are given in his two books (Smith, 1953; 1979). The holotype is in the Natural
History Museum, London under BM(NH) 1976.6.28:1, was collected in April 1976, is apparently an immature male and is 35.5 mm standard length. Locality data is given below.
Greenwood (1976) placed this species in the catchall genus Noemacheilus (correctly
spelled Nemacheilus) pending an adequate revision of the subfamily Nemacheilinae and since little purpose would
be served by erecting a new genus for a fish with such features as eyelessness and depigmentation found in common with other unrelated cave-dwelling fishes. Greenwood (1976) considers its relationship to
lie with species in the subgenus or genus Paracobitis as does Nalbant and Bianco (1998).
Kottelat (2012) tentatively follows the latter authors. Key characters
The only eyeless, depigmented nemacheilid reported from Iran.
Morphology
Dorsal fin with 3 unbranched and 7 branched rays, anal fin with 3 unbranched and 5 branched rays, pectoral fin with 10 branched rays and pelvic fin with 5-6 branched rays. In specimens seen by me
dorsal fin with 7(5) branched rays, anal fin with 5(5) branched rays, pectoral fin with 10(5) branched rays and pelvic fin with 5(5) branched rays. The caudal fin is unusual in having only 14(5) branched
rays. Vertebrae 37-38. check on my fish? The barbels are short with the middle pair the longest. There are no scales. The lateral line is interrupted irregularly and there are more pores on the posterior
half of the body. A long adipose fin is present dorsally, most obvious in young, and a weaker ventral ridge is present. The mouth is subterminal, lips are weakly to moderately papillose, and there is a
horny ridge on the dentaries and on the upper jaw. The middle pair of barbels is the longest. The internarial flap is long. Gill membranes are broadly attached to the isthmus. The gut is short with a
single transverse loop.
Sexual dimorphism
Unknown.
Colour
A dead white with the red of blood visible as a pale pink cast and a deeper red at the gills visible through the gill cover. Small, straw-coloured fat globules are visible under the skin of formalin
preserved fish over the whole head and body with concentrations in the orbits and the fin bases, particularly the dorsal and anal bases. The viscera are visible through the body wall. There is no
peritoneal pigment.
Size
Attains 64.5 mm total length.
Distribution
Found only at "Kaaje-ru" above the garden "Bagh-e Loveh", "Lowa" or "Levan" (probably Loven at 33°04'N, 48°37'E) which is about 4 km from kilometre 382 on the
railway from Bandar Shapur and approximately 12 km north of the railway station Tang-e Haft. The stream below the cave locality is the "Ab-e Serum" which runs into the "Ab-e Zezar"
which is a tributary of the Dez River. The locality is at 33°04'38.6"N, 48°35'33.1"E in Lorestan Province. Further locality details are given in Bruun and Kaiser (1948) and under the cyprinid
Iranocypris typhlops.
Zoogeography
Endemic to Iran, its relationships to other species are unknown. It shares the cave habitat with another eyeless species, Iranocypris typhlops, a member of the family Cyprinidae. This blind cave
species is placed in a world-wide context by Proudlove (1997a; 1997b).
Habitat
Known only from a well-like but natural outlet of a subterranean
system. The outlet overflows to form a small stream from January to
May (Smith, 1979) during the snow-melt period in the Zagros Mountains
but in April to June this flow ceases (the precise timing of flow and
its cessation is estimated from villager's comments and scientific
visits and also varies with precipitation). The pictures above show flowing
water in May 2010. The well area is about 5
by 3 m and gradually decreases as the year progresses. Divers
descended to a depth of 60 feet (= 18.3 m) in 1977 in the "well" until the
resurgence narrowed (Farr, 1977). A rope was let down by R. Mehrani (pers. comm.,
2000) and reached 23 m before the rope ran out and yet it was not at
the bottom. Smith (1979) reports divers descending to 60-70 feet (18.3- 21.3 m). The
pool shelves deeply under the cliff rearwards but the whole pool
surface is exposed to light. There is no vegetation in the pool except
for some encrusting algae on the rocky sides. The shale fragments
forming the outermost floor of the pool have a thin layer of mud on
them which may contain algae.
It seems probable that a complex of flooded but narrow and
inaccessible passages is the habitat of this species and the well is
merely the surface manifestation of this complex (Bruun and Kaiser,
1948; Smith, 1978; Banister, 1992). There is a smaller pool (about 2 m
across narrowing rapidly inside) and flowing exit stream lower down
the gorge, about 50 m away from the main locality, where an Iranocypris
typhlops
was seen but not caught in December 2000 (Smith (1979) also
tentatively reports sighting a fish here). This is assumed to be
evidence of the interconnectivity of subterranean passages. The main
pool was not flowing at this time. The stream from the smaller pool
increases in flow downstream, possibly tapping more groundwater, and
eventually has a moderate flow. No fish were seen in it. The stream
falls over a high waterfall (estimated at 10-15 m high by Smith (1979)
which seems about right) so the well localities are isolated from the
local fishes in the main river. The main river houses Garra rufa
and Nemacheilus species s.l.. The stream shows evidence of recent higher
flow which tends to confirm overflow from the main well.
Sampling in December 2000 recorded a water temperature of 18.5°C, pH 7.5 and
a conductivity of 334 µS. More photographs of the habitat can be seen under the
account of Iranocypris typhlops (Cyprinidae).
A captive specimen attempted to climb out of a glass tank, almost its whole body being out of the water before it slid back. It did not hide from or
react to light and spent most of the time resting on the tank bottom, moving along the bottom or more often swimming actively around the tank (Greenwood, 1976).
Amir Hosin Zalaghi reports that this species swims faster than Iranocypis
tyhplops (pers. comm., 10 August 2010).
Age and growth
Unknown.
Food
Unknown but a captive specimen was fed on mosquito larvae.
Reproduction
Unknown.
Parasites and predators
Unknown but there are probably no predators in the cave environment.
Economic importance
Robins et al. (1991) list this species as important to North Americans. Importance is based on its use in textbooks.
Conservation
A fine of 10,000 rials is imposed specifically for illegal fishing of this species (Anonymous, 1977-1978), more recently 100,000 rials (N. Najfpour pers. comm., 2008). It is on the IUCN 1994 Red List
of Threatened Animals as one of two rare fish species from Iran (see also Iranocypris typhlops) and is on the 2000 IUCN Red List as VU D2 (Vulnerable, acute restriction in its area of occupancy,
also on subsequent Red Lists; see also Proudlove (2001)). Coad (2000a), using 18 criteria, found this species to be one of the top 4 threatened species of freshwater fishes in Iran.
B. Sandford (in litt., 1979) considered this fish to be endangered. The cave appeared to be a recently collapsed system and the network of fissures could be quite small. Coupled with recent
collecting the number of extant specimens may be quite low.
This species is much rarer than the co-occurring Iranocypris typhlops, by at least an order of magnitude. Including the holotype, about 14 specimens are known to have been collected
(see below; plus 2 in Muze-ye Melli-ye Tarikh-e Tabi'i, Tehran (MMTT 1227-1228) and 6 collected by students of A. Abdoli, Shahid Beheshti University, Tehran in 2001).
Further work
?
Sources
Type material: Holotype of Noemacheilus smithi (BM(NH) 1976.6.28:1), see above.
Iranian material: CMNFI 2007-0123, 5, 24.1-52.9 mm standard length, type locality, 28 January 1977.
Further information on the habitat is in the account of Iranocypris typhlops.
Paracobitis vignai
Common names
sagmahi-ye Sistan.
[Sistan loach].
Systematics
The species is named for Professor Augusto Vigna Taglianti, La Sapienza University, Rome. The holotype is 89.0 mm standard length (86.5 mm standard length when measured by me) collected from
"Nahr Taheri, Zabol, Seistan" on 9 October 1977 by A. Vigna and is deposited in the (Department of Zoology, University of Naples (IZA 7838). Paratypes are from the same locality and number 24
specimens, 35.0-78.0 mm standard length (IZA 7839) and 7 specimens, 44.0-49.0 mm standard length (Institutul Stiinte Biologice, Bucharest, ISBB uncatalogued). One specimen is in the American Museum of
Natural History (AMNH 40946), presumably a paratype from one of the preceding
series and 3 specimens are in the Canadian Museum of Nature, Ottawa under CMNFI
2012-0007
(paratypes from IZA 7839, 39.0-63.4 mm standard length). Kottelat (2012)
gives a publication date of 1999 but the journal is dated November 1998. Key characters
This species is scaleless and modally has 9 branched pectoral fin rays in
contrast to P. rhadinaea with some scales and modally 10 pectoral rays.
Morphology
Dorsal fin branched rays 6-8 (80% with 7 rays in original description, n = 20), anal fin with 5-6 branched rays (95% with 5 rays), pectoral fin with 8-10 branched rays (85% with 9 rays) and pelvic fin
with 6-7 branched rays (95% with 7 branched rays). Total vertebrae number 44-45.The lateral line extends to the base of the caudal fin. The body is slender and compressed, particularly posteriorly. The head is long and the eyes are
small. The dorsal fin origin is at mid-body (snout tip to caudal fin base). The mouth is arched with strongly furrowed lips that have few papillae. Mental lobes are reduced.
The caudal fin is deeply forked. The dorsal adipose crest or keel on the caudal
peduncle is well-developed. The stomach is syphonal and the
intestine is straight. The anus is placed well anterior to the anal fin origin. The gas bladder capsule has globular chambers and a short duct.
Sexual dimorphism
Unknown.
Colour
The head and body are greyish, lighter ventrally. Dark spots along the back and flank may be occasionally fused. Fine spots are scattered in a reticulated pattern on the body. The dorsal and caudal
fins have dots in rows while other fins are colourless. The caudal fin base has a distinct blackish bar.
Size
Reaches 89.0 mm standard length.
Distribution
Endemic to Sistan.
Zoogeography
Nalbant and Bianco (1998) consider this species to be an epigean form of the blind cave fish P. smithi. The differences are in head shape and mouth and lip morphology, apart from the eyes and
pigment loss typical of ipogean P. smithi.
Habitat
Details are unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
The distinction of another Paracobitis species in Sistan, an area of
interconnected waterways through periodic flooding, could be questioned. The
chief distinguishing features from P. rhadinaea are the absence of scales
(a somewhat variable character in respect of presence and the scales are often
deciduous) and a modal count of 9 pectoral fin branched rays (some rhadinaea
have 9 rays). Fresh material for molecular studies would help re-affirm this
taxon. P. ghazniensis from higher in the Helmand River basin is
distinguishable on colour pattern (flank bars), pelvic fin rays (6 as opposed to
7 in rhadinaea and vignai), caudal peduncle length (mostly
6.4-7.8 in standard length, compared to 5.4-6.2 in rhadinaea and
vignai), and is in a water body isolated at times from the main
Helmand River basin. Sources
Type material: The holotype (IZA 7838) and paratypes (IZA 7839) of Paracobitis vignai,
see above.
Paracobitis sp. Common names
None. Systematics
Paracobitis species are known and described from the Caspian Sea and
Namak Lake basins, far to the north of specimens found in the Kor River basin of
Fars. The latter material represents a new taxon, as yet undescribed.
Multivariatr analyses separate the few Kor River specimens from Caspian and
Namak fish but with no single character or small suite of characters diagnostic
and useable in a key. Key characters
Distribution is the key character. Morphology
Dorsal fin branched rays 7(3), anal fin branched rays
5(3), pectoral fin branched rays
9(1), 10(1) or 11(1), pelvic fin branched rays 5(3), predorsal fin vertebrae 14 (2), abdominal vertebrae 26(1)
or 27(1), caudal vertebrae
17(2), and total vertebrae 43(1) or 44(1).
Sexual dimorphism
Unknown. Colour
Two specimens are faded but the material has evidence of blotches typically
found in Paracobitis species, extending onto the adipose crest, an
irregular and broad caudal fin base bar, pigment on the rays of the dorsal and
caudal fins, and blotches extending to the lower flank posteriorly. Size
Reaches 81.5 mm standard length, possibly larger as only three specimens were
caught.
Distribution
This species is found in the Kor River basin of Fars Province, southern Iran.
Localities include the Kor River proper and a tributary stream. Zoogeography
The relationships of this taxon could lie to the north in the Namak and
Caspian basins or possibly to the west in the Tigris-Euphrates basin. Material
from the latter was not available for study but other taxa from the Kor River
basin show affinities with Mesopotamian ones. Habitat
Unknown in detail. Two fish were collected from a qanat stream at 21°C where
the bottom was gravel and mud with encrusting and submergent plants. Age and growth
Unknown. Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
This species is too small and rare to be of direct economic importance.
Conservation
Since this species is known only from a limited number of specimens, further
studies are needed to ascertain its abundance and distribution. Further work
This taxon needs diagnosis and description. Molecular studies would help
define its distinction from P. malapterura.Another Paracobitis
species may well exist in the Tigris-Euphrates basin (Coad, 2010) but no
material is available - it would throw light on the distinction of the Kor River
population. More collections are needed to record information on biology and distribution.
Source Iranian material: CMNFI 1977-0510A, 2, 74.2-81.5 mm standard length,
Fars, stream in front of Naqsh-e Rostam (29°59`30`N, 52°54È); CMNFI 2008-0255,
1, 51.0 mm standard length, Fars, Kor River (30°00`N, 52°44`58È). Genus Paraschistura
This genus is recently described and not all species have been examined and
ascribed to it or related genera. Many were formerly included in the genus
Schistura McClelland, 1838. The species in this genus are small and are distributed from interior water
bodies of Turkmenistan and from Iranian Baluchestan east to the upper reaches of
the Indus River in Afghanistan and Pakistan. Four species are reported from
Iran. The genus is characterised in particular by a dark black spot or strip always
present at the base of the anterior dorsal fin rays, found only in one other
genus in Iran, Metaschistura (which in contrast has a well-developed caudal peduncle
crest). Note that other genera may have a dark brown blotch or part of a saddle
on the anterior dorsal fin base but this is never the deep black spot of this
genus. The genus is also characterised by an elongate body with dorsal and ventral
profiles almost parallel. The belly is not rounded. The body is compressed anteriorly and the head is compressed or depressed. The caudal peduncle is short
and moderately deep. The snout is usually blunt. The upper lip is furrowed and
is continuous or has a slight median interruption. Lips are simple, being
plicate to almost smooth. The processus dentiformis on the upper jaw is
well-developed in many species and there is a corresponding notch on the lower
jaw but the processus can be absent. Nostrils are closely spaced. The dorsal fin
usually has 7 branched rays and the anal fin 5 branched rays. The caudal fin is
slightly to deeply emarginated. There is no adipose crest or, if present, it is
weakly-developed near the caudal fin and not supported by procurrent rays of the
caudal fin. The lateral line is incomplete, may be very short and rarely reaches
the end of the anal fin level. Scales are absent or rarely present but weakly
developed on the caudal peduncle. A pelvic axillary lobe is present but is short
and does not usually extend beyond the pelvic fin base. Prokofiev (2009) lists various osteological characters that help diagnose the
genus including the absence of a bony bridge between the parietal and pterotic
bones which separates the species from Schistura sensu lato. There is no
preethmoid I bone in the skull. Externally, there is no bony roof to cst,
the fork of cst-cio is not ossified and it is not fused to the
skull. The cst is the supratemporal commissure running across the top of
the head at the rear and cio is the temporal part of the infraorbital
head canal. The free part of the swimbladder is absent, the right and left lobes
of its bony capsule are fully divided by the manubrium, and the fifth trunk
vertebra does not take part in support of the capsule. There is no sexual dimorphism. Colour pattern is distinctive with dark bars
of varying number and form being present. The bar at the caudal fin base is
usually much darker than anterior bars, and is never in the form of a spot.
There is no stripe along the flank although a dark line is rarely present. Paraschistura alta Described from "Afghanistan, Kajkai, Helmand river drainage, north east of Girisk". No Iranian record.
Kottelat (2012) gives a publication date of 1999 but the journal is dated
November 1998, and he retains this species in Schistura.
Paraschistura baluchiorum Possibly a synonym of P. bampurensis according to Nalbant and Bianco
(1998). The type locality is Panjgur on the Rakhshan River of the Hamun-i Mashkel basin in Pakistani Baluchistan and it is also recorded from Afghanistan
in the Helmand River drainage. A cotype is in the Naturhistorisches Museum Wien
under NMW 19851 and is 46.0 mm standard length. Not recorded from Iran by specimens although Abdoli (2000) reports it from the Simish River and a river just north of it, both in the Mashkel basin.
Prokofiev (2009) places this species in Schistura. Paraschistura bampurensis
Common names
sagmahi-ye Bampur.
[Bampur loach]
Systematics
Publication date is given as 1900 in Berg (1949), Bănărescu and Nalbant (1966)
and Kottelat (2012). This may be correct if the volume appeared late as the volume of the publication is for the year 1899; but
note that a footnote and the plate both bear the year 1899.
Nemacheilus baluchiorum Zugmayer, 1912 is possibly a synonym. (Berg (1949) places this species in synonymy with Nemacheilus montanus - see below). Bănărescu and Nalbant (1966)
and Nalbant and Bianco (1998) place S. bampurensis in the subgenus or genus Schistura
but Kottelat (2012) has it in Paraschistura. Berg (1949) placed P. bampurensis in Nemacheilus montanus (McClelland, 1839) but Bănărescu and Nalbant (1966) recognise bampurensis as distinct on its incomplete lateral
line, focal zone of scales larger, dorsal fin positioned more anteriorly, deeper body, and colour pattern without regular bands (Bănărescu and Nalbant (1966: fig 12) for three pattern varieties).
Observations were made by me on the faded syntypes of N. montanus from Simla, 62.8-68.7 mm standard length (BM(NH) 1860.3.19:118-119)
as well as ZISP 8298, 9 specimens 25.3-51.1 mm standard length. Additional characters which distinguish bampurensis and
montanus are as follows. The preorbital process is very strongly developed in N. montanus, being almost as deep as the eye and extending almost an eye length below the lower orbit margin,
extending almost twice the distance of the process in bampurensis types
and being less curved and close under the eye. N. montanus has a slight but obvious keel on the back before the caudal fin while bampurensis are humped there but usually not keeled. The
caudal fin is dark at the base in montanus, not so in bampurensis or not as solid, wide and dark. The flank bars are oblique with the top forward in contrast to
bampurensis, and are less numerous than in bampurensis (5-10
versus 11-22 total, from under dorsal fin insertion to caudal fin but
excluding any caudal fin base bar). N. montanus types have faded bars but
are clearly fewer.
The syntypes of Nemacheilus bampurensis are in the Zoological Institute, St. Petersburg under catalogue numbers ZISP 11698 and 11699 at the localities, dates and number of specimens in Latin as
follows respectively:- "Kjagur prope urb. Bazman. 4. VII (6)" and "Kaskin prope urb. Bazman. 6.VII (4)" (Nikol'skii, 1899). Berg (1949) gives both these localities as between Bazman
and Bampur. However, ZISP 11698 comprises 9 specimens, 35.1-44.6 mm standard length and ZISP 11699 comprises 4 specimens not listed as types on the jar, 36.8-44.5 mm standard length.
Key characters
The male has a characteristic, moveable protuberance directed downward on the preorbital bone at the antero-ventral corner of the eye. It lies close to the eye, extends slightly below the lower orbit,
and curves partly around the orbit.
Morphology
Dorsal fin with 2-4 unbranched rays (anteriormost embedded in flesh and
difficult to see) and 6-7 branched rays, anal fin with 2-3 unbranched and 4-5 branched rays,
pelvic fin branched rays 6-7, and total vertebrae 34-36. Lateral line incomplete, ending in front of the dorsal fin level.
Scales well-developed but the anterior third to a quarter of the body is scaleless. The posterior nostril is ovoid, slanting postero-dorsally. Barbels are large and the third pair is usually the largest
although in some fish the second pair is the largest (Saadati, 1977). The lower lip is divided, lips are corrugated, and the upper jaw process is rounded, overlapping the lower one. There is a fleshy
pelvic axillary process. Caudal fin slightly emarginate.
The type series (ZISP 11698) has dorsal fin branched rays 6(3) or 7(6); anal fin branched rays 4(1) or 5(8); pectoral fin branched rays 9(8) or 10(1); pelvic fin branched rays 6(2) or 7(7); and flank
bars 14(2), 15(3), 17(1), 19(2) or 22(1).
Meristic counts for Iranian material:- Dorsal fin branched rays 6(3) or 7(73), anal fin branched rays 4(1) or usually 5, pectoral fin branched rays 8(2), 9(59) or 10 (10), pelvic fin branched rays 6(7),
7(51) or 8(2), total vertebrae 34(19), 35(17) or 36(4), and flank bars 10(1), 11(7), 12(6), 13(12), 14(6), 15(8), 16(3), 17(1), 18(2), 19(2) and 22(1).
Sexual dimorphism
See above under Key characters. Males also have tubercles on the pectoral fin rays and on the operculum (see figure in Berg (1949)). The first branched pectoral fin ray is greatly expanded,
2-3 times broader than the second ray, which itself may be expanded a little.
Colour
Body yellowish to a light olive-green with 9-18 dark, brownish or chestnut-brown bands. The caudal fin has 3-4 dark wavy bars and a bar at its base, the dorsal fin 2-3 dark bars and a black spot at the
anterior fin base. Fins are a light orange or pinkish, particularly the caudal fin.
Size
Reaches 5.3 cm.
Golzarianpour et al. (2011) gives a total length 6.7 cm (but see below
under Age and growth). Distribution
Berg (1949) and Abdoli (2000) record this species from the Dasht-e Lut basin without specific localities, questionably from the Tigris River basin (presumably based on Bănărescu
and Nalbant (1966), see below), the middle and lower Bampur and Halil rivers of the
Hamun-e Jaz Murian basin, and the Sarbaz and Nikshahr rivers of the eastern Makran.
Specific localities include Kjagur and Kaskin near Bazman (Nikol'skii, 1899),
both between Bazman and Bampur (Berg, 1949), Shur Ab near Kuh-i Birk (Berg
(1949) and the Bampur River near Bazman. Bănărescu and Nalbant (1966) report this species from Shapur, 12 km north of Kazerun and from Shah Bazan on a tributary of the Ab-i-Diz, and "probably most of Iran". This
seemed inherently unlikely as the type locality is in Baluchestan and the material
was later described as a new species, P. nielseni q.v.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Maroon River with a (intercept) being 0.008 and b (slope)
3.11.
Note that if this is the Marun River in the northern Persian Gulf basin, then
the species is probably not the P. bampurensis of Baluchestan. Food
Unknown.
Reproduction
A female measuring 4.8 cm and caught in late February carried fairly well-developed eggs (Berg, 1949).
Parasites and predators
Unknown.
Econmic importance
None.
Conservation
This species is reported from several localities in Baluchestan, including those remote from human influence, and does not seem to be in any danger.
Further work
The biology of this species needs to be studied.
Sources
Type material: Syntypes of Nemacheilus bampurensis (ZISP 11698 and 11699), see above.
Iranian material: CMNFI 1979-0312, 14, 24.9-41.5 mm standard length, Baluchestan, Bampur River 8 km west of Iranshahr (27º11'N, 60º36'E);
CMNFI 1979-0313, 5, 31.1-39.9 mm standard length, Baluchestan, Bampur River at Bangharabad (27º20'N, 60º46'E);
CMNFI 1979-0315, 17, 20.0-44.8 mm standard length, Baluchestan, Bampur River 2 km north of Karevandar (27º51'N, 60º46'E);
CMNFI 1979-0317, 28, 19.9-45.8 mm standard length, Baluchestan, Sarbaz River at Bondan (26º35'N, 61º13'E);
CMNFI 1979-0318, 1, 23.8 mm standard length, Baluchestan, Sarbaz River at Huvar (26º09'N, 61º27'E);
CMNFI 1979-0323, 5, 20.7-31.4 mm standard length, Baluchestan, Sarbaz River (ca. 26º26'N, ca. 61º16'E);
CMNFI 1979-0326, 12, 19.3-35.0 mm standard length, Baluchestan, stream in Oghin River drainage (ca. 26º35'N, ca. 60º02'E);
CMNFI 1979-0327, 10, 21.2-37.4 mm standard length, Baluchestan, stream in Nikshahr River drainage (26º32'N, 59º57'E);
CMNFI 1979-0329, 14, 21.1-33.4 mm standard length, Baluchestan, stream at Zaminbandan
(27º02'N, 61º20'E).
Paraschistura chrysicristinae Described from the Tigris River basin in Turkey but no Iranian record.
Paraschistura kessleri
Common names
sagmahi-ye Bejestan, sagmahi-ye Kessler.
[golets kesslera or Kessler's loach in Russian; sundali in Pakistan].
Systematics
Nemacheilus prashari Hora, 1933 is possibly a synonym, along with its subspecies (q.v.). Banarescu and Mirza (1965) have comparisons of Nemacheilus lindbergi (which is later
regarded as a subspecies of Nemacheilus (= Parachistura) prashari, q.v.) with
P. kessleri where most characters overlap in varying degrees. Bănărescu
and Nalbant (1966) place P. kessleri in the subgenus Schistura and Nalbant and Bianco (1998) place it in the genus Schistura. Berg (1949) refers
P. prashari to a subspecies of
P. kessleri.
Kottelat (2012) has this species and P. prashari as distinct taxa. Nemacheilus kessleri turcomanus Nikol'skii, 1947 is reported from Turkmenistan east of the Iranian border in the Murgab River drainage (Kushk River near Kushk). Bănărescu and Nalbant
(1966) consider that the differences are too slight to warrant a separate subspecies.
Berg (1948-1949) considered P. kessleri to be close to P. sargadensis, differing in having the snout slanting steeply in front of the eyes (rounded in sargadensis - this is
generally true but exceptions occur with sharply downturned snouts in sargadensis examined by me), dorsal origin midway between the snout tip and the caudal fin base (further back in
sargadensis - but see below, same in both species; and in a series of sargadensis of varying size small fish have a longer predorsal distance than dorsal origin to caudal base distance, in
large fish a shorter predorsal distance, and in intermediate-sized fish an about equal predorsal distance, so this character appears to be highly variable and unreliable for separating
these species), rudimentary caudal peduncle crest (weak to clearly pronounced crest - ?check on my sargadensis), fewer and less sharply pronounced flank bars (10-12 total, broad bars in kessleri
seen by me while sargadensis has 13 or more thinner bars - ?check on my sargadensis), longer intestine (?), and pelvic fin origin just behind the level of the dorsal fin origin (under dorsal fin
origin or slightly in advance - kessleri types seen by me had the pelvic fin origin almost exactly under the dorsal fin origin). Generally
P. sargadensis agrees with the description of
P. kessleri except for pigmentation (?I need to run type measurements I have against sargadensis noting that Ghazni fish may be a different species and comparing gut length/shape and bladder
capsule (see Mirza et al. (1981)).
Nemacheilus kessleri was described from Nushki in the Pishin Lora River basin of Afghanistan (Menon, 1987). Four syntypes are in the Natural History Museum, London (BM(NH) 1886.9.21:177-180,
38.3-48.4 mm standard length) and 4 syntypes (now 3) are in the Zoological Survey of India, Calcutta (ZSI 11487-11490) (Eschmeyer et al., 1996). The holotype of Nemacheilus kessleri turcomanus
is in the Zoological Museum of Moscow State University (MMSU P.5734) with apparently a paratype under MMSU P.5734 and another paratype under MMSU P.5735 (Eschmeyer et al., 1996). The
Zoological Museum of Moscow University (ZMMU; their acronym) has 3 specimens under P-5734 and 1 specimen under P-5735, all syntypes (Pavlinov and Borissenko, 2001).
Key characters
Characterised by Menon (1987) as a member of the subgenus Schistura with 7 branched dorsal fin rays, no scales, forked caudal fin, short lateral line not reaching mid-body and no sexual
dimorphism, and additionally by and Mirza et al. (1981) and Bănărescu and Nalbant (1995) as a member of the genus Schistura part of the kessleri-lindbergi group with the
above characters and also small size, rather compressed head, emarginate or slightly forked caudal fin and a reduced dentiform process.
Morphology
Dorsal fin unbranched rays 2-4 (anteriormost rays embedded in flesh and hard
to distinguish), branched rays 7, anal fin unbranched rays 2-3, branched rays 5, pectoral fin branched rays 8-11, pelvic fin branched rays 6-7,
usually 7, and total vertebrae 37-39. Dorsal fin origin midway between the
nostrils and the caudal fin base according to Berg (1948-1949) but the types examined by me clearly have the dorsal fin origin closer to the snout (predorsal distance when stepped back from dorsal fin
origin overlaps the rays of the caudal fin). Scales are absent. The lateral line ends under the dorsal fin (above the tip of the pectoral fin in Menon (1987) ? check on fish, Menon quote is correct). No
adipose fin but a rudimentary crest may be evident. Caudal fin slightly emarginate to quite deeply forked. Pelvic fin origin somewhat behind the level of the dorsal fin origin. The snout is blunt; in the
types it falls gently at the nostrils in 3 fish, abruptly in 1 fish; and in 3 fish from Ghazni, Afghanistan abruptly in 2 fish and less so in the third, all apparently independent of size. Both lips are
fringed, the upper with a slight interruption, the lower with a wide interruption. The dentiform process is reduced (Berg (1949), well-developed in Menon (1987) reduced in Ban and Nal 1995 ? check on
fish). Barbels are variably developed or absent in some individuals, a condition illustrated by Hora (1933). The posterior part of the intestine has a single loop.
Sexual dimorphism
There is no sexual dimorphism.
Colour
The back and flank are crossed by 10-14 irregular, brownish bands, becoming
less band-like and more irregular on the caudal peduncle. There is a dark spot at the base of the 3 anteriormost dorsal fin rays.
There are 1-2 bands or rows composed of small spots on the distal part of the
dorsal fin, or the rows may not be clearly defined with pigment in elongate
patches along rays and the fin margin clear. The anal fin may have an anterior basal spot. There is a narrow blackish band at the caudal fin base and 2-3 narrower bands on the fin itself. The sides of the head are minutely spotted. Bănărescu and Nalbant (1966) consider that the fish reported by Berg (1949: fig. 54) from Kelat Margh in Neh-i-Bendan do not have the typical colour pattern of this species. Berg (1949) describes
6-8 chestnut-brown bars on the posterior part of the body, none anteriorly.
Size
Reaches 8.7 cm total length (Patimar et al., 2010).
Distribution
Found from the Lora River basin of Pakistan through Afghanistan to the Murgab River basin of Turkmenistan and in eastern Iran
at such localities as Keljate-Marg in Zirckuch region and the Neh-i Bendan
district (?Berg check localities for modern spelling). For Iran, Abdoli (2000) lists middle and upper Kashaf River of the Tedzhen
River basin, questionably in the Bejestan basin, and in the Simish and Mashkid rivers, and river north of them in the Mashkid River basin. Nalbant and Bianco (1998) also cite the Mashkel Lake, northern
Sistan.
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Patimar et al. (2010) examined fish from the Zanglanlou River in
northeast Iran and found maximum age for both sexes was 4+ years. Growth
was positively allometric and sex ratio was 1:1.2 in favour of females. Food
Unknown.
Reproduction
Patimar et al. (2010) found reproduction occurred in April-May with
the highest GSI in April. Maximum egg diameter was 1.46 mm, aboslute fecundity
reached 1246 eggs and up to 1285.71 eggs in relation to total weight. Parasites and predators
Unknown.
Economic importance
None.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus kessleri (BM(NH) 1886.9.21:177-180), see above.
Comparative material: BM(NH) 1944.4.1:7-9, 3, 42.0-57.6 mm standard length, Afghanistan, Ghazni (ca.
33°33’N, 68°26’E).
Paraschistura lindbergi Found in the Mashkel (= Mashkid) River basin of Pakistan on the southeastern border of Iran (see
P. prashari for further discussion). This species was described from a rivulet connected to the
drainage of the Farah River, Siaw, between Farah and Dilaram, Afghanistan. No Iranian record although Nalbant and Bianco (1998) record a specimen from near the Iranian border in the "Mashkel Lake
system". Originally described in the genus Noemacheilus.
Paraschistura nielseni Common names
sagmahi-ye Nielsen.
[Nielsen's loach].
Systematics
Note that P 27110 (33.7 mm SL, my measurement here and below) "Shah Bazan, W. Iran, 16. IV. 1937. E. Kaiser" is listed as the holotype in the Zoological Museum, University of Copenhagen
(ZMUC), not 27109 as in the original, published description (observed in 1999). The Shah Bazar locality in the description is presumably a misprint for Shah Bazan. Paratypes are 27109 (25.8 mm SL) from
"Shah Bazan..." etc as above (probably reversed with the holotype), and 27105 "lok 44" (30.7 mm SL), 27106 "lok 54" (12.7 mm SL), 27107 "lok 54" (29.5 mm SL),
27117 "lok 54" (37.3 mm SL) from "Shapur, 15.03.1937. E. Kaiser. W. Iran". The published description gives the locality of Shapur as "12 km north-west of Kazerun".
The size of one paratype (12.7 mm) is smaller than any cited in the type description. The catalogue number of one fish in a drawing in the original description (i.e. 27108) is not in that description
nor in jars in ZMUC. There has evidently been some confusion over the type series.
Named for Dr. Jorgen G. Nielsen, Department of Ichthyology, Zoological Museum, University of Copenhagen.
Kottelat (2012) gives a publication date of 1999 but the journal is dated
November 1998. This species was previously reported as Nemacheilus bampurensis by Bănărescu and Nalbant (1966) based on this type series.
The dorsal fin spot is reported as absent or present in this paper and the type
description respectively (see below). Presence of this spot is a character of
Paraschistura species and needs to be clarified. Key characters
This species has a relatively short body with a short head and blunt snout. The lateral line is incomplete ending just anterior to the dorsal fin origin. Scales have a large, eccentric focus. The
caudal fin is distinctly emarginate. The body is yellowish with 7-17 dusky brown
bars. The dorsal fin has 7 branched rays. Morphology
Meristics from type series:-
dorsal fin branched rays 7(5), anal fin branched rays 5(5), pectoral fin branched rays 9(4), 10(1), pelvic fin branched rays 6(1), 7(4), and vertebrae 33(1), 34(4), 35(1). The body is compressed,
especially posteriorly. The eyes are small and positioned in the middle or anterior portion of the head. The mouth is slightly arched and the upper jaw has a reduced processus dentiformis. Lips are
slightly furrowed and the mental lobes are reduced. The maxillo-mandibular barbels are the longest. The nostrils are near the eyes with a relatively long tube on the anterior opening. The dorsal fin
origin is at the middle of the body or slightly posterior. The pelvic fin insertion is just below the dorsal fin origin. The caudal fin is emarginate or slightly forked with rounded lobes. The anterior
part of the body is scaleless. The stomach is syphonal and there is a single intestine loop. The gas bladder capsule has two globular chambers connected by a relatively long duct.
Sexual dimorphism
A 37.3 mm standard length specimen from the type series (ZMUC 27117) has the first pectoral fin branched ray broadened with wide band of tubercles and rays 2-4 decreasingly tuberculate. This male has
a preorbital prolongation or flap. Paratype ZMUC 27105 has tubercles on up to 6 branched rays of the pectoral fin in broad bands, gradually decreasing in extent on more medial rays.
Colour
The head and body are yellowish, darker and more intense along the upper flank. Bars on the body are sometimes irregular or interrupted and number 7-17. The caudal fin has a bar at its base. The
anterior dorsal fin base has a round dark spot (Nalbant and Bianco (1998),
appears as an extension of saddle, a character of Oxynoemacheilus in
illustrations); or no spot or a small and pale brown spot
(Bănărescu and Nalbant (1966), appears as a distinctive spot of
the Paraschistura type in illustrations - both based on the same
material). Size
Reaches 54.9 mm total length and 45.0 mm standard length.
Distribution
This species is endemic to Iran, known from the Tigris River and Gulf basins
as in the type description cited above. Zoogeography
Nalbant and Bianco (1998) regard this species as the westernmost member of the genus
Paraschistura (but see dorsal fin spot problem). Habitat
Unknown.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Sabzab River with a (intercept) being 0.030 and b (slope)
2.94.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Population trends and numbers unknown.
Further work
More collections are needed to record information on biology and distribution.
Sources
Type material: The holotype (ZMUC P 27110) and paratypes (ZMUC P 27105, 27106, 27107, 27109, 27117) of Schistura nielseni, see above and note possible confusion in numbers.
Paraschistura prashari Reported from the Mashkel (= Mashkid) River basin of Pakistan on the southeastern border of Iran and from the Farah River basin in the Sistan drainage of Afghanistan as Nemacheilus prashari
lindbergi Banarescu and Mirza, 1965 (or as a distinct species, lindbergi, in Schistura or Nemacheilus in Mirza, Nalbant and Banarescu (1981) and Menon (1987)) and from the
Helmand River basin in the Sistan drainage of Afghanistan as Nemacheilus prashari haarlovi Bănărescu and Nalbant, 1966 (Banarescu and Mirza, 1965; Bănărescu and Nalbant, 1966;
Mirza, Bănărescu and Nalbant, 1969; Coad, 1981c; Mirza, Nalbant and Banarescu, 1981). Possibly a synonym of
P. kessleri as suggested by Berg (1949). No Iranian record.
Paraschistura sargadensis
Common names
sagmahi-ye Sarhad.
[Turkmenskii golets or Turkmen loach in Russian; perhaps Sarhadd loach should be used in English, see below].
Systematics
The syntypes of Nemacheilus sargadensis are in the Zoological Institute, St. Petersburg under catalogue number ZISP 11700 with locality data in Latin as "Sija-Rischan in sargado. 20.VIII
(6)" (Nikol'skii, 1899) and in Russian in Berg (1949) "Zia-rishan, Province of Sarhad in Kerman, S.W. Iran, border with Beluchistan, not far from Kuh-e Taftan volcano, 1 IX 1898, N.
Zarudnyi". The catalogue data in ZISP give the date as 20.VIII. There are 31 fish, 31.0-53.7 mm standard length, in rather poor shape, some stained dark brown, others uniformally decoloured or drab.
Sarhad is the "frontier" or "land at the upper boundary", an area of plains or broad valleys and isolated mountains around Khash, for example, and a term used generally for the
mountainous plateau of Baluchestan: Saadati's (1977) statement that the Sarhad is unlocatable is in error.
Nemachilus turcmenicus Berg, 1932 is described from "einem Bache unweit von der Eisenbahnstation Gjaurs in Turkmenistan (Transkaspien)" near the Iranian border
and alternatively from the Kelte-chinar River (Cherokh River) near Gyaurs
(37°47'N, 58°44'E), Turkmenistan (Catalog of Fishes). Three syntypes, 31.7-33.8 mm
standard length, are in ZISP 11064. Berg (1948-1949) synonymises turcmenicus with sargadensis but Bănărescu
and Nalbant (1966) consider it to be a valid subspecies, adding a third, paludani, from the Kabul River drainage of Afghanistan. Later Mirza, Nalbant and Banarescu (1981) separate paludani
as a distinct species in a different species group from P. sargadensis, since
P. sargadensis has scales, an almost forked caudal fin, a feeble dentiform process and irregular bars on the
flank, all in contrast to P. paludani.
Given the remote localities of turcmenicus and sargadensis they
are unlikely to be synonyms (Kottelat, 2012). Berg (1948-1949; 1949) and Saadati (1977) consider this species to be close to
P. kessleri (q.v.), possibly a synonym. Nalbant and Bianco (1998) place this species in the genus
Schistura.
Key characters
The caudal peduncle is deeper than in Schistura bampurensis and it
lacks the protuberance under the eye in males. Morphology
Dorsal fin with 2-5 unbranched and 6-8 branched rays, anal fin with 2-3 unbranched and 4-6, usually 5, branched rays, pectoral fin branched rays 7-10, and pelvic fin branched rays 6-7 for
P.
sargadensis turcmenica (Berg, 1932a; 1948-1949; Nikolaev, 1993). Body scaleless (scaled according to Mirza, Nalbant and Banarescu (1981) based on fish from the Kul River, Hormozgan ? check on my fish
as this would distinguish from kessleri). Caudal fin moderately emarginate (deeply emarginate, almost forked in Mirza, Nalbant and Banarescu (1981)). Caudal peduncle depth 1.6-2.2 in caudal peduncle
length. Dorsal fin origin nearer caudal base than snout tip and slightly behind the pelvic fin origin. A dorsal adipose fin may be present or absent. Dentiform process on upper jaw, if present,
rudimentary.
Meristics for Iranian specimens:- branched dorsal fin rays 6(1) or 7(105), anal fin branched rays 5(106), pectoral fin branched rays 9(11), 10(84) or 11(11), pelvic fin branched rays 6(1), 7(94) and
8(11), and flank bars 11(12), 12(8), 13(13), 14(11), 15(8), 16(5), 17(5) or 18(5).
Sexual dimorphism
Unknown.
Colour
Body colour is brown backed with greenish tinges or grey, with 13-14 dark bands, in some fish hardly visible (and irregular in form according to Mirza, Nalbant and Banarescu (1981)). The bands are
broad, slightly broader than the interspaces. A dark stripe runs along the midline of the back. The caudal fin has 2 dark bars and the dorsal fin an anterior dark spot at its base. A dark band is present
at the caudal base. Young fish have a row of dark speckles along the lateral line.
Size
Reaches 6.5 cm.
Distribution
Found in the streams of the northern slope of the Kopetdag in Turkmenistan as Nemacheilus sargadensis turcmenicus, possibly extending into northern Iran. In southern Iran it is found in the
Hormuz, Hamun-e Jaz Murian, Hamun-e Mashkid and Makran basins (Nalbant and Bianco, 1998)
including at Tangeh Sarreh, Baluchestan, Shah Abbas qanat at Assadabad and the
Kul River near Darab.
Zoogeography
See family account.
Habitat
Unknown.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Halil River with a (intercept) being 0.007 and b (slope)
3.12. Age and growth
Maximum age in the subspecies turcmenica does not exceed 1.5 years (Nikolaev, 1993).
Food
Important food items in the subspecies turcmenica are larval mayflies, caddis flies, chironomids, dragonflies and detritus (Nikolaev, 1993).
Reproduction
Eggs in the subspecies turcmenica reach 1.24 mm in diameter (Nikolaev, 1993).
Parasites and predators
Unknown.
Economic importance
None.
Conservation
?
Further work
?
Sources
Type material: Syntypes of Nemacheilus sargadensis (ZISP 11700), see above.
Iranian material: CMNFI 1979-0210, 37, 21.0-35.4 mm standard length, Kerman, river west of Istor (29º21'N, 56º08'E)?to
locate; CMNFI 1979-0215, 14, 27.7-51.7 mm standard length, Kerman, Kharan River drainage at Baft (29º14'N, 56º37'E);
CMNFI 1979-0218, 8, 32.8-47.8 mm standard length, Kerman, Kharan River drainage (28º53'N, 56º55'E);
CMNFI 1979-0220, 1, 37.3 mm standard length, Kerman, jube 2 km south of Jiroft (28º39'N, 57º43'E);
CMNFI 1979-0221, 19, 24.3-46.8 mm standard length, Kerman, river in Halil River drainage (28º51'N, 57º52'E);
CMNFI 1979-0308, 11, 22.2-40.1 mm standard length, Kerman, river 44 km from Baft (29º02'N, 56º50'E);
CMNFI 1979-0338, 68, 17.0-38.6 mm standard length, Baluchestan, Tahlab River drainage 8 km from Mirjaveh (28º58'N, 61º24'E);
CMNFI 1979-0339, 5, 23.7-30.9 mm standard length, Baluchestan, Tahlab River drainage 16 km from Mirjaveh (28º56'30"N, 61º21'E);
CMNFI 1979-0340, 25, 15.9-37.5 mm standard length, Baluchestan, jube at Ladiz
(28º55'N, 61º18'E); CMNFI 1979-0419, ?; CMNFI 2008-0138, ?.
Genus Triplophysa
This genus contains loaches of the High Asian region numbering over 110 species.
The region centres on the Tibetan plateau with some species found in north China
to the east and in Central Asia to the west. The largest nemacheilines are found
in this genus. One species is reported from Iran. The date of publication of the
genus is given as 1874 in Kottelat (2012), a lapsus.
The body is elongate with a rounded belly and the caudal peduncle is slightly
compressed or highly depressed, and is often very elongate or whip-like. The
upper lip is deeply furrowed and there is no processus dentiformis on the upper
jaw. The dorsal fin has 6-9 branched rays and is emarginate. There is no adipose
crest on the caudal peduncle although a small ridge may be present in some
species. The caudal fin is slightly to deeply forked, or is truncate. There is
no pelvic axillary lobe. Scales are absent and the lateral line is usually
complete. The swimbladder is reduced to two lateral parts and a posterior
chamber which is rudimentary or with a secondarily developed bladder lying free
in the abdominal cavity. A reduced swimbladder is an advantage in fast water,
reducing buoyancy. The gut is short or long, a simple s-shape or a spiral. The
colour pattern is usually mottled rather than banded. Males have a raised
tuberculate area below the nostrils extending from the lip corner to the
anterior eye margin, separated by a groove from a lower tuberculate area.
Pectoral fin rays have thickened and tuberculate pads on their dorsal surface.
Prokofiev (2009) summarises osteological characters in his key and these
include possession of 3 pectoral fin radials, if 4 are present then the 2
external ones are more or less dilated, flattened and at least partially overlie
each other, and the presence of the preethmoid I bone in the skull. Prokofiev
(2010) adds other osteological characters. Triplohysa farwelli Described from the "Helmand river, Afghanistan" in the Sistan basin but without any definite locality; it may be remote from the Sistan lowlands of Iran (Hora, 1935). Originally described
under Nemacheilus. No Iranian record.
Triplophysa griffithii Described erroneously as from Assam but the type locality is the Arghandab River near Kandahar in the Helmand River drainage of the Sistan basin (Hora, 1929; 1935; Bănărescu and Nalbant,
1966). Originally described in Nemachilus (sic). No Iranian record but see T. stolickai.
Triplophysa stolickai
Common names
sagmahi-ye Pamir.
[Tibetskii golets or Tibetan loach, Pamirskii golets or Pamirs loach, both in Russian; singhat in Pakistan; Stoliczka's loach].
Systematics
Often spelt stoliczkae, (e.g. in Annandale and Hora (1920)), the original spelling is Stoličkai, which becomes stolickai in Latin where accents on letters are not used
(Kottelat, 2012).
This species is placed in the genus Triplophysa by Menon (1987) and Bănărescu and Nalbant (1995).
Nemacheilus akhtari Vijayalakshmanan, 1950 described from "Farakhollum, about 10 miles South of Gardan Diwar, Helmund river" (Farakhulm at 34°31'N, 68°08'E),
Afghanistan in the Sistan basin may be a synonym of Nemacheilus (= Triplophysa) griffithii (Günther, 1868) according to Bănărescu and Nalbant (1966)
or T. stolickai according to Prokofiev (2007). There is no Iranian record.
This is a highly variable species originally described from streams around Lake Tsumureri in Rupshu Province of western Tibet and also found in the upper reaches of such rivers as the Hwang,
Brahmaputra and Yangtse. It is reported further west from the Aral Sea basin, the upper Indus River basin, the upper Helmand River basin in Afghanistan and probably in Sistan in Iran. Prokofiev
(2007) considers the systematics of the loaches in the stolickai group to be the most complicated in High Asian species. Berg (1948-1949) gives a series of figures which plainly show the
variability in body form and pigmentation of this species. Such a wide distribution and variability may be indicative of several taxa being confused under this name.
Regan (1906) refers specimens from Sistan to Nemacheilus stenurus Herzenstein, 1888
(= Triplophysa stenura). Annandale and Hora (1920) consider stenurus not to be a distinct species but Hora (1922)
compares stenurus with Nemacheilus tenuis (see below) and notes that stenurus has a continuous and entire lower lip (slightly indented in ZISP 7355 examined by me -
wide interruption and greatly plicated in tenuis) and the dorsal fin origin is closer to the snout than the caudal fin base (equidistant or nearer the caudal base in tenuis).
Specimens from Sistan labelled as Nemacheilus stenurus by Regan (1906) are then misidentified T. stolickai according to Annandale and Hora (1920). Since N. stenurus was
described from the sources of the Yangtse Kiang in China and is a more recent name, it is probably not relevant to Iranian loaches.
Berg (1948-1949) places Nemacheilus tenuis Day, 1877 in stolickai as var. tenuis
but notes that it occurs together with the type form in the Gunt River of the Pamirs in Tadjikistan as well as in Sistan, and thereby the implication is that this variety is not a subspecies (since
subspecies do not occur together). Berg (1948-1949) also has the subspecies Nemacheilus stenurus uranoscopus Kessler, 1872 described from the Zeravshan River in Uzbekistan as possibly occurring in
the Helmand River basin, presumably the upper reaches, but he includes Nemacheilus tenuis Day under this subspecies too.
Note that the publication by Day is dated 1876, apparently published 1877
according to Eschmeyer et al. (1996). N. tenuis is the type
species of the subgenus Indotriplophysa Prokofiev, 2010 elevated to a
genus by Kottelat (2012). Kottelat (2012) notes the widely disjunct localities
of the types of this species suggesting that two species may be involved. Annandale and Hora (1920) initially place Sistan specimens in Nemacheilus stolickai but later Hora (1922) places them in Nemacheilus tenuis. Hora (1922) restricts the name
stolickai to fish from the Indus River basin and considers that the various wide-ranging reports of stolickai refer to various other species. See also Vijayalakshmanan (1950) for
comparisons with some nominal Afghan species in the Helmand basin.
The fish from Sistan examined by Annandale and Hora (1920) and assigned to T. stolickai are in two forms - one with a thin caudal peduncle, the stenurus type which they do not recognise
as specifically distinct, and one with a thick caudal peduncle which they place in var. leptosoma Herzenstein, 1888 (note that varieties are not recognised by the International Code of Zoological
Nomenclature). The former has a minimum caudal peduncle depth in caudal peduncle length of 5.3-7.8, mean 6.8 based on 3 fish from a table in Annandale and Hora (1920) and the one fish seen by me, and
the latter has values of 2.9-3.6, mean 3.1 from the table in Annandale and Hora (1920). Annandale and Hora (1920) point out that this character is dimorphic but is neither related to sex nor
"race" and that gut loop development also varies but independently of the caudal peduncle character. The specimen I examined from Sistan has a stenurus form of caudal peduncle
(value 7.6), the dorsal fin origin is about equidistant between the snout tip and caudal base and so agrees with neither stenurus nor stolickai (see table in Vijayalakshmanan, 1950)), the
head length in standard length (4.5) falls within the limits of stolickai (4.2-4.8) and not stenurus (4.9-5.5) (see table in Vijayalakshmanan, 1950)), and the lip grooves resemble another
species, Nemacheilus akhtari Vijayalakshmanan, 1950, described from the Helmand River at Farakhollum about 10 miles south of Gardan Diwar in Afghanistan. The conclusion I reach here is that an
individual fish in this group of species or forms can have a mix of characters used by authors to separate and define species.
The resolution of this problem, the correct name for Sistan loaches, other than the distinctive P. rhadinaea and P. vignai, requires extensive material from the whole range of the nominal
species involved for dissection and comparison of gut shape, for analysis of sexual dimorphism and individual variability in such morphometric characters as caudal peduncle breadth and thickness and
position of the dorsal fin, as well as in determining new and, hopefully, definitive characters. Conservatively, I refer Sistan fish to stolickai, the oldest available name, while recognising that
tenuis may be a distinct species or subspecies and be the fish found in Sistan, or even that the fish in Sistan are an unnamed taxon or taxa. The Iranian and other material available to me does not
permit a resolution of this wide ranging problem.
Syntypes of Cobitis Stoličkai are in the Naturhistorisches Museum Wien under NMW 48436 (5 fish), NMW 48439 (1) and NMW 50477 (4).
A syntype, or at least material examined by Day, of Nemacheilus tenuis is in the Naturhistorisches Museum Wien under NMW 48477 (Eschmeyer et al., 1996).
A syntype of Nemacheilus stenurus, 135.2 mm standard length, is in the Zoological Institute, St. Petersburg, Russia under ZISP 7355. Other types are ZISP 7256 (4), 7354 (3, now 2), 7355 (1),
and BM(NH) 1891.10.7.33 (1, 64.9 mm standard length) (formerly in ZISP).
SEE Prokofiev (2007) for tenuis and stoliczkai and other data ? Key characters
The elongate yet rounded caudal peduncle without an adipose fin distinguishes this species from P. rhadinaea and P. vignai the only other
nemacheilids recognised in Sistan. The depth of the
caudal peduncle just behind the end of the anal fin is the same as the width.
Morphology
Dorsal fin with 2-4 unbranched rays (anteriomost ones small and hidden in
flesh) and 6-9, usually 7-8, branched rays, anal fin with 2-3 unbranched and 5 branched rays, pectoral fin with 8-12 branched rays, and pelvic fin with 6-8 branched rays.
Total vertebrae 44. Lateral
line complete and distinctive. Scales are absent. Caudal peduncle long and slender or short and deep (see above). Head length in standard length 4.0-4.8. Eye diameter in head length 4.8-5.5, mean 5.2 in
Annandale and Hora (1922) for Sistan fish (note that Day (1876; 1878) gives eye diameter as 8 in head length but Steindachner (1866) in his original description gives 5.5). Snout blunt. Barbels are
reported as the maxillary ones reaching behind the eye, the rostral ones shorter; the specimen from Sistan had the maxillary and longest rostral barbel about equal in length, the maxillary reaching back
to about mid-eye level. Lips are rugose, deeply incised and may be fimbriated. The lower lip is fimbriate and interrupted in the middle. There is a narrow, elongate post-labial groove with well-marked
ridges on each side extending back on the mid-line of the lower head (in one specimen seen by me). The pectoral fin tip is formed by the third and fourth branched rays. Dorsal fin origin nearer the snout
tip than the caudal base in Iranian specimens or equidistant while elsewhere in the range of this species it is nearer the caudal base (but dorsal origin is individually quite variable in
Nemacheilidae).
Caudal fin slightly emarginate, lower lobe longer than upper. A well-marked groove between the anus and the anal fin is absent. The gut length is slightly longer than the fish itself with two loops.
Chromosome number is 2n=50 for fish from Kyrgyzstan (Klinkhardt et al., 1995).
Sexual dimorphism
Mature males have the first 3-6 pectoral fin rays thickened and tuberculate. A raised tuberculate area below the nostrils extends from the lips to the eye and is separated by a groove from a tuberculate
area below (see Fig. 2B in Bănărescu and Nalbant (1995)).
Colour
The flank bears irregular dark spots which may coalesce into a stripe, or is marbled dark green or black. There are dark brown saddles over the back. Fins have rows of pigment, best developed on
the dorsal and caudal.
Size
Reaches 16.5 cm.
Distribution
Found from Tibet to northern Kashmir, Turkestan and Sistan. In Iran, it is reported from the Hirmand River delta of Sistan (Annandale, 1921).
Zoogeography
See family account.
Habitat
Unknown.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None.
Conservation
Biology and numbers are poorly known so an assessment of conservation status
cannot be made.
Further work
The biology of this species needs study.
Sources
Type material: Syntypes of Nemacheilus stenurus (ZISP 7355 and BM(NH) 1891.10.7.33), see above.
Comparative material: BM(NH) 1905.4.7:3, 1, 87.7 mm standard length, Sistan, Helmand River, collected by H. McMahon and from the Indian Museum, Calcutta (the size of this fish is at variance with the
6 specimens measured by Annandale and Hora (1920:181) but appears to be from the collections made by the Seistan Arbitration Commission of 1902-1904 and is possibly the seventh specimen referred
to in the text. This specimen has been identified as Triplophysa griffithii by S. O. Kullander and has old labels as Nemacheilus stenurus and Nemacheilus tenuis).
Genus Turcinoemacheilus This genus contains a single species and is only recently
recorded from Iran. The characters of the genus are the same as the species. Turcinoemacheilus kosswigi
Common names
None.
Systematics
Originally described from Kapozik Kadun, Hakkari, Turkey. The holotype is ZMH
H1884 and 6 paratypes are ZMH H1885. Key characters
The pelvic fin origin lies in front of the dorsal fin origin and the anus
lies closer to the pelvic fins base than the anal fin base, distinguishing this
species from all other Iranian nemacheilids. Morphology
The body is of uniform depth and almost cylindrical. Dorsal fin with 2-4 unbranched rays and 7 branched rays, anal fin with 1-4
unbranched rays and 5 branched rays, pectoral fin with 7-9 branched rays, and
pelvic fin with 5-7 branched rays. The lateral line is short with 18-19 pores,
ending before the dorsal fin origin level. The lateral line pores are larger
than in other species in Iran. The body is scaleless. Eyes are small
and far apart. There is no processus dentiformis. All fins are
small and more or less rounded. The dorsal fin origin is behind the level of the
posterior margin of the pelvic fins. The caudal fin is weakly emarginate. The lips are simple and smooth, the
head short and flat and there is no keel on the caudal peduncle. The greater
distance between dorsal and ventral fin, longer snout length, lower body depth
and narrow body width are viewed as adaptations to fast flowing streamlets (Golzarianpour
et al., 2009).
The pectoral fins are large and horizontal and act as vacuum suckers (and
possibly the pelvic fins too) (Breil and Bohlen, 2001).
There is a fairly large fleshy pelvic axillary lobe. Sexual dimorphism
None. Colour
Overall colour is brown to yellowish. The back has a brown line. There are a series of irregular flank blotches (ca. 12)
extending from the back to the mid- or lower flank but not the belly. These are weakly
expressed anteriorly or merge into the general dark pigmentation there. There is a bar
or arc of dark pigmentation at the caudal fin base.
Fins are not spotted. Size
Attains 5.9 cm total length (Golzarianpour et al., 2011). Distribution
Reported from the Sezar River, a tributary of the Dez River at 33°28'N,
49°03'E by Golzarianpour et al. (2009) and in the northern Gulf basin (Nalbant
et al., 2010). Also found in the Tigris River
and its tributaries and the upper Euphrates River basin including the Göksu
River in Turkey (Breil and Bohlen, 2001; Golzarianpour et al., 2009). Zoogeography
An endemic species in the Tigris-Euphrates basin, its relationships with
other nemacheilids is uncertain. Habitat
Gravel beds with a strong current are favoured, the slender body allowing
this fish to exploit spaces between the gravel. Capture sites in Turkey had
clear to muddy water. Aquarium specimens showed thigmotaxis, fixing themselves
in place by erecting all fins and pressing against opposing structures. In a
strong current, they attached to the substrate with the pectoral fins using
suction. Fish even left the aquarium water, a few centimetres above the
waterline, attached to the glass with only the tail tip in the water. Splashing
with water moved the head and body but the pectoral fins remained attached (Breil
and Bohlen, 2001).The Iranian specimens were
caught at 1456 m altitude. Age and growth
Unknown.
Golzarianpour et al. (2011) give a length-weight relationship for fish
from the Sezar River with a (intercept) being 0.008 and b (slope)
2.89. Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Tajbakhsh et al. (2012) record metacercaria of Posthodiplostomum
cuticola and Allocreadium sp. (Digenea) and Procamallanus sp.
(Nematoda) in fish from the Tigris River basin in Iran. Economic importance
None.
Conservation
Biology and numbers are poorly known so an assessment of conservation status
cannot be made.
It is widely distributed and probably more common than current knowledge
indicates. Golzarianpour et al. (2009) point out that dam construction
and human activities may threaten the species. Further work
The biology of this species needs study.
Sources
Based on Golzarianpour et al. (2009). This family comprises the salmons, trouts, charrs and whitefishes
and contains 11 genera and about 213 species found in cooler water in the Northern
Hemisphere (Nelson, 2006; Eschmeyer and Fong, 2011). The distribution map in Berra (2001) is too extensive in
the south of Iran. There are only two species native to Iran but several
others have been introduced with varying degrees of success.
Whitefishes may be placed in their own family, Coregonidae, but views
differ (see Nelson, 1994; 2006).
Members of this family are characterised by numerous, deeply
embedded scales on the body but not the head; an adipose fin; a
lateral line with relatively numerous and often quite small scales; 7-20 branchiostegal rays; 3 upturned vertebrae at the tail
fin; a large swimbladder; usually numerous pyloric caeca (11-210);
gill membranes free from the isthmus; a small dorsal fin with few rays
(less than 17); a pelvic axillary process; young usually with parr
marks (bars along the flank); and a tetraploid karyotype.
Family members are important in fish culture in all the cooler
waters world-wide.
This genus is characterised by small, almost toothless mouth, 115
or fewer scales in the lateral line, gill rakers often long and slender, there
is a single flap between the nostrils, and no bright and complex colour patterns
in adults and no parr marks in young, forked caudal fin. Numerous named species
in North America and Eurasia (FishBase gives 217 in Coregonus alone,
August 2007), variously recognised as full species or not.
Coregonus lavaretus
Common names
safid mahi (= white fish at Karaj Dam), safid mahi juibarye (=
brook or rivulet white fish), koregon or coregon.
[European whitefish, powan, houting, skelly, gwyniad, lavaret, pollan].
Systematics
Salmo lavaretus was originally described from Lake Bourget,
France (Kottelat, 1997). There are numerous subspecies and
infrasubspecific names for populations of this fish in northern Europe
(Berg, 1948-1949). The origin of the Iranian specimens is unknown so
it is not clear which subspecies or species they belong to and accordingly
information on biology is of a general nature.
Key characters
This species is the only one in its genus reported from Iran and is
recognisable by the generic characters above.
Morphology
The mouth is small, inferior and lacks evident teeth. Lateral line
scales are 84-105. The dorsal fin has 3-5 unbranched and 8-13 branched
rays, the anal fin has 3-5 unbranched and 11-15 branched rays and the
caudal fin has a strong fork. Gill rakers are sparse and short or long
and dense, denticulated or not, and number 21-56, with means and form
varying between subspecies. Vertebrae number 56-64. The chromosome
number is 2n=80 (Klinkhardt et al., 1995).
Sexual dimorphism
Males may bear rows of elongate tubercles on the scales.
Colour
The back and upper head is a dark bluish-grey, blue-green or dark
green, flanks are greenish-grey to silvery and the belly is silvery to
yellowish-white. Fine speckles may be apparent on the body. The snout
is black. The iris is white. The dorsal, anal, adipose and caudal fins
are dark while the pectoral and pelvic fins are only dark at the tips.
Size
Attains 97.0 cm and 10.0 kg.
Distribution
The native distribution of this species is in northern Europe and
Siberia, in particular the drainages of the Baltic, North, Barents and
White seas with isolated populations in central Europe in mountainous areas.
Fingerlings were released into the Karaj and Latian reservoirs near
Tehran from 1965-1968 after hatching from eggs imported from Europe (Armantrout,
1980). Walczak (1972) reports some existing still in the Karaj
Reservoir but none were found in the Latian. Ahmadi et al. (2011) record
this species as still present in the Karaj (= Amir Kabir) Dam reservoir, the
only locality for it in Iran. It was also introduced to
the Manjil Dam on the Safid River (Griffiths et al., 1972) but
this reservoir is drained to remove excess silt and no fishery exists
(J. Holčík, pers. comm., 1992). There is no evidence of reproduction. Saadati
(1977) states that they were established in both reservoirs. Also
reported from the Farahnaz Reservoir, Markazi. Abdoli (2000)
depicts the Karaj River and the Abhar River as harbouring this species.
Zoogeography
An exotic species not naturally occurring in Iran.
Habitat
This species is typically an inhabitant of large and deep lakes
where its oxygen requirments are quite high. However it is tolerant of
warm water and even a measure of pollution. Spawning may occur in
rivers tributary to the lake. Anadromous forms occur but are rare in
full seawater.
In lakes during the day, it is found at depths of 20-30 m or on the
bottom if water is shallower. At night it may rise as far as the
surface following the migrating plankton or to the waters edge in the
shallows. Schools form in sub-littoral areas during the spawning
season and strandings may occur.
Age and growth
Maximum age attained is 20+ years although in some populations most
fish are 1+ to 3+ years old while in others most fish are 7+ to 10+
years old. Under good conditions young can attain 10-12 cm after one
year and 15-20 cm after two years of life. Maturity is attained at 3-5
years and 25-35 cm.
Food
Plankton is the principal food item, with benthic crustaceans being
taken in brackish water and crustaceans, molluscs and insect larvae in
rivers. This species cannibalises its own eggs and eats the eggs of
other fishes. Feeding may vary through the year, planktonic
crustaceans being taken in summer and benthic invertebrates in winter.
Reproduction
There is a spawning migration which may occur in summer but more
usually peaks in autumn. Spawning takes place in summer, autumn or
winter, varying with the subspecies or form. Summer spawning takes
place in deeper water than winter spawning. In lakes, the adult males
enter the spawning grounds at dusk, these grounds being gravelly
shoals off headlands or on offshore reefs. Some populations spawn over
sand or even mud. Females move in each night as they ripen. The yellow
eggs are 2-3 mm in diameter and slightly adhesive. They stick to
gravel and are protected from predators by falling in crevices between
the gravel. Up to 82,250 eggs may be laid. Eggs incubate for 90-100
days during winter at an optimum temperature of 6°C or less.
Parasites and predators
None reported from Iran.
Economic importance
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and as food
in Europe and Russia. It has been widely introduced to reservoirs.
Ahmadi et al. (2011) collected fry from the Karaj Dam and fed them in
rearing cages with three diets, rotifers (Brachionus plicatilis), a
commercial salmonid diet, and a an equal mixture of both. Total feeding rate had
a profound positive impact on survival for all three treatments. The mixed feed
had an easier prediction of survival rate perhaps because it better satisfied
nutritional needs. Conservation
No conservation of this exotic species is advisable.
Further work
The survival of this species in Iran should be verified.
Sources
Based on general European literature.
Iranian material: None.
Comparative material: BM(NH) 1986.11.14:1-3, 3, ca. 312-330 mm standard
length, England, Cumbria, Lake Haweswater (no other locality data).
Genus Oncorhynchus Members of this genus are found naturally in the Northern Pacific
Ocean and its drainages, migrating between fresh and salt water. Some
live permanently in fresh water. There are about 12 species and two have
been successfully introduced to Iran.
This genus is characterised by a large mouth, in adults extending
back to the level of the posterior eye margin or further, scales are small, ca.
100 or more in the lateral line, lateral line scales are as large or larger than
scales in adjacent rows and overlap with scales in front and behind, evident
teeth in adults, teeth are present on the head and shaft of the vomer bone in
the roof of the mouth, usually dark parr marks in young but adults without
snow-white leading edge to lower fins, and usually black spots on the body or
caudal fin or both.
These salmons spawn once and then die. They are important commercially as food and as sport fishes.
Oncorhynchus gorbuscha
Introduced to the former Soviet Caspian Sea basin in 1963 but not observed subsequently (Baltz, 1991). No Iranian record.
Oncorhynchus keta
Common names
azad mahi keta (= keta free fish or keta salmon, azad mahi being
the Farsi for trout or salmon), mahi-ye azad-e chum.
[chum salmon, dog salmon, keta, summer chum].
Systematics
Salmo keta was originally described from rivers of Kamchatka, Russia.
Key characters
This species is distinguished by having 124-153 lateral line
scales, 13-17 principal anal fin rays, flanks and caudal fin without
distinct black spots, short, stout gill rakers 18-26 and pyloric caeca 135-249.
Morphology
Dorsal fin with 10-14 principal rays, pectoral rays 14-16 and
pelvic rays 10-11. Scales are horizontal ovals with a central focus
and no radii. Circuli are fine but are lost or only partly developed
on the flesh-covered exposed part of the scale. The gill rakers reach
just beyond the first to just beyond the second raker when appressed.
The pelvic axillary scale is very elongate. The gut is s-shaped. The
chromosome number is 2n=74 (Klinkhardt et al., 1995).
Sexual dimorphism
Breeding males develop hooked jaws and large teeth and a slight
hump in front of the dorsal fin.
Colour
Marine fish are steel-blue on the back and upper flank, silvery on
the flank and silvery-white on the belly. The upper flank and back may
have fine black speckles but no spots. The pectoral, pelvic, anal and
caudal fins have dark edges. Spawning males in fresh water are dark
olive to black above, greyish-red to brick-red on the flank with
greenish to purplish bars or blotches and a dark grey belly. Calico is
the characteristic colour of dominant males. The anal and pelvic fins
are often tipped with white. Females are less strongly marked. Young
chum are iridescent, mottled green on the back and silvery iridescent
green on the flanks and belly. There are 6-14 parr marks which do not
descend much below the lateral line and are narrower than the space
between them. Fins are clear to white.
Size
Reaches 120.0 cm and 20.8 kg.
Distribution
Found from Alaska to California on the Pacific coast of North
America and also in eastern Arctic Siberia, the Beaufort Sea and south
to the Sea of Japan, the widest distribution of any Pacific salmon.
This species was introduced to the former Soviet Caspian Sea in 1962-1970
in an attempt to offset losses of Salmo caspius which
was cut off from its spawning grounds by dams. It was considered as
ideal because it spawns in the lower reaches of rivers and dams would
not affect its spawning migration, it returns to spawn after 2 or 3
years and so some year-classes could escape detrimental conditions in
any one year, and because it produces downstream migrants at a smaller
size and age than the native salmonid (Magomedov, 1970; 1978; Baltz,
1991). 7.5 million fertilised eggs were transported from the Amur
River and Sakhalin Island in the former Soviet Far East and incubated at the Samur Fish Farm, Dagestan. 5,850,000 fry were released.
Walczak (1972) reported capture of a specimen in Iranian waters in
1971, presumably a stray from the former Soviet stocking programmes. Holčík and
Razavi (1992) record only two specimens from Iran, one in 1964 and
one in 1972, taken between Bandar Anzali and the Astara River on the
Iranian coast of the Caspian Sea. They also seem to indicate another
specimen taken after 1972. Jolodar and Abdoli (2004) report it from Astara and
the Anzali regions, indicating no large scale spread.
Zoogeography
A species introduced to Iranian waters by man.
Habitat
In North America, chum salmon enter streams on the spawning
migration and often travel less than 150 km, stopping at the first
barrier as they are not strong jumpers. Some fish even spawn in tidal
areas. However some rivers, like the Yukon, have a run which travels
about 3200 km and takes from early June to the end of September. A
migratory speed of up to 115 km/day has been recorded with bursts of
speed to 4.6 m/s. Most runs are in the fall but some are in the
summer. Very rarely a chum will become trapped in a lake if an outlet
stream dries up but normally adult chum are only found in fresh waters
on the spawning run.
Age and growth
Reproduction occurs at ages 1+ to 2+, with spawning fish at 2+
predominating in Caspian populations from former Soviet waters. In
their native streams in the Far East fish are in their fourth and
fifth years of life. 1500 adults were caught returning to one river to
spawn in the Caspian in 1966, considered impressive by former Soviet
biologists. Accelerated growth and maturity in transplanted fish is
well documented (Magomedov, 1970). There is a 15C°
difference between the Caspian and native water temperatures for
spawning and egg development. Life span in Canada is about 7 years,
perhaps 9 years, with fish in British Columbia maturing at 1-6 years
with age 3 fish dominant. They spend 2-7 years at sea before returning
to spawn. Males grow faster and larger than females.
Food
Food in the sea includes crustaceans, worms, molluscs, squids,
jellyfish and fishes. Young fish in the sea take zooplankton. Adults
on the spawning run do not feed. Young fish in fresh water eat aquatic
insects, such as chironomids, mayflies and caddisflies, as well as
crustaceans, worms and terrestrial insects.
Reproduction
Transplantations into the former Soviet Caspian Sea basin were not very
successful because of a lack of suitable spawning streams (McNeil,
1979). Spawning runs into some former Soviet streams were recorded from the
first half of September to the end of October, the same periods as in
the native habitat (Magomedov, 1970). Males arrived first, the mass
run was composed more of females and there were more males at the end
of the run. Since a single male will spawn with several females, early
male arrival on the spawning grounds may promote successful
fertilisation. The absolute fecundity of a 3+ salmon was 2739 eggs in
the Caspian.
Females excavate a redd by lying on their sides and lashing the
tail. The redd is a trough up to about half a metre deep and up to 3.2
m long and 2.1 m wide bordered by a ridge of gravel at the downstream
end. In some cases no redd is excavated and eggs are shed over and
between boulders. Females may excavate more than one redd and males
may spawn with more than one female. A female and one dominant male
lie in the redd, gape their mouths, vibrate and release eggs and
perm. The dominant male may have several accessory males accompanying
him. The female dislodges gravel at the upstream end of the redd to
cover the eggs. The orange eggs are up to 7.8 mm in diameter (perhaps
9.5 mm when fertilised) and each female may shed up to 7779 in the
Pacific basin. The adult fish die after spawning and may live only a
week after first entering fresh water. The fry emerge from the gravel
in March-May in North America, some remaining for several weeks in
fresh water or immediately migrating to the sea.
Parasites and predators
Various insects, fish, birds and mammals prey on both young and adult chum.
Economic importance
It is too rare to have any economic importance in Iran. Total
catches in the North Pacific Ocean have been as high as 69.2 million
fish annually. In Japan it is very important in an ocean ranch industry.
Conservation
The presence of this species in Iranian waters is the result of
former Soviet attempts to acclimatise it to the Caspian Sea as a potential
replacement for declining stocks of Salmo caspius. The
stocking programme lasted from 1962 to 1979 and rivers along the
Dagestan coast showed mass spawning but this became rare. Pollution by
wood from forestry operations in Dagestan, absence of suitable gravel
beds, low salinity in the Caspian Sea and heavy surf on the Dagestan
coast may be responsible for the heavy mortality (Magomedov, 1978; Holčík
and Razavi, 1992). As an exotic species, there is no need for
conservation of chum salmon.
Further work
Reports of this species in Iranian waters should be documented.
Sources
Bakkala (1970) summarised the biology of this species in North
American waters and Salo in Groot and Margolis (1991) over its whole range.
Iranian material: None.
Oncorhynchus kisutch Introduced to the Caspian Sea basin but not subsequently observed and no Iranian records (Holčík and Razavi, 1992).
Oncorhynchus mykiss
Courtesy of Iraj Hashemzade

Two photographs above courtesy of Amir Hosin Zalaghi, 11-19 May 2010
Nalbant and Bianco, 1998

Prokofiev, 2009
(Nalbant and Bianco, 1998)
(Zugmayer, 1912)
(Nikol'skii, 1899)
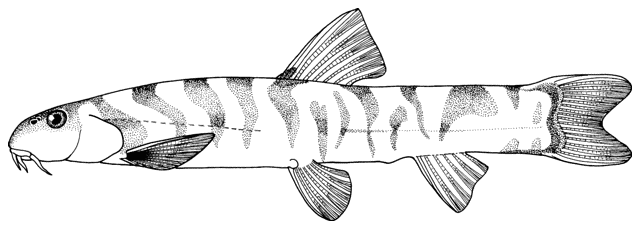
(Nalbant, 1998)
(Günther, 1889)
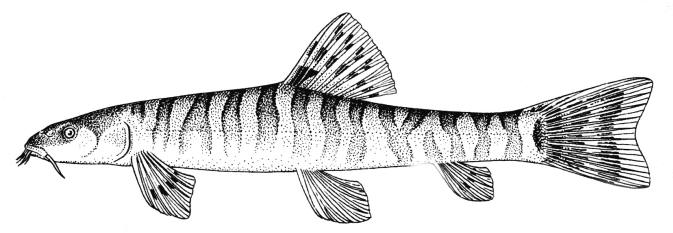
(Banarescu and Mirza, 1965)
(Nalbant and Bianco, 1998)
(Hora, 1933)
(Nikol'skii, 1899)
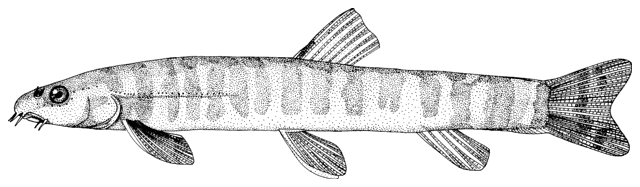
Rendahl, 1933
(Hora, 1935)
(Günther, 1868)
(Steindachner, 1866)

Banarescu and Nalbant, 1964
Banarescu and Nalbant, 1964


(Linnaeus, 1758)
Suckley, 1861
(Walbaum, 1792)
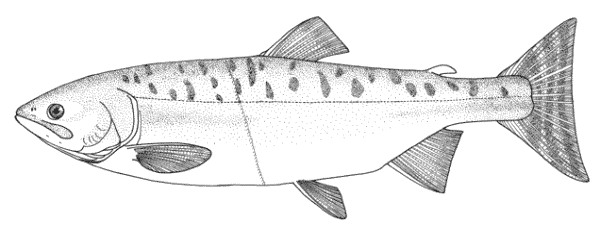
(Walbaum, 1792)
(Walbaum, 1792)
(Walbaum, 1792)
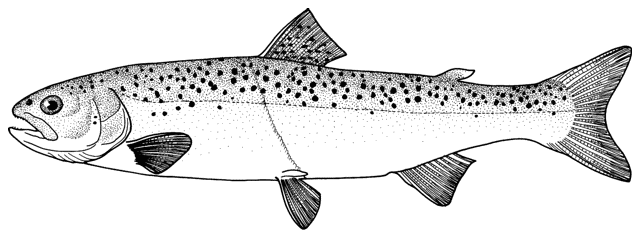

photograph Brian W. Coad
Common names
قزل آل (= gazalala, ghezel ala, qezel ala or kizil ala; probably in confusion with native Salmo
caspius,
these words meaning literally red spots or red spot fish but used for trout and salmon species in Iran),
[mykiss in Russian; rainbow trout, steelhead trout].
Systematicss
Salmo mykiss was originally described from Kamchatka, Russia.
Formerly known as Salmo gairdneri Richardson, 1836 (see
Bailey and Robins, 1988; Smith and Stearley, 1989). Placed in the
genus Parasalmo Vladykov, 1972 by Reshetnikov et al.
(1997) and Mednikov et al. (1999) but Osinov (1999) has
reservations since allozyme data show specific level differences with Oncorhynchus
although further studies were deemed necessary.
Key characters
This species is distinguished by having 100-161 lateral line
scales, 8-12 principal anal fin rays, the vomer bone in the roof of
the mouth has teeth on its head and shaft, no red spots on the body
but only small dark ones and radiating rows of black spots on the
dorsal and caudal fins, and no teeth at the tongue base.
Morphology
Dorsal fin principal rays 10-12, pectoral rays 11-17 and pelvic
rays 9-10. Gill rakers 16-22. Pyloric caeca 27-80. The chromosome
number is 2n=58-62 (Klinkhardt et al., 1995).
Sexual dimorphism
Breeding males have an elongated snout, the lower jaw is hooked and the roof of the mouth is white.
Colour
Overall colour is very variable. Stream fish are darker and more
colourful (rainbows) than lighter, silvery lake or sea fish
(steelhead). Some sea-run and lake fish have small orange to red marks
below the lower jaw. The back and upper flank are steel-blue,
greenish, silvery-olive or even brown, flanks and belly are silvery,
grey, white or yellow-green. The side of the head and the flank are
characteristically pink. The flank has a broad pink to red or lilac
stripe with small black spots. The adipose fin has a black margin and
a few spots. Pectoral, pelvic and anal fins may have a few spots and
are dusky without any strong markings. Pectoral and pelvic fins are
often orange-red. Spawning fish are very dark and the flank stripe is
dark red or purple. The young have 5-13 dark, oval parr marks centred
on the lateral line with the spaces between the marks wider than the
marks. There are 5-10 parr marks on the back in front of the dorsal
fin. The upper flank has some dark spots. The dorsal fin is tipped
white or orange and has a dark leading edge, sometimes broken up into
spots. The adipose fin has a black margin. The anal fin has a white or
orange tip. Some adults in streams do not lose their parr marks. A
golden form occurs rarely on fish farms in Lorestan and elsewhere is
farmed specifically.
Sizee
Reaches 122.0 cm, possibly 150 cm, and 35.0 kg as sea-run or lake fish but smaller in streams.
Distribution
Found in western Canada and from Japan and Alaska to Mexico. Widely
introduced outside this natural range world-wide in suitable waters. They have been introduced to the
former Soviet part of the Caspian
Sea in 1973 and several hundred returning adults were reported in 1975 (McNeil, 1979; Baltz, 1991).
Rainbow trout were introduced to Iran about 1966 for hatchery
production as a commercial product (MacCrimmon, 1971; 1972). They are
now widespread in Iran, stocks being maintained by hatchery
introductions and sometimes natural reproduction, wherever temperature
regimes are suitable in the higher reaches of the Alborz and Zagros
mountains (Walczak, 1972; Anonymous, 1977; B. Sandford, in litt.,
1979; Y. Keivany, in litt., 1992; Ghorbani Chafi, 2000; personal visits to fish farms).
B. Sandford considered few populations became established because of competition with large
populations of omnivorous cyprinids which also take fish eggs,
presumably of trout too. Sandford cited viable populations in the Madar Su, a small stream in the former Mohammad Reza Shah
Park (see below), Tar Lake in the Alborz (also recorded from Tar Lake by Riahi (1996)),
Gahar Lake in the Zagros and the Qara Chai west of Hamadan.
Introductions to Neuer or Neur Lake near Ardabil, Ghorighol Lake,
Rebeshahr and the Ab-e Bazuft were all failures (although Walczak
(1972), Saadati (1977) and R. Mehrani (pers. comm., 2000) report several successful stockings
including the Ab-e Bazuft, Gahar Lake, Namrud, Dez River and Jajehrud).
Also reported from the Gorgan and Haraz rivers and Gorgan Bay (the latter escapees
from cages)(Kiabi et al., 1999), Madar Su, Golestan National Park (Kiabi et al., 1994),
from the Farahnaz Reservoir, Markazi, Namrud in Semnan, Golestan
River, Karaj River, Amir Kabir Dam (www.iran-doe.org/Special/Alborz.htm), Shah
Abbas Dam west of Esfahan, Darius-e Kabir Dam north of Shiraz, Karaj River,
Zayandeh near Esfahan, Rebeshahr southeast of Yasuj (Anonymous, 1977; Y. Keivany, in litt.,
1992). An attempt to culture this species in cages in the Avan River, 7 km from
Alamut, in 1994 failed and fish escaped (Nialmir, 2001). Also in the Sardab Rud (Jalali et al., 2005).
An attempt to establish a run of steelhead, the migratory form, in
the Chalus River from the Caspian Sea was apparently unsuccessful (Armantrout, 1980).
This species was stocked in the 1970s by the Iranian Department of the Environment in
the Doogh or Madar Su, head stream of the Gorgan River in Golestan
National Park and "Neur" Lake in East Azarbayjan near Ardabil in the
Caspian Sea basin; in Ghorigol Lake (east of Tabriz) and the Liqvan Chay in the
Lake Orumiyeh basin; the Karaj River, the Jajrud, the Namrud, Tar Lake northeast of Tehran, Lake Lasem
near Tehran, the Lar River, probably in the Chashmeh Do Barare, Ab Kharsang,
Varangarud and Baragon near Tehran, all in the Namak Lake basin; the Karun
River, Mohammad Reza Shah Dam, Rebeshahr River southeast of Yasuj, the Ab-e Bazuft in 1975 (established), Gahar
Lake in the headwaters of the Dez River and its outlet stream (established, caught by A. Abdoli in
1995), at farms in the Rostamabad area discharging into the Kuhrang stream of
the Karun River basin, and an isolated section of the Dez River formerly fishless (established),
all in the Tigris River basin; the Shah Abbas Dam west of Esfahan, in the Zianrud
(presumably the Zayandeh River of the Esfahan basin) where a reproducing population
was established; in the Dorudzan (Dariush-e Kabir) Dam near Shiraz (104,000 in Esfand
1350)(Surber, 1969; Anonymous, 1977; Armantrout, 1980; Petr, 1987; Abdoli, 1993b;
Fadaeifard et al., 2012).
Qanats in Markazi Province were stocked with 930,000 trout (presumably this
species) in 2006 (www.iranfisheries.net, downloaded 28 July 2006).The qanats
were in Shazand (2 qanats), Arak (4), Delijan (1) and Khomein (1).
Abdoli (2000) lists the Dasht-e Kavir and Kerman-Na'in basins generally, the upper Kashaf River in the
Tedzhen River basin, the Gorgan, lower Neka, middle and lower Babol, and Heraz
rivers in the Caspian Sea basin; the upper Talkheh,
Tatavi and Zarrineh rivers in the Lake Orumiyeh basin, the Abhar stretch of the Shur
and the upper and middle Karaj rivers of the Namak Lake basin; and the Khersan,
upper Dez and Kashkan rivers of the Tigris River basin, and the upper and middle Zayandeh
River of the Esfahan basin.
Zoogeography
An exotic species introduced as a food fish.
Habitat
Rainbows are found in rivers or streams where there are pools and
riffles. Some live in lakes and are called Kamloops trout in Canada
while others run to sea and are called steelheads. They can tolerate
temperatures up to 24°C, warm for a trout, but prefer temperatures below 20°C.
Sea-run fish spend about 1-4 years usually in inshore waters at middle
to surface depths after 1-4 years in fresh water. Some fish (half-pounders)
return to streams after a few months at sea. Summer steelhead have
spent one winter at sea and return in summer (April-October) to spawn
next spring while winter steelhead are larger and return from December
to April peaking in January to spawn in March and April. Aquaculture of trout in
Iran is affected by drought conditions reducing water flow to farms. Production
was higher in 1999 compared to the previous year but this was because 82 new
farms were opened; average production per farm fell (Foghi, 2004).
Age and growth
Life span varies with habitat, up to 11 years in some lake fish but
only 3-4 years in many streams and small lakes. Growth varies with
habitat including such factors as length of sea, stream or lake life,
years before spawning, available food supplies, latitude, altitude,
temperature regime, competition with other salmonids, and so on.
Ageing these fish may be difficult because of the complicated life
history pattern of stream and lake residency. Maturity is also
variable with habitat. Some males mature at 9 months in fish
introduced to warm southern waters and some females only at 8 years,
but generally maturity is reached at 3-5 years in Canada, for example,
with males maturing a year earlier than females. Neur Lake fish had a
growth rate of 17 lbs (7.72 kg) in 3 years, more than doubling in
size, because of the excellent food supply of shrimp. In Tar Lake,
where food resources were poor, mostly surface insects, fish grew to
only 3-5 lbs (1.4-2.3 kg) in 2 years (Anonymous, 1977). In the Karaj
Reservoir, one-year-old fish with a length of 8-10 cm grew to 25 cm in
one year although the available food was only plankton.
Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based
on 111 Iranian fish measuring 27.5-57.0 cm standard length. The a-value
was 0.0161 and the b-value 3.044 (a b-value < 3 indicating a fish
that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Food
Food includes plankton, crustaceans, aquatic and terrestrial
insects, snails, leeches, salmon eggs, and other fishes. The fish
eaten enhance growth and the species taken depends on what is
available. In Lorestan, this species thrives in rivers where Gammarus
is numerous, e.g. near Dow Ab-e Aleshtar. Fish and squid are taken at
sea along with some crustaceans. Abdoli (2000) lists Trichoptera, Plecoptera,
Chironomidae, Ephemeroptera, Ceratopogonidae, Odonata, Simuliidae, Coleoptera,
Decapoda and Amphipoda as food in Iran.
Reproduction
Spawning takes place from March to August but is usually in spring.
North American Great Lakes fish may spawn from late December to late
April. Water temperature for spawning usually exceeds 10°C
but may be 5-13°C. A female excavates a redd by lying on her side and thrashing her tail.
Redd excavation occurs during the day and night and dimensions are
usually longer and deeper than the female's body. A male courts a
female by rubbing his snout and body against her, by vibrating, by
swimming over her in the redd and by pressing against her. Several
males are found around each female but one male is dominant. The
spawning act lasts 5-8 seconds with the pair parallel in the redd
pressed together, both fish gape, arch and vibrate. Other males may
shed sperm. The female covers the eggs with gravel by dislodging it
from the upstream end of the redd. Most spawning takes place in the
morning and evening and nests may be abandoned during the day. Females
construct several redds and may spawn with several males. Eggs are
orange or pink, 5.0 mm in diameter and up to 12,749 in number.
However, egg numbers are usually a few hundreds to thousands per
female. The eggs hatch in about 8 weeks and fry generally emerge in
June to August from spring spawnings. Repeat spawning can occur for up
to 5 years.
Nematollahi and Azari Takami (2002) studied the quality and quantity of
seminal fluid in cultured male broodstock specimens of this species at the Karaj
Fish Farm. Differences were found between stocks in sperm motility and concentration.
Parasites and predators
A wide variety of other fishes and birds feed on this species and
there have been extensive studies on parasites and diseases of this
commercially important species. Akhlagi (1999, www.mondialvet99.com,
downloaded 31 May 2000) reports infectious pancreatic and
haemotopoitic necrosis in fish from Bovir Ahmadi va Kohkiluyeh Province but not in Fars Province. Liver and intestinal submucosa
degeneration and kidney necrosis were observed in diseased fish.
Prearo and Ghittino (1993) record a case of lipid liver degeneration in cultured
trout. Asadzadeh Mangili (2000) records ichthyobodosis in 3 cm fry from a
farm in Bovir Ahmadi va Kohkiluyeh. The mortality rate was
40% and infection rate with Ichthyobodo was 100%. Rainbow trout
fed on raw marine fishmeal powder in Fars Province were found to be
more exposed to vibiosis (pathogenic Vibrio anguillarum)(Ghazi
and Akhlaghi, 1998). Diplostomiasis (infection with Diplostomum
spathaceum) is reported from cultured trout in West Azarbayjan (Asadzadeh
Manjili and Ghorbanzad, 1999). Zorriehzahra (2002) reports on diseases in farmed
fry and Zorriehzahra et al. (2002) record enteric redmouth disease in
farmed fish around Tehran. Akhondzadeh et al. (2002) and Akhondzadeh Basti and Zahrae Salehi (2003) show that the
psychotropic pathogen Listeria monocytogenes is found in market and fish
farm samples of this species. Soltani and Rostami (1997) record a mortality level of about 16% for farmed
trout in northern Iran infected with a Cytophaga/Flexibacter-like
bacterium. Soltani et al. 1999) report a yersinosis-like infection in
farmed Iranian rainbow trout. Jalali et al. (2005) summarise the occurrence of Gyrodactylus
species in Iran and record G. derjavini from fish in the Sardab-rud.
Asadzadeh Mangili (2001) describes gas bubble disease from fish farms in
Kermanshah Province. Mortality occurred mostly in fish at 100-200g and symptoms
included inappetition, skin darkening, aggregation of fish at inlets and outlets
in the ponds, and swimming near the surface. In 20% of the samples, unilateral
or bilateral exophthalmia was observed. High day-night fluctuations in the
oxygen and carbon dioxide content of the pond water was observed through
excessive plant growth. Abdi et al. (2006) recorded the first mortality
from acanthocephalosis with Pomphorhynchus laevis in fish farms in Maku,
West Azarbayjan. Ebrahimzadeh Mousavi et al. (2007) isolated 12 species
of fungi from eggs in Mazandaran hatcheries.
Barzegar et al. (2008) record eye parasites from this fish including the
protozoan Ichthyophthirius multifilis and the digenean Diplostomum
spathaceum.
Miar et al. (2008) examined fish in Valasht Lake and the Chalus River,
Mazandaran and found the protozoans Trichodina trutta,
Ichthyophthirius multifilis and Chilodonella hexastica as well as the
metazoan Gyrodactylus derjavini. Khosravi et al. (2010) found 5
species of Saprolegniaceae fungi on eggs in two hatcheries in Kermanshah
Province, three species for the first time in Iran.
Barzegar and Jalali
(2009) reviewed crustacean parasites in Iran and found Lernaea sp. on
this species.
Mood et al. (2011) record Ichthyophthirius multifilis,
Trichodina sp., Chilodonella sp., Gyrodactylus sp. and
Dactylogyrus sp. in fish from coldwater fish farms of Mazandaran. Economic importance
This species is commercially farmed in Iran for local sale.
Experimental studies in the 220 ha "Neur", "Neuer"
or "Noor" Lake near Ardabil have shown very good production
of this species. A growth rate of 17 lbs (7.72 kg) in 3 years was
reported (Anonymous, 1977). A yield of 160 kg/ha was obtained and a
catch of 35,000 kg reported (Saadati, 1977) but this was unusual in
that there were no other fish and food supplies were extensive. The
lake is subject to winter-kill and requires stocking and/or helixers
to maintain open water in winter (Bullock, 1971; Nehring, 1973b;
1973c; 1973d; 1974b; 1974c; 1974d; 1974e; 1974f; 1975b; 1975c; 1975d;
1975e; 1975f; 1975g; Nehring et al., 1974; Abbasi, 1974; 1975;
Boettcher, 1974a; 1974b; Sanford, 1975; Harrington, 1976).
Kohnehchahry and Heydarpur (1973) outline methods of raising trout
using submerged cages which they believe would be suitable for Iranian
waters. Cage culture has been tried in Gorgan Bay, the fish escaping
during several storms and numbering in the millions (B. Kiabi, pers.
comm., 1994; www.ramsar.org/ram_rpt_37e.htm, downloaded 28 July 2000).
They have also been cage cultured in Valasht Lake, Mazandaran where 12
cages were expected to produce 22 tonnes of fish (Abzeeyan,
Tehran, 5(5):IV, 1994). Nezami et al. (2000) give a
total production of fish farms in Gilan, Mazandaran and Golestan provinces
(along the Caspian Sea) as 851 t.
The "Kelardasht" or Kalerdasht" Fish Farm (= Shaheed
Bahonar Trout Farm of Shilat, the Iranian Fisheries Company, near
Kelardasht) has an annual production capacity of 2,500,000 fingerlings
of 2 g. Various other fish farms in Iran produce rainbow trout for
stocking (Surber, 1969; Krasznai, 1987; Petr, 1987). More recently,
Bartley and Rana (1998b) give a figure for the Kelardasht Farm of 3
million fingerlings from a pool of 2000 brood fish at a cost of 600
million rials. White (1988) reported that 60 ha of trout ponds and
raceways produced 1000 t a year (see also under Cyprinus
carpio). The largest fish farm in Lorestan produced 500 t a year
in 2000. Edwards (1989) reported production of 1750 t from 20 licensed
farms with a further 1000 t from unlicensed farms. Fingerling
"trout" production (probably rainbow trout) in government
hatcheries was 0.57 million in 1984, 1.81 million in 1985, 1.57
million in 1986, 3.02 million in 1987, 0.50 million in 1988, 4.23
million in 1989, 4.34 million in 1990, 1.90 million in 1991, and 2.00
million in 1992 (Emadi, 1993a). Fingerling production in 1996 was 6
million (Bartley and Rana, 1998a). Production of this trout increased
27% per year betwen 1991 and 1996, the raceway area for trout
increased from 80,000 to 166,000 sq m between 1992 and 1996 and
production increased from 775 to 1900 t (Rana and Bartley,
1998a). For comparison, the figures for carps (probably Chinese carps)
exceeded 100 million and for mahi safid (Rutilus frisii kutum)
it was 145 million. However there are also private commercial
hatcheries producing about 750 t of trout each year. The Jajrud
Hatchery near Tehran produced 1 million fingerlings as early as 1968
as well as several thousand marketable sized fish (Surber, 1969); and
there has been a considerable expansion of hatchery capacity in Iran
since that time. Rainbow trout production is only about 5% of total
aquaculture production although value per metric tonne is U.S.$14,000
while for carp it is U.S.$10,000. Trout production in Ilam Province was
estimated at 3.3 million fish based on 21 farms (Tehran Times, 8 August 2005,
downloaded 20 January 2006).
Rana and Bartley (1998a; 1998b) outline trout aquaculture methods
and problems in Iran. Most culture of this trout takes place in the
Alborz and Zagros mountains which are cooler because of altitude.
Farms are small, producing less than 50 tonnes per year. Raceways are
used for breeding, rearing of larvae and growing out. Eyed eggs may be
imported from Scotland and Norway but this may be banned in future
because of disease risks. Increased Iranian expertise and costs may
also favour production of eyed eggs in Iran. Survival to the eyed
stage attains 80% and to the alevin stage 70%. The private sector
produced 10 million fingerlings in 1996. The Manzanarbad Trout Farm
near Kelardasht sold 2 million fingerlings in 1997 and produced 25 t
for local consumption from 2000 sq m of concrete raceways (Bartley and
Rana, 1998b). The Mahi Sera Fish Farm at Karaj produces market sized
fish from 20,000 brood stock (Bartley and Rana, 1998b). These are
replaced every 2-3 years to maintain growth rates. About 5000 fish are
saved each year as next year's brood stock based on size, form and
colour. Milt from 4 males is used to fertilise eggs from one female.
In 1997, the farm produced 185 t of fish each weighing about 250
g (= 800,000 fish). Sale price is 10,000-12,000 rials/kg. The
production cycle is 9-10 months at 13°C.
Trout are fed commercial pellets with a conversion efficiency of
1:1.1-1.4 (wet:dry weight). Growth to marketable size (about 30 cm and
at least 225 g) takes 9-14 months depending on temperature (2-13°C)
at different localities. Feed deficiencies, poor genetic stocks and
low temperatures contribute to slow growth in some farms. Production
in raceways is nevertheless about 15kg/cu m, a good efficiency. Cost
of fingerlings to grow out farms is 120 rials for a 1 g fry, 250 rials
for a 5 g fry and 15 rials/g for heavier fish (Bartley and Rana, 1998b).
Zorriezhara (2003) notes that there will be 40 trout farms along the Haraz
River on the Caspian coast producing over 30 million eyed eggs, over 20 million
fry and over 100 tonnes of grow-out. Effluents from rainbow trout farms have a significant adverse impact
downstream on nitrate, nitrite and phosphate and on conductivity as shown by a
study of the Zaringol Stream, a branch of the Gorgan River (Mirrasooli et al.,
2012). Fadaeifard et al. (2012) also found impacts but observed that it
was possible to construct fish farms at about 1.5 km intervals discharging into
the Kuhrang stream in the upper Karun River basin, this distance being dependent
on stream discharge and self-purification of the stream. There is no consistent monitoring of released fish so the success
of stocking is unknown. The Dorudzan Reservoir about 100 km from
Shiraz has received 400,000 fry in 1970, 2 million in 1972, 300,000
from Australia in 1974, 100,000 in 1984 and 100,000 in 1985 (Petr,
1987). Experimental fishing in 1976 gave a poor return, perhaps
through competition for space with native e Capoeta species,
heavy poaching during migration into the inflowing river, and lowering
of the reservoir level. Eggs are no longer imported and the supplies
are spawned in Iran (Emadi, 1993b).
Artificial breeding of this species at the Shahid Motahary Fish
Hatchery in Yasuj has been carried out 4-5 months earlier than normal
using changes in light regime and hormones. The object of this work
was to produce fingerlings throughout the year, increase production
and save on production costs (Abzeeyan, Tehran, 7(5):IV, 1996).
Dehghani and Akbari (1997) have successfully carried out gonadectomies
on this species under field conditions.
A production of 5.5-8.0 tonnes of rainbow trout in rice paddies
during winter has been recorded (Iranian Fisheries Research
Organization Newsletter, 22:2, 2000).
Production in Ilam Province was estimated at to be 3.3 million fish by the end
of Iranian year 2005 (http://agriculturenews.faorne.net,
downloaded 11 April 2006). However, some farms are not economical in Ilam (Rezaei
and Darvishi, 2007).
Forghani et al. (2010) studied culture of rainbow trout fingerlings in
shrimp ponds at Abadan and found a survival rate of 86.7% with a food conversion
rate of 1.59. Culture of this species in earthern ponds at Bafgh in Yazd Province
used running, saline ground water unsuitable for drinking or
agriculture. Production was 2-4 tonnes/ha in 0.40-0.75 ha ponds with
salinity at 10-15‰, well water temperature 24ºC
reduced to usually less than 20ºC, with a water replacement of 5-10% per day for
a period of 5-6 months in autumn and winter. The highest growth performance was
from fish fed 35% protein, 430 Kcal/100g energy diet and 20.6% lipid with P/E
ratio of 81.4 mg protein/Kcal energy. Aeration had no significant effects on
growth (Iranian Fisheries Research Oraganization Newsletter, 25:4, 2000;
54 & 55:4, 2009; Ahmadi and Alizadeh, 2004; Bahabadi, 2006).
A total of 500,000 fingerlings in fish ponds were released in a two month period in 2005, expected to result in 220 t
of product (www.growfish.org/Iranianreport.html).
Gonad development was found to be accelerated in brackish water, males maturing
two months earlier than in fresh water and ovarian development also being
accelerated in Yazd fish (Fahlati Marvast et al., 2003; Fahlati Marvast et al., 2006).
Edwards (1989) gives details on culturing this species and the
problems to overcome. Abtahi et al. (2002) found clove oil had no
significant difference with MS222, another anaesthetic used in fish farms. Sajedi et al. (2003) have examined mtDNA in Iranian
hatchery stocks to measure inter- and intra-populational diversity as an aid in
fishery management by selection of the best broodstocks. Studies on the best
foods and conditions for this trout in Iran have been undertaken by various
authors. Kenari and Mirzakhani (2005) examined the effects of using Artemia
urmiana from Lake Urmia (= Orumiyeh) enriched with n-3 HUFA, a fatty acid not present in the brine
shrimp, as a larval food. Growth and survival was better than with non-enriched
shrimp or with artificial feed. All-female diploid and triploid rainbow trout
have been produced in Iran, such trout being unable to reproduce if escaping
from fish farms (Johari, 2005). Ahmadi et al. (2005) used the Caspian Sea
amphipod, Pontogammarus maeoticus, in rainbow trout food as a means of
improving muscle pigmentation, the reddish colour from carotenoids in this
dietary item enhancing market value. Carotenoid deposition was higher in males
(R. Khodarahami in 5th International Symposium on Sturgeon, Iranian Fisheries
Research Organization, 9-13 May 2005, Ramsar). Alavi Yeganeh et al. (2004) studied the use
of gammarid powder as a dietary item and its effects in reducing stress in trout
larvae, finding a 10% supplement to be effective. Supplementary levels of 2 and
4% of the amphipod were effective in improving flesh colour. Fani (2006a) found
fishmeal to have higher digestible and metabolizable energy than soybean meal as
feed. Fani (2006b) examined the use of oil by-product from soybeans on weight
gain, feed conversion ratio and flesh chemical composition, recommending 10% oil
by-product be added to the diet. Shadnoush (2006) showed that corn meal could be
used as a nutrient in trout diet, improving weight and length. Kalbassi et al.
(2004) induced tetraploidy using heat shock. Johari et al. (2006)
investigated production of all-female trout using sex-reversed males and found
this to be one of the best methods. Zamani et al. (2006) showed that
chromosome manipulation (triploidy) had no effects on digestive enzyme activity.
The decline in caviar production has led to a project in Iran to use trout eggs,
test being made on egg tenderness, colour, smell taste and shelf life ( (Iranian Fisheries Research
Organization Newsletter, 58 & 59:1, 2009). In addition to these reports, there have been a wide variety of studies in
Iran on methods and treatments relevant to culturing rainbow trout, briefly
summarised here. Others may be searched for in the Bibliography by common or
scientific name of the fish. Sharifian (1998) studied increases in cortisol hormone levels
in trout as a measure of stress caused by handling and induced spawning;
Akhlaghi (2000c) on immunology of suspected viral diseases in cultured trout,
namely infectious haematopoitic and pancreatic necrosis; Akhlagi
et al. (2000) on using powdered clove tree as an anaesthetic; Mirvaghefi et al. (2000) on hydrogen peroxide
for fungal control on eggs; Alizadeh et al. (2001) on the effects of
dietary energy levels on growth and carcass composition in trout maintained in
brackish water; Nafisi Behabadi et al. (2001) on using poultry by-product
meal as a dietary replacement for fish meal fed to trout maintained in brackish
water; Soltani et al. (2001) on the anaesthetic effect of clove flower
extract under various temperatures and pH; Vahdati et al. (2001) on optimal conditions
for phagocytic activity of granulocytes; Vahdati et al. (2001) on
crowding stress and haematological parameters; Vahdati et al. (2001) on the
effect of cortisol on phagocytic activity of macrophages; Akhlagi (2002) on vaccination
against streptococcosis; Azari Takami et al. (2002)
and Dorafshan et al. (2006) examined induction of gynogenesis by UV
radiation; Dorafshan et al. (2002) induced spawning by use of a GnRH
analogue; Soltani et al. (2002) on fry mortality syndrome in farmed fish
in Tehran and Lorestan provinces; Yakhchali (2002) on the abundance of the parasite
Ichthyophthirius multifilis on coldwater fish farms (which produce trout
mostly); Fallahi et al. (2003) on isolating and identifying infectious
haematopoietic necrosis virus from farmed fish; Farhangi et al. (2003) on the use of natural zeolites for reducing
ammonia toxicity; Johari et al. (2003) on sperm traits and fertilisation performance of
normal males and neomales; Ebrahimi (2004; 2005) on the deleterious effects of copper, cadmium and
zinc, pollutants, on sperm anatomy and motility; Lorestany et al. (2004) on activating
solutions and their effects on sperm motility and fertilisation rate; Sharifrohani (2004) on the use
of plant essential oils as an antifungal agent for fish eggs in aquaculture; Sheikhi
Moghaddam et al. (2004) on the use of alvita (sodium di-acetate) as a
fungicide and bactericide; Bazyar Lakeh et al. (2005) on the effect of dietary astaxanthin on fertilisation rate; Azari
Takami et al. (2005) on the nutritional effects on trout larvae of vitamin
C-enriched Artemia urmiana nauplii in relation to growth, survival and
environmental stress; Esmaeili et al. (2006) induced meiotic gynogenesisi
by thermal shocks, the yield being best using shock 35 and 50 minutes after
fertilisation; Fallahi et al. (2006) on the serological diagnosis
of infectious haematopoietic necrosis disease in fry; Gholipour et al. (2006) found a density of 62 fish
per square metre was optimal in concrete ponds for weight gain and feed
conversion; Hosseini Najd Geramei and Irani (2006) studied the effects of
photoperiod on growth, survival and and feeding of larvae; Johari and Kalbassi (2006) on alterations in the red blood cells of
triploid trout; Mirzakhani et al. (2006) studied the feeding of n-3HUFA enriched
Artemia nauplii as food for larvae used to increase resistance to
environmental stress from pH and temperature; Mirzakhani and Mahmood Abad (2006) found feeding n-3HUFA enriched Artemia
nauplii to larvae increased resistance to environmental stress (pH and
temperature); Niksirat et al. (2006) found that unfertilised eggs can be
held in coelomic fluid outside the body for at least 48 hours without any other
treatment; Rohani et al. (2006) on using Geranium herbarum
essence to control fungi on eggs, Safari and Boldaji (2006) examined use of canola meal as a partial
replacement for fishmeal to reduce cost of diets; Sear et al. (2006)
evaluated sperm in relation to weight, length and condition factor of the fish; Tala et al. (2006) on
gonad morphology after administration of 17α-methyl testosterone, female trout
becoming functional males; Tala et al. (2006) on histological changes to
the gonads after application of 17α-methyl testosterone, ranging from functional
gonads to intersexes and sterility; Yarahmadi and Moghadasi (2006)
on use of decapsulated cysts of Artemia urmiana in the larval diet; Zorriehzahra (2006) on fry
mortality syndrome from 52 fish farms and hatcheries across Iran; Emtiazjou and Sheykhi Moghadam (2007) on
the use of Alvita (sodium di-acetate) for removing fungi from eggs as an
alternative to the carcinogen malachite green; Geramy et al. (2007) on photoperiod on growth, survival and feeding
parameters in larvae; Hosseinie Najd et al.
(2007) on initial different foods on subsequent growth of larvae; Kalbasi and
Lorestani (2007) investigated the effect of various solutions on the duration of
sperm motility, coelomic fluid without blood being the best; Khajeh and Peyghan
(2007) evaluated blood serum parameters in fish cultured in earthen ponds and
showed age-related changes; Latifi et al. (2007) found that citric acid
retarded deterioration of trout during refrigerated storage; Lorestani et al.
(2007) correlated various sperm quality measures of broodstocks with fish of
different ages; Meshkat Rouhani et al. (2007) found that a lower protein
content (40%) when the carbohydrate to lipid ratio in diet is 0.5 gave the most
favourable conditions for growth; Mohagheghi Samarin et al. (2007) on the effect of
temperature on oocyte quality and therefore stripping time; Naem et al.
(2007) on treatment of diplostomiasis using praziquantel; Nafisi Bahabadi (2007)
found that high energy feeds for trout reared in brackish water were more
effective and commercial; Rezaei et al. (2007) on bacterial changes and the biogenic amines found in
farmed trout stored on ice, Soltani et al. (2007, 2007) on vaccination against,
and immunological responses to,
Streptococcus iniae infection; Naji et al. (2007) on the effects of 17ß-estradiol
valerat on sex reversal on sac fry larvae; Rahmati Leeshei et al. (2007)
on the effect of phytase enzyme on digestibility of four vegetative
foods; Safari and Boldaji (2007) on dietary lipid level effects on growth, feed
utilisation and composition; Tabandeh and Akhlaghi (2007) on efficacy of conventional disinfectants on
isolated Streptococcus iniae from diseased fish, sodium iodide and sodium
hypochlorite being most efficient although biofilms lowered efficiency;
Varirizadeh et al. (2007) on using the gonadotropin releasing
hormone agonist (GnRHa) in emulsified form to stimulate onset of ovulation;
Yousefian and Rezvani (2007) found that abnormalities were not significantly
higher in brother-sister matings than controls; Zamani et al. (2007) on the activity of various digestive enzymes at
initiation of exogenous feeding in comparison to Salmo caspius, activity
being higher in rainbow trout; Zargarian et al. (2007) on using 2000
units/kg diets of phytase with at least 35% replacement of fish meal by soybean
meal in farming trout; Akhlaghi and Sharifi Yazdi (2008) identified
Yersinia ruckeri, the causative agent of enteric redmouth disease, in
cultured fish from Fars Province; Alevi Yaganeh et al. (2008) on
use of Gammarus powder as a dietary supplement for larvae; Alishahi
(2008) on vaccinating trout against the ciliate Ichthyophthirius multifilis; Faghani et al.
(2008) on the immunostimulatory effects of alginic acid and anti-streptococcus
vaccine on growth rate, feed conversion ratio, condition factor and
survival rate; Johari et
al. (2008) on red blood cell alterations in triploids; Karbassi et
al. (2008) on the environmental management of aquaculture in raceways at
Sarab Gerdu; Manouchehri and Akrami (2008) on a new automatic sorter for large
amounts of fish; Mohagheghi Samarin et al. (2008) on eyeing and hatching
rates under cold temperatures; Motalebi et al. (2008) on production of
aflatoxins by molds in improperly stored fish meal used as food for trout; Nafisi Bahabadi and Soltani (2008) on dietary energy levels and feeding
rates in fingerlings; Safari and Boldaji (2008) on replacing soybean meal with
canola meal in diets; Shadnoush et al. (2008) on using acorn meal as a
pellet binder and stabiliser; Shafaeipour et al. (2008) on the effects of dietary canola meal
on physiology and biochemistry as it can be used to replace substantial levels
of fish meal in food; Yousefian (2008) on genetic parameters of
growth; Yousefian and Nejati (2008) on inbreeding depression shown as variations
in percentage egg hatchability, survival of fry and weights; Zargar et al.
(2008) on the distribution of infectious haematopoietic necrosis in five
provinces; Iranian Fisheries Research
Organization Newsletter (56:42, 2008) on use of the growth hormone
somatotropin to increase body weight, daily weight gain and length; Akbari et
al. (2009) on feeding larvae with HUFA and vitamin C enriched Artemia
urmiana nauplii; Fazaeili et al. (2009) on deposition of pigments
from plants in various tissues (feed conversion ratio and specific growth rate
were affected by the experimental diets); Gashti (2009)
determined that machine trimming of fillets produced less waste than hand
trimming, although hand trimming was faster; Heydarnezhad and Purser (2009)
assessed food anticipatory behaviour in fish hand-fed in small raceways; Imani et al. (2009) on
feeding deprivation and re-feeding, showing that fish could tolerate and
compensate for starvation; Jafarian et al. (2009) on the increase in
growth performance and feeding efficiency in larvae fed probiotic larvae with
Daphnia magna meal; Lorestani et al. (2009) on
the interaction of male broodstock age and different dilutions on the production
rate of eyed eggs; Mahmoudzadeh et al. (2009) on
the effect of synthetic and alfalfa pigments on tissue pigmentation (feed
conversion ratio and specific growth rate were affected by the experimental
diets); Mousavi et al. (2009) on the antifungal activity on trout eggs of
a combination of essential oils from various herbs; Mousavi et al. (2009)
on identification of 7 species of Saprolegniaceae fungi from infected trout
eggs; Omran and Hedayatifard (2009) on fatty acid composition; Pooramini et
al. (2009) on use of yeast as a probiotic and its affect on growth, survival
and carcass quality; Rafaei et al. (2009) on the bioaccumulation of
copper, zinc iron and manganese in eggs during incubation, found to be lower
than EPA standards; Shafaeipour et al. (2009) on effects of canola meal
on growth, body composition and biochemical parameters and its use to replace
fish meal in diets; Soltani et al. (2009) on the use of Zataria
multiflora (Persian thyme) essential oil and its effects on egg hatchability
and survival of larvae compared with hydrogen peroxide and malachite green used
as anti-fungal agents; Soltani et al. (2009) on the experimental
pathology of Streptococcus sp.; Iranian Fisheries Research
Organization Newsletter (56:2, 2008 and 58 & 59:2, 2009) on culturing trout
using Silo tanks which are easier to maintain and have higher production than
cement tanks; Sourinezhad et al. (2009) on suppression of ovary development in
triploids and increase in body growth; Tavakoli and Akhlaghi (2009) on changes in serum and blood factors
after infection with the pathogen Aeromonas hydrophila; Akbary et al. (2010) comparing live food and an artificial
diet on survival, growth and body composition in larvae, the former two factors
being better with live food; Akhlaghi and Nouroziasi (2010) on identifying
probiotic vibrios to combat pathogenic ones by stimulating immunity; Chegeni
et al. (2010) on formulated diet with Artemia nauplii in the
diet to improve growth and survival of larvae; Dadgar et al. (2010) on cottonseed meal as a
total replacement for soybean meal; Dorofshan et al. (2010) on using
microsatellite makers to detect and asses hybridization with Salmo caspius; Fadaeifard et al. (2010) on iron and
lead amounts in farmed fish, their food and influent water in Chahar Mahall va
Bakhtiari being no threat to human health; Ghovati et al. (2010) on on
screening broodstock using microsatellite markers associated with body weight; Haghparast et al. (2010) on the
antioxidant properties of various sodium salts on refrigerated sticks; Kalbassi
et al. (2010) on reduced sperm quality when broodstock are stripped
weekly rather than biweekly; Mahboobi Soofiani et al. (2010) on
starvation effects on morphology and haematology; Meshkini
et al. (2010) on various combinations of Artemia decapsualted
cysts, nauplii and concentrated feed on growth and survival of larvae, nauplii
and nauplii plus feed being the best; Mohammadtaheri et al. (2010) used
microsatellite markers to screen broodstock for resistance to the IHN virus. Moogouei et al. (2010) on the effects of physico-chemical
parameters on growth in a raceway culture system; Zorriehzahra et al.
(2010) on hematological and biochemical parameters of fry in western Mazandaran,
helpful in diagnosing some diseases; Akbary
et al. (2011) on fatty acid and vitamin C enriched Artemia nauplii
fed to trout larvae and the effects on growth, survival and resistance to high
temperature stress; Alinezhad et al. (2011) on
contamination of pellet feed with fungi and aflatxoxin B1; Ansari
et al. (2011) on the improving effects of natural (algal) over synthetic
astaxanthin in food on egg quality; Badzohreh et al. (2011) on the
supplement β-glucan (Macrogard) having a positive effect on growth and enhancing
efficacy of anti-Streptococcus iniae vaccine; Banaee et al. (2011)
on oral administration of the herbal medicine silymarin (an extract from milk
thistle seeds) affecting blood parameters at higher doses (800 mg/kg); Faramarzi et al. (2011) on longer
photoperiods increasing growth and feed utilization; Gholipour
Kanani et al. (2011) on anaesthetic effects of methanesulfonate, clove
oil and electroanaesthesia on lysozyme activity, with the first having the least
effect; Golchinfar et al. (2011) on activity of digestive enzymes during
fry development in relation to optimal aquaculture feeding; Haghighi et al.
(2011) on the improving effects of oral recombinant bovine somatotropin on
growth performance; Jafarian et al. (2011) on comparison of commercial
microbial products and those isolated from sturgeon guts on growth and survival
of larvae; Jalali et al. (2011) on gene expression; Kiaalvandi
et al. (2011) on the use of different fish meals as the main protein
sources for growth; Liaghat et al. (2011) on how the acquired immune
status of survivors of natural Streptococcus iniae infection was not
altered by immunization; Mavadati and Habibian (2011) on the comparative effects of
clove oil and 2-phenoxyethanol as safe anaesthetics; Mohammadzadeh and Rezaei
(2011) on dipping whole fish in green tea extract as an anti-oxidant to increase
storage on ice; Noori et al. (2011) on long photoperiods being effective
in growth and retardation of gonadal development in females; Oraei et al. (2011) on gamma irradiation and its lack
of effect on fatty acid composition in fillets; Rahimi et al. (2011) on
triploid hybrids with Salmo caspius not having salinity tolerance; Rami et al. (2011) on
hormonal sex reversal using synthetic estrogen as all-female stocks are
economically advantageous, growing larger and maturing later than males;
Salamatdoustnobar and Ghorbani (2011) and Salamatdoustnobar et al. (2011)
on using prebiotics to improve meat quality and growth; Sheikholeslami et al.
(2011) on use of inulin as a prebiotic and the resulting increased resistance to
Streptococcus bacteria; Tahmasebi et al.
(2011) on dietary nucleotides havig positive effects on serum complements and
resistance to Streptococcus iniae; Vahidi and Ghodratizadeh
(2011) on the enhancement of immune response with a chitin-supplemented diet;
Zolfaghari et al. (2011) on on determining shelf-life of fresh fillets (5
days at 4°C); Bakhtiyari et al. (2012) on varying dietary protein levels
at different feeding times and the effect on nitrogenous waste production in a
semi-recirculating rearing system; Moahmmadi Azarm et al. (2012) on
soybean and egg lecithin effects on growth, survival and resistance to anoxia
stress of alevins, with the latter being best; Moradyan et al. (2012) on low stocking density having better effects on
alevin survival and growth; Saeedi Far
et al. (2012) on the effects of diazinon, an organophosphate pesticide, on
haematological indices of fry; Zarghami et al. (2012) evaluated
oxytetracycline resistance of Aeromonas hydrophila isolated from reared
trout, which resistant bacterial infection or resistant genes could be
transferred to humans; etc. Numerous further studies on this
economically important species in Iran are not all documented here. The Department of the Environment has arranged fishing matches for
this trout in the Lar Dam since 1994 (www.iran-doe.org/Special/LAR.htm,
downloaded 29 December 2000).
Robins s et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaculture and aquaria,
as food, in sport and in textbooks. Rainbows have been used
extensively as research animals as their requirements are well known
and they are readily available from hatcheries. Hatchery fish often
have reduced or absent fins and deformed mouths. Farmed fish are sold
frozen world-wide, the most important trout in this regard. Rainbow
trout are one of the top few sport fishes in North America and of
great commercial importance because of the money spent on gear,
accommodation, transport, etc. by anglers in pursuit of this fish.
Many books and articles have been written on the methods and joy of
catching this trout. The flesh is excellent eating fresh or smoked and
may be red if food is mostly invertebrates or white if food is fishes.
Conservation
Alborz trout have escaped and made their way to the Caspian Sea but
the numbers and impact are unknown (Bartley and Rana, 1998b). This
species is an exotic and does not need conservation but escapees may
affect survival of native species.
Further work
Bartley and Rana (1998b) make various recommendations for the
aquaculture of this species in Iran. The establishment of reproducing
populations in the wild should be carefully monitored because of the
dangers for native species posed by this exotic. Triploid all-female
trout should be considered.
Sources
Sources are summaries of biology in North America such as Scott and
Crossman (1973). Observed on trout farms in Iran but there is little data on escaped Iranian populations.
Iranian material: no preserved material.
Members of this genus comprise about 25 nominal species found in North
America and Eurasia including the Black, Caspian and Aral seas basins
and the upper, and cooler, reaches of neighbouring basins in Iran and
Turkey including the Euphrates River, and the Orumiyeh and Namak lakes (Berg,
1948-1949). A single, variable species was recognised throughout Europe and east
to Afghanistan, including Iran (Salmo trutta Linnaeus, 1758) but recent
studies indicate a greater diversity (Turan et al., 2011).
These
fishes have teeth on the shaft and head of the vomer bone in the roof of the
mouth (may be lost with age), the jaw is long, reaching to the posterior eye
margin or beyond, scales are small usually more than 100 in the lateral line,
colour is pale silvery with dark markings or spottings, anal fin rays (all
counted) number 15 or less (16 or more in Oncorhynchus), and there are
various unique osteological characters.
These are famous sport fishes and were once caught in commercial quantities. Their biology has been studied extensively
and numerous books and papers have been written about them.
Salmo salar Introduced to the Caspian Sea (Mamaev, 2002). No Iranian record.
Salmo caspius

Duane Raver, U.S. Fish and Wildlife Service
قزل آلاي رنگين كمان (qezel ala-ye ranginkhaman,
meaning rainbow trout).
Linnaeus, 1758
Kessler, 1877
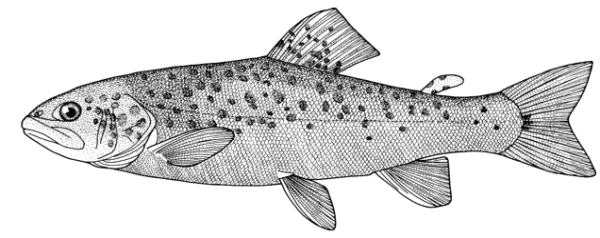



Hevigh River, Caspian Sea basin,
July 2010, courtesy of K. Abbasi

Lar Dam,
September 2008, courtesy of K. Abbasi
Common names آزاد ماهي (azad mahi or mahi-ye azad = free fish, used in Farsi
for trout and salmon), ماهي آزاد (= mahi azad), azad mahi qezelala-ye daryacheh khazar (= Caspian Sea
trout), mahi azad Darya-ye Khazar, mahi azad setareh-i Darya-ye Khazar,
all for the subspecies caspius; قزل آلا (= gazalala, ghezel ala or
kizil ala, meaning red spots), قزل آلاي خال قرمز
(= qezelala-ye khalqermez; the Turkic qezel ala is
used in Farsi for trout and hence this last means "trout with red
spots" although literally it is a tautonym, "red spots with
red spots"), mahi azad-ye kezel ala-ye ilbumi (= native salmon
trout), qezel ala khalqermez yabumi, qezel ala-ye juibary (= brook
trout), all apparently for freshwater residents; mahi azade khal sorkh; qezel ala-ye
Liqvanchai (for trout in the Lake Urmia basin).
[gizilxalli or ala-balyk for freshwater residents and gizil balig
or kizil-balyk for sea-run fish in Azerbaijanian; Kaspi azatmahysy or kumja in
Turkmenian; ala-balukh Armenia; Kaspiiskii losos' or Caspian salmon for sea-run fish, and ruch'evaya
forel or brook trout and pestrushka for freshwater residents in
Russian; alabalik in Turkish; massialé in Kurdish; brown trout, brook trout, river trout, Caspian trout].
Systematics
Salmo Trutta was originally described from European rivers. Salmo
caspius Kessler, 1877 was described from the Bozh'em-Promysl (or
Bozhii Promysel) fishing grounds on the Kura River, Azerbaijan with 3
syntypes in the Zoological Institute, St. Petersburg (ZISP). Salmo
spurius Pallas, 1814 from the Terek River in the Caspian Sea
basin, Russia and Salmo lacustris var. Romanovi
Kavraiskii, 1896 from Lake Tabiszchuri in Transcaucasia are usually
regarded as synonyms of Salmo trutta. Salmo trutta ezenami
Berg, 1948 is a subspecies from Lake Ezenam in Dagestan (ZISP 28356)(Dorofeyeva
and Salmanov (2001)) though it may be deserving of species rank (Reshetnikov et al.,
1997). Salmo trutta ciscaucasicus Dorofeyeva, 1967 (see also
Dorofeyeva (1997) and Dorofeyeva and Salmanov (2001)) is described from the Keyranchay (ZISP 26244) and
is found in drainages of the western shore of the Caspian Sea, except
for the Kura River and, presumably, excepting Iranian rivers.
The native brown trout (freshwater populations) and Caspian salmon
(sea-run populations) of Iran were both usually referred to Salmo
trutta caspius Kessler, 1877 in the Caspian Sea and Namak Lake
basins (Saadati, 1977). Derzhavin (1929b) examined two small fish from
the Karaj River of the Namak Lake basin and recognised them as typical
"brook trout" (Salmo trutta). Nümann (1969)
considers that southern Alborz trout originate from Caspian stocks.
Some authors call the species Salmo fario caspius Kessler,
1877, e.g. Dadikyan (1986). Fricke et
al. (2007) and Naseka and Bogutskaya (2009) regard caspius as a full species.
Shirangi et al. (2011) used microsatellite DNA to compare spring and fall
migratory forms in Iran. Spring fish are more fusiform and silvery than fall
fish and this was thought to indicate a genetic difference. However, they found
low genetic differentiation and conclude that only one population exists in the
southern Caspian Sea. Tortonese (1954), Kuderskii (1974) and Kottelat (1997) discuss the
confused nomenclature of this species. Boulenger (1896), Berg
(1948-1949) and Derzhavin (1929b) consider Namak Lake basin trout to
be e Salmo trutta macrostigma (Dumeril, 1858), originally
described from Algeria as a distinct species. Turan et al. (20120 note
that Salmo macrostigma (Duméril, 1858) is restricted to Algeria. The Namak trout were
supposedly derived from Mediterranean populations via Persian Gulf
drainages but Saadati (1977) dismisses this on the grounds of a close
similarity between Namak and Caspian trout and the apparent absence of
trout from the Zagros Mountains and Persian Gulf drainages of Iran.
Osinov (1988) could not determine a Mediterranean route based on
electrophoretic evidence.
In the Lake Orumiyeh basin, apparently restricted to the "Lighvanchai"
(probably the Liqvan Chay, the town of Liqvan being at 37°50'N, 46°26'E),
is a population of brown trout which has been referred to as a
distinct subspecies (Anonymous, 1977). It has not been formally
described. Saadati (1977) considers all Iranian trout to be S. t.
caspius, with those in the Namak Lake basin being a distinct
"race", with some slight differences to typical S. t.
caspius (see below), and those trout in the Lake Orumiyeh basin
could be a new subspecies.
Earlier authors consider the Caspian basin species to be the same
as the Atlantic salmon, Salmo salar Linnaeus, 1758, but blood
serum electrophoresis (Ostroumova, 1970) support its position as Salmo
trutta (and presumably caspius) as does karyology and osteology (Dorofeyeva, 1965; 1967)
and enzyme electrophoresis (Osinov, 1984; 1988). Osinov and Bernatchez
(1996) consider trout from the Caspian Sea basin to be part of their
"Danubian" grouping, brown trout from the Black, Caspian and
Aral sea basins as opposed to an "Atlantic" grouping from
the Baltic, Barents and White sea basins (and also a tributary of the
upper Volga River in the Caspian Sea basin), based on allozyme and
mtDNA studies. Vera et al. (2011) concur. Novikov et al. (2008) examined allozyme variability in
trout populations from Iran and found them to be similar but diverging
significantly from other populations in the Caspian Sea basin. Vatandoust et
al. (2008) demonstrated population differences between rivers in Mazandaran
using meristic and morphometric characters. Turan et al.
(2009) described two new species of trouts from northern Anatolian streams, one
resident and the other migratory. A similar situation may occur in Caspian
streams where there are the two life history types, although the authors caution
that each situation should be investigated individually. They advocate a more
cautious treatment of trout taxonomy as such results have important implications
for management and conservation. Vera et al. (2011) found two main
clusters, using microsatellite data, connected by gene flow among river basins
in Iran,
presumably by anadromous fish. Jamshidi and Kalbassi (2011) examined random
amplified polymorphic DNA markers for spring- and fall-run trout from rivers
along the Caspian shore of Iran, and recognised them as conspecific. Hashemzadeh
Segherloo et al. (2012) used DNA and morphology to compare south Caspian,
Orumiyeh (including Liqvan Chai fish) and Namak populations. They concluded that
the Caspian and Orumiyeh populations did not differ significantly but the Namak
population represented a unique haplotype. All haplotypes fell within the
Danubian phylogenetic grouping as distinct members. The Namak haplotype may have
a centre of origin in the Caspian basin. A artificial hybrid of Salmo trutta caspius with Oncorhynchus
mykiss is reported on by Pourgholam and Noruzy Moghadam (1996).
Key characters
The dark-spotted back, light halos around some of the dark-coloured flank
spots, caudal fin not or only weakly spotted, teeth on the vomer shaft, and only
9-15 total anal fin rays are distinctive.
? from S. trutta? Morphology
Salmanov (1990) has demonstrated that brook, lake and sea forms of
trout from the Caspian Sea basin differ in head and body proportions
and in fin shapes although no populations in Iran were considered or
given taxonomic status. Osinov (1988) maintains that migratory and
non-migratory populations are the same species.
In the Caspian Sea basin dorsal fin with 3-5 unbranched and 7-12
branched rays, anal fin with 2-5 unbranched and 6-10 branched rays,
pectoral fin with 10-14 branched rays, and pelvic fin with 6-9
branched rays. Lateral line scales 108-134 (Salavatian et al., 2011;
Salavatian et al., 2011). Scale rows between end of the base of the
adipose fin and the lateral line 15-17, mode 16 (Turan et al., 2012). Gill rakers 15-23. Pyloric caeca 26-61. Vertebrae 55-61. Scales in lateral series for S. caspius 140-160, vertebrae 59-61 (Derzhavin, 1929b; Behnke, 1965).
See also below under Zoogeography. The chromosome number is
2n=78-84 (Klinkhardt et al., 1995), 2n=80 (Annual Report,
1995-1996, Iranian Fisheries Research and Training Organization,
Tehran, p. 61, 1997; Kalbassi et al., 2006) or 2n=80-82 (Dorofeeva, 1998).
The chromosome formula is 14M + 10SM + 56T with NF=104 (Kalbassi et al., 2006).
Meristic values for Iranian specimens are:- dorsal fin branched rays 9(1), 10(5) or 11(1), anal fin
branched rays 8(7), pectoral fin branched rays 12(7), pelvic fin branched
rays 7(1) or 8(6), and total gill rakers 17(3), 18(3) or 19(1).
The Liqvan Chay fish are similar to S. caspius in all
except colour, gill rakers being somewhat higher (18-22, mean 20.2)
and lateral series scales being higher (132-154, mean 142) but sample sizes were small.
Salavatian et al. (2011, 2011) found the Lar Reservoir population to be
distinct morphometrically and meristically from fish from the Republic of
Azerbaijan. Sexual dimorphism
Abdurakhmanov (1962) reports on two populations form Azerbaijan
where he found head length, predorsal distance, snout length and lower
jaw length to be greater in males and postorbital length greater in
females for both populations, while body depth, dorsal and anal fin
heights, and pectoral and pelvic fin lengths are greater in males and
eye diameter, pelvic-anal fin distance, head depth and interorbital
width are greater in females for one population.
Colour
A specimen from the Anzali Mordab had a dark brown-grey back and
top of the head with some round black spots, silvery-yellowish sides
with 3-4 longitudinal rows of small red spots with whitish halos, a
large black spot on the preoperculum and several smaller spots on the
operculum. The belly and lower head surface were white with minute
dark melanophores. The dorsal fin was a pale grey with 3-4 horizontal
rows of black spots and 1-2 series of 4-8 ellipsoid red spots
vertically. The adipose fin was overall yellowish with some grey
pigment while the upper half of the fin was more orange in colour. The
pectoral, pelvic and anal fins were a pale yellowish or nearly
colourless with grey pigment. The anterior 2-3 rays were coloured
orange. The caudal fin was greyish with the upper and lower marginal
rays dark orange. All fins lacked spots except one red spot observed
on the dorsal fin of a larger fish (19.8 cm total length). Smaller
fish (13.7-16.2 cm fork length) had 12-14 parr marks. The red flank
spots are recognised in the name "kizil ala". Generally in
the sea, this species has numerous, dark, x-shaped spots on silvery
flanks although some individuals have almost none. The fish caught by
Holmes (1845) mentioned below had a dirty olive-green back, dark brown
and red spots on the flanks, a roseate tint to the flanks, and a
golden belly. The migratory form was dark blue on the back, silvery on
the flanks and belly, and had dark spots on the flanks. Turan et al.
(2012) state that this species has few, black spots on the body, ocellated with
a narrow light ring, restricted to the back and upper part of the flank (about
upper third in fish larger than 200 mm SL), and absent from the mid-dorsal area
in front of thr dorsal fin.
Prosek (2003) illustrates in colour a trout from the Tigris River in Turkey,
from the Caspian Sea basin in Turkey and Armenia, and from the Liqvan Chay.
The Liqvan Chay population has 54-400 red to orange spots along its
flank (compared to only 30-50 generally in European brown trout)
giving it a ruby-red sheen (Anonymous, 1977). Only one Liqvan Chay
fish had less than 100 red spots based on 13 fish examined by Saadati
(1977) compared to a range of 27-134 for 13 S. t. caspius from
the Shah Neshin River near Ardabil, where only 2 fish had more than
100 spots. The spotting pattern consists of profuse black and red
spots and is distinct from all other Salmo trutta sensu lato which have
fewer and larger spots (Saadati, 1977). Colour photographs, courtesy
of Asghar Abdoli of the Agricultural and Natural Resources University,
Gorgan, of 3 fish from the Liqvan Chay dated 4 October 1994 with a
total length of 27.0 cm showed two fish with about 87 red spots while
the other had about 200 (accurate counts not possible because of body
curvature and silvery reflections). There was considerable variation
in spot size among the three fish, the one with most spots having
generally smaller spots which give the sheen referred to above, while
the other two fish have more discrete and larger spots. It is unknown
whether the Liqvan Chay population has been contaminated with other
stocks of Salmo trutta.
Size
The sea-run Caspian trout attains a larger size than freshwater
populations. Attains 51.0 kg and 1.5 m but most seen in Iran were 10-15 kg (Walczak,
1972). Length reaches 118 cm and weight 21 kg in Iranian samples but
such large fish are rare in Iran (Farid Pak, 1968b). Most Iranian
catches are 55-105 cm and 1.8-12.7 kg (Farid-Pak, no date). Holmes
(1845) reported one fish caught at the mouth of the "Mazzur"
River weighing 16 lbs (7.3 kg) and measuring 38.5 inches total length
(0.94 m). Nümann (1969) reports fish almost 1 m long in the Karaj
Reservoir in 1967. A fish 78 cm long and weighing 8 kg was worthy of
note in 1996 (Anonymous, 1996c).
Distribution
The Caspian salmon is found in the Caspian Sea and enters Iranian
rivers to spawn as well as being resident in Gilan, Mazandaran and Golestan
provinces (Sheil, 1856; Nedoshivin and Iljin, 1929; Kozhin, 1957; Nümann, 1966;
R. J. Behnke files; Armantrout, 1980; Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 28, 1996; Abbasi et al.,
1999; Abdoli, 2000). Formerly known from the
Anzali Mordab and its tributaries (Holčík and Oláh, 1992).
Reported prolifically from the Seh Hazar, Chalus and Babul
rivers (Fortescue, 1920). Found in the Safid River, Kargan River near Hashtpar,
Chalus and Babol rivers. Rare in the Aras (Berg, 1948-1949). Abundant
in the upper reaches of the Gorgan (G. S. Karelin cited in Berg
(1948-1949) although Karelin only visited the lower Gorgan himself). Reported
from Gorgan Bay, the southeast Caspian Sea, southwest Caspian Sea and
south-central Caspian Sea and from the Tajan,
Babol, Haraz, Sardab, Shirin, Ashek, Kelyareh, Nav, Tonekabon, Pol-e Rud, Lar,
Elarm, Ab-Safid, Kamardasht, Delichai, and Safid rivers (Kiabi et al., 1999;
Vatandoust et al., 2008; Abdoli and Naderi, 2009; Salavatian et al.,
2011 Shirangi et al., 2011). Jolodar and Abdoli (2004) give its distribution in the Caspian basin as in
upstream waters from the Aras to the Tajan, absent in the Atrak and Gorgan
rivers. Also found in the Jajrud (Ouseley, 1819-1823), in the Karaj River (Fortescue,
1924; Derzhavin, 1929b; Berg, 1949), and from the vicinity of Damavand (? Damavand
River) (Fraser, 1825), and numerous other localities in the Namak Lake basin
(see Armantrout (1980) for localities from the 1970s and Abdoli (2000) for more
recent general distributions). However Nümann
(1969) seems to indicate that fish in the Karaj River were stocked
about 100 years previously from Caspian Sea rivers.
Reports from the
Zagros Mountains in Iran are uncertain and those from mountains near
Kerman even more so! (Walczak, 1972). These last two records have not
been confirmed by specimens and with introduction of exotic material
may now be difficult to verify. Fraser (1834) reports that "Trouts
are found in several of the streams of Azerbijan and Kurdistan".
It may be worth noting that Heckel (1843b:995) reports that the
collector Theodor Kotschy "did not find trout....in the mountains
of Kurdistan. Since we know our collector's diligence, we doubt that
trout occurs there" but in (1846-1849a:254) Heckel states
"we must add one trout (Salmo), which tastes excellently
according to the report presented by our traveller; that trout occurs
relatively frequently in the mountains of Kurdistan, but has not been
seen by us". This is merely confusing: did Kotschy later find
trout or change his account, or did Heckel recall Kotschy's accounts incorrectly?
Turan et al. (2011) describe a new species of trout, Salmo tigridis,
from the Tigris River basin in Turkey and it is possible that this species
occurs in Iranian tributaries of that basin. It has 19-20, mode 19, scale rows
between the end of the adipose fin base and the lateral line (27-32, mode 28 in
S. caspius), a deeper and stouter caudal peduncle with depth 11.5-12.6%
SL, mean 11.8% (10.0-11.5, mean 10.4), fewer gill rakers 17-19 (19-21), a
shorter maxillary in males at 8.7-9.2% SL, mean 8.9% (9.4-11.2, mean 10.4), and
in general body colouration being grey in males and greenish to yellowish in
female (brownish) The Liqvan Chai in the Lake Orumiyeh basin contains a distinctive
trout and Liqvan Chay trout were stocked in the Ab-e Bazuft of the upper Karun
River (Tigris River) basin in the autumn of 1975 but this
failed (B. Sandford, in litt., 1979; Prosek, 2003).
Zoogeography
The origin of Iranian trout is probably from the north via glacial
lakes during the last or earlier ice ages and via the Volga River
system or, possibly, from the west via the Black and Mediterranean sea
basins (Tortonese, 1954; Nümann, 1969; Kuderskii, 1974; Osinov, 1984;
Farid-Pak, 1991). The initial colonisation of the Caspian Sea by this
trout apparently occurred much earlier than the late glacial period on
electrophoretic evidence (Osinov, 1988). The question of a northern
origin remains open however on present biochemical evidence (Osinov
and Bernatchez, 1996). The division between their Atlantic and
Danubian groupings occurred about 0.5-0.9 million years ago based on
their data, perhaps 0.7-2.0 million years ago when the division of
European and North American groups of salmonids are taken into
account. These authors note that populations within the Caspian Sea
basin have unique gene pools, suggestive of reproductive isolation,
and these fish are not simply the subspecies S. trutta caspius.
Bernatchez (2001) considers all Caspian trout to belong to the Danubian or Ponto-Caspian
lineage, one of five lineages, and that the centre of origin was probably from
drainages associated with the Cauacasus region of the Black Sea. The major
demographic expansion of this lineage probably occurred about 270,000-290,000
years ago. Bernatchez (2001) considers that populations of each sea basin
occupied by the Danubian lineage should be recognised as distinct evolutionary
lineages but he does not give them names.
Trout in the Zagros Mountains may be e Salmo trutta macrostigma
(Dumeril, 1858), if this is a valid subspecies, while some authors
consider Zagros trout to be S. t. caspius on zoogeographical
grounds but pure samples of this population have never been examined
systematically (Behnke, 1965). Boulenger (1896) and Berg (1948-1949;
1949) consider trout in the upper Euphrates and in the Namak Lake
basin to be S. t. macrostigma. Namak Lake trout are then
Pliocene relicts of this Mediterranean subspecies from an invasion via
the Persian Gulf region. However, the Namak Lake fishes generally show
evident affinities with the Caspian Sea basin ichthyofauna and the
trout themselves are very similar in appearance.
The Namak Lake basin trout are generally held in Iran to have been
planted by the royal family in the nineteenth century (Saadati, 1977).
The royal family had sporting camps on the Lar River and the
"Varang-e Rud", a tributary of the Karaj River. Saadati
(1977) compares 30 specimens from each of these two localities and
found characters to be similar except for pyloric caeca (45-58, mean
51 in the Karaj sample, 35-46, mean 39 in the Lar sample) and in
scales in lateral series (131-146, mean 138) and 121-134, mean 127
respectively). He concluded that Karaj River fish are native, of
relatively recent origin from the Caspian Sea basin, and only slightly divergent.
Saadati (1977) also reports on a population of trout in the Mordugh
Chai (probably Mordaq Chay) in the Lake Orumiyeh basin. This
population was typical S. caspius with only 17-38 red spots
on the flank. It may have been of recent origin via a headwater
transfer as the tributaries to the Caspian Sea basin are found nearby.
A human agency may have been involved, always a complicating factor in
the distribution of fish which are of sporting or commercial interest.
Habitat
Mahi azad live in the Caspian Sea proper and migrate up rivers to
spawn. Moosari (1996) describes migration into the Tonekabon River of
Iran. Young mahi azad stay in rivers for 2 years. There are also
land-locked populations such as that of the Lar River near Damavand
Mountain and this species may also be found in cool to cold lakes.
Areas with clean gravel are required for reproduction. Temperatures
above 15°C cause egg mortality. Adults can survive up to 29°C,
18-24°C is the optimum range and 12.4-17.6°C the preferred range. Atai Mehr
et al. (2006) found juveniles to survive salinities of 0 to 12.5 g/l over
120 hours, indicative of their adaptability to changing environments. The best
weight and water salinity for release of cultured juveniles was 10 g and 8.0-12.5 g/l.
Bourani et al. (2010) found that weight groups of 10, 15 and 20 g could
carry out hypo-osmoregulation of water the salinity of the Caspian Sea but that
this ability is not evident in 5 g fish. In the sea, they inhabit coastal areas at 40-50 m with distinct
stocks centred on the basins of the major rivers. Migrations occur from Iranian
shores to Dagestan (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
The Liqvan Chay population females only begin to mature in their
third year and only 50% of males are mature in their second year. This
population appears to be in good condition compared to other
populations, e.g. in the Lar River, and these figures probably
represent more natural values. A population estimate was 1246 fish per
km compared to 11,380 per km in part of the Lar River where fish had
concentrated to avoid severe drought. The life span in the Lar is 6
years (Nehring, 1975a).
There are stocks of dwarf males in Caspian rivers which do not
descend to the sea.
Farid Pak (1968b) found the smallest sexually mature fish in the
Caspian Sea was 53 cm long and the largest 105 cm. Most fish in the
commercial catch of the Caspian Sea were 5-9 years old in the 1950s
(Farid-Pak, no date). Caspian Sea fish may mature as early as 1 year for males
under riverine conditions but most fish mature at 3-9 years, depending on the
river (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Afraei et al. (2000) examined 190 fish from the Tonekabon River and found
age groups 0+ to 4+. Most fish were in the 2+ age group. Maximum size was 175 mm
and 84.5 g. Condition factors were 1.268 for males and 1.257 for females.
See also below under Reproduction.
Niksirat and Abdoli (2009) and Abdoli et al. (2011) found a decline in length from 77.6 cm in 1947 to
59.8 cm in 2007 (22.4%), in weight from 4880.2 g to 2486.8 g (49.5%), in
absolute fecundity from 7041.8 in 1947 to 4526.1 in 1973 and 2941.2 in 1986, and
in relative fecundity from 1451.4 per kg body weight in 1947 to 1372.6 in 1973
and 1199.8 in 1986 (only latter value significantly different in relative
fecundity). Size classes over 75 cm have disappeared from the population over
the past 60 years and mature samples were less than 50 cm. Various factors cause
such declines, including fishing mortality and overfishing of prey such as
Clupeonella spp. (and their loss from the exotic comb jelly), an important
food which helps produce more eggs. Kheyrandish et al. (2010) examined
fish from six rivers in Mazandaran and found five age classes (0+ to 4+) with
the most frequent being 1+ and 2+. Condition factor ranged from 0.58 to 1.47.
Females dominated over males in 5 of 6 rivers, the highest sex ratio being 6.7
females:1 male in the Pajimiane River and 0.8:1 in the Khojirood. There were
significant correlations between fish length and depth and fish length and width
of rivers surveyed. Fazli et al. (2011) studied trout from the Lar Dam
and found a significantly different male:female sex ratio at 1:1.84, a life span
up to 6 years with age 4 the largest age group, a length-weight regression of W
= 0.028L2.706 for females, W = 0.034L2.624 for males
showing negative allometric growth for both sexes and W = 0.029L2.710
for the population, growth parameters were L∞ = 45.0 cm, K = 0.27 yr-1,
t0 = -0.23 yr, instantaneous coefficients for natural, total and
fishing mortality were estimated to be 0.40 yr-1, 0.75 yr-1,
and 0.35 yr-1and the exploitation rate was 0.47 These results
indicate a fast growth and a population in good condition in the study year of
2004. Nümann (1969) found Alborz trout to reach 26 cm at the end of 3
years, corresponding to the growth rate for central European fish.
Food
Freshwater populations are known to eat mayfly larvae in Iran as
well as other aquatic insects and crustaceans and terrestrial
insects taken at the surface. Larger specimens elsewhere are known to
take fish and crayfish, and more rarely frogs, salamanders and rodents
and this probably occurs in Iran too. Tonekabon River fish (Afraei et al., 2000) fed most
intensively in spring and least intensively in autumn. The main prey was
Simulium, Plecoptera and Ephemeroptera. Salavatian et al. (2011)
examined fish from the the Elarm, Ab-Sefid, Kamardasht and Delichayi rivers in
Lar National Park where food was Chironomidae pupae and larvae (91.5%),
Coleoptera (6.4%) and others 2.1%. Cannibalism was rare In the sea, young feed on amphipods, mysids and shrimps while
adults take common and anchovy kilkas, silversides and shads.
Reproduction
This fish runs up rivers in October-November and March-May in Iran
(Roux, 1961b). Holčík
and Oláh (1992) record migrations into the Anzali Mordab and a run up
the Pasikhan and Siahdarvishan rivers from late September to the end
of December. A second run occurred in April-May but was much smaller.
Holmes (1845) reports "salmon" in rivers of Gilan from June to the end
of October. Bartley and Rana (1998b) report the spawning run on the
Tonekabon River to be from September to November and spawning to be
from November to December. Spawning occurs on riffles of sand and gravel beds. Spawners are 8-12 years old and are mature
at 700 g. About 1500 eggs are produced for each kilogramme of female. The fish
examined from the Tonekabon River (Afraei et al., 2000) had an
absolute fecundity of 168-379 eggs, average 268 eggs.
Maximum egg diameter is 6.1 mm, number of eggs per gram reaches an
average of 14.7 and absolute fertility reaches 13,468 eggs in Iranian
fish which are considerably smaller than Kura River fish in Azerbaijan (Farid Pak, 1968b).
Alborz trout are ripe at 2-3 years while Caspian Sea fish spend 2-3
years in rivers, then 2 years in the sea before re-entering rivers to
spawn at 60-70 cm. Spawning can occur more than once in a life span (Nümann, 1969).
In the Kura River of Azerbaijan there are two races. One enters the
river in October and travels no higher than the middle reaches 600-700
km from the mouth, is sexually mature and does not weigh more than 12
kg, and spawns in the same year. The second begins to enter the Kura
in October but most fish run in November and December, this race spawns in the upper
reaches about one year later (8-11 months) after a long migration of over 1000
km, and attains 51 kg. There are two races also in Iranian rivers (Berg,
1959). The Kura salmon may have up to 45,000 eggs of up to 6.5 mm
diameter. Kura salmon spawn only once between ages 5 and 9 years. Some
Kura salmon become smolts and migrate to sea in the first year of life
but most descend in their second year.
Parasites and predators
Mokhayer (1976b) records the protozoan Trichodina from the
gills of trout (? this species) in the Karaj River, the cestodes Eubothrium
crassum and E. rugosum, the acanthocephalan Corynosoma
caspicum, and the annelid Piscicola geometra.
Jalali et al. (2005) and Malmberg et al. (2007) summarise the occurrence of Gyrodactylus
species in Iran and record G. derjavini from fish in the Sardab-rud.
Sattari et al. (2005) surveyed this species in the
Chesli and Khorma rivers, recording Cystidicoloides ephemeridarum.
Sattari et al. (2004) record the nematode Cystidicoloides
ephemeridarum sp. from this species in Gilan. Economic importance
In a survey of the Lar River in June, RaLonde and Walczak (1970b)
found 22 fishermen had caught 222 trout with an average length of
19.85 cm, comparable with an earlier survey by Nümann (1964). The
largest fish was 27 cm long. The catch was 2.56 fish per fisherman per
hour, a good rate of success (but see older records below). This rate
and the production of trout in this river was expected to decline
drastically with construction of a dam on the Lar. Sport fishermen
took 50,000 trout from the Lar River in 1967 (Surber, 1969).
Malek-Eizadi (1993) gives a recent account, in Farsi, of the Lar trout
and apparently confirms its decline. Floor (2003) cites older records of fishing
in the Lar River. Salavatian et al. (2011) indicate that 17,862 licences
were issued for summer 2006 fishing in Lar Reservoir. Azad mahi were caught in weirs, nets and with long, 3-pronged forks year
round but the principal seasons were spring and autumn (Holmes, 1845; Floor,
2003).
The catch in the Safid River region was largest from February to
April, with a maximum in the middle of March. In 1912-1913, a total of
1180 fish were caught in the Safid River, Anzali and Astara regions.
Weights reached 10-12 kg, average 7.5 kg (Nedoshivin and Iljin (1929)
cited in Berg (1948-1949)). Fortescue (1920) reported 1500-2500
"salmon" from the Seh Hazar at Shahsevar, averaging 8-10 lbs
(3.63-4.54 kg) each and seldom exceeding 18 lbs (8.17 kg). Nevraev (1929) reports on catches in
various regions of Iran in the early years of the twentieth century.
In the Astara region from 1901-1902 to 1913-1914, the catch ranged in
numbers from 236 to 1563 and in the Safid River region from 1916-1917
to 1917-1918 the range was 185-663 fish. Yearly catches of mahi azad
in the Anzali region alone have reached as high as 3,037 kg in
1939/1940 and as low as 90 kg in 1946/1947 before commercial fishing
was prohibited (Vladykov, 1964; Walczak, 1972). The Food and
Agriculture Organization, Rome reported only 1 tonne of
"salmonoids" caught in Iran for each of the years 1983
to 1985, presumably the Caspian salmon. Occasional catches are taken in
sturgeon nets of fish 1-4 kg in the Anzali region and elsewhere along
the Iranian coast (Holčík and Oláh, 1992). Many fish are taken by poachers in traps and nets
used to block spawning streams. Salehi (2008a) notes a decline in catches of this
species from 13 t in 1995 to less than 3 t in 2005 and most of the catch is
based on stocking rather than natural reproduction. There is a state supported stocking programme but growth rate is slow. High summer temperatures
(28°C) in the Caspian Sea would affect cage culture and survival (Rana and Bartley, 1998a).
Salmo trutta is reported
to be ichthyootoxic although there are no reports for S. caspius (Coad,
1979b). Conservation
Pietro delle Valle, who traveled in Iran from 1616 for 7 years,
reported trout from rivulets in Ardabil in the Caspian Sea basin (see
Pinkerton, 1758-1826, volume IX:84), a city not now noted for so
salubrious an environment for salmonids.
Ouseley (1819-1823) dined while at Tehran on dried and salted azad
mahi two feet long (0.61 m) from the Caspian Sea for breakfast one day
in November 1811 and on fresh trout (later referred to as
"kizl-áleh") from the Jajrud for dinner.
Vigne (1842) recorded catching six or seven dozen a day in the Lar
River near Tehran, Charles Murray reported in 1858 that the stream
"abounds so much in trout that I frequently kill 50 in an hour
with a fly" (recorded in Wright (1977)), Valentine Baker (cited in Prosek
(2003)) caught 50-60 trout a day up to 4 lb in weight, and Mounsey (1872)
caught 450 fish up to 1.5 lbs (0.68 kg) in 3 days using flies.
Anderson (1880) found that the Lar River "abounded in
trout". They were still numerous there in the 1970s (Nehring,
1975a). However this population was unusual in that growth rates and
population densities fluctuated drastically from year to year,
probably because spawning habitat and nursery areas were the only
adequate features of this river. Features lacking were feeding areas,
stable banks and stream bed, good plant cover along the banks to
supplement productivity, good physical and chemical water quality and
water levels, and presence of predators as controls on excess
reproduction. As a result, recruitment of young-of-the-year was high
and led to interspecific competition, earlier sexual maturity, and
stunting. Overgrazing in the Lar Protected Area (formerly a National
Park) has affected the survival of trout in this region since this
reduces the availability of insect food, and in 2000 this situation
was exacerbated by the prolonged drought (Imamai, 2000).
Numbers of this species have declined drastically as evidenced by
catch records. The catch decreased 100 times since 1959 to the early 1970s and smaller fish taken
in beach seines were used by fishermen to eat as their market value was low.
In the 1970s, the Shahsavar River was assessed as the best in Iran for this
species as the river was in good condition. However, even this river was blocked
in at least two areas for capturing fish and almost all adults were taken for
"culture" by Shilat. A take of 300 fish provide as many eggs as 20
should because of poor treatment (15°C, , Saprolegnia infections). The
Anzali Mordab was surveyed half a mile from a guard station and 11 gill nets
were found, all illegal, in a half mile stretch in half an hour (Carl Bond Archives, Oregon State
University, Corvallis). Niksirat and Abdoli (2009) report gill nets used in
estuaries catch migrating fish illegally and they are also caught as bycatch in
the Rutilus frisii kutum fishery, but not released. About 2-3 times the
the legal fishery for artificial reproduction is taken by poaching. Attempts to increase natural reproduction by raising
young to a size more likely to survive have been attempted in Iran
(Andersskog, 1970). The "Kelardasht" (or Shaeed Bahonar)
Fish Farm (part of Shilat, the Iranian Fisheries Company) on the
"Sandar" River has a production capacity of 100,000
fingerlings of 15-20 g but this has never been achieved (Woynarovich,
1985; Krasznai, 1987). It takes about 2 years before fingerlings reach
15-20 g and 10-15 cm, large enough to release. Brood stock are
collected from the Tonekabon River during the September to November
spawning migration. Spawners are 8-12 years old and mature at 700 g.
New brood stock are collected each year although stock from previous
years are kept as backup. In 1997, 500 fish were caught in a 3:1 ratio
of males to females. Eggs from two females are mixed with milt from
two males. Egg yield is about 1500 eggs/kg. Growth rate is slow in
this species, compounded by low rearing temperatures of 2-17°C.
Fingerlings take 10 months to reach 10 g (Bartley and Rana, 1998b). In
a study of the Tonekabon River migration pattern, the following
conditions are found to be favourable for release of cultured fish:
release size should be at least 15 g, release point 500-700 m away
from the estuary, release time March and October, and for one-year-old
fish smolt release is favoured over parr as the former would migrate
to the sea (Annual Report, 1994-1995, Iranian Fisheries Research
and Training Organization, Tehran, p. 28, 1996). Fingerling
production by government hatcheries was 0.01 million in 1986, 0.05
million in 1988, 0.10 million in 1989, 0.16 million in 1990, and 0.20
million in 1991 and in 1992 (Emadi, 1993a). Fingerling production in
1995 was 0.8 million and in 1996 was 0.42 million (Bartley and Rana,
1998a; 1998b). In 2002 and 2003, 344,000 and 325,000 fingerlings were released
at a cost of 2125 and 2288 million rials respectively (Salehi, 2008a). The "Gharasoo" Research Station in Sari is
using cage culture of this species (Madbaygi, 1993b). Smolts weighing
25 g on average were released into cages and fed on locally prepared
food (Iranian Fisheries Research and Training Organization
Newsletter, 4:5, 1994). Culture of a rapid-growing triploid sea
trout has been studied in Iran (Annual Report, 1994-1995, Iranian
Fisheries Research and Training Organization, Tehran, p. 32, 1996;
Annual Report, 1995-1996, Iranian Fisheries Research and Training
Organization, Tehran, p. 67, 1997).
Sayyad Borani et al. (2006) studied the effects of weight on the
osmoregulatory ability of juveniles in an attempt to determine the ideal weight
for release (see also above under Habitat). Weight classes were 5, 10, 15 and 20 g and the three larger classes
were capable of osmoregulation in Caspian Sea water. Mojazi Amiri et al.
(2005) detailed the histology of the digestive tract from hatching to parr stage
in order to better understand the digestive system capabilities and enhance
rearing. Asaeian et al. (2006) studied brood fish taken from the early
mid and late migrations, finding those from the early period were more suitable
for offspring production, passing through smoltification more rapidly. However,
they noted that fish from all periods should be used to maintain genetic
diversity. Bahrkazemi et al. (2006) traced the histochemical changes in
the digestive tract from hatching to parr stage. Javaheri Baboli et al.
(2006) examined the effects of n-3 HUFA enriched Artemia nauplii as a
starter food and showed it was no better than newly hatched Artemia. Sarvi et al. (2006), Hatef et al. (2007), Niksirat
et al. (2007) and Sarvi Moghanloo et al. (2007), have studied cryopreservation of sperm, sperm and seminal
plasma composition, and in vitro storage of unfertilized ova in Iran as
means to help conserve wild populations. Research into osmotic regulation
determined that fingerlings 10 g or above have a higher chance of survival (Iranian Fisheries Research Organization
Newsletter, 53:4, 2008). Ataei Mehr et al. (2007) studied the effects
of weight and salinity on the number and size of Malphigian bodies in juvenile
kidneys which has relevance for release of cultured fish in fresh and salt
water. Saber et al. (2007) found that for larvae a
diet with 50% protein and 4600cal/g energy was appropriate. Jamalzadeh et al.
(2008) established haematological and serum biochemical indices for smolts,
juveniles and breeders. Zamani et al. (2007) found differences in
activity of some digestive enzymes in the stomach, pyloric caeca and intestine
of parr and of smolt. Bahrekazemi et al. (2009) studied the
relation between egg viability, egg and ovarian fluid composition and time of
stripping in fish at the Kelardasht Hatchery. Eyeing and hatching rate declined
with over-ripening time although larval abnormalities remained constant.
The best time to take eggs was up to 10 days post-ovulation. Mahmoudi et al.
(2009) fed different levels of dietary nucleotides showed positive effects on
growth performance and a decrease in liver demolition. Ramezani (2009) fed
fish experimental diets and found a better feed conversion ratio and specific
growth rate at lower protein levels, 50% protein supporting maximum growth.
Bahre Kazemi et al. (2010) investigated the use of biochemical and
histological parameters as egg quality biomarkers. Hajirezaee et al.
(2010a; 2010b, 2010c) examined the chemical and physical properties of seminal fluid and its effect
on sperm motility, and effects on semen characteristics of various stripping
frequencies. Oulad et al. (2010) looked at the effects of different
levels of dietary nucleotides on osmoregulation of pyloric caeca in in
juveniles. Rahbar et al. (2010, 2011) examined three female broods (4,
5 and 6 years old) and found that the 6 year olds showed maximum average
fertilisation (97.5%), survival rate until eyed stage (92%), hatching (93%) and
survival rate until absorption of the yolk sac (94.5%). Ahmadi et al.
(2011) experimentally infected trout with the bacterial pathogen Aeromonas
hydrophila and studied blood and tissue changes. Maniei et al. (2011)
investigated the selective use of body fatty acids by parr under feeding,
starvation and re-feeding regimes. Sotoudeh et al. (2011) found that 4%
dietary phosphatidylcholine had positive effects on growth, nutrition factors
and liver fatty acid composition of alevins. Amiri Moghaddam et al.
(2012) found starvation had negative effects on ion regulatory capacity of parr
and re-feeding did not fully compensate for this. Sourinejad et al.
(2012) examined the effects of milt volume equalization, which did not result in
a balanced contribution of breeders and also produced large variations in
contribution to progeny. Toorchi et al. (2012) found that larger juveniles performed
better in response to salinity challenges, concluding that releasing large
juveniles in stocking programmes in estuaries was safe but that smaller
juveniles should be released into rivers or be acclimatised to estuarine
conditions before release there. Salehi (2008a) carried out a cost
factor analysis for fingerling production at the Kelardasht Hatchery in Iran and
found labour costs to be 52% and feed 16%. The average cost of production of a
fingerling was U.S.$0.84 (6269-7123 rials), more than that for sturgeon
(1753-2028) and kutum (54-121). Higher costs are probably associated with lack
of broodstock, lower production quantities and the scale of the hatchery. The
rate of return at age 3-4 years was estimated at 0.5% of those released
annually. RaLonde and Walczak (1970b) and Walczak (1972) lists the following
reasons for decline of this species: lowering of the Caspian Sea level
making spawning migrations difficult, habitat changes including
siltation, pollution, irrigation dams, and unscreened irrigation
intake canals, and poaching. Both adults and freshly released
fingerlings are heavily poached (Petr, 1987). In the Lar and Karaj
rivers about 1960 professional fishermen took trout using nets,
chemicals and explosives until laws regulating seasons and equipment
were passed and game wardens hired to enforce them (Surber, 1969).
Streams running into the Caspian have their lower 1-3 km dried up by
May through irrigation demands and trout are unable to reach the sea.
Stronger enforcement of laws by game guards, education of the public,
particularly local people, habitat protection and improvement, and a
rational exploitation system are required to protect this species.
Firouz (1974; 1976) reported that the sea-run form was a major
commercial species 25 years prior to his account but the deteriorating
environment coupled with dynamiting, netting and trapping severely
reduced populations. This fish is now completely absent from the
Anzali Mordab (Holčík and Oláh, 1992). Waters where populations survived were designated
"Protected Rivers" in an effort to manage this species
effectively. There are special licence requirements, bag limits and
seasons which anglers must observe. A fine of 10,000 rials is imposed
specifically for illegal fishing of this species (Anonymous, 1977-1978) and each
fisherman is now limited to 3 fish per day with the season lasting from 11 July
to 10 September for two days a week (Salavatian et al., 2011). The Liqvan Chay population has been confined to this single river
by, it is believed, destruction of habitat through agriculture and
domestication of sheep and goats (Anonymous, 1977). De Mecquenem
(1908) said trout were abundant in the upper reaches of rivers in the
Lake Orumiyeh basin. In 1975, 500 specimens of this subspecies were
transplanted into the Ab-e Bazoft near Shiraz in an effort to conserve
it (Anonymous, 1977).
Caspian salmon used to enter the Kura River basin, with 20% running
up the Aras River on the Iranian border. Most spawning beds on the
Aras became inaccessible in the 1950s with the construction of the
Bagramtapinskaya Dam. The Mingechaurskaya Dam on the Kura cut off 80%
of that rivers spawning beds in 1951. The former Soviets built salmon
hatcheries to offset the losses but these proved to have a low
efficiency. The young fish had a high mortality because of release at
unsuitable high temperatures, release in poor feeding areas, and
exposure to water intakes. In addition, the released fish were too
small or too few to undergo significant smoltification and migration
to ensure subsequent reproduction. Under natural conditions only a
small proportion of juveniles undergo smoltification (Bakshtanskii et al., 1973).
Kiabi et al. (1999) and Jalali et al. (2009) consider S. caspius to be
critically endangered in the south Caspian Sea basin according to IUCN
criteria. Criteria include sport fishing, few in numbers, habitat
destruction, medium range (25-75% of water bodies), absent in other
water bodies in Iran, and absent outside the Caspian Sea basin. Kiabi et al. (1999) also consider S. t. fario to be vulnerable in the south Caspian
Sea basin according to IUCN criteria. Criteria include sport fishing,
medium numbers, habitat destruction, medium range (25-75% of water
bodies), present in other water bodies in Iran, and present outside
the Caspian Sea basin. Coad (2000a), using 18 criteria, found this species
to be one of the top 4 threatened species of freshwater fishes in Iran. Nezami
et al. (2000) consider this species to be endangered because of
overfishing, habitat destruction and spawning ground degradation. Mostafavi
(2007) lists it as vulnerable in the Talar River, Mazandaran.
Critically endangered in Turkey (Fricke
et al., 2007). Jalali et al. (2009) recommend reduction in pollution,
waters where fish are released should be designated Protected Rivers, cage
culture should be enhanced, spawners from different populations should be used
to enhance genetic diversity in restocked populations, and gamete storage
protocols could be applied to commercial aquaculture or to a biological
conservation programme. Shirangi et al. (2011) note that successful
propagation is of fall run fish and the numbers of spring run fish have
declined. Osinov and Bernatchez (1996) note that populations within the
Caspian Sea basin have unique gene pools, suggestive of reproductive
isolation, and these fish cannot be managed as a single subspecies, S.
trutta caspius. Re-introductions and artificial maintenance of
native populations could lead to loss of diversity.
Yousefian et al. (2012) found a high heritability of body weight and
length using broodstock of different age and weight. Further work
Togan et al. (1995) show that two Turkish populations of
brown trout are genetically distinct, at a level often found between
species. Genetical analysis of suspected surviving pure populations of
Iranian Salmo caspius should be carried out to determine which
stocks should be receive special attention for conservation.
Sources
Iranian material:- CMNFI 1970-0551, 5, 109.9-166.3 mm standard length, Gilan, Ghaleh River near Fowman (37º13'N, 49º19'E);
CMNFI 1979-0086, 2, 76.5-145.5 mm standard length, Mazandaran, Hasanabad River tributary to Chalus River (no other locality data);
CMNFI 1980-0133, 3, 116.8-139.2 mm standard length, Markazi, Karaj fish hatchery (no other locality data);
CMNFI 1980-0158, 1, 93.3 mm standard length, Markazi, Polurd River at Damavand Mountain after Ab-e Ali (35º51'N, 52º04'E);
CMNFI 2007-0125, 3, 91.2-169.3 mm standard length, Markazi, Luniz River in Karaj River basin (no other locality data);
CMNFI 2007-0126, 7, 95.2-158.3 mm standard length, Azarbayjan-e Khavari, Aldarvish River, Sabalan Mountain (no other locality data);
BM(NH) 1877.7.5:1, 7, 168.1-194.7 mm standard length, Tehran (no other locality data); BM(NH) 1908.8.7:27-28, 2, 129.0-145.7 mm standard length, north slope of Elburz
Mountains near Tehran (no other locality data).
Salmo tigridis This species is recently
described from the Tigris River basin of Turkey (Çatak Stream - and see below
under Salmo trutta for some biology). It probably exists in Iranian
waters but requires confirmation by specimens.
The photograph below is a fish from the Tigris River basin.

Chesli River, Gilan, June 2010,
courtesy of K. Abbasi

Turan, Kottelat and Bektaş, 2011
Salmo trutta
Common names
See above under Salmo caspius where general names for trout will
encompass this species.
[Brown trout, German brown trout, European brown trout, sea trout, brownie, Loch Leven
trout, Von Behr trout, spotted
trout and liberty trout]. Systematics See above under Salmo caspius. Key Characters
?This species is a relative of the Atlantic Salmon and is distinguished from it by the upper jaw extending beyond the eye in adults
(and below rear half of the eye in young rather than the centre in young), the gill cover has many spots, dorsal fin principal rays
usually 10-14, and orange to rusty-red spots are often present on adult flanks.
Morphology
Principal anal fin rays 9-12, pectoral rays 13-14 and pelvic rays 9-10. Lateral line scales 110-136 and pyloric caeca 30-60. Males
develop a hooked lower jaw when spawning and the brown colour becomes more intense and golden.
Sexual dimorphism Males develop a hooked lower jaw when spawning and
the brown colour becomes more intense and golden.
Colour
Overall colour is a light to golden brown with silvery flanks and a white to yellowish belly. The back, flanks, side of the head and
dorsal and adipose fins bear black or dark brown spots, often with a lighter halo of orange, pink or red, and the flank has pink or red
spots. Only brown trout have both light and black spots on the flanks. The caudal fin may have spots restricted to its upper lobe but
lacks the overall radiating spots of rainbow trout. The adipose fin is orange to orange-red, the only family member with this colouration. Sea run or lake fish are more silvery, obscuring some of the spots. Spots may be x- or y-shaped. The red flank spots
usually have blue halos. Young have 7-14, narrow parr marks and a few red spots
along the lateral line. The adipose fin is orange with a light margin.
Size
Reaches 1.4 m and reputedly 50 kg for sea-run fish. Note that as the taxonomy
of trouts changes this maximum may not apply. Distribution
Brown trout were artificially planted in Gahar Lake of the upper
Dez River of the Tigris River basin where viable populations
existed in the 1970s in both the upper and lower lake, and more recently (B. Sandford, in
litt., 1979; R. Mehrani, pers. comm., 2000). European brown trout
were planted in the Caspian Sea and Namak Lake
basin and established south of Dorud in the Zagros, and in the Zayandeh
River dam but their origin is unknown (Coad and Abdoli, 1993). Trout were also
introduced to the Karun River basin and the Zayandeh River Dam (Y. Keivany, in litt., 1992).
Trout are also recorded from the the Lake Orumiyeh basin in the upper Talkheh,
Zarreineh and Tatavi rivers (Abdoli, 2000) but whether these are introduced is
not certain.
Zoogeography This species is introduced in Iran.
Habitat
Brown trout are mostly stream and river dwellers although some are in lakes and ponds. They can tolerate warmer and more turbid
waters than brook trout and only the tainbow trout is more tolerant among salmonids. Brown
trout may survive in areas no longer
suitable for Brook Trout because deforestation has increased stream temperatures and agriculture and industry have increased pollution
and turbidity. Ideally there should be overhanging and submerged vegetation, coarse gravel and cover such as logs, boulders and
undercut banks, growth is better in alkaline waters (20-200 mg L-1, total dissolved solids 800 mg L-1 for
culturing this species), a depth of 7-58 cm is needed for spawning, a winter flow of less than 15 cm sec-1 is required,
preferred temperatures are 10.0-17.6°C (other reports give 21.1°C and temperatures up to 24°C are tolerated) and for spawning 6.7-8.9°C,
dissolved oxygen is optimal at 7-9 mg L-1 and pH range tolerated is 5.0-9.5 although the optimal range is 6.8-7.8.
Age and Growth
Life span is over 18 years with maturity attained at 2-4 years.
Çetinkaya (1999)
found the fish in the Çatak stream, of the upper Tigris River, Turkey to have an
age range of 1-8 years, a fork length of 8.4-39.0 cm, a weight of 6.7-756 g, and
a sex ratio 2.45:1 male:female. This population was described as S. tigridis
(see below) and is not the same taxon as
introduced populations in Iran. Food
Food is aquatic and terrestrial insects, crustaceans, molluscs, various fishes, frogs, salamanders and even small mammals. Most
food is taken as drift, the trout positioned in slower water behind a rock and darting out into faster current to seize prey.
Predators include other fishes, birds, water-snakes and otters. Çetinkaya (1999)
found the diet of fish in the Çatak stream, of the upper Tigris River, Turkey to
be Trichoptera, Ephemeroptera, Gammarus, chironomid pupae and larvae, and
Plecoptera. Reproduction
Spawning occurs from October to January depending on locality, usually at
7-9°C but as low as 2°C or as high as 14°C. It usually
takes place in gravel stream riffles but may occur on rocky shores of lakes. The female excavates a redd into which the spawning pair
deposit eggs and sperm while gaping and quivering over a 4 second period. The female covers the redd with gravel after spawning to
protect the eggs. Subsequent spawning occurs, usually after a 10 hour interval. Occasionally a community redd is excavated by a number
of fish spawning close together. Eggs are up to 5.0 mm in diameter and each female can contain 20,865. Young may spend about 2 years
in their natal stream before going to a lake but some fish remain permanently in streams.
Çetinkaya (1999)
found the fish in the Çatak stream, of the upper Tigris River, Turkey, to be
sexually mature at age 4 and 16-17 cm fork length with an individual fecundity
of 2349 eggs. Parasites and predators None known in Iran. Economic importance
This trout is a valuable and popular sport fish in Europe, The benefits
of stocking brown trout include its difficulty of capture (and thus appeal to anglers), its longer contribution to the fishery than
brook trout and rainbow trout, better survival and growth than other trouts, and its tolerance of warmer waters. There is an extensive
European literature on angling methods and it is reputed to be a wilier fish than
brook trout, for example. They tend to bite best in the late evening or at dawn, especially
when large. These trout are caught on worms, crayfish, lures and flies. Brown trout have white to pink flaky flesh and are excellent
eating. Flesh colour changes with age, to pink, and is related to diet.
Robins et al. (1991) list this species as important to North
Americans. Importance is based on its use in aquaria and aquaculture,
as food, in sport, in textbooks and because it has been widely
introduced outside its natural range. Salmo trutta is reported
to be ichthyootoxic although there are no reports for Iran or for S. caspius (Coad, 1979b; see also under the genus Schizothorax
for symptoms of this egg poisoning).
Conservation None required fro an exotic species. Further work
The distribution and biology of this trout in Iran needs detailed investigation
to separate it from native taxa. Sources Based on general accounts of
the species. Genus Salvelinus The charr genus comprises many species in Europe, northern Asia and
North America whose systematics have not been fully worked out. FishBase lists
83 nominal species (August 2007). The charrs have numerous small scales 105 or
more in lateral series), teeth on the jaws, palatines and tongue, teeth are
present on the head but not the shaft of the vomer bone in the roof of the
mouth, scales in the lateral line are smaller than surrounding scales and have
little or no overlap with scales before and behind, the body has light pink, red
or cream spots, and the lower fins have snow-white leading edges.
Salvelinus fontinalis
Common names
ماهي آزاد چشمه اي (= mahi azad
cheshmehi or azad mahi cheshmehi, meaning spring free fish, i.e. spring salmon, free
fish being used for salmon and trout species in Farsi), qezel ala-ye juibary (= brook trout).
[brook trout, brook charr, speckled trout].
Systematics
Salmo fontinalis was originally described from the vicinity of New York city.
Key characters
This species is characterised by 109-132 lateral line scales, 8-13
anal fin principal rays, light-coloured spots on the body, teeth on
the head of the vomer bone in the roof of the mouth, pectoral, pelvic
and anal fins with a white leading edge followed by contrasting black,
truncate caudal fin, dorsal and caudal fins have wavy, dark lines and
blotches and the back has dark or light green or cream, worm-track
markings (vermiculations).
Morphology
Dorsal fin principal rays 9-14, pectoral rays 10-15 and pelvic rays
7-10. Scales are minute, horizontal and irregular ovals with a central
focus, few circuli and no radii. The pelvic axillary scale is
well-developed. Gill rakers 13-22, reaching the second adjacent raker
whem appressed. Pyloric caeca 20-55. The gut is s-shaped. The
chromosome number is 2n=84 (Klinkhardt et al., 1995).
Sexual dimorphism
Spawning males develop a hooked lower jaw or kype.
Colour
The back is olive-green to dark brown or blackish fading to a
silvery-white belly. Flanks have a red to yellow tint. Flank spots are
pale but there are also small, red spots with blue halos. The
pectoral, pelvic and anal fins are yellow, orange, or reddish behind
the white and black leading edges. Sea-run fish have a blue-green back
and silvery flanks with a purplish tinge. Spots are obscured except
for the red ones. Brook trout in large lakes are also more silvery
than stream resident fish. The jaw tips and the roof of the mouth are
blackish. Spawning males are much brighter in overall colour and have
an orange-red lower flank and upper belly, bordered below by black on
each side which delimits the white belly. Young have 6-12 brown parr
marks, the widest equal to eye diameter, and small red, yellow or blue
flank spots. The white leading edge to the lower fins is apparent.
Size
Attains a reputed 86.0 cm and 8.0 kg, possibly 9.39 kg, but most are smaller.
Distribution
In North America this species is found from the shores of Hudson
Bay and Labrador south in marine waters to Cape Cod in the east and
Georgia in the Appalachian Mountains, west through all the Maritime
provinces, Québec and Ontario (except the extreme west) to northeast
Manitoba. Widely introduced to western Canadian provinces, the U.S.A.,
South America, Europe, Asia and Australasia.
A private hatchery on the Jajrud imported over 1 million brook
trout eggs which were raised to fingerling size only for most to be
lost in a flood in 1968. Some were planted in the Jajrud and in the
Latian Reservoir in the Namak Lake basin. Survival remains unknown. Also
recorded from the Sardab and Chalus rivers of the Caspian Sea basin (Annual Report, 1994-1995, Iranian Fisheries
Research and Training Organization, Tehran, p. 26, 1996) but this
is possibly a misidentification for Oncorhynchus mykiss.
Zoogeography
An exotic species from North America, not closely related to native Iranian salmonids.
Habitat
Brook trout are found in cool waters of streams and lakes, usually less than 20°C,
adults preferring 14-16°C. Spawning requires temperatures below 15°C, and below 9°C
for optimal hatching. The upper lethal limit is 25°C for adults and 20°C
for newly hatched fish. pH range is 4.1-9.5. Pools, underneath banks,
under overhanging bushes or behind rocks are favoured spots. During
summer months they retreat to deeper water, to about 8 m, in lakes.
Some populations run to sea in Hudson Bay and Atlantic Canada. They
stay in coastal waters, not moving more than a few kilometres from
their natal stream. Populations in the North American Great Lakes live
and feed mostly in the lake and run up natal streams to spawn.
Age and growth
Maximum life span is over 20 years but most reach only 5 years in
North America. Maturity is attained at 2-3 years, some males at 1
year. Stunting is common in small streams while sea run fish grow
faster than freshwater ones. Optimum growth temperatures are 10-19°C.
Food
Food in North America includes aquatic and terrestrial insects,
molluscs, various fishes, frogs, and even snakes, mice, voles and
shrews. Stream-dwelling fish feed heavily on drifting aquatic
organisms during spring run-off but in summer as drift decreases
surface insects become important. Most feeding occurs in the early
morning and late evening although some food is taken throughout the
day. Diet shifts in response to competition with other species. Sea
run fish take various marine invertebrates and fishes. Sea run adults
in spring and summer eat crustaceans and fish in lower estuarine areas
while young are in the upper estuary eating crustaceans and insects.
During the fall in the river adults eat little and during winter back
in the estuary consumed mostly crustaceans. There is thus a division
of food resources between young and adults.
Reproduction
Spawning in North America takes place from August to December,
earlier in the north and later in the south. Sea-run charr enter their
natal stream in spring and summer even though spawning occurs in fall.
Each year they spend 1.5-3 months feeding in the sea. The spawning
ground is usually gravelly streams but may be lake shoals if there is
some current or spring outflow to keep eggs oxygenated. Spring flows
are preferred even in streams. Males arrive on the spawning ground
first and defend a territory. Both sexes will rush at other fish
entering the redd area. The female cleans a redd of debris by turning
on her side and lashing her tail. Redd depth between stones is tested
by inserting the anal fin. Redd construction may take up to 2 days
with work being carried out both by day and night. Courtship involves
gentle pushes, touches and strokes of the female by the male. The
female is ready to spawn when she crouches in the redd with her
genital area between the stones. The male arches his body and may
press the female against the redd bottom, both fish vibrate and eggs
and sperm are shed. Accessory or sneaky males may rush in to shed
sperm. The female lashes her tail to push eggs into the gravel and
then dislodges gravel with her anal fin to cover the eggs to depths as
great as 20 cm. Yellow-orange eggs are up to 5.0 mm in diameter and
number up to perhaps 17,000 per female although averages range from
the low hundreds to a few thousand. Both sexes may spawn again with
other fish. The eggs develop over winter, taking 165 days at 2.8°C
but only 47 days at 10°C. Temperatures above 11°C will kill the eggs.
Parasites and predators
Brook trout are cannibals on their eggs and young and are eaten
themselves by other fishes, water snakes, turtles, various birds and otters.
Economic importance
Brook trout are very popular sport fish in North America caught on
lures, live baits and flies. These trout are easier to catch than Salmo
trutta sensu lato and take a wider range of lures. They fight well but are
often quite small and do not leap spectacularly like some other
members of the salmon family.
The ready availability of this salmonid has made it a useful
experimental fish for various physiological, biochemical,
toxicological and other studies. Some reared stocks however show
deformed or lost fins and distorted mouths.
Conservation
This introduced species is not in need of conservation but in fresh
water their preference for cool and clear water makes them susceptible
to loss if waters are dammed, channelised and polluted or if banks are
eroded deforested and overgrazed, conditions not uncommon in Iran.
Further work
The survival of stocks of this species in Iran should be verified
and the effects of this exotic on native species researched.
Sources
Based on general summaries on North American populations such as
those in Scott and Crossman (1973) and Becker (1983) as no Iranian material to
hand. Iranian
populations have not been studied.
Genus Stenodus The inconnu genus contains a single species found in waters
draining to the Arctic Ocean in Eurasia and North America and in the Caspian Sea.
Its characters are essentially those in the species description; a
small bone lacking a head canal on the outer side of the lower jaw at
the joint of the articular and dentary is distinctive. Branchiostegals number 8-11.
Stenodus leucichthys
Common names
safid mahi (= white fish), آزاد ماهي (azad mahi or free fish), azad
mahi Volga (= Volga free fish, or
Volga salmon, free fish being used in Farsi for salmon), زيبا (= ziba), mahi-ye ziba or
ziba mahi (= beautiful fish, in northwest Gilan, e.g. at Astara),
mahi-e-azad-e-ziba, inkonu.
[belorybitsa in Russian; nelma or azatmahy in Turkmenian; inconnu, conny, white salmon, Caspian inconnu].
Systematics
Salmo leucichthys was originally described from the Volga
and Ural rivers, Caspian Sea and Kamchatcka, Russia.
The type subspecies in the Caspian Sea basin is the one found in
Iran. The subspecies Stenodus leucichthys nelma (Pallas, 1773)
of White Sea, Siberian and northwestern North American drainages is more common.
Key characters
The characters of the species are those of the genus.
Morphology
Dorsal fin branched rays 8-13 after 2-6 unbranched rays and anal
fin branched rays 9-16 after 2-5 unbranched rays. Lateral line scales
88-121, scales above the lateral line 8-13, and scales below the
lateral line 10-12. There is a pelvic axillary scale. Scales bear
numerous fine circuli, the focus is slightly subcentral posterior, the
anterior scale margin is wavy but irregular in outline and the scale
is generally rounded. There are no radii. There is a single flap between the
nostrils.Gill rakers number 17-27 and
reach the fourth adjacent raker when appressed. Pyloric caeca number
191-193 and the gut is s-shaped. Vertebrae number 64-70.
Sexual dimorphism
Males develop tubercles on the head and sides of the abdomen during spawning.
Colour
Overall colour is silvery without spots.
Size
Reaches 1.5 m and 28 kg, rarely 40 kg, possibly to 48 kg. On the coast of Dagestan the
average size was 7.6-10.8 kg in the 1920s (Berg, 1948-1949).
Distribution
The subspecies is restricted to the Caspian Sea and its drainage.
Rare in Iranian waters with 5 specimens caught and preserved from 5-7
km east of Bandar-e Anzali in the Caspian Sea from 1984-1990. Several
dozen fish have been caught each year in Iran, starting in the late
1960s, all from between the mouths of the Safid and Astara rivers (Holčík
and Razavi, 1992). Reported from the southeast Caspian Sea, southwest
Caspian Sea and south-central Caspian Sea (Kiabi et al., 1999; Abdoli and
Naderi, 2009).
Zoogeography
This species is found naturally in the Caspian Sea but was
generally believed to inhabit only the northern, and rarely the
western, parts to the north of 40°N.
Rare specimens were reported by Kazancheev (1981) from the coasts of
Dagestan and Azerbaijan in early summer. It probably penetrated into
the Caspian Sea from the Arctic Ocean basin during the last glacial
period via ponded lakes.
Habitat
Inconnu live in the Caspian Sea proper at depths less than 60-65 m (optimally
25-45 m) and migrate up rivers to
spawn, in the Volga for 3000 km. Fat content of fish at Astrakhan at
the Volga mouth was 26% of total weight but by the time fish reached
the spawning grounds in the Ufa River this had fallen to 14%, and
after spawning to 1.5% for females and 1.6% for males. Off Iran they
are caught mostly above depths of 25 m from October until the end of
December. Some are caught in beach seines in shallow water (Holčík
and Razavi, 1992) but elsewhere are known from depths down to 46 m. It
is said to move into the central and southern Caspian in summer and
returns north when water temperatures fall below 8-10°C
in the first half of September (Berg, 1948-1949). This contrasts with
the catch records for Iran. Fingerlings tolerate temperatures up to 25ºC
in culture ponds but in the sea it is found at temperatures below 20ºC
(Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Age and growth
Life span is at least 12 years in the Volga population for females
and 8 years for males. Iranian fish were up to 6 years old (Holčík
and Razavi, 1992). Spawning females are predominately 6-8 years and
males 5-6 years old (Letichevskiy, 1981). Females on the Volga
spawning grounds are 85-105 cm, 5.5-9.5 kg while males are 75-100 cm, 3.5-7.5 kg.
Food
There is no feeding on the spawning migration, the fish relying
solely on their stored reserves of fat. Fat declined in one study from
21% before, to 2% after, spawning. In the Caspian Sea it feeds on
fishes such as gobies (Gobiidae), Rutilus rutilus (presumably R.
caspicus), Atherina
boyeri (= caspia), young Sander, common and anchovy kilkas, and shads. In
the sea, adults feed on kilka and silversides (97-99%)(Caspian
Sea Biodiversity Database, www.caspianenvironment.org).
Young feed on zooplankton 7-10 days after hatching but are predatory
in their second month. Fry descend to the Caspian Sea to feed until
they are sexually mature.
Reproduction
Spawning takes place in October and November at 0.2-6.0°C
after an ascent up north Caspian rivers. The Volga River run peaks in
December and March. Fecundity reaches 390,000 eggs of up to 2.4 mm
diameter. Eggs average 26% of the female's weight. The eggs are
slightly adhesive and shed on the river bottom. Spawning occurs only
once or twice in the life cycle of most fish although exceptional
females may spawn 3 times. Spawning occurs at intervals of 2-3 years.
Incubation takes 180-200 days and hatching takes place in spring. The
young immediately descend to the sea.
Parasites and predators
Eggs are eaten by Gobio species and by Lota lota.
Economic importance
This species has no economic importance in Iran but was a
significant member of former Soviet fisheries, taken both in the sea and on
its spawning migration. Up to 110,000 fish were taken annually in the
Volga-Caspian region. It has a rich, delicate flesh with a fat content of 18-26% and is a favoured
smoked product (Caspian Sea Biodiversity Database, www.caspianenvironment.org).
Conservation
This species was listed as threatened in Southwest Asia by Holloway
(1976) and endangered in the Caspian Sea by
Fricke et al. (2007).
Kottelat and Freyhof (2007) state that it is maintained only by stocking and is
on the brink of extinction. The population of this species, spawning principally in the
Volga, declined drastically after regulation of this river. In the
1960s, the population was estimated to be only 2000 fish but it has
recovered to 17,000 fish through artificial rearing (Mina, 1992). In
the two decades 1961-1980, fish hatcheries in the Volga Delta raised
72.4 million inconnu. Gravel spawning grounds were also constructed
(Letichevskiy, 1981). It has been proposed for inclusion in the
"Red Book of the U.S.S.R." which forms the basis for
measures to protect species (Pavlov et al., 1985). Lelek (1987)
classified it as endangered. Kiabi et al. (1999) consider this
species to be data deficient in the south Caspian Sea basin according
to IUCN criteria. Criteria include commercial fishing, sport fishing,
few in numbers, bodies), limited range (less than 25% of water
bodies), absent in other water bodies in Iran, and present outside the
Caspian Sea basin.
Further work
Further records of this species should be recorded to document the presence
and spread of this fish in Iranian waters.
Sources
Morphology based on Holčík and Razavi (1992) for Iranian specimens.
Iranian material: None. Comparative material: BM(NH) 1878.12.26:23, 1,
199.5 mm standard length, Bremen (no other locality data); BM(NH) 1899.7.25:22,
1, 215.5 mm standard length, Russia, River Obi (no other locality data). The tooth-carps, killifishes or pupfishes are small fishes found in fresh, brackish and sea water. There are 9 genera and about 121 species found in tropical to warm temperate climates almost
world-wide (Eschmeyer and Fong, 2011). Iran has
9 described species, with more to be discovered.
This family is characterised by a moderately elongate and compressed body, rather cyprinid-like but with jaw teeth (hence the common and scientific names), body and head covered with scales, no
spines in the fins, no barbels, the mouth is very protractile and armed with comb-like teeth, the lower jaw is strong and robust as the dentary is expanded medially, 4-6 branchiostegal rays, gill
membranes free from the isthmus, lateral line absent or reduced to points, caudal fin rounded or truncate, pectoral fins set low on the body (in contrast to Poeciliidae), dorsal and anal fins short, no
adipose fin, no pyloric caeca, and no gonopodium (egg layers, in contrast to the livebearer subfamily in the Poeciliidae).
Wildekamp (1993) gives a general account of cyprinodontid species. These fish can be maintained in aquaria, and are very popular with aquarists. Specific requirements where known are given under each
species. Moderate to hard fresh water is used and for water with a total hardness less than 10dGH, sea salt can be added (about one teaspoon per 10 litres). Summer temperatures of 20-25°C are
good and although higher temperatures make the fish more active, they also age the fish more rapidly. Winter temperatures below 20°C imitate nature and are recommended. Weekly partial water changes are
suggested and live and frozen fish foods and even flake foods are eaten. Breeding occurs throughout the year in aquaria kept above 15°C, eggs are deposited on fine-leaved plants (thread
algae, Java moss or yarn mops) or even on aquarium filter sponges and gravel. Egg development takes 8-20 days, fry take about 2 days to absorb their yolk sac and will then consume baby brine shrimp.
These fish can be kept in outdoor ponds in milder climates (http://ark.aka.org/Eurasian.htm, downloaded 1 December 2003).
These tooth-carps are found around the shores of the Mediterranean, in Southwest Asia and as far as northeastern India and Somalia. There are about 25 known species.
Sethi (1960) advocated placing Aphanius in a separate family, Aphaniidae, but this did not find general acceptance.
The genus Aphanius Nardo, 1827 has been used for these tooth-carps for many years. However, Lazara (1995) designated Lebias fasciata Valenciennes, 1821 as the type species for
Lebias Goldfuss, 1820, making Lebias a subjective senior synonym of Aphanius. On this basis Lebias must be used rather than Aphanius but Lazara's type species
designation is invalid (Kottelat and Wheeler, 2001). Since Lazara (1995) could have chosen another species as type species, the change involves a large number of species and these species are
threatened and listed in various legislation, a petition is before the International Commission on Zoological Nomenclature to suppress Lazara's designation (Kottelat, 1997; Kottelat and Wheeler, 2001;
Wildekamp, 2001; Villwock et al., 2002). Wildekamp et al. (1999) present evidence that Lebias is a synonym of Cyprinodon.
Literature on these Mediterranean and Southwest Asian fishes may appear under either of these names or under Cyprinodon Lacepède, 1803, the latter now restricted to American species.
Bănărescu (1995) disagrees with Parenti's (1981) relationship of Aphanius to South American Orestias (see also Parker and Kornfield (1995), Stevenson (1997) and Costa (1997)
for conflicting views).
This genus is characterised by a thick oval body, large to moderate cycloid scales, the head flattened on top, a small, superior mouth with tricuspid teeth, the upper jaw bordered by the premaxillaries
only, lateral line system present only on the head, dorsal fin positioned somewhat posteriorly with1-2 unbranched rays and 7-13 branched rays, anal fin rays 1-2 unbranched and 7-14 branched, dorsal and
anal fins larger in males than in females, dorsal fin inserted opposite the anal fin origin (in contrast to Gambusia), and colouration of males and females distinct. Seyfali et al. (2002)
has 2n=48 for fish from the Damghan basin which Coad and Abdoli (2000b) suggest may be a distinct taxon.
Kamal et al. (2009, 2009) studied some biological characteristics of the Damghan population. Aphanius is the only genus in the family currently recognised in Iran. However Parenti (1981) distinguishes derived members of the genus Aphanius as `Aphanius' without
formally describing a new genus. One of the distinguishing features of `Aphanius' is the reduction of cephalic sensory pores to neuromasts, a character found in A. mento, A. sophiae
and A. vladykovi in Iran.
Wildekamp et al. (1999) review the Turkish species which show scale reductions not seen in Iranian populations. Hrbek et al. (2002) outline the historical biogeography of the species
complex in central Anatolia, Turkey using mtDNA, testing the hypothesis of geographic speciation driven by early Pliocene orogenic events that may well be paralleled in Iran. Hrbek and Meyer (2003)
studied the phylogeny of Eurasian tooth-carps using mtDNA and observed a western Tethys Sea clade (all species except those listed below) with a middle Oligocene divergence into Iberian
Peninsula and Atlas Mountains, and Turkey and Iran sections. Late Miocene orogenic events were correlated with a large amount of genetic differentiation in Turkey (and presumably Iran). An eastern Tethys
Sea clade (dispar, ginaonis, mento, sirhani) had an Oligocene divergence into a freshwater clade inhabiting the Arabian Peninsula and neighbouring areas and a euhaline
clade inhabiting coastal areas from Pakistan to Somalia. Speciation is predominately vicariant-based but ecological factors played a significant role.
These fishes are known generally as gour-e khar in Farsi (= literally "striped donkey" which means zebra here although usually in Iran this term refers to the wild ass or onager),
kapurdandandar or kopurdandandar (= tooth-carp), or even آفانيوس (= Aphanius). Saefali (1999) is a recent study of these fishes in Farsi.
Iranian Aphanius away from coastal drainages are thought to be relicts of the Tethys Sea (Kosswig, 1955a; 1967; Bianco, 1995), having been trapped by the rising Iranian Plateau, rather than
invaders from the coast (Krinsley, 1970). Fossil tooth-carps, Aphanius kirgisicus and A. longipinnis have been described from Kyrgyzstan in Miocene deposits, far inland from the distribution
of living species (Yakovlev, 1959). Priem (1908) describes a fossil cyprinodont from the Miocene of Iran.
The systematics of the Iranian populations would repay careful study using biochemical and genetic techniques coupled with aquarium studies on behaviour and cross breeding. Certain populations,
isolated from others in discrete drainage basins, appear to be different on the basis of colour patterns yet show little morphological variation, e.g. the Damghan population, see Coad and Abdoli (2000b),
Saefali (1999) and Seyfali et al. (2002), the most northerly and easterly population in Iran characterised by large spots in females; populations in Fars outside the Lake Maharlu and Kor River
basins; and populations in the Namak Lake basin - all yet to be investigated in detail (Coad, 2000b). Since these patterns are used in mate recognition, the
populations could be distinct taxa. Huber (1996) has noted in cyprinodonts from Africa that many taxa can only be separated on the basis of colour in life, other characters overlapping. He suggests a
cyprinodont species definition which includes possession of at least one stable phenotypic characteristic, and this could be colour of the male fish. He does recommend that genetic isolation be
demonstrated where possible by karylogy, biochemical techniques or breeding experiments.
Seifali et al. (2003) compare A. sophiae and A. vladykovi
meristically and morphometrically. Saifali et al. (2004) found a suite of morphometric characters to be different between samples of A. vladykovi and fish from Qomy-Abad in Tehran Province, the Namak Lake basin.
The mosquitofish (Gambusia) is a niche competitor and may well eliminate these tooth-carps. Generally, Aphanius prefers springs, lakes, marshes, sea shores and hot springs. Flowing
water is usually avoided but they can be found near the source of springs flowing into salt lakes, constrained by hypersaline waters downstream. However they are tolerant of high salinities. They occur
in schools but males are aggressive to other males. Males are brightly coloured, often striped, while females are subdued with spots or faint bars.
A related Mediterranean species is reputed to be ichthyootoxic but this has not been demonstrated for Iranian species (Coad, 1979b). Symptoms of this egg poisoning are summarised under the genus
Schizothorax.
These small fishes are used in the aquarium trade as they tolerate a wide range of temperatures and salinities and are particularly colourful. As early as 1912, J. P. Arnold in Germany noted the
survival of imported Iranian Aphanius (possibly A. farsicus) in aquaria at temperatures of 14-27°C, with a variety of plants such as Myriophyllum, Nitella, Riccia,
Cabomba, Salvinia and filamentous algae, with daphnia, cyclops, enchytraeids and red mosquito larvae as food, and egg laying apparently preferred on the Salvinia with 8-12 days for
hatching at 27°C.
Aphanius sp. from the Namak Lake is recorded as having the parasite Clinsotomum complanatum (Hosseinie, 1987).
Aphanius from Damghan were infected with the trematode Tetracotyle
sp. (Gholami et al., 2011).
Kamal et al. (2007; 2009) and Bakhtiyari et al. (2011) give some life history data on the Damghan and Shour
River of Eshtehard (west of Tehran) populations.
Food in the Shour River was mostly chironomids while crustaceans including
Daphnia were eaten at Damghan. Stable environmental conditions at Damghan
allowed more prey selection and a higher condition factor.
The Damghan fish lived longer and had a higher egg diameter while Shour
River fish had a higher L∞ and a shorter
reproductive period, again probably reflecting environmental constraints. Aphanius
arakensis
Common nameses
Kapour-e-dandandar-e-Arak.
[Arak tooth-carp]. Systematics
The
holotype is a male, 31.5 mm SL from Arak, Namak Lake basin, 34º00’N, 49º50’E,
in the Zoological Museum of Shiraz University, Collection of Biology Department (ZM-CBSU
10999), and paratypes are
35 males, 22.6-32.7 mm SL, and 35 females, 22.5-34.1 mm SL, same locality as
holotype (ZM-CBSU 11000). The species
is named for the city of Arak, the capital of Markazi Province.. Key characters
This species is distinguished by distribution and by
pigmentation, males having 12-19, commonly 15-16, flank bars and females with
prominent pigmentation along the mid-flank. Morphology
Total dorsal fin rays 11-14, total anal fin rays 10-12, branched pectoral fin
rays 14-18, branched pelvic fin rays 6-8, lateral series scales 27-32 (also
given as 28-32), caudal peduncle scales 10-13, and total gill rakers 8-10.
Lateral series scales and flank bar numbers are statistically different from
other species. The otolith has a short but high antirostrum, a wide excisura and
a ventral rim with some small, drop-like processes. Molecular evidence also
defines the species with 19 apomorphies. Sexual dimorphism
See colour description. Colour
Lighter flank bars in males are narrow with the interspaces broader than the
bars and becoming wider posteriorly. The head is grey on top and the body
overall is dark from numerous melanophores although the belly is pale. The
dorsal, anal and caudal fins have a white margin with some darker pigment
proximally making a strong contrast. The first dorsal fin rays are dark and
membranes may all be dark too in some fish. The anal fin also can have darkened
membranes but this not as pronounced as in the dorsal fin. The bulk of the
caudal fin is pale or whitish but membranes may be lightly pigmented. The
pectoral and pelvic fins are yellowish. Most fish have dark blotches at the
dorsal and anal fin bases. Females are greyish on the back with the belly and
lower head surface light. The chin and sides of the head are speckled with
melanophores. There is a line of relatively dark melanophores below the eye. All
fins are white. The flanks have dark, irregular blotches or spots from the body
middle to the caudal peduncle. Small falnk spots may line up along the mid-flank
as a row from behind the head to the caudal fin base. A lozenge-shaped spot is
found at the central caudal peduncle base. Size
Attains
34.1 mm standard length. Distribution
Zoogeography
This taxon is the sister-species of A. sophiae from the Kor River
basin to the south. Habitat
The type series was collected from a small, shallow polluted pond, 6 x 4 m,
fed from a nearby spring. The shores had Juncus and Typha but
there was no vegetation in the pond. The pond bottom was muddy with some gravel.
The water was almost stagnant, with a temperature of 23°C. This was the only
fish species in the pond and the tooth-carp was also present in neighbouring
springs. Age and growth
Unknown. Food
Unknown. Reproduction
Unknown.
Parasites and predators
Unknown.
Economic importance
None at present in Iran but a possibly a species of interest to aquarists. Conservation
This species is known from a restricted area and the type locality has
anthropogenic pollution. It may well be threatened. Further work
The distribution of this taxon in the Namak Lake basin should be explored
further and threats to its existence assessed. Sources Based on Teimori et al. (2012).
Type material: See above. Aphanius dispar

Bazoft River, upper Karun River
basin, August 2009, courtesy of K. Abbasi
Linnaeus, 1758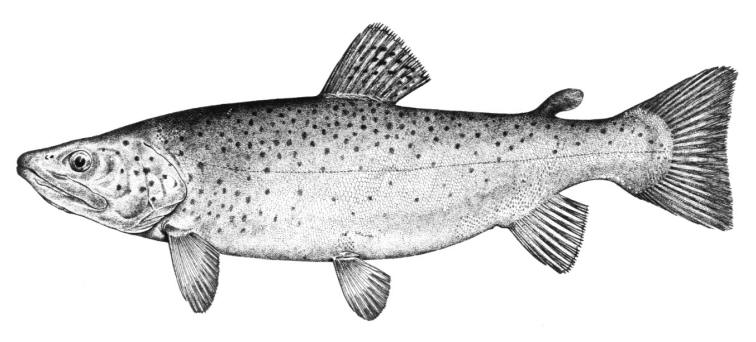

European Salmo trutta, Duane Raver, U.S. Fish and Wildlife Service
Richardson, 1836
(Mitchill, 1814)
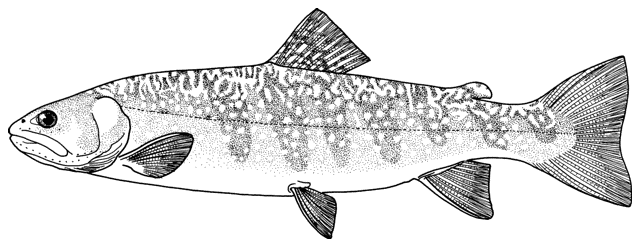

Duane Raver, U.S. Fish and Wildlife Service
Richardson, 1836
(Güldenstaedt, 1772)
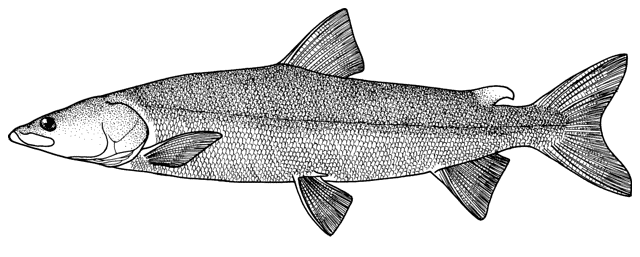
Teimori,
Esmaeili, Gholami, Zarei and Reichenbacher, 2012
(Rüppell, 1829)
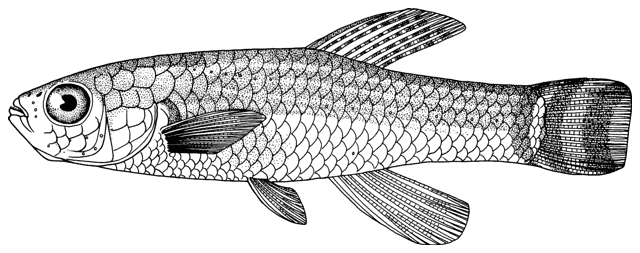
Male.jpg)
Female, courtesy of Z. Gholami, H. R. Esmaeili and M. Ebrahimi
Male, courtesy of J. E. Randall

Common names
gour-e khari, kopurdandandar-e balehbolband (= tooth-carp with striped fin), kapurdandan-e balmband,
mahi dom parchami.
[harsun. batrikh or batrikh motakayer in Arabic; nambal in Pakistan; high-finned pupfish, Arabian killifish, mother of pearl fish].
Systematics
A detailed synonymy is given by Villwock et al., (1983), Wildekamp et al. (1986) and Wildekamp (1993). Hoedeman (1951) proposed a new genus for this species, Aphaniops, based on
the absence of a dermal sheath or genital pouch around the anterior anal fin rays, only 8-9 dorsal fin rays in contrast to 10-14 rays in Aphanius and 7-8 pelvic fin rays in contrast 5-7 rays. It
has not found general acceptance.
The type subspecies is found in all Iranian drainages (Krupp, 1983) although Aphanius dispar stoliczkanus (Day, 1872) has been recorded in earlier literature as the subspecies of these drainages
except the Tigris River basin for which Aphanius dispar richardsoni (Boulenger, 1907) is reported (Berg, 1949). Aphanius dispar richardsoni is limited to the Dead Sea valley of Israel and
western Jordan by Villwock et al. (1983) and Wildekamp (1993). Cyprinodon stoliczkanus Day, 1872 is regarded as a synonym of Aphanius dispar dispar by Krupp (1983) while Berg (1949)
places Bampur River, Baluchestan fish in this subspecies as a southern representative of Aphanius dispar with 6-9, rarely 10 dorsal fin rays as opposed to 9-10 rays in Aphanius d. dispar.
Hrbek and Meyer (2003) note that, based on their mtDNA study, the monophyly of A. dispar is strongly rejected and it does not constitute a species in terms of the phylogenetic species concept.
Reichenbacher et al. (2009) found that isolated populations in Saudi
Arabia may be capable of evolving into new species in a short period of time,
based on otolith morphology - evidently there is still much to be learned about
this nominal species. Hybrids with Aphanius dispar richardsoni are reported from Israel (Goren and Rychwalski, 1978).
The lectotype of Lebias dispar as designated by Villwock et al. (1983) is in the Senckenberg Museum Frankfurt under SMF 821 with paralectotypes SMF 1988 (10). Further paralectotypes in the
Natural History Museum, London under BM(NH) 1860.11.9:152 listed as from Abyssinia (3 fish, 44.1-49.6 mm standard length) (Eschmeyer et al., 1996) but a label in the jar states that "status
as types doubtful, types in SMF range from 26.1-35.8 mm SL, locality in description and types given as 'Red Sea'".
Types of Cyprinodon stoliczkanus Day, 1872 are in the Zoological Survey of India, Calcutta (ZSI 1477, ZSI 1478), the Natural History Museum, London (BM(NH) 1889.2.1.2065-74, originally 21
fish, now 14 fish, 14.4-29.4 mm standard length when examined in September 2007) and the Australian Museum, Sydney (AMS B.7730-7731) (Whitehead and Talwar, 1976; Eschmeyer et al.,
1996; Ferraris et al., 2000). This species was originally described from a "stream at the village of Joorun, and also at Lodai, along the edge of the Rann" (= the salt water Rann of
Cutch, Sind, India).
The lectotype of Cyprinodon richardsoni as designated by Krupp and Schneider (1989) is in the Natural History Museum, London under BM(NH) 1856.5.2:4 with paralectotypes under BM(NH) 1856.5.2:5
(8 fish) (12.9-25.0 mm standard length, the largest being the lectotype) (Eschmeyer et al., 1996; personal observations).
Krupp (1983) did not find the variation in teeth numbers between different populations and subspecies observed by Berg (1949).
Keivany et al. (2011) examined 16 populations from southern Iran
meristically and found significant differences in 10 of 11 characters but their
wide overlaps prevented population differentiation.
Teimori et al. (2012) used otolith morphology to show vicariance events
in the Persian Gulf area during the last glacial maximum (21,000-18,000 B.P.),
dispersal during the Early Holocene rise in sea level (11,000-8000 B.P.), and
Holocene to Present interruption of gene flow at the Strait of Hormuz have
shaped intraspecific differentiation of this species. Key characters
The colour pattern is distinctive.
Morphology
Scales along the flank 24-35. Scales are squarish with an almost vertical anterior margin and protruding anterior corners, parallel dorsal and ventral margins and a rounded posterior margin with tiny
teeth. The anterior margin has a small central protuberance with shallow indentations above and below or almost straight margins to each anterior corner. Radii are present on the anterior field,
numbering 10-20 but mostly 14-16, and are almost horizontal and parallel. The exposed part of the scale has dimples rather than circuli. Circuli are few. The focus is subcentral posterior. There is
no pelvic axillary scale. Total dorsal fin rays 7-11, usually 9-10, total anal fin rays 8-12, usually 10-11 (the number of unbranched rays in the dorsal and anal fins varies from 1-3), total pectoral rays
12-18 and total pelvic rays 6-7, usually 7. Vertebrae 24-29. Al-Hassan (1982b) reports on abnormalities in the vertebrae including loss of the posterior part of the centrum and fusions. Teeth are
tricuspid with the central cusp concave at its tip and only slightly longer than the lateral cusps (Goren and Rychwalski, 1978), although in some fish seen by me the tip is rounded. There are 12-20 teeth
per jaw (Krupp, 1983). Gill rakers 11-22, modally 13-16, reaching the second adjacent raker when appressed. Some gill raker counts are difficult to make accurately as those at the anterior arch end are
minute and those at the dorsal end are partially concealed in flesh. The gut is coiled ?see my ginaonis paper. Reichenbacher et al. (2007,
2009) give descriptions of otolith morphology. Chromosomes
number 2n=48 (Klinkhardt et al., 1995; Esmaeili et al., 2008).
Karyotype 16Sm + 32St and arm number 32 (Esmaeili et al., 2008).).
Meristic values for Iranian specimens:- total dorsal fin rays 8(1), 9(39) or 10(10); total anal fin rays 9(2), 10(42) or 11(6); total pectoral fin rays 14(2), 15(16), 16(28), 17(3) or 18(1); total
pelvic fin rays 6(6) or 7(44); lateral series scales 24(2), 25(5), 26(11), 27(17), 28(7), 29(2), 30(1), 31(2), 33(2) or 35(1); total gill rakers 15(13), 16(16), 17(16) or 18(3); and total vertebrae ?.
Sexual dimorphism
Males have longer fins than females and are more brightly coloured. The dorsal fin is twice as long in the male and reaches the caudal fin when appressed. When expanded it is widely flared and
distinctive as is the enlarged anal fin.
Colour
Breeding males are brown-grey, grey or black-brown with iridescent blue-white flank spots and white and brown to light orange or light blue, irregular, narrow bars. The head has blue and orange tinges
and in particular there is an orange spot on the operculum postero-dorsally. Lips are blue-white. The flank over the pectoral fin has electric blue spots. The anterior belly becomes blue with pearl spots.
Scales have a dark margin. The dorsal fin is spotted light blue on a bright orange background and is barred. Barring may be irregular and in overall view in breeding males appears as speckling. Pectoral,
anal and pelvic fins are lemon-yellow. The anal fin has barring on the posterior 4 rays in breeding males. The caudal fin has 2-3 dark and light blue alternating bars, the last bar being yellow. The bars
are crescent-shaped, with concave side posterior. Males outside the breeding season are less brightly coloured with silvery on the flanks with a grey or black-brown back and irregular flank bars. Male
specimens from the Bampur River basin in Iran have a body speckled silvery-white to light blue, weak tail bars, blue and orange tinges to the head, an orange spot on the operculum and an orange-yellow
edge to the anal fin.
Young have pale brown flank bars on the caudal peduncle. Some young may have a few spots on the flank rather than bars. Females are brown-grey to silvery with 8-20 narrow flank bars and a dark-brown
back. The flank bars are dark brown and may have a tinge of orange, the interspaces silvery. Flank bars may number as few as
6 or 7 (Talwar and Jhingran, 1991; Keivany and Ghorbani, 2012). Females also have the orange operculum spot and
the blue spots over the pectoral fin. Female fins are hyaline. The peritoneum is brown to black.
Ross (1980) notes that Arabian specimens are brighter in waters with algae and plants; those found over sand and mud are duller, an effective camouflage. He also records variant colour patterns with
blue tails, or an overall gold colour, or with green or turquoise flank spots.
Size
Reaches 8.0 cm, but this may be an error as Krupp (1983) points out. He found fish only to 5.2 cm standard length although in Carpenter et al. (1997) 8 cm is given as a maximum. Wildekamp
(1993) records fish to 7.0 cm for males and 6.5 cm for females in total length. Fish from Ain Al Adhari, Bahrein reach 7.3 cm total length. Generally fish in fresh water are smaller than marine specimens.
Distribution
Found from Egypt, eastern Sudan and Ethiopia, and in the Red Sea through southern Southwest Asia and east to Rajasthan. Also in the eastern Mediterranean Sea (Kornfield and Nevo, 1976). The
subspecies Aphanius dispar richardsoni is found in the Dead Sea rift valley.
A specimen in the Naturhistorisches Museum Wien from the hot spring at Ginao (NMW 13799) may be mislabelled as several other collections from this hot spring are all clearly A. ginaonis.
This species is found in Iranian fresh waters from the Tigris basin to the Makran basin in water bodies draining to the Persian Gulf and Sea of Oman, islands in the Gulf such as Qeshm (OSU
8120), and in the endorheic
Hamun-e Jaz Murian basin. It is reported from the Shapur and Dalaki rivers (Gh. Izadpanahi, pers. comm., 1995;Bibak
et al., 2012), Gudar River east of Anveh, Mehran River below Bastak, Sarageh River north of
Bandar Abbas, Galashkerd Lake near Minab, and the Kol. Shur, Hasan Langi and
Ghale-gazii rivers in Hormozdgan. Abdoli (2000) illustrates distributions in the Arvand, lower Zohreh (=
Hendijan), lower Hilleh, Mand, Kul, Hasan Langi and Minab rivers, lower reaches of rivers in the Makran basin from the Jagin to Bahu Kalat, the lower Halil and Bampur Rivers in the
Hamun-e Jaz Murian
basin, and the endorheic Mashkid River. Keivany and Ghorbani (2012) map
distribution from Khuzestan to Baluchestan in waters draining to the Persian
Gulf, as well as the Hamun-e Jaz Murian, Mashkid and southern Lut internal
basins. They also document a record more remote from these distributions, 50 km
northwest of Ilam, in the Sartang-e Bijar River of the Tigris River basin
(33°41'24"N, 45°51'48"E). Zoogeography
Villwock et al. (1983) contend that an ancestor of A. dispar was present in the middle Miocene Transgression connecting the Mediterranean Sea to the Indian Ocean. The mesohaline
conditions, which developed in the Mesopotamian basin, were a good prerequisite for the development of A. dispar since, apart from some euryhaline species, there would be no competitors.
A. dispar spread from Mesopotamia through the Persian Gulf to India and the Red Sea. During the Pleistocene, it colonised inland waters.
Habitat
Highly tolerant of salinity, this species is found from the sea to fresh water habitats, including landlocked basins. Slightly saline waters in aquaria seem to discourage parasites (Andrews, 1983).
Salinities as high as 145‰ are tolerated (Lotan, 1971) and this fish can be moved abruptly from fresh to hypersaline water. Adaptation to high salinity waters involves a reduced osmotic
permeability of the gills and an increase in transportation of sodium chloride by the intestine (Skadhauge and Lotan, (1974). Lotan (1969, 1971, 1973) and Skadhauge and Lotan (1974) describe sodium,
chloride and water balance, and osmoregulation in this species. Plaut (1999) found that salinities above ~250% seawater seem to decrease swimming capabilities and routine activity rate and a
decrease in resting metabolic rate indicated stress. This species has been reported to survive in waters 38.4°C in Oman and only began to die at 45.1-46.0°C (Haas, 1982). Fish survived up to 40°C in
aquaria without acclimation (Lewis, 1970). Populations survive in isolated sections of streams with little food because of histological changes in the stomach (Ba-Omar et al. (1998).
Aphanius dispar occurs in shallow water and among vegetation over sand, rock or soft detritus bottoms.
Frenkel (1995) describes the effects of environmental factors on growth and reproduction in this species in Israel. Frenkel and Goren (1997) showed fish to be tolerant of a wide range of environmental
variables as measured against the gonado-somatic index (GSI) and ovary maturation stages. The GSI was not affected by temperature over a range of 18 to 37°C while maturation increased from 18 to
27°C but remained the same between 27 and 37°C. For salinities between 0‰ and 56‰ differences were only found in mean GSIs at the two extremes and at 0‰ ovaries contained only primordial germ cells.
GSIs and maturation stages only differed in feeding experiments when fish were deprived of food or fed at equal or greater than 1% of body weight per day. A decrease in photoperiod from 14L:10D (14
hours light, 10 hours dark) to 10L:14D caused a decrease in oocyte maturation stages and ovaries were no longer suitable for spawning. In aquaria, a pH of 7.5-8.5, temperatures above 27°C and water
hardness greater than 200 p.p.m. and the addition 4 heaped tablespoons of kosher salt per ten gallons of water (38 litres) favoured reproduction (Mackowiak, 1988). Alkahem (1989) found 50% mortality in
96 hours when fish were exposed to pH levels of 3.5-3.9.
Feulner (1998) reports that United Arab Emirate fish "hover" in the water column with the tail slightly curved to one side, presumably a feeding behaviour. Males and females in aquaria
swam in separate schools (Al-Daham et al., 1977).
Age and growth
Sexual maturity in ideal conditions may be attained as early as 7 months but full size takes a year. The length-weight relationship in southern Iraq was W = 1.2301 x 10-4
L2.4544 for males and W = 2.8357 x 10-6 L3.5112 for females. The average condition factor for males was 1.8900 and for females 1.6952. For a given length group males are
heavier than females and condition factors are higher for males (Huq et al. 1977). Muhsin (1987) studied the effect of differing food supply on the weight and chemical composition in females of
this species taken from the Al-Asafiah River, Basrah, Iraq but examined in tanks. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 41 Iranian fish measuring 2.37-3.56
cm total length. The a-value was 0.0012 and the b-value 3.214 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating
a fish that becomes more rotund as length increases).
Alavi-Yeganeh et al. (2011?check published paper date) give length-weight
data on this species from two localities in Iran with a (intercept) being
0.0108 and b (slope) being 3.332 for all localities and sexes combined. Food
Iranian specimens have guts packed with filamentous algae and detrital remains. Al-Daham et al. (1977) found food in southern Iraq to be predominately filamentous algae and diatoms, with some
copepods, rotifers and, in one instance, a small winged insect. In contrast, Shafi and Shalli (1986) report a diet of beetles, ephemeropteran nymphs, rotifers, filamentous algae and plants
in southern Iraq. Younis et al. (2001b) found Shatt al Arab, Iraq fish fed on amphipods and copepods (29.9%), organic detritus (11.6%), and algae and diatoms (7.1%). In aquaria, Al-Daham et
al. (1977) found this species to prefer animal foods in contrast to Aphanius mento which preferred plant foods. Al-Akel et al. (1987) have observed the selective feeding behaviour of
this species at Al-Khurj, Saudi Arabia. Certain species of filamentous algae are selected over others, e.g. the blue-green alga Oscillatoria, the green algae Ulothrix, Spirogyra and
Crucigenia, the desmid Cosmarium, and the diatoms Cocconeis, Diatoma, Navicula and Rhoicosphania, and algal spores and zygotes. Haas (1982) observed this species in Oman to pick
at rocks and other substrates, take items at the surface, chase small fish and eat insect larvae. Gut contents are mainly desmids and diatoms, some filamentous algae and rarely insect larvae and one
snail. This species is an omnivorous surface feeder (Carpenter et al., 1997).
Reproduction
A female in spawning condition approached a male and appeared to feed on a plant or plant-encrusted rock in fish studied by Haas (1982) in Oman. Feulner (1998) reports fluttering of the tail by male
fish. The male places his chin on her nape and 2-3 eggs are spawned rapidly. The female presses her vent against the plant or rock vertical surface, the male arches his back into an s-shape and presses
his body and anal fin against hers and clasps her with his dorsal fin. Eggs are released singly with a quiver. The time elapsed from chin on nape to egg release is as short as 1-2 seconds. Reproduction
occurs throughout the day and throughout the year in Oman with 69% of females having ripe eggs (up to 41) in April-June. Peak spawning in southern Iraq is April to June when only a small proportion of
eggs in the single ovary are fully developed eggs (Al-Daham et al., 1977). Shafi and Shalli (1986) record up to 73 mature eggs in southern Iraq specimens with a diameter of 220 microns. Breeding
season was May-July. In Saudi Arabia mature and developing oocytes are observed in fish during the whole year. The egg cycle may be more than one year with spawning in March and April (El-Hawawi and
Al-Imam, 1984). On the Mediterranean coast of Egypt, spawning occurs from March to September with a peak in July and August. Maximum egg diameter is 2.2 mm and size at maturity for females is 30 mm total
length (Lotan and Ben-Tuvia, 1996). Iranian specimens have large eggs (2.0 mm) on 16 March but young fish (8.9 mm standard length) were caught on 26 November suggesting reproduction is almost year round.
Bibak et al. (2012) examined reproduction in the Dalaki River, Bushehr
and found females to predominate (390 fish versus 140), males had a maximum
length of 49 mm and females 39 mm, and gonadosomatic indices indicated a
spawning period of April to June, with peaks in each of these months of 6.449
and 6.632. Spawning areas have some water flow and vertical surfaces of plants or algae-encrusted rocks. Males defend a territory about 30 cm wide against other males and will display with erect fins if
approached. Parallel swimming, head to head confrontations, rapid circling, and flank bites were observed by Haas (1982). Two males will swim and chase each other in circles until one swims away with
fins depressed. Males will change their territory after one day defending it. In Israel, males of Aphanius dispar richardsoni do not show any territorial behaviour (Goren and Rychwalski, 1978).
This fish will spawn in a few months of birth in aquaria (Ross, 1987). Three pairs of fish yielded about 40 eggs per day in aquaria and these eggs hatched in two weeks at 27°C (Mackowiak, 1988).
Parasites and predators
This species is reported to be heavily infested with metacercariae of the trematode Clinostomum complanatum in eastern Saudi Arabia, up to 86.6% incidence with up to 41 metacercariae per fish,
the infestation being higher in females than males (Kalantan et al., 1987).
Gholami et al. (2011) record the trematode Clinostomum complanatum
from fish in the Mehran River near Rudan and Gholami et al. (2011) report the cestode Ligula intestinalis in
fish from the Mehran River near Bastak. Economic importance
Al-Akel et al. (1987) suggest the use of this species as a control agent for filamentous algae.
Steven (1913) reported that this fish was responsible for the absence of mosquito larvae from streams in Karachi which otherwise appeared to be good breeding places. Christophers and Shortt
(1920-1921) thought that tooth-carps in southern Mesopotamia were a significant factor in lowering the malaria rate, presumably mostly this species. Sharma and Al-Daham (1979) compared the efficiency of
this tooth-carp with the mosquitofish for mosquito control under experimental conditions. The tooth-carp is more efficient when males and females were together than mosquitofish since males of the latter
expend time and energy defending territory. Mosquitofish are more active in pursuit of mosquito larvae than the tooth-carp when filamentous algae was present. Cloudy conditions and lower temperatures
slow consumption and both species prefer pupae to larvae of mosquitos, with females taking more pupae than males. The tooth-carp could consume nearly twice the amount of mosquitos than the mosquitofish
over a given period of time. Overall the tooth-carp compares favourably with the mosquitofish as a destroyer of the malaria-carrying mosquito. Homski et al. (1994) under laboratory conditions
found that Aphanius dispar is more successful than the mosquitofish in preying on the third and fourth instars and on pupal stages of mosquitos while the mosquitofish, with a smaller relative mouth
size, is more successful on the first two instars. Small fish of both species eat first instars exclusively. However, Aphanius dispar eats more second instar larvae under a vegetation cover since
the larger Aphanius are more capable of penetrating shallow water than mosquitofish. Aphanius dispar occurs in shallower water and among more vegetation than the open water mosquitofish.
The two species can be used to complement each other in control of mosquitos.
Ataur-Rahim (1981) found that this species will eat mosquito larvae in water storage tanks in Riyadh, Saudi Arabia. Fifteen fish consumed all larvae at a mean density of 5/100 sq cm water surface area
in a tank 3 by 2 m in a day. One fish, 5.2 cm long, ate 61 mosquito larvae. Haas (1982) fed this species mosquito larvae in the presence of abundant algae as an alternative food. An average of 96 larvae
are eaten each day. Etemadfar et al. (1983) studied the potential use of this species for malaria control in southern Fars, Bushehr and Hormozgan provinces. Louis et al. (1988) detail a
control strategy against malaria using this species in Djibouti as do Fletcher et al. (1992) in Ethiopia and Fareed and Baban (1995) in Yemen. Fletcher et al. (1992) showed a decrease in
sites harbouring mosquito larvae from 34% without the fish to 1.6% with the fish stocked.
Chandra et al. (2008) briefly reviews the use of this species in
biocontrol. The studies of Frenkel and Goren (1997) in Israel show that the reproduction of this species can be controlled such that it has potential for mass production and could be used in mosquito control.
Carpenter et al. (1997) state that this species is used in mosquito control. Ross (1987) gives details of aquarium care and breeding of this species.
Conservation
Al-Johany and Yousuf (1993) show that Gambusia affinis has a wider range of temperature tolerance than this species and is better suited for a desert habitat in Saudi Arabia. The exotic
Gambusia is therefore a threat to A. dispar.
Al-Kaheh-Al-Balawi et al. (2008) found that Saudi Arabian populations are
declining from reduced availability of food, habitat degradation, chemical
contamination, introduction of exotics and exploitation and some of these
factors doubtless obtain in Iran. Further work
This widespread species has been reported from a number of springs in southern Iran and, like A. ginaonis, may be distinct at some level. Molecular work would be useful in determining
the taxa involved and evolution in hot springs. The utility of this species in mosquito control in smaller water bodies of Iran where it also occurs naturally could be explored. Al-Daham et al.
(1977) found that this species in aquaria ate mosquito larvae "eagerly" compared to "very eagerly" for Gambusia holbrooki and "not eaten" for Aphanius mento.
Sources
Type material:
Iranian material: NMW 13799, 1, ?;
CMNFI 1979-0129, 1, 31.3 mm standard length, Fars, spring about 2 km north of Farrashband (28º54'N, 52º04'E);
CMNFI 1979-0137, 106, 8.9-37.7 mm standard length, Fars, stream outside Lar (27º40'N, 54º32'30"E);
CMNFI 1979-0138, 1, ? mm standard length, Fars-Hormozgan border, Rasul River drainage (ca. 27º32'N, ca. 54º58'30"E);
CMNFI 1979-0139, 2, ? mm standard length, Fars-Hormozgan border, Rasul River drainage (ca. 27º25'30"N, ca. 54º59'E);
CMNFI 1979-0140, 3, ? mm standard length, Hormozgan, Kul River drainage (27º14'N, 55º46'30"E);
CMNFI 1979-0141, 6, ? mm standard length, Hormozgan, Kul River at road bridge (27º17'30"N, 56º03'30"E);
CMNFI 1979-0142, 10, ? mm standard length, Hormozgan, Baghu River at Baghu (27º17'N, 56º28'E);
CMNFI 1979-0143, 118, ? mm standard length, Hormozgan, Hasan Langi River drainage (27º21'N, 56º50'30"E);
CMNFI 1979-0145, 1, ? mm standard length, Hormozgan, Geru River (26º55'N, 57º01'30"E);
CMNFI 1979-0148, 21, ? mm standard length, Hormozgan, Sarzeh River (27º30'30"N, 56º15'30"E);
CMNFI 1979-0151, 45, ? mm standard length, Hormozgan, Shur River drainage near Kahkom (28º02'N, 55º53'E);
CMNFI 1979-0177, 37, ? mm standard length, Hormozgan, Sarzeh River drainage (27º33'N, 56º14'E);
CMNFI 1979-0179, 13, ? mm standard length, Hormozgan, Sarzeh River drainage (27º36'N, 56º15'E);
CMNFI 1979-0181, 9, ? mm standard length, Hormozgan, Kul River drainage (27º17'30"N, 56º03'30"E);
CMNFI 1979-0182, 31, ? mm standard length, Hormozgan, stream on road to Bandar-e Lengeh (27º14'N, 55º46'30"E);
CMNFI 1979-0185, 24, ? mm standard length, Hormizgan, stream in Rasul Rievr drainage (27º06'N, 55º45'E);
CMNFI 1979-0187, 2, 26.2-33.2 mm standard length, Hormozgan, Sar Khun oasis (27º23'30"N, 56º26'E);
CMNFI 1979-0312, 7, ? mm standard length, Baluchestan, dam on Bampur River (27º11'N, 60º36'E);
CMNFI 1979-0313, 1, ? mm standard length, Baluchestan, Bampur River at Bangharabad (27º20'N, 60º46'E);
CMNFI 1979-0328, 18, ? mm standard length, Baluchestan, jube 2 km south of Bampur River near Bampur (27º10'30"N, 60º21'E);
CMNFI 1979-0352, 2, ? mm standard length, Khuzestan, marsh in Jarrahi River drainage (30º33'30"N, 48º48'E);
CMNFI 1979-0354, 8, ? mm standard length, Khuzestan, Karun River tributary (30º31'N, 48º19'E);
CMNFI 1979-0355, 1, ? mm standard length, Khuzestan, Karun River tributary (30º35'N, 48º22'E);
CMNFI 1979-0362, 2, ? mm standard length, Khuzestan, jube in Karkheh River drainage (31º42'N, 48º33'E);
CMNFI 1979-0379, 4, ? mm standard length, Khuzestan, Dez River (32º12'N, 48º27'E);
CMNFI 1979-0381, 1, ? mm standard length, Khuzestan, stream west of Shushtar (ca. 32º10'N, ca. 48º35'E);
CMNFI 1979-0384, 7, ? mm standard length, Khuzestan, Ab-e Shur drainage (32º00'N, 49º07'E);
CMNFI 1979-0403, 25, check size range ?19.7-37.0 mm standard length, Bushehr, stream 23 km south of Kaki (ca. 28º11'N, ca. 51º43'E);
CMNFI 1979-0406, 48, 15.6-35.8 mm standard length, Hormozgan, stream near Bandar-e Charak (26º48'N, 54º18'E);
CMNFI 1979-0407, 3, ? mm standard length, Hormozgan, stream 30 km from Barvedun (26º51'N, 54º37'E);
CMNFI 1979-0408, 106, 14.6-48.4 mm standard length, Hormozgan, Mehran River (27º04'N, 54º35'E);
CMNFI 1979-0410, 10, ? mm standard length, Hormozgan, Mehran River (26º53'N, 55º17'E);
CMNFI 1979-0414, 12, ? mm standard length, Hormozgan, pool on road to Tiab (27º05'N, 57º02'E);
CMNFI 1979-0415, 1, ? mm standard length, Hormozgan, stream 18 km south of Genu (27º17'30"N, 56º20'E);
CMNFI 2007-0053, 4, ? mm standard length, Hormozgan, Sarzeh River (ca. 27º36'N, ca. 56º15'E);
CMNFI 2007-0057, 16, ? mm standard length, Hormozgan, Mehran River 4 km below Bastak (ca. 27º05'N, ca. 54º05'E);
CMNFI 2007-0058, 3, ? mm standard length, Fars, headwaters of Gowdar River (ca. 27º24'N, ca. 54º16'E);
OSU 8129, 1, 54.5 mm standard length, Hormozgan, Qeshm Island salt spring (no other locality data);
BWC95-30, uncatalogued, 3, ?, Khuzestan, Kupal (31º15'N, 49º10'E);
BWC98-6, uncatalogued, 24, ?, Hormozgan, Ab-e Garm-e Khamir (ca. 26º59'N, ca. 55º35'E);
BWC2000-6, uncatalogued, ?, ?, Khuzestan, Dez River (32º14.663'N, 48º20.112'E);
BWC2000-15, uncatalogued, ?, ?, Khuzestan, stream south of Shushtar (31º41.867'N, 48º59.581'E).
Comparative material:- BM(NH) 1920.3.3:193-202, 12, 21.0-30.5 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1920.3.6:9-10, 3, 17.1-26.8 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1949.7.21:1-11, 12, 18.4-42.4 mm standard length, Iraq, Bahr-el-Milk (32º35'N, 43º50'E);
BM(NH) 1974.5.22:30, 1, 35.2 mm standard length, Iraq, Basra (30º30'N, 47º47'E); BM(NH) 1974.5.22:31-32, 2, 37.4-44.4 mm standard length, Iraq, Al Faw (). Aphanius
farsicus
Mehran River, courtesy of H. R. Esmaeili
Teimori, Esmaeili and Reichenbacher, 2011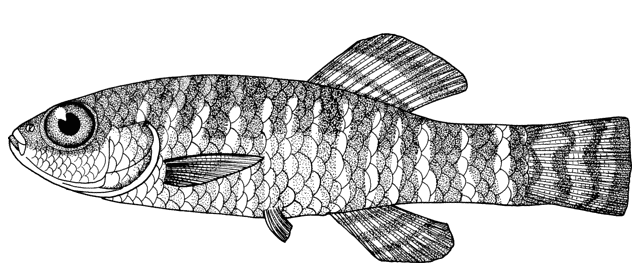
Male
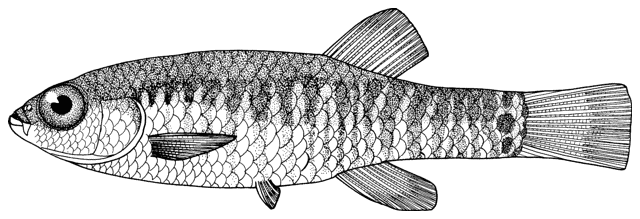
Female
Cyprinodon persicus male after Jenkins (1910)

Male, courtesy of Z. Gholami, A. Gholamifard and R. Zarei

Female, courtesy of Z. Gholami, A. Gholamifard and R. Zarei Common names
Kapour-e dandandar-e Farsi (Farsi tooth-carp (sic), correctly Fars
tooth-carp as it was named for the province not the language), gour-e khar, kopurdandan-e Irani (= Iranian tooth-carp
as persicus).
[Persian pupfish or toothcarp, but see also A. sophiae].
Systematics
Cyprinodon blanfordii Jenkins, 1910 is potentially a synonym by authors (see Coad, 1996a). Hanko
(1924) placed Cyprinodon blanfordi and C. persicus in Cyprinodon chantrei Gaillard, 1895 but this is an error.
Jenkins (1910) described Cyprinodon blanfordii ("East of Shiraz, South Persia")
and C. persicus ("Spring on the edge of Shiraz Lake, Southern Persia"). The
first nominal species has so generalised a locality as to be of very uncertain provenance. It
could conceivably be in the Shiraz valley, the Kor River valley or outside either of these (see below under A. sophiae where it is argued that is is a synonym of this species). The second nominal
species is clearly from the valley of Shiraz and its salt lake Maharlu, an endorheic
basin.
The valid name for Lake Maharlu basin Aphanius was given previously as persicus
in this website. Gaudant (2011) showed that Brachylebias Priem, 1908 is a
junior synonym of Aphanius Nardo, 1827. Teimori et al. (2011) show
that Brachylebias persicus Priem, 1908, a Late Miocene species from
northwest Iran, is distinct from the modern species and introduce A. farsicus
as a replacement name for A. persicus (Jenkins, 1910).
The Jenkins types in Calcutta have not been examined by me. Cyprinodon blanfordii has page priority but the
locality is vague and the fish have a pigment pattern description and illustration which is spotted on the flank with a lozenge-shaped spot at the caudal fin base, thus appearing to be a female A.
sophiae.
Material described and illustrated by Villwock (1960) as A. sophiae are this species.
Three syntypes of Cyprinodon blanfordi (ZSI F9416 to ZSI F9418), two syntypes of Cyprinodon persicus (ZSI F9403 and ZSI
F9404) are in the Zoological Survey of India, Calcutta (Menon and Yazdani, 1968).
Key characters
Females of this species are barred where all other Aphanius species in this area of southern Iran have spotted females. The distribution is apparently limited to the Lake Maharlu basin near
Shiraz.
Morphology
Although populations are isolated in springs and streams around Lake Maharlu, Coad (1996a) found them to be relatively homogenous.
Lateral line scales 24(3), 25(34), 26(69), 27(176), 28(100) or 29(22). Scales above the lateral line 4-6, scales between lateral line and the anal fin 4-8, scales between the lateral line and the
pelvic fin 5-9, and scales around the caudal peduncle 12-17. Esmaeili et al. (2007) determined a chromosome number of 2n=48 with 11 pairs of submetacentric and 13 pairs of subtelocentric
chromosomes and an arm number of NF=70. Karyotype 16Sm + 32St and arm number 32
(Esmaeili et al., 2008). Total dorsal fin rays 8(1), 10(8), 11(97), 12(204), 13(89), or 14(7); total anal fin rays 9(3), 10(111), 11(225), 12(64) or 13(3); total pectoral fin rays 13(9), 14(82), 15(211), 16(94), 17(9) or
18(1); total pelvic fin rays 4(11), 5(181) or 6(213); total gill rakers 9(5), 10(51), 11(220), 12(108), 13(19) or 14(3); abdominal vertebrae 9(1), 10(1), 11(27), 12(355), or 13(22); and caudal
vertebrae 12(1), 14(16), 15(285) or 16(104).
Sexual dimorphism
Most apparent in colour and pigmentation detailed below. Females are longer and heavier than males (Esmaeili and Shiva, 2006).
Colour
"The basic colouration is bright shining silvery white. The entire body, with the exception of the dorsal crest and the belly, is tigered with small brown spots; in some specimens these spots
seem to be arranged in three irregular longitudinal rows of which the uppermost extends as a band across the operculum and to the eyes; all fins are uniformly yellow with a reddish border. In males the
spots are more dense, in females they are paler and scarcer, only the 2-3 at the base of the caudal fin are dark brown" (Heckel (1847b) on A. sophiae). "The colour of this small
fish with its enviable crystal teeth is uniformly brown, at least it is now in ethyl alcohol, with a silvery-white throat and belly; laterally on the tail there are a few scattered, darker spots, and a
deeper black spot is located on the last scales anteriorly of the caudal fin. All fins are blackish without any markings" (Heckel (1847b) on A. crystallodon). "Silvery, brownish on
back, brown on top of the head, with numerous small spots of brownish, arranged in three or more, more or less irregular, longitudinal series, the median of which, on the lateral line, commonly ends in a
black spot on the middle base of the caudal. On some this median series is entirely composed of blackish spots. Rarely the spots are somewhat confluent into longitudinal bands. Brownish specimens show a
longitudinal band of silvery along the middle of the flank. The form described as A. crystallodon has spots only towards the base of the caudal, around the black spot usually ending the median
series" (Garman, 1895).
The flank in females bears numerous alternating light and dark bars, the light bars varying in width from about one half to twice that of dark bars. The bars gradually merge with background
pigmentation anteriorly on the flank and while clearly defined on the rear flank are difficult to distinguish anteriorly.
The caudal fin spot in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally, single, smaller, dark, subsidiary spots may be found antero-dorsally and
antero-ventrally to the basal spot or scattered spots may be found irregularly before and behind the basal spot.
Males have a pigmentation very similar to that of A. sophiae and the description here is identical. Some minor observed variation is attributed to variation in size and maturity of the fish
compared. Males have light flank bars half the width or much narrower than alternating dark bars. The margins of the dorsal, anal and caudal fins are clear while the rest of these fins is dark. Some fish
have up to 3, but usually 2, thin, light bars on the basal half of the caudal fin; these are generally larger fish. The margin of the lower half of the pectoral fin has concentrations of pigment on the
membranes such that this area is darker than the rest of the fin. The anal fin is darkest posteriorly where pigment is concentrated on membranes. The distal third of the fin is pigmented to form a dark
band, becoming lighter proximally. The dorsal fin is the darkest fin (except for the clear margin) and the anterior base is the darkest part of the fin. Bands are not always evident but pigment spots are
large proximally. Some fish have 2, sometimes 3, thin light bars at the base separated by thin dark bars and paralleling the body profile while others have none. The dorsal fin base may have instead a
series of lighter spots, sometimes irregular and not paralleling the body profile.
Size
Reaches 59.5 mm standard length (Esmaeili and Shiva, 2006).
Distribution
This species is restricted to the Lake Maharlu basin near Shiraz.
Zoogeography
This species is found only within the endorheic Lake Maharlu basin, Coad (1996a) suggests that tooth-carps in inland waters may have risen with the post-Pliocene uplift of the Zagros Mountains rather
than being the result of relatively recent inter-basin dispersal.
Habitat
This species is found in fresh streams and springs and in springs of varying saline content or saline influence from hypersaline chloride Lake Maharlu. The lake at 124‰ is an impassable barrier
to dispersal but is very shallow and dries out periodically, e.g. in 1967 (Cornwallis, 1968a). The larger springs and their streams can then meet and transfer fishes on the dried-out lake bed. Some springs
are 27 km apart along the lake margin and have probably had no contact or fish exchange over many generations. This is especially the case for the smaller springs which emerge from the ground with a
diameter of only 1 m and restricted flow. When lake water rises, lower springs are inundated with hypersaline water and the fishes are killed. The spring is presumably recolonised from a higher spring
when lake level falls.
Fish placed in pure lake water die within minutes. Springs discharging directly into the lake contain tooth-carps but the fish do not venture into the lake. Some fish, if disturbed or deliberately
harassed, will swim out for a short distance into the hypersaline lake water but they blanch and rapidly retreat to fresher water (Coad, 1996a).
Esmaeili and Shiva (2006) found the bottom of these water bodies to be muddy but the water was clear and slow-running. Conditions in October at three different sites were 16.9-19.0ºC, pH 6.70-6.74,
dissolved oxygen 3.96-6.11 mg/l, nitrate 0.9-1.6 mg/l, nitrite 0.029-0.062 mg/l, phosphate 0.35-0.65 mg/l and ammonium 1.55-2.60 mg/l.
Age and growth
In the studies of Esmaeili and Shiva (2006), the sex ratio was 1.67:1 for females:males, highly significant, the age groups were 0+ to 3+ years, and positive allometric growth was determined (b
value significantly greater than 3).The condition factor for females was highest in February and March and lowest in September while in males it was lowest in December and increased until February.
Fishes smaller than 25 mm had an equal sex ratio, suggesting selective predation on males, or better survival or longevity of females. Esmaeili and Ebrahimi (2006) give a significant length-weight
relationship based on 62 fish measuring 1.86-4.27 cm standard length. The a-value was 0.0222 and the b-value 3.395 (a b-value < 3 indicating a fish that becomes less rotund as
length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Alavi-Yeganeh et al. (2011?check published paper date) give length-weight
data on this species from Kaftarak spring with a (intercept) being
0.0097and b (slope) being 3.303 for sexes combined. Food
Unknown but presumably similar to other tooth-carps.
Reproduction
Esmaeili and Shiva (2006) found the gonadosomatic index in females to increase from November to June, decreasing slowly from late June to November. The reproductive period lasted six months. Males had
two peaks, in April and August. Egg diameters reached 1.71 mm, absolute fecundity ranged from 45 to 250 eggs (average 115.7 eggs) and relative fecundity was 21.6 to 244.1 with a mean of 90.01 per gram
body weight. Even small fish had hydrated eggs and, with the extended reproductive period, shows adaptation to an unstable habitat.
Monsefi et al. (2009) found that this species is a batch spawner in the
Barm-e Shur spring with a spawning period from April to November. This species
lives in unstable environments such as temporary lagoons or very small pools and
batch spawning of relatively large eggs gives a greater chance of survival. Parasites and predators
Mokhayer (1989) reported metacercariae of the eye fluke, Diplostomum spathaceum from Aphanius sophiae, probably this species, in Iran. The fluke can cause complete blindness and death in
commercially important species of fish. This fish was also infested with yellow grub, Clinostomum complanatum. González-Solís et al. (1997) report Contracaecum sp. larvae from this
species in the Lake Maharlu drainage, Fars.
Economic importance
This species has no current economic importance although it would make an excellent aquarium fish. Villwock (1959) gives details of maintaining what seems to be this species under aquarium conditions,
gradually transferring them from a mixture of salty and magnesium sulphate water to stagnant tap water. They prefer some admixture of sodium chloride and magnesium sulphate which matches their natural
habitat (2% synthetic sea water plus a tablespoon of epsom salts per 25 litres water). Aquatic plants such as Myriophyllum and Cabomba are also necessary for breeding as the eggs are
attached to them but artificial substitutes can be used. Yellowish transparent eggs are 1.5 mm and are deposited usually individually, rarely in small groups.
Larvae feed on Artemia salina nauplii and adults on daphnia, red mosquito larvae and tubifex. Male-male interactions include mock fights with spread fins and close swimming. The loser hides in the
algae and is not strongly pursued by the victor. These interactions, and those between sexes, are not strongly developed and aquaria can be stocked at 15-20 fish per 25 litres. Y. Keivany notes (pers.
comm., 2004) that this species is difficult to maintain in aquaria.
Conservation
The populations are found in a variety of springs and streams around Lake Maharlu. Some of these localities are threatened by water abstraction, road construction and pollution but a number of
localities are small and/or saline and not under threat.
Further work
Studies on aquaria maintenance would enable it to become established in the aquarium trade and help its conservation.
Sources
Type material: None seen.
Iranian material: CMNFI 1979-0017, ?47, ?17.9-35.5 mm standard length, Fars, stream at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 1979-0018, 75, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0033, 1, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0034, ?124, 14.8-38.7 mm standard length, Fars, spring on shore of Lake Maharlu (29º27'N, 52º44'E);
CMNFI 1979-0035, 17, ? mm standard length, Fars, spring south of Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0038, 16, ? mm standard length, Fars, spring near Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0039, 91, 15.9-37.9 mm standard length, Fars, spring on shore of Lake Maharlu (29º27'N, 52º44'E);
CMNFI 1979-0041, 61, 20.8-41.6 mm standard length, Fars, spring on shore of Lake Maharlu (29º23'N, 52º48'E);
CMNFI 1979-0042, 28, ? mm standard length, Fars, spring on shore of Lake Maharlu (29º23'N, 52º48'E);
CMNFI 1979-0046, 5, 18.1-32.2 mm standard length, Fars, qanat at Barm-e Dalak (ca. 29º35'N, ca. 52º38'E);
CMNFI 1979-0047, 41, 11.3-32.1 mm standard length, Fars, spring source of Ab-e Paravan marshes (ca. 29º34'N, ca. 52º42'E);
CMNFI 1979-0048, ?45, ?15.3-39.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0049, 12, 17.2-38.5 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0050, 27, 18.3-28.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'30"N, ca. 52º49'30"E);
CMNFI 1979-0051, 3, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º30'N, ca. 52º52'E);
CMNFI 1979-0052, 16, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º30'N, ca. 52º52'E);
CMNFI 1979-0064, 10, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'30'N, ca. 52º49'30"E);
CMNFI 1979-0065, 8, ? mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º31'N, ca. 52º50'E);
CMNFI 1979-0066, ?31, 15.6-42.9 mm standard length, Fars, spring on shore of Lake Maharlu (ca. 29º32'N, ca. 52º48'E);
CMNFI 1979-0110, 2, ? mm standard length, Fars, lake in Park-e Shahr, Shiraz (29º38'N, 52º32'E);
CMNFI 1979-0112, 24, 11.9-45.9 mm standard length, Fars, stream at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 1979-0118, 12, ? mm standard length, Fars, Barm-e Shur (29º28'N, 52º41'30"E);
CMNFI 1979-0119, 75, ? mm standard length, Fars, steram at Pol-e Fasa (29º29'N, 52º38'30"E);
CMNFI 2004-0006 (GenBank AY593484, AY593493), 15, ?22.2-41.8 mm standard length, Fars, Nasrabad Spring near Imamzadeh Ibrahim (29º35'09"N, 52º39'08"E);
Aphanius ginaonis
Common names
gour-e khar, kopurdandan-e Genu or kapurdandan-e Geno (= Genow tooth-carp).
[Holly's pupfish, Genow pupfish, Gheno pupfish].
Systematicscs
Cyprinodon ginaonis was originally described from "Hei$e Quelle vom Djebel Ginao nordlich von Bender Abbas, südöstliches Persien". This is the Ab Garm-e Ganow at 27°26-28'N,
56°18-20'E, north of the Iranian port of Bandar Abbas at the Straits of Hormuz.
Berg (1949) places this species in the synonymy of Aphanius dispar stoliczkanus (see A. dispar above) and Villwock et al. (1983) regard it tentatively as a synonym of Aphanius
dispar. Wildekamp (1993) is of the opinion that it may be a subspecies of Aphanius dispar. Hrbek and Meyer (2003) using mtDNA found this species to be deeply nested within the A. dispar
clade. Villwock (2004) has used cross-breeding experiments that demonstrate this taxon and A. dispar are comparable to intraspecific crosses in other taxa. However, fin ray counts are
non-overlapping and Reichenbacher et al. (2007; 2009) demonstrate differences in otolith morphology
and affirm its validity as a species. Holly (1929b) reports a specimen of Cyprinodon dispar from the same spring. I was unable to
verify the co-occurrence of two species in the hot spring through my own samples. Holly's specimen of A. dispar is in the Naturhistorisches Museum Wien (NMW 13799) but there are no field notes to
confirm its locality with accuracy. It may have been collected in an adjacent stream. A. ginaonis is regarded here as a good species on morphological grounds (see below).
Four syntypes are in the Naturhistorisches Museum Wien (NMW 13800-13803). Holly (1929a) in his original description refers to 3 syntypes. One of these 4 fish was selected as a lectotype by F. Krupp
(NMW 13800) and measures 21.8 mm standard length while the remainder measure 17.8-23.2 mm standard length.
Key characters
This species is distinguished from Aphanius dispar, the only other tooth-carp in this part of southern Iran by its locality and by having fewer total dorsal fin rays (5-7 as opposed to 8-11,
mostly 9-10 in A. dispar from neighbouring drainages).
Morphologygy
Total dorsal fin rays 5(16), 6(38) or 7(6). Gut coiling is highly variable between individuals and is complex (illustrated in Coad (1980b)). Reichenbacher et al. (2007,
2009) give descriptions of
otolith morphology. Chromosome number 2n=48, karyotype 14Sm + 34St and arm
number 31 (Esmaeili et al., 2008). Esmaeili and Gholami (2007) give
details of scale ultrastructure. Saefali (1999) has D4-7, Pelvic5-7, Pect 13-17, A6-10, ll25-32,vert 26-30, bars10-22, gr13-21 SEE DATA SHEETS
Sexual dimorphism
Females have a higher mean numbers of anal fin rays, total vertebrae and flank bars, and longer pelvic fins (Coad, 1980b). Seifali and Sheidai (2001) found rostrum to pelvic fin distance, rostrum to
anal fin distance, weight, total distance (? total length), head depth, head distance (? length) and body depth to differ between the sexes.
Esmaeili and Gholami (2007) found that female scales were larger than those in
males. Colour
Males have a brighter colouration than females although colouration is individually variable within sexes. The back is mottled black. The dorsal, anal, pectoral and pelvic fins are a light orange.
Flank bars are alternately black tinged with orange and grey-white, light blue or light orange. The side of the head anteriorly is iridescent light blue to green and the opercle is dorsally orange,
postero-ventrally blue. The iris is iridescent green and the anterior ventral head surface is light blue. The peritoneum is black.
Preserved specimens have two bars on the male caudal fin with the fin margin hyaline. These bars are usually straight. Some fish have 1-3 bars or a forked bar. Females lack these bars and their caudal
fin rays are lightly speckled. The male dorsal fin has 4-5 horizontal rows of spots, fading dorsally. These are not always well-developed and then consist of irregular, black pigmentation on the rays and
membranes. The dorsal fin pigmentation in females is weak. The anal and pelvic fins have little or no pigment. The pectoral fins of males carry pigment concentrated on the rays, weakly speckled in females.
Flank bars in females vary from weak to obvious, the light intermediate bars not as highly coloured as in males nor as well defined.
Size
Reaches 4.5 cm total length.
Distribution
This species was described by Holly (1929a) from the Ab Garm-e Ganow at 27°26-28'N, 56°18-20'E (various reports give differing latitude-longitudes for this well-known locality, hence the range). Ab
Garm literally means "hot water" in Farsi and is used to denote a hot spring. Ganow means "foul water". The spring lies at an altitude of about 135 m (Moghadam, 1974) on the slopes of
the Ganow Mountain (= transliterated as Ginao in German), hence its scientific name.
Zoogeography
The occurrence of sympatric A. dispar would confirm the species distinction of this species.
Habitat
The species is common within the bounds of the hot spring stream, numbering in the thousands in the 1970s, but no population trends have been recorded (but see below). Fish are found particularly along
the stream margin and in many minor subsidiary springs which emerge a few metres from the main spring. Some fish are found in water as shallow as 1 cm but temperatures are 37-40°C (when shade air
temperature was 20°C at 1400 hours on 27 January). The main spring itself issues from the ground at 30 litres second-1 and 41°C and drains as a stream about 10-15 m wide. A fault, over which
the spring stream pours, isolates the fish from those downstream. Coad (1980b) summarises water chemistry
and Reichenbcher et al. (2009) and Golmoradizadeh et al.
(2012) give recent descriptions of the habitat. The water is clear and colourless but there is a strong sulphur odour. The stream bed is
composed of stones and pebbles covered by lime-green to dark blue-green algae. Moghadam (1974) gives details of the algae species.
Age and growthth
Essentially unknown although fish are larger later in the year (11.8-31.4 mm standard length in January and 15.6-34.7 mm in March). Esmaeili and Ebrahimi (2006) give a significant length-weight
relationship based on 33 fish measuring 1.70-3.17 cm standard length. The a-value was 0.0210 and the b-value 3.375 (a b-value < 3 indicating a fish that becomes less rotund as
length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Alavi-Yeganeh et al. (2011?check published paper date) give
length-weight data on this species with a (intercept) being 0.0076 and
b (slope) being 3.384 for sexes combined.
Golmoradizadeh et al. (2012) gave growth
parameters of W(t) = 0.012TL3.42 for females and W(t) =
0.0101TL3.38 for males. Females showed an asymptotic total length (TL∞)
of 53.03 mm, a growth coefficient (K) of 0.15 year-1, t0
1.01 year, and natural mortality coefficient (M) 0.49 year-1.
Males showed an asymptotic total length (TL∞) of 48.83 mm, a growth
coefficient (K) of 0.21 year-1, t0 0.44
year, and natural mortality coefficient (M) 0.62 year-1 (note
that the values for M are reversed in the abstract compared to the text
by sex - the text value is given here). Females attained an age of 5+ years and
males 4+ years. Maturity was attained at age 2+ years or older. Sex ratio was
1:1.33 (male: female) and significantly different. Food
Gut contents are the lime green algae typical of the hot spring and chironomids.
Reproduction
Temperature is constant and daylength may then be important in triggering reproduction, although fishes caught on 27 January and 21 March have mature eggs of similar size (ca. 1.6-1.7 mm) suggesting
an extended reproductive period. There was a strong correlation between absolute
fecundity and weight (Golmoradizadeh et al., 2012). Parasites and predators
Unknown.
Economic importance
None except related species are important in the aquarium trade and this is a potential economic use.
Conservation
Coad (1980b) listed this species as rare although it should be classed as vulnerable under the revised IUCN Red List Categories (IUCN, 1996) according to Coad (1998a).
The site is easily accessible by an asphalt road and is close to the large city of Bandar Abbas. Two hammams (bath houses) are in operation, one for men and one for women, the spring water is drained
off to irrigate date palms, and local people also use the site for bathing, washing kitchen utensils and food containers, and washing clothes so there is input of soap and food debris (personal
observations; A. R. Zinaei and H. R. Esmaeili, Payam-e Noor University, Bandar Abbas, 6 August 1997). The area is not under direct management by environment officials although in 1999 press reports
referred to planned research studies aimed at preventing extinction of this taxon (IRNA, 29 September 1999) and the spring does lie in the Genu Protected Area (Biosphere Reserve) described by
Zehzad et al. (1997).
The habitat could deteriorate if too much soap and food debris enter the stream. A single catastrophic event, such as a chemical spill, water diversion or construction activity, could eliminate the
species. Recent construction has severely affected the population with only 1-2 specimens being caught and the population being replaced by A. dispar (H. R. Esmaeili, pers. comm., September 2007).
Reichenbcher r et al. (2009) suggest that A. dispar specimens
have been added to the spring by local people to increase the population size.
Otolith differences between samples taken in 201 and 2009 suggest hybridisation. Local people using the spring should be educated on its ecological significance and alternative facilities for bathing and washing established. Roadside access to the spring should be prevented. The
population structure and reproductive regime of the fishes could be analyzed to determine if they are susceptible to abrupt changes.
Further work
This species differs from A. dispar in a number of characters but this may merely be a consequence of the unusual environment of the hot spring. Cross-breeding experiments would be very
revealing and would demonstrate or disprove reproductive isolation and hybrid fertility. The population is very important as the only species in a unique habitat, lending itself to studies of speciation,
adaptation and variation in response to high temperatures.
Sources
Type material: see above, NMW 13800-13803.
Topotypes: CMNFI 1979-0175, 77, 11.8-31.4 mm standard length. check size range;
CMNFI 1979-0416, 7, ? mm standard length, Hormozgan, Ab Garm-e Ganow below falls (ca. 27º26'N, ca. 56º20'E);
CMNFI 1979-0417, 44, ? mm standard length, Hormozgan, Ab Garm-e Ganow, side springs and edge of mainstream (ca. 27º26'N, ca. 56º20'E); CMNFI 2007-0054, 13, ? mm standard length, Hormozgan, Ab Garm-e Ganow (ca. 27º26'N, ca. 56º20'E);
Aphanius isfahanensis
Common names
كپوردندان اصفهان (= kapurdandan-e Esfahan or Esfahan tooth-carp).
Systematics
The holotype is in the Canadian Museum of Nature, Ottawa under CMNFI 2004-0001, male, 25.0 mm SL, Esfahan Province, Zayandeh Rud (Zayandeh River) at Varzaneh Bridge, 32º25'32"N, 52º39'14"E,
1 July 2002, Y. Keivany and S. Asadollah and paratpes are under CMNFI 2004-0002, 18 males, 20.8–30.9 mm SL, 18 females, 22.0–38.4 mm SL, of 49 total (13 not used in meristic and morphometric
analyses, total range in size 12.1-38.4 mm standard length), same locality as holotype; AMNH 233639, 1 male, 25.2 mm SL, 1 female, 21.6 mm SL, same locality as holotype; MRAC 2004-05-P-01-02, 1 male, 25.2
mm SL, 1 female, 21.6 mm SL, same locality as holotype; GenBank accession numbers AY593488, AY593489, AY593497, and AY593498.
Key characters
Distribution and colour pattern identify this species. Males can be distinguished from those of all other Iranian species by having distinct black edge on the dorsal, anal, and pelvic fins. The dorsal
fin is covered with a high density of black blotches. Females can be distinguished from females of A. sophiae and A. vladykovi by having flank-bars rather than spots. It can also be
distinguished from A. farsicus by less well-defined bars terminating at a mid-flank stripe and a relatively light gray stripe at the caudal-fin base rather than a black spot or blotch. Flank-bars
are also characteristic of females of Aphanius dispar, however.
Morphology
Morphometric data are given by Hrbek et al. (2007) but these characters cannot be used for a simple distinction from other Aphanius in Iran but only in multivariate space. This species
is clearly distinguished at the genetic level from all other species of Aphanius. It has 82 molecular apomorphies – 19 transversions, two transversions/transitions (depending on comparison), and
61 transitions – that show fixed character state differences to homologous characters analyzed in A. sophiae, A.
farsicus (formerly persicus), and A. vladykovi from Iran. Thirty-seven of these character
states are also apomorphies when compared to A. anatoliae, A. danfordii, A. villwocki, A. asquamatus, and A. fasciatus from Turkey.
Dorsal fin rays 11-14, anal fin rays 10-13, pectoral fin rays 13-16, pelvic fin rays 4-6, lateral line scales 25-29, total gill rakers 10-13, precaudal vertebrae 10-13 and caudal vertebrae 15-18.
Chromosome number 2n=48, karyotype 12Sm + 36St and arm number 30 (Esmaeili i et
al., 2008). Sexual dimorphism
See colour below.
Colour
Male flank bars number 8-12, mean 10.2, significantly less than in A. sophiae at 10-21, mean 14.4. The bars are broad with interspaces about equal or slightly narrower. The bars extend from
behind the head to the tail. Anterior bars fade on the belly, whereas, posteriorly on the caudal peduncle, they encircle the body. Dorsally, the head is dark gray and the body is lighter but still
heavily pigmented with melanophores; the belly lacks pigmentation. The sides of the head are densely speckled with melanophores, more thinly on the ventral side; in most specimens the chin is darker than
the rest of the ventral head surface. The eye is bounded ventrally and postero-ventrally by a thin line of black pigment. The defining male coloration is the black margins of the pelvic, anal, and dorsal
fins. The dorsal and anal fins may present a halo effect, the margins being so dark in relation to the rest of these fins. The tips and outer margin of the pelvic fin are blackish. The anal
fin has a broad, blackish margin with the rest of the fin light cream-colored. The dorsal fin has the blackest margin. The rest of the dorsal fin is variably blotched, the blotches being much lighter than
the fin margin. Most specimens have a contrasting pigmentless area just below the fin margin. The pectoral fin has sparse pigmentation along rays and ventrally on the interradial membranes
but lacks the concentrated black pigmentation seen on the pelvic fins. The caudal fin rays and membranes are sparsely pigmented, and the whole margin may be blackish but in most fish pigment is restricted
to the upper and lower margins, the lower margin only, or is absent. Large females have a grayish dorsal surface to the head, a lighter back and upper flank covered with scattered melanophores, and a
mid-flank stripe terminating on the caudal peduncle in a blackish, short stretch covering up to three scales (Fig. 2b). This short stretch of pigment is present in all females, faint in some, rarely
forming a blotch and in some small fish tapering anteriorly. The flank stripe may be broken into a series of blotches in some smaller females, or it may be continuous as in large fish. Starting anterior
to the belly there is a ventrolateral series of thin bars (up to ten) separated by cream colored interspaces 1–3 bars wide. These are absent in some smaller females, which may only have blotches at various
levels present in this region. Even some large females have faint flank pigmentation so that bars and the stripe are weakly expressed. At about the origin of the anal fin, the flank bars may continue onto
the caudal peduncle in regular form or become irregular, breaking up into blotches. Anteriorly the bars terminate ventrally at about the level of the lower edge of the pectoral fin. The belly and lower
head have sparse pigment although the chin and sides of head are speckled with melanophores. The eye is bounded ventrally and posteroventrally by a thin line of black pigment. Fins lack any distinctive
pigment pattern. Fin rays are outlined with melanophores, and interradial membranes of the caudal and anal fins have melanophores at varying degrees of density. The dorsal fin has the most interradial
pigmentation, particularly near, but clear of the fin base.
Size
Attains 38.4 mm standard length, 42.8 mm total length (Alavi-Yeganeh et al.,
2011?check published paper date). Distribution
The type locality is near the town of Varzaneh on the lower reaches of the Zayanadeh River, about 30 km upriver from the terminal sump, the Gav Khuni marsh.
Zoogeography
An estimate of a 4.8 MYA divergence of A. isfahanensis and A. sophiae + A. persicus
(now farsicus) is given by Hrbek et al. (2007). This divergence time is in accord with the hypothesis
of a near-simultaneous diversification of ~5 MYA of organisms occupying different geological units of central Iran.
Habitat
The type locality had a water temperature of 27ºC, pH was 6.7, the water was brackish, conductivity was 10.9 mS, dissolved solids were 5450 ppm, dissolved oxygen was 12.3 mg/L, river width was 50 m,
and capture depth was 0.5 m. Current was slow, and there was no cover. Gambusia holbrooki was captured at the same locality.
Bleher (20110 notes that the type locality was dry at Varzaneh Bridge and
only found specimens in an artificial circulating spring channel 50 m long in
southern Esfahan. Age and growth
Unknown.
Alavi-Yeganeh et al. (2011?check published paper date) give length-weight
data on this species from two localities with a (intercept) being 0.0120
and b (slope) being 3.035 for all localities and sexes combined. Food
Unknown.
Reproduction
Unknown.
Parasites and predators
Gholami et al. (2009) record the trematode Diplostomum spathaceum
from the eyes of this fish. Economic importance
None at present but a potential aquarium species. Breeding and aquarium conditions are similar to A. mento although it is reputedly difficult to breed.
Conservation
Known only from the type locality, which is not protected, this species needs a conservation assessment.
Further work
The biology of this species requires study.
Sources
Type material: See above, CMNFI 2004-0001, CMNFI 2004-0002, AMNH 233639, MRAC 2004-05-P-01-02.
Aphanius mento
Male
Common names
gour-e khar, kopurdandandar-e Arvand (= Arvand River tooth-carp), kopurdand-e Irani.
[batrikh qabras in Arabic; Persian pupfish, Persian minnow, Black Persian minnow, Persian killie, Arvand pupfish, chin killifish, pearl-spotted killifish].
Systematics
Lebias Cypris Heckel, 1843 is a synonym. Krupp (1984a) gives a detailed synonymy of this species but considered cypris to have priority. Klee (1967) and Lazara (1989) point out that
Garman (1895), in a work dated in July of that year, as first revisor placed cypris in mento. This decision has priority over Gaillard (1895), a work with no exact date of publication, in
which mento was placed in cypris, according to the Zoological Code of Nomenclature (Ride et al., 1985). The type locality for both Lebias mento and Lebias cypris is
"Mossul" according to Heckel (1843b).
Hybrids with h Aphanius dispar richardsoni are reported from Israel (Goren and Rychwalski, 1978).
Parenti (1981; 1984) places this species in the genus "Aphanius", i.e. distinct from true Aphanius without defining and naming a new genus. This genus is more closely related
to the Anatolian Kosswigichthys Sözer, 1942 and the South American Orestias Valenciennes in Cuvier and Valenciennes, 1846 than true Aphanius. Hrbek and Meyer (2003) note that, based
on their mtDNA study, this species diverged from the ancestor of the A. dispar clade at an early date, 26.87±1.31 MYA. They speculate that the ancestor of mento invaded the northern
perimeter of the Arabian plate as it was abutting Laurasia some time after the separation of their eastern and western clades of Aphanius (see above) before the closing of the Tethys Sea. It later
spread through the present-day Tigris-Euphrates and into the Levant and southern Turkey.
The 5 syntypes of Lebias cypris are in the Naturhistorisches Museum Wien (NMW 59598) with 1 specimen selected as a lectotype by Krupp and Schneider (1989), the other 4 being paralectotypes. The
syntypes of Lebias mento are (NMW 62105 (4 fish), 59042 (5) and 22624-22632 (9). Eschmeyer et al. (1996) list NMW 21699-704 (6) and NMW 59832 (21) as possible syntypes. The Vienna catalogue
lists 5 specimens of Lebias cypris and 8 of Lebias mento.
Key characters
The adult colour pattern is distinctive and dorsal fin ray counts are usually higher than in A. dispar.
Morphology
Scales along side of body 23-28, total dorsal fin rays 9-14, usually 12-14, total anal fin rays 9-13, pectoral rays 12-16, and pelvic rays 4-6, usually 5. Frontal scalation is E-type (Hoedemann, 1958).
Flank scales are squarish to a vertical oval. The former shape has a vertical anterior margin which may be wavy or slightly convex, upper and lower anterior corners rounded but square-cut, dorsal and
ventral margins parallel, and the posterior margin rounded. The oval scales have all margins rounded. The focus is central to subcentral posterior, circuli are fine although coarser on the posterior field,
radii are restricted to the anterior field and are almost horizontal and parallel. There is no pelvic axillary scale. Total gill rakers 11-15, rakers being spinulose and reaching beyond the adjacent raker
when appressed. Vertebrae 25. Teeth are tricuspid with a long and pointed central cusp (Goren and Rychwalski, 1978). There are 14-16 teeth on the lower and 10-12 on the upper jaw (Berg, 1949). The gut is
s-shaped. Reichenbacher et al. (2007) give a description of otolith morphology. Chromosome number 2n=44 or 48 (Wildekamp, 1993; Klinkhardt et al., 1995).
Sexual dimorphism
See below under Colour.
Colour
Adult, breeding males are a dark blue-black to dark brown or almost black with iridescent blue-white to silvery spots regularly-arranged on the fins as curved lines, and irregularly on the body
(sometimes as irregular vertical bars and sometimes the spots are vertically elongate) (Richter, 1989; preserved material from Iraq; Goren and Rychwalski, 1978). The edges of the gill cover are
orange-red (Wildekamp et al. (1999). Male colour fades in low light conditions and in winter males have silvery flanks with a dark brown back. Spots are silvery-blue on the upper flank and
are not as numerous as in the spawning male. Females are grey-brown or grey-white to silvery with large golden blotches or silvery to blue spots and dark dots. Scales along the mid-flank usually have a
dark border (Wildekamp et al. (1999). Fins in females are hyaline. Body colour is reportedly heightened in brackish water (Grimes, 1974). The peritoneum is silvery with dense but fine melanophores.
Sizeze
Reaches 8.4 cm total length or more (Güçlü and Küçük, 2008). Distribution
Found in the Orontes (= Asi) and Tigris-Euphrates basins, the Levant in coastal and Dead Sea basins, western Jordan, and in southern Turkey in Mediterranean basins as well as in central Turkey.
Tigris-Euphrates records are relatively rare (see map in Wildekamp (1993)). The species was described from Mosul in northern Iraq and there are records from the region of Basrah in southern Iraq near the
border with Khuzestan. Wildekamp (1993) has one record apparently mapped in southern Iran near the border with Iraq. This is the only record from Iran although Abdoli (2000) indicates the Arvand River on
a map.
Zoogeography
See Systematics above and under the genus.
Habitat
This species has been reported to survive in waters up to 38°C and as low as 0°C. pH at 7.0-8.5 has been reported as ideal for aquaria maintenance. Aquarists report it as a robust species favouring
hard and alkaline water with some salt added (Thiermann, 1978; Kostich, 1979; Phillips, 1985). Grimes (1974) considers it to be quarrelsome in aquaria like other Aphanius and Allen
(1988) also noted that males are very aggressive. It inhabits fresh or slightly brackish water of springs, streams and lakes, usually near shore where males establish territories in vegetation. It
generally prefers habitats dense in vegetation.
Age and growth
Life span is about 3 years with maturity in aquaria at 4-6 months. The length-weight relationship in southern Iraq was W = 6.0001 x 10-5 L2.6403 for males and W = 2.3028 x
10-4 L2.235 for females. The average condition factor for males was 2.0499 and for females 2.3121. The condition factor increases with increase in length in females and decreases in
males (Huq et al. 1977).
Güçlü and Küçük (2008) examined a population of this species in a spring in the
Mediterranean Sea basin of Turkey and found fish in age groups 0 to 7. They also
give length-weight relationships and a von Bertalanffy growth equation. Food
Al-Daham et al. (1977) found filamentous algae and diatoms were the most important foods in southern Iraq. Shafi and Shalli (1986) report a diet of beetles, ephemeropteran nymphs and algae in
southern Iraq. Aquarium specimens prefer meaty foods such as brine shrimps over flake foods (Grimes, 1974) but flake food is taken (Allen, 1988). Curiously, Al-Daham et al. (1977) for their
southern Iraqi fish in aquaria found this species to prefer plant food. Males approached food items individually and prevented other fish from taking the food. Females were also solitary though less
aggressive than the males. However Gambusia holbrooki out-competed male A. mento for food, on one occasion even snatching a Gambusia embryo from the mouth of an A. mento.
Güçlü and Küçük (2008) found their Turkish fish to feed principally on
Gammarus sp and Palaemon sp. Reproduction
Anderson (1966), Lutman (1982) and Richter (1989) observed spawning in aquaria. There is a dominant male which is more deeply and richly coloured than other males. The dominant male defends a spawning
territory, fighting other males. Males will defend separate spawning mops if these are made available in aquaria. The dominant male will pursue a female and places his larger dorsal fin over the her back.
Several spawnings will follow and females will spawn with more than one male. Spawning can occur near the surface. These fish can spawn at six months of age. Peak spawning in southern Iraq is April to
June when only a small proportion of eggs in the single ovary contained fully developed eggs (Al-Daham et al., 1977). However, Shafi and Shalli (1986) state that it breeds in southern Iraq in
May-July. There are up to 71 highly-adhesive, eggs of 170 microns diameter. Aquarium spawned eggs hatch in 7-14 days, depending on temperature, 10 days at 25°, 3 days in the 80s°F, or 6-7 days in the low
70s°F (accounts vary). Allen (1988) found egg production to be higher in aquaria when the equivalent of seven tablespoons of salt was added per gallon at a water temperature of 25°C. A minimum of 20 eggs
per day were produced.
Byniak (1979) describes the effects of day length and temperature on the reproductive cycle of females in this species in Israel.
Parasites and predators
Unknown.
Economic importance
An aquarium fish with breeding details given by Zipay (1961), Anderson (1966), Lutman (1982), Richter (1989), Semeit (1999) and www.killi-data.org/ (downloaded 21 September 2007). The species is
hardy and fry are easily raised, given large enough tanks and an easily defensible area for the male to guard eggs. However, populations stop breeding in aquaria after 6-8 generations
(www.killi-data.org/, downloaded 21 September 2007). Blaustein and Byard (1993) show that this species will prey on mosquito larvae in a laboratory situation and has potential in control of this vector
of malaria, at least in vegetated habitats.
Conservationon
The presence of this species in Iran needs confirmation by field work and until this is done and its distribution, population numbers and biology worked out, its conservation status cannot be assessed.
Endangered in Turkey (Fricke et al., 2007).
Further work
See above.
Sources
Iranian material: None.
Comparative material: BM(NH) 1920.3.3:203-222, 22, 24.9-36.0 mm standard length, Iraq, Basra (30º30'N, 47º47'E);
BM(NH) 1949.7.21:12, 24.4 mm standard length, Iraq, Bahr-el-Milh Lake east of Karbala (32º35'N, 43º50'E);
BM(NH) 1981.10.6:19-23, 5, 26.6-31.8 mm standard length, Iraq, Qarmat Ali, Basra (30º30'N, 47º47'E);
BM(NH) no catalogue number, 4, 19.7-31.7 mm standard length, Iraq, Basra Liwa (no other locality data);
uncatalogued, 1, 31.2 mm standard length, Iraq, Khawr az Zubayr (no other locality data).
?Iraq fish from Hussain
Aphanius mesopotamicus
(Holly, 1929)
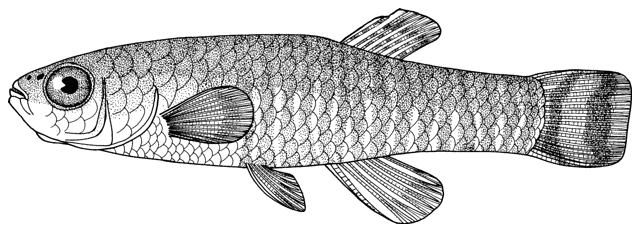
Male
Hrbek, Keivany and Coad, 2007.jpg)
Courtesy of M. Ebrahimi, A Teimori and Z. Gholami.jpg)
Courtesy of M. Ebrahimi, A Teimori and Z. Gholamimi
(Heckel, 1843)
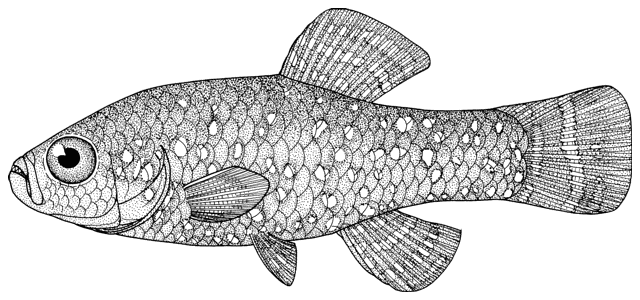
Coad, 2009

Male paratype
21.7 mm SL above, female holotype below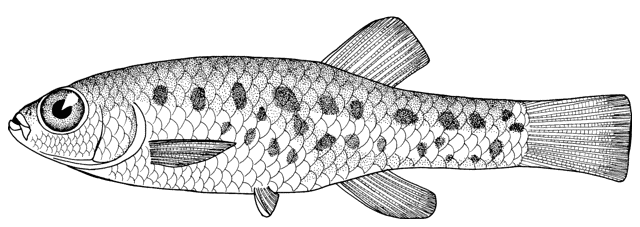
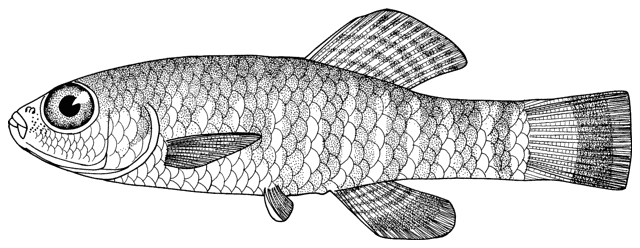
Common nameses
None.
Mesopotamian tooth-carp.
Systematics
The holotype is a female, 29.3 mm SL from Khuzestan, canal branch of Karkheh River, 31º40’N, 48º35’E (CMNFI 1979-0360A) and paratypes are 37 specimens, 14.6-29.1 mm SL, same locality as above (CMNFI 1979-0360B) and 6 specimens, 15.1-20.5 mm SL, from Khuzestan, Karkheh River branch at Abdolkhan, 31º52’30”N, 48º20’30”E (CMNFI 1979-0364). The species is named for Mesopotamia (the land between the rivers) referring to the Tigris-Euphrates basin where the species is found. .
Key characters
This species is distinguished by distribution and by pigmentation, males having clear margins to the unpaired fins, no bars on the caudal fin and 10-15 clearly defined flank bars, females bear irregular blotches or spots on the flank.
Morphology
Total dorsal fin rays 11-13, total anal fin rays 10-12, branched pectoral fin rays 13-15, branched pelvic fin rays 5-6, lateral series scales 25-29, total gill rakers 10-14, precaudal vertebrae 11-13 and caudal vertebrae 14-17.
Sexual dimorphism
See colour description.
Colour
Males have clear margins to the unpaired fins, no bars on the caudal fin and have 10-15 clearly defined flank bars. The dorsal surface of the head and the upper flank are more heavily pigmented with melanophores than more ventral areas. The belly and lower head are unpigmented. The chin and snout have dense melanophores and a rim of melanophores underscores the eye in both sexes. The dorsal, anal and caudal fins in males have wide clear margins. This is also seen in the material from Basrah, Iraq (BM(NH) 1982.9.2:326-328). The material from Basrah figured by Berg (1949) has distinct light margins in some printed versions of the figures. The caudal fin is darker just proximal to the clear margin, lighter in mid-fin and dark again at the base. The dorsal fin has irregular pigmentation on the membranes and, to a lesser extent, on the rays. The pigmentation may involve an overall darker colour in contrast to the light margin or may have some pattern to it. The pattern is often elongate and short blotches with no regular arrangement and sometimes may appear as up to 5 wavy and oblique bands. Dark pigmentation is found just behind the first ray on the fin membrane. The anal fin is darkest just proximal to the clear margin. Up to the last 6 membranes of the anal fin are dark and this pigment may be broken up in as many as 4 elongate bars along each membrane. A similar pattern is found in some dorsal fins and the general effect on both fins is that the postero-dorsal (anal fin) and postero-ventral (dorsal fin) parts of these fins are the darkest. The dorsal, anal and caudal fins generally have more pigment on the membranes than the rays and in some this is quite distinctive, making the rays stand out.
The pectoral and pelvic fins in males are generally clear or somewhat milky and opaque and lack melanophores. The distal parts of the membranes between the last 5 rays of the pectoral fin and the small membrane area of the pelvic fins can be pigmented. Males have flank bars circling the caudal peduncle and reaching the anal fin base but fading ventrally on the lower part of the anterior flank, not reaching the ventral margin of the belly and becoming progressively shorter and less distinct the more anterior they are. Bars are 2-5 times broader than the pale interspaces.
Females have a similar head and dorsal and ventral body pigmentation but it is much lighter than in males. Fins have little or no pigmentation. The proximal third to half of the dorsal fin rays and membranes, particularly the anterior ones, have pigment in some fish but this is weakly expressed compared to the condition in males. Some fish have a few faint melanophores lining the anal fin rays.s.
The most distinctive feature in females is a spot, oval to lozenge-shaped, at the central base of the caudal fin. Its concentration of melanophores is much higher than any other feature. The flank in females can have up to 14 thin, dark, wavy and irregular vertical patches of pigment. These patches may be interrupted in their vertical extent and are weakly expressed anteriorly. They fade ventrally, ending above the lower edge of the caudal peduncle and above the anal fin base, and are absent on the lower anterior flank. The patches are light and not as contrasting with the lighter interspaces as the bars found in males. Patches are thin, half to one third of the width of the interspaces. Very often the patches are broken up into spots and elongate blotches of various sizes, and a regular barred appearance is not usual. The spots and blotches are all smaller than the eye size by at least half. Material from Basrah, Iraq figured by Berg (1949) has the spots emphasised but material from Basrah (BM(NH) 1982.9.2:326-328) examined for this study has a more blotchy appearance and spots are not well-defined.Size
Attains 29.3 mm standard length.
Distribution
Found in the southern Karkheh River basin of Iran and at Qarmt 'Ali at the northern part of Basrah on the Shatt al Arab, southern Iraq. Possibly also at Shalili-ye Bala or Shalili-ye Pa'in (ca. 31º58'N, 48º53'E), near Shushtar, Karun River basin of Iran from a record in Berg (1949) from Shellali (ZISP 25446). Keivany et al. (2012) captured fresh material from a Karkheh River branch 10 km west of Hoor-Al-Azim Wetland (31º32'59.4"N, 48º09'48.3'E, not shown on map below).
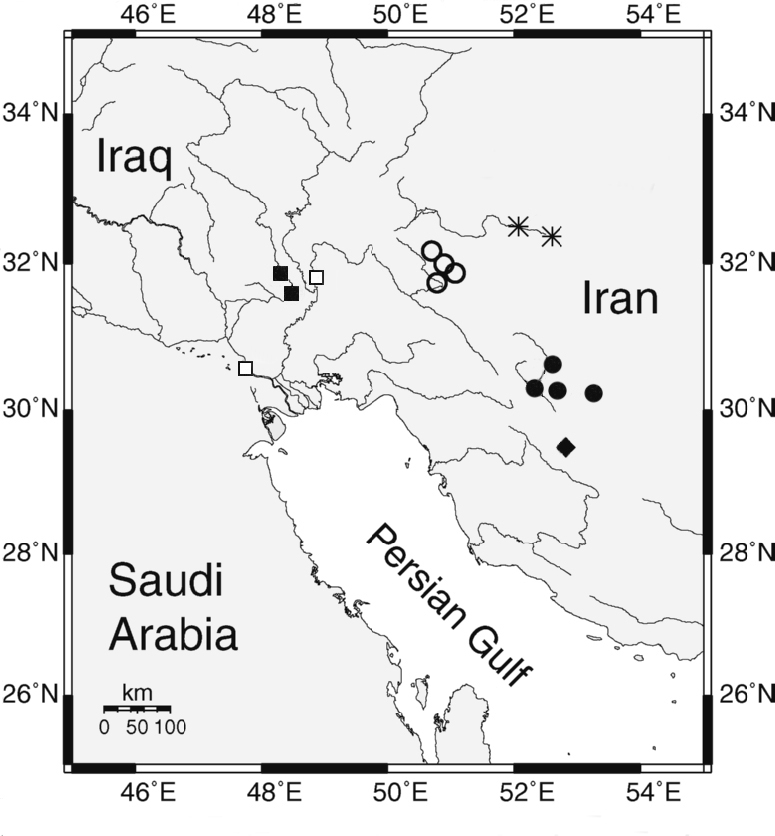
Distribution map of Aphanius in Mesopotamia and adjacent areas. Solid squares type series of A. mesopotamicus (lower one CMNFI 1979-0360A and B, upper 1979-0364), open squares additional, non-type material of A. mesopotamicus (BM(NH) 1982.9.2:326-328 and ZISP 25393 (lower left) and ZISP 25446 (adjacent to type series)), open circles A. vladykovi, stars A. isfahanensis, solid circles A. sophiae, and diamond A. farsicus (map modified after Hrbek et alal. (2006)).
Zoogeography
This species is found in the Tigris River basin and its morphology shows evidence of relationship with species of internal basins in Iran.
Habitat
The habitat of the new species is known only from field notes made at the time of capture in Iran on the Khuzestan plains. The two localities are a river and a canal branching from that river. The 25 m wide river had a water temperature of 22°C at 1205 hours, pH 6.0, conductivity 1.0 milliSiemens, a mud bottom and the principal plant materials were rushes and reeds. The 30 m wide canal had a water temperature of 15°C at 0930 hours, pH 6.0, conductivity 1.8 milliSiemens, a mud bottom and the principal plant material was filamentous green algae. Keivany et al. (2012) captured material in a river branch with calm water flow on a sandy bed with Ceratophyllum, Typha and Phragmites, water temperature 24.4°C, dissolved oxygen 8.8 mg/l at 106% saturation, pH 8.23, conductivity 2367 µS and total dissolved solids 1181 mg/l.
Age and growth
Unknown.
Food
Unknown.
Reproduction
Unknown.
Parasites and predators
None recorded in Iran.
Economic importance
None at present in Iran but a possibly a species of interest to aquarists.
Conservation
This species is known only from museum collections and recent efforts to capture fresh material were unsuccessful. These attempts should be repeated and the numbers and biology of the species assessed.
Further work
The biology and continued presence of this species in Iranian waters requires study.
Sources
Based on Coad (2009).
Type material: See above.
Comparative material: BM(NH) 1982.9.2:326-328, 4, 20.2-25.3 mm SL, Iraq, Qarmat `Ali, Basrah (30°34’N, 47°46’E).
Aphanius
pluristriatus
(Jenkins, 1910)

Male, courtesy of H. R. Esmaeili
Common names Kapour-e-dandandar-e-Mond.
[Multi-striped (Mond) tooth-carp] Systematicscs
Cyprinodon
pluristriatus Jenkins, 1910 was often referred to the synonymy of A. sophiae but is found "East of Shiraz, stream running to Fussa, Southern Persia, 5,000 feet". Assuming this to be
Fasa and using topographic maps, the locality is in the Mand River basin
draining to the Persian Gulf. Recently, Esmaeili et al. (2012) have
re-discovered and re-described this species from the Zarjan Spring system in the Mand (or Mond) River basin of Fars near Fasa, presumed to be the type locality. Four syntypes of Cyprinodon pluristriatus
(ZSI F9408 to ZSI F9411) are in the Zoological Survey of India, Calcutta (Menon and Yazdani, 1968), although Jenkins (1910) has ZSI F9408-9412.
Key characters
Distribution, a smaller caudal peduncle index, higher number of flank bars on
average, lower number of gill rakers and higher J-scale index separate this
species from other southern Iranian Aphanius species (Esmaeili et al.,
2012). Morphology
Morphological and meristic characters overlap with other Aphanius
species examined by Esmaeili et al. (2012), q.v. for
details, although the both sexes of this species are separated by discriminant
function analyses. For example, gill rakers number 8-11 with mean values of 9.84
for males and 9.58 for females (ranges for other species are 8-13 for A.
farsicus, means 10.47 for males and 10.43 for females; 10-13 for A.
isfahanensis, 10.78 and 11.16; and 9-12 for A. sophiae, 10.66 and
10.71). Sexual dimorphism
See colour description. Colour
The dorsal, caudal and anal fins in males have a pale margin, proximal to
which the fin is dark with the bulk of the fin lighter in colour. The main part
of these fins has numerous rows of spots. The lower edge of the pectoral fin is
black. The dark fin margins and spots are absent in females. Males have
alternating thin white and brown flank bars, the white bars numbering 11-17 with
a significantly higher mean (13.84) than A. farsicus (9-16 bars,
mean11.56), A. isfahanensis (9-13, 10.67) and A. sophiae (8-15,
11.89) (Esmaeili et al., 2012). Females have thin or thick, dark, wavy
and irregular short bars or blotches of pigments on the flank, some approaching
a spot shape. An oval to lozenge-shaped dark spot is at the central base of the
caudal fin and this may be broken up into several spots. Both males and females
have small and irregularly arranged spots on the operculum.
Size
Not given
Distribution
Specimens were known only from the Zarjan Spring system in the Mand River basin
of Fars near Fasa, but possibly exist in a qanat near Jahrom (Esmaeili et al.,
2012). Zoogeography
Esmaeili et al. (2012) suggest that this species diverged from an
Aphanius population in the ancient Kor River system. In the Quaternary, a
Palaeo-Kor River crossed the type locality of A. pluristriatus and drained
to the Persian Gulf via the Mand River. In the late Quaternary (20,000-10,000
years ago) or Holocene (6000-2000 years ago), this exorheic drainage was closed
due to tectonic uplift of the Zagros Mountains. The Kor River basin became
endorheic and isolated from the exorheic Mand River basin. The Kor and Mand
Aphanius populations diverged to become sophiae and pluristriatus
respectively. Habitatat
Unknown in detail. Age and growth
Unknown. Food
Unknown. Reproduction
Unknown.
Parasites and predators
None recorded.
Economic importance
None at present in Iran but a possibly a species of interest to aquarists. Conservation
This species is known only from the Zarjan Spring system but has not been
collected post-2003 because of drought and competition with such exotics as
Xiphophorus hellerii (Esmaeili et al., 2012). It could be extinct and
a thorough survey of all possible habitats should be made. Further work
See above under Conservation.
Sources
Esmaeili et al. (2012), Zarjan being at 29°03'39.44N, 53°36'59.23E
(H. R. Esmaeili, pers. comm., 5 March 2012). Aphanius sophiae
Common names
gour-e khar, kopurdandan-e safiyeh or kopurdandan-e sufieh (= sufi or sophy tooth-carp).
[batrikh sophiae in Arabic; Persian minnow, Cypris pupfish, but this seems inapt].
Systematics
The type locality of Lebias Sophiae is in "lauen Salzquellen bei Persepolis" according to Heckel (1847b).
Lebias punctatus Heckel, 1847 is listed from "Nemek-Deria oder
Salzsee, in welchen sich unter Schiraz die Quellen des Saadi ergiessen"
(Nemek-Deria
or
salt lake
below Shiraz into which the Saadi springs flow), i.e. Lake Maharlu in the Shiraz
Valley, the spring at Sa'adi's Tomb being a tributary, if somewhat distant (Heckel, 1847b).
Lebias
crystallodon is from "grossen Salzsee Nemek-Deria, unter Schiraz" (Heckel, 1847b).
Both punctatus and crystallodon are synonyms (Günther, 1859-70; Garman, 1895; Gaillard, 1895; Hanko, 1924; Berg, 1949; Coad, 1996a).
The
original catalogue, handwritten by Heckel in Vienna, gives the type locality of
sophiae as "Salzquellen bei Persepolis" which is in agreement with Heckel's publication. The catalogue is more equivocal for the two nominal species,
punctatus and crystallodon, listing only "Nemek Deria".
The labels in jars containing L. crystallodon and L. punctatus were written under Franz Steindachner's curatorship and are not original (H. Ahnelt, in litt., 1987). Nemek Deria (=
salt lake in Farsi) is a general term employed for the type of terminal water body seen in both the Maharlu and Kor River basins. The situation was further confused by Berg (1949) who listed the locality
of punctatus as "Lake Niriz in Shiraz". Lake Niriz (= Neyriz or Bakhtegan) is the terminal sump for the Kor River.
All material of Aphanius from the Kor River basin examined fresh by me comprises fish with bars along the flank and fish with spots. The barred fish are males and the spotted fish females as
confirmed by dissection. Males and females do not segregate in the field and it seems very unlikely that the material examined by Heckel from several collections would be comprised solely of males (I found
sample sizes as low as 5 to contain both males and females). The males and females may have been separated and allocated different collection localities. The material described as L. punctatus is
then female L. sophiae and I strongly suspect that it was misunderstood as coming from the Shiraz valley and the locality in Heckel (1847b) is incorrect. All female Aphanius from the
Shiraz valley are barred and the name punctatus is singularly inappropriate and would not be applied to them (see illustrations in Villwock (1960; 1977)). L. crystallodon could be a female
L. sophiae with weak spotting on the flanks, again mislabelled as from the Shiraz valley. Günther (1866) considers crystallodon to be a post-spawning female of L. sophiae and
Gaillard (1895) also considers it to be a female L. sophiae. Berg (1949) places L. sophiae as the male and L. punctatus and L. crystallodon the females of L. sophiae.
Cyprinodon blanfordiiii Jenkins, 1910 may well be a synonym of A. sophiae (see above under A.
farsicus for other views). The locality is vague, "East of Shiraz, South
Persia" and could lie in the Lake Maharlu basin (occupied only by A. farsicus, the females of which are barred on the flank), the Kor River basin (A. sophiae only), or outside either of
these, in rivers draining to the Persian Gulf. The illustration shows a finely-spotted fish with a lozenge-shaped larger spot at the tail base, pigmentation found in A. sophiae.
Parenti (1981; 1984) places Aphanius sophiae in the genus "Aphanius", i.e. distinct from true Aphanius without defining and naming a new genus. This genus is more closely
related to the Anatolian Kosswigichthys and the South American Orestias than true Aphanius.
The syntypes of Lebias sophiae are in the Naturhistorisches Museum Wien and include a wide range of material of which the following is part:-
Lectotype and paralectotypes (as labelled in 1997): NMW 14496 (6 fish measuring 25.8-30.4 mm standard length, one of which measuring 29.3 mm standard length is the lectotype as designated by F. Krupp,
24 October 1984 according to a jar label), NMW 22616-22623 (8, 26.1-29.0 mm standard length), NMW 60327 (8, 25.2-31.1 mm standard length), NMW 68283 (8, 18.9-27.6 mm standard length, partially dried and
distorted), NMW 75067 (7, 24.7-27.4 mm standard length), and probable syntypes NMW 14760 (67) and NMW 15056 (7). The catalogue in Vienna lists 40 fish in one column and 12 fish in the adjacent column, the
latter possibly meant to be the type series as it is written more boldly but which fish these were cannot now be ascertained. All these fish are males.
Eschmeyer et al., (1996) list syntypes under NMW 14496 (6), NMW 33616-23 (8) (not noted above), NMW 60327 (8), NMW 68283 (8), NMW 75067 (7) and also 9 fish in the Museum für Naturkunde,
Universität Humboldt, Berlin under ZMB 31377 (not noted above). The Berlin material is listed as ex 16140, is all male and 20.3-26.7 mm standard length.
Syntypes of Lebias punctatus comprise the following material:-
Lectotype and paralectotypes (as labelled in 1997): NMW 76509 (7 fish, measuring 29.9-36.0 mm standard length, one of which measuring 36.0 mm standard length is the lectotype as designated by F. Krupp,
24 October 1984 according to a jar label), NMW 15070 (5, ca. 29.9-35.6 mm standard length, dried and distorted), NMW 15156 (14, 19.2-32.1 mm standard length), NMW 59837 (5, 26.6-35.7 mm standard length),
and probable syntypes NMW 59609 (6, ca. 21.4-34.7 mm standard length, smallest dried). The Vienna catalogue lists only 12 fish opposite this name. These fish are all females.
The holotype of Lebias crystallodon measuring 40.1 mm standard length is NMW 15175 as the description and catalogue agree there is only one fish. The specimen is decoloured and in poor condition.
Key characters
The large, dark, usually lozenge-shaped spot at the caudal fin base in females with fine spotting on the flanks is distinctive for tooth-carps in this area of southern Iran.
Morphology
Lateral line scales 25(1), 27(7), 28(14), 29(17), 30(12) or 31(2). Scales above the lateral line 4-7, scales between lateral line and the anal fin 5-8, scales between the lateral line and the pelvic
fin 6-9, and scales around the caudal peduncle 15-20.
Total dorsal fin rays 11(1), 12(11), 13(27), 14(12) or 15(2); total anal fin rays 10(1), 11(18), 12(27) or 13(7); total pectoral fin rays 14(1), 15(7), 16(31), 17(10), 18(3) or 19(1); total pelvic fin
rays 5(9) or 6(44); total gill rakers 10(2), 11(28), 12(21) or 13(2); abdominal vertebrae 11(11), 12(37), or 13(5); caudal vertebrae 15(3), 16(33) or 17(17); and total vertebrae 27(8), 28(29) or 29(16).
Cephalic sensory pores are reduced to a series of neuromasts. Older literature on chromosome number was 2n=48 but is based on fish from outside the Kor River basin to which the species is restricted
here (Klinkhardt t et al., 1995). Esmaeili et al. (2007) determined a chromosome number of 2n=48 with 14 pairs of submetacentric and 10 pairs of subtelocentric chromosomes and an arm number
of NF=76. Karyotype 8Sm + 40St and arm number 280 (Esmaeili et al.,
2008). Sexual dimorphism
Males have more bands on the flank and are darker and more intensely coloured than females. Male dorsal, anal and caudal fins are a deep black with narrow, silvery-white borders and the anal fin has a
few silvery spots at its base. Females have brown dorsal, anal and caudal fins with 3-4 rows of black spots and the anal fin also has 2 cross rows of white silvery spots near the base. The caudal fin spot
in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally, single, smaller, dark, subsidiary spots may be found antero-dorsally and antero-ventrally to
the basal spot or scattered spots may be found irregularly before and behind the basal spot. Male flank bars 10(4), 11(1), 12(2), 13(3), 14(5), 15(4), 16(6), 17(3), 18(1) or 21(1).
Eleven of 21 morphometric characters are significantly different between the sexes including head length, head and body depths, fin lengths, and caudal peduncle shape (Coad, 1998i).
Colour
"The body colouration is basically dark brown; the body, with the exception of the forward dorsal portion and the belly, is marked by 12-17 white, silvery shining vertical lines or narrow bands.
Those individuals which have the most bands and are also darker and more intensively coloured seem to be males; their vertical fins are deep black with narrow silvery-white borders, and only the anal fin
has a few silvery spots at the base. The lighter individuals which are also slightly higher and wider, we believe to be the females; they have brown vertical fins with 3-4 cross rows of black spots;
their anal fins are marked with 2 cross rows of white silvery spots at the base" (Heckel, 1847b).
The caudal fin spot in females can be oval, tear-drop shaped or elongate but is usually in the form of a lozenge. Occasionally single, smaller, dark, subsidiary spots may be found antero-dorsally and
antero-ventrally to the basal spot or scattered spots may be found irregularly before and behind the basal spot.
The flank spots in females are much lighter than the caudal base spot and are from a half to much less in size, usually much smaller than half size. Spots are usually rounded but can be oval and
vertically elongate. Spots are independent of scale arrangement on the lower half of the flank, particularly posteriorly and may form 2-3 poorly defined to distinct longitudinal rows. Upper flank scales
have a crescent of pigment in mid-scale, leaving the margin and anterior base mostly free of pigment, or pigment may fill each exposed scale surface almost entriely except for the posterior margin, and
thus appear as spots. Females have purplish tints on mid-flank and yellowish tints on the lower flank, upper flank, head top and chin.
Recent collections and photographs (see above) show females from the Kor River
basin without the characteristic small spots of the type series and collections
made in the 1970s. It is not known whether the is natural variation or the
result of translocation of specimens.
Males have a pigmentation very similar to that of A. farsicus and the description here is identical. Some minor observed variation is attributed to variation in size and maturity of the fish
compared. Males have light flank bars half the width or much narrower than alternating dark bars. The margins of the dorsal, anal and caudal fins are clear while the rest of these fins is dark. Some fish
have up to 3, but usually 2, thin, light bars on the basal half of the caudal fin; these are generally larger fish. The margin of the lower half of the pectoral fin has concentrations of pigment on the
membranes such that this area is darker than the rest of the fin. The anal fin is darkest posteriorly where pigment is concentrated on membranes. The distal third of the fin is pigmented to form a dark
band, becoming lighter proximally. The dorsal fin is the darkest fin (except for the clear margin) and the anterior base is the darkest part of the fin.
Bands are not always evident but pigment spots are large proximally. Some fish have 2, sometimes 3, thin light bars at the base separated by thin dark bars and paralleling the body profile while others
have none. The dorsal fin base may have instead a series of lighter spots, sometimes irregular and not paralleling the body profile.
Sizeze
Reaches 5.8 cm total length (Alavi-Yeganeh et al., 2011?check
published paper date).
Distribution
This species is restricted to the Kor River basin in Fars.
Zoogeography
As noted in the genus account, Aphanius species in Southwest Asia are regarded as relicts of the Tethys Sea. The mountain populations of A. sophiae (and A. vladykovi) may well
have risen with the post-Pliocene uplift of the Zagros Mountains. Since Aphanius species have a low dispersal ability (Kosswig, 1967) it is considered unlikely that these small fishes dispersed
from lowland populations into an existing, high mountain range represented today by the Zagros.
Bobek (1963) suggests that there may have been an outflow from the Kor River basin to the Persian Gulf at the south-east corner which was cut off at the end of the Pleistocene by alluvial fans.
Krinsley (1970) maintains that any outlet was closed by the late Pliocene. Evidence for the isolation time of Kor River basin tooth-carps is equivocal.
Habitat
Tooth-carps identified as this species has been reported to survive in waters up to 39°C and salinities as high as 130‰. In aquaria lives at 5-37°C. It is found in springs, streams and small pools
and lakes in fresh and saline waters.
Age and growth
Unknown. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 64 fish measuring 2.18-4.64 cm standard length. The a-value was 0.0343 and the b-value 2.787
(a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases).
Alavi-Yeganeh et al. (2011?check published paper date) give length-weight
data on this species from six localities with a (intercept) being
0.0087and b (slope) being 3.333 for all localities and sexes combined. Food
Unknown in detail.
Reproduction
Unknown. Incubation is said to be 10 days at 25°C but this is a general citation for Aphanius spp. in aquaria.
Parasites and predators
Unknown.
Economic importance
This species has importance as an aquarium species, being colourful and hardy.
Conservation
Construction of irrigation canals and drying of some springs because of man-induced changes in the water table may threaten populations of this species.
Further work
The distribution of this species and its population numbers should be documented by field work. Molecular and chromosomal techniques would prove useful information for relating this species with others
from neighbouring basins and throughout central Iran. Its biology is poorly known under natural conditions.
Sources
Type material: See above, Lebias sophiae (NMW 14496, 22616-22623, 60327, 68283, 75067, 14760 and 15056, Lebias crystallodon (NMW 15175), and Lebias punctatus
(NMW 76509, 15070, 15156, 59837 and 59609).
Iranian material:- CMNFI 1979-0025, 1, 31.6 mm standard length, Fars, Kor River at Marv Dasht (29º51'N, 52º46'30"E);
CMNFI 1979-0059, 30, 18.1-39.3 mm standard length, Fars, Pulvar River 8 km south of Sivand (30º01'30"N, 52º57'E);
CMNFI 1979-0061, 3, 23.2-32.1 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0062, 6, 24.0-42.0 mm standard length, Fars, spring 17 km south of Sa'adatabad (30º05'N, 53º00'E);
CMNFI 1979-0067, 1, 25.7 mm standard length, Fars, qanat at Zarqan (ca. 29º46'N, ca. 52º43'E);
CMNFI 1979-0071, 6, ? mm standard length, Fars, qanat stream on road to Ramjerdi (ca. 30º00'N, ca. 52º38'E);
CMNFI 1979-0091, 3, 23.2-32.1 mm standard length, Fars, stream tributary to Pulvar River (30º04'N, 53º01'E);
CMNFI 1979-0117, , mm standard length, ; fish?
CMNFI 1979-0155, 2, ? mm standard length, Fars, spring at Gavonoo (28º47'N, 54º22'E);ID?
CMNFI 1979-0292, 6, 26.5-34.4 mm standard length, Fars, Lapu'i spring near Zarqan (29º48'N, 52º39'E);
CMNFI 1979-0342, 1, 39.4 mm standard length, Fars, Kor River at Band-e Amir (29º49'N, 52º51'E);
CMNFI 1979-0498, 6, 17.4-25.2 mm standard length, Fars, spring in Kor River basin (30º05'N, 52º27'E);
CMNFI 2004-0003 (GenBank AY593483, AY593492), 47, ?20.7-28.8 mm standard length, Fars, Abdolmahdi Spring (30º06'12"N, 52º58'38"E);
CMNFI 2004-0004 (GenBank AY593482, AY593492), 38, ?19.5-34.0 mm standard length, Fars, Malasskuh Spring (29º52'04"N, 52º29'20"E);
uncatalogued (GenBank AY593481, AY593490), 6, 25.7-37.0 mm standard length, Fars, Dolatabad Spring (29º43'05"N, 52º50'11"E);
Aphanius vladykovi
Male
Female
and male, Mand River basin courtesy of
H. R. Esmaeili
(Heckel, 1847)
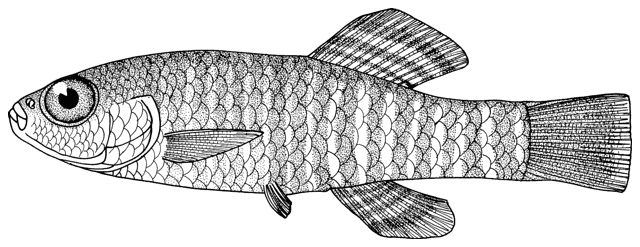
Male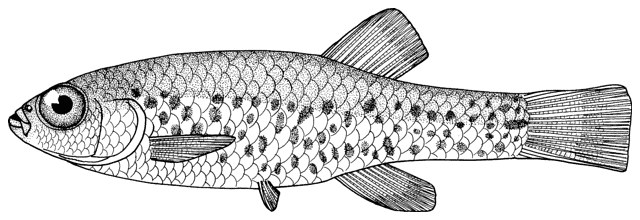
Female
Cyprinodon blanfordii female after
Jenkins (1910)0)
Male courtesy of H. R. Esmaeili, A. Parsi, Z. Gholami and M.
Tahami
Male courtesy of H. R. Esmaeili, A. Parsi, Z. Gholami and M.
Tahami
Female courtesy of H. R. Esmaeili, A. Parsi, Z. Gholami and M.
Tahami
Coad, 1988
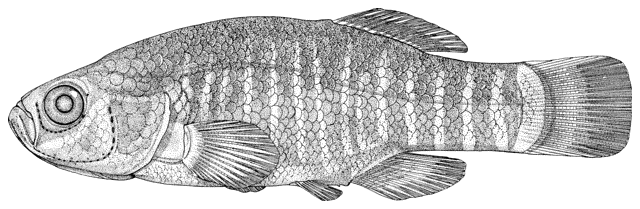
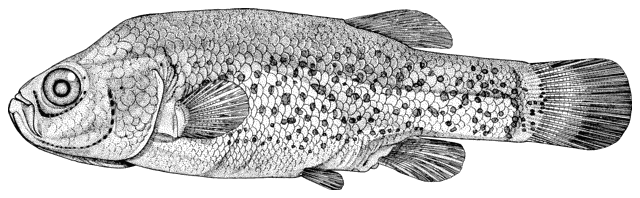
Female
Male, 

Female, courtesy of H. R. Esmaeili

Female, Gandoman, courtesy of H. R. Esmaeili
Common names
gour-e khar, mahi-e gour-e khari (= "striped donkey" or zebra fish), kopurdandan-e Zagros (= Zagros tooth-carp), mahi parchami (= flag fish).
[Zagros pupfish].
Systematics
The type locality is in the Shahrestan-e Bakhtiari va Chahar Mahall in a large pool, 3 km west of Boldaji at 31°57'N, 51°01'E. The male holotype, 36.6 mm standard length, is in the Canadian Museum of Nature, Ottawa under CMNFI 1979-0247 with 35 male and 16 female paratypes from the same locality under CMNFI 1979-0247A and 1 male paratype from a stream 3 km east of Boldaji at 31°55'N, 51°05'E under CMNFI 1979-0248. Villwock (2004) has used cross-breeding experiments that demonstrate this species, A. sophiae and A. dispar are genetically isolated and distinct species.
Key characters
This species is distinguished from all other Aphanius in Iran and Southwest Asia by the higher number of normal-sized lateral line scales (36-47). Other members of Aphanius, and the related Kosswigichthys, have 35, usually 30, or less in the lateral series. The high number of scales is matched only by the genus Anatolichthys Kosswig and Sozer, 1945 of southwest Anatolia. Anatolichthys is polymorphic for scale counts which reach as high as 55 and shows scale loss, perhaps associated with the bitter, salty lakes in which it lives (Coad, 1988i). Male dorsal fin colour is much darker and the anal fin is light compared to A. sophiae, which most closely resembles A. vladykovi. Females lack the typical large spot at the base of the caudal fin found in A. sophiae.
Morphology
Scales are relatively small and numerous over the whole body as exemplified by scale counts. Scales are regularly arranged, embedded and imbricate. Anterior flank scales are a vertical oval with circuli and radii restricted to the posterior field. Numbers of circuli and radii are body and scale dependent and so are fewer in this species than in A. sophiae. There are up to 21 teeth in the upper jaw and teeth are in a single row in each jaw. Gill rakers are short, just reaching the adjacent raker when appressed. All fins are rounded distally and the anal fin is enclosed by a fleshy sheath, most apparent in large females but is also present in males. The gut has a single large loop. Cephalic sensory pores are reduced to a series of neuromasts. Keivany (2003) gives details of cephalic osteology. Pazooki et al. (2008) and Mardani Karani et al. (2008) examined interlocality morphological variation, finding one of four populations to be more distinctive and 55 of 72 characters different respectively. Esmaeili et al. (2009) give a chromosome number of 2n = 48 and a karyotype of 8 submetacentric and 40 subtelocentric chromosomes with an arm number of 28.
Lateral line scales 36(2), 37(8), 38(15), 39(6), 40(10), 41(6), 42(3), 43(1), 46(1) or 47 (1). Scales above the lateral line 6-9, scales between lateral line and the anal fin 8-12, scales between the lateral line and the pelvic fin 11-15, and scales around the caudal peduncle 20-25.
Total dorsal fin rays 11(4), 12(21), 13(27) or 14(1), total anal fin rays 11(13), 12(25) or 13(15), total pectoral fin rays 14(4), 15(18), 16(25) or 17(6), total pelvic fin rays 5(9), 6(42), 7(1) or 8(1), total gill rakers 10(8), 11(41), or 12(4), and total vertebrae 27(2), 28(24), 29(26) or 30(1). Saefali (1999) records 8-11 gill rakers, 9-14 anal rays, and 9-15 dorsal rays.
Sexual dimorphism
Colour is sexually dimorphic and is outlined below. Ten of 21 morphometric characters are significantly different between the sexes including head length, head and body depths, fin lengths, and caudal peduncle shape (Coad, 1998i). Females attain larger sizes than males (76 mm versus 58 mm total length (Keivany and Soofiani, 2004a; b)).
Colour
Live adult males bear creamy bars on yellowish flanks. The pelvic fins and the edge of the caudal fin are yellow, the anal fin has an orange-yellow edge and the pectoral fins are orange-yellow. The dorsal fin is white with a wide blue-black band in the centre and a narrow blue-black band at the base. In some males the basal band is absent. Live adult females have a slight bluish tinge to their flanks and flank spots are brown.
In preserved specimens, the dorsal fin bands are dark and remain darker in preservative than the pigmentation seen in Aphanius sophiae which is more diffuse, tending to several horizontal rows of speckles, and which is not formed into a strongly distinct band or bands. Other fins are lightly pigmented, being finely speckled with melanophores with the dorsal and pectoral fins the darkest. The anal fin is fleshy and not hyaline but pigmentation is light and not dark as in A. sophiae. There is some tendency to formation of one or two narrow bars on the anal fin, paralleling the base, but these are weakly developed or absent in most fish. A mid-dorsal stripe is not apparent, perhaps because the dorsal flanks and back are darker than in females. Flank bars are light alternating with darker broad bars. The lighter bars become broader posteriorly. There is a light, wide bar at the base of the caudal fin which is not included in the count of male flank bars. Male flank bars 9(3), 10(8), 11(8), 12(10), 13(3), or 14(5). The sides of the head and upper and lower jaws are pigmented with scattered melanophores while the underside of the head is not. The belly is also free of extensive melanophores.
The most characteristic feature of females is the large number of scattered spots on the flank, extending from behind the head to the base of the tail. These spots do not extend to the dorsal flank or the back. The distinctive, large and often lozenge-shaped spot on the central caudal fin base of A. sophiae is absent. The upper back and flank are more heavily pigmented than the flanks (except for the flank spots). The lower surface of the head and the belly are melanophore free as in males and the sides of the head and the upper and lower jaws are heavily pigmented. The dorsal fin is finely pigmented on the rays and membranes and is generally darker than the caudal fin which is also finely pigmented. The other fins have very little pigment but all are fleshy and not hyaline. There is some suggestion of a thin horizontal stripe near the base of the dorsal fin in the largest specimens but this is never well developed.
Teeth are hyaline or discoloured with brown in patches. The peritoneum is dark brown.
Size
Reaches 7.6 cm (Keivany and Soofiani, 2004b).
Distribution
This species is found near Boldaji and in the Chaghakor Wetland in the upper reaches of the Karun River basin. Abdoli (2000) indicates its presence on a map in the upper Marun River and the upper Khersan River (the latter in the Karun basin).
Zoogeography
The origins of this mountain species have been discussed above under the account for A. sophiae.
It is found only in the uppermost reaches of the Karun River basin which drains to the head of the Persian Gulf. In this locality it is isolated from populations of Aphanius in the lowlands by a series of tangs which block gene flow. Oberlander (1965) in studying the origin of Zagros drainage patterns found that streams did not follow the line of least resistance but often incise the higher parts of mountain ridges which are anticlines composed of Miocene Asmari limestone. This situation arises because the Zagros ridges were eroded by a superposed drainage pattern already antecedent to the exhumation of overlying sediments of later Miocene marls and evaporites. The trend of the Zagros ridges is in a NW-SE direction and streams often cross this direction at right angles. This has resulted in the development of the tangs, narrow clefts in the Zagros ridges with very swift waters, which may be up to 2400 m deep with vertical sides rising from the water surface for 300 m, effective barriers to small fishes.
Habitat
This species is found high in the Zagros Mountains (the type locality lies at an altitude of about 2380 m). It is found in small pools, streams and marshes, in fresh water. The type locality is an artificially dammed pool about 300 m wide with cloudy water and a mud and pebble bottom. Capture depth was 40 cm. The shore was grassy and the pool contained large amounts of Myriophyllum and marginal rushes. At 1515 hours on a warm, windy and sunny day, water temperature was 29°C, pH was 6.5 and conductivity was 0.2 mS. A second locality in Coad (1988i) was a mud-bottomed stream 3-4 m wide with pools up to 1 m deep. At 1545 hours water temperature was 22°C, pH was 6.2 and conductivity was 0.45 mS. Current was slow and the stream had moderate amounts of Myriophyllum. Keivany and Soofiani (2004b) reporting on the habitat at the Madar-Dokhtar spring near Gandoman, found water conductivity of 240-280 μS, pH 6.9-8.5 and total dissolved solids125-138 mg/l. The bottom was muddy, interspersed with pebbles, and water is clear (but cloudy in the river). Myriophyllum and Potamogeton were used for spawning. Fish survive 30‰ and 30°C in aquaria.
Pazooki et al. (2008) found females to be more numerous than males, and the sexes lived in mixed schools. Occasionally larger males would chase smaller males but then both would rejoin the school. Males are territorial and defended against fish and beetles up to twice their size. The species preferred areas free from vegetation. These authors found the species easily tolerated to 33°C in aquaria for long periods, and the optimum temperature for the species is probably 21-24°C.
A mass mortality of this species was observed by A. Abdoli in late March or early April 1994 at Chagh-khor wetland near Boldaji.
Bagheri (1999) describes the macrofauna and environment of the Chaghakhour Lagoon where this species occurs.
Age and growth
Keivany and Soofiani (2004b) show maximum age is 2+ years. Esmaeili and Ebrahimi (2006) give a significant length-weight relationship based on 319 fish measuring 1.46-4.64 cm standard length. The a-value was 0.0309 and the b-value 3.062 (a b-value < 3 indicating a fish that becomes less rotund as length increases and a b-value >3 indicating a fish that becomes more rotund as length increases). Alavi-Yeganeh et al. (2011?check published paper date) give length-weight data on this species from four localities with a (intercept) being 0.0087and b (slope) being 3.414 for all localities and sexes combined.
Food
Abdoli (2000) reports Daphnia and Gammaridae as food. The main food in the Madar-Dokhtar spring is Gammarus, but a specimen from a river also had some algae, Cyclops sp., snail eggs and fish larvae. Mouth structure would lead to the conclusion that this species is a surface feeder but it concentrated on the dominant crustacean in the Madar-Dokhtar spring (Keivany and Soofiani, 2004b). Pazooki et al. (2008) found chironomids, dipterans, Diaptomus, Daphnia, diatoms and filamentous algae in guts and considered this species to prefer an animal diet.
Reproduction
Mature males chase the female in aquaria, pushing her into a corner, and showing pectoral fin flipping and shivering. Eggs were laid in a batch of 3-10. Absolute fecundity in the Madar-Dokhtar spring examined by Keivany and Soofiani (2004b) was 220-650 eggs, relative fecundity was 45-155 per gram body mass and working fecundity (free and ripe eggs) was 110 eggs absolute and 73 relative. Size of ripe eggs was 0.8-1.2 mm. Eggs are adhesive and attch to plants in small patches, from 3 to 30. Early April was the spawning peak. Eggs hatched at 9-13 days at 21-22ºC. Young reached 11.0-27.2 mm after three months in an aquarium.
Parasites and predators
Barzegar et al. (2004) examined this species for parasites in fish from the Beheshtabad river in Chahar Mahall va Bakhtiari Province and found Lernaea cyprinacea. Keivany and Soofiani (2004b) found it to be susceptible to trichodiniasis and ichthyophthiriasis in aquaria. Barzegar et al. (2008) record eye parasites from this fish including the digeneans Diplostomum spathaceum, Tylodelphys clavata and Ornithodiplostomum sp. Raissy (2008) also investigated parasites in this species from the Gandoman Lagoon. Barzegar and Jalali (2009) reviewed crustacean parasites in Iran and found Lernaea cyprinacea on this species.
Economic importance
This pretty little fish has great potential as an aquarium species (Coad and Keivany, 1998; Keivany and Soofiani, 2004b). It has been maintained in an aquarium for several months, tolerating temperatures of 30°C but probably favouring 20-22°C. pH values were 8-9. Water changes with their associated variations in conditions had no affect on the fish. The pupfish survived 3-4 hours longer than a Capoeta species when the latter was treated with formalin in a shared tank for a Saprolegnia infection and the tank was inadvertently left for 6-8 hours without a water change. Male pupfish are very aggressive and frequently attack other fishes of the same size or even larger. They ate all the coexisting guppy larvae in the aquarium, even those aged 2-3 weeks. The pupfish was fed crushed, dried freshwater shrimp and sometimes live shrimp although they had difficulty handling live shrimp. They were also observed biting and destroying vegetation in the aquarium, although it is unclear whether they actually ate any. It is now available in aquarium stores in Germany (Oliver Lucanus, pers. comm., 23 January 2004) and is kept during summer months in garden ponds, reproducing there successfully (Thomas Schulz, pers. comm., 14 January 2005).
Conservation
The distribution of the species is apparently quite limited, in waters neighbouring the towns of Boldaji and Shahr-e Kord. This should be confirmed by extensive field work in this poorly explored area of the Zagros Mountains. A limited distribution renders this species liable to significant loss or even extinction if habitats are disturbed or destroyed. The type locality, it should be noted, is an artificially dammed pond.
Significantly, a mass mortality of this species was observed by A. Abdoli in late March or early April 1994 at Chagha Khur wetland although the cause is unknown. M. Raissy (pers. comm., 1 July 2009) reports parasitic infections (7 species, including the digenean Ornithodiplostomum in most organs) as a cause for depopulation in Choghakhor (Chagha Kur) lagoon.
An additional threat is escapes of cultured rainbow trout (Oncorhynchus mykiss). Trout culture is being rapidly developed in this region and the trout are known predators on native fishes.
Further work
The distribution of this species in the Zagros needs to be clarified.
Sources
Type material: See above, CMNFI 1979-0247, CMNFI 1979-0247A and CMNFI 1979-0248.
Iranian material: CMNFI 2004-0005, ?, ?, mm standard length, ; uncatalogued (GenBank AY593486, AY593495), 4, 25.6-37.0 mm standard length, Chahar Mahall va Bakhtiari, Ebrahimabad (31º52'30"N, 51º10'10"E); uncatalogued (GenBank AY593487, AY593496), 2, 21.5-28.7 mm standard length, Chahar Mahall va Bakhtiari, Taqanak Bridge (32º12'35"N, 50º49'29"E); uncatalogued (GenBank AY593485, AY593494), 5, 31.0-49.8 mm standard length, Chahar Mahall va Bakhtiari, Madar-Dokhtar spring (31º52'12"N, 51º08'29"E).
© Brian W. Coad (www.briancoad.com)